chapter 128 Neuropathic Dysfunction of the Lower Urinary Tract
Many of the clinical problems seen in pediatric urology are the result of neurologic lesions that affect lower urinary tract function. As pediatric urology developed in the latter half of the 20th century, urinary diversion initially was the mainstay of treatment for these children with either intractable incontinence or normal or abnormal upper urinary tracts (Smith, 1972). The advent of clean intermittent catheterization (CIC) in the early 1970s by Lapides and associates (1972), refinements in techniques of urodynamic studies in children (Gierup and Ericsson, 1970; Blaivas et al, 1977; Blaivas, 1979), and the development of surgical modalities in conjunction with CIC to manage incontinence dramatically changed the way this group of children was traditionally managed. Most pediatric urologic centers consider functional assessment of the lower urinary tract as an integral element in the evaluation process and almost if not as important as radiographic visualization in characterizing and managing these abnormal conditions (McGuire et al, 1981; Bauer et al, 1984). The natural outcome of early functional investigation has been the advocacy of proactive or early aggressive management in the children who are at risk for urinary tract deterioration based on specific hostile urodynamic parameters (Perez et al, 1992; Edelstein et al, 1995; Kaufman et al, 1996). This has resulted in (1) a statistically significant decrease in upper urinary tract deterioration, compared with those children followed expectantly in the past, and (2) a dramatic reduction in the need for augmentation cystoplasty (Wu et al, 1997; Kaefer et al, 1999; Bauer, 2000). Furthermore, there is now evidence that molecular changes occur intracellularly when prophylactic treatment is begun as early as possible (Park et al, 1999; Ohnishi et al, 2000). Because urodynamic testing has become such an integral part of any discussion of the subject, the testing process as it applies to children is discussed first and then various neurologic abnormalities that are prevalent in children are elaborated upon. For purposes of minimizing ambiguity the terminology used in this chapter is based on the International Continence Society (ICS) standardization of terminology documents (Abrams et al, 2002, 2003; Neveus, 2006).
Urodynamic Evaluation
Before a urodynamic study is performed it is important for the child and the family to have full knowledge of the procedure. Therefore an explanation of the test is sent to each family before the appointment. Attempts are made to minimize anxiety in children by providing reasons for specific portions of the test and explaining exactly what the child will experience. Premedication is rarely given. Parents are instructed to make sure the child has a bowel movement the night before the study.
In males a topical anesthetic cream is applied to the perineum about 45 minutes before the electromyography (EMG) needle is inserted to record sphincter activity. If possible, the child is instructed to come to the urodynamic suite with a full bladder to obtain an initial representative uroflow. The time of the child’s previous urination (or catheterization) is noted to calculate an average rate of urine production per unit of time. The flowmeter is located in a private bathroom that contains a one-way mirror so that voiding can be viewed unobtrusively. This allows the investigator to see what position the child assumes when voiding and whether a Credé or a Valsalva maneuver is employed by the child to help empty.
Next, the nurse reviews the test and shows the child all the equipment in an attempt to make the patient feel as comfortable as possible. The child is catheterized with either a No. 7 Fr or a No. 11 Fr triple-lumen urodynamic catheter (Cook Urological, Inc, Spencer, IN)—the smaller, the better—after a small amount of liquid lidocaine (Xylocaine) (1%) has been injected into the urethra and held in place for a moment or two. First, the intravesicular pressure is recorded. Then the bladder is drained and the residual urine is carefully measured, yielding a pressure at residual volume (Kaefer et al, 1997). Sometimes it is necessary to aspirate the catheter to get the accurate volume of residual urine, especially if the bladder is underactive (particularly if the child is taking anticholinergic medication) or has been previously augmented and secretes mucus (because it may not drain completely after insertion of a catheter).
A small balloon catheter is passed into the rectum at this time to measure intra-abdominal pressure during the cystometrogram to identify artifacts of motion and monitor increases in abdominal pressure during the filling and emptying phases of the study (Bates et al, 1970; Bauer, 1979).
Before the bladder is filled, a urethral pressure profile is sometimes obtained by infusing saline through the side-hole channel at a rate of 2 mL/min as the catheter is withdrawn at a rate of 2 mm/sec (Yalla et al, 1980). Many centers have eliminated urethral pressure profile measurements in favor of monitoring detrusor leak point pressure (the subtracted detrusor pressure at which the child leaks when the bladder is filled to its functional or maximal capacity). Abdominal leak point pressure is the abdominal pressure at which the child leaks when straining or coughing but that is not as accurate as the detrusor leak point pressure, which is a proportional reflection of outlet resistance (McGuire et al, 1996).
External urethral sphincter EMG is performed using a 24-gauge concentric needle electrode (Diokno et al, 1974; Blaivas et al, 1977b) inserted perineally in boys or paraurethrally in girls and advanced into the skeletal muscle component of the sphincter until individual motor unit action potentials are seen or heard on a standard EMG recorder. Alternatively, perineal electrodes (Maizels and Firlit, 1979) or abdominal patch electrodes (Koff and Kass, 1982) have been used to record the bioelectric activity in the sphincter muscle. Disagreements exist concerning the accuracy of these surface electrode measurements, compared with those obtained with needle electrodes, particularly during voiding. The intactness of sacral spinal cord function is easily measured with needle electrodes by (1) looking at the characteristic waveform of individual motor unit action potentials when the patient is relaxed and the bladder is empty, (2) performing and recording the responses to bulbocavernosus and anal stimulation and to Credé and Valsalva maneuvers, (3) asking the patient to voluntarily contract and relax the external sphincter, and (4) seeing the reaction of the sphincter to filling and emptying of the bladder (Fig. 128–1) (Blaivas et al, 1977a; Blaivas, 1979b).
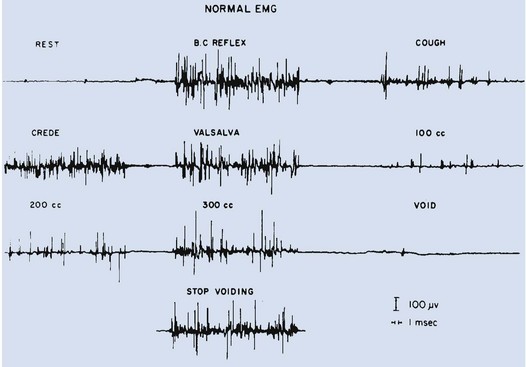
Figure 128–1 Normal reaction of the external urethral sphincter on electromyogram to all the sacral reflexes and to bladder filling and emptying.
(From Bauer SB. Urodynamic evaluation and neuromuscular dysfunction. In: Kelalis PP, Kin LR, Belman AB, editors. Clinical pediatric urology. 2nd ed, vol. 1. Philadelphia: WB Saunders; 1985. p. 283–310.)
The rate of bladder filling per minute is selected by first calculating the child’s predicted or known capacity (see Chapter 127) and then dividing the result by 10 in order to fill the bladder slowly. In children with myelodysplasia, the average bladder capacity (in milliliters) fits the following formula: 24.5 × age in years + 62 (Palmer et al, 1997). It has been shown that more rapid filling rates may yield falsely low levels of detrusor compliance and may minimize an overactive detrusor (Turner-Warwick, 1975; Joseph, 1992). The bladder is filled slowly with saline warmed to 37° C to avoid this problem (Chin-Peuckert et al, 2004). When it is important to determine very mild degrees of poor compliance, even slower rates of filling are used to measure its true incidence (Kerr et al, 1994; Yeung et al, 1995; Zermann et al, 1997). Some investigators have recorded pressure at residual volume to measure natural filling pressure as the most accurate means of denoting poor compliance (Kaefer et al, 1997; Walter et al, 1998). During filling, it is helpful to try to divert the child’s attention by asking unrelated questions, reading a story aloud, or showing a movie or cartoon. If the examiner wishes to elicit detrusor overactivity, the child is asked to cough (Mayo, 1978); alternatively, a cold solution may be instilled at a rapid rate. Most urodynamic parameters do not change with a child’s position (sitting vs. supine). However, the sitting position is preferred for the detection of detrusor overactivity and incontinence (Lorenzo et al, 2007).
The study is not considered complete until the child urinates and voiding pressures are measured in those children who urinate on their own. The small size of the urodynamic catheter does not seem to affect micturition pressures adversely, even in very young children. The normal voiding pressure varies from 55 to 80 cm H2O in boys and from 30 to 65 cm H2O in girls (Blaivas et al, 1977b; Blaivas, 1979a; Gierup et al, 1979). Infants tend to have higher voiding pressures than older children. Sometimes it is difficult to get the child to void; patience and time are needed in this situation. Placing a urine collection device under the buttocks of the child when supine or dripping tepid water on the genital area often stimulates the child sufficiently to begin urinating. Once the child has voided, it is important to do the following:
Video-urodynamics has gained popularity since its introduction in 1970 (Bates et al, 1970). Visualization of the bladder and bladder neck during filling and of the urethra during voiding has added a dimension for more accurately integrating all aspects of lower urinary tract function and characterizing an abnormality (Glazier et al, 1997). Incompetency of the bladder neck or pelvic floor or the location of any posterior urethral obstruction can be correlated with pressure measurements and external urethral sphincter EMG activity recorded simultaneously (Zerin et al, 1990). Sometimes, aberrations noted on pressure monitoring of sphincter EMG tracings can be confirmed, enhancing these findings (Passerini-Glazel et al, 1992; Weerasinghe and Malone, 1993). Ambulatory urodynamics has become available and feasible in children with the development of microtransducers mounted on small catheters positioned in the bladder (McInerney et al, 1991; Kulseng-Hanssen and Klevmark, 1996).
Often, because it is appropriate to see the effects of drug administration, studies are repeated under similar circumstances several weeks to months later. This is especially true when one is trying to treat reduced detrusor compliance or overactivity medically. To determine the maximal effect, the usual dose of most drugs is taken 2 to 3 hours before the test is performed a second time. The most commonly used drugs that affect lower urinary tract function are listed in Table 128–1, along with their appropriate dose ranges.
Table 128–1 Drugs That Affect Lower Urinary Tract Function
| TYPE | Dosage† | |
|---|---|---|
| Minimum | Maximum | |
| Cholinergic | ||
| Bethanechol (Urecholine) | 0.7 mg/kg tid | 0.8 mg/kg qid |
| Anticholinergic | ||
| Propantheline (Pro-Banthine) | 0.5 mg/kg bid | 0.5 mg/kg qid |
| Oxybutynin (Ditropan) | 0.2 mg/kg bid | 0.2 mg/kg qid |
| Glycopyrrolate (Robinul) | 0.01 mg/kg bid | 0.03 mg/kg tid |
| Hyoscyamine (Levsin) | 0.03 mg/kg bid | 0.1 mg/kg tid |
| Tolterodine (Detrol) | 0.01 mg/kg bid | 0.04 mg/kg bid |
| Trospium (Sanctura)* | 10 mg/day | 20 mg/day |
| Solifenacin (Vesicare)* | 5 mg/day | 10 mg/day |
| Darifenacin (Enablex)* | 7.5 mg day | 15 mg day |
| Propiverine (Detrunorm)* | 0.1 mg/kg bid | 0.4 mg/kg bid |
| α-Sympathomimetic | ||
| Phenylpropanolamine | 2.5 mg/kg tid | 2.5 mg/kg qid |
| Ephedrine | 0.5 mg/kg tid | 1.0 mg/kg tid |
| Pseudoephedrine | 0.4 mg/kg bid | 0.9 mg/kg tid |
| α-Sympatholytic | ||
| Prazosin (Minipress) | 0.05 mg/kg bid | 0.1 mg/kg tid |
| Phenoxybenzamine | 0.3 mg/kg bid | 0.5 mg/kg tid |
| β-Sympatholytic | ||
| Propranolol | 0.25 mg/kg bid | 0.5 mg/kg bid |
| Smooth Muscle Relaxant | ||
| Flavoxate (Urispas) | 3.0 mg/kg bid | 3.0 mg/kg tid |
| Dicyclomine (Bentyl) | 0.1 mg/kg tid | 0.3 mg/kg tid |
| Other | ||
| Imipramine (Tofranil) | 0.7 mg/kg bid | 1.2 mg/kg tid |
* Studies evaluating safe dosing in children have not been completed.
Neurospinal Dysraphisms
Myelodysplasia
The most common cause of neurogenic bladder dysfunction in children is abnormal development of the spinal canal and internecine spinal cord. Formation of the spinal cord and vertebral column begins at about the 18th day of gestation. Closure of the canal proceeds in a caudal direction from the cephalad end and is complete by 35 days. The exact mechanism that results in closure and what produces a dysraphic state are yet to be elucidated, but numerous factors have been implicated. The incidence was reported as 1 per 1000 births in the United States (Stein et al, 1982), but there has been a definite decrease in this rate in the past 20 years (Laurence, 1989; Lary and Edmonds, 1996; CDC, 2004; Williams et al, 2005). With the advent of prenatal screening, many affected fetuses are being identified and the pregnancies terminated before 16 weeks of gestation, thereby lowering the number of children born with this disease (Palomaki et al, 1999). The addition of folate supplements to the diet of women of childbearing age has also reduced the incidence of spina bifida worldwide (Committee on Genetics, American Academy of Pediatrics, 1999). The incidence of myelodysplasia (per 1000 live births) in the general population is 1.0, for a mother with one affected child it is 20 to 50, and for a patient with myelodysplasia it is 40 (Scarff and Fronczak, 1981; Stein et al, 1982). Therefore when the disease is already present in a family, the Medical Research Council Vitamin Study Group recommends that women of childbearing age take 4000 µg (4.0 mg) of folic acid per day beginning at least 2 months before the time they plan on becoming pregnant (Committee on Genetics, American Academy of Pediatrics, 1999). There is strong evidence that folate deficiency can lead to a myelodysplastic abnormality de novo; therefore, maternal ingestion of 400 µg of folate per day in all women of childbearing age can reduce the incidence of spina bifida by 50% (Laurence et al, 1981; MRC Vitamin Study Research Group, 1991; Czeizel and Dudas, 1992; Centers for Disease Control and Prevention, 2004). It is now mandatory that enriched grain products be fortified with folic acid (U.S. Food and Drug Administration, 1996). Since these recommendations have been followed worldwide, there has been a reduction in neural tube defects in the United States (Williams et al, 2005) and elsewhere (Botto et al, 2005). In some cases, however, despite increased folic acid intake, women may still give birth to a child with spina bifida owing to antibodies that have developed in response to the increased folate ingestion, thus negating its salutary effect (Rothenberg et al, 2004).
Pathogenesis
Myelodysplasia is an all-inclusive term used to describe the various abnormal conditions of the vertebral column that affect spinal cord function. More specific labels for each abnormality include the following: a meningocele occurs when just the meninges but no neural elements extend beyond the confines of the vertebral canal; in a myelomeningocele, neural tissue, either nerve roots or portions of the spinal cord, has evaginated with the meningocele (Fig. 128–2); the term lipomyelomeningocele denotes that fatty tissue has developed with the cord structures and both are extending with the protruding sac. Myelomeningocele accounts for more than 90% of all open spinal dysraphic states (Stark, 1977). Most spinal defects occur at the level of the lumbar vertebrae, with the sacral, thoracic, and cervical areas, in decreasing order of frequency, less affected (Bauer et al, 1977) (Table 128–2). An overwhelming number of meningoceles are directed posteriorly, but on rare occasions they may protrude anteriorly, particularly in the sacral area. Usually the meningocele is made up of a flimsy covering of transparent tissue, but it may be open and leaking cerebrospinal fluid. For this reason urgent repair is necessary, with sterile precautions being followed in the interval between birth and spinal canal closure. In 85% of affected children there is an associated Arnold-Chiari malformation, in which the cerebellar tonsils have herniated down through the foramen magnum, obstructing the fourth ventricle and preventing the cerebrospinal fluid from entering the subarachnoid space surrounding the brain and spinal cord.
Table 128–2 Spinal Level of Myelomeningocele
| LOCATION | INCIDENCE (%) |
|---|---|
| Cervical–high thoracic | 2 |
| Low thoracic | 5 |
| Lumbar | 26 |
| Lumbosacral | 47 |
| Sacral | 20 |
Key Points: Neurogenic Bladder Dysfunction
In the past several years, several centers in the United States have begun closing the defect in fetuses between 19 and 25 weeks of gestation in an attempt to improve the neurologic defect in these children (Adzick et al, 1998; Bruner et al, 1999; Holzbeierlein et al, 2000). Thus far, this procedure has not altered the incidence of abnormal findings in the lower extremities (Farmer et al, 2003) or on urodynamic assessment in the postnatal period (Koh et al, 2003), but, surprisingly, aqueduct obstruction and, consequently, hydrocephalus has not occurred at birth to the same incidence or extent (Johnson et al, 2003). It is possible that leakage of cerebrospinal fluid from the open spinal column accounts for herniation of the posterior brainstem down the foramen magnum, leading to the development of hydrocephalus.
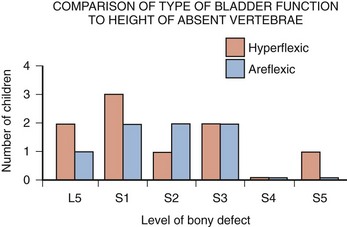
The neurologic lesion produced by the myelomeningocele can be variable, depending on what neural elements, if any, have everted with the meningocele sac. The bony vertebral level often provides little or no clue to the exact neurologic level or lesion produced. The height of the bony level may differ from the highest extent of the neurologic lesion for one to three vertebrae in either direction (Bauer et al, 1977). Furthermore, there may be differences in function from one side of the body to the other at the same neurologic level and from one neurologic level to the next, owing to asymmetry of the affected neural elements. It is important to remember that no two children have the same neurourologic defect. In addition, in 20% of affected children a vertebral bony or intraspinal abnormality occurs more cephalad than the vertebral defect and meningocele, and this can affect function in those portions of the cord. Children with thoracic or upper lumbar meningoceles often have complete reconstitution of the spine in the sacral area, and these individuals frequently have intact sacral reflex arc function involving the sacral spinal roots (in the authors’ series of patients with high-level lesions, 74% of neonates and 54% of older children were affected thusly) (Pontari et al, 1995). In fact, it is more likely that children with upper thoracic or cervical lesions will have just a meningocele and no myelomeningocele. Children with neurologic deficits at S1 or lower may also manifest a variety of findings on urodynamic testing, ranging from normal function to upper and/or lower motor neuron lesions involving either the bladder and/or the external urethral sphincter (Dator et al, 1992). Upper motor neuron lesions are characterized by an overactive detrusor, exaggerated sacral reflexes, absence of voluntary control over sphincter function, detrusor-sphincter dyssynergia (DSD), and no EMG evidence of denervation potentials in the sphincter (White and Klauber, 1976; Koff and DeRidder, 1977; Guzman et al, 1983; Capitanucci et al, 1997). The bladder tends to be thick walled (or trabeculated) with a closed bladder neck on voiding cystourethrography (VCUG) or ultrasonography. A lower motor neuron lesion is noted when an acontractile detrusor and partial or complete denervation of the external urethral sphincter with diminished or absent sacral reflexes may be seen. In these instances the bladder is usually small and smooth walled with an open bladder neck (Wilmshurst et al, 1999). The presence or absence of the bulbocavernosus reflex is an indicator (although not a foolproof one) of an upper or a lower motor neuron lesion, respectively (Schlussel et al, 1994). Approximately 10% of neonates with myelomeningocele exhibit no abnormality on urodynamic testing (Tarcan et al, 2001). Finally, the differing growth rates of the vertebral bodies and the elongating spinal cord add a dynamic factor in the developing fetus that further confounds the picture (Lais et al, 1993). Twenty-four percent of children with normal lower urinary tract function at birth develop upper motor neuron changes over time (Tarcan et al, 2001). Superimposed on all this is the Arnold-Chiari malformation, which can have a profound effect on the brainstem and pontine mesencephalic center, areas that are involved in control of lower urinary tract function.
Therefore the neurologic lesion produced by this condition influences lower urinary tract function in a variety of ways and cannot be predicted just by looking at the spinal abnormality or the neurologic function of the lower extremities. Even careful assessment of the sacral area may not provide sufficient information to make a concrete inference. As a result, urodynamic evaluation in the neonatal period is now the standard of care at most pediatric centers in the United States, because it not only provides a clear picture of the function of the sacral spinal cord and lower urinary tract but also has predictive value in infants at risk for future urinary tract deterioration and progressive neurologic change (McGuire et al, 1981; Van Gool et al, 1982; Bauer, 1984b; Sidi et al, 1986). The advent of hostility scores and the reliability of urodynamic risk factors have led many clinicians to initiate prophylactic therapy in those neonates considered to be at risk for urinary tract deterioration (Perez et al, 1992). There are some, however, who do not consider urodynamic testing an important factor but rely solely on physical examination and radiologic assessment to direct their decision-making process (Hopps and Kropp, 2003), a concept not shared by many.
Neonatal Assessment
Ideally, it would be best to perform urodynamic testing immediately after the infant is born, but the risk of spinal infection and the urgency for closure have not made this a viable option. It was accomplished in one study, however, and the results showed that 1 in 30 children (3.2%) experienced a change in neurologic status as a result of the spinal canal closure (Kroovand et al, 1990). Therefore, renal ultrasonography and measurement of residual urine are performed as early as possible after birth, either before or immediately after the spinal defect is closed. Urodynamic studies are delayed until it is safe to transport the child to the urodynamic suite and place him or her on the back or side for the test. The initial urodynamic study should be performed within the first 3 months of life. If the infant cannot empty the bladder after a spontaneous void or with a Credé maneuver, CIC is begun even before urodynamic studies are conducted. If the Credé maneuver is effective in emptying the bladder, it is performed on a regular basis instead of using CIC until the lower urinary tract can be fully investigated. The normal bladder capacity in the neonatal period is 10 to 15 mL; therefore, a residual urine volume of less than 5 mL is acceptable. Other tests that should be performed in the neonatal period include a urinalysis and culture, serum creatinine determination, and careful neurologic examination of the lower extremities.
Once the spinal closure has healed sufficiently, a renal ultrasonogram is performed to reassess upper urinary tract architecture. Next, VCUG and urodynamic study are conducted. These studies meet several objectives: they provide baseline information about the radiologic appearance of the upper and lower urinary tracts as well as the condition of the sacral spinal cord and the central nervous system (CNS); they provide information that can be compared with findings on subsequent assessments, so that early signs of deterioration of urinary tract function and drainage, or of progressive neurologic denervation, can be detected; they help to identify infants at risk for urinary tract deterioration as a result of a poorly compliant or overactive detrusor or outflow obstruction from DSD, which predetermines the need to initiate prophylactic measures before any deterioration in upper urinary tract architecture and function actually takes place; and they help the physician to counsel parents about the child’s future bladder and sexual function (McGuire et al, 1981; Bauer et al, 1984; Bauer, 1984a; Sidi et al, 1986; Lais et al, 1993). If an abnormality is detected on renal ultrasonography or vesicoureteral reflux is demonstrated, a dimercaptosuccinic acid (DMSA) renal scan is recommended.
Findings
Fifteen to 20 percent of neonates have an abnormal urinary tract on radiologic examination when first evaluated (Bauer, 1985); 3% have hydroureteronephrosis secondary to spinal shock, probably from the spinal canal closure (Chiaramonte et al, 1986), and 15% have abnormalities that developed in utero as a result of abnormal lower urinary tract function in the form of outlet obstruction (Bauer, 2003).
