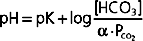CHAPTER 24 Control of Respiration
We breathe without thinking, and we can willingly modify our breathing pattern and even hold our breath. Control of ventilation, which is discussed in this chapter, includes the generation and regulation of rhythmic breathing by the respiratory center in the brainstem and its modification by the input of information from higher brain centers and from systemic receptors. The goal of breathing, from a mechanical perspective, is to minimize work and, from a physiological perspective, to maintain blood gases and, specifically, to regulate arterial PCO2. Another goal of breathing is to maintain acid-base balance in the brain by regulating arterial PCO2. Automatic respiration begins at birth. In utero, the placenta, not the lung, is the organ of gas exchange in the fetus. Its microvilli interdigitate with the maternal uterine circulation, and O2 transport and CO2 removal from the fetus occur by passive diffusion across the maternal circulation.
VENTILATORY CONTROL: AN OVERVIEW
There are four major sites of ventilatory control: (1) the respiratory control center, (2) central chemoreceptors, (3) peripheral chemoreceptors, and (4) pulmonary mechanoreceptors/sensory nerves. The respiratory control center is located in the medulla oblongata of the brainstem and is composed of multiple nuclei that generate and modify the basic ventilatory rhythm. The center consists of two main parts: (1) a ventilatory pattern generator, which sets the rhythmic pattern, and (2) an integrator, which controls generation of the pattern, processes input from higher brain centers and chemoreceptors, and controls the rate and amplitude of the ventilatory pattern. Input to the integrator arises from higher brain centers, including the cerebral cortex, hypothalamus, amygdala, limbic system, and cerebellum.
Central chemoreceptors are located in the central nervous system just below the ventrolateral surface of the medulla. These central chemoreceptors detect changes in the PCO2 and pH of interstitial fluid in the brainstem, and they modulate ventilation. Peripheral chemoreceptors are located on specialized cells in the aortic arch (aortic bodies) and at the bifurcation of the internal and external carotid arteries (carotid bodies) in the neck. These peripheral chemoreceptors sense the PO2, PCO2, and pH of arterial blood, and they feed information back to the integrator nuclei in the medulla through the vagus nerves and carotid sinus nerves, which are branches of the glossopharyngeal nerves. Pulmonary mechanoreceptors and sensory nerve stimulation, in response to lung inflation or to stimulation by irritants or release of local mediators in the airways, modify the ventilatory pattern.
The collective output of the respiratory control center to motor neurons located in the anterior horn of the spinal column controls the muscles of respiration, and this output determines the automatic rhythmic pattern of respiration. Motor neurons located in the cervical region of the spinal column control the activity of the diaphragm through the phrenic nerves, whereas other motor neurons located in the thoracic region of the spine control the intercostal muscles and the accessory muscles of respiration.
In contrast to automatic respiration, voluntary respiration bypasses the respiratory control center in the medulla. The neural activity controlling voluntary respiration originates in the motor cortex, and signaling passes directly to motor neurons in the spine through the corticospinal tracts. The motor neurons to the respiratory muscles act as the final site of integration of the voluntary (corticospinal tract) and automatic (ventrolateral tracts) control of ventilation. Voluntary control of these muscles competes with automatic influences at the level of the spinal motor neurons, and this competition can be demonstrated by breath holding. At the start of the breath hold, voluntary control dominates the spinal motor neurons. However, as the breath hold continues, the automatic ventilatory control eventually overpowers the voluntary effort and limits the duration of the breath hold. Motor neurons also innervate muscles of the upper airway. These neurons are located within the medulla near the respiratory control center. They innervate muscles in the upper airways through the cranial nerves. When activated, they dilate the pharynx and large airways at the initiation of inspiration.
RESPONSE TO CO2
Ventilation is regulated by PCO2, PO2, and pH in arterial blood. Arterial PCO2 is the most important of these regulators. Both the rate and depth of breathing are controlled to maintain PaCO2 close to 40 mm Hg. In a normal awake individual, there is a linear rise in ventilation as arterial PCO2 reaches and exceeds 40 mm Hg (Fig. 24-1). Changes in PaCO2 are sensed by central and peripheral chemoreceptors, and they transmit this information to the medullary respiratory centers. The respiratory control center then regulates minute ventilation and thereby maintains arterial PCO2 within the normal range. In the presence of a normal PAO2, ventilation increases by about 3 L/min for each millimeter rise in PaCO2. The response to an increase in PACO2 is further increased in the presence of a low PaO2 (Fig. 24-2). With a low Pao2, ventilation is greater for any given PACO2, and the increase in ventilation for a given increment in PACO2 is enhanced (the slope is greater).
