CHAPTER 23 Periodontal Microbiology
The human fetus inside the uterus is sterile but as soon as it passes through the birth canal, it acquires vaginal and fecal microorganisms.105 Within 2 weeks, a nearly mature microbiota is established in the gut of the newborn baby. After weaning (>2 years), the entire human microbiota is formed and comprises a very complex collection of hundreds of different types of bacteria, totalling approximately 1014 microbial cells.248 From this moment on, our body contains 10 times more bacteria than human cells. It has been estimated that for a normal, healthy human being, the bacterial population comprises 2 kg of the total body weight. This is fascinating if one realizes that the average human brain weighs only about 1.4 kg.
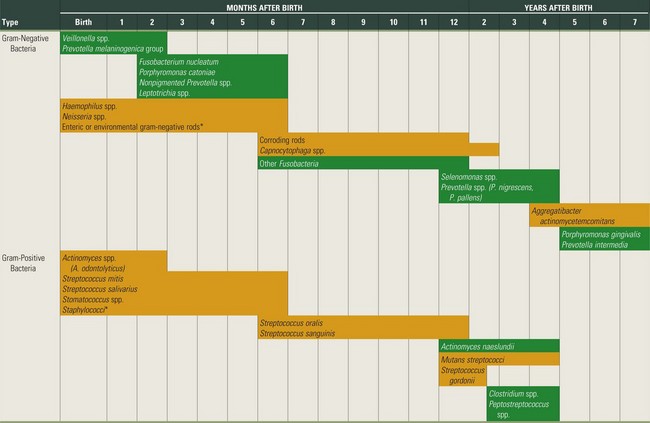
The colonization of the oral cavity also starts close to the time of birth (Table 23-1). Within hours after birth, the sterile oral cavity will be colonized by low numbers of mainly facultative and aerobic bacteria.368 From the second day, anaerobic bacteria can be detected in the infant’s edentulous mouth.89,319 The number of oral bacteria increases gradually as a result of exposure to external environmental microbial sources.179,271,319 Streptococcus salivarius and Streptococcus mitis (Figure 23-1, A) have been identified as the first and most dominant oral microbes to colonize the oral cavity of newborn infants.177,178,197 Veillonella spp. (Figure 23-1, B), Neisseria spp., Actinomyces spp. (Figure 23-1, C and D) and Staphylococcus spp. are also among the first colonizers of the oral cavity. After tooth eruption, a more complex oral microbiota is established. The species colonizing the teeth after eruption include S. sanguinis (Figure 23-1, D), Lactobacillus spp. (Figure 23-1, E), and S. oralis. Oral streptococci, including S. oralis, S. anginosus, Mutans streptococci, and S. gordonii (Figure 23-1, F), are commonly reported to be present after the first year of life.49,53,271,319 In addition, anaerobes, including Fusobacterium spp. (Figure 23-1, G) and Prevotella spp. (Figure 23-1, H and I), can also be detected in young children.49,179 In later childhood, the bacterial diversity and numbers in the oral cavity increase as more teeth erupt and provide more areas for the adherence and retention of bacteria.32 Because of the paucity of longitudinal studies, relatively little is known about the first time of colonization by key microbes found in the oral cavity of children and adults.197 It is estimated that more than 500 different species are capable of colonizing the adult mouth and that any individual typically harbors 150 or more different species.250,363 When one thinks about bacteria, one almost immediately associates them with different pathologies. However, most oral bacteria are harmless commensals under normal circumstances. This means that this microbiota lives in harmony with its host, but under specific conditions (increased mass and/or pathogenicity, suppression of commensal or beneficial bacteria and/or reduced host response), disease can occur. The importance of the commensal microbiota is clearly illustrated by the development of yeast infections when the normal oral microbiota is reduced, for example, after a longer period of systemic antibiotic usage.410 Additionally, it has been shown that aggressive periodontitis is associated with a loss of colonization of S. sanguinis.371
TABLE 23-1 Colonization of the Oral Cavity
Adapted from Kononen E: Oral Dis 5:278, 1999; Kononen E: Ann Med 32:107, 2000.
Amenable periods for the establishment of most frequent bacterial species or groups (prevalence >25%) in infants’ mouths. Anaerobic bacteria are indicated in green, and aerobic or facultative bacteria are indicated in orange.
Bacteria indicated with an asterisk (*) decrease in prevalence with age.

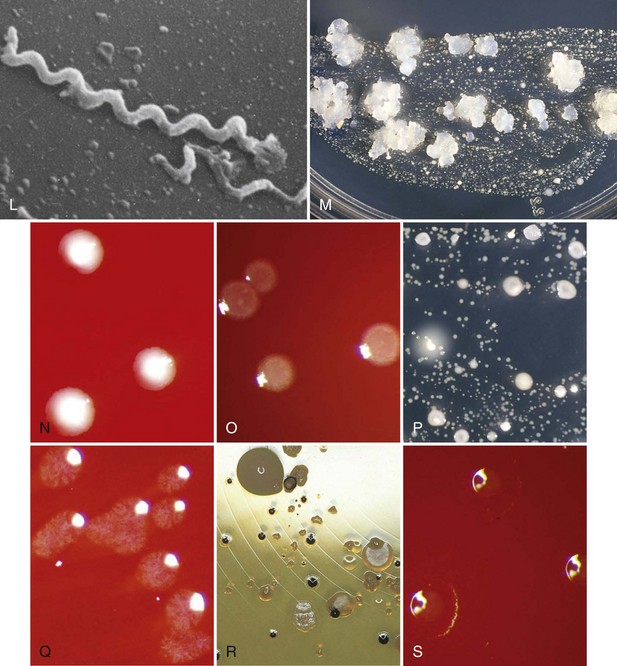
Figure 23-1 Various periodontal and cariogenic species grown on agar plates. A, Streptococcus mitis are gram-positive, fast-growing, facultatively anaerobic bacteria that are easy to culture on a blood-agar plate. A clear halo surrounding the colonies appears through hemolytic activity. B, Veillonella parvula are anaerobic gram-negative small cocci. They form small transparent colonies (<1.0 mm) after 48 hours of incubation. C, Actinomyces viscosus are microaerophilic to anaerobic gram positive rods with possible branches (pseudomycelium). They form slimy white spherical colonies in 48 hours. D, Typical colony morphology of Streptococcus sanguinis (right) and Actinomyces odontolyticus (left). E, Lactobacillus spp. will typically grow on Rogosa agar as a sesame seed. F, Streptococcus gordonii are facultative anaerobic gram-positive cocci. On a blood plate, colonies of 1 to 3 mm are formed within 48 hours. These bacteria are α-hemolytic, resulting in the formation of a clear halo surrounding the colony. G, This selective agar plate containing crystal violet and erythromycin (CVE-agar plate) will allow Fusobacterium nucleatum to grow as a round, flat rhizoid, opaque purple colony. H, Detailed picture of Porphyromonas gingivalis (green-brown) and Prevotella intermedia (black) on a classic nonspecific blood-agar plate. I, Prevotella nigrescens forms like Prevotella intermedia, a black pigmented colony on a blood agar plate. It is a strictly anaerobic bacterium whose growth is limited to an oxygen-free environment. J, Detailed picture of Parvimonas micra (small white colony) next to Porphyromonas gingivalis (green-brown colony) on a classic non-specific blood-agar plate. K, Detailed picture of Aggregatibacter actinomycetemcomitans grown on a selective agar plate containing tryptic soy, horse serum, bacitracin and vancomycin (TSBV-agar plate). L, It is extremely difficult to culture Treponema denticola (spirochete) on an agar plate and therefore not possible to identify this bacteria with classic culture. Phase-contrast microscope, the dark-field microscope, or the electron microscope are often used to visualize this bacterium. Identification and quantification is only possible through DNA analysis. M, On a selective agar plate containing trypticase yeast-extract, cystine, sucrose, and bacitracin (TYCSB agar plate), Streptococcus mutans will grow as a sugar cube. N, Eubacterium nodatum colony morphology strongly depends on its substrate. Its growth is very slow, and it is an obligate anaerobic gram-positive rod. O, Tannerella forsythia are fastidious bacteria and are therefore difficult to culture. This organism grows on a blood-agar plate as a smooth white colony with a faded edge. The bacteria are strictly anaerobic. P, Typical colony morphology of Streptococcus sobrinus on TYCSB agar (colony with white halo). Q, Capnocytophaga are slowly growing bacteria that require an elevated CO2 concentration for their growth. They are facultative anaerobic rods. R, Campylobacter rectus grows on a Hammond plate as small, smooth opaque, round colonies with a black color. S, Eikenella corrodens has a variable colony morphology and shows different biochemical and serologic reactions. Because of the difficult determination with classic culture, identification and quantification through DNA techniques is very suitable for this organism. E. corrodens cells are facultative anaerobic gram-negative rods.
(Images A, B, C, F, I, L, N, O, Q, and S courtesy ADD Clinident, Malden, The Netherlands).
It is obvious that the periodontal microbiota is extremely complex. Since it affects the host, the oral environment and periodontal treatment and since it is affected by the host, the oral environment and periodontal treatment, a profound knowledge of periodontal microbiology is necessary.
The Oral Cavity from A Microbe’s Perspective
In general, with the exception of those microorganisms that are present in feces and in secretory fluids, all bacteria maintain themselves within their host by adhering to a surface. This principle also applies to the oral cavity. From an ecologic viewpoint, the oral cavity, which communicates with the pharynx, should be considered as an “open growth system” with an uninterrupted ingestion and removal of microorganisms and their nutrients. A dynamic equilibrium exists between the adhesion forces of microorganisms and a variety of removal forces originating from (1) swallowing, mastication, or blowing the nose; (2) tongue and oral hygiene implements; (3) the wash-out effect of the salivary, nasal, and crevicular fluid outflow; and (4) active motion of the cilia (nasal and sinus walls). Most organisms can only survive in the oropharynx when they adhere to either the soft tissues or the hard surfaces (teeth, dentures, and implants).
The ability of a bacterium to adhere to its host is crucial for the induction of infectious diseases, such as gingivitis or periodontitis.310 Oral bacteria and especially pathogenic bacteria, such as Porphyromonas gingivalis (Figure 23-1, H and J) and Aggregatibacter actinomycetemcomitans (Figure 23-1, K), have a large battery of virulence factors, one of which is the ability to adhere to hard intraoral surfaces (teeth, restorative and prosthetic materials, and perimucosal implants) and/or to the oral mucosae, which have a significant variety of epithelial characteristics (Figure 23-2).56,58,103,357
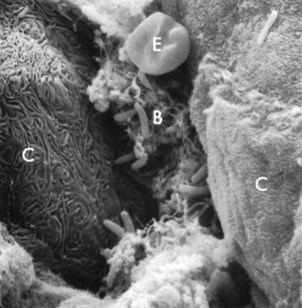
Figure 23-2 Scanning electron micrograph of epithelial intercellular spaces containing bacterial plaque (B) enmeshed in a fibrinlike material. C, Epithelial cells; E, erythrocyte. The cells to the left show signs of necrosis. (×4000.)
Several different types of surfaces are available for bacterial adhesion: soft tissues, such as mucosae, skin, and cornea, and hard tissues, such as teeth and nails. On the basis of physical and morphologic criteria, the oral cavity can be divided into six major ecosystems (also called niches), each with the following distinct ecologic determinants:
Table 23-2 summarizes several publications on the detection frequency of periodontopathogens in these different niches. Most species (with the exception of spirochetes (Figure 23-1, L) are able to colonize all of them. Some periodontopathogens (Fusobacterium nucleatum (Figure 23-1, G) and Prevotella intermedia (Figure 23-1, H) are involved in the etiology of tonsillitis and most periodontopathogens are able to colonize the maxillary sinus.36,407
TABLE 23-2 Intraoral Habitats (Periodontal Pocket, Buccal Mucosa, Tongue, Saliva, Tonsils, and Supragingival Plaque) for Periodontopathogenic and Cariogenic Species
The soft tissue surfaces are not passive bystanders in the process of bacterial adhesion and colonization.146 They employ a variety of mechanisms to prevent adhesion of pathogenic organisms. One of the most important mechanisms is shedding. The high turnover rate of the intraoral epithelial cells, especially of the gingiva, prevents the permanent accumulation of large masses of microorganisms on these surfaces. In essence, this is a natural cleansing mechanism. However, bacteria can adhere to host cells and form a commensal relationship, beneficial for both parties.
The host vaginal epithelial cells, for example, supply glucose for the colonized lactobacilli, which in turn produce acid. A lowering of the pH prevents the growth of many other species that have deleterious effects on the vaginal environment.317 These endogenous bacteria and their products can thus be a considered a necessary and beneficial component of a healthy body.
