Chapter 18 Specialized antenatal investigations
Antenatal assessment of the fetus is now a mainstream aspect of care for all pregnancies. In early to mid-pregnancy, a number of tests for fetal anomaly are offered to all mothers as part of the NHS Antenatal and Newborn Screening Programmes. Later in pregnancy, monitoring of fetal well-being may be required. Advances in technology mean that fetal assessments have become increasingly sophisticated and more widespread. For instance, ultrasound scan machines are now able to detect a number of abnormalities in the first trimester of pregnancy. Some centres already offer this facility. Inevitably, increased testing results in increased information for parents. Generally, test findings give a degree of reassurance, but they can also result in significant anxiety and devastating decisions about whether to end the pregnancy. Increased childbearing among older mothers (Langford 1992) has resulted in more age-related chromosomal abnormalities. Consequently, there are a number of factors that contribute to an ever-increasing emphasis on antenatal fetal assessments.
Social and psychological impact of fetal investigations
Pregnancy is a profound and life-changing event. During this time, the mother has to adapt physically, socially and psychologically to the forthcoming birth of her child. Many women feel more emotional than usual (Raphael-Leff 1991) and may have heightened levels of anxiety (e.g. Dragonas & Christodoulou 1998, Tindall 1997). As Hawkey (1998) states, the increasing availability of fetal investigations has been shown to cause women even greater anxiety and stress. Any feelings of excitement and anticipation can quickly change when the mother is introduced to the idea that she is ‘at risk’ of having a baby with a particular problem (Fisher 2006).
There is evidence that mothers nearing the end of their reproductive years (with a higher risk of chromosomal abnormality) experience pregnancy in a way that is different to younger women. Older mothers are often more anxious and have fewer feelings of attachment to the fetus at 20 weeks of pregnancy (Berryman & Windridge 1995). Psychologists, sociologists and health professionals now generally accept the finding that high-risk women delay attachment to the fetus until they receive reassuring test results. Rothman (1986) classically termed this the ‘tentative pregnancy’, in a study of women undergoing amniocentesis.
Anxiety caused by consideration of possible fetal abnormality may be accompanied by moral or religious dilemmas. Tests that can diagnose chromosomal or genetic abnormalities also carry a risk of procedure-induced miscarriage. Many parents agonize about whether to subject a potentially normal fetus to this risk in order to obtain this information. Parents may then need to consider whether they wish to terminate or continue with an affected pregnancy. Some religious authorities only support prenatal testing so long as the integrity of the mother and fetus are maintained. There are also opposing views about the legitimacy of terminating a pregnancy, even when a serious disorder has been diagnosed. Such dilemmas are an unfortunate but inevitable cost of the choices associated with some fetal investigations.
Despite this, there are important advantages to the acquisition of knowledge about the fetus before birth. First, society greatly values the freedom of individuals to choose. People are encouraged to accept some responsibility when making decisions about treatment options, in partnership with healthcare professionals. A second advantage is that reproductive autonomy may be increased. Women can choose for themselves whether they wish to embark upon the lifelong care of a child with special needs. This may be viewed as empowering and as a means of preventing later suffering and hardship for child and family alike.
In summary, prenatal testing is a two-edged sword. It enables midwives and doctors to give people choices that were unheard of in previous generations and that may prevent much suffering. However, in some circumstances they actually increase the amount of anxiety and psychological trauma experienced in pregnancy. The long-term effects of such trauma on family dynamics are not currently understood.
The midwife’s role and responsibilities
All midwives need to have a broad understanding of fetal investigations because they are responsible for offering, interpreting and communicating the results. The Midwives Rules and Standards (NMC 2004) state that midwives ‘should enable the woman to make decisions about her care based on her individual needs, by discussing matters fully with her’ (p 16). Some midwives specialize in discussing complex testing issues with parents and become antenatal screening coordinators. The UK National Screening Committee (2004) recommends that dedicated screening coordinators oversee the running of screening programmes in every Trust. Screening coordinators also provide specialist advice and ensure that there is a line of referral for women whose needs are not met by routine services (Ferguson 2001).
When offering tests, it is necessary for the midwife to present and discuss the options, so that women can make a choice that best suits their circumstances and preferences. Midwives commonly recommend antenatal tests such as infectious disease screening, full blood count or cardiotocograph for reduced fetal movements. However, tests for fetal anomaly require a non-directive approach that enables the mother to make an informed choice (Clarke 1994). Consent must be obtained prior to all tests and this must be documented (National Institute for Clinical Excellence (NICE) 2003, UK National Screening Committee 2004). The information required to obtain consent is shown in Box 18.1 (DH 2001). It is noteworthy that obtaining consent is a process and not usually a one-off event. This means that tests should be discussed on more than one occasion and mothers should be given time to consider their options.
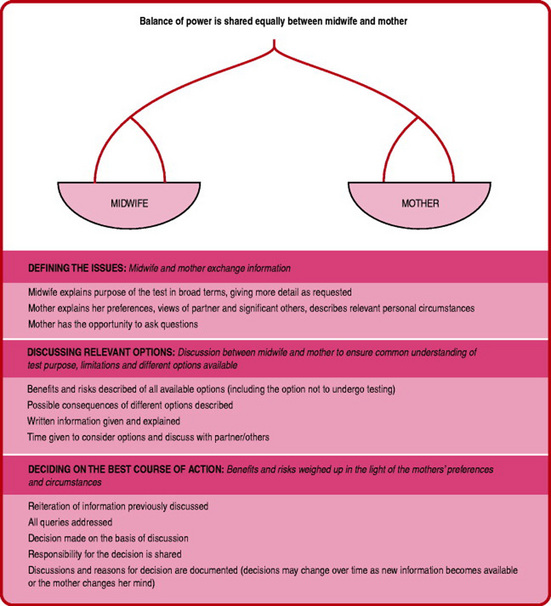
Midwives are required to discuss options for testing in a manner that enables shared decision making (Sullivan 2005). This means that the midwife and mother both share information and are jointly accountable for the decisions that are made. There may be mixed feelings about the final decision. Sometimes, it is helpful to consider what the mother’s worst-case scenario would be, as that can help to decide the best way forward. Attention should be paid to documenting all discussions carefully for future reference and for continuity of care. The process for shared decision-making is shown in Figure 18.1.
Issues to consider when presenting information
When discussing tests, it is important to understand the motivations and thought processes of pregnant women. The motivation for testing is often different for mother and practitioner. Whereas the medical indication for testing is to identify fetal anomalies, mothers commonly accept these tests in order to gain reassurance that their fetus is normal (Farrant 1985). Mothers often think that fetal anomaly tests such as ultrasound scans are an integral or mandatory part of their antenatal care. They may also be unaware of the reasons for performing the test and this can compound the shock of finding problems or abnormalities (Health Technology Assessment 2000).
It is also evident that, when feeling anxious or under stress, people are less able to remember the information portrayed (Ingram & Malcarne 1995). Parents may feel vulnerable and less able to ask questions. This may lead to dissatisfaction with the quality of communications with healthcarers. Since an unborn fetus is something of an enigma to parents, this may increase anxiety and sensitivity to real or imaginary cues. For example, professionals practising non-directive counselling may be perceived as evasive and as concealing bad news. One particular aspect of counselling that has been criticized by parents is the portrayal of risk estimates (Al-Jader et al 2000).
There is much evidence that people do not make consistent decisions about undertaking tests in pregnancy on the basis of the risk information received. For instance, a mother with a risk of Down syndrome of 1:200 may perceive herself to be at a very high risk and may request amniocentesis. However, others may view that same risk as very low. We do not fully understand the way in which parents interpret risk information, although it is clear that personal circumstances, preferences and beliefs are an integral part of this process. For this reason, it is vital that the midwife begins a consultation by investigating how mothers feel about testing and what they already know.