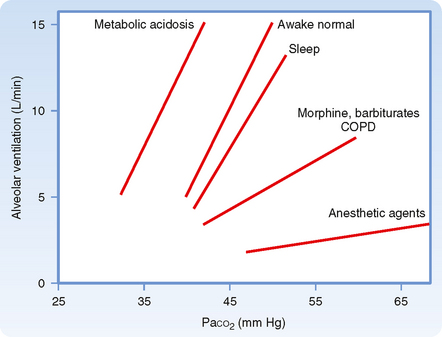
Figure 24-1 Relationship between PaCO2 and alveolar ventilation in awake normal states, during sleep, after narcotic ingestion and deep anesthesia, and in the presence of metabolic acidosis. Both the slopes of the response (sensitivity) and the position of the response curves (threshold, the point at which the curve crosses the x axis) are changed, thus indicating differences in ventilatory responses and response thresholds.
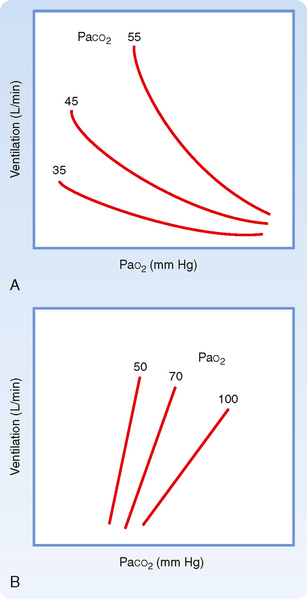
Figure 24-2 The effects of hypoxia (A) and hypercapnia (B) on ventilation as the other respiratory gas partial pressure is varied. A, At a given PaCO2, ventilation increases more and more as PaO2 decreases. When PaCO2 is allowed to decrease (the normal condition) during hypoxia, there is little stimulation of breathing until PO2 falls below 60 mm Hg. The hypoxic response is mediated through the carotid body chemoreceptors. B, The sensitivity of the ventilatory response to CO2 is enhanced by hypoxia.
The slope of the minute ventilation response as a function of the inspired CO2 is termed the ventilatory response to CO2 and is a test of CO2 sensitivity. It is important to recognize that this relationship is amplified by low O2 (Fig. 24-2, B). The enhanced responsiveness to low O2 occurs because different mechanisms are responsible for sensing PO2 and PCO2 in the peripheral chemoreceptors. Thus, the presence of both hypercapnia and hypoxemia (often called asphyxia when both changes are present) has an additive effect on chemoreceptor output and on the resulting ventilatory stimulation.
The ventilatory drive or response to changes in PCO2 can be reduced by hyperventilation and by drugs, such as morphine, barbiturates, and anesthetic agents, that depress the respiratory center and decrease the ventilatory response to both CO2 and O2 (Fig. 24-1). In these instances, the stimulus is inadequate to stimulate the motor neurons that innervate the muscles of respiration. It is also depressed during sleep.
In addition, the ventilatory response to changes in PCO2 is reduced if the work of breathing is increased, which can occur in individuals with chronic obstructive pulmonary disease (COPD) (Fig. 24-1). This effect occurs primarily because the neural output of the respiratory center is less effective in promoting ventilation as a result of the mechanical limitation to ventilation.