Also, in periodontal pockets, studies have shown high numbers of bacteria attached to pocket epithelial cells in vivo. In areas of gingival inflammation, there is an increased number of adhering bacteria.79,393 These adhering bacteria can also infiltrate the pocket wall in relatively large numbers and reach the underlying stroma (Figure 23-3).96,231,327 In general, there is a positive correlation between the adhesion rate of pathogenic bacteria to different epithelia and the susceptibility of that patient to certain infections.262
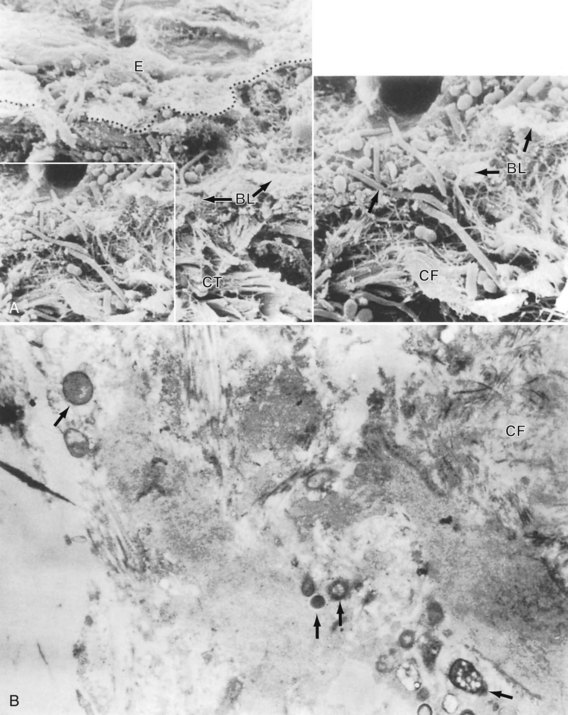
Figure 23-3 Bacterial penetration into the pocket wall in advanced periodontitis. A, The penetration through the pocket epithelium (E) and basement lamina (BL) into the connective tissue (CT) (arrows). CF, Collagen fibers. B, Connective tissue–associated bacteria in advanced periodontitis.
(A courtesy Dr. R. Saglie; A and B from Nissengard RJ, Newman MG: Oral microbiology and immunology, ed 2, Philadephia, 1994, Saunders.)
Females prone to urinary tract infections, for example, harbor five times more bacteria per cell in adhesion assays of Escherichia coli to different epithelial cells of their urogenital tract (periurethral, vaginal, or uroepithelial cells). Similar observations have been made for the adhesion of S. pneumoniae to nasopharyngeal epithelial cells of children prone to recurrent otitis media infections, as well as for the adhesion of Haemophilus influenzae to buccal cells of subjects prone to acute bronchitis.69,381
There are some indications that the same may be true for periodontal infections. Isogai and co-workers reported a significantly lower adherence rate of P. gingivalis and Prevotella intermedia strains to gingival epithelial cells in rats, which were resistant to gingivitis when compared to susceptible rats.153 An in vitro study on cultured human pocket epithelial cells (Figure 23-4) showed a similar tendency when patients resistant to periodontitis were compared to patients with severe periodontal breakdown.299
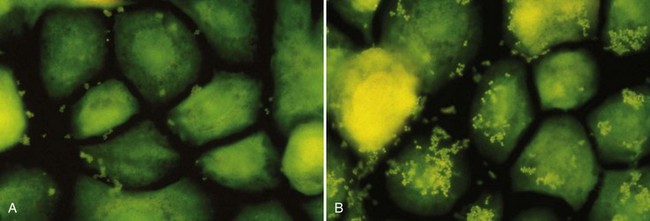
Figure 23-4 Microscopic confirmation of significant difference in adhesion capacity of P. gingivalis (small green dots) to epithelial cells from resistant patient (left) versus a patient suffering from severe periodontitis (right).
In contrast to soft tissues, teeth and nails are the only naturally occurring nonshedding surfaces. Artificial nonshedding surfaces of medical importance are prosthetic devices such as catheters, artificial joints, dental implants, and heart valves. From a microbiologic viewpoint, teeth and implants are unique for two reasons: (1) they provide a hard nonshedding surface that allows the development of extensive structured bacterial deposits and (2) they form a unique ectodermal interruption. There is a special seal of epithelium (junctional epithelium) and connective tissue between the external environment and the internal parts of the body. The accumulation and metabolism of bacteria on these hard surfaces is considered the primary cause of caries, gingivitis, periodontitis, periimplantitis, and sometimes, bad breath.
In the periodontal pocket, different strategies contribute to bacterial survival such as swimming in the crevicular fluid, adhesion to the pocket epithelium, and when dentine is encountered, the colonization of the dentine tubules.290 The crevicular fluid with its constant outflow does not favor the maintenance of unattached bacteria in the periodontal pocket.
It has been suggested that teeth are the primary habitat for periodontopathogens. Indeed, soon after full-mouth tooth extraction in patients with severe periodontitis, key pathogens, such as A. actinomycetemcomitans and P. gingivalis, disappeared from the oral cavity as determined by bacterial culturing techniques.71 Prevotella intermedia and other black-pigmented Prevotella spp. could, however, remain but at lower detection frequencies and numbers (see Table 23-2).
The same applies to edentulous infants or full denture wearers in whom significant proportions of periodontopathogens have been recorded, with the exception of A. actinomycetemcomitans and P. gingivalis.70,179 Therefore teeth were considered as a “port d’ entrée” for periodontopathogens.
However, recent studies using molecular tools to detect and quantify oral bacteria seem to indicate that A. actinomycetemcomitans and P. gingivalis are not entirely eradicated after full-mouth extraction. They may remain colonizers of the oral cavity, but when teeth are lost, their relative numbers decrease.304
Cariogenic species, on the contrary, were thought to be relatively restricted to solid surfaces (see Table 23-2). For that reason S. mutans (Figure 23-1, M) was often called an obligate periphyte.380 In some studies, this species was only detected from the time that the deciduous teeth erupted in the oral cavity.50 In a longitudinal observation of adults with severe dental caries, the cariogenic species fell below detection level after full-mouth extraction but reappeared a few days after denture insertion.51 Based on these reports and on their own observations, Caufield and Gibbons concluded that most of the S. mutans cells in the saliva or on the tongue are derived from the biofilm present on the teeth and that the mucosae could not act as a reservoir for the infection of teeth by those organisms.54 They suggested that there is a “window of infectivity” for the acquisition of S. mutans at a mean age of 26 months (range 9 to 44 months).53 This observation has been supported by a few clinical studies, which showed that initial colonization of S. mutans varied between 7 to 36 months, which is a time period coinciding with eruption of the primary teeth.6,49,99 On the other hand, longitudinal studies by Wan and co-workers411,412 and Law and Seow196 showed that there was increasing S. mutans colonization with increasing ages of the children, without any discrete window of infectivity. There is now clinical evidence that S. mutans can be detected in mouths of predentate children before eruption of the first tooth.241,411,413
Bacteria and Their Biofilm Mode of Living
To many people, the word “microbiologist” conjures images of someone in a laboratory coat swilling a flask of bacterial cells that are growing happily as pure cultures in nutrient-rich broth. In nature, bacteria rarely find life so easy. The major struggle faced by bacteria lies in obtaining sufficient nutrients to support growth. Competition is rife, and most microbial communities contain many different species, sometimes a hundred or more, sharing the same site. Nutrients tend to concentrate at interfaces, and consequently the densest bacterial populations are located at interfaces of various kinds. In aquatic ecosystems, the major interfaces are the solid-liquid boundaries, such as the surfaces of submerged rocks or soil particles, and the air-water interface that is present in open systems, such as oceans or lakes. Bacteria grow well in the laboratory at the solid-gas interface on the surface of an agar plate. However, desiccation rapidly kills most bacterial cells, and microbial growth at solid-gas interfaces is therefore generally restricted to areas where moisture is available. The human body provides several interfaces that support microbial populations, of which the most important in healthy individuals are the skin, gut, mouth, and female urogenital tract. As mentioned earlier, the oral cavity is somewhat unique in this regard since it provides hard, nonshedding surfaces (teeth) that are accessible for microbial colonization.
The importance of surfaces for microbial growth was recognized as early as the 1920s, when a number of workers independently noted that bacteria growing on glass slides submerged in soil were different from those that could be cultured in broth.195 However, it was not until around 50 years later that sessile microbial populations were considered to be sufficiently different from free-living microorganisms to merit their own name, and the term “biofilms” was coined (Figure 23-5 and Figure 23-6, B and C). Biofilms are composed of microbial cells encased within a matrix of extracellular polymeric substances (EPS) such as polysaccharides, proteins, and nucleic acids.

Figure 23-5 Vertical section through a 4-day human plaque sample. An intraoral device designed for in vivo generation of plaque biofilms on enamel was used. Confocal microscopy enabled visualization of the section of plaque without the dehydration steps used in conventional histologic preparations. Notice the open fluid filled channels (white arrows) that traverse from the plaque surface through the bacterial mass (M; grey-white areas) to the enamel surface. An area in which the bacterial mass appears to attach to the enamel surface (A) is indicated. Scale bar = 25 µm.
(From Wood SR, Kirkham J, Marsh PD, et al: J Dent Res 79:21, 2000.)
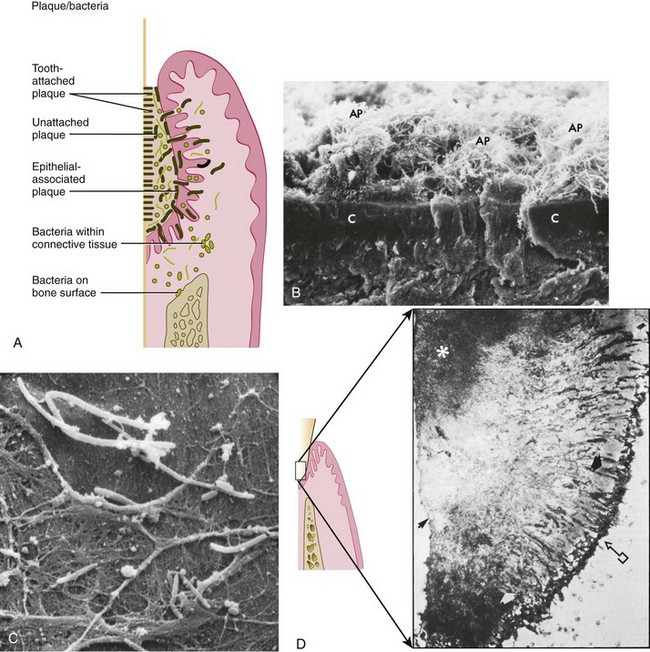
Figure 23-6 A, Diagram depicting the plaque-bacteria association with tooth surface and periodontal tissues. B, Scanning electron photomicrograph of cross-section of cementum (C) with attached subgingival plaque (AP). Area shown is within a periodontal pocket. C, Scanning electron micrograph of cocci and filaments associated with surface of pocket epithelium in a case of marginal gingivitis. (Magnification ×3000.) D, Left, Diagrammatic representation of the histologic structure of subgingival plaque. Right, Histologic section of subgingival plaque. Arrow with box, Sulcular epithelium. White arrow, Predominantly gram-negative unattached zone. Black arrow, Tooth surface. Asterisk, Predominantly gram-positive attached zone.
(B courtesy Dr. J. Sottosanti, La Jolla, CA.)
Biofilm bacteria are often up to 1000 times more resistant to antimicrobial agents than their planktonic counterparts.8,89 Bacteria growing in multispecies biofilms interact closely with neighboring cells. Sometimes, these interactions are mutually beneficial, as is the case when one organism removes another’s waste products and utilizes them to make energy. In other instances, bacteria compete with their neighbors by secreting antibacterial molecules such as inhibitory peptides (bacteriocins) or hydrogen peroxide (H2O2). In addition, the biofilm mode of growth facilitates cell-cell signalling and deoxyribonucleic acid (DNA) exchange between bacteria. It is clear that microbial ecology within biofilm communities is highly complex and in many cases, is only beginning to be understood.
Biofilms are heterogenous: variations in biofilm structure exist within individual biofilms and between different types of biofilms. However, a number of structural features that are common to many biofilms have been noted. For example, biofilms frequently contain microcolonies of bacterial cells. Water channels are commonly found in biofilms, and these can form a primitive circulatory system, removing waste products and bringing fresh nutrients to the deeper layers of the film. Surface structures, such as fronds, can dissipate the energy of fluid flowing over the biofilm. In mixed-species biofilms, there is often heterogeneity in the distribution of different species. Steep chemical gradients exist, such as oxygen or pH, and these produce distinct microenvironments within the biofilm.
