Metabolic and Neuromechanical Problems of Urinary Intestinal Diversion
Problems that result from interposition of intestine in the urinary tract may be conveniently divided into three areas for the purposes of discussion: metabolic, neuromechanical, and technical-surgical. Metabolic complications are the result of altered solute reabsorption by the intestine of the urine that it contains. Neuromechanical aspects involve the configuration of the gut, which affects storage volume and contraction of the intestine that may lead to difficulties in storage. Finally, technical-surgical complications involve aspects of the procedure that result in surgical morbidity; these have been discussed after each section on the technical aspects of urinary intestinal diversion. The following is a discussion of metabolic and neuromechanical problems.
Metabolic Complications
Metabolic complications include electrolyte abnormalities, altered sensorium, abnormal drug metabolism, osteomalacia, growth retardation, persistent and recurrent infections, formation of renal and reservoir calculi, problems ensuing from removal of portions of the gut from the intestinal tract, and development of urothelial or intestinal cancer. Many of these complications are a consequence of altered solute absorption across the intestinal segment. The factors that influence the amount of solute and type of absorption are the segment of bowel used, the surface area of the bowel, the amount of time the urine is exposed to the bowel, the concentration of solutes in the urine, the renal function, and the pH of the fluid.
Electrolyte Abnormalities
Serum electrolyte complications and the type of electrolyte abnormalities that occur are different, depending on the segment of bowel used. If stomach is employed, a hypochloremic metabolic alkalosis may occur. If jejunum is the segment used, hyponatremia, hyperkalemia, and metabolic acidosis occur. If the ileum or colon is used, a hyperchloremic metabolic acidosis ensues. Other electrolyte abnormalities that have been described include hypokalemia, hypomagnesemia, hypocalcemia, hyperammonemia, and elevated blood urea nitrogen and creatinine. Specific abnormalities for each segment of intestine are detailed.
When stomach is used, a hypochloremic, hypokalemic metabolic alkalosis may ensue. This is generally not a significant problem unless the patient has concomitant renal failure, in which case there is a significant impairment of bicarbonate excretion or the patient is significantly dehydrated (Kurzrock et al, 1998). The metabolic alkalosis on occasion can be severe and life threatening (syndrome of severe metabolic alkalosis) (Table 85–10). This syndrome has been reported in patients with normal renal function. When it is fully manifested, lethargy, respiratory insufficiency, seizures, and ventricular arrhythmias may occur (Gosalbez et al, 1993). These symptoms are usually preceded by vomiting resulting in dehydration. A pronounced hypochloremic, hypokalemic metabolic alkalosis ensues. Patients are generally successfully treated with an H2 blocker to reduce proton secretion by the gastric segment and rehydration. In life-threatening circumstances, arginine hydrochloride infusion has been employed to rapidly restore acid-base balance. On occasion, when the H2 blockers are ineffective, the proton pump blocker omeprazole has been successfully employed. Rarely, omeprazole is ineffective and if the life-threatening metabolic alkalosis persists, the gastric segment must be removed (Gosalbez et al, 1993).
Table 85–10 Syndromes of Electrolyte Disturbances in Patients in Whom Bowel Is Interposed in the Urinary Tract

The role the serum concentration of gastrin plays appears pivotal in the syndrome. In the severe cases that have been reported, there is generally an elevated serum gastrin level. We have shown that serum gastrin levels are significantly correlated with systemic bicarbonate concentration in gastrocystoplasty patients; the greater the gastrin level, the more severe the metabolic alkalosis (Tanrikut and McDougal, 2004). When volume depletion, hypochloremia, and hypokalemia result from vomiting in those who normally have elevated circulating gastrin levels and a persisting long-standing metabolic alkalosis, the patient is at greater risk for development of the syndrome of severe metabolic alkalosis and manifestation of the symptoms outlined before. Thus persistent loss of protons from the gastric-augmented bladder with net addition of bicarbonate to the systemic circulation, alteration of normal homeostatic mechanisms for acute changes in acid-base balance, and impaired ability of even a normal kidney to excrete bicarbonate in the face of hypochloremia, hypokalemia, and increased circulating aldosterone levels (due to the dehydration) create a vicious circle in which normal homeostatic mechanisms are circumvented. Indeed, elevated aldosterone levels have been reported in this syndrome (Gosalbez et al, 1993). Hypokalemia, hypochloremia, and increased aldosterone levels impair the kidney’s ability to excrete excess bicarbonate. These perturbations coupled with a continued addition of bicarbonate from the gastric segment produce the extreme electrolyte abnormalities noted in the patients described. In view of the sigmoid correlation of serum bicarbonate to gastrin levels in patients with gastric segments in the urinary tract, patients at most risk for development of the syndrome of severe metabolic alkalosis are those whose serum gastrin concentrations exceed 120 ng/L because on this portion of the curve, small additional increments in gastrin concentration result in large increases in serum bicarbonate (Fig. 85–40). On the other hand, patients with serum gastrin concentrations below 100 ng/L can have significant increases in gastrin levels with little change in serum bicarbonate. Failure to properly empty the diversion with overdistention of the gastric segment would be expected to increase the serum gastrin level because stretch is a stimulus for gastrin release. For those who have either an elevated resting gastrin level in excess of 120 ng/L or impaired renal function, both patient and physician should be made aware of the consequences of dehydration and distention of the segment.
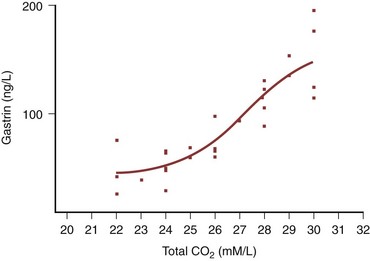
Figure 85–40 Sigmoid relationship of serum gastrin level to serum bicarbonate. Notice that over the physiologic range of normal gastrin levels, 10 to 120 ng/L, there is little change in serum bicarbonate. However, at levels of serum gastrin in excess of 120 ng/L, small changes in serum gastrin levels result in large changes in serum bicarbonate.
Electrolyte disorders that occur when jejunum is used for urinary intestinal diversion, particularly when proximal jejunum is used, include hyponatremia, hypochloremia, hyperkalemia, azotemia, and acidosis (see Table 85–10). These disorders result from an increased secretion of sodium and chloride with an increased reabsorption of potassium and hydrogen ions. This excessive loss of sodium chloride carries with it water, and thus the patient becomes dehydrated. The dehydration results in hypovolemia, which increases renin secretion and thereby aldosterone production (Golimbu and Morales, 1975). Aldosterone production may also be stimulated by hyperkalemia. The high levels of renin-aldosterone facilitate sodium reabsorption by the kidney and potassium loss, which produces a urine low in sodium content and high in potassium. This, when presented to the jejunum, results in a favorable concentration gradient for loss of sodium by the jejunum and increased reabsorption of potassium, thus perpetuating the abnormalities.
These electrolyte abnormalities result in lethargy, nausea, vomiting, dehydration, muscle weakness, and elevated temperature. If the abnormalities are allowed to persist, the patient may become moribund and finally die. This syndrome may be exacerbated by administration of hyperalimentation solutions. The mechanism by which hyperalimentation solutions exacerbate this syndrome in patients with jejunal intestine interposed in the urinary tract is unclear (Bonnheim et al, 1984). The severity of the syndrome depends on the location of the segment of jejunum that is used. The more proximal the segment, the more likely the syndrome is to develop. Its incidence varies from a low of 25% (Klein et al, 1986) to the majority of patients demonstrating significant abnormalities. Severe abnormalities may occur in as little as 4% when short segments are employed (Fontaine et al, 1997). Treatment of the disorder is rehydration with sodium chloride and correction of the acidosis with sodium bicarbonate. Provided that renal function is normal, the hyperkalemia is corrected by renal secretion. On occasion, a diuretic may be helpful to correct the hyperkalemia. After restoration of normal electrolyte balance, long-term therapy involves oral supplements with sodium chloride. A thiazide diuretic has also been useful in selected cases to control hyperkalemia during the long term (Hasan et al, 1994).
The electrolyte abnormality that occurs with the ileum and colon is hyperchloremic metabolic acidosis (see Table 85–10). This acidosis occurs to some degree in most patients who have ileum or colon interposed in the urinary tract but is generally of a minor degree. Its clinical significance when it is of a minor degree at this time is unknown. Hyperchloremic acidosis has been reported with a frequency of 68% of patients (19 of 28 patients; 10 of the 19 cases were severe enough to require treatment) with ileal conduits (Castro and Ram, 1970). In another study, 70% of patients with ileal conduits observed for 4 years or more had a decreased serum bicarbonate concentration (Malek et al, 1971). A more current study places the incidence at about 25% (Nieuwenhuijzen et al, 2008) with about 4% requiring hospitalization for correction (Studer et al, 2006). Severe electrolyte disturbances occur to a much lesser degree. It has been reported to be a major problem in 18% of patients (8 of 45) with intestinal cystoplasties (Whitmore and Gittes, 1983), in 10% of patients (17 of 178) with ileal conduits (Schmidt et al, 1973), and in 80% of patients (112 of 141) with ureterosigmoidostomies (Ferris and Odel, 1950). In continent diversions involving either ileum and cecum or cecum alone, most patients have an elevated serum chloride and depressed serum bicarbonate (Ashken, 1987; McDougal et al, 1989). Sixty-five percent of patients with Mainz pouches require alkali therapy to maintain a normal acid-base balance (Thuroff et al, 1987). Early reports of patients with continent diversions made of ileum have a much lower incidence of electrolyte problems, in the range of 10% to 15% (see Table 85–3) (Allen et al, 1985; Boyd et al, 1989). Symptoms in those in whom the syndrome is severe include easy fatigability, anorexia, weight loss, polydipsia, and lethargy. Those with ureterosigmoidostomies also have an exacerbation of diarrhea.
These electrolyte abnormalities, if significant and allowed to persist, result in major metabolic abnormalities, to be discussed subsequently. In and of themselves, however, they may be lethal because severe electrolyte abnormalities have contributed to death of patients (Heidler et al, 1979).
The mechanism of hyperchloremic metabolic acidosis is due to the ionized transport of ammonium. Ammonium substitutes for sodium in the Na+-H+ antiport. The exchange of the weak acid NH4 for a proton is coupled with the exchange of bicarbonate for chloride. Thus ammonium chloride is absorbed across the lumen into the blood in exchange for carbonic acid (i.e., CO2 and water). Ammonium may also gain entry to the blood from bowel lumen through potassium channels (McDougal et al, 1995).
