Surgical Reconstruction of Bladder Exstrophy
Sweetser and colleagues (1952) initially described a staged surgical approach for bladder exstrophy. Four to 6 days before bladder closure, bilateral iliac osteotomies were performed. Epispadias repair was performed as a separate procedure. The continence procedure was limited to freeing the fibers from the intrasymphyseal band and wrapping this band around the urethra at the time of closure to increase outlet resistance.
The initial staged approach to functional bladder closure included three separate stages: bladder, abdominal wall, and posterior urethral closure; bladder neck reconstruction and antireflux procedure; and later epispadias repair. This approach was recommended for most cases of exstrophy reconstruction beginning in the early 1970s (Cendron, 1971; Jeffs et al, 1972; Williams and Keaton, 1973; Gearhart and Jeffs, 1989b). Although this procedure was successful, it has been significantly modified to include bladder, abdominal wall closure, and posterior urethral closure well onto the penis in the newborn period with bilateral innominate and vertical iliac osteotomy, if indicated; epispadias repair at 6 months to 1 year of age; and bladder neck reconstruction along with antireflux procedure at age 4 to 5 years, when the child has achieved an adequate bladder capacity for bladder neck reconstruction and is motivated to participate in a postoperative voiding program (Gearhart and Jeffs, 1998).
Other methods of treatment of the newborn with bladder exstrophy have been offered. Grady and Mitchell (1999) proposed combining bladder exstrophy closure with epispadias repair in the newborn period. Baka-Jakubiak (2000) recommended newborn exstrophy closure alone and combined bladder neck reconstruction and epispadias repair when the child reaches a satisfactory age for participation in a voiding program. Kelly (1995) and colleagues have recommended a staged repair in which no osteotomy is used and supplanted by a second-stage “radical soft tissue mobilization” before later urethral repair. Schrott and colleagues (1984) recommended bladder closure, ureteral reimplantation, epispadias repair, and bladder neck reconstruction in the newborn period. Lastly, Stein and colleagues (1999) recommended ureterosigmoidostomy in the newborn period with abdominal wall and bladder closure. This chapter offers a comprehensive look at all of these repairs, their outcomes, and most importantly overall complications and those specific to the different types of repairs.
Evaluation and Management at Birth
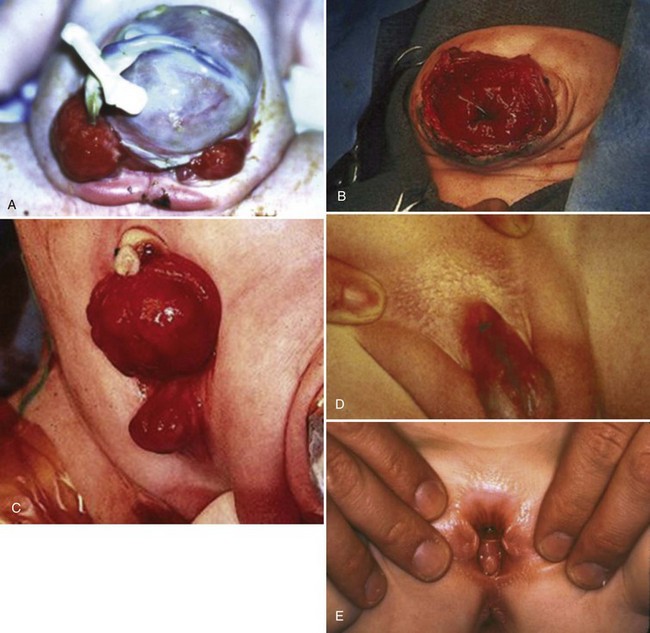
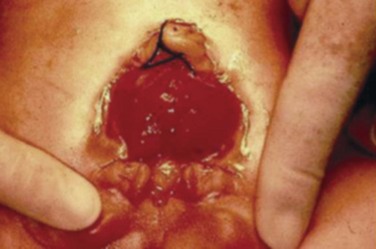
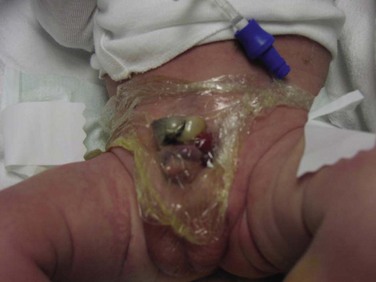
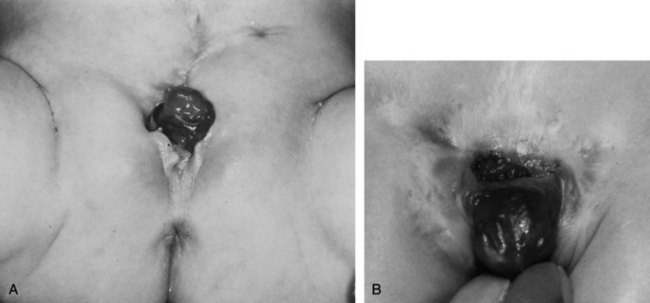
At birth, although the bladder mucosa is usually smooth, pink, and intact, it is also sensitive and easily denuded. In the delivery room the umbilical cord should be tied with 2-0 silk close to the abdominal wall so that the umbilical clamp does not traumatize the delicate mucosa and cause excoriation of the bladder surface. The bladder can then be covered with a nonadherent film of plastic wrap (e.g., Saran Wrap) (Fig. 124–16) to prevent sticking of the bladder mucosa to clothing or diapers. In addition, each time the diaper is changed the plastic wrap should be removed, the bladder surface irrigated with sterile saline, and clean plastic wrap placed over the bladder surface area.
The distraught parents need reassurance at this stage. Counseling of the parents and decisions regarding eventual therapy should begin prenatally if the condition is diagnosed by prenatal ultrasonography. The parents should be educated by a surgeon with a special interest and experience in managing cases of bladder or cloacal exstrophy. An exstrophy support team should be available and should include a pediatric orthopedic surgeon, pediatric anesthesiologist, social workers, nurses with special interest in bladder exstrophy, and a child psychiatrist with expertise and experience in genital anomalies. The Association of Bladder Exstrophy Children, based in La Salle, MI, has a website for parents and family members to obtain further information about this condition. In addition, should the parents desire, websites are available in some major exstrophy centers for further information.
Although it is true that parents need to be educated as fully as possible about the exstrophy condition, this is especially true regarding the sex of rearing males with bladder exstrophy. We have seen several families who were made even more distraught by being told that their child had ambiguous genitalia in addition to bladder exstrophy and that the child would need a change of gender. The need for changing the sex of rearing in classic bladder exstrophy is almost nonexistent in the male infant.
Cardiopulmonary and general physical assessment measures can be carried out in the first few hours of life. Radionuclide scans and ultrasound studies can provide evidence of renal structure, function, and drainage, even in the first few hours of life before the patient undergoes closure of the exstrophy defect.
Circumstances may be less than ideal at birth. A thorough neonatal assessment may have to be deferred until transportation to a major children’s medical center can be arranged. In these days of modern transportation, no child is ever more than a few hours away from a neonatal center with full diagnostic and consultative services. During travel the bladder should be protected by a plastic membrane, as in the nursery, to prevent damage to the delicate newborn bladder mucosa.
Selection of Patients for Immediate Closure
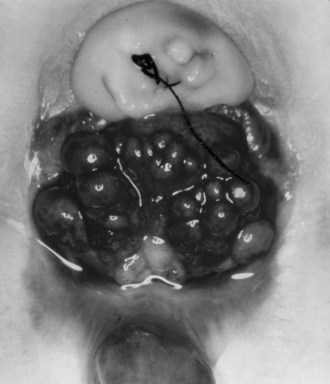
Successful treatment of exstrophy with functional closure demands that the potential for success in each child be carefully considered at birth. The size and functional capacity of the detrusor muscle are important considerations for the eventual success of functional closure. Correlation between apparent bladder size and the potential bladder capacity must not be confused. In minor grades of exstrophy that approach the condition of complete epispadias with incontinence, the bladder may be small yet may demonstrate acceptable capacity, either by bulging when the baby cries or by indenting easily when touched by a sterile gloved finger in the operating room with the child under anesthesia. Sometimes a good bit of previously unappreciated bladder can be discovered behind the fascia under examination with anesthesia (Gearhart and Jeffs, 1998). Once the bladder is relieved of surface irritation and repeated trauma, the small bladder can enlarge and increase in capacity with the absence of sphincter activity and with minimal outlet resistance. The exstrophied bladder that is estimated at the time of birth to have a capacity of 5 mL or more and demonstrates elasticity and contractility can be expected to develop useful size and capacity after successful bladder, posterior urethral, and abdominal wall closure with early epispadias repair (Gearhart and Jeffs, 1998).
Small Exstrophy Bladder Unsuitable for Newborn Closure
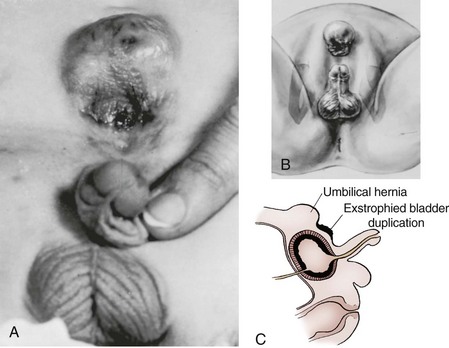
A small, fibrotic bladder patch that is stretched between the edges of the small triangular fascial defect without elasticity or contractility cannot be selected for the usual closure procedure (Gearhart and Jeffs, 1998) (see Fig. 124–10). Examination with the patient under anesthesia may at times be required to assess the bladder adequately, particularly if considerable edema, excoriation, and polyp formation have developed between birth and the time of assessment. Decisions regarding the suitability of bladder closure or the need for waiting should be made only by surgeons with a great deal of experience in the exstrophy condition (Gearhart and Jeffs, 1998). Neonatal closure, even when the bladder is small, can allow assessment of bladder potential, provides an initial step in genital reconstruction, and is helpful in reassuring the family. Some conditions preclude primary closure, including penoscrotal duplication, ectopic bowel within the extruded bladder (a relative contraindication), a hypoplastic bladder, and significant bilateral hydronephrosis.
In a recent review by Lakshmanan and colleagues (2008) of cases at our institution, it was found on initial judgment that the bladder was too small for closure in 34 patients evaluated at birth. There were 27 males and 6 females who underwent delayed closure at a mean age of 13.2 months. Osteotomy was performed on 29 (85%). All had a successful delayed primary closure. Eleven (41%) of the boys had a simultaneous epispadias repair. Sixty one percent developed sufficient capacity for bladder neck reconstruction and 39% are continent. Compared with newer data by Novak and colleagues (2010), these rates are over double the continence rates seen in bladder neck repair after failed primary closure and successful secondary closure.
Thus waiting for the bladder template to grow for 6 to 12 months in the child with a small bladder is not as risky as submitting a small bladder template to closure in an inappropriate setting, resulting in dehiscence and allowing the fate of the bladder to be sealed at that point. If the bladder does not grow to sufficient size after 6 to 12 months, other options include excision of the bladder and a nonrefluxing colon conduit or ureterosigmoidostomy. Another alternative involves urinary diversion with a colon conduit and placing the small bladder inside to be used later for the posterior urethra in an Arap-type procedure. Lastly, if the bladder is small and the presentation is for late primary closure, bladder augmentation, ureteral reimplantation, and an outlet procedure, in addition to a continent urinary stoma, can be considered.
Osteotomy
Children born with bladder exstrophy have an exposed bladder and a wide diastasis of the pubic rami, resulting in an open pelvic ring. The musculoskeletal function appears to be normal throughout childhood; the gait is characterized by external foot progression, which lessens with growth, even if osteotomy is not performed.
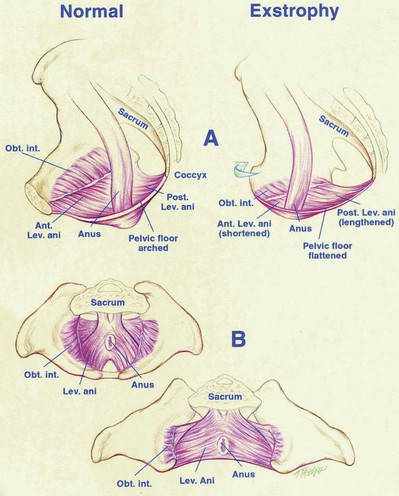
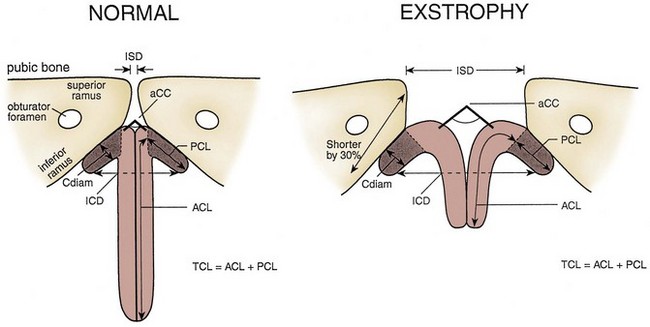
Several types of pelvic osteotomies have been developed to help close the pelvic ring, decrease the stress on the abdominal wall during initial exstrophy closure, and improve the outcome of future genitourinary reconstruction. Shultz was the first researcher known to the authors to describe bilateral posterior iliac osteotomy as part of a two-stage repair of bladder exstrophy. This early osteotomy was shown to lower rates of wound dehiscence and help obtain a more secure and better genitourinary reconstruction than was formerly obtained with reconstruction without osteotomy. The success in achieving continence has been reported to be related to the reduction in the diastasis and has been thought to result from better approximation of the muscles of the pelvic floor around the urethra.
Patients may undergo pelvic osteotomy at any stage of exstrophy repair if the diastasis prevents attainment of these urologic goals. In general, the authors rarely perform osteotomy on newborns unless the diastasis is over 4 cm because the laxity of the sacroiliac ligaments allows closure of the defect without undue tension.
Types of osteotomies that have been used include bilateral osteotomy of the superior pubic ramus, diagonal osteotomy of the iliac wing, and anterior innominate osteotomy with or without posterior iliac osteotomy. The combined anterior innominate and vertical osteotomy was developed for several reasons. The osteotomy is performed with the patient in the supine position as is the urologic repair, thus avoiding the need to turn the patient. The anterior osteotomy also allows placement of an external fixator under direct vision. A greenstick-type closing-wedge osteotomy of the ilium is also performed adjacent to the sacrum in most patients, creating two large bony fragments that are easily movable.
Following failure of initial closure and in children with significant pubic diastasis, as seen in cloacal exstrophy, osteotomy is also required. Pubic approximation may not be possible in a single step at the time of abdominal closure when the diastasis is extensive. The use of staged closure of the pelvis following osteotomy has been used successfully in this circumstance. This technique has been used with great success for the treatment of children presenting with extreme diastasis, even in younger patients.
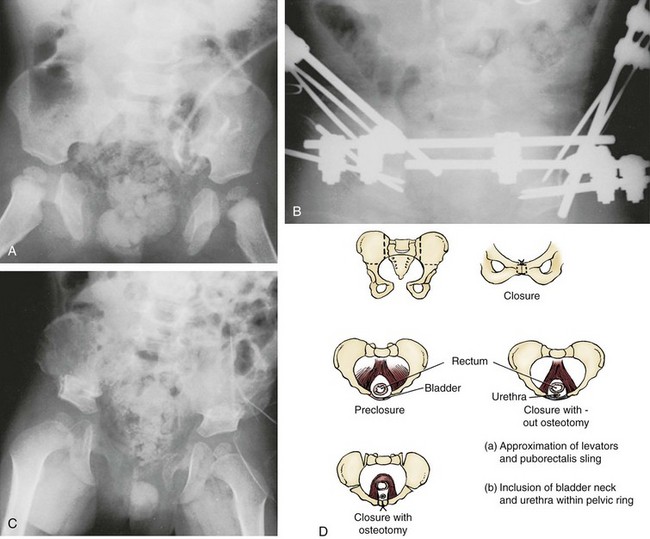
Pelvic osteotomy performed at the time of initial closure confers several advantages including (1) easy approximation of the symphysis with diminished tension on the abdominal wall closure and elimination of the need for fascial flaps; (2) placement of the posterior vesicourethral unit deep within the pelvic ring, enhancing bladder outlet resistance; and (3) bringing the large pelvic floor muscles near the midline, where they can support the bladder neck and aid in eventual urinary control (Fig. 124–17). After pubic approximation with osteotomy, some patients show the ability to stop and start the urinary stream, experience dry intervals, and in some cases become completely continent (Gearhart and Jeffs, 1991a). In a review of a large number of patients referred to our institution after failed exstrophy procedures, it was found that a majority of the patients who had partial or complete dehiscence of the bladder or major bladder prolapse had not undergone a prior osteotomy at the time of initial bladder closure (Gearhart et al, 1993b). The authors recommend performing bilateral transverse innominate and vertical iliac osteotomy when bladder closure is performed after 72 hours of age (Fig. 124–18). In addition, if the pelvis is not malleable or if the pubic bones are more than 4 cm apart at the time of initial examination under anesthesia, osteotomy should be performed, even if closure is done before 72 hours of age. A well-coordinated surgery and anesthesia team can perform osteotomy and proceed to bladder closure without undue loss of blood or risk of prolonged anesthesia in the child. However, it must be realized that osteotomy together with posterior urethral and bladder closure and abdominal wall closure is a 5- to 7-hour procedure in these infants.