Microbial populations on the surfaces of teeth (dental plaque) are excellent examples of biofilm communities (Figure 23-7). The architecture of a dental plaque biofilm has many features in common with other biofilms. It is heterogeneous in structure, with clear evidence of open fluid-filled channels running through the plaque mass.64,65,428 (see Figure 23-5). Nutrients make contact with the sessile (attached) microcolonies by diffusion from the water channels to the microcolony, rather than from the matrix. The bacteria exist and proliferate within the intercellular matrix through which the channels run. The matrix confers a specialized environment, which distinguishes bacteria that exist within the biofilm from those that are free-floating, the so-called planktonic state in solutions such as saliva or crevicular fluid. The biofilm matrix functions as a barrier. Substances produced by bacteria within the biofilm are retained and concentrated, which fosters metabolic interactions among the different bacteria.
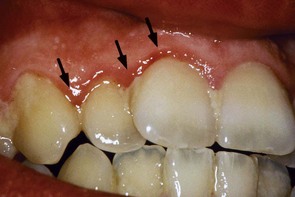
Figure 23-7 Clinical picture of 10-day-old supragingival plaque. The first signs of gingival inflammation (arrows) are becoming visible.
The intercellular matrix consists of organic and inorganic materials derived from saliva, gingival crevicular fluid (GCF), and bacterial products.
Organic constituents of the matrix include polysaccharides, proteins, glycoproteins, lipid material, and (perhaps) DNA. Albumin, probably originating from crevicular fluid, has been identified as a component of the plaque matrix. The lipid material consists of debris from the membranes of disrupted bacterial and host cells, and possibly food debris. Glycoproteins from saliva are an important component of the pellicle that initially coats a clean tooth surface, but they also become incorporated into the developing plaque biofilm. Polysaccharides produced by bacteria also contribute to the organic portion of the matrix. They play a major role in maintaining the integrity of the biofilm.
The inorganic components of plaque are predominantly calcium and phosphorus, with trace amounts of other minerals such as sodium, potassium, and fluoride. The source of inorganic constituents of supragingival plaque is primarily saliva. As the mineral content increases, the plaque mass becomes calcified to form calculus (Figure 23-8). Calculus is frequently found in areas of the dentition adjacent to salivary ducts (e.g., the lingual surface of the mandibular incisors and canines and the buccal surface of the maxillary first molars), reflecting the high concentration of minerals available from saliva in those regions. The inorganic components of subgingival plaque are derived from crevicular fluid (a serum transudate). Calcification of subgingival plaque also results in calculus formation (Figure 23-9). Subgingival calculus is typically dark green or dark brown, probably reflecting the presence of blood products associated with subgingival hemorrhage.
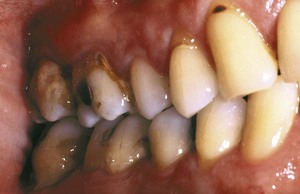
Figure 23-8 Supragingival calculus is depicted on the buccal surface of maxillary molars adjacent to the orifice for the parotid duct.
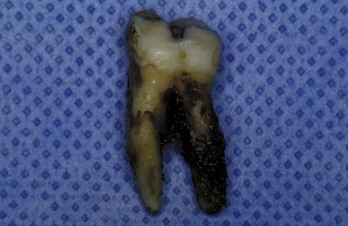
Figure 23-9 Dark-pigmented deposits of subgingival calculus are shown on the distal root of an extracted lower molar.
The importance of these biofilms for oral diseases, such as dental caries and periodontitis, together with the relative ease with which tooth surface biofilms can be accessed, has led to dental plaque becoming one of the most highly studied biofilm systems. It is hoped that by understanding the mechanisms involved in the accumulation of dental plaque and the transition from health to disease, it will be possible to improve our control over these processes and to further restrict plaque-associated oral diseases.
Structure of a Mature Dental Plaque Biofilm
Dental plaque (see Figure 23-7) is defined clinically as a structured, resilient yellow-grayish substance that adheres tenaciously to the intraoral hard surfaces, including removable and fixed restorations.29 The tough extracellular matrix makes it impossible to remove plaque by rinsing or the use of sprays. Plaque can thus be differentiated from other deposits that may be found on the tooth surface such as materia alba and calculus. Materia alba refers to soft accumulations of bacteria, food matter, and tissue cells that lack the organized structure of dental plaque and are easily displaced with a water spray. Calculus is a hard deposit that forms by mineralization of dental plaque and is generally covered by a layer of unmineralized plaque (Table 23-3).
TABLE 23-3 Differences Between Tooth Deposits
| Materia Alba | Dental Plaque | Calculus |
|---|---|---|
Dental plaque is composed primarily of microorganisms. One gram of plaque (wet weight) contains approximately 1011 bacteria.339,360 The number of bacteria in supragingival plaque on a single tooth surface can exceed 109 cells. In a periodontal pocket, counts can range from 103 bacteria in a healthy crevice to >108 bacteria in a deep pocket. Using highly sensitive molecular techniques for microbial identification, it has been estimated that more than 500 distinct microbial phylotypes can be present as natural inhabitants of dental plaque.1 In fact, this number may be much greater.
Large-scale pyrosequencing of DNA from plaque populations indicates that there may be as many as 19,000 species-level phylotypes (essentially, distinct species in all but name) in dental plaque.165
Any individual may harbor 150 or more different species. Nonbacterial microorganisms that are found in plaque include archaea, yeasts, protozoa, and viruses.62,203
Dental plaque is broadly classified as supragingival or subgingival based on its position on the tooth surface toward the gingival margin.
Supragingival plaque typically demonstrates a stratified organization of a multilayered accumulation of bacterial morphotypes (Figure 23-10). Gram-positive cocci and short rods predominate at the tooth surface, whereas gram-negative rods and filaments, as well as spirochetes, predominate in the outer surface of the mature plaque mass.
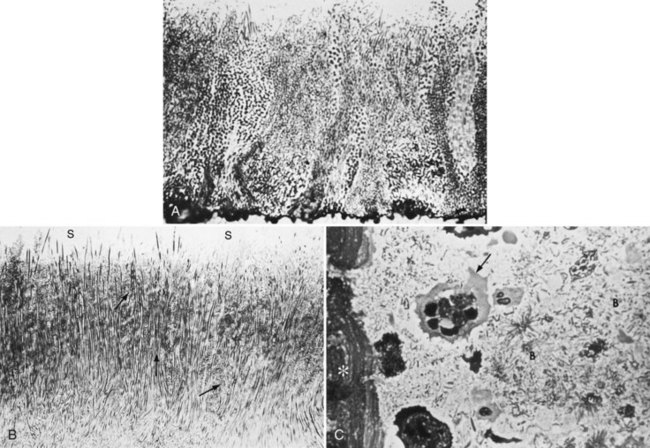
Figure 23-10 A, 1-day-old plaque. Microcolonies of plaque bacteria extend perpendicularly away from tooth surfaces. B, Developed supragingival plaque showing overall filamentous nature and microcolonies (arrows) extending perpendicularly away from tooth surface. Saliva-plaque interface shown (S). C, Histologic section of plaque showing nonbacterial components such as white blood cells (arrow) and epithelial cells (asterisk), interspersed among bacteria (B).
(Courtesy Dr. Max Listgarten, Philadelphia, PA.)
In general, the subgingival microbiota differs in composition from the supragingival plaque, primarily because of the local availability of blood products and a low reduction-oxidation (redox) potential, which characterizes the anaerobic environment.
Many periodontopathogens are indeed fastidious strict anaerobes and as such may contribute little to the initiation of disease in shallow gingival pockets. In deep periodontal pockets, however, they find their preferred habitat.
The identification of bacteria within intact dental plaque represents a significant challenge. Most of the previously cited studies on dental plaque microbiota used techniques that involved disruption of the dental plaque matrix followed by microbial culture (Figure 23-11) or culture-independent identification. Techniques have been developed that allow the specific visualization of individual bacteria within mixed populations. In these methods, specific labeling is achieved using nucleic acid probes (fluorescence in situ hybridization [FISH]) or specific antibodies (immunofluorescence). FISH has been applied to identify particular species within plaque samples scraped from periodontal pockets.109,264,404 With this methodology, it is even possible to image bacteria that have never been cultured in the laboratory such as members of the phylum Synergistetes (Figure 23-12). Supragingival biofilms have proved somewhat easier to visualize than subgingival plaque. Biofilms can be cultured on retrievable enamel chips held in intraoral devices in the mouths of volunteers. The enamel pieces can then be removed and processed for FISH or immunofluorescence. Images of biofilms can be captured by confocal laser scanning microscopy, which produces three-dimensional representations of the biofilm architecture. Labeling with nucleic acid probes requires desiccation of the sample before hybridization, and therefore some structural information may be lost. Nevertheless, this technique has been applied successfully to determine the spatial relationships between Actinomyces naeslundii and Streptococcus spp. in dental plaque.77 In these studies, A. naeslundii cells were frequently observed juxtaposed to Streptococcus spp. cells. Many strains of Actinomyces coaggregate with oral streptococci and therefore the spatial organization of these organisms within dental plaque may be influenced by adhesin-receptor interactions.175 In fact, a protein adhesin of A. naeslundii has been shown to colocalize with a cognate oral streptococcal receptor polysaccharide in in situ biofilms using specific antibody (immunofluorescence) labeling (Figure 23-13).266 These studies clearly demonstrate that the interactions identified between bacteria isolated in the laboratory are relevant to the dental plaque biofilm.
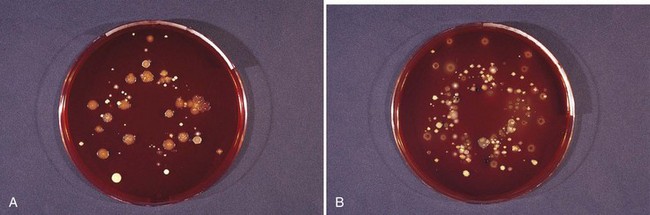
Figure 23-11 A plaque sample grown under aerobic (A) and anaerobic (B) conditions. A comparison of the culture plates shows different bacteria and different colony morphologies.
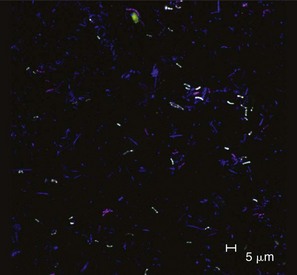
Figure 23-12 Identification of uncultured Synergistetes in subgingival dental plaque. Plaque was removed from deep (6 to 10 mm) periodontal pockets of volunteers with localized or generalized severe periodontitis. Bacteria were transferred to microscope slides and intracellular DNA was hybridized with nucleic acid probes for all eubacteria (blue), Synergistetes cluster A (red), or a specific member of the Synergistetes cluster A, “3.3/BH007” (green). Synergistetes 3.3/BH007 appear white (blue + red + green) and other Synergistetes cluster A appear purple (blue + red). Synergistetes cluster A were commonly detected in healthy and periodontally diseased sites, even though these organisms have never been cultured in the laboratory. Bar = 5 µm.
(Courtesy of S. R. Vartoukian and W. G. Wade, King’s College London Dental Institute, London. For further details, see Vartoukian SR, Palmer RM, Wade WG: Appl Environ Microbiol 75:3777, 2009.)
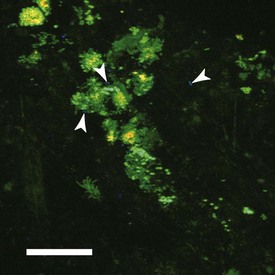
Figure 23-13 Visualization of specific bacteria in dental plaque biofilms using specific antisera. Biofilms were developed on the surface of enamel chips held in the mouths of volunteers for 8 hours. Samples were labeled with acridine orange (stains all bacteria green), antibodies against streptococcal cell surface polysaccharides (orange), and antibodies against Actinomyces oris type 1 fimbriae (blue). A. oris cells (some are indicated by white arrowheads) and polysaccharide-bearing streptococci can be seen in juxtaposition with other bacteria, indicating that mixed-species interactions are common in dental plaque biofilms. Bar = 20 µm.
(Courtesy of R. J. Palmer Jr, NIDCR, Bethesda, MD.)