The treatment of hyperchloremic metabolic acidosis involves administration of alkalizing agents or blockers of chloride transport. Alkalinization with oral sodium bicarbonate is effective in restoring normal acid-base balance. Oral administration of bicarbonate may not be tolerated particularly well, however, because it can produce considerable intestinal gas. An effective alternative is sodium citrate and citric acid solution (Bicitra or Shohl solution) used together; however, many patients do not care for the taste. Potassium citrate, sodium citrate, and citric acid solution (Polycitra) may be used instead if excessive sodium administration is a problem because of cardiac or renal disease and if potassium supplementation is desirable or at least not harmful. In those patients in whom persistent hyperchloremic metabolic acidosis occurs and in whom excessive sodium loads are undesirable, chlorpromazine or nicotinic acid may be used to limit the degree of the acidosis. These agents used alone do not correct the acidosis in humans, but they limit its development and thus reduce the need for alkalinizing agents. Chlorpromazine and nicotinic acid inhibit cyclic adenosine monophosphate and thereby impede chloride transport. Chlorpromazine may be given in a dose of 25 mg three times a day. On occasion, as much as 50 mg three times a day may be necessary, but at such doses, side effects are not uncommon. Chlorpromazine should be used with care in adults because there are many untoward side effects including tardive dyskinesia. Nicotinic acid may be given in a dose of 400 mg three or four times a day. The drug should not be used in patients with peptic ulcer disease or significant hepatic insufficiency. Side effects that may be observed include exacerbation of liver dysfunction, exacerbation of peptic ulcer disease, headaches, and double vision. Flushing and dermatitis are not uncommon and generally disappear as the patient becomes adapted to the drug.
Hypokalemia and total-body depletion of potassium may occur in patients with urinary intestinal diversion. This is more common in patients with ureterosigmoidostomies than it is in patients who have other types of urinary intestinal diversion (Geist and Ansell, 1961). In one study, patients with ureterocolonic diversions had a 30% reduction in total-body potassium, whereas those with ileal conduits had, as a group, no significant alteration in total-body potassium; individually, however, some had as much as a 14% reduction in total-body potassium (Williams et al, 1967). Patients with continent diversions have also been noted to have a decrease in total-body potassium. The patients most susceptible to total-body potassium depletion are those with long-standing uncorrected metabolic acidosis (Stein et al, 1998). The potassium depletion is probably due to renal potassium wasting as a consequence of renal damage, osmotic diuresis, and gut loss through intestinal secretion. The last-mentioned (probably quantitatively) plays a relatively minor role. Indeed, it has been shown that ileal segments exposed to high concentrations of potassium in the urine reabsorb some of the potassium, whereas colon is less likely to do so (Koch et al, 1990). Thus those with ileum interposed in the urinary tract likely blunt the potassium loss by the kidney, whereas those with colon do not, thus explaining why patients with ureterosigmoidostomies and ureterocolonic diversions are more likely to have total-body potassium depletion. When the depletion is severe, the patient may develop a flaccid paralysis. In treating these patients, one must remember that if the hypokalemia is associated with severe hyperchloremic metabolic acidosis, treatment must involve both replacement of potassium and correction of the acidosis with bicarbonate. If the acidosis is corrected without attention to potassium replacement, severe hypokalemia may occur, marked flaccid paralysis may develop, and significant morbidity may ensue (Koff, 1975).
Because the bowel transports solutes and because its membrane is not particularly watertight, osmolality generally re-equilibrates across the bowel wall. Thus attempts to deprive a patient of water and determine osmolality as a reflection of renal function are inappropriate because the bowel alters the osmotic content. The bowel also makes the contents more alkaline, and therefore it is impossible to determine the ability of the kidney to acidify simply by measuring urinary pH in patients with urinary intestinal diversion. Finally, because urea and creatinine are reabsorbed by both the ileum and the colon, serum concentrations of urea and creatinine do not necessarily accurately reflect renal function (Koch and McDougal, 1985; McDougal and Koch, 1986).
Histologic alterations of the intestine may occur over time when urine is chronically exposed to the mucosa. Villous atrophy and the formation of pseudocrypts may occur, particularly in the ileum. These changes are patchy because there is normal ileal mucosa interspersed between these abnormalities. Submucosal inflammatory infiltrates may also be observed. There appear to be fewer changes in the colonic mucosa during the long term. In the colon, a decrease in the size of goblet cells has been described. In time, some transport processes may be altered, with some solutes less actively transported, whereas other processes of solute transport remain active (Philipson et al, 1983). The ability to establish a hyperchloremic metabolic acidosis, however, appears to be retained by most segments of ileum and colon over time. In an experimental study, chronic exposure of intestine to urine resulted in a decreased number of transporters, but those that remained were perfectly functional (Grocela and McDougal, 1999).
Altered Sensorium
Alteration of the sensorium may occur as a consequence of magnesium deficiency, drug intoxication, or abnormalities in ammonia metabolism. Patients who develop magnesium deficiency do so either secondary to nutritional depletion or in relation to magnesium wasting by the kidney in much the same way that calcium wasting occurs (see later). Alterations in the sensorium have also occurred because of diabetic hyperglycemia; however, this is not a consequence of the intestinal diversion. In such patients, reabsorption of urinary glucose can result in hyperglycemia without demonstrable glucosuria (Onwubalili, 1982). Perhaps the more common cause of an altered sensorium is a consequence of altered ammonia metabolism. Ammoniagenic coma in patients with urinary intestinal diversion has been reported in those with cirrhosis (Silberman, 1958), those with altered liver function without underlying chronic liver disease (McDermott, 1957), and those with normal hepatic function as determined by serum enzyme activities (Mounger and Branson, 1972; Kaufman, 1984; Perez-Fidalgo, 2007). The syndrome is most commonly associated with decreased liver function, however, and even in those cases in which normal liver function has been reported, the crude methods by which it was assessed in these reports have been unable to confirm the absence of subtle alterations in liver function. The syndrome is most commonly found in patients with ureterosigmoidostomies but has been reported for those with ileal conduits as well (McDermott, 1957).
The treatment of ammoniagenic coma involves draining the urinary intestinal diversion, either with a rectal tube in the case of ureterosigmoidostomy or with a Foley catheter in those with a continent diversion so that the urine does not remain exposed to the intestine for extended periods. Neomycin is administered orally to reduce the ammonia load from the enteric tract, and protein consumption is curtailed, thus limiting the nitrogen load to the patient until serum ammonium levels return to normal. In severe circumstances, arginine glutamate, 50 g in 1000 mL of 5% dextrose in water, may be given intravenously. This complexes the ammonia by providing substrate for the formation of glutamine (Silberman, 1958). Lactulose may be given orally or by rectum (Edwards, 1984). This complexes the ammonia in the gut and prevents its absorption.
Abnormal Drug Absorption
Drug intoxication has been reported in patients with urinary intestinal diversion. Drugs more likely to be problematic are those absorbed by the gastrointestinal tract and excreted unchanged by the kidney. Thus the excreted drug is re-exposed to the intestinal segment, which then reabsorbs it, and toxic serum levels develop. This has been reported for phenytoin (Dilantin) (Savarirayan and Dixey, 1969) and has been seen for certain antibiotics that are excreted unchanged. Although chemotherapy is generally well tolerated by patients with conduits, methotrexate toxicity has been documented in a patient with an ileal conduit (Bowyer and Davies, 1986).
A more recent study suggests that in patients with normal renal function, both those with and without continent diversions tolerate chemotherapy well. The authors studied 23 patients with continent diversions and 19 with ileal conduits who received cisplatin, methotrexate, and vinblastine. The authors concluded that there was no difference in toxicity in patients without diversions, those with ileal conduits, and those with continent diversions. Indeed, the patients with continent diversions did not have a Foley catheter placed during the chemotherapy infusion. However, if one looks carefully at the data, it is clear that there is in fact an increased toxicity in the continent diversion group, although it did not achieve statistical significance (Srinivas et al, 1998). In those patients receiving antimetabolites, it is prudent to carefully monitor the patient for toxic products that are excreted in the urine and capable of intestinal absorption lest lethal toxic serum levels develop. Moreover, in patients with continent diversions who are receiving chemotherapy, consideration should be given to draining the pouch during the time the toxic drugs are being administered.
Osteomalacia
Osteomalacia or renal rickets occurs when mineralized bone is reduced and the osteoid component becomes excessive. Osteomalacia has been reported in patients with colocystoplasty (Hassain, 1970), ileal ureters (Salahudeen et al, 1984), colon and ileal conduits, and, most commonly, ureterosigmoidostomies (Harrison, 1958; Specht, 1967). The cause of osteomalacia may be multifactorial but commonly involves acidosis. With persistent acidosis, the excess protons are buffered by the bone with release of bone calcium. With its release, it is excreted by the kidney. Support for the theory that chronic acidosis is causative in osteomalacia comes from those patients in whom correction of the acidosis results in remineralization of the bone (Richards et al, 1972; Siklos et al, 1980). It has also been shown, however, that major alterations in serum bicarbonate are not necessary for the development of the syndrome (Koch and McDougal, 1988; McDougal et al, 1988). Moreover, some patients with osteomalacia secondary to urinary intestinal diversion do not have bone demineralization corrected with restoration of normal acid-base balance. These patients have been found to manifest vitamin D resistance that is independent of the acidosis. It is likely that this resistance is of renal origin. Resistance can be overcome by supplying 1-α-hydroxycholecalciferol, a vitamin D metabolite that is much more potent than vitamin D2. By providing this substrate in excess amount, remineralization of bone occurs (Perry et al, 1977). Also, it has been shown that reabsorption of urinary solutes may play a role in increasing calcium excretion by the kidney. Sulfate filtered by the kidney inhibits calcium reabsorption and results in both calcium and magnesium loss by the kidney. Thus if the gut increases its sulfate reabsorption and requires the kidney to increase sulfate excretion, this results in hypercalciuria and hypermagnesuria (McDougal and Koch, 1989). Finally, there is some evidence to indicate that the calcium-to-parathormone ratio is altered, suggesting that a resistance to parathormones develops during the long term (Tanrikut and McDougal, 2004). Osteomalacia in urinary intestinal diversion may be due to persistent acidosis, vitamin D resistance, and excessive calcium loss by the kidney. It appears that the degree to which each of these contributes to the syndrome may vary from patient to patient.
It is clear that a number of metabolic problems have been obviated when meticulous attention is paid to correction of the abnormalities prospectively. If a base deficit of more than 2.5 mEq/L is corrected, some investigators have found no evidence of bone mineral density abnormalities (Stein et al, 1998). Indeed, the type of diversion does not seem to make a difference when the acidosis is taken into account and corrected (Kawakita et al, 1996). Others suggest that there is no difference in continent diversions and ileal conduits (Campanello et al, 1996); however, in such selected series, the distribution of acidosis across both groups is generally identical. It is clear that if the groups are large enough and not preselected, there is an increased incidence of acidosis in continent diversion patients. Indeed, if patients are followed long enough some will develop bone mineral density abnormalities, particularly in those who are acidotic over the long term (Incel et al, 2006). The take-home message is that if one pays meticulous attention to correction of the acidosis, bone mineral density abnormalities will probably not become a problem.
Patients who develop osteomalacia generally complain of lethargy; joint pain, especially in the weight-bearing joints; and proximal myopathy. Analysis of serum chemistries reveals that the calcium concentration is either low or normal. The alkaline phosphatase level is elevated, and the phosphate level is low or normal (Harrison, 1958). The treatment as indicated earlier involves correction of the acidosis and dietary supplementation of calcium. If this does not result in remineralization of the bone, the active form of vitamin D may be administered. If this is not successful, the more active metabolite of vitamin D3, 1-α-hydroxycholecalciferol, should be administered.
