Workplace Safety
After completion of this chapter, the reader will be able to:
• Describe both the short-term and long-term effects of waste anesthetic gas on persons working in health care environments.
• Recognize ways in which the release of waste anesthetic gases may be minimized.
• Describe proper procedures for handling and transporting compressed gas cylinders.
• Outline the precautions necessary for handling potentially hazardous injectable agents.
Veterinary technicians may participate in the anesthetic management of several thousand animals during the course of their careers. It is therefore essential that the technician be familiar with the human safety considerations involved in veterinary anesthesia. These can be divided into three categories: (1) hazards of waste anesthetic gas, (2) safety considerations for handling compressed gas cylinders, and (3) hazards associated with potent injectable agents.
This chapter outlines the precautions that the anesthetist can take to reduce, as much as possible, the health risks of working with anesthetic equipment, injectable drugs, and compressed gases.
HAZARDS OF WASTE ANESTHETIC GAS
Concerns have been raised regarding the possible adverse effects resulting from exposure of hospital personnel to waste anesthetic gas and vapors. The term waste anesthetic gas refers to nitrous oxide, halothane, isoflurane, and other anesthetic vapors that are breathed out by the patient or that escape from the anesthetic machine. These vapors are breathed inadvertently by all personnel working in areas where animals are anesthetized or are recovering from inhalation anesthesia. Significant exposure to anesthetic vapors can also occur when emptying or filling anesthetic vaporizers. In addition, short-term exposure to high levels of anesthetic vapors can occur because of an accidental spill of liquid anesthetic.
Waste gas concentrations are usually expressed in parts per million (abbreviated ppm). If the concentration of halothane in room air is 33 ppm, this means that out of every 1 million molecules of air, 33 are halothane. (The rest are chiefly nitrogen, oxygen, and carbon dioxide.) Although at first glance 33 ppm appears to be a small amount, this is the level at which the average person can smell the odor of halothane in room air. It is also more than 15 times the recommended maximum concentration that should be present in a veterinary hospital.
The concentration of waste anesthetic gas that is hazardous to humans is surprisingly difficult to determine with exactness.
Since the first study of waste anesthetic gas was published in 1967, many investigators have investigated the toxic effects of isoflurane, halothane, methoxyflurane, nitrous oxide, and other anesthetic agents on operating room personnel. Although some of the evidence is contradictory, it is suspected that exposure to high levels of waste anesthetic gas is associated with a higher than normal incidence of some health problems. The suspected health hazards can be divided into two categories: (1) short-term problems that occur during or immediately after exposure to these agents and (2) long-term problems that may become evident days, weeks, or years after exposure.
Short-Term Effects
The short-term problems associated with breathing waste gas appear to arise from a direct effect of anesthetic molecules on brain neurons. Persons working in environments with a high level of waste gas have reported symptoms such as fatigue, headache, drowsiness, nausea, depression, and irritability. Although these symptoms usually resolve spontaneously when the affected person leaves the area, the frequent occurrence of these symptoms may indicate that excessive levels of waste gas are present and that a potential for long-term toxicity exists.
Long-Term Effects
Long-term inhalation of air polluted with waste gas may be associated with serious health problems, including reproductive disorders, liver and kidney damage, bone marrow abnormalities, and chronic nervous system dysfunction. Although current evidence suggests that the risk of these disorders is not high in normal veterinary practice settings, every person working in an environment in which waste gas is present should be informed of the potential for adverse health effects.
The mechanism of long-term anesthetic gas toxicity is not fully understood but is thought to be the result of toxic metabolites produced by the breakdown of anesthetic gases within the liver and their subsequent excretion by the kidneys. These metabolites include inorganic fluoride or bromide ions, oxalic acid, and free radicals, all of which are known to have harmful effects on animal tissues. It is widely accepted that anesthetic agents that are retained by the body and metabolized are likely to have greater long-term toxicity than those that are quickly eliminated through the lungs. For this reason, isoflurane is thought to be the least toxic inhalation agent in common use (0.2% of the amount inhaled is retained and metabolized), followed by sevoflurane (approximately 3% is retained and metabolized). In contrast, approximately 15% to 20% of the halothane and 40% to 50% of the methoxyflurane administered to a patient or inhaled by the anesthetist are retained within the body fat, to be metabolized in the liver and excreted through the kidneys over the next few hours to days. Metabolites of halothane have been recovered from the urine of human patients as long as 20 days after anesthesia. Human patients who inhale 50% nitrous oxide for 1 hour have been shown to have more than 100 ppm nitrous oxide in their expired breath for the next 3 hours. In the same way, the anesthetist who inhales waste anesthetic gas may retain the gas or its metabolites for a considerable period. For example, anesthetists may show traces of halothane in their breath 64 hours after administering this gas to a patient.