Urodynamic studies in the neonatal period have shown that 63% of infants have bladder contractions. This is also true for an equivalent number of children with upper lumbar or thoracic lesions in whom the sacral spinal cord is spared, 50% of whom have detrusor overactivity (Pontari et al, 1995). Thirty-seven percent have an acontractile detrusor with compliance during filling that is either good (20%) or poor (17%) in this subgroup (Bauer et al, 1984; Bauer, 2003). EMG assessment of the external urethral sphincter demonstrates an intact sacral reflex arc with no evidence of lower motor neuron denervation in 40% of neonates; partial denervation is seen in 24%; and complete loss of sacral cord function is noted in 36% (Lais et al, 1993; Bauer, 2003).
A combination of bladder contractility and external sphincter activity results in three categories of lower urinary tract dynamics: synergic (26%), dyssynergic with and without poor detrusor compliance (37%), and complete denervation (36%) (Fig. 128–3) (Bauer et al, 1984; Sidi et al, 1986; Bauer, 2003). DSD occurs when the external sphincter fails to decrease or actually increases its activity during a detrusor contraction or a sustained increase in intravesical pressure as the bladder is filled to capacity (Blaivas et al, 1986). Frequently, a poorly compliant bladder with high intravesical pressure occurs in conjunction with a dyssynergic sphincter, resulting in a bladder that empties only at high intravesical pressure (Van Gool et al, 1982; Sidi et al, 1986). Synergy is characterized by complete silencing of the sphincter during a detrusor contraction or when capacity is reached at the end of filling. Voiding pressures are usually within the normal range. Complete denervation is noted when no bioelectric potentials are detectable in the region of the external sphincter at any time during the micturition cycle or in response to sacral stimulation or a Credé maneuver.
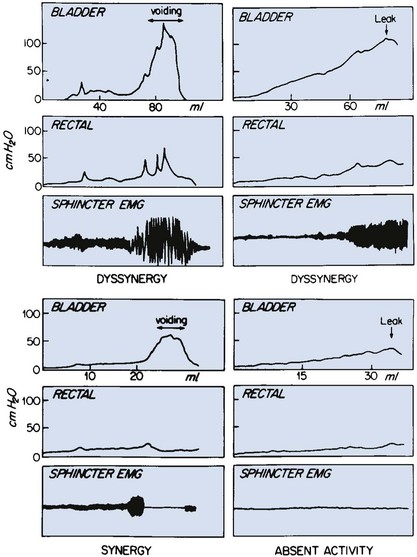
Figure 128–3 Various patterns of urodynamic findings on electromyography (EMG) in neonates with myelodysplasia. Note that a hypertonic detrusor with a nonrelaxing sphincter is also labeled dyssynergy.
(From Bauer SB. Early evaluation and management of children with spina bifida. In: King LR, editor. Urologic surgery in neonates and young infants. Philadelphia: WB Saunders; 1988. p. 252–64.)
The categorization of lower urinary tract function in this way has been extremely useful because it reveals which children are at risk for urinary tract changes, who should be treated prophylactically, who need close surveillance, and who can be monitored at shorter or longer intervals. Within the first 3 years of life, 71% of neonates with DSD had urinary tract deterioration on initial assessment or subsequent studies whereas only 17% of synergic children and 23% of completely denervated individuals developed similar changes (Fig. 128–4). Infants in the synergic group who showed deterioration did so only after they converted to a dyssynergic pattern of sphincter function. Among the infants with complete denervation, the ones who showed deterioration were those who had increased levels of urethral resistance, presumably caused by fibrosis of the skeletal muscle component of the external sphincter. Therefore, it appears that outlet obstruction is a major contributor to the development of urinary tract deterioration in these children (Fig. 128–5). Poor detrusor compliance plays an important role in this regard, especially when outlet resistance exceeds 40 cm H2O (McGuire et al, 1981; Landau et al, 1994; Tanaka et al, 1999). Detrusor compliance seems to be worse in children with high levels of outlet resistance (Ghoniem et al, 1989). Bloom and colleagues (1990) and then Park and associates (2001) noted an improvement in compliance when outlet resistance was reduced after gentle urethral dilation in these children; however, the reasons for this change are unclear, and the long-term effect of this maneuver on ultimate continence and lower urinary tract function remains uncertain.
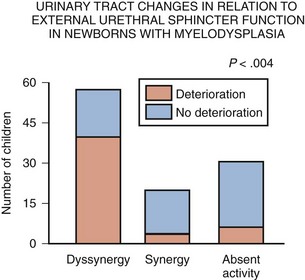
Figure 128–4 Urinary tract deterioration is related to outflow obstruction and is most often associated with dyssynergy. Children with synergy converted to dyssynergy, and patients with complete denervation developed fibrosis with a fixed high outlet resistance in the external sphincter, before any changes occurred in the urinary tract.
(From Bauer SB. Early evaluation and management of children with spina bifida. In: King LR, editor. Urologic surgery in neonates and young infants. Philadelphia: WB Saunders; 1988. p. 252–64.)
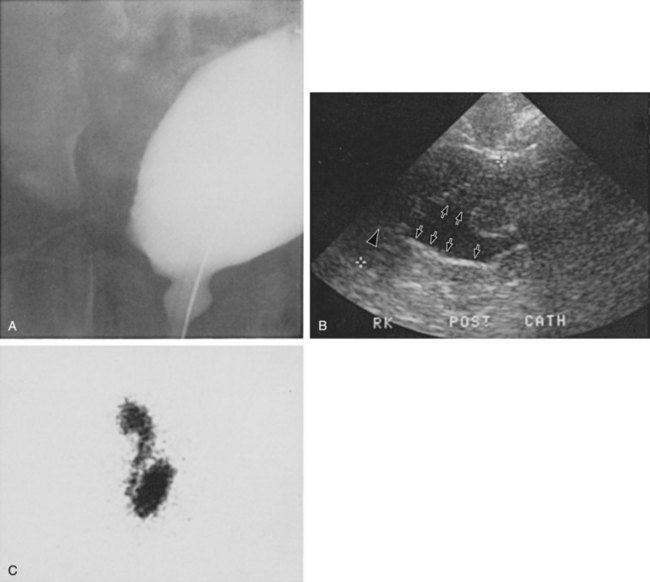
Figure 128–5 A, A voiding cystourethrogram in a female neonate with dyssynergy and elevated voiding pressures demonstrates no reflux and a smooth-walled bladder. Her initial renal echogram was normal. She was started on clean intermittent catheterization and oxybutynin chloride (Ditropan) but did not respond. Within 1 year she developed right hydronephrosis (B, arrows) and severe reflux, evident on a radionuclide cystogram (C).
It may be that detrusor filling pressures need to be looked at in a more critical way to determine whether they are an important factor in upper urinary tract deterioration. Landau and colleagues (1994) developed the concept of low detrusor filling pressure (<30 cm H2O) at specific volumes adjusted for age and not at maximal capacity. Applying this idea, they noted significantly improved sensitivity in predicting upper urinary tract deterioration. The ability to predict accurately which neonates are at risk for urinary tract deterioration prompted the initiation of prophylactic therapy with CIC and anticholinergic drugs (Geranoitis et al, 1988; Kasabian et al, 1992). Long-term success has been achieved in preventing reflux and hydronephrosis and in reducing the need for augmentation cystoplasty, from between 27% and 41% to between 11% and 17% (Edelstein et al, 1995; Wu et al, 1997; Kaefer et al, 1999). Others believe that aggressive observation and prompt intervention yields similar long-term results with less morbidity from catheterization (Teichman et al, 1994), but only time will delineate which avenue of treatment is most efficacious (Tanaka et al, 1999; Hopps and Kropp, 2003).
Key Points: Neonatal Assessment for Neurogenic Bladder Dysfunction
Management Principles
Because expectant treatment has revealed that infants with outlet obstruction in the form of DSD are at considerable risk for urinary tract deterioration, the idea of treating these children prophylactically has emerged as an important alternative. When CIC is begun in the neonatal period, it is easy for parents to master, even in uncircumcised boys, and for children to accept as they grow (Joseph et al, 1989). Complications such as meatitis, epididymitis, and urethral injury are rarely encountered, and urinary infections occur in fewer than 30% (Kasabian et al, 1992), although asymptomatic bacteriuria can be seen in almost 70% (Schlager et al, 2001).
CIC alone or in combination with anticholinergic agents, when detrusor filling pressures exceed 40 cm H2O and voiding pressures are higher than 80 to 100 cm H2O, resulted in an incidence of urinary tract deterioration of only 8% to 10% (Geranoitis et al, 1988; Kasabian et al, 1992; Edelstein et al, 1995). This represents a significant drop in the occurrence of detrimental changes compared with children observed expectantly (McGuire et al, 1981; Bauer et al, 1984; Sidi et al, 1986; Teichman et al, 1994; Wu et al, 1997). Oxybutynin is administered in a dose of 0.2 to 0.4 mg/kg divided two or three times daily with no apparent side effects. On rare occasions when an overactive or poorly compliant bladder fails to respond to these measures, augmentation cystoplasty may be needed with cutaneous vesicostomy almost never required (Duckett, 1974; Mandell et al, 1981).
Key Points: Management Principles
Neurologic Findings and Recommendations
The neurologic lesion in myelodysplasia is a dynamic disease process in which changes take place throughout childhood (Epstein, 1982; Reigel, 1983; Venes and Stevens, 1983; Oi et al, 1990), especially in early infancy (Spindel et al, 1987) and later at puberty (Begger et al, 1986), when the linear growth rate accelerates again. When a change is noted on neurologic, orthopedic, or urodynamic assessment, radiologic investigation of the CNS often reveals (1) tethering of the spinal cord (Fig. 128–6), (2) a syringomyelia, a dilation and fluid collection within the spinal cord, (3) increased intracranial pressure due to a shunt malfunction, or (4) partial herniation of the brainstem and cerebellum. Children with completely intact or only partially denervated sacral cord function are particularly vulnerable to progressive changes. Magnetic resonance imaging (MRI) is the test of choice because it reveals anatomic details of the spinal column and CNS (Just et al, 1990). However, it is not a functional study and when used alone it cannot provide exact information about a changing neurologic lesion.
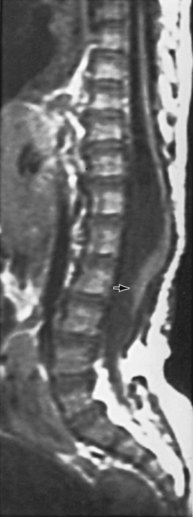
Figure 128–6 MR image in a 9-year-old girl who developed a tethered cord after myelomeningocele repair reveals the conus opposite the L3–4 vertebrae (arrow).
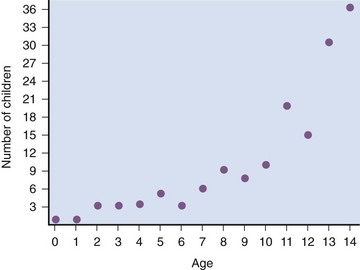
Sequential urodynamic testing on a yearly basis beginning in the neonatal period and continuing until the child is 5 years old provides a means of carefully monitoring these children to detect signs of change, thus offering the hope that early detection and neurosurgical intervention may help to arrest or even reverse a progressive pathologic process. Changes occurring in a group of neonates monitored in this manner involved both the sacral reflex arc and the pontine-sacral reflex interaction (Fig. 128–7) (Lais et al, 1993). Most children who experience such changes tend to do so in the first 3 years of life (Phuong et al, 2002) (Fig. 128–8). Twenty-two of 28 children in whom the neurologic picture became worse underwent a second neurosurgical procedure; 11 of them had a beneficial effect from the surgery and showed improvement in urethral sphincter function (Lais et al, 1993). Infants with only sacral level deficits have a 47% risk of deterioration (Dator et al, 1992). Even children with normal neurourologic function at birth have a 32% risk of developing a tethered spinal cord with the development of DSD with or without detrusor overactivity (Tarcan et al, 2001).
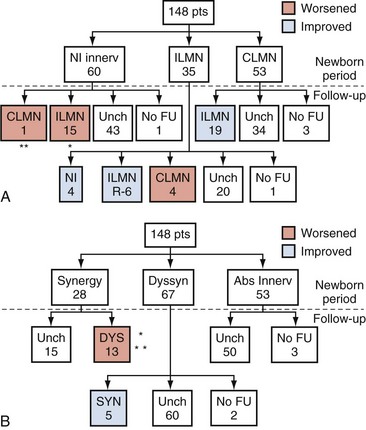
Figure 128–7 Changes in innervation of the purely sacral (A) and pontine-sacral (B) reflex arc pathways that occurred in a group of children with myelodysplasia who were monitored with sequential urodynamic studies beginning in the newborn period. ILMN, incomplete lower motor neuron lesion; CLMN, complete lower motor neuron lesion; NL, normal innervation; Unch, unchanged; FU, follow-up; DYS, dyssynergy; Syn, synergy. A, The double asterisk indicates 1 patient changed from synergy to dyssynergy and the single asterisk indicates 4 of 15 patients so changed. B, The single asterisk indicates 1 patient changed from normal to partial and then complete denervation, and the double asterisk indicates 4 patients changed from normal to partial denervation.
(From Lais A, Kasabian NG, Dyro FM, et al. Neurosurgical implications of continuous neuro-urological surveillance of children with myelodysplasia. J Urol 1993;150:1879–83.)
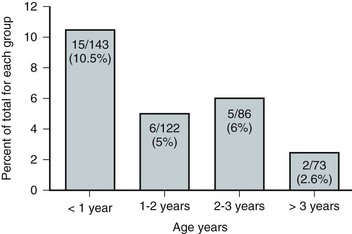
Figure 128–8 The propensity for a change in urethral sphincter innervation is greatest in the first year of life.
(From Lais A, Kasabian NG, Dyro FM, et al. Neurosurgical implications of continuous neuro-urological surveillance of children with myelodysplasia. J Urol 1993;150:1879–83.)
As a result of these developments it is recommended that all infants with myelodysplasia be monitored according to the guidelines set forth in Table 128–3. It is not enough to look at just the radiologic appearance of the urinary tract; scrutiny of the functional status of the lower urinary tract is important as well. In addition to the reasons cited previously, it may be necessary to repeat a urodynamic study if the upper urinary tract dilates secondary to impaired drainage from a poorly compliant detrusor.
Table 128–3 Surveillance in Infants with Myelodysplasia*
| SPHINCTER ACTIVITY | RECOMMENDED TESTS | FREQUENCY |
|---|---|---|
| Intact–synergic | Postvoid residual volume | q 4 mo |
| US | q 12 mo | |
| UDS | q 12 mo | |
| Intact–dyssynergic† | US | q 12 mo |
| UDS | q 12 mo | |
| VCUG or RNC‡ | q 12 mo | |
| Partial denervation | Postvoid residual volume | q 4 mo |
| US | q 12 mo | |
| UDS§ | q 12 mo | |
| VCUG or RNC‡ | q 12 mo | |
| Complete denervation | Postvoid residual volume | q 6 mo |
| US | q 12 mo |
US, renal ultrasonography; UDS, urodynamic study; VCUG, voiding cystourethrography; RNC, radionuclide cystography.
† Patients receiving intermittent catheterization and anticholinergic agents.
‡ If detrusor hypertonicity or reflux is already present.
§ Depending on degree of denervation.
Beyond the age of 5 years renal ultrasonography every 18 months until puberty and then every 2 years thereafter is recommended. Urodynamic studies are indicated to evaluate the development of new neurologic symptoms, new hydronephrosis, or changes in continence. VCUG should be done if recurrent urinary tract infections are experienced.
Management of Reflux
Vesicoureteral reflux occurs in 3% to 5% of neonates with myelodysplasia, usually in association with poor detrusor compliance, detrusor overactivity, and/or DSD (Flood et al, 1994). It is rare to find reflux in any neonate without these urodynamic findings (Bauer, 1984b; Geranoitis et al, 1988; Edelstein et al, 1995). If left untreated, the incidence of reflux in these infants at risk increases with time until 30% to 40% are afflicted by 5 years of age (Bauer, 1984a; Seki et al, 1999). Prophylactic treatment that lowers detrusor filling and voiding pressures with anticholinergic agents and empties the bladder by means of CIC significantly reduces this rising incidence of reflux (Edelstein et al, 1995).
In children with reflux grades 1 to 3 (International Classification) who void spontaneously or have complete lesions with little or no outlet resistance and empty their bladder completely, management consists solely of prophylaxis with antibiotics to prevent recurrent infection. In children with high-grade reflux (grade 4 or 5), CIC is begun to ensure complete emptying. Children who cannot empty their bladder spontaneously, regardless of the grade of reflux, are treated with CIC to empty the bladder efficiently. Children with poor detrusor compliance with or without hydroureteronephrosis are also started on anticholinergic agents to lower intravesical pressure and ensure adequate upper urinary tract decompression (Flood et al, 1994). When reflux is managed in this manner there has been a dramatic response, with reflux resolving in 30% to 55% of individuals (Kass et al, 1981; Bauer, 1984b; Joseph et al, 1989; Flood et al, 1994; Agarwal et al, 1997; Hopps and Kropp, 2003). Although bacteriuria can be seen in as many as 56% of children on CIC it is not harmful except in the presence of high-grade reflux, because symptomatic urinary infection and renal scarring rarely occur with lesser grades of reflux (Kass et al, 1981; Cohen et al, 1990).
Credé voiding should be avoided in children with reflux, especially those with a reactive external urethral sphincter. In this circumstance, the Credé maneuver results in a reflex response in the external sphincter that increases urethral resistance and raises the pressure needed to expel urine from the bladder (Barbalais et al, 1983) (Fig. 128–9). This has the effect of aggravating the degree of reflux and accentuating its water-hammer effect on the kidneys. Vesicostomy drainage (Duckett, 1974; Mandell et al, 1981) is rarely required today but is reserved for those infants (1) who have such severe reflux that CIC and anticholinergic medication fail to improve upper urinary tract drainage, (2) whose parents cannot adapt to the catheterization program, or (3) who are not good candidates for augmentation cystoplasty (Morrisroe et al, 2005).
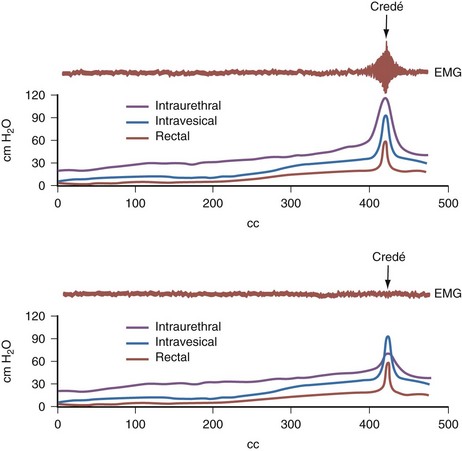
Figure 128–9 When the external sphincter is reactive (top), a Credé maneuver produces a reflex increase in electromyographic (EMG) activity of the sphincter and a concomitant rise in urethral resistance, resulting in high voiding pressure. A child whose sphincter is denervated and nonreactive (bottom) will not have a corresponding rise in EMG activity, urethral resistance, or voiding pressure. A Credé maneuver here will not be detrimental.
(From Bauer SB. Early evaluation and management of children with spina bifida. In: King LR, editor. Urologic surgery in neonates and young infants. Philadelphia: WB Saunders; 1988. p. 252–64.)
The indications for antireflux surgery in this group of children are not very different from those applicable to children with normal bladder function. They include recurrent symptomatic (febrile) urinary infection while receiving adequate antibiotic therapy and appropriate catheterization techniques; persistent hydroureteronephrosis despite effective emptying of the bladder and lowering of intravesical pressure; severe reflux with an anatomic abnormality at the ureterovesical junction; reflux that persists into puberty; and the presence of reflux in any child undergoing surgery to increase bladder outlet resistance. Although some clinicians do not advocate reimplanting ureters in patients with low-grade reflux who are undergoing augmentation cystoplasty to lower intravesical pressure, this concept is not universally accepted (Nasrallah and Aliabadi, 1991).
Jeffs and colleagues (1976) were the first to show that antireflux surgery can be very effective in children with neurogenic bladder dysfunction as long as it is combined with measures to ensure complete bladder emptying. Before this observation was made, the results of ureteral reimplantation were so dismal that most physicians treating these children advocated urinary diversion as a means of managing reflux (Smith, 1972; Cass, 1976). Since the advent of CIC, success rates for antireflux surgery have approached 95% (Kass et al, 1981; Woodard et al, 1981; Bauer et al, 1982; Kaplan and Firlit, 1983). Bilateral surgery for unilateral disease need not be done, because contralateral reflux does not occur postoperatively (Bauer, 1984a). The endoscopic injection of various materials has altered the management of reflux in children with myelomeningocele (Elder et al, 2004; Schlussel, 2004). A recent meta-analysis demonstrated that the success rate of endoscopic treatment is less in children with neurogenic bladder than those with normal bladder function (62% vs. 74%) (Elder et al, 2004). Studies comparing the effectiveness of open surgical to endoscopic management in this population show a significantly greater success rate for traditional open procedures (61% to 72.5% vs. 84.3% to 95.5%) (Engel, 1997; Granata, 1999). Thus, the endoscopic approach is a reasonable alternative to ureteroneocystotomy; however, the long-term outcomes are not defined.
Continence and Prevention of Upper Urinary Tract Deterioration
The primary goals of management for children with myelomeningocele are the attainment of social continence and preservation of normal renal function. These goals can be achieved for most children with a combination of medical and surgical therapies.
Urinary continence is becoming an increasingly important issue that demands attention at an early age. It has been shown that children who are continent have higher ratings in self-confidence and social acceptance than boys and girls who are wet; thus this issue becomes paramount as the children grow up and attend school (Moore et al, 2004). Initial attempts at achieving continence include CIC and drug therapy designed to maintain low intravesical pressures and a reasonable level of urethral resistance (Figs. 128-10 and 128-11). Although these measures can be initiated on a trial-and-error basis, it is more efficient to use exact treatment protocols based on specific urodynamic findings. As a result, urodynamic testing is performed if initial attempts with CIC and oxybutynin fail to achieve continence. Without urodynamic studies it is hard to know whether (1) a single drug is effective, (2) the dose should be increased, (3) a second drug should be added to the regimen, or (4) alternative methods of treatment (i.e., augmentation cystoplasty) should be contemplated.
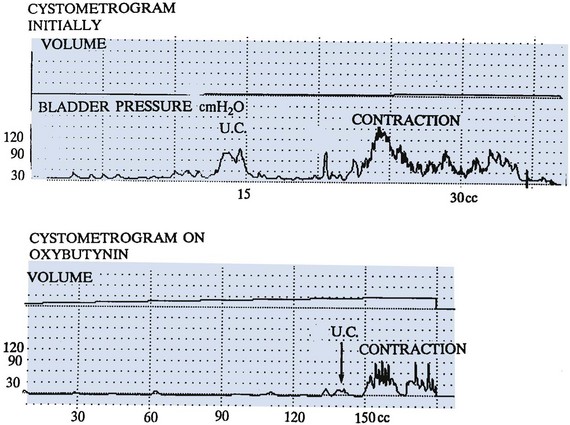
Figure 128–10 Oxybutynin is a potent anticholinergic agent that dramatically delays detrusor contractions and lowers contraction pressure, as demonstrated on these two graphs. U.C., uninhibited contraction.