Growth and Development
Considerable evidence suggests that urinary intestinal diversion has a detrimental effect on growth and development. In a study of 93 myelodysplasia patients observed for 17 to 23 years, significant aberrations in growth were noted when morphometric parameters were analyzed. Anthropometric measurements in those with urinary intestinal diversion showed a decrease in linear growth in all indices measured, with a statistically significant decrease in biacromial span and elbow-hand length (Koch et al, 1992).
Patients with long-term urinary diversions are more susceptible to fractures and to complications after orthopedic procedures. When myelodysplastic patients with ileal conduits were compared with a similar group of patients who retained their bladder and were on intermittent catheterization, the patients with an ileal conduit had an increased number of fractures, as well as malunions and nonunions after orthopedic procedures (Koch et al, 1992). It was found that more patients with urinary intestinal diversion fell below the tenth percentile than did patients who were treated with intermittent catheterization. There was, in fact, no difference in height and weight between the two groups studied (Koch et al, 1992; McDougal, 1992).
There is also experimental evidence for impaired linear growth in urinary intestinal diversion. Rats with unilateral ureterosigmoidostomies observed long term demonstrated significantly decreased femoral bone length compared with nondiverted controls (Koch and McDougal, 1988). Thus it appears clear that although obvious alterations in growth and development do not occur, when carefully studied, patients who have urinary intestinal diversions constructed in childhood and who maintain these diversions for more than 10 years have significant changes in linear growth.
Infection
An increased incidence of bacteriuria, bacteremia, and septic episodes occurs in patients with bowel interposition. A significant number of patients with intestinal cystoplasty develop pyelonephritis, and 13% have septic and major infectious complications (Kuss et al, 1970). The episodes are more frequent after colocystoplasty than ileocystoplasty (Kuss et al, 1970). In a contemporary series 4% of patients were admitted to a hospital for sepsis (Studer et al, 2006). Acute pyelonephritis occurs in 10% to 17% of patients with colon and ileal conduits (Schmidt et al, 1973; Schwarz and Jeffs, 1975; Hagen-Cook and Althausen, 1979). Approximately 4% of patients (8 of 178) with ileal conduits die of sepsis (Schmidt et al, 1973).
Patients with conduits have a high incidence of bacteriuria. Indeed, approximately three quarters of ileal conduit urine specimens are infected (Guinan et al, 1972; Middleton and Hendren, 1976; Elder et al, 1979). It is clear that some patients are merely colonized at the distal end of the conduit because the incidence of positive cultures can be markedly diminished by culture of the proximal portion of the loop by a double-catheter technique (Smith, 1972). Many of these patients, however, show no untoward effects and seem to do well with chronic bacteriuria. Deterioration of the upper tracts is more likely when the culture becomes dominant for Proteus or Pseudomonas. Thus patients with relatively pure cultures of Proteus or Pseudomonas should be treated, whereas those with mixed cultures may generally be observed, provided they are not symptomatic. Patients with continent diversions also have a significant incidence of bacteriuria and septic episodes (McDougal, 1986). Indeed, two thirds of patients with Kock continent diversions have positive cultures (Kock, 1987). The reasons for the increased incidence of bacteriuria and sepsis are unclear, but it is likely that the intestine is incapable of inhibiting bacterial proliferation, in contrast to the urothelium. Thus intestine that normally lives symbiotically with bacteria when interposed in the urinary tract serves as a source for ascending infection and septic complications. Moreover, the intestine may make the urine less bacteriostatic and thereby promote the growth of bacteria. Distention of the intestinal segment may aid in translocation of bacteria across the intestine and into the blood. Studies have shown some changes in intestinal mucosal immunologic bacterial defense mechanisms; however, for the most part, they seem to be preserved (Wullt et al, 2004).
Stones
One of the consequences of persistent infection is the development of magnesium ammonium phosphate stones. Indeed, the great majority of stones formed in patients with urinary intestinal diversions are composed of calcium, magnesium, and ammonium phosphate. Those most susceptible to development of renal calculi are patients who have hyperchloremic metabolic acidosis, pre-existing pyelonephritis, and urinary tract infection with a urea-splitting organism (Dretler, 1973). The incidence of renal stones is 3% to 4% in patients with colon conduits (Althausen et al, 1978; Hagen-Cook and Althausen, 1979) and 10% to 12% in those with ileal conduits (Schmidt et al, 1973). In those with continent cecal reservoirs, there is a 20% incidence of calculi within the reservoir (Ashken, 1987). The stones may be due to persistent infection with alkalinization of the urine, persistent hypercalciuria for reasons described previously, and alterations of urinary excretion products by the intestine. A major cause of calculus formation in conduits and pouches is a foreign body such as a staple or nonabsorbable suture, on which concretions form. In intestinal reservoirs, alterations in bowel mucosa may also serve as a nidus for stone formation. Finally, alterations in intestinal mucus, particularly in the presence of infection or obstruction, may serve as a nidus or more importantly may interfere with emptying and thereby exacerbate infection and stone formation (N’Dow et al, 2004).
Intestinal Motility, Short Bowel, and Nutritional Problems
Many nutritional problems may occur from the loss of significant intestinal absorptive surface resulting from removal of substantial portions of the gut for construction of a urinary intestinal diversion. In patients with a significant loss of ileum, vitamin B12 malabsorption has been reported and results in anemia and neurologic abnormalities. Vitamin B12 deficiency has been shown to occur in 10 of 41 patients who received preoperative radiotherapy before radical cystectomy and ileal ureterostomy (Kinn and Lantz, 1984) and 21% of children following ileocystoplasty have low serum levels of B12 (Rosenbaum et al, 2008). Loss of significant portions of ileum also results in malabsorption of bile salts. Because the ileum is the major site of bile salt reabsorption, the lack of reabsorption allows bile salts entry into the colon, which causes mucosal irritation and diarrhea. Also, loss of the ileum results in the loss of the “ileal break.” The ileal break is a mechanism whereby gut motility is reduced when lipids come in contact with the ileal mucosa so that increased absorption can occur. With the loss of ileum, the lipid does not result in decreased motility and is presented unmetabolized to the colon, which may result in fatty diarrhea.
It has been difficult to demonstrate untoward effects of low serum B12 levels in these patients. The liver stores enough B12 to supply the body’s requirement for 3 to 5 years without oral intake. Thus pathologic problems would not be expected to manifest themselves for many years and have rarely been reported. Moreover, low serum levels of Vitamin B12 do not necessarily correlate with metabolic deficiency. To assess whether or not there is a metabolic impact it is necessary to measure serum levels of homocysteine and/or methylmalonic acid. Because vitamin B12 serves as a coenzyme in the metabolic pathways of homocysteine and methylmalonic acid, their measurement is a sensitive indicator of whether the low vitamin B12 level is significant. These measurements have not been done in this group of patients, and thus it is unclear at this time as to the importance of vitamin B12 measurements in these patients. Many physicians, however, empirically give patients who have had intestinal diversions of ileum in place for more than 5 to 7 years parenteral vitamin B12 as a precaution.
Loss of the ileocecal valve may have a number of untoward effects. Because of the loss of the valve, reflux of large concentrations of bacteria into the ileum may occur, resulting in small intestinal bacterial overgrowth. This may result in nutritional abnormalities that involve interference with fatty acid reabsorption and bile salt interaction. With the lack of absorption of fats and bile salts, these are presented to the colon and result in diarrhea. Moreover, reflux of bacteria into the small bowel may result in bile salt deficiency. Also, the lack of fat absorption may result in deficiencies of the fat-soluble vitamin A, osteomalacia due to lack of vitamin D, and complexing of calcium with the fats to form soaps and thus lack of its absorption. The ileocecal valve also serves as a break, and an intact valve prolongs transit time of the small bowel and enhances absorption. Thus its loss may contribute to nutritional abnormalities. Some have advocated reconstruction of the valve mechanism between ileum and colon when the ileocecal segment is used for reconstruction.
Loss of a significant portion of jejunum may result in malabsorption of fat, calcium, and folic acid; however, significant portions of jejunum are rarely used for urologic reconstructive procedures. Loss of the colon may result in diarrhea because of lack of fluid and electrolyte absorption, loss of bicarbonate because of its increased secretion in the ileum and lack of reabsorption, and dehydration because of the loss of fluids.
Of concern when intestinal segments are used in urinary reconstruction is the effect removal of a segment of intestine from the alimentary tract might have on intrinsic bowel function. Indeed, removal of major segments from the alimentary tract may cause nocturnal bowel movements, fecal urgency, fecal incontinence, diarrhea, and nutritional deficiencies (Riddick et al, 2004). A study compared patients with ileal conduits with those who had segments used for clam cystoplasty and not surprisingly found that those with clam cystoplasty had a 40% incidence of significant bowel problems. It is known that there is an association between detrusor instability and irritable bowel, perhaps accounting for the incidence of untoward disorders of bowel function in this series (N’Dow et al, 1998). Thus there is a need for heightened awareness of bowel dysfunction in patients with detrusor instability in whom bowel segments are to be used. A recent report of patients who had an ileocystoplasty noted that 7% had significant diarrhea (Blaivas et al, 2005). One should warn patients who will have major portions of the intestinal tract used in the reconstruction that bowel problems may ensue.
Cancer
The incidence of cancer development in patients with ureterosigmoidostomy varies between 6% and 29%, with a mean of 11% (Schipper and Decter, 1981; Stewart et al, 1982; Zabbo and Kay, 1986). There is generally a 10- to 20-year delay before the cancer becomes manifest. On histologic examination, the tumors include adenocarcinoma, adenomatous polyps, sarcomas, and transitional cell carcinoma. Case reports of tumors developing in patients with ileal conduits, colon conduits, bladder augmentations, rectal bladder, neobladders, and ileal ureters have been described (Austen and Kalble, 2004). Anaplastic carcinomas and adenomatous polyps have been reported in patients with ileal conduits. Adenocarcinoma has developed in patients with colon conduits; adenocarcinoma, undifferentiated carcinoma, sarcomas, and transitional cell carcinomas have developed in patients with bladder augmentations with both ileum and colon (Filmer, 1986).
The etiologic mechanism of the development of the carcinoma is not understood. Whether the tumor arises from transitional epithelium or colonic epithelium is unclear. Because most of the tumors are adenocarcinomas, it has been assumed that the tumor arises from the intestinal epithelium. Adenocarcinomas have been shown to arise from transitional cell epithelium exposed to the fecal stream in experimental animals (Aaronson et al, 1989). Furthermore, studies show that the ureters in ureterosigmoidostomy patients have an exceedingly high incidence of dysplasia (Aaronson and Sinclair-Smith, 1984). Moreover, if the transitional epithelium is removed from the enteric tract, adenocarcinomas do not develop. If the urothelium is left in contact with the intestinal mucosa, however, even though the diversion is defunctionalized and the area is not bathed in urine, adenocarcinoma may still develop. This is illustrated by a case report in which a patient who had a ureterosigmoidostomy that was defunctionalized with a conduit subsequently developed cancer 9 months later. The distal ureters at the sigmoid were left in situ. Twenty-two years later, the patient developed cancer at the site of the ureterointestinal anastomosis (Schipper and Decter, 1981). This suggests that when ureterointestinal anastomoses are defunctionalized, they should be excised rather than merely ligated and left in situ. Other evidence including cell staining techniques suggests that the colon is the primary organ of origin (Mundy, personal communication, 1991). Whether the urothelium or intestine is the primary site of origin, it seems likely that tumors can arise from both tissues.
