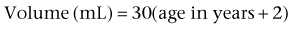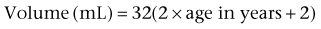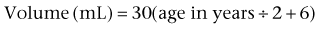chapter 129 Urinary Tract Reconstruction in Children
The goal of this chapter is to review techniques for lower urinary tract reconstruction in pediatric patients and the principles that guide their use. In general, such intervention is taken to reestablish or to construct anew a system that protects renal function, avoids significant infection, and eventually provides for urinary continence. Those, in simplistic terms, are functions of a “normal” urinary tract.
The scope of the chapter is large. Many specific techniques are presented in detail, particularly those used primarily in the pediatric population. When techniques have been used more extensively in adults, descriptions are brief with focus on adaptations for and results of use in children. Urinary diversion, both temporary and permanent with bowel, is discussed elsewhere in this text. Augmentation cystoplasty is reviewed in detail from preoperative evaluation and surgical techniques to long-term results and newer alternatives. In few areas of urology have intestinal segments been used more extensively than for bladder augmentation in pediatric patients. This experience with augmentation cystoplasty established the groundwork for later work with continent urinary diversions and orthotopic neobladders.
Complex patients with bladder dysfunction may have bladder neck and external sphincteric problems as well. This chapter covers techniques to increase native outflow resistance in the pediatric population. Certain techniques, such as Young-Dees-Leadbetter bladder neck repair, are presented elsewhere in this section of this text. Others (i.e., sling, collagen injection, and artificial urinary sphincter) have been used more extensively in adults; again, in that setting, the focus in this chapter is on adaptations of those procedures for children. Perhaps the most important contribution affecting lower urinary tract reconstruction was the introduction of clean intermittent catheterization (CIC) by Lapides and colleagues (1972, 1976). Because many pediatric patients with bladder and sphincteric dysfunction will not void adequately after reconstruction, a reliable means for easy catheterization without discomfort is an important part of their care. The work of Mitrofanoff (1980) stimulated interest in continent abdominal wall stomas within pediatric urology, and several effective techniques have since been developed. That experience serves as a nice lead into a discussion of continent urinary diversion in children because construction of an effective efferent limb that provides continence and a reliable means for catheterization is often the most challenging aspect of such diversion. “Pure” continent urinary reservoirs, such as Indiana and Kock pouches, are occasionally performed in children, and that experience is reviewed. More frequently in children, some of the patient’s native urinary tract is used in the reconstruction. This is done to maintain as much urothelium-lined tissue in the urinary tract as possible, to minimize the amount of needed bowel, and, potentially at least, to minimize the morbidity to the patient. The result then is a spectrum of repairs between bladder augmentation and continent urinary diversion but rarely a continent urinary reservoir in the classic sense.
Several decades ago, many such reconstructions were performed after previous, permanent urinary diversion with use of bowel (Hendren, 1998). Today, however, few children are initially treated with permanent diversion. Most reconstructive procedures are now undertaken primarily to correct a problem in the native urinary tract (hydronephrosis, infection, incontinence) unresponsive to medical management or after temporary diversion. Children with bladder and sphincteric dysfunction are among the most complex seen in pediatric urology; among others, patients with diagnoses such as exstrophy, persistent cloaca and urogenital sinus, posterior urethral valves, bilateral single ectopic ureters, and prune-belly syndrome may be involved. For most pediatric urologists, patients with myelomeningocele make up the majority of patients requiring this type of surgical intervention. Consequently, many of the results discussed herein focus on the neurogenic population.
Many children with anomalies affecting the bladder and outlet are managed so that surgical intervention is not necessary, and a primary goal of pediatric urologists is to minimize the number of children requiring many of these techniques. Once conservative, medical therapy fails, surgical reconstruction remains an important and effective tool. When reconstruction is considered, it is imperative that the patient be thoroughly evaluated. Each child is unique, and the particular pathophysiologic changes must be understood so that surgical techniques available may be used thoughtfully to optimize results while minimizing morbidity. The most important factor influencing the outcome of urinary tract reconstruction in children is the commitment of the patient and family to achieving good care. Determining that commitment may at times be difficult, but its importance should not be underestimated.
The “Functional” Urinary Tract
The renal pelves and ureters should empty effortlessly into the bladder without any increase in pressure or element of obstruction. The normal ureterovesical junction prevents vesicoureteral reflux. Bladder physiology can be characterized as two different dynamic phases: passive and active. During the passive storage phase the bladder functions as a reservoir, allowing an appropriate volume of urine to be stored without leakage while maintaining low pressure. In the active voiding phase the bladder contracts for elimination of urine.
Basic Bladder Function
Passive: Storage
Appropriate urinary storage requires a reservoir that is compliant and of age-appropriate capacity. Age-based capacity may be estimated by formulas proposed by Koff (1983):
or by Kaefer and associates (1997c) for children younger than 2 years:
and for children older than 2 years:
Compliance is defined as the change in bladder volume divided by the change in pressure. Normally, the bladder is considered a highly compliant vesicle in that it will accommodate an increasing volume of urine without a corresponding increase in intravesical pressure. Multiple factors contribute to this property. Initially, the bladder is in a collapsed state that allows the storage of urine at low pressure by simple unfolding. As it expands, detrusor properties of elasticity and viscoelasticity take effect. Elasticity allows the detrusor muscle to stretch without an increase in tension until it reaches a critical volume. This volume should be greater than the expected bladder capacity. The viscoelastic bladder property allows a subtle continuous pressure change that occurs with bladder filling. It is associated with a small rise in pressure as the bladder stretches, balanced by a corresponding rapid pressure decay (Zinner et al, 1976; Wagg and Fry, 1999). With slow natural bladder filling there should be no net change in bladder pressure until capacity is reached. This viscoelastic bladder property is also defined as stress relaxation and can be overcome when the rate of bladder filling exceeds normal parameters, resulting in a pattern consistent with poor compliance (Mundy, 1984; Finkbeiner, 1999). This artifact of testing is often noted when urodynamic assessment is performed at an excessive filling rate for the child’s age or size (Bauer and Joseph,1990; Joseph, 1992). These properties of elasticity and viscoelasticity will eventually be overcome in every child, and at that point the bladder pressure rapidly rises. Favorable dynamics for urine storage include a thin bladder wall with an appropriate composition of muscle and collagen allowing expression of normal elastic and viscoelastic properties. Factors adversely affecting normal compliance include detrusor hypertrophy, fibrosis, outlet obstruction, and recurrent urinary tract infections (Mundy, 1984; Joseph, 1994).
Continence during urinary storage requires a closed bladder neck at times supported by a contracted external urinary sphincter. Fixed obstruction, neurogenic dysfunction, and chronic inflammation can affect any or all of these passive parameters, resulting in resting bladder hostility and clinical manifestations of poor compliance, upper tract deterioration, and incontinence (Brading, 1997).
Active: Voiding
Under normal conditions the active phase of voiding requires the bladder to contract after descent of the bladder neck (Morrison, 1997). Reflexive opening of the bladder neck and sequential relaxation of the external urinary sphincter allow low-pressure balanced voiding and complete elimination of urine. Again, obstruction, neurogenic dysfunction, and chronic infection can cause physiologic changes preventing coordinated function of the detrusor, bladder neck, and external sphincter, defined as dyssynergy (Mundy et al, 1985). A poorly functioning external sphincter from denervation fibrosis may also prevent appropriate relaxation, causing elevated voiding pressure against a fixed outlet. Finally, detrusor pathophysiologic changes may prevent a sustained, coordinated bladder contraction and full elimination of all urine.
Dysfunction
Upper Tracts
It is critical to understand the dynamics of the entire urinary tract before any major reconstructive procedure. Careful evaluation is imperative. In the presence of hydronephrosis, upper tract obstruction must be excluded. With severe and long-standing bladder problems, particularly those involving poor bladder compliance and emptying, upper tract obstruction is often secondary to the bladder abnormality. Nuclear renography with a catheter in the bladder to keep the bladder empty and at low pressure may be useful to rule out a primary upper tract problem. Antegrade perfusion studies with fluoroscopy and pressure measurement are occasionally necessary. Certainly, if upper tract obstruction is present it should be corrected at the time of bladder-sphincter reconstruction.
Much like obstruction, vesicoureteral reflux in the presence of a bladder abnormality may be primary or secondary. Differentiation between the two can be difficult. On occasion the history is helpful. If reflux was not present initially in a patient with neurogenic dysfunction but developed later, it is typically secondary to bladder hostility. Most reflux in patients with neurogenic dysfunction requiring bladder augmentation is likely to be secondary; reflux in patients with other problems (exstrophy, prune-belly syndrome, or posterior urethral valves) may be either primary or a fixed secondary problem if it persists until the time that bladder reconstruction is necessary. Previous work has suggested that reflux truly secondary to bladder dysfunction may not need surgical correction if the bladder is adequately managed. In those reports, neurogenic bladder patients requiring augmentation did not undergo reimplantation for secondary reflux, and virtually all of the low-grade reflux resolved with augmentation alone (Nasrallah and Aliabadi, 1991; Morioka et al, 1998; Lopez Pereira et al, 2001; Soylet et al, 2004; Juhasz et al, 2008). It is interesting to speculate whether reflux is even a significant problem if a large, compliant bladder is achieved (Soylet et al, 2004). Bacteria may ascend without reflux after reconstruction (Gonzalez and Reinberg, 1987). Experience with certain forms of continent diversion has not shown an increased risk of pyelonephritis in the absence of any antireflux mechanism if the reservoir is adequate (Helal et al, 1993; Pantuck et al, 2000). Although the authors agree that most low-grade secondary reflux is likely to resolve with adequate treatment of the bladder alone, our preference is to correct reflux at the time of bladder reconstruction, unless it is low grade or clearly secondary, because the morbidity is low. After bladder reconstruction, many patients have chronic bacteriuria, particularly those who catheterize to empty, and the absence of reflux, at least theoretically, may decrease the likelihood of ascent to the kidney. Caution must be taken when the treatment of chronically dilated and scarred ureters is considered. Correction of reflux in that setting is certainly appropriate, but one must be careful not to trade reflux for more problematic obstruction. Overaggressive tapering or tunneling of such ureters may be fraught with complications.
Dysfunction in the upper urinary tract is usually manifested by hydronephrosis, pyelonephritis, or impairment of renal function. When such problems are present in patients with lower tract dysfunction, thoughtful evaluation and treatment are necessary. All problems should be addressed at reconstructive surgery to provide the best result for the patient.
Bladder Dysfunction
Bladder dysfunction is a composite of physiologic abnormalities, and it is helpful to assess each component of passive and active bladder function independently. Elevated passive filling pressure becomes clinically pathogenic when a pressure higher than 40 cm H2O is chronically reached (McGuire et al, 1981; Wang et al, 1988; Weston et al, 1989). Pressures at this level sustained for a time impair ureteral drainage, which may result in pyelocalyceal changes, hydroureteronephrosis, and decreased glomerular filtration rate. In addition, persistent elevation in filling pressure can result in acquired vesicoureteral reflux (Sidi et al, 1986a; Cohen et al, 1990).
Pharmacologic management can play a role in decreasing filling pressure, particularly when hyperreflexic detrusor contractions are present. A combination of medications and intermittent catheterization has a positive impact, particularly in children with neurogenic dysfunction (Rink and Mitchell, 1984; Aslan et al, 2002; Verpoorten et al, 2008). Bladders that are poorly compliant because of irradiation or chronic inflammatory processes are not as likely to respond in a positive fashion to this form of therapy. When compliance is unaffected by medical management, augmentation cystoplasty will be required to improve the storage characteristics. It is expected that detrusor contractions resulting in effective emptying will be significantly diminished after augmentation. With rare exceptions, intermittent catheterization should be taught to and accepted by the patient and caretaker before urinary reconstruction is contemplated.
One of the most important contributions in the care of children with bladder dysfunction came with the acceptance of CIC described by Lapides and colleagues (1972, 1976) based on the work of Guttmann and Frankel (1966). The effective use of CIC has allowed the application of augmentation and lower tract reconstruction to groups of patients who had not previously been candidates. The principle of intermittent catheterization allows the reconstructive surgeon to aggressively correct storage problems by providing an adequate reservoir and good outflow resistance. Good spontaneous voiding, although a goal, is not imperative because catheterization can be used for emptying. Intermittent catheterization can maintain a physiologic state of complete emptying on a regular basis.
Urinary incontinence is a prominent sign of bladder dysfunction. Continence is based on outflow resistance generated by the bladder neck and external urinary sphincter. Outflow resistance must remain greater than resting bladder pressure during storage throughout normal daily activity. When outflow resistance is diminished because of an abnormal bladder neck and external urinary sphincter, incontinence often will occur. Pharmacologic management with α-adrenergic agents can enhance outflow resistance when needed, but operative reconstruction is more commonly required in that setting.
When incontinence occurs during the filling phase because of poor outlet resistance, it is essential to evaluate not only the bladder neck and external urinary sphincter but also the detrusor characteristics. Clinical experience has shown that once appropriate resistance is achieved at the bladder neck through operative intervention, adverse detrusor characteristics may become unmasked and result in high-pressure urinary storage or uninhibited contractions not previously documented (Bauer et al, 1986; Churchill et al, 1987; Dave et al, 2008b). For that reason, provocative urodynamic assessment with occlusion of the bladder neck is important before any bladder neck reconstruction in an attempt to identify children who will be at risk.
Normal synergistic voiding occurs when the bladder neck descends, relaxes, and opens followed by relaxation of the external urinary sphincter and subsequent detrusor contraction. The cascade results in low-pressure voiding. Dysfunctional voiding during this active bladder phase occurs from discoordinated activity of the bladder neck, external urinary sphincter, and detrusor and can result in urinary incontinence. In addition, when neurogenic dysfunction leads to detrusor-sphincter dyssynergy, high-pressure voiding results during a period of time, having a negative impact on the bladder and upper urinary tract (Mundy et al, 1982; Bauer et al, 1984). A similar clinical situation occurs with a fixed, fibrotic external sphincter. Initial treatment usually involves pharmacologic management and mechanical elimination of urine from the bladder through CIC in an attempt to bypass the abnormal voiding mechanics.
Other Considerations
Renal function should be assessed in any patient undergoing bladder reconstruction, particularly if hydronephrosis or severe renal scarring is present. Demos (1962) and Koch and McDougal (1985) have demonstrated that urinary solutes, particularly chloride, are absorbed from urine in contact with the mucosa of small and large bowel. For patients with normal renal function, the kidneys are able to handle the reabsorbed load of chloride and acid without obvious difficulty. Patients with decreased renal function, however, may develop significant metabolic acidosis secondary to such reabsorption. If acidosis exists preoperatively, it will invariably worsen if urine is stored in small or large intestinal segments (Mitchell and Pizer, 1987). The first component of renal function to deteriorate after obstruction or infection is typically concentrating ability. Patients with compromised function may generate enormous volumes of urine. The bladder volume achieved through bladder reconstruction must accommodate the patient’s urinary output for an acceptable time, usually about 4 hours. Patients with renal failure or other medical problems may conversely develop oliguria. Low urinary output may affect an augmented bladder or bowel reservoir because there is greater potential for collection and inspissation of mucus. There is also less urine for dilution and buffering of gastric secretions if stomach is used.
Abnormal function of other organ systems also influences the risk of bladder reconstruction with use of intestinal segments. Reabsorption of ammonia by large or small intestinal segments in contact with urine may be dangerous for patients with hepatic failure (McDougal, 1992a). Some medications excreted in urine may be reabsorbed by bowel mucosa (Savauagen and Dixey, 1969). Therefore, liver function tests and arterial blood gas studies may be appropriate for some patients. Careful history should be taken of the patient’s preoperative bowel function; this is particularly true of adult patients who may have acquired, or secondary, gastrointestinal problems. Obviously, short-gut syndrome is a concern among patients with cloacal exstrophy, prior bowel resections, or a history of significant irradiation. A history of chronic diarrhea or fecal incontinence preoperatively should signal concern about use of the ileocecal valve in urinary reconstruction.
A critical factor to consider is the commitment of the patient and family. Urinary incontinence, at times, protects some patients from infection and upper tract deterioration. Effective storage can put the patient at risk for such problems if emptying is not accomplished on a regular basis. All must be aware of the responsibility that goes along with bladder reconstruction and urinary continence.
The timing of appropriate surgical intervention may vary dramatically among patients. It is sometimes necessary to perform early reconstruction when the upper tracts and renal function are threatened. This situation most often occurs in the presence of high outflow resistance and poor bladder compliance. More commonly, intervention may be undertaken later to achieve urinary continence. The age at which urinary incontinence becomes socially unacceptable varies among patients and families.
When appropriate, it is beneficial for the patient and family to wait for bladder reconstruction until all needs of the patient are identified. With intervention because of infection or hydronephrosis this is not always possible. When reconstruction is undertaken to achieve continence it is most efficient to identify all reconstructive issues and to address them with one operation. Good urodynamic assessment is usually necessary to determine whether a procedure to increase outflow resistance is necessary in addition to bladder augmentation or replacement. Introduction of intermittent catheterization preoperatively is mandatory in that it allows the patient to demonstrate the ability and desire to do so on a regular basis. It is also helpful in determining whether a continent abdominal wall stoma may help improve the reliability of catheterization and increase the patient’s independence. Likewise, particularly in the neurogenic population, it is advantageous to identify the patient who may also benefit from an antegrade colonic enema. It is certainly better for the patient and surgeon to address all of these issues at one operative sitting rather than with sequential procedures that may add morbidity.
Evaluation of the Patient
Each patient should have upper tract imaging before bladder reconstruction. Most patients with significant bladder problems will have had routine ultrasonography as part of their surveillance. If hydronephrosis is present, obstruction and vesicoureteral reflux should be sought with a functional renal study and voiding cystography. Nuclear renography with a catheter in the bladder is usually adequate to rule out a primary upper tract obstruction. Reflux should be excluded with a voiding study, although that study may be done as part of videourodynamics. In the presence of any hydronephrosis, each patient should have determinations of serum electrolytes, blood urea nitrogen, and serum creatinine. For patients with elevation of the serum creatinine concentration or significant hydronephrosis, a 24-hour urine collection for both creatinine clearance and urine volume should be obtained.
Urodynamics
Bladder Dynamics: Capacity and Compliance
Urodynamic assessment of the lower urinary tract plays an essential role in considering bladder reconstruction. It can provide reproducible results in infants and children but requires meticulous attention to detail (Joseph, 1994). Several mechanical factors may adversely influence urodynamics data, creating artifacts that can have a negative impact on the validity of the evaluation if they are not recognized. Testing is typically performed with transurethral catheter placement in this population of patients. The size of the catheter can lead to the appearance of elevated leak or voiding pressure and the inability to empty well, particularly in infants and young boys (Decter and Harpser, 1992). Suprapubic catheter placement circumvents this problem but is not practical in most cases. The testing medium and infusion rate can influence the results. Carbon dioxide is not as reliable as fluid infusion, particularly in evaluation of bladder compliance and capacity. The most common fluids used for testing are saline and iodinated contrast medium, both of which provide reproducible results (Joseph, 1993). Use of testing media at body temperature is also appropriate (Joseph, 1996). End filling pressure, and therefore bladder compliance, can be dramatically affected by simply changing the filling rate (Joseph, 1992). Bauer (1979) has suggested that cystometrography be performed at a fill rate of no more than 10% of the predicted bladder capacity per minute.
Sphincter Dynamics: Outflow Resistance
The bladder neck and external urinary sphincter work in synergy, but only one is required to maintain urinary continence. Often, neurogenic dysfunction leads to abnormalities of both the bladder neck and external urinary sphincter, resulting in diminished outlet resistance during storage or dyssynergic function with voiding. Monitoring of external urinary sphincter electrical activity is required to evaluate coordinated voiding and dyssynergic detrusor-sphincter activity. Perineal surface electrodes, abdominal wall sensors, anal plugs, vaginal monitors, electrical wires, and concentric needle electrodes have all been used for electromyography (Joseph, 1996). In children with neurogenic dysfunction, a concentric needle electrode or dual needle electrodes placed through a 25-gauge needle increase accuracy in measuring sphincter activity (Blaivas et al, 1977; Joseph, 1996).
The functional length of the external urinary sphincter also plays a role in outflow resistance, and its measurement can be undertaken with urethral pressure profilometry. Unfortunately, the short length and small diameter of the pediatric urethra make this study technically more difficult to perform than in the adult because the mechanical pulling device is not practical for pediatric use. To assess urethral pressure profile, a constant infusion of testing medium at a rate of 2 mL/min with use of a continuous Harvard pump is required (Joseph, 1996) to eliminate pressure wave artifacts noted with the standard roller ball infusion pumps. Hand withdrawal of the catheter is done while every 5 mm is marked on the recording strip. With practice, reliable measurements can be obtained. There is limited value in comparing specific urethral pressure profile results for a patient against a standard uroflow nomogram; however, a preoperative urethral pressure profile for a given patient provides baseline information that can be beneficial in assessing the intraoperative and postoperative functional urethral length.
Some surgeons use leak point pressure to evaluate outflow resistance, the bladder pressure causing leakage per urethra, which can be determined during passive filling and Valsalva maneuver. Fluoroscopy can be informative during those events. The leak point pressure may be artifactually elevated by the urodynamics catheter in a small male urethra (Decter and Harpser, 1992). Much work remains to determine how well such measurable parameters correlate and identify which patients require a procedure on the outlet to achieve adequate continence.
Bladder Emptying
The patient’s ability to empty the bladder before reconstruction should be assessed carefully. Useful parameters related to bladder emptying include synergistic relaxation of the external sphincter on electromyography, urinary flow rates, and measurements of postvoid residual urine. Neurologically normal patients who are able to empty the bladder well preoperatively are much more likely to do so after reconstruction than are patients who have neurogenic dysfunction or those who are unable to empty well preoperatively. No test ensures that a patient will be able to void spontaneously and empty well after bladder augmentation or other reconstruction. Therefore, all patients must be prepared to perform CIC postoperatively. The native urethra should be examined for the ease of catheterization. Ideally, the patient should learn CIC and practice it preoperatively until the patient, family, and surgeon are comfortable that catheterization can and will be done reliably. Physical and psychosocial limitations of the patient must be considered in regard to the ability to self-catheterize and to perform self-care. Failure to catheterize and empty reliably after bladder reconstruction may result in upper tract deterioration, urinary tract infection, or bladder perforation despite a technically perfect operation. Most patients who may catheterize per native urethra or an abdominal wall stoma will overwhelmingly prefer the latter (Horowitz et al, 1995).
Preparation of the Patient
Bladder and sphincter reconstructive procedures remain some of the most challenging in urology. The general status of patients should be optimized so that they have the best chance to achieve a good result with the least risk of morbidity. Each patient’s general nutritional and hydration status should be determined and corrected, if necessary, before surgery. Coexisting medical problems, particularly cardiac and pulmonary, should be well managed preoperatively.
Bowel Preparation
Historically, each patient has undergone preoperative bowel preparation to minimize the potential risk of surgery if the use of any bowel is contemplated. Even when ureterocystoplasty or other alternatives are planned, intraoperative findings may dictate the need for use of a bowel segment. A clear liquid diet for 2 days before bowel preparation aids in clearing solid stool. The patient should then undergo full mechanical bowel preparation the day before surgery. Such bowel preparations have usually been done in the hospital (O’Donnell, 2007); however, recent trends find this changing to an outpatient setting. Major reconstructive procedures often require many hours of operative time with large fluid shifts. It is critical that the patient be well hydrated at the time of surgery. There should be a low threshold for the use of intravenous fluids during the preoperative day. The use of oral antibiotics in bowel preparation, once dogma, is now a matter of personal preference (Breckler et al, 2007). Special attention must be paid to the bowel preparation of patients with neurogenic dysfunction. Most of these patients have chronic constipation. Good bowel cleansing is difficult in such patients and should be done aggressively. Several days of oral cathartics and a clear liquid diet at home may be helpful. Patients with renal insufficiency should be observed carefully during bowel preparation for dehydration and electrolyte disturbances.
Theoretically, gastric contents are sterile, and parenteral antibiotics and a routine bowel preparation are not necessary before gastrocystoplasty. In one study (Gundeti et al, 2006) it was suggested that routine bowel preparation may not be necessary before ileocystoplasty in children.
