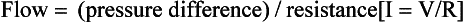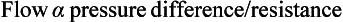Physiology of the cardiovascular system
In this chapter, you will learn to:
• Discuss the organization of the cardiovascular system
• Describe the different layers and types of valves of the heart
• Describe the conduction system and how a cardiac action potential is generated
• Explain the regulation of cardiac output and the factors that influence it
• Discuss the structure of blood vessels and the factors by which their size is regulated
• Explain how the cardiovascular system is regulated via intrinsic and extrinsic mechanisms
• Describe how the cardiovascular system regulates blood flow in specialized tissues
OVERVIEW OF THE CARDIOVASCULAR SYSTEM
The cardiovascular system (CVS) consists of the heart and tissues (blood vessels). It is involved in circulating blood and lymph through the body.
Organization of the organs and structures
The heart is a hollow, muscular, pumping organ. It beats, on average, 72 times per minute in an adult and more quickly in newborn infants and young children. The heart has two sides: left and right. Each side is further separated into a ventricle and an atrium (Fig. 5.1). The right atrium receives deoxygenated blood from the venous system and delivers it to the right ventricle. The ventricle then contracts and sends the blood to the lungs, where it is re-oxygenated and carbon dioxide is released. The left atrium receives the oxygenated blood from the lungs and delivers it to the left ventricle, which subsequently contracts and pumps it to the organs of the body, where the oxygen is removed and carbon dioxide enters. The cycle then repeats. Valves are present in the heart to prevent the blood flowing the wrong way.
Blood vessels
Arteries primarily transport oxygenated blood away from the heart. They are highly elastic, tube-like structures that stretch and recoil to maintain blood flow and pressure (Fig. 5.2). Arteries branch and become smaller (arterioles) until they reach a precapillary sphincter that regulates the flow of blood into the capillaries (Fig. 5.3). Capillaries are thin-walled vessels that facilitate the exchange of nutrients and waste products, as well as oxygen and carbon dioxide, with the body tissues. Capillaries merge into very small veins (venules), which connect to form veins that transport blood back to the heart.
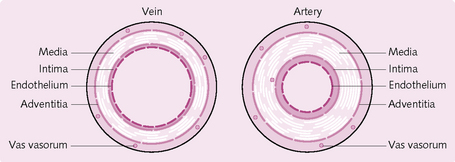
Fig. 5.2 Basic structure of a vein and artery (see also Fig. 5.13).
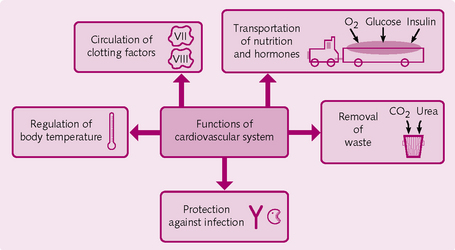
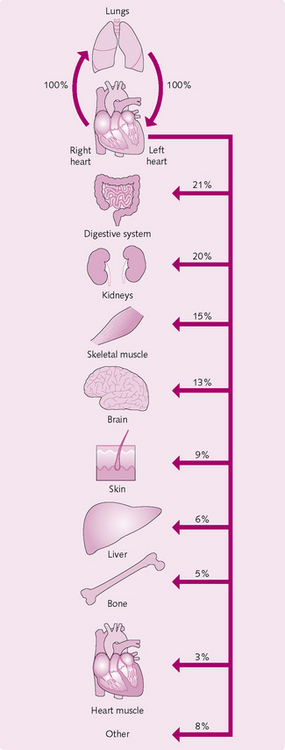
FUNCTIONS OF THE CARDIOVASCULAR SYSTEM
The functions of the cardiovascular system are outlined in Figure 5.4.
• Transports nutrients (oxygen, amino acids, glucose, water, etc.) and removes waste products (carbon dioxide, urea, creatinine).
• Protects the body against infection by circulating immunological cells and mediators to sites of disease.
• Circulation of clotting factors and cells to stop bleeding after injury.
• Transports hormones [insulin, antidiuretic hormone (ADH), etc.] to target cells and organs; the cardiovascular system also secretes its own hormone [atrial natriuretic peptide (ANP)].
THE HEART
The heart is located in the mediastinum, which is a mass of tissue that extends from the sternum to the vertebral column and is flanked by the two lungs. The heart is placed in the middle of the mediastinum and has the following relations:
Layers of the heart
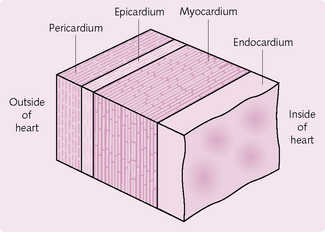
The heart wall has three layers: pericardium, myocardium and endocardium (Fig. 5.5).
Pericardium
This comprises an outer fibrous sac, covering the whole heart, and an inner double layer of serous pericardium. The fibrous layer is a tough, inelastic connective tissue that protects the heart, preventing it from overstretching and stabilizing it within the mediastinum.
The serous pericardium has two layers. The parietal pericardium is attached to the fibrous layer. The visceral layer (the epicardium) adheres to the outer surface of the heart. Between these two layers, the serous pericardium produces a thin film of fluid that reduces friction as the heart moves while contracting.
Heart chambers
The heart has four chambers (see Fig. 5.1); two atria superiorly and two ventricles inferiorly. A series of sulci – grooves – on the surface of the heart mark the boundaries between the chambers. Within these grooves are the coronary vessels that carry the blood supply to the heart muscle. The thickness of the walls of the myocardium is related to function. The atria are thin walled, as they have only to deliver the blood to the adjacent ventricles. The walls of the ventricles are a lot thicker because they pump blood further; the walls of the left ventricle are the thickest because it transmits blood all around the body.
