Infection Control and the Dental Radiographer
After completion of this chapter, the student will be able to do the following:
• Define the key terms associated with infection control
• Describe the rationale for infection control
• Describe the three possible routes of disease transmission
• Describe the conditions that must be present for disease transmission to occur
• Discuss personal protective equipment (PPE), hand hygiene, sterilization and disinfection of instruments, and the cleaning and disinfection of the dental unit and environmental surfaces.
• Describe the infection control procedures that are necessary before x-ray exposure.
• Describe the infection control procedures that are necessary during x-ray exposure
• Describe the infection control procedures that are necessary for digital imaging
• Describe the infection control procedures that are necessary after x-ray exposure
• Describe the infection control procedures that are necessary for processing
• Discuss film handling in the darkroom—with and without barrier envelopes
• Discuss film handling without barrier envelopes using the daylight loader of an automatic processor
Infectious diseases present a significant hazard in the dental environment, and dental professionals are at an increased risk for acquiring such diseases. Therefore, infection control is a major concern in dentistry. Infection control protocols are used in dentistry to minimize the potential for disease transmission. To protect themselves as well as their patients, dental professionals must understand and use infection control protocols.
The infection control practices used in dentistry apply to radiographic procedures as well. The purpose of this chapter is to present the rationale for infection control and the associated terminology, to review the guidelines from the Centers for Disease Control and Prevention (CDC), and to describe in detail the step-by-step infection control procedures used in dental radiography.
Infection Control Basics
To understand infection control practices, the dental professional must first understand the purpose of infection control and the terminology that is frequently used in infection control protocols.
Rationale for Infection Control
The primary purpose of infection control procedures is to prevent the transmission of infectious diseases. Infectious diseases may be transmitted from a patient to the dental professional, from the dental professional to a patient, and from one patient to another patient. The use of recommended infection control guidelines can greatly reduce the transmission of infectious diseases.
Before the dental professional can use infection control practices to prevent disease transmission, an understanding of how disease transmission occurs in the dental environment is necessary. Disease transmission involves pathogens; a pathogen is a microorganism capable of causing disease. Dental professionals and dental patients may be exposed to a variety of pathogens that are present in oral or respiratory secretions. These pathogens may include the following:
• Cold and flu viruses and bacteria
• Herpes simplex virus (HSV-1, HSV-2)
In the dental environment, the general routes of disease transmission can be described as follows:
• Direct contact with pathogens present in saliva, blood, respiratory secretions, or lesions
• Indirect contact with contaminated objects or instruments
• Direct contact with airborne contaminants present in spatter or aerosols of oral and respiratory fluids
For an infection to occur by one of these routes of transmission, the following three conditions must be present:
Effective infection control practices are intended to alter one of these three conditions, thereby preventing disease transmission.
Infection Control Terminology
An understanding of the terminology related to infection control is important for the dental professional. The following terms are frequently used in discussions of infection control, in the infection control literature, and in infection control protocols:
Antiseptic: A substance that inhibits the growth of bacteria. This term is often used to describe handwashing or wound-cleansing procedures.
Asepsis: The absence of pathogens, or disease-causing microorganisms. This term is often used to describe procedures that prevent infection (e.g., aseptic technique).
Bloodborne pathogens: Pathogens present in blood that cause diseases in humans.
Disinfect: Use a chemical or physical procedure to inhibit or destroy pathogens. Highly resistant bacterial and mycotic (fungal) spores are not killed during disinfection procedures.
Disinfection: The act of disinfecting.
Exposure incident: A specific incident that involves contact with blood or other potentially infectious materials and that results from procedures performed by the dental professional.
Infectious waste: Waste that consists of blood, blood products, contaminated sharps, or other microbiologic products.
Occupational exposure: Contact with blood or other infectious materials that involves the skin, eye, or mucous membranes and that results from procedures performed by the dental professional.
Parenteral exposure: Exposure to blood or other infectious materials that results from piercing or puncturing the skin barrier (e.g., a needle-stick injury results in parenteral exposure).
Personal protective equipment (PPE): Includes protective attire, gloves, mask, and eyewear.
Sharps: Any objects that can penetrate the skin, including, but not limited to, needles and scalpels.
Standard precautions: Measures that include a standard of care designed to protect health care personnel and patients from pathogens that can be spread by blood or any other body fluid, excretion, or secretion.
Sterilize: The use of a physical or chemical procedure to destroy all pathogens, including highly resistant bacteria and mycotic spores.
