chapter 85 Use of Intestinal Segments in Urinary Diversion
Reconstructive urologic surgery frequently requires use of bowel for ureteral substitutes, bladder augmentation, or bladder replacement. In rare cases, gastrointestinal segments may also function as urethral or vaginal substitutes. The stomach, jejunum, ileum, and colon each have a potential use in these various procedures. The appropriate use of these intestinal segments requires a thorough knowledge of their surgical anatomy, the methods of preparing the intestine for an operative event, and the techniques for isolating segments of intestine and reconstituting continuity of the enteric tract. Also crucial to success is an understanding of the technical procedures and potential complications of incorporating the intestine into the urinary tract. With this knowledge, reconstruction of the urinary tract may be performed with the proper segment of intestine in the least morbid way. This chapter reviews the technical aspects involved in the use of intestinal segments in urologic surgery that are germane to all types of reconstructive procedures. We review the potential acute and long-term difficulties and complications of the use of intestinal segments in reconstructing the genitourinary tract.
Surgical Anatomy
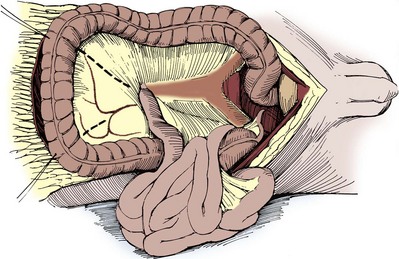
Please see the Expert Consult website for this section, including Figures 85-1 and 85-2.![]()
The segments that bowel urologists use most frequently are the ileum, colon, and rectum. Less commonly, the jejunum and stomach are employed in reconstructive procedures. Successful surgical mobilization of these structures and constructing them into their new role requires a thorough knowledge of the surgical anatomy of the vascular supply and metabolic function of each portion of the intestinal tract.
Stomach
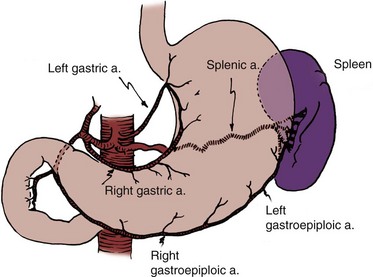
The stomach is a vascular organ that receives its blood supply primarily from the celiac trunk (Fig. 85–1). Three branches of the celiac axis give rise to the majority of the arterial supply of the stomach.
The right gastroepiploic artery anastomoses with the left gastroepiploic artery, and both supply the greater curve of the stomach. By use of the gastroepiploic vessels, a pedicle of stomach may be mobilized to the pelvis. The pedicle may consist of the entire antrum pylori or a wedge of the fundus.
The blood supply for these segments is based on either the left or right gastroepiploic artery, depending on the portion of stomach employed. On occasion, the left gastroepiploic artery is atretic at some point in its course and does not provide an adequate blood supply. Under these circumstances, the right gastroepiploic artery must be employed. When a wedge of fundus is employed, it should not include a significant portion of the antrum and should never extend to the pylorus or all the way to the lesser curve of the stomach. When the blood supply is based on the left gastroepiploic artery, the short gastric vessels that course from the gastroepiploic artery to the stomach are ligated along the greater curve proximal to the pedicle to the origin of the gastroepiploic artery. The omentum is left attached to the gastroepiploic vessels and helps secure and support them. It may be necessary for proper pedicle mobility to detach the omentum from the colon along the avascular plane located at the point of its attachment to the transverse colon. If an antrectomy is performed, a Billroth I anastomosis reconstitutes gastrointestinal continuity. The stomach has a thick seromuscular layer that can be easily separated from the mucosa should a submucosal ureteral reimplantation be necessary.
Small Bowel
The small bowel is about 22 feet long; however, it may vary from 15 to 30 feet in length. Its largest diameter is in the duodenum; the lumen becomes smaller in the more distal portions, reaching its smallest diameter in the ileum, approximately 12 inches from the ileocecal valve. About two fifths of the small bowel is jejunum, whereas the distal three fifths is ileum. There is no definite demarcation between the two; however, each possesses several unique properties that allow the surgeon to distinguish one from the other intraoperatively. The ileum, being more distal in location, has a smaller diameter. It has multiple arterial arcades, and the vessels in the arcades are smaller than those in the jejunum. The ileal mesentery is also thicker than the jejunal mesentery. In contrast, the jejunal diameter is larger, the arterial arcades are usually single, and the vessels composing them are larger in diameter. The arcades anastomose one with another and give off straight vessels, which enter the bowel and form an anastomotic network within the bowel wall. It has been shown experimentally that up to 15 cm of small bowel can survive laterally to a straight vessel. Thus theoretically, the mesentery could be cleaned from the small bowel for a length of 15 cm without necrosis of the end. In general, however, it is unwise to assume that more than 8 cm of small bowel will survive away from a straight vessel. The arcades receive their blood from the superior mesenteric artery. When segments of jejunum or ileum are isolated, the mesentery should be transected in such a way that the isolated intestinal segment receives its blood supply from an arcade supplied by a palpable artery of substance that courses through the base of the mesenteric pedicle.
Two portions of the small bowel may lie within the confines of the pelvis and, as such, may be exposed to pelvic irradiation and pelvic disease: the last 2 inches of the terminal ileum, which is often fixed in the pelvis by ligamentous attachments, and the 5 feet of small bowel beginning approximately 6 feet from the ligament of Treitz, the mesentery of which is the longest of the entire small bowel. As such, this portion of the small bowel can descend into the pelvis. In a postirradiated patient, one should try to avoid use of these two segments of the small intestine in any reconstructive procedure.
Colon
The large bowel is divided into the cecum, ascending colon, transverse colon, left colon, sigmoid colon, and rectum. Portions of the large bowel are fixed or retroperitoneal, and other segments lie free within the peritoneal cavity. The cecum, on rare occasion, may lie free within the abdominal cavity and therefore may have great mobility. In general, however, it is fixed in the right lower quadrant. Two accessory peritoneal bands bind the cecum and distal ileum to the retroperitoneum and lateral abdominal wall. One band arises from the distal ileum, attaches to the cecum, and is fixed to the retroperitoneum. A second band arises from the cecum and fixes the cecum to the posterior abdominal wall laterally. The remainder of the ascending colon is fixed to the right posterior abdominal wall to the level of the hepatic flexure, at which point the hepatocolic ligament secures this portion of the colon to the liver. The transverse colon lies free within the abdominal cavity and is fixed in the left upper quadrant at the splenic flexure by the phrenocolic ligament. The transverse colon is attached to the stomach by the gastrocolic omentum. The descending colon is fixed to the lateral abdominal wall; however, the sigmoid colon may or may not lie free within the abdominal cavity. The rectosigmoid colon’s most cephalad portion is intraperitoneal, and as its distal, more caudad portions are approached, it becomes retroperitoneal and finally subperitoneal.
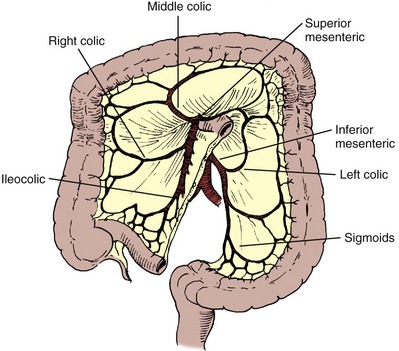
The colon receives its blood supply from the superior mesenteric artery, inferior mesenteric artery, and internal iliac arteries (Fig. 85–2). The major arteries supplying the colon and rectum include the ileocolic, right colic, middle colic, left colic, sigmoid, superior hemorrhoidal, middle hemorrhoidal, and inferior hemorrhoidal arteries. These arteries anastomose one with the other to form the arc of Drummond and allow considerable leeway in mobilizing the colon. The middle colic artery arises from the first portion of the superior mesenteric artery and generally ascends the transverse mesocolon to the right of midline. The right colic artery usually arises just below the middle colic artery from the superior mesenteric artery and courses to the right colon. It may arise, however, from the ileocolic artery or directly from the middle colic artery. If it arises from the ileocolic artery, mobilization of the distal ascending colon is facilitated so that this portion of the colon can easily be brought into the deep pelvis. On occasion, however, it is necessary to sever the right colic artery at its origin to mobilize the distal portion of the ascending colon to the pelvis. This is particularly true if the right colic artery originates from the middle colic artery. The ileocolic artery is the terminal portion of the superior mesenteric artery and supplies the last 6 inches of ileum and ascending colon. The left colic artery arises from the inferior mesenteric artery, and then the inferior mesenteric artery gives off four to six sigmoid branches, the last of which becomes the superior hemorrhoidal artery. This anastomoses with the middle hemorrhoidal artery, a branch of the internal iliac artery, which in turn anastomoses with the inferior hemorrhoidal artery, the terminal branch of the internal pudendal artery. The middle sacral artery, which originates directly from the aorta, may supply the posterior aspect of the rectum.
Three weak points involving the vascular supply to the colon have been described. Sudeck’s critical point, which is located between the junction of the sigmoid and superior hemorrhoidal arteries, was thought to be a particularly tenuous anastomotic area such that if the colon were transected in this region, the anastomosis would heal with difficulty because the blood supply might be compromised. Similarly, the midpoints between the middle colic and right colic arteries and between the middle colic and left colic arteries also have somewhat tenuous anastomotic communications. Although anastomoses in these areas generally heal well, provided the principles of proper technique are adhered to, it is usually wise to select an area for the anastomosis to one side of these points.
The ascending colon is mobilized first by transecting the cecal and distal ileal fibrous attachments to the lateral abdominal wall and retroperitoneum described previously and then by detaching it from the lateral abdominal wall along the avascular line of Toldt. This is a bloodless plane, provided the colonic mesentery is not violated. The transverse colon is mobilized by dividing the gastrocolic omentum (along the avascular plane of its attachment to the colon), the hepatocolic ligament (which may have some small vessels coursing through it), and the phrenocolic ligament. The descending colon is mobilized, much like the right colon, by incising the avascular line of Toldt lateral to the colon. With these attachments taken down, there is considerable mobility of the colon. Further mobility is gained by isolating a pedicle of the intestinal segment on the basis of one of the major arterial vessels described earlier.
Selecting the Segment of Intestine
Please see the Expert Consult website for this section.![]()
The stomach, jejunum, ileum, and colon have unique properties, each of which has special advantages and disadvantages. The selection of the proper intestinal segment should be based on the patient’s condition, renal function, history of previous abdominal procedures, and type of diversion or substitution required. The stomach has been employed as a replacement for bladder, for augmentation cystoplasty, as a conduit, and for continent diversions (Bihrle et al, 1989; Abdel-Azim, 2003; DeFoor, 2003; Bissada, 2004). The advantage of the stomach over other intestinal segments for urinary intestinal diversion is that it is less permeable to urinary solutes, it has a net excretion of chloride and protons rather than a net absorption of them, and it produces less mucus. Urodynamically, it behaves like other intestinal segments. When it is used in urinary reconstruction, electrolyte imbalance rarely ensues in patients with normal renal function, although a hypochloremic metabolic alkalosis has been described. The incidence of bacteriuria has been reported to be as low as 25%, much less than the 60% to 80% incidence reported for ileal and colon segments. However, more recent data from our institution suggest that there is no difference in bacteriuria among any of the segments. The urine, which usually has a pH of 6 to 7, does not generally result in an increased incidence of peristomal skin problems. The authors have also noted that in bladder augmentation patients, there is little difference in urinary pH between gastric and ileal augmentations. Serum gastrin levels are generally normal or minimally elevated, depending on what portion of the stomach is used and how much (Leong, 1978; Adams et al, 1988). Although exclusion of the antrum from the gastrointestinal tract has not resulted in elevated serum gastrin levels and an ulcer diathesis clinically (Lim et al, 1983), antral exclusion experimentally results in elevated circulating gastrin levels, which may cause major intestinal ulcerative problems in the postoperative period (Tiffany et al, 1986).
Rarely, severe ulcerative complications have been reported in cases that have employed stomach for urinary reconstruction (Reinberg, 1992; Tainio, 2000). Long-term H2 or proton-pump inhibition should be considered for these patients. When the antral portion of stomach is employed, reconstitution is generally by a Billroth I anastomosis. Complications with Billroth I gastroduodenostomy are well documented. The antrum should not be employed if the fundus is available. Early complications of the use of portions of the stomach for reconstruction include gastric retention due to atony of the stomach or edema of the anastomosis; hemorrhage, most commonly originating from the anastomotic site; hiccups secondary to gastric distention; pancreatitis as a consequence of intraoperative injury; and duodenal leakage. Delayed complications include dumping syndrome, steatorrhea, small stomach syndrome, increased intestinal transit time, bilious vomiting, afferent loop syndrome, hypoproteinemia, and megaloblastic or iron deficiency anemia. Postoperative bowel obstruction occurs with an incidence of 10% (2 of 21 patients) (Leong, 1978). Gastroduodenal and gastroureteral leaks have also been reported, occasionally resulting in a fatal outcome (Leong, 1978).
The use of stomach for urinary intestinal diversion may be considered when the use of other intestinal segments in a patient with a decreased amount of intestine would result in serious nutritional problems. One advantage of using stomach segments in the patient with severe abdominal adhesions is that the area of the stomach is generally adhesion free and easily mobilized. Complications specific to the use of stomach include the hematuria-dysuria syndrome and severe metabolic alkalosis associated with respiratory distress in some patients (see “Metabolic Complications” later).
The jejunum is usually not employed for reconstruction of the urinary system because it may result in severe electrolyte imbalance. In general, diseases that would make the ileum inappropriate for use also make the jejunum inappropriate for use. Rarely, it is the only segment available. Under these circumstances, as distal a segment of jejunum as possible should be employed to minimize the electrolyte problems.
The ileum and colon are used most often for urinary tract reconstruction and have been employed in all types of reconstructive procedures. The ileum is mobile and of small diameter, has a constant blood supply, and serves well for ureteral replacement and the formation of conduits. Loss of significant portions of the ileum results in nutritional problems because of lack of vitamin B12 absorption, diarrhea because of lack of bile salt reabsorption, and fat malabsorption. On occasion, the mesenteric fat is excessive, making mobility and anastomosis difficult. Also, the mesentery may be so short that it is difficult to mobilize the ileum into the deep pelvis. Postoperative bowel obstruction occurs in up to 10% of patients who have segments isolated from the ileum for urinary tract reconstruction (Varkarakis, 2006). As many as half of the obstructions occur in the early postoperative period (Schwarz and Jeffs, 1975).
The colon requires mobilization from its fixed positions to give it the mobility necessary for use in urinary reconstruction. It has a larger diameter than the ileum and is usually easily mobilized into any area of the abdomen or pelvis. In patients who have received pelvic irradiation, portions of the right, transverse, and descending colon may be used confidently with the knowledge that they have not been exposed to the radiation therapy. Removal of segments of colon from the enteric tract results in fewer nutritional problems than does removal of segments of ileum, provided the ileocecal valve is not violated. Should the ileocecal valve be used, diarrhea, excessive bacterial colonization of the ileum with malabsorption, and fluid and bicarbonate loss may occur. The incidence of postoperative bowel obstruction with colon is 4%, less than that occurring with ileum. Both ileal and colon segments result in the same type of electrolyte imbalance with similar frequencies. An antireflux ureterointestinal anastomosis by the submucosal tunnel technique is easier to perform with use of the colon. In general, ileum and colon are comparable and have few differences, which does not argue strongly for the selection of one over the other except under special circumstances.
Bowel Preparation
It has been a long-held tenet of elective intestinal surgery that bowel preparation is appropriate. The bacterial population in the stomach is relatively low, but in the remaining segments of the bowel including the jejunum, ileum, and colon, there are high bacterial counts. Early studies had suggested that bowel anastomoses in the patients whose intestinal tract had not been prepared before surgery had increased wound infection rates, increased intraperitoneal abscesses, and an anastomotic dehiscence rate greater than in those patients who have had proper bowel preparation before surgery (Irvin and Goligher, 1973; Dion et al, 1980). Other studies showed that mechanical preparation resulted in collapsed bowel at the time of surgery, which was shown to reduce the incidence of anastomotic leaks (Christensen and Kronborg, 1981). Studies have recently begun to question the widely held belief that bowel preparation is mandatory. In meta-analyses of randomized clinical trials of anastomotic leakage during colon and rectal surgery, researchers found that there was no support for the conclusion that bowel preparation reduces anastomotic leak rates and other complications (Guenaga et al, 2003; Slim, 2009). There are suggestions that mechanical bowel preparation may actually increase the rate of anastomotic leakage and wound complications (Guenaga et al, 2005).
In experimental animals, it has been shown that an anastomosis with vascular compromise at the anastomotic line, which would normally result in perforation, heals if the bowel has been properly prepared with antibiotics. Also, solid feces may place strain on the anastomosis in the early phase of healing and result in ischemia with subsequent perforation. Complications that result from bacterial contamination are a major cause of morbidity and mortality in patients undergoing urologic procedures. Infectious complications after radical cystectomy that are a direct result of fecal contamination may occur in 18% to 20% of patients who undergo radical cystectomy and include wound infections, peritonitis, intra-abdominal abscesses, wound dehiscence, anastomotic dehiscence, and systemic sepsis (Bracken et al, 1981). More recent series suggest that current management practices appear to have made a substantial improvement with perioperative infectious complications of 7% (Stein et al, 2004). In another contemporary series of radical cystectomy with continent or ileal loop urinary diversion in 167 patients, there was an infection complication rate of 7.2% (Mansson et al, 2003). A 5.2% rate of infectious complications was reported in another contemporary series (Cookson et al, 2003).
There are two aspects to bowel preparation, mechanical and antibiotic. Both methods attempt to reduce the complication rate from intestinal surgery. The mechanical preparation reduces the amount of feces, whereas the antibiotic preparation reduces the bacterial count. The bacterial flora in the bowel consists of aerobic organisms, the most common of which are Escherichia coli and Enterococcus faecalis, and anaerobic organisms, the most common of which are Bacteroides species and Clostridium species. The bacterial concentration ranges from 10 to 105 organisms per gram of fecal content in the jejunum, 105 to 107 in the distal ileum, 106 to 108 in the ascending colon, and 1010 to 1012 in the descending colon.
Mechanical Bowel Preparation
A mechanical bowel preparation reduces the total number of bacteria but not their concentration. Thus the same number of organisms is present per gram of fecal content (Nichols et al, 1972). Therefore spilling enteric contents during the procedure may be less likely with the mechanically prepared bowel because there is less of it to spill; however, once spilled, cubic centimeter for cubic centimeter, the inoculum is the same as if the bowel had not been prepared. Recent analysis has suggested, however, that there may in fact be an increase in bacterial contamination in patients who have undergone bowel preparation (Fa-Si-Oen et al, 2005).
