CHAPTER 25 Nonsurgical Retreatment
Nonsurgical root canal therapy has become a routine procedure in modern dentistry. Recent technical and scientific advances in endodontics have resulted in the retention of millions of teeth that would otherwise be lost. Even as recent advances in surgical and prosthetic restorative care have made tooth replacement less onerous than in the past, it is universally accepted that a natural tooth with a good prognosis is a superior choice to loss and replacement.
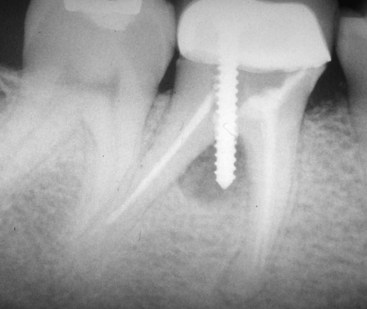
Unfortunately, not all treatments result in optimum long-term healing. Given the large numbers of treatments performed, the very small rate of unsuccessful outcomes translates into relatively large numbers of patients requiring further treatment. Clinicians should be able to diagnose persistent endodontic disease and be aware of the options for treatment. If clinicians wish to approach treating these teeth, they should have the appropriate armamentarium and be capable of performing these very specialized techniques at the highest level (Fig. 25-1). Also, clinicians must always have a scientifically sound, evidence-based rationale for every treatment decision that is made so they may best serve the patients who entrust them with their care. The purpose of this chapter is to provide information to allow the reader to maximize the likelihood of success in the treatment of persistent endodontic disease.
Etiology of Posttreatment Disease
In the past, undesirable outcomes of endodontic therapy were described as failures. Clinicians quote failure rates based on published success/failure studies. Using the words “success” and “failure” may be a holdover from a time when clinicians felt they needed to congratulate themselves on their successes and blame themselves for the failures of their treatment endeavors. This thought process does not reflect reality and can be potentially destructive. There are many instances where treatments performed at the highest level of clinical competence result in an undesirable outcome, and there are other instances where a procedure is performed well below a scientifically acceptable standard and yet provides long-term success.179,180 We must begin to dissect the science from emotion and ego, and this separation may start with nomenclature. Friedman states that “most patients can relate to the concept of disease-treatment-healing, whereas failure, apart from being a negative and relative term, does not imply the necessity to pursue treatment.”218 He has suggested using the term posttreatment disease to describe those cases that would previously have been referred to as treatment failures. This will be the term used in the remainder of this chapter to describe persistent or reintroduced endodontic disease.
Almost 16 million root canal procedures were performed in 199926; with success rates varying between 86% and 98%,54,55 it has proven to be a very reliable treatment option. Conversely, the incidence of posttreatment disease, although small, translates into a very large number of cases where further treatment is needed. When faced with such a situation, the clinician must determine the etiology of the persistent pathosis and devise a rationale and strategy for treatment.
There are many causes for “failure” of initial endodontic therapy that have been described in the endodontic literature (Fig. 25-2). These include iatrogenic procedural errors such as poor access cavity design, untreated canals (both major and accessory),235 canals that are poorly cleaned and obturated,34,96 complications of instrumentation (ledges, perforations, or separated instruments),148 and overextensions of root filling materials.141 Coronal leakage114,127,172,182,207,219 has also been blamed for posttreatment disease, as has persistent intracanal and extracanal infection143,191,203 and radicular cysts.139 These etiologies may be obvious at the time of diagnosing the diseased root-filled tooth, or they may remain uncertain until the completion of successful therapy. Occasionally, the cause of posttreatment disease may take years to become discernable or may ultimately never be known. The most important causative factors for the clinician, however, are those related to treatment planning and determining prognosis. To effectively plan treatment, the clinician may place the etiologic factors into four groups148 (Fig. 25-3):
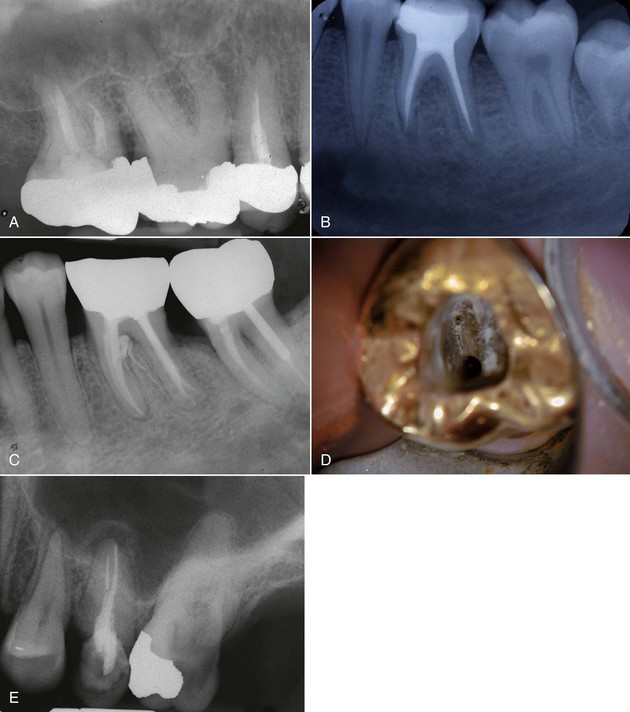
FIG. 25-2 Clinical presentations of posttreatment disease. A, Canals that are poorly cleaned, shaped, and obturated. B, Mesial canal with apical transport, ledge, and zip perforation. C, Strip perforation of the mesial root. D, Missed MB2 canal in an upper molar. E, Suspected coronal leakage of bacteria and a separated file.
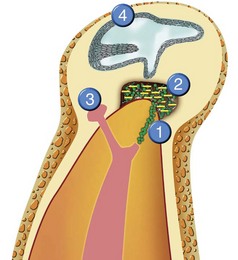
FIG. 25-3 The causes of posttreatment disease. 1, Intraradicular microorganisms. 2, Extraradicular infection. 3, Foreign body reaction. 4, True cysts.
(Modified from Sundqvist G, Figdor D: In Orstavik and Pitt-Ford Essential Endodontology, New York, 1998, Blackwell; diagram courtesy of DENTSPLY Tulsa Dental, Tulsa, OK.)
1. Persistent or reintroduced intraradicular microorganisms When the root canal space and dentinal tubules are contaminated with microorganisms or their byproducts, and if these pathogens are allowed to contact the periradicular tissues, apical periodontitis ensues. As stated earlier, inadequate cleaning, shaping, obturation, and final restoration of an endodontically diseased tooth can lead to posttreatment disease. If initial endodontic therapy does not render the canal space free of bacteria, if the obturation does not adequately entomb those that may remain, or if new microorganisms are allowed to reenter the cleaned and sealed canal space, posttreatment disease can and usually does occur. In fact, it has been asserted that persistent or reintroduced microorganisms are the major cause of posttreatment disease.140 Many iatrogenic treatment complications, such as creation of a ledge or separation of an instrument, result in persistence of bacteria in the canal system. It is not the complication itself that results in persistent disease but the inability to remove or entomb the microorganisms present that creates the pathologic state. Although infected root canals of endodontically untreated teeth generally contain a polymicrobial, predominantly anaerobic flora,202 cultures of infected, previously root-filled teeth produce very few or even one single species. The infecting flora are predominantly gram positive and not anaerobic. A very commonly isolated species is Enterococcus faecalis,60,158 which has been shown to be very resistant to canal disinfection regimes.14,30 Interestingly, if the previous root canal treatment is done so poorly that the canal space contains no obturating material in the apical half of the root canal space, its flora is more typical of the untreated necrotic infected pulp than that of classic “failed” root canal therapy.148 Though posttreatment disease has been primarily blamed on bacteria in the root canal system, fungi, notably Candida albicans, are found frequently in persistent endodontic infections and may be responsible for the recalcitrant lesion.189
2. Extraradicular infection Occasionally, bacterial cells can invade the periradicular tissues either by direct spread of infection from the root canal space via contaminated periodontal pockets that communicate with the apical area,187 extrusion of infected dentin chips,84 or by contamination with overextended, infected endodontic instruments.226 Usually, the host response will destroy these organisms, but some microorganisms are able to resist the immune defenses and persist in the periradicular tissues, sometimes by producing an extracellular matrix or protective plaque.220 It has also been shown143,191,203 that two species of microorganisms, Actinomyces israelii and Propionibacterium propionicum can exist in the periapical tissues and may prevent healing after root canal therapy.
3. Foreign body reaction Occasionally, persistent endodontic disease occurs in the absence of discernable microorganisms and has been attributed to the presence of foreign material in the periradicular area. Several materials have been associated with inflammatory responses, including lentil beans186 and cellulose fibers from paper points.108 In the seemingly endless debate about which endodontic obturation technique is superior, there has been much discussion about the effect of overextended root canal filling materials upon apical healing. Outcomes assessments generally show that filling material extrusion (root filling flush to the radiographic apex or gross overextension) leads to a lower incidence of healing.148,190 Many of these cases involved not only overextension but also inadequate canal preparation and compaction of the root filling, whereby persistent bacteria remaining in the canal space could leak out. Gutta-percha and sealers are usually well tolerated by the apical tissues, and if the tissues have not been inoculated with microorganisms by vigorous overinstrumentation, healing in the presence of overextended filling materials can still occur.59,119,148
4. True cysts Cysts form in the periradicular tissues when retained embryonic epithelium begins to proliferate due to the presence of chronic inflammation. The epithelial cell rests of Malassez are the source of the epithelium, and cyst formation may be an attempt to help separate the inflammatory stimulus from the surrounding bone.157 The incidence of periapical cysts has been reported to be 15% to 42% of all periapical lesions,139,196 and determining whether periapical radiolucency is a cyst or the more common periapical granuloma cannot be done radiographically.20 There are two types of periapical cysts: the periapical true cyst and the periapical pocket cyst. True cysts have a contained cavity or lumen within a continuous epithelial lining. With pocket cysts, the lumen is open to the root canal of the affected tooth. True cysts, owing to their self-sustaining nature, probably do not heal following nonsurgical endodontic therapy96,142 and usually require surgical enucleation (Fig. 25-4).
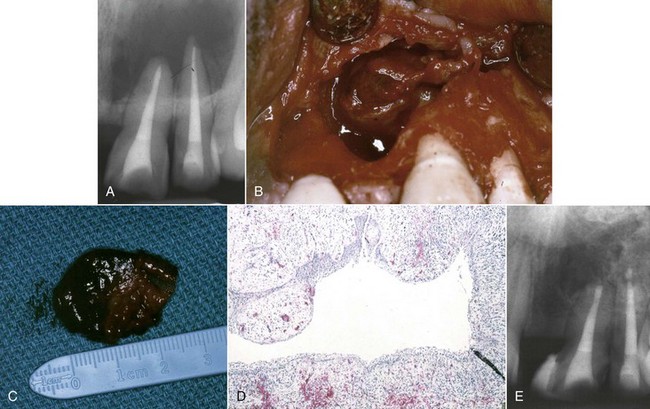
FIG. 25-4 A, Apparently good nonsurgical retreatment with large persistent lesion. B, Surgical exposure of apical lesion in situ. C, Large lesion removed in toto. D, Histopathologic section confirming cystic nature of the lesion. E, Four-year postoperative film showing apical scar formation due to the large size of the lesion. The teeth were asymptomatic and in function.
When a patient presents with posttreatment disease, clinical decision making depends upon determining the cause of the persistent disease and then making an assessment of how best to treat the pathologic condition. In the following section, the reader will be presented with a rationale and methods for performing endodontic diagnosis that will allow for the greatest likelihood of a successful outcome.
Diagnosis of Posttreatment Disease
It has been stated that “there may be different ways of treating a disease; however, there can be but one correct diagnosis”.7 The proper diagnosis is probably the most important portion of any endodontic procedure. This is not as bold a statement as one may first suspect when consideration is given to what the patient may undergo if treatment is performed based on an incorrect diagnosis (Fig. 25-5). To make a correct diagnosis, clinicians must rule out non-odontogenic etiology, perform all appropriate tests, properly interpret the patient’s responses to these tests, derive a definitive diagnosis, and decide on treatment options. When performing a diagnosis in endodontic cases where there is no history of previous endodontic therapy, both a pulpal and periradicular diagnosis are necessary. In cases of persistent disease, the diagnosis may not be straightforward; the clinician may be dealing with partially treated pulp canals, missed canals, or many other types of problems associated with the previous treatment. These must be included in the diagnostic description for each case.
FIG. 25-5 This patient was misdiagnosed for years and underwent unnecessary endodontic therapy. The actual cause of the patient’s complaint was nondental pain.
(Courtesy Dr. Ramesh Kuba.)
Endodontic diagnosis has been thoroughly discussed in Chapter 1, and the reader is referred there for further details on these procedures. The diagnostic method requires collecting subjective information, developing objective findings, and using these to arrive at a diagnosis and plan of treatment.
The subjective information is collected by questioning the patient and then actively listening to the responses. Of particular interest in cases of suspected posttreatment disease is whether the patient recalls the use of aseptic techniques during the previous endodontic therapy. If a rubber dam was not used, for example, and this can be confirmed with a call to the previous clinician, nonsurgical retreatment will almost certainly be necessary. The canals can be assumed to be contaminated regardless of how esthetically pleasing the previously filled case may appear on the radiograph. The diagnostician should be careful to avoid or to minimize communicating to the patient any negative feelings he or she has toward the previous treatment, however bad it may seem. This approach allows the patient to become more comfortable with the current clinician and the proposed corrective treatment. An irate patient is an irate patient, and negativity will color their emotional state, level of trust, and ability to accept current or future treatment plans. If the patient asks a direct question about the previous treatment, an honest answer is necessary, but avoid the temptation to imply superiority by disparaging the former clinician. To state the situation honestly and correctly without being inflammatory, use a sentence such as, “Well, it may be that your previous dentist (endodontist) had some difficulty with that tooth. Let’s see if we can figure out what could have been the problem.”
Following a thorough review of the patient’s health history, the next step is to gather all of the objective information needed to obtain an accurate diagnosis, including clinical and radiographic examinations. The clinical examination should include a visual extraoral and intraoral examination and a thorough periodontal evaluation. Visual examination is greatly aided by magnification and illumination, which can allow the clinician to identify significant conditions invisible to the naked eye, such as very fine fractures on root surfaces (Fig. 25-6). Exposed dentin from recession and narrow-based probing defects may be the result of an endodontic infection draining through the sulcus; however, they are sometimes indicative of vertical root fracture (for further information, please refer to Chapter 1). The presence of occlusal wear facets indicates the presence of occlusal trauma that may complicate diagnosis and treatment outcome by predisposing the tooth to fracture75; this condition has been associated with posttreatment disease.97
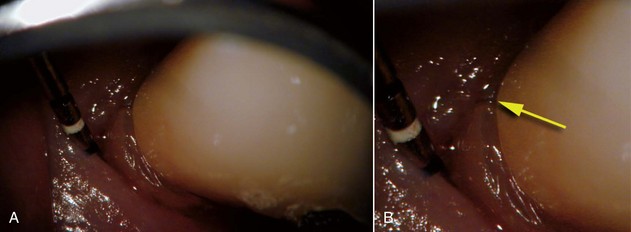
FIG. 25-6 A, Buccal aspect of a premolar with posttreatment disease. B, Higher magnification reveals a vertical fracture
(Courtesy Dr. Jay Rosenthal.)
Radiographic assessment is obligatory. Even though radiographs may be a critical aid to the clinician, they should never be the sole support for a conclusive diagnosis. They are only one piece of the puzzle in determining endodontic etiology.49 In cases with previous endodontic therapy, radiographs are very useful in evaluation of caries, defective restorations, periodontal health, the quality of the obturation, existence of missed canals, impediments to instrumentation, periradicular pathosis, perforations, fractures,209 resorption, and canal anatomy. If films are used, radiographs should be properly exposed and have a sharp, clear image. They should include the tooth and surrounding tissues, and multiple angulated films should be used to determine endodontic etiologies, using the “buccal object moves most rule” (Fig. 25-7). Bitewing radiographs are useful in determining periodontal bone height and to look for caries or fractures. All sinus tracts should be traced with a cone of gutta-percha followed by a radiograph to localize their origin.96
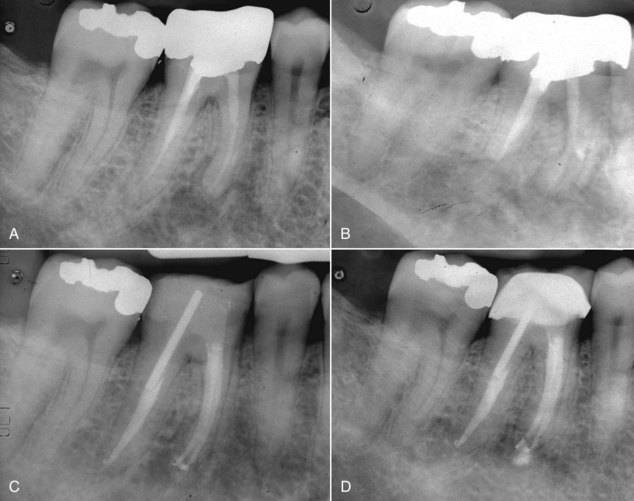
FIG. 25-7 A, Posttreatment disease. Previous endodontic therapy performed 3 years previously. B, Distal angle radiograph reveals asymmetry, indicating the presence of an untreated mesiobuccal canal. C, Immediate postobturation film showing treated MB canal. D, Fourteen-month postoperative view. The patient was asymptomatic.
Comparative testing is the next procedure performed to collect objective information about the pulpal and periradicular status. Most useful are the periradicular tests that include percussion, bite, and palpation.218 These will allow the diagnostician to begin developing a sense of the status of the periradicular tissues. Such tests are of great importance any time an endodontic diagnosis is needed. However, they are of even greater importance when evaluating teeth that have been previously treated with endodontic therapy, owing to the lack of significant and consistent evidence that can be gained from pulp vitality tests in these cases. If a tooth exhibits percussion tenderness, it may be due to persistent endodontic disease, but recent trauma or occlusal trauma may also cause this finding,75 as can periodontal disease.218
Pulp vitality tests are often of little value when examining teeth with previous endodontic therapy, but if the patient’s chief complaint reveals the need for these tests, they must be performed. When there is vital tissue remaining in the canals of a previously root-filled tooth, either by way of a completely missed canal or from an improperly cleaned canal, patients may complain of sensitivity to heat or cold.75 Pulp vitality tests should then be performed to assess the situation. They are also useful in testing adjacent and opposing nonendodontically treated teeth to rule out those as etiologies for poorly localized pain. Once the tissue is removed from the pulp chamber after root canal therapy, the results of these tests should almost always be negative, even with radicular pulp remaining. Thus, a negative response with previously treated teeth is not necessarily conclusive, but a positive response usually means there is responsive pulp tissue remaining in the tooth.75 Care is always warranted in interpreting pulp test results, however, since false-positive and false-negative results may occur.180 As with cold tests, the same limits apply to heat tests as far as the reasons for false results and accuracy relative to retreatment cases.
The remaining pulp vitality tests, electric pulp test, test cavity, and direct mechanical dentin stimulation are of even less value than thermal testing when evaluating teeth that have already received endodontic therapy. These are usually precluded by the existing restoration or endodontic therapy.
When all diagnostic information is collected, a diagnosis must be developed. It is important to record the diagnosis in the patient’s record so that anyone reading the record can discern the clinician’s rationale for treatment. The pulpal diagnosis will usually be recorded as previous endodontic treatment, but the periradicular diagnosis will vary depending on the clinical picture presented. In the case of previous endodontic treatment, however, a brief note about the suspected etiology of the persistent disease is warranted.
Treatment Planning
Once the diagnosis is complete, the cause of the persistent disease will usually become apparent. At this point in the clinical process, information must be given to the patient by the clinician as to what treatment options are available and the likely outcomes of each choice. The patient is then allowed to make a decision based upon his or her own perceptions of the options, not by the clinician’s opinion about what is “best” for the patient. The reader is reminded, however, that if the cause of the posttreatment condition remains unknown despite a thorough diagnostic workup, any decision results in an empirical, trial-and-error type of treatment. This approach should be avoided if possible, and prior to definitive treatment, consultation with an endodontist or other colleague is in order. This consultation may be as simple as a brief conversation or even referral of the patient, but a second opinion is extremely useful in these situations. Because of the interdisciplinary nature of modern dentistry, consultation with other clinicians who are treating the patient often becomes a necessity to enhance the potential for successful treatment outcomes.
Occasionally a patient will have persistent symptoms that mimic posttreatment disease, but these symptoms are actually the result of nonendodontic conditions such as occlusal trauma, concurrent periodontal disease, or nondental pain conditions. Appropriate diagnostic procedures should allow the clinician to sift through these options and treat accordingly.
The patient harboring true endodontic posttreatment disease has four basic options for treatment:
The first option is to do nothing with the condition and allow it to take its course (Fig. 25-8). This approach is sometimes a useful, short-term option if the etiology of the condition remains unknown and the clinician feels that another diagnostic sampling would help with diagnosis. Even though most clinicians would find this approach to be less than desirable as a long-term course of action, the decision belongs to the patient. The clinician is bound, however, to ensure that the patient has complete information about what will happen if nothing is done. The events in the progression of the disease and a reasonable timeline are necessary, and the conversation needs to be thoroughly documented in the patient record to avoid possible subsequent accusations of abandonment. The question of whether the clinician is required to follow up with the patient or dismiss them from the practice is one that each clinician must make based upon the clinician’s experience, judgment, and knowledge of the patient.
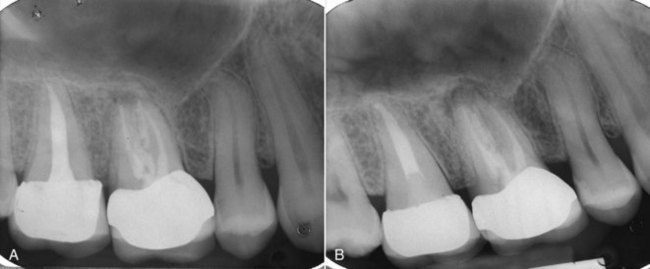
FIG. 25-8 A, Radiograph indicating presence of asymptomatic persistent apical periodontitis 7 years after initial treatment. The patient elected no treatment at that time. B, Six-year follow-up. Lesion has enlarged, and the tooth has become symptomatic.
Extraction of the tooth is usually considered a viable option. Recent advances in both prosthetic reconstruction techniques and dental implantology have made extraction and replacement a more desirable option in certain cases where previously “heroic” (read: expensive with an unknown prognosis) methods were needed to “save” the tooth. This alternative, however, provides results that are inferior, more expensive, and much more time consuming than preserving the natural tooth. The average titanium root-form implant restoration can take up to 6 months to finish, not counting site preparation prior to the implant, which can add months more. Despite published long-term success rates for dental implants,2 postimplant disease does occur2,71,72 (Fig. 25-9) and can leave the patient with very few options. The cost of implant treatment is high and usually not covered under dental benefit plans, so the net financial impact on the patient is large. Implant esthetics can be inferior to that of natural teeth in the esthetic zone of the mouth, and there are patients that are just not candidates for implant procedures.2 Fixed partial dentures are another replacement alternative with a very long history of successful use, but there are negative outcomes possible also. Most concerning to the endodontist is the likelihood that retainer fabrication procedures will result in endodontic disease of the abutment teeth79 that may potentially occur at a rate of up to 10%130,224 (Fig. 25-10). Removable partial dentures are a less desirable option to the patient because they are generally less comfortable, usually require a long period of patient adaptation, and frequently result in damage to adjacent oral tissues (teeth, gingiva, and mucosa) if not meticulously cleaned. Owing to these factors, patient compliance with removable dentures is relatively low, and their use is declining. Occasionally a patient will choose to have a tooth extracted and not pursue replacement. This decision is usually disastrous for the patient, but there are a few situations where this choice is a reasonable alternative. Diseased maxillary second molars with no opposing tooth, or with an opposing tooth in class I or class III occlusion that articulates with another tooth, may be extracted without concern for future inappropriate movement of the remaining teeth, which can be so occlusally and periodontally damaging. In most instances, however, removal of a tooth will result in the need for replacement, and unless the tooth is hopelessly unrestorable, retaining the tooth with endodontic procedures is better for the patient.
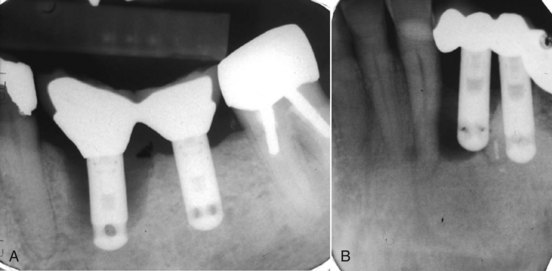
FIG. 25-9 A, Classic periimplantitis. The implant needed removal. B, Another periimplantitis. Note the endodontically treated root tip apical to the implant that may have contributed to the persistent disease. Perhaps apicoectomy should have been performed.
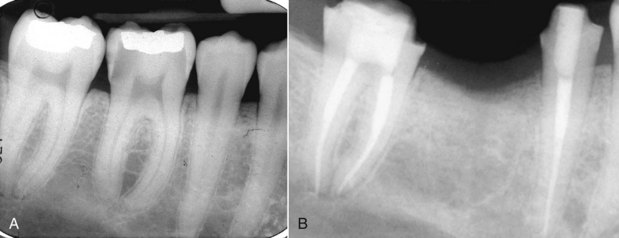
FIG. 25-10 A, Preoperative film showing deep caries approaching the pulp. The patient’s holistic dentist advised extraction and replacement rather than endodontic therapy to retain the tooth. B, Fixed partial denture fabrication procedures resulted in irreversible pulpitis on both abutments, requiring endodontic therapy.
Various situations may render a tooth unrestorable (Fig. 25-11), but the line of demarcation between restorable and unrestorable is a movable one, depending on who is evaluating the tooth. There are several widely agreed upon situations that render a tooth unrestorable. These include extensive caries or coronal fracture approaching or entering the furcation or the biologic width. This situation may render preprosthetic periodontal procedures ineffective (leaving a furcation involvement or poor crown-to-root ratio, for example) or worse, removes bone that would otherwise be useful for implant procedures. Terminal periodontal disease (extensive pocketing or mobility) or root fracture generally result in loss of the tooth despite all efforts at treatment. If the patient has a life-threatening endodontic infection with extensive trismus, most oral surgeons are going to extract the tooth rather than allow less aggressive management. Some previously root-filled teeth may have endured procedural complications, such as an irretrievable separated instrument or irreparable ledge formation. In combination with the proximity to vital anatomic structures, such as the inferior alveolar canal, endodontic retreatment, either surgical or nonsurgical, may not be feasible. Extraction may be the only option. These situations are fortunately quite rare, and in most instances, teeth presenting with posttreatment disease can be retained with endodontic procedures.
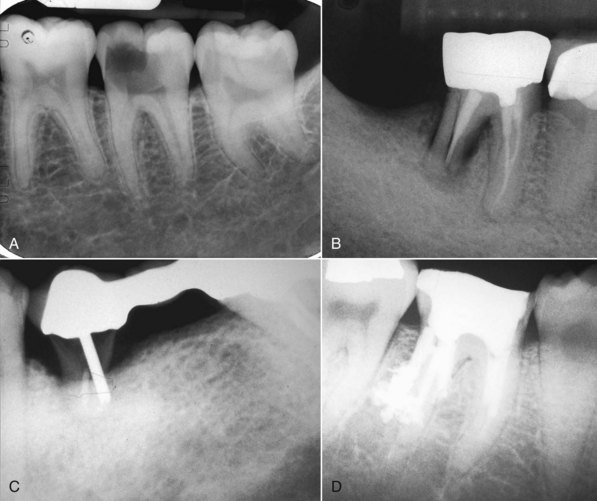
FIG. 25-11 A, Deep caries approaching the furcation and the biologic width. Necessary crown-lengthening surgery would open the furcation to bacterial invasion and persistent periodontal disease. B, Distal root vertical fracture resulting in a split root. C, Severe caries and post perforation. Inadequate root structure remaining to restore. D, Multiple distal root perforations so weaken the root as to make it unrestorable. Note: Cases A, B, and D could have resective endodontic surgery such as hemisection, but long-term prognosis is poorer than for extraction and replacement.28,111
Once the decision has been made to retain the tooth, there are several choices for treatment. These can be grouped together into either nonsurgical or surgical endodontic treatments. The surgical options can be further broken down into periradicular curettage, apical root resection (with or without root filling), root amputation or hemisection, and intentional replantation (extraction/replantation).76,147 A situation sometimes arises that will require both nonsurgical and surgical types of treatment to effect healing. The American Association of Endodontists has published guidelines that may help the clinician with clinical decision making.5 However, the choice of which option to undertake will be determined by the clinician’s experience, knowledge, patient considerations, and the preoperative diagnosis. If the etiology of the posttreatment disease can be made known, the choices become more obvious. In a previous section, four basic etiologies were presented. If the suspected etiology is in the first group, which is persistent or reintroduced microorganisms, several choices are available. If the cause of the posttreatment disease is persistent extraradicular infection, foreign body reaction, or the presence of a true cyst, nonsurgical root canal therapy has little likelihood of allowing healing to occur, and surgical methods should be employed.148 The problem for the clinician is that in most instances, it cannot be determined which of these etiologies exist, so the treatment becomes more empirical.
