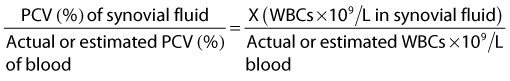Chapter 65Infectious Arthritis and Fungal Infectious Arthritis
 Infectious Arthritis
Infectious Arthritis
Classic clinical signs of infectious arthritis are heat and swelling and rapid development of non–weight-bearing lameness, often in less than 24 hours. The suspicion of joint infection increases if a predisposing risk factor is evident, such as prematurity, a high sepsis score, or multisystemic disease in a foal1,2 or preceding joint injection in an adult horse. Fracture and nonarticular infection (cellulitis or foot abscess) need to be differentiated from infectious arthritis, because in these diseases, acute, severe lameness also develops.3,4
Causes
Musculoskeletal infection was reported to cause death in 5.2% of 2468 foals.5 Yearly morbidity was 27.4% (677 foals), and morbidity attributed to musculoskeletal infection was 2.1%. Septicemia was the second most common cause of death, and hematogenous spread was the most common cause of infectious arthritis.6 Bacteremia with infectious arthritis in foals decreased survival.7 The risk of infection was highest in the first 30 days postpartum and was lowest in practices that assessed the efficacy of transfer of passive immunity. Isolation of Salmonella species from synovial fluid and systemic disease were associated with an unfavorable prognosis for survival.1
In two retrospective studies of 153 mature horses8,9 the most common causes of synovial infection were traumatic wounds (36.5% for joints and 55% for tendon sheaths), injections (34.1% and 22%), postoperative infection (1.0% for arthroscopy,10 19.8% overall for joints), and idiopathic causes (9.5% and 22%).9 Standardbreds,9 draft breeds,10 and the tarsocrural joint9,10 were overrepresented in adult horses with joint infection, reflecting a greater number of joint injections in these breeds and this joint.
Of 424 bacterial isolates from 233 horses with joint, tendon sheath, or bone infection, 91% were aerobic or facultative anaerobes. The most common organisms were Enterobacteriaceae (28.8%), followed by streptococci (13%) and staphylococci (11.8%).11 In foals, Enterobacteriaceae including Escherichia coli and Staphylococcus species1,12 were more likely to be isolated. Staphylococci, specifically Staphylococcus aureus, are the most common organisms isolated from infections occurring after surgery or injections. Foals or horses with infectious arthritis secondary to penetrating wounds are likely to have multiple bacterial infections. Clostridium species have been isolated from foals13 and were the most common anaerobes isolated, particularly from wounds near the hoof.11 Fungal or mycobacterial organisms are a rare cause of infectious arthritis but can be considered pathogens if identified in pure culture more than once (see later).14,15 Reactive arthritis in foals with septicemia from Rhodococcus equi or subsequent to injection reactions can be confused with infectious arthritis, but lameness usually is not prominent, and synovial fluid nucleated cell counts are often within normal limits.16,17
Examination and Initial Management
Potential infectious arthritis must be considered an emergency and is treated most effectively with early diagnosis. A systematic approach should include hematological examination; measurement of plasma fibrinogen; synovial fluid analysis, including cytological examination, Gram stain, and synovial fluid culture (up to 5 mL of synovial fluid in a broth culture bottle); and radiography.3 In foals, particularly those with abnormal sepsis scores, blood culture should be performed simultaneously. In adult horses, systemic blood examination is less rewarding than in foals, particularly in the early phases of clinical signs. In horses with experimental infectious arthritis, elevations in leukocyte count and total protein and fibrinogen concentrations have been found to take several days to develop and were changed significantly from baseline values for individual horses, but they remained in the normal range for all horses.18 Before arthrocentesis is performed, surgical scrub materials, sterile gloves, needles, syringes, broth culture bottles, ethylenediamine tetraacetic acid (EDTA) or heparin tubes for cytological examinations, smear slides for Gram stain, and a dose of antimicrobial agents to instill directly into the joint after sampling should be available (see the following discussion).
Adult horses should be sedated. Foals can be placed in lateral recumbency with administration of an α2-agonist and synthetic narcotic combination. Although data suggest that aseptic preparation of unclipped hair may be adequate and may significantly decrease bacterial counts on the skin,19 clipping the site for arthrocentesis is strongly recommended. In horses with periarticular wounds, arthrocentesis should be performed well away from the wound to avoid the risk of joint contamination, if the joint is not contaminated already from the wound. To determine if a joint and wound communicate, the clinician should infuse 50 to 200 mL of balanced electrolyte solution into the joint after a synovial fluid sample has been obtained for culture and cytological examination. The clinician should watch closely for leakage of fluid from the wound. This is easier than using blue dye injections or contrast radiography. The joint should be drained and antibiotic instilled into the joint after samples have been obtained and any lavage or injection of fluid completed. Samples should be submitted immediately for evaluation.
