Physiology of the musculoskeletal system
In this chapter, you will learn to:
• Describe the basic structure of the skeleton
• Discuss the structure and function of the different types of muscles
• Explain the mechanism of muscle contraction
• Discuss the different sources of energy used in muscle contraction
• Outline the central and peripheral control of the musculoskeletal system
• Describe the structure of long bones and the different types of cells involved in bone remodelling
OVERVIEW OF THE MUSCULOSKELETAL SYSTEM
The musculoskeletal system has many important functions, which are facilitated by its structure.
Structure
The musculoskeletal system consists of muscles, skeleton, joints and connective tissue.
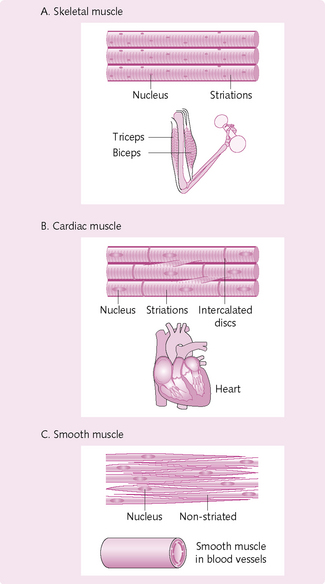
Muscle types
The three different types of muscle have the following features (Fig. 4.1):
The skeleton
The skeleton has four components:
Cartilage: This is a semi-rigid form of connective tissue and is present at points of mobility, e.g. costal cartilage, which attaches the ribs to the sternum.
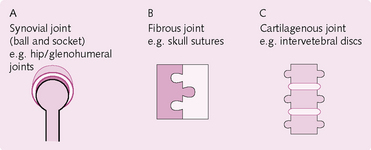
Joints: A joint is the point of union between two or more bones. The three main types (Fig. 4.2) of joint are classified according to the material used to adjoin the bones:
1. Synovial: the most common type of joint, which permits free movement between the adjoining bones. Synovial joints are united by an articular capsule, e.g. knee, elbow, hip, shoulder.
2. Fibrous joints: these are united by fibrous tissue. Movement is usually restricted, e.g. skull sutures, interosseous membrane between radius/ulna and tibia/fibula.
3. Cartilaginous joints: these are united by hyaline or fibrocartilage, e.g. intervertebral discs.
SKELETAL MUSCLE: STRUCTURE AND FUNCTION
Three types of muscle are present in the body: skeletal, cardiac and smooth. Cardiac and smooth muscle are discussed in Chapter 5. The following text relates to skeletal muscle.
Microstructure
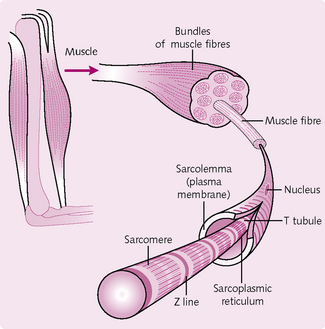
Skeletal muscle consists of a number of individual muscle fibres grouped together in bundles called fasciculi (Fig. 4.3). These are bounded by a coat of connective tissue called the epimysium. Connective tissue is also present in the muscle body which transfers the mechanical force generated by the muscle to the skeleton.
Skeletal muscle fibres
Long, thin cylindrical cells containing many nuclei. They are up to 30 cm in length and 10–100 mm in diameter.
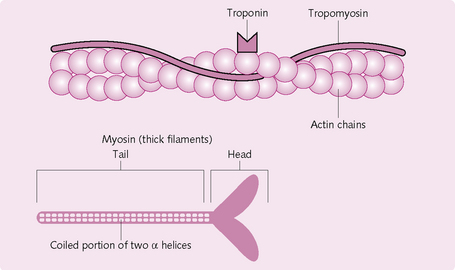
Myofibrils: Filamentous bundles found in individual muscle fibres; they run along the entire length of the fibre. Myofibrils consist of a system of longitudinal thin and thick filaments arranged in a regular pattern. The thin filaments consist mainly of the protein actin, which is arranged spirally along the filamentous protein tropomyosin. At regular intervals (40 nm) there is a regulatory protein complex made up of troponins C, I and T. Thick filaments consist mainly of the protein myosin. These too are arranged in a regular manner, with each thick filament surrounded by six thin filaments.
The overlap of thick and thin filaments gives the muscle its striated appearance. The A band (dark in colour) contains myosin filaments overlapping actin filaments. It has a high reflective index under light microscopy and can refract polarized light (i.e. it is anisotropic). The I band (light in colour) contains only actin filaments and has a lower refractive index (isotropic). In the centre of each I band is a Z line, a disc of material running across the muscle fibre and joining one myofibril to another.
Sarcoplasmic reticulum: This is the muscle fibre’s equivalent of endoplasmic reticulum. The sarcoplasmic reticulum (SR) runs longitudinally along the myofibrils and wraps around groups of myofibrils.
Sarcolemma: The cell membrane surrounding the muscle cell. At each Z line, the sarcolemma invaginates to form a T tubule. Where the T tubule and SR meet, the SR enlarges to form the terminal cisternae. A triad is a T tubule sandwiched between two SRs. This plays an important role in excitation–contraction coupling.
Ion balance and the resting membrane potential
The ionic balance between the intracellular fluid (ICF) and extracellular fluid (ECF) (Fig. 4.4) of a muscle cell is maintained by:
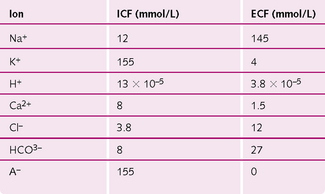
Fig. 4.4 Distribution of ions in the intracellular (ICF) and extracellular fluid (ECF) of muscle cells.
At rest, the ionic difference across the sarcolemma creates a resting potential of −90 mV. This difference in charge is maintained by a Na+/K+-ATPase, which moves two K+ into the cell and three Na+ ions out, thus creating a net negative charge inside the cell.
Neuromuscular junction
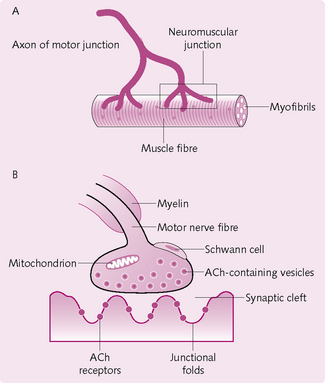
The skeletal muscle fibres are innervated by large, myelinated nerve fibres that arise from the anterior horns of the spinal cord. The nerve fibres branch and, when they reach the muscle fibre, lose their myelin sheath and end in a small swelling (terminal bouton). This nerve terminal together with the underlying membrane on the target cell (i.e. skeletal muscle cell) constitute the neuromuscular junction (NMJ; Fig. 4.5). The space between the two is the synaptic cleft. Each muscle fibre has only one NMJ.
Presynaptic nerve terminal/terminal bouton
• Synaptic vesicles: these contain the neurotransmitter acetylcholine (ACh).
• Mitochondria: ATP is required to power the action potential and to synthesize ACh.
• Active zones: these are the sites of neurotransmitter release. They lie opposite a junctional fold in the postsynaptic membrane.
• Voltage-activated Ca2+ channels: these are adjacent to active zones and open in response to an AP. Ca2+ then enters and causes the vesicles to migrate to the active zones and release the neurotransmitter.
Postsynaptic membrane
The membrane on the other side of the synapse – the postsynaptic membrane – contains:
• Acetylcholine receptors (AChR): the binding of two ACh molecules to an AChR induces a conformational change in the receptor, which results in the opening of the voltage-gated Na+ channels.
• Voltage-gated Na+ channels: located throughout the junctional folds and in membranes adjacent to the NMJ. They open when the neurotransmitter ACh binds to AChR.
• Acetylcholinesterase: the enzyme that breaks down ACh into acetate and choline. It is found in the junctional folds.
Transmission across the synapse
1. Nerve impulse reaches the NMJ.
2. Voltage-gated Ca2+ channels open and Ca2+ diffuses into the terminal bouton.
3. The Ca2+ attracts the ACh vesicles to the neural membrane.
4. The vesicles fuse with the neural membrane and empty their ACh into the synaptic cleft.
5. The binding of two molecules of ACh to an AChR causes the channel in the receptor to open.
6. Na+ enters the channel and creates a potential change at the muscle membrane, called the end-plate potential (EPP).
7. This EPP initiates an AP at the muscle membrane and causes muscle contraction.
8. The ACh in the synaptic space continues to activate the AChR, so when muscular contraction is no longer required the ACh must be removed. This is done by two means:
• acetylcholinesterase is attached to the basal lamina; this enzyme breaks ACh into acetic acid and choline
• a small amount of ACh diffuses out of the synaptic space and is broken down by plasma cholinesterases.
9. Recycled choline reacts with acetyl CoA from the mitochondria and, after catalysis with acetyltransferase, is reformed as ACh and packaged into vesicles in the terminal bouton.
Excitation–contraction coupling
The skeletal muscle fibre is so large that an AP spreading along its surface membrane causes almost no current flow deep within the fibre. To overcome this, transverse tubules (T tubules) penetrate the muscle fibre and transmit the AP. In response to an AP, the sarcoplasmic reticulum in the myofibrils releases Ca2+, which causes the muscle fibre to contract. This is known as excitation–contraction coupling.
SKELETAL MUSCLE: CONTRACTION
Myosin
Myosin comprises six polypeptide chains, two of which are heavy and the remaining four light. The heavy chains intertwine to form a double helix, one end of which is folded into a globular structure known as a head; the other end is the tail (Fig. 4.6).
The four light chains attach to the myosin head and control function during muscle contraction. The head also has ATPase activity, which is required to cleave ATP to provide the energy required in contraction.
Each myosin filament is made up of 200 or more individual myosin molecules, which are bundled together. The bundled tails form the body, part of the helix forms the arm, which has the head attached to it. The protruding tail and head form the cross-bridges with the actin filament.
Actin filament
Each actin filament consists of three protein components: actin, tropomyosin and troponin.
Actin: Double-stranded F-actin comprises the backbone of the actin filament. Each F strand is made up of G-actin molecules. Attached to these is one molecule of ADP, which is the attachment site for the myosin cross-bridges.
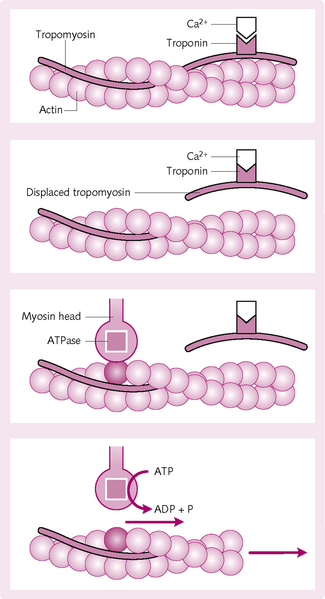
Mechanism of contraction (Fig. 4.7)
1. Ca2+ ions combine with troponin C.
2. The combination of Ca2+ and troponin C causes a conformational change in the troponin complex.
3. This displaces the tropomyosin from its resting position over the actin active sites.
4. The myosin heads from the cross-bridges attach to the actin active site.
5. ATP is hydrolysed by the ATPase on the myosin head to ADP. The energy released causes the head to tilt (power stroke) and drag the actin filament with it.
6. The cycle then repeats, with the head breaking away from the active actin site and attaching to a new site further along.
7. The cycles continue until the actin filament pulls the Z membrane up against the ends of the myosin filaments or until the load on the muscle becomes too great for further pulling to occur.
Relaxation
During AP repolarization, Ca2+ is actively pumped back into the SR. This causes the tropomyosin to obstruct the myosin binding sites on the actin myosin heads, so the actin and myosin filaments cannot attach. The filaments then slide back to their original position, passively and without the expenditure of energy.
Bioenergetics of muscle contraction
The mechanism of muscle contraction requires energy for:
• The power stroke, which is the actual muscle contraction.
• Pumping Ca2+ from the sarcoplasm into the SR when contraction is over.
• Pumping Na+ and K+ ions through the muscle fibre membrane to maintain an appropriate ionic environment for the propagation of action potentials.
Sources of energy
• Phosphocreatine: carries a high-energy phosphate bond similar to that of ATP. It can be used to phosphorylate ADP to ATP by the enzyme creatine kinase.
• Glycogen: is stored in muscle cells. Rapid enzymatic breakdown to pyruvic acid and lactic acid liberates energy that converts ADP to ATP. There are two advantages to this mechanism:
• it can occur even in the absence of oxygen, although only for about 1 minute, as there would be too much of a build-up of lactic acid to go on longer than this
• ATP formation is 2.5 times greater via glycolysis than when foodstuffs react with oxygen.
• Oxidative metabolism: combining oxygen with various foodstuffs (fats, carbohydrates and proteins) liberates ATP. Around 95% of all energy derived is from this source.
MUSCLE FUNCTION AND MOTOR CONTROL
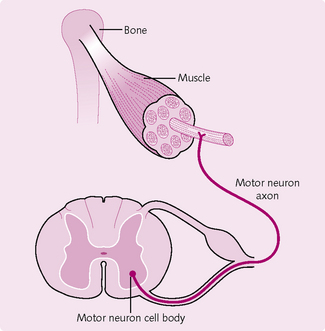
A motor unit consists of a group of muscle fibres and the motor neuron that innervates them. Each muscle fibre is innervated by a single motor neuron (Fig. 4.8). However, the axons of each motor neuron can branch and innervate many muscle fibres. The number of muscle fibres innervated by a single motor neuron axon is functionally important. All the muscle fibres in a single motor unit contract and relax together. Muscles requiring fine control, such as the laryngeal, therefore have fewer muscle fibres per motor neuron. The opposite is true for muscle involved in gross movements. This motor unit: muscle fibre ratio also dictates the strength of contraction.
Muscle mechanics
There are two types of muscle contraction (Fig. 4.9):
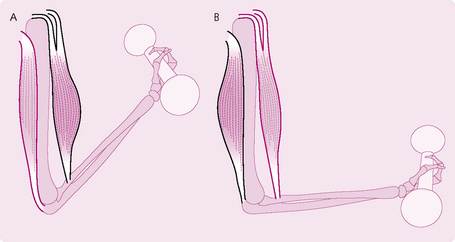
Fig. 4.9 Types of muscle contraction (A) isotonic (B) isometric. In isotonic contraction the muscle contracts and either shortens or lengthens. In isometric contraction, the muscle neither shortens or lengthens, but it does contact.
1. Isometric: the muscle does not shorten during contraction. An example would be if you held a dumb-bell steady with your arm outstretched. These movements are important in maintaining posture.
2. Isotonic: the muscle shortens during contraction, while the muscle tension remains constant. This is divided into:
Maintaining muscle contraction
Twitch contraction: This refers to a single contraction from a single AP. As this is insufficient to cause any useful movement, summation occurs to add together the twitches and cause contraction. There are two types of summation (Fig. 4.10):
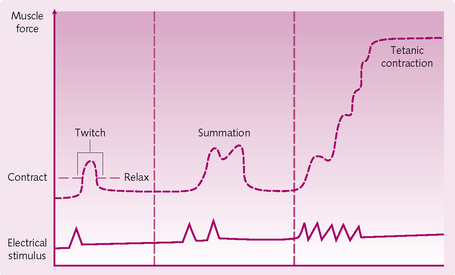
Fig. 4.10 Maintaining muscle contractions. (From Sherwood L, Human Physiology: From Cells to Systems, Brooks/Cole Pub Co., 2001).
1. Multiple fibre summation: this is the increase in the number of motor units contracting simultaneously to produce sustained contraction.
2. Frequency summation/tetanization: initially, twitches follow each other sequentially at low frequency. The frequency of stimulation increases until the twitches overlap and contraction occurs. However, there is still time between contractions for partial relaxation (unfused tetanization). When a critical period is reached the repeated contractions merge with each other appearing smooth and continuous with no relaxation in between.
Length–tension relationship
The force, or tension, of muscle contraction depends on the length of the sarcomeres before contraction. There is an optimal range of sarcomere length at which the force of contraction is at its maximum. To understand this, think about the structure of muscle: if muscle is overstretched, the sarcomere will overstretch. This will minimize the overlap between the actin and myosin heads, thus reducing tension. Similarly, if the sarcomere is shortened, the actin–myosin interactions decline and, again, this reduces contraction. This principle is important in heart failure, in which the chambers of the heart (i.e. the myocardium) enlarge.
The majority of resting muscle fibres are held very close to their optimum length via attachments to bones and tendons. This ensures no overstretching occurs.
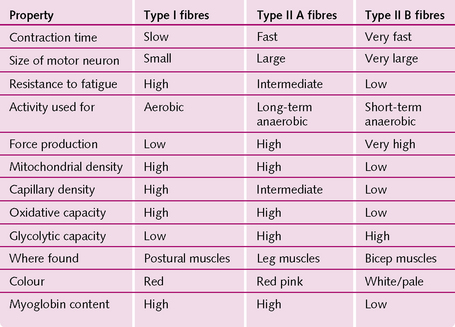
Types of muscle fibre
Most mammals have three types of muscle – fast (fast is divided into fast and very fast) and slow – which relate to their speed of contraction. The extraocular muscles are the fastest, followed by the jaw (40 ms) and those in the hand and feet (50 ms). The slowest are the gastrocnemius (100 ms) and soleus (120 ms).
Slow muscle has a greater density of capillaries, higher resting blood flow, is red in colour because of a high myoglobin content, and is more resistant to fatigue than fast muscle. Fast muscle is predominately white in colour, with less myoglobin but more of the glycogen and enzymes responsible for glycolysis and anaerobic metabolism. Fast muscle is capable of intense activity but fatigues rapidly (Fig. 4.11).
Muscle plasticity
This refers to the continuous remodelling of muscles in the body to match function. Many different factors can be adjusted, such as the muscle length, diameter, vascular supply and strength. To a small degree, fibre types can even be altered:
• Hypertrophy: when the total mass of the muscle increases through the enlargement of pre-existing cells. The main cause of this is continuous maximal contraction of the muscle, as in weight training.
• Hyperplasia: an increase in muscle mass through an increase in the actual number of muscle cells. The main cause is the splitting of the muscle fibres. It is much less common than hypertrophy.
• Atrophy: a lack of muscle stimulation, e.g. nerve denervation or muscle disuse causes a reduction in the fibre size.
Central control of movement
Central control of movement is achieved by three key regions in the brain: motor cortex, basal ganglia and cerebellum.
Motor cortex
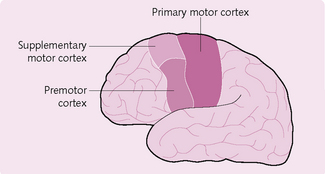
Anatomically, the motor cortex is divided into three main areas (Fig. 4.12):
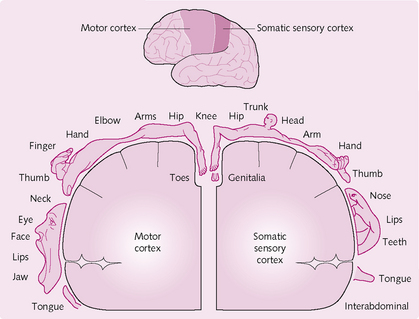
Primary motor cortex: This lies in the frontal lobe (Fig. 4.13) and is involved in the planning and ongoing control of voluntary movements requiring the coordination of several muscles. It projects to all contralateral body motor neurons and its innervation is represented by a motor homunculus, which demonstrates how some regions are generously supplied by motor neurons (e.g. lips, finger tips) whereas others (e.g. knee) are not. The primary motor cortex receives inputs from the supplementary cortex, premotor area, cerebellum and somatosensory cortex.
Supplementary cortex: This lies anterior to the primary motor cortex and projects to distal muscles. It is involved in programming motor sequences and bimanual coordination. Input is received from the basal ganglia via the thalamus, and also from the contralateral supplementary motor area.
Premotor cortex: This projects to the brainstem, reticulospinal and corticospinal tracts. It has a role in getting the body into to the right initial posture before movement. It also receives input from the cerebellum and basal ganglia via the thalamus, and from the primary sensory and visual cortices.
Basal ganglia
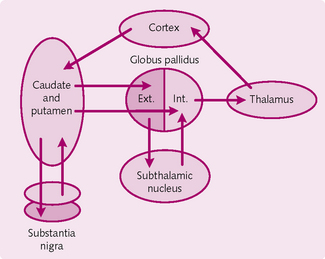
The basal ganglia comprise five structures on either side of the brain: caudate nucleus, putamen, globus pallidus, subthalamic nucleus and substantia nigra. The ganglia have the following functions:
• Regulation of complex/skilled patterns of motor activity in association with the corticospinal system, e.g. writing numbers sequentially, suturing a wound during surgery.
• Cognitive control of motor pattern sequences, e.g. seeing and recognizing that something is imminently dangerous and running away from it.
• Control of the timing and magnitude of movements: for example writing the word ‘physiology’ slowly or quickly; or even writing it in large and small letters. For both examples, the proportion of the letters will be the same.
• Saccadic eye movements via connections to the frontal eye fields.
Circuits of the basal ganglia (Fig. 4.14): Collectively, the caudate and putamen are known as the striatum. They form the main input complex of the basal ganglia, containing identical cell types and receiving input from the motor, sensory, thalamus and limbic areas. They project to the globus pallidus and substantia nigra. The caudate regulates eye movements and cognition, whereas the putamen is concerned with preceding or anticipating body movements.
The globus pallidus is separated into two regions: internal (GPi) and external (GPe). This helps regulate muscle tone for specific bodily actions.
The subthalamic nucleus receives inputs from and projects to the GPe. It also has an excitatory output to the GPi.
Neurons from the substantia nigra release dopamine, which helps regulate subconscious activity in the muscles. An absence of these substantia nigra cells leads to Parkinson’s disease.
Cerebellum
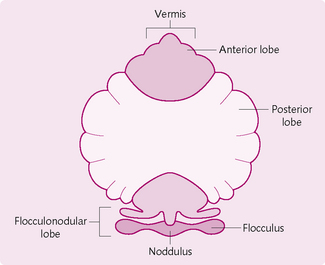
The cerebellum (Fig. 4.15) is connected to the brainstem on either side by the superior, middle and inferior peduncles. Functionally, it has three parts:
1. Flocculonodular lobe/vestibulocerebellum: involved in equilibrium, posture and eye movements.
2. Spinocerebellum (vermis + paravermis): projects to the brainstem and regulates axial and proximal limb muscles.
3. Lateral part of the cerebellum/neocerebellum: interacts with the motor cortex to plan and programme movements.
Organization can be separated into input axons, processing interneurons and output neurons.
Input axons: These comprise the mossy and climbing fibres. Excitatory neurons send collaterals to the deep nuclei and pass to the cortex:
• Granule cells: receive input from the mossy fibres and innervate the Purkinje cells. Their long axons are known as parallel fibres.
• Golgi cells: inhibitory interneurons located in the granular layer. Receive input from the parallel and mossy fibres and Purkinje cells. Axons output to the dendritic cells.
• Basket cells: inhibitory interneurons: receive signals from the parallel fibres and project to many Purkinje cells.
• Stellate cells: similar to basket cells but are located more superficially.
Peripheral motor control
Proprioceptors allow us to know the position of our head and limbs in space even with our eyes closed. These are present in tendons and muscles and give us an indication to the extent of the muscle contraction. There are two main types of proprioceptor found in the musculoskeletal system: muscle spindles and Golgi tendon organs (GTO).
Muscle spindles
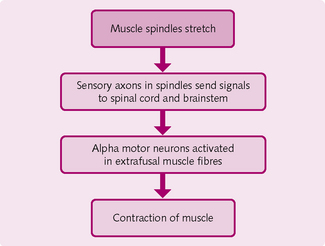
These spindle-shaped organs lie parallel to the skeletal muscle fibres. They consist of intrafusal (within a spindle) muscle fibres surrounded by sensory nerve endings. This structure is then enclosed by a connective tissue capsule and attaches the spindle via the endomysium and perimysium. They are more concentrated in muscles that produce fine movements, such as the ocular muscles.
Muscle spindles measure the extent of muscle stretch (Fig. 4.16). They stimulate the attached sensory endings, which propagate to the cerebral cortex sensory areas and cerebellum. In the former region the incoming information tells the body where in space the moved part is. Information sent to the cerebellum is used to coordinate the contraction of muscle.
At the end of the intrafusal fibres are gamma (g) motor neurons. The brain regulates the sensitivity of the muscle spindles through these nerves.
Extrafusal muscle fibres surround the muscle spindle. These are innervated by alpha (a) motor neurons, which are large-diameter A fibres. It is via these that the activation of the muscle spindles causes the muscle to contract.
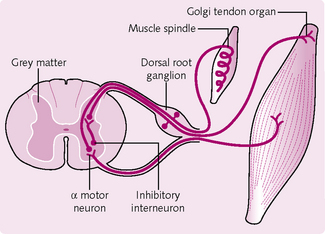
Golgi tendon organs
These are present at the muscle–tendon junction (Fig. 4.17). They comprise bundles of collagen fibres encapsulated by a connective tissue layer. Sensory nerve endings enter the capsule and wrap around the collagen fibres. The GTOs protect the tendons and muscles from damaging excessive tension by tendon reflexes. These reflexes cause the muscle to relax and so decrease the tension on the muscle.
Posture
This describes the relative positions of the trunk, head and limbs in space. The skeleton cannot stand straight without the support of coordinated muscle activity. These muscles are controlled by higher centres and reflex pathways, such as the stretch and crossed extensor reflex.
Balance is an important component of posture. Humans are relatively tall and balance on a small base represented by the feet. The centre of gravity is just above the pelvis. To maintain stability, the centre of gravity must be within the base of support. If the centre of gravity moves out of the base of support then one will fall over. Postural reflexes operate to counteract this.
Crossed extensor reflex
When one leg flexes and lifts off the ground, the opposite leg extends more strongly to support the shifted weight of the body. The centre of gravity is shifted so it remains over the base of support.
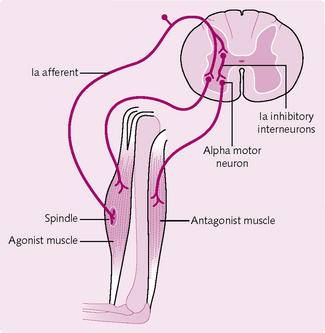
Stretch reflex
The muscle spindle stretches when the muscle does (Fig. 4.18), monitors the change in length and signals to the CNS. This instigates the stretch reflex, which attempts to resist the change in muscle length by causing the stretched muscle to contract.
The eyes, vestibular apparatus and somatic receptors provide afferent information for the postural reflexes. They send signals to the brainstem and spinal cord, which initiate muscular contraction via the a motor neurons.
Vestibular system
Changes in head position and acceleration are detected by the vestibular system. Afferent nerves from the neck muscles and cervical vertebrae direct impulses towards the vestibular nuclei, which can influence antigravity and axial musculature via the spinal cord. Eye movements are also initiated to help maintain balance.
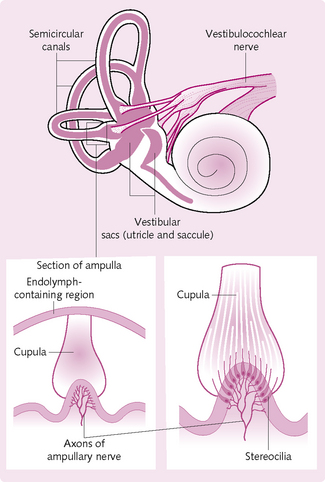
Receptor system/vestibular apparatus
This consists of the three semicircular canals and the two sac-like swellings called the utricle and saccule, all of which lie within the temporal bone.
Semicircular canals
The semicircular canals (Fig. 4.19) contain the semicircular ducts, which detect angular acceleration during rotation of the head along three perpendicular axes. Receptor cells in the ducts contain hair-like stereocilia, which are enclosed by a cupula comprising gelatinous-like material in the dilated portion of the duct (the ampulla). During head movement, the semicircular ducts and hair cells move along with it. The endolymph within the ampulla lags behind and causes the stereocilia to bend. This generates a receptor potential, which propagates along the vestibulocochlear cranial nerve (CN VIII).
Utricle and saccule
These structures provide information about linear acceleration and changes in head position relative to gravity. They contain stereocilia-covered receptors covered by a gelatinous material in which small stones (otoliths) are embedded. These otoliths move during movement and disrupt the stereocilia, causing a receptor potential signalling to CN VII.
Vestibular nuclei
The vestibular nuclei on the floor of the fourth ventricle and medulla receive input from the CN VIII. They then project to limb muscles via the medial and lateral vestibulospinal tracts. They also connect to the thalamus, cerebellum, contralateral vestibular nuclei and cranial nerve nuclei 3, 4 and 6. These help maintain eye position in the presence of head movement.
BONE
The skeletal system is usually divided into axial skeleton and appendicular skeleton. The axial skeleton includes the bones that form the axis of the body, such as skull, vertebrae, sternum and ribs. The appendicular skeleton includes the bones that form appendages of the body, such as femur, fibula, tibia, humerus, radius, ulna and phalanges.
Classification of bone
Bones are classified by their shape:
• Long bones: are longer than they are wide. Consist of a shaft (diaphysis) and variable number of ends (epiphyses). The ends are composed of spongy bone surrounded by a thin layer of compact bone. They are usually curved so as to absorb the stress of the body. Examples: femur, radius, ulna, tibia, fibula.
• Short bones: length and width are similar. Mainly cancellous bone surrounded by a layer of compact bone covered by periosteum. Hyaline cartilage covers the articulating surfaces. Examples: carpal bones (except pisiform), tarsal (except calcaneus).
• Irregular bones: cancellous bone covered with thin compact bone. Examples: vertebrae and sphenoid.
• Sesamoid bones: shaped like a sesame seed. These bones develop in close proximity to tendons where there is a lot of stress and friction. They protect the tendons from excessive wear and tear and also change the direction of pull of a tendon. Examples: quadriceps femoris and patella.
• Sutural bones: not classified by shape but by location. Located within joints called sutures. Examples: cranial, sagittal, coronal, occipital sutures.
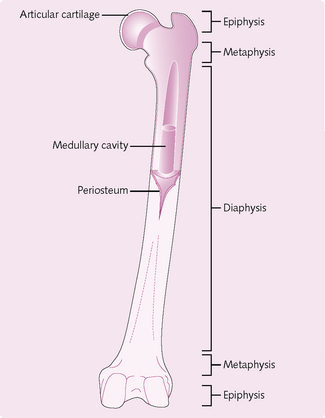
Structure of long bones (Fig. 4.20)
• Epiphysis: distal ends of the shaft.
• Metaphysis: mature bone – joins the diaphysis and epiphysis.
• Articular cartilage: thin layer of hyaline cartilage covering the epiphysis where the bones in the joint articulate. Reduces friction and acts as a shock absorber.
• Periosteum: surrounds that part of the bone that is not covered by non-articular cartilage. Contains bone-forming cells that allow the bone to thicken. Protects and nourishes bone. Provides attachment site for ligaments and helps in fracture repair.
• Medullary cavity: contains bone marrow in adults. Lined by a thin, single layer of bone cells and connective tissue (endosteum).
Histology of bone
Bones consists of a matrix surrounded by cells. The matrix is constituted of:
The salts are mainly hydroxyapatite (calcium phosphate and calcium carbonate). Also present are magnesium hydroxide, fluoride and sulphate. These salts are deposited in the matrix, crystallize and harden to form bone (calcification).
Bone formation, growth and remodelling
All bones are derived from mesenchyme. They then ossify via one of two methods: intramembranous and endochondral ossification (see below). Following ossification, the immature bone is continually remodelled by osteoclasts and osteoblasts until it matures. Hormones regulate these mechanisms.
Ossification
This is the conversion of fibrous tissue or cartilage into bone.
• Mesenchymal cells migrate to the site of eventual bone formation.
• The cells condense, align and secrete an organic framework of extracellular matrix (ECM), i.e. the osteoid (or ground substance).
• Osteoblasts (differentiated mesenchymal cells) line the osteoid and begin to deposit calcium salts (bone matrix).
• The bone matrix is now a mixture of organic ECM and the inorganic salt component of the developing bone; the mineralized organic strands are termed trabeculae.
• Both the matrix and the trabeculae have the properties of strength and flexibility.
• As the trabeculae increase in thickness, consecutive growth rings called lamellae are seen.
• Cycles of osteoid secretion and mineralization by the mesenchymal cells and osteoblasts add to the lamellae.
• A lattice structure forms when multiple trabeculae within the developing bone contact one another.
• Bones that do not completely fill-in and contain lattice structures are called primary cancellous bones; bones that fill-in are called compact bones. Most – but not all – bones are a mixture of the two, containing a compact outer surface and a cancellous interior.
• Mesenchymal cells migrate to the site of eventual bone formation and transform into chondrocytes.
• These produce cartilage, which takes the shape of the ensuing bone.
• Chondrocytes secrete a loose ECM comprising collagen and mucopolysaccharides.
• The chondrocytes are prised apart and become encapsulated.
• The cartilage is also surrounded by a layer of connective tissue cells (the perichondrium) that is also derived from mesenchyme.
• Within the body of cartilage, the encapsulated cells die and the matrix erodes.
• At this point, the cartilage begins to be replaced with bone.
• Blood vessels invade the cartilage.
• The outer layer of mesenchyme cells is now called the periosteum.
• The periosteum is identical to the perichondrium except for its location.
• As the cartilage is degraded, strands of remaining cartilage act as templates for osteoblasts, which secrete more ECM that undergoes calcification.
• Those areas that are completely calcified are compact bone, whereas those that are not are cancellous.
Functions of bone
Calcium is important for blood coagulation, muscle contraction and the stability of the nervous system. Ninety-nine percent of the body’s calcium is in bone incorporated in hydroxyapatite. In plasma 45% is in the ionised form (Ca2+) with the remainder being bound to plasma proteins and other solutes. It is imperative that there is tight control of body calcium and so there is a continuous interchange between plasma and bone. Hormones are inherent in this regulation.
Parathyroid hormone: Parathyroid hormone (PTH) is a peptide secreted from the parathyroid glands in response to low blood calcium concentration. It increases blood calcium by:
Calcitonin: This peptide is secreted by the parafollicular cells of the thyroid gland and decreases plasma calcium concentration by:
Vitamin D: Vitamin D increases the absorption of calcium from the small intestine (mainly) and the kidney, and also increases osteoclast activity. Vitamin D can be ingested via foods but the majority is produced in the epidermis via a number of reactions mediated by ultraviolet light.
A lack of vitamin D can lead to demineralization and poor calcification of bone. This is called osteomalacia in adults and rickets in children.
Haemopoiesis
Haemopoiesis is the formation of red blood cells (RBCs) in the bone marrow. There are two types of bone marrow: red and yellow. Red marrow actively forms RBCs whereas yellow marrow is full of fat and thus inactive. When increased RBC production is required, yellow marrow can be converted to red.
 Objectives
Objectives There are three types of muscle: skeletal, cardiac and smooth.
There are three types of muscle: skeletal, cardiac and smooth.