Urine Culture
All patients should have a urine culture performed several days before bladder reconstruction. The bladder should not be opened with infected urine, which will spill intraperitoneally. Any patient with a positive preoperative urine culture should undergo treatment with either oral or intravesical antibiotics and have a second culture documenting sterile urine before the procedure. Many pediatric patients undergoing augmentation cystoplasty have spina bifida and a ventriculoperitoneal shunt. With a negative urine culture and good bowel preparation the incidence of shunt infection or problems should be low (Yerkes et al, 2001; Hayashi et al, 2008).
Cystoscopy
Preoperative cystoscopy may be the final step in evaluating the native bladder, outflow, or ureteral orifices in pediatric patients. Endoscopy should be performed immediately before bladder reconstruction under the same anesthetic. In adult patients with interstitial cystitis or irritative bladder symptoms requiring augmentation cystoplasty, cystoscopy should be performed well before augmentation to rule out urothelial carcinoma in situ. Bladder biopsy and cytologic examination of the urine may be helpful in excluding tumor. On the basis of history, an occasional patient may warrant endoscopic or radiographic gastrointestinal evaluation.
Key Points: Evaluation and Preparation
Antireflux
Ureteral reimplantation into a native bladder, whether the ureter is dilated or not, is a standard procedure familiar to all pediatric urologists and reconstructive surgeons. The long-term success rate is good, and complications are rare. Such reimplantation is certainly preferable and usually possible during lower tract reconstruction.
Transureteroureterostomy and Single Ureteral Reimplantation
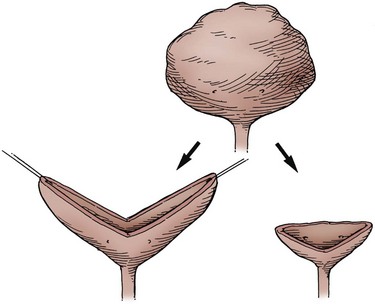
On occasion, the urinary bladder is so small that it is inadequate for bilateral reimplantation, and alternatives must be considered. It is preferable to reimplant both ureters separately, although if the native urinary bladder is small and adequate for only a single ureteral tunnel, transureteroureterostomy and a single reimplant may be helpful (Fig. 129–1). Typically, the better ureter should be implanted into the bladder, draining the other across into it. The crossing ureter should be mobilized to swing gently across the abdomen to the recipient side in a smooth course without tension. It should be carefully mobilized with all of its adventitia and as much periureteral tissue as possible to preserve blood supply. Care must be taken not to angulate the crossing ureter immediately beneath the inferior mesenteric artery. The crossing ureter is widely anastomosed to the posteromedial aspect of the recipient ureter. The recipient is not mobilized or brought medially to meet the end of the other ureter. Transureteroureterostomy, when it is fashioned appropriately, is successful and carries minimal risk for leakage or obstruction (Hodges et al, 1963; Hendren and Hensle, 1980; Noble et al, 1997; Mure et al, 2000). A history of previous calculi remains a relative contraindication to transureteroureterostomy.
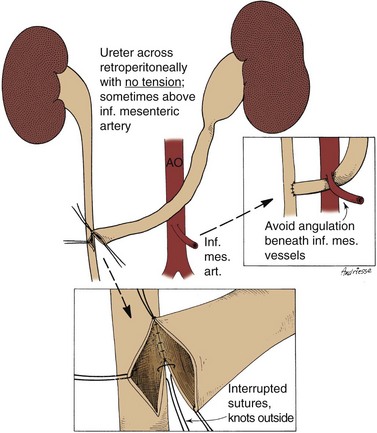
Figure 129–1 Technique for transureteroureterostomy. AO, aorta; Inf. mes. art., inferior mesenteric artery.
(Courtesy of W. Hardy Hendren.)
The manner in which the bladder is opened may optimize its use for a single reimplant. Rather than incision of the bladder in the anterior midline, a wide, anterior U-shaped incision based cephalad can be made. This potentially elongates the bladder as a posterior plate that can be brought to one side or the other to meet a single ureter. Incision of the bladder in this way may also be useful in placing a continent catheterizable stoma to the umbilicus when the native bladder otherwise will not reach that far. For ureteral reimplantation after such an incision, a psoas hitch of the bladder fixes the bladder in position for a long, straight ureteral tunnel.
Psoas Hitch
Fixation of the bladder to the psoas muscle allows precise control of both the length and direction of a ureteral reimplant. Securing the bladder in this manner helps the bladder reach a short ureter or development of a long tunnel when necessary. A psoas hitch should prevent any angulation of the ureter with bladder filling. Such angulation may be particularly problematic or obstructive with a dilated and scarred ureter. The bladder should be secured to the psoas muscle and fascia with nonabsorbable sutures. Those sutures must not enter the bladder lumen or stones will occur. Broad, shallow purchases of the psoas muscle and fascia should be used to hold long term without trapping the sciatic nerve. The hitching sutures should not be tied so tightly that they cut through either bladder or psoas muscle. The contralateral bladder pedicle may be divided to increase bladder mobility and the length of the hitch on occasion. In general, the ureteral reimplantation should be performed before the bladder psoas hitch (Fig. 129–2).
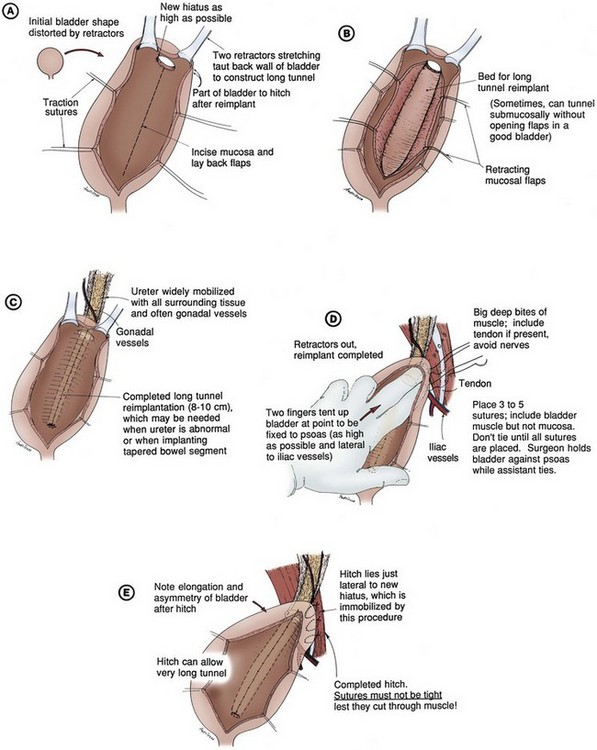
Figure 129–2 A to E, Technique for psoas hitch, which allows the construction of a long reimplantation tunnel and prevents angulation of the ureter or tapered bowel segment when the bladder fills. Monofilament nonabsorbable suture material is used to fix the bladder to psoas muscle. Care must be taken to avoid entering the bladder, which might cause stone formation on a suture.
(Courtesy of W. Hardy Hendren.)
Antireflux with Intestinal Segments
Necessity of ureteral reimplantation into an intestinal segment may occasionally determine the segment to be used for bladder augmentation or replacement. Long experience with ureterosigmoidostomy and colon conduit diversion has established an effective means of antireflux into a colonic segment. A flap valve mechanism can be constructed by tunneling the ureter beneath a taenia. Important principles learned from ureterosigmoidostomy include a direct mucosa-to-mucosa anastomosis and a submucosal tunnel of adequate length. This technique, familiar to most urologists, has provided favorable long-term results since the 1950s (Nesbit, 1949; Goodwin et al, 1953; Leadbetter and Clarke, 1954) and is based on the initial work of Coffey (1911). Implantation may be done from inside the reservoir with the intestinal segment open or from without after the intestinal segment has been completely reconfigured and closed.
If a gastric segment is used for bladder augmentation or replacement, ureters may be reimplanted into the stomach in a manner remarkably similar to that used in the native bladder. It is easy to form a submucosal tunnel with good muscle backing. The same principles for choosing the length of tunnel relative to the width of the ureter are used as with bladder. Construction of an effective antireflux mechanism into an ileal segment is more difficult. The split-nipple technique described by Griffith may prevent reflux at least at low reservoir pressure (Turner-Warwick and Ashken, 1967; Patil et al, 1976; Stone and MacDermott, 1989; Sagalowsky, 1995). A short longitudinal incision is made in the distal ureter and the ureteral wall turned back on itself. The nipple should be at least twice as long as the width of the ureter. The cuff is stabilized by suturing the ureter to itself. The adventitia of the ureter immediately proximal to the cuff is then approximated to the full thickness of the ileal wall at the hiatus so that the cuff protrudes into the lumen. Le Duc and associates (1987) described a technique in which the ureter is brought through a hiatus in the ileal wall. From that hiatus the ileal mucosa is incised and the edges are mobilized to construct a trough for the ureter. The spatulated ureter is laid into the trough and approximated to the mucosa at the distal end. The ileal mucosa is sutured to the lateral edges of the ureter and should eventually grow over it. Long-term results with these two techniques have been conflicting but generally have not proved as reliable as a tunneled ureterocolonic anastomosis in preventing reflux (Patil et al, 1976; Le Duc et al, 1987; Rowland, 1996; Bihrle, 1997), with one exception (Lugagne et al, 1997). It is also possible to construct an antirefluxing, serosa-lined tunnel between two limbs of ileum as described by Abol-Enein and Ghoneim (1999; Soygur et al, 2005).
Reinforced nipple valves of ileum have been used extensively for antireflux with the Kock pouch (Skinner et al, 1989). After several modifications by Skinner, good long-term results have been achieved. This technique requires a relatively long segment of ileum and use of permanent staples. Attempts have been made to secure the nipple long term without staples or mesh (Hanna and Bloiso, 1987; Gosalbez and Gousse, 1998; Tsuchiya et al, 2004). Maintenance of the intussuscepted cuff is the key to a successful result. The same forces that compress the nipple to achieve antireflux or continence tend to evert or destabilize it. An ileal nipple valve may be particularly useful with short, dilated ureters; an isoperistaltic segment of ileum may be left with the nipple to replace a short ureter. Likewise, the antireflux mechanism is based on the ileal segment and not the ureter. Consequently, a very dilated ureter may be anastomosed to the ileum without tapering. In some neobladders, an isoperistaltic limb of ileum is positioned between the reservoir and ureters to discourage reflux, at least at low pressures (Studer and Zingg, 1997).
Bladder Neck Reconstruction
One of the greatest technical challenges facing the surgeon in bladder reconstruction is providing adequate outflow resistance. The bladder neck may be incompetent due to neurogenic dysfunction secondary to spinal dysraphism. Other underlying pathologic conditions, such as exstrophy, bilateral ectopic ureters, and ureteroceles, are unique problems that must be considered in addressing outlet resistance. Many operative techniques have been described for bladder neck reconstruction, indicating that no single option is best for all patients (Kryger et al, 2000; Cole et al, 2003; Lemelle et al, 2006; Dave et al, 2008a). Khoury and associates (2008) have challenged the need for bladder neck reconstruction in the incontinent neurogenic patient with severe bladder trabeculation. The necessity of preoperative evaluation and thorough knowledge of the patient’s specific physiologic limitations cannot be overstated.
The capability for a sustained bladder contraction to result in complete emptying by voiding or the absence of hyperreflexic contractions may influence the technique selected for gaining outlet resistance. Any operative technique that increases outlet resistance may do so at the expense of detrusor contractibility. For reasons not clearly defined, increasing outlet resistance can change the behavior of the detrusor into a noncompliant hostile environment (Bauer et al, 1986; Burbige et al, 1987; Churchill et al, 1987). Provocative cystometry may identify only some patients at risk, and close postoperative observation is mandatory (Kronner et al, 1998b).
A spectrum of techniques are available for repair; these encompass tightening of the bladder neck, construction of a flap valve mechanism, placement of artificial or autologous bulking agents, and the artificial urinary sphincter. The selected option should be individualized to the patient’s pathologic process, needs, and personal goals.
An important consideration is the patient’s ability to empty well before reconstruction and the likelihood that the patient can afterward. Some of these repairs may prohibit spontaneous voiding. In many patients, particularly patients with neurogenic dysfunction also requiring bladder augmentation, voiding cannot be expected and the major concern is providing adequate outflow resistance. For those with other diagnoses, especially if augmentation cystoplasty is not necessary, spontaneous voiding remains a key goal.
The following discussion covers a variety of operative techniques used to achieve urinary continence through bladder neck and external urinary sphincter reconstruction. Most of the results are based on experience in individuals with spinal dysraphism, but the techniques may be used with other pathogenic conditions. All techniques have a learning curve, which necessitates careful analysis of results and forthright reporting. An evidence-based review of operative bladder neck procedures found assessment of results to be limited by several factors, including the lack of a true, consistent definition of “success” and “continence,” consideration of patients with and without concomitant bladder augmentation, and evaluation of small populations of patients with mixed pathologic conditions (Joseph et al, 2003).
Young-Dees-Leadbetter Repair
The Young-Dees-Leadbetter bladder neck reconstruction is one of the most recognized operative techniques to increase outlet resistance. The original Young procedure has evolved and remains of primary consideration in reconstruction of the exstrophic bladder neck (Ferrer et al, 2001). It has been used in patients with many diagnoses.
Technique
Young’s initial description (1919) of excising a portion of the bladder neck and significantly tightening the bladder neck over a silver probe was modified by Dees (1949), who extended the length of excised tissue through the trigone. Leadbetter (1964) followed by elevating the ureters off the trigone, placing them in a more cephalad position on the bladder floor. This allowed tubularization of the trigone and further enhanced lengthening of the urethra. A detailed description and illustrations are found in Chapter 124.
Results
Reports of success with the Young-Dees-Leadbetter bladder neck reconstruction in children with neurogenic sphincter dysfunction are limited, not only in the number of patients but also in overall improvement. Tanagho (1981) and Leadbetter (1985) independently reviewed their long-term results and showed minimal success in individuals with neurogenic dysfunction. They speculated that the lack of success was due to a lack of muscle tone and activity in the wrapped muscle related to the underlying neurogenic problem. Many patients in the early series did not undergo bladder augmentation, possibly compromising the continence achieved. Contrary to the results reported in exstrophy patients, the majority of individuals with neurogenic deficiency of the bladder neck will require bladder augmentation and intermittent catheterization. Sidi and colleagues (1987b) documented a 4-hour continence interval in 7 of 11 patients after such repair, although 10 required catheterization and 9 required augmentation. Five of the 7 needed reoperation to achieve continence. This small series represents one of the more recent long-term results of the Young-Dees-Leadbetter reconstruction in children with neurogenic dysfunction because it has largely fallen out of favor. In an attempt to enhance the Young-Dees-Leadbetter procedure, Mitchell and Rink (1983) described the addition of external support and compression achieved through the placement of a silicone sheath around the reconstructed bladder neck. This was somewhat done to establish a plane for future placement of an artificial sphincter cuff, if necessary. In place, it seemed to improve the function of the repair by either improving coaptation or by maintaining the proximal repair in a better anatomic position. Unfortunately, most of the thicker Silastic sheaths eventually eroded and disrupted the repairs (Kropp et al, 1993). Quimby and colleagues (1996) used a thinner Silastic sheath without the same risk of erosion. They also wrapped omentum between the repair and Silastic wrap. The authors reported better results for continence and thought that placement of an artificial sphincter cuff was much easier when needed.
Donnahoo and coworkers (1999) reviewed one of the largest series of the repair used in treatment of neurogenic incontinence (38 children, 25 of whom were girls). A primary repair was performed in 24 children, a secondary procedure in 6, and a primary repair in conjunction with a silicone sheath in 8. Partial continence was achieved after the initial repair in 26 children (68%). All children with the silicone sheath were initially continent, but erosion occurred in 5. More critically, 35 (92%) of the children required augmentation cystoplasty to become continent. The authors found that although continence could be achieved with this technique, it was at the expense of augmentation cystoplasty and multiple procedures. Their results are similar to those reported by Cole and associates (2003).
Fascial Sling
Sling procedures were developed in an attempt to increase resistance at the bladder neck. Both artificial and natural tissue have been used with similar technique. Resultant coaptation and elevation of the bladder neck should cause approximation of opposing epithelial surfaces and increased outlet resistance that is greater than the resting bladder pressure and the pressure achieved during stressful activity or Valsalva behavior. With sling coaptation, the bladder neck remains fixed; and although a strong detrusor contraction can establish a voiding pressure leading to urine flow, it rarely allows adequate bladder emptying in the face of anatomic or neurologic problems. The majority of pediatric patients who undergo a sling procedure must be prepared for intermittent catheterization. The resistance achieved with bladder neck slings can potentially be overcome by hyperreflexic bladder contractions or elevated pressure due to diminished bladder compliance. Therefore simultaneous bladder augmentation has again been reported in 55% to 100% of patients who achieve urinary continence after a sling procedure (Bauer et al, 1989; Elder, 1990; Decter, 1993; Kakizaki et al, 1995; Perez et al, 1996a; Dik et al, 1999, 2003; Walker et al, 2000; Bugg and Joseph, 2003; Cole et al, 2003; Godbole et al, 2003). Snodgrass and associates (2007) have reviewed their series of 30 consecutive patients with neurogenic incontinence who underwent bladder neck fascial sling only. They conclude continence can be achieved without upper tract damage and the adverse effect of enterocystoplasty. Others have cautioned taking this approach (Dave et al, 2008b). Alternatives to fascia, such as an expanded fluorocarbon polymer (Gore-Tex), have been used in a similar fashion, although early continence has not been maintained (Godbole and Mackinnon, 2004). Good early results have been noted with small intestinal submucosa and other biodegradable scaffolds (Colvert et al, 2002; Misseri et al, 2005).
Technique
The bladder neck is exposed by clearing fatty tissue overlying the bladder neck and the lateral endopelvic fascia. An incision is made within the endopelvic fascia for approximately 2 cm. The junction between the bladder neck and proximal urethra can be identified by placing a transurethral catheter into the bladder and gently pulling down on the catheter to lodge the balloon at the bladder neck. By blunt dissection, a plane between the posterior bladder neck and vagina in girls or rectal wall in boys is developed. The proper plane may be more easily developed from the cul-de-sac by dissecting behind the bladder and ureters from above (Lottmann et al, 1999; Badiola et al, 2000). If the landmarks are not easily defined, as in a secondary repair, the dissection becomes difficult. It may be appropriate to open the bladder to help prevent inadvertent dissection into the urethra or posterior structures. Storm and associates (2008) reported on a robotic-assisted laparoscopic approach for posterior bladder neck dissection and placement of the sling. This may become more appealing as robotic technology expands into bladder reconstruction. Dik and colleagues (2003) proposed a transvaginal approach, eliminating the need to open the bladder or to dissect between the bladder neck and anterior vagina.
When fascial tissue is used the technique is based on that described by McGuire and Lytton (1978) for stress urinary incontinence. Rectus abdominis fascia 1 cm in width and an appropriate length is harvested. This fascia can be taken in either vertical or horizontal fashion, depending on the initial skin incision. Fascia from other sites has been used in a similar fashion but requires a second incision. Often poor nutritional states and prior abdominal procedures limit the availability of rectus fascia, resulting in the use of alternative material such as small intestinal submucosa, cadaveric tissue, biodegradable scaffolds, and bladder wall (Colvert et al, 2002; Misseri et al, 2005; Albouy et al, 2007). All are generally secured to the anterior rectus fascia on either side. Autologous fascial tissue has been used, combining the benefits of a compressive wrap and suspension of the proximal urethra and bladder neck. Several variations of fascial placement and configuration have been described (Woodside and Borden, 1982; McGuire et al, 1986; Elder, 1990; Perez et al, 1996a; Bugg and Joseph, 2003; Dik et al, 2003). When fascial slings and wraps are used for neurogenic sphincter incontinence there is not as much concern for making a wrap or sling that is too tight because most patients are preferentially managed with CIC.
Results
Fascial slings have been used more extensively and with better results in girls with neurogenic sphincter incompetence, although some success has recently been reported in boys. Overall long-term success with fascial slings in the neurogenic population has varied greatly from 40% to 100% (Kryger et al, 2000; Castellan et al, 2005; Misseri et al, 2005; Snodgrass et al, 2007). A variation thought to contribute to a higher success includes a circumferential fascial wrap around the bladder neck. A circumferential wrap may equalize the compressive pressure over a greater surface area of bladder neck and posterior urethra (Walker et al, 1995; Strang et al, 2006). With a 360-degree wrap, simultaneous suspension has also been applied (Bugg and Joseph, 2003). Success rates have varied so much that it is difficult to determine whether any modification of the sling accounts for an increase in continence. Most patients who have undergone a fascial sling or wrap have also had simultaneous bladder augmentation. Success of the sling, as with most repairs, appears to be improved with augmentation cystoplasty in this patient population by almost all reports (Castellan et al, 2005). Perez and associates (1996a) reviewed the outcome of sling cystourethropexy in 39 children, 15 of whom were boys. One of four techniques was performed. When postoperative continence was evaluated on the basis of age, sex, underlying diagnosis, preoperative urodynamics, surgical technique, and enterocystoplasty, only concomitant enterocystoplasty was predictive of a successful outcome.
Contrary to the Silastic sheath, fascial sling erosion rarely occurs. Gormley and coworkers (1994) reported a revision rate with fascial slings of 15%. Placement of a fascial sling does not eliminate the possibility of later placement of an artificial urinary sphincter (Decter, 1993; Barthold et al, 1999). It is not unreasonable to consider placement of a fascial sling in a child with a marginally competent bladder neck and posterior urethra if the child is undergoing augmentation cystoplasty and already requires intermittent catheterization.
Bladder Neck Bulking Agents
Vorstman and colleagues (1985) reported one of the initial descriptions of injection therapy with a bulking agent into the bladder neck of incontinent children. The initial enthusiasm for use of polytetrafluoroethylene was quickly tempered because of concern about migration of particles to regional and distant sites including pelvic nodes, lungs, brain, kidney, and spleen found in animal models (Malizia et al, 1984). The technique remains of interest, and several alternatives to polytetrafluoroethylene have been assessed, including glutaraldehyde cross-linked collagen, dextranomer/hyaluronic acid copolymer, and polydimethylsiloxane (Leonard et al, 1990a; Guys et al, 2006; Knudson et al, 2006; Lottmann et al, 2006; Dean et al, 2007). Bovine collagen should not be used in latex-sensitive children with spina bifida because the product is not latex free (Kryger et al, 2000). In an attempt to achieve an ideal substance for injection, investigation is ongoing with autologous cartilage cells harvested from a separate site and then grown in an alginate matrix for endoscopic implantation (Bent et al, 2000). Although preliminary results have been obtained in adult women with stress incontinence, the material has not achieved an enthusiastic use in pediatrics. Whether this or other materials will be an appropriate alternative for neurogenic sphincter incontinence in children is yet to be seen.
In an attempt to alleviate the risks with injection of a foreign biologic product, alternatives to collagen and bovine have been investigated. Polydimethylsiloxane is one such agent. It is composed of sterile solid textured silicone particles, with an average size of 200 µg, suspended in a biologic hydrogen carrier. The large size of the particles should virtually eliminate lymphatic and distant migration (Beisang and Ersek, 1992; Guys et al, 1999; Halachmi et al, 2004; Guys et al, 2006). Contemporary reports favor the use of dextranomer/hyaluronic acid (Lottmann et al, 2006; Dean et al, 2007; Dyer et al, 2007).
Technique
Endoscopic exposure is used to localize the proximal urethra and bladder neck. Injection can be done directly through the working channel of the endoscope, often one with an offset lens system. Ideal placement of the material is in a subepithelial space, mobilizing the epithelium toward the lumen of the bladder neck. When it is completed in a circumferential fashion, adequate epithelial coaptation may occur that effectively raises outlet resistance. Alternatively, periurethral injection in women with a long needle placed from the perineum or through a suprapubic approach has been used. Dean and associates (2007) advocate simultaneous antegrade and retrograde endoscopic injection. Evidence is lacking whether the exact approach affects success, but accurate placement is important and transurethral injection generally preferred.
