Chapter 8 Placenta and Fetal Membranes
The fetal part of the placenta and fetal membranes separate the embryo or fetus from the endometrium. The chorion, amnion, umbilical vesicle (yolk sac), and allantois constitute the fetal membranes. An interchange of substances (e.g., nutrients and oxygen) occurs between the maternal and fetal blood through the placenta. The vessels in the umbilical cord connect the placental circulation with the fetal circulation.
Placenta
The placenta is a fetomaternal organ that has two components:
• A fetal part that develops from part of the chorionic sac
• A maternal part that is derived from the endometrium (inner layer of uterine wall)
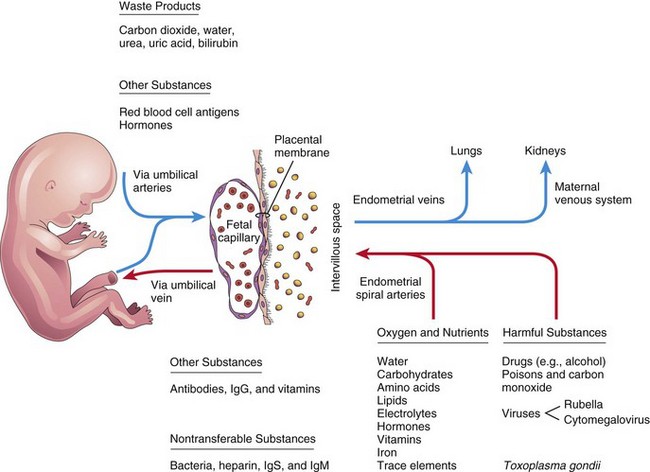
The placenta and umbilical cord function as a transport system for substances passing between the mother and the fetus. Nutrients and oxygen pass from the maternal blood through the placenta to the fetal blood, and waste materials and carbon dioxide pass from the fetal blood through the placenta to the maternal blood. The placenta and fetal membranes perform the following functions and activities: protection, nutrition, respiration, excretion, and hormone production. Shortly after birth, the placenta and fetal membranes are expelled from the uterus as the afterbirth.
Decidua
The decidua is the gravid (pregnant) endometrium, the functional layer of the endometrium in a pregnant woman that separates from the remainder of the uterus after parturition (childbirth).
Three regions of the decidua are named according to their relation to the implantation site (Fig. 8-1):
• Decidua basalis—the part of the decidua deep to the conceptus that forms the maternal part of the placenta
• Decidua capsularis—the superficial part of the decidua overlying the conceptus
• Decidua parietalis—the remaining intervening parts of the decidua
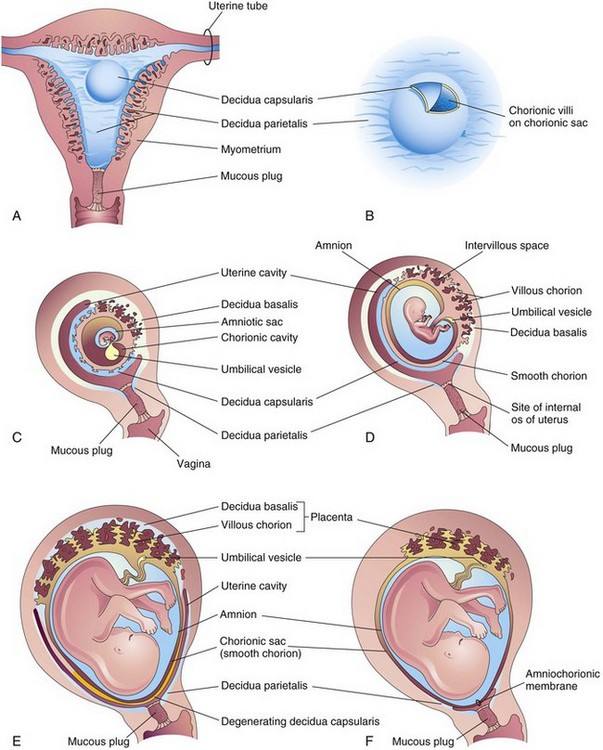
Figure 8–1 Development of placenta and fetal membranes. A, Coronal section of the uterus showing elevation of the decidua capsularis and the expanding chorionic sac at 4 weeks. B, Enlarged illustration of the implantation site. The chorionic villi were exposed by cutting an opening in the decidua capsularis. C to F, Sagittal sections of the gravid (pregnant) uterus from the 5th to 22nd weeks, showing the changing relationship of the fetal membranes to the decidua. In F, the amnion and the chorion are fused with each other and with the decidua parietalis, thereby obliterating the uterine cavity.
In response to increasing progesterone levels in the maternal blood, the connective tissue cells of the decidua enlarge to form pale-staining decidual cells. These cells enlarge as glycogen and lipid accumulate in their cytoplasm. The cellular and vascular changes in the decidua that result from pregnancy are referred to as the decidual reaction. Many decidual cells degenerate near the chorionic sac in the region of the syncytiotrophoblast and, together with maternal blood and uterine secretions, provide a rich source of nutrition for the embryo. Decidual regions, clearly recognizable during ultrasonography, are important in diagnosing early pregnancy.
Development of Placenta
Early placental development is characterized by the rapid proliferation of the trophoblast and development of the chorionic sac and chorionic villi. By the end of the third week, the anatomic arrangements necessary for physiologic exchanges between the mother and the embryo have been established. By the end of the fourth week, a complex vascular network develops in the placenta, allowing maternal-embryonic exchanges of gases, nutrients, and metabolic waste products. Chorionic villi cover the entire chorionic sac until the beginning of the eighth week (Figs. 8-1D and 8-2). As this sac grows, the villi associated with the decidua capsularis are compressed, reducing the blood supply to them. These villi soon degenerate, producing a relatively avascular bare area, the smooth chorion. As these villi disappear, those associated with the decidua basalis rapidly increase in number, branch profusely, and enlarge (Fig. 8-3). This bushy part of the chorionic sac is known as the villous chorion or chorion frondosum (Figs. 8-1E and 8-4).
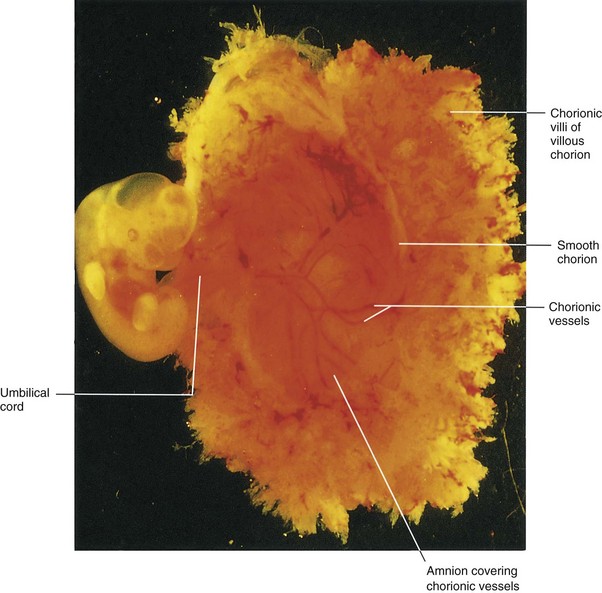
Figure 8–2 Lateral view of a spontaneously aborted embryo at Carnegie stage 14, approximately 32 days. The chorionic and amniotic sacs have been opened to show the embryo.
