Chapter 4 Second Week of Development
Implantation of the blastocyst is completed during the second week of development. As this process takes place, changes occur, producing a bilaminar embryonic disc composed of two layers, the epiblast and hypoblast (Fig. 4-1A). The embryonic disc gives rise to germ layers that form all the tissues and organs of the embryo. Extraembryonic structures forming during the second week include the amniotic cavity, amnion, umbilical vesicle (yolk sac), connecting stalk, and chorionic sac.
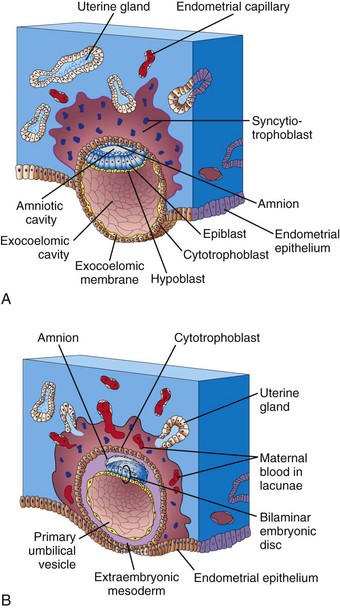
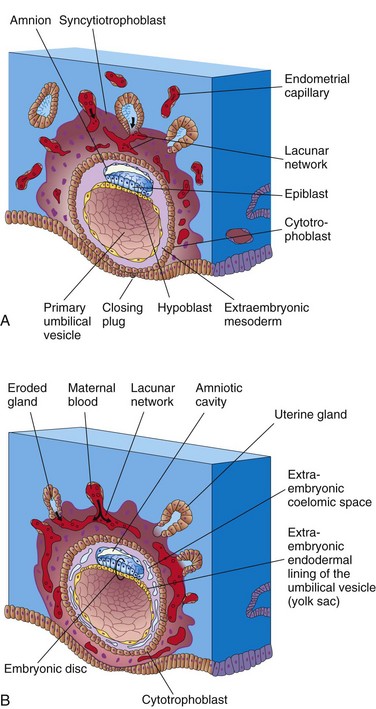
Figure 4–1 Implantation of blastocyst. The actual size of the conceptus is approximately 0.1 mm. A, Illustration of a section of a partially implanted blastocyst (approximately 8 days after fertilization). Note the slitlike amniotic cavity. B, Illustration of a section through a blastocyst at approximately 9 days.
Implantation of the blastocyst begins at the end of the first week and normally occurs in the endometrium, usually superiorly in the body of the uterus and slightly more often on the posterior than on the anterior wall. The actively erosive syncytiotrophoblast invades the endometrial connective tissue that supports the uterine capillaries and glands. As this occurs, the blastocyst slowly embeds itself in the endometrium. Syncytiotrophoblastic cells from this region displace endometrial cells in the central part of the implantation site. The endometrial cells undergo apoptosis (programmed cell death), which facilitates implantation. Proteolytic enzymes produced by the syncytiotrophoblast are involved in this process. The uterine connective tissue cells around the implantation site become loaded with glycogen and lipids. Some of these cells—decidual cells—degenerate adjacent to the penetrating syncytiotrophoblast. The syncytiotrophoblast engulfs these degenerating cells, providing a rich source of embryonic nutrition. As the blastocyst implants, more trophoblast contacts the endometrium and continues to differentiate into two layers (see Fig. 4-1A):
• The cytotrophoblast, a layer of mononucleated cells that is mitotically active. It forms new trophoblastic cells that migrate into the increasing mass of syncytiotrophoblast, where they fuse and lose their cell membranes.
• The syncytiotrophoblast, a rapidly expanding, multinucleated mass in which no cell boundaries are discernible.
The syncytiotrophoblast produces a hormone, human chorionic gonadotropin (hCG), which enters the maternal blood in the lacunae in the syncytiotrophoblast (see Fig. 4-1B). hCG maintains the development of spiral arteries in the myometrium and formation of the syncytiotrophoblast. It also forms the basis for pregnancy tests. Highly sensitive radioimmunoassays are available for detecting hCG at the end of the second week even though the woman is probably unaware that she is pregnant.
Formation of Amniotic Cavity and Embryonic Disc
As implantation of the blastocyst progresses, changes occurring in the embryoblast result in the formation of a flattened, almost circular, bilaminar plate of cells—the embryonic disc—consisting of two layers (Figs. 4-1A and 4-2B):
• The epiblast, the thicker layer, consists of high, columnar cells related to the amniotic cavity.
• The hypoblast, the thinner layer, consists of small, cuboidal cells adjacent to the exocoelomic cavity (the primordium of the umbilical vesicle).
Concurrently, a small cavity appears in the embryoblast, which is the primordium of the amniotic cavity (see Fig. 4-1A). Soon, amniogenic (amnion-forming) cells called amnioblasts separate from the epiblast and organize to form a thin membrane, the amnion, which encloses the amniotic cavity.