Although it is generally accepted that exposure to isoflurane or sevoflurane is associated with fewer health risks than exposure to other halogenated anesthetics, safety concerns (including National Institute for Occupational Safety and Health [NIOSH] recommendations and Occupational Safety and Health Administration [OSHA] regulations) apply to all anesthetics.
Effects on Reproduction
There is some evidence that high concentrations of waste anesthetic gas can adversely affect the reproductive system. In a comprehensive survey of nurse and physician anesthetists, the American Society of Anesthesiologists found that the risk of spontaneous abortion in this group was 1.3 to 2 times that of the normal population. Another study showed that the frequency of spontaneous abortion among working hospital anesthetists (18.2%) was higher than that observed among nonworking anesthetists (13.7%) and a control group (14.7%). The same study showed that 12% of the working anesthetists interviewed were infertile, compared with 6% of the control group. A more recent study (Shirangi and colleagues, 2008) showed a twofold increase in the risk of spontaneous abortion in women exposed to unscavenged waste gas for 1 or more hours per week. In contrast, a very large study of 11,000 operating room personnel showed no correlation between hours worked in the operating room and miscarriage (Spence and colleagues, 1977). Studies of veterinary personnel (Shuhaiber and co-workers, 1999; Johnson and co-workers, 1987; and Schenker and co-workers, 1990) made similar findings.
Some studies have suggested a link between exposure to anesthetic gases and an increased risk of congenital abnormalities in the children of pregnant operating room personnel. One study reported a 16% incidence of congenital abnormalities in children of practicing nurse-anesthetists, compared with a 6% incidence in a control group. Reported problems included microcephaly, mental retardation, low birth weight, and heart defects. Other studies have failed to show a statistically significant correlation between waste gas exposure and an increased incidence of congenital abnormalities, and the evidence linking waste gas exposure and congenital anomalies is generally weaker than that linking waste gas exposure to spontaneous abortion. One report (Hoerauf and colleagues, 1999) suggests that exposure to even trace levels of waste anesthetic gas could cause genetic damage, as indicated by an increased incidence of sister chromatid exchanges. However, the study group was small, and other recent studies have so far failed to confirm these findings.
Interpreting or comparing the results obtained by these and other studies is difficult because there are wide variations in the types of anesthetics used, the amount of waste gas exposure, and the availability of control measures (such as scavengers). In most cases, operating room personnel were exposed to several agents simultaneously, and it is difficult to determine which agent or combination of agents was responsible for the adverse effects. It appears, however, that nitrous oxide is a potential reproductive hazard, because rats with prolonged exposure to high levels of nitrous oxide had abnormalities in sperm morphology, reduced ovulation, fetal resorption, and abnormal fetal development.
Oncogenic Effects
Given that waste anesthetic gases may exert their adverse reproductive effects by altering DNA, investigators have attempted to determine whether these agents have the potential to cause other DNA-related changes, such as neoplasia. Several studies undertaken in the 1970s appeared to suggest that operating room personnel have an increased incidence of some types of cancer. These studies, however, have been criticized for inappropriate data collection and statistical analysis, and it is now generally thought that none of the commonly used inhalant anesthetic agents (isoflurane, halothane, sevoflurane) is carcinogenic at the levels found in veterinary hospitals.
Effects on the Liver
Several studies have investigated the incidence of liver disorders in personnel exposed to waste anesthetic gas. Halothane, in particular, is recognized as being potentially hepatotoxic. Metabolism of halothane in certain rare anesthetized individuals produces toxic by-products that may result in massive hepatic necrosis, termed halothane hepatitis.
The possible adverse effects of waste anesthetic gas on the liver were suggested by a study showing that the risk of liver disease in hospital operating room personnel is 1.5 times that of the general population. However, it is difficult to say with certainty whether the increased incidence of liver disease is associated with exposure to halothane or other waste anesthetic gas or the result of other occupational hazards, such as viral hepatitis.
Effects on the Kidney
It is well established that methoxyflurane has the potential to cause renal toxicity in human beings anesthetized with this agent, but the risk to operating room personnel has been more difficult to assess. Studies have indicated that there is a 1.2- to 1.4-fold increase in renal disease in female operating room personnel and a 1.2- to 1.7-fold increase in renal disease in female dental assistants compared with the general population. It has not been determined whether this increase is the result of the effect of methoxyflurane, nitrous oxide, other anesthetic agents, or other occupational factors working alone or in combination.
Neurologic Effects
Because the mechanism of action of anesthetic agents involves their effect on neurons, various studies have investigated the effect of waste anesthetic gas on the central nervous system. It has been suggested that exposure to high levels of anesthetics produces a decline in performance of motor skills and short-term memory. However, the threshold at which they begin to affect performance has not been established.