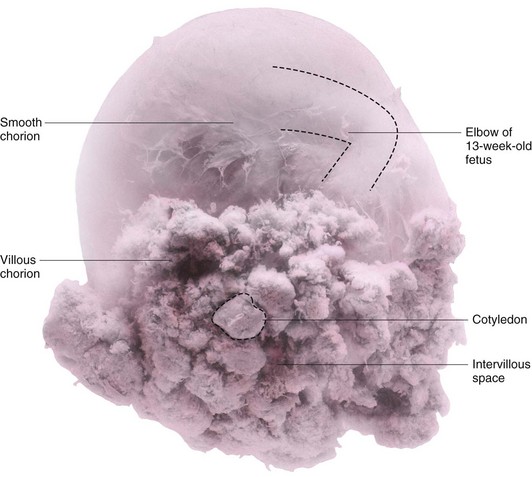
Figure 8–3 A human chorionic sac containing a 13-week fetus. The villous chorion is where chorionic villi persist and form the fetal part of the placenta. In situ, the cotyledons were attached to the decidua basalis and the intervillous space was filled with maternal blood.
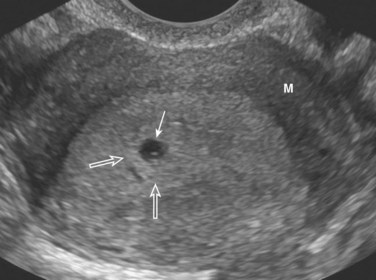
Figure 8–4 Endovaginal axial scan of a pregnant uterus showing a 3-week chorionic sac (arrow) in the posterior endometrium (decidua). There is a bright (echogenic) ring of chorionic villi (open arrows) around the sac. M, Myometrium.
(Courtesy E. A. Lyons, M.D., Professor of Radiology, Obstetrics and Gynecology, and Anatomy, University of Manitoba, Health Sciences Centre, Winnipeg, Manitoba, Canada.)
Ultrasonography of the Chorionic Sac
The size of the chorionic sac is useful in determining the gestational age of embryos in pregnant women with uncertain menstrual histories. Growth of the chorionic sac is extremely rapid between the 5th and 10th weeks of development. Modern ultrasound devices permit detection of the chorionic sac when it has a median diameter of 2 to 3 mm (Fig. 8-4). Chorionic sacs with this diameter indicate a gestational age of approximately 32 days.
Fetomaternal Junction
The fetal part of the placenta (villous chorion) is attached to the maternal part of the placenta (decidua basalis) by the cytotrophoblastic shell, the external layer of trophoblastic cells on the maternal surface of the placenta (see Fig. 8-5). The chorionic villi, which are attached firmly to the decidua basalis through the cytotrophoblastic shell, anchor the chorionic sac to the decidua basalis. Endometrial arteries and veins pass freely through gaps in the cytotrophoblastic shell and open into the intervillous space.
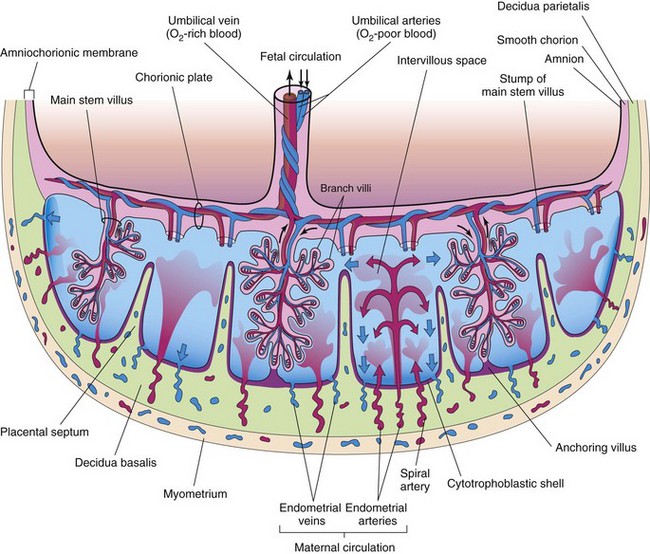
Figure 8–5 Illustration of a transverse section through a full-term placenta, showing (1) the relation of the villous chorion (fetal part of placenta) to the decidua basalis (maternal part of placenta); (2) the fetal placental circulation; and (3) the maternal placental circulation. Maternal blood flows into the intervillous spaces in funnel-shaped spurts from the spiral arteries, and exchanges occur with the fetal blood as the maternal blood flows around the branch villi. The inflowing arterial blood pushes venous blood out of the intervillous space and into the endometrial veins. Note that the umbilical arteries carry poorly oxygenated fetal blood (shown in blue) to the placenta and that the umbilical vein carries oxygenated blood (shown in red) to the fetus. Only one stem villus is shown in each cotyledon, but the stumps of those that have been removed are indicated. Arrows indicate direction of maternal (red and blue) and fetal (black) blood flow.
The shape of the placenta is determined by the shape of the persistent area of chorionic villi (Fig. 8-1F). Usually this is a circular area, giving the placenta a discoid shape. As the chorionic villi invade the decidua basalis during placental formation, decidual tissue is eroded to enlarge the intervillous space. This erosion produces several wedge-shaped areas of decidua—placental septa—that project toward the chorionic plate (Fig. 8-5). The placental septa divide the fetal part of the placenta into irregular convex areas called cotyledons (Fig. 8-3). Each cotyledon consists of two or more stem villi and many branch villi.
The decidua capsularis, the layer overlying the implanted chorionic sac, forms a capsule over the external surface of the sac (Fig. 8-1A to D). As the conceptus enlarges, the decidua capsularis bulges into the uterine cavity and becomes greatly attenuated. Eventually, parts of the decidua capsularis make contact and fuse with the decidua parietalis, thereby slowly obliterating the uterine cavity (Fig. 8-1E and F). By 22 to 24 weeks, reduced blood supply to the decidua capsularis causes it to degenerate and disappear.
Intervillous Space
The intervillous space of the placenta contains maternal blood, which is derived from the lacunae that developed in the syncytiotrophoblast during the second week of development (see Fig. 4-1C). This large, blood-filled space results from the coalescence and enlargement of these lacunar networks. The intervillous space of the placenta is divided into compartments by the placental septa; however, free communication occurs between the compartments because the septa do not reach the chorionic plate (Fig. 8-5), the part of the chorion associated with the placenta. Maternal blood enters the intervillous space from the spiral arteries in the decidua basalis; these arteries pass through gaps in the cytotrophoblastic shell and discharge blood into the intervillous space. This large space is drained by endometrial veins that also penetrate the cytotrophoblastic shell. The numerous branch villi, arising from stem villi, are continuously showered with maternal blood as it circulates through the intervillous space. The blood in this space carries oxygen and nutritional materials that are necessary for fetal growth and development. The maternal blood also contains fetal waste products, such as carbon dioxide, salts, and products of protein metabolism.