The choice of nonsurgical retreatment versus apical surgery becomes the focus of the decision in most instances. Outcomes assessment studies provide some help in making this decision. The reported healing rates of nonsurgical retreatment range between 74% and 98%,55 but with apical surgery alone, only 59% heal completely.148 When apical surgery is preceded by orthograde retreatment, however, the incidence of complete healing rises to 80%.148 In general, nonsurgical retreatment will be the preferred choice, since it seems to provide the most benefit with the lowest risk. It has the greatest likelihood of eliminating the most common cause of posttreatment disease: intraradicular infection. Nonsurgical retreatment is usually less invasive than surgery and has a less traumatic postoperative course. There is less likelihood of incurring damage to adjacent vital structures such as nerves, adjacent teeth, and sinus cavities. However, nonsurgical retreatment may be more costly than surgical treatment, especially if large restorations must be sacrificed during disassembly procedures prior to the retreatment. In addition, the amount of time needed for retreatment is usually longer than surgical intervention. There are times when the clinician may not be able to achieve the complete elimination of microorganisms from the canal space, and complete obturation may not be possible. Apical surgery is chosen, therefore, when nonsurgical retreatment is not possible or when the risk-to-benefit ratio of nonsurgical retreatment is outweighed by that of surgery.124,218
There are many factors to consider when deciding whether to retreat surgically or nonsurgically. The patient must be fully aware of the proposed treatment and the alternatives, and he or she must be motivated to follow through with all treatment, including the final restoration. The patient must have adequate time to undergo the required procedures. If they do not, then apical surgery alone may be indicated, although the patient must be made aware of the potentially compromised nature of the treatment. The clinician must be armed with the best equipment and knowledge available, and critical self-evaluation should allow the experienced clinician to know what they can treat and what they cannot. The tooth must be restorable and retreatable. Attempting nonsurgical retreatment on teeth where there is little likelihood of improving the previous treatment provides little benefit to the patient. Thus, in disease situations where there is an apparently adequate root filling and no evidence of coronal leakage, surgery may be indicated. If the previous treatment falls below any acceptable standard and there is no evidence of apical periodontitis, then there is no indication for any treatment unless a new coronal restoration is planned. In that case, conservative retreatment is indicated, and the reported success rates are very high.55,148 If there has been a previous procedural complication, such as a ledge that cannot be bypassed or a separated instrument that cannot be removed, surgery may become a better option. Most times, it is still prudent to attempt the retreatment, since ledges or separated instruments that appear impenetrable on diagnostic films can frequently be bypassed. Even if they cannot, nonsurgical retreatment can enhance the success of subsequent apical surgery, as noted earlier. The clinician must be careful not to worsen the situation by overly vigorous attempts to treat the previous complication; root perforation, worsening of a ledge, or another separated instrument may be the result. Previous failed apical surgery should be retreated nonsurgically and then followed up, since many surgical failures are due to poorly cleaned and filled canal systems163 (Fig. 25-12). In many instances, performing the surgery a second time can be avoided altogether. If there is evidence of root fracture (narrow-based probing defect and/or a J-shaped radiolucency encompassing the root apex and progressing in a coronal direction,208 (Fig. 25-13) nonsurgical retreatment would be unlikely to improve that situation. Apical exploratory surgery may be necessary that could result in root resection or even extraction of the tooth.
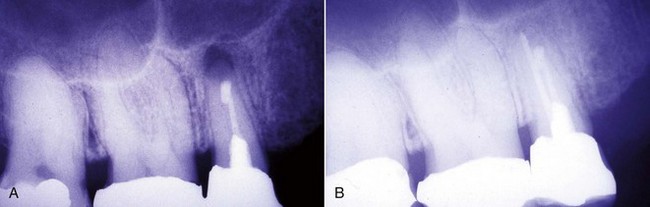
FIG. 25-12 A, Posttreatment disease following apical surgery. Off-center positioning of the root filling indicates the presence of a second untreated canal. B, One year following nonsurgical retreatment showing complete healing.
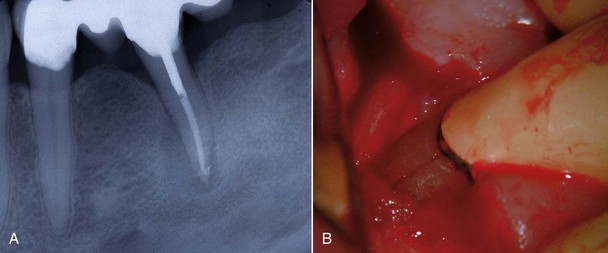
FIG. 25-13 A, The J-shaped radiolucency possibly indicates root fracture. B, Exploratory surgery confirms presence of vertical root fracture.
Each case should be approached as a unique set of considerations that must be reviewed and interpreted prior to selecting a treatment method. Once the selected option is undertaken, the prudent clinician is always watchful because additional pieces of information can be discovered during treatment that may modify previous decisions.
Nonsurgical Endodontic Retreatment
The primary difference between nonsurgical management of primary endodontic disease versus posttreatment disease is the need to regain access to the apical area of the root canal space in the previously treated tooth. After that, all of the principles of endodontic therapy apply to the completion of the retreatment case. Coronal access needs to be completed, all previous root filling materials need to be removed, canal obstructions must be managed, and impediments to achieving full working length must be overcome. Only then can cleaning and shaping procedures be instituted that will allow for effective obturation and case completion. The remainder of this chapter will be devoted to these topics in the order they generally present themselves to the clinician treating the previously root-filled tooth.
Coronal Access Cavity Preparation
Retreatment access has been called coronal disassembly163,164 because of the frequent need to take apart or remove the previous coronal and radicular restoration. Following initial endodontic therapy, most teeth require and receive a full coverage restoration, and many times that restoration is supported by a post and core. Coronal-radicular access for retreatment is much more complicated in these cases when compared to endodontically treated teeth that have been minimally restored. The goal of the access preparation is to establish straight-line access to the root canal system while conserving as much tooth structure as possible. The ideal access preparation allows for instruments to enter the canals without being deflected by the access cavity walls. This is reasonably easy to achieve when the tooth is completely intact and a pulp chamber is present, since surface and internal anatomic landmarks can guide the search for the canals. Unfortunately, when endodontic retreatment is necessary, the tooth structure has almost always been altered and is commonly quite misrepresentative of the original anatomy of the tooth.
When presented with a tooth in need of retreatment that has a full-coverage restoration, the decision for the clinician becomes whether to attempt to preserve the restoration or plan its replacement. This decision is made simpler if there is a defect or caries associated with the restoration or if the treatment plan calls for a new crown. The old one is simply removed and replaced later in the treatment sequence (Fig. 25-14). When the crown is considered to be satisfactory, the decision becomes more complex. If the restoration is maintained, the cost for replacement can be avoided, isolation is easier, the occlusion is preserved, and the esthetics will be minimally changed. Even if the crown requires replacement, the clinician may elect to retain it during the endodontic retreatment to allow for better isolation with the rubber dam. Unfortunately, retreatment may be more difficult with the crown in place, because this could lead to an increased chance for an iatrogenic mishap due to restricted visibility. In addition, removal of canal obstructions such as posts will be more difficult, and there is an increased chance the clinician may miss something important such as hidden recurrent caries, a fracture, or an additional canal. To preserve the restoration, two approaches can be taken: access through the crown or crown removal and replacement when retreatment is completed. The simplest choice is to prepare an access cavity through the existing crown, although there is a significant risk of damaging the restoration, resulting in the need to replace it.137 This risk must be communicated to the patient prior to instituting therapy. If the clinician decides to access through the existing restoration, there are several choices of access burs to use, depending on what material the preparation will be cut through. If the access will be primarily cut through metal (amalgam alloy or cast metal) or composite resin, carbide fissure burs such as the #1556 are usually chosen. With many restorations, it is advisable to consider using a combination of burs to achieve access. For example, when a porcelain fused to metal (PFM) crown is encountered, a round diamond is used to cut through the porcelain layer. Once the metal substructure is encountered, an end-cutting bur, such as the Transmetal bur (DENTSPLY Maillefer, Tulsa, OK) or the Great White bur (SS White, Burs Inc, Lakewood, NJ), can be used to cut through to and remove the core material efficiently. An important consideration for the clinician is the potential for porcelain fracture, which may occur during the preparation or possibly at a later date after completion of the treatment. This damage is especially common with porcelain jacket crowns. Restorations fabricated completely of porcelain are becoming more and more popular, thus creating added concern owing to the increased likelihood of crack formation during access. Porcelain is a glass, and drilling through this material will create many microfractures, which in turn may weaken the structure of the restoration, making it more prone to future failure.80 Copious coolant water spray and the use of diamond burs are recommended during access through porcelain to minimize occurrence of this event.206 In a novel approach, researchers165 recently showed that compared to the use of drills, air abrasion produced almost no defects in the porcelain structure of all ceramic crowns when used for endodontic access. It was, however, significantly more time consuming to access through the crown this way.
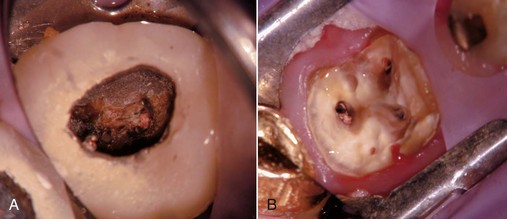
FIG. 25-14 A, Limited visibility and access with crown present. B, Enhanced visibility and access with crown off. Note the isolation achieved using a Silker-Glickman clamp and sealing putty.
If the decision is made to remove the crown for reuse, the visibility is increased, allowing for much easier removal of canal obstructions and a decrease in the potential for operator error; however, rubber dam clamp placement and tooth isolation may become a bigger problem. Also, despite all of the varying techniques and armamentaria available for removal of an existing restoration, the procedure remains unpredictable and many times can also result in damage to the restoration or the inability to remove it at all.
The clinician must decide how to remove the crown. If the crown is of no value, even as a temporary, the clinician can take the easiest road and simply cut it off. If the crown is to be preserved, a more conservative approach must be used. Two considerations that may influence the decision about removal of a crown or bridge are what material the restoration is made of and what is it cemented with. Conservative removal efforts are difficult with traditional, all-metal restorations cemented with nonbonded cements. This situation is even more of a concern lately because of the increasing popularity of tooth-colored restorations, mainly different types of porcelain or PFM restorations, which are being bonded to the tooth. These restorations are less likely to withstand the stresses of removal than those composed completely of metal, and restorations that are bonded are much more difficult to remove because of the adhesive strengths of bonding agents. Each new generation of bonding agent is stronger than the previous, making removal increasingly more difficult as cosmetic dentistry advances.
There have been many devices developed specifically for the conservative removal of crowns. Some of the more commonly used devices are forceps that have been designed specifically for crown removal, such as the K.Y. Pliers (GC America, Alsip, IL) (Fig. 25-15), which uses small replaceable rubber tips and emery powder to enable a firm grasp of the crown without damaging it. Other instruments of this type include the Wynman Crown Gripper (Miltex, York, PA), the Trial Crown Remover (Hu-Friedy, Chicago, IL), and the Trident Crown Placer/Remover (CK Dental Industries, Orange, CA). Unfortunately, a crown that has been cemented with long-term cement or has been bonded to the tooth will usually not be removed with one of these instruments. There are also forceps designed specifically to engage the margins of the crown while using an adjacent tooth as a fulcrum. Squeezing the handles together will cause the crown to be elevated off of the tooth. The Roydent Bridge Remover (Roydent Dental Products, Johnson City, TN) works in this fashion and can be effective in crown removal, but care must be taken to avoid damage to fine, fragile margins, especially on porcelain crowns. Another type of instrument can be engaged under the margin, and a subsequent impact delivered at this site will dislodge the restoration. The Easy Pneumatic Crown and Bridge Remover (Dent Corp, White Plains, NY) and the Coronaflex (KaVo, Lake Zurich, IL) create this impact from compressed air, whereas the Morrell Remover (Henry Schein, Melville, NY) applies the force manually using a sliding weighted handle. The ATD Automatic Crown & Bridge Remover (J. Morita, Irvine, CA) uses vibrations to break the crown-to-preparation bond, and the Crown-A-Matic (Peerless International, S. Easton, MA) delivers a shock impulse to loosen the crown. As mentioned before, crown margin damage may result as can inadvertent extraction of the tooth if the periodontium is compromised159 (see Fig. 25-15, E). A different approach to conservative crown removal involves drilling a small hole through the crown in order to allow a device to thread a screw through the hole. This approach creates a lifting force that separates the crown and the tooth. The instruments that work in this manner are the Metalift (Classic Practice Resources, Baton Rouge, LA), the Kline Crown Remover (Brassler, Savannah, GA), and the Higa Bridge Remover (Higa Manufacturing, West Vancouver, BC, Canada). Although very effective on metal crowns, these instruments may cause damage to porcelain occlusal surfaces on PFM restorations, so their use on anterior teeth and all porcelain restorations is generally precluded.
FIG. 25-15 A, KY Pliers (GC America, Alsip, IL) and supplied emery powder. B, Roydent Bridge Remover (Roydent Dental Products, Johnson City, TN). C, CoronaFlex Kit (KaVo Dental Corp, Lake Zurich, IL). D, Top, Crown-A-Matic (Peerless International, N. Easton, MA); Bottom, Morrell Crown Remover (Henry Schein Inc, Melville, NY) with interchangeable tips. E, Tooth inadvertently extracted using a crown/bridge remover. Endodontic therapy was performed, and the tooth was replanted, a procedure known as unintentional replantation. F, Kline Crown Remover (Brasseler USA, Savannah, GA).
Another interesting technique designed to remove a crown without causing damage is performed using the Richwil Crown & Bridge Remover (Almore, Portland, OR). This material is a water-soluble resin, which is softened using warm water (Fig. 25-16). The small block of material is placed on the crown to be removed, and the patient bites into this material until the resin cools and hardens, at which point the patient opens the mouth, generating enough force to pull the crown off. Care must be taken by the clinician to avoid using this technique when the opposing tooth is extensively restored, since the opposing restoration may inadvertently be removed during the procedure. None of these techniques works in every case, and they may produce damage to the restoration being removed or possibly others. These are, however, methods that are available and may work while permitting reuse of the restoration.
Post Removal
Once the access is prepared, it is very common to encounter a post, inasmuch as posts are frequently used in the restoration of endodontically treated teeth. There are many different types of posts the clinician may encounter during retreatment (Fig. 25-17). These can be classified into two categories: prefabricated posts and custom-cast posts. Historically, cast posts were more commonly used than prefabricated posts, but over the past 2 decades, cast posts have become much less popular.177 The main reason for this decrease is the convenience of placing the prefabricated post immediately after post preparation as opposed to waiting for a laboratory to fabricate the casting. There is also less likelihood of the interappointment contamination that frequently occurs with temporary post/core/crowns that are needed for cast/custom post-and-core fabrication. Prefabricated posts come in a variety of shapes, designs, and materials. The shapes can be subclassified into two groups: parallel sided or tapered. The design of posts also can be subclassified into active (threaded), passive, vented, fluted, and acid-etched groups. There are also many materials that have been used to fabricate posts: stainless steel, gold, titanium, ceramic, zirconium, and fiber-reinforced composite posts. Cast posts, which are fabricated in a laboratory, will always be made up of precious or non-precious metal alloys. These posts will also come in a variety of shapes and configurations, since they are custom manufactured for each root in which they are placed. Most of these will have some degree of taper, and many will be cast in one piece with the core included.
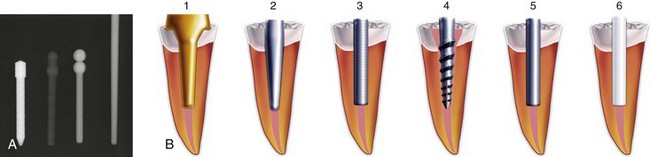
FIG. 25-17 A, Relative radiopacities of post materials (left to right): stainless steel, fiber post, titanium post, gutta-percha. B, Diagrammatic representation of post types: 1, custom cast; 2, tapered; 3, parallel; 4, active; 5, passive/metal; 6, passive/nonmetal.
(Diagrams Courtesy DENTSPLY Tulsa Dental, Tulsa, OK.)
In addition to the shape, design, and material of posts, there are two more very important factors that will have some influence on the clinician’s ability to remove them. These factors are the adhesive material used to cement the post and the location in the arch of the tooth that requires post removal.
The same concerns regarding cements that were discussed in the section on crown removal apply to post removal. The main consideration is whether the post was cemented with traditional cement or bonded with a composite resin and dentin-bonding agent. Several post systems on the market today, such as the ProPost (DENTSPLY Tulsa Dental, Tulsa, OK), use acid-etched metal posts that are bonded into the canal with cements such as Panavia (Kuraray America, Houston, TX) or C&B Metabond (Parkell Inc, Edgewood, Farmingdale, NY). Removal of these posts is extremely difficult and occasionally impossible regardless of which technique is used.70 A study62 has shown that heat generation with ultrasonic vibration may help to decrease retention of resin-cemented posts, but concern for heat-generated periodontal ligament damage may preclude this technique.177
With regard to location, the more posterior in the arch, the more difficult the post is to remove. This predicament is a result of accessibility. The more accessible the tooth is, the easier the post is to remove, since the clinician will have more techniques and instruments available to use.1 Also, the more anterior the tooth is, the less the opposing occlusion will interfere with post removal.
Post Removal Techniques
After the initial access and the post to be removed has been located, the clinician is faced with the decision of how to remove it. There have been many techniques developed for the sole purpose of post removal. Regardless of which technique is chosen, there is one simple yet extremely important rule to follow: it is not only what is removed, but what is left behind that is important. This rule applies to the removal of all intracanal obstructions. The reason for this rule is to make sure that the remaining tooth, after removal of the obstruction, can be restored predictably with a good long-term prognosis. For example, there is little use in successfully removing a post and leaving behind a root that is eggshell thin and prone to fracture (Fig. 25-18).
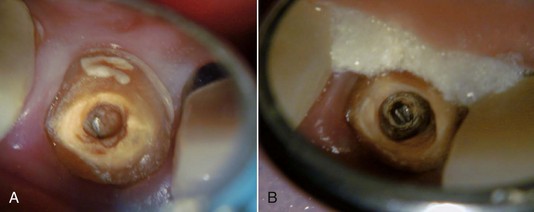
FIG. 25-18 A, Broken post (incisal view before excavation). B, Root has been so thinned and weakened by excavation procedures, restorability is questionable.
The first step in post removal is to expose it properly by removal of all adjacent restorative materials. With preformed posts, the bulk of the core material around the post and within the chamber can be removed with a high-speed handpiece using cylindrical or tapered carbide or diamond burs. When the majority of the restorative material is removed, a less aggressive instrument, such as a tapered bur in a slow-speed handpiece or a tapered midsized ultrasonic tip, should be used to remove the last of the embedding core material. This process is greatly facilitated by use of magnification and illumination. Once there is minimal restorative material remaining, a smaller-sized ultrasonic instrument should be used to minimize the risk of removing unnecessary tooth structure or thinning the post. The more post that is left, the more options for removal, and the more tooth structure that is left, the more options for restoration. At this point, a high-speed bur is too risky to use. When the core is cast in one piece with the post, a high-speed instrument can perform this process to generate a shape that can facilitate removal.
Once the post is well isolated and freed from all restorative materials, the clinician can begin the retrieval process. There are many instruments and kits on the market that can be used to remove posts; however, prior to using one, the retention of the post should be reduced. The clinician can usually continue to use the same medium-sized ultrasonic tip that helped get to this point. Using this instrument at the interface between the post and the tooth (the cement line) and constantly moving it around the circumference of the post will disrupt the cement structure along the post/canal wall interface and decrease post retention, facilitating removal16,29,103—although the effects of ultrasonic vibration may be minimal in reducing retention of well-fitted, long, large-diameter titanium posts.17 Titanium has a lower modulus of elasticity than stainless steel, so it may dampen the ultrasonic vibrations, which may decrease the effectiveness of the ultrasonic; however, a study83 failed to duplicate this effect. Nonetheless, care should be taken not to push the ultrasonic tip against the post with too much force, because this will dampen the ultrasonic wave and actually reduce the effectiveness of this technique. Taking away a small amount of the dentin around the coronal aspect of the post is not critical at this time and will aid in the reduction of post retention without unduly weakening the root. If the root is thin and the amount of space between the cement line and the root surface is restricted, the size of the tip that can be used may be limited. Unfortunately, the smaller tips are not only less effective for post removal, they are also more prone to breakage. At this point, the ultrasonic handpiece should be used with copious air/water spray as a coolant. Owing to the heat that can be generated from this procedure, the tip should be removed from the access every 10 to 15 seconds to allow the use of an air/water syringe to clean not only the area of debris but also reduce the temperature produced that could potentially cause damage to the periradicular tissues.177 If a rubber dam is in place, the area around the post may be flooded with a solvent such as chloroform prior to activating the ultrasonic instrument; this will help dissolve the cement around the post. Using a solvent in conjunction with removal of cemented obstructions may prove beneficial, since the ultrasonic energy produced will set up shock waves in the solvent and make it penetrate deeper into the canal space, exerting a faster solvent action on the cement.67
Using an ultrasonic instrument in this fashion is not simply helpful in reducing post retention, it may also prove to be all that is needed to remove the post. Many times, after judicious use of the ultrasonic instrument, the post will loosen and actually spin out of the preparation, completing post removal (Fig. 25-19). In addition, if post removal cannot be accomplished in this manner, the resulting post exposure will be very beneficial in contributing to the predictable use of other techniques. Many of the instruments to be discussed involve using a trephine bur to shape the coronal end of the post, and ultrasonic exposure will facilitate this process. Another instrument to consider for exposing and loosening a post is the Roto-Pro bur (Ellman International, Oceanside, NY) (Fig. 25-20). There are three shapes available, all of which are six-sided, non-cutting tapered burs that are used in a high-speed handpiece around the circumference of the post. The vibrations created when the non-cutting flutes come in contact with the post decrease the retention of the post, facilitating its removal.
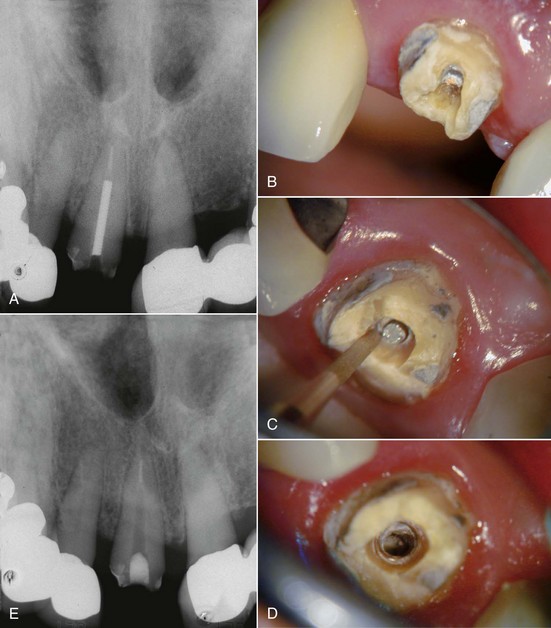
FIG. 25-19 A, Radiograph of fractured post. B, Fractured post, labial view. C, Ultrasonic troughing. D, Post removed by ultrasonic alone. E, Check film confirming complete post removal.
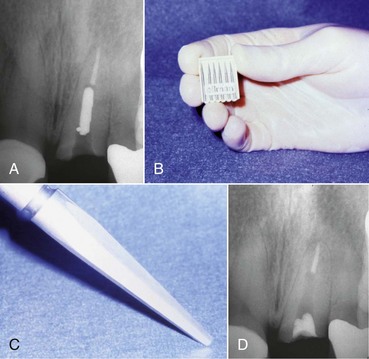
FIG. 25-20 A, Radiograph of fractured post. B, Roto-Pro Kit (Ellman International, Oceanside, NY). C, Roto-Pro Bur. D, Post removed by vibration of the instrument alone.
If retention reduction does not remove the post, some form of vice is needed to pull the post from its preparation. Many post removal kits are available on the market today with varying degrees of effectiveness. One such device is the Gonon Post Removing System (Thomas extracteur de pivots, FFDM-Pneumat, Bourge, France) that is a very effective instrument for removing parallel or tapered, nonactive preformed posts.125,167 This kit utilizes a hollow trephine bur that is aligned with the long axis of the post and placed over its newly exposed end. The trephine then cuts in an apical direction, shaving off the post’s outer layer, not only to remove tooth structure adjacent to the post but also to reduce the circumference of the post to a specific size and shape. This procedure is necessary to allow a specific, matched-size extraction mandrel to create or tap a thread onto the exposed milled portion of the post. Once the extraction mandrel with its associated washer/bumpers (Fig. 25-21) is attached to the post, the extraction forceps or vice is applied to the tooth and post. Turning the screw on the handle of the vice applies a coronal force in a similar fashion as a corkscrew removes a cork from a bottle of wine. This method is effective because all the force is applied to the bond between the tooth and the post, ideally in the long axis of the root. The main problem with this technique is the size of the vice that can make access in the molar region and between crowded lower incisors difficult. Also, if the extraction force applied is not directed in the long axis of the root, root fracture may occur.32
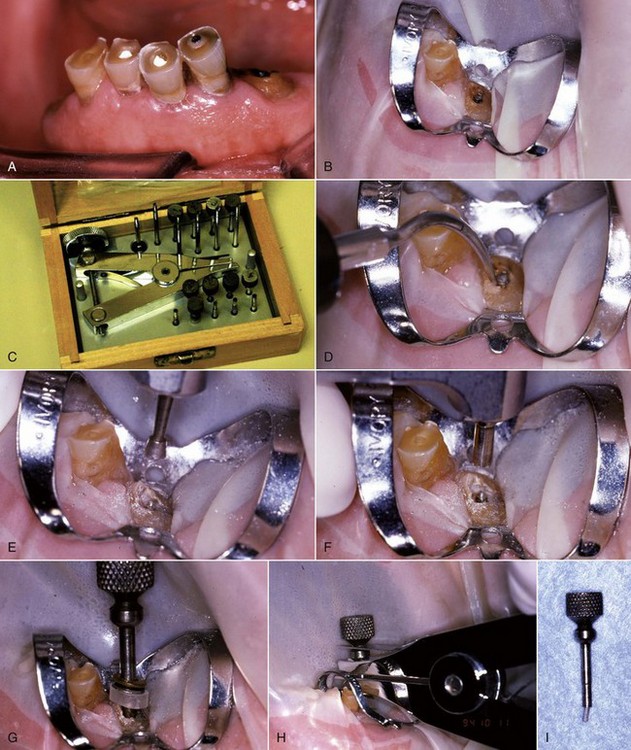
FIG. 25-21 Gonon Post Removal Technique. A, Fractured post in a lower incisor. B, Tooth isolated with a rubber dam. C, Gonon Kit (Dent Corp, White Plains, NY). D, Ultrasonic exposure of the post. E, Domer bur creating a shape the trephine bur can engage. F, Trephine bur milling the post. G, Extraction device tapping a thread onto the post. Note the three bumpers needed to protect the tooth from the vice. H, Vice applied. Turning the screw on the vice opens the jaws, creating the extraction force. I, Post removed.
The Thomas Screw Post Removal Kit (Thomas extracteur de pivots, FFDM-Pneumat) (Fig. 25-22) is an instrument designed specifically for the removal of active or screw posts. The trephine burs are identical to those used with the Gonon Post Removal System, but the extraction mandrels are threaded in the opposite direction. The mandrels are reverse threaded to enable them to tap onto the screw post in a counterclockwise direction so that continued torquing force while creating the thread will unscrew the post.
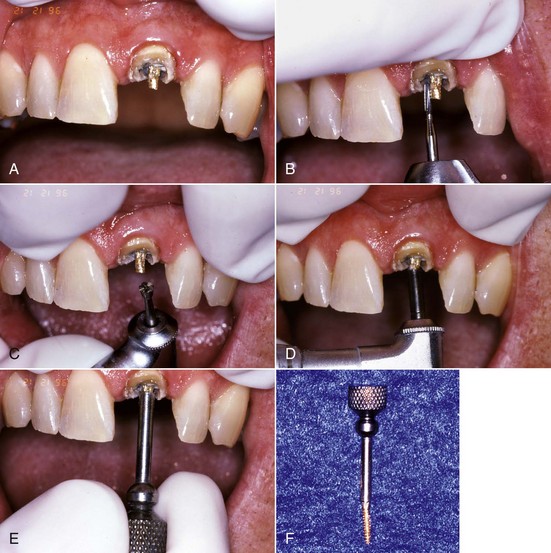
FIG. 25-22 Thomas screw post removal technique. A, Broken screw post. B, Head of post being contoured to a roughly cylindrical shape. C-D, Thomas Post Removal Kit. E, Domer bur creating a shape the trephine bur can engage. F, Trephine bur milling the post. G, Application of counterclockwise rotational force using the wrench. H, Post removed.
The Ruddle Post Removal System (Sybron SybronEndo, Orange, CA)163 (Fig. 25-23) and the Universal Post Remover (Thomas extracteur de pivots, FFDM-Pneumat) were designed to combine the properties of both the Gonon and Thomas Kits. Both of these very similar kits are not only useful in the removal of parallel or tapered passive types of posts but also in removing screw posts. They can even be adapted to remove large separated instruments in the coronal straight portion of a large canal. These kits also use a trephine bur to machine the post to a specific size that will dictate which mandrel to use. These mandrels tap in the counterclockwise direction so that the same taps can be used for both passive and active posts. Once the mandrel is tapped onto the post, the extraction jaws, or vice, can be applied and activated, enabling removal of passive posts; or the tap is continuously rotated counterclockwise to unthread screw-type posts.