Some studies have indicated that exposure to even low concentrations of anesthetic gas mixtures (e.g., nitrous oxide 500 ppm and halothane 15 ppm for a period of 4 hours) results in decreased cognitive and motor skills. Chronic exposure to nitrous oxide has been associated with increased risk of neurologic disease: a study of female dental assistants exposed to high levels of nitrous oxide showed them to have a 1.7- to 2.8-fold increase in the incidence of neurologic disease compared with the normal population. Dentists and dental assistants exposed to high levels of waste nitrous oxide reported muscle weakness, tingling sensations, and numbness.
Assessment of Risk
Despite the alarming list of potential health hazards, the average veterinary technician working in a veterinary clinic is not necessarily at high risk. It is difficult to determine a clear-cut assessment of risk for several reasons, including the following:
• Caution must be used in interpreting the epidemiologic evidence provided by these studies. The evidence produced by various studies (or within one study) is sometimes contradictory. For example, some studies have failed to show any association between the incidence of spontaneous abortion or congenital abnormalities and a history of exposure to waste gas, whereas other studies suggest the opposite. This discrepancy may arise from inconsistencies in the way in which the studies were conducted. Studies vary widely in the type of anesthetics to which the personnel are exposed, the duration and level of waste gas exposure, and the control measures available (such as a scavenging system). Most anesthetists surveyed had been exposed to several different agents, and the investigators were unable to determine which agent(s) were responsible for the observed adverse health effects.
• Although many epidemiologic studies indicate an increased incidence of health problems in persons working in an environment where exposure to waste gas occurs, it does not necessarily follow that the anesthetic gases themselves are the causative agents. Other chemicals such as ethylene oxide, or exposure to x-radiation, or other factors present in the operating room or dentist’s office may contribute to increased incidence of health disorders.
• Many of the early studies have been faulted for low response rates, lack of verification of reported outcomes, and the possibility of bias. Some commentators have observed that the increased risks observed are small and could be a result of uncontrolled variables.
• Most studies of the adverse effects of waste anesthetic gases do not measure the level of waste gas present in the working environment. Epidemiologic studies do not give information about the use of scavengers and procedures that reduce waste gas pollution. Without this information, interpretation of the findings of any study is difficult.
The American Society of Anesthesiologists Task Force on Trace Anesthetic Gases (1999) suggests that although adverse health effects are associated with chronic exposure to high levels of waste anesthetic gas, studies have failed to demonstrate an association between the low levels of waste anesthetic gas normally found in scavenged hospitals and adverse effects on hospital employees. The Task Force concluded that even at the maximum allowable dose of isoflurane, halothane, or nitrous oxide, there was no evidence of significant damage to the gonads, liver, kidney, or other organs, even in long-term studies. No data suggest that waste anesthetic gases are a danger to hospital employees (including pregnant women) working in an effectively scavenged environment.
Most authorities and regulatory agencies agree that exposure to high levels of waste anesthetic gas should be avoided and that controls should be introduced to reduce exposure. After reviewing the available literature, NIOSH recommended that the concentration of any volatile gas anesthetic (including halothane, methoxyflurane, and isoflurane) not exceed 2 ppm when used alone and not exceed 0.5 ppm when used with nitrous oxide. It is also suggested the nitrous oxide concentration not exceed 25 ppm. These levels were chosen because they were thought to be the lowest levels realistically achievable given current technology. The British Government Health Service Advisory Committee (1991) recommends a maximum of 100 ppm nitrous oxide, 50 ppm isoflurane, and 10 ppm halothane (average reading over an 8-hour period).
Measurement of Waste Gas Levels
Surveys of human and veterinary hospitals reveal a wide variation in the levels of waste anesthetic gas present in different locations within the clinic (Tables 13-1 and 13-2). The halothane concentration in the air of unscavenged surgery suites in human hospitals has been reported to be as high as 85 ppm, and concentrations of nitrous oxide have been measured as high as 7000 ppm. Veterinary hospitals using scavengers usually have isoflurane or halothane levels of 1 to 20 ppm and nitrous oxide levels of 50 to 200 ppm. Higher levels may be present if scavengers are not in use. As expected, air samples taken from surgery suites, surgical preparation rooms, and anesthetic recovery rooms are more likely to contain waste gas than samples taken elsewhere in the clinic. During the anesthetic period itself, the level of waste gas is highest immediately adjacent to the anesthetic machine, but the actual level varies according to the following factors.
TABLE 13-1
Waste Anesthetic Gas (Halothane) Concentrations in Various Locations within the Veterinary Hospital (Semiclosed Circuit, 1 L/Min Oxygen Flow)
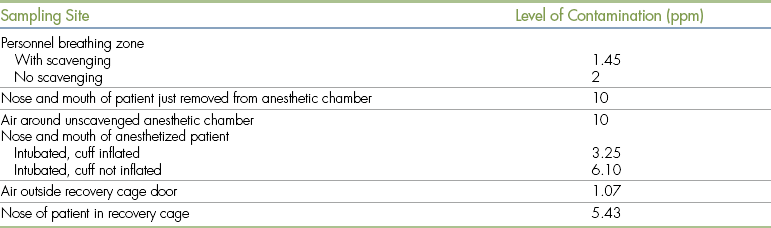
Modified from Short CE, Harvey RC: Anesthetic waste gases in veterinary medicine, Cornell Vet 73:363, 1983.
