chapter 91 Benign Prostatic Hyperplasia
Etiology, Pathophysiology, Epidemiology, and Natural History
Benign prostatic hyperplasia (BPH) is a pathologic process that contributes to, but is not the sole cause of, lower urinary tract symptoms (LUTS) in aging men. Despite intense research efforts in the past 5 decades to elucidate the underlying etiology of prostatic growth in older men, cause-and-effect relationships have not been established. For example, androgens are a necessary but not a clearly causative aspect of BPH. Previously held ideas that the clinical symptoms of BPH (prostatism) are simply due to a mass-related increase in urethral resistance are too simplistic. It is now clear that a significant portion of LUTS is due to age-related detrusor dysfunction and other conditions such as polyuria, sleep disorders, and a variety of systemic medical conditions unrelated to the prostate-bladder unit.
Historically, voiding symptoms have been related to obstruction of the bladder outlet (Chapple et al, 2008). The traditional association in men is with the prostate, the so-called symptoms of “prostatism.” However, it is well recognized that voiding symptoms poorly correlate with underlying pathophysiology (de la Rosette et al, 1998). Similar symptoms can also be produced by any other form of obstruction, such as a urethral stricture or, conversely, by poor function of the lower urinary tract in circumstances in which there is impaired detrusor contractility. This has led to the recognition that, although LUTS commonly may be related to bladder outlet obstruction (BOO) as a result of benign prostatic obstruction (BPO), which is often associated with benign prostatic enlargement (BPE) resulting from the histologic condition of BPH, this is not invariably the case. For example, women also commonly present with voiding symptoms (Irwin et al, 2006). Failure to empty can be related to an outlet obstruction or to detrusor underactivity of the bladder or to a combination of both. Postmicturition symptoms, such as postvoid dribbling, occur in both sexes but most often in men, in whom these symptoms are highly common, are very troublesome, and cause significant interference with quality of life (Reynard et al, 1996). Storage symptoms are currently largely encompassed by the term overactive bladder (OAB) syndrome, which is defined as urgency, frequency, nocturia, and urgency incontinence and which is believed to be correlated with an underlying detrusor overactivity (Abrams et al, 2003). These symptoms tend to be more bothersome than voiding symptoms, especially if they are associated with incontinence. Storage symptoms in both sexes are commonly associated with urinary infections or, more rarely, with other conditions, such as bladder stones, carcinoma, or carcinoma in-situ in the bladder.
This understanding can be shown as partially overlapping populations (Fig. 91–1). Whereas many men older than the age of 40 will develop histologic hyperplasia (i.e., BPH), not all will have bothersome LUTS. Of those, some will and others will not develop measurable BPE. It is common for men to have BPE without having LUTS and vice versa. BOO may also be present with or without LUTS and with or without BPE; and in some cases BOO exists in men with BPH (e.g., from stricture) (Roehrborn, 2008).
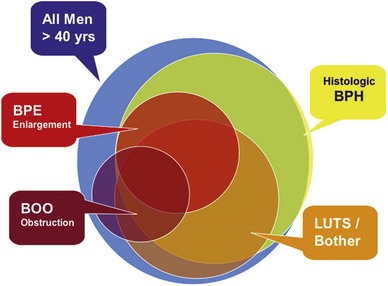
Figure 91–1 Diagram showing the relationship between histologic hyperplasia of the prostate (BPH), lower urinary tract symptoms (LUTS), benign prostate enlargement (BPE), and bladder outlet obstruction (BOO). The size of the circles does not represent actual proportions but rather illustrates the partial overlap between the different disease definitions.
(From Roehrborn CG. Pathology of benign prostatic hyperplasia. Int J Impot Res 2008;20[Suppl. 3]:S11–8.)
Undoubtedly the constellation of cellular pathologic processes that give rise to the symptoms of LUTS is far more complex than we currently realize. Only by unraveling these complexities, however, will we be able to design alternative strategies to treat successfully and possibly prevent the adverse impact of BPH on lower urinary tract function.
Etiology
Histopathologically BPH is characterized by an increased number of epithelial and stromal cells in the periurethral area of the prostate and thus correctly referred to as hyperplasia and not hypertrophy, a term often found in the older literature. The observation of new epithelial gland formation is normally seen only in fetal development and gives rise to the concept of embryonic reawakening of the stroma cell’s inductive potential (Cunha et al, 1983; Isaacs, 2008). The precise molecular etiology of this hyperplastic process is uncertain. The observed increase in cell number may be due to epithelial and stromal proliferation or to impaired programmed cell death leading to cellular accumulation. Androgens, estrogens, stromal-epithelial interactions, growth factors, and neurotransmitters may play a role, either singly or in combination, in the etiology of the hyperplastic process.
Hyperplasia
In a given organ, the number of cells, and thus the volume of the organ, is dependent on the equilibrium between cell proliferation and cell death (Isaacs and Coffey, 1987). An organ can enlarge not only by an increase in cell proliferation but also by a decrease in cell death. Although androgens and growth factors stimulate cell proliferation in experimental models, the relative role of cell proliferation in human BPH is questioned because there is no clear evidence of an active proliferative process. Although it is possible that the early phases of BPH are associated with a rapid proliferation of cells, the established disease appears to be maintained in the presence of an equal or reduced rate of cell replication. Increased expression of antiapoptotic pathway genes (e.g., bcl2) supports this hypothesis (Kyprianou et al, 1996; Colombel et al, 1998) Androgens not only are required for normal cell proliferation and differentiation in the prostate but also actively inhibit cell death (Isaacs, 1984). In the dog, experimental BPH can be produced by androgens combined with estradiol (Walsh and Wilson, 1976; DeKlerk et al, 1979; Berry et al, 1986a; Juniewicz et al, 1994). Despite a significant increase in gland size there is actually a reduction in the rate of DNA synthesis compared with untreated controls (Barrack and Berry, 1987), indicating that androgens and estrogens both inhibit the rate of cell death. Neural signaling pathways, especially α-adrenergic pathways, may also play a role in balancing cell death and cell proliferation (Anglin et al, 2002).
The hyperplasia results in a remodeling of the normal prostatic architecture (Untergasser et al, 2005). Epithelial budding from preexisting ducts and the appearance of mesenchymal nodules characterize the early stages of the process, but the tissue phenotype of patients with established disease is highly variable.
BPH may be viewed as a stem cell disease (Barrack and Berry, 1987). Presumably, dormant stem cells in the normal prostate rarely divide, but when they do, they give rise to a second type of transiently proliferating cell capable of undergoing DNA synthesis and proliferation, thus maintaining the number of cells in the prostate. When the proliferating cells mature through a process of terminal differentiation they have a finite life span before undergoing programmed cell death. In this paradigm the aging process induces a block in this maturation process so that the progression to terminally differentiated cells is reduced, reducing the overall rate of cell death. Indirect evidence for this hypothesis comes from the observation that secretion, one parameter of epithelial cell differentiation, decreases with age, suggesting that the number of differentiated cells capable of secretory activity may be decreasing (Isaacs and Coffey, 1987). A survey of human BPH specimens for a marker of cellular senescence (senescence-associated β-galactosidase [SA-β-gal]) demonstrated a higher portion of senescent epithelial cells in men with large prostates, suggesting that an accumulation of those cells may play a role in the development of prostate enlargement (Choi et al, 2000). More recent studies support the hypothesis that impaired cell senescence may play a significant role in the etiology of BPH (Castro et al, 2003).
Hormones may exert their influence over the stem cell population not only with advancing age but also during embryonic and neonatal development (Naslund and Coffey, 1986). The size of the prostate may be defined by the absolute number of potential stem cells present in the gland, which in turn may be dictated at the time of embryonic development. Studies in animal models have suggested that early imprinting of prostatic tissue by postnatal androgen surges is critical to subsequent hormonally induced prostatic growth. As with the hormonal regulation of adult prostatic tissues, sex steroid hormones may exert their imprinting effect directly or indirectly through a complex series of signaling pathways (Lee and Peehl, 2004).
Role of Androgens
Although androgens do not cause BPH, the development of BPH requires the presence of testicular androgens during prostate development, puberty, and aging (McConnell, 1995; Marcelli and Cunningham, 1999). Patients castrated before puberty or who are affected by a variety of genetic diseases that impair androgen action or production do not develop BPH. It is also known that prostatic levels of dihydrotestosterone (DHT) as well as the androgen receptor (AR) remain high with aging despite the fact that peripheral levels of testosterone are decreasing. Moreover, androgen withdrawal leads to partial involution of established BPH (Peters and Walsh, 1987; Isaacs, 2008).
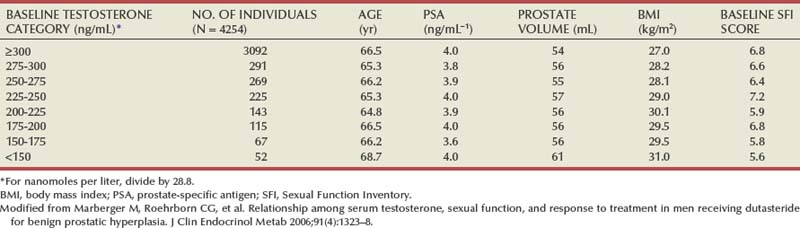
If normal ranges are assumed, there is no clear relationship between the concentration of circulating androgens and prostate size in aging men. In the Olmsted County Study of Urinary Symptoms and Health Status Among Men cohort (median age 60.9 years) serum bioavailable testosterone levels were found to decline with increasing age while the estradiol/bioavailable testosterone ratio increased (Roberts et al, 2004). Bioavailable testosterone correlated negatively and estradiol/bioavailable testosterone ratio positively with prostate volume, but this association was much less apparent after age adjustment. Baseline data from a large BPH medical therapy study confirmed the absence of a relationship between serum testosterone, serum prostate-specific antigen (PSA), and prostate volume (Marberger et al, 2006) (Table 91–1).
Table 91–1 Absence of Significant Relationship between Serum Testosterone and Serum PSA and Prostate Volume
In the brain, skeletal muscle, and seminiferous epithelium, testosterone directly stimulates androgen-dependent processes. In the prostate, however, the nuclear membrane bound enzyme steroid 5α-reductase converts the hormone testosterone into DHT, the principal androgen in this tissue (see Fig. 91–1) (McConnell, 1995). Ninety percent of total prostatic androgen is in the form of DHT, principally derived from testicular androgens. Adrenal androgens may constitute 10% of total prostatic androgen, although the importance of this stored hormone source in the etiology of BPH is negligible. Inside the cell, both testosterone and DHT bind to the same high-affinity androgen receptor protein (Chatterjee, 2003). DHT is a more potent androgen than testosterone because of its higher affinity for the AR. Moreover, the DHT-receptor complex may be more stable than the testosterone receptor complex. The hormone receptor then binds to specific DNA binding sites in the nucleus, which results in increased transcription of androgen-dependent genes and ultimately stimulation of protein synthesis (Andriole et al, 2004). Conversely, androgen withdrawal from androgen-sensitive tissue results in a decrease in protein synthesis and tissue involution. Besides inactivation of key androgen-dependent genes (e.g., PSA), androgen withdrawal leads to the activation of specific genes involved in programmed cell death (Kyprianou and Isaacs, 1989; Martikainen et al, 1990). Despite the importance of androgens in normal prostatic development and secretory physiology, there is no evidence that either testosterone or DHT serves as the direct mitogen for growth of the prostate in older men. Indeed, neither hormone is mitogenic to cultured prostatic epithelial cells (McKeehan et al, 1984). In the rat ventral prostate, differential gene expression experiments failed to demonstrate direct activation of mitogenic pathways (Wang et al, 1997). However, many growth factors and their receptors are regulated by androgens (see later). Thus the action of testosterone and DHT in the prostate is mediated indirectly through autocrine and paracrine pathways.
Androgen Receptors
The prostate, unlike other androgen-dependent organs, maintains its ability to respond to androgens throughout life. In the penis, AR expression decreases to negligible rates at the completion of puberty (Roehrborn et al, 1987; Takane et al, 1991a, 1991b). Thus, despite high circulating levels of androgen, the adult penis loses its ability for androgen-dependent growth. If the penis maintained high levels of AR throughout life, presumably the organ would grow until the time of death. In contrast, AR levels in the prostate remain high throughout aging (Barrack et al, 1983; Rennie et al, 1988). In fact, there is evidence to suggest that nuclear AR levels may be higher in hyperplastic tissue than in normal controls. Age-related increases in estrogen, as well as other factors, may increase AR expression in the aging prostate, leading to further growth (or to a decrease in cell death), despite decreasing levels of androgen in the peripheral circulation and “normal” levels of DHT in the prostate.
The potential role of AR mutations, polymorphisms, or other alterations in the pathogenesis of BPH is unclear (Chatterjee, 2003). A polymorphism in the number of CAG repeats (short vs. control) in the AR gene has been associated with larger prostate size and an increased risk of surgery (Giovannucci et al, 1999a, 1999b). However, another study from the Netherlands showed no relationship between the number of CAG repeats and BPH (Bousema et al, 2000). The later study also found no relationship between BPH and vitamin D receptor polymorphisms, although one Japanese study suggested an association (Habuchi et al, 2000). A more recent study of U.S. men showed a positive correlation between short CAG repeats and prostate volume, but a study of Finnish men found that short CAG repeats were significantly less common in men with BPH compared with control subjects (Mononen et al, 2002). Given the significant variation in reported findings, if short CAG repeats play a role in BPH pathogenesis it is likely to be minor (Hoke and McWilliams, 2008).
Dihydrotestosterone and Steroid 5α-Reductase
Intraprostatic DHT concentrations are maintained but not elevated in BPH. Initial studies of resected prostatic tissue suggested that prostatic DHT levels were higher in the hyperplastic gland than in normal control tissues. However, the controls used for these early studies were largely accident victims. Ongoing metabolism of DHT after death lowers the level of this androgen in cadaveric tissues. This was clearly shown in a study by Walsh and colleagues (1983) in which prostatic surgical specimens from men without BPH were used as the control. These investigators demonstrated that DHT levels are the same in hyperplastic glands as in normal glands. However, the aging prostate maintains a high level of DHT as well as a high level of AR; thus the mechanism for androgen-dependent cell growth is maintained. There is little question that androgens have at least a permissive role in the development of the disease process.
Two types of steroid 5α-reductase have been discovered, each encoded by a separate gene (Russell and Wilson, 1994). Type 1 5α-reductase, the predominant enzyme in extraprostatic tissues, such as skin and liver, is normally expressed in the 5α-reductase deficiency syndrome and is inhibited by dutasteride (Avodart) but not substantially by finasteride (Proscar). Type 2 5α-reductase is the predominant prostatic 5α-reductase, although it is also expressed in extraprostatic tissues. Mutations in the type 2 isoform are responsible for the clinical phenotype observed in the 5α-reductase deficiency syndrome. It is exquisitely sensitive to inhibition by finasteride and dutasteride (Carson and Rittmaster, 2003). Clearly, the type 2 isoform is critical to normal development of the prostate and hyperplastic growth later in life. The role of type 1 5α-reductase in normal and abnormal prostate growth remains to be defined. There is growing evidence to suggest that the type 1 isoform may play a more important role in prostate cancer compared with BPH, because increased levels of messenger RNA (mRNA) expression, protein, and functional enzymes have been demonstrated in prostate cancer (Thomas et al, 2008). Given that finasteride produces prostate size reduction identical to that with dual type 1/type 2 inhibitors and roughly equivalent to that with castration, it is unlikely that type 1–derived DHT is critical to hyperplastic growth.
Immunohistochemical studies with type 2 5α-reductase specific antibodies show primarily stromal cell localization of the enzyme (Thigpen et al, 1993; Silver et al, 1994). Epithelial cells uniformly lack type 2 protein, and some basal epithelial cells stain positively. Type 1 5α-reductase protein could not be detected in BPH or prostate cancer using initially available antibodies, although trace levels of type 1 mRNA have been seen in normal prostates, BPH, and cancer (Shirakawa et al, 2004). A study with a selective type 1 antibody demonstrated positive staining in only 7% of BPH cases (Thomas et al, 2003). In the same study, type 1 enzymatic activity was found in only 2 of 29 BPH specimens.
These data demonstrate that the stromal cell plays a central role in androgen-dependent prostatic growth and that type 2 5α-reductase within the stromal cell is the key androgenic amplification step. Thus a paracrine model for androgen action in the gland (Fig. 91–2) is evident. In addition, it is possible that circulating DHT produced in the skin and liver may act on prostate epithelial cells in a true endocrine fashion (McConnell, 1995). If dual type 1/type 2 5α-reductase inhibition has clinical utility over selective type 2 inhibitors, it is likely to be due to inhibition of peripherally produced DHT.
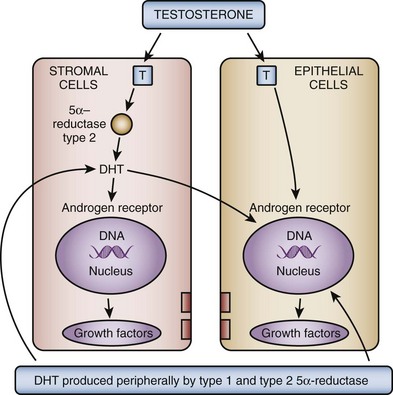
Figure 91–2 Testosterone (T) diffuses into the prostate epithelial and stromal cell. T can interact directly with the androgen (steroid) receptors bound to the promoter region of androgen-regulated genes. In the stromal cell a majority of T is converted into dihydrotestosterone (DHT)—a much more potent androgen—which can act in an autocrine fashion in the stromal cell or in a paracrine fashion by diffusing into epithelial cells in close proximity. DHT produced peripherally, primarily in the skin and liver, can diffuse into the prostate from the circulation and act in a true endocrine fashion. In some cases the basal cell in the prostate may serve as a DHT production site, similar to the stromal cell. Autocrine and paracrine growth factors may also be involved in androgen-dependent processes within the prostate.
(From Roehrborn CG. Pathology of benign prostatic hyperplasia. Int J Impot Res 2008;20[Suppl. 3]:S11–8.)
Immunohistochemical studies in open enucleated BPH specimens show considerable intraprostatic and interprostatic 5α-reductase, making studies of its distribution based on single or one-time biopsy material very difficult (Sherwood et al, 2003).