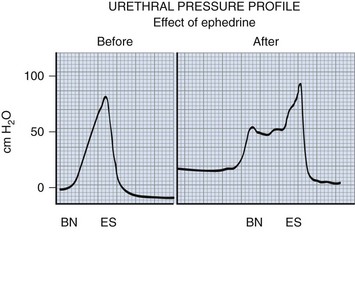
Figure 128–11 α-Sympathomimetic agents potentially have their greatest effect in the bladder neck region, where the highest concentration of α-adrenergic receptor sites exists. They can raise outlet resistance and improve continence in many individuals. BN, bladder neck; ES, external sphincter.
At this point, if poor detrusor compliance and/or overactivity have not been dealt with effectively the dose of the anticholinergic drug may be increased. If this is not successful, another anticholinergic agent may be combined with or given instead of oxybutynin (see Table 128–1). A recent study in adults with neurogenic bladder has shown the addition of a second anticholinergic agent to an existing double-dose anticholinergic agent resulted in improved urodynamic parameters and continence in 85% of patients who had failed single-agent therapy with minimal adverse effects (Amend, 2008). Glycopyrrolate is the most potent oral anticholinergic drug available today, but it may have the typical belladonna-like side effects common to all these drugs. Tolterodine, a newly approved anticholinergic drug, is equally effective as oxybutynin in reducing detrusor overactivity and improving compliance, but with fewer side effects, especially constipation (Goessl et al, 2000). Hyoscyamine produces fewer side effects still, but its potency is even less. Intravesical instillation of oxybutynin has been proven to be successful in lowering detrusor pressure and has fewer side effects compared with oral administration; serum concentrations may reach similar levels regardless of the route of intake (Greenfield and Fera, 1991; Massad et al, 1992). Inconvenience of administration, however, seems to preclude its long-term use (Kasabian et al, 1994). Trospium chloride, a recently approved quaternary ammonium compound with antimuscarinic activity that has a high binding affinity for muscarinic receptors M1, M2, and M3 but not nicotinic or cholinergic receptors holds promise because it has a more direct effect on detrusor function with fewer systemic side effects than anticholinergic agents (Rovner, 2004; Doroshyenko et al, 2005; Kim et al, 2005). Solifenacin, darifenacin, and propiverine are muscarinic receptor antagonists with high affinity for the M3 receptor. Propiverine also has calcium channel–blocking effects. These three novel agents may reduce adverse events; however, their safety and effectiveness has not yet been established in children. If conservative management with medications and CIC fail to achieve continence or prevent upper urinary tract deterioration, more aggressive intervention is warranted. In up to 80% of children, nocturnal bladder emptying has been shown to improve hydronephrosis, bladder compliance and capacity, urinary tract infections, and continence (Koff, 2005).
Key Points: Continence in Neurogenic Bladder Dysfunction
If urodynamic testing reveals that urethral resistance is inadequate to maintain continence because either the sphincter fails to react to increases in abdominal pressure or its resistance drops with bladder filling, α-sympathomimetic agents are added to the regimen (see Table 128–1); phenylpropanolamine is the most effective drug in this regard.
Some children will ultimately require surgical intervention. A persistent poorly compliant or overactive detrusor may be treated either with enterocystoplasty (Mitchell and Piser, 1987; Sidi et al, 1987; Hernandez et al, 1994), autoaugmentation (Cartwright and Snow, 1989a, 1989b), or a combination thereof (Duel et al, 1998). Sigmoid, cecum, and small intestine, in that order, have been used to enlarge the bladder. The ileocecal segment is avoided in children with myelodysplasia because removing it might aggravate the bowel dysfunction in these children. Detubularization of the bowel is needed to minimize the intrinsic contractions of the intestinal segment and prevent it from causing intractable incontinence once it has been added to the bladder (Goldwasser et al, 1987; Hinman, 1988). Concerns have surfaced about gastric segments because they can cause hyponatremic hypochloremic metabolic alkalosis (Gosalbez et al, 1993) or the hematuria dysuria syndrome (Nguyen et al, 1996). Except for children with progressive or end-stage renal failure, gastrocystoplasty has not been recommended as a routine form of augmentation (Leonard et al, 2000; Plaire et al, 2000).
With the long view of hindsight it is becoming evident that augmentation cystoplasty with any segment of the gastrointestinal tract can lead to long-term consequences in acid-base balance, vitamin B12 deficiency, fat absorption, renal function, bone metabolism, growth retardation, recurrent urinary tract infection, stone formation, and even cancer risk (Gilbert and Hensle, 2005). Consequently, there is considerable interest in developing new techniques for replacing the bladder without the need for bowel substitution (i.e., tissue-engineered autologous augments; see also Chapter 19).
The well-documented development of malignancy in patients with intestinal and gastric bladder augmentation mandates careful surveillance of these patients. Most studies note a 10-year minimum lag time between the augmentation and presentation of disease. Thus, 10 years after augmentation an annual surveillance cystoscopy with site-directed biopsies at the anastomotic suture line is recommended (Soergel et al, 2004; Castellan et al, 2007; Vemulakonda et al, 2008). Symptoms and signs such as new-onset hydronephrosis, hematuria, or bladder wall thickening warrant prompt evaluation (Castellan et al, 2007; Vemulakonda et al, 2008).
If bladder neck or urethral resistance is insufficient to allow adequate storage capacity, several operations are available to improve this continence mechanism. Bladder neck reconstruction can be undertaken in a variety of ways, including the Young-Dees or Leadbetter procedures (Young, 1919; Dees, 1949; Leadbetter, 1964) or the Kropp approach (Kropp and Angwafo, 1986), modified by Salle to make catheterization easier (Salle et al, 1994). A fascial sling procedure suspending the bladder neck and buttressing it against the undersurface of the pubis has been advocated enthusiastically by several clinicians (McGuire et al, 1986; Raz et al, 1988; Bauer et al, 1989). Artificially derived material, small intestine submucosa (SIS) (Colvert et al, 2002), and a rectus muscle “fascial” wrap around the bladder neck (Walker et al, 2000) have been as effective as rectus fascia in increasing bladder neck resistance and their effectiveness does not seem to dissipate over time (Elder, 1990; Castellan et al, 2005). Even though it is applicable to males it has not been as effective as in females (Nguyen et al, 2001; Castellan et al, 2005). Each of these operations, however, necessitates the use of CIC to empty the bladder postoperatively. It is most effective when combined with augmentation cystoplasty to ensure an adequate reservoir with good compliance (Castellan et al, 2005). The use of a bladder neck sling in conjunction with continent cutaneous catheterizable channel has been proposed to avoid bladder augmentation in some patients with neurogenic bladder. These children have a similar improvement in their continence level but may have a shorter interval between catheterization and require more anticholinergic agents than children with slings and augmentation (Snodgrass et al, 2009). However, this approach is controversial because of the potential risk of bladder deterioration related to increasing bladder pressures and lack of sufficient long-term follow-up. The artificial sphincter (Barrett and Furlow, 1982; Light and Scott, 1983; Light et al, 1983) also increases bladder outlet resistance, and its mechanism of action allows emptying at low urethral pressure. Any patient who can empty his or her bladder before the device is implanted should be able to do so afterward without the need for CIC. In fact, this is the only bladder neck procedure that allows for spontaneous voiding yet is compatible with CIC when needed (Herndon et al, 2003). However, children who have had bladder augmentation or an artificial sphincter placed before puberty are much more likely to require CIC to empty the bladder postpubertally (Catti, 2008). Poor detrusor compliance can develop postoperatively if the preoperative cystometrogram is not carefully scrutinized for signs of increased filling pressure (Woodside and McGuire, 1982; Bauer et al, 1986), and this may lead to progressive renal failure if not treated in a timely fashion with medication or augmentation cystoplasty (Bauer et al, 1993). Long-term results with the artificial sphincter have shown that it is a viable option in children with neurogenic bladder dysfunction (Bosco et al, 1991; Levesque et al, 1996; Kryger et al, 1999; Kryger et al, 2001; Herndon et al, 2003); however, device removal may be required in 9% to 20% (Lopez Pereira et al, 2006; Bauer, 2008; Catti, 2008).
An alternative, minimally invasive option to increase bladder outlet resistance is the endoscopic injection of bulking agents in the bladder neck (Ben-Chiam et al, 1995). Various bulking agents have been used. Early reports are usually promising; however, long-term results are less favorable (Perez et al, 1996; Silveri et al, 1998; Guys et al, 2001). Long-term data on newly developed injectable agents are not available (Caione and Capozza, 2002; Misseri et al, 2005).
Urinary diversion, once considered a panacea for children with myelodysplasia, has turned out to be a Pandora’s box of new clinical problems (Schwarz and Jeffs, 1975; Shapiro et al, 1975). Pyelonephritis and renal scarring, calculi, ureterointestinal obstruction, strictures of the conduit, and stomal stenosis are often encountered in children who are monitored on a long-term basis. Although antirefluxing colon conduits seem to have fewer complications, they are still not ideal. In the past 15 years very successful attempts have been made to reverse a urinary tract diversion in children who probably would not have undergone the procedure today (Hendren, 1973, 1990). Few children undergo urinary diversion now; if they do, it is in association with a continent catheterizable stoma.
Continent urinary diversion with complete closure of the bladder neck (or compression via a fascial sling) has been used to provide a better quality of life for those with intractable urethral incompetence despite bladder outlet surgery or to make it easier to catheterize those individuals who cannot easily catheterize themselves via their native urethra (MacNeily et al, 2005).
Several operations have been devised, but the ones that achieved the most publicity initially were the Kock pouch in adults (Kock, 1971; Skinner et al, 1987) and the Indiana reservoir in children (Rowland et al, 1987). Mitrofanoff (1980) created a continence mechanism by tunneling one end of the vermiform appendix into the bladder, as if reimplanting the ureter to prevent reflux, with the other end being brought out through the skin as a continent catheterizable stoma. This principle has been extended to the ureter. After the ureter is transected at the pelvic brim, a proximal transureteroureterostomy is performed and the cut end of the distal segment is brought to the skin as a stoma (Duckett and Snyder, 1986). Continence is achieved by the intramural detrusor tunnel of the ureter. Other narrow structures (e.g., fallopian tube, a rolled strip of stomach, a tapered or reconfigured ileal segment) have been implanted either into the native bladder or along the tinea of a detubularized portion of sigmoid or cecum when the latter act as a urinary reservoir (Woodhouse et al, 1989; Reidmiller et al, 1990; Bihrie et al, 1991; Montie et al, 1997). The success rate for achieving continence has been excellent, approaching 85%, primarily owing to the flap valve effect of the intramural tunnel (Hinman, 1990; Watson et al, 1995; Kaefer et al, 1997; Gerharz et al, 1998). For this reason, it is now the preferred method for continent urinary diversion (Harris et al, 2000).
An alternative option for achieving continence and improving urodynamic parameters is the intravesical injection of botulinum-A toxin, which is a neurotoxin protein produced by Clostridium botulinum that acts by inhibiting acetylcholine release, resulting in muscle paralysis. The commonly injected dose ranges from 5 to 12 IU/kg, with a maximum total dose of up to 360 IU. Sixty-five to 87 percent of children who have failed conservative medical management and CIC have achieved dryness with a botulinum-A injection. Improvements in urodynamic parameters include decreased maximum detrusor pressure (33% to 57%); increased maximum cystometric capacity (34% to 165%); and improved compliance (121% to 183%) (Schulte-Baukloh et al, 2002; Riccabona et al, 2004; Altaweel et al, 2006; Kajbafzadeh et al, 2006). The duration of response ranges from 6 to 10.5 months. It appears that repeated injections continue to be effective with the interval between repeated injections increasing with each series of injections (Schulte-Baukloh et al, 2005; Altaweel et al, 2006). Complete lack of a response to botulinum-A injection has been linked to the presence of botulinum-A antibodies (Schulte-Baukloh et al, 2008).
The artificial somatic-autonomic reflex pathway is a promising new therapeutic approach to restore bladder function in children with spina bifida. The procedure involves a limited laminectomy and a lumbar ventral root to S3 ventral root microanastomosis. The L5 dorsal root is left intact as the afferent branch of the somatic-autonomic reflex pathway after axonal regeneration (Xiao et al, 2005). In a series of 20 children with spina bifida, 17 developed satisfactory bladder control and continence within 12 months of the procedure. Those children with DSD and overactivity demonstrated nearly normal storage and synergic voiding on follow-up urodynamic studies (Xiao, 2005). A recent study at an independent center confirmed early favorable results with improved continence and spontaneous voiding in 30% of the spina bifida patients (median age 8 years) who underwent intradural lumbar to sacral motor root microanastomosis. Postoperative complications included prolonged wound drainage, ipsilateral footdrop, and temporary lower extremity muscle weakness (Peters et al, 2010). Thus this novel approach appears to have promising results when preformed by experts. The long-term results and general application of the procedure are yet to be determined.
Sexuality
Sexuality in this population is becoming an increasingly important issue as more individuals reach adulthood and want to marry or to have intimate relationships (Cromer et al, 1990). Investigators are looking into the concerns, fears, self-imagery, and desires of teenagers and young adults and at the ability of males to procreate and females to bear children (Bomalaski et al, 1995). However, few studies are available that look critically at sexual function in these patients. Mental disability, poor manual dexterity, lack of education regarding normal and abnormal sexual function, invasion of personal privacy, and overprotective parents often prevent independent behavior and, as a result, lead to poor understanding of sexual issues (Joyner et al, 1998; Woodhouse, 2005).
In several studies, researchers interviewed groups of teenagers and reported that 28% to 40% of them had had one or more sexual encounters and almost all of them had a desire to marry and ultimately to bear children (Cromer et al, 1990; Palmer et al, 1999a). In one study, 72% of male subjects claimed they were able to have an erection, and two thirds of these were able to ejaculate (Decter et al, 1997). Medical therapy has been shown to be effective in this population. Sildenafil improved erectile function in 80% of men with meningomyelocele in a randomized, double-blind, placebo-controlled trial (Palmer et al, 2000). Other studies revealed that 70% to 80% of myelodysplastic women were able to become pregnant and to have an uneventful pregnancy and delivery, although urinary incontinence in the latter stages of gestation was common in many, as was delivery by cesarean section (Laurence and Beresford, 1975; Cass et al, 1986; Bomalaski et al, 1995; Arata et al, 2000). In the same studies, 17% to 39% of male subjects claimed that they were able to father children and another 25% had a good prognosis for fathering them (Laurence and Beresford, 1975; Bomalaski et al, 1995; Decter et al, 1997). It is more likely that men will have problems with erectile and ejaculatory function because the sacral spinal cord is frequently involved, whereas reproductive function in women, which is under hormonal control, is not affected. Men with neurologic lesions at S1 or lower are likely to have normal or adequate reproductive sexual function, but only 50% of those with lesions above that level have adequate function (Woodhouse, 1994, 2005). Poor semen quality (Reilly and Oates, 1992) and Sertoli cell–only histology on testis biopsy (Glass and Soni, 1999) have been reported as reasons (in addition to erectile dysfunction) for infertility in males with spina bifida.
The degree of sexuality is inversely proportional to the level of neurologic dysfunction (Joyner et al, 1998; Palmer et al, 1999b). Until recently, however, this subject has been taboo (Decter et al, 1997). Sexual identity, education, and social mores have been taken out of the realm of secrecy and are now openly discussed. However, parents of children with spina bifida are more overprotective than parents of normal children and are less willing to grant autonomy to them for adequate peer and sexual development (Holmbeck et al, 2002); this tends to lead to less self-assurance regarding social interaction with the opposite sex and diminished sexual identity. Boys reach puberty at an age similar to the age for normal males, whereas breast development and menarche tend to start as much as 2 years earlier than usual in myelodysplastic compared with normal girls. The cause of this early hormonal surge is uncertain, but it may be related to changes in pituitary function in girls secondary to hydrocephalus (Hayden, 1985).
Bowel Function
There is no unanimity regarding the ideal bowel management program for myelodysplastic children. The external anal sphincter is innervated by the same (or similar) nerves that modulate the external urethral sphincter, whereas the internal anal sphincter is under the influence of more proximal nerves from the sympathetic nervous system. In addition, the internal anal muscle reflexively relaxes in response to anal distention. Consequently, bowel incontinence is frequently unpredictable and is not associated with the attainment of urinary continence; it is often related to the consistency of fecal material and how rapidly the anal area refills after an evacuation, the degree of intactness of sacral cord sensation and motor function, and reflex reactivity of the external anal sphincter muscle (Younoszai, 1992).
A majority of pediatricians managing these children believe that a regular and efficient bowel emptying regimen is paramount and mandatory. Most programs begin at about 1 year of age, but the best method of attaining these objectives is still controversial. Usually the children are placed on diets that are intended to create formed but not severely constipated stool. Roughage in the form of fruits and bran and stool softeners (in older children) are given to achieve this goal. Suppositories that help evacuate the rectum are used on a regular basis to help train the lower bowel to fill and empty. Some physicians believe that enemas are more effective in evacuating a greater portion of the lower bowel, but one problem with them is the difficulty of retaining the solution when the anal sphincter muscle is lax. Biofeedback training programs to strengthen the external anal sphincter are no more effective than a good bowel management program in attaining fecal continence (Loening-Baucke et al, 1988). Whereas electrostimulation of the bowel has had a variable effect in fecal continence, ranging from 0% to 36% (Marshall and Boston, 1997; Palmer et al, 1997), intravesical bladder stimulation has had a more pronounced effect on bowel continence (Han et al, 2004). When a carefully constructed bowel regimen is adhered to, most children with myelomeningocele can achieve some degree of fecal continence (Myers, 1984), but the uncertainty of accidents always makes this a tenuous situation. Often the urinary incontinence is effectively managed with CIC, drugs, and/or surgery but episodes of fecal soiling remain a problem, particularly in socially minded adolescents (Hayden, 1985; Krogh et al, 2003). When diet, medications, and manual evacuation fail to achieve predictable bowel emptying without soiling, a continent cutaneous pathway from the lower abdominal wall to the cecum may be created using the vermiform appendix; this is called the ACE procedure, for Antegrade Continence Enema (Griffiths and Malone, 1995). When the appendix is unavailable, a small segment of the bowel may be reconfigured to act as a conduit. Alternatively, a button cecostomy provides effective access for antegrade continence enemas (Duel, 1999). Enemas consisting of either Golytely, saline, or tap water, sometimes in combination with bisacodyl, are instilled daily or every other day to evacuate the colon. Cleansing the colon in this manner has resulted in complete continence in 89% of children in whom it has been tried (Squire et al, 1993; Gerharz et al 1997; Yerkes et al, 2002). Older children readily become independent in managing their bowel function, leading to improved self-esteem and sociability (Bau et al, 2001; Aksnes et al, 2002).
Lipomeningocele and Other Spinal Dysraphisms
Diagnosis
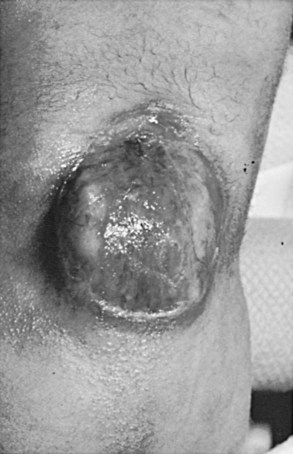
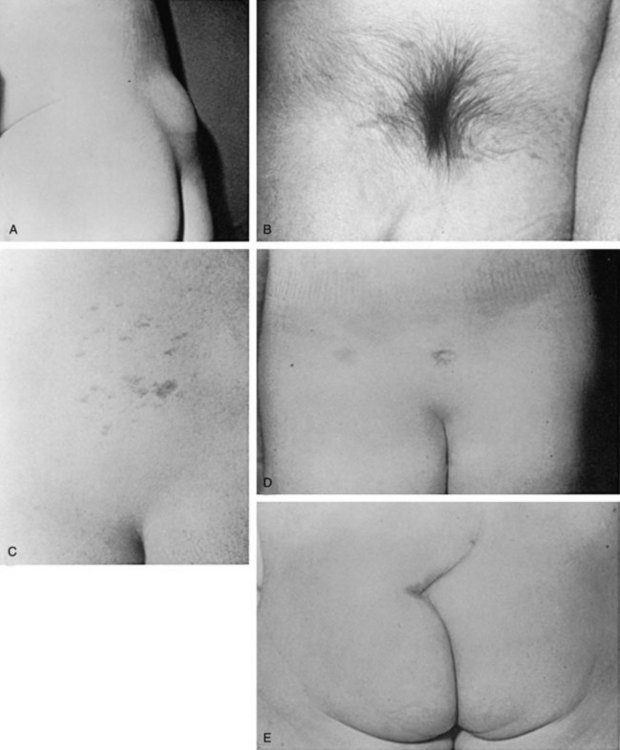
There is a group of congenital defects that affect the formation of the spinal column but do not result in an open vertebral canal (James and Lassman, 1972) (Table 128–4). They occur once in 4000 live births, but with the ease of MRI screening of children with suspected lesions the incidence of these defects is increasing (Bruce and Schut, 1979). In a recent study the incidence of lipomeningocele in families was calculated to be 0.043% (Sebold et al, 2005). These lesions can be very subtle and have no obvious outward signs, but in more than 90% of children there is a cutaneous abnormality overlying the lower spine (Anderson, 1975; Pierre-Kahn et al, 1997). This varies from a small dimple or skin tag to a tuft of hair, a dermal vascular malformation, a very noticeable subcutaneous lipoma, or an asymmetrically curving gluteal cleft (Fig. 128–12). In addition, on careful inspection of the legs, one may note a high arched foot or feet; hammer toes or claw toes; a discrepancy in muscle size, shortness, and decreased strength in one leg compared with the other, typically at the ankle; and/or a gait abnormality, especially in older children (Dubrowitz et al, 1965; Weissert et al, 1989; Jindal and Mahaptra, 2000). Absent perineal sensation, back pain, and secondary incontinence after a period of dryness are common symptoms in older children and young adults (Linder et al, 1982; Yip et al, 1985; Weissert et al, 1989). Lower urinary tract function is abnormal in 40% to 90% of affected older individuals, with the incidence of an abnormality increasing proportionately with age (Mandell et al, 1980; Koyangi et al, 1997; Pierre-Kahn et al, 1997; Sarica et al, 2003). The child may experience difficulty with toilet training, urinary incontinence after an initial period of dryness once toilet trained (especially during the pubertal growth spurt), recurrent urinary infection, and/or fecal soiling. Occasionally, some patients without an obvious back lesion escape detection until they develop urinary (66%) or lower extremity (19%) symptoms or back pain (14%) after puberty caused by delayed traction on the spinal cord (Satar et al, 1995).
Table 128–4 Types of Occult Spinal Dysraphisms
Findings
When these children are evaluated in the neonatal period or early infancy, a majority have a perfectly normal neurologic examination (Atala et al, 1992). Urodynamic testing, however, reveals abnormal lower urinary tract function in about one third of infants younger than 18 months of age (Keating et al, 1988) (Fig. 128–13). Such studies may provide the only evidence of a neurologic injury involving the lower spinal cord (Keating et al, 1988; Foster et al, 1990; Atala et al, 1992, Satar et al, 1997; Nogueira et al, 2004). When present, the most likely abnormality is an upper motor neuron lesion characterized by an overactive detrusor and/or hyperactive sacral spinal cord reflexes (Fone et al, 1997; Pierre-Kahn et al, 1997); mild forms of DSD are rarely noted. Lower motor neuron signs with denervation potentials in the sphincter or an acontractile detrusor occur in only 10% of young children.