If the patient is younger than 72 hours old and examination under anesthesia reveals that the pubic bones are malleable and able to be brought together easily in the midline by medial rotation of the greater trochanters, the patient can undergo closure without osteotomy. However, no chances should be taken with a decision of this magnitude. If there is any doubt, an osteotomy should be performed.
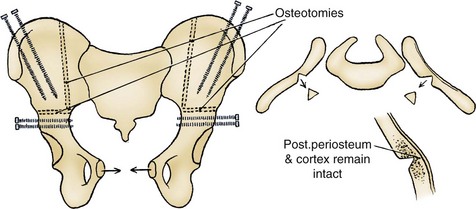
The most frequently used osteotomy today is the bilateral anterior innominate and vertical iliac osteotomy, popularized by Gearhart, Sponseller, and colleagues in 1996 (Gearhart et al, 1996b). This approach improves the ease of symphyseal approximation in the patient with exstrophy compared with posterior approaches, which require turning the patient. In our experience, this osteotomy is superior to the pubic mobilization seen with simple bilateral transverse anterior innominate osteotomy or even pubic ramotomy. With the ease of approximation obtained with this combined osteotomy, tension on the midline abdominal closure is lessened and the rates of bladder dehiscence and bladder prolapse are markedly decreased (Gearhart and Jeffs, 1998). In addition, pelvic closure allows approximation of the levator ani to strengthen the puborectalis sling, with positioning of the bladder neck and posterior urethra deep within the pelvic ring and improved continence rates. Besides the ease of approximation, combined osteotomy was developed for three reasons: (1) Osteotomy is performed with the patient in the supine position, as is the urologic repair, thereby avoiding the need to turn the patient; (2) the anterior approach to this osteotomy allows placement of an external fixator device and intrafragmentary pins under direct vision; and (3) the cosmetic appearance of this osteotomy is superior to that of the posterior iliac approach (Gearhart et al, 1996b).
In the posterior approach there was occasional malunion of the ileum, the blood loss was greater in the posterior approach, and the need to turn the patient intraoperatively from the prone to the supine position was always worrisome. The results of the combined horizontal transverse innominate osteotomy and vertical iliac osteotomy are so satisfactory that the approach is now being used in all primary closures of bladder exstrophy that require pelvic osteotomy (Gearhart et al, 1996b).
Combined osteotomy is performed by placing the patient in the supine position, preparing and draping the lower body below the costal margins, and placing soft absorbent gauze over the exposed bladder. The pelvis is exposed from the iliac wings inferiorly to the pectineal tubercle and posteriorly to the sacroiliac joints. The periosteum and sciatic notch are carefully elevated, and a Gigli saw is used to create a transverse innominate osteotomy exiting anteriorly at a point halfway between the anterior-superior and anterior-inferior spines (see Fig. 124–18). This osteotomy is created at a slightly more cranial level than that described for a Salter osteotomy in order to allow placement of external fixator pins in the distal segments. In addition to the transverse osteotomy, the posterior ileum may be incised from the anterior approach in an effort to correct the deformity more completely. This is important because anatomic studies have shown that the posterior portion of the pelvis is also externally rotated in patients with exstrophy, and as patients age they lose elasticity of the sacroiliac ligaments. For this part of the osteotomy, an osteotome is used to create a closing wedge osteotomy vertically and just lateral to the sacroiliac joints. The proximal posterior iliac cortex is left intact and used as a hinge (see Fig. 124–18). This combination osteotomy easily corrects the abnormalities in both the anterior and posterior segments of the pelvis.
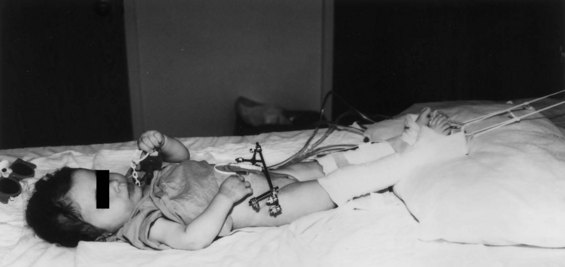
Two fixator pins are placed in the inferior osteotomized segment, and two are placed in the wing of the ileum superiorly. Radiographs are obtained to confirm pin placement, soft tissues are closed, and the urologic procedure is performed (see Fig. 124–17). At the end of the procedure, the pelvis is closed with a suture between the two pubic rami. The external fixators are then applied between the pins to hold the pelvis in a correct position. In a newborn with less than optimal amounts of cancellous bone, only one pin is placed inferiorly and superiorly in the wing of the ileum, whereas older children have two pins in each bone wing.
Radiographs are taken 7 to 10 days after surgery to look for complete reduction of the symphyseal diastasis. If this diastasis has not been completely reduced, the right and left sides can be gradually approximated by means of the fixator bars over several days. Longitudinal skin traction is used to keep the legs still (Fig. 124–19). The patient remains supine in traction for approximately 4 weeks to prevent dislodgement of tubes and destabilization of the pelvis. The external fixator is kept on for 4 to 6 weeks, until adequate callus is seen at the site of the osteotomy (see Fig. 124–15B and C). Postoperatively, in newborns who undergo closure without osteotomy in the first 48 to 72 hours of life, the baby is immobilized in modified Bryant traction in a position in which the hips have 90 degrees of flexion. When modified Bryant traction is used, the traction is employed for 4 weeks (Fig. 124–20). A horizontal mattress suture of No. 2 nylon is placed between the fibrous cartilage of the pubic rami and tied anteriorly to the pubic closure at the time of bladder closure. Evidence obtained by Sussman and colleagues (1997) from biomechanical testing in an intact piglet pelvic model revealed that all methods of pubic approximation were weak compared with the intact symphysis. However, the best technique with the strongest load-to-failure ratio was a No. 2 nylon horizontal mattress suture. Should this suture work loose or cut through the tissues during healing, the anterior placement of a knot in the horizontal mattress suture ensures that it will not erode into the urethra and interfere with the bladder or urethral lumen.
Complications of Osteotomy and Immobilization Techniques
Complications of immobilization can include failure of the closure and skin breakdown with resultant ulceration. Meldrum and colleagues (2003) reviewed a series of 86 patients in whom initial exstrophy closure failed. Most had been immobilized with a “mummy wrap” or spica cast. Successful closure was noted in 97% of those immobilized with an external fixator and modified Buck traction. In addition, in those failing initial closure, six had severe skin ulcerations from the mummy wrapping or spica cast and two of these children required later skin grafting.
Sponseller and colleagues (2001) reported on a total of 86 combined bilateral anterior innominate and vertical iliac osteotomies performed in 88 children. Ten of these children had cloacal exstrophy, and 72 had bladder exstrophy with at least 2 years of clinical follow-up (mean 4.8 years). Complications included seven cases of transient left femoral nerve palsy, which resolved fully by 12 weeks after surgery. There were no cases of right femoral nerve palsy, although the same surgeon performed the same technique on both sides. Patients with transient femoral nerve palsy were on bed rest for the first 6 to 8 weeks; use of a knee immobilizer was necessary for the remaining 6 weeks until resolution. Other complications included three cases with delayed ileal union, one case of superficial infection of the ileal femoral incision that required irrigation and débridement, one case of transient right thigh abductor weakness, one infection of the ileum around a pin site requiring irrigation and débridement, and one case of transient right peroneal palsy. Almost all patients had skin inflammation around the pins, particularly those in the proximal (iliac crest) segments. This was always controlled with the use of oral antibiotics. In a recent paper by Satsuma and colleagues (2006), comparisons were made between patients who underwent posterior iliac or the combined osteotomy. Pubic approximation was better, and the mean recurrence far less in the combined transverse innominate and posterior iliac osteotomy. Thus the combined approach corrected and maintained the pelvic ring with fewer complications than a post-pelvic osteotomy.
Whichever type of osteotomy is used, pelvic ring closure not only allows midline approximation of the abdominal wall structures but also allows the levator ani and puborectalis muscles to lend potential support to the bladder outlet, thus increasing resistance to urinary outflow (see Fig. 124–17D) (Sponseller et al, 1991; Gearhart et al, 1993b, 1996b; Schmidt et al, 1993; McKenna et al, 1994). Furthermore, a continence procedure can be performed later on the bladder neck and urethra deep within the closed pelvic ring at a distance from the surface without independent movement of the two halves of the pubis. The urethra and bladder neck are set more deeply in the true pelvis, in a more normal relationship than when acutely angulated.
When good callus formation is seen on radiography, the fixating device and pins are removed with the patient under light sedation. The age of the patient plays a role in the amount of correction of the diastasis that is maintained over time. On review of the previously described types of osteotomy, both classic and cloacal exstrophy patients gained approximation, although the former group gained greater correction toward normal (Gearhart et al, 1996b). Greater postoperative diastasis, as well as less optimal bone density in the newborn, contributes to the greater difficulty in obtaining and maintaining closure of the pelvic bone deformity over time.
The authors’ impression is that partial recurrence of diastasis in classic exstrophy occurs by two mechanisms. First, the pelvis may partially derotate owing to early loosening of pins before the time of osteotomy healing; this is seen mostly in infants. In the older child, increased bone density allows more rigid external fixation and thus better maintenance of the corrected position. Second, there is long-term undergrowth of the ischiopubic segment, which has been shown to be 33% smaller than normal in the adult with exstrophy, as the pelvis grows. Pubic diastasis increases with growth in the patient with uncorrected exstrophy. Therefore even with some loss of approximation, significant correction remains in comparison with the unoperated state. The authors regard the main role of osteotomy to be relaxation of tension on the bladder, posterior urethra, and abdominal wall repair during healing. Therefore they use osteotomy rarely in newborns and young infants because ligament laxity allows the pelvis to be closed without tension. However, it becomes essential in the older child with a failed exstrophy repair, in the patient with cloacal exstrophy, and in a newborn with a wide diastasis and excellent bladder template. In patients undergoing combined exstrophy closure and epispadias repair, osteotomy allows the pubis to be joined, making it easier for the corpora to be brought over the closed proximal urethra (Gearhart et al, 1998). In a recent large series of failed exstrophy reclosures, 56 patients were found who underwent repeat pelvic osteotomy (Nelson et al, 2006). All of the reclosures were successful, and 95% had a normal gait after reoperative osteotomy. There were no femoral or sciatic nerve palsies and only five local pin site infections, which were easily treated. Thus even repeat pelvic osteotomy is safe in experienced hands.
Surgical Options in the Newborn with Classic Bladder Exstrophy
Although the modern staged repair of exstrophy (MSRE) currently enjoys widespread popularity, other methods have been described for the primary reconstruction of exstrophy. This section discusses the other types of repair, their use, and application. The outcomes and complications are discussed in separate sections because many are similar and their correction the same.
Warsaw Approach
First described by Baka-Jakubiak in 2000, this procedure involved closing the bladder, posterior urethra, pubis, and abdominal wall at the time of primary closure with or without osteotomy but always with appropriate immobilization. Osteotomy is used in all patients older than 72 hours or in any with a diastasis greater than 5 cm. A spica cast is used for 3 weeks and an elastic bandage for 3 further weeks. When the bladder had achieved suitable capacity (>70 mL) and the child was interested in continence, bladder neck repair was performed along with epispadias repair. Baka-Jakubiak has used this approach in more than 100 with classic exstrophy and complete epispadias. The intersymphyseal band is routinely divided to allow better visualization of the bladder neck and posterior urethra region. An additional proposed benefit of this procedure is that the bladder neck and posterior urethral unit is straighter at the second procedure and this allows easier catheterization and cystoscopy following reconstruction. A 10% complication rate, mainly urethral fistula or stricture, was noted in this procedure.
Kelly Repair
The radical soft tissue mobilization procedure was developed by Kelly in the late 1980s and early 1990s as a means to improve on the technique described by Ansell and to obviate pelvic osteotomies that were often required to obtain a tension-free closure. It is a multistaged repair that includes (1) closure of the bladder dome and hernia repair at birth, followed at age 3 to 6 months by (2) reconstruction of the proximal urethra with associated sphincteric tissue with penile lengthening and creation of a penoscrotal urethrostomy in males; and (3) repair of the resulting penoscrotal hypospadiac around 3 years of age. The unique aspects relate to the second stage, where a more radical mobilization of the pelvic floor muscles is performed. Specifically, the dissection includes the periosteum of the ischium and pubis where one encounters the attachment of the voluntary and involuntary sphincter muscles, as well as the pudendal nerves and vessels. The muscles are then wrapped around the reconstructed proximal urethra in an attempt to provide a continence mechanism. The neourethra itself is constructed from the urethral plate on the dorsum of the penis after it is dissected from the corporal bodies, much as in the penile disassembly technique. However, unlike the penile disassembly technique, the meatus is always brought to the penoscrotal junction and later brought distally.
Complete Repair
Developed by Mitchell, the complete repair combines the standard bladder closure with the “penile disassembly” technique for epispadias repair in an effort to decrease the number of procedures required for reconstruction and potentially provide continence without the need for formal bladder neck reconstruction (Grady and Mitchell, 1999). The urethral plate is dissected from the corporal bodies, and the urethra and bladder plate are moved posteriorly into the pelvis. Mitchell emphasizes the importance of dissecting the perineal membrane from the pubis, allowing the bladder and posterior urethra to be set deep into the pelvis. This feature is common to all properly performed exstrophy reconstructions and helps ensure success. The dissection of the urethral plate from the corpora leaves 60% to 75% of patients with hypospadias (Hafez and El-Sherbiny, 2005), and 50% of children require ureteral reimplantation in the first year of life (Grady and Mitchell, 1999).
Mainz Repair
Hohenfelner and colleagues first began using ureterosigmoidostomy for both failed exstrophy patients and for patients with small bladder templates, which led to its use as a technique in primary exstrophy closures. Beginning in 1964, this was begun in all newborns with exstrophy irrespective of bladder template size at birth. At the age of 2, ureterosigmoidostomy is performed. Some of the remnant bladder is made into a small seminal receptaculum, and the penis is reconstructed during the same procedure. In girls, the external genitalia are reconstructed and anterior fixation of the uterus is performed. In both boys and girls, cosmetic correction is usually necessary later. However, this usually means only one further operation. In 1996 Fisch and Hohenfelner modified this technique with the introduction of the Mainz Sigma pouch. This was designed to reduce intracolonic pressure and to maintain better fecal continence. Most of the patients are immediately started on oral alkalinization.
Erlangen Approach
This approach is clearly the most involved of any of the primary closure techniques. In the Erlangen approach developed by Schrott and popularized by Roesch, if the bladder template is deemed of adequate size, the “total” repair is done at 8 weeks of age. If the template is too small at birth, the bladder only is closed with bilateral groin exploration, closure of the pubis, epispadias repair, and no osteotomy. In the classic Erlangen “total repair,” the bladder is closed along with bilateral reimplantations, bladder neck plasty, bilateral groin exploration, epispadias repair, pubic closure, no osteotomy, and an epidural catheter for 5 days. Osteotomy is only done in cloacal exstrophy and reoperative closures. Thus the Erlangen repair is truly a “complete repair” encompassing all phases of exstrophy repair in one setting (Ebert et al, 2010).
Regardless of the modern method of reconstruction chosen for the newborn, certain surgical principles remain: (1) radical mobilization of the bladder and posterior urethra from surrounding tissue; (2) combined closure with epispadias repair in only carefully selected patients; (3) tension-free closure of the abdominal wall and pelvic bones with adjunctive osteotomy if needed; and (4) definition of strict criteria for selection of patients to undergo newborn closure.
Modern Reconstruction of Bladder Exstrophy
The primary objective in functional closure is to convert the bladder exstrophy into a complete epispadias with the urethra well onto the penis with completion of the epispadias in only highly select patients. The resultant incontinence with balanced posterior outlet resistance not only preserves renal function but also stimulates bladder growth. Typically, epispadias repair is now performed around 6 months of age, after testosterone stimulation. Bladder neck repair usually occurs when the child is 4 to 5 years of age, has an adequate bladder capacity, and, most importantly, is ready to participate in a postoperative voiding program. Many repairs such as the Erlangen, Kelly, and complete primary repair of exstrophy (CPRE) were purported to establish continence without the need for bladder neck repair, but recent reports have shown the need for an outlet procedure in most patients (Gearhart et al, 2007; Shoukry et al, 2009).