Results
The durability and success of bladder neck and proximal urethral injection remain in doubt for the pediatric population, particularly those with neurogenic dysfunction. True continence, as defined by a 4-hour dry period between voidings or catheterization, has been reported to be at most 64% and has ranged as low as 5% (Leonard et al, 1990a; Capozza et al, 1995; Bomalaski et al, 1996; Perez et al, 1996b; Sundaram et al, 1997; Silveri et al, 1998; Guys et al, 2001, 2006; Godbole et al, 2003; Halachmi et al, 2004; Lottmann et al, 2006; Dean et al, 2007). Several factors play a role in the outcome, one of which is a history of any previous operative bladder neck repair. Success is enhanced by elevation of the epithelium of the bladder neck, which may be compromised by scarring from previous operative procedures. The concept of a minimally invasive operation used to enhance a marginal result gained from a more formal bladder neck repair is enticing; unfortunately, the data are lacking to show that bladder neck injection is of lasting value in that setting.
Sundaram and colleagues (1997) reported on the efficacy and durability of glutaraldehyde cross-linked bovine collagen in 20 children, 12 of whom had neurogenic sphincter dysfunction. More than half of the children required two or three independent injections. Success was achieved in only 1 patient (5%), who was considered dry; 5 had some improvement, and 10 had either no change or transient improvement of only 2 to 90 days. Thus collagen therapy only delayed the ultimate need for bladder neck reconstruction.
Submucosal bladder neck injection of bovine dermal collagen was used by Perez and coworkers (1996b) in 32 patients. Continence was achieved after a single injection in only 20% of the children with neurogenic dysfunction. Complications were limited to febrile urinary infections, one episode of urinary retention, and worsening incontinence in 2 patients. The authors concluded that even though their success was limited the low morbidity and ease of placement justified submucosal injection in selected children.
Guys and associates (2006) treated 49 children with polydimethylsiloxane, 41 of whom had neurogenic bladder neck and sphincter incontinence. The level of continence continued to deteriorate through 18 months, then stabilized. At an average follow-up of 73 months, success was achieved in 16 (33%) and 7 (14%) were improved. Godbole and colleagues (2003), Halachmi and associates (2004), and Dyer and coworkers (2007) independently came to the conclusion that regardless of the material injected (polytetrafluoroethylene, collagen, polydimethylsiloxane, dextranomer/hyaluronic acid), short-term success is not long lasting.
Kitchens and colleagues (2007) evaluated the use of dextranomer/hyaluronic acid to correct incontinence in patients who had previously undergone bladder neck reconstruction. They achieved success or improvement in 70% of select patients at 17 months, indicating this may be an alternative to other salvage therapy.
Overall, the cost of the bulking agents can be excessive and there does not appear to be any financial benefit over a formal repair (Kryger et al, 2000). At present, bulking agents play a limited role for increasing outlet resistance and should be reserved for a select group of patients. The exact criteria that define that group have not been established. Patients with marginal native outflow resistance are probably better candidates than are those with minimal preoperative function.
Artificial Urinary Sphincter
The artificial urinary sphincter has been recognized as a device that can result in prompt continence in select children while preserving their ability to void spontaneously. The artificial urinary sphincter was introduced by Scott in 1974. The general concept and design of the initial model have been retained; however, improvements and enhancements have evolved that have positive impact on the long-term success of the artificial urinary sphincter. The current 800 model includes a seamless, pressurized balloon reservoir, nonkink tubing, and changes in the cuff that facilitate its placement and effectiveness with coaptation of the bladder neck and proximal urethra (Light and Reynolds, 1992; Barrett et al, 1993; Lai et al, 2007). Alternatives to the AS800 model have also been explored (Vilar et al, 2004).
Technique
Placement of the cuff should be at the level of the bladder neck in all female patients and prepubertal boys (Fig. 129–3). It is also the most desirable and effective location in pubertal boys and adult men with neurogenic sphincter incompetence. The bulbar urethra can be used as an alternative site in adult men with mature spongiosum. Levesque and associates (1996) have indicated that age is not a factor in placement of the cuff around the bladder neck. They found that children do not outgrow the artificial urinary sphincter as they progress through puberty and that replacement of the cuff is not routinely necessary. The artificial urinary sphincter cuff can be positioned around intestinal segments used in total urinary reconstruction but is more susceptible to erosion there. Several authors have described the successful placement of the cuff around a bowel segment, particularly when omentum is interposed between the cuff and the segment (Burbige et al, 1987; Weston et al, 1991; Light et al, 1995).
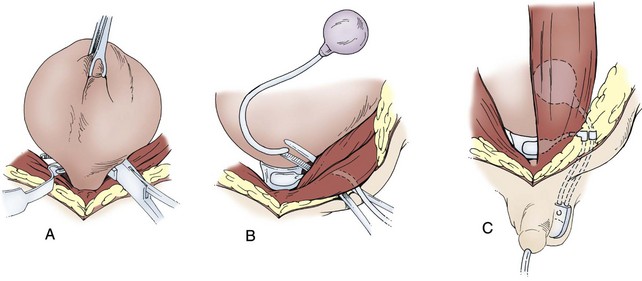
Figure 129–3 Artificial urinary sphincter placement in children. A, The cuff is placed around the bladder neck in prepubertal children. Dissection may be facilitated by palpation of a urethral catheter and balloon or by a posterior approach. If dissection is difficult, the bladder can be opened. B, The reservoir is placed beneath the rectus muscle. C, A tunnel is then made into the scrotum or labia for positioning of the pump on the same side as the reservoir. Tubing connections are made ventral to the rectus fascia, and the device is initially left deactivated.
Placement of an artificial sphincter in children is the same as that described for adults. Development of the proper plane for the cuff is virtually identical to that described for a fascial sling. The cuff should be sized snugly, but not tightly, around the bladder neck. Obviously, a sterile environment is critical in considering placement of the artificial urinary sphincter to avoid infection. For that reason, preoperative antibiotics are a necessity, and confirmation of sterile urine is required. With those precautions there is freedom to open the bladder in dissecting around the bladder neck. This will often facilitate the dissection and ensure proper placement. The AS800 model has a locking mechanism in the pump that permits the artificial urinary sphincter to be deactivated and activated without a second operative procedure. Experience has shown that leaving the unit deactivated with the cuff deflated after placement allows formation of a pseudocapsule around the cuff and decreases the risk of erosion (Furlow, 1981; Sidi et al, 1984). The noncycled artificial urinary sphincter occasionally may provide enough resistance for continence, eliminating the disadvantages of activation (Herndon et al, 2004a).
Results
There are substantial short- and long-term data regarding continence after placement of the artificial urinary sphincter, with the largest series presented by Herndon and associates (2003). In investigation of continence, it must be placed in context of the cost experienced by the patient defined by mechanical malfunctions resulting in secondary operative procedures and more catastrophic complications, such as device infection and erosion. Dramatic improvement regarding the need for secondary procedures has occurred with the technical refinements in the device. Ten- to 15-year long-term follow-up of the artificial urinary sphincter in children has been reported (Levesque et al, 1996; Kryger et al, 1999, 2001; Castera et al, 2001; Hafez et al, 2002; Herndon et al, 2003; Lopez Pereira et al, 2006; Ruiz et al, 2006). All groups report an impressive continence rate of 80% and a functioning sphincter in 95% of patients. These reports are consistent with older series in children reporting continence rates of 75% to 90% and a functioning sphincter in 85% to 97% (Nurse and Mundy, 1988; Gonzalez et al, 1989a; Bosco et al, 1991; O’Flynn and Thomas, 1991; Aprikian et al, 1992; Singh and Thomas, 1996; Simeoni et al, 1996; Catti et al, 2008). Herndon and colleagues (2003) presented the most comprehensive long-term data. They achieved overall continence in 86% of 142 patients with an average follow-up of 10 years. Age at implementation does not appear to affect continence (Kryger et al, 2001).
Whereas the artificial urinary sphincter is one of the few surgically created continence mechanisms that does not negatively affect spontaneous voiding, intermittent catheterization remains an important adjunct in approximately 75% of children with neurogenic sphincter incompetence and can be performed successfully through the cuff (Diokno and Sonda, 1981; Gonzalez et al, 1995; Levesque et al, 1996; Kryger, 1999, 2001; Castera et al, 2001; Hafez et al, 2002; Herndon et al, 2003). As boys approach puberty, spontaneous voiding may become progressively inadequate. It has been speculated that growth of the prostate causes an increase in native outlet resistance. Kaefer and associates (1997a) evaluated increases in cuff size to facilitate spontaneous voiding in boys. In their limited series, they did not find that up-sizing restored the ability to void spontaneously. Jumper and colleagues (1990) reported on prostatic development and sexual function in pubertal boys with spinal dysraphism who had been treated with the artificial urinary sphincter. They found that the artificial sphincter did not alter sexual development, prostatic growth, or morphologic features.
Herndon and associates (2003) reported device malfunction in 64% with the pre-AS800 model and 30% with the AS800. Sphincter erosion was similar for the pre-AS800 and AS800, occurring at 19% and 16%, respectively, in their experience. Fastidious attention to detail and sterile technique diminish the risk of infection but do not eliminate it. When infection occurs without erosion, the unit can be removed and later replaced (Nurse and Mundy, 1988). Infections are minimized by sterilization of the urine preoperatively, meticulous cleaning of the wound site, preoperative bowel preparation, perioperative parenteral antibiotics, and copious antibiotic wound irrigation. Newer cuff design and a 6-week delay in activation of the device help formation of a thickened pseudocapsule that substantially decreases bladder neck and proximal urethral erosion. Kryger and associates (1999) indicated that erosion can be virtually eliminated when the cuff is placed as the primary treatment for bladder neck incompetence. They and others (Aliabadi and Gonzalez, 1990; Gonzalez et al, 1995; Simeoni et al, 1996; Levesque et al, 1996; Castera et al, 2001; Hafez et al, 2002; Herndon et al, 2003) noted that the risk of erosion substantially increases after previous failed repairs but this has not been the experience of others (Ruiz et al, 2006). Identification of the correct plane between the bladder neck and vagina in female patients or rectum in male patients preserves the vascularity of the bladder neck and proximal urethra and may decrease the rate of erosion (Aliabadi and Gonzalez, 1990). Initial exposure through a posterior bladder approach as described by Lottmann and coworkers (1999) may be helpful. Shankar and colleagues (2001) suggested that there is an advantage to exposure of the bladder neck with a transperitoneal approach by decreasing the potential of bleeding from the prostatic venous plexus and improving visualization of the rectal wall.
Levesque and associates (1996) evaluated the long-term outcome of the artificial urinary sphincter on the basis of date of insertion and location of the placement. Before 1985 the artificial urinary sphincter had been inserted in 36 children. Between 1985 and 1990, an additional 18 children underwent placement. In the original group, 24 of the 36 sphincters were in place and 22 functional; 12 had required at least one revision. The mean survival of the device was 12.5 years. Success rates at 5 and 10 years were 75% and 72%, respectively. In the group implanted after 1985, 78% retained a functioning sphincter. The overall continence rate in both groups was 59%; sphincter survival probability at 10 years was approximately 70%. There was no difference found between failure rates in males and females with the exception that female patients who had previously undergone bladder neck surgery were more likely to suffer an erosion. The ability to void independently without the use of intermittent catheterization was retained in 36 children (67%). Those findings are supported by contemporary series (Levesque et al, 1996; Kryger, 1999, 2001; Castera et al, 2001; Hafez et al, 2002; Herndon et al, 2003; Lopez Pereira et al, 2006; Ruiz et al, 2006; Catti et al, 2008).
Upper urinary tract changes including hydronephrosis have been reported to occur in up to 15% of children after placement of the artificial urinary sphincter (Light and Pietro, 1986; Churchill et al, 1987; Gonzalez et al, 1995; Levesque et al, 1996; Kryger et al, 1999). In extreme cases, renal insufficiency has resulted. It is now recognized that occlusion of the bladder neck in children with neurogenic sphincter incompetence can result in the unmasking or development of detrusor hostility manifested by a decrease in bladder compliance or increase in detrusor hyperreflexia (Bauer et al, 1986). Careful preoperative urodynamic assessment helps identify only some of the children who are at risk (Kronner et al, 1998b). When hostile bladder characteristics are found preoperatively, anticholinergic medications can be beneficial for hyperreflexic contractions but augmentation cystoplasty is usually required for diminished compliance. Churchill and associates (1987) showed that favorable parameters can be maintained after placement of the artificial urinary sphincter; however, close observation is still recommended in any child undergoing bladder neck reconstruction to identify any early deterioration in bladder dynamics before upper tract changes.
Some children undergoing sphincter placement need bladder augmentation as well, and the timing of the two procedures may be questioned because of the concern for artificial urinary sphincter infection. Light and colleagues (1995) reported a 50% infection rate with simultaneous augmentation compared with 9.5% when the procedures were staged. On the contrary, a contemporary review by Miller and coworkers (1998) found that infection necessitating removal of the device occurred in only 2 of 29 such patients (7%). This low rate is similar to that noted by others (Strawbridge et al, 1989; Gonzalez et al, 1989b). Several reports have evaluated various factors and found that the intestinal segment selected for augmentation appeared to be the only parameter affecting results; gastric augmentation was the least offensive regarding infection (Ganesan et al, 1993; Miller et al, 1998; Holmes et al, 2001). Gonzalez and associates (2002) reported an alternative technique using a seromuscular colocystoplasty and simultaneous placement of the artificial urinary sphincter. They achieved continence in 89% without the need for additional procedures and no deterioration of the upper urinary tract.
The AS800 is the subject of most reviews when the artificial urinary sphincter is discussed, but alternative devices have been reported (Lima et al, 1996; de O Vilar et al, 2004). An alternative device is a one-piece adjustable cuff connected to an injection port for inflation. The injection port is placed subcutaneously and made available for percutaneous access. This allows for adjusting the pressure within the cuff in order to achieve continence. It is particularly convenient for individuals with limited ability to actively pump the AS800. The periurethral constrictor is wider and more fluid than the AS800. It can be deactivated and reactivated by tapping the subcutaneous port.
The ultimate benefits of the artificial urinary sphincter lie in its ability to achieve a high rate of continence while maintaining the potential for spontaneous voiding. For practical purposes, when intermittent catheterization is required along with augmentation cystoplasty, use of native tissue for continence eliminates the long-term concern for infection or erosion and the risk of mechanical failure.
Urethral Lengthening
Young’s original description of bladder neck reconstruction (1919) consisted of two components: excision of a segment of anterior urethral bladder neck tissue and narrowing of the adjacent remaining posterior portion. This, however, ultimately led to failure because the tubularized segment remained unsupported within the bladder. Refinements by Dees (1949) and Leadbetter (1964) maximized good muscle tone at the bladder neck and extension of the urethral tube through the trigone.
With similar principles, Tanagho (1981) described a cephalad-based anterior detrusor wall tube. Closure of the tubularized bladder neck formed circularly oriented muscle fibers that Tanagho described as a sphincter mechanism. However, he cautioned against the use of this technique in the neurogenic population. Because of potential breakdown of that tubularized bladder neck and poor results, other techniques have been developed on the basis of the concept of a flap valve mechanism for urinary retention. Kropp and Angwafo (1986) described urethral lengthening and construction of a flap valve for neurogenic bladder neck and sphincter dysfunction. The technique is based on an anterior detrusor wall tube that is kept in continuity with the urethra, tubularized, and implanted into a submucosal tunnel within the trigone. Conceptually, this is effective; however, difficulty with catheterization is a common problem and significant concern.
Technique
A Foley catheter is placed intravesically and the bladder filled to capacity. The bladder is exposed through either a midline or low transverse abdominal incision. The bladder neck is then identified with application of gentle catheter traction. A 6 × 2-cm rectangular flap based on the bladder neck and urethra is then isolated. Stay sutures are placed, and the flap is mobilized in continuity with the proximal urethra. The detrusor musculature at the bladder neck is then divided, separating the bladder and urethra, or the muscle may be left intact at the 5- and 7-o’clock positions. The anterior vaginal wall is exposed in girls; the seminal vesicles are exposed in boys. The rectangular strip based on the urethra is tubularized posteriorly around the urethral catheter with a continuous absorbable suture. The distal portion of the tubularized strip should be approximated in an interrupted fashion to facilitate excision of excessive tissue without jeopardizing the suture line. A capacious submucosal tunnel through the trigone is then developed posteriorly for the proximal neourethra (Fig. 129–4). A wide tunnel is required to prevent kinking at the level of the bladder neck, which will impede catheterization. It is important to eliminate dead space at the entrance of the urethra into the bladder, and this can be accomplished by placement of lateral anchoring sutures in the region of the bladder neck. The detrusor tube must be pulled straight through the tunnel without curve or deviation to facilitate catheterization. Waters and colleagues (1997) and Kropp (1999) have not found it necessary to reimplant all ureters in a cephalic location; they now typically reimplant only refluxing ureters (Kropp, 1999). When the bladder is closed, the lateral wings in the region of the bladder neck are approximated and incorporate adventitia of the tubularized urethra. This enhances a watertight closure and is continued for 2 to 3 cm anteriorly, often up to the area of augmentation. The tubularized neourethra should be long enough to reach the true lumen of the bladder, where it is exposed to pressure as an effective flap valve.
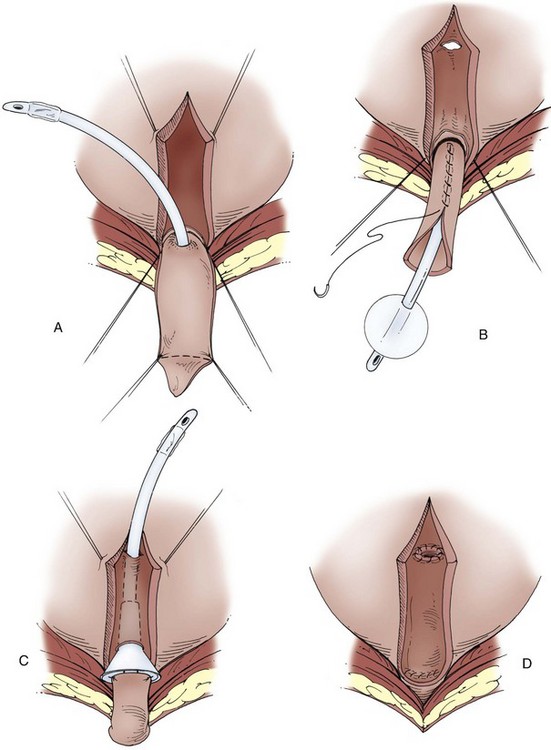
Figure 129–4 Kropp anterior detrusor tube. A, An anterior flap of bladder (2 cm wide × 5 to 6 cm long) is developed in continuity with the urethra. B, The anterior flap is tubularized over a catheter. A tunnel beneath the mucosa of the trigone is made between the ureteral orifices for the length of the tube. C, The tubularized flap is brought through the tunnel. D, The detrusor tube is secured to the floor of the trigone with interrupted absorbable suture in a straight course.
Because of the difficulties with catheterization, modifications of the Kropp bladder neck procedure have been described. Belman and Kaplan (1989) suggested a simplified approach. They harvested a rectangular strip from the anterior bladder wall similar to that described by Kropp. The lateral and posterior muscles at the bladder wall are not incised, however, and the proximal urethra and bladder are not separated. The flap is tubularized over an 8-Fr catheter. The epithelium on the floor of the bladder is incised, contrary to the tunnel made by Kropp. The tube is placed within the trough with the proximal meatus secured on the floor of the bladder. The epithelial edges of the trough are then secured to the lateral aspect of the tube. As in the initial description, the suture line for tubularization of the urethra is posterior against trigonal muscle. Closure of the bladder begins with reapproximation of the lateral walls of the bladder to the tube until the bladder edges meet. The remaining portion of the bladder is covered by an augmentation. Regardless of any modification to the Kropp bladder neck procedure, catheterization potentially remains problematic and therefore the patient should be prepared for a catheterizable abdominal-vesicle channel.
Results
Snodgrass (1997) examined the results in 23 children, 22 of whom had neurogenic sphincter incompetence and noted continence in more than 90% of the children. The most common complication was difficult catheterization, particularly in boys. Less than half of the boys in Snodgrass’ series catheterize through the native urethra; the majority do so through an abdominal wall stoma. Postoperative vesicoureteral reflux was identified in 9 of 18 children, and Snodgrass speculated that this was due to lateral retraction of the ureters. The recommendation was made to leave the posterior bladder wall open and flat in receiving the augmentation to prevent this distortion. Kropp (1999) has not had as much problem with catheterizations in his patients and likewise has achieved a high rate of continence without a high incidence of new reflux.
Some patients with an effective flap valve mechanism constructed by urethral lengthening will virtually never leak per urethra. This potentially puts them at risk for upper tract deterioration or bladder rupture, particularly if they do not or cannot catheterize reliably. Snodgrass (1997) thought that his modification was beneficial in that it resulted in a shorter intravesical tunnel for the neourethra, allowing leakage per urethra with overfilling.
Pippi-Salle Procedure
In an attempt to maximize the benefits of the Kropp technique and to decrease problems with catheterization, Salle (1994) reported an anterior bladder onlay flap. With this technique the posterior wall of the neourethra is intact, theoretically providing less potential hang-up during catheterization. Modifications have been made since the first report to improve flap viability, to minimize fistula formation, and to extend the indications for the procedure beyond that of the neurogenic bladder (Salle et al, 1997).
Technique
An anterior, full-thickness bladder wall flap 5 × 1 cm is mobilized to the bladder neck with 0.1 cm of its epithelial edges excised to avoid overlapping suture lines. Two parallel incisions down to the level of the muscle are made in the mucosa of the trigone from the native bladder neck. The anterior flap is secured to the midline strip of trigone mucosa to form a tube of lengthened urethra with absorbable suture. The muscle on either side of the posterior epithelial strip may be incised superficially to provide an edge to which the muscle of the anterior flap may be approximated with a second layer of suture in an effort to avoid fistula (Fig. 129–5). The more lateral mucosa of the trigone is mobilized and closed over the midline urethra. Distally, the muscle of the bladder neck is wrapped fairly tightly around the urethra with closure. More proximally, the lengthened urethra should extend well into the lumen of the bladder.
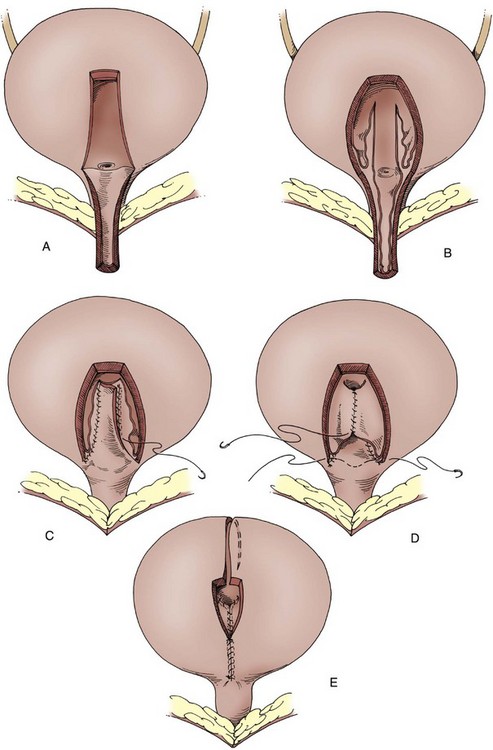
Figure 129–5 Pippi-Salle anterior detrusor tube onlay. A, An anterior detrusor tube (1 cm × 4 to 5 cm) is mobilized to the level of the bladder neck. B, A central strip on the floor of the bladder is developed by incision of the epithelium on either side. C, The anterior detrusor flap is secured to the strip in a two-layer fashion, approximating the epithelium with the first layer and muscle with the second. D, The lateral mucosa of the trigone is mobilized and secured over the lengthened urethra. Distally, the bladder is closed to itself and should incorporate a portion of the anterior detrusor flap to ensure a watertight seal. E, After distal closure the remaining portion of the bladder is left open if augmentation cystoplasty is necessary.
Results
Initial complications of this procedure included persistent incontinence, urethrovesical fistula, and partial necrosis of the intravesical neourethra. Widening the base of the urethra at the level of the bladder neck may decrease these problems. Children who have previously undergone bladder surgery can have a secondary Salle repair if the anterior bladder flap is lateralized slightly to avoid any old midline suture line and increased potential for scarring or necrosis.