If the joint fluid is grossly cloudy, turbid, or flocculent, broad-spectrum antibiotics initially should be given intravenously (IV), and ingress and egress or through-and-through lavage of the joint should be performed until diagnosis of infectious arthritis can be confirmed. Antibiotic should be instilled into the joint after lavage has been completed. If a wound or puncture is the inciting cause, endoscopic evaluation of the synovial cavity is recommended. In one study of 95 contaminated or infected joints, 43% had intraarticular foreign material found at endoscopy.20
Diagnosis
Gross evaluation of the synovial fluid can be informative. If newspaper print cannot be read through the fluid sample, it probably has a cell count of at least 30 × 109 nucleated cells per liter. Fluid from infected joints is usually turbid, cloudy, and watery. Flocculence develops in chronically infected joints or joints that have been invaded with a needle or surgery. Blood contamination makes gross assessment of synovial fluid difficult, and determining if infection is present without clinicopathological analysis is often impossible. Serosanguineous fluid commonly is obtained from infected joints (Figure 65-1). An estimate of the amount of blood (packed cell volume [PCV)] or red blood cell count) can be made to correct for the number of nucleated cells contaminating the sample (hemorrhage from arthrocentesis or leakage of blood from the infection):
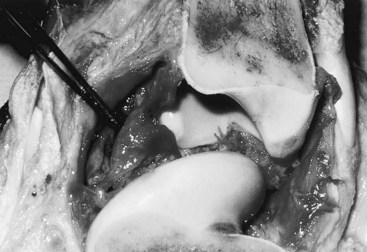
Fig. 65-1 At postmortem examination, hemorrhagic synovitis associated with acute joint infection is seen in this tarsocrural joint.
where WBCs are white blood cells.
Synovial fluid nucleated cell count, differential cell count, and total protein concentration are the most useful parameters to evaluate in diagnosing infectious arthritis. Although other biomarkers, such as lactate, metalloproteinases, and myeloperoxidase activity, have been found to be increased in synovial fluid from infected joints, most correlate with the nucleated cell count.18,21,22 Synovial fluid nucleated cell counts in excess of 30 × 109 cells/L, with more than 80% neutrophils, or total protein concentration in excess of 40 g/L are considered consistent with infection, particularly if these synovial fluid parameters correlate with clinical signs and predisposing circumstances.3,23 The likelihood of isolating the causative organism is correlated positively to the nucleated cell count—that is, higher nucleated cell counts are associated with greater isolation rates.23 Approximately 25% of samples from horses suspected of having infectious arthritis have bacteria on Gram stain, confirming infection and offering the added benefit of assisting with identifying the organism and the initial selection of an antimicrobial agent.18
For horses that do not meet obvious criteria for infectious arthritis, assessment of all parameters is necessary to conclude that the joint is infected. It is important to note that joints can be infected with nucleated cell counts lower than 30 × 109/L. Infection should be suspected if nucleated cell counts are 10 × 109/L to 30 × 109/L and fluid is not serosanguineous, clinical signs of substantial lameness exist, fluid is flocculent (coagulating cells may falsely lower the measured cell count), a predisposing cause for infection is present (septicemia, surgery, or joint injection), and protein concentration exceeds 40 g/L. Previous intraarticular treatment with corticosteroids may delay onset of clinical signs and confuse interpretation of nucleated cell counts and total protein concentration in the acute phase.24,25
Synovial fluid protein levels continue to increase in chronically infected joints to more than 60 g/L in some horses.18 Few other differential diagnoses, other than infection, produce significant elevations in total protein concentration (>50 g/L). Isolation of a pure culture of a single organism, particularly a known pathogen, such as a coagulase-positive Staphylococcus species, almost always indicates infection is present, even if the nucleated cell count is low.
In horses with chronic infectious arthritis refractory to aggressive treatment, nucleated cell counts can be low (5 to 10 × 109/L), but organisms, usually Staphylococcus, can still be isolated. In these horses the synovial fluid protein values are often high (>50 g/L) and other clinical signs of infectious arthritis (lameness, effusion, and heat) persist. In my experience these are horses that developed infectious arthritis after injection with a corticosteroid, were treated with systemic antibiotics and lavage but not aggressive drainage, have a nidus of infected subchondral bone that keeps seeding the joint, or have a joint with severe cartilage erosion.
The distribution of nucleated cells in synovial fluid is an important aid to diagnosis. In horses with early infectious arthritis, nucleated cells almost always consist of more than 80% neutrophils and commonly more than 90% neutrophils. The neutrophils usually appear healthy and not degenerate, although in overwhelming or aggressive infection, degeneration of neutrophils is seen. If synovial fluid has less than 75% neutrophils, infection is usually resolving.
