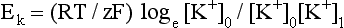Physiology of the nervous system
In this chapter, you will learn to:
• Describe the divisions of the nervous system
• State the different cell types and their arrangement within the nervous system
• Describe the action potential and the different ions involved
• Explain the different types of synaptic transmission
• Describe in detail the process of chemical transmission
• Describe the types of neurotransmitter
• Discuss the different sensory receptors in the skin and mucous membranes
• Explain how pain is regulated peripherally and centrally
• Relate the peripheral and central control of pain to how the most common forms of analgesia work
• Describe the different divisions of the brainstem and their functions
• Discuss the structure and function of the visual, auditory, olfactory and gustatory systems
This chapter provides a general overview of neurophysiology and of the sensory systems. The neurophysiology of motor control and higher functions is covered in Crash Course: Nervous System.
OVERVIEW OF THE NERVOUS SYSTEM
The nervous system receives information about the internal and external environments from the different sensory organs. In turn, it influences how the body responds. The nervous system functions at three main levels:
1. Sensory division: sensory/afferent receptors (e.g. pain receptors, receptors for vision and hearing) provide information to the brain about the environment.
2. Information processing: the brain collates this information and decides what action to take via its motor system.
3. Motor division: the motor/efferent system responds appropriately via contraction of muscle and/or gland secretion in the body.
Anatomy of the nervous system
The nervous system is divided into two broad parts (Fig. 3.1):
Peripheral nervous system
The PNS has two main divisions: the somatic and autonomic nervous systems.
Somatic nervous system: The somatic nervous system contains the sensory (afferent) and motor (efferent) nerves that supply the skin, muscles and joints. Single neurons relay information between the CNS and the target cells. For example, touching something painful stimulates a sensory (afferent) nerve, which carries the information that the hand is touching something painful to the CNS to be processed. A signal from the CNS then travels down the motor (efferent) pathway to the muscle, which responds, e.g. it contracts and moves the hand out the way.
CELLULAR PHYSIOLOGY OF THE NERVOUS SYSTEM
Neurons: structure and function
The basic cell of the nervous system is the neuron. These are excitable cells, which transmit information in the form of action potentials (see later) to other excitable cells. The neuron has four main regions (Fig. 3.2):
1. Soma: the main body of the neuron that contains the organelles (see Chapter 1).
2. Dendrites: a branching network of soma that communicates with nearby cells.
3. Axon: a long extension from the soma that transmits the action potential to the terminal boutons.
4. Terminal boutons: swellings that form the presynaptic membrane; they contain the neurotransmitters that propagate the action potential to other excitable cells.
Neuronal excitation and inhibition
An electrical potential gradient exists across the nerve cell membrane. This gradient is caused by the relative permeability of the cell membrane to sodium ions (Na+) in the extracellular fluid and potassium ions (K+) in the intracellular fluid. The value of this potential determines whether an action potential is fired.
Resting potential
There is a potential difference of −70 mV across the membrane of a resting neuron, the inside being negative relative to the outside. This is due to a greater concentration of K+ ions inside the cell, which results in a negative inside and a positive outside and hence a potential difference (diffusion potential).
Diffusion takes place until an equilibrium state, in which the electrical force attracting the positive K+ into the cells is equal to the chemical force of the concentration gradient, is reached. The electrical potential (Ek) at this equilibrium point can be calculated using the Nernst equation:
Where R = the ideal gas constant [8.314510 J K−1 mol−1]; T = thermodynamic temperature (+273 K); F = Faraday’s constant [96485.3 C mol−1]; Z = ionic valency (+1 for K+); [K+]o = concentration of K+ outside cell; and [K+]i = concentration of K+ inside cell.
In fact, this equation suggests that the value of Ek is not −70 mV but −90 mV. The explanation for the difference is that other ions, such as Na+, diffuse across the cell membrane in the opposite direction to the K+. The Goldman–Hodgkin–Katz equation is similar to the Nernst equation but, as it takes ions other than K+ into consideration, is far more accurate.
There is always some unregulated leakage of Na+ into the cell and K+ out of the cell along the concentration gradient, but this is counteracted by an energy-dependent Na+/K+-ATPase exchange pump, which stabilizes the resting membrane potential. This pump ejects three Na+ in exchange for every two K+ transported into the cell.
Action potentials
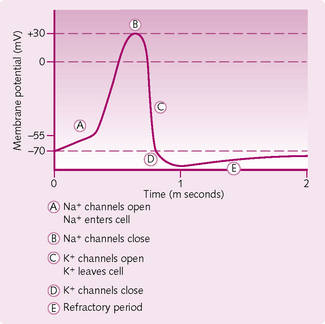
An action potential (AP) is the feature of muscle and nerve cells that results in self-propagating membrane depolarization (Fig. 3.3). When a nerve cell is stimulated the electrical potential across the membrane changes. If the stimulus is strong enough and reaches a certain value (threshold potential), an AP is generated. APs have three distinct properties:
1. They are all or nothing: this means that an AP will occur when the threshold potential has been exceeded. Increasing the stimulus further does not change the shape or size of the AP.
2. They have a refractory period: during an AP it is either impossible or very difficult to stimulate a second AP. There are two types of refractory period:
• absolute: in the early part of the AP no further stimulus is possible
• relative: a further AP is possible but a larger-than-normal stimulus is required.
3. They are self-propagating: once the AP has been initiated it spreads throughout the excitable tissue.
Ionic mechanism of an action potential:
In 1952, Hodgkin and Huxley published a series of papers in which they investigated membrane currents through the membrane of the squid giant axon. Theirs was the first ever recording of the AP and forms the basis of neuroscience today.
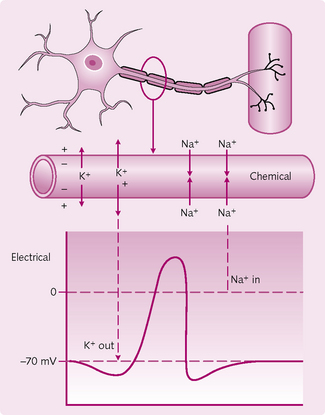
The propagation of a nerve impulse along an axon begins when synapses on a neuron receive neurotransmitters from adjacent nerve endings (Fig. 3.4). This causes the electrical potential across the cell membrane to change, setting off a chain of events that results in the action potential.