Amniochorionic Membrane
The amniotic sac enlarges more quickly than the chorionic sac. As a result, the amnion and smooth chorion soon fuse to form the amniochorionic membrane (see Fig. 8-1F). This composite membrane fuses with the decidua capsularis and, after disappearance of this part of the decidua, adheres to the decidua parietalis. It is the amniochorionic membrane that ruptures during labor. Preterm rupture of this membrane is the most common event leading to premature labor. When the amniochorionic membrane ruptures, the amniotic fluid escapes through the cervix and vagina.
Placental Circulation
The many branch chorionic villi provide a large surface area where materials may be exchanged across the very thin placental membrane interposed between the fetal and maternal circulations (Fig. 8-6B and C). It is through the branch villi that the main exchange of material between the mother and the fetus takes place. The placental membrane consists of extrafetal tissues.
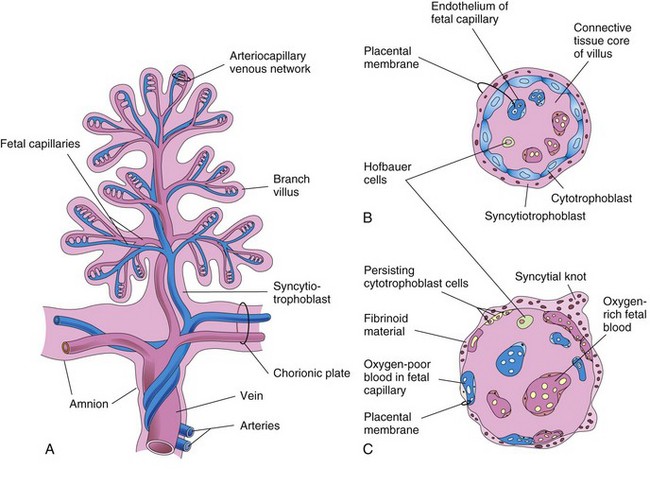
Figure 8–6 A, Illustration of a stem chorionic villus showing its arteriocapillary-venous system. The arteries carry poorly oxygenated fetal blood and waste products from the fetus, whereas the vein carries oxygenated blood and nutrients to the fetus. B and C, Sections through a branch villus at 10 weeks’ gestation and at full term, respectively. The placental membrane, composed of extrafetal tissues, separates the maternal blood in the intervillous space from the fetal blood in the capillaries in the villi. Note that the placental membrane becomes very thin at full term. Hofbauer cells are believed to be phagocytic cells.
Fetoplacental Circulation
Poorly oxygenated blood leaves the fetus via the umbilical arteries (Figs. 8-5 and 8-7). At the attachment of the umbilical cord to the placenta, these arteries divide into a number of radially disposed chorionic arteries that branch freely in the chorionic plate before entering the chorionic villi (Fig. 8-5). The blood vessels form an extensive arteriocapillary venous system within the chorionic villi (Fig. 8-6A) that brings the fetal blood extremely close to the maternal blood (Fig. 8-7). This system provides a very large surface area for the exchange of metabolic and gaseous products between the maternal and fetal blood. Normally, no intermingling of fetal and maternal blood occurs. The well-oxygenated fetal blood in the fetal capillaries passes into thin-walled veins that follow the chorionic arteries to the site of attachment of the umbilical cord, where they converge to form the umbilical vein. This large vessel carries oxygen-rich blood to the fetus.
Maternal-Placental Circulation
The maternal blood enters the intervillous space through 80 to 100 spiral arteries in the decidua basali (Fig. 8-5). The entering blood is at considerably higher pressure than that in the intervillous space, so it spurts toward the chorionic plate. As the pressure dissipates, the blood flows slowly around the branch villi, allowing an exchange of metabolic and gaseous products with the fetal blood. The blood eventually returns through the endometrial veins to the maternal circulation (Fig. 8-7). Reductions of uteroplacental circulation result in fetal hypoxia (decreased level of oxygen) and IUGR (intrauterine growth restriction). The intervillous space of the mature placenta contains approximately 150 ml of blood that is replenished three or four times each minute.
Placental Membrane
The placental membrane (placental barrier) consists of the extrafetal tissues that separate the maternal and fetal blood. Until approximately 20 weeks, the placental membrane consists of four layers (Fig. 8-6B): syncytiotrophoblast, cytotrophoblast, connective tissue of the villus, and endothelium of the fetal capillaries.
After the 20th week, microscopic changes occur in the branch villi that result in the cytotrophoblast becoming attenuated in many villi. Eventually, cytotrophoblastic cells disappear over large areas of the villi, leaving only thin patches of syncytiotrophoblast. As a result, the placental membrane at full term consists of only three layers in most places (Fig. 8-6C). In some areas, the placental membrane becomes markedly thinned. At these sites, the syncytiotrophoblast comes in direct contact with the endothelium of the fetal capillaries to form a vasculosyncytial placental membrane.
Only a few substances, endogenous or exogenous, are unable to pass through the placental membrane. The membrane acts as a true barrier only when the molecule or organism has a certain size, configuration, and charge. Most drugs and other substances in the maternal plasma pass through the placental membrane and are found in the fetal plasma (Fig. 8-7).
During the third trimester, numerous nuclei in the syncytiotrophoblast of the villi aggregate to form syncytial knots (nuclear aggregations) (Fig. 8-6C). These knots continually break off and are carried from the intervillous space into the maternal circulation; some may lodge in capillaries of the maternal lungs, where they are rapidly destroyed by local enzyme action. Toward the end of pregnancy, fibrinoid material also forms on the surfaces of the villi.
Placental Metabolism
The placenta synthesizes glycogen, cholesterol, and fatty acids, which serve as sources of nutrients and energy for the embryo or fetus. Many of the metabolic activities of the placenta are critical for its other two major activities: transport and endocrine secretion.
Placental Transport
The transport of substances in both directions between the placenta and the maternal blood is facilitated by the large surface area of the placental membrane. Almost all materials are transported across the placental membrane by one of the following four main transport mechanisms: simple diffusion, facilitated diffusion, active transport, and pinocytosis.
Passive transport by simple diffusion is usually characteristic of substances moving from areas of higher to lower concentration until equilibrium is established. Facilitated diffusion requires a transporter but no energy.
Active transport against a concentration gradient requires energy. This mechanism of transport may involve carrier molecules that temporarily combine with the substances to be transported. Pinocytosis is a form of endocytosis in which the material being engulfed is a small amount of extracellular fluid. Some proteins are transferred very slowly through the placenta by pinocytosis.