The epiblast forms the floor of the amniotic cavity and is continuous peripherally with the amnion. The hypoblast forms the roof of the exocoelomic cavity and is continuous with the cells that migrated from the hypoblast to form the exocoelomic membrane. This membrane surrounds the blastocystic cavity and lines the internal surface of the cytotrophoblast.
The exocoelomic membrane and cavity soon become modified to form the primary umbilical vesicle (the primary yolk sac). The embryonic disc then lies between the amniotic cavity and the primary umbilical vesicle (Fig. 4-1B). The outer layer of cells from the umbilical vesicle endoderm forms a layer of loosely arranged connective tissue, the extraembryonic mesoderm (Fig. 4-1B).
As the amnion, embryonic disc, and primary umbilical vesicle form, isolated cavities called lacunae appear in the syncytiotrophoblast (see Figs. 4-1B and 4-2). The lacunae are soon filled with a mixture of maternal blood from ruptured endometrial capillaries and cellular debris from eroded uterine glands. The fluid in the lacunae, sometimes called embryotroph, passes to the embryonic disc by diffusion. The communication of the eroded uterine vessels with the lacunae represents the beginning of uteroplacental circulation. When maternal blood flows into the lacunae, oxygen and nutritive substances become available to the extraembryonic tissues over the large surface of the syncytiotrophoblast. Oxygenated blood passes into the lacunae from the spiral endometrial arteries in the endometrium; deoxygenated blood is removed from the lacunae through endometrial veins.
The 10-day embryo is completely embedded in the endometrium (Fig. 4-2A). For approximately 2 more days, there is a defect in the endometrial epithelium that is filled by a closing plug, a fibrinous coagulum of blood. By day 12, an almost completely regenerated uterine epithelium covers the closing plug (Fig. 4-2B).
As the conceptus (the embryo and its membranes) implants, the endometrial connective tissue cells undergo a transformation known as the decidual reaction resulting from cAMP and progesterone signaling. The cells swell because of the accumulation of glycogen and lipid in their cytoplasm, and they are then known as secretory decidual cells. The primary function of the decidual reaction is to provide an immunologically privileged site for the conceptus.
In a 12-day embryo, adjacent syncytiotrophoblastic lacunae have fused to form lacunar networks (Fig. 4-2B), the primordia of the intervillous space of the placenta (see Chapter 8). The endometrial capillaries around the implanted embryo become congested and dilated to form sinusoids, which are thin-walled terminal vessels that are larger than ordinary capillaries. The syncytiotrophoblast then erodes the sinusoids and maternal blood flows into the lacunar networks. The degenerated endometrial stromal cells and glands, together with the maternal blood, provide a rich source of material for embryonic nutrition. Growth of the bilaminar embryonic disc is slow compared with the growth of the trophoblast.
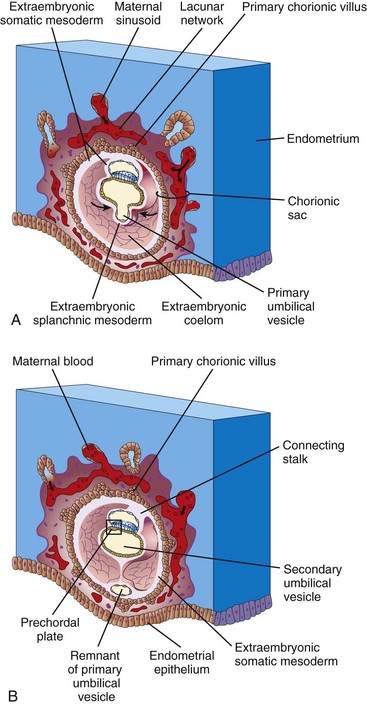
As changes occur in the trophoblast and endometrium, the extraembryonic mesoderm increases and isolated extraembryonic coelomic spaces appear within it (Fig. 4-2B). These spaces rapidly fuse to form a large, isolated cavity, the extraembryonic coelom (Fig. 4-3A). This fluid-filled cavity surrounds the amnion and the umbilical vesicle, except where they are attached to the chorion by the connecting stalk. As the extraembryonic coelom forms, the primary umbilical vesicle decreases in size and a smaller, secondary umbilical vesicle forms (Fig. 4-3B). During formation of the secondary umbilical vesicle, a large part of the primary umbilical vesicle is pinched off. The human umbilical vesicle (yolk sac) contains no yolk. It may have a role in the selective transfer of nutritive materials to the embryonic disc.