Polymorphisms in type 2 5α-reductase (SRD5A2) have been reported, but their linkage to BPH is uncertain. The SRD5A2 gene on chromosome 2p23 frequently encompasses A49T and V89L substitutions and a TA dinucleotide repeat polymorphism. The 89L allele has been associated with lower enzyme activity, whereas the 49T allele has been associated with higher activity. Longer TA repeats are associated with mRNA instability and thus decreased enzyme activity. The number of L alleles, but not testosterone alleles or TA repeats, in one study correlated significantly with the presence of BPH (Salam et al, 2005). In the Olmsted County population, consistent associations between SRD5A2 genotypes and BPH were not demonstrated, although there was a weak correlation between V89L polymorphisms and prostate volume (Roberts et al, 2005).
Androgen withdrawal may partially exert its effect on the prostate through vascular effects (Buttyan et al, 2000). Castration induces acute and drastic vasoconstriction of blood vessels in the rat prostate (Hayek et al, 1999). This effect does not appear to be mediated through vascular endothelial growth factor (Burchardt et al, 2000). There is indirect evidence to suggest that abnormalities in the prostatic vascular system produced by other disease states (e.g., diabetes) may be a risk factor of BPH (Parsons et al, 2006; Parsons, 2007).
Role of Estrogens
There is animal model evidence to suggest that estrogens play a role in the pathogenesis of BPH; the role of estrogens in the development of human BPH, however, is less clear. In the dog, in which estrogens act synergistically with androgens to produce experimental BPH, estrogen appears to be involved in induction of the AR (Moore et al, 1979). Estrogen may, in fact, “sensitize” the aging dog prostate to the effects of androgen (Barrack and Berry, 1987). The canine prostate contains an abundance of high-affinity estrogen receptor (ER). In the dog, estrogen treatment stimulates the stroma, causing an increase in the total amount of collagen (Berry et al, 1986a, 1986b). There are at least two forms of ER. ER-α is expressed by prostate stromal cells, and ER-β is expressed by prostate epithelial cells (Prins et al, 1998). The estrogenic response of the prostate is determined by the type of ER present within the prostatic cells. Experiments in knockout mice suggest a “constraining influence” of estrogens on the prostate (Krege et al, 1998). In-vitro studies suggest that upregulation of ER-α in cultured prostate stromal cells is also associated with upregulation of fibroblast growth factor (FGF)-2, FGF-7, and other growth factors; the addition of androgens downregulated the ER and various stroma-derived growth factors (Smith et al, 2000, 2002).
Different actions may be mediated by the stromal ER-α and epithelial ER-β (Prins and Korach, 2008). Evidence also indicates that estrogen action mediated through the separate receptors may contribute to the etiology and progression of multiple prostate-diseased states (Table 91–2). These findings provide new avenues and alternative approaches for the treatment of prostate diseases including prostate cancer with novel therapies directed at ERs or estrogen metabolism. Because the two types of ERs may play distinct and perhaps opposing roles in many diseases of the prostate, including cancer progression, it is possible that receptor-specific agonists and antagonists may prove beneficial in therapeutic strategies in future clinical trials.
Table 91–2 Comparison of ER-α and ER-β Expression and Activities in the Prostate Gland
| ER-α | ER-β | |
|---|---|---|
| Localization | Stromal | Epithelial |
| Proliferation | Epithelial squamous metaplasia | Antiproliferative |
| Stromal proliferation | ||
| Differentiation | Epithelial dysplasia | Prodifferentiation |
| Immune response | Anti-inflammatory | |
| Antioxidant | ||
| Expression | Dysregulated in prostate cancer | Dysregulated in prostate cancer |
| Silenced in early cancers | ↓ Organ-confined disease | |
| Re-emergence with progression | ↑ In metastatic prostate cancer |
ER, estrogen receptor.
From Prins GS, Korach KS. The role of estrogens and estrogen receptors in normal prostate growth and disease. Steroids 2008;73(3):233–44.
Serum estrogen levels increase in men with age, absolutely or relative to testosterone levels. There is also suggestive evidence that intraprostatic levels of estrogen are increased in men with BPH. Patients with larger volumes of BPH tend to have higher levels of estradiol in the peripheral circulation (Partin et al, 1991). In the Olmsted County study cohort, in men with above median levels of bioavailable testosterone, the serum estradiol level correlated positively with prostate volume, even after adjusting for age (Roberts et al, 2004). Data on obesity, serum testosterone, estradiol, and prostate volume are conflicting (Zucchetto et al, 2005). Although there are relatively low concentrations of classic high-affinity ERs in human BPH (Farnsworth, 1996; Sciarra and Toscano, 2000), there may be a sufficient amount for biologic activity.
From experimental studies with aromatase inhibitors it appears that decreases in intraprostatic estrogen in animal models may lead to reduction in drug-induced stromal hyperplasia (Farnsworth, 1996, 1999). At present, however, the role of estrogens in human BPH is not as firmly established as the role of androgens. Species variation and cause-and-effect relationships are problematic.
There are high levels of progesterone receptor in the normal and hyperplastic prostate. However, the role of the progesterone receptor in normal prostatic physiology as well as in BPH remains to be defined.
Regulation of Programmed Cell Death
Programmed cell death (apoptosis) is a physiologic mechanism crucial to the maintenance of normal glandular homeostasis (Kerr and Searle, 1973). Cellular condensation and fragmentation precede phagocytosis and degradation, during which the apoptotic cell is phagocytosed by neighboring cells and degraded by lysosomal enzymes. Apoptosis occurs without activation of the immune system but requires both RNA and protein synthesis (Lee, 1981). In the rat prostate, active cell death occurs naturally in the proximal segment of the prostatic ductal system in the presence of normal concentrations of plasma testosterone (Lee et al, 1990). Androgens (presumably testosterone and DHT) appear to suppress programmed cell death elsewhere in the gland. After castration, active cell death is increased in the luminal epithelial population as well as in the distal region of each duct. Tenniswood (1992) suggested that there is regional control over androgen action and epithelial response, with androgens providing a modulating influence over the local production of growth regulatory factors that varies in different parts of the gland. Members of the transforming growth factor-β (TGF-β) family are likely candidates for this regulatory step (Martikainen et al, 1990).
In the rat prostate, at least 25 different genes are induced after castration (Montpetit et al, 1986). Normal glandular homeostasis requires a balance between growth inhibitors and mitogens, which respectively restrain or induce cell proliferation but also prevent or modulate cell death. Abnormal hyperplastic growth patterns, such as BPH, might be induced by local growth factor or growth factor receptor abnormalities, leading to increased proliferation or decreased levels of programmed cell death.
Stromal-Epithelial Interaction
There is abundant experimental evidence to demonstrate that prostatic stromal and epithelial cells maintain a sophisticated paracrine type of communication. The growth of canine prostate epithelium can be regulated by cellular interaction with the basement membrane and stromal cells. Isaacs and Coffey (1987), using a marker of canine prostatic epithelial cell function, demonstrated that epithelial cells grown on plastic quickly lose their ability to secrete this protein. In addition, the cells begin to grow rapidly and change their cytoskeletal staining pattern. In contrast, if the cells are grown on prostatic collagen they maintain their normal secretory capacity and cytoskeletal staining pattern and do not grow rapidly. This is strong evidence that one class of stromal cell excretory protein (i.e., extracellular matrix) partially regulates epithelial cell differentiation. Thus BPH may be due to a defect in a stromal component that normally inhibits cell proliferation, resulting in loss of a normal “braking” mechanism for proliferation. This abnormality could act in an autocrine fashion to lead to proliferation of stromal cells as well.
Further evidence of the importance of stromal-epithelial interactions in the prostate comes from the elegant developmental studies of Cunha, which demonstrate the importance of embryonic prostatic mesenchyme in dictating differentiation of the urogenital sinus epithelium (Cunha et al, 1980, 1983, 2003; Cunha and Donjacour, 1987; Cunha, 1994, 1996). The process of new gland formation in the hyperplastic prostate suggests a “reawakening” of embryonic processes in which the underlying prostatic stroma induces epithelial cell development (McNeal, 1990). Many of the prostatic stromal-epithelial interactions observed during normal development and in BPH may be mediated by soluble growth factors or by the extracellular matrix (ECM), which itself has growth factor–like properties. This model is even more intriguing, given the cellular localization of 5α-reductase (and thus DHT production) in the prostate stromal cell (Silver et al, 1994).
The complexity of the stromal/ECM/epithelial relationship is revealed in studies of the ECM signaling protein CYR61. CRY61 (an early immediate response gene) is an ECM-associated protein that promotes adhesion, migration, and proliferation of epithelial and stromal cells. A variety of growth factors increase the expression of CYR61 in both cell types, and the suppression of CYR61 expression by an antisense oligonucleotide significantly affects normal cell morphology (Sakamoto et al, 2003, 2004a, 2004b). CRY61 expression is significantly increased in human BPH tissues and is induced by lysophosphatidic acid (an endogenous lipid growth factor).
As our understanding of stromal-epithelial cell relationships in the prostate increases it is possible that therapies may be designed to induce regression of established BPH by modulating these autocrine/paracrine mechanisms.
Growth Factors
Growth factors are small peptide molecules that stimulate, or in some cases inhibit, cell division and differentiation processes (Steiner, 1995; Lee and Peehl, 2004). Cells that respond to growth factors have on their surface receptors specific for that growth factor that in turn are linked to a variety of transmembrane and intracellular signaling mechanisms. Interactions between growth factors and steroid hormones may alter the balance of cell proliferation versus cell death to produce BPH (Fig. 91–3). Lawson’s group was the first to demonstrate that extracts of BPH stimulate cellular growth. This putative prostatic growth factor was subsequently found on sequence analysis to be basic fibroblastic growth factor (bFGF) (Story et al, 1989). Subsequently, a variety of growth factors have been characterized in normal, hyperplastic, and neoplastic prostatic tissue. In addition to bFGF (FGF-2), acidic FGF (FGF-1), Int-2 (FGF-3), keratinocyte growth factor (KGF, FGF-7), transforming growth factor-β (TGF-β), and epidermal growth factor (EGF) have been implicated in prostate growth. TGF-β is a potent inhibitor of proliferation in normal epithelial cells in a variety of tissues. In models of prostatic cancer there is evidence that malignant cells have escaped the growth inhibitory effect of TGF-β (McKeehan and Adams, 1988). Similar mechanisms may be operational in BPH (Salm et al, 2000), leading to the accumulation of epithelial cells (Kundu et al, 2008). Growth factors may also be important in modulating the phenotype of the prostate smooth muscle cell (Peehl and Sellers, 1998).
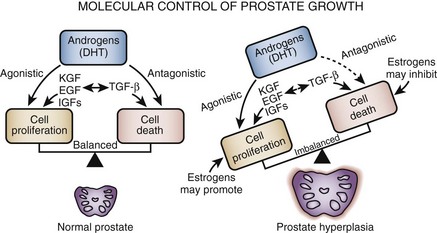
Figure 91–3 Prostate hyperplasia is probably due to an imbalance between cell proliferation and cell death. Androgens play a necessary but probably permissive role. Growth factors are more likely to be sites of primary defects.
There is mounting evidence of interdependence between growth factors, growth factor receptors, and the steroid hormone milieu of the prostate (Rennie et al, 1988; Lee and Peehl, 2004). Although data on the absolute level of growth factor and growth factor receptors in hyperplastic as opposed to normal tissue conflict, it is likely that growth factors play some role in the pathogenesis of BPH. However, further research is necessary to establish the role of growth factors in a disease process in which cellular proliferation is not obvious.
If cellular proliferation is a component of the BPH process, it appears that growth stimulatory factors such as the FGF-1, FGF-2, FGF-7, and FGF-17 families, vascular endothelial growth factor (VEGF), and insulin-like growth factor (IGF) may play a role, with DHT augmenting or modulating the growth factor effects. In contrast, TGF-β, which is known to inhibit epithelial cell proliferation, may normally exert a restraining influence over epithelial proliferation that is lost or downregulated in BPH (Wilding et al, 1989; Sporn and Roberts, 1990, 1991; Peehl et al, 1995; Cohen et al, 2000; Lee and Peehl, 2004). TGF-β1 is a potent mitogen for fibroblasts and other mesenchymal cells but is also an important inhibitor of epithelial cell proliferation (Roberts and Sporn, 1993). TGF-β1 also regulates ECM synthesis and degradation and can induce cells to undergo apoptosis. In addition, TGF-β upregulates the production of bFGF-2, which is known to be an autocrine growth factor for prostate stromal cells (Story et al, 1993) and at least on one prostate smooth muscle cell line (PSMC1), TGF functions as an autocrine mitogen (Salm et al, 2000). Thus upregulation of TGF-β1 (which is expressed in prostate stromal cells) during BPH would favor expansion of the stromal compartment.
Indirect evidence to support this view comes from studies of reconstituted mouse prostate (Yang et al, 1997). Interestingly, the observation that TGF-β1 may regulate smooth muscle contractile protein expression suggests that TGF-β isoforms may be physiologic regulators of prostatic smooth muscle function (Orlandi et al, 1994). Cohen and colleagues (2000) found that stromal cells isolated from BPH specimens exhibited a blunted TGF-β growth inhibition relative to normal stromal cells and that the blunted response appeared to be due to a reduction in TGF-mediated increase in IGF binding protein 3 (IGFBP-3) expression. TGF-β may stimulate the overexpression of versican (chondroitin sulfate proteoglycan 2) in the ECM through inhibition of key metalloproteases (ADAMTS lineage) that normally degrade versican, leading to accumulation in the ECM (Cross et al, 2006). An increased risk for BPH was described in patients with a codon 10 polymorphism in TGF-β (Li et al, 2004).
The first evidence of increased FGF-2 levels in BPH came from the studies of Begun and coworkers (1995), who demonstrated a twofold to threefold elevation of FGF-2 in BPH compared with histologically normal glands. Further studies have demonstrated that both FGF-2 and FGF-7 are overexpressed in BPH tissues (Ropiquet et al, 1999). The major target of FGF-2 is thought to be the stroma itself (autocrine), although transgenic mice overexpressing FGF-2 develop glandular epithelial hyperplasia (Konno-Takahashi et al, 2004). KGF, a member of the FGF family (FGF-7), is produced in prostatic stromal cells (Yan et al, 1992). However, cell surface receptors for stroma-derived KGF are expressed exclusively in epithelial cells. As a result, FGF-7 (or a homolog) is the leading candidate for the factor mediating the stromal cell–based hormonal regulation of the prostatic epithelium. There is direct evidence that FGF-7 plays this role in the androgen-dependent mesenchymal-epithelial interactions involved in development of the seminal vesicle (Alarid et al, 1994). Abnormalities in stromal FGF-7 production or epithelial FGF-7 receptor could promote epithelial cell proliferation. Indirect evidence supporting this hypothesis comes from a study of transgenic mice overexpressing FGF-7 that develop atypical prostatic hyperplasia (Kitsberg and Leder, 1996). McKeehan’s laboratory demonstrated that FGF-10, a homolog of FGF-7, is expressed at high levels in the rat prostate, specifically in stromal cells of smooth muscle origin (Lu et al, 1999; Nakano et al, 1999). FGF-10 expression is increased by androgens and may have a mitogenic effect on prostate epithelium. Others studies suggest that cells expressing FGF-7 are localized in the stroma immediately adjacent to the epithelium, suggesting that the epithelial cells may induce FGF-7 expression. The paracrine factor most likely responsible for this effect is cytokine interleukin (IL)-1α (Giri and Ittmann, 2000; Lee and Peehl, 2004).
Some investigators have speculated that local hypoxia in the prostate (perhaps from atherosclerosis or other vascular events) is the initial event that induces FGF production (Lee and Peehl, 2004). Further growth of BPH nodules could impede blood flow, leading to further hypoxia (Parsons et al, 2008; Parsons and Kashefi, 2008). Hypoxia leads to upregulation of hypoxia inducible factor-1, which in turn increases the secretion of FGF-2 and FGF-7 from stromal cells.
Other growth factors implicated in BPH include FGF-17 (Polnaszek et al, 2004), FGF-10, and VEGF (Walsh et al, 2002). It remains difficult to ascertain which of the growth factors and growth factor receptors are key mediators of the BPH disease process and which are bystanders.
A unique animal model provides additional evidence that FGF-like factors may be involved in the etiology of BPH. A transgenic mouse line expressing the Int-2/FGF-3 growth factor demonstrated androgen-sensitive epithelial hyperplasia in the male mouse prostate histologically similar to human and canine BPH (Tutrone et al, 1993).
Insulin-like growth factors, binding proteins, and receptors also appear to be important modulators of prostatic growth, at least as it relates to cell growth in culture (Peehl et al, 1995; Lee and Peehl, 2004). A transgenic mouse model with overexpression of IGF-1 demonstrated prostate gland enlargement (Konno-Takahashi et al, 2003). Studies of BPH tissue demonstrate a higher concentration of IGF-2 in the periurethral area than in the peripheral zone (Monti et al, 2001). A study of Chinese men demonstrated a significant correlation between circulating IGF-1 and IGFBP-3 level and BPH (Dahle et al, 2002), but a study of the Olmsted County cohort failed to demonstrate any relationship between serum IGF-1 and prostate volume (Roberts et al, 2003).
Other Signaling Pathways
Sympathetic signaling pathways are important in the pathophysiology of LUTS, as is evident from the use of drugs interfering with the adrenergic nervous system such as α-adrenergic receptor blockers, which are highly effective for the treatment of LUTS (American Urological Association, 2003). In addition, there is increasing evidence that sympathetic pathways may be important in the pathogenesis of the hyperplastic growth process (McVary et al, 1994, 2005). α-Adrenergic blockade, in some model systems, can induce apoptosis (Anglin et al, 2002). α-Adrenergic pathways can also modulate the smooth muscle cell phenotype in the prostate (Lin et al, 2000). All the components of the renin-angiotensin system (RAS) are present in prostatic tissue and may be activated in BPH (Dinh et al, 2001, 2002; Fabiani et al, 2001). Either with or without sympathetic modulation, local RAS pathways may contribute to cell proliferation and smooth muscle contraction.
The early growth response gene-1 (EGR1) transcription regulation pathway was found to be active in a BPH cell line (Mora et al, 2005). Also of interest is the finding that α2-macroglobulin, a large protein that binds PSA and many growth factors, is very highly expressed in human prostate and is upregulated in BPH (Lin et al, 2005). Trapping and inactivation of inhibitory molecules could promote growth pathways.
Potential Role of Inflammatory Pathways and Cytokines in Benign Prostatic Hyperplasia
An additional source of growth factors in human BPH tissue may be the inflammatory cell infiltrates seen in many men with BPH. In the 1990s, descriptive studies suggested a link between inflammation and BPH-related growth. Theyer and associates (1992) reported extensive infiltration of human BPH tissues by activated T cells. Peripheral blood and tumor infiltrating T cells are known to express VEGF, a potent epithelial mitogen (Blotnik et al, 1994; Freeman et al, 1995). T cells are known to produce and secrete a variety of other growth factors, including HB-EGF and bFGF/FGF-2. Thus T cells present in the local prostate environment were thought to be capable of secreting potent epithelial and stromal mitogens that promote stromal and glandular hyperplasia.
