Chapter 54Pathogenesis of Osteochondrosis
Osteochondrosis: Definitions and Terminology
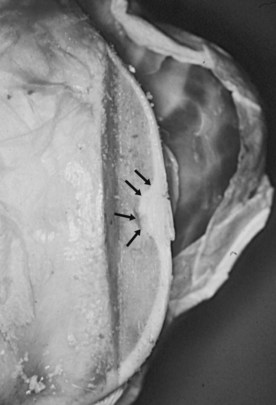
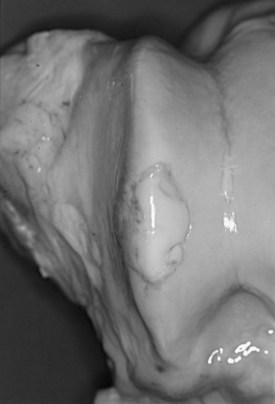
Equine osteochondrosis (OC) is characterized by focal failures of endochondral ossification that typically occur in well-defined predilection sites. Lesions that result from external trauma or infection are not generally regarded as OC.1,2 The thickened, retained growth cartilage that characterizes typical lesions (Figure 54-1) in the articular-epiphyseal cartilage complex (AECC) may be complicated by development of fissures that extend from the deepest layers of the lesion to the articular surface. Cartilaginous or osteochondral fragments may then detach from the parent bone, forming intraarticular fragments.3 Once lesions extend to the articular surface, thereby causing inflammation of the joint, the condition may be referred to as osteochondritis.4,5 The term osteochondritis dissecans (OCD) is usually reserved for lesions in which a dissecting flap of tissue is present (Figure 54-2).3 Although it has been proposed that the term dyschondroplasia should be used in place of OC,3,6 the term OC remains in widespread use and is used in this chapter to refer to the primary lesion. Dyschondroplasia is used only when referring to work by authors who prefer this term. A condition similar to equine OC occurs in a number of other animal species, as well as in people.1
Endochondral Ossification
Endochondral ossification is the process by which growing cartilage is systematically replaced by bone to form the growing skeleton.7 This process occurs at three main sites: the physis, the epiphysis, and the cuboidal bones of the carpus and tarsus. Chondrocytes in the physis can be divided into a series of layers or zones (Figure 54-3). The zone farthest from the metaphysis is the resting or reserve zone. Adjacent to this is the proliferative zone, in which chondrocytes divide. These cells progress to the hypertrophic zone, in which they enlarge and form ordered columns. During this stage the chondrocytes become surrounded by extracellular matrix that gradually becomes mineralized in the zone of provisional calcification. The chondrocyte columns are then invaded by metaphyseal blood vessels, and bone forms on the residual columns of calcified cartilage. This mixture of calcified cartilage and immature bone (primary spongiosa) is then gradually remodeled to produce the mature bone of the metaphysis.7 Endochondral ossification, which continues throughout the period of growth, also occurs in the AECC at the ends of long bones (Figure 54-4).8 The chondrocytes of the AECC that are closest to the articular surface produce articular cartilage, whereas those cells closer to the epiphysis participate in endochondral ossification in the same manner as occurs in the physis. It is generally accepted that the growth cartilages of both the physis and the AECC are susceptible to OC.1,3,8-11

Fig. 54-3 Sagittal section of the epiphysis, metaphysis, and diaphysis of growing bone, with sequential histological sections showing the different zones of the physis (metaphyseal growth cartilage). 1, Articular-epiphyseal growth cartilage complex; 2, secondary ossification center in the epiphysis; 3, metaphyseal growth cartilage; 4, primary ossification center in the diaphysis. Toluidine blue stain.
(Courtesy Gustavo Hernández-Vidal, Faculty of Veterinary Medicine, Universidad Autónoma de Nuevo León, Monterrey, Mexico.)
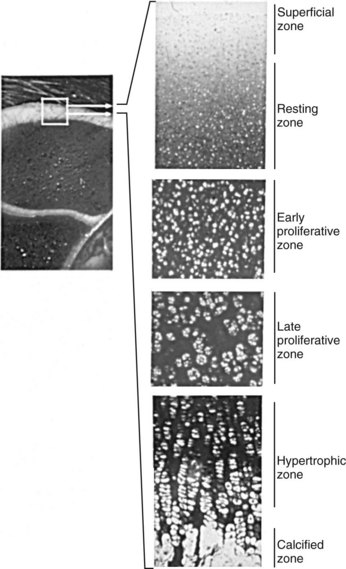
Fig. 54-4 Sagittal section of the epiphysis with sequential histological sections showing the different zones of the articular-epiphyseal cartilage complex. Toluidine blue stain.
(Courtesy Gustavo Hernández-Vidal, Faculty of Veterinary Medicine, Universidad Autónoma de Nuevo León, Monterrey, Mexico.)
