CHAPTER 63 SUPPLEMENT B Tissue Engineering in Periodontal Plastic Surgery
For patients who desire to avoid the palatal donor site morbidity associated with the subepithelial connective tissue graft (SECTG) and free gingival graft (FGG), tissue engineering methods hold a powerful alternative. Many patients who desire these alternative methods to autogenous periodontal soft tissue grafting, however, also expect predictable outcomes. Two recent systematic database review articles evaluated the existing evidence on mucogingival procedures and encouraged consideration of predictable modalities with patient-centered outcomes.2,15 It is essential that clinicians understand what the patient both truly desires from treatment and considers an aesthetically appealing outcome. If clinicians can meet the patient’s aesthetic needs and create stable periodontal health, all while avoiding the palatal donor site, the patient perceives that the trauma suffered was less, and procedures with these attributes become valuable methods to conduct clinical practice and future investigation. When superiority or equivalence to traditional therapy is not established with new products or alternative treatments, the outcome may still be more attractive to the patient because there is less trauma suffered. Validated tissue engineering applications for periodontal plastic procedures to obtain root coverage, increase attached gingiva, and provide keratinized tissue, all without using a donor site, may present outcomes that are more attractive to the patients and worthy of the surgeons’ consideration.
Tissue Engineering Alternatives for Mucogingival Procedures
Active Engineering
Barrier membranes and ADM offer wonderful solutions with an unlimited source and positive outcomes in the literatures as a GTR technology or tissue replacement graft, respectively.1,17-19 Barrier membranes allow certain cell types to proliferate but do not offer an active component. ADM used in periodontal plastic surgery holds no active component to influence wound healing and therefore is also considered a component in passive engineering. A large body of literature provides clinical and histological evidence of periodontal regeneration from biologically active materials for the intrabony lesion.5,13,16,21 This evidence can be used to support the exploration of these active materials for application in mucogingival surgery. As referenced in Chapter 61, a vast amount of literature supports tissue-engineered regeneration for periodontal defects, as well as implant site development. Randomized controlled clinical trials and human histologic evidence are needed to claim true regeneration and efficacy using new technologies. The remainder of this chapter concentrates on the novel approaches utilizing tissue engineering for periodontal mucogingival applications.
Periodontists have been practicing tissue engineering for decades, beginning with GTR, which is a form of passive tissue engineering that excluded certain cell types and created an engineered wound left to heal with the appropriate cell types.14 The ability to introduce biologic mediators, growth factors, and morphogens, all of which are actively influencing the physiology of wound repair, is now possible. The periodontist’s basic science knowledge of wound healing and the defined phases of inflammation have influenced and inspired the evaluation of biologic agents placed into the healing sites. Periodontists have led the research on tissue engineering applications in health care. We continue to search for the mechanism of action and optimal kinetics for tissue engineering methods in periodontics, and this understanding is sure to gain clarity.
Tissue engineering in periodontics and all of dentistry holds the future of wound repair and will continue to evolve into a valuable treatment variable that the clinician can influence and upregulate. It is important, however, to understand our limitations when it comes to influencing the complex wound healing scenario. The role of inflammation, existing cytokines, growth factors, extracellular matrices, and collagen synthesis must be considered when evaluating tissue engineering methods. Furthermore, having the correct factor at the appropriate time, at an effective concentration, and for the proper duration are issues that need further investigation and clinical validation. Just as we strive to understand the host role in the pathogenesis of periodontal disease, we must also take into consideration the host influence, both positive and negative, on wound healing after regenerative procedures.