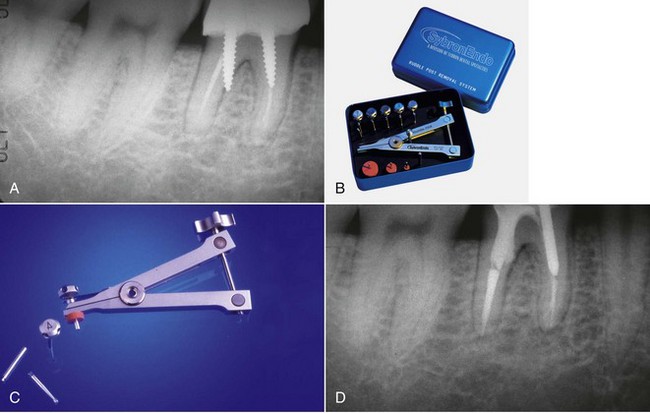
FIG. 25-23 A, Perforated post requiring removal. B-C, Ruddle post removal kit. D, Post removed and perforation repaired.
(B Courtesy SybronEndo, Orange, CA.)
Another device that works in a similar fashion as the Gonon and the Ruddle Post Removal System is the JS Post Extractor (Roydent Dental Products). The biggest advantage of this kit is the size. This is the smallest of the kits that work using a pulling action, which may help with cases where access is difficult. However, this kit does have one disadvantage: a smaller variety of trephine burs and extraction mandrels than some of the others. Therefore, the size of the post may be a limiting factor.
Another post removal device is the Post Puller, also known as the Eggler Post Remover (Automaton-Vertriebs-Gesellschaft, Germany)200 (Fig. 25-24). This device works in a similar manner as some of the others, except there are no trephine burs or extraction mandrels. The design of this instrument enables it to be used more efficiently with the crown removed. In addition, the design also allows this instrument to be used for cases in which the post and core are cast as one unit. This device consists of two sets of jaws that work independently of one another. With this device, both the post and the tooth are reduced to allow attachment of the post puller. Since there are no trephine burs, this reduction is done with a high-speed handpiece and bur. Next, the first set of jaws are attached to the post while the second set of jaws push away from the tooth in line with the long axis of the tooth, removing the post from the canal.200 Care must be taken to align the pulling forces of this instrument with the long axis of the root to prevent fracture32; this technique is not recommended for the removal of screw posts. In a survey of the Australian and New Zealand Academy of Endodontists, this was the most commonly used technique for post removal.31 However, in a survey of the American Association of Endodontists, it was one of the least used techniques.201 Clearly, techniques that are common in one country are not always that common in another.
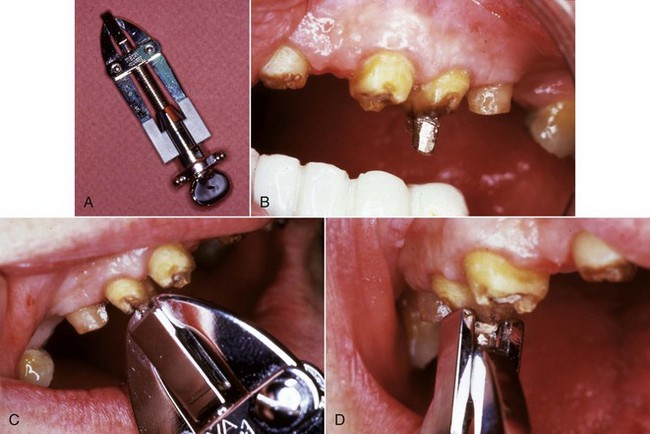
FIG. 25-24 A, Eggler Post Remover (Automaton-Vertriebs-Gesellschaft, Germany). B, Post has been contoured with a high-speed bur. C, Eggler Post Remover grasping the post. D, Elevating the post.
(From Stamos DE, Gutmann JL: Revisiting the post puller. J Endod 17:466, 1991.)
The recent increased popularity of cosmetic dentistry has created an impetus towards the use of tooth-colored posts that are fabricated from ceramic, zirconium, or various types of fiber-reinforced composite. Unfortunately, as with all posts, cosmetic posts also will need to be removed periodically. Neither the use of the Gonon Kit nor ultrasonic instruments allows for removal of fiber posts. The use of a high-speed bur to channel down through the post may result in a high rate of root perforation.154,177 The use of the Largo Bur (DENTSPLY Limited, Surrey, UK)64 and the Peeso drill154 to remove these posts has been advocated, and most of the post manufacturers have removal burs in the kit.40 These manufacturers’ removal kits have been shown to be more efficient at removing their own fiber posts than the use of diamond burrs and ultrasonics.120 In addition, a new bur, the GyroTip (MTI Precision Products, Lakewood, NJ), has been designed for the specific purpose of removing fiber-reinforced composite posts (Fig. 25-25). These drills consist of a heat-generating tip designed to soften the matrix that binds the fibers within the fiber-reinforced post. The fibers within the post are parallel, which assists the axial travel of the drill through the center of the post. The fluted zone of the drill allows the fibers to be safely removed, creating access to the root canal filling. Above the fluted zone, a layer of plasma-bonded silica carbide reduces the heat generation that would otherwise occur if a smooth carbide surface were rotating in contact with enamel and/or dentin. This abrasive zone also provides for a straight-line access preparation and facilitates the placement of a new post. Ceramic and zirconium posts are usually impossible to retrieve. They are more fragile than metal posts, and though ceramic posts may be removed by grinding them away with a bur (a procedure with a high risk of root perforation), zirconium has a hardness approaching that of diamond and cannot be removed by this method.177
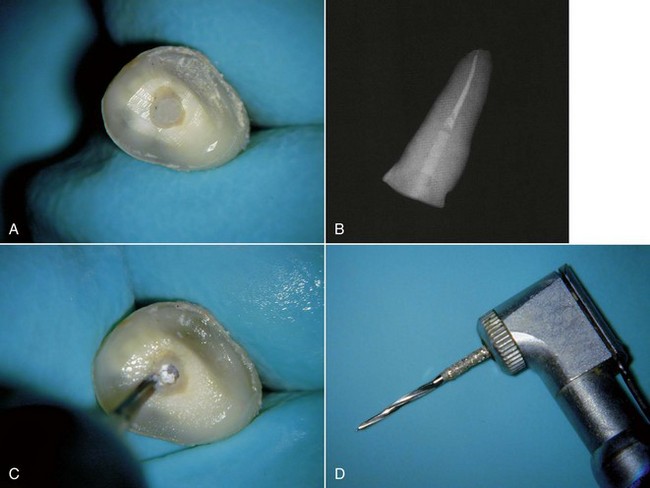
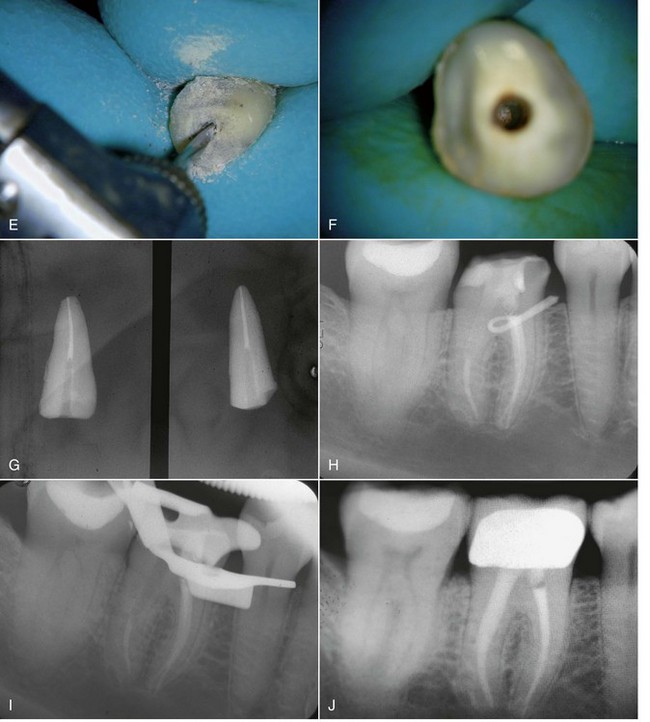
FIG. 25-25 GyroTip technique (MTI Precision Products, Lakewood, NJ). A, Broken fiber post in an extracted tooth. B, Radiograph of test tooth with post in place. C, Creating a pilot hole. D, GyroTip instrument. E, GyroTip cutting through the fiber post. Note alignment with long axis of post. F-G, Post removed. H, Clinical case showing fiber post perforation into furcation area. I, Post removed with the GyroTip. J, One-year follow-up of MTA repair.
Regardless of the post type or retrieval method used, once the post has been removed, the final step in exposing the underlying root filling material is to ensure that none of the post cement remains in the apical extent of the post space. This step can be easily accomplished by visualizing the cement using magnification and illumination and then using a straight ultrasonic tip to expose the underlying canal filling.
Potential Complications of Post Removal
As with many dental procedures, post removal has risks. These risks include fracture of the tooth, leaving the tooth unrestorable, root perforation, post breakage, and inability to remove the post.201 An additional concern is ultrasonically generated heat damage to the periodontium.177
Even though there may still be some who feel posts strengthen teeth, it is widely accepted that they do not.177 Actually, it has been shown that post preparation alone weakens teeth.221 It seems obvious that any additional work that may require removal of further tooth structure will weaken the tooth, increasing the likelihood of fracture. An in vitro study showed that cracks can form in radicular dentin during post removal using both the Gonon Kit and using ultrasonics, but there was no significant difference between these two groups and teeth with posts that were not removed.4 The authors speculated that the potential for vertical root fracture might be increased, but the clinical significance of this remains unknown. A more recent study, however, concluded that the incidence of root fracture during post removal was extremely low and that with good case selection, post removal is in fact a very predictable procedure.1 But if post removal would also leave the remaining tooth structure in a state that may not be predictably restored with a good prognosis, and if this situation can be predicted ahead of time, surgery may be the preferred treatment option.
Perforation is an additional possible complication that can happen during post removal, especially if the post is removed by simply attempting to drill it out with high-speed burs.154 If perforation occurs, the clinician should repair it immediately; the prognosis will worsen as the time between perforation and repair lengthens.21,102,182 Once a perforation occurs, the clinician must reconsider the prognosis and determine whether or not the tooth should be salvaged. Terminating the procedure and pursuing a different treatment option could be considered at this point. Extraction and replacement with an implant and/or a fixed prosthesis was a treatment option prior to initiating the retreatment, and some may consider this treatment the best option once a perforation has occurred. However, with the recent development of mineral trioxide aggregate (Pro-Root MTA, DENTSPLY Tulsa Dental), perforations can be repaired with a favorable prognosis.160 The techniques and materials for perforation repair will be discussed in detail in a later section of this chapter.
Another complication is separation of the post, causing removal of the coronal segment and leaving a small portion of the post with even less accessibility. This separation will decrease the likelihood of removal and occurs more frequently when attempting to retrieve titanium posts.177
The use of ultrasonic energy for prolonged periods of time can generate excessive amounts of heat. The heat generated can cause damage to the surrounding periodontium.67,177 This damage may be as serious as both tooth and permanent bone loss (Fig. 25-26). For this reason, stopping periodically to cool off the area with a water spray is necessary. This will be discussed in detail in a later section.
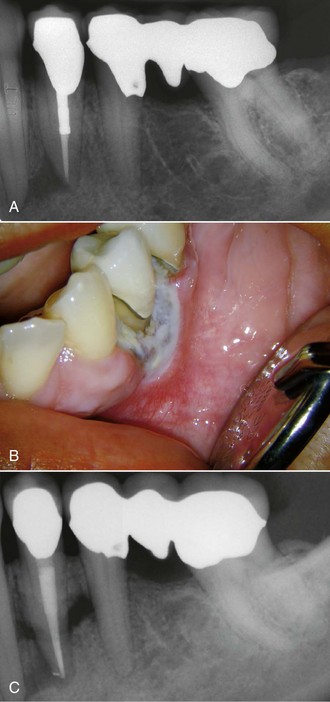
FIG. 25-26 Tissue damage from heat generated by ultrasonic application to a post during removal. The ultrasonic tip was applied to the post for no more than 5 minutes at high power, with the assistant applying a constant water spray. A, Preoperative radiograph. B-C, These images were taken 1 month after retreatment. Note sloughing bone visible on C. The tooth was lost 1 month later.
(From Schwartz RS, Robbins JW: Post placement and restoration of endodontically treated teeth: a literature review. J Endod 30:289, 2004.)
If the clinician is unable to remove the post, he or she will be faced with a decision of what to do. This decision is based on whether the post is being removed for restorative purposes or due to the persistence of disease. If the reason is for restorative purposes and the clinician can adequately restore the tooth with the existing post or post segment, then he or she should do so. If the tooth cannot be properly restored without removal of the post and placement of a new post, extraction and replacement with an implant and/or fixed prosthesis will be needed. If the reason for post removal is the persistence of disease, the tooth should be treated surgically and restored as well as possible.
Regaining Access to the Apical Area
Once the coronal-radicular access is made and all posts and obstructing restorations have been removed, the clinician must regain access to the apical area by removing the previous root-filling materials (Fig. 25-27). This part of nonsurgical retreatment is complicated by the large variety of types of root fillings used. Today, the majority of root fillings are performed using gutta-percha in various forms, but many other materials have been and are still being used. Silver points were very popular until the 1970s, and various types of pastes are unfortunately still in use. The authors have seen cases of definitive root filling with phenol-soaked paper points and sometimes no root filling at all. New materials such as Resilon (Resilon Research LLC, Madison, CT), a soft polyester material that is bonded into the canal space, are coming on the market all the time. Though all root-filling materials have their advocates and their critics, the only certainty is that all will have some incidence of persistent disease and will need retreatment.
During the diagnostic phase, it is very important to ascertain the nature of the root filling to minimize surprises when attempting retreatment. Sometimes this is readily apparent, but in other instances, this determination may require contacting the previous clinician to discover what type of root filling was used. Occasionally this information cannot be determined until canal entry, so extreme caution should be used when performing access so as not to possibly remove parts of the root filling that may be useful in its removal, such as the core material in solid core obturators.
Gutta-percha Removal
One of the great advantages of using gutta-percha for root filling is its relative ease of removal. When the canal contains gutta-percha and sealer or a chloropercha filling, it is relatively easy to remove this material using a combination of heat, solvents, and mechanical instrumentation.58,163 Upon access, it is usually relatively easy to find the treated canal orifices with the visible pink gutta-percha material inside. Initial probing with an endodontic explorer into the material can help rule out the possibility that there is a solid core carrier. If there is a plastic carrier, then heat should not be used to remove the coronal gutta-percha (more on this later). If there is no carrier, heat is applied using an endodontic heat carrier that has been heated to a cherry red glow in a torch. Unfortunately, the carrier begins to cool upon removal from the flame, so many endodontists are now using other heat sources—such as the Touch ’n Heat (SybronEndo) or DownPak (Hu-Friedy, Chicago, IL) (Fig. 25-28, A)—to provide constant, consistent heat application to soften the gutta-percha in the coronal portion of the canal.75 Care must be exercised to not overheat the root, which can cause damage to the periodontal ligament.115,169,170 Heat should be applied in a short burst to allow the instrument to penetrate the gutta-percha mass, followed by cooling, which will cause the material to adhere to the heat carrier, facilitating its removal (Figure 25-28, B). After removing as much gutta-percha as possible with the heated instrument, then remove any remaining coronal material with small Gates-Glidden drills, taking care not to overenlarge the cervical portion of the canal. However, since the previously treated tooth may have had an underprepared cervical third of the canals, these drills can also be used to flare the coronal aspect in an anticurvature direction to facilitate enhanced straight-line access to the apical third of the canal and create a reservoir for potential solvent use.129 Again probe the canal, this time using a #10 or #15 K-file. It is sometimes possible to remove or bypass the existing cones of gutta-percha if the canal has been poorly obturated, thus eliminating the need for solvents.199 If that is not possible, a gutta-percha solvent must be used to remove the remaining material in the apical portion of the canal.
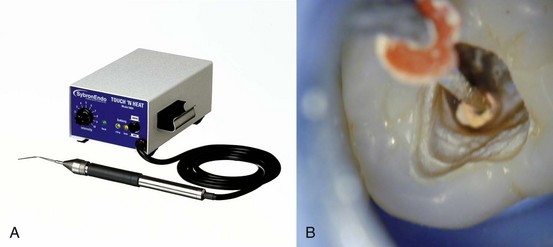
FIG. 25-28 A, Touch ’n Heat instrument. B, Gutta-percha adhering to the Touch ’n Heat tip as it cools.
(A Courtesy SybronEndo, Orange, CA.)
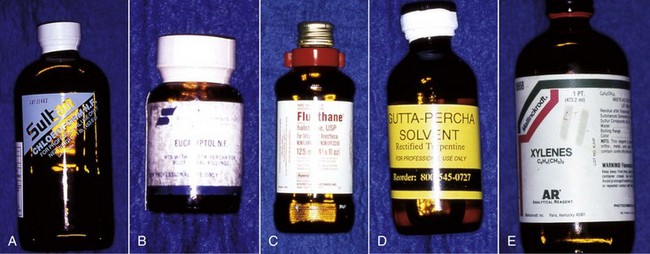
Several solvents have been recommended to dissolve and remove gutta-percha for retreatment (Fig. 25-29), including chloroform,132 methylchloroform,228 eucalyptol,242 halothane,90,110 rectified turpentine,104 and xylene.78 All of the solvents have some level of toxicity,12,35 so their use should be avoided if possible; however, a solvent is usually needed to remove well-condensed gutta-percha. The most popular solvent is chloroform, since it dissolves the gutta-percha rapidly and has a long history of clinical use. In 1976, the U.S. Food and Drug Administration (FDA) banned the use of chloroform in drugs and cosmetics after a report of suspected carcinogenicity.222 There was no associated ban on its use in dentistry,132 but the report did result in the search for alternatives, some of which are listed. When used carefully, chloroform is regarded as a safe and effective endodontic solvent.35,132 All of the others generally have been reported to be less effective or have some other drawback that limits their use. Xylene and eucalyptol dissolve gutta-percha slowly and only approach the effectiveness of chloroform when heated.238 Rectified turpentine has a higher level of toxicity than chloroform12 and produces a very pungent odor in the operatory. Halothane has been shown to be as effective a solvent as chloroform in several studies,90,110 but a more recent study indicated that the time for removal of the root filling was longer than using chloroform.231 The increased cost and volatility of halothane and the potential for idiosyncratic hepatic necrosis make it less desirable to use as a gutta-percha solvent.35 Although methylchloroform is less toxic than chloroform, it is also less effective as a solvent for gutta-percha.228 Both halothane and chloroform have been shown to affect the chemical composition of dentin43,105 and may affect bonding strengths of adhesive cements to the altered dentin.44 The clinical significance of these effects remains unknown. The evidence for the carcinogenicity of chloroform in humans is suspect,132 but with careful use, its toxicity may be eliminated as a risk factor to both the patient35 and the personnel in the operatory.3 As such, its continued use as a gutta-percha solvent is recommended.
Using an irrigating syringe, the selected solvent is introduced into the coronal portions of the canals, which will then act as a reservoir for the solvent. Then, small hand files (sizes #15 and #20) are used to penetrate the remaining root filling and increase the surface area of the gutta-percha to enhance its dissolution. This procedure can be facilitated by using precurved rigid files such as the C+ File (DENTSPLY Maillefer, Johnson City, TN) (Fig. 25-30), which can penetrate the gutta-percha mass more efficiently than the more flexible types of K-files. The newly introduced C+ file is a stainless steel, end-cutting hand file that is twisted from a square blank. The secret to its stiffness is that the taper varies along the shaft, giving it the rigidity and strength to cut through well-condensed gutta-percha efficiently. The gutta-percha must be removed carefully, however, to avoid overextending the resultant mixture of gutta-percha and solvent beyond the confines of the canal to minimize the risk of severe postoperative pain.75 Unfortunately, electronic apex locators, which in non-retreatment situations are very accurate,106 seem to frequently misread the working length when gutta-percha is initially being removed. This clinical observation may be a result of the file being covered with chloropercha that may affect its conductivity. It has been shown that apex locators may be less accurate in retreatment situations,227 but in this study, the error was that readings indicated a working length that was too short. In a more recent study, an apex locator built into a rotary handpiece indicated working lengths that were too long in simulated retreatment situations.223 To avoid overextending root filling materials into the periodontium, it is recommended that a radiograph be made to gain a preliminary measurement when the estimated length is approached. Well into the retreatment, after the root fillings have been thoroughly removed, the apex locator will regain its accuracy if a clean file is used. Once the working length is reached, progressively larger-diameter hand files are rotated in a passive, nonbinding, clockwise reaming fashion to remove the bulk of the remaining gutta-percha until the files come out of the canal clean (i.e., with no pink material on them). Frequent replenishment of the solvent should be used, and when the last loose fitting instrument is removed clean, the canal is flooded with the solvent, which then acts as an irrigant. The solvent is then removed with paper points. The wicking action of the absorbent points163 will remove much of the remaining film of gutta-percha and sealer that remains adherent to the canal walls and in the irregularities of the canal system.234 Cleanliness of canals after gutta-percha removal is not improved by using a microscope10; however, using kinked small files, the clinician should probe the canal wall looking for irregularities that may harbor the last remnants of gutta-percha. These irregularities can usually be felt rather than seen and should be cleaned using this method.75

FIG. 25-30 C+ files. These rigid instruments remove gutta-percha more efficiently than more flexible types of K-files.
It should be noted that there exists a glass ionomer–based endodontic sealer (Ketac-Endo, 3M, Pymble, Australia) that is used in conjunction with gutta-percha.156 This sealer is virtually insoluble in both chloroform and halothane230 and must be retreated by removing the gutta-percha and then using ultrasonics to débride the canal walls. Canal cleanliness can approach that of other gutta-percha retreatment cases, but it is difficult and time consuming to achieve this result.56,136
Overextended gutta-percha removal can be attempted by inserting a new Hedstrom file into the extruded apical fragment of root filling, using a gentle clockwise rotation to a depth of 0.5 to 1 mm beyond the apical constriction. The file is then slowly and firmly withdrawn with no rotation, removing the overextended material133 (Fig. 25-31). This technique works frequently, but care must be taken not to force the instrument, or further extrusion of the gutta-percha or separation of the file may result. The overextended apical fragment should not be softened with solvent, because this application can decrease the likelihood of the Hedstrom file getting a solid purchase, hindering retrieval.199
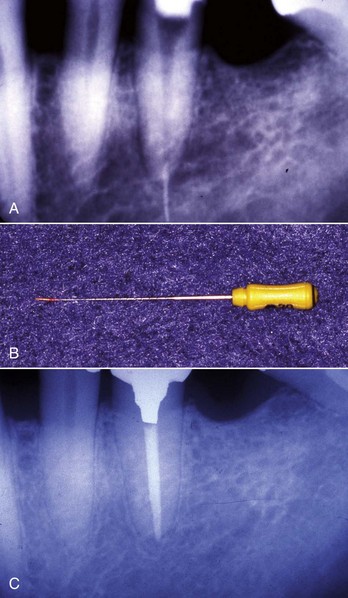
FIG. 25-31 Removal of overextended gutta-percha. A, Preoperative radiograph showing overextended filling material. B, A small Hedstrom file pierces the overextended material and retrieves it. C, Eighteen-month reevaluation. The tooth is asymptomatic.
Using rotary systems to remove gutta-percha in the canals has been advocated for their enhanced efficiency and effectiveness in removing gutta-percha from treated root canals.163 This has generally been borne out in the literature. There are several types of mechanical rotary systems available for gutta-percha removal, including rotary file systems such as the ProFile (DENTSPLY Limited) (Fig. 25-32), a mechanical push-pull, quarter-turn file system, the Canal Finder (Endo Technique Co, Tustin, CA), and dedicated gutta-percha removal instruments, such as the GPX (Brasseler, Savannah, GA), the ProTaper Universal retreatment files (DENTSPLY Limited) (Fig. 25-33), and the Mtwo R (Sweden and Martina, Padova, Italy). These engine-driven instruments mechanically chop up the gutta-percha and sealer while thermoplasticizing the root filling mass via frictional heat to aid in removal. A survey of Australian dentists showed that 54% of the respondents who perform endodontic retreatment used rotary instrumentation to remove gutta-percha either always (15%) or sometimes (39%), with an increased likelihood of rotary gutta-percha removal if the clinician had more experience with the use of these instruments.151 In vitro studies have generally shown these systems to be efficient in that they generally need less time to remove the bulk of the gutta-percha filling material than hand removal,* although in two studies, they were slower to remove the root filling than hand filing.13,93 Assessments of canal cleanliness and extruded apical debris generally indicated that there were no overall differences between hand and mechanical gutta-percha removal.† In one study using Quantec SC instruments (Tycom, Irvine, CA), however, it was found that hand files with solvent cleaned canals more effectively.19 This finding has been repeated recently using the ProTaper retreatment files,77 but in another study in the same journal issue, ProTaper retreatment files were found to leave canals cleaner than hand files.66 Clearly, this is an area where further research is warranted. It is recommended that after rotary gutta-percha removal, subsequent hand instrumentation is needed to remove the residual obturating materials completely from the canal. In several studies of mechanical gutta-percha removal, fracture of the mechanical instruments or the tooth root occurred.‡ This result was reported to occur less frequently when the instrument rotary speed was increased from 350 to 1500 rpm.24 One study showed no separation or other canal defects when using dedicated retreatment files, the ProTaper Universal and Mtwo R instruments.194 The dedicated retreatment files have end-cutting tips to enhance penetration and removal of the root filling mass, increasing their efficiency210 (see Fig. 25-33, B). This in combination with flute design and techniques advocated may be the reason for the potential reduced risk of separation. While the mechanical gutta-percha removal systems may provide enhanced efficiency, the increased risk of instrument separation, further complicating retreatment, may outweigh this benefit. Dedicated retreatment files may reduce this risk.
FIG. 25-32 Nickel-titanium rotary Profile thermoplasticizing and removing gutta-percha. Optimum rotary speed is 1500 rpm.
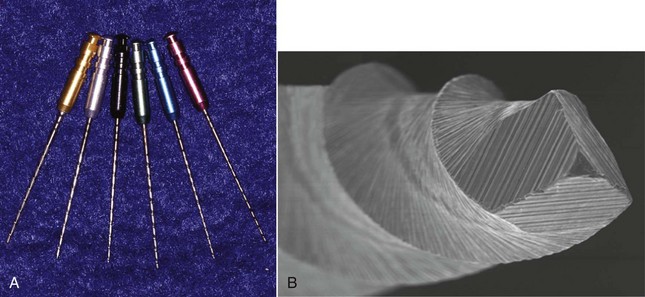
FIG. 25-33 Rotary gutta-percha removal instruments. A, Brasseler GPX Instruments. B, ProTaper Universal retreatment file has a cutting tip for enhanced penetration of the root filling materials.
(B Courtesy DENTSPLY Tulsa Dental, Tulsa, OK.)
†References 13, 52, 88, 89, 94, 145, 166, 193, 213, and 244.
‡References 11, 19, 24, 89, 93, and 213.
Use of the Nd:YAG laser to remove gutta-percha from root-filled teeth has been investigated in vitro.225 The time taken to remove the gutta-percha was within the range of other studies of mechanical gutta-percha removal, and the addition of solvents did not improve the performance of the laser. As in most other studies, gutta-percha in varying amounts was left in the canals after laser removal. Root surface temperatures did increase, so without further investigation proving safety and efficacy, laser gutta-percha removal cannot be recommended at this time.
Resilon (Resilon Research LLC) (Fig. 25-34) is a thermoplastic polyester polymer that is bonded into the canal space using an unfilled resin bonding system (Epiphany; Pentron). It is also marketed as RealSeal (Sybron Endodontics, Orange, CA). It has been advocated as a root canal obturating material to replace traditional gutta-percha and sealer because of its apparent enhanced sealing ability185 and potential to strengthen root resistance to fracture as a result of internal bonding.212 Resin-bonded obturation systems have been advocated in the past,118 but the inability to retreat canals filled with this obturating material has prevented its widespread use. The Resilon polymer itself is reported by the manufacturer to be soluble in chloroform and may be removed by heat application, a behavior that is similar to gutta-percha. Recent studies have shown that the polycaprolactone polymer of the Resilon is removed easily and leaves canal walls cleaner than removal of gutta-percha and AH+ sealer,38,39,47,175 although this finding has been disputed. Hassanloo et al. found that there was less residue on the walls when removing gutta percha/AH+ if the materials were allowed to set for a longer period.82 This finding has been corroborated175,211 and indicates that there may be a temporal effect that introduces a methodological bias in these studies. There may also be a problem with removal of the unfilled Epiphany resin sealer, especially since the sealer tags have been shown to penetrate deep into dentinal tubules185 and presumably also into anatomic ramifications of the canal that need cleaning during retreatment. More research into this interesting material and technique is warranted, especially to determine the best technique for retreatment. After the Resilon core has been removed using heat and chloroform, the authors would recommend the use of a resin solvent such as Endosolv-R (Septodont, Kent, UK) (Fig. 25-35) to attempt elimination of the unfilled resin sealer prior to instrumentation.