The highest incidence of cancer occurs when the transitional epithelium is juxtaposed to the colonic epithelium and both are bathed by feces (Shands et al, 1989). Nitrosamines, known mutagens, are produced in rats with ureterosigmoidostomy (Cohen et al, 1987), but there appears at least at this juncture no convincing evidence to support a primary role for them in the genesis of the tumor. An abnormal pattern of colonic mucin secretion has been demonstrated in patients with ureterosigmoidostomy, but its significance is unclear (Iannoni et al, 1986). Induction of specific enzymes associated with carcinoma has also been demonstrated. Ornithine decarboxylase, an enzyme that has been found to be elevated in malignant colonic mucosa, is also elevated in experimental animals with vesicosigmoidostomy (Weber et al, 1988). The role of epidermal growth factor and other growth factors is currently being investigated. Evidence suggests that these may at least play a role in development, if not in induction. At this time, the cause of the genesis of cancer in urinary intestinal diversion is not known. Because its incidence is significant in patients with ureterosigmoidostomies, they should have routine colonoscopies on a scheduled periodic basis.
Neuromechanical Aspects of Intestinal Segments
Both small bowel and colon contract to propel luminal contents in an aboral direction. The ability to propel luminal contents is a consequence of muscle activity, as well as coordinated nerve activity. Both the small bowel and the colon have an outer longitudinal layer of muscle and an inner circular layer. There is also a muscularis mucosa, which is immediately beneath the mucosa and may extend into the villi. The outer and inner layers of muscle, however, play the major role in peristalsis. In the colon, the outer longitudinal layer of muscle condenses to form three taeniae coli. The bowel receives its parasympathetic innervation from the vagus. It is also innervated by the sympathetic nervous system. The nerves lie between the circular and longitudinal layers of muscle. The enteral nervous system operates autonomously, and therefore one can denervate the intestine and not affect the coordinated contractions. These contractions are termed activity fronts and may be stimulated by feeding, or they may be inhibited by exposure of the lumen to various substances (i.e., lipid in the ileum decreases ileal motility). Two aspects of neuromechanical properties are particularly germane to urinary intestinal diversion: volume-pressure relationships and motor activity.
Volume-Pressure Considerations
The volume-pressure relationships depend on the configuration of the bowel. If one splits the bowel segment and turns it back on itself, the volume may be doubled if the ends are not closed (Fig. 85–41). In reconstruction of intestinal segments for the urinary tract, however, one must close the ends. Thus the limit of doubling the volume is never quite reached. Indeed, the greater the ratio of length to diameter, the greater the volume change when the ends are closed. If the ends are closed when a ratio of 1 : 3.5 diameter to length is reached, splitting the segment no longer increases the volume. By splitting most segments, one is, in fact, increasing the volume by about 50%. The goal in reconfiguring the bowel is to achieve a spherical storage vessel. This configuration has the most volume for the least surface area. By increasing the volume, it has been suggested that pressure relationships within the confines of the intestine are reduced. This is based on Laplace’s law, which states that for a sphere, the tension of its wall is proportional to the product of the radius and pressure. Thus theoretically, for a given wall tension, the greater the radius, the smaller the generated pressure. This is desirable in an attempt to prevent deterioration of the upper tracts or incontinence. This relationship (Laplace’s law), however, may not be accurately reflected for intestinal segments because they are not perfectly spherical, and the intestinal wall does not conform to Hooke’s law but rather demonstrates viscoelastic properties, which tend to distort the relationship between pressure applied at the wall and tension generated in it. In any event, it seems desirable to attempt to construct as spherical a container as possible if one is attempting to make a reservoir.
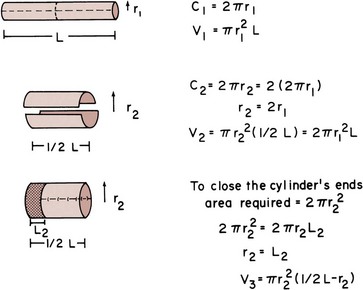
Figure 85–41 Effect of “detubularization.” The bowel is split on its antimesenteric border and divided in two. When the two segments are placed together, the circumference is doubled, thus doubling the volume. Closing the ends of the cylinder requires a reduction in its length equal to the radius of the end. This limits the increase in volume that occurs by reconfiguration.
Over time, the volume capacity of segments increases. This occurs only if they are frequently filled. Their volume decreases with time if they are nonfunctional (Kock et al, 1978). Over time, it can be demonstrated that there is a marked accommodation in volume of pouches made from intestine. For ileal pouches, it has been shown that the capacity increases sevenfold after 1 year (Berglund et al, 1987). As the reservoirs increase in volume, there is a significant increase in smooth muscle thickness of the bowel wall (Philipson et al, 1983).
Motor Activity
It has been suggested that splitting the bowel on its antimesenteric border discoordinates motor activity and thereby causes a lesser intraluminal pressure. Clearly, the ideal situation is to provide the patient with a spherical vessel that has few or ineffective contractions of its walls. It can be demonstrated in experimental animals that by splitting the bowel wall on its antimesenteric border and reconfiguring it, acutely there is a marked interruption of coordinated activity fronts, which during a period of 3 months return to their normal coordinated state (Concepcion et al, 1988). This has also been demonstrated clinically: Initially after reconfiguration of the bowel (detubularization), coordinated activity fronts have been shown to decrease. During extended periods, however, many of the peristaltic waves (activity fronts) reappear and can be readily demonstrated (Fig. 85–42).
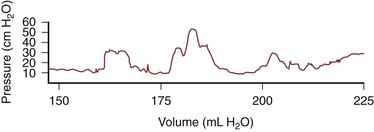
Figure 85–42 Pressure waves recorded 1 year postoperatively in a patient with a continent diversion constructed from detubularized ileum and right colon. Notice that the coordinated pressure waves are of magnitude and frequency similar to those found in a normal colonic or ileal segment.
The literature is contradictory with respect to the effect of detubularization on segments of ileum and colon used to construct storage vessels for continent diversions. Pressure within the lumen of bowel that has both ends closed may be increased by adding volume or reducing the size of the bowel through contractions of its wall. Because the bowel wall is freely permeable to water, the higher osmotic content of urine obligates movement of water into the bowel lumen. Most patients with continent diversions excrete 2 to 4 L/day (McDougal, 1986). In evaluating whether motor activity is the primary determinant of intravesical pressure, one must be cognizant of fluid volume changes. Also, as indicated previously, early reports of detubularized segments would be expected to differ from later reports when coordinated activity fronts in these segments return.
These facts are often forgotten, and because pressure measurements are used to infer motor activity, rather than its direct measurement as reflected by changes in bowel wall tension, it is not difficult to understand why there are so many contradictions reported in the literature. Detubularization of ileal segments has been reported by some to decrease motor activity at a year compared with immediately postoperatively (Berglund et al, 1987), whereas others have noted increased motor activity at 1 year. Involuntary pressure waves occur in 25% of patients with Kock pouches. Maximum intravesical pressures average 41 cm H2O in these pouches (Chen et al, 1989). Ileum has also been shown to have fewer activity fronts per unit of time than cecum (Berglund et al, 1986). Cecum has been observed to have the same number of activity fronts 1 year postoperatively, but the amplitude of the pressure waves has been observed to decrease over time (Hedlund et al, 1984). Maximum pressures in normal cecum have been shown to range from 18 to 100 cm H2O (Jakobsen et al, 1987), whereas detubularized cecum has been shown to have pressures that range between 5 and 25 cm H2O 1 year postoperatively (Hedlund et al, 1984). Others, comparing ileum to cecum, find no difference in pressure generated after a year (Hedlund et al, 1984). The Mainz pouch, which employs both ileum and cecum, has an average pressure at capacity of 39 cm H2O with a maximum pressure of 63 cm H2O (Thuroff et al, 1987). Thus reconfiguring bowel usually increases the volume, but its long-term effect on motor activity and wall tension is unclear at this time. It has been the authors’ observation that some patients with orthotopic bladders after a number of years of spontaneous voiding require intermittent catheterization. In these patients the bowel segment has become flaccid, and the ability of the patient to generate intraluminal pressure by a Valsalva maneuver is limited.
Key Points: Urinary Intestinal Diversion
Summary
This chapter has addressed complications both independent of and dependent on the specific type of urinary intestinal diversion. Each unique type of diversion has its own set of individual complications. Moreover, the procedure preceding the urinary intestinal diversion also has a set of complications that must be added to those described previously. It is clear that with current modalities of urinary intestinal diversion, long-term complications significantly contribute to mortality and morbidity. Many patients who have intestinal diversion after an extirpative procedure for cancer, however, die of the cancer rather than of these long-term complications. Those for whom a urinary intestinal diversion has been constructed for benign disease and those who are cured of cancer are most likely to experience long-term morbid complications. The knowledge of the frequency of these complications and the correct performance of preoperative preparation, surgical technique, and postoperative care, as outlined in this chapter, should provide the best chance for the least mortality and morbidity in patients undergoing urinary intestinal diversion.
Guenaga K, Matos D, Castro A, et al. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev 2005;CD001544.
Koch MO, McDougal WS, Hall MC, et al. Long-term effects of urinary diversion: a comparison of myelomeningocele patients managed by clean, intermittent catheterization and urinary diversion. J Urol. 1992;147:1343-1347.
Kristjansson A, Davidsson T, Mansson W. Metabolic alterations at different levels of renal function following continent urinary diversion through colonic segments. J Urol. 1997;157:2099-2103.
McDougal WS. Metabolic complications of urinary intestinal diversion. J Urol. 1992;147:1199-1208.
Nelson R, Edwards S, Tse B. Prophylactic nasogastric decompression after abdominal surgery. Cochrane Database Syst Rev 2005;CD004929.
Nichols RL, Condon RE, Gorback SL, Nyhus LM. Efficacy of preoperative antimicrobial preparation of the bowel. Ann Surg. 1972;176:227-232.
Ram E, Sherman Y, Weil R, et al. Is mechanical bowel preparation mandatory for elective colon surgery? A prospective randomized study. Arch Surg. 2005;140:285-288.
Tanrikut C, McDougal WS. Acid-base and electrolyte disorders after urinary diversion. World J Urol. 2004;22:168-171.
Aaronson IA, Constantinides CG, Sallie LP, Sinclair-Smith CC. Pathogenesis of adenocarcinoma complicating ureterosigmoidostomy: experimental observations. Urology. 1989;29:538-543.