Guidelines for Infection Control Practices
In 2003, the CDC released a publication entitled Guidelines for Infection Control in Dental Health Care Settings, which provides updated infection control practices for dentistry.
The recommended infection control practices are applicable to all settings in which dental treatment is provided (Box 15-1). These guidelines must be observed in conjunction with the practices and procedures for worker protection required by the Occupational Safety and Health Administration (OSHA) in its final rule on Occupational Exposure to Bloodborne Pathogens. The recommended infection control practices that directly relate to dental radiography procedures include the following:
• Handwashing and care of hands
• Sterilization or disinfection of instruments
• Cleaning and disinfection of dental unit and environmental surfaces
Personal Protective Equipment
All dental professionals must wear protective clothing (e.g., gown, lab coat, uniform) to prevent skin and mucous membrane exposure when contact with blood or other body fluids is anticipated. Protective clothing must be changed daily or changed more frequently if it is visibly soiled. Dental professionals must remove all protective garments before leaving the dental office, and the garments should be laundered according to the manufacturer’s instructions.
Gloves
All dental professionals must wear medical latex or vinyl gloves to prevent skin contact with blood, saliva, or mucous membranes. The dental professional must wear new gloves for each patient. Gloves must also be worn when touching contaminated items or surfaces. Nonsterile gloves are recommended for examinations and nonsurgical procedures; sterile gloves are recommended for all surgical procedures.
In preparation for treating each patient, hands must be washed before gloves are worn. After treating each patient or after exiting the patient treatment area, the dental professional must remove and discard the gloves and wash hands immediately. Dental professionals must always wash their hands and put on new gloves between patients. During treatment, gloves must be removed and changed whenever they are torn, cut, or punctured. Gloves should never be washed before use or disinfected for reuse. Washing or disinfection causes defects and diminishes the barrier protection provided by the gloves.
Masks and Protective Eyewear
Whenever spatter and aerosolized sprays of blood and saliva are likely, all dental professionals must use surgical masks and protective eyewear, or chin-length plastic face shields, to protect the eyes and face. The mask, when used, must be changed between patients or during treatment if it becomes wet or moist. After treatment, face shields and protective eyewear must be washed with appropriate cleaning agents. When visibly soiled, such equipment should be disinfected between patients.
Hand Hygiene and Care of Hands
In dental radiography, hand hygiene is a general term that applies to routine handwashing, antiseptic hand-wash, and antiseptic hand-rub techniques. Indications for hand hygiene include the following:
• Before and after treating each patient (e.g., before glove placement and after glove removal)
• After removing gloves that are torn, cut, or punctured and before putting on new gloves
• After contact of bare hands with inanimate objects likely to be contaminated by blood, saliva, or respiratory secretions
In dental radiography, three different types of hand hygiene may be practiced.
• Routine hand wash: Water and nonantimicrobial soap (i.e., plain soap) for 15 seconds
• Antiseptic hand wash: Water and antimicrobial soap (e.g., chlorhexidine, iodine and iodophors, chloroxylenol [PCMX], triclosan) for 15 seconds
• Antiseptic hand rub: Alcohol-based product until the hands are dry
For more information on hand hygiene including hand lotions and how to store hand care products, please visit the CDC at http://www.cdc.gov/OralHealth/infectioncontrol/faq/hand.htm
Care of Hands
All dental professionals must take precautions to avoid hand injuries during dental procedures. Dental professionals with exudative or “weeping” lesions on their hands must refrain from all direct patient contact and from handling patient care equipment until the condition has resolved.
Sterilization and Disinfection of Instruments
All instruments in the dental practice can be classified into one of the following categories, depending on the risk of transmitting infection and the need to sterilize the instrument between uses:
Critical instruments: Instruments that are used to penetrate soft tissue or bone are considered critical and must be sterilized after each use. Examples include forceps, scalpels, bone chisels, scalers, and surgical burs. In dental radiography, no critical instruments are used.
Semicritical instruments: Instruments that contact but do not penetrate soft tissue or bone are classified as semicritical. These devices must also be sterilized after each use. If the instrument can be damaged by heat and sterilization is not feasible, high-level disinfection is required. Beam alignment devices are an example of a semicritical instrument used in dental radiography.
Noncritical instruments: Instruments or devices that do not come in contact with mucous membranes are considered noncritical. Because little risk of transmitting infection from noncritical devices exists, intermediate-level or low-level infection techniques are required for their care between patients. Examples in dental radiography include the position-indicating device (PID) of the dental x-ray tubehead, the exposure button, the x-ray control panel, and the lead apron.
