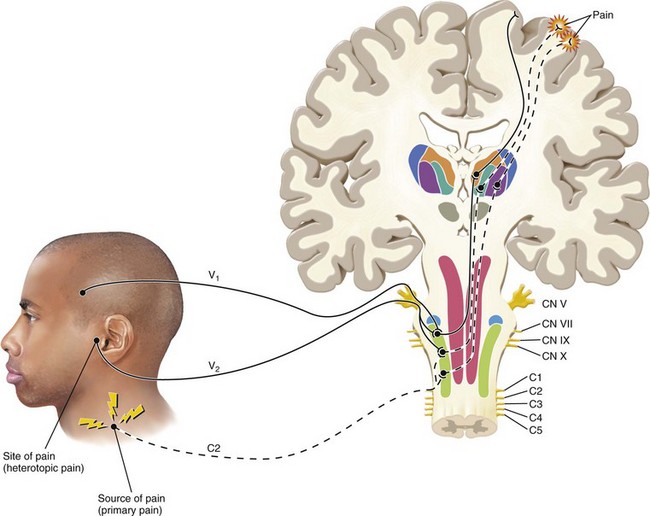
FIG. 3-3 Illustration of pain that is referred from an area innervated by one nerve (C2) to an area innervated by a different nerve (V2). Note that this phenomenon occurs secondary to the convergence of different neurons onto the same second-order neuron in the trigeminal nucleus. The sensory cortex perceives two locations of pain. One area is the trapezius region that represents the source of pain. The second area of perceived pain is felt in the temporomandibular joint area, which is only a site of pain, not a source of pain. This pain is heterotopic (referred).
(Redrawn from Okeson JP: Bell’s orofacial pains, ed 5, Chicago, 1995, Quintessence Publishing.)
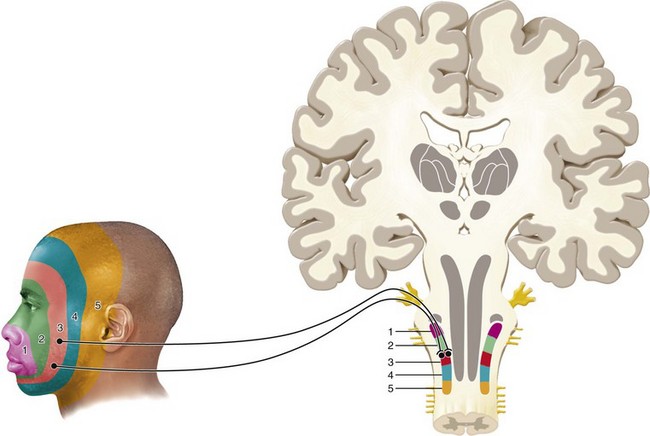
FIG. 3-4 Illustration of the laminated pattern of innervation from orofacial structures into the trigeminal nucleus. These laminated patterns commonly reflect the patterns of referred pains felt in the orofacial structures.
(Redrawn from Okeson JP: Bell’s orofacial pains, ed 5, Chicago, 1995, Quintessence Publishing.)
CHAPTER 3 Diagnosis of Nonodontogenic Toothache
An unthinking dentist is a bad dentist. Perfect technique misapplied is at least as unconscionable as sloppy work.
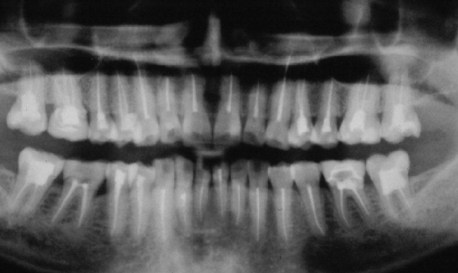
FIG. 3-1 Pantomogram of a patient who has undergone several endodontic procedures without resolution of her chief complaint.
(Courtesy Dr. Jeffrey Okeson, Lexington, Kentucky.)
A nonodontogenic toothache is, of course, an oxymoron. How can one have a toothache that is not odontogenic in etiology? The answer lies in the differentiation of the person’s perception of where they sense their pain, termed the site of the pain, from the location of a pathophysiologic process giving rise to the pain that may or may not be in the same region, termed the source of the pain. This concept of the attribution of pain to an anatomic region that is different from the location of the etiologic process is generically known as “referred pain phenomenon” and is known to occur in multiple areas of the body. Thus, a nonodontogenic toothache has a source of pain that is not the tooth indicated by the patient, thereby clearly demonstrating the diagnostic challenge being presented.
Pain is common. It causes human suffering and has significant socioeconomic effects. Pain is a motivator that provokes individuals to seek care. But protracted chronic pain debilitates and can significantly impair the quality and productivity of a person’s life. One survey revealed that 66% of respondents reported experiencing pain or discomfort over a 6-month period. Significantly, 40% of respondents reported that this pain affected them to a “high degree.”16 A study published in 2003 estimated the lost productive work time attributed to common pain conditions among active workers to cost $61.2 billion per year.104 One investigator reported that over a 6-month period of time, 22% of Americans experienced at least one of five types of facial pain. Of these pains, the most common type (12.2%) was toothache.71
Although toothache is the most common pain entity occurring in the facial region,71 it is clear that many other types of pain can occur in the same general area. A primary responsibility of a dental practitioner is to diagnose pathologic entities associated with the oral cavity and masticatory apparatus. Many of these pathologic entities have pain as a primary component of their presentation. Because dental practitioners are sought out daily for the alleviation of odontogenic pain, it is imperative for them to have a basic working knowledge of other types of facial pain in order to make an accurate diagnosis and properly select care for patients. It is paramount to realize that not all pain entities presenting as toothache are of odontogenic origin. The presenting toothache may be a heterotopic symptom of another disorder. A heterotopic symptom is perceived to originate from a site that is different from the tissue that is actually the source of the pain. This is in contrast to primary pain, in which the perceived site of pain is the actual tissue from which the pain originates. Before discussing pain entities that mimic toothache it is helpful to have an understanding of the neurobiologic mechanisms of orofacial pain.
Review of Neuroanatomy
Somatic Structures
To understand the pathways by which orofacial pain occurs, one must first gain a basic understanding of the structures involved in its transmission to higher brain centers. Structures of the orofacial region can be divided into two broad categories: somatic and neural structures. Somatic structures are those that make up the different nonneural tissues and organs. The somatic structures can be further anatomically divided into superficial and deep structures. Superficial structures include the skin, mucosa, and gingiva; pain that arises from these superficial structures is usually well localized (e.g., a sharp explorer penetrating the gingiva results in well-localized pain). Deep structures include musculoskeletal and visceral tissues. Pain from these deep structures is typically poorly localized and diffuse.
Neural Structures
Neural structures involved in the perception of pain include the afferent (toward the brain) and efferent (away from the brain) regulation of somatic structures. Transmission of nerve impulses from orofacial structures to the brain is via the peripheral nervous system, whereas modulation and interpretation of these impulses into what we feel as pain occur in the central nervous system. Pain can arise solely from either central or peripheral nervous tissue but heterotopic pain, which is often involved with nonodontogenic toothache, likely requires central modulation to occur.
Peripheral Nervous System
Pain arises as a result of tissue damage, or the potential for tissue damage, and is transmitted via terminal nerve fibers known as primary afferent nerve fibers. Two major classes of nociceptive (or pain-sensing) primary afferent nerve fibers can detect potentially damaging noxious stimuli: the A-delta and C fibers. Both fiber types have a wide distribution throughout the skin, oral mucosa, and tooth pulp. In addition, separate classes of nerve fibers exist that are involved in detecting nonnoxious stimuli such as vibration and proprioception. Such fibers can be found in the periodontal ligament, skin, and oral mucosa and include the A-beta fibers.
Primary Afferent Neurons
Detection and encoding of noxious stimuli for the orofacial region are performed primarily by the trigeminal, or fifth cranial, nerve. The majority of cell bodies of the trigeminal sensory fibers are in the trigeminal ganglion located on the floor of the middle cranial fossa. The peripheral axons of the trigeminal ganglion run in three divisions—the ophthalmic (V1), maxillary (V2), and mandibular (V3)—which innervate most of the oral mucosa, the temporomandibular joint, the anterior two thirds of the tongue, the dura of the anterior and middle cranial fossae, the tooth pulp, gingiva, and periodontal membrane.
In the peripheral nervous system, these neurons or nerves are referred to as primary afferent (i.e., sensory) fibers. The primary afferent fibers can broadly be divided into A-beta fibers, which transmit light touch or proprioceptive information, and A-delta and C fibers, which encode pain. The tooth is densely innervated by afferent nerve fibers, which are believed to transmit mainly pain in response to thermal, mechanical, or chemical stimuli. The vast majority of dental nerves are C fibers that innervate the central pulp, and most of which terminate beneath the odontoblasts.20
A-beta Fibers
The rapidly conducting myelinated neurons that respond to light touch are called A-beta fibers. Under normal conditions, activation of the A-beta fibers by high-intensity stimulation results in low-frequency output in the central nervous system. Activation of A-beta fibers normally is interpreted as nonpainful mechanical stimulation109 or “prepain.”20 A-beta fibers have been shown to undergo phenotypic changes that allow them to encode painful stimuli under inflammatory conditions.84
A-delta Fibers
The A-delta fibers are lightly myelinated, have a faster conduction velocity than C fibers, and are believed to transmit a sharp or pricking sensation. A-delta fibers respond primarily to noxious mechanical stimuli rather than to chemical or thermal stimuli. Other A-delta fibers may be polymodal (responding to mechanical, chemical, and thermal stimuli)10 or respond only to cold/mechanical68 or hot/mechanical noxious stimuli.34
In the tooth pulp, A-delta fibers traverse the odontoblastic layer and terminate in the dentinal tubules.22 Because of their location and their sensitivity to mechanical stimulation, A-delta fibers are believed to respond to stimuli that result in movement of fluid within the dentinal tubules (e.g., osmotic, mechanical probing or thermal stimuli applied to the external surface of the tooth).15 Consistent with the hypothesized mechanism of dentinal pain is the fact that the stimuli that cause dentinal fluid movement result in a sharp pain associated with A-delta fiber activation.81 When intense noxious stimuli activate the A-delta fibers, the input to the central nervous system consists of high-frequency action potentials.
C Fibers
The C fibers are unmyelinated, have slower conduction velocity, and are associated with a dull, aching, or burning sensation. Most C fibers are polymodal, responding to mechanical, thermal, and chemical stimuli. Because of the difference in conduction velocities, A-delta fibers are believed to transmit early, shooting pain, whereas C fibers would transmit late, dull pain. Noxious stimuli that exceed the receptor threshold of these nociceptive primary afferent terminals result in action potentials that travel centrally, signaling tissue damage. In the pulp tissue, the more centrally located C fibers respond to thermal, mechanical, and chemical stimuli, and are believed to be sensitized by inflammation.34 All visceral structures are innervated primarily by afferent fibers conducting nociceptive information such as that carried by A-delta and C fibers.
Central Nervous System
The primary afferent fibers are responsible for the transduction and transmission of sensory information to higher brain centers and they do so by synapsing on neurons located within the trigeminal nucleus, which spans the midbrain and cervical spinal cord. This point marks the beginning of the central nervous system and is the point at which processing of pain information begins (Fig. 3-2).
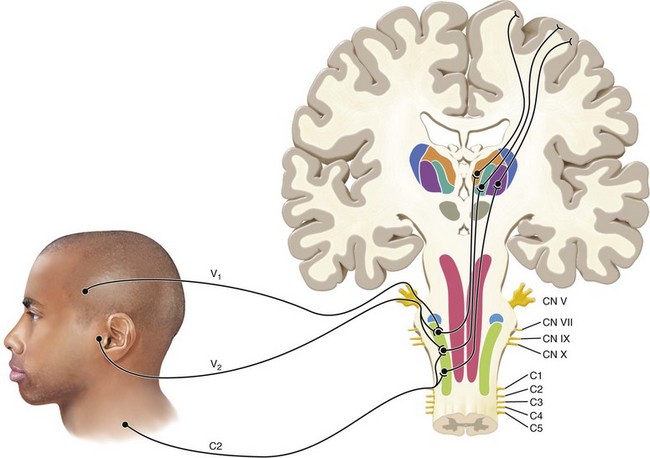
FIG. 3-2 A graphic depiction of the trigeminal nerve entering the brainstem. The primary afferent neuron synapses with a second-order neuron in the trigeminal nucleus. The second-order neuron carries pain information to the thalamus from which it is sent to the cerebral cortex for interpretation.
(Redrawn from Okeson JP: Bell’s orofacial pains, ed 5, Chicago, 1995, Quintessence Publishing.)
Just as there are different types of sensory neurons in the periphery, in the trigeminal nucleus there are also different types of neurons that receive nociceptive input from the periphery. The ascending neurons located in the trigeminal nucleus are known collectively as second-order or projection neurons and can be subdivided into three distinct groups of neurons based on the type of information they receive: (1) low-threshold mechanoreceptors, (2) nociceptive-specific and (3) wide dynamic range neurons.