Characteristics of Osteochondrosis
Equine OC characteristically manifests as one or two lesions that occur in known predilection sites. In this so-called “typical pattern” of the disease, lesions are often bilaterally symmetrical, although only one lesion may cause clinical signs.12 The femoropatellar (lateral and medial femoral trochlear ridges, lateral facet of patella), tarsocrural (cranial aspect of the intermediate ridge and medial malleolus of the distal aspect of the tibia, lateral and medial trochlear ridges of the talus), scapulohumeral (glenoid fossa and humeral head), and metacarpophalangeal and metatarsophalangeal joints (midsagittal ridge and condyles of the third metacarpal or metatarsal bone) are affected most commonly. OC of the elbow, hip, and cervical vertebral joints has also been described,12,13 but lesions in these sites are less common and the etiology is more controversial.12 This typical pattern of OC contrasts with that of the atypical pattern in which animals show numerous articular (and sometimes physeal) lesions.12,14 Predilection and nonpredilection sites may be affected in these horses, and bilaterally symmetrical lesions are absent or infrequent. A third pattern of lesion distribution, the mixed pattern, describes horses in which both typical and atypical lesions are present.14
OC may manifest very early in life. For example, lesions of the cranial aspect of the intermediate ridge of the tibia have been identified in foals that are less than 1 month old.15-17 Lesions of the lateral trochlear ridge of the femur may appear later (3 to 4 months of age),17 but lesions at this site, on the medial femoral condyle, and in the tarsocrural and fetlock joints all develop before approximately 7 or 8 months of age.18,19 The studies from which these conclusions were drawn were conducted in a range of breeds. In contrast, recent radiological data suggest that the prevalence of OC may increase substantially after approximately 1 year of age in South German Coldbloods.20 Further work is necessary to establish whether there are breed differences in the timing of lesion development.
It is important to realize that most osteochondral lesions identified radiologically in young horses heal without intervention17,21-23 and that lesions that lead to clinical signs represent only a small fraction of the total. Thus many of the lesions identified in postmortem studies would never have become clinically relevant and may not even be evident radiologically. The age at which lesions are capable of repair appears to depend on the joint involved. A longitudinal study involving Dutch Warmbloods determined that OC lesions of the hock (the cranial aspect of the intermediate ridge of the tibia and the lateral trochlear ridge of the talus) that were still present at 5 months of age never regressed.17 Five months was thus designated as the “age of no return” for tarsocrural lesions. In contrast, lesions of the lateral trochlear ridge of the femur did not become permanent until the animal was 8 months of age.17
The features of equine OC lesions were reported as early as 1947.9 Since then the gross and histological characteristics of the condition have been described and defined by numerous authors. Early descriptions characterized OC as a lack of chondrocyte differentiation that prevented provisional calcification of the matrix and invasion of the cartilage by blood vessels.3 Necrosis was described as a secondary change.3 A more recent histological study that examined AECC samples from the lateral trochlear ridge of the femur of horses ranging in age from 270 days’ gestation to 4 years defined OC (“dyschondroplasia”) as the presence of cartilage cores (i.e., cartilage extending into subchondral bone).24 This study identified two types of lesion that could be differentiated on the basis of type VI collagen immunoreactivity. However, both types showed evidence of chondrocyte clusters and chondronecrosis. Lesions of one type (group A) showed disruption of the normal sequential transition of chondrocytes through the stages of proliferation and maturation and were characterized by accumulation of large numbers of small, rounded chondrocytes, apparently arrested at the prehypertrophic stage. In contrast, group B lesions showed alteration in the staining pattern of mineralized cartilage and adjacent subchondral bone and complete absence of invading capillaries into newly formed bone.24,25 Differentiation of lesions into subcategories was not reported in a recent histological study in which necrosis of growth cartilage was described as a common feature of OC lesions.15 This study was carried out using material from the distal tibiae of foals that were all 5 months old or younger (the age range during which the disease is initiated at this site).17 The results suggested that chondrocyte necrosis precedes matrix change (identified histologically as relative eosinophilia and pallor in hematoxylin-eosin–stained sections and as pallor in toluidine blue–stained sections), delayed ossification, and fissure formation. Moreover, these authors considered chondrocyte clusters to be a sign of attempted repair rather than a primary change.15
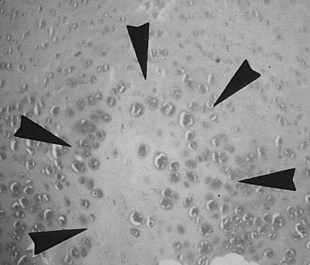