In the past 5 years, specific inflammatory mediator pathways have been studied in detail to elucidate the potential role of these pathways in BPH pathogenesis. A large number of cytokines and their receptors are seen in BPH tissue (Konig et al, 2004). Specifically, significant levels of IL-2, IL-4, IL-7, IL-17, interferon-γ (IFN-γ), and their relevant receptors are found in BPH tissue (Kramer et al, 2002; Steiner et al, 2003a, 2003b). IL-2, IL-7, and IFN-γ stimulate the proliferation of prostatic stromal cells in vitro. Prostatic epithelial cell senescence results in increased expression of IL-8, which can promote proliferation of nonsenescent epithelial and stromal cells (Castro et al, 2004). Macrophage inhibitory cytokine-1 is expressed in normal prostate tissue but significantly downregulated in BPH (Kakehi et al, 2004; Taoka et al, 2004). Chronic inflammation in BPH is also associated with focal upregulation of cyclooxygenase 2 (COX-2) in the glandular epithelium (Wang et al, 2004). To date, however, no firm cause-and-effect relationships have been established between prostatic inflammation and related cytokine pathways and stromal-epithelial hyperplasia.
An excellent recent review of BPH as a potentially autoimmune disease was published by Kramer and colleagues (2007), and Figure 91–4 illustrates the immunologic key features of chronic inflammation in BPH and the present interpretation of these changes in the development and progression of BPH.
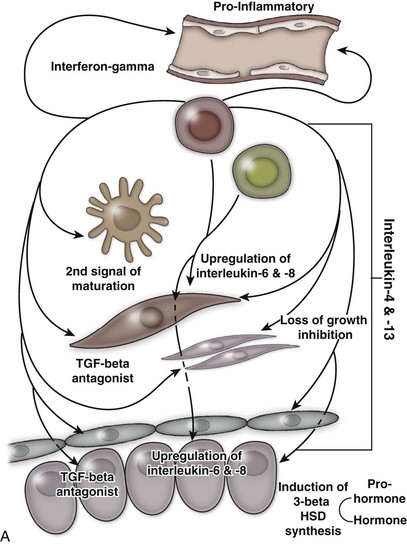
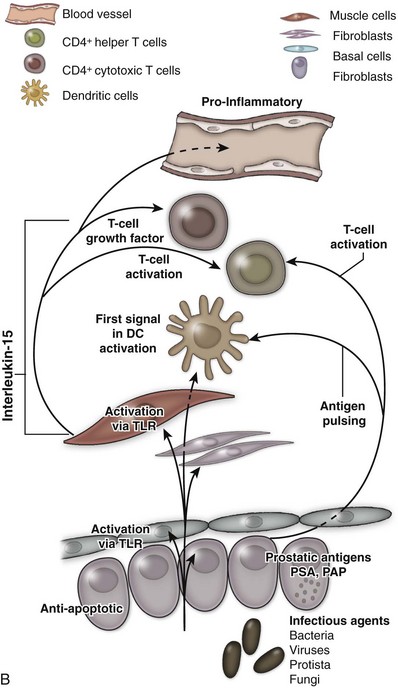
Figure 91–4 A, Tissue effect of T cell–derived proinflammatory cytokines on the pathogenesis and progression of immune inflammation and stromal growth in the aging prostate (T cells indicated in red). B, Role of smooth muscle cells (indicated in red) in maintenance and propagation of immune infiltration in the aging prostate. PSA, prostate-specific antigen; PAP, prostatic acid phosphatase; TLR, toll-like receptor.
(From Kramer G, Mitteregger D, et al. Is benign prostatic hyperplasia [BPH] an immune inflammatory disease? Eur Urol 2007;51[5]:1202–16.)
Genetic and Familial Factors
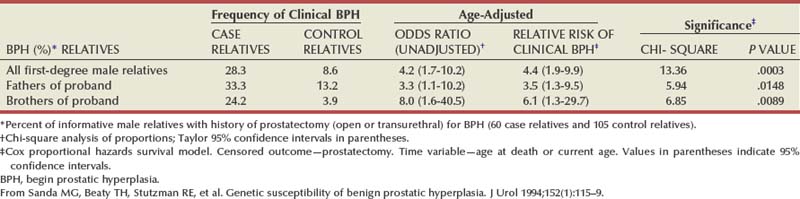
There is substantial evidence that BPH has an inheritable genetic component. Sanda and colleagues conducted a retrospective case-control analysis of surgically treated BPH patients and control subjects at Johns Hopkins Hospital (Partin et al, 1994; Sanda et al, 1994). The BPH patients were men whose resected prostate weights were in the highest quartile (>37 g) and whose age at prostatectomy was in the lowest quartile. The hazard-function ratio for surgically treated BPH among first-degree male relatives of the BPH cases compared with the first-degree male relatives of the controls was 4.2 (95% confidence interval [CI], 1.7 to 10.2), demonstrating a very strong relationship (Table 91–3). The results did not appear to be due to differences in health-seeking behavior between the two groups. A segregation analysis showed that the results were most consistent with an autosomal dominant inheritance pattern. Utilizing this model, approximately 50% of men undergoing prostatectomy for BPH when younger than 60 years of age could be attributable to an inheritable form of disease. In contrast, only about 9% of men undergoing prostatectomy for BPH when older than 60 years of age would be predicted to have a familial risk. In addition, monozygotic twins demonstrate a higher concordance rate of BPH than dizygotic twins (Partin et al, 1994).
In a community-based cohort study of more than 2000 men, Roberts and colleagues (1995) found an elevated risk of moderate to severe urologic symptoms in men with a family history of an enlarged prostate and a family history of BPH compared with those with no history. Analysis of the subjects who participated in the U.S. finasteride clinical trial identified 69 men who had three or more family members with BPH, including the proband (Sanda et al, 1997). Regression analysis demonstrated that familial BPH was characterized by large prostate size, with a mean prostate volume of 82.7 mL in men with hereditary BPH compared with 55.5 mL in men with sporadic BPH. Serum androgen levels and the response to 5α-reductase inhibition were similar in familial and sporadic BPH. A more recent familial aggregation study in the finasteride database confirmed that a strong family history of early-onset and large prostate volume is more likely to be associated with inheritance of risk than symptom severity or other factors (Pearson et al, 2003).
These studies clearly demonstrate the presence of a familial form of BPH and suggest the presence of a gene contributing to the pathogenesis of the disease. The studies of Meikle and coworkers (1997, 1999) also support a genetic basis for BPH. Preliminary studies demonstrate evidence of DNA mutations (White et al, 1990), DNA hypomethylation (Bedford and van Helden, 1987), and abnormalities of nuclear matrix protein expression (Partin et al, 1993), miscellaneous genetic polymorphisms (Werely et al, 1996; Konishi et al, 1997; Habuchi et al, 2000), and abnormal expression of the Wilms tumor gene (WT1) (Dong et al, 1997) in human BPH. However, the specific gene or genes involved in familial BPH or that contribute to the risk of significant prostatic enlargement in sporadic disease remain to be elucidated.
Other Etiologic Factors
Androgens and soluble growth factors are clearly not the only important factors for the development of BPH. All mammalian prostates studied have testosterone, DHT, and AR as well as most of the known growth factor signaling pathways; however, only dog and man develop BPH. Interestingly, another glandular organ that remains androgen responsive throughout life, the seminal vesicle, does not develop hyperplasia. Obviously, other mechanisms or cofactors must be present in these two unique species making them susceptible to the disease. Nonandrogenic substances from the testis, perhaps transmitted through the vas deferens or deferential blood vessels, for example, may play some role (Dalton et al, 1990). Rats with intact testes treated with exogenous androgen demonstrate a greater degree of prostatic growth than castrated rats treated with androgen. Sutkowski and coworkers (1993) have demonstrated that human spermatocele fluid is mitogenic to both human prostatic epithelial and stromal cells in culture. Similar results have been seen in castrated versus testes-intact dogs treated with exogenous androgen and exogenous testosterone and estradiol combination (Juniewicz et al, 1994). In addition to increases in prostate weight, the incidence of histologic BPH was significantly higher in the dogs with intact testes. Grayhack and colleagues (1998) have identified a putative substance that may be a candidate for such a factor.
Prolactin has long been speculated to play a role in BPH because of the known effects of this hormone on prostate cells in vitro. Transgenic mice overexpressing the prolactin gene develop significant enlargement of the prostate (Wennbo et al, 1997). However, despite the documented presence of prolactin receptors in the human prostate and low circulating levels of the hormone, the role of prolactin in human prostate disease is unclear.
Molecular profiling, fingerprinting, microarrays, and high-throughput screening tools have uncovered new genes, as well as known genes not previously associated with BPH. Preliminary findings from the Getzenberg laboratory (Prakash et al, 2002; Sakamoto et al, 2004a, 2004b; Shah et al, 2004; Minnery and Getzenberg, 2005) and other groups (Fromont et al, 2004; Dhanasekaran et al, 2005) suggest that new markers for BPH and new therapeutic targets will be found in the next few years.
Pathophysiology
The pathophysiology of BPH is complex (Fig. 91–5). Prostatic hyperplasia increases urethral resistance, resulting in compensatory changes in bladder function. However, the elevated detrusor pressure required to maintain urinary flow in the presence of increased outflow resistance occurs at the expense of normal bladder storage function. Obstruction-induced changes in detrusor function, compounded by age-related changes in both bladder and nervous system function, lead to urinary frequency, urgency, and nocturia, the most bothersome BPH-related complaints. Thus, an understanding of BPH pathophysiology requires detailed insight into obstruction-induced bladder dysfunction.
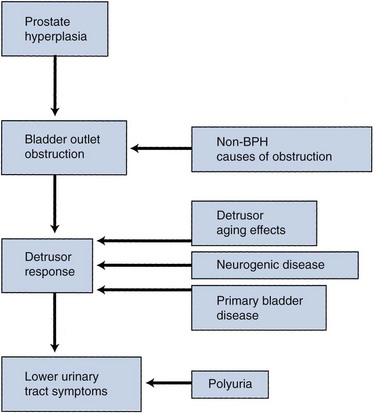
Figure 91–5 The pathophysiology of benign prostatic hyperplasia (BPH) involves complex interactions between urethral obstruction, detrusor function and dysfunction, and urine production.
Pathology
Anatomic Features
McNeal (1978) demonstrated that BPH first develops in the periurethral transition zone of the prostate (Fig. 91–6). The transition zone consists of two separate glands immediately external to the preprostatic sphincter. The main ducts of the transition zone arise on the lateral aspects of the urethral wall at the point of urethral angulation near the verumontanum. Proximal to the origin of the transition zone ducts are the glands of the periurethral zone that are confined within the preprostatic sphincter and course parallel to the axis of the urethra. All BPH nodules develop either in the transition zone or in the periurethral region (McNeal, 1978, 1990). Although early transition zone nodules appear to occur either within or immediately adjacent to the preprostatic sphincter, as the disease progresses and the number of small nodules increases they can be found in almost any portion of the transition or periurethral zone. However, the transition zone also enlarges with age, unrelated to the development of nodules.
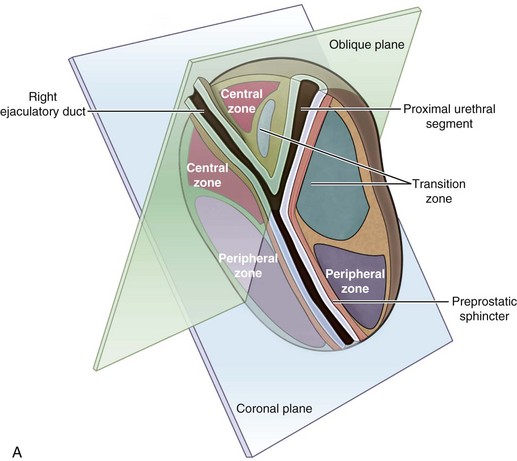
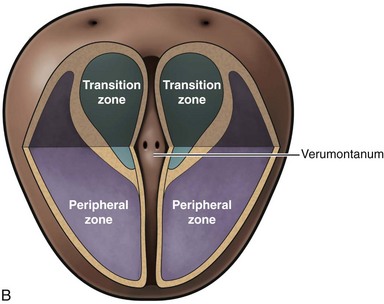
Figure 91–6 Zonal anatomy of the prostate as first described by McNeal (1978). Sagittal (A) and coronal (B) sections of the prostate showing peripheral zone, transition zone, central zone, the verumontanum, the proximal urethral segment, as well as preprostatic sphincter and ejaculatory duct.
(From Roehrborn CG. Pathology of benign prostatic hyperplasia. Int J Impot Res 2008;20[Suppl. 3]:S11–8.)
One of the unique features of the human prostate is the presence of the prostatic capsule, which plays an important role in the development of LUTS (Caine and Schuger, 1987). In the dog, the only other species known to develop naturally occurring BPH, symptoms of bladder outlet obstruction and urinary symptoms rarely develop because the canine prostate lacks a capsule. Presumably the capsule transmits the “pressure” of tissue expansion to the urethra and leads to an increase in urethral resistance. Thus the clinical symptoms of BPH in man may be due not only to age-related increases in prostatic size but also to the unique anatomic structure of the human gland. Clinical evidence of the importance of the capsule can be found in series that clearly document that incision of the prostatic capsule (transurethral incision of the prostate) results in a significant improvement in outflow obstruction, despite the fact that the volume of the prostate remains the same.
The size of the prostate does not correlate with the degree of obstruction. Thus other factors such as dynamic urethral resistance, the prostatic capsule, and anatomic pleomorphism are more important in the production of clinical symptoms than the absolute size of the gland. In some cases, predominant growth of periurethral nodules at the bladder neck gives rise to the “middle lobe” (Fig. 91–7). The middle lobe must be of periurethral origin because there is no transition zone tissue in this area. It is not clear whether middle lobe growth occurs at random in men with BPH or whether there is an underlying genetic susceptibility to this pattern of enlargement.
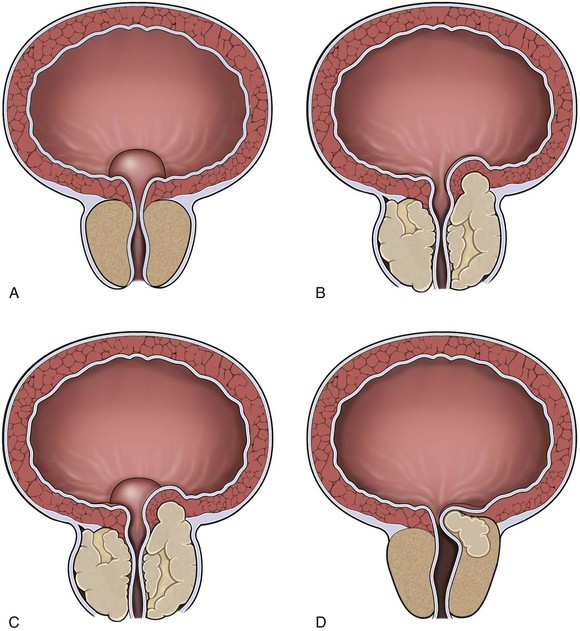
Figure 91–7 Diagrams of hyperplastic prostatic tissue obstructing the prostatic urethra forming “lobes.” A, Isolated middle lobe enlargement. B, Isolated lateral lobe enlargement. C, Lateral and middle lobe enlargement. D, Posterior commissural hyperplasia (median bar).
(After Randall [1931], from Roehrborn CG. Pathology of benign prostatic hyperplasia. Int J Impot Res 2008;20[Suppl. 3]: S11–8.)
Histologic Features
BPH is a hyperplastic and not a hypertrophic process, that is, there is a net increase in the number of cells and not in the size of the cells. Histologic studies document an increase in the cell number (McNeal, 1990). In addition, thymidine uptake studies in the dog clearly indicate an increase in DNA synthesis in experimentally induced BPH (Barrack and Berry, 1987). The term benign prostatic hypertrophy is pathologically incorrect.
McNeal’s studies (1990) demonstrate that the majority of early periurethral nodules are purely stromal in character. These small stromal nodules resemble embryonic mesenchyme with an abundance of pale ground substance and minimal collagen. It is unclear whether these early stromal nodules contain mainly fibroblast-like cells or whether differentiation toward a smooth muscle cell type is occurring. In contrast, the earliest transition zone nodules represent proliferation of glandular tissue that may be associated with an actual reduction in the relative amount of stroma (Fig. 91–8). The minimal stroma seen initially consists primarily of mature smooth muscle, not unlike that of the uninvolved transition zone tissue. These glandular nodules are apparently derived from newly formed small duct branches that bud off from existing ducts, leading to a totally new ductal system within the nodule. This type of new gland formation is quite rare outside embryonic development. This proliferative process leads to a tight packing of glands within a given area as well as an increase in the height of the lining epithelium. There appears to be hypertrophy of individual epithelial cells as well. Again, the observed increase in transition zone volume with age appears to be related not only to an increased number of nodules but also to an increase in the overall size of the zone.
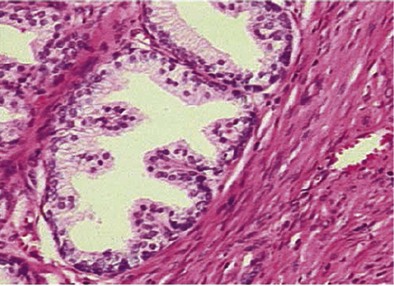
Figure 91–8 Stromoglandular hyperplasia of the prostate showing glandular (upper left) and stromal (lower right) tissue (hematoxylin and eosin stain).
(From Roehrborn CG. Pathology of benign prostatic hyperplasia. Int J Impot Res 2008;20[Suppl. 3]:S11–8.)
During the first 20 years of BPH development, the disease may be predominantly characterized by an increased number of nodules, and the subsequent growth of each new nodule is generally slow. Then a second phase of evolution occurs in which there is a significant increase in large nodules. In the first phase, the glandular nodules tend to be larger than the stromal nodules. In the second phase, when the size of individual nodules is increasing, the size of glandular nodules clearly predominates.
There is significant pleomorphism in stromal-epithelial ratios in resected tissue specimens. Studies from primarily small resected glands demonstrate a predominance of fibromuscular stroma (Shapiro et al, 1992). Larger glands, predominantly those removed by enucleation, demonstrate primarily epithelial nodules (Franks, 1976). However, an increase in stromal-epithelial ratios does not necessarily indicate that this is a “stromal disease”; stromal proliferation may well be due to “epithelial disease.”