Managing Solid Core Obturators
Solid core canal obturation systems, such as Thermafil, Dens-Fil, and the GT Obturator (DENTSPLY Tulsa Dental), have become very popular since their introduction several years ago. After cleaning and shaping procedures are completed, the clinician heats a solid core obturator (alpha-phase gutta-percha surrounding a core that is attached to a handle) in an oven and places the carrier in the canal. The solid core carries the gutta-percha down into the canal and condenses it while the material is cooling. This system provides a rapid and simple technique for warm gutta-percha endodontic obturation. As with any obturating material, retreatment will be necessary occasionally.
Retreatment of solid core materials is considered to be more complex and difficult than removal of gutta-percha alone, owing to the presence of the solid carrier within the mass of gutta-percha. The nature of the carrier will determine the method used and complexity of the retrieval. There are two types of carriers found in these systems: metal (stainless steel or titanium) and plastic. The plastic carriers are smooth sided, as are some brands of metal carriers; however, most metal carriers are fluted and resemble endodontic hand files with a layer of gutta-percha on the outside. The fluted metal carriers present an exceptional challenge to the retreating clinician, since many times they are improperly inserted and either wedged or screwed into the canal to make up for inadequate canal shaping or the lack of skilled use of the size-verifying techniques available. This makes them especially difficult to remove. Once the carrier has been placed, it is cut off in the pulp chamber using a bur, and the tooth is restored. The level at which the metal carrier is severed is important in its retrieval. If it is cut down to the level of the canal orifice, retrieval is difficult,75 so the prudent clinician plans for retrievability by severing the handle from the carrier, leaving 2 to 3 mm of carrier exposed in the access above the pulp chamber floor to allow easier removal if retreatment is ever needed. Unfortunately, this is not always the case. The technique of placing a nick in the midcanal level of the carrier to allow the clinician to rotate the carrier handle and sever the obturator deep in the canal is sometimes used to allow creation of a post space, but the rotational force used to create the “twist-off” apical plug can engage the flutes of a metal carrier, increasing the complexity of removal if retreatment is needed.243
It is advantageous to determine prior to initiating treatment whether there is a solid core obturation in the root-filled tooth. The preoperative radiograph may show this, since stainless steel carriers will exhibit a fluting effect on the radiograph (Fig. 25-36); however, titanium carriers rarely are distinguishable from gutta-percha, and plastic ones never are. In most instances, the clinician finds that they are dealing with a carrier-based obturator after initial access to the pulp chamber. This is why, as was stated in an earlier section, careful access and probing of the root-filling material is necessary when entering a canal. If there is a carrier, it will be detected as either a metallic structure embedded in the gutta-percha mass or a black spot, indicating a plastic carrier. Occasionally the carrier may be found embedded in the coronal core material, so careful excavation with small burs and straight, tapered ultrasonic tips may be necessary to preserve the carrier intact to help with removal.75
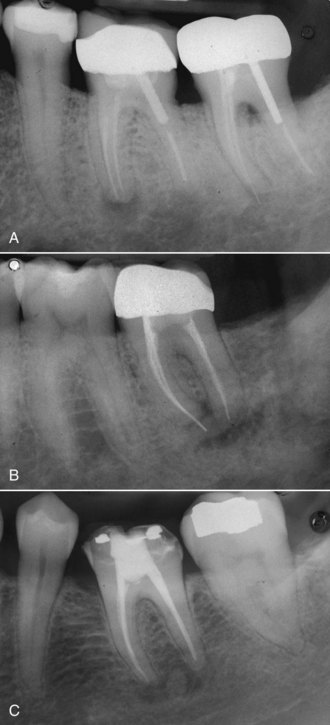
FIG. 25-36 Comparison of radiographic appearances of three different obturating materials. A, Gutta-percha. B, Stainless steel Thermafil carrier (note subtle fluting effect in the fill). C, Plastic Thermafil carrier.
Removal of a metal carrier is accomplished with initial use of heat application to the carrier that can soften the gutta-percha surrounding it, facilitating its removal with Peet silver point forceps (Silvermans, New York, NY) or modified Steiglitz forceps (Pulpdent Corporation, Watertown, MA)75,173,232,233 (Fig. 25-37). Often there is not enough of the carrier remaining in the access to grasp with forceps, and removal will require solvent application and removal of the surrounding coronal gutta-percha using small hand instruments, usually followed by ultrasonic excavation around the carrier and removing it like a separated instrument75,163 (Fig. 25-38), as described in a later section of this chapter. Care should be exercised to avoid excessive heat generation during this procedure. This is also the case if the metal carrier has been sectioned for post space preparation. The metal carrier has been shown to be much more difficult to remove than plastic ones,53,243 frequently resulting in nonretrieval. Fortunately, their use in endodontic therapy has been declining.
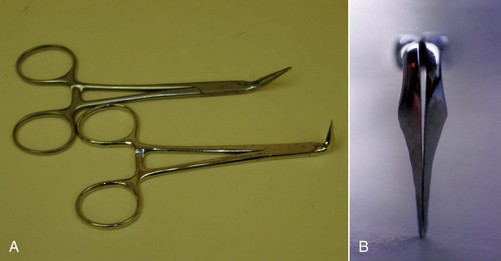
FIG. 25-37 A, Steiglitz forceps (Pulpdent Corporation, Watertown, MA) in 45- and 90-degree head angles. B, Tips of the Steiglitz forceps ground to a thinner contour to create the “modified” instrument. This allows deeper penetration into the tooth to enhance removal of obstructions.
(B Courtesy Dr. Daniel Erickson.)
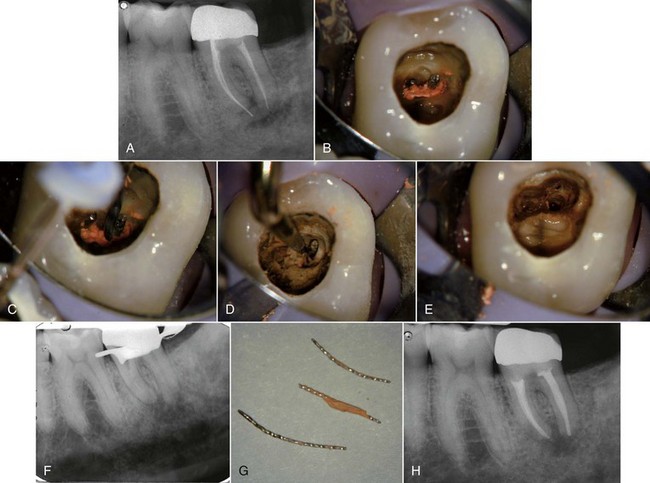
FIG. 25-38 Metal carrier retreatment. A, Preoperative radiograph. B, Metal carriers exposed by careful excavation of gutta-percha. C, Use of the Touch ’n Heat instrument to heat the carriers and soften the gutta-percha. This allowed removal of one of the carriers using modified Steiglitz forceps. The other could not be removed using heat or solvents. D, Ultrasonic troughing around the carrier to facilitate grasping it with forceps. E-F, Carriers removed and confirmed with a radiograph. G, Metal carriers showing gutta-percha still adhering to them. H, Final obturation of the tooth.
Removal of plastic carriers is similar to removal of gutta-percha root fillings, except that generally, heat should be avoided to minimize the likelihood of damaging the carrier.75 The older Thermafil plastic carriers were made of two different materials depending on their size. In the smaller sizes (up to size #40) the material used was Vectra, which is insoluble in available solvents; the larger sizes used polysulfone, which is soluble in chloroform.152 Solvents, on the other hand, seem not to affect the newer GT plastic carriers, so their use can be recommended18 (Fig. 25-39). The access is flooded with a solvent such as chloroform, and the gutta-percha surrounding the carrier is removed with hand files in a larger to smaller sequence (e.g., #25, #20, #15, etc.), each file progressively penetrating deeper around the carrier. The solvent should be replenished frequently, and when a #08 file can penetrate to the apical extent of the carrier and there is little remaining gutta-percha, a larger Hedstrom file is inserted into the canal alongside the plastic carrier and gently turned clockwise to engage the flutes. When the file is withdrawn, it invariably brings the carrier with it, and the rest of the gutta-percha and sealer removal proceeds as described earlier. Care must be taken to avoid overstressing the Hedstrom file. It should not be “screwed” into the canal, or separation of the file or the carrier may result.18 Occasionally, grasping pliers will be needed to remove the carrier if it is accessible95,163 after the gutta-percha has been removed. Another potential problem with retrieval is present if the carrier has been overextended beyond the apical foramen during the previous root canal treatment. This overextension may make it prone to separation and unable to be retrieved, potentially resulting in the need for apical surgery.92
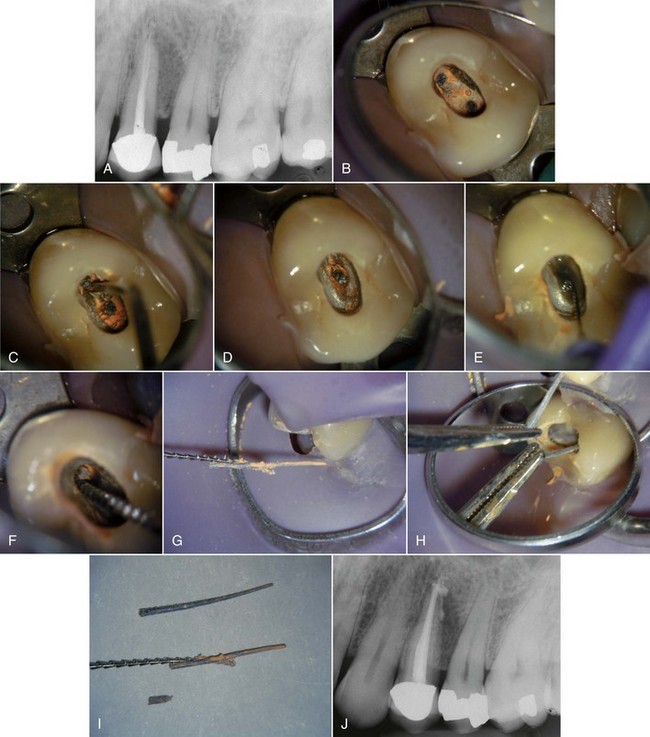
FIG. 25-39 Plastic carrier retreatment. A, Preoperative radiograph. At this stage, the nature of the root filling is unknown. B, Plastic carriers visible in the access as two black spots in the gutta-percha mass. C, Gutta-percha in the chamber is carefully removed from the carriers. D, Carrier is exposed. E, Chloroform solvent is placed into the chamber, and a small file is worked alongside the carriers to remove the gutta-percha. F-G, A Hedstrom file is gently screwed into the canal alongside the carrier and withdraws it on removal. H, A hemostat removes the other carrier. I, Plastic carriers removed. J, Final obturation with Resilon and Epiphany sealer.
A technique for plastic carrier removal has been described using a System B HeatSource (SybronEndo) to soften the gutta-percha surrounding the carrier without melting the carrier itself.236 The temperature is set at 225° C, and the heat plugger is placed buccal and lingual to the carrier, after which #50 to #55 Flex-R hand files are placed around the carrier and braided to engage the carrier and remove it. This technique has been shown to require significantly less time to remove the carrier compared to using solvent.236 Concerns regarding heat generation in the periradicular tissues have been raised,121 with the authors concluding that caution should be exercised when using this technique. When other techniques have been unsuccessful, and the plastic carrier has been sectioned apical to the orifice, resulting in limited access, the clinician may attempt to retrieve the carrier by placing a heated System B tip directly into it. As apical pressure is maintained, the heat is turned off. This allows the plastic carrier to adhere to the tip while cooling and may result in its removal upon withdrawal of the heat tip (Fig. 25-40).
Rotary instruments have been advocated for use in removal of plastic carriers and gutta-percha, and a recent study showed that removal of plastic carriers was successful in all but one of the teeth obturated with them.11 Unfortunately, root fracture occurred in the lone specimen that did not have a successful retrieval, and two instances of rotary instrument fracture also occurred in the study. As with gutta-percha removal, the clinician must carefully weigh the risks of rotary carrier removal against the perceived benefits.162
Once the carrier has been removed, the remaining gutta-percha and sealer must be removed from the canal, and like removal of root-filling materials described previously, there is no technique that completely removes all materials from the canal system. Canal cleaning may be even more difficult when removing carrier-based obturations, since the more highly processed gutta-percha used with these carriers may be more difficult to remove than other forms of the material. One study described a sticky film of gutta-percha and sealer adhering to the canal walls upon removal of metal carrier obturators and found more of it than if the canals had been obturated with lateral condensation.243 Their findings have not been corroborated, however, and other studies have shown no difference in residual debris remaining after carrier-based removal.53,95,243 It is important to remove as much of the residual gutta-percha and sealer from the pulpal anatomic spaces as possible, so flooding the canal with solvent and “wicking” it out with paper points is also recommended for carrier-based retreatment.163
Paste Retreatment
Various pastes have been used as root canal obturating materials, especially outside North America. There are such a wide variety of paste compounds used in endodontics that it is impossible to categorize them all. The individual clinician who is using it formulates most pastes, so the ultimate composition of a paste found in a tooth with persistent disease is generally indiscernible. Many of the pastes used, such as N2 or RC2B, contain formaldehyde and heavy metal oxides, making them toxic and a potential danger to the patient’s health, both local and systemic if overextended beyond the confines of the root canal system.22,150 None has the potential to seal the canal effectively, and many render a tooth impossible to retreat,176 so their use is strongly discouraged. On radiographic examination, they can usually be discerned by their lack of radiopacity, the presence of voids, and evidence of inadequate canal shaping and poor length control (Fig. 25-41). When a paste fill is suspected or found in a tooth, a telephone call to the previous treating dentist should be made if possible to find out the exact formulation of the paste, since this information may help in its removal.
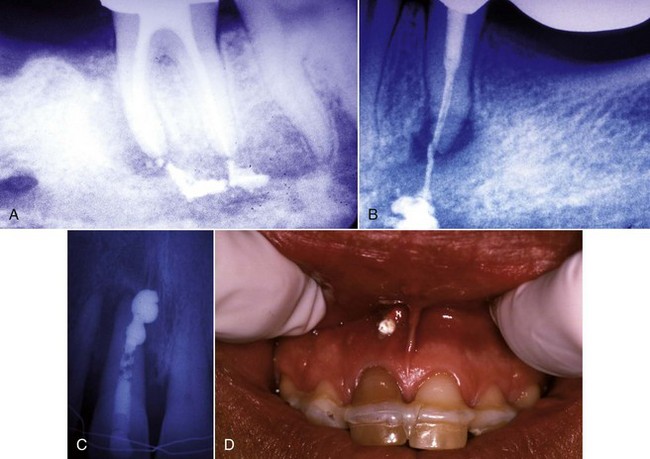
FIG. 25-41 Example of poor length control with paste root fillings. A, Overextended paste filling into the inferior alveolar canal. B, Overextended paste filling into the mental foramen. C, Overextended paste filling extending through a perforation in an upper central incisor. D, Clinical appearance of the case in part C. Note the material extending out through a sinus tract.
For purposes of retreatment, paste fills can be categorized as soft or hard, and all should be considered potentially toxic. Great care should be exercised when removing the paste to avoid overextension, potentially severe postoperative pain,73 and possible paresthesia/dysesthesia from the paste’s potential neurotoxicity.25,183 Soft pastes are generally easy to remove using crown-down instrumentation with copious sodium hypochlorite irrigation to minimize extrusion.75 Greater difficulty arises when the paste is set hard.176 Since the nature of the paste remains unknown, removing it becomes an empirical process. Following access preparation and coronal orifice exposure, the paste is probed with an endodontic explorer and files. If hard and impenetrable, then the coronal paste can be removed with burs58 or a straight, tapered ultrasonic tip in the easily accessible straight portions of the canal, using magnification and illumination.163 Once the canal curvature is reached, further use of this method will result in damage to the canal walls and possible perforation. Precurved small hand files are inserted to probe the apical area. Many times the density of the paste filling material decreases in the apical extent of the fill, so penetration to the apex may be possible.163 If not, a solvent must be used to attempt to soften the remaining paste. The choice of solvent is usually made by trial and error, starting with chloroform. If that does not soften the material in a reasonable amount of time and does not allow penetration with small files, then the chloroform is wicked out of the canal and another solvent is chosen. There are two frequently used solvents for paste fills: Endosolv-E and Endosolv-R (Septodont, Kent, UK) (see Fig. 25-35). The Endosolv-E is selected if the paste contains zinc oxide and eugenol, and the Endosolv-R is chosen for resin-based pastes. The obvious problem is that the nature of the paste is usually unknown at the time of removal, so contacting the previous clinician (if possible) can help with this choice. Otherwise, the choice is just a guess. The chosen solvent should be placed in the access, and attempts should be made to penetrate the paste with hand or ultrasonic files. Care must be taken to avoid creating a ledge or other defect in the canal that may preclude successful retreatment. The progress is frequently slow,61 and the clinician may elect to leave some solvent in the canals between appointments to soften the paste.164 Care should be taken in the choice of temporary restoration, since the solvent left in the canal may also soften the temporary, potentially leading to breakdown of the interappointment seal.135
Ultrasonically activated files have been advocated for use in penetrating hard-set pastes in the curved apical segments of canals101,109 (Fig. 25-42). The ultrasonic energy breaks up the paste, and the irrigation floats the fragments in a coronal direction until the apical terminus is reached.58 This technique is reported to be very time consuming, and care must be exercised to avoid instrument separation, perforations, or alteration of canal morphology. On occasion, despite all best efforts, the paste cannot be removed from the tooth, so apical surgery or extraction should be considered in these cases.73
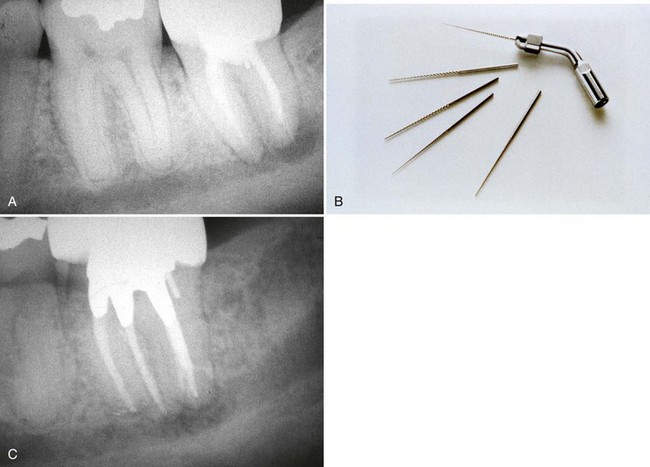
FIG. 25-42 A, Preoperative radiograph of a hard paste root filling exhibiting a short fill, inadequate seal, and periapical radiolucency. Note the proximity of the inferior alveolar canal. B, Ultrasonic files like this were used to break up the hard paste in the apical third of the canal, allowing removal. C, Seventeen-month postoperative follow-up. The patient is asymptomatic and has no paresthesia.
Biocalex 6.9 (currently known as Endocal 10) (Fig. 25-43) is a hard-setting calcium oxide paste that has been popular in Europe for over 30 years but is now being used in North America since its recent FDA approval.69 The paste seems to seal well, but there is an unacceptably high incidence of root fracture due to the large amount of expansion on setting.69 Retreatment will be complicated by the hard-setting nature of this material, but since it is a calcium oxide paste, ethylenediamine tetra-acetic acid (EDTA) may soften it, facilitating its removal. Because EDTA also softens dentin, care must be taken not to gouge or ledge the canal walls during retreatment, and root fractures must be suspected as a potential complicating factor in the outcome.
Silver Point Removal
Historically, the use of silver points for endodontic therapy has been extremely popular and quite successful because of their ease of handling and placement, ductility, radiopacity, and because silver appears to have some antibacterial activity.178 However, over the past few decades, the usage of silver points has dramatically diminished. Presently they are considered a deviation from the standard of care. The main reason for this change is because they corrode over time (Fig. 25-44), and the apical seal may be lost.23 Also, silver points do not produce an acceptable three-dimensional seal of the canal system; rather, they simply produce a plug in the apical constriction while not sealing the accessory canals that are frequently present123,174 (Fig. 25-45). The corrosion of silver points occurs when they come into contact with tissue fluids and certain chemicals used in endodontics, including sodium hypochlorite and some sealers.74 This corrosion produces chemicals such as silver sulfide, silver sulfate, silver carbonate, and silver amine hydrate,181 which have been shown to be cytotoxic in tissue culture.178 Corrosion occurs mainly at the apical and coronal portions of the points, indicating that leakage is responsible.181 Gutta-percha root-filling techniques do not suffer from these disadvantages and have replaced the use of silver points in endodontics. Owing to this decrease in usage over the past quarter century, the quantity of cases the clinician will come across that will require silver point removal has also decreased. Nevertheless, there are still occasions when their removal will be necessary.
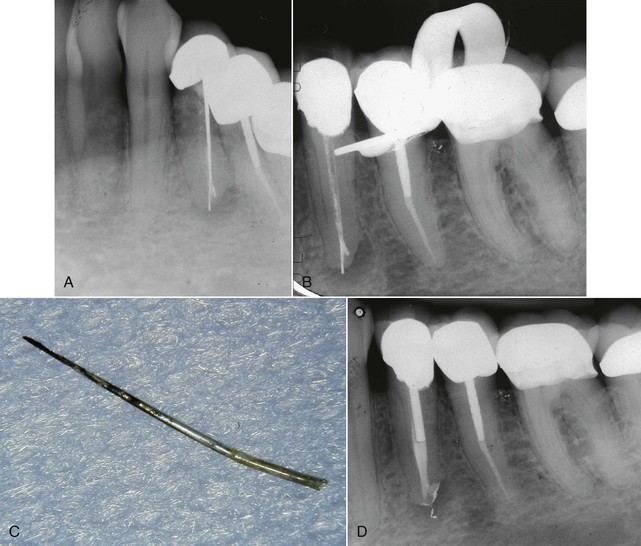
FIG. 25-44 A, Persistent disease in a silver point filled tooth. B, Silver point removed. Note the radiopaque material in the apical portion of the canal system. This represents corrosion products remaining in the canal and a possible separated apical segment of the cone. C, Removed silver point showing black corrosion products adhering to the apical one half. D, Crown-down instrumentation prevents extrusion of most of the corrosion products into the periradicular tissues.
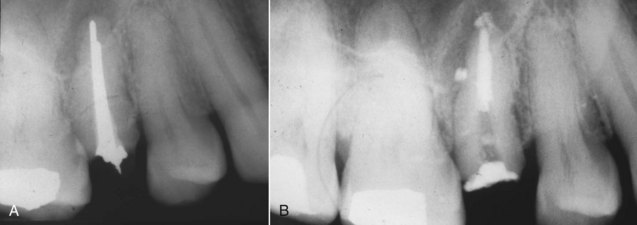
FIG. 25-45 A, Preoperative view of a silver point case with persistent disease. Note that the periapical radiolucency extends coronally on the distal aspect of the root end, indicating the presence of an unfilled lateral canal. B, Postobturation radiograph showing the cleaned and filled distal canal branch.
Many of the same techniques described for removing separated instruments in the following section apply to the removal of silver points. Silver points have a minimal taper, are smooth-sided, and corrosion may loosen the cone within the preparation. Therefore, the clinician should encounter a much easier time removing them than would be the case with separated instruments, which may be mechanically engaged into canals. Silver point canal preparation techniques produced a milled, round preparation in the apical 2 to 3 mm of the canal. Coronal to that, the clinician will frequently find space between the round silver point and the flared canal walls that can usually be negotiated with hand files, facilitating point removal.163
The first step in removal of silver points is to establish proper access. Frequently, the coronal portion of the cone is embedded in the core material. This material must be carefully removed with burs and ultrasonics, taking care to not remove any of the silver point within the access cavity preparation. The more of the silver point the clinician has to work with, the more predictable will be its removal. Once proper access is established, the clinician should flood the access preparation with a solvent such as chloroform to soften or dissolve the cement, enabling easier removal. An endodontic explorer or small file may be used to carry the solvent down along the silver point to dissolve as much of the cement as possible. The chamber can be rinsed and dried, and this step may be repeated, since fresh solvent enhances the efficiency of cement removal. At this point, the easiest technique, which is also very predictable, is to grasp the exposed end of the silver point with a Stieglitz pliers (Henry Schein, Melville, NY) (see Fig. 25-37) or other appropriate forceps and gently pull it out of the access cavity preparation. If too much extraction force is needed, the point may separate, so slow force application is advised. The clinician will need a variety of sizes and angles of forceps available to deal with the variety of cases that will need to be treated. Occasionally, the forceps may not get a good purchase on the silver cone and will slip off. In these instances, gripping the cone with the forceps and then gripping the forceps in a hemostat or needle driver to increase the squeezing force of the forceps will allow removal of the cone75 (Fig. 25-46). If the silver point is held tight by the frictional fit in the preparation, indirect ultrasonics may be employed to loosen it. The silver point is retained in a pair of forceps, and ultrasonic energy is applied to the forceps, not the point (Fig. 25-47). This transmits energy down the cone and may loosen it. If there is not much of the silver point exposed in the chamber, the clinician can attempt to remove it using the Caufield silver point retrievers (Miltex Inc. York, PA). This instrument is a spoon with a groove in the tip (Fig. 25-48) that can engage the exposed end of the silver point so it may be elevated from the canal or possibly elevated to the point where it may be grasped by forceps.75 The Caufield silver point retrievers are available in three sizes: 25, 35, and 50.
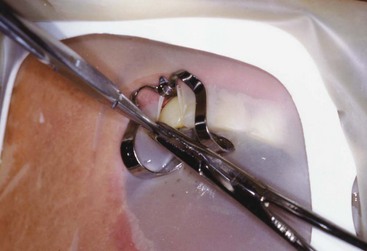
FIG. 25-46 Removal of a highly retentive silver point using a needle driver to squeeze the tips of the Steiglitz forceps. This applies increased gripping force to aid in removal.
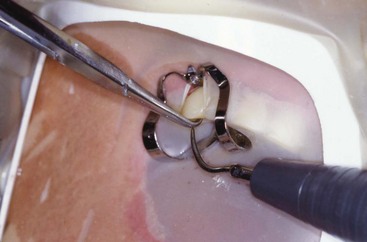
FIG. 25-47 Application of indirect ultrasonic energy to a silver point by placing the ultrasonic tip against forceps that are holding the silver point.
FIG. 25-48 Caufield elevator tip, useful in gripping and elevating silver points that are protruding a small amount into the pulp chamber.
If the silver point cannot be dislodged by the forgoing techniques, the clinician should consider using Hedstrom files to remove the silver point. The Hedstrom file technique requires at least some coronal length of canal space around the silver point to be negotiated first.122 The sealer is dissolved as previously mentioned, then files are negotiated as far apically as possible in two to three areas around the silver point. If only one space can be negotiated, this technique may still be effective. The spaces surrounding the silver point are carefully instrumented to size 15, and small Hedstrom files are gently screwed in as far as possible apically. To prevent breakage, they should not be screwed in too tightly. The flute design of the Hedstrom file allows for much better engagement into the silver point, compared to other file designs. The files are then twisted together and pulled out through the access (Fig. 25-49). If the first attempt fails, this technique may be repeated, possibly using larger Hedstrom files. If this technique does not completely remove the silver point from the canal, it may still be dislodged to the point where it can be grasped by forceps and removed.
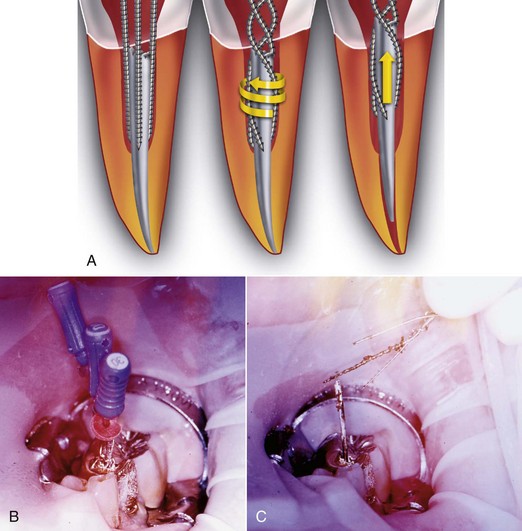
FIG. 25-49 A, Diagram illustrating the braiding of Hedstrom files around a silver point. By twisting the braided files, a gripping force is applied which aids in removal of the obstruction. B, Small files being braided around a silver point. C, Pulling coronally with the braided files removes the silver point.
(A Courtesy DENTSPLY Tulsa Dental, Tulsa, OK.)
If the clinician needs to expose more of the silver point to enable removal, the use of trephine burs and microtubes or ultrasonics may be necessary.58 Trephine burs are used in the same manner as described for separated instrument removal; however, the clinician must be more careful when using ultrasonic instruments for retrieval of silver points. When using ultrasonics for post removal or separated instrument removal, the tip of the ultrasonic instrument can be placed at the interface between the obstruction and the canal wall. Although applying ultrasonic energy directly to a post or file may prove beneficial in vibrating them loose, silver points are much softer. If ultrasonic instruments are applied directly to them, the portion in contact may be shredded, leaving a smaller segment to work with because elemental silver rapidly erodes during mechanical manipulation.163 The ultrasonic instrument is used on tooth structure circumferentially around the silver point. This is a very delicate process requiring a microscope or other powerful source of magnification. The energy supplied by the careful use of the ultrasonic instrument can safely expose silver points as well as break up the cement around them.