TABLE 13-2
Sources of Anesthetic Gas Contamination
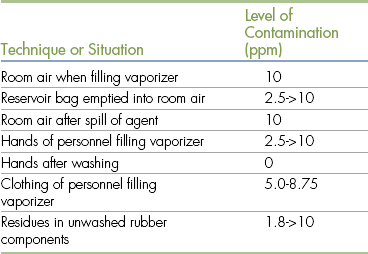
Modified from Short CE, Harvey RC: Anesthetic waste gases in veterinary medicine, Cornell Vet 73:363, 1983.
Duration of Anesthesia: The longer the anesthetic machine is in use, the higher the waste gas concentration in the room air. For example, if a surgery room is used for several procedures in one morning, the waste gas levels may slowly increase, reaching a peak at the end of the final surgery.
Flow Rate of Carrier Gas: Higher flow rates may lead to more waste gas pollution. For example, if the oxygen flow rate is 2 L/min, the room will contain more waste gas than if the flow rate were 500 mL/minute, unless a very effective gas scavenger is in use.
Anesthetic Machine Maintenance: Leak testing and periodic maintenance of the anesthetic machine are important in reducing the escape of anesthetic gas from the machine, which contributes to room air pollution.
Use of an Effective Scavenging System: When a circle system (rebreathing system) with no scavenging system is used, anesthetic gas mixed with oxygen is vented through an open pop-off valve at a rate approximately equal to the oxygen flow rate (usually 500 mL to 3 L per minute). If a non-rebreathing system such as a Bain circuit is in use, the gas exits through the relief valve or reservoir bag outlet. Either way, in the absence of a scavenging system, all of the waste gas enters the room air.
Anesthetic Techniques Used: Mask inductions and anesthetic chambers may release high levels of waste gas, as a considerable quantity of air can leak around the mask or be released when an anesthetic chamber is opened.
Room Ventilation: Room ventilation is the only means of eliminating waste gases that leak from anesthetic machines or are exhaled around an endotracheal tube or mask (scavengers are unable to retrieve these gases once they enter the air). Rooms with a ceiling fan, wall fan, or other ventilating device generally have lower levels of waste gas. It is advised that all rooms in which anesthetic gases are released have at least 15 air changes per hour (20 air changes is the preferred number). Open windows and doors also reduce waste gas levels in surgery rooms, although this may not be consistent with principles of hospital design and sterile technique.
Anesthetic Spills: The highest levels of waste gas contamination are associated with spills of anesthetic liquids. Liquid anesthetic rapidly evaporates when it is poured or spilled, and this produces a very large amount of concentrated anesthetic vapor that rapidly mixes with room air. Accidental spillage of only 1 mL of liquid halothane, for example, will evaporate to form 200 mL of vapor, with a concentration of 1,000,000 ppm. Liquid anesthetic spilled on the skin can also be absorbed into the circulation.
Reducing Exposure to Waste Anesthetic Gas
Given the potential health hazards associated with exposure to high levels of waste anesthetic gas, it is in the technician’s best interest to minimize exposure as much as possible. The American College of Veterinary Anesthesiologists recommends that any veterinary facility using inhalant anesthetics should institute and maintain a control program for waste anesthetic gases.
If proper equipment, techniques, and procedures are used, it is possible to reduce waste gas exposure to a level well below the NIOSH standards. This can be achieved through several means, including using a gas scavenging system, testing equipment for leaks, and using techniques and procedures that minimize exposure to waste gas.
Use of a Scavenging System
A scavenger consists of tubing attached to the anesthetic machine pop-off valve (or in the case of a non-rebreathing system, to the outlet port or tail of the reservoir bag). The function of a scavenger is to collect waste gas from the machine and conduct it to a disposal point outside the building. The installation and consistent use of an effective gas scavenging system are the most important steps in reducing waste gas exposure. A 1982 survey of veterinary hospitals showed that scavenging reduced waste halothane concentrations in the hospitals surveyed by 64% to 94%.
From the regulatory perspective, OSHA’s Hazard Chemical Standard (1910.1200) requires the employer to install adequate engineering controls to ensure that occupational exposure to any chemical never exceeds the permissible exposure limit. In the case of waste anesthetic gas, this is difficult or impossible to achieve in a veterinary clinic unless a waste anesthetic gas scavenger or activated charcoal canister is used on every anesthetic machine. Scavenging should include the exhaust not only from the anesthetic machine but also from ancillary equipment including non-rebreathing systems such as the Bain circuit or Ayre’s T-piece, ventilators, anesthetic chambers, and capnometers.