Transfer of Gases
Gases, such as oxygen, carbon dioxide, and carbon monoxide, cross the placental membrane by simple diffusion. Interruption of oxygen transport for several minutes endangers the survival of the embryo or fetus. The efficiency of the placental membrane approaches that of the lungs for gas exchange. The quantity of oxygen reaching the fetus is generally flow-limited, rather than diffusion-limited; hence, fetal hypoxia results primarily from factors that diminish either uterine blood flow or fetal blood flow through the placenta. Nitrous oxide, an inhalation analgesic and anesthetic, also readily crosses the placenta.
Nutritional Substances
Water is rapidly exchanged by simple diffusion and in increasing amounts as pregnancy advances. Glucose produced by the mother and the placenta is quickly transferred to the embryo or fetus by facilitated diffusion. Very small amounts of maternal cholesterol, triglycerides, and phospholipids are transferred. Although free fatty acids are transported, the amount transferred appears to be relatively small. Amino acids cross the placenta to the fetus in high concentrations by active transport. Vitamins cross the placental membrane and are essential for normal development. A maternal protein, transferrin, crosses the placental membrane and carries iron to the embryo or fetus. The placental surface contains special receptors for this protein.
Hormones
Protein hormones do not reach the embryo or fetus in significant amounts, except for a slow transfer of thyroxine and triiodothyronine. Unconjugated steroid hormones cross the placental membrane relatively freely. Testosterone and certain synthetic progestins also cross the placenta.
Electrolytes
Electrolytes are freely exchanged in significant quantities, each at its own rate. When a mother receives intravenous fluids with electrolytes, they also pass to the fetus and affect the fetal water and electrolyte status.
Maternal Antibodies
The fetus produces only small amounts of antibodies because of its immature immune system. Some passive immunity is conferred on the fetus by placental transfer of maternal antibodies. Only immunoglobulin G is transferred across the placenta (receptor-mediated transcytosis). Maternal antibodies confer fetal immunity for diseases such as diphtheria, smallpox, and measles; however, no immunity is acquired to pertussis (whooping cough) or chickenpox.
Waste Products
Urea and uric acid pass through the placental membrane by simple diffusion. Conjugated bilirubin (which is fat soluble) is easily transported by the placenta and is quickly cleared.
Drugs and Drug Metabolites
Most drugs and drug metabolites cross the placenta by simple diffusion. Drugs taken by the mother can affect the embryo or fetus, directly or indirectly, by interfering with maternal or placental metabolism. Some drugs cause major birth defects (see Chapter 19). Fetal drug addiction may occur after maternal use of drugs such as heroin, and newborn infants may experience withdrawal symptoms. Most drugs used for the management of labor readily cross the placental membrane. Depending on their dose and timing in relation to delivery, these drugs may cause respiratory depression of the newborn infant. Neuromuscular blocking agents, such as succinylcholine, that might be used during operative obstetrics, cross the placenta in only very small amounts. All sedatives and analgesics affect the fetus to some degree. Inhaled anesthetics can also cross the placental membrane and affect fetal breathing if used during parturition.
Infectious Agents
Cytomegalovirus, rubella, and coxsackie viruses, as well as viruses associated with variola, varicella, measles, and poliomyelitis, may pass through the placental membrane and cause fetal infection. In some cases, as with the rubella virus, severe congenital anomalies may result (see Chapter 19). Maternal infection with Treponema pallidum causes fetal syphilis and Toxoplasma gondii produces destructive changes in the brain and eyes of the fetus.
Placental Endocrine Synthesis and Secretion
Using precursors derived from the fetus, the mother, or both, the syncytiotrophoblast of the placenta synthesizes protein and steroid hormones. Protein hormones synthesized by the placenta include the following:
• Human chorionic gonadotropin (hCG)
• Human chorionic somatomammotropin, or human placental lactogen
The glycoprotein hCG, similar to luteinizing hormone, is first secreted by the syncytiotrophoblast during the second week. The hCG maintains the corpus luteum, preventing the onset of menstrual periods. The concentration of hCG in the maternal blood and urine rises to a maximum by the eighth week and then declines. The placenta also plays a major role in the production of steroid hormones (i.e., progesterone and estrogens). Progesterone is essential for the maintenance of pregnancy.
Hemolytic Disease of the Newborn
Small amounts of fetal blood may pass to the maternal blood through microscopic breaks in the placental membrane. If the fetus is Rh-positive and the mother is Rh-negative, the fetal cells may stimulate the formation of anti-Rh antibody by the mother’s immune system. This antibody passes to the fetal blood and causes hemolysis of fetal Rh-positive blood cells and anemia in the fetus. Some fetuses with hemolytic disease of the newborn, or fetal erythroblastosis, do not make a satisfactory intrauterine adjustment. They may die unless delivered early or given intraperitoneal, or intravenous transfusions of packed Rh-negative blood cells to maintain the fetus until after birth. Hemolytic disease of the newborn is relatively uncommon now because Rh immunoglobulin given to the mother usually prevents development of this disease in the fetus.
Uterine Growth during Pregnancy
The uterus of a nonpregnant woman lies in the pelvis. It increases in size during pregnancy to accommodate the growing fetus. As the uterus enlarges, it increases in weight and its walls become thinner. During the first trimester the uterus expands out of the pelvic cavity, and by 20 weeks, it usually reaches the level of the umbilicus. By 28 to 30 weeks, the uterine fundus reaches the epigastric region, the area between the xiphoid process of the sternum and the umbilicus.
Parturition
Parturition (childbirth) is the process during which the fetus, placenta, and fetal membranes are expelled from the mother (Fig. 8-8). Labor is the sequence of uterine contractions that results in dilation of the cervix and delivery of the fetus and placenta from the uterus. The factors that trigger labor are not completely understood, but several hormones are related to the initiation of contractions. The fetal hypothalamus secretes corticotrophin-releasing hormone, stimulating the pituitary gland to produce adrenocorticotrophic hormone (ACTH). ACTH causes the suprarenal (adrenal) cortex to secrete cortisol, which is involved in the synthesis of estrogens.
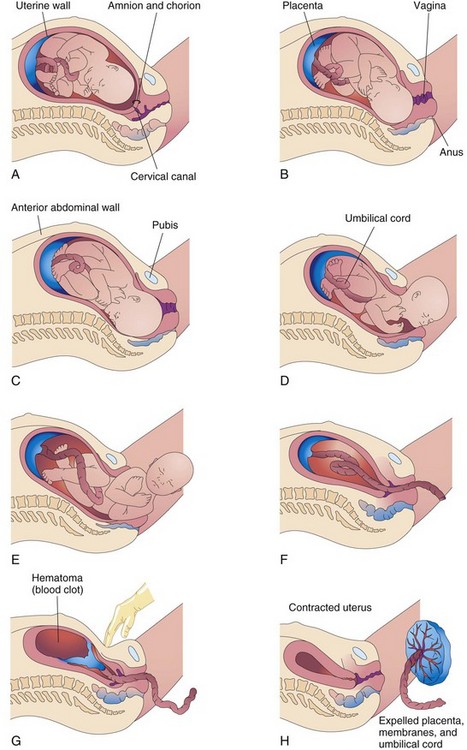
Figure 8–8 Illustrations of parturition. A and B, The cervix is dilating during the first stage of labor. C to E, The fetus is passing through the cervix and vagina during the second stage of labor. F and G, As the uterus contracts during the third stage of labor, the placenta folds and pulls away from the uterine wall. Separation of the placenta results in bleeding and the formation of a large hematoma (mass of blood). Pressure on the abdomen facilitates placental separation. H, The placenta is expelled and the uterus contracts.
