Chapter 21 Medical disorders associated with pregnancy
Pregnancy may be complicated by a variety of disorders and conditions that can profoundly affect the woman and her family. Care for women with pre-existing medical disorders (PEMD) should ideally take place before conception in multidisciplinary pre-pregnancy clinics. This process should begin during adolescence with discussions about family planning, contraception and pregnancy. A complete medical history and assessment of health at this time, including obtaining up-to-date investigations, enables a risk assessment for pregnancy to be made. These risks should be discussed with the woman and her family so that appropriate choices can be made.
The pathophysiology of these disorders may adversely affect the pregnancy. Similarly, the physiological changes occurring in pregnancy may modify the clinical course of these disorders and their management. Women with PEMD have high-risk pregnancies and a collaborative multidisciplinary approach is recommended to ensure careful monitoring of both the woman and her fetus.
Introduction
During pregnancy, women with PEMD require regular review by the multidisciplinary team hence total midwife-led care is inappropriate, however it is important that they have ‘routine’ midwifery input and support. The frequency of visits at hospital clinics will depend on the underlying condition and it must be decided early in pregnancy if all care is at the hospital or if shared with the community team. Women who miss appointments should be immediately contacted and followed up. Women should be counselled about potentially worrying symptoms and advised to attend hospital if such symptoms develop or if they feel unwell. Equally midwives and doctors need to be aware and recognize the clinical signs and symptoms of deteriorating maternal health (NICE 2007). Recognition of acute illness is often delayed and its subsequent management may be inappropriate leading to late referral to senior medical care, admission to a critical care unit and may lead to maternal death, particularly where the initial standard of care is sub-optimal (Lewis 2007).
Labour and birth in women with PEMD can be a time of additional challenges. Timing and mode of birth should be carefully planned and should take place in a hospital with neonatal facilities. Promoting normality is key although invasive monitoring may be required in symptomatic women and those with severe disease. In general, vaginal birth is preferred and a caesarean section should only be undertaken if clinically indicated (Bridges et al 2003).
Where there is chronic illness this can have a profound effect on the physical, psychological, sexual and social aspects of women’s lives. Involvement of the woman and her family in decisions regarding her care engenders feelings of autonomy and control over a condition that has the potential to result in the medicalization of childbirth. Midwives have a role in supporting women and their families, ensuring that their needs are met and that the pregnancy is treated as normal, so far as is possible (Harrison et al 2003).
Cardiac disease
In most pregnancies, heart disease is diagnosed before pregnancy. There is, however, a small but significant group of women who will present at an antenatal clinic with an undiagnosed heart condition. Although heart disease complicates <1% of maternities, it continues to contribute significantly to maternal morbidity and mortality and is the leading cause of maternal death overall in the UK (Lewis 2007). Heart disease can be broadly classified into ‘congenital’ and ‘acquired’. Those more likely to be seen in pregnancy are described below.
Congenital heart disease
The most common congenital heart diseases (CHD) found in pregnancy are atrial septal defect (ASD), ventricular septal defect (VSD), patent ductus arteriosus (PDA), pulmonary stenosis, aortic stenosis and tetralogy of Fallot. The majority of these lesions will have been corrected surgically in childhood resulting in a growing population of women with CHD compatible with pregnancy. Uncorrected lesions may cause pulmonary hypertension, cyanosis and severe left ventricular failure and are therefore high risk for pregnancy. CHD is also associated with increased fetal complications linked with the maternal functional class and the degree of cyanosis. These include fetal loss, intrauterine growth restriction, pre-term birth and an increased risk of fetal CHD (Head & Thorne 2005).
Particularly high risk cardiac conditions for pregnancy include:
Acquired heart disease
Rheumatic heart disease
Rheumatic heart disease (RHD) has declined progressively in Europe and North America but worldwide it remains the most common cardiac problem. RHD causes inflammation and scarring of the heart valves and results in valve stenosis, plus or minus regurgitation. The mitral valve is most often affected with stenosis, occurring in two-thirds of cases. This condition is often diagnosed because of severe breathlessness and tiredness for the first time during pregnancy – particularly in immigrant or refugee women who have not had access to medical care. Most women with valvular heart disease can be managed medically which aims to reduce the work rate of the heart. During pregnancy, this involves bed rest, oxygen therapy and the use of cardiac drugs e.g. diuretics (reduce fluid load), digoxin (reduces and regulates the heart rate) and heparin (reduces risk of thromboembolic disease). Women with more severe symptomatic disease may require surgical intervention such as balloon valvoplasty or valve replacement, although both of these procedures carry a degree of maternal and fetal mortality. Antibiotic prophylaxis is recommended for all women with valvular lesions during labour (Gelsen et al 2007, Prasad & Ventura 2001).
Myocardial infarction and ischaemic heart disease
Myocardial infarction (MI) and ischaemic heart disease (IHD) are uncommon cardiac complications but are an increasing cause of maternal death in the UK. Identifiable risk factors include increasing maternal age, obesity, diabetes, pre-existing hypertension, smoking, family history and inequalities in health (BCS 2007, Lewis 2007). A myocardial infarction is most likely to occur in the third trimester and peripartum period when haemodynamic changes are having their maximum effect and there is a higher risk of thrombotic events due to the hypercoagulability induced by hormonal changes. In the immediate postpartum period, spontaneous coronary artery dissection is the most common cause of MI. Typically, women present with ischaemic chest pain in the presence of an abnormal ECG and elevated cardiac enzymes although these signs and symptoms may be masked during labour and birth (Ray et al 2004). Atypical features include abdominal or epigastric pain and vomiting. Primary percutaneous transluminal coronary angioplasty (PTCA) which improves the patency of blocked arteries is first line therapy for this condition (Baird & Kennedy 2006).
Aortic dissection (acute)
Aortic dissection (acute) may occur in pregnancy in association with severe hypertension (systolic >160 mmHg) due to pre-eclampsia, coarctation of the aorta or connective tissue disease such as Marfan’s syndrome. The woman typically presents with severe chest or intrascapular pain. Early diagnosis using computed tomography chest scan or MRI or transoesophageal echocardiogram is critical as maternal mortality is high (Lewis 2007, Ray et al 2004).
Endocarditis
Endocarditis is an inflammation of the heart usually involving the heart valves. Although rare in pregnancy, it is one of the most serious complications of heart disease. Women with valvular heart disease, prosthetic valves, a previous history of endocarditis, periodontal disease and intravenous substance misusers are particularly vulnerable to this condition. Streptococcal organisms are the most common cause and give rise to the subacute form of the disease. Acute endocarditis is due to more virulent organisms such as Staphylococcus aureus, Streptococcus pneumoniae and Neisseria gonorrhoeae. Primary prevention includes recognition of risk factors and use of strategies to minimize bacteraemia, e.g. good dental hygiene, avoidance of drug misuse, early treatment of sepsis and administration of antibiotic prophylaxis to women with high risk cardiac conditions (Stuart 2006).
Peripartum cardiomyopathy
Peripartum cardiomyopathy is a relatively rare but potentially fatal disease; mortality rates range from 25% to 50% with a significant number of deaths occurring shortly after the onset of signs and symptoms. Diagnosis is made within a specific period of time, occurring between the last month of pregnancy and the first 5 months postpartum and commonly women have no previous history of heart disease. It is associated with older and multiparous women, hypertension, pre-eclampsia, obesity and diabetes. It has also been linked to myocarditis, viral infection, long-term oral tocolytic therapy and cocaine misuse. Inflammation and enlargement of the myocardium (cardiomegaly) give rise to left ventricular heart failure and thromboembolic complications. Treatment involves use of medication (oxygen, diuretics, vasodilators) to decrease pulmonary congestion and fluid overload, inotropic agents to improve myometrial contractility and anticoagulation therapy. As the cardiomegaly resolves there should be a corresponding improvement in the woman’s condition but this process may take up to 6 months and there is a risk of recurrence in a subsequent pregnancy. In some women, left ventricular dysfunction persists and unless a heart transplant is performed mortality will be high (Palmer 2006, Pryn et al 2006).
Changes in cardiovascular dynamics during pregnancy
In normal pregnancy the haemodynamic profile alters in order to meet the increasing demands of the growing fetoplacental unit (see Ch. 14). Normal, healthy pregnant women are able to adjust to these physiological changes quite easily. In women with coexisting heart disease, however, the added workload can precipitate complications. The three peak periods of cardiovascular stress (28–32 weeks of pregnancy, during labour, 12–24 hrs postpartum) are the most critical and life threatening for women with heart disease (Cox et al 2005).
Recognition of cardiac compromise
Many of the symptoms of normal pregnancy resemble those of heart disease. The symptoms and signs of cardiac compromise include: fatigue, shortness of breath (dyspnoea), difficulty in breathing unless upright (orthopnoea), palpitations, bounding/collapsing pulse, chest pain, development of peripheral oedema, distended jugular veins and progressive limitation of physical activity. Severity of cardiac disease may be classified by the degree of functional compromise (Table 21.1).
Table 21.1 The New York Heart Association’s functional classification of cardiac disease
| Class | Definition |
|---|---|
| Class I | No limitation of physical activity. Ordinary activity does not cause undue fatigue, palpitations, dyspnoea or angina |
| Class II | Slight limitation of physical activity. Comfortable at rest. Ordinary physical activity results in fatigue, palpitations, dyspnoea or angina |
| Class III | Marked limitation of physical activity. Comfortable at rest. Less than ordinary physical activity results in fatigue, palpitations, dyspnoea or angina |
| Class IV | Inability to carry on any physical activity without discomfort. Symptoms of cardiac insufficiency or angina may be present even at rest, and are intensified by activity |
| Dobbenga – Rhodes & Pride 2006 | |
Diagnosis
Along with the signs and symptoms, physical assessment and laboratory tests can assist with the diagnosis of cardiac disease and determine the type of lesion together with an assessment of current functional capacity. These may include:
Risks to mother and fetus
The majority of pregnancies complicated by maternal heart disease can be expected to have a favourable outcome for both mother and fetus. The risk for morbidity and mortality depends on: (1) the nature of the cardiac lesion, (2) its affect on the functional capacity of the heart and (3) the development of pregnancy-related complications such as hypertensive disorders of pregnancy, infection, thrombosis and haemorrhage. Congestive heart failure precipitated by the altered haemodynamic state is a serious complication that may result in maternal death and can occur at any time during pregnancy (Lewis & Drife 2004). Adverse fetal effects are the result of decreased uterine blood flow or decreased maternal oxygenation. This can lead to spontaneous abortion, intrauterine growth restriction, fetal hypoxia, preterm birth and intrauterine death. If either parent has a congenital heart defect this may be inherited by their offspring (Tan 1999).
Preconception care
Women with known heart disease should seek advice from a cardiologist and an obstetrician before becoming pregnant. Preconception and antenatal counselling offer women information regarding the risks of the pregnancy to themselves and their baby, enables assessment of their functional status and whether pregnancy should be avoided. The woman’s condition should be optimized and general health advice given by the midwife.
Antenatal care
The symptoms of normal pregnancy can mimic the signs and symptoms of heart disease, e.g. dypsnoea on exertion, orthopnoea, palpitations, dizziness, fainting, a bounding pulse, tachycardia, peripheral oedema, distended jugular veins and alterations in heart sounds. Maternal investigations should be carried out prior to and at the onset of pregnancy to gain baseline referral points.
Management
Management requires a multidisciplinary approach involving midwives, obstetricians, cardiologists and anaesthetists (Badawy & El-Metwally 2001). During the antenatal period women with heart disease are monitored more frequently than healthy pregnant women. The aim is to maintain a steady haemodynamic state and prevent complications, as well as promote physical and psychological well-being. Visits to a joint clinic run by a cardiologist and obstetrician are usually every 2 weeks until 30 weeks’ gestation and weekly thereafter until birth. At each visit the woman is asked about cardiac symptoms and whether there is any limitation on her activities. The severity of the heart lesion is assessed by clinical examination.
Physical and psychological care
The midwife can give advice with regard to modifying and adjusting physical activity during pregnancy. Some women will need to commence maternity leave earlier than anticipated. In late pregnancy, women may require admission to hospital for rest and close monitoring. Psychological support by the midwife is important. Where possible, care in the community is preferable, although the importance of adequate rest should be emphasized.
Dietary advice
Guidance should be given about what constitutes a well-balanced diet. Cholesterol, sodium-rich foods and salt should be restricted. Weight gain should be monitored in these women as excess weight gain will place additional strain on the heart. Compliance with taking iron and folic acid supplementation is important for preventing anaemia.
Prevention of infection
Infections often cause a pyrexia and tachycardia, which will put an added strain on the heart. In addition the infective organism can cause further damage in women with heart lesions by causing endocarditis. The midwife needs to advise the woman about how she may identify respiratory, urinary and vaginal infections and the necessity of seeking treatment as quickly as possible. An early dental examination is important to detect and treat caries and gum disease, which may precipitate endocarditis. Prophylactic antibiotic therapy is recommended for women who are at high risk of endocarditis (Endocarditis Working Party of the British Society of Antimicrobial Chemotherapy 1990). All invasive procedures should be carried out using a strict aseptic technique and the number of vaginal examinations in labour should be kept to a minimum.
Antithrombotic therapy
The hypercoagulable state in pregnancy increases the risk of thromboembolic disease in women who have arrhythmias, mitral valve stenosis or who have had mechanical cardiac valve replacements.
The treatment of women requiring antithrombotic therapy during pregnancy is difficult. Warfarin is commonly used in the non-pregnant state but is teratogenic in early pregnancy and is associated with a high fetal loss rate. It also predisposes the woman and her fetus to haemorrhage when used in the third trimester. Subcutaneous low molecular weight heparins, such as enoxaparin, are useful for thromboprophylaxis (Ellison 2000) but may not be suitable for women with mechanical heart valves. The advice of a haematologist should be sought. High thromboembolic support stockings should be worn if the woman is admitted for rest and assessment. They should also be worn during labour and in the immediate postnatal period.
Intrapartum care: the first stage of labour
A coordinated team approach with good communication between the midwife, obstetrician, cardiologist, neonatologist, anaesthetist, the woman and her family is essential. Women with heart disease may have uncomplicated labours. Vaginal birth is preferred unless there is an obstetric indication for caesarean section. The advantages of a vaginal birth are: less blood loss, greater haemodynamic stability, avoidance of surgical stress and less chance of postoperative infection and pulmonary complications (Ray et al 2004). Optimal management involves monitoring the maternal condition to include: temperature, pulse, respiration, blood pressure, fluid intake and urine output. Observation of the fetal condition is also required using electronic fetal monitoring. Continuous electrocardiography (ECG) is recommended in nearly all cases and pulse oximetry may be utilized to assess arterial haemoglobin saturation, which may be reduced in women with heart disease owing to disruption of normal gas exchange between the lungs and blood. If oxygen saturation levels fall below 92%, oxygen therapy will be required. The use of invasive haemodynamic studies via an arterial line may be required in women with moderate to severe heart disease. Blood and urine tests are undertaken during labour to determine the haematological and metabolic changes.
Fluid balance
Fluid management requires judicial use of intravenous fluids and fluid may need to be restricted. Overload will lead to an increase in the circulating blood volume and development of pulmonary oedema and congestive heart disease (Witcher & Harvey 2006).
Pain relief
The midwife should help the woman to use the techniques that she has learned for coping with stress. An epidural may be the analgesia of choice as it is an effective form of analgesia that decreases cardiac output and heart rate. It causes peripheral vasodilatation and decreases venous return, which alleviates pulmonary congestion. Nitrous oxide and oxygen and pethidine are usually considered safe, but it is important to consult a doctor before administering any form of pain-relieving drug to a woman with a heart condition (Durbridge et al 2006).