Bladder, Urethral, and Abdominal Wall Closure
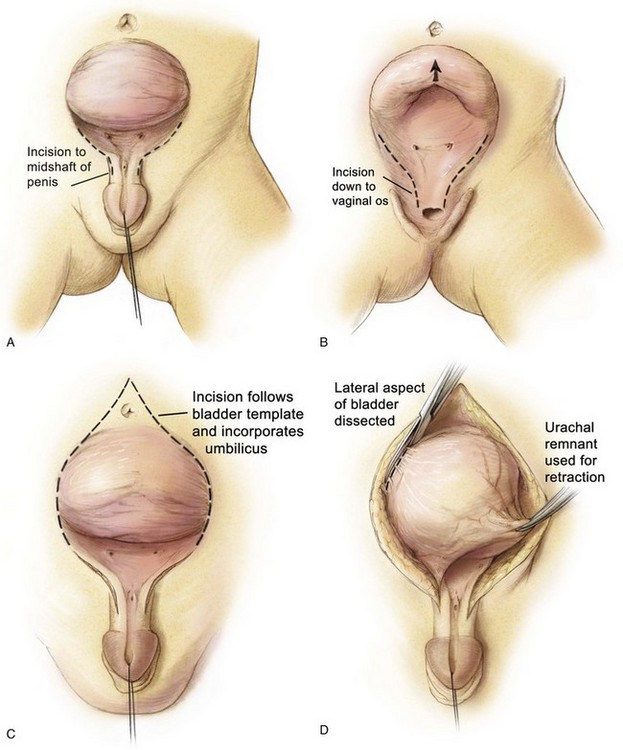
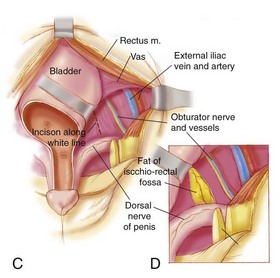
Various steps in primary bladder closure are illustrated in Figure 124–21. A strip of mucosa 2 cm wide, extending from the distal trigone to below the verumontanum in the male and to the vaginal orifice in the female, is outlined for prostatic and posterior urethral reconstruction in the male and adequate urethral closure in the female. The male urethral groove may be adequate, in which case no transverse incision of the urethral plate need be performed for urethral lengthening (see Fig. 124–21A). The authors tend to not incise a urethral plate unless the length of the urethral groove from the verumontanum to the urethral glans is so short that it interferes with eventual penile length and produces dorsal angulation. If so, the urethral groove is lengthened after the manner of Johnston (1974) or Duckett (1977). The drawings in Figure 124–21B and C show marking of the incision just above the umbilicus, down the junction of the bladder and paraexstrophy skin, to the level of the urethral plate.
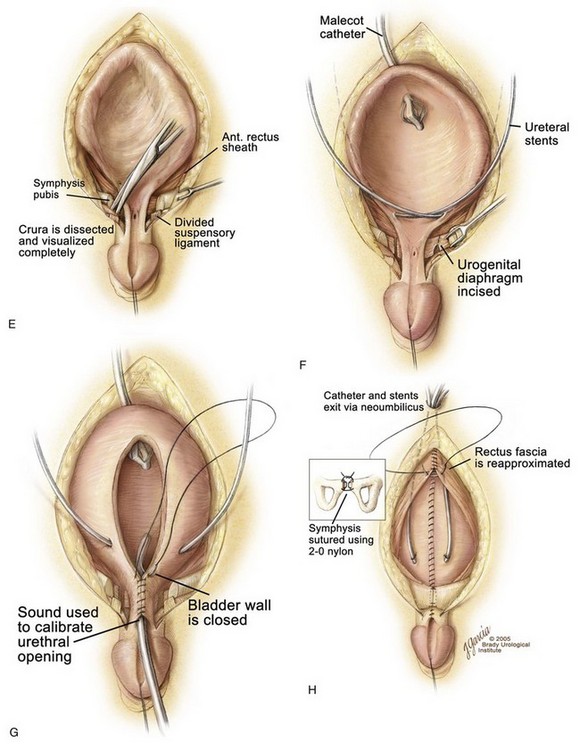
An appropriate plane is entered just above the umbilicus, and a plane is established between the rectus fascia and the bladder (see Fig. 124–21D). The umbilical vessels are doubly ligated and incised and allowed to fall into the pelvis. The peritoneum is taken off the dome of the bladder at this point so that the bladder can be placed deep into the pelvis at the time of closure. The plane is continued caudally down between the bladder and rectus fascia until the urogenital diaphragm fibers are encountered bilaterally. The pubis is encountered at this junction, and a double-pronged skin hook can be inserted into the bone at this time and pulled laterally to accentuate the urogenital diaphragm fibers. This helps the surgeon radically incise these fibers among the bladder neck, posterior urethra, and pubic bone (see Fig. 124–21E). Gentle traction on the glans at this point shows the insertion of the corporal body on the lateral inferior aspect of the pubis. These urogenital diaphragm fibers are taken down sharply with electrocautery down to the pelvic floor in their entirety (see Fig. 124–21F). If this maneuver is not performed adequately, the posterior urethra and bladder will not be placed deeply into the pelvis, and when the pubic bones are brought together, the posterior vesical unit will be brought anteriorly into an unsatisfactory position for later Cantwell-Ransley epispadias repair. If the urethral plate is left in continuity, it must be mobilized up to the level of the prostate in order to create as much additional urethral and penile length as possible. Further urethral lengthening can be performed at the time of epispadias repair.
Penile lengthening is achieved by exposing the corpora cavernosa bilaterally and freeing the corpora from their attachments to the suspensory ligaments on the anterior part of the inferior pubic rami. However, because Silver and colleagues (1997b) showed that there is a 50% shortage of length of the corporal bodies in exstrophy versus normal controls, any penile length that is obtained is more correction of chordee and change in angulation of the penis rather than true penile lengthening. The wide band of fibers and muscular tissue representing the urogenital diaphragm is detached subperiosteally from the pubis bilaterally (see Fig. 123–21F). Reluctance to free the bladder neck and urethra well from the inferior ramus of the pubis moves the neobladder opening cephalad should any separation of the pubis occur during healing, thus increasing the chance of bladder prolapse. The mucosa and muscle of the bladder, bladder neck, and urethra are then closed well onto the penis in the midline anteriorly (see Fig. 123–21G). This should be tapered over a 10- to 12-Fr sound comfortably. The size of the opening should allow enough resistance to aid in bladder adaptation and to prevent prolapse but not enough outlet resistance to cause upper tract changes. The posterior urethra and bladder neck are buttressed with a second layer of local tissue if possible. If a CPRE is performed, a penile disassembly is done with many of the patients being made hypospadiac. The bladder is drained by a suprapubic non-latex Malecot catheter for a period of 4 weeks. The urethra is not stented in order to avoid necrosis with accumulation of secretions in the neourethra. Stents provide drainage during the first 10 to 14 days after closure because swelling caused by the pressure of closure of a small bladder can obstruct the ureters and give rise to obstruction and transient hypotension. If there are no problems with the stents during healing, the authors leave the stents in as long as 2 to 3 weeks.
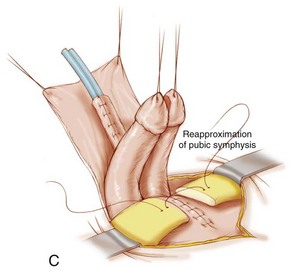
When the bladder and urethra have been closed and the drainage tubes placed, pressure over the greater trochanters bilaterally allows the pubic bones to be approximated in the midline. Horizontal mattress sutures are placed in the pubis and tied with a knot away from the neourethra (see Fig. 123–21H). Often, it is possible to use another stitch of No. 2 nylon at the most caudal insertion of the rectus fascia onto the pubic bone. This maneuver adds to the security of the pubic closure. A V-shaped flap of abdominal skin at a point corresponding to the normal position of the umbilicus is tacked down to the abdominal fascia, and a drainage tube exits this orifice. The method described by Hanna (1986) is our most commonly performed procedure. Before and during the procedure, the patient is given broad-spectrum antibiotics in an attempt to convert a contaminated field into a clean surgical wound.
If the decision is made at this point that the urethral groove is to be transected, the groove is cut distal to the verumontanum with continuity maintained between the thin, mucosal, non–hair-bearing skin adjacent to the posterior urethra and bladder neck and the skin and mucosa of the penile skin and glans. Flaps in the area of the thin skin are subsequently moved distally and rotated to reconstruct the urethral groove, resurfacing the penis dorsally. The corporal bodies are not brought together at this juncture because later Cantwell-Ransley repair is planned.
Combined Bladder Closure and Epispadias Repair
The modern staged repair of bladder exstrophy has yielded consistently good cosmetic and functional results, and the utilization of osteotomy has improved the potential for successful initial closure and later continence. In an effort to decrease costs, decrease the morbidity associated with multiple operative procedures, and possibly affect continence, there has been interest in performing single-stage reconstruction or combining procedures in appropriately selected patients. This technique was first described by Lattimer and Smith (1966) but was abandoned in the 1970s because of high complication and failure rates. The technique was revisited by Gearhart and Jeffs (1991a) for failed exstrophy closures and more recently by Grady and Mitchell for newborn patients (1999). In the combined exstrophy and epispadias repair, bladder closure is combined with the modified Cantwell-Ransley epispadias repair (Gearhart et al, 1998; Baird et al, 2005c). This technique can be applied to both delayed primary closure and failed closures. As with the complete repair, many require early ureteral reimplantation, but none were made hypospadiac.
Results have now emerged in groups of boys undergoing single-stage reconstruction (bladder closure and epispadias repair) in infancy (Gearhart et al, 1998; Baird et al, 2005c). In our opinion, this technique should be limited to boys of older age (older than 4 to 6 months) because of evidence indicating that potential complication of these combined procedures is significant loss of penile and corporal tissue that makes further reconstruction problematic (Husmann and Gearhart, 2004). Patients should be carefully selected, and utilization of these extensive procedures by the occasional exstrophy surgeon is not recommended. Selection should take into account phallic size and length, depth of the urethral groove, and size of the bladder template in those with delayed primary closures, as well as perivesical and urethral scarring in those who have undergone a prior failed closure (Gearhart and Jeffs, 1991a; Gearhart et al, 1998; Baird et al, 2005c).
Management after Primary Closure
The initial step of the MSRE converts a patient with exstrophy into one with penile epispadias and incontinence. Before removal of the suprapubic tube, 4 weeks after surgery, the bladder outlet is calibrated by a urethral catheter or a urethral sound to ensure free drainage. A complete ultrasound examination is obtained to ascertain the status of the renal pelves and ureters, and appropriate urinary antibiotics are administered because all patients have reflux after closure. Residual urine is estimated by clamping the suprapubic tube, and specimens for culture are obtained before the patient leaves the hospital and at subsequent intervals to detect infection and ensure that the bladder is empty. If the initial ultrasound examination shows good drainage, upper tract imaging by ultrasonography is repeated 3 months after discharge from the hospital and at intervals of 6 months to 1 year during the next 2 to 3 years to detect any upper tract changes caused by reflux, infection, or obstruction. Prophylactic antibiotics should be continuous because all patients with bladder exstrophy, once closed, have vesicoureteral reflux. If a useful continence interval has resulted from the initial closure, a further operation for incontinence may not be required; however, this situation is quite unusual. In the authors’ experience with more than 85 primary closures, this has occurred in three patients, all of whom were girls. After the conversion from exstrophy to complete epispadias with incontinence, the bladder gradually increases in capacity as inflammatory changes in the mucosa resolve.
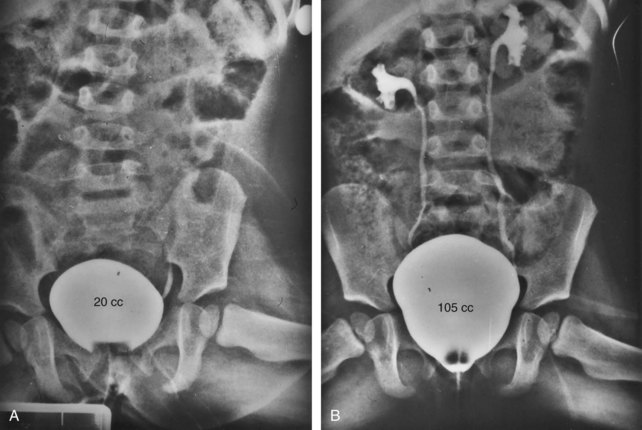
Cystoscopy and cystography at yearly intervals are used to evaluate the degree of reflux noted in almost 100% of patients and to provide an estimate of bladder capacity (Gearhart and Jeffs, 1998). Even in a completely incontinent patient, bladder capacity gradually increases to a point at which the bladder can be distended at cystography to its true capacity. This must be done under anesthesia in young children because the values obtained differ markedly from those obtained when trying to fill the bladder of a crying, squirming infant on an x-ray table (Gearhart and Jeffs, 1998). If the bladder has not achieved a capacity of at least 30 mL by 1 to 2 years, concern must be voiced to the parents about the overall ability of the bladder to undergo a continence procedure. Currently, the only parameters available to predict overall success are the size of the bladder template at birth and a successful primary closure with absence of infections.
Should bladder outlet resistance be such that urine is retained within the bladder and reflux and ureteral dilatation develop with infected urine, it may be necessary to dilate the urethra or to begin intermittent catheterization (Baker et al, 1999). Sometimes the posterior urethral obstruction can be such that it requires a transurethral incision of the stricture to maintain an adequate posterior urethral outlet. If bladder outlet resistance persists and infections continue, an antireflux procedure may be required as early as 6 months to 1 year after initial closure (Mathews and Gearhart, 2003). After primary closure with the CPRE repair, 50% will require ureteral reimplantation in the first year after closure. Because of this problem, some units are trying ureteral reimplantation at the time of bladder closure but the numbers of patients treated in this fashion is small (Braga et al, 2008a). If severe upper tract changes occur, surgical revision of the bladder outlet by advancing skin flaps into the orifice or even patching the stricture may be necessary to prevent scarring and further obstruction. As mentioned previously, transurethral incision of the urethral stricture to obtain a balanced outlet should be tried before surgical revision. Judgment is required to know when to avoid attempts at functional closure and to know when to return to urinary diversion as a means to preserve renal function. This change of plan is seldom necessary if an adequate outlet has been constructed at the initial closure and if careful attention has been paid to the details of follow-up of the bladder and posterior urethra. An important caveat is that if there are recurrent urinary tract infections (UTIs) and if the bladder is distended on ultrasonography, cystoscopy should be performed and the posterior urethra should be carefully examined anteriorly for erosion of the intrapubic stitch, which may be the cause of the recurrent infections (Baker et al, 1999). If the intrapubic stitch is seen in the posterior urethra, a small suprapubic incision should be made and the stitch should be removed, or, if it can be grasped, it should be removed transurethrally. Husmann and colleagues (1990) have shown acceptable levels of upper tract function after primary closure as long as prophylactic antibiotics were used after initial closure and elevated urinary residuals were kept below 50 mL.
Selected Technical Aspects of Other Methods of Closure
Kelly Repair
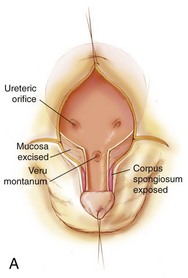
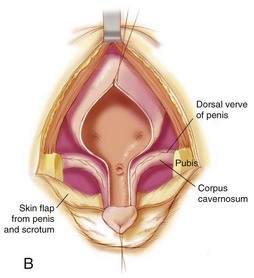
In the first stage of the Kelly repair the bladder is closed up to the level of the posterior urethra with no attempt to bring the pubic bones into apposition. The second step of the Kelly repair, which differs from other types of repair, is the “radical soft tissue mobilization.” This is accomplished by making an incision around the old bladder closure and by making parallel incisions on the urethral plate extending proximally to halfway between the ureteric orifices and the verumontanum. This maneuver helps expose the corpora laterally as they move over toward their insertion onto the pubis. The mucosa lateral to the urethra is excised, as well as some of the mucosa of the bladder (Fig. 124–22A and B). The initial lateral extension of the incision exposes the dorsal nerves of the penis and the attachment of the corpora to the pubis (Fig. 124–22B). The urethral plate is mobilized from the corporal bodies and moved to below them to create a penoscrotal hypospadias.
Mitchell Repair
The Mitchell repair, as in all closures, is best performed in the newborn period. The closure technique is much the same as in all methods of closures as far as preoperative evaluation and postoperative care. The main difference is that the urethra is separated from its attachments to the underlying corporal bodies and pelvic diaphragm. Mitchell purports that this allows better posterior positioning of the bladder neck and posterior urethra into the pelvis. This combined bladder closure with epispadias repair was originally thought to be sufficient for the patient to achieve urinary continence, but this has been found not to be the case in that most of these patients require bladder neck repair (Shoukry et al, 2009).
