chapter 93 Minimally Invasive and Endoscopic Management of Benign Prostatic Hyperplasia
The prostate continues to be central to urologic practice in terms of the volume of cases that arise involving a disorder of that organ, be it benign, malignant, or inflammatory. Because of this, the amount of money available from grant-giving authorities for research into prostatic diseases is probably the highest for any research on the genitourinary tract, and so inevitably our interest in and understanding of the pathogenesis of problems relating to prostatic conditions have increased greatly. This has led to increasingly effective therapies for some prostatic abnormalities, but after the initial advances in the management of benign prostatic hyperplasia (BPH) of 10 to 12 years ago, how far have things moved forward? And, although groups of patients are continually being identified who would benefit from individual or combined medical treatments, can we say that the same has been happening in our development of minimally invasive therapies?
One thing that is certain is that in our investigation of medical therapy of BPH the identification of groups of patients who would best respond to different drugs has taken many large trials with long-term follow-up and careful statistical powering to demonstrate equivalence or a predefined difference. One always has the feeling that drug trials keep moving forward, leading to a greater understanding of the condition itself and of the ideal treatment for BPH. It can be said with some degree of confidence that the advent of medical management has reduced greatly the indications for and the number of patients undergoing transurethral resection of the prostate (TURP) and has also strongly influenced the wish of patients to have TURP, in that they would now prefer to try most other types of treatment before agreeing to undergo this procedure.
As the pharmacologic management of BPH was flourishing, many new minimally invasive therapies were being introduced. The huge success that greeted the development of technologic innovations in the management of renal calculous disease with percutaneous nephrolithotomy and extracorporeal shockwave lithotripsy prompted most of us to believe that the same could be achieved with BPH.
The yardstick against which the new minimally invasive therapies were measured was, of course, TURP. This was a very significant technologic innovation when it was introduced, and it went through a considerable amount of evolution before it reached the high levels of excellence that it has reached today. We know that it does not seem to produce as good results as the open prostatectomy, but it is not far behind. This statement is made without there having been a randomized trial between the two procedures, and comments on the safety and efficacy of one against the other are not evidence based. However, TURP can be viewed as a highly effective treatment with an acceptable complication rate, so much so that it is now accepted as the “gold standard,” that is, the standard against which other minimally invasive therapies must be measured and the standard that it is hoped they can achieve.
One of the slightly disappointing things is that although many of these therapies have been introduced, in spite of the fact that they were originally welcomed as a significant advance in the treatment of BPH, in the longer term they have not turned out to be so. In many cases, the conclusion that they were of value was based on a series of poorly constructed short-term trials in which intention-to-treat analysis of the results may not have been rigidly adhered to. However, the demise of some of the earlier technologies has led to strengthening and evolution of the newer ones, all of which has been beneficial for patients with BPH.
It should also be remembered why it is that the new minimally invasive techniques have been introduced. Ideally, they have been popularized and developed in the hope that they will have less complications than TURP, less requirement for anesthesia, and a shorter hospital stay and if possible will be less expensive. If the treatment is as effective as TURP, it should be ahead on one of these counts, and if it is not as efficacious, a rationale for its use should be devised, in addition to attempts at the identification of the patients most likely to benefit from it. Its exact therapeutic positioning in the management of BPH should be found.
The discussion presented here focuses on the current approach to the minimally invasive and endoscopic management of BPH. The literature has been critically and fairly reviewed, and the information outlined has relied heavily on evidence-based principles. When level I evidence is available, such as a meta-analysis or systematic literature review, the reader’s attention will be drawn to it. The purpose of all of this is to present the data so that readers will be able to make a valued judgment on the various treatment options, thus helping in their urologic practice.
Intraprostatic Stents
One of the earliest attempts to find less traumatic methods of treating symptomatic BPH was the introduction of either temporary or permanent intraprostatic stents, and, to a degree, they are still being used. They were first introduced as a method of treating certain cardiovascular conditions; and since the work of Dotter (1969), they have also been part of the treatment for peripheral vascular disease. They have since been used to treat stenosis of the coronary, femoral, and renal arteries (Maass et al, 1982; Dotter et al, 1983; Wright et al, 1985; Palmaz et al, 1987). Other types of obstruction have also been treated by the placement of stents: vena caval obstruction (Furui et al, 1990), bronchial obstruction (Mair et al, 1990), and tracheal stenosis (Skapshay et al, 1989). Even cervical stenosis (Luesley et al, 1990) and lacrimal duct obstruction (Hurwitz, 1989) have been treated in this manner. It is clear that stents have a role to play in treating obstruction in different parts of the body, but the exact role needs to be defined accurately. In the case of coronary artery disease, for example, a trial comparing stents with coronary artery bypass grafting is still awaited. In the case of the prostate, it took some time before it was realized that the role of stents was rather a limited one and that they would not replace TURP in every patient.
One of the few innovations that have occurred has been the introduction of a new temporary prostatic stent, which is based on a modification of the Foley catheter. This is described in detail later.
The idea of using stents for splinting the lobes of the prostate was derived from their original use in the cardiovascular system, where they are used to prevent arterial restenosis after angioplasty. Fabian (1980) first described the use of stents in urology when he suggested their usefulness in the treatment of outlet obstruction secondary to enlargement of the prostate. At some time after this the use of stents was advocated in the treatment of urethral strictures, and, subsequent to this, the use of prostatic stents became widespread, with the introduction of many different types: stents are now available in different lengths, diameters, materials, and designs.
As mentioned earlier, when stents were introduced as a treatment for symptomatic BPH, their exact role was uncertain and tended to be overstated as perhaps something that could be used in virtually every case. Eventually it became clear that their major role was likely to be found in the management of patients who were unfit for surgery, in either the short or the long term, in which the alternative would have been months or, indeed, a lifetime of indwelling urethral catheterization.
Along with these changes in the indications for the use of stents have emerged two basic types of stent: that which is put in for a short time and can be removed with ease and that which is placed as a permanent type of treatment and can be removed only with much greater difficulty.
Temporary Stents
Temporary stents are tubular devices that are made of either a nonabsorbable or a biodegradable material. They remain in the prostatic urethra for a limited period of time; they neither become covered by the urethral epithelium nor become incorporated into the urethral wall. The nonabsorbable stents need to be removed every 6 to 36 months, depending on which type of material is used. They can usually be removed, and, if necessary, replaced, without difficulty with the patient under topical anesthesia with sedation.
Temporary stents are designed for short-term use, to relieve bladder outlet obstruction (BOO), and to act as an alternative to an indwelling urethral or suprapubic catheter in high-risk patients considered unfit for surgery. In such patients these temporary stents permit normal micturition with an acceptable side effect profile. Success rates have been reported in the range of 50% to 90%. They are easy to reposition or replace, but catheterization or cystoscopy cannot be performed while the stent is in place. Complications such as encrustation, migration, breakage, stress incontinence, and bacteriuria have been reported in varying degrees of frequency.
Spiral Stents
First-Generation Stents
The Urospiral (Porges) and the Prosta Kath (Pharma-Plast) are examples of spiral stents. The former is made of 21-Fr stainless steel and varies between 40 and 80 mm in length. The latter is also made of 21-Fr stainless steel but is gold plated in an attempt to prevent encrustation. It varies in length between 35 and 95 mm. Neither stent should remain in the prostatic urethra for longer than 12 months.
A spiral stent should be inserted with a 21-Fr panendoscope using either a 30-degree or a 0-degree lens under direct vision and with the aid of a grasping forceps. It may also be inserted over a catheter guide under ultrasound visualization (Nordling et al, 1989).
In the original series reported by Nordling and colleagues (1989), the Prosta Kath was inserted under topical anesthesia in 45 patients with acute or chronic retention. Ultrasonography was used in 35 patients and endoscopy in 6 to facilitate stent placement. Retention was relieved in 41 of the 45 patients, leading the authors to advocate its use in high-risk patients.
Ozgur and coworkers (1993) reported re-establishment of voiding with the Urospiral with good results after 4 months of follow-up in 31 patients who were unfit for surgery. The Urospiral was also used in 10 patients with advanced prostate cancer (Anson et al, 1993). All had retention or severe obstruction. The patients were started on antiandrogens after stent insertion. The stent was removed 3 months later, and all patients were reported as voiding satisfactorily, although one patient subsequently required a limited TURP. In another report, 18 high-risk patients with BPH had a Urospiral inserted (Karaoglau et al, 1992). All voided without difficulty and with complete bladder emptying. However, the complications were rather high: hematuria in 2, migration in 1, and infection in 8.
In 87 patients declared unfit for TURP, Thomas and colleagues (1993) reported an experience extending over 4 years using the Prosta Kath. Sixty-four patients presented with acute urinary retention, and, after treatment, 57 voided successfully whereas 7 failed to void. A further 14 patients presented with chronic urinary retention; 5 of these voided satisfactorily, but 9 required alternative therapy. Complications included hematuria with clot retention (5%), stent migration (15%), recurrent urinary tract infections (10%), and encrustation (4%). These findings of relatively high complications are also reported by other authors (Nordling et al, 1989; Harrison and De Souza, 1990). Braf and coworkers (1996) observed 55 men for between 12 and 16 months, 32 of whom were treated with the Prosta Kath and 23 with the Urospiral. Ten patients failed in the Prosta Kath group, and 8 patients failed in the Urospiral group. Complications in both groups include encrustation, urinary tract infection, migration, stricture formation, and failure to void. In the largest number of patients reported from one center Nordling and associates reported on the use of the Prosta Kath in 318 patients. They divided the complications into none, moderate, and severe. In the patients who were described as having severe complications, stress or urgency incontinence occurred in 63, emptying problems in 8, and frequency or nocturia (more than three times per night) or both in 57.
Second-Generation Stents
Other spiral stents have been developed as second-generation models in an attempt to overcome the problems of the first-generation stents just described while maintaining the efficacy and ease of insertion. These are the Memokath and the Prosta Coil.
The Memokath (Engineers and Doctors A/S, Copenhagen, Denmark) is made of nitinol, a nickel-titanium alloy, which has the property of shape memory. It is malleable and heat expandable at a temperature of 45° C to 50° C. Like other temporary stents, it is easy to insert and maintains its expanded position in the prostatic urethra. With the earlier model of the Memokath, epithelial hyperplasia at the apex of the prostate was reported in some cases, but modifications have since been made. The later model ensured close contact of the wires even in the expanded position, thus reducing the possibility of hyperplastic growth of the urethral epithelium through the gaps in the expanded spiral. The Memokath is soft and malleable when cooled, returning to its original shape when heated to the preceding temperatures. Of 22-Fr caliber, it has a length of 35 to 95 mm. It permits the passage of a flexible cystoscope and may be left in place for up to 36 months.
The Prosta Coil is a self-expanding and self-retaining stent made of a nickel-titanium alloy (Instent, Minneapolis, MN). It is inserted by being mounted on a 17-Fr delivery catheter under fluoroscopy; once released from the mounting it takes the form of a wave-shaped tube whose diameter varies from 24 to 30 Fr. Its length is from 40 to 80 mm, and it can be left in position for up to 36 months.
The Memokath was reported as being used in 30 patients who were unfit for surgery or who refused it; the success rate was 80%. Normal voiding is described as having occurred in all patients. The peak urinary flow rate (PFR, also Qmax) reached a mean of 16 mL/sec immediately after insertion (Poulsen et al, 1993). However, there was a wide range of PFRs, from 4 to 25 mL/sec. Unfortunately, 3 patients later developed urinary retention at 5 days, 2.5 months, and 5 months, respectively, after insertion. Two more stents needed to be removed because of hyperplastic growth of the epithelium. A total of 24 stents were still in place at 3 months. Nordling (1996) updated these authors’ experience after inserting 64 of the modified Memokath stents. Similar successful voiding was reported. The complications that patients described as severe were few, with urgency incontinence being the most common (10 patients). However, moderate symptoms were relatively more common.
Results are available from a long-term study conducted in the United Kingdom. The Memokath was inserted in men who were either permanently or temporarily unfit for TURP, in most cases because of severe respiratory or cardiovascular disease. In this study, 211 men had 217 stents inserted over an 8-year period. In the same time frame, 1511 TURPs were performed. The mean age of patients having stents was 80.2 years, and in the TURP group it was 70.2 years. The patients who had stents fitted experienced an improvement in the mean International Prostate Symptom Score (IPSS) from 20.3 to 8.2 in just 3 months, with results being maintained for 7 years. However, these results must be viewed in the light of the fact that 38% died with stents in place, 34% remain alive with their stents, and 23% had stent removal because of failure. Migration occurred in 13%, and 16% required repositioning. This study suggests that long-term success can be achievable using the Memokath but that failure can also be anticipated in a significant minority (Perry et al, 2002).
The Prosta Coil has been used in a small series of patients with short follow-up (Yachia et al, 1994). The mean follow-up was 14 months, with a range of 2 to 28 months. There were initial irritative urinary symptoms that were reported as having disappeared within 1 month. The mean PFR at the most recent follow-up was 21.3 mL/sec (with a range of 15 to 36 mL/sec) and the mean IPSS was 9 (with a range of 6 to 12).
Thus spiral stents were among the earliest type of temporary stents to be introduced, and they have developed to a large degree from the original designs. New models have reduced such complications as encrustation and urothelial hyperplasia, but stress incontinence and urgency incontinence still occur, as does displacement of the stent. However, they are easy to insert and do not require general anesthesia. Although the follow-up is relatively short term, in most of the reported series, good success rates are reported. The place of spiral stents is clearly that of a nontraumatic therapy for urinary retention in patients unfit for surgery, and they have a reasonable chance of a successful outcome.
It is hard to predict whether further design changes will take place. The earlier type of spiral stents had the advantage of being less expensive. The newer models are made of compounds aimed to reduce complications or prevent stent migration but are all more expensive than the earlier models. With a limited market and with a restricted acceptability, further developments in this type of stent are unlikely.
Polyurethane Stents
Polyurethane stents are also known as intraurethral catheters. There are three types: the intraurethral catheter (Angiomed, Germany), the Barnes stent (CR Bard, Covington, GA), and the trestle stent (Microvasive, Boston Scientific, Natick, MA).
Intraurethral Catheter
The intraurethral catheter, the first to be introduced, was reported initially by Nissenkorn (1991). It is made of a type of polyurethane known as Puroflex and has a fixed 16-Fr caliber. Its length varies from 40 to 60 mm, and it can be left in place for up to 6 months. It has a double device at its proximal end shaped like the head of a de Pezzer or Malecot catheter. It has a nylon string at its distal end and a flared split end proximally, which sits in the bladder. It is inserted under topical anesthesia using a 22-Fr cystoscope. The nylon string is cut after placement, and any positional adjustment required can be performed by the use of a grasping forceps. Eighty-five devices were inserted into 73 patients, and, of these, 60 patients had an indwelling catheter for 1 week to 3 years before insertion. Nissenkorn (1991) described a successful outcome in 63 patients who believed that their quality of life was considerably better than it had been when they had an indwelling catheter. He therefore believed that this was suitable for use in such patients, with a high likelihood of success.
A later study reported the use of the Nissenkorn intraurethral catheter in 43 patients (Sassine and Schulman, 1994). Once again, the patients treated had developed urinary retention and were unfit for surgery but also had a short life expectancy. Thirty-six of the 43 patients were able to void satisfactorily after stent insertion. The intraurethral catheter should not be inserted in the presence of bladder stones or anything else likely to block or have a ball-valve effect on the device.
One of the potential uses of temporary prostatic stents is as an expedient to overcome the early retentive effects of heat treatment to the prostate. Clearly, an objection to this might be that such a therapeutic strategy effectively doubles the cost of the treatment and so makes it less attractive. However, it is an attractive way of overcoming an early complication of heat treatment, which might have been seen by some as a significant limitation of heat treatment. The Barnes stent and the trestle stent may have such an application.
Barnes Stent
What has been called the Barnes stent is made of polyurethane, has a 16-Fr caliber, and is of a single length. It also has a de Pezzer end proximally, but this time a single one. It is thus a modification of the original intraurethral catheter. It was used in 25 patients who underwent endoscopic laser ablation of the prostate (ELAP). Twenty-two of the 25 voided immediately. Early stent migration occurred in 1 patient, but late migration did not occur. The stent was inserted with ease, could be removed with ease at 12 weeks, and was inexpensive. PFRs improved from 8 mL/sec before ELAP to 16.5 mL/sec at 6 weeks with the stent in place (Barnes et al, 1996). The Nissenkorn catheter has also been used safely and with equal success after laser therapy (Nissenkorn et al, 1996).
Trestle Stent
The trestle stent or prostatic bridge catheter has been described and consists of two tubes and an interconnecting thread. The tube that lies in the prostate has a 22-Fr diameter and has a 30-degree angulation. The length is 75 mm, and it has a smooth tip: It is to be used in prostates with a volume of less than 80 mL. The connecting thread is 25 mm, which passes through the distal sphincteric mechanism. The second tube lies in the bulbar urethra and is 35 mm long. It is inserted with the patient under topical anesthesia using a delivery system comprising a positioning stylet, an inflatable balloon with injection cannula, and an outer pusher tube. The technique is described in detail by Djavan and colleagues (1999).
In a report from Devonec and Dahlstrand (1998) the results of its use in 52 patients after high-energy transurethral microwave therapy (TUMT) were described. Tolerance was good in 32 patients, acceptable in 13, and poor in 6. Retrograde ejaculation occurred in eight. PFRs reached 14.6 mL/sec on the day of removal of the device. The device was left in place for 1 month, but the improvement in PFR was maintained at 1 year. Djavan and colleagues (1999) also described its use in 54 patients who had received high-energy TUMT. The device was left in place for up to 1 month, and it was found that the incidence of post-treatment retention was prevented, with concurrent early but significantly improved symptom scores and PFRs. Toleration was high, with 48 of 54 devices remaining in place for 1 month. Early removal was required because of urinary retention in 3 and migration in 3.
The intraurethral catheter or its more recent variations are being used in a rather different context than the spiral stents. They have been used successfully, albeit in a small number of nonrandomized short-term studies, with few problems related to tolerance. A further cost-efficacy evaluation is required, in addition to larger multicenter randomized controlled comparative trials.
The Spanner
The spanner has a design very similar to the proximal 4 to 6 cm of a Foley catheter. It includes a balloon to prevent displacement, a port for urine drainage that lies proximal to the balloon, and a reinforced stent of varying length that spans most of the prostatic urethra (Fig. 93–1).

Figure 93–1 Pro Vu device used for transurethral needle ablation of the prostate.
(Courtesy of Neo Vitalis Ltd, Southport, UK.)
In the first study using this device, the stent was inserted under topical anesthesia in 30 patients (Corica et al, 2004), of whom 5 had been in urinary retention. The stents remained in place for a mean of 57 days. The mean PFR improved by 42% from 8.2 to 11.6 mL/sec. The overall mean IPSS decreased from 22.3 to 7.1, a 68% difference. The adverse events were few, and the device was found to be stable and patent at the end of the study.
This article describes an early open study designed to test the efficacy, safety, and stability of the device. It could be criticized in that it was composed of a group of patients whose characteristics were not adequately described, with no entry criteria defined. It is, however, an interesting new device that may well be important for many patients as a temporary method of bypassing prostatic obstruction.
Biodegradable Stents
The concept of stents that can be put in place after a procedure that has a high incidence of secondary and temporary obstruction has been mentioned earlier in the context of stents being inserted after laser or high-energy TUMT. These stents are removed some weeks later. With the biodegradable stent the concept is brought one step further; the stents do not need to be removed, and eventually they disappear by biodegrading. This interesting idea was first introduced in urology when Kemppainen and colleagues (1993) used a biodegradable stent in rabbits after urethrotomy. The idea has now been extended to the ureter (Schlick and Planz, 1998; Lumiaho et al, 1999; Clayman, 2000), after endoscopic urethroplasty (Oosterlinck and Talja, 2000), and in coronary artery disease (Tamai et al, 2000).
Further experimental studies have shown that biodegradable stents can potentially be used as a bridge across the prostate after minimally invasive procedures, without the necessity of having to remove them later (Petas et al, 1997a, 1998; Laaksovirta et al, 2002; Vaajanen et al, 2003).