CONTROL OF VENTILATION: THE DETAILS
The Respiratory Control Center
When the brain is transected experimentally between the medulla and the pons, periodic breathing is maintained, thus demonstrating that the inherent rhythmicity of breathing originates in the medulla. Although no single group of neurons in the medulla has been found to be the breathing “pacemaker,” two distinct nuclei within the medulla are involved in generation of the respiratory pattern (Fig. 24-3). One nucleus is the dorsal respiratory group (DRG), which is composed of cells in the nucleus tractus solitarius located in the dorsomedial region of the medulla. Cells in the DRG receive afferent input from the 9th and 10th cranial nerves, which originate from airways and the lung and are thought to constitute the initial intracranial processing station for this afferent input. The second group of medullary cells is the ventral respiratory group (VRG), located in the ventrolateral region of the medulla. The VRG is composed of three cell groups: the rostral nucleus retrofacialis, the caudal nucleus retroambiguus, and the nucleus paraambiguus. The VRG contains both inspiratory and expiratory neurons. The nucleus retrofacialis and the caudally located cells of the nucleus retroambiguus are active during exhalation, whereas the rostrally located cells of the nucleus retroambiguus are active during inspiration. The nucleus paraambiguus has inspiratory and expiratory neurons that travel in the vagus nerve to the laryngeal and pharyngeal muscles. Discharges from cells in these areas excite some cells and inhibit other cells.
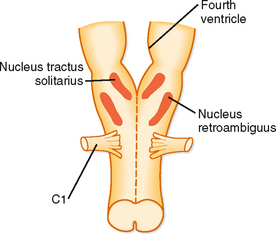
Figure 24-3 The respiratory control center is located in the medulla (the most primitive portion of the brain). The neurons are mainly in two areas called the nucleus tractus solitarius and the nucleus retroambiguus.
At the level of the respiratory control center, inspiration and exhalation involve three phases—one inspiratory and two expiratory (Fig. 24-4). Inspiration begins with an abrupt increase in discharge from cells in the nucleus tractus solitarius, the nucleus retroambiguus, and the nucleus paraambiguus, followed by a steady ramplike increase in firing rate throughout inspiration. This leads to progressive contraction of the respiratory muscles during automatic breathing. At the end of inspiration, an off-switch event results in a marked decrease in neuron firing, at which point exhalation begins. At the start of exhalation (phase I of expiration), a paradoxical increase in inspiratory neuron firing slows the expiratory phase down by increasing inspiratory muscle tone and expiratory neuron firing. This inspiratory neuron firing decreases and stops during phase II of exhalation. Although many different neurons in the DRG and VRG are involved in ventilation, each cell type appears to have a specific function. For example, the Hering-Breuer reflex is an inspiratory inhibitory reflex that arises from afferent stretch receptors located in the smooth muscles of the airways. Increasing lung inflation stimulates these stretch receptors and results in early exhalation by stimulating the neurons associated with the off-switch phase of inspiratory muscle control. Thus, rhythmic breathing depends on a continuous (tonic) inspiratory drive from the DRG and an intermittent (phasic) expiratory drive from the cerebrum, thalamus, cranial nerves, and ascending sensory tracts in the spinal cord.
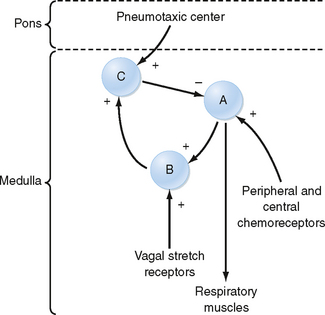
Figure 24-4 The basic wiring diagram of the brainstem ventilatory controller. The signs of the main output (arrows) of the neuron pools indicate whether the output is excitatory (+) or inhibitory (−). Pool A provides tonic inspiratory stimuli to the muscles of breathing. Pool B is stimulated by pool A and provides additional stimulation to the muscles of breathing, and pool B stimulates pool C. Other brain centers feed into pool C (inspiratory cutoff switch), which sends inhibitory impulses to pool A. Afferent information (feedback) from various sensors acts at different locations: chemoreceptors act on pool A and intrapulmonary sensory fibers act via the vagus nerves on pool B. A pneumotaxic center in the anterior pons receives input from the cerebral cortex, and it modulates the pool C group.
