Chapter 48 Metabolic and endocrine disorders and drug withdrawal
Metabolic disturbances in the newborn
Many metabolic abnormalities can occur in the newborn particularly in pre-term or growth restricted infants; by far the most common problem is hypoglycaemia. Some other common problems are also highlighted here.
Glucose homeostasis
In utero the healthy fetus has a constant supply of glucose via the placenta. Following birth, this supply of nutrient ceases and there is a fall in glucose concentration (Srinivasan et al 1986). However at the same time, endocrine changes (decrease in insulin and a surge of catecholamines and release of glucagon) result in an increase in glycogenolysis (breakdown of glycogen stores to provide glucose), gluconeogenesis (glucose production from the liver), ketogenesis (producing ketones, an alternative fuel) and lipolysis (release of fatty acids from adipose) bringing about an increase in glucose and other metabolic fuel. Problems arise in the newborn when either there is a lack of glycogen stores to mobilize (pre-term and growth restricted infants) or excessive insulin production (infants of diabetic mothers) or when infants are sick and have poor supply of energy and increased requirements.
Low glucose concentrations are a potential problem in the newborn infant because if there is a lack of fuel or nutrient available for the brain, cerebral dysfunction and potentially brain injury may occur. The problem for those caring for newborn infants is not only to identify infants who are at risk and treat them appropriately but also to avoid excessive treatment and investigation in infants who are normal and where intervention is not required.
Hypoglycaemia
Definition of hypoglycaemia
The definition of hypoglycaemia is controversial and many different definitions can be found in the literature (Koh et al 1988a). The problem is that defining a specific level of blood glucose is unhelpful because an infant’s ability to compensate and use alternative fuels may be as important as the specific glucose concentration. However, pragmatically a specific level is helpful for management purposes. The consensus appears to favour a cut-off value in the newborn of 2.6 mmol/L although the evidence for the use of this level is not strong. This figure comes mainly from two studies (Koh et al 1988b, Lucas et al 1988). Koh et al demonstrated abnormal sensory-evoked brain stem potentials in a small number of term infants. This did not occur in any infants where the blood glucose was above 2.6 mmol/L whether or not symptoms were present (Koh 1988b). In addition, and perhaps more importantly, in a retrospective study of pre-term infants the neurological outcome was less good if the blood glucose concentration had been <2.6 mmol/L on ≥5 days during the neonatal period (Lucas et al 1988). These studies suggest that levels of blood glucose concentration above 2.6 mmol/L are likely to be safe but they do not take into account infants’ ability to compensate for low glucose concentrations. Lower values may be safe in some infants.
Symptoms of hypoglycaemia
Any infant who has symptomatic hypoglycaemia has a glucose concentration that is too low and this should be treated whatever the exact glucose level. The symptoms of hypoglycaemia are lethargy, poor feeding, seizures and decreased consciousness level. Jitteriness is commonly ascribed to hypoglycaemia but is a common symptom in the newborn and alone should not be used as an indication for measuring blood glucose concentration.
Normal term infants
It is likely that healthy term infants are able to tolerate low blood glucose concentrations using compensatory mechanisms and use alternative fuels such as ketone bodies, lactate or fatty acids (Hawdon et al 1992). These infants may have blood glucose concentrations as low as 2.0 mmol/L without any ill effects because, if responding normally, they are likely to have increased ketone body concentrations so that fuel is available for the brain (Hawdon et al 1992). Term infants who are breastfed are a group who are particularly likely to have low blood glucose concentrations probably because of the low energy content of breastmilk in the first few postnatal days. However these infants have higher ketone body concentrations to compensate (Hawdon et al 1992) and they are unlikely therefore to suffer any ill effects. Unfortunately however, routine measurements of ketone body concentrations are not readily available and when glucose measurements are made in these infants it becomes difficult for practitioners to resist giving treatment that may involve supplementary formula feeding or even intravenous dextrose at the expense of breastfeeding. This should obviously be avoided unless there are other clinical indications for intervention.
Because of their ability to compensate, clinically well appropriately grown full term infants who are feeding do not require monitoring of their glucose concentration. Doing so would result in many infants being inappropriately treated.
Infants at risk of neurological sequelae of hypoglycaemia
Infants where monitoring and treatment should be considered are those in whom counter-regulation may be impaired for some reason. These groups of infants are:
Diagnosis, prevention and management of hypoglycaemia
Term infants who are admitted to the postnatal ward and are feeding should not have measurements of blood glucose unless they are symptomatic. In particular breastfeeding advice and intervention should not be based on blood glucose concentrations.