There are three stages to the generation of an AP:
1. The threshold: to initiate an AP, the stimulus must increase the membrane potential to about 20 mV above the resting potential of −70 mV. When this happens, Na+ enters the neuron via voltage-gated Na+ channels.
2. Upstroke (depolarization): if the threshold is reached then more voltage-gated Na+ channels open and further repolarization takes place (positive feedback). The membrane potential rises towards 0 mV and then overshoots to +30 mV.
3. Downstroke (repolarization): once 0 mV is passed, the positive intracellular charge resists further Na+ entry and the slow-inactivation gates begin to close. The slow voltage-gated K+ channels are fully open at +30 mV and K+ leaves the cell and the internal negativity of the membrane is returned. There is a period of hyperpolarization when the voltage-gated K+ channels are not fully closed permitting additional K+ to leave — the absolute refractory period. Restoration of the resting membrane potential occurs when voltaged-gated Na+ and K+ channels are closed. The Na+/K+-ATPase continues to actively pump Na out of and K into the cell throughout the AP.
Myelinated and unmyelinated fibres
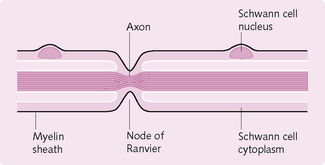
Myelin comprises proteins and lipids (i.e. fatty substances) that form a sheath around some nerves (Fig. 3.5) and increases the speed of transmission of impulses. There are roughly twice as many myelinated fibres as unmyelinated.
Myelin sheaths in the PNS are formed by Schwann cells, which envelop the naked axon and produce a cellular membrane containing the lipid substance sphingomyelin. This insulates the axon, decreases the ion flow through the membrane and consequently reduces its capacitance.
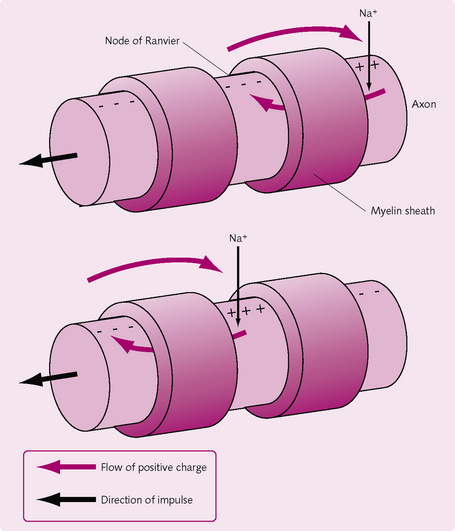
Between every two Schwann cells there is a small, exposed area of axon where ions can still flow between the axon and extracellular fluid; these are the nodes of Ranvier. APs jump from node to node by saltatory conduction (Fig. 3.6). The importance of saltatory conduction is twofold:
Demyelination
Lack of myelin can cause a number of diseases, of which multiple sclerosis and Guillain–Barré syndrome are the most important:
• Multiple sclerosis: this disease of unknown aetiology causes multiple plaques of demyelination within the brain and spinal cord. Clinical features include optic neuropathy, diplopia (double vision), vertigo (dizziness) and spastic paraparesis.
• Guillain–Barré syndrome: this is thought to have an autoallergic basis and follows 1–3 weeks after a viral infection. It is a demyelinating neuropathy that results in the patient complaining of weak distal muscles and/or distal weakness, which progress more proximally with time. It can sometimes lead to respiratory failure.
Synaptic transmission
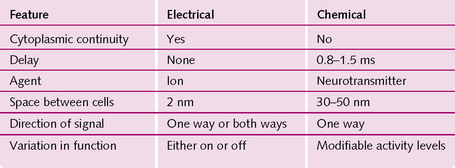
A synapse is the gap between the end of a nerve fibre and the target cell, across which nerve impulses pass. There are two types (Fig. 3.7):
1. Chemical synapse: the action potential travels along the first (presynaptic) neuron and causes release of a neurotransmitter substance at the synapse. This diffuses across the synapse to the other (postsynaptic) neuron. It acts on the postsynaptic membrane receptors, causing either their excitation or inhibition.
2. Electrical synapse: these have direct channels (usually gap junctions) that conduct ions (electricity) from one cell to another.
THE PROCESS OF NERVOUS TRANSMISSION
There are two types of transmission:
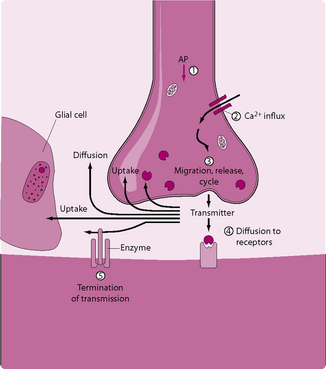
Chemical transmission (Fig. 3.8)
1. The AP spreads over the presynaptic terminal. Depolarization causes opening of voltage-gated calcium channels in the presynaptic membrane.
2. Large numbers of Ca2+ ions enter the terminal boutons of the presynaptic membrane.
3. As the result of the influx of Ca2+ ions, neurotransmitter-containing vesicles migrate towards the end of the presynaptic terminal, using an actin cytoskeleton. When they reach the synapse, they exocytose and release their contents in the synaptic cleft.
4. The postsynaptic neuronal membrane contains large numbers of receptor proteins. The neurotransmitter diffuses across the cleft and binds to these receptors. Once bound, the neurotransmitter causes a change in the postsynaptic membrane potential and can activate either iontrophic receptors or second-messenger systems.
5. Enzymes in the synaptic cleft inactivate the neurotransmitter.
Ionotrophic receptors are coupled with an ion channel can either inhibit or stimulate the postsynaptic neuron. There are two types:
1. Cation/excitatory postsynaptic potential (EPSP) ionotrophic receptors: these allow Na+ to pass into the postsynaptic neuron, causing excitation and propagation of the AP down the postsynaptic neuron.
2. Anion/inhibitory postsynaptic potential (IPSP) ionotrophic receptors: these allow Cl− to enter, which inhibits the postsynaptic neuron.
Second-messenger transmission
Ion channels close within milliseconds and so do not permit prolonged postsynaptic neural changes as is required in memory formation. The second-messenger system allows a prolonged response via second messengers such as cAMP or calmodulin.