Techniques that may be useful clinically in the future for diagnosis include polymerase chain reaction (PCR) analysis for detecting base pairs of bacterial or viral DNA in the synovial fluid26,27 and determining enzyme and cytokine release that may be compatible with infection.28-30 Benefits of PCR include rapid (<24 hours), sensitive testing that can detect selected species of bacteria in the presence of antimicrobial drugs. PCR diagnostic techniques are sensitive because they amplify small quantities of bacterial DNA. Inherent in high sensitivity, however, is a possible high false-positive rate, because of skin contamination or contamination with bacteria at the time of arthrocentesis. Future clinical use and evaluation of this technique are expected.
Identification of enzyme/cytokine ratios or quantity may be specific for infection, because infection is the largest joint challenge that exists. Knowledge of the presence and interaction of mediators and inhibitors in joints is growing rapidly and is an active area of research. For further information on this aspect of joint infection, the reader is referred to other publications.28,30
It is important to determine whether bone or physeal involvement is concomitant, particularly in foals with refractory infectious arthritis. In most foals with bone involvement, radiolucent changes occur rapidly and are often detectable within 1 week after onset of clinical signs. In most bones, radiological changes may be apparent in 7 to 10 days, but if the small cuboidal bones of the carpus or tarsus are involved, the clinician may face an even greater diagnostic challenge. Infarction associated with infection may slow bone resorption, and evidence of radiolucency may lag for several days.
Nuclear scintigraphy may be useful to locate foci of infected bone, particularly in identifying involvement of subclinical sites of infection in polyarthritic foals, but the technique has practical restrictions. Normal bone scans or radiolabeled white cell studies can be performed (see Chapter 19). The foal becomes radioactive, and handling of the foal, blood samples, and synovial fluid samples poses a small risk to attendants. In addition, if the joints surrounding the small bones are infected, the resolution of the scan may be inadequate to identify specific bone involvement. Magnetic resonance imaging offers promise of improved identification of osteomyelitis31,32 and has become available at many referral hospitals. Osteomyelitis is present in up to 59% of foals with infectious arthritis, but a favorable prognosis still can be achieved with treatment,1 although the prognosis for athletic function is reduced when adjacent bone is involved or infectious arthritis is protracted.
In adult horses with acute-onset lameness associated with a wound, radiological examination should be performed to rule out the presence of radiodense foreign material or concurrent traumatic osseous injury. In the absence of a wound, if lameness has already been present for some days, radiological examination is warranted to determine if there are any bony changes, which will prompt early arthroscopic evaluation and debridement. Radiological examination is also indicated in a horse that has failed to show progressive improvement after instigation of appropriate management. The presence of radiolucent areas is indicative of likely bone infection, which warrants a more guarded prognosis, especially if progressive.
Antimicrobial Therapy
Systemic Therapy
Intravenous antimicrobial therapy should be initiated immediately, before bacterial culture results are available. Systemic administration of broad-spectrum antimicrobial drugs should be combined with the local administration of antimicrobial agents. Most common combinations include penicillin with an aminoglycoside or a third-generation cephalosporin such as ceftiofur sodium or cefotaxime.33 In a retrospective study of equine musculoskeletal infections, gentamicin and amikacin were effective against 85% and 95% of equine isolates, respectively, indicating that they are good choices for initial combination therapy.11 Antimicrobial drugs given orally should be reserved for infection that is resolving, because gastrointestinal absorption is erratic, and blood and tissue levels are lower. Oral enrofloxacin is used to treat chronic bone and synovial infections in mature horses without reported incident, but enrofloxacin is not currently approved for use in horses. Enrofloxacin (Baytril 100) administered IV at 5 mg/kg once daily is effective and safe in mature (3 years of age and older) horses.34 Enrofloxacin may cause lameness and cartilage lesions in foals and should not be used.35 Enrofloxacin should not be administered to lactating mares, because the milk may concentrate the drug and subject the foal to chondrotoxic doses. (Editor’s note [MWR]: In several horses orally administered enrofloxacin has not been effective at managing infectious arthritis, even when testing has suggested that in vitro susceptibility is present.)
Antimicrobial drugs should be given IV and in general at a dosage interval that maximizes peak serum levels and sustains trough levels that are at or above the minimum inhibitory concentration (MIC) for the isolated organism. Serum antimicrobial peak and trough measurements should be periodically checked to maximize effectiveness and minimize toxicity (see the following discussion).