Central Chemoreceptors
A chemoreceptor is a receptor that responds to a change in the chemical composition of blood or other fluid around it. Central chemoreceptors are specialized cells on the ventrolateral surface of the medulla. Chemoreceptors are sensitive to the pH of the surrounding extracellular fluid. Because this extracellular fluid is in contact with cerebrospinal fluid (CSF), changes in the pH of CSF affect ventilation by acting on these chemoreceptors
CSF is an ultrafiltrate of plasma that is secreted continuously by the choroid plexus and is reabsorbed by the arachnoid villi. Because it is in contact with the extracellular fluid in the brain, the composition of CSF is influenced by the metabolic activity of the cells in the surrounding area and the composition of the blood. Although the origin of CSF is plasma, the composition of CSF is not the same as that of plasma because the blood-brain barrier exists between the two sites (Fig. 24-5). The blood-brain barrier is composed of endothelial cells, smooth muscle, and the pial and arachnoid membranes, and it regulates the movement of ions between blood and CSF. In addition, the choroid plexus also determines the ionic composition of CSF by transporting ions into and out of CSF. The blood-brain barrier is relatively impermeable to H+ and HCO3− ions, but it is very permeable to CO2. Thus, the PCO2 in CSF parallels the arterial PCO2 tension. CO2 is also produced by cells of the brain as a product of metabolism. As a consequence, the PCO2 in CSF is usually a few mm Hg higher than that in arterial blood, so the pH is slightly more acidic (7.33) than in plasma (Table 24-1).
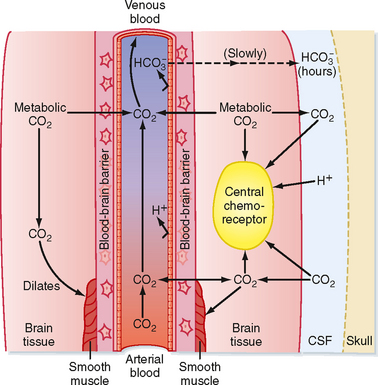
Figure 24-5 CO2 and the blood-brain barrier. Arterial CO2 crosses the blood-brain barrier and rapidly equilibrates with CSF CO2. H+ and HCO3− ions cross the barrier slowly. Arterial CO2 combines with metabolic CO2 to dilate the smooth muscle. When compared with arterial blood, the pH of CSF is lower and the PCO2 is higher, with little protein buffering.
Table 24-1 Normal Values for the Composition of Cerebrospinal Fluid and Arterial Blood
| CSF | Arterial | |
|---|---|---|
| pH | 7.33 | 7.40 |
| PCO2 (mm Hg) | 44 | 40 |
| HCO3− (mEq/L) | 22 | 24 |
The Henderson-Hasselbalch equation relates the pH of CSF to [HCO3−]:
where α is the solubility coefficient (0.03 mmol/L/mm Hg) and pK is the negative logarithm of the dissociation constant for carbonic acid (6.1). The Henderson-Hasselbalch equation demonstrates that an increase in CSF PCO2 will decrease the pH of CSF at any given [HCO3−]. The decrease in pH will stimulate the central chemoreceptors and thereby increase ventilation. Thus, CO2 in blood regulates ventilation by its effect on the pH of CSF. The resulting hyperventilation reduces the PCO2 in the blood, and therefore in CSF, and returns the pH of CSF toward a normal value. Furthermore, cerebral vasodilation accompanies an increase in arterial PCO2, and this enhances the diffusion of CO2 into CSF. By contrast, an increase in CSF [HCO3−] will increase the pH of CSF at any given PCO2.
Changes in arterial PCO2, by altering pH, activate homeostatic mechanisms that return the pH back toward normal. The blood-brain barrier regulates the pH of CSF by adjusting the ionic composition and [HCO3−] of CSF. These changes in CSF [HCO3−], however, occur slowly, over a period of several hours, whereas changes in CSF PCO2 can occur within minutes. Thus, compensation for changes in the pH of CSF requires hours to fully develop.