Infants at risk of neurological complications of hypoglycaemia can be easily identified from the above categories and the following infants should be monitored:
Prevention is important in these infants and they should therefore have:
There is no advantage to checking the blood glucose concentration earlier than this as long as there are no symptoms as it is likely to be low and the appropriate treatment at that stage is to feed the baby. If there are symptoms the glucose should be checked and treatment given immediately. Breastfed infants are particularly difficult in this situation as it is important to avoid supplemental feeding with formula to promote successful breastfeeding but the risks associated with significant hypoglycaemia in at risk infants out-weigh this advantage.
If the blood glucose concentration is <2.6 mmol/L then feed should be given at an increased volume and decreased frequency (2-hourly or even hourly). This may require supplementary feeding with formula milk in infants who are breastfed and/or nasogastric tube (NGT) feeding. Breastmilk can also be expressed to give via an NGT.
If the blood glucose concentration remains low despite these measures and there is an adequate feed volume intake then intravenous treatment with dextrose is required. It is important in this situation that enteral feeding is continued as feeding contains much more energy than 10% glucose and it promotes ketone body production and metabolic adaptation.
If the blood glucose concentration is >2.6 mmol/L before the second and the third feed than glucose monitoring can be discontinued but feeding should continue at 3-hourly intervals.
In infants where enteral feeding is contraindicated for some reason then i.v. 10% dextrose at least 60 mL/kg per day should commence.
Hyperglycaemia
Hyperglycaemia is much less of a clinical problem than hypoglycaemia and occurs predominantly in pre-term and severely growth restricted infants. It is also seen in term infants in response to stress especially following perinatal hypoxia-ischaemia, surgery or drugs (especially corticosteroids). In general no treatment is required.
In pre-term infants it is usually a transient phenomenon related to the infants immature glucoregulation or inability to deal with excessive glucose intakes. In general, treatment is not required unless there is significant loss of glucose in the urine that may cause an osmotic diuresis. If treatment is required the rate of glucose infusion can be decreased but there may be some advantages in this situation of giving an intravenous insulin infusion. This allows glucose input to continue and sufficient calories to continue to be given and may result in better weight gain (Collins et al 1991).
Electrolyte imbalances in the newborn
Postnatal weight loss, fluid and electrolyte changes
In the first few days after birth all babies lose weight due to a loss of extracellular fluid. This diuresis and loss of weight is associated with cardiopulmonary adaptation; it occurs rapidly in healthy babies but may be delayed in those with respiratory distress syndrome. As extracellular fluid is lost there is a net loss of both water and sodium over these first few days after birth, although the infant’s serum sodium should remain within the normal range. The normal infant should lose up to 10% of its birth weight. This weight loss is physiological and should be expected.
Sodium
Sodium is normally excreted via the kidney, controlled by the renin–angiotensin system. This control mechanism is functional in the pre-term infant but loss of sodium may occur in pre-term infants because of renal tubule unresponsiveness. Term breastmilk has relatively little sodium (<1 mmol/kg per day) showing that the normal newborn can preserve sodium via the kidney in order to maintain growth. Normal sodium requirements are 1–2 mmol/kg per day in term infants and 3–4 mmol/kg per day in pre-term infants.
Changes in serum sodium reflect changes in sodium and water balance. In order to assess changes in sodium concentration it is important to know an infant’s weight: hypernatraemia in the presence of a loss of weight suggests dehydration whereas when there is weight gain it is due to fluid and sodium overload. Hyponatraemia in the presence of weight gain represents fluid overload whereas a low sodium with inappropriate weight loss represents sodium depletion. The normal serum sodium concentration is 133–146 mmol/L (Green & Keffler 1999).
Hyponatraemia
Hyponatraemia is either due to fluid overload or sodium depletion. The latter may be due to inadequate intake or excessive losses.
Fluid overload
In the first few days after birth this is the commonest cause of a low sodium concentration. It is commonly seen in infants receiving intravenous (i.v.) fluids or in infants with oliguric renal failure.
Appropriate treatment is to limit the fluid intake whilst maintaining normal sodium intake with appropriate intravenous fluids.
Sodium depletion
This is commonest in pre-term infants after the first few days after birth due to renal losses but is also common in term infants on diuretics (usually for cardiac failure).