Within the last 10 years, we have seen tissue engineering applications in periodontics including: live cell therapy,8,6,20,11 fully synthetic recombinant growth factors,12,10 and tissue engineered animal-derived biologic factors.7,4 All have been used to facilitate soft and sometimes hard tissue regeneration during periodontal mucogingival procedures. For the first time we have evidence from these randomized controlled clinical trials showing the efficacy of tissue engineering applications during mucogingival surgery to obtain root coverage and/or increased attached and keratinized gingiva. In 2003, McGuire and Nunn7 published results of a single center randomized controlled clinical trial comparing a coronally advanced flap with EMD to a SECGT. The results showed no statistical difference in percentage of root coverage between test and control, and it was concluded that within the limitations of this single-center trial that EMD with a coronally advanced flap is a valid alternative to the gold standard.7 In 2006, McGuire and Scheyer9 published a case series using a growth factor mediated procedure, including rhPDGF, β-TCP, and a collagen wound dressing to obtain root coverage comparable to the SECTG, which led to a controlled clinical trial. This randomized controlled clinical trial using the growth factor–mediated procedure to obtain clinical and histologic evidence of root coverage and regeneration when compared to the SECTG was published.10,12 These landmark studies provided “proof of principle” that in four of four human histology block sections, true periodontal regeneration (alveolar bone, periodontal ligament, and new cementum) was possible with rhPDGF-BB + β-TCP + a collagen wound dressing as evidenced by an example in Supplement B Figure 63-1. Furthermore, clinical efficacy was shown comparable to the connective tissue graft (CTG). Histologic evidence of root coverage verifies that this tissue engineering method offers true periodontal regeneration, which is not expected with the gold standard SECTG.
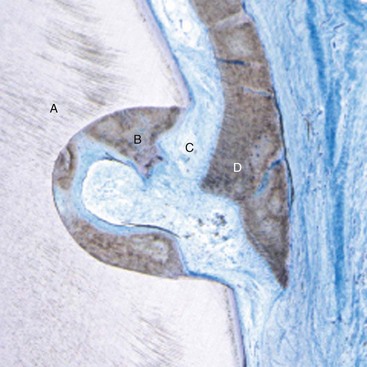
Supplement B Figure 63-1 Human histology from root coverage graft performed with recombinant human platelet-derived growth factor-BB (rhPDGF-BB) + beta-tricalcium phosphate (β-TCP) + collagen wound dressing. A, Tooth root with histologic reference notch made at the pregrafted alveolar crest. B, New cementum. C, Periodontal ligament. D, New bone.
(From McGuire MK, Scheyer ET, Nevins M, et al: Int J Perio Rest Dent 29:7-21, 2009.)
We have also witnessed tissue engineering applications that establish a functional zone of attached and keratinized gingiva for the treatment of recession apical to the recession defect. In 2005, McGuire and Nunn published a pilot study evaluating the safety and efficacy of a living tissue-engineered HFDDS (Dermagraft, Advanced Tissue Science, La Jolla, CA) compared to a gingival autograft. Although the autograft produced a greater band of keratinized tissue, the test group represented the first attempt to use an “off the shelf” tissue-engineered material capable of generating attached and keratinized gingival.20 In 2007, McGuire and Scheyer11 published a pilot study comparing a tissue-engineered BLCT (Apligraf, Organogenesis, Inc, Canton, MA) to an FGG with promising enough results to warrant a multicentered controlled clinical trial that is soon to be published. From the pilot study, we have evidence of de novo formation of attached and keratinized gingiva with the placement of a live cell therapy device without donor site surgery. In this randomized, controlled clinical trial, the test material was capable of generating up to 2.72 mm of keratinized gingiva, and in more than 75% of the subjects, greater than 2 mm of keratinized tissue was developed after a 6-month follow-up visit.11 This pilot study supports further research of the BLCT to produce de novo keratinized gingiva without the use of a traditional autograft. More powerful data are being analyzed from the multicenter trial which had a similar study design and will likely allow for the commercial use of this material.