It is obvious from this short summary that histological definitions and descriptions of OC vary, making accurate identification of lesions difficult.24 Even chondrocyte clusters (Figure 54-5), considered by many to be one of the most consistent findings in OC lesions,24,26 are not pathognomonic for the disease,15,24 and, unless used to define the condition, necrosis is not a universal finding.25,27 It is thus not surprising that the relevance to OC of some of the lesions studied has been questioned.28 The matter is complicated further by the limited repertoire of responses that bone and cartilage can mount to injury and developmental abnormalities12 and by the fact that many lesions are not identified until they have reached the chronic stage. The features of such chronic lesions may represent the results of secondary change and attempted repair, rather than primary osteochondrotic change. The results of studies based on such lesions may thus be misleading. This situation has resulted in a wide-ranging, heterogeneous, and somewhat confusing body of literature, in which equine OC has been ascribed to genetic, dietary, endocrine, biomechanical, traumatic, ischemic, and toxic causes.12,18,26,29-33
Relationship among Physeal Dysplasia (Physitis), Subchondral Bone Cysts, and Osteochondrosis
The relationship between OC and physeal dysplasia (physitis or epiphysitis) is poorly defined. Retained cartilage has long been regarded as a possible cause of physeal dysplasia,16,34 and OC lesions of the physis have been described in clinically affected horses (thickened and irregular metaphyseal growth cartilage)5,10 and in foals with experimentally induced dyschondroplasia (retained cartilage cores).26,35 If OC truly is a generalized or multifocal disturbance of endochondral ossification, it would seem logical that metaphyseal growth cartilage has the potential for involvement. However, some have questioned whether articular and physeal lesions have the same cause and have proposed that physitis be regarded as a manifestation of developmental orthopedic disease but not of OC.12 Moreover, the results of a recent study suggest that many enlargements of the distal aspect of the third metacarpal and metatarsal bones, including those with evidence of increased metaphyseal cartilage thickness, represent physiological remodeling rather than a pathological process.36 The role of OC in the pathogenesis of equine physeal dysplasia thus remains unclear. However, it should be noted that a similar pathogenic mechanism (failure of blood supply to growth cartilage) has been proposed for both articular OC and physeal lesions in pigs.1 Physitis is covered in more detail in Chapter 57 and is not discussed further here.
The relationship among OC, subchondral bone cysts, and osseous cystlike lesions is also controversial. In contrast to OCD lesions, which are most commonly found on the nonloaded margins of high-motion joints, bone cysts typically occur in the central, loaded areas of joints.2 Originally interpreted as a manifestation of retained cartilage of the AECC,10,37-39 many cysts are now thought to be traumatic in origin.40,41 This proposed cause was demonstrated experimentally by the successful induction of cysts in the medial femoral condyle (a predilection site) after creation of a linear slit in the articular cartilage followed by normal weight bearing.42 OC may thus be only one of several possible causative mechanisms,12,40,43 and cysts may result from a number of nonspecific articular injuries sustained at a load-bearing site.12
Proposed Causative Factors
Numerous studies aimed at determining the cause and pathogenesis of equine OC have been performed, and many of the factors investigated have been shown to influence the incidence of osteochondral lesions. However, because OC cannot yet be diagnosed on anything other than morphological grounds, it is unclear whether the lesions induced by some of these potential causative factors are the same as those that occur under “natural” conditions. A pertinent example is low copper intake, which has been shown to induce osteochondral lesions44 but which is not believed to be a causative factor in most horses with naturally occurring OC.1,45
It is generally accepted that equine OC has a multifactorial cause.1,25,45 This renders more difficult the task of unraveling the pathogenesis of the disease. Further complications are introduced by the presence of interrelationships among a number of proposed causative factors. For example, a horse’s growth rate may be affected by its genetic background,46 its plane of nutrition,47 and possibly also by its hormonal response to carbohydrate intake,48 all factors that have been implicated in the pathogenesis of OC.
Another topic that requires clarification is the balance between generalized or systemic and local factors. OC has historically been described as a generalized failure of endochondral ossification.16 However, the propensity for lesions to occur in a small number of bilaterally symmetrical predilection sites argues strongly that local factors are important in the cause. It remains to be determined whether lesions can be induced solely by these local factors or whether the presence of an underlying generalized disorder (e.g., biomechanically or biochemically inferior tissue) is a prerequisite for lesion development. The following discussion summarizes major findings relating to the pathogenesis of equine OC.
Involvement of Cartilage Canals
There has been much interest recently in the role of cartilage canals in the pathogenesis of equine OC. Cartilage canals are channels that invade the epiphyseal growth cartilage from the surrounding perichondrial plexus.49 The arterioles, venules, and capillaries that they contain assist in the nutrition of epiphyseal cartilage, much of which is too far from synovial fluid to obtain nutrients by diffusion.1 Under normal circumstances the cartilage canals become obliterated in a regionally staggered sequence29,49 as endochondral ossification (and therefore growth) ceases. Studies performed to date have found no evidence of cartilage canals in foals after approximately 6 or 7 months of age.18,27,50 There is an apparent anatomical association between prolonged dependence of cartilage on a vascular supply and predisposition to OC.29
A number of investigations have highlighted an association between the presence of cartilage canals and development of OC. However, the proposed pathogenic mechanism differs among studies. A report published in 1995 that involved examination of tissue from 35 horses younger than 18 months of age found that cartilage canals containing patent blood vessels were present in all samples taken from OC predilection and nonpredilection sites in foals younger than 3 weeks of age.18 Overall, 34% of the horses had lesions of OC in the medial femoral condyle, lateral femoral trochlear ridge, and/or distal aspect of the tibia, and the prevalence rose to 56% in horses 3 weeks to 5 months of age. All the lesions seen in this age group (3 weeks to 5 months) were associated with necrotic cartilage canal blood vessels. Lesions found in horses 7 months of age and older had extensive involvement of subchondral bone and bone marrow and were considered to be chronic. The authors concluded that OC lesions develop before 7 months of age and that ischemic necrosis of cartilage is involved in the pathogenesis of the condition.18
This conclusion is supported by the results of more recent work conducted using tissue from the distal tibiae and tarsi of foals 5 months of age or younger.15,29,51 From the results of a histopathological study, it was concluded that early OC lesions manifest as areas of cartilage canal and chondrocyte necrosis within the proliferative zone.15 As the ossification front advances, these lesions become incorporated in the chondro-osseous junction where the necrotic chondrocytes and altered matrix are believed to represent conditions unfavorable for vascular invasion and replacement by bone (Figure 54-6). Vascular perfusion techniques were used to show that as foals grow the midportion of each cartilage canal becomes incorporated into the ossification front.29 Anastomoses then form between canal vessels and subchondral vessels at this point.29 As a result, tissue that is nourished by the vessels in the end of the cartilage canal farthest from the perichondrium comes to be nourished by subchondral vessels. The histological lesions identified were consistently characterized by the presence of necrotic growth cartilage in association with necrotic cartilage canal vessels located at the point where the vessels cross the ossification front, suggesting that the vessels are prone to failure at this point.29 These results are supported by the results of micro–computed tomography analysis.51
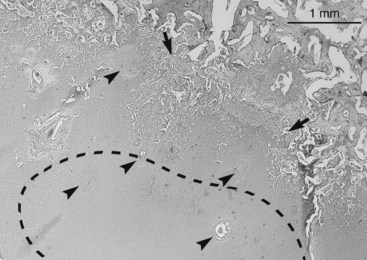
Fig. 54-6 Histological section from the intermediate ridge of the tibia of a 3-week-old foal showing an area of chondrocyte necrosis within the proliferative zone (stippled outline) and a band of chondronecrosis within the ossification front (between arrows). Both are associated with necrotic cartilage canals (arrowheads). Toluidine blue stain.
(Courtesy Osteochondrosis Research Group at the Equine Clinic, Norwegian School of Veterinary Science, Oslo, Norway.)
An earlier study proposed a different mechanism for involvement of cartilage canals in the pathogenesis of OC. In this investigation, which involved horses that were in some cases considerably older than those in the previous study (≤15 months), nonnecrotic dyschondroplastic lesions were associated with cartilage canals containing patent vessels.27 This finding led the investigators to propose that in some circumstances the presence of cartilage canals may be associated with failure of chondrocytes to hypertrophy and that this may be the initiating lesion. It was suggested that blood vessels within cartilage canals may expose local growth cartilage to any imbalance of systemic hormones (such as that induced by a high-energy diet) and that chronicity of lesions accounted for the difference between these findings27 and those of studies in which necrosis is a dominant feature. It should be noted that proximity of osteochondral lesions to the remnants of cartilage canals has been reported in many studies, but is not a universal finding.52
Body Size and Growth Rate
The widespread anecdotal belief that OC is more prevalent in large horses and those with a rapid growth rate gained support from the results of a Swedish survey involving 77 Standardbred (STB) foals.53 This study reported a positive relationship between radiologically evident OC of the tarsocrural joint and body weight at birth, body weight during the growth period, average daily weight gain, and skeletal frame size. Seven of the eight foals that developed tarsocrural OC were sired by the same stallion, but the relationship among OC, body measurements, and growth rate was still present when affected and unaffected foals by this sire were considered in isolation.
In postmortem studies, positive associations were reported between various measures of body size and number and severity of OC or OC-like lesions in the limbs and cervical vertebrae of 12-month-old animals of various breeds47 and between recent average daily weight gain and tarsocrural osteochondral lesions in 5-month-old Thoroughbred (TB) foals.54 However, this latter study found no significant association between body weight and the frequency or severity of lesions, a finding that was echoed in a recent study involving Hanoverian foals.55
Further evidence for a relationship among OC, body size, and growth rate comes from a study performed in Warmbloods.56 Weight and height were measured from birth to 5 or 11 months of age in 43 foals with a presumed genetic predisposition to OC of the femoropatellar or tarsocrural joints. The foals’ stifle and hock joints were evaluated radiologically, macroscopically, and histologically. Development of femoropatellar OC was associated with a higher overall rate of weight gain and greater final body weight and height. Moreover, the period during which weight gain of the OC-positive foals was significantly higher than that of the OC-negative foals coincided with the period during which femoropatellar joint lesions become visible radiologically.17 In contrast to the previously described findings in STBs,53 no relationship existed between tarsocrural OC and body size or growth rate in this population of Dutch Warmbloods.56 A similar lack of correlation between surgical OC lesions and body weight has been reported in TBs.57
Nutrition
A number of dietary factors have been implicated in the pathogenesis of OC. These include digestible energy, phosphorus, and copper.
Digestible Energy and Protein
The results of a French study have suggested that a high plane of nutrition does not in itself predispose to development of OC, as long as the ration is balanced.47 However, a high nutrient intake was implicated in the pathogenesis of OC for many years10 and the topic has been explored in a number of studies. One landmark trial investigated the effects on skeletal development of feeding 129% of National Research Council (NRC, 1989)58 recommendations for digestible energy or 126% of NRC recommendations for crude protein to foals approximately 5 months of age.26 A control group received 100% of NRC recommendations for energy and protein. After 12 to 16 weeks on the experimental diets, all foals were euthanized, growth plates and the growth cartilage of the AECC were examined, and a definitive diagnosis of dyschondroplasia was made only when a retained core of cartilage was identified histologically. Dyschondroplasia occurred in two of 12 control foals (17%), four of six foals on the high-protein diet (67%), and all 12 foals on the high-energy diet. The lesions in the foals in the high-protein group were minor and were mainly single lesions of the growth plates, with no AECC involvement. No significant difference in incidence occurred between the control and high-protein groups. In contrast, many of the foals in the high-energy group had lesions of the AECC and the growth plates, and the difference in incidence between the control and high-energy groups was significant.
The effects of overfeeding on endochondral ossification within the growth plate have been described. Twelve TB weanlings aged 6 to 8 months were randomly assigned to receive diets containing 70%, 100%, or 130% of the National Research Council recommendations (1978)59 for digestible energy and protein.60 All diets contained 100% of the recommended levels of calcium and phosphorus. Biopsies of the distal radial physis obtained after 8 months showed that physeal thickness was directly proportional to digestible energy level. Moreover, the physes of the overfed horses showed many features similar to those of OC: the reserve and hypertrophic zones were enlarged, the hypertrophic cartilage had lost its normal columnar organization, and metaphyseal capillaries appeared unable to penetrate this abnormal hypertrophic cartilage. It was concluded that the lesions associated with overfeeding were similar to those caused by hypothyroidism and that the link between dietary excess and OC is mediated by endocrine factors.60
Subsequent work showed that ingestion of a high-energy meal is associated with accelerated insulin secretion, decreased thyroxine (T4) secretion, and accelerated conversion of T4 to triiodothyronine (T3).61 Insulin, T3, and T4 play a role in controlling the terminal differentiation of chondrocytes,62,63 and it is possible that the transient postprandial hypothyroxemia induced by diets high in energy could adversely affect osteochondral development. Moreover, studies conducted on equine tissue suggest that insulin may promote the survival or depress the differentiation of chondrocytes in growth cartilage, potentially reducing the rate at which cells enter the terminal phases of hypertrophy and leading to accumulation of prehypertrophic chondrocytes.64
The possibility of a relationship between abnormal insulin levels and osteochondral lesions is supported by the finding that postprandial plasma glucose and insulin levels are significantly higher in young horses with OC than in unaffected animals,57,65 an association that may be mediated, at least in part, by the glycemic index of the feed.57 An association between feeds with a high glycemic index and increased plasma levels of insulin-like growth factor I (IGF-I) has been observed during periods of rapid growth.66 IGF-I, which is structurally similar to insulin, is primarily involved in the control of growth and differentiation,67 including regulation of chondrocyte growth and endochondral ossification.64,68 However, recent data show that there is cross-talk between the insulin and IGF-I pathways, and that IGF-I participates in metabolic activities such as promotion of glucose uptake.67 The suggestion that a high glycemic index feeding regimen and osteochondrotic risk may be linked via increased IGF-I levels is contradicted by a study in which OC-positive foals were found to have significantly lower IGF-I activity than those without the disease.69 The significance of the IGF-I pathway in the pathogenesis of OC thus remains unclear.
Calcium and Phosphorus
The effects of overfeeding phosphorus (388% of NRC [1989] recommendations), calcium (342%), and both calcium and digestible energy (342% and 129%, respectively) for 16 to 18 weeks was assessed in foals aged 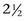 to
to 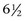 months at the start of the study.35 The diets containing excessive phosphorus and calcium provided 100% of NRC recommendations for digestible energy. Histologically confirmed dyschondroplastic lesions were found in numerous joints and growth plates in two control foals (17%), five foals fed excess phosphorus (83%), two foals fed excess calcium (33%), and six foals fed excess digestible energy and calcium (100%). The lesions were more numerous and severe in the foals fed high-phosphorus or high–digestible energy and high-calcium diets than in the control foals or those fed high-calcium diets only. Dyschondroplasia is thus not induced by diets high in calcium and is not alleviated by excessive calcium in foals fed excessive energy. The apparent association between excessive dietary phosphorus and abnormal endochondral ossification may be mediated by acidosis35 or by osteoporosis-induced weakening of the subchondral bone plate.45
months at the start of the study.35 The diets containing excessive phosphorus and calcium provided 100% of NRC recommendations for digestible energy. Histologically confirmed dyschondroplastic lesions were found in numerous joints and growth plates in two control foals (17%), five foals fed excess phosphorus (83%), two foals fed excess calcium (33%), and six foals fed excess digestible energy and calcium (100%). The lesions were more numerous and severe in the foals fed high-phosphorus or high–digestible energy and high-calcium diets than in the control foals or those fed high-calcium diets only. Dyschondroplasia is thus not induced by diets high in calcium and is not alleviated by excessive calcium in foals fed excessive energy. The apparent association between excessive dietary phosphorus and abnormal endochondral ossification may be mediated by acidosis35 or by osteoporosis-induced weakening of the subchondral bone plate.45
Copper
Copper is a necessary cofactor of lysyl oxidase, the enzyme that catalyzes the oxidative deamination of lysine and hydroxylysine residues to their corresponding aldehydes. This is a necessary step in the formation of pyridinoline cross-links in collagen and elastin. Low-copper diets have been associated with an increase in the soluble fraction of articular collagen, reduced collagen cross-linking of cartilage and bone, and an increased incidence of osteochondral lesions in growing foals.44,70 A plausible mechanistic link thus exists between low copper levels and the development of OC, via the formation of biomechanically weak cartilage and bone. However, the clinical significance of this proposed pathogenic mechanism in the cause of naturally occurring OC remains unclear.
Several early studies showed an association between primary or secondary copper deficiency and equine osteochondral lesions.70-72 However, the lesions reported—which included intrachondral splitting through the hypertrophic zone and denudation of subchondral bone—were generally much more widespread and severe than typically occur under field conditions.70-72 Moreover, retention of cartilage was not a major pathological feature.70 This link between copper deficiency and development of osteochondral lesions prompted investigations into the effects on equine skeletal development of copper supplementation during late gestation and the early growing period. In one such study, 21 pregnant mares were assigned to a control group (13 ppm dietary copper) or a supplemented group (32 ppm) during the last 3 to 6 months of pregnancy and the first 3 months of lactation.32 The foals were subsequently fed diets containing 15 ppm (control) or 55 ppm (supplemented) copper for up to 6 months. The copper content of the control foals’ diet was close to NRC recommendations. OC was defined as thickening of cartilage within the physis or AECC. Compared with the supplemented foals, 6-month-old control foals had nearly twice as many lesions in the physes and more than five times as many in the AECC. The most notable lesions in the control group were cartilage thickening and separation of the cranial aspect of the intermediate ridge of the tibia.32
A separate study investigated the effects of feeding diets containing 8 and 25 ppm copper to 3-month-old foals for 6 months.44 Cartilage and bone lesions were rare in the foals fed 25 ppm copper. In contrast, the majority of the foals fed 8 ppm copper were severely affected with numerous lesions. Cartilaginous flaps and cartilage thinning, erosion, and eburnation comprised the majority of the lesions, many of which were associated with microfractures within the physes and primary spongiosa of the long bones and cervical vertebrae. Biochemical analysis of tissues from four low-copper–diet foals with OCD-like lesions revealed significantly fewer pyridinoline cross-links in articular cartilage, physeal cartilage, and bone than in tissues from a group of six foals (four supplemented, two on low-copper diets) with no lesions. This study thus demonstrated a link among low dietary copper, inferior collagen quality, and OCD-like lesions.44
The pathological condition induced in these two studies by dietary copper levels of 8 and 15 ppm appeared similar to naturally occurring OC grossly, radiologically, and in many cases histologically.32,44 However, the relevance to the field situation of the lesions recorded remains unknown. Foals fed the lower amount of dietary copper in these studies typically had numerous lesions, many of which were present in the cervical vertebrae.32,44 This lesion distribution would thus be described as atypical.12,14 Moreover, it is evident that relatively high levels of dietary copper (25 and 55 ppm) do not completely prevent osteochondral lesions,32,44 and that relatively low levels (4 to 11 ppm) do not always result in foals with numerous osteochondral abnormalities.26,52,73 Supplementation of foals’ diets with copper is thus not a panacea, nor is it always effective.73 Provision of copper supplements to pregnant mares has also generally been unsuccessful in reducing the incidence of osteochondral lesions at 5 to 6 months of age,52,54 although one study did show a positive effect.73
Heredity
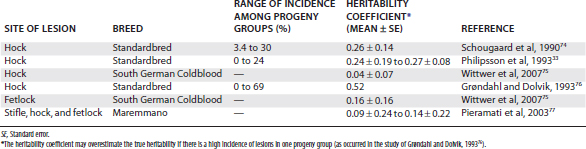
Heritability studies of equine OC have been completed in a number of breeds. The proportion of the total variation in incidence attributed to genetic factors is expressed as the heritability coefficient, and values of up to 0.52 have been reported for OC of the hock (Table 54-1).33,74-77 Although the standard errors are in some studies substantial and the range of heritability coefficients reported is quite wide (a factor that may be attributed, in part, to variation in the mathematical approaches used),75,77 these findings suggest that at least some manifestations of OC have a genetic component.
Recent genome-wide searches for markers associated with OC have identified significant quantitative trait loci on a number of equine chromosomes in Hanoverians and South German Coldbloods.78,79 Further study has revealed a significant association between a single nucleotide polymorphism in the AOAH gene and fetlock OC in South German Coldbloods,80 but a role in osteochondral development or repair for the protein encoded by this gene (acyloxyacyl hydrolase) has not been identified.
In spite of the success of these early genomic studies, it remains likely that OC is a polygenic disorder81 that develops from the superimposition of environmental factors on a susceptible genetic background. Moreover, genetic factors may influence the development of disease either directly or via influences on other factors that appear to be associated with OC such as conformation, growth rate, and the hormonal response to ingestion of food.53,56,57,65 Analysis of large numbers of genotypes will therefore be required for successful identification of the genes involved,82 and identification of a simple set of genetic markers is a goal that may well prove elusive.
An alternative strategy for reducing the incidence of OC is to exclude affected mares and stallions from breeding programs. The results of genomic and heritability studies suggest that OC lesions at different anatomical sites may be genetically related,75,78 and this raises the possibility that it may be possible to reduce the overall incidence of the disease by such a strategy of selective breeding. The results of mathematical modeling suggest that the incidence of OC in Maremmano horses could be reduced from 16% to 2% over five generations by active selection of both stallions and mares free of the disease.77 Researchers who simulated the effects of various selection strategies in Dutch Warmbloods came to a similar conclusion,83 estimating that the incidence of OC would decrease from 25% to 13% over 50 years (2.4% reduction per generation) as a result of two alternative strategies: (1) active selection for OC-negative mares and stallions, and (2) progeny testing of stallions. The model output concluded that these strategies were more effective than selection of OC-negative stallions only (17% incidence at 50 years) and no active selection (21%).
Gender
Early reports suggested that the incidence of equine OC was substantially higher in males than females.9,10 Nonsignificant relationships between male gender and OC were reported in epidemiological studies in which male/female incidence ratios of 1.6 : 1 and 1.4 : 1 were found.84,85 However, a controlled study involving Warmbloods reported no gender predisposition for OC of the tarsocrural or femoropatellar joints,56 and similar findings were reported in TBs.54 In contrast, a study involving South German Coldbloods found a twofold higher risk for OC of the hock and fetlock joints in female horses,20 and a study involving feral horses found typical OC lesions only in fillies.86
Exercise
The role of exercise in the pathogenesis of OC has been investigated experimentally, with conflicting results. In one study the incidence of OC was compared in foals subjected to low- and high-exercise regimens from ages 3 to 24 months.87 All foals were housed in groups or kept in boxes and allowed paddock exercise for 2 to 4 hours per day. In addition, the low-exercise group received 15 to 45 minutes of walking exercise per day. The high-exercise group received 15 to 45 minutes of walking and trotting, plus eight to 20 gallop sprints of short duration (10 to 15 seconds). At the end of the study, hock and stifle joints were examined clinically and radiologically. OC was detected in only three (6%) of the 50 foals in the high-exercise group, but in 13 (20%) of the 66 foals in the low-exercise group, demonstrating a significant protective effect of high-intensity, short-duration exercise on the incidence of OC.
These findings were not supported by the results of a subsequent extensive investigation into the effects of exercise on musculoskeletal development. Forty-three foals with a presumed genetic predisposition to OC of the femoropatellar or tarsocrural joints were subjected to one of three exercise regimens: pasture exercise only, confinement to a box stall, and confinement to a box stall with an increasing number of gallop sprints.23 These exercise regimens were imposed from 1 week of age to 5 months, and the incidence of OC, including subchondral bone cysts, was determined at postmortem examination in a subset of the foals at 5 months of age (eight per group). The remaining foals were subjected to the same light exercise regimen for an additional 6 months before euthanasia at 11 months of age. Lesions were found in all 5-month-old foals.23 Frequency was highest in the tarsocrural joints (1.9 lesions per foal), with a lower incidence in the femoropatellar or femorotibial (1.0), cervical intervertebral (1.0), and metatarsophalangeal joints (0.6). Exercise did not influence the number of lesions, although there was a tendency for lesions to be more severe in the box-rested foals. Exercise also appeared to influence the type and distribution of lesions within the stifle joint: the foals subjected to box rest were more likely to develop subchondral bone cysts (femoral condyles), whereas the trained foals tended to develop OC or OCD lesions (lateral trochlear ridge of the femur). This difference suggests an effect of mechanical loading on lesion development because the lateral trochlear ridge is loaded by the patella during exercise,12 and subchondral bone cysts tend to develop at the point of maximum load bearing during the support phase of the stride.23,40,43 It was concluded that exercise has no role in the pathogenesis of OC, although it may alter the appearance and distribution of lesions.23 It should be noted that other studies conducted on tissues from these foals suggested that a regimen of box rest supplemented with short bouts of high-intensity exercise (gallop sprints) had deleterious long-term effects on chondrocyte metabolism and viability88,89 and cannot be recommended for optimal musculoskeletal development.
Trauma and Biomechanical Force
The roles of trauma and biomechanical force in the pathogenesis of osteochondral lesions are not well established. It is generally agreed that biomechanical forces are responsible for converting an OC lesion into a dissecting OCD lesion,5,10,16 although little solid evidence exists to support this assertion. More pertinent is establishment of the roles of trauma and biomechanical force as primary causative factors. The consistent location of typical OC lesions within joints does suggest involvement of physical factors in the pathogenesis.90 It has been proposed that physeal dysplasia could result from excessive force on normal tissues or from the superimposition of normal force on structurally deficient tissues,34 and a similar hypothesis has been suggested for the development of articular OC.14,28 Many of the factors discussed previously could lead to generation of abnormal skeletal tissues, and abnormally high forces could result from excessive or inappropriate exercise, excessive body weight, or poor conformation.34 A possible relationship between severe outward rotation of the hindlimb and tarsocrural OC was noted in a study of growing STB foals,53 but proof of a causative effect requires further research.
The Role of Enzymes and Signaling Peptides
There is evidence that disturbances in the expression or function of a number of enzymes and/or signaling peptides may be involved in the pathogenesis of OC. Cathepsins and matrix metalloproteinases (MMPs) are proteases that are involved in the degradation of collagen and other extracellular matrix components. Cathepsin B is present in the AECC of normal growing horses, particularly in the hypertrophic zone,52,91,92 and is abundant in the chondrocyte clusters that are commonly found in OC lesions.52,91,93 Increased MMP activity was identified in OC lesions, particularly in the deep zone adjacent to subchondral bone, around chondrocyte clusters, and in lines that radiate from the deep zone toward the articular surface.94 Other alterations in collagen metabolism that have been identified in association with the presence or severity of OC include changes in serum levels of biomarkers of collagen degradation and synthesis, reduced hydroxylysyl pyridinoline cross-linking (unassociated with copper deficiency), and reduced total collagen content of cartilage.95-97
The potential roles of parathyroid hormone–related protein (PTHrP) and Indian hedgehog (Ihh) in the pathogenesis of OC have been evaluated. These signaling molecules, which regulate chondrocyte differentiation and hypertrophy in growth cartilage via a negative feedback loop,98 are expressed at significantly higher levels in osteochondrotic cartilage than in control tissue, and it has been proposed that this may result in retention of prehypertrophic cartilage and delayed endochondral ossification.99,100 However, identification of reduced levels of Gli1 (the primary transcription factor for Ihh) in diseased cartilage suggests that further work in this area is necessary.100
Another peptide that may be involved in the pathogenesis of OC is transforming growth factor–β1 (TGF-β1), a signaling peptide that is particularly important in controlling mammalian endochondral ossification101,102 and that has been shown to stimulate PTHrP expression.103,104 Reduced TGF-β1 expression has been identified in the AECC of the lateral trochlear ridge of the femur in horses with dyschondroplasia at this site, and it was suggested that this may result in cessation of chondrocyte hypertrophy and an accumulation of prehypertrophic chondrocytes.105 However, the clinical significance of these findings has not been established. In this work, as in all other investigations into the pathogenesis of OC, it is important that distinctions be made between primary and secondary change whenever possible.
Toxic Causes of Osteochondral Lesions
Foals exposed to excessive amounts of zinc and zinc and cadmium in combination have been found to develop generalized, severe osteochondral lesions.31,106-108 The gross lesions described in these reports were characterized by separation of articular cartilage from subchondral bone. The role of cadmium in the pathogenesis of these lesions is unclear.31,106,108 However, the effect of excessive dietary zinc is likely to be mediated by secondary copper deficiency.109 These environmental contaminants are clearly toxic causes of osteochondral lesions and are not considered to be factors in the pathogenesis of naturally occurring OC.
Summary
A range of conditions results in failure of endochondral ossification and retention of cartilage cores at the cartilage-bone interface. However, opinions regarding pathogenesis of OC still differ widely on the major factors involved in the cause of the naturally occurring condition. The situation is complicated by the apparently multifactorial nature of the condition,1,5,8,25,45 by the fact that environmental influences and genetic susceptibility apparently combine to determine the final outcome,53 and by the superimposition of secondary changes on primary lesions. However, it is hoped that a renewed focus on the examination of early lesions may allow development of a unified theory on the pathogenesis of the disease.