The primary central site of termination for nociceptive fibers is the subnucleus caudalis located in the most caudal region of the trigeminal nucleus,34,50,120 which anatomically and functionally resembles the dorsal horn of the spinal cord and has been referred to as the medullary dorsal horn.50 Four major components of nociceptive processing are located in the dorsal horn of the subnucleus caudalis: central terminals of afferents, local circuit neurons (interneurons), projection neurons, and descending neurons.62 Within the subnucleus caudalis, the A-delta and C fibers terminate primarily in the outer laminae (I and IIa) and lamina V. Local circuit neurons are composed of islet cells (which are thought to be inhibitory) and stalked cells (which are believed to be excitatory).33 Combined, the local circuit neurons may modulate nociceptive transmission from the primary afferents to the projection neurons.
The fourth component of the dorsal horn is the terminal endings of descending neurons. The descending neurons originate in the nucleus raphe magnus (NRM), the medullary reticular nuclei, and the locus ceruleus (LC). Descending brainstem neurons release serotonin (from the NRM) and/or norepinephrine (from the LC), which may inhibit the activity of projection neurons directly or by activating local opioid interneurons. These neurons are responsible for the endogenous abatement of pain; blockade of their activity results in increased pain transmission and reduced pain thresholds.
Second-Order Neurons
Projection neurons have axons that cross to the contralateral medulla to ascend in the trigeminothalamic tract and project to the ventral posterior medial and intralaminar nuclei of the thalamus, where additional neurons project to the cortex. Projection neurons involved in the transmission of painful stimuli can be divided into two classes: wide dynamic range and nociceptive-specific neurons. Wide dynamic range neurons receive input from mechanoreceptors, thermoreceptors, and nociceptors, whereas nociceptive-specific neurons are excited solely by nociceptors. These two types of projection neurons may be responsible for signaling the severity and location of pain, respectively.69
Multiple primary afferent neurons may synapse on a single projection (i.e., convergence). This occurs to a much greater degree in deep tissues as opposed to cutaneous tissues. Primary afferent fibers of nontrigeminal origin such as those derived from vagus, glossopharyngeal, facial, and cervical spinal ganglia have been shown to converge and synapse onto trigeminal projection neurons located as far caudal as spinal level C4.64 This phenomenon of convergence may result in the clinical finding of pain that radiates beyond an area of tissue injury. Convergence may also explain why pain appears to be associated with a site other than the injured area. Interestingly, when projection neurons receive input from superficial and deep structures, the more superficial inputs usually predominate.99 Thus, pain originating from deep structures would typically be referred to superficial areas (e.g., pain originating from the jaw muscles would typically be referred to the face rather than deeper structures).
Autonomic Nervous System
The entire sympathetic innervation of the orofacial region is supplied by the stellate ganglia, which is located bilaterally at the level of the seventh cervical vertebra. Under normal conditions sympathetic stimulation has no influence on sensory function. However, afferent sympathetic fibers in an area of trauma may become involved in the response to pain and may also play a role in chronic pain states. Specifically, C fibers in the area of partial nerve injury may become responsive to sympathetic nerve stimulation. The modulation of nociception by the sympathetic nervous system has been shown such that release of pain neurotransmitters may be altered in the presence of sympathetic agonists and by blockade of the sympathetic nervous system, using antagonists.61 Whether the effects of sympathetic nerve fibers on pain transmission are direct (via homeostatic regulation) or indirect remain unclear. The parasympathetic division of the autonomic nervous system has not been shown to be involved in the development or modulation of pain.
Review of Neurophysiology
Peripheral Sensitization
After tissue insult there is an inflammatory reaction that often produces pain. The severity of pain that follows is related to several conditions of the injury, such as the type, extent, and location; the innervation of the tissue; and the phase of the inflammation. In the nociceptive system, tissue injury can manifest itself as increased responsiveness and/or reduced thresholds to a noxious stimulus, referred to as hyperalgesia. Hyperalgesia can be partially accounted for by sensitization of nociceptors (primary hyperalgesia), and by central nervous system mechanisms (secondary hyperalgesia).
In the absence of tissue damage, activation of C or A-delta fibers produces a transient pain. This pain is believed to serve as a physiologic warning. When there is tissue injury, afferent fibers may be activated by lower intensity stimuli than usual, and the quality of pain may be more persistent and intense. This phenomenon is due, in part, to sensitization of nociceptors, including an increase in spontaneous activity.
At the site of tissue injury there are a number of inflammatory mediators that can directly or indirectly sensitize primary afferent nociceptors (see Chapter 19 for more details). These inflammatory mediators may be released from the local tissue cells, circulating and resident immune cells, vasculature and endothelial smooth muscle cells, and peripheral nervous system cells.
Central Sensitization
After peripheral tissue injury there is an afferent barrage from C fibers resulting from peripheral tissue inflammation, decreased afferent thresholds, and spontaneous firing of afferent fibers. When a second-order neuron receives a prolonged barrage of nociceptive input, the second-order neuron may also become sensitized. This results in a phenomenon referred to as central sensitization.13 The result of central sensitization is enhanced processing (i.e., amplification) of neural impulses that are being transmitted to higher brain centers. Two effects of central sensitization are secondary hyperalgesia and referred pain.
Secondary hyperalgesia is an increased response to painful stimulation at the site of pain resulting from central nervous system changes. This is in contrast to primary hyperalgesia, which is a lowered pain threshold resulting from sensitization of peripheral neurons. Secondary hyperalgesia might be felt in superficial (e.g., gingiva or skin) or deep structures (e.g., muscles or teeth).
Terminology
In general, as research progresses and uncovers new ways we look at pain, the terminology changes. This can introduce some confusion, especially when older terms are used. Therefore, it may be helpful to present contemporary definitions of some of the basic terms and review some of the previously mentioned terms (Box 3-1).
Pain
An unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in term of such damage73
Neuropathic Pain
Pain arising as a direct consequence of a lesion or disease affecting the somatosensory system73,111
Peripheral Sensitization
Increased responsiveness and reduced thresholds of nociceptors to stimulations of their receptive fields73
Central Sensitization
Increased responsiveness of nociceptive neurons in the central nervous system to their normal or subthreshold afferent input73
Heterotopic Pain
Any pain that is felt in an area other than its true source is heterotopic pain. There are three types of heterotopic pain: referred, central, and projected.87 Referred pain is pain felt in an area innervated by a nerve different from the one that mediates the primary pain. Referred pain cannot be provoked by stimulation of the area where the pain is felt; rather, it is brought on by manipulation of the primary source of pain (Fig. 3-3). In addition, referred pain cannot be arrested unless the primary source of pain is anesthetized. The referral of pain tends to occur in a laminated fashion (Fig. 3-4). This is because peripheral nociceptors enter the spinal trigeminal tract in a laminated fashion. As a result there are general referral patterns in the face. In addition, the referral of pain is usually in a cephalad or upward direction. This is evidenced clinically in that pain from mandibular molars typically is referred to maxillary molars, as opposed to premolars or incisors
Clinical Entities That Can Present as Toothache
Sources of Odontogenic Toothache
Before considering heterotopic pains that may present as toothache, it is important to fully understand odontogenic pain as a primary source for toothache. There are only two structures that serve as sources for primary odontogenic pain. These structures are the pulp–dentin complex and the periradicular tissues. The innervation of the pulp is similar to that of other deep visceral tissues and in various states of pathosis will have pain characteristics similar to deep visceral tissues. The primary nociceptors of the pulp that respond to inflammation are the slow-conducting, high-threshold C fibers. Because their threshold is high, C fibers do not respond to normal or nonpathologic dentinal stimulation. C fibers typically conduct pain that is associated with tissue damage. In addition, C fibers respond in a threshold manner that can be termed “all or nothing.” For example, a slightly cold stimulus that is below the C fiber threshold will fail to produce any sensation. Only when a stimulus is intense enough to reach the threshold will the C fiber fire, resulting in the sensation of pain.
Pulpal pain is mediated by C fibers and is dull, aching, or throbbing in nature. This is in contrast to the quick short sharp sensation produced by A-delta fibers that mediate dentinal pain. Therefore, when pulp testing, it is meaningful to note not only whether the patient perceived the stimulus but also the nature of the stimulus perceived. A simple notation of “s” (short) to indicate a response more typical of an A-delta fiber (dentinal pain), or a “p” (prolonged) to indicate the response was more indicative of a C fiber response (pulpal pain).
Tissue inflammation can result in sensitization of nerve fibers. When peripheral nociceptors (e.g., pulpal C fibers) are sensitized, the threshold of firing in response to a given stimulus (e.g., temperature and pressure) is lowered. In states of sensitization these nociceptors can be provoked with a less intense stimulus. The threshold for excitation is still “all or nothing” but the required level of stimulation has decreased. These fibers can become so sensitized that they may fire at as low a temperature threshold as body temperature,81 normally not sufficient to stimulate a C fiber. In fact, they can become so sensitized that they will fire in response to the normal pulse pressure of cardiac contraction, eliciting a complaint of “I can feel my heartbeat in my tooth” or “my tooth is throbbing.” Sensitized C fibers can even fire without provocation, resulting in spontaneous pain.
Typical of deep visceral tissues, the pulpal nociceptors demonstrate a high degree of convergence in the central nervous system. In a study of cat brain, 74% of the neurons tested in the subnucleus caudalis showed convergence from multiple tooth pulps.19 In addition, the dental pulp lacks proprioceptive neurons. The high degree of convergence from pulp tissue and the lack of proprioceptive information provided are the key factors in why purely pulpal pain can be so difficult for patients to localize. In addition to reducing localization of pain, convergence increases the referral of pain to tissues not actually affected by the inflammation. The fact that there is convergence of neurons from the pulps of mandibular teeth with those of maxillary teeth can result in pain from a mandibular pulpitis being referred to the maxillary arch. Because pulpal pain may be poorly localized by the patient, it is important for the clinician to localize the source of the pain. This is often accomplished through the use of tests that are employed in an attempt either to reproduce the eliciting stimulus of a patient’s pain or to eliminate the pain. For example, pulpal pain should be aggravated with hot or cold stimulation and should be eliminated or significantly reduced with local anesthetic.
Unlike pulpal pain, pain of periradicular origin is easier to localize. Mechanoreceptors are numerous in the periodontal ligament (PDL) and are most densely concentrated in the apical one third.74 Once inflammation from pulpal disease extends into the periodontal ligament patients are able to locate the source of the pain much more readily. As a musculoskeletal structure the PDL responds to noxious stimulation in a graded fashion. That is, the degree of discomfort a patient feels in relation to their periradicular pain is dependent on the degree of peripheral sensitization and the amount of provocation to this structure. A sensitized PDL will be uncomfortable to a patient if percussed lightly but more uncomfortable if percussed heavily. This is known as a graded response. It is, therefore, appropriate to record periradicular testing such as percussion and palpation in terms of degrees of tenderness (vs. “all or nothing”). As with pulpal pain, pain of periradicular origin should also have an identifiable etiology. Periradicular pain tends to be dull, aching, or throbbing and should resolve completely with local anesthesia. If pain of suspected periradicular origin is nonresponsive to local anesthetic, it is a strong indication that the pain may be nonodontogenic in origin.
The tooth is unique in the human body in that it has a visceral-like component, the pulp, and a musculoskeletal component, the periodontal ligament. Therefore, odontogenic pain can have a wide variety of presentations. Tooth pain can be diffuse or well localized, mild or intense, spontaneous or provoked with various stimuli applied at various intensities. The quality can vary between sharp and dull, aching or throbbing. This potential for extreme variability makes it possible for toothaches to mimic or be mimicked by many other types of pain that occur in the head and neck. In addition, because both the pulp tissue and periodontal ligament can be categorized as deep somatic tissue, continued nociceptive input from odontogenic pain has a great propensity to produce central excitatory effects such as secondary hyperalgesia, referred pain, secondary co-contraction of muscles, myofascial trigger points, and autonomic changes. These effects play a large role in adding to the complexity of diagnosing odontogenic pain and differentiating tooth pain from other sources in the region.
Sources of Nonodontogenic Toothache
This chapter provides information for the dental clinician to aid in the identification of toothaches with a nonodontogenic etiology. The clinician must have a thorough knowledge of all possible causes of orofacial pain, which includes both odontogenic and nonodontogenic conditions. This knowledge will prevent misdiagnosis and allow for proper treatment selection and referral if necessary. For information about treatment of these disorders other references should be used.