Aminoglycoside administered once daily may be most effective, producing a large serum aminoglycoside concentration and a greater bactericidal effect than more frequent administration.36 The aminoglycoside postantibiotic effect (duration of sustained antimicrobial killing) is concentration dependent. With a single daily dose, trough levels are lower than with repeated administration, thus lowering the risk of toxicity. Using in vivo models of infectious osteomyelitis, improved efficacy of gentamicin was demonstrated using once-daily administration (6.6 mg/kg body mass IV) compared with three-times-a-day administration (2.2 mg/kg body mass IV). In normal horses, gentamicin (administered at 6.6 mg/kg body mass once daily for 10 days) did not induce signs of nephrotoxicity and prolonged the postantibiotic effect.37 Therefore gentamicin and other aminoglycosides should be administered once daily in horses. However, the pharmacokinetics of aminoglycosides are altered in septic and premature hypoxic foals.38 Serum drug concentrations should be measured to adjust dosage intervals. Sepsis score and creatinine concentration are inversely correlated to amikacin clearance in foals and could be useful indicators of altered drug disposition and delayed clearance.39 The dosage interval may need to be lengthened beyond 24 hours in these foals to avoid toxicity because of higher trough concentrations. In septic neonatal foals, administration of gentamicin at 3.3 mg/kg twice daily IV produced peak serum concentrations of more than 6 mcg/mL and trough concentration less than 2 mcg/mL in all foals without toxicity or development of new sites of infection.40
The drug must have excellent diffusion into the joint.8 Most antimicrobial drugs penetrate the synovium in therapeutic concentrations when administered systemically at recommended doses.4 Concentrations of aminoglycosides actually may be greater in inflamed joints compared with normal joints.41 If systemic blood trough levels drop below the MIC for that antimicrobial drug, synovial fluid concentrations may also drop below the MIC. Local antimicrobial drug administration (intraarticular), used to sustain high drug concentrations at the site of infection, may be most effective in both killing bacteria and penetrating organic debris.
Most positive synovial fluid cultures will be so within 24 hours after onset of infection, and a Gram stain can be immediately helpful. An additional 24 hours is usually needed to confirm the identity and susceptibility pattern of the organism. In my opinion, a broad-spectrum antimicrobial drug should be administered initially and continued at least until clinical signs begin to resolve substantially. Some joints may contain single or several organisms that were not isolated, particularly if infection was caused by a wound or by septicemia. Joints may be open, or repeatedly invaded during treatment, or a foal may be at risk for continued showers of bacteremia, thereby increasing the risk of a shift in causative organism in the middle of treatment.
Local Therapy
For years local administration of antimicrobial agents was considered taboo, because solutions varied in pH and were believed to be injurious to tissues. The deleterious effects of local antibiotic administration were greatly overemphasized, and one of the most substantial advances in the management of horses with infectious arthritis and osteomyelitis has been the implementation of local antimicrobial therapy. High tissue concentration of antimicrobial agents causes rapid elimination of joint and bone infections.42,43 Many innovative methods are being explored to provide high local antimicrobial drug levels. Administration of systemic antimicrobial agents remains an important adjunctive therapy, but it is recognized that tissue concentrations at the site of infection may be considerably lower than those achieved with local administration of drugs (at or below the MIC for the organism), and therefore systemic administration is less effective.44
Direct Local Infusion of Antimicrobial Drugs
Intraarticular injection of antimicrobial drugs into an infected joint every 24 hours is effective for early infectious arthritis. Intracarpal administration of 150 mg gentamicin has been found to maintain synovial fluid gentamicin concentrations well above the MIC for most equine pathogens (2 mcg/mL) for 24 hours.42,45 Most antimicrobial drugs, including penicillin, the cephalosporins, and aminoglycosides, are minimally irritating.