Ideally, scavenging systems should be professionally installed when the veterinary clinic is built. However, it is not difficult to assemble and install an effective scavenging system in an established veterinary hospital. Scavenger parts may be purchased or can be readily assembled with simple materials. The hose or tubing of the scavenger may be constructed from plastic tubing, polyvinyl chloride (PVC) pipe, or other gas-impermeable material. Most modern anesthetic machines have fittings that allow easy connection to a scavenging system. Older machines can be retrofitted with adapters that can be connected to a scavenger hose. It is important to choose the type of pop-off connection that matches the type of scavenging system used (high vacuum, low vacuum, or passive). The international standard for scavenger system attachments for anesthetic machines is 30 mm.
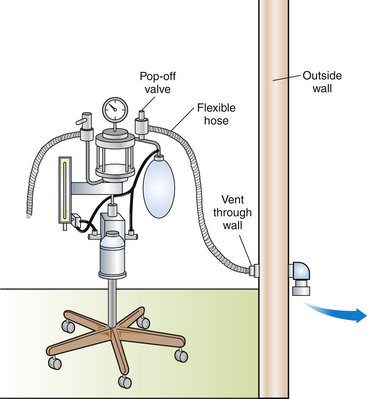
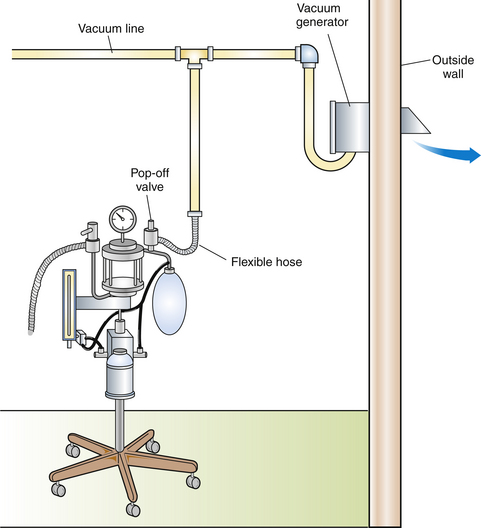
Scavenging systems may be passive or active (Figures 13-1 and 13-2). An active system uses suction created by a vacuum pump or fan to draw gas into the scavenger, whereas a passive system uses the positive pressure of the gas in the anesthetic machine to push gas into the scavenger. The most commonly used type of passive system discharges waste gas to the outdoors through a hole in the wall. This system is best suited for rooms adjacent to the exterior of the building and is ineffective for interior rooms where the distance to the outlet is more than 20 feet (7 m).
Although both active and passive scavenging systems appear to be effective when correctly assembled and operated, the most efficient system appears to be an active one with a dedicated vacuum pump. However, active scavenging systems are more costly to install than passive systems, they require more maintenance, and the operator must remember to turn on the system each day.
Once the waste gas has been collected by an active or passive system, it must be expelled outside of the building, away from doors, windows, and air intakes. Waste gas collected in the tubing should be totally confined within the scavenger hose from the pop-off valve to the point of discharge and must not be recirculated into the building air. Scavenger hoses that discharge gas on the floor of the surgery room or into an attic or a basement, or that conduct the waste gas into a recirculating central vacuum system or recirculating ventilation exhaust, merely contaminate all building rooms with the waste gas.
Normally, use of a scavenging system with an anesthetic machine does not alter the operation of the machine. The anesthetist should, however, be aware of two potential difficulties that can occur when a scavenging system is present:
1. When an active scavenging system is used, the anesthetist should prevent the negative pressure (vacuum) from the scavenger from being excessively applied to the breathing circuit. If this is allowed to occur, the reservoir bag will collapse. Many anesthetic machines are equipped with a negative pressure relief valve, adjacent to the pop-off valve, which opens automatically if negative pressure is detected in the circuit. The open valve admits room air to the circuit, thereby ensuring that a vacuum does not develop. When a machine that is not equipped with a negative pressure relief valve is used, the anesthetist should ensure that the reservoir bag is at least partially inflated with air or oxygen at all times.
2. If either a passive or active scavenging system is in use, the anesthetist must be aware that an obstruction may occur and block waste gas entry into the system. If this happens, gas will accumulate within the anesthetic circuit. This situation is analogous to operating a machine with a closed pop-off valve and may result in excessive pressure developing within the circuit and the patient’s lungs. To avoid this, many machines have scavenger interfaces that are equipped with a positive pressure relief valve that opens automatically if excessive pressure builds up within the circuit.
It is sometimes impractical to use a scavenger when it is in a specialized room such as the x-ray room or when a mobile anesthetic machine is used. In these situations, either anesthesia can be maintained with an injectable agent, or an anesthetic machine with an activated charcoal cartridge can be used (e.g., F/Air canister, AM Bickford, Inc). To effectively absorb anesthetic vapors, however, these cartridges must be replaced after 12 hours of use or after a weight gain of 50 g. Additional drawbacks of these units are their inability to absorb nitrous oxide and their relative inefficiency at flow rates greater than 2 L/min.