The environmental parameters of the subgingival region differ from those of the supragingival region. The gingival crevice or pocket is bathed by the flow of crevicular fluid, which contains many substances which bacteria may use as nutrients. Host inflammatory cells and mediators are likely to have considerable influence on the establishment and growth of bacteria in the subgingival region. Both morphologic and microbiologic studies of subgingival plaque reveal distinctions between the tooth-associated and soft tissue-associated regions of subgingival plaque (see Figure 23-6, A to C).209,253
The tooth-associated cervical plaque, adhering to the root cementum, does not markedly differ from that observed in gingivitis. At this location, filamentous microorganisms dominate, but cocci and rods also occur. This plaque is dominated by gram-positive rods and cocci, including S. mitis, S. sanguinis, Actinomyces oris (a new species containing strains that were formerly classified as A. naeslundii), A. naeslundii, and Eubacterium spp. (see Figure 23-1, N). However, in the deeper parts of the pocket, the filamentous organisms become fewer in numbers, and in the apical portion they seem to be virtually absent. Instead, the microbiota is dominated by smaller organisms without a particular orientation.209 The apical border of the plaque mass is separated from the junctional epithelium by a layer of host leukocytes, and the bacterial population of this apical tooth-associated region shows an increased concentration of gram-negative rods (see Figure 23-6, D).
The layers of microorganisms facing the soft tissue lack a definite intermicrobial matrix and contain primarily gram-negative rods and cocci, as well as large numbers of filaments, flagellated rods, and spirochetes. Studies on plaque associated with crevicular epithelial cells indicate a predominance of species such as S. oralis, S. intermedius, Parvimonas micra (formerly Micromonas micra and Peptostreptococcus micros), P. gingivalis, Prevotella intermedia, Tannerella forsythia (see Figure 23-10), and F. nucleatum.76,79 Host tissue cells (e.g., white blood cells and epithelial cells) may also be found in this region (see Figure 23-10, C). Bacteria are also found within the host tissues, such as in the soft tissues (see Figure 23-3), and within epithelial cells (Figure 23-14), as well as in the dentinal tubules (Figure 23-15).326,327

Figure 23-14 A to C show images of z-section No. 39 from a stack of 74 0.2-µm z-sections (magnification ×600; the scale bar in A also applies to B and C). Buccal epithelial cells (BEC) in this field were double-labeled with the EUB338 universal probe (A) and the A. actinomycetemcomitans-specific probe (B). The cell in the center of A contained a large mass of brightly fluorescent intracellular bacteria (red arrow). Other cells in the field contained smaller bacterial masses (not marked). B shows that a portion of the large mass labeled with the universal probe also hybridized with the A. actinomycetemcomitans-specific probe (green arrow). Images from A and B were superimposed (C), confirming that bacteria labeled with both probes (yellow arrow) were adjacent to other bacteria labeled only with the universal probe (red arrow). D presents a three-dimensional reconstruction of the same field. Bacteria recognized only by the universal probe are shown in solid red, whereas colocalization of the A. actinomycetemcomitans and universal probes is depicted by a green wireframe over a red interior. Reconstructed BEC surfaces are presented in blue. The red and green colors are muted when bacterial masses are intracellular, and brighter when bacteria appear to project out of the surface. The angle of view was rotated along the z-axis, and the image was zoomed. The large mass that appeared to have a lobular structure in z-section No. 39 was seen to be a cohesive unit containing A. actinomycetemcomitans in direct proximity to other species (red and green arrows).
(From Rudney JD, Chen R, Sedgewick GJ: J Dent Res 84:59-63, 2005.)
The composition of the subgingival plaque depends on the pocket depth. The apical part is more dominated by spirochetes, cocci and rods, whereas in the coronal part more filaments are observed.
The site specificity of plaque is significantly associated with diseases of the periodontium. Marginal plaque, for example, is of prime importance in the initiation and development of gingivitis. Supragingival plaque and tooth-associated subgingival plaque are critical in calculus formation and root caries, whereas tissue-associated subgingival plaque is important in the tissue destruction that characterizes different forms of periodontitis. Biofilms also form on artificial surfaces exposed to the oral environment such as prostheses and implants. A large series of papers compared the microbiota in pockets around teeth with those around implants of partially edentulous patients. The similarities were striking.*
Accumulation of a Dental Plaque Biofilm
The process of plaque formation can be divided into several phases: (1) the formation of the pellicle on the tooth surface, (2) initial adhesion/attachment of bacteria, and (3) colonization/plaque maturation.
Formation of the Pellicle
All surfaces in the oral cavity, including hard and soft tissues, are coated with a layer of organic material known as the acquired pellicle. The pellicle on tooth surfaces consists of more than 180 peptides, proteins, and glycoproteins, including keratins, mucins, proline-rich proteins, phosphoproteins (e.g., statherin), histidine-rich proteins, and other molecules that can function as adhesion sites (receptors) for bacteria.346,347,432 Salivary pellicle can be detected on clean enamel surfaces within 1 minute after introduction into the mouths of volunteers.134 By 2 hours, the pellicle is essentially in equilibrium between adsorption and detachment, although further pellicle maturation can be observed for several hours.
Transmission electron microscopy shows the pellicle to be composed of two layers: a thin basal layer that is very difficult to remove even with harsh chemical and mechanical treatments, and a thicker globular layer, up to 1 µm or more, that is easier to detach.134,135 From these observations, it can be concluded that dental enamel is permanently covered with an acquired pellicle from the moment that teeth erupt.
Consequently, bacteria that adhere to tooth surfaces do not contact the enamel directly but interact with the acquired enamel pellicle. However, the pellicle is not merely a passive adhesion matrix. Many proteins retain enzymatic activity when incorporated into the pellicle, and some of these, such as peroxidases, lysozyme, and α-amylase, may affect the physiology and metabolism of adhering bacterial cells.129,131,132,133
Recently, Walker and co-workers reported that dental plaque samples will only produce in vitro biofilms if the surface on which they were grown contained a salivary pellicle belonging to the patient who donated the plaque sample. No biofilm could be grown on a pellicle coming from a different subject.409
Initial Adhesion/Attachment of Bacteria
Toothbrushing removes most but not all bacteria from the exposed surfaces of teeth.394 However, recolonization begins immediately, and bacteria can be detected within 3 minutes of introducing sterile enamel into the mouth.130
The initial steps of transport and interaction with the surface are essentially nonspecific (i.e., they are the same for all bacteria). The proteins and carbohydrates that are exposed on the bacterial cell surface become important once the bacteria are in loose contact with the acquired enamel pellicle. It is the specific interactions between microbial cell surface “adhesin” molecules and receptors in the salivary pellicle that determine whether a bacterial cell will remain associated with the surface. Only a relatively small proportion of oral bacteria possess adhesins that interact with receptors in the host pellicle, and these organisms are generally the most abundant bacteria in biofilms on tooth enamel shortly after cleaning. Over the first 4 to 8 hours, 60% to 80% of bacteria present are members of the genus Streptococcus.77,258 Other bacteria commonly present at this time include species that cannot survive without oxygen (obligate aerobes), such as Haemophilus spp. and Neisseria spp., as well as organisms that can grow in the presence or absence of oxygen (facultative anaerobes) including Actinomyces spp. and Veillonella spp.1,74 These species are considered the “primary colonizers” of tooth surfaces. The primary colonizers provide new binding sites for adhesion by other oral bacteria. The metabolic activity of the primary colonizers modifies the local microenvironment in ways that can influence the ability of other bacteria to survive in the dental plaque biofilm. For example, by removing oxygen, the primary colonizers provide conditions of low oxygen tension that permit the survival and growth of obligate anaerobes.
The initial steps in colonization of teeth by bacteria can be summarized as in the following.
Phase 1: Transport to the Surface
The first stage involves the initial transport of the bacterium to the tooth surface. Random contacts may occur, for example, through brownian motion (average displacement of 40 µm/hour), through sedimentation of microorganisms, through liquid flow (several orders of magnitude faster than diffusion), or through active bacterial movement (chemotactic activity). It should be noted, however, that relatively few oral bacteria are motile and forces, such as saliva flow or mechanical contact, between oral soft tissues and teeth are almost certainly more important than swimming for bringing the primary colonizing bacteria into contact with teeth.
Phase 2: Initial Adhesion
The second stage results in an initial, reversible adhesion of the bacterium. This is initiated when the bacterial cell comes into close proximity to the surface (separation distance approximately 50 nm). Long- and short-range forces, including van der Waals attractive forces and electrostatic repulsive forces, operate at this distance. The behavior of bacterial cells can be reasonably described by the Derjaguin-Landau-Verwey-Overbeek (DLVO) theory of colloid stability.140 According to this theory, the total interaction energy (also called the total Gibbs energy [GTOT]), is the sum of the attractive forces (GA) and the electrostatic repulsion (GR). At the physiologic ionic strength of saliva, the van der Waal’s forces result in a net attraction of bacterial cells at distances of tens of nm from the surface. Approximately 10 nm from the surface, there is a secondary net energy minimum. Electrostatic repulsion prevents bacterial cells from getting even closer to the surface. At distances of approximately 10 nm from the surface, bacterial cells are reversibly bound. It is thought that stronger binding at this point is the consequence of interactions between bacterial adhesins and receptors in the salivary pellicle. It has been estimated that 10 to 50 ligand-receptor interactions are required to attain essentially irreversible binding of a bacterial cell to the pellicle.43
It is important to note that although the DLVO theory presents a neat picture of the initial stages of bacterial adhesion, in reality even these early steps in adhesion are extremely complex. Bacteria are not perfect spheres, and many cells possess structures, such as fimbriae, that protrude from the cell surface. Lewis acid-base interactions (hydrophobicity) also influence cell-surface interactions.140 In addition, microbial cell surfaces are not uniformly coated with a negative charge. There may be regions of the cell surface that are positively charged and for these areas electrostatic interactions with a negatively charged surface will tend to be attractive. Therefore it is difficult to predict how initial attachment will be affected by modifying the prevailing environment.
Phase 3: Strong Attachment
After initial adhesion, a firm anchorage between bacterium and surface is established. On a rough surface, bacteria are better protected against shear forces so that a change from reversible to irreversible binding may occur more easily and more frequently. The substratum surface free energy becomes important, since the water film between the interacting surfaces has to be removed before short-range forces can be involved. The binding between bacteria and pellicle is mediated by specific adhesins on the bacterial cell surface (usually proteins) and complementary receptors (proteins, glycoproteins, or polysaccharides) in the acquired pellicle. Many proteins in the acquired pellicle can act as receptors for streptococci including α-amylase, acid proline-rich proteins, statherin, and salivary agglutinin glycoprotein gp340.334 The specific adhesins of primary colonizing bacteria have been the subject of many investigations, since these represent potential targets for interfering with the buildup of dental plaque. One of the best-characterized interactions is the binding between antigen I/II family adhesins of oral streptococci and gp340.156,157,283 Antigen I/II family adhesins are 160 to 180 kDa proteins expressed on the surfaces of many oral streptococci, including S. mutans, S. sobrinus (see Figure 23-1, P), S. gordonii, and S. intermedius. Gp340 is present in fluid-phase saliva and is a component of the salivary pellicle. In the fluid phase, interactions between antigen I/II proteins and gp340 result in aggregation of bacteria. Large clumps of bacterial cells do not stick well to surfaces and are probably removed by swallowing.206 In contrast, bacterial binding to immobilized gp340 results in retention of cells within the biofilm. Interestingly, recognition of fluid-phase and immobilized gp340 by streptococci appears to involve different mechanisms.221 It is likely that gp340 changes conformation on adhesion to a surface and exposes different receptors for bacterial binding. Similar conformational changes have been noted for a number of host proteins, including proline-rich proteins and statherin.86,110 Selective binding of fluid-phase or surface-bound proteins may modulate the ability of bacterial cells to colonize salivary pellicle-coated surfaces.
Colonization and Plaque Maturation
The primary colonizing bacteria (Table 23-4) adhered to the tooth surface provide new receptors for attachment by other bacteria, in a process known as “coadhesion.”175 Together with growth of adherent microorganisms, coadhesion leads to the development of microcolonies (Figure 23-16) and eventually to a mature biofilm.
TABLE 23-4 Overview of Primary and Secondary Colonizers in Dental Plaque
| Primary colonizers | |
| Secondary colonizers |
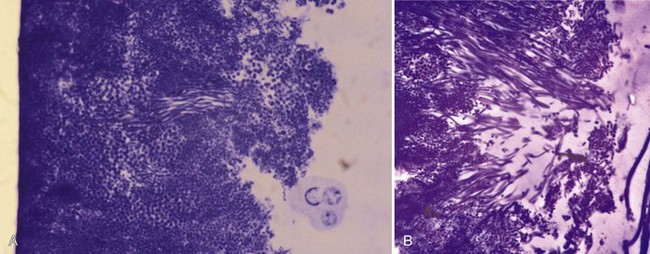
Figure 23-16 When a single microorganism adheres to the tooth surface, it can start to multiply and slowly forms a microcolony of daughter cells. These views were taken after plaque formation on a plastic strip (e.g., as shown in Figure 23-26), glued to a tooth surface.