Development of Chorionic Sac
The end of the second week is characterized by the appearance of primary chorionic villi (Figs. 4-3A and 4-4A and C). Proliferation of the cytotrophoblastic cells produces cellular extensions that grow into the overlying syncytiotrophoblast. The cellular projections form primary chorionic villi, the first stage in the development of the chorionic villi of the placenta. The extraembryonic coelom splits the extraembryonic mesoderm into two layers (Fig. 4-3A and B):
• The extraembryonic somatic mesoderm, which lines the trophoblast and covers the amnion
• The extraembryonic splanchnic mesoderm, which surrounds the umbilical vesicle
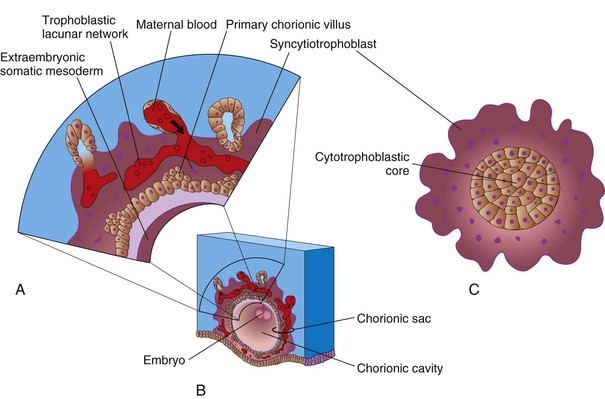
Figure 4–4 A, Illustration of a section of the wall of the chorionic sac. B, Illustration of a 14-day conceptus showing the chorionic sac and the chorionic cavity. C, Transverse section through a primary chorionic villus.
The growth of these cytotrophoblastic extensions is believed to be induced by the underlying extraembryonic somatic mesoderm. The extraembryonic somatic mesoderm and the two layers of trophoblast form the chorion. The chorion forms the wall of the chorionic sac (Fig. 4-3A). The embryo, amniotic sac, and umbilical vesicle are suspended in the chorionic cavity by the connecting stalk (Figs. 4-3B and 4-4B). Transvaginal ultrasonography (endovaginal sonography) is used to measure the diameter of the chorionic sac. This measurement is valuable for evaluating early embryonic development and pregnancy outcome.
Extrauterine Implantation Sites
Blastocysts may implant outside the uterus resulting in ectopic pregnancies; most ectopic implantations occur in the uterine tube (Figs. 2-2B and 4-5A and B). Ectopic tubal pregnancy occurs in approximately 1 in 200 pregnancies in North America. A woman with a tubal pregnancy has the usual signs and symptoms of pregnancy, but she may also experience abdominal pain (from distention of the uterine tube), abnormal bleeding, and irritation of the pelvic peritoneum.
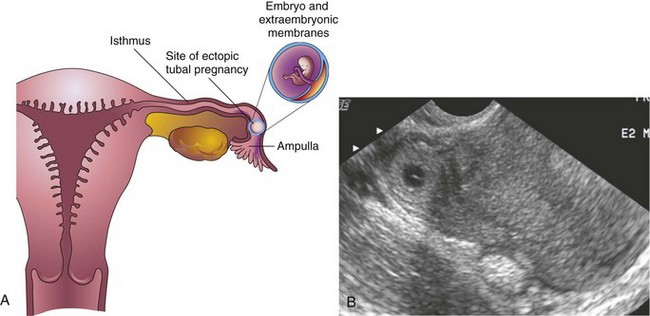
Figure 4–5 A, Coronal section of the uterus and uterine tube illustrating an ectopic pregnancy in the ampulla of the uterine tube. B, Endovaginal axial scan of the uterine fundus and isthmic portion of the right uterine tube The ring-like mass is a 4-week ectopic chorionic (gestational) sac in the tube (arrow)
(B, Courtesy E. A. Lyons, MD, Department of Radiology, Health Sciences Centre, University of Manitoba, Winnipeg, Manitoba, Canada.)
The causes of tubal pregnancy are often related to factors that delay or prevent transport of the cleaving zygote to the uterus (e.g., blockage of the uterine tube). Ectopic tubal pregnancies usually result in rupture of the uterine tube and hemorrhage into the peritoneal cavity during the first 8 weeks, followed by death of the embryo.
Inhibition of Implantation
The administration of relatively large doses of estrogen (“morning-after pills”) for several days, beginning shortly after unprotected sexual intercourse, usually does not prevent fertilization, but it often prevents implantation of the blastocyst. Normally, the endometrium progresses to the luteal phase of the menstrual cycle as the zygote forms, undergoes cleavage, and enters the uterus. A large amount of estrogen, however, disturbs the normal balance between estrogen and progesterone that is necessary to prepare the endometrium for implantation.
An intrauterine device inserted into the uterus through the vagina and cervix usually interferes with implantation by causing a local inflammatory reaction. Some intrauterine devices contain slow-release progesterone, which interferes with the development of the endometrium so that implantation does not usually occur. Copper-based IUDs appear to inhibit sperm tubal migration, while levonorgestrol-based IUDs alter the quality of cervical mucus and endometrial development.
Clinically Oriented Questions
1. What is meant by the term implantation bleeding? Is this the same as menses (menstrual fluid)?
2. Can a drug taken during the first 2 weeks of pregnancy cause abortion of the embryo?
3. Can an ectopic pregnancy occur in a woman who has an intrauterine device?
4. Can a blastocyst that implants in the abdomen develop into a full-term fetus?