In addition, clinical studies have been performed that examine the use of biodegradable stents after various procedures (Talja et al, 1995; Dahlstrand et al, 1997; Petas et al, 1997b). The benefit is that these stents prevent the development of obstruction that can occur after laser procedures; however, the use of the second procedure in association with, for example, laser prostatectomy takes away a great deal from the value of the first procedure, not least in terms of cost-effectiveness. There are three types of biodegradable stents.
A randomized study (Petas et al, 1997b) compared self-reinforced polyglycolic acid biodegradable spiral stents (group 1), no device (group 2), or an indwelling catheter (group 3) after visual laser ablation of the prostate. The procedure was performed on 72 men, and 27 were in group 1, 23 in group 2, and 22 in group 3. Voiding began at a median of 1 day in group 1 and a median of 6 days in group 2; the indwelling catheter was required for an average of 6.5 days in group 3, and voiding commenced a median of 6 days after this. The authors found, as they had in previous in-vitro studies, that the stent degraded into small fragments of polymer debris that were passed out in the urine. They commented that voiding became more obstructed at 3 to 4 weeks postoperatively, presumably from degradation and sloughing of the stent, but they found that this was only a transient effect.
In another study, Dahlstrand and colleagues (1997) evaluated the same polyglycolic acid stent after high-energy TUMT with the Prostasoft 2.5. They compared the use of the stent in 15 patients against a further 15 patients in whom a standard 16-Fr urethral catheter was inserted. The mean duration of catheterization was 14.1 days, with a standard deviation of 4.1 days; this was obviously prevented by the stent, which did not cause problems, even when it was degrading.
In a rather innovative way, Knutson and colleagues (2003) described the use of a biodegradable polyglycolic acid stent to assess the risk of post-TURP incontinence in patients with combined BOO and overactive bladder. In 37 patients with severe overactive bladder and moderate to severe BOO this biodegradable stent was inserted into the prostatic urethra; 25 noticed either no leakage or minor leakage and 19 have had TURP with good results. Twelve of the 37 had a major problem with incontinence after the insertion of the stent. There was a small complication rate related to stent insertion.
These stents are definitely of interest for the future, but their exact place in the treatment of BPH needs to be established, with larger and longer-term studies. In addition, the problem related to the overall cost must be defined accurately; otherwise, the value of the primary procedure will be questioned.
Permanent Stents
With permanent stenting of the prostate, the urologist is attempting to treat definitively and permanently patients who present with symptomatic BPH. To be of proven value this type of treatment, like any other, must be shown to be at least comparable to TURP. The initial enthusiasm for permanent stents has been replaced by relative silence in the literature at present. Permanent stents were initially introduced as treatment for recurrent urethral strictures and were subsequently used in patients with lower urinary tract symptoms (LUTS). In urologic terms, permanent stents are being used preferentially for the treatment of detrusor-sphincter dyssynergia (Chancellor et al, 1999; Chartier-Kastler et al, 2000; Gajewski et al, 2000), postbrachytherapy BOO (Konety et al, 2000), anastomotic strictures and urinary incontinence after radical prostatectomy (Meulen et al, 1991), and complex urethral strictures (Tillem et al, 1997). There have been no reports in the recent literature that relate to the long-term follow-up of the patients originally treated with permanent stents, and there has been no indication of new interest in their use.
UroLume
The UroLume endourethral prosthesis (American Medical Systems, Minnetonka, MN) is a woven tubular mesh that maintains its position in the urethra by outward external pressure, thus maintaining the patency of the prostatic urethra. The original 42-Fr device was made of metal superalloy and varied in length from 1.5 to 4.0 cm. It is inserted with a special 21-Fr deployment tool using a 0-degree panendoscope. Gradually, epithelialization occurs, ideally in a smooth manner, covering the individual wires of the mesh. The stent can be removed by securing about 5 mm of the distal aspect in the jaws of a grasping forceps and then pulling the distal end inside a resectoscope sheath to minimize any possibility of urethral trauma while removing it.
The original stent tended to shorten while it expanded outward, leading to its replacement by a stent less likely to do so. However, this stent tended to migrate more easily, and a further modification was required. In addition to these changes the delivery tool was modified, leading to the present, more satisfactory model.
Chapple and colleagues (1990) reported on the initial experience with the UroLume. Twelve patients who were considered to be a poor risk for surgery presented with LUTS; 9 of the 12 patients presented with urinary retention. The results were encouraging, with 11 of 12 voiding satisfactorily for a mean follow-up of 8.2 months. The mean PFR after the procedure was 13.6 mL/sec. Further encouragement came from the low complication rate, consisting mainly of short-term irritative voiding symptoms, with only 1 of the 12 being dissatisfied because of severe urgency and frequency (the patient was subsequently found to have detrusor instability). A further study in a similar group of unfit patients was performed by McLoughlin and colleagues (1990). All 19 patients in their study group presented in urinary retention, and all voided satisfactorily after the stent was inserted under local anesthesia.
In a larger, multicenter open trial from the United States, Oesterling and colleagues (1994) reported on 126 men who presented either with moderate or severe LUTS (95 men) or with urinary retention (31 men). There were strict inclusion and exclusion criteria in the trial design, but fitness for surgery was not among them. In the nonretention group, 80 of 95 were evaluable at 12 months and 52 at 24 months; the Madsen symptom score decreased from 14.0 to 5.9 and 5.4, respectively. In the retention group, 24 of the 31 patients evaluable at 12 months had a mean symptom score of 6.1. In the nonretention group, the PFR increased from 9.1 to 13.0 and 13.1 mL/sec, respectively, with the retention group having a mean PFR of 11.7 mL/sec at 12 months. Difficulties with insertion were experienced in 16% of cases; irritative voiding symptoms occurred in 10%.
Guazzoni and coworkers (1994) described a European study using the modified UroLume stent (the so-called less shortening variety described earlier). Once again, the strict inclusion and exclusion criteria did not refer to fitness for surgery, and at this time the stent was being presented as a proposed therapy for prostatic obstruction, not necessarily only for unfit patients. In this multicenter study, 135 healthy patients (91 with LUTS, 44 with urinary retention) were treated. In the nonretention group, 74 of 91 patients were evaluable at 12 months. The mean Madsen-Iversen symptom score had decreased from 14.1 to 6.4, but the tight standard deviations of the mean observed in the U.S. study (0.4) were not seen in this study (5.1); the PFR improved from 9.3 to 15.7 mL/sec at 12 months (with a very wide standard deviation of 6.5, unlike that in the U.S. study). In the retention group, 34 of 44 were evaluable at 12 months; the mean symptom score was 4.5 and the mean PFR was 13.1 mL/sec. The complications were well described but were found to be significant in the long term.
In a British study (Bajoria et al, 1995), 44 men fit for TURP accepted as an alternative a second-generation UroLume stent. The stent was inserted in 44 patients, who either were in urinary retention or had urodynamically proven outflow obstruction. The results achieved were similar to those reported by Guazzoni and colleagues (1994), but there was also a relatively high complication rate. Both sets of authors noted epithelial hyperplasia and migration of the stent in addition to irritative urinary symptoms and painful ejaculation. The second-generation less shortening stent was not recommended for general use, and a third-generation stent was then produced. However, Bajoria and colleagues (1995) strongly suggested that permanent stents should still be considered as being under evaluation rather than for general use.
In a multicenter study of 96 men who were unfit for prostatic surgery, 73 presented in acute urinary retention and 11 in chronic retention. All but 6 were able to void immediately after stent insertion; 2 required a second stent, and 4 required a period of suprapubic catheter drainage. At 12 months, the PFR was 15 mL/sec in the retention group and 18.1 in the nonretention group. Severe irritative symptoms were seen in the majority of patients for up to 3 months, and encrustation was encountered in 15 of 27 patients who underwent cystoscopy (Williams et al, 1993).
The results of a long-term analysis of the UroLume Wallstent have been published by Masood and coworkers (2004). The stent was inserted in 62 patients with moderate or severe LUTS secondary to BPH. The 5- and 12-year follow-up was completed by 22 and 11 patients, respectively. Death occurred in 21 patients (34%), and the stent was removed in 29 patients (47%), the vast majority of these removals occurring in the first 2 years. The authors concluded that this is a safe treatment but that cases must be carefully selected and that it should be performed only by experienced hands.
The use of the UroLume stent can be seen to have some application in patients with prostatic obstruction, particularly if they are unfit. However, interest in this type of stent seems to have waned somewhat because of the use of other types of less invasive treatments, which appear more satisfactory to patient and urologist alike.
Memotherm
The Memotherm (Angiomed, Germany) is a stent of nickel-titanium alloy that is expandable to 42 Fr with heat. When it is cooled, it can easily be compressed and distorted, but when warmed to body temperature it expands to a flexible cylinder and does not shorten. It is made from a woven single wire, which makes it easy to remove; traction unravels the wire. It is manufactured in lengths varying between 1.5 and 8.0 cm and is available in a prepacked sterile delivery service. It is inserted under direct vision with a 0-degree telescope.
Williams and White (1995) reported on 48 men with LUTS and urodynamic findings suggestive of bladder outflow obstruction. The results were disappointing. Only 37 patients were able to void immediately after stent insertion, the others requiring a suprapubic catheter for up to 8 weeks. Symptomatic improvement occurred in many, but complications, including stent migration, were relatively high. Thirteen of the 48 patients required removal of their stents. These authors suggested that the results were not appropriate to encourage marketing of the device.
Gesenberg and Sintermann (1998) used the Memotherm in 123 patients considered to be at high risk for prostatic surgery; 46 of these presented in urinary retention. Of the 123 patients, only 52 were evaluable at 12 months. The mean PFR increased from 7.4 to 13.0 mL/sec (with a standard deviation of 6.2), and the IPSS improved from 24.0 to 8.8 (SD 6.2). The authors noted a considerable improvement in quality of life. However, the complication rate was relatively high, with recurrent infections and urgency symptoms in 56%, urothelial hyperplasia in 34%, and urethral stricture in 10%. There was a high number of re-treatments, and the authors suggested that there may be an additional role for medical treatment in some of these patients.
These results have not appeared to be convincing large numbers of urologists to use these stents on patients with symptomatic BPH, but modifications may occur. Heat-expandable stents are widely used in cardiovascular conditions and in biliary stenosis.
Other Permanent Stents
The ASI stent (Advanced Surgical Instruments) was evaluated in several centers (Kirby et al, 1992; Kaplan et al, 1995). It was introduced on a balloon, which was then inflated, thus expanding the stent. The early results suggested an improvement in symptom score (44%) and PFR (22%), but complications also occurred that made it less attractive for general use. It has since been withdrawn from production.
The Ultraflex stent (Boston Scientific, Natick, MA) is made of nickel-titanium alloy that also has a capacity to expand to a caliber of 42 French when exposed to body heat. It is available in lengths varying from 2 to 6 cm. There have been reports of its use in patients with prostatic obstruction, but it has been studied in a group of patients with detrusor-sphincter dyssynergia (Chartier-Kastler et al, 2000), and the incidence of epithelial hyperplasia and migration was encouragingly low.
Summary
Originally, permanent prostatic stents were introduced as a definitive treatment for prostatic obstruction, particularly (but not in every study) for patients unfit for prostatic surgery who presented with urinary retention. Patients were able to void satisfactorily in most cases, but complications were relatively high. One stent has been removed from the market, one has not yet been reported on as a treatment for prostatic problems, variable results have been reported on another, and the most frequently investigated, the UroLume, has not received recent attention in the literature as a specific treatment for BPH.
Temporary stents are receiving widespread attention, but the original idea that they should be used as a temporary expedient to overcome outflow problems in the medically unfit population is being modified. The newer stents, whether biodegradable or not, are being viewed as possible methods of overcoming the temporary retention that can occur secondary to treatments such as laser therapy or high-energy TUMT.
Transurethral Needle Ablation of the Prostate
Heat treatment of whatever kind to the prostate is intended to reduce outflow resistance and the volume of the obstruction by increasing the temperature within the prostate and inducing necrosis of prostatic tissue. The aim is to increase prostatic temperature to in excess of 60° C. Transurethral needle ablation of the prostate (TUNA) uses low-level radiofrequency (RF) energy that is delivered by needles into the prostate and that produces localized necrotic lesions in the hyperplastic tissue. It has previously been used to ablate cardiac nerve bundles in the Wolff-Parkinson-White syndrome (Calkins et al, 1992) and to destroy malignant tissue (Rossi et al, 1995; Zlotta et al, 1995). It has also been used to treat chronic cervical zygapophyseal joint pain (Lord et al, 1996). The advantage of TUNA is that it can be delivered under topical anesthesia to patients with symptomatic BPH, causing very precise and reproducible lesions within the prostate.
Delivery of Radiofrequency Energy
The TUNA system (Medtronic, Inc, Minneapolis, MN) consists of a special catheter attached to a generator. At the end of the catheter are two adjustable needles that are withdrawn into two adjustable shields made from Teflon. The needles are advanced into the prostatic tissue and can be placed accurately into the required position.
The generator produces a monopolar RF signal of 490 kHz, which allows excellent penetration and uniform tissue distribution. The patient has a grounding pad placed over the sacrum, and the current passes toward this through the prostatic tissue. In other words, tissue heating is created because of tissue resistance to the current as it flows from the active to the return electrode. The active electrode has a small surface area, with the RF current being concentrated in an area immediately surrounding it. The return electrode is large, and so the diffusion of the RF current is greater. This arrangement allows heat to be concentrated near the active electrode early, thus accurately controlling the tissue effect. The size of the lesion caused by RF relates to the position and depth of insertion of the electrode as well as the power used and the duration of the treatment.
RF produces molecular or ionic agitation with collision of particles that relates to the frequency of the energy, and this results in a central hot core inside the prostate and away from the urethra (Schulman et al, 1993). The limited distance dissipation reinforces the safety of the procedure because RF can be applied to tissue only by direct contact, with heat being generated proportional to one over the fourth power of the radius. If the power generated is too high, the prostate rapidly desiccates with a rise in tissue impedance, preventing the desired heating effect. Therefore the appropriate energy level required to produce the localized necrotic lesion must be found, preventing the increase in tissue impedance resulting in prostatic charring around the needle caused by excessive generation of energy (Schulman et al, 1993). Interestingly, heat is lost by convection, and so increased vascularity can have an effect on the degree of localization of the lesion. RF is very much affected by blood flow and has almost no effect on vessels larger than 2 to 3 mm in diameter (Organ, 1976).
There is a difference in the method of tissue heating brought about by RF and, for example, microwave application. Microwaves treat a broad area and can penetrate tissue more deeply than RF. The central temperature is therefore lower than with RF to maintain safe heat levels at the treatment rim. Therefore treatment with microwaves takes longer than RF to produce coagulative necrosis. RF, however, has a much hotter central area with a very quick decline in temperature as the distance increases from the treatment needle. This results in faster generation of the necrotic lesion but of a smaller area (Perlmutter et al, 1993).
Experimental Studies
In a series of preliminary studies on animals and ex-vivo human prostates it has been shown that the TUNA system can create 1-cm necrotic lesions without difficulty in the prostate with no damage to rectum, bladder base, or distal prostatic urethra (Goldwasser et al, 1993; Ramon et al, 1993). Other studies showed that the lesions are accurate with sharp delineation from the untreated areas (Schulman et al, 1993). These authors also showed that the lesion appeared first as a hemorrhagic lesion along the needle path, with slight discoloration in the surrounding area. Necrosis was maximal at 7 days, with fibrosis having developed by 15 days.
In an elegant neurohistochemical study, Zlotta and colleagues (1997) removed prostates from patients scheduled for prostatectomy 1 to 46 days after TUNA. Immunohistochemistry was used with anti–S-100 protein and neuron-specific enolase for nerve staining and anti–prostate-specific antigen and antidesmin for glandular and muscle cells. They showed that the maximal lesion size ranged from 10 × 7 to 20 × 10 mm2 and that there was destruction of all tissue components. The lesions were accurately positioned 0.3 to 1.0 cm from the urethra, which remained undamaged. In the treated area there was an absence of staining for prostate-specific antigen, smooth muscle actin, and α-adrenergic neural tissue. Even in specimens removed 24 hours after TUNA, no positively staining nerve cells were seen in the treated areas.
It has also been shown (Issa et al, 1996) that there may be sequential damage to different types of nerve endings. Nitric oxide synthase receptors were found to be most vulnerable to thermal damage, which occurred earliest, with damage to the α-adrenergic receptors maximal at 1 to 2 weeks.
The temperatures achieved in the largest area have been studied by Rasor and coworkers (1993) using an infrared temperature monitor in an ex-vivo animal model. They showed that the central core of the lesion around the tip of the lesion reached 90° to 100° C. Treatment times of 5 to 7 minutes were required to produce coagulation necrosis in the treatment zone. Dosimetry studies have shown that the temperatures at the edge of the zone were 50° C.
Instruments
The generator produces low-level monopolar RF waves of about 490 kHz, which produce temperatures of about 100° C in the target area. The RF generator is connected to the TUNA catheter, which has changed somewhat in design since it was first manufactured.
The TUNA catheter is, in fact, a specifically designed endoscopic instrument. This has evolved from what was a device through which a panendoscope lens could be inserted. A number of other changes have also been made. The most up-to-date version of the TUNA catheter is called the Pro Vu system, and part of this device is reusable, unlike previous models (Fig. 93–2; see also Fig. 93–1). The new system also contains a markedly improved optical system. Previously, the needles were introduced either blindly or under transrectal ultrasound (TRUS) guidance, which had the advantage in the latter case of seeing how close to the surrounding tissues the needles lay. However, an adequate visualization of the needle entering the prostate tissue gives the urologist a better idea of the treatment area. The final change concerns the angle between the catheter and the needles, which is at present not fixed. This means that a high bladder neck that is hypertrophied or a genuine median lobe enlargement can now be treated easily by this technique.
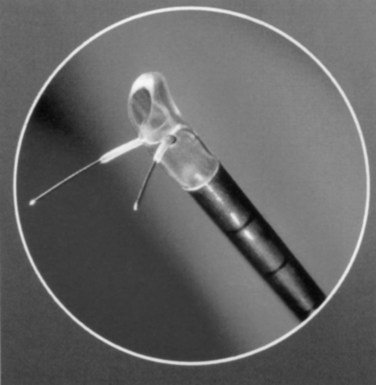
Figure 93–2 Deployment of radiofrequency needles in transurethral needle ablation of the prostate.
(Courtesy of Neo Vitalis Ltd, Southport, UK.)
The configuration of the needles within their protective sheath ensures that the treatment area is deep inside the prostatic lobes, and this ensures that the prostatic urethra is spared. Because there is a limited number of nerve endings in the prostatic glandular tissue and because the higher concentration of nerve endings immediately underlying the urethral epithelium remains undisturbed, topical anesthesia can be used with the patient experiencing only moderate discomfort. In addition, postprocedural irritative urinary symptoms are kept to a minimum.
Treatment
The patient is placed in the lithotomy position and topical anesthesia with 2% intraurethral lidocaine (Xylocaine) is achieved. The best result is attained by applying a penile clamp for 10 minutes and by giving intravenous sedation if required. There is a variation from institution to institution as to the optimum form of anesthesia; for many, what is described previously is satisfactory, but some prefer spinal or even general anesthesia, particularly for their initial experience with the technique. In other cases, a local prostatic block, either transperineally or transrectally, may be the method of choice.
With the new modifications the TUNA catheter is advanced under vision with the 0-degree fiberoptic telescope, which also allows the urologist to see the needles being advanced accurately into the prostate. The exact position within the prostate of the needle tip can be visualized by TRUS. Preprocedural assessment of the size of the prostate by this method allows calculation of the length of deployment of the needle within the prostate; this can also be done at the beginning of the procedure, as described earlier, with the ultrasound showing the exact position of the needle tip. It is important to remember that the thermal lesion may extend for up to 5 to 6 mm beyond the position of needle deployment with lesions measuring up to 20 × 10 mm. When the position is deemed satisfactory, the Teflon shield on the proximal part of the needle is advanced to protect the urethral epithelium and the underlying tissue. Therefore the tip of the needle should not lie within 5 to 6 mm of the outer rim of the prostate, and the urethra is protected by the shields that extend from 5 to 6 mm from the TUNA catheter itself.