In many cases, a silver point may have been sectioned deep in the canal to allow post space preparation. In these cases, where the most coronal portion of the silver point is well below the orifice, the use of Gates-Glidden burs to obtain straight-line access to the most coronal extent of the point may be necessary. The burs should be used in a brushlike manner, cutting on the outstroke while applying gentle pressure in an anticurvature direction to decrease the risk of root perforation. Following this, techniques that involve the use of an end-cutting trephine bur to remove tooth structure around the point and then use of an extraction device to remove it may be employed (Fig. 25-50). There are many kits that use these principals with slight variations from one another, including the Endo Extractor (Brasseler),198 the Masserann Kit (Medidenta International, Woodside, NY), and the Extractor System (Roydent, Troy, MI) (Fig. 25-51). Additional techniques that are effective for removing silver points include the S.I.R. (Separated Instrument Retrieval) System (Vista Dental Products, Racine, WI), the use of a dental injection needle with a 0.14-mm wire, the use of stainless steel tubing with a Hedstrom file,163 and the Instrument Removal System (DENTSPLY Tulsa Dental, Tulsa, OK).
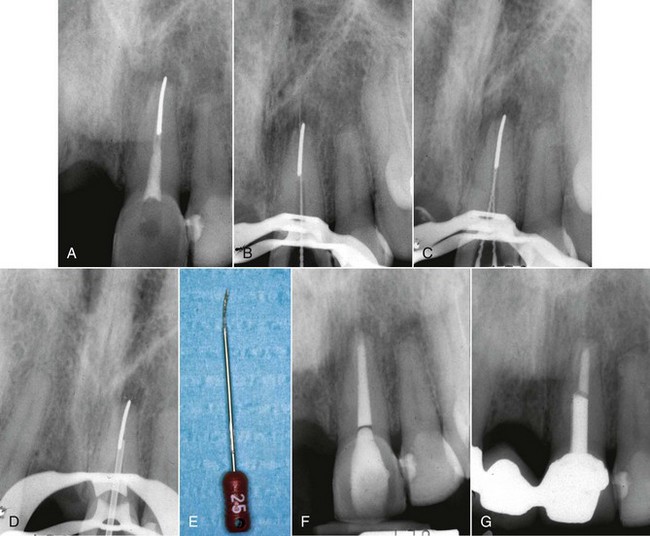
FIG. 25-50 Twist-off silver point case. A, Preoperative radiograph showing apical periodontitis and a split silver cone (twist-off) obturation technique. B, The cone was initially bypassed but could not be loosened. C, The braided Hedstrom file technique was attempted but was unsuccessful. D, A Brasseler Endo Extractor tube is cemented to the cone with cyanoacrylate cement. E, The silver point is removed. F, Immediate postobturation radiograph. G, One-year follow-up showing apical healing.
(From Gutmann JL, Dumsha TC, Lovdahl PE (eds): Problem solving in endodontics: prevention, identification, and management, ed 4, St Louis, Mosby, 2006.)
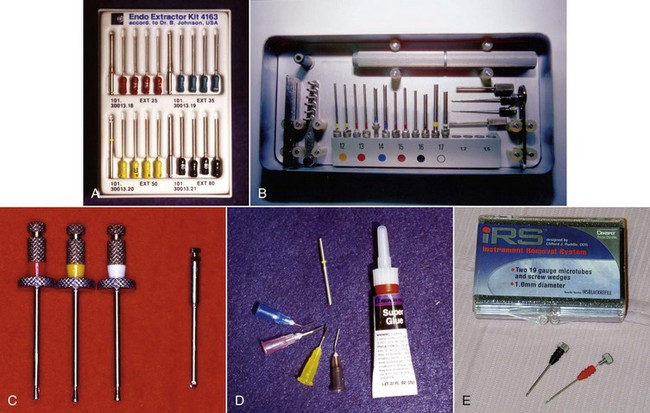
FIG. 25-51 A, Brasseler Endo Extractor kit (Brasseler USA, Savannah, GA). B, Masserann kit (MICRO-MEGA Dentalvertrieb GmbH & Co. KG, Germany). C, Roydent Extractor System (Roydent Dental Products, Johnson City, TN). D, Separated Instrument Retrieval System (SIR) (Vista Dental Products, Racine, WI). E, Instrument Removal System (IRS) (DENTSPLY Tulsa Dental, Tulsa, OK).
(B Courtesy Dr. Daniel Erickson.)
These kits are not only effective in the removal of silver points but also in the removal of separated instruments. Because of this common approach to removing both during endodontic retreatment, these techniques will be discussed in detail in the following section on separated instrument removal.
After the silver point is removed, it is very important that subsequent instrumentation procedures be performed in a crown-down manner to minimize extrusion of the silver corrosion products into the periradicular tissues to decrease the occurrence of painful acute flare-ups. This goal is complicated by the fact that ledges are frequently encountered at the level of the apical extent of the silver point as a result of the type of milled preparation that was frequently used in this technique. Managing ledges will be discussed in a following section of this chapter.
Occasionally the apical portion of a silver point will separate upon the removal attempt. If it cannot be bypassed or removed, the case should be completed and followed carefully (Fig. 25-52). Apical surgery or extraction could be necessary in the future (Fig. 25-53).
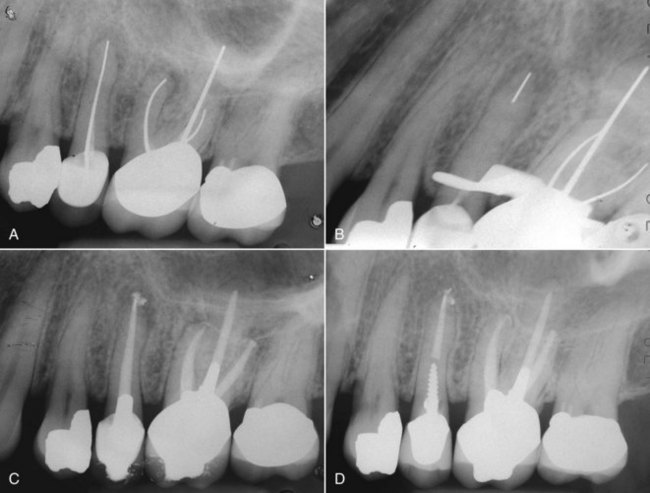
FIG. 25-52 Case illustrating healing despite inability to remove a separated silver point. A, Preoperative radiograph showing persistent disease in an upper premolar and molar. B, Working film showing the separated cone that could not be retrieved despite extensive clinical efforts. C, Final obturation after two-appointment procedure using a calcium hydroxide interappointment medicament. D, Four-year follow-up showing apical healing.
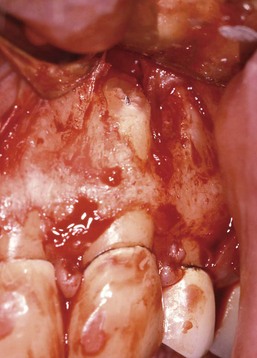
FIG. 25-53 If silver point retreatment is unsuccessful, apical surgery may be needed. Note the silver point visible on the resected root end. If the point cannot be pulled out in a retrograde direction, ultrasonic root-end preparation may be complicated by its presence; root-end preparation using rotary burs may be necessary.
Removal of Separated Instruments
Causes of Instrument Separation
Occasionally during nonsurgical root canal therapy, an instrument will separate in a canal system, blocking access to the apical canal terminus. This instrument is usually some type of file or reamer but can include Gates-Glidden or Peeso Drills, lentulo spiral paste fillers, thermomechanical gutta-percha compactors, or the tips of hand instruments such as explorers or gutta-percha spreaders. During retreatment, it may be obvious after completing the diagnostic phase that there is a separated instrument in the canal system, or it may only become apparent after removal of the root filling materials (Fig. 25-54). It is useful to expose a check radiograph after removal of the root filling to see if there is any metallic obstruction in the canal. Regardless of which type of instruments the clinician uses, whether stainless steel or nickel-titanium, and how they are used, by hand or engine driven, the potential for separation exists. The incidence of hand instrument separation has been reported to be 0.25%98; for rotary instruments, it ranges from 1.68% to 2.4%.98,237
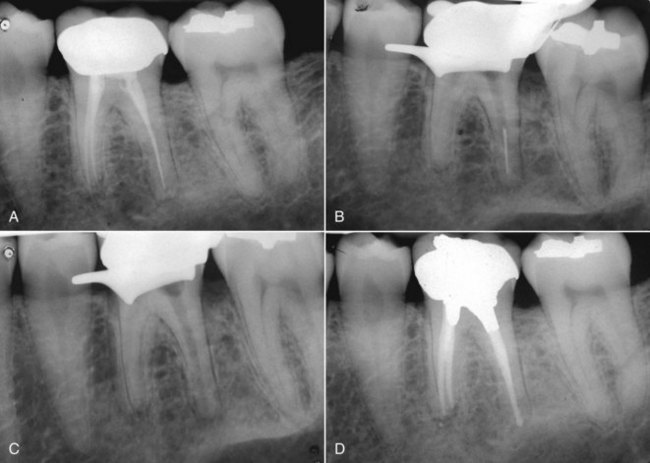
FIG. 25-54 A, Preoperative radiograph of a tooth with symptomatic posttreatment disease. B, Although not readily apparent on the preoperative film, there is a separated nickel-titanium instrument in the distal canal. C, Check film showing that ultrasonics has removed the separated file. D, Thirteen-month recall film. The patient was asymptomatic.
The most common causes for file separation are improper use, limitations in physical properties, inadequate access, root canal anatomy, and possibly manufacturing defects.
A very common cause for instrument separation is improper use. Included in this are overuse or not discarding an instrument and replacing it with a new one when needed. The following is a list of guidelines for when to discard and replace instruments.75
Another type of improper use is to apply too much apical pressure during instrumentation,218 especially when using rotary nickel-titanium files. This pressure can lead to deflection of the instrument within the canal system or increased frictional binding against the canal walls that can overstress the metal, resulting in separation. Regardless of which type of files the clinician uses, they should never use them in a dry canal, because attempting to instrument a dry canal will cause excessive frictional stresses on an instrument.218 Continual lubrication of the canal with either irrigating solutions or lubricants is required.75 This will reduce the frictional resistance as well as increase the efficiency of the instrument. All files have flutes that have the ability to build up with dentin shavings, which will decrease the efficiency of the instrument, leading to greater frictional forces and ultimately separation. Therefore, files should be periodically removed and cleaned during the instrumentation process.
Inadequate access cavity preparations can lead to many problems, one of which is excessive or unnecessary force applied to the instrument if it is not allowed to enter the canal freely without interference from the access cavity walls. If the file is in contact with the access cavity wall during instrumentation, the chance for separation is greatly increased. Inadequately enlarged access preparations also increase the number and severity of curvatures the file must negotiate. This under prepared access can lead to the creation of an iatrogenic S curve that can overstress the instrument. This situation is especially hazardous when using rotary instrumentation, since traversing an S curve greatly stresses the rotating file, leading to separation (Fig. 25-55).
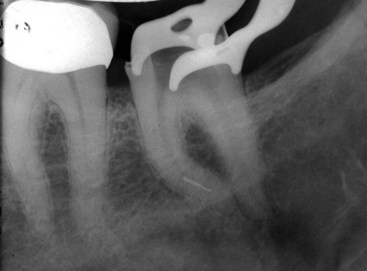
FIG. 25-55 Complicated canal anatomy can increase stress on rotary instruments, leading to separation such as in this S-shaped canal.
Anatomy, such as abrupt curvatures or anatomic ledges, increases the likelihood of instrument fracture. When the file’s progress is hindered, it is quite natural to try to force it further. This approach will rarely result in the file advancing along the naturally occurring path and indeed may result in file separation, perforation, or ledge creation.
Some clinicians would like to blame instrument separation on manufacturing defects; however, this has never been shown to be of clinical relevance and is quite rare.218
The best treatment for the separated instrument is prevention. If proper techniques for cleaning and shaping of the root canal system are followed, file separation should be an infrequent occurrence. Nevertheless, an occasional event may take place. When instrument separation occurs, a radiograph should be taken immediately.218 This radiograph will not only confirm the separation, it will give the clinician information that may aid in removal, such as location, size of the file segment, root canal anatomy, and ultimately the possibility of removal. The patient should be advised of the accident and its effect on the prognosis.36 In addition, when a file separates, as with other procedural accidents, detailed documentation is necessary for medicolegal considerations,218 and the remaining segment of the file should not be discarded but placed in a coin envelope and kept in the patients record.36
Prognosis
A separated instrument does not necessarily mean surgery or loss of the tooth. Actually, the prognosis may not be reduced at all, depending on what stage of instrumentation the separation occurs, the preoperative status of the pulp and periradicular tissues, and whether or not the file can be removed or bypassed.197 The presence of a separated instrument in the canal in itself does not predispose the case to posttreatment disease. Rather, it is the presence of any necrotic, infected pulp tissue that remains in the apical canal space that determines the prognosis. The outcome is better if the canal was instrumented to the later stages of preparation when the separation occurs.218 If the preoperative pulp was vital and uninfected (irreversible pulpitis, for example), and there was no apical periodontitis, the presence of the separated instrument should not affect the prognosis.37 If the file can be removed without excessive overenlargement of the canal or causing an additional iatrogenic mishap such as a perforation, the prognosis will not be affected. Bypassing the instrument and incorporating it into the obturation should also have no affect on the prognosis. However, if the instrument cannot be removed or bypassed in a tooth with a necrotic infected pulp and apical periodontitis, the prognosis will be uncertain. These cases should be followed closely. If symptoms persist, apical surgery or extraction should be considered.218,240
The potential to remove a separated instrument depends on many factors that should be considered during the diagnostic workup. The location of the separated instrument is of critical importance. If the separated instrument extends into the straight coronal portion of the canal, retrieval is likely, but if the instrument has separated deep in the canal and the entire broken segment is apical to the canal curvature, orthograde removal will not be possible, and attempts to do so could lead to a much higher rate of iatrogenic complication.184,195 If there is persistent disease and the file cannot be bypassed safely, either apical surgery or extraction will be necessary. Because of the need to enlarge the coronal radicular access, root curvatures, external root concavities, and root thickness all will be important factors to consider when deciding which treatment option will provide the best chance of long-term success. Teeth with thin roots and deep external root concavities have a greater likelihood of being perforated during the coronal radicular access, so surgery should be considered as an alternative to orthograde instrument retrieval. The type of material the separated instrument is made of will affect the chances of removal. Nickel-titanium files tend to shatter when ultrasonic energy is applied to them, hindering removal, whereas stainless steel instruments are more robust and more easily removed with ultrasonics.163
Removal Techniques
Many different instruments and techniques will be discussed in this section, all of which are important to include in the armamentarium for separated instrument removal. None, however, is more important than the operating microscope (Fig. 25-56). This instrument will not only increase visibility by the use of magnification and light, it will also increase the efficiency and safety of almost all of the techniques to be discussed. The use of a headlamp and magnifying loupes will help with the removal of many canal impediments, but the enhanced lighting and magnification the operating microscope offers has caused a quantum leap in visualization.107 Many of the techniques to be described should not even be attempted without the use of this valuable tool.205
FIG. 25-56 The surgical operating microscope is not only invaluable in helping to remove separated instruments, it is in fact a necessary tool for these procedures.
Once the patient has been advised of the treatment options and the decision has been made to attempt removal, the clinician’s first choice in treatment will be based on the location of the instrument. If the file is clinically visible in the coronal access and can be grasped with an instrument such as a hemostat or Stieglitz Pliers (Henry Schein, Melville, NY) (see Fig. 25-37), these should be used to obtain a firm hold of the file and extract it out through the access cavity preparation. Many different sizes and angles of forceps are available, and almost all are necessary in order to have the ability to remove obstructions from the many different angles and levels of accessibility presented to the clinician. These will work well if the object is loose fitting within the canal and if the clinician has good access. However, establishing a firm purchase can sometimes be difficult without removing excessive tooth structure. Once a purchase onto the file has been achieved, it is best to pull it from the canal with a slight counterclockwise action. This action will unscrew the flutes that are engaged in the dentin as the file is being removed. This is the easiest technique for removal of a separated file. Unfortunately, many files separate at a point where these forceps cannot be used.
Frequently a file will separate at a point deeper in the canal where visibility is difficult. To remove separated root canal instruments predictably, the clinician must create straight-line coronal radicular access. Either removing the crown or creating a large access cavity preparation establishes adequate coronal access to allow the use of the appropriate instruments. Straight-line radicular access can be created with the use of modified Gates-Glidden drills. These drills may be ground down or sectioned with a bur at their maximum cross-sectional diameter. This process will create a circumferential staging platform to facilitate ultrasonic use (Fig. 25-57).163 A recent study showed that use of a similarly modified Lightspeed nickel-titanium rotary instrument (Lightspeed Technology Inc, San Antonio, TX) created a staging platform that was more centered in curved canals than the Gates-Glidden drills.99
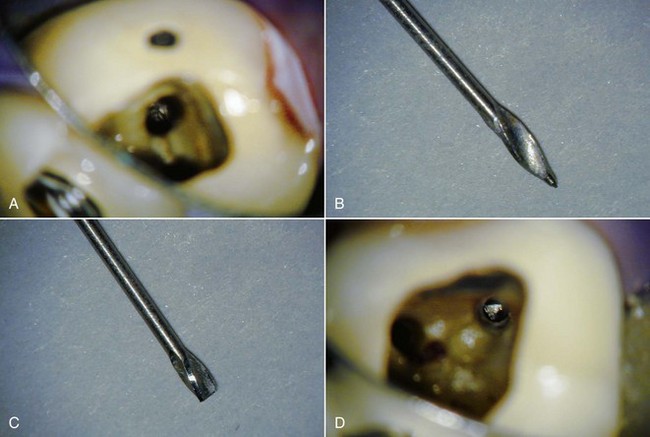
FIG. 25-57 A, Separated instrument in the mesiobuccal canal of a molar. B, Unmodified Gates-Glidden drill. C, Modified instrument. The tip has been ground off to the maximum diameter of the cutting head. D, Staging platform created in the straight coronal section of the canal. Note the enhanced visibility and the triangular cross-section of this rotary instrument.
Ultrasonic instruments have been shown to be very effective for the removal of canal obstructions.33,138,163 The ultrasonic tip is placed on the staging platform between the exposed end of the file and the canal wall and is vibrated around the obstruction in a counterclockwise direction that applies an unscrewing force to the file as it is being vibrated. This technique will help with removing instruments that have a clockwise cutting action. If the file had a counterclockwise cutting action (such as the hand GT files), then a clockwise rotation will be needed. The energy applied will aid in loosening the file, and occasionally the file will appear to jump out of the canal. It is prudent to cover the orifices of the adjacent open canals with cotton or paper points to prevent the removed file fragment from falling into them, causing further case complication163 (Fig. 25-58). There are many different sizes and angles of ultrasonic tips available for this purpose, but in general the deeper in the canal the obstruction is, the longer and thinner an ultrasonic tip must be. It should be remembered that long, thin tips must be used on very low power settings to prevent tip breakage (Fig. 25-59). Occasionally, if the separated instrument can be bypassed, the use of ultrasonic files can loosen it. Care must be taken, however, to avoid ultrasonic file separation or root perforation.87 As mentioned previously, nickel-titanium instruments often break up into fragments when subjected to the energy supplied by an ultrasonic instrument. The clinician may be tempted to use this information to their advantage by applying the tip of the ultrasonic directly onto nickel-titanium files. Although this method may work, the chance of pushing the separated file further into the canal or beyond the apical foramen may increase the risk of this technique.
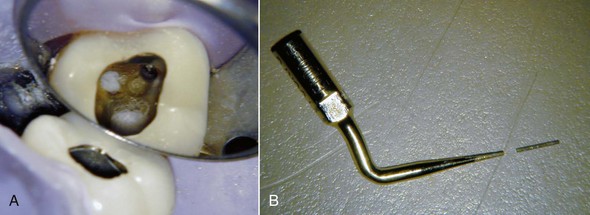
FIG. 25-58 A, Cotton pellets protecting the orifices of the other canals when ultrasonic obstruction removal is needed. B, Ultrasonic tip separated during excavation around separated instrument. If the adjacent canals were not protected, further unnecessary complication could result.
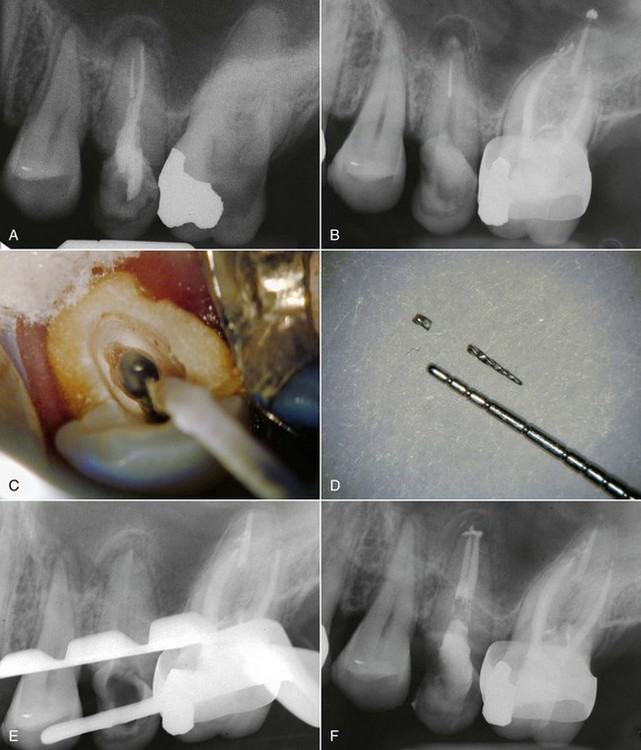
FIG. 25-59 A, Preoperative radiograph showing a separated file in the palatal canal, potential coronal leakage, and apical periodontitis. B, Check film showing the separated instrument after gutta-percha removal. C, Photograph showing the separated instrument in the palatal canal and a paper point in the buccal canal to protect it. D, Separated nickel-titanium file removed. Note that it is in two pieces—a result typical when applying ultrasonic energy to nickel-titanium. E, Check film showing that the file has been completely removed. F, Final canal obturation.
If the direct application of ultrasonic energy does not loosen the separated instrument sufficiently to remove it, the fragment must be grabbed and retrieved. This is accomplished with a variety of techniques, most using some variant of a microtube. The staging platform is further reduced by ultrasonics until enough of the separated instrument is exposed to retrieve (about 2 to 3 mm).163 This reduction must be done carefully to avoid root perforation. One relatively simple microtube technique is to use a short piece of stainless steel tubing that is pushed over the exposed end of the object. A small Hedstrom file is then pushed between the tube and the end of the object, using a clockwise turning motion that produces a good mechanical lock between the separated instrument, the tube, and the Hedstrom file. The three connected objects can then be removed by pulling them in a coronal direction204 (Fig. 25-60).
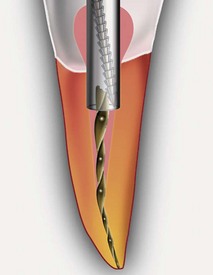
FIG. 25-60 Tube and Hedstrom file removal technique. The tube is slipped over the obstruction, and a Hedstrom file is gently screwed into the space between the tube and the obstruction. Pulling the tube and Hedstrom file together can withdraw the obstruction.
(Diagram courtesy DENTSPLY Tulsa Dental, Tulsa, OK.)
Another technique is to use a 25-gauge dental injection needle along with a 0.14-mm diameter steel ligature wire. The needle is cut to remove both the beveled end and the opposite end so it no longer extends beyond the hub. Both ends of the wire are then passed through the needle from the injection end until they slide out of the hub end, creating a wire loop that extends from the injection end of the needle. Once the loop has passed around the object to be retrieved, a small hemostat is used to pull the wire loop up and tighten it around the obstruction, then the complete assembly is withdrawn from the canal.161 Occasionally, a larger diameter tube and thinner 0.11-mm ligature wire will facilitate assembly of this extractor (Fig. 25-61).
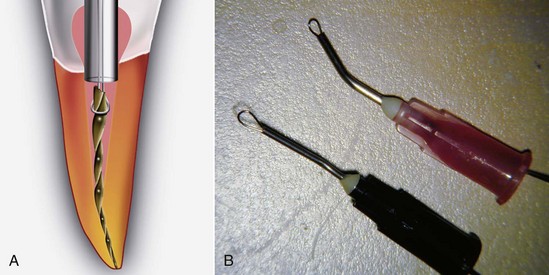
FIG. 25-61 A, Diagram illustrating the wire-loop-and-tube method of obstruction removal. The wire loop is carefully placed around the obstruction, tightened, then removed. B, Larger-diameter tubes and smaller-diameter 0.11 mm ligature wire enhances the efficiency of this technique.
(A Courtesy DENTSPLY Tulsa Dental, Tulsa, OK.)
Another effective technique, especially in cases where access or obtaining an adequate purchase on the file is difficult, is to use an end-cutting trephine bur to remove tooth structure around the file and then use an extraction device to remove it. There are many kits that use these principles with slight variations from one another, including the Endo Extractor (Brasseler USA Inc), the Masserann Kit (Medidenta International Inc), and the Extractor System (Roydent Dental Products) (see Fig. 25-51).
The Endo Extractor kit includes a cyanoacrylate adhesive which is used to bond a hollow tube to the exposed end of the file for removal. This kit also includes four sizes of trephine burs and extractors. The most important factor in using this kit is the snugness of fit between the extractor tube and the obstruction. It has been shown that even with only 1 mm of overlap between the extractor tube and the obstruction, if there is a snug fit, the bond created with the cyanoacrylate may be strong enough to remove many obstructions. However, the recommended amount of overlap between the tube and the obstruction is 2 millimeters. The time needed for the adhesive to set to ensure adequate bond strength for removal is 5 minutes for a snug fit and 10 minutes for a loose fit.65,198 One disadvantage of this instrument is that the trephine burs are much larger than their ISO equivalents, so the manufacturer has also added a separate smaller trephine bur that is sold separately from the kit. This bur corresponds better with the smaller extractors and removes less dentin, which can decrease the likelihood of weakening the root and the risk of fracture.52 Another disadvantage is that the burs cut very aggressively when new but dull rather quickly. When new, their aggressive cutting may lead to perforation or even separation of the obstruction, so great care is needed when using this instrument (Fig. 25-62). Once the separated instrument has been removed, the extractors may be reused, either by using the debonding agent included in the kit to remove the embedded instrument from the extractor tube or by simply cutting the extraction device with a bur beyond the extent that the separated instrument has penetrated.
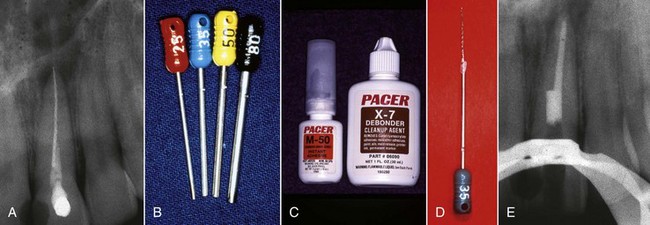
FIG. 25-62 A, Separated file wedged into an upper incisor. B, Brasseler Endo Extractor tubes. C, Cyanoacrylate cement and debonding agent. D, Separated file pulled out by the bonded tube. E, Final obturation. Note the excessive amount of tooth structure removal by the trephine bur that was needed to bond the tube.
The Masserann technique has also been recommended for the removal of separated instruments.131 This technique is similar to the Endo Extractor in that it uses trephine burs and a specific extraction device. This kit comes with a very convenient gauge that aids in predicting the size of the bur and the extractor to be used, and it contains many different sizes of trephine burs. In addition, the trephine burs with this kit cut in a counterclockwise direction that provides an unscrewing force on separated files. The extraction mandrels have an internal stylus that wedges the file against the internal wall of the mandrel, allowing the obstruction to be removed. Although effective, this technique may require removal of an excessive amount of radicular dentin,58 leading to root weakening and the risk of perforation241; therefore, this instrument must be used with caution.
The Extractor System from Roydent comes with only one bur and three extraction devices. The bur is very conservative and removes a minimal amount of tooth structure, enabling access to the obstruction. The extractor tubes are also quite small and will only work for the removal of small obstructions. The extractor surrounds the obstruction with six prongs that can be tightened onto the object, enabling removal. This works in the same way a drill chuck tightens onto a drill bit (Fig. 25-63). The disadvantages of this kit are the lack of variety of instruments, the possibility of separating the obstruction with the bur, and the potential problem of breakage of the prongs in the extractor if they are submitted to bending rather than applying strict tensile force during removal.

FIG. 25-63 Close-up view of the Roydent Extractor tip. The tip is placed over the separated instrument and tightened to grasp the obstruction.
Two techniques have been designed specifically for removing instruments in conjunction with the operating microscope: the Cancellier instrument and the Mounce extractor (SybronEndo) (Fig. 25-64). The Cancellier instrument works in a very similar manner to the Brasseler Endo Extractor in that they are used in conjunction with cyanoacrylate adhesive to bond onto the separated end of the instrument. Unlike the Brasseler extractors, the Cancellier extractors are attached to a handle that enables them to be used without blocking visibility when using the operating microscope. The Brasseler extractors are finger instruments that interfere with the line of sight the microscope requires. There are no trephine burs in the Cancellier kit; rather, it is used in conjunction with ultrasonic exposure of the separated instrument. There are four sizes of extractor tubes available, each of which corresponds to specific file sizes. The Mounce extractors are also hand instruments that enable usage with the operating microscope. These instruments are similar to a ball burnisher with slots cut into the ball end. These slots are cut at various angles and are designed to slide onto the broken end of the file. Cyanoacrylate is used to bond the extractor to the file, allowing removal. This instrument can be used when the separated file is lying against the canal wall, but the ball tip is relatively large and is only useful in retrieving instruments that are in the most accessible coronal portion of the canal.
