Chapter 3 First Week of Development
A zygote, formed by the union of a sperm and an oocyte, is a highly specialized, totipotent cell. It contains chromosomes and genes derived from the mother and father. The zygote divides many times and is progressively transformed into a multicellular human being through cell division, migration, growth, and differentiation.
Fertilization
The usual site of fertilization is in the ampulla of the uterine tube (see Fig. 2-2B). If the oocyte is not fertilized here, it slowly passes along the tube into the cavity of the uterus, where it degenerates and is resorbed.
Fertilization is a complex sequence of coordinated molecular events that begins with the contact between a sperm and an oocyte (Fig. 3-1) Fertilization ends with the intermingling of maternal and paternal chromosomes at metaphase of the first mitotic division of the zygote (see Fig. 2-5). Carbohydrate- and protein-binding molecules on the surface of the gametes are involved in sperm chemotaxis and gamete recognition, as well as in the process of fertilization.
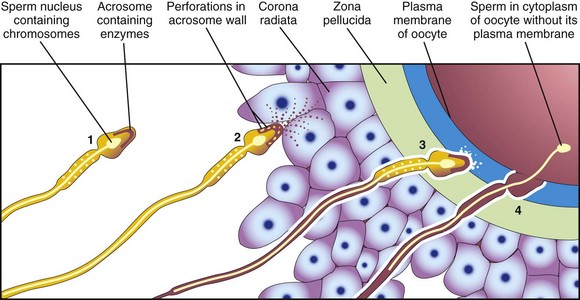
Figure 3–1 Acrosome reaction and sperm penetration of an oocyte. 1, Sperm during capacitation. 2, Sperm undergoing the acrosome reaction. 3, Sperm forming a path through the zona pellucida. 4, Sperm entering the cytoplasm of the oocyte.
Phases of Fertilization
The phases of fertilization follow (Figs. 3-1 and 3-2):
• Passage of a sperm through the corona radiata of the oocyte. Dispersal of the follicular cells of the corona radiata results mainly from the action of the enzyme hyaluronidase, which is released from the acrosome of the sperm. Tubal mucosal enzymes also appear to assist hyaluronidase. Additionally, movements of the tail of the sperm are important during penetration of the corona radiata.
• Penetration of the zona pellucida. The formation of a pathway through the zona pellucida for the sperm results from the action of enzymes released from the acrosome. The proteolytic enzyme acrosin (as well as esterases and neuraminidase) appear to cause lysis of the zona pellucida, thereby forming a path for the sperm to follow to the oocyte.
• Fusion of the plasma cell membranes of the oocyte and sperm. Once the fusion occurs, the contents of cortical granules are released into the perivitelline space, resulting in changes in the zona pellucida. This change prevents other sperms from entering. The cell membranes break down at the area of fusion. The head and tail of the sperm then enter the cytoplasm of the oocyte, but the plasma membrane and mitochondria of the sperm remains behind (see Figs. 3-1 and 3-2A).
• Completion of the second meiotic division of the oocyte. The oocyte completes the second meiotic division and forms a mature oocyte and a second polar body (see Fig. 3-2A). The nucleus of the mature oocyte becomes the female pronucleus.
• Formation of the male pronucleus. Within the cytoplasm of the oocyte, the nucleus of the sperm enlarges to form the male pronucleus. The tail of the sperm degenerates (see Fig. 3-2B). During growth, the male and female pronuclei replicate their DNA (Fig. 3-2C).
• Breakdown of the pronuclear membranes. Condensation of the chromosomes, arrangement of the chromosomes for mitotic cell division, and the first cleavage division of the zygote occur (see Figs. 3-2D and 3-3A). The combination of 23 chromosomes in each pronucleus results in a zygote with 46 chromosomes.