Aaronson IA, Sinclair-Smith CC. Dysplasia of ureteric epithelium: a source of adenocarcinoma in ureterosigmoidostomy? Z Kinderchir. 1984;39:364-367.
Abdel-Azim MS, Abdel-Hakim AM. Gastrocystoplasty in patients with an areflexic low compliant bladder. Eur Urol. 2003;44:260-265.
Abol-Enein H, Ghoneim MA. A novel uretero-ileal reimplantation technique: the serous lined extramural tunnel. A preliminary report. J Urol. 1994;151:1193-1197.
Abol-Enein H, Ghoneim MA. Functional results of orthotopic ileal neobladder with serous-lined extramural ureteral reimplantation: experience with 450 patients. J Urol. 2001;165:1427-1432.
Abratt RP, Wilson JA, Pontin AR, Barnes RD. Salvage cystectomy after radical irradiation for bladder cancer: prognostic factors and complications. Br J Urol. 1993;72:756-760.
Adams MC, Mitchell ME, Rink RC. Gastrocystoplasty: an alternative solution to the problem of urological reconstruction in the severely compromised patient. J Urol. 1988;140:1152-1156.
Albert DJ, Persky L. Conjoined end-to-end uretero-intestinal anastomosis. J Urol. 1971;105:201-204.
Allen T, Peters PC, Sagalowsky A. The Camey procedure: preliminary results in 11 patients. World J Urol. 1985;3:167.
Althausen AF, Hagen-Cook K, Hendren WHIII. Nonrefluxing colon conduit: experience with 70 cases. J Urol. 1978;120:35-39.
Arabi Y, Dimock F, Burdon DW, et al. Influence of bowel preparation and antimicrobials on colonic microflora. Br J Surg. 1978;65:555-559.
Ashken MH. Urinary cecal reservoir. In: King LR, Stone AR, Webster GD, editors. Bladder reconstruction and continent urinary diversion. Chicago: Year Book; 1987:238-251.
Atan A, Konety BR, Nangia A, Chancellor MB. Advantages and risks of ileovesicostomy for the management of neuropathic bladder. Urology. 1999;54:636-640.
Austen M, Kalble T. Secondary malignancies in different forms of urinary diversion using isolated gut. J Urol. 2004;172:831-838.
Barbalias GA, Liatsikos EN, Karnabatidis D, et al. Ureteroileal anastomotic strictures: an innovative approach with metallic stents. J Urol. 1998;160:1270-1273.
Barqawi A, de Valdenebro M, Furness PD3rd, et al. Lessons learned from stomal complications in children with cutaneous catheterizable continent stomas. BJU Int. 2004;94:1344-1347.
Bartlett JG. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med. 2002;346:334-339.
Baum ML, Anish DS, Chalmers TC, et al. A survey of clinical trials of antibiotic prophylaxis in colon surgery: evidence against further use of no-treatment controls. N Engl J Med. 1981;305:795-799.
Beckley S, Wajsman Z, Pontes JE, Murphy G. Transverse colon conduit: a method of urinary diversion after pelvic irradiation. J Urol. 1982;128:464-468.
Beddoe AM, Boyce JG, Remy JC, et al. Stented versus nonstented transverse colon conduits: a comparative report. Gynecol Oncol. 1987;27:305-313.
Beloosesky Y, Grinblat J, Weiss A, et al. Electrolyte disorders following oral sodium phosphate administration for bowel cleansing in elderly patients. Arch Intern Med. 2003;163:803-808.
Benchekroun A. The ileocecal continent bladder. In: King LR, Stone AF, Webster GD, editors. Bladder reconstruction and continent urinary diversion. Chicago: Year Book; 1987:224-237.
Berglund B, Kock NG, Myrvold HE. Volume capacity and pressure characteristics of the continent cecal reservoir. Surg Gynecol Obstet. 1986;163:42-48.
Berglund B, Kock NG, Norlen L, Philipson BM. Volume capacity and pressure characteristics of the continent ileal reservoir used for urinary diversion. J Urol. 1987;137:29-34.
Bihrle R, Foster RS, Steidle CP, et al. Creation of a transverse colon-gastric composite reservoir: a new technique. J Urol. 1989;141:1217-1220.
Bissada NK, Keane T, Caczmarek AT, et al. Experience with coapted gastric tube outlet and composite gastrointestinal reservoir for continent cutaneous urinary diversion. J Urol. 2004;171:229-231.
Blaivas JG, Weiss JP, Desai P, et al. Long-term followup of augmentation enterocystoplasty and continent diversion in patients with benign disease. J Urol. 2005;173:1631-1634.
Bonnheim DC, Petrelli NJ, Steinberg A, Mittelman A. The pathophysiology of the jejunal conduit syndrome and its exacerbation by parenteral hyperalimentation. J Surg Oncol. 1984;26:172-175.
Borglund E, Nordstrom G, Nyman CR. Classification of peristomal skin changes in patients with urostomy. J Am Acad Dermatol. 1988;19:623-628.
Bowyer GW, Davies TW. Methotrexate toxicity associated with an ileal conduit. Br J Urol. 1986;60:592.
Boyd SD, Schiff WM, Skinner DG, et al. Prospective study of metabolic abnormalities in patients with continent Kock pouch urinary diversion. Urology. 1989;33:85-88.
Bracken RB, McDonald MW, Johnson DE. Cystectomy for superficial bladder cancer. Urology. 1981;18:459-463.
Bricker EM. Bladder substitution after pelvic evisceration. Surg Clin North Am. 1950;30:1511-1521.
Bucher P, Gervaz P, Soravia C, et al. Randomized clinical trial of mechanical bowel preparation versus no preparation before elective left-sided colorectal surgery. Br J Surg. 2005;92:409-414.
Campanello M, Herlitz H, Lindstedt G, et al. Bone mineral and related biochemical variables in patients with Kock ileal reservoir or Bricker conduit for urinary diversion. J Urol. 1996;155:1209-1213.
Canin-Endres J, Salky B, Gattorno F, et al. Laparoscopically assisted intestinal resection in 88 patients with Crohn’s disease. Surg Endosc. 1999;13:595-599.
Castro JE, Ram MD. Electrolyte imbalance following ileal urinary diversion. Br J Urol. 1970;42:29-32.
Catena F, La Donna M, Gagliardi S, et al. Stapled versus hand-sewn anastomoses in emergency intestinal surgery: results of a prospective randomized study. Surg Today. 2004;34:123-126.
Chang TW. Antibiotic-associated injury to the gut. In: Berk JE, editor. Gastroenterology. Philadelphia: WB Saunders; 1985:2585-2590.
Chassin JL, Rifkind KM, Sussman B, et al. The stapled gastrointestinal tract anastomosis: incidence of postoperative complications compared with the sutured anastomoses. Ann Surg. 1978;188:689-696.
Chavez DR, Snyder PM, Juravsky LI, Heaney JA. Recurrent ileal conduit hemorrhage in an elderly cirrhotic man. J Urol. 1994;152:951-953.
Checile G, Klein EA, Bauer L, et al. Functional equivalence of end and loop ileal conduit stomas. J Urol. 1992;147(3):582-586.
Chen KK, Chang LS, Chen MT. Urodynamic and clinical outcome of Kock pouch continent urinary diversion. J Urol. 1989;141:94-97.
Christensen PB, Kronborg O. Whole-gut irrigation versus enema in elective colorectal surgery: a prospective, randomized study. Dis Colon Rectum. 1981;24:592-595.
Clark PB. End-to-end ureteroileal anastomoses for ileal conduits. Br J Urol. 1979;51:105-109.
Clarke JS, Condon RE, Bartlett JG, et al. Preoperative oral antibiotics reduce septic complications of colon operations. Ann Surg. 1977;186:251-259.
Classen DC, Evans RS, Pestotnik SL, et al. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. N Engl J Med. 1992;326:281-282.
Clayman RV, Reddy P, Nivatvongs S. Acute pseudo-obstruction of the colon: a serious consequence of urologic surgery. J Urol. 1981;126:415-417.
Cohen MS, Hilz ME, Davis CP, Anderson MD. Urinary carcinogen (nitrosamine) production in a rat animal model for ureterosigmoidostomy. J Urol. 1987;138:449-452.
Concepcion RS, Koch MO, McDougal WS, Richards WO. Detubularized intestinal segments in urinary tract reconstruction: why do they work? Abstr Am Urol Assoc. 1988:592.
Cookson MS, Chang SS, Wells N, et al. Complications of radical cystectomy for nonmuscle invasive disease: comparison with muscle invasive disease. J Urol. 2003;169:101-104.
Cordonnier JJ. Ureterosigmoid anastomosis. J Urol. 1950;63:276-285.
Cornud F, Lefebvre JF, Chretien Y, et al. Percutaneous transrenal electro-incision of ureterointestinal anastomotic strictures: long-term results and comparison of fluoroscopic and endoscopic guidance. J Urol. 1996;155:1575-1578.
Costello AJ, Johnson DE. Modified autosuture technique for ileal conduit construction in urinary diversion. Aust N Z J Surg. 1984;54:477-482.
Davies BJ, Allareddy V, Konety BR. Effect of postcystectomy infectious complications on cost, length of stay, and mortality. Urology. 2009;73:598-602.
De Carli P, Micali S, O’Sullivan D, et al. Ureteral anastomosis in the orthotopic ileal neobladder: comparison of two techniques. J Urol. 1997;157:469-471.
DeFoor W, Minevich E, Reeves D, et al. Gastrocystoplasty: long-term followup. Urology. 2003;170:1647-1649.
De Ganck J, Everaert K, Van Laecke E, et al. A high easy-to-treat complication rate is the price for a continent stoma. BJU Int. 2002;90:240-243.
DeGiorgio R, Knowles CH. Acute colonic pseudo-obstruction. Br J Surg. 2009;96:229-239.
Delgado GE, Muecke EC. Evaluation of 80 cases of ileal conduits in children: indications, complications and results. J Urol. 1973;109:210.
Didolkar MS, Reed WP, Elias EG, et al. A prospective randomized study of sutured versus stapled bowel anastomoses in patients with cancer. Cancer. 1986;57:456-460.
DiMarco DS, LeRoy AJ, Thieling S, et al. Long-term results of treatment for ureteroenteric strictures. Urology. 2001;58:909-913.
Dion YM, Richards GK, Prentis JJ, Hinchey EJ. The influence of oral versus parenteral preoperative metronidazole on sepsis following colon surgery. Ann Surg. 1980;192:221-226.
Donat SM, Slaton JW, Pisters LL, Swanson DA. Early nasogastric tube removal combined with metoclopramide after radical cystectomy and urinary diversion. J Urol. 1999;162:1599-1602.
Dretler SP. The pathogenesis of urinary tract calculi occurring after conduit diversion: I. Clinical study; II. Conduit study; III. Prevention. J Urol. 1973;109:204-209.
Edwards RH. Hyperammonemic encephalopathy related to ureterosigmoidostomy. Arch Neurol. 1984;41:1211-1212.