Removal of the transmitter substance
To prevent continued action, the neurotransmitter must be removed from the synapse. This is done by:
Summation
The excitation of a single presynaptic terminal on the surface of a neuron will rarely cause an AP. This is because even though an EPSP might be generated, on its own it is insufficient to pass the threshold. Many EPSPs need to summate to reach the threshold and produce an AP. There are two mechanisms for this:
Neurotransmitters
Rapidly acting neurotransmitters
Small, rapidly acting neurotransmitters are involved in an acute response, such as the transmission of signals to the brain and motor signals back to the muscles. These neurotransmitters are synthesized in the cytosol of presynaptic terminals.
Acetylcholine: ACh is secreted in the motor cortex, basal ganglia, motor neurons innervating skeletal muscle, preganglionic neurons of the autonomic nervous system and postganglionic neurons of both the sympathetic and parasympathetic nervous systems. It can have both inhibitory and excitatory effects. Reduced ACh concentrations in certain parts of the brain are associated with Alzheimer’s disease.
Norepinephrine (noradrenaline): This is secreted by the locus caeruleus in the pons, where it is involved in the control of sleep. It is also the main neurotransmitter of ganglion cells in the sympathetic nervous system, which excite some organs (e.g. myocardium to contract stronger) and inhibit others (e.g. lung bronchi to dilate). Norepinephrine also causes constriction of peripheral blood vessels. Blockage of its removal at synapse is the mode of action of tricyclic antidepressants.
Dopamine is secreted by neurons that originate in the substantia nigra, the area of the brain that initiates movement. A lack of dopamine is responsible for Parkinson’s disease.
Amino acids: There are two types:
1. Excitatory, e.g. glutamate and aspartate: glutamate is the most common amino acid and stimulates two receptors; NMDA and AMPA. The former is thought to be involved in the formation of memory.
2. Inhibitory, e.g. GABA and glycine: GABA is secreted by nerve terminals present in the basal ganglia, cerebellum and the spinal cord. Glycine is mainly present in the spinal cord, particularly in the interneurons.
Slow-acting neuropeptide transmitters
These are larger in size than the rapidly acting transmitters and cause more prolonged responses, e.g. long-term changes in the numbers of receptors and synapses. They are manufactured in the neuronal cell body and have a broad range of functions, e.g. pain modulation by opioids.
SENSATION AND PAIN
Sensation
The term ‘modalities’ describes the different types of sensation, of which there are two:
• Somatic: provide information from sensory receptors in the skin and mucous membranes and include:
• tactile, e.g. touch, vibration and pressure
• proprioception, which tells the brain the relative positions of the body in space.
2. Special: these include smell, vision, hearing and balance. These are discussed later.
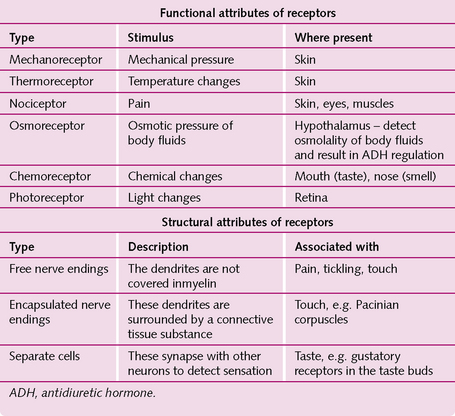
Sensory receptors (Fig. 3.9)
The different types of sensory receptor monitor particular stimuli and can be classified functionally or structurally (Fig. 3.10).
The mechanism of sensation: For example, as demonstrated by an increase in external temperature:
1. The receptor is stimulated, e.g. the increase in temperature stimulates the skin thermoreceptors.
2. The receptor converts this into a graded potential in a neuron.
3. When this reaches threshold it triggers an action potential.
4. The action potential propagates towards the CNS via afferent fibres, e.g. temperature signals travel through the spinothalamic tract.
5. Certain regions of the cerebral cortex, e.g. the hypothalamus, process the information.
6. The cerebral cortex sends signals via efferent fibres to particular regions of the body to respond to the stimulus, e.g. if the body is too hot then sympathetic tone is reduced and the blood vessels dilate passively.
Adaptation: During a continued stimulus, the generator potential decreases in amplitude causing fewer and fewer action potentials. Consequently, the perception of sensation declines. An example is when you go into your flatmate’s room and it has that pungent smell(!). After a while you can’t smell it any more. This is not because the smell has gone but because your olfactory system has adapted.
Somatic sensations
These arise from stimulation of sensory receptors in the skin and mucous membranes:
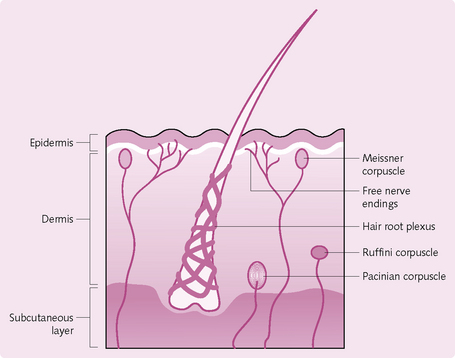
Tactile receptors: Tactile sensation includes touch, pressure, vibration, itching and tickling. It is sensed by a combination of the following receptors (the first three activate encapsulated large-diameter myelinated A fibres):
• Meissner corpuscles: comprising free nerve endings. These are egg-shaped masses in the skin dermal papillae. They sense fine touch and predominate in the finger tips, clitoris, lips, nipples and tip of the penis.
• Mechanoreceptors: e.g. Merkel discs, flattened free nerve endings in the stratum basale of skin, for fine touch sensation.
• Ruffini corpuscles: stretch receptors are present deep in dermis, ligaments and tendons.
• Hair root plexus: found in hairy skin, detect movements of hair.
• Pacinian corpuscles: large, multilayered connective tissue capsules. They are found in the dermis and subcutaneous layer, where they detect pressure.
• Free nerve endings: small-diameter, unmyelinated C fibres stimulated by chemicals such as bradykinin, for tickle and itch sensation.
Photoreceptors: Photoreceptors are light-sensitive proteins involved in the function of photoreceptor cells. An example is the rhodopsin in the retina.
Chemoreceptors: A chemoreceptor is a cell, or a group of cells, that transduces a chemical signal into an action potential. There are two types: distance and direct. An example of distance chemoreceptors are olfactory receptor neurons in the olfactory system. Examples of direct chemoreceptors include taste buds in the gustatory system and carotid bodies that detect changes in pH inside the body.