For additional protection, masks with activated charcoal filters can be worn by personnel who are at special risk (such as pregnant workers). Like the activated charcoal canister for anesthetic machines, these are not effective in filtering out nitrous oxide vapors; however, organic vapor cartridges effectively absorb isoflurane, sevoflurane, halothane, and other anesthetic gases and vapors. Masks with cartridges designed for particulate matter do not absorb anesthetic vapors and should not be used.
Equipment Leak Testing
Although the installation of a scavenging device is the most important step in reducing anesthetic gas pollution, there are many other procedures and techniques that significantly reduce the anesthetist’s risk of exposure. One of these is leak testing of anesthetic machines. Gas leaks from anesthetic machines are a significant source of operating room pollution and are not reduced by a scavenging system. A 1988 study of veterinary hospitals showed that 55% of the anesthetic machines tested had significant leaks. Leakage may occur from any part of the machine in which nitrous oxide or gas anesthetic is present.
The most common problems that can result in waste gas leakage are the following:
• The connections for nitrous oxide gas lines are not tightly secured.
• Rings, washers, and other seals joining nitrous oxide gas tanks to the machine hanger yokes are missing, worn, or out of position.
• The covering over a unidirectional valve is not tightly closed.
• The carbon dioxide absorber canister is not securely sealed. Leaks are often caused by improper positioning of the canister or by the presence of absorbent granules on the seals around the canister.
• The connection between the pop-off valve and scavenger is not airtight.
• Breathing hoses, the reservoir bag, or the endotracheal tube have holes or are not securely connected to the machine.
• The vaporizer cap was not replaced after the vaporizer was last filled.
In some cases the presence of a leak is obvious—there may be an audible hiss, the odor of anesthetic, or a jet of air coming out of the reservoir bag or hose. However, small leaks are often undetected unless the anesthetist regularly performs a leak test.
Two types of leak tests are commonly done in veterinary clinics: high-pressure tests and low-pressure tests.
1. High-pressure tests check for high-pressure leaks arising between the gas tanks and the flow meter, where the pressure is 50 pounds per square inch (psi) or greater. These leaks release only pure oxygen or nitrous oxide, without volatile anesthetic.
2. Low-pressure tests check for anesthetic gases that escape from the anesthetic machine itself, where the pressure of gas within the machine is approximately 15 psi. Low-pressure leaks may arise in any part of the anesthetic machine or breathing circuit that does not fit together tightly or that develops a hole. Low-pressure leaks release waste anesthetic gas as well as oxygen (and nitrous oxide, if in use).
The type of leak test used on an anesthetic machine depends on the type of carrier gas used: if both nitrous oxide and oxygen are used, both a high-pressure and a low-pressure test should be done. If oxygen alone (without nitrous oxide) is used, it is necessary to do only a low-pressure test. Release of oxygen into the room through a high-pressure leak does not affect the air quality, although it wastes oxygen and may empty the tank prematurely.
Low-pressure tests should be performed before use of the machine each day. High-pressure tests on nitrous oxide tanks should be performed once weekly and whenever the nitrous oxide tank is changed. Details of high- and low-pressure tests are given in Procedure 13-1, p. 361.
For both non-rebreathing and rebreathing systems, the location of leaks may be determined by listening for the hiss of escaping air or by using a detergent solution as described in Procedure 13-1. It is important to never try to stop the flow of gas from a high-pressure leak by putting a hand over the leaking part. Once the source of a leak has been identified, it can often be fixed by tightening a connection or replacing a part. If the leak cannot be fixed, the machine should not be used until it is serviced by qualified personnel. Frequent, routine servicing of the anesthetic machine and breathing circuit by qualified personnel is helpful in detecting equipment problems that can lead to leaks but is not an adequate substitute for daily leak testing of the machine by the anesthetist.
Anesthetic Techniques and Procedures
The anesthetist, by his or her choice of anesthetic techniques, has considerable control over the amount of waste gas released into the room air. One survey of human hospitals found that faulty work practices accounted for 94% to 99% of waste anesthetic gas released in scavenged operating rooms.
The steps in Procedure 13-2, p. 362, are recommended to minimize waste gas release.
Monitoring Waste Gas Levels
It is advisable to periodically monitor waste anesthetic gas levels to ensure that the NIOSH-recommended levels are not exceeded. Monitoring waste gas levels is particularly important if a hospital employee becomes pregnant and is still working around anesthetized animals. Waste gas monitoring is also advisable if hospital employees frequently detect the odor of anesthetic gas, or if there are special concerns about waste gas levels (e.g., if the clinic is using induction chambers). If professional monitoring is required, an accredited industrial hygiene laboratory can be contacted for assistance. (Industrial hygienists are found in the yellow pages of the telephone directory under “occupational safety.”) An occupational hygienist will usually visit the hospital to evaluate ventilation and scavenging techniques and to interview the anesthetist regarding procedures used to minimize waste gas release. Air samples should be collected from all areas in which anesthetics are used, and the level of waste gas in the collected air should be determined with an infrared spectrometer. The cost of such a visit ranges from $250 to $700.