Appropriate intraarticular doses of antimicrobial drugs are not scientifically identified, but anecdotally up to 500 mg gentamicin, 250 mg amikacin, 1 × 106 units of sodium penicillin, 500 mg cefazolin, and 500 mg ceftiofur sodium have been used without reported difficulties. Fluoroquinolones should not be used because at high concentrations the drugs are toxic to chondrocytes.46
Topical lavage of an infected joint or osteomyelitic bone is beneficial for the removal of infected organic debris, destructive enzymes, and neutrophils, but the inclusion of antimicrobial or antiseptic compounds in the lavage solution is still of questionable benefit. An increase in the local antimicrobial drug concentration is expected after lavage with antimicrobial drugs, but most of the drug leaves the joint with the lavage solution. Injection of antimicrobial drugs at the termination of lavage is probably more efficient. Use of antiseptics and potentiated antiseptics (EDTA and Tris buffer) in lavage may kill surface bacteria, but sustained killing is expected to be limited.47 Even dilute antiseptic compounds such as 0.05% chlorhexidine can be irritating to equine joints.48
Antimicrobial-Impregnated Biomaterials
One of the most practical methods for maintaining slow but effective release of antimicrobial agents in bone and joint infections is the intraarticular insertion of impregnated polymethylmethacrylate (PMMA) beads.49 In a study of 1085 open limb fractures in people, the postoperative infection rate was significantly reduced, from 12% to 3.7%, when aminoglycoside PMMA beads were inserted at surgery.50 PMMA impregnated with aminoglycoside and cefazolin was used in horses with open fractures and with bone, implant, and joint infections, and survival rate was about 60%.51,52 In 12 horses with infectious arthritis treated with gentamicin-impregnated PMMA beads and lavage, including six with osteomyelitis, 92% survived.53 Several antimicrobial drugs were shown to elute from PMMA in active concentrations, including aminoglycosides such as amikacin and gentamicin, and fluoroquinolones such as ciprofloxacin.43,53-55 Use of fluoroquinolones in PMMA is not currently recommended in joints, because the concentrations expected to be released with local therapy may be toxic to equine chondrocytes.46
Implants (beads) can be prepared in the operating room, or beads can be gas sterilized and stored for future use. The antimicrobial compound (1 or 2 g of powder or liquid) is mixed thoroughly with 20 to 40 g of PMMA (medical grade) and shaped as desired. Beads can be placed on suture material to assist with retrieval from the joint. The beads can be placed into the joint through an arthrotomy, which is usually left open to drain. If infection recrudesces, existing beads can be exchanged with fresh ones or with those impregnated with different antimicrobial agents. Small beads (4 × 6 mm) can be placed through arthroscopic portals or cannulas, thus avoiding arthrotomy. Arthrotomy, however, can also assist with joint drainage if left open to heal by contraction and epithelialization. In my experience, if infection is rapidly eliminated, the beads can be difficult if not impossible to retrieve. Beads frequently migrate to a joint pouch and become enveloped by synovium. If the arthrotomy heals quickly, the beads may need to be removed surgically. Occasionally beads well removed from articular surfaces can be left in place permanently. Long-term sequelae appear minimal, but studies to date have not been performed. Complications with PMMA implants include the use of too many or too large beads, causing soft tissue trauma and pain, failure to exchange beads in unresolving infection, and placement of beads under tendons and on articular cartilage. If osteomyelitis exists, lesions can be debrided and lavaged, and beads can be implanted directly into the bone bed. Beads placed directly into bone lesions are usually removed without difficulty.
Addition of antimicrobial compounds to PMMA alters the biomechanics of PMMA, particularly when liquid forms are used. For purposes of treating joint infection, accuracy in drug elution and biomechanical properties of the PMMA are not critical. Use of the PMMA in orthopedic implants may require more precise preparation of implant material. The elution rate of antimicrobial drugs varies with size and shape of the implant, the amount of antimicrobial agent impregnated, and the type and form of antimicrobial drug selected and depends on thorough and even mixing of the formulation.
Antimicrobial-Impregnated Biodegradable Drug Delivery Systems
Although PMMA offers advantages for local antibiotic delivery, its permanency is not ideal, particularly for certain tissue types. Many biodegradable compounds have been investigated as possible implants for antimicrobial drug delivery, most notably collagen,56,57 dl–lactide-glycolide copolymers, polyanhydrides, polylactide, sebacic acid, tricalcium phosphate and calcium carbonate bone cement, and plaster of Paris.58-63 Because of the ongoing degradation a greater amount and duration of antimicrobial drug release can be achieved. In a study using rabbits with infectious osteomyelitis, bacterial counts were significantly lower in those treated with gentamicin-polyanhydride implants than in those treated with gentamicin-PMMA implants.60
In horses, 50 : 50 dl–lactide-glycolide copolymers and poly(dl)lactide impregnated with gentamicin that eluted for 10 days eliminated infection of synovial explants in vitro without significant detrimental effects on synovial cell function (hyaluronan production), morphology, or viability.58 In horses with experimentally induced Staphylococcus joint infection, intraarticular treatment with C44 fatty acid–sebacic acid (1 : 1) beads impregnated 20% with gentamicin as the sole treatment effectively eliminated joint infection in 33% of joints by day 3 and 66% of joints by day 13.62 Lameness significantly improved. Gentamicin concentration peaked at 82 mcg/mL at 24 hours after insertion of beads and remained higher than 10 mcg/mL for 12 days (range of 5 to 41 times the MIC for the organism).62 Currently these implants are being investigated for clinical use, but they are not yet commercially available. Plaster of Paris beads may offer a practical option for degradable implants, but biocompatibility studies should be performed.63