The number of lesions depends on the size of the prostate, but because there are two needles each time power is switched on in the generator two lesions are produced. It is usually advised that one pair of lesions should be used to treat 20 g of prostate tissue; it can also be expressed in terms of length, with one pair of lesions, or treatment plane, being used for less than 3 cm of prostatic urethral length, two planes for 3 to 4 cm, and one extra plane of treatment for every extra centimeter of urethral length. The procedure is then repeated in the opposite lobe.
The RF power that is delivered is 2 to 15 W for 5 minutes per lesion. In earlier models the treatment was begun at a low power, but now the whole thermal process is automated, with temperature levels at treatment areas preset. The temperature at the tip of the needle varies from 80° C to 100° C. The urethral temperature is kept below 46° C, and the temperature in the lesion is sustained for the treatment period.
At the end of the procedure, some urologists like to leave a urethral catheter in overnight; others do not put in a catheter and allow the patients to go home after they have voided and feel that they are emptying their bladder.
Clinical Results
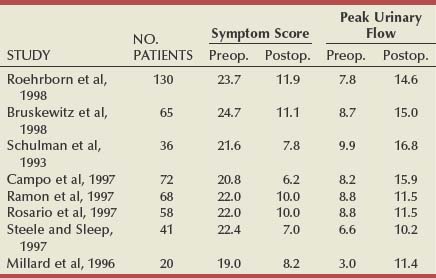
Table 93–1 shows the results of the total world experience in the use of TUNA. There is a wide variety in the number of patients in each series and in the length of follow-up. Of note is that most are open series, with a minority of randomized studies (Issa and Oesterling, 2000). Although the inclusion and exclusion criteria are the same in most series, they are not exactly the same and are not as strict as those in studies testing α-adrenergic blockers and 5α-reductase inhibitors. The size of the studies varies from 12 to 130 patients, and, in many cases, the number of patients observed for the longest period of time consists of less than 50% of the original sample, which makes it difficult to draw definite conclusions. However, when looking at them all together, a number of points can be considered.
A total of 546 patients have been observed for 12 months, with an increase in the mean PFR by an average of 6 mL/sec, representing an average improvement of 77%. The mean symptom score decreased by an average of 13.1 symptom units, which is an average improvement of 58%. Although there was consistency of the general improvements, there was a range. The greatest improvement in the mean PFR was 9.2 mL/sec (Giannakopoulos et al, 1996), and in the mean symptom score it was 15.4 symptom units (Steele and Sleep, 1997). The least improvement in mean PFR was 2.7 mL/sec (Rosario et al, 1997) and in symptom score it was 10.8 (Millard et al, 1996). Although the numbers in patients observed for longer are less (176 for 24 months and 88 for 36 months), the mean improvements appear to be maintained (Issa and Oesterling, 2000).
In the U.S. randomized trial comparing TUNA with TURP, 65 patients were treated by TUNA and 56 by TURP (Bruskewitz et al, 1998). At 1 year, 59 of the TUNA group (90%) and 47 of the TURP group (84%) were available for evaluation. In the TUNA group the symptom score improved from 24.7 to 11.1 (13.6 symptom units) and the PFR from 8.7 to 15.0 mL/sec (6.3 mL/sec). In the TURP group, the symptom score improved from 23.3 to 8.3 (15.0 symptom units) and the PFR improved from 8.4 to 20.8 mL/sec (12.4 mL/sec). The reasons for patients being lost to follow-up are clearly stated, but it was because of ineffectiveness in only 2 patients (3.1%) of the TUNA group and none of the patients treated by TURP. The treatment was found to be effective and safe. The complication rate of TUNA was low, in both the short and the long term; the most commonly reported adverse events were bleeding (32.3%), urinary tract infection (7.7%), and urethral stricture (1.5%). There was no adverse effect of any kind on sexual function in patients treated by TUNA.
There are some additional long-term studies that are of interest. Zlotta and coworkers (2003) entered 188 consecutive patients into a study; there were 5-year data on 121 of these. At the 5-year assessment, 41 of the 176 patients who were evaluable (2 dead, 10 lost to follow-up) required additional treatment after TUNA (23.3%). Although there were 5-year data on 121 patients, and this was defined as a 5-year follow-up, the 10 patients on whom there were only 4-year data were also included, giving a total of 131 patients. The mean IPSS decreased from 20.9 to 8.7, with tight standard deviations but a range at long-term follow-up of 2 to 20. The PFR improved from 8.6 to 12.1 mL/sec with a range at long-term follow-up of 6.5 to 19.2 mL/sec, once again giving the impression of quite a wide scatter of results. Although the drop in the mean IPSS is 12.2, which is an impressive decrease, the improvement in PFR is a less impressive decrease, being 3.5 mL/sec and comparable to that achieved by medical management at the same time point.
In another study from the United States (Hill et al, 2004), 121 men were enrolled in a prospective, randomized, multicenter clinical trial; 65 (54%) were randomly selected to receive TUNA and 56 (46%) were selected to receive TURP. It was reported that 9 of the 65 men (14%) required further intervention in the TUNA cohort, compared with 1 of the 56 men (2%) in the TURP cohort. The requirement for additional medical management in the TUNA cohort was not stated, presumably explaining the difference observed between this study and that described in the previous paragraph. Although this was reported as a 5-year study, only 18 of the 65 men in the TUNA group (28%) and 22 of the 56 (39%) in the TURP were evaluable at 5 years. This makes the 5-year data difficult to interpret despite the highly significant statistical differences described by the authors. At 3 years, the mean IPSS had decreased from 24.0 to 15.2 in the TUNA group and from 24.1 to 10.1 in the TURP group. The mean PFR had improved from 8.8 to 13.0 mL/sec in the TUNA group and from 8.8 to 19.1 mL/sec in the TURP group.
A cost comparison of medical management and TUNA for treating BPH over a 5-year period was performed by Naslund and colleagues (2005). They constructed a cost analysis model using published costs for tamsulosin, finasteride, TUNA, and TURP. They found that over the 5 years tamsulosin was less expensive than TUNA and that finasteride cost the same as TUNA. Combination therapy was more expensive, reaching a break-even point at 2 years and 7 months of treatment. The authors also calculated that TURP is more expensive than TUNA for achieving improvements in IPSS but less expensive for improving PFR.
A meta-analysis of trials of TUNA for treating symptomatic BPH has been reported (Boyle et al, 2004). Meta-analyses are dependent on the data that are put into them. In the case of TUNA, the trials analyzed were often poorly constructed, with inadequate numbers, and not randomized. In fact, there were 2 randomized trials, 2 nonrandomized observational protocols, and 10 single-aim observational studies. One thing that was consistent, however, was that in all studies the patients had severe LUTS, the mean IPSS at entry being greater than 20. The effect of TUNA was to halve the mean IPSS at 1 year. The shortage of long-term studies makes it difficult to draw specific conclusions, but although there was a tendency for the IPSS to increase in the long term the 50% decrease was maintained. The PFR increased by about 70% from baseline to 1 year. It tended to decline over time, but a 50% or greater improvement was maintained. One question has not been answered: although the mechanism of action of TUNA is an adequate explanation for the early improvements, how can it explain any positive long-term effect?
Pressure-Flow Studies
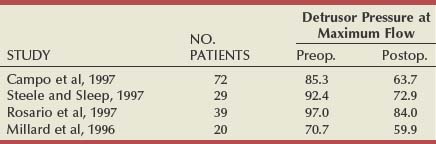
There have been six studies that examined relief of urodynamically proven obstruction as an end point, and these are summarized in Table 93–2. The number of patients in each group varies considerably (from 12 to 108), as does the length of follow-up. The largest number of patients are evaluable at 3 months (253), with smaller numbers having been observed to 12 months (140) or even longer. All patients had pressure-flow studies performed before and after treatment by TUNA.
At 3 months the average detrusor pressure (Pdet) at PFR was 85.4 cm H2O, which 3 months after treatment had decreased to an average of 64.8 cm H2O (average decrease of 20%). The range at 3 months was 53.2 to 79.0 cm H2O. It is difficult to draw too many conclusions from these average figures because of the variability in each series, but they do point to the fact that there is a decrease in the Pdet at PFR in most series, if not quite relieving obstruction reliably in every case. At 12 months the average Pdet at PFR was 91.6 before treatment and 73.5 after treatment. In each series there was a small decrease in the difference that had been observed at 3 months.
In another urodynamic study (Minardi et al, 2001), a small number of patients (24) were observed to 24 months. The authors showed that there was initially no change in the prostatic volume or the prostate-specific antigen levels and that pressure-flow studies showed a reduction in the mean opening pressure and Pdet at PFR. This led them to speculate that the ideal patient for TUNA was a man younger than 70 years with a prostatic volume of less than 6 mL, with a pretreatment Pdet at PFR of less than 60 cm H2O and residual volume of less than 100 mL.
Adverse Effects
The adverse effects that occurred in the U.S. randomized study (Bruskewitz et al, 1998) have been alluded to earlier. By far the most common complication reported, however, is post-treatment urinary retention, occurring at a rate between 13.3% and 41.6%. It can be expected that within the first 24 hours about 40% of patients experience urinary retention. The second most common adverse event reported is that of irritative voiding symptoms, occurring in about 40% of patients in the early period after treatment. Given the mechanism of the TUNA treatment, this high rate is surprising, but the symptoms are usually mild, lasting between 1 and 7 days.
Urinary tract infection was not commented on in every series, but it was reported in up to 3.1% of patients. It is advisable to give a prophylactic antibiotic to cover the treatment, using whatever regimen the particular urologic department uses for other endoscopic procedures. In this way symptomatic infections leading to epididymo-orchitis or significant sepsis can be completely prevented. Urethral strictures are uncommon, with the highest rate reported as being 1.5%. Hematuria occurs in a large number of treated patients but is almost always mild and short lasting. Patients who are taking aspirin, in whatever dosage, should be advised to refrain from taking it for 7 days before the procedure.
Sexual dysfunction is rare after TUNA. Urinary incontinence has not been reported in any series.
Reoperation
The reoperation rate must be compared with that of TURP. Although a 14% requirement for reoperation because of lack of efficacy of the primary treatment with TUNA may seem low, it occurred in less than 2 years (Schulman and Zlotta, 1995). In addition, the 12.7% incidence reported by Steele and Sleep (1997) occurred inside a 2-year period. In the multicenter study reported by Ramon and colleagues (1997), 9 of 76 patients were deemed to have experienced failure of the procedure because of an absence of improvement in PFR. Eight of these patients had no symptomatic improvement, but 5 had an improvement in quality of life.
Indications
The patient most likely to benefit from TUNA would be one who had lateral lobe enlargement and a prostate of 60 g or less (Naslund, 1997). Larger glands can be treated, but more time has to be spent treating each 1-cm segment. Patients with larger prostates, purely bladder neck hypertrophy, or median lobe enlargement are not ideal patients to be treated in this way, but they can be treated; for example, median lobe enlargement can be treated by rotating the TUNA catheter so that the needles point posteriorly, with special care being taken in assessing the depth of their penetration into the prostate.
Summary
The TUNA procedure is simple to perform, and the technology is improving all the time. As long as it is performed carefully, with special emphasis on placement of needles and awareness of the depth of their penetration, complications can be kept to a minimum. With the combination of improved endoscopic visualization of the needles and placement using TRUS, efficacy can be high, with an average improvement in the mean symptom score of 13.1 symptom units and in the mean PFR of 6 mL/sec to be expected at 12 months. A treatment by some other modality can be expected in 12.7% to 14% of patients within 2 years. TUNA can be given with the patient under topical anesthesia or local prostatic or perineal block, and so it is useful in poor-risk cases also. About 40% of patients have retention within the first 24 hours. The long-term efficacy of the treatment has not been clearly evaluated, with no large series of patients having long-term follow-up.
Transurethral Microwave Therapy
Transurethral microwave therapy has been much evaluated in the past decade and has been widely used. Many urologists have a high regard for its usefulness in treating patients with LUTS, but, for many others, it has no place in the therapeutic line-up. TUMT has been examined clinically in many centers throughout the world, although the very large number of patients who have been entered into open noncomparative studies may surprise some urologists. The rationale for its effect on symptoms has also been studied carefully by authors from different centers, which is unlike that of many of the other so-called minimally invasive treatment modalities. In addition there has been an evolution in the technology of TUMT (Figs. 93-3 and 93-4), from low-energy to high-energy application, perhaps indicating that this technique has a future in the treatment of LUTS.
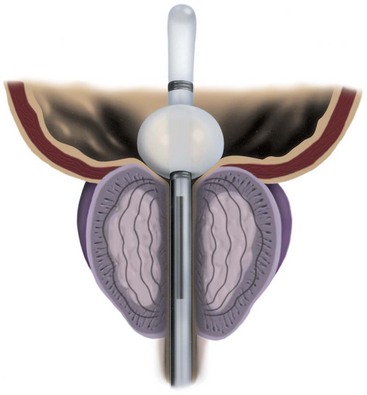
Figure 93–4 The Prostatron antenna, treating the prostatic transition zone.
(Courtesy of Urologix, Inc, Minneapolis, MN.)
The current transurethral method has developed from the early transrectal devices that supplied heat ranging from 42° C to 44° C. The results with this early form of treatment were rather disappointing, and transurethral catheters were developed that would allow higher temperatures to be used while cooling the urethral mucosa. In one of these devices currently used, the Prostatron, the cooling fluid in the catheter maintains the urethral temperature at about 44° C or lower while producing temperatures within the prostate of up to 70° C. The early experimental studies (Magin et al, 1980; Harada et al, 1985; Leib et al, 1986) showed that microwave energy could create high temperatures in the prostate of laboratory animals without damaging the surrounding structures.
Early clinical results using transrectal probes suggested some efficacy (Yerushalmi et al, 1985; Servadio et al, 1987), but overall results were disappointing when tested against sham controls and did not demonstrate that further use of this type of treatment would be beneficial to patients (Zerbib et al, 1992). Later changes resulted in the transurethral method of applying microwave treatment, and this has been further developed, leading ultimately to the sophisticated machines that are being used today. The two most commonly used are the Prostatron and the Targis, but there are several others, all producing similar effects. The Prostatron has three different types of software programs, ranging from relatively low to higher energy: the Prostasoft 2.0, 2.5, and, more recently, 3.5.
Method of Action
The effect of TUMT on prostatic tissue has been studied widely, and a number of different theories, none mutually exclusive, have been presented. These cover heat changes and differential blood flow in the prostate, damage to the sympathetic nerve endings, and induction of apoptosis.
Temperature and Blood Flow
Several studies have been performed that described the interstitial thermal mapping in canine and human subjects (Astrahan et al, 1991; Kaplan et al, 1992; Roehrborn et al, 1992; Devonec et al, 1993). These have all shown that the temperature varies in the prostatic adenoma, with the area around the urethra being relatively spared from high temperatures and with surrounding tissues such as the rectum also being unaffected. Some of these studies involved a limited number of measurement sites, the use of periodic rather than continuous temperature monitoring, and difficulties with positioning of the temperature probes.
In an extensive study involving careful and accurate placement of probes, Larson and Collins (1995a) measured temperature gradients within the prostate. Their interstitial thermal mapping method involved the placement of thermosensors using a biplane ultrasound imaging system; this allowed accurate needle placement in both the anteroposterior and the longitudinal dimensions. In their study they increased the microwave power by 5 W, reaching a maximum of 36 W in 11 minutes and then maintaining an average power of 31.4 W. In one representative patient, urethral temperature reached a nadir of 21.7° C, then rose progressively to 40.1° C, and remained between 38.7° C and 41.1° C for the duration of the treatment. Rectal temperature did not show an initial decline but remained at 37.7° C to 39.1° C throughout. Prostatic temperature at a distance of 5 mm radially from the urethra reached 62.4° C about 7 minutes after the initial power maximum and remained between 60.9° C and 68.7° C. At 10 mm radially from the urethra, the average temperature was 50.5° C, whereas at 15 mm it was indistinguishable from urethral or rectal temperature (Larson and Collins, 1995a; Larson et al, 1998b).
Osman and colleagues (2000) performed a study in 13 patients in which they recorded maximum mean peak temperatures of 66.8° ± 13° C at 4 mm from the urethra using the Targis device. No temperature higher than 45° C was recorded beyond 15 mm on either side of the urethra anteroposteriorly or beyond 16 mm on either side of the urethra transversely. Using gadolinium-enhanced magnetic resonance imaging (MRI), they found postprocedural perfusion defects with means of 28.1% ± 2.1% of the total gland and 63.6% ± 34% of the transition zone volume.
Histopathologic changes were found to be related to the temperature rises in different parts of the prostate (Larson et al, 1996). They showed that tissue exposed to a minimum of 45° C for about 60 minutes suffered hemorrhagic necrosis with uniform extirpation of tissue. The border between viable and necrotic tissue was sharply defined. The histologic changes were related to temperature rises within the prostate, and it was also believed that differences in the thermal sensitivity between stromal and epithelial elements appeared unlikely to account for differences in treatment outcomes. However, there has been some disagreement about these findings. In an evaluation with MRI, Nordenstam and coworkers (1996) did not find that there was significant necrosis in the prostate. Similarly, D’Ancona and colleagues (1999), using the high-energy Prostasoft hardware (Larson and colleagues used the Targis T3), found that histologic parameters were moderately predictive of response; large prostates and prostates with a high epithelium to stroma ratio responded better to high-energy TUMT.
With the use of color Doppler ultrasonography, blood flow changes were estimated during microwave treatment in 2 patients with BPH (Larson and Collins, 1995b). At rest, a comparatively low level of blood flow was seen. As heat energy was delivered, the blood flow rose in a marked and sustained manner. This increase was seen in the posterior half of the prostate, including the peripheral zone and the posterior half of the transition zone. Marked recruitment of posterior and periurethral vessels was noted. It was also believed that peripheral resistance within the prostatic vessels was reduced. Therefore these authors in a series of studies linked temperature changes to histologic and blood flow findings. However, the blood flow alterations were performed in only 2 patients, and experimental confirmation with more accurate methods of blood flow estimation is required.
In another study, Larson and coworkers (1998a) showed that in terms of heating patterns, the Targis antenna (operating at a frequency of 902 to 1928 MHz) gave a more efficient delivery of thermal energy than the Prostatron antenna (operating at a frequency of 1296 MHz).
Sympathetic Nerve Degeneration
In a histologic study, 10 patients underwent TUMT and some days later had an open prostatectomy (Perachino et al, 1993). Multiple prostatic samples were taken for examination from the periurethral prostatic tissue to a depth of 2.5 cm. The samples were stained with hematoxylin and eosin and with S-100, neuron-specific enolase, and vimentin. The authors found microabscesses, epithelial necrosis, and vasculitis in the prostate. Compared with controls, it was found that nerve fibers were disrupted, with axons rarely being seen. This study suggested that thermal damage to the adrenergic fibers was behind the improvement in symptoms after TUMT. The authors did not attempt to quantify their findings. They also believed that the variable response sometimes noted was due to the variable numbers of nerve endings seen in different parts of the prostate.
Support for this theory came from an investigation into human prostatic α1-adrenergic receptor density after TUMT. Radioligand-binding assays using 3H-prazosin were performed on prostatic tissue from 25 patients, 10 of whom had received TUMT. Binding was saturable, and a single class of high-affinity binding sites was identified in all cases. In the controls (untreated by TUMT), the mean α1-adrenergic receptor density was 96.4 fmol/mg compared with 71.3 fmol/mg in those who had undergone TUMT, a statistically significant difference. The mean dissociation constants were 0.56 in both groups (Bdesha et al, 1996). These results suggested that there was a significant reduction in prostatic α1-adrenergic receptor density in the region of the prostate that had been subjected to maximal heating.
Another study has been performed to test this hypothesis, but using a different methodology. Ten patients had TUMT 1 week before scheduled TURP. Biopsy samples were taken, 5 to 6 mm deep, and stained with hematoxylin and eosin and with anti–PGP 9.5, which is a nonspecific neural marker gene product that is immunoreactive to all neurons. With this method, nerve fibers were found in the urethral epithelial layer, lamina propria, and smooth muscle layer of controls. Almost all of the TUMT biopsy samples had nerve fibers in the epithelial layer and the lamina propria, but there were no nerve fibers in the smooth muscle layer in virtually all of the specimens (Brehmer et al, 2000).