Cell-cell adhesion between genetically distinct oral bacteria also occurs in the fluid phase (i.e., in saliva).
In the laboratory, interactions between genetically distinct cells in suspension result in clumps, or “coaggregates,” that can easily be seen with the naked eye (Figure 23-17).
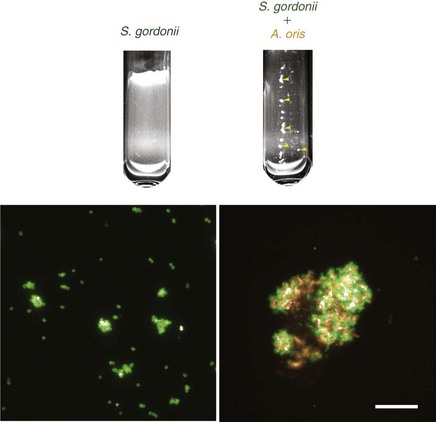
Figure 23-17 Coaggregation between Streptococcus gordonii DL1 and Actinomyces oris MG1 in vitro. A monoculture of S. gordonii appears uniformly turbid. Microscopically, cells labeled with specific anti-DL1 antibodies (green) are in small chains or clumps. Following addition of A. oris, cells clump together to form macroscopic coaggregates (yellow arrows). Under the microscope, S. gordonii (green) are evenly distributed throughout the coaggregates with A. oris (orange). Bar = 20 µm.
(Image reproduced in part from Jakubovics NS, Gill SR, Iobst SE, et al: J Bacteriol 190:3646, 2008.)
Coaggregation is a direct interaction and is distinct from agglutination, which occurs when cells are stuck together by molecules in solution. At least 18 genera from the oral cavity have shown some form of coaggregation.173 All oral bacteria possess surface molecules that foster some sort of cell-cell interaction (Figure 23-18).174 The initial stages of coaggregation or coadhesion are essentially the same as the first steps involved in bacterial binding to surfaces: bacterial cells come into contact through passive or active transport and bind weakly through nonspecific hydrophobic, electrostatic, and Van der Waals forces.78,95,170,174 These steps can be dramatically accelerated in vitro by vigorously mixing dense suspensions of bacterial cells.175 Strong cell-cell binding is then determined by the presence of adhesin proteins or carbohydrates on one partner, and complementary receptor proteins or carbohydrates on the other. Note that adhesin-receptor interactions are mediated by the fundamental physicochemical forces (hydrophobic, electrostatic, and Van der Waals), but they are highly specific.
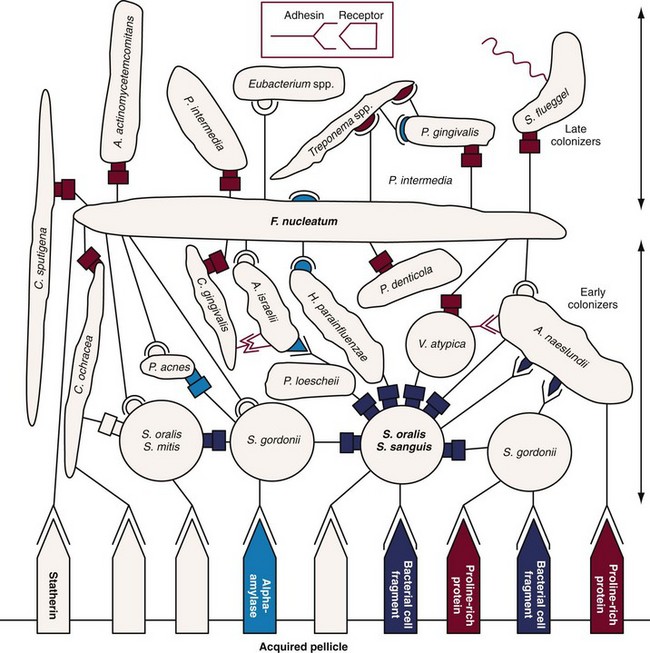
Figure 23-18 Diagrammatic representation of initial plaque formation. Early colonizers bind to receptors in the pellicle. Each adherent cell becomes in turn the nascent surface and bridge for additional species (secondary colonizers). The complementary sets of adhesin receptor symbols (example in box) represent the various kinds of coaggregations, as well as the interactions with molecules in the pellicle. The symbol with a stem (adhesin) represents a cellular component that is heat inactivated (cell suspension heated to 85° C for 30 minutes) and sensitive to protease treatment. The cell type exhibiting the complementary symbol (receptor) is insensitive to either treatment. The symbols with a rectangular shape represent lactose-inhibitable coaggregations, others are lactose-noninhibitable.
(Adapted from Kolenbrander PE, London J: J Bacteriol 175:3247, 1993.)
Different species, or even different strains of a single species, have distinct sets of coaggregation partners (see Figure 23-18 online). Fusobacteria coaggregate with all other human oral bacteria while Veillonella spp., Capnocytophaga spp. (see Figure 23-1, Q) and Prevotella spp. bind to streptococci and/or actinomyces.174,176,421 Each newly accreted cell becomes itself a new surface and therefore may act as a coaggregation bridge to the next potentially accreting cell type that passes by.
Many coaggregations between strains of different genera are mediated by lectin-like adhesins (proteins that recognize carbohydrates) and can be inhibited by lactose and other galactosides. The significance of coaggregation in oral colonization has been documented in studies on biofilm formation in vitro as well as in animal model studies.31,234
Well-characterized interactions of secondary colonizers (see Table 23-4) with early colonizers include the coaggregation of F. nucleatum with S. sanguinis, Prevotella loescheii with A. oris, and Capnocytophaga ochracea with A. oris.162,164,416,417,418 Streptococci show intrageneric coaggregation, allowing them to bind to the nascent monolayer of already bound streptococci.148,172,259,349
Secondary colonizers (see Table 23-4), such as Prevotella intermedia, Prevotella loescheii, Capnocytophaga spp., F. nucleatum, and P. gingivalis do not initially colonize clean tooth surfaces but adhere to bacteria already in the plaque mass.174 The transition from early, supragingival dental plaque to mature plaque growing below the gingival margin involves a shift in the microbial population from primarily gram-positive organisms to high numbers of gram-negative bacteria. Therefore, in the later stages of plaque formation, coaggregation between different gram-negative species is likely to predominate. Examples of these types of interactions are the coaggregation of F. nucleatum with P. gingivalis or Treponema denticola (see Figure 23-1, L).166,171,176
The concept that coaggregation is important during the formation of oral biofilms opens new perspectives especially for the use of probiotics. Special examples of coaggregations are the corn-cob formation (Figure 23-19), in which streptococci adhere to filaments of Corynebacterium matruchotii or Actinomyces spp., and the test-tube brush composed of filamentous bacteria to which gram-negative rods adhere.60,209,251
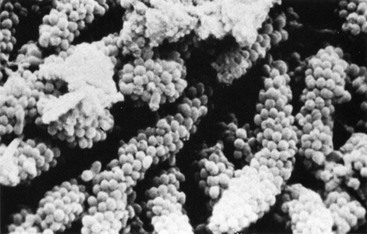
Figure 23-19 Long-standing supragingival plaque near the gingival margin demonstrates “corn cob” arrangement. A central gram-negative filamentous core supports the outer coccal cells, which are firmly attached by interbacterial adherence or coaggregation.
An analysis of more than 13,000 plaque samples, looking for 40 subgingival microorganisms using a DNA-hybridization methodology, defined color-coded “complexes” of periodontal microorganisms that tend to be found together in health or disease. The composition of the different complexes was based on the frequency with which different clusters of microorganisms were recovered, and the complexes were color-coded for easy conceptualization (Figure 23-20).364 Interestingly, the early colonizers are either independent of defined complexes (A. naeslundii, A. oris) or members of the yellow (Streptococcus spp.) or purple complexes (A. odontolyticus (see Figure 23-1, D). The microorganisms primarily considered secondary colonizers fell into the green, orange, or red complexes. The green complex includes Eikenella corrodens, A. actinomycetemcomitans serotype a, and Capnocytophaga spp. The orange complex includes Fusobacterium, Prevotella, and Campylobacter spp. (see Figure 23-1, R). The green and orange complexes include species recognized as pathogens in periodontal and nonperiodontal infections. The red complex consists of P. gingivalis, T. forsythia, and T. denticola. This complex is of particular interest because it is associated with bleeding on probing.364 The existence of complexes of species in plaque is another reflection of bacterial interdependency in the biofilm environment.
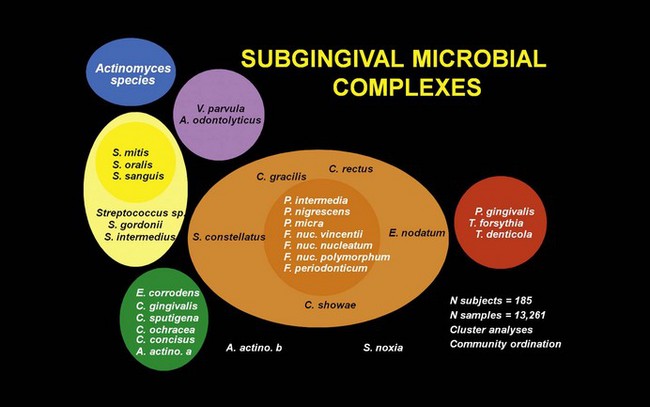
Figure 23-20 Diagram of the association among subgingival species The data were derived from 13,261 subgingival plaque samples taken from the mesial aspect of each tooth in 185 adult subjects. Each sample was individually analyzed for the presence of 40 subgingival species using checkerboard DNA-DNA hybridization. Associations were sought among species using cluster analysis and community ordination techniques. The complexes to the left are comprised of species thought to colonize the tooth surface and proliferate at an early stage. The orange complex becomes numerically dominant later and is thought to bridge the early colonizers and the red complex species which become numerically more dominant at late stages in plaque development.
(Adapted from Socransky SS, Haffajee AD, Cugini MA, et al: J Clin Periodontol 25:134,1998.)
Factors Affecting Supragingival Dental Plaque Formation
Clinically, early undisturbed plaque formation on teeth follows an exponential growth curve when measured planimetrically.298 During the first 24 hours starting from a clean tooth surface, plaque growth is negligible from a clinical viewpoint (<3% coverage of the vestibular tooth surface, which is an amount nearly undetectable clinically). This “lag time” is due to the fact that the microbial population must reach a certain size before it can be easily detected by the clinician. During the following 3 days, coverage progresses rapidly to the point where, after 4 days, on average 30% of the total coronal tooth area will be covered with plaque (see Video 23-1: Plaque Growth online).
This movie shows undisturbed plaque formation on a central incisor after professional plaque removal over a period of 96 hours in the absence of oral hygiene. The plaque was visualized with erythrosin. After professional plaque removal, it takes, even in the absence of oral hygiene, at least 36 hours before plaque becomes detectable. Plaque first develops in irregularities on the tooth surface and at the gingival margin. Once established, the speed of plaque formation increases over time. Nevertheless, even in the absence of oral hygiene, no more than one-fourth of the tooth surface is covered after 96 hours of undisturbed plaque growth.
Several reports have shown that the microbial composition of the dental plaque will change with a shift toward a more anaerobic and a more gram-negative flora, including an influx of fusobacteria, filaments, spiral forms, and spirochetes (Figure 23-21). This was beautifully illustrated in experimental gingivitis studies.375,387 In this ecologic shift within the biofilm, there is a transition from the early aerobic environment characterized by gram-positive facultative species to a highly oxygen-deprived environment in which gram-negative anaerobic microorganisms predominate. Bacterial growth in older plaque is much slower than in newly formed dental plaque, presumably because nutrients become limiting for much of the plaque biomass.415
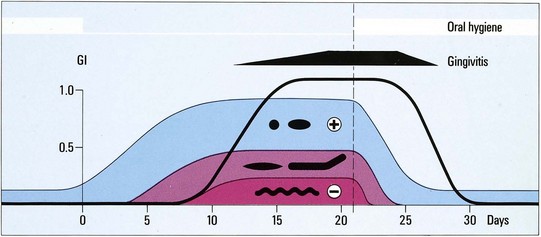
Figure 23-21 Artistic impression of the 1965 published classic experimental proof of the bacterial etiology of gingivitis using the experimental gingivitis model. In plaque-free subjects with clinically non-inflamed gingiva, plaque will slowly develop on the teeth when all mechanical plaque control is stopped. With time, the plaque composition changes. During the first few days, the plaque is mainly composed of gram positive (+) cocci and rods. Later on, the composition is shifted toward more gram-negative species, more rods, filaments, and finally gram-negative (−) spirochetes appear (see morphotypes in black). Within a few days, a mild gingivitis ensues (black line, gingival index according to Löe & Silness, GI=1). From the moment that proper plaque control is re-established (vertical line), the plaque composition returns to the initial situation and the symptoms of gingivitis disappear.