Peristaltic contractions of the uterine smooth muscle are elicited by oxytocin, which is released by the maternal neurohypophysis of the pituitary gland. This hormone is administered clinically when it is necessary to induce labor. Oxytocin also stimulates the release of prostaglandins that, in turn, stimulate myometrial contractility by sensitizing the myometrial cells to oxytocin. Estrogens also increase myometrial contractile activity and stimulate the release of oxytocin and prostaglandins.
Stages of Labor
Labor is a continuous process, but clinically it is divided into three stages:
• Dilation begins with progressive dilation of the cervix (Fig. 8-8A and B), and ends with complete dilation of the cervix. During this phase, regular contractions of the uterus occur less than 10 minutes apart. The average duration of the first stage is approximately 12 hours for first pregnancies (primigravidas) and approximately 7 hours for women who have had a child previously (multigravidas).
• Expulsion begins when the cervix is fully dilated and ends with delivery of the infant (Fig. 8-8C to E). During this stage, the fetus descends through the cervix and vagina. As soon as the fetus is outside the mother, it is called a newborn infant, or neonate. The average duration of this stage is 50 minutes for primigravidas and 20 minutes for multigravidas.
• Placental separation begins as soon as the infant is born and ends with the expulsion of the placenta and fetal membranes (Fig. 8-8F to H). A hematoma forms deep to the placenta, separating it from the uterine wall. The placenta and fetal membranes are then expelled. Contractions of the uterus constrict the spiral arteries, preventing excessive uterine bleeding. The duration of this stage is approximately 15 minutes. A retained or adherent placenta—one not expelled within 1 hour of delivery—is a cause of postpartum bleeding.
Placenta and Fetal Membranes after Birth
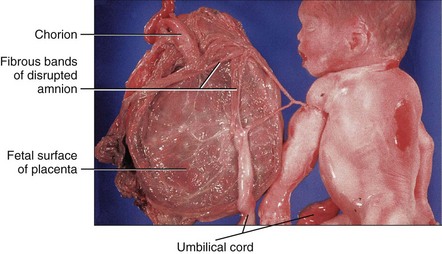
The placenta is commonly discoid, with a diameter of 15 to 20 cm and a thickness of 2 to 3 cm (Fig. 8-9). The margins of the placenta are continuous with the ruptured amniotic and chorionic sacs.
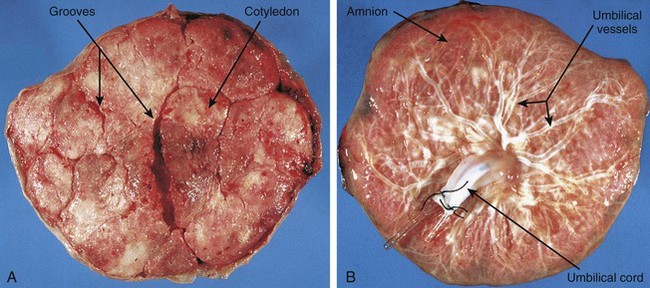
Figure 8–9 Placentas and fetal membranes after birth, shown approximately one third their actual size. A, Maternal surface, showing cotyledons and the grooves around them. Each convex cotyledon consists of a number of main stem villi with their many branch villi. The grooves were occupied by the placental septa when the maternal and fetal parts of the placenta were together (see Fig. 8-5). B, Fetal surface showing blood vessels running in the chorionic plate deep to the amnion and converging to form the umbilical vessels at the attachment of the umbilical cord.
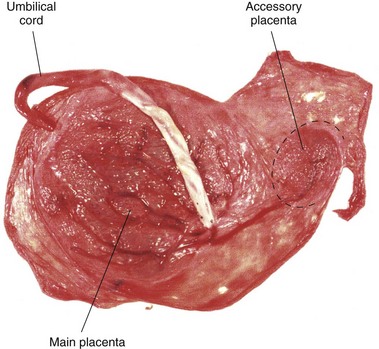
Variations in Placental Shape
As the placenta develops, chorionic villi usually persist only where the villous chorion is in contact with the decidua basalis. When villi persist elsewhere, several variations in placental shape occur, such as accessory placenta (Fig. 8-10). Examination of the placenta, prenatally by ultrasonography or postnatally by gross and microscopic study, may provide clinical information about the causes of placental dysfunction, intrauterine growth restriction, fetal distress and death, and neonatal illness. Postnatal placental examination can also determine whether the expelled placenta is intact. Retention of cotyledons or an accessory placenta in the uterus causes postpartum uterine hemorrhage.
Placental Abnormalities
Abnormal adherence of the chorionic villi to the myometrium of the uterine wall is called placenta accreta (Fig. 8-11). When chorionic villi penetrate the myometrium all the way to the perimetrium (peritoneal covering), the abnormality is called placenta percreta. Third-trimester bleeding is the most common presenting sign of these placental abnormalities. After birth, the placenta does not separate from the uterine wall, and attempts to remove it may cause severe hemorrhage that is difficult to control. When the blastocyst implants close to or overlying the internal os of the uterus, the abnormality is called placenta previa. Late pregnancy bleeding can result from this placental abnormality. In such cases, the fetus is delivered by cesarean section because the placenta blocks the cervical canal.
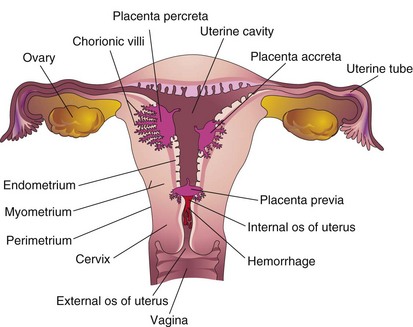
Figure 8–11 Placental abnormalities. In placenta accreta, there is abnormal adherence of the placenta to the myometrium (muscle layer). In placenta percreta, the placenta has penetrated the full thickness of the myometrium. In placenta previa, the placenta overlies the internal os of the uterus, blocking the cervical canal.
Maternal Surface of Placenta
The cobblestone appearance of the maternal surface of the placenta is produced by slightly bulging villous areas—the cotyledons—which are separated by grooves formerly occupied by placental septa (Fig. 8-9A).
Fetal Surface of Placenta
The umbilical cord usually attaches near the center of the fetal surface, and its epithelium is continuous with the amnion adhering to the chorionic plate of the placenta (Fig. 8-9B). The chorionic vessels radiating to and from the umbilical cord are visible through the smooth, transparent amnion. The umbilical vessels branch on the fetal surface, forming the chorionic vessels, which enter the chorionic villi (Fig. 8-5).