Conventional bowel preparations commonly used in the past tended to exhaust the patient and exacerbate nutritional depletion because they generally required a 3-day preparation period of insufficient calorie intake (Table 85–1). The use of elemental diets has been advocated to clean the colon of feces while not compromising the nutritional status of the patient. Unfortunately, they have not proved useful because the elemental diets do not empty the colon of feces, and they do not reduce the bacterial flora (Arabi et al, 1978). In an attempt to reduce the time required for intestinal preparation and to obviate low-calorie intakes, whole-gut irrigation has been used. Originally, whole-gut irrigation was performed by placement of a nasogastric tube (NGT) into the stomach and infusion of 9 to 12 L of lactated Ringer solution or normal saline during a several-hour period. These fluids were subsequently replaced with 10% mannitol, which was equally successful in ridding the bowel of its fecal content; however, the mannitol served as a bacterial nutrient and thereby facilitated microbial growth (Hares and Alexander-Williams, 1982). These solutions have largely been replaced by a polyethylene glycol–electrolyte solution. Whole-gut irrigation may be exhausting to the patient and may, in fact, result in a fluid gain, particularly when either saline or mannitol is used. Whole-gut irrigation is contraindicated in patients with an unstable cardiovascular system, patients with cirrhosis, patients with severe renal disease, patients with congestive heart failure, or those with an obstructed bowel. Whole-gut irrigation has been found to be no more effective than conventional preparations in reducing wound infections and septic complications (Christensen and Kronborg, 1981), even though there is a reduction of aerobic flora compared with the conventional preparations (van den Bogaard et al, 1981). The advantages of the whole-gut irrigation are that it gives the patient dietary freedom, there is a short preparation time, and it eliminates the enema. Its disadvantages are that it may result in the patient’s exhaustion, it is rather rigorous, and it does result on occasion in fluid overload.
The polyethylene glycol–electrolyte lavage solution (GoLYTELY or the more palatable NuLYTELY) is an effective lavage agent in preparing the gut for elective colon and rectal surgery, as well as for urologic surgery in which bowel is used. For the adult, 20 to 30 mL/min or approximately 1 to 1.5 L/hr for 3 hours is given either orally or through a small-caliber NGT placed into the stomach. If it is taken by mouth, it is better tolerated if the solution is chilled. The administration of GoLYTELY is stopped when the rectal effluent is clear and there is no particulate matter in it or when 4 L of fluid has been given. This preparation in the adult has been as effective as conventional preparations. The septic complications with its use are approximately 4%. An inadequate preparation occurs in 5% of the patients using this modality (Wolff et al, 1988). For children, even those younger than 1 year, GoLYTELY may be used at a rate of 20 to 40 mL/kg/hour and given until the rectal effluent is clear and free of particulate matter (Tuggle et al, 1987). Metoclopramide (Reglan), 10 mg, in adults is often given simultaneously to control nausea.
Bowel preparation can increase metabolic complications and cause electrolyte disturbances, which could affect surgical care. Caution must be exercised in elderly and debilitated patients receiving sodium phosphate preparation; the sodium phosphate preparation has been shown to cause significant derangements in potassium, calcium, and phosphorus levels in frail individuals (Beloosesky et al, 2003). Phosphate nephropathy has recently been recognized as a serious complication of oral sodium phosphate (OSP) bowel preparation (Markowitz, 2005). Caution should be used in prescribing OSP to patients with underlying renal insufficiency or treated with nephrotoxic medications. The only study in postsurgical complications comparing sodium phosphate with polyethylene glycol found no significant difference in complication rates (Oliveira et al, 1997). One study suggested that polyethylene glycol is better tolerated by elderly patients and causes less disruption in potassium and sodium levels (Seinela et al, 2003). Oral electrolyte solution rehydration may prevent some of the complications of bowel preparation (Tjandra and Tagkalidis, 2004).
A number of studies have questioned the efficacy of mechanical bowel preparation. Some have suggested that a limited mechanical bowel preparation is all that is necessary; others have questioned even the need for a mechanical bowel preparation. In one study, 2 L of polyethylene glycol plus metoclopramide was compared with the administration of 4 L of polyethylene glycol solution. There was no difference in surgical complication rate or the extent to which the bowel was clean (Grundel et al, 1997). In another study, when 4 L of polyethylene glycol was compared with 90 mL of sodium phosphate, there was no significant difference in surgical complication rate (Oliveira et al, 1997). Two meta-analyses have found that there is an increased anastomotic dehiscence rate with preoperative mechanical bowel preparation (Wille-Jorgensen et al, 2003; Bucher et al, 2005). Polyethylene glycol may be the agent responsible for the increased rate of complications, but other preparation strategies have not been adequately analyzed (Slim et al, 2004). A recent meta-analysis of randomized trials comparing mechanical bowel prep to no bowel prep before elective colorectal surgery found no difference between the groups for anastomotic leakage, abdominal abscess, or wound sepsis (Slim, 2009). No study has adequately addressed the issue of the need for debulking of the intestine before laparoscopic approaches to intestinal surgery. It is extremely important to note that in these studies, the administration of intravenous antibiotics was crucial in keeping the complication rate low. Moreover, it is important to note that in these studies there was limited exposure of the intestine as the patients underwent elective bowel resections, unlike urologic procedures in which long segments of the intestine are opened or interposed in a urinary tract that is normally free of fecal contents.
Antibiotic Bowel Preparation
There has been considerable recent controversy as to whether the addition of antibiotics in elective colon and small bowel surgery reduces mortality and morbidity significantly. The long-held practice of mechanical and oral antibiotic bowel preparation dates to the 1970s. In one study, the septic complication rate was reduced from 68% in the control group to 8% in the antibiotic group (Washington et al, 1974). Most series, however, report a lesser incidence of reduction in wound infection, generally from 35% without antibiotics to 9% with their use (Clarke et al, 1977). Others have suggested that the mortality rate drops from 9% to 3% with the use of antibiotics (Baum et al, 1981). It is clear that the use of antibiotics protects vulnerable bowel in that it may allow the tenuous anastomosis to survive. Other studies, however, have shown that without the use of oral antibiotics in mechanically prepared bowel in elective surgery, the septic complication rate is comparable with those studies using antibiotics and the rate of Clostridium difficile colitis was lower without oral antibiotics (Wren, 2005). In the presence of a bowel obstruction, however, oral antibiotics are of little value because they do little good in sterilizing the bowel. The disadvantages of antibiotics include postoperative increase in the incidence of diarrhea; pseudomembranous enterocolitis; theoretical increased incidence of tumor implantation at the suture line that is not germane to urologic surgery; monilial overgrowth resulting in stomatitis, thrush, and diarrhea; and, with prolonged use, malabsorption of protein, carbohydrate, and fat. The antibiotics most commonly used for bowel preparation include kanamycin, which is the best single agent; neomycin and erythromycin base; and neomycin and metronidazole (Table 85–2). With an appropriate antibiotic preparation, enteric organisms are reduced to 102 per gram of feces (Nichols et al, 1972). A contemporary randomized trial of patients undergoing elective colonic surgery found the lowest fecal bacterial concentrations when patients had preoperative mechanical bowel preparation, oral neomycin, and supplemental “synbiotic” treatment (to provide benign flora to the intestine). No difference in clinical infections was seen between patients prepared with or without oral antibiotic bowel preparation (Reddy, 2007).
Perioperative intravenous antibiotics appear to be the most important means of preventing infectious complications of intestinal surgery. Systemic antibiotics must be given before the operative event if they are to be effective. Ideally, antibiotics should be given between 1 and 2 hours before the start of surgery (Classen et al, 1992). They appear to be most effective against the anaerobic flora and apparently reduce the complications caused by these organisms (Dion et al, 1980). Perioperative systemic antibiotics, when added to the oral regimen, reduced the septic complication rate from 15% to 20% to half that rate in several series (Hares and Alexander-Williams, 1982; Gottrup et al, 1985). Other studies, however, have shown no effect of systemic cephalosporin, for example, in reducing septic complications (Wolff et al, 1988). If perioperative antibiotics are given, they should be effective against anaerobes because it is complications from these organisms against which perioperative antibiotics appear to be particularly effective. Third-generation cephalosporins have been advocated as an appropriate systemic antibiotic. Other recent studies support the use of both oral and systemic antibiotic prophylaxis before intestinal surgery (Lewis, 2002). It is clear that preoperative antibiotics reduce postoperative complications. Most agree that preoperative intravenous antibiotics are important, and many advocate discontinuing the use of oral antibiotics as the incidence of C. difficile diarrhea is increased and there appears to be no advantage provided preoperative intravenous antibiotics are given within an hour of the operative event (Wren et al, 2005).
It is the authors’ preference not to perform a vigorous mechanical bowel preparation allowing the patient a normal diet until one day preoperatively, at which time a clear liquid diet is begun and citrate of magnesia is taken as a cathartic. No oral antibiotic bowel preparation is performed; however, the patient is given intravenous antibiotics 1 hour before the incision.
Diarrhea and Pseudomembranous Enterocolitis
Antibiotic bowel preparations may result in diarrhea and pseudomembranous enterocolitis. Pseudomembranous enterocolitis is the more severe form of a spectrum of diarrhea. Clinically, this occurs after a bowel preparation in the postoperative period and is heralded by abdominal pain and diarrhea usually in the absence of fever or chills. As the symptoms and infection become more severe, systemic toxicity supervenes. These patients can develop a toxic megacolon, and if this occurs, the mortality may exceed 15% to 20%. Historically, pseudomembranous enterocolitis was thought to be due to staphylococcus, but there was, in fact, little evidence to support that organism as the etiologic agent. It is now clear that C. difficile plays a significant role in the majority of cases. C. difficile elaborates at least two toxins that cause diarrhea and enterocolitis. C. difficile does not invade the bowel, and it is not normally a significant inhabitant of the fecal flora. It is held in check by other bacteria that inhibit its growth. Thus antibiotics destroy the bacteria that inhibit the growth of C. difficile and thereby allow it to flourish. The toxin produces a diffuse inflammatory response with cream-colored plaque formation, erythema, and edema of the bowel wall. On microscopic examination, the villi appear to be intact and there is a polymorphonuclear leukocyte infiltrate of the submucosa (Bartlett, 2002).
As the disease progresses, large areas of mucosa may slough and areas of the bowel are denuded of their mucosa. The lesions may involve the colon, in which case it is called pseudomembranous enterocolitis, or the small bowel, in which case it is called pseudomembranous enteritis, or they may involve both. The diagnosis is suspected by the symptoms or endoscopy and confirmed by culture of the organism or identification of its toxin. Because culture takes a prolonged time, it is more expeditious and therefore clinically useful to confirm the diagnosis by identifying the toxin produced by C. difficile. Once the diagnosis has been made, treatment involves the administration of vancomycin or metronidazole and discontinuance of other antibiotics that the patient is receiving. Vancomycin or metronidazole is effective in most cases. Rarely, toxic megacolon supervenes, requiring subtotal colectomy as a lifesaving procedure (Chang, 1985).
Intestinal Anastomoses
Regardless of the type of anastomosis or the methods used to perform it, certain fundamental principles must be observed to minimize morbidity and mortality from intestinal surgery. In urologic procedures in which gut is used, the most common cause of mortality and morbidity within the immediate postoperative period relates to complications involving the bowel, either with the enteroenterostomy or with the segment interposed in the urinary tract. Therefore it cannot be overemphasized that great care must be taken and proper techniques used in handling bowel in urologic procedures. Unfortunately, the portion of the procedure that involves mobilization of the intestine and reanastomosis often follows a rather lengthy urologic endeavor and is performed when the surgical team is not fresh. Therefore the following principles should be so firmly ingrained in the surgeon that they are performed without the need to recall each one specifically.
The first principle of proper technique for intestinal anastomoses is adequate exposure. The intestine should be mobilized sufficiently so that the anastomosis may be performed without struggling for exposure. If possible, it is preferable to mobilize the intestine sufficiently so that the anastomosis can be performed on the anterior abdominal wall. The area of the anastomosis should be walled off from the rest of the abdominal cavity with Mikulicz pads. This is important so that any inadvertent enteric spills are not distributed throughout the abdominal cavity. The mesentery must be cleared from the bowel segments to be anastomosed for a suitable distance (usually 0.5 cm) from the intestinal clamps or staple line at the severed ends so that good serosal apposition may be achieved without interposed mesentery. Sufficient serosa must be exposed so that the seromuscular sutures or staples can be placed directly in the serosa without traversing the mesentery.
The second principle of performing a proper anastomosis is to maintain a good blood supply to the severed ends of the bowel. The blood supply may be compromised by construction of an anastomosis under tension, excessive dissection or mobilization of the bowel, excessive use of the electrocautery, and tying of the sutures so tight that the intervening tissue is strangulated. A cut margin of bowel that is pink and bleeds freely suggests that the blood supply has not been compromised; however, hemostasis must be ensured before beginning the anastomosis. The site of transection is selected at a point where the blood supply is adequate to both segments. The mesentery should be transilluminated so that the blood supply may be defined before transection of the bowel segment. In urologic surgery, the location of the transection is elective so that an area may be selected in which excellent arcades supply both sections of the transected segment. The area must be selected with an eye to how deep the mesenteric transection must be for proper segment mobility. After location of the appropriate area where the mesentery is to be transected, it is cleaned from the serosa, severed between mosquito clamps, and tied with 4-0 silk sutures. Alternatively, the mesentery may be transected with the LDS staple device (Covidien Surgical, Mansfield, MA), a bipolar cautery device, or ultrasonic shears.
The third principle involves prevention of local spillage of enteric contents. The best way of preventing spills is to operate on bowel properly prepared (i.e., devoid of feces and collapsed). Stripping of the enteric contents between the fingers both cephalad and caudad from the proposed transection site and application of a noncrushing occlusive clamp across the bowel make a spill even less likely. The clamp should prevent enteric contents from exiting the cut ends of the bowel without interference with the mesenteric blood supply. After linen-shod clamps are applied and the area is walled off, Allen clamps are applied to the bowel and the bowel is transected between the Allen clamps. An anastomotic staple device may be used to transect the bowel at this point in place of Allen clamps (see later). Local spills and local sepsis have an adverse effect on the healing anastomosis, and it is for this reason that noncrushing occlusion clamps, in addition to an adequate bowel preparation, are advisable. If a spill does occur, it should be caught in the Mikulicz pads if the bowel has been properly walled off as described previously. The isolated segment that is to be used in the reconstructive procedure should be irrigated thoroughly with copious amounts of normal saline. The segment should be walled off. The irrigant is placed in one end of the segment and caught in a kidney basin as it exits the other end. This should be continued until the efflux is clear. This procedure prevents local spills during the ureterointestinal anastomosis and other aspects of reconstruction.
The fourth principle, germane to all intestinal anastomoses, is that there should be an accurate apposition of serosa to serosa of the two segments of bowel to be anastomosed. The anastomosis should be watertight and performed without tension. The bowel must be handled gently with the use of noncrushing forceps. The anastomotic line should be inverted and not everted. There is considerable controversy about this issue in that an everted anastomosis has been shown to heal with few complications. It is clear that when marginal conditions occur, an inverted anastomosis is more likely to remain intact than is the everted anastomosis.
The fifth principle is not to tie the sutures so tight that the tissue is strangulated. Obviously, the sutures must bring the serosa of the two segments firmly together. Nonabsorbable sutures used for the anastomosis result in a stronger anastomotic line in the early healing phase compared with absorbable sutures, but the difference is minimal and probably not particularly significant.
The final principle involves realignment of the mesentery of the two segments of bowel to be joined. These should be parallel to each other and ensure that there is no twist on completion of the anastomosis.
Factors that significantly contribute to anastomotic breakdown include poor blood supply, local sepsis induced by fecal spillage, drains placed on an intra-abdominal anastomosis, and anastomosis performed in irradiated bowel. Poor blood supply and local sepsis cause ischemia. Drains placed on the anastomosis increase the likelihood of an anastomotic leak, and an anastomosis performed in irradiated bowel is more likely to result in an anastomotic failure than one performed in nonirradiated tissue. The importance of careful technique and adherence to these principles is emphasized by the fact that in one series of urinary intestinal diversion, 75% of the lethal complications that occurred in the postoperative period were related to the bowel. Eighty percent of these patients had received radiation before the intestinal surgery (Mansson et al, 1979).
Types of Anastomoses
Intestinal anastomoses may be performed with use of sutures or staples. Properly performed, both have similar complication rates (Catena, 2004). In selected circumstances, however, one method may have advantages over the other. Because there is a high rate of stones that form on surgical staples (Woodhouse, 2004), absorbable suture should be used for intestinal segments that are exposed to urine (e.g., suturing intestine to renal pelvis or bladder, closing the proximal end of a conduit [Costello and Johnson, 1984], and forming an intestinal pouch for urine).
Enteroenterostomy by a Two-Layer Suture Anastomosis
A 3-0 silk holding suture is placed on the mesenteric border just beneath the Allen clamps traversing both segments to be anastomosed, and a second suture is placed on the antimesenteric border similarly just beneath the Allen clamps (Fig. 85–3). It is important that the mesentery is cleaned sufficiently so that these sutures are placed in the serosa under direct vision. A row of silk sutures is placed 2 mm apart between the two holding sutures. This is accomplished by rotating the two Allen clamps away from each other, thus apposing the serosal surfaces. Sutures must traverse the muscularis but should not traverse the full thickness of the bowel. After all sutures have been placed, each is tied and the tails of all the sutures are cut, except those at each end; these are used as holding sutures. The Allen clamps are removed, and hemostasis is achieved, if necessary, with the light application of electrocautery. A 3-0 double-ended chromic intestinal suture is placed in the posterior suture line through all layers and tied to itself. Each end of the suture is then run in a locking fashion away from the midpoint until the mesenteric and antimesenteric borders are approached. As the lateral aspects of the bowel are approached, the suture is converted to a Connell suture (Fig. 85–4), which proceeds onto the anterior bowel wall. The sutures meet anteriorly in the midline and are tied together. The anterior serosa is then apposed with interrupted 3-0 silk sutures. The noncrushing occlusive clamps are removed, and the mesentery is closed with interrupted 3-0 silk sutures.
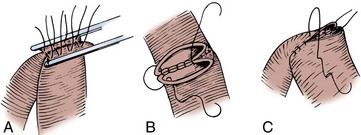
Figure 85–3 Two-layer suture anastomosis. A, Two holding sutures of 3-0 silk have been placed at the mesenteric and antimesenteric border, and the posterior wall is approximated with seromuscular sutures of 3-0 silk. B, A 3-0 intestinal chromic suture is placed through the full thickness of the bowel posteriorly, tied to itself, and run to the lateral borders with a continuous locking suture. At the lateral borders, it is converted to a Connell suture. C, The Connell suture brings the anterior margins together, inverting the suture line. The anastomosis is completed by placement of horizontal mattress seromuscular sutures of 3-0 silk over the anterior suture line (not depicted).