Importance of Prostatic Smooth Muscle
Regardless of the exact proportion of epithelial to stromal cells in the hyperplastic prostate, there is no question that prostatic smooth muscle represents a significant volume of the gland (Shapiro et al, 1992) (Fig. 91–9). Although the smooth muscle cells in the prostate have not been extensively characterized, presumably their contractile properties are similar to those seen in other smooth muscle organs. The spatial arrangement of smooth muscle cells in the prostate is not optimal for force generation; however, there is no question that both passive and active forces in prostatic tissue play a major role in the pathophysiology of BPH. The factors that determine passive tone in the prostate remain to be elucidated. The series elastic elements in the stromal and epithelial cells and (most important) the ECM contribute to passive tissue force, independent of active smooth muscle contraction. However, stimulation of the adrenergic nervous system clearly results in a dynamic increase in prostatic urethral resistance. Blockade of this stimulation by α-receptor blockers clearly diminishes this response. However, α-adrenergic blockade does not decrease passive tension in the prostate, which may be an equal determinant of urethral resistance.
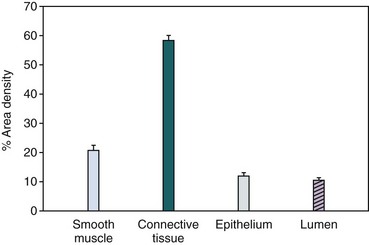
Figure 91–9 Prostate sections obtained from men with symptomatic benign prostatic hyperplasia were analyzed by double immunoenzymatic staining and quantitative image analysis. The percent area density of smooth muscle and connective tissue is significantly greater than glandular epithelium and glandular lumen area density (mean ± SEM).
(From Shapiro E, Becich MJ, et al. The relative proportion of stromal and epithelial hyperplasia is related to the development of symptomatic benign prostate hyperplasia. J Urol 1992;147:1293–7.)
Several additional observations on the prostatic stromal/smooth muscle cell are important. It is generally assumed that the stromal cells are resistant to the effects of androgen withdrawal. In short-term studies, androgen ablation appears to affect primarily the epithelial cell population. In general, however, stromal cells have much slower turnover rates than epithelial cells. If the effect of androgen ablation is primarily to increase cell death rates, a decrease in stromal cell numbers may not be appreciated until a year or more of therapy. Thus further study is required to determine whether the stromal cell is really resistant to androgen withdrawal. Likewise, it cannot be assumed that hormonal therapy has no effect on the stroma even if stromal cell volumes are not decreased. In a variety of smooth muscle cell systems (e.g., vascular and myometrial), contractile proteins, neuroreceptors, and ECM proteins are regulated by a variety of hormones and growth factors. In vitro, androgens have been shown to modulate the effects of α-agonists on prostate smooth muscle cells (Smith et al, 2000). Thus a given therapy may affect stromal cell function without decreasing the absolute number or volume of cells.
Studies of human tissue samples by Lin and colleagues (2000) have clearly shown that the smooth muscle cells from men with BPH have a significant downregulation of smooth muscle myosin heavy chain and a significant upregulation of nonmuscle myosin heavy chain. This myosin expression pattern is typical of dedifferentiated smooth muscle and indicates either proliferation or loss of normal modulation pathways.
Active smooth muscle tone in the human prostate is regulated by the adrenergic nervous system (Roehrborn and Schwinn, 2004). The α1-adrenoreceptor nomenclature has been standardized (Hieble et al, 1995) to reconcile differences in nomenclature based on pharmacologic and molecular studies. Receptor binding studies clearly demonstrate that α1A is the most abundant adrenoreceptor subtype present in the human prostate (Lepor et al, 1993a, 1993b; Price et al, 1993). Moreover, the α1A receptor clearly mediates active tension in human prostatic smooth muscle. It is still unclear whether other factors may regulate smooth muscle contraction. Endothelin and endothelin receptors (Kobayashi et al, 1994a, 1994b; Imajo et al, 1997; Walden et al, 1998) have been reported in human prostate. However, the physiologic role of this potent contractile agent in prostate smooth muscle function remains to be defined. Various components of the kallikrein-kinin system (e.g., bradykinin) may play a role in the regulation of both smooth muscle proliferation and contraction in the prostate (Walden et al, 1999; Srinivasan et al, 2004).
The presence of type 4 and type 5 phosphodiesterase isoenzymes in the prostate and the detrusor muscle of the bladder implies that phosphodiesterase inhibitors may be appropriate candidate therapies for BPH-related LUTS (Uckert et al, 2001, 2008, 2009). In fact, placebo-controlled trials have verified a beneficial effect of commercially available drugs for the treatment of erectile dysfunction in men with LUTS and BPH (McVary et al, 2007; Roehrborn et al, 2008; Stief et al, 2008).
The role of adrenergic stimulation in the prostate may exceed simple smooth muscle contraction. Adrenergic neurotransmitters are known to regulate expression of contractile protein genes in cardiac myocytes (Kariya et al, 1993) and to be involved in the development of cardiac hypertrophy (Matsui et al, 1994). Interestingly, evidence suggests that testosterone may regulate the expression of adrenergic receptors, at least in the kidney (Gong et al, 1995). It is possible that adrenergic neurotransmitters may play a role in prostatic smooth muscle cell regulation as well as contraction (Smith et al, 2000). α-Adrenergic blockade in patients with documented BPH leads to a significant downregulation of normal contractile protein gene expression, specifically smooth muscle myosin heavy chain.
Autonomic nervous system overactivity may contribute to LUTS in men with BPH. McVary and coworkers (2005) demonstrated that autonomic nervous system activity, as measured by a standard set of physiologic tests, plasma, and urinary catecholamines, correlates positively with symptom score and other BPH measures. Serum norepinephrine increase after tilt predicted prostate size (transition zone).
The Bladder’s Response to Obstruction
Current evidence suggests that the bladder’s response to obstruction is largely an adaptive one. However, it is also clear that many lower tract symptoms in men with BPH or prostate enlargement are related to obstruction-induced changes in bladder function rather than to outflow obstruction directly. Approximately one third of men continue to have significant voiding dysfunction and mostly storage symptoms after surgical relief of obstruction (Abrams et al, 1979). Obstruction-induced changes in the bladder are of two basic types. First, the changes that lead to detrusor instability or decreased compliance are clinically associated with symptoms of frequency and urgency. Second, the changes associated with decreased detrusor contractility are associated with further deterioration in the force of the urinary stream, hesitancy, intermittency, increased residual urine, and (in a minority of cases) detrusor failure. Acute urinary retention should not be viewed as an inevitable result of this process. Many patients presenting with acute urinary retention (AUR) have more than adequate detrusor function, with evidence of a precipitating event leading to the obstruction.
Much of our knowledge of the detrusor’s response to obstruction is based on experimental animal studies. Limited information is available on the natural history of the human bladder’s response to obstruction. It has been demonstrated that the major endoscopic detrusor change, trabeculation, is due to an increase in detrusor collagen (Gosling and Dixon, 1980; Gosling et al, 1986). Severe trabeculation is associated with significant residual urine (Barry et al, 1993), suggesting that incomplete emptying may be due to increased collagen rather than impaired muscle function. Severe trabeculation, however, is seen in fairly advanced disease. In experimental animal models the initial response of the detrusor to obstruction is the development of smooth muscle hypertrophy (Levin et al, 1995, 2000). It is likely that this increase in muscle mass, although an adaptive response to increased intravesical pressure and maintained flow, is associated with significant intracellular and extracellular changes in the smooth muscle cell that lead to detrusor instability and in some cases impaired contractility. Obstruction also induces changes in smooth muscle cell contractile protein expression, impaired energy production (mitochondrial dysfunction), calcium signaling abnormalities, and impaired cell-to-cell communication (Levin et al, 1995, 2000).
There is considerable evidence that the response of the detrusor smooth muscle cell to stress (increased load related to outlet obstruction) is not as adaptive as the response of skeletal muscle to stress. In the latter case, a relatively normal repertoire of contractile protein genes are upregulated and an increased number of normally organized contractile units assemble in the muscle cell. In the detrusor smooth muscle cell, load-induced hypertrophy leads to a change in myosin heavy chain isoform expression (Lin and McConnell, 1994; Cher et al, 1996) and to a significant alteration in the expression of a variety of thin filament-associated proteins (Mannikarottu et al, 2005a, 2005b, 2006). Taken together, these observations strongly suggest that smooth muscle cells revert to a secretory phenotype in response to obstruction-induced hypertrophy. One consequence of this phenotypic switch is increased ECM production. The detrusor smooth muscle cell is a key contributor to the complex of symptoms associated with prostatic obstruction. Additional research in this area is required (Christ and Liebert, 2005).
In experimental animal models, unrelieved obstruction is associated with the development of significant increases in detrusor ECM (collagen) (Levin et al, 1995, 2000). This also appears to be the case in the human, although cause-and-effect relationships have not been established (Gosling et al, 1986). In addition to obstruction-induced changes in the smooth muscle cell and ECM of the bladder there is increasing evidence that obstruction may modulate neural-detrusor responses as well (Steers et al, 1990, 1999; Clemow et al, 1998, 2000). Altered neural control of micturition has been noted in aging rats, including reduced bladder contractility, impaired central processing, and altered sensation (Chai et al, 2000).
Independent of obstruction, aging produces some of the same changes in bladder function, histology, and cellular function (Nordling, 2002). There is suggestive evidence from animal models that atherosclerosis and the resultant chronic bladder ischemia or hypoxia induced by other mechanisms (e.g., increased bladder wall tension) may contribute to bladder pathology (Tarcan et al, 1998; Azadzoi et al, 1999, 2003, 2008; Azadzoi, 2003).
Key Points: Etiology and Pathophysiology
Epidemiology and Natural History
Definitions
The study of epidemiology determines the distribution and determinants of diseases in man. From this evolve the components of descriptive epidemiology, which is the description of disease incidence, mortality and prevalence by person, place, and time, and analytical epidemiology, which is the search for determinants of disease risk that may serve to increase prospects for prevention (Oishi et al, 1989). Epidemiologists assess and compare rates of diseases within one population stratified by sex, age, and other demographic and socioeconomic parameters and between populations of different culture, ethnicity, lifestyles, and diet.
The following definitions of rates are important to understand:
Although for highly fatal conditions (those with a high fatality and mortality rate) the incidence rate (i.e., the rate of people developing the condition in a year) is of pre-eminent interest, for conditions such as BPH, which are rather benign in their course, the prevalence rate (i.e., the number of men having the condition at a given point in time) is of greater interest.
There is no globally accepted epidemiologic definition of BPH; thus prevalence and incidence rates must be viewed in the context of the definitions chosen by the investigator reporting the data (Barry, 1990a, 1990b). The prevalence of BPH thus can be calculated based on histologic criteria (autopsy prevalence) or on clinical criteria (clinical prevalence). Because the clinical definitions vary widely, it is easier to compare the autopsy or histologic prevalence of BPH. Despite the low mortality and fatality rates for BPH in modern series a review of these data is interesting and revealing from a historical point of view.
Lastly, descriptive epidemiologic studies can be divided into cross-sectional (a population stratified by baseline parameters is assessed one single time to determine if and how certain measures change depending on the parameter of interest) and longitudinal (a population is assessed at baseline and in regular intervals to study the changes in parameters of interest stratified by age or other demographic criteria) studies. It is obviously easier to perform cross-sectional studies owing to the cost and logistical difficulties involved with longitudinal follow-up of a cohort over time. The majority of studies addressing LUTS and clinical BPH are cross-sectional. Furthermore, there are by definition no longitudinal autopsy studies of any condition and histologically based longitudinal studies are exceedingly difficult to perform owing to the need for repetitive tissue procurement.
Descriptive Epidemiologic Studies
Histologic or Autopsy Prevalence
The definition of BPH is the presence of stromoglandular hyperplasia on a surgical specimen or in the case of autopsy series in whole prostates removed after death from men not dying of prostate conditions. The 1984 landmark study by Berry and associates (1984) summarized the data from five studies demonstrating that no men younger than the age of 30 had evidence of BPH and that the prevalence rose with each age group, peaking at 88% in men in their 80s. Figure 91–10 demonstrates the age-stratified prevalence based on several rigorously performed autopsy studies in the United States, England, Austria, Norway, Denmark, China, Japan, and India. The prevalence increases rapidly in the fourth decade of life, reaching nearly 100% in the ninth decade. It is striking that the age-specific autopsy prevalence is remarkably similar in all populations studied regardless of ethnic and geographic origin (Moore, 1943; Swyer, 1944; Franks, 1954; Karube, 1961; Harbitz and Haugen, 1972; Haugen and Harbitz, 1972; Pradhan and Chandra, 1975; Holund, 1980; Berry et al, 1984; Carter and Coffey, 1990).
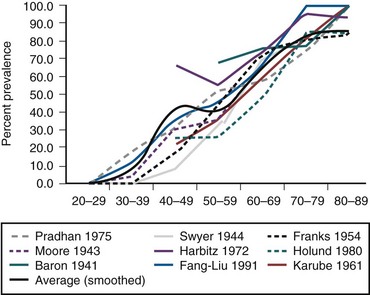
Figure 91–10 Age-stratified autopsy prevalence of histologic benign prostatic hyperplasia from different series and (smoothed) average.
(Data from Moore, 1943; Swyer, 1944; Franks, 1954; Karube, 1961; Harbitz and Haugen, 1972; Haugen and Harbitz, 1972; Pradhan and Chandra, 1975; Holund, 1980; Berry et al, 1984; Carter and Coffey, 1990.)
Cross-Sectional Studies of Clinical Prevalence
Descriptive epidemiology relies on the presence of a single universally accepted definition of disease. The definitions of BPH, however, have undergone several changes in the past decade, and at present no single criterion can be applied. In the past the term prostatism was used, incorrectly referring to the prostate as the sole source of the typical LUTS found in aging men. Tage Hald pointed out that there are at least three inter-related phenomena that can be assessed independently, namely, the symptoms (formerly called “prostatism”), enlargement of the prostate gland, and presence of obstruction (Nielsen et al, 1994). In a given patient, all three, two of the three, or only one of the three entities might be present. Paul Abrams coined the term lower urinary tract symptoms to replace the old and inappropriate term prostatism (Chapple et al, 2008). When evaluating elderly men, one can therefore stratify them by the level of LUTS into mildly, moderately, and severely symptomatic according to a standardized symptom severity and frequency questionnaire (Barry et al, 1992a). The same patients then can be further classified based on the degree of prostatic enlargement as measured by digital rectal examination (DRE), transrectal ultrasonography (TRUS), or magnetic resonance imaging (MRI) and lastly by the presence and degree of BOO as measured by flow rate recordings or invasive pressure flow studies. The diagram in Figure 91–1 attempts to illustrate the difficulties in using different disease definitions. Of all men older than the age of 40, a certain proportion will develop histologic hyperplasia of the prostate, that is, BPH. Of those, some will develop LUTS, while others may have LUTS due to reasons other than BPH (e.g., overactive bladder or other bladder- and detrusor-related conditions, urethral stricture, stones, inflammation). Prostate enlargement occurs in some but again not all men with histologic BPH and LUTS, and some men with enlarged glands may not have any symptoms at all. Lastly, urodynamically proven obstruction may be present in men who have either one or several or all of histologic BPH, LUTS, and enlarged glands, whereas others yet may have obstruction without having any evidence of BPH (e.g., urethral stricture, prostate cancer, primary bladder neck sclerosis). In addition to the mere enumeration of symptoms by frequency of occurrence, the bother associated with the symptoms, interference with activities of daily living, and the impact the symptoms have on quality of life are important distinguishing characteristics.
Accordingly, when studying the prevalence of clinical BPH—admittedly an imprecise term describing the just-described constellation of LUTS, bother, interference, quality of life impact, with or without enlargement, obstruction, and so on—disease definitions may be applied that take either one or several of these items into consideration. For the subsequent discussion it is important to recognize that very few clear cutoff points have been established that allow differentiation between whether disease is absent or present (e.g., one might argue that a prostate volume over 30 mL constitutes clinical BPH, but others might argue for a higher or lower cutoff point with similar observations for symptoms or degrees of obstruction). Thus, rather than describing truly the prevalence of a “disease” in populations, one can describe the distribution of certain attributes of such disease in different populations stratified by age. Figure 91–11 illustrates the different estimates of prevalence of “disease” when different definitions are applied ranging from autopsy prevalence to a combination of clinical threshold parameters and insurance examination data (Berry et al, 1984; Garraway et al, 1991; Chute et al, 1993; Gu et al, 1994; Jolleys et al, 1994; Bosch et al, 1995a; Guess, 1995; Moon et al, 1995; Overland et al, 2001).
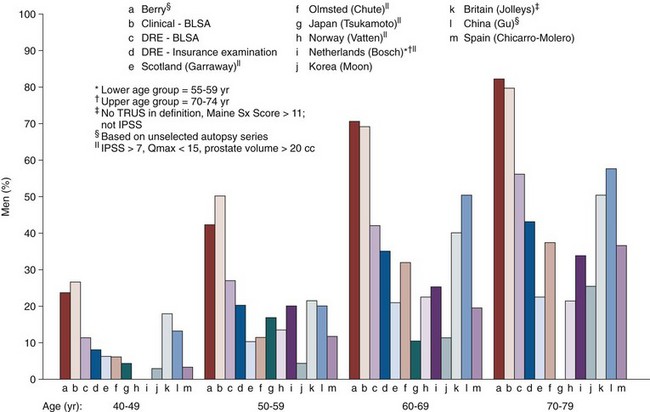
Figure 91–11 Prevalence of disease using autopsy series, clinical diagnosis, low maximum flow rate, palpable prostatic enlargement by digital rectal examination (DRE), and community-based studies. BLSA, Baltimore Longitudinal Study of Aging; IPSS, International Prostate Symptom Score; TRUS, transrectal ultrasonography.
(Data from Berry et al, 1984; Garraway et al, 1991; Chute et al, 1993; Gu et al, 1994; Jolleys et al, 1994; Bosch et al, 1995a; Moon et al, 1995; Overland et al, 2001.)