Consensus on the exact taxonomy with diagnostic criteria and their interrelationships among various orofacial pain disorders has not been established. Various health care professions involved in the diagnosis and treatment of such pains have used different terms in the literature. This of course can, and has, led to confusion, especially within what we refer to as neuropathic pain. The terms used in the literature are diverse, and overlap in meaning to an unknown degree; for example, phantom tooth pain and atypical odontalgia are used interchangeably. At other times the literature uses the same terms to describe seemingly different disorders; for example, trigeminal neuralgia has the connotation of an idiopathic pain disorder characterized either as intense, intermittent lightning bolt–type pain within one or more distributions of the trigeminal nerve, or as continuous pain that is often mild to moderate in intensity that arises in association with injury to a specific branch of the trigeminal nerve. Efforts have resulted in a working diagnostic framework for neuropathic pains.111 Our classification scheme uses this framework to enhance clarity of communication and follow consensus, even though the application of these criteria to pains that present in the orofacial region is known to be associated with misclassification.30
Overall, one can classify the nonodontogenic reasons for toothache into five broad groups of pain disorders:
Musculoskeletal and Somatic Pain
Myofascial Pain
Although any deep somatic tissue type in the head and neck has the propensity to induce central excitatory effects and therefore cause referral of pains to teeth, pains of muscular origin appear to be the most common.40 Myofascial pain (MFP) emanates from small foci of hyperexcitable muscle tissue. Clinically these areas feel like taut bands or knots and are termed trigger points.110 Typically the pain is described as a diffuse, constant, dull, aching sensation; this may lead the clinician to a misdiagnosis of pulpal pain. Another potentially misleading characteristic of masticatory muscle pain is that patients may report pain when chewing. This feature is similar to pain that is periradicular, not pulpal in origin. On further investigation, it should become clear that the pain is triggered by contraction of masticatory muscles rather than loading of periodontal ligaments. Palpation of the muscles of mastication should reproduce the pain whereas percussion of the teeth should not. The intensity of the pain will increase and can be perceived in a distant site. Myofascial pain that is perceived to emanate from a tooth is a referred type of heterotopic pain. That is, the pain is felt in an area other than the nerve branch that innervates the trigger point. Typically muscles that refer pain to teeth are the masseter, temporalis, and lateral pterygoid; muscles of the neck and nonmuscular deep structures of the face can also be a source for this type of pain.118
Although the definitive pathogenesis of MFP is unknown, authors have theorized that muscles may become disturbed through injury or sustained contraction such as clenching.39,88 Clinically this muscular contraction might occur as a parafunctional habit or as a protective response by localized muscle to an ongoing deep noxious input such as dental pain. Considering this theory and what is witnessed clinically, trigger points appear to be induced or aggravated by toothache. It also appears that trigger points can persist after the toothache has been resolved. This can be confusing for the clinician and frustrating for the patient. It is important to realize the relationship of these two entities. MFP can mimic toothache and toothaches may induce the development of MFP.
Toothaches of myofascial origin may arise with or without evidence of pulpal or periapical pathosis. Definitive diagnosis is based on lack of symptoms after pulp testing and percussion and/or palpation sensitivity, and/or failure to resolve symptoms with local anesthetic blockade. In contrast, jaw function and palpation of the masticatory muscle(s) will elicit toothaches of myofascial origin. Typically, local anesthetic infiltration into the trigger point(s) will lead to resolution of symptoms.
Some common therapeutic modalities used to treat myofascial pain include deep massage, relaxation techniques, “spray and stretch,” muscle relaxants, and trigger point injections. Deep massage and relaxation techniques have the advantage of being noninvasive and easily administered. Spray and stretch involves application of a vapor coolant spray to the skin overlying the trigger point followed by a gentle stretching of the muscle. Trigger point injections are used for both the diagnosis and treatment of myofascial pain. Specifically, if the pain complaint is diminished on injection of the trigger point(s) then the source of the pain has been confirmed. The therapeutic efficacy of a trigger point injection varies. Some patients might experience lasting relief with one injection or several, whereas others may not. See the later section Additional Tests for further information about trigger point injections.
Pain of Sinus and/or Nasal Mucosal Origin
Sinus/nasal mucosal pain is another source of pain that can mimic toothache.2,3,25,114 Sinus pain can exhibit symptoms of fullness or pressure below the eyes, but is generally not particularly painful unless the nasal mucosa is also affected.32 Pain from the nasal mucosa tends to be dull and aching and can also have a burning quality typical of visceral mucosal pain. In general, these pains are of viral, bacterial, or allergic etiology. Other symptoms consistent with these types of disease (e.g., congestion and/or nasal drainage) should be noted in the patient history.
Typical of deep visceral tissues, sinus/nasal mucosal pain can induce central excitatory effects such as secondary hyperalgesia, referral of pain, and autonomic changes. It is this tendency that gives sinus/nasal pain the ability to masquerade as toothache. Secondary hyperalgesia, seen clinically as a concentric spread of pain beyond the area of tissue injury, will result in tenderness of the mucosa in the area of the maxillary sinuses as well as tenderness to percussion of several maxillary teeth. Teeth tender to percussion and palpation suggest periradicular inflammation. Autonomic sequelae might present as edema and/or erythema in the area, which could be suggestive of a dental abscess. However, when an etiology for pulpal and therefore periradicular pathosis is absent, sinus/nasal mucosal disease should be suspected. Other symptoms of sinus disease include sensitivity to palpation of structures overlying sinuses (i.e., paranasal tenderness) and a throbbing or increased pain sensation when the head is placed lower than the heart. Dental local anesthetic blockade will not abate sinus/nasal mucosa pain although topical nasal anesthetic will.
Patients with suspected sinus/nasal mucosal disease should be referred to an otolaryngologist for further diagnosis and treatment. Physical examination as well as adjunctive tests may be necessary for definitive diagnosis. Tests may include nasal cytologic and ultrasound studies and the use of nasal endoscopes, in addition to imaging tests such as radiology and computed tomographic imaging.31 Treatment of sinus/nasal mucosa pain is dependent on the etiology (e.g., bacterial, viral, allergic, or obstructive),
Salivary Gland Pain
Pain referred from one or more of the salivary glands may be perceived as tooth pain; this has not been encountered in clinical practice by the authors, but has been reported to present as a nonodontogenic toothache.70,94 Because the primary somatosensory innervation of the salivary glands comes from the mandibular branch, it is conceivable that such a presentation will occur most often in mandibular teeth.
Neurovascular Pain
Neurovascular pains, otherwise and interchangeably referred to as headache disorders, have qualities similar to pulpal pain. These types of pain can be intense, often pulsatile, and are known to occur only in the head. The International Headache Society (Oxford, UK) has developed a classification system that is widely accepted even though validation studies of these criteria have yet to be published. The interested reader should consult the classification system for more details on this topic.49 Primary neurovascular pain disorders are thought to be a referred pain phenomenon, meaning that intracranial branches of the trigeminal nerve become sensitized via incompletely understood mechanisms and the associated pain and symptoms are perceived in the somatic structures of the head. Most commonly people report pain presenting in the forehead, back of the head, and temples but also in the sinuses, jaws, and teeth. The current understanding of the pathophysiology of headaches implies that dental disease and treatments are not likely to be a cause of a person developing a headache disorder, but rather, because the same neuroanatomic circuitry is involved, these aspects of dentistry can be thought of as an inciting event, similar to the analogy that exercise producing increased demands on the cardiovascular system can be an inciting event for an acute myocardial infarction. For this reason, the dental clinician should be aware of the diagnostic status of their patients, because patients with headache disorders are likely to experience more peritreatment pain complications that are related to the innate hyperexcitability of the trigeminal nervous system in these people.
Of most interest to the dental clinician are the primary headache disorders, which comprise the bulk of the headache disorders that occur within the population and have been reported to present as nonodontogenic toothache. To simplify thinking, these primary headache disorders can be grouped into three major subdivisions: (1) migraine, (2) tension-type headache, and (3) cluster headache and other trigeminal autonomic cephalalgias (TACs).
Migraine is a common headache, with about 18% of females and 6% of males experiencing this type of headache.72,105 It is associated with significant amounts of disability, which is the motivating factor that brings the patient to seek care and the reason why this type of headache is the one most often seen in medical clinics.107 Migraine has been reported to present as toothache4,23,29,46,82,86 and is likely the most common neurovascular disorder to do so. In addition to this, people with migraine headaches are thought of as having increased regional pain sensitivity that has diagnostic and treatment implications for the clinician.85
Migraine headaches typically last between 4 and 72 hours. They tend to be unilateral in presentation and pulsatile in quality, with a moderate to severe intensity to the pain. Patients may also experience nausea and/or vomiting, as well as photophobia or phonophobia, which are different from toothache. The headache is usually aggravated with routine physical activity, such as walking up stairs. Caffeine/ergotamine compounds have been used widely in the past as abortive agents for migraine headaches, but in contemporary times they have been replaced with triptans, such as sumatriptan and rizatriptan.79 Of note: Migraine headaches may partially or fully abate with the use of nonsteroidal antiinflammatory medications in a similar fashion as toothaches.
Tension-type headache is the most frequent headache disorder experienced, with a wide range of reported prevalence (41% to 96%).96,100 The concept of tension-type headache pain presenting as toothache has not been reported in the literature, to our knowledge, likely because the construct of what a tension-type headache is has not been clearly defined. Some research supports the notion that a tension-type headache has a significant musculoskeletal component to the pain,106 whereas other research suggests otherwise. Tension-type headaches are likely a heterogeneous group of similarly presenting head pains that have overlapping pathophysiologic mechanisms, which has led some researchers to consider aspects of tension-type headache to be the same as musculoskeletal orofacial pain, otherwise known as temporomandibular disorders (TMDs).48
Cluster headaches and other TACs are rare neurovascular disorders that are strictly unilateral pains defined by the concurrent presentation of at least one ipsilateral autonomic symptom, such as nasal congestion, rhinorrhea, lacrimation, eyelid edema, periorbital swelling, facial erythema, ptosis, or miosis, that occurs with the pain. The major distinguishing features between these headache disorders are the duration and frequency of the pain episodes, as well as the gender most often afflicted. Cluster headache is the most common of the group, occurring in men three to four times more often than in women, with pain episodes lasting between 15 minutes and 2 hours that occur at a frequency of eight episodes per day to one every other day. These headaches come in clusters, with active periods of 2 weeks to 3 months,49 thus the name. Elimination of pain after 10 minutes’ inhalation of 100% oxygen is diagnostic for cluster headache43; sublingual ergotamine and sumatriptan are also effective acute treatment for cluster headache.36 Paroxysmal hemicrania, which has a 3:1 female predilection, presents with characteristics similar to those of cluster headache but with a frequency of more than five per day lasting 2 to 30 minutes.49 This headache disorder has a 100% response to indomethacin but is refractory to other treatments,56 which underscores the need for obtaining an accurate diagnosis from an experienced clinician.
From a nonodontogenic perspective, cluster headache4,11,18,45 and almost all the other TACs have been reported in the literature to present as nonodontogenic toothache.4,8,9,27,77,91,98 The concurrent autonomic features, such as discoloration or swelling in the anterior maxilla, might compound the diagnostic problem by suggesting tooth abscess. It is important to note that neurovascular headaches tend to be episodic with complete remission between episodes, whereas toothache pain usually has at least some background pain that stays between any exacerbations. Provocation of the tooth should not result in a clear increase in pain, but cause a slight alteration because this tissue has become hypersensitized. Local anesthetic is unpredictable in these cases and can mislead the clinician. Management by the typical clinician is to determine that the pain is not of odontogenic origin and then to refer the patient to an appropriate care provider. Other neurovascular disorders not classified as primary headaches have been reported to present as nonodontogenic toothache, such as cough headache.78 One would not expect a dental clinician who does not have a specific focus on orofacial pain to arrive at such a specific diagnosis, but rather to be aware of and sensitive to the fact that more obscure headache disorders exist and should be considered in the differential diagnosis of a nonodontogenic toothache that is not easily classified.
Neuropathic Pain
All previously described pain entities can be classified as somatic pain. That is, they are a result of noxious stimulation of somatic structures. These impulses are transmitted by normal neural structures and their clinical characteristics are related to stimulation of normal neural structures. Neuropathic pain actually arises from abnormalities in the neural structures themselves. The clinical examination generally reveals no somatic tissue damage and the response to stimulation of the tissue is disproportionate to the stimulus. For this reason, neuropathic pains can be misdiagnosed as psychogenic pain simply because a local cause cannot be visualized. There are many ways to categorize neuropathic pain in the orofacial region. For the purposes of this chapter and ease of discussion, neuropathic pain is divided into four subcategories: neuralgia, neuroma, neuritis, and neuropathy. It should be acknowledged that these subcategories are arbitrary and that the different subcategories are not mutually exclusive.
Neuralgia
As alluded to previously, not all uses of the term “neuralgia” refer to what is often thought of as the classic trigeminal neuralgia or tic douloureux. Sometimes the term “neuralgia” is used to describe pain felt along a specific peripheral nerve distribution, such as with postherpetic neuralgia and occipital neuralgia, as opposed to a focus of pain disorders that have similar characteristics and are thought to have common underlying pathophysiologic mechanisms. When used in the generic sense to describe pains that present intraorally, it can lead to a great deal of confusion.