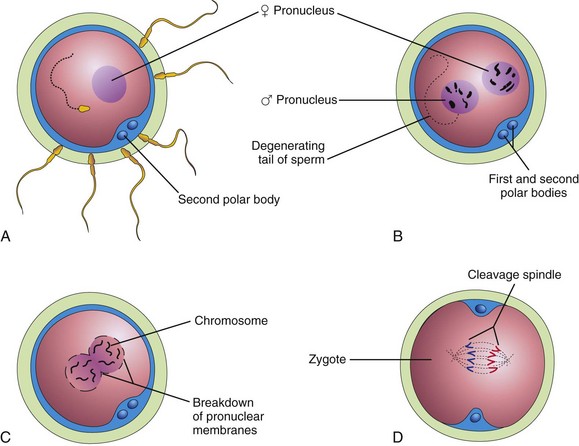
Figure 3–2 Illustrations of fertilization. A, A sperm has entered the oocyte and the second meiotic division has occurred, resulting in the formation of a mature oocyte. The nucleus of the oocyte is now the female pronucleus. B, The sperm head has enlarged to form the male pronucleus. C, The pronuclei are fusing. D, The zygote has formed.
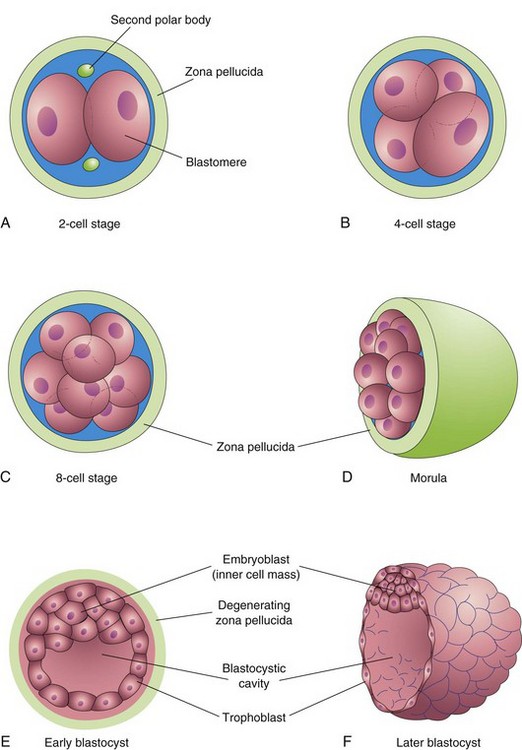
Figure 3–3 Illustrations showing cleavage of the zygote and formation of the blastocyst. A–D show various stages of cleavage. The period of the morula begins at the 12- to 32-cell stage and ends when the blastocyst forms. E and F show sections of blastocysts. The zona pellucida disappears by the late blastocyst stage (5 days). Although cleavage increases the number of blastomeres, note that each of the daughter cells is smaller than the parent cells. As a result, there is no increase in the size of the developing embryo until the zona pellucida degenerates.
Results of Fertilization
• Stimulates the secondary oocyte to complete the second meiotic division, producing the second polar body
• Restores the normal diploid number of chromosomes (46) in the zygote
• Results in variation of the human species through mingling of maternal and paternal chromosomes
• Determines the chromosomal sex of the embryo; an X-bearing sperm produces a female embryo and a Y-bearing sperm produces a male embryo
• Causes metabolic activation of the oocyte, which initiates cleavage of the zygote
The zygote is genetically unique because half of its chromosomes come from the mother and half are derived from the father. This mechanism forms the basis for biparental inheritance and variation of the human species. Meiosis allows independent assortment of maternal and paternal chromosomes among the germ cells. Crossing over of chromosomes, by relocating segments of the maternal and paternal chromosomes, “shuffles” the genes, thereby producing a recombination of genetic material (see Fig. 2-6).
Cleavage of Zygote
Cleavage consists of repeated mitotic divisions of the zygote, resulting in a rapid increase in the number of cells, now called blastomeres. Division of the zygote begins approximately 30 hours after fertilization. These blastomeres become smaller with each cleavage division (Fig. 3-3A to D). During cleavage, the zygote is still surrounded by the zona pellucida.