Symptom Severity and Frequency
From a pragmatic point of view, studies of symptom severity and frequency are of greatest importance in a disease that is rarely fatal and is characterized by its effect on the quality of life. The development, validation, translation with cultural and linguistic validation of the standardized, self-administered seven-item American Urological Association (AUA) Symptom Index (AUASI, also known as the International Prostate Symptom Score [IPSS]) has been a pivotal event in the clinical research of LUTS and BPH (Barry et al, 1992a, 1992b; O’Leary et al, 1992). With the total score running from 0 to 35 points, patients scoring 0 to 7 points are classified as mildly symptomatic, those scoring from 8 to 19 points as moderately symptomatic, and those scoring 20 to 35 points as severely symptomatic. The instrument is an integral part of virtually every epidemiologic study as well as treatment studies in the field, and the availability of validated translations in many common languages allows cross-cultural comparisons of unprecedented scope. Socioeconomic factors do not seem to influence responses to the questionnaire (Moon et al, 1994), and fundamentally similar responses are obtained when the questionnaire is self-administered, read to the patient, mailed in or administered in some other way (Barry et al, 1995a), or readministered in a scrambled format (Barnboym et al, 1999). However, there is no question that subtle differences in comprehension of the translated questionnaire as well as different perception of the symptoms, willingness to admit to the symptoms, acceptance of symptoms as a natural sign of aging, and other factors are at least partially the cause for cross-cultural differences in symptom severity reported in the literature. Figure 91–12 shows the prevalence of at least moderate to severe symptoms stratified by decade of life as reported in 11 cross-sectional population-based studies from around the world (Garraway et al, 1991; Chute et al, 1993; Hunter et al, 1994, 1996; Norman et al, 1994; Bosch et al, 1995a; Moon et al, 1995; Tsukamoto et al, 1995; Sagnier et al, 1996; Overland et al, 2001). A very large international investigation of LUTS in Asian men was undertaken by Homma and associates (1997), in which 7588 men from Japan, China, Taiwan, Korea, the Philippines, Thailand, Singapore, Pakistan, India, and Australia were queried. The finding of 18%, 29%, 40%, and 56% of men in their 40s, 50s, 60s, and 70s, respectively, having moderate to severe symptoms is in line with the other studies reported both from Asia and from Europe and North America. In addition to the major community-based studies listed, other studies have been published with similar findings but often done under less stringent conditions (Nacey et al, 1995; Tay et al, 1996). Despite the significantly different proportion of men admitting to moderate to severe symptoms, a clear trend toward an increase in symptom scores with advancing age is noticeable in all reported studies.
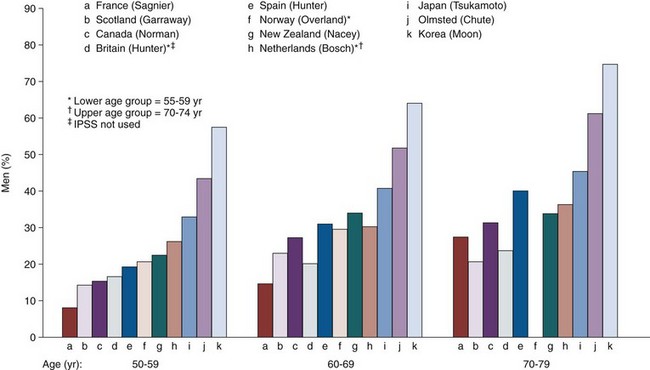
Figure 91–12 Prevalence of at least moderate to severe symptoms stratified by decade of life as reported in cross-sectional population-based studies from around the world.
(Data from Garraway et al, 1991; Chute et al, 1993; Hunter et al, 1994; Norman et al, 1994; Bosch et al, 1995a. Moon et al, 1995; Tsukamoto et al, 1995; Hunter et al, 1996; Sagnier et al, 1996; Homma et al, 1997; Overland et al, 2001).
Bother, Interference, and Health-Related Quality of Life (HRQOL)
Bother and interference with activities of daily living due to LUTS are equally if not more important than the enumeration of symptom frequency and severity alone (Garraway et al, 1993b; Roberts et al, 1994b). Embarrassment due to symptoms has been found to be an important determinant in seeking medical care (Roberts et al, 1994b). LUTS and clinical BPH affect overall quality of life as measured, for example, with the Medical Outcomes Trust Short-Form (SF-36) only marginally (Hunter et al, 1995). Accordingly, several instruments were developed and validated to assess bother, interference, and the disease-specific quality of life and sexual function (Epstein et al, 1992; Barry et al, 1995b; Hansen et al, 1995; Lukacs et al, 1995; O’Leary et al, 1995; Donovan et al, 1996).
These instruments have not been as widely applied to cross-sectional descriptive epidemiologic studies as compared with the IPSS. However, as observed for symptom severity and frequency, both bother and interference scores increase with advancing age, and disease-specific HRQOL measures are significantly worse in men with higher symptom frequency and severity ratings in population-based studies done in Olmsted County in the United States, in Forth Valley in Scotland, in France, and in a small fishing village in Japan (Shimamaki-Mura) (Girman et al, 1998).
Prostate Size
Prostate size can be estimated by DRE, although the reliability across observers is in general considered poor (Roehrborn et al, 1997). A recent analysis of the PLCO trial verified the significant errors associated with the DRE-assessed prostate size (Pinsky et al, 2006). In addition, DRE tends to underestimate true prostate size as determined by TRUS or other imaging modalities. The magnitude of the underestimation increases with increasing prostate size from 25% up to 50% or more (Roehrborn et al, 1997). For the purpose of epidemiologic studies TRUS and MRI measurements are preferred, although MRI measurements are somewhat expensive when attempting cross-sectional examinations of populations. TRUS volume measurements using the prolate ellipsoid volume formula are the most widely accepted measure of prostate volume with reasonable statistical performance characteristics, particularly when performed by a single or several well-trained examiners (Sech et al, 2001). Three-dimensional TRUS evaluation appears to provide even more accurate measurements, although such technology is not available in urologists’ offices for the most part (Giubilei et al, 2005).
In general, in all cross-sectional studies prostate volume as assessed by TRUS has been found to increase slowly but steadily with advancing age. The slight differences in the absolute volume measures and the different slopes of increase with advancing age may be caused by differences in the population examined as follows (Fig. 91–13).
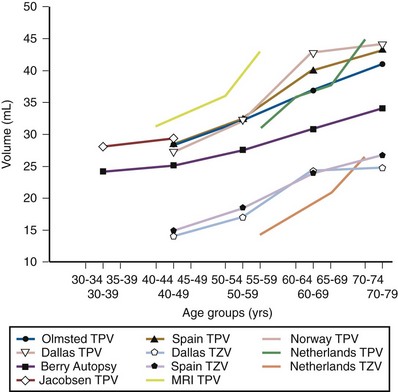
Figure 91–13 Mean estimates for total prostatic volume (TPV) and transitional zone volume (TZV) based on one autopsy series (Berry et al, 1984), one series of baseline MRI measurements in men with alopecia (Roehrborn et al, 2000b), and cross-sectional population-based studies (Torp-Pedersen et al, 1988; Oesterling et al, 1993; Bosch et al, 1994; Benaim et al, 1998; Chicharro-Molero et al, 1998; Overland et al, 2001).
A cohort of 344 men between 40 and 60 years old with no evidence of BPH enrolled in an alopecia study had baseline endorectal coil MRI measurements done (Roehrborn et al, 2000b). Mean total prostate volume (TPV) increased from 31.3, 33.7, 36.1, to 43.1 mL in increments of 5 years. MRI tends to yield prostate volume measurements approximately 10% larger compared with TRUS. A series of 100 men aged 40 to 80 years without BPH or LUTS underwent TRUS measurements of TPV and transition zone volume (TZV). TPV increased from 22.1, 29.1, 41.5, to 43.2 mL by decade, and TZV from 7.2, 9.9, 19.0, to 19.6 mL (Benaim et al, 1998). A cross-sectional study of 611 Norwegian men from 55 to 70 years of age exhibited increases in TPV from 26.5, 31.0, to 32.0 mL in increments of 5 years (Overland et al, 2001), and Jakobsen and associates (1988) examined patients between 30 and 50 years by TRUS, the mean TPV being 23.9 and 25.7 mL per decade of life. TPV and TZV were assessed in 1104 men older than the age of 40 years in a Spanish cross-sectional study, yielding increases from 23.4 to 41.9 mL for TPV and from 7.9 to 21.9 mL for TZV (Chicharro-Molero et al, 1998). Finally, the baseline data from the Olmsted County study provided data on men from 40 to 79 years of age (Oesterling et al, 1993).
Overall, TPV increases in these cross-sectional studies from approximately 25 mL for men in their 30s to 35 to 45 mL for men in their 70s, whereas TZV increases from 15 mL to 25 mL for similarly aged men. The similarities between the TPV and TZV measurements done in the different studies are striking particularly when considering the different measurement methods used (see Fig. 91–13).
Measures of Obstruction
Subvesical obstruction only can be measured by invasive pressure-flow studies, whereas nonintubated free flow rates provide at best an indirect measure for the probability of obstruction being present (Abrams, 1995). Unfortunately, no large-scale cross-sectional studies have been done employing pressure-flow studies owing to the invasive and costly nature of the test and it is unlikely that significant datasets will ever become available.
It is commonly accepted that a maximum flow rate of less than 10 mL/sec indicates a high probability of obstruction, whereas a flow rate of greater than 15 mL/sec indicates a low probability, with the 10- to 15-mL/sec range presenting an intermediate range. The utility of this categorization is reduced by several observations. First, the maximum flow rate is somewhat dependent of the voided volume (Girman et al, 1993). This has prompted some to propose a nomogram to correct for this phenomenon; however, at present no single nomogram is accepted universally (Von Garrelts, 1956, 1957, 1958; Scott and McIlhaney, 1961; Beck and Gaudin, 1969; Susset et al, 1973; Siroky et al, 1979, 1980; Drach and Steinbronn, 1986; Haylen et al, 1998). A high degree of diurnal variability (Golomb et al, 1992) and within-subject day-to-day variability (Barry et al, 1995c) further reduces the utility of flow rate recording in defining disease.
Aging men and women experience a decrease in the maximum urinary flow rate, which is nearly linear. In the Olmsted County study the median flow rate decreased from 20.3 mL/sec for men 40 to 44 years old to 11.3 mL/sec for men 75 to 79 years old (Girman et al, 1993), and similar declines have been reported from other cross-sectional studies. When applying combined thresholds such as at least moderate symptoms of more than 7 points on the IPSS score and a maximum flow rate of less than 15 mL/sec, in the Olmsted County study 17% of men in their 50s, 27% of men in their 60s, and 35% of men in their 70s would fall into such category (Jacobsen et al, 1995b) versus 14.4%, 28.6%, and 38.7% of men in the respective age groups in the Spanish population-based study (Chicharro-Molero et al, 1998).
Analytical Epidemiologic Studies
Analytical epidemiologic studies address the search for determinant of a disease. Inasmuch as a clear disease definition is lacking, such a search could attempt to find determinants for LUTS, prostate growth, and/or BOO, consistent with the previously outlined concepts. The presence of functioning testes at time of puberty (hormonal factor) as a required permissive element has long been established and accepted (McConnell, 1991), and age has been shown to be the most critical determinant of all aspect of this complex entity. Numerous other demographic and environmental factors have been suggested as risk factors or contributors to the disease process. When evaluating the associations identified one has to critically ask whether other factors could contribute to the association without their being a cause-and-effect relationship. For example, it seems intuitive that the husbands and family members of nurses are more likely to seek medical care and practice preventive medicine. Therefore the detection rates of prostate cancer in a cohort of nurses’ husbands might be higher not due to a truly higher incidence rate of cancer but due to an increased rate of seeking medical attention and an increase in the number of diagnosed cases of cancer. Such pitfalls are abundant in epidemiologic studies and have to be suspected and carefully ruled out.
Religion
The case-control study by Morrison (1978), the cohort study by Lytton and colleagues (1968), and the Normative Aging Study (Glynn et al, 1985) all revealed the Jewish religion to be associated with a higher prostatectomy rate (2.2- to 2.6-fold increase). The fact, however, that the Jewish religion is not associated with an increase in the diagnosis of BPH allows the speculation that Jewish patients are more likely to see a physician or to be offered surgical therapy.
Socioeconomic Factors
Araki and associates (1983) found higher rates of BPH in higher income groups, whereas, in contrast, Glynn and coworkers (1985) reported higher rates of surgery in lower income groups. One might argue that higher income groups might have better access to health care whereas lower income groups might submit more readily to the suggestion of a surgical procedure. Education and socioeconomic status do not influence responses to the IPSS (Moon et al, 1994; Badia et al, 2001), but they do appear to influence both expectations from treatment for BPH and perception of improvement, with patients in higher income strata requiring a larger drop in symptom scores after treatment to perceive subjectively similar levels of improvement (Padley et al, 1997). This finding suggests at least some impact of socioeconomic factors if not in the growth of the prostate or measures of obstruction but likely in the perception of symptoms. Data from the Olmsted County study suggest a relationship between care-seeking behavior and physician visit and retirement status. Bivariate analysis suggested significant associations between a propensity to seek care for physical reasons and retirement (odds ratio [OR], 2.0; 95% CI, 1.1 to 2.6), age of 65 years or more (OR, 1.9; 95% CI, 1.5 to 2.4), incomplete high school education (OR, 1.6; 95% CI, 1.1 to 2.2), and an annual income of less than $25,000 (OR, 1.4; 95% CI, 1.1 to 1.9). Multivariable logistic regression analysis demonstrated that retired men were more likely to have a high propensity to seek care (OR, 1.7; 95% CI, 1.2 to 2.4), with the other variables no longer-being significant (Roberts et al, 1997a).
Recently our understanding of the impact of some socioeconomic factors on LUTS and BPH had deepened with the help of two studies, the European Prospective Investigation into Cancer and Nutrition (EPIC) and the Epidemiology of Lower Urinary Tract Symptoms (EpiLUTS), and the Boston Area Community Health (BACH) studies (Rosen et al, 2008; Kaplan et al, 2009) (Table 91–4). It is likely that the longitudinally designed BACH study with a balanced enrollment of men and women stratified by age and race/ethnicity will contribute in the future significantly to our understanding of LUTS in both men and women. A symptom-based cluster analysis was performed in both cohorts, and remarkably similar clusters of symptoms were identified, allowing for an in-depth analysis of their association with comorbidities and other factors (Rosen et al, 2008). In the BACH study, decreasing household income and increasing difficulties in paying for health care were associated with increasingly more severe symptom clusters (Hall et al, 2009).
Table 91–4 Overview of the EpiLUTS and BACH Studies
| EpiLUTS | BACH |
|---|---|
| Description | |
| Cross-sectional, population-representative, Internet-based survey conducted in the United States, the United Kingdom, and Sweden in 30,000 (U.S., 20,000; U.K., 7500; Sweden, 2500) men and women aged 40-99 years (mean age, 56.6 years) | Population-based epidemiologic survey among 5503 randomly selected adults residing in Boston aged 30-79 years in three race/ethnic groups (2301 men, 3202 women; 1767 black, 1877 Hispanic, 1859 white respondents) |
| LUTS Prevalence | |
| 1 LUTS at least sometimes: 72.3% (men), 76.3% (women) 1 LUTS at least often: 79.99% (men), 52.5% (women) |
LUTS (AUASI ≥8): 18.7% (men), 18.6% (women); increased with age (10.5% for 30-39 years to 25.5% for 70-79 years), no difference by race/ethnicity (16.2%-19.3%) |
| Bother | |
| Rates of bother were lower for LUTS classified as at least “sometimes” than those classified as at least “often” However, leaking urine during sexual activity, which was reported infrequently, was highly bothersome (82.1% men; 87.2% women), whereas terminal dribble, which was reported frequently, was less bothersome (40.6% men; 40.2% women) |
Mean bother scores were higher in those with LUTS (vs. no LUTS) and for women vs. men |
| Treatment Seeking | |
| Treatment seeking was low but most common in those in the voiding + storage + postmicturition subgroup: 29.1% men, 27.5% women; prescription medication use was also highest in this subgroup (17.6% and 10.4%, respectively) | Prescription medication use for urinary symptoms was low even among those with AUASI ≥8, at 9.8% (men) and 2.1% (women) |
| Comorbidities | |
| Comorbid conditions were most common in the voiding + storage + postmicturition subgroup with significant associations for LUTS with arthritis, asthma, chronic anxiety, depression, diabetes (men only), heart disease, irritable bowel syndrome, neurologic conditions, recurrent UTI, and sleep disorders; childhood nocturnal enuresis was significantly associated with most LUTS subgroups | LUTS (AUASI ≥8) was associated with heart disease, diabetes (men only), and depression |
AUASI, American Urological Association Symptom Index; LUTS, lower urinary tract symptoms; UTI, urinary tract infection.
From Kaplan SA, Roehrborn CG, et al. Implications of recent epidemiology studies for the clinical management of lower urinary tract symptoms. BJU Int 2009;103(Suppl. 3):48–57.
Sexual Activity and Vasectomy
Ekman (1989) suggested that the increase in the fibromuscular stroma is a result of sexual activity, and many authors since then have attempted to find relevant associations. Morrison (1992) reported a 49% reduction in risk for prostatectomy in widowed versus single men. A similar association could not be verified by other authors. Cross-sectional data from the Olmsted County study suggest that the frequency of ejaculation has no effect on LUTS, peak urinary flow rates, or prostate volume; the apparent protective association appears to be an artifact caused by the confounding effects of age (Jacobsen et al, 2003). The decrease in sexual ability and frequency of sexual activity with advancing age, exactly when the prevalence of BPH increases, in fact might suggest a reverse relationship, namely, a causative effect of BPH on sexual function (Altwein and Keuler, 1992).
Recent evidence suggests a strong correlation between the severity of LUTS and impairment of sexual function, that is, erectile dysfunction (ED) as well as ejaculatory disturbances (Boyle et al, 2003; Chung et al, 2003; Rosen et al, 2003). Cross-sectional questionnaire-based data from various countries suggest that ED and ejaculatory disturbances increase with advancing age but also within each decade of life with increasing LUTS severity (Fig. 91–14). Although these correlations do not necessarily imply a causative mechanism, it is possible that similar pathophysiologic events underlie the development of both problems in the aging male, the most obvious being ischemia in both the genital and lower urinary tract organs (McVary, 2005). A logistical regression analysis of the EpiLUTS data documented as well a strong relationship between various domains of sexual and ejaculatory dysfunction and LUTS (Wein et al, 2009) (Table 91–5).