Professional monitoring services are not always necessary. Clinic employees can inexpensively monitor waste gas levels using detector tubes or badges. Badges may detect only one chemical, such as halothane, isoflurane, or nitrous oxide, or may be sensitive to all halogenated anesthetics. The badges (called passive dosimeters) are uncapped at the beginning of the exposure period, then worn by personnel in the surgical preparation room, operating room, or recovery area for a timed period when anesthetic gases are being used (usually an exposure time of 2 to 8 hours is chosen). Alternatively, the badge may be placed in a room for area monitoring. After exposure, the badge is recapped and returned to the supplier (usually an industrial health and safety supply house or a company specializing in OSHA compliance) for analysis. Results are given as a time-weighted average in parts per million. Cost, including analysis, is approximately $50 to $70 per badge.∗
SAFE HANDLING OF COMPRESSED GASES
There is a potential for fire in any room where oxygen or nitrous oxide is used. Oxygen and nitrous oxide are not flammable; however, both support combustion and cause fuels to burn more readily. Even static electricity can cause fires in areas in which oxygen and flammable materials are used together. (This is one of the reasons why ether, which is extremely flammable, is no longer used in anesthesia.) It is recommended that no flames or sources of ignition (e.g., matches, lighters, or Bunsen burners) be present in any room in which oxygen or nitrous oxide cylinders are stored or used. For obvious reasons, smoking also must be prohibited in all rooms in which oxygen is stored or used.
Use and Storage of Compressed Gas Cylinders
Tanks of compressed gas can be viewed as a storehouse of tremendous amounts of energy, waiting for release. If gas is released too quickly, nearby personnel may be injured. Persons connecting compressed gas cylinders to an anesthetic machine or gas piping system should wear impact-resistant goggles to protect their eyes from jets of gas. If a cylinder leak occurs, never use your hand to try to stop the leak.
Gas may also be suddenly released when the tank is turned on. When turning on a compressed air tank that is connected to the anesthetic machine, use the appropriate wrench and turn the valve slowly to the full open position. Keep your head and face away from the valve outlet, pressure gauge, and pressure relief device.
If a cylinder is damaged, the sudden release of gas can have catastrophic consequences. If, for example, a cylinder is punctured or is knocked over and the regulator (the metal attachments at the top of the tank) or cylinder neck is broken off the tank, the force of the gas suddenly escaping from the tank may cause it to move like a rocket through a wall or roof. To prevent this occurrence, chain or belt large cylinders to a wall and always store them in an upright position. Valve caps should be used on all large cylinders that are not connected to gas lines to protect the valves from damage.
Gas cylinders should be stored away from emergency exits or areas with heavy traffic. If a large cylinder must be moved to another location, a handcart should be used; do not drag or roll the cylinder.
Full tanks should be kept separate from empty tanks and also should be clearly labeled for quick identification. The use of tear-off labels helps eliminate confusion regarding the empty, in-use, or full status of a given compressed air cylinder. The current status of the tank is given on the outermost section of the label (see Figure 4-23). Cylinders should be used in the order in which they are received (i.e., first in, first out).
ACCIDENTAL EXPOSURE TO INJECTABLE AGENTS
All anesthetic agents are potentially toxic to personnel handling them. Skin exposure, eye splash, or oral ingestion of injectable drugs or inhalation agents may be hazardous (or even fatal).
The injectable drugs of most concern are the opioids used for the restraint and capture of wildlife, including etorphine (Immobilon, M99) and carfentanil (Wildnil). Etorphine has 10,000 times the potency of morphine and is absorbed readily through mucous membranes or broken skin. Exposure may also occur through accidental injection, eye splash, or oral ingestion.
Human exposure to even a minute amount of these agents by any route can cause rapid onset of unconsciousness, respiratory failure, and death. The following precautions should be taken:
• Never handle potent opioids such as etorphine unless you have been adequately trained in their safe use, potential adverse effects, and treatment in case of exposure.
• Never work alone when using potent opioids.
• Wear gloves. If exposure occurs, immediately wash skin and clothing with cold water.
• A reversal agent (diprenorphine, naloxone, naltrexone) must be drawn up and ready for use. Note that up to three bottles (12 mg) of naloxone may be necessary to antagonize a single drop of these opioids. It is advisable to have a prearranged plan for on-site treatment of exposed personnel, including provision for cardiovascular and respiratory support.
• Dispose of needles and syringes in a closed container immediately after use.