Hypernatraemia
Increased sodium concentration is almost always due to water depletion and loss of extracellular fluid but can also rarely be due to an excessive sodium intake. These causes can again be easily differentiated by weighing an infant to assess the change since birth.
Water depletion
This is rare in term babies but does occur occasionally in infants with an inadequate intake of breast-milk. It is more common in pre-term infants.
For the midwife this is perhaps the most important cause of hypernatraemia. The incidence has been estimated as 2.5/10 000 live births and it typically occurs in term infants of breastfeeding primiparous mothers (Oddie et al 2001). It can be associated with significant morbidity and even mortality (Edmondson et al 1997), however, it can be prevented with sufficient assistance and supervision of feeding. Babies typically present at 5–9 days of age with lethargy and poor feeding. They have lost >15% of their birthweight and are usually significantly jaundiced. The serum sodium concentration can be between 150 and 200 mmol/L.
In general many infants are not weighed during this period. Mothers who are breastfeeding can be discouraged by the fact that their infant has lost weight despite a good technique and this can serve to undermine breastfeeding no matter how carefully the physiology of the phenomenon is explained. Additionally (particularly in primiparous mothers) lactogenesis is only just becoming established between 48 and 72 hrs. Thus the volume of milk transferred to the infant is still rising sharply between 72 and 96 hrs of age. However, weighing babies during this period can be very useful when a baby is unwell or if there are concerns about intake and fluid and electrolyte balance. It has been suggested that routine weighing of infants may be useful to prevent dehydration and hypernatraemia in breastfed infants with referral to hospital if weight loss exceeds 10% (van Dommelen et al 2007).
The infant’s fluid deficit can be calculated from the loss in weight and this is then replaced by gradual rehydration over 24–48 hrs. Feeding can continue but i.v. treatment is often required with normal saline and dextrose. Assistance with lactation can then be given to continue to promote breastfeeding.
Potassium
Potassium is the major intracellular cation. A low serum concentration therefore implies significant potassium depletion. Abnormalities in serum potassium concentration are important because they can cause significant arrhythmias. Potassium concentrations can be severely affected by measurement technique and any haemolysis of the blood sample especially from capillary sampling is likely to lead to a falsely high value.
Hyperkalaemia
Treatment is to remove all potassium supplements from i.v. fluids, and to consider giving calcium resonium rectally, calcium gluconate i.v., sodium bicarbonate to increase pH and i.v. glucose and insulin. In general these measures will only be required where there is a serum potassium that is very high (>8 mmol/L) and/or evidence of an abnormal ECG or arrhythmias.
Calcium
Calcium metabolism is closely linked to phosphate metabolism and these are very important minerals in relation to bone development. This is of particular importance in pre-term infants as they need much higher concentrations of phosphate and calcium. These are given as intravenous supplements, by supplementing breastmilk with fortifier (Lucas et al 1996) or by giving specific pre-term milk formulae rather than term formula.
High serum calcium concentrations are unusual but there are rare but important causes of low serum calcium. The normal serum concentration is 2.2–2.7 mmol/L but this must be interpreted with the serum albumin concentration as serum calcium is bound to albumin therefore a low albumin concentration will lead to a falsely low serum value.
Calcium concentrations fall within 18–24 hrs of birth as the infant’s supply of placental calcium ceases but accretion into bone continues. In the past, hypocalcaemia during the first week after birth used to be caused by giving unmodified cow’s milk. This has a high phosphate concentration and a relatively low calcium concentration that depressed the serum calcium concentration and caused seizures. This is now rare with modern formula feeds.
Hypocalcaemia can cause seizures, tremors, jitteriness, lethargy, poor feeding and vomiting. Severe symptoms can be treated by i.v. replacement of calcium. Longer-term management depends on the cause.
Inborn errors of metabolism in the newborn
Background/Incidence
Inborn errors of metabolism (IEM) are rare inherited disorders occurring in approximately 1 in 5000 births. They result mainly from enzyme deficiencies in metabolic pathways leading to an accumulation of substrate, leading to toxicity. In utero, the placenta provides an effective dialysis system for most disorders, removing toxic metabolites. Most affected babies are therefore initially born in good condition with normal birth weight. A high index of suspicion is needed when evaluating an acutely ill neonate, as many disorders are treatable and early diagnosis and institution of therapy can reduce morbidity. It has been estimated that 20% of infants presenting with sepsis in the absence of risk factors have an inborn error of metabolism.
