CHAPTER 38 Periodontal Therapy in the Female Patient
Throughout a woman’s life cycle, hormonal influences affect therapeutic decision making in periodontics. Historically, therapies have been gender biased. However, the advent of new research has provided keener appreciation of the unique systemic influences on oral, periodontal, and implant tissues. Oral health care professionals have greater awareness of and better capabilities for dealing with hormonal influences associated with the reproductive process. Periodontal and oral tissue responses may be altered, creating diagnostic and therapeutic dilemmas. Therefore it is imperative that the clinician recognize, customize, and appropriately alter periodontal therapy according to the individual woman’s needs based on the stage of her life cycle.
This chapter reviews phases of the female life cycle from puberty through menopause. Periodontal manifestations, systemic effects, and clinical management are presented.
Puberty
Puberty occurs between the average ages of 11 to 14 in most women. The production of sex hormones (estrogen and progesterone) increases, then remains relatively constant during the remainder of the reproductive phase. Also, the prevalence of gingivitis increases, without an increase in the amount of plaque. Gram-negative anaerobes, especially Prevotella intermedia, have been implicated in association with puberty gingivitis. Kornman and Loesche56 postulated that this anaerobic organism may use ovarian hormone as a substitute for vitamin K growth factor. Levels of black-pigmented Bacteroides, especially P. intermedia (formerly known as Bacteroides intermedius), are thought to increase with increased levels of gonadotropic hormones in puberty. Capnocytophaga species also increase in incidence, as well as in proportion. These organisms have been implicated in the increased bleeding tendency observed during puberty.
Recent studies associated with pubertal gingivitis indicate proportionately elevated motile rods, spirochetes, and P. intermedia.79 Statistically significant increases in gingival inflammation and in the proportions of P. intermedia and P. nigrescens have been seen in pubertal gingivitis.83 A recent study of 11- to 17-year-old adolescents found higher levels of Actinobacillus actinomycetemcomitans and Fusobacterium nucleatum, which were associated with bleeding indices, probing depth, and attachment loss.69
During puberty, periodontal tissues may have an exaggerated response to local factors. A hyperplastic reaction of the gingiva may occur in areas where food debris, materia alba, plaque, and calculus are deposited. The inflamed tissues become erythematous, lobulated, and retractable. Bleeding may occur easily with mechanical debridement of the gingival tissues. Histologically, the appearance is consistent with inflammatory hyperplasia.
During the reproductive years, women tend to have a more vigorous immune response, including higher immunoglobulin concentrations, stronger primary and secondary responses, increased resistance to the induction of immunologic tolerance, and a greater ability to reject tumors and homografts.114 Allergy, sensitivity, and asthma occur more often in young men, but after puberty, women become more susceptible than their male counterparts.
Management
During puberty, education of the parent or caregiver is part of successful periodontal therapy. Preventive care, including a vigorous program of oral hygiene, is also vital.5 Milder gingivitis cases respond well to scaling and root planing, with frequent oral hygiene reinforcement. Severe cases of gingivitis may require microbial culturing, antimicrobial mouthwashes and local site delivery, or antibiotic therapy. Periodontal maintenance appointments may need to be more frequent when periodontal instability is noted.
The clinician should recognize the periodontal manifestations and/or intraoral lesions reflected by systemic diseases (i.e., diabetes).24,91 Thorough review of the patient’s medical history and medical referral should occur when deemed necessary. The clinician should be aware of the effects of chronic regurgitation of gastric contents on intraoral tissues; this age group also is susceptible to eating disorders, namely, bulimia and anorexia nervosa. Perimolysis (smooth erosion of enamel and dentin), typically on the lingual surfaces of maxillary anterior teeth, varies with the duration and frequency of the behavior.16 Also, enlargement of the parotid glands (occasionally sublingual glands) has been estimated to occur in 10% to 50% of patients who “binge and purge.”73 Therefore a diminished salivary flow rate may also be present, which will increase oral mucous membrane sensitivity, gingival erythema, and caries susceptibility.
Menses
Periodontal Manifestations
During the reproductive years, the ovarian cycle is controlled by the anterior pituitary gland. The gonadotropin follicle-stimulating hormone (FSH) and luteinizing hormone (LH) are produced from the anterior pituitary gland. The secretion of gonadotropins also depends on the hypothalamus. Ongoing changes in the concentration of the gonadotropins and ovarian hormones occur during the monthly menstrual cycle (Figure 38-1). Under the influence of FSH and LH, estrogen and progesterone are steroid hormones produced by the ovaries during the menstrual cycle. During the reproductive cycle, the purpose of estrogen and progesterone is to prepare the uterus for implantation of the egg.
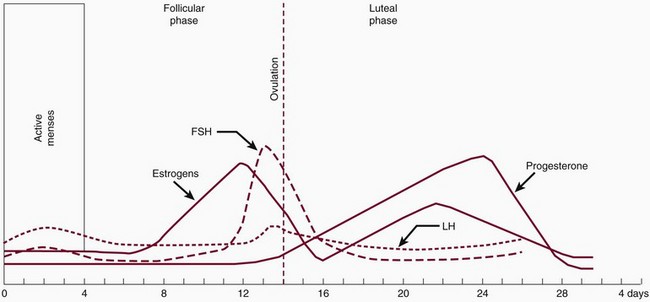
Figure 38-1 Female reproductive cycle. Note the peak levels of progesterone and estrogen compared with follicle-stimulating hormone (FSH) and luteinizing hormone (LH).
The monthly reproductive cycle has two phases. The first phase is referred to as the follicular phase. Levels of FSH are elevated, and estradiol (E2), the major form of estrogen, is synthesized by the developing follicle and peaks approximately 2 days before ovulation. The effect of estrogen stimulates the egg to move down the fallopian tubules (ovulation) and stimulates proliferation of the stroma cells, blood vessels, and glands of the endometrium.
The second phase is called the luteal phase. The developing corpus luteum synthesizes both estradiol and progesterone. Estrogen peaks at 0.2 ng/ml and progesterone at 10.0 ng/ml to complete the rebuilding of the endometrium for implantation of the fertilized egg. The corpus luteum involutes, ovarian hormone levels drop, and menstruation ensues. It has been postulated that ovarian hormones may increase inflammation in gingival tissues and exaggerate the response to local irritants. Gingival inflammation seems to be aggravated by an imbalance or increase in sex hormones. Numerous studies have demonstrated in vitro and in vivo that sex hormones affect and modify the actions of cells of the immune system. In addition, evidence suggests that the interaction between estrogen and cells of the immune system can have nonimmune regulatory effects.7,20 Possible mechanisms have been suggested for the increase in hormonal gingival interaction in the menstrual cycle. Tumor necrosis factor alpha (TNF-α), which fluctuates during the menstrual cycle13; elevated prostaglandin E2 (PGE2) synthesis75; and angiogenetic factors, endothelial growth factors, and receptors may be modulated by progesterone and estrogen, contributing to increases in gingival inflammation during certain stages of the menstrual cycle.
Progesterone has been associated with increased permeability of the microvasculature, altering the rate and pattern of collagen production in the gingiva,70 increasing folate metabolism,95,124 and altering the immune response. During menses, progesterone increases from the second week, peaks at approximately 10 days, and dramatically drops before menstruation. (Note that this is based on a 28-day cycle; individual cycles are variable.) Progesterone plays a role in stimulating the production of prostaglandins that mediate the body’s response to inflammation. PGE2 is one of the major secretory products of monocytes and is increased in inflamed gingiva. Miyagi et al76 found that the chemotaxis of polymorphonuclear leukocytes (PMNs, neutrophils) was enhanced by progesterone but reduced by estradiol. Testosterone did not have a measurable effect on PMN chemotaxis. The researchers suggested that the altered PMN chemotaxis associated with gingival inflammation may be caused by the effects of sex hormones. Physiologic, experimental, and clinical data confirm differences in immune responses between the two sexes.133
Gingival tissues have been reported to be more edematous during menses and erythematous before the onset of menses in some women. A recent study reported higher gingival indices during ovulation and before menstruation despite reported increases in oral symptoms during menses.71 In addition, an increase of gingival exudate has been observed during the menstrual period and is sometimes associated with a minor increase in tooth mobility.39 The incidence of postextraction osteitis is also higher during initiation of menses. No significant hematologic laboratory findings accompany this, other than a slightly reduced platelet count and a slight increase in clotting time.
When the progesterone level is highest (during luteal phase of cycle), intraoral recurrent aphthous ulcers,33 herpes labialis lesions, and candidal infections occur in some women as a cyclic pattern. Because the esophageal sphincter is relaxed by progesterone, women may be more susceptible to gastroesophageal reflux disease (GERD) during this time of the cycle as well. Symptoms of GERD include heartburn, regurgitation, and chest pain, and when reflux is severe, some patients develop unexplained coughing, hoarseness, sore throat, gingivitis, and asthma.108
Management
During the peak level of progesterone (about 7 to 10 days before menstruation), premenstrual syndrome (PMS) may also occur. There appears to be no significant difference in the estrogen and progesterone levels between women with PMS and those without PMS. However, women with PMS seem to have lower levels of certain neurotransmitters such as enkephalins, endorphins, γ-aminobutyric acid (GABA), and serotonin. Depression, irritability, mood swings, and difficulty with memory and concentration may be symptoms of neurotransmitter reduction. Patients are more sensitive and less tolerant of procedures, have a heightened gag reflex, and may have an exaggerated response to pain.
Increased gingival bleeding and tenderness associated with the menstrual cycle require closer periodontal monitoring. Periodontal maintenance should be titrated to the individual patient’s need and if problematic, 3- to 4-month intervals should be recommended. An antimicrobial oral rinse before cyclic inflammation may be indicated. Emphasis should be placed on oral hygiene. For the patient with a history of excessive postoperative hemorrhage or menstrual flow, scheduling surgical visits after cyclic menstruation is prudent. Anemia is common, and appropriate consultation with a physician and recent laboratory tests, when indicated, should be maintained.
During PMS, many women exhibit physical symptoms that include fatigue, sweet and salty food cravings, abdominal bloating, swollen hands or feet, headaches, breast tenderness, nausea, and gastrointestinal upset. GERD may make it more uncomfortable for the patient to lay fully supine, especially after a meal, and the woman may have a more sensitive gag reflex. The clinician should be aware that nonsteroidal antiinflammatory drugs (NSAIDs), infection, and acidic foods exacerbate GERD. Patients taking over-the counter antacids, H2-receptor antagonists (cimetidine, famotidine, nizatidine, ranitidine), prokinetic agents (cisapride, metoclopramide), and proton pump inhibitors (lansoprazole, omeprazole, pantoprazole, or rabeprazole)109 may be GERD patients. These medications interact with some antibiotics and antifungals, and thus a review of their pharmacology is necessary. Fluoride rinses and trays, frequent periodontal debridement, and avoidance of mouthwashes with high alcohol content may reduce the associated gingival and caries sequelae.
PMS is often treated by antidepressants. Selective serotonin reuptake inhibitors (SSRIs) are generally the first-line choice because they have fewer side effects than other antidepressants, do not require blood monitoring, and are safe in overdoses. Women with PMS taking the SSRI fluoxetine were reported to have a 70% response rate. Fluoxetine was ranked the fifth most dispensed prescription (new and refills) in the United States (US) in 1998, but when the patent was lifted, its sales slowed. However, overall SSRIs ranked second in total dollar sales in the 2000s. (Sertraline was ranked twelfth and is the drug of choice for treatment of PMS.141) The clinician should be aware that patients taking fluoxetine have increased side effects with highly protein-bound drugs (e.g., aspirin), and the half-life of diazepam and other central nervous system (CNS) depressants is increased. Additional common SSRIs are fluvoxamine, paroxetine, and citalopram. Other antidepressants that may be prescribed are the selective serotonin and norepinephrine reuptake inhibitors (SNRIs), tricyclics, trazodone, mirtazapine, nefazodone, and maprotiline.
The PMS patient may be difficult to treat because of emotional and physiologic sensitivity. The dentist should treat the gingival and oral mucosal tissues gently. Gauze pads or cotton rolls should be moistened with a lubricant, chlorhexidine rinse, or water before placing them in the aphtha-prone patient. Careful retraction of the oral mucosa, cheeks, and lips is necessary in patients prone to aphthous or herpetic lesions. Because the hypoglycemic threshold is elevated, the clinician should advise the patient to have a light snack before her appointment. Of menstruating women, 70% have PMS symptoms, but only 5% meet the strict diagnostic criteria.
Pregnancy
Periodontal Manifestations
The link between pregnancy and periodontal inflammation has been known for many years. In 1778, Vermeeren discussed “toothpains” in pregnancy. In 1818, Pitcarin101 described gingival hyperplasia in pregnancy. Despite awareness regarding pregnancy and its effect on periodontal disease, only recently has evidence indicated an inverse relationship to systemic health. Current research implies periodontal disease may alter the systemic health of the patient and adversely affect the well-being of the fetus by elevating the risk for low-birth-weight, preterm infants.
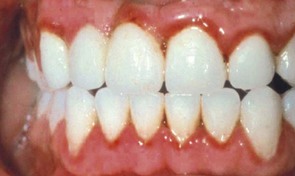
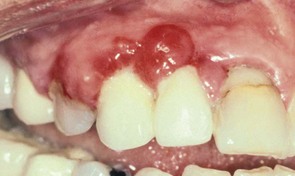
In 1877, Pinard100 recorded the first case of “pregnancy gingivitis.” Only recently has periodontal research begun to focus on causative mechanisms. The occurrence of pregnancy gingivitis is extremely common, occurring in 30% to 100% of all pregnant women.44,61,65,112 It is characterized by erythema, edema, hyperplasia, and increased bleeding. Histologically, the description is the same as for gingivitis. However, the etiologic factors are different despite clinical and histologic similarities. Cases range from mild-to-severe inflammation (Figure 38-2), which can progress to severe hyperplasia, pain, and bleeding (Figures 38-3 and 38-4).
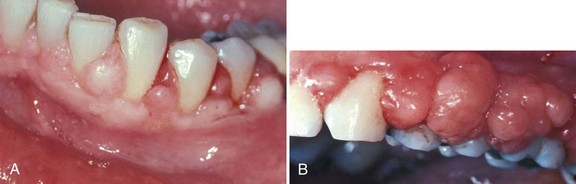
Figure 38-4 Severe pregnancy gingivitis with hyperplasia in patients with poorly controlled non–insulin-dependent diabetes mellitus. A, Moderate gingival enlargement. B, Severe gingival enlargement.
Other growths that resemble pregnancy granulomas must be ruled out, such as central giant cell granulomas or underlying systemic diseases. Periodontal status before pregnancy may influence the progression or severity as the circulating hormones fluctuate. The anterior region of the mouth is affected more often, and interproximal sites tend to be most involved.25 Increased tissue edema may lead to increased pocket depths and may be associated with transient tooth mobility.102 Anterior site inflammation may be exacerbated by increased mouth breathing, primarily in the third trimester, from pregnancy rhinitis. The gingiva is the most common site involved (approximately 70% of all cases), followed by the tongue and lips, buccal mucosa, and palate.112 An increase in attachment loss may represent active periodontal infection accelerated by pregnancy.60
Pyogenic granulomas (“pregnancy tumors,” pregnancy epulis) occur in 0.2% to 9.6% of pregnancies. They are clinically and histologically indistinguishable from pyogenic granulomas occurring in nonpregnant women or in men. Pyogenic granulomas appear most often during the second or third month of pregnancy. Clinically, they bleed easily and become hyperplastic and nodular. When excised, the lesions usually do not leave a large defect. They may be sessile or pedunculated and ulcerated, ranging in color from purplish red to deep blue, depending on the vascularity of the lesion and degree of venous stasis.10 The lesion classically occurs in an area of gingivitis and is associated with poor oral hygiene and calculus. Alveolar bone loss is usually not associated with pyogenic granulomas of pregnancy.
Role of Pregnancy Hormones
Subgingival Plaque Composition
Epidemiologic studies indicate a relationship between the level of home care and the severity of gingival inflammation. It appears that the association between signs of gingival inflammation and the amount of plaque is greater after parturition than during pregnancy. An alteration in the compositions of subgingival plaque occurs during pregnancy. As mentioned, Kornman and Loesche57 found that during the second trimester, gingivitis and gingival bleeding increased, without an increase in plaque levels. Bacterial anaerobic/aerobic ratios increased, in addition to proportions of Bacteroides melaninogenicus and P. intermedia (2.2% to 10.1%). The authors suggested that estradiol or progesterone can substitute for menadione (vitamin K) as an essential growth factor for P. intermedia but not Porphyromonas gingivalis or Eikenella corrodens. There was also an increase in P. gingivalis during the 21st through 27th weeks of gestation, but this was not statistically significant. The relative increase in the numbers of P. intermedia may be a more sensitive indicator of an altered systemic hormonal situation than clinical parameters of gingivitis.117 A recent study concluded subgingival levels of bacteria associated with periodontitis did not change. P. gingivalis and Tannerella forsythia counts were higher and associated with bleeding on probing at week 12.2
Periodontal Disease and Preterm, Low-Birth-Weight Infant
Although the greatest majority of studies support a causal relationship regarding the hypothesis that periodontitis during pregnancy poses an increased risk for adverse pregnancy outcomes, there are conflicting results. Variation in study results may be due to confounding factors, effect modifiers, population studied, timing of intervention or evaluation, and severity of periodontal disease caused by varying definitions.
Several systematic reviews indicate periodontal disease113,128,135,137 may adversely effect pregnancy outcomes. Intervention trials have shown a positive effect with periodontal therapy and reduction of adverse pregnancy outcomes,49,67,68,90,122 but three large multicenter trials in the US did not support these results.74,87,118 Studies do indicate that routine nonsurgical periodontal therapy after the first trimester is not associated with adverse pregnancy outcomes.74
Initially, Offenbacher et al89 provided evidence that untreated periodontal disease in pregnant women may be a significant risk factor for preterm (<37 weeks’ gestation), low-birth-weight (<2500 g) infants. The relationship between genitourinary tract infection and preterm, low-birth-weight (PLBW) infants is well documented in human and animal studies. Periodontal researchers, suspecting periodontal disease as another source of infection, found that otherwise low-risk mothers of PLBW infants had significantly more periodontal attachment loss than control mothers having normal-weight infants at birth.
The current opinion is that the correlation of periodontal disease to PLBW births may occur as a result of infection and is mediated indirectly, principally by the translocation of bacterial products such as endotoxin (lipopolysaccharide [LPS]) and the action of maternally produced inflammatory mediators.36 Jared et al47 noted in utero fetal exposure to oral pathogens increases the risk of neonatal intensive care unit admission and length of stay. Biologically active molecules, such as PGE2 and TNF-α, which are normally involved in normal parturition, are raised to artificially high levels by the infection process, which may foster premature labor.3 Gram-negative bacteria in periodontal diseases therefore may permit selective overgrowth or invasion of gram-negative bacteria within the genitourinary tract.
Gingival crevicular fluid (GCF) levels of PGE2 were positively associated with intraamniotic PGE2 levels (p = 0.018), suggesting that gram-negative periodontal infection may present a systemic challenge sufficient to initiate the onset of premature labor as a source of LPS, or through stimulation of secondary inflammatory mediators such as PGE2 and interleukin-1 beta (IL-1β).21 Offenbacher et al88 suggested a dose-response relationship for increasing GCF PGE2 as a marker of current periodontal disease activity and decreasing birth weight. Four organisms associated with mature plaque and progressing periodontitis—T. forsythia, P. gingivalis, A. actinomycetemcomitans, and Treponema denticola—were detected at higher levels in PLBW mothers compared with normal-birth-weight controls (see Chapter 28). Despite research supporting the association of periodontal disease and PLBW,22,23 more studies with improved methodology are needed to assess the validity of the association.
Preeclampsia
A systematic review of preeclampsia and periodontitis indicated an increased risk during pregnancy. Preeclampsia is a life-threatening condition in late pregnancy characterized by high blood pressure and excess urine protein. High C-reactive protein levels also are associated with preeclampsia in this population.111,115,127
Maternal Immunoresponse
The maternal immune system is thought to be suppressed during pregnancy. This response may allow the fetus to survive as an allograft. Documentation of immunosuppressive factors in the sera of pregnant women can be noted by marked increase of monocytes (which in large numbers inhibit in vitro proliferative responses to mitogens, allogenic cells, and soluble antigen),126 and pregnancy-specific βl-glycoproteins contribute to diminished lymphocyte responsiveness to mitogens and antigens.11 In addition, a decrease in the ratio of peripheral T helper cells to T suppressor cells (CD4/CD8) has been reported to occur throughout pregnancy.104 These changes in maternal immunoresponsiveness suggest an increased susceptibility to developing gingival inflammation. In one study, the gingival index was higher, but percentages of T3, T4, and B cells appeared to decrease in peripheral blood and gingival tissues during pregnancy compared with a control group.1 Other studies report decreased PMN (neutrophil) chemotaxis, depression of cell-mediated immunity, phagocytosis, and a decreased T-cell response with elevated ovarian hormone levels, especially progesterone.103 Decreased in vitro responses of peripheral blood lymphocytes to several mitogens and to a preparation of P. intermedia have been reported.12,66,86 Also, evidence suggests a decrease in the absolute numbers of CD4+ cells in peripheral blood during pregnancy compared with the number of these cells postpartum.78,87 Lapp et al59 suggest that high levels of progesterone during pregnancy affect the development of localized inflammation by downregulation of IL-6 production, rendering the gingiva less efficient at resisting the inflammatory challenges produced by the bacteria. A recent study indicated live preterm birth is associated with decreased levels of immunoglobulin G (IgG) antibody to periodontal pathogens in women with periodontitis when assessed during the second trimester but not associated with birth outcomes.27
Also, ovarian hormone stimulates the production of prostaglandins, in particular PGE1 and PGE2, which are potent mediators of the inflammatory response. With the prostaglandin acting as an immunosuppressant, gingival inflammation may increase when the mediator level is high.30,92 Kinnby et al54 found that high progesterone levels during pregnancy influenced plasminogen activator inhibitor type 2 (PAI-2) and disturbed the balance of the fibrinolytic system. Because PAI-2 serves as an important inhibitor of tissue proteolysis, this research implies that components of the fibrinolytic system may be involved in the development of pregnancy gingivitis.
During pregnancy, sex hormonal levels rise dramatically (Box 38-1). Progesterone reaches levels of 100 ng/ml, 10 times the peak luteal phase of menses. Estradiol in the plasma may reach 30 times higher levels than during the reproductive cycle. In early pregnancy and during the normal ovarian cycle, the corpus luteum is the major source of estrogen and progesterone. During pregnancy the placenta begins to produce estrogens and progesterone.
BOX 38-1 Etiology of Gingival Responses to Elevated Estrogen and Progesterone during Pregnancy
Subgingival Plaque Composition
Maternal Immunoresponse
Estrogen may regulate cellular proliferation, differentiation, and keratinization, whereas progesterone influences the permeability of the microvasculature,62,63 alters the rate and pattern of collagen production, and increases the metabolic breakdown of folate (necessary for tissue maintenance).139 High concentration of sex hormones in gingival tissues, saliva, serum, and GCF also may exaggerate the response.
Regulation of most cellular processes by hormones occurs through the interaction of these products with intracellular receptors. The resulting effects depend on the concentration of unbound hormone diffused through the cell membrane. Vittek et al130 have demonstrated specific estrogen and progesterone receptors in gingival tissues, providing direct biochemical evidence that this tissue may function as a target organ for sex hormones. Muramatsu and Takaesu81 found increasing concentration of sex hormones in saliva from the first month and peaking in the ninth month of gestation, along with increasing percentages of P. intermedia. Probing depth, number of gingival sites with bleeding, and redness increased until 1 month postpartum. Also, evidence indicates sex hormone concentration in GCF, providing a growth media for periodontal pathogens.
Other Oral Manifestations of Pregnancy
As previously mentioned, perimolysis (acid erosion of teeth) may occur if “morning sickness” or esophageal reflux is severe and involves repeated vomiting of the gastric contents. Severe reflux may cause scarring of the esophageal sphincter, and the patient may become a more likely candidate for GERD later in life.
Xerostomia is a frequent complaint among pregnant women. One study found this persistent dryness in 44% of pregnant participants.28
A rare finding in pregnancy is ptyalism, or sialorrhea. This excessive secretion of saliva usually begins at 2 to 3 weeks of gestation and may abate at the end of the first trimester. The etiology of ptyalism has not been identified, but it may result from the inability of nauseated gravid women to swallow normal amounts of saliva, rather than from a true increase in saliva production.19
Because pregnancy places the woman in an immunocompromised state, the clinician must be aware of the patient’s total health. Gestational diabetes, leukemia, and other medical conditions may appear during pregnancy.
Clinical Management
A thorough medical history is an imperative component of the periodontal examination, especially in the pregnant patient. Because of immunologic alterations, increased blood volume (ruling out mitral valve prolapse and heart murmurs), and fetal interactions, the clinician must diligently and consistently monitor the patient’s medical and periodontal stability. Medical history dialog should include pregnancy complications, previous miscarriages, and recent history of cramping, spotting, or pernicious vomiting. The patient’s obstetrician should be contacted to discuss her medical status, periodontal or dental needs, and the proposed treatment plan.
Establishing a healthy oral environment and maintaining optimal oral hygiene levels are primary objectives in the pregnant patient. A preventive periodontal program consisting of nutritional counseling and rigorous plaque control measures in the dental office and at home should be reinforced.
Plaque Control
The increased tendency for gingival inflammation during pregnancy should be clearly explained to the patient so that acceptable oral hygiene techniques may be taught, reinforced, and monitored throughout pregnancy. Scaling, polishing, and root planing may be performed whenever necessary during pregnancy. Some practitioners avoid the use of high-alcohol-content antimicrobial rinses in pregnant women and prefer to use non–alcohol-based oral rinses.
Prenatal Fluoride
The prescribing of prenatal fluoride supplements has been an area of controversy. Although two studies have claimed beneficial results,37,38others suggest that the clinical efficacy of prenatal fluoride supplements is uncertain and that the mechanism by which prenatal fluorides might impart cariostasis is unclear.98 The American Dental Association (ADA) does not recommend the use of prenatal fluoride because its efficacy has not been demonstrated. The American Academy of Pediatric Dentistry supports this position as well. The American Academy of Pediatrics has no stated position on prescribing prenatal fluorides.
Treatment
Elective Dental Treatment
Other than good plaque control, it is prudent to avoid elective dental care if possible during the first trimester and the last half of the third trimester. The first trimester is the period of organogenesis, when the fetus is highly susceptible to environmental influences. In the last half of the third trimester, a hazard of premature delivery exists because the uterus is very sensitive to external stimuli. Prolonged chair time may need to be avoided because the woman is most uncomfortable at this time. Further, supine hypotensive syndrome may occur. In a semireclining or supine position, the great vessels, particularly the inferior vena cava, are compressed by the gravid uterus. By interfering with venous return, this compression will cause maternal hypotension, decreased cardiac output, and eventual loss of consciousness. Supine hypotensive syndrome can usually be reversed by turning the patient on her left side, thereby removing pressure on the vena cava and allowing blood to return from the lower extremities and pelvic area. A preventive 6-inch soft wedge (rolled towel) should be placed on the patient’s right side when she is reclined for clinical treatment.
Early in the second trimester is the safest period for providing routine dental care. The emphasis at this time is on controlling active disease and eliminating potential problems that could arise in late pregnancy. Major elective oral or periodontal surgery may be postponed until after delivery. “Pregnancy tumors” that are painful, interfere with mastication, or continue to bleed or suppurate after mechanical debridement may require excision and biopsy before delivery.
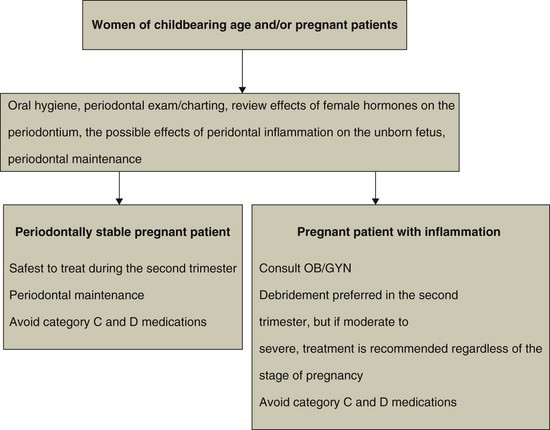
The American Academy of Periodontology (www.perio.org) has developed a position statement regarding the need for providing proper periodontal therapy for pregnant patients (Figure 38-5). Because of research indicating possible impact on the fetus, the presence of acute infection, abscesses, or other potential disseminating sources of sepsis may warrant prompt intervention irrespective of the stage of pregnancy.4
Dental Radiographs
The safety of dental radiography during pregnancy has been well established, provided features such as high-speed film, filtration, collimation, and lead aprons are used. However, it is most desirable not to have any irradiation during pregnancy, especially during the first trimester, because the developing fetus is particularly susceptible to radiation damage.64 When radiographs are needed for diagnosis, the most important aid for the patient is the protective lead apron. Studies have shown that when an apron is used during contemporary dental radiography, gonadal and fetal radiation is virtually immeasurable.9
Even with the obvious safety of dental radiography, x-ray films should be taken selectively during pregnancy and only when necessary and appropriate to aid in diagnosis and treatment. In most cases, only bite-wing, panoramic, or selected periapical films are indicated.
Medications
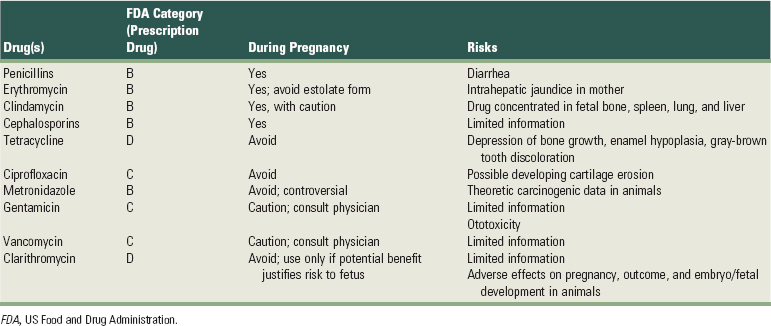
Drug therapy in the pregnant patient is controversial because drugs can affect the fetus by diffusion across the placenta. Prescriptions should be only the duration absolutely essential for the pregnant patient’s well-being and only after careful consideration of potential side effects. The classification system established by the US Food and Drug Administration (FDA) in 1979 to rate fetal risk levels associated with many prescription drugs provides safety guidelines (Box 38-2). The prudent practitioner should consult references, such as Briggs et al’s Drugs in Pregnancy and Lactation15 and Olin’s Drug Facts and Comparisons,93 for information on the FDA pregnancy risk factor associated with prescription drugs. Ideally, no drug should be administered during pregnancy, especially the first trimester.64 However, it is sometimes impossible to adhere to this rule. Fortunately, most common drugs in dental practice can be given during pregnancy with relative safety, although there are a few important exceptions. Tables 38-1, 38-2, and 38-3 present general guidelines for anesthetic and analgesic, antibiotic, and sedative-hypnotic drugs, respectively.105,125
BOX 38-2 FDA Drug Classification System Based on Potential for Causing Birth Defects
TABLE 38-1 Local Anesthetic and Analgesic Administration during Pregnancy
| Drug | FDA Category (Prescription Drug) | During Pregnancy |
|---|---|---|
| Local Anesthetics* | ||
| Lidocaine | B | Yes |
| Mepivacaine | C | Use with caution; consult physician. |
| Prilocaine | B | Yes |
| Bupivacaine | C | Use with caution; consult physician. |
| Etidocaine | B | Yes |
| Procaine | C | Use with caution; consult physician. |
| Articaine | B | Yes; no blocks |
| Analgesics | ||
| Aspirin | C/D, third trimester | Caution; avoid in third trimester. |
| Acetaminophen | B | Yes |
| Ibuprofen | B/D, third trimester | Caution; avoid in third trimester. |
| Codeine† | C | Use with caution; consult physician. |
| Hydrocodone† | B | Use with caution; consult physician. |
| Oxycodone† | B | Use with caution; consult physician. |
| Propoxyphene | C | Use with caution; consult physician. |
FDA, US Food and Drug Administration.
* Can use vasoconstrictors if necessary.
TABLE 38-3 Sedative-Hypnotic Drug Administration during Pregnancy
| Drug(s) | FDA Category | During Pregnancy |
|---|---|---|
| Benzodiazepines | D | Avoid |
| Barbiturates | D | Avoid |
| Nitrous oxide | Not assigned | Avoid in first trimester; otherwise use with caution; consult physician. |
FDA, US Food and Drug Administration.
In particular, antibiotics are often needed in periodontal therapy. The effect of a particular medication on the fetus depends on the type of antimicrobial, dosage, trimester, and duration of the course of therapy.94 Research regarding subgingival irrigation and local site delivery in relation to the developing fetus is inadequate at this date.
Breastfeeding
Usually, there is a risk that the drug can enter breast milk and be transferred to the nursing infant, in whom exposure could have adverse effects (Tables 38-4 and 38-5). Unfortunately, there is little conclusive information about drug dosage and effects through breast milk; however, retrospective clinical studies and empiric observations coupled with known pharmacologic pathways allow recommendations to be made.64 The amount of drug excreted in breast milk is usually not more than 1% to 2% of the maternal dose; therefore it is highly unlikely that most drugs have any pharmacologic significance for the infant.136
TABLE 38-4 Local Anesthetic and Analgesic Administration during Breastfeeding
| Drug | During Breastfeeding |
|---|---|
| Local Anesthetics | |
| Lidocaine | Yes |
| Mepivacaine | Yes |
| Prilocaine | Yes |
| Bupivacaine | Yes |
| Etidocaine | Yes |
| Procaine | Yes |
| Analgesics | |
| Aspirin | Avoid |
| Acetaminophen | Yes |
| Ibuprofen | Yes |
| Codeine | Yes |
| Hydrocodone | No data |
| Oxycodone | Yes |
| Propoxyphene | Yes |
TABLE 38-5 Antibiotic and Sedative-Hypnotic Administration during Breastfeeding
| Drug(s) | During Breastfeeding |
|---|---|
| Antibiotics* | |
| Penicillins | Yes |
| Erythromycin | Yes |
| Clindamycin | Yes, with caution |
| Cephalosporins | Yes |
| Tetracycline | Avoid |
| Ciprofloxacin | Avoid |
| Metronidazole | Avoid |
| Gentamicin | Avoid |
| Vancomycin | Avoid |
| Sedative-Hypnotics | |
| Benzodiazepines | Avoid |
| Barbiturates | Avoid |
| Nitrous oxide | Yes |
* Antibiotics have the risk of diarrhea and sensitization in the mother and infant.
The mother should take prescribed drugs just after breastfeeding and then avoid nursing for 4 hours or more, if possible,64,119 to decrease the drug concentration in breast milk.
Oral Contraceptives
Women may have responses to oral contraceptives (OCs) similar to those seen in pregnant patients. Mullally et al found that current users of OCs had poorer periodontal health.80 An exaggerated response to local irritants occurs in gingival tissues. Inflammation ranges from mild edema and erythema to severe inflammation with hemorrhagic or hyperplasic gingival tissues. It has been reported that more exudate is present in inflamed gingival tissues of OC users than in pregnant women.116,140 Investigators have suggested several mechanisms for the heightened response in gingival tissues. Kalkwarf53 reported that the response may be caused by an altered microvasculature, increased gingival permeability, and increasing synthesis of prostaglandin. PGE, a potent mediator of inflammation,31 appears to rise significantly with increasing levels of sex hormones. Jensen et al51 found dramatic microbial changes in pregnant and OC groups compared with a nonpregnant group. A sixteenfold increase in Bacteroides species was noted in the OC group versus the nonpregnant group, despite no statistically significant clinical differences in gingival index or GCF flow. The authors stated that the increased female sex hormones substituting for the naphthoquinone requirement of certain Bacteroides species were most likely responsible for this increase.
The OC-associated gingival inflammation may become chronic (versus the acute inflammation of pregnancy) because of the extended periods that women are exposed to elevated levels of estrogen and progesterone.55,97 Some have reported that the inflammation increases with prolonged use of OCs. Kalkwarf53 did not find that duration of use made a significant difference; however, the brand used resulted in different responses. Further studies need to be performed in relation to dosage, duration, and type of OC used in association with the periodontium. The concentration of female sex hormones in current OCs is significantly less than that of the 1970s, with the same level of contraceptive efficacy.
Salivary composition changed notably in patients taking OCs in studies from the 1970s. A decreased concentration of protein, sialic acid, hexosamine fucose, hydrogen ions, and total electrolytes has been reported. Salivary flow rates were increased in one report72 and decreased in 30% of subjects in another report.29
The dental literature reports that women taking OCs experience a twofold to threefold increase in the incidence of localized osteitis after extraction of mandibular third molars.120 The higher incidence of osteitis is these patients may be attributed to the effects of OCs (estrogens) on clotting factors. However, a number of studies refutes these findings.18 Evidence thus far is inconclusive on osteitis after third molar extraction and OC use. Also, a spotty melanotic pigmentation of the skin may occur with OC use. This suggests a relationship between the use of OCs and the occurrence of gingival melanosis,45 especially in fair-skinned individuals.
Management
Medical histories should include OCs under medications, and an oral dialog should include questions regarding OCs in women of childbearing age. The patient should be informed of the oral and periodontal side effects of OCs and the need for meticulous home care and compliance with periodontal maintenance. Treatment of gingival inflammation exaggerated by OCs should include establishing an oral hygiene program and eliminating local predisposing factors. Periodontal surgery may be indicated if resolution after initial therapy (scaling and root planing) is inadequate. It may be advisable to perform extraction of teeth (especially of third molars) on nonestrogenic days (days 23 to 28) of the OC cycle to reduce the risk of a postoperative localized osteitis34; however, evidence of this association is inconclusive and warrants further investigation.
Although the results from animal studies have demonstrated antibiotic interference adversely affecting contraceptive sex hormone levels, several human studies have failed to support such an interaction.8,35,82,85 This issue is controversial, and antibiotics possibly could render OCs ineffective in preventing pregnancies. In 1991, an ADA report stated that all women of childbearing age should be informed of possible reduced efficacy of steroid OCs during antibiotic therapy and advised women to use additional forms of contraception during short-term antibiotic therapy.6 During long-term antibiotic therapy, they should consult their physician about using high-dose OC preparations. Although only research regarding oral manifestations attributed to OCs has been reported in the literature, the same effects presumably could occur with the use of contraceptive implants. Similarly, the remote possibility of reduced efficacy of the contraceptive implant with concurrent antibiotic administration also exists, and women can adhere to the same precautions as with OC use.
Menopause
Female life expectancy is 80+ years, so many women will live 40% of their lives in menopause. This cohort represents a large number of the patients that present in clinical practices. Therefore dental clinicians must be aware of the effects of reduced hormones on the periodontal tissues, as well as the systemic changes that may manifest.
Throughout a woman’s lifetime, the number of oocytes steadily diminishes. Menopause is associated with symptoms of estrogen deficiency. Estradiol levels fall gradually in the years before menopause. Levels of FSH and LH begin to rise, and levels of sex hormones begin to fluctuate. This stage of “perimenopause” is characterized by increasing ovarian unresponsiveness, and thus sporadic ovulation ensues. Anovulatory cycles indicate low estradiol and progesterone because of absent corpus luteum function.
Oral Changes
It is important for the clinician to recognize the effect of hormonal alterations on the oral cavity as well as systemic and psychologic changes. Oral changes in menopause include thinning of the oral mucosa, oral discomfort (“burning mouth”), gingival recession, xerostomia, altered taste sensation, alveolar bone loss, and alveolar ridge resorption.
Fluctuations of sex hormones during menopause have been implicated as factors in inflammatory changes in the human gingiva, hypertrophy, or atrophy. Estrogen affects cellular proliferation, differentiation, and keratinization of the gingival epithelium. Hormone receptors have been identified in basal and spinous layers of the epithelium and connective tissue, implicating gingiva and other oral tissues as targets to manifest hormone deficiencies. Sex steroids are known to have a direct effect on connective tissue, with estrogens increasing the intracellular fluid content. Estrogen deficiency can lead to a reduction in collagen formation in connective tissues, resulting in a decrease in skin thickness.134Alterations in collagen affect tissues such as joints, hair, nails, and glands. Mohammed et al77 noted significantly increased recession in postmenopausal patients with low bone density. A recent study of postmenopausal women indicated that those who were overweight with T. forsythia infections were more likely to have oral bone loss.14
Osteopenia and osteoporosis have been associated with the menopausal patient. Osteopenia is a reduction in bone mass caused by an imbalance between bone resorption and formation, favoring resorption and resulting in demineralization and osteoporosis. Osteoporosis is a disease characterized by low bone mass and fragility and a consequent increase in fracture risk.131 In most women, peak bone mass occurs between 20 and 30 years of age, then declines. Menopause accelerates declining bone mass.129 An estimated 25 million Americans have osteoporosis, 80% of whom are female. Ongoing studies are examining the association of postmenopausal primary osteoporosis with mandibular and maxillary bone mineral density (BMD), tooth loss, alveolar ridge atrophy, and clinical periodontal attachment loss41,48; however, problems in extrapolation and application of data arise as a result of small sample size, study design, and inadequately controlled confounding factors and limited understanding of the relationship between the two diseases. The effects of hormone replacement therapy (HRT) or estrogen replacement therapy (ERT) on the oral bone and tooth loss also are under investigation. Evidence indicates a probable association between osteoporosis and maxillary tooth loss, as well as alveolar bone loss.50,96,106,107
Studies show the greatest association when evaluating tooth loss and alveolar ridge atrophy with osteoporosis.26,42,138 There is also less risk of tooth loss when postmenopausal women are placed on HRT.40,121 Improved BMD with HRT has been a recognized benefit. Krall et al found that the odds of being edentulous were reduced by 6% for each 1-year increase in duration of HRT use.58 It is also apparent that there is increased alveolar ridge resorption in edentulous patients with osteoporosis.
Current knowledge regarding the effects of osteoporosis/osteopenia on periodontal diseases and alveolar bone loss are inconclusive. Articles have presented an association of osteoporosis in postmenopausal women with periodontitis, attachment loss, and gingival recession.46,123 Greater alveolar crestal height loss is noted with osteoporosis and osteopenia.77,132 However, studies evaluating the association of clinical attachment loss and osteoporosis have mixed results. Several studies indicate decreasing BMD was associated with increased clinical attachment loss.78,110,138 Others have found weak or no significant association of systemic BMD and clinical attachment loss.32,99 Further clinical trials with greater numbers, longer time intervals, male patients, and accounting for confounding variables needs to be performed. Most studies indicate improved periodontal status in women on HRT/ERT.17,107,121 Increased alveolar bone mass with associated improved alveolar crestal height, reduced clinical attachment loss, or reduced periodontal inflammation has been reported with patients on hormone therapy. A recent review found that research supports the idea that osteoporosis independently influences alveolar bone height loss and that strategies for reducing osteoporosis may help retard alveolar bone loss.52
Clinical Management
It is the clinician’s responsibility to review the patient’s medical history and keep information up to date. Because of possible alterations in oral soft and osseous tissues during perimenopause and after menopause, appropriate questioning regarding hormone changes should be performed and documented. The many available therapies for HRT/ERT, from prescriptions to holistic approaches, need to be followed. Many medications may alter clotting times, prolong the effects of other medications, and interfere with absorption or effectiveness of prescription medications. If gingival and mucosal tissue thinning occurs, soft tissue augmentation may be performed.
Brushing with an extrasoft toothbrush using the “toe” or “heel” of the brush may prevent “scrubbing” the thinning gingiva. Dentifrices with minimal abrasive particles should be used. Rinses should have low alcohol concentration. During periodontal maintenance, root surfaces should be debrided gently with minimal soft tissue trauma. Oral pain may result from thinning tissues, xerostomia, inadequate nutritional intake, or hormone depletion. In patients with oral symptoms who receive HRT, symptoms may be significantly reduced.
If the patient is susceptible to osteoporosis (menopausal, Caucasian or Asian, smoker, minimal physical activity, low calcium intake, thin build or low body weight [<58 kg], systemic disease associated with predisposition, and genetic history),48 the dentist should consult the patient’s physician as to the risks versus benefits of HRT/ERT and calcium/vitamin D supplementation for the individual patient. Sodium fluoride, bisphosphonates (e.g., alendronate), selective estrogen receptor modulators, and parathyroid hormone may be other therapies for the osteoporotic patient. Close monitoring of the patient’s periodontal stability, performing titrated periodontal maintenance, informing the patient about potential risks of hormone depletion on the oral tissues, and consulting the treating physician are advised. The National Institutes of Health (NIH; 1994 Conference on Optimal Calcium Intake) recommends 1000 mg of elemental calcium per day for premenopausal women and 1500 mg/day for postmenopausal women (Box 38-3). Vitamin D3 recommendations are 400 IU daily for premenopausal women and 800 IU for postmenopausal women.
BOX 38-3 National Institutes of Health (NIH) Consensus Conference Recommendations for Optimal Calcium Intake
| Premenopausal women (25-50 years old): Postmenopausal women (estrogen therapy): Postmenopausal women (no estrogen therapy): Men (25-65 years old): Women and men >65 years old: |
1000 mg/day 1000 mg/day 1500 mg/day 1000 mg/day 1500 mg/day |
To date, no data are available regarding success or failure with periodontal regeneration procedures in osteoporotic versus nonosteoporotic individuals. Also, no scientific data are available to contraindicate the use of osseointegrated implants in osteoporotic patients, despite articles stating osteoporosis as a risk factor. Much research is needed to address the increasing number of patients who may present to periodontal practices with osteoporosis or osteopenia, most of whom will be undiagnosed.
Conclusion
Clinical periodontal therapy includes an understanding of the clinician’s role in the total health and well-being of female patients. Dentists do not treat localized infections without affecting other systems (and the fetus or the breastfed infant). Therefore female patients may present with periodontal and systemic considerations that alter conventional therapy.
The cyclic nature of the female sex hormones often is reflected in the gingival tissues as initial signs and symptoms. Medical histories and dialogs should include thoughtful investigation of the individual patient’s problems and needs. Questioning should reflect hormonal stability and medications associated with regulation. Patients should be educated regarding the profound effects the sex hormones may play on periodontal and oral tissues, as well as the consistent need for home and office removal of local irritants.
Research regarding female issues and medical/periodontal therapy is in process. In the near future, information regarding specific management and etiology of sex hormone–mediated infections will enhance our ability to provide quality care to our patients.
![]() Science Transfer
Science Transfer
Variations in the levels of circulating female hormones have a significant effect on periodontal health. Pregnancy can change inflammatory reactions to dental plaque in the most dramatic fashion with accentuation of vascular proliferation leading to increased inflammation and a hyperplastic gingival response. Puberty can have a similar but usually smaller effect, and changes during menstrual cycles and the use of hormone-based birth control tablets give the smallest changes in gingival health. There is also an increase in the risk of low-birth-weight preterm infants in patients with active periodontal inflammation so all pregnant women should be evaluated periodontally, and appropriate phase I therapy should be performed.
Dentists should consult the Food and Drug Administration (FDA) classification of medications that can or cannot be used in pregnancy and during breastfeeding before beginning periodontal treatment. Consultation with the patient’s physician should be part of the management of pregnant and lactating patients.
1 Aboul-Dahab OM, el-Sherbiny MM, Abdel-Rahman R, et al. Identification of lymphocytes subsets in pregnancy. Egypt Dent J. 1994;40:653.
2 Adriaens LM, Alessandri R, Sporri S, et al. Does pregnancy have an impact on the subgingival microbiota? J Periodontol. 2009;80:72.
3 American Academy of Periodontology. Position paper: periodontal disease as a potential risk factor for systemic disease. J Periodontol. 1998;69:841.
4 American Academy of Periodontology. Statement regarding periodontal management of the pregnant patient. J Periodontol. 2004;75:495.
5 American Dental Association (ADA), Council on Access, Prevention, and Interpersonal Relations. Women’s oral health issues. Chicago: ADA; 1995.
6 American Dental Association Health Foundation Research Institute, Department of Toxicology. Antibiotic interference with oral contraceptives. J Am Dent Assoc. 1991;122:79.
7 Aschkenazi S, Naftolin F, Mor G. Menopause, sex hormones and the immune system. Menopause Manag. 2000;9:6.
8 Back DJ, Orme M. Pharmacokinetic drug interactions with oral contraceptives. Clin Pharmacokinet. 1990;18:472.
9 Bean LRJr, Devore WD. The effects of protective aprons in dental roentgenography. Oral Surg. 1969;28:505.
10 Bhashkar SN, Jacoway JR. Pyogenic granuloma: clinical features, incidence, histology, and results of treatment report of 242 cases. J Oral Surg. 1966;24:391.
11 Bischof P, Lauber K, Girard JP, et al. Circulating levels of pregnancy proteins and depression of lymphoblastogenesis during pregnancy. J Clin Lab Immunol. 1983;12:93.
12 Brabin BJ. Epidemiology of infection in pregnancy. Rev Infect Dis. 1985;7:579.
13 Brannstrom M, Friden BE, Jasper M, Norman RJ. Variations in peripheral blood levels of immunoreactive tumor necrosis factor alpha throughout the menstrual cycle and secretion of TNF-a from the human corpus luteum, Curr J Obstet Gynecol. Reprod Biol. 1999;83:213.
14 Brennan RM, Genco RJ, Wilding G. E, et al: Bacterial species in subgingival plaque and oral bone loss in postmenopausal women. J Periodontol. 2007;78:1051.
15 Briggs GG, Freeman RK, Yaffe SJ. Drugs in pregnancy and lactation, ed 4. Baltimore: Williams & Wilkins; 1994.
16 Brown S, Bonifaz DZ. An overview of anorexia and bulimia nervosa and the impact of eating disorders on the oral cavity. Compend Contin Educ Dent. 1993;14:1594.
17 Civitelli R, Pilgram TK, Dotson M, et al. Alveolar and postcranial bone density in postmenopausal women receiving hormone/estrogen replacement therapy: A randomized, double-blind, placebo-controlled trial. Arch Intern Med. 2002;162:1409.
18 Cohen ME, Simecek JW. Effects of gender-related factors on the Incidence of localized alveolar osteitis. Oral Surg Oral Med Oral Pathol. 1995;79:416.
19 Cruikshank O, Hayes PM. Maternal physiology. In: Gabbe S, Niebyl JR, Simpson JL, editors. Pregnancy in obstetrics: normal and problem pregnancies. New York: Churchill Livingstone, 1986.
20 Cutolo M, Sulli A, Seriolo B, et al. Estrogens, the immune response and autoimmunity. Clin Exp Rheumatol. 1995;13:217.
21 Damare SM, Wells S, Offenbacher S. Eicosanoids in periodontal diseases: potential for systemic involvement. Adv Exp Med Biol. 1997;433:23.
22 Dasanayake AP. Poor periodontal health of the pregnant woman as a risk. Ann Periodontol. 1998;3:206.
23 Davenport ES, Williams CE, Sterne JA, et al. The East London study of maternal chronic periodontal disease and preterm low birth weight infants: study design and prevalence. Ann Periodontol. 1998;3:213.
24 Dakovic D, Pavlovic MD. Periodontal disease in children and adolescents with type 1 diabetes in Serbia. J Periodontol. 2008;79:987.
25 DeLiefde B. The dental care of pregnant women, NZ. Dent J. 1984;80:41.
26 Drozdzowska B, Pluskiewicz W, Michno M. Tooth count in elderly women in relation to their skeletal status. Maturitas. 2006;55:126.
27 Ebersole JL, Novak MJ, Michalowicz BS, et al. Systemic immune responses in pregnancy and periodontitis: relationship to pregnancy outcome in the obstetrics and periodontal therapy (OPT) study. J Periodontol. 2009;80:953.
28 El-Ashiry G. Comparative study of the influence of pregnancy and oral contraceptives on the gingivae. Oral Surg. 1970;30:472.
29 El-Ashiry G, El-Kafrawy AH, Nasr MF, et al. Effects of oral contraceptives on the gingiva. J Periodontol. 1971;42:273.
30 El-Attar TM. Prostaglandin F2 in human gingiva in health and disease and its stimulation by female sex steroids. Prostaglandins. 1976;11:331.
31 El-Attar TM, Roth GD, Hugoson A. Comparative metabolism of 4-C progesterone in normal and chronically inflamed human gingival tissue. J Periodontal Res. 1973;8:79.
32 Famili P, Cauley J, Suzuki JB, Weyant R. Longitudinal study of periodontal disease and edentulism with rates of bone loss in older women. J Periodontol. 2005;76:11.
33 Ferguson MM, Carter J, Boyle P. An epidemiological study of factors associated with recurrent aphthae in women. J Oral Med. 1984;39:212.
34 Fleisher ABJr, Resnick SD. The effect of antibiotics in the efficacy of oral contraceptives. Arch Dermatol. 1980;125:1582.
35 Fraser IS, Jansen RP. Why do inadvertent pregnancies occur in oral contraceptive users? Effectiveness of oral contraceptive regimens and interfering factors. Contraception. 1983;27:531.
36 Gibbs RS, Romero R, Hillier SL, et al. A review of premature birth and subclinical infections. Am J Obstet. 1992;166:1515.
37 Glenn FB. Immunity conveyed by a fluoride supplement during pregnancy. J Dent Child. 1977;44:391.
38 Glenn FB, Glenn WDIII, Duncan RC. Fluoride tablet supplementation during pregnancy for caries immunity: a study of the offspring produced. Am J Obstet Gynecol. 1982;143:560.
39 Grant D, Stern J, Listgarten M. The epidemiology, etiology, and public health aspects of periodontal disease. In: Grant D, Stern J, Listegarten M, editors. Periodontics. St Louis: Mosby, 1988.
40 Grodstein F, Colditz GA, Stamfer MJ. Post-menopausal hormone use and tooth loss: A prospective study. J AM Dent Assoc. 1996;127:370.
41 Grossi SG, Jeffcoat MK, Genco RJ. Osteopenia, osteoporosis, and oral disease. In: Rose LR, Genco RJ, Cohen DW, et al, editors. Periodontal medicine. New York: BC Decker, 2000.
42 Gur A, Nas K, Kayhan O, et al. The relation between tooth loss and bone mass in postmenopausal osteoporotic women in Turkey: a multicenter study. J Bone Miner Metab. 2003;21:43.
43 Gusberti FA, Mombelli A, Lang NP, et al. Changes in subgingival microbiota during puberty. J Clin Periodontol. 1990;17:685.
44 Hanson L, Sobol SM, Abelson T. The otolaryngologic manifestations of pregnancy. J Fam Pract. 1986;23:151.
45 Hertz RS, Beckstead PC, Brown WJ. Epithelial melanosis of the gingiva possibly resulting from the use of oral contraceptives. J Am Dent Assoc. 1980;100:173.
46 Inagaki K, Kurosu Y, Sakano M, Yamamoto G, et al. Oral osteoporosis: a review and its dental implications. Clin Calcium. 2007;17:157.
47 Jared H, Boggess KA, Moss K, et al. Fetal exposure to oral pathogens and subsequent risk for neonatal intensive care admission. J Periodontol. 2009;80:878.
48 Jeffcoat MK. Osteoporosis: a possible modifying factor in oral bone loss. Ann Periodontol. 1998;3:312.
49 Jeffcoat MK, Hauth JC, Geurs NC, et al. Periodontal disease and preterm birth: results of a pilot intervention study. J Periodontol. 2003;74:1214.
50 Jeffcoat MK, Lewis CE, Reddy MS, et al. Postmenopausal bone loss and its relationship to oral bone loss. Periodontol 2000. 2000;23:94.
51 Jensen J, Lilijmack W, Blookquist C. The effect of female sex hormones on subgingival plaque. J Periodontol. 1981;52:599.
52 Kaye EK. Bone health and oral health. J Am Dent Assoc. 2007;138:616.
53 Kalkwarf KL. Effect of oral contraceptive therapy on gingival inflammation in humans. J Periodontol. 1978;49:560.
54 Kinnby B, Matsson L, Astedt B. Aggravation of gingival inflammatory symptoms during pregnancy associated with the concentration of activator inhibitor type 2 (PAI-2) in gingival fluid. J Periodontal Res. 1996;31:271.
55 Knight GM, Wade AB. The effects of oral contraceptives on the human periodontium. J Periodontol Res. 1974;9:18.
56 Kornman K, Loseche JF. Direct interaction of estradiol and progesterone with Bacteroides melaninogenicus. J Dent Res. 1979;58A:10.
57 Kornman KS, Loesche WJ. The subgingival flora during pregnancy. J Periodontol. 1980;15:111.
58 Krall EA, Dawson-Hughes B, Hannan MT, et al. Postmenopausal estrogen replacement and tooth retention. Compend Contin Educ Dent Suppl. 1998;22:S17.
59 Lapp CA, Thomas ME, Lewis JB. Modulation by progesterone of interleukin-6 production by gingival fibroblasts. J Periodontol. 1995;66:279.
60 Lieff S, Boggess KA, Murtha AP, et al. The oral conditions and pregnancy study: periodontal status of a cohort of pregnant women. J Periodontol. 2004;75:116.
61 Levin RP. Pregnancy gingivitis. Md State Dent Assoc. 1987;30:27.
62 Lindhe J, Branemark P. Changes in microcirculation after local application of sex hormones. J Periodontal Res. 1967;2:185.
63 Lindhe J, Branemark P. Changes in vascular permeability after local application of sex hormones. J Periodontal Res. 1967;2:259.
64 Little JW, Falace DA. Dental management of the medically compromised patient, ed 4. St Louis: Mosby; 1993.
65 Löe H, Silness J. Periodontal disease in pregnancy. 1. Prevalence and severity. Acta Odontol Scand. 1984;21:533.
66 Lopatin DE, Kornman KS, Loesche WJ. Modulation of immunoreactivity to periodontal disease-associated microorganisms during pregnancy. Infect Immun. 1980;28:713.
67 Lopez NJ, Da Silva I, Ipinza J, et al. Periodontal therapy reduces the rate of preterm low birth weight in women with pregnancy-associated gingivitis. J Periodontol. 2005;76:2144.
68 Lopez NJ, Smith PC, Guiterrez J. Periodontal therapy may reduce the risk of preterm low bith weight in women with periodontal disease: a randonmized controlled trial. J Periodontol. 2002;73:2011.
69 Lu SY, Shi Q, Yang SH. Bacterial analysis of subgingival plaque in adolescents. ZhonghuaKou Qiang Yi Xue Za Zhi. 2008;43:737. (Chinese)
70 Lundgren D, Magnssen B, Lindhe J. Connective tissue alterations in gingiva of rats treated with estrogens and progesterone. Odontology. 1973;24:49.
71 Machtei EE, Mahler D, Sanduri H, Peled M. The effect of the menstrual cycle on periodontal health. J Periodontol. 2004;75:408.
72 Magnusson T, Ericson T. Hugoson A: The effect of oral contraceptives on some salivary substance in women. Arch Oral Biol. 1975;20:119.
73 Mandel L, Kaynar A. Bulimia and parotid swelling: a review and case report. J Oral Maxillofac Surg. 1992;50:1122.
74 Michalowicz BS, Di Angelis AJ, Novak MJ, et al. Examining the safety of dental treatment in pregnant women. J Am Dent Assoc. 2008;139:685.
75 Miyagi M, Morishita M, Iwamoto Y. Effect of sex hormones on production of prostaglandin E2 by human peripheral monocytes. J Periodontol. 1993;64:1075.
76 Miyagi M, Aoyama H, Morishita M, et al. Effects of sex hormones on chemotaxis of polymorphonuclear leukocytes and monocytes. J Periodontol. 1992;63:28.
77 Mohammed AR, Brunsvold M, Bauer R. The strength of association between systemic postmenopausal osteoporosis and periodontal disease. Int J Prosthet. 1996;9:479.
78 Mohammad AR, Hooper DA, Vermilyea SG, et al. An investigation of the relationship between system bone density and clinical periodontal status in post-menopausal Asian-American women. In Dent J. 2003;53:121.
79 Mombelli A, Rutar A, Lan NP. Correlation of the periodontal status 6 years after puberty with clinical and microbiological conditions during puberty. J Clin Periodontol. 1995;22:300.
80 Mullally BH, Coulter WA, Hutchinson JD, et al. Current oral contraceptive status and periodontitis in young adults. J Periodontol. 2007;78:1031.
81 Muramatsu Y, Takaesu Y. Oral health status related to subgingival bacterial flora and sex hormones in saliva during pregnancy. Bull Tokyo Dent Coll. 1994;35:139.
82 Murphy AA, Zacur HA, Charache P, et al. The effect of tetracycline on levels of oral contraceptives. Am J Obstet Gynecol. 1991;164:28.
83 Nakagawa S, Fujii H, Machida Y, et al. A longitudinal study from prepuberty to puberty of gingivitis: correlation between the occurrence of Prevotella intermedia and sex hormones. J Clin Periodontol. 1994;21:658.
84 Novak MJ, Novak KF, Hodges JS, et al. Periodontal bacterial profiles in pregnant women: response to treatment and associations with birth outcomes in the obstretics and peridontal therapy (OPT) study. J Periodontol. 2008;79:1870.
85 Neely JL, Abate M, Swinker M, et al. The effect of doxycycline on serum levels on ethinyl estradiol, norethindrone, and endogenous progesterone. Obstet Gynecol. 1991;77:416.
86 O’Neil TC. Maternal T-lymphocyte response and gingivitis in pregnancy. J Periodontol. 1979;50:178.
87 Offenbacher S, Beck J, Jared H, et al. Maternal oral therapy to reduce obstetric risk (MOTOR): a report of a multi-centered periodontal therapy randomized-controlled trial on rate of preterm delivery. Am J Obstetr & Gynec. 2008;199:S2.
88 Offenbacher S, Jared HL, O’Reilly PG, et al. Potential pathogenic mechanisms of periodontitis associated pregnancy complications. Ann Periodontol. 1998;3:233.
89 Offenbacher S, Katz V, Fertik G, et al. Periodontal infection as a possible risk factor for preterm low birthweight. J Periodontol. 1996;67(suppl):1103.
90 Offenbacher S, Lin D, Strauss R, et al. Effects of periodontal therapy durig pregnancy on periodontal status, biologic parameters, and pregnancy outcomes: a pilot sutdy. J Periodontol. 2006;77:2011.
91 Oh Tj, ber R, Wang L: Periodontal dieasese in the child and adolescent. J Clin Periodontol. 2002;29:400.
92 Ojanotko-Harri AO, Harri MP, Hurrita HP, et al. Altered tissue metabolism of progesterone in pregnancy gingivitis and granuloma. J Clin Periodontol. 1991;18:262.
93 Olin BR. Drug facts and comparisons. St Louis: Kluwer; 1994.
94 Otomo-Corgel J. Systemic considerations for female patients. In Antibiotic/antimicrobial use in dental practice. Tokyo: Quintessence; 1990.
95 Pack AR, Thomson ME. Effects of topical and systemic folic acid supplementation on gingivitis in pregnancy. J Clin Periodontol. 1980;7:402.
96 Paganini-Hill A. Benefits of estrogen replacement therapy on oral health: the leisure world cohort. Arch Intern Med. 1995;155:2325.
97 Pankhurst CL. The influence of oral contraceptive therapy on the periodontium: duration of drug therapy. J Periodontol. 1981;52:617.
98 Pediatric Dentistry special issue: Reference manual. Pediatr Dent. 16(7), 1994.
99 Pilgram TK, Hildebolt CF, Dotson M. Relationships between clinical attachment level and spine and hip bone mineral density: data from healthy postmenopausal women. J Periodontol. 2002;73:298.
100 Pinard A. Gingivitis in pregnancy. Dent Register. 1877;31:258.
101 Pitcarin J. A case of disease of the gums [that] occurred during pregnancy. Dubing Hosp Rep. 1818;2:309.
102 Raber-Durlacher JE, van Steenbergen TJ, van der Velden U. Experimental gingivitis during pregnancy and postpartum: clinical, endocrinological and microbiological aspects. J Clin Periodontol. 1994;21:549.
103 Raber-Durlacher JE, Leene W, Palmer-Bouva CC, et al. Experimental gingivitis during pregnancy and postpartum: immunohistochemical aspects. J Periodontol. 1993;64:211.
104 Raber-Durlacher JE, Zeylemaker WP, Meinesz AA, et al. CD4 to CD8 ratio and in vitro lymphoproliferative responses during experimental gingivitis in pregnancy and postpartum. J Periodontol. 1991;62:663.
105 Reese RE, Betts RF. Handbook of antibiotics, ed 2. Boston: Little, Brown; 1993.
106 Reinhardt RA, Payne JB, Maze C, et al. Gingival fluid IL-beta in postmenopausal females on supportive periodontal therapy: a longitudinal 2-year study. J Clin Periodontol. 1998;25:1029.
107 Reinhardt RA, Payne JB, Maze CA, et al. Influence of estrogen and osteopenia/osteoporosis on clinical periodontitis in postmenopausal women. J Periodontol. 1999;70:823.
108 Robb-Nicholson C. PMS: it’s real. Harv Women Health Watch. 1994;1(11):2.
109 Robb-Nicholson C. Gastroesophageal reflux disease. Harv Women Health Watch. 1999;4(6):4.
110 Ronderos M, Jacobs DR, Himes JH, Pihlstrom BL. Associations of periodontal disease with femoral bone mineral density and estrogen replacement therapy: cross-sectional evaluation of U.S. Adults from NHANES III. J Clin Periodontol. 2000;27:778.
111 Ruma M, Moss K, Jared H. Maternal periodontal disease, systemic inflammation and eclampsia. Am J Obstet Gynecol. 2008;19:389.
112 Samant A, Malik CP, Chabra SK, et al. Gingivitis and periodontal disease in pregnancy. J Periodontol. 1976;47:415.
113 Scannapieco FA, Bush RB, Paju S. Periodontal disease as a risk factor for adverse pregnancy outcomes: a systematic review. Ann Periodontol. 2003;8:70.
114 Schuurs A, Verheul H. Effects of gender and sex steroids on the immune response and autoimmune disease. J Steroid Biochem. 1990;35:157.
115 Siqueira FM, Cota LO, Costa JE. Maternal periodontitis as a potential risk variable for preeclampsia: a case-control study. J Periodontol. 2008;79:207.
116 Sooriyamoorthy M, Gower DB. Hormonal influences on gingival tissues: relationship to periodontal disease. J Clin Periodontol. 1989;16:201.
117 Sridama V, Pacini F, Yang SL, et al. Decreased levels of helper T cells: a possible cause of immunodeficiency in pregnancy. N Engl J Med. 1982;307:352.
118 Srinivas SK, Aauuel MD, Stamilio DM, et al. Periodontal disease and adverse pregnancy outcomes: Is there an association? Am J Obstet Gynecol. 2009;200:297.
119 Steinberg BJ. Sex hormonal alterations. In Rose LD, Kay D, editors: Internal medicine for dentistry, ed 2, St Louis: Mosby, 1990.
120 Sweet JB, Butler DP. Increased incidence of postoperative localized osteitis in mandibular 3rd molar surgery associated with patients using oral contraceptives. Am J Obstet Gynecol. 1977;127:518.
121 Taguchi A, Sandada M, Suei Y, et al. Effect of estrogen use on tooth retention, oral bone height, and oral bone porosity in Japanese postmenopausal women. Post menopausal estrogen replacement and tooth retention. Menopause. 2004;11:556.
122 Tarannum F, Faizuddin M. Effect of periodontal therapy on pregnancy outcome in women affected by periodontitis. J Periodontol. 2007;78:2095.
123 Tezal M, Wactawski-Wende J, Grossi SG, et al. The relationship between bone mineral density and periodontitis in postmenopausal women. J Periodontol. 2000;71:149.
124 Thomson ME, Pack AR. Effects of extended systemic and topical folate supplementation on gingivitis in pregnancy. J Clin Periodontol. 1982;9:275.
125 Turner M, Aziz SR. Management of the pregnant oral and maxillofacial surgery patient. J Oral Maxillofac Surg. 2002;60:1479.
126 Valdimarsson H, Mulholland C, Fridriksdottir V, et al. A longitudinal study of leukocyte blood counts and lymphocyte responses in pregnancy: a marked early increase of monocyte-lymphocyte ratio. Clin Exp Immunol. 1983;53:437.
127 Vergnes JN. Studies suggest an association between maternal periodontal diseases and pre-eclampsia. Evid Based Dent. 2008;9:46.
128 Vetorre MV, Leal M, Leao AT, et al. The relationship between peridontitis and preterm low birthweight. J Dent Res. 2008;87:73.
129 Vinco L, Prallet B, Chappard D, et al. Contributions of chronological age, age at menarche and menopause and of anthropometric parameters to axial and peripheral bone densities. Osteoporosis Int. 1992;2:153.
130 Vittek J, Gordon G, Rappaport C, et al. Specific progesterone receptors in rabbit gingiva. J Periodontal Res. 1982;17:657.
131 Wactawski-Wende J, Grossi SG, Trevisan M, et al. The role of osteopenia in oral bone loss and periodontal disease. J Periodontol. 1996;67:1076.
132 Wactawski-Wende J, Hauwmann E, Hovey K, et al. The association between osteoporosis and alveolar crestal height in postmenopausal women. J Periodontol. 2000;76(11 Suppl):2116.
133 Whitacre CC, Reingold SC, O’Looney PA. A gender gap in autoimmunity. Science. 1999;283:1277.
134 Whitehear MI, Whitcroft SI, Hillard TC. An atlas of the menopause. New York: Parthenon; 1993.
135 Wimmer G, Pihlstrom BL. A critical assessment of adverse pregnancy outcomes and periodontal disease. J Clin Periontol. 2008;35:380.
136 Wilson JT, Brown RD, Cherek DR, et al. Drug excretion in human breast milk: principles, pharmacokinetics and projects consequences. Clin Pharmacokinet. 1980;5:1.
137 Xiong X, Buekens P, Fraser WD, et al. Periodontal disease and adverse pregnancy outcomes: a systemic review. Obstetr & Gyn Surv. 2006;61:307.
138 Yoshihara A, Seida Y, Hanada N, et al. The relationship between bone mineral density and the number of remaining teeth in community-dwelling older adults. J Oral Rehabil. 2005;32:735.
139 Zachariasen RD. Ovarian hormones and oral health: pregnancy gingivitis. Compend Contin Educ Dent. 1989;10:508.
140 Zachariasen RD. The effects of elevated ovarian hormones on periodontal health: oral contraceptives and pregnancy. Women Health. 1993;20(2):21.