Salle and coworkers (1997) found that continence was achieved in 12 of 17 patients (70%) for more than 4 hours. Catheterization difficulties occurred in only 3 of 17 children, 1 of whom subsequently underwent an appendicovesicostomy. Fistula formation at the base of the flap between the proximal, intravesical urethra and bladder occurred in 2 children and resulted in recurrent incontinence. This problem appears to be diminished by making a wider base to the flap and generously trimming the epithelial edges. Jawaheer and Rangecroft (1999) reported a diurnal continence rate of 61% for 3 hours or longer with the Salle procedure. However, only 44% of their patients were dry through the night. Less trouble with catheterization has occurred relative to the Kropp technique, and it rarely remains a problem. Continence rates have not been quite as high in most series (Rink et al, 1994; Mouriquand et al, 1995; Cole et al, 2003).
Canales and coworkers (2006) capitalized on both the Kropp and Pippi-Salle techniques. They evaluated a miniature intravesical urethral lengthening. The primary difference relates to a diminished tunnel (3 cm) and radius of the anterior detrusor tube (8 Fr) with the intent of enhancing resistance, maintaining a popoff mechanism, limiting the use of bladder tissue, and avoiding the need for ureteral reimplantation. At follow-up of 2.5 years, 8 of 9 patients were dry, had normal upper tracts, and had an average leak point pressure of 71 cm H2O.
Bladder Neck Division
The ultimate procedure to increase bladder outlet resistance is to divide the bladder neck so that it is no longer in continuity with the urethra. It must be accompanied by construction of a continent abdominal wall stoma and should be performed only in patients who will reliably catheterize. If effective, it prevents any leakage or popoff per urethra and potentially increases the risk for upper tract deterioration or bladder rupture if emptying is not performed. Division is seldom performed as a primary procedure but may be considered if the previously mentioned repairs fail (Khoury et al, 1999; Bergman et al, 2006).
Such a procedure effectively moves the reconstructive effort into the realm of continent urinary diversion. The bladder neck is abandoned from a physiologic standpoint, although the bladder may be used as part of the reservoir. Effective division of the bladder neck is not a simple procedure. The bladder must be aggressively mobilized away from the urethra. The bladder should be closed distally in several layers after the posterior lip of bladder neck and trigone are rolled anteriorly and widely separated from the distal urethra. Omentum has generally been interposed. Without these steps, fistulization and leakage per urethra can occur (Khoury et al, 1999; Nguyen and Baskin, 2003). With aggressive mobilization, the fistula rate should be low (Thomasch et al, 2009).
Augmentation Cystoplasty
The initial approach to the patient for augmentation cystoplasty is similar regardless of the bowel segment to be used. Cystoscopy should be performed preoperatively to identify any unsuspected anatomic abnormalities that may affect the surgery or postoperative care. If other bladder procedures, such as ureteral reimplantation, are to be performed, the bladder is left full after cystoscopy. If only augmentation is indicated, the bladder is emptied to allow easy access to the peritoneal cavity.
Key Points: Bladder Neck Reconstruction
As a general rule, a midline incision is preferred for intestinal cystoplasty, although these procedures can be performed through a lower transverse incision if there has been no previous abdominal surgery. Laparoscopic assistance with mobilization of the intestine may allow augmentation through a smaller, lower incision (Hedican et al, 1999). Augmentation cystoplasty may eventually be performed completely by laparoscopy with or without use of a robotic system (Lorenzo et al, 2007a, 2007b; Wang et al, 2007; Passerotti et al, 2008). Associated bladder procedures should be performed before the peritoneal cavity is opened to minimize third-space fluid loss. For gastrocystoplasty the incision needs to extend from the pubis to xiphoid to allow more cephalad exposure.
Management of the Native Bladder
In the past it had been recommended that the majority of the “diseased” bladder be excised in preparation for augmentation. This meant removal of the supratrigonal bladder, leaving only a small cuff of higher bladder for anastomosis to the intestinal segment. Despite the cuff, a relatively small area is left for anastomosis to the bowel segment; most of the bowel is approximated to itself. Most surgeons now preserve the native bladder as long as it is widely opened to prevent a narrow-mouthed anastomosis, which can result in the augmentation segment’s behaving like a diverticulum (Fig. 129–6). A sagittal incision to bivalve the bladder is generally useful (Fig. 129–7). The incision is carried from a point several centimeters cephalad to the bladder neck anteriorly to a position just above the trigone posteriorly. Such an incision allows a technically easier anastomosis to the bowel segment and leaves the native bladder to add to the overall capacity. A greater circumference for the anastomosis can be provided, if needed, by opening the bladder in a stellate fashion with a second transverse incision into the two bladder halves. There have been reports of severe penile or perineal pain in sensate boys after augmentation with preservation of the native bladder (Phelps and Malone, 2004). Although four such patients required secondary excision of the bladder, similar problems have not been frequent enough to warrant routine excision at the time of augmentation.
Management of Intestinal Segments
Hinman (1988) and Koff (1988) have demonstrated the advantages of opening a bowel segment on its antimesenteric border, with subsequent detubularization and reconfiguration of that intestinal segment. Reconfiguration into a spherical shape provides multiple advantages, including maximization of the volume achieved for any given surface area, blunting of bowel contractions, and improvement of overall capacity and compliance. All intact, tubular intestinal segments have been noted to generate pressures of 60 to 100 cm H2O with contractions, including ileum (Kock, 1969; Light and Engleman, 1985; Fowler 1988; Camey et al, 1991). Detubularization lowered the maximal contractile pressure from 63 to 42 cm H2O in the right colon and 81 to 28 cm H2O with use of ileum (Goldwasser et al, 1987). Furthermore, a shorter intestinal segment can be used to achieve the same capacity than when it is left in tubular form. Detubularization and reconfiguration should always be performed during augmentation cystoplasty.
Mathematical models based on the length and width of the bowel segment used may predict the volume needed but are cumbersome (Rink and Mitchell, 1990). Depending on the volume needed, 20 to 40 cm of ileum or approximately 20 cm of colon is generally used for cystoplasty. This somewhat depends on the volume of the native bladder being augmented. If the cystoplasty is being done on a bladder of moderate volume that generates high pressure by uninhibited contractions, less bowel is necessary than for one that is tiny in capacity. Unless it is otherwise contraindicated, the surgeon should err by making the bladder too large rather than too small. Appreciation of the patient’s urinary volumes also should influence the size of the bladder required. Patients with upper tract damage, particularly to concentrating ability, may make huge volumes of urine and require a larger capacity.
Ileocystoplasty
Goodwin and associates (1959) were among the first to demonstrate the numerous ways of anastomosing a patch of ileum to the native bladder after the ileum was detubularized and reconfigured to achieve the most spherical shape possible.
Technique
A segment of ileum at least 15 to 20 cm proximal to the ileocecal valve should be selected. The distal portion of terminal ileum is unique from a physiologic standpoint and should be avoided. The isolated segment should be 20 to 40 cm in length, depending on the patient’s size, native bladder capacity, and desired final capacity. With short ureters, an extra tail of isoperistaltic ileum can be useful to reach the foreshortened ureters. This requires construction of an ileal nipple valve to prevent reflux as in the Kock or hemi-Kock pouch. This type of construction may require up to 60 cm of small intestine. The segment to be used should have an adequate mesentery to reach the native bladder without tension.
After selection of the appropriate segment, the mesentery is cleared from the bowel at either end for a short distance to make a window. The bowel is divided at those ends, and a hand-sewn ileoileostomy or stapled anastomosis is performed. The mesenteric window at the bowel anastomosis is closed to prevent an internal hernia. The harvested ileal segment is irrigated clear with 0.25% neomycin solution and opened on its antimesenteric border (Fig. 129–8A). The ileum is folded in a U shape most commonly, although longer segments can be folded further into an S or W configuration. The ileum is anastomosed to itself with running absorbable sutures (Fig. 129–8B). The suture line should approximate the full thickness of ileum to ileum while inverting the mucosa. If the bladder was not opened previously, it is incised in a sagittal plane. The anastomosis of the ileum to native bladder is easily done when it is started posteriorly. The anastomosis may be done in a one- or two-layer fashion, always using absorbable suture and inverting the mucosa to the lumen (Fig. 129–8C). Permanent sutures should never be used for any cystoplasty because they serve as a nidus for stone formation.
Figure 129–8 A, Ileocystoplasty. A 20- to 40-cm segment of ileum at least 15 cm from the ileocecal valve is removed and opened on its antimesenteric border. Ileoileostomy reconstitutes the bowel. B, The opened ileal segment should be reconfigured. This can be done in a U, S, or W configuration. It can be further folded as a cup patch. C, The reconfigured ileal segment is anastomosed widely to the native bladder.
A suprapubic tube is brought out through the native bladder when possible and secured. The anterior aspect of the anastomosis is then completed. A drain is placed near the bladder and brought out of the pelvis through a separate stab incision. It should be removed promptly if it is not draining urine, particularly in neurogenic patients with a ventriculoperitoneal shunt. The wound is thoroughly irrigated and the abdomen is closed in layers.
Cecocystoplasty and Ileocecocystoplasty
Couvelaire described the use of the cecum for augmentation cystoplasty in 1950, and numerous reports of simple cecocystoplasty have appeared since then. Presently, cecocystoplasty is an uncommon operative procedure and is not discussed because it has largely been replaced by various forms of ileocecocystoplasty. With this technique, the cecum is opened, reconfigured, and used to augment the bladder alone, leaving a segment of ileum to reach the ureters or for construction of a continent abdominal wall stoma based on imbrication of the ileocecal valve and proximal ileum. Conversely, the ileal segment can be opened and used as a patch on the cecal segment before augmentation cystoplasty. Many modifications of the technique exist, but all start with mobilization of the cecum and right colon by incision of the peritoneum along the white line of Toldt up to the hepatic flexure. Fifteen to 30 cm of the terminal ileum is used. The length of the ileal segment depends on the technique employed. As with all intestinal cystoplasties, before division of the bowel segment it should be noted that it will reach the bladder without tension (Fig. 129–9A).
Figure 129–9 Ileocecocystoplasty. A, An ileocecal segment is selected. The length of segment chosen depends on the technique employed. After removal, it is opened on the antimesenteric border (dashed lines). B, The opened ileal and cecal segments are anastomosed to form a cup in the standard ileocecal cystoplasty.
Technique
The isolated ileocecal segment is irrigated clear with neomycin solution and opened on its antimesenteric border through the ileocecal valve for its entire length. In the typical ileocecal augmentation the ileal and cecal segments are of equivalent length such that the borders of the open segment can be anastomosed and then folded on themselves to form a cup cystoplasty (Fig. 129–9B). The anastomosis of the reconfigured segments is done in either a one- or two-layer closure with absorbable suture. The opening should be left large enough to provide a wide anastomosis to the bivalved bladder. If more volume is necessary, the ileal segment can be significantly longer, allowing it to be folded before anastomosis to the cecum. The Mainz ileocecocystoplasty uses an ileal segment twice the length of the cecal segment. The opened edge of the cecal portion is anastomosed to the first portion of the ileal segment. The first and second portions of the ileal segment are next approximated. The compound ileocecal patch is then anastomosed to the bladder (Fig. 129–10). The mesenteric window is closed, and a suprapubic tube is placed through the native bladder and secured through the abdominal wall.
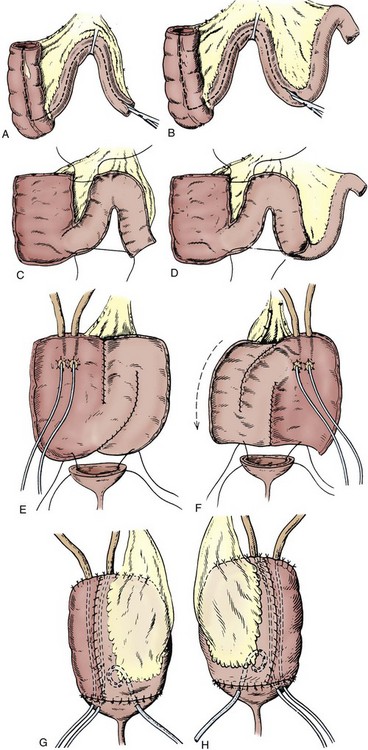
Figure 129–10 The Mainz ileocecocystoplasty. A and B, The ileal segment is twice the length of the cecal segment. C and D, It is opened on the antimesenteric border. E and F, The ureters can be reimplanted into the opened cecal segment if necessary. G and H, The ileocecal segment is anastomosed to the native bladder.
(From Thuroff JW, et al. In: King LR, Stone AR, Webster GD, editors. Bladder reconstruction and continent urinary diversion. Chicago: Year Book; 1987.)
Appendix
One potential advantage of ileocecocystoplasty is the presence of the appendix. Particularly in children the appendix is useful in construction of a reliable continent abdominal wall stoma. The appendix may be removed with a small cuff of cecal wall and tunneled into the native bladder or a taenia of the cecal segment to provide a continence mechanism. Likewise, it may be left in situ and the base safely tunneled beneath a taenia. If the appendix is not to be used, an appendectomy is performed with standard ileocecocystoplasty.
Ileocecal Valve
The ileocecal segment has been used extensively in the adult population undergoing reconstruction and bladder replacement. It has been used less frequently so in children because the majority of patients undergoing augmentation cystoplasty do so for neurovesical dysfunction. Those patients generally have neurapraxic bowel dysfunction as well. Removal of the ileocecal valve in such children can result in intractable diarrhea (Gonzalez and Cabral, 1987; King, 1987). Use of the ileocecal valve in such patients should be avoided unless other advantages of the segment outweigh the risk of diarrhea and fecal incontinence.
There are potential advantages to the use of the ileocecal segment. Antireflux tunnels can easily be performed into the taenia of the cecum when necessary. Again, for the short ureter, a tail of ileum can be left intact to bridge the gap and the imbricated ileocecal valve used for antireflux. The same imbrication technique can be used to construct a continent abdominal wall stoma as with the appendix. Cain and associates (Cain and Husmann, 1994; Cain et al, 1999) have proposed use of the ileocecal segment for augmentation with the plicated ileal segment brought to the abdominal wall as a catheterizable stoma as in the Indiana pouch.
Sigmoid Cystoplasty
Use of the sigmoid colon for augmentation cystoplasty was first reported by Lemoine in 1912 (Charghi et al, 1967) and continues to be used commonly. Because of the strong unit contractions of the sigmoid, it is imperative to detubularize and reconfigure the segment to provide maximal compliance and disruption of contractions.
Technique
Fifteen to 20 cm of sigmoid colon is identified and mobilized. The mesentery is transilluminated to identify the vascular arcade to the segment. After identification of this blood supply the surgeon must ensure that the segment can reach the bladder without tension. If so, the bowel segment is divided between clamps and a colocolostomy performed (Fig. 129–11A). The remainder of the abdominal cavity is carefully packed to prevent contamination from the open sigmoid segment. Detubularization and reconfiguration are done in a fashion determined by the surgeon’s preference. The sigmoid patch is anastomosed to the bivalved bladder in a manner similar to that previously described for ileocystoplasty. Again, a large suprapubic tube is brought out through the native bladder and secured to the bladder and skin exit sites. Drains are placed as previously noted.
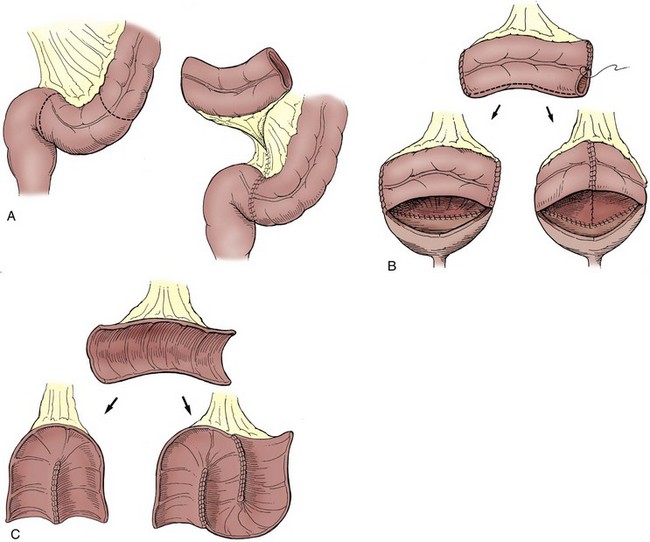
Figure 129–11 Sigmoid cystoplasty. A, A sigmoid segment of adequate length is removed from the gastrointestinal tract, and a colocolostomy is performed. B, In the Mitchell technique the two opened ends are closed. The antimesenteric border is incised, and the segment is anastomosed to the bivalved bladder. It may be rotated 180 degrees to allow an easy fit. C, The opened sigmoid segment can be reconfigured into a U or S configuration, which may lower pressure.
Reconfiguration of Sigmoid
Sigmoid colon segments are generally reconfigured in one of two ways. Mitchell (1986) suggested closing the two ends and then opening the segment longitudinally opposite its blood supply. The segment easily fits on the bivalved bladder in either the sagittal or coronal plane (Fig. 129–11B). More radical reconfiguration, and perhaps breakup of unit contractions, may be achieved by folding the sigmoid segment in a U shape similar to that described for ileocystoplasty (Sidi et al, 1987a) (Fig. 129–11C). A slightly longer segment of sigmoid may be necessary for effective reconfiguration in this manner.
Gastrocystoplasty
Two basic techniques exist for use of stomach in bladder augmentation.
Technique with Use of Gastric Antrum
Leong and Ong (1972) described the use of the entire gastric antrum with a small rim of body for bladder replacement. With their technique the left gastroepiploic artery is always used as a vascular pedicle. If the right gastroepiploic artery is dominant and the left vessel ends high of the greater curvature, a strip of body along the greater curvature from the left gastroepiploic artery to the antrum is maintained and provides adequate blood supply (Leong, 1988). Continuity of the upper gastrointestinal tract is restored by a Billroth I gastroduodenostomy.
Technique with Use of Gastric Body
A gastric wedge based on the midportion of the greater curvature may be used (Adams et al, 1988) (Fig. 129–12A). The gastric segment used in this technique is made up mainly of body and consequently has a higher concentration of acid-producing cells. The right or left gastroepiploic artery may be used as a vascular pedicle to this segment. The right artery is commonly dominant and thus more frequently used. The wedge-shaped segment of stomach includes both the anterior and posterior wall. The segment used may be 10 to 20 cm along the greater curvature, depending on the patient’s age and size as well as the needed volume (Fig. 129–12B). The incision into the stomach is stopped just short of the lesser curvature to avoid injury to branches of the vagus nerve controlling the gastric outlet. Branches of the left gastric artery just cephalad to the apex of this incision are suture ligated in situ before incision to avoid significant bleeding. Parallel atraumatic bowel clamps are placed on either side of the gastric incisions to avoid excessive bleeding or spillage of gastric contents. Alternatively, the stomach may be incised by use of a gastrointestinal stapling device that places a double row of staples on each side of the incision (Mitchell et al, 1992). The staple lines, however, must eventually be excised. The native stomach is closed in two layers with permanent sutures on the outer seromuscular layer.
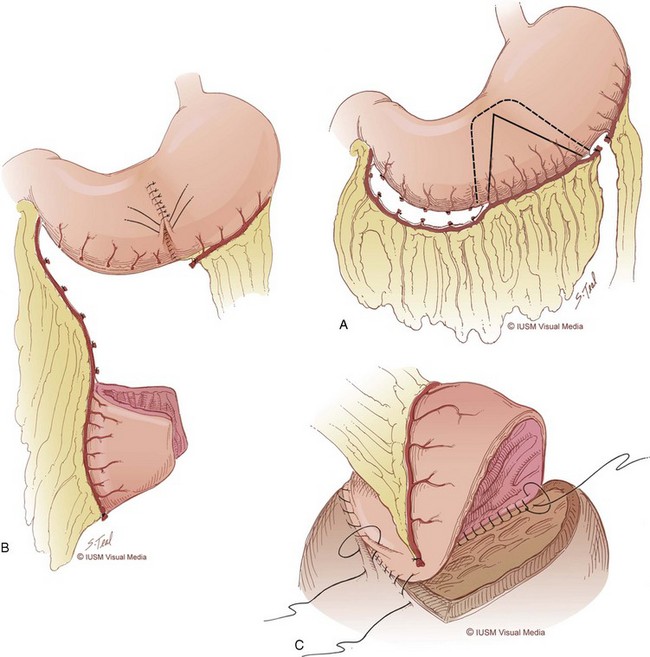
Figure 129–12 Gastrocystoplasty with use of the body. A, Gastric segment of body is mobilized on the right gastroepiploic artery. The left vessel may also be used; neither vessel as a pedicle should be free floating through the peritoneum. B, A longer gastric segment along the greater curvature with wider apex provides more surface area for augmentation. C, The gastric segment is anastomosed to the bivalved bladder with the mucosa inverted. (© Indiana University Medical Illustration Department.)
Branches of the gastroepiploic artery to the antrum on the right or to the high corpus on the left are divided to provide mobilization of the gastroepiploic pedicle. For the eventual pedicle to be long enough to reach the bladder, the appropriate segment may be higher on the greater curvature if the right vessel is used as a pedicle or lower if it is based on the left. The vascular pedicle, with omentum, should not be free floating through the abdomen. The segment and pedicle may be passed through windows in the transverse mesocolon and mesentery of the distal ileum and carefully secured to the posterior peritoneum. Despite careful consideration for an adequate pedicle length, on occasion the gastric segment initially may not reach the bladder without tension. Either gastroepiploic artery may be mobilized closer to its origin for further length. The first few branches from the gastroepiploic artery to the isolated gastric segment may also be divided. Because of the rich submucosal arterial plexus in the stomach, devascularization of the isolated segment does not result. Rarely, it may be necessary to approximate some of the isolated gastric segment to itself in one corner. The gastric segment should be approximated to the native bladder with one or two layers of absorbable sutures, with care taken to invert the mucosa (Fig. 129–12C).
Raz and colleagues (1993) and Lockhart and associates (1993) have described the use of a much longer, more narrow segment of stomach based along the greater curvature. Use of this segment, which includes both body and antrum, somewhat narrows the lumen of the stomach along its entire length except at the fundus and pylorus. Raz and colleagues have isolated this segment with a gastrointestinal stapler so that the native stomach is never open. The segment used in both of these series is similar to that first described by Sinaiko (1956), the first surgeon to use stomach in bladder replacement. Postoperative bladder and gastric drainage is no different from that described for intestinal cystoplasty. Histamine-2 (H2) blockers are often given in the early postoperative period to promote healing (Rink et al, 2000).
Postoperative Management
Early Management
Care of patients after cystoplasty is similar regardless of the gastrointestinal segment used in the procedure. Typically, all patients have been maintained on nasogastric decompression until bowel function recovers, although in two studies (Gundeti et al, 2006; Erickson et al, 2007) it was suggested that nasogastric suction may not be necessary after ileocystoplasty. Attention to fluid and electrolyte management is important because third-space losses may be significant after extensive reconstructive surgery.
Continuous drainage of the bladder is achieved by suprapubic cystostomy. Mucus production from small or large bowel may be excessive and can potentially occlude the drainage catheter. The suprapubic tube should be irrigated at least three times daily and whenever drainage is slowed by mucus. Extravesical drains may be removed after several days if drainage of urine is not apparent. The drains are generally removed more promptly in patients with a ventriculoperitoneal shunt to avoid potential infection. Some surgeons prefer a cystogram before discharge of the patient; others wait approximately 3 weeks for the study before clamping the suprapubic tube. All patients should begin on CIC every 2 to 3 hours during the day and one or two times at night after bladder healing is documented. The suprapubic tube is removed after catheterization is successfully under way and well tolerated. The duration between catheterizations is gradually increased during several weeks but should not exceed 4 to 5 hours during the day. Patients without neurologic impairment may eventually attempt to void spontaneously. All should check postvoid residual volumes and continue catheterizations if the residual urine amounts are significant.
Late Management
Routine radiographic surveillance of the upper urinary tract is indicated at 6 weeks, 6 months, and 1 year after augmentation cystoplasty. Most such surveillance may be done with ultrasonography. Serum electrolyte, blood urea nitrogen, and creatinine determinations along with urine cultures are performed several times in the first year after surgery. Not all positive urine cultures need to be treated in patients on CIC. Certainly, symptomatic cystitis or infections involving urea-splitting organisms should be cleared. Evaluation by ultrasonography and serum chemistries is then appropriate once a year. Eventually, yearly endoscopy for tumor surveillance may be performed. No consensus exists about the initiation or frequency of cystoscopic examination; however, we generally begin such surveillance approximately 5 years after augmentation. Husmann (2009) has suggested that surveillance may be of greater yield in patient populations at greater risk such as valve patients that are on immunosuppression after transplantation or exstrophy patients with their inherent increased risk for bladder adenocarcinoma. There is no experience demonstrating that routine endoscopy is cost effective or successful in this population.