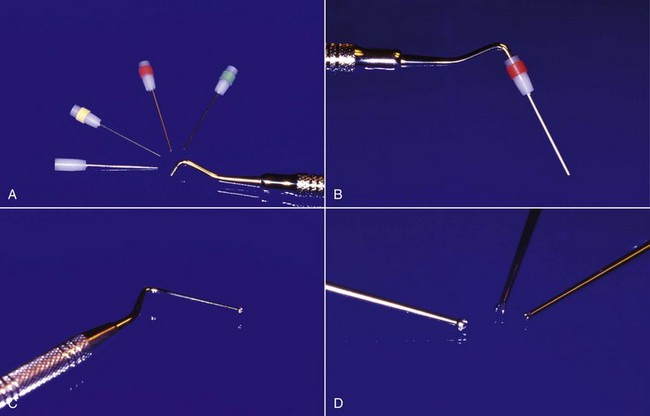
FIG. 25-64 A, The Cancellier Kit (SybronEndo, Orange, CA) with four tube sizes available. B, The Cancellier instrument is used with superglue to bond the obstruction, but its design allows for greater visibility during use. C, The Mounce instrument. D, Varying tip sizes for the Mounce instrument.
Another device designed specifically for the purpose of separated file removal is the Instrument Removal System (DENTSPLY Tulsa Dental, Tulsa, OK) (see Fig. 25-51). This kit consists of two different sizes of extraction devices that are tubes with a 45-degree bevel on the end and a side cutout window. Each tube has a corresponding internal stylus or screw wedge. Prior to usage of this instrument, 2 to 3 mm of the obstruction is exposed by troughing around it with an ultrasonic instrument. Once the file is exposed, the appropriate size microtube is selected and slid into place over the obstruction. Once in place, the screw wedge is turned counterclockwise to engage and displace the head of the obstruction through the side window. The assembly is then removed.163 This instrument is very useful in the straight portion of the canal, but it is difficult to force large-diameter separated files through the cutout window, hampering their removal (Fig. 25-65).
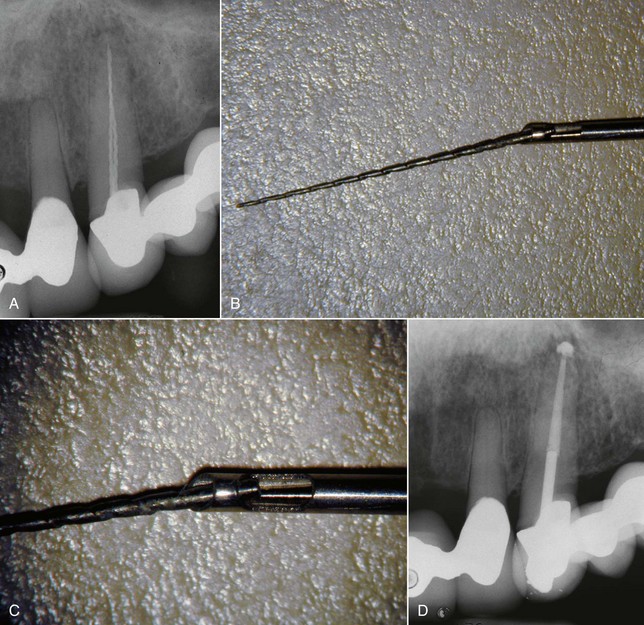
FIG. 25-65 A, Preoperative radiograph showing two separated instruments in one tooth. B, IRS instrument with a removed file. C, Note that large-size files are difficult to push through the cutout window. D, Postoperative radiograph.
The S.I.R. (Separated Instrument Retrieval) System (Vista Dental Products) (see Fig. 25-51) is another microtube method of separated instrument retrieval. Like the Cancellier instrument, it utilizes extractor tubes bonded onto an obstruction, enabling removal. Once the obstruction is exposed using ultrasonics or the trephine burs from one of the other kits described, the bendable dead-soft tubes are bonded onto it. Once the adhesive is set, the obstruction is removed through the access cavity preparation. Included in this kit are the necessary bonding agent, a bottle of accelerator, five different sizes of tubes, assorted fulcrum props, and a hemostat. The accelerator causes the bonding agent to set almost instantaneously. The ability to bend these tubes allows for access in most areas of the mouth. A hemostat allows the clinician to establish a firm purchase onto the tube, creating the ability to lever the bonded obstruction out of the canal. A vinyl autoclavable instrument prop provides protection for the next most anterior tooth, which is to be used as a fulcrum. If the clinician has access to grasp the extractor with their fingers to remove the extractor/obstruction unit, the hemostat may not be necessary.
Heat Generation During Retreatment Procedures
There are many procedures in endodontic therapy that can generate heat, but perhaps the area with the greatest risk of heat-related tissue damage occurs during nonsurgical retreatment. Use of heat to soften canal filling materials to aid in their removal115,121 and use of ultrasonics to dislodge posts168 and separated instruments81,126 can potentially generate enough heat to raise the temperature of the external root surface by 10° C or more. Temperature elevations of the periodontal ligament in excess of 10° C can cause damage to the attachment apparatus.9,45,170,171
The greatest danger of heat-related damage occurs with the use of ultrasonic energy to dislodge foreign objects in the canal space to gain access to the apical portion. As noted earlier, this has recently become an extremely important and useful part of the clinician’s armamentarium because of the ability of ultrasonic energy application to conserve tooth structure while removing the obstruction. These instruments have allowed clinicians to perform predictable retreatment when surgery would have been previously indicated. As with most instruments used in dentistry, however, these devices must be used with caution. In vitro research and clinical case reports imply that they have the potential to damage the tooth attachment apparatus as a result of the heat generated during usage.27,67,177 One in vitro study has showed that ultrasonic vibration for post removal without coolant can cause root surface temperature increases approaching 10° C in as little as 15 seconds.41 Thermal damage to the periradicular tissues may be so serious as to result in both tooth loss and permanent bone loss (see Fig. 25-26). This is not to say that ultrasound energy should be avoided for the removal of canal obstructions, since it is many times the only way to reach the apical area of the canal.
The factors that may contribute to a heat-induced injury are the length of the post, post diameter, post material, and type of luting cement. Studies to establish heat-reduction protocols based on these variables are needed. Some have proposed that the dentin thickness between the outer surface of the post and the root surface may affect root surface temperature rise,68,96 and one study showed this.168 However, a more recent study has shown that the dentin thickness is statistically insignificant as a factor in root surface temperature rise.86 One possible mitigating factor would be the effect of the periradicular blood supply, which could act as a heat sink, dissipating the generated thermal energy and helping to prevent injury. This may be why the effect of seemingly similar conditions of ultrasonic application can have such differing results on different patients. Clearly, more in vivo research is needed in this area.
It has become accepted that the heat-induced damage to periradicular tissues during the use of ultrasound energy for post removal is time dependent.27,91 Studies have suggested that cooling the ultrasonic tips can greatly reduce heat buildup,27,91 but this reduces the efficiency of debonding resin-bonded posts.41,62 The amount of time the clinician can use these instruments safely is very difficult to determine; specific evidence-based protocols using in vivo research have yet to be established. The authors feel that any specific recommendations regarding rest intervals between usage of ultrasound energy, ways of monitoring heat buildup, or duration of consistent activation cannot be made based on the available research at the time of publication. However, the authors do feel strongly with regards to several recommendations for the use of ultrasound energy during the removal of canal obstructions, and they are as follows:
Prudent clinicians must take extreme care when applying ultrasound energy to a canal obstruction. It has been shown that even with the use of water coolant, root surfaces can increase in temperature rapidly.41,168 Until evidence-based heat-reduction protocols are developed, caution when using instruments that can generate heat in the periodontal ligament is required.
Management of Canal Impediments
Following removal of all root-filling materials, further progress to the apical constriction may be prevented by the presence of a block or a ledge in the apical portion of the canal. Most of these impediments are iatrogenic mishaps resulting from vigorous instrumentation short of the appropriate working length and failure to confirm apical patency regularly during instrumentation. A blocked canal contains residual pulp tissue (sometimes necrotic, often fibrosed or calcified) and packed dentinal “mud” in the apical several millimeters of the canal system.163 This debris is frequently infected, resulting in persistent disease, and must be removed if possible. A ledge is the result of placing non-precurved, end-cutting instruments into curved canals and filing with too much apical pressure.75,100 It is a type of canal transportation that results in a canal irregularity on the outside of the canal curvature that is difficult or impossible to bypass. The canal space apical to the ledge is not thoroughly cleaned and sealed, so ledges frequently result in posttreatment disease. The best treatment for blocked and ledged canals, as with all iatrogenic problems, is prevention. If the clinician is careful and attentive during the instrumentation process, the chance for an impediment to develop is minimized. When the clinician becomes careless or hurried, problems occur. The reader is referred to Chapter 9 for strategies to prevent blocks and ledges. During the treatment planning phase, blocks and ledges may be detectible on radiographs as a root filling short of the ideal working length, and the patient should be warned that they might prove impenetrable and require future apical surgery or extraction.57 This should not deter the clinician from choosing nonsurgical retreatment, however. In a recent study, 74% of teeth showing short root fillings were successfully negotiated to adequate length, with the authors stating that presence of a short fill should not be considered a technical contraindication to retreatment.48 The clinical encounter usually occurs after removal of the previous root-filling material when apical advancement of small files is impeded. At this point, the clinician may be unaware of which type of impediment exists, but a common approach to management is helpful in the early stages of the process. The coronal portion of the canal should be enlarged to enhance tactile sensation and remove cervical and middle third obstructions in the canal space. The canal should be flooded with irrigant, and instrumentation to the level of the impediment should be accomplished using non–end-cutting rotary files, such as the Lightspeed (Lightspeed Technology), the Profile or GT instruments (DENTSPLY Limited), or the K-3 instrument (SybronEndo) in a crown-down manner. This procedure will enlarge and flare the canal space coronal to the impediment while minimizing the likelihood of worsening any ledge present.
At this point, the impediment should be gently probed with a precurved #8 or #10 file to determine if there are any “sticky” spots that could be the entrance to a blocked canal. A directional silicone stop should be used so the clinician knows in which direction the tip of the instrument is pointing, which helps in visualizing the three-dimensional layout of the canal system. Frequently, evacuating the irrigant and using a lubricant such as RC Prep (Premier, Plymouth Meeting, PA) or Pro-Lube (DENTSPLY Tulsa Dental) will enhance the ability to place the small file into the apical canal segment. If repeated, gentle apical pressure or “pecking” of the hand file against the blockage results in some resistance when withdrawing the instrument on the outstroke (“stickiness”), the clinician should continue to peck at the sticky spot until further apical advancement is accomplished.163 This is frequently a slow and tedious process. The endeavor can be made more efficient by using precurved stiff files such as the C+ file (DENTSPLY Maillefer), but there is a risk of deviating from the original canal path, creating a ledge, and ultimately a false canal leading to zip perforation.75 It is prudent to make a working radiograph when some apical progress has been made to confirm the placement of the instrument into the suspected apical extent of the canal. The clinician should resist the urge to rotate the file excessively. If the tip of a small instrument is tightly bound in the blocked segment of the canal and the tip has been worked by pecking, it is prone to fracture in the apical area, further complicating the case.163 The separated file tip is frequently irretrievable, and surgery or extraction may be the result. Dropping down to the next smaller size file and using a very gentle reciprocal rotational motion (“twiddling”) will aid in advancement through the blocked canal. Frequently, as apical progress is being made, the clinician will be using an electronic apex locator to gauge the proximity of the apical constriction. Unfortunately, the apex locator is sometimes not able to give an accurate reading in a blocked canal, and because of the continued sticky feel that occurs even as the instrument bypasses the foramen and penetrates apical tissues, an overextension may result. To prevent this complication—with its attendant risk of a painful postoperative flare-up—when the estimated working length is reached, a working length radiograph is necessary.129 Once apical working length is achieved, apical patency should be confirmed, and gentle, short-amplitude 1- to 2-mm push/pull strokes should be made until the file can pass freely to the apical constriction (Fig. 25-66).
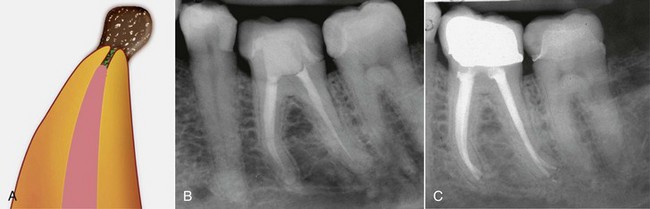
FIG. 25-66 A, Diagrammatic representation of a canal block. Fibrotic and/or calcified pulp and debris that is potentially infected remains in the apical segment of the canal when the canal is instrumented short of the apical constriction. B, Preoperative radiograph showing obturation short of ideal length. The patient was symptomatic, and the canals were blocked. C, Three months post treatment. Treatment took a total of 3.5 hours over three appointments because of the time-consuming nature of bypassing blocked canals.
(A Courtesy DENTSPLY Tulsa Dental, Tulsa, OK.)
If after a reasonable amount of time, no sticky spot can be found, the clinician must consider the possible presence of a ledge, despite possibly not detecting it on the preoperative radiograph. The main problem with ledges is that instruments will invariably find their way to the ledge, whereas finding the original canal is many times impossible. Ledges feel like a hard brick wall, short of length when encountered, and care must be used to prevent worsening them by indiscriminately burrowing into them.218 To manage a ledge, the tip of a small #08 or #10 file has a small bend placed in it 1 to 2 mm from the end,100,218 so the tip of the file forms an approximately 45-degree angle with the shaft of the instrument. The directional stop is oriented to the bend, and the file is carefully negotiated to the level of the ledge. Since ledges form mainly on the outside of curvatures, the directional stop (and thus the bent tip of the file) is turned in the direction of the suspected apical curvature away from the ledge (Fig. 25-67). The file tip is slowly scraped along the internal wall of the canal curve slightly coronal to the level of the ledge,218 looking for another sticky spot. This spot will be the entrance to the apical canal segment, and gentle reciprocal rotation will usually allow the file to be negotiated to the canal terminus. Confirm with a radiograph. Once the ledge has been bypassed, short-amplitude push/pull and rotational forces, keeping the file tip apical to the ledge, will be needed to clean and enlarge the apical canal space. When the file can be easily negotiated around the ledge, anticurvature filing will enable the clinician to blend the ledge into the canal preparation (Fig. 25-68). Many times this cannot be completely accomplished,218 but so long as the apical segment can be cleaned and obturated, the prognosis should not be adversely affected.
FIG. 25-67 A, Diagrammatic representation of a ledged canal. Potentially infected debris remaining in the apical segment can result in posttreatment disease. B, Attempting to bypass the ledge with a small file having a 45-degree bend in the tip. Note that the opening to the apical canal segment is on the inside of the canal curvature and coronal to the level of the ledge.
(A Courtesy DENTSPLY Tulsa Dental, Tulsa, OK.)
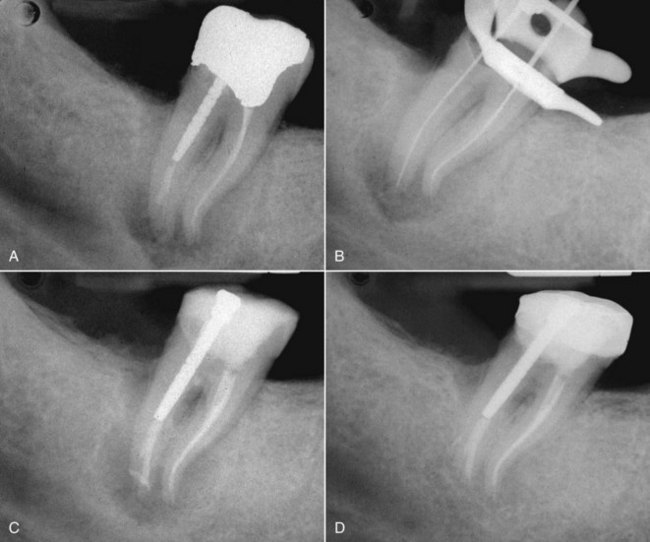
FIG. 25-68 A, Preoperative radiograph showing a distal canal ledge with a small amount of sealer that has entered the apical segment. The ledge prevented proper cleaning and sealing of the canal system, resulting in posttreatment disease. B, The ledge has been bypassed. The attempt is made to blend the ledge into the contour of the prepared canal wall. C, Final obturation showing the filled ledge and apical segment. D, Thirteen-month recall showing healing. The patient was then directed to have a definitive coronal restoration placed.
The use of Greater Taper (GT) NiTi hand files (DENTSPLY Tulsa Dental) for the blending of ledges has been advocated.163 The advantage these instruments have is that they are non–end cutting, and their rate of taper is two to six times that of conventional 0.02 tapered files, so they can do the work of multiple 0.02 tapered hand files. Once the ledge has been bypassed and the canal can be negotiated with a conventional size #15 or #20 K-file, a GT hand file is selected. The K-file creates a pilot hole so the tip of the GT file can passively follow this glide path beyond the ledge. The GT file must have a tip diameter of 0.2 mm (#20) and a taper that will vary depending on the requirements of the preparation. The largest taper that will enter the apical segment is used; however, these instruments must be precurved, which presents a challenge since they are made from nickel-titanium alloy. To precurve this superelastic shape-memory alloy, a file-bending tool such as the Endo Bender Pliers (SybronEndo) is needed. The pliers grasp the tip of the instrument, and the file is overcurved between 180 and 270 degrees to plastically deform the alloy. At this time, the appropriate tapered GT file is then carried into the canal, and the rubber stop is oriented so that the instrument’s precurved working end can bypass and move apical to the ledge. The GT file is then worked to length, and the ledge is either reduced or eliminated (Fig. 25-69).
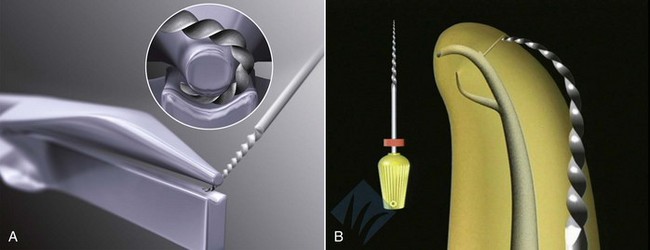
FIG. 25-69 A, Endobender Pliers (SybronEndo) used to over bend a nickel-titanium Hand GT file (DENTSPLY Tulsa Dental). B, GT hand file can hold a bend to allow it to bypass ledges.
(Courtesy Dr. Steve Buchanan.)
If the canal blockage or ledge cannot be negotiated, then the canal space coronal to the impediment should be cleaned, shaped, obturated, and coronally sealed. The patient must be informed of this complication, the guarded prognosis, and the need for regular reevaluation (Fig. 25-70). If symptoms of posttreatment disease arise subsequently, apical surgery or extraction will be needed.163,218
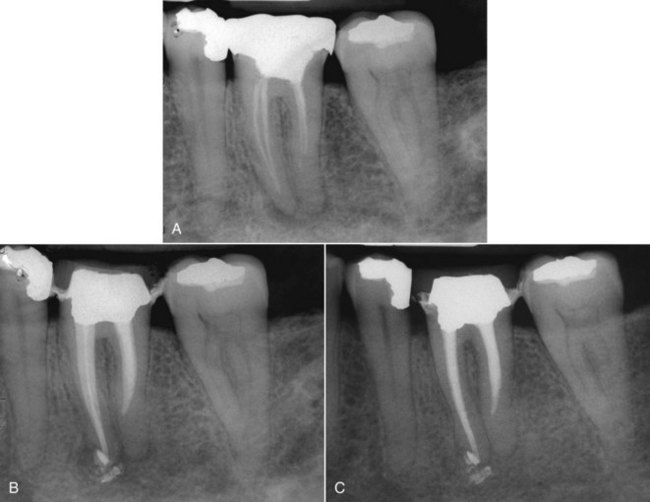
FIG. 25-70 A, Preoperative radiograph showing mesial canal blockages and potential distal root ledging with accompanying posttreatment disease. B, Final film showing mesial blockages bypassed but inability to negotiate beyond the distal ledge. The patient elected to pursue no further treatment at this time. C, One-year recall. Despite not achieving all the aims of conventional endodontic therapy, periradicular healing is apparent. The patient is asymptomatic and will now begin the final restoration with the knowledge that apical surgery may be needed in the future.
Finishing the Retreatment
After regaining the apical extent of the canal system, routine endodontic procedures are instituted to complete the retreatment. Any missed canals must be found using magnification, microexcavation techniques, and most importantly, the knowledge of canal anatomy that is discussed in another section of this text (Fig. 25-71). One cannot find a canal unless one suspects it is there. Cleaning and shaping procedures must focus on a crown-down approach to minimize extrusion of irritants into the periradicular tissues, and also must emphasize enlargement of the apical portion of the preparation to ensure complete removal of apical debris. These aims are best accomplished using technique hybridization during instrumentation, keeping the goals of the retreatment procedure in mind. These matters are covered in detail in Chapter 9.
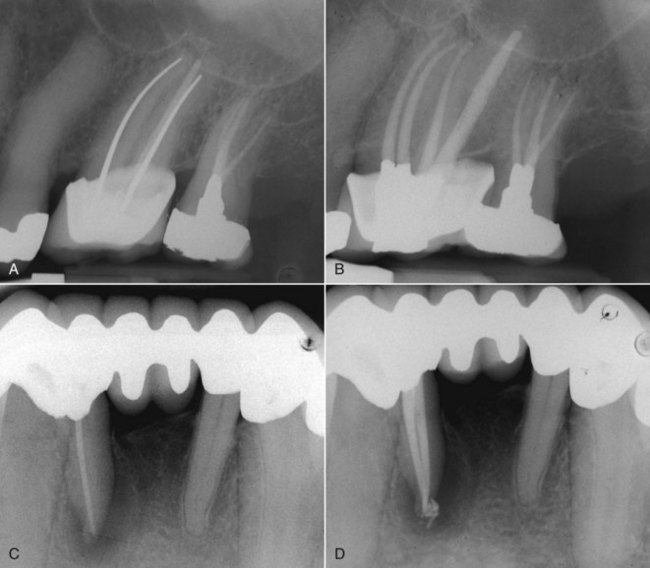
FIG. 25-71 Frequently missed canals that may result in posttreatment disease. A, Missed second mesiobuccal canal (MB2) in an upper molar. B, Final obturation showing cleaned, shaped, and filled MB2 canal. C, Missed lingual canal on a lower incisor results in posttreatment disease. D, Immediate postoperative film showing management of the missed canal.
Canal disinfection procedures are, however, paramount after the completion of cleaning and shaping. Since the primary cause of posttreatment disease is usually microbial,140,218 and these microbes (i.e., Enterococcus faecalis) are frequently resistant to traditional canal disinfection regimes,14 every effort must be made to eliminate these organisms from the canal system. This effort is complicated by the fact that no instrumentation regime can predictably remove the entire previous root filling from the canal space after retreatment.10 This leaves areas where microbes can reside underneath fragments of root filling materials and remain protected from standard antimicrobial canal irrigants such as sodium hypochlorite. Whether the canal space can be adequately disinfected when completing treatment in one visit or whether an interappointment medicament such as calcium hydroxide is needed is still a matter of debate, and the reader is directed to the appropriate chapter of this text for details of the problem. It is important to keep in mind, however, that teeth that require retreatment also require the highest level of disinfection possible to ensure the most favorable outcome.
Repair of Perforations
Occasionally, posttreatment endodontic disease will be the result of root perforation.96 Root perforations are created pathologically by resorption and caries and iatrogenically during root canal therapy (zip, strip, and furcation perforations) or its aftermath (e.g., post preparation perforation)160 (Fig. 25-72). When they are present, perforations may usually be found during the diagnostic phase as areas where the root filling materials, or possibly restorative materials such as posts, are found radiographically to have left the confines of the presumed canal space and approach or cross the radiographic interface between the dentin and the periodontal ligament. Angled radiographs are of paramount importance in determining whether a perforation exists and locating which surface or surfaces of the root have been perforated. This information is necessary when deciding upon treatment options. Frequently, cervical and occasionally midroot perforations are associated with epithelial downgrowth and subsequent periodontal defects, so thorough periodontal assessment is required128,182 (Fig. 25-73). If there were no evidence of posttreatment disease associated with the defect or tooth, then no treatment would be indicated. If, however, there is evidence of periradicular periodontitis, repair may be instituted in one of two ways, either nonsurgically by approaching the defect internally through the tooth, or surgically by using an external approach through the periradicular tissues.160 In general, if all other factors are considered equal, internal nonsurgical perforation repair will be the preferred method, since it is usually less invasive, produces less destruction of periradicular tissues via the surgical access wound needed, and isolation from microbes and disinfection is usually enhanced. If, however, the defect is readily accessible surgically, and disassembly of the existing restorations would impose an unacceptably high cost and long treatment time to the patient, surgical repair should be selected. If a longstanding defect has a periodontal lesion that has formed around it, surgery (perhaps with guided-tissue regeneration) will usually be needed. In most of these cases, nonsurgical retreatment and internal perforation repair prior to surgery will be beneficial to the treatment outcome. A multidisciplinary approach will be required, usually in consultation with the restorative dentist, a periodontist, and perhaps an orthodontist.163,229
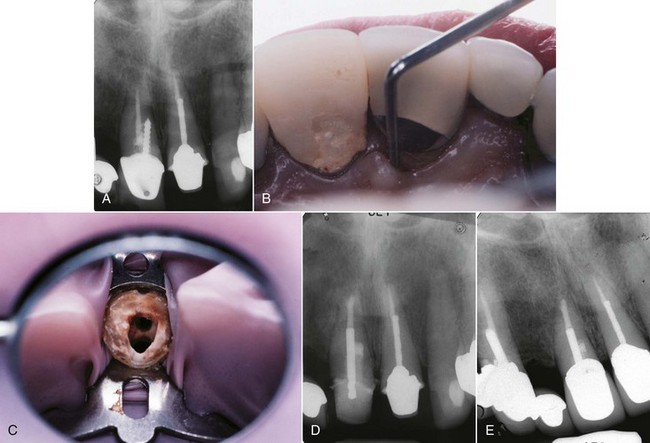
FIG. 25-73 A, Mesially angulated preoperative radiograph showing a palatally oriented post perforation in an upper incisor. B, An 8-mm narrow-based probing defect on the mesiopalatal corner of the tooth. C, Following coronal disassembly, the true canal can be seen lying in a facial direction relative to the palatal post preparation. D, The perforation was repaired with an external matrix of Colla-Cote and MTA. Subsequently, in conjunction with a periodontist, periodontal flap surgery was used to remove periodontal disease etiology from the longstanding pocket, and guided tissue regeneration procedures were instituted. E, Three-year reevaluation. The tooth is asymptomatic, and mesiopalatal probing depth is 4 mm.
Factors that affect the prognosis of perforation repair include location of perforation, time delay before perforation repair, ability to seal the defect, and previous contamination with microorganisms.112,182,188 Generally, the more apical the perforation site, the more favorable is the prognosis, but the converse is true for the repair procedure itself. The difficulty of the repair will be determined by the level at which the perforation occurred. If the defect is in the furcal floor of a multirooted tooth or in the coronal third of a straight canal (access perforation), it is considered easily accessible. If it is in the middle third of the canal (strip or post perforations), difficulty increases, and in the apical third of the canal (instrumentation errors), predictable repair is most challenging; frequently apical surgery will be needed.
Immediate repair is better than delayed repair, since delay can cause breakdown of the periodontium, resulting in endo-perio lesions that are difficult to manage,110,186 and elimination of microbial contamination of the defect and sealing it properly are critical to success. Many materials have been advocated for the repair of perforations in the past, but none provided predictable healing after treatment. Commonly used materials include amalgam, Super EBA cement (Bosworth, Skokie, IL), various bonded composite materials, and more recently, mineral trioxide aggregate (ProRoot MTA, DENTSPLY Tulsa Dental)163 (Fig. 25-74).
FIG. 25-74 ProRoot MTA is a medical grade of Portland cement that has had the arsenic removed so it can be used in the human body. It is the material of choice for endodontic perforation repair.
(Courtesy DENTSPLY Tulsa Dental, Tulsa, OK.)
Since the recent introduction of MTA for perforation repair, the choice of which repair material to use is more clear.128,160 MTA has many advantages over other restoratives when being used for perforation repair. This material seals well144,239 even when the cavity preparation is contaminated with blood.214 It is very biocompatible,149,155,215,217 rarely eliciting any response from the periradicular tissues, and a cementum-like material has been consistently shown to grow directly on the material after placement.85,155 MTA has also been shown to have a high degree of clinically favorable long-term outcomes when used as a perforation repair material.128,160 The main disadvantage of MTA is the long time required for setting216 that makes this material inappropriate for transgingival defects such as those associated with cervical resorption. If the material is in contact with oral fluids, it will wash out of the defect prior to setting, so a more rapid-setting resin ionomer such as Geristore (Den-Mat, Santa Maria, CA) is recommended for lesions that cross the gingival margin15,42,173 (Fig. 25-75). MTA is available in the original gray-colored formulation and a newer, more esthetic off-white color for treatments in the esthetic zone of the mouth, although there is little research on the differences between the two formulations. Their sealing ability seems comparable,51 but questions remain as to whether white MTA exhibits the same biocompatibility153 and will have the same long-term success as the older variety.