There are also common biases in the way people interpret risk information. The midwife should be aware of these in order to help parents choose the most appropriate course of action. For example, people tend to view an event as more likely if they can easily imagine or recall instances of it. This means that a mother whose friend or neighbour has a baby with Down syndrome may be sensitized to this possibility and overestimate the chances of it happening to her. In reality, her risk remains unchanged. Mothers who work with infirm people, or those with a disability, are most likely to seek prenatal diagnosis (Sjogren 1996). Perhaps these mothers are easily able to imagine the lifelong commitment of caring for a child with special needs. This common bias in risk perception is important because it means that some mothers may not easily be reassured by reiteration of the fact that the risk of abnormality may be comparatively rare.
Explaining risk
The way in which the midwife tells a mother about risk will also greatly influence how that risk is perceived. For example, a mother who is told that her risk of a particular condition is 1 in 10 may be more alarmed than if she had been informed that there was a 90% chance of normality. This is known as the ‘framing’ effect (Kessler & Levine 1987).
People vary considerably in the ways that they consider and understand risk, so it is important that this information is presented in a variety of ways. As such, a midwife discussing a 1 in 100 risk of a disorder should also point out the fact that 99% or 99 out of 100 similar people will not experience that disorder. This may help people cope when considering tests or when anxiously awaiting results.
There are other general considerations to take into account when providing information (Hunter 1994):
If a test is undertaken in pregnancy, it is good practice to ensure that parents are clear about how, when and from whom they will be able to obtain the result. If possible, there should be some options available.
Antenatal fetal tests
Broadly speaking, there are two types of test offered to pregnant women. They are known as screening or diagnostic tests.
Screening tests
Screening tests aim to identify individuals who are most likely to be affected by a named disorder. This makes it possible to target further investigations towards those with the greatest apparent need. Mothers who undergo screening tests will be classified as above or below an action limit for follow-up investigations. Traditionally, this classification has been known as ‘screen positive’ or ‘screen negative’. However, this terminology has caused problems with interpretation. As such, many mothers given a ‘screen positive’ result have assumed that there is a positive certainty that they have an affected pregnancy. Likewise, a ‘screen negative’ result has been interpreted as the exclusion of a problem. In fact, positive or negative in this context simply means that the chances of a problem are higher or lower than specified by an action limit. For this reason, screening results are now referred to as ‘high(er) risk’ or ‘low(er) risk’.
It is also important to note that the action limit (or the dividing point between high and low risk) is usually defined in line with the level of resources available for follow-up procedures. There is no agreed scientific means of calculating what defines high risk. Consequently, some mothers within the low-risk group will be sufficiently anxious to request follow-up, whereas some who are categorized as high risk will not wish to pursue subsequent investigations.
The performance of a screening test is defined in a number of ways:
National fetal screening programmes have been developed for a range of congenital abnormalities. These include fetal anomaly ultrasound screening, Down syndrome screening and haemoglobinopathy screening. These screening programmes should be offered to all pregnant women.
Diagnostic tests
Diagnostic tests are performed in order to confirm or disprove the presence of a particular abnormality. They may be offered as a consequence of screening test results. However, the diagnosis may not give certainty as to the severity of the disorder or the quality of life of a particular individual. Responses to a diagnosis will vary, according to cultural, social, moral and religious beliefs. Furthermore, we are not currently able to diagnose all fetal abnormalities and some will not be manifested until childhood or even adulthood. Examples of diagnostic tests include amniocentesis, chorionic villus sampling (CVS) and ultrasound scans (scans can be used to diagnose some structural anomalies such as spina bifida and they can also be used to screen for clue signs of disorders such as Down syndrome).
Fetal anomaly screening from maternal serum
Down syndrome screening
Down syndrome (also referred to as Down’s syndrome) is the most common cause of severe learning difficulty in children. In the absence of antenatal screening, around 1 in 700 births would be affected (Kennard et al 1995). While some children with Down syndrome learn literacy skills and lead semi-independent lives, others remain completely dependent. Around one in three of these babies are born with a serious heart defect. The average life expectancy is about 60 years, although most people develop pathological changes in the brain (associated with Alzheimer’s disease) after the age of 40 (Kingston 1994).
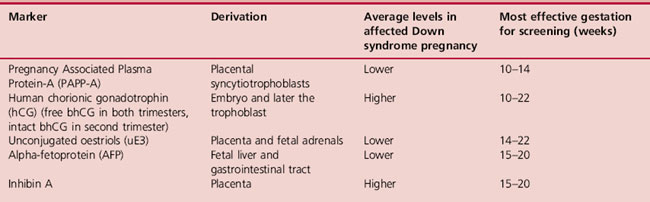
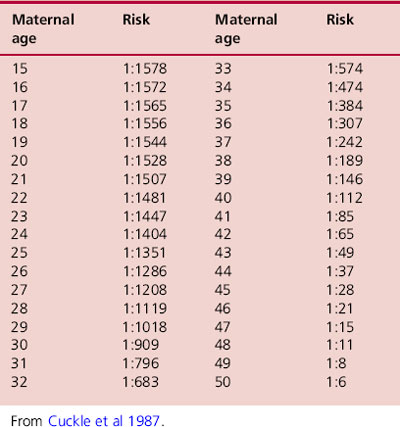
Maternal blood contains a number of hormones that can be used to assess the risk of Down syndrome. A growing number of hormones have been shown to have different average levels in Down syndrome compared with unaffected pregnancies and are therefore known as biochemical markers. These are shown in Table 18.1. One of the difficulties with using biochemical markers for screening is the fact that their levels fluctuate and are dependant on a number of factors, such as maternal weight. Consequently, a more accurate risk estimate can be obtained if several markers are used in combination. Levels of biochemical markers are never diagnostic for Down syndrome. The incidence of Down syndrome is also related to maternal age, so this is also factored into risk calculations. Older mothers are more likely to receive a higher risk result. The risk of Down syndrome by maternal age is shown in Table 18.2.
Table 18.2Estimated risk of having a Down syndrome birth according to maternal age at birth (calculated using eight surveys)
Biochemical markers, combined with maternal age, form part of the national Down syndrome screening programme. The UK National Screening Committee (2004) standards state that, whatever markers are used, tests should have a detection rate of >75% with a false positive rate of <3%. The cut-off point between higher and lower risk is 1:250. Some centres also add ultrasound markers (see Nuchal translucency measurement, p. 299) to maternal age and biochemical screening tests. This greatly enhances the reliability and safety of Down syndrome screening. The aim is to maximize the detection rate, while minimizing the false positive results. This is very important because women with higher-risk results can only find out whether the baby is affected by undergoing invasive procedures such as amniocentesis or CVS. These tests carry a risk of causing a procedure-induced miscarriage. The types and safety levels of different screening tests are shown in Table 18.3, which shows increasing safety and effectiveness as more markers are added together.
It is noteworthy that the serum integrated and integrated tests are performed in two stages, with first and second trimester testing. Some clinicians find this concept problematic because it necessitates withholding test results until all phases of the testing process have been completed. Research is currently underway to explore the feasibility of this model in mainstream practice. One possible modification is known as contingency screening. A two-phase testing process occurs, but women above or below certain thresholds receive their results after the first phase. Higher risk mothers are offered diagnostic testing at this point and lower risk mothers are informed that their risk is low. Women in the middle range proceed to second trimester testing, since further information is advantageous in determining the risk classification.