Results and Complications
The effect of cystoplasty on the patient should be considered in two main categories. One must first consider the effect of removal of a relatively small portion of the gastrointestinal tract for use in urinary reconstruction. Any more than rare development of gastrointestinal problems would be prohibitive, even if results were perfect from the standpoint of the urinary bladder. Second, the effect of augmentation cystoplasty on the urinary bladder must be reviewed. The primary goal of augmentation is to provide a compliant urinary reservoir. Therefore, the main consideration after augmentation is the storage pressure and capacity that are achieved. Any other effect on the urinary bladder is a side effect or complication that exists because bowel is not a perfect physiologic substitute for native bladder. It is clear that approximately one third of patients will require further surgery after augmentation cystoplasty because of various problems (Metcalfe et al, 2006).
Gastrointestinal Effects
Postoperative bowel obstruction is uncommon after augmentation cystoplasty, occurring in approximately 3% of patients after augmentation (Gearhart et al, 1986; King, 1987; Mitchell and Pizer, 1987; Hollensbe et al, 1992; Rink et al, 1995a). The rate of obstruction is equivalent to that noted after conduit diversion or continent urinary diversion (McDougal, 1992b). Delicate handling of tissues, closure of mesenteric windows, and elimination of sites of internal herniation help avoid obstruction. Occasional series have suggested differing rates of bowel obstruction, depending on the segment used. These differences have not been consistent in most series and therefore are not likely to be significant; the incidence of bowel obstruction is low regardless of the gastrointestinal segment used and should not influence the choice of a particular segment for enterocystoplasty.
Reports of chronic diarrhea after bladder augmentation alone have been rare. Diarrhea can occur after removal of large segments of ileum from the gastrointestinal tract, although the lengths of the segments typically used for augmentation are rarely problematic unless other problems coexist. Much longer segments required for continent urinary diversion may increase the risk. The use of a typical colonic segment for augmentation only rarely results in a change in bowel function. Removal of a segment from the gastrointestinal tract including the ileocecal valve is more likely to cause diarrhea. Some children with neurogenic impairment depend on controlled constipation for fecal continence. Removal of the ileocecal valve from the gastrointestinal tract may significantly decrease bowel transit time. Loss of the valve can also allow bacterial backflow into the ileum, and the organisms may interfere with fat and vitamin B12 metabolism. Studies have noted chronic diarrhea in 10% to 23% of patients with neurogenic dysfunction after displacement of the ileocecal valve (King, 1987; Roth et al, 1995), although the risk may be lower for carefully selected patients (Husmann and Cain, 1999).
Ileum is the sole site of vitamin B12 absorption. Removal of the distal ileum from the gastrointestinal tract may therefore result in vitamin B12 deficiency and megaloblastic anemia. Certainly, the terminal 15 to 20 cm of ileum should not be used for augmentation, although problems may arise even if that segment is preserved (Waters et al, 1987; Steiner and Morton, 1991; Racioppi et al, 1999). Again, the risk is greater if longer segments of ileum are used as with continent diversion. Thirty-five percent of patients observed for 5 years after a Kock pouch were found to be deficient in vitamin B12 in one series (Akerlund et al, 1989). In general, the length of ileum used for augmentation is less than half of that used for a Kock pouch, so that vitamin B12 deficiency seems unlikely after routine bladder augmentation. Canning and associates (1989) evaluated 26 patients after bladder augmentation or replacement and found no patients with either fat malabsorption or vitamin B12 deficiency. Only 3 patients, however, were observed longer than 3 years, and longer observation is necessary because existing body vitamin B12 storage may last considerably longer (Stein et al, 1997a). Eventually, determination of vitamin B12 levels or routine injections of the vitamin may be appropriate after ileocystoplasty.
Early satiety may occur after gastrocystoplasty but usually resolves with time. Disorders of gastric emptying should be extremely rare, particularly when gastric body is used.
Bladder Compliance after Augmentation
An early lesson of past clinical experience with augmentation cystoplasty is the value of detubularization and reconfiguration of the bowel segment (Hinman, 1988; Koff, 1988). Bowel in its native, tubular form continues to display peristalsis or mass contraction. The tubular form does not maximize the volume achieved for the surface area of bowel used. Hinman (1988) demonstrated with a mathematical model that the maximum volume achieved for a given surface area occurs when a sphere is formed. No finished cystoplasty is a perfect sphere but should approach it as nearly as possible.
Some surgeons with extensive experience in augmentation cystoplasty and continent diversion have concluded that ileum is superior to other segments in terms of compliance after augmentation, although controlled experimental examination of similarly sized and used bowel or gastric segments is lacking (Goldwasser and Webster, 1986; Rink and McLaughlin, 1994; Studer and Zingg, 1997). On the contrary, one report has suggested superior results with colon compared with ileum (Shekarriz et al, 2000) when a longer colonic segment reconfigured into a U shape was utilized. Good results have been achieved with all segments in most cases, and it is more important to use a bowel segment well than to choose a particular bowel segment for every patient.
Most problems with pressure after augmentation cystoplasty occur from uninhibited contractions, apparently in the bowel segment. It is extremely rare not to achieve an adequate capacity or flat tonus limb unless a technical error has occurred with use of the bowel segment. On occasion, a small, scarred pelvis may prevent adequate expansion of the augmented bladder. When pressure contractions occur in the bladder after augmentation they are often noted in a rhythmic or sinusoidal pattern occasionally with increasing amplitude (Fig. 129–13). Contractions that begin at low amplitude later in filling and progress only near capacity may be of no clinical significance at all. Early contractions of higher pressure may occasionally result in persistent incontinence, delayed perforation, hydronephrosis, or vesicoureteral reflux. If patients have such clinical problems after augmentation, repeated urodynamic testing is necessary. One cannot assume the bladder is compliant after augmentation. Rhythmic contractions have been noted postoperatively with all bowel segments, although ileum seems the least likely to demonstrate remarkable urodynamic abnormalities and stomach the most likely.
Figure 129–13 Rhythmic, sinusoidal contractions (top) may occur after bladder augmentation, in this case with stomach. Contractions of significant amplitude early in filling occasionally require secondary augmentation. After secondary augmentation with ileum (bottom), urodynamics show that contractions still occur but are much lower in pressure and occur later in filling.
After bladder augmentation or replacement, some urodynamic evaluation has suggested that colonic segments, whether cecum or sigmoid, still generate more pressure than ileum despite detubularization (Berglund et al, 1987; Jakobsen et al, 1987; Thuroff et al, 1987; Lytton and Green, 1989; Studer and Zingg, 1997). Other work has suggested that pressure contractions from the colon decrease with time (Hedlund et al, 1984; Sidi et al, 1986b). Nonetheless, Goldwasser and coworkers’ review (1987) of enterocystoplasty demonstrated contractions above 15 cm H2O in 42% of patients after ileocystoplasty versus 60% of those after colocystoplasty. Significant contractions, defined as those above 40 cm H2O at a volume of less than 200 mL, were not noted in any of the ileal augmentations but persisted in 10% of colocystoplasties. Their work agreed with that of Berglund and colleagues (1987) and Studer and Zingg (1997) in suggesting that ileal reservoirs have lower basal pressures and less motor activity. None of these studies critically controlled for the size of the bowel segment or the technique in which it was used. In fact, a canine model of partial bladder replacement using identically sized segments failed to demonstrate in vitro or in vivo any differences between the gastrointestinal segments (Nelson et al, 2005).
Rhythmic contractions after gastrocystoplasty have been noted in up to 62% of patients (Adams et al, 1988; Atala et al, 1993a; Gosalbez et al, 1993a; Roth et al, 2000). The segment of stomach initially described for augmentation with the body was much smaller than segments of ileum or colon commonly used for cystoplasty. Use of a slightly larger gastric segment that is longer along the greater curvature results in improved urodynamics after augmentation with less prominent contractions (Adams et al, 1995; Kurzrock et al, 1998; Koraitim et al, 1999; DeFoor et al, 2003b). Leong (1988) has suggested that an antral segment of stomach is less likely to demonstrate such contractions.
In perhaps the largest experience with pediatric bladder augmentation, Hollensbe and associates at Indiana University found that approximately 5% of several hundred patients had significant uninhibited contractions after augmentation cystoplasty causing clinical problems (Hollensbe et al, 1992). Pope and Rink (1999) found that 6% of more than 300 patients required secondary augmentation of a previously augmented bladder for similar problems in long-term follow-up. With longer follow-up, the same group has eventually performed reaugmentation in 9% of patients (Metcalfe et al, 2006). These secondary augmentations represent true failures of the primary cystoplasty, not from any side effect or complication but from failure to achieve the objective—capacity and compliance. In that series, sigmoid colon followed by stomach and then ileum were more likely to require reaugmentation. A colonic segment closed at the ends and not generally reconfigured otherwise was typically used in that experience. Other studies have suggested that stomach is more likely than colon to require secondary intervention (El-Ghoneimi et al, 1998).
Metabolic Complications
Chloride Absorption and Acidosis
The first recognized metabolic complication related to storage of urine within intestinal segments was the occasional development of hyperchloremic metabolic acidosis after ureterosigmoidostomy (Ferris and Odel, 1950). Patients with this metabolic derangement were noted to have fatigue, weakness, anorexia, and polydipsia. Koch and McDougal (1985) demonstrated the mechanisms by which acid is absorbed from urine in contact with intestinal mucosa. Resorption in the form of ammonium results in chronic acid loading. Patients with normal renal function are generally able to handle the resorbed load of chloride and acid without frank acidosis. Mitchell and Piser (1987) noted that essentially every patient after augmentation with an intestinal segment had an increase in serum chloride concentration and a decrease in serum bicarbonate level, although full acidosis was rare if renal function was normal. Similar trends of increased serum chloride and decreased bicarbonate have been noted with ileal conduits (Malek et al, 1971) and continent urinary reservoirs (Allen et al, 1985; McDougal, 1986; Ashken, 1987; Thuroff et al, 1987; Boyd et al, 1989). More severe acidosis and electrolyte disturbances requiring treatment have been reported despite normal renal function (Schmidt et al, 1973; Whitmore and Gittes, 1983). Such derangements may be debilitating to the patient if they are not recognized and treated, and death of patients has been reported (Heidler et al, 1979). Hall and colleagues (1991) noted that there is an increase in the urinary acid load with wasting of bone buffers even in the absence of frank acidosis. Such wasting may result in bone demineralization and could potentially cause retarded growth in children after augmentation cystoplasty (Abes et al, 2003; Hafez et al, 2003; Vajda et al, 2003). Patients with acidosis should receive bicarbonate therapy. Whether all patients with intestine in the lower urinary tract might benefit from supplemental bicarbonate is controversial. Nurse and Mundy (1989) have suggested that arterial blood gas values may be more sensitive than serum bicarbonate or chloride levels for detecting acidosis. Stein and associates (1998) thought that measurements of arterial blood gas for base deficit allowed early treatment of acidosis and avoidance of bone demineralization. In severe cases of acidosis, chloride transport can be blocked with chlorpromazine and nicotinic acid.
Although jejunum is rarely used for bladder reconstruction, storage of urine in this segment results in a unique metabolic pattern of hyponatremic, hypochloremic, and hyperkalemic metabolic acidosis. The problem is often associated with significant hypovolemia.
Gastric mucosa is a barrier to chloride and acid resorption and, in fact, secretes hydrochloric acid (Piser et al, 1987). This difference was the primary factor in the initial consideration of stomach for use in the urinary tract. This secretory nature was shown to be of benefit in azotemic animals during acid loading (Piser et al, 1987; Kennedy et al, 1988). Serum chloride does decrease and serum bicarbonate increases slightly after gastrocystoplasty, whether antrum or body is used in patients with normal and impaired renal function (Adams et al, 1988; Ganesan et al, 1991; Kurzrock et al, 1998). In 21 patients with renal insufficiency, serum bicarbonate improved in all patients except one after gastrocystoplasty, and many patients requiring oral bicarbonate therapy before cystoplasty did not do so after gastrocystoplasty (Ganesan et al, 1991). A similar benefit has been noted in a smaller group of patients with renal failure (Sheldon and Gilbert, 1991).
Patient Growth
Delayed or slowed growth in some children after intestinal cystoplasty has previously been recognized (Wagstaff et al, 1991, 1992; Mundy and Nurse, 1992). A delay in linear growth was noted in 20% of almost 200 pediatric patients without any gross biochemical abnormalities. However, no control patients were included in the series, and body habitus and growth are difficult to measure and predict in children with myelodysplasia. Such patients make up the majority in most series of augmentation cystoplasty. Gros and colleagues (2000) evaluated growth in exstrophy patients. Patients requiring augmentation were matched retrospectively with a similar group not requiring bladder augmentation. Hydronephrosis was not mentioned in the patients but was unlikely with a diagnosis of exstrophy. Only 1 patient was noted to have acidosis in either group. Other factors that might affect growth, such as urinary tract infection, were not controlled. Of 17 patients with adequate measurements before and after augmentation cystoplasty, 14, or 82%, had a decline in percentile height postoperatively. The decline corresponded to a 1.5-inch decrease in expected height. The pattern of growth was significantly different between patients with and without augmentation in the series. That series is small, and no evaluation of familial growth patterns or ultimate height was possible; however, the findings are worrisome, particularly because Feng and coworkers (2002) noted similar differences in exstrophy patients. In the absence of any serum abnormalities the exact mechanism of delayed growth was not evident, although it seems likely to be related to chloride absorption and subclinical acidosis (Koch and McDougal, 1988; Bushinsky, 1989; Hochstetler et al, 1997). It should be noted that Taskinen and coworkers (2008) found no adverse effect on longitudinal growth after bladder augmentation in the exstrophy population. Three recent series did show the effect of bowel cystoplasty on bone mineral density in some patients (Abes et al, 2003; Hafez et al, 2003; Vajda et al, 2003), although Mingin and associates (2002) noted no such changes. One must be careful to determine whether any such changes are due to augmentation cystoplasty or the underlying pathologic process (Boylu et al, 2006; Taskinen et al, 2007). Better analysis of subtle metabolic alterations after enterocystoplasty may establish better understanding of the effect on growth, minimize changes, or aid in early treatment to avoid the complication (Brkovic et al, 2004).
Alkalosis
The secretory nature of gastric mucosa may at times be detrimental to the patient and can result in two unique complications of gastrocystoplasty. Severe episodes of hypokalemic, hypochloremic metabolic alkalosis that followed acute gastrointestinal illnesses have been noted in 5 of 37 patients after gastrocystoplasty (Hollensbe et al, 1992). The episodes were significant enough to require hospitalization in all cases and were recurrent in 2 patients. Three of the 5 patients suffering the complication had renal insufficiency and would not have been good candidates for augmentation with other segments because of acidosis. Ganesan and associates (1991) noted similar episodes of alkalosis in 5 of 21 patients with renal insufficiency after gastrocystoplasty. Those patients with the primary indication for consideration of gastrocystoplasty may be the ones at greatest risk for this unusual complication. It has been proposed that the alkalosis results from ongoing chloride loss from the gastric segment in the bladder in the presence of decreased oral intake. McDougal (1992a) suggested that the decreased ability to excrete bicarbonate from an impaired kidney may compound the problem. Gosalbez and associates (1993b) demonstrated persistently increased fractional excretion of chloride despite profound hypochloremia, suggesting that inappropriate gastric secretion is likely to be the primary mediator. One patient in their series eventually required resection of three fourths of the gastric segment in the bladder because of recurrent problems with alkalosis, and several required therapy with H2 blockers or H+,K+ pump inhibitors.
All patients and families should be made aware of this potential problem because it has been reported to occur intermittently in between 3% and 24% of patients. A composite reservoir of stomach and ileum or colon may provide a more metabolically neutral reservoir (McLaughlin et al, 1995; Austin et al, 1997, 1999, 2001), although they have typically been constructed in only complex circumstances. Duel and associates (1996) have used stomach and colon together to advantage in a staged fashion for oncology patients; patients initially diverted with a colon conduit after cystectomy had a composite reservoir constructed later after cure from tumor was ensured.
Hematuria-Dysuria Syndrome
Acid secretion by gastric mucosa may result in another unique problem after gastrocystoplasty: the hematuria-dysuria syndrome. Mitchell’s group has characterized this syndrome well (Nguyen et al, 1993; Plaire et al, 1999). Virtually all patients after gastrocystoplasty with normal sensation have occasional hematuria or dysuria with voiding or catheterization beyond that which is expected with other intestinal segments (Leonard et al, 1999). All patients should be warned of this potential problem, although these symptoms are intermittent and mild in most patients and do not require treatment. The problem has led one group to recommend avoiding gastrocystoplasty in patients with bladder exstrophy (El-Ghoneimi et al, 1998). The dysuria is certainly not as problematic in patients with neurogenic dysfunction. In the experience of Nguyen and colleagues (1993), 36% of patients have developed signs or symptoms of the hematuria-dysuria syndrome after gastrocystoplasty; 14% of patients have required treatment with medications, including 9% on a regular basis. They believe that patients who are incontinent or have decreased renal function are at increased risk. Others have noted a similar requirement for short-term and chronic medical therapy (Hollensbe et al, 1992; Adams et al, 1995). In the authors’ experience and that of Nguyen and colleagues, the symptoms of the hematuria-dysuria syndrome respond well to H2 blockers and H+ pump blockers. Bladder irrigation with baking soda may also be effective. It has been demonstrated that urine pH may decrease remarkably after meals following gastrocystoplasty (Bogaert et al, 1995). The signs and symptoms of the hematuria-dysuria syndrome are most likely secondary to acid irritation. Recent work has suggested that Helicobacter pylori may play a role in this complication as it may in acid complications in the native stomach (Celayir et al, 1999). Such problems can occur but are less frequent after antral cystoplasty where there is a smaller load of parietal cells (Ngan et al, 1993).
Acid in the urine may also cause external irritation. Leong first noted glanular excoriation after gastrocystoplasty in a patient with voiding symptoms (Ngan et al, 1993). Similar meatal irritation has been noted in other patients after gastrocystoplasty; most have had significant dysuria. Nguyen and associates (1993) noted skin excoriation in 8 of 57 patients after gastrocystoplasty; all 8 patients had some element of urinary incontinence. It is imperative to achieve reliable urinary continence in patients undergoing gastrocystoplasty because urine leakage may result in the exposure of the skin to gastric secretions and in gastric secretions that are poorly diluted and buffered. Such dilution is important; Reinberg and coworkers (1992) reported a perforation of a gastric segment in a defunctionalized bladder after gastrocystoplasty. They then evaluated the influence of urine on gastrocystoplasties in dogs (Castro-Diaz et al, 1992). The animals developed marked inflammation of the gastric segment and native bladder after construction of a dry gastrocystoplasty; three of nine dogs developed ulceration and perforation. Use of H2 blockers resulted in some protection for the animals; however, such a clinical situation should certainly be avoided. Rare perforations and ulcerations have been noted clinically without defunctionalization (El-Ghoneimi et al, 1998; Mingin et al, 1999b).
Mucus
Intestinal segments continue to produce mucus after placement in the urinary tract. The proteinaceous material can potentially impede bladder drainage during voiding or CIC, particularly in pediatric patients in whom the use of small-caliber catheters is necessary. Mucus may serve as a nidus for infection or stone formation when it remains in the bladder for long periods. Mucus production often increases after cystoplasty in the presence of cystitis. Kulb and associates (1986) have shown experimentally in dogs that colonic segments produce more mucus than ileum does and that gastric segments produce the least amount. This has been noted clinically as well. Most patients do not require any routine bladder irrigations for mucus after gastrocystoplasty. Villous atrophy in the ileum has been documented after long-term placement in the urinary tract. It has been suggested that such atrophy may result in decreased mucus production (Gearhart, 1987), although laboratory demonstration of any decrease in production with time has not been evident (Murray et al, 1987). Hendren and Hendren (1990) noted a decrease in mucus production from colonic segments over years; however, others have not been impressed with such changes (Rink et al, 1995a). Glandular atrophy in colonic mucosa has not been noted histologically (Mansson et al, 1984). Routine use of daily bladder irrigations to prevent mucus buildup may minimize complications of enterocystoplasty, such as urinary tract infection and calculi (Hensle et al, 2004).
Urinary Tract Infection
Bacteriuria is common after intestinal cystoplasty, particularly among patients requiring intermittent catheterization (Gearhart et al, 1986; Hendren and Hendren, 1990; King, 1991). Recent experience with bowel neobladders has demonstrated that patients who are able to spontaneously void to completion frequently maintain sterile urine. It appears that the use of CIC is a prominent factor in the development of bacteriuria in patients after augmentation cystoplasty. Bacteriuria has been noted even when patients are maintained on daily oral antibiotics or antibiotic irrigation (Gearhart et al, 1986; Casale et al, 1999). In Hirst’s (1991) experience, persistent or recurrent bacteriuria occurred in 50% of patients augmented with sigmoid colon versus 25% of those after ileocystoplasty. Hollensbe and coworkers (1992) noted bacteriuria much more commonly in patients requiring CIC regardless of the segment considered. The incidence of symptomatic cystitis after cystoplasty probably depends on the length of follow-up and the diligence with which symptoms are sought. All patients and families should be told to expect some signs or symptoms of cystitis. Recurrent episodes of symptomatic cystitis requiring treatment occurred in 23% of patients after ileocystoplasty, 17% after sigmoid cystoplasty, 13% after cecocystoplasty, and 8% after gastrocystoplasty at Indiana University (Hollensbe et al, 1992). Febrile urinary tract infections occurred in 13% of those 231 patients after augmentation. The same trend among different bowel segments was noted for febrile infections, although there was no statistically significant difference among the various segments. The incidence of pyelonephritis after augmentation cystoplasty, as long as upper tract problems are corrected, is similar to that noted for conduit diversion, whether refluxing or not (McDougal, 1992b). Recurrent infections resulting in deterioration of renal function in the absence of other problems have been rare after effective augmentation. Infections may occasionally be more problematic in an immunocompromised patient (Alfrey et al, 1997).
Not every episode of asymptomatic bacteriuria requires treatment in patients performing CIC. Bacteriuria should be treated for significant symptoms, such as incontinence or suprapubic pain, and perhaps for hematuria, foul-smelling urine, or remarkably increased mucus production. Bacteriuria should be treated if the urine culture demonstrates growth of a urea-splitting organism that may lead to stone formation. To minimize infection, patients requiring CIC must perform it on a regular basis to avoid increased reservoir pressures and work to empty the bladder completely. This process may require periodic irrigation of mucus as well as patience with the catheter in place. Special care must be taken by patients catheterizing through a continent abdominal wall stoma. Such patients may have more difficulty completely emptying the bladder from a nondependent stoma. Most can do so with effort (Ludlow et al, 1995). Although catheterization is not routinely a sterile technique, proper clean technique should be emphasized.
Calculi
Another long-term complication of augmentation cystoplasty is bladder calculus formation. In the early 1990s, several series reported calculi in 18% of patients after augmentation cystoplasty (Hendren and Hendren, 1990; Hirst, 1991). Blyth and associates (1992) noted calculus formation in 30% of such patients; they found that patients catheterizing through an abdominal wall stoma have the highest risk, probably because of incomplete emptying. Palmer and associates (1993) noted urolithiasis in 52% of patients after augmentation cystoplasty. Metcalfe and colleagues (2006) noted a 15% rate of bladder stone formation in 500 patients with long-term follow-up after enterocystoplasty; the reasons for these remarkable differences are not clear. The majority of bladder stones in this population of patients are struvite, and bacteriuria has been thought to be an important risk factor. Any infection with a urea-splitting organism should therefore be treated aggressively. All patients requiring CIC, particularly those who have already formed stones, should make every effort to empty the bladder completely with each catheterization. If stones are found in patients voiding spontaneously after augmentation, the adequacy of emptying should be reevaluated. The association of urinary stasis with stone formation is well established. Routine bladder irrigations to avoid buildup of inspissated mucus may remove a nidus for stone formation. The group at Indiana University and others have stressed irrigations and asked patients and families to routinely irrigate the bladder several times a day after augmentation (Rink et al, 1995a; Hensle et al, 2004). Compliance with such irrigations may lower the frequency of stone formation.