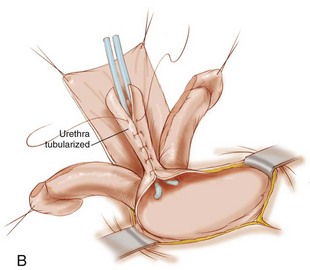
The closure is begun in the standard fashion, but the incision is carried out onto the urethral plate taking care to preserve its blood supply and avoid corporal injury. The penis is then disassembled into three components: (1) right corpus; (2) left corpus; and (3) urethral wedge (Fig. 124–23A). The dissection moves cranially, and the intersymphyseal band is incised. This is also accomplished as in the MSRE by going medial to the inferior aspect of the pubis and cutting these fibers from their posterior urethral attachment all the way to the levator hiatus (Gearhart et al, 2007). After standard closure of the bladder, the urethra is closed and an attempt to move the urethra to the tip of the penis is made (Fig. 124–23B). If it does not reach the tip of the penis, the urethra is taken to the base of the penis in a hypospadiac position in a majority of patients (Fig. 124–23C).
Penile and Urethral Closure in Exstrophy
Epispadias Repair
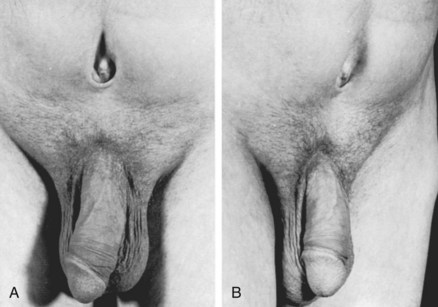
Historically, bladder neck reconstruction was performed before penile and urethral reconstruction. However, an increase in bladder capacity in patients with extremely small bladder capacities after epispadias repair prompted a change in the management program (Gearhart and Jeffs, 1989a) (Fig. 124–24A and B). In a group of patients with a small bladder capacity after initial closure, there was a mean increase of 55 mL in males in only 22 months after epispadias repair. However, with MSRE, the epispadias repair is now performed at about 6 months of age in all patients. With this modification, possibly all patients can achieve an appropriate capacity at the time when they are ready physically and mentally to undergo bladder neck reconstruction. Because most boys with exstrophy have a somewhat small penis and a shortage of available penile skin, all patients undergo testosterone stimulation before urethroplasty and penile reconstruction (Gearhart and Jeffs, 1987).
Many techniques have been described for reconstruction of the penis and urethra in patients with classic bladder exstrophy. Current methods of epispadias repair in bladder exstrophy are the Cantwell-Ransley repair (1989), the modified Cantwell-Ransley repair (1992, 1995), and the penile disassembly technique described by Mitchell and Bagli (1996).
Regardless of the surgical technique chosen for reconstruction of the penis in bladder exstrophy, four key concerns must be addressed to ensure a functional and cosmetically pleasing penis. These concerns are (1) correction of dorsal chordee, (2) urethral reconstruction, (3) glanular reconstruction, and (4) penile skin closure.
Although it is possible to achieve some penile lengthening with release of chordee at the time of initial closure, it is often necessary to perform formal penile elongation with release of chordee at the time of urethroplasty in exstrophy patients. Data of Silver and colleagues (1997b) clearly showed that this is more an apparent lengthening of the penis than a true lengthening because the anterior corporal bodies in exstrophy patients have 50% less length than those of age-matched controls in adult life. Certainly, all remnants of the suspensory ligaments and old scar tissue from the initial bladder closure must be excised. Also, further dissection of the corpora cavernosa from the inferior pubic ramus can be achieved. It is often surprising how little is accomplished in freeing the corporal bodies from the pubis at the time of initial exstrophy closure (Gearhart, 1991).
Lengthening of the urethral groove is also essential. Whether or not paraexstrophy skin flaps are used at the time of bladder closure, further lengthening is necessary. This can range from something as simple as Cantwell’s original procedure to transection of the urethral plate and replacement of the genital tissue. However, in modern techniques of epispadias repair, replacement of the urethra is not typically performed. In the penile disassembly technique described by Mitchell and Bagli (1996), the urethral plate is dissected completely from the glans; in more than 70% of patients it is not long enough to reach the tip of the penis, and as a result hypospadias is present with further reconstruction needed at another setting (Hafez and El-Sherbiny, 2005). The authors believe that because of the marked length disparity in male children with exstrophy compared with normal males, complete detachment of the urethra from the glans is an unnecessary step in light of the small amount of length that is obtained with this maneuver and the near certainty of creating hypospadias that, with the paucity of skin in this condition, can be difficult to repair and many end up with a coronal urethral opening.
Chordee
Besides lengthening of the urethral groove, dorsal chordee must be addressed. To release dorsal chordee, one may lengthen the dorsomedial aspect of the corpora by incision and anastomosis of the corpora themselves (Gearhart et al, 1992). Also, length can be gained by placement of a dermal graft to allow lengthening of the dorsal aspect of the corpora (Woodhouse, 1986). Another technique to improve dorsal chordee before urethroplasty is shortening or medial rotation of the ventral corpora (Koff and Eakins, 1984). However, most of these techniques, especially grafting, are reserved for patients who are seen in adolescent and adult years for epispadias surgery with the need for some increased penile length and correction of residual chordee.
Urethral Reconstruction
Urethral reconstruction is an important aspect of external genital reconstruction in exstrophy. This can be accomplished by many previously reported methods. Tubularization of the dorsal urethral groove as a modified Young urethroplasty has been abandoned in the authors’ practice because of the high incidence of urethrocutaneous fistula and the less than optimal cosmetic appearance of the penis (Lepor et al, 1984). In the past, interposition of a free graft of genital or extragenital skin, or even bladder mucosa, has been used (Hendren, 1979). In addition, ventral transverse island flaps and double-faced island flaps have been used with some success for urethral reconstruction in exstrophy patients (Thomalla and Mitchell, 1984; Monfort et al, 1987). However, modern techniques of epispadias repair associated with bladder exstrophy use tubularization of the urethral plate, moving the urethral plate under the corporal bodies after closure to lessen the incidence of urethrocutaneous fistula and also to give the penis a more downward deflection and more easily catheterizable urethral channel (Surer et al, 2001a). With the penile disassembly technique, interrupted sutures are often used to try and get the urethra to the tip of the penis again after it is completely detached with resultant hypospadias in many.
Penile Skin Closure
If skin closure continues to be a problem in genital reconstruction, owing to the paucity of skin associated with this condition, a Z-plasty incision and closure at the base of the penis prevents skin contraction and upward tethering of the penis. The ventral foreskin can be split in the midline and brought to the dorsum as lateral preputial flaps for coverage of the penile shaft. If the flaps are a bit asymmetrical, a staggered dorsal suture line results, with less upward tethering. Alternatively, a buttonhole can be created in the ventral foreskin and simply transposed to the dorsum for additional penile skin coverage.
Modified Cantwell-Ransley Repair
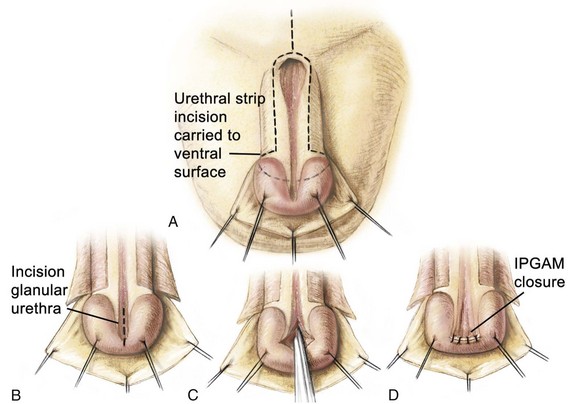
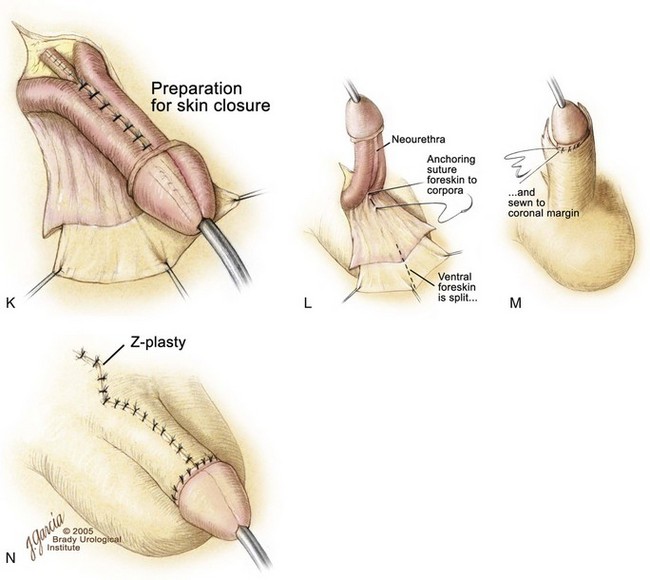
The authors’ preference for urethroplasty and penile reconstruction, if the urethral groove has adequate length, is the modified Cantwell-Ransley repair (Gearhart et al, 1992, 1995c, 2004) (Fig. 124–25). Currently, in the modern applications of the staged reconstruction of bladder exstrophy, epispadias repair is performed when the child is 6 months to 1 year of age. The modified Cantwell-Ransley procedure is begun by placing an island stitch through the glans as a traction stitch. Incisions are made over two parallel lines marked previously on the dorsum of the penis that outline an 18-mm-wide strip of urethral mucosa, extending from the prostatic urethral meatus to the tip of the penis (see Fig. 124–25A). For this procedure, a deep vertical incision is made in the urethral plate distally. The incision is then closed with 6-0 polyglycolic sutures in a transverse fashion (see Fig. 124–25B to D). This procedure flattens the distal urethral plate and advances the urethra to the tip of the phallus so that it will be in excellent glanular position when the glanular wings are closed over the reconstructed urethra. Glanular mucosal areas of the dorsal glans are excised adjacent to the urethral strip, and thick glandar flaps are constructed bilaterally (see Fig. 124–25F). Lateral skin flaps are mobilized and undermined. A Z-incision of the suprapubic area permits wide exposure and division of the suspensory ligaments and old scar tissue from initial exstrophy closure (see Fig. 124–25F).
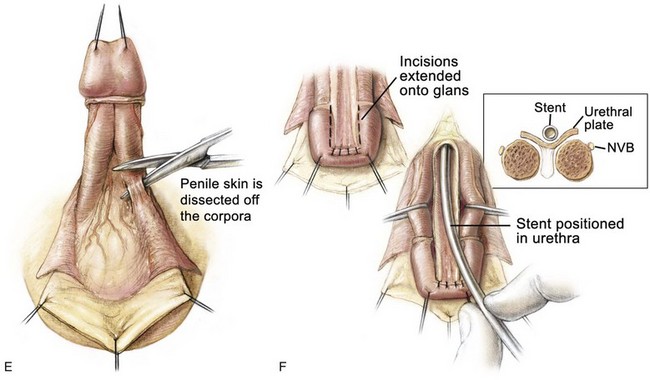
Ventral penile skin is taken down to the level of the scrotum (see Fig. 124–25E). Care is taken to preserve the mesentery to the urethral plate, which arises proximally and extends upward between the corporal blood supply to the urethral plate. Dissection of the corpora is begun ventrally with dissection on the surface of Buck fascia covering the corporal bodies. The plane is followed closely until one exits on the dorsum of the penis between the corpus spongiosum and the corporal body, first on one side and then on the other (see Fig. 124–25F and G). The loops are placed around the corporal bodies, and the dissection is extended proximally on the corpora to dissect the urethral plate free from the corporal bodies up to the level of the prostate. Although one might expect difficulties when dissecting proximally where the paraexstrophy skin flaps had been sutured to the urethral plate, this has not been encountered in the authors’ experience, and dissection is kept just on the corporal bodies while proceeding proximally. The urethral plate is also dissected distally past the level of the junction of the glans with the corporal bodies. In this manner, adequate mobilization is obtained, and it is not difficult to bring the corporal bodies over the urethra at the level of the corona. This almost separates the penis into three components: the two corpora and the urethral plate (see Fig. 124–25F). Complete penile disassembly is not undertaken, and the distal-most 1-cm attachment of the mucosa plate to the glans is left intact (Surer et al, 2001a; Gearhart et al, 2004).
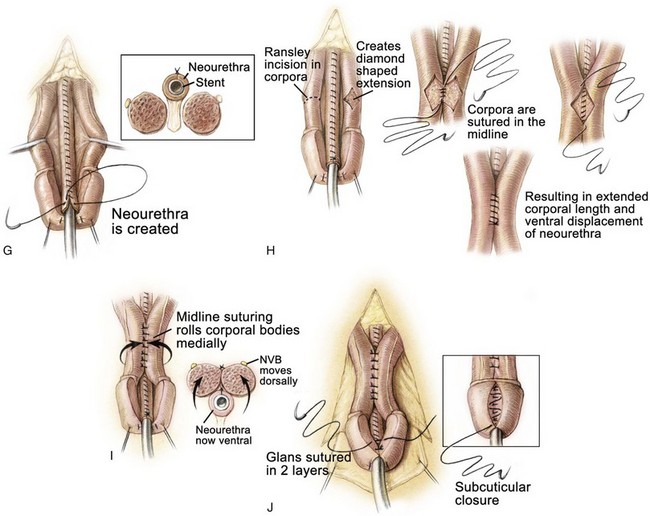
The neurovascular bundles, situated between Buck fascia and the corporal wall, are typically left intact in young patients if rotation of the corporal bodies over the urethra effectively straightens the penis. If not, the neurovascular bundles are dissected free from the corporal bodies, with vessel loops being placed around these structures so that the neurovascular bundles will not be compromised when incisions are made in the corpora and the corpora are rotated medially over the neourethra (see Fig. 124–25H). After the corporal bodies are incised or rotated over the urethra, the urethral strip is closed in a linear manner from the prostatic opening to the glans over an 8-Fr silicone stent with 6-0 polyglycolic sutures. After this is accomplished, incisions are made in the corporal bodies at the point of maximum curvature, opening a diamond-shaped effect in the erectile tissue (see Fig. 124–25H). The corpora are then closed over the neourethra with two running sutures of 5-0 polydiaxone, and the diamond-shaped defects in the adjacent area of the corpora are sutured to each other. This procedure effectively displaces the urethra ventrally in a normal position. This not only causes the downward deflection of the penis but also allows some additional length by dorsal rotation and approximation of the corporal bodies over the neourethra. After the urethra has been transferred to the ventrum, further sutures of 4-0 polyglycolic acid are placed between the corporal bodies to bury the urethra further, especially at the level of the junction of the glans and the corporal bodies at the corona (see Fig. 124–25I).
The glans wings are then closed over the glanular urethra using subcuticular sutures of 5-0 polyglycolic acid, and the glans epithelium is closed with 6-0 polyglycolic acid sutures (see Fig. 124–25I). The ventral skin is then brought up and sutured to the ventral edge of the corona, and the flaps are fashioned to provide adequate coverage and lengthening of the dorsum of the penis. The skin is reapproximated with interrupted 5-0 or 6-0 polyglycolic sutures (see Fig. 124–25K to M). A Z-plasty at the base of the penis is closed with interrupted 5-0 or 6-0 polyglycolic acid sutures. A silicone stent is left indwelling in the neourethra to provide drainage for 10 to 12 days (see Fig. 124–25N).
Postoperative Problems
Postoperative pain and bladder spasms after extensive external genital reconstructive surgery require a combined effort of the pediatric anesthesia pain service and the surgical service. Controlling bladder spasms is paramount because they are associated with urinary extravasation and fistula formation. All of our patients have a caudal epidural catheter placed at the time of surgery to help with postoperative pain control and bladder spasms. In addition, oxybutynin is started immediately after surgery to decrease the incidence of bladder spasms and enhance the patient’s comfort. At the time of discharge, the plastic dressing on the penis is left intact and the child is discharged with narcotics, antispasmodics, and appropriate broad-spectrum antibiotic coverage.
Penile Disassembly Epispadias Repair
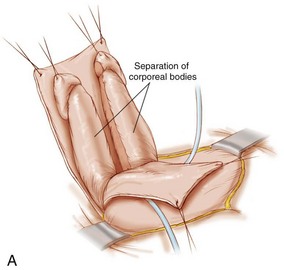
This technique of epispadias repair was developed by Mitchell and Bagli (1996). It has now been incorporated in the CPRE exstrophy repair for primary closure in the newborn. The authors feel this approach better recapitulates the anatomy of the penis and allows better placement of the bladder/urethra into the pelvis.
The penis is dissected into three components—the right and left corpora with their associated hemiglans and the urethral wedge (urethral plate with its associated spongiosa) (see Fig 124–23). The dissection as in the Cantwell-Ransley repair begins on the ventral aspect of the penis. The dissection moves medially just above Buck fascia but is taken down to the tunica albuginea of the corpora. Care is taken to preserve the spongiosum with the urethral wedge, and this dissection is carried posteriorly to the area of the bladder neck. The penis is then separated into the three previously mentioned components. Several authors have found problems getting the reconstructed urethra back to the tip of the glans and have recommended interrupted suturing of the urethral plate or leaving the most distal part of the urethral plate attached to the glans as in the Cantwell-Ransley repair. Others have found the need to make the patients hypospadiac and then perform a later hypospadias repair due to a shortened urethral plate. The rate of hypospadias created has been reported to be from 30% to 70% of cases. If the urethra can be brought up to each hemiglans, an orthotopic meatus is configured. The glans is brought together using interrupted mattress sutures of PDS followed by horizontal mattress sutures of 7-0 nonfilament sutures to bring the glanular epithelium together. The urethra is matured to the glans with interrupted sutures of 7-0 braided polygalactin sutures. The corpora are then brought over the reconstructed urethra with interrupted sutures. Skin closure is similar to that of standard hypospadiac with fine absorbable sutures. If concomitant exstrophy closure is to be done, the dissection then moves proximally into the pelvis to be able to move the structures into the pelvis (see section on CPRE repair).