Acceptable methods of sterilization include steam under pressure (autoclave), dry heat, and chemical vapor. The instructions of the manufacturers of the instruments and sterilizer must be followed. Proper functioning of sterilization cycles must be verified by periodic use of a biologic indicator, such as the spore test.
The U.S. Environmental Protection Agency (EPA) has classified certain chemicals as “sterilants–disinfectants.” These EPA-registered chemicals are classified as high-level disinfectants and can be used to disinfect heat-sensitive semicritical dental instruments.
Cleaning and Disinfection of Dental Unit and Environmental Surfaces
After each patient has been treated, dental unit surfaces and countertops that may have been contaminated with blood or saliva must be thoroughly cleaned with disposable toweling, using an appropriate cleaning agent and water as necessary. All surfaces must then be disinfected with a suitable chemical germicide.
EPA-registered chemical germicides labeled as both hospital disinfectants and tuberculocidals are classified as intermediate-level disinfectants and are recommended for all surfaces that have been contaminated. Intermediate-level disinfectants include phenolics, iodophors, and chlorine-containing compounds.
EPA-registered chemical germicides that are labeled only as hospital disinfectants are classified as low-level disinfectants and are recommended for general housekeeping purposes, such as cleaning floors and walls.
Infection Control in Dental Radiography
In dentistry, treatment to all patients must be provided after taking standard precautions. Standard precautions include a standard of care designed to protect health care personnel and patients from pathogens that can be spread by blood or any other body fluid, excretion, or secretion. The same infection control procedures must be used for each patient. No exceptions exist, and no “extra” precautions should be used on any patients. Specific infection control procedures pertain to dental radiography and must be used for each patient.
The areas designated for the exposure and processing of dental radiographs are not routinely associated with the spatter of blood or saliva; however, transmission of infectious diseases is still possible if the equipment, supplies, film packets, digital sensors, or cassettes used for the radiographic procedure are contaminated. Therefore, specific infection control procedures that pertain to dental radiography must be used before, during, and after film exposure (Box 15-2), as well as during film processing (Procedure 15-1).
Infection Control Procedures Used Before Exposure
Before dental x-ray receptors are exposed, the treatment area must be prepared using aseptic techniques. Necessary supplies and equipment must also be prepared. After such preparations, the dental radiographer can seat the patient. At that time, the dental radiographer can also complete the final infection control procedures that are necessary before exposure.
Preparation of Treatment Area
The dental professional must prepare the surfaces that are likely to be touched during the radiographic procedure. All these surfaces should be covered with impervious, disposable materials such as plastic wrap, plastic-backed paper, or aluminum foil. This provides adequate protection while eliminating the need for surface cleaning and disinfection between patients. If disposable materials are not used, after the radiographic procedures have been completed, all contaminated areas must be disinfected with disinfecting products, following the manufacturer’s instructions. Examples of surfaces that must be covered or disinfected include the following:
X-Ray Machine: The tubehead, PID, control panel, and exposure button must all be covered or disinfected.
Dental Chair: The headrest as well as the headrest adjustment and chair adjustment controls must be covered or disinfected.
Preparation of Supplies and Equipment
The dental professional must also have ready all anticipated supplies and equipment, such as film, sensors, sterilized beam alignment devices, and other miscellaneous items, and must make these available in the work area.
Film: Dental x-ray films should be dispensed from a central supply area in a disposable container (e.g., coin envelope, paper cup). Commercially available plastic barrier envelopes that fit over intraoral films can be used to protect the film packets from saliva and minimize contamination after exposure of the film. Intraoral films may be inserted and sealed in plastic barrier envelopes (e.g., ClinAsept Barrier Envelopes, manufactured by Carestream Health, Inc.) before dispensing films from a central supply area (Figure 15-1).
Digital Sensors: The sensors/receptors (see Chapter 25) used in digital radiography cannot be heat sterilized. In order to avoid cross-contamination, both barrier techniques and disinfection are required. The CDC recommends cleaning and disinfecting the sensor with an EPA-registered intermediate-level disinfectant after removing the barrier and before using it on another patient. Because sensors and digital radiography components vary by manufacturer and can be expensive, manufacturers should be consulted regarding specific disinfection products and procedures. As with dental x-ray film, sensors used in indirect digital imaging should also be dispensed from a central supply area. These sensors must be wrapped in plastic barrier envelopes to protect the sensor from saliva and contamination, much like the barriers used for intraoral films (Figure 15-2). Dental offices using direct digital imaging require plastic disposable sleeves to cover both the sensor and the wire connection (Figure 15-3).