(From Loe H, Theilade E, Jensen SB: Experimental gingivitis in man, J Periodontol 36:177, 1965.)
Topography of Supragingival Plaque
Early plaque formation on teeth follows a typical topographic pattern (Figure 23-22), with initial growth along the gingival margin and from the interdental space (areas protected against shear forces). Later, a further extension in the coronal direction can be observed.239,302 This pattern may fundamentally change when the tooth surface contains irregularities that offer a favorable growth path (Figure 23-23). Plaque formation can also start from grooves, cracks, perikymata, or pits.
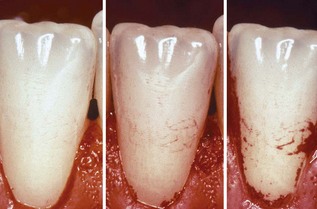
Figure 23-22 Clinical illustration of the typical topography of plaque growth. Initial growth starts along the gingival margins and from the interdental spaces (areas protected against shear forces), to further extend in a coronal direction. This pattern may fundamentally change, for example if the tooth surface contains irregularities (such as those evident in Figure 23-23).
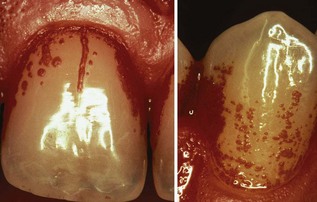
Figure 23-23 Important surface irregularities (crack on central upper incisor, several small pits on canine) are also responsible for the so-called individualized plaque growth pattern.
Scanning electron microscopy studies clearly revealed that the early colonization of the enamel surface starts from surface irregularities in which bacteria escape shear forces, thereby permitting them the time needed to change from reversible to irreversible binding.
By multiplication, the bacteria subsequently spread out from these starting up areas as a relatively even monolayer. Surface irregularities are also responsible for the so-called “individualized” plaque growth pattern (see Figure 23-23), which is reproduced in the absence of optimal oral hygiene.239,240 This phenomenon illustrates the importance of surface roughness in plaque growth, which should lead to proper clinical treatment options.
Rough intraoral surfaces (e.g., crown margins, implant abutments, and denture bases) accumulate and retain more plaque and calculus in terms of thickness, area, and colony-forming units.289 Ample plaque also reveals an increased maturity/pathogenicity of its bacterial components, characterized by an increased proportion of motile organisms and spirochetes, and/or a denser packing of them (Figures 23-24 and 23-25). Smoothing an intraoral surface decreases the rate of plaque formation. Below a certain surface roughness (Ra < 0.2 µm), however, further smoothing does not result in an additional reduction in plaque formation.27,291 There seems to be a threshold level for surface roughness (Ra around 0.2 µm), above which bacterial adhesion will be facilitated.26 Although surface free energy and surface roughness are two factors influencing plaque growth, the latter predominates (see Figure 23-24 and 23-25).
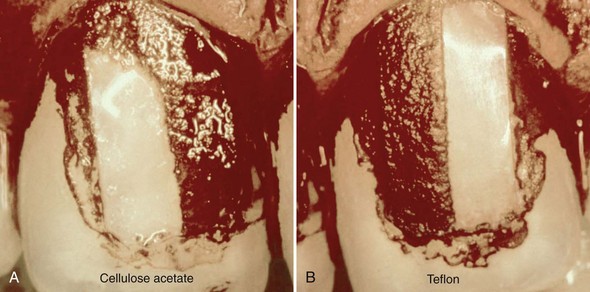
Figure 23-24 Photographs showing the clinical impact of surface roughness and surface free energy on de novo plaque formation. Two small strips were glued to the central upper incisors of a patient who refrained from oral hygiene for 3 days. Each strip was divided in two halves, a rough region (Ra 2.0 µm) located mesially, and a smooth region (Ra 0.1 µm) distally located. The left strip was cellulose acetate (medium surface free energy [sfe]: 58 ergcm-2) and the right strip Teflon (low sfe: 20 ergcm-2). Plaque was disclosed with 0.5% neutral red solution. The smooth regions show the decrease in biofilm formation because of the low sfe; the rough regions demonstrate the predominance of surface roughness (i.e., more plaque with no difference between the two surfaces), even with different sfe.
(From Quirynen M, Listgarten MA: Clin Oral Implants Res 1:8, 1990.)
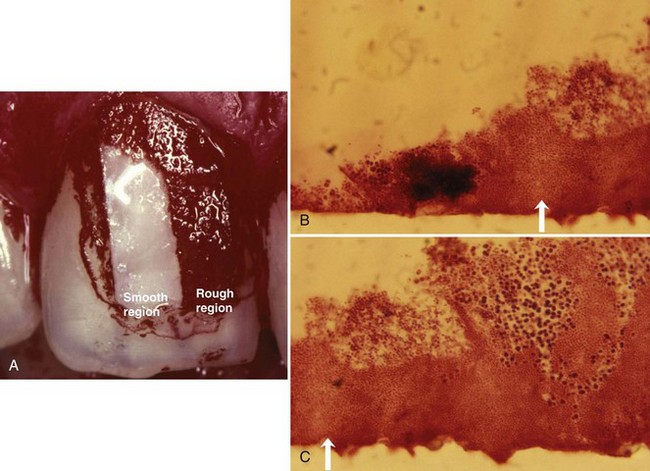
Figure 23-25 A, Small plastic strip, divided in half (a rough region, Ra 2.0 µm located mesially and a smooth region Ra 0.1 µm, distally located), had been glued to the central upper incisors of a patient who refrained from oral hygiene for 3 days. B and C, After removal, the strip had been cut in small slices for microscopic evaluation. It is obvious that the rough part (C) contains a thicker plaque layer than the smooth part (B). Arrow shows border between rough and smooth surface.
Individual Variables Influencing Plaque Formation
The rate of plaque formation differs significantly between subjects, differences that might overrule surface characteristics. A distinction is often made between “heavy” (fast) and “light” (slow) plaque formers (see Video 23-2: Difference in Plaque Growth between Heavy and Light Plaque Former online).
Difference in Plaque Growth between Heavy and Light Plaque Former.
This movie shows undisturbed plaque formation after professional plaque removal over a period of 96 hours in the absence of oral hygiene. The left pane shows the visible plaque formation in a heavy, fast plaque former. The right pane shows the same process in a light, slow plaque-forming patient. Clear differences are noted in the amount of plaque formed after 96 hours between patients.
Simonsson and co-workers selected from a group of 133 individuals one group of heavy and one group of light plaque formers.345 Both groups were investigated for clinical, biochemical, biophysical, and microbiologic variables. In a comparative analysis, there were only minor differences between the groups, and no single variable was considered as the only explanation for the great differences in the rate of plaque formation.
A multiple regression analysis showed that the clinical wettability of the tooth surfaces, the saliva-induced aggregation of oral bacteria, and the relative salivary flow conditions around the sampled teeth explained 90% of the variation. Moreover, the saliva from light plaque formers reduced the colloidal stability of bacterial suspensions of, for example, S. sanguinis.344
In a study by Zee et al, de novo plaque formation was followed on small enamel blocks that were bonded onto the teeth of slow and heavy plaque formers.437 After 1 day, the heavy plaque formers showed more plaque with a more complex supragingival structure. However, from days 3 to 14, there were no discernible differences between both groups, except for a more prominent intermicrobial matrix in the group of fast growers. In another study by the same group of investigators, qualitative differences in the composition of the plaque between slow and rapid plaque formers were detected.436 Rapid plaque formers demonstrated higher proportions of gram-negative rods (35% versus 17%) in 14-day-old plaque. The intersubject variation in plaque formation can also be explained by factors such as diet, chewing fibrous food, smoking, the presence of copper amalgam, tongue and palate brushing, the colloid stability of bacteria in the saliva, antimicrobial factors present in the saliva, the chemical composition of the pellicle, and the retention depth of the dentogingival area.*
Variation within the Dentition
Within a dental arch, large differences in plaque growth rate can be detected. In general, early plaque formation occurs faster: in the lower jaw (when compared to the upper jaw); in molar areas; on the buccal tooth surfaces, when compared to palatal sites (especially in the upper jaw); and in the interdental regions when compared to the buccal or lingual surfaces.102,194,288
Impact of Gingival Inflammation and Saliva
Several studies clearly indicate that early in vivo plaque formation is more rapid on tooth surfaces facing inflamed gingival margins than on those adjacent to healthy gingivae.294,306,307 These studies suggest that the increase in crevicular fluid production enhances plaque formation. Probably, some substance(s) from this exudate (e.g., minerals, proteins, or carbohydrates) favor both the initial adhesion and/or the growth of the early colonizing bacteria. Additionally, it is known that during the night, plaque growth rate is reduced by some 50%.302 This seems surprising, since one would expect that reduced plaque removal and the decreased salivary flow at night would enhance plaque growth. The fact that the supragingival plaque obtains its nutrients mainly from the saliva appears to be of greater significance than the antibacterial activity of saliva.47
Although older studies were contradictory, more recent papers clearly indicate that a subject’s age does not influence de novo plaque formation. In a study by Fransson et al.97 no differences could be detected in de novo plaque formation between a group of young (20 to 25 years of age) and older (65 to 80 years of age) subjects who abolished mechanical tooth cleaning measures for 21 days, neither in amount nor in composition.97
This observation largely confirms data by Holm-Pedersen et al and data by Winkel et al.147,425
The developed plaque in the older patient group resulted, however, in a more severe gingival inflammation, which seems to indicate an increased susceptibility to gingivitis with aging.
Many clinicians still believe that plaque is removed spontaneously from the teeth such as during eating. However, based on the firm attachment between bacteria and surface, this seems unlikely. Even in the occlusal surfaces of the molars, plaque remains, even after chewing fibrous food (carrots, apples, or chips). The inefficiency of the spontaneous plaque removal is neatly illustrated by the clinical pictures in Figure 23-26, taken before and after dinner, starting from 4 days of undisturbed plaque formation. Only negligible differences in plaque extent could be observed.
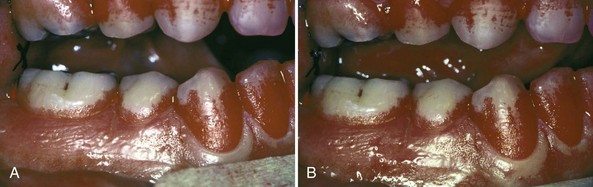
Figure 23-26 Lower premolars and molars from a dental student who refrained from oral hygiene for 100 hours to evaluate undisturbed plaque formation. A is before dinner, and B is after dinner, eating fibrous food. Nearly no reduction in plaque extension could be observed, illustrating the absence of spontaneous plaque removal.
De Novo Subgingival Plaque Formation
It is technically impossible to record the dynamics of subgingival plaque formation in an established dentition for the simple reason that one cannot sterilize a periodontal pocket. Some early studies, using culturing techniques, examined the changes within the subgingival microbiota during the first week after mechanical debridement and reported an only partial reduction around 3 logs (from 108 bacterial cells to 105 cells), followed by a rapid regrowth towards nearly pretreatment levels (−0.5 log) within 7 days.111,136,229 The fast recolonization was explained by several factors. A critical review of the effectiveness of subgingival debridement, for instance, revealed that a high proportion of treated tooth surfaces (5% to 80%) still harbored plaque and/or calculus after scaling. These remaining bacteria were considered the primary source for the subgingival recolonization.276 Some pathogens penetrate the soft tissues or the dentinal tubules and eventually escape instrumentation (see Figure 23-15).4,108,320 In a beagle dog study, Leknes et al studied the extent of subgingival colonization in 6 mm pockets with smooth or rough root surfaces.201 They also observed that smooth surfaces harbored significantly less plaque and concluded that subgingival irregularities shelter submerged microorganisms. Moreover, biopsies of the soft tissues showed an elevated proportion of inflammatory cells in the junctional epithelium (and the underlying connective tissue) facing the rough surfaces.200
Finally, the same group reported higher rates of attachment loss around teeth with groves in the root surface.199
The introduction of oral implants, especially of the two-stage type, provides a new experimental set-up. When the transmucosal part of the implant (the abutment) is inserted on top of the osseointegrated endosseous part, a new “pristine” surface is created on which the intraoral translocation of bacteria can be investigated.291 Recent papers demonstrated that a complex subgingival microbiota, including most periodontopathogens, is established within 1 week after abutment insertion. This is followed by a slow increase in the number of periodontopathogens.101,303,304 Oral implants have also been used as a model to study the impact of surface roughness on subgingival plaque formation.* Smooth abutments (Ra < 0.2 µm) were found to harbor 25 times fewer bacteria than rough ones, with a slightly higher density of coccoid (i.e., nonpathogenic) cells. The subgingival microbiota was also largely dependent on the remaining presence of teeth and the degree of periodontitis in the remaining natural dentition (for review see Quirynen et al293). These observations highlight the importance of intraoral bacterial translocation for subgingival biofilms.