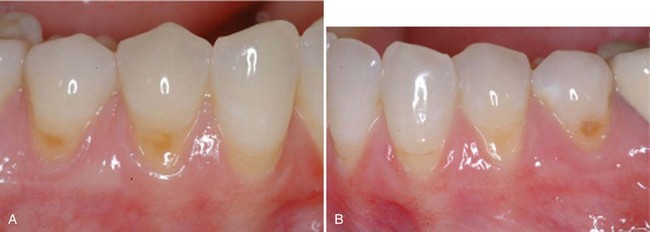
Not only are tissue engineering applications being evaluated for tissue gains above and below the recession defect but also for more challenging defects. The reconstruction of the open interproximal space remains one of the greatest challenges for periodontists. In a landmark study by McGuire and Scheyer,8 autologous fibroblasts were injected into the interdental papilla in a method to atraumatically augment the deficient gingival papilla. Although this method has not been thoroughly validated, treatment outcomes reduced open interproximal spaces and improved aesthetics in the maxillary anterior region (Supplement B Figure 63-2). No long-term evidence exists for tissue stability with this method, but this pilot study shows promise for an innovative study design for a tissue engineering application in dentistry. For example, this technology allows for the concept of “banking” autologous fibroblasts for future use or research. This therapy could be compared to the multiple extra oral procedures (i.e., Botox, collagen injections, and other dermal fillers) performed in plastic surgery and dermatology in which long-term soft tissue stability is not expected. This novel approach in periodontics needs further investigation, but the idea of multiple doses to maintain positive outcomes is not unrealistic to achieve much desired patient-centered outcomes.
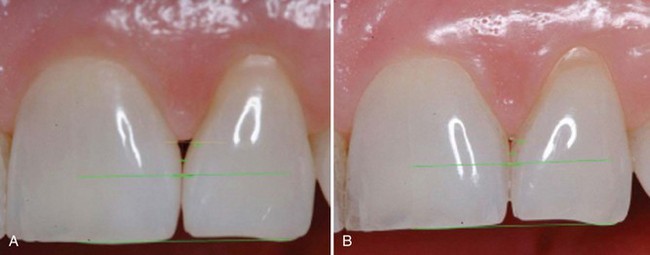
Supplement B Figure 63-2 A, Preoperative view of open interproximal space. B, Four months after three injections into the papilla with the patient’s own expanded and concentrated fibroblast. Note the improved papillary form. Also note green horizontal lines used for image analysis.
(From McGuire MK, Scheyer ET: J Periodontal 78:4-17, 2007.)
Because the literature routinely uses the SECTG as the gold standard for root coverage, many other techniques and materials have been compared to the SECTG. All of these methods are technically similar to the coronally advanced flap with varying materials secured underneath the flap.1,3,7,19,20 Providing successful root coverage and increasing attached and keratinized gingiva with tissue engineering methods requires the operator’s respect for the principal surgical techniques he or she has learned from the literature and in this text. If the surgeon combines these sound principles with tissue engineering methods he or she can optimize treatment outcomes, reduce trauma, and provide patient preference by eliminating a donor site.
The methods described in Supplement B Figures 63-3 to 63-13 are from some of the clinical trials mentioned and help the reader understand how to apply tissue engineering agents to traditional surgical therapy.
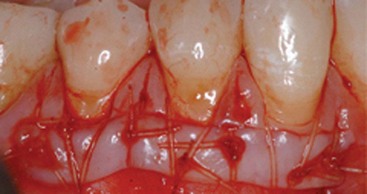
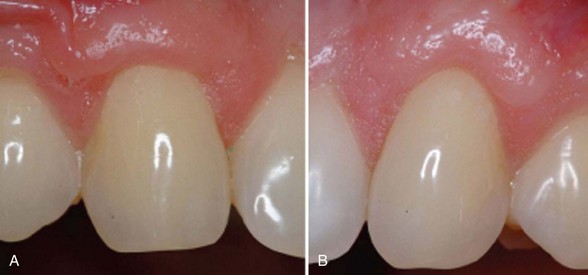
Supplement B Figure 63-3 A, Before view of test tooth displaying advanced gingival recession. B, Six-month postoperative view of test tooth treated with recombinant human platelet-derived growth factor-BB (rhPDGF-BB) + beta-tricalcium phosphate (β-TCP) + collagen wound dressing. C, Tooth #6 was the control tooth treated with a subepithelial connective tissue graft (SECTG). Note both test and control teeth have not had postgrafting gingivoplasty.