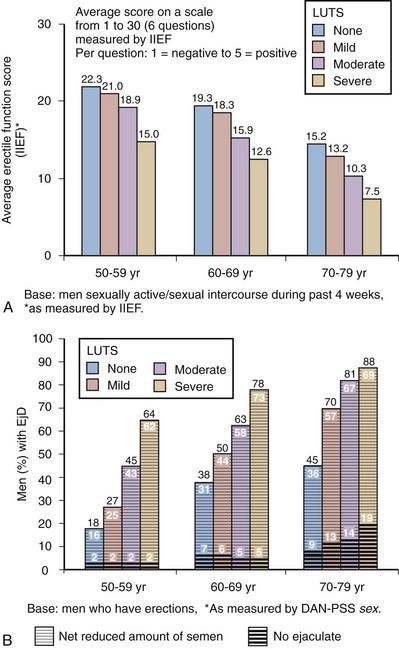
Figure 91–14 Erectile function (A) decreases and ejaculatory disturbances (B) increase with advancing age, but within each decade of life they also decrease and increase, respectively, with increasing severity in lower urinary tract symptoms (LUTS). DAN-PSS, Danish Prostatic Symptom Score; IIEF, International Index of Erectile Function.
(From Rosen R, Altwein J, et al. Lower urinary tract symptoms and male sexual dysfunction: the multinational survey of the aging male [MSAM-7]. Eur Urol 2003;44[6]:637–49.)
Table 91–5 Logistic Regression of Decreased Sexual Enjoyment and Activity as Well as Predictors of Erectile Dysfunction (ED), Ejaculatory Dysfunction (EjD), and Premature Ejaculation (PE)
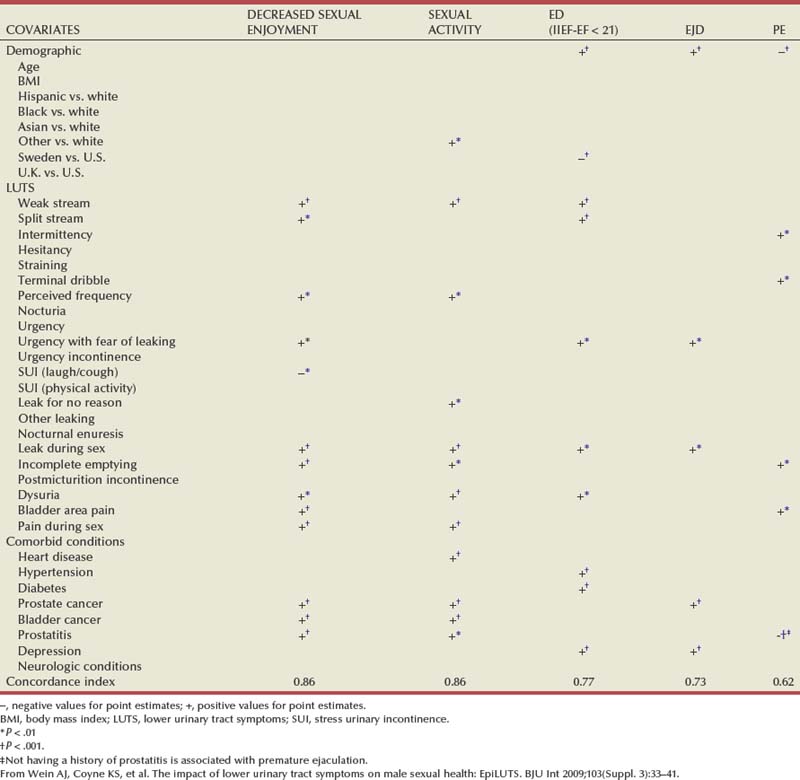
Sidney (1987) analyzed the Kaiser Permanente Database and initially found a risk ratio of 1.2 for the diagnosis of BPH among men who had a vasectomy. However, after 5 years of follow-up the risk ratio was 0.97 and not significant. There does not appear to be a relationship between vasectomy and BPH development or prostate size (Jakobsen et al, 1988); and in the Massachusetts Male Aging Study, Meigs and colleagues (2001) did not find that vasectomy increased the risk of being diagnosed with BPH.
Alcohol and Liver Cirrhosis
Alcohol may decrease plasma testosterone levels and production and increase clearance of testosterone (Chopra et al, 1973). Despite this hypothetical reason for a lower incidence of BPH, inverse relationships have been described. Both Morrison (1992) and Sidney and colleagues (1991b) reported by multivariate analysis an adjusted risk ratio of 0.49 and an age-adjusted risk ratio of 0.75, respectively, for the risk of having surgery for BPH when taking in more than three glasses of alcohol per day. However, one might argue that the poorer health in heavy drinkers might bias the physician against surgery. Glynn and coworkers (1985), in fact, found no increased risk for either the clinical diagnosis or surgical rates. A recent analysis of the Prostate Cancer Prevention Trial (PCPT) showed as well an inverse relationship between a diagnosis of BPH and alcohol intake, that is, a somewhat protective effect (Kristal et al, 2008).
Of five studies examining the relationship between liver cirrhosis and BPH based on autopsy material, four found a lower prevalence of BPH in men with cirrhosis (Bennett et al, 1950; Stumpf and Wilens, 1953; Robson, 1966; Frea et al, 1987) while one study—admittedly with some design flaws—found a higher prevalence (Wu, 1942). Because most cirrhosis cases are alcohol induced, the separation of the effects of alcohol versus cirrhosis is virtually impossible.
Hypertension
The sympathetic nervous system plays via α-adrenergic fibers and receptors an important role in both hypertension and the symptoms of BPH. However, because both hypertension and LUTS/BPH increase with advancing age, it is difficult to prove a causal relationship between these two conditions (Boyle and Napalkov, 1995). The previously cited studies by Glynn and Sidney and their colleagues (Glynn et al, 1985; Sidney et al, 1991b) did not find an association. In a small clinic cohort study with methodologic flaws, Pressler and coworkers (1997) reported an increase in the incidence of hypertension from 15%, to 18% and 31% for men with mild, moderate, and severe symptoms. Autonomic hyperactivity has been implicated in the development of both LUTS and ED in the aging male, but conclusive clinical data are lacking (McVary et al, 2005). In the EpiLUTS study both heart diseases and hypertension were associated with more severe LUTS constellations (Coyne et al, 2009). Further studies are needed to better understand the common underlying pathophysiologic mechanisms and a potential cause-and-effect relationship.
Smoking
Smoking cigarettes appears to increase both testosterone and estrogen levels owing to the nicotine level and thus should have a positive and inductive effect on the development of BPH. However, because severe smoking causes other health problems, surgery rates are probably a poor indicator for a possible correlation because physicians are biased against surgery in heavy smokers. Seitter and Barrett-Connor (1992) indeed found no correlation between smoking and prostatectomy rates, and Glynn and colleagues (1985) failed to identify a correlation between smoking and the clinical diagnosis of BPH. Sidney and associates (1991b) followed 16,000 men over 15 years and found a negative correlation between being a smoker at baseline and subsequent risk of prostatectomy. Daniell (1993b) examined the records of 345 patients who underwent prostatectomy and found smaller gland volumes in smokers and a lower age-adjusted prevalence of ever-smokers compared with a control group. Roberts and associates (1994a) examined this issue in the Olmsted County study and later (1997b) in a Japanese population. In the Olmsted County study only 16% of the more than 2000 men were current smokers, which appears low but understandable considering the population mix in Rochester, Minnesota. A biphasic association was found, with light to moderate smokers being less likely to have moderate to severe LUTS and heavy smokers at least the same risk as never smokers.
A recent update from the Olmsted County study suggests that smoking is associated with decreased urinary flow rates and moderate to severe symptoms but not with enlarged prostate volume or increasing serum PSA (Rule et al, 2005). This is in contrast to earlier studies suggesting a relationship between smoking and prostate size (Kupeli et al, 1997). In EpiLUTS an ex-smoker had an increased risk of having more severe symptom constellations and a man who had never smoked had a decreased risk, while the risk for a current smoker was not a factor (Coyne et al, 2009).
Any relationships between current or past smoking and LUTS/BPH are likely to be weak and of limited clinical significance.
Physical Activity, Diet, Obesity, Body Mass Index (BMI), and the Metabolic Syndrome
Chyou examined 33 food items in relationship to prostatectomy rates and found only beef intake significantly associated (Chyou et al, 1993). Araki and associates (1983) reported increased clinical diagnosis of BPH in men with higher milk consumption and lower consumption of green and yellow vegetables. Overall there is no convincing evidence for any individual diet factor to play a major role in the development of LUTS/BPH.
The relationships between LUTS/BPH and obesity, BMI, and the metabolic syndrome have recently been of great interest (Hammarsten et al, 1998, 1999, 2001; Rohrmann et al, 2005; Gupta et al, 2006; Kasturi et al, 2006). Metabolic syndrome is a clinical constellation of metabolic abnormalities, including obesity, glucose intolerance, dyslipidemia, and hypertension that increases the risk of cardiovascular disease and results primarily from modifiable risk factors, particularly physical inactivity and dietary practices endemic to Westernized societies (Haffner and Taegtmeyer, 2003). There are plausible biologic considerations: adipose tissue is the main source of aromatization of testosterone to estrogen, and men with lower BMI have higher serum testosterone levels (Eldrup et al, 1987).
BMI was negatively associated with surgically treated BPH in the Kaiser Permanente cohort study (Sidney et al, 1991b), and with a clinical diagnosis of BPH in the normative aging study (Glynn et al, 1985). In contrast, in a study of 68 men the average prostate weight increased both with age and with increasing obesity together with an increase in serum estradiol levels (Soygur et al, 1996). Daniell (1993a) also found larger adenomas in more obese men undergoing prostatectomy. Both latter studies report a positive correlation between obesity and prostate size, but none between obesity and symptom severity. Several caveats must be mentioned: DRE is less likely to yield a diagnosis of BPH and prostate enlargement in very obese patients owing to anatomic obstacles, and patients with high BMI may be biased against surgical interventions.
Men aged 40 to 75 years who were participants in the Health Professionals Follow-up Study and who were without prior diagnosis of cancer or prostatectomy provided data on weight, height, and waist, and hip circumferences. After adjustment for age, smoking, and BMI, abdominal obesity was related to prostatectomy (OR = 2.38) and with frequent urinary symptoms among those without prostatectomy (OR = 2.00). BMI, hip circumference, and waist-to-hip ratio were not associated with BPH independently of waist circumference. These results suggest that abdominal obesity in men may increase the frequency and severity of urinary obstructive symptoms and may increase the likelihood that such obese men will undergo a prostatectomy (Giovannucci et al, 1994).
Hammarsten and Hogstedt (1999) examined 250 patients with LUTS and found non–insulin-dependent diabetes mellitus, hypertension, tallness, obesity, high insulin level, and low high-density lipoprotein cholesterol levels to be risk factors for the development of BPH. They suggest a causal relationship between high insulin levels and the development of BPH and hypothesize increased sympathetic nerve activity in men with BPH. In a hospital-based case-control study being overweight was modestly inversely related to BPH. The hypothesis of reduced testosterone levels in obese individuals may explain the different BPH risk and need to be tested (Zucchetto et al, 2005). Parsons (2007) performed a comprehensive literature review and found factors that potentially increase the risk of BPH and LUTS include obesity and diabetes whereas factors that potentially decrease the risk include increased physical activity and moderate alcohol consumption (Table 91–6). Parsons and Kashefi (2008) performed also a comprehensive literature review and found eight studies (n = 35,675) were eligible for a pooled analysis to study the effect of physical activity levels stratified into light, moderate, and vigorous categories, with a sedentary category for reference. Compared with the sedentary group, the pooled ORs for BPH or LUTS were 0.70, 0.74, and 0.74 for men engaging in light, moderate, and heavy physical activity, respectively. Physical activity thus appears to reduce the risks of BPH and LUTS. Similar findings of increased likelihood of LUTS with increasing BMI and decreasing likelihood with greater physical activity were also reported from the EpiLUTS study (Coyne et al, 2009).
Table 91–6 Cohort Studies of Modifiable Risk Factor Associated with Decreased or Increased Risk of LUTS and/or BPH
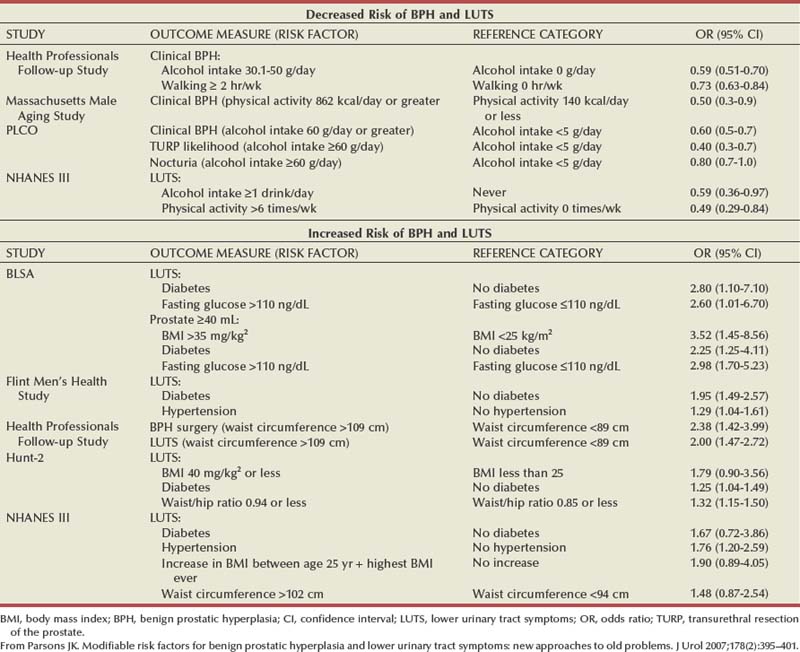
The evidence at present suggests a positive relationship between lack of physical activity, obesity, BMI, and other measures of the metabolic syndrome and both LUTS and BPH (including prostate volume), whereas increased physical activity appears to have a protective effect.
Medications
Very limited information is available. Cold medications containing α-sympathomimetic drugs exacerbate LUTS by the expected effect on the smooth muscles of the bladder outlet. Recently a careful analysis of the data from the Olmsted County study demonstrated that daily use of antidepressants, antihistamines, or bronchodilators is associated with a 2- to 3-point increase in the IPSS compared with age-matched nonusers and daily use of antidepressants is associated with a decrease in the age-adjusted flow rate (Su et al, 1996).
Correlations between Parameters
As noted, all relevant parameters such as symptom severity and frequency, bother, interference, disease-specific HRQOL, maximum flow rate, and prostate volume tend to worsen with advancing age. However, reported correlations between these parameters as well as urodynamic pressure-flow studies are in general weak, with some exceptions. Strong correlations exist as one might expect between subjective measures such as symptom severity and frequency (IPSS score), bother, disease-specific HRQOL, and interference scores (Barry et al, 1995b; Girman et al, 1999) (Fig. 91–15).
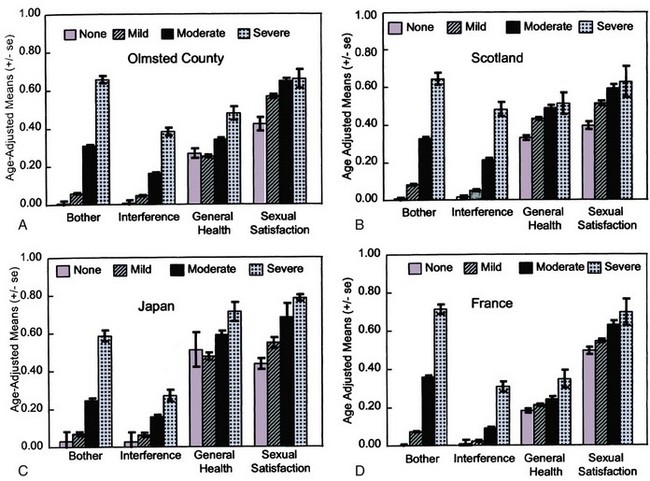
Figure 91–15 A-D, Age-adjusted means of disease-specific HRQOL measures after transformation to a 0 to 1 scale stratified by levels of symptom severity and frequency (IPSS score).
(From Girman CJ, Jacobsen SJ, et al. Health-related quality of life associated with lower urinary tract symptoms in four countries. Urology 1998;51[3]:428–36.)
Weak numerical correlations can arise artifactually, regardless of whether true physiologic relationships exist (Girman, 1998). Correlations are further affected by intraindividual variability of measured parameters, day-to-day variability, measurement errors associated with the technique or equipment, or the natural history of the condition itself (Bruskewitz et al, 1982; Diokno et al, 1992; Barry et al, 1995c; Sagnier et al, 1996).
It is furthermore crucial to consider the population utilized to evaluate correlations. A correlation is easier identified if patients exhibiting the full spectrum of the parameters of interest are included. In most LUTS/BPH studies, however, patients are excluded based on thresholds imposed, making it more difficult for a significant correlation to emerge. There are many examples in clinical medicine in which weak numerical correlations exist but strong clinical relationships are well accepted (Wilson and Cleary, 1995).
The absence of meaningful baseline correlations for symptoms, flow rate, and prostate volume in a tightly controlled population of men with LUTS and BPH enrolled in a BPH treatment study is shown in Table 91–7.
Table 91–7 Correlation Table between Baseline Parameters in a BPH Treatment Study of 2800 Men Older Than Age 50 Years, an IPSS Score >12, Peak Flow Rate (Qmax) <15 mL/sec, Serum PSA between 1.5 and 10 ng/mL, and TPV >30 mL (TZV Not Specified)
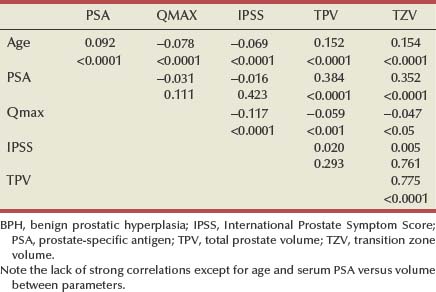
An example of how restriction of the full range of parameters affects correlations is shown in Figure 91–16. Volunteers without known prostatic diseases were asked to fill in an AUASI score and perform a flow rate test. When all data are considered a clear relationship exists, with the maximum flow rate decreasing with increasing symptom severity (r = 0.4; P < .05). However, when only considering those patients typically seen in a BPH study, namely, with a score above 10 points and a maximum flow rate between 5 and 15 mL/sec, the correlation within that cohort exhibiting a limited range of observation on both scales is very weak (r = 0.08; not significant [NS]), and the regression line becomes flat.
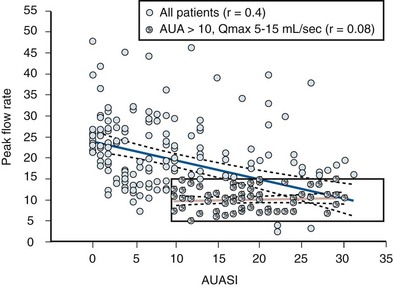
Figure 91–16 Correlation and regression (95% confidence interval) between symptom score and maximum flow rate in volunteers (r = 0.4; P < .05). When only those volunteers are considered with a symptom score above 10 points and a flow rate between 5 and 15 mL/sec ( ), the correlation is virtually absent (r = 0.08; NS) as indicated by a flat regression line (black). AUASI, American Urological Association Symptom Index.