Induction of Apoptosis
Seven patients, all with glands weighing more than 100 g, were selected for open prostatectomy, and all had TUMT with the ECP (Comair, Sweden) 915-MHz equipment 2 hours to 1 week before surgery. Specimens were stained with hematoxylin and eosin, and apoptosis was verified by the terminal deoxynucleotidyl nick-end labeling (TUNEL) technique in sections showing histologic changes suggestive of apoptosis, such as pyknotic nuclei and chromatin segregation. Necrotic areas were frequently seen in the prostate to a depth of 4 to 5 cm. Outside these necrotic areas, normal and apoptotic areas were interspersed, the latter confirmed by TUNEL. The author found that the area of tissue damage seen after TUMT was relatively small compared with the volume of the prostate (Brehmer, 1997). The heat was implicated as the cause of the apoptosis, but there was no speculation about the exact mechanism whereby it brought this about.
In another study, Brehmer and Svensson (2000) performed culture of prostatic stromal cells from patients undergoing TURP. These were then stained for several cytoskeletal proteins and assessed for apoptosis by light microscopy of cells stained with the Giemsa nuclear stain, by transmission electron microscopy, and by measurement of caspase-3–like activity, the latter being one of the main effects of apoptosis. The cell cultures were exposed to moderate hyperthermia (47° C). Twenty-four hours after heat exposure, 76% of the cells were apoptotic, with only 14% of the cells being necrotic. The caspase activity (indicating increased apoptosis) had increased to about sixfold by 24 hours after heat treatment. The application of moderate heat for a longer period was found to be the most effective way of inducing apoptosis. Higher temperatures for shorter times resulted in a greater degree of necrosis. It was believed that, in vivo, other factors might modify the apoptotic result, such as stromal-epithelial interactions or heat dissipation by increased blood flow.
Thus these studies have shown that there may be several factors involved in the mode of action of TUMT. High temperatures cause necrosis of prostatic cells, whereas lower temperatures for longer periods of application induce programmed cell death or apoptosis. It is possible that the greater prostatic blood flow that has been found during treatment may be an attempt to dissipate the heat and that this may modify the induction of apoptosis. There is also evidence that TUMT causes disruption of α1-adrenergic receptor nerves in the smooth muscle of the prostate, another possible cause of the beneficial effect of TUMT, but this effect depends on the number of nerve endings in the treated area of the prostate. A further study investigating the combination of these findings and assessing the relative contribution of many of them was reported by Bolmsjo and coworkers (1996).
Clinical Results
The literature on TUMT is characterized by a large number of open studies, much more than any other technology-based treatment for BPH. In addition, many of them are short-term studies; some of those that purport to be longer term have in fact only a small percentage of patients who have reached what might be called long-term follow-up. However, there are also many carefully performed comparative studies, both against sham treatment and against TURP. The lesson that can be learned from these studies about TUMT is that machines delivering higher power yield better results than those delivering lower power. At the same time it might be said that this use of higher energy makes the procedure more complicated in that there is a greater requirement for sedation or analgesia.
Two of the earliest reports investigated, in a small number of patients, the use of the lower energy Prostatron device. In the first of these, 37 patients were treated and at 3 months had an improvement in Boyarsky symptom score from 12 to 8 and an increase in the PFR from 8.4 to 10.8 mL/sec (Devonec et al, 1991). These results were statistically significant if perhaps not quite so clinically impressive. In another study, 19 patients with LUTS who were not in retention were treated (Carter et al, 1991), but, at 12 weeks, only 9 of these patients were evaluable. The mean symptom score had improved from 12.0 to 2.8 and the mean PFR from 8.2 to 14.3 mL/sec. However, this study could be criticized for the fact that fewer than half of the patients were evaluable at 12 weeks and that the standard deviations of the mean PFRs were very large. The results were elegantly presented in terms of histopathology and technique and spurred many urologists to assess the TUMT in their own departments.
At this stage in the development of the technique the concept was specifically that heat treatment even with lower energy caused necrosis of prostatic tissue. The other ideas about sympathetic nerve damage and apoptosis were introduced later.
Open Studies
In an attempt to find medium- to long-term efficacy results, only studies with a follow-up of 1 year or greater are reviewed. A study performed with the Prostatron 2.0 software (Blute et al, 1993) evaluated 150 patients; 44% of these required oral or intravenous sedation. There were 150 patients, and results were presented from 118 who had Madsen symptom scores before treatment and at 12 months and from 104 who had PFRs. The American Urological Association (AUA) Symptom Index (AUA-7 or AUASI, equivalent to the IPSS) score improved from a mean of 13.7 to 5.4 and the PFR improved from 8.5 to 11.3 mL/sec. A total of 43 patients (36%) required catheterization for urinary retention; 63% required catheterization for 1 week or less, but it was necessary for more than 30 days in 4 patients. In another study with the same device, 30 patients were treated and 20 of these were evaluable at 1 year. The mean symptom score improvement was from 16.5 to 6.9, and the PFR improved from 7.2 to 10.7 mL/sec (Homma and Aso, 1993). The study reported in 1996 by Baba and coworkers entered 135 patients into treatment with the same device, and although they refer to “durability of response,” it can be seen that less than 20% of the total are followed to 2 years and less than 50% for 12 months. They noted that by 2 years 61 patients had been lost to follow-up. The same 3 mL/sec improvement as just mentioned was noted in those evaluable at 1 year.
A 4-year open study observed 187 patients treated with the Prostatron software 2.0 (Hallin and Berlin, 1998). At the end of the 4-year period, 56 were evaluable, although only 9 were truly lost to follow-up because the fate of all the rest was known. A total of 97 patients had needed further treatment for their LUTS. The satisfaction rate was 62% at 1 year, 34% at 2 years, and 23% at 4 years, with 66% having required further treatment and 11% being dissatisfied at the same time point. The authors believed that the patients who would respond best to this treatment were those with a PFR of 10 mL/sec or more or those with an initial irritative score in the lower range. The fact that many patients do not respond to TUMT treatment but that some do for long periods of time was commented on. The patterns of treatment after the introduction of TUMT in Sweden were reported on by Blomqvist and coworkers (1997), who found that the number of TUMT procedures performed in Sweden increased rapidly after the technique was introduced but then decreased equally rapidly. Three years after the introduction of drugs for BPH, the number of men receiving drug treatment was greater than the number of men having both TURP and TUMT annually.
Pressure-flow analysis has also been performed on patients being treated by the lower-energy TUMT (De la Rosette et al, 1995; Tubaro et al, 1995). These authors found that there was no noticeable change in the Pdet at PFR at 6 months but that there was a marginally greater improvement (although not statistically better) in those with constrictive obstruction as opposed to those with compressive obstruction. Finally, a series of patients being treated with the Prostatron 2.0 were observed to 5 years (Keijzers et al, 1998). Of the 231 patients treated, only 89 patients reached 5 years without requiring further treatment for BPH. The total number of patients treated in six European centers was 1092. Overall, the improvement in PFR was between 2 and 3 mL/sec and the re-treatment rate relatively high (Francisca et al, 1999).
It was thought that by increasing the energy the results achieved with TUMT could be improved. Of 85 patients treated with the Prostasoft 2.5 protocol, 74 were evaluable at 1 year (De Wildt et al, 1996a). The mean Madsen symptom score improved from 13.9 to 5.8, and the mean PFR increased from 9.4 to 14.9 mL/sec. The Pdet at PFR improved from a mean of 63.6 to a mean of 38.9 cm H2O at 26 weeks. A further study of 116 patients using the same protocol was reported by a European group (De la Rosette et al, 1996), with 67 patients having reached 52 weeks of observation. The Madsen score improved from 13.6 to 4.9 and the PFR from 9.6 to 14.5 mL/sec. More recently, the Prostasoft 3.5 protocol has been introduced and has been found to be well tolerated (Francisca et al, 2000b). Early results of its use in a small number of patients in urinary retention have indicated some value (Floratos et al, 2000).
A large open study was performed into the use of the 30-minute algorithm for high-energy TUMT with the Prostasoft 3.5 protocol (De La Rosette et al, 2000). Of 108 men treated, 86 were evaluable at 6 months (with only 41 at 1 year). At 6 months, the IPSS had improved from 20.0 to 9.3 and the PFR improved from 9.5 to 14.6 mL/sec. The Pdet at PFR decreased from 58.7 cm H2O at baseline to 46.9 cm H2O at 6 months. As reflected on the Abrams-Griffiths nomogram, this represented a shift from the obstructed to the equivocal zone for most patients. The mean duration of catheterization after treatment was 17.9 days, with a range of 6 to 62 days. The authors commented that at 3 months all treatment-related complications had disappeared. It was noted that the faster treatment was an improvement on previous methods from the viewpoint of tolerability to patients but had the same subjective and objective improvement as the Prostasoft 2.5 protocol.
In a study using the high-energy T3 Urologix device it was found that at 1 year there was a 63% improvement in symptom score and a 64% improvement in PFR. Urodynamic analysis showed that there was a better improvement in patients with marginal obstruction rather than unequivocal obstruction (Javlé et al, 1996).
A full urodynamic evaluation of the Prostalund Compact Device was carried out by Alivizatos and coworkers (2005). In this study 38 patients were evaluated, 19 of whom had an indwelling catheter. The treatment lasted a mean of 43.1 minutes, and the maximum intraprostatic temperature achieved was 58.7° C; a mean of 18.4 ± 14.3 g of prostatic tissue was destroyed. All of the urodynamic parameters improved in the patients without a pretreatment catheter. The mean changes were IPSS from 21.5 to 6.5, PFR from 7.2 to 18.1 mL/sec, and Pdet at PFR from 87.5 to 48.4 cm H2O. In those who had a pretreatment catheter, the PFR at 3 months was 13.2 mL/sec. This is a small series, but the results are encouraging.
To complete the equipment types the Core Therm–monitored feedback TUMT was used in 102 patients, but only early results were presented, and, of note, the median catheter time was 8.5 days (David et al, 2004). The results of the Cooled Thermo Cath (Urologix) have been published in abstract form (Roehrborn et al, 2005), and again in a small relatively short-term study it is hard to draw too many conclusions, although at 6 months the symptom score improved from a mean of 21.0 to 8.3, with a less encouraging improvement in PFR, from 7.8 to 12.5 mL/sec.
In a prospective cohort study, 31 patients with acute urinary retention were treated with the Targis machine (Djavan et al, 1999a). By 4 weeks, 29 of the 31 were able to void, with a median time to spontaneous voiding being 3.0 weeks. The flow rate gradually improved to a maximum at 12 weeks. The authors suggested that this type of treatment is helpful in patients who present with acute urinary retention but are unsuitable for surgical treatment.
A further study attempted to determine the efficacy of high-energy TUMT in treating patients with medically refractory urinary retention secondary to BPH (Kellner et al, 2004). This was a relatively small series of 39 patients, all of whom received treatment. The mean prostate volume was quite large (75.2 mL), but the standard deviation was 57.6. The treatment was unsuccessful in 18%. Of the 32 patients (82%) in whom success was achieved there was a mean of 1.6 voiding trials, and these patients required a catheter for a mean period of 4.1 weeks after treatment. Only 6 patients (15%) were able to stop their medication for BPH. The follow-up for this study was relatively short, 18 ± 10.2 months.
Berger and colleagues (2003) evaluated the use of TUMT in a similar but larger group of patients. A total of 78 patients in poor general health presented with acute urinary retention secondary to BPH with a mean prostatic volume of 53.9 mL. The mean follow-up was 34 months (range 6 to 42 months), with 38.5% lost to follow-up (30 patients), but early results in these were evaluable. Three months after the procedure, 68 (87.1%) were able to urinate spontaneously. Of the 68, 5 (7.3%) developed a further episode of retention within 2 years. The catheter was in place for a mean of 21 days, with 6 patients requiring prolonged catheterization. It can be seen therefore that treating patients in acute urinary retention with TUMT is a possibility, but the requirement for maintaining medical therapy is a cost burden that may limit its acceptability.
Long-term results are becoming available. Trock and colleagues (2004) reported the long-term pooled analysis of results using the Targis cooled thermotherapy system. Although the article is entitled results of 3 months to 4 years, only 171 of 540 patients had symptoms evaluable at 4 years and only 67 of 524 had a PFR result. In fact, there is no parallel between the number of patients who had a symptom score result or a PFR result at any given time point with a significant dropoff in the number of flow rate results occurring at 2 years. The 4-year results must be viewed with some caution; the mean symptom score fell from 20.91 to 11.54, and the mean PFR rose from 7.91 to 10.94 mL/sec. It was not possible to conclude how many patients dropped out of the study because of lack of efficacy or how many required alternative treatment. In another study using the same technology Miller and colleagues (2003) reported on 5-year data. Of note was that they previously reported on their 1-year and 3-year results in separate publications, but even in this report only 132 of 150 were evaluable at 1 year, 90 of 150 at 3 years, and 59 of 150 at 5 years. The results are similar to those of the multicenter trial mentioned earlier. Other long-term results have been published by Ohigashi and colleagues (2002), showing that in their experience using the Prostatron the durability of successful results was limited.
In another 2-year study (Thalmann et al, 2002), 200 patients with urodynamically proven outflow obstruction were found to have derived benefit from TUMT. However, as observed clearly by the authors, 13 (6.5%) were lost to follow-up, 15 (7.5%) had died, and 43 (21.5%) required a second or third treatment (TUMT or TURP). Of the 10 patients who required a repeated TUMT, only 4 voided successfully without obstruction and without requiring another intervention.
TUMT versus Sham Procedure
A short-term, 3-month study compared TUMT (21 patients) with the Prostasoft 2.0 to a sham procedure (19 patients) (Ogden et al, 1993). The mean Madsen score changed from 14.2 to 12.8 in the sham group and 14.5 to 4.3 in the TUMT group. The PFR changed from 8.6 to 9.2 mL/sec in the sham group and from 8.5 to 13.0 mL/sec in the TUMT group. A further study was performed in which 50 patients were randomly assigned to TUMT or sham treatment (De la Rosette et al, 1994). Twenty-four of the 25 patients in each group were evaluable at 12 weeks. In the sham group, the mean Madsen score had changed from 12.1 to 8.2 and in the TUMT group it changed from 13.2 to 5.9. The PFR had changed from 9.7 to 9.5 mL/sec in the sham group and from 9.6 to 13.0 mL/sec in the TUMT group. In a U.S. study, 110 patients were randomly assigned to sham treatment (35 patients) or TUMT (75 patients) (Blute et al, 1996). At 3 months the sham-treated patients had changed their symptom score from 14.9 to 10.8 and the TUMT patients’ scores had changed from 13.9 to 6.3. The PFR had changed in the sham group from 7.4 to 9.4 mL/sec and in the TUMT group from 7.2 to 11.5 mL/sec.
A randomized controlled trial compared TUMT with the Prostatron 2.0 to a sham procedure (Nawrocki et al, 1997). There were three groups: group 1 received standard TUMT; group 2, a sham treatment; and group 3, no treatment. Patients were observed for 6 months after treatment. In group 1, the AUASI score improved from 19 to 9.5, in group 2 from 17.5 to 9.5, and in group 3 from 18 to 17. The PFR changed from 8.83 to 9.94 mL/sec in group 1, from 9.44 to 9.49 in group 2, and from 8.79 to 8.47 mL/sec in group 3. That is, there was no difference in the degree of improvement in PFR whether the patient had the machine switched on or off or had no treatment. However, the symptomatic improvement was greater than with no treatment, but once again it did not matter whether the machine was on or off.
In a further study, using the Dornier microwave, 147 patients received TUMT and 73 received a sham procedure (Roehrborn et al, 1998). The AUASI score changed from 23.9 to 18.0 in the sham group and from 23.6 to 12.7 in the TUMT group. PFR changed from 8.1 to 9.8 mL/sec in the sham group and from 7.7 to 10.7 mL/sec in the TUMT group, all observations being made at 6 months. Using the T3 Targis, 125 were treated with TUMT and 44 with a sham procedure and results reviewed at 6 months (Larson et al, 1998b). The sham group showed a symptom score improvement from 21.3 to 14.3 and the TUMT group improved from 20.8 to 10.5. The PFR increased from 7.8 to 9.8 mL/sec in the sham group and to 11.8 mL/sec in the TUMT group.
A further sham-controlled study was performed using the ECP system (Brehmer et al, 1999). In this study, patients’ results were reviewed at 1 year, and a PFR improvement of 3.6 mL/sec was noted in the TUMT group, with a change of 0.4 mL/sec in the sham group. The changes in symptom score were similar in that there was a relatively smaller improvement in the sham group.
Finally, it is appropriate to include the results of a study in which α-adrenergic blockers were given either adjuvantly or neoadjuvantly in addition to TUMT with the Targis machine. It was found that the use of this treatment improved the early results achieved in the treatment of LUTS (Djavan et al, 1999b).
Sham-controlled studies are helpful in assessing whether the improvement noted after a specific treatment is merely a placebo effect. They are sometimes difficult to justify in that they involve a nontreatment arm, but several have been performed in the treatment of LUTS. In one, results were reviewed at 3 months; and those patients who had had a sham treatment and were not improved were given definitive TUMT (De Wildt et al, 1996b). Virtually all of the sham-controlled studies showed a greater improvement in the TUMT group except one (Nawrocki et al, 1997), in which the results suggested a placebo improvement only. In a further study, in which TUMT was compared with urethral catheterization the changes in symptom score and PFR were no different at 3 months (Mulvin et al, 1994).
TUMT versus TURP
Comparative studies between TUMT and TURP can help to define the exact place TUMT occupies in the management of BPH. For example, the weight of evidence was that it had a therapeutic benefit when compared with sham treatment or when viewed singly. However, it was unlikely that it would compete realistically with TURP in terms of symptomatic or flow rate improvement, and a more valuable approach might have been to concede the battle in terms of efficacy and concentrate almost entirely on post-treatment morbidity.
The earliest comparative trial against TURP randomly assigned 39 patients to TUMT and 40 patients to TURP (Dahlstrand et al, 1993). One-year follow-up was possible in 25 and 22 patients, respectively. The mean Madsen symptom score improved from 11.2 to 2.7 in the TUMT group and from 13.3 to 0.9 in the TURP group. The PFR improved from 8.0 to 12.3 mL/sec in the TUMT group and from 7.9 to 17.7 mL/sec in the TURP group. Both of these differences are statistically significant in favor of TURP-treated patients. Retrograde ejaculation occurred in 25% of the sexually active patients treated by TURP and in none of those treated by TUMT. In addition, the urethral stricture rate was 7.5% in the TURP group but zero in the TUMT group. The authors concluded that the improvements were not as great as with TURP but that the complication rate was much lower, although a detailed evaluation of all the complications would have been interesting.
In a follow-up study the results achieved by these treatments in 69 patients were evaluated at 2 years (Dahlstrand et al, 1997). There were 31 patients treated by TUMT and 30 treated by TURP who had reached 2 years of follow-up. In the TUMT group the Madsen-Iversen score had improved from 12.1 to 2.3 and in the TURP group from 13.6 to 1.2. The PFR increased from 8.6 to 12.3 mL/sec in the TUMT group and from 8.6 to 17.6 mL/sec in the TURP group. The authors carried out pressure-flow studies 6 months after treatment and found that the decrease in infravesical outflow resistance was greater after TURP than after TUMT. However, they also found that when the obstruction was calculated in terms of urethral resistance there was a statistically significant decrease in both groups. This is in keeping with other observations on pressure-flow and urethral resistance changes after TUMT, when previous studies had suggested that TUMT induced a change from constrictive to compressive obstruction, with an increased elasticity in the prostatic urethra (Höfner et al, 1993). The long-term complications were not discussed.