(From McGuire MK, Scheyer ET, Schupbach P: J Periodontol 80:550-564, 2009.)
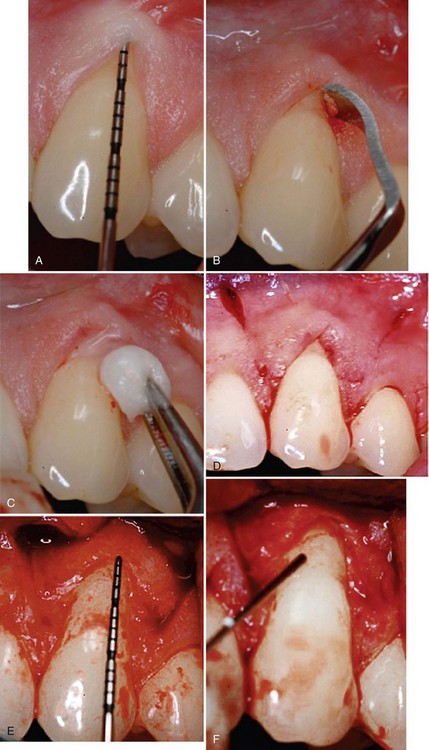
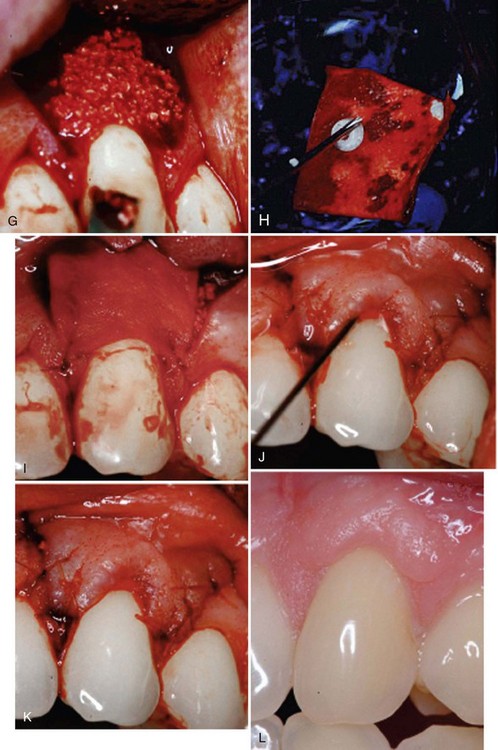
Supplement B Figure 63-4 A, A study test tooth with preoperative 3 mm of recession and probing depth of 1 mm. This tooth will be treated with recombinant human platelet-derived growth factor-BB (rhPDGF-BB) + beta-tricalcium phosphate (β-TCP) + collagen wound dressing. B, Root surface preparations with a chisel. C, Root surface preparation with ethylenediaminetetraacetic acid (EDTA). D, Horizontal and vertical beveled incisions. E, Alveolar bone crest 6.5 mm from cement-enamel junction. F, Root surface exposed to rhPDGF. G, rhPDGF saturated β-TCP placed over the root surface. H, Collagen wound dressing saturated with rhPDGF. I, Collagen dressing sutured with resorbable 6-0 plain gut sutures to the papillary connective tissue bed. J, Remaining rhPDGF is expressed over the closed wound. K, Vertical incisions closed with 5-0 chromic gut sutures. L, Four-week postoperative photograph.
Supplement B Figure 63-5 A, Six-month postoperative view of control tooth #6 treated with subepithelial connective tissue graft (SECTG). B, Six-month postoperative view without tissue plasty.
Supplement B Figure 63-6 A, Preoperative control site to be treated with free gingival graft. B, Preoperative view of test site to be treated with bilayered cell therapy (BLCT).
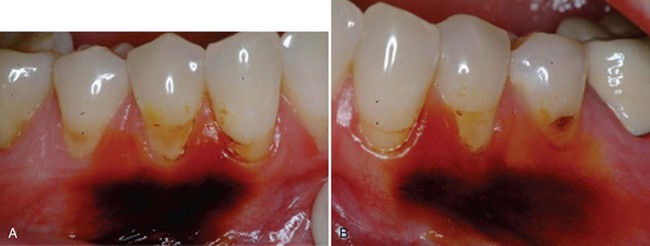
Supplement B Figure 63-7 A, Preoperative control site stained with Schiller’s iodine to better delineate the mucogingival junction. B, Test site stained with Schiller’s iodine.
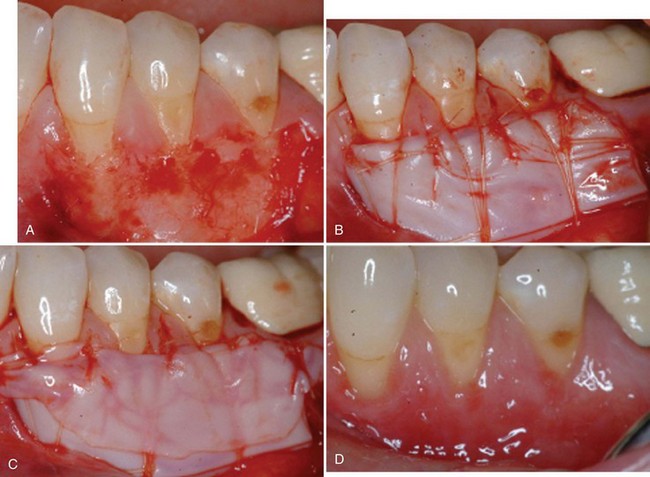
Supplement B Figure 63-9 A, Partial thickness graft bed preparation at the test site. B, Stabilization of bilayered cell therapy (BLCT) with 5-0 plain gut sutures. C, A fourth layer of BLCT is secured to the papilla to serve as an active wound dressing. D, One-week postoperative view of test site.
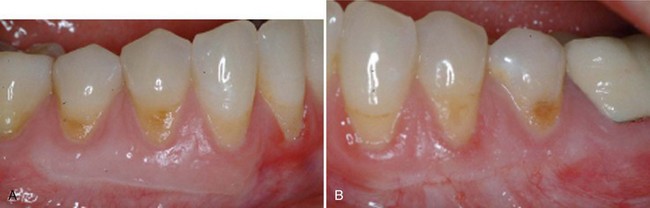
Supplement B Figure 63-10 A, Six-month postoperative view of control site. B, Six-month postoperative view of test site.
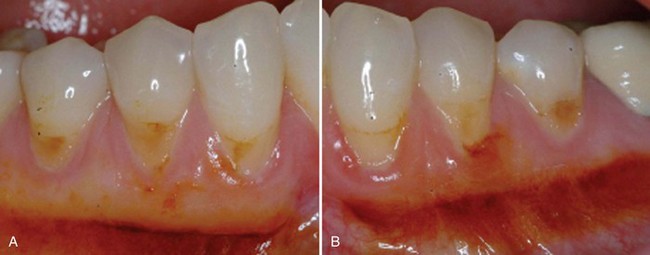
Supplement B Figure 63-11 A, Control site stained with Schiller’s iodine. B, Test site stained with Schiller’s iodine. Note de novo formation of attached and keratinized gingiva.
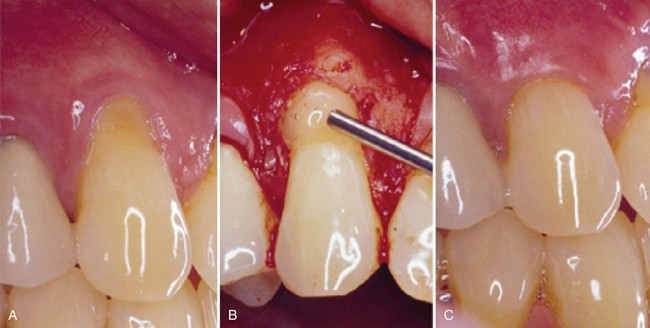
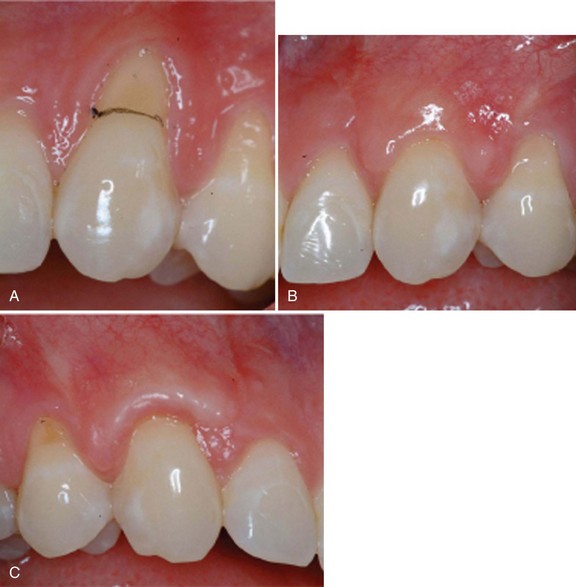
Supplement B Figure 63-12 A, Pretreatment recession defect of 3 mm to be treated with enamel matrix derivative (EMD). B, Application of EMD to the root surface before coronally advancing the flap with 5-0 plain gut sutures. C, A 1-year follow-up with 100% root coverage and increased keratinized gingiva.
(Courtesy Dr. Michael K. McGuire.)
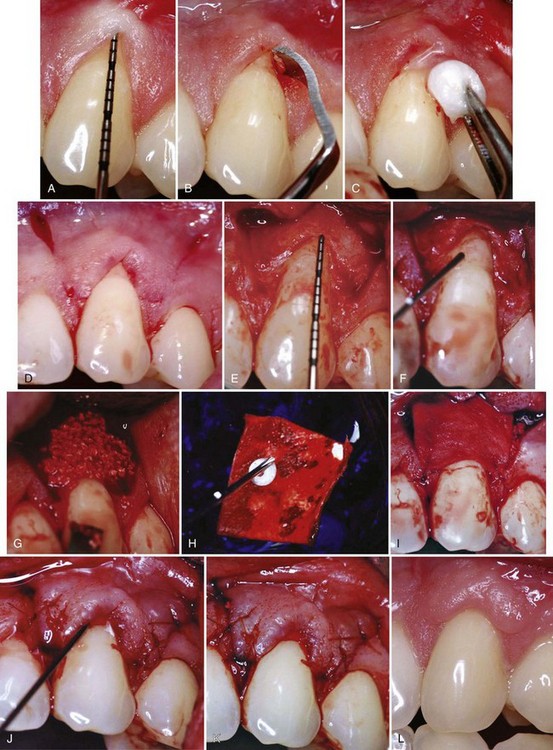
Supplement B Figure 63-13 A, A study test tooth with preoperative 3 mm of recession and probing depth of 1 mm. This tooth will be treated with recombinant human platelet-derived growth factor (rhPDGF) + tricalcium phosphate (β-TCP) and a collagen wound dressing. B, Root surface preparations with a chisel. C, Root surface preparation with ethylenediaminetetraacetic acid (EDTA). D, Horizontal and vertical beveled incisions. E, Alveolar bone crest 6.5 mm from cementoenamel junction (CEJ). F, Root surface exposed to rhPDGF. G, rhPDGF saturated TCP placed over the root surface. H, Collagen wound dressing saturated with rhPDGF. I, Collagen dressing sutured with resorbable 6-0 plain gut sutures to the papillary connective tissue bed. J, Remaining rhPDGF is expressed over the closed wound. K, Vertical incisions closed with 5-0 chromic gut sutures. L, Four week postoperative photograph.