Elder DD, Moisey CU, Rees RWM. A long-term follow-up of the colonic conduit operation in children. Br J Urol. 1979;51:462-465.
Emmott D, Noble MJ, Mebust WK. A comparison of end versus loop stomas for ileal conduit urinary diversion. J Urol. 1985;133:588-590.
Evans AJ, Manhire AR, Bishop MC. Duplex ureters: a pitfall during ileal conduit urinary diversion. Br J Urol. 1994;73:214-215.
Fa-Si-Oen PR, Verwaest C, Buitenweg J, et al. Effect of mechanical bowel preparation with polyethyleneglycol on bacterial contamination and wound infection in patients undergoing elective open colon surgery. Clin Microbiol Infect. 2005;11:158-160.
Fazio VW, Jagelman AG, Lavery IC, McGonagle BA. Evaluation of the Proximate-ILS circular stapler: a prospective study. Ann Surg. 1985;201:108-114.
Ferris DO, Odel HM. Electrolyte pattern of blood after ureterosigmoidostomy. JAMA. 1950;142:634-641.
Filmer RB. Malignant tumors arising in bladder augmentations and ileal and colon conduits. Soc Pediatr Urol Newslett. 1986. December 9
Flanigan RC, Kursh ED, Persky L. Thirteen year experience with ileal loop diversion in children with myelodysplasia. Am J Surg. 1975;130:535-538.
Fontaine E, Barthelemy Y, Houlgatte A, et al. Twenty-year experience with jejunal conduits. Urology. 1997;50:207-213.
Franks ME, Hrebinko RLJr. Technique of parastomal hernia repair using synthetic mesh. Urology. 2001;57:551-553.
Friedman RM, Flashner SC, King LR. Effectiveness of a handsewn nipple valve for reflux prevention in bladder reconstruction. J Urol. 1992;147:441-443.
Geist RW, Ansell JS. Total body potassium in patients after ureteroileostomy. Surg Gynecol Obstet. 1961;113:585-590.
Ghitulescu GA, Morin N, Jetty P, et al. Revisiting the biofragmentable anastomotic ring: is it safe in colonic surgery? Can J Surg. 2003;46:92-98.
Gill IS, Kaouk JH, Meraney AM, et al. Laparoscopic radical cystectomy and continent orthotopic ileal neobladder performed completely intracorporeally: the initial experience. J Urol. 2002;168:13-18.
Gill IS, Rackley RR, Meraney AM, et al. Laparoscopic enterocystoplasty. Urology. 2000;55:178-181.
Gill IS, Savage SJ, Senagore AJ, et al. Laparoscopic ileal ureter. J Urol. 2000;163:1199-1202.
Glassman DT, Docimo SG. Concealed umbilical stoma: long-term evaluation of stomal stenosis. J Urol. 2001;166:1028-1030.
Golimbu M, Morales P. Jejunal conduits: technique and complications. J Urol. 1975;113:787-795.
Gonzalez R, Reinberg Y. Localization of bacteriuria in patients with enterocystoplasty and nonrefluxing conduits. J Urol. 1987;138:1104-1105.
Goodwin WE, Harris AP, Kaufman JJ, Beal JM. Open, transcolonic ureterointestinal anastomosis. Surg Gynecol Obstet. 1953;97:295-330.
Gosalbez RJr, Woodard JR, Broecker BH, Warshaw B. Metabolic complications of the use of stomach for urinary reconstruction. J Urol. 1993;150:710-712.
Gottrup F, Diederich P, Sorensen K, et al. Prophylaxis with whole gut irrigation and antimicrobials in colorectal surgery: a prospective randomized double-blind clinical trial. Am J Surg. 1985;149:317-322.
Grocela JA, McDougal WS. Ammonia transport in the intestine chronically exposed to urine: is it reduced over time? Urology. 1999;54:373-376.
Grundel K, Schwenk W, Bohm B, Muller JM. Improvements in mechanical bowel preparation for elective colorectal surgery. Dis Colon Rectum. 1997;40:1348-1352.
Guenaga KF, Matos D, Castro AA, et al. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev 2003;CD001544.
Guenaga K, Matos D, Castro A, et al. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev 2005;CD001544.
Guilliotreau J, Game X, Mouzin M, et al. Radical cystectomy for bladder cancer: morbidity of laparoscopic versus open surgery. J Urol. 2009;181:554-559.
Guinan PD, Moore RH, Neter E, Murphy GP. The bacteriology of ileal conduit urine in man. Surg Gynecol Obstet. 1972;134:78-82.
Hagen-Cook K, Althausen AF. Early observations on 31 adults with nonrefluxing colon conduits. J Urol. 1979;121:13-16.
Hares MM, Alexander-Williams J. The effect of bowel preparation on colonic surgery. World J Surg. 1982;6:175-181.
Harrison AR. Clinical and metabolic observations on osteomalacia following ureterosigmoidostomy. Br J Urol. 1958;30:455-461.
Hasan ST, Coorsh J, Tapson JS. Use of bendrofluazide in the management of recurrent jejunal conduit syndrome. Br J Urol. 1994;73:101-102.
Hassain M. The osteomalacia syndrome after colocystoplasty: a cure with sodium bicarbonate alone. Br J Urol. 1970;42:243-245.
Hautmann RE, Egghart G, Frohneberg D, Miller K. The ileal neobladder. J Urol. 1988;139:39-42.
Hayashi T, Ikai K, Kiriyama T, et al. Percutaneous intrapelvic pressure registration in patients with ureterointestinal urinary diversion. Urology. 1986;28:176-178.
Hedlund H, Lindstrom K, Mansson W. Dynamics of a continent caecal reservoir for urinary diversion. Br J Urol. 1984;56:366-372.
Heidler H, Marberger M, Hohenfellner R. The metabolic situation in ureterosigmoidostomy. Eur Urol. 1979;5:39-44.
Hensle TW, Burbige KA. Bladder replacement in children and young adults. J Urol. 1985;133:1004-1010.
Herget-Rosenthal S, Marggraf G, Hüsing J, et al. Early detection of acute renal failure by serum cystatin C. Kidney Int. 2004;66:1115-1122.
Hill JT, Ransley PG. The colonic conduit: a better method of urinary diversion? Br J Urol. 1983;55:629-631.
Hirdes WH, Hoekstra I, Vlietsra HP. Hammock anastomoses: a nonrefluxing ureteroileal anastomosis. J Urol. 1988;139:517-518.
Ho KM, Fawcett DP. Parastomal hernia repair using the lateral approach. BJU Int. 2004;94:598-602.
Hohenfellner R, Black P, Leissner J, Allhoff EP. Refluxing ureterointetinal anastomosis for continent cutaneous urinary diversion. J Urol. 2002;168:1013-1017.
Husmann DA, McLorie GA, Churchill BM. Nonrefluxing colonic conduits: a long-term life-table analysis. J Urol. 1989;142:1201-1203.
Iannoni C, Marcheggiano A, Pallone F, et al. Abnormal patterns of colorectal mucin secretion after urinary diversion of different types: histochemical and lectin binding studies. Hum Pathol. 1986;17:834-840.
Incel N, Inc NA, Uygur MC, et al. Effect of Stanford pouch and ileal conduit urinary diversions on bone density and metabolism. Int Urol Nephrol. 2006;38:447-451.
Irvin TT, Goligher JC. Aetiology of disruption of intestinal anastomosis. Br J Surg. 1973;60:461.
Jacobs JA, Young JDJr. The Strickler technique of ureterosigmoidostomy. J Urol. 1980;124:451-454.
Jaffe BM, Bricker EM, Butcher HRJr. Surgical complications of ileal segment urinary diversion. Ann Surg. 1968;167:367-376.
Jakobsen H, Steven K, Stigsby B, et al. Pathogenesis of nocturnal urinary incontinence after ileocaecal bladder replacement: continuous measurement of urethral closure pressure during sleep. Br J Urol. 1987;59:148-152.
Jex RK, van Heerden JA, Wolff BG, et al. Gastrointestinal anastomoses: factors affecting early complications. Ann Surg. 1987;206:138-141.
Kaidar-Persno O, Rosenthal RJ, Wexner SD, et al. Compression anastomosis: history and clinical considerations. Am J Surg. 2008;195:818-826.
Kamizaki H, Cass AS. Conduit and renal pelvic pressure after ileal and colonic urinary diversion in dogs. Invest Urol. 1978;16:27-32.
Kaufman JJ. Ammoniagenic coma following ureterosigmoidostomy. J Urol. 1984;131:743-745.
Kawakita M, Arai Y, Shigeno C, et al. Bone demineralization following urinary intestinal diversion assessed by urinary pyridinium cross-links and dual energy x-ray absorptiometry. J Urol. 1996;156:355-359.
King LR. Protection of the upper tracts in undiversion. In: King LR, Stone AF, Webster GD, editors. Bladder reconstruction and continent urinary diversion. Chicago: Year Book; 1987:127-153.
Kinn A, Lantz B. Vitamin B12 deficiency after irradiation for bladder carcinoma. J Urol. 1984;131:888-890.
Klein EA, Montie JE, Montague DK, et al. Jejunal conduit urinary diversion. J Urol. 1986;135:244-246.
Koch MO, Gurevitch E, Hill DE, McDougal WS. Urinary solute transport by intestinal segments: a comparative study of ileum and colon in rats. J Urol. 1990;143:1275-1279.
Koch MO, McDougal WS. Determination of renal function following urinary diversion through intestinal segments. J Urol. 1985;133:517-520.
Koch MO, McDougal WS. Bone demineralization following ureterosigmoid anastomosis: an experimental study in rats. J Urol. 1988;140:856-859.
Koch MO, McDougal WS, Hall MC, et al. Long-term effects of urinary diversion: a comparison of myelomeningocele patients managed by clean, intermittent catheterization and urinary diversion. J Urol. 1992;147:1343-1347.
Kock NG. The development of the continent ileal reservoir (Kock pouch), an application in patients requiring urinary diversion. In: King LR, Stone AF, Webster GD, editors. Bladder reconstruction and continent urinary diversion. Chicago: Year Book; 1987:269-290.
Kock NG, Nilson AE, Nilsson LO, et al. Urinary diversion via a continent ileal reservoir: clinical results in 12 patients. J Urol. 1982;128:469-475.
Kock NG, Nilson AE, Norlen L, et al. Changes in renal parenchyma and the upper urinary tract following urinary diversion via a continent ileum reservoir: an experimental study in dogs. Scand J Urol Nephrol. 1978;49(Suppl.):11-22.
Koff SA. Mechanisms of electrolyte imbalance following urointestinal anastomoses. Urology. 1975;5:109-114.
Kouba E, Sands M, Lentz A, et al. A comparison of Bricker versus Wallae ureteroileal anastomosis in patients undergoing urinary diversion for bladder cancer. J Urol. 2007;178:945-949.
Kozlowski PM, Wang PC, Winfield HN. Laparoscopic repair of incisional and parastomal hernias after major genitourinary or abdominal surgery. J Endourol. 2001;15:175-179.