Stones have been noted after the use of all intestinal segments, with no significant difference noted between small and large intestine. Struvite stones are less likely after gastrocystoplasty (Kaefer et al, 1998; Kronner et al, 1998a), probably because of decreased mucus production and acid that minimizes bacteriuria. Uric acid calculi have rarely been noted in the bladder after gastrocystoplasty (Kaefer et al, 1998). Clearly, any foreign body will serve as a nidus for stone formation; the use of permanent suture or staples in the urinary tract should be avoided during enterocystoplasty. Khoury and associates (1997) looked for metabolic problems in patients after augmentation and noted low urinary citrate levels in patients with and without stones. They thought that poor emptying and mucus buildup were more significant factors.
Tumor Formation
A well-recognized complication of ureterosigmoidostomy has been the development of tumors, primarily adenocarcinoma, at the ureterocolonic anastomotic site. In Husmann and Spence’s (1990) review of reported tumors after ureterosigmoidostomy the latency for development of such tumors averaged 26 years and ranged from 3 to 53 years. Adenocarcinomas were the prominent tumors that developed, but benign polyps and other types of carcinoma were also found. Eraklis and Folkman (1978) estimated that the risk for development of such tumors is increased by 7000-fold over that of age-matched controls after ureterosigmoidostomy. The exact basis for the increased risk is unknown; however, N-nitroso compounds thought to originate from a mixture of urine and feces may be carcinogenic. These compounds have been noted in the urine of patients with conduit diversion and augmentation (Treiger and Marshall, 1991). Husmann and Spence (1990) suggested that those compounds are more likely to be enhancing agents rather than a lone cause of tumor development. It has been proposed that inflammatory reaction at the anastomotic site may induce growth factor production, which in turn increases cellular proliferation.
Filmer and Spencer (1990) identified 14 patients who have developed adenocarcinoma in an augmented bladder, and several more have been reported since then. Nine of those tumors occurred after ileocystoplasty and 5 after colocystoplasty. One study has noted a relatively high incidence of tumor after gastrocystoplasty (Castellan et al, 2007). Experimental work in the rat demonstrated hyperplastic growth in the augmented bladder with use of all intestinal segments; no segment showed any particular increased risk (Klee et al, 1990; Buson et al, 1993; Spencer et al, 1993; Little et al, 1994). The applicability of such findings to humans is uncertain. The long latency noted for tumor development after ureterosigmoidostomy suggests that short-term follow-up after augmentation cystoplasty is not adequate to evaluate tumor formation. The earliest reported tumor after augmentation was found only 4 years after cystoplasty (Carr and Hershown, 1997). Patients undergoing augmentation cystoplasty should be made aware of a potential increased risk for tumor development. Yearly surveillance of the augmented bladder with endoscopy should eventually be performed; the latency period until such procedures are necessary is not well defined (Vajda et al, 2002). The earliest reported tumor after augmentation was found only 4 years after cystoplasty (Carr and Herschorn, 1997). Transitional cell carcinoma, hyperplasia, and dysplasia have also been noted near the anastomosis in humans (Gregoire et al, 1993; Barrington et al, 1997; Soergel et al, 2004). Transitional cell carcinomas associated with augmentation cystoplasty have been aggressive; often the patients have metastatic disease at presentation (Metcalfe et al, 2006; Higuchi et al, 2010). Urothelium adjacent to the anastomosis has been demonstrated to be genetically unstable on biopsy in one study (Appanna et al, 2007). Urine cytology or flow cytometry may ultimately become useful in surveillance.
Delayed Spontaneous Bladder Perforation
Perhaps the most disturbing complication of augmentation cystoplasty is delayed bladder perforation. Patients presenting with spontaneous perforation after augmentation cystoplasty are generally quite ill with abdominal pain, distention, and fever. Sepsis has been common. Nausea, decreased urine output, and shoulder pain from diaphragmatic irritation have also been noted. Perforations have been found in evaluation of asymptomatic pelvic masses (Pope et al, 1999). Patients with neurogenic dysfunction often have impaired lower abdominal sensation and present later in the course of the illness; severe sepsis and death have occurred. Patients with perforation after gastrocystoplasty often present promptly because of acid irritation. A high index of suspicion for perforation is necessary. Contrast cystography is diagnostic in most cases (Braverman and Lebowitz, 1991; Rosen and Light, 1991; Bauer et al, 1992) (Fig. 129–14). Thorough technique is important to identify as many true positives as possible with cystography (Braverman and Lebowitz, 1991). Some reports of perforations have noted a significant false-negative rate on cystography (Rushton et al, 1988; Sheiner and Kaplan, 1988; Pope et al, 1999) and suggested that ultrasonography and computed tomography improve diagnostic accuracy. They recommended that one of those studies be done in any child thought to have a perforation if the initial cystogram is normal.
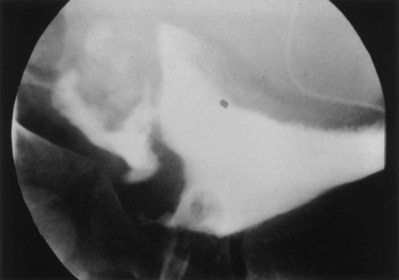
Figure 129–14 After complete filling, a sagittal view on the cystogram demonstrates a spontaneous bladder perforation on the post-drain view.
Etiology
The etiology of delayed perforations within a bowel segment is unknown. It has been suggested that perforation might be secondary to traumatic catheterization in some cases (Elder et al, 1988; Rushton et al, 1988). Perforation of a bladder not previously augmented has been recognized after CIC (Reisman and Preminger, 1989). It seems unlikely that catheterization trauma is the lone cause in most patients. The location of the perforations has been variable among patients and even in a single patient with multiple perforations. Perforations have occurred after augmentation in patients who did not catheterize at all. Others have suggested that trauma to the bowel because of fixed adhesions resulting in shearing forces with emptying and filling may result in perforation (Elder et al, 1988). Chronic, transmural infection of the bladder wall has also been proposed as a cause. Histologic examination of bowel segments adjacent to areas of perforation has noted necrosis, vascular congestion, hemorrhage, and hemosiderin deposition compatible with chronic bowel wall ischemia (Crane et al, 1991). Chronic overdistention of the bladder might result in such ischemia. Decreased perfusion in dog bowel used for augmentation can be induced experimentally with high intravesical pressure (Essig et al, 1991). Those changes were noted more prominently at the antimesenteric border of the bowel. Chronic ischemia may thus play a significant role in at least some delayed bladder perforations. Anderson and Rickwood (1991) have reported perforations occurring in bladders with significant uninhibited contractions after augmentation, as have others (Pope et al, 1998). High outflow resistance may maintain bladder pressure rather than allow urine leakage and venting of the pressure, potentially increasing ischemia (Martinez del Castillo et al, 2005). Hyperreflexia alone is not likely to be a solitary cause of perforation; the complication was essentially never recognized in the era before bowel detubularization and reconfiguration when persistent pressure contractions were more common after augmentation cystoplasty. Once bowel is reconfigured, however, it may be more susceptible to ischemia if high pressure does persist. Failure to perform CIC on a regular basis when needed to empty may exacerbate such issues (DeFoor et al, 2003a; Martinez del Castillo et al, 2005).
The majority of patients suffering perforations after augmentation cystoplasty have had myelodysplasia. The incidence of perforation has been lower in series of patients with other diagnoses requiring bladder reconstruction (Hendren and Hendren, 1990). The role of neurogenic dysfunction in the etiology of these perforations is unclear. No matter what the etiology there is likely to be some field effect on the entire segment. Once a patient has suffered a spontaneous perforation, the chance of recurrence is significant (Hollensbe et al, 1992; Martinez del Castillo et al, 2005; Metcalfe et al, 2006). One fourth of patients with ruptures in one very large series had a recurrence (Metcalfe et al, 2006). Consideration must eventually be given to removal of the original segment and replacement by another after repeated perforation.
Incidence
Early postoperative leaks from the bowel-to-bowel or bowel-to-bladder anastomoses after augmentation cystoplasty are rare and represent a technical error or problem with early healing. Delayed perforations more commonly occur within the bowel segment itself and represent a problem with long-term storage of urine within an intestinal segment. There may be no particular increased risk of one intestinal segment over another. At Indiana University, perforations were noted in 43 of 500 patients (8.6%) undergoing cystoplasty (Metcalfe et al, 2006) an average of 4.3 years after augmentation. Analysis of this experience suggested that the use of sigmoid colon was the only significantly increased risk. Several other large series of patients with sigmoid cystoplasty have noted a low incidence of delayed perforation (Sidi et al, 1987a; Hendren and Hendren, 1990; Shekarriz et al, 2000). At Children’s Hospital Boston, the incidence of perforation after augmentation was highest in ileum (9.3%) and less frequent in ileocecal, sigmoid, and gastric segments (Bauer et al, 1992).
With inconsistent differences across multiple large series it is unlikely that any given enteric segment is at significantly increased risk for perforation and that likely multiple factors influence the risk for the complication. Pope, Rink, and the Indiana group (1999) still believe strongly from their experience that sigmoid colon has a greater risk for perforation based on a four-time higher rate than ileum there. Some other experienced surgeons think the same (Ransley, 1998). The highest rate of perforation noted has been 16.6% in one relatively small series (Martinez del Castillo et al, 2005); however, it is reasonable to present an expected risk of about 9% to patients and families based on experience at Indiana University (Metcalfe et al, 2006).
Treatment
The standard treatment of spontaneous perforation of the augmented bladder is surgical repair, as it is for intraperitoneal rupture of the bladder after trauma. There are reported series of conservative management for suspected perforation (Slaton and Kropp, 1994). Conservative management including catheter drainage, antibiotics, and serial abdominal examinations was successful in 87% of the patients, although only 2 of the 13 patients with suspected ruptures had x-ray documentation unequivocally identifying a perforation. Even patients who do well with conservative management during the acute episode often require eventual surgical intervention (Pope et al, 1999). Such management may be a consideration in a stable patient with sterile urine. The surgeon should certainly have a low threshold for surgical exploration and repair. The majority of patients with perforations have myelodysplasia and present late in the course of the disease because of impaired sensation. Increasing sepsis and death of the patient may result from a delay in diagnosis or treatment.
Pregnancy
Good success has been reported for female patients after bladder augmentation, bladder neck repair, or continent urinary diversion in terms of continence and upper tract preservation. Little note has been made of fertility issues. Limited information exists regarding the outcome of pregnancy in women who have undergone augmentation cystoplasty, because many of these patients are just reaching childbearing age. Older women undergoing bladder replacement for oncologic reasons often are rendered infertile by the nature of the disease and treatment.
Three major issues are raised in the context of pregnancy and bladder reconstruction: how the expanding uterus will affect the pedicle of the reconstruction; how to manage bacteriuria during pregnancy; and whether cesarean section is needed in all or some of these patients and, when it is performed, how to manage the pedicle of the augmentation patch.
Experience during pregnancy related to the pedicle of a prior bladder augmentation is limited. Hatch and colleagues (1991) and Schumacher and coworkers (1997) have noted that the mesenteric pedicle to bladder augmentations did not appear to be stretched at the time of cesarean section. In those cases the pedicle was not located near the exposed anterior uterus but deflected laterally. Schilling and colleagues (1996) recognized a difference between urinary diversion and augmentation. With diversion, the pedicle of the intestinal segment extended cranially and laterally away from the uterus, whereas the pedicle covered the uterus after bladder augmentation. Neither process prevented the rise of the uterus during pregnancy. Those authors speculated that the mesentery underwent changes that enabled deflection or stretch without any adverse effect on circulation. Loss of the augmentation due to mechanical effect on the pedicle from the enlarging uterus has not been reported. Pregnancy itself has not had a reported detrimental effect on the augmentation other than temporary incontinence in some patients, particularly during the third trimester. Successful pregnancy with spontaneous vaginal delivery has been observed in a few cases with bladder augmentations (Quenneville et al, 2003) and other forms of urinary reconstruction, such as ureterosigmoidostomy and ileal conduit urinary diversion (Pedlow, 1961; Asif and Uehling, 1969). Schumacher and coworkers (1997) reviewed their experience with pregnancy and delivery in patients who had undergone continent catheterizable ileocecal diversion and found no major complications. It is likely that this would also be true for other forms of reconstruction (Wren et al, 2003). Urgency and late difficulty with catheterization have been noted with Kock continent ileostomies (Ojerskog et al, 1988) and can occur with any continent abdominal wall stoma (Greenwell et al, 2003).
Urinary tract infections may be problematic during pregnancy in women who have undergone urinary reconstruction including bladder augmentation. Ureteral dilation, increased residual urine, and diminished tone to the upper tract may all be important risk factors (Hill et al, 1990; Hatch et al, 1991). Asymptomatic bacteriuria is usually treated immediately in pregnancy women because of an increased rate of progression to symptoms and pyelonephritis and because of the potential significant consequences of pyelonephritis. Asymptomatic bacteriuria is often not treated routinely in a woman on intermittent catheterization after cystoplasty when not pregnant. There is little consensus about the role of antibiotics and the risk of asymptomatic bacteriuria in the same population once pregnant (Thomas and Adams, 2009). Antibiotics, if used, must not be teratogenic.
Patients with preexisting hydronephrosis may be at increased risk not only for infection but also for worsening hydronephrosis. Some may require stent or nephrostomy tube placement (Gitlin et al, 2002). The patients at greatest risk during pregnancy after reconstruction are those with renal insufficiency. Some may demonstrate deterioration of function or permanent failure (Jones and Hayslett, 1996). Uterine prolapse has been noted to occur at a higher rate with pregnancy, particularly in exstrophy patients (Kennedy et al, 1993). Because of such concerns, Stein and associates (1994) suggested fixation of the uterus during reconstructive surgery.
The need for cesarean section is probably not universal for all women with bladder reconstructions. Adequate progression of spontaneous vaginal delivery, however, may require flaccid, distensible pelvic tissues, which may not be present after extensive pelvic surgery. It is unknown if tissues fixed from previous operative repairs can undergo the trauma of delivery and resume the same level of function as before the pregnancy. The authors’ bias would be that women having undergone extensive bladder neck repair should consider cesarean delivery, particularly if the progression toward spontaneous vaginal delivery is slowed or difficult at all. Although there are reports of spontaneous vaginal delivery in the presence of an artificial urinary sphincter (Fishman and Scott, 1993; Creagh et al, 1995), the presence of such a prosthetic device raises a concern for erosion with a long, difficult delivery.
If cesarean section is required or selected, it is imperative to protect the augmentation or continent stoma and its vascular pedicle. The anterior uterus can typically be exposed atraumatically, although some time and patience may be required to protect the bladder. Such exposure may be more difficult if multiple abdominal stomas are present. The reconstructive urologist familiar with the patient and her anatomy should be present during cesarean section to maximize safety.
Choice of Segment
The use of bowel for augmentation of the bladder was first described experimentally by Tizzoni and Foggi in 1888 and in humans by Mikulicz in 1898 (Orr et al, 1958). Extraordinary advances have been made in the use of the bowel in urinary tract reconstruction since then. Bladder augmentation is used for patients with bladder dysfunction related to a small-capacity, noncompliant reservoir. Enterocystoplasty improves bladder capacity and compliance in most cases when medical management fails. It is obvious from the preceding discussion that there is no one single bowel segment that is perfect for augmentation in all patients. All gastrointestinal segments have been used and continue to be used with good results. Unremitting medical problems are relatively rare after augmentation cystoplasty if used appropriately in well-selected patients. No one bowel segment has a clear advantage over another when all such problems are considered. Diagnosis, anatomy, and physiology may suggest that one bowel segment is preferable for a particular patient. Each surgeon interested in augmentation cystoplasty should be familiar with the advantages and disadvantages of each segment in different settings.
In many routine cases, any gastrointestinal segment may be chosen for cystoplasty purely on the basis of the personal preference and familiarity of the surgeon. The surgeon’s experience and confidence in using a segment are important. The authors believe that no one bowel segment is the best choice in all patients and that optimal results are achieved when the bowel segment is chosen on the basis of the needs of the particular patient. The segment must then be used correctly. Ileum is preferred if there is no clear advantage to or reason for use of another segment. Stomach is reserved for children with renal insufficiency and acidosis, short-gut syndrome, and heavy irradiation. Sigmoid cystoplasty is used in select patients without reservation; clearly, good results can be expected for most patients with any segment if it is used properly.
Key Points: Augmentation Cystoplasty
Alternatives to Gastrointestinal Cystoplasty
Largely because of the complications and side effects just reviewed, alternative methods that can achieve a large-capacity, compliant reservoir remain attractive. Efforts have covered the spectrum from synthetic materials and autologous grafts to construction of a bladder diverticulum (autoaugmentation) and to various forms of neural stimulation. Some of these alternatives appear to hold promise, but none has stood the test of time for true comparison to intestinal cystoplasty.
An ideal tissue for increasing capacity and improving compliance would have transitional epithelium to be relatively impermeable and avoid metabolic changes. The lining would also not produce mucus and carry no increased potential for tumor development. The ability to augment the bladder without violation of the peritoneal cavity would also decrease potential morbidity. Two such alternative procedures are ureterocystoplasty and autoaugmentation. With ureterocystoplasty there is good muscle backing of transitional epithelium; collagen eventually backs the transitional mucosa of an autoaugmentation.
Ureterocystoplasty
It has been noted for years that in patients with posterior urethral valves, unilateral reflux may behave like a “popoff” valve to lower intravesical pressures and protect the contralateral upper tract (Hoover and Duckett, 1982; Rittenberg et al, 1988; Kaefer et al, 1995). In many of these patients the refluxing ureter is massively dilated, draining a poorly functioning or nonfunctioning kidney. It was a logical extension to use this ureteral tissue to augment the bladder.
Technique
Ureterocystoplasty may be performed through a midline, intraperitoneal incision. This incision provides access to the intestine should mobilization of the ureter for augmentation be unsatisfactory. Bellinger (1993), Dewan and colleagues (1994a), and Reinberg and associates (1995) have shown that ureterocystoplasty can be done through two incisions, remaining completely extraperitoneal. The general technique is the same. A standard nephrectomy is performed with great care to preserve the renal pelvic and upper ureteral blood supply. All adventitia and periureteral tissue is swept from the peritoneum toward the ureter during mobilization to protect the ureteral blood supply. Proximally, this blood supply typically arises medially. As the ureter enters the true pelvis the blood supply arises posterior and laterally. After mobilization of the ureter into the pelvis the bladder is opened in the sagittal plane. Posteriorly, this incision has typically been carried off-center directly into and through the ureteral orifice of the ureter used for cystoplasty. The ureter is not detached from the bladder but is opened longitudinally along its entire length, with care taken to avoid its main blood supply (Fig. 129–15). The incision in the bladder and distal ureter should avoid branches of the superior vesical artery that serves as an important blood supply to the mobilized ureter. The ureter is folded on itself, and the ureter-to-ureter and ureter-to-bladder anastomosis is performed with running absorbable suture. A suprapubic tube is left indwelling through the native bladder for 3 weeks during healing. After cystography documents the absence of leakage, CIC is started. Patients, particularly those without neurogenic dysfunction, may attempt to void spontaneously, although all must prove that they can empty appropriately by checking postvoid residual urine amounts.
Figure 129–15 Ureterocystoplasty. A, After nephrectomy, the bladder is bivalved, with the posterior aspect of the incision carried off the midline to enter the ureteral orifice. The ureter is not detached from the bladder. B, The ureter is opened opposite its main blood supply. C, The ureter is reconfigured as in intestinal cystoplasty before anastomosis to the bladder.
Alternatively, the bladder incision can be stopped approximately 2 cm from the orifice and a similar length of distal ureter left in situ and intact without incision. The resulting small loop of intact ureter does not cause clinical problems or adversely affect the end volume in a significant manner (Adams et al, 1998). This modification of technique is easier and may be safer in that it avoids potential injury to the blood supply of the mobilized ureter near the ureterovesical junction.
Results
Early reports suggested that ureterocystoplasty could be achieved after nephrectomy by bivalving the bladder through the vesicoureteral junction and laying in the open ureter (Mitchell et al, 1992; Bellinger, 1993). Later reports modified and improved the procedure by noting that with meticulous care the vascularity to the entire renal pelvis could be preserved, allowing more tissue for cystoplasty (Churchill et al, 1993; Landau et al, 1994; McKenna and Bauer, 1995; Reinberg et al, 1995). As with intestinal cystoplasty, folding the ureter into a more spherical configuration maximizes the volume that can be achieved. If a massively dilated ureter drains a functioning kidney the distal ureter alone may be used for augmentation; the proximal ureter is either reimplanted into the bladder or anastomosed to the contralateral ureter (Bellinger, 1993).
Numerous series have reported good results after augmentation with use of ureter, some with follow-up as long as 8 years. The upper tracts have remained stable or improved in virtually all patients. Complications have been uncommon, with only a rare early extravasation of urine reported (Churchill et al, 1993). Landau and colleagues (1994) compared age-matched and diagnosis-matched children having ureterocystoplasty or ileocystoplasty. The total mean bladder capacity was 470 mL in the ureterocystoplasty group and 381 mL in the ileocystoplasty group. Bladder volumes at 30 cm H2O were 413 mL and 380 mL after ureterocystoplasty and ileocystoplasty, respectively. Ureter effectively enhanced both volume and compliance. There was one failure in the group that occurred in a child in whom the distal ureter did not provide enough volume. Work has shown that one dilated ureter typically is enough for cystoplasty (Zubieta et al, 1999).
The main disadvantage to ureterocystoplasty is the limited population of patients with a nonfunctioning kidney draining into a megaureter. McKenna and Bauer (1995) have reported the use of a normal-sized ureter. The ultimate success of ureterocystoplasty with normal ureter requires further follow-up, particularly since Gonzalez (1999) has stated that one fourth of his patients with posterior urethral valves have failed ureterocystoplasty with a dilated ureter because of their huge urinary volume. Husmann and colleagues (2004) noted even worse results if the ureter used was less than 1.5 cm in diameter. Atala and colleagues (1994) presented an experimental technique to slowly dilate a normal ureter for later use. Work continues to develop such a technique that is clinically applicable for patients (Stifelman et al, 1998; Desai et al, 2003).
Autoaugmentation
Techniques and Results
Cartwright and Snow (1989a, 1989b) described an ingenious method to improve bladder compliance and capacity with use of native urothelial tissue. In their procedure, known as autoaugmentation, they excised the detrusor muscle over the dome of the bladder, leaving the mucosa intact to protrude as a wide-mouth diverticulum. Initially, they made a midline incision through the bladder muscle (Fig. 129–16A). With the bladder distended with saline so that mucosa bulged from the incision, the muscle was then mobilized and excised laterally in each direction (Fig. 129–16B). The lateral edges of the detrusor muscle were then secured to the psoas muscle bilaterally to prevent collapse of the diverticulum (Fig. 129–16C). The early experience of these investigators noted improved compliance in most, and increased capacity in some, patients (Cartwright and Snow, 1989a).
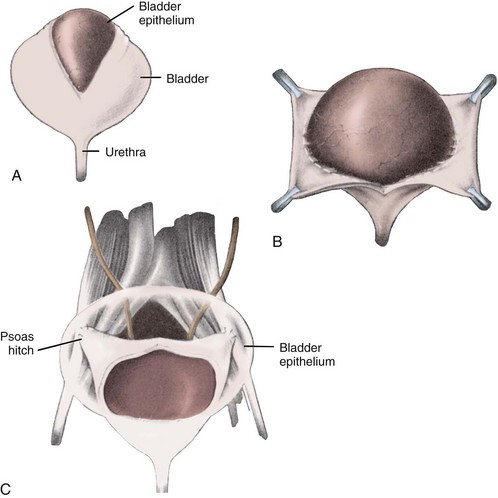
Figure 129–16 Autoaugmentation. A, The detrusor is incised. B, Detrusor is stripped and excised from mucosa. C, The detrusor muscle is anchored bilaterally so that the mucosa bulges with bladder filling.
(From Cartwright PC, Snow BW. Bladder autoaugmentation: early clinical experience. J Urol 1989;142:505.)