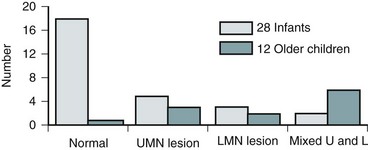
Figure 128–13 Most neonates with an occult spinal dysraphism have normal lower urinary tract function, whereas older children tend to have both upper motor neuron (UMN) and lower motor neuron (LMN) lesions.
In contrast, practically all individuals older than 3 years of age who have not been operated on or in whom an occult dysraphism has been belatedly diagnosed have either an upper or lower motor neuron lesion or a combination thereof on urodynamic testing (92%) (see Fig. 128–13) or neurologic signs of lower extremity dysfunction (Yip et al, 1985; Kondo et al, 1986; Keating et al, 1988; Atala et al, 1992; Satar et al, 1997; Nogueira et al, 2004). When such children were observed expectantly from infancy after the diagnosis was made, 58% experienced deterioration of their disorder within 2 years (Andar et al, 1997; Cornette et al, 1998). There does not seem to be a preponderance of one type of lesion over another (i.e., upper vs. lower motor neuron); and often the child shows signs of both (Hellstrom et al, 1986; Kondo et al, 1986). In one study of children older than 3 years of age, 43% had denervation in the sphincter and 52% detrusor areflexia, with a total of 81% having an abnormality (Satar et al, 1995).
Key Points: Lipomeningocele and Other Spinal Dysraphisms
Pathogenesis
Various occult spinal dysraphic lesions produce different neurourologic findings. When they do cause an abnormality, lipomas of the cauda equina invariably cause an upper motor neuron lesion (70%), alone or in combination with a lower motor neuron deficit (30%) (Satar et al, 1997). The split cord syndrome results in an isolated upper or lower motor neuron lesion in 25% each or a combined lesion in 50% (Proctor et al, 2000).
The reason for this difference in neurologic findings may be related to (1) compression on the cauda equina or sacral nerve roots by an expanding lipoma or lipomeningocele (Yamada et al, 1983), (2) tension on the spinal cord from tethering secondary to differential growth rates in the bony vertebrae and neural elements while the lower end of the cord is held in place by the lipmoa or by a thickened filum terminale (Dubrowitz et al, 1965), or (3) fixation of the split lumbosacral cord by an intravertebral bony spicule or fibrous band (Pang, 1992; Pang et al, 1992; Andar et al, 1997). The overt stretching that invariably occurs when there is a forcible flexion and/or extension of the spinal cord with normal movement leads to changes in oxidation/reduction of cytochrome oxidase, most notably in the lumbosacral spinal neurons when there is no intraspinal pathologic process (Yamada et al, 2004; Henderson et al, 2005). Under normal circumstances the conus medullaris ends just below the L2 vertebra at birth and recedes upward to T12 by adulthood (Barson, 1970). When the cord does not “rise” or is fixed in place owing to one of these lesions, ischemic injury may ensue (Yamada et al, 1981, 2004). Correcting the lesion in infancy has resulted not only in stabilization but also in improvement in the neurologic picture in many instances (Koyangi et al, 1997, Cornette et al, 1998; Proctor et al, 2000) (Fig. 128–14). Sixty percent of infants with abnormal urodynamic findings preoperatively revert to normal postoperatively, with improvement noted in 30%; 10% become worse with time. In older children there is a less dramatic change after surgery, with only 27% becoming normal, 27% improving, 27% stabilizing, but 19% actually becoming worse with time (see Fig. 128–14) (Keating et al, 1988; Satar et al, 1997). Older children with an overactive detrusor tend to improve, whereas those with acontractile bladders do not (Hellstrom et al, 1986; Kondo et al, 1986; Flanigan et al, 1989). Finally, 5% to 27% of children operated on in early childhood develop secondary tethering when observed for several years, suggesting that early surgery has both beneficial and sustaining effects in patients with this condition (Pierre-Kahn et al, 1997; Satar et al, 1997; Proctor et al, 2000).
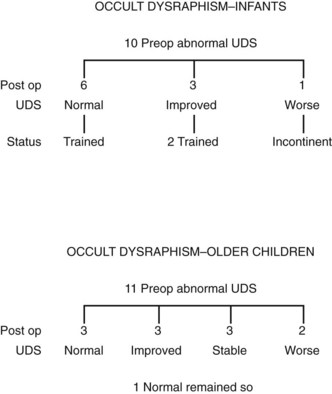
Figure 128–14 The potential for recoverable function is greatest in infants (6 of 10, 60%) and less so in older children (3 of 11, 27%). The risk of damage to neural tissue at the time of exploration to those with normal function is small (2 of 19, 11%). UDS, urodynamic study.
As a result of these findings, it is apparent that urodynamic testing may be the only way to document that an occult spinal dysraphism is actually affecting lower spinal cord function (Keating et al, 1988; Khoury et al, 1990; Sarica et al, 2003). The serial use of electromyography of the external urethral sphincter using a needle electrode to monitor individual motor unit action potentials provides a precise mechanism for measuring changes in innervation that may occur over time. Some investigators have shown that posterior tibial somatosensory evoked potentials are an even more sensitive indicator of tethering and should be an integral part of the urodynamic evaluation (Roy et al, 1986). The implication of these findings lies in the fact that early detection and early intervention can either reverse or at least stabilize the progression of the lesion, which does not happen in older children (Yamada et al, 1983; Tami et al, 1987; Kaplan et al, 1988) and offers a degree of protection from subsequent tethering (Pierre-Kahn et al, 1997; Satar et al, 1997; Proctor et al, 2000), which seems to be a frequent occurrence when the lesion is not dealt with expeditiously in infancy (Chapman, 1982; Seeds and Jones, 1986).
Recommendations
In addition to MRI studies (Tracey and Hanigan, 1990), urodynamic testing including EMG of the external urethral sphincter should be performed in every child who has a questionable cutaneous or bony abnormality of the lower spine, especially if there is a radiologic abnormality of the spinal cord (Packer et al, 1986; Campobasso et al, 1988; Hall et al, 1988; Meyrat et al, 2003). This test provides the most accurate measure of sacral spinal cord function at diagnosis and provides a basis for comparison with subsequent studies when the children are either operated on or carefully observed. In children younger than 3 months of age the vertebral bones have not ossified; thus a window of opportunity exists for ultrasonography to be a useful screening tool in visualizing the spinal canal (Fig. 128–15) (Raghavendra et al, 1983; Scheible et al, 1983). At this age there is good correlation between the ultrasound imaging and MRI findings; however, if a spinal cord abnormality is identified the latter provides for a better definition of the spinal cord lesion. Consequently, ultrasonography should not be used as the definitive imaging modality (Hughes et al, 2003). Older children with an occult spinal cord lesion may have urologic symptoms in 20% of cases (Hsieh et al, 2006), and 50% to 60% may have abnormal urodynamic findings preoperatively (Geurra et al, 2006). Resolution of the abnormal urodynamic parameters is noted in 50% to 60% of cases after detethering (Hseih et al, 2006; Geurra et al, 2006). Therefore, urodynamic study is recommended for all children with an occult spinal dysraphism before and after spinal cord detethering procedures.
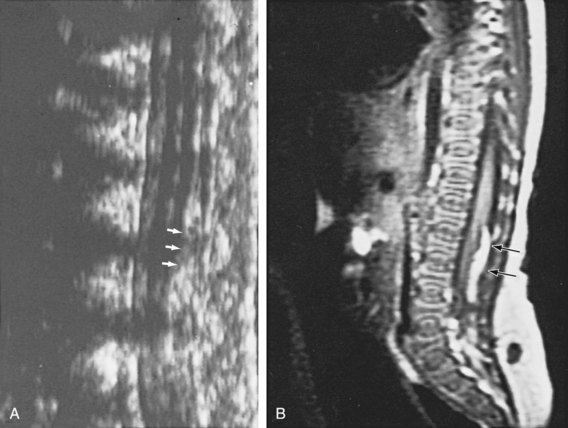
Figure 128–15 A, During the first few months of life, ultrasonography can clearly demonstrate intravertebral anatomy because the posterior arches have not completely ossified. Note that the spinal cord along with its central canal is displaced anteriorly (white arrows) beginning at L3 because of an intradural lipoma. B, The MR image is juxtaposed to confirm the ultrasound findings. The longitudinal white intraspinal mass (black arrows) is the lipoma; the longitudinal gray mass is the spinal cord.
In the past, most of these conditions were treated by excising the superficial skin lesion, without dissecting further into the spinal canal to remove or repair the entire abnormality. Currently, most neurosurgeons advocate laminectomy and removal of the intraspinal process as completely as possible without injuring the nerve roots or cord to release the tether and prevent further injury with subsequent growth (Linder et al, 1982; Kondo et al, 1986; Kaplan et al, 1988; Foster et al, 1990; Atala et al, 1992; Pierre-Kahn et al, 1997; Proctor et al, 2000; Jindal and Mahapatra, 2000; Nogueira et al, 2004).
Sacral Agenesis
Sacral agenesis has been defined as the absence of part or all of two or more lower vertebral bodies. The cause of this condition is still uncertain, but teratogenic factors may play a role, because insulin-dependent diabetic mothers have a 1% chance of giving birth to a child with this disorder. Conversely, 16% or more of children with sacral agenesis have a mother who is insulin dependent with diabetes mellitus (Passarge and Lenz, 1966; Guzman et al, 1983; Wilmshurst et al, 1999). Often the mothers have only gestational insulin-dependent diabetes. The disease has been reproduced in chicks by exposing embryos to insulin (Landauer, 1945; White and Klauber, 1976). Maternal insulin-antibody complexes have been noted to cross the placenta, and their concentration in the fetal circulation is directly correlated with macrosomia (Menon et al, 1990). It is possible that a similar cause-and-effect phenomenon occurs in sacral agenesis. There is evidence that a deletion of the seventh chromosome (7q36) leading to the absence of a transcription factor may be responsible for this anomaly (Papapetrou et al, 1999). In addition, maternal drug exposure (i.e., minoxidil) has been reported to cause sacral agenesis (Rojansky et al, 2002).
In familial cases of sacral agenesis associated with the Currarino syndrome (presacral mass, sacral agenesis, and anorectal malformation), deletions in chromosome 7 (7q) resulting in HLXB9 genetic mutations have been found (Ross et al, 1998). A mutation in HLXB9, a homeodomain gene of a 403 amino acid protein that appears to be responsible for neural plate infolding, has been identified in 20 of 21 patients with familial cases and in 2 of 7 sporadic cases of Currarino syndrome (Hagan et al, 2000; Kochling et al, 2001). Heterozygote carriers within these families have also been identified (Lynch et al, 2000). Thus, sacral agenesis may represent one point on a spectrum of abnormalities that encompass sacral meningoceles and anorectal malformations (Bernbeck et al, 2004).
Diagnosis
The presentation is bimodal, with more than three fourths of the disorders being detected in early infancy and the remainder discovered between 4 and 5 years of age (Wilmshurst et al, 1999). With the increased use of prenatal ultrasonography, it is being diagnosed with increased frequency before birth. Sacral vertebral bony ossification begins at 15 weeks, so it is possible to detect the lesion after 18 weeks of gestation on an ultrasound examination and then to confirm it by fetal MRI (De Biasio et al, 2003). When not detected prenatally or at birth, the diagnosis is often delayed until failed attempts at toilet training bring the child to the attention of a physician. Sensation, including that in the perianal dermatomes, is usually intact, and lower extremity function is normal (Koff and DeRidder, 1977; Jakobson et al, 1985; Capitanucci et al, 1997). Because these children have normal sensation and little or no orthopedic deformity in the lower extremities (although high arched feet or claw toes or hammer toes may be present), the underlying lesion is often overlooked. In fact, 20% escape detection until the age of 3 or 4 years (Guzman et al, 1983). The only clue, besides a high index of suspicion, is flattened buttocks and a low, short gluteal cleft (Bauer, 1990) (Fig. 128–16). Palpation of the coccyx is helpful in detecting the absent vertebrae (White and Klauber, 1976). The diagnosis is most easily confirmed with a lateral film of the lower spine, because this area is often obscured by the overlying gas pattern on an anteroposterior projection (White and Klauber, 1976; Guzman et al, 1983) (Fig. 128–17). MRI has been used to visualize the spinal cord in these cases; its imaging reveals a sharp cutoff of the conus at T12 as a consistent finding (Pang, 1993; Diel et al, 2001) (Fig. 128–18).
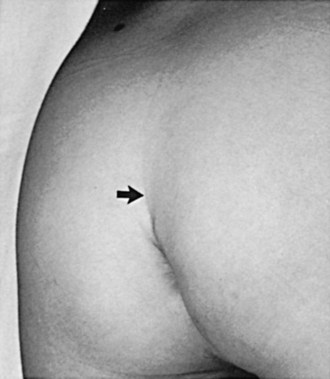
Figure 128–16 Characteristically, in sacral agenesis the gluteal crease is short and is seen only inferiorly (below arrow) because of the flattened buttocks.
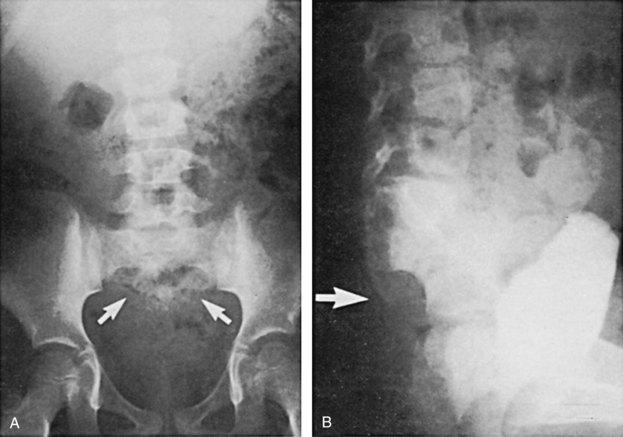
Figure 128–17 The diagnosis of partial or complete sacral agenesis (arrows) is easily confirmed on an anteroposterior (A) or lateral (B) radiograph of the spine if bowel gas obscures the sacral area.
(From Bauer SB. Early evaluation and management of children with spina bifida. In: King LR, editor. Urologic surgery in neonates and young infants. Philadelphia: WB Saunders; 1988. p. 283–310.)
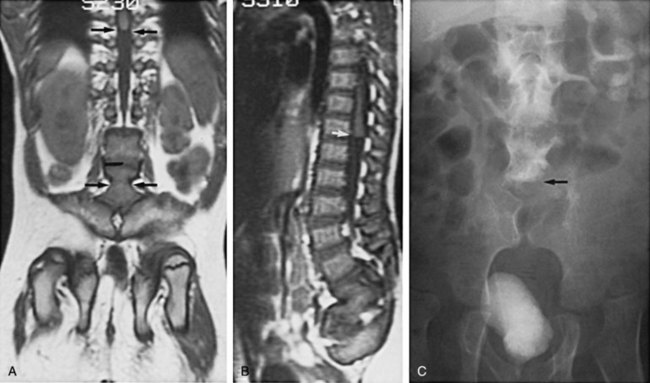
Figure 128–18 Coronal (A) and sagittal (B) MR images in a 10-year-old boy with sacral agenesis beginning at L5. Note the squared lower limit of the cord adjacent to T11 (A, upper arrows; B, white arrow) and the two sacroiliac joints (A, lower arrows), which are in the midline because of absence of the sacrum. C, An anteroposterior radiograph from an excretory urogram shows no vertebral bodies below L4 (arrow).
Key Points: Sacral Agenesis
Findings
On urodynamic evaluation an almost equal number of individuals manifest either an upper or a lower motor neuron lesion (35% vs. 40%, respectively); 25% have no sign of denervation (Guzman et al, 1983; Boemers et al, 1994). The number of affected vertebrae does not seem to correlate with the type of motor neuron lesion present (Boemers et al, 1994) (Fig. 128–19). The injury appears to be stable and rarely shows signs of progressive denervation as the child grows. Sacral sensation is relatively spared, even in the presence of extensive sacral motor deficits (Boemers et al, 1994; Schlussel et al, 1994). Urinary tract infection may be detected in 75% of children over time, with vesicoureteral reflux diagnosed in 37%. Reflux is most likely to occur in those with an upper (75%) (irrespective of whether they have synergy or dyssynergy) versus a lower (40%) motor neuron lesion (Schlussel et al, 1994; Wilmshurst et al, 1999).
Recommendations
Because most children who are diagnosed with this condition have a neurologic deficit, urodynamic testing is mandatory at the time of diagnosis. Renal ultrasonography and nuclear or conventional cystography should be included as part of the evaluation process, especially if the child has a history of urinary tract infection or findings of an upper motor neuron lesion on urodynamic testing. Additional imaging studies may be required based on the child’s history and findings from baseline studies.
Management depends on the specific type of neurourologic dysfunction seen on urodynamic testing. Anticholinergic agents should be given to children with upper motor neuron findings of detrusor overactivity, whereas CIC and α-sympathomimetic medications may have to be given to individuals with lower motor neuron deficits who cannot empty their bladders or stay dry between catheterizations. When anticholinergic medication is ineffective in controlling the overactive detrusor, augmentation cystoplasty may be required to attain an adequate organ for urinary storage. Failure of α-sympathomimetic agents may require either endoscopic injection of bulking agents or even artificial urinary sphincter implantation to increase bladder outlet resistance. The bowels manifest a similar picture of dysfunction and need as much characterization and treatment as the lower urinary tract. Anorectal manometry has identified abnormalities in the internal anal sphincter and in voluntary anal sphincter squeeze pressure, leading to weakness of the muscle and concomitant fecal incontinence (Morera and Nurko, 2003). It is important to identify these individuals as early as possible so that they can become continent and out of diapers at an appropriate age, thus avoiding the social stigma of fecal or urinary incontinence.
Imperforate Anus
Imperforate anus is a condition that can occur alone or as part of a constellation of anomalies that have been called the VATER or VACTERL syndrome (Barry and Auldist, 1974). This mnemonic denotes all the regions or structures that can possibly be affected (vertebral, anal, cardiac, tracheo-esophageal fistula, renal, limb). The incidence of anorectal malformations varies between 1 in 4000 to 1 in 5000 live births (Templeton and O’Neill, 1986). There is a male predominance of 1.5:1. The majority of female patients have low lesions, whereas males tend to have high ones (Santulli et al, 1971). Several classification systems have been devised, but the most commonly used one is the Wingspread Workshop on Anorectal Malformations (Table 128–5) (Shaul and Harrison, 1997). This format attempts to divide the lesions into high, intermediate, or low, depending on whether the rectum ends above, at, or below the levator ani muscle, respectively. The relative incidence for the various levels of the defect is 36% high, 14% intermediate, 47% low, and 1% a cloacal lesion. Imperforate anus may be part of a spectrum of hindgut abnormalities that include sacral agenesis and even a presacral mass or meningocele recently labeled as Currarino syndrome (Currarino et al, 1981; Hagan et al, 2000), which has genetic implications (Ross et al, 1998).
Table 128–5 Wingspread Classification of Anorectal Malformations
| FEMALE | MALE | |
|---|---|---|
| High | Anorectal agenesis | Anorectal agenesis |
| With rectovaginal fistula | With rectourethral (prostatic) fistula | |
| Without fistula | Without fistula | |
| Rectal atresia | Rectal atresia | |
| Intermediate | Rectovestibular fistula | Rectovestibular urethral fistula |
| Rectovaginal fistula | Anal agenesis without fistula | |
| Anal agenesis without fistula | ||
| Low | Anovestibular fistula | Anocutaneous fistula |
| Anocutaneous fistula | Anal stenosis | |
| Anal stenosis | Rare malformation | |
| Cloacal malformation | ||
| Rare malformation |
Associated Findings
A fistulous communication with the lower urinary tract is a common occurrence, related in part to the sex of the child and the extent of the lesion: 87% of male and 79% of female infants with a high or intermediate lesion have a connection, whereas 7% of male and 10% of female children with a low lesion have such a communication (Templeton and O’Neill, 1986; Holschneider et al, 1994). Urinary tract abnormalities have been noted in 26% to 52% of affected children, with renal agenesis (primarily left-sided) and vesicoureteral reflux as the most common associated findings (Parrott, 1985). Again, the highest incidence of an abnormality is in those children with a high (70%) versus a low (infralevator) (35%) lesion (Shaul and Harrison, 1997; Emir and Soylet, 1998), with boys more prone than girls to having an anomaly (50% vs. 29%) (Metts et al, 1997). Spinal bony abnormalities range in incidence from 30% to 44%, but patients with a high lesion are more likely to be affected (48% to 54%) than those with a low lesion (15% to 27%) (Carson et al, 1984; Tsakayannis and Shamberger, 1995; Long et al, 1996). Spinal cord abnormalities including a tethered cord, thickened or fatty filum terminale, and a lipoma have been noted in 18% to 50% of patients, with the incidence varying proportionately in relation to the height of the rectal lesion (Shaul and Harrison, 1997). Not all patients with a spinal cord abnormality have a bony defect, so intraspinal imaging is mandatory to ensure the presence of a normal spinal cord, especially in cases of high lesions (Rivosecchi et al, 1995). On occasion, an abnormal spinal cord can be found in patients even without one of these commonly associated other anomalies. Neurogenic bladder dysfunction is a frequent finding (Kakizaki et al, 1994), usually manifesting as incontinence, but its occurrence is rare when no spinal cord malformation exists (Hulthen de Medina et al, 2004) (Fig. 128–20). It often manifests when the child is older and the parents have difficulty with toilet training. It is not certain whether the dysfunction is caused by a spinal cord defect or by trauma to the nerves supplying the lower urinary tract or the pelvic floor musculature occasioned during surgery to repair the spinal cord or rectal defect (Parrott and Woodard, 1979). With the advent of the Peña posterior sagittal midline approach, the latter etiology is less likely (Peña, 1986; Boemers et al, 1994). Despite careful dissection techniques, 37% still have long-term problems with fecal incontinence (Peña and Hong, 2000).
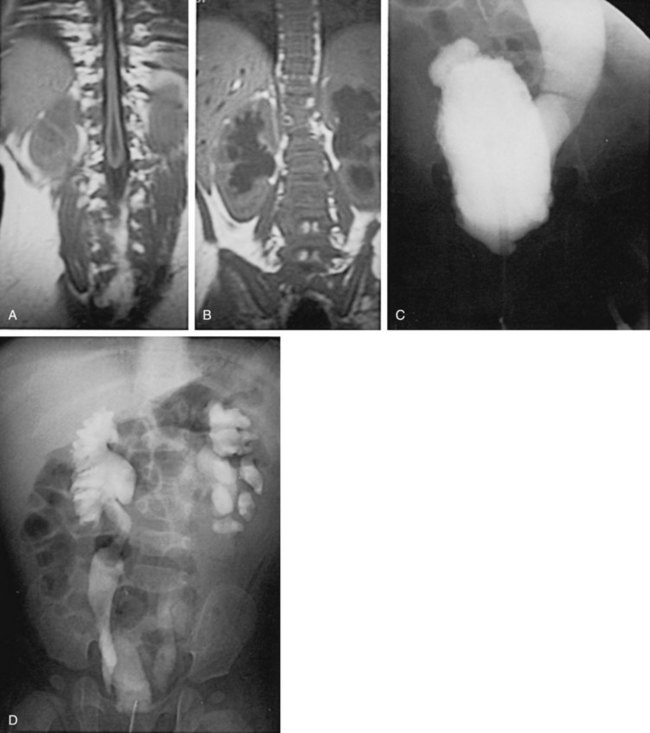
Figure 128–20 A and B, A 1-year-old girl with an imperforate anus and bony vertebral abnormalities has a tethered cord and bilateral hydronephrosis on MRI. C, Voiding cystourethrogram reveals significant trabeculation and reflux on the left. D, Excretory urogram demonstrates bilateral hydronephrosis secondary to the reflux on the left and a ureterovesical junction obstruction on the right. Subsequent urodynamic study revealed poor detrusor compliance and dyssynergy.
Neonatal Assessment
Initial evaluation in the neonatal period should include a careful inspection of the perineum looking for a fistulous site from the bowel, an examination of the upper and lower extremities, and an assessment of the spine and spinal cord (Carson et al, 1984; Mosiello et al, 2003). A prone cross-table lateral image with the child held in that position for 3 minutes and a radiopaque marker placed on the perineum will help delineate the distance from the end of the rectum to the skin in order to classify the extent of the rectal defect. Gas, meconium, or a gram-negative infection in the urine strongly suggests a rectal/urinary tract fistula, which then necessitates a divided colostomy very early in the postnatal period. Once the child is stable clinically, radiologic investigation of the urinary tract is begun, first with renal ultrasonography and then with VCUG, to define the presence of an abnormality and discover any clues for possible neurogenic bladder dysfunction. Vertebral bony anomalies, especially sacral level lesions, often signify an underlying spinal cord abnormality (Boemers et al, 1996). Thus any bony abnormality warrants ultrasonography of the spine if the child is younger than 3 months of age because the vertebral bodies have not ossified and a spinal MRI to rule out any abnormal intraspinal process (see Fig. 128–20) (Barnes et al, 1986; Tunell et al, 1987; Emir and Soylet 1998; Karrer et al, 1988; Mosiello et al, 2003). Urodynamic studies including sphincter electromyography are reserved for those children with either a bony abnormality of the spine, a spinal cord defect, or the telltale signs of dysfunction on VCUG or renal ultrasonography (Taskinen et al, 2002; Mosiello et al, 2003). These studies should be conducted early in infancy before the child has had any definitive surgery for the imperforate anus and again after a pull-through operation has been performed on the rectum to determine, respectively, the true incidence of neurogenic bladder dysfunction and any changes that might have occurred as a result of the surgery. The presence of an abnormality on urodynamic testing in early infancy may warrant either intervention at that time to correct a spinal cord defect or watchful waiting to determine whether the lesion is progressive. Urodynamic studies are repeated subsequently or performed for the first time if secondary urinary or fecal incontinence ensues (Taskinen et al, 2002).
The most common finding on urodynamic testing is an upper motor neuron lesion with an overactive detrusor and/or DSD (Boemers et al, 1994; Taskinen et al, 2002), but a lower motor neuron lesion with an acontractile detrusor and a denervated sphincter may be seen as well (see Fig. 128–20) (Greenfield and Fera, 1991; Taskinen et al, 2002). EMG assessment of the perianal musculature at this time helps determine the exact location of the future anus. In addition, it can provide precise information about the innervation to the levator ani muscle and its competence to function as a sphincter. The posterior midline approach espoused by Peña (1986) minimizes the chances of injuring the pelvic nerves that innervate the pelvic floor muscles and therefore reduces the risk of an iatrogenic cause for neurogenic bladder dysfunction.
Central Nervous System Insults
Cerebral Palsy
Pathogenesis
Cerebral palsy is a nonprogressive injury of the brain occurring in the perinatal period that results in either a neuromuscular disability, a specific symptom complex, or cerebral dysfunction (Kuban and Leviton, 1994). Its incidence is approximately 1.5 per 1000 births, but the incidence is increasing as smaller and younger premature infants survive in intensive care units (Kuban and Leviton, 1994). The condition is usually caused by a perinatal infection (e.g., sepsis, meningoencephalitis) or by a period of anoxia (or hypoxia) that affects the CNS (Nelson and Ellenberg, 1986; Naeye et al, 1989). It most commonly appears in infants who were premature, weighed less than 2 kg at birth, had intraventricular hemorrhage, and received mechanical ventilation for a prolonged time in the postnatal period and in premature infants weighing more than 2 kg who experienced a neonatal seizure (Kim et al, 1999). Maternal urinary infection in the later stages of pregnancy increases the risk of having an affected child by four to five times (Polivka et al, 1997).
Diagnosis
Affected children have delayed gross motor development, abnormal fine motor performance, altered muscle tone, abnormal stress gait, and exaggerated deep tendon reflexes. These findings can vary substantially, from obvious to very subtle when no discernible lesion is present, unless a careful neurologic examination is performed. These abnormalities may not manifest in the postnatal period, but they do become evident over time, because myelination of axons and maturation of neurons in the basal ganglia are required before spasticity, dystonia, and athetosis become apparent (Kyllerman et al, 1982). The lesions are classified according to which extremities are involved (monoplegia, hemiplegia, diplegia, and quadriplegia) and what kind of neurologic dysfunction is present (spastic, hypotonic, dystonic, athetotic, or a combination thereof). Among the more overtly affected individuals, spastic diplegia is the most common of the five types of dysfunction that characterize this disease, accounting for almost two thirds of the cases (Kuban and Leviton, 1994).
Findings
Most children with cerebral palsy develop total urinary control. Incontinence is a feature in some, but the exact incidence has never been truly determined (McNeal et al, 1983; Decter et al, 1987). In a recent survey of questionnaires sent to over 600 families with an affected child aged 4 to 18 years, 23.5% had persistent urinary incontinence (Roijen et al, 2001). When urinary continence was achieved, its development was delayed when compared with normal children of similar age. With further analysis of the data, 80% of children with spastic diplegia, 54% with tetraplegia, and 38% with low intellectual capacity attained continence. The presence of incontinence is often related to the extent of the physical impairment, because the handicap prevents the child from getting to the bathroom on time, causing an episode of wetting (Murphy et al, 1995). A number of children have such a severe degree of mental retardation that they are not trainable. They either do not recognize the need to void or cannot relate this fact to caregivers early enough to be toileted successfully (Roijen et al, 2001). The majority, however, have sufficient intelligence to learn basic societal protocols with patient and persistent handling. When continence is achieved (usually at a later than expected age), diurnal continence is attained first with nocturnal continence occurring within the subsequent year (Roijen et al, 2001). Therefore, urodynamic evaluation is reserved for children who appear to be trainable and do not seem to be hampered too much by their physical impairment but who have not achieved continence by late childhood or early puberty.
In one review (Decter et al, 1987), urodynamic studies were performed in 57 children with cerebral palsy (Table 128–6). Forty-nine (86%) had the expected picture of a partial upper motor neuron type of dysfunction, with exaggerated sacral reflexes, an overactive detrusor and/or DSD (Fig. 128–21), even though they manifested voluntary control over voiding. Six (11%) of the 57 had evidence of both upper and lower motor neuron denervation with an acontractile detrusor or abnormal motor unit potentials on sphincter EMG assessment (Table 128–7). Most of the children who exhibited these latter findings were found to have an episode of cyanosis in the perinatal period when their records were analyzed on a retrospective basis (Table 128–8). Therefore, a lower motor neuron lesion may be seen in addition to the expected upper motor neuron dysfunction. Similar findings have been noted subsequently (Mayo, 1992; Reid and Borzyskowski, 1993).
Table 128–6 Lower Urinary Tract Function in Cerebral Palsy
| TYPE | NO. PATIENTS | % |
|---|---|---|
| Upper motor neuron lesion | 49 | 86 |
| Mixed upper + lower motor neuron lesion | 5 | 9.5 |
| Incomplete lower motor neuron lesion | 1 | 1.5 |
| No urodynamic lesion | 2 | 3 |
Adapted from Decter RM, Bauer SB, Khoshbin SJ, et al. Urodynamic assessment of children with cerebral palsy. J Urol 1987;138:1110.
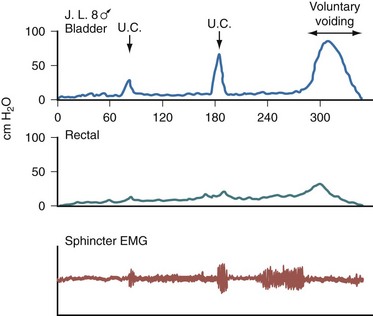
Figure 128–21 Electromyogram (EMG) of an 8-year-old boy with spastic diplegia shows a typical partial upper motor neuron type bladder with uninhibited contractions (U.C.) associated with increased sphincter activity but normal voiding dynamics at capacity. Wetting is caused by these contractions when they are unaccompanied by the heightened sphincter activity.
Table 128–7 Urodynamic Findings in Cerebral Palsy*
| TYPE OF LESION | NO. PATIENTS |
|---|---|
| Upper Motor Neuron | |
| Uninhibited contractions | 35 |
| Detrusor sphincter dyssynergy | 7 |
| Hyperactive sacral reflexes | 6 |
| No voluntary control | 3 |
| Small-capacity bladder | 2 |
| Hypertonia | 2 |
| Lower Motor Neuron | |
| Excessive polyphasia | 5 |
| ↑ Amplitude + ↑ duration potentials | 4 |
* Some patients had more than one finding.
Adapted from Decter RM, Bauer SB, Khoshbin S, et al. Urodynamic assessment of children with cerebral palsy. J Urol 1987;138:1110.
Table 128–8 Perinatal Risk Factors in Cerebral Palsy
| FACTOR | UPPER MOTOR NEURON LESION (NO. PATIENTS) |
LOWER MOTOR NEURON LESION (NO. PATIENTS) |
|---|---|---|
| Prematurity | 10 | 1 |
| Respiratory distress/arrest/apnea | 9 | 2 |
| Neonatal seizures | 5 | — |
| Infection | 5 | — |
| Traumatic birth | 5 | — |
| Congenital hydrocephalus | 3 | — |
| Placenta previa/abruption | 2 | 2 |
| Hypoglycemia ± seizures | 2 | — |
| Intracranial hemorrhage | 2 | — |
| Cyanosis at birth | 1 | 3 |
| No specific factor noted | 15 | — |
Adapted from Decter RM, Bauer SB, Khoshbin S, et al. Urodynamic assessment of children with cerebral palsy. J Urol 1987;138:1110.
In two more recent reviews of 65 children with cerebral palsy undergoing urodynamic studies (Bross et al, 2004; Karaman et al, 2005), abnormal bladder and urethral sphincter function was found in almost all. In the first study involving 29 (Bross et al, 2004), 23 had incontinence and/or urinary tract infection whereas 6 were asymptomatic; urodynamic studies demonstrated poor compliance in 21 of the 23 symptomatic and 2 of the 6 asymptomatic children, an overactive detrusor in all symptomatic and 5 of 6 asymptomatic children, and high leak point pressure in 16 of 23 and 4 of 6 symptomatic and asy-mptomatic children, respectively. VCUG revealed bladder trabeculation in 58% and reflux in 9% of symptomatic children, and trabeculation in 50% but no reflux in the asymptomatic children. In the other study (Karaman et al, 2005), 66% had dysfunctional voiding symptoms, including 47.2% with daytime incontinence and 44.4% with difficulty urinating. Neurogenic detrusor overactivity was present in 47.2%, and dyssynergy between the bladder and sphincter was noted in 11%. In a meta-analysis of all published reports of urodynamic findings in children with cerebral palsy, detrusor overactivity was seen in 80%, dyssynergy between the bladder and sphincter in 5%, and normal function in only 12% (Decter et al, 1987; Mayo and Burns, 1990; Reid et al, 1993; Bross et al, 2004; Karaman et al, 2005). From these reports it is apparent that urodynamic studies can be very revealing in all children, whether they have urinary symptoms (Bross et al, 2004), but are especially useful if frequent toileting or initial anticholinergic treatment fails to improve incontinence, the child develops urinary infection, or renal ultrasonography reveals hydronephrosis. Upper urinary tract imaging is usually normal even in children with upper motor neuron lesions (Brodak et al, 1994).
Key Points: Cerebral Palsy
Recommendations
Treatment usually centers on abolishing the overactive detrusor using anticholinergic medication, but residual urine must be monitored closely to ensure complete evacuation with each void. CIC may be required for those who cannot empty their bladder. Selective dorsal rhizotomy has improved bladder capacity, reduced the detrusor overactivity, and increased compliance, thus rendering the patient more continent, in a selected group of children who failed to respond to less invasive measures (Houle et al, 1998). The risk of neurourologic deterioration from this procedure is extremely low, with less than 5% developing incontinence if careful mapping of dorsal nerve roots are identified (Huang, 1997; Steinbok and Schrag, 1998; Liu et al, 1999). Upper and lower urinary tract imaging is not recommended unless urinary tract infection has occurred (Reid and Borzyskowski, 1993).
Traumatic Injuries to the Spine
Diagnosis
Despite increased exposure to and the potential for traumatic spinal cord injuries, such injuries are rarely encountered in children. The incidence in Sweden is 2.6 cases per million children per year (Augutis and Levi, 2003). The incidence tends to increase geometrically with age (Anderson and Schutt, 1980) (Fig. 128–22). When an injury does occur, it is more likely to happen in a male than a female child, and it is usually the result of a motor vehicle or bicycle accident (24% to 52%), a fall from a high place, a gunshot wound, or a diving or sports incident (Cass et al, 1984; Hadley et al, 1988; Decter and Bauer, 1993; Brown et al, 2001; Augutis and Levi 2003; Cirak et al, 2004). The type of causative injuries varies with age, with infants more prone to an injury from a motor vehicle accident (71%), toddlers and children injured secondary to a fall (48% and 34%, respectively) and adolescents having a sports-related event (29%) (Cirak et al, 2004). An injury may also occur iatrogenically after surgery to correct scoliosis, kyphosis, or other intraspinal processes or congenital aortic anomalies or after ligation of a patent ductus arteriosus (Cass et al, 1984; Batista et al, 1995). Neonates are particularly prone to a hyperextension injury during a high forceps delivery (Adams et al, 1988; Lanska et al, 1990). Among younger children, girls are affected as often as boys (Ruge et al, 1988).
Pathogenesis
Spinal cord injuries in children are intrinsically different from those in adults because of a variety of factors, including the mechanism of injury and the difference in configuration of the brainstem and spinal cord in children compared with adults. In addition, the horizontal versus vertical orientation of the facet joints in vertebral bodies that predisposes to anteroposterior subluxation in children, the delayed supportive effect of the paraspinous musculature and ligaments, and the relative heaviness of the head, which causes a fulcrum of maximal flexion of the upper cervical region in infants and young children, all contribute to a high degree of hypermobility that places the child’s spinal cord at risk for ischemic necrosis (Decter and Bauer, 1993).
Key Points: Traumatic Injuries to the Spine
Findings
The lower urinary tract dysfunction that ensues is not likely to be an isolated event but is usually associated with loss of sensation and paralysis of the lower limbs. Radiologic investigation of the spine may not reveal any bony abnormality, although momentary subluxation of osseous structures resulting from the elasticity of the vertebral ligaments can result in a neurologic injury (Pollack et al, 1988). This condition has been seen only in children (usually younger than 8 years old) and has been labeled spinal cord injury without radiologic abnormality (SCIWORA) (Pang and Wilberger, 1982; Pang and Pollack, 1989). Overall, SCIWORA can account for up to 38% of spinal cord injuries in children (Brown et al, 2001). Myelography and computed tomography show swelling of the cord below the level of the lesion (Adams et al, 1988; Lanska et al, 1990). Often, what appears to be a permanent lesion initially turns out to be a transient phenomenon with time. Although sensation and motor function in the lower extremities may be restored relatively quickly, the dysfunction involving the bladder and rectum may persist considerably longer.
During the acute phase of the injury the bladder is often acontractile and the urethral sphincter nonreactive, although normal-appearing bioelectric potentials can be recorded on sphincter EMG (spinal shock). Over a variable period of time, detrusor contractility and sphincter reactivity return as spinal cord edema subsides. With this return of function, an overactive detrusor and bladder-sphincter dyssynergy develop if the lateral reticulospinal cord pathways to and from the brainstem have been disrupted. When the lesion affects the cauda equina there is probably little to no return of bladder or urethral sphincter function. Sacral sensation and peripheral reflexes are not good indicators of ultimate lower urinary tract function (Shenot et al, 1998). Over time, the predominant urodynamic pattern in patients with a thoracic-level lesion is an overactive detrusor with DSD, high voiding pressures, eventual hydronephrosis, and vesicoureteral reflux. Often children exhibit a highly compliant bladder for a portion of bladder filling but then have C-fiber–mediated, small, ineffective rhythmic contractions of the detrusor with simultaneous waxing and waning of external urethral sphincter activity. Some urinary leakage may occur with these contractions, but in general the bladder does not empty with them. Patients with an upper thoracic or cervical lesion are likely to exhibit autonomic dysreflexia with a spontaneous discharge of α1-stimulants during bladder filling and with contractions of the detrusor. Monitoring of blood pressure and availability of α-antagonists are mandatory during VCUG or urodynamic studies (Perkash, 1997; Vaidyanathan et al, 1998b).
Management
If urinary retention occurs immediately after the injury, an indwelling Foley catheter is passed into the bladder and left in place for as short a time as possible, until the patient’s condition is stable and aseptic intermittent catheterization can be started safely on a regular basis (Guttmann and Frankel, 1966; Barkin et al, 1983). There is no difference in the incidence of urinary infection or in the development of stones in patients using sterile versus clean catheterization techniques to empty the bladder (Prieto-Fingerhut et al, 1997; VanHala et al, 1997). Rates of infection range as high as 60% to 80% (Biering-Sorensen et al, 1999), and stone formation occurs in 1.5% to 3% within the first 5 years after the trauma (Donnellan and Bolton, 1999; McKinley et al, 1999). When the child starts to void again, the timing of catheterization can be regulated so that it is used as a means of measuring the residual urine after a spontaneous void. Residual urine volumes of 25 mL or less are considered safe enough to allow reducing the frequency or even stopping the catheterization program (Barkin et al, 1983). After 4 to 6 weeks, if there is no improvement in lower urinary tract function, urodynamic studies are conducted to determine whether the condition is the result of spinal shock or an actual nerve root or spinal cord injury. An acontractile detrusor is not uncommon under these circumstances (Iwatsubo et al, 1985). On the other hand, EMG recording of the sphincter often reveals normal motor units without fibrillation potentials but absent sacral reflexes and a nonrelaxing sphincter with bladder filling, a sign that transient spinal shock has occurred (Iwatsubo et al, 1985). The outcome of this situation is guarded but good, because most cases resolve completely as edema of the cord in response to the injury subsides, leaving no permanent damage (Iwatsubo et al, 1985; Fanciullacci et al, 1988).
If and when bladder function returns and emptying is incomplete, it has been shown in the cat (Xiao et al, 1999) that peripheral L7 dermatome stimulation initiates a micturition reflex without DSD. In a preliminary study of 15 adults with spinal cord injury, detrusor overactivity, and DSD, the creation of an artificial somatic/CNS/autonomic reflex pathway resulted in satisfactory bladder control in 67% of patients. Follow-up urodynamic study revealed a change to almost normal storage and synergic voiding without DSD (Xiao et al, 2003). A more recent study showed satisfactory bladder control in 75% of patients with complete suprasacral spinal cord injury within 6 to 12 months. However, the mean and median time since the injury was 9 months, so the time from injury may have been a factor in this study (Lin et al, 2009). Incomplete emptying may be enhanced by the judicious use of α-sympatholytic agents (Al-Ali et al, 1999). The goal is balanced voiding at pressures lower than 40 cm H2O, which reduces the 30% risk for urinary tract deterioration seen in poorly managed patients (Giannantoni et al, 1998; Kim et al, 1998). If this cannot be achieved, then CIC is continued. Anticholinergics, either orally or intravesically (Vaidyanathan et al, 1998a; Wein, 1998), or capsaicin (an inhibitor of C-fiber stimulation [Wiart et al, 1998]) have been added and are effective in reducing an overactive detrusor, but at the cost of significant side effects. Alternative treatments that have been effective to ensure complete emptying at low pressure include external urethral sphincterotomy (Kim et al, 1998), urethral stent placement (Chancellor et al, 1999), or injection of botulinum-A toxin into the external sphincter (Schurch et al, 1997, 2000; Kuo, 2003; Tai et al, 2005). In some cases, a continent catheterizable abdominal urinary stoma may be created to facilitate self-catheterization in patients with low cervical or upper thoracic lesions who cannot easily catheterize themselves, in order to minimize the embarrassment of periodic genital organ exposure when care providers need to repeatedly catheterize the bladder (Sylora et al, 1997).
Recommendations
Most permanent traumatic injuries involve either the upper thoracic or the cervical spinal cord but some affect the cauda equina region. The sacral cord injury most likely produces a lower motor neuron deficit of the striated urethral sphincter that usually leads to low pressure bladder emptying with little risk of upper urinary tract deterioration. However, it probably necessitates medical and/or surgical therapy to achieve continence. On the other hand, upper spinal cord injuries produce an upper motor neuron type lesion with an overactive detrusor and DSD. The potential danger from this outflow obstruction is obvious (Donnelly et al, 1972). Substantial residual urine volumes, high-pressure reflux, urinary infections, and their sequelae are the leading causes of long-term morbidity and mortality in patients with spinal cord injury (Giannantoni et al, 1998). Even patients who voluntarily urinate and are continent or who involuntarily but spontaneously void to completion are not immune to urinary tract changes (Decter and Bauer, 1993). Urodynamic studies are imperative to identify which patients are at risk (Barkin et al, 1983). These studies should be performed within 2 to 3 months after the injury, again 6 to 9 months later, and possibly at 2 years after the trauma to determine the stability of lower urinary tract function, the need for continued CIC, and whether adjuvant drug or surgical therapy should be added (or continued) to achieve good long-term success. When these measures are employed judiciously, effective management can be achieved (Pannek et al, 1997). Renal ultrasonography early in the course and VCUG if signs of bladder outlet obstruction are present on urodynamic testing or if recurrent urinary infection ensues are also recommended. Radionuclide cystography is indicated if the patient has repeated urinary tract infection or develops hydronephrosis (Phillips et al, 1997). Because stone formation can be insidious, periodic imaging of the kidneys and bladder is necessary. Early identification and proper management may prevent the signs and effects of bladder outlet obstruction before they become apparent on radiographic examination of the urinary tract (Pearman, 1976; Ogawa et al, 1988; Watanabe et al, 1996).
Bauer SB, Colodny AH, Retik AB. The management of vesico-ureteral reflux in children with myelodysplasia. J Urol. 1982;128:102.
Bauer SB, Hallet M, Khoshbin S, et al. The predictive value of urodynamic evaluation in the newborn with myelodysplasia. JAMA. 1984;152:650.
Blaivas JG, Labib KB, Bauer SB, Retik AB. A new approach to electromyography of the external urethral sphincter. J Urol. 1977;117:773.
Edelstein RA, Bauer SB, Kelly MD, et al. The long-term urologic response of neonates with myelodysplasia treated proactively with intermittent catheterization and anticholinergic therapy. J Urol. 1995;154:1500.
Guzman L, Bauer SB, Hallet M, et al. The evaluation and management of children with sacral agenesis. Urology. 1983;23:506.
Kaefer M, Pabby A, Kelly M, et al. Improved bladder function after prophylactic treatment of the high risk neurogenic bladder in newborns with myelomeningocele. J Urol. 1999;162:1068-1071.
Kaefer M, Rosen A, Darbey M, et al. Pressure at residual volume: a useful adjunct to standard fill cystometry. J Urol. 1997;158:1268-1271.
Lapides J, Diokno AC, Silber SJ, Lowe BS. Clean intermittent self-catheterization in the treatment of urinary tract disease. J Urol. 1972;107:458.
McGuire EJ, Woodside JR, Borden TA, Weiss RM. The prognostic value of urodynamic testing in myelodysplastic patients. J Urol. 1981;126:205.
Mitrofanoff P. Cystometric continente trans-appendiculaire dans le traitement de vessies neurologiques. Chir Pediatr. 1980;21:297.
Palmer LS, Richards I, Kaplan WE. Age related bladder capacity and bladder capacity growth in children with myelomeningocele. J Urol. 1997;158:1261-1264.
Satar N, Bauer SB, Scott RM, et al. Late effects of early surgery on lipoma and lipomeningocele in children less than two years old. J Urol. 1997;157:1434-1437.
Spindel MR, Bauer SB, Dyro FM, et al. The changing neuro-urologic lesion in myelodysplasia. JAMA. 1987;258:1630.
Neuropathic Dysfunction of the Lower Urinary Tract
Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function. Neurourol Urodyn. 2002;21:167-178.
Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Urology. 2003;61:37-49.
Bauer SB. The effects and challenges of bladder outlet obstruction [editorial]. J Urol. 2000;163:3.
Bauer SB, Hallet M, Khoshbin S, et al. The predictive value of urodynamic evaluation in the newborn with myelodysplasia. JAMA. 1984;152:650.
Blaivas JG. A critical appraisal of specific diagnostic techniques. In: Krane RJ, Siroky MB, editors. Clinical neurourology. Boston: Little, Brown; 1979:69-110.
Blaivas JG, Labib KB, Bauer SB, Retik AB. Changing concepts in the urodynamic evaluation of children. J Urol. 1977;117:777.
Edelstein RA, Bauer SB, Kelly MD, et al. The long-term urologic response of neonates with myelodysplasia treated proactively with intermittent catheterization and anticholinergic therapy. J Urol. 1995;154:1500.
Gierup J, Ericsson NO. Micturition studies in infants and children: intravesical pressure, urinary flow and urethral resistance in boys with intravesical obstruction. Scand J Urol Nephrol. 1970;4:217.
Kaefer M, Pabby A, Kelly M, et al. Improved bladder function after prophylactic treatment of the high risk neurogenic bladder in newborns with myelo-meningocele. J Urol. 1999;162:1068-1071.
Kaufman AM, Ritchey ML, Roberts AC, et al. Decreased bladder compliance in patients with myelomeningocele treated with radiologic observation. J Urol. 1996;156:2031-2033.
Lapides J, Diokno AC, Silber SJ, Lowe BS. Clean intermittent self-catheterization in the treatment of urinary tract disease. J Urol. 1972;107:458.
McGuire EJ, Woodside JR, Borden TA, Weiss RM. The prognostic value of urodynamic testing in myelodysplastic patients. J Urol. 1981;126:205.
Nevéus T, von Gontard A, Hoebeke P, et al. The standardization of terminology of lower urinary tract function in children and adolescents: report from the Standardisation Committee of the International Children’s Continence Society. J Urol. 2006;176:314-324.
Ohnishi N, Horan P, Levin SS, Levin RM. Intermittent catheterization limits rabbit bladder dysfunction in response to partial outlet obstruction. J Urol. 2000;163:292-295.
Park JM, Bauer SB, Freeman MR, Peters CA. Oxybutynin chloride inhibits proliferation and suppresses gene expression in bladder smooth muscle cells. J Urol. 1999;162:1110-1114.
Perez LM, Khoury J, Webster D. The value of urodynamic studies in infants less than one year old with congenital spinal dysraphism. J Urol. 1992;148:584.
Smith ED. Urinary prognosis in spina bifida. J Urol. 1972;108:115.
Wu HY, Baskin LS, Kogan BA. Neurogenic bladder dysfunction due to myelomeningocele: neonatal versus childhood treatment. J Urol. 1997;157:2295-2297.
Bates CP, Whiteside CG, Turner-Warwick RT. Synchronous cine/pressure/flow/cysto-urethrography with special reference to stress and urge incontinence. Br J Urol. 1970;42:714.
Bauer SB. Pediatric neurourology. In: Krane RJ, Siroky MB, editors. Clinical neurourology. Boston: Little, Brown; 1979:275-294.
Blaivas JG. A critical appraisal of specific diagnostic techniques. In: Krane RJ, Siroky MB, editors. Clinical neurourology. Boston: Little, Brown; 1979:69-110.
Blaivas JG. EMG: other uses. In: Barrett DM, Wein AJ, editors. Controversies in neuro-urology. New York: Churchill Livingstone; 1979:103-116.
Blaivas JG, Labib KB, Bauer SB, Retik AB. A new approach to electromyography of the external urethral sphincter. J Urol. 1977;117:773.
Blaivas JG, Labib KB, Bauer SB, Retik AB. Changing concepts in the urodynamic evaluation of children. J Urol. 1977;117:777.
Chin-Peuckert L, Rennick JE, Jednak R, et al. Should warm infusion solution be used for urodynamic studies in children? J Urol. 2004;172:1657-1661.
Diokno AC, Koff SA, Bender LF. Periurethral striated muscle activity in neurogenic bladder dysfunction. J Urol. 1974;112:743.
Gierup J, Ericsson NO, Okmain L. Micturition studies in infants and children. Scand J Urol Nephrol. 1979;3:1.
Glazier DB, Murphy DP, Fleisher MH, et al. Evaluation of the utility of video-urodynamics in children with urinary tract infection and voiding dysfunction. Br J Urol. 1997;80:806-808.
Joseph DB. The effect of medium-fill and slow-fill cystometry on bladder pressure in infants and children with myelodysplasia. J Urol. 1992;147:444.
Kaefer M, Rosen A, Darbey M, et al. Pressure at residual volume: a useful adjunct to standard fill cystometry. J Urol. 1997;158:1268-1271.
Kerr LA, Bauer SB, Staskin DR. Abnormal detrusor function precipitating hydronephrosis identified by extended voiding cystometry. J Urol. 1994;152:89.
Koff SA, Kass EJ. Abdominal wall electromyography: a noninvasive technique to improve pediatric urodynamic accuracy. J Urol. 1982;127:736.
Kulseng-Hanssen S, Klevmark B. Ambulatory urodynamic monitoring in women. Scand J Urol Nephrol Suppl. 1996;30:27.
Lorenzo AJ, Wallis MC, Cook A, et al. What is the variability in urodynamic parameters with position change in children? Analysis of a prospectively enrolled cohort. J Urol. 2007;178:2567-2570.
Maizels M, Firlit CF. Pediatric urodynamics: clinical comparison of surface vs. needle pelvic floor/external sphincter electromyography. J Urol. 1979;122:518.
Mayo ME. Detrusor hyperreflexia: the effect of posture and pelvic floor activity. J Urol. 1978;119:635.
McGuire EJ, Cespedes RD, O’Connell HE. Leak-point pressures. Urol Clin North Am. 1996;23:253-262.
McInerney PD, Harris SA, Pritchard A, Stephenson TP. Night studies for primary diurnal and nocturnal diuresis and preliminary results of “clam” ileocystoplasty. Br J Urol. 1991;67:42.
Palmer LS, Richards I, Kaplan WE. Age related bladder capacity and bladder capacity growth in children with myelomeningocele. J Urol. 1997;158:1261-1264.
Passerini-Glazel G, Cisternino A, Camuffo MC, et al. Video-urodynamic studies of minor voiding dysfunction in children: an overview of 13 years’ experience. Scand J Urol Nephrol Suppl. 1992;141:70.
Turner-Warwick RT. Some clinical aspects of detrusor dysfunction. J Urol. 1975;113:539.
Walter JS, Wheeler JSJr, Markley J, et al. Home monitoring of bladder pressure and volume in individuals with spinal cord injury and multiple sclerosis. J Spinal Cord Med. 1998;21:7-14.
Weerasinghe N, Malone PS. The value of video-urodynamics in the investigation of neurologically normal children who wet. Br J Urol. 1993;71:539.
Yalla SV, Sharma GV, Barsamian EM. Micturitional static urethral pressure profile method of recording urethral pressure profiles during voiding and implications. J Urol. 1980;124:649.
Yeung CK, Godley ML, Duffy PG, Ransley PG. Natural filling cystometry in infants and children. Br J Urol. 1995;76:531.
Zerin JM, Lebowitz RL, Bauer SB. Descent of the bladder neck: a urographic finding in denervation of the urethral sphincter in children with myelodysplasia. Radiology. 1990;174:833-836.
Zermann DH, Lindner H, Huschke T, Schubert J. Diagnostic value of natural fill cystometry in neurogenic bladder in children. Eur Urol. 1997;32:223-228.
Adams MC, Mitchell ME, Rink RC. Gastrocystoplasty: an alternative solution to the problem of urological reconstruction in the severely compromised patient. J Urol. 1988;140:1152.
Adzick NS, Sutton LN, Crombleholme TM, Flake AW. Successful fetal surgery for spina bifida. Lancet. 1998;352:1675-1676.
Agarwal SK, McLorie GA, Grewal D, et al. Urodynamic correlates of resolution of reflux meningomyelocele patients. J Urol. 1997;158:580.
Aksnes G, Diseth TH, Helseth A, et al. Appendicostomy for antegrade enema: effects on somatic and psychosocial functioning in children with myelomeningocele. Pediatrics. 2002;109:484.
Altaweel W, Jednack R, Bilodeau C, Corcos J. Repeated intradetrusor botulinum toxin type A in children with neurogenic bladder due to myelomeningocele. J Urol. 2006;175(3 Pt. 1):1102-1105.
Amend B, Hennenlotter J, Schäfer T, et al. Effective treatment of neurogenic detrusor dysfunction by combined high-dosed antimuscarinics without increased side-effects. Eur Urol. 2008;53:1021-1028.
Arata M, Grover S, Dunne K, Bryan D. Pregnancy outcome and complications in women with spina bifida. J Reprod Med. 2000;45:743-748.
Barbalais GA, Klauber GT, Blaivas JG. Critical evaluation of the Credé maneuver: a urodynamic study of 207 patients. J Urol. 1983;130:720.
Barrett DM, Furlow WL. The management of severe urinary incontinence in patients with myelodysplasia by implantation of the AS791/792 urinary sphincter device. J Urol. 1982;128:44.
Bau MO, Younes S, Aupy A, et al. The Malone antegrade colonic enema isolated or associated with urological incontinence procedures: evaluation from patient point of view. J Urol. 2001;165:2399-2403.
Bauer SB. Vesico-ureteral reflux in children with neurogenic bladder dysfunction. In: Johnston JH, editor. International perspectives in urology, vol. 10. Baltimore: Williams & Wilkins; 1984:159-177.
Bauer SB. Myelodysplasia: newborn evaluation and management. In: McLaurin RL, editor. Spina bifida: a multidisciplinary approach. New York: Praeger; 1984:262-267.
Bauer SB. The management of spina bifida from birth onwards. In: Whitaker RH, Woodard JR, editors. Paediatric urology. London: Butterworths; 1985:87-112.
Bauer SB. Bladder neck reconstruction. In: Glenn JF, Graham SD, editors. Urologic surgery. Philadelphia: JB Lippincott; 1990:509-522.
Bauer SB. Urodynamics in myelodysplasia. Presented at the Bladder and Bowel Dysfunction in Myelodysplasia Symposium, Aachen, Germany, April 3, 2003.
Bauer SB. Long-term efficacy of artificial urinary sphincters in children. J Urol. 2008;180:441.
Bauer SB, Colodny AH, Retik AB. The management of vesico-ureteral reflux in children with myelodysplasia. J Urol. 1982;128:102.
Bauer SB, Hallet M, Khoshbin S, et al. The predictive value of urodynamic evaluation in the newborn with myelodysplasia. JAMA. 1984;152:650.
Bauer SB, Kelly MD, Darbe M, Atala A. Late onset detrusor instability in patients with the artificial urinary sphincter [abstract 597]. Presented at the annual meeting of the American Urologic Association, San Antonio, May 18, 1993.
Bauer SB, Labib KB, Dieppa RA, et al. Urodynamic evaluation in a boy with myelodysplasia and incontinence. Urology. 1977;10:354.
Bauer SB, Peters CA, Colodny AH, et al. The use of rectus fascia to manage urinary incontinence. J Urol. 1989;142:516.
Bauer SB, Reda EF, Colodny AH, Retik AB. Detrusor instability: a delayed complication in association with the artificial sphincter. J Urol. 1986;135:1212.
Begger JH, Meihuizen de Regt MJ, Hogen Esch I, et al. Progressive neurologic deficit in children with spina bifida aperta. Z Kinderchir. 1986;41(Suppl. 1):13.
Ben-Chiam J, Jeffs RD, Peppas DS, Gearhart JP. Submucosal bladder neck injections of glutaraldehyde cross-linked bovine collagen for treatment of urinary incontinence in patients with exstrophy/epispadias complex. J Urol. 1995;154:862-864.
Bihrie R, Klee LW, Adams MC, et al. Transverse colon-gastric tube composite reservoir. Urology. 1991;37:36-40.
Blaivas JG, Sinka HP, Zayed AH, et al. Detrusor-sphincter dyssynergia: a detailed electromyographic study. J Urol. 1986;125:545.
Bloom DA, Knechtel JM, McGuire EJ. Urethral dilation improves bladder compliance in children with myelomeningocele and high leak point pressures. J Urol. 1990;144:430.
Bomalaski MD, Teague JL, Brooks B. The long-term impact of urologic management on the quality of life in children with spina bifida. J Urol. 1995;156:778.
Bosco PJ, Bauer SB, Colodny AH, et al. The long-term follow-up of artificial urinary sphincters in children. J Urol. 1991;146:396.
Botto LD, Lisi A, Robert-Gnansia E, Erickson JD. International retrospective cohort study of neural tube defects in relation to folic acid recommendations: are the recommendations working? Obstet Gynecol Surv. 2005;60:563-565.
Bruner JP, Tulipan N, Paschall RL, et al. Fetal surgery for myelomeningocele and the incidence of shunt dependent hydrocephalus. JAMA. 1999;282:1819-1825.
Caione P, Capozza N. Endoscopic treatment of urinary incontinence in pediatric patients: 2-year experience with dextranomer/hyaluronic acid copolymer. J Urol. 2002;168:1868.
Capitanucci ML, Silveri M, Nappo S, et al. Total sacral agenesis and neurogenic bladder dysfunction. Pediatr Med Chir. 1997;19:113.
Cartwright PC, Snow BW. Bladder autoaugmentation: early clinical experience. J Urol. 1989;142:505.
Cartwright PC, Snow BW. Bladder autoaugmentation: partial detrusor excision to augment bladder without use of bowel. J Urol. 1989;142:1050.
Cass AS. Urinary tract complications of myelomeningocele patients. J Urol. 1976;115:102.
Cass AS, Bloom BA, Luxenberg M. Sexual function in adults with myelomeningocele. J Urol. 1986;136:425.
Castellan M, Gosalbez R, Perez-Brayfield M, et al. Tumor in bladder reservoir after gastrocystoplasty. J Urol. 2007;178(4 Pt. 2):1771-1774.
Castellan M, Gosalbez R, Labbie A, et al. Bladder neck sling for treatment of neurogenic incontinence in children with augmentation cystoplasty: long-term results. J Urol. 2005;173:2128-2131.
Catti M, Lortat-Jacob S, Morineau M, Lottmann H. Artificial urinary sphincter in children—voiding or emptying? An evaluation of functional results in 44 patients. J Urol. 2008;180:690-693.
Centers for Disease Control and Prevention. Spina bifida and anencephaly before and after folic acid mandate—United States, 1995-1996 and 1999-2000. MMWR Morb Mort Wkly Rep. 2004;53:362-365.
Chiaramonte RM, Horowitz EM, Kaplan GA, et al. Implications of hydronephrosis in newborns with myelodysplasia. J Urol. 1986;136:427.
Cohen RA, Rushton HG, Belman AB, et al. Renal scarring and vesicoureteral reflux in children with myelodysplasia. J Urol. 1990;144:541.
Colvert IIIJR, Kropp BP, Cheng EY, et al. The use of small intestinal submucosa as an off-the-shelf urethral sling material for pediatric urinary incontinence. J Urol. 2002;168:1872-1876.
Committee on Genetics, American Academy of Pediatrics, Desposito F (chairperson). Folic acid for the prevention of neural tube defects. Pediatrics. 1999;104:325-327.
Cromer BA, Enrile B, McCoy K, et al. Knowledge, attitudes and behavior related to sexuality in adolescents with chronic disability. Dev Med Child Neurol. 1990;32:602.
Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by preconceptual vitamin supplementation. N Engl J Med. 1992;321:1832.
Dator DP, Hatchett L, Dyro EM, et al. Urodynamic dysfunction in walking myelodysplastic children. J Urol. 1992;148:362-365.
Decter RM, Furness PD, Nguyen TA, et al. Reproductive understanding, sexual functioning and testosterone levels in men with spina bifida. J Urol. 1997;157:1466-1468.
Dees JE. Congenital epispadias with incontinence. J Urol. 1949;62:513.
Doroshyenko O, Jetter A, Odenthal KP, Fuhr U. Clinical pharmacokinetics of trospium chloride. Clin Pharmacokinet. 2005;44:701-720.
Duckett JW. Cutaneous vesicostomy in childhood. Urol Clin North Am. 1974;1:485.
Duckett JW, Snyder HMIII. Continent urinary diversion: variations of the Mitrofanoff principle. J Urol. 1986;136:58.
Duel BP, González R. The button cecostomy for management of fecal incontinence. Pediatr Surg Int. 1999;15:559-561.
Duel B, Gonzalez R, Barthold JS. Alternative techniques for augmentation cystoplasty. J Urol. 1998;159:998-1005.
Edelstein RA, Bauer SB, Kelly MD, et al. The long-term urologic response of neonates with myelodysplasia treated proactively with intermittent catheterization and anticholinergic therapy. J Urol. 1995;154:1500.
Elder JS. Periurethral and pubovaginal sling repair for incontinence in patients with myelodysplasia. J Urol. 1990;144:434.
Elder JS, Diaz M, Caldamone AA, et al. Endoscopic therapy for vesicoureteral reflux: a meta-analysis. Presented at the annual meeting of the American Academy of Pediatrics Section on Urology, San Francisco, abstract #53, October 10, 2004.
Engel JD, Palmer LS, Cheng EY, Kaplan WE. Surgical versus endoscopic correction of vesicoureteral reflux in children with neurogenic bladder dysfunction. J Urol. 1997;157:2291-2294.
Epstein F. Meningocele: pitfalls in early and late management. Clin Neurosurg. 1982;30:366.
Farmer DL, von Koch CS, Peacock WJ, et al. In utero repair of myelomeningocele: experimental pathophysiology, initial clinical experience, and outcome. Arch Surg. 2003;138:872-878.
Flood HD, Ritchey ML, Bloom DA, et al. Outcome of reflux in children with myelodysplasia managed by bladder pressure monitoring. J Urol. 1994;152:1574.
Geranoitis E, Koff SA, Enrile B. Prophylactic use of clean intermittent catheterization in treatment of infants and young children with myelomeningocele and neurogenic bladder dysfunction. J Urol. 1988;139:85.
Gerharz EW, Tassadaq T, Pickard RS, et al. Transverse retubularized ileum: early clinical experience with a new second line Mitrofanoff tube. J Urol. 1998;159:525-528.
Gerharz EW, Vik V, Webb G, et al. The in situ appendix in the Malone antegrade continent enema procedure for faecal incontinence. Br J Urol. 1997;79:985.
Ghoniem GM, Bloom DA, McGuire EJ, Stewart KL. Bladder compliance in meningocele children. J Urol. 1989;141:1404.
Gilbert SM, Hensle TW. Metabolic consequences and long-term complications of enterocystoplasty in children. J Urol. 2005;173:1080-1086.
Glass C, Soni B. Sexual problems of disabled patients. BMJ. 1999;318:518-521.
Goessl C, Sauter T, Michael T, et al. Efficacy and tolerability of tolterodine in children with hyperreflexia. Urology. 2000;55:414-418.
Goldwasser B, Barrett DM, Webster GD, Kramer SA. Cystometric properties of ileum and right colon after bladder augmentation, substitution and replacement. J Urol. 1987;138:1007.
Gosalbez RJr, Woodard JR, Broecker BH, Warshaw B. Metabolic complications of the use of stomach for urinary reconstruction. J Urol. 1993;150:710.
Granata C, Buffa P, Di Rovasenda E, et al. Treatment of vesico-ureteric reflux in children with neuropathic bladder: a comparison of surgical and endoscopic correction. J Pediatr Surg. 1999;34:1836-1838.
Greenfield SP, Fera M. The use of intravesical oxybutynin chloride in children with neurogenic bladder. J Urol. 1991;146:532.
Griffiths DM, Malone PS. The Malone antegrade continence enema. J Pediatr Surg. 1995;30:68-71.
Guys JM, Fakhro A, Louis-Borrione C, et al. Endoscopic treatment of urinary incontinence: long-term evaluation of the results. J Urol. 2001;165:2389.
Guzman L, Bauer SB, Hallet M, et al. The evaluation and management of children with sacral agenesis. Urology. 1983;23:506.
Han SW, Kim MJ, Kim JH, et al. Intravesical electrical stimulation improves neurogenic bowel dysfunction in children with spina bifida. J Urol. 2004;171:2648.
Harris CF, Cooper CS, Hutcheson JC, Snyder IIIHM. Appendicovesicostomy: the Mitrofanoff procedure—a 15-year perspective. J Urol. 2000;163:1922-1926.
Hayden P. Adolescents with meningomyelocele. Pediatr Rev. 1985;6:245.
Hendren WH. Reconstruction of the previously diverted urinary tracts in children. J Pediatr Surg. 1973;8:135.
Hendren WH. Urinary tract refunctionalization after long-term diversion. Am Surg. 1990;212:478.
Hernandez RD, Hurwitz RS, Foote JE, et al. Nonsurgical management of threatened upper urinary tracts and incontinence in children with myelomeningocele. J Urol. 1994;152:1582.
Herndon CDA, Rink RC, Shaw MBK, et al. The Indiana experience with artificial urinary sphincters in children and young adults. J Urol. 2003;169:650-654.
Hinman FJr. Selection of intestinal segments for bladder substitution: physical and physiological characteristics. J Urol. 1988;139:519.
Hinman FJr. Functional classification of conduits for continent diversion. J Urol. 1990;144:27.
Holzbeierlein J, Pope JC, Adams MC, et al. The urodynamic profile of myelodysplasia in childhood with spinal canal closure during gestation. J Urol. 2000;164:1336.
Holmbeck GN, Johnson SZ, Wills KE, et al. Observed and perceived parental overprotection in relation to psychological adjustments in preadolescents with a physical deformity. J Consult Clin Psych. 2002;70:96-110.
Hopps CV, Kropp KA. Preservation of renal function in children with myelomeningocele managed with basic newborn evaluation and close followup. J Urol. 2003;169:305.
Jeffs RD, Jones P, Schillinger JF. Surgical correction of vesico-ureteral reflux in children with neurogenic bladder. J Urol. 1976;115:449.
Johnson MP, Sutton LN, Rintoul N, et al. Fetal myelomeningocele repair: short-term clinical outcomes. Am J Obstet Gynecol. 2003;189:482-487.
Joseph DB, Bauer SB, Colodny AH, et al. Clean intermittent catheterization in infants with neurogenic bladder. Pediatrics. 1989;84:78.
Joyner BD, McLorie GA, Khoury AE. Sexuality and reproductive issues in children with myelomeningocele. Eur J Pediatr Surg. 1998;8:29-34.
Just M, Schwarz M, Ludwig B, et al. Cerebral and spinal MR findings in patients with post repair myelomeningocele. Pediatr Radiol. 1990;20:262.
Kaefer M, Pabby A, Kelly M, et al. Improved bladder function after prophylactic treatment of the high risk neurogenic bladder in newborns with myelomeningocele. J Urol. 1999;162:1068-1071.
Kaefer M, Tobin M, Hendren WH, et al. Continent urinary diversion: the Children’s Hospital experience. J Urol. 1997;157:1394-1399.
Kajbafzadeh AM, Moosavi S, Tajik P, et al. Intravesical injection of botulinum toxin type A: management of neuropathic bladder and bowel dysfunction in children with myelomeningocele. Urology. 2006;68:1091-1096.
Kaplan WE, Firlit CF. Management of reflux in myelodysplastic children. J Urol. 1983;129:1195.
Kasabian NG, Bauer SB, Dyro FM, et al. The prophylactic value of clean intermittent catheterization and anticholinergic medication in newborns and infants with myelodysplasia at risk of developing urinary tract deterioration. Am J Dis Child. 1992;146:840.
Kasabian NG, Vlachiotis JD, Lais A, et al. The use of intravesical oxybutynin chloride in patients with detrusor hypertonicity and detrusor hyperreflexia. J Urol. 1994;151:944.
Kass EJ, Koff SA, Lapides J. Fate of vesico-ureteral reflux in children with neuropathic bladders managed by intermittent catheterization. J Urol. 1981;125:63.
Khoury AE, Hendrick EB, McLorie GA, et al. Occult spinal dysraphism: clinical and urodynamic outcome after division of the filum terminale. J Urol. 1990;144:426.
Kim Y, Yoshimura N, Masuda H, et al. Antimuscarinic agents exhibit local inhibitory effects on muscarinic receptors in bladder afferent pathways. Urology. 2005;65:238-242.
Kock NG. Ileostomy without external appliances: a survey of 25 patients provided with intra-abdominal intestinal reservoir. Ann Surg. 1971;173:545.
Koh CJ, DeFilippo RL, Borer JG, et al. Lower urinary tract function after fetal closure of myelomeningocele. J Urol. 169, 2003. Supplement Abstract Book 106. Abstract #411, Chicago, April 27
Koff SA, DeRidder PA. Patterns of neurogenic bladder dysfunction in sacral agenesis. J Urol. 1977;118:87.
Koff SA, Gigax MR, Jayanthi VR. Nocturnal bladder emptying: a simple technique for reversing urinary tract deterioration in children with neurogenic bladder. J Urol. 2005;174(4 Pt. 2):1629-1631.
Krogh K, Lie HR, Bilenber N, Laurberg S. Bowel function in Danish children with myelomeningocele. APMIS (Suppl). 2003;109:81.
Kroovand RL, Bell W, Hart LJ, Benfeld KY. The effect of back closure on detrusor function in neonates with myelodysplasia. J Urol. 1990;144:423.
Kropp KA, Angwafo FF. Urethral lengthening and reimplantation for neurogenic incontinence in children. J Urol. 1986;135:533.
Kryger JV, Spencer Barthold J, Fleming P. The outcome of artificial urinary sphincter placement after a mean of 15 years’ follow-up in a paediatric population. Br J Urol. 1999;83:1026-1031.
Kryger JV, Leverson G, Gonzalez R. Long-term results of artificial urinary sphincters in children are independent of age at implantation. J Urol. 2001;165:2377-2379.
Lais A, Kasabian NG, Dyro FM, et al. Neurosurgical implications of continuous neuro-urological surveillance of children with myelodysplasia. J Urol. 1993;150:1879-1883.
Landau EH, Churchill BM, Jayanthi VR, et al. The sensitivity of pressure specific bladder volume versus total bladder capacity as a measure of bladder storage dysfunction. J Urol. 1994;152:1578.
Lary JM, Edmonds LD. Prevalence of spina bifida at birth—United States, 1983-1990: a comparison of two surveillance systems. MMWR Morb Mortal Wkly Rep. 1996;45:15-26.
Laurence KM. A declining incidence of neural tube defects in UK. Z Kinderchir. 1989;44(Suppl. 1):51.
Laurence KM, Beresford A. Continence, friends, marriage and children in 51 adults with spina bifida. Dev Med Child Neurol. 1975;17(Suppl. 35):128.
Laurence KM, James M, Miller MH, et al. Double-blind randomized controlled trial of folate treatment before conception to prevent recurrence of neural tube defects. BMJ. 1981;282:1509.
Leadbetter GWJr. Surgical correction for total urinary incontinence. J Urol. 1964;91:261.
Leonard MP, Dharamsi N, Williot PE. Outcome of gastrocystoplasty in tertiary pediatric urology practice. J Urol. 2000;164:947-950.
Levesque PE, Bauer SB, Atala A, et al. Ten-year experience with artificial urinary sphincter in children. J Urol. 1996;156:625.
Light JK, Hawila M, Scott FB. Treatment of urinary incontinence in children: the artificial sphincter vs. other methods. J Urol. 1983;130:518.
Light JK, Scott FB. Use of the artificial urinary sphincter in spinal cord injury patients. J Urol. 1983;130:1127.
Loening-Baucke V, Deach L, Wolraich M. Biofeedback training for patients with myelomeningocele and fecal incontinence. Dev Med Child Neurol. 1988;30:781.
López Pereira P, Somoza Ariba I, Martínez Urrutia MJ, et al. Artificial urinary sphincter: 11-year experience in adolescents with congenital neuropathic bladder. Eur Urol. 2006;50:1096-1101.
MacNeily AE, Morrell J, Secord S. Lower urinary tract reconstruction for spina bifida—does it improve health-related quality of life? J Urol. 2005;174:1637-1643.
Mandell J, Bauer SB, Colodny AH, Retik AB. Cutaneous vesicostomy in infancy. J Urol. 1981;126:92.
Marshall DF, Boston VE. Altered bladder and bowel function following cutaneous electrical field stimulation in children with spina bifida: interim results of a randomized double-blind placebo-controlled trial. Eur J Pediatr Surg. 1997;7:41-43.
Massad CA, Kogan BA, Trigo-Rocha FE. The pharmacokinetics of intravesical and oral oxybutynin chloride. J Urol. 1992;148:548.
McGuire EJ, Wang CC, Usitalo H, Savastano J. Modified pubovaginal sling in girls with myelodysplasia. J Urol. 1986;135:94.
McGuire EJ, Woodside JR, Borden TA, Weiss RM. The prognostic value of urodynamic testing in myelodysplastic patients. J Urol. 1981;126:205.
Misseri R, Casale AJ, Cain MP, Rink RC. Alternative uses of detranomer/hyaluronic acid copolymer: the efficacy of bladder neck injection for urinary incontinence. J Urol. 2005;174:1691-1694.
Mitchell ME, Piser JA. Intestinocystoplasty and total bladder replacement in children and young adults: follow-up of 129 cases. J Urol. 1987;138:1140.
Mitrofanoff P. Cystometric continente trans-appendiculaire dans le traitement de vessies neurologiques. Chir Pediatr. 1980;21:297.
Montie PR, Lara RC, Dutra MA DeCarvalho JR. New techniques for construction of efferent conduits based on the Mitrofanoff principle. Urology. 1997;49:112.
Moore C, Kogan BA, Parekh A. Impact of urinary incontinence on self-concept in children with spina bifida. J Urol. 2004;171:1659-1662.
Morrisroe SN, O’Connor RC, Nanigian DK, et al. Vesicostomy revisited: the best treatment for the hostile bladder in myelodysplastic children? BJU Int. 2005;96:397-400.
MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet. 1991;338:131.
Myers GJ. Myelomeningocele: the medical aspect. Pediatr Clin North Am. 1984;31:165.
Nasrallah PF, Aliabadi HA. Bladder augmentation in patients with neurogenic bladder and vesicoureteral reflux. J Urol. 1991;146:563.
Nguyen DH, Mitchell ME, Horowitz M, et al. Demucosalized augmentation gastrocystoplasty with bladder autoaugmentation in pediatric patients. J Urol. 1996;156:206-209.
Nguyen HT, Bauer SB, Diamond DA, Retik AB. Rectus fascial sling for the treatment of neurogenic sphincter incontinence in boys: is it safe and effective? J Urol. 2001;166:658-661.
Oi S, Yamada H, Matsumoto S. Tethered cord syndrome versus low-placed conus medullaris in an overdistended cord following initial repair for myelodysplasia. Childs Nerv Syst. 1990;6:264.
Palmer JS, Kaplan WE, Firlit CF. Erectile dysfunction in patients with spina bifida is a treatable condition. J Urol. 2000;164(3 Pt. 2):958-961.
Palmer JS, Kaplan WE, Firlit CF. Sexuality of the spina bifida male and female: anonymous questionnaires [abstract 13]. Presented at the annual meeting of the Section on Urology of the American Academy of Pediatrics. Washington (DC): October 9, 1999a.
Palmer JS, Kaplan WE, Firlit CF. Erectile dysfunction in spina bifida is treatable. Lancet. 1999;354:125-126.
Palmer LS, Richards I, Kaplan WE. Transrectal electrostimulation therapy for neuropathic bowel dysfunction in children with myelomeningocele. J Urol. 1997;157:1449-1452.
Palomaki GE, Williams JR, Haddow JE. Prenatal screening for open neural-tube defects in Maine. N Engl J Med. 1999;340:1049-1050.
Park JM, McGuire EJ, Koo HP, et al. External urethral sphincter dilation for the management of high risk myelomeningocele: 15 year experience. J Urol. 2001;165:2383.
Perez LM, Khoury J, Webster GD. The value of urodynamic studies in infants less than one year old with congenital spinal dysraphism. J Urol. 1992;148:584.
Perez LM, Smith EA, Parrott TS, et al. Submucosal bladder neck injection of bovine dermal collagen for stress urinary incontinence in the pediatric population. J Urol. 1996;156:633-636.
Peters KM, Girdler B, Turzewski C, et al. Outcomes of lumbar to sacral nerve rerouting for spina bifida. J Urol. 2010;184:702-707.
Phuong LK, Schoeberl KA, Raffel C. Natural history of tethered cord in patients with meningomyelocele. Neurosurgery. 2002;50:989-995.
Plaire JC, Snodgrass WT, Grady RW, Mitchell ME. Long-term followup of the hematuria-dysuria syndrome. J Urol. 2000;164:921-923.
Pontari MA, Keating M, Kelly MD, et al. Retained sacral function in children with high level myelodysplasia. J Urol. 1995;154:775.
Raz S, Ehrlich RM, Ziedman EJ, et al. Surgical treatment of the incontinent female patient with myelomeningocele. J Urol. 1988;139:524.
Reilly JM, Oates RD. Preliminary investigation of the potential fertility status of postpubertal males with myelodysplasia. J Urol. 1992;147:75.
Riccabona M, Korn M, Schindler M, et al. Botulinum-A toxin injected into the detrusor: a safe alternative in the treatment of children with myelomeningocele with detrusor hyperreflexia. J Urol. 2004;171:845-848.
Riedmiller H, Bürger R, Müller S, et al. Continent appendix stoma: a modification of the Mainz pouch technique. J Urol. 1990;143:1115-1117.
Reigel DH. Tethered spinal cord. Concepts Pediatr Neurosurg. 1983;4:142.
Rothenberg SP, da Costa MP, Sequeira JM, et al. Autoantibodies against folate receptors in women with a pregnancy complicated by a neural-tube defect. N Engl J Med. 2004;350:134.
Rovner ES. Trospium chloride in the management of overactive bladder. Drugs. 2004;64:2433-2446.
Rowland RG, Mitchell ME, Birhle R, et al. Indiana continent urinary reservoir. J Urol. 1987;137:1136.
Salle JLP, Amarante FA, Silveira ML, et al. Urethral lengthening with anterior bladder wall flap for urinary incontinence: a new approach. J Urol. 1994;152:803.
Scarff TB, Fronczak S. Myelomeningocele: a review and update. Rehab Lit. 1981;42:143.
Schlager TA, Clark M, Anderson S. Effect of a single-use sterile catheter for each void on the frequency of bacteriuria in children with neurogenic bladder on intermittent catheterization for bladder emptying. Pediatrics. 2001;108:E71.
Schlussel R. Cystoscopic correction of reflux. Curr Urol Rep. 2004;5:127.
Schlussel RN, Bauer SB, Kelly MD, et al. The clinical and urodynamic findings in 35 patients with sacral agenesis [abstract 412]. Presented at the annual meeting of the American Urological Association, San Francisco, May 16, 1994.
Schulte-Baukloh H, Bigalke H, Miller K, et al. Botulinum neurotoxin type A in urology: antibodies as a cause of therapy failure. Int J Urol. 2008;15:407-415.
Schulte-Baukloh H, Knispel HH, Stolze T, et al. Repeated botulinum-A toxin injections in treatment of children with neurogenic detrusor overactivity. Urology. 2005;66:865-870.
Schulte-Baukloh H, Michael T, Schobert J, et al. Efficacy of botulinum A toxin in children with detrusor hyperreflexia due to myelomeningocele: preliminary results. Urology. 2002;59:325-328.
Schwarz GR, Jeffs RD. Ileal conduit urinary diversion in children: computer analysis of follow-up from 2 to 16 years. J Urol. 1975;114:285.
Seki N, Akazawa K, Senoh K, et al. An analysis of risk factors for upper urinary tract deterioration in patients with myelodysplasia. Br J Urol. 1999;84:679.
Shapiro SR, Lebowitz RL, Colodny AH. Fate of 90 children with ileal conduit urinary diversion a decade later: analysis of complications, pyeloplasty, renal function and bacteriology. J Urol. 1975;114:289.
Sidi AA, Aliabadi H, Gonzalez R. Enterocystoplasty in the management and reconstruction of the pediatric neurogenic bladder. J Pediatr Surg. 1987;22:153.
Sidi AA, Dykstra DD, Gonzalez R. The value of urodynamic testing in the management of neonates with myelodysplasia: a prospective study. J Urol. 1986;135:90.
Silveri M, Capitanucci ML, Mosiello G, et al. Endoscopic treatment for urinary incontinence in children with a congenital neuropathic bladder. Br J Urol. 1998;82:694-697.
Skinner DG, Lieskovsky G, Boyd SD. Construction of a continent ileal reservoir (Kock pouch) as an alternative to cutaneous urinary diversion: an update after 250 cases. J Urol. 1987;137:1140.
Snodgrass W, Keefover-Hicks A, Prieto J, et al. Comparing outcomes of slings with versus without enterocystplasty for neurogenic urinary incontinence. J Urol. 2009;181:2709-2716.
Smith ED. Urinary prognosis in spina bifida. J Urol. 1972;108:115.
Soergel TM, Cain MP, Misseri R, et al. Transitional cell carcinoma of the bladder following augmentation cystoplasty for the neuropathic bladder. J Urol. 2004;172(4 Pt. 2):1649-1651.
Spindel MR, Bauer SB, Dyro FM, et al. The changing neuro-urologic lesion in myelodysplasia. JAMA. 1987;258:1630.
Squire R, Kiely EM, Carr B, et al. The clinical application of the Malone antegrade colonic enema. J Pediatr Surg. 1993;28:1012-1015.
Stark GD. Spina bifida: problems and management. Oxford (UK): Blackwell Scientific; 1977.
Stein SC, Feldman JG, Freidlander M, et al. Is myelomeningocele a disappearing disease? Pediatrics. 1982;69:511.
Tanaka H, Kakizaki H, Kobayashi S, et al. The relevance of urethral resistance in children with myelodysplasia: its impact on upper urinary tract deterioration and the outcome of conservative management. J Urol. 1999;161:929-932.
Tarcan T, Bauer S, Olmedo E, et al. Long-term follow-up of newborns with myelodysplasia and normal urodynamic findings: is it necessary? J Urol. 2001;165:564-567.
Teichman JMH, Scherz HC, Kim KD, et al. An alternative approach to myelodysplasia management: aggressive observation and prompt intervention. J Urol. 1994;152:807.
U.S. Food and Drug Administration. Food standards: amendment of standards of identity for enriched grain products to require addition of folic acid. Fed Reg. 1996;61:8781-8797.
Van Gool JD, Juijten RH, Donckerwolcke RA, Kramer PP. Detrusor-sphincter dyssynergia in children with myelomeningocele: a prospective study. Z Kinderchir. 1982;37:148.
Vemulakonda VM, Lendvay TS, Shnorhavorian M, et al. Metastatic adenocarcinoma after augmentation gastrocystoplasty. J Urol. 2008;179:1094-1096.
Venes JL, Stevens SA. Surgical pathology in tethered cord secondary to meningomyelocele repair. Concepts Pediatr Neurosurg. 1983;4:165.
Walker RD, Eerhard M, Starling J. Long-term evaluation of rectus fascial wrap in patients with spina bifida. J Urol. 2000;164:485-486.
Watson HS, Bauer SB, Peers CA, et al. Comparative urodynamics of appendiceal and ureteral Mitrofanoff conduits in children. J Urol. 1995;154:878.
White RI, Klauber GT. Sacral agenesis: analysis of twenty-two cases. Urology. 1976;8:521.
Williams LJ, Rasmussen SA, Flores A, et al. Decline in the prevalence of spina bifida and anencephaly by race/ethnicity: 1995–2002. Pediatrics. 2005;116:580-586.
Wilmshurst JM, Kelly R, Borzyskowski M. Presentation and outcome of sacral agenesis: 20 years’ experience. Dev Med Child Neurol. 1999;41:806-812.
Woodard JR, Anderson AM, Parrott TS. Ureteral reimplantation in myelodysplastic children. J Urol. 1981;126:387.
Woodhouse CRJ. The sexual and reproductive consequences of congenital genitourinary anomalies. J Urol. 1994;152:645.
Woodhouse CRJ. Myelomeningocele in young adults. Br J Urol. 2005:223-230.
Woodhouse CRJ, Malone PR, Cumming J, Reilly TM. The Mitrofanoff principle for continent urinary diversion. Br J Urol. 1989;63:53.
Woodside JR, McGuire EJ. Techniques for detection of detrusor hypertonia in the presence of urethral sphincter incompetence. J Urol. 1982;127:740.
Wu H-Y, Baskin LS, Kogan BA. Neurogenic bladder dysfunction due to myelomeningocele: neonatal versus childhood treatment. J Urol. 1997;157:2295-2297.
Xiao CG, Du MX, Li B, et al. An artificial somatic-autonomic reflex pathway procedure for bladder control in children with spina bifida. J Urol. 2005;173:2112-2116.
Yerkes EB, Rink RC, Cain MP, Casale AJ. Use of a Monti channel for administration of antegrade continent enemas. J Urol. 2002;168:1883-1885.
Young HH. An operation for the cure of incontinence of urine. Surg Gynecol Obstet. 1919;28:84.
Younoszai MK. Stooling problems in patients with myelomeningocele. South Med J. 1992;85:718.
Lipomeningocele and Other Spinal Dysraphisms
Andar UB, Harkness WF, Hayward RD. Split cord malformations of the lumbar region: a model for the neurosurgical management of all types of “occult” spinal dysraphism? Pediatr Neurosurg. 1997;26:17-24.
Anderson FM. Occult spinal dysraphism: a series of 73 cases. Pediatrics. 1975;55:826.
Atala A, Bauer SB, Dyro FM, et al. Bladder functional changes resulting from a lipomeningocele repair. J Urol. 1992;148:592.
Barson AJ. The vertebral level of termination of the spinal cord during normal and abnormal development. J Anat. 1970;106:489.
Bruce DA, Schut L. Spinal lipomas in infancy and childhood. Brain. 1979;5:92.
Campobasso P, Galiani E, Verzerio A, et al. A rare cause of occult neuropathic bladder in children: the tethered cord syndrome. Pediatr Med Chir. 1988;10:641.
Chapman PH. Congenital intraspinal lipomas: anatomic considerations and surgical treatment. Childs Brain. 1982;9:37.
Cornette L, Verpooten C, Lagae L, et al. Tethered spinal cord in occult spinal dysraphism: timing and outcome of surgical release. Neurology. 1998;50:1761-1765.
Dubrowitz V, Lorber J, Zachary RB. Lipoma of the cauda equina. Arch Dis Child. 1965;40:207.
Flanigan RF, Russell DP, Walsh JW. Urologic aspects of tethered cord. Urology. 1989;33:80.
Fone PD, Vapnek JM, Litwiller SE, et al. Urodynamic findings in the tethered spinal cord syndrome: does surgical release improve bladder function? J Urol. 1997;157:604-609.
Foster LS, Kogan BA, Cogan PH, Edwards MSB. Bladder function in patients with lipomyelomeningocele. J Urol. 1990;143:984.
Guerra LA, Pike J, Milks J, et al. Outcome in patients who underwent tethered cord release for occult spinal dysraphism. J Urol. 2006;176(4 Pt. 2):1729-1732.
Hall WA, Albright AL, Brunberg JA. Diagnosis of tethered cord by magnetic resonance imaging. Surg Neurol. 1988;30(Suppl. 1):60.
Hellstrom WJ, Edwards MS, Kogan BA. Urologic aspects of the tethered cord syndrome. J Urol. 1986;135:317.
Henderson FC, Geddes JF, Vaccaro AR, et al. Stretch-associated Injury in cervical spondylotic myelopathy. Neurosurgery. 2005;56:1101-1103.
Hsieh MH, Perry V, Gupta N, et al. The effects of detethering on the urodynamics profile in children with a tethered cord. J Neurosurg. 2006;105(5 Suppl.):391-395.
Hughes JA, de Bruyn R, Patel K, et al. Evaluation of spinal ultrasound in spinal dysraphism. Clin Radiol. 2003;58:227.
James CM, Lassman LP. Spinal dysraphism: spina bifida occulta. New York: Appleton-Century-Crofts; 1972.
Jindal A, Mahapatra AK. Spinal lipomatous malformations. Indian J Pediatr. 2000;67:342.
Kaplan WE, McLone DG, Richards I. The urologic manifestations of the tethered spinal cord. J Urol. 1988;140:1285.
Keating MA, Rink RC, Bauer SB, et al. Neuro-urologic implications of changing approach in management of occult spinal lesions. J Urol. 1988;140:1299.
Khoury AE, Hendrick EB, McLorie GA, et al. Occult spinal dysraphism: clinical and urodynamic outcome after diversion of the filum terminale. J Urol. 1990;144:426.
Kondo A, Kato K, Kanae S, Sakakibara T. Bladder dysfunction secondary to tethered cord syndrome in adults: is it curable? J Urol. 1986;135:313.
Koyangi I, Iwasaki Y, Hida K, et al. Surgical treatment supposed natural history of the tethered cord with occult spinal dysraphism. Childs Nerv Syst. 1997;13:268-274.
Linder M, Rosenstein J, Sklar FH. Functional improvement after spinal surgery for the dysraphic malformations. Neurosurgery. 1982;11:622.
Mandell J, Bauer SB, Hallett M, et al. Occult spinal dysraphism: a rare but detectable cause of voiding dysfunction. Urol Clin North Am. 1980;7:349.
Meyrat BJ, Tercier S, Lutz N, et al. Introduction of a urodynamic score to detect pre- and postoperative neurological deficits in children with a primary tethered cord. Childs Nerv Syst. 2003;19:716.
Nogueira M, Greenfield SP, Wan J, et al. Tethered cord in children: a clinical classification with urodynamic correlation. J Urol. 2004;172:1677.
Packer RJ, Zimmerman RA, Sutton LN, et al. Magnetic resonance imaging of spinal cord diseases of childhood. Pediatrics. 1986;78:251.
Pang D. Split cord malformation: II. Clinical syndrome. Neurosurgery. 1992;31:481-500.
Pang D, Dias M, Ahab-Barmada M. Split cord malformation: I. A unified theory of embryogenesis for double spinal cord malformations. Neurosurgery. 1992;31:451-481.
Pierre-Kahn A, Zeral M, Renier D, et al. Congenital lumbosacral lipomas. Childs Nerv Syst. 1997;13:298-334.
Proctor M, Bauer SB, Scott MR. The effect of surgery for the split spinal cord malformation on neurologic and urologic function. Pediatr Neurosurg. 2000;32:13-19.
Raghavendra BN, Epstein FJ, Pinto RS, et al. The tethered spinal cord: diagnosis by high-resolution real-time ultrasound. Radiology. 1983;149:123.
Roy MW, Gilmore R, Walsh JW. Evaluation of children and young adults with tethered spinal cord syndrome: utility of spinal and scalp recorded somatosensory evoked potentials. Surg Neurol. 1986;26:241.
Sarica K, Erbagci A, Yagci F, Yurtseven C. Multidisciplinary evaluation of occult spinal dysraphism in 47 children. Scand J Urol Nephrol. 2003;37:329-334.
Satar N, Bauer SB, Scott RM, et al. Late effects of early surgery on lipoma and lipomeningocele in children less than two years old. J Urol. 1997;157:1434-1437.
Satar N, Bauer SB, Shefner J, et al. The effects of delayed diagnosis and treatment in patients with an occult spinal dysraphism. J Urol. 1995;154:754.
Scheible W, James HE, Leopold GR, Hilton SW. Occult spinal dysraphism in infants: screening with high-resolution real-time ultrasound. Radiology. 1983;146:743.
Sebold CD, Melvin EC, Siegel D, et al. Recurrence risks for neural tube defects in siblings of patients with lipomyelomeningocele. Genet Med. 2005;7:64-67.
Seeds JW, Jones FD. Lipomyelomeningocele: prenatal diagnosis and management. Obstet Gynecol. 1986;67(Suppl.):34.
Tami S, Yamada S, Knighton RS. Extensibility of the lumbar and sacral cord: pathophysiology of the tethered cord in cats. J Neurosurg. 1987;66:116.
Tracey PT, Hanigan WC. Spinal dysraphism: use of magnetic resonance imaging in evaluation. Clin Pediatr. 1990;29:228.
Weissert M, Gysler R, Sorensen N. The clinical problem of the tethered cord syndrome: a report of three personal cases. Z Kinderchir. 1989;44:275.
Yamada S, Knierim D, Yonekura M, et al. Tethered cord syndrome. J Am Paraplegic Soc. 1983;6(Suppl. 3):58.
Yamada S, Zincke DE, Sanders D. Pathophysiology of “tethered cord syndrome.”. J Neurosurg. 1981;54:494.
Yamada S, Won DJ, Yamada SM. Pathophysiology of tethered cord syndrome: correlation with symptomatology. Neurosurg Focus. 2004;16:E6.
Yip CM, Leach GE, Rosenfeld DS, et al. Delayed diagnosis of voiding dysfunction: occult spinal dysraphism. J Urol. 1985;124:694.
Bernbeck B, Schurfeld-Furstenberg K, Ketteler K, et al. Unilateral pulmonary atresia with total sacral agenesis and other congenital defects. Clin Dysmorphol. 2004;13:47.
Boemers TM, van Gool JD, de Jong TP, Bax KM. Urodynamic evaluation of children with caudal regression syndrome (caudal dysplasia sequence). J Urol. 1994;151:1038.
De Biasio P, Ginocchio G, Aicardi G, et al. Ossification timing of sacral vertebrae by ultrasound in the mid-second trimester of pregnancy. Prenat Diagn. 2003;23:1056.
Diel J, Ortiz O, Losada RA, et al. The sacrum: pathologic spectrum, multimodality imaging, and subspecialty approach. RadioGraphics. 2001;21:83.
Hagan DM, Ross AJ, Strachan T, et al. Mutation analysis and embryonic expression of the HLXB9 Currarino syndrome gene. Am J Human Genet. 2000;66:1504.
Jakobson H, Holm-Bentzen M, Hald T. Neurogenic bladder dysfunction in sacral agenesis and dysgenesis. Neurourol Urodyn. 1985;4:99.
Kochling J, Karbasiyan M, Reis A. Spectrum of mutations and genotype-phenotype analysis in Currarino syndrome. Eur J Hum Genet. 2001;9:599.
Landauer W. Rumplessness of chicken embryos produced by the injection of insulin and other chemicals. Exp Zool. 1945;98:65.
Lynch SA, Wang Y, Strachan T, et al. Autosomal dominant sacral agenesis: Currarino syndrome. J Med Genet. 2000;37:561.
Menon RK, Cohen RM, Sperling MA, et al. Transplacental passage of insulin in pregnant women with insulin-dependent diabetes mellitus. N Engl J Med. 1990;323:309.
Morera C, Nurko S. Rectal manometry in sacral agenesis. J Pediatr Gastroenterol Nutr. 2003;37:47.
Pang D. Sacral agenesis and caudal spinal cord malformations. Neurosurgery. 1993;32:755.
Papapetrou C, Drummond F, Reardon W, et al. A genetic study of the human T gene and its exclusion as a major candidate gene for sacral agenesis with anorectal atresia. J Med Genet. 1999;36:208-213.
Passarge E, Lenz K. Syndrome of caudal regression in infants of diabetic mothers: observations of further cases. Pediatrics. 1966;37:672.
Rojansky N, Fasouliotis SJ, Ariel I, et al. Extreme caudal agenesis. J Reprod Med. 2002;47:241.
Ross AJ, Ruiz-Perez V, Wang Y, et al. A homeobox gene, HLXB9, is the major locus for dominantly inherited sacral agenesis. Nat Genet. 1998;20:358.
Barnes PD, Lester PD, Yamanashi WS, Prince JR. MRI in infants and children with spinal dysraphism. Am J Radiol. 1986;147:339.
Barry JE, Auldist AW. The Vater syndrome. Am J Dis Child. 1974;128:769.
Boemers TM, van Gool JD, de Jong TP, Bax KM. Urodynamic evaluation of children with the caudal regression syndrome (caudal dysplasia sequence). J Urol. 1994;151:1038.
Boemers TM, de Jong TP, van Gool JD, et al. Urologic problems in anorectal malformations: II. Functional urologic sequelae. J Pediatr Surg. 1996;31:634.
Carson JA, Barnes PD, Tunell WP, et al. Imperforate anus: the neurologic implication of sacral abnormalities. J Pediatr Surg. 1984;19:838.
Currarino G, Coln D, Votteler T. Triad of anorectal, sacral, and presacral anomalies. AJR Am J Roentgenol. 1981;137:395-398.
Emir H, Soylet Y. Neurovesical dysfunction in patients with anorectal malformations. Eur J Pediatr Surg. 1998;8:95.
Greenfield SP, Fera M. Urodynamic evaluation of the patient with imperforate anus: a prospective study. J Urol. 1991;146:539.
Hagan DM, Ross AJ, Strachan T, et al. Mutation analysis and embryonic expression of the HLXB9 Currarino syndrome gene. Am J Hum Genet. 2000;66:1504.
Holschneider AM, Pfrommer W, Gerresheim B. Results in the treatment of anorectal malformations with special regard to the histology of the rectal pouch. Eur J Pediatr Surg. 1994;4:303-309.
Hulthen de Medina V, Mellstam L, Amark P, et al. Neurovesical dysfunction in children after surgery for high or intermediate anorectal malformations. Acta Pediatr. 2004;93:43.
Kakizaki H, Nonomura K, Asano Y, et al. Preexisting neurogenic voiding dysfunction in children with imperforate anus: problems in management. J Urol. 1994;151:1041.
Karrer FM, Flannery AM, Nelson MDJr, et al. Anal rectal malformations: evaluation of associated spinal dysraphic syndromes. J Pediatr Surg. 1988;23:45.
Long FL, Hunter JV, Mahboubi S, et al. Tethered cord and associated vertebral anomalies in children and infants with imperforate anus: evaluation with MR imaging and plain radiography. Radiology. 1996;200:377-382.
Metts JC, Kotkin L, Kasper S, et al. Genital malformations and coexistent urinary tract or spinal anomalies in patients with imperforate anus. J Urol. 1997;158:1298-1300.
Mosiello G, Capitanucci ML, Gatti C, et al. How to investigate neurovesical dysfunction in children with anorectal malformations. J Urol. 2003;170:1610-1613.
Parrott T. Urologic implications of anorectal malformations. Urol Clin North Am. 1985;12:13-21.
Parrott T, Woodard J. Importance of cystourethrography in neonates with imperforate anus. Urology. 1979;13:607.
Peña A. Posterior sagittal approach for the correction of anal rectal malformations. Adv Surg. 1986;19:69.
Peña A, Hong A. Advances in the management of anorectal malformations. Am J Surg. 2000;180:370.
Rivosecchi M, Lucchetti MC, Zaccara A, et al. Spinal dysraphism detected by magnetic resonance imaging in patients with anorectal anomalies: incidence and clinical significance. J Pediatr Surg. 1995;30:488.
Ross AJ, Ruiz-Perez V, Wang Y, et al. A homeobox gene, HLXB9, is the major locus for dominantly inherited sacral agenesis. Nat Genet. 1998;20:358.
Santulli TV, Schullinger JN, Kiesewetter WB, et al. Imperforate anus: a survey from the members of the Surgical Section of the American Academy of Pediatrics. J Pediatr Surg. 1971;6:484-487.
Shaul DB, Harrison EA. Classification of anorectal malformation: initial approach, diagnostic test, and colostomy. Semin Pediatr Surg. 1997;6:187-195.
Taskinen S, Valanne L, Rintala R. Effect of spinal cord abnormalities on the function of the lower urinary tract in patients with anorectal abnormalities. J Urol. 2002;168:1147-1149.
Templeton JM, O’Neill JAJr. Anorectal malformations. In: Welch KJ, Randolph JG, Ravitch MM, et al, editors. Pediatric surgery. Chicago: Year Book Medical Publishers; 1986:1022-1037.
Tsakayannis DE, Shamberger RC. Association of imperforate anus with occult spinal dysraphism. J Pediatr Surg. 1995;30:1010-1012.
Tunell WP, Austin JC, Barnes TP, Reynolds A. Neuroradiologic evaluation of sacral abnormalities in imperforate anus complex. J Pediatr Surg. 1987;22:58.
Brodak PP, Sherz HC, Packer M, Kaplan GW. Is urinary screening necessary for patients with cerebral palsy? J Urol. 1994;152:1586.
Bross S, Pomer S, Doderlein L, et al. Urodynamic findings in patients with infantile cerebral palsy. Aktuelle Urol. 2004;35:54.
Decter RM, Bauer SB, Khoshbin S, et al. Urodynamic assessment of children with cerebral palsy. J Urol. 1987;138:1110.
Houle AM, Vernot O, Jednak R, et al. Bladder function before and after sacral rhizotomy in children with cerebral palsy. J Urol. 1998;160:1088-1091.
Huang JC, Deletis V, Vodusek DB, et al. Preservation of pudendal afferents in sacral rhizotomies. Neurosurg. 1997;41:411.
Karaman MI, C Kaya, Caskurlu T, et al. Urodynamic findings in children with cerebral palsy. Int J Urol. 2005;12:717-720.
Kim JN, Namburg R, Chang W, et al. Prospective evaluation of perinatal risk factors for cerebral palsy and delayed development in high risk infants. Yonsei Med J. 1999;40:363-370.
Kuban KCK, Leviton A. Cerebral palsy. N Engl J Med. 1994;330:188.
Kyllerman M, Bogen B, Bensch J, et al. Dyskinetic cerebral palsy: I. Clinical categories, associated neurological abnormalities and incidences. Acta Paediatr Scand. 1982;71:543.
Liu M, Hu TZ, Wei FK. A review on the treatment of spastic cerebral palsy with selective posterior rhizotomy. Chin J Reparative Reconstr Surg. 1999;13:183.
Mayo ME. Lower urinary tract dysfunction in cerebral palsy. J Urol. 1992;147:419.
Mayo ME, Burns MW. Urodynamic studies in children who wet. Br J Urol. 1990;65:641-645.
McNeal DM, Hawtrey CE, Wolraich ML, Mapel JR. Symptomatic neurogenic bladder in a cerebral-palsied population. Dev Med Child Neurol. 1983;25:612.
Murphy KP, Molnar GE, Lankasky K. Medical and functional status of adults with cerebral palsy. Dev Med Child Neurol. 1995;37:1075-1084.
Naeye RL, Peters EC, Bartholomew M, Landis R. Origins of cerebral palsy. Am J Dis Child. 1989;143:1154.
Nelson KB, Ellenberg JH. Antecedents of cerebral palsy. N Engl J Med. 1986;315:81.
Polivka BJ, Nickel JT, Wilkins IIIJR. Urinary tract infection during pregnancy: a risk factor for cerebral palsy? J Obstet Gynecol Neonatal Nurs. 1997;26:405-413.
Reid CJD, Borzyskowski M. Lower urinary tract dysfunction in cerebral palsy. Arch Dis Child. 1993;68:739.
Roijen LE, Postema K, Limbeek VJ, et al. Development of bladder control in children and adolescents with cerebral palsy. Dev Med Child Neurol. 2001;43:103.
Steinbok P, Schrag C. Complications after selective sacral rhizotomy for spasticity in children with cerebral palsy. Pediatr Neurosurg. 1998;28:300-313.
Traumatic Injuries to the Spine
Adams C, Babyn PS, Logan WJ. Spinal cord birth injury: value of computed tomographic myelography. Pediatr Neurol. 1988;4:109.
Al-Ali M, Salman G, Rasheed A, et al. Phenoxybenzamine in the management of neuropathic bladder following spinal cord injury. Aust N Z J Surg. 1999;69:660-663.
Anderson JM, Schutt AH. Spinal injury in children: a review of 156 cases seen from 1950 through 1978. Mayo Clin Proc. 1980;55:499.
Augutis M, Levi R. Pediatric spinal cord injury in Sweden: incidence, etiology and outcome. Spinal Cord. 2003;41:328.
Barkin M, Dolfin D, Herschorn S, et al. The urologic care of the spinal cord injury patient. J Urol. 1983;129:335.
Batista JE, Bauer SB, Shefner JM, et al. Urodynamic findings in children with spinal cord ischemia. J Urol. 1995;154:1183.
Biering-Sorensen F, Nielans HM, Dorflinger T, Sorensen B. Urological situation five years after spinal cord injury. Scand J Urol Nephrol. 1999;33:157-161.
Brown RL, Brunn MA, Garcia VF. Cervical spine injuries in children: a review of 103 patients treated consecutively at a level 1 pediatric trauma center. J Pediatr Surg. 2001;36:1107.
Cass AS, Luxenberg M, Johnson CF, Gleich P. Management of the neurogenic bladder in 413 children. J Urol. 1984;132:521.
Chancellor MB, Bennett C, Simoneau AR, et al. Sphincteric stents versus external sphincterotomy in spinal cord injured men: prospective randomized multicenter trial. J Urol. 1999;161:1893-1898.
Cirak B, Ziegfeld S, Knight VM, et al. Spinal cord injuries in children. J Pediatr Surg. 2004;39:607.
Decter RM, Bauer SB. Urologic management of spinal cord injury in children. Urol Clin North Am. 1993;20:475.
Donnellan SM, Bolton DM. The impact of contemporary bladder management techniques on struvite calculi associated with spinal cord injury. BJU Int. 1999;84:280-285.
Donnelly J, Hackler RH, Bunts RC. Present urologic status of the World War II paraplegic: 25-year follow-up comparison with status of the 20-year Korean War paraplegic and 5-year Vietnam paraplegic. J Urol. 1972;108:558.
Fanciullacci F, Zanollo A, Sandri S, Cantanzaro F. The neuropathic bladder in children with spinal cord injury. Paraplegia. 1988;26:83.
Giannantoni A, Scivoletti G, DiStasi SM, et al. Clean intermittent catheterization and prevention of renal disease in spinal cord injured patients. Spinal Cord. 1998;36:29-32.
Guttmann L, Frankel H. The value of intermittent catheterization in the early management of traumatic paraplegia and tetraplegia. Paraplegia. 1966;4:63.
Hadley MN, Zabramski JM, Browner CM, et al. Pediatric spinal trauma: review of 122 cases of spinal cord and vertebral column injuries. J Neurosurg. 1988;68:18.
Iwatsubo E, Iwakawa A, Koga H, et al. Functional recovery of the bladder in patients with spinal cord injury: prognosticating programs of an aseptic intermittent catheterization. Acta Urol Japan. 1985;31:775.
Kim YH, Kattan NW, Boon TB. Bladder leak point pressure: the measure for sphincterotomy success in spinal cord injured patients with external detrusor sphincter dyssynergia. J Urol. 1998;159:493-496.
Kuo H-C. Botulinum A toxin urethral injection for the treatment of lower urinary tract dysfunction. J Urol. 2003;170:1908-1912.
Lanska MJ, Roessmann U, Wiznitzer M. Magnetic resonance imaging in cervical cord birth injury. Pediatrics. 1990;85:760.
Lin H, Hou C, Zhen X, Xu Z. Clinical study of reconstructed bladder innervation below the level of spinal cord injury to produce urination by Achilles tendon–to–bladder reflex contractions. J Neurosurg Spine. 2009;10:452-457.
McKinley WO, Jackson AB, Cardenas DD, DeVivo JM. Long-term medical complications after traumatic spinal cord injury: a regional model systems analysis. Arch Phys Med Rehabil. 1999;80:1402-1410.
Ogawa T, Yoshida T, Fujinaga T. Bladder deformity in traumatic spinal cord injury patients. Acta Urol Jpn. 1988;34:1173.
Pang D, Pollack IF. Spinal cord injury without radiographic abnormalities in children: the SCIWORA syndrome. J Trauma. 1989;29:654.
Pang D, Wilberger JEJr. Spinal cord injury without radiographic abnormalities in children. J Neurosurg. 1982;57:114.
Pannek J, Diederichs W, Botel U. Urodynamically controlled management of spinal cord injury in children. Neurourol Urodyn. 1997;16:285-292.
Pearman JW. Urologic follow-up of 99 spinal cord injury patients initially managed by intermittent catheterization. Br J Urol. 1976;48:297.
Perkash I. Autonomic dysreflexia and detrusor-sphincter dyssynergia in spinal cord injury patients. J Spinal Cord Med. 1997;20:365-370.
Phillips JR, Jadvar H, Sullivan G, et al. Effect of radionuclide renograms on treatment of patients with spinal cord injuries. AJR Am J Roentgenol. 1997;169:1045-1047.
Pollack IF, Pang D, Sclabassi R. Recurrent spinal cord injury without radiographic abnormalities in children. J Neurosurg. 1988;69:177.
Prieto-Fingerhut T, Banovac K, Lynne CM. A study comparing sterile and non-sterile urethral catheterization in patients with spinal cord injury. Rehabil Nurs. 1997;22:299-302.
Ruge JR, Sinson GP, McLone DG, et al. Pediatric spinal injury: the very young. J Neurosurg. 1988;68:25.
Schurch B, Hodler J, Rodic B. Botulism A toxin as a treatment of detrusor-sphincter dyssynergia in patients with spinal cord injury: MRI controlled transperineal injections. J Neurol Neurosurg Psychiatry. 1997;63:474-476.
Schurch B, Strohrer M, Kramer G, et al. Botulinum-A toxin for treating detrusor hyeperreflexia in spinal cord injured patients: a new alternative to anticholinergic drugs? J Urol. 2000;164:692-697.
Shenot PJ, Rivas DA, Watanabe T, Chancellor MB. Early predictors of bladder recovery and urodynamics after spinal cord injury. Neurourol Urodyn. 1998;17:25-29.
Sylora JA, Gonzalez R, Vaughn M, Reinberg Y. Intermittent self-catheterization by quadriplegic patients via a catheterizable Mitrofanoff channel. J Urol. 1997;151:48-50.
Tai CF, Roppolo JR, de Groat WC. Response of external urethral sphincter to high frequency electrical stimulation of pudendal nerve. J Urol. 2005;174:782-786.
Vaidyanathan S, Soni BM, Brown E, et al. Effect of intermittent urethral catheterization and oxybutynin bladder instillation on urinary continence status and quality of life in a select group of spinal cord injury patients with neuropathic bladder dysfunction. Spinal Cord. 1998;36:409-414.
Vaidyanathan S, Soni BM, Sett P, et al. Pathophysiology of autonomic dysreflexia: long-term treatment with terazosin in adult and pediatric spinal cord injury patients manifesting recurrent dysreflexic episodes. Spinal Cord. 1998;36:761-770.
VanHala S, Nelson VS, Hurvitz EA, et al. Bladder management in patients with pediatric onset neurogenic bladders. J Spinal Cord Med. 1997;20:410-415.
Watanabe T, Rivas DA, Chancellor MB. Urodynamics of spinal cord injury. Urol Clin North Am. 1996;23:459.
Wein AJ. Pharmacologic options for the overactive bladder. Urology. 1998;51(2A Suppl.):43-47.
Wiart L, Joseph PA, Petit H, et al. The effects of capsaicin on the neurogenic hyper-reflexic detrusor: a double blind placebo controlled study in patients with spinal cord disease: preliminary results. Spinal Cord. 1998;36:95-99.
Xiao CG, deGroat WC, Godec CJ, et al. “Skin-CNS-bladder” reflex pathway for micturition after spinal cord injury and its underlying mechanisms. J Urol. 1999;162:936-942.
Xiao CG, Du MX, Dai C, et al. An artificial somatic-central nervous system-autonomic reflex pathway for controllable micturition after spinal cord injury: preliminary results in 15 patients. J Urol. 2003;170(4 Pt. 1):1237-1241.