Pain
Pain serves as a protective function by alerting the body to tissue damage. Nociceptors are the receptors for pain and found almost everywhere. They are activated by thermal, mechanical or chemical stimuli.
1. Fast: occurs within 0.1 s of application of the stimulus:
2. Slow: occurs 1–2 s after application of the stimulus and persists:
Pain can be superficial somatic (in the skin), deep somatic (in deeper structures like muscles, joints and tendons) and visceral organ pain, which can occur away from the organ that is damaged, i.e. referred pain. This is due to the damaged visceral organ and the referred region being innervated by the same spinal cord segment, e.g. liver damage refers to shoulder tip. In this example, the damaged liver presses on the diaphragm, which is innervated by the C3, C4 and C5 nerve branches, which also supply sensation to the shoulder tip.
Regulation of pain
• Peripheral regulation: activity in the low-threshold mechanoreceptors can inhibit the spinothalamic nerve impulses, e.g. rubbing the area of pain.
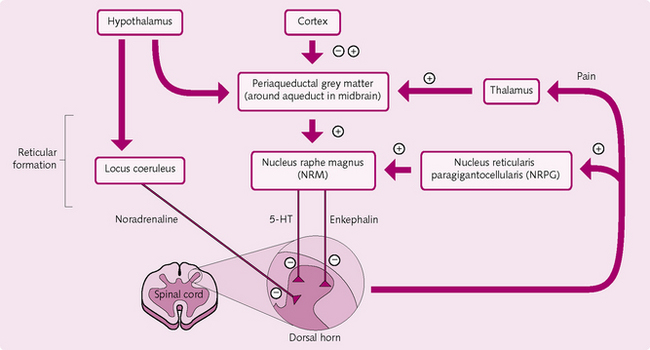
• Central regulation (Fig. 3.11): the CNS has its own pain control areas:
• periaqueductal grey areas of the midbrain and pons
• raphe magnus nucleus: located in the lower region of the pons and superior medulla
• reticular formation regions in the dorsal horn: nucleus reticularis paragigantocellularis and locus coeruleus – stimulation of opiate and 5-HT receptors in these areas can inhibit the pain pathway and cause analgesia.
Analgesia
Three classes of opioid receptor are found throughout the CNS:
Opiate-like peptides are derived from three large molecules: proenkephalin, propiomelanocortin and prodynorphin. They act on the m and d-opioid receptors in the spinal cord, brainstem and hypothalamus to block pain signals. This is endogenous analgesia.
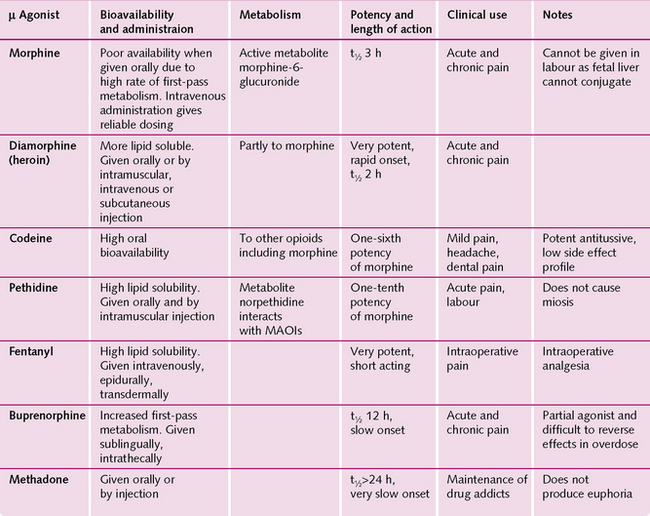
The opioid morphine mimics endogenous opioid peptides and stimulates the m-receptors, resulting in non-endogenous analgesia, euphoria, sedation and respiratory depression. All opioids have certain side-effects (Fig. 3.12):
• Respiratory depression: reducing the sensitivity of the brainstem to arterial PCO2 (PaCO2).
• Constipation: due to reduced motility of the gastrointestinal tract.
Repeated administration of morphine causes:
• Tolerance: decreased responsiveness to the drug so that more must be taken to achieve the same effect.
• Dependence: both physical (where a withdrawal causes physical symptoms, e.g. influenza) and psychological (drug-seeking behaviour).
Overdose with opiates can result in a comatose patient, respiratory depression and pin-point pupils. Treatment is with intravenous m-antagonists such as naloxone.
The brainstem
The brainstem is sandwiched between the spinal cord and the diencephalon. It consists of the medulla oblongata, pons and midbrain. It is here that the cranial nerves, and their respective nuclei, originate.
Medulla oblongata
The medulla oblongata contains three important functional regions:
1. White matter: contains all the sensory (ascending) and motor (descending) tracts:
• the pyramids are bulges of white matter consisting of large motor tracts; around 90% of these tracts decussate (cross over) to the opposite side of the body, which is the reason why the left brain controls the right side of the body and vice versa.
2. Olives: small olive-shaped structures, just lateral to the pyramids; proprioceptive impulses enter the medulla oblongata here and are then conducted to the cerebellum for processing.
3. Nuclei: regions of grey matter where neurons synapse with each other. There are many different types:
• gracile: both the gracile and cuneate nuclei are implicated in sensations of touch and in consciousness
• cuneate: is also involved in proprioception, pressure and vibration
• cranial nerve nuclei: there are between 8 and 12
• vital body function nuclei: regulate the cardiovascular, respiratory, vomiting and coughing centres.
The pons
The word ‘pons’ means bridge. This 2.5-cm structure rests superior to the medulla and connects different parts of the brain via bundles of axons, which can be part of either the ascending or the descending tract. The nuclei for cranial nerves (CN) V, VI, VII and VIII, and the nerves that relay information from the cerebellum to the cerebral cortex, are also present in the pons.
Midbrain
This extends from the pons to the diencephalon and contains:
• Cerebral peduncles: contain motor axons from the cerebrum to the spinal cord (corticospinal/pontine/bulbar) and sensory axons from the medulla to the thalamus.
• Colliculi: four elevations in the posterior part of the midbrain:
• superior × 2: some of the optic tracts enter here and regulate reflex eye movements, e.g. scanning, papillary reflex, accommodation reflex and the reflexes that occur with the eyes, head and neck with regard to visual stimuli
• inferior × 2: conduct information from the auditory pathway to the thalamus.