Beam Alignment Devices: Beam alignment devices should be packaged in sterilized bags and dispensed from a central supply area.
Miscellaneous Items: Other items include cotton rolls that can be used to stabilize receptor placement and paper towels that can be used to remove saliva from exposed receptors. A disposable container (e.g., paper cup or bag) labeled with the patient’s name is necessary to collect the exposed receptors. All miscellaneous items should be dispensed from a central supply area.
Preparation of the Patient
The dental professional can seat the patient following preparation of the treatment area, supplies, and equipment. After seating the patient, the dental radiographer must complete the procedures discussed next before washing hands and putting on gloves.
Chair Adjustment: The chair must be positioned so that the patient is seated upright. The height of the chair should be adjusted to a comfortable working height for the dental radiographer.
Headrest Adjustment: The headrest must be adjusted to support the patient’s head. The patient’s head should be positioned with the maxillary arch parallel to the floor.
Preparation of the Dental Radiographer
After patient preparation and before x-ray exposure of the patient, the dental radiographer must complete some final infection control procedures.
Infection Control Procedures Used During Exposure
Once gloves have been put on and exposure begins, the dental radiographer should take special care to touch only covered surfaces. The best way the dental radiographer can minimize contamination is to touch as few surfaces as possible. During and immediately after exposure, the dental radiographer must handle each receptor in a manner consistent with comprehensive infection control guidelines.
Drying of Exposed Receptors: After each receptor has been placed in the patient’s mouth, exposed, and removed, it must be dried with a paper towel to remove excess saliva.
Collection of Exposed Receptors: Once dried, each receptor must be placed in a disposable container (paper bag or cup) labeled with the patient’s name. This container is used to collect and transport the exposed receptors to the darkroom or scanning area and must not be touched by gloved hands. To prevent fogging caused by scatter radiation, the container should not be placed in a room where additional receptors are being exposed. In addition, exposed receptors should never be placed in the dental radiographer’s lab coat or uniform pocket.
Beam Alignment Devices: During exposure, beam alignment devices should be transferred from the covered work area to the patient’s mouth and then back to the same area. Contaminated instruments should never be placed on an uncovered countertop.
Interruptions During Exposure: If the dental radiographer is interrupted (e.g., by a telephone call) and must leave the room during exposure of receptors, the radiographer must remove the gloves and wash hands before leaving the area. He or she must then rewash hands and put on new gloves before resuming the procedure.
Infection Control Procedures Used After Exposure
Immediately after the completion of receptor exposures, all contaminated items must be discarded, and any uncovered areas must be disinfected. Contaminated items must be handled in a manner consistent with recommended infection control guidelines.
Disposal of Contaminated Items: All contaminated items (cotton rolls, bite-wing tabs, cups, bags, and protective coverings) must be disposed of following local and state environmental regulations. Contaminated items must be discarded while the dental radiographer is still wearing gloves; this includes disposable materials found on protected surfaces as well. The dental radiographer must carefully unwrap all covered surfaces; the surfaces that are wrapped should not be touched by gloved hands. Ideally, the disposal of all contaminated items should take place in the presence of the patient.
Beam Alignment Devices: While still wearing gloves, the dental radiographer must remove the contaminated beam alignment devices from the treatment area and place them in an area designated for contaminated instruments.
Handwashing: After removal and disposal of all contaminated items, the radiographer must remove the gloves and discard them and wash hands.
Infection Control Procedures Used for Processing
After the exposure of x-ray receptors, specific infection control guidelines must be followed while transporting the receptors to the darkroom, handling them, and processing them.
Film Transport: As previously described, films contaminated with saliva must be placed in a labeled disposable container after exposure. The disposable container should never be touched by gloved hands. Only after removing gloves, washing hands, dismissing the patient, and cleaning the area, should the dental radiographer carry the disposable container holding the contaminated films to the darkroom.
Darkroom Supplies: Paper towels and gloves, which are necessary for handling films before they are processed, must be available in the darkroom. Paper envelopes, paper cups, or film mounts labeled with the patient’s name are used to hold films after processing, and these also should be available in the darkroom.