Positioning
Cardiac output is influenced by the position of the woman during labour. Women with heart disease are particularly sensitive to aortocaval compression by the gravid uterus in the supine position. It may be necessary to recommend an upright position or left lateral position for some women to adopt during labour and the birth, according to their individual condition.
Pre-term labour
Beta sympathomimetic drugs widely used for the treatment of preterm labour are contraindicated in women with heart disease, since they cause tachycardia and predispose to pulmonary oedema. Consideration may be given in individual cases if it is safe to use oxytocin antagonists.
Induction
The least stressful labour for a woman with cardiac disease will be spontaneous in onset. Prostaglandins should be used with caution as they are potent vasodilators and cause a marked increase in cardiac output. Oxytocin by intravenous infusion causes a degree of fluid retention and it is important for the midwife to keep a careful record of fluid balance if this is used.
The second stage of labour
This should be short without undue exertion on the part of the mother. Prolonged pushing with held breath (the Valsalva manoeuvre), may be dangerous for a woman with heart disease. It raises the intrathoracic pressure, pushes the blood out of the thorax and impedes venous return, with the result that cardiac output falls. The midwife should encourage the woman to breathe normally and follow her natural desire to push, giving several short pushes during each contraction. Forceps or ventouse may be used to shorten the second stage. Care should be taken when the woman is in the lithotomy position, where the lower part of the body is higher than trunk, as this produces a sudden increase in venous return to the heart, which may result in heart failure (Durbridge et al 2006). A wedge should be used to avoid aortocaval compression.
The third stage of labour
This is usually actively managed owing to the increased risk of postpartum haemorrhage (PPH). Oxytocin is the drug of choice but its use in the prevention of PPH must be balanced against the risk of oxytocin-induced hypotension and tachycardia in women with cardiovascular compromise (Lewis & Drife 2004). Administration should follow the guidance in the British National Formulary (BMA & RPS 2001) and when given as an intravenous bolus the drug should be given slowly in a dose that should not exceed 5 IU. Ergot-containing preparations such as ergometrine are contraindicated in cardiac conditions which would be worsened by a rise in blood pressure.
Postnatal care
The first 48 hrs following birth are critical for the woman with significant heart disease. The heart must be able to cope with the extra volume of blood (autotransfusion) from the uterine circulation as well as the increased venous return following relief of aortocaval compression of the uterus. Conversely, total blood volume may be diminished by loss at birth and during the postnatal period and the heart will need to compensate if blood flow is impaired due to postpartum haemorrhage. Close monitoring of haemodynamic changes is required at this time and the midwife should identify early signs of infection, thrombosis or pulmonary oedema (Ramsay 2006). Breastfeeding should be encouraged as cardiac output is not affected by lactation although drug therapy for specific heart conditions may need to be reviewed for safety during breastfeeding. The midwife provides support with breastfeeding as is usual (see Ch. 41), with emphasis placed on the need for adequate rest and a dietary intake with sufficient calories to support breastfeeding. Discharge planning is particularly important for women with heart disease. The midwife can evaluate the help and support that will be available in the home during the postnatal period. Relatives and friends often fulfill this need but community support services should be considered if necessary. The woman and her partner should discuss with the cardiologist and obstetrician the implications of a future pregnancy and be given appropriate contraceptive advice (Thorne et al 2006).
Respiratory disorders
Asthma
The prevalence of active asthma in the UK is 5.8% (Pinnock & Shah 2007) and affects 3–12% of pregnant women worldwide with 5.8% of women hospitalized for asthma during pregnancy (Murphy et al 2006). Prevalence is increasing mainly due to environmental factors such as: change in indoor environment, smoking, family size, pollution and diet (Rees 2005).
Effect of asthma on pregnancy
Pregnancy does not consistently affect asthmatic status; some women experience no change in symptoms whereas others have a distinct worsening of the disease. The mechanisms that contribute to the varying changes in asthma during pregnancy are not well understood, although increases in maternal circulating hormones (cortisol, oestradiol and progesterone), altered β2-adrenoreceptor responsiveness and immune function or the presence of a female fetus may be involved (Murphy et al 2005). When asthma is well controlled maternal and fetal outcomes are similar to those in women without asthma. Women with severe disease and those who have poor control of asthma seem to have an increased incidence of adverse maternal and neonatal outcomes including preterm labour (Schatz et al 2006).
Diagnosis
The characteristic symptoms of asthma are: chest tightness, dyspnoea, wheezing and coughing. Measuring peak expiratory flow (PEF) using a PEF meter is a useful tool for making a diagnosis and determining how well a person’s asthma is controlled (Booker 2007). PEF monitors the level of resistance in the airways caused by inflammation or bronchospasm, or both and values are lower than predicted in people with asthma. A range of normal values can be predicted for each person according to sex, height and age. Knowledge of the usual PEF and self-monitoring at home will enable a person with asthma to determine when to take or increase their medication and when to seek medical attention. Hospital admission is usually required if the PEF is <50% of the normal value and the person is too breathless to complete sentences.
Management
The BTS & SIGN (2005) guidelines recommend a stepwise approach to management of asthma. Therapy should be initiated to establish quick control of the asthma symptoms and then stepped down to the minimum medication necessary to maintain control. Treatment relies on inhaled bronchodilators and inhaled steroids with or without oral steroids. Nebulized drugs are given during acute attacks of asthma.
Antenatal care
Care should ideally be provided jointly between the midwife, GP, chest physician and obstetrician. At the booking interview the midwife should be able to discuss with the woman the frequency and severity of her asthma, family history, any known asthma triggers and current treatment. The main anxiety for women and those providing care relates to the use of asthma medication and its effect on the fetus. In general, the medications used in the treatment of asthma, including systemic steroids, are considered safe to use in pregnancy (BTS & SIGN 2005). It is crucial that therapy is maintained during pregnancy as a severe asthma attack may result in a deterioration in the maternal condition and a reduction in the oxygen supply to the fetus. Respiratory tract infections should be diagnosed and treated promptly in order to prevent an acute asthma attack. If during the pregnancy there are any difficulties in controlling the symptoms of asthma the woman should be admitted to hospital.
Intrapartum care
An increase in cortisone and adrenaline (epinephrine) from the adrenal glands during labour is thought to prevent attacks of asthma during labour (BTS & SIGN 2005). If an asthma attack does occur this should be treated in the usual way. Women should continue their usual asthma medications during labour and it is important that they remain well hydrated. Maternal and fetal condition should be monitored closely, namely: respiratory function, pulse oximetry, oxygen therapy and continuous fetal heart rate monitoring. All forms of pain relief may be used although regional anaesthesia reduces hyperventilation and the stress response to pain. It is also advocated for operative delivery as it avoids the potential complications of ventilating people with asthma (Kuczkowski 2005). Certain aspects of labour management in women with asthma require special attention. These include: the use of β2-adrenergic antagonists for the treatment of hypertension and the use of ergometrine or carboprost (prostaglandin F2a) for the management of postpartum haemorrhage. These drugs may cause bronchospasm and should be avoided or used with caution (Kuczkowski 2005). Oxytocin and prostaglandin E2 are safe to use for the induction of labour (BTS & SIGN 2005). Women who have received corticosteroids in pregnancy (>7.5 mg prednisolone/day for >2 weeks prior to the onset of labour) should receive parenteral hydrocortisone 100 mg 6–8-hourly during labour (BTS & SIGN 2005).
Cystic fibrosis
Cystic fibrosis (CF) is an autosomal recessive multi-system disorder which significantly reduces life expectancy (Rowe et al 2005). People with CF develop chronic obstructive lung disease (poor peak flow, reduced forced expiratory volume (FEV1) and decreased oxygen saturation). Obstruction of the pancreatic ducts leads to a loss of acinar cells and replacement by fibrous tissue and fat. Loss of pancreatic function causes poor digestion, malnutrition and the development of type 1 diabetes. Many women with CF now live into their reproductive years in good health and although fertility may be slightly reduced, principally because of alteration in the chemical make-up of the cervical mucus, pregnancies are possible.
Pre-pregnancy care
One in 25 people carry the defective gene and therefore if the partner is a carrier there is a one in two chance that their children will have CF. Specific changes in respiratory, cardiac and pancreatic function as well as increased nutritional demands during pregnancy pose a serious health risk for many women with CF and should be assessed prior to pregnancy (McMullen et al 2006).
Antenatal care
Midwifery, obstetric, dietetic, medical, nursing and physiotherapy expertise are essential. Specific assessment includes pulmonary function tests, arterial blood gases, sputum culture, liver function tests, glucose tolerance test, chest radiogram, electrocardiogram, echocardiogram and monitoring of weight gain. Compliance with antibiotic therapy must be stressed as the potential risks to the fetus are outweighed by the risk of the mother developing a severe lung infection. In addition, it is important to pay attention to nutrition and CF-related diabetes, the risks of which increase with age and are more likely to be problematic in pregnancy (Bussey & Mittelstaedt 2003).
Intrapartum care
During labour close monitoring of cardiorespiratory function will be required and an anaesthetist should be involved at an early stage. Fluid and electrolyte management requires careful attention as women with CF may easily become hypovolaemic from the loss of large quantities of sodium in sweat. Epidural analgesia is the recommended form of pain relief in labour and general anaesthesia should be avoided because of the potential risks from respiratory complications (Cameron & Skinner 2005, Holdcroft & Thomas 2000).
Postnatal care
Women should be cared for in a high dependency unit as cardiorespiratory function often deteriorates following birth. Sodium concentration in breastmilk has been found to be similar to women without CF and therefore breastfeeding is not contraindicated. However, in order for breastfeeding to be successful women need to be well nourished and maintain an adequate calorie intake. As CF is the UK’s most common life-threatening inherited disease, it is recommended that universal neonatal testing is undertaken as part of the UK newborn blood spot screening programme (UKNSPC 2005).
Pulmonary tuberculosis
Tuberculosis (TB) is an air-borne infectious disease caused by the tubercule bacillus, Myobacterium tuberculosis. It is transmitted through inhalation of infected air-borne droplets from a person with infectious TB. It may also be contracted from infected cattle through the consumption of milk and dairy products that have not been pasteurized. The lungs are the organ most commonly affected (pulmonary TB) although it may spread to other areas parts of the body typically bones, joints and the lymphatic, genitourinary and central nervous system (extrapulmonary TB). All forms of TB are notifiable under the Public Health (Control of Disease) Act 1984 and therefore primary healthcare workers including midwives are among the first to be involved in the prevention, screening and treatment of TB (NICE 2006, Royal College of Physicians 2006). Contributory factors leading to the increasing incidence of this disease include: (1) women and children who have immigrated to the UK from areas where TB is endemic, principally South-Asia and sub-Saharan African countries, (2) the development of drug-resistant organisms and (3) increases in adults and children who have become infected with HIV (HPA 2007). Most cases occur in inner city areas where additional social factors such as poverty, homelessness, substance misuse, poor nutrition and crowded living conditions contribute to the transmission of the disease (HPA 2007, Kothari et al 2006). TB is primarily a disease of poverty and almost all cases are preventable.
Diagnosis
The onset of primary TB is often insidious and the symptoms are non-specific: fatigue, malaise, loss of appetite, loss of weight, alteration in bowel habit and low grade fever. These can be interpreted as usual symptoms occurring in pregnancy leading to a delay in diagnosis (Kothari et al 2006). The classic symptoms of chronic cough, intermittent fever, night sweats, haemoptysis, dyspnoea and chest pain occur quite late in the disease process and are often absent when the TB is extrapulmonary.
Measures to increase awareness about TB in the immigrant population and in the community, and to provide access to medical care could reduce delays in diagnosis (DH 2007). The presence of risk factors requires assessment via the Mantoux tuberculin skin test and/or an interferon-γ (secreted by lymphocytes in the presence of antigens to TB) test. A thorough history and physical examination should also be undertaken. A positive tuberculin test should be further evaluated with a chest X-ray; abdominal shielding for this procedure keeps fetal exposure to a minimum. Microscopic examination and culture of sputum are also required to confirm active mycobacterial infection and identify drug sensitivity (HPA 2006, Ormerod 2001). Once active TB has been diagnosed, the need for contact tracing must be assessed and testing and treatment of asymptomatic household and other close contacts implemented in order to prevent spread of the disease (NICE 2006).
Management
All pregnant women with TB should be under the care of a specialist physician and a named key worker with full training in the disease who manage the clinical aspect of the woman’s treatment (NICE 2006). It is important that they work collaboratively with the midwife, GP and obstetrician. The key to a successful outcome is to ensure that the woman is involved in treatment decisions and adheres to the prescribed treatment. Figueroa-Damian & Arredondo-Garcia (2001) found that maternal morbidity and mortality are significantly higher where active TB remains untreated and when treatment is started late in pregnancy. In addition, neonates of women with TB have a higher risk of prematurity and perinatal death and low birth weight.
Standard anti-tuberculous therapy is considered safe in pregnancy (HPA 2006). TB is treated in two phases. The first involves taking rifampicin, isoniazid, pyrazinamide and ethambutol daily for 2 months. In the second (continuation) phase, rifampicin and isoniazid are taken for a further 4 months (NICE 2006). Congenital deafness has been reported in infants with exposure to streptomycin in utero and therefore this anti-tuberculous drug is avoided in pregnancy. Part of the midwife’s role during this time is to assist the named key worker to ensure that women are compliant with the drug therapy and understand the importance of adhering to the regimen in order to cure the disease and prevent the bacillus becoming resistant to the drugs. The majority of women will require minimum supervision and a monthly review will be sufficient to monitor progress (Kothari et al 2006). For those women at risk of non-adherence care is planned individually and thrice weekly directly observed therapy (DOTS) may be required (NICE 2006).
Attention should also be placed on rest, good nutrition and education with regard to preventing the spread of the disease. TB usually becomes non-infectious by 2 weeks of treatment. Where possible the treatment is undertaken in the woman’s home to disrupt family life as little as possible. Some women may require admission to hospital because of the severity of the illness or adverse effects of drug therapy, for obstetric reasons such as the onset of labour, for social reasons or for further investigations. Risk assessment should be made in order to determine appropriate infection control measures.
Providing all TB bacteria are killed, the person with TB is cured. In a small number of people, the disease can return if not all bacteria have been killed. This is more likely to occur where there is poor/no compliance with drug treatment or where there is multi-drug resistant (MDR) TB.
Postnatal care
Following birth, babies born to mothers with infectious TB should be protected from the disease by the prophylactic use of isoniazid syrup (5 mg/kg per day) and pyridoxine (5–10 mg/day) for 6 weeks and then to be tuberculin tested. If negative, the neonatal Bacille Calmette–Guérin (BCG) vaccination should be given and drug therapy discontinued. If the tuberculin test is positive the baby should be assessed for congenital or perinatal infection and drug therapy continued if these are excluded (HPA 2006). The baby cannot be infected by the mother via the breastmilk unless she has tuberculous mastitis. Additionally the concentration of the anti-tuberculous drugs in breastmilk is insufficient to cause harm in the neonate, therefore in the majority of cases breastfeeding should be encouraged (HPA 2006).
Caring for a child at home makes great demands on the mother and extra help should be arranged if possible. Midwives should explain that poor nutrition, stress and overtiredness will encourage a recurrence of active disease. Family planning advice is an integral part of postnatal and preconception care and it is advisable for a woman with TB to avoid further pregnancies until she has been disease-free for at least 2 years. When choosing her method of family planning, the woman needs to be aware that rifampicin reduces the effectiveness of oral contraception (BMA & RPS 2001). Long-term medical and social follow-up is necessary.
The outcome for both mother and baby is improved by early diagnosis and effective treatment. Midwives are pivotal in ensuring compliance with drug therapy and providing general health education during pregnancy in order to reduce the incidence of TB in both the obstetric and the general population.
Renal disease
A knowledge of the changes in renal physiology and function in healthy pregnancy is crucial to the midwife’s understanding of the impact of pregnancy on existing renal disease and the predisposition women have to develop urinary tract infections (see Ch. 14).
Urinary tract infection
Urinary tract infection (UTI) is the presence of significant bacteria in a clean-catch or catheter specimen of urine, most commonly described as a colony of at least 100 000 bacteria/mL of urine. Infection in the lower urinary tract may originate in the urethra (urethritis) or bladder (cystitis) and if untreated ascend into the upper urinary tract and affect the kidneys (pyelonephritis). Symptoms of a lower urinary tract infection include burning or pain on urination (dysuria), frequent passing of small amounts of urine (frequency), a change in the smell of the urine, the presence of blood in the urine (haematuria) and discomfort in the suprapubic area. The presence of fever (pyrexia >38 °C), rigors, tachycardia, nausea and vomiting leading to dehydration, pain and tenderness over the kidney area is indicative of pyelonephritis.
Acute pyelonephritis occurs in 1–2 % of pregnant women (Norman et al 2001) and Sharma & Thapa (2007) found that it most commonly occurs: in nulliparous women; the younger age group (20–29 years) and at the end of the second/beginning of the third trimester and the puerperium. Examination of the urine shows it to be cloudy with the presence of white blood cells (leucocytes) and the infecting organism is often Escherichia coli.
UTIs in pregnancy need to be treated promptly to prevent the development of maternal morbidity (chronic renal insufficiency, transient renal failure, acute respiratory distress syndrome (ARDS), sepsis and shock) as well as fetal morbidity and mortality (pre-labour rupture of membranes, chorioamnionitis, preterm labour and birth) (Gilstrap & Ramin 2001, Vazquez & Villar 2003).
Management
The diagnosis of UTI/pyelonephritis is usually made on presenting symptoms and dipstick urinalysis using nitrite and leucocyte detectors (Bent et al 2002); this may be confirmed by urine microscopy and culture. Vaginal infections and sexually transmitted diseases such as Chlamydia trachomatis may mimic symptoms of UTI and should be excluded. Referral to a doctor is required and in cases of pyelonephritis admission to hospital is usual in order that intravenous antibiotics can be administered. During the early stages of the illness the woman will feel quite ill. Severe nausea and vomiting will lead to dehydration and intravenous fluids may be required. A record of fluid balance is maintained to assess renal function. The midwife should provide general nursing care; this will include regular observation of temperature, pulse, blood pressure and respiratory rate. The temperature should be reduced by tepid sponging and antipyretics. Uterine activity should be monitored to detect the onset of pre-term labour. The midwife should also take steps to prevent complications of immobility such as deep vein thrombosis. This requires use of antithrombotic stockings and the doctor may prescribe low dose heparin therapy.
Antibiotic therapy is effective in curing urinary tract infections although there is insufficient data to recommend any one specific treatment regime. Many different drugs may be used, given by oral or i.v. route with the course of treatment dependent on the drug used. Repeat cultures should be done 2 weeks after completion of the course of treatment and monthly until birth in order to ensure there is no recurrence. Women who develop recurrent UTI may require prophylactic antibiotic treatment throughout pregnancy. Follow-up examination of the renal system (excretion urography) is often undertaken 3 months postnatally as persistent or recurrent infection, with or without symptoms, may be associated with an abnormality of the renal tract.
Asymptomatic bacteriuria
All pregnant women should be screened for bacteriuria using a clean voided specimen of urine at their first antenatal visit. A diagnosis of asymptomatic bacteriuria (ASB) (significant bacteriuria without symptoms of UTI) is made when there are >100 000 bacteria/mL of urine. ASB occurs in 2–10% of pregnant women as a result of the physiological changes in the urinary tract during pregnancy. If ASB is not identified and treated, 20–30% of these women will develop a symptomatic urinary tract infection such as cystitis or pyelonephritis. Treatment with antibiotics is recommended to reduce the incidence of symptomatic kidney infection and pregnancy complications (Smaill & Vazquez 2007).
Chronic renal disease
When a woman has renal disease, most often the outcome of the pregnancy depends on the severity of rather than type of kidney disease. In order to determine the impact of pregnancy on a woman with chronic renal disease, the following factors need to be considered:
If the renal disease is under control maternal and fetal outcome is usually good. In some instances renal function may deteriorate and the chance of pregnancy complications subsequently rises. Renal disease combined with hypertension is associated with fetal growth restriction, pre-term birth and increased perinatal mortality. Pregnant women with mild renal insufficiency (serum creatinine [Scr] <125 μmol/L or 1.4 mg/dL) have relatively few complications of pregnancy. Moderate or severe renal insufficiency (Scr 125–250 μmol/L or 1.4–2.6 mg/dL) in pregnancy may accelerate the underlying disease and reduce fetal survival. Complications are frequent and include a rise in hypertension, high grade proteinuria (urinary excretion >3 g in 24 hrs) and loss of renal function, which may persist up to 1 year following birth. Around 10% of cases will progress to end-stage renal failure necessitating dialysis during or shortly after pregnancy; this is most likely to occur when the Scr is >250 μmol/L or 2.8 mg/dL at the beginning of pregnancy (Davison 2001).
Care and management
Assessment of renal function prior to conception is important in order to advise a couple appropriately on the risks of pregnancy. The aim of pregnancy care is to detect deterioration in renal function. This will necessitate more frequent attendance for antenatal care and close liaison between the midwife, obstetrician and nephrologist. Renal function can be assessed on a regular basis by measuring serum urate levels, serum electrolyte and urea, 24 hrs creatinine clearance and serum creatinine. Urinalysis is undertaken for glycosuria, proteinuria and haematuria. Regular urine cultures will detect infection and advice should be given regarding the signs and symptoms so that women can seek treatment early. The emergence and severity of hypertension and pre-eclampsia are monitored by recording blood pressure, undertaking urinalysis and utilizing pre-eclampsia blood screening tests. A full blood count will detect anaemia as the production of erythropoietin is suppressed in chronic renal disease. Fetal surveillance includes fortnightly ultrasound scans from 24 weeks, Doppler blood flow studies and monitoring fetal activity. Admission to hospital is advised when there is evidence of fetal compromise, if renal function deteriorates and proteinuria increases or the blood pressure rises. If the maternal condition becomes life-threatening, the risks and benefits of continuing with the pregnancy need to be discussed with the woman and her family.
Women on haemodialysis/peritoneal dialysis
Women who develop end-stage renal failure prior to or during pregnancy may require dialysis. End-stage renal failure results in hypothalamic-gonadal dysfunction causing infertility; however, dialysis lessens the hormonal dysfunction and those who conceive and continue a pregnancy are at significant risk for adverse maternal and fetal outcomes. Pregnancy will increase the length and frequency of dialysis required in order to achieve a serum urea below 20 mmol/L. Higher levels are associated with an increased risk of fetal demise (Norman et al 2001). During dialysis, it is important to prevent fluid overload and the development of hypertension, which will be influenced by other factors, such as electrolyte imbalance. The anaemia of chronic renal disease is exacerbated and this may require erythropoietin (Epo) therapy and blood transfusions to resolve. Hypertension and superimposed pre-eclampsia are common maternal complications. Many pregnancies in dialysed patients end in early spontaneous abortion, therapeutic abortion and pre-term birth with only 40–50% of pregnancies resulting in a successful outcome (Davison 2001).
Renal transplant
Renal transplantation reverses abnormal renal, endocrine and sexual functions and therefore women are more likely to become pregnant following a renal transplant. Preconception advice is particularly important as the woman must be in optimal health before embarking on a pregnancy. It is advisable for her to wait a minimum of 2 years before attempting pregnancy as this allows time for the success of the graft to be evaluated and pregnancy outcome is more likely to be successful (Davison 2001). During pregnancy women are monitored closely by the multidisciplinary team. Clinic visits are likely to be more frequent, during which time renal function including urinalysis, blood pressure, haemoglobin levels and the status of the graft are assessed. Close monitoring of the fetus is also required to detect fetal growth restriction. Immunosuppressive therapy is usually continued during pregnancy although the effect on the pregnancy and the fetus is unknown (Davison 2001). It is likely, however, to make the woman more vulnerable to infection. The newborn baby will also be more prone to infection as immunosuppressive therapy reduces the transmission of maternal antibodies to the fetus.
Kuvacic et al (2000) identified the following specific factors that appear to contribute to the higher rate of therapeutic and spontaneous abortion, pre-term birth and low birth weight infants in pregnancies following renal transplant:
The anaemias
Anaemia is a reduction in the oxygen-carrying capacity of the blood; this may be caused by a decrease in red blood cell (RBC) production, or reduction in haemoglobin (Hb) content of the blood, or a combination of these. It is often defined by a decrease in Hb levels to below the normal range of 13.5 g/dL (men), 11.5 g/dL (women) and 11.0 g/dL (children and pregnant women) (Higgins 2000). The effect on the individual will depend on the severity and speed of onset of the anaemia and the degree to which the oxygen-carrying capacity of the blood is diminished. Signs and symptoms include pallor of the mucous membranes, fatigue, dizziness and fainting, headache, exertional shortness of breath, tachycardia and palpitations. Severe anaemia is defined as Hb <7 g/dL and requires medical treatment. Very severe anaemia is defined as a Hb <4 g/dL; in pregnant women this is a medical emergency due to the risk of congestive heart failure and maternal death (WHO 2001). It is estimated that nearly half of the pregnant women in the world are considered to be anaemic; 52% in non-industrialized as compared with 23% in industrialized countries, and is a contributory factor to women developing health problems and dying during pregnancy and childbirth (WHO 2001).
Physiological anaemia of pregnancy
During pregnancy the maternal plasma volume gradually expands by 50%, or an increase of approximately 1200 mL by term. The total increase in RBCs is 25%, or approximately 300 mL. This relative haemodilution produces a fall in Hb concentration, which reaches its lowest level during the second trimester in pregnancy and then rises again in the third trimester. These changes are not pathological but are considered to represent a physiological alteration of pregnancy necessary for the development of the fetus. Fetal outcomes appear to mirror this U-shaped curve, with an increased incidence of low birthweight and pre-term birth in mothers who have either a very low or very high haemoglobin concentration (Rasmussen 2001). A low Hb level is likely to affect the ability of the maternal system to transfer sufficient oxygen and nutrients to the fetus. High Hb levels are considered to reflect poor plasma volume expansion as found in some pathological conditions such as pre-eclampsia (Yip 1996).
Iron deficiency anaemia in pregnancy
Iron deficiency anaemia (IDA) is the most common cause of anaemia in pregnant women worldwide. It is defined as a condition in which there are no mobilizable iron stores and compromised supply to body tissues. The more severe stages of iron deficiency are associated with anaemia but even mild-moderate iron deficiency will have adverse consequences. During pregnancy iron deficiency increases the risk of haemorrhage, sepsis, maternal mortality, perinatal mortality and low birthweight (WHO 2001). IDA in women is usually due to:
In non-industrialized countries other common causes include hookworm infestation, infections such as amoebic dysentery, malaria due to Plasmodium falciparum and haemoglobinopathies.
Iron and pregnancy
When giving advice in early pregnancy regarding the dietary intake of iron, the midwife needs to take into consideration how the intake of iron may be affected by social, religious and cultural preferences. She also needs to explain how iron is absorbed and identify the optimal sources of iron (bioavailability). The absorption of iron is complex and tends to decrease during the first trimester and then rises throughout the remainder of the pregnancy and during the first months of the puerperium. Iron absorption is also influenced by the bioavailability of iron in the diet. Iron is most easily absorbed in the form found in red meat and wholegrain products such as wholemeal bread (haem iron). Where the diet is mainly vegetarian (non-haem), iron is of low bioavailability. Absorption of iron is inhibited by tea and coffee but enhanced by ascorbic acid, which is present in orange juice and fresh fruit (Bothwell 2000). It is estimated that a median amount of 840–1210 mg of iron needs to be absorbed over the course of the pregnancy (Beard 2000). The demand for absorbed iron increases from 0.8 mg/day in early pregnancy to 6 mg/day in late pregnancy owing to the increase in maternal Hb, and in oxygen consumption by both mother and fetus, fetal growth and deposition of iron, placental circulation, the replacement of daily loss through stools, urine and skin, the replacement of blood lost at birth and in the postnatal period and lactation (Bothwell 2000). WHO (2001) data on the prevalence of anaemia in women suggest that the normal dietary intakes of iron are insufficient to meet these requirements for the majority of women and recommend that all pregnant women should be given iron and folic acid daily in pregnancy.
Assessing iron status in pregnancy
Assessing iron status in pregnancy can be difficult (see Ch. 14). A low haemoglobin (Hb) concentration indicates anaemia is present but it does not reveal the cause. The mean cell volume (MCV = the average volume occupied by the red cell, normal value 80–95 fL) and the mean cell haemoglobin concentration (MCHC) indicating how well filled with Hb the cells are (normal value 32–36 g/dL), are usually used to identify the cause of anaemia. Iron deficiency anaemia is microcytic (low MCV) and hypochromic (low MCHC). By the time the Hb falls, the iron stores will already be depleted. Relative total iron body stores can also be assessed by measuring serum ferritin (iron storage protein) levels (normal range 15–300 μg/L). Serum ferritin levels fall in proportion to the decrease in iron stores and will show changes before the Hb level falls (Higgins 2000) although in late pregnancy this test becomes unreliable as serum ferritin levels diminish even when bone marrow iron is present (WHO 2001).
Management
Decisions about whether to prescribe prophylactic iron supplements during pregnancy in order to maintain the Hb at 11 g/dL remain controversial. A number of authors have contributed to the debate (e.g. Beard 2000, Beaton 2000, Bothwell 2000, Rasmussen 2001, Scholl & Reilly 2000, Strong 2006).
The first-line treatment for women with iron deficiency anaemia is oral iron preparations 120–240 mg/day in divided doses. Common iron preparations include ferrous sulphate, 200 mg tablets containing 60mg of available iron, and ferrous gluconate, 300 mg tablets containing 35 mg of available iron. There are gastrointestinal side-effects of oral iron therapy that women need to be aware of. These are largely dose related and include nausea, epigastric pain and constipation. These discomforts can be reduced by taking iron supplements after meals. Some women may find one form of iron salts more tolerable than another; slow release preparations, although more expensive, are relatively free from side-effects.
Iron can also be given intramuscularly or intravenously bypassing the gastrointestinal tract. This can be beneficial in women who are unable to take, tolerate or absorb oral preparations. Intramuscular iron is given in the form of iron sorbitol. The injection should be given using a ‘Z technique’ deep in to the muscle to prevent staining and irritation at the injection site. Injections should not be given in conjunction with oral iron as this enhances the toxic effects such as headache, dizziness, nausea and vomiting.
Iron dextran is given as total dose intravenous infusion. The dosage is calculated by taking account of bodyweight and the Hb concentration deficit. Side-effects include allergic reaction, which may take the form of severe anaphylactic shock. The infusion should therefore be administered slowly under close surveillance. Joint pain occurring within 24 hrs of the infusion is not uncommon.
Oral, intramuscular and intravenous administrations of iron result in similar rates of increase in the Hb concentration. An increase of 0.8 g/dL per week is usual irrespective of the route of administration. Blood transfusion is rarely used to treat iron deficiency anaemia in pregnancy. It may be considered where there is an inadequate amount of time to treat severe anaemia prior to birth.
In women with iron deficiency anaemia, oral iron supplementation should continue postnatally particularly if they are breastfeeding. Blood tests should be repeated at the 6-week postnatal check and further investigation undertaken should iron deficiency anaemia remain.
Folic acid deficiency anaemia
Folic acid is needed for the increased cell growth of both mother and fetus but there is a physiological decrease in serum folate levels in pregnancy. Anaemia is more likely to be found towards the end of pregnancy when the fetus is growing rapidly. It is also more common during winter when folic acid is more difficult to obtain and in areas of social, economic and nutritional deprivation. The MRC Vitamin Study Research Group (1991) found a positive correlation between folate deficiency and the development of neural tube defects in the fetus.
The cause of folic acid deficiency anaemia is primarily a reduced dietary intake or reduced absorption, or a combination of these. In some instances, there may be excessive demand and loss of folic acid. In haemolytic anaemia, there is an increased demand for the production of new red cells and consequently for folic acid. Multiple pregnancy also results in an increased demand. Some drugs may interfere with the utilization of folic acid, e.g. anticonvulsants, sulphonamides and alcohol.
Investigation
The signs and symptoms are varied and may be mistaken as ‘minor disorders of pregnancy’, such as pallor, lassitude, weight loss, depression, nausea and vomiting, glossitis, gingivitis and diarrhoea. Examination of the red cell indices will reveal that the red cells are reduced in number but enlarged in size. This condition is termed macrocytic or megaloblastic anaemia. The MCV rises, the MCHC may remain the same but as there are fewer cells the Hb level falls.
Management
The risk of folic acid deficiency can be reduced by advising pregnant women on foods that are high in folic acid (see Ch. 13). Following the MRC trial in 1991, the DoH Expert Advisory Group recommended a folic acid supplement of 0.4 mg/day (DoH 1992). Some women require extra folate supplements from early pregnancy in order to prevent megaloblastic anaemia. The recommended daily supplement is 5–10 mg orally in the following circumstances:
Vitamin B12 deficiency anaemia
Deficiency of vitamin B12 also produces a megaloblastic anaemia. Vitamin B12 levels fall during pregnancy but anaemia is rare because the body draws on its stores. Deficiency is most likely in vegans, who eat no animal products at all, and should therefore take vitamin B12 supplements during pregnancy (Strong 2006).
Haemoglobinopathies
This term describes inherited conditions where the haemoglobin is abnormal. Haemoglobin consists of a group of four molecules, each of which has a haem unit made up of an iron porphyrin complex and a protein or globin chain. A total of 97% of adult Hb (HbA) has two α- and two β-chains; the remaining 3% is HbA2 and is composed of two α- and two δ-chains. Fetal Hb (HbF) has two α- and two γ-chains; by 6 months of age this has been replaced by adult haemoglobin. The type of globin chain is genetically determined. Defective genes lead to the formation of abnormal haemoglobin; this may be as a result of impaired globin synthesis (thalassaemia syndromes) or from structural abnormality of globin (haemoglobin variants such as sickle cell anaemia). These conditions prevail in certain geographical areas because the heterozygous (trait) form of thalassaemia and sickle cell offers some protection against malaria. It is found mainly in people whose families come from Africa, the West Indies, the Middle East, the eastern Mediterranean and Asia (Higgins 2000). As these conditions are inherited, and in the homozygous form can be fatal, screening of the population at risk should be carried out. Blood is examined by electrophoresis, which detects the different types of haemoglobin. Prospective parents who are known to have (or carry genes for) abnormal haemoglobin need genetic counselling in order to help them make an informed decision regarding contraception, pregnancy and prenatal diagnostic techniques (NHS Sickle Cell and Thalassaemia Screening Programme Centre 2006).
Thalassaemia
This condition is most commonly found in people of Mediterranean, African, Middle and Far Eastern origin. The basic defect is a reduced rate of globin chain synthesis in adult haemoglobin. This leads to ineffective erythropoiesis and increased haemolysis with a resultant inadequate haemoglobin content. The red cell indices show a low Hb and MCHC level but raised serum iron level. Definitive diagnosis may require DNA analysis. The severity of the condition depends on the number of abnormal genes. There are also different types of thalassaemia (Box 21.1). The key issues should be: test everyone (as the gene pools are thoroughly mixed up); partner testing when the mother is positive; offer couples who are both positive early prenatal diagnosis, as some combinations of haemoglobinopathies are fatal and others very serious, to enable termination choice to be made.
Box 21.1 Types of thalassaemia and their inheritance
α-thalassaemia major = four defective α genes
α-thalassaemia intermedia = three defective α genes
α-thalassaemia minor = two or one defective α genes
Beta thalassaemia major
The defective genes present result in severe haemoglobin deficiency, which may result in cardiac failure and death in early childhood. In those that survive, the ineffective erythropoiesis and increased haemolysis cause hypersplenism. A splenectomy is often performed in order to increase RBC survival and reduce the need for frequent blood transfusions. Blood transfusions increase the possibility of survival to childbearing age. Until recently, the high mortality rate made pregnancy in transfusion-dependent thalassaemia rare. However, advances in paediatric and haematological management have resulted in good maternal and fetal outcomes in women with β-thalassaemia major.
Alpha and beta thalassaemia minor
α- and β-thalassaemia minor is the most common problem encountered among pregnant women. This heterozygous condition produces an anaemia that is similar to iron deficiency in that the Hb, the MCV and the MCH are all lowered. A definitive diagnosis needs DNA analysis. A deficiency in iron is not, however, usually a problem because RBCs are broken down more rapidly than normal and the iron is stored for future use. In pregnancy, oral iron and folate supplements are necessary in order to maintain the iron stores. Parenteral iron should never be given. Blood transfusions may be required if the haemoglobin is thought to be inadequate for the stress of labour and blood loss at birth (Strong 2006).
Sickle cell disorders
Sickle cell disorders are found most commonly in people of African or West Indian origin. Defective genes produce abnormal haemoglobin alpha or beta chains; the resulting Hb is called HbS. In sickle cell anaemia (HbSS, or SCA) abnormal genes have been inherited from both parents, whereas in sickle cell trait (HbAS) only one abnormal gene has been inherited (Khattab et al 2006a). The introduction of a national antenatal and neonatal screening programme for haemoglobinopathies in England will improve healthcare and reduce childhood morbidity and mortality significantly (NHS Sickle Cell and Thalassaemia Screening Programme Centre 2006).
Sickle cell anaemia
Sickle cells have an increased fragility and shortened life span of 17 days, which results in chronic haemolytic anaemia and causes episodes of ischaemia and pain, known as attacks of pain or sickle cell crises. Sickling crisis may occur whenever oxygen concentration is low. Precipitating factors that affect oxygen uptake include psychological stress, cold climate and extreme temperature changes, smoking-induced hypoxia, strenuous physical exertion and fatigue, respiratory disease, infection and pregnancy. When subjected to low oxygen tension, HbS contracts damaging the cell and causing it to assume a sickle shape. Sickle cells easily adhere to the vascular epithelium causing a reduced blood flow, vascular obstruction and hypoxia. This results in acute pain, particularly in the bones, joints and abdominal organs.
Painful episodes last for a few hours to a few days and are often poorly managed and undertreated by nursing, midwifery and medical staff in pregnancy. Effective management of sickle cell crisis can be achieved by:
Blood transfusion therapy is key to the management of sickle cell anaemia as it increases the oxygen carrying capacity of blood by increasing the haemoglobin concentration and decreasing the percentage of sickle haemoglobin. However, due to the risk of developing red cell antibodies and iron overload the indications for the use of blood transfusion therapy include: acute chest syndrome, heart failure, multiorgan failure, stroke, splenic sequestration and aplastic crisis. New therapies include the use of drugs such as hydroxyurea, which inhibits the development of sickle cells by increasing fetal haemoglobin production. Bone marrow and stem cell transplantation can cure SCA but is still in the early stages of development and not generally available (Claster & Vichinsky 2003, Khattab et al 2006b, National Institutes of Health 2002). Life expectancy for people with sickle cell anaemia has improved considerably over the last decade although overtime major organ damage results in a chronic illness requiring long term physical and psychosocial support (Okpala et al 2002, Thomas & Taylor 2002).
Maternal and fetal effects. There have been significant decreases in both maternal and perinatal mortality. Women with SCA are at an increased risk for medical complications during pregnancy. Maternal risks include antenatal and postnatal attacks of pain, infections, pulmonary complications, anaemia, pre-eclampsia and caesarean section. Fetal and neonatal complications include spontaneous abortion, pre-term birth, intrauterine growth restriction and perinatal death (Sergeant et al 2004, Sun et al 2001).
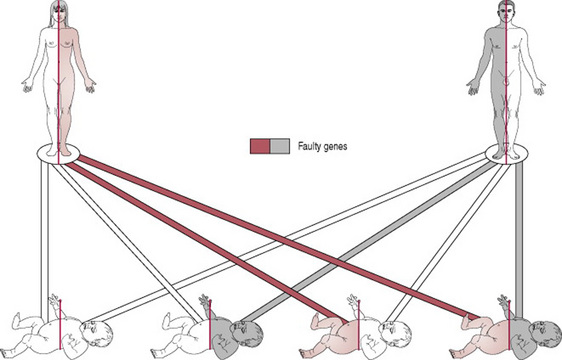
Preconception care. All women should be offered haemoglobin electrophoresis in early pregnancy and for any with positive results it is important to have their partner tested. Where both parents have abnormal results, counselling regarding prenatal diagnosis should be offered at a dedicated obstetric/haematology clinic. If both parents are carriers for HbS (i.e. heterozygous) there is a one in four chance that the fetus will inherit the more serious condition, sickle cell anaemia (Fig. 21.1) (Zack-Williams 2007).
Antenatal care. Antenatal care aims to minimize the maternal and fetal complications as 35–50% of women with SCA will experience sickle crises during pregnancy which requires hospital admission and treatment (Rahimy et al 2000, Sun et al 2001). Monitoring of pregnancy is performed at frequent intervals by a multidisciplinary team involving a midwife, obstetrician, haematologist, specialist nurse and physician, maintaining close liaison and discussion with the woman and her family. Midwives have an important role in providing information and education about SCA and how it affects pregnancy, particularly in emphasizing the factors that may precipitate sickle crisis. In addition to the tests routinely performed as part of antenatal care, initial blood tests should include screening for red cell antibodies, haemoglobin electrophoresis, serum iron, total iron-binding capacity, serum ferritin levels, liver function tests, blood urea nitrogen and serum creatinine. Regular monitoring of the haemoglobin concentration is required throughout pregnancy; this is usually in the range of 6–9 g/dL. Fluid intake should be well maintained to prevent dehydration and it is important to detect bacterial infections, particularly genitourinary and respiratory infections, at an early stage. Good nutrition and folic acid supplements assist in maintaining a steady haematological state. The use of prophylactic blood transfusions to improve the outcome of pregnancy remains controversial. The potential benefits for the pregnancy have to be offset against the disadvantages of transfusions. Most authors recommend a policy of transfusing only when indicated, for example in symptomatic anaemia, severe anaemia with a haematocrit <18%, sickle crisis, cardiac failure or prior to caesarean section. Fetal assessment includes regular ultrasound scans to assess fetal growth. If growth restriction is identified, then more intensive monitoring will be required utilizing biophysical profiles and uterine Doppler blood flow studies (Dauphin-McKenzie et al 2006, Rahimy et al 2000).
Intrapartum care. Timing and mode of birth is gauged according to maternal and fetal health, induction of labour or caesarean section should be performed for obstetric reasons. Good pain relief and adequate hydration are essential to reduce the risk of a sickle crisis; an epidural is usually recommended. Oxygen therapy via a nasal prong or mask is advised to maintain adequate oxygenation and improve cardiac function. Prophylactic antibiotics may be considered to prevent infection. Prolonged labour should be avoided and active management or a caesarean section may be advised on clinical grounds. CTG monitoring is recommended, especially where there is intrauterine growth restriction.
Postnatal care. Women with SCA are at a high risk of developing thromboembolic disorders therefore early ambulation and wearing anti-embolic stockings should be encouraged. To prevent puerperal sepsis, antibiotic cover is continued throughout the postnatal period. Neonatal screening of babies must be undertaken by obtaining a sample of neonatal capillary or venous blood at birth. Those with positive results should be followed-up by a haematologist. In order to prevent the high incidence of infant mortality from sickle cell anaemia, early diagnosis combined with prophylaxis against infection, parental education and adequate follow-up are recommended (NHS Sickle Cell and Thalassaemia Screening Programme Centre 2006).
Sickle cell trait is usually asymptomatic. The blood appears normal, although the sickle screening test is positive. The woman may be mildly anaemic in pregnancy and folate 5 mg daily is recommended to help erythropoiesis.
Combinations of abnormal haemoglobins
Sickle cell disease can be combined with other disorders and therefore individual advice from a haematologist is necessary.
Other rare inherited disorders
Glucose-6-phosphate dehydrogenase
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is an X-linked, hereditary genetic defect. G6PD is an enzyme necessary for the survival of the red cell; when it is deficient RBCs are destroyed in the presence of certain substances. These include fava beans, sulphonamides, vitamin K analogues, salicylates and camphor (found in products such as ‘Vicks VapoRub’). To prevent haemolysis these substances should be avoided (Cappellini & Fiorelli 2008).
Diabetes mellitus
Diabetes is a chronic and progressive disorder which has a significant impact on almost all aspects of life. Approximately 2–5% of pregnancies involve women with diabetes (NICE 2008). The St Vincent Declaration of the European Association for the Study of Diabetes and of the UK Task Force (SVD Working Party 1990) was an agreement to work towards achieving pregnancy outcome for women with diabetes equal to that of women without diabetes. Research by the Confidential Enquiry into Maternal and Child Health (CEMACH) found that babies of women with diabetes in England, Wales and Northern Ireland are five times more likely to be stillborn, three times more likely to die in their first months of life and twice as likely to have a major congenital abnormality. Additionally, the study showed that nearly one in three mothers with diabetes have type 2 diabetes and that the risk for poor outcome for these women is similar to those with type 1 diabetes (CEMACH 2005, Macintosh et al 2006). Critical to reducing the incidence of poor outcome is the need for good preconception care, near normal glycaemic control before and around conception and throughout pregnancy. Efforts to improve outcome should also focus on identifying at risk groups, particularly women who have difficulties accessing appropriate care either because of language difficulties or unfamiliarity with the healthcare system (CEMACH 2007). Midwives are increasingly the initial contact in early pregnancy and have a vital role, working collaboratively with the woman and the obstetric and diabetes team, to ensure that women with diabetes have a ‘positive experience of pregnancy and childbirth and receive care that promotes their physical health and well-being and optimizes the health of their babies’ (DH 2001, p 35).
The term ‘diabetes mellitus’ (DM) describes a metabolic disorder that affects the normal metabolism of carbohydrates, fats and protein. It is characterized by hyperglycaemia and glycosuria resulting from defects in insulin secretion, or insulin action, or both. The classic signs and symptoms are excessive thirst (polydipsia), excessive urinary excretion (polyuria) and unexplained weight loss. The long-term effects of DM are reflected in the development of macrovascular and microvascular disease producing coronary heart disease, peripheral arterial disease, kidney disease (diabetic nephropathy), loss of vision (diabetic retinopathy) and nerve damage (diabetic neuropathy). It can also affect mental health and well-being.
A normal fasting blood glucose of <6.1 mmol/L is regulated by the pancreatic hormones insulin and glucagon. Following the ingestion of carbohydrates, the rising blood glucose stimulates the pancreas to secrete insulin, which reduces blood glucose. Falling blood glucose levels induce glucagon production, which prevents further glucose reduction. The combined action of these two hormones maintains the blood glucose within normal limits.
Hyperglycaemia is usually the result of insulin deficiency or when there is a high secretion of hormones antagonistic to insulin action; severe hyperglycaemia (blood glucose >25.0 mmol/L) may result in diabetic ketoacidosis, coma or death.
Hypoglycaemia is defined as a blood glucose <2.2 mmol/L. Symptoms of a falling blood glucose include tremor, sweating and tachycardia. Severe hypoglycaemia, particularly in neonates, can result in fits, coma and death. Repeated severe episodes of hypoglycaemia are associated with the risk of permanent brain damage (Higgins 2001).
Classification
Type 1 diabetes
This occurs when β cells in the islets of Langerhans in the pancreas are destroyed, stopping insulin production. Insulin therapy is required in order to prevent the development of ketoacidosis, coma and death. It presents more commonly in childhood, but can occur at any age and in some cases is attributable to an autoimmune process (WHO 1999).
Type 2 diabetes
This results from a defect in the action of insulin. Insulin therapy is not always needed. The risk of developing this type of diabetes increases with age, obesity and lack of physical activity. It occurs more frequently in women with prior gestational diabetes mellitus and in individuals with hypertension. Its frequency varies between different racial or ethnic groups and there is some suggestion of a genetic predisposition (WHO 1999).
Gestational diabetes mellitus
Gestational diabetes mellitus (GDM). This is defined as carbohydrate intolerance resulting in hyperglycaemia of variable severity, with its onset or first recognition during pregnancy (WHO 1999).
Impaired glucose regulation
This includes impaired glucose tolerance (IGT) and impaired fasting glycaemia (IFG), which are metabolic states intermediate between normal glucose homeostasis and diabetes. IGT is categorized as carbohydrate metabolism resulting in slightly raised post-meal blood glucose levels of >7.8 mmol/L. IFG refers to fasting glucose concentrations that are lower than those required to diagnose diabetes but higher than the ‘normal’ reference range (i.e. >6.1 mmol/L but <7.0 mmol/L). Individuals with impaired glucose regulation are at increased risk of developing diabetes and cardiovascular disease (WHO 1999).
The demographic pattern of diabetes is changing with increasing numbers of young people diagnosed as having type 1 diabetes and the number of people diagnosed as having type 2 diabetes increasing predominantly among people in Black, Asian or other minority ethnic groups (DH 2001, Hotu et al 2004). Among pregnancies complicated by diabetes, 25% of cases involve pre-existing type 1 diabetes, 10% involve pre-existing type 2 diabetes and, the remaining 65% involve gestational diabetes which may or may not resolve after pregnancy.
Diagnosis
WHO (1999) recommend the following criteria for diagnosing diabetes:
Monitoring diabetes
The main objective of diabetic therapy is to maintain blood glucose levels as near to normal as possible and to reduce the risk of long-term complications. People with diabetes are therefore encouraged to monitor their blood glucose concentration regularly by obtaining a finger-prick sample of capillary blood and using reagent test strips (e.g. BM test) with or without a reflectance glucose meter. Blood glucose can also be estimated by testing urine for glucose using reagent strips, although this is less accurate than the blood test and not recommended in pregnancy. Long-term blood glucose control can be determined by undertaking a laboratory test to measure glycosylated haemoglobin (HbA1c). Around 5–8% of haemoglobin in the red blood cells carries a glucose molecule and is said to be glycosylated. The degree of haemoglobin glycosylation is dependent on the amount of glucose the red blood cells have been exposed to during their 120-day life. A random blood test measuring the percentage of haemoglobin that is glycosylated will reflect the average blood glucose during the preceding 1–2 months. The higher the HbA1c the poorer is the blood sugar control. Good diabetic control is defined as an HbA1c of <6.5% (Higgins 2001).
Carbohydrate metabolism in pregnancy
Pregnancy is characterized by several factors that produce a diabetogenic state so that insulin and carbohydrate metabolism is altered in order to make glucose more readily available to the fetus. Increasing levels of oestrogen, progesterone and prolactin produce progressive hyperplasia of the pancreatic β-cells resulting in the secretion of 50% more insulin (hyperinsulinaemia) by the third trimester. However, progesterone, human placental lactogen and cortisol are insulin antagonists and reduce the effectiveness of insulin. This is considered to be a ‘glucose-sparing mechanism’, which enables large quantities of glucose to be taken up by the maternal circulation and transferred to the fetus via the placenta by a process known as ‘facilitated diffusion’. After the placenta is delivered, insulin resistance and requirements decrease rapidly and the pre-pregnancy sensitivity to insulin is restored. Gestational diabetes is most likely to emerge during the third trimester when the extra demands on the pancreatic beta cells precipitate glucose intolerance. Women with diabetes do not have the capacity to increase insulin secretion in response to the altered carbohydrate metabolism in pregnancy and therefore glucose accumulates in the maternal and fetal system leading to significant morbidity and mortality.
Pre-pregnancy care
The National Service Framework (NSF) for Diabetes: Standards (DH 2001) and the NICE guideline on diabetes in pregnancy (NICE 2008) set out standards and key interventions that will reduce the risks of adverse pregnancy outcomes. Women with diabetes who are planning to become pregnant should be informed that establishing good glycaemic control before conception and continuing throughout pregnancy will reduce but not eliminate the risk of miscarriage, congenital malformation (predominately cardiac anomalies, urogenital anomalies and neural tube defects [NTD]), stillbirth and neonatal death.
Pre-pregnancy care aims to achieve optimal glycaemic control in women with diabetes and provides an opportunity for genetic counselling, diabetes care, preconception care and family planning options (Hofmanova 2006). A comprehensive obstetric, gynaecological and diabetic history is essential and assessment is made of current diabetic control. Individualized targets for self-monitoring of blood glucose should be agreed with women with pre-existing diabetes taking into account the risk of hypoglycaemia. If safely achievable, women should aim for an HbA1cbelow 6.1% (NICE 2008). Women with type 1 diabetes will need their insulin dosage reviewed and an explanation given of the adjustments that will be required during pregnancy. Women with type 2 diabetes who have poor glycaemic control will require insulin during pregnancy and those taking oral hypoglycaemics will need to change to insulin to prevent the possibility of teratogenesis. Pregnancy may lead to a deterioration of diabetes and for this reason, the presence of renal, cardiovascular or retinal changes need to be assessed. Angiotensin-converting enzyme (ACE) inhibitors used to control hypertension in women with diabetes are contraindicated in pregnancy because of possible teratogenesis and therefore alternative antihypertensive therapy such as methyldopa or nifedipine is required. Nutritional and dietary advice should be given as weight gain will influence the insulin requirements and affect its absorption. Folic acid supplementation, 5 mg/day, is recommended when planning a pregnancy and should be continued until 12 weeks pregnant to reduce the risk of having a baby with a NTD. General health measures, including checking rubella status and smoking cessation, need to be discussed in addition to giving advice regarding the effect of diabetes on pregnancy and of pregnancy on diabetes (DH 2001, Diabetes UK 2005, NICE 2008, SIGN 2001).
Antenatal care
Antenatal care for women with diabetes should be provided by a multidisciplinary team in a joint diabetes and antenatal clinic (NICE 2008). The woman is seen as often as required in order to maintain good glycaemic control; this may entail 2–4 weekly visits until 28 weeks’ gestation and then 1–2 weekly until term. Glycaemic control is particularly difficult to maintain in early pregnancy owing to the effects of pregnancy on diabetes and this may be exacerbated by other pregnancy disorders such as nausea and vomiting. Blood glucose levels should be monitored frequently (four times a day using a calibrated blood glucose meter correctly) and insulin levels adjusted to achieve a fasting blood glucose of between 3.5 and 5.9 mmol/L and 1 hr postprandial <7.8 mmol/L (NICE 2008). Additional estimations of blood glucose control such as HbA1c (or fructosamine) measurements are also required. Intensive glycaemic control means that women with diabetes are also more likely to become severely hypoglycaemic, particularly at night-time and in gestational weeks 10–20. Loss of hypoglycaemic awareness and warning symptoms are also common (Temple 2007). Women and their relatives need to be warned of this and advice should be given regarding recognition, management and treatment of hypoglycaemia. Temple (2007) recommends the following treatments for hypoglycaemia: 3–5 glucose tablets, 250–300 mL isotonic Lucozade, 75–120 mL carbonated lemonade or 4–6 jelly babies repeated as necessary. This should be followed by one piece of fruit, one or two plain biscuits or a piece of bread. In addition, a glucagon kit should be supplied and the woman’s partner and relatives instructed on how to use it.
Dietary advice and monitoring is continued throughout pregnancy as the need for carbohydrate increases as the fetus grows. A diet that is high in fibre is beneficial as carbohydrates are released slowly and therefore a more constant blood glucose level can be achieved. Glycosuria is common in pregnancy owing to the increased glomerular filtration rate (GFR) and decreased renal threshold. Women with diabetes have a predisposition to urinary and vaginal infections during pregnancy; these should be discussed with the midwife so women can recognize the signs and symptoms and seek treatment as soon as possible.
Pre-existing vascular disease will increase the risk of a woman with diabetes developing hypertensive disorders in pregnancy. It will also cause a deterioration of diabetic retinopathy; retinal assessment should therefore be undertaken in the first and third trimesters. Renal function should also be monitored and referral made to a nephrologist if the serum creatinine is 120 μmol/L or more or total protein excretion exceeds 2 g/day (NICE 2008).
In view of the increased risk of congenital malformations, a detailed anomaly ultrasound scan should be performed at 20 weeks’ gestation. It is also recommended that fetal echocardiography is undertaken at 20 weeks to detect any cardiac abnormalities. Serum screening for Down syndrome is altered with maternal diabetes and care should be taken when interpreting the results.
Fetal growth must be observed carefully because of the risk of growth restriction due to maternal vascular disease, pre-eclampsia, or a combination of both. Poor glycaemic control will result in fetal macrosomia. A dating ultrasound scan should be undertaken in the first trimester (8–10 weeks). Monitoring of fetal growth should be offered monthly from 28 weeks’ gestation. Serial ultrasound should also assess fetal well-being in late pregnancy through monitoring of amniotic fluid volume.
As far as possible, the woman monitors her diabetes at home and diabetic care is provided on an outpatient basis. It is important that the midwife assesses the progress of the pregnancy in the normal way in order to detect any complications. Hospital admission may be required because of poor glycaemic control, a destabilizing illness or obstetric complications.
Intrapartum care
For women with uncomplicated diabetes the spontaneous onset of labour and normal birth is recommended but this should take place in a hospital with neonatal intensive care facilities (SIGN 2001). Routine induction of labour or planned caesarean section before 38 weeks’ gestation is not recommended as it does not reduce the perinatal mortality rate, is more likely to result in neonatal respiratory morbidity and contributes to the high caesarean section rate for women with diabetes. However, Diabetes UK (2005) and NICE (2008) recommend that labour should commence after 38 completed weeks’ gestation in order to minimize the risk of late fetal death. Poor glycaemic control or a deterioration in the maternal or fetal condition may necessitate earlier, planned birth. Induction of labour may also be considered where the fetus is judged to be macrosomic (birth weight >4000 g). Fetal lungs mature more slowly when the mother is diabetic and it is important to take this into account if early induction of labour is planned. In addition, steroids such as dexamethasone, which may be used to aid lung maturation and surfactant production, will increase insulin requirements in women with diabetes.
The aim of intrapartum care is to maintain normoglycaemia in labour (i.e. 4.0–7.0 mmol/L) by use of an insulin infusion titrated according to blood glucose levels. Maternal hyperglycaemia (>8 mmol/L) leads to an increase in fetal insulin production, which will cause neonatal hypoglycaemia. Fetal compromise is more common as placental blood flow is reduced and glycosylated haemoglobin decreases oxygen carriage in women with diabetes. In addition, maternal ketoacidosis may result from dehydration and unstable diabetes. If the mother becomes acidotic, ketones will cross the placenta and affect the fetal acid–base status. CTG monitoring is recommended and fetal blood sampling should be utilized if acidosis is suspected.
Postpartum care
Immediately after the third stage of labour the insulin requirements will fall rapidly to pre-pregnancy levels. The insulin infusion rate should be reduced by at least 50%. Carbohydrate metabolism returns to normal very quickly and women can resume their pre-pregnancy insulin regimen as soon as they are able to eat. Women with type 2 diabetes who were previously on oral hypoglycaemics or dietary control need to be reviewed prior to recommencing therapy. Monitoring of blood glucose levels should continue during this interim period. Breastfeeding should be encouraged in all women with diabetes. An additional carbohydrate intake of 40–50 g is recommended and insulin therapy may need to be adjusted accordingly. Operative birth, together with diabetes, predisposes these women to infection and delayed healing. The administration of antibiotics may be a useful preventative measure in this instance. All women should be offered contraceptive advice so that optimum glycaemic control is achieved prior to planning the next pregnancy. The issues governing choice of contraception for women with diabetes are similar to those for women without diabetes; all contraceptive methods are considered safe, acceptable and effective. Women with diabetes should be reviewed at 6 weeks, ideally at a combined diabetes/obstetric clinic especially if they are breastfeeding.
Neonatal care
All babies should remain with their mothers unless there is a specific medical indication for admission to a neonatal unit (CEMACH 2004, CEMACH 2007, DH 2001, Diabetes UK 2005, NICE 2008). The development of complications in the neonate is related to maternal hyperglycaemia during pregnancy leading to fetal hyperinsulinaemia. This will result in the following neonatal problems: macrosomia (see Chs 33 and 45 for birth difficulties); hypoglycaemia (see Ch. 48); polycythaemia (see Ch. 43); and respiratory distress syndrome (see Ch. 44).
Gestational diabetes
The incidence of gestational diabetes (GD) varies widely across different ethnic groups. In Caucasians, it is 1–2%, in Afro-Caribbeans 2–3% and in Asians 4–5% (Lowy 1997). An agreement as to what is considered a ‘normal’ blood glucose level in pregnancy and at what level maternal and fetal morbidity ensues remains elusive. Hence, the significance of GDM is difficult to determine (Mitchell 2001). The strongest evidence suggests that fetal macrosomia and caesarean section rates are increased. In the longer term, there appears to be an association between raised glucose levels in utero and the development of obesity and diabetes in later life. There is also evidence to suggest that women who develop GDM are at risk of developing type 2 diabetes (Virjee et al 2001). Davey & Hamblin (2001) identify that some women are at high risk of developing GDM and there may be some benefit in selective screening for GDM in women where the following risk factors are identified:
NICE (2008) guidance for screening and diagnosis of GDM includes:
Treatment will depend on the blood glucose levels. The midwife should involve both the diabetic nurse (or midwife) specialist and dietician in dietary interventions to regulate carbohydrate intake and restrict fat and sugars. Advice regarding exercise in pregnancy will be of benefit and smoking cessation strategies where appropriate. Grossly abnormal results are likely to require insulin therapy. Blood glucose monitoring should continue on a regular basis throughout pregnancy in order to detect hyperglycaemia. Fetal macrosomia is the main complication and therefore fetal growth and well-being should be closely monitored for the remainder of the pregnancy. Decisions can then be made about the optimal mode and time of birth. Following birth the baby should be closely monitored for hypoglycaemia. If the woman is on insulin therapy, this is withdrawn immediately after the birth of the baby. It is recommended that a postnatal OGTT is performed at 6 weeks; if the results are abnormal then appropriate referral should be made. Those with normal glucose levels require advice regarding the implications for future pregnancies and the development of type 1 or type 2 diabetes. If the woman adopts a healthy lifestyle and avoids obesity this risk may be reduced.
Epilepsy
Epilepsy is a common serious chronic neurological disorder. MacDonald et al (2000) estimate that approximately 7 out of 1000 people have epilepsy. It can affect anyone at any age but the disorder commonly develops before 20 years of age, 30% of cases occurring in early childhood. Due to the wide variation of epileptic syndromes this results in significant difficulties in the diagnosis, management and treatment of the condition and morbidity mortality can be high (APPG 2007).
Between 2003–2005, 11 women died as a result of epilepsy (Lewis 2007). Key to a successful pregnancy includes ensuring that:
Aetiology
An epileptic seizure results from abnormal electrical activity in the brain, which is manifest by brief disturbances of sensory, motor and autonomic function. These disturbances recur spontaneously and are classified according to the parts of the brain affected. Seizures may be described as partial, usually arising from the temporal or frontal lobe of the brain, or generalized, resulting from disturbances involving both halves of the brain. General seizures may be further classified as absence seizures (petit mal), myoclonic seizures, tonic-clonic seizures (grand mal), atonic seizures and status epilepticus (Taylor 2000).
The cause of epilepsy in most instances is unknown. There is some suggestion of a genetic component, which in certain circumstances predisposes an individual to epileptic seizures. In other cases, an underlying cause such as hypoglycaemia, encephalitis, meningitis, cerebral hypoxia, toxicity from alcohol or drugs, or structural damage or abnormality of the brain may result in epilepsy. A number of ‘trigger’ factors have been identified that may precipitate an attack in those who have been diagnosed with epilepsy; these include emotional stress, sleep deprivation/physical exhaustion, increased body temperature (fever, hot steamy environments), environmental factors (strobe lighting, noise), non-compliance with drug therapy. In women, the hormonal changes at the onset of menstruation may trigger epileptic seizures (Jackson 2006) and women with pre-existing epilepsy may find that seizures cluster peri-menstrually (Reddy 2007).
Diagnosis
Identification of the type and cause of epilepsy is important in the treatment of epilepsy, there are however approximately 30 different epileptic syndromes and over 38 different types of seizure. Diagnosis is therefore complex and involves a number of investigations: taking a clear history including eyewitness accounts is fundamental; blood tests to determine haematology, biochemistry and toxicology assays; neuroimaging such as magnetic resonance imaging (MRI) and computerized tomography (CT) to identify any structural abnormalities and neurological lesions; electroencephalogram (EEG) to classify seizure type by identifying the origin of the abnormal electrical discharge and telemetry which uses video and EEG to observe and identify the seizure type; neuropsychological assessment to evaluate any learning disability and cognitive dysfunction (NICE 2004).
Treatment
The aim of treatment is to identify the cause of the seizure and provide appropriate therapy to prevent recurrence. For the majority of people the control of seizures can be achieved through the use of one antiepileptic drug (AED). In some individuals a combination of drugs (polytherapy) may be required and a few will require surgery. Most of the drugs have side-effects, which include drowsiness, sedation, nausea and skin rashes; these are most notable when the drug is first taken or if the dosage is too high. The ideal treatment therefore is a single AED prescribed at the lowest effective dose. AED therapy must be started under the guidance of a specialist physician and will need to be reviewed at regular intervals and at a minimum once a year. Women who take AEDs have a 4% chance of having a baby with a major congenital malformation and therefore the dosage and type of drug will need to be reviewed and adjusted in pregnancy in order to reduce this risk (NICE 2004).
Health education
The provision of appropriate advice in primary care and prompt referral to secondary care is vital for people with epilepsy. Midwives and other health professionals have an important role in the education of women and their families and for ensuring that they are provided with a range of information including:
Effect of epilepsy on the fetus and neonate
In general, women whose epilepsy is well controlled have few problems in pregnancy and can expect a normal, uncomplicated pregnancy and birth. Some women may experience an increase in seizures and the risk of complications in pregnancy is increased when epilepsy is poorly controlled (EURAP Study Group 2006). This is often due to non-compliance with the drug regimen, sleep deprivation during pregnancy and the decline in plasma concentrations of the AED as the pregnancy progresses. Prolonged and/or serial seizures during pregnancy increase the risk of fetal morbidity and mortality caused by hypoxia or placental abruption. There is also an increased risk of maternal death. Particular emphasis should be placed on the first aid measures that should be adopted following an epileptic seizure in order to prevent aspiration, the dangers of hot baths inducing fainting and consequent drowning and the risk of SUDEP (Lewis 2007). The majority of women on antiepileptic drugs have physically normal babies, however evidence suggests there is a two–four-fold increased risk of major congenital malformations in babies of women with epilepsy which is directly related to the type and number of drugs the woman is taking (Morrow et al 2006).
Pre-pregnancy care
Preconception advice is essential for women with epilepsy and a review of AED therapy is crucial before women consider becoming pregnant (Lewis & Smith 2006). In some instances, the gradual withdrawal of AED therapy may be considered prior to pregnancy in order to reduce the risk of congenital malformation in the fetus. This may be possible where the woman (a) suffers from seizures that are unlikely to harm the fetus such as absence, partial or myoclonic or (b) has been seizure free for more than two years and a recurrence is unlikely (Tomson & Hiilesmaa 2007). Folic acid supplementation (5 mg/day) should be commenced before pregnancy and continued throughout pregnancy to prevent congenital malformation and the development of anaemia (NICE 2004).
Antenatal care
Pregnancy has no effect on seizure control and most women with epilepsy will remain seizure free (EURAP Study Group 2006). Close monitoring of the maternal and fetal condition is required and antenatal care should be provided by a multidisciplinary team which includes a named midwife, obstetrician and a neurologist or physician with a specialist interest in epilepsy in pregnancy (Lewis 2007). Care should include a detailed anomaly scan at 18–22 weeks. Epilepsy is not an indication for early induction of labour or elective caesarean section.
Intrapartum care
The EURAP Study Group (2006) found that labour and birth carry an increased risk for tonic-clonic seizures with 2–5% of women with epilepsy having seizures at this time. Careful observation and monitoring of the maternal and fetal condition by the midwife is required through labour and the early postnatal period. AEDs should be administered as scheduled throughout labour and it is important to prevent the development of possible ‘trigger’ situations such as sleep deprivation, hypoglycaemia, stress, hyperventilation and anaemia, all of which may arise during the course of labour. Women with epilepsy should be offered the same choices for pain relief in labour as other women, including epidural analgesia.
Postnatal care
Following birth, women with epilepsy may be at an increased risk of seizures due to fluctuating hormone levels and sleep disturbance. Safety precautions in the home should be discussed with the woman and her partner. This will include giving advice about how to minimize risks when feeding, bathing, changing and transporting the baby.
AEDs cross the placenta freely and decrease production of Vitamin K leading to the risk of Vitamin K deficiency bleeding in the newborn (Ch. 45). This can be prevented by routine administration of oral vitamin K (20 mg/day) to the mother from 36 weeks’ gestation and to the baby (1 mg i.m.) shortly after birth (NICE 2004). Breastfeeding is generally safe but specific information must be obtained for each agent. How much AED passes into breastmilk must be considered, as well as the rate of clearance by the neonate. Some AEDs have a sedative effect, causing drowsy babies less efficient at feeding and gaining weight more slowly. Advice should be sought from a neonatologist and some breastfeeds may need to substituted with formula feeds. The British National Formulary (JFC 2007) will indicate which AEDs are contraindicated when a women is breastfeeding.
AED therapy should be reviewed soon after birth by the neurology team and the dosage adjusted to pre-pregnancy levels if it has been increased. Future pregnancy plans should be discussed and appropriate contraceptive advice given. All methods of contraception are available to women with epilepsy, although oral contraceptives are less effective with some AEDs as they induce hepatic enzymes which metabolize oestrogen faster. Women taking these AEDs will require oral contraceptives with a higher dosage of oestrogen (i.e. >50 mg oestrogen) in order to prevent the risk of an unplanned pregnancy due to contraceptive failure (Gilmour-White 2000, NICE 2004).
Autoimmune disease
Autoimmune disease arises from a disruption in the function of the immune system of the body, resulting in the production of antibodies against the body’s own cells. Antigens normally present on the body’s cells stimulate the development of autoantibodies, which, unable to distinguish the self antigens from non-self or foreign antigens, act against the body’s cells to cause localized and systemic reactions. The cause of these conditions is unknown but it is thought to be multifactorial with genetic, environmental, hormonal and viral influences. Many autoimmune diseases are more prevalent in women, particularly between puberty and the menopause, which suggests that female hormonal factors may play a role. They broadly fall into two groups:
These disorders are characterized by periods of remission interrupted by periods of crisis, which may require hospitalization. This cyclical variation appears to be related to some external factors, for example excessive emotional stress. Treatment is aimed at lessening the severity of the symptoms rather than effecting a cure. Mild cases usually respond to anti-inflammatory drugs; more severe illnesses may require steroids or immunosuppressant therapy.
Systemic lupus erythematosus
Systemic lupus erythematosus (SLE), or lupus, is an autoimmune, connective tissue disorder with a wide range of clinical manifestations. Connective tissue is found throughout the body; therefore SLE produces multisystem disorders affecting muscles, bone, skin, blood, eyes, nervous system, heart, lungs and kidneys. Ethnic groups, particularly those with African or Asian ancestry are at greatest risk of developing the disorder and one of the highest prevalence is seen in the UK Afro-Caribbean populations (D’Cruz et al 2007). Most people with SLE have a normal life expectancy and serious complications are rare. Infection is the major cause of mortality at all stages of SLE; early deaths are usually due to active SLE and late deaths are attributed to thromboembolic disorders (Ruiz-Irastorza et al 2001).
Diagnosis
The diagnosis of SLE is based on a collection of signs and symptoms particularly when joint pain, skin conditions and fatigue occur in combination or evolve over time. The initial manifestation of SLE is often arthritis accompanied by fever, fatigue, malaise, weight loss, photosensitivity and anaemia. A wide range of skin lesions are seen and an erythematous facial ‘butterfly’ rash is characteristic of the disorder. Depending on the organs involved, inflammatory conditions such as pruritus, pericarditis, glomerulonephritis, neuritis and gastritis may arise. Renal disease and neurological abnormalities are the most serious manifestations of the disease. Blood tests are used to confirm the diagnosis and comprise full blood count, erythrocyte sedimentation rate (ESR) and testing for antinuclear antibody (ANA). There is often normochromic normocytic anaemia, the ESR is elevated even when the disease is in remission and >95% of people with SLE will have ANA (D’Cruz et al 2007).
Antiphospholipid syndrome (Hughes syndrome)
Antiphospholipid syndrome (APS) is a prothrombotic disorder characterized by arterial and/or venous thrombosis, recurrent spontaneous miscarriage, neurological disease including stroke in the presence of circulating antiphospholipid antibodies (aPL). Approximately 30–40% of women with SLE have aPL antibodies and some will develop APS. A blood test will detect aPL and lupus anticoagulant. APS in conjunction with SLE increases the risk of these women developing thromboembolic disorders in pregnancy and is associated with a higher risk of pregnancy loss, intrauterine growth restriction, placental insufficiency, pre-eclampsia and pre-term birth. Reducing the risk of thrombosis through the use of antithrombolytic therapy during pregnancy significantly improves the chance of a successful pregnancy outcome (Khamashta 2006, Tincani et al 2006).
Effects of SLE on pregnancy
As SLE occurs primarily in women during their childbearing years it is likely to complicate pregnancy and it may arise for the first time in pregnancy. The effect of SLE on pregnancy is variable although lupus flares (worsening of SLE symptoms) are common. The frequency of the flares is lower in women with mild and well-controlled disease and providing the disease is in remission at the time of conception, it is less likely that it will become active during the course of the pregnancy. Exacerbation of SLE with major organ involvement (such as the kidneys and central nervous system) may occur in approximately 20% of cases (Classen et al 1998).
Overall pregnancies in SLE women have an increased incidence of adverse pregnancy outcome. Approximately one-third will result in fetal loss owing to spontaneous abortion, therapeutic abortion, intrauterine death or stillbirth. Maternal renal disease and the presence of aPL have been found to be significant predictors of fetal loss, development of pre-eclampsia and intrauterine growth restriction. The rate of pre-term birth and intrauterine growth restriction is closely related to the incidence of pre-eclampsia and many women with SLE give birth before 37 weeks (Yasmeen et al 2001).
Neonatal lupus syndrome is rare but may occur as a result of the transplacental passage of maternal IgG autoantibodies. The neonate presents with a mild form of lupus that is transient and resolves when the antibodies are cleared in a few months following birth. A more severe form of the disease results in fetal anaemia, leucopenia and thrombocytopenia. When anti-Ro and/or anti-La antibodies have passed to the fetus, then there is a risk of developing congenital heart block (CHB), which is permanent and carries significant morbidity and mortality. Over 60% of affected children require lifelong pacemakers (Tincani et al 2006).
Preconception care
Women with SLE should be counselled about planning a pregnancy in order to allow time to optimize their health prior to pregnancy. The management of SLE should start before conception so that baseline assessments and alterations to drug therapy can be undertaken. It is recommended that the disease has been in remission for at least 6 months prior to conception. SLE in conjunction with pulmonary hypertension, renal nephritis or APS confers a high risk of maternal morbidity and mortality (Mackillop et al 2007).
Antenatal care
Women should be referred as soon as possible to a centre that specializes in the care of people with lupus disorders. Antenatal care should be provided by a multidisciplinary team in combined clinics. The frequency of antenatal visits is dependent on the severity of the disease, but women with SLE may have additional social and psychological needs requiring consistent midwifery care and support. A minimum of monthly visits until 28 weeks, fortnightly visits to 36 weeks and then weekly visits is recommended (Khamashta 2006). Baseline investigations include: full blood count; urea, creatinine and electrolytes; liver function tests; immunological blood tests to detect antibodies; blood pressure; urinalysis and 24 hrs urine collection for creatinine clearance and total protein to assess renal function is also recommended (Mackillop et al 2007).
An early first trimester scan is undertaken to confirm fetal viability and an anomaly scan is performed at 18–20 weeks. Women with SLE and APS are offered a fetal cardiac anomaly scan at 24 weeks’ gestation and echocardiography to detect CHB at intervals in the second and third trimesters.
In view of the high incidence of intrauterine fetal death, careful monitoring of fetal growth and well-being should begin at 28–32 weeks. This should include serial ultrasound examinations for fetal growth, placental Doppler studies and amniotic fluid volume, as well as CTG. Doppler assessment of uterine artery blood flow studies at 20–24 weeks may also be undertaken to predict pre-eclampsia and intrauterine growth restriction (Khamashta 2006).
During pregnancy, the aim is to control disease activity and achieve clinical remission while keeping drug therapy to a minimum. Avoidance of emotional stress and the promotion of a healthy lifestyle may play a part in reducing the likelihood of flares or exacerbations of SLE arising during pregnancy. Alternative therapies and low impact exercise may be utilized by women to reduce the effects of pain, joint stiffness and fatigue. Simple analgesics such as paracetamol and codeine derivatives may be used for symptomatic relief. Women who have a mild form of the disease or are in remission require minimal to no medication. Mild flares with joint pain, skin lesions and fatigue respond well to low dose prednisolone (up to 10 mg/day). Antimalarial drugs are effective as maintenance therapy in women with frequent flares and hydroxychloroquine is considered safe to use in pregnancy. Advanced renal disease and women with more severe SLE will require higher doses of prednisolone and immunosuppression. Women with SLE and APS have associated recurrent miscarriage, thrombosis and thrombocytopenia and it is recommended that treatment with anticoagulants such as low dose aspirin and/or heparin is commenced as soon as pregnancy is diagnosed (Mackillop et al 2007). Thromboprophylaxis promotes successful embryonic implantation in the early stages of pregnancy and protects against thrombosis of the uteroplacental vasculature after successful implantation.
Intrapartum care
Generally, intrapartum care falls into the high risk category and women with SLE should be cared for in hospital although a normal labour and vaginal birth should be the aim. Close liaison is required between all healthcare professionals involved: the midwife, obstetrician, rheumatologist, anaesthetist, paediatrician and haematologist. The woman and her family should continue to be involved in the development of the care pathway and the decision making process.
Women with SLE are particularly prone to infection, hypertension, thrombocytopenia and thromboembolic disorders. Careful hand-washing, strict aseptic techniques with invasive procedures and limiting the number of vaginal examinations will reduce the risk of infection. Close monitoring of the maternal condition is required by the midwife, obstetrician and anaesthetist to evaluate cardiac, pulmonary and renal function. Blood tests should be undertaken to screen for haematological conditions, which may lead to clotting disorders. Comfort measures, nursing interventions and the use of TED stockings can reduce the risk of pressure sores and the development of deep vein thrombosis. Women who have been on long-term and/or high doses of steroid therapy will require parenteral steroid cover during labour. SLE may compromise the uteroplacental circulation therefore continuous fetal monitoring in conjunction with fetal blood gas estimation is recommended (Classen et al 1998).
Postpartum care
During the immediate postpartum period, the midwife should observe closely for: signs of SLE flares that may occur as a result of the stress of labour, signs and symptoms of infection, pre-eclampsia, renal disease, thrombosis and neurological changes. Careful consideration needs to be given to breastfeeding as most of the drugs used to treat SLE are excreted in breastmilk: paracetamol is the drug of choice for postpartum analgesia; low dose steroids and hydroxychloroquine are considered safe; immunosuppressive therapy is contraindicated; large doses of aspirin should be avoided and non-steroidal antiinflammatory drugs (NSAIDs) are contraindicated when breastfeeding jaundiced neonates.
The midwife has a role in advising women with regard to her contraceptive options as the choice for a woman with SLE may be limited. Combined oral contraception increases the risk of hypertension, thrombosis and SLE flares. Low dose oestrogen combined pills may be considered in women with well-controlled SLE without a history of thromboembolic disease or APS. Intrauterine contraceptive devices are associated with an increased risk of infection in SLE women. Progestogens and barrier methods represent the safest options and may be suitable for those women where other methods are contraindicated (Mok & Wong 2001).
Thyroid disease
Thyroid disease is the second most common endocrine disorder affecting women of reproductive age. The thyroid gland comprises two lobes connected by the isthmus. Follicular cells within the lobes produce the thyroid hormones. Thyroxine (T4) and tri-iodothyronine (T3) are iodine-containing hormones, which are essential for normal body growth in infancy and childhood and affects the metabolic rate of the body. The thyroid gland also produces calcitonin, which is required for calcium metabolism. The production and release of T3 and T4, is regulated by thyroid-stimulating hormone (TSH), which is secreted through a negative feedback mechanism by the anterior pituitary gland. Production of the thyroid hormones depends on dietary consumption of iodine and calcium. After digestion and synthesis the thyroid hormones become bound to a transport protein called thyroid-binding globulin (TBG) and are stored within the thyroid. Stored thyroid hormone is capable of supplying the body with the required amount of hormone for 2–3 months. When released into the circulation 99% of T4 and T3 are bound to plasma proteins and serve as a reservoir or store of thyroid hormones in the body. Less than 1% remains as ‘free’ (unbound to protein) T4 and T3, which can be utilized metabolically and act as indicators of the thyroid level in the body, stimulating the release of TSH when T4 and T3 levels fall (Rashid & Rashid 2007). In pregnancy, hypothalamic and pituitary regulation maintain normal levels of TSH; however, thyroid function is affected by four factors that increase the basal metabolic rate by 20%:
Clinical assessment of thyroid dysfunction is difficult as pregnancy-related symptoms are similar to hyperthyroidism and hypothyroidism. Thyroid function can be assessed by biochemical tests that measure, free thyroxine (FT4), free T3 (FT3) and TSH (Higgins 2000).
Hyperthyroidism
Hyperthyroidism (also called thyrotoxicosis) occurs in 0.1–0.2% of pregnancies (Lao 2005). The most common cause of hyperthyroidism in pregnancy is Graves’ disease. This is an autoimmune disorder that results in TSH receptor stimulating antibody (TSHRAb) activation of the thyroid gland. The gland becomes enlarged and secretes an increased amount of thyroid hormone. The metabolic processes of the body are accelerated resulting in sweating, tachycardia, dyspnoea, diarrhoea, mood lability and fatigue. Clinical diagnosis may be difficult as the physiological signs and symptoms that pregnant women normally exhibit may mask this condition. Maternal and fetal complications include miscarriage, placenta abruption, pre-term labour and birth, pre-eclampsia and intrauterine growth restriction (Rashid & Rashid 2007).
A serious complication of untreated or poorly controlled hyperthyroidism is thyroid storm. This may occur spontaneously or be precipitated by infection, surgery or stress such as labour and birth. It is characterized by signs and symptoms associated with an extreme hypermetabolic state: hyperthermia (>41 °C) leading to dehydration, tachycardia, acute respiratory distress and cardiovascular collapse. This is a medical emergency requiring the administration of oxygen, use of antipyretics, cooling blanket, hydration, antibiotics and drug therapy to stop the production and reduce the effect of thyroid hormone. Thyroid storm is a rare occurrence in pregnancy but carries a high risk of maternal heart failure, fetal or neonatal hyperthyroidism and stillbirth hence intensive maternal and fetal monitoring will be required (Sheffield & Cunningham 2004).
Treatment
Treatment of hyperthyroidism is achieved through the use of antithyroid medication. Propylthiouracil (PTU), methimazole and carbimazole may be used in pregnancy. PTU is the drug of choice as less of it crosses the placenta and only small amounts are found in breastmilk. The aim of treatment is to use the lowest dose possible as these drugs may cause goitre and hypothyroidism in the fetus. During childbirth the midwife should be aware of factors that may precipitate thyroid storm, such as infection, the stress of labour and caesarean section. The woman should be seen monthly by the endocrinologist for clinical evaluation and monitoring of her thyroid levels. Fetal well-being should also be monitored closely (Rashid & Rashid 2007).
Hypothyroidism
Hypothyroidism occurs as the result of decreased activity of the thyroid gland and occurs in 2.5% of pregnancies and may lead to maternal and neonatal complications as well as being a cause of infertility. The most common cause of hypothyroidism in pregnancy is autoimmune thyroiditis (Hashimoto’s disease). It may also be induced following treatment for Graves’ disease. Slowing of the body’s metabolic processes may occur giving rise to mental and physical lethargy, excessive weight gain, constipation, cold intolerance and dryness of the skin. However, the symptoms may be non-specific and the condition can be difficult to diagnose.
Thyroid hormone is essential for human brain development and the fetus obtains its hormone almost entirely from its mother; reduced availability for fetal requirements results in impaired neurological development in childhood (Haddow et al 1999, Pop et al 2003). In addition, untreated hypothyroidism in pregnancy is associated with increased risk of miscarriage, pre-eclampsia, fetal growth restriction, placental abruption, perinatal mortality and neonatal morbidity (Lao 2005). Women should be encouraged to increase their dietary iodine intake during pregnancy. A study undertaken by Kibirige et al (2004) in the north-east of England found that 40% of pregnant women had iodine deficiency.
It is important to identify and treat hypothyroidism with daily thyroxine as early as possible in order to improve pregnancy outcome. Following birth, thyroid status in the neonate should be checked to identify whether neonatal hypothyroidism is present. There is no contraindication to breastfeeding but the dose of thyroxine may need adjustment postpartum because of maternal weight loss following childbirth.
Postpartum thyroiditis
This is an autoimmune disorder and is a form of Hashimoto’s thyroiditis. It occurs in 10% of women within 12 months following childbirth (Lazarus & Premawardhana 2005). It is a transient thyroid disorder, characterized by a period of mild hyperthyroidism 1–4 months after the birth of her baby, followed by a phase of hypothyroidism. In both phases the disorder presents with fatigue and a painless goitre; the condition may also mimic postpartum depression. Treatment is not required as recovery is usually spontaneous but the disorder tends to recur in subsequent pregnancies and may progress to permanent hypothyroidism (Muller et al 2001).
Screening for thyroid disorders
Due to the maternal obstetric complications and reduced neonatal and child neurological development, it is recommended by some authors that a screening programme for thyroid dysfunction should be undertaken in early pregnancy (Lazarus & Premawardhana 2005, Rashid & Rashid 2007). The evidence base for this is currently being assessed; interim measures to improve pregnancy outcome include: optimum iodine nutrition during pregnancy and identifying women with (a) known thyroid disease and (b) increased risk of thyroid disease, e.g. those with other autoimmune disorders. All babies in the UK are screened for congenital hypothyroidism as part of the newborn blood spot programme (UKNSPC 2005).
APPG (All Party Parliamentary Group on Epilepsy). Wasted money, wasted lives. The human and economic cost of epilepsy in England. Joint Epilepsy Council of UK and Ireland, Leeds. 2007. Online. Available www.jointepilepsycouncil.com.
Badawy AM, El-Metwally AG. Cardiac disease during pregnancy: who will manage? Journal of Obstetrics and Gynaecology. 2001;21(1):36-38.
BCS (British Cardiovascular Society). British Cardiovascular Society working group report and recommendations for Women’s Heart Health. London: BCS, 2007.
Baird S, Kennedy B. Myocardial infarction in pregnancy. Journal of Perinatal & Neonatal Nursing. 2006;20(4):311-321.
Beard JL. Effectiveness and strategies of iron supplementation during pregnancy. American Journal of Clinical Nutrition. 2000;71:1288S-1294S.
Beaton GH. Iron needs during pregnancy: do we need to rethink out targets? American Journal of Clinical Nutrition. 2000;72:265S-271S.
Bent S, Nallamothu BK, Simel DL, et al. Does this woman have an acute uncomplicated urinary tract infection? Journal of the American Medical Association. 2002;287(20):2701-2710.
BMA, RPS (British Medical Association, Royal Pharmaceutical Society of Great Britain). British National Formulary 42. London: BMJ Books, 2001.
Booker R. Peak expiratory flow measurement. Nursing Standard. 2007;21(39):42-43.
Bothwell TH. Iron requirements in pregnancy and strategies to meet them. American Journal of Clinical Nutrition. 2000;72(1):257S-264S.
Bridges EJ, Womble S, Wallace M, et al. Haemodynamic monitoring in high-risk obstetric patients, I – Expected haemodynamic changes in pregnancy. Critical Care Nurse. 2003;23(4):53-62.
BTS (British Thoracic Society), SIGN (Scottish Intercollegiate Guidelines Network). British Guideline on the Management of Asthma – a national clinical guideline, BTS, SIGN, London, 2005. Online. Available www.brit-thoracic.org.uk www.sign.ac.uk.
Bussey CG, Mittelstaedt EA. Pregnant with cystic fibrosis. AWHONN Lifelines. 2003;7(1):40-46.
Cameron AJD, Skinner TAJ. Case report – Management of a parturient with respiratory failure secondary to cystic fibrosis. Anaesthesia. 2005;60:77-80.
Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. 2008;371:64-74.
CEMACH (Confidential Enquiry into Maternal and Child Health). Maternity services in 2002 for women with type 1 and type 2 diabetes. England, Wales and Northern Ireland. London: RCOG, 2004.
CEMACH. (Confidential Enquiry into Maternal and Child Health) Pregnancy in women with type 1 and type 2 diabetes in 2002–2003. England, Wales and Northern Ireland. London: RCOG, 2005.
CEMACH (Confidential Enquiry into Maternal and Child Health). Diabetes in pregnancy: are we providing the best care? Findings from a National Enquiry: England, Wales and Northern Ireland. London: CEMACH, 2007.
Classen SR, Paulson PR, Zacharias SR. Systemic lupus erythematosus: perinatal and neonatal implications. Journal of Obstetric, Gynaecological and Neonatal Nursing Sept/Oct. 1998:493-500.
Claster S, Vichinsky EP. Managing sickle cell disease. British Medical Journal. 2003;327:1151-1155.
Cox P, Boris W, Gogarten W, et al. Maternal cardiac disease. Current Opinion in Anaesthesiology. 2005;18(3):257-262.
Dauphin-McKenzie N, Gilles JM, Jacques E, et al. Sickle cell anaemia in the female patient. Obstetrical and Gynaecological Survey. 2006;61(5):343-352.
Davey RX, Hamblin PS. Selective versus universal screening for gestational diabetes mellitus: an evaluation of predictive risk factors. Medical Journal of Australia. 2001;174:118-121.
Davison JM. Renal disorders in pregnancy. Current Opinion in Obstetrics and Gynaecology. 2001;13:109-114.
D’Cruz DP, Khamashta MA, Hughes GRV. Systemic lupus erythematosus. Lancet. 2007;369:587-596.
DH (Department of Health). The National Service Framework for Diabetes (England) Standards. London: The Stationery Office, 2001.
DH (Department of Health). National Service Framework for Long-term Conditions. London: The Stationery Office, 2005.
DH (Department of Health). Tuberculosis prevention and treatment: a toolkit for planning, commissioning and delivering high quality services in England. London: The Stationery Office, 2007.
Diabetes UK. Recommendations for the management of pregnant women with diabetes (including gestational diabetes), Diabetes UK, London, 2005. Online. Available www.diabetes.org.uk.
Dobbenga-Rhodes YA, Pride AM. Assessment and education of women with cardiac disease in pregnancy. Journal of Perinatal and Neonatal Nursing. 2006;20(4):295-302.
DoH (Department of Health), Scottish Office Home and Health Department, Welsh Office, Department of Health & Social Services, Northern Ireland. Folic acid and the prevention of neural tube defects. Report from an expert advisory group. Lancashire: Health Publications Unit, 1992.
Durbridge J, Dresner M, Harding KR, et al. Pregnancy and cardiac disease – peripartum aspects. In: Steer PJ, Gatzoulis MA, Baker P, editors. Heart disease in pregnancy. London: RCOG Press, 2006.
Ellison J, Walker ID, Greer IA. Antenatal use of enoxaparin for prevention and treatment of thromboembolism in pregnancy. British Journal of Obstetrics and Gynaecology. 2000;107:1116-1121.
Endocarditis Working Party of the British Society of Antimicrobial Chemotherapy. Antibiotic prophylaxis of infective endocarditis. Recommendations. Lancet. 1990;335:88-90.
EURAP Study Group. Seizure control and treatment in pregnancy. Observations from the EURAP epilepsy pregnancy registry. Neurology. 2006;66:354-360.
Figueroa-Damian R, Arredondo-Garcia JL. Neonatal outcome of children born to women with tuberculosis. Archives of Medical Research. 2001;32(1):66-69.
Gelsen E, Gatzooulis M, Johnson M. Valvular heart disease. British Medical Journal. 2007;335:1042-1045.
Gilmour-White S. Epilepsy. In: Lee A, Inch S, Linnigan D, editors. Therapeutics in pregnancy and lactation. Oxford: Radcliffe Medical, 2000.
Gilstrap LC, Ramin SM. Urinary tract infections during pregnancy. Obstetric and Gynaecology Clinics of North America. 2001;28(3):581-591.
Haddow JE, Palomaki GE, Allan WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. New England Journal of Medicine. 1999;341(8):549-555.