Kramolowsky EV, Clayman RV, Weyman PJ. Endourological management of ureteroileal anastomotic strictures: is it effective? J Urol. 1987;137:390-394.
Kramolowsky EV, Clayman RV, Weyman PJ. Management of ureterointestinal anastomotic strictures: comparison of open surgical and endourological repair. J Urol. 1988;139:1195-1198.
Kristjansson A, Davidsson T, Mansson W. Metabolic alterations at different levels of renal function following continent urinary diversion through colonic segments. J Urol. 1997;157:2099-2103.
Kurzrock EA, Baskin LS, Kogan BA. Gatrocystoplasty: long-term follow up. J Urol. 1998;160:2182-2186.
Kuss R, Bitker M, Camey M, et al. Indications and early and late results of intestino-cystoplasty: a review of 185 cases. J Urol. 1970;103:53-63.
Lashley DB, Saxon RR, Fuchs EF, et al. Bleeding ileal conduit stomal varices: diagnoses and management using transjugular transhepatic angiography and embolization. Urology. 1997;50:612-614.
Laven BA, O’Connor RC, Steinberg GD, Gerber GS. Long-term results of antegrade endoureterotomy using the holmium laser in patients with ureterointestinal strictures. Urology. 2001;58:924-929.
Leadbetter WF, Clarke BG. Five years’ experience with uretero-enterostomy by the “combined” technique. J Urol. 1954;73:67-82.
Le Duc A, Camey M, Teillac P. An original antireflux ureteroileal implantation technique: long-term follow-up. J Urol. 1987;137:1156-1158.
Leong CH. Use of stomach for bladder replacement and urinary diversion. Ann R Coll Surg Engl. 1978;60:283-289.
Leslie A, Steele RJ. The interrupted serosubmucosal anastomosis—still the gold standard. Colorectal Dis. 2003;5:362-366.
Lewis RT. Oral versus systemic antibiotic prophylaxis in elective colon surgery: a randomized study and meta-analysis send a message from the 1990s. Can J Surg. 2002;45:173-180.
Lim STK, Lam SK, Lee NW, et al. Effects of gastrocystoplasty on serum gastrin level and gastric acid secretion. Br J Surg. 1983;70:275-277.
Lockhart JL, Bejany DE. Antireflux ureteroileal reimplantation: an alternative for urinary diversion. J Urol. 1987;137:867-870.
Loening SA, Navarre RJ, Narayana AS, Culp DA. Transverse colon conduit urinary diversion. J Urol. 1982;127:37-39.
Madersbacher S, Schmidt J, Eberle JM, et al. Long-term outcome of ileal conduit diversion. J Urol. 2003;169:985-990.
Maffezzini M, Campodonico F, Canepa G, et al. Current perioperative management of radical cystectomy with intestinal urinary reconstruction for muscle-invasive bladder cancer and reduction of the incidence of postoperative ileus. Surg Oncol. 2008;17:41-48.
Magnus RV. Pressure studies and dynamics of ileal conduits in children. J Urol. 1977;118:406-407.
Malek RS, Burke EC, DeWeerd JH. Ileal conduit urinary diversion in children. J Urol. 1971;105:892-900.
Mansson W, Colleen S, Forsberg L, et al. Renal function after urinary diversion: a study of continent caecal reservoir, ileal conduit, and colonic conduit. Scand J Urol Nephrol. 1984;18:307-315.
Mansson W, Colleen S, Stigsson L. Four methods of uretero-intestinal anastomoses in urinary conduit diversion. Scand J Urol Nephrol. 1979;13:191-199.
Mansson W, Davidsson T, Konyves J, et al. Continent urinary tract reconstruction—the Lund experience. BJU Int. 2003;92:271-276.
Markowitz GS, Stokes MB, Radhakrishnan J, D’Agati VD. Acute phosphate nephropathy following oral sodium phosphate bowel purgative: an underrecognized cause of chronic renal failure. J Am Soc Nephrol. 2005;16:3389-3396.
Matsuura T, Tsujihashi H, Park YC, et al. Assessment of the long-term results of ileocecal conduit urinary diversion. Urol Int. 1991;46:154-158.
Mattei A, Birkhaeuser FD, Baermann C, et al. To stent or not to stent perioperatively the ureteroileal anastomosis of ileal orthotopic bladder substitutes and ileal conduits? Results of a prospective randomized trial. J Urol. 2008;179:582-586.
McDermott WVJr. Diversion of urine to the intestines as a factor in ammoniagenic coma. N Engl J Med. 1957;256:460-462.
McDougal WS. Bladder reconstruction following cystectomy by ureteroileo-colourethrostomy. J Urol. 1986;135:698-701.
McDougal WS. Metabolic complications of urinary intestinal diversion. J Urol. 1992;147:1199-1208.
McDougal WS, Koch MO. Accurate determination of renal function in patients with intestinal urinary diversion. J Urol. 1986;135:1175-1178.
McDougal WS, Koch MO. Effect of sulfate on calcium and magnesium homeostasis following urinary diversion. Kidney. 1989;35:105-115.
McDougal WS, Koch MO, Flora MD. Ammonium metabolism in urinary intestinal diversion. Abstr Am Assoc Genitourin Surg. 1989:45.
McDougal WS, Koch MO, Shands CIII, Price RR. Bony demineralization following urinary intestinal diversion. J Urol. 1988;140:853-855.
McDougal WS, Stampfer DS, Kirley S, et al. Intestinal ammonium transport by ammonium hydrogen exchange. J Am Coll Surg. 1995;181:241-248.
Medina CA, Caridi JG, Wajsman Z. Massive bleeding from ileal conduit peristomal varices: successful treatment with the transjugular intrahepatic portosystemic shunt. J Urol. 1998;159:200-201.
Menon M, Yu GW, Jeffs RD. Technique for antirefluxing ureterocolonic anastomosis. J Urol. 1982;127:236-237.
Mesrobian HJ, Kelalis PP, Kramer SA. Long-term follow-up of 103 patients with bladder exstrophy. J Urol. 1988;139:719-722.
Middleton AWJr, Hendren WH. Ileal conduits in children at the Massachusetts General Hospital from 1955 to 1970. J Urol. 1976;115:591-595.
Mounger EJ, Branson AD. Ammonia encephalopathy secondary to ureterosigmoidostomy: a case report. J Urol. 1972;108:411-412.
Mutchnik SE, Hinson JL, Nickell KG, Boone TB. Ileovesicostomy as an alternative form of bladder management in tetraplegic patients. Urology. 1997;49:353-357.
N’Dow J, Leung HY, Marshall C, Neal DE. Bowel dysfunction after bladder reconstruction. J Urol. 1998;159:1470-1475.
N’Dow J, Pearson J, Neal D. Mucus production after transposition of intestinal segments into the urinary tract. World J Urol. 2004;22:178-185.
Nelson R, Edwards S, Tse B. Prophylactic nasogastric decompression after abdominal surgery. Cochrane Database Syst Rev 2005;CD004929.
Nesbit RM. Ureterosigmoid anastomosis by direct elliptical connection: a preliminary report. J Urol. 1949;61:728-734.
Nguyen DH, Reinberg Y, Gonzalez R, et al. Outcome of renal transplantation after urinary diversion and enterocystoplasty: a retrospective controlled study. J Urol. 1990;144:1349-1351.
Nichols RL, Condon RE, Gorback SL, Nyhus LM. Efficacy of preoperative antimicrobial preparation of the bowel. Ann Surg. 1972;176:227-232.
Nieuwenhuijzen JA, deVries RR, van der Poel HG, et al. Urinary diversions after cystectomy: the association of clinical factors, complications and functional results of four different diversions. Eur Urol. 2008;53:834-842.
Oliveira L, Wexner SD, Daniel N, et al. Mechanical bowel preparation for elective colorectal surgery. A prospective, randomized, surgeon-blinded trial comparing sodium phosphate and polyethylene glycol-based oral lavage solutions. Dis Colon Rectum. 1997;40:585-591.
Onwubalili JK. Overt diabetes mellitus without glycosuria in a patient with cutaneous ureteroileostomy. BMJ. 1982;284:1836-1837.
Pagano F. Ureterocolonic anastomoses: description of a technique. J Urol. 1980;123:355-356.
Pagano F, Cosciani-Cunico S, Dal Bianco M, Zattoni F. Five years of experience with a modified technique of ureterocolonic anastomosis. J Urol. 1984;132:17-18.
Rosenbaum DH, Cain MP, Kaefer M, et al. Ileal enterocystoplasty and B12 deficiency in pediatric patients. J Urol. 2008;179:1544-1548.
Palascak P, Bouchareb M, Zachoval R, et al. Treatment of benign ureterointestinal anastomotic strictures with permanent ureteral Wallstent after Camey and Wallace urinary diversion: long-term follow-up. J Endourol. 2001;15:575-580.
Pantuck AJ, Han KR, Perrotti M, et al. Ureteroenteric anastomosis in continent urinary diversion: long-term results and complications of direct versus nonrefluxing techniques. J Urol. 2000;163:450-455.
Park HK, Kwak C, Byun SS, et al. Early removal of nasogastric tube after cystectomy with urinary diversion: does postoperative ileus risk increase? Urology. 2005;65:905-908.
Patil U, Glassberg KI, Waterhouse K. Ileal conduit surgery with nippled ureteroileal anastomoses. Urology. 1976;7:594-597.
Perez-Fidalgo JA, Chirivella Gonzalez I, Gunther S, et al. Hyperammonaemic encephalopathy, possible complication after urinary diversion in radical cystectomy. Review of the literature with regard to clinical case. Actas Urol Esp. 2007;31:394-399.
Perry W, Allen LN, Stamp TCB, Walker PG. Vitamin D resistance in osteomalacia after ureterosigmoidostomy. N Engl J Med. 1977;297:1110-1112.
Philipson BM, Kock NG, Jagenburg R, et al. Functional and structural studies of ileal reservoir used for continent urostomy and ileostomy. Gut. 1983;24:392-398.
Pitts WRJr, Muecke EC. A 20-year experience with ileal conduits: the fate of the kidneys. J Urol. 1979;122:154-157.
Potter SR, Charambura TC, Adams JB2nd, et al. Laparoscopic ileal conduit: five-year follow-up. Urology. 2000;56:22-25.
Poulakis V, Witzsch U, De Vries R, Becht E. Cold-knife endoureterotomy for nonmalignant ureterointestinal anastomotic strictures. Urology. 2003;61:512-517.
Ravi R, Dewan AK, Pandey KK. Transverse colon conduit urinary diversion in patients treated with very high dose pelvic irradiation. Br J Urol. 1994;73:51-54.
Reddy BS, MacFie J, Gatt M, et al. Randomized clinical trial of effect of synbiotics, neomycin and mechanical bowel preparation on intestinal barrier function in patients undergoing colectomy. Br J Surg. 2007;94:546-554.
Regan JB, Barrett DM. Stented versus nonstented ureteroileal anastomoses: is there a difference with regard to leak and stricture? J Urol. 1985;134:1101-1103.