Peripheral Chemoreceptors
The carotid and aortic bodies are peripheral chemoreceptors that respond to changes in arterial PO2 (not the O2 content), PCO2, and pH, and they transmit afferent information to the central respiratory control center. The peripheral chemoreceptors are the only chemoreceptors that respond to changes in PO2. The peripheral chemoreceptors are also responsible for approximately 40% of the ventilatory response to CO2. These chemoreceptors are small, highly vascularized structures. They consist of type I (glomus) cells that are rich in mitochondria and endoplasmic reticulum. They also have several types of cytoplasmic granules (synaptic vesicles) that contain various neurotransmitters, including dopamine, acetylcholine, norepinephrine, and neuropeptides. Afferent nerve fibers synapse with type I cells, and they transmit information to the brainstem through the carotid sinus nerve (carotid body) and vagus nerve (aortic body). Type I cells are the cells primarily responsible for sensing PO2, PCO2, and pH. In response to even small decreases in arterial PO2 there is an increase in chemoreceptor discharge, which enhances respiration. The response is robust when arterial PO2 decreases below 75 mm Hg. Thus, ventilation is regulated by changes in arterial and CSF pH via effects on peripheral and central chemoreceptors (Fig. 24-6).
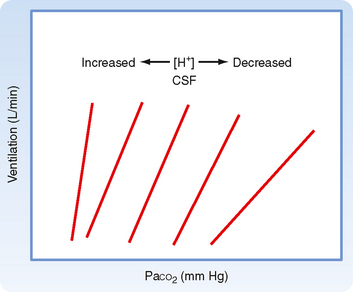
Figure 24-6 The ventilatory response to PCO2 is affected by the [H+] of CSF and brainstem interstitial fluid. During chronic metabolic acidosis (e.g., diabetic ketoacidosis), the [H+] of CSF is increased and the ventilatory response to inspired PCO2 is increased (steeper slope). Conversely, during chronic metabolic alkalosis (a relatively uncommon condition), the [H+] of CSF is decreased and the ventilatory response to inspired PCO2 is decreased (reduced slope). The positions of the response lines are also shifted, thus indicating altered thresholds.
Imagine flying from New York City to Denver. The barometric pressure in New York is about 760 mm Hg, whereas in the mountains surrounding Denver, Colorado, it is 600 mm Hg. At sea level, the PO2 in arterial blood is approximately 95 mm Hg (using the alveolar air equation [see Chapter 22], PAO2 = [(760 − 47) × 0.21] − [40/0.8] = 100 mm Hg. If the alveolar-arterial PO2 difference [AaDO2] is 5 mm Hg, PaO2 = 100 − 5 = 95 mm Hg). In the CSF, pH would be about 7.33, PCO2 would be 44 mm Hg (arterial PCO2 + CO2 produced by metabolism of brain cells), and HCO3− would be approximately 22 mEq/L.
There is an abrupt decrease in PIO2 when you arrive in the mountains (PIO2 = [600 − 47] × 0.21 = 116 mm Hg), and alveolar and arterial O2 decrease (PAO2 = 116 − [40/0.8] = 66 mm Hg; PaO2 = 61 mm Hg, assuming no change in AaDO2). This decrease in arterial O2 stimulates the peripheral chemoreceptors and thereby increases ventilation. The increase in ventilation decreases arterial PCO2 and elevates arterial pH. The result of this increase in ventilation is to minimize the hypoxemia by increasing PAO2. (For example, assume that PACO2 decreases to 30 mm Hg. Then, PAO2 = [(600 − 47) × 0.21] − [30/0.8] = 78 mm Hg, a 12–mm Hg increase in PAO2.)
The decrease in arterial PCO2 also decreases the PCO2 of CSF. Because [HCO3−] is unchanged, the pH of CSF increases. This increase in the pH of CSF attenuates the rate of discharge of the central chemoreceptors and decreases their contribution to the ventilatory drive. Over the next 12 to 36 hours, [HCO3−] in CSF decreases as acid-base transporter proteins in the blood-brain barrier reduce [HCO3−]. Consequently, the pH of CSF returns toward normal. Central chemoreceptor discharge increases and minute ventilation is further increased. At the same time that [HCO3−] in CSF decreases, HCO3− is gradually excreted from plasma by the kidneys. This results in a gradual return of arterial pH toward normal. Peripheral chemoreceptor stimulation increases further as arterial pH becomes normal (peripheral chemoreceptors are inhibited by the elevated arterial pH). Finally, within 36 hours of arriving at high altitude, minute ventilation increases significantly. This delayed response is greater than the immediate effect of the hypoxemia on ventilation. This further increase in ventilation is due to both central and peripheral chemoreceptor stimulation. Thus, by the end of the weekend, both arterial pH and CSF pH are approaching normal; minute ventilation is increased, arterial PO2 is decreased, and arterial PCO2 is decreased.