The higher-energy Prostatron 2.5 was also evaluated against TURP in a 6-month study (Ahmed et al, 1997). After TUMT the AUASI score changed from 18.5 to 5.3, and, after TURP, it changed from 18.4 to 5.2. The PFR actually worsened slightly from 10.1 to 9.1 mL/sec after TUMT and improved from 9.5 to 14.6 mL/sec after TURP. Pressure-flow studies showed a change from 98.5 to 105.6 cm H2O in the TUMT group and from 96.7 to 48.8 mL/sec in the TURP group. Obviously, in this study not only did TUMT not relieve obstruction but it also was associated with a marginal worsening of flow rate. However, in spite of this, the improvement in symptom score was exactly comparable to the symptom score of TURP at 6 months. Complications were higher in the TURP group, but prolonged catheter time (either clean intermittent or indwelling) was more marked in the TUMT group.
A further comparative study was performed using the Prostatron 2.5 device and comparing results with those of TURP in a prospective randomized trial (D’Ancona et al, 1997). A total of 31 patients underwent TUMT and 21 underwent TURP, of whom 26 and 18, respectively, were evaluable at 12 months. The Madsen symptom score improved from 13.3 to 4.2 in the TUMT group and from 13.8 to 2.8 in the TURP group. The PFR improved from 10.0 to 16.9 mL/sec in the TUMT group and from 9.3 to 18.6 mL/sec in the TURP group. Pressure-flow studies showed that whereas 62% of the TUMT group and 76% of the TURP group were obstructed before their respective treatments, at 6 months 40% and 15%, respectively, were still considered to be obstructed. All of the TUMT procedures were done as day cases, and the average number of days in hospital for patients having TURP was 4.1. The length of catheterization was 4.1 days for TURP with a range of 4 to 5 days and was 12.7 days for TUMT with a range of 6 to 35 days. The incidence of urinary tract infections was 4% and 16%, respectively. Irritative voiding symptoms were present in 19% of those who underwent TURP and 29% of those who were treated by TUMT.
Later evaluation of these results took place at a mean of 2.4 years, although 17 of 31 patients who had TUMT and 12 of 21 who had TURP were all who remained in the study. Eight of the original 31 TUMT patients and 1 of the 21 who had TURP required alternative treatment. The Madsen score improved from 13.3 to 5.8 in the TUMT group and from 13.8 to 3.6 in the TURP group. The PFR increased from 9.3 to 15.1 mL/sec in the TUMT group and from 9.3 to 19.1 mL/sec in the TURP group (D’Ancona et al, 1998).
A full quality of life study was performed on 147 patients randomly assigned to either TUMT with the Prostasoft 2.5 protocol or TURP. It was found that both treatments had a positive effect on various aspects of quality of life. The areas of perception of urinary difficulties and daily activities particularly were improved. However, TURP caused a greater improvement in both quality of life and clinical outcome, with neither treatment influencing sexual function (Francisca et al, 2000a).
Overall, the main complication, particularly with the higher energy TUMT, was prolonged catheterization and, with it, urinary infection. It would seem that sexual side effects such as erectile dysfunction and retrograde ejaculation were significantly less common than after TURP.
There have been two systematic reviews of the literature, which give level I evidence concerning the value of this technique. It must be remembered that these reviews are only as good as they are allowed to be by the data put into them. One of the issues characterizing the entire technology in BPH literature is the large number of poorly constructed trials that have been performed.
The first of these systematic reviews came from an Australian group attached to the Royal Australian College of Surgeons (Wheelahan et al, 2000) and covers trials performed up to 1999. It found that TUMT offered less morbidity than TURP but was not as effective as TURP in improving symptoms or reducing BOO. TUMT was associated with a lengthy period of catheterization after treatment, thus causing the higher rates of urinary tract infection and urinary retention. The durability of TUMT, which showed that 23% of patients remained satisfied with a single such treatment after 4 years, was not particularly convincing. The authors concluded that it was not possible to determine the safety and efficacy of TUMT because of the poor-quality evidence base.
The second systematic review came from the United States and included all the trials from the previous review up to 1999 but added four other papers up to 2002 (Hoffman et al, 2004). The authors were specific in including only randomized trials of TUMT and TURP and found that this meant only 6 of 26 possible trials could be reviewed, with 540 participants (322 TUMT, 218 TURP). Without being directly critical of the standard of these trials, the authors outlined clearly the shortcomings of each of them, showing a lack of uniformity that makes it difficult to draw definite conclusions. The pooled mean PFR increased from 7.9 to 13.5 mL/sec after TUMT and from 8.6 to 18.7 mL/sec after TURP. Only two studies reported a mean PFR after TUMT that had reached 15 mL/sec or greater. The authors were unable to find statistically significant differences between low-energy and high-energy systems. Only four studies provided a follow-up of 12 months or greater with 85% to 93% of patients available for follow-up. There was an absence of comprehensive reporting of adverse perioperative events in most of the trials, but the findings of previous systematic reviews in this regard appeared to remain the same. The very definite conclusion once again was that TURP produced greater improvement in symptom scores and PFR and that fewer men required re-treatment for BPH than with TUMT. The recommendation was that further research was required to evaluate the long-term efficacy and safety of TUMT.
Summary
TUMT is not as effective as TURP in improving the objective signs of outflow obstruction, in terms of either PFR or Pdet at PFR. However, it does seem to have a measurable effect on the prostate because the elasticity of the urethra is increased after treatment.
The symptomatic improvement that occurs after TUMT seems to be energy related. However, the surprising finding is that even when no objective signs of improvement are seen in comparative trials, the improvement in symptom score caused by TUMT can be considerable. Whether this is a placebo effect or not is unclear because in the two trials in which it occurred the results are given only from the 6-month time point, at which the placebo effect would be at its maximum. Long-term results from these two trials are needed.
Complications are less than with TURP, with prolonged catheterization and infection being the most common. However, the fact that the procedure can be performed as a day case with the patient under mild sedation is an important positive side of the procedure. The success associated with this treatment cannot be guaranteed in every patient, as can be seen from the relatively high number of nonresponders in many series. Why this should be is unclear, but several possible explanations have been given and were outlined earlier.
It would seem that the higher-energy devices will be used preferentially to those producing lower energy, but long-term studies, particularly multicenter studies, would be particularly helpful in being able to assess them fully. Because it is clear that TUMT does not outperform TURP, it is to be hoped that trials will be aimed specifically at assessing the exact place of TUMT in the treatment of symptomatic BPH and that the standard of trials will improve.
Lasers
The introduction of the laser as a treatment option for BPH was greeted by urologists and patients with a high degree of expectancy. However, as with other types of minimally invasive therapy, an initial excess of optimism gave way to realism, with the development of a clearer view of the indications for this type of treatment and, indeed, of its limitations. Newer modifications have taken place, allowing even an endoscopic “enucleation” to be performed. When the original laser techniques were introduced, it was hard to predict this being possible, and so it is reasonable to suggest that with technologic advances happening at an increasingly fast rate the laser may well play a greater role in the management of BPH in the future. However, although TURP is now recognized as the gold standard, it was not always the case; in fact, it took TURP several decades to achieve this exalted status. It may be that the absence of randomized clinical trials delayed the ultimate recognition of TURP as the treatment against which all others must be compared. For this reason, large multicenter randomized clinical trials comparing the laser with other modalities are needed for this technology to achieve widespread acceptance; these must be adequately statistically powered and have a sufficiently long follow-up time. In addition, it would probably be a more satisfactory approach if the authors of these trials tried to find the exact role of the laser in the treatment of BPH rather than suggesting that it is a method that will supplant TURP. It is likely that both methods of treatment will have a role to play, but that of the laser still needs to be accurately defined (Fitzpatrick, 1998, 2000).
The volume of papers in the literature on lasers has increased beyond all expectations in the past decade. This reflects a continuing interest in the technology and a belief on the part of urologists that the laser will be the way that symptomatic BPH should be treated. Many of the older technologies are now of no interest to urologists, but it is to be hoped that the somewhat uncritical approach that was taken toward the earlier lasers will not be taken toward the new favorites, the holmium laser and the photoselective KTP laser.
Types of Laser
As is well known, “laser” stands for light amplification by the stimulated emission of radiation. The mechanism of the operating principle of lasers is a process known as stimulated emission of radiation (Nau et al, 2000). In the laser, a flashlamp gives out high-intensity light, which then bombards a resonator cavity with photons. These excite electrons in the resonator cavity to higher energy status. Most of the photons that come from the flashlamp to the resonator cavity are wasted in the form of heat, with less than 5% being absorbed. For this reason, lasers used in the treatment of BPH require rather elaborate cooling devices.
The electrons in the resonator cavity, which are excited by the bombardment of photons, are caused to jump to higher or “excited state” orbitals. Because of the instability of these excited state orbitals there is a very rapid decay of the electrons, which emit a photon. This process is known as spontaneous emission of radiation. However, the emitted photon has the energy required to interact with other excited state atoms. If this interaction happens, further electron orbital decay and photon emission are induced. This photon has the same characteristics and travels in the same direction as the incident photon. This is known as stimulated emission of radiation and, as stated earlier, is the operating principle of lasers. These photons leave the resonator cavity as a coherent laser beam.
There are four types of laser that can be used to treat the prostate.
Neodymium : Yttrium-Aluminum-Garnet Laser
The neodymium : yttrium-aluminum-garnet (Nd : YAG) laser emits light at a wavelength of 1064 nm, and its active medium consists of neodymium atoms in an yttrium-aluminum-garnet rod. Because this light is poorly absorbed by water and body pigments it can penetrate tissues relatively deeply. This poor absorption in a fluid medium causes thermal coagulation of the surface tissue and of areas just under the surface. The tissue that has been coagulated becomes white, and hemostasis is total. Subsequently, the coagulated tissue sloughs, and this may occur over a period of some weeks. It may take up to 3 months to achieve complete healing.
One of the more recent modifications of laser application is the process of tissue vaporization and desiccation, and this can be achieved with the Nd : YAG laser. This technique requires a higher energy density for a longer period of time, and surface carbonization occurs, leading to increased laser light absorption superficially and a limitation of the depth of penetration, further increasing the energy density.
Potassium-Titanyl-Phosphate Laser
The potassium-titanyl-phosphate (KTP) laser uses a KTP crystal to double the frequency of an Nd : YAG laser and produces a 532-nm wavelength. This provides an intermediate level of coagulation and vaporization. Only half the depth of tissue penetration is reached compared with that of the Nd : YAG laser. However, the consequent higher energy per unit tissue volume produced may increase tissue vaporization and desiccation. This may be used as an advantage in that the prostate and bladder neck may be incised with the KTP laser.
Holmium : Yttrium-Aluminum-Garnet Laser
The holmium : yttrium-aluminum-garnet (Ho : YAG) laser emits light at a frequency of 2100 nm. The energy is emitted in a series of rapid pulses over a few milliseconds, the Q-switched laser. This is unlike the continuous wave of the Nd : YAG or KTP lasers. Whereas the same type of standard optical fiber can be used for the Nd : YAG or the KTP laser, a different flexible optical fiber is required for the Ho : YAG laser. Because it produces a cutting effect by vaporization of the tissue water, its hemostatic properties are less than those of the continuous wave lasers.
Diode Laser
With conventional lasers, less than 5% of the electrical input is converted into laser light, and this inefficiency means that conventional lasers require high-energy cooling devices and radiators, thus increasing the size of the machine. The high gain of the diode laser allows the more efficient use of the photons that are generated. Thus the available diode lasers used for medical purposes are small and portable, and special connections are not required.
Methods of Delivery
The energy from lasers can be delivered as follows:
Energy levels can be varied, depending on the type of laser, to allow coagulation or evaporation to occur, as alluded to earlier. As with any form of heat treatment, an increase in the temperature of the tissue being treated has different effects, and it is important to be able to control and localize this temperature rise. When the temperature is between 45° C and 50° C, desiccation of tissue occurs, and then, as the temperature increases from 50° C to 100° C, coagulation begins and becomes irreversible. When the temperature exceeds 100° C, the tissue boils and there is resultant carbonization and vaporization.
Coagulation alone may make the procedure imprecise, with danger to neighboring tissue such as the distal sphincter mechanism. During the process of vaporization, tissue water is converted to steam and miniexplosions occur within tissues, increasing the mechanical rupture. The vaporization is determined by the intensity of laser application to tissue rather than its duration. Once the cells become coagulated, laser energy penetrates through them less well, halting the forward process of ablation and increasing backscatter and surrounding coagulation (Stein and Narayan, 2000).
Delivery Systems and Laser Techniques
The laser experience has shown that it is possible to adapt a technique and then evolve it almost completely into something new while still using the same basic form of energy. For example, the evolution from the transurethral ultrasound–guided laser-induced prostatectomy (TULIP) device (Intrasonic, Burlington, MA) to the currently used interstitial and contact devices has been relatively quick but demonstrates clearly the wish on the part of investigators to remove imperfections and to improve results. The aim to find a truly minimally invasive technique is encapsulated in this evolution.
There are two ways in which lasers can have an effect on the prostate, either by coagulation or by vaporization. In the first of these coagulation is achieved at temperatures of 70° C to 90° C. The Nd : YAG laser supplies a wide scatter of power over a relatively large surface area with deep penetration. The second way is by vaporization, which raises the temperature to several hundred degrees Celsius. The power density is high, delivered by a narrow beam. Vaporization can be achieved by using a laser tip that increases the absorption of power by the prostate. Therefore the factors determining whether coagulation or vaporization occurs are essentially the power density of the laser beam itself, the total energy delivered, and the time for which it is applied.
The TULIP device was one of the earliest laser systems and consisted of a characteristic balloon, which maintained the distance between the laser beam and the prostate. Also contained in the tip was a 7.5-MHz ultrasonic transducer and an Nd : YAG laser. The probe was moved from the bladder neck to the verumontanum and was then moved to another position, so that, in this longitudinal manner, the entire circumference of the prostate could be treated. The TULIP device has not withstood the test of time in spite of many improvements made to the original model. It is likely that the reason is that urologists prefer being able to visualize a technique directly rather than indirectly by ultrasonography.
Side-Firing Laser
Probably the first study of the side-firing laser was performed by Costello and colleagues (1992) using a canine model. The energy used was 60 W for a duration of 60 seconds in four quadrants of the prostate, which caused coagulative necrosis. The canine prostate is different from the human prostate, but it served as an indicator to how the procedure could be performed in humans. Considerable improvements have been made since that time in terms of the laser fibers, the formula for dosimetry, and the technique used.
A large volume of work has been performed on the side-firing laser systems, and this has up to now dominated the literature. The fiber bends the laser beam at various angles, and this is accomplished by reflection or refraction, depending on the fiber used.
Reflective systems use a gold-plated mirror or solid gold tip to deflect the beam. The angle of reflection varies from 45 to 105 degrees, with 90 degrees being the most common. There is a further divergence depending on the design of the reflector mechanism that also is variable. This is important because it determines the power density by establishing the size of the laser spot on the prostate. Reflective systems tend to absorb more energy than refractive systems. At present, this type of system is more suited to tissue coagulation. Refractive systems either are glass capped or use a quartz tip to deflect the beam. The angle of divergence is narrow, allowing either coagulation or vaporization.
The most popular method using the side-firing laser is that of coagulation. Some surface vaporization occurs, but most of the tissue is coagulated, followed later by sloughing, which lasts for some weeks. The power settings have been recommended as 40 to 60 W for 60 seconds or even 90 W for 60 seconds. This latter higher power may cause mainly vaporization. In this situation the lack of coagulative necrosis means an absence of tissue sloughing. A hybrid procedure has also been described that combines coagulation of the prostate with contact incision through the prostate.
The technique has also varied in description. One method is the use of a quadrant spot application, or even a sextant approach for larger glands, extending every 1 to 2 cm along the prostate from bladder neck to verumontanum (Kabalin, 1993; Norris et al, 1993; Kabalin et al, 1995). The beam is applied until the treated area becomes white. It is then moved onto a normally colored area, which is then treated until the entire surface of the prostate gland has been rendered white. After an initial increase in prostate size from edema, sloughing of the tissue takes place, with the tissue being passed urethrally.
Another technique has been described (Nau et al, 2000). The treatment begins proximal or distal to the bladder neck, dependent on whether retrograde ejaculation is a factor of importance. If the procedure is begun 1 cm distal to the bladder neck, it can reduce the incidence of this complication from up to 15% to zero, according to these authors. They scan the laser beam either longitudinally or radially. That is, from the beginning of their treatment in the region of the bladder neck, the fiber is scanned distally at a rate of 1 mm/sec to the level of the verumontanum; it is then returned to the starting point and rotated slightly so that the next line of tissue that is coagulated is immediately beside the previously treated line. This is continued until the entire prostate appears white.
Alternatively, a radial scanning technique can be used. With this method, the probe is rotated through 360 degrees at a speed of 1 mm/sec, starting in the region of the bladder neck. The fiber is then withdrawn 5 mm distally and the circumferential treatment continued. This is repeated at 5-mm distances until the level of the verumontanum is reached.
Contact Laser
Although the technique of the side-firing laser is well described and the results are well known, a number of possible criticisms have led to further evolution of the laser technique. For example, there is difficulty in controlling the distribution of energy and predicting the eventual result; the side effects of post-treatment retention and urethral discomfort with irritable bladder symptoms have also deterred many urologists from using it. These criticisms have led to the evolution of other methods, one of which is the use of the contact laser, which removes some tissue immediately, thereby decreasing post-treatment voiding difficulties (McNicholas and Singh, 2000).
Contact laser tips are made of synthetic sapphire and can be made in different shapes, either conventionally round or in the shape of a wedge. Initially, the size of the tips was 1.5 mm (Watson et al, 1994). The success of these led to the introduction of tips of 5 mm (Bartsch et al, 1994) and most recently to tips of 10 mm (McNicholas and Hines, 2000). A removable side-firing contact probe has been introduced. This allows the surgeon to remove the contact tip and replace it with a side-firing free-beam device.
Because the size of the contact tip exceeds the size of the instrument channel of the cystoscope, it is usual to advance the fiber until it protrudes through the end of the instrument and then to attach the contact tip. It is then advanced along the length of the urethra. Special instruments to facilitate the passage of this type of laser have been designed (Press and Smith, 1995).
Once in position, the contact tip should be placed just above the verumontanum and 20 W power applied at this level. After allowing this power to raise the temperature at the treatment position and visually obvious tissue contraction to occur, the tip is advanced proximally in the curve of the prostate to the bladder neck, taking care not to undermine it. This procedure is then repeated, from verumontanum to bladder neck, by rotating the instrument so that a further channel can be cut adjacent to the previous one. At the end of the procedure, a bladder neck incision can be performed if required (McNicholas and Hines, 2000).
Interstitial Laser
The purpose of this method of applying laser energy is to reduce post-treatment voiding difficulty and urethral obstruction because the integrity of the prostatic urethra can be preserved. A small fiber is introduced into the prostate, usually transurethrally, and energy from either the Nd : YAG or the diode laser at low power heats the prostate and induces coagulative necrosis. The necrotic tissue is removed by the process of tissue repair and is not sloughed off and passed urethrally as with other methods.
The laser energy is applied to the prostate by one of three possible fibers: (1) a bare fiber, (2) a bare fiber within a cannula with saline solution irrigation, and (3) a fiber with a distal diffusor tip.
In a study by McNicholas and colleagues (1993) it was shown that the size of the intraprostatic lesion is dependent on the power applied, the duration of treatment, and the vascularity of the tissue. In addition, the ratio of fibrovascular to epithelial tissue is important. The aim of interstitial treatment is to create spherical lesions of 1 to 2 cm diameter within the prostate. The prostate should be heated slowly to avoid carbonization, which limits the effect of the treatment and prevents further coagulative necrosis.
Two types of laser generators are used for interstitial laser therapy. The Dornier ITT system (Dornier, Germering, Germany, and Kennesaw, GA) uses the Nd : YAG laser to generate 1064-nm energy with a power range of 2 to 100 W. The other generator is the Indigo LASEROPTIC system (Indigo Medical, Johnson & Johnson, Cincinnati, OH). This generator is portable, weighing about one eighth as much as the Dornier system. It is a diode laser generator, which uses a diode pump source and gallium-aluminum-arsenide to generate a 830-nm wavelength with a power range of 2 to 20 W.
The laser fiber is inserted through a standard cystoscope and is passed transurethrally into the prostatic adenoma. With the use of the Nd : YAG interstitial laser, it is left in each position for 10 minutes at 5 to 7 W. The diode laser requires a 3-minute treatment session at each location. The length of the procedure depends on the size of the gland and the type of laser system being used.