Regional Perfusion
Regional perfusion can be used to deliver therapeutic concentrations of antimicrobial agents to a selected region of the limb, usually using the venous system for drug administration with horses under anesthesia or sedated. Drugs also can be administered using an intraosseous route.64 In either case a tourniquet is applied proximal to the infected bone or joint for approximately 30 minutes (Figure 65-2). Concentrations of gentamicin obtained in synovial fluid rose to a peak of 589 mcg/mL immediately after regional perfusion and declined to 4.8 mcg/mL at 24 hours.65 This technique was used to decrease WBC numbers in infected synovial fluid in experimentally induced equine arthritis and is most practical if applied when the horse is anesthetized for other treatment of infection, such as debridement and lavage, when intraosseous infusion can be performed simultaneously.66 Advantages of this technique include the use of antimicrobial drugs that may be toxic if administered systemically (vancomycin)67 or directly into the joint (enrofloxacin),68 the ability to repeat daily injections, and distribution to the entire limb including bone. Limitations include the development of vasculitis, difficult identification of the vein, and the discomfort of a tourniquet and infusion of antimicrobial drugs. Perineural analgesia can be performed. Intraarticular antimicrobial drug concentrations using intravenous regional infusion are not as high as those obtained with direct intraarticular injection.45,69
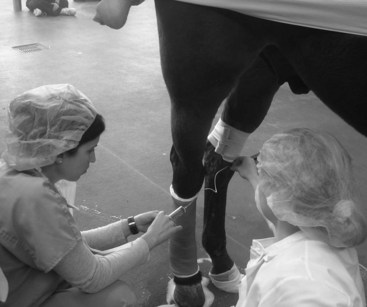
Fig. 65-2 Use of regional limb perfusion to deliver an antimicrobial drug into an infected tarsocrural joint. The tourniquet above the joint permits retrograde infusion of the saphenous vein. Concentrations of antimicrobial drugs above the minimum inhibitory concentration for most organisms that cause musculoskeletal infection can be maintained for 24 hours. The tourniquet is maintained for 30 minutes and then removed.
Bone concentration of antimicrobial agents is similar after intraarticular injection or regional perfusion.69 In foals with infectious arthritis and adjacent osteomyelitis, either delivery method should effectively provide bone antimicrobial concentrations, but for foals with numerous joint or bone sites affected, regional perfusion offers an advantage. Intraarticular administration relies on drug diffusion through cartilage or local blood supply to reach bone. Caution should be used when applying tourniquets in foals, because even short durations of application can induce clinical signs of distal limb ischemia. In foals with compromised bone blood flow from swelling and toxic effects of infection, this may be detrimental. I have seen two foals develop severe osteonecrosis after repeated regional perfusion procedures, a complication attributed to tourniquet application.
Balloon Constant Rate Infusion Systems
Constant rate infusion (CRI) of antimicrobial drugs, with or without local anesthetic solution, can be administered by commercial systems designed to deliver a constant rate of a therapeutic agent. These systems typically come as kits, and tubing is placed intraarticularly, usually at the initial arthroscopic evaluation and debridement of the infected joint. The reservoir (balloon) for the therapeutic agent(s) can be secured to the limb and aseptically refilled as needed (Figure 65-3). These systems have been reported to provide a clinical response and long-term outcome similar to other techniques for treatment of equine infectious synovitis.70 Use of local anesthetic solution in CRI systems is controversial for joints because both lidocaine hydrochloride and mepivacaine hydrochloride have demonstrated chondrocyte toxicity when in direct contact in culture.71,72
Joint Drainage and Debridement
Joint lavage and drainage are vital for the effective management of infectious arthritis.1,4,6,8,20 Ideally, arthroscopic evaluation should be performed to remove foreign material and fibrin and to assess cartilage health. Removal of angry, infected synovium may reduce bacterial count and a source of infection. Healthy synovium should not be removed, because it may help combat the infection and may normalize joint health. In horses, maximal synovectomy has been found to be no better than open arthrotomy for management of infectious arthritis.73 After synovectomy villous structures may not be regained for many months.73-76 Repeated through-and-through lavage using needles placed on opposite sides of a joint can be effective in eliminating infection in some horses, but the lavage fluid may completely bypass some areas of a joint, allowing the infection to persist. Arthroscopy permits more thorough lavage of the entire joint, and together with a form of continued joint drainage, provides the most complete and rapid method to remove infective material and to estimate the extent of damage.4,8,20
Arthroscopic portals can be enlarged to function effectively as open arthrotomy incisions for continued drainage, or closed suction drainage can be initiated. The goal of either technique is continued joint decompression and drainage. Arthrotomies usually heal without complication and with minimal scarring. With continued infection, arthrotomies stay open and may develop excessive granulation or fibrous tissue, but this complication may occur with other methods of chronic drainage as well. Open drainage in joints that cannot be bandaged, such as the stifle, can be performed by tying up the horses using crossties or an overhead wire. Adhesive bandages applied using ether can be used to cover open drainage sites. I have not seen substantial permanent complications associated with arthrotomy and open drainage. Open arthrotomy can be used to insert PMMA beads for treatment of osteomyelitis or in horses with refractory infection.