), the correlation is virtually absent (r = 0.08; NS) as indicated by a flat regression line (black). AUASI, American Urological Association Symptom Index.
The correlations observed between symptoms, flow rate and prostate volume in true community-based population studies without artificially imposed entry criteria are consequently somewhat higher than those seen in BPH clinic or trial populations. In the Olmsted County study, after adjusting for age, the odds of moderate to severe symptoms were 3.5 times greater for men with prostatic enlargement (>50 mL) than for men with smaller prostates, whereas the odds were similarly increased (2.4-fold) for men not achieving a peak urinary flow rate of 10 mL/sec (Girman et al, 1995). Men with enlarged prostates were about twice as likely to have bother due to symptoms (OR 2.4) or activity interference (OR 1.8) relative to men with smaller prostates (Girman et al, 1999). In a similar study from the Netherlands, Bosch and colleagues (1995a) reported numerically weak but statistically significant correlations between the IPSS and total prostate volume (r = 0.19, P < .001), peak flow rate (r = −0.18, P < .001), and postvoid residual urine volume (r = 0.25, P < .001).
Although weak correlations between prostate volume and symptoms as well as flow rate have been accepted, recently attention focused on correlations between the transition zone of the prostate and physiologic measures. A stronger correlation between transition zone and symptoms (r = 0.48, P = .03), and peak urine flow (r = −0.34, P = .05) was reported first by Kaplan and colleagues (1995), who also showed a significant correlation between transition zone index (TZV/TPV) and symptoms (r = 0.75), peak urine flow (r = −0.71), and—somewhat unexpectedly—detrusor pressure at peak urine flow (r = 0.43). These data were generated from a relatively small cohort of 61 men with BPH. Invasive pressure-flow studies are not performed in community-based studies, and thus comparative data from population-based studies cannot be reviewed. However, the finding of a relationship between measures of obstruction and prostate volume is rare, with most authors denying such relationship in series of BPH clinic or trial patients (Bosch et al, 1995c; Yalla et al, 1995; Ezz el Din et al, 1996; Witjes et al, 1997; d’Ancona et al, 1998; Homma et al, 1998; Kuo, 1999; Steele et al, 2000). Data from the Olmsted County study suggest no stronger correlation between symptoms and peak flow rates with transition zone volume (r = 0.17, P = .001 and r = −0.20, P < .001, respectively) than with total volume (r = 0.16, P < .001 and r = −0.16, P < .001, respectively). The transition zone index weakly correlated with AUASI score (r = 0.08, P = .103) and peak urinary flow rate (r = −0.08, P = .0823) (Corica et al, 1999). In a large BPH treatment trial the TZV also did not correlate stronger with other measures compared with the TPV.
The correlation between serum PSA and prostate volume, both total and transition zone, has recently been described in greater detail (Roehrborn et al, 1999b, 1999c). Although the individual variability is significant, precluding an accurate prediction of prostate volume by serum PSA in individual patients, there is a strong log linear relationship between these parameters that can be shown in both population-based as well as clinical studies (Hochberg et al, 2000; Morote et al, 2000; Hedelin et al, 2005). The relationship is further influenced by patients’ age, with older patients having a greater increase in prostate volume per unit of serum PSA (Fig. 91–17). In Asian men similar relationships exist; however, in general both prostate volume and serum PSA tend to be smaller/lower (see Fig. 91–17B) (Gupta et al, 2005). Relationships between other PSA-derived parameters have been examined, and certain subforms of PSA (BPSA) have been shown to be stronger related to BPH compared with total serum PSA values (Canto et al, 2004).
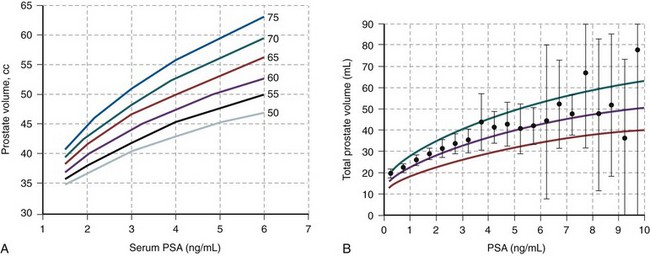
Figure 91–17 Prediction of prostate volume based on serum prostate-specific antigen (PSA) stratified by age in white men (A) and in Japanese men (B).
(A, From Roehrborn CG, Boyle P, Gould AL, Waldstreicher J. Serum prostate-specific antigen as a predicator of prostate volume in men with benign prostatic hyperplasia. Urology 1999;53:581–9. B, From Gupta A, Aragaki C, Gotoh M, et al. Relationship between prostate specific antigen and indexes of prostate volume in Japanese men. J Urol 2005;173:503–6.)
With the exception of age, correlations between various measures of LUTS and BPH are modest in community-based population studies and weak in BPH clinic and trial populations. The relationship between serum PSA and prostate volume is moderate and influenced by age and race and ethnic origin. Neither symptoms nor flow rate nor prostate volumes measures can predict presence and degree of obstruction reliably.
Natural History of Untreated Benign Prostatic Hyperplasia
The natural history of a disease process refers to the prognosis of the disease over time. In other words, the measurement of changes in parameters of interest and the incidence rates of significant outcomes constitute what is commonly referred to as the natural history of a disease. It is important for clinicians to gain as good an understanding as possible of the natural history of any illness, because the benefits and risks of any therapeutic interventions should always be balanced with the risks involved in just watching the disease (i.e., the natural history). In fact, the degree to which natural history must be studied depends on the seriousness of the disease, as well as the risks involved with the therapeutic intervention. For example, it proves to be crucial to understand the natural history of abdominal aortic aneurysms and how their prognosis is determined by their size, to adequately counsel patients regarding surgical interventions, which have considerable risks in themselves.
Clinical Parameters and Outcomes of Interest
Table 91–8 lists the beneficial and harmful changes in measurable parameters and outcomes, which are of interest in both the natural history and treatment of LUTS and BPH. They may be divided further into direct or biologic and indirect or proxy outcomes (Eddy, 1990, 1992). The biologic or direct outcomes are those that are immediately noticeable by the patients (e.g., symptom changes, infections, death), whereas the indirect or proxy outcomes are not directly noticeable but may predict future changes in direct outcomes (e.g., changes in pressure-flow parameters may predict episode of retention). The parameters and outcomes are dichotomous (yes or no, e.g., retention or no retention), categorical (grades of severity, e.g., grades of obstruction measured by pressure-flow studies), or continuous (scales of severity, e.g., symptom score, maximum flow rate). Accordingly, the most common ways to measure such outcomes are probabilities or rates of occurrences for dichotomous or categorical outcomes and measures of central tendency (mean or median) and variability (standard deviation [SD] or standard error [SE] or confidence intervals [CI]) for outcomes measured by continuous scales.
Methods of Studying Natural History of Benign Prostatic Hyperplasia
The natural history of BPH can theoretically be evaluated by studies of a variety of designs:
There are problems associated with any of the approaches. Concerning the watchful waiting cohorts, the first question to be resolved is whether it is ethical (or feasible) to enroll symptomatic men in such a study even if the disease studied is not fatal. Secondly, the very fact that the patients had an initial contact and presumably subsequent contacts with health care providers in the course of the study will bias them, leading to changes in outcome parameters of interest presumably different from those observed in an age-matched cohort of men similar in all parameters at baseline but who did not choose to participate (a cohort one might call in analogy to genetic language “wild type”). Furthermore, many diagnosed men will in the course of such natural history study become more and more symptomatic and desire and receive treatment, making them ineligible to further participate in the study and thus reducing the number of men available for analysis. Last, a very important bias is introduced by the fact that patients in such cohorts are selected based on thresholds imposed by inclusion and exclusion criteria. In general, patients are selected to participate who are more symptomatic, that is, those with higher symptom scores and lower maximum flow rates. By the principle of trial conduct patients then repeat the symptom and flow rate assessment during follow-up (or after treatment). However, the same inclusion/exclusion criteria are not applied to the follow-up measurements, which leads to a unilateral regression to the mean, suggesting (falsely) an improvement in the parameter of interest.
To determine the effect that the stringency of pretrial screening has on the outcome of placebo treatment, a cohort of 145 volunteers were invited to fill in the IPSS score and perform a flow rate recording twice within a month without receiving any therapy or instructions whatsoever (Sech et al, 1998). Although many patients experienced either an increase or a decrease in both parameters, the mean values did not change significantly (IPSS 12.1 vs. 11.7 points, peak flow rate 17.7 vs. 17.4 mL/sec; NS). However, when typical BPH trial conditions were applied and only those patients considered for analysis who had an IPSS score above (>7, >10, >15) and a flow rate below a certain threshold (<15, <12, <10 mL/sec), a unilateral regression to the mean took place, by which the “more” symptomatic volunteers (i.e., patients) experienced still considerable natural variability on the occasion of the second assessment but the net effect was toward “improvement,” that is, lower scores and higher flow rates. For example, when only considering patients with IPSS scores greater than 10 points, the mean difference between first and second assessment was between 19.9 versus 18.8 points or −1.1 (P < .05). Similarly, when only patients with a peak flow rate less than 12 mL/sec were considered, the mean difference between the first and second assessment was 9.3 versus 10.9 mL/sec or +1.6 mL/sec (P < .01) (Table 91–9).
Table 91–9 Mean ± Standard Deviation (SD) and Range for American Urological Association Symptom Index for Test No. 1 and Test No. 2, Mean Difference between the Two Tests, and the 95% Confidence Interval (CI) for the Difference, for All Subjects and for Subjects Censored Based on Test No. 1 >7 Points, >10 Points, and >15 Points
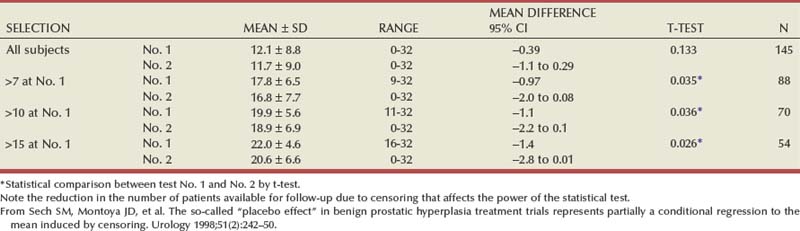
This experiment clearly illustrates that a unilateral regression to the mean induced and controlled by the stringency of the inclusion criteria can result in a significant “improvement” in any parameter for which a threshold is set at the baseline screening. Such purely mathematical effect is likely at work in many if not all studies in which the outcome parameters are measured using a numerical scale of some sort and when baseline screening criteria are applied.
Control groups of men being randomized to receive either no treatment or placebo or sham treatment while being compared with a matched group of men receiving active therapeutic interventions also suffer from a variety of biases. As the watchful waiting cohorts, they have initial and constant contact with health care providers, they know from the design of the study that they are randomized to the perceived less effective or ineffective arm, and thus may have a lower threshold than community-dwelling men to cross over to active therapy. On the other hand, owing to a sense of obligation, they may elect to be observed until the trial is over and then choose an active treatment. They are also subject to the regression to the mean bias discussed earlier because they were subjected to inclusion/exclusion criteria.
Longitudinal population-based studies, although difficult and expensive to conduct, are likely the best vehicle to understand the natural history of the disease. The regression to the mean bias does not apply, because the participants are not selected based on inclusion and exclusion thresholds. Because no formal diagnosis is established, the natural history may take place unencumbered by preconceived notions about symptoms, end points of trials, and anything else leading to a bias in a watchful waiting cohort of diagnosed and thus labeled men or in a control group of diagnosed men. One must, however, realize, that the very need to monitor the natural history, that is, the need to ask questions and perform tests and interventions, conflicts with the notion of the “natural history,” and thus some biases are likely to be unavoidable even in the most ideal setting.
Watchful Waiting Studies
Observing cohorts of men diagnosed with LUTS and/or BPH conservatively (watchful waiting cohort) was attempted by some investigators in the past, but the few published reports are of limited scientific rigor and informational value and have significant shortcomings. Inclusion and exclusion criteria are poorly defined, follow-up and compliance are poor, assessment instruments are either not defined or insufficient, and patient accounting is incomplete. The studies provide answers regarding the probability of symptom changes and the magnitude of flow rate changes.
Five such studies were reported between 1919 and 1988 with a total enrollment of 456 patients and a follow-up ranging from 3 to 6 years (Clarke, 1919; Craigen et al, 1969; Birkhoff et al, 1976; Ball et al, 1981; Kadow et al, 1988). A change in symptom status was reported for all 456 patients, whereas none of the studies utilized a quantitative symptom severity scale. Data on urinary flow rate and residual urine were available for 223 and 197 patients, respectively. The peak urinary flow rate deteriorated in 66% and improved in 20%. Residual urine increased (35%), decreased (37%), and remained unchanged (28%) in about the same number of all patients. The mean change in peak flow rate (in those patients for whom data are available) was +2.2 (from a mean of 9.0 to 11.2) mL/sec or 24%, whereas the mean change in residual urine was +37 (from a mean of 115 to 152) mL or 32%. Data on symptom improvement were reported as a dichotomous outcome (improved vs. not improved). The mean probability for any improvement in symptom severity in these studies is 42.5% (90% CI 30.8 to 54.8) as calculated by meta-analytical methods (Eddy and Hasselblad, 1992).
A superior approach, namely, to follow a no treatment control group of men diagnosed with LUTS and BPH, was chosen by Wasson and coworkers (1995). A total of 556 men with moderate symptoms and a Madsen-Iversen symptom score between 10 and 20 points (0-27 point scale) were randomized to either undergo transurethral resection of the prostate (TURP) or be watched conservatively. There were 47 treatment failures (defined as death, recurrent infection, residual urine volume more than 350 mL, development of bladder calculus, incontinence, a symptom score of 24 or higher, or a doubling of serum creatinine level from baseline) in the watchful waiting study arm (N = 276) versus 23 in the surgery study arm (N = 280) over 3 years of follow-up (relative risk 0.48; 95% CI 0.3 to 0.77). Sixty-five (24%) men assigned to watchful waiting underwent surgery during follow-up, 20 of them for treatment failure. The majority of these men were classified as having more bother at baseline, and about 40% of patients in this category experienced an improvement in the degree of bother from urinary difficulties, a proportion strikingly similar to the overall estimate of 42.5% from the natural history studies. At 3 years of follow-up the patients randomized to watchful waiting who did not fail or cross over to surgery had a small but noticeable improvement in nearly all outcomes measured including a +0.4 mL/sec increase in the maximum flow rate. More recently a 60-month update was reported providing data on 966 patient-years of follow-up on the TURP patients and 990 patient-years on the watchful waiting patients (Flanigan et al, 1998). The treatment failure rate was 21% for the watchful waiting and 10% for the TURP groups, and all outcomes were better for the TURP-treated patients (Table 91–10). During follow-up, 76 (27%) of watchful waiting patients crossed over to TURP, and the 5-year Kaplan-Meier estimate of the crossover rate was 36% and twice as many patients crossed over electively as compared with post-treatment failure protocol dictated crossover. The most significant factor predicting elective crossover was high baseline bother due to urinary symptoms (Fig. 91–18). When separately analyzed, the patients crossing over from watchful waiting to TURP had fewer improvements in outcome parameters compared with those randomized to TURP initially (Table 91–11). In many instances the differences between the groups were statistically and clinically significant. A reduction in symptom score by −11 points (high baseline symptom group) and an improvement in maximum flow rate by 8.7 mL/sec (low baseline flow rate group) after TURP is expected; however, in those men undergoing TURP after an unsuccessful trial of watchful waiting the improvements were only −8 points and +4.7 mL/sec. This allows the speculation that during the period of watchful waiting before crossover some irreversible damage might have occurred preventing symptom and flow rate improvements to the same level as observed in those men initially treated by TURP.
Table 91-10 Extended Follow-up Treatment Outcomes by Intention to Treat in the Veterans Affairs Cooperative Study Comparing Watchful Waiting with TURP
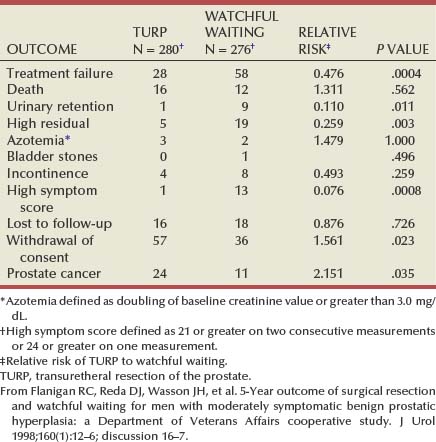
Figure 91–18 Percent of patients crossing over from watchful waiting (WW) to transurethral resection of the prostate (TURP) by baseline bother scores and elective versus post-treatment failure protocol dictated.
(From Flanigan RC, Reda DJ, et al. 5-Year outcome of surgical resection and watchful waiting for men with moderately symptomatic benign prostatic hyperplasia: a Department of Veterans Affairs cooperative study. J Urol 1998;160[1]:12–6; discussion 16–7.)
Table 91-11 Changes from Baseline to 60 Months of Follow-up for Patients Initially Randomized to TURP (1), Randomized and Remaining on Watchful Waiting (2), and Those Randomized to Watchful Waiting but Crossing Over to TURP at Some Point during Their Follow-up (3)
Djavan and colleagues (2004) followed a group of 397 men presenting to a urology clinic with mild LUTS, defined as scoring less than 8 points on the IPSS. These men were followed for 4 years beginning with a watchful waiting protocol and were re-evaluated every 3 months for 48 months. The cumulative incidence of clinical progression defined as worsening of the IPSS with migration from mild to moderate (IPSS 8 to 18 points) or severe symptoms (IPSS 19 to 35 points) was assessed. The likelihood of migrating or transitioning from one health state to another was 6%, 13%, 15%, 24%, 28%, and 31% at 6, 12, 18, 24, 36, and 48 months, respectively.
Placebo and Sham Control Groups in Randomized Trials
Data from control groups treated with placebo or sham and compared with matched groups of patients treated with medications or devices are available to address many of the parameters and outcomes of interest.