Hanna NJ, Black M, Sander JW, et al. Related death: epilepsy – death in the shadows. The National Sentinel Clinical Audit of Epilepsy. The Stationery Office, London, 2002.
Harrison MJ, Kushner KE, Benzies K. Women’s satisfaction with their involvement in health care decisions during high-risk pregnancy. Birth. 2003;20(2):109-115.
Head CEG, Thorne SA. Congenital heart disease in pregnancy. Postgraduate Medical Journal. 2005;81:292-298.
Higgins C. Understanding laboratory investigations. A text for nurses and healthcare professionals. Blackwell Science, Oxford, 2000.
Higgins C. Diagnosing diabetes: blood glucose and the role of the laboratory. British Journal of Nursing. 2001;10(4):230-236.
Hofmanova I. Pre-conception care and support for women with diabetes. British Journal of Nursing. 2006;15(2):90-94.
Holdcroft A, Thomas TA. Principles and practice of obstetric anaesthesia and analgesia. Oxford: Blackwell Science, 2000.
Hotu S, Carter B, Watson PD, et al. Increasing prevalence of type 2 diabetes in adolescents. Journal of Paediatric Child Health. 2004;40(4):201-204.
HPA (Health Protection Agency). Pregnancy and tuberculosis. London: HPA, 2006. Guidance for Clinicians
HPA (Health Protection Agency). Tuberculosis in the UK. Annual report on TB surveillance and control in the UK 2007. HPA, London, 2007.
Jackson M. Epilepsy in women: a practical guide to management. Practical Neurology. 2006;6:166-179.
JFC (Joint Formulary Committee). British National Formulary 53; Appendix 5. London: British Medical Journal and RPS Publishing, 2007.
Khamashta MA. Systemic lupus erythematosus and pregnancy. Best Practice and Research in Clinical Rheumatology. 2006;20(4):685-694.
Khattab AD, Rawlings B, Ibitsam SA. Care of patients with haemoglobin abnormalities: history and biology. British Journal of Nursing. 2006;15(18):994-998.
Khattab AD, Rawlings B, Ibitsam SA. Care of patients with haemoglobin abnormalities: nursing management. British Journal of Nursing. 2006;15(19):1057-1062.
Kibirige MS, Hutchinson S, Owen CJ, et al. Prevalence of maternal dietary iodine insufficiency in the north east of England: implications for the fetus. Archives of Disease in Childhood – Fetal and Neonatal edn. 2004;89:436-439.
Kothari A, Mahadevan N, Girling J. Tuberculosis and pregnancy – results of a study in a high prevalence area in London. European Journal of Obstetrics, Gynaecology and Reproductive Biology. 2006;126:48-55.
Kuczkowski KM. Labor analgesia for the parturient with respiratory disease: what does the obstetrician need to know? Archives of Gynaecology and Obstetrics. 2005;272:160-166.
Kuvacic I, Sprem M, Skrablin S, et al. Pregnancy outcome in renal transplant recipients. International Journal of Gynaecology and Obstetrics. 2000;70(3):313-317.
Lao TT. Thyroid disorders in pregnancy. Current Opinion in Obstetrics and Gynaecology. 2005;17:123-127.
Lazarus JH, Premawardhana LD. Screening for thyroid disease in pregnancy. Journal of Clinical Pathology. 2005;58:449-452.
Lewis G, Drife J, editors. Why mothers die 2000–2002. The Sixth Report of Confidential Enquiries into Maternal Deaths in the United Kingdom. RCOG Press, London, 2004.
Lewis G, editor. Saving mothers’ lives: reviewing maternal deaths to make motherhood safer – 2003–2005. The Seventh Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. CEMACH, London, 2007.
Lewis S, Smith D. Counselling of women with epilepsy on anti-epileptic drugs: the value of nurse-led clinics. British Journal of Neuroscience Nursing. 2006;2(7):356-359.
Lipscomb KJ, Clayton Smith J, Clarke B, et al. Outcome of pregnancy in women with Marfan’s syndrome. British Journal of Obstetrics and Gynaecology. 1997;104:201-206.
Lowy C. Diabetes and pregnancy. Medicine. 1997;25(7):57-58.
MacDonald BK, Cockerell OC, Sander JW, et al. The incidence and lifetime prevalence of neurological disorders in a prospective community-based study in the UK. Brain. 2000;123:665-667.
Macintosh MCM, Fleming KM, Bailey JA, et al. Perinatal mortality and congenital anomalies in babies of women with type 1 or type 2 diabetes in England, Wales and Northern Ireland: population based study. British Medical Journal. 2006;333:177-180.
Mackillop LH, Germain SJ, Nelson-Piercy C. Systemic lupus erythematosus. British Medical Journal. 2007;335:933-936.
McMullen AH, Pasta DJ, Frederick PD. Impact of pregnancy on women with cystic fibrosis. Chest. 2006;129:706-711.
Mitchell M. Gestational diabetes: a controversial concept. British Journal of Midwifery. 2001;91:26-34.
Mok CC, Wong RW. Pregnancy in systemic lupus erythematosus. Postgraduate Medical Journal. 2001;91:26-34.
Morrow J, Russell A, Guthrie E, et al. Malformation risk of antiepileptic drugs in pregnancy: a prospective study from the UK Epilepsy and Pregnancy Register. Journal of Neurology, Neurosurgery and Psychiatry. 2006;77:193-198.
MRC (Medical Research Council) Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet. 1991;338:131-137.
Muller AF, Drexhage HA, Berghout A. Postpartum thyroiditis and autoimmune thyroiditis in women of childbearing age; recent insights and consequences for antenatal and postnatal care. Endocrinology Review. 2001;22:605-630.
Murphy VE, Gibson PG, Smith R, Clifton VL. Asthma during pregnancy: mechanisms and treatment implications. European Respiratory Journal. 2005;25:731-750.
Murphy VE, Clifton VL, Gibson PG. Asthma exacerbations during pregnancy: incidence and association with adverse pregnancy outcomes. Thorax. 2006;61:169-176.
National Institutes of Health (National Heart, Lung and Blood Institute). The management of sickle cell disease, NIH, Bethesda, 2002. Online. Available www.nhlbi.nih.gov.
NHS Sickle Cell and Thalassaemia Screening Programme Centre. NHS Sickle Cell and Thalassaemia Screening Programme. Standards for the linked antenatal and newborn screening programme. NHS Sickle Cell and Thalassaemia Screening Programme Centre, Kings College, London, 2006.
NICE (National Institute for Health and Clinical Excellence). The epilepsies. The diagnosis and management of the epilepsies in adults and children in primary and secondary care. NICE, London, 2004. Clinical Guideline No. 20
NICE (National Institute for Health and Clinical Excellence). Tuberculosis. Clinical diagnosis and management of tuberculosis, and measures for its prevention and control. NICE, London, 2006. Clinical Guideline No. 33
NICE (National Institute for Health and Clinical Excellence). Acutely ill patients in hospital. London: NICE, 2007. Clinical Guideline No. 50
NICE (National Institute for Health and Clinical Excellence). Diabetes in pregnancy: management of diabetes and its complications from preconception to the postnatal period. London: RCOG Press, 2008. Clinical Guideline No. 63
Norman JC, Davison JM, Lindheimer MD. Renal disorders in pregnancy. Contemporary Clinical Gynaecology and Obstetrics. 2001;1:59-67.
NSE (National Society for Epilepsy). Information on epilepsy: first aid for epilepsy. 2007. Online. Available www.epilepsy.nse.org.
Okpala I, Thomas V, Westerdale N, et al. The comprehensive care of sickle cell disease. European Journal of Haematology. 2002;68:157-162.
Ormerod P. Tuberculosis in pregnancy and the puerperium. Thorax. 2001;56:494-499.
Palmer DG. Peripartum cardiomyopathy. Journal of Perinatal & Neonatal Nursing. 2006;20(4):324-332.
Pinnock H, Shah R. Asthma – BMJ Masterclass for GPs. British Medical Journal. 2007;334:847-850.
Pop VJ, Brouwers EP, Vader HL, et al. Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3 year follow-up study. Clinics in Endocrinology. 2003;59:282-288.
Prasad AK, Ventura HO. Valvular heart disease and pregnancy. Postgraduate Medicine. 2001;110(2):69-88.
Pryn A, Bryden F, Reeve W, et al. Cardiomyopathy in pregnancy and caesarean section: Four case reports. International Journal of Anesthesia. 2006;16:68-73.
Rahimy MC, Gangbo A, Adjou R, et al. Effect of active prenatal management on pregnancy outcome in sickle cell disease in an African setting. Blood. 2000;96(5):1685-1689.
Raja SG, Basu D. Pulmonary hypertension in congenital heart disease. Nursing Standard. 2005;19(50):41-49.
Ramsay M. Management of the puerperium in women with heart disease. In: Steer PJ, Gatzoulis MA, Baker P, editors. Heart disease in pregnancy. London: RCOG Press, 2006.
Ramsay MM. Normal values in pregnancy, 2nd edn. London: Saunders, 2000.
Rashid M, Rashid MH. Obstetric management of thyroid disease. Obstetrical and Gynecological Survey. 2007;62(10):680-688.
Rasmussen KN. Is there a causal relationship between iron deficiency or iron-deficiency anaemia and weight at birth, length of gestation and perinatal mortality? Journal of Nutrition. 2001;131(25–22):590S-603S.
Ray P, Murphy GJ, Shutt LE. Recognition and management of maternal cardiac disease in pregnancy. British Journal of Anaesthesia. 2004;93(3):428-439.
Rees J. ABC of asthma – Prevalence. British Medical Journal. 2005;331:443-445.
Reddy DS. Perimenstrual catamenial epilepsy. Women’s Health. 2007;3(2):195-206.
Rowe SM, Miller S, Sorscher EJ. Mechanisms of disease – cystic fibrosis. New England Journal of Medicine. 2005;352(19):1992-2001.
Royal College of Physicians/The National Collaborating Centre for Chronic Conditions. Tuberculosis. Clinical diagnosis and management of tuberculosis, and measures for its prevention and control. RCP, London, 2006.
Ruiz-Irastorza G, Khamashta MA, Castellino G, et al. Systemic lupus erythematosus. Lancet. 2001;357:1027-1032.
Sergeant GR, Look Loy L, Crowther M, et al. Outcome of pregnancy in homozygous sickle cell disease. American College of Obstetricians and Gynecologists. 2004;103(6):1278-1285.
Schatz M, Dombrowski MP, Wise R, et al. Spirometry is related to perinatal outcomes in pregnant women with asthma. American Journal of Obstetrics and Gynecology. 2006;194:120-126.
Scholl T, Reilly T. Anaemia, iron and pregnancy outcome. Journal of Nutrition. 2000;130:443S-447S.
SIGN (Scottish Intercollegiate Guidelines Network). Management of diabetes in pregnancy. Edinburgh: SIGN, 2001.
SIGN (Scottish Intercollegiate Guidelines Network). Diagnosis and management of epilepsy in adults. Edinburgh: SIGN, 2003.
Sharma P, Thapa L. Acute pyelonephritis in pregnancy: a retrospective study. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2007;47:313-315.
Sheffield JS, Cunningham FG. Thyrotoxicosis and heart failure that complicates pregnancy. American Journal of Obstetrics and Gynecology. 2004;190:211-217.
Siu SC, Colman JM. Heart disease and pregnancy. Heart. 2001;85:710-715.
Smaill F, Vazquez JC. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database of Systematic Reviews. 2007. Online. Available www.cochranedatabase.co.uk, Issue 2:CD000490
Stoneman A. Mother in mind, Epilepsy Action, Leeds, 2005. Online. Available www.epilepsy.org.uk.
Strong J. Anaemia and white blood cell disorders. In James DK, Steer PJ, Weiner CP, et al, editors: High risk pregnancy management options, 3rd edn, Philadelphia: W B Saunders, 2006.
Stuart G. Maternal endocarditis. In: Steer PJ, Gatzoulis MA, Baker P, editors. Heart disease and pregnancy. London: RCOG Press, 2006.
Sun PM, Wilburn W, Raynor BD, et al. Sickle cell disease in pregnancy: Twenty years of experience at Grady Memorial Hospital, Atlanta, Georgia. American Journal of Obstetrics and Gynecology. 2001;184(6):1127-1130.
SVD (Saint Vincent declaration) Working Party. Diabetes care and research in Europe: the St Vincent declaration. Diabetic Medicine. 1990;7:360.
Tan J. Diagnosis of unsuspected heart disease in pregnancy. Contemporary Reviews in Obstetrics and Gynaecology. 1999;10(2):85-91.
Taylor M. Managing epilepsy: a clinical handbook. Oxford: Blackwell Science, 2000.
Temple R. Hypoglycaemia during pregnancy. Diabetes update. Diabetes UK Autumn. 2007:38-42.
Thomas VJ, Taylor LM. The psychosocial experience of people with sickle cell disease and its impact on the quality of life: qualitative findings from focus groups. British Journal of Psychology. 2002;7(3):345-363.
Thorne S, MacGregor A, Nelson-Piercy C. Risks of contraception and pregnancy in heart disease. Heart. 2006;92:1520-1525.
Tincani A, Bompane D, Danieli E, et al. Pregnancy, lupus and antiphospholipid syndrome (Hughes Syndrome). Lupus. 2006;15:156-160.
Tomson T, Hiilesmaa V. Epilepsy in pregnancy. British Medical Journal. 2007;335:769-773.
UKNSPC (UK Newborn Screening Programme Centre). Health professional handbook for newborn blood spot screening in the UK, UKNSPC, London, 2005. Online. Available www.newbornscreening-bloodspot.org.uk.
Vazquez JC, Villar J. Treatments for symptomatic urinary tract infections during pregnancy. Cochrane Database of Systematic Reviews. 2003. Online. Available www.cochranedatabase.co.uk, Issue 4:CD002256
Virjee S, Robinson S, Johnston DG. Screening for diabetes in pregnancy. Journal of the Royal Society of Medicine. 2001;94:502-509.
WHO (World Health Organization). Definition, diagnosis and classification of diabetes mellitus and its complications. Report of a WHO consultation. Part 1: diagnosis and classification of diabetes mellitus. Geneva: WHO, 1999.
WHO (World Health Organization). Iron deficiency anaemia: assessment, prevention and control. A guide for programme managers. Geneva: WHO, 2001.
Witcher PM, Harvey CJ. Modifying labor routines for the woman with cardiac disease. Journal of Perinatal & Neonatal Nursing. 2006;20(4):303-310.
Yasmeen S, Wilkins EE, Field NT, et al. Pregnancy outcomes in women with systemic lupus erythematosus. Journal of Maternal Fetal Medicine. 2001;10(2):91-96.
Yip R. Iron supplementation in pregnancy: is it effective? American Journal of Clinical Nutrition. 1996;63:853-855.
Zack-Williams D. Sickle cell anaemia in pregnancy and the neonates: ethical issues. British Journal of Midwifery. 2007;14(4):205-209.
Blackburn ST, Loper DL. Maternal, fetal and neonatal physiology: a clinical perspective, 3rd edn. W B Saunders, Philadelphia, 2007.
Greer IA, Nelson Piercy C, Walters B, editors. Maternal medicine: medical problems in pregnancy. Churchill Livingstone, London, 2007.
de Swiet M, editor. Medical disorders in pregnancy, 4th edn, London: Blackwell, 2002.
Nelson Piercy C. Handbook of obstetric medicine, 3rd edn. London: Taylor Francis, 2006.
Robson SE, Waugh JNS, editors. Medical disorders in pregnancy: a manual for midwives. Blackwell, Oxford, 2008.