This procedure has since been modified by a number of surgeons, each providing a different name for the procedure, depending on whether the detrusor muscle is simply incised or excised to make the diverticulum. In an effort to determine if incision or excision provided superior results, Johnson and colleagues (1994) performed 16 vesicomyotomies and 16 vesicomyectomies in rabbits after previously reducing the bladder capacity. Functional bladder capacity in the animals increased by 43.5%, and there was no statistical difference between the two techniques. They then performed vesicomyotomies (incision) in 12 patients with neurogenic bladder dysfunction and demonstrated a mean increase in capacity of 40% and a mean decrease in leak point pressure of 33% (Stothers et al, 1994). They concluded that detrusor excision offered no advantage over incision. All patients demonstrated some increase in capacity (15% to 70%), and no patient in early follow-up clinically deteriorated and required enterocystoplasty.
Detrusorectomy, leaving a small cap of muscle at the dome through which a suprapubic tube can be placed, has been proposed by Landa and Moorehead (1994). They have been concerned that although these procedures usually improve compliance, increase in volume is “modest at best,” a concern shared by others (Snow and Cartwright, 1996; Cartwright and Snow, 1998). In a report of 12 detrusorectomies, five patients were considered to have excellent results, two had acceptable results, and one was lost to follow-up. There were four failures (one hydronephrosis, two persistent incontinence, one worsening renal insufficiency); three patients have undergone gastrocystoplasty or ileocystoplasty (Landa and Moorehead, 1994). Overall, only 52% of patients had a good result with autoaugmentation; 20% had a poor outcome (Snow and Cartwright, 1996). Reoperative enterocystoplasty was not hampered by the prior detrusorectomy. The urothelial diverticulum at the time of augmentation cystoplasty was noted to be thick and fibrous, similar to a leather bag.
There are obvious advantages to autoaugmentation and its variants. Native urothelial tissue is used. It is an extraperitoneal procedure, which shortens operative time and avoids the risks of intestinal surgery and adhesions. Autoaugmentation is compatible with CIC and does not seem to complicate subsequent intestinal cystoplasty when it is necessary.
Complications from the procedures are generally uncommon. Perforation, a major concern after intestinal cystoplasty, has not been reported. Inadvertent opening of the mucosa during the procedure can make subsequent mobilization more difficult and may promote prolonged postoperative extravasation. Such extravasation usually stops with bladder drainage (Landa and Moorehead, 1994; Stothers et al, 1994). Prolonged drainage, however, may result in compromised results from collapse of the diverticulum. If concomitant ureteral reimplantation or bladder neck surgery is necessary, various authors have recommended that it be done first and the bladder then closed before detrusorectomy (Stothers et al, 1994).
Ehrlich and Gershman (1993) first reported laparoscopic myotomy (incision). A laparoscopic approach uses a smaller incision and perhaps shortens postoperative hospitalization; it may make effective fixation of the detrusor muscle in an open fashion to allow good bulging more difficult.
Concerns
The main disadvantage of autoaugmentation is a limited increase in bladder capacity such that adequate preoperative volume may be the most important predictor of success (Landa and Moorehead, 1994). If the maximum capacity and the volume of urine held at 40 cm H2O are similar the patient may be better served by immediate intestinal cystoplasty. It is of note that some patients have demonstrated clinical improvement after these procedures without a significant change in urodynamics. The reason for the improvement in that setting is unknown.
In most series of autoaugmentation, no matter what the technique, it has been noted in occasional patients or concern has existed at the time of the initial procedure that adequate expansion was not achieved. In most such cases it was elected to proceed with enterocystoplasty immediately at the time (Landa and Moorehead, 1994). These patients are not included in the failure rate of autoaugmentation. The patient and surgeon must be prepared for such an event on occasion. Stohrer’s group (1999) as well as Leng and associates (1999) reported good results with the technique among patients with hyperreflexia. In terms of capacity, even if adequate expansion is achieved initially, there is concern that any improvement may not persist long term (Dewan et al, 1994b). In animals the surface area of the autoaugmentation site was noted to decrease approximately 50% at 12 weeks. Progressive thickening and contracture of the site have been noted because of collagenous infiltrate (Johnson et al, 1994). Milam (2000) has noted that almost half of his adult patients with hyperreflexia who had a good early result after autoaugmentation have failed with longer follow-up. Similar delayed failures have been noted in pediatric patients (Lindley et al, 2003), including one series with very poor results (MacNeily et al, 2003).
At this point, autoaugmentation should probably be considered only in patients who have reasonable capacity but poor compliance due to uninhibited contractions. If a significant increase in capacity is needed, autoaugmentation is unlikely to be as definitive as other techniques.
Seromuscular Enterocystoplasty
Based on concerns about collagen deposition and contraction around autoaugmentation, efforts have been made to cover the bulging urothelium with demucosalized enteric segments. The use of enteric segments without bowel mucosa within the bladder is not new. As far back as the 1950s, seromuscular augmentation cystoplasty was performed with the serosal side of the bowel turned to the bladder lumen (Shoemaker and Marucci, 1955; Shoemaker et al, 1957). Urothelium soon covered the serosa. Gonzalez’s group experienced good results with reversed seromuscular cystoplasty in rats, as did others with rabbits (Celayir et al, 1995), but contracture of the patch occurred in larger animals (De Badiola et al, 1991; Long et al, 1992). Others left the exposed submucosa facing the bladder lumen and noted reepithelialization with urothelium in animals (Oesch, 1988; Salle et al, 1990). Despite the reepithelialization, patch contracture occurred (Salle et al, 1990). Several recent series have reevaluated demucosalized augmentation in humans after taking care to preserve the submucosa. Better results have been noted (Lima et al, 1998, 2004; de Badiola et al, 1999; Dayanc et al, 1999), although regrowth of metaplastic enteric mucosa was found in the second study. Early placement of a silicone balloon or mold may help prevent contracture (Lima et al, 2004).
Techniques and Results
To avoid contracture, a combination of autoaugmentation after detrusorectomy and coverage with a demucosalized enteric segment has now been used. This has been done to potentially preserve the advantages of both procedures. In a similar fashion the combination has been undertaken with both colon and stomach. Buson and colleagues (1994) used reconfigured, demucosalized sigmoid colon placed over the urothelium (seromuscular colocystoplasty lined with urothelium). They and others noted that the intestinal submucosa should be preserved to avoid contracture (Buson et al, 1994; Vates et al, 1997) (Fig. 129–17). This procedure has been performed clinically with early reports of good results in most patients (Gonzalez et al, 1994). Postoperative bladder capacity increased an average of 2.4-fold (139 to 335 mL) in 14 of 16 patients, and end filling pressure decreased from an average of 51.6 to 27.7 cm H2O. Two patients experienced failure of the procedure and required ileocystoplasty; their urodynamic data were excluded. Two other patients developed an hourglass deformity (Gonzalez et al, 1994). Endoscopic biopsy of the segments has been interesting. Of 10 biopsies performed in the series, in 1 urothelium with islands of colonic mucosa was noted and in 2 others only colon mucosa was found. Removal of all of the enteric mucosa is important when sigmoid is used to prevent mucoceles or overgrowth of intestinal mucosa (Gonzalez et al, 1994; Lutz and Frey, 1994). Dewan and associates (1997) thought that preservation of the submucosa eventually promoted regrowth of bowel mucosa. The interaction of the two different tissues will be interesting to follow. The long-term effects on the urothelium by the seromuscular segment and vice versa are unknown. Work has shown that persistent transitional lining will protect from metabolic problems and mucus production (Denes et al, 1997).
Figure 129–17 Seromuscular enterocystoplasty with use of sigmoid colon. Detrusor incision is performed as in autoaugmentation; however, the bulging mucosa is covered with a demucosalized segment of sigmoid colon.
(From Buson H, Manivel JC, Dayanc M, et al. Seromuscular colocystoplasty lined with urothelium: experimental study. Urology 1994;44:745.)
Dewan and Byard (1993) and Close and associates (2004) alternatively used demucosalized stomach to cover an autoaugmentation, first in sheep and then in patients. Early results showed improved bladder function both clinically and by urodynamics (Horowitz and Mitchell, 1993; Dewan et al, 1994c; Horowitz et al, 1994; Robinson et al, 1994), although long-term results are not as encouraging (Carr et al, 1999). These procedures are technically more demanding than simple augmentation or autoaugmentation and are associated with more blood loss and a longer operative time (Gonzalez et al, 1994; Horowitz et al, 1994). Increased bleeding is particularly true when stomach is used. These urothelium-lined, seromuscular augmentations are theoretically attractive. Thus far, the failure and reoperation rate after such procedures remains higher than that noted for standard enterocystoplasty (Vates et al, 1997; Carr et al, 1999; Shekarriz et al, 2000). The results have been reported with colon (Shekarriz et al, 2000). Those results may be partially attributed to the learning curve with a new, complex procedure. Longer follow-up and more experience are necessary to determine whether the complication rate will decrease with experience or increase because of problems with the combination.
Bladder Regeneration
Other efforts to find alternatives to intestinal cystoplasty in the 1950s included the use of alloplastic materials for bladder substitution (Gleeson and Griffith, 1992; Kanematsu et al, 2007; Lewis et al, 2007; Yamzon et al, 2008). Early research efforts met with limited success due to foreign body complications but provided the foundation for regenerative medicine, which uses cells and biomaterials to establish regrowth of healthy tissue. This led to a focus on biodegradable, collagen-rich tissues serving as a scaffold for bladder regeneration (Kelami, 1971; Fishman et al, 1987; Kambic et al, 1992; Kropp et al, 1995a, 1995b, 2004; Zhang et al, 2004; Harrington et al, 2008). Successful regeneration using this technology has been produced in the laboratory with either “unseeded” or cell “seeded” regenerative growth. Atala and associates (2006) provide the first reported data using a seeded biodegradable construct for augmentation in the neurogenic population. Seven children underwent autologous augmentation. No major complications were reported. Encouraging subjective response to the augmentation was obtained, but uniform urodynamic improvement was not universally appreciated. This work by Atala and associates has provided the foundation for an industry-supported prospective multi-institution investigation using a neobladder construction (Joseph et al, 2009). The study enrolled 11 children, and 10 underwent neobladder augmentation. All 10 patients have completed the primary 12-month endpoint. Results similar to Atala and associates’ initial findings have been encountered. Subjective improvement has been reported by some families but has not been correlated with objective urodynamic changes (Joseph et al, 2009). Although these results remain promising, to date an objective successful clinical outcome using autologous regenerative tissue for bladder augmentation is limited. Using autologous tissue from a neuropathic source may potentiate growth of abnormal tissue, but an alternative cell source for regenerative tissue such as stem cells may circumvent that concern. However, the clinical use of stem cells carries its own set of obstacles (Aboushwareb and Atala, 2008; Aitken and Bägli, 2009; Soler et al, 2009). Further investigation into the clinical applicably of regenerative tissue engineering is required. This technology remains poised to significantly alter the field of reconstructive bladder surgery (Atala et al, 1992, 1993b; Cilento et al, 1994; Kropp et al, 1995b; Oberpenning et al, 1997; Probst et al, 1997; Fauza et al, 1998; Kropp, 1998; Yoo et al, 1998; Reddy et al, 2000; Lai et al, 2002; Zhang et al, 2004; Wood and Southgate, 2008; Aitken and Bägli, 2009; Soler et al, 2009).
A Decreasing Necessity?
Although augmentation cystoplasty works well for most patients who require it, and work on alternatives to bowel cystoplasty may lower morbidity for the patient, a primary goal for every pediatric urologist is to minimize the number of patients needing cystoplasty. Newer medical regimens and neuromodulation may prove effective for some patients who presently do not respond to conservative measures (Aslan and Kogan, 2002). Xiao (2005) continues to refine an artificial somatic-autonomic reflex pathway in children with neurogenic bladder due to spinal cord injury and spina bifida. Through a limited laminectomy, the dorsal nerve root of L5 is identified and transected and an anastomosis is made with the distal end of the S3 ventral root. Scratching the dermatome supported by L5 creates reflex stimulation of the bladder initiating a contraction. Improvement in bladder compliance may occur. Other centers are exploring this technique (Peters et al, 2010). No matter what the diagnosis, earlier and more aggressive treatment of bladder dysfunction may minimize the insult to the bladder and maximize recovery as well as ultimate bladder function. Early urodynamic evaluation of boys with posterior urethral valves may identify treatable bladder problems and improve the prognosis from the standpoint of the kidneys and bladder (Misseri et al, 2002). Grady and colleagues (2003) have suggested that complete primary repair of bladder exstrophy results in early bladder cycling that improves eventual bladder function and decreases the likelihood of augmentation cystoplasty.
With perhaps the most compelling evidence to date, Kaefer and associates (1999b) found that only 17% of patients with hostile neurogenic bladder dysfunction treated immediately on diagnosis required augmentation cystoplasty compared with 41% of similar patients treated expectantly. Although the series included no collaborative urodynamic data and might be subject to lag time bias, the authors thought that there was a significant difference in the outcomes for the two groups. Because there are no prospective, randomized trials evaluating early evaluation and treatment of pediatric bladder dysfunction, recommendations that expectant treatment may lower the necessity for augmentation cystoplasty (Bauer, 2003; Mitchell, 2003) remain a personal bias rather than a scientific fact. Thus far, early bladder management has not decreased the rate of augmentation cystoplasty (Lendvay et al, 2006). Critical, prospective evaluation of such treatment is needed and will, it is hoped, demonstrate how to manage these patients. It is likely that such improvements will minimize the need for cystoplasty but not completely remove it.
Improving Quality of Life?
Reconstruction to achieve continence has been assumed to improve health-related quality of life. Early evaluation of small numbers of patients undergoing reconstruction including augmentation cystoplasty do not always show improved status on objective questionnaires compared with preoperative studies or control patients without surgery despite what generally would be considered to be good clinical results (Macneily et al, 2005; Parekh et al, 2006). Patient-reported scores also do not always correlate with those noted by their parents. Most evaluation of surgical techniques to date has focused on results and complications from the perspective of surgeons. Future evaluation should include objective, patient-reported consideration. Tools to acquire that information must be validated for longitudinal study of these patients and their disease processes.
Key Points: Alternatives to Gastrointestinal Cystoplasty
Continent Urinary Diversion
The frequency with which continent urinary diversion is performed in children depends on one’s definition of continent diversion. In adults, total bladder replacement is not uncommon after cystectomy for transitional cell carcinoma. This has led to extensive experience with continent urinary diversion and orthotopic neobladders that allow spontaneous voiding. Tumors resulting in cystectomy among children are much less common. It is in that setting, and the occasional child in whom the bladder is congenitally absent or so small as to be virtually useless, that a pure continent urinary diversion in the classic sense of an Indiana or Kock pouch might be performed. Very good results with continent diversion in children have been achieved, equivalent to those reported in adults (Stein et al, 2005). Orthotopic neobladders in children are performed infrequently. Neurogenic or anatomic problems at the outlet prevent spontaneous voiding in many cases. Even among patients having undergone cystectomy for tumors, irradiation can interfere with voiding. On occasion, a child with neurogenic dysfunction may be a candidate for orthotopic bladder substitution (Stein et al, 2000, 2005). It is not clear how many of those patients with neurogenic dysfunction can be expected to void adequately.
More frequently in children, a series of continent diversion have included patients undergoing a mix of the techniques discussed in this chapter. Some authors have defined combinations of bladder augmentation, continent abdominal wall stoma, and some procedure at the outlet as continent diversions (Kaefer et al, 1997b). Division and closure of the bladder neck to prevent incontinence per native urethra has typically meant inclusion. Certainly, maximal use of the native urinary tract is beneficial to the child. Much like urinary refunctionalization, these procedures typically have been performed in complex patients with multiple problems that must be addressed, often after numerous previous surgeries.
Considerations
The amount of bowel used in continent urinary diversion may vary according to the patient. Total bladder replacement requires much more intestine than simple augmentation. Typically, a 40-cm segment of small bowel is used for an ileal reservoir in a Kock pouch compared with the 20 cm often used for augmentation. Likewise, the entire right colon with the hepatic flexure may be used in an Indiana pouch with or without a patch of small intestine, whereas only 15 to 20 cm of colon is needed for colocystoplasty. Because of the potential morbidity associated with use of a larger intestinal segment the native bladder is often incorporated in children if it provides any significant volume. To do so, however, may require repair of the outlet if outflow resistance is low. Otherwise, incontinence per native urethra will result.
Imbrication of the ileocecal valve and terminal ileum has proved to be a simple and reliable means for construction of an effective efferent limb in continent diversion among adults and children. Despite reports to the contrary in select patients (Husmann and Cain, 1999), concern about fecal incontinence secondary to use of the ileocecal valve persists for patients with neurogenic dysfunction. The flap valve continence mechanism provides numerous alternatives for those surgeons with such concerns in continent diversion. The good results achieved with the appendix or tapered intestinal segments has led to their increased use in recent years.
Whereas maintenance of the native urethra for catheterization is ideal, it may not be appropriate or possible in all individuals. As indicated previously, reconstructive bladder neck procedures are often subject to difficulty with catheterization. Children with neurogenic sphincter incompetence may have associated neurologic limitations that prevent easy access to the native urethra. This is particularly true for the wheelchair-bound child. For children without neurologic deficits, normal sensation in the native urethra can prevent compliance with a routine catheterization schedule because of discomfort. For these reasons, a continent catheterizable stoma provides an adequate and sometimes a more reliably useful alternative.
Continence Mechanisms and Catheterizable Segments
Ureterosigmoidostomy and Its Variants
Ureterosigmoidostomy can be an effective form of continent urinary diversion in some patients. Its major advantage is the potential for spontaneous emptying by evacuation of urine with stool. The significant complications of hyperchloremic acidosis, infection, hydronephrosis, and the development of colonic malignant neoplasms have led to disfavor and disuse, particularly in the United States. Although good results with standard ureterosigmoidostomy have been reported in some children, the procedure is rarely performed as originally described. The morbidity of acidosis and upper tract changes may increase with time, making those complications particularly worrisome for children with a long life expectancy. The significant risk of adenocarcinoma commits the patients to a lifetime of close surveillance for an avoidable tumor. It is unlikely that ureterosigmoidostomy, as classically described, will regain acceptance for children.
Several modifications of ureterosigmoidostomy have been developed in an attempt to decrease the significant complication rate. The most basic of the modifications is the sigma rectum or the Mainz II pouch. The colon is incised along the antimesenteric border for 6 cm both proximal and distal to the rectosigmoid junction. The ureters are implanted in an antirefluxing fashion, and that portion of colon is then reconfigured and closed. This is done to create a rectosigmoid reservoir of lower pressure to protect the upper tracts. The Mainz II pouch has been used in children primarily with bladder exstrophy (Stein et al, 1997a). Continence in appropriately selected patients is good, although acidosis is still a significant problem because of exposure of the entire colon to urine (Fisch et al, 1996; Gerharz et al, 1999; Mingin et al, 1999a). The potential for development of adenocarcinoma still exists with this technique. Long-term follow-up is necessary to determine if the reconfiguration of the sigmoid in the area of ureteral implantation is truly effective for protection of the upper tracts. Stricture at the ureterocolonic anastomosis has been the most common complication in relatively short follow-up (Fisch et al, 1996).
In an effort to control the amount of colon to which urine is exposed, Kock and associates (1988) described a colorectal valve to confine urine to the distal segment. The intussuscepted nipple valve is stabilized with permanent staples. The distal rectal segment is opened and patched with ileum to lower pressure. With short-term follow-up, the valve is effective in that the authors noted no necessity for sodium bicarbonate or potassium citrate therapy (Kock et al, 1988), a finding duplicated by others (Mahran et al, 1999). An increased risk for tumor development probably still exists with this modification because the ureterocolic anastomoses are still exposed to an admixture of stool and urine. Urodynamic evaluation has revealed low pressure in the rectal reservoir (Kock et al, 1988). Long-term follow-up of its use in children is needed to see if it is truly less morbid in terms of infection and upper tract deterioration. If placement of a colorectal valve avoids most complications of metabolic acidosis from ureterosigmoidostomy and construction of a reservoir with lower pressure better protects the upper tracts, then the last remaining major concern about ureterosigmoidostomy is tumor development. The concern is significant; of 94 children observed in Boston after ureterosigmoidostomy, adenocarcinoma developed in 7, of whom 4 died of the tumor (Rink and Retik, 1991). Kock and coworkers (1988), followed by Skinner and associates (1989), used a hemi-Kock pouch to augment the distal rectal segment. A colorectal valve was made to isolate urine to the distal colonic reservoir. The afferent nipple valve kept stool away from the ureteroileal anastomoses, perhaps lowering the risk for tumor development. Simoneau and Skinner (1995) reported their results with the procedure in 15 patients, including 4 children. Their complication rate, both early and late, was relatively high. This is not surprising, considering the relatively complex nature of the procedure. They did think that the pediatric patients were better suited for the procedure. Rink and Retik (1991) suggested that the rectum could be augmented with a nonrefluxing ileocecal conduit in a similar fashion.
Before any variant of ureterosigmoidostomy is considered, competence of the anal sphincter must be ensured. Tests used to assess sphincter integrity include manometry, electromyography, and practical evaluation of the ability to retain an oatmeal enema in the upright position for a time without soilage. Incontinence of a mixture of stool and urine results in foul soilage and must be avoided. Consequently, most patients with neurogenic dysfunction, who are incapable of fecal continence in the presence of diarrhea, are not candidates for these procedures. Procedures that separate the fecal and urinary streams within the rectal sphincter have been described but have not been widely used among children.
Nipple Valves
The greatest experience with nipple valves used to achieve urinary continence has been with the Kock pouch. Skinner and associates (1989) made a series of modifications to aid in maintenance of the efferent nipple. Despite experience and use of these modifications, a failure rate of 15% or higher can be expected (Benson and Olsson, 1998) (Fig. 129–18). Several authors have reported a reoperative rate of approximately 33% with the Kock pouch, most frequently related to the efferent nipple (deKernion et al, 1985; Waters et al, 1997). Equivalent results with the nipple valve and a Kock pouch have been achieved in children (Hanna and Bloiso, 1987; Skinner et al, 1988; Kaefer et al, 1997b; Abd-El-Gawad et al, 1999). The last report noted a significant incidence of hyperchloremic acidosis and new hydronephrosis, although those complications were likely due to the complex nature of the patients rather than the particular continent diversion used.
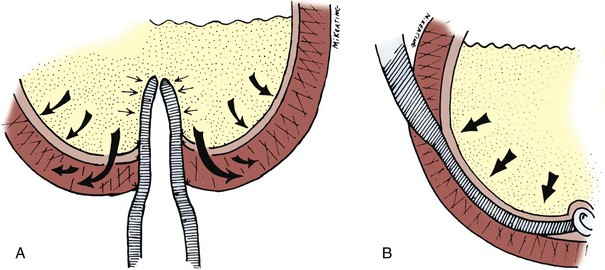
Figure 129–18 Continence mechanisms. A, The same pressures that create continence tend to efface nipple valves. B, This is not true of flap valves, which have been more reliable in pediatric reconstruction.
Intussuscepted nipple valves have also been used with colonic and ileocolonic reservoirs, particularly the Mainz I pouch. Again, evolution of the nipple valve in the Mainz pouch occurred over time (Thuroff et al, 1986, 1988; Hohenfellner et al, 1990; Stein et al, 1995). Most recently, the intussuscepted ileum was fixed with staples, passed through the intact ileocecal valve, and fixed again. Much as with the Kock pouch, the incidence of incontinence decreased with experience and modifications. The Mainz I pouch has been used in children with good results and low rates of incontinence with the latest modifications (Stein et al, 1995, 1997a, 2000; Steiner et al, 1998). Maintenance of normal upper tracts has been good, and metabolic problems are rare (Stein et al, 1997b). In fact, improvement of preexisting hydronephrosis has been more common than worsening. For the past several years, the group in Mainz has used a flap valve mechanism and the appendix for a continent catheterizable stoma with less incontinence (Stein et al, 1995; Gerharz et al, 1997).
Flap Valves and the Mitrofanoff Principle
Mitrofanoff (1980) described a continence mechanism with use of the appendix and ureter to make a flap valve. He recognized that any tubular structure could be implanted effectively into a low-pressure reservoir. This continence mechanism circumvents many of the secondary potential complications associated with harvesting of the ileocecal valve or use of other gastrointestinal segments.
The foundation for the success of the Mitrofanoff principle is based on construction of a submucosal tunnel for a supple, small-diameter conduit. As the reservoir fills, the rise in intravesical pressure is transmitted through the epithelium and to the implanted conduit, coapting its lumen (Fig. 129–19). The appendix is an ideal natural tubular structure that can be safely removed from the gastrointestinal tract without significant morbidity. The small caliber of the appendix facilitates construction of a short functional tunnel with the bladder wall. Experience has shown that continence can be achieved with only a 2-cm appendiceal tunnel (Kaefer and Retik, 1997). Whether in a continent urinary reservoir or native bladder the appendix has been used as an efferent limb with very good results (Hampel et al, 1995; Jayanthi et al, 1995; Kaefer et al, 1997b; Mollard et al, 1997; Cain et al, 1999). The appendix has been particularly useful in children because it is relatively longer and the abdominal wall generally thinner. The flap valve is probably the most reliable of all of the surgically constructed continence mechanisms. This is good in terms of continence when the patient is catheterized reliably. Many patients with good flap valves will virtually never leak per stoma. This potentially puts them at greater risk for upper tract deterioration or spontaneous rupture of the bladder or reservoir if catheterization is not performed.
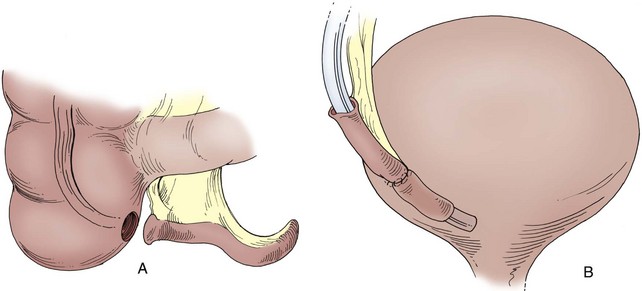
Figure 129–19 Appendicovesicostomy. A, The appendix is harvested with a cuff of cecum on a wide pedicle based on the appendiceal artery. A flap of cecum can be harvested in continuity with the appendix and tubularized to increase the length. B, The distal appendix is tunneled into the bladder to provide continence after the end is amputated. The proximal end of the appendix, the cecal cuff, is brought to the umbilicus or right lower quadrant as a catheterizable stoma.
If the appendix is used in situ as a continence mechanism in a continent urinary reservoir, the reservoir by necessity will include the right colon. Duckett and Snyder (1986, 1987) used the right colon and appendix with good results in children. The mesoappendix, in most cases, allows mobilization of the appendix for use in the native bladder or virtually any reservoir.
Technique
Appropriate preoperative planning is required to position the skin incision to allow adequate mobilization of the appendix to the bladder. In most children this can be achieved through a low midline or a transverse incision. On occasion, the cecum may be high in the abdomen. Mobilization of the ascending colon along the line of Toldt may be required to gain mobilization of the appendix and its mesentery. Cadeddu and Docimo (1999) have used laparoscopy to aid in mobilization of the ascending colon and cecum. Once the cecum has been mobilized the base of the appendix is amputated, leaving a small cuff of cecum with the appendix. Use of the cuff at the stoma may decrease the risk of stenosis. The cecum is closed in a fashion similar to an open appendectomy. If the length of the appendix is marginal, then a greater portion of the cecum can be harvested, which effectively increases the functional length of the appendix (Cromie et al, 1991). A portion may be tubularized with the appendix (Bruce and McRoberts, 1998).
After harvesting, a location is selected for implantation of the appendix into the bladder. The location is based on the length of the appendix, the mobility of the bladder, and the location for the appendiceal stoma. Typically, the distal end of the appendix is tunneled into a posterolateral position within the bladder (see Fig. 129–19).
The base of the appendix is brought to the abdominal wall in a location chosen preoperatively to suit the patient. If a patient wears a supportive brace, stomal positioning must take this into account. The appendix should be brought up to reach the skin without tension, and care must be taken not to twist the pedicle or to occlude it as it passes through the abdominal wall fascia. The base of the appendix often can be hidden within the umbilicus, which allows elimination of a small but obvious abdominal stoma. Because of the small circular diameter of the appendiceal base, stomal stenosis is common; and techniques have been described to prevent this problem. Various flaps have been described as a method for avoiding stomal stenosis (Keating et al, 1993; Kajbafzadeh et al, 1995; Kaefer and Retik, 1997; Frane-Guimond et al, 2006; Berrettini et al, 2008; Landau et al, 2008). The VQZ stoma-plasty and its variations have been described in an attempt to prevent exposing intestinal mucosa from the catheterizable channel and decreasing the risk of stomal stenosis (Frane-Guimond et al, 2006; Berrettini et al, 2008; Landau et al, 2008). Regardless of the technique, stomal stenosis will remain a primary complication. Chronic nighttime catheterization with a stent placed into the channel but not reaching the bladder may further decrease the risk of superficial stenosis (Mickelson et al, 2009). For prevention of kinking and problems with catheterization it is advisable to maintain as short a conduit as possible. Securing the appendix and bladder wall to the peritoneum beneath the fascia will help diminish the problem of conduit kinking with reservoir filling. When appendix or any catheterizable stoma is used, it is advisable to repeatedly catheterize the channel after each step in reconstruction to confirm easy passage. If the catheter does not pass easily into the reservoir, the preceding step needs to be revised. Problems with catheterization by the surgeon during the operative procedure usually result in more difficulty for the patient afterward. It is also beneficial to catheterize at variable reservoir volumes to confirm proper fixation of the reservoir and the absence of any kinking. A 6- to 10-Fr Silastic stent is typically left for 10 to 14 days within the efferent catheterizable channel during the healing process. It is advisable for the surgeon to personally catheterize the efferent limb before allowing the patient or other family members to do so. Catheterization should be repeated at least every 4 hours during the day for reservoir drainage, maintenance of patency, and minimalization of the risk of stomal stenosis.
The appendix may not be available for use in all patients because of previous appendectomy, its location or length, congenital absence, involvement with adhesions, or its use for continence enemas. Histologic abnormalities of the appendix have been reported to occur in as many as 30% of patients (Liebovitch et al, 1992) and to increase with age. They rarely are of enough clinical significance to preclude use (Mulvihill et al, 1983).
Results
Several papers have reviewed large series of patients after appendicovesicostomy (Kaefer et al, 1997b; Cain et al, 1999, Harris et al, 2000; Thomas et al, 2006; Welk et al, 2008). The populations of patients have been typical of most pediatric groups, the majority having neurogenic dysfunction. Inability to use the appendix, other than because it was needed in situ for antegrade continence enemas, has been rare. The results, in terms of continence, have been superb, usually above 95% (Gerharz et al, 1997, 1998; Kaefer and Retik, 1997d; Mor et al, 1997; Suzer et al, 1997; Gosalbez et al, 1998; Cain et al, 1999; Castellan et al, 1999; Liard et al, 2001; Thomas et al, 2006; Welk et al, 2008). Incontinence is a rare event with the Mitrofanoff principle and may result from inadequate length of the flap valve mechanism or persistently elevated reservoir pressure. Urodynamic evaluation is required to evaluate the cause of the incontinence. Injection of collagen or other biomaterials is a possible treatment for inadequate outflow resistance, with success reported up to 50% but long-term durability unknown (Welk et al, 2008). A more formal approach with takedown and revision of the leaking Mitrofanoff valve is usually required (Kaefer and Retik, 1997d). The most common complication has been stomal stenosis, which has generally occurred in 6% to 10% of patients (Thomas et al, 2006; Welk et al, 2008). Such stenosis resulting in difficult catheterization may occur early in the postoperative course and require formal revision (Harris et al, 2000). Another recognized complication has been appendiceal perforation. Most problems with stomal stenosis and creation of a false passage (perforation) occur in the first few years after reconstruction (Thomas et al, 2006). Stricture and necrosis, particularly of cecal extensions of the appendix, have occurred rarely. Abdominal stomas may be associated with a higher risk of reservoir calculi because of the potential for incomplete emptying.
Follow-up in the recent series has averaged approximately 4 years. Patients from Mitrofanoff’s early experience (1980) should now have follow-up of more than 20 years, suggesting that the appendix may provide a durable alternative for pediatric patients with a long life expectancy, a finding also noted by others (Cain et al, 1999; Harris et al, 2000; Liard et al, 2001). Whereas the majority of the experience with the Mitrofanoff technique has been in children, similar outcomes with successful durable continence has been achieved in the adult population (Gowda et al, 2008; Van der Aa et al, 2009).
Alternatives
When the appendix is unavailable for use, other tubular structures can provide a similar mechanism for catheterization and continence. Mitrofanoff (1980) described a similar technique with ureter (Kaefer et al, 1997b). Care must be taken to preserve the distal blood supply to prevent ischemia. Refluxing ureters have even been used after extravesical reimplantation (Ashcraft and Dennis, 1986; Duel et al, 1996; Kaefer et al, 1997b). Stomal stenosis seems to be more problematic with use of the ureter compared with the appendix, possibly owing to compromised blood supply. In addition, distention of the ureter due to catheter passage has caused discomfort in some individuals (Duckett and Lofti, 1993).
Woodhouse and MacNeily (1994) as well as others have used the fallopian tube, which can accommodate catheterization. Stenosis again appears to be a significant problem with the fallopian tube. In addition, the effect on fertility should be considered when there is a normal ipsilateral gonad. Bihrle and associates (1991) fashioned a gastric tube mobilized on the gastroepiploic artery for implantation in continent reservoirs. Acid irritation of the skin was a problem in some patients. They then used a tapered segment of ileum as well (Adams et al, 1992). By narrowing the segment longitudinally along the mesenteric border with permanent staples in series, they were able to construct a uniform tube of adequate length that was easy to catheterize and provided good continence rates. Others (Hasan et al, 1994; Woodhouse and MacNeily, 1994) have had similar good results. It is ideal for such tubes to be long enough to reach from the reservoir to skin without tension; however, they should then be kept as short and straight as possible to facilitate easy catheterization. Any unnecessarily long catheterizable channel that is mobile within the abdomen can potentially kink and result in difficult catheterization or perforation.
Monti and colleagues (1997) have been credited with a novel modification of the tapered intestinal segment that can be reimplanted according to the Mitrofanoff principle. Recognition should also be directed to another report of this procedure by Yang (1993). A very short (1 to 2 cm) segment of small bowel is opened longitudinally along the antimesenteric border and then closed transversely (Fig. 129–20). By this reconfiguration, the initial circumference of the segment is converted to length, and the original length to circumference, of the reconfigured tube. A uniform tube is made with a small mesentery toward the center. The two ends are devoid of mesentery, making them easy to tunnel into bladder and bring through the abdominal wall. If the first incision is made directly at the antimesenteric border, both limbs are of equal length. By making the incision well around one side, one limb may be made much longer than the other. Experience has shown that one need not achieve a long tunnel in the bladder or reservoir to achieve continence with such a small tube, and the longer limb has typically been used to reach the skin through a thick abdominal wall.
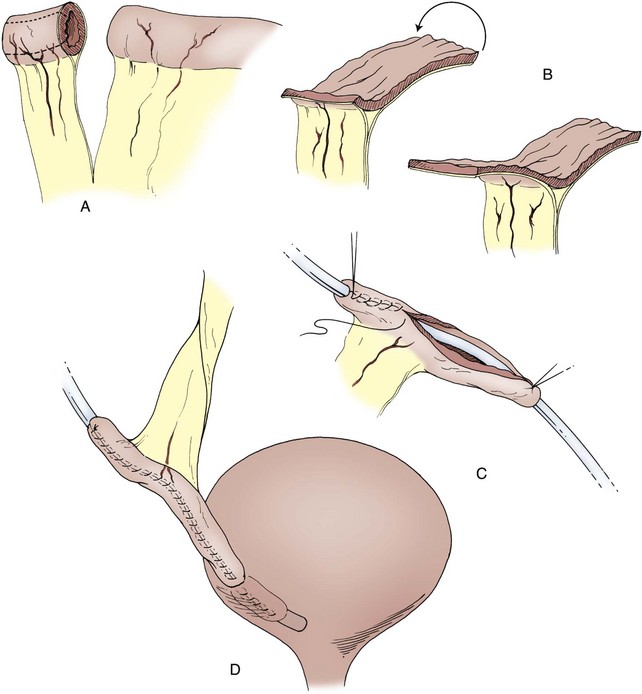
Figure 129–20 Yang-Monti technique to efficiently construct a catheterizable tube. A, A 2-cm length of ileum is harvested either independently or next to a segment for augmentation cystoplasty. The segment is opened horizontally and tubularized in a longitudinal fashion. B, If the segment is opened directly on the antimesenteric border, two equal-length limbs result with a central mesenteric pedicle. Initial incision may be made to one side to form a shorter limb for implantation in the bladder and a longer one to be brought through the abdominal wall. C, The reconfigured ileal segment is tubularized over a 12-Fr catheter in a two-layer technique with absorbable sutures. D, A continent catheterizable stoma is constructed.
Very good results have been achieved with the Yang-Monti tube as a catheterizable stoma, and it is certainly an efficient use of bowel (Thomas et al, 2006). Some surgeons have suggested that it may be easier to catheterize than an ileal segment tapered longitudinally because circular mucosal folds are redirected longitudinally in the direction of catheter passage. Stomas constructed with ileum may have a lower rate of stomal stenosis than those made with appendix (Kaefer et al, 1999a). The one potential disadvantage of the Yang-Monti tube is that it remains relatively short and may not reach the skin without tension in obese patients. Despite extensive use of skin flaps, such tension may lead to stomal stenosis. Two separate reconfigured tubes can be anastomosed together for better length (Kaefer and Retik, 1997). Casale (1999) has used an initial segment that was twice as long that was partially split in the middle and then opened in a spiral fashion on opposite sides to make a longer strip that could be tubularized in continuity. Narayanaswamy and colleagues (2001) noted difficulty with catheterizations through Yang-Monti channels in 28% of a large series of patients due to “pouchlike dilatation.” Such a problem has been avoidable in most series.
Ileocecal Valve
Use of the ileocecal valve as a continence mechanism began with Gilchrist (1950) and was popularized by the Indiana group (Rowland et al, 1985; Bihrle, 1997). Various modifications exist. In general, a short segment of terminal ileum, whether imbricated or tailored, is used as an efferent limb. This segment should be kept as short and straight as possible to facilitate easy intermittent catheterization. Continence is based on the imbricated ileocecal valve, not the length of the efferent limb. The imbrication is usually secured with interrupted, permanent sutures, involving the very distal ileum and ileocecal valve, and the imbrication is carried well onto the cecum. The reservoir itself is constructed from reconfigured right colon up through the hepatic flexure, with or without a patch of ileum. Standard ureterocolonic anastomoses are done beneath the taenia to prevent reflux. Staplers using absorbable staples have been used for reconfiguration of the reservoir (Kirsch et al, 1996) and may shorten the operative time for reconstruction.
The Indiana pouch has been used in children with excellent results, as it has in adults. Besides the appendix, this continence mechanism is perhaps the simplest and has the shortest learning curve to achieve reliable results. Continence rates as high as 95% have been reported with preservation of normal upper tracts (Rowland et al, 1985; Lockhart et al, 1990; Rink and Bihrle, 1990; Hensle and Ring, 1991; Rowland, 1995; Kaefer et al, 1997b). Rare reports have noted higher rates of incontinence (Canning, 1998). Husmann and Cain (1999) have used the cecal segment for bladder augmentation and the efferent limb to construct a continent catheterizable stoma with equally good results. They noted a low incidence of detrimental effect on gastrointestinal function in a select group of patients with neurogenic dysfunction.
Hydraulic Valves
Benchekroun (1982, 1989) developed an interesting hydraulic valve as a continence mechanism that was modified by Guzman and coworkers (1989). Urine from the reservoir and any pressure generated there is allowed to enter a sleeve of ileum around the catheterizable channel. Compression of the inner tube theoretically provides continence, and early results were encouraging. Initial continence rates approached 75% and then 90% with a single revision (Benchekroun et al, 1989). Others have not been able to duplicate those results (Sanda et al, 1988; Leonard et al, 1990b), and the valve has largely been abandoned.
Koff and associates (1989) added hydraulic compression to the terminal ileum of an Indiana pouch by anastomosing a segment of tubular ileum to the cecum and then wrapping it around the efferent limb. The procedure has not gained popularity, probably because of the excellent success of the standard Indiana pouch.
Continent Vesicostomy
Yachia (1997) described construction of a bladder tube fashioned from a wide flap of the anterior bladder wall. No attempt was made to produce continence at the level of the bladder. The continence mechanism was fashioned by weaving the bladder tube through the rectus muscle to produce compression and continence. Continence in the small, short-term series was reported at 100% but has not been duplicated.
Hanna and colleagues (1999) described a continence mechanism based on either a flap of bladder or intestinal tissue fashioned after prior enterocystoplasty. A rectangular flap in continuity with the bladder is tubularized over a 14- to 16-Fr catheter. The bladder is plicated around the proximal 3 cm of the tube with nonabsorbable suture to make a type of nipple similar to gastric fundoplication. No significant morbidity has been identified in a limited series of five children, and all children have remained dry on intermittent catheterization. Macedo and Srougi (2000) described a similar continence mechanism made at the time of initial augmentation. They achieved acceptable continence in eight of their first nine patients. Their technique is potentially appealing for patients requiring augmentation and having no appendix because of the simplicity; however, continence is based on a type of nipple valve that historically has been difficult to keep fixed. The same forces that create continence tend to efface the continence mechanism.
Casale (1991) has described a form of continent vesicostomy in which the continence mechanism is based on a flap valve made from a tubularized strip of bladder mucosa. It is particularly suitable when the bladder is compliant and of large capacity. An anterior detrusor strip is also used to construct a catheterizable limb.
Technique
Parallel incisions 3 cm apart are made into the anterior bladder and used to form a long rectangular flap. The abdominal wall should be measured to ensure that the strip is long enough to reach the skin without tension. The full-thickness strip is tubularized down to the bladder, typically in two layers. The muscle portion is left broad to come around without tension and provide good blood supply. The mucosa may be trimmed in width before tubularization to avoid redundancy. A strip of mucosa within the bladder, 2 to 3 cm in length and 1.5 cm in width, is incised in a direct line and in continuity with the mobilized bladder tube. The edges of this strip are mobilized until it can be tubularized along its entire length. It may be beneficial to mobilize only one edge over to the other side, which allows one to avoid overlapping suture lines. Casale (1991) originally incised the mucosa transversely at the end of the intravesical strip to be tubularized; Rink and associates (1995b) then suggested that it could be left intact (Fig. 129–21). The bladder mucosa from either side of the tube is then mobilized and closed over the mucosal tube to create a flap valve. More extensive mobilization of the side opposite that mobilized for the inner tube allows closure without overlapping suture lines, which may help avoid fistula formation and incontinence. A soft stent is usually left through the tube for 3 weeks during healing to prevent stenosis. It does tend to close if it is not catheterized regularly and may be even more susceptible than other catheterizable channels to stomal stenosis (Cain et al, 2002; Thomas et al, 2005).
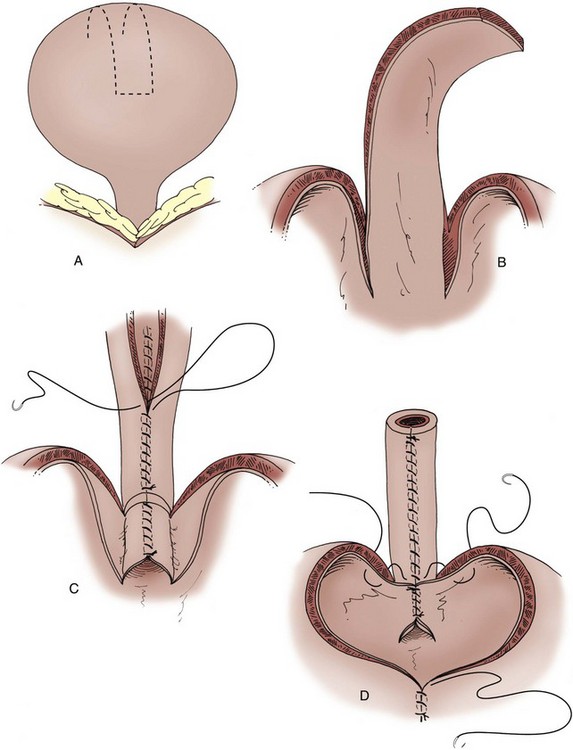
Figure 129–21 Casale continent catheterizable stoma. A, Parallel incisions are made in the bladder dome, forming a full-thickness bladder strip. B, The epithelium of the bladder is then incised for an additional 2.5 cm. The edges of the epithelium are mobilized, allowing tubularization. C, The epithelium is tubularized from the bladder out to the tip of the strip with an absorbable suture. The muscle of the bladder strip is then tubularized with an absorbable suture. D, The lateral edges of the epithelium within the bladder are reapproximated over the tubularized bladder segment with absorbable suture. The bladder is then closed with absorbable suture, incorporating the bladder tube with the initial sutures to prevent kinking.
Results
Continence rates have been good, as with most flap valve mechanisms (Cain et al, 1999, 2002). Stomal stenosis remains a significant problem—45% in the experience at Indiana University (Cain et al, 2002). Skin flaps and avoidance of tension to reach the skin may minimize this risk but not remove it. Advantages include avoidance of an intraperitoneal procedure and bowel anastomosis; the appendix can be reserved for use with enemas. It does use some bladder and decreases capacity, which may not be appropriate for some patients.
Results with Pediatric Continent Diversion
Continent urinary reservoirs have been performed in children with very good results, equivalent to those achieved for adults. Construction of an adequate reservoir and an antireflux mechanism has generally been straightforward. For review of issues concerning compliance of the reservoir or mechanisms by which reflux can be prevented, the reader may review earlier parts of this chapter or the information on continent urinary diversion in adults elsewhere in this textbook. The most challenging aspect of continent diversion remains construction of an efferent limb that provides reliable continence and easy catheterization. The continence mechanism most familiar to pediatric urologists is the flap valve. The appendix is simple to use, suitable for most children, and associated with very good continence rates. If the appendix is not present or is to be used for antegrade colonic enemas, tapered intestinal segments provide a nice alternative. Nipple valves are the most complex continence mechanism and therefore their creation requires a longer learning curve. Continence rates of approximately 85% can be expected with stapled nipple valves (Kaefer et al, 1997b; Benson and Olsson, 1998), even with extensive experience. With use of the other efferent limbs, continence rates above 90% and approaching 95% have routinely been reported in children (Duckett and Snyder, 1986; Hensle and Ring, 1991; Kaefer et al, 1997b; Surer et al, 2003). Those continence rates have been achieved with preservation of the upper tracts. With proper selection of patients and appropriately performed continent diversion, hydronephrosis in children is rare postoperatively. Particularly if catheterization is not performed reliably, new hydronephrosis can occur (Abd-El-Gawad et al, 1999). There is no evidence that children suffer more hydronephrosis after continent diversion compared with conduit diversion (Stein et al, 2000).
Children undergoing continent diversion have a long life expectancy. The incidence of complications after continent diversion will probably increase with longer follow-up. Those patients will be subject to the same complications discussed at length for bladder augmentation. All of those complications, including infection, hydronephrosis, calculi, spontaneous perforation, and tumor, have been reported after continent diversion in adults if not in children. These are largely a function of the use of intestine as a urinary reservoir. Because more intestine is usually required in continent diversion than in bladder augmentation, the incidence of complications may ultimately be higher than with simple augmentation as follow-up increases. Certainly, serum changes of increased chloride, decreased bicarbonate, and acidosis have been noted in some patients after continent diversion (Allen et al, 1985; Ashken, 1987; Thuroff et al, 1987; Boyd et al, 1989; McDougal, 1992a). Spontaneous perforation has occurred in up to 1.5% of patients (Mansson et al, 1997).
The most common complication in pediatric continent diversion thus far has been stomal stenosis. Such stenosis occurs more commonly at the umbilicus and when appendix is used compared with tapered ileal segments (Fichtner et al, 1997; Kaefer et al, 1999a). Various skin flaps may be placed into the terminal end of the appendix or intestinal segment to lower the rate of stenosis but do not eliminate it (Kajbafzadeh et al, 1995). Bladder calculi have also been common problems (Surer et al, 2003).
Key Points: Continent Urinary Diversion