When performing serum screening, it is essential that the gestation is accurately assessed. The scan dates should be used in preference to menstrual history, since scans give a more accurate indicator of the time of conception. Crown–rump length or biparietal diameter scan measurements should be used. Femur length is not a reliable indicator of gestational age. Serum screening is not indicated in multiple pregnancies because it is impossible to know how the levels relate to each fetus. Mothers with multiple pregnancies should be offered nuchal translucency scanning as an alternative. Serum levels also change with maternal weight (i.e. they are more concentrated in women with lower body mass and circulating volume). It is therefore important to weigh the mother on the day of the test.
Neural tube defect screening and AFP
Traditionally, α-fetoprotein (AFP) has been used as a biochemical marker for neural tube defects such as spina bifida. This test has been superseded by the use of ultrasound to diagnose a range of neural tube defects. However, many centres still report high AFP levels at 15–20 weeks because this can be a prognostic indicator for other problems. Around 2% of mothers have a raised AFP level (Kennard et al 1995). Reasons include multiple pregnancy, incorrect gestation and threatened miscarriage. Raised AFP levels can be predictive of intrauterine growth restriction and pre-eclampsia. These pregnancies are therefore considered by some to be high risk and some centres offer increased fetal growth and fetal well-being monitoring in the third trimester.
Haemoglobinopathy screening
The NHS antenatal and newborn screening programmes include antenatal screening for fetal haemoglobinopathies. This should be linked with the newborn bloodspot screening programme, which tests for sickle cell disease. Haemoglobinopathies are inherited disorders of haemoglobin and are more prevalent in certain racial groups (NHS Sickle Cell and Thalassaemia Programme 2004; see Ch. 21).
Currently, antenatal screening is organized differently in different areas, based on population prevalence. High prevalence areas offer all pregnant women electrophoresis screening for haemoglobin variants and thalassaemia trait. Low prevalence areas use a family origins questionnaire to determine genetic ancestry for the last two generations (or more if possible). Women with genetic ancestry that includes high-risk racial groups are then offered electrophoresis testing. If the mother is found to be a haemoglobinopathy carrier, partner testing should be offered. Genetic ancestry is also important when interpreting screening results. It is also important to establish maternal iron levels when carrier status is suspected, since iron deficiency can give rise to similar red cell appearances. Haemoglobinopathies are recessively inherited, so the fetus would have a 1 in 4 chance of inheriting the disorder and a 1 in 2 chance of being a carrier. Diagnostic CVS or amniocentesis should be offered when both parents are carriers of a haemoglobinopathy.
Ultrasound scans for fetal testing
Pregnant women are offered two ultrasound scans in pregnancy. These include an early pregnancy scan and an 18–20-week fetal anomaly screening scan. Ultrasound scanning enables assessment and monitoring of many aspects of the pregnancy. It is used in order to screen for and to diagnose fetal abnormalities. Ultrasound works by transmitting sound at a very high pitch, via a probe, in a narrow beam. When the sound waves enter the body and encounter a structure, some of that sound is reflected back. The amount of sound reflected varies according to the type of tissue encountered. For example, fluid does not reflect sound and appears as a black image. Conversely, bone reflects a considerable amount of sound and appears as white or echogenic. Many structures appear as different shades of grey. Generally, pictures are transmitted in ‘real time’, which enables fetal movements to be seen.
Safety aspects of ultrasound
Ultrasound has been used as a diagnostic imaging tool since the 1950s, so we are now into the third generation of scanned babies. It seems reasonable to assume that any major adverse effects of this technology would have become apparent before now. However, modern machines have higher resolutions and indications for ultrasound scanning have greatly increased. This means that levels of exposure to ultrasound have increased in pregnancy and there is little research into the effects. Ultrasound should be used with respect and only when there is good indication.
Ultrasound waves have been shown to cause tissue heating, primarily within the first 40 s of exposure (Bosward et al 1993). There are also conflicting data regarding a possible association with low birth weight (Newnham et al 1993, Salvesen 1997), with dyslexia (Stark et al 1984) and with non-right-handedness (Salvesen et al 1993). Consequently, care should be taken to limit exposure time and the thermal indices should be controlled (European Committee of Medical Ultrasound Safety 2006). The use of ultrasound for entertainment or as a ‘keep sake’ is not recommended. Ultrasound is a diagnostic tool, but diagnosis can only be as reliable as the expertise of the operator and the quality of the machine. As Wood (2000) states, abnormalities may be missed or incorrectly diagnosed if the operator is inexperienced or inadequately trained.
Women’s experiences of ultrasound
In general, women experience ultrasound as a pleasurable opportunity to have visual access to their unborn baby (Sandelowski 1994). Indeed, ultrasound scans have been shown to increase psychological attachment to the fetus (Sedgman et al 2006). Parents have a profound curiosity about their baby and a scan can turn something nebulous into something which seems much more real as a living individual (Furness 1990). This can be particularly important for a woman’s partner and family, who do not have the immediate physical experience of the pregnancy. Women tend to regard their scan as providing a general view of fetal well-being: the fact that the fetus is alive, growing and developing. However, this reassurance is temporary and begins to wear off after a few weeks (Clement et al 1998). Mothers may then seek other forms of reassurance (e.g. monitoring fetal movements, auscultation of the fetal heartbeat). This initial reassurance may also create an enthusiasm for scans when there is no clinical indication.
However, scans may also cause considerable anxiety, particularly if there is a suspected or actual problem with the fetus. There is evidence to suggest that women who miscarry after visualization of the fetus on scan may feel a heightened sense of anguish because the fetus seemed more real. This may also be the case for parents considering termination of pregnancy on the grounds of fetal abnormality. However, others may view their scan as a treasured memory of the baby they lost (Black 1992).
The identification of fetal abnormality in the antenatal period has differing psychological effects for parents when the pregnancy is to continue. Some parents have reported feeling grateful that they were able to prepare for the birth of a child with a disability (Chitty et al 1996). However, others have reported feelings of wishing they had not known about their child’s problems before birth because this created a powerful image of the fetus as a ‘monster’. Some parents reported this to be far worse than the reality of caring for the baby after birth (Turner 1994). It is necessary for midwives to be mindful of the powerful psychological effects ultrasound scans have on pregnant women and their families, if sensitive and appropriate care is to be given at this potentially distressing time.
The midwife’s role concerning ultrasound scans
As for all procedures, mothers should be fully informed about the purpose of the scan. Information should be given about which conditions are being checked for and which problems the scan would be unable to detect. Because of the pleasurable aspect of seeing the fetus, ultrasound scans have traditionally been tests that mothers undertake willingly, without prior discussion and consideration of potential consequences. It is advisable to remind mothers gently of the medical indications for scans, so that they can decide whether or not they wish to undergo a procedure that may bring unwelcome news. Women should be aware that ultrasound scans are optional and not an inevitable part of their care.
There is evidence that, although some mothers may find this information disturbing, most feel that this is outweighed by the positive aspects of seeing the baby and gaining reassurance (Oliver et al 1996). Indeed, extra information about the purpose of the scan has been shown to increase women’s understanding and satisfaction with the amount of information received, while the proportion of women accepting a scan (99%) appears to remain unchanged (Thornton et al 1995).
The Royal College of Obstetricians and Gynaecologists recommends that, wherever scans are performed, a midwife or counsellor with a particular interest or expertise in the area should be available to discuss difficult news. Discussion about the implications of this should also take place with an obstetrician within one working day (RCOG 2000). Effective multidisciplinary team working and communication are therefore essential. It is also good practice for the midwife to liaise with the primary healthcare team, who would normally carry out the majority of antenatal care. With the increasing use of client-held records, mothers may have more opportunity to scrutinize the written results of their scan. Midwives may increasingly be called upon to explain and discuss these findings, both in hospital and in the community setting.