Properly managed closed suction drainage is useful for large joints such as the tarsocrural and scapulohumeral joints, using a flat, fenestrated, latex drain. The drain should be tunneled under the skin to exit at a site removed from the joint. Negative pressure is applied using a large syringe, with a large guarded needle passed through the syringe to keep the plunger retracted.77 The syringe is evacuated several times a day, and the drain is left in place until only small volumes of fluid are collected or the fluid appears relatively normal grossly and cytologically, and lameness improves. If the suction system fails, the drain is removed.
After initial debridement, lavage, and establishment of drainage, the joint can be flushed and local antimicrobial drug therapy applied daily, usually for 3 days. If clinical improvement is dramatic, further lavage is optional, but local antimicrobial injection should continue for 3 days. If clinical improvement continues, local antimicrobial therapy is discontinued. The joint is then rested for 24 hours and the synovial fluid is reassessed. Ideally, follow-up synovial fluid contains fewer than 15 × 109 nucleated cells/L, but irritation from aggressive lavage and intraarticular antimicrobial compounds can result in higher cell counts.
Clinical improvement is assessed by evaluating rectal temperature, lameness, and heat over the joint surface. The synovial fluid nucleated cell counts should decline slowly over the next 7 days if infection remains under control. If nucleated cell counts rise again or if lameness recurs, lavage and local antimicrobial therapy can be reinstituted. Having to perform two series of lavage procedures (3 days each time) in foals is typical.
Articular Osteomyelitis
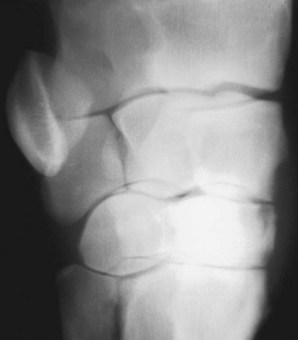
Fifty percent of foals with infectious arthritis also have osteomyelitis or bone necrosis.1,8,9,13 Usually bone is infected, but bone infarction can also occur. These foals are best treated using arthroscopic debridement, and joint infection usually clears within days of removal of infected bone. The importance of arthroscopic debridement cannot be overemphasized. In young foals with severe joint distention, lesions that would not normally be amenable to arthroscopic evaluation and debridement become accessible, such as those involving the tibial plateau (Figure 65-4), caudal aspect of the humeral head, or coxofemoral joint. In my experience infection or necrosis of the tarsal bones and some aspects of the carpal bones is the most difficult to treat, because they are not directly accessible using arthroscopy. Insertion of PMMA beads close to the distal tarsal joints and prolonged systemic antimicrobial administration may be successful.
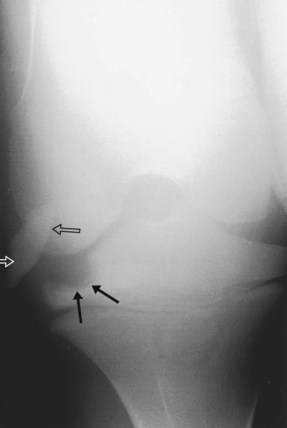
Fig. 65-4 Craniocaudal radiographic image of a foal’s stifle joint demonstrating radiolucency of the medial tibial plateau (solid black arrows) that was debrided arthroscopically and a singular polymethylmethacrylate bead (open arrows) was placed intraarticularly.
In adult horses with articular osteomyelitis it is critical to debride the infected bone as soon as the condition is recognized radiologically (Figure 65-5). In chronic, progressive, infectious osteomyelitis in adult horses, arthrodesis, joint resection, or stimulated ankylosis with a bone graft may be the only way to eliminate joint pain.78-81 Infection of implants does not always occur. Bone infection can resolve with prolonged antimicrobial drug therapy, but joint immobilization is important to assist with elimination of infective organisms.