Absence of an Umbilical Artery
In approximately 1 in 200 newborn infants, only one umbilical artery is present (Fig. 8-12), a condition that may be associated with chromosomal and fetal abnormalities. Absence of an umbilical artery is accompanied by a 15% to 20% incidence of cardiovascular anomalies in the fetus. Absence of an artery results from either agenesis or degeneration of this vessel early in development.
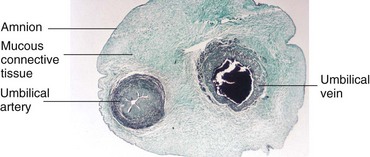
Figure 8–12 Transverse section of the umbilical cord. Note that the cord is covered by a single-layered epithelium derived from the enveloping amnion. It has a core of mucous connective tissue. Observe also that the cord has one umbilical artery and one vein. Usually, there are two arteries.
(Courtesy Professor V. Becker, Pathologisches Institut der Universität, Erlangen, Germany.)
Umbilical Cord
The umbilical cord is usually 1 to 2 cm in diameter and 30 to 90 cm in length (Fig. 8-10). Doppler ultrasonography may be used for prenatal diagnosis of the position and structural abnormalities of the umbilical cord. Long cords have a tendency to prolapse through the cervix or to coil around the fetus. Prompt recognition of prolapse of the cord is important because, during delivery, it may be compressed between the presenting body part of the fetus and the mother’s bony pelvis, causing fetal anoxia. If the deficiency of oxygen persists for more than 5 minutes, the infant’s brain may be damaged.
The umbilical cord usually has two arteries and one vein surrounded by mucoid connective tissue (Wharton jelly). Because the umbilical vessels are longer than the cord, twisting and bending of the cord is common. The cord frequently forms loops, producing false knots that are of no significance; however, in approximately 1% of pregnancies, true knots form in the umbilical cord. These may tighten and cause fetal death secondary to fetal anoxia (Fig. 8-13C). In most cases, the knots form during labor as a result of the fetus passing through a loop of the cord. Because these knots are usually loose, they have no clinical significance. Simple looping of the cord around the fetus occasionally occurs. In approximately one fifth of all deliveries, the cord is loosely looped around the neck without causing increased fetal risk.
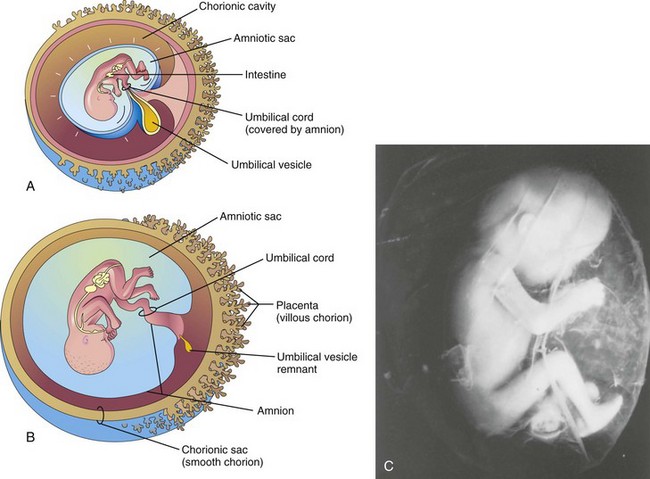
Figure 8–13 Illustrations of how the amnion enlarges, fills the chorionic sac, and envelops the umbilical cord. Observe that part of the umbilical vesicle (yolk sac) is incorporated into the embryo as the primordial gut. Formation of the fetal part of the placenta and degeneration of the chorionic villi are also shown. A, At 10 weeks. B, At 20 weeks. C, A 12-week fetus within its amniotic sac (shown actual size). The fetus and its membranes aborted spontaneously. It was removed from its chorionic sac with the amniotic sac intact.
Amnion and Amniotic Fluid
The amnion forms a fluid-filled, membranous amniotic sac that surrounds the embryo and fetus. As the amnion enlarges, it gradually obliterates the chorionic cavity and forms the epithelial covering of the umbilical cord (Fig. 8-13A and B). Amniotic fluid plays a major role in fetal growth and development. Initially, most amniotic fluid is derived from maternal tissue fluid by diffusion across the amniochorionic membrane from the decidua parietalis (see Fig. 8-5). Some fluid is secreted by amniotic cells. Later, there is diffusion of fluid through the chorionic plate from blood in the intervillous space of the placenta. Before keratinization (formation of keratin) of the skin occurs, a major pathway for passage of water and solutes in tissue fluid from the fetus to the amniotic cavity is through the skin. Fluid is also secreted by the fetal respiratory and gastrointestinal tracts and enters the amniotic cavity. Beginning in the 11th week, the fetus contributes to the amniotic fluid by expelling urine into the amniotic cavity.
The water content of amniotic fluid changes every 3 hours. Large amounts of water pass through the amniochorionic membrane into the maternal tissue fluid and into the uterine capillaries. An exchange of fluid with fetal blood also occurs through the umbilical cord and at the site where the amnion adheres to the chorionic plate on the fetal surface of the placenta (see Figs. 8-5 and 8-9B); thus, amniotic fluid is in balance with the fetal circulation.
Amniotic fluid is swallowed by the fetus and absorbed by the fetal respiratory and digestive tracts. It has been estimated that during the final stages of pregnancy, the fetus swallows up to 400 ml of amniotic fluid daily. The fluid is absorbed by the gastrointestinal tract and passes into the fetal bloodstream. The waste products cross the placental membrane and enter the maternal blood in the intervillous space. Excess water in the fetal blood is excreted by the fetal kidneys and returned to the amniotic sac through the fetal urinary tract.
Virtually all of the fluid in the amniotic cavity is water, in which undissolved material (such as desquamated fetal epithelial cells) is suspended. Amniotic fluid contains approximately equal portions of dissolved organic compounds and inorganic salts. Half of the organic constituents are protein; the other half is composed of carbohydrates, fats, enzymes, hormones, and pigments. As pregnancy advances, the composition of the amniotic fluid changes as fetal urine is added. Because fetal urine enters the amniotic fluid, fetal enzyme systems, amino acids, hormones, and other substances can be studied by examining fluid removed by amniocentesis. Studies of cells in the amniotic fluid permit the detection of chromosomal abnormalities.
Disorders of Amniotic Fluid Volume
A low volume of amniotic fluid—oligohydramnios—results, in some cases, from placental insufficiency, with diminished placental blood flow. Preterm rupture of the amniochorionic membrane is the most common cause of oligohydramnios. In the presence of renal agenesis (failure of kidney formation), the lack of fetal urine in the amniotic fluid is the main cause of oligohydramnios. A similar decrease in amniotic fluid occurs with obstructive uropathy (urinary tract obstruction). Complications of oligohydramnios include fetal abnormalities (pulmonary hypoplasia, facial defects, and limb defects) caused by fetal compression by the uterine wall.