You now return home. When you land in New York, the inspired PO2 returns to normal, and the hypoxic stimulus to ventilation is removed. Arterial PO2 returns to normal and the peripheral chemoreceptor stimulation to ventilation decreases. This increases arterial [CO2] toward normal, which in turn increases CSF [CO2]. This increase is associated with a decrease in the pH of CSF as [HCO3−] in CSF is now reduced and ventilation is augmented. Over the next 12 to 36 hours, acid-base transporters in the blood-brain barrier transport HCO3− back into CSF, and the pH of CSF gradually returns toward normal. Similarly, the pH of blood decreases as arterial PCO2 rises because arterial [HCO3−] decreases. This stimulates the peripheral chemoreceptors, and minute ventilation remains augmented. Over the next 12 to 36 hours, the kidney increases blood [HCO3−] (see Chapter 36), arterial pH returns to normal, and minute ventilation returns to normal.
Pulmonary Mechanoreceptors
Chest Wall and Lung Reflexes
Several reflexes that arise from the chest wall and lungs affect ventilation and ventilatory patterns (Table 24-2). The Hering-Breuer inspiratory-inhibitory reflex is stimulated by increases in lung volume, especially those associated with an increase in both ventilatory rate and tidal volume. This stretch reflex is mediated by vagal fibers, and when elicited, it results in cessation of inspiration by stimulating the off-switch neurons in the medulla. This reflex is inactive during quiet breathing and appears to be most important in newborns. Stimulation of nasal or facial receptors with cold water initiates the diving reflex. When this reflex is elicited, apnea, or cessation of breathing, and bradycardia occur. This reflex protects individuals from aspirating water in the initial stages of drowning. Activation of receptors in the nose is responsible for the sneeze reflex.
The aspiration or sniff reflex can be elicited by stimulation of mechanical receptors in the nasopharynx and pharynx. This is a strong, short-duration inspiratory effort that brings material from the nasopharynx to the pharynx, where it can be swallowed or expectorated. The mechanical receptors responsible for the sniff reflex are also important in swallowing by inhibiting respiration and causing laryngeal closure. Only newborn infants can breathe and swallow simultaneously, which allows more rapid ingestion of nutrients.
The larynx contains both superficial and deep receptors. Activation of the superficial receptors results in apnea, cough, and expiratory movements that protect the lower respiratory tract from aspirating foreign material. The deep receptors are located in the skeletal muscles of the larynx, and they control muscle fiber activation, as in other skeletal muscles.
Sensory Receptors and Reflexes
There are three major types of sensory receptors located in the tracheobronchial tree that respond to a variety of different stimuli and result in changes in the lung’s mechanical properties, alterations in the respiratory pattern, and the development of respiratory symptoms. Inhaled dust, noxious gases, or cigarette smoke stimulates irritant receptors in the trachea and large airways that transmit information through myelinated vagal afferent fibers. Stimulation of these receptors results in an increase in airway resistance, reflex apnea, and coughing. These receptors are also known as rapidly adapting pulmonary stretch receptors. Slowly adapting pulmonary stretch receptors respond to mechanical stimulation, and they are activated by lung inflation. They also transmit information through myelinated, vagal afferent fibers. The increased lung volume in people with obstructive pulmonary disease stimulates these pulmonary stretch receptors and delays the onset of the next inspiratory effort. This explains the long, slow expiratory effort in these individuals, and it is essential to minimize dynamic, expiratory airway compression. Finally, specialized sensory receptors occur in the lung parenchyma and respond to chemical or mechanical stimulation in the lung interstitium. These receptors are called juxtaalveolar or J receptors. They transmit their afferent input through unmyelinated, vagal C fibers. They may be responsible for the sensation of dyspnea (abnormal shortness of breath) and the rapid, shallow ventilatory patterns that occur in interstitial lung edema and some inflammatory lung states.
Somatic receptors are also located in the intercostal muscles, rib joints, accessory muscles of respiration, and tendons, and they respond to changes in the length and tension of the respiratory muscles. Although they do not directly control respiration, they do provide information about lung volume and play a role in terminating inspiration. They are especially important in individuals with increased airway resistance and decreased pulmonary compliance because they can augment muscle force within the same breath. Somatic receptors also help minimize the chest wall distortion during inspiration in newborns, who have very compliant rib cages.
EXERCISE
The ability to exercise depends on the capacity of the cardiac and respiratory systems to increase delivery of O2 to tissues and remove CO2 from the body. Ventilation increases immediately when exercise begins, and this increase in minute ventilation closely matches the increase in O2 consumption and CO2 production that accompanies exercise (Fig. 24-7). Ventilation is linearly related to both CO2 production and O2 consumption at low to moderate levels (Fig. 24-7). During maximal exercise, a fit individual can achieve an O2 consumption of 4 L/min with a minute volume of 120 L/min, which is almost 15 times the resting level.
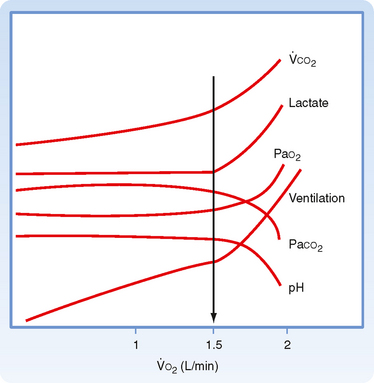
Figure 24-7 Oxygen consumption ( O2) as a function of the metabolic changes that occur during exercise. The anaerobic threshold (arrow) is the point at which the illustrated variables change and is due to lactic acidosis.
O2) as a function of the metabolic changes that occur during exercise. The anaerobic threshold (arrow) is the point at which the illustrated variables change and is due to lactic acidosis.
Exercise is remarkable because of the lack of significant changes in blood gases. Except at maximal exertion, changes in arterial PCO2 and PO2 are minimal during exercise. Arterial pH remains within normal values during moderate exercise. During heavy exercise, arterial pH begins to fall as lactic acid is liberated from muscles during anaerobic metabolism. This decrease in arterial pH stimulates ventilation that is out of proportion to the level of exercise. The level of exercise at which sustained metabolic (lactic) acidosis begins is called the anaerobic threshold (Fig. 24-7).
ABNORMALITIES IN THE CONTROL OF BREATHING
Changes in the ventilatory pattern can occur for both primary and secondary reasons. During sleep, approximately a third of normal individuals have brief episodes of apnea or hypoventilation that have no significant effects on arterial PO2 or PCO2. The apnea usually lasts less than 10 seconds, and it occurs in the lighter stages of slow-wave and rapid eye movement (REM) sleep. In sleep apnea syndromes, the duration of apnea is abnormally prolonged, and it changes arterial PO2 and PCO2. There are two major categories of sleep apnea (Fig. 24-8). The first is obstructive sleep apnea (OSA). OSA is the most common of the sleep apnea syndromes, and it occurs when the upper airway (generally the hypopharynx) closes during inspiration. Although the process is similar to what happens during snoring, it is more severe, obstructs the airway, and causes cessation of airflow.
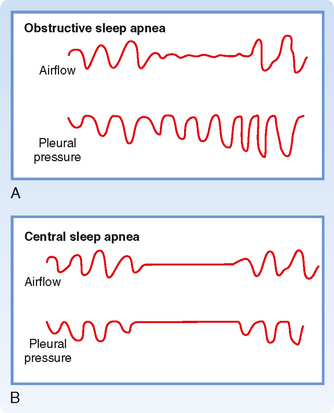
Figure 24-8 The two main types of sleep apnea. A, In obstructive sleep apnea, the pleural pressure oscillations increase as CO2 rises. This indicates that resistance to airflow is very high as a result of upper airway obstruction. B, Central sleep apnea is characterized by no attempt to breathe, as demonstrated by no oscillations in pleural pressure.
The clinical history of individuals with Obstructive Sleep Apnea (OSA) is very similar among patients. A spouse usually reports that the individual snores. The snoring becomes louder and louder and then stops while the individual continues to make vigorous respiratory efforts (Fig. 24-8). The individual then awakens, falls back to sleep, and continues the same process repetitively throughout the night. Individuals with OSA awaken when the arterial hypoxemia and hypercapnia stimulate both peripheral and central chemoreceptors. Respiration is restored briefly before the next apneic event occurs. Individuals with OSA can have hundreds of these events each night that interrupt sleep.