The position of the laser tip within the prostate can be visualized by the use of TRUS. It is possible that conventionally positioning the fiber under vision will place it too close to the prostatic urethra, risking urethral damage and edema with resultant post-treatment voiding problems. Issa and colleagues (1998) have modified this using the Indigo laser so that the fiber can enter the prostate at a greater angle, thus keeping it away from the prostatic urethra.
There is no definite agreement about how much tissue should be ablated, with some investigators using a lower number of treatment sites, paying particular attention to the position of the tip of the fiber. Others suggest a more aggressive approach, such as one lesion per 5 to 7 mm3 (Arai et al, 1996).
Prostatectomy with Holmium Laser
The vaporization techniques described earlier using the Nd : YAG laser with high-energy-density beams at high power (60 to 100 W) at a wavelength of 1064 nm have been effective in removing small amounts of tissue immediately during the procedure. However, it is a relatively inefficient way to do this because of the high power required. The Ho : YAG laser beam is absorbed by water (unlike the Nd : YAG beam) at a wavelength of 2140 nm and causes considerable tissue vaporization.
This technique was first evaluated in canine experiments in 1992 (Johnson et al, 1992) and subsequently with improved technology in 1996 by Kabalin. In the latter study the mean transverse diameter of the defect was almost 2 cm. A surrounding rim of 2 mm of tissue coagulation was also produced.
The methods of using the Ho : YAG laser have evolved because of several modifications in both technology and methodology (Webb et al, 1993; Dennstedt et al, 1995; Gilling et al, 1995, 1996, 1999). The technique has passed through simple vaporization to combined endoscopic laser ablation of the prostate (CELAP) and is now used to resect large pieces of prostatic tissue.
The dual-wavelength Versa Pulse Select laser (Coherent, Inc., Santa Clara, CA) was used, and a maximal power of 60 W in a pulsed mode was used to resect tissue. A 26-Fr continuous-flow resectoscope was modified, and an end-firing bare fiber was passed through it; its position was stabilized by passing it through a 6-Fr ureteral catheter. The median lobe was first resected by initial bilateral bladder neck incisions and then with a transverse incision just proximal to the verumontanum. The median lobe was then undermined to the bladder neck and detached back into the bladder. The lateral lobes were removed by making incisions at 1 and 5 o’clock and then at 7 and 11 o’clock and then beginning distally and undermining proximally by detaching them backward into the bladder. If the lobes were large, they could be cut into smaller pieces. They were retrieved from the bladder with a modified grasping forceps, or more recently using a morcellator, and by irrigation (Fig. 93–5).
Clinical Results
Part of the difficulty with trials using surgical instruments such as the laser is that a placebo-based randomized controlled trial cannot be performed. This effectively means that to test efficacy the laser must be compared against what is accepted as the best method to treat LUTS endoscopically, which is recognized as TURP. Unfortunately, and particularly with the laser (whose name implies modernity and success in the public’s mind), this does not exclude a pre-existing bias that might be present toward the laser. In addition, and this is similar to the other technologies for treating BPH, none of the comparative trials is as carefully statistically powered as those testing medical management of BPH. It might be said that a large number of the open studies are too large and that a comparative study should have been introduced at an earlier stage. However, there have been many trials, both open and comparative, and a large amount of relevant information is available.
Transurethral Ultrasound-Guided Laser-Induced Prostatectomy
TULIP has ceased to be used by urologists, but because it was the first to be introduced and is currently out of favor because it has been surpassed by instruments that are easier to use rather than because of its lack of efficacy, it is worth looking briefly at the results achieved with its use.
The initial results came from the TULIP National Human Cooperative Study Group (McCullough et al, 1993; McCullough, 1994). These showed that in a small subset observed for 6 months only (68 of 242 patients) there was a 78% improvement in PFR (6.7 to 11.8 mL/sec), although, as can be seen, the final mean PFR is still rather low, in spite of treatment. There was also a 68% improvement in symptom score. However, 4% of the patients underwent TURP, and irritative symptoms and urgency lasted for 2 to 3 months in a high percentage of patients. When patients were observed up to 1 year, it could be seen that the incidence of post-treatment retention was 22%, with a stricture rate of 5% and bladder neck contractures in 1%.
Schulze and coworkers (1995) carried out the procedure in 89 patients who were observed for a maximum of 1 year. The mean PFR increased from 7 to 15 mL/sec but was reported as exceeding 20 mL/sec in about half. The modified Boyarsky symptom score decreased from 17 to 5 at 1 year. Although it was efficacious in this group, the patients were chosen only as candidates for TURP rather than having strict inclusion and exclusion criteria.
Chatzopoulos and colleagues (1996) evaluated 38 patients with a maximal follow-up of 30 months after the TULIP procedure. The authors pointed out that the mean symptom score decreased by 54% and PFR increased by 112%. However, an improvement in symptom score was seen in 43.6% and in the PFR in 41%, but in only 28.2% was there an improvement in both. In addition, reoperation was required in 16% and the incidence of complications was considered high.
In a further study (Horninger et al, 1997) it was found that the efficacy of TULIP, contact laser, and interstitial laser was similar. However, because simpler machines were becoming available, interest waned in the TULIP and techniques with an improved side effect profile were sought.
Side-Firing Laser
The side-firing laser has several advantages over the TULIP technique, related to the ease with which it can be used. The fact that it can be passed through an endoscope and the effect that the procedure is having can be visualized (at least on the urethral surface) make it especially attractive to the urologist. When viewing the results achieved with this technology, the reader must remember that ultimately it should be compared with TURP in terms of efficacy and side effects, to say nothing of cost-effectiveness.
The initial study (Costello et al, 1992), looking at 6-week results in 17 patients, found an increase in the PFR from 5 to 9 mL/sec and an improvement in the Madsen-Iversen symptom score from 15 to 4. Costello and coworkers (1994) reported on 33 patients observed for 3 months. They showed that the PFR increased from 8.5 to 15.2 mL/sec and that the AUASI score improved from 21.5 to 9.5. That the short-term results were encouraging was also supported by a further study (Norris et al, 1993). One hundred eight patients were observed for a maximum of 6 months; the PFR increased from 7.6 to 12 mL/sec, and the AUASI score improved from 22.3 to 9.2. These studies were noncomparative and were performed essentially to find out whether the technique might be submitted to trials against TURP. It can be seen that there is a variation to the PFR improvement in the important series just cited.
However, the longer-term study of Kabalin (1996) suggested that the results could be maintained. In 227 patients the PFR was 7.3 mL/sec before treatment and the improvement to 15.2 mL/sec at 3 months was still attained through 1 year (17 mL/sec), 2 years (18.3 mL/sec), and 3 years (18.5 mL/sec). The AUASI score decreased from 20.3 to 10 at 3 months and to 5.7 in those who had been observed to 3 years. Therefore, although a symptom score of 10 after treatment might have appeared disappointing because it is still in the moderately symptomatic range, the fact that it had further decreased to 8 at 1 year and 8.6 at 2 years, with a further drop to 5.7 at 3 years, showed the benefit of long-term follow-up.
The efficacy of visual laser ablation of the prostate (VLAP) has been subjected to urodynamic analysis. James and colleagues (1995) reported on 79 patients waiting for TURP who were treated by VLAP as an alternative treatment. They had preoperative symptom scores and urodynamic evaluation, and these were repeated 3 months after treatment. The AUASI score decreased from 21.4 to 9.4, and the PFR increased from 6.5 to 10.6 mL/sec. The Pdet at PFR decreased from 74 to 54.2 cm H2O. In another carefully performed urodynamic study (Te Slaa et al, 1995), results were assessed preoperatively and at 6 months after the procedure. The IPSS decreased from 21.3 to 5.3, and the PFR increased from 7.9 to 17.8 mL/sec. The percentage of patients demonstrating urodynamically proven obstruction decreased from 80% before treatment to 5% at 6 months after treatment.
All of these were, of course, open studies, but they did lead to a number of comparative studies, in each case against TURP. In 60 patients observed for 6 months it was found that VLAP gave a significantly better result in terms of symptom score than TURP but that TURP gave a significantly greater improvement in PFR (Sengor et al, 1996). However, in a multicenter trial in the United States at six investigational sites, a longer follow-up took place (Cowles et al, 1995). In this study, 115 men who did not have urinary retention were randomly assigned to TURP (59 patients) or VLAP (56 patients); initially, all of these men were observed for 1 year after treatment. The authors showed that VLAP required less operating time (23.4 against 45.2 minutes) and a shorter hospitalization (1.8 against 3.1 days). The AUASI score improved by 13.3 in the TURP group and by 9.0 in the VLAP group, and the PFR improved by 7.0 mL/sec in the TURP group and by 5.3 mL/sec in the VLAP group. The IPSS improvement was statistically significantly better in the patients who had TURP. The authors noted a quality of life improvement in 93.0% of the patients who had TURP and in 78.2% of those who had VLAP.
A further comparative study showed 1-year results in 151 patients (Anson et al, 1995). In this trial, 75 patients were randomly assigned to TURP and 76 to VLAP. In 1 year, the AUASI score had improved from 18.2 to 5.1 in the TURP group and from 18.1 to 7.7 in the VLAP group; the PFR increased from 10.0 to 21.8 mL/sec (improvement of 11.8) in the TURP group and from 9.5 to 15.4 mL/sec (improvement of 5.9) in the VLAP group. These authors and those of the previously mentioned trials concluded on the evidence described that VLAP was a safe and viable or useful alternative therapy to TURP.
However, the last-mentioned study has been re-evaluated at 5 years (McAllister et al, 2000). Of the original 151 patients, 109 could be traced; of these, 69 patients had not required further treatment. In those in whom further treatment was not required and who had not been lost to follow-up, VLAP had produced results comparable with those of TURP. Unfortunately, because this was less than 50% of the original sample, it is difficult to be absolutely certain which patients respond better than others to VLAP. Of the original group treated, 26 patients had required further surgical treatment: 8 of 51 (16%) TURP patients and 18 of 47 (38%) VLAP patients. These authors then admitted that the figures did not support their original conclusion that VLAP could be offered to every patient with BPH.
A urodynamic study was performed on 90 patients having VLAP and 43 having TURP (Jung et al, 1996). This suggested that TURP eliminated obstruction in all patients but that the grade of obstruction was lowered to normal in those treated by VLAP having prostate sizes of 50 mL or less.
The complication rates vary from center to center. The figures related to blood loss and blood transfusion are absolutely clear. Neither the U.K. study (Anson et al, 1995) nor the U.S. study (Cowles et al, 1995) had any patient treated with VLAP requiring a blood transfusion. In fact, two studies have shown that VLAP can be performed on patients either on full anticoagulation therapy or having abnormal coagulation indices because of hematologic disorders (Costello and Crowe, 1994; Kingston et al, 1995). It is also certain that VLAP causes neither “TURP syndrome” nor any particular effect on serum sodium levels (Cummings et al, 1995).
The retrograde ejaculation rate varies from 27% to 33%, urethral stricture rate varies from 0% to 1.8%, and bladder neck contracture occurs in 4.4% (Anson et al, 1995; Cowles et al, 1995). The U.K. study showed a higher incidence of post-treatment dysuria (15% at 3 months) and bacteriuria (28 of 76 patients). The development of post-treatment urinary retention was 30.4% in the patients treated by VLAP by Cowles and colleagues (1995). This has led them and other authors to recommend catheterization after VLAP for 4 to 7 days.
The incidence of post-treatment retention has led some urologists to advocate a “hybrid” technique, in which a side-firing laser is first used to coagulate the adenoma and then a contact laser is introduced to perform bladder neck incisions, usually with a KTP : YAG laser for the latter procedure. Carter and coworkers (1999) found that this technique gave statistically similar improvements in both symptom score and PFRs at 1 year to TURP in a randomized study involving 204 patients. These results were still maintained when viewed at 18 months (Pearcy et al, 1999). In a further but smaller study of 45 patients, TURP was found to be more effective than the hybrid treatment in prostates larger than 40 mL; 38% of patients who had received the hybrid treatment were discharged with a suprapubic catheter, whereas all TURP patients were able to void at the time of discharge from hospital (Tuhkanen et al, 1999).
Contact Laser
In a study performed in the United States (Narayan et al, 1995), 100 patients were treated by transurethral evaporation of the prostate (TUEP) using the Nd : YAG laser and were stratified according to gland size: in 41 it was less than 40 mL, in 39 it was 41 to 80 mL, and in 20 it was greater than 80 mL. The mean improvement of PFR at 6 months was 9.9 mL/sec in the first group and 9.2 mL/sec in the third group; the mean improvement in AUASI score was 14.6 in the first group and 16.2 in the third group at the same time. The authors believe that the efficacy of the treatment was independent of the size of the prostate gland and also showed that it was effective in patients who presented in acute urinary retention. In this and in another study (Fournier et al, 1996), the length of time that patients required indwelling catheters after treatment was 2.9 to 4.7 days.
In a urodynamic study comparing contact laser vaporization of the prostate with TURP, patients with prostate glands smaller than 40 mL were evaluated before treatment and at 6 months. There was a similar statistical improvement in both symptom scores and PFRs in both groups. The authors also found that both treatments were equally effective in relieving obstruction; the mean Pdet at PFR was 38.3 cm H2O in the contact laser vaporization group and 31.3 cm H2O in the TURP group (Tuhkanen et al, 1999).
The Oxford Laser Prostate Trial was first reported on by Keoghane and colleagues in 1996. A total of 148 patients were randomly assigned to contact laser (72 patients) or to TURP (76 patients). At 3 months the AUASI score had improved by 7.3 in the laser arm and by 11.9 in the TURP arm, a statistically significant difference in favor of TURP. However, the authors also found that at 3 months there was an equal distribution in bothersome score in both groups. They also found an equal effect on loss of sexual function in both groups (Keoghane et al, 1996). At 3 years the improvement in the mean symptom score was 11.0 for the laser patients and 12.0 for the TURP patients. These improvements were virtually the same as had been noticed at 1 year, indicating that patients had a symptom improvement roughly equal to that of TURP and equally maintained for 3 years. The improvement in mean PFR in the two groups showed a different trend: at 1 year it was 9.4 for TURP and 6.2 for laser, at 2 years it was 4.9 for TURP and 5.2 for laser, and at 3 years it was 2.1 for TURP and 1.8 for laser. That is, the pre-laser PFR was 11.8 mL/sec and at 3 years this was 13.4, having been 17.1 at 1 year. The same downward trend was noticed in PFRs after TURP, which also reached a maximum at 1 year. In spite of a gradual but obvious decrease in PFR after both types of treatment there was no such decrease in symptom scores. At 3 years, 18% of the laser patients and 9% of the TURP patients required reoperation (Keoghane et al, 2000).
Interstitial Laser
McNicholas and colleagues introduced the topic of interstitial lasers with a description of their use in an experimental study (1993). Further experimental results were described by Muschter and coworkers, and, in addition, the first large clinical experience was described (1994). They had 221 patients with LUTS treated with interstitial laser and observed for 12 months. The results were encouraging, with a mean AUASI score improvement from 25.4 before treatment to 6.1 at 12 months. In addition, the PFR increased from 7.7 to 17.8 mL/sec. The authors found that the prostate volume (measured by TRUS) decreased by 40%. A further large open study confirmed these results; sexual side effects were few, but irritative symptoms occurred in 12.6% and urethral strictures in 4% (Muschter and Hofstetter, 1995). A further but much smaller study reported 3-month results in 16 patients; the AUASI score decreased from 16.5 to 5.8 and the PFR increased from 8.8 to 11.9 mL/sec (Orovan and Whelan, 1994). A short-term open study on 61 patients revealed a similarly low improvement in the PFR at 3 months, from 6.7 to 10.0 mL/sec, whereas the AUASI score improved from 18.9 to 7.7 (Arai et al, 1996).
All of these open studies show that, at 3 months, the improvements in PFR are less than might have been expected, demonstrating the importance of longer-term studies and the relative lack of value of short-term studies; they also show that large numbers of patients can be treated safely with this technique with a good side effect record, considerably improving on previous laser techniques. However, the results of a comparative trial, against TURP or TUNA, would make interesting reading and would also give valuable clinical information.
In an excellent summary of the procedure, Muschter (1999) reviewed the reported literature, indicating that the interstitial laser technique had been performed on a total of 785 patients. The length of follow-up was very variable, but improvement in symptom score for 70% was noted overall (range, 32% to 92%). The average improvement in PFR was 98% (range, 35.2% to 203%). The decrease in prostate volume ranged from 8.3% to 41.6%, and the re-treatment rate at 12 months ranged from 0% to 15.4%.
All of these results are with the interstitial Nd : YAG laser, and results with the more recently introduced diode interstitial laser have not been reported as frequently. Although comparative trials with the diode laser are ongoing, short-term results of small open studies indicate a safe and reliable technology. At 3 months, 25 patients thus treated had an improvement in symptom score from 20.6 to 6.9; the PFR increased from 9.1 to 20.3 mL/sec (De la Rosette et al, 1997). A further open study reported on results in 104 patients reviewed at 1 year (Conn et al, 1999). The AUASI score showed a mean improvement from 22.4 to 8.3 and the PFR from 8.6 to 14.2 mL/sec. The mean catheterization time after treatment was 4.8 days.
Transurethral interstitial laser coagulation (TUILC) was compared in a small randomized trial with TUMT and TURP (Norby et al, 2002) and was found to be effective but associated with a relatively high morbidity (64%), principally urinary tract infection. In another small noncomparative trial, TUILC combined with minimal TURP was assessed in 41 patients with BOO caused by BPH (Corvin et al, 2002). The authors believed that this combination could reduce the morbidity associated with TURP, which was irrigation fluid absorption and blood loss. The case was not made particularly strongly, because of the absence of a comparator group, that there was an unacceptably high morbidity associated with TURP.
Kursh and coworkers (2003) described a randomized multicenter trial comparing TUILC and TURP with a 2-year follow-up. They found that TUILC performed well, and although the authors concluded that TUILC was an acceptable alternative to TURP it was noted that 16% of the TUILC group required TURP within the first year. In another small randomized trial, Liedberg and colleagues (2003) randomly assigned 20 to a TUILC group and 11 to a TURP group. These authors found a significantly greater improvement in symptom score and PFR in the TURP group and a greater morbidity rate in the TUILC group. Urinary infections occurred in 13 TUILC patients (65%) as against 1 TURP patient. Post-treatment catheterization was required for 24 days after TUILC and 2 days after TURP. In a small study of 28 patients in acute urinary retention, TUILC was performed with urinary infection in 2 and a mean catheterization time after TUILC of 6.3 days (Nishizawa et al, 2003). The mean prostatic volume decreased by 19.4% at 6 months, and the IPSS and PFR were 8.0 and 11.2 mL/sec, respectively, at 3 months. In a study of 82 patients observed for a mean of 48.4 months, 29 (35%) were re-treated, 18 (22%) had TURP, and 11 (13%) had additional pharmacotherapy (Terada et al, 2004).
Holmium : Yttrium-Aluminum-Garnet Laser
The Ho : YAG laser was introduced for use in a number of urologic conditions (Webb et al, 1993) and was then used in combination with the Nd : YAG laser in the treatment of BPH (Gilling et al, 1995).
Prostate resection with the Ho : YAG laser was first reported on 84 patients with a mean follow-up of 4.3 months (range, 1.5 to 9); the mean prostate volume was 50 mL, and the mean resection time was 35.1 minutes (Gilling et al, 1996). Recatheterization was required in 2 patients before they left the hospital and in a further 2 within the first week but was in every case short term. The side effects were minimal, with irritative symptoms and dysuria being negligible. The mean AUASI score improved from 21.3 to 4.1 at 3 months, and the PFR increased from 7.5 to 19.3 mL/sec at 3 months.
A combined review of data in a single center in New Zealand and a single center in the United Kingdom showed that the procedure had been safely performed in 967 patients (Mackey et al, 1998). Of these, 503 had been observed to 3 months, and the mean AUASI score had improved from 20.6 to 7.0 and the PFR increased from 8.8 to 21.1 mL/sec. Because only 33% of the total number had been observed to 6 months, it is difficult to draw conclusions from the results, but the improvements were being maintained (AUASI score, 4.8; PFR, 22.3 mL/sec).
These encouraging results from large open studies led to a comparative trial between two centers in New Zealand and the United States. Patients were randomly assigned to TURP (59 patients) or Ho : YAG prostatic resection (61 patients). In a well-described study, 102 patients were observed to 1 year. The AUASI score improved from 21.9 to 4.2 in the Ho : YAG group and from 23.0 to 4.3 in the TURP group at 1 year. At the time point, the PFR had increased from 8.9 to 25.2 mL/sec in the holmium group and from 9.1 to 20.4 mL/sec in the TURP group. All cases were urodynamically studied; the mean Pdet at PFR decreased from 75.9 to 35.2 cm H2O at 6 months in the holmium group and from 83.4 to 39.2 cm H2O in the TURP group. Complications were low and were equal in both groups. Prolonged catheterization was not required in either group (Gilling et al, 1999).
This type of treatment can also be applied to large prostates. A total of 43 patients with prostates larger than 100 g were treated by Ho : YAG prostate resection. The mean catheter time was 19.7 hours, and the mean hospitalization time was 28.4 hours. At 6 months follow-up the AUASI score had improved from 23.5 to 2.8 and the PFR increased from 9.0 to 24.8 mL/sec.
The Ho : YAG laser has been used in both smaller and larger prostates. These are several descriptions of holmium laser enucleation of the prostate (HoLEP) in this context. Thirty-eight men with a mean prostatic volume of less than 60 mL were treated as day cases (Larner et al, 2003). This treatment was found to be safe and effective with outcomes equivalent to those expected from TURP, but it must be remembered that this was not a comparative trial. In another study, Ho : YAG laser bladder neck incision was compared with HoLEP as an outpatient procedure (Aho et al, 2005). HoLEP was superior in producing relief of obstruction, and fewer patients required recatheterization or reoperation. Catheter time, hospital stay, and postoperative morbidity were found to be similar. Both techniques were believed to be effective, but the bladder neck incision was quicker to perform. Using the slightly older holmium technology, holmium laser ablation of the prostate (HoLAP), there was a 7-year follow-up from a study of 79 patients (Tan et al, 2003a). Only 43% of the sample were available for follow-up, the mean AUA score of the remainder was 10.0, and the mean PFR was 16.8 mL/sec. The reoperation rate was 15%.
There have been a number of studies of the use of HoLEP in large prostates. The definition of a large prostate varies, as does the type of trial. Two studies from the same unit in the United States evaluated HoLEP in patients with prostates weighing more than 75 g and more than 125 g, respectively (Kuo et al, 2003; Matlaga et al, 2006). Of note was the tissue retrieval rate of 1.09 g/min for this technique, with an operative time of 94.7 minutes, a range of 27 to 263 minutes for enucleation, and a further mean of 33.4 minutes for morcellation. If the long operating time was a negative, the fact that the mean postoperative catheterization was 15.1 hours and the mean length of hospitalization was 26.0 hours was a strong positive.
A number of randomized trials comparing HoLEP with TURP have been performed. The statistical powering of these studies was not commented on in many cases, leading the reader to question the outcomes of the studies. However, the comments of the authors were all clearly in favor of the value of HoLEP to patients with LUTS. Tan and colleagues (2003b) found that HoLEP was superior to TURP in terms of time of catheterization, hospital stay, and amount of tissue removed, but it took almost twice as long as TURP to perform. It was also superior to TURP in relieving urodynamically proven obstruction at 6 months, but there was no significant difference in the improvement in AUASI score and PFR. Exactly the same findings were produced in other randomized trials (Kuntz et al, 2004a, 2004b; Montorsi et al, 2004). The most recent holmium laser technology, holmium laser resection of the prostate (HoLRP), was subjected to a randomized trial against TURP (Westenberg et al, 2004). There were 120 patients, of whom 73 completed a 48-month assessment. The authors found no difference between the two techniques in terms of urodynamic indices, potency, continence, and symptom scores. As in the other studies, HoLRP took much longer to perform than TURP but was associated with less perioperative morbidity, catheter time, and hospital stay.
There have been three systematic literature reviews, one specifically of holmium laser prostatectomy (Tooher et al, 2004) and two of laser prostatectomy in general (Wheelahan et al, 2000; Hoffman et al, 2004). These reviews showed that there were three randomized controlled trials comparing HoLRP and TURP and two comparing HoLEP and TURP. As with the other minimally invasive procedures, the majority of the studies were nonrandomized comparative studies or case series. The quality of the available evidence was found to be poor in most cases; most studies were lacking information on statistical powering, methods of randomization, allocation concealment, and blinding; had short follow-up periods; and had significant losses to follow-up. All three studies suggested that it was impossible to draw any long-term conclusions but that the laser techniques offered an option other than TURP for treating symptomatic BPH. However, the report of Wheelahan and colleagues (2000), which did not look at holmium laser therapy, photoselective vaporization of the prostate, or interstitial laser therapy, stated that safety or efficacy could not be determined owing to an incomplete and poor evidence base.
Photoselective KTP Laser
Photoselective vaporization of the prostate (PVP) using a high-power 80-W KTP laser (Greenlight PV Laser System, Laserscope, San Jose, CA) has produced an alternative laser technology seen by many as an exciting new advance. KTP/532 laser energy is delivered by a side-firing glass fiber through a 27-Fr continuous-flow resectoscope. Sterile water irrigation is used, and the procedure is performed with the use of spinal anesthesia. The end point of the procedure is the production of a TURP-like cavity that has resulted from complete vaporization of the prostatic adenoma.
The only comparative trial thus far reported is an experimental one using a porcine kidney model, where ex-vivo blood-perfused kidneys were used to verify the hemostatic effect of KTP laser vaporization against TURP-like tissue resection. The laser technique was associated with highly significantly decreased bleeding with larger coagulation zones (Reich et al, 2004).
The remainder of the reported literature indicates a low level of evidence on which to base clinical decision-making. Kumar (2005) treated 18 patients and found a mean 51% reduction in prostate volume, but the follow-up was only 2.8 months. Sulser and coworkers (2004) described their early experience with PVP in 65 patients. The mean operating time was 57 minutes and the mean prostatic volume before treatment was 49 mL. All patients were catheter free by 1 month, but this was only a short-term 3-month observational study. PFR increased from a mean of 7.7 to 18.2 mL/sec, and IPSS decreased from 18.5 to 7.2. There were no major complications. Hai and Malek (2003) reported on only 10 patients who had a mean prostate volume decrease of 27%. There was a significant improvement in PFR and AUASI.
There has been a prospective clinical trial from six American medical centers on 139 men with symptomatic BOO related to BPH, and the 1-year efficacy and safety data have been reported (Te et al, 2004). The mean symptom score had decreased at 1 year from 23.9 to 4.3 and the mean PFR increased from 7.8 to 22.6 mL/sec. No significant complications were noted. The authors conceded that although the results were encouraging, long-term follow-up data were required.
PVP has been performed in 64 men with symptomatic BPH who had large-volume prostates (>60 mL). The mean operative time was over 2 hours (123 minutes), and 62 of the 64 patients were discharged within 23 hours. At 12 months the mean IPSS had decreased from 18.4 to 6.7 and the mean PFR had improved from 7.9 to 18.9 mL/sec (Sandhu et al, 2004). There has also been a report of PVP being performed in 66 high-risk patients, who had an American Society of Anesthesiologists score of 3 or greater; 29 patients were treated with ongoing oral anticoagulants or had severe bleeding disorders (Reich et al, 2005). No major complications occurred, nor was blood transfusion required. The average catheterization time was 1.8 days. The mean IPSS improved from 20.2 to 6.5 at 1 year, and the mean PFR improved from 6.7 to 21.6 mL/sec at 1 year.
Summary
The use of the laser to treat symptomatic BPH has evolved from the relatively cumbersome TULIP technique to other methods that require smaller pieces of equipment or that can treat a wider range of prostates. The difficulties in treating large prostates have been overcome using the newer technology. The minimal invasiveness of the laser has been improved, and the complications, particularly prolonged catheterization, bacteriuria, and urethral strictures, have been lowered very considerably. Cost-effectiveness still remains a question in urologists’ minds, but it would appear that quality of life improvement is as good as that achieved with TURP (Arai et al, 2000). Further long-term results from comparative studies are required from multicenter groups, which it is hoped would also answer the question of a higher reoperation rate seen in some long-term laser studies.
Recently, a systematic literature review assessed the evidence presented between 2006 and 2008 for the 532-nm photoselective laser vaporization and holmium laser enucleation of the prostate (Naspro et al, 2009). Although the authors suggested that both of these techniques are promising alternatives to both TURP and open prostatectomy, the quality of the trials was not universally high and the follow-up relatively short in many of them, particularly in the case of the KTP laser. More high-quality randomized controlled trials with adequate follow-up are required.
Transurethral Resection of the Prostate
History
TURP, as we know it today, was developed in the United States in the 1920s and 1930s. Nesbit (1975) pointed out that there were several significant factors important in its development: (1) the invention of the incandescent lamp by Edison in 1879; (2) the cystoscope, developed independently by Nitze and Lieter in 1887; and (3) the development of the fenestrated tube by Hugh Hampton-Young, which allowed the obstructing tissue to be sheared off blindly. Other important factors were the invention of the vacuum tube in 1908 by De Forest, which allowed the constant production of high-frequency electrical current that could be used in resecting tissue. In 1926, Bumpus combined the cystoscope and the tubular punch. Also, at that time, Stearns developed the tungsten loop that could be used for the resection. This was put together by McCarthy in 1932, using a foroblique lens so that he could resect the tissue under direct vision using a wire loop.
In the 1970s the development of the fiberoptic lighting system, together with the Hopkins (1976) rod lens wide-angle system, significantly improved visualization for endoscopic surgery. Previously, the optical system was a series of small lenses placed in a rigid tube. In the Hopkins rod lens wide-angle system the air spaces were replaced by solid glass rods. The spacer tubes were shorter, resulting in minimal obstruction and increased admission of light.
Over the years, TURP, as a treatment modality for obstructing BPH, gained popularity throughout the world. It is now considered the gold standard for the surgical management of BPH. In the 1986 National Health Survey, 96% of patients had TURP when they had surgery done for BPH. It was estimated that 350,000 Medicare patients had TURP that year. However, today there are many medical and surgical alternatives to transurethral prostatectomy, and the number of TURPs has fallen to less than 200,000 per year in the Medicare age group.
Another factor that may have influenced the incidence of transurethral prostatectomy was the formal development of care guidelines for patients with BPH (McConnell et al, 1994). In 1989, the Omnibus Budget Reconciliation Act created the Federal Agency for Health Care Policy and Research (AHCPR). Patient care guidelines were demanded by Congress, and two of the first selected were the diagnosis and management of BPH. It was the BPH Guideline Panel’s recommendation that patients with minimal symptoms should undergo watchful waiting and that if intervention was to be considered in patients who were more symptomatic the patient should be informed of the harms and benefits of each therapeutic modality and participate actively in making the decision, not only whether to intervene but which treatment modality would be his choice. Many patients opted for a less invasive procedure, although less effective than TURP.
Indications
In 1968, Lytton and coworkers (1968) estimated that the chance of a 40-year-old man having a prostatectomy in his lifetime was approximately 10%. However, Glynn and colleagues in 1985 raised the estimate to 29%. Arrighi and associates (1991), in reviewing the Baltimore Longitudinal Study on Aging (BLSA), believed that men older than 60 years had a 39% risk of requiring surgery in the next 20 years; men 50 to 59 years of age, a 24% chance; and men 40 to 49 years of age, a 13% chance.
There are very few studies of the natural history of patients who are seen initially because of modest symptoms of prostatism without an absolute indication for intervention (i.e., acute refractory urinary retention, recurrent infections). Ball and coworkers (1981) observed 97 patients for 5 years and found that the patients’ symptoms were essentially the same in 52% and worse in only 16.5%. The urodynamic studies revealed little change in that group, and only 1.6% of the patients developed retention. Conversely, Birkhoff and associates (1976) observed 26 patients for 3 years and found a 50% to 70% worsening of the patients’ subjective symptoms and 71% deterioration in objective criteria. Furthermore, acute retention was unpredictable.
The most common reasons for recommending intervention in a patient with symptoms of BOO and irritability are that the symptoms are moderate to severe, bothersome, and interfere with the patient’s quality of life. Mebust and colleagues (1989) noted that 90% of patients undergoing TURP had symptoms of prostatism but 70% had another indication as well (e.g., acute urinary retention, occurring in 27%).
In 1989, the AUA initiated the Guideline Panel for Diagnosis and Management of Benign Prostatic Hyperplasia. Recognizing the significance of assessing the patients’ symptoms, a system score was developed by the AUA’s Measurement Committee, Winston Mebust, Chairman. Barry (1992) was the senior author of this questionnaire, which came to be known as the AUA-7 Symptom Index (shortened to AUASI and now also known as the IPSS) (Table 93–3). Other scoring systems have been developed, such as those by Madsen and Iversen (1983), Boyarsky and associates (1977), and the International Continence Society Study on BPH. However, the AUASI was validated as to its clarity, test-retest reliability, internal consistency, and criteria validity. The AHCPR took over the AUA BPH Guideline Panel in 1990. The Guideline Panel subsequently recommended a formal assessment of the patients’ symptoms, using any scoring system but preferring the AUASI. Patients with mild symptoms (with a score of 0 to 7) were assigned to watchful waiting; those with moderate (8 to 19) or severe (20 to 35) symptoms would undergo further testing or treatment, or both. The AUASI is not disease specific. The questionnaire has been given to older women, who obviously do not have a prostate but may have bladder dysfunction, resulting in a significant score. The AUASI was considered to be part of the patient’s initial evaluation but did not make the diagnosis of BPH.
The AUASI was adopted at the World Health Organization Consultation on BPH in Paris in 1991 (Mebust et al, 1991). In addition to the AUASI, one global quality of life question was added—“If you were to spend the rest of your life with your urinary condition, just the way it is now, how would you feel about it?” Responses ranged from “delighted” to “terrible.” Therefore, not only should the patient’s severity of symptoms be considered in deciding whether intervention is warranted, but also how much the patient is being bothered by his symptoms and how they affect his overall quality of life. It should be pointed out that patients having symptoms secondary to an obstructing prostate may have a variation in their symptoms over time. Ball and Smith (1982) noted that 31% of the patients they were observing had improvements in their symptoms. More recently there have been efforts to develop a concise questionnaire to evaluate further the degree to which the condition is bothersome or the impact on quality of life of patients with symptoms from BPH. Such an example is the BPH Impact Index (Barry et al, 1995) (Table 93–4).
Although symptoms constitute the primary reason for recommending intervention, in patients with an obstructing prostate there are some absolute indications. These are acute urinary retention, recurrent infection, recurrent hematuria, and azotemia. A postvoid residual urine has been used by some urologists as an indication, but, as pointed out by Bruskewitz and coworkers (1982), there can be extreme variability within a given patient as to the amount of postvoid residual when this factor is assessed repetitively over a period of time. Furthermore, there is no information on the amount of postvoid residual urine that represents the point “that if nothing is done, irreparable damage to the bladder will occur.” It was believed by the AHCPR BPH Panel (McConnell et al, 1994) that it might be of some use in following patients who are assigned to watchful waiting. In the AHCPR BPH Guideline, the patient’s history is taken with focus on the urinary tract. Many of these patients have other comorbidity problems, and Mebust and colleagues (1989) noted that only 23% did not have a significant medical problem before surgery. The most common were pulmonary disorders (14.5%), gastrointestinal disorders (13.2%), myocardial infarction (12.5%), arrhythmia (12.4%), and renal insufficiency (4.5%). Therefore, a general medical evaluation is warranted.
A urinalysis is also recommended to be sure that the patient’s symptoms are not related to infection. A digital rectal examination should be done, taking into consideration the consistency of the prostate, to help the urologist in determining whether the patient has cancer, and also for an estimate of size. The size of the prostate might be important in selecting what type of surgical therapy would be warranted (e.g., transurethral incision of the prostate or, in the very large prostate, an open prostatectomy). A serum creatinine value is also to be obtained. It was noted by Mebust and colleagues (1989) that patients with a serum creatinine level greater than 1.5% had a 25% incidence of postoperative complications versus 17% in those who had a normal creatinine level.
A number of tests are considered to be optional for the urologist in evaluating the patient with moderate to severe symptoms from BPH. Many consider uroflowmetry to be the single best noninvasive urodynamic test to detect lower tract obstruction. However, it should be pointed out that the patient’s obstructive symptoms, when evaluated with a formal questionnaire, correlate poorly with uroflowmetry measurements. Furthermore, there is no specific cutoff point at which one can state that the patient is definitely obstructed. A PFR less than 15 mL/sec does not differentiate between outflow obstruction and detrusor impairment. Pressure-flow studies are recommended as an optional test. This is one of the best ways to evaluate a patient’s degree of obstruction and detrusor function, particularly when the diagnosis is unclear. However, it is invasive, is uncomfortable for the patient, requires expensive equipment, and requires considerable experience in performing so that the results are reproducible.
A number of tests are not recommended, such as a filling cystometrogram. Although this test can demonstrate uninhibited bladder contractions, it is invasive and does not give much more information than can be obtained from the patient’s history of bladder irritability symptoms. Cystoscopy, done routinely in the physician’s office as a diagnostic procedure, is not recommended. The only reason for performing cystoscopy in the urologist’s office is if the urologist needs to know the size of the prostate and its configuration in making a recommendation about the type of therapy that would be useful for the patient (e.g., transurethral incision if a small prostate or an open prostatectomy if a very large prostate). If it is critical for the urologist to know the exact size when deciding whether the patient should have an open prostatectomy, TRUS is more precise than cystoscopy. Upper tract imaging is also not recommended to be performed routinely. Rather, it should be reserved for patients with hematuria or renal insufficiency or for those with a history of urinary infection, urinary tract surgery, or stones.
Anesthesia
Transurethral surgery of the prostate is usually performed with the use of a general or spinal anesthetic. However, Sinha and associates (1986) have reported doing TURP with the patient under local anesthesia. Birch and colleagues (1991) have recommended performing TURP using sedation and local anesthesia. However, they pointed out that the gland size should be less than 40 g. Nielsen and associates (1981) noted no difference in blood loss when the surgery was done with either an epidural or a general anesthetic. McGowan and Smith (1980) evaluated spinal anesthesia versus general anesthesia and found no difference in blood loss, postoperative morbidity, or mortality.
Use of an anesthetic should be individualized. During the procedure, the anesthetist should monitor the patient’s tissue oxygen saturation, electrocardiogram, and, if warranted, a serum sodium level, in addition to the usual parameters.
Perioperative Antibiotics
Urinary tract infections can be found in 8% to 24% of BPH patients preoperatively. The infection should be treated before surgery. Prophylactic use of antibiotics, usually a single dose just before the start of surgery, has become the norm; and postoperative sepsis has been kept to a minimum in this way. In patients who have preoperative bacteriuria or a catheter in situ before surgery, the antibiotics should be continued until the catheter at time of surgery is removed. It is recommended that patients should be given a first-generation cephalosporin in combination with gentamicin before the initiation of surgery.
Monopolar versus Bipolar Techniques
Traditionally, TURP has been performed using monopolar technology with 1.5% glycine or mannitol as nonhemolytic fluids for irrigation. This technique has been used for a very long time with considerable success, but concerns about TUR syndrome have led to the introduction of bipolar TURP.
The standard monopolar TURP is now being challenged by the use of bipolar resection (Gyrus PlasmaKinetic System, Gyrus Medical, Maple Grove, MD). The rationale for the introduction of this system is that the complications of standard monopolar TURP need to be reduced to improve acceptability by patients. There has been a series of studies with the Gyrus system (Botto et al, 2001; Dunsmuir et al, 2003) and with the Vista CTR system (ACMI Corp.) (Singh et al, 2005). In a small retrospective study, 18 patients had conventional monopolar TURP, and 26 had bipolar TURP. The point was made by the authors that there have been few innovations in TURP technique in recent years and that this latest change has allowed earlier removal of the catheter and earlier discharge from hospital. The fact that all of these studies are short term, nonrandomized, and in some cases retrospective means that the observations on relative complication rates must be taken with some caution. However, this new technology may have something to offer. The Gyrus bipolar system consists of a generator with 200-W capability, an RF range of 320 to 450 kHz, and a voltage range of 254 to 350 V. There is a plasmakinetic resectoscope with a TUR loop of 80/20 platinum/iridium alloy electrode with the active and return electrode on the same axis (axipolar) separated by a ceramic insulator (Botto et al, 2001; Patel and Adshead, 2004).
In a systematic review of the literature and meta-analysis of randomized controlled trials, Mamoulakis and colleagues (2009) compared the two techniques. They found 16 randomized controlled trials with 1406 patients involved. Unfortunately, the overall trial quality was poor. A lack of information about allocation concealment and blinding outcome assessors was very frequently observed and the absence of prevention of statistical errors of bias because of underpowered studies was also commonly noted. Long-term efficacy evaluation of bipolar TURP was not possible and although the numbers of TUR syndrome and postoperative clot retention were significantly less than in monopolar TURP, owing to the relatively small patient volume in each treatment arm, in absolute numbers there was not a great deal of difference between the two techniques. Bipolar TURP may well turn out to be the technology of the future, but it is hoped there will be a number of well-designed multicenter randomized controlled trials with a longer follow-up and a cost analysis to prove this.
Surgical Technique
The surgical technique employed has been described in detail in previous editions of this textbook (Fitzpatrick and Mebust, 2002). It is not necessary to describe the technique in detail, but it is appropriate to show the diagrams of the technique described (Figs. 93-6 to 93-12).
Figure 93–6 A to C, First stage of resection of the prostate. The resection is begun at the 12-o’clock position, and the tissue at the bladder neck and the adjacent adenoma are resected in quadrants.
Figure 93–7 A, The midportion of the gland is resected starting at the 12-o’clock position and carrying it down to the 9-o’clock position. B and C, The sagittal and coronal section views are shown.
Figure 93–8 A, The resection is now begun at the 12-o’clock position, and the left side of the patient’s gland in the midfossa is resected down to the 3-o’clock position. B and C, Sagittal and coronal sections are shown.
Figure 93–9 A, The midportion of the gland is resected farther down from the 9-o’clock position to the 6-o’clock position. B and C, Sagittal views.
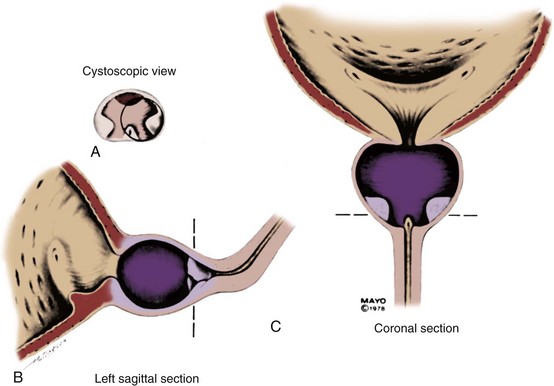
Figure 93–10 A to C, The tissue remaining at the apex is now resected. It is begun by initiating the resection next to the verumontanum and carrying it toward the 12-o’clock position.
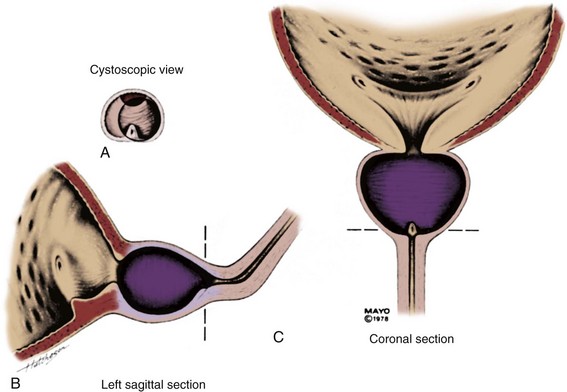
Figure 93–12 A to C, The remaining residual tissue is cleared from the patient’s left side, leaving an unobstructed view from the verumontanum through the bladder neck into the bladder.
Various surgical techniques have been developed by urologists for removing the adenoma. Every surgical technique employs the principle that the resection should be performed in a routine step-by-step manner. Another technique is demonstrated in Figures 93-13 to 93-15.
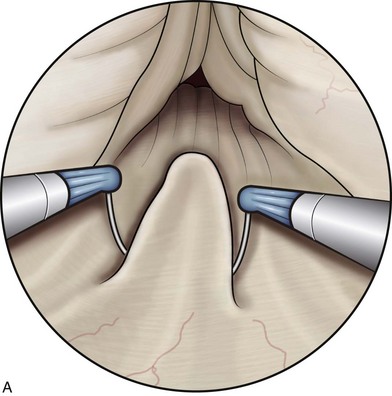
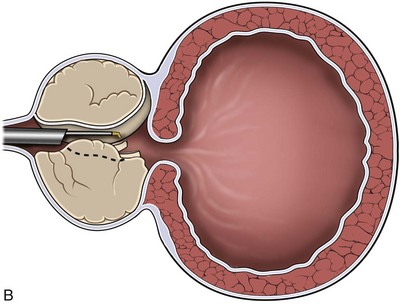
Figure 93-13 A and B, Resection usually begins at the proximal portion of the middle lobe at the 6-o’clock position. The resectoscope is placed just proximal to the verumontanum and the resection performed always controlling the end point of each cut. It is necessary to be aware of the position of the verumontanum to see that the lower part of the cut is not extending below this level or otherwise damage to the sphincter mechanism may occur.
(Modified from May F, Hartung R. Surgical atlas: transurethral resection of the prostate. BJU Int 2006;98:921–34.)
Figure 93-14 A and B, Resection should be performed with long cuts toward the verumontanum. A large overhanging middle lobe should be resected with special care. It is important to make short cuts in the region of the bladder neck, because the surgeon might not be aware that he or she is cutting down the trigone toward the ureteral orifices. Subsequent cuts are made down to the peripheral tissue, which is recognized as a rather fibrous structure compared with the granular appearance of the prostatic adenoma.
(Modified from May F, Hartung R. Surgical atlas: transurethral resection of the prostate. BJU Int 2006;98:921–34).
Figure 93-15 A and B, Resection in smaller adenomas is now carried directly to the side lobe. It depends on the preference of the surgeon whether to begin on the left and then to resect the other side or vice versa.
(Modified from May F, Hartung R. Surgical atlas: transurethral resection of the prostate. BJU Int 2006;98:921–34).
Management of Intraoperative Problems
Hemostasis
The amount of intraoperative bleeding depends on the size of the prostate, the length of time required to resect the adenoma, and, to a degree, the surgeon’s skill. Arterial bleeding is controlled by electrocoagulation. This should be done as one completes each stage of the resection, before moving on to the next stage. After the catheter is inserted, at the end of the surgical procedure, the irrigation fluid should be light pink. If the irrigation fluid has a continued red color, one should suspect arterial bleeding. The surgeon should reinsert the resectoscope and coagulate the arterial bleeding. Venous bleeding is apparent at the end of the procedure, when on irrigating the catheter the return is initially clear but then dark blood later oozes from the catheter. Venous bleeding can be controlled by filling the bladder with 100 mL of irrigating fluid and placing the catheter on traction for 7 minutes at the operating table. The balloon of the catheter is overinflated to 50 mL of fluid.
Extravasation, or perforation of the prostatic capsule, occurs in about 2% of patients. The symptoms of extravasation are restlessness, nausea, vomiting, and abdominal pain, despite spinal anesthesia. Pain is usually localized to the lower abdomen and back. If extravasation is suspected, the operation should be terminated as rapidly as possible but hemostasis must be secured. Bleeding must be controlled, even as the extravasation is increased, because simultaneous postoperative management of extravasation and hemorrhage is difficult. Cystography may provide information about the diagnosis and, to some degree, the extent of the extravasation. Over 90% of these patients can be managed simply by urethral catheter drainage and cessation of the operative procedure. If there is extensive extravasation and concern about infecting the perivesical tissue, suprapubic drainage should be instituted.
Transurethral Resection Syndrome
In the AUA cooperative study (Mebust et al, 1989), TUR syndrome occurred in 2% of the patients. The syndrome was characterized by mental confusion, nausea, vomiting, hypertension, bradycardia, and visual disturbance. Usually, the patients do not become symptomatic until the serum sodium concentration reaches 125 mEq/dL. The risk is increased if the gland is larger than 45 g and the resection time is longer than 90 minutes.
In 1950, several studies were undertaken to determine the amount of fluid absorbed during TURP. Hagstrom (1955) weighed patients preoperatively and postoperatively and calculated that approximately 20 mL/min of fluid was absorbed by the patient. However, there was significant variation in patients. Oester and Madsen (1969), using a double-isotope technique, demonstrated that the average amount of fluid absorbed by the patient was 1000 mL and that one third of this fluid was absorbed intravenously. Madsen and Naber (1973) demonstrated that pressure in the prostatic fossa and the amount of fluid absorbed were dependent on the height of the fluid by the patient. They noted that when the height of the fluid was changed from 60 to 70 cm, fluid absorption was greater than twofold. They also reported that approximately 300 mL/min of fluid was needed for a good vision field. This could not be achieved when the fluid level was below 60 cm H2O. Harrison and colleagues (1956) believed that the TUR syndrome was related to dilutional hyponatremia. Certainly, the syndrome can be reduced by the administration of 3% saline solution. However, other factors have been suggested as a possible cause. Glycine is metabolized to glycolic acid and ammonium. Ammonium intoxication has been suggested as a possible cause of the TUR syndrome or direct toxic effect of the glycine. Nevertheless, it is our belief that the TUR syndrome is secondary to dilutional hyponatremia. This can be corrected by giving the patient 200 mL of 3% saline solution very slowly over a period of time.
Conversely, one can attempt to calculate the amount of water overload by determining the serum sodium preoperatively and postoperatively, knowing the patient’s weight, and determining the amount of extracellular fluid. Stalberg and coworkers (1992), in patients undergoing TURP with general anesthesia, tagged the irrigating fluid with 1% ethanol and measured the amount of ethanol excreted in the breath. This is believed to be a very effective and quick way of determining which patients are becoming overloaded with fluid. In patients who have a large gland or when the operating time is being prolonged, a serum sodium value is routinely obtained, although the patient is still undergoing surgery. When patients demonstrate a drop in serum sodium level, diuretics (e.g., furosemide [Lasix]) are administered. The serum sodium concentration is measured in the postoperative recovery area, and, if need be, a further dose of diuretic is administered.
Intraoperative Priapism
During the surgical procedure a penile erection may occur, which may obviate the surgery unless a penile urethrostomy is performed. This has usually been managed by injecting an α-adrenergic agent directly into the corpora cavernosa. The solution of ephedrine or phenylephrine is usually diluted (e.g., 0.3 mL 1% phenylephrine diluted to 3 mL with normal saline solution for 100 µg/1 mL) (Lee et al, 1995).
Outcomes
Patients’ Symptoms
In developing the guideline for the diagnosis and management of BPH, the AHCPR Guideline Panel (McConnell et al, 1994) asked patients what was the most significant factor they considered in deciding what type of therapy should be used in treating their BOO secondary to BPH. The foremost factor was relief of symptoms.
In reviewing the literature, the AHCPR Guideline Panel used meta-analysis to combine the various clinical studies. They noted that the chance of improvement of patients’ symptoms after TURP was 70% to 96% confidence interval (CI), with a mean of 88%. The magnitude of reduction in symptom score was 85%. This was significantly better than with less invasive procedures.
Mortality
Over the past 50 to 60 years there has been a gradual reduction in the immediate postoperative mortality rate associated with TURP. Perrin and colleagues (1976) found a mortality rate of 5%, reported in the 1930s. Holtgrewe and Valk (1962) reported 2.5%; Melchior and associates (1974), 1.3%; and Mebust and coworkers (1989), 0.2%. This was the mortality rate noted at 30 days. The number of patients noted in each of these three studies was well over 2000.
Roos and colleagues (1989), using insurance claim data from England, Denmark, and Manitoba, Canada, noted that the death rate at 90 days was significantly higher compared with an open prostatectomy. They noted an early postoperative death rate of 2.9% in a study involving almost 40,000 patients. A meta-analysis of other series showed a mortality rate of 1.18%. However, including the study by Roos and colleagues with other reports, the mean estimate was 1.5% (90% CI, 0.5% to 0.3%).
In more recent studies, Ala-Opas and colleagues (1993) found no immediate mortality in a series of over 400 patients undergoing TURP. Chute and associates (1991), in an epidemiologic study, compared patients who had TURP with those who did not have TURP and found that the mortality rate over time was identical. Montorsi and coworkers (1993) compared a group of patients who underwent either TURP or open prostatectomy and found the mortality rate in each group was identical. Concato and colleagues (1992) compared a group of patients retrospectively, undergoing TURP or prostatectomy; and, when corrected for comorbidity, the mortality rate was the same for both groups. However, a chart review had been conducted by Malenka and associates (1990) looking at comorbidity factors in the Canadian population. They could not identify comorbidity factors to account for the apparent difference in mortality rate between TURP and open prostatectomy.
Fuglsig and coworkers (1994) age matched a group of patients who had undergone TURP with those who had not, and the mortality rate for the two groups was identical. The mortality rate could not be influenced by many factors, such as the type of hospital where the surgery was performed, the skill of the surgeon, the size of the gland, the operating time, or, again, comorbidity factors.
In reviewing the cause of death in the Canadian series it was found to be secondary to cardiac disease. In the patients who were studied in Denmark, death resulted from pulmonary complications. This suggests that the observation by Roos and associates (1989) was truly related to comorbidity being significant in those undergoing TURP as opposed to those who underwent an open prostatectomy.
The immediate morbidity rate after TURP was reported by Mebust and coworkers (1989) as 18%. This was similar to the rates reported by Holtgrewe and Valk (1962) and Melchior and associates (1974). Although the incidence was unchanged, in the Mebust report the incidence of significant complications (e.g., acute pyelonephritis) was not as high as that noted in the previous studies. The most common complications in the immediate postoperative period were failing to void (6.5%), bleeding requiring transfusion (3.9%), and clot retention (3.3%). The AHCPR BPH Guideline Panel (McConnell et al, 1994) found a mean immediate postoperative complication rate of 14.95% with a 90% CI of 5.2% to 30.7%. In more recent studies, the immediate postoperative complication rate has been noted by Plentka and associates (1991) (3.1%), Estey and colleagues (1993) (7.8%), and Chute and coworkers (1991) (4.2%). However, these are all retrospective studies.
Wasson and associates (1995) reported on a prospective randomized study in which patients were assigned to either watchful waiting or TURP. Ninety-one percent of the men had no complication during the first 30 days after surgery. Specifically, there was no difference between either group in the incidence of urinary incontinence or impotence. The most frequent complications noted were a need for replacement of urinary catheter (4%), perforation of the prostatic capsule (2%), and hemorrhage requiring transfusion (1%). At the end of 3 years of follow-up, the mortality rate was the same for each group. Twenty-three patients in the treatment group were considered treatment failures, and 47 patients were in the watchful waiting group. The failure rate in the watchful waiting group was 6.1 per 100 man-years of follow-up compared with 3 per 100 man-years of follow-up in the surgery group (P = .002). The higher rate in the watchful waiting group was largely attributed to a higher incidence of three outcomes: (1) intractable urinary retention (2.9% vs. 0.9%), (2) a high volume of residual urine (5% vs. 1.1%), and (3) a high urinary symptom score (4.3% vs. 0.4%). In those undergoing surgery there was improvement in PFR, reduction in the severity of symptoms, and reduction in the degree to which the symptoms bothered the patient. The authors noted that the outcomes of surgery were best for the men who were most bothered by urinary symptoms at baseline. This study refutes the uncontrolled observations in prior retrospective series that TUR frequently leads to incontinence and impotence, and it confirms that the incidence of short-term complications and re-treatment after surgery is low.
There are few data on the long-term outcomes of patients undergoing TURP. In the study conducted by Wasson and colleagues of 280 men undergoing surgery, at 3-year follow-up, 9 men had vesical neck contracture requiring endoscopic surgery, 9 had a urethral stricture that required dilatation, and 8 underwent a second TUR, 4 because of adenocarcinoma. Bruskewitz and coworkers (1986), in observing a series of patients over 3 years, noted that at 1 year most patients’ symptoms improved (84%) and 10% remained approximately the same. At 3 years, 75% had improved and 13% remained the same. The change in symptomatology was the development of urge incontinence occurring in those patients several years after surgery. Only 1 of the 84 patients required a repeated resection, resulting in an incidence of 2%. However, 10% of the patients developed a vesical neck contracture.
Meyhoff and Nordling (1986) noted that 90% of their patients were considered to have satisfactory results at 5 years after TURP. Flow rates were improved from preoperative values, although there had been a slight decline in flow rates over the 5-year period. They did note an 8% re-resection rate, but all patients were noted to have especially small glands, suggesting that these might have been postoperative vesical neck contractures, as had been noted in the prior studies.
Ala-Opas and associates (1993) found that 92% of the patients were satisfied with the results of their surgery 6.5 years after the procedure had been done. Montorsi and associates (1993), in a group of patients observed for 5 years, found that 95% of the patients were unobstructed and subjectively satisfied about their urinary status.
In a large U.K. study, entitled The National Prostatectomy Audit (Emberton et al, 1995, 1996; Neal, 1997), 5361 patients who had undergone prostatectomy were reviewed by questionnaire. The findings were significant because of the large number of patients who responded, representing 89% of all those who had undergone the operation in four health regions of the United Kingdom. It was found that patients who were most bothered by their symptoms had the best response to surgery. The degree of preoperative evaluation varied greatly from urologist to urologist. Men who waited longer for surgery had worse symptoms by the time of their operation. Older men and those of a higher social class were more likely to undergo prostatectomy with fewer symptoms. Twelve percent of men throughout the study were undergoing surgery for the second time, and the clinical course of men having a second operation differed considerably from that of men having a first procedure.
A large study from Australia assessed the mortality and prostate cancer risk in 19,598 men after surgery for BPH. This was a population-based cohort of men in Western Australia operated on over a 17-year period (Holman et al, 1999). It was found that at 10 years the relative survival was 116.5% in patients who had TURP and 123.5% in patients after open prostatectomy. However, the relative risk of 1.20 fell to 1.10 (0.99 to 1.23) after adjustment for comorbidity. It was concluded that any excess mortality risk from TURP was small and clinically unimportant.
Another study found that just looking at mortality after TURP without attention to other issues could be misleading (Hargreave et al, 1996). A total of 81,997 men underwent prostatectomy in Scotland between 1968 and 1989. Although the study confirmed the increased risk of late mortality after TURP compared with open prostatectomy, it was found that limitations in the coding of the comorbidities and the absence of coding of more subtle aspects of the condition of the patient that may influence the choice between different types of prostatectomy mean that the differential mortality after the two procedures could reflect preoperative selection rather than the effects of the procedure itself.
Finally, in a review of 166 patients older than 80 years who had undergone TURP, with a mean follow-up of 60 months, it was found that 88.5% had an American Society of Anesthesiologists operative risk classification of III or IV, indicating a poor risk. All patients had at least one serious associated medical disease. Early complications occurred in 25.9%, late complications in 13.2%, and reoperation in 4.2%. The mortality rate within 30 days of operation was 1.2%, and 43 patients died during the follow-up period. The authors compared the Kaplan-Meier survival curves of the group under review with the expected survival rate of the age-matched population and found no statistical difference (Matani et al, 1996).
Summary
TURP was introduced as a method of treatment that was less invasive than open prostatectomy. Although evidence-based medicine now considers it imperative that a new treatment should be compared in a randomized fashion against a known standard, this did not happen with TURP. Thus it took at least 50 years for it to be accepted as the established treatment and it took even longer for its exact role in the therapeutic armamentarium to be found. Although comparative trials evaluating minimally invasive treatments against TURP in terms of efficacy have lost that particular battle in the majority of cases, the complication rate after TURP has not been thus compared. When looked at without the benefit of comparison in a randomized trial, TURP is still an incomparable treatment for BPH, particularly if the patient has a high “bother” score, has recurrent urinary tract infections caused by incomplete bladder emptying, or has outflow obstruction when measured by pressure-flow urodynamic studies.