Film Handling with and without Barrier Envelopes: Commercially available barrier envelopes help to minimize contamination in the darkroom. Procedure 15-1 lists the recommended film-handling steps when exposed films are protected by barrier envelopes (Figure 15-4) and when films are not protected by barrier envelopes (Figure 15-5). These same steps can be applied to the handling and scanning of storage phosphor sensors used with indirect digital imaging.
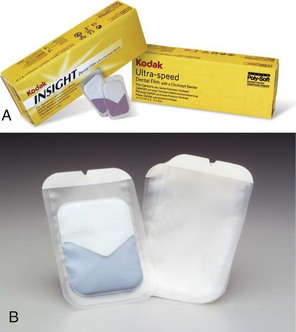
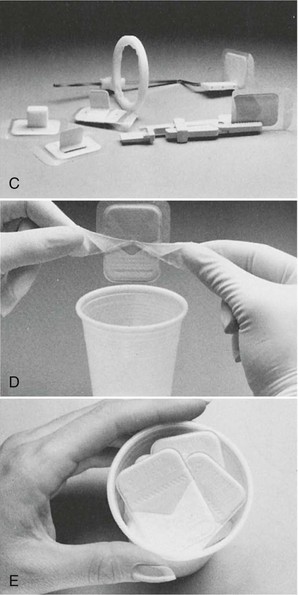
FIGURE 15-4 A, Packaging for barrier film. B, Protected and unprotected film. C, Bite-wing and periapical applications of protected film. D, Film packet removal from envelope without contamination. E, Uncontaminated film ready for processing. (Courtesy Carestream Health, Inc. Rochester, NY.)
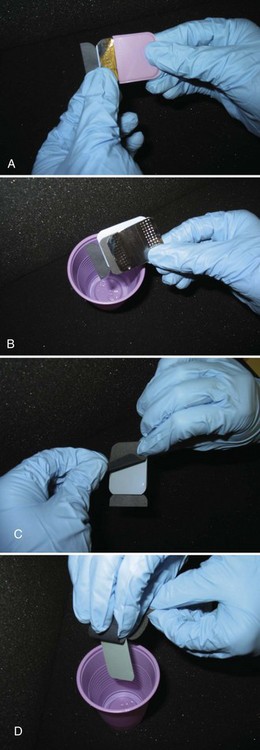
FIGURE 15-5 Steps used to open a size 2 film packet without contaminating film. A, Method for removing films from packet and not touching them with contaminated gloves. Open tab, and slide lead foil and black interleaf paper from wrapping. B, Rotate contaminated film packet away from black paper and foil and discard. C, Peel back paper wrapping away from film. D, Allow film to fall into a clean cup.
Disinfection of Darkroom: Darkroom countertops and any areas touched by gloved hands must be disinfected with an EPA-registered hospital-grade disinfectant.
Daylight Loader Procedures: Infection control procedures for processing film without barrier envelopes in automatic film processors equipped with daylight loaders include the following:
1. Place the paper cup and the vinyl or nonpowdered gloves in the daylight loader compartment.
2. Place the container with contaminated films next to the cup.
3. Close the daylight loader lid, and push hands through openings.
5. Take one contaminated film out of the container.
6. Open film packets as described in Film Handling without Barrier Envelopes (see Procedure 15-1).
7. Allow the film to drop onto the processor film feed slot area. (Do not touch the film with gloved hands.)
8. Dispose of film packet wrappings in the paper cup.
9. After all film packets have been opened, remove gloves and place them in the cup.
10. Feed all unwrapped films into the processor.
11. Remove hands from the daylight loader.
13. Lift the daylight loader lid to remove and discard the cup with contaminated wrappings and the container that held contaminated films.
14. Label the film mount, paper cup, or envelope with the patient’s name, and use it to collect processed films.
Summary
• To protect themselves as well as their patients, dental professionals must understand and use infection control protocols. The primary purpose of infection control procedures is to prevent the transmission of infectious diseases.
• Disease transmission involves pathogens, or microorganisms that are capable of causing disease. In dentistry, disease transmission may occur as a result of one of the following:
• Direct contact with pathogens in saliva, blood, respiratory secretions, or lesions
• Indirect contact with contaminated objects or instruments
• Direct contact with airborne contaminants present in spatter or aerosols or oral and respiratory fluids
• For infection to occur, three conditions must be present: (1) susceptible host, (2) pathogen with sufficient infectivity and numbers to cause infection, and (3) portal of entry for pathogen to infect host.