Tissue engineering methods that require the surgeon to think beyond the phases of wound healing, host physiology, and patient biologic and systemic factors should be included in the decision tree if the clinician desires optimal clinical outcomes. Furthermore, the surgeon’s desire to reduce trauma and morbidity warrant his or her investigation of alternatives to harvesting autogenous tissue grafts. The evidence in the literature for mucogingival procedures provides support for the CTG and/or EMD with a coronally advanced flap as the current gold standard during mucogingival root coverage procedures.2 In our quest for new technologies to reduce trauma during the coronally advanced flap and thus expand our clinical abilities, we must further investigate treatment methods such as ADM and biologic factors, as well as materials that could carry growth factors to the surgical site and maintain their action. At some point, recombinant growth factors could eliminate both the need for donor sites and the use of exogenous materials inserted into the human body.
Although not yet investigated, the idea of injecting a group of growth factors on a time-released basis that could communicate with each other and with autologous host cells is extremely intriguing. Obviously, the wound healing cascade ending with regeneration is complex, and the addition of just one cell, growth factor, or matrix is unlikely to provide the complete answer in our quest for ideal regenerative outcomes. The search for new methods and techniques that improve wound healing, patient care, efficiency, and all that can be influenced during regenerative techniques is a realistic goal. Our ability and responsibility to research and utilize tissue engineering applications in periodontics is crucial for the progression and evolution of periodontal plastic and aesthetic surgical techniques.
Clinical evidence exists to support the use of tissue engineering to influence wound healing in the complex periodontal wound. Tissue engineering methods can allow for a positive response to our therapy creating more predictable results with histological evidence of regeneration. With these emerging technologies, we are improving our outcomes and providing our patients a less traumatic method of treatment by allowing us to avoid the morbidity of the autogenous donor site.
Cost-benefit analysis and an evaluation of patient-centered outcomes have enabled periodontal researchers to begin developing the evidence to support these tissue-engineered applications. An assessment of recent systematic database review articles led researchers to conclude that patient-centered outcomes must be considered with mucogingival procedures.2,15 Our focus on patient-centered outcomes in therapy can ensure that our patients have all the information necessary to make decisions about their treatment options that are supported by evidence of success. With the literature background and cases presented in this chapter, the reader can better appreciate the applications of tissue engineering in periodontal plastic and aesthetic surgical procedures.
The periodontal community is fortunate to have advanced the scientific and clinical evidence for advancing technologies that can influence our ability to improve patient care, facilitate wound healing, and allow for outcomes that are comparable to the gold standard. Future research should focus on obtaining additional evidence when using growth factors and live cell technology during periodontal therapy to eliminate donor site morbidity. Basic science studies should be completed to better understand the mechanism of action and wound healing kinetics of growth factor–mediated and live-cell therapy. Identification of optimal carrying agents for cells and factors allows us to best harness the technology. With recombinant technologies and novel live-cell therapies, the field of periodontology has a bright future but must remain focused on evidence-based decisions to maintain the long-term predictability of successful outcomes. Patient-centered outcomes should be considered when making clinical decisions to improve the patient’s perception of care.
1 Aichelmann-Reidy ME, Yukna RA, Evans GH, et al. Clinical evaluation of acellular allograft dermis for the treatment of human gingival recession. J Periodontol. 2001;72(8):998-1005.
2 Cairo F, Pagliaro U, Nieri M. Treatment of gingival recession with coronally advanced flap procedures: A systematic review. Journal of Clinical Periodontology. 2008;35(s8):136-162.
3 Cortelllini P, Clauser C, Pini-Prato GP. Histologic assessment of new attachment following the treatment of a human buccal recession by means of a guided tissue regeneration procedure. J Periodontol. 1993;64:387-391.
4 McGuire MK, Cochran DL, Nunn ME. Evaluation of human recession defects treated with coronally advanced flaps and either enamel matrix derivative or connective tissue. II. Histological evaluation. J Periodontol. 2003;74:1126-1135.