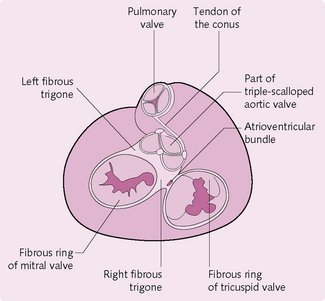
Heart valves
The heart valves (Fig. 5.6) respond to pressure changes in the heart and ensure that blood travels in one direction. There are two main groups:
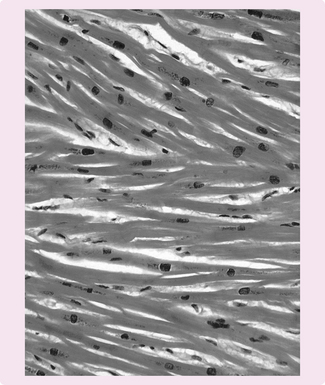
Cellular physiology of the heart
The myocardium comprises cardiac myocytes (muscle cells) (Fig. 5.7), which have the following features:
• Striated and branched network.
• 50–100 micrometres in length and 10–20 micrometres in diameter.
The myocytes are connected by intercalated discs that help the cells adhere at desmosomes (proteoglycan bridges that glue cells together). Between these are gap junctions made of connexin proteins. These pores allow electrical conductivity through the myocardium.
Like skeletal muscle, the myocytes contain actin and myosin filaments – the contractile components of the heart. The actin and myosin form a similar network to that in skeletal muscle (see Chapter 4), with M and Z lines and A, H and I bands. At the Z line, the sarcolemma forms the transverse tubular structure, which enables rapid electrical conduction by activating the whole contractile apparatus. The sarcoplasmic reticulum stores the calcium required for muscle contraction.
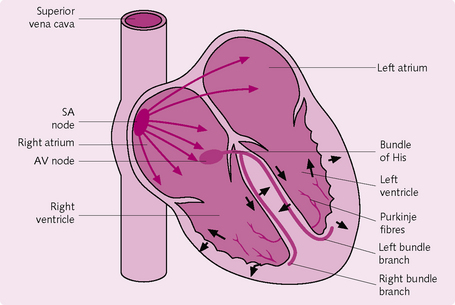
Conduction system of the heart
The continual beating of the heart is due to autorhythmic fibres, which repeatedly generate action potentials. These fibres act as a pacemaker to regulate the rhythm of contraction and ensure that the heart beats in a coordinated fashion.
One beat of the heart is conducted as follows (Fig. 5.8):
1. Spontaneous depolarization in the sinoatrial node (SA node) results in a pacemaker potential. The SA node is located in the right atrium near the entrance of the superior vena cava.
2. When SA depolarization reaches threshold it generates an action potential (AP) in the myocytes.
3. The AP propagates to both atria via the gap junctions. This causes the atria to contract in synchrony and pump blood into the ventricles.
4. The AP travels along the cardiac muscle to the atrioventricular (AV) node, which is located in the septum, sandwiched by the two atria.
5. The AP then enters the bundle of His, which carries it from the atria to the ventricles.
6. The AP enters the left and right bundle branches, which extend through the interventricular septum towards the apex of the heart.
7. The AP eventually reaches the Purkinje fibres – a network of fine fibres through the ventricular walls. The AP causes synchronous contraction of the ventricles, pushing blood from the right ventricle into the pulmonary artery and from left ventricle into the aorta via the semilunar valves.
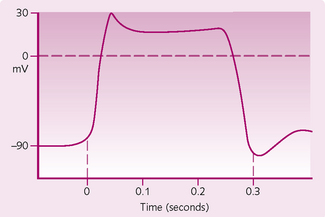
Action potentials
Myocyte action potential: The three key stages to the formation of an AP in the contractile fibres (Fig. 5.9) are as follows:
1. Rapid depolarization: the resting membrane potential of a cardiac cell is −90 mV because the cells are more permeable to K+ than to Na+ (see Chapter 3). When threshold is reached and an AP occurs, voltage-gated Na+ channels open for a few milliseconds, allowing the entry of Na+. This produces a rapid depolarization and the fast upstroke. The channels then close.
2. Plateau: voltage-gated slow Ca2+ channels then open in the membrane of the sarcoplasm reticulum, allowing the influx of Ca2+ to the cell. This phase influences the strength of contraction. Concurrently, K+ channels close, causing a decrease in K+ permeability and a brief depolarization. The Ca2+ influx is also responsible for the refractory period of the cell, making it less likely to start another contraction. This is important, as contraction needs to be co-ordinated or the heart will not have enough time to refill with blood.
3. Repolarization: voltage-gated K+ channels open, which increases the efflux of K+. This restores the resting membrane potential of −90 mV and causes the muscle to relax.
Sinoatrial node action potential: The SA node AP is different from the myocyte AP. It has an unstable resting membrane potential, so when it reaches the threshold value it triggers an AP. The upstroke is due to the entry of Ca2+. The SA node is regulated by the autonomic nervous system and the hormones epinephrine (adrenaline) and thyroxine: these alter the rate at which the threshold potential is achieved.
THE CARDIAC CYCLE
The cardiac cycle is the sequence of pressure and volume changes that occur with one heartbeat.
Events of the cardiac cycle (Fig. 5.10)
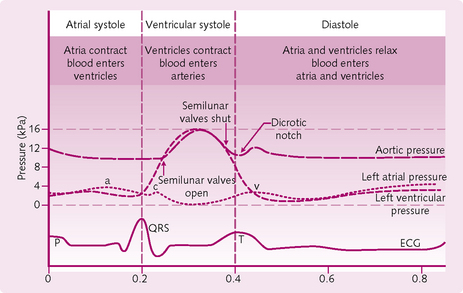
Fig. 5.10 Events in the cardiac cycle with the corresponding ECG and atrial and ventricular pressures. a, atrial systole; c, closure of the mitral valve and its bulging into the left atrium; v, atrial filling against closed mitral valve.
1. Atrial and ventricular filling (diastole): all the chambers of the heart are relaxed and there is passive filling of both the atria and ventricles.
2. Atrial systole (lasts for 0.1 s): the atria contract and add about 25 mL to the relaxed ventricles, producing a final volume of ∼130 ml. The ventricles are still relaxed at this stage.
3. Ventricular systole (lasts for 0.3 s): both ventricles contract. The pressure inside the ventricles increases and closes the atrioventricular (AV) valves. For about 0.06 s both the semilunar (SL) and AV valves are shut. This is when the cardiac muscle fibres are contracting but not yet shortening (isovolumetric contraction).
4. When the ventricular pressure exceeds aortic and pulmonary pressure, both SL valves open (ventricular ejection) and blood is expelled into the aorta and pulmonary trunk. The amount of blood ejected per ventricle (around 70 mL) is called the stroke volume. The volume remaining in the ventricles after ventricular systole is called the end-systolic volume.
Regulation of cardiac output
• Cardiac output (CO) = the volume of blood ejected by one ventricle into its respective artery each minute. Hence pulmonary blood flow = systemic blood flow in the aorta.
• Stroke volume (SV) = the volume of blood ejected in one ventricular contraction.
• Heart rate (HR) = number of ventricular contractions in one minute.
• End-diastolic volume (EDV) = volume of blood in ventricle immediately prior to contraction.
• End systolic volume (ESV) = volume of blood in ventricle after contraction.
• Central venous pressure (CVP) = pressure of blood in the great veins as it enters the right atrium.
• Total peripheral resistance (TPR) = resistance of blood flow in the circulatory system.
Three things directly affect CO:
In a typical adult male, SV averages 70 mL/min and HR 75 mL/min, so CO = 5.25 L/min. This value is close to the total blood volume. So, effectively, it takes 1 minute for the whole circulation to flow through the pulmonary and systemic systems (Fig. 5.11).
Preload
This is how much the heart stretches prior to contraction. Within reason, the more the heart stretches, the stronger will be the force of contraction. This is known as Starling’s law of the heart. The more blood that enters the heart during diastole, the more the heart will stretch and hence the greater the contraction. Preload is determined by the duration of diastole and rate of venous return. The longer the diastole, the more the ventricles fill. Furthermore, the more blood is returned to the heart by the venous system, the more it will fill and strengthen contraction.
Contractility
Contractility can be affected by many factors, such as length of fibre and inotropic (referring to the strength of contraction of the heart) agents. Positive inotropes (increase contractility) usually work by increasing Ca2+ inflow during the action potential. These include:
• Norepinephrine (noradrenaline) binds to myocyte β1-adrenergic receptors and increases Ca2+ via G protein activation.
Negative inotropes decrease contractility. Examples include:
Afterload
This is the pressure that must be overcome before the semilunar valve opens. Anything that increases afterload will cause stroke volume to decrease as less blood will be ejected and more will remain in the ventricles. Hypertension and atherosclerosis are conditions that increase the afterload. Any increase in afterload causes the ventricles to work harder to overcome the increased resistance and hence hypertrophy of ventricular muscle occurs. This can eventually lead to muscle weakness and failure. Right ventricular failure due to obstructed pulmonary circulation (cor pulmonare) is a common complication of emphysema and chronic bronchitis.
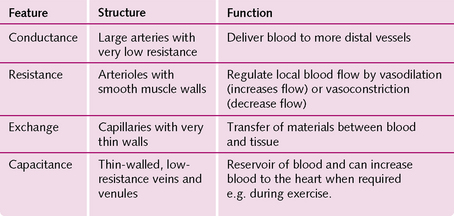
STRUCTURE AND FUNCTION OF THE BLOOD VESSELS
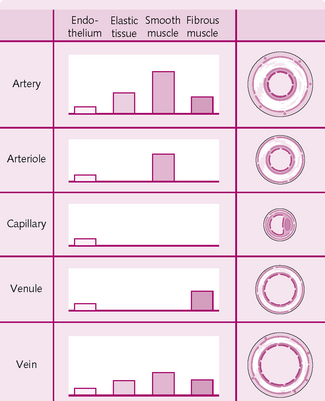
The main types of vessel in the circulatory system are the elastic arteries, muscular arteries, arterioles, capillaries, postcapillary venules, muscular venules and veins. Their properties are shown in Figure 5.12.
Arteries
Structure of the arterial wall
There are two types of artery:
• Muscular arteries: contribute a very small proportion to the overall resistance and so do not really regulate blood flow.
The arterial wall has three layers:
• Tunica interna: endothelium lining – makes contact with blood.
• Tunica media: elastic fibres for compliance, smooth muscle fibres.
The tunica media has a high proportion of elastic fibres and relatively thin walls (Fig. 5.13). Examples are the aorta, the brachiocephalic artery, and the common carotid artery.
Arterioles
These are smaller than the arteries, from which they branch. The walls of the arterioles contain variable amounts of smooth muscle and elastic tissue. They function to regulate – via vasodilation and vasoconstriction – how much blood goes to the capillaries. They are known as resistance vessels and act as precapillary sphincters.
Capillaries
These join the arterioles and venules and lie close to almost every organ in the body. The concentration of capillaries varies with the metabolic demand of the tissue. Those with high demands, e.g. heart muscle, have a far higher number than those with lower metabolic needs, e.g. tendons, ligaments.
Capillaries function to exchange fluids containing nutrients and waste products from the blood and adjacent cells.
Venules
These collect the blood from the capillaries and transmit it to the veins. The venules closest to the capillaries tend to be porous, allowing phagocytic white blood cells to exit the bloodstream to the site of infection.
Veins
These have the same structure as the arteries, i.e. three layers of tunica. However, the intima and media are a lot thinner and there is no external elastic lamina (see Fig. 5.13). The tunica externa, which consists of collagen, elastin and smooth muscle fibres, forms the majority of the vein wall. The lumen of a vein is far larger than the arterial lumen.
The lumen of veins in the lower limbs contains valves made from tunica externa. These function to prevent the backflow of blood and to encourage flow back to the heart.
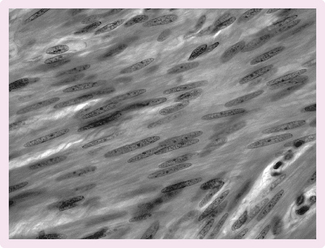
Vascular smooth muscle (Fig 5.14)
Smooth muscle is activated involuntarily and is found in the vascular system and many of the visceral organs, e.g. stomach, intestine, bladder.
Smooth muscle contraction
• Increase in Ca2+ from interstitium and sarcoplasm reticulum.
• The regulatory protein calmodulin binds to incoming cytosol Ca2+.
• The calmodulin–Ca2+ complex activates the light chain of the enzyme myosin kinase.
• This phosphorylates the myosin head using ATP.
• The myosin head then binds to the actin and contraction is achieved.
Smooth muscle relaxation
For relaxation to occur, the intracellular concentration of Ca2+ must decrease. This can be achieved by the following mechanisms:
Smooth muscle tone
Smooth muscle can contract for long periods of time. This is important in the gastrointestinal system, where prolonged contraction is needed for peristalsis. This is achieved by the Ca2+ entering and leaving the smooth muscle cells very slowly.
Haemodynamics
The volume of blood travelling through a tissue per unit time is deemed to be its blood flow. Ohm’s law states:
So where the volume ends up depends on two factors:
Pressure changes: Blood travels from an area of high pressure to one of low pressure. The pressure drops as blood moves from the aorta and arteries into the arterioles. Most of the pressure drop occurs at the arterioles. The pressure in the large arteries (e.g. the brachial artery, which is used when assessing blood pressure with a syphygmomanometer) is a good measure of pressure in the aorta.
Resistance to blood flow: As with all liquids, blood flows along the path of least resistance. This depends on three factors:
1. Lumen diameter: Poiseuille’s law states that resistance is proportional to 1/r4 where r = radius. So the smaller the diameter of the lumen, the greater is its resistance. If the lumen radius is 5 mm, the resistance will be 1/54 = 0.0016. If the vessel diameter was 2 mm then the resistance would be a lot more: 0.0625.
2. Blood viscosity: Newton defined viscosity as a ‘lack of slipperiness’. Poiseuille’s law states that resistance is proportional to viscosity. Viscosity is influenced by the ratio of red blood cells (RBC) and proteins (globulins and albumins) and the fluid component (plasma) in blood. The higher the RBC or protein concentration relative to the plasma, the greater the viscosity: an example is polycythaemia, in which there is an increase in numbers of RBCs. Dehydration can also increase viscosity as there is a decrease in the fluid component of plasma, thereby increasing the cell/protein concentration.
3. Vessel length: resistance to blood flow is directionally proportional to the length of the blood vessels. The longer the vessel, the greater the resistance.
Haemodynamics of the venous system
Venules and veins are thin-walled, distensible capacitance vessels that act as a reservoir of blood for cardiac filling. The volume in the venous system depends on the venous pressure and the active wall tension.
Posture and gravity
When a person moves from supine to a standing position, gravity increases the blood pressure in the vessels below the heart. The body has a number of mechanisms to push blood back up to the heart:
• Venous valves: these permit the blood to flow in one direction towards the heart. The blood pressure in the veins rises because blood is continually flowing into the veins from the capillaries. The increase in pressure will cause pooling of blood in the legs, which will decrease central venous pressure and cardiac output. Baroreceptor-mediated reflex mechanisms that cause vasoconstriction to maintain blood pressure are brought into play.
• Skeletal muscle pump: this is based on an alternating compression and relaxation of veins. Contraction of the leg muscles compresses the veins, which pushes the blood towards the heart and maintains central venous pressure. Drainage of blood back to the feet is prevented by valves in the veins.
• Respiratory pump: this is based on similar principles to the leg muscle pump. During inspiration the diaphragm flattens and descends. This causes negative intrathoracic pressure and an increase in intra-abdominal pressure. Consequently, the veins in the abdomen are compressed and cause blood to flow into the thoracic cavity, thereby maintaining cardiac output.
Capillary dynamics and transport of solutes
The majority of capillaries comprise a single layer of endothelial cells resting on a basement membrane: no smooth muscle or elastic tissue is present in the walls. The aim of the cardiovascular system is to maintain blood flow through capillaries and allow capillary exchange.
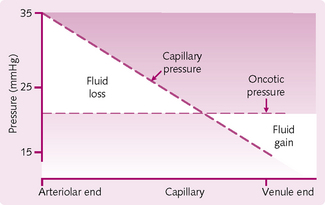
Starling’s forces and capillary exchange
The plasma and the interstitial fluid (ISF) exchange readily through the capillary system. The primary forces that govern this exchange are hydrostatic pressure (the blood pressure within the capillaries) and the colloid osmotic pressure (COP) of plasma (see Fig. 5.15 and Fig. 2.5).
Hydrostatic pressure: The capillary wall acts as a filtration barrier. Most of the fluid within the capillaries is retained, although some filters through pores between the cells, driven by the pressure difference between the capillary plasma and the ISF. Water and small solutes pass readily through these pores. The net effect of the hydrostatic pressure alone is movement of water and solute from plasma to the ISF. However, the capillary walls are impermeable to the plasma proteins and lipids. Under normal conditions, these stay within the plasma.
At the arteriolar end of the capillary, the hydrostatic pressure is higher than the oncotic pressure, so there is fluid movement from plasma to interstitium. The hydrostatic pressure decreases along the length of the capillary. At the arteriolar end, the pressure is usually about 35 mmHg; at the venule end of the capillary, the pressure is around 15 mmHg.
Osmotic forces in the capillaries: The capillary wall is impermeable to plasma proteins so they generate an osmotic pressure, drawing fluid into the capillary. Furthermore, as these proteins are negatively charged, they tend to hold additional cations in the plasma, further enhancing an osmotic gradient between the plasma and the interstitial fluid. The combined effect results in a pressure that draws water out of the interstitium and into the plasma. This pressure (COP) is also known as the oncotic pressure (Fig. 5.15).
So hydrostatic pressure tends to cause fluid to leave the plasma, and oncotic pressure pulls it back; the two forces tend to balance each other. They can be altered by certain conditions:
• An increase in pulmonary capillary pressure from cardiac failure will prevent fluid from leaving lung tissue, causing pulmonary oedema (left-sided failure).
• In liver disease or extreme starvation, plasma protein concentration will decline, resulting in a fall in COP. More fluid will move out into the interstitium, causing oedema.
• Capillary damage will result in an inflammatory response, with mast cells releasing histamine. This causes an increase in permeability, allowing protein to leave and so decreasing the COP. Again, this results in oedema of the site involved.
Capillary transport
The exchange of substances between the capillaries and the ISF occurs via three mechanisms:
1. Simple diffusion through the endothelial cell membrane
This applies to simple substances, such as CO2, O2, glucose, amino acids and hormones.
2. Diffusion through pores and fenestrations
Pores and fenestrations are present in the cell membrane and permit the diffusion of water-soluble substances such as glucose, amino acids and ions. Lipid-soluble substances, such as steroid hormones, can diffuse directly through the lipid bilayer.
The blood–brain barrier is unusual in that the endothelial cell junctions are arranged tightly. This allows greater regulation of substances obtaining access to the brain.
Capillary pores in the liver, where many substances are synthesized, are large (fenestrated capillaries) to allow easy exchange of materials and secretion.
LYMPH AND THE LYMPHATIC SYSTEM
Approximately eight litres of fluid comprising solutes and plasma proteins are filtered from the microcirculation daily. This is returned to the blood via the lymphatic system.
Structure
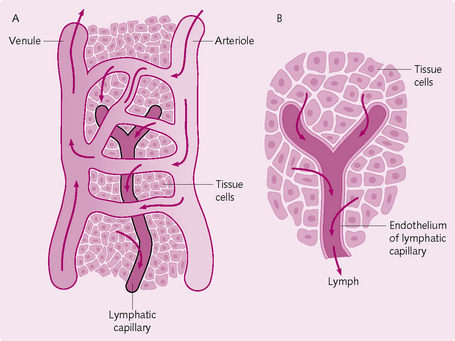
The lymphatic system comprises lymph fluid, lymphatic vessels, lymphatic tissue and red bone marrow (Fig. 5.16). The lymph capillaries are blind-ending, bulbous tubes with a monolayer of endothelial cells between the cells. The endothelial cells contain a door-like structure that permits lymph to flow in the lymph capillaries, but in only one direction.
The lymph capillaries merge to form a network of collecting lymphatics vessels. These contain smooth muscle cells, which help to move the lymph along. During its passage through the lymph vessels, lymph flows through lymph nodes containing B and T cells, where the lymphatic fluid is presented to the immune system. Efferent vessels from the nodes unite to form lymph trunks (lumbar, intestinal, mediastinal, subclavian, jugular, bronchomediastinal). The lymph then passes either through the left (thoracic) or right lymphatic ducts into venous blood.
The sequence of lymphatic fluid flow is: blood capillaries → interstitial spaces (IF) → lymphatic capillaries (lymph) → lymphatic vessels (lymph) → lymphatic ducts (lymph) → subclavian veins (blood).
The skeletal muscle and respiratory pump enhance the lymph flow.
Functions of the lymphatic system
• Drainage of surplus interstitial fluid and returning this to the blood.
• Transporting dietary lipids: lymphatic vessels are responsible for transporting the fat-soluble vitamins (A, D, E and K) from the gut into the blood.
• Immunity: the lymph presents foreign antigens to the immune system, which reacts appropriately.
CONTROL OF THE CARDIOVASCULAR SYSTEM
Control of blood vessels
The vascular system delivers nutrients to tissues in the body. The amount of flow required, and the consumption, vary depending on the region involved. Mechanisms that regulate blood flow through the arterioles must therefore be present.
Intrinsic control
Intrinsic control mechanisms are independent of nerves and hormones and are the means by which tissues and organs alter their own arteriolar resistances.
Local temperature: This mainly regulates flow through skin. High temperature causes vasodilatation in skin arterioles and veins. At temperatures of 12–15°C a2-adrenoceptors are stimulated and vasoconstriction occurs. Below 0°C, neurotransmitter release is paralysed and the vessels dilate slowly, resulting in paradoxical cold vasodilatation; eventually the vessels warm up owing to the flow of blood, and vasoconstriction occurs.
Transmural pressure: This is the pressure across the wall of the vessel. It can be affected by external and internal pressures:
• External: high external pressure, such as during muscle contraction, can impair blood flow.
• Internal: initially, an increase in blood pressure causes the vessel to distend. The smooth muscle stretches to produce a contracted response. The vessel constricts, reducing flow within the vessel. This is known as the myogenic response and is a mechanism of autoregulation.
Local metabolites: Changes in the concentration of various metabolites can cause vasodilatation:
• Hypoxia: decreased arterial blood PO2 (PaO2).
• Acidosis: due to CO2 and lactate.
Different tissues react differently to these factors. For example, the coronary vessels mainly react to hypoxia and adenosine, whereas cerebral vessels are influenced by K+, H+ and PCO2.
Cytokines: The old name for cytokines is autacoids. They are chemical substances that are produced and are effective locally:
• Histamine: this inflammatory mediator causes vasodilatation in arterioles (H1 receptor mediated) and vasoconstriction and increased permeability in veins (H2 receptor mediated).
• Bradykinin: release of this causes vasodilatation via nitric oxide (NO) and an increase in vessel permeability via Ca2+.
• 5-hydroxytryptamine (5-HT or serotonin): this causes vasoconstriction and is present in platelets, intestinal wall and the CNS.
• Prostaglandins (PGs): macrophages, leukocytes, fibroblasts and endothelium produce these inflammatory mediators. They are synthesized from arachidonic acid by cyclo-oxygenase and are inhibited by non-steroidal anti-inflammatory drugs (NSAIDs) such as aspirin. PGF causes vasoconstriction whereas PGE and PGI2 are vasodilators.
• Thromboxane A2: this activates platelets and is important in haemostasis by causing vasoconstriction.
• Leukotrienes: leukocytes produce these inflammatory mediators from arachidonic acid catalysed by lipoxygenase. They cause vasoconstriction and increased vascular permeability.
• Platelet-activating factor (PAF): this inflammatory mediator causes vasodilatation, increased vascular permeability and vasospasm in hypoxic coronary vessels.
Endothelium-dependent relaxation and contraction: The endothelium produces an array of products that influence the patency of blood vessels:
• Endothelium-derived relaxing factor (EDRF): this endothelium-produced factor was discovered to be NO, the production of which is stimulated by thrombin, bradykinin, substance P, adenosine diphosphate, acetylcholine and histamine. NO is cleaved from L-arginine by the enzyme NO synthase. It then diffuses into smooth muscle cells and activates the cGMP messenger system, causing relaxation and vasodilatation.
• Endothelin: a peptide that has a potent, long-lasting vasoconstrictor action that is released in response to stretch, thrombin and epinephrine. It acts locally but appears to have a role in regulation of systemic blood pressure.
• Endothelium-derived constricting factor (EDCF): a putative factor produced in larger arteries to cause vasoconstriction in response to stretch and/or hypoxia.
Autoregulation: This is where tissue perfusion remains relatively constant despite blood pressure changes. Consider the following:
So, to maintain flow, any pressure change must be opposed by a resistance change. For example, an increase in pressure causes arteriolar vasoconstriction, thereby increasing resistance. It takes 30–60 s for the effect to take place, so there is usually an increase in flow with a pressure increase before steady state is achieved.
Autoregulation occurs only over a limited pressure range. It is an intrinsic feature of the vessels and is independent of nervous control. Autoregulation can be reset to work at a new level by, say, an increase in sympathetic drive. The mechanisms for autoregulation are:
• Myogenic response: increased pressure produces constriction of the vessel, opposing the rise in pressure and stabilizing blood flow.
• Vasodilator washout: this is the effect that blood flow has on the concentration of local vasodilator metabolites. If blood flow increases, these metabolites are washed away faster, causing the vessel to constrict, thereby increasing resistance and slowing flow.
In the heart, any increase in coronary arterial pressure causes a rise in tissue PO2. This leads to vasoconstriction, which autoregulates coronary flow.
Autoregulation is also important in the renal artery to maintain the glomerular filtration rate (GFR). If the GFR is too fast, the nephron will not be able to reabsorb sufficient ions and water.
Hyperaemia: Hyperaemia means an increase in blood flow; there are two types:
• Metabolic/functional/active hyperaemia: is due to metabolites released by exercising muscle (e.g. CO2, H+, K+, adenosine) causing vasodilatation and a decrease in vascular resistance locally.
• Reactive/post-ischaemic hyperaemia: when the blood supply to an organ or tissue is completely occluded, a profound transient increase in blood flow occurs as soon as the vessel becomes patent again. During the period of absent flow, the arterioles in the affected organ dilate due to the local metabolites, e.g. prostaglandins. Reactive hyperaemia enables resupply to ischaemic tissue. It is a temporary process and decays exponentially. It can also be painful after a prolonged period of ischaemia.
Ischaemic reperfusion injury: Reactive hyperaemia is impaired after a prolonged interruption of blood flow. Reperfusion of the ischaemic tissue causes formation of superoxide (O2−) and hydroxide radical (OH−), K+ increase from damaged tissue and an increase in Ca2+ ions in the cell. All these damage the tissue and vessel wall, resulting in further occlusion.
It is thought that reperfusion injury exacerbates damage to the myocardium, intestine and brain following ischaemia.
Extrinsic control
The nervous system is mainly responsible for the short-term control of the vasculature (Fig. 5.17).
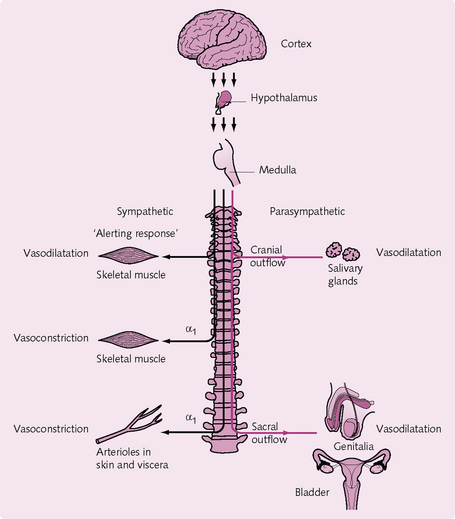
Fig. 5.17 Overview of nervous control of the vasculature. The sympathetic tracts are shown in black and the parasympathetic tracts in red.
The sympathetic nervous system:
Vasoconstrictor nerves: These innervate the vascular smooth muscles of the resistance and capacitance vessels. A basal level of activity of these nerves is responsible for vessel tone at rest. The neurotransmitter involved is noradrenaline (norepinephrine), which acts on a1-receptors on vascular smooth muscle to cause contraction. During an increase in sympathetic drive:
Vasodilator nerves: These innervate some skeletal muscle and sweat glands. In skeletal muscle, the neurotransmitter acetylcholine (ACh) acts on muscarinic receptors and causes vasodilatation.
In sweat glands, stimulation of these nerves via the neurotransmitter vasoactive intestinal peptide (VIP) produces sweating and cutaneous vasodilatation.
Hormonal control
The following is a brief account of the hormones involved in regulating vascular tone. Further details of these hormones can be found in Crash Course: Endocrine and Reproductive Systems.
Adrenaline (epinephrine) and noradrenaline (norepinephrine): The old names for epinephrine and norepinephrine are adrenaline and noradrenaline, respectively. These hormones are secreted by the adrenal medulla in response to flight, fight or frolic situations, with epinephrine secretion being three times as much as that of norepinephrine. Both are β-adrenoceptor agonists, and so increase heart rate and myocardium contractility. They also stimulate alpha-receptors, causing vasoconstriction.
Epinephrine is a potent vasoconstrictor in most organs and a vasodilator in skeletal muscle, myocardium and liver. This is because there are more β-receptors in these latter tissues, and epinephrine has a higher affinity for these receptors. Thus it can be used to stimulate the heart without causing an overall increase in peripheral resistance and consequently blood pressure.
Norepinephrine has higher affinity for a-receptors and so causes vasoconstriction. The effects on the heart include increased contractility, stroke volume and heart rate. Blood pressure rises as a result because the vasodilatory effects of epinephrine do not fully counteract the vasoconstrictor effects combined with the increased cardiac output.
Antidiuretic hormone: ADH is a peptide produced by the hypothalamus in response to an increase in plasma osmolality; it is released into the bloodstream by the posterior pituitary gland. ADH causes the kidney to retain water and results in vasoconstriction in most tissues. However, vasodilatation occurs in the heart and brain, which ensures these vital tissues remain perfused.
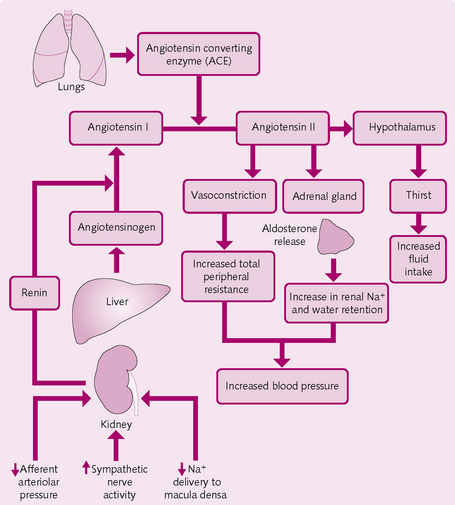
Renin–angiotensin–aldosterone system (RAA) (Fig. 5.18): The enzyme renin, produced by the juxtaglomerular cells of the kidney, converts liver α-globulin, angiotensinogen, to angiotensin I. In the lungs, angiotensin-converting enzyme (ACE) converts angiotensin I to angiotensin II. This:
Atrial natriuretic peptide: The stretching of the atria that occurs when there is an increase in blood volume stimulates the atria to secrete atrial natriuretic peptide (ANP). ANP acts to relax the glomerular mesangial cells, increase GFR and hence salt and water excretion. Decreased salt and water reabsorption through its actions on proximal convoluted tubules and, more importantly, medullary collecting ducts have been reported. ANP also has a small vasodilating effect.
CARDIOVASCULAR RECEPTORS AND CENTRAL CONTROL
The cardiovascular centre (CVC) in the medulla oblongata regulates heart rate, stroke volume and regional blood flow via neural, hormonal and local negative-feedback systems. Impulses from the CVC travel via sympathetic and parasympathetic nerves (nucleus ambiguus) to stimulate or inhibit the heart, respectively. Similarly, the vasomotor centre of the CVC can vasoconstrict or dilate vessels via sympathetic nerves. Information from the CVC can be relayed to the hypothalamus.
Hypothalamus
This has four areas of interest:
1. Depressor area: this can produce the baroreceptor reflex.
2. Defence area: this is responsible for the fight/flight response and governs sympathetic outflow.
3. Temperature-regulating area: this regulates skin vascular tone and sweating.
4. Vasopressin-secreting area: vasopressin travels through nerve axons to the pituitary.
Baroreceptor reflexes
Baroreceptors are sensitive to pressure and play a fundamental role in short-term blood pressure control; they also respond to vessel wall stretch. They are found in the aorta, internal carotid arteries and other large arteries in the neck and chest. The two main baroreceptor reflexes are the carotid sinus and aortic reflexes, which produce continuous impulses at normal vessel-wall tone. The impulses from the receptor travel via the glossopharyngeal nerve (carotid sinus) and vagus nerve (aortic arch) to the CVC in the medulla oblongata. An increase in firing causes the medulla to decrease the heart rate and total peripheral resistance by increasing parasympathetic and/or decreasing sympathetic drive.
The above mechanisms serve to reduce the blood pressure. Carotid sinus massage can be used on patients to apply pressure to the carotid bodies and so slow the heart rate if the patient has paroxysmal supraventricular tachycardia.
When the baroreceptors are not stretched, for example because of rapid blood loss, the CVC is not as stimulated and the end result is an increase in sympathetic drive, which causes:
Ultimately, these increase cardiac output, total peripheral resistance and the circulating volume, all of which serve to return blood pressure to normal.
The sensitivity of the arterial baroreceptors is decreased by:
• Age: the compliance of the arterial vessels falls so that it is more difficult to stretch.
• Chronic hypertension: distensibility of the arterial wall is decreased.
The level of blood pressure that the baroreceptor takes as the set point can be adjusted by central or peripheral mechanisms:
• Central mechanisms: during exercise, the corresponding increase in blood pressure does not cause a bradycardia as a new blood pressure set point has been initiated. The neurons that drive inspiration inhibit cardiac vagal nerves, so blocking baroreceptor impulses and causing a decreased vagal drive.
• Peripheral: persistent hyper- or hypotension results in the resetting of the blood pressure level.
Cardiopulmonary receptors
Numerous cardiopulmonary receptors are connected to afferent fibres innervating the heart, great veins and pulmonary arteries. When these are stimulated they cause bradycardia, vasodilatation and hypotension. There are three main types:
1. Venoatrial stretch receptors
Located where the great veins join the atria, venoatrial stretch receptors are connected to myelinated vagal fibres. Stimulation produces a reflex tachycardia by increasing sympathetic drive to the pacemaker and increasing salt and water excretion.
2. Unmyelinated mechanoreceptor fibres
These are located in the atria and left ventricle and travel in the vagal and sympathetic nerves. Large distension stimulates these receptors, causing an inhibitory effect. Reflex bradycardia and peripheral vasodilatation occurs.
3. Chemosensitive fibres
Some unmyelinated vagal and sympathetic afferents are chemosensitive. They can be stimulated in response to substances such as bradykinin. Respiration also increases, causing bradycardia and peripheral vasodilatation.
4. Chemoreceptors
Chemoreceptors are located in close proximity to the baroreceptors of the carotid sinus and aortic arches in small structures known as the carotid and aortic bodies. They are nerve terminals, which are sensitive to changes in the level of O2 (hypoxia), CO2 (hypercapnia) and H+ (acidosis). They send impulses to the CVC, which stimulates arterioles and veins to vasoconstrict and so increases the blood pressure. Impulses are also sent to the respiratory centre (RC) to modify the rate of breathing.
REGULATION OF CIRCULATION IN INDIVIDUAL TISSUES
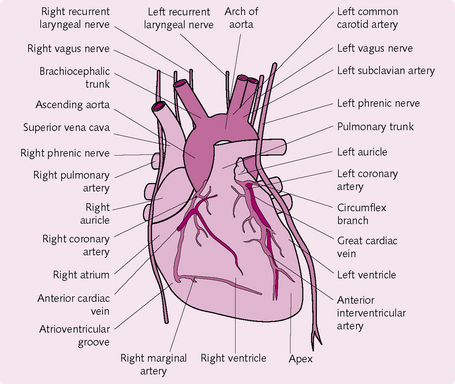
The coronary arterial system comprises the right and left coronary arteries, which supply the bulk of the myocardium (Fig. 5.19).
Oxygen demand in the mycocardium is very high, at 9 mL/min/100 g, and this rate can increase fivefold during exercise. The coronary circulation adapts to this via:
• High capillary density: the large number of capillaries means that there is a higher degree of flow and a shorter distance for exchange of O2.
• Myoglobin: this red-coloured protein is found in muscle cells, where it binds diffusing oxygen molecules. The myocardium has a high proportion of myoglobin, so oxygen extraction from the capillaries is high.
• Anastomoses between the coronary arteries: these ensure adequate blood supply if some arteries become occluded.
Skeletal muscle
The delivery of nutrients and removal of waste products needs to increase during exercise. This is aided by:
• Capillary density: this varies depending on the muscle type. White muscle fibres involved in phasically active muscles (e.g. biceps) have a lower capillary density than postural muscles (e.g. soleus).
• Myoglobin: levels are higher in tonic muscles than in phasic muscles.
• Hormones: epinephrine (adrenaline) is a potent vasodilator, which acts on β2-adrenoceptors in smooth muscle cells.
• The skeletal muscle pump: this encourages venous return to the heart by pushing blood through one-way valves in the veins.
Cutaneous circulation
The skin can be a source of blood reserve. Initially, during exercise, epinephrine and norepinephrine cause blood vessels in the skin to constrict and divert more blood to skeletal muscle and the heart.
Temperature is regulated by the rate of blood flow through the skin. Exposed areas of skin – the fingers, toes, palms, soles, lips, nose and ears – contain numerous, well-innervated arteriovenous anastomoses (AVA). The hypothalamus regulates these via the sympathetic nerves. If the core temperature is too high, the AVAs open, blood flow in the skin increases and heat loss occurs via conduction and convection. This occurs in the later stages of exercise as the body core warms up. The converse happens if the core temperature drops.
In exposed areas, such as the extremities, prolonged exposure to low temperatures causes a paradoxical vasodilation. This is probably due to the low temperature impairing sympathetic vasoconstriction; it ensures the skin is not damaged. However, cold vasodilation can also cause a rapid heat loss.
Hypovolaemia results in the release of angiotensin II, ADH and epinephrine, which causes blood vessels in the skin to vasoconstrict and produces the pale skin colour seen in shock.
Reactive hyperaemia occurs when there is a period of obstruction to blood flow to the skin. Once the obstruction is overcome and blood flow is re-established, the rate of flow increases to supply the starved region. This, along with the skin’s high tolerance to hypoxia, prevents ischaemic damage.
Cerebral circulation
As an organ, the brain has the third highest rate of oxygen consumption (3.3 mL/100 g/min) in the human body. Grey matter has little tolerance to hypoxia and loss of consciousness follows within as little as 9 s of ischaemia. As a result, the cerebral circulation is strictly regulated at the expense of other tissues to ensure an adequate flow. The factors affecting cerebral blood flow are:
• Intracranial pressure (ICP): a rise in ICP puts pressure on the arteries and restricts blood flow. The venous pressure also rises, which effectively compresses the cerebral vessels.
• Cerebral arteriole resistance: this is determined by metabolites, hormones, circulating peptides and vasomotor nerves.
Autoregulation is widespread in the brain and aims to keep flow at a relatively constant level despite variations in perfusion pressure. However, autoregulation ceases when blood pressure falls below 60 mmHg.
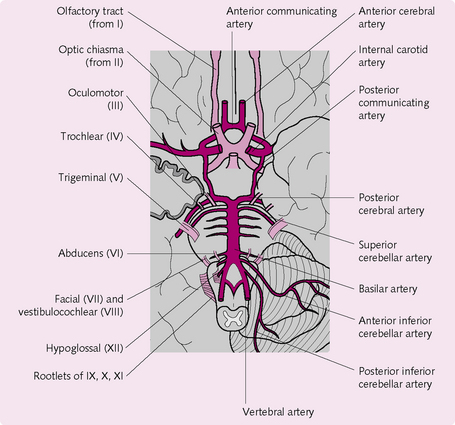
The anatomy of the cerebral circulation is an important component in the maintenance of blood flow (Fig. 5.20). The circle of Willis is the site of anastomosis of the large blood vessels supplying the brain. Its circular structure ensures that if blood flow through one vessel is obstructed blood can access the deficient area. Furthermore, capillary density in the brain is also very high.
Pulmonary circulation
The pulmonary circulation has the following features:
• Pulmonary arterioles are shorter, thinner walled and more distensible than systemic vessels so the pulmonary vascular resistance and pressure are very low.
• Thin-walled vessels facilitate gaseous exchange.
• There is no autoregulation of blood flow, although the pulmonary vessels still react to systemic mediators, e.g. epinephrine (adrenaline).
Renal circulation
The kidneys have the second highest oxygen consumption (6 mL/100 g/min) after the heart. Renal blood flow is autoregulated within certain limits to ensure adequate glomerular filtration rate and is dependent on:
Mesenteric circulation
The intestines are supplied by the superior and inferior mesenteric arteries, of which there are extensive anastomoses. Blood flow to the mucosa responds to changes in metabolic activity. The arrival of food causes a hyperaemia and is due to local hormones (gastrin, cholecystokinin), products of digestion (amino acids, glucose) and an increase in vagal activity.
This rise in blood flow produces a tachycardia and an increase in cardiac output. Conversely, blood flow to skeletal muscle decreases.
 Objectives
Objectives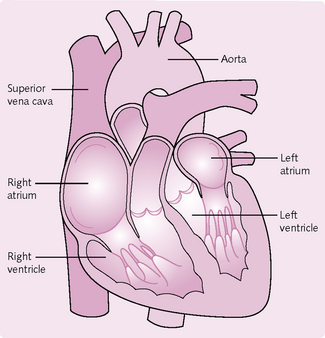
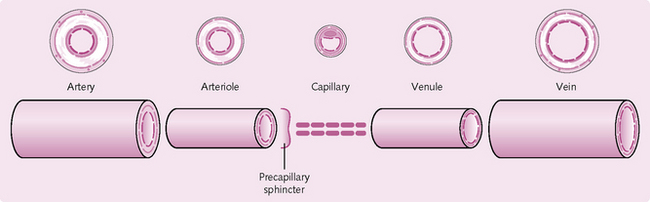
 Arteries transport oxygenated blood away from the heart and veins transport deoxygenated blood towards the heart except the pulmonary circulation. The pulmonary vein carries oxygenated blood towards the heart, and vice versa for the pulmonary artery.
Arteries transport oxygenated blood away from the heart and veins transport deoxygenated blood towards the heart except the pulmonary circulation. The pulmonary vein carries oxygenated blood towards the heart, and vice versa for the pulmonary artery.