Complications of OSA include sleep deprivation, polycythemia, right-sided cardiac failure (cor pulmonale), and pulmonary hypertension secondary to the recurrent, hypoxic events. OSA is common in individuals with obesity and in those with excessive compliance of the hypopharynx, upper airway edema, and structural abnormalities of the upper airway.
The second sleep apnea syndrome is called central sleep apnea. This variant of apnea occurs when the ventilatory drive to the respiratory motor neurons decreases. Individuals with central sleep apnea have repeated episodes of apnea every night, during which they make no respiratory effort (Fig. 24-8). The degree of hypercapnia and hypoxemia in individuals with central sleep apnea is less than that in individuals with OSA, but the same complications (polycythemia, etc.) can occur when central sleep apnea is recurrent and severe.
Cheyne-Stokes ventilation is another abnormality of ventilatory control that is characterized by varying tidal volume and ventilatory frequency (Fig. 24-9). After a period of apnea, tidal volume and respiratory frequency increase progressively over several breaths, and then they progressively decrease until apnea occurs. This irregular breathing pattern is seen in some individuals with central nervous system diseases, head trauma, and increased intracranial pressure. It is also present on occasion in normal individuals during sleep at high altitude. The mechanism of Cheyne-Stokes respiration is not known. In some individuals it appears to be due to slow blood flow in the brain associated with periods of overshooting and undershooting ventilatory effort in response to changes in PCO2.
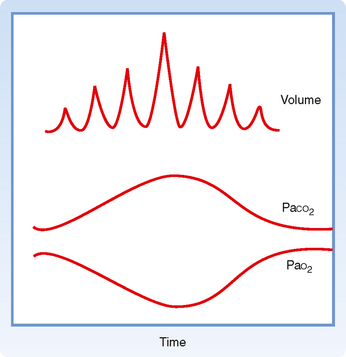
Figure 24-9 In Cheyne-Stokes breathing, tidal volume and consequently arterial blood gases wax and wane. Generally, Cheyne-Stokes breathing is a sign of vasomotor instability, particularly low cardiac output.
Central alveolar hypoventilation (CAH), also known as Ondine’s curse, is a rare disease in which voluntary breathing is intact but abnormalities in automaticity exist. It is the most severe of the central sleep apnea syndromes. As a result, people with CAH can breathe as long as they do not fall asleep. For these individuals, mechanical ventilation or, more recently, bilateral diaphragmatic pacing (similar to a cardiac pacemaker) can be lifesaving.
Sudden infant death syndrome (SIDS) is the most common cause of death in infants in the first year of life outside the perinatal period. Although the cause of SIDS is not known, abnormalities in ventilatory control, particularly in CO2 responsiveness, have been implicated. Placing infants on their back to sleep (reducing the potential for CO2 rebreathing) has dramatically decreased (but not eliminated) the death rate from this syndrome.
Apneustic breathing is another abnormal breathing pattern that is characterized by sustained periods of inspiration separated by brief periods of exhalation (Fig. 24-10, C). The mechanism of this ventilatory pattern appears to be a loss of inspiratory-inhibitory activities resulting in the augmented inspiratory drive. The pattern sometimes occurs in individuals with central nervous system injury.
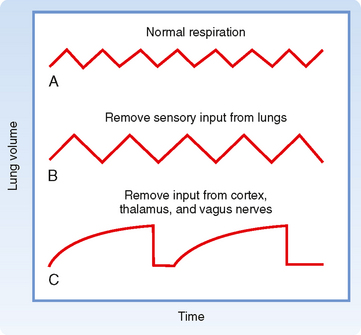
Figure 24-10 Some patterns of breathing. A, Normal breathing at about 15 breaths/min. B, The effect of removing sensory input from various lung receptors (mainly stretch) is to lengthen each breathing cycle and increase tidal volume so that alveolar ventilation is not significantly affected. C, When input from the cerebral cortex and thalamus is also eliminated together with vagal blockade, the result is prolonged inspiratory activity broken after several seconds by brief expirations (apneusis).