Patient group
The mode of inheritance is usually autosomal recessive, therefore family history is crucial and the following features should be sought:
Clinical examination however is usually normal. The features below may be seen in isolation with many diagnoses, however multiple features indicate that an underlying IEM should be seriously considered:
Diagnosis
The following tests are a basic first step in investigation:
Many other investigations may be necessary and useful but in general investigations need to be discussed with a consultant biochemist or paediatrician with an interest in metabolic disorders.
Principles of emergency management are to reduce load on affected pathways by removing toxic metabolites and stimulating residual enzyme activity. Hypoglycaemia is corrected, adequate ventilatory support and hydration are maintained, convulsions are treated and significant metabolic acidosis is treated with i.v. sodium bicarbonate, and electrolyte abnormalities are corrected. In general antibiotics are frequently given as infection may have precipitated metabolic decompensation and occasionally dialysis may also be required (Wraith and Walker 1996).
Phenylketonuria
Phenylketonuria (PKU) is important, first because it is a treatable cause of brain injury and second it is possible to successfully screen for it during the first week of life in order to identify affected individuals and treat them appropriately to produce a favourable outcome.
PKU is an autosomal recessive disorder that has an incidence of approximately 1 in 10 000 in the UK. Babies with PKU are born in good condition but begin to be affected by their condition during the first few weeks/months after birth. Untreated it leads to severe mental retardation (IQ<30). However, if it is identified early (within the first 3 weeks), it can be treated by a diet specifically restricted in phenylalanine. The common type is caused by the absence of or reduction in an enzyme in the liver that converts phenylalanine to tyrosine (phenylalanine hydroxylase). This leads to a build up of phenylpyruvic acid that is toxic to the brain.
PKU is particularly suitable for mass screening because there is a simple widely available diagnostic test and because treatment is effective. Midwives collect the blood sample for PKU screening in the UK between days 5–8 after birth. It is commonly collected on the Guthrie Card along with a sample for screening for congenital hypothyroidism (see later). The level of phenylalanine is analysed and babies with increased levels need to be prescribed a low phenylalanine diet and have further assessment to determine whether they are affected by the ‘classic’ type or other variants.
If it is treated early the prognosis for PKU is good and normal intelligence can result. In females, a return to the low phenylalanine diet is essential prior to conception and during pregnancy. This is because fetal brain injury may result from exposure to high concentrations of phenylalanine and its metabolites in the mother.
Galactosaemia
Galactosaemia is a disorder of carbohydrate metabolism that is autosomal recessive in inheritance and has an incidence of 1 in 60 000. It is caused by an absence or severe deficiency of the enzyme galactose-1-phosphate uridyltransferase (often referred to as Gal-I-P UT). This enzyme is important for converting galactose to glucose and since milk’s main sugar lactose is a disaccharide containing glucose and galactose, infants with this condition rapidly become affected when fed either human breastmilk or cow’s milk formulae. The metabolite that builds up and is harmful is galactose-1-phosphate.
The clinical signs and symptoms of the disorder are those of liver failure and renal impairment. They tend to present with vomiting, hypoglycaemia, jaundice, bleeding, acidosis, failure to gain weight and hypotonia during the first few days after birth. Another important clinical feature sometimes present is cataracts. Affected babies may also present with septicaemia (particularly E. coli) due to damage to intestinal mucosa by high levels of galactose in the gut. Galactosaemia is an important differential diagnosis to consider when dealing with an infant with unresponsive hypoglycaemia and prolonged or severe jaundice.
Infants with galactosaemia will have galactose but not glucose in their urine. The diagnosis therefore can be made by looking for urine-reducing substances (i.e. galactose) using a Clinitest, whereas a urine test for glucose will be negative. Confirmation of the diagnosis is by assay of the enzyme level (Gal-I-P UT) within red blood cells.
Treatment is with a lactose free milk formula and this must be commenced as soon as the diagnosis is suspected. This results in a rapid correction of the abnormalities. However cataracts and mild brain injury have occurred even when galactosaemic infants have been fed lactose-free milk from birth.
Screening for this disorder is possible but many infants will have presented before the screening test is available and there is little evidence to suggest that diagnosis at or soon after birth gives a better long-term outlook than diagnosis by rapid screening of the symptomatic neonate.
Endocrine disorders in the newborn
Endocrine problems in the newborn are relatively rare but may be serious, even life threatening but are nearly always treatable so identification and diagnosis is important. Disorders of blood glucose homeostasis have already been described so this section will concentrate on other endocrine abnormalities that may present in the newborn.