Characteristics of Biofilm Bacteria (Life in “Slime City”)
Metabolism of Dental Plaque Bacteria
The majority of nutrients for dental plaque bacteria originate from saliva or GCF, although the host diet provides an occasional but nevertheless important food supply. The transition from gram-positive to gram-negative microorganisms observed in the structural development of dental plaque is paralleled by a physiologic transition in the developing plaque.
The early colonizers (e.g., Streptococcus and Actinomyces species) use oxygen and lower the redox potential of the environment, which then favors the growth of anaerobic species.75,408 Many of the gram-positive early colonizers use sugars as an energy source. The bacteria that predominate in mature plaque are anaerobic and asaccharolytic (do not break down sugars), and use amino acids and small peptides as energy sources.217
Laboratory studies have demonstrated many metabolic interactions among the different bacteria found in dental plaque (Figure 23-27).
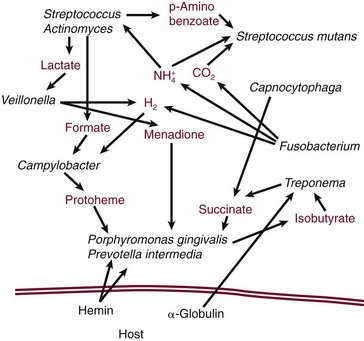
Figure 23-27 Schematic illustration of metabolic interactions among different bacterial species found in plaque, and between the host and plaque bacteria. These interactions are likely to be important to the survival of bacteria in the periodontal environment.
(Based on Carlsson J: Microbiology of plaque associated periodontal disease. In Lindhe J, ed: Textbook of clinical periodontology, Munksgaard, 1983; Munksgaard International Publishers; Grenier D: Infect Immun 60:5298, 1992; Loesche WJ: Periodontics 6:245, 1968; Walden WC, Hentges DJ: Appl Microbiol 30:781, 1975.)
For example, lactate and formate are byproducts of the metabolism of streptococci and actinomyces species and may be used in the metabolism of other plaque microorganisms, including Veillonella spp. and A. actinomycetemcomitans.41,84
The growth of P. gingivalis is enhanced by metabolic byproducts produced by other microorganisms, such as succinate from C. ochracea and protoheme from Campylobacter rectus (see Figure 23-1, R).113,114,233 Overall, the total plaque population is more efficient than any one constituent organism at releasing energy from the available substrates.422
Metabolic interactions occur also between the host and plaque microorganisms.
The bacterial enzymes that degrade host proteins result in the release of ammonia, which may be used by bacteria as a nitrogen source.48 Hemin iron from the breakdown of host hemoglobin may be important in the metabolism of P. gingivalis.33
Increases in steroid hormones are associated with significant increases in the proportions of P. intermedia found in subgingival plaque.181 These nutritional interdependencies are probably critical to the growth and survival of microorganisms in dental plaque and may partly explain the evolution of highly specific structural interactions observed among bacteria in plaque.
Communication Between Biofilm Bacteria
Bacterial cells do not exist in isolation. In a biofilm, bacteria have the capacity to communicate with each other. One example of this is quorum sensing, in which bacteria secrete a signaling molecule that accumulates in the local environment and triggers a response such as a change in the expression of specific genes once they reach a critical threshold concentration. The threshold concentration is reached only at a high-cell density, and therefore bacteria sense that the population has reached a critical mass, or quorum. There is some evidence that intercellular communication can occur after cell-cell contact and in this case, may not involve secreted signaling molecules.155 Two types of signalling molecules have been detected from dental plaque bacteria: peptides released by gram-positive organisms during growth and a “universal” signal molecule autoinducer 2 (AI-2). Peptide signals are produced by oral streptococci and are recognized by cells of the same strain that produced them. Responses are induced only when a threshold concentration of the peptide is attained, and thus the peptides act as cell density, or quorum, sensors. Local concentrations of signalling molecules may be enhanced in biofilms if the signals become trapped in the biofilm matrix. The streptococcal peptides are known as competence-stimulating peptides, since the major response to these signals is the induction of competence, a physiologic state whereby cells are primed for DNA uptake and incorporation.
In some species, such as S. mutans, a small proportion of the cells in a population respond to competence-stimulating peptides by lysing.275 Lysis is considered to be an altruistic behavior that helps to disseminate genetic information throughout the population of S. mutans cells.
In contrast to the strain-specific competence-stimulating peptides, AI-2 is produced and detected by many different bacteria. Detection of AI-2 produces wide-ranging changes in gene expression, in some cases affecting up to one-third of the entire genome.376
Little is known about the specific functions of AI-2 in oral biofilms. However, this molecule has been demonstrated to play a role in mutualistic interactions between S. oralis and A. oris (A. naeslundii).315 Thus, in an in vitro model system, neither S. oralis nor A. oris formed biofilms in monoculture. When cultured together, these organisms grew abundantly on surfaces to form thick, confluent biofilms. This mutualistic behavior was only observed when AI-2 was present: disrupting the gene for AI-2 in S. oralis abrogated mutualistic growth. These data demonstrate that AI-2 is produced and sensed by oral bacteria and suggest that interbacterial communication is important for the development of dental plaque.
Quorum sensing therefore appears to play diverse roles in, for example, modulating the expression of genes for antibiotic resistance, encouraging the growth of beneficial species to the biofilm, and discouraging the growth of competitors.
Interactions between Dental Plaque Bacteria
Several clinical studies followed the detection frequency/relative proportion of cariogenic species after periodontal therapy. They all suggest a relative increase in the number, as well as the detection frequency of S. mutans up to 8 months after mechanical debridement.73,296 In a cross-sectional study, subgingival plaque samples from chronic periodontitis patients were tested for the presence and levels of S. mutans and putative periodontal pathogens.395 They divided the patients into four groups based on the stage of periodontal treatment: untreated, after initial periodontal therapy, maintenance phase without periodontal surgery, and a group of patients after periodontal surgery. The prevalence of mutans streptococci in the four study groups was equivalent. The shift toward a more cariogenic flora observed after initial and surgical periodontal therapy could be explained by a subgingival outgrowth by S. mutans occupying spots that became available after periodontal therapy (e.g., increased number of free adhesion/receptor sites), the creation of a new ecosystem in the subgingival area (a more anaerobic environment, changes in redox potential, pH, and nutrition) that allows/facilitates the growth of S. mutans species, and/or a down growth of S. mutans from the supragingival area in which the species could survive in the saliva. Additionally, Socransky and co-workers showed that the total numbers of P. gingivalis, T. forsythia, and T. denticola are reduced 2 weeks after scaling and root planing, and concomitantly several orange complex organisms are also reduced in number at this point. However, the proportion of S. sanguinis in the population increased 2 weeks after treatment by scaling and root planing in conjunction with azithromycin or metronidazole and 3 months after treatment with doxycycline. Although these observations might be due to an antagonistic relationship between S. sanguinis and periodontal pathogens, they might also reflect the relatively high intrinsic antibiotic resistance of oral streptococci.
There is some evidence from laboratory studies that nonpathogenic organisms in subgingival dental plaque can modify the behavior of periodontal pathogens.
For example, long and short fimbriae of P. gingivalis are required for adhesion and biofilm formation. The expression of long fimbriae is downregulated in the presence of S. cristatus, and short fimbriae are downregulated by S. gordonii, S. mitis, or S. sanguinis.207,268 Changes in bacterial physiology after transitions from monoculture to mixed-species communities may be quite wide ranging.
Using a proteomics approach to probe the phenotype of P. gingivalis, it has been shown that the expression of almost 500 P. gingivalis proteins is changed in model oral microbial communities containing S. gordonii and F. nucleatum.186 At present, it is not clear how these changes affect the interaction between P. gingivalis and the host.
In multispecies biofilms in which many bacteria are juxtaposed to cells of different species, interactions between genetically distinct microorganisms can be mutually beneficial (see Figure 23-4, A). However, there are many examples of competitive interactions between different bacteria (Figure 23-28).
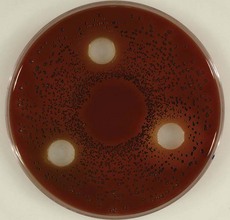
Figure 23-28 The growth of Prevotella intermedia (black colonies) is inhibited by the presence of S. mitis, S. salivarius, and S. sanguinis (in the white holes). This is represented by a zone of no growth (halo) around the white holes. This is a typical example of growth inhibition induced by streptococci.
For example, S. mutans produces antimicrobial peptides that have broad activity against bacteria in vitro.184 Other oral streptococci compete with S. mutans by excreting the strongly oxidizing molecule H2O2.184
In fact, S. oligofermentans can convert lactic acid produced by S. mutans into H2O2, which then kills the S. mutans cells.390
In subgingival biofilms, oxygen is scarce and therefore H2O2 production by bacteria is low. Oxidizing agents may be more important in the interaction between host and pathogen, since reactive oxygen species are a major component of the neutrophil response to bacteria.
Another example of competitive interaction exists between streptococci and periodontopathogens. S. sanguinis, S. salivarius, and S. mitis have been shown to inhibit hard and soft tissue colonization of A. actinomycetemcomitans (see Video 23-3: Colonization Inhibition online), P. gingivalis, and Prevotella intermedia in vitro.351,383,397
This movie shows the colonization of a glass surface by a green fluorescent protein labeled A. actinomycetemcomitans (green dots) strain over a 3-hour period. The colonization was visualized in a flow cell mounted on a confocal laser scanning microscope. The left panels show the colonization of a virgin surface while the middle and right panel show the colonization of a glass surface that was coated with either S. mitis or S. sanguinis. It is clearly visible that both streptococci inhibit A. actinomycetemcomitans colonization over time. S. sanguinis was clearly the best inhibitor of A. actinomycetemcomitans colonization.
Surprisingly, when these bacteria were injected in freshly root-planed periodontal pockets in vivo, a significant inhibitory effect was observed on the recolonization of the periodontal pocket by periodontopathogens.384 These observations highlight the importance of investigating nonpathogenic bacteria in periodontal biofilms, in addition to studying the (putative) pathogens, and might open doors for new treatment approaches.
Recent studies also show that these interactions also can influence the host. Although in the study by Teughels and co-workers, no clinical effect was expected, a reduction in bleeding on probing was noted in pockets that received the streptococci.384 At that time, this was explained by the establishment of a more host-compatible microbiota. However, an interaction with the host via the immune system seems an additional interesting hypothesis. Oral streptococci have been shown to modulate bacteria-host interactions involving both F. nucleatum and A. actinomycetemcomitans. Thus S. cristatus and other oral streptococci attenuate the ability of F. nucleatum to stimulate interleukin-8 (IL-8) production by host oral epithelial cells.438 Sliepen and co-workers recently showed similar results for the streptococci that were used in the study of Teughels and co-workers.352
Another interesting example is the S. gordonii–induced increased expression of a complement resistance protein, ApiA, in A. actinomycetemcomitans, resulting in increased resistance to killing by host serum.309
Biofilms and Antimicrobial Resistance
Bacteria growing in microbial communities adherent to a surface do not “behave” the same way as bacteria growing suspended in a liquid environment (in a planktonic or unattached state). For example, the resistance of bacteria to antimicrobial agents is dramatically increased in the biofilm.8,65,139,284 Almost without exception, organisms in a biofilm are 1000 to 1500 times more resistant to antibiotics than in their planktonic state. The mechanisms of this increased resistance differ from species to species, from antibiotic to antibiotic, and for biofilm growing in different habitats.
It is generally accepted that the resistance of bacteria to antibiotics is affected by their nutritional status, growth rate, temperature, pH, and prior exposure to subeffective concentrations of antimicrobial agents.39,40,423 Variations in any of these parameters will thus lead to a varied response to antibiotics within a biofilm. An important mechanism of resistance appears to be the slower rate of growth of bacterial species in a biofilm, which makes them less susceptible to many but not all antibiotics.18,37,65,431 The biofilm matrix, although not a significant physical barrier to the diffusion of antibiotics, does have certain properties that can retard antibiotic penetration.