Pain Management
Nonsteroidal Antiinflammatory Drugs
In a rabbit model of infectious arthritis caused by Staphylococcus, use of nonsteroidal antiinflammatory drugs (NSAIDs) with antimicrobial drugs significantly reduced joint swelling, prostaglandin E2 release, and collagen joint destruction compared with the administration of antimicrobial drugs alone.82 In mature horses phenylbutazone administration (4.4 to 8.8 mg/kg body mass daily) is commonly used to reduce pain and joint inflammation. Risk of laminitis in the supporting limbs of lame horses is an important consideration in treatment of infectious arthritis. Cyclooxygenase-2 (COX-2) inhibitors are approved for use in horses and seem promising at reducing the risk of toxic side effects, such as gastrointestinal ulcers and nephrotoxicity, seen with currently marketed NSAIDs.83 COX-2 inhibitors reduce the production of inducible prostaglandin E2 without suppressing the constitutive form of prostaglandin E2 that protects epithelial mucosa homeostasis. Administration of NSAIDs in horses with infectious arthritis must be titrated to allow for the accurate assessment of joint pain as an indicator of clinical response to treatment. Most currently available NSAIDs marketed for use in horses, or used off label in horses, have been demonstrated to suppress equine synovial membrane inflammation and prostaglandin E2 release in culture experiments.84,85
Dimethyl sulfoxide (DMSO) solution (10% to 40%) has been used experimentally to treat synovitis and clinically to treat infectious arthritis in horses.4 Lavage or intraarticular injection of up to 40% DMSO does not appear to have clinically significant negative effects on articular cartilage. DMSO is a free radical scavenger, antiseptic, and analgesic and may inhibit chemotaxis of inflammatory cells. Practically, DMSO can be used in lavage fluid, particularly in the last liter of lavage. Because of the high solubility of DMSO, some residual drug probably is absorbed into the synovium during lavage.
Epidural Narcotics
Placement of indwelling epidural catheters and chronic infusion of α2-agonists and narcotics such as morphine have been useful in alleviating hindlimb lameness and in horses with severe pain secondary to infectious arthritis (see Chapter 85).86,87 In horses with chronic osteomyelitis or refractory infectious arthritis, this technique may be useful in preventing recumbency or supporting limb laminitis.
Topical Treatment, Bandaging, and Alternative Therapy
Diclofenac topical medications can be directly applied to swollen and painful joints as antiinflammatory agents. DMSO applied topically to horses with experimentally induced carpal synovitis has significantly reduced synovial fluid nucleated cell counts.88 DMSO was detectable in the synovial fluid of five of six horses and in the plasma of one of six horses, indicating its penetration of the tissues from topical application.88 Topical application over infected joints may reduce synovitis.
Pressure bandages applied to reduce swelling also can reduce joint pain. Edema formation can be overwhelming in horses with infected joints and produce stiffness, discomfort, elevated synovial fluid production (joint pressure), and poor joint clearance of infected material through the lymphatic system. Pressure bandages should be sterile, particularly when covering open arthrotomy incisions.
Once acute inflammation has resolved, passive flexion is indicated to enhance lymph flow, improve drainage, reduce edema, and enhance joint range of motion. Capsulitis associated with infection can be a substantial complication, and physiotherapy to restore joint function can be critical to improving long-term outcome and joint motion.
Prognosis
Adult horses with infectious arthritis have a good prognosis for survival and for return to athletic function, provided that infection is recognized early. In most horses infection is eliminated.4,8,9,53 Preexisting osteoarthritis and articular cartilage damage are the most common reasons for failure to return to performance. Prompt recognition, aggressive drainage and lavage, and early treatment with local antimicrobial drugs have steadily improved prognosis to more than 80%8 and hopefully success rates close to 100% will be possible. Aggressive surgical debridement and use of implantable elution materials for chronic administration and multiple site delivery of antimicrobial agents have improved prognoses. The prognosis in foals is more guarded. In one study septicemia, osteomyelitis, and hypogammaglobulinemia resulted in a lower prognosis for life in foals compared with adults, and infection was eliminated in only 50%.9 In other studies of neonates1,13 and adult horses,8 elimination of infection was achieved in 70%,1,8 50% survived,8,13 and 30% reached racing performance.1
Future Treatments
Newer biodegradable polymers impregnated with antimicrobial drugs for direct joint insertion through an arthroscopic portal could greatly enhance the process of sustained drug delivery. Arthroscopic instrumentation can be used to insert the beads into the joints of standing horses.
Medical therapies for intraarticular drug therapy in the future may focus on prevention of S. aureus adhesion. A prominent feature of S. aureus virulence is the production of a bacterial surface marker that recognizes adhesive matrix molecules in collagen, precipitating bacterial adherence.89,90 Monoclonal antibody, receptor antagonists, and vaccination challenges reduce the risk of acquiring infection with S. aureus in experimental animals.89,91 These therapies may also be useful in the horse, because S. aureus is the most common cause of joint infection in adult horses.
 Fungal Infectious Arthritis
Fungal Infectious Arthritis
Literature Review
Unlike bacterial infectious arthritis, which occurs commonly in horses,1-4 there are few reports in the equine literature describing infectious arthritis caused by fungi. As with bacteria, fungi can invade a synovial structure by direct inoculation from an exogenous source (secondary to trauma, surgery, or joint injection), by hematogenous spread, or by direct extension from an adjacent focus.5 Predisposing factors to fungal infectious arthritis in people include previous (often repeated) intraarticular injection of glucocorticoids, severe systemic illness, long-term administration of systemic