After the eight-cell stage, the blastomeres change their shape and tightly align themselves against each other—compaction. This phenomenon may be mediated by cell surface adhesion glycoproteins. Compaction permits greater cell-to-cell interaction and is a prerequisite for segregation of the internal cells that form the inner cell mass (see Fig. 3-3E). When there are 12 to 32 blastomeres, the conceptus is called a morula. The inner cells of the morula—the embryoblast or inner cell mass—are surrounded by a layer of flattened blastomeres that form the trophoblast. An immunosuppressant protein—the early pregnancy factor—is secreted by the trophoblastic cells and appears in the maternal serum within 24 to 48 hours after implantation. The early pregnancy factor forms the basis for a pregnancy test applicable during the first 10 days of development.
Formation of Blastocyst
Shortly after the morula enters the uterus (approximately 4 days after fertilization), uterine fluid passes through the zona pellucida to form a fluid-filled space—the blastocystic cavity—inside the morula (see Fig. 3-3E). As fluid increases in the cavity, the blastomeres are separated into two parts:
• The trophoblast, the thin outer cells that give rise to the embryonic part of the placenta
• The embryoblast, a discrete group of blastomeres that is the primordium of the embryo
At this stage, the conceptus, or embryo, is called a blastocyst. The embryoblast now projects into the blastocystic cavity, and the trophoblast forms the wall of the blastocyst (see Fig. 3-3E and F). After the blastocyst has floated in the uterine fluid for approximately 2 days, the zona pellucida degenerates and disappears. Shedding of the zona pellucida has been observed in vitro. The shedding permits the blastocyst to increase rapidly in size. While floating freely in the uterine cavity, the blastocyst derives nourishment from secretions of the uterine glands.
Approximately 6 days after fertilization, the blastocyst attaches to the endometrial epithelium (Fig. 3-4A). As soon as it attaches to the epithelium, the trophoblast starts to proliferate rapidly and differentiate into two layers (Fig. 3-4B):
• The cytotrophoblast, the inner layer of cells
• The syncytiotrophoblast, the outer layer consisting of a multinucleate protoplasmic mass formed by the fusion of cells
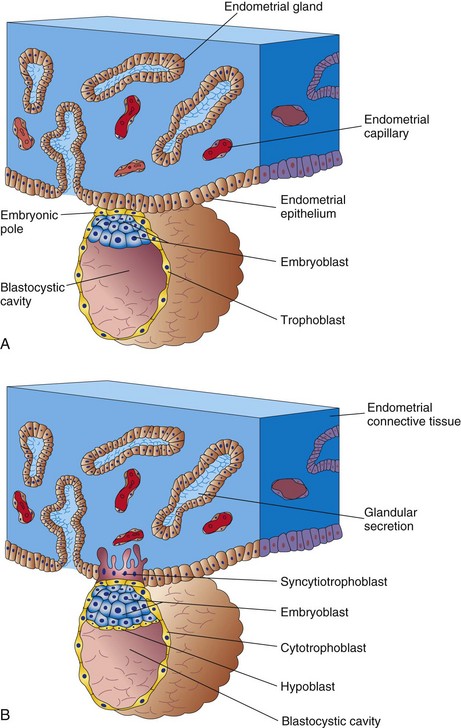
Figure 3–4 Attachment of the blastocyst to the endometrial epithelium during the early stages of its implantation. A, At 6 days, the trophoblast is attached to the endometrial epithelium at the embryonic pole of the blastocyst. B, At 7 days, the syncytiotrophoblast has penetrated the epithelium and has started to invade the endometrial connective tissue.
The fingerlike processes of the syncytiotrophoblast extend through the endometrial epithelium and invade the endometrial connective tissue. By the end of the first week, the blastocyst is superficially implanted in the compact layer of the endometrium and is deriving its nourishment from the eroded maternal tissues. The highly invasive syncytiotrophoblast rapidly expands adjacent to the embryoblast—the embryonic pole (Fig. 3-4A). The syncytiotrophoblast produces proteolytic enzymes that erode the maternal tissues, enabling the blastocyst to “burrow” into the endometrium. At the end of the first week, a cuboidal layer of cells, called the hypoblast, appears on the surface of the embryoblast, facing the blastocystic cavity (see Fig. 3-4B).