Although deviations are not uncommon, trigeminal neuralgia is characteristically an intense, sharp shooting pain that is most often unilateral. Ipsilateral to the perceived location of the symptoms is an area that, on stimulation such as light touch, elicits sharp shooting pain. The area that elicits the pain is referred to as a trigger zone, and it can be in the distribution of the resultant pain or in a different distribution—but is always ipsilateral. While most patients present with a characteristic trigger zone, not all patients will present with this finding. An important characteristic of trigger zones is that the response to the stimulus is not proportional to the intensity of the stimulus. That is, slight pressure on a trigger zone results in severe pain. In addition, once triggered, pain typically subsides within a few minutes until triggered again. This is in contrast to odontogenic pain, which may come and go but does not do so in such a predictable and repeatable manner. Finally, the trigger for odontogenic pain is an area that has no sensory abnormalities (e.g., dysesthesia or paresthesia).
Trigger zones for trigeminal neuralgia tend to be related to areas of dense somatosensory innervation, such as the lips and teeth. For this reason, triggers that elicit this type of pain may include chewing and may lead both the patient and clinician to think of a diagnosis of odontogenic pain. In addition, because the trigger involves peripheral input, anesthetizing the trigger zone may result in a diminution of symptoms. This can be very misleading to the clinician if the assumption is that local anesthetic blocks only odontogenic pain.
Because symptoms can be quite severe, patients may consent to or even insist on treatment even though the clinical findings do not definitively support an odontogenic etiology. The possibly misleading symptoms, along with the willingness of the patient to consent to what may seem to be desperate measures, emphasize the importance of a thorough history and clinical evaluation. The absence of a dental etiology for the symptoms (e.g., large restorations, dental trauma, or recent dental treatment) in the presence of the characteristic sharp shooting pain should alert the clinician to consider trigeminal neuralgia in the differential diagnosis. In general, these individuals should be referred to a neurologist or orofacial pain/oral medicine clinician for a complete diagnostic workup and treatment, because case series have suggested 15% to 30% of patients have secondary reasons related to their pain,51,119 such as brain tumors and multiple sclerosis.
Trigeminal neuralgia typically presents in individuals more than 50 years of age. The etiology is thought to be inflammation in one of the trunks of the gasserian ganglion, possibly as a result of carotid artery pressure. Individuals with multiple sclerosis will develop trigeminal neuralgia more frequently than the general population. For this reason, a person less than 40 years of age who develops trigeminal neuralgia should also be screened for multiple sclerosis122 or other intracranial pathosis.51 The two general treatment options for trigeminal neuralgia are pharmacologic and surgical procedures. Because of the possible complications associated with surgery, this form of treatment is usually considered only after attempting pharmacologic therapies. Several medications, including carbamazepine, baclofen, gabapentin, and more recently pregabalin and oxcarbazepine, have been used to treat trigeminal neuralgia. Drugs aimed at relieving nociception, such as nonsteroidal antiinflammatory agents, have no significant benefit in these patients; nor do opioid-based analgesics. Clinical trials support carbamazepine as a first-line drug for treating trigeminal neuralgia.6 In patients who experience pain relief from carbamazepine, the effect is usually rapid; most will report a decrease in severity of symptoms within the first couple of days.
What is thought to be a variation of trigeminal neuralgia, and may also mimic toothache, is pretrigeminal neuralgia. Pretrigeminal neuralgia, as suggested by the name, has been described as symptoms that are different from those of classic trigeminal neuralgia but that respond to pharmacotherapy like classic trigeminal neuralgia and, over time (usually weeks to 3 yr), take on the classical characteristics of trigeminal neuralgia. The definitive features include the presence of a dull aching or burning pain that is less paroxysmal in nature but still triggered by a light touch within the orofacial region, with variable periods of remission.42 The subsequent onset of true neuralgic pain may be quite sudden or may appear several years later,88 which emphasizes the need for long-term follow-up of these patients to obtain an accurate final diagnosis.
Neuroma
The term “neuroma” has been around for many years and is often overused in an attempt to describe other types of neuropathic pain. A traumatic neuroma, also known as an amputation neuroma, is a proliferative mass of disorganized neural tissue at the site of a traumatically or surgically transected nerve. A part of the diagnosis, therefore, is confirmation of a significant event that would account for the damage to the nerve. Symptoms will not develop until the neural tissue on the proximal stump has had time to proliferate, typically about 10 days after the event. Tapping over the area of a neuroma elicits volleys of sharp electrical pain (i.e., Tinel’s sign) similar to trigeminal neuralgia. In contrast to trigeminal neuralgia, there should be a zone of anesthesia peripheral to the area of the neuroma92 that can be identified by checking for loss of pinprick sensibility, such as with the use of an explorer. Treatment involves pharmacologic management, often via local measures, and surgical coaptation of the nerve with prognosis being variable and dependent on adequate distal nerve tissue and time interval between injury and reconstruction.123 Therefore, early recognition and referral are of key importance to prevent significant distal nerve degeneration.66 Although neuromas most commonly develop in the area of the mental foramen, lower lip, and tongue, there is some evidence that they can also form in extraction sites and after pulpal extirpation. Neuromas were found to form in extraction sites between 4 and 6 months after removal of the tooth in an experimental animal model.60 Although not all neuromas that form are painful, this could be a potential explanation for ongoing pain in extraction sites after healing has appeared to occur.92 It is interesting to ponder the possibility of neuroma formation in deafferentation injuries such as pulpectomy and the implications this might have on continued PDL sensitivity after adequate root canal treatment. For treatment of neuromas that are not amenable to surgical correction, see the later section Neuropathy.
Neuritis
Neuritis is a condition caused by inflammation of a nerve or nerves secondary to injury or infection of viral or bacterial etiology. In general, pain from a virally induced neuritis, such as recurrent herpes simplex or herpes zoster, will be associated with skin or mucosal lesions (Fig. 3-5). This presentation does not result in much of a diagnostic challenge, but pain can precede the vesicular outbreak by many days or even weeks.41 Because neuritic disorders are caused by reactivation of a virus that has been dormant in the trigeminal ganglion, they are considered projected pain with distribution within the dermatomes innervated by the affected peripheral nerves. The nerves affected by the virus may solely supply deeper tissues and therefore may not produce any cutaneous lesions. In the absence of skin or mucosal lesions a viral neuritis can be difficult to diagnose41,53,58 and should be considered in the differential diagnosis of a patient with a history of primary herpes zoster infection. Bacterial infection of the sinuses or dental abscess can also cause neural inflammation that may result in pain. This pain occurs simultaneously with pain of the infected tissues and usually dissipates once the etiology is addressed. In susceptible individuals, virally or bacterially induced neuritis may produce a postinfection neuropathy of the infected nerve. The pain is fairly constant and can be dull, aching, and burning. Also, the pain may be accompanied by allodynia, a painful response to normally nonnoxious stimulation such as light brushing of the skin. Oral acyclovir has become the most common treatment for acute herpetic outbreaks and been shown to be efficacious in decreasing the duration and severity of pain after herpes zoster infection. Efficacy is based only on administration in the prevesicular, not the vesicular, stage.103 Addition of prednisolone to acyclovir produces only slight benefits over acyclovir alone. Neither acyclovir alone nor its combination with prednisolone appears to reduce the frequency of postherpetic neuralgia.116
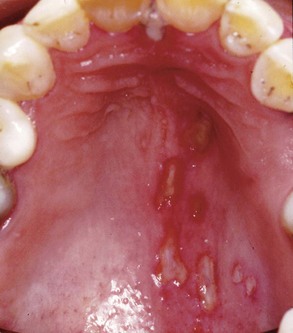
FIG. 3-5 Herpes zoster involving the maxillary division of the left trigeminal nerve of the palate of a 45-year-old male. He complained of a deep, diffuse dull ache of his maxillary left quadrant for 1 week before this vesicular outbreak.
Localized traumatic injury can also induce neuritis. This injury can be chemical, thermal, or mechanical in nature. A classic endodontic example of a chemical injury to a nerve is the overextension of a highly neurotoxic paraformaldehyde-containing paste (e.g., Sargenti paste) onto the inferior alveolar nerve. Chemical trauma can be due to certain toxic components of the endodontic filling materials such as eugenol, irrigating solutions such as sodium hypochlorite, or intracanal medicaments such as formocresol (Fig. 3-6).80 Mechanical compression in addition to thermal trauma may be a factor when thermoplasticized material is overextended, using an injectable44 or carrier-based technique. Mechanical nerve trauma is more commonly associated with oral surgical procedures such as orthognathic surgery and third molar extraction.
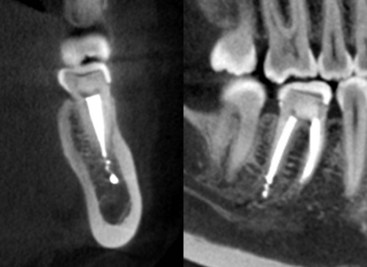
FIG. 3-6 Extrusion of the filling material from the distal canal of tooth #30 of a 36-year-old female. Her complaint was of extreme pain after completion of the root canal treatment followed by sharp, burning continuous pain that could be made worse by a light touch of the tooth.
Neuropathic complications have also been documented after mandibular implant surgery at a rate of 5% to 15% with permanent neuropathies resulting in approximately 8% of these cases.57 It is unfortunate that traumatic neuritis is often misdiagnosed as a posttreatment chronic infection and that the area is re-entered and debrided. Additional surgical insult further traumatizes the nerve, prolonging the already present nociceptive barrage, which puts the patient at an increased risk of developing central hyperalgesia. Undiagnosed and mistreated cases of acute neuritis not only lead to unnecessary dental procedures but may also aggravate the neuritis and, therefore, the neuritic pain has a greater chance of becoming chronic.
Neuritic pain typically is a persistent, nonpulsatile burning and is often associated with sensory aberrations such as paresthesia, dysesthesia, or anesthesia. The pain can vary in intensity, but when stimulated, the pain provoked is disproportionate to the stimulus.
Treatment of acute neuritis is based on its etiology. In instances of chemical trauma (e.g., Sargenti paste) where there is obvious irritant present, surgical debridement of the nerve to remove any substance that can continue to irritate the nerve is an important aspect of treatment. With a neuritis secondary to mechanical compression (e.g., implant placement) of a nerve, nerve decompression by removal of the implant fixture is indicated. Such localized, acute, traumatically induced neuritis is inflammatory in nature and, therefore, can also benefit from supportive pharmacotherapies such as steroids. For management of neuritis that is not responsive to the previously cited treatments, medications used to treat neuropathic pain may be used (see Neuropathy).
Neuropathy
In this chapter we use the word neuropathy as the preferred term for localized, sustained nonepisodic pain secondary to an injury or change in a neural structure. Historically, other terms have been used including atypical facial pain. This term suggests pain that is felt in a branch of the trigeminal nerve and that does not fit any other pain category. If pain of an unknown source is perceived in a tooth it may be labeled atypical odontalgia. If the pain persists after the tooth has been extracted it is referred to as phantom tooth pain. The major limitation in the use of all these terms is that they merely suggest an area where a pain of unknown etiology exists and completely lack any information regarding their pathophysiology. Although each of these terms has been extensively described in the literature,75,76 probably none of them actually represents one discrete condition but rather a collection of various conditions. At present, a subcommittee of the International Association for the Study of Pain (IASP, Seattle, WA) has put on hold the development of diagnostic criteria and terminology for orofacial pain because the research evidence does not clearly indicate preferred terminology based on presumed mechanisms. Until these criteria are published the use of old terms, and the confusion they bring, will persist.
Once a nerve has been sensitized via injury or disease it may remain so and present as a peripherally sensitized nerve. This peripheral sensitization and the ongoing pain (nociceptive barrage) that accompanies it can induce changes in the central or sympathetic nervous systems. Peripheral sensitization, central sensitization, and sympathetic enhancement all can potentially impact the clinical presentation of a neuropathy. A typical clinical course of someone with an undiagnosed neuropathy might consist of treatment for a toothache. When the pain does not resolve with nonsurgical root canal treatment, it might then be followed by apical surgery and then perhaps an extraction. The extraction site might then be explored and debrided in a misguided attempt to remove any potential source of the patient’s ongoing pain. After each treatment, there tends to be a reduction of the pain for a short time and then a return to its original, or even increased, intensity level. It is likely that this is a result of a new neural injury consisting of reorganization and resprouting that increases the inhibition of nerve firing for a time. Surgical approaches to neuropathies are not effective because they do not desensitize the nerve. On the contrary, surgical intervention may aggravate the situation by inflicting an additional neural injury in the periphery and contributing to the already present nociceptive input. This intervention therefore puts the patient at increased risk of developing worse peripheral sensitization, a new-onset central sensitization, or a sympathetic component to their pain. This statement is not meant to refer to situations with concurrent nerve trunk compression or other type of physical or chemical irritation present.
A diagnosis of neuropathy is based primarily on history and examination. The history should reveal some inflammation-inducing event (see the earlier sections Neuritis and Neuroma) although the nature of the initial insult is not always identified. Typically, the examination is grossly unremarkable with no evidence of local tissue damage. However, constant pain of varying degrees of intensity will be reported in a focal area. This area may be hyperalgesic or allodynic. That is, noxious stimulation to the area will be perceived as more painful or nonnoxious stimulation will now be perceived as painful. Neuropathic disorders have a predilection for women rather than men but can affect both genders. These patients are usually more than 30 years of age and may have a history of migraine.102 In the orofacial region neuropathies are most commonly seen in the maxillary premolar area and molar region.54
Neuropathies can be classified on the basis of their clinical presentation and response to therapies. Peripheral neuropathy may develop after sensitization of a peripheral nerve and presents clinically as described previously. Diagnosis of peripheral neuropathy is based on its favorable response to peripheral neural blockade. Treatment is directed at decreasing the sensitization of peripheral nerves and reducing ectopic neuronal firing. Topical as well as systemic medications can be used to treat cutaneous peripheral neuropathies. Some topical medications include topical anesthetics and capsaicin-containing compounds as well as transdermal preparations of nonsteroidal antiinflammatory drugs (NSAIDs), sympathomimetic agents, and N-methyl-d-aspartate (NMDA) receptor-blocking agents.90
The clinical presentation of a central neuropathy is similar to that of a peripheral neuropathy. After sensitization of peripheral nerves and the accompanying nociceptive barrage, the pain is nonremitting and lacks evidence of tissue insult. Unlike its peripheral counterpart, allodynia and secondary hyperalgesia are clearly present. That is, the area of pain is significantly larger than the initial site of injury. The most telling sign that a neuropathy has taken on a more central component is that local anesthetics are no longer effective. Therefore, the treatment must be directed toward the central processing of pain. This is done with medications such as NMDA receptor agonists (ketamine), gabapentin, tricyclic antidepressants, and opioids. The prognosis for a central neuropathy is not as good as for a peripheral neuropathy, as central neuropathic pain tends to become more refractory with time. Treatment is often based on the management of pain rather than its cure.
The last variation of neuropathic pain is sympathetically enhanced or maintained pain. In cases of sympathetically maintained pain (SMP), peripheral nerve fibers upregulate the expression of adrenergic receptors, making them responsive and sensitive to sympathetic input. SMP may also have a central component, whereby constant sympathetic drive alters neuronal excitability. Neuronal injury may induce sprouting of sympathetic axons into the trigeminal spinal nucleus because basket-like formations of sympathetic fibers have been reported around the cell bodies of sensory neurons in the dorsal root ganglia.117 Increases in sympathetic drive, such as with stress and fever, may aggravate SMP. Diagnosis of sympathetically maintained pain is based on blocking sympathetic outflow to the affected region via sympathetic nerve blocks. In the orofacial region this would require a stellate ganglion block. The block is considered diagnostic for SMP if the block is effective in decreasing the patient’s pain. Multiple blocks can also be used as a form of therapy. Other therapies include drugs that target peripheral α2-adrenoceptors (agonists) or α1-adrenoceptors (antagonists), such as guanethidine, phentolamine, and clonidine.
Psychogenic Toothache
Psychogenic toothache falls within a group of mental disorders known as somatoform. The name is derived from the fact that the patient has somatic complaints, yet lacks a physical cause. Because these patients lack a physical cause for pain they will also present without local tissue changes. Patients with somatoform disorder are not fabricating the symptoms, nor are they seeking conscious benefit. It is important to make a distinction between somatoform disorders and factitious or malingering disorders.5 In factitious disorders there are physical and/or psychologic symptoms that are produced by the individual and are under voluntary control. Malingering is similar to factitious disorder with the added characteristic that the symptoms are presented for obvious and recognizable benefit. This diagnostic category poses a significant diagnostic challenge. Lacking evidence of local tissue damage is typical of heterotopic pain entities previously discussed in this chapter. It is important to emphasize that psychogenic pain is rare. When arriving at this diagnosis it is critical that all other potential diagnoses have been ruled out. The diagnosis of psychogenic toothache is one of exclusion and is based on the clinician’s awareness of other heterotopic pain characteristics and behavior. Of particular note are centrally emanating pains, cardiac pain, and neurovascular and neuropathic pain.
Psychogenic pain is known to be precipitated by severe psychologic stress. These pains present a general departure from the characteristics of any other pain condition. That is, they may not fit normal anatomic distributions or physiologic patterns. The pain may be felt in multiple teeth and the pain may jump around from one tooth to another. The intensity of pain reported tends to be more severe than is reflected by the patient’s level of concern about their condition. Their response to therapy is variable, including a lack a response or an unusual or expected response. Early identification of psychogenic pain and referral to a psychologist or psychiatrist is necessary to avoid irreversible and unnecessary dental treatment.
Toothache Referred From a Distant Organic Source
A variety of different pathologies that seem to be unrelated have been reported to present as nonodontogenic toothache.89,94 The only common link that can be identified is that the involved tissues are innervated by branches of cranial nerves, and hence nociceptive input is processed in the trigeminal nucleus. Therefore, conceivably, any somatic structure with cranial nerve innervation has the potential to cause pain that is perceived by the patient as a toothache. For this reason, once dentoalveolar etiologies for such pain have been ruled out, all possible sources of nonodontogenic pain including distant pathology should be considered in the differential diagnosis. Several of these types of organic pathologies that have been reported to present as toothache are described in the following sections.
Cardiac and Thoracic Structures
Cardiac pain has been cited as the cause of nonodontogenic toothache in a number of case reports.7,35,55,67,83,112 Classically, cardiac pain presents as a crushing substernal pain that most commonly radiates to the left arm, shoulder, neck, and face. Although not as common, anginal pain may present solely as dental pain, generally felt in the lower left jaw.14 Similar to pain of pulpal origin, cardiac pain can be spontaneous and diffuse with a cyclic pattern that fluctuates in intensity from mild to severe. The pain can also be intermittent and the patient may be completely asymptomatic at times. The quality of cardiac pain when referred to the mandible is chiefly aching and sometimes pulsatile. Cardiac pain may be spontaneous or increased with physical exertion, emotional upset, or even the ingestion of food.7 Cardiac pain cannot be aggravated by local provocation of teeth. Anesthetizing the lower jaw or providing dental treatment will not reduce the pain. It can be decreased with rest or a dose of sublingual nitroglycerin. Diagnosis of cardiac pain, along with immediate referral, is mandatory to avoid impending myocardial infarction.
Besides pain of cardiac origin, other chest structures have been reported to produce nonodontogenic toothache pain. Various cancerous lesions of the lungs have been described to present a mandibular pain, on both the ipsilateral and contralateral sides of the tumor,21,52 as well as diaphragmatic pain mediated via the phrenic nerve.12
Intracranial Structures
Space-occupying lesions within and around the brain are known to impinge on structures innervated with somatosensory fibers, such as the dural and perivascular tissues, causing pain. These pains are highly variable, with a common complaint being headache or head pain. Just as intracranially derived pain may be referred to the face and jaws in neurovascular disorders, it may also present as a toothache.1,113 To outline the vast differences in clinical features of such pain, intracranial lesions have also been reported to cause trigeminal neuralgic pain in response to treatment of what was first thought to be toothache.26 This extreme variability has been observed by one of the authors, which leads to the recommendation that if local etiologic factors are not readily identified in a patient with toothache symptoms, magnetic resonance brain imaging should be considered.
Throat and Neck Structures
Nonodontogenic toothache has been reported to arise from various structures of the neck, but these reports are sparse and hence it is not possible to draw conclusions regarding how patients with these pain-provoking disorders may present. Squamous cell carcinoma of the lateral pharyngeal surface presenting as ipsilateral mandibular molar pain has been observed by one of the authors; this finding is consistent with previous reports of non-odontogenic pain being associated with smooth muscle tumors of a similar location.115 Vascular structures of the neck have also been implicated in the production of toothache symptoms, with a report of a patient initially presenting for dental care when pain was from the result of a life-threatening carotid artery dissection.97
Craniofacial Structures
Clinically, pain from other craniofacial structures has been observed as being the most common reason for organic pathologies presenting as nonodontogenic toothache, likely because these structures are innervated by branches of the trigeminal nerve. Tumors in the maxillary sinus24,37,121 and jaw,108 as well as metastatic disease, particularly within the mandible,28,47,93,101 have been reported. The clinical presentation of symptoms is highly variable, but a common feature is sensory loss along the distribution of the nerve, the result of pain arising from nerve impingement. This underscores the need for regional imaging techniques, such as pantomography or computed tomography (CT) (as opposed to just periapical radiographs), especially in patients who have a history of cancer. One must also not forget that nerve impingement anywhere along the distribution of the trigeminal nerve, even within the cranial vault itself,17 can elicit nonodontogenic tooth pain.
Vascular structures within the craniofacial region have also been reported to present as nonodontogenic toothache, with arteritis being the pain-provoking pathology.59,63 These pains have been described as a continuous dull pain that can sometimes be made worse with jaw function. The stereotypical presentation include a history of eyesight changes, such as blurred vision, and the examination feature of pulseless, indurated temporal arteries that are painful to palpation. A laboratory finding of an elevated erythrocyte sedimentation rate (ESR) is suggestive of the disorder, and diagnosis is confirmed by temporal artery biopsy. Treatment includes administration of corticosteroids and, because permanent blindness is a possible sequela if cranial arteries are left unmanaged, immediate referral to the appropriate medical colleague.
Taking a Patient’s History
Pain diagnosis is largely based on the patient’s subjective history; however, it is rare that patients will give all pertinent diagnostic information about their pain of their own accord. Often it is necessary to carefully extract the details of the patient’s pain complaint through systematic and thorough questioning. This is known as “taking a history” and it involves both careful listening and astute questioning. Figure 3-7 is an example of a basic diagnostic workup for odontogenic pain. It can be easily used to obtain histories of typical odontogenic pain by circling all descriptors that apply and then filling in the remaining blanks. As the details of a patient’s pain complaint are gathered, the clinician should be mentally progressing though an algorithm of possible diagnoses, as each detail should lend itself to one type of pain over another. After completing a thorough and accurate history of the complaint(s) (Fig. 3-8), often the diagnosis has already been narrowed down to one particular pain entity. This is particularly true with odontogenic pain. The only question that will remain is “which tooth is it?” It is critical to keep in mind that whereas patients will provide information about the perceived site of pain, it is the clinician’s examination that will reveal the true source of their pain. With more complicated pain complaints the clinician may have a list of possible diagnoses. This is known as a differential diagnosis. This differential will serve to guide the examination and testing in an effort to confirm one diagnosis while ruling out all others. If after completing the subjective examination all items on the differential are outside the clinician’s scope of practice, then the clinician should continue the examination until he or she has a firm idea of the possible diagnosis so that a proper referral can be made. In addition, it is paramount that all odontogenic sources have been ruled out and that this information is communicated to the health care provider to which the patient is referred. If no differential can be formulated after the history has been taken, then the history should be redirected to the patient to confirm that the information is complete and accurate. If the patient is unable to provide sufficient information regarding their pain complaint then it may be helpful to have them keep a pain history, detailing the aspects of their pain on a daily basis. Of utmost importance is to avoid treatment when the diagnosis is uncertain. Diagnostic therapy (i.e., “let’s do a root canal treatment and see if it helps”) may result in costly treatment that does not improve the patient’s condition, and could be a factor in aggravating and perpetuating a patient’s pain. Treatment should always specifically address a diagnosis.
A complete medical history along with current medications and drug allergies should always be ascertained. It is also important to make note of demographic information, as patients of certain genders and ages are more at risk for some disorders compared with others.
Recording a patient’s chief complaint in their own words is a medical legal necessity but falls short of comprising a thorough pain history. A complete history begins with a patient’s general pain complaint, for example, “My tooth hurts.” Patients may have more than one pain complaint, for example, “My tooth hurts and it is starting to make my jaw hurt.” All pain complaints should be noted and investigated separately. Understanding the specific components of the complaints makes it possible to discern the relationship between the complaints. That is, either the complaints are wholly separate and there are two types of pathosis present, or one source of pain is merely creating a heterotopic pain that is wholly secondary to the first.
Begin with determining the location at which the patient perceives the pain. Aspects of the location involve localization and migration. Pain should be definable as either well localized or diffuse and either superficial or deep. Easily localized superficial pain tends to be cutaneous or neurogenic. Musculoskeletal pain is felt deeply and is more localizable once it is provoked. Deep, diffuse pain is suggestive of deep somatic pain, be it visceral or musculoskeletal. Both tissue types are involved in a high degree of nociceptor convergence in the trigeminal nucleus and, therefore, much more likely to be involved in creating heterotopic pain. Typical referral patterns of deep somatic pain tend to follow peripheral dermatomes that reflect the laminations in the trigeminal nucleus. Referred pain also tends to occur in a cephalad direction. Therefore, referred pain from a deep somatic tissue such as tooth pulp, cardiac tissue, or skeletal muscle will respect this pattern. Pain that spreads distally along a nerve branch is much more indicative of a projected type of heterotopic pain. Projected pains imply a neurogenic source and possibly one that is secondary to impingement from intracranial pathosis. Recall that superficial sources of pain are not likely to be involved in referral, so if a patient is indicating that the pain is superficial and spreading this is highly suggestive of a neurogenic rather than a cutaneous source.
Assessment of the intensity of pain is easily accomplished using a verbal analog scale. This question is best phrased, “On a scale of one to ten, zero being no pain and ten being the worst pain you can imagine, how bad is your pain?” Not only can intensity provide insight about pain type; it can also help guide posttreatment pain management as well as provide a baseline for response to therapies.
Identifying the onset of pain may provide information regarding etiology. Question if the onset followed a particular event such as a dental appointment or a traumatic injury. Beware of these relationships, as they can also be misleading. Having a temporal correlation does not necessarily ensure a cause-and-effect relationship. The onset of pain may be either gradual or sudden. Severe pain of sudden onset can signal a more serious problem. Pain that has been present over a protracted period to time, particularly if the pain has been unchanging, is highly suggestive of a nonodontogenic pain source.
Other temporal aspects of pain include frequency and duration. The question should be asked: How often does the pain occur and how long does it last? These temporal aspects may establish patterns that point more clearly to one condition over another.
Progression of the patient’s pain over time should be noted. Whether pain is better, worse, or unchanging since its onset should be broken down into three factors: frequency, intensity, and duration. Static pain that does not change over time is typically not odontogenic in origin.
The quality of pain, that is, “what it feels like,” is a critical aspect of a pain history. Knowledge of pain characteristics as they relate to tissue types is essential. Pain quality can be difficult for patients to describe and it is often necessary to provide them with a list of descriptors from which to choose. In instances of odontogenic pain the list is fairly short. The deep visceral and musculoskeletal components of a tooth limit true odontogenic pain to having qualities that are either dull, aching, or throbbing. If there is an aspect of sharpness to the pain it is helpful to understand whether the sharpness is stabbing in nature, which would be more indicative of A-delta fiber-mediated dentinal pain, or whether it is electrical in nature, which would be more indicative of a neuralgia. Some common examples of pain descriptors and their respective pain types are listed in Table 3-1.
TABLE 3-1 Examples of Pain Descriptors
| Origin | Quality of Pain |
|---|---|
| Muscular | Dull, aching |
| Neurogenic | Shocking, burning |
| Vascular | Throbbing, pulsatile |
Factors that precipitate or aggravate the patient’s pain complaint are of key importance in diagnosis. Not only do aggravating factors suggest the tissue types that may be involved, but they also aid in directing the objective tests. When gathering information, it is important to be specific. If a patient reports pain while eating, keep in mind that many structures are stimulated during mastication, such as muscles, temporomandibular joints (TMJs), mucosa, PDLs, and potentially pulps. Be specific as to the aggravating factor. The lack of any aggravating factors is an indication that the pain is not of odontogenic origin.
Alleviating factors can provide insight as to the nature of the pain. If the pain is relieved by a medication it is critical to know the specific medication, its dosage, and the degree to which the pain was attenuated. It is equally important to know what has no effect on the intensity of the pain. For example, a pain that is mid-level in intensity yet completely unresponsive to antiinflammatory drugs suggests a pain that is not inflammatory in origin.
Associated factors such as swelling, discoloration, and numbness must be ascertained, as well as their correlation with symptoms. Swelling of acute onset is suggestive of an infection, and its concurrent pain would be of inflammatory origin. Swelling that comes and goes with the intensity of pain suggests an autonomic component. The same can be said for discoloration, such as redness. Numbness or any other type of sensory aberrations should be recorded. If the altered sensation is a major component of the pain complaint then it should be investigated separately and its relationship to the pain determined. Pains that occur with sensory aberrations tend to have a strong neurogenic component.
If there is more than one pain complaint present, an effort should be made during the subjective history to determine the relationship of the complaints. One pain might serve as an aggravating factor to the other. There may be a correlation as to the onset, intensity, or progression of the complaints. Also, keep in mind that patients may actually have more than one type of pathosis occurring concurrently and there may be no relationship whatsoever. Ask whether there have been any previous similar complaints and, if so, what happened. Recurrence of similar pains might reveal a pattern that lends itself to a particular pain diagnosis.
It is critical to gain knowledge of any prior consultations that have occurred. Details regarding the type of clinician, actual workup performed, and diagnosis rendered will be helpful in narrowing down a differential. Any treatment that was performed should be ascertained and its effect on the chief complaint.
Patient Examination
As stated previously, the purpose of the history is to gather information about a patient’s pain complaint in order to formulate a list of possible diagnoses based on specific pain characteristics. Poor or improper symptom analysis will lead to a false differential and any testing will therefore have limited meaning. Performing a general examination including extraoral, intraoral, and hard and soft tissue assessment is requisite to confirm the health of various structures and to identify possible pain-producing etiologies. When a patient presents with a toothache, the pain is usually of odontogenic origin. Diagnostic procedures are often limited to confirming a suspect tooth rather than identifying a nonodontogenic source of pain. Standard pulpal and periradicular tests serve to aid in both ruling in odontogenic pain and therefore ruling out nonodontogenic pain as a diagnosis. Remember that the site of pain is determined by patient history, but the true source of the pain should be revealed with testing. If the chief complaint cannot be reproduced with standard tests then additional tests may be necessary to narrow down a differential diagnosis. For detail on general examinations and standard tests, please refer to Chapter 1.
Additional Tests
Further tests should be chosen with forethought in an effort to develop a workable differential that can guide the clinician toward a meaningful consultation or an appropriate referral for the patient. These tests may consist of palpation or provocation of various structures, sensory testing, or diagnostic blocks. The application of these tests is not covered in detail in this chapter. For more information about the application and interpretation of these tests, please consult other sources.
Palpation and percussion are common tests to differentiate odontogenic pain from pain of sinus origin. Palpation of the sinuses consists of firm pressure placed over the involved sinus (usually maxillary). In addition, pain of sinus origin may be provoked with a lowering of the patient’s head.
If pain of muscular origin is suspected then an attempt to reproduce this pain can be done by palpation of the muscles of mastication or provocation via functional manipulation. The temporalis, deep and superficial masseter, medial pterygoid, and digastric muscles should be palpated in an effort to discover tenderness or trigger points that reproduce the pain complaint. The medial pterygoid is only partially accessible to palpation and may need to be functionally tested by stretching the muscle (opening wide) or contracting the muscle (biting firmly). The lateral pterygoid may be difficult if not impossible to palpate intraorally and therefore is more appropriately assessed by functional manipulation. Pain emanating from this muscle may be increased by protruding the jaw against resistance. Exacerbating the chief complaint by muscular function provides a strong indication of a myofascial source of pain.
Because of the complexity of innervation and the occurrence of heterotopic pain in the orofacial region it may be difficult to definitively determine the origin of pain by testing alone. It cannot be stressed enough that primary pain should not only be provoked by local manipulation but also be relieved by anesthetic blocking. In diagnostic anesthesia the relief of pain has a typical onset and duration, depending on the particular anesthetic used. In addition, the pain should be completely diminished or else suspicion of a central component or a coexisting disorder should arise. The use of diagnostic anesthesia may be necessary and useful in augmenting the diagnostic workup (see Fig. 3-9, A and B). Topical anesthetic can be helpful in the investigation of cutaneous pain and peripheral neuropathies. Anesthetic injection including peripheral nerve blocks can be used to determine whether the etiology of the pathosis is peripheral to the area of the block. Pain that persists after the onset of usual signs of anesthesia suggests a central component to the patient’s pain. The patient’s history and general examination are of key importance in differentiating between the pain of a central neuropathy and the central pain emanating from an intracranial mass.
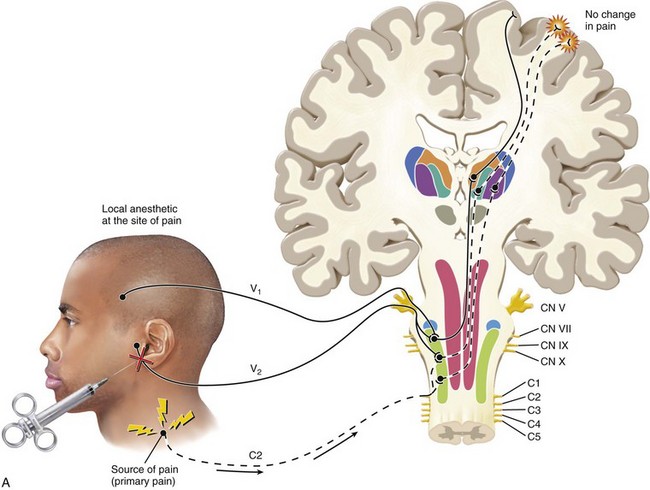
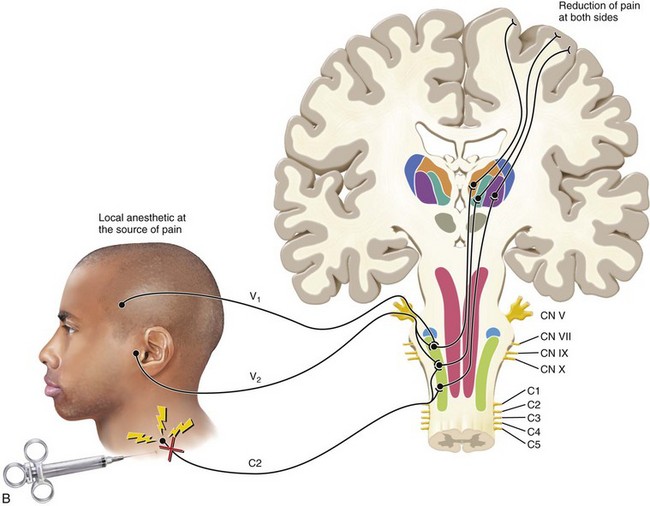
FIG. 3-9 A, Local anesthesia at the site of pain fails to reduce the pain. B, Local anesthesia at the source of pain reduces the pain at the source as well as at the site.
(Redrawn from Okeson JP: Bell’s orofacial pains, ed 5, Chicago, 1995, Quintessence Publishing.)
Pain that is primarily muscular, as suggested by trigger points discovered on examination, can be further investigated by local anesthetic injection into the trigger point. Trigger point injections are typically performed with either a 27- or 25-gauge needle and a minimally myotoxic anesthetic such as 2% lidocaine or 3% mepivacaine without a vasoconstrictor. Myofascial trigger point injections may result in temporary relief from pain at the trigger point as well as at the site of referral.
Sympathetic efferent activity can play a role in the enhancement or maintenance of chronic pain. In the head and neck, sympathetic activity flows through the stellate ganglion located bilaterally near the first rib. In patients in whom there is suspicion of a sympathetic component to their pain, a stellate ganglion block can be used to provide diagnostic insight. This is usually done by a trained anesthesiologist. An effective block delivered to the stellate ganglion will interrupt sympathetic outflow to the ipsilateral side of the face, resulting in a partial Horner’s syndrome. This is evidenced by flushing, congestion, lacrimation, miosis of the pupil, ptosis, and anhidrosis.65 A sympathetic blockade that diminishes or eliminates a pain state may serve to guide future treatment such as repeated blocks or systemic treatment with sympathetically active drugs (e.g., clonidine and prazosin).95
Neurologic conditions, both peripheral and central, can present as pain in the orofacial region. A role of the clinician is to help rule out gross neurologic conditions secondary to intracranial pathosis. Patients with systemic complaints such as nausea, dizziness, or changes in one of the special senses should raise suspicion of intracranial pathosis. A neurologic screening examination including a gross sensory and motor evaluation of cranial nerves ii–xii should be performed. For details on cranial nerve examination, please refer to other sources.38 Investigation of sharp/dull differentiation as well as light touch discrimination between the different branches of the trigeminal nerve can provide insight as to location and etiology of pathosis.
Case Studies
Case 1
A 56-year-old male presents with a chief complaint of “This tooth still hurts and it’s getting worse. It even hurts when I smile.” His medical history reveals a history of angina secondary to 70% occlusion of his right coronary artery. He also reports a history of hypercholesterolemia. He has no history of myocardial infarction and denies any other significant medical history. The patient is taking lovastatin (Mevacor, 400 mg/day), nifedipine (Procardia, 60 mg once per day), and atenolol (50 mg once per day). He has no known drug allergy.
The patient was referred by a periodontist for evaluation of continued pain associated with tooth #3. He had been receiving periodontal maintenance therapy for generalized moderate adult periodontitis for more than 5 years. He had root canal treatment and a mesial buccal root amputation of tooth #3 as treatment for a localized area of advanced periodontitis 6 months ago.
Subjective History
After careful questioning, it becomes apparent that the patient is experiencing pain having two different qualities: an intermittent ache associated with tooth #3 and an intermittent sharp shooting pain also associated with tooth #3. The intermittent dull ache was of gradual onset 9 months ago. This pain was unaffected by the nonsurgical root canal treatment and root amputation. This pain has increased in frequency, intensity, and duration over the last 3 months. There is no temporal component. The dull ache is aggravated by biting and by the occurrence of the sharp shooting pain. The sharp shooting pain had a sudden onset 6 months ago. It has also increased in frequency, intensity, and duration without a temporal component. It can occur spontaneously or when “smiling big.” The patient reports that the sharp shooting pain can also be aggravated by pressing lightly on his face in the area overlying #3, but not by pressing intraorally on #3.
Examination
The coronal portion of the amputated mesiobuccal root had been restored with IRM (Intermediate Restorative Material; DENTSPLY Caulk, Milford, DE). No cracks, fractures, sinus tracts, or swelling is detected. There are generalized probing depths of 4 mm throughout the upper right sextant. Tooth #3 has an 8-mm-broad probing defect mesially with bleeding on probing. For the results of clinical testing, see Table 3-2.
A periapical radiograph (Fig. 3-10) shows evidence of prior nonsurgical root canal treatment and mesiobuccal root amputation of tooth #3. Mild to moderate horizontal bone loss is evident in the quadrant. No radiographic evidence of caries or apical radiolucencies is noted.
FIG. 3-10 Periapical radiograph showing prior nonsurgical root canal treatment and mesiobuccal root amputation of tooth #3.
Additional tests: In the absence of a clear etiology, a more extensive extraoral examination is performed. Cranial nerves ii–xii are intact. The sharp shooting pain is predictably produced with light brushing of the skin over the area of tooth #3. This examination increases the patient’s subjective complaint of a dull ache associated with tooth #3. With the likelihood of two possible sources of pain existing, a diagnostic anesthetic block of tooth #3 is performed: buccal infiltration of tooth #3 with 27 mg of 3% mepivacaine without epinephrine. After 3 minutes the patient no longer reports a dull ache at tooth #3 and he is nontender to percussion. His sharp shooting pain can still be initiated with light brushing of the skin over the area of tooth #3 and continues to cause a dull ache in the area of tooth #3. Diagnoses of trigeminal neuralgia and advanced localized adult periodontitis of tooth #3 are made. The patient is referred to a neurologist for evaluation and treatment. The diagnosis of trigeminal neuralgia is confirmed and he is placed on carbamazepine at 100 mg/day.
Case 2
A 28-year-old male presents with a chief complaint of “My teeth on the right side hurt.” His past medical history is not significant. He denies any systemic disease and has no known drug allergies. He is currently taking 600 mg of ibuprofen as needed for pain. He is taking no other medications. The patient was referred by his general clinician for evaluation of pain associated with his teeth on the right side.
Subjective History
After careful questioning, it is determined that the patient is experiencing pain of two different types. The most distressing pain to the patient is a diffuse, right-sided, constant low-grade dull ache (3/10 on a verbal analog scale [VAS]). The onset was gradual, beginning 2 years ago. The pain has recently increased in intensity and duration. This pain is aggravated by opening wide and increases in intensity after the occurrence of a sharp pain that is induced by biting down. There is no notable temporal component and no attempts have been made by the patient to obtund the pain.
His other pain type had a sudden onset approximately 4 months ago. This pain is localized to the area of the right first molars. It is an intermittent sharp shooting pain (8/10 on VAS) that occurs when biting.
Examination
Tooth #3 has an occlusal amalgam with cracks evident on the mesial marginal ridge and the buccal groove. Tooth #30 has an occlusal amalgam and cracks are noted on the mesial and distal marginal ridges of the tooth. There are no swellings or sinus tracts and no probing greater than 4 mm on the right side. A periapical radiograph demonstrates no evidence of caries or apical radiolucencies. The patient’s sharp pain is reproduced with a bite test applied to the ML cusp of tooth #30. After the bite test he reports an intensifying of his dull ache. For the results of clinical testing see Table 3-3.
Thirty seconds after pulp testing has been completed the patient again reports an intensifying of his dull ache.
Additional tests: In consideration of an uncertain diagnosis a more extensive examination is performed. Palpation and provocation tests of the muscles of mastication reveal a trigger point in his right deep masseter. Palpation of this trigger point results in immediate intensifying of his “toothache.” A trigger point injection of 3% mepivacaine without epinephrine is done in an effort to clarify a diagnosis. After a trigger point injection, all tests are repeated. Palpation of the trigger point no longer produces pain. The bite test and cold test still produce a short sharp pain but are no longer followed by a dull ache.
Diagnosis of reversible pulpitis secondary to a cracked tooth #31, and myofascial pain of the right masseter, are made. The patient is given home care instructions for treatment of his myofascial pain and he is referred to his general clinician for cuspal coverage of both teeth #3 and #30.
Summary
As clinicians who are frequently called on to diagnose and treat complaints of orofacial pain, it is important to have a thorough knowledge of odontogenic and nonodontogenic causes of orofacial pain. The basis for this knowledge begins with an understanding of the anatomy and physiology of the pain system, and how alterations in this system can result in pain that is poorly localized and therefore misdiagnosed. A realization that pain does not always originate in the structures in which they are felt, along with an understanding of the neurobiologic basis of heterotopic pain, is necessary to ensure accurate diagnosis of orofacial pain.
There are several indicators that a toothache may be nonodontogenic in origin. Red flags for nonodontogenic pain include toothaches that have no apparent etiology for pulpal or periradicular pathosis; pain that is spontaneous, poorly localized, or migratory; and pain that is constant and nonvariable. In addition, pain that is described as burning, pricking, or “shocklike” is less likely to be pulpal or periradicular in origin.
A thorough pain history and examination of dental and nondental structures are essential to differentiate between odontogenic and nonodontogenic sources of pain. Examples of key components of the pain history and examination are included in this chapter for reference. In addition, the chapter has focused on the more common nonodontogenic sources of orofacial pain. As stated previously, the role of the dental clinician is to diagnose and treat disorders of the oral cavity and masticatory structures. In the event that a nondental pathosis is suspected, a differential diagnosis of probable disorders is essential as part of a referral to a more appropriate health care provider. In addition, an understanding of any potential role or interaction of dental structures in the patient’s pain complaint should be communicated as part of the referral.
1. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 17-1988. A 40-year-old man with a cerebral mass one month after severe pain in the region of an upper tooth. N Engl J Med. 1988;318:1116.
2. Abu-Bakra M, Jones NS. Prevalence of nasal mucosal contact points in patients with facial pain compared with patients without facial pain. J Laryngol Otol. 2001;115:629-632.
3. Albin R, Wiener J, Gordon R, et al. Diagnosis and treatment of pansinusitis: report of case. J Oral Surg. 1979;37:604-607.
4. Alonso AA, Nixdorf DR. Case series of four different headache types presenting as tooth pain. J Endod. 2006;32:1110-1113.
5. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV). Washington, DC: American Psychiatric Association; 1994. p. 447
6. Backonja M. Use of anticonvulsants for treatment of neuropathic pain. Neurology. 2002:10.
7. Batchelder B, Krutchkoff DJ, Amara J. Mandibular pain as the initial and sole clinical manifestation of coronary insufficiency. J Am Dent Assoc. 1987:115.
8. Benoliel R, Sharav Y. Paroxysmal hemicrania: case studies and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:285-292.
9. Benoliel R, Sharav Y. SUNCT syndrome: case report and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:158-161.
10. Besson J, Chaouch A. Peripheral and spinal mechanisms of nociception. Physiol Rev. 1987;67:67.
11. Bittar G, Graff-Radford SB. A retrospective study of patients with cluster headaches. Oral Surg Oral Med Oral Pathol. 1992;73:519-525.
12. Blows WT. Diaphragmatic cramp as a possible cause of noncardiac chest pain and referred mandibular pain. J Neurosci Nurs. 1999;31:187-190.
13. Bonica J. The management of pain. Philadelphia: Lea & Febiger; 1990.
14. Bonica J. The management of pain with special emphasis on the use of analgesic block in diagnosis, prognosis and therapy. Philadelphia: Lea & Febiger; 1953.
15. Brannstrom M, Johnson G, Nordenvall KJ. Transmission and control of dentinal pain: resin impregnation for the desensitization of pain. J Am Dent Assoc. 1979;99:612.
16. Brattberg J, Thorslund M, Wickman A. The prevalence of pain in a general population: the results of a postal study in a county in Sweden. Pain. 1989;37:215.
17. Brazis PW, Wharen RE, Czervionke LF, et al. Hemangioma of the mandibular branch of the trigeminal nerve in the Meckel cave presenting with facial pain and sixth nerve palsy. J Neuroophthalmol. 2000;20:14.
18. Brooke RI. Periodic migrainous neuralgia: a cause of dental pain. Oral Surg Oral Med Oral Pathol. 1978;46:511-516.
19. Broton J, Hu JW, Sessle BJ. Effects of temporomandibular joint stimulation on nociceptive and nonnociceptive neurons of the cat’s trigeminal subnucleus caudalis (medulla dorsal horn). J Neurophysiol. 1988;59:1575.
20. Brown A, Beeler WJ, Kloka AC, et al. Spatial summation of pre-pain and pain in human teeth. Pain. 1985;21:1.
21. Buddery DJ. Mandible pain. Br Dent J. 2003;194:121.
22. Byers M. Dental sensory receptors. Int Rev Neurobiol. 1984;25:39.
23. Campbell JK. Facial pain due to migraine and cluster headache. Semin Neurol. 1988;8:324-331.
24. Chan YW, Guo YC, Tsai TL, et al. Malignant fibrous histiocytoma of the maxillary sinus presenting as toothache. J Chin Med Assoc. 2004;67:104.
25. Chen YH, Tseng CC, Chao WY, et al. Toothache with a multifactorial etiology: a case report. Endod Dent Traumatol. 1997;13:245-247.
26. Cirak B, Kiymaz N, Arslanoglu A. Trigeminal neuralgia caused by intracranial epidermoid tumor: report of a case and review of the different therapeutic modalities. Pain Physician. 2004;7:129.
27. Delcanho RE, Graff-Radford SB. Chronic paroxysmal hemicrania presenting as toothache. J Orofac Pain. 1993;7:300-306.
28. Dewan K, Owens J, Silvester K. Maintaining a high level of suspicion for recurrent malignant disease: report of a case with periapical involvement. Int Endod J. 2007;40:900.
29. Dodick DW. Migraine with isolated face pain: a diagnostic challenge. Cephalalgia. 2007;27:1199-2000.
30. Drangsholt MT, Svennson P, Nguyen KL: Which orofacial pain conditions are neuropathic? A systematic review using NEUPSIG ’08 criteria. 12th World Congress on Pain, International Association for the Study of Pain 2008; 17-22 August Glasgow, UK.
31. Druce H. Diagnosis of sinusitis in adults: history, physical examination, nasal cytology, echo, and rhinoscope. J Allergy Clin Immunol. 1992;90:436.
32. Druce H, Slavin RG. Sinusitis: critical need for further study. J Allergy Clin Immunol. 1991;88:675.
33. Dubner R, Bennet G. Spinal and trigeminal mechanisms of nociception. Annu Rev Neurosci. 1983;6:381.
34. Dubner R, Hayes RL, Hoffman DS. Neural and behavioral correlates of pain in the trigeminal system. Res Publ Assoc Res Nerv Ment Dis. 1980;58:63-72.
35. Durso BC, Israel MS, Janini ME, et al. Orofacial pain of cardiac origin: a case report. Cranio. 2003;21:152-153.
36. Ekborn K. Treatment of cluster headache: clinical trials, design and results. Cephalalgia. 1995;15:33.
37. Ellinger RF, Kelly WH. Maxillary sinus lymphoma: a consideration in the diagnosis of odontogenic pain. J Endod. 1989;15:90.
38. Evans RW. Saunders manual of neurologic practice. Philadelphia: Elsevier Science; 2003.
39. Fricton J. Masticatory myofascial pain: an explanatory model integrating clinical, epidemiological and basic science research. Bull Group Int Research Sci Stomatol Odontol. 1999;41:14.
40. Fricton JR. Critical commentary: a unified concept of idiopathic orofacial pain: clinical features. J Orofacial Pain. 1999;13:185-188.
41. Fristad I, Bardsen A, Knudsen GC, et al. Prodromal herpes zoster—a diagnostic challenge in endodontics. Int Endod J. 2002;35:1012.
42. Fromm G, Graff-Radford SB, Terrence CF, et al. Pre-trigeminal neuralgia. Neurology. 1990;40:1493.
43. Gallagher R, Mueller L, Ciervo CA. Increased prevalence of sensing types in men with cluster headaches. Psychol Rev. 2000;87:555.
44. Gatot A, Peist M, Mozes M. Endodontic overextension produced by injected thermoplasticized gutta-percha. J Endod. 1989;15:273.
45. Gaul C, Gantenbein AR, Buettner UW, et al. Orofacial cluster headache. Cephalalgia. 2008;28:903-905.
46. Gaul C, Sandor PS, Galli U, et al. Orofacial migraine. Cephalalgia. 2007;27:950-952.
47. Gaver A, Polliack G, Pilo R, et al. Orofacial pain and numb chin syndrome as the presenting symptoms of a metastatic prostate cancer. J Postgrad Med. 2002;48:283.
48. Glaros AG, Urban D, Locke J. Headache and temporomandibular disorders: evidence for diagnostic and behavioural overlap. Cephalalgia. 2007;27:542-549.
49. Goadsby PJ. The international classification of headache disorders. Oxford: Blackwell Publishing; 2004.
50. Gobel S, Falls W, Hockfield S, et al. The division of the dorsal and ventral horns of the mammalian caudal medulla into eight layers using anatomical criteria. In: Anderson DJ, Matthews B, editors. Pain in the trigeminal region: proceedings of a symposium held in the Department of Physiology, University of Bristol, England on 25–27 July 1977. Amsterdam: Elsevier Press; 1977:443.
51. Goh B, Poon CY, Peck RH. The importance of routine magnetic resonance imaging in trigeminal neuralgia diagnosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:424-429.
52. Goldberg HL. Chest cancer refers pain to face and jaw: a case review. Cranio. 1997;15:167-169.
53. Goon WW, Jacobsen PL. Prodromal odontalgia and multiple devitalized teeth caused by a herpes zoster infection of the trigeminal nerve: report of case. J Am Dent Assoc. 1988;116:500-504.
54. Graff-Radford SB, Solberg WK. Atypical odontalgia. J Craniomandib Disord. 1992;6:260-265.
55. Graham LL, Schinbeckler GA. Orofacial pain of cardiac origin. J Am Dent Assoc. 1982;104:47-48.
56. Greenberg DA, Aminoff MJ, Simon RP. Clinical neurology. New York: McGraw Hill; 2002.
57. Gregg J. Neuropathic complications of mandibular implant surgery: review and case presentations. Ann R Australas Coll Dent Surg. 2000;15:176.
58. Gregory WBJr, Brooks LE, Penick EC. Herpes zoster associated with pulpless teeth. J Endod. 1975;1:32-35.
59. Guttenberg SA, Emery RW, Milobsky SA, et al. Cranial arteritis mimicking odontogenic pain: report of case. J Am Dent Assoc. 1989;119:621-623.
60. Hansen H. Neuro-histological reactions following tooth extractions. Int J Oral Surg. 1980;9:411.
61. Hargreaves K, Bowles WR, Jackson DL. Intrinsic regulation of CGRP release by dental pulp sympathetic fibers. J Dent Res. 2003;82:398.
62. Hargreaves K, Dubner R. Mechanisms of pain and analgesia. Amsterdam: Elsevier Press; 1991.
63. Hellmann DB. Temporal arteritis: a cough, toothache and tongue infarction. JAMA. 2002;287:2996-3000.
64. Jacquin MF, Renehan WE, Mooney RD, et al. Structure–function relationships in rat medullary and cervical dorsal horns. I. Trigeminal primary afferents. J Neurophysiol. 1986;55:1153-1186.
65. Kisch B. Horner’s syndrome, an American discovery. Bull Hist Med. 1951;25:284.
66. Kraut R, Chahal O. Management of patients with trigeminal nerve injuries after mandibular implant placement. J Am Dent Assoc. 2002;133:1351.
67. Kreiner M, Okeson JP, Michelis V, et al. Craniofacial pain as the sole symptom of cardiac ischemia: a prospective multicenter study. J Am Dental Assoc. 2007;138:74-79.
68. Lamotte R, Campbell JN. Comparison of responses of warm and nociceptive C-fiber afferents in monkey with human judgements of thermal pain. J Neurophysiol. 1978;41:509.
69. LeBars D, Dickenson AH, Besson JM, et al. Aspects of sensory processing through convergent neurons. In: Yaksh TL, editor. Spinal afferent processing. New York: Plenum Press, 1986.
70. Li Y, Li HJ, Huang J, et al. Central malignant salivary gland tumors of the jaw: retrospective clinical analysis of 22 cases. J Oral Maxillofac Surg. 2008;66:2247-2253.
71. Lipton J, Ship JA, Larach-Robinson D. Estimated prevalence and distribution of reported orofacial pain in the United States. J Am Dent Assoc. 1993;124:115.
72. Lipton RB, Stewart WF, Diamond S, et al. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41:646-657.
73. Loeser JD, Treede RD. The Kyoto protocol of IASP basic pain terminology. Pain. 2008;137:473-477.
74. Long A, Loeschr AR, Robinson PP. A quantitative study on the myelinated fiber innervation of the periodontal ligament of cat canine teeth. J Dent Res. 1995;74:1310.
75. Marbach J, Raphael KG. Phantom tooth pain: a new look at an old dilemma. Pain Med. 2000;1:68.
76. Melis M, Lobo SL, Ceneviz C, et al. Atypical odontalgia: a review of the literature. Headache. 2003;43:1060.
77. Moncada E, Graff-Radford SB. Benign indomethacin-responsive headaches presenting in the orofacial region: eight case reports. J Orofac Pain. 1995;9:276-284.
78. Moncada E, Graff-Radford SB. Cough headache presenting as a toothache: a case report. Headache. 1993;33:240-243.
79. Mondell B. A review of the effects of almotriptan and other triptans on clinical trial outcomes that are meaningful to patients with migraine. Clin Ther. 2003;25:331.
80. Morse D. Infection-related mental and inferior alveolar nerve paresthesia: literature review and presentation of two cases. J Endod. 1997;23:457.
81. Nahri M. The neurophysiology of the teeth. Dent Clin North Am. 1990;34:439.
82. Namazi MR. Presentation of migraine as odontalgia. Headache. 2001;41:420-421.
83. Natkin E, Harrington GW, Mandel MA. Anginal pain referred to the teeth: report of a case. Oral Surg Oral Med Oral Pathol. 1975;40:678-680.
84. Neumann S, Doubell TP, Leslie T, et al. Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons. Nature. 1996;384:360.
85. Nixdorf DR, Velly AM, Alonso AA. Neurovascular pains: implications of migraine for the oral and maxillofacial surgeon. Oral Maxillofac Surg Clin North Am. 2008;20:221-235.
86. Obermann M, Mueller D, Yoon MS, et al. Migraine with isolated facial pain: a diagnostic challenge. Cephalalgia. 2007;27:1278-1282.
87. Okeson JP. Bell’s Orofacial Pains, 6th ed. Chicago: Quintessence Publishing; 2004.
88. Okeson JP. Orofacial Pain: Guidelines for Assessment, Diagnosis and Management. Chicago: Quintessence Publishing; 1996.
89. Okeson JP, Falace DA. Nonodontogenic toothache. Dent Clin North Am. 1997;41:367-383.
90. Padilla M, Clark GT, Merrill RL. Topical medications for orofacial neuropathic pain: a review. J Am Dent Assoc. 2000;131:184.
91. Pareja JA, Antonaci F, Vincent M. The hemicrania continua diagnosis. Cephalalgia. 2001;21:940-946.
92. Peszkowski M, Larsson AJ. Extraosseous and intraosseous oral traumatic neuromas and their association with tooth extraction. J Oral Maxillofac Surg. 1990;48:963.
93. Pruckmayer M, Glaser C, Marosi C, et al. Mandibular pain as the leading clinical symptom for metastatic disease: nine cases and review of the literature. Ann Oncol. 1998;9:559.
94. Quail G. Atypical facial pain—a diagnostic challenge. Aust Fam Physician. 2005;34:641-645.
95. Raja S, Davis KD, Campbell JN. The adrenergic pharmacology of sympathetically-maintained pain. J Reconstr Microsurg. 1992;8:63.
96. Rasmussen BK, Jensen R, Schroll M, et al. Epidemiology of headache in a general population—prevalence study. J Clin Epidemiol. 1991;44:1147-1157.
97. Roz TM, Schiffman LE, Schlossberg S. Spontaneous dissection of the internal carotid artery manifesting as pain in an endodontically treated molar. J Am Dent Assoc. 2005;136:1556-1559.
98. Sarlani E, Schwartz AH, Greenspan JD, et al. Chronic paroxysmal hemicrania: a case report and review of the literature. J Orofac Pain. 2003;17:74-78.
99. Schaible H-G Basic mechanisms of deep somatic pain Wall and Melzack’s textbook of pain 5th ed 2006 Elsevier Churchill Livingstone London
100. Schwartz BS, Stewart WF, Simon D, et al. Epidemiology of tension-type headache. JAMA. 1998;279:381-383.
101. Selden HS, Manhoff DT, Hatges NA, et al. Metastatic carcinoma to the mandible that mimicked pulpal/periodontal disease. J Endod. 1998;24:267.
102. Sicuteri F, Nicolodi M, Fusco BM, et al. Idiopathic pain as a possible risk factor for phantom tooth pain. Headache. 1991;31:577-581.
103. Soltz-Szots J, Tyring S, Andersen PL, et al. A randomized controlled trial of acyclovir versus netivudine for treatment of herpes zoster. International Zoster Study Group. Antimicrob Chemother. 1998;41:549.
104. Stewart W, Ricci JA, Chee E, et al. Lost productive time and cost due to common pain conditions in the US workforce. J Am Med Assoc. 2003;290:2443.
105. Stewart WF, Lipton RB, Celentano DD, et al. Prevalence of migraine headache in the United States: relation to age, income, race, and other sociodemographic factors. JAMA. 1992;267:64-69.
106. Svennson P. Muscle pain in the head: overlap between temporomandibular disorders and tension-type headaches. Curr Opin Neurol. 2007;20:320-325.
107. Tepper SJ, Dahlof CG, Dowson A, et al. Prevalence and diagnosis of migraine in patients consulting their physician with a complaint of headache: data from the Landmark Study. Headache. 2004;44:856-864.
108. Thomas G, Pandey M, Mathew A, et al. Primary intraosseous carcinoma of the jaw: pooled analysis of world literature and report of two new cases. Int J Oral Maxillofac Surg. 2001;30:349.
109. Torebjork H, Lundbegr LE, LaMotte RH. Pain, hyperalgesia and activity in nociceptive C units in humans after intradermal injection of capsaicin. J Physiol. 1992;448:749.
110. Travell J, Simons D. Myofascial pain and dysfunction: the trigger point manual. Baltimore: Williams & Wilkins; 1983.
111. Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630.
112. Tzukert A, Hasin Y, Sharav Y. Orofacial pain of cardiac origin. Oral Surg Oral Med Oral Pathol. 1981;51:484-486.
113. Uehara M, Tobita T, Inokuchi T. A case report: toothache caused by epidermoid cyst manifested in cerebellopontine angle. J Oral Maxillofac Surg. 2007;65:560.
114. Webb DJ, Colman MF, Thompson K, et al. Acute, life-threatening disease first appearing as odontogenic pain. J Am Dent Assoc. 1984;109:936-938.
115. Wertheimer-Hatch L, Hatch GFIII, Hatch KF, et al. Tumors of the oral cavity and pharynx. World J Surg. 2000;24:395-400.
116. Wood M, Johnson RW, McKendrick MW, et al. A randomized trial of acyclovir for 7 days or 21 days with and without prednisolone for treatment of acute herpes. N Engl J Med. 1994;330:896.
117. Woolf C. Phenotype modification of primary sensory neurons: the role of nerve growth factor in the production of persistent pain. Philos Trans R Soc Lond B Biol Sci. 1996;351:441.
118. Wright EF. Referred craniofacial pain patterns in patients with temporomandibular disorder. J Am Dent Assoc. 2000;131:1307-1315.
119. Yang J, Simonson TM, Ruprecht A, et al. Magnetic resonance imaging used to assess patients with trigeminal neuralgia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:343-350.
120. Yokota T. Neural mechanisms of trigeminal pain. In: Proceedings of the Fourth World Congress on pain. New York: McGraw-Hill; 1985.
121. Yoon JH, Chun YC, Park SY, et al. Malignant lymphoma of the maxillary sinus manifesting as a persistent toothache. J Endod. 2001;27:800.
122. Zakrzewska J. Diagnosis and differential diagnosis of trigeminal neuralgia. Clin J Pain. 2002;18:14.
123. Zuninga J. Surgical management of trigeminal neuropathic pain. Atlas Oral Maxillofac Surg Clin North Am. 2001;9:59.