Reinberg Y, Manivel JC, Froemming C, Gonzalez R. Perforation of the gastric segment of an augmented bladder secondary to peptic ulcer disease. J Urol. 1992;148:369-371.
Richards P, Chamerlain MJ, Wrong OM. Treatment of osteomalacia of renal tubular acidosis by sodium bicarbonate alone. Lancet. 1972;2:994-997.
Richie JP. Intestinal loop urinary diversion in children. J Urol. 1974;111:687-689.
Richie JP, Skinner DG. Urinary diversion: the physiological rationale for nonrefluxing colonic conduits. Br J Urol. 1975;47:269-275.
Riddick ACP, Turner WH, Mills RD. Bowel function after urinary diversion. World J Urol. 2004;22:210-214.
Rothenberg SS. Laparoscopic segmental intestinal resection. Semin Pediatr Surg. 2002;11:211-216.
Safadi B. Laparoscopic repair of parastomal hernias: early results. Surg Endosc. 2004;18:676-680.
Salahudeen AK, Elliott RW, Ellis HA. Osteomalacia due to ileal replacement of ureters: report of two cases. J Urol. 1984;131:335-337.
Samuel JD, Bhatt RI, Montague RJ, et al. The natural history of postoperative renal function in patients undergoing ileal conduit diversion for cancer measured using serial isotopic glomerular filtration rate and 99mtechnetium-mercaptoacetyltriglycine renography. J Urol. 2006;176:2518-2522.
Savarirayan F, Dixey GM. Syncope following ureterosigmoidostomy. J Urol. 1969;101:844-845.
Schipper H, Decter A. Carcinoma of the colon arising at ureteral implant sites despite early external diversion: pathogenetic and clinical implications. Cancer. 1981;47:2062-2065.
Schmidt JD, Bucksbaum HJ, Nachtsheim DA. Long-term follow-up, further experience with and modifications of the transverse colon conduit in urinary tract diversion. Br J Urol. 1985;57:284-288.
Schmidt JD, Hawtrey CE, Flocks RH, Culp DA. Complications, results, and problems of ileal conduit diversions. J Urol. 1973;109:210-216.
Schwaibold H, Friedrich MG, Fernandez S, et al. Improvement of ureteroileal anastomosis in continent urinary diversion with modified LeDuc procedure. J Urol. 1998;160:718-720.
Schwarz GR, Jeffs RD. Ileal conduit urinary diversion in children: computer analysis of follow-up from 2 to 16 years. J Urol. 1975;114:285-288.
Seinela L, Pehkonen E, Laasanen T, et al. Bowel preparation for colonoscopy in very old patients: a randomized prospective trial comparing oral sodium phosphate and polyethylene glycol electrolyte lavage solution. Scand J Gastroenterol. 2003;38:216-220.
Sener SF, Imperato JP, Blum MD, et al. Technique and complications of reconstruction of the pelvic floor with polyglactin mesh. Surg Gynecol Obstet. 1989;168:475-480.
Shands CIII, McDougal WS, Wright EP. Prevention of cancer at the urothelial enteric anastomotic site. J Urol. 1989;141:178-181.
Shapiro SR, Lebowitz R, Colodny AH. Fate of 90 children with ileal conduit urinary diversions a decade later: analysis of complications, pyelography, renal function, and bacteriology. J Urol. 1975;114:289-295.
Siklos P, Davie M, Jung RJ, Chalmers TM. Osteomalacia in ureterosigmoidostomy: healing by correction of the acidosis. Br J Urol. 1980;52:61-62.
Silberman R. Ammonia intoxication following ureterosigmoidostomy in a patient with liver disease. Lancet. 1958;2:937-939.
Skinner DG, Lieskovsky G, Boyd S. Continent urinary diversion. J Urol. 1989;141:1323-1327.
Slim K, Vicaut E, Launay-Savary M-V, et al. Updated systematic review and meta-analysis of randomized clinical trials on the role of mechanical bowel preparation before colorectal surgery. Ann Surg. 2009;249:203-2009.
Slim K, Vicaut E, Panis Y, et al. Meta-analysis of randomized clinical trials of colorectal surgery with or without mechanical bowel preparation. Br J Surg. 2004;91:1125-1130.
Smith ED. Follow-up studies on 150 ileal conduits in children. J Pediatr Surg. 1972;7:1-10.
Specht EE. Rickets following ureterosigmoidostomy and chronic hyperchloremia. J Bone Joint Surg. 1967;49:1422-1430.
Srinivas S, Mahalati K, Freiha FS. Methotrexate tolerance in patients with ileal conduits and continent diversions. Cancer. 1998;82:1134-1136.
Starr A, Rose DH, Cooper JF. Antireflux ureteroileal anastomosis in humans. J Urol. 1975;113:170-174.
Stein JP, Dunn MD, Quek ML, et al. The orthotopic T pouch ileal neobladder: experience with 209 patients. J Urol. 2004;172:584-587.
Stein JP, Freeman JA, Esrig D, et al. Complications of the afferent antireflux valve mechanism in the Kock ileal reservoir. J Urol. 1996;155:1579-1584.
Stein JP, Skinner DG. The craft of urologic surgery: the T pouch. Urol Clin North Am. 2003;30:647-661.
Stein R, Fisch M, Andreas J, et al. Whole-body potassium and bone mineral density up to 30 years after urinary diversion. Br J Urol. 1998;82:798-803.
Stein R, Fisch M, Stockle M, et al. Colonic conduit in children: protection of the upper urinary tract 16 years later? J Urol. 1996;156:1146-1150.
Stephenson AJ, Gillis IS. Laparoscopic radical cystectomy for muscle-invasive bladder cancer: pathological and oncological outcomes. BJU Int. 2008;102:1296-1301.
Stewart M, Macrae FA, Williams CB. Neoplasia and ureterosigmoidostomy: a colonoscopy survey. Br J Surg. 1982;69:414-416.
Strickler WL. A modification of the combined uretero-sigmoidostomy. J Urol. 1965;93:370-373.
Studer UE, Burkland FC, Schumacher TM, et al. Twenty years experience with an ileal orthotopic low pressure bladder substitute—lessons to be learned. J Urol. 2006;176:161-166.
Sullivan JW, Grabstald H, Whitmore WFJr. Complications of ureteroileal conduit with radical cystectomy: review of 336 cases. J Urol. 1980;124:797-801.
Tainio H, Kylmala T, Tammela TL. Ulcer perforation in gastric urinary conduit: never use a gastric segment in the urinary tract if there are other options available. Urol Int. 2000;64:101-102.
Tanrikut C, McDougal WS. Acid-base and electrolyte disorders after urinary diversion. World J Urol. 2004;22:168-171.
Tapper D, Folkman J. Lymphoid depletion in ileal loops: mechanism and clinical implication. J Pediatr Surg. 1976;11:871-880.
Thuroff JW, Alken P, Hohenfellner R. The MAINZ pouch (mixed augmentation with ileum and cecum) for bladder augmentation and continent diversion. In: King LR, Stone AF, Webster GD, editors. Bladder reconstruction and continent urinary diversion. Chicago: Year Book; 1987:252.
Tiffany P, Vaughan ED, Marion D, Amberson J. Hypergastrinemia following antral gastrocystoplasty. J Urol. 1986;136:692.
Tjandra JJ, Tagkalidis P. Carbohydrate-electrolyte (E-Lyte) solution enhances bowel preparation with oral fleet phosphor-soda. Dis Colon Rectum. 2004;47:1181-1186.
Tuggle DW, Hoelzer DJ, Tunell WP, Smith EI. The safety and cost-effectiveness of polyethylene glycol electrolyte solution bowel preparation in infants and children. J Pediatr Surg. 1987;22:513-515.
Turkolmez K, Baltaci S, Gogus C, et al. Results of the ureteral reimplantation with serous-lined extramural tunnel in orthotopic ileal W-neobladder. Int J Urol. 2004;11:368-373.
Turner-Warwick RT, Ashken MH. The functional results of partial, subtotal, and total cystoplasty with special reference to ureterocaecocystoplasty, selective sphincterotomy and cystocystoplasty. Br J Urol. 1967;39:3-12.
van den Bogaard AE, Weidema W, Hazen MJ, Wesdorp RI. A bacteriological evaluation of three methods of bowel preparation for elective colorectal surgery. Antonie Van Leeuwenhoek. 1981;47:86-88.
Varkarakis IM, Chrisofos M, Antoniou N, et al. Evaluation of findings during re-exploration for obstructive ileus after radical cystectomy and ileal-loop urinary diversion: insight into potential technical improvements. BJU Int. 2006;99:893-897.
Waidelich R, Rink F, Kriegmair M, et al. A study of reflux in patients with an ileal orthotopic bladder. Br J Urol. 1998;81:241-246.
Wallace DM. Uretero-ileostomy. Br J Urol. 1970;42:529-534.
Washington JA, Dearing WH, Judd ES, Elveback LR. Effect of preoperative antibiotic regimen in development of infection after intestinal surgery: prospective, randomized double-blind study. Ann Surg. 1974;180:567-572.
Weber TR, Westfall SH, Steinhardt GF, et al. Malignancy associated with ureterosigmoidostomy: detection by ornithine decarboxylase. J Pediatr Surg. 1988;23:1091-1094.
Wendel RG, Henning DC, Evans AT. End-to-end ureteroileal anastomosis for iliac conduits: preliminary report. J Urol. 1969;102:42-43.
Whitmore WFIII, Gittes RF. Reconstruction of the urinary tract by cecal and ileocecal cystoplasty: review of a 15 year experience. J Urol. 1983;129:494-498.
Wiesner C, Stein R, Pahernik S, et al. Long-term followup of the intussuscepted ileal nipple and the in situ, submucosally embedded appendix as continence mechanisms of continent urinary diversion with the cutaneous ileocecal pouch (Mainz Pouch I). J Urol. 2006;176:155-160.
Wille-Jorgensen P, Guenaga KF, Castro AA, et al. Clinical value of preoperative mechanical bowel cleansing in elective colorectal surgery: a systematic review. Dis Colon Rectum. 2003;46:1013-1020.
Williams RE, Davenport TJ, Burkinshaw L, Hughes D. Changes in whole body potassium associated with uretero-intestinal anastomoses. Br J Urol. 1967;39:676-680.
Wolff BG, Beart RWJr, Dozois RR, et al. A new bowel preparation for elective colon and rectal surgery: a prospective, randomized clinical trial. Arch Surg. 1988;123:895-900.
Woodhouse CRJ, Robertson WG. Urolithiasis in electrocystoplasties. World J Urol. 2004;22:215-221.
Wren SM, Ahmed N, Jamal A, et al. Preoperative oral antibiotics in colorectal surgery increase the rate of Clostridium difficile colitis. Arch Surg. 2005;140:752-756.
Wullt B, Agace W, Mansson W. Bladder, bowel and bugs—bacteriuria in patients with intestinal urinary diversion. World J Urol. 2004;22:186-195.
Zabbo A, Kay R. Ureterosigmoidostomy and bladder exstrophy: a long-term follow-up. J Urol. 1986;136:396-398.