In Vitro Fertilization and Embryo Transfer
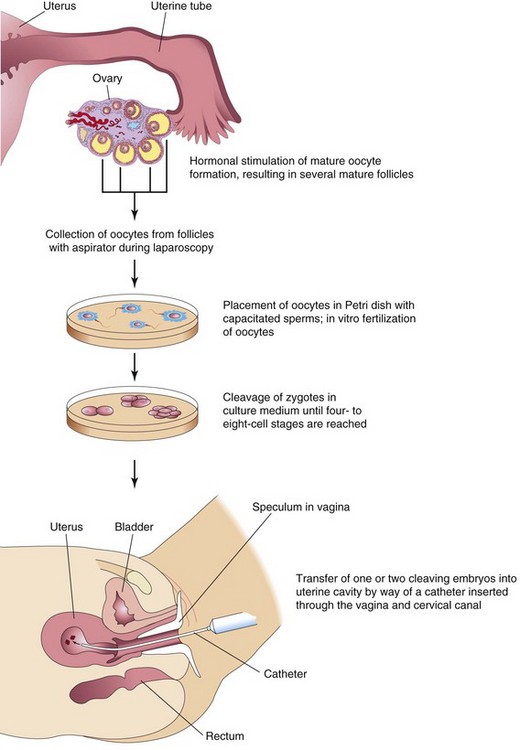
The process of in vitro fertilization (IVF) of oocytes and transfer of either the dividing zygotes or a blastocyst into the uterus has provided an opportunity for many couples who are infertile. The first of these IVF babies was born in 1978. The steps involved in IVF and embryo transfer are summarized in Figure 3-5. The incidence of multiple pregnancies is higher with IVF than when pregnancy results from normal ovulation. The incidence of spontaneous abortion of transferred embryos is also higher with IVF.
The technique of intracytoplasmic sperm injection involves injecting a sperm directly into the cytoplasm of the mature oocyte. This procedure is invaluable in cases of infertility resulting from blocked uterine tubes or oligospermia (reduced number of sperms).
Preimplantation Diagnosis of Genetic Disorders
Using currently available techniques, a cleaving zygote known to be at risk for a specific genetic disorder may be diagnosed before implantation during IVF. The sex of the embryo can be determined from a blastomere taken from a six- to eight-cell zygote and analyzed by DNA amplification of sequences from the Y chromosome. This procedure has been used to determine chromosomal sex in cases in which a male embryo would be at risk for a serious X-linked disorder. The polar body may also be tested for disorders when the mother is the carrier.
Abnormal Embryos and Spontaneous Abortions
Many early embryos abort spontaneously. The early implantation stages of the blastocyst are critical periods of development that may fail to occur because of inadequate production of progesterone and estrogen by the corpus luteum (see Fig. 2-8). Clinicians occasionally see a patient whose last menstrual period was delayed by several days and whose last menstrual flow was unusually profuse. Very likely, such patients have had an early spontaneous abortion. The overall early spontaneous abortion rate is believed to be approximately 45%. Early spontaneous abortions occur for a variety of reasons, an important one being the presence of chromosomal abnormalities.
Clinically Oriented Questions
1. Although women do not commonly become pregnant after they are 48 years old, very elderly men may still be fertile. Why is this? Is there an increased risk of Down syndrome or other congenital anomalies in the child when the father is older than 50 years of age?
2. Are there oral contraceptives for men? If not, what is the reason?
3. Is a polar body ever fertilized? If so, does the fertilized polar body give rise to a viable embryo?
4. What is the most common cause of spontaneous abortion during the first week of development?
5. Could a woman have dissimilar twins as a result of one oocyte being fertilized by a sperm from one man and another one being fertilized by a sperm from another man?
6. When referring to a zygote, do the terms cleavage and mitosis mean the same thing?
7. How is the cleaving zygote nourished during the first week?
8. Is it possible to determine the sex of a cleaving zygote developing in vitro? If so, what medical reasons would there be for doing so?