• Nuclei substantia nigra: darkly pigmented, neurons extend from here to the basal ganglia, where they release dopamine and regulate subconscious muscle activities. The absence of these neurons causes Parkinson’s disease.
• Red nuclei: cerebellar and cerebral neurons synapse here and regulate muscular movements.
• Cranial nerve nuclei 3 (oculomotor) and 4 (trochlear) are present here.
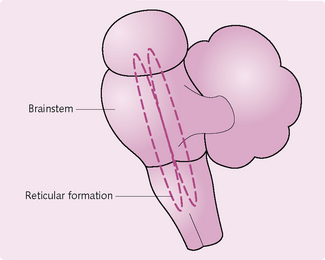
Reticular formation
The reticular formation (Fig. 3.13) is a loosely arranged network of white and grey matter in the brainstem. It has the following functions:
• Motor control: via modulation of spinal interneurons and the transmission of information to the cerebellum.
• Sensory control: exerts some control over activity in spinal reflex arcs. It is also important in the regulation of pain perception.
• Visceral control: involved in the regulation of the respiratory and cardiovascular systems.
• Control of consciousness: a certain region of sensory axons, which project to the cerebral cortex (reticular activating system), maintains consciousness. Damage to this region results in prolonged coma.
The brainstem and autonomic function
The autonomic nervous system (ANS) regulates most of the visceral body functions, e.g. vessel tone, gastrointestinal motility, sweating. The ANS is activated by centres located in the spinal cord, brainstem and hypothalamus. These structures relay signals to the particular visceral organ, eliciting a response via the two major subdivisions of the ANS:
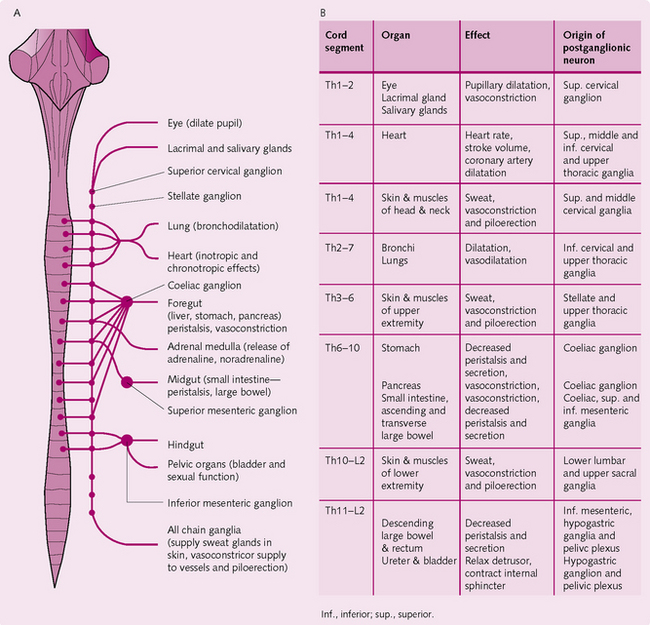
Sympathetic nervous system (Fig. 3.14): The sympathetic fibres originate in the spinal cord between cord segments T1 and L2. Each sympathetic pathway from the spinal cord to the tissues comprises two neurons, one pre- and one postganglionic. The cell body of each preganglionic neuron lies in the intermediolateral horn of the spinal cord and the fibres pass from here to the anterior cord root in the corresponding spinal nerve.
When the spinal nerve leaves the spinal canal, the sympathetic fibres pass through the white ramus into the sympathetic chain ganglia. The course of the fibre can then:
• Communicate with postganglionic neurons in the ganglion it enters.
• Move up or down in the chain and synapse with other ganglia in the chain.
• Migrate through the chain and radiate out from the chain to synapse in a peripheral sympathetic ganglion.
The level at which the sympathetic fibres exit from the chain determines which region of the body they innervate.
Some preganglionic fibres pass, without synapsing, through the intermediolateral horn cells of the spinal cord to the sympathetic chain. They then travel via the splanchnic nerves to terminate in the adrenal medullae. These fibres influence the secretion of epinephrine (adrenaline) and norepinephrine (noradrenaline).
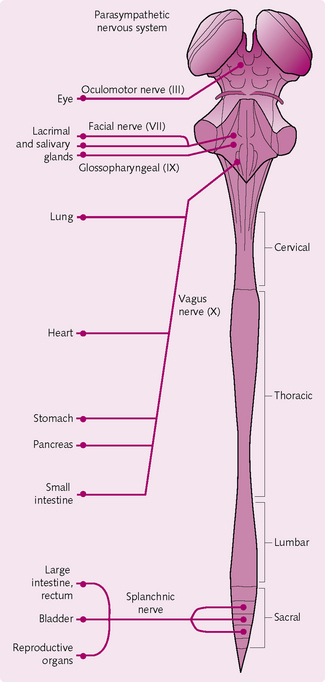
Parasympathetic nervous system (Fig. 3.15): The parasympathetic fibres leave the CNS via cranial nerves III, VII, IX and X and spinal nerves S2–S4. Around 75% of the fibres exit via cranial nerve X (the vagus nerve) and distribute to the entire thorax and abdominal regions.
As with the sympathetic system, the parasympathetic system has both pre- and postganglionic neurons. However, the majority of preganglionic fibres reach their target organ uninterrupted and the parasympathetic system thus has longer preganglionic fibres. The postganglionic neurons tend to be located in the wall of the target organ and, after synapsing with the preganglionic fibres, innervate the organ tissues.
Enteric nervous system: The neurons for this third division of the ANS are located in the walls of the gastrointestinal tract, pancreas and gall bladder. The enteric nervous system comprises two plexi:
1. Meissner’s/the submucosal plexus: which lies between the mucous membrane and circular muscle layer: This regulates gastrointestinal secretion and blood flow.
2. Auerbach’s/the myenteric plexus: which is present between the longitudinal and circular muscle layers: This controls the gastrointestinal movements.