Placebo Control Groups
For the Guidelines for the Diagnosis and Treatment of BPH published by the Agency for Health Care Policy and Research in 1994, data on 1417 patients treated in 45 placebo arms of placebo-controlled trials were analyzed (McConnell et al, 1994). Table 91–12 allows a direct comparison between the watchful waiting studies discussed earlier and these placebo groups, both summarized by meta-analytical techniques. For none of the examined parameters a significant difference between the two treatment modalities can be identified. However, several points deserve discussion. As opposed to the longer-term follow-up in the watchful waiting studies, the placebo studies are part of short- to mid-term medical treatment trials ranging from 3 days to 52 weeks in duration (mean 13 weeks). In all these studies the patients are blinded as to the treatment arm and thus have in most cases at least a 50 : 50 (or better in case of 2 : 1 or 3 : 1 randomization) chance of receiving an active drug. Dropping out of such studies because of failure does not have the same ramification as it has in watchful waiting studies in which the patients willingly assumed a conservative treatment approach knowing that it might fail (i.e. they might fail and go on to active treatments). In contrast, in some placebo-controlled studies a promise—either tacit or openly—is made stating that after the conclusion of the trial the patient would be eligible for either “free” active treatment or would be “moved up” on the surgical waiting list (this phenomenon is unique to those studies conducted in the United Kingdom). The inactive preparation given should theoretically add to the placebo effect and thus improve the outcome above those noted for watchful waiting studies. The probability for a patient to experience improvement, however, is estimated to be about 40% in the uncontrolled watchful waiting studies, the randomized watchful waiting versus TURP study, and the combined placebo arms. The changes in peak flow rate and residual urine volume are similar and similarly small for these three groups as well. It becomes evident that 40% of patients report some improvement, and the minimal fluctuation of peak flow rate and residual urine represent the “placebo effect” background against which the more substantial changes in these parameters achieved by active treatment modalities must be seen.
Table 91–12 Comparison of Outcomes after Watchful Waiting and Placebo Treatment
| OUTCOMES | WATCHFUL WAITING | PLACEBO |
|---|---|---|
| Total no. of patients | 456 | 1417 |
| Probability for flow rate increase | 19.7% | 35.8% |
| Probability for no change in flow rate | 14.2% | 41.1% |
| Probability for flow rate decrease | 66.1% | 23.1% |
| Mean pre- and post-treatment flow rate (mL/sec) | 9.0-11.2 | 9.1-9.7 |
| Difference (mL/sec) and percent change | +2.2/+24.4% | +0.6/+6.6% |
| Probability for decrease in residual urine | 35.0% | 38.0% |
| Probability for residual urine to remain unchanged | 28.0% | 26.1% |
| Probability for increase in residual urine | 37.0% | 35.9% |
| Mean pre- and post-treatment residual urine (mL) | 115-152 | 87-76 |
| Difference (mL) and percent change | +37/+32.2% | −11/−12.6% |
| Probability for symptom improvement | 41.7% | 41.7% |
| Probability for symptoms to remain unchanged | 25.8% | 53.5% |
| Probability for symptom worsening | 32.4% | 4.7% |
| Mean probability for symptom improvement* | 41.7% | 41.7% |
| 90% CI for symptoms improvement* | 30.8-54.8% | 26.3-65.1% |
CI, confidence interval.
* Calculated by confidence profile method using hierarchical Bayes (Eddy and Hasselblad, 1992).
Modified from McConnell, JD, Barry MJ, et al. Benign prostatic hyperplasia: diagnosis and treatment. Clinical practice guideline no. 8. Rockville (MD): U.S. Department of Health and Human Services, Agency for Health Care Policy and Research, Public Health Service; 1994. p. 1–17.
Sham Control Arms of Device Treatment Trials for Benign Prostatic Hyperplasia
In recent years a multitude of minimally invasive device treatments for BPH have been developed and tested in randomized, sham-controlled, open, single, or even double-blinded trials. Although the majority of these trials compare various types of heat treatments (transrectal or transurethral hyperthermia or thermotherapy) with a sham treatment, one investigator compared balloon dilation with “sham” cystoscopy alone in a randomized, double-blinded trial involving 33 men with BPH (Lepor et al, 1992b).
The outcomes of the balloon dilation versus cystoscopy trial, one multicenter trial using transrectal or transurethral hyperthermia in comparison with a sham control (Abbou et al, 1994), and five transurethral microwave thermotherapy (TUMT) trials and their respective sham control arms can be used for analyses (Blute et al, 1993; Ogden et al, 1993a, 1993b; Bdesha et al, 1994; De la Rosette et al, 1994). Although the mean pretreatment severity score differs from trial to trial, the mean improvements in symptom severity in the sham groups ranged from 5.2% to 15.6% (on a 100% scale) while the active thermotherapy treated patients had improvements ranging from 27.0% to 37.8%, or in most cases twice the improvement as the sham control. The multicenter hyperthermia trial represents the exception, in that the actively treated cohort has an improvement similar to the sham control, which is well within the range of the other sham control trials. The changes in peak urinary flow rate follow a similar pattern. With few exceptions the changes in peak flow rate are either very modest improvements or deteriorations (0.5, 0.6, −0.2, −1.0 mL/sec), while the active treatment arms report substantial improvements with the exception of the hyperthermia trial.
Placebo/Sham Effect and Baseline Symptom Severity
An area of considerable interest is the question to what degree the placebo/sham effect depends on the baseline status of the patients. This could pertain to baseline symptom severity, baseline bother, quality of life, baseline flow rate, and all other imaginable parameters. Although few investigators have thus far reported data stratified by baseline parameters, results from a multicenter, placebo-controlled, 12-month α-adrenergic blocker trial (terazosin [Hytrin]) can be analyzed (Roehrborn et al, 1996). Although the active drug–treated cohort has almost twice the improvement within each stratum, the placebo-treated patients had improvements ranging from 1.4 points (4.6%) to 7.5 points (21.4%) in order of increasing baseline severity score. The placebo improvement for the placebo cohort overall was 3.3 points (10.6%).
A similar increase in the placebo effect with increasing baseline symptom severity has been reported for patients treated with finasteride in phase 3 trials (Gormley et al, 1992). It might be speculated that the baseline symptom severity may also affect the sham effect seen in device trials. This phenomenon might be due to increased expectations in patients with more severe baseline symptoms or simply due to a regression to the mean.
Natural History and Disease Progression in Long-Term Placebo Groups
The distinction between changes in parameters and rates of outcomes observed in placebo and sham control groups versus the longitudinal population-based studies becomes blurred when the placebo control is carried out over a period of time long enough to allow natural history changes to take place and confound the situation.
The Proscar Long-Term Efficacy and Safety Study (PLESS) followed a cohort of more than 3000 men with moderate symptoms and enlarged prostate glands randomized to treatment with finasteride, 5 mg, daily versus placebo over 4 years (McConnell et al, 1998). In most control arms, both placebo and sham, the combined placebo effect interfering with the natural history of the disease is maintained for the entire duration of the study. In this 4-year trial, however, both the mean symptom score and mean maximum flow rate slowly drifted back to baseline after a typical initial placebo response (McConnell et al, 1998). The changes occurring in measurable parameters after the initial placebo effect has taken place thus can be considered to represent the natural history of the disease. The rates of outcomes such as AUR or surgery, as well as changes in prostate volume, which are less or not at all susceptible to the placebo effect, are also valid measures of the natural history of the disease.
The almost 1500 men in the placebo arm of this trial allowed for a detailed analysis of the placebo response and the subsequent natural history stratified by baseline parameters. It had been shown that in men with BPH there is a relatively strong correlation between prostate volume and serum PSA. In PLESS only 10% of patients underwent baseline and yearly volume measurements by endorectal coil MRI. Based on the assumption that serum PSA would be a good proxy parameter for prostate volume, the analysis was initially performed for serum PSA and based on all 1500 men treated with placebo.
When stratifying the population by serum PSA into tertiles, or thirds, of patients with PSA levels from 0 to 1.3, 1.4 to 3.2, and 3.3 to 10 ng/mL, three distinctly different patterns regarding symptom score and maximum flow rate changes emerge (Fig. 91–19) (Roehrborn et al, 1999a). Whereas the initial placebo response in the lowest PSA tertile for both symptom and flow rate was maintained over the entire 4 years of follow-up, the middle tertile experienced a slow deterioration of symptoms back to baseline and in essence the natural history and progression of disease negated any flow rate gains. In the highest PSA tertile the symptom score increased steadily over time following an initial placebo response by −1.5 points. Over the subsequent years the score increased by 0.5 points/yr, bringing it at the end of the study back to the original baseline level. The initial response in terms of flow rate improvement was completely negated by the progression/natural history after 2 years, and at the end of the study this group of patients registered a net worsening of the flow rate by a mean of −1.0 mL/sec (see Fig. 91–19B). Similar results regarding the changes in symptom and maximum flow rate over time were obtained when the 150 patients for whom prostate volume measurements were available were divided into tertiles (14 to 41 mL, 42 to 57 mL, 58 to 150 mL). The PLESS study also assessed periodically bother due to urinary symptoms, disease-specific quality of life, aspects of sexual function, and sense of well-being. Surprisingly, serum PSA at baseline also predicts after an initial placebo response the rate of deterioration of bother, quality of life, and certain aspects of sexual function (Bruskewitz et al, 1999) (Fig. 91–20).
Figure 91–19 Mean changes for symptom (A) and peak flow rate (B) in placebo-treated patients in Proscar Long-Term Efficacy and Safety Study study stratified by baseline serum prostate-specific antigen (PSA) levels.
(From Roehrborn CG, Boyle P, et al. Serum prostate-specific antigen (PSA) is a reliable surrogate for prostate volume. Urology 1999;53:581–9.)
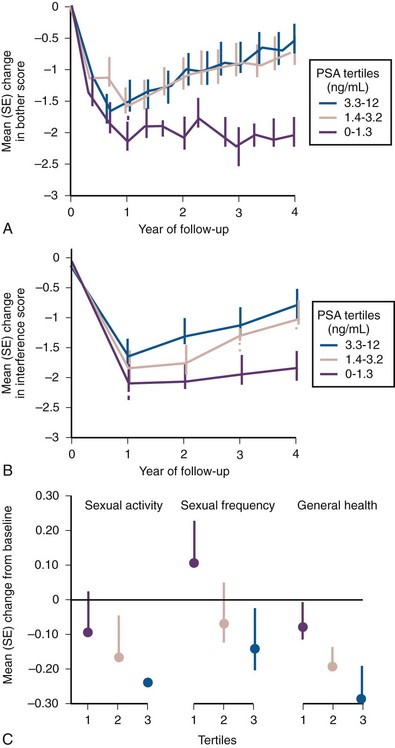
Figure 91–20 Mean changes for bother (A), interference with activities of daily living (B), and for sexual activity, frequency, and perception of general health (C) in placebo-treated patients in Proscar Long-Term Efficacy and Safety Study study stratified by baseline serum prostate-specific antigen (PSA). SE, standard error.
(From Bruskewitz R, Girman CJ, et al. Effect of finasteride on bother and other health-related quality of life aspects associated with benign prostatic hyperplasia. PLESS Study Group. Proscar Long-term Efficacy and Safety Study. Urology 1999;54[4]:670–8.)
The Medical Therapy of Prostatic Symptoms (MTOPS) study followed 3045 men treated with either placebo versus doxazosin (Cardura) versus finasteride versus combination therapy for 4 to 5 years and was designed as a progression study (Bautista et al, 2003; McConnell et al, 2003). The composite end point captured either a worsening of 4 points or more on the IPSS score verified twice within 4 weeks, AUR, socially unacceptable incontinence, recurrent urinary tract infection, or renal insufficiency due to BPH (Table 91–13). The worsening of 4 points or more was chosen to reflect a clinically meaningful worsening of LUTS symptomatology. Despite an overall numerical improvement in the IPSS by −4.9 points in the placebo group (intention to treat cohort), about 14% of placebo-treated patients experienced a verified worsening by 4 points or more over the course of the study for an incidence of 3.6/100 patient-years.
The PLESS study and its placebo-control group allowed the study of natural history and disease progression in a cohort selected for moderate symptoms and other evidence of disease (in contrast to a population-based study) on the background of the initial combined placebo responses. In MTOPS, approximately 14% of placebo-treated patients experienced a worsening of 4 or more points in the IPSS over 4 years of follow-up.
Relationship between Placebo/Sham Effect and Perception of Improvement
Barry and colleagues (1995d) reported an important observation by assessing the relationship between changes in the AUASI score and patients’ global rating of improvement in over 1200 men treated in a medical treatment trial for BPH. They noted that a mean decrease in AUASI score of 3.1 points was associated with a slight improvement; however, this relationship was strictly dependent of the baseline value. For patients to perceive a slight, moderate, or marked improvement, increasing drops in the AUASI score were required with increasing baseline symptom severity. For patients starting with a lower versus higher baseline score, the drop had to be −7.4 versus −15.3 points, respectively, to perceive a marked improvement, −4.0 versus −8.7 for a moderate improvement, −1.9 versus −6.1 for a slight improvement, −0.2 vs −2.0 for no improvement, and +3.3 versus +1.2 for worsening to be perceived.
Longitudinal Population-Based Studies
Diokno and coworkers (1992) provided estimates of the prevalence, incidence, and remission of LUTS in 803 community-dwelling men 60 years or older. The annual incidence of prostate surgery was 2.6% and 3.3% during years 1 and 2 of follow-up. The prevalence of at least one symptom was 35%, with annual incidence rates during years 1 and 2 of follow-up as 16.4% and 16.1%. Remission was also noted in that 22.9% of those having severe symptoms at baseline were asymptomatic at follow-up. Table 91–14 details the changes in symptom severity from one survey to the next. The tendency for fluctuation and spontaneous remission of symptoms as well as the regression to the mean become evident from an analysis of these data.
Garraway and coworkers conducted a study of LUTS in four villages in the Forth Valley in Scotland in men 40 to 79 years old, defining BPH as a prostate volume of over 20 mL by TRUS and a maximum flow rate of less than 15 mL/sec and applying a lower threshold to the symptom severity (Garraway et al, 1993a, 1993b; Guess CG et al, 1993; Tsang and Garraway, 1993). By this definition the prevalence of BPH increased at baseline from 14% in men in their 40s to 43% in men in their 60s, and about one half of men reported interference with activities of daily living due to LUTS and BPH. One- and 3-year follow-up data have been reported (Lee et al, 1996). From baseline to 3 years the proportion of men with moderate symptoms increased from 34% to 45% and significant increases in symptom level occurred for 8 of 12 symptoms queried as well as for the AUASI (increase from 6.37 to 7.88 from baseline to year 3, P < .0001) (Lee et al, 1996). Parallel to symptom frequency and severity, bothersomeness of symptoms increased over time (Fig. 91–21) and the proportion of men reporting interference with two or more activities increased from 26% to 41%, with three or more activities from 18% to 27%, and with four or more activities from 15% to 18%. This study clearly demonstrated LUTS and BPH being a progressive disease, albeit with a very flat slope of progression.
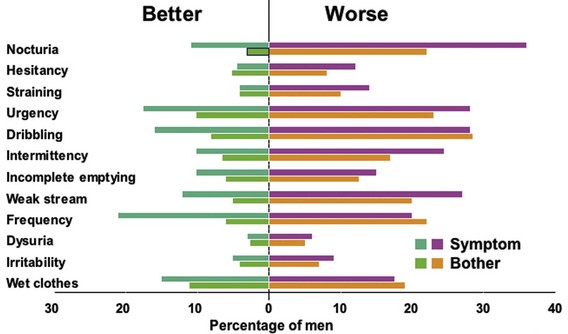
Figure 91–21 Changes in urinary symptoms and bothersomeness status between baseline and 3 years in a Scottish population-based study.
(From Lee AJ, Russell EB, et al. Three-year follow-up of a community-based cohort of men with untreated benign prostatic hyperplasia. Eur Urol 1996;30[1]:11–7.)
The most informative natural history study to date is the Olmsted County Study of Urinary Symptoms and Health Status Among Men, which has given us much information about prevalence and severity of urinary symptoms, bother, worry and embarrassment, quality of life due to symptoms, and the relationship between symptoms and other parameters such as flow rates, prostate volume, and PSA (Oesterling et al, 1993; Girman et al, 1994, 1995; Guess et al, 1995; Jacobsen et al, 2003; Sarma et al, 2003; Rule et al, 2005).
With continued follow-up of this cohort, data have emerged regarding the longitudinal changes in symptoms and flow rate over time in this population-based study. Of 904 men reporting none to mild symptoms (AUASI, 0 to 7 points) at baseline, 118 reported moderate to severe symptoms (AUASI, > 7 points) at 18 months and 196 did so at 42 months follow-up (Jacobsen et al, 1995a). However, 47 men who had developed moderate to severe symptoms at 18 months had none to mild symptoms at 42 months. At 42 months of follow-up an average increase in the IPSS of 0.18 (95% CI 0.13 to 0.24) points per year of follow-up was recorded. The average annual symptom score slope and variability in slope increased with patient age at baseline from a mean of 0.05 ± 1.06 (standard deviation) per year among men in their 40s to 0.44 ± 1.35 per year for men in their 60s and decreased to 0.14 ± 1.42 per year for men in their 70s (Jacobsen et al, 1996). More recently, 92-month data showed an annual change of 0.34 point/yr, with 31% of all men reporting at least a 3-point increase. The greatest annualized increase was observed in men in their 60s with 0.6 point/yr (Rhodes et al, 2000).
In addition, 6-year follow-up data on peak flow rate measurements in a subset of about 500 men showed a median peak urinary flow rate slope decrease of −2.1% per year (25th percentile −4.0, 75th percentile −0.6). Peak urinary flow rate declined more rapidly with decreasing baseline rate and increasing baseline age, prostate volume, and symptom severity (all P = .001). When the variables were simultaneously adjusted for each other, a rapid decline (negative slope 4.5% or greater per year) was more likely in men 70 years old or older and those with a rate less than 10 mL/sec at baseline compared with those 40 to 49 years old and those with a rate of 15 mL/sec or greater, respectively. Prostate volume and symptom severity were not statistically significant predictors of a rapid decline in peak urinary flow rate when variables were considered simultaneously (Roberts et al, 2000).
Based on TRUS the growth of the prostate in these men 40 to 79 years old was estimated to be about 0.6 mL per year or 6 mL per decade of life. However, prostate growth followed an exponential growth pattern with a slope estimate of 0.4 mL per year for men aged 40 to 59 years at baseline and of 1.2 mL per year for those 60 to 79 years at baseline (Rhodes et al, 1995, 1999). An updated analysis revealed a median growth rate of about 1.9% per year independent of age and symptoms. However, a higher baseline serum PSA and larger prostate volume predicted greater annualized volume increases.