Thyroid disorders
The thyroid gland produces hormones that have an effect on the metabolic rate in most tissues. They are also essential for normal neurological development. Thyroid stimulating hormone (TSH) is produced by the anterior pituitary gland and this stimulates production of T3 and T4 by the thyroid gland with a feedback mechanism to the anterior pituitary.
Hypothyroidism
The incidence of hypothyroidism in the newborn is 1 in 3500. There are several possible causes for hypothyroidism in the newborn including abnormalities in gland formation (thyroid dysgenesis), defects in hormone synthesis (dyshormonogenesis) and rarely secondary pituitary causes. The latter causes a decrease or lack of TSH, whereas primary (thyroid) causes result in very high TSH values. The presentation is however the same although this has implications for screening.
Infants with hypothyroidism tend to be large, postmature and have a large posterior fontanelle. They have coarse features and often have an umbilical hernia. These features are often missed, however, and this is why screening for this disorder is so important. Untreated infants develop impaired motor development with growth failure, a low IQ, impaired hearing and language problems. With treatment the physical signs of hypothyroidism do not appear but the intellectual and neurological prognosis is poor unless treatment is started within the first few weeks of life but this should always occur when infants are detected by screening.
Screening
Screening for hypothyroidism involves measuring TSH on a blood spot taken along with the screening test for PKU at 5–8 days of age. This method detects almost all cases, however, it cannot detect cases caused by secondary (pituitary) hypothyroidism that will have a low TSH. This condition is however much less common with an incidence of 1 in 60–100 000 (Fisher et al 1979).
Hyperthyroidism
Graves disease is an autoimmune disorder that causes hyperthyroidism. Neonatal hyperthyroidism occurs relatively rarely but is possible when the mother has or has had Graves disease. It occurs not because of neonatal autoantibodies but as a result of the transfer of maternal thyroid stimulating immunoglobulins. These are autoantibodies that are produced and act in the same way as TSH. This can occur when a mother has active, inactive or treated Graves disease (Teng et al 1980). Thyrotoxicosis in the fetus can lead to pre-term labour, low birth weight, stillbirth and fetal death but only a small percentage of infants of mothers with Graves disease become symptomatic
In the neonate the symptoms are irritability, jitteriness, tachycardia, prominent eyes, sweating, excessive appetite and weight loss. These symptoms may be present immediately after birth or presentation may be delayed for as long as 4–6 weeks (Skuza et al 1996). Infants therefore need to be observed for this period and treatment will be required with antithyroid medication if there are symptoms.
Adrenal disorders
The adrenal glands are vital for normal function of many systems within the body. They are divided into a medulla and a cortex. The medulla produces catecholamines these help to maintain blood pressure and are produced at times of stress. Abnormalities of function of the adrenal medulla are not described in the newborn. The adrenal cortex produces three groups of hormones: glucocorticoids, mineralocorticoids and sex hormones that have distinct functions. Glucocorticoids regulate the general metabolism of carbohydrates, proteins and fats on a long-term basis. They have a particular role in modifying the metabolism in times of stress. Mineralocorticoids regulate sodium, potassium and water balance. The sex hormones are responsible for normal development of the genitalia and reproductive organs. Abnormalities in function of the glands represent the functions of these different groups of hormones.
Adrenocortical insufficiency
This is caused by congenital hypoplasia, adrenal haemorrhage, enzyme defects or can be secondary to pituitary problems. It generally presents with symptomatic hypoglycaemia, poor feeding, vomiting, poor weight gain and even prolonged jaundice. Infants may have hyponatraemia, hypoglycaemia, hyperkalaemia and acidosis. Treatment is by i.v. therapy with glucose and electrolytes replacement of corticosteroid and mineralocorticoid hormones is then required.
Adrenocortical hyperfunction
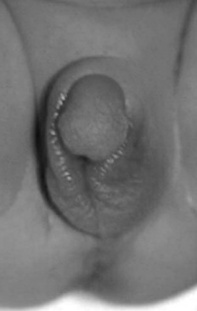
This may occur in the form of congenital adrenal hyperplasia (CAH). This is the name given to a group of inherited disorders that are due to deficiency of enzymes responsible for hormone production within the adrenal. The most common enzyme deficiency results in an excess of androgenic hormones but a deficiency of glucocorticoid and mineralocorticoids often also occurs. These disorders can cause abnormalities in the formation of the genitalia leading to ambiguous genitalia (virilization of females or inadequate virilization of males), and symptoms of adrenal insufficiency (vomiting, diarrhoea, vascular collapse, hypoglycaemia, hyponatraemia, hyperkalaemia) (see Fig. 48.1).
The classification of disorders of sexual differentiation has been revised in recent years. For more information see the consensus statement by Hughes et al (2006).
It is important to make a prompt diagnosis. The genetic sex must be determined (chromosome analysis) and it is important not to assign a sex until the diagnosis has been established. The biochemical diagnosis is made by analysing urine and plasma for steroid hormone metabolites. Treatment is as for adrenocortical insufficiency by replacement of glucocorticoid and mineralocorticoid hormones. Virilized girls may also require surgical intervention to correct the genital abnormalities.
Pituitary disorders
Pituitary insufficiency is rare in the newborn. It may occur in association with other abnormalities, particularly midline developmental defects. Presentation is with signs of glucocorticoid deficiency (hypoglycaemia), prolonged jaundice or signs of hypothyroidism. Growth hormone deficiency generally causes hypoglycaemia but no other signs in the newborn. When it is recognized treatment is with replacement of the missing hormones.
Parathyroid disorders
The parathyroid glands are responsible for control of calcium metabolism but abnormalities of the parathyroids are rare causes of hypo- or hypercalcaemia in the newborn. When hypoparathyroidism does occur it may be familial or may occur in association with deletions of chromosome 22 (22q11 deletion or DiGeorge syndrome). The symptoms associated with hypocalcaemia are detailed above.
Effects of maternal drug abuse/ use during pregnancy on the newborn
The incidence of drug use within the population has a large geographical variation. As a result the incidence of drug withdrawal symptoms amongst infants also has a markedly varying incidence. Inner city areas are more likely to be affected but even within cities large variation is seen in the incidence of problems.
Opiates and other drugs cross the placenta and the fetus during pregnancy is likely to be exposed to the same peaks and troughs of drug exposure that the mother is. Withdrawal may be manifested before birth. The increased incidence of fetal distress may be related in part to drug withdrawal during labour but the effects of drugs and withdrawal on the fetus and newborn are obviously related to the timing of drug doses.
Infants born to mothers who have used illicit drugs during pregnancy are at risk of withdrawal symptoms but there are many other problems associated with these pregnancies and infants that must be considered as well as the obvious problem of withdrawal symptoms. Other problems that are more common in these pregnancies are:
Attendance for antenatal care and supervision during pregnancy may be improved by speciality midwifery support and community liaison. It is important to identify these women during pregnancy in order to try to prevent some of the above problems and offer appropriate support. Identification during pregnancy also allows screening for infectious diseases and this is particularly important for hepatitis B and HIV where treatments are available to decrease the chance of the newborn being affected.
Symptoms
Many drugs have been reported to cause problems of withdrawal in the newborn. The most common seen in the UK are opiates in the form of heroin and methadone but barbiturates, benzodiazepines cocaine and amphetamines are also frequently seen. Multidrug use is common and usually leads to prolonged difficult withdrawal. Each drug has a different half-life and this leads to different patterns of withdrawal symptoms. In general methadone produces symptoms for longer periods than heroin (Herzlinger et al 1977) but benzodiazepines may also contribute to this (Sutton & Hinderliter 1990).
The symptoms most frequently seen are jitteriness, irritability and constant high-pitched crying. Infants often fail to settle between feeds and are hyperactive. When feeds are offered they often feed voraciously although some infants have poor sucking. Vomiting is common. Diarrhoea and an irritant nappy rash are also often seen. Sneezing and yawning are also symptoms and episodes of high temperature in the absence of infection. In rare circumstances infants may also have seizures.
Several scoring systems have been developed to help to guide when to give pharmacological treatment (Finnegan et al 1975). These scoring systems aim to make the assessment more objective, however, most of the symptoms and their severity are difficult to quantify. Infants assessed for signs of drug withdrawal by a scoring system are less likely to be inappropriately treated and may have a shorter hospital stay. It is important not to over-treat infants with drugs as the long-term effects of treatment are not clear and treatment may then be difficult to withdraw but also treatment in many maternity hospitals means admitting the infant to the neonatal unit and therefore moving the baby away from the mother. On the other hand infants who are withdrawing appear to be in discomfort that we obviously want to relieve and the long-term effects of withdrawal symptoms are also unclear. Personally I find that the most useful symptom is whether infants settle and sleep between feeding. If they do then pharmacological treatment may be unnecessary.
Treatment
Treatment can be divided into general care given to these infants and pharmacological treatment. It is important, if at all possible, to keep the infant with his/her mother. Bonding with and care of these infants by their mother is vital to encourage. The mother is likely to be feeling upset and guilty because of the infant’s symptoms and there are frequently already social problems involved with these families. Involving parents in the care of the infant is difficult when he/she provides little positive behavioural return for their effort. Breastfeeding can be encouraged as long as there is no evidence of HIV or on-going drug use that precludes this (cocaine, heroin). This includes methadone although some recommend limiting this to mothers who are taking a dose which is <20 mg/day (Committee on Drugs, American Academy of Pediatrics 1989).
A quiet environment with reduced light and noise is helpful in keeping stimuli to a minimum. Swaddling is useful and feeds may need to be given frequently. These infants often will take large volumes of milk which is acceptable as long as vomiting is not a problem. Rocking or cradling are also useful adjuncts.
Pharmacological treatment
Several different treatments have been recommended in the past. Previously, the four drugs recommended for use were paregoric (a mixture of alcohol and opiate), phenobarbitone, diazepam and chlorpromazine (Committee on Drugs, American Academy of Pediatrics 1983). A number of randomized trials have been performed attempting to assess the use of various drugs in the treatment of neonatal abstinence syndrome (NAS) (Theis et al 1997). It seems logical to treat opiate withdrawal with opiates and now the two most commonly used treatments are oral methadone and oral morphine. These appear to control withdrawal seizures much more effectively (Rivers 1999). They can be given in increasing doses if necessary until symptoms are controlled and then the dose gradually reduced. A possible dosing regimen for oral morphine is shown below:
The dose is reduced every 24 hrs if the baby is feeding well and settling better between feeds. If the feeding and settling does not improve or profuse watery stools and profuse vomiting continue other treatment needs to be considered. Other medication may sometimes be useful, e.g. clonazepam for benzodiazepine use or chloral hydrate as a general sedative.
Cocaine
Cocaine deserves special mention because its effects on the newborn are different. It is a larger problem in the USA than in the UK but the incidence of its use during pregnancy is unknown. It is only present in maternal urine for 24 hrs after exposure therefore detection is difficult (Zuckerman et al 1989). It can produce significant withdrawal symptoms but these are often less severe and less troublesome than with other drugs but it is associated with many other harmful effects on the fetus (Fulroth et al 1989). These include significant fetal growth restriction, brain injury due to haemorrhage or infarction (Hadeed & Siegel 1989), abnormalities of brain development, limb reduction defects and gut atresias. A correlation between cocaine exposure, small head size and developmental scores has been reported (Chasnoff et al 1992).
Discharge and long-term effects
Discharge must be planned with the involvement of other support agencies. This may involve a planning meeting involving all agencies involved in the care of the mother and baby.
Although it seems intuitive that exposure to drugs in utero would cause neurodevelopmental impairment this is not borne out by carefully controlled studies (Lifschitz et al 1985). This implies that impairment in intellectual outcome in these children relates to other adverse prenatal and postnatal factors. Infants born to these mothers are smaller and have smaller head circumferences (Kandall et al 1976). However it is difficult to be certain about the exact causes of any long-term harmful effects because so many factors are involved all of which are interlinked. These include:
Chasnoff IJ, Griffith DR, Freier C, et al. Cocaine/polydrug use in pregnancy: two year follow up. Pediatrics. 1992;89:284-289.
Collins JWJr, Hoppe M, Brown K, et al. A controlled trial of insulin infusion and parenteral nutrition in extremely low birth weight infants with glucose intolerance. Journal of Pediatrics. 1991;118(6):921-927.
Committee on Drugs, American Academy of Pediatrics. Neonatal drug withdrawal. Pediatrics. 1983;72:895-902.
Committee on Drugs, American Academy of Pediatrics. Transfer of drugs and other chemicals into human milk. Pediatrics. 1989;84:924-936.
CEMACH (Confidential Enquiry into Maternal and Child Health). Pregnancy in women with Type 1 and Type 2 diabetes in 2002–2003, England, Wales and Northern Ireland. London: CEMACH, 2005.
Edmondson MB, Stoddard JJ, Owens LM. Hospital admission with feeding related-problems after early postpartum discharge of normal newborns. Journal of the American Medical Association. 1997;278:299-303.
Finnegan LP, Kron RE, Connaughton JF, et al. Assessment and treatment of abstinence in the infant of the drug dependent mother. International Journal of Clinical Pharmacology. 1975;12:19-32.
Fisher DA, Dussault JH, Foley TP, et al. Screening for congenital hypothyroidism: results of screening one million North American infants. Journal of Pediatrics. 1979;94:700-705.
Fulroth R, Phillips B, Durand D. Perinatal outcome of infants exposed to cocaine and/or heroin in utero. American Journal of Diseases of Children. 1989;143:905-910.
Green A, Keffler S. Neonatal biochemical reference ranges. In: Rennie JM, Roberton NRC, editors. Textbook of neonatology. 3rd edn. Edinburgh: Churchill Livingstone; 1999:1408-1414.
Hadeed AJ, Siegel SR. Maternal cocaine use during pregnancy: effect on the newborn infant. Pediatrics. 1989;84:205-210.
Herzlinger RA, Kandall SR, Vaughan HG. Neonatal seizures associated with narcotic withdrawal. Journal of Pediatrics. 1977;91:638-641.
Hawdon JM, Ward Platt MP, Aynsley-Green A. Patterns of metabolic adaptation for pre-term and term infants in the first neonatal week. Archives of Disease in Childhood. 1992;67:357-365.
Hughes IA, Houk C, Ahmed SF, et al. Consensus statement on management of intersex disorders. Archives of Disease in Childhood. 2006;91:554-563.
Kandall SR, Albin S, Lowinson J. Differential effects of maternal heroin and methadone use on birthweight. Pediatrics. 1976;58:681.
Koh THHG, Eyre JA, Aynsley-Green A. Neonatal hypoglycaemia – the controversy regarding definition. Archives of Disease in Childhood. 1988;63:1386-1388.
Koh THHG, Aynsley-Green A, Tarbit M, et al. Neural dysfunction during hypoglycaemia. Archives of Disease in Childhood. 1988;63:1353-1358.
Koran G, Graham K, Shear H, et al. Bias against the null hypothesis: the reproductive hazards of cocaine. Lancet. 1989;ii:1440-1442.
Lifschitz MH, Wilson GH, Smith EO, et al. Factors affecting head growth and intellectual function in children of drug addicts. Pediatrics. 1985;75:269-274.
Lucas A, Morley R, Cole TJ. Adverse neurodevelopmental outcome of moderate neonatal hypoglycaemia. British Medical Journal. 1988;297:1304-1308.
Lucas A, Fewtrell MS, Morley R, et al. Randomized outcome trial of human milk fortification and developmental outcome in pre-term infants. American Journal of Clinical Nutrition. 1996;64:142-151.
Oddie S, Richmond S, Coulthard M. Hypernatraemic dehydration and breast feeding: a population study. Archives of Disease in Childhood. 2001;85:318-320.
Rivers R. Infants of drug-addicted mothers. In: Rennie JM, Roberton NRC, editors. Textbook of neonatology. 4th edn. Edinburgh: Churchill Livingstone; 1999:443-451.
Skuza KA, Sills IN, Rapaport R. Prediction of neonatal hyperthyroidism in infants born to mothers with Graves’ disease. Journal of Pediatrics. 1996;128:264-267.
Srinivasan G, Pildes RS, Cattamanchi G, et al. Plasma glucose values in normal neonates: a new look. Journal of Pediatrics. 1986;109:114-117.
Sutton LR, Hinderliter SA. Diazepam abuse in pregnant women on methadone maintenance. Implications for the neonate. Clinical Pediatrics (Philadelphia). 1990;29:108-111.
Teng CS, Tong TC, Hutchinson JH, et al. Thyroid stimulating immunoglobulins in neonatal Graves’ disease. Archives of Disease in Childhood. 1980;55:894-895.
Theis JGW, Selby P, Ikizler Y, et al. Current management of the neonatal abstinence syndrome: a critical analysis of the evidence. Biology of the Neonate. 1997;71:345-356.
van Dommelen P, van Wouwe JP, Breuning-Boers JM, et al. Reference chart for relative weight change to detect hypernatraemic dehydration. Archives of Disease in Childhood. 2007;92:490-494.
Wraith JE, Walker JH. Inherited metabolic disorders diagnosis and initial management. Manchester: Willink Biochemical Genetics Unit, Royal Manchester Children’s Hospital, 1996.
Zuckerman B, Frank DA, Hingson R, et al. Effects of maternal marijuana and cocaine use on fetal growth. New England Journal of Medicine. 1989;320:762-768.