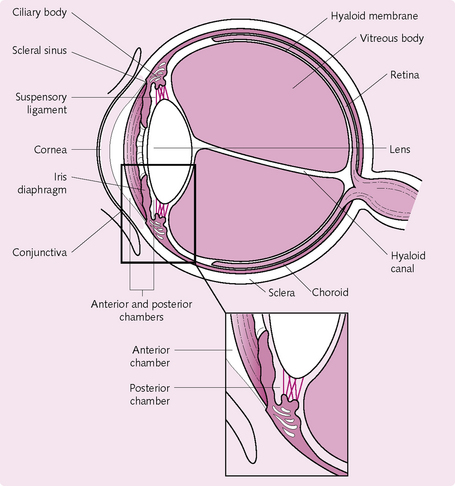
THE VISUAL SYSTEM (FIG. 3.16)
• Anterior chamber: the space between the cornea and the iris is filled with aqueous humor.
• Choroid: the vascular portion of the posterior segment of the eye; it provides nutrition to the retina.
• Ciliary body: aqueous fluid is produced here.
• Ciliary muscles: these smooth muscles attach the ciliary body to the lens via the zonular ligaments.
• Conjunctiva: lines the inner surface of the eyelids.
• Cornea: clear tissue in front of the eye. It has an epithelial surface layer; a stroma, which comprises most of the corneal tissue; a tough layer called Desçemet’s membrane and an endothelium on the inner surface.
• Iris: the coloured part of the eye. Consists of dilator and constrictor muscles and vascular tissue. The pupil is the opening in the centre of the iris.
• Lacrimal apparatus: group of structures that produces and drains lacrimal fluid (tears). The lacrimal glands secrete lacrimal fluid, which drains into the lacrimal ducts. It then enters the nasolacrimal duct and finally drains away into the nasal cavity.
• Lens capsule: a fibrous capsule over the lens.
• Lens: ball-like structure that changes shape to focus the image on the retina.
• Lids: provide protection for the eye and the glands within the eyelid.
• Limbus: where the cornea meets the sclera.
• Meibomian glands: secrete an oily substance that helps stabilize the tear film.
• Optic disc: that part of the fundus where all the nerve fibres from the retina meet to form the optic nerve.
• Optic nerve (cranial nerve II): sensory nerve that runs from the retina to various parts of the brain.
• Posterior chamber: the space between the iris and the lens; filled with aqueous fluid.
• Retina: nervous tissue that lines the posterior three-quarters of the eyeball. Photoreceptive cells in the retina convert the light image into a bioelectrical signal for the brain to understand.
• Sclera: the fibrous shell of the eye.
• Vitreous: a jelly-like material that fills the posterior segment of the eye.
• Zonular ligaments: the ligaments connecting the ciliary muscle to the perimeter of the lens, stabilizing the position of the lens.
Anatomy
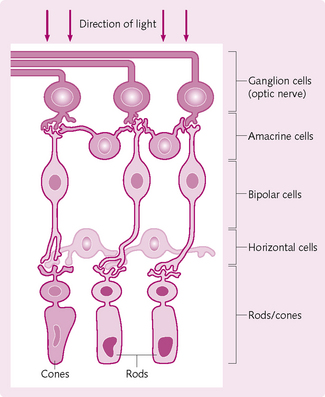
The retina forms the inner surface of the eyeball. It has two layers:
1. Deep – pigmented – layer: this melanin-coated epithelial sheet prevents the scattering of light rays and focuses them on the retina.
2. Superficial – neural – layer: processes the incoming light source and sends information to the optic nerve. This information is analysed in the brain and an image is formed. The neural layer is divided into three cell levels:
These levels (Fig. 3.17) are separated by two synaptic cell layers where synaptic contacts are made. Two other types of cell are present: horizontal and amacrine cells. These alter the signals from the photoreceptive cells, bipolar cells and ganglion cells.
Photoreceptive cells
Two cell types transduce light rays into receptor potentials:
1. Rods: contain the blue–green light-absorbing photopigment rhodopsin. Dim light stimulates the rods, enabling to see with minimal light, but not in colour.
2. Cones: react to bright light and produce colour vision. The cones contain three different types of photopigment, which absorb blue, green and yellow–orange light. These photopigments are composed of two parts: opsin (glycoprotein) and retinol (a vitamin A derivative). Each photopigment contains a different variant of opsin, which permits the absorption of different wavelengths of light and hence the different colours.
Processing visual stimuli
Seeing in the dark: Sodium ions enter the photoreceptor via ligand (cGMP)-gated Na+ channels. This depolarizes the rods and cones to about −30 mV. (Remember, to elicit an action potential the resting membrane potential must be at least −70 mV.) The −30 mV partial depolarization causes release of glutamate, which triggers IPSPs that hyperpolarize bipolar cells and prevent transmission to the ganglion cells.
Seeing when light is present and isomerization occurs: The ospin undergoes a conformational change, which reduces cGMP levels. This causes the ligand-gated Na+ channels to close and the membrane potential becomes closer to −70 mV. This hyperpolarization decreases glutamate and prevents the inhibition of the ganglion cell, which transmits a signal to the optic nerve and to the optic region of the brain where it is analysed.
Optics
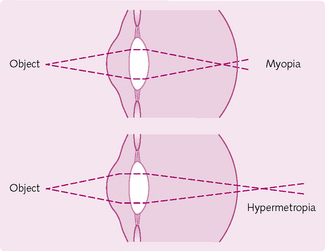
Light that enters the eye is refracted by the cornea and – to a lesser degree – by the lens so that it is focused exactly on the retina (convergence). If the rays do not meet together at the same retinal point then the image produced is unfocused (Fig 3.18). If images of near objects fall on the retina but images of far objects do not, then the person is nearsighted (myopic). Conversely, a person is farsighted (hypermetropic) if images of far objects focus on the retina but images of near images do not.
Accommodation
Objects that are very close to the eye hit the cornea at a greater angle and so must be refracted more to meet at the retina. The lens is responsible for this extra refraction. The ciliary muscles contract, causing the zonular fibres to relax. This eases the tension on the lens and it becomes shorter and broader in shape, and refracts the rays so they meet the same point on the retina.
Visual acuity
This is how precisely an image is seen: the greater the precision the higher the acuity. The area of greatest acuity in the retina is the central fovea, which contains only cones.
Clinically, visual acuity is tested by using a Snellen chart. This contains letters in decreasing size. Patients stand 20 feet away and, if they can see the letters one would normally see at 20 feet, then they are said to have 20/20 vision.