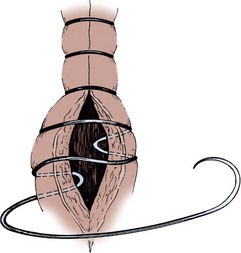
Figure 85–4 Connell suture. The suture traverses the bowel from serosa to mucosa and then from mucosa to serosa on the same side of the anastomosis. The suture is then placed on the opposite side of the anastomosis “outside in-inside out.” The sequence is repeated until the two segments are approximated.
Patency of the anastomosis is ensured by palpating the anastomosis with the thumb and forefinger and feeling an annulus of tissue around the fingers. This anastomotic technique is employed when the antrum pylorus is removed and intestinal continuity is restored by a Billroth I procedure. It is also the most secure of all the anastomoses and should be used when one is forced to do an anastomosis under less than ideal circumstances.
Enteroenterostomy by a Single-Layer Suture Anastomosis
The single-layer anastomosis for reapproximating bowel is an excellent technique with a low complication rate, that is, a 0.2% anastomotic leakage rate compared with an 8.4% anastomotic leakage rate for a stapled anastomosis in one large series (Leslie and Steele, 2003).
The mesenteries of the two segments of bowel to be anastomosed are aligned, and a 3-0 silk suture is passed through the seromuscular layers of both segments on the mesenteric side; a second suture is similarly placed on the antimesenteric side. The mesenteric suture is tied, and the antimesenteric suture is left untied. The Allen clamps are removed, and hemostasis is achieved with light electrocautery. The critical point of the anastomosis, where most leaks occur, is at the mesenteric border. Leaking generally occurs because the sutures are placed carelessly or the serosa has not been cleaned of mesentery sufficiently so that the sutures are placed through it under direct vision. Because this mesenteric border is the critical area, it is approached first. Two 3-0 silk sutures are placed through the full thickness of the bowel on either side of the mesenteric holding suture. These sutures are placed in such a way as to include more serosa than mucosa, thus causing inversion of the suture line (Fig. 85–5A). Some prefer to use a Gambee stitch at this point, which involves placing the suture through the full thickness of the bowel followed by traversing a small segment of mucosa of each segment of bowel before exiting through the full thickness of the bowel of the other segment (Fig. 85–5B). The two bowel sutures on the mesenteric border are tied, with care taken to invert the suture line, thus apposing serosa. Then 3-0 silk sutures are placed 2 mm apart, both on the anterior and posterior walls, inverting the suture line, thus apposing the serosa of the two bowel segments to each other. On approaching the antimesenteric holding suture, several sutures are placed before all are tied. A patent anastomosis is confirmed by feeling the annulus with the thumb and forefinger as described previously.
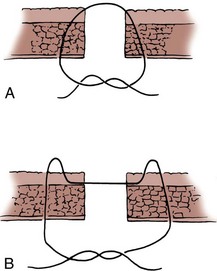
Figure 85–5 A, When it is properly placed, the suture through the intestine should include more serosa than mucosa. B, The Gambee stitch. The suture is placed through the full thickness of the bowel, the mucosa is traversed, and then the mirror image procedure is performed on the segment to be anastomosed.
End-to-Side Ileocolic Sutured Anastomosis
The transected end of the colon is closed in the following manner (Fig. 85–6). A 3-0 silk suture is placed beneath the Allen clamp on the mesenteric border, and a second suture is placed on the antimesenteric border. These are tied. A 3-0 chromic suture is placed beneath the clamp in a horizontal mattress fashion. Beginning at the mesenteric border, it is tied to itself and the horizontal mattress suture is placed until the antimesenteric border is reached, at which point the suture is again tied to itself. The clamp is removed, and an over-and-over suture is performed with the same chromic suture throughout the full thickness of the bowel until returning to the point of origin (i.e., the mesenteric border is approached). At this point, the suture is again tied to itself. The suture line is buried by approximating the serosa on each side with interrupted 3-0 silk sutures placed 2 mm apart. Our preference is to close the end of the colon similarly to the way one closes the proximal end of a conduit. After a 3-0 silk suture is placed through the serosa on the antimesenteric and mesenteric sides, the clamp is removed and a 3-0 chromic suture is placed through all layers at the mesenteric and antimesenteric end. A Connell suture is used—the two chromic sutures meet in the middle and are tied together. Seromuscular sutures of 3-0 silk are placed to appose the serosal margins.
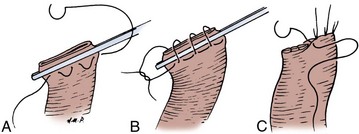
Figure 85–6 Closure of the proximal end of the intestine. A, A 3-0 chromic suture is tied to itself at the antimesenteric border and placed beyond the intestinal clamp in a horizontal mattress fashion until the mesenteric border is reached. The suture is then tied to itself at this point. B, The intestinal clamp is removed, and an over-and-over suture through the full thickness of the bowel returns the chromic suture to its point of origin, where it is again tied to itself. C, Interrupted horizontal mattress seromuscular sutures of 3-0 silk invert the chromic suture line.
The mesenteries are aligned, and the ileal serosa is sutured with interrupted 3-0 silk sutures to the colonic serosa 2 mm below a taenia (Fig. 85–7). The taenia is incised the length of the diameter of the ileum adjacent to it. As described earlier for the two-layer anastomosis, a 3-0 double-ended intestinal chromic suture is placed through all layers of the colon and ileum in the midpoint of the posterior wall and run in a locking fashion laterally to either side of the incision in the taenia. At the most lateral border, the suture is converted to a Connell suture and the anterior wall is closed. Seromuscular sutures of 3-0 silk placed from ileum to colon bury the anterior suture line. The mesentery is reapproximated.
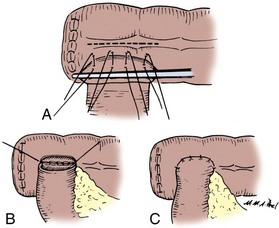
Figure 85–7 End-to-side anastomosis. A, The serosa of the ileum is sutured to the serosa of the colon 2 to 3 mm below a taenia. B, The taenia is opened for a distance sufficient to accommodate the diameter of the ileum. A 3-0 chromic suture is placed through all layers on the posterior wall, tied to itself, run in a locking fashion to both borders, and converted to a Connell suture laterally, thus completing the inversion anteriorly. C, The anterior margin of serosa is reapproximated with interrupted horizontal mattress sutures of 3-0 silk.
Ileocolonic End-to-End Sutured Anastomosis with Discrepant Bowel Sizes
A 3-0 silk suture is placed on the mesenteric border of the ileum and colon (Fig. 85–8). A second 3-0 silk suture is placed on the antimesenteric border of the colon immediately beneath the Allen clamp. The other end of the suture is placed on the antimesenteric border of the ileum at a distance proximal to the Allen clamp so that the serosal lengths between the two sutures of both ileal and colon segment are equal. Thus an equal amount of ileal serosa is applied to the length of colonic serosa bordering the severed end of bowel. In the seromuscular layers of ileum and colon, 3-0 silk sutures are placed 2 mm apart, thus apposing the serosa of the ileum to the colon. The Allen clamps are removed. Hemostasis is achieved, and the antimesenteric border of the ileum is incised to the level of the most proximal suture in the ileum. Thus the bowel lumens are now of identical size. With a 3-0 chromic double-ended intestinal suture, the posterior row is run in a locking fashion, laterally converting to a Connell suture, and the anterior row is completed. Seromuscular sutures of 3-0 silk bury the anterior suture line.
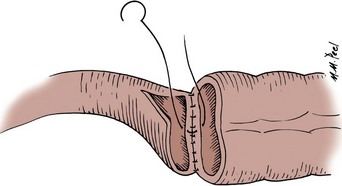
Figure 85–8 Anastomosis of discrepant-sized bowel. A seromuscular suture of 3-0 silk is placed adjacent to each end of the lumen on the mesenteric side. A second 3-0 silk seromuscular suture is placed adjacent to the lumen on the colon and on the antimesenteric border proximal to the cut end of the small bowel at a distance sufficient that when the antimesenteric border is incised, the lumens are the same size. Interrupted seromuscular sutures of 3-0 silk are then placed at 2-mm intervals between the two holding sutures. The small bowel is opened on its antimesenteric border until the opening in the small bowel is the same size as the opening in the colon. A 3-0 chromic suture is placed through all layers, tied to itself, and run laterally in a running locking fashion. At the borders, it is converted to a Connell suture, thus inverting the anterior margin. The anastomosis is completed with interrupted horizontal mattress 3-0 silk sutures that bring the seromuscular layers together anteriorly. This is similar to the closure depicted in Figure 85–3.
Stapled Anastomoses
The theoretical benefits of a stapled anastomosis are that it provides for a better blood supply to the healing margin, there is reduced tissue manipulation, there is minimal edema with uniformity of suture placement, a wider lumen is constructed, there is greater ease and less time involved in performing the anastomosis, and the length of postoperative paralytic ileus is reduced. When they are placed in intestine through which urine traverses, however, stapled anastomoses employing nonabsorbable staples frequently cause stone formation and should be avoided (Costello and Johnson, 1984; Woodhouse, 2004).
The TA (transverse anastomosis) stapled anastomosis everts the suture line. Because staples close in a B and do not crush the tissue, theoretically they prevent ischemia at the suture line. This may be obvious when a staple line is used to transect the bowel and bleeding continues to occur. The bleeding points may be lightly electrocoagulated or tied off with fine absorbable suture. Stapled bowel anastomoses have been shown to be as efficacious as hand-sewn anastomoses because both have similar complication rates. They usually require less time to perform when the techniques are properly learned, but for prolonged procedures, they save little if any time when the length of time for the whole procedure is taken into account. In a large prospective, randomized trial in which a two-layer closure was compared with a staple closure, it was found that the complication rate was the same, but the time required to complete the stapled anastomosis was 10 minutes less than that for the hand-sewn anastomosis; when the total operative time was compared between the two, it was the same (Didolkar et al, 1986). A comparison of complications between sutured and stapled anastomoses reveals a leak and fistula rate of 2.8% for stapled and 3% for sutured anastomoses (Chassin et al, 1978). The clinically significant leak rate, however, is only 0.9% (Fazio et al, 1985). A 4.5% incidence of stapled anastomotic leakage has been reported during ileal conduit construction (Costello and Johnson, 1984).
Thus the use of staples or sutured anastomosis is at the discretion of the surgeon. A stapled anastomosis appears to be superior to a hand-sewn anastomosis in an esophageal-intestinal anastomosis and a low rectal anastomosis. In these two areas, the circular stapler allows a more precise anastomosis than is often possible with hand-sewn techniques. Because these are not problems of urologic surgery, staples are used if that is the preference of the surgeon. The one area in urology where the authors believe the stapling device is superior is in the ileocolonic end-to-side anastomosis. With use of the circular stapling device, a widely patent anastomosis can be achieved expeditiously.
Three staple instruments are commonly employed in intestinal reconstruction: the linear stapler, the anastomotic stapler, and the circular stapler. The linear stapler places a double or triple row of staggered staples in a straight line. Depending on the cartridge and instrument chosen, various lengths of staple lines and heights of the closed staples may be chosen. The length is selected according to how long one wants the staple line to be. The height of staple is selected according to the tissue to be stapled. Vascular and pulmonary tissues require staples with a closed height of 1 mm (open height of 2.5 mm). Most intestinal anastomoses are performed with medium staples, which have a closed height of approximately 1.5 mm (open height of 3.5 mm). On occasion, for thick tissues, large staples are required that have a closed height of 2 mm (open height of 4.8 mm). If there is any doubt in selecting the staple size, the tissue thickness may be measured with a special instrument used for this purpose. In general, tissues less than 1 mm or more than 3 mm in thickness are not amenable to the use of staples.
The anastomotic stapler places two linear double rows of staggered staples. When the knife is advanced, the staple line is divided. The height of the staples is also chosen according to the tissue to be transected.
The circular stapler places two concentric, staggered circular staple rows and cuts the tissue within the circle completely from the surrounding tissue. It may be selected in various diameters and with various heights of staples. The diameter and height are selected according to the tissue to be anastomosed. The diameter to be selected is determined by sizing the diameter of the tissue to be stapled. Special sizers are available for this maneuver. In most intestinal anastomoses in urology, the height of the closed staple is 1.5 to 2 mm. The following is a description of various types of stapled anastomoses.
Ileocolonic Anastomosis with the Circular Stapling Device
The mesenteric borders are cleared for a distance of 1.5 cm from the cut end of both the colon and the ileum (Fig. 85–9). Holding sutures of 3-0 silk are placed on the mesenteric and antimesenteric border of the colon. Two other holding sutures are placed on the medial and lateral walls of the colon, midway between the mesenteric and antimesenteric sutures. A purse-string suture of 2-0 polypropylene (Prolene) is placed around the ileum no more than 2 mm from the cut end. It is important to take small bites of mucosa to avoid bunching of tissue. Sutures must be placed evenly to avoid a gap. A purse-string instrument can be used for this step, if preferred. The ileal diameter is determined with sizers so that the correct circular stapler diameter instrument may be chosen—usually 25 mm. A purse-string suture is also placed in a circle, 1 cm in diameter, through which a taenia traverses on the medial aspect of the colon. A stab wound is made in the center of the colonic purse-string suture. The distal anvil of the circular stapler is removed, and the instrument is placed through the open end of the colon with its post passed out the stab wound made in the center of the purse-string on the medial wall of the colon. The purse-string is tied tight. The top anvil is then secured to the post, and the ileum is placed over it. The ileal purse-string is tied.
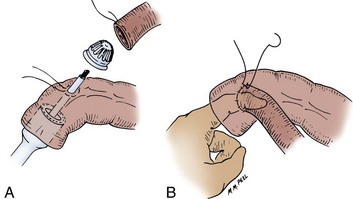
Figure 85–9 Stapled circular anastomosis. A, A purse-string suture of 2-0 polypropylene (Prolene) is placed around the circumference of the small bowel, and a second purse-string suture 1 cm in diameter is placed on a medial taenia 5 to 6 cm from the open end of the colon. The anvil is removed. A stab wound is made in the center of the purse-string suture in the colon, and the circular stapler is introduced through the end of the colon, with its post thrust through the stab wound. B, The anvil is placed on the post and introduced into the end of the small bowel, the purse-string sutures in the small bowel and colon are tied snugly around the post, and the circular stapler is approximated with a gap of 1.5 to 2 mm, with care taken not to include any mesentery in the gap. The anastomosis is completed by placement of interrupted silk sutures around the circumference of the anastomosis.
Care must be taken to align the mesenteries at this point. The instrument is approximated with a staple gap of 1.5 to 2 mm. Care must be taken not to catch fat or mesentery in the gap. The instrument is fired and removed by rotatory movement from the colon. Two doughnuts of tissue should be identified on the instrument, and they should have their complete circumference intact with no gaps. With a finger in the open end of the colon and through the anastomosis, seromuscular sutures of 3-0 silk are placed 3 to 4 mm apart around the circumference of the anastomotic line.
The transected end of the colon may be closed by the suture technique or by the use of staples. If the end is to be closed with sutures, one 3-0 chromic suture is brought out the mesenteric border and another out the antimesenteric border, and both are tied to themselves with the knots on the inside of the bowel. The two sutures are run toward each other with a Connell stitch until they meet, and then they are tied to each other. The suture line is inverted by placement of a second row of 3-0 silk seromuscular sutures. If staples are preferred, the holding sutures are held up and a linear stapler is applied across the open end. Excess tissue is trimmed and the stapler removed. By holding the holding sutures up, one is secure in applying the staple line to the serosa and mucosa circumferentially around the bowel. Some invert the staple line with seromuscular sutures of 3-0 silk. This is not necessary, however. The mesentery between the two segments is now approximated with interrupted 3-0 silk sutures.
End-to-End Stapled Anastomosis: Ileal-Ileal or Ileocolonic Anastomosis
The antimesenteric border of the two bowel segments to be joined is approximated with a 3-0 silk suture 5 to 6 cm from the cut ends of the bowel (Fig. 85–10). A holding suture is placed through both segments of bowel at their cut ends at the midpoint of the antimesenteric borders. Stay sutures are placed at the mesenteric border of each bowel segment, and two other sutures midway between the mesenteric and antimesenteric border on the lateral aspects of the bowel are also placed. The anastomotic stapler is positioned in the lumens of both segments of bowel along the antimesenteric border. The antimesenteric holding suture is pulled up adjacent to the stapler. The anastomotic stapler is locked in place, the staples are fired, and the knife is advanced. The staple lines are inspected for bleeders, which if persistent should be suture ligated with an absorbable suture. It is important for several 3-0 silk sutures to be placed at the apex of the stapled and cut antimesenteric incision. At this point, slight tension on the anastomotic line can place undue stress on the staple margin and cause a leak. The holding sutures are held up, and a linear stapler is placed across the open end of bowel and fired. Care must be taken so that the staples include the serosa in its entire circumference. Excess bowel tissue is excised flush with the instrument before it is disengaged. The mesentery is then reapproximated.
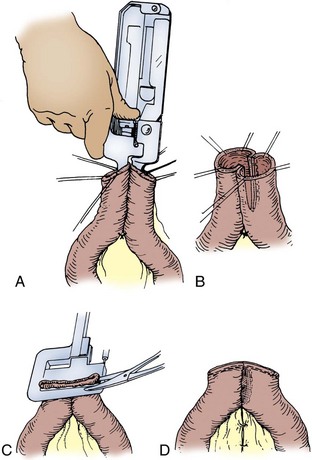
Figure 85–10 Stapled end-to-end anastomosis. A, A 3-0 silk suture is placed 5 to 6 cm from the cut ends of the intestine on the antimesenteric borders of both intestinal segments and tied. Holding sutures are placed around the circumference of both intestinal lumens, one suture securing together the antimesenteric borders of both intestinal segments. The linear anastomotic stapler is placed into the lumens, secured and locked in place, and fired. The knife is advanced. B, The appearance of the intestinal anastomosis after firing of the staple device. C, The open end of the two intestinal segments is closed with a linear stapler by elevation of the holding sutures while the linear stapler is applied so that the circumferences of the mucosa and serosa are incorporated in the staple margin. D, The anastomosis is completed by closure of the mesentery with interrupted 3-0 silk sutures.
Laparoscopic Anastomoses
Laparoscopic approaches to cystectomy and augmentation cystoplasty have been reported (Gill et al, 2000a, 2002; Guilliotreau, 2009). In these procedures of urinary diversion with continent neobladder or ileal ureter substitution, a purely laparoscopic technique is possible for bowel resection with the use of endoscopic stapling devices. Proper placement of the trocars is dependent on the need to mobilize the bowel and the procedure for which the bowel is to be used. Trocar placement for mobilization of the distal ileum is illustrated in Figure 85–11. The technique primarily involves the use of endoscopic linear cutting and stapling devices, which can be used to divide small bowel and its mesentery (Figs. 85-12 and 85-13). Reanastomosis purely intracorporeally can be accomplished with the same endo-GIA stapler to reconstitute bowel continuity with a side-to-side functional end-to-end anastomosis (Gill et al, 2000b; Potter et al, 2000). An endoscopic TA stapler may be used to complete the bowel closure.