A high volume of amniotic fluid is termed polyhydramnios. Most cases of polyhydramnios (60%) are idiopathic (of unknown cause); 20% of cases are caused by maternal factors, whereas 20% are fetal in origin. Polyhydramnios may be associated with severe anomalies of the central nervous system, such as meroencephaly (anencephaly) (see Chapter 16). With other anomalies, such as esophageal atresia, amniotic fluid accumulates because it cannot pass to the fetal stomach and the intestines for absorption.
Significance of Amniotic Fluid
• Permits uniform external growth of the embryo
• Acts as a barrier to infection
• Permits fetal lung development
• Prevents adherence of the amnion to the embryo
• Cushions the embryo against injuries by distributing impacts that the mother may receive
• Helps to control embryonic body temperature by maintaining a relatively constant temperature
• Enables the fetus to move freely, thereby aiding muscular development (e.g., in the limbs)
• Assists in maintaining homeostasis of fluid and electrolytes
Umbilical Vesicle
The umbilical vesicle (yolk sac) can be observed sonographically early during the fifth week of gestation. At 32 days, the umbilical vesicle is large (see Fig. 8-1C). By 10 weeks, the umbilical vesicle has shrunk to a pear-shaped remnant approximately 5 mm in diameter (Fig. 8-13A). By 20 weeks, the umbilical vesicle is very small (Fig. 8-13B).
Significance of Umbilical Vesicle
The umbilical vesicle is nonfunctional as far as yolk storage is concerned, but its presence is essential for several reasons:
• It has a role in the transfer of nutrients to the embryo during the second and third weeks before the uteroplacental circulation is established.
• Blood first develops in the well-vascularized, extraembryonic mesoderm covering the wall of the umbilical vesicle beginning in the third week (see Chapter 5), and it continues to develop there until hematopoietic activity begins in the liver during the sixth week.
• During the fourth week, the dorsal part of the umbilical vesicle is incorporated into the embryo as the primordial gut (see Fig. 6-1). Its endoderm, derived from the epiblast, gives rise to the epithelium of the trachea, bronchi, lungs, and alimentary tract.
• Primordial germ cells appear in the endodermal lining of the wall of the umbilical vesicle in the third week and subsequently migrate to the developing gonads (see Chapter 13). They differentiate into the spermatogonia in males and the oogonia in females.
Allantois
Although the allantois is not functional in human embryos, it is important for three reasons:
• Blood formation occurs in its wall during the third to fifth weeks of development.
• Its blood vessels become the umbilical vein and arteries.
• The intraembryonic portion of the allantois runs from the umbilicus to the urinary bladder, with which it is continuous (see Fig. 13-11E). As the bladder enlarges, the allantois involutes to form a thick tube, the urachus (see Fig. 13-11G). After birth, the urachus becomes a fibrous cord, the median umbilical ligament, which extends from the apex of the urinary bladder to the umbilicus.
Premature Rupture of Fetal Membranes
Premature rupture of the amniochorionic membrane is the most common event leading to premature labor and delivery and the most common complication resulting in oligohydramnios. Loss of amniotic fluid removes the major protection the fetus has against infection. Rupture of the membrane may cause various fetal anomalies that constitute amniotic band syndrome, or amniotic band disruption complex. These anomalies are associated with a variety of abnormalities, ranging from constriction of digits to major scalp, craniofacial, and visceral defects. The cause of these anomalies is probably related to constriction by encircling amniotic bands (Fig. 8-14).
Fetal Membranes in Multiple Pregnancies
Multiple gestations are associated with higher risks of fetal morbidity and mortality than single gestations. The risks are progressively greater as the number of fetuses increases. In North America, twins naturally occur approximately once in every 85 pregnancies, triplets approximately once in every 902 pregnancies, quadruplets approximately once in every 903 pregnancies, and quintuplets approximately once in every 904 pregnancies.
Twins and Fetal Membranes
Twins that originate from two zygotes are dizygotic (DZ) twins (fraternal twins) (Fig. 8-15), whereas twins that originate from one zygote are monozygotic (MZ) twins (identical twins) (Fig. 8-16). The fetal membranes and placentas vary according to the origin of the twins. Approximately two thirds of twins are dizygotic, and the rate of DZ twinning increases with maternal age.
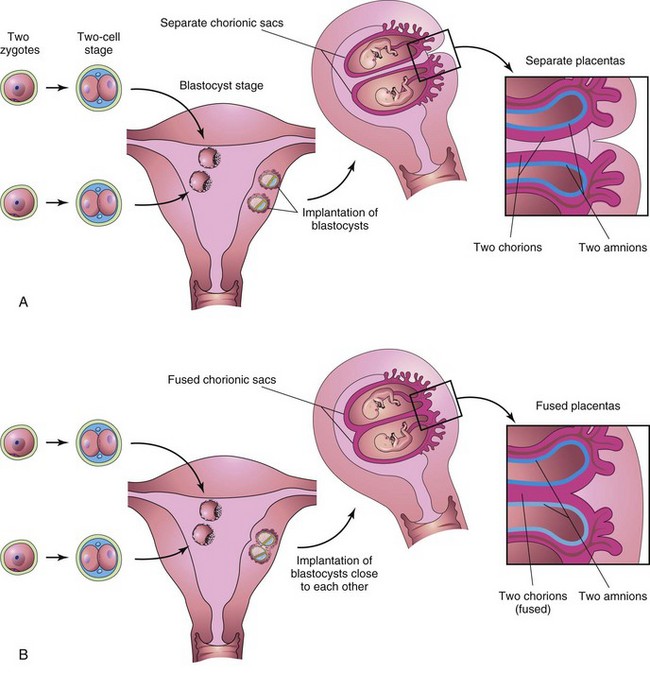
Figure 8–15 Dizygotic twins developing from two zygotes. The relationship of the fetal membranes and placentas are shown for instances in which the blastocysts implant separately (A) and the blastocysts implant close together (B). In both cases, there are two amnions and two chorions.
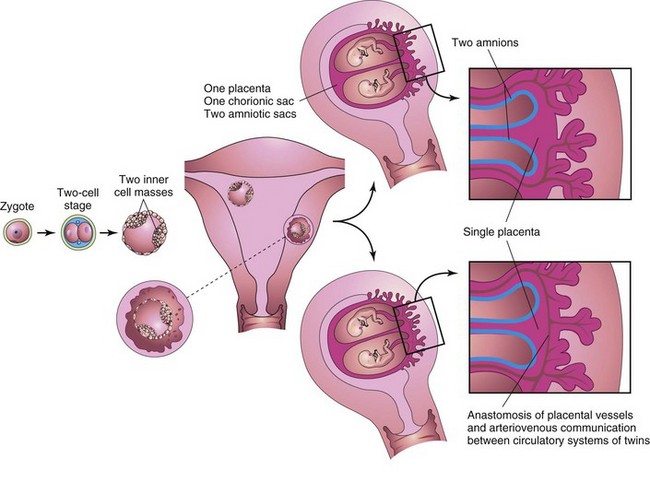
Figure 8–16 Illustrations of how approximately 65% of monozygotic twins develop from one zygote by division of the inner cell mass. These twins always have separate amnions, a single chorionic sac, and a common placenta. If there is anastomosis of the placental vessels, one twin may receive most of the nutrition from the placenta (see Fig. 8-17).