First trimester pregnancy scans
All women should be offered a first trimester scan. The purpose of this is to establish:
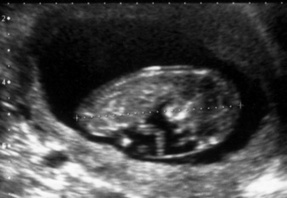
There is evidence to suggest that at least one scan is beneficial, mainly in reducing the need to induce labour for post-maturity (Neilson 1999). A gestation sac can usually be visualized from 5 weeks’ gestation and a small embryo from 6 weeks. Until 13 weeks, gestational age can be accurately assessed by crown–rump length (CRL) measurement (the length of the fetus from the top of the head to the end of the sacrum). This is demonstrated in Figure 18.2. Care must be taken to ensure that the fetus is not flexed at the time of measurement. Mothers are asked to attend with a full bladder, since this aids visualization of the uterus at an early gestation.
Measurement of nuchal translucency at 10–14 weeks
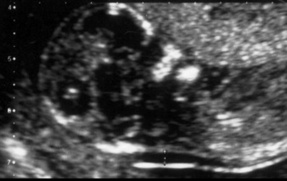
Additional information about the fetus can be gained by measuring the nuchal translucency (NT) at 10–14 weeks’ gestation. This is the thickness of the subcutaneous collection of fluid at the back of the neck, as shown in Figure 18.3. During the last decade, a series of studies have reported that increased NT is associated with chromosomal abnormalities, as well as other structural and genetic disorders (Nicolaides et al 1999). This information can be used in combination with maternal age and biochemical markers for Down syndrome.
The main advantage of this test is that it offers an early way of assessing the mother’s risk for Down syndrome. In general, mothers greatly value the opportunity for early information about Down syndrome, so that they could consider the option of termination before they are visibly pregnant and can feel their baby’s movements. Increased NT is also associated with other structural (mainly cardiac) and genetic syndromes, so increased pregnancy surveillance could be arranged. However, a disadvantage of this knowledge is that parents may suffer considerable anxiety until later scans offer some degree of reassurance.
Another potential disadvantage is that early identification of chromosomally abnormal pregnancies may mean that parents are faced with a decision about whether to terminate a pregnancy that may be destined to miscarry naturally. Approximately 40% of affected fetuses die between 12 weeks’ gestation and term (Nicolaides et al 1999). Some parents may experience more feelings of guilt after a termination than they would have done had the pregnancy spontaneously miscarried.
Second trimester ultrasound scans
After 13 weeks of pregnancy, gestational age is primarily assessed using the biparietal diameter (BPD). This is the measurement between the two parietal eminences of the fetal skull. It is a very useful measurement during the second trimester, but becomes less accurate towards the end of pregnancy because the shape of the head may alter. Limbs are also measured, most notably the femur.
The detailed fetal anomaly screening scan
This scan is usually performed at 18–20 weeks of pregnancy, since visualization of fetal anatomy is more difficult before that time (Drife & Donnai 1991). The purpose of this scan is to reassure the mother that the fetus has no obvious structural anomalies that fall into the following categories:
Detection rates vary considerably, but it is thought that around 50% of significant abnormalities are identified at this time (Boyd et al 1998). This is influenced by the expertise of the sonographer and quality of the equipment. There may also be technical difficulties, such as fetal position, multiple pregnancy or maternal obesity.
Also, some structural problems do not have associated sonographic signs. An example of this would be tracheo-oesophageal fistula (an opening between the trachea and the lower oesophagus). Moreover, some fetal abnormalities, such as hydrocephalus and bowel obstructions, may not appear until later in pregnancy. Diagnosis may therefore be missed. Average detection rates for some abnormalities are presented in Table 18.4. It is vital that mothers are fully aware of the precise purpose and limitations of the detailed scan. The comprehensive range of structures to be examined during the detailed scan are presented in Box 18.2. Examination of the four chambers of the fetal heart is shown in Fig. 18.4.
Table 18.4 Detection rates of fetal abnormalities, if present, on detailed ultrasound scan
| Problem | Chance of being seen (%) |
|---|---|
| Spina bifida | 90 |
| Anencephaly | 99 |
| Hydrocephalus | 60 |
| Major congenital heart problems | 25 |
| Diaphragmatic hernia | 60 |
| Exomphalos/gastroschisis | 90 |
| Major kidney problems | 85 |
| Major limb abnormalities | 90 |
| Cerebral palsy | Never seen |
| Autism | Never seen |
| Down syndrome | May be associated with heart or bowel problems in about 40% |
Box 18.2 Features examined on detailed fetal ultrasound scans
From RCOG 2000
Markers for chromosomal abnormality
Markers are minor sonographic clue signs, which may increase the chance that the fetus has a chromosomal abnormality (most are associated with Down syndrome). They are seen in many normal fetuses (at least 5%) and, when isolated, are of dubious value (Whittle 1997). Examples of such markers include the following:
The strength of association between each individual marker and Down syndrome varies considerably. As such, an increased nuchal fold increases the chances of an affected pregnancy by 10 times the background risk. Conversely, echogenic foci in the heart increase the chances only by a marginal factor of 1.2 (Snijders & Nicolaides 1996). The UK National Screening Committee recommends that the Down syndrome screening risk should not be altered on the basis of a single ultrasound marker, unless it is at least as significant as an increased nuchal fold. It is also noteworthy that there is much debate about the validity of some of the above markers, particularly echogenic foci in the heart. It is possible that sonographers will not continue to report some of these markers in the future. Likewise, many new markers are emerging and may be incorporated into national screening programmes in the future. One example is the absence of nasal bone.
Advantages and disadvantages of fetal anomaly scans
Providing the sonographer has sufficient expertise, many lethal or severely disabling conditions can be detected during the 18–20 week scan. There is also an increase in first trimester diagnosis. Although this means that parents may be faced with difficult and unexpected decisions, it may be that later psychological trauma and physical suffering can be prevented. Furthermore, many parents are offered reassurance that no obvious abnormalities were seen. There is also evidence that, for neonates requiring early surgical or paediatric interventions, prior knowledge of the abnormality allows a plan of care to be evolved in advance of the birth. The mother can then give birth in a unit with appropriate facilities. This has been shown to reduce morbidity in the cases of gastroschisis (an abdominal wall defect, adjacent to the umbilicus, allowing the intestines and other abdominal organs to protrude outside the body), cardiac abnormalities and intestinal obstruction (Chang et al 1991, Romero et al 1989).
However, there is also the potential for false positive findings that are not confirmed postnatally. In particular, markers for chromosomal abnormalities may cause considerable anxiety without having any clinical significance for the baby. Indeed, they may be regarded as variants of normal, particularly if the fetal chromosomes are proven to be normal (see ‘A testing time’, Box 18.3).
(Note: the validity of ‘echogenic focus’ as a marker for Down syndrome has become increasingly debatable since this scan was performed. This makes the passage even more poignant. Also, rapid diagnostic technologies would mean that results would nowadays usually be available after 1–2 days. This greatly reduces the long period of uncertainty that women faced at that time.)
I was called upon some time ago to outline my experiences of antenatal investigations to a midwives’ research group, I did this and thought at the time that I was bearing my soul to 200 people that I had never met before. I can only guess at the readership of this textbook and so with great trepidation, I will attempt to give a taste of what antenatal investigations are like from the parents’ side of the fence and hopefully ‘bring alive’ some of the traumas and dilemmas involved. The research, the facts, the paper evidence are there for all to see, whereas the emotions, considerations and ‘angst’ are less tangible; here is what it feels like to experience the process of diagnostic testing.