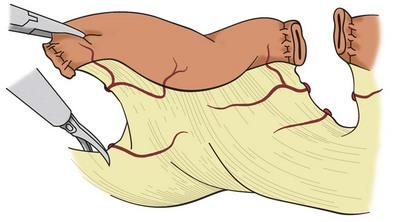
Figure 85–12 Laparoscopic mobilization of a small bowel segment for use in reconstruction. The visceral peritoneum is divided sharply; then the mesenteric vessels are ligated and divided. Staplers, ultrasonic shears, or bipolar cautery devices may be used to divide the mesenteric vessels.
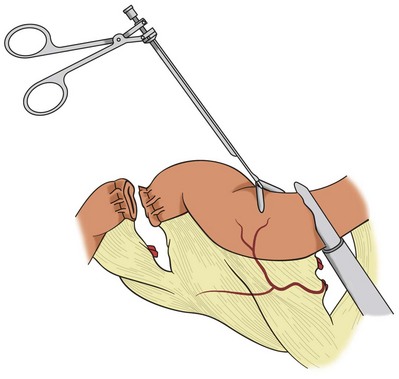
Figure 85–13 The endoscopic gastrointestinal stapler divides the intestinal segment. The staple line must be excised from the segment intended for use in the urinary reconstruction.
A laparoscopic ileal conduit can be performed with these techniques. The ureterointestinal anastomosis is performed with freehand laparoscopic suturing. One of the abdominal trocar sites is used to draw the bowel segment through the abdominal wall for stoma construction. Completely laparoscopic orthotopic ileal neobladder has been reported (Gill et al, 2002). The neobladder is constructed by freehand running suture with use of laparoscopic techniques (Fig. 85–14). The results of laparoscopic bowel anastomoses suggest that these anastomoses are safe, but no large series that compares open with laparoscopic approaches for bowel anastomosis has been published (Canin-Endres et al, 1999; Rothenberg, 2002).
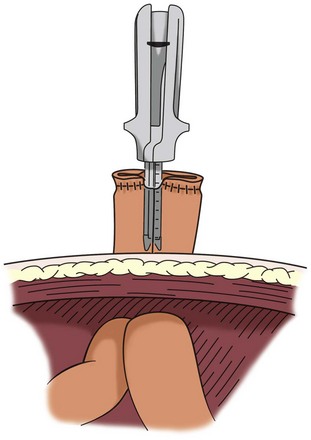
Figure 85–14 Many urologic procedures in which intestinal segments are used for reconstruction involve extirpative surgery in which a small laparotomy is employed for specimen removal. In this case the small laparotomy is used to deliver the bowel segment outside the abdomen. Any form of anastomosis may be employed by this technique, which also reduces the chance of spillage of intestinal contents.
Other approaches to laparoscopic intestinal surgery include laparoscopic mobilization of the bowel segment and exteriorization of the segment; the anastomosis of the bowel and ureteral anastomoses are then performed in an open fashion through a small laparotomy incision (Fig. 85–15). This is now the preferred approach for many performing laparoscopic cystoprostatectomy. Purely laparoscopic intestinal anastomoses in radical cystectomy are associated with a much higher complication rate from intestinal and ureteral anastomotic leaks than an exteriorized approach (Stephenson, 2008). In radical cystectomy, a small incision is already required for intact specimen removal. An open approach therefore allows direct tactile assessment of the anastomoses.
Compression Anastomoses and the Biofragmentable Ring
There is a long history of experimental work on compression anastomoses. These technologies may facilitate natural orifice and minimally invasive surgery. With the most clinical evidence, the biofragmentable ring has proven safe, effective, and time efficient. Several studies have shown that these intestinal anastomoses are as safe as hand-sewn or stapled anastomoses (Ghitulescu et al, 2003). Other compression devices have been devised but have not gained in popularity (Kaidar-Persno, 2008).
Postoperative Care
The patient should not be allowed to begin oral alimentation until bowel function returns after surgery. Coordinated small bowel activity begins within hours after the operative event, and stomach activity may return as early as 24 hours postoperatively. Clear liquids may be begun when the paralytic ileus resolves and bowel activity resumes. If clear liquids are tolerated, the diet may be advanced. This sequence of events generally takes 1 to 4 days. If the nutritional condition of the patient is impaired preoperatively, a postoperative complication delays oral feeding, or the paralytic ileus is still present on the fifth postoperative day, the patient should receive intravenous nutrition that supplies the total calorie requirement (hyperalimentation). It is preferable to begin the hyperalimentation the day after surgery if any of these complications are anticipated. Once started, it is discontinued only when oral intake is sufficient to satisfy the body’s calorie requirements. Some have advocated the use of a jejunal feeding tube for the early institution of intestinal feeding (Maffezzini et al, 2008).
The use of nasogastric or gastrostomy decompression during the postoperative period of ileus is somewhat controversial. In a prospective study of elective intestinal anastomoses in which 274 patients had postoperative gastric decompression and 261 patients were given nothing by mouth until bowel activity resumed, there was no significant difference in major intestinal complications between the two groups; however, those who did not have gastric decompression showed a much greater incidence of abdominal distention, nausea, and vomiting. It must be understood that only healthy patients with no complications were entered into the study. Sixty percent of the patients initially entered were excluded. Specific exclusion criteria included emergency surgery with peritonitis, extensive fibrous adhesions, enterotomies, previous pelvic irradiation, intra-abdominal infection, pancreatitis, chronic obstruction, prolonged operating time, and difficult endotracheal intubation (Wolff et al, 1988). Meta-analysis of randomized studies of postoperative nasogastric decompression suggests that bowel function returns earlier without a tube. In this study, nonsignificant trends were seen toward fewer pulmonary complications but more wound complications when an NGT was not used (Nelson et al, 2005).
Because of the limitations in the available studies, it is still recommended to use nasogastric decompression in all but the most medically fit patients. Vomiting in the postoperative period increases the risk of aspiration and morbidity in the compromised patient. Moreover, tube decompression allows the administration of ice chips by mouth before enteric activity resumes, thus enhancing the patient’s comfort. If the patient has severe pulmonary disease, decompression by placement of a gastrostomy tube at the time of surgery facilitates pulmonary toilet and enhances comfort. In patients who have significant comorbid conditions such as sepsis, consideration should be given to administration of a histamine (H2) blocker and an antacid via the stomach tube every 2 hours as needed to keep the gastric pH above 5 during the period of ileus. By keeping the gastric contents alkaline in these critically ill patients in the postoperative period, the incidence of gastric stress ulceration is markedly reduced.
Because many studies have shown that the absence of an NGT in the postoperative period after intestinal surgery does not increase intestinal anastomotic complications compared with patients in whom NGTs are used, there is a trend to reduce the length of time the NGT is in place. In patients who underwent a radical cystectomy and who were given metoclopramide 10 mg intravenously every 6 hours, until taking fluids orally, the authors found that three quarters of the NGTs could be removed in less than a day. Compared with the standard regimen of NGT drainage, the group given metoclopramide had a much earlier return of bowel function (Donat et al, 1999). More recent studies also support the safety of early NGT removal (Park, 2005).
Complications of Intestinal Anastomoses
The complications that follow anastomoses include leakage of fecal contents, sepsis, wound infections, abdominal abscesses, hemorrhage, anastomotic stenosis, pseudo-obstruction of the colon (Ogilvie syndrome), and intestinal obstruction. These untoward events increase morbidity and are frequently major contributors to mortality. The complication rates for elective colocolonic and ileocolonic anastomoses performed in prepared bowel with contemporary technique are intestinal leak, 2%; hemorrhage, 1%; and stenosis or obstruction, 4%. These complications require reoperations in 1% of the patients and result in death in 0.2% of patients (Jex et al, 1987).
Fistulas
Fistulas in the postoperative period are of two types, fecal and urinary. These generally occur within the first several weeks after the operative event. They frequently result in sepsis and markedly increase morbidity and mortality. Fecal fistulas occur in 4% to 5% of patients (Sullivan et al, 1980; Beckley et al, 1982). Sepsis is a common complication of these untoward events and carries with it a mortality of 2% (1 of 47 patients) (Hill and Ransley, 1983).
Sepsis and Other Infectious Complications
Wound infections, pelvic abscesses, and wound dehiscences may complicate the immediate postoperative period. Although wound dehiscences and pelvic abscesses are rare complications, morbid wound infections occur with an incidence of 5% (3 of 62 patients) (Loening et al, 1982). Many of these complications may be averted by operating on a properly prepared bowel, by walling off the intestine with Mikulicz pads while the anastomosis is being completed, and by irrigating the intestinal segment to be used in the reconstruction until it is free of any residual enteric contents before it is manipulated and its contents are spilled in the abdomen and pelvis. The overall septicemia rate after radical cystectomy is currently 3.6% with a 17% mortality rate (Davies, 2009).
Bowel Obstruction
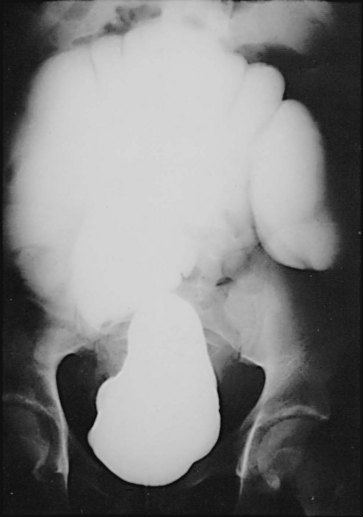
The incidence of intestinal obstruction after abdominal procedures for urinary intestinal diversion differs according to whether the stomach, ileum, or colon is used for the diversion. In patients who have had a segment of stomach or ileum removed for the diversion, there is a 10% incidence of postoperative bowel obstruction requiring treatment. When the colon is used, the incidence of postoperative obstruction requiring an operation is 5% (Table 85–3). Half of the bowel obstructions occur in the early postoperative period. In one series, after radical cystectomy and ileal conduit, 15% of the patients had a mild obstruction in the first 6 months that responded to conservative management, whereas 3% required an operation to relieve the obstruction during this period. The occurrence of obstruction after this 6-month period was much less frequent (Sullivan et al, 1980). More recently, a 10.5% incidence of reoperation for bowel obstruction was noted in a large series of radical cystectomy patients (Varkarakis, 2006). Bowel obstruction can be a morbid event: A significant number of patients who develop obstruction after an ileal conduit and require an operation die. The most common cause of the obstruction is adhesions, followed by recurrent cancer. These two causes account for the great majority of the cases. Volvulus and internal hernia account for far fewer cases (Jaffe et al, 1968). Rarely, severe stenosis or obstruction at the anastomotic suture line occurs. Stenosis is a result of edema, poor technique, or performing the anastomosis on ischemic bowel (Fig. 85–16); obstruction is a result of improper technique.
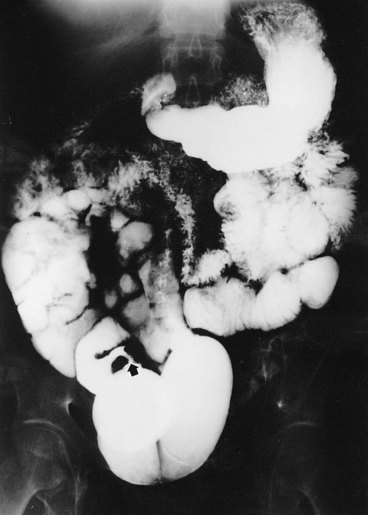
Figure 85–16 Upper gastrointestinal tract series illustrates a small bowel stricture (arrow) at the ileoileostomy after ileal conduit urinary diversion. At the time of the initial ileoileostomy, the anastomotic suture line appeared bluish. At subsequent exploration, a bowel lumen of only 2 mm was found. The serosa at the anastomotic site was fibrotic.
The incidence of postoperative bowel obstruction may be reduced by using nonirradiated bowel, performing the anastomosis on well-vascularized bowel, closing all apertures, reperitonealizing the isolated segment, decompressing the gastrointestinal tract for an adequate time, placing omentum over the anastomosis, and reconstituting the pelvic floor after exenterative surgery. The isolated segment is reperitonealized by tacking its antimesenteric border to the lateral abdominal sidewall peritoneum. The proximal mesenteric border should be tacked to the posterior parietal peritoneum because failure to obliterate this potential space has resulted in entrapment of bowel, causing a bowel obstruction. Placing the sigmoid colon in the area may close the pelvic space left after an anterior exenteration. This effectively prevents small bowel from herniating into the raw pelvis. Omentum may also be mobilized and used to fill any space the sigmoid colon does not fill. In a total exenteration, sufficient sigmoid colon is not available and the omentum is often not bulky enough to fill the pelvis and thus prevent small bowel from filling the denuded pelvis. This situation is of particular concern in patients who must receive postoperative pelvic irradiation. The bowel may be kept out of the pelvis in these patients by reconstructing the pelvic floor with polyglactin mesh. The mesh is sutured along the posterior pelvic brim to the sacral promontory and presacral fascia and laterally to the adventitia of the iliac vessels. Laterally and anteriorly, it is sewn to the peritoneum two thirds of the distance between the pubis and umbilicus. Omentum is then brought down, placed over the mesh, and sutured in position. This effectively excludes the bowel from the pelvis for 4 to 6 weeks while postoperative irradiation is being administered (Sener et al, 1989).
Hemorrhage
Hemorrhage is a rare complication of intestinal anastomoses. It is much more likely to occur when stomach is used and a Billroth I anastomosis is constructed. It is usually due to failure to secure bleeding at the time of anastomosis or anastomotic ulcers that develop on the suture line.
Intestinal Stenosis
Intestinal stenosis occurs at two distinct times: in the immediate postoperative period and during the long term. Intestinal stenosis in the immediate postoperative period is due to technical mishaps or edema. Edema resolves by continuing the intestinal decompression, whereas technical mishap requires a reoperation. During the long term, it is likely due to ischemia or perienteric infection. Figure 85–16 shows an upper gastrointestinal tract series of a severe intestinal stenosis caused by ischemia. At the time of the ileoileostomy, the suture line was blue. Chronic symptoms of partial small bowel obstruction occurred in the postoperative period.
Pseudo-obstruction
Pseudo-obstruction of the colon, or Ogilvie syndrome, on rare occasion may complicate the early postoperative period. Its cause is not understood. Pseudo-obstruction usually occurs within the first 3 days postoperatively in patients with multiple medical illnesses. The patient complains of severe abdominal pain, and a radiograph of the abdomen reveals a dilated cecum. A gentle water-soluble contrast enema eliminates the possibility of a mechanical obstruction. In the presence of a rising leukocyte count or cecum that is increasing in size and exceeds 12 to 15 cm in diameter, rupture may be imminent. Under these circumstances, an emergency cecostomy should be performed (Clayman et al, 1981). In less acute circumstances, endoscopic decompression may be attempted (DeGiorgio, 2009).
Complications of the Isolated Intestinal Segment
Intestinal Stricture
Strictures of intestinal segments are usually late complications primarily occurring in conduits, although they have been described in ileal ureters as well. The stricture is thought to be a consequence of lymphoid depletion of the intestine exposed to urine. The lymphoid depletion contributes to persistent infection, which may result in midloop stricture, bacterial seeding of the upper tracts, and renal deterioration (Tapper and Folkman, 1976). Because of the persistent infection and lack of intestinal resistance to the detrimental action of bacteria, submucosal edema with fibrosis and stricture formation occurs. The intestinal segment may also be blocked by encroachment of hypertrophied mesenteric lymph nodes. Hypertrophied mesenteric lymph nodes, submucosal lymphoid depletion, edema, and fibrosis are commonly found when intestinal segments that have been chronically exposed to urine are examined pathologically.
Elongation of the Segment
Another complication of the intestinal segment is elongation, occasionally resulting in massive enlargement. When this occurs in conduits or ureteral substitutes, there is usually a distal obstruction. In continent diversions, it may signal failure to catheterize the pouch frequently enough. If allowed to persist, the increased pressure may result in deterioration of renal function. Enlargement and elongation of the intestine may also result in a volvulus of the segment (Fig. 85–17).
Abdominal Stomas
Two types of stomas may be made on the anterior abdominal wall: those that are flush with the skin and those that protrude. The flush stoma is preferable for the continent type of diversion in which intermittent catheterization is carried out and over which a small dressing is placed. The stoma that protrudes is preferable when a collection device is worn. A properly protruding stoma worn with an appliance results in a lesser incidence of stomal stenosis and a better appliance fit with fewer peristomal skin problems. There are two types of protruding stomas: the end stoma and the loop end ileostomy. Most complications of stomas are the result of technical errors in their construction. Therefore to minimize such complications, specific technical points must be rigidly followed.
The site of the stoma should be selected preoperatively. This is done by marking the stomal site with the patient in the sitting position, as well as in the supine position; care is taken to place it over the rectus muscle at least 5 cm away from the planned incision line. The point chosen should be well away from skin creases, scars, the umbilicus, belt lines, and bone prominences. A site in which radiotherapy has previously injured the area should be avoided. All stomas should be placed through the belly of the rectus muscle and be located at the peak of the infraumbilical fat roll. If the stoma is placed lateral to the rectus sheath, a parastomal hernia is likely to occur. The bowel should traverse the abdominal wall perpendicular to the peritoneal lining (i.e., it should come straight out). One should avoid trimming fat or epiploic appendages from around the margin of the stoma, and the appliances should be applied in the operating room.
The stoma should be brought through a circular incision made at the predetermined site. A perfectly circular opening in the skin may be made by placing the finger hole of a Kelly clamp at the desired point and grasping the skin in the center of the hole with a Kocher clamp. Pulling up on the Kocher and pushing the handle of the Kelly against the abdominal wall allows for a small button of skin to be removed with a single pass of the knife. This makes a perfectly circular opening in the skin. However, the tendency to remove too much skin is great, resulting in a circular opening that is too large. To avoid this complication, one should not cut the skin flush with the Kelly clamp but rather immediately beneath the Kocher clamp. The subcutaneous tissue is left intact. This is spread, not excising any fat, because the fat falls back adjacent to the bowel and eliminates any dead space. Kocher clamps are placed on the fascia in the incision and pulled medial so that when the fascia and peritoneum are incised, they are incised directly over the skin line and thus do not result in angulation of the gut when the abdominal incision is closed. The fascia is incised in a cruciate manner, and the rectus muscles are spread. The peritoneum is incised. The opening should accommodate two fingers snugly.
Nipple Stoma: “Rosebud”
A Babcock clamp is placed through the opening, and the bowel is grasped and brought out for a distance of 5 to 6 cm to make a nipple of about 2 to 3 cm in length (Fig. 85–18). Two 3-0 chromic sutures are placed through the seromuscular layer of the bowel and the peritoneum on the anterior abdominal wall. Alternatively, the serosa may be sutured to the fascia with two 2-0 chromic sutures. The mesentery is aligned in its normal anatomic direction before the serosa is sutured to the peritoneal wall. The ileum is usually curved concave toward the mesentery. If this is severe, the mesentery may be partially incised 1 cm from the bowel wall (Fig. 85–19). Thus a portion of mesentery is preserved along the entire length of the bowel. This should straighten the curve in the bowel significantly if not completely. Four 3-0 chromic sutures are placed in quadrants through the full thickness of the bowel edge and through the seromuscular layer of the bowel 3 to 4 cm from the cut edge and then through the subcuticular skin layer. Sutures should not traverse the full thickness of the skin but should be placed through the subcuticular and subdermal layers only.
Figure 85–18 A and B, Nipple stoma. Five to 6 cm of intestine are brought through the abdominal wall. The serosa is scarified, and quadrant 3-0 chromic sutures are placed through the full thickness of the distal end of the intestine. Each suture is placed in the seromuscular layer 3 cm proximal and then secured to the dermis before it is tied.
Figure 85–19 If it is tethered by the mesentery, the bowel may be straightened by incising the mesentery several centimeters away from the serosa and parallel to the bowel.
When the sutures are tied, the bowel is everted and forms a nipple. A more secure nipple may be made by performing multiple myotomies through the seromuscular layer of the bowel above the skin line before construction of the nipple. The myotomies adhere serosa to serosa and reduce the risk of stomal retraction. This is particularly appropriate for patients who are obese.
Flush Stoma
Quadrant sutures of 3-0 chromic are placed through the full thickness of the bowel and subsequently passed through the subdermal layer of the skin and tied. Several sutures are placed between the quadrant sutures from bowel to subdermal skin. This constructs a flush stoma that has a 1-mm raised margin.
Loop End Ileostomy
Obese patients have a thick abdominal wall and often a thick, short ileal mesentery. This makes construction of an end ileal stoma extremely difficult. The loop end ileostomy obviates some of these problems and is usually easier to perform than the ileal end stoma in the patient who is obese (Fig. 85–20). To construct this type of stoma, the distal end of the ileum is closed as described previously for closing the proximal end of an intestinal segment and a loop is brought up through the belly of the rectus muscle and onto the anterior abdominal wall. This avoids bringing the mesenteric border onto the abdominal wall and prevents one side of the ileostomy from being involved with mesentery. A slightly larger skin opening is required than for the end stoma. A 3-cm disk of skin is removed. The subcutaneous tissue is spread, the fascia incised, the rectus spread, and the peritoneum incised as described earlier. The opening should admit two fingers comfortably. The loop may be pulled through the opening in the abdominal wall by passing an umbilical tape through a small opening in the mesentery at a distance from the distal end that is sufficient to leave that end in the abdomen when the loop has been pulled through the abdominal wall. By gentle traction on the umbilical tape, the loop is brought onto the abdomen.
Figure 85–20 Loop end ileostomy. A, After the distal end of the loop is closed and the bowel is drawn through the rent in the abdominal wall, the bowel is held in place by a rod passed through the mesentery. The mesentery is realigned, and the peritoneum is sutured to the serosa of the bowel circumferentially. B, A transverse incision is made in the bowel four fifths of the loop distance cephalad. C, The cephalad portion of the stoma is simply sutured to the dermal layer of skin with interrupted 3-0 chromic sutures. D, On the inferior aspect of the incision, 3-0 chromic sutures are placed through the full thickness of the cut edge, then through the seromuscular layer, and then through the dermis. This everts the caudal portion of the stoma.
The distal portion of the bowel is brought through the opening such that the closed end lies cephalad to the body of the segment. When a sufficient amount of loop protrudes beyond the skin edge, a small rod is placed through the hole in the mesentery at the apex of the loop and holds the bowel on the anterior abdominal wall during suturing. If the rent in the rectus muscle is too large, it may be closed with interrupted 0 chromic sutures from within the abdomen. The serosa is sutured to the peritoneum on the anterior abdominal wall. The bowel wall is opened in a transverse direction at a point four fifths of the distance cephalad to the most caudal portion of the loop. With 3-0 chromic sutures, the full thickness of the caudal incision in the bowel is sutured back to itself (serosa) and then to the dermis as in the rosebud technique. The cephalad nonfunctional opening is sutured directly to the dermis. The rod is sutured to the skin and left in place for 7 days.
The Turnbull loop stoma results in a lesser incidence of stomal stenosis but a higher incidence of parastomal hernias (Emmott et al, 1985). Overall, the two types of stoma are functionally equivalent over time (Chechile, 1992). Stomas for the colon may be constructed in much the same way as end stomas for the ileum. Their suturing, however, is usually more flush than everted.
Complications of Intestinal Stomas
Complications of the abdominal stoma are the single-most common problem encountered in the postoperative period after urinary intestinal diversion. Early complications of abdominal stomas include bowel necrosis, bleeding, dermatitis, parastomal hernia, prolapse, obstruction, stomal retraction, and stomal stenosis. At some point, virtually every patient has one of these complications. Many of these complications can be reduced by proper construction of the stoma. If periodic visits with the enterostomal therapist are made, products for skin care are appropriately used, nonirritative stomal adhesives are employed, the urine in the collection device is maintained acidic, and properly fitting collection devices are used, most stomal complications can be significantly reduced and many eliminated.
Parastomal skin lesions may be classified as irritative, which are manifested by hypopigmentation, hyperpigmentation, and skin atrophy; erythematous erosive lesions, which appear as macular lesions, scaling of the skin, and loss of the epidermis; and pseudoverrucous, which are wartlike lesions (Borglund et al, 1988).
Stomal stenosis has been reported, on average, in 20% to 24% of patients with ileal conduits and 10% to 20% of patients with colon conduits (see Table 85–3). This incidence has been considerably reduced by better attention to stomal care and better-fitting appliances. Stomal stenosis is less for loop stomas than for end stomas. Parastomal hernias occur rarely (1% to 4%) with end stomas but are more likely to occur with loop stomas, with reported incidences ranging from 4% to 20%. Stenosis of catheterizable stomas is high, reaching to more than 50% in children (Barqawi et al, 2004). Stoma-related complications appear to be more common in an umbilically placed stoma versus one in the abdominal wall (De Ganck et al, 2002). Others have reported excellent long-term results with few complications in the concealed umbilical stoma (Glassman and Docimo, 2001).
Bleeding, stomal stenosis, and dermatitis can be markedly reduced by attention to parastomal skin care and by the use of a properly fitting appliance around a protruding stoma. The other complications are minimized by proper surgical technique.
Parastomal hernia occurs frequently after ileal loop urinary diversion, with an incidence of 2% to 6.6%. It can be effectively treated with open repair (Franks and Hrebinko, 2001; Ho and Fawcett, 2004). Laparoscopic approaches have also been reported with mixed results. One series reported a 56% failure rate within 6 months of laparoscopic parastomal hernia repair with Gore-Tex mesh (Safadi, 2004). This contrasts with the high success rate reported in another small series (Kozlowski et al, 2001).
Massive bleeding from the conduit occasionally occurs, usually as a result of varices. There have been several interesting reports of patients who have developed ileal conduit varices generally as a result of hepatic dysfunction and portal hypertension. Treatment by transhepatic portal-systemic shunt and transhepatic angiography and embolization is a relatively minimally invasive technique that has promise and is worthwhile using in these difficult patients (Lashley et al, 1997; Medina et al, 1998).
Ureterointestinal Anastomoses
The ureter may be anastomosed to the colon or small bowel in a refluxing or nonrefluxing anastomosis. There is considerable controversy as to whether a nonrefluxing or refluxing anastomosis is desirable in urinary tract reconstruction. Deterioration of the upper tracts for ileal and colon conduits has been reported in 10% to 60% of the patients (Koch et al, 1992; Samuel, 2006). In one series, 49% of the upper tracts showed changes after conduit diversion, 16% of which had an increase of the blood urea nitrogen of 10 mg/dL or more (Schwarz and Jeffs, 1975). Deterioration of the upper tracts is usually a consequence of lack of ureteral motility, infection, or stones and less commonly due to obstruction at the ureteral-intestinal anastomosis. Because bacteriuria occurs in almost all conduits and because the intestine certainly does not inhibit and may, in fact, promote bacterial colonization, many have suggested that a nonrefluxing anastomosis would minimize the incidence of renal deterioration.
The evidence that suggests a nonrefluxing system in urinary intestinal diversions is desirable comes from several observations. In a group of patients who had nonrefluxing colon conduits constructed, those whose anastomoses remained nonrefluxing had a lesser incidence of renal deterioration than did those in whom the antireflux anastomosis failed. Follow-up for 9 to 20 years revealed that 79% (22 of 28 patients) of the refluxing renal units deteriorated, whereas only 22% (11 of 51 patients) of the nonrefluxing units deteriorated (Elder et al, 1979; Husmann et al, 1989). Others have reported that in continent diversions, the majority of patients who experience reflux show upper tract dilation and deterioration, whereas few show upper tract deterioration when a nonrefluxing anastomosis is present (Kock et al, 1978). Similar findings have been reported in experimental animals.
If a nonrefluxing ureteral-colonic conduit diversion is constructed, only 7% of the renal units show evidence of pyelonephritic scarring after 3 months. If a refluxing anastomosis is constructed, 83% of the renal units show scarring. Half of the conduits in both groups have significant bacteriuria (Richie and Skinner, 1975). Others have not found the same high incidence of renal deterioration associated with ureteral-intestinal reflux. One group of investigators studying colon conduits noted no difference in the incidence of renal deterioration regardless of whether the colon conduit demonstrated reflux; 17% (5 of 29) of nonrefluxing renal units showed deterioration compared with 18% (5 of 27) of refluxing units (Hill and Ransley, 1983). In another series, only 3 of 135 renal units with refluxing ureteral-intestinal anastomoses that were unobstructed showed evidence of renal deterioration (Shapiro et al, 1975). A more recent study compared refluxing and nonrefluxing ureteral-intestinal anastomoses in 58 patients with conduit diversions; 56 renal units were refluxing and 60 renal units were nonrefluxing. There was no difference in renal deterioration or in pyelonephritis between the two groups. Ureteral-intestinal stricture formation occurred in 2% of refluxing units as opposed to 13% of nonrefluxing units (Pantuck et al, 2000). A high-capacity, low-pressure reservoir may not require antirefluxing anastomoses (Hohenfellner, 2002).
It does not appear that conduit pressures are transmitted to the renal pelvis. The pressure within the renal pelvis in refluxing conduit diversions is not elevated above normal, and it is not dependent on the segment of bowel used (i.e., ileum or colon) (Magnus, 1977; Kamizaki and Cass, 1978; Hayashi et al, 1986). Peristaltic ureteral contractions apparently dampen pressure transmission from intestine to renal pelvis, attesting to the importance of normal ureters. When bowel is substituted for the ureter, it does not appear that it makes any difference whether there is reflux at the bladder. The voiding pressure is blunted by the distensible bowel segment. Indeed, in one study of continent diversions in which a segment of ileum formed the pouch to which the ureters were anastomosed, radionuclide voiding cystography failed to detect reflux to the kidneys (Waidelich et al, 1998). Moreover, there is no difference in ileal and colon conduits between those who experience reflux and those who do not in renal function measured 2 to 5 years postoperatively (Mansson et al, 1984). Also, the successful construction of an antirefluxing anastomosis does not prevent bacterial colonization of the renal pelvis. In six of eight patients with nonrefluxing enterocystoplasties and one patient with a nonrefluxing colon conduit in whom the absence of reflux was documented by loopogram, percutaneous renal pelvic aspiration revealed positive cultures (Gonzalez and Reinberg, 1987). One stated advantage of a refluxing anastomosis in patients who have urothelia and are prone to malignant change is that the upper tracts may be observed by periodic introduction of contrast material into the conduit. From these studies, it appears that reflux associated with impaired ureteral peristalsis in the presence of bacteriuria or obstruction results in renal deterioration, but it has not been established for either conduit or continent diversions that reflux associated with the normal ureter in the absence of obstruction is detrimental to the adult kidney.
Although many techniques have been described to make the various types of ureterointestinal anastomoses, certain basic surgical principles are germane to all the anastomoses regardless of type. Only as much ureter as needed should be mobilized so that there is no redundancy or tension on the anastomosis. Mobilization should not strip the ureter of its periadventitial tissue because it is in this tissue that the ureter’s blood supply courses. The ureter should be cleaned of its adventitial tissue only for 2 to 3 mm at its most distal portion where the ureter-intestinal mucosa anastomosis is to be performed. The ureterointestinal anastomosis must be performed with fine absorbable sutures, which are placed so that a watertight mucosa-to-mucosa apposition is constructed. The bowel should be brought to the ureter and not vice versa (i.e., the ureter should not be extensively mobilized so that it can be brought into the wound to the bowel lying on the anterior abdominal wall). At the completion of the anastomosis, the bowel should be fixed to the abdominal cavity, preferably adjacent to the site of the ureterointestinal anastomosis. If possible, the anastomosis should be retroperitonealized or a pedicle flap of peritoneum should be placed over the anastomosis.
In those diversions in which the intestinal stoma is brought to the abdomen and the proximal end of the bowel fixed to the retroperitoneum, there are two places where the bowel may be conveniently fixed to the retroperitoneum without jeopardizing mesenteric blood supply. The most convenient point of fixation is below the root of the small bowel mesentery at the level of the pelvic brim. The ureterointestinal anastomosis may be retroperitonealized at the level of the pelvic brim, thus fixing the bowel segment to the posterior body wall. In those situations in which the ureters are short, a more cephalad fixation to the posterior peritoneum may be accomplished by placing the proximal end in the right upper quadrant cephalad to the takeoff of the right colic artery and immediately below the duodenum. This is a relatively avascular area and places the intestine fairly close to the right and left kidneys, thus reducing the length of ureter required to reach the intestinal segment.
Perhaps one of the most difficult complications of ureterointestinal anastomoses to manage is a stricture. Strictures are generally caused by ischemia, a urine leak, radiation, or infection. The incidence of urine leak for all types of ureterointestinal anastomoses is 3% to 5% (see Table 85–3). This incidence of leak can be reduced nearly to zero if soft Silastic stents are used. In one series of ureterointestinal anastomoses done at the same institution, the nonstented group had a 2% anastomotic leak and a 4% stricture rate. When non-Silastic rigid stents were used, there was a 10% incidence of stricture. When a soft Silastic stent was used, however, there were no strictures or leaks (Regan and Barrett, 1985). In a similar series in which colon conduits were constructed after gynecologic exenterative operations, the nonstented group had an 18% leak rate and an 18% stricture rate, whereas those who had been stented had a 3% leak rate and an 8% stricture rate (Beddoe et al, 1987). Early postoperative metabolic complications were reduced in a randomized study of stented versus nonstented anastomoses (Mattei, 2008). Thus the evidence indicates that modern soft Silastic stents are effective in reducing the leak rate, subsequent stricture formation, and postoperative complications. Better technique and better suture materials have also reduced the incidence of stricture in nonrefluxing anastomoses. A series of nonrefluxing ureter-colon anastomoses reported an 8% incidence of stricture formation (Stein et al, 1996).
In constructing a submucosal tunnel in those procedures in which a nonrefluxing anastomosis is made, it is often helpful to inject saline with a 25-gauge needle submucosally to raise the mucosa away from the seromuscular layer. This makes dissection considerably easier (Menon et al, 1982).
These principles of surgical technique are common to all ureterointestinal anastomoses. Each type of ureterointestinal anastomosis, however, has specific technical points unique to its construction. Techniques involving ureter-colon anastomoses are discussed first, followed by ureter–small bowel anastomoses.
Ureterocolonic Anastomoses
Combined Technique of Leadbetter and Clarke
This method establishes a nonrefluxing ureterocolonic anastomosis by employing a submucosal tunnel. The technique combines the ureterocolonic anastomosis of Nesbit, which is a refluxing elliptical anastomosis to the intestine, with the tunneled technique of Coffey (Fig. 85–21) (Leadbetter and Clarke, 1954).
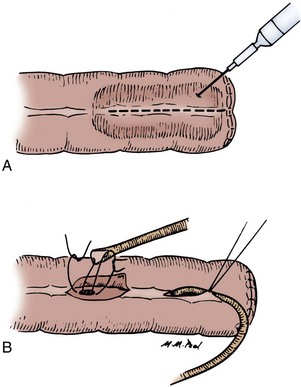
Figure 85–21 Leadbetter-Clarke ureterointestinal anastomosis. A, Injection of the submucosal tissues with saline facilitates the dissection. B, A linear incision is made in the taenia, the taenia is raised, and the mucosa is identified. A small button of mucosa is removed, and the ureter is spatulated and then sutured to the mucosa with 5-0 PDS. The seromuscular layer is sutured over the ureter, with care taken not to compromise or occlude the ureter.
The anterior taenia is incised obliquely for 2.5 to 3 cm as close to the mesenteric border as possible. The mucosa is dissected off the muscularis for the entire length of the incision. At the distal end of the incision in the taenia, the mucosa is picked up with a fine Adson forceps and a small button is excised. The ureter is spatulated for 5 to 7 mm such that an elliptical anastomosis may be made. The ureter is sewn mucosa to mucosa with 5-0 polydioxanone sutures (PDS) by either interrupted sutures with the knots tied on the outside or a running suture. If the suture line is to be run, it is wise to begin the anastomosis at the apex of the ureter. This suture is tied, and the posterior row is run to the most distal portion of the ureter, which is subsequently tied. A second running suture completes the anterior aspect. The seromuscular layer is then reapproximated loosely over the ureter in such a way as to allow “the ureter [to] lie in the bowel as a hammock without being compressed” (Leadbetter and Clarke, 1954). The bowel should be fixed to the peritoneum so that there is no tension on the ureters.
The complications reported with this procedure include a leak rate of 2.5%; deterioration of the upper tracts, which varies between 4.3% and 25%; and a stricture rate that varies between 8% and 14% (Table 85–4).
Transcolonic Technique of Goodwin
This method establishes a nonrefluxing ureterocolonic anastomosis by construction of a submucosal tunnel (Fig. 85–22). By this technique, the anastomosis is performed from within the bowel (Goodwin et al, 1953). If it is performed in bowel in continuity with the gastrointestinal tract, a noncrushing occlusive clamp is applied across the bowel cephalad to the desired point of the ureterointestinal anastomosis. This clamp is placed loosely about the bowel so as not to occlude the arterial supply in the mesentery.
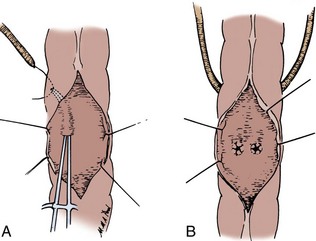
Figure 85–22 Transcolonic technique of Goodwin. A, The bowel is opened on its anterior surface; a small rent in the mucosa is made; and with a mosquito hemostat, the mucosa is raised from the submucosa extending laterally. A 3- to 4-cm tunnel is made before the clamp exits the serosal wall. The ureter is grasped and pulled into the submucosal tunnel. B, Both ureters have been drawn into the bowel through their submucosal tunnels before each is spatulated and circumferentially sutured to the mucosa. These sutures should also incorporate a portion of the muscularis for security. Where the ureter enters the colonic sidewall adjacent to the mesentery, the adventitia of the ureter is secured to the colonic serosa with interrupted 5-0 polydioxanone sutures.
A vertical incision is made in the bowel anteriorly, and the desired point of entrance of the ureter into the bowel is identified. A 0.5-cm incision is made in the posterior mucosa, and with use of a curved hemostat the mucosa is dissected from the submucosal layer in an oblique fashion coursing from medial to lateral. The hemostat is passed beneath the mucosa for a distance of approximately 3 to 4 cm and then brought through the serosa. A traction suture that has been placed on the ureter is then grasped with the hemostat, and the ureter brought into the colon. Both ureters should be brought into the bowel before they are sutured to the mucosa. The ureters should lie without tension or angulation. A No. 5 feeding tube is passed through the ureter to be sure that there is no kinking as it passes through the bowel wall. The redundant ureter is excised, and its end is spatulated and sewn with interrupted 5-0 PDS to the mucosa, with care taken to include with the mucosa some muscularis so that the ureter is securely fixed in place. A Silastic stent is placed up both ureters. As the ureters come through the serosa from without the bowel, the adventitia of the ureter is sutured to the serosa of the colon with two 4-0 PDS sutures. The anterior bowel wall is closed in two layers.
The reported results with this technique appear to be satisfactory. However, specific reliable data on the complication rate are not available.
Strickler Technique
This method establishes a nonrefluxing ureterocolonic anastomosis by construction of a submucosal tunnel (Fig. 85–23) (Strickler, 1965; Jacobs and Young, 1980). A 1-cm incision is made on the margin of the taenia. The technique originally described removal of a 2-mm button of seromuscular tissue. A 2-cm tunnel is formed laterally beneath the seromuscular layer with a hemostat. The seromuscular layer is incised, with care taken not to tent up the mucosa and inadvertently incise it. The holding suture in the ureter is grasped and drawn throughout the submucosal tunnel. The ureter is spatulated for 0.5 cm. A button of mucosa is removed, and the full thickness of the ureter is sewn to the mucosa of the bowel with either interrupted or running 5-0 PDS. The serosa is reapproximated over the ureter with 4-0 silk sutures. The serosal suture line is perpendicular to the course of the ureter. Where the ureters enter the serosa, they are also fixed with interrupted 4-0 PDS sutures. A lateral peritoneal flap is placed over the anastomosis.
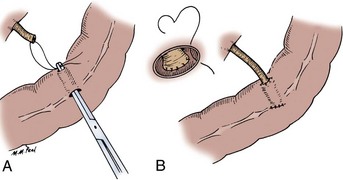
Figure 85–23 Strickler ureterointestinal anastomosis. A, A small linear incision is made in the taenia, and the submucosa is dissected from the mucosa laterally. After a distance of 3 to 4 cm is achieved, a small hole is made in the serosa and the ureter is drawn through. B, A button of mucosa is excised, and the ureter is spatulated and sutured to the mucosa with 5-0 polydioxanone sutures. The rent in the taenia is closed with interrupted sutures, and an adventitial suture at the ureter’s entrance point into the colon secures it to the serosa of the colon.
The advantage of this anastomosis is that because the taeniae do not need to be aligned, one can form the tunnel according to the normal course of the ureter and avoid angulation. This technique reliably prevents reflux but results in a stricture rate of approximately 14% (see Table 85–4).
Pagano Technique
This method establishes a nonrefluxing ureterointestinal anastomosis by construction of a submucosal tunnel (Fig. 85–24) (Pagano, 1980). The taenia is incised for a length of 4 to 5 cm, and the seromuscular layer is separated from the mucosa on both sides of the taenia laterally as far as the mesenteric border. The ureter is brought in one end (i.e., the distal end) laterally and laid in the 4- to 5-cm tunnel paralleling the mesenteric border. A button of mucosa is excised, and the ureters are spatulated and sutured to the mucosa with either interrupted or running 5-0 PDS. The seromuscular layer is then closed loosely with silk sutures in the midline. Each suture includes the seromuscular layer of the taenia and the mucosa in the midline.
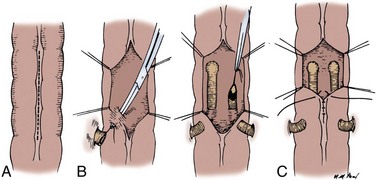
Figure 85–24 Pagano ureterointestinal anastomosis. A, A linear incision is made in the taenia between 4 and 5 cm in length. B, The submucosa is dissected from the mucosa laterally on both sides to the level of the mesentery. The ureters are drawn into the submucosal tunnel distally and sutured to the mucosa with 5-0 polydioxanone sutures proximally. C, The serosa is reapproximated, with incorporation of the mucosa in the midline.
This technique has a reported low complication rate. The leakage rate is approximately 3%, the stricture rate is 6%, and the reflux rate is approximately 6% (see Table 85–4) (Pagano et al, 1984).
Cordonnier and Nesbit Technique
These techniques use no tunnel and are direct refluxing anastomoses of the ureter to the colon (Nesbit, 1949; Cordonnier, 1950). They are not desirable for ureterosigmoidostomies. They are performed in much the same way a Bricker anastomosis would be performed for the small bowel (see later).
Small Bowel Anastomoses
There is a variety of ureter–small bowel anastomoses, which are of two basic types: end-to-side or end-to-end. The end-to-side anastomoses may be constructed in a refluxing or nonrefluxing manner.
Bricker Anastomosis
The Bricker anastomosis is a refluxing end-to-side ureter–small bowel anastomosis that is simple to perform and has a low complication rate (Fig. 85–25) (Bricker, 1950). Although originally described for the small bowel, it may be employed in any suitable intestinal segment.
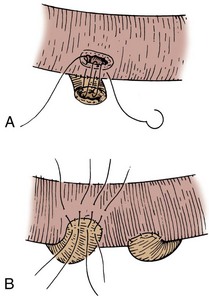
Figure 85–25 Bricker ureterointestinal anastomosis. A, The adventitia of the ureter is sutured to the serosa of the bowel. A small full-thickness serosal and mucosal plug is removed. Interrupted 5-0 polydioxanone sutures approximate the ureter to the full thickness of the mucosa and serosa. B, The anterior layer is completed by interrupted sutures placed through the adventitia of the ureter and the serosa of the small bowel.
In the original description, the adventitia of the ureter was sutured with interrupted silk sutures to the serosa of the bowel. The mucosa and serosa were incised; a small mucosa plug was removed; and with fine absorbable chromic sutures, the full thickness of the ureter was sewn to the mucosa of the bowel. The anterior layer of ureteral adventitia was then sewn with interrupted silk sutures to the serosa of the bowel. A less cumbersome method of performing this anastomosis is to excise a small button of seromuscular tissue and mucosa, spatulate the ureter for 0.5 cm, and suture the full thickness of the ureter to the full thickness of the bowel (i.e., mucosa and seromuscular layer to ureteral wall) with either interrupted or running 5-0 PDS. The anastomosis is stented with a soft Silastic catheter.
The stricture rate for this anastomosis varies between 4% and 22% (average of 6%). The leak rate is approximately 3% in the absence of stents (see Table 85–4).
Wallace Technique
A frequently employed anastomotic technique is that of Wallace, in which the end of the intestine is sutured to the end of the ureter (Fig. 85–26) (Wallace, 1970; Albert and Persky, 1971). This is a refluxing anastomosis. The intestinal segment employed may be either small bowel or colon. There are three basic types of anastomoses:
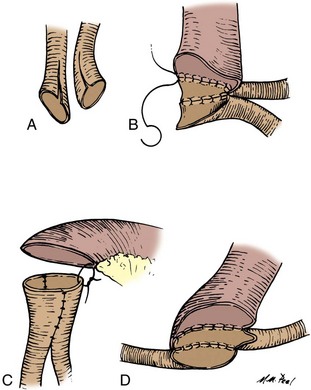
Figure 85–26 Wallace ureterointestinal anastomosis. A, Both ureters are spatulated and laid adjacent to each other. B, The apex of one ureter is sutured to the apex of the other ureter with 5-0 polydioxanone sutures (PDS). The posterior medial walls of both ureters are then sutured together with interrupted or running 5-0 PDS, the knots tied to the outside. The lateral ureteral walls are then sutured to the intestine. C, A Y-type anastomosis is formed by completing the anterior row of the anterior lateral ureteral walls of the ureters as shown in B and then suturing the ends of the ureters directly to the intestine. D, The head-to-tail anastomosis involves suturing the apex of one ureter to the end of the other. The posterior medial walls are sewn together, and then the ends and lateral walls are sewn to the intestine.
The ureters are spatulated for 1.5 to 2 cm, and fine 5-0 PDS sutures are used for each anastomosis.
For the first anastomosis, a fine suture is placed at the apex of each ureter with the knot tied to the outside. The posterior medial ureteral walls are sutured together, and the anterior lateral walls are sutured directly to the bowel with interrupted 5-0 PDS. Where the suture line of the end of the ureters comes to the bowel, a horizontal mattress suture is placed to make the anastomosis watertight. If a Y type of anastomosis is desired, after the posterior ureteral walls are sutured together as described previously, the anterior walls of the ureters are sutured together, and the end of the composite anastomosis is sutured to the bowel. Again, where the suture lines meet the bowel, a horizontal mattress suture is placed so that the anastomosis is watertight. The head-to-tail anastomosis involves suturing the end of one ureter to the apex of the other. The posterior medial walls of the two ureters are approximated. The anterior lateral walls are sutured to the bowel.
The Wallace anastomosis has the lowest complication rate of any of the ureterointestinal anastomotic techniques. Stricture formation is approximately 3%, deterioration of the upper tracts is about 4%, and leakage is about 2% (see Table 85–4). The Wallace technique is not recommended for patients who have extensive carcinoma in situ or who have a high likelihood of recurrent tumor in the ureter. A recurrence of tumor at the anastomotic line in one ureter would block both ureters, causing uremia from bilateral obstruction.
Tunneled Small Bowel Anastomosis
This method attempts to establish a nonrefluxing anastomosis by construction of a submucosal tunnel (Fig. 85–27) (Starr et al, 1975). Two 0.5-cm incisions are made in the serosa on the antimesenteric border 2.5 cm apart at right angles to the long axis of the bowel. The seromuscular layer is then gently separated from the mucosa with a blunt hemostat. The ureter is pulled through one incision, a button of mucosa is removed over the other incision, and the ureter is spatulated and sutured to the mucosa with interrupted 5-0 PDS. The serosa is then closed with interrupted 4-0 silk, and the adventitia of the ureter is sutured at its entrance through the serosa of the bowel to the serosa.
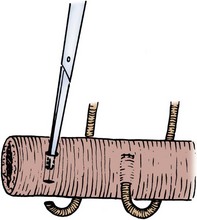
Figure 85–27 Tunneled small bowel anastomosis. A small transverse incision is made in the small bowel, and a second transverse incision 3 cm lateral to it is also made. The submucosal tunnel is made, a button of mucosa is removed, and the ureter is drawn through the tunnel and sutured directly to the mucosa. The rent in the serosa is closed, and an adventitial ureteral suture is placed and secured to the serosa at the ureter’s entrance to the small bowel.
Good results have been reported, but this technique has not been widely used. Therefore long-term follow-up is not available.
Split-Nipple Technique
This method attempts to establish a nonrefluxing anastomosis by employing a nipple mechanism. It may be applied to either the small or large bowel. This technique was described by Griffiths and involves formation of a nipple in the ureter and implantation into the small bowel (Fig. 85–28) (Turner-Warwick and Ashken, 1967). A 0.5-cm longitudinal incision in the ureter is made, and the ureteral wall is turned back on itself, constructing a nipple at least twice as long as its width. The cuff is stabilized at the corners with sutures. A button of seromuscular and mucosal tissue is removed, and the ureter is then placed into the bowel such that it protrudes through the mucosa. The adventitia just proximal to the point where the ureter has been sewn to itself is sutured to the full thickness of the bowel wall with interrupted 5-0 PDS. The anastomosis is stented.
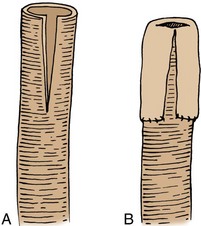
Figure 85–28 A and B, Split-nipple technique. The ureter is spatulated and turned back on itself, and the end of the ureter is secured to the adventitia of the ureter with interrupted 5-0 polydioxanone sutures.
In one series, this type of anastomosis prevented reflux in more than 50% of the patients. In other subsequent series, approximately 80% of patients had a nonrefluxing anastomosis with an acceptably low incidence of stenosis (see Table 85–4). There is a stricture rate of about 7% (De Carli et al, 1997).
Le Duc Technique
This method establishes a nonrefluxing anastomosis by laying the ureter onto the interior of the bowel wall, eventually resulting in a submucosal tunnel when it is re-epithelialized (Fig. 85–29) (Le Duc et al, 1987). This technique has been used to prevent reflux in the ureter–small intestine anastomosis. Excellent exposure is required, and therefore the small bowel needs to be opened along its antimesenteric border for a length of approximately 5 cm. The mucosa is incised for a length of 3 cm beginning 2 cm proximal to the cut edge of the bowel. It is important to begin the mucosal tunnel away from the cut edge of the bowel to allow enough distal bowel for closure without jeopardizing the entrance point of the ureter. The ureter is then brought through the serosa at the most distal portion of the mucosal sulcus, laid in the trough, spatulated, and sutured to the proximal end of the sulcus with interrupted 5-0 PDS using the full thickness of ureteral wall and anchored to the muscularis and mucosal layers of the bowel. The mucosa of the sulcus of the bowel is then sutured to the adventitia of the ureter. The mucosa of the bowel should not be sutured over the ureter but rather to its lateral aspect. The idea is that the mucosa eventually grows over the top of the ureter. Where the ureter enters the small bowel, its adventitia is sutured to the bowel serosa with 4-0 silk sutures. Stents are placed in the ureter, and their passage must be unimpeded to ensure that there is no angulation. The bowel should be fixed to the body wall near the site of the ureteral implantation so that the ureters do not angulate.
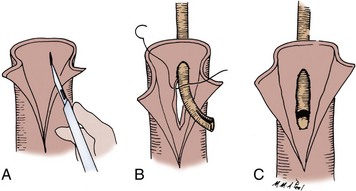
Figure 85–29 Le Duc ureterointestinal anastomosis. A, The small bowel is opened for approximately 4 to 5 cm. A longitudinal rent in the mucosa is made and the mucosa raised. B, At the distal end of the mucosal rent, a hole is made in the serosa, and the ureter is then drawn through. The entrance of the ureter through the serosa should be at least 2 cm proximal to the cut end of the bowel to allow sufficient bowel length to close the end. C, The ureter is spatulated and sutured to the mucosa and muscle layers. The mucosa is not reapproximated over the top of the ureter but rather sutured to the side of it.
The complication rate for this technique is relatively low, although the follow-up is also relatively short. Initial reports suggested that it carried with it an 87% incidence of maintaining an antireflux valve with a 5% incidence of stricture and a 2% incidence of leak (Schwaibold et al, 1998). A modification of the Le Duc technique was prompted by a 15% incidence of stricture when longer follow-up was available. With the modified technique, the stricture rate was 3% with  years of follow-up. The technique involves construction of a 3-cm mucosal sulcus to the submucosa. The ureter is brought in the proximal sulcus transmurally and anchored to the serosa externally. The ureter is spatulated for 1.5 cm, and only the spatulated portion is sewn to the muscularis of the bowel. The proximal intraluminal ureter is not fixed to the bowel (see Table 85–4).
years of follow-up. The technique involves construction of a 3-cm mucosal sulcus to the submucosa. The ureter is brought in the proximal sulcus transmurally and anchored to the serosa externally. The ureter is spatulated for 1.5 cm, and only the spatulated portion is sewn to the muscularis of the bowel. The proximal intraluminal ureter is not fixed to the bowel (see Table 85–4).
Hammock Anastomosis
This type of anastomosis involves conjoining the ureters and implanting them into the small bowel in a nonrefluxing manner. The small bowel is closed at its proximal end, and three 10-cm longitudinal incisions separated by 1 to 2 mm are made through the seromuscular layer to the mucosa. These incisions are crosshatched by multiple incisions. This serves as a hammock. The ureters are conjoined as in the Wallace technique and sutured to the intestinal mucosa. The ureters are buried by closing the intestinal wall over the top of them with seromuscular sutures of 3-0 polyglycolic acid suture (Hirdes et al, 1988).
With this technique, there is a 6% incidence of ureteroileal stenosis and approximately a 20% incidence of reflux. Follow-up, however, is relatively short.
Ureter–Small Bowel Anastomosis Employing Serosal Compression of the Extramural Ureter as an Antireflux Mechanism
This technique is primarily applicable to a continent diversion in which detubularized small bowel is employed and an antireflux ureterointestinal anastomosis is desired. It requires two segments of small bowel to be juxtaposed to each other. The ureter is laid between the two small bowel serosal surfaces, which are sutured posterior and anterior to the extramural portion of the ureter. The technique is as follows.
The posterior wall of the extramural tunnel is composed of the two segments of small bowel sutured together with 3-0 silk (Fig. 85–30A and B). The two segments of small bowel are opened along their antimesenteric borders. The ureter is spatulated and sutured to the mucosal-seromuscular layer with 5-0 absorbable sutures. The edges of the opened small bowel are then reapproximated over the anterior ureter with absorbable sutures (Fig. 85–30C), thus making a serosal tunnel of 4 cm in length. When the bowel is distended, it then applies pressure to the extramural ureter, thus preventing reflux (Abol-Enein and Ghoneim, 1994).
Figure 85–30 Ureter–small bowel anastomosis. A, Small bowel aligned so that four serosal surfaces are apposed in preparation for construction of two serosal compression tunnels. Serosal surfaces of the different limbs adjacent to the mesentery are sutured together with silk sutures. B, The serosal surfaces of the afferent limbs adjacent to the mesentery are sutured together with silk sutures. The antimesenteric borders of the apposed bowel segments are opened, thus making a trough. C, The ureters are laid in the trough and sutured to the seromuscular-mucosal wall at its distal end (illustrated on the right ureter), and the seromuscular-mucosal layer is closed over the ureter anteriorly (illustrated on the left ureter).
With an average of 3 years of follow-up, the anastomotic stricture rate is 4% and the failure of the antireflux mechanism is 3% (Abol-Enein and Ghoneim, 2001; Turkolmez et al, 2004).
Intestinal Antireflux Valves
Another technique of preventing reflux into the ureter involves construction of an antireflux mechanism with bowel distal to the ureterointestinal anastomosis. The ureter is sutured by the technique of either Bricker or Wallace (as described earlier) to the end of the bowel, and the bowel is used to make a one-way valve. Unlike with individual ureterointestinal antirefluxing anastomosis, when these valves fail or stenose, both kidneys are affected. Three basic types of antireflux mechanisms commonly employed with use of the bowel are ileocecal intussusception, ileoileal intussusception, and ileal nipple valve placed into colon.
Intussuscepted Ileocecal Valve
The mesentery is cleaned from the ileum for a length of 8 cm beginning at the cecum and coursing proximal (Fig. 85–31). At least 5 cm of ileum proximal to the detached mesentery must be intact to ensure intestinal viability. Thus the ileum should not be transected less than 13 cm from the ileocecal junction. A No. 22 catheter is placed through the ileum into the cecum. The ileal serosa is scarified either by multiple cross-incisions with a knife or with the electrocautery unit. The 8-cm segment is intussuscepted over the catheter into the cecum. The intussuscepted ileum is secured to the cecal wall with 3-0 silk sutures placed circumferentially 2 mm apart.
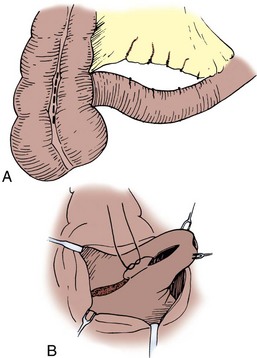
Figure 85–31 Ileocecal intussusception. A, An 8-cm segment of ileal mesentery is cleaned from the serosa beginning at the ileocecal junction. At least 5 cm of mesentery remains attached to the proximal ileum. An incision is made along a taenia at the level of the ileocecal valve. B, The ileum is intussuscepted over a No. 22 French catheter into the cecum under direct vision. The mucosa of the intussuscepted segment is incised, and the mucosa of the cecum adjacent to it is also incised. The muscle coats of both segments are sutured together. The serosa of the ileum is secured to the serosa of the cecum with interrupted 3-0 silk sutures placed circumferentially (not depicted).
The valve has a moderate tendency to fail because the intussusception has a significant chance of reduction. In one series, the antireflux mechanism remained intact in 55% of the patients during the long term (Hensle and Burbige, 1985). The intussusception may be made more secure by employing a modification described by King. The mesentery is cleaned as described before. The cecum is opened along a taenia, and the ileum is intussuscepted over the catheter under direct vision. Where the intussusception lies adjacent to the cecal wall, mucosa of the intussuscepted ileum and the cecal mucosa adjacent to it are incised down to muscle. The muscles are sewn together with interrupted 3-0 chromic suture. Long-term follow-up in eight patients reveals maintenance of the antireflux valve in seven with the modified technique (King, 1987; Friedman et al, 1992). The Mainz group advocates stapling the intussuscepted ileum to the ileocecal valve to prevent reduction. In their series 82% of patients maintain valve competence; however, there is a 20% incidence of stone formation (Wiesner et al, 2006).
Intussuscepted Ileal Valve
The mesentery is cleaned from an 8-cm segment of ileal serosa (Fig. 85–32). There must be 5 cm of ileal mesentery proximal and distal to the cleared segment to ensure proper blood supply. The ureters are sewn to the proximal end of ileum. The distal end of ileum is opened along its antimesenteric border to within 2 to 3 cm of the cleared mesentery to provide adequate exposure and direct visualization of the intussuscepted segment. A Babcock clamp is placed into the lumen of the bowel, and a portion of bowel wall is grasped by invaginating it into the clamp with a finger. The ileal segment is intussuscepted by pulling on the Babcock clamp with gentle constant traction. If there is resistance, the mesentery is generally too bulky and it must be defatted carefully before trying again to intussuscept the segment. A 5-cm intussuscepted segment should protrude. The gastrointestinal stapler without the knife or the linear stapler, from which the distal five to eight staples have been removed, is used to secure the intussusception in place; three rows with the gastrointestinal stapler or four rows with the linear stapler are placed in quadrants. The staple size should be 4.8 mm. The proximal staples are important in securing the intussusception and preventing its reduction, whereas the distal staples are less effective and more likely to be exposed to urine and thus facilitate stone formation. It is for this reason that the distal staples are removed from the staple cartridge before it is placed in the stapler and before the intussusception is stapled. With the cautery unit, the mucosa of the intussusception is incised along its length. Adjacent to this incision, another is made in the mucosa of the ileum. The muscularis of both is exposed and sewn together with interrupted 3-0 chromic suture. The distal serosa is then sutured proximally to the serosa of the intussuscepted segment circumferentially with 3-0 nonabsorbable sutures. This is meant to secure the intussusception and to prevent its reduction with failure of the antireflux mechanism. The valve is successful in preventing reflux 90% of the time (Kock et al, 1982; Skinner et al, 1989).
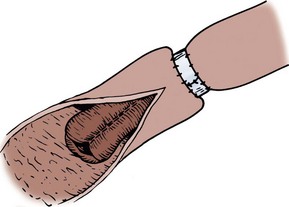
Figure 85–32 Intussuscepted ileal nipple valve. Eight centimeters of ileal mesentery are cleaned from the serosa. The ileum distally is opened within 2 to 3 cm of the rent in the mesentery. Five centimeters of ileum are intussuscepted and secured by placement of staples in quadrants. The ileal mucosa is incised adjacent to an incision in the intussuscepted segment, and the two muscle coats are sutured together with interrupted 3-0 chromic suture. The serosa of the intussuscepted segment is sutured circumferentially to the base of the ileum, into which the proximal segment is intussuscepted with interrupted silk suture.
Because the intussuscepted nipple valve as an antireflux mechanism has resulted in a 10% complication rate (5% stone formation on the exposed staples, 4% stenosis, and 1% prolapse [Stein et al, 1996]), modifications have been developed in an attempt to reduce these untoward outcomes. One such modification has been described by the University of Southern California group. An 8- to 10-cm isolated segment of ileum is tapered distally and laid between two segments of small bowel in which their serosal walls adjacent to the mesentery are sutured together. This serves as the posterior support for the isolated segment. The apposed bowel is opened along its antimesenteric border; lateral flaps are constructed adjacent to the segment and closed over its anterior aspect, thus making a serosal trough in which 4 cm of the segment of tapered ileum is positioned (Stein and Skinner, 2003). When the pouch is closed, a nipple valve is constructed. The concept is that as pressure in the pouch increases, the walls of the tapered nipple valve are compressed, thus preventing reflux. The authors call their modification a T pouch. At the time of this writing, long-term outcomes are not available.
Nipple Valve
The simplest intestinal antireflux valve to make is the nipple valve with use of ileum (Fig. 85–33). The mesentery is cleared from the last 8 cm of the cut end of the ileum. The distal 6 cm of serosa is scarified by multiple cross-striations and then turned back on itself to form a nipple. The nipple should be at least 4 to 4.5 cm in length. The end of the inverted ileum is then sutured to itself with interrupted 4-0 PDS. An incision on the taenia large enough to accommodate the segment is made. A No. 22 catheter is placed through the segment, and its serosa is sutured to the colon serosa circumferentially with interrupted 3-0 silk sutures placed 2 mm apart. The long-term success rate for this type of valve is unknown but would appear to be less than with the other two methods.
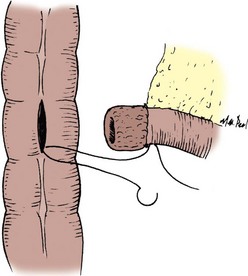
Figure 85–33 Nipple valve. Approximately 8 cm of mesentery are cleaned from the distal end of the ileum, and the serosa is scarified and then turned back on itself to form a nipple of approximately 4 cm in length. The end of the ileum is sutured to itself with interrupted 4-0 PDS. A rent is made in the colon through a taenia, and the nipple valve is placed through the rent and secured with circumferential interrupted 4-0 PDS through the full thickness of the colon and the seromuscular layer of the ileum.
Complications of Ureterointestinal Anastomoses
The complications that occur with ureterointestinal anastomoses include leakage, stricture, reflux in those anastomoses that were performed to prevent reflux, and pyelonephritis. In a review of the various types of procedures, it appears that of the colonic antirefluxing procedures, the Pagano technique offers the lowest incidence of stricture with an acceptable incidence of reflux. With respect to small bowel antireflux procedures, the Le Duc procedure and the ureterointestinal serosal apposition procedure seem to offer the lowest incidence of stricture with the highest success rates in preventing reflux. With respect to stricture formation and leakage, it appears the Wallace technique has the best results. In a comparison of the Bricker, the Wallace, and the nipple valve, however, in one series there was no difference in complication rate among any of the procedures. In another contemporary series the Wallace technique had the lowest incidence of stricture (Kouba et al, 2007). All had an incidence of approximately 29% of some form of obstruction in the long term (Mansson et al, 1979). In a more recent study, there appeared to be no difference between rate of reflux and stricture when a Bricker anastomosis was compared with a split-cuff nipple. The incidence of stricture was 7% for both types (De Carli et al, 1997).
Urinary Fistula
Urinary fistulas invariably occur within the first 7 to 10 days postoperatively with an incidence of 3% to 9% (see Table 85–4) (Beckley et al, 1982; Loening et al, 1982). The incidence of urinary intestinal leak is markedly reduced by the use of soft Silastic stents (Mattei et al, 2008). A urinary intestinal leak may cause periureteral fibrosis and scarring with subsequent stricture formation.
Stricture
In general, the antirefluxing techniques have a higher incidence of stricture. Patients are at risk for ureterointestinal strictures for the life of the anastomosis and must be observed on a scheduled periodic basis. A stricture has been reported to develop 13 years after the procedure (Shapiro et al, 1975). Ureteral strictures also occur away from the ureterointestinal anastomosis. This stricture is most common in the left ureter and is usually found as the ureter crosses over the aorta beneath the inferior mesenteric artery. It has been suggested that this is due to too-aggressive stripping of adventitia and angulation of the ureter at the inferior mesenteric artery.
Once a stricture has developed, various techniques may be used to rectify the situation. The most successful is re-exploration, with removal of the stenotic segment and reanastomosis of the ureter to the bowel by one of the aforementioned techniques. A number of studies have compared open surgical correction of ureterointestinal anastomotic strictures with endourologic methods. In general, open repair has a success rate of approximately 75% at 3 years versus 15% for balloon dilation with similar follow-up (DiMarco et al, 2001). Open surgical methods may be morbid and difficult procedures. Endourologic procedures employing balloon dilation have not proved to be durable, and therefore many have employed either cold knife incisions or laser incisions. When several series employing endourologic methods are combined, there is a 50% to 60% success rate with 2 years of follow-up. Strictures occurring in less than 1 year from the original procedure, strictures 1.5 cm or longer, and left-sided strictures have less favorable outcomes from endourologic methods (Kramolowsky et al, 1987, 1988; Cornud et al, 1996; Laven et al, 2001; Poulakis et al, 2003). These data must be viewed with caution because longer follow-up generally results in additional recurrences. In selected cases, metallic stents have been employed, which might be a reasonable approach in a patient with a limited life expectancy, thus avoiding a major open operation (Barbalias et al, 1998). A study in which nonmalignant ureterointestinal strictures were stented reported that all patients were successfully treated with 2 years of follow-up; one patient developed a stone on the stent (Palascak et al, 2001).
Pyelonephritis
Acute pyelonephritis occurs both in the early postoperative period and during the long term. Its incidence is approximately 10% to 20% in patients diverted with ileal conduits and 9% in those diverted with antirefluxing colon conduits (see Table 85–3). These complications cause considerable morbidity and in fact are associated with significant mortality. In one series of intestinal segments in the urinary tract, 8 of 178 patients died of sepsis (Schmidt et al, 1973). That these complications may result in delayed mortality is indicated by the fact that 2 of 115 children and 3 of 127 adults died of septic complications 5 to 14 years after intestinal diversion (Pitts and Muecke, 1979). When sepsis is associated with decreasing renal function and uremia, the morbidity and mortality are markedly increased.
Table 85–4 summarizes the complications and success rates for the various types of anastomoses. The table is derived from composite reports in the literature in which specific anastomoses were described and from which the data could be accurately analyzed. Because of these two requirements, it is not possible to comment, for example, on the incidences of reflux or leakage among various anastomotic types inclusively. These complications can be minimized by adherence to the principles of ureterointestinal surgery discussed earlier.
Renal Deterioration
The incidence of renal deterioration after conduit urinary intestinal diversion has varied from 10% to 60% (Koch et al, 1992; Madersbacher et al, 2003). This variance is perhaps due to the fact that many reports include both renal units that were abnormal and those that were normal before diversion. In analyzing abnormal renal units before diversion and documenting progressive disease, it is difficult to be sure whether the urinary diversion caused the progression or whether progression is due to the intrinsic abnormality for which the diversion was constructed. When the incidence of renal deterioration is determined by comparing renal units that were normal before diversion and then deteriorated postoperatively, 18% of patients who have ileal conduits show progressive deterioration versus 13% who have nonrefluxing colon conduits (see Table 85–3). Twenty percent of patients with nonrefluxing continent ileocecal bladders show some evidence of deterioration of the upper tracts when they are observed during the long term (Benchekroun, 1987). This deterioration leads to a 10% incidence of azotemia in children with ileal conduits (Schwarz and Jeffs, 1975) and a 12% (5 of 41 patients) incidence of renal failure in patients with colon conduits constructed for benign disease (Elder et al, 1979).
The incidence of both sepsis and renal failure is greater in patients with ureterosigmoidostomy than in those with conduits. Sepsis and renal failure may occur either in the immediate postoperative period or many years later. The most common cause of death in patients who have had a ureterosigmoidostomy for more than 15 years is acquired renal disease (i.e., sepsis or renal failure). In this group of patients, approximately 10% to 22% die of these disorders (Zabbo and Kay, 1986), some as late as 27 years after diversion (Mesrobian et al, 1988). In patients with ileal conduits, about 6% ultimately die of renal failure (Richie, 1974).
Renal Function Necessary for Urinary Intestinal Diversion
The amount of renal function required to effectively blunt the reabsorption of urinary solutes by the intestinal segment and to prevent serious metabolic side effects depends on the type of urinary intestinal diversion constructed (i.e., the amount of bowel to be used and the length of time the urine is exposed to the intestinal mucosa). Thus a greater degree of renal function is necessary for retentive (continent) diversions than for short conduit diversions. Patients who have a glomerular filtration rate (GFR) on average above 40 mL/min generally tolerate a continent diversion reasonably well. Indeed, in one study, two groups of patients were analyzed: those with GFRs around 100 mL/min (range, 91 to 112) and those with GFRs around 55 mL/min (range, 36 to 69). Both seemed to tolerate the metabolic load well with a minimal development of metabolic acidosis. There was a slightly increased incidence of metabolic acidosis in the group with low GFR, as would be expected; however, in this group, distal tubule function was excellently maintained as demonstrated by ammonium chloride loading. This observation points out that GFR is not the sole determining factor that allows the body to manage intestinal diversion. Indeed, GFR is but one factor. Just as important is distal tubule function. This article confirms that when distal tubule function is normal and the GFR is above 40 mL/min, patients do extremely well (Kristjansson et al, 1997).
There are five components of renal function: renal blood flow, glomerular filtration, tubule transport, concentration and dilution, and glomerular permeability. Aspects of renal function that must be specifically addressed are GFR, best measured by inulin clearance; ability of the tubule to acidify, determined by ammonium chloride loading; concentrating ability, determined by water deprivation; and glomerular permeability, reflected by urine protein concentrations. In general, patients with normal urine protein content who have a serum creatinine concentration below 2.0 mg/dL do well with intestine interposed in the urinary tract. Serum cytostatin C is a promising serum protein that reflects GFR more accurately than creatinine; however, its usefulness in urinary intestinal diversion has not yet been determined (Herget-Rosenthal et al, 2004). At a level of serum creatinine below 2 mg/dL, renal blood flow, GFR, tubule transport, and concentrating and diluting ability are relatively well preserved. In patients whose serum creatinine concentration exceeds 2 mg/dL and who are being considered for retentive diversion or in whom long segments of intestine will be used, a more detailed analysis of renal function is necessary. If the patient can achieve a urine pH of 5.8 or less after an ammonium chloride load, has a urine osmolality of 600 mOsm/kg or greater in response to water deprivation, has a GFR that exceeds 35 mL/min, and has minimal protein in the urine, the patient may be considered for a retentive diversion.
Urinary Diversion
This section deals with specific types of conduit urinary diversions. Fundamentally, there are two types of conduits: (1) those using the small bowel, which includes the jejunum or ileum, and (2) those in which a portion of large bowel is used. Conduits made of stomach have been described but are rarely indicated and may carry with them difficult problems of stomal maintenance. Their construction is not discussed here. Each type of conduit has specific indications and advantages, and for each there are specific complications. Some complications, however, are similar among all types. The indications for a conduit are the need for urinary diversion: after a cystectomy; because of a diseased bladder; before transplantation in a patient who has a bladder that cannot adequately receive the transplant ureter; and for dysfunctional bladders that result in persistent bleeding, obstructed ureters, poor compliance with upper tract deterioration, and inadequate storage with total urinary incontinence.
Preparation
Regardless of whether small or large bowel is used for the conduit, although subject to debate as noted previously, it is the authors’ preference that all patients have a bowel preparation as outlined earlier.The reason for this is that large segments of bowel are exposed to the urinary tract and in some cases require detubularization, thus exposing the abdominal cavity to a large amount of solid fecal material if the bowel is unprepared. The specific types of ureterointestinal anastomosis and stomal construction and their complications are also described in previous sections. This section describes features that are unique to the construction of the conduit. The complications cited for each conduit also depend on the length of follow-up and the concomitant procedure performed.
Ileal Conduit
In this procedure, a portion of distal ileum is chosen. It is the simplest type of conduit diversion to perform and is associated with the fewest intraoperative and immediate postoperative complications. It is not advisable to use ileum for a conduit in patients with a short bowel syndrome, in patients with inflammatory small bowel disease, and in those whose ileum has received extensive irradiation, often as a consequence of prior radiation therapy for a pelvic malignant neoplasm.
Procedure
A segment 10 to 15 cm in length is selected 10 to 15 cm from the ileocecal valve. The cecum and ileal appendage (i.e., that portion of the distal ileum fixed to the retroperitoneum) are mobilized. The ileal mesentery is transilluminated, and a major arcade identified to the segment selected. With a mosquito clamp, the mesentery immediately beneath the bowel is penetrated, and the bowel is encircled with a vessel loop. An area at the base of the mesentery that is to one side of the feeding vessel is selected, and a second vessel loop is placed through the mesentery. At this juncture, the peritoneum overlying both sides of the mesentery is incised from bowel vessel loop to the base of mesentery vessel loop. With mosquito clamps, the tissue is clamped, severed, and tied with 4-0 silk. A portion of mesentery 2 cm in length is cleaned away from the bowel beneath the mesenteric incision. This procedure is repeated at the other end of the selected segment. The base of the mesentery should be as wide as possible and the mesenteric windows not excessive (generally about 5 cm in length) to prevent ischemia of the segment. Allen clamps are placed across the bowel in an angled fashion such that the antimesenteric portion is shorter than the mesenteric portion. (Some prefer to transect the bowel with an anastomotic stapler.) Thus a triangular piece of bowel is removed and discarded.
The isolated ileal segment is placed caudad, and an ileoileostomy is performed as described earlier (Fig. 85–34). The mesenteric window of the ileoileostomy is closed with interrupted 3-0 silk sutures. The isolated segment is then flushed with copious amounts of saline until the irrigant is clear, at which point the ureters are brought out the retroperitoneum in the right lower quadrant. To accomplish this, the left ureter must be brought over the great vessels and posterior to the sigmoid mesentery to the rent in the posterior peritoneum. This may be done by mobilizing the cecum cephalad to identify the right ureter. The left ureter may be identified by incising the line of Toldt of the left descending colon (Fig. 85–35). This dissection allows anastomosis of the ileal segment as proximal as needed to the ureter. Indeed, the ileum may be anastomosed directly to the renal pelvis on both sides if necessary (Fig. 85–35C). After a cystectomy, the ureters are identified caudad to the iliac vessels and may be conveniently traced cephalad similar to the previous description. The ureteral-ileal anastomoses are performed as described previously. These anastomoses are stented.
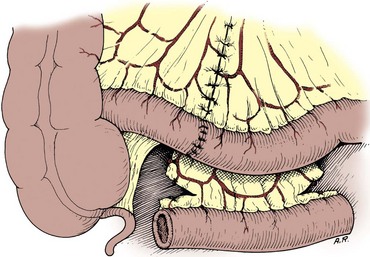
Figure 85–34 The isolated segment of ileum is placed caudal to the ileoileostomy. An incision on the mesentery of the isolated segment 1 cm from the bowel wall straightens the end. This is generally not necessary unless the mesentery is excessively bulky.
Figure 85–35 A, The cecum and root of the mesentery are incised. By blunt dissection, the cecum, ascending colon, and small bowel are mobilized cephalad. B, The right and left ureters are identified. The left ureter is more easily identified by mobilizing the left colon. C, The left colon is mobilized by incising the line of Toldt. This degree of mobilization allows anastomosis of the ileal segment to as proximal a position to the ureter as needed. This figure depicts a left renal pelvic and right ureterointestinal anastomosis.
A convenient method of introducing the stent through the loop to the opening in the bowel is illustrated in Figure 85–36. The base of the conduit is fixed to the retroperitoneum in the right lower quadrant by suturing the posterior peritoneum to the conduit, thus effectively retroperitonealizing the ureterointestinal anastomosis. The stoma is made as described previously. The authors prefer to suture the loop segment to the lateral peritoneal wall, thus obviating any chance of herniating small bowel lateral to the conduit. Many prefer to bring the segment directly to the anterior abdominal wall, however, thus allowing bowel to descend caudad on either side of the loop.
Figure 85–36 The ureterointestinal anastomoses should be stented with soft Silastic stents. These stents may be conveniently introduced with a Yankauer suction instrument from which the tip has been removed. The suction instrument is introduced by way of the distal end of the segment to the desired location of the ureteral anastomosis. When one cuts down on the Yankauer tip, its end protrudes through the bowel at the desired site. The stent is threaded through the suction instrument and the instrument removed.
Complications
Early and late postoperative complications are listed in Table 85–5. It is difficult to clearly ascribe these complications solely to construction of the conduit because many are reported in patients undergoing a cystectomy as well. These incidences in Table 85–5 are therefore expected to reflect the high end of the spectrum. Bleeding may occur from either the stoma or the conduit itself. Approximately 10% of patients have stomal bleeding. In 4%, it originates from the loop beneath the fascia (Delgado and Muecke, 1973). Bleeding that is extremely difficult to manage may be due to cirrhosis and varices. In this situation, life-threatening bleeding from the conduit may occur. To stop the bleeding, portal decompression may be required (Chavez et al, 1994). A less morbid method involves percutaneous transhepatic portal shunt or transhepatic angiography with embolization (Lashley et al, 1997; Medina et al, 1998). Indeed, this has been successful in several reported cases. Complications not listed include hypertension, renal failure, decreased renal function, and death. These complications in large part depend on the concomitant procedure performed, the length of follow-up, and the status of the kidneys before diversion. During the long term (20 years), 7% of patients have renal failure requiring dialysis, and 60% show deterioration of the upper tracts (Koch et al, 1992). After salvage cystectomy, complications are increased so that approximately one third of patients have one of the early complications (Abratt et al, 1993). Also, the complication rate is increased in patients in whom an intestinal segment is used in those requiring renal transplantation (Nguyen et al, 1990).
Table 85–5 Complications: Ileal Conduit*
| EARLY | LATE | |
|---|---|---|
| Urine leak | 2% (9/356) | |
| Bowel leak | ||
| Sepsis | 3% (7/230) | 3% (4/142) |
| Acute pyelonephritis | 3% (21/700) | 18% (133/726) |
| Wound infection | 7% (17/230) | 2% (4/178) |
| Wound dehiscence | 3% (11/326) | |
| Gastrointestinal bleed | 2% (2/90) | |
| Abscess | 2% (3/168) | |
| Prolonged ileus | 6% (14/230) | |
| Conduit bleed | 2% (3/178) | 10% (18/178) |
| Intestinal obstruction | 3% (18/610) | 5% (42/878) |
| Ureteral obstruction | 2% (14/610) | 6% (56/878) |
| Parastomal hernia | 2% (9/454) | |
| Stomal stenosis | 3% (143/486) | |
| Stone formation | 7% (59/822) | |
| Excessive conduit length | 9% (26/276) | |
| Metabolic acidosis | 13% (27/206) | |
| Conduit infarction | 2% (2/90) | |
| Volvulus | 7% (2/268) | |
| Conduit stenosis | 3% (11/320) | |
| Conduit-enteric fistula | <1% |
* Incidence as a percentage of the total number of reported cases from the literature. Numbers in parentheses represent the number of cases from which the percentage is derived.
One should be cautious in identifying duplex ureters. A failure to identify a second ureter on one side results in intraperitoneal urine leak and can cause excessive morbidity (Evans et al, 1994). The duplex ureters may be dealt with by implanting them separately if they are of sufficient caliber, or they may be spatulated, sewn together, and then implanted into the ileal conduit as a single unit (Fig. 85–37).
Jejunal Conduit
The jejunum has the largest diameter of the small bowel and the longest mesentery. Jejunal conduits generally have not had a high rate of acceptance because of perceived electrolyte abnormalities. A more recent report of patients, most of whom have been observed for more than 5 years, has shown that the bulk of electrolyte problems are minor—only about 4% in this series had severe hyponatremic metabolic acidosis. Renal calculi (12%), parastomal hernia (6%), and pyelonephritis (4%) constituted the majority of the remaining complications (Fontaine et al, 1997). The advantage of using the jejunum is that it avoids irradiated bowel and ureter. It is difficult to make a case for the jejunal conduit except in circumstances in which it is inadvisable to use either colon or ileum. However, this series does point out that when necessary, one can successfully use jejunum as a conduit. This might occur in patients who have extensive irradiation that involves the ileum, those with severe adhesions of the ileum and absence of the large bowel, and those who have an absent colon with inflammatory disease of the distal small bowel. The contraindications to its use are severe bowel nutritional disorders and the presence of another acceptable segment.
Procedure
The procedure is similar to that for an ileal conduit. A 10- to 15-cm segment of jejunum is isolated 15 to 25 cm from the ligament of Treitz as described for the ileal conduit. One should plan for the stoma to be in the upper quadrant, generally the left upper quadrant. The remainder of the technique is as described for the ileal conduit.
Complications
The early and long-term complications are similar to those listed for ileal conduit except that the electrolyte abnormality is a hyperkalemic, hyponatremic metabolic acidosis instead of the hyperchloremic metabolic acidosis of ileal diversion (Table 85–6). The treatment of the jejunal syndrome consists of administration of sodium chloride and sodium bicarbonate. Thiazides may also be used and are helpful in allaying the hyperkalemia (Hasan et al, 1994).
Table 85–6 Complications: Jejunal Conduit*
| EARLY | LATE | |
|---|---|---|
| Urine leak | 14% (3/21) | |
| Wound dehiscence | 5% (1/21) | |
| Acute pyelonephritis | 10% (2/21) | |
| Gastrointestinal bleed | 4% (1/27) | |
| Electrolyte abnormalities | 27% (17/62) | |
| Stomal stenosis | 7% (2/27) | |
| Bowel obstruction | 7% (2/27) | |
| Ureteral stricture | 12% (5/41) | |
| Enteric fistula | 2% (4/140) |
* Incidence as a percentage of the total number of reported cases from the literature. Numbers in parentheses represent the number of cases from which the percentage is derived.
Colon Conduit
Three types of colon conduits are commonly used: transverse, sigmoid, and ileocecal. Each has specific indications with advantages and disadvantages.
The transverse colon is used when one wants to be sure that the segment of conduit employed has not been irradiated in individuals who have received extensive pelvic irradiation. It is also an excellent segment when an intestinal pyelostomy needs to be performed. The sigmoid conduit is a good choice in patients undergoing a pelvic exenteration who will have a colostomy. Thus no bowel anastomosis needs to be made. It also allows nonrefluxing submucosal reimplantation and provides for an easily placed left-sided stoma when that is desirable.
The use of sigmoid colon is contraindicated with disease of this segment or when the hypogastric arteries have been ligated and the rectum has been left in situ. The latter circumstance may result in sloughing of the rectum or its mucosa because its blood supply of necessity is interrupted. It is also unwise to use this segment in individuals with extensive pelvic irradiation because it has probably been included in the radiation fields.
An ileocecal conduit has the advantage of providing a long segment of ileum when long segments of ureter need replacement, as well as the advantage of providing colon for the stoma. It is also used in situations in which free reflux of urine from the conduit to the upper tracts is thought to be undesirable. Contraindications to the use of transverse, sigmoid, and ileocecal conduits include the presence of inflammatory large bowel disease and severe chronic diarrhea.
Procedure
Transverse Colon
The segment may be isolated on the right or middle colic arteries, most commonly the latter (Fig. 85–38). The gastrocolic ligament is taken down, and the omentum is dissected from the portion of colon that is to be isolated. The splenic and hepatic flexures should be mobilized next. The proper length of segment is determined by taking into consideration the desired location of the stoma and the length of available ureters. In general, a length of 15 cm is sufficient. It is important not to isolate a segment that is too short and therefore incapable of reaching the retroperitoneum in such a position that a tension-free ureterocolonic anastomosis may be performed and retroperitonealized. The segment is isolated between bowel clamps, and a two-layer colocolostomy or stapled anastomosis is performed as outlined earlier. The segment is placed caudad to the anastomosis. If a colopyelostomy is to be performed, the segment should be placed cephalad to the bowel anastomosis. The isolated segment is irrigated with copious amounts of saline until the effluent is clear. The proximal end is closed with a running Connell suture of 3-0 chromic and a second layer of Lembert sutures of 3-0 silk. The ureterocolic anastomoses are then performed (see earlier), and the end is anchored to the retroperitoneum close to the midline. The stoma is usually placed in the right upper quadrant but may be placed anywhere in the abdomen if indicated.
Sigmoid Colon
The sigmoid colon is mobilized by incising its peritoneal attachments and the line of Toldt along the descending colon. The segment is isolated on the sigmoid vessels and placed lateral to the sigmoid colon (Fig. 85–39). The anastomosis of the sigmoid colon and ureterocolic anastomosis are as described for the transverse colon.
Figure 85–39 A, The sigmoid colon is freed of any peritoneal attachments. B, The segment is isolated and placed laterally. Bowel continuity is restored and the mesenteric window closed. C, The ureters are anastomosed to the colon and stented.
(A to C, from Hinman F Jr. Atlas of urologic surgery. Philadelphia: WB Saunders; 1989.)
Ileocecal Conduit
The ileocecal conduit is based on the terminal branches of the superior mesenteric artery (i.e., ileocecal artery). The segment is placed caudad, and an ileum-ascending colon anastomosis is performed as described previously. The stoma is placed in the right lower quadrant. The ileocecal valve may be reinforced to ensure prevention of reflux. This is described earlier (see Fig. 85–31). For the ureterointestinal anastomoses, see previously as well.
Complications
Early and late complications after a transverse colon (Beckley et al, 1982; Ravi et al, 1994; Schmidt et al, 1985), sigmoid, or ileocecal conduit are listed in Tables 85-7 to 85-9. As is true for the small bowel, complications not listed including death, renal failure, and renal deterioration depend on the concomitant procedure performed and the length of follow-up. Interestingly, early reports suggested a lower incidence of renal deterioration with colon conduits, but some recent series suggest that the incidence of these complications is about the same. However, there continue to be proponents of the colon conduit in that during the long term, there appears to be a 7.6% incidence of pyelonephritis and a 78% incidence of preservation of the upper tracts (Stein et al, 1996).
Table 85–7 Complications: Transverse Colon Conduit*
| EARLY | LATE | |
|---|---|---|
| Urine leak | 8% (11/137) | 8% (2/25) |
| Acute pyelonephritis | 11% (8/75) | |
| Wound infection | 5% (5/92) | |
| Wound dehiscence | 7% (8/109) | |
| Abscess | 5% (3/62) | |
| Prolonged ileus | 6% (2/30) | |
| Ureteral stricture | 6% (5/84) | 17% (37/215) |
| Bowel obstruction | 3% (1/30) | 2% (2/109) |
| Parastomal hernia | 4% (5/114) | |
| Stones | 11% (11/98) | |
| Enterocutaneous fistula | 2% (1/62) | |
| Stomal stenosis | 2% (1/62) | |
| Stomal prolapse | 11% (6/56) | |
| Metabolic acidosis | 12% (3/26) |
* Incidence as a percentage of the total number of reported cases from the literature. Numbers in parentheses represent the number of cases from which the percentage is derived.
Table 85–8 Complications: Sigmoid Conduit*
| EARLY | LATE | |
|---|---|---|
| Urine leak | 1% (1/70) | |
| Wound infection | 1% (1/70) | |
| Wound dehiscence | 1% (1/70) | |
| Acute pyelonephritis | 7% (5/70) | |
| Bowel obstruction | 6% (4/70) | |
| Ureteral stricture | 9% (6/70) | |
| Stones | 4% (3/70) | |
| Parastomal hernia | 3% (2/70) | |
| Stomal stenosis | 3% (2/70) |
* Incidence as a percentage of the total number of reported cases from the literature. Numbers in parentheses represent the number of cases from which the percentage is derived.
Table 85–9 Complications: Ileocecal Conduit*
| EARLY | LATE | |
|---|---|---|
| Urine leak | 6% (9/147) | |
| Bowel leak | 3% (5/147) | |
| Gastrointestinal bleed | 1% (1/147) | |
| Wound dehiscence | 7% (11/147) | |
| Acute pyelonephritis | 14% (20/147) | |
| Bowel obstruction | 3% (5/147) | 10% (14/147) |
| Stomal prolapse | 16% (24/147) | |
| Parastomal hernia | 5% (7/147) | |
| Stomal stenosis | 2% (3/147) | |
| Stones | 5% (8/147) | |
| Fecal fistula | 2% (3/147) |
* Incidence as a percentage of the total number of reported cases from the literature. Numbers in parentheses represent the number of cases from which the percentage is derived.
Complications of the ileocecal conduit in one reported series occurred in 21% of patients (Matsuura et al, 1991). In this series, complications of the ileal conduit were compared with those of the ileocecal conduit, and there appeared to be no difference in the frequency of early and late postoperative complications. Early complications included urinary leakage, bowel obstruction, fecal leakage, acute renal failure, fulminant hepatitis, pneumonia, gastrointestinal bleeding, hemorrhage, perforation of ileum, heart failure, and wound dehiscence. Late complications included stomal prolapse, acute pyelonephritis, bowel obstruction, urinary stones, parastomal hernia, incisional hernia, stomal stenosis, and fecal leakage. There was no difference in the incidence of deterioration of the upper tracts with either form of diversion. Of some note is that at high pressures, a large portion of the ileocecal conduits experienced reflux. At low pressures, however, there was minimal or no reflux. Whenever a portion of colon is used for a conduit, chronic diarrhea may be a consequence.
Ileal Vesicostomy
An ileal vesicostomy uses spatulated ileum and a generous transverse cystotomy to decompress the bladder and to allow an appliance to be used on the abdomen. This procedure is particularly well suited to spinal cord injury patients or those with significant neurologic disease. The concept is that patients with a neurogenic bladder have an easier job of caring for themselves with an abdominal stoma. Patients who are particularly good candidates are those with significant detrusor-external sphincter dyssynergia. Those who have detrusor hyperreflexia, particularly women, may have an increased incidence of incontinence. The complications of the procedure include urethral incontinence requiring closure of the urethra in 20% of patients, stomal stenosis, and bladder and renal calculi.
The procedure is performed by spatulating an ileal segment and performing a generous transverse cystotomy. The spatulated ileum is sutured to the bladder with absorbable suture, and the distal segment is brought to the abdominal wall by fashioning a rosebud stoma. This results theoretically in a low-pressure reservoir. Its appeal is that if indicated at a later date, the patient’s anatomy can be converted back to normal (Mutchnik et al, 1997; Atan et al, 1999).
Management Common to All Conduits
All anastomoses are stented with Silastic stents. A convenient method for introducing the stents through the conduit and into the ureter is illustrated in Figure 85–36. They are removed individually on the fourth to sixth postoperative days. If there is no increase in drainage, the Jackson-Pratt closed-suction drain is removed. All conduits are retroperitonealized, with the ureterointestinal anastomosis being placed in the retroperitoneum. This may be accomplished by suturing the posterior peritoneum to the serosa of the conduit above the ureterointestinal anastomosis. A drain may then be laid into the retroperitoneum. The authors prefer to drain the ureterointestinal anastomosis with a Jackson-Pratt closed-suction drain laid in the retroperitoneum 3 to 4 cm away from the anastomosis. The peritoneal cavity should not be drained.
All patients are given nothing by mouth until bowel function returns. A progressive diet is instituted after confirmation of bowel activity. It has been the authors’ practice to use NGT decompression in all patients having a bowel anastomosis. In reported surgical series, it is clear that there are advantages and disadvantages of NGT decompression after intestinal surgery. Without its use, vomiting is more common. With its use, pulmonary complications are more frequent. For those individuals with severe respiratory disease, consideration should be given to performing a gastrostomy. All patients have compression boots applied as prophylaxis for pulmonary embolus. The authors have not employed heparin or warfarin (Coumadin) prophylaxis in this group of patients.