The study of twins is important in human genetics because it is useful for comparing the effects of genes and environment on development. If an abnormal condition does not show a simple genetic pattern, comparison of its incidence in MZ and DZ twins may show that heredity is involved.
Dizygotic Twins
Because they result from the fertilization of two oocytes by two sperms, DZ twins may be of the same sex or different sexes. For the same reason, they are no more alike genetically than brothers or sisters born at different times. DZ twins always have two amnions and two chorions (Fig. 8-15A), but the chorions and placentas may be fused (Fig. 8-15B). DZ twinning shows a hereditary tendency. The recurrence risk in families with one set of DZ twins is approximately triple that of the general population. The incidence of DZ twinning shows considerable racial variation, ranging from 1 in 500 in Asian populations, to 1 in 125 in white populations, to as high as 1 in 20 in some African populations.
Monozygotic Twins
Because they result from the fertilization of one oocyte and develop from one zygote (Fig. 8-16), MZ twins are of the same sex, are genetically identical, and are similar in physical appearance. Physical differences between MZ twins are environmentally induced, such as by anastomosis of the placental vessels, resulting in differences in blood supply from the placenta (Fig. 8-17). MZ twinning usually begins in the blastocyst stage, approximately at the end of the first week, and results from division of the embryoblast into two embryonic primordia (Fig. 8-16). Subsequently, two embryos, each in its own amniotic sac, develop within one chorionic sac and share a common placenta, a monochorionic-diamniotic twin placenta. Uncommonly, early separation of the embryonic blastomeres (e.g., during the two- to eight-cell stage) results in MZ twins with two amnions, two chorions, and two placentas that may or may not be fused (Fig. 8-18). In such cases, it is impossible to determine, from the membranes alone, whether the twins are monozygotic or dizygotic.
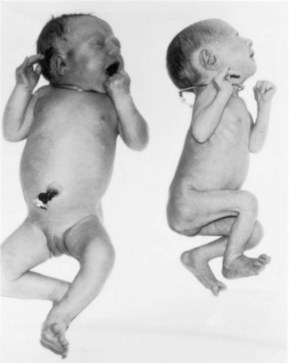
Figure 8–17 Monozygotic, monochorionic-diamniotic twins. Note the wide discrepancy in size resulting from an uncompensated arteriovenous anastomosis of the placental vessels. Blood was shunted from the smaller twin to the larger one, producing the twin transfusion syndrome.
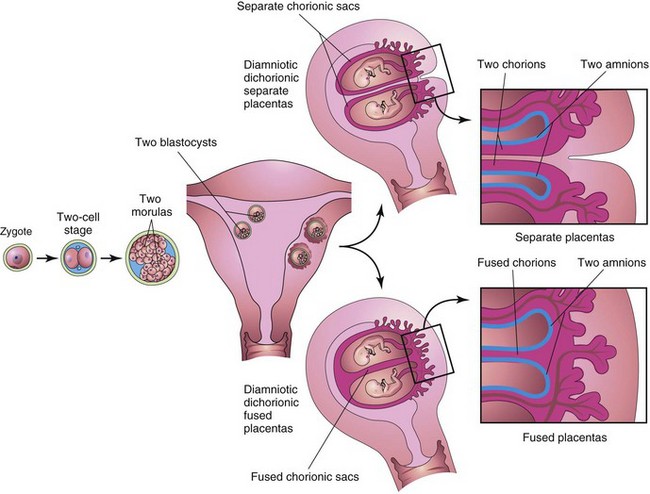
Figure 8–18 Illustrations of how approximately 35% of monozygotic twins develop from one zygote. Separation of the blastomeres may occur at any point from the two-cell stage to the morula stage, producing two identical blastocysts. Each embryo subsequently develops its own amniotic and chorionic sacs. The placentas may be separate or fused. In most cases, there is a single placenta resulting from secondary fusion, whereas in fewer cases, there are two placentas. In the latter cases, examination of the placenta suggests that they are dizygotic twins. This explains why some monozygotic twins are incorrectly classified as dizygotic twins at birth.
Twin Transfusion Syndrome
Twin transfusion syndrome occurs in 15% to 30% of monochorionic-diamniotic MZ twins. Arterial blood may be preferentially shunted from one twin through arteriovenous anastomoses in the placenta into the venous circulation of the other twin. The donor twin is small, pale, and anemic (see Fig. 8-17), whereas the recipient twin is large and polycythemic (i.e., having a higher than normal red blood cell count). The placenta shows similar abnormalities; the part of the placenta supplying the anemic twin is pale, whereas the part supplying the polycythemic twin is dark red. In lethal cases, death results from anemia in the donor twin and from congestive heart failure in the recipient twin.
Zygosity of Twins
Establishment of the zygosity of twins has become important, particularly because of the introduction of tissue and organ transplantation (e.g., bone marrow transplants). Twin zygosity is now determined by molecular testing. Any two people who are not MZ twins are virtually certain to show differences in some of the large number of DNA markers that can be studied.
Late division of the early embryonic cells (i.e., division of the embryonic disc during the second week) results in MZ twins with one amniotic sac and one chorionic sac. A monochorionic-monoamniotic twin placenta is associated with a fetal mortality rate approaching 50%. The umbilical cords are frequently so entangled that circulation of the blood through their vessels ceases, and one or both fetuses die. Ultrasonography plays an important role in the diagnosis of twin pregnancies and the management of various conditions that may complicate MZ twinning, such as intrauterine growth restriction, intrauterine fetal distress, and premature labor.
Other Types of Multiple Births
• Two zygotes and consist of identical twins and a singleton
• Three zygotes and be of the same sex or of different sexes, in which case the infants are no more similar than infants from three separate pregnancies
Similar combinations occur in quadruplets, quintuplets, sextuplets, and septuplets.
Conjoined Twins
If the embryonic disc does not divide completely, various types of conjoined (MZ) twins may form. These twins are named according to the regions of the body that are attached; for example, thoracopagus indicates anterior union of the thoracic regions. In some cases, the twins are connected to each other by skin only or by cutaneous and other tissues, such as fused livers. Some conjoined twins can be separated successfully by surgery. The incidence of conjoined twins is 1 in 50,000 to 1 in 100,000 births.
Clinically Oriented Questions
1. What is meant by the term stillbirth? Do older women have more stillborn infants?
2. An infant was born dead, reportedly because of a “cord accident.” What does this mean? Do these accidents always kill the infant? If not, what defects may be present?
3. What is the scientific basis of the home pregnancy tests that are sold in drugstores?
4. What is the proper name for what laypeople sometimes refer to as the bag of waters? What is meant by a dry birth? Does premature rupture of this “bag” induce the birth of the infant?
5. What does the term fetal distress mean? How is the condition recognized? What causes fetal distress?
6. Some say that twins are born more commonly to older mothers. Is this true? Others maintain that twinning is hereditary. Is this correct?