Female Exstrophy
The principles of repair of both female and male exstrophy are similar. However, in the female the authors recommend the following: (1) radical dissection of the bladder and urethra from surrounding structure; (2) dissection of the lateral aspects of the posterior vesicourethral and vaginal unit from their attachments to the pelvic floor; (3) tension-free closure of the abdomen, bladder/urethra, and external genitalia; and (4) judicious use of osteotomy and proper immobilization of the pelvis and extremities.
As in the male, if the pelvic bones are separated more than 4 cm or are not malleable under anesthesia, an osteotomy is then performed. The transverse innominate osteotomy along with vertical iliac osteotomy is commonly used in our practice. Intrafragmentary pins are placed, and the fixator is applied after the soft tissue surgery has been completed. An interesting finding in more than 928 exstrophies in the authors’ database is that even with a large bladder template the diastasis tends to be narrower than in males. Thus female exstrophy patients are easier to close and do not require an osteotomy as commonly as males.
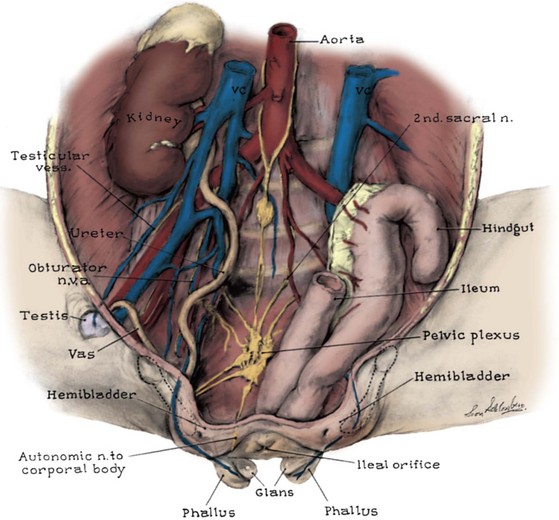
The vagina is prepped with an iodine prep so that if it is violated during the dissection, it can simply be closed. In the closure, the authors start their dissection along the medial aspect of the clitoral/corporal halves and move deeply into the pelvis. Pinpoint electrocautery at a low setting is used to limit both bleeding and tissue injury. The dissection of the vagina proceeds laterally and posteriorly. The urethrovaginal septum is left intact to preserve its blood supply. As in the male, the dissection proceeds posterior and downward until the levator hiatus is reached with the urogenital diaphragm fibers lateral and posterior to the vesicourethral plate and vaginal incision. This maneuver allows placement of the unit deeply into the pelvis, and more importantly it keeps it from being displaced anteriorly when the pelvic bones are brought together.
The bladder is closed with a single figure-of-eight layer of 3-0 polygalactin suture to maximize postclosure bladder volume. The urethra is closed with a single layer of 4-0 to 5-0 polygalactin depending on the thickness of the tissue. An indwelling suprapubic tube is brought out through the dome of the bladder along with two small feeding tubes, which act as stents and are left in place for 4 full weeks and exit the neoumbilicus. No tubes are brought out through the neourethra because this can be associated with wound infection and prolapse or dehiscence. A No. 2 nylon suture in a horizontal mattress fashion is used to bring the pubic bones into apposition. Once the pubic bones are brought together, a routine subcutaneous and skin closure is performed. A mons plasty is then performed, and the subcutaneous tissues of the clitorides are brought together with 5-0 polygalactin and the epithelium with 6-0 polygalactin sutures. If needed, a Y-V labioplasty is performed for better exteriorization of the vaginal introitus.
If an osteotomy has been performed, the external fixator is then placed and a pelvic radiograph obtained. If pin and fixator placement are optimal, then the infant is placed in modified Buck traction for 4 weeks. If an osteotomy was not performed, the infant is placed in Bryant traction for 4 weeks. Postoperative care is the same as in the male with excellent pain control delivered by an indwelling epidural catheter and bladder spasms controlled by both the epidural and oral oxybutinin.
Continence and Antireflux Procedure
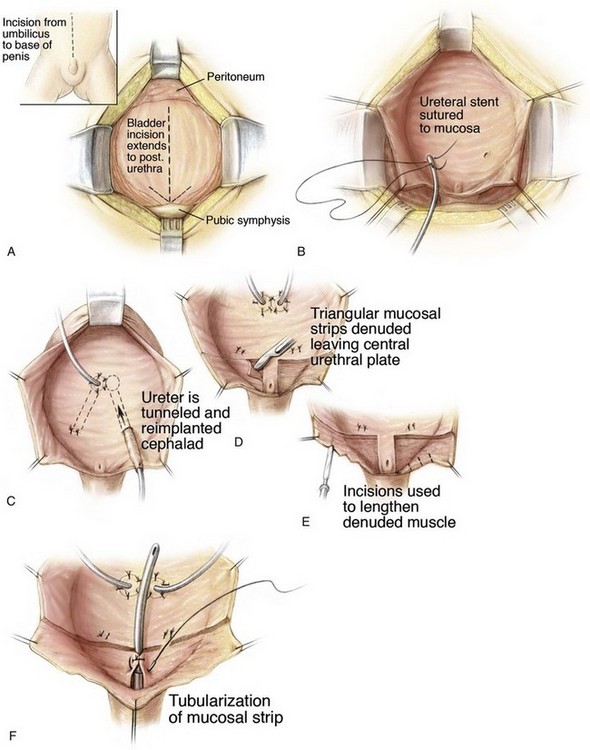
Although some modern exstrophy repairs claim to establish suitable continence without formal bladder neck repair, many patients are referred to our unit still incontinent after only prior newborn repair or after newborn repair and multiple injections of various substances into the bladder neck area. Likewise, other groups have reported their outcomes with newborn repair and most recommended some sort of outlet procedure. In our unit each child has a cystoscopy and gravity cystogram under anesthesia yearly after newborn closure to assess bladder growth. Prior data by Gearhart and Jeffs (1989b) have shown that after newborn exstrophy closure there should be an average increase in bladder capacity by 54 mL in 24 months. Recently, Purves and colleagues (2009b) found that, in selected exstrophy patients who underwent closure, epispadias repair, and bladder neck reconstruction at the authors’ institution, a median bladder capacity of 100 mL was more common in the group that was completely dry after bladder neck reconstruction. Most of these children were 4 to 5 years of age and were ready emotionally, maturationally, and intellectually to participate in a postoperative voiding program. Continence and antireflux procedures performed at the authors’ institution are illustrated in Figure 124–26. The bladder is open through a transverse incision at the bladder neck with a vertical extension. The later midline closure of this incision is the width of the bladder neck and enlarges the vertical dimension of the bladder, which in exstrophy is often short (see Fig. 124–26A). A Cohen transtrigonal ureteral reimplantation or a cephalotrigonal reimplantation is performed to move the ureter across the bladder, above the trigone, or to direct the ureter cephalad up onto the edge of the trigone (see Fig. 124–26B and C) (Canning et al, 1992). In addition, if the ureters are low on the trigone, there is a need to move the ureteral hiatus higher on the trigone. The hiatus is simply cut in a cephalad direction, and cross-trigonal reimplants are performed at the upper aspect of the trigone (see Fig. 124–26C).
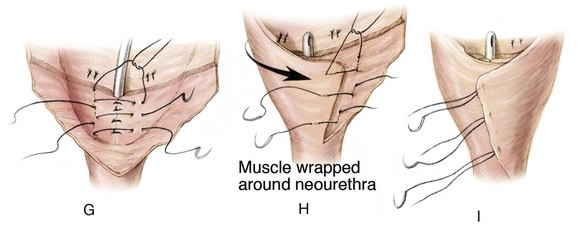
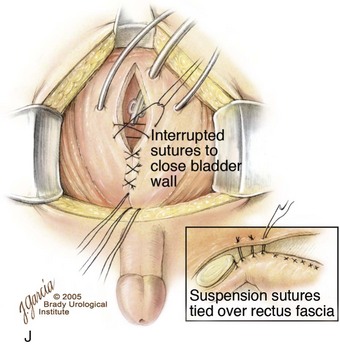
The continence procedure is begun by selecting a posterior strip of mucosa 15 to 18 mm wide and 30 mm long that extends distally from the midtrigone to the prostate or posterior urethra (see Fig. 124–26D). Bladder muscle lateral to the mucosal strip is denuded from the mucosa. It is often helpful at this juncture to use one or two epinephrine-soaked sponges to aid in the control of bleeding and the visualization of the denuded area. Tailoring of the denuded lateral muscle triangles is aided by multiple small incisions on the free edge bilaterally that allow the area of the reconstruction to assume a more cephalad position (see Fig. 124–26E). These muscle flaps are not only smaller but also not incised transversely at their cephalad extent as described in the original Young-Dees procedure. The authors believe that, if these flaps are incised medially at the cephalad border only where they join the floor of the bladder, there is a significant risk of denervation and ischemia that will harm the bladder neck repair. The basic premise is to create a mucosa-lined tube inside a muscular funnel that narrows from its junction with the floor of the bladder that extends caudally. The edges of the mucosa and underlying muscle are closed with interrupted sutures of 4-0 polyglycolic acid (see Fig. 124–26F). The adjacent denuded muscle flaps are overlapped and sutured firmly in place with a 3-0 polydiaxone suture to provide reinforcement of the bladder neck and urethral reconstruction (see Fig. 124–26G to I). An 8-Fr urethral stent may be used as a guide during urethral reconstruction, but it is removed after the bladder neck reconstruction is completed. After the bladder neck repair is completed, the repair is suspended to the rectus fascia (see Fig. 124–26J; see inset).
Very radical dissection of the bladder, bladder neck, and posterior urethra is required, not only within the pelvis but also from the posterior aspect of the pubic bar to provide enough mobility for the bladder neck reconstruction. This maneuver allows adequate bladder neck narrowing and tightening of the bladder neck repair and subsequent anterior suspension of the newly created posterior urethra and bladder neck. In patients who present after CPRE repair in the newborn period and who had a vesicocutaneous fistula after closure, great care must be taken anteriorly during mobilization of the bladder neck because the tissues are more adherent to the back of the intrasymphyseal bar than in those who underwent bladder neck tailoring as typically seen in that repair. If visualization of the posterior urethra is problematic, the intrasymphyseal bar can be cut, thus providing a widened field of exposure. The intrasymphyseal bar is approximated with 2-0 sutures of polydioxanone. If the intrasymphyseal bar is cut, mobility of the child should be restricted in the postoperative period to allow proper healing of the intrasymphyseal bar.
Postoperative Care
Ureteral stents are placed in the reimplanted ureters and brought out through the wall of the bladder, and the bladder is drained by suprapubic tube, which is left indwelling for a 3-week period. At the end of 3 weeks the suprapubic tube is clamped, and the patient is allowed to attempt to void. Initially, the tube should not be clamped for more than 1 hour. If voiding does not occur, the child is given an anesthetic and an 8-Fr Foley catheter is placed. This is left in place for 5 days and removed, and then another voiding trial is begun. This part of the postoperative period is most demanding on the patient, family, and surgeon. Some children require several catheter placements before voiding is initiated. If the child can empty the bladder satisfactorily, the suprapubic tube is removed. Frequent bladder and renal ultrasound examinations are required in the first few months after bladder neck repair.
Modern Initial Repair of Bladder Exstrophy: Outcomes and Results
The use of functional reconstruction in bladder exstrophy has resulted in dramatic improvement in the success of reconstruction. Several recent series (Purves et al, 2008, 2009b; Grady and Mitchell, 1999) have demonstrated the success and applicability of early newborn closure with or without pelvic osteotomy to bladder exstrophy. Other important but older series have shown acceptable continence rates with preservation of renal function in a majority of patients treated in early life (Table 124-1).
Table 124–1 Urinary Continence after Functional Bladder Closure
Two of the most reliable predictors of eventual urinary continence are the size of the bladder template at birth and a successful primary closure. Regardless of the technique used, a complication-free newborn closure of the abdomen, pelvis, bladder, and proximal/complete urethra paves the way for an optimal long-term result. A large series by Surer and colleagues (2001b) has demonstrated in a large group of exstrophy patients who underwent early closure with importance of a successful primary closure. Sixty-eight patients (57 males and 11 females) were referred for bladder neck reconstruction after primary closure at many other centers. Twenty percent had concomitant pelvic osteotomy at the time of closure. The majority of patients were closed within the first 72 hours of life. Mean capacity at the time of bladder neck repair was 121 mL. Eighty-three percent are continent and voiding per urethra. This application of early successful closure and follow-up reconstruction by a second surgeon shows convincingly that a successful primary closure is one of the most important determinants of eventual bladder capacity and compliance regardless of who originally performed the repair.
Initial Closure
Long-term data on all modern types of exstrophy repair can be difficult to obtain. Nonetheless, this section deals with what has been gleaned from the recent literature. Most data come from the MSRE and CPRE groups. In a large series reported by Purves and colleagues (2008), 189 patients who had undergone primary closure between 1988 and 2004 were extracted. The records of 131 patients (95 males) who underwent MSRE with a modified Cantwell-Ransley repair by a single surgeon in 1988-2004 were reviewed with a minimum of 5-year follow-up. Complications from bladder closure and additional procedures to correct these complications are presented in Table 124–2. The importance of a successful initial closure is emphasized by Oesterling and Jeffs (1987) and Husmann and colleagues (1989), who found that the onset of continence was quicker and the continence rate higher in those who underwent a successful primary closure with or without osteotomy. In addition, Novak and colleagues (2010) reported on patients with bladder exstrophy who underwent more than one attempt at primary closure. If a patient underwent two closures, the chance of having an adequate bladder capacity for bladder neck repair was 60% and the chance of voided continence was 17% overall. Patients who underwent three closures had only a 50% chance of an adequate capacity and less than 16% chance of voided continence. In an evaluation of this select group it was found that at the time of primary closure, 80% of patients had no form of pelvic osteotomy. Thus the chance of obtaining an adequate bladder capacity and eventual continence after more than one exstrophy closure is markedly diminished. These poor results underline the paramount importance of a secure abdominal, bony pelvis, and posterior vesicourethral unit with or without epispadias repair in the newborn exstrophy.
Table 124–2 Urologic/Orthopedic/Neurologic Complications in 185 Primary Bladder Closures
| UROLOGIC COMPLICATIONS |
NO. OF PATIENTS |
| Bladder prolapse/dehiscence |
6 |
| Post-outlet obstruction |
4 |
| Suprapubic tube removal |
2 |
| Intrapubic stitch erosion |
4 |
| Febrile urinary tract infection |
6 |
| Wound infection |
3 |
| Bladder calculi |
5 |
| Orthopedic Complications |
|
| Nonunion osteotomy |
2 |
| Leg length inequity |
1 |
| Persistent joint pain |
1 |
| Pelvic osteomyelitis |
1 |
| Pinsite infection |
3 |
| Pressure sore |
1 |
| Neurologic Complications |
|
| Femoral nerve palsy |
4 |
Also, compared with many of the other repairs, data are available from several units on their results with the CPRE repair. In a recent series by Shnorhavorian and coworkers (2008) there were 2 instances of dehiscence of the fascia, and 9 of 37 developed a vesicocutaneous fistula. Complications seen in the CPRE repair are much as those seen in the MSRE. However, some that are particular to this repair have been seen. A large series of vesicocutaneous fistula was reported from a single institutional study, in which some patients had an osteotomy and others did not (Shoukry et al, 2009). In addition, several series have reported the need for early ureteral reimplantation after closure and the occurrence of significant upper tract changes in many patients (Grady and Mitchell, 1999). This has prompted the call for ureteral reimplantation at the time of exstrophy closure by one group (Braga et al, 2008a).
Although most published series are not large, the incidence of bladder prolapse and dehiscence is reported to be small. In a recent series of primary CPRE repairs by Shoukry and colleagues (2009), the incidence of major prolapse and dehiscence was 15.8% even with the use of osteotomy. In this author’s series, we have seen a number of referred patients with complete dehiscence and major bladder prolapse after newborn CPRE repair. However, the complicating factors we have seen, along with the previously mentioned factors, have been significant losses of soft tissue including partial or complete loss of the glans in nine patients and the loss of the urethrovaginal septum in two. In a series by Hammouda (2003), 5 of 42 patients had ischemic loss of glans tissue after CPRE repair. Thus the complications of closure between the CPRE and MSRE are similar but more serious if soft tissue loss occurs.