Dark and light adaptation
After a person has been sitting in a bright room for a while, much of the photochemical in the rods and cones will have been converted to retinol (which is subsequently converted to vitamin A) and opsins. This reduction in the amount of photosensitive chemical reduces the sensitivity of the eye to light even further.
Conversely, sitting in a dark room for a while results in the photochemical being converted back into light-sensitive pigments, so much dimmer levels of light can be detected.
Central visual processing
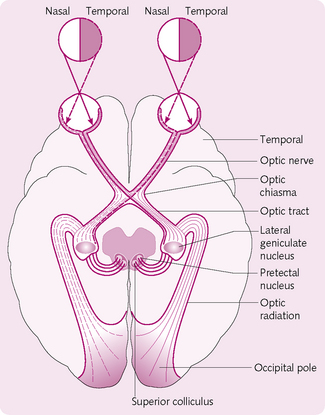
After they have left the eyes, the optic nerves pass through the optic chiasma, where some nerve fibres cross and travel to the opposite side of the brain from which the visual stimuli came (Fig. 3.19). After the chiasma, the axons become the optic tract, which ends in one of the following locations:
• Lateral geniculate nucleus (LGN): the retinal fibres terminate in six discrete layers of the LGN: ipsilateral fibres in layers 2, 3 and 5 and contralateral fibres in layers 1, 4 and 6. A given area of the retina projects to a certain level in the LGN. This organization is preserved all the way to the visual cortex.
• Pretectal nuclei and superior colliculus in the midbrain: activate the pupillary light reflex and govern rapid directional eye movements.
• Suprachiasmatic nucleus in the hypothalamus: involved with the control of circadian rhythms.
The visual cortex/Brodmann’s area cortical area 17
The primary visual cortex lies in the medial occipital cortex and contains six layers of cells arranged in millions of vertical hypercolumns, each representing a different function, e.g. orientation columns enable lines to be discriminated; ocular dominance columns enable the perception of the depth of an object; there are also colour-detecting regions. All this information is processed individually and then brought together to form an image. Although it is unclear precisely how these cells interact to form an image, the process no doubt involves excitatory and inhibitory interplay between the cells.
THE AUDITORY SYSTEM
Sound waves are changes in pressure that are transmitted through the air; sound is defined by the amplitude (loudness) and frequency (pitch) of the sound waves. Amplitude is measured in decibels (dB), which is a logarithmic scale; frequency is measured in hertz (Hz), with the normal range being between 20 and 20 000 Hz.
The auditory system, which converts sound waves into electrical signals, consists of two main areas:
The ear
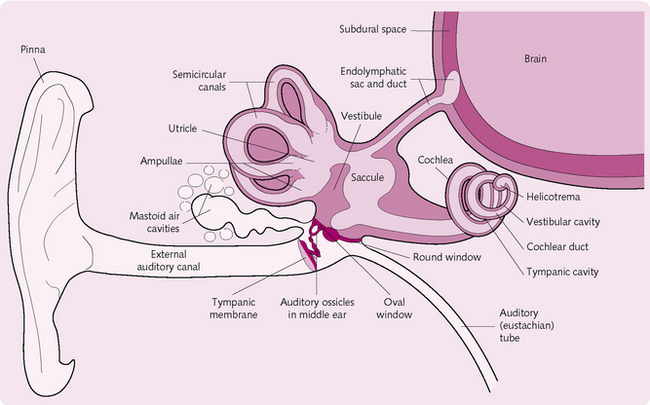
The ear is divided into three regions (Fig. 3.20).
The external ear
This consists of the pinna, external auditory canal and eardrum. It collects the sounds and transmits them to the external auditory meatus, from where they strike the eardrum (tympanic membrane).
The middle ear
This small, epithelium-lined, air-filled cavity contains the auditory ossicles. These are three small bones called the malleus, incus and stapes (or hammer, anvil and stirrup, respectively). The malleus is attached to the inside surface of the eardrum and to the incus, which articulates with the stapes. They transmit sound to the inner ear.
The muscles in the middle ear include the tensor tympani, which limits extreme movements of the eardrum to prevent excessive (and damaging) vibrations in the inner ear, and the stapedius, which attenuates large vibrations on the stapes to protect the oval window where the sound will travel.
The eustachian/pharyngotympanic/auditory tube connects the middle ear and the oropharynx. It allows pressure in the middle ear to equal atmospheric pressure. This permits sound vibrations to travel freely.
The round and oval windows are openings that separate the middle from the inner ear.
The inner ear
This contains two distinct functional divisions, the cochlea (for hearing) and the vestibule and semicircular canals (for balance); these resemble tunnels within the temporal bone. Within the canals is a series of membranous sacs (labyrinths) containing sensory epithelium and endolymph (a fluid). The canals are further surrounded by another fluid (perilymph). The content of endolymph is similar to that of intracellular fluid, whereas perilymph resembles extracellular fluid.
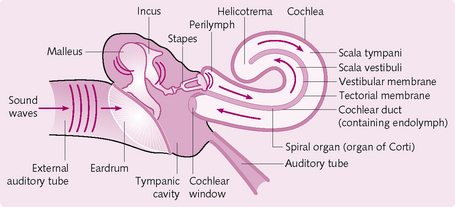
The cochlea resembles a snail’s shell. It is divided into three channels, the scala vestibuli, scala tympani and scala media/cochlear duct. The scala media contains endolymph; the others are bathed in perilymph. The floor of the scala media is formed by the basilar membrane, which contains hair cells covered with stereocilia on their surfaces. Attached to the scala media is a gelatinous structure (the tectorial membrane) which lies above the inner and outer hair cells and comes into contact with their stereocilia.
The organ of Corti is the functional unit of hearing and contains the sound receptors that convert mechanical vibrations into nerve impulses. The epithelium of the organ of Corti consists of supporting cells and hair cells. The bases of the hairs are surrounded by the afferent fibres of the cochlear nerve. The tips of the hairs protrude into the endolymph, the longest of them being embedded in the overlying, gel-like tectorial membrane.
Axons from the auditory nerve synapse at the base of the hair cells and leave the cochlear and temporal bone through the internal auditory canal to the brainstem.