For example, strongly charged or chemically highly reactive agents can fail to reach the deeper zones of the biofilm because the biofilm acts as an ion-exchange resin removing such molecules from solution.106,378
In addition, extracellular enzymes such as β-lactamases, formaldehyde lyase, and formaldehyde dehydrogenase may become trapped and concentrated in the extracellular matrix, thus inactivating some antibiotics (especially positively charged hydrophilic antibiotics).
Some antibiotics, such as the macrolides, which are positively charged but hydrophobic, are unaffected by this process.
Recently, “super-resistant” bacteria were identified within a biofilm. These cells have multidrug resistance pumps that can extrude antimicrobial agents from the cell.37 Since these pumps place the antibiotics outside the outer membrane, the process offers protection against antibiotics that target, for example, cell wall synthesis. The penetration and efficacy of antimicrobials against biofilm bacteria are critical issues for the treatment of periodontal infections.
Antibiotic resistance may be spread through a biofilm by intercellular exchange of DNA. The high density of bacterial cells in a biofilm facilitates the exchange of genetic information among cells of the same species and across species and even genera. Conjugation (the exchange of genes through a direct interbacteria connection formed by a sex pilus), transformation (movement of small pieces of DNA from the environment into the bacterial chromosome), plasmid transfer, and transposon transfer have all been shown to occur in biofilms.
Bacterial Transmission and Translocation
Transmission of pathogens, from one locus to another, is an important aspect of infectious diseases. In theory, such transmission may jeopardize the outcome of periodontal therapy but also transmission of pathogens from one person to another might be important in terms of disease transmission. The significance of such an intraoral or a vertical or horizontal transmission is, however, difficult to prove and/or to quantify.
One of the first questions in this regard is: “are oral bacteria transmissible between humans?” Molecular fingerprinting techniques clearly illustrated that periodontal pathogens are transmissible within members of a family434 (Figure 23-29).
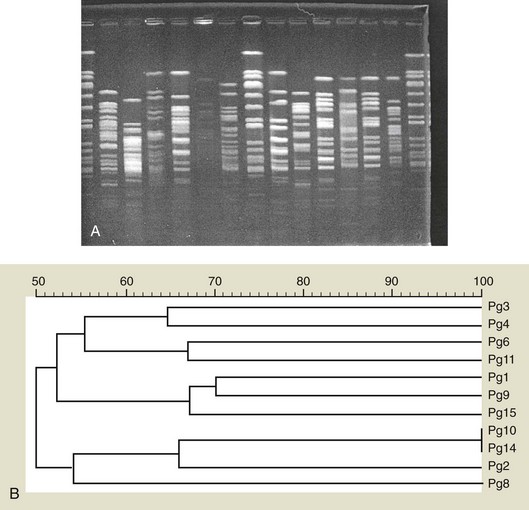
Figure 23-29 A, Band pattern of DNA isolated from multiple Porphyromonas gingivalis strains after pulsed-field gel electrophoresis. Lanes 1, 8, and 15 contain a reference strain DNA (Staphylococcus aureus) cut with SmaI (a restriction endonuclease) in lanes 2 to 7 and 9 to 14; 12 different P. gingivalis strains are compared on their genetic similarity. The genetic similarity is expressed in a dendrogram (B). Bacteria are considered genetically similar when their band pattern is similar for 80%.
This bacterial transmission between subjects (and even between animals and human beings) should not be confused with contagion (the term contagious referring to the likelihood of a microorganism being transmitted from an infected to an uninfected host to create disease). Asikainen and co-workers used a genetic fingerprinting method termed arbitrarily primed-polymerase chain reaction (PCR) to genotype A. actinomycetemcomitans isolates from family members.19 In 11 of 12 families, they found identical A. actinomycetemcomitans genotypes among family members. This suggests transmission of this microorganism among family members.
However, it was more often encountered that children carried a genotype identical to one of the parents than that spouses carried an identical genotype. This indicates that in terms of A. actinomycetemcomitans transmission, vertical transmission is more important than horizontal transmission. Similar observations were made for the transmission of cariogenic species from mother to child.168 Vertical or horizontal transmission of P. gingivalis has rarely been observed.401
The existence of an “intraoral” translocation of bacteria (from one niche to another) has been investigated more thoroughly.
Intraoral transmission of bacteria was first examined in cariology. Loesche and co-workers showed that streptomycin-resistant strains of S. mutans, grown on a dental inlay, were spontaneously transmitted to the neighboring teeth (probably via saliva) and could even reach the contralateral quadrant after transmission with a dental explorer.219 Previously, Edman and co-workers had been successful in implanting the previously mentioned species in two volunteers by means of inoculated dental flosses.82 Comparable observations have been made for periodontopathogens.
Christersson and co-workers demonstrated a translocation of A. actinomycetemcomitans via periodontal probes in localized juvenile periodontitis patients.57 They were able to successfully colonize previously noninfected pockets with A. actinomycetemcomitans by a single course of probing with a probe previously inserted in an infected pocket of the same patient. Although the colonization was only temporary, the question remained whether the colonization could not have become permanent if the site had offered more suitable growth conditions (e.g., a deep bleeding pocket, as frequently encountered immediately after initial periodontal therapy). It is well established that colonization of an already established microbial niche by a new species is difficult since it is hampered by a variety of microbial interactions. Therefore intraoral translocation to and colonization of sterile surfaces might be different from translocation and colonization of already colonized surfaces. Within the oral cavity, such sterile surfaces are not naturally occurring. However, at the moment of abutment placement on a dental implant, a sterile subgingival pocket is created. A large series of papers compared the microbiota in pockets around teeth with that in periimplant pockets in partially edentulous patients and reported a striking similarity.* Several recent studies have investigated the subgingival colonization of such “sterile” periimplant pockets in partially edentulous patients. It was shown that translocation to and colonization of these pockets is extremely fast. Periodontopathogens could already be detected in this niche 30 minutes after abutment placement.101 The initial colonization of these pockets with periodontopathogens is completed within 1 week. After 2 weeks there are only minor additional changes in this newly developed microbiota.101,303,305 These additional changes reflect primarily changes in the numbers of species. Based on the previously mentioned similarity between the microbial populations associated with teeth and implants, it has been suggested that at least in partially edentulous patients, teeth might act as a reservoir for the (re)colonization of the subgingival area around implants. This hypothesis was supported by Sumida and co-workers who detected similar intrasubject pulsed field gel electrophoresis patterns for P. gingivalis from teeth and implants (indicating that these locations contained precisely the same P. gingivalis strains), compared to large intersubject variations.374
The clinical significance of intraoral translocation can be derived from several studies.
Nowzari and co-workers evaluated the amount of guided tissue regeneration and membrane contamination after the treatment of mandibular bony defects in either a group of patients with a healthy periodontium in the remaining dentition, or a group of patients with multiple deep pockets and numerous pathogens.257 The healthy group showed significantly less membrane contamination both immediately after insertion and at their removal after 6 weeks. The healthy group showed concurrently significantly more clinical gain in attachment (3.4 mm versus 1.4 mm).
Mombelli and co-workers compared the clinical and microbial changes when tetracycline fibers (local application of antibiotics) were applied only to the two deepest pockets in the mouth (without further treatment to the remaining pockets) to the changes obtained when all teeth were cleaned and every pocket with a depth >3 mm was treated.245 After 6 months, significant “additional” improvements (clinical, as well as microbiologic) were recorded in the group of patients with the more global approach. Their pockets showed a probing depth reduction (1.7 mm) and attachment gain (0.7 mm) that was significantly higher than in the pockets of patients with some remaining pockets (0.9 and 0.3 mm, respectively). The authors concluded that most likely pathogens were transferred via saliva from infected untreated periodontal lesions or other niches to the treated site(s).
Quirynen and co-workers used a treatment strategy called “one-stage, full-mouth disinfection” to investigate the impact of intraoral translocation on the outcome of nonsurgical periodontal therapy.292 This strategy attempted to suppress translocation of periodontopathogens from all their intraoral habitats (mucous membranes, tongue, and saliva) to root-planed periodontal pockets. Several comparative studies between the one-stage, full-mouth approach and the standard therapy (root planing per quadrant with 2 week intervals that allows translocation) from the Leuven group illustrated the impact of bacterial translocation in relation to the obtained gain in attachment, pocket depth reduction, and changes in the microbiota.293
Nonbacterial Inhabitants of The Oral Cavity
Oral microbiology is often reduced to oral “bacteriology.” However, the oral cavity comprises a much more diverse microbiota than merely a bacterial one. Viruses, fungi, archaea, and protozoa can be encountered in the oral cavity of humans. Many of these species can reside in the oral cavity, similar to oral bacteria, in harmony with the host as commensals, but they also can cause several oral diseases.
Viruses
Viral diseases of the oral mucosa and the perioral region are often encountered in dental practice. Viruses are important ulcerogenic and tumorigenic agents of the human mouth. The finding of an abundance of mammalian viruses in periodontitis lesions may suggest a role for viruses in more oral diseases than previously recognized.341 A more in-depth review can be found in Slots.356
In contrast to most bacteria, viruses replicate only when present within eukaryotic (animals, plants, protists, and fungi) or prokaryotic (bacteria and archaea) cells, and not on their own. The extracellular virion particle ranges in size from 20 nm to 300 nm and consists of either DNA or ribonucleic acid (RNA) contained within a protective protein coat or capsid (Figure 23-30).
Figure 23-30 Electron micrographic picture of herpes simplex virus type 1, enveloped (left) and naked capsids (right). (Magnification ×198,000.)
(From Fields BN, Knipe DM: In Fields BN, eds: Fields’ virology, New York, 1985, Raven Press.)
Some viruses have an additional envelope comprising a lipid bilayer derived from the outer cellular membrane, the internal nuclear membrane, or the endoplasmic reticulum membrane of the infected cell. Four major viral families are associated with the main viral oral diseases of adults, as follows:
Herpesviruses
Herpesvirus virions vary in size from 120 to 250 nm and consist of a double-stranded linear DNA molecule surrounded by an icosahedral capsid, a proteinaceous tegument, and a lipid-containing envelope with embedded viral glycoproteins.273 Eight human herpesvirus species with distinct biologic and clinical characteristics have been described: herpes simplex virus-1, herpes simplex virus-2, varicella-zoster virus, Epstein-Barr virus (EBV), human cytomegalovirus (hCMV), human herpesvirus-6, human herpesvirus-7, and human herpesvirus-8 (see Table 23-5). Typically, after a primary infection, each herpesvirus subfamily maintains latent infection in specific cell population(s). In the oral cavity, an infection with herpesviruses can present itself as a primary herpetic stomatitis, herpes labialis (cold sores), chickenpox (varicella), or shingles (zoster). In contrast to a chronic infection, this latency is not associated with the production of infectious virions. Additionally, a single individual can simultaneously show herpesvirus latent infection in some cells and active viral infection in other cells. Herpesviruses express proteins during the normal lytic and latent viral life cycle that can interfere with activities of the innate and adaptive immune systems and alter the cellular environment. The alteration of the host defense ensures a lifelong persistence of the viruses in the infected host and can contribute to disease development. Herpesvirus active infections can remain asymptomatic but still give rise to viral shedding, or can cause illness ranging from classic infectious diseases to benign and malignant tumors, especially in immunocompromised hosts such as patients with acquired immunodeficiency syndrome (AIDS) and organ transplant recipients.
Papillomaviruses
The papillomaviruses comprise a group of small, epitheliotropic, nonenveloped, icosahedral, double-stranded and circular DNA viruses (see Table 23-6). Human papillomaviruses are grouped within 5 genera and comprise more than 100 types. They can induce benign lesions of the skin (warts) and mucous membranes (condylomas) but they can also cause epithelial malignancies such as cancer of the uterine cervix, certain types of anal cancer, vulvar cancer, penile cancer, laryngeal cancer, and oral cancer. Type 16 papillomaviruses have been implicated in one-third of oropharyngeal squamous cell carcinomas and show a particularly strong relationship with cancer of the tonsils.128,145 Head and neck cancers related to papillomaviruses exhibit relatively high mortality rates despite early diagnosis and treatment.
Picornaviruses/Enteroviruses
Picornaviruses are nonenveloped, single-stranded, positive-sense RNA viruses with a virion diameter of approximately 30 nm.265 This family of viruses consists of nine genera and contains several serious pathogens, including poliovirus, hepatitis A virus, rhinovirus, and the hand, foot and mouth disease virus (see Table 23-7).
The enteroviruses form a specific genus in the picornavirus family. They include Coxsackievirus, echovirus, poliovirus, and human enterovirus. Enteroviruses are common human pathogens that are associated with a broad spectrum of clinical presentations ranging from asymptomatic infections, various enanthems and exanthems, respiratory diseases, aseptic meningitis, severe illness in newborns and immunocompromised hosts, and fatal outcomes. They replicate in the alimentary tract.261