FIG. 25-75 Geristore Kit (Den-Mat, Santa Maria, CA). This and other resin ionomers have been advocated for cervical perforation repair, owing to good biocompatibility and the shorter, more controlled setting times that make them useful for transgingival root fillings.
If the perforation is to be repaired nonsurgically through the tooth, coronal-radicular access to the defect is prepared as stated previously (Fig. 25-76). First, the root canals are located and preliminarily instrumented to create enough coronal shape to allow them to be protected from blockage by the repair material. The defect is cleaned and sometimes enlarged with the use of ultrasonics or appropriate rotary drills such as the Gates-Glidden to remove any potentially contaminated dentin surrounding the perforation. Use of a disinfectant irrigating solution such as sodium hypochlorite should be considered if the perforation is not so large as to allow the irrigant to significantly damage the periradicular tissues. If the perforation is large, sterile saline should be used as an irrigant, and disinfection of the margins of the defect is performed using mechanical dentin removal. Investigators8 have advocated the use of copious flushing of the defect with 2.5% hypochlorite, but in light of the potential severe complications of hypochlorite overextension through a perforation,63 extreme care should be used. After the defect has been cleaned, vigorous bleeding may result. Hemostasis should be undertaken using collagen (Colla-Cote, Integra Life Sciences, Plainsboro, NJ) (see Fig. 25-76, B), calcium sulfate (Capset, Lifecore Biomedical, Chaska, MN), or calcium hydroxide163; however, astringents such as ferric sulfate should be avoided, since the coagulum they leave behind may promote bacterial growth and may compromise the seal of the repair.117
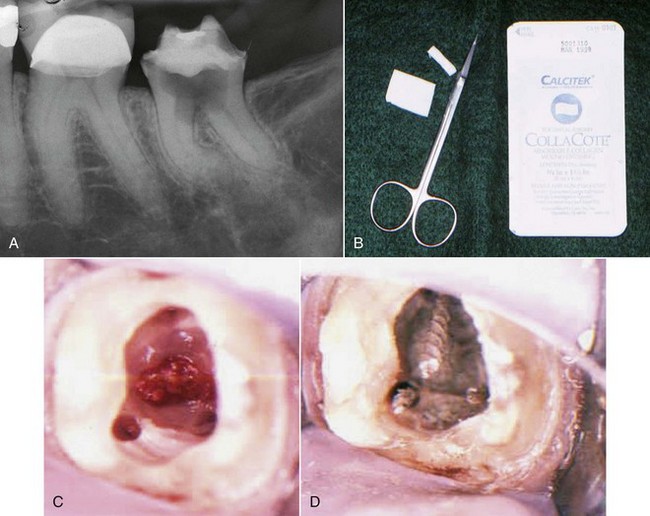
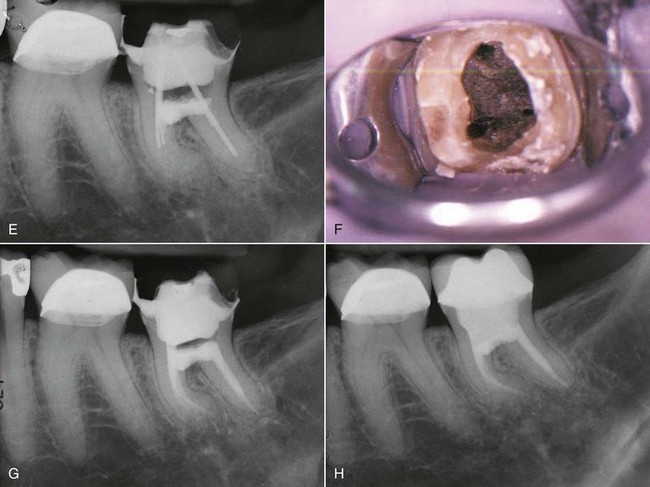
FIG. 25-76 A, Large furcal perforation created during an attempt at endodontic access. B, Colla-Cote (Integra Life Sciences, Plainsboro, NJ) to be used as an external matrix material to recreate the external root contour. C, Canals have been found, preliminarily instrumented, and the external matrix has been placed. D, Canals are protected from being blocked using large endodontic files cut off above the orifice. MTA has been placed into the defect. E, Radiograph showing the initial repair with MTA recreating the vault of the furcation. F, On the second appointment, the blocking files are removed with difficulty, since the MTA has flowed into the flutes and set. At this time, endodontic therapy is completed as normal. G, Postobturation radiograph. Note the radiolucency in the furcal vault, which represents the Colla-Cote external matrix material. H, Nineteen-month reevaluation. The patient is asymptomatic, and there is evidence of healing in the furcal vault area.
When the bleeding has been controlled, some easily removable material should be placed over the entrances to the deeper portion of the canals to prevent the repair material from blocking reaccess to the apical terminus. The canals may be protected with cotton, gutta-percha cones, paper points, or shredded collagen. The use of severed files is not recommended, because removal of the files after placement of the repair material is very difficult, since the material tends to lock into the instrument’s flutes (see Fig. 25-76, F). After protecting the canals, the perforation site is inspected to determine whether an external matrix is needed to ensure a proper contour of the restoration.116 If the surrounding bone is closely adapted to the defect margins, minimal to no matrix material will be necessary. If the perforation is associated with a large osseous defect, this must be filled with an external matrix to minimize overcontouring of the repair restoration. The matrix material should be a biocompatible, usually absorbable material such as collagen, freeze-dried demineralized bone allograft (FDDB), hydroxylapatite, gelfoam, or calcium sulfate.160,163 Care must be exercised so that the external matrix material is not condensed so forcefully that it damages adjacent vital structures, such as the mental nerve or the floor of the sinus.
Following preparation of the defect, the repair material is placed. It may be carried in a small syringe or amalgam carrier, and it is condensed with pluggers or microspatulas. In the case of MTA in an accessible defect, the butt end of paper points make an excellent compactor, since they can wick some of the water out of the material, giving it a firmer consistency and aiding compaction. When the MTA has been placed, a moist cotton pellet is placed over it to hydrate the material, and the tooth is sealed to allow the MTA to set. Upon reentry, the material should be set hard and well retained in the perforation site192 (see Fig. 25-76, F). If there is an overextension of the material beyond the normal external root contour, it seems not to affect the prognosis of the repair.8,160
If the perforation is deeper in the canal, the objectives and principles of repair as just outlined all apply, except that access to the defect is more complicated (Fig. 25-77). Protecting the canal from blockage is somewhat more difficult, and placement of the repair material requires the enhanced vision provided by the surgical operating microscope. Ideally, the canal should be fully shaped prior to the repair attempt,163 and a canal patency protector should be placed apical to the defect. In some of these instances, protecting the canal can be accomplished by using a severed file, notwithstanding the previous warning, since not only can it protect the canal from blockage, it can also be used as an indirect carrier for transmitting ultrasonic energy to the MTA, causing it to “slump” into the defect when direct compaction is impossible. The file is placed into the canal to a level well below the defect, and the MTA is carried to place. Once compaction has been performed as well as possible, the coronal extent of the file is touched with an ultrasonic tip to vibrate the MTA into the defect. After this is accomplished, the file must be vigorously instrumented in a short, 1- to 2-mm amplitude push/pull motion to free it from the placed MTA so that it can be easily removed after the material is set163 (Fig. 25-78). There is some evidence that ultrasonic placement of MTA may enhance the seal against bacteria in an apexification model,113 although other researchers have not agreed with this conclusion and found poorer canal wall adaptation when filling the apical extent of canals using ultrasonic compaction compared with hand filling.6 Further study of ultrasonic MTA placement is warranted, but clinical observation suggests that this method has merit.
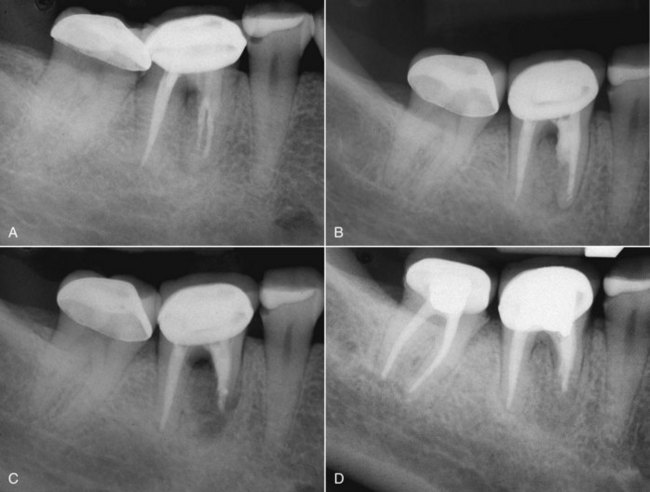
FIG. 25-77 Midroot-level perforation repair. A, Preoperative film showing mesial strip perforation with bone loss. B, Nonsurgical internal repair with inability to negotiate canals to the apical terminus and MTA overextension into the furca. C, Apical and perforation repair surgery performed. D, One-year follow-up showing complete healing.
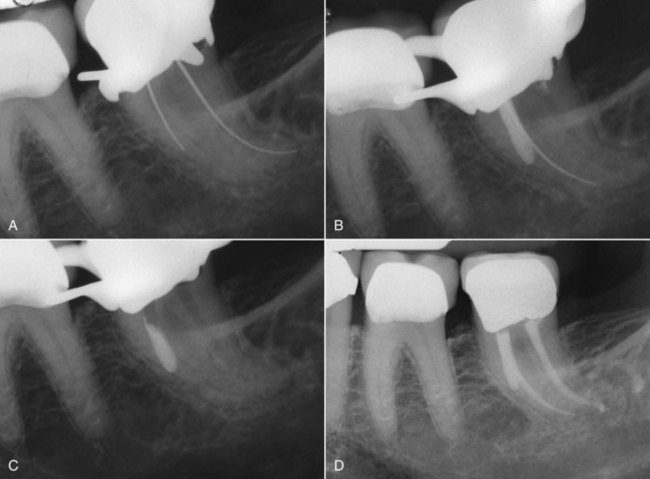
FIG. 25-78 A, This patient was in extreme pain following initial endodontic instrumentation by her dentist. Midroot-level perforation found on access. B, The original canal was found and protected with an endodontic file. MTA was vibrated using ultrasonic energy applied to the file, and it flowed into the defect. Then the file is moved in a push/pull manner to dislodge it from the MTA prior to closure. Note that the defect was intentionally enlarged to allow for more predictable application of the MTA. C, On the second appointment, the file is withdrawn easily, since it was detached from the MTA repair material. Now the endodontic therapy is concluded as normal. The patient has been asymptomatic since the end of the first appointment. D, Follow-up at 27 months, showing complete healing.
If the perforation is in the apical portion of the canal, it is usually due to a procedural accident during instrumentation of a curved canal and is invariably accompanied by a block or ledge. This type of perforation is the most difficult to repair, since repair not only involves cleaning and sealing the defect but also finding, cleaning, and filling the apical canal segment. All of the aforementioned techniques for managing blocks and ledges are required to find and clean the apical canal segment. When this has been accomplished, the decision is made as to whether the canal should be filled with MTA or with gutta-percha and sealer. The MTA is undoubtedly more effective in sealing the canal (especially if it cannot be dried) and is much more biocompatible, but carrying it predictably to the apical extent of a curved canal is problematic. If a holding file is placed in the apical canal segment to anticipate eventual gutta-percha placement after the MTA repair material is set, the presence of the file precludes consistent extension of the MTA into the apical end of the defect, even when ultrasonically vibrated. If a holding file is not placed, the MTA will also flow into the prepared apical segment, which may not be completely three-dimensionally obturated. Generally, whichever choice is made, the outcome will be unpredictable, so the patient must be advised that regular reevaluation is necessary, and apical surgery or extraction may ultimately be needed.
Prognosis of Retreatment
When the proper diagnosis has been made and all of the technical aspects of retreatment are carefully performed, orthograde retreatment can be highly successful (Fig. 25-79). The prognosis depends to a large extent on whether apical periodontitis exists prior to retreatment.146 In a recent systematic review of outcomes studies,55 investigators report that in the absence of prior apical periodontitis, the incidence of healed cases after both initial treatment and orthograde retreatment ranges from 92% to 98% up to 10 years after treatment. When prior apical periodontitis is present, the incidence of healing drops to 74% to 86%, regardless of whether initial treatment or orthograde retreatment was performed. The authors state that this “similar potential to heal after initial treatment and orthograde retreatment challenges the historic perception of the latter having a poorer prognosis than the former.”55
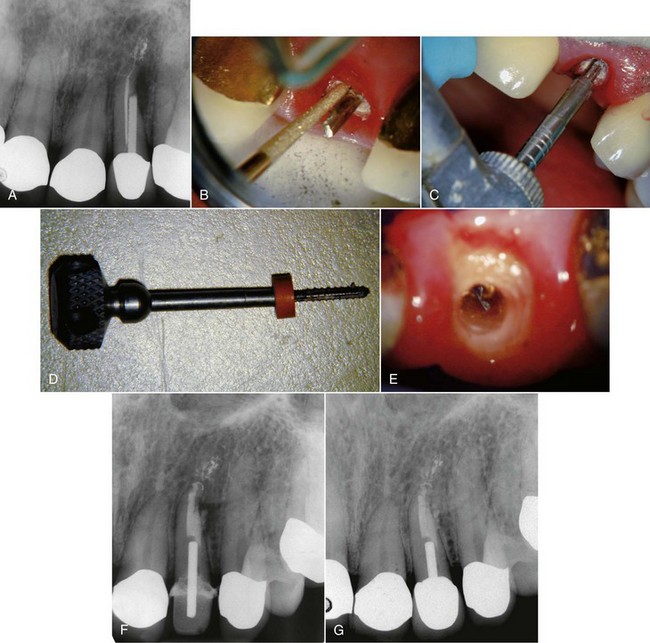
FIG. 25-79 A, Preoperative radiograph showing midroot-level post perforation and associated periradicular periodontitis. B, The crown was removed, and ultrasonic energy was applied to the post. C, Using the trephine bur from the Ruddle Post Removal kit to mill the endo of the post. D, The screw post is removed using the wrench from the Ruddle kit. E, After removal of the post and gutta-percha, a plastic solid core carrier is found in the canal and removed using the techniques described in this chapter. F, Postoperative radiograph showing MTA perforation repair, canal seal with gutta-percha, and post and core fabrication. G, Thirteen-month recall showing healing around the perforation repair site.
Unfortunately, these numbers mean that the desired outcome will not occur in potentially a fourth of retreatment cases. Many techniques and devices for endodontic retreatment have been mentioned here to aid the clinician. However, none of this will guarantee success. Even when strict endodontic principles and fundamentals are followed, the result may be persistent posttreatment disease. When healing does not occur, the clinician is faced with the decision of what to do next. The choice is between four treatment options: observation, endodontic surgery, extraction-replantation, or extraction.
Many times a tooth that has persistent apical periodontitis may remain in asymptomatic function for an extended period of time, a state that has been referred to as functional retention of the tooth.55 If the patient’s goal of treatment is not necessarily complete healing of the tooth but simply to retain it in function and without pain, then regular evaluation by the clinician is warranted. If signs and symptoms of worsening infection (e.g., progressive enlargement of a periapical radiolucency, pain, periodontal pocket formation, or sinus tract eruption) occur, then further treatment may be needed. However, many teeth classified early on as uncertain healing may indeed be retained for many years.134
Endodontic surgery (Fig. 25-80) is a very predictable procedure55,76 that can be performed on most teeth, but there are some anatomic and medical concerns regarding treatment planning for this procedure which are covered in detail in Chapter 21. Extraction-replantation (Fig. 25-81), also referred to as intentional replantation,147 is another treatment option. This involves extraction of the tooth and performing the apicoectomy and root-end filling while the tooth is out of the patient’s mouth, followed by replantation and splinting if indicated. This procedure is also discussed in detail in another chapter. Extraction and replacement should be the treatment of last resort, to be selected only when the tooth has been shown to be irreparable. If the decision is made to extract the tooth, usually replacement will be necessary to prevent shifting of the dentition with its attendant problems. Replacement can be with an implant, a fixed partial denture, or a removable partial denture.
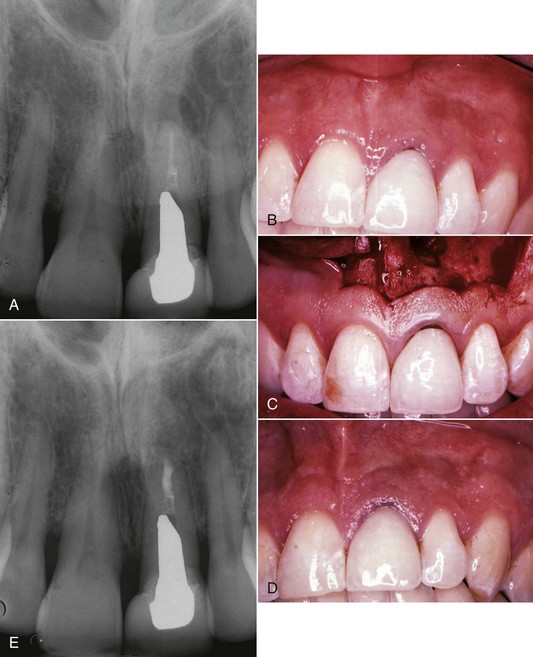
FIG. 25-80 A-B, Preoperative images showing posttreatment disease in the upper left central incisor and a large custom cast post. The patient elected to leave the post and perform surgery rather than risk damage to his new crown. C, Submarginal rectangular flap design. D, Three-week follow-up showing excellent soft-tissue healing. E, Eighteen-month follow-up showing excellent healing of the periradicular tissues.
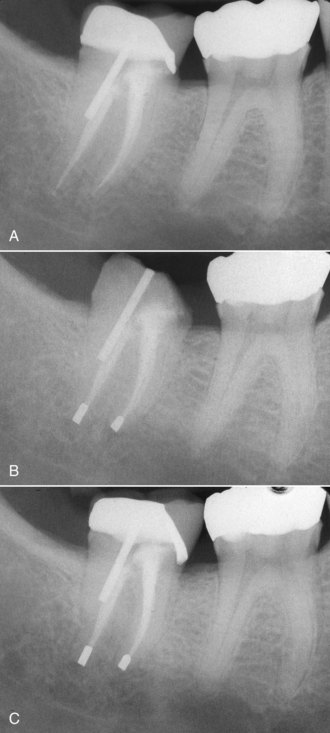
FIG. 25-81 A, This lower second molar remained symptomatic despite nonsurgical retreatment. Surgery was precluded by the poor access and proximity of the inferior alveolar canal. B, Intentional replantation was performed using amalgam alloy retrograde fillings. Better materials such as MTA or Super-EBA are available now. C, Fifteen-month reevaluation showing apical healing. The patient was asymptomatic.
Summary
Posttreatment endodontic disease does not preclude saving the involved tooth. In fact, the vast majority of these teeth can be returned to health and long-term function by current retreatment procedures. In most instances, the retreatment option provides the greatest advantage to the patient, since there is no replacement that functions as well as a natural tooth. Armed with the information in the preceding section, appropriate armamentaria, and the desire to do what is best for the patient, the clinician will provide the foundation for long-term restorative success.
1. Abbott PV. Incidence of root fractures and methods used for post removal. Int Endod J. 2002;35:63.
2. ADA Council on Scientific Affairs. Dental endosseous implants: an update. J Am Dent Assoc. 2004;135:92.
3. Allard U, Andersson L. Exposure of dental personnel to chloroform in root-filling procedures. Endod Dent Traumatol. 1992;8:155.
4. Altshul JH, Marshall G, Morgan LA, Baumgartner JC. Comparison of dentinal crack incidence and of post removal time resulting from post removal by ultrasonic or mechanical force. J Endod. 1997;23:683.
5. American Association of Endodontists. Guide to clinical endodontics. Chicago: American Association of Endodontists; 2004.
6. Aminoshariae A, Hartwell GR, Moon PC. Placement of mineral trioxide aggregate using two different techniques. J Endod. 2003;29:679.
7. Amsterdam M. Periodontal prosthesis. Twenty-five years in retrospect. Alpha Omegan. 1974;67:8.
8. Arens DE, Torabinejad M. Repair of furcal perforations with mineral trioxide aggregate: two case reports. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:84.
9. Atrizadeh F, Kennedy J, Zander H. Ankylosis of teeth following thermal injury. J Periodont Res. 1971;6:159.
10. Baldassari-Cruz LA, Wilcox LR. Effectiveness of gutta-percha removal with and without the microscope. J Endod. 1999;25:627.
11. Baratto Filho F, Ferreira EL, Fariniuk LF. Efficiency of the 0.04 taper ProFile during the re-treatment of gutta-percha-filled root canals. Int Endod J. 2002;35:651.
12. Barbosa SV, Burkard DH, Spångberg LS. Cytotoxic effects of gutta-percha solvents. J Endod. 1994;20:6.
13. Barrieshi-Nusair KM. Gutta-percha retreatment: effectiveness of nickel-titanium rotary instruments versus stainless steel hand files. J Endod. 2002;28:454.
14. Basrani B, Tjaderhane L, Santos JM, et al. Efficacy of chlorhexidine- and calcium hydroxide-containing medicaments against Enterococcus faecalis in vitro. Oral Surg. 2003;96:618.
15. Behnia A, Strassler HE, Campbell R. Repairing iatrogenic root perforations. J Am Dent Assoc. 2000;131:196.
16. Berbert A, Filho MT, Ueno AH, Bramante CM, Ishikiriama A. The influence of ultrasound in removing intraradicular posts. Int Endod J. 1995;28:100.
17. Bergeron BE, Murchison DF, Schindler WG, Walker WA3rd. Effect of ultrasonic vibration and various sealer and cement combinations on titanium post removal. J Endod. 2001;27:13.
18. Bertrand MF, Pellegrino JC, Rocca JP, Klinghofer A, Bolla M. Removal of Thermafil root canal filling material. J Endod. 1997;23:54.
19. Betti LV, Bramante CM. Quantec SC rotary instruments versus hand files for gutta-percha removal in root canal retreatment. Int Endod J. 2001;34:514.
20. Bhaskar SN. Periapical lesion—types, incidence, and clinical features. Oral Surg. 1966;21:657.
21. Bhaskar SN, Rappaport HM. Histologic evaluation of endodontic procedures in dogs. Oral Surg Oral Med Oral Pathol. 1971;31:526.
22. Block RM, Lewis RD, Hirsch J, Coffey J, Langeland K. Systemic distribution of N2 paste containing 14C paraformaldehyde following root canal therapy in dogs. Oral Surg Oral Med Oral Pathol. 1980;50:350.
23. Brady JM, del Rio CE. Corrosion of endodontic silver cones in humans: a scanning electron microscope and x-ray microprobe study. J Endod. 1975;1:205.
24. Bramante CM, Betti LV. Efficacy of Quantec rotary instruments for gutta-percha removal. Int Endod J. 2000;33:463.
25. Brodin P. Neurotoxic and analgesic effects of root canal cements and pulp-protecting dental materials. Endod Dent Traumatol. 1988;4:1.
26. Brown LJ, Nash KD, Johns BA, Warren M. The Economics of Endodontics. Chicago: American Association of Endodontists; 2003.
27. Budd JC, Gekelman D, White JM. Temperature rise of the post and on the root surface during ultrasonic post removal. Int Endod J. 2005;38:705.
28. Buhler H. Evaluation of root-resected teeth. Results after 10 years. J Periodontol. 1988;59:805.
29. Buoncristiani J, Seto BG, Caputo AA. Evaluation of ultrasonic and sonic instruments for intraradicular post removal. J Endod. 1994;20:486.
30. Bystrom A, Claesson R, Sundqvist G. The antibacterial effect of camphorated paramonochlorophenol, camphorated phenol and calcium hydroxide in the treatment of infected root canals. Endod Dent Traumatol. 1985;1:170.
31. Castrisos T, Abbott PV. A survey of methods used for post removal in specialist endodontic practice. Int Endod J. 2002;35:172.
32. Castrisos TV, Palamara JE, Abbott PV. Measurement of strain on tooth roots during post removal with the Eggler post remover. Int Endod J. 2002;35:337.
33. Chenail BL, Teplitsky PE. Orthograde ultrasonic retrieval of root canal obstructions. J Endod. 1987;13:186.
34. Chugal NM, Clive JM, Spangberg LS. Endodontic infection: some biologic and treatment factors associated with outcome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:81.
35. Chutich MJ, Kaminski EJ, Miller DA, Lautenschlager EP. Risk assessment of the toxicity of solvents of gutta-percha used in endodontic retreatment. J Endod. 1998;24:213.
36. Cohen S, Schwartz S. Endodontic complications and the law. J Endod. 1987;13:191.
37. Crump MC, Natkin E. Relationship of broken root canal instruments to endodontic case prognosis: a clinical investigation. J Am Dent Assoc. 1970;80:1341.
38. Cunha RS, De Martin AS, Barros PP, et al. In vitro evaluation of the cleansing working time and analysis of the amount of gutta-percha or Resilon remnants in the root canal walls after instrumentation for endodontic retreatment. J Endod. 2007;33:1426.
39. de Oliveira DP, Barbizam JV, Trope M, Teixeira FB. Comparison between gutta-percha and resilon removal using two different techniques in endodontic retreatment. J Endod. 2006;32:362.
40. de Rijk WG. Removal of fiber posts from endodontically treated teeth. Am J Dent. 2000;13:19B.
41. Dominici JT, Clark S, Scheetz J, Eleazer PD. Analysis of heat generation using ultrasonic vibration for post removal. J Endod. 2005;31:301.
42. Dragoo MR. Resin-ionomer and hybrid-ionomer cements: part II, human clinical and histologic wound healing responses in specific periodontal lesions. Int J Periodont Restorative Dent. 1997;17:75.
43. Erdemir A, Eldeniz AU, Belli S. Effect of gutta-percha solvents on mineral contents of human root dentin using ICP-AES technique. J Endod. 2004;30:54.
44. Erdemir A, Eldeniz AU, Belli S, Pashley DH. Effects of solvents on bonding to root canal dentin [abstract]. J Dent Res (Spec Iss A). 2002;81:241.
45. Eriksson AR, Albrektsson T. Temperature threshold levels for heat-induced bone tissue injury: a vital-microscopic study in the rabbit. J Prosthet Dent. 1983;50:101.
46. Ettrich CA, Labossiere PE, Pitts DL, Johnson JD. An investigation of the heat induced during ultrasonic post removal. J Endod. 2007;33:1222.
47. Ezzie E, Fleury A, Solomon E, Spears R, He J. Efficacy of retreatment techniques for a resin-based root canal obturation material. J Endod. 2006;32:341.
48. Farzaneh M, Abitbol S, Friedman S. Treatment outcome in endodontics: the Toronto Study. Phases I and II: orthograde retreatment. J Endod. 2004;30:627.
49. Fava LR, Dummer PM. Periapical radiographic techniques during endodontic diagnosis and treatment. Int Endod J. 1997;30:250.
50. Ferreira JJ, Rhodes JS, Ford TR. The efficacy of gutta-percha removal using ProFiles. Int Endod J. 2001;34:267.
51. Ferris DM, Baumgartner JC. Perforation repair comparing two types of mineral trioxide aggregate. J Endod. 2004;30:422.
52. Fors UG, Berg JO. Endodontic treatment of root canals obstructed by foreign objects. Int Endod J. 1986;19:2.
53. Frajlich SR, Goldberg F, Massone EJ, Cantarini C, Artaza LP. Comparative study of retreatment of Thermafil and lateral condensation endodontic fillings. Int Endod J. 1998;31:354.
54. Friedman S, Abitbol S, Lawrence HP. Treatment outcome in endodontics: the Toronto Study. Phase 1: initial treatment. J Endod. 2003;29:787.
55. Friedman S, Mor C. The success of endodontic therapy—healing and functionality. Calif Dent Assoc J. 2004;32:493.
56. Friedman S, Moshonov J, Trope M. Efficacy of removing glass ionomer cement, zinc oxide eugenol, and epoxy resin sealers from retreated root canals. Oral Surg Oral Med Oral Pathol. 1992;73:609.
57. Friedman S, Stabholz A. Endodontic retreatment—case selection and technique. Part 1: criteria for case selection. J Endod. 1986;12:28.
58. Friedman S, Stabholz A, Tamse A. Endodontic retreatment—case selection and technique. 3. Retreatment techniques. J Endod. 1990;16:543.
59. Fristad I, Molven O, Halse A. Nonsurgically retreated root-filled teeth—radiographic findings after 20-27 years. Int Endod J. 2004;37:12.
60. Fukushima H, Yamamoto K, Hirohata K, Sagawa H, Leung KP, Walker CB. Localization and identification of root canal bacteria in clinically asymptomatic periapical pathosis. Journal of Endodontics. 1990;16:534.
61. Gambrel MG, Hartwell GR, Moon PC, Cardon JW. The effect of endodontic solutions on resorcinol-formalin paste in teeth. J Endod. 2005;31:25.
62. Garrido ADB, Fonseca TS, Alfredo E, Silva-Sousa YTC, Sousa-Neto MD. Influence of ultrasound, with and without water spray cooling, on removal of posts cemented with resin or zinc phosphate cements. J Endod. 2004;30:173.
63. Gernhardt CR, Eppendorf K, Kozlowski A, Brandt M. Toxicity of concentrated sodium hypochlorite used as an endodontic irrigant. Int Endod J. 2004;37:272.