5 McGuire MK, Kao RT, Nevins M, Lynch SE. rhPDGF-BB promotes healing of periodontal defects: 24-month clinical and radiographic observations. Int J Periodontics Restorative Dent. 2006;26:223-231.
6 McGuire MK, Nunn ME. Evaluation of the safety and efficacy of periodontal applications of a living tissue engineered human fibroblast-derived dermal substitute. I. Comparison to the gingival autograft: A randomized, controlled pilot study. J Periodontol. 2005;76:867-880.
7 McGuire MK, Nunn ME. Evaluation of human recession defects treated with coronally advanced flaps and either enamel matrix derivative or connective tissue. I. Comparison of clinical parameters. J Periodontol. 2003;74:1110-1125.
8 McGuire MK, Scheyer ET. A randomized double-blind placebo-controlled study to determine the safety and efficacy of cultured and expanded autologous fibroblast injections for the treatment of interdental papillary insufficiency associated with the papilla priming procedure. J Periodontal. 2007;78:4-17.
9 McGuire MK, Scheyer ET. Comparison of recombinant human platelet-derived growth factor—BB plus beta tricalcium phosphate and a collagen membrane to subepithelial connective grafting for the treatment of recession defects. A case series. Int J Periodontics Restorative Dent. 2006;26:127-133.
10 McGuire MK, Scheyer ET, Nevins M, Schupbach P. Evaluation of human recession defects treated with coronally advanced flaps and either purified recombinant human platelet-derived growth factor-BB (rhPDGF) with beta tricalcium phosphate (β-TCP) or connective tissue: A histological and micro-CT examination. Int J Perio Rest Dent. 2009;29:7-21.
11 McGuire MK, Scheyer ET, Nunn ME, Levin PT. A pilot study to evaluate a tissue-engineered bilayered cell therapy as an alternative to tissue from the palate. J Periodontol. 2008;79:1847-1856.
12 McGuire MK, Scheyer ET, Schupbach P. Growth factor-mediated treatment of recession defects: A randomized controlled trial and histologic and microcomputed tomography examination. J Periodontol. 2009;80:550-564.
13 Nevins M, Giannobile WV, McGuire MK, et al. Platelet-derived growth factor stimulates bone fill and rate of attachment level gain: Results of a large multi-center, randomized controlled trial. J Periodontol. 2005;76:2205-2215.
14 Nyman S, Lindhe J, Karring T, Rylander H. New attachment following surgical treatment of human periodontal disease. J Clin Periodontol. 1982;9:290-296.
15 Oates TW, Robinson M, Gunsolley JC. Surgical therapies for the treatment of gingival recession. A systematic review. Ann Periodontol. 2003;8:303-320.
16 Sculean A, Donos N, Blaes A, et al. Comparison of enamel matrix proteins and bioresorbable membranes in the treatment of intrabony periodontal defects. A split-mouth study. J Periodontol. 1999;70:255-262.
17 Tal H, Moses O, Zohar R, et al. Root coverage of advanced gingival recession: A comparative study between acellular dermal matrix allograft and subepithelial connective tissue grafts. J Periodontol. 2002;73(12):1405-1411.
18 Tatakis DN, Trombelli L. Gingival recession treatment: Guided tissue regeneration with bioabsorbable membrane versus connective tissue graft. Journal of Periodontology. 2000;71:299-307.
19 Wang HL, Bunyaratavej P, Labadie M, et al. Comparison of two clinical techniques for treatment of gingival recession. Journal of Periodontology. 2001;72:1301-1311.
20 Wilson TG, Nunn ME, McGuire MK. Evaluation of the safety and efficacy of periodontal applications of a living tissue engineered human fibroblast-derived dermal substitute. Part II. Comparison to the subepithelial connective tissue graft. A randomized, controlled pilot study. J Periodontol. 2005;76:881-889.
21 Velasquez-Plata D, Scheyer ET, Mellonig JT. Clinical comparison of an enamel matrix derivative used alone or in combination with a bovine-derived xenograft for the treatment of periodontal osseous defects in humans. J Periodontol. 2002;73:433-440.