Data from Baka-Jakubiak (2000) and colleagues from Warsaw involved 100 primary closures. Complete dehiscence occurred in 31 patients, of whom 24 had no osteotomy and 7 a posterior iliac osteotomy only. Of those who were closed at less than 72 hours (n = 47) and no osteotomy performed, dehiscence occurred in 13 patients. All were immobilized with a modified spica “chair” cast for 3 weeks and then an elastic bandage for 3 weeks. The authors currently recommend osteotomy for all newborns with a diastasis greater than 5 cm and in those closed after 72 hours.
In a recent publication of the Erlangen repair by Rosch and colleagues (1997) of 100 closures, the complications were generally mild with urethrocutaneous fistulae in 2. Minimal hydronephrosis occurred in 20%, and 3% had severe hydronephrosis requiring further surgery. There were no incidents of bladder prolapse or dehiscence. Osteotomy was not used in any patient, but a sophisticated coaptation technique involving the obturator foramen was used in all patients. In a recent report by Kelly and colleagues (2008) of 26 patients, there was a reported incidence of bladder prolapse requiring treatment in 25%. Soft tissue loss of glanular tissue was noted in 2 of 26 patients.
Interest in the outcomes of exstrophy closure has expanded to interest in the economic outcomes of the treatment of this major birth defect and who should be doing these types of operations in the newborn. In a paper by Nelson and colleagues (2005), high-volume hospitals (those closing more than five exstrophies per year) had lower overall costs per patient than low-volume hospitals (fewer than five exstrophies per year). In addition, Nelson and colleagues (2005) found that successful newborn closure had overall markedly less costly inflation-adjusted hospital charges than reclosures due to shorter operating times and shorter length of stay.
A corollary to these papers was one from Meldrum and colleagues (2005), who found that when exstrophy closures failed and had to be reclosed, the success rates and ultimate continence were better among fellowship-trained pediatric urologists rather than other surgeons (general urologists, non–fellowship-trained pediatric urologists, and general pediatric surgeons).
In the evaluation of this group of selected exstrophy patients, it was found that at the time of initial closure 19 of 23 patients had no form of osteotomy. Six of the patients had obtained bladder capacity suitable for bladder neck reconstruction; three were dry, and three were incontinent. Bladder size was inadequate in nine patients after being monitored for bladder growth. The chance of obtaining an adequate bladder capacity and eventual continence after more than one closure attempt is markedly diminished. These less than satisfactory results underline the paramount importance of a secure abdominal wall, posterior urethra, and bladder closure in these complex cases. Lastly, the importance of early initial closure was emphasized by data from Husmann and colleagues (1989) showing that only 10% of the patients who undergo bladder closure before 1 year of age but 40% of those who have the procedure at a later age require eventual augmentation.
Epispadias Repair
Although urinary incontinence remains the most significant problem for patients with classic bladder exstrophy and epispadias, anxiety about inadequate and unattractive genitalia still poses the greatest concern to male patients. The authors began using the modified Cantwell-Ransley repair in patients with classic exstrophy or epispadias in 1988 and have reported their early experience (Gearhart et al, 1992, 1995c, 2004).
Since 1988, the modified Cantwell-Ransley repair has been performed for 129 male patients with either classic bladder exstrophy (97 patients) or epispadias (32 patients) (Baird et al, 2005b). At the time of surgery, the patients’ age ranged from 1 to 18 years with an average age of 19 months. Of the 97 patients with bladder exstrophy, 31 had a short urethral groove requiring paraexstrophy skin flaps for penile lengthening at the time of initial bladder exstrophy closure. Of the 32 epispadiac patients, 26 had penopubic and 6 had penile epispadias at presentation.
This technique was used for primary urethroplasty in 106 patients with bladder exstrophy and 32 with epispadias. The modified Cantwell-Ransley repair was used as a secondary procedure after failed urethroplasty in 15 patients with exstrophy and 8 with epispadias and was combined with reclosure of bladder exstrophy in 18 patients. Early epispadias repair was performed when the patients were 6 months to 1 year of age. However, because of concerns about getting the urethra deeper under the corpora at the glanular level, beginning in 1994 we further modified the Cantwell-Ransley repair by detaching the mucosal plate from the corona except for the distal 0.5 to 1 cm of the plate. Intramuscular testosterone enanthate in oil was given to all children at a dose of 2 mg/kg at approximately 5 weeks and again at 2 weeks before surgery.
One hundred twenty patients had a horizontal or downward-angled penis while standing. The incidence of urethrocutaneous fistula in the immediate postoperative period was 16%, and at 3 months it was 12%. Nine patients developed a urethral stricture of the proximal anastomotic site, and 12 had minor skin separation of the dorsal skin closure. Cystoscopy with catheterization in 120 patients revealed an easily negotiable channel in all. Eight patients required further penile-straightening surgery. Fifteen patients older than 16 years had engaged in satisfactory intercourse, and all reported orgasms and ejaculation with a straight penis on erection. One patient reported that his penis was shorter following surgery.
Modern penile reconstructive techniques should create a straight and functional penis with a glanular meatus, an easily catheterizable neourethral channel (if needed), and an acceptable cosmetic appearance. Many adolescents considered their odd-appearing genitalia with a short, widened penis that deviated upward to be a greater psychosocial problem than incontinence, and therefore every effort should be made to restore the penis to a normal condition. In 1989, Ransley and colleagues introduced a concept to release dorsal chordee by incision and anastomosis of the dorsomedial aspect of the corpora over the urethra and urethral meatotomy at the distal end of the glans, to move the meatus to a more normal position and secure good direction of the urinary stream (IPGAM). Ransley’s group reported their long-term experience with 95 patients in whom the modified Cantwell-Ransley repair was used: Fistula occurred in only 4% of patients, and a urethral stricture in only 5% (Kajbafzadeh et al, 1995).
Dissection of the urethral strip to inside the glans penis provides a ventral position of the urethra and the glans and submerges the urethra well below the corpora at the glans level. This approximation of raw surface of glanular tissues dorsally over the urethra is clearly why the incidence of fistula in the area of the corona is rare compared with the Young repair. Fistulae in the authors’ patients usually appear at the base of the penis, where the urethra comes up proximally between the corporal bodies. In modern exstrophy reconstructive techniques, most surgeons try to preserve the urethral plate at the time of exstrophy closure. Interestingly, the authors do not find a significant difference for fistula formation between those in whom paraexstrophy skin flaps were used at the time of initial closure and those in whom they were not. Despite the positive findings with regard to fistula formation, the use of paraexstrophy skin flaps has been noted to be associated with the development of strictures. Use of these flaps is therefore limited to selected patients in whom penile lengthening cannot be achieved with standard techniques.
Papers from several institutions have reported their results with the Mitchell-Bagli penile disassembly technique. Complications are similar to other methods of epispadias repair (i.e., fistula and urethral stricture) (Zaontz et al, 1998; Kibar et al, 2009a). Although not a complication, a high percentage is made hypospadiac as the completely dissected urethral plate fails to reach the tip of the glans. The rate of being made hypospadiac has been reported from 38% to 83% (Mitchell and Bagli, 1996; El-Sherbiny and Hafez, 2005). As mentioned in the section on exstrophy closure, reports of ischemic loss of the glans, urethral plate, and corpora have been reported by Hammouda (2003) and Husmann and Gearhart (2004). Repair of the hypospadias in these patients has been reported by the Seattle group as not difficult or associated with major complications. However, data from Hafez and colleagues (2005) and Gearhart and Baird (2005) show difficulties that can be associated with these repairs. In an attempt to deal with the issues and prevent them, El-Sherbiny and Hafez (2005) have modified the Mitchell repair as with the modified Cantwell-Ransley repair (Gearhart and Baird, 2005) to keep the urethral plate attached to the distal glans to obviate the need for making the child hypospadiac at the time of isolated epispadias repair or when associated with CPRE.
In the authors’ opinion, none of the current epispadias repairs offers any significant gain in penile length by removal of the entire urethral plate from the glans or even the use of a free graft. Data reported by Silver and colleagues (1997b) clearly showed that, although anterior corporal length is significantly less in patients with exstrophy, the posterior corporal length is normal. These findings suggest that penile lengthening procedures at the time of epispadias repair improve apparent penile length and straighten the penis but do not transfer additional tissue (i.e., length) to the corporal bodies. The authors have observed that the modified Cantwell-Ransley repair effectively corrects corporal chordee and adds some penile length. It can be hoped that dorsal penile curvature, which is often seen during the growth spurt at puberty, will be resisted. After significant experience with adolescent exstrophy males with significant dorsal chordee, the authors agree that movement of the neurovascular bundles along with incision and grafting of the resultant defect gives better results long term than incision and corpora cavernostomy. In the patients in whom corporal rotation is used without corporal incision and anastomosis, the neurovascular bundle is left intact and not dissected from its bed. However, if incision and anastomosis are necessary, mobilization of the neurovascular bundle is required. Typically in the authors’ experience, incision and rotation are used only for older patients with marked chordee. Although review of findings reveals that almost all penises are straight or deflected downward, many of these patients are still young children. The long-term assessment of penile and urethral reconstruction in exstrophy patients by the modified Cantwell-Ransley repair has shown that in patients with bladder exstrophy and epispadias there is some increase in penile length, and a relatively straight penis with an adequate urethral caliber, which is adequate to void and ejaculate through, can be achieved with minimal morbidity. Long-term reports with the penile disassembly technique have also demonstrated a reasonably straight penis (Grady et al, 2003).
Bladder Neck Repair
Bladder neck reconstruction results in the exstrophy population have been reported by several groups. Some large experiences have come from the European groups in Lyon and Paris. Mouriquand and colleagues (2003) reported on 80 children with bladder exstrophy and 25 with incontinent epispadias. Follow-up ranged from 1 to 11 years. Forty-five percent of the group with exstrophy and 52% of those with epispadias had a dry interval greater than 3 hours. Although the continence rate was low, many of the exstrophy patients were not closed until 6 to 12 months of age. Also, many had their epispadias repair after bladder neck reconstruction, a factor known to influence both eventual capacity and continence. Lottmann and colleagues (1998) presented a long-term follow-up study of Cendron’s exstrophy patients who underwent complete reconstruction. With the Young-Dees repair they achieved urinary continence in 71% of male patients and 53% of females. Overall continence was 65% with a mean follow-up of 12 years after bladder neck repair. Series from North America using mainly the classic Young-Dees-Leadbetter repair reported continence rates ranging from 60% to 82% (Husmann et al, 1989; Mergurian et al, 1991; Perlmutter et al, 1991; Franco et al, 1994; McMahon et al, 1996; Chan et al, 2001; Cole et al, 2003) (see Fig. 124–2). The most important long-term factor gleaned from a review of all these series is the fact that bladder capacity at the time of bladder neck reconstruction is an important determinant of eventual success.
Records of 95 patients who underwent all stages of MSRE by a single surgeon at our institution between 1988 and 2004 were reviewed by Purves and colleagues (2008). Sixty-seven patients with bladder neck reconstruction and minimum 5-year follow-up were available for analysis. The current voiding status of each patient was obtained from parental or patient interview or direct observation by the nursing and physician staff. The patients were categorized as spontaneous voiding not on intermittent catheterization and were assigned a status of (1) completely dry day and night; (2) socially continent, being dry at least 3 hours during the day with occasional wet nights; or (3) wet being dry for less than 3 hours during the day and wet at night (Table 124–3).
Of the 67 male patients who underwent bladder neck repair, the mean age for primary closure was 4 months (range 6 hours to 4 months). The mean age at bladder neck repair was 4.8 years (range 40 to 60 months), and these patients had a mean bladder capacity before repair of 98 mL (range 75 to 185 mL). Of the 67 patients, 47 (70%) are continent and voiding urethrally without the need for augmentation or intermittent catheterization. Seven patients (10%) had social continence with occasional wet nights. The renal units of all patients who underwent bladder neck repair were evaluated by intravenous pyelography or ultrasound postoperatively on multiple occasions to assess preservation of renal function after the outlet procedure. One patient had reflux and hydronephrosis after the outlet procedure and bilateral reimplantation and developed left pyelonephritis with resultant mild scarring. DMSA revealed nearly normal bilateral function. Conservative follow-up revealed resolution of the reflux with time. One patient developed ureteral obstruction and required reoperative reimplantation. Prolonged outlet obstruction required cystoscopy and placement of an 8-Fr catheter in 19 patients, and prolonged suprapubic drainage was required in 13 patients. Thirteen (19%) failed bladder neck repair completely, six underwent continent diversion, and seven awaited further surgery. The mean time to daytime continence was 14 months (range 4 to 23 months), and the mean time to nighttime dryness was 23 months (range 11 to 34 months). No correlation was found between age at bladder neck reconstruction and age at achieving continence.
The findings in this series were that continence was more likely in patients who underwent primary exstrophy closure before 72 hours of age or after 72 hours of age with an osteotomy. These results coincide with those of Husmann and colleagues (1989) who found that patients who underwent delayed closure without osteotomy showed a continence rate of only 10%. There was another revealing factor in this study. Bladder capacity at the time of bladder neck repair gave a strong indication of not only who would be dry but also who would be dry sooner. If the patients were divided into those with a preoperative bladder capacity greater than or less than 100 mL, results showed that the group with the larger capacity had a high rate of continence (42/50 dry and voiding versus 5/17 in the lower-capacity group). In addition, the mean time to dryness in the higher- and lower-capacity group was 10 months and 21 months, respectively (14 months in the group overall).
In a similar series by Purves and Gearhart (2008), the results in females mirrored those of the male group with a slightly higher continence rate of 74% (dry day and night) and social continence again in 10%. Again, patients with capacity greater than 100 mL had better outcomes. In a phenomenon not seen in the males, six patients (20%) became continent after bladder and pelvic closure alone.
A continence procedure should be delayed until the bladder reaches a capacity of 100 mL, and the child is motivated to be dry and participate in a postoperative voiding program. The onset of continence is an interesting phenomenon in these patients. The vast majority achieve daytime dryness within 12 months after bladder neck repair. A few patients gain a longer daytime dry interval during the second year after their outlet procedure. However, patients who are not dry within 2 years are considered incontinent and failures. The onset of nighttime continence varies but takes longer than the time needed for daytime continence and can be 2 to 3 years. Caione and colleagues (1999) showed that use of desmopressin acetate (DDAVP) can increase the onset and number of dry nights in these patients. In the series by Purves and Gearhart (2008), 25% of patients required either DDAVP or oxybutynin to establish nighttime dryness.
Urodynamic evaluation by Dave and colleagues (2001) showed that patients with good continence after bladder neck repair had high cystometric bladder capacity and compliance compared with those who were incontinent. However, unstable contractions were seen in both groups. Also, higher-end filling pressures were reported and those patients had a high incidence of nonobstructive hydronephrosis. These findings in addition to those of Bolduc and colleagues (2002) indicate that careful lifelong follow-up is essential in these patients after successful childhood reconstruction.
Other Modern Exstrophy Repairs: Continence Outcomes
Warsaw Approach
The long-term reported outcomes of this repair include 36 patients with classic exstrophy and 37 with epispadias. Eighty-nine percent of patients with epispadias were continent during the day, but more than 40% were still wet at night. Seventy-five percent of patients with classic exstrophy had daytime continence, but nine had occasional wet nights. Eleven boys required short-term intermittent catheterization, which was easily performed by the patient and family. All but two began voiding within 3 to 5 months, and only two have continued with intermittent catheterization. Complications in seven boys included two urethrocutaneous fistulas and five urethral strictures (Baka-Jakubiak, 2000). All of these responded to urethral dilatation or endoscopic incision. There were no instances of abdominal wall dehiscence or glanular or corporal loss. Ureteral reimplantation was not performed at the time of bladder neck reconstruction and epispadias repair, but many patients required later reimplantation for gradually worsening hydronephrosis.
Compared with this experience, Mathews and Gearhart (2003) reported on a group of patients who had ureteral reimplantation performed at the time of bladder neck reconstruction and epispadias repair. None of these patients developed reflux or worsening hydronephrosis. Baka-Jakubiak (2000) recommends performance of this combined procedure if the bladder capacity is documented to be above 100 mL and the penis is large enough for epispadias repair. Follow-up urodynamic studies demonstrated the presence of normal detrusor function in most, although some patients developed high voiding pressures and some had poor detrusor contractility. If poor detrusor contractility was noted, prolonged intermittent catheterization was required and high voiding pressures were managed with anticholinergic therapy. Most patients were managed later in life, and the standard addition of ureteral reimplantation at the time of reconstruction should probably be universally performed.