Events involved in hearing (Fig. 3.21)
1. The pinna channels sound waves to the external auditory meatus.
2. The sound hits the eardrum, causing it to vibrate.
3. The vibration is transmitted from the malleus to the incus and finally to the stapes.
4. The vibrating stapes pushes the oval window membrane in and out.
5. The moving oval window causes the perilymph in the cochlea to form a wave, which travels through the cochlea.
6. The fluid thrill passes the basilar membrane, causing it to move in a wave-like pattern that travels the length of the cochlea and dissipates at the round window.
7. As the basilar membrane is displaced by the perilymph wave, the stereocilia at the apex of each inner and outer hair cell move.
8. Mechanical movement can open or close cation channels in the stereocilia. Opening results in an inward K+ current, graded depolarization and the release of a neurotransmitter (glutamate) that causes the cochlear fibres to transmit a faster stream of impulses to the brain.
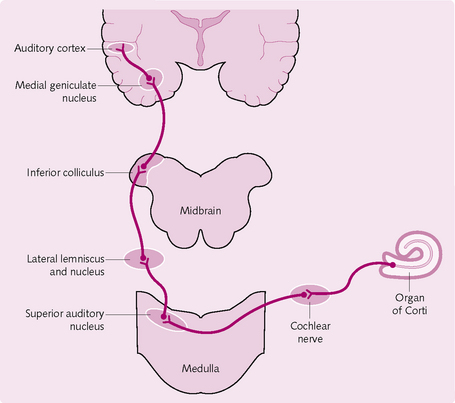
Central auditory processing: the neural pathway
• Nerve fibres from the organ of Corti enter the cochlear nuclei in the medulla oblongata (Fig. 3.22).
• Here they synapse with second-order neurons and the majority travel to the opposite side of the brain (a minority stay ipsilateral), where they terminate in the superior olivary nucleus (SON).
• The fibres then pass to the lateral lemniscus. Some terminate in this nucleus, but many pass through and synapse in the inferior colliculus.
• They then travel to and synapse in the medial geniculate nucleus. Finally, the pathway continues as the auditory radiation, finally ending in the auditory cortex.
The auditory cortex
The auditory cortex is located in the superior temporal gyrus of the temporal lobe. It is divided into two separate areas: primary auditory cortex (PAC) and secondary auditory cortex (SAC). The PAC is stimulated by impulses form the medial geniculate body; the SAC receives impulses from the PAC.
Approximately six tonotopic maps have been found in both association cortices. An association cortex is part of the cerebral cortex involved with advanced steps of sensory information processing. Tonotopic maps are associative areas of the brain that performs a topology preserving mapping of acoustic frequencies on the auditory cortex. Each map discriminates certain characteristics of sounds. For instance, one distinguishes sound frequencies, another tells you where the sound is coming from and so on.
THE OLFACTORY SYSTEM
The sensations of smell and taste arise from interactions between certain molecules and specific receptors. Signals are then sent to the limbic and other higher cortical areas, which analyse the incoming information.
Anatomy of the olfactory system
The epithelial lining of the nasal cavity contains three types of cell:
Physiology of the olfactory system
Odours enter the nasal cavity and stimulate the nasal cilia on the olfactory receptor. This is linked to a second-messenger system that opens Na+ channels. The influx of Na+ results in depolarization and a subsequent action potential. This propagates along the olfactory nerve (CN I), which terminates below the frontal lobes of the cerebrum and lateral to the crista galli in olfactory bulbs. Within these bulbs, the olfactory receptors synapse with second-order neurons to form the olfactory tract. This projects to the lateral olfactory area in the temporal lobe. Further connections exist between this lateral olfactory area to the hypothalamus, thalamus, limbic system and frontal lobe. Thus smell excites many areas of the brain, which explains why some smells can evoke memories and others can cause nausea.
THE GUSTATORY SYSTEM
Anatomy of the gustatory system
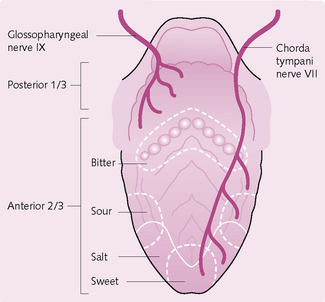
The receptors for this system are the taste buds, of which there are approximately 10 000. They predominate on the epithelial surface of the tongue, soft palate, pharynx and epiglottis. On the tongue they are found in three types of papillae: circumvallate, fungiform and foliate. They detect four modalities; salt, sour, bitter and sweet. Each taste bud contains three types of cell:
Physiology of the gustatory system
Chemicals (e.g. food) make contact with the microvillus on the GRC and result in depolarization with the release of a neurotransmitter substance (Fig. 3.23). The signal for depolarization varies for each taste modality:
• Saltiness: Na+ from salty food enters the GRC via Na+ channels. This causes the influx of Ca2+, which results in the release of neurotransmitter.
• Sourness: H+ from acid in the sour substance either enters Na+ channels or blocks K+ channels, causing depolarization.
• Sweetness: molecules bind to a receptor site coupled with a G protein. This causes an increase in cAMP, which activates protein kinase A. This blocks K+ channels, causing depolarization.
• Bitterness: molecules either block K+ channels directly or act via second messengers to cause depolarization.
Once the neurotransmitter is released it stimulates the first-order neurons in the gustatory pathway. These include CN VII (which serves the anterior two-thirds of the tongue), CN IX (serving the posterior third of the tongue) and CN X (the pharynx and epiglottis).
The afferent neurons enter the medulla, where they synapse in the gustatory nucleus. They then propagate to the thalamus, hypothalamus and limbic system. From the thalamus, some project to the parietal lobe, which gives the conscious perception of taste.
 Objectives
Objectives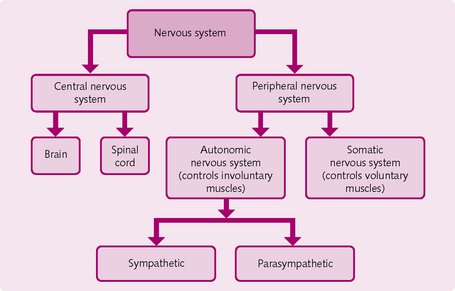
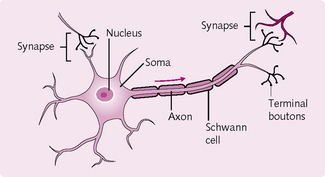
 The four main regions of the neuron are the: soma, dendrites, axon and terminal boutons.
The four main regions of the neuron are the: soma, dendrites, axon and terminal boutons.