• The CDC’s Guidelines for Infection Control in Dental Health Care Settings (2003) outlines specific infection control measures that pertain to dentistry, including PPE, hand hygiene, and sterilization and disinfection of instruments.
• The recommended infection control practices are applicable to all settings in which dental treatment is provided. The same infection control procedures must be used for each patient, with no exceptions and no extra precautions for select patients.
• Infection control procedures before x-ray exposure include the preparation of (1) the treatment area (x-ray machine, dental chair, work area, lead apron), (2) the supplies and equipment (film, digital sensors, beam alignment devices, other items), (3) the patient (chair and headrest adjustment, lead apron), and (4) the radiographer (handwashing, gloves).
• Infection control practices during exposure involve drying and collecting exposed receptors, reassembly of beam alignment devices, and dealing with interruptions properly.
• Infection control procedures after exposure include disposal of contaminated items, removal of beam alignment devices, handwashing, lead apron removal, and surface disinfection.
• Infection control practices during processing involve film transport, darkroom supplies, film handling with and without barrier envelopes, disinfection of the darkroom, and daylight loaders.
American Academy of Oral and Maxillofacial Radiology. Infection control guidelines for dental radiographic procedures. Oral Surg Oral Med Oral Pathol. 1992;73:248.
Centers for Disease Control and Prevention. Guidelines for infection control in dental health care settings. MMWR. 2003;52(RR-17):1–61.
Cottone, JA, Terezhalmy, GT, Molinari, JA. Infection control in dental radiology. In: Practical infection control in dentistry. Philadelphia: Lea & Febiger; 1991.
Cottone, JA, Terezhalmy, GT, Molinari, JA. Rationale for practical infection control in dentistry. In: Practical infection control in dentistry. Philadelphia: Lea & Febiger; 1991.
Cottone, JA, Terezhalmy, GT, Molinari, JA. Appendix B. In: Practical infection control in dentistry. Philadelphia: Lea & Febiger; 1991.
Frommer, HH, Savage-Stabulas, JJ, Infection control in dental practice. Radiology for the dental professional, ed 8, St. Louis, Mosby, 2005.
White, SC, Pharoah, MJ, Radiographic quality assurance and infection control. Oral radiology: principles and interpretation, ed 6, St. Louis, Mosby, 2009.
American Dental Association. ADA Statement on Infection Control in Dentistry. http://www.ada.org/1857.aspx.
OSAP. Dentistry’s Resource for Infection Control and Safety. http://www.osap.org/.
Infection Control Checklist. http://www.osap.org/?page=ChartsChecklists.
Matching
For questions 1 to 10, match each definition with one term.
________ 1. Use of a chemical or physical procedure to destroy all pathogens, including spores
________ 2. Microorganism capable of causing disease
________ 3. Exposure to infectious material resulting from procedures performed by the dental professional
________ 4. Exposure to infectious materials that results from piercing or puncturing the skin
________ 5. Use of a chemical or physical procedure to destroy all pathogens, except spores
________ 6. Instrument used to penetrate soft tissue or bone
________ 7. Instrument that contacts but does not penetrate soft tissue or bone
________ 8. Instrument that does not contact mucous membranes
________ 9. Waste that consists of blood, blood products, contaminated sharps, and other microbiologic products
Fill in the Blank
Multiple Choice
________ 14. Identify the false statement concerning protective clothing:
a. It must be worn by all dental professionals.
b. It must be worn to prevent contact with infectious materials.
________ 15. Identify the false statement concerning gloves:
a. Gloves must be worn by all dental professionals.
b. Gloves must be washed before use.
________ 16. Identify the false statement concerning masks and protective eyewear:
a. Masks and protective eyewear are optional for dental radiographic procedures.
b. Masks must be changed between patients.
c. When visibly soiled, protective eyewear must be disinfected between patients.
d. Protective shield must be worn during radiographic procedures.
________ 17. Identify the true statements concerning handwashing. Hands must be washed:
________ 18. Examples of critical instruments include:
________ 19. EPA-registered chemical germicides labeled as both hospital disinfectants and tuberculocidal agents are classified as:
________ 20. EPA-registered chemical germicides labeled only as hospital disinfectants are classified as:
Essay
21. Describe the infection control procedures that are necessary before x-ray exposure.
22. Describe the infection control procedures that are necessary during x-ray exposure.
23. Describe the infection control procedures that are necessary after x-ray exposure.
24. Describe the infection control procedures that are necessary for film processing.
25. Discuss film handling in the darkroom, with and without barrier envelopes.