Other injectable agents that may be hazardous include the cyclohexamines (ketamine, tiletamine), which have been reported to cause disorientation, excitement and other behavioral changes, dizziness, and unconsciousness after an accidental eye splash. Human exposure to alpha2-agonists (e.g., xylazine, detomidine, medetomidine, or dexmedetomidine) by injection or skin contact may cause profound sedation, hypotension, bradycardia, respiratory depression, and coma.
Safety precautions for prevention of exposure to any injectable agent include the use of personal protective equipment if there is a risk of spillage or eye splash, careful loading of syringes, and proper disposal of used needles and syringes. If accidental exposure occurs, the exposed person should receive prompt first aid (eye wash, flushing of exposed skin with large amounts of water, respiratory support) and subsequent transport to a medical center.
KEY POINTS
1. Anesthesia presents several potential health risks to hospital personnel, including exposure to waste anesthetic gas and hazardous injectable agents and accidents associated with handling of compressed gas cylinders.
2. Waste anesthetic gases and vapors are breathed by all personnel working in areas in which animals are anesthetized or are recovering from inhalation anesthesia. Filling or emptying vaporizers and the cleanup of accidental spills also may result in significant exposure.
3. Exposure to waste anesthetic gas is associated with short-term problems such as fatigue, headache, drowsiness, nausea, depression, and irritability.
4. Long-term exposure to waste anesthetic gases may be associated with reproductive disorders, liver and kidney damage, and nervous system dysfunction, although the evidence of epidemiologic studies is sometimes contradictory and difficult to interpret. Most authorities recommend that exposure to high levels of waste anesthetic gas be avoided, particularly by pregnant women.
5. Anesthetics such as methoxyflurane and halothane, which undergo significant hepatic metabolism and renal excretion, are considered to be a greater hazard than anesthetics that are minimally eliminated by these routes (isoflurane, sevoflurane).
6. Volatile waste anesthetic gases apparently do not have oncogenic (cancer-causing) effects.
7. The National Institute for Occupational Safety and Health (NIOSH) recommendations for waste anesthetic gas concentrations limit exposure to 2 parts per million (ppm) or less for halogenated anesthetics (0.5 ppm or less if nitrous oxide is concurrently used). Surveys of veterinary clinics show wide variations in waste gas levels, depending on the sampling site, scavenging system, and anesthetic techniques used.
8. Installation and use of an effective gas scavenging system greatly reduces waste gas exposure. Caution should be used to prevent the scavenger from applying negative pressure to the breathing circuit.
9. Equipment leak testing should be done on a daily basis to detect and allow correction of leakage from the anesthetic machine and compressed air cylinders. Tests should be done on both the high-pressure and low-pressure components of the machine.
10. Certain anesthetic techniques are associated with excessive release of waste gas. These include the use of anesthetic chambers, masks, and uncuffed endotracheal tubes. Procedures such as turning off the vaporizer before disconnecting the animal from the machine are helpful in reducing waste gas contamination of hospital air.
11. Vaporizers should be filled and emptied with care, using appropriate equipment and protective clothing.
12. Waste gas levels may be monitored by professional occupational hygienists or by the use of detector badges.
13. Compressed air cylinders should be transported, used, and stored with care. Special hazards include risk of fire in areas in which cylinders are stored and the risk of sudden release of pressurized gas from damaged cylinders.
14. Potent injectable opioids such as etorphine have considerable potential to cause serious, even fatal reactions. Special training is necessary to safely handle these agents, and a narcotic reversing agent must be readily available in case of human exposure.
REVIEW QUESTIONS
1. Waste anesthetic gases are a potential hazard to personnel, but problems that arise are only of long-term nature.
2. Long-term toxicity of inhalation anesthetics is thought to be caused by the release of toxic metabolites during the breakdown of these drugs within the body.
3. The anesthetic most clearly associated with neurologic and adverse reproductive effects is:
4. In the United States, the National Institute for Occupational Safety and Health (NIOSH) recommends that the levels of waste anesthetic gases for anesthetics such as isoflurane, halothane, or methoxyflurane should not exceed ___ ppm.
5. The odor of halothane may be detected by a person when the levels reach a minimum of ___ ppm.
6. Rooms in which animals are recovering from anesthesia may be highly contaminated with waste gas.
7. Which of the following can be used to effectively monitor waste anesthetic gas levels?
8. How often should a test for low-pressure leaks be conducted?
9. The safest way to transport a large high-pressure tank, such as an oxygen tank, is by:
10. How many air changes per hour should be available in a room in which waste anesthetic gases are present?
For the following questions, more than one answer may be correct.
11. A technician may reduce the amount of waste gases by:
a. Using cuffed endotracheal tubes
b. Ensuring that the anesthetic machine has been tested for leaks
12. To conduct a low-pressure test on an anesthetic machine (circle system), you must: