CHAPTER 28 Impact of Periodontal Infection on Systemic Health
Knowledge of the pathogenesis of periodontal diseases has evolved markedly over the last 50 years.55 Periodontal disease is an inflammatory disease initiated by bacterial pathogens. Environmental, physical, social, and host stresses may affect and modify disease expression through a multitude of pathways. Certain systemic conditions can affect the initiation and progression of gingivitis and periodontitis (see Chapters 24 and 27). Systemic disorders affecting neutrophil, monocyte/macrophage, and lymphocyte function result in altered production or activity of host inflammatory mediators.55,86 These alterations may manifest clinically as early onset of periodontal destruction or a more rapid rate of destruction than would occur in the absence of such disorders.
Evidence has also shed light on the converse side of the relationship between systemic health and oral health, that is, the potential effects of inflammatory periodontal diseases on a wide range of organ systems. This field of periodontal medicine addresses the following important questions:
Pathobiology of Periodontitis
Our understanding of the pathogenesis of periodontitis has changed remarkably over the last 30 years.55,87,90 Nonspecific accumulation of bacterial plaque was once thought to be the cause of periodontal destruction, but it is now recognized that periodontitis is an infectious disease associated with a small number of predominantly gram-negative microorganisms that exist in a subgingival biofilm.34 Furthermore, the importance of the host in disease initiation and progression is clearly recognized. Although pathogenic bacteria are necessary for periodontal disease, they are not sufficient alone to cause the disease. A susceptible host is also imperative. In a host who has relatively low susceptibility to disease, bacterial pathogens may have no clinical effect. This may be due to a particularly effective host immunoinflammatory response that eliminates pathogenic organisms while minimizing destruction of native tissues. Conversely, in a host with relatively high disease susceptibility, marked destruction of periodontal tissues may result.
Recognition of the importance of host susceptibility opens a door to understanding the differences in the onset, natural history, and progression of periodontitis seen throughout the scientific literature. Because of differences in host susceptibility, not all individuals are equally vulnerable to the destructive effects of periodontal pathogens and the immunoinflammatory response to those organisms. Thus patients may not necessarily have similar disease expression despite the presence of similar bacteria. Likewise, the response to periodontal treatment may vary depending on the wound-healing capacity and susceptibility of the host to further disease progression. The importance of host susceptibility is clearly evident in the medical literature. For example, respiratory tract pathogens may have minimal effect on many individuals, but in a susceptible host, such as an elderly patient, these same pathogens may cause life-threatening respiratory tract illnesses.
There are many systemic conditions that can modify the host’s susceptibility to periodontitis. For example, patients with immune suppression may not be able to mount an effective host response to subgingival microorganisms, resulting in more rapid and severe periodontal destruction. Conversely, individuals with a significant increase in production of proinflammatory mediators may respond to periodontal pathogens with an exuberant inflammatory response that results in destruction of periodontal tissues. Although the potential impact of many systemic disorders on the periodontium is well documented, recent evidence suggests that periodontal infection may significantly enhance the risk for certain systemic diseases or alter the natural course of systemic conditions.75,104 Conditions in which the influences of periodontal infection are documented include coronary heart disease (CHD) and CHD-related events such as angina and infarction, atherosclerosis, stroke, diabetes mellitus, preterm labor, low-birth-weight delivery, and respiratory conditions such as chronic obstructive pulmonary disease75,89 (Box 28-1).
Focal Infection Theory Revisited
Research in the area of periodontal medicine marks a resurgence in the concept of focal infection. In 1900, William Hunter, a British physician, first developed the idea that oral microorganisms were responsible for a wide range of systemic conditions that were not easily recognized as being infectious in nature.82,116 He claimed that restoration of carious teeth instead of extraction resulted in the trapping of infectious agents under restorations. In addition to caries, pulpal necrosis, and periapical abscesses, Hunter also identified gingivitis and periodontitis as foci of infection. He advocated extraction of teeth with these conditions to eliminate the source of sepsis. Hunter believed that teeth were liable to septic infection primarily because of their structure and their relationship to alveolar bone. He stated that the degree of systemic effect produced by oral sepsis depended on the virulence of the oral infection and the individual’s degree of resistance. He also believed that oral organisms had specific actions on different tissues and that these organisms acted by producing toxins, resulting in low-grade “subinfection,” which produced systemic effects over prolonged periods. Finally, Hunter believed that the connection between oral sepsis and resulting systemic conditions could be shown by removal of the causative sepsis through tooth extraction and observation of the improvement in systemic health. Because it explained a wide range of disorders for which there was no known explanation at the time, Hunter’s theory became widely accepted in Britain and eventually the United States, leading to wholesale extraction of teeth.
The focal infection theory fell into disrepute in the 1940s and 1950s when widespread extraction, often of the entire dentition, failed to reduce or eliminate the systemic conditions to which the supposedly infected dentition had been linked.116 The theory, while offering a possible explanation for perplexing systemic disorders, had been based on very little, if any, scientific evidence. Hunter and other advocates of the theory were unable to explain how focal oral sepsis produced these systemic maladies. They were also unable to elucidate possible interactive mechanisms between oral and systemic health. Furthermore, the suggested intervention of tooth extraction often had no effect on the systemic conditions for which patients sought relief. However, Hunter’s ideas did encourage extensive research in microbiology and immunology.
Evidence-Based Clinical Practice
Many of the precepts of the focal infection theory are being revived today in light of recent research demonstrating links between oral and systemic health. However, in order for the “hypothesis not to fall into disrepute for a second time, there must be no unsubstantiated attributions, no theories without evidence.”82 Today’s era of evidence-based medicine and dentistry provides an excellent environment in which to examine the possible relationships between oral infection and systemic disorders. To establish a relationship between conditions A and B, different levels of evidence must be examined. All scientific evidence is not given the same weight.43,78,83 The stronger the evidence, the more likely it is that a true relationship exists between the conditions. Table 28-1 describes these various levels of evidence.
TABLE 28-1 Evaluation of Evidence
For example, in examining the relationship between elevated cholesterol levels and CHD-related events, the literature might initially consist entirely of case reports or similar anecdotal information in which individual patients with recent myocardial infarction (MI) are found to have elevated cholesterol levels. These anecdotal reports suggest a possible relationship between elevated cholesterol and MI, but the evidence is weak. The case reports may lead to cross-sectional studies in which a large subject population is examined to determine whether those individuals who had an MI have higher cholesterol levels than other individuals (control subjects) who did not have an MI. Ideally, these cross-sectional studies are controlled for other potential causes or factors associated with MI such as age, gender, and smoking history. In other words, the subjects with a previous MI would be retrospectively “matched” with subjects of similar age, gender, and smoking history. Then their cholesterol levels would be examined for similarities or differences. Significantly higher cholesterol level in subjects with a previous MI compared with those without MI offers stronger evidence than case reports, and it further substantiates a possible link between elevated cholesterol and MI.
Even stronger evidence is provided by longitudinal studies, in which subject populations are examined over time. For example, a group of subjects might periodically have cholesterol levels evaluated over several years. If individuals with elevated cholesterol levels have a significantly higher rate of MI over time compared with subjects with normal cholesterol levels, even stronger evidence is available to substantiate the link between cholesterol and MI. Finally, intervention trials may be designed to alter the potentially causative condition and to determine the effect of this change on the resultant condition. For example, patients with elevated cholesterol may be divided into two groups: a group who uses a cholesterol-lowering drug or diet and a control group who has no intervention. These two groups might also be compared with a third group with normal cholesterol levels. Over time, the rate of MI in each group would be determined. If the group receiving the cholesterol-lowering regimen has a significantly lower rate of MI than the group with continued elevations in cholesterol level, strong evidence of a link between cholesterol and MI would be established.
Finally, the highest level of evidence is the systematic review. A systematic review is not a standard literature review in which the articles selected for review are based on the desires and search methods chosen by the author, often for convenience. In a systematic review, the topic in question is selected before the review begins. For example, the authors may state the question as, “Compared to subjects not taking cholesterol-lowering medications, do subjects taking such medications demonstrate a difference in the rate of myocardial infarction?” A specific search strategy is then determined to reveal as much potential data as possible to answer the stated question. The authors state specifically why research papers were included or excluded from the review. If possible, the data are subjected to metaanalysis, a statistical method that combines the results of multiple studies that address a similar research hypotheses. This provides a more robust evaluation of the overall data than one can glean from individual research articles.
At each level of evidence, it is important to determine whether a biologically plausible link exists between conditions A and B. For example, if case reports, cross-sectional studies, longitudinal studies, and intervention trials all support the link between cholesterol levels and MI, the following questions remain:
These studies evaluate the mechanisms by which conditions A and B might be linked and provide explanatory data that further substantiate the association between the two conditions.
The focal infection theory, as proposed and defended in the early part of the twentieth century, was based on almost no evidence. Only the occasional case report and other anecdotes were available to substantiate the theory. Although explanatory mechanisms were proposed, none was validated with scientific research. Unfortunately, this theory predated current concepts of evidence-based clinical practice, leading to unnecessary extraction of millions of teeth. Currently, in reexamining the potential associations between oral infections and systemic conditions, it is important to determine what evidence (1) is available, (2) is still needed to substantiate the associations, and (3) validates the possible mechanisms of association. This chapter reviews current knowledge relating periodontal infection to overall systemic health.
Subgingival Environment as Reservoir Of Bacteria
The subgingival microbiota in patients with periodontitis provides a significant and persistent gram-negative bacterial challenge to the host, one that is met by a potent immunoinflammatory response.84 These organisms and their products, such as lipopolysaccharide (LPS), have ready access to the periodontal tissues and to the circulation via the sulcular epithelium, which is frequently ulcerated and discontinuous. Even with treatment, complete eradication of these organisms is difficult, and their reemergence is often rapid. The total surface area of pocket epithelium in contact with subgingival bacteria and their products in a patient with generalized moderate periodontitis has been estimated to be approximately the size of the palm of an adult hand, with even larger areas of exposure in cases of more advanced periodontal destruction.90 Bacteremias are common after mechanical periodontal therapy and also occur frequently during normal daily function and oral hygiene procedures.29,57,76 Just as the periodontal tissues mount an immunoinflammatory response to bacteria and their products, systemic challenge with these agents also induces a major vascular response.23,40,93 This host response may offer explanatory mechanisms for the interactions between periodontal infection and a variety of systemic disorders.
Periodontal Disease and Mortality
The ultimate medical outcome measure is mortality. A number of studies suggest an increased mortality rate is associated with inflammatory periodontal diseases.25,28,49,97 The Normative Aging Study examined 2280 healthy men every 3 years for more than 30 years after baseline clinical, radiographic, laboratory, and electrocardiographic examinations. A subset of this population was examined in the Veterans Affairs (VA) Dental Longitudinal Study to determine age-related changes in the oral cavity and to identify risk factors for oral disease. Clinical examinations were performed, and alveolar bone level measurements determined from full-mouth radiographs. The mean percentage of alveolar bone loss and the mean probing depth were determined for each subject.
A study of data from this subject population sought to determine whether periodontal disease status was a significant predictor of mortality independent of other baseline characteristics within the population.28 From the original sample of 804 dentate, medically healthy subjects, a total of 166 died during the study. Periodontal status at the baseline examination was a significant predictor of mortality independent of other factors such as smoking, alcohol use, cholesterol levels, blood pressure, family history of heart disease, education level, and body mass. For those subjects with the most alveolar bone loss, averaging more than 21% alveolar bone loss at baseline, the risk of dying during the follow-up period was 70% higher than for all other subjects. Interestingly, alveolar bone loss increased the risk of mortality more than smoking (52% increased risk), a well-known risk factor for mortality. A later follow-up of these same subjects confirmed a higher incidence of CHD-related events such as MI and unstable angina among men under the age of 60 with alveolar bone loss than those without bone loss.25 In these studies, periodontitis preceded and increased the risk of mortality. However, this only establishes an association, not causation. It is possible that periodontal disease reflects other health behaviors not evaluated in this study, rather than acting as a specific cause of mortality. In other words, patients with poor periodontal health may also have other risk factors that increase mortality rate (e.g., smoking).
In examining research that suggests oral health status as a possible risk factor for systemic conditions, it is important to recognize when other known risk factors for those systemic conditions have been accounted for in the analysis. Host susceptibility factors that place individuals at risk for periodontitis may also place them at risk for systemic diseases such as cardiovascular disease. In these patients the association may actually be between the risk factors rather than between the diseases. For example, periodontitis and cardiovascular disease share such risk factors as smoking, age, race, male gender, and stress. Genetic risk factors may also be shared.54 In the VA Dental Longitudinal Study, smoking was an independent risk factor for mortality. When examining the data to determine if periodontal status was a risk factor, smoking status and other known risk factors for mortality were removed from the equation to allow independent evaluation of periodontal status. Other studies support the association between poor oral health and an increased risk for mortality.97 In a prospective longitudinal study of subjects with type 2 diabetes, those with severe periodontitis had 3.2 times the risk of death from ischemic heart disease or kidney disease compared to subjects without periodontitis or with only slight periodontitis after adjusting for other risk factors including age, sex, duration of diabetes, glycemic control, macroalbuminuria, body mass index, serum cholesterol concentration, hypertension, and current smoking.97
Periodontal Disease and Coronary Heart Disease/Atherosclerosis
To further explore the periodontal disease and CHD/atherosclerosis association, investigators have studied specific systemic disorders and medical outcomes to determine their relationship to periodontal status. CHD-related events are a major cause of death. MI has been associated with acute systemic bacterial and viral infections, and MI is sometimes preceded by influenzalike symptoms.70,106 Is it possible that oral infection is similarly related to MI? Traditional risk factors, such as smoking, dyslipidemia, hypertension, and diabetes mellitus, do not explain the presence of coronary atherosclerosis in a large number of patients. Localized infection resulting in a chronic inflammatory reaction has been suggested as a mechanism underlying CHD in these individuals.77
In cross-sectional studies of patients with acute MI or confirmed CHD compared with age- and gender-matched control patients, MI patients had significantly worse dental health (periodontitis, periapical lesions, caries, or pericoronitis) than did controls.48,66,69 This association between poor dental health and MI was independent of known risk factors for heart disease, such as age, cholesterol levels, hypertension, diabetes, and smoking. Because atherosclerosis is a major determinant of CHD-related events, dental health has also been related to coronary atheromatosis. Mattila et al67 performed oral radiographic examinations and diagnostic coronary angiography on men with known CHD and found significant correlation between the severity of dental disease and the degree of coronary atheromatosis. This relationship remained significant after accounting for other known risk factors for coronary artery disease (CAD). Similarly, Malthaner et al65 found an increased risk of angiographically defined CAD in subjects with greater bone loss and attachment loss; however, after adjusting for other known cardiovascular risk factors, the relationship between periodontal status and CAD was no longer statistically significant. There is evidence that the extent of periodontal disease may be associated with CHD. For example, there may be a greater risk for CHD-related events, such as MI, when periodontitis affects a greater number of teeth in the mouth, compared with subjects having periodontitis at fewer teeth.5
Cross-sectional studies thus suggest a possible link between oral health and CHD; however, such studies cannot determine causality in this relationship. Rather, dental diseases may be indicators of general health practices. For example, periodontal disease and CHD are both related to lifestyle and share numerous risk factors, including smoking, diabetes, and low socioeconomic status. Bacterial infections have significant effects on endothelial cells, blood coagulation, lipid metabolism, and monocytes/macrophages. The research of Mattila et al66 showed that dental infections were the only factors, other than the classic and well-recognized coronary risk factors, that were associated independently with the severity of coronary atherosclerosis.
Longitudinal studies provide compelling data on this relationship. In a 7-year follow-up study of the patients from the study of Mattila and colleagues, dental disease was significantly related to the incidence of new fatal and nonfatal coronary events, as well as to overall mortality.68 In a prospective study of a national sample of adults, subjects with periodontitis had a 25% increase in the risk for CHD compared with those with no or minimal periodontal disease, after adjusting for other known risk factors.24 Among younger males (age 25 to 49), periodontitis increased the risk of CHD by 70%. The level of oral hygiene was also associated with heart disease. Patients with poor oral hygiene, as indicated by higher debris and calculus scores, had a twofold increased risk for CHD.
In another large prospective study, 1147 men were followed for 18 years.9 During that time, 207 men (18%) developed CHD. When periodontal status at baseline was related to the presence or absence of CHD-related events during follow-up, a significant relationship was found. Subjects with greater than 20% mean bone loss had a 50% increased risk of CHD compared with those with up to 20% bone loss. The extent of sites with probing depth greater than 3 mm was strongly related to the incidence of CHD. Subjects with probing depths greater than 3 mm on at least half their teeth had a twofold increased risk, whereas those with probing depths greater than 3 mm on all teeth had more than a threefold increased risk of CHD. This study and others in which the periodontal condition is known to have preceded the CHD-related events support the concept that periodontal disease is a risk factor for CHD, independent of other classic risk factors. Not all studies, however, support this concept; some show little independent effect of periodontal status on the risk for CHD, after adjusting for commonly accepted cardiovascular risk factors.44,45 It is particularly difficult to control for smoking as a confounding variable in these studies because it is such an important risk factor for both periodontal disease and cardiovascular disease. This confounding influence of smoking makes it difficult to clarify the significance of the relationship between the diseases.
Perhaps the best evidence available comes from systematic reviews of studies examining the relationship between periodontal infection and cardiovascular diseases. Janket et al47 performed a metaanalysis of periodontal disease as a risk factor for future cardiovascular events and found an overall 19% increased risk of such events in individuals with periodontitis. The increase in risk was greater (44%) in people under age 65. Although this increased risk is fairly modest, the extensive prevalence of periodontal disease in the population may increase the significance of the risk from a public health perspective. An extensive systematic review by Scannapieco et al98 concluded that a moderate degree of evidence exists to support an association between periodontal disease and atherosclerosis, MI, and cardiovascular disease but that causality is unclear. Importantly, insufficient evidence exists to show that treatment of periodontal disease has any impact on the risk of heart disease. Intervention trials are needed to make this determination.
Effect of Periodontal Infection
Periodontal infection may affect the onset or progression of atherosclerosis and CHD through both causal and noncausal mechanisms (i.e., by both direct and indirect pathways). Periodontitis and atherosclerosis both have complex etiologic factors, combining genetic and environmental influences. In addition to smoking, the diseases share many risk factors and have distinct similarities in basic pathogenic mechanisms.
Ischemic Heart Disease
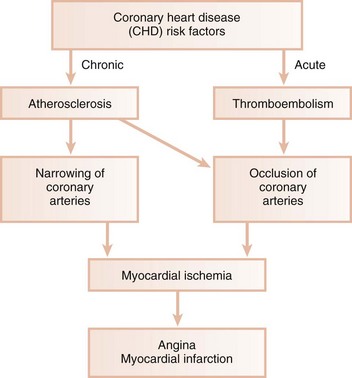
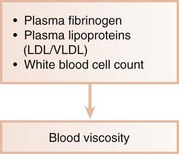
Ischemic heart disease is associated with the processes of atherogenesis and thrombogenesis (Figure 28-1). Increased viscosity of blood may promote major ischemic heart disease and cerebrovascular accident (stroke) by increasing the risk of thrombus formation.62 Fibrinogen is a major factor in promoting this hypercoagulable state. Fibrinogen is the precursor to fibrin, and increased fibrinogen levels increase blood viscosity. Increased plasma fibrinogen is a recognized risk factor for cardiovascular events and peripheral vascular disease63 (Figure 28-2). Elevated white blood cell (WBC) count is also a predictor of heart disease and stroke, and circulating leukocytes may promote occlusion of blood vessels. Coagulation factor VIII/von Willebrand factor (vWF) has likewise been associated with the risk of ischemic heart disease.94
Systemic Infections
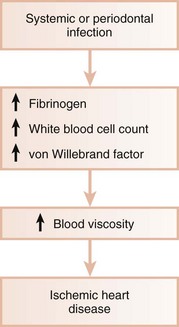
Systemic infections are known to induce a hypercoagulable state and to increase blood viscosity (Figure 28-3). Fibrinogen levels and WBC counts are often increased in patients with periodontal disease.15,56 Individuals with poor oral health may also have significant elevations in coagulation factor VIII/vWF antigen, increasing the risk of thrombus formation. Thus periodontal infection may also promote increased blood viscosity and thrombogenesis, leading to an increased risk for central and peripheral vascular disease.
Daily Activity
Routine daily activities, such as mastication and oral hygiene procedures, result in frequent bacteremia with oral organisms.57 Periodontal disease may predispose the patient to an increased incidence of bacteremia, including the presence of virulent gram-negative organisms associated with periodontitis. An estimated 8% of all cases of infective endocarditis are associated with periodontal or dental disease, without a preceding dental procedure.26 The periodontium, when affected by periodontitis, also acts as a reservoir of endotoxin (LPS) from gram-negative organisms. Endotoxin can pass readily into the systemic circulation during normal daily function, inducing damage to the vascular endothelium and precipitating many negative cardiovascular effects. In a study of the incidence of endotoxemia after simple chewing, subjects with periodontitis were four times more likely to have endotoxin present in the bloodstream than subjects without periodontitis. furthermore, the concentration of endotoxin present was more than fourfold greater in those with periodontitis than in healthy subjects.29
Thrombogenesis
Platelet aggregation plays a major role in thrombogenesis, and most cases of acute MI are precipitated by thromboembolism. Oral organisms may be involved in coronary thrombogenesis. Platelets selectively bind some strains of Streptococcus sanguis, a common component of supragingival plaque, and Porphyromonas gingivalis, a pathogen closely associated with periodontitis.38,39 Aggregation of platelets is induced by the platelet aggregation–associated protein (PAAP) expressed on some strains of these bacteria.95 In animal models, intravenous infusion of PAAP-positive bacterial strains resulted in alterations of heart rate, blood pressure, cardiac contractility, and electrocardiogram (ECG) readings consistent with MI. Platelet accumulation also occurred in the lungs, leading to tachypnea. No such changes were seen with infusion of PAAP-negative strains. PAAP-positive bacteria caused aggregation of circulating platelets, resulting in formation of thromboemboli and the resultant cardiac and pulmonary changes. Thus periodontitis-associated bacteremia with certain strains of S. sanguis and P. gingivalis may promote acute thromboembolic events through interaction with circulating platelets.
Atherosclerosis
Atherosclerosis is a focal thickening of the arterial intima, the innermost layer lining the vessel lumen, and the media, the thick layer under the intima consisting of smooth muscle, collagen, and elastic fibers (Figure 28-4).95 Early in the formation of atherosclerotic plaques, circulating monocytes adhere to the vascular endothelium. This adherence is mediated through several adhesion molecules on the endothelial cell surface, including intercellular adhesion molecule-1 (ICAM-1), endothelial leukocyte adhesion molecule-1 (ELAM-1), and vascular cell adhesion molecule-1 (VCAM-1).11,52 These adhesion molecules are upregulated by a number of factors, including bacterial LPS, prostaglandins, and proinflammatory cytokines. After binding to the endothelial cell lining, monocytes penetrate the endothelium and migrate under the arterial intima. The monocytes ingest circulating low-density lipoprotein (LDL) in its oxidized state and become engorged, forming foam cells characteristic of atheromatous plaques. Once within the arterial media, monocytes may also transform to macrophages. A host of proinflammatory cytokines, such as interleukin-1 (IL-1), tumor necrosis factor alpha (TNF-α), and prostaglandin E2 (PGE2), are then produced, which propagate the atheromatous lesion. Mitogenic factors, such as fibroblast growth factor and platelet-derived growth factor, stimulate smooth muscle and collagen proliferation within the media, thickening the arterial wall.63 Atheromatous plaque formation and thickening of the vessel wall narrow the lumen and dramatically decrease blood flow through the vessel.95 Arterial thrombosis often occurs after an atheromatous plaque ruptures. Plaque rupture exposes circulating blood to arterial collagen and tissue factor from monocytes/macrophages that activate platelets and the coagulation pathway. Platelet and fibrin accumulation forms a thrombus that may occlude the vessel, resulting in ischemic events such as angina or MI. The thrombus may separate from the vessel wall and form an embolus, which may also occlude vessels, again leading to acute events such as MI or cerebral infarction (stroke).
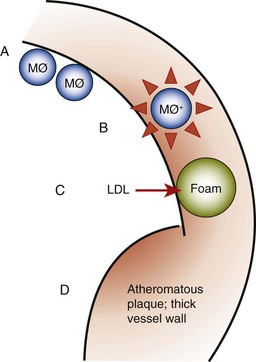
Figure 28-4 Pathogenesis of atherosclerosis. A, Monocytes/macrophages adhere to vascular endothelium. B, Monocytes/macrophages penetrate into arterial media, producing proinflammatory cytokines and growth factors. C, Ingestion of oxidized low-density lipoprotein (LDL) enlarges monocytes to form foam cells. D, Smooth muscle proliferation and plaque formation thicken vessel wall and narrow lumen. MØ+, Hyperinflammatory monocyte/macrophage phenotype.
Role of Periodontal Disease in Myocardial or Cerebral Infarction
In animal models, gram-negative bacteria and associated LPS cause infiltration of inflammatory cells into the arterial wall, proliferation of arterial smooth muscle, and intravascular coagulation. These changes are identical to those seen in naturally occurring atheromatosis. Patients with periodontitis are at increased risk for thickening of the walls of major coronary arteries.8 In several studies of atheromas obtained from humans during endarterectomy, more than half the lesions contained periodontal pathogens, and many atheromas contained multiple different periodontal species.14,36,121 Periodontal diseases result in chronic systemic exposure to products of these organisms. Low-level bacteremia may initiate host responses that alter coagulability, endothelial and vessel wall integrity, and platelet function, resulting in atherogenic changes and possible thromboembolic events (Figure 28-5).
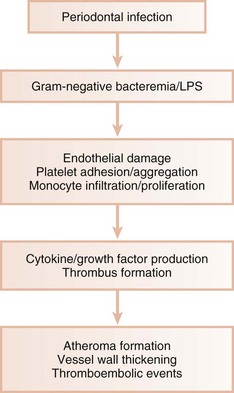
Figure 28-5 Influence of periodontal infection on atherosclerosis. Periodontal pathogens and their products result in damage to vascular endothelium. Monocytes/macrophages enter the vessel wall, producing cytokines that further increase inflammatory response and propagate the atheromatous lesion. Growth factor production leads to smooth muscle proliferation in the vessel wall. Damaged endothelium also activates platelets, resulting in platelet aggregation and potentiating thromboembolic events. LPS, Lipopolysaccharide.
Research has clearly shown a wide variation in host response to bacterial challenge. Some individuals with heavy plaque accumulation and high proportions of pathogenic organisms appear relatively resistant to bone and attachment loss. Others develop extensive periodontal destruction in the presence of small amounts of plaque and low proportions of putative pathogens. Patients with abnormally exuberant inflammatory responses often have a hyperinflammatory monocyte/macrophage phenotype (MØ+). Monocytes/macrophages from these individuals secrete significantly increased levels of proinflammatory mediators (e.g., IL-1, TNF-α, PGE2) in response to bacterial LPS compared with patients with a normal monocyte/macrophage phenotype. Patients with aggressive periodontitis, refractory periodontitis, and type 1 diabetes mellitus often possess the MØ+ phenotype,10 which appears to be under both genetic and environmental control. The monocyte/macrophage cell line is intimately involved in the pathogenesis of both periodontal disease and atherosclerosis. Diet-induced elevations in serum LDL levels upregulate monocyte/macrophage responsiveness to bacterial LPS. Thus elevated LDL levels, a known risk factor for atherosclerosis and CHD, may increase secretion of destructive and inflammatory cytokines by monocytes/macrophages. This may result not only in propagation of atheromatous lesions but also in enhanced periodontal destruction in the presence of pathogenic organisms. This is one example of a potential shared mechanism in the pathogenesis of cardiovascular and periodontal diseases. The presence of an MØ+ phenotype may place patients at risk for both CHD and periodontitis (Figure 28-6). Periodontal infections may contribute to atherosclerosis and thromboembolic events by repeatedly challenging the vascular endothelium and arterial wall with bacterial LPS and proinflammatory cytokines. Vascular monocytes/macrophages in patients with an MØ+ phenotype meet this challenge with an abnormally elevated inflammatory response that may directly contribute to atherosclerosis and may precipitate thromboembolic events.75
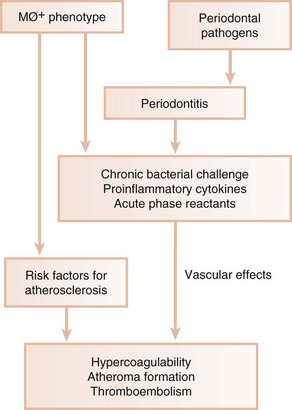
Figure 28-6 Cardiovascular and periodontal consequences of hyperresponsive monocyte/macrophage phenotype. In combination with other risk factors, the MØ+ phenotype predisposes to both atherosclerosis and periodontitis. Bacterial products and inflammatory mediators associated with periodontitis affect vascular endothelium, monocytes/ macrophages, platelets, and smooth muscle and may increase blood coagulability. This may further increase atherosclerosis and result in thromboembolism and ischemic events.
Cardiovascular diseases are increasingly recognized as having a major systemic inflammatory component, further emphasizing possible similarities with inflammatory periodontal diseases.95 As such, detection of systemic inflammatory markers plays an increasingly important role in risk assessment for vascular events such as MI and cerebral infarction. Acute-phase proteins, such as C-reactive protein (CRP) and fibrinogen, are produced in the liver in response to inflammatory or infectious stimuli and act as inflammatory markers.93 CRP induces monocytes/macrophages to produce tissue factor, which stimulates the coagulation pathway and increases blood coagulability. Increased fibrinogen levels may contribute to this process. CRP also stimulates the complement cascade, further exacerbating inflammation.
Elevations in serum CRP and fibrinogen levels are well-accepted risk factors for cardiovascular disease.92,93 Recent efforts have focused on periodontitis as a potential trigger for systemic inflammation. Serum CRP and fibrinogen levels are often elevated in subjects with periodontitis compared with subjects without periodontitis.19,59,117 These acute-phase proteins may act as intermediary steps in the pathway from periodontal infection to cardiovascular disease (see Figures 28-5 and 28-6). Thus periodontal diseases may have both direct effects on the major blood vessels (e.g., atheroma formation) and indirect effects that stimulate changes in the cardiovascular system (e.g., elevation of systemic inflammatory responses).
Interesting supportive evidence for these mechanisms can be derived from intervention trials in which serum levels of inflammatory mediators and markers are assessed before and after treatment aimed at decreasing periodontal inflammation. For example, in subjects with chronic periodontitis, serum levels of IL-6 and CRP are reduced after scaling and root planing.20 Inflammatory periodontal disease is also associated with altered vascular endothelial function compared to periodontally healthy individuals.21 Altered vascular endothelial function is a major risk factor for acute thrombotic events. After scaling and root planing, with a resultant decrease in periodontal inflammation, markers of vascular health also improve significantly over time.21,112 Functional assessment of vascular endothelial function also returns to normal after scaling and root planing.102,112 These results suggest that periodontal inflammation adversely affects the health of the vascular endothelium, whereas a reduction in inflammation improves endothelial health. Whether these changes directly impact the risk of acute cardiovascular events remains to be determined in prospective, controlled clinical intervention trials over long periods of time; these studies do not currently exist.
Periodontal Disease and Stroke
Ischemic cerebral infarction, or stroke, is often preceded by systemic bacterial or viral infection. In one study, patients with cerebral ischemia were five times more likely to have had a systemic infection within 1 week before the ischemic event than were nonischemic control subjects.31 Recent infection was a significant risk factor for cerebral ischemia and was independent of other known risk factors such as hypertension, history of a previous stroke, diabetes, smoking, and CHD. Interestingly, the presence of systemic infection before the stroke resulted in significantly greater ischemia and a more severe postischemic neurologic defect than did stroke not preceded by infection.32 Stroke patients with a preceding infection had slightly higher levels of plasma fibrinogen and significantly higher levels of CRP than those without infection.
Periodontal Infection Associated with Stroke
In case-control studies, poor dental health was a significant risk factor for cerebrovascular ischemia. In one study, bleeding on probing, suppuration, subgingival calculus, and the number of periodontal or periapical lesions were significantly greater in male stroke patients than in controls.107 Overall, 25% of all stroke patients had significant dental infections versus only 2.5% of controls. This study supports an association between poor oral health and stroke in men under age 50. In another study, men and women age 50 and older who had a stroke had significantly more severe periodontitis and more periapical lesions than nonstroke controls.33 Poor dental health was an independent risk factor for stroke. In a longitudinal study over 18 years, subjects with greater than 20% mean radiographic bone loss at baseline were almost three times as likely to have a stroke than subjects with less than 20% bone loss.9 Periodontitis was a greater risk factor for stroke than was smoking and was independent of other known risk factors. Both large epidemiologic studies and systematic reviews of the evidence suggest an approximate threefold increased risk of stroke in subjects with periodontitis.47,118
Stroke is classified as either hemorrhagic or nonhemorrhagic stroke. Nonhemorrhagic stroke is usually caused by thromboembolic events and cerebrovascular atherosclerosis, while hemorrhagic stroke often results from a vascular bleed such as an aneurysm. Periodontal disease has been associated primarily with increased risk of nonhemorrhagic stroke. As previously discussed, periodontal infection may contribute directly to the pathogenesis of atherosclerosis by providing a persistent bacterial challenge to arterial endothelium, contributing to the monocyte/macrophage-driven inflammatory process that results in atheromatosis and narrowing of the vessel lumen. Furthermore, periodontal infection may stimulate a series of indirect systemic effects, such as elevated production of fibrinogen and CRP, which serve to increase the risk of stroke (see Figures 28-5 and 28-6). Finally, bacteremia with PAAP-positive bacterial strains from the supragingival and subgingival plaque can increase platelet aggregation, contributing to thrombus formation and subsequent thromboembolism, the leading cause of stroke.75
Periodontal Disease and Diabetes Mellitus
The relationship between diabetes mellitus and periodontal disease has been extensively examined. It is clear from epidemiologic research that diabetes increases the risk for and severity of periodontal diseases.58 The biologic mechanisms through which diabetes influences the periodontium are discussed in Chapter 27. The increased prevalence and severity of periodontitis typically seen in patients with diabetes, especially those with poor metabolic control, led to the designation of periodontal disease as the “sixth complication of diabetes.”58 In addition to the five “classic” complications of diabetes (Box 28-2), the American Diabetes Association (ADA) has officially recognized that periodontal disease is common in patients with diabetes, and the Association’s Standards of Care include taking a history of current or past dental infections as part of the physician’s examination.3 In fact, the ADA’s 2009 Standards for Medical Care include assessment of a patient’s history of “dental disease” as part of the recommended medical history in diabetic patient evaluation.2 In addition, the Standards specifically recommend the physician refer patients with diabetes to a dentist for oral examination.
Although many studies have examined the effects of diabetes on the periodontium, fewer have examined the effect of periodontal infection on control of diabetes.73 Such studies are difficult to perform because of the influence of ongoing medical management on diabetes control during the study. The following questions remain:
In a longitudinal study of patients with type 2 diabetes, severe periodontitis was associated with significant worsening of glycemic control over time.108 Individuals with severe periodontitis at the baseline examination had a greater incidence of worsening glycemic control over a 2- to 4-year period than did those without periodontitis at baseline. In this study, periodontitis is known to have preceded the worsening of glycemic control. Periodontitis has also been associated with the classic complications of diabetes. Diabetic adults with severe periodontitis at baseline had a significantly greater incidence of kidney and macrovascular complications over the subsequent 1 to 11 years than did diabetic adults with only gingivitis or mild periodontitis.111 This was true despite both groups having similar glycemic control. One or more cardiovascular complications occurred in 82% of patients with severe periodontitis versus 21% of patients without severe periodontitis. Again, severe periodontitis preceded the onset of clinical diabetic complications in these subjects.
In diabetic patients with periodontitis, periodontal therapy may have beneficial effects on glycemic control.73 This may be especially true for patients with relatively poor glycemic control and more advanced periodontal destruction before treatment. Fifty years ago, the potential benefits of periodontal therapy were first described in young adults with type 1 diabetes and severe periodontitis.115,116 Treatment with scaling and root planing, surgery, selected tooth extraction, and systemic antibiotics resulted in decreased insulin demand. In a more recent case series evaluating scaling and root planing combined with systemic doxycycline therapy for 2 weeks, a small group of type 1 diabetic patients with improved periodontal health also had significant improvement in glycemic control (Figure 28-7).80 Conversely, those individuals who demonstrated little beneficial clinical effect from periodontal treatment had no change in glycemic control. It is common to observe wide interindividual variability in responses to various medical management approaches in patients with type 1 diabetes. Similarly, wide variability in the impact of periodontal therapy on glycemic control can be expected in the type 1 diabetic population. For example, a study of periodontal therapy in 65 people with type 1 diabetes and chronic periodontitis showed highly variable responses.110 While there was an overall improvement in periodontal health after therapy, approximately 35% of subjects had an improvement in glycemic control after treatment, 37% had no significant change, and 28% showed a worsening of glycemic control. In this study, subjects were divided into those with better baseline glycemic control (hemoglobin A1c [HbA1c] values <8.5%) and those with poorer glycemic control (HbA1c 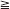 8.5%). Interestingly, twice as many subjects showed improved glycemic control among those whose glycemic control before periodontal treatment was poor compared to those among whom baseline glycemic control was good. Thus, the clinician may anticipate a greater glycemic response to periodontal therapy among those type 1 diabetes patients whose glycemic control is relatively poor than among those whose glycemic control is already good before periodontal treatment.
8.5%). Interestingly, twice as many subjects showed improved glycemic control among those whose glycemic control before periodontal treatment was poor compared to those among whom baseline glycemic control was good. Thus, the clinician may anticipate a greater glycemic response to periodontal therapy among those type 1 diabetes patients whose glycemic control is relatively poor than among those whose glycemic control is already good before periodontal treatment.
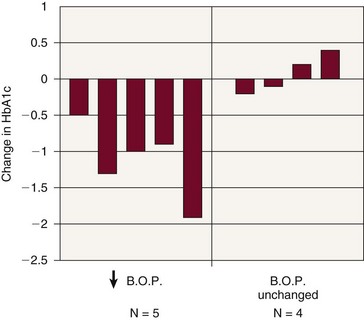
Figure 28-7 Periodontal treatment: effects on glycemic control. In five patients, reductions in periodontal inflammation after mechanical therapy combined with systemic doxycycline were accompanied by improved glycemic control (decreased glycated hemoglobin values, HbA1c). In four patients with no improvement in periodontal health, no improvement in glycemic control occurred. B.O.P., Bleeding on probing (a measure of periodontal inflammation).
(Adapted from Miller LS, Manwell MA, Newbold D, et al: J Periodontol 63:843, 1992.)
In a classic placebo-controlled study of individuals with poorly controlled type 2 diabetes and severe periodontitis, scaling and root planing combined with systemic doxycycline for 14 days was compared with similar treatment combined with systemic placebo.35 All patient groups had significant improvements in periodontal status, with reduced probing depths and bleeding on probing. Those treated with doxycycline had a greater reduction in the prevalence of P. gingivalis, which was more sustained over time. The doxycycline-treated patients also demonstrated significant improvement in glycemic control 3 months after treatment, which gradually reverted to baseline levels at 6 months. Placebo-treated subjects had no significant improvement in glycemic control. These studies suggest that the combination of subgingival mechanical debridement and systemic doxycycline may result in short-term improvement in glycemia in diabetic patients with severe periodontitis and poor metabolic control. Conversely, individuals with moderately well-controlled or well-controlled diabetes and periodontitis who are treated by mechanical therapy alone may demonstrate no significant changes in glycemic control, despite improvement in their periodontal condition.
In studies of subjects treated by mechanical therapy without adjunctive use of antibiotics, significant changes in glycemic control are less common.1,16,105 Many patients in these studies had relatively good glycemic control before treatment, so less impact on metabolic control might be expected. However, some studies show improvement in glycemic control after scaling and root planing without antibiotics. In one such study, a group of well-controlled type 2 diabetic subjects with only gingivitis or mild periodontitis received scaling and root planing without antibiotics.53 Three months after treatment, the subjects showed a 50% reduction in bleeding on probing that was accompanied by a significant improvement in glycemic control. A group of control subjects who received no periodontal treatment showed no change in glycemic control 3 months later, as one might expect given the continued presence of periodontal inflammation.
Although routine use of systemic antibiotics in treatment of chronic periodontitis is not justified, patients with poorly controlled diabetes and severe periodontitis may constitute one patient group for whom such therapy is appropriate. Antibiotics remain an adjunct to the necessary mechanical removal of plaque and calculus. The mechanisms by which adjunctive antibiotics may induce positive changes in glycemic control when combined with mechanical debridement are unknown at this time. Systemic antibiotics may eliminate residual bacteria after scaling and root planing, further decreasing the bacterial challenge to the host. Tetracyclines are also known to suppress glycation of proteins and to decrease activity of tissue-degrading enzymes such as matrix metalloproteinases (MMPs). These changes may contribute to improvement in metabolic control of diabetes. A low-dose form of doxycycline (20 mg) has been introduced with the specific purpose of reducing the production of collagen-degrading MMPs (see Chapter 48). This dose has no antibiotic effects and does not induce antimicrobial resistance with long-term use. Because diabetes is associated with greatly elevated production of collagenase, low-dose doxycycline has been used in treatment of periodontitis in diabetic subjects. Further research is indicated to determine the adjunctive benefit of such host modulation therapies in the diabetic population because little published evidence exists today.
Periodontal Infection Associated with Glycemic Control in Diabetes
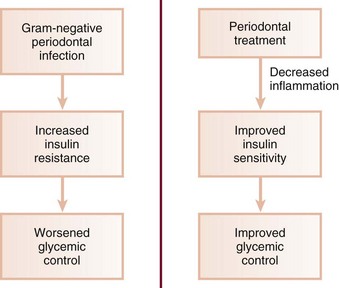
An understanding of the effects of other infections is useful in delineating the mechanisms by which periodontal infection influences glycemia. It is well known that systemic inflammation plays a major role in insulin sensitivity and glucose dynamics. As discussed previously, periodontal diseases can induce or perpetuate an elevated systemic chronic inflammatory state, as reflected in increased serum CRP, IL-6, and fibrinogen levels seen in many people with periodontitis.20,59 Inflammation induces insulin resistance, and such resistance often accompanies systemic infections. For example, acute nonperiodontal bacterial and viral infections have been shown to increase insulin resistance and aggravate glycemic control.96,120 This occurs in individuals with and without diabetes. Systemic infections increase tissue resistance to insulin through a variety of mechanisms, preventing glucose from entering target cells, causing elevated blood glucose levels, and requiring increased pancreatic insulin production to maintain normoglycemia. Insulin resistance may persist for weeks or even months after the patient has recovered clinically from their illness. In the individual with type 2 diabetes, who already has significant insulin resistance, further tissue resistance to insulin induced by infection may considerably exacerbate poor glycemic control. In type 1 patients, normal insulin doses may be inadequate to maintain good glycemic control in the presence of infection-induced tissue resistance. It is possible that chronic gram-negative periodontal infections may also result in increased insulin resistance and poor glycemic control.34 In patients with periodontitis, persistent systemic challenge with periodontopathic bacteria and their products may act similar to well-recognized systemic infections (Figure 28-8). This mechanism would explain the worsening of glycemic control associated with severe periodontitis. Periodontal treatment designed to decrease the bacterial insult and reduce inflammation might restore insulin sensitivity over time, resulting in improved metabolic control. The improved glycemic control seen in several studies of periodontal therapy would support such a hypothesis.
Periodontal Disease and Pregnancy Outcome
Low-birth-weight (LBW) infants (<2500 g at birth) are 40 times more likely to die in the neonatal period than normal-birth-weight (NBW) infants.71 LBW infants who survive the neonatal period are at increased risk for congenital anomalies, respiratory disorders, and neurodevelopmental disabilities. The social and financial costs of LBW infants are enormous, and an emphasis on prevention of low birth weight is preferred to the high-cost intensive care often required to allow survival of LBW infants.
The primary cause of LBW deliveries is preterm labor or premature rupture of membranes (PROM). Factors such as smoking, alcohol, or drug use during pregnancy, inadequate prenatal care, race, low socioeconomic status, hypertension, high or low maternal age, diabetes, and genitourinary tract infections increase the risk of preterm LBW delivery. However, these risk factors are not present in approximately one fourth of preterm LBW cases, leading to a continued search for other causes.30,85
Research has examined the relationship between maternal infection and preterm labor, PROM, and LBW delivery. The true extent of this relationship is difficult to determine because the majority of maternal infections may be subclinical. Genitourinary tract infections have been associated with adverse pregnancy outcomes. Women with bacteriuria have increased rates of preterm delivery, and antibiotic treatment of bacteriuria has resulted in a significant decrease in preterm delivery rates compared with placebo treatment.41,85 Vaginal colonization with group B streptococci or Bacteroides species increases the risk of PROM, preterm delivery, and LBW infants.72
Bacterial vaginosis is the most common vaginal disorder in women of reproductive age. It is caused by changes in the vaginal microflora in which normally predominant facultative lactobacilli are replaced by Gardnerella vaginalis; anaerobic organisms, including species of Prevotella, Bacteroides, Peptostreptococcus, Porphyromonas, and Mobiluncus; and other organisms.42 Bacterial vaginosis is a known risk factor for preterm labor, PROM, and LBW delivery.34,114 The incidence of preterm birth is approximately three times greater in women with bacterial vaginosis compared to those without.4 In fact, treatment of bacterial vaginosis with metronidazole in pregnant women resulted in decreased preterm birth rates compared with placebo treatment.81
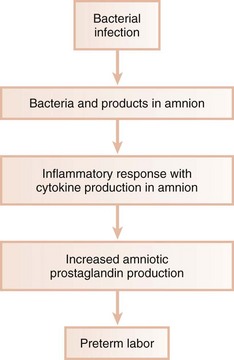
The exact mechanism by which vaginal colonization or genitourinary tract infection may cause PROM and preterm labor is not known.114 The primary mechanism has traditionally been thought to be ascending infection from the vagina and endocervix. Endotoxin (LPS) and bioactive enzymes produced by many organisms associated with vaginosis may directly injure tissue, as well as induce release of proinflammatory cytokines and prostaglandins. Throughout normal gestation, amniotic prostaglandin levels rise steadily until a sufficient threshold is reached that induces labor and delivery. Maternal infection may cause increased amniotic prostaglandin production and may result in labor-inducing levels being achieved before full gestation. In addition to prostaglandins, various proinflammatory cytokines (e.g., IL-1, IL-6, or TNF) have been found in the amniotic fluid of women with preterm labor. Women with preterm labor often have culture-positive amniotic fluid, even in the absence of clinical infection. Of culture-positive patients, the species most often isolated is Fusobacterium nucleatum.41 Although F. nucleatum is occasionally isolated from the vaginal flora in bacterial vaginosis, its prevalence in women with preterm labor is much greater than in vaginosis. F. nucleatum is even less frequently isolated from the vaginal flora of women without bacterial vaginosis. Many of the other species isolated from amniotic fluid in women with preterm labor are those often found in bacterial vaginosis, which supports an ascending route of infection. However, the frequency of F. nucleatum detection suggests other possible routes of infection. Some investigators have suggested infection by a hematogenous route from a location in which the organism is often detected.41 F. nucleatum is a common oral species highly prevalent in patients with periodontitis and could reach the amniotic fluid by hematogenous spread from the oral cavity. This route is also suggested by the occasional isolation of Capnocytophaga species in the amniotic fluid of women with preterm labor, an organism rarely isolated from the vagina but common in the oral cavity. The species and subspecies of F. nucleatum isolated from amniotic fluid cultures in women with preterm labor more closely match those found in subgingival plaque than strains identified from the lower genital tract. In addition to hematogenous spread, another possible route of infection is by oral-genital contact, with transfer of oral organisms to the vagina.41
Although direct effects of microorganisms may play an important role in many cases of preterm labor, PROM, and LBW delivery, indirect mechanisms may also be operative.85 Bacterial infection of the chorioamnion, or extraplacental membrane, may lead to chorioamnionitis, a condition strongly associated with PROM and preterm delivery. However, many cases of histologic chorioamnionitis demonstrate negative bacterial cultures, indicating that infection is not the sole cause of this condition. It is likely that an indirect mechanism may be active in which the cascade of host products produced in response to infection is often responsible for preterm labor. Maternal infection may lead to the presence of amniotic bacterial products, such as LPS from gram-negative organisms, which stimulate production of host-derived cytokines in the amnion and decidua (Figure 28-9). These cytokines, including IL-1, TNF-α, and IL-6, stimulate increased prostaglandin production from the amnion and decidua, leading to onset of preterm labor. A premature rise in PGE2 and PGF2α is characteristic of preterm labor, regardless of whether clinical or subclinical maternal genitourinary tract infection is detected.
The question then arises as to what stimulates the increased cytokine levels and resultant increased prostaglandin levels seen in preterm delivery in patients with no evidence of genitourinary infection. Many cases of preterm LBW could result from infections of unknown origin such as infections originating in areas other than the genitourinary tract.
Periodontitis is a remote gram-negative infection that may play a role in LBW infants. As discussed previously, periodontopathic organisms and their products may have wide-ranging effects, most likely mediated through stimulation of host cytokine production in target tissues. Animal studies suggest that remote reservoirs of gram-negative organisms and their products may have a negative impact on pregnancy outcome. P. gingivalis implanted in subcutaneous chambers during gestation caused significant increases in TNF-α and PGE2 levels.18 This localized subcutaneous infection resulted in a significant increase in fetal death and a decrease in fetal birth weight for those that remained viable, compared with control animals that were not inoculated. There was a significant correlation between both TNF-α and PGE2 levels, as well as fetal death and growth retardation. These data suggest that a remote, nondisseminated infection with P. gingivalis may result in abnormal pregnancy outcomes in this model.
Decreased fetal birth weight and increased fetal death were also seen after intravenous injections with LPS derived from P. gingivalis.17 This effect was greatly increased when P. gingivalis LPS was administered before mating and during gestation, indicating that repeated immunization with P. gingivalis LPS does not provide protection during pregnancy but potentiates the negative effects of LPS exposure during gestation. P. gingivalis-induced experimental periodontitis in animal models resulted in decreased fetal birth weight and increased amniotic fluid levels of TNF-α and PGE2 (Figure 28-10).85 This provides direct evidence that periodontal infection can affect the fetal environment and pregnancy outcome.
Figure 28-10 Amniotic fluid levels in experimental periodontitis. Experimental periodontitis resulted in increased amniotic fluid levels of tumor necrosis factor alpha (TNF-α) and prostaglandin E2 (PGE2) in the pregnant hamster model, providing evidence that periodontal infection can affect the fetal environment.
(From Offenbacher S, Jarad HL, O’Reilly PG, et al: Ann Periodontol 3:233, 1998.)
These animal studies have led to examination of the potential effects of periodontitis on pregnancy outcome in humans. This relationship has been examined in numerous studies from many countries around the world. In an initial case-control study of 124 women in the United States (93 cases with at least one LBW delivery and 31 controls with at least one NBW delivery), Offenbacher et al86 found that women having LBW infants had significantly greater clinical attachment loss than women having NBW infants. After adjusting for known risk factors for LBW delivery, women with periodontitis resulting in greater than 3 mm of attachment loss in at least 60% of sites had a 7.5-fold increased risk of having a LBW infant. In fact, periodontitis contributed to more preterm LBW cases than did smoking or alcohol use during pregnancy. In a large prospective study of more than 1300 pregnant women, subjects with generalized periodontitis had a fivefold increased risk of preterm birth before 35 weeks of gestation and a sevenfold increased risk of delivery before 32 weeks compared with women without periodontitis.51 These studies and others22,60 indicate a strong association between periodontal infection and adverse pregnancy outcomes. However, not all studies demonstrate such a relationship. For example, a large case-control study including over 900 Brazilian women showed no significant differences in periodontal status among women having normal deliveries compared to those having preterm and/or LBW deliveries.7
Excellent systematic reviews have recently been published examining the wide range of studies evaluating the relationship between periodontitis and pregnancy outcome. A metaanalysis of 17 studies involving over 7000 subjects confirmed a significant relationship between periodontitis and preterm and/or LBW infants.113 The pooled estimate for the risk of having a preterm and/or LBW infants in mothers with periodontal disease was 2.83 compared to mothers without periodontal disease. In another systematic review of 25 studies, 18 studies showed a significant association between periodontal disease and increased risk of adverse pregnancy outcome, whereas 7 studies showed no such association.119
One potential mechanism by which periodontal disease could impact pregnancy outcome is by systemic dissemination of periodontal pathogens. In a cross-sectional study, women having LBW infants had significantly higher levels of Aggregatibacter (formerly Actinobacillus) actinomycetemcomitans, Tannerella forsythia, P. gingivalis, and Treponema denticola in their subgingival plaque than did the control women having NBW infants.85 Studies of fetal immunoglobulin M (IgM) antibody levels have found a remarkably high fetal antibody seropositivity to oral bacteria.64 This indicates that the fetal immune response is activated in utero by bacterial antigens derived from the oral cavity. In fact, preterm babies have been shown to have a threefold increased prevalence of IgM seropositivity to many of the classic periodontopathogens when compared to full-term babies.64 In particular, 20% of preterm infants had a positive IgM response to Campylobacter rectus, compared to 6.3% of term infants. The prevalence of fetal IgM seropositivity to other periodontopathogens, such as P. intermedia, P. micros, and F. nucleatum, was fourfold to eightfold higher in preterm infants compared to normal term neonates. This suggests that the immune system of preterm infants is often challenged by organisms from the mother’s oral cavity.
Women having LBW infants also have higher levels of gingival crevicular fluid (GCF), PGE2, and IL-1.85 In primiparous women (those experiencing a first birth), GCF levels of PGE2 were inversely related to their infants’ birth weight. Women with higher PGE2 levels in GCF had smaller and more premature infants. GCF levels of IL-1 and PGE2 have been shown to correlate highly with intraamniotic IL-1 and PGE2 levels. In fact, measuring GCF levels of these inflammatory mediators has been suggested as a less invasive means of screening expectant mothers for elevated amniotic IL-1 and PGE2 levels than amniocentesis.85 Thus women having LBW infants often have a higher prevalence and severity of periodontitis, more gingival inflammation, higher levels of putative periodontal pathogens, and an elevated subgingival inflammatory response compared with women having NBW infants; furthermore, the increased bacterial challenge may have broader systemic effects, resulting in an elevated challenge to the fetal immune system.
Periodontal disease may increase the risk for preeclampsia. This hypertensive disorder affects about 5% to 10% of pregnancies and is a major cause of perinatal and maternal morbidity and mortality. Preeclampsia has multiple potential etiologies, several of which involve vascular changes in the placenta that are similar to those seen in atherosclerosis. The presence of periodontitis during pregnancy or a worsening of periodontal disease during pregnancy is associated with a 2.0- to 2.5-fold increased risk for preeclampsia.12 In one study, women with severe preeclampsia were 3.8 times more likely to present with extensive periodontal disease than were normotensive women, while women with mild preeclampsia were 2.4 times more likely to have extensive periodontal disease than were normotensive women.13
Several intervention trials have examined the effects of treating periodontal disease during gestation, rather than waiting until after parturition to provide needed care. In a study of 351 pregnant women with periodontitis, women who received scaling and root planing before 28 weeks’ gestation, followed by prophylaxis every 2 weeks until parturition, had an LBW rate of 1.8%.61 Conversely, women who did not receive periodontal therapy during gestation but instead were treated after parturition had an LBW rate of 10.1%. Similarly, Jeffcoat et al50 found a reduced preterm birth rate in women who received mechanical periodontal therapy during gestation. Conversely, other studies have shown that providing periodontal treatment during pregnancy improves periodontal health but has minimal impact on pregnancy outcome.79 Given the conflicting evidence, it is currently unclear whether periodontal therapy provided during gestation has a beneficial effect on pregnancy outcome. What is clear is that scaling and root planing provided during the second and third trimester of gestation is a safe procedure. Every intervention trial published to date shows that scaling and root planing during pregnancy is associated with either a decrease or no change in adverse pregnancy outcome rates. Not a single study has shown an increase in adverse outcome rates. Thus the clinician should feel comfortable providing scaling and root planing during the second and third trimester of pregnancy.
Periodontal Disease and Chronic Obstructive Pulmonary Disease
Chronic obstructive pulmonary disease (COPD) is characterized by airflow obstruction resulting from chronic bronchitis or emphysema. Bronchial mucous glands enlarge, and an inflammatory process occurs in which neutrophils and mononuclear inflammatory cells accumulate within the lung tissue.37,109 About 14 million Americans have COPD, and tobacco smoking is the primary risk factor.
COPD shares similar pathogenic mechanisms with periodontal disease. In both diseases, a host inflammatory response is mounted in response to chronic challenge by bacteria in periodontal disease and by factors, such as cigarette smoke, in COPD. The resulting neutrophil influx leads to release of oxidative and hydrolytic enzymes that cause tissue destruction directly. Recruitment of monocytes and macrophages leads to further release of proinflammatory mediators.
Less is known about the clinical relationship between periodontal disease and COPD compared with CHD and other systemic conditions. In analyzing data from a longitudinal study of more than 1100 men, alveolar bone loss was associated with the risk for COPD.37 Over a 25-year period, 23% of subjects were diagnosed with COPD. Subjects with more severe bone loss at the baseline dental examination had a significantly increased risk of subsequently developing COPD compared with subjects with less bone loss. The increase in risk was independent of age, smoking status, and other known risk factors for COPD. Individuals with poor oral hygiene have also been found to be at increased risk for chronic respiratory diseases such as bronchitis and emphysema.100 Conversely, a large epidemiologic study revealed no significant overall association between periodontal disease and COPD.46 In nonsmokers and former smokers, even severe periodontitis did not increase the risk for COPD. In current smokers, however, the presence of severe periodontitis was associated with an increased risk of COPD. These results suggest that smoking may act as a major “effect modifier” in the relationship between COPD and periodontal disease and that this modifier’s presence may be required to generate an effect. A recent systematic review of available evidence stated that insufficient evidence exists on the potential association between COPD and periodontal disease.114 Therefore more research is needed in this area.
Periodontal Disease and Acute Respiratory Infections
The upper respiratory passages are often contaminated with organisms derived from the oral, nasal, and pharyngeal regions. Conversely, the lower airways, in which gas exchange occurs, are generally maintained free of microorganisms by a combination of host immune factors and mechanical clearance through the cough reflex, ciliary transport of aspirated contaminants, and movement of secretions from the lower airways into the trachea.109 Pneumonia is an infection of the lungs caused by bacteria, viruses, fungi, or mycoplasma and is broadly categorized as either community-acquired or hospital-acquired pneumonia. A wide variety of bacteria may cause pneumonia, and the spectrum of offending organisms differs greatly between community-acquired and hospital-acquired infections.
Community-acquired bacterial pneumonia is caused primarily by inhalation of infectious aerosols or by aspiration of oropharyngeal organisms. Streptococcus pneumoniae and Haemophilus influenzae are most common, although numerous other species may be found, including anaerobic bacteria.88 Antibiotic therapy is highly successful in resolution of most cases of community-acquired bacterial pneumonia. To date, no associations have been found between oral hygiene or periodontal disease and the risk for acute respiratory conditions such as pneumonia in community-dwelling individuals.100
The same cannot be said for individuals in the hospital setting. Hospital-acquired (nosocomial) bacterial pneumonia has a very high morbidity and mortality rate. The incidence of nosocomial pneumonia is highest in severely ill patients such as those in intensive care units or on ventilatory support. Although nosocomial pneumonia is most often caused by gram-negative aerobic organisms, many cases are the result of infection by anaerobic bacteria, including those typically found in the subgingival environment.
![]() Science Transfer
Science Transfer
Clinicians have the responsibility to treat periodontal disease not only to maintain oral health but also to reduce the risk of systemic diseases related to active periodontal inflammation. The relationship between periodontal disease and arteriosclerosis is particularly important. Patients with periodontal disease are at greater risk for arteriosclerotic coronary artery disease (CAD) than patients with healthy periodontal tissues. There is also a connection between periodontal disease and thromboembolic events such as stroke. Diabetic patients may have improved diabetic status once their active periodontal disease is treated.
Dental clinicians need to inform patients of these risks and can play a role in enhancing the quality and longevity of life for their patients. Also, dentists need to communicate with physicians so that susceptible patients are evaluated for periodontal disease and receive appropriate treatment. Once successful periodontal therapy is completed, appropriate long-term maintenance therapy is essential to consolidate the reduction of the risk of systemic diseases.
Hospital-acquired pneumonia is usually caused by aspiration of oropharyngeal contents.99 Oropharyngeal colonization with potential respiratory pathogens (PRPs) is generally a transient phenomenon; however, such colonization increases during hospitalization. The longer the hospital stay, the greater the prevalence of PRPs. PRPs are found predominantly in the gastrointestinal tract and may be passed through esophageal reflux into the oropharynx, where they colonize. Subsequent aspiration may lead to pneumonia. Patients whose posterior oropharynx becomes colonized with PRPs have a significantly increased risk of developing nosocomial pneumonia compared with those without oropharyngeal colonization by PRPs.101 Dental plaque has been shown to serve as a reservoir of PRPs; such colonization in plaque results in a persistent source of potential aspiration.27
Selective decontamination is a technique that combines systemic antibiotics with orally administered nonabsorbable antibiotics in an attempt to eradicate PRPs from the digestive tract and oropharynx and thereby minimize the risk of nosocomial respiratory infections. The technique is used primarily in patients who are intubated and on mechanical ventilators. Selective decontamination significantly decreases the incidence of nosocomial pneumonia.103 Decontamination of only the digestive tract does not reduce the incidence of pneumonia, but decontamination of the oropharynx alone does.91 This provides further evidence that the oropharynx is the primary site of PRP colonization, with subsequent aspiration of causative organisms leading to pneumonia.
PRPs may also originate in the oral cavity, with dental plaque serving as a reservoir of these organisms.6 Poor oral hygiene is common in the hospital and nursing home settings, especially in severely ill patients.101 PRPs are more often isolated from supragingival plaque and buccal mucosa of patients in intensive care units than in outpatient settings. Thus organisms that are not routinely found in dental plaque become plaque colonizers after prolonged hospitalization. Subgingival plaque may also harbor PRPs, and putative periodontal pathogens have been associated with nosocomial pneumonia. Furthermore, anaerobic organisms from periodontal pockets may serve as the primary inoculum for suppurative respiratory diseases (e.g., pulmonary abscesses) that have significant morbidity and mortality.
Systematic review of the evidence has determined that interventions used to improve oral hygiene, such as mechanical toothbrushing and chemical antimicrobial rinses, have the potential to decrease the risk of nosocomial pneumonia in high-risk patients such as those in intensive care units or those on ventilators.6,99 Improved oral hygiene by the patient, as well as frequent oral health care provided professionally, reduces both the incidence and the progression of respiratory diseases in older patients living in nursing homes and has a major impact on those in intensive care units in which the risks for nosocomial pneumonia are markedly elevated.
Periodontal Medicine in Clinical Practice
The concept of periodontal diseases as localized entities affecting only the teeth and supporting apparatus is oversimplified. Rather than being confined to the periodontium, inflammatory periodontal diseases may have wide-ranging systemic effects. In most persons, these effects may be relatively inconsequential or at least not clinically evident. In susceptible individuals, however, periodontal infection may act as an independent risk factor for systemic disease and may be involved in the basic pathogenic mechanisms of these conditions. Furthermore, periodontal infection may exacerbate existing systemic disorders.
Periodontal Disease and Systemic Health
Proper use of the knowledge of potential relationships between periodontal disease and systemic health requires the dental professional to recognize the oral cavity as one of many interrelated organ systems. An infection the size of one’s palm on the leg of a pregnant woman would be a major concern to the patient and her health care provider, given the potential negative consequences of this localized infection on fetal and maternal health. A similar suppurating infection on the foot of a person with diabetes would be cause for immediate evaluation and aggressive treatment, knowing the effects of such infections on metabolic control of diabetes.
Periodontal infection must be viewed in a similar manner. Periodontitis is a gram-negative infection often resulting in severe inflammation, with potential intravascular dissemination of microorganisms and their products throughout the body. However, periodontitis tends to be a “silent” disease until destruction results in acute oral symptoms. Most patients, as well as many medical professionals, do not recognize the potential infection that may exist within the oral cavity.
Patient Education
Patient education is a priority. Only 30 years ago, the factors involved in CHD were unclear. At present, however, it would be difficult to find an individual who was unfamiliar with the link between cholesterol and heart disease. This change was precipitated by research clearly demonstrating the increased risk for heart disease in individuals with high cholesterol levels, followed by intensive education efforts to spread the message from the scientific community to the public at large. It is important to recognize that high cholesterol levels have not been shown to cause heart disease in all individuals, but rather significantly increase the risk of disease. Cholesterol has also been demonstrated to have a biologically plausible role in the pathogenesis of CHD.
Similarly, patient education efforts in the realm of periodontal medicine must emphasize the inflammatory nature of periodontal infections, the increased risk for systemic disease associated with the infection, and the biologically plausible role periodontal infection may play in systemic disease. Few individuals had their cholesterol levels evaluated until the knowledge of the link between cholesterol and heart disease became widespread. Likewise, increased appreciation of the potential effects of periodontal infection on systemic health may result in increased patient demand for periodontal evaluation.
Enhanced community awareness may be derived from newspapers, magazines, and other lay sources. However, the most reliable origin of information should be the dental and medical professions through daily contact with patients. The pregnant woman usually knows that infections may adversely affect her pregnancy. Individuals with diabetes generally know that infections impair glycemic control. However, many of these patients do not know that occult periodontal infections can have the same effect as more clinically evident infections. The dentist is responsible for diagnosing periodontal infections, providing appropriate treatment, and preventing disease recurrence or progression. Because many medical professionals are unfamiliar with the oral cavity and oral health research, dentists must reach out to the medical community to improve patient care through education and communication.74 Likewise, patients must be educated in disease prevention. Just as patients know that lowering cholesterol levels may decrease their risk for heart disease, prevention of periodontal infection should be emphasized. A physician would be remiss if he or she did not provide education on decreasing cholesterol, losing weight, and ceasing a smoking habit to a patient at risk for CHD. Likewise, controlling the risk factor of periodontal infection requires the dentist to emphasize personal and professional preventive measures focused on thorough oral hygiene and regular recall.
Summary
Does periodontal disease cause CHD, COPD, or adverse pregnancy outcomes? This question may only be answered based on the evidence currently available, with the full knowledge that conclusions may change as future evidence dictates. Periodontal disease may increase the risk for many systemic disorders. Biologically plausible mechanisms support the role of periodontal infection in these conditions, but periodontal infection should not be presented as the cause of such systemic diseases any more than cholesterol is said to cause heart disease. Periodontal infection is one of many potential risk factors for a number of systemic conditions. Fortunately, it is a readily modifiable risk factor, unlike age, gender, and genetic influences.
The focal infection theory of the early twentieth century was widely and appropriately discredited when treatment based on the theory, consisting almost solely of tooth extraction, had no effect on the underlying diseases that oral sepsis supposedly caused. Similarly, the clinical utility of our current knowledge base is only now evolving. Future research will further delineate the role of periodontal infection in systemic health. The associations between periodontal infection and conditions, such as LBW delivery, diabetes, cardiovascular and cerebrovascular diseases, and respiratory diseases, may be further substantiated. Longitudinal studies and intervention trials are needed before any causative role can be assigned.
The emerging field of periodontal medicine offers new insights into the concept of the oral cavity as one system interconnected with the whole human body. For many years the dental profession has recognized the effects of systemic conditions on the oral cavity. Only now, however, are dental professionals beginning to understand more fully the impact of the periodontium on systemic health.
Finally, it is important to appreciate the differences between the science and art of dental practice as it relates to periodontal medicine. Science is based, in general, on means and standard deviations (or standard errors). Thus science may determine that, on average, diabetic patients with periodontal disease have poorer glycemic control than diabetic patients who are periodontally healthy. However, the patient sitting in the dental chair at any given point in time may or may not be a “mean” patient, that is, she may demonstrate the same relationship that science has determined to exist as an average within the population, or she may not. She may lie somewhere within or outside the standard deviation. She may have extremely poor glycemic control that is directly related to her extensive periodontal inflammation, or she may have average glycemic control, or she may have good glycemic control. The clinical practice of periodontal medicine recognizes that while using an evidence-based approach is absolutely key to the modern dentistry, each patient is an individual and he or she may not always fit the average determined by science.
1 Aldridge JP, Lester V, Watts TLP, et al. Single-blind studies of the effects of improved periodontal health on metabolic control in type 1 diabetes mellitus. J Clin Periodontol. 1995;22:271-275.
2 American Diabetes Association Standards of Medical Care in Diabetes—2009. Diabetes Care. 2009;32(suppl 1):s13-s61.
3 American Diabetes Association. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(suppl 1):s5-s20.
4 Andrews WW, Hauth JC, Goldenberg RL. Infection and preterm birth. Am J Perinatol. 2000;17:357-365.
5 Arbes SJJr, Slade GD, Beck JD. Association between extent of periodontal attachment loss and self-reported history of heart attack: an analysis of NHANES III data. J Dent Res. 1999;78:1777-1782.
6 Azarpazhooh A, Leake JL. Systematic review of the association between respiratory diseases and oral heath. J Periodontol. 2006;77:1465-1482.
7 Bassani DG, Olinto MT, Kreiger N. Periodontal disease and perinatal outcomes: a case-control study. J Clin Periodontol. 2007;34:31-39.
8 Beck JD, Elter JR, Heiss G. Relationship of periodontal disease to carotid artery intima-media wall thickness: the Atherosclerosis Risk in Communities (ARIC) study. Arterioscler Thromb Vasc Biol. 2001;21:1816.
9 Beck JD, Garcia RG, Heiss G, et al. Periodontal disease and cardiovascular disease. J Periodontol. 1996;67:1123-1137.
10 Beck JD, Offenbacher S, Williams R, et al. Periodontitis: a risk factor for coronary heart disease? Ann Periodontol. 1998;3:127-141.
11 Beck JD, Offenbacher S. The association between periodontal diseases and cardiovascular diseases: a state-of-the-art review. Ann Periodontol. 2001;6:9-15.
12 Boggess KA, Lieff S, Murtha AP, et al. Maternal periodontal disease is associated with an increased risk for preeclampsia. Obstet Gynecol. 2003;101:227-231.
13 Canakci V, Canakci CF, Yildrim A, et al. Periodontal disease increases the risk of severe pre-eclamsia among pregnant women. J Clin Periodontol. 2007;34:639-645.
14 Chiu B. Multiple infections in carotid atherosclerotic plaques. Am Heart J. 1999;138(suppl):s534-s536.
15 Christan C, Dietrich T, Hagewald S, et al. White blood cell count in generalized aggressive periodontitis after non-surgical therapy. J Clin Periodontol. 2002;29:201-206.
16 Christgau M, Pallitzsch KD, Schmalz G, et al. Healing response to non-surgical periodontal therapy in patients with diabetes mellitus: clinical, microbiological and immunological results. J Clin Periodontol. 1998;25:112-124.
17 Collins JG, Smith MA, Arnold RR, et al. Effects of a Escherichia coli and Porphyromonas gingivalis lipopolysaccharide on pregnancy outcome in the golden hamster. Infect Immun. 1994;62:4652-4655.
18 Collins JG, Windley HWIII, Arnold RR, et al. Effects of a Porphyromonas gingivalis infection on inflammatory mediator response and pregnancy outcome in hamsters. Infect Immun. 1994;62:4356-4361.
19 Craig RG, Yip JK, So MK, et al. Relationship of destructive periodontal disease to the acute-phase response. J Periodontol. 2003;74:1007-1016.
20 D’Aiuto F, Parkar M, Andreou G, et al. Periodontitis and systemic inflammation: control of the local infection is associated with a reduction in serum inflammatory markers. J Dent Res. 2004;83:156-160.
21 D’Aiuto F, Parkar M, Tonetti MS. Acute effects of periodontnal therapy on bio-markers of vascular health. J Clin Periodontol. 2007;34:124-129.
22 Dasanayake AP. Poor periodontal health of the pregnant woman as a risk factor for low birth weight. Ann Periodontol. 1998;3:206-212.
23 DeNardin E. The role of inflammatory and immunological mediators in periodontitis and cardiovascular disease. Ann Periodontol. 2001;6:30-40.
24 DeStefano F, Andra RF, Kahn HS, et al. Dental disease and risk of coronary heart disease and mortality. Br Med J. 1993;306:688-691.
25 Dietrich T, Jimenez M, Krall Kaye EA, Vokonas PS. Age dependent associations between chronic periodontitis: eduntulism and risk of coronary heart disease. Circulation. 2008;117:1668-1674.
26 Drangsholt MT. A new causal model of dental diseases associated with endocarditis. Ann Periodontol. 1998;3:184-196.
27 El-Solh AA, Pietrantoni C, Bhat A, et al. Colonization of dental plaques. A reservoir of respiratory pathogens for hospital-acquired pneumonia in institutionalized elders. Chest. 2004;126:1575-1582.
28 Garcia RI, Krall EA, Vokonas PS. Periodontal disease and mortality from all causes in the VA Dental Longitudinal Study. Ann Periodontol. 1998;3:339-349.
29 Geerts SO, Nys M, De MP, et al. Systemic release of endotoxins induced by gentle mastication: association with periodontitis severity. J Periodontol. 2002;73:73-78.
30 Gibbs RS. The relationship between infections and adverse pregnancy outcomes: an overview. Ann Periodontol. 2001;6:153-163.
31 Grau AJ, Buggle F, Heindl S, et al. Recent infection as a risk factor for cerebrovascular ischemia. Stroke. 1995;26:373-379.
32 Grau AJ, Buggle F, Steichen-Wiehn C, et al. Clinical and biochemical analysis in infection-associated stroke. Stroke. 1995;26:1520-1526.
33 Grau AJ, Buggle F, Ziegler C, et al. Association between acute cerebrovascular ischemia and chronic and recurrent infection. Stroke. 1997;28:1724-1729.
34 Grossi SG, Mealey BL, Rose LF. Effect of periodontal infection on systemic health and well-being. In: Rose LF, Mealey BL, Genco RJ, Cohen DW, editors. Periodontics: medicine, surgery and implants. St Louis: Elsevier, 2004.
35 Grossi SG, Skrepcinski FB, DeCaro T, et al. Treatment of periodontal disease in diabetics reduces glycated hemoglobin. J Periodontol. 1997;68:713-719.
36 Haraszthy VI, Zambon JJ, Trevisan M, et al. Identification of periodontal pathogens in atheromatous plaques. J Periodontol. 2000;71:1554-1560.
37 Hayes C, Sparrow D, Cohen M, et al. The association between alveolar bone loss and pulmonary function: the VA Dental Longitudinal Study. Ann Periodontol. 1998;3:257-261.
38 Herzberg MC, Meyer MW. Dental plaque, platelets and cardiovascular diseases. Ann Periodontol. 1998;3:151-160.
39 Herzberg MC, Meyer MW. Effects of oral flora on platelets: possible consequences in cardiovascular disease. J Periodontol. 1996;67:1138-1142.
40 Herzberg MC. Coagulation and thrombosis in cardiovascular disease: plausible contributions of infectious agents. Ann Periodontol. 2001;6:16-19.
41 Hill GB. Preterm birth: associations with genital and possibly oral microflora. Ann Periodontol. 1998;3:222-232.
42 Hill GB. The microbiology of bacterial vaginosis. Am J Obstet Gynecol. 1993;169:450-454.
43 Hujoel P. Grading the evidence: the core of EBD. J Evid Based Dent Pract. 2008;8:116-118.
44 Hujoel PP, Drangsholt M, Spiekerman C, Derouen TA. Examining the link between coronary heart disease and the elimination of chronic dental infections. J Am Dent Assoc. 2001;132:883-889.
45 Hujoel PP, Drangsholt M, Spiekerman C, DeRouen TA. Periodontal disease and coronary heart disease risk. JAMA. 2000;284:1406-1410.
46 Hyman JJ, Reid BC. Cigarette smoking, periodontal disease, and chronic obstructive pulmonary disease. J Periodontol. 2004;75:9-15.
47 Janket S, Baird AE, Chuang S, Jones JA. Meta-analysis of periodontal disease and risk of coronary heart disease and stroke. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:559-569.
48 Janket S, Qvarnstrom M, Muerman JH, et al. Asymptomatic dental score and prevalent coronary heart disease. Circulation. 2004;109:1095-1100.
49 Jansson L, Lavstedt S, Frithiof L. Relationship between oral health and mortality rate. J Clin Periodontol. 2002;29:1029-1034.
50 Jeffcoat MJ, Hauth JC, Guers N, et al. Periodontal disease and preterm birth: results of a pilot intervention study. J Periodontol. 2003;74:1214-1218.
51 Jeffcoat MK, Guers NC, Reddy MS, et al. Periodontal infection and preterm birth: results of a prospective study. J Am Dent Assoc. 2001;132:875-880.
52 Kinane DF. Periodontal disease’s contributions to cardiovascular disease: an overview of potential mechanisms. Ann Periodontol. 1998;3:142-150.
53 Kiran M, Arpak N, Unsal E, Erdogan MF. The effect of improved periodontal health on metabolic control in type 2 diabetes mellitus. J Clin Periodontol. 2005;32:266-272.
54 Kornman KS, Duff GW. Candidates genes as potential links between periodontal and cardiovascular diseases. Ann Periodontol. 2001;6:48-57.
55 Kornman KS. Mapping the pathogenesis of periodontitis: a new look. J Periodontol. 2008;79:1560-1568.
56 Kweider M, Lowe GD, Murray GD, et al. Dental disease, fibrinogen and white cell counts: links with myocardial infarction? Scott Med J. 1993;38:73-74.
57 Lockhart PB, Brennan MT, Sasser HC, et al. Bacteremia associated with toothbrushing and dental extraction. Circulation. 2008;117:3118-3125.
58 Löe H. Periodontal disease: the sixth complication of diabetes mellitus. Diabetes Care. 1993;16(suppl 1):329-334.
59 Loos BG, Craandijk J, Hoek FJ, et al. Elevation of systemic markers related to cardiovascular diseases in the peripheral blood of periodontitis patients. J Periodontol. 2000;71:1528-1534.
60 Lopez NJ, Smith PC, Gutierrez J. Higher risk of preterm birth and low birth weight in women with periodontal disease. J Dent Res. 2002;81:58-63.
61 Lopez NJ, Smith PC, Gutierrez J. Periodontal therapy may reduce the risk of preterm low birth weight in women with periodontal disease: a randomized controlled trial. J Periodontol. 2002;73:911-924.
62 Lowe GD, Lee AJ, Rumley A, et al. Blood viscosity and risk of cardiovascular events: the Edinburgh Artery Study. Br J Haematol. 1997;96:168-173.
63 Lowe GD. Etiopathogenesis of cardiovascular disease: hemostasis, thrombosis, and vascular medicine. Ann Periodontol. 1998;3:121-126.
64 Madianos PN, Murtha AP, Boggess KA, et al. Maternal periodontitis and prematurity. Part II: maternal infection and fetal exposure. Ann Periodontol. 2001;6:175-182.
65 Malthaner SC, Moore S, Mills M, et al. Investigation of the association between angiographically defined coronary artery disease and periodontal disease. J Periodontol. 2002;73:1169-1176.
66 Mattila KJ, Nieminen MS, Valtonen VV, et al. Association between dental health and acute myocardial infarction. Br Med J. 1989;298:779-781.
67 Mattila KJ, Valle MS, Nieminen MS, et al. Dental infections and coronary atherosclerosis. Atherosclerosis. 1993;103:205-211.
68 Mattila KJ, Valtonen VV, Nieminen M, et al. Dental infection and the risk of new coronary events: prospective study of patients with documented coronary artery disease. Clin Infect Dis. 1995;20:588-592.
69 Mattila KJ. Dental infections as a risk factor for acute myocardial infarction. Eur Heart J. 1993;14:51-53.
70 Mattila KJ. Viral and bacterial infections in patients with acute myocardial infarction. J Intern Med. 1989;225:293-296.
71 McCormick MC. The contribution of low birth weight to infant mortality and childhood morbidity. N Engl J Med. 1985;312:82-90.
72 McDonald HM, O’Loughlin JA, Jolley P, et al. Vaginal infections and preterm labour. Br J Obstet Gynecol. 1991;98:427-435.
73 Mealey BL, Oates TW. Diabetes mellitus and periodontal diseases. J Periodontol. 2006;77:1289-1303.
74 Mealey BL. The interactions between physicians and dentists in managing the care of patients with diabetes mellitus. J Am Dent Assoc. 2008;139(suppl):4s-7s.
75 Mealey BL. Influence of periodontal infections on systemic health. Periodontology 2000. 1999;21:197-209.
76 Mealey BL. Periodontal implications: medically compromised patients. Ann Periodontol. 1996;1:256-321.
77 Mehta JL, Saldeen TG, Rand K. Interactive role of infection, inflammation and traditional risk factors in atherosclerosis and coronary artery disease. J Am Coll Cardiol. 1998;31:1217-1225.
78 Merijohn GK, Bader JD, Frantsve-Hawley J, Aravamudhan K. Clinical decision support chairside tools for evidence-based dental practice. J Evid Based Dent Pract. 2008;8:119-132.
79 Michalowicz BS, Hodges JS, DiAngelis AJ, et al. Treatment of periodontal disease and the risk of preterm birth. New Engl J Med. 2006;355:1885-1894.
80 Miller LS, Manwell MA, Newbold D, et al. The relationship between reduction in periodontal inflammation and diabetes control: a report of 9 cases. J Periodontol. 1992;63:843.
81 Morales WJ, Schorr S, Albritton J. Effect of metronidazole in patients with preterm birth in preceding pregnancy and bacterial vaginosis: a placebo-controlled double-blind study. Am J Obstet Gynecol. 1994;171:345-347.
82 Newman HN. Focal infection. J Dent Res. 1996;75:1912-1919.
83 Newman MG, Caton JG, Gunsolley JC. The use of the evidence-based approach in a periodontal therapy contemporary science workshop. Ann Periodontol. 2003;8:1-11.
84 Offenbacher S, Barros SP, Beck JD. Rethinking periodontal inflammation. J Periodontol. 2008;79:1577-1584.
85 Offenbacher S, Jarad HL, O’Reilly PG, et al. Potential pathogenic mechanisms of periodontitis-associated pregnancy complications. Ann Periodontol. 1998;3:233-250.
86 Offenbacher S, Katz V, Fertik G, et al. Periodontal disease as a possible risk factor for preterm low birth weight. J Periodontol. 1996;67:1103-1113.
87 Offenbacher S. Periodontal diseases: pathogenesis. Ann Periodontol. 1996;1:821-878.
88 Ostergaard L, Anderson PL. Etiology of community-acquired pneumonia: evaluation by transtracheal aspiration, blood culture, or serology. Chest. 1993;104:1400-1407.
89 Page RC, Beck JD. Risk assessment for periodontal diseases. Int Dent J. 1997;47:61-87.
90 Page RC. The pathobiology of periodontal diseases may affect systemic diseases: inversion of a paradigm. Ann Periodontol. 1998;3:108-120.
91 Pugin J, Auckenthaler R, Lew DP, et al. Oropharyngeal decontamination decreases incidence of ventilator-associated pneumonia: a randomized, placebo-controlled, double-blind clinical trial. JAMA. 1991;265:2704-2710.
92 Ridker PM, Rifai N, Rose L, et al. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557-1565.
93 Ridker PM, Silvertown JD. Inflammation, C-reactive protein and atherothrombosis. J Periodontol. 2008;79:1544-1551.
94 Ridker PM. Fibrinolytic and inflammatory markers for arterial occlusion: the evolving epidemiology of thrombosis and hemostasis. Thromb Haemost. 1997;78:53-59.
95 Ross RL. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;342:115-126.
96 Sammalkorpi K. Glucose intolerance in acute infections. J Intern Med. 1989;225:15-19.
97 Saremi A, Nelson RG, Tulloch-Reid M, et al. Periodontal disease and mortality in type 2 diabetes. Diabetes Care. 2005;28:27-32.
98 Scannapieco FA, Bush RB, Paju S. Associations between periodontal disease and risk for atherosclerosis, cardiovascular disease, and stroke: a systematic review. Ann Periodontol. 2003;8:38-53.
99 Scannapieco FA, Bush RB, Paju S. Associations between periodontal disease and risk for nosocomial bacterial pneumonia and chronic obstructive pulmonary disease: a systematic review. Ann Periodontol. 2003;8:54-69.
100 Scannapieco FA, Papandonatos GD, Dunford RG. Associations between oral conditions and respiratory disease in a national sample survey population. Ann Periodontol. 1998;3:251-265.
101 Scannapieco FA, Stewart EM, Mylotte JM. Colonization of dental plaque by respiratory pathogens in medical intensive care patients. Crit Care Med. 1992;20:740-745.
102 Seinost G, Wimmer G, Skerget M, et al. Periodontal treatment improves endothelial dysfunction in patients with severe periodontitis. Am Heart J. 2005;149:1050-1054.
103 Selective Decontamination of the Digestive Tract Trialist’s Collaborative Group: Meta-analysis of randomised controlled trials of selective decontamination of the digestive tract. Br Med J. 1993;307:525-532.
104 Seymour GJ, Ford PJ, Cullinan MP, et al. Relationship between periodotnal infections and systemic disease. Clin Microbiol Infect. 2007;13(suppl 4):3-10.
105 Smith GT, Greenbaum CJ, Johnson BD, et al. Short-term responses to periodontal therapy in insulin-dependent diabetic patients. J Periodontol. 1996;67:794-802.
106 Spodick DH, Flessas AP, Johnson MM. Association of acute respiratory symptoms with onset of acute myocardial infarction: prospective investigation of 150 consecutive patients and matched control patients. Am J Cardiol. 1984;53:481-482.
107 Syrjanen J, Peltola J, Valtonen V, et al. Dental infections in association with cerebral infarction in young and middle-aged men. J Intern Med. 1989;225:179-184.
108 Taylor GW, Burt BA, Becker MP, et al. Severe periodontitis and risk for poor glycemic control in patients with non– insulin-dependent diabetes mellitus. J Periodontol. 1996;67:1085.
109 Terpenning M. The relationship between infection and chronic respiratory diseases: an overview. Ann Periodontol. 2001;6:66-70.
110 Tervonen T, Lamminsalo S, Hiltunen L, et al. Resolution of periodontal inflammation does not guarantee improved glycemic control in type 1 diabetic subjects. J Clin Periodontol. 2009;36:51-57.
111 Thorstensson H, Kuylensteirna J, Hugoson A. Medical status and complications in relation to periodontal disease experience in insulin-dependent diabetics. J Clin Periodontol. 1996;23:194.
112 Tonetti MS, D’Aiuto F, Nibali L, et al. Treatment of periodontitis and endothelial function. New Engl J Med. 2007;356:911-920.
113 Vergnes J-N, Sixou M. Preterm low birth weight and maternal periodontal status: a meta-analysis. Am J Obstet Gynecol. 2007;196:135.e1-135.e7.
114 Williams C, Davenport E, Sterne J, et al. Mechanisms of risk in preterm low- birthweight infants. Periodontology 2000. 2000;23:142-150.
115 Williams RC, Mahan CJ. Periodontal disease and diabetes in young adults. JAMA. 1960;172:776.
116 Williams RC. Understanding and managing periodontal diseases: a notable past, a promising future. J Periodontol. 2008;79:1552-1559.
117 Wu T, Trevisan M, Genco RJ, et al. Examination of the relation between periodontal health status and cardiovascular risk factors: serum total and high density lipoprotein cholesterol, C-reactive protein, and plasma fibrinogen. Am J Epidemiol. 2000;151:273-282.
118 Wu T, Trevisan M, Genco RJ, et al. Periodontal disease and risk of cerebrovascular disease: the first National Health and Nutrition Examination Survey and its follow-up study. Arch Intern Med. 2000;160:2749-2755.
119 Xiong X, Beukens P, Fraser WD, et al. Periodontal disease and adverse pregnancy outcomes: a systematic review. BJOG. 2006;113:135-143.
120 Yki-Jarvinen H, Sammalkorpi K, Koivisto VA, et al. Severity, duration and mechanism of insulin resistance during acute infections. J Clin Endocrinol Metab. 1989;69:317-323.
121 Zaremba M, Gorska R, Suwalski P, Kowalski J. Evaluation of the incidence of periodontitis-associated bacteria in the atherosclerotic plaque of coronary blood vessels. J Periodontol. 2007;78:322-327.
Suggested Reading
Kornman KS. Mapping the pathogenesis of periodontitis: a new look. J Periodontol. 2008;79:1560-1568.
Williams RC. Understanding and managing periodontal diseases: a notable past, a promising future. J Periodontol. 2008;79:1552-1559.
Scannapieco FA, Bush RB, Paju S. Associations between periodontal disease and risk for atherosclerosis, cardiovascular disease, and stroke: a systematic review. Ann Periodontol. 2003;8:38-53.
Ross RL. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;342:115-126.
Beck JD, Offenbacher S. The association between periodontal diseases and cardiovascular diseases: a state-of-the-art review. Ann Periodontol. 2001;6:9-15.
D’Aiuto F, Parkar M, Andreou G, et al. Periodontitis and systemic inflammation: control of the local infection is associated with a reduction in serum inflammatory markers. J Dent Res. 2004;83:156-160.
Tonetti MS, D’Aiuto F, Nibali L, et al. Treatment of periodontitis and endothelial function. New Engl J Med. 2007;356:911-920.
Mealey BL, Oates TW. Diabetes mellitus and periodontal diseases. J Periodontol. 2006;77:1289-1303.
Xiong X, Beukens P, Fraser WD, et al. Periodontal disease and adverse pregnancy outcomes: a systematic review. BJOG. 2006;113:135-143.
Scannapieco FA, Bush RB, Paju S. Associations between periodontal disease and risk for nosocomial bacterial pneumonia and chronic obstructive pulmonary disease: a systematic review. Ann Periodontal. 2003;8:54-69.