64. Gesi A, Magnolfi S, Goracci C, Ferrari M. Comparison of two techniques for removing fiber posts. J Endod. 2003;29:580.
65. Gettleman BH, Spriggs KA, ElDeeb ME, Messer HH. Removal of canal obstructions with the Endo Extractor. J Endod. 1991;17:608.
66. Giuliani V, Cocchetti R, Pagavino G. Efficacy of ProTaper universal retreatment files in removing filling materials during root canal retreatment. J Endod. 2008;34:1381.
67. Glick DH, Frank AL. Removal of silver points and fractured posts by ultrasonics. J Prosthet Dent. 1986;55:212.
68. Gluskin AH, Ruddle CJ, Zinman EJ. Thermal injury through intraradicular heat transfer using ultrasonic devices: precautions and practical preventive strategies. J Am Dent Assoc. 2005;136:1286.
69. Goldberg RA, Kuttler S, Dorn SO. The properties of Endocal 10 and its potential impact on the structural integrity of the root. J Endod. 2004;30:159.
70. Gomes AP, Kubo CH, Santos RA, Santos DR, Padilha RQ. The influence of ultrasound on the retention of cast posts cemented with different agents. Int Endod J. 2001;34:93.
71. Goodacre CJ, Bernal G, Rungcharassaeng K, Kan JY. Clinical complications with implants and implant prostheses. J Prosthet Dent. 2003;90:121.
72. Goodacre CJ, Kan JY, Rungcharassaeng K. Clinical complications of osseointegrated implants. J Prosthet Dent. 1999;81:537.
73. Gound TG, Marx D, Schwandt NA. Incidence of flare-ups and evaluation of quality after retreatment of resorcinol-formaldehyde resin (‘Russian Red Cement’) endodontic therapy. J Endod. 2003;29:624.
74. Gutierrez JH, Villena F, Gigoux C, Mujica F. Microscope and scanning electron microscope examination of silver points corrosion caused by endodontic materials. J Endod. 1982;8:301.
75. Gutmann JL, Dumsha TC, Lovdahl PE, editors. Problem solving in endodontics: prevention, identification, and management, ed 4, St. Louis: Mosby, 2006.
76. Gutmann JL, Harrison JW. Surgical endodontics, 2nd ed. St. Louis: Ishiyaku EuroAmerica; 1994.
77. Hammad M, Qualtrough A, Silikas N. Three-dimensional evaluation of effectiveness of hand and rotary instrumentation for retreatment of canals filled with different materials. J Endod. 2008;34:1370.
78. Hansen MG. Relative efficiency of solvents used in endodontics. J Endod. 1998;24:38.
79. Hargreaves K. Seltzer and Bender’s the dental pulp, ed 2. Chicago: Quintessence; 2010.
80. Haselton DR, Lloyd PM, Johnson WT. A comparison of the effects of two burs on endodontic access in all-ceramic high lucite crowns. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:486.
81. Hashem AA. Ultrasonic vibration: temperature rise on external root surface during broken instrument removal. J Endod. 2007;33:1070.
82. Hassanloo A, Watson P, Finer Y, Friedman S. Retreatment efficacy of the Epiphany soft resin obturation system. Int Endod J. 2007;40:633.
83. Hauman CHJ, Chandler NP, Purton DG. Factors influencing the removal of posts. Int Endod J. 2003;36:687.
84. Holland R, De Souza V, Nery MJ, de Mello W, Bernabe PF, Otoboni Filho JA. Tissue reactions following apical plugging of the root canal with infected dentin chips. A histologic study in dogs’ teeth. Oral Surg Oral Med Oral Pathol. 1980;49:366.
85. Holland R, Filho JA, de Souza V, Nery MJ, Bernabe PF, Junior ED. Mineral trioxide aggregate repair of lateral root perforations. J Endod. 2001;27:281.
86. Horan BB, Tordik PA, Imamura G, Goodell GG. Effect of dentin thickness on root surface temperature of teeth undergoing ultrasonic removal of posts. J Endod. 2008;34:453.
87. Hulsmann M. Removal of fractured instruments using a combined automated/ultrasonic technique. J Endod. 1994;20:144.
88. Hulsmann M, Bluhm V. Efficacy, cleaning ability and safety of different rotary NiTi instruments in root canal retreatment. Int Endod J. 2004;37:468.
89. Hulsmann M, Stotz S. Efficacy, cleaning ability and safety of different devices for gutta- percha removal in root canal retreatment. Int Endod J. 1997;30:227.
90. Hunter KR, Doblecki W, Pelleu GBJr. Halothane and eucalyptol as alternatives to chloroform for softening gutta-percha. J Endod. 1991;17:310.
91. Huttula AS, Tordik PA, Imamura G, Eichmiller FC, McClanahan SB. The effect of ultrasonic post instrumentation on root surface temperature. J Endod. 2006;32:1085.
92. Ibarrola JL, Knowles KI, Ludlow MO. Retrievability of Thermafil plastic cores using organic solvents. J Endod. 1993;19:417.
93. Imura N, Kato AS, Hata GI, Uemura M, Toda T, Weine F. A comparison of the relative efficacies of four hand and rotary instrumentation techniques during endodontic retreatment. Int Endod J. 2000;33:361.
94. Imura N, Zuolo ML, Ferreira MO, Novo NF. Effectiveness of the Canal Finder and hand instrumentation in removal of gutta-percha root fillings during root canal retreatment. Int Endod J. 1996;29:382.
95. Imura N, Zuolo ML, Kherlakian D. Comparison of endodontic retreatment of laterally condensed gutta-percha and Thermafil with plastic carriers. J Endod. 1993;19:609.
96. Ingle JI, Bakland LK, Baumgartner JC, editors. Endodontics, ed 6, New York: BC Decker, 2008.
97. Iqbal MK, Johansson AA, Akeel RF, Bergenholtz A, Omar R. A retrospective analysis of factors associated with the periapical status of restored, endodontically treated teeth. Int J Prosthodont. 2003;16:31.
98. Iqbal MK, Kohli MR, Kim JS. A retrospective clinical study of incidence of root canal instrument separation in an endodontics graduate program: a PennEndo database study. J Endod. 2006;32:1048.
99. Iqbal MK, Rafailov H, Kratchman SI, Karabucak B. A comparison of three methods for preparing centered platforms around separated instruments in curved canals. J Endod. 2006;32:48.
100. Jafarzadeh H, Abbott PV. Ledge formation: review of a great challenge in endodontics. J Endod. 2007;33:1155.
101. Jeng HW, ElDeeb ME. Removal of hard paste fillings from the root canal by ultrasonic instrumentation [published erratum appears in J Endod 1987 Dec;13(12):565]. J Endod. 1987;13:295.
102. Jew RC, Weine FS, Keene JJJr, Smulson MH. A histologic evaluation of periodontal tissues adjacent to root perforations filled with Cavit. Oral Surg Oral Med Oral Pathol. 1982;54:124.
103. Johnson WT, Leary JM, Boyer DB. Effect of ultrasonic vibration on post removal in extracted human premolar teeth. J Endod. 1996;22:487.
104. Kaplowitz GJ. Using rectified turpentine oil in endodontic retreatment. J Endod. 1996;22:621.
105. Kaufman D, Mor C, Stabholz A, Rotstein I. Effect of gutta-percha solvents on calcium and phosphorus levels of cut human dentin. J Endod. 1997;23:614.
106. Kim E, Lee SJ. Electronic apex locator. Dent Clin North Am. 2004;48:35.
107. Koch K. The microscope. Its effect on your practice. Dent Clin North Am. 1997;41:619.
108. Koppang HS, Koppang R, Solheim T, Aarnes H, Stolen SO. Cellulose fibers from endodontic paper points as an etiological factor in postendodontic periapical granulomas and cysts. J Endod. 1989;15:369.
109. Krell KV, Neo J. The use of ultrasonic endodontic instrumentation in the re-treatment of a paste-filled endodontic tooth. Oral Surg Oral Med Oral Pathol. 1985;60:100.
110. Ladley RW, Campbell AD, Hicks ML, Li SH. Effectiveness of halothane used with ultrasonic or hand instrumentation to remove gutta-percha from the root canal. J Endod. 1991;17:221.
111. Langer B, Stein SD, Wagenberg B. An evaluation of root resections. A ten-year study. J Periodontol. 1981;52:719.
112. Lantz B, Persson PA. Periodontal tissue reactions after root perforations in dog’s teeth. A histologic study. Odontol Tidskr. 1967;75:209.
113. Lawley GR, Schindler WG, Walker WA, Kolodrubetz D. Evaluation of ultrasonically placed MTA and fracture resistance with intracanal composite resin in a model of apexification. J Endod. 2004;30:167.
114. Lazarski MP, Walker WA3rd, Flores CM, Schindler WG, Hargreaves KM. Epidemiological evaluation of the outcomes of nonsurgical root canal treatment in a large cohort of insured dental patients. J Endod. 2001;27:791.
115. Lee FS, Van Cura JE, BeGole E. A comparison of root surface temperatures using different obturation heat sources. J Endod. 1998;24:617.
116. Lemon RR. Nonsurgical repair of perforation defects. Internal matrix concept. Dent Clin North Am. 1992;36:439.
117. Lemon RR, Steele PJ, Jeansonne BG. Ferric sulfate hemostasis: effect on osseous wound healing. Left in situ for maximum exposure. J Endod. 1993;19:170.
118. Leonard JE, Gutmann JL, Guo IY. Apical and coronal seal of roots obturated with a dentine bonding agent and resin. Int Endod J. 1996;29:76.
119. Lin LM, Skribner JE, Gaengler P. Factors associated with endodontic treatment failures. J Endod. 1992;18:625.
120. Lindemann M, Yaman P, Dennison JB, Herrero AA. Comparison of the efficiency and effectiveness of various techniques for removal of fiber posts. J Endod. 2005;31:520.
121. Lipski M, Wozniak K. In vitro infrared thermographic assessment of root surface temperature rises during Thermafil retreatment using system B. J Endod. 2003;29:413.
122. Lovdahl PE. Endodontic retreatment. Dent Clin North Am. 1992;36:473.
123. Luks S. Gutta percha vs. silver points in the practice of endodontics. N Y State Dent J. 1965;31:341.
124. Maalouf EM, Gutmann JL. Biological perspectives on the non-surgical endodontic management of periradicular pathosis. Int Endod J. 1994;27:154.
125. Machtou P, Sarfati P, Cohen AG. Post removal prior to retreatment. J Endod. 1989;15:552.
126. Madarati AA, Qualtrough AJ, Watts DC. Factors affecting temperature rise on the external root surface during ultrasonic retrieval of intracanal separated files. J Endod. 2008;34:1089.
127. Madison S, Swanson K, Chiles SA. An evaluation of coronal microleakage in endodontically treated teeth. Part II. Sealer types. J Endod. 1987;13:109.
128. Main C, Mirzayan N, Shabahang S, Torabinejad M. Repair of root perforations using mineral trioxide aggregate: a long-term study. J Endod. 2004;30:80.
129. Mandel E, Friedman S. Endodontic retreatment: a rational approach to root canal reinstrumentation. J Endod. 1992;18:565.
130. Martin JA, Bader JD. Five-year treatment outcomes for teeth with large amalgams and crowns. Oper Dent. 1997;22:72.
131. Masserann J. Entfernen metallischer Fragmente aus Wurzelkanalen (Removal of metal fragments from the root canal). J Br Endod Soc. 1971;5:55.
132. McDonald MN, Vire DE. Chloroform in the endodontic operatory. J Endod. 1992;18:301.
133. Metzger Z, Ben-Amar A. Removal of overextended gutta-percha root canal fillings in endodontic failure cases. J Endod. 1995;21:287.
134. Molven O, Halse A, Fristad I, MacDonald-Jankowski D. Periapical changes following root-canal treatment observed 20-27 years postoperatively. Int Endod J. 2002;35:784.
135. Moshonov J, Peretz B, Ben-Zvi K, Cohenca N, Rotstein I. Effect of gutta-percha solvents on surface microhardness of IRM fillings. J Endod. 2000;26:142.
136. Moshonov J, Trope M, Friedman S. Retreatment efficacy 3 months after obturation using glass ionomer cement, zinc oxide-eugenol, and epoxy resin sealers. J Endod. 1994;20:90.
137. Mulvay PG, Abbott PV. The effect of endodontic access cavity preparation and subsequent restorative procedures on molar crown retention. Aust Dent J. 1996;41:134.
138. Nagai O, Tani N, Kayaba Y, Kodama S, Osada T. Ultrasonic removal of broken instruments in root canals. Int Endod J. 1986;19:298.
139. Nair PN. New perspectives on radicular cysts: do they heal? Int Endod J. 1998;31:155.
140. Nair PN, Sjogren U, Krey G, Kahnberg KE, Sundqvist G. Intraradicular bacteria and fungi in root-filled, asymptomatic human teeth with therapy-resistant periapical lesions: a long-term light and electron microscopic follow-up study. J Endod. 1990;16:580.
141. Nair PN, Sjogren U, Krey G, Sundqvist G. Therapy-resistant foreign body giant cell granuloma at the periapex of a root-filled human tooth. J Endod. 1990;16:589.
142. Nair PN, Sjogren U, Schumacher E, Sundqvist G. Radicular cyst affecting a root-filled human tooth: a long-term post-treatment follow-up. Int Endod J. 1993;26:225.
143. Nair PNR, Schroeder HE. Periapical actinomycosis. J Endod. 1984;10:567.
144. Nakata TT, Bae KS, Baumgartner JC. Perforation repair comparing mineral trioxide aggregate and amalgam using an anaerobic bacterial leakage model. J Endod. 1998;24:184.
145. Nearing MV, Glickman GN. Comparative efficacy of various rotary instrumentation systems for gutta-percha removal [Abstract]. J Endod. 1999;24:295.
146. Ng YL, Mann V, Gulabivala K. Outcome of secondary root canal treatment: a systematic review of the literature. Int Endod J. 2008;41:1026.
147. Niemczyk SP. Re-inventing intentional replantation: a modification of the technique. Pract Proced Aesthet Dent. 2001;13:433.
148. Orstavik D, Pitt-Ford TR, editors. Essential Endodontology. Prevention and treatment of apical periodontitis. New York: Wiley-Blackwell, 2008.
149. Osorio RM, Hefti A, Vertucci FJ, Shawley AL. Cytotoxicity of endodontic materials. J Endod. 1998;24:91.
150. Ozgoz M, Yagiz H, Cicek Y, Tezel A. Gingival necrosis following the use of a paraformaldehyde-containing paste: a case report. Int Endod J. 2004;37:157.
151. Parashos P, Messer HH. Questionnaire survey on the use of rotary nickel-titanium endodontic instruments by Australian dentists. Int Endod J. 2004;37:249.
152. Parker H, Glickman GN. Solubility of plastic Thermafil carriers. J Dent Res. 1993;72:188.
153. Perez AL, Spears R, Gutmann JL, Opperman LA. Osteoblasts and MG-63 osteosarcoma cells behave differently when in contact with ProRoot MTA and White MTA. Int Endod J. 2003;36:564.
154. Peters SB, Canby FL, Miller DA. Removal of a carbon-fiber post system [abstract PR35]. J Endod. 1996;22:215.
155. Pitt-Ford TR, Torabinejad M, McKendry DJ, Hong C-U, Kariyawasam SP. Use of mineral trioxide aggregate for repair of furcal perforations. Oral Surg Oral Med Oral Pathol. 1995;79:756.
156. Ray H, Seltzer S. A new glass ionomer root canal sealer. J Endod. 1991;17:598.
157. Regezi JA, Sciubba JJ, Jordan RCK, editors. Oral pathology. Clinical pathologic correlations, ed 4, St. Louis: Saunders, 2008.
158. Rocas IN, Siqueira JFJ, Santos KR. Association of Enterococcus faecalis with different forms of periradicular diseases. J Endod. 2004;30:315.
159. Roda R. Clinical Showcase—unintentional replantation: a technique to avoid. J Can Dent Assoc. 2006;72:133.
160. Roda RS. Root perforation repair: surgical and nonsurgical management. Pract Proced Aesthet Dent. 2001;13:467.
161. Roig-Greene JL. The retrieval of foreign objects from root canals: a simple aid. J Endod. 1983;9:394.
162. Royzenblat A, Goodell GG. Comparison of removal times of Thermafil plastic obturators using ProFile rotary instruments at different rotational speeds in moderately curved canals. J Endod. 2007;33:256.
163. Ruddle CJ. Non-surgical endodontic retreatment. In: Cohen S, Burns RC, editors. Pathways of the pulp. 8th ed. St. Louis: Mosby; 2002:875.
164. Ruddle CJ. Nonsurgical retreatment. J Endod. 2004;30:827.
165. Sabourin CR, Flinn BD, Pitts DL, Gatten TL, Johnson JD. A novel method for creating endodontic access preparations through all-ceramic restorations: air abrasion and its effect relative to diamond and carbide bur use. J Endod. 2005;31:616.
166. Sae-Lim V, Rajamanickam I, Lim BK, Lee HL. Effectiveness of ProFile .04 taper rotary instruments in endodontic retreatment. J Endod. 2000;26:100.
167. Sakkal S, Gauthier G, Milot P, Lemian L. A clinical appraisal of the Gonon post-pulling system. J Can Dent Assoc. 1994;60:537.
168. Satterthwaite JD, Stokes AN, Frankel NT. Potential for temperature change during application of ultrasonic vibration to intra-radicular posts. Eur J Prosthodont Restor Dent. 2003;11:51.
169. Saunders EM. In vivo findings associated with heat generation during thermomechanical compaction of gutta-percha. 1. Temperature levels at the external surface of the root. Int Endod J. 1990;23:263.
170. Saunders EM. In vivo findings associated with heat generation during thermomechanical compaction of gutta-percha. 2. Histological response to temperature elevation on the external surface of the root. Int Endod J. 1990;23:268.
171. Saunders EM, Saunders WP. The heat generated on the external root surface during post space preparation. Int Endod J. 1989;22:169.
172. Saunders WP, Saunders EM. Coronal leakage as a cause of failure in root-canal therapy: a review. Endod Dent Traumatol. 1994;10:105.
173. Scherer W, Dragoo MR. New subgingival restorative procedures with Geristore resin ionomer. Pract Periodont Aesthet Dent. 1995;7:1.
174. Schilder H. Filling root canals in three dimensions. Dent Clin North Am. 1967:723. Nov
175. Schirrmeister JF, Meyer KM, Hermanns P, Altenburger MJ, Wrbas KT. Effectiveness of hand and rotary instrumentation for removing a new synthetic polymer-based root canal obturation material (Epiphany) during retreatment. Int Endod J. 2006;39:150.
176. Schwandt NW, Gound TG. Resorcinol-formaldehyde resin ‘Russian Red’ endodontic therapy. J Endod. 2003;29:435.
177. Schwartz RS, Robbins JW. Post placement and restoration of endodontically treated teeth: a literature review. J Endod. 2004;30:289.
178. Seltzer S. Endodontology: Biologic considerations in endodontic procedures, 2nd ed. Philadelphia: Lea & Febiger; 1988.
179. Seltzer S, Bender IB. Cognitive dissonance in endodontics. Oral Surg Oral Med Oral Pathol. 1965;20:505.
180. Seltzer S, Bender IB, Ziontz BA. The dynamics of pulp inflammation: correlations between diagnostic data and actual histologic findings in the pulp. Oral Surg. 1963;16:846.
181. Seltzer S, Green DB, Weiner N, DeRenzis F. A scanning electron microscope examination of silver cones removed from endodontically treated teeth. Oral Surg Oral Med Oral Pathol. 1972;33:589.
182. Seltzer S, Sinai I, August D. Periodontal effects of root perforations before and during endodontic procedures. J Dent Res. 1970;49:332.
183. Serper A, Ucer O, Onur R, Etikan I. Comparative neurotoxic effects of root canal filling materials on rat sciatic nerve. J Endod. 1998;24:592.
184. Shen Y, Peng B, Cheung GS. Factors associated with the removal of fractured NiTi instruments from root canal systems. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:605.
185. Shipper G, Orstavik D, Teixeira FB, Trope M. An evaluation of microbial leakage in roots filled with a thermoplastic synthetic polymer-based root canal filling material (Resilon). J Endod. 2004;30:342.
186. Simon JH, Chimenti RA, Mintz GA. Clinical significance of the pulse granuloma. J Endod. 1982;8:116.
187. Simon JH, Glick DH, Frank AL. The relationship of endodontic-periodontic lesions. J Periodontol. 1972;43:202.
188. Sinai IH. Endodontic perforations: their prognosis and treatment. J Am Dent Assoc. 1977;95:90.
189. Siqueira JF, Sen BH. Fungi in endodontic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:632.
190. Sjogren U, Hagglund B, Sundqvist G, Wing K. Factors affecting the long-term results of endodontic treatment. J Endod. 1990;16:498.
191. Sjogren U, Happonen RP, Kahnberg KE, Sundqvist G. Survival of Arachnia propionica in periapical tissue. Int Endod J. 1988;21:277.
192. Sluyk SR, Moon PC, Hartwell GR. Evaluation of setting properties and retention characteristics of mineral trioxide aggregate when used as a furcation perforation repair material. J Endod. 1998;24:768.
193. So MV, Saran C, Magro ML, Vier-Pelisser FV, Munhoz M. Efficacy of ProTaper retreatment system in root canals filled with gutta-percha and two endodontic sealers. J Endod. 2008;34:1223.
194. Somma F, Cammarota G, Plotino G, Grande NM, Pameijer CH. The effectiveness of manual and mechanical instrumentation for the retreatment of three different root canal filling materials. J Endod. 2008;34:466.
195. Souter NJ, Messer HH. Complications associated with fractured file removal using an ultrasonic technique. J Endod. 2005;31:450.
196. Spatafore CM, Griffin JAJr, Keyes GG, Wearden S, Skidmore AE. Periapical biopsy report: an analysis of over a 10-year period. J Endod. 1990;16:239.
197. Spili P, Parashos P, Messer HH. The impact of instrument fracture on outcome of endodontic treatment. J Endod. 2005;31:845.
198. Spriggs K, Gettleman B, Messer HH. Evaluation of a new method for silver point removal. J Endod. 1990;16:335.
199. Stabholz A, Friedman S. Endodontic retreatment—case selection and technique. Part 2: treatment planning for retreatment. J Endod. 1988;14:607.
200. Stamos DE, Gutmann JL. Revisiting the post puller. J Endod. 1991;17:466.
201. Stamos DE, Gutmann JL. Survey of endodontic retreatment methods used to remove intraradicular posts. J Endod. 1993;19:366.
202. Sundqvist G. Ecology of the root canal flora. J Endod. 1992;18:427.
203. Sundqvist G, Reuterving CO. Isolation of Actinomyces israelii from periapical lesion. J Endod. 1980;6:602.
204. Suter B. A new method for retrieving silver points and separated instruments from root canals. J Endod. 1998;24:446.
205. Suter B, Lussi A, Sequeira P. Probability of removing fractured instruments from root canals. Int Endod J. 2005;38:112.
206. Sutherland JK, Teplitsky PE, Moulding MB. Endodontic access of all-ceramic crowns. J Prosthet Dent. 1989;61:146.
207. Swanson K, Madison S. An evaluation of coronal microleakage in endodontically treated teeth. Part I. Time periods. J Endod. 1987;13:56.
208. Tamse A, Fuss Z, Lustig J, Kaplavi J. An evaluation of endodontically treated vertically fractured teeth. J Endod. 1999;25:506.
209. Tamse A, Kaffe I, Lustig J, Ganor Y, Fuss Z. Radiographic features of vertically fractured endodontically treated mesial roots of mandibular molars. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:797.
210. Tasdemir T, Er K, Yildirim T, Celik D. Efficacy of three rotary NiTi instruments in removing gutta-percha from root canals. Int Endod J. 2008;41:191.
211. Tasdemir T, Yildirim T, Celik D. Comparative study of removal of current endodontic fillings. J Endod. 2008;34:326.
212. Teixeira FB, Teixeira EC, Thompson JY, Trope M. Fracture resistance of roots endodontically treated with a new resin filling material. J Am Dent Assoc. 2004;135:646.
213. Teplitsky PE, Rayner D, Chin I, Markowsky R. Gutta percha removal utilizing GPX instrumentation. J Can Dent Assoc. 1992;58:53.
214. Torabinejad M, Higa RK, McKendry DJ, Pitt Ford TR. Dye leakage of four root end filling materials: effects of blood contamination. J Endod. 1994;20:159.
215. Torabinejad M, Hong C, Pitt Ford TR. Tissue reaction to implanted Super-EBA and mineral trioxide aggregate in the mandible of guinea pigs: a preliminary report. J Endod. 1995;21:569.
216. Torabinejad M, Hong CU, McDonald F, Pitt Ford TR. Physical and chemical properties of a new root-end filling material. J Endod. 1995;21:349.
217. Torabinejad M, Hong CU, Pitt Ford TR, Kettering JD. Cytotoxicity of four root end filling materials. J Endod. 1995;21:489.
218. Torabinejad M, Walton RE, editors. Principles and practice of endodontics, ed 4, St. Louis: Saunders, 2009.
219. Torabinejad M, Ung B, Kettering JD. In vitro bacterial penetration of coronally unsealed endodontically treated teeth. J Endod. 1990;16:566.
220. Tronstad L, Barnett F, Cervone F. Periapical bacterial plaque in teeth refractory to endodontic treatment. Endod Dent Traumatol. 1990;6:73.
221. Trope M, Maltz DO, Tronstad L. Resistance to fracture of restored endodontically treated teeth. Endod Dent Traumatol. 1985;1:108.
222. United States Drug Administration. Chloroform used as an ingredient (active or inactive) in drug products. Federal Register No. 26845. Washington DC: US Government Printing Office; 1976.
223. Uzun O, Topuz O, Tinaz C, Nekoofar MH, Dummer PM. Accuracy of two root canal length measurement devices integrated into rotary endodontic motors when removing gutta-percha from root-filled teeth. Int Endod J. 2008;41:725.
224. Valderhaug J, Jokstad A, Ambjornsen E, Norheim PW. Assessment of the periapical and clinical status of crowned teeth over 25 years. J Dent. 1997;25:97.
225. Viducic D, Jukic S, Karlovic Z, Bozic Z, Miletic I, Anic I. Removal of gutta-percha from root canals using an Nd:YAG laser. Int Endod J. 2003;36:670.
226. Weiger R, Manncke B, Werner H, Lost C. Microbial flora of sinus tracts and root canals of non-vital teeth. Endod Dent Traumatol. 1995;11:15.
227. Welk ARDMD, Baumgartner JCDDSP, Marshall JGDMD. An in vivo comparison of two frequency-based electronic apex locators. J Endod. 2003;29:497.
228. Wennberg A, Orstavik D. Evaluation of alternatives to chloroform in endodontic practice. Endod Dent Traumatol. 1989;5:234.
229. White C, Bryant N. Combined therapy of mineral trioxide aggregate and guided tissue regeneration in the treatment of external root resorption and an associated osseous defect. J Periodontol. 2002;73:1517.
230. Whitworth JM, Boursin EM. Dissolution of root canal sealer cements in volatile solvents. Int Endod J. 2000;33:19.
231. Wilcox LR. Endodontic retreatment with halothane versus chloroform solvent. J Endod. 1995;21:305.
232. Wilcox LR. Thermafil retreatment with and without chloroform solvent. J Endod. 1993;19:563.
233. Wilcox LR, Juhlin JJ. Endodontic retreatment of Thermafil versus laterally condensed gutta-percha. J Endod. 1994;20:115.
234. Wilcox LR, Krell KV, Madison S, Rittman B. Endodontic retreatment: evaluation of gutta-percha and sealer removal and canal reinstrumentation. J Endod. 1987;13:453.
235. Wolcott J, Ishley D, Kennedy W, Johnson S, Minnich S, Meyers J. A 5 yr clinical investigation of second mesiobuccal canals in endodontically treated and retreated maxillary molars. J Endod. 2005;31:262.
236. Wolcott JF, Himel VT, Hicks ML. Thermafil retreatment using a new “System B” technique or a solvent. J Endod. 1999;25:761.
237. Wolcott S, Wolcott J, Ishley D, et al. Separation incidence of protaper rotary instruments: a large cohort clinical evaluation. J Endod. 2006;32:1139.
238. Wourms DJ, Campbell AD, Hicks ML, Pelleu GBJr. Alternative solvents to chloroform for gutta-percha removal. J Endod. 1990;16:224.
239. Yatsushiro JD, Baumgartner JC, Tinkle JS. Longitudinal study of the microleakage of two root-end filling materials using a fluid conductive system. J Endod. 1998;24:716.
240. Yeo JF, Loh FC. Retrograde removal of fractured endodontic instruments. Ann Acad Med Singapore. 1989;18:594.
241. Yoldas O, Oztunc H, Tinaz C, Alparslan N. Perforation risks associated with the use of Masserann endodontic kit drills in mandibular molars. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:513.
242. Zakariasen KL, Brayton SM, Collinson DM. Efficient and effective root canal retreatment without chloroform. J Can Dent Assoc. 1990;56:509.
243. Zuolo ML, Imura N, Ferreira MO. Endodontic retreatment of thermafil or lateral condensation obturations in post space prepared teeth. J Endod. 1994;20:9.
244. Zuolo ML, Kherlakian N, Imura N. Effectiveness of nickel titanium rotary and hand instrumentation in endodontic retreatment [Abstract]. J Endod. 1996;22:209.