Erlangen Approach
In a recent review by Ebert and colleagues (2010), who have reported on 100 patients, using this technique has occurred in both newborns and failed closures. Ninety-one children with exstrophy (69 boys and 22 girls) and nine with complete epispadias (7 boys and 2 girls) have had this procedure performed. The complete single-stage repair was performed in 47 children and included pelvic closure without osteotomy, bladder neck reconstruction, an antireflux procedure, and epispadias repair using the Cantwell-Ransley technique. An additional 53 patients had primary reconstruction elsewhere and then had bladder neck reconstruction and epispadias repair performed. Continence was defined as dryness for more than 3 hours and no nocturnal enuresis. Partial continence was defined as dry for 1 to 3 hours or longer with occasional stress incontinence or wet nights. Patients dry for less than 1 hour were considered incontinent. Among the patients undergoing single-stage repair, 72% (34 of 39) are dry voiding per urethra, 2 on clean intermittent catheterization (CIC), and 3 on CIC following augmentation. Four patients are partially continent, and two are dry on desmopressin. Four patients are incontinent, and three have had continent diversion. Of 53 patients who had primary closure elsewhere, 55% are continent and 7 have been augmented. Fourteen are partially continent and 10 are incontinent, 4 of whom have had continent diversion. The authors have emphasized the superiority of this repair for primary rather than failed closures.
Complete Repair
Various series have been published about outcomes including the need for ureteral reimplantation in the first year of life, the number of patients requiring hypospadias repair after this procedure, and the complication rates of this procedure. However, little has been published about the long-term continence results of the need for bladder neck reconstruction in this group of patients. Borer and colleagues (2005) reported that ultimately 65% to 70% of patients undergoing CPRE in the newborn period will require a bladder outlet procedure. In an update series, Borer and colleagues (2005) found that four of six patients with long-term follow-up have suitable continence after bladder neck repair. In a large series by Hafez and colleagues (2005), some of whom were delayed or failed primary closures, 84% of males and 50% of females required bladder neck repair to be dry. Some of these require concomitant bladder augmentation to be dry. In another large series by Shoukry and colleagues (2009), none were continent after CPRE alone and most required augmentation to be dry. More importantly, early dry intervals did not translate into later continence.
New data from Shnorhavorian and coworkers (2008) reviewed their total experience with the CPRE repair. This was a total of 39 patients since 1989. Daytime voided continence was achieved in 74% of males and females with 4 years of follow-up. However, only 20% and 43% of boys and girls, respectively, achieved continence without the need for bladder neck repair. Eighteen percent achieved dryness with bladder neck injection only. In a recent series of CPRE patients referred for reclosure or after successful closure for bladder neck reconstruction, the results were interesting (Gearhart et al, 2007). All patients were operated on by an experienced senior surgeon who does not routinely do newborn CPRE repairs. None of the 14 patients who underwent successful CPRE in the newborn period was continent for any appreciable interval after closure. Of the 19 patients who underwent bladder neck reconstruction after complications with their newborn closure, only 25% were voiding from the urethra and continent. In the group with a successful primary closure (n = 14), 57% were dry day and night and 28% were dry during the day only (Purves et al, 2009a). Of interest, all patients who were dry after bladder neck reconstruction had a successful primary closure with pelvic osteotomy and hypospadias repair before 1 year of age. None required ureteral reimplantation before bladder neck repair. These data clearly show that in the majority of cases, “complete repair” patients will need to undergo bladder neck repair. However, as in all of the major repairs, in a successful primary closure with fewer bladder violations with interval surgeries, the chance for voided continence, although reasonable, still needs to be improved.
Other methods have combined epispadias repair with bladder exstrophy in the male patient. In a series of 38 boys with classic exstrophy, Baird and colleagues (2005c) evaluated patients with either failed or delayed primary closures. The complications were those seen with routine exstrophy repairs including urethrocutaneous fistula and urethral strictures. Three boys were dry with combined closure alone. Nineteen boys went on to modified Young-Dees-Leadbetter bladder neck repair. During both day and night, 12 of 19 were totally dry. These data, as well as the data of Mitchell and Grady, clearly show that epispadias repair and exstrophy closure can be combined with acceptable results. However, the complications are real and can portend the loss of any chance at volitional voiding and should only be performed by experienced exstrophy surgeons and not the occasional surgeon.
Kelly Repair
There are not many papers with long-term follow-up of the Kelly technique. However, a recent presentation by the Melbourne group gives the best and most current results that can be found with this repair. Data was collected in children older than 4 years. Complete continence was defined as dry for more than 3 hours day and night (with two or fewer wet nights per months). Partial continence was dry for 2 or more hours during the day and three or more wet nights per month and/or stress incontinence. Twenty-four of 31 Kelly patients voided spontaneously, and 17 of 31 voided in an unaided fashion (without IC or augmentation). Overall continence was 71% with 3 of 17 (18%) who voided in an unaided fashion enjoying complete continence, Nine of 17 (53%) had partial continence. The 18% dry and voiding rate compares favorably with that recently reported by Shnorhavorian and colleagues (Jarzebowski et al, 2009).
Exstrophy Reconstruction Failures
Failed Closure
After any form of repair, failures can manifest as complete bladder dehiscence, bladder prolapse, neourethral stricture and obstruction, and soft tissue loss. Meldrum and colleagues (2005) reported on a select group of children who failed exstrophy reconstruction before referral to a tertiary care facility. In the cohort of 101 children, 51 had primary surgical management performed by a fellowship-trained pediatric urologist, 18 by a general urologist, 6 by a pediatric surgeon, and 9 by an unknown surgeon. Following successful reclosure, 38 patients eventually developed adequate bladder capacity for bladder neck reconstruction, and only 26% (10) eventually achieved dryness. These data emphasize the need for initial successful reconstruction and suggest that individuals undertaking this reconstruction should be comfortable with the complexity of repair. It is prudent for the surgeon who may see only a few patients with this condition to consider referral of these complex management situations to a center where special expertise and experience exist.
In a recent large series by Schaeffer and colleagues (2008), 185 patients who underwent closure by one of two surgeons were reviewed for both major and minor complications (see Table 124–2). Sixty-three underwent osteotomy at the time of primary closure. There were 14 major complications (11%) and 27 minor complications (14%). Major urologic complications included bladder prolapse or dehiscence in six male patients (3%), all which were successfully reclosed. Major orthopedic complications including nonunion in two patients, leg length inequality in one, and persistent joint pain in one developed in 4 of 63 patients (6%) who underwent osteotomy. Major neurologic complications included femoral nerve palsy in four patients (2%). There were 21 minor urologic complications (11%) including posterior bladder outlet obstruction in four cases, urethrocutaneous fistula in two, suprapubic tube removal in two, intrapubic stitch erosion in four, febrile UTI in six, and surgical site infection in three. Six patients (3%) had minor orthopedic complications including pelvic osteomyelitis in one, pin site infection in three, and a pressure sore from immobilization in one.
Dehiscence, which may be precipitated by incomplete mobilization of the pelvic diaphragm and inadequate pelvic immobilization postoperatively, wound infection, abdominal distention, or urinary tube malfunction, necessitates a 6-month recovery period before a second attempt at closure can be made (Gearhart and Jeffs, 1991a; Gearhart et al, 1993b) (Fig. 124–27A). Dehiscence and prolapse have also been reported following the CPRE and may be associated with glandar, corporal, urethral plate, and other major soft tissue loss (Fig. 124–27B). Tension-free reclosure with osteotomy and immobilization are important factors in initial and subsequent closures. Unfortunately, the chance of obtaining adequate bladder capacity for bladder neck plasty and eventual continence after multiple closures is markedly diminished (Gearhart et al, 1996a). Similarly, bladder prolapse is considered a failure and requires bladder reclosure or revision. In a recent series from a single institution of 122 patients who underwent reclosure with a mean follow-up of 14 years, the voided continence rate was 16% (Novak et al, 2010). In a patient with significant bladder prolapse or dehiscence, at the time of secondary closure the authors combine epispadias repair with bladder, posterior urethra, and abdominal wall closure (Gearhart et al, 1998). The patient is given testosterone enanthate intramuscularly 5 weeks and 2 weeks before surgical repair and undergoes an osteotomy with concomitant bladder, urethral, and abdominal wall closure. Major soft tissue loss that is seen following CPRE requires extensive preoperative planning and may require use of buccal mucosal or full-thickness grafts or tissue expansion (Gearhart and Baird, 2005).
Gearhart and Baird (2005) reported on 38 boys undergoing combined exstrophy closure and epispadias repair along with osteotomy, and 30 had failed prior reconstruction. Complications in this group were limited to urethrocutaneous fistulae and urethral strictures. Although the results after reclosure are not as good as those obtained with a successful primary closure, they are respectable and may obviate the need for bladder augmentation.
Erosion of the intrapubic stitch into the posterior urethra during the healing process can present as a UTI, increasingly dry diapers, or high residual urine in the immediate postoperative state before removal of the suprapubic tube. In any of these cases or if any hydronephrosis is seen on postoperative ultrasound, immediate cystoscopy should be performed to rule out obstruction. If the stitch has eroded into the urethra and the child is older than 4 months, it can be removed easily through a small suprapubic incision. If it occurs early, the authors usually leave the postoperative tube for 8 to 12 weeks before stitch removal.
Neourethral stricture is often associated with paraexstrophy skin flap use, pubic suture reaction, erosion, or the use of urethral stents. This may be somewhat subtle; however, warning signs consist of UTIs, detectable increased bladder volumes on ultrasonography, bladder stones, prolonged dry intervals, and unexplained rectal prolapse. In a review by Baker and colleagues (1999), posterior urethral obstruction was found in 41 patients. Most episodes occurred within 60 days after primary closure. If diversion was used for longer than 6 months, the ultimate fate of the bladder was augmentation. If diversion was used for less than 6 months, most reconstructions were ultimately bowel free. The authors have also seen outlet obstruction including complete obliteration after both the CPRE and Kelly repair (Hernandez et al, 2008). Whether this is due to ischemic damage to the urethral plate or technical error is unclear. Regardless, because there is a paucity of long-term data from these two repairs, the outlet must be monitored carefully as with all exstrophy patients after primary closure. Ultimately, posterior urethral obstruction after exstrophy closure markedly decreased the success of any repair. This complication presents a significant risk to the upper urinary tract and should be detected early.
Although all closed exstrophy bladders have vesicoureteral reflux, upper tract deterioration is the ultimate fate of significant outlet obstruction. At this point the management includes urethral dilation (incision), open urethroplasty, or upper tract diversion. If renal function is compromised, the choice must achieve unquestionable free drainage to allow the upper tracts and kidneys to recover fully. Further bladder neck or urethral reconstruction should not be performed until the posterior urethral stricture is clearly repaired and free drainage has been achieved. After CPRE repair, up to 50% of patients will require bilateral ureteral reimplantation during the first year following their operation because of UTIs and increasing hydronephrosis.
Despite satisfactory closure, some bladders never achieve adequate capacity to act as functional bladders. It has become clear that multiple bladder closures, bladder prolapse, dehiscence, bladder calculi, recurrent infections, and vesicostomy have a negative impact on the potential of the exstrophy bladder (Silver et al, 1997b). The authors have used transurethral injection of collagen around the bladder neck to increase outlet resistance and stimulate the bladder to grow. However, in our hands this has not been as successful as reported by Caione and colleagues (Caione et al, 1993a, 1993b) from Italy. If the bladder does not grow to a sufficient size for bladder neck reconstruction, bladder augmentation is recommended in this situation. If the bladder neck or urethra or both are problematic, a catheterizable continent stoma with or without bladder neck plasty or transection is performed, along with augmentation (Gearhart et al, 1995b).
Failed Bladder Neck Repair
Early successful bladder closure with or without pelvic osteotomy with a good bladder template is the first step to achieve voided continence. Early epispadias repair with good bladder growth (>100 mL) is the second step in this sequence. Proper patient selection with a motivated family and, lastly, proper execution in the operating room are the final steps in this journey. Nonetheless, missteps can occur and there remains a subgroup of children with urinary incontinence after bladder neck reconstruction secondary to (1) inadequate outlet resistance; (2) a small bladder capacity (lack of growth after bladder neck reconstruction); (3) decreased compliance; and (4) a combination of these factors.
If urinary continence, defined as a 3-hour dry interval, is not achieved within 2 years after bladder neck reconstruction, failure to achieve dryness has resulted. Occasionally, the dry interval is nearly acceptable for daytime dryness (i.e., longer than 2 hours). In these situations, collagen injections into the bladder neck can move the dry interval up to 3 hours, but more than one injection may be required (Ben-Chaim et al, 1995a). Data from Lottmann with the use of dextranomer implant (Deflux) have shown improvement in the dry interval when used following partial success with bladder neck reconstruction. Some successes have been seen with repeated Young-Dees-Leadbetter repair if the bladder neck is patulous, the bladder capacity is adequate, and urodynamic evaluation reveals a stable bladder (Gearhart et al, 1991). In a series by Gearhart and colleagues (1991), only 50% of patients with a failed prior bladder neck reconstruction were candidates for reoperative surgery. In this highly select group, continence rates were acceptable after reoperative bladder neck reconstruction. All of these patients had excellent bladder capacity (>100 mL) and were stable on urodynamics before the redo repair. A majority of bladder neck failures require eventual augmentation or continent diversion. The artificial urinary sphincter has been used with some success in patients who have a good bladder capacity. However, in most of these failures the bladder capacity is small and augmentation is required. At the time of reoperative surgery, either the bladder neck is transected proximal to the prostate with a Mitrofanoff substitution or a continence procedure such as an artificial sphincter or collagen injection, or both, are performed. In the authors’ extensive experience with failed bladder neck reconstructions, most of the patients have had several surgeries and need to be dry. In a recent large series of 31 patients who had undergone prior bladder neck reconstruction, studied by Novak and colleagues (2009), most patients had undergone multiple prior repairs to achieve continence. Bladder neck transaction along with enterocystoplasty was performed with initial continence achieved in 86%. Seven patients underwent further bladder neck surgery, and 90% achieve dryness by intermittent catheterization. One renal unit was lost, nonobstructive hydronephrosis developed in 8, and bladder stones formed in 30%.
Delayed urinary continence has been reported at the onset of puberty in some males and has been attributed to prostatic growth. On MRI, the prostate of exstrophy patients is clover shaped and absent anteriorly, and its mean weight and maximal cross section and volume are normal (Gearhart et al, 1992). Therefore it is doubtful that growth of this abnormally configured prostate can have much impact on later urinary continence after failed bladder neck repair.
Failed Genitourethral Reconstruction
Common complications of modern epispadias repair include urethrocutaneous fistula formation. This has been reported in as little as 4% and up to 19% of cases (Kajbafzadeh et al, 1995; Surer et al, 2001a). Urethral tortuosity with difficult catheterization or strictures is uncommon with modern epispadias repair. Since the application of the penile disassembly for epispadias reconstruction, newer, more significant complications have been noted (Hammouda, 2003). Gearhart and Baird (2004) have reported loss of the glans, corpora, or both in addition to loss of penile skin and the urethral plate. Whether this is secondary to surgical misadventure, vagaries in the blood supply of the penis, or the intrinsic difficulties in the procedure remains a topic of debate. Reconstruction of these complications has required additional techniques including tissue expansion, full-thickness skin grafting, buccal mucosal grafting, and other complex techniques. In some patients with significant losses, tissue engineering may eventually provide the material for final cosmetic reconstruction.
At an older age, unsightly penile scars and a short phallus may prompt further surgical intervention. Scar excision can be closed in a plastic fashion if enough penile skin is available. Otherwise, flaps or full-thickness skin grafts can be used. In severe cases, tissue expanders can be placed under the penile skin and gradually inflated over 6 weeks to allow more penile skin and obviate the need for grafting. Freeing all scar tissues and suspensory ligament tissue can maximize available penile length. A dorsal dermal corporal graft or ventral corporal plication or rotation may additionally help lengthen and correct any chordee. However, it must be recognized that the exstrophy penis, when compared with age- and race-matched controls, is congenitally deficient in anterior corporal tissue as assessed by MRI (Silver et al, 1997b). Therefore overly aggressive attempts at penile lengthening may result only in corporal denervation and devascularization without additional lengthening.
Alternative Techniques of Reconstruction
Not all children with bladder exstrophy are candidates for primary repair at initial evaluation because of a small bladder plate or significant hydronephrosis. Additional reasons for seeking other methods of treatment include failure of initial closure with a small remaining bladder or failure of continence surgery, or both. Excluding the patients in whom initial treatment fails, this discussion deals with options available when modern repair is not chosen by the surgeon or for other reasons has not been suitable.
Ureterosigmoidostomy
Whichever urinary diversion is chosen, the upper tracts and renal function are initially normal. This allows the reimplantation of normal-sized ureters in a reliable, nonrefluxing manner into the colon or other suitable reservoir. Historically, ureterosigmoidostomy was the first form of diversion to be popularized for patients with exstrophy. Although the initial series was associated with multiple metabolic problems, results improved markedly with newer techniques of reimplantation (Zarbo and Kay, 1986; Koo et al, 1996). Ureterosigmoidostomy is favored by some because of the lack of an abdominal stoma. However, this form of diversion should not be offered until one is certain that anal continence is normal and after the family has been made aware of the potential serious complications including pyelonephritis, hyperkalemic acidosis, rectal incontinence, ureteral obstruction, and delayed development of malignancy (Spence et al, 1979; Duckett and Gazak, 1983). More recently, ureterosigmoidostomy has been proposed again as an initial treatment of bladder exstrophy with acceptable continence and renal preservation on follow-up of 10 years or longer. In a recent long-term study by Gobet and colleagues (2009) of 42 patients, 50% had their ureterosigmoidostomy in place and were fecally continent at 50 years of age. One had suffered a colonic malignancy, and 60% had normal renal function.