My husband and I knew early on in my pregnancy that I was expecting twins; having digested in some measure that awesome news, we nervously attended the hospital for my detailed scan. Everything seemed fine, the babies were assessed and all the checks made, except the position of the lower twin meant that the spine could not be seen clearly. We waited outside, I drank yet more water, though my bladder was bursting already, hoping that the baby would change position. We were euphoric, two baby girls on the way, how pleased our families would be, getting carried away, we even started to consider possible names: Amy, Alice, Emily, Lucy … (Fig. 18.9).
We re-entered the room to continue the scan and among the, ‘yes, that’s fine, there’s the other leg, etc.’, we detected a different intonation in the radiographer’s voice as she said, ‘Ah, echogenic foci, there’s a small marker near the baby’s heart, a calcium deposit. It’s probably nothing, but I’ll tell you about it anyway even though some radiographers would not even mention it. An echogenic focus is a soft indicator of Down syndrome’. The words hit us like a hammer; there was nothing remotely ‘soft’ about what we had just heard. In literally a few minutes and the length of a hospital corridor the effect of that discovery was to have a devastating impact on us and our families.
We were ushered to the other end of the corridor where the facts were explained to us. We were told percentage risk factors for women of a certain age and Downrisk factors associated with amniocentesis; fact followed fact followed fact. That I was carrying twins complicated matters. Do we have both babies tested? How significantly does that increase the risk of miscarriage? What if one baby is fine and the other shows signs of abnormality? Would we be able to cope with a handicapped child? Can one baby be aborted? Question followed question followed question. We were given all the known evidence associated with our predicament and, while in our rational minds we understood the notion of professional ethics, we most desperately craved the one thing we could not have, a considered expert medical opinion. Fine, we knew about all the research, but how could we, with so little medical knowledge or familiarity with such situations, even begin to make such a monumental decision?
The journey home was a silent one; to say that we hardly knew how we got from A to B is not to overstate our sense of confusion and trauma. My parents were in the middle of a meal when we arrived. Having delivered the news about two grand-daughters there followed the giant, 10 feet tall letters of BUT … The effect was instantaneous; not another bite was eaten.
There followed 3 days of complete bewilderment as we tried to cope with the dilemma we faced and the monstrous decisions we had to make. We considered a million times the avenues available and canvassed everyone’s opinion: family, friends, work colleagues, my local midwife, my health visitor, people we knew in the medical profession. Despite comforting and reassuring words, no respite came as we were forced to acknowledge any decisions were going to be exclusively ours; how desperately we needed advice!
We decided to have the amniocentesis on one baby and even now, the reasons as to why we chose this option are not clear, just as they were not at the time. Intuition, selfishness, whim, we really did not know ifwe were doing the right thing. In our minds, facts and research belong in textbooks; we were people craving guidance.
Funnily enough I did not find the actual test too awful. As we waited our turn, a member of the consultant’s team shared with us similar cases she had dealt with where the results had been favourable and I will never forget her words when she said, ‘I’ve got a good feeling about this, I’m sure everything will be fine’. This was the most human of comments from a professional that we had the pleasure to hear; it truly was like manna from heaven. Of course we knew that she could not possibly anticipate the results of the test, but her words at least made us feel there was some hope. Over the next 2 weeks we were to cling to those words time and time and time again. As we left the unit, we were told, ‘give us a ring in a couple of weeks’, and for what seemed like much longer than 2 weeks we were casually cast adrift to contemplate and imagine the results of the test.
Without wishing to jargonize or be drawn into using a cliché-ridden phrase, those 2 weeks were literally nightmarish. The comments people had made played over and over and over in our minds. ‘I’ll tell you this but it’s probably nothing … echogenic-foci … soft indicator of Down … I’ve got a good feeling about this …’. We merely functioned at work, nothing more; our lives were on hold and we were both emotionally and physically drained. We shrank from conversation with others and between ourselves on occasions; we considered innumerable times the possible outcomes. We slept fitfully; one night I sat on the stairs sobbing as I contemplated death, funerals and the fact that our babies may never exist in our world. My own midwife made a poignant and frighteningly accurate comment when she said that I was ‘mourning’ my babies – she was right. I was numb, we both were!
When the day for the result finally arrived there was absolutely no possibility that I could have made the call to the hospital. My husband was to phone up and then relay the news to me at work, but there were safety measures! Bad news, no call, no test results, no call, this gave me a get-out and ensured that hope could live on. To say I shook as I took my husband’s phone call is a massive understatement and upon hearing the good news, I broke down, utterly and completely. The pent-up emotion of the previous 2 weeks poured out. That I hugged the headteacher, ignoring, forgetting or simply not caring about protocol, is an indication of the level of euphoria and relief I felt.
Our daughters were born on the 18 January 1999, weighing in at 5 lb 15 oz and 6 lb 11 oz (Fig. 18.10).
Many, many issues were contemplated during this ‘testing’ experience – among them, can research be too advanced? A few years ago it is unlikely that such a marker would have been detected and a trouble-free pregnancy would have ensued! Most significantly, the role of counselling and support offered to women undergoing diagnostic tests must be considered as a vital component in the whole process.
I do not know if I am a better or different person as a result of my experience of diagnostic testing. I do know, however, that it is forever etched in my mind and heart; talking and writing about it even now feels incredibly emotional, vivid and raw.
There is also the problem of defining the prognosis for some recognizable abnormalities. In summary, the 18–20 week scan appears to confer psychological and health improvement benefits in some cases, but also has the capacity to cause great anxiety and distress. Care must be taken to ensure that parents are fully informed of the purpose, benefits and limitations of ultrasound scans before they consent to this procedure.
Third trimester pregnancy scans
In general, late pregnancy scans are performed in response to a specific clinical need and not as a screen of the low risk pregnant population. However, fetal abnormalities may come to light or be reassessed at this time. Many late scans are performed as a means of monitoring fetal well-being, growth and development.
Fetal growth
Many scans are performed in order to detect instances when growth deviates from the norm. Fetuses with excessive growth (macrosomia) have increased perinatal mortality and morbidity. There may be cephalopelvic disproportion or shoulder dystocia, with consequent birth asphyxia and trauma. In most cases, there is no apparent cause, but there is sometimes an association with maternal diabetes mellitus. Serial growth measurements are indicated in this latter group.
Fetuses may be small because they are preterm or because they are small for dates. Sometimes, these two problems overlap. In general, growth-restricted fetuses can be divided into two groups, symmetrical and asymmetrical growth restriction.
Symmetrical growth restriction
Most symmetrically small fetuses are entirely normal and may be genetically predetermined to be small. However, in some instances, this may be caused by chromosomal abnormalities, infection or environmental factors such as maternal substance misuse.
Asymmetrical growth restriction
These fetuses have a head size appropriate for gestational age, but thin bodies. This is generally caused by placental insufficiency, whereby the placenta is unable to provide sufficient nourishment for the fetus. Glycogen stores in the liver are reduced, so there are less energy reserves for the fetus during labour. Asymmetrically growth-restricted fetuses are therefore more likely to suffer antenatal or perinatal asphyxia, or both. Other potential problems include hypoglycaemia, hypothermia and premature birth.
In order to assess fetal growth, the gestational age must be accurately assessed on scan before 24 weeks. Women at high risk of having an abnormally grown fetus should have serial scans – often at 28, 32 and 36 weeks. Where there is a particular concern, growth may be measured every 2 weeks. The most important measurements are head circumference and abdominal circumference. In this way, trends in fetal growth can be assessed.
Biophysical profiling
Another measure of fetal well-being is the fetal biophysical profile. This is based on recognized fetal adaptations that occur as placental function declines. A score is calculated on the basis of five criteria (Manning et al 1980). These are listed as follows:
Doppler ultrasonography
Placental blood flow can be assessed using the Doppler shift (a change in the frequency of ultrasound wave reflection, according to the speed and direction of blood flow). Compromises in maternofetal circulation can be identified. Abnormalities in Doppler measurements may be detected before growth becomes impaired and can be used as a prognostic indicator. Doppler analysis of the umbilical artery is the only test that improves outcomes in high-risk pregnancies (Neilson & Alfirevic 2005). This is shown in Figure 18.6.
Findings from growth scans, biophysical profile scores and CTG recordings should be considered collectively, taking into account the full clinical picture and obstetric history.
Invasive diagnostic tests
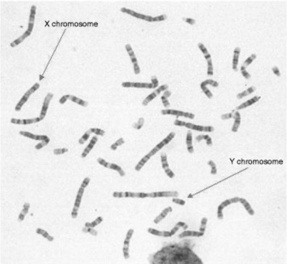
If mothers are found to have an increased risk of chromosomal or genetic problems, they may wish to undergo a diagnostic procedure. The two most frequently used tests are chorionic villus sampling (CVS) and amniocentesis. They should be performed in specialist centres, by obstetricians with specific training and expertise. These tests provide the opportunity to examine the fetal karyotype (the number and structure of chromosomes, visible through a microscope during mitotic metaphase (see Fig. 18.7) or DNA analysis for particular gene mutations, or both.
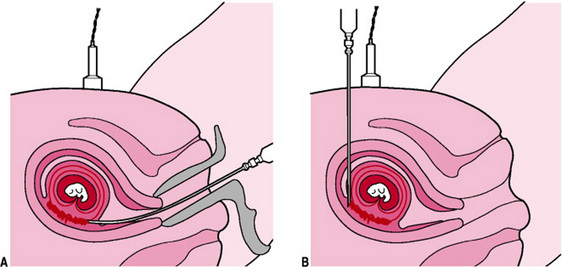
Chorionic villus sampling
Chorionic villus sampling is the acquisition of chorionic villi (placental tissue) under continuous ultrasound guidance. Chorionic villi originate from the same cells as the fetus and therefore generally have the same genes and chromosomes. This may be performed at any stage after 10 weeks of pregnancy.
Access may be achieved transcervically (until 13 weeks’ gestation) or via the transabdominal route. When CVS is performed transcervically, a catheter is introduced through the cervix and is guided into the chorion frondosum. Suction is then applied to an attached syringe and 10–40 mg of tissue are aspirated. This procedure is represented in Figure 18.8.
The transabdominal approach involves the insertion of a needle into the maternal abdomen. Under ultrasound guidance, the needle is pushed through the uterine wall and into placental tissue. Tissue is aspirated via an attached syringe. Villi are carefully separated from maternal decidua under a dissection microscope, prior to cytogenetic (chromosome) analysis.
In general, there are two stages to the reporting of results. The initial result is issued after 24–48 hrs and counts specific chromosomes (e.g. to test for Down syndrome). Cells are then cultured for 14–21 days, to confirm the initial results and to allow more detailed cytogenetic examination of the chromosome structure. DNA (for genetic) or biochemical (for metabolic) diagnoses can also be obtained from chorionic villi tissue.
The main advantage of CVS is that this is the earliest way mothers can obtain definitive information about the chromosomal/genetic status of the fetus. Mothers who are known carriers of particular disorders often have high recurrence risks and so are anxious until their results are known. If the news is not good and mothers wish to end their pregnancy, a surgical procedure can be performed. After 12–13 weeks, terminations are usually induced medically, in order to reduce the risk of causing cervical incompetence. This process of induction and vaginal birth can be very distressing. Decisions about termination of pregnancy may also become more difficult as the pregnancy progresses, since the mother begins to feel fetal movements and becomes visibly pregnant. Most women considering diagnostic procedures prefer to have an early test (Abramsky & Rodeck 1991). The main disadvantage with CVS is the procedure-induced risk of miscarriage. This is 0.5–2%, depending upon the experience of the operator (National Electronic Library for Health 2001) and occurs because of infection or bleeding. Also, ambiguous results are obtained in 1% of samplings (Holzgreve et al 1999).
Early CVS has been associated with limb reduction abnormalities. However, a large WHO international trial found the incidence of these abnormalities to be equal to the background rate (6/10 000), providing CVS was performed after 10 weeks. If CVS is performed prior to this gestation (i.e. during organogenesis), there is a higher chance that it may be harmful. Consequently, the WHO recommends 10 weeks as the earliest safe gestation for the procedure (Froster & Jackson 1996). In general, if an experienced operator with sufficient counselling support performs CVS, it is an effective and safe means of gaining early information about the fetus.
Amniocentesis
This is usually performed after 15 weeks’ gestation, since early amniocentesis has a higher loss rate than early CVS (Nicolaides et al 1994). The procedure involves transabdominal insertion of a fine needle into the amniotic fluid cavity, under continuous ultrasound guidance; 15 mL of amniotic fluid are aspirated. Cytogenetic, molecular (DNA) and biochemical analyses are possible. Amniocytes are often examined. These comprise cells that have been shed from several fetal sites, including skin, lungs and renal tract. The risk of procedure-induced miscarriage is 1% (National Electronic Library for Health 2001), although 2–3% of mothers have some leakage of amniotic fluid (Simpson et al 1981). Miscarriage is usually caused by infection or spontaneous rupture of the membranes at the puncture site.
Amniocentesis has traditionally been performed more commonly than CVS, mainly because more obstetricians had the required training. However, recent clinical governance initiatives have resulted in a move towards performing invasive procedures only in specialist centres. This is important because procedure loss rates are so dependent on the operator. In the second trimester of pregnancy, CVS and amniocentesis have similar risks and benefits. The miscarriage risks are comparable and, in most cases, both tests provide the required information.
Recent advances in cytogenetic techniques mean that mothers can obtain an initial set of results (usually for Down syndrome) and then a full culture result after 2–3 weeks.
Fetal blood sampling
The use of this technique has declined in recent years because improved molecular and cytogenetic techniques allow more diagnoses to be made from chorionic villi or amniotic fluid. However, fetal blood may be advantageous when there are ambiguous findings from placental tissue. Also, when there is Rhesus isoimmunization, it may be necessary to determine the fetal haemoglobin. When this is low, an intrauterine transfusion may be performed. Blood can be sampled from the umbilical cord or intrahepatic umbilical vein. The latter is less risky, as there are no umbilical arteries in close proximity. The loss rate also depends upon the gestation and condition of the fetus. In uncomplicated procedures after 20 weeks, the loss rate is around 1% (Holzgreve et al 1999).
Rapid diagnostic techniques
Rapid diagnostic technologies are now available to women who undergo invasive testing. There are two main techniques in use today, FISH and QF-PCR.
Fluorescent in situ hybridization (FISH)
A specific probe ‘paints’ the chromosomes to be examined. For example, for Down syndrome, the probe appears fluorescent under a microscope when in contact with chromosome 21. Cells are examined to determine whether there are two or three signals for this chromosome. Two is the normal count, whereas three indicates Down syndrome. Since many mothers undergo testing because of a high risk serum screening result or identification of markers on scan, they are very anxious to obtain quick results. For this reason, the use of FISH has greatly enhanced service delivery. See Plate 1 for an example of this technique.
Quantitative fluorescence-polymerase chain reaction (QF-PCR, sometimes referred to as PCR)
This is a molecular technique for replicating DNA. Fetal DNA is extracted from the cells and is then multiplied. Fluorescent dyes are used to identify gene regions and to enable analysis. This technique is commonly used for ‘genetic fingerprinting’ and for paternity testing.
New and emerging technologies
Fetal imaging techniques
Ultrasound scans in pregnancy have been discussed at length in this chapter, since they are important fetal investigations. Women generally see two-dimensional (2D) images of their unborn baby. However, there is a growing market for three-dimensional ultrasound imaging (3D). As such, multiple images are stored digitally and then shaded to produce life-like pictures. This technique can assist the diagnosis of surface structural anomalies, such as cleft lip and spina bifida. However, there are a growing number of commercial enterprises that offer 3D or 4D (3D in real time) ‘bonding’ scans. This is a matter of concern, since such services have minimal regulation and vary considerably in their quality and marketing techniques. The level of pre- and post-test advice is also questionable in some cases.
Magnetic resonance imaging (MRI) has also been applied in the examination of the fetus over the last two decades. This technique has not been widely applied because ultrasound can give similar diagnostic information at a lower cost. However, MRI may have a contribution to make, particularly when examining the brain. There is evidence that this may provide additional information and change the counselling and management for a significant number of pregnancies where brain abnormalities are suspected (Levine et al 1997). A further possible application is that MRI could offer an alternative to postmortem following termination or perinatal death. This may offer information to parents who decline postmortem because of its invasive nature (Brookes & Hall-Craggs 1997). MRI may also be useful in providing serial brain images following asphyxia in the newborn. This may give us a better understanding of the evolution of brain injury (Maalouf et al 1998). More recently, MRI imaging has been used to refine the diagnosis of diaphragmatic hernias and sacrococcygeal teratomas (Kumar & O’Brien 2004).
Fetal cells in the maternal circulation
There has been extensive research into the use of fetal cells in maternal blood for genetic diagnosis. This would be helpful to mothers who want diagnostic information without the risk of procedure-induced miscarriage of a potentially normal fetus. Some progress has been made, such as fetal sexing and rhesus typing from maternal blood. However, this technology is not yet sufficiently well developed for wider application to general clinical care.
Fetal therapy
There are certain instances when therapeutic interventions may improve fetal prognoses. For instance, therapeutic amniocentesis may be performed to drain excess liquor. This may reduce the likelihood of pre-term labour when the uterus is large-for-dates. Therapeutic amniocentesis is also sometimes performed in monochorionic twin pregnancies with twin-to-twin transfusion syndrome (discordant placental circulation and consequent discordant growth and liquor volume – if severe, this has a poor prognosis for both babies). Liquor is drained from the largest sac; this sometimes helps equal the pressures between the twins and allows both fetuses to grow satisfactorily.
Another treatment that is sometimes used is laser treatment to reduce pathological placental flow between the twins. Shunts can also be inserted into the fetus in order to drain pathological collections of fluid. These include ascites, renal obstruction and hydrocephalus. However, while there may be some improvement in outcome, much will depend on the underlying cause of the obstruction.
Conclusion
Fetal investigations are an integral aspect of antenatal care. Scientists and clinicians have developed a range of new diagnostic and imaging technologies. Some of these have been incorporated into national screening programmes and standards of care. The midwife must therefore ensure that women are informed about the benefits and risks associated with these technologies, so that they can make choices to suit their requirements. Undoubtedly, testing technologies profoundly influence women’s experiences of pregnancy and their early attachment to their unborn child. Midwives therefore have a duty to prepare women for tests through sensitive and accurate communications and then to support parents in their assimilation of information and decision-making once the results are known.
Abramsky L, Rodeck CH. Women’s choices for fetal chromosome anomalies. Prenatal Diagnosis. 1991;11:23-28.
Al-Jader LN, Parry Langdon N, Smith RJ. Survey of attitudes of pregnant women towards Down syndrome screening. Prenatal Diagnosis. 2000;20:23-29.
Berryman JC, Windridge KC. Motherhood after 35. A report of the Leicester motherhood project. Leicester University, Nestlé, Leicester, 1995.
Black RB. Seeing the baby: the impact of ultrasound technology. Journal of Genetic Counselling. 1992;1:45-54.
Bosward KL, Barnett SB, Wood AK, et al. Heating of guinea pig fetal brain during exposure to pulsed ultrasound. Ultrasound in Medicine and Biology. 1993;19(5):415-424.
Boyd PA, Chamberlain P, Hicks NR. Six-year experience of prenatal diagnosis in an unselected population in Oxford. Lancet. 1998;352:1577-1581.
Brookes JS, Hall-Craggs MA. Postmortem perinatal examination: the role of magnetic resonance imaging. Ultrasound in Obstetrics and Gynaecology. 1997;9(3):145-147.
Chang AC, Huhta JC, Yoon GY. Diagnosis, transport and outcome in fetuses with left ventricular outflow tract obstruction. Journal of Thoracic and Cardiovascular Surgery. 1991;102:841-848.
Chitty L, Barnes C, Berry C. Continuing with the pregnancy after a diagnosis of lethal abnormality. British Medical Journal. 1996;313:701-702.
Clarke A. Genetic counselling. Practice and principles. Routledge, London, 1994.
Clement S, Wilson J, Sikorski. Women’s experiences of antenatal ultrasound scans. In: Clement S, editor. Psychological perspectives on pregnancy and childbirth. Edinburgh: Churchill Livingstone; 1998:117-132.
Cuckle HS, Wald NJ, Thompson SG. Estimating a woman’s risk of having a pregnancy associated with Down syndrome using her age and serum alpha feto-protein level. British Journal of Obstetrics and Gynaecology. 1987;94:387-402.
Department of Health. Good practice in consent implementation guide: consent to examination or treatment, Department of Health Publications (Crown Copyright), London, 2001. Online. Available www.doh.gov.uk/consent.
Department of Health. Model of best practice. Down syndrome screening, Department of Health, London, 2003. Online. Available www.screening.nhs.uk/downs/model_bestpractice.pdf.
Dragonas T, Christodoulou GN. Prenatal care. Clinical Psychology Review. 1998;18(2):127-142.
Drife JO, Donnai D. Antenatal diagnosis of fetal abnormalities. London: Springer-Verlag, 1991.
European Committee of Medical Ultrasound Safety (ECMUS). Clinical safety statement for diagnostic ultrasound. 2006. Online. Available www.efsumb.org.
Farrant W. Who’s for amniocentesis? The politics of prenatal screening. In: Homans H, editor. The sexual politics of prenatal screening. London: Gower Press, 1985.
Ferguson P. Skimming the surface: antenatal screening and testing. RCM Midwives Journal. 2001;4(8):262-264.
Fisher J. Pregnancy loss, breaking bad news and supporting parents. In: Sullivan A, Kean L, Cryer A, editors. Midwife’s guide to antenatal investigations. London: Elsevier; 2006:31-42.
Froster UG, Jackson L. Limb defects after chorionic villus sampling: results from an international registry, 1992–1994. Lancet. 1996;347:489-494.
Furness ME. Fetal ultrasound for entertainment? Medical Journal of Australia. 1990;153(7):371.
Hawkey M. Psychological impacts on pregnancy: from hormones to genes. British Journal of Midwifery. 1998;6(5):310.
Health Technology Assessment. Ultrasound screening in pregnancy: a systematic review of the clinical effectiveness, cost-effectiveness and women’s views. Southampton: The National Coordinating Centre for HTA, 2000.
Holzgreve W, Tercanli S, Surbek D, et al. Invasive diagnostic methods. In: Rodeck CH, Whittle MJ, editors. Fetal medicine: basic science and clinical practice. Edinburgh: Churchill Livingstone; 1999:417-434.
Hunter M. Counselling in obstetrics and gynaecology. Leicester: British Psychological Society Books, 1994.
Ingram R, Malcarne V. Cognition in depression and anxiety. Same, different or a little of both. In: Craig K, Dobson K, editors. Anxiety and depression in adults and children. London: Sage; 1995:37-56.
Kennard A, Goodburn S, Golightly S, et al. Serum screening for Down syndrome. Royal College of Midwives Journal. 1995;108(1290):207-210.
Kessler S, Levine E. Psychological aspects of genetic counselling IV. The subjective assessment of probability. American Journal of Medical Genetics. 1987;28:361-370.
Kingston HM. ABC of clinical genetics. London: BMJ Publishing, 1994.
Kumar S, O’Brien A. Recent developments in fetal medicine. British Medical Journal. 2004;328(7446):1002-1006.
Langford J. Over 35 and at risk? New Generation. 1992;11(4):4-5.
Levine D, Barnes PD, Madsen JR, et al. Fetal central nervous system anomalies: MR imaging augments sonographic diagnosis. Radiology. 1997;204(3):635-642.
Maalouf EF, Counsell S, Battin M, et al. Magnetic resonance imaging of the neonatal brain. Hospital Medicine. 1998;59:41-45.
Manning FA, Platt LD, Sipros L. Antepartum fetal evaluation: development of a fetal biophysical profile. American Journal of Obstetrics and Gynaecology. 1980;136:787-795.
National Electronic Library for Health. Down syndrome screening. Invasive diagnosis. 2001. Online. Available www.nelh.nhs.uk/screening.
Neilson JP. Ultrasound for fetal assessment in early pregnancy. The Cochrane review. The Cochrane Library. Update Software, Oxford, 1999.
Neilson JP, Alfirevic Z. Doppler ultrasound for fetal assessment in high-risk pregnancies. The Cochrane Database of Systematic Reviews, Issue 1, 2005.
Newnham JP, Evans SF, Mehael CA, et al. Effects of frequent ultrasound during pregnancy: a randomised controlled trial. Lancet. 1993;342:887-891.
NHS Sickle Cell and Thalassaemia Screening Programme. Online. Available www.sickleandthal.org.uk, 2004.
NICE (National Institute for Clinical Excellence). Antenatal care. Routine care for the healthy pregnant woman, National Collaborating Centre for Women’s and Children’s Health, RCOG Press, London, 2003. Online. Available www.rcog.org.uk/resources/Public/pdf/Antenatal_Care.pdf.
Nicolaides K, de Lourdes BM, Patel F, et al. Comparison of chorionic villus sampling and amniocentesis for fetal karyotyping at 10–13 weeks gestation. Lancet. 1994;344:435-439.
Nicolaides KH, Souka AP, Noble PL. Fetal nuchal translucency at 10–14 weeks of gestation. In: Rodeck C, Whittle MJ, editors. Fetal medicine. Basic science and clinical practice. London: Churchill Livingstone; 1999:573-580.
NMC (Nursing and Midwifery Council). Midwives rules and standards. London: NMC, 2004.
Oliver S, Rajan L, Turner H, et al. A pilot study of ‘informed choice’ leaflets on positions in labour and routine ultrasound. York: NHS Centre for Reviews and Dissemination, 1996.
Raphael-Leff J. Psychological processes of childbearing. London: Chapman & Hall, 1991.
RCOG (Royal College of Obstetricians and Gynaecologists). Routine ultrasound screening in pregnancy. Protocol, standards and training. Supplement to ultrasound screening for fetal abnormalities report of the RCOG working party, RCOG, London, 2000. Online. Available www.rcog.org.uk/index.asp?PageID=1185#20week.
Romero R, Ghidini A, Costigan K, et al. Prenatal diagnosis of duodenal atresia: does it make any difference? Obstetrics and Gynaecology. 1989;71:739-741.
Rothman B. The tentative pregnancy. How amniocentesis changes the experience of motherhood. New York: Norton Paperbacks, 1986.
Salvesen KA. Epidemiology of diagnostic ultrasound exposure during human pregnancies. BMUS Bulletin November. 1997:32-34.
Salvesen KA, Vatten LJ, Eik-Nes SH, et al. Routine ultrasonography in utero and subsequent handedness and neurological development. British Medical Journal. 1993;307:159-164.
Sandelowski M. Channel of desire: fetal ultrasonography in two-use contexts. Qualitative Health Research. 1994;4:262-280.
Sedgman B, McMahon C, Cairns D, et al. The impact of two-dimensional versus three dimensional ultrasound exposure on maternal-fetal attachment and maternal health behavior in pregnancy. Ultrasound in Obstetrics and Gynecology. 2006;27:245-251.
Simpson JL, Socol MI, Aladam S. Normal fetal growth despite persistent amniotic fluid leakage after genetic amniocentesis. Prenatal Diagnosis. 1981;1:277-279.
Sjogren B. Psychological indications for prenatal diagnosis. Prenatal Diagnosis. 1996;16:449-454.
Snijders R, Nicolaides K. Ultrasound markers for fetal chromosomal defects. London: Parthenon, 1996.
Stark C, Orleans M, Haverkamp A, et al. Short and long-term risks after exposure to diagnostic ultrasound in utero. Obstetrics and Gynaecology. 1984;63:194-200.
Sullivan A. Skilled decision making: the blood supply of midwifery practice. In: Raynor M, Marshall J, Sullivan A, editors. Decision making in midwifery practice. London: Elsevier, 2005.
Thornton JG, Hewison J, Lilford RJ, et al. A randomised trial of three methods of giving information about prenatal testing. British Medical Journal. 1995;311:1127-1130.
Tindall N. Psychology of childbearing. Midwifery practice guides 6. Hale, Cheshire: Books for Midwives Press, 1997.
Turner L. Problems surrounding late prenatal diagnosis. In: Abramsky L, Chapple J, editors. Prenatal diagnosis. The human side. London: Chapman & Hall, 1994.
UK National Screening Committee. Antenatal screening working standards. National Down Syndrome Screening Programme for England. Online. Available www.screening.nhs.uk/downs/working-standards.pdf, 2004.
Wald NJ, Rodeck C, Hackshaw AK, et al. First and second trimester antenatal screening for Down syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS). Health Technology Assessment. 2003;7(11):1-77.
Whittle MJ. Ultrasonographic ‘soft markers’ of fetal chromosomal defects. British Medical Journal. 1997;314:918.
Wood P. Safe and (ultra)sound some aspects of ultrasound safety. Royal College of Midwives Journal. 2000;3(2):48-50.
Sullivan A, Kean L, Cryer A, editors. Midwifes’ guide to antenatal investigations. London: Elsevier, 2006.
A practical guide for midwives to use when discussing and interpreting antenatal test results. Covers maternal and fetal investigations
Screening Choices: A learning resource for health professionals offering antenatal and newborn care. Prepared for UK National Screening Committee by Homerton School of Health Studies and Jill Rogers Associates, www.screening.nhs.uk/cpd/webfolder/web_nsc.html Training resource covering genetics, understanding and communicating risk, informed choice, the parent perspective.
DIPEx – Patient experiences website, www.dipex.org/Experiences.aspx
Includes a range of pregnancy and screening experiences from the woman’s perspective. Includes video clips of interviews with women who talk about their experiences