Stein and colleagues (1999) treated a group of 128 patients with bladder exstrophy-epispadias with the Mainz technique for ureterosigmoidostomy. The Mainz Sigma pouch is constructed, and the ureters are reimplanted in a nonrefluxing manner. The abdominal wall is reconstructed and the bladder is closed as a seminal receptacle above the prostate. The penis is then reconstructed at a later date. D’elia and colleagues (2004) reported on 26 patients with the exstrophy-epispadias complex who showed excellent continence rates with long-term upper tract preservation when compared with standard ureterosigmoidostomy. Continence was achieved in 95% of patients with a rectal reservoir. Their recommendation for treatment in a number of patients with severely impaired renal function was that a colonic conduit was the best method of choice for diversion. In the patients with a normal or slightly dilated upper tract and intact anal sphincters, a Mainz rectal reservoir was recommended. Although the group from Mainz reported no cancer in a long-term follow-up study of patients who underwent ureterosigmoidostomy, the risk for malignancy still exists and must be carefully considered in a young child. Patients who have had mixing of urine and feces at any time during reconstruction remain at high risk for the development of cancers (Smeulders and Woodhouse, 2001). Bladder exstrophy patients who have failed an initial reconstruction and have polyposis at the time of repeated closure may also be at higher risk for the later development of malignancy (Novak et al, 2005). This is especially true in today’s mobile society, in which careful long-term follow-up may be difficult to guarantee.
Continent Urinary Diversion in the Exstrophy Patient
In modern pediatric urology there is usually little need for incontinent urinary diversion in the patient with bladder exstrophy. In a young child with a bladder that is too small to close, or in a failed closure where the bladder template is too small to reclose, the authors recommend a nonrefluxing colon conduit. This protects the kidneys from vesicoureteral reflux, and undiversion can be performed when clinically indicated at an older age.
Advances in the reconstruction of the lower urinary tract in the past several years have been applied to the patient with exstrophy (Cervellione et al, 2008). Most commonly, further reconstruction is required in the patient in whom bladder neck reconstruction has failed. If a patient does not meet the criteria for bladder neck reconstruction (i.e., inadequate bladder capacity or inability to conform to a voiding regimen), deferring surgery is advisable. Patients in whom bladder neck reconstruction fails are most often destined to augmentation cystoplasty and continent urinary diversion. Surer and colleagues (2003) reported on 91 patients with the exstrophy-epispadias complex who underwent continent urinary diversion. Eight patients had bladder exstrophy, eight had cloacal exstrophy, and three had complete epispadias. The majority had undergone prior failed bladder neck reconstruction (n = 62). Seventy-nine patients (87%) had exstrophy closure before referral, 53 had also undergone bladder neck reconstruction, and 29 patients had never reached adequate capacity for bladder neck reconstruction. Ten of the 53 patients had undergone one prior attempt, 35 had two prior attempts, and 8 had three prior attempts at bladder neck reconstruction. A combined augmentation cystoplasty, continent urinary diversion, and bladder neck closure was performed in 59 patients (65%), and reaugmentation and continent urinary diversion was performed in 18 children. Ileocystoplasty was used in 41 patients, and sigmoid cystoplasty was performed in 30 patients. The appendix was used as the continent urinary diversion in 67 patients. Continence using intermittent catheterization through the stoma was achieved in 93% of children with the most common complication reported being that of bladder stones, noted in 26%. Although low, failures can occur with continent urinary diversion in exstrophy (Frimberger et al, 2003). The most common mode of failure was a deintussusception of a nipple valve, inadequate tunnel for an appendiceal channel, or continued bladder neck incompetence. The vast majority of these complications can be successfully repaired. Equally successful outcomes have been reported by Baird and colleagues (2005a) in adolescents who had undergone a mean of eight prior procedures before their definitive repair.
Continence can be achieved in most patients with exstrophy following failure of bladder neck reconstruction. This continence is typically obtained through augmentation cystoplasty and revision of the bladder neck reconstruction or closure. If a patient is unlikely to develop adequate capacity for eventual bladder neck reconstruction, moving early to continent urinary diversion is preferable. One long-term concern has been the risk of malignancy in these patients over the long term. Recent data by Higuchi and colleagues (2010) revealed that the overall rate of malignancy is low unless associated with coexisting carcinogenic stimuli (prolonged tabacco/chronic immunosuppression) or the inherent risk associated with bladder exstrophy.
Key Points: Bladder Exstrophy—Priorities in Management
•
Size and quality of bladder template
•
Extent of pubic diastasis and malleability of the pelvis
•
Length and width of urethral plate
Cloacal Exstrophy
Cloacal exstrophy includes a spectrum of abnormalities but is primarily an anterior abdominal wall defect. A reported incidence of 1 : 200,000 to 1 : 400,000 makes this one of the rarer urologic abnormalities (Hurwitz et al, 1987). Although prior reports have suggested a male/female ratio of 2 : 1 (Gearhart and Jeffs, 1998), a large study by Boyadjiev and colleagues (2004a) indicated that the sex ratio may be 1 : 1. Most cases are sporadic, and isolated incidences of unbalanced translocations have been reported to be potentially causative (Thauvin-Robinet et al, 2004); however, one report noted recurrence in siblings, perhaps indicating a more multifactorial etiology. Associated defects of the neurospinal axis, intestinal tract, and urogenital and skeletal systems are frequently noted. When neurospinal defects and omphalocele coexist with cloacal exstrophy, the term OEIS complex (omphalocele, exstrophy, imperforate anus, spinal defects) has been used (Keppler-Noreiul, 2001). Advances in critical care of children have resulted in most patients surviving into adult life and modified the focus to improving quality of life (Mathews et al, 1998).
Anatomic Considerations
The classic constellation of anomalies that are noted in children with cloacal exstrophy includes exstrophy of the bladder, complete phallic separation, wide pubic diastasis, exstrophy of the terminal ileum between the two halves of the bladder, a rudimentary hindgut, imperforate anus, and the presence of an omphalocele (Fig. 124–28). Many children have associated spinal defects, and various lower extremity malformations may be noted (Loder and Dayioglu, 1990; Jain and Weaver, 2004). The urogenital anomalies in cloacal exstrophy are similar to those seen with classic bladder exstrophy (see earlier), although they are typically of greater severity.
Neurospinal Abnormalities
Abnormalities of the spinal cord or vertebral column, or both, have been noted in 85% to 100% of children with cloacal exstrophy (Appignani et al, 1994; McLaughlin et al, 1995). Although most patients have lumbar myelodysplasia (80%), thoracic defects may be noted in 10% with the remaining having sacral defects. In a large series of 34 patients from a single center with cloacal exstrophy and associated spinal defects, Mathews and colleagues (1998) noted lipomeningocele in 17, myelomeningocele in 8, and spina bifida and isolated cord tethering in 7 and 2 patients, respectively. The nearly universal incidence of spinal abnormalities has led some authors to suggest MRI for all patients with a diagnosis of cloacal exstrophy (Cohen, 1991). Spinal ultrasound evaluation, which is easily performed, has been noted to be comparable with MRI for the diagnosis of spinal anomalies in infants with cloacal exstrophy (Dick et al, 2001). Karrer and colleagues (1988) noted that only one in five children with spinal dysraphism noted on ultrasonography had a sacral abnormality visible on the skin surface.
The embryologic basis for the neurospinal defects associated with cloacal exstrophy has been postulated to be secondary to problems with the disruption of the tissue of the dorsal mesenchyme rather than failure of neural tube closure (McLaughlin et al, 1995). Alternatively, it has been suggested that the defects that lead to the formation of cloacal exstrophy may lead to the developing spinal cord and vertebrae being pulled apart (Cohen, 1991). Functional deficits can range from patients who have almost normal sensation of the pelvis and lower extremity to patients who are wheelchair bound. The presence of a clinically significant neurologic anomaly was found to affect negatively the development of continence (Husmann et al, 1999) and led to a significant reduction in continence when compared with patients with bladder exstrophy. Only 1 of 13 neurologically impaired patients in this series developed voided continence.
The neuroanatomic dissections performed by Schlegel and Gearhart (1989) further indicate that the neuroanatomic landmarks in the infant with cloacal exstrophy are different from those in the normal newborn with the autonomic bladder innervation being derived from a more medial location (see Fig. 124–28). This potentially puts the nerve supply in jeopardy during initial bladder dissection and reconstruction and may leave the bladder neuropathic following reconstruction (Husmann et al, 1999). Innervation to the duplicated corporal bodies arises from the sacral plexus, travels in the midline, perforates the interior portion of the pelvic floor, and courses medially to the hemibladders (Schlegel and Gearhart, 1989). Innervation abnormalities were also noted at a histologic level in the studies by Rosch and colleagues (1997). When compared with those in patients with bladder exstrophy, the neural elements identified on immunohistochemical evaluation were found to show significant structural abnormalities.
Skeletal System Abnormalities
Anomalies of the skeletal system are universally noted in children with cloacal exstrophy (Suson et al, 2009). The pelvic defects that are seen with classic bladder exstrophy are noted with greater severity in the patient with cloacal exstrophy. Sponseller and colleagues (1995), studying patients with the exstrophy complex, noted that the posterior segment of the pelvis in children with cloacal exstrophy was angled farther posteriorly and there was a greater likelihood of asymmetry between the two sides. Similarly, the anterior segment of the pelvis had more severe degrees of external rotation. The actual length of the bone segments, however, was similar between those with cloacal and classic exstrophy. The interpubic distance (diastasis) in children with cloacal exstrophy was noted to be almost twice that of children with classic bladder exstrophy. Malformation of the sacroiliac joints and side-to-side asymmetry were also noted. These issues become of increasing importance, and most children with cloacal exstrophy require osteotomy for successful reconstruction. Stec and colleagues (2003) noted that, microscopically, the bones in children with cloacal exstrophy were similar to those in normal controls and were developing at a similar rate, indicating that the potential for growth was also similar.
Vertebral anomalies that were not associated with myelodyplasia were noted in 8 of 37 children with cloacal exstrophy (Mathews et al, 1998). Loder and Dayioglu (1990) noted vertebral anomalies in three of five children with cloacal exstrophy. Absence or shortening of limbs was also noted, as were clubfoot malformations. Skeletal and limb anomalies were also reported by Diamond (1990) in 12% to 65% of cases. The vast majority were clubfoot deformities, although absence of feet, severe tibial or fibular deformities, and congenital hip dislocations were commonly noted in this group of patients. A similar high incidence of foot abnormalities and greater than normal abduction of the hips was noted in a study by Greene and colleagues (1991).
Intestinal Tract Abnormalities
Gastrointestinal tract anomalies occur in virtually all patients with cloacal exstrophy. In Diamond’s series (1990), the incidence of omphalocele was 88% and a majority of all series reported an incidence of 95% or greater. In the series reported by Mathews and colleagues (1998), 100% had an omphalocele. Omphaloceles do vary in size and usually contain small bowel or liver, or both. Immediate closure of the omphalocele defect in the newborn period is advised to prevent subsequent rupture.
Hurwitz and colleagues (1987), in a large review of cloacal exstrophy patients, reported a 46% incidence of associated gastrointestinal tract anomalies, with malrotation, duplication anomalies, and anatomically short bowel occurring with equal frequency. A hindgut remnant of varying size is also noted in most patients (Sawaya et al, 2010). Hurwitz noted a 23% incidence of short gut syndrome, which is compatible with the 25% incidence reported by Diamond (Hurwitz et al, 1987; Diamond, 1990). It now seems well accepted that short gut syndrome may occur in the presence of normal small bowel length, suggesting absorptive dysfunction and emphasizing the absolute need to preserve as much large bowel as possible. If not used for incorporation into the fecal stream, the hindgut remnant may be preserved for use in urogenital tract reconstruction (Mathews et al, 1998).
Genitourinary Abnormalities
Müllerian anomalies have been frequently noted in conjunction with cloacal exstrophy. The most commonly reported müllerian anomaly was uterine duplication, seen in 95% of patients (Diamond, 1990). The vast majority of these patients had partial uterine duplication, predominantly a bicornate uterus. Vaginal duplication occurred in 65% of cases, and vaginal agenesis was seen in 25% to 50% of patients. In a report by Hurwitz and colleagues (1987), cases of complete duplication of the uterus and fallopian tubes associated with both vaginal duplication and vaginal agenesis were noted. Gearhart and Jeffs (1991b) recommended preservation of all müllerian duplication anomalies for possible utilization in reconstructing the lower urinary tract.
Upper urinary tract anomalies occurred in 41% to 60% of patients in Diamond’s review. The most common anomalies were pelvic kidney and renal agenesis, both occurring in up to one third of patients. Hydronephrosis and hydroureter were common, occurring in one third of patients. Multicystic dysplastic kidneys and fusion anomalies were seen less frequently. Ectopic ureters draining to the vasa in the male and into the uterus, vagina, or fallopian tubes in the female were also reported (Diamond, 1990). A similar incidence of upper tract defects was noted by Mathews and colleagues (1998). Ureteral duplication, congenital stricture, and megaureter were also reported.
Genital anomalies in the male have typically included complete separation of the two phallic halves and accompanied separation of the scrotal halves. Asymmetry of these structures can also be seen and provide additional challenges to successful reconstruction. Testes may be noted in the scrotum but are frequently noted to be undescended, and associated inguinal hernias are a common finding. Girls typically have widely divergent clitoral halves.
The lower urinary tract is typically composed of two exstrophied hemibladders flanking the exstrophied intestinal segment. Each bladder half usually drains the ipsilateral ureter and is closely related to the ipsilateral phallic segment. Variations of anatomy, however, are frequently seen and every patient has unique anatomic features.
Additional System Anomalies
Life-threatening cardiovascular and pulmonary anomalies are rarely seen in cloacal exstrophy. Reported cases included two patients with cyanotic heart disease and one with aortic duplication. A bilobed lung was reported in two patients and an atretic right upper lung in one. Also, Schlegel and Gearhart (1989) reported caval duplication in their anatomic dissection of a patient with cloacal exstrophy.
Because of the complexity and the multisystem nature of cloacal exstrophy, Hurwitz and coauthors (1987) have devised a grid for the clarification of anatomy in each patient and to permit planning for reconstruction (Fig. 124–29). This permits the standard form of cloacal exstrophy to be separated from variants and allows the soft tissue components of the defect to be described systematically.
Prenatal Diagnosis
Since its initial description in the early 1980s, further refinements in the prenatal diagnosis of cloacal exstrophy have occurred (Meizner and Bar-Ziv, 1985). These authors indicated that the three main criteria used to identify the diagnosis were a large midline infraumbilical anterior abdominal wall defect, lumbosacral myelomeningocele, and failure to visualize the urinary bladder. Chitrit and colleagues (1993) reported the diagnosis of monozygotic twins with cloacal exstrophy detected during antenatal ultrasound screening. Since these initial reports, there have been only occasional case reports of prenatal diagnosis of cloacal exstrophy, and only 15% of patients with this anomaly have been diagnosed by prenatal ultrasonography, according to the literature. With the marked improvements in survival of patients with cloacal exstrophy in the past 20 years and the common application of fetal ultrasonography, early diagnosis may permit appropriate prenatal counseling for parents and expedite postnatal care.
Austin and colleagues (1998) reviewed 20 patients with this abnormality, expanded on the diagnostic findings, and proposed major and minor criteria for the prenatal diagnosis of cloacal exstrophy on the basis of the frequency of occurrence rather than the severity of individual findings. A criterion was considered major if it was present in more than 50% of cases. The gestational age at diagnosis of cloacal exstrophy ranged from 15 to 32 weeks (mean, 22 weeks). Major diagnostic criteria included nonvisualization of the bladder in 91%, a large midline infraumbilical anterior wall defect or a cystic anterior wall structure in 82%, an omphalocele in 77%, and a myelomeningocele in 68%. Minor criteria included lower extremity defects in 23%, renal anomalies in 23%, ascites in 41%, widened pubic arches in 18%, narrow thorax in 9%, hydrocephalus in 9%, and a single umbilical artery in 9%. Hamada and colleagues (1999) reported a single case in which ultrasonography revealed a wavy cordlike segment of soft tissue protruding from the anterior abdominal wall of the fetus below the umbilicus. This was found to be prolapsed terminal ileum, which resembled the trunk of an elephant. The authors suggested that this sonographic image be added to the criteria described by Austin and colleagues (1998) for making a prenatal diagnosis of cloacal exstrophy.
Universal use of antenatal ultrasonography has permitted diagnosis of cloacal exstrophy to be made at a time when parental counseling may lead to possible pregnancy termination. Extensive parental counseling regarding the significant anatomic anomalies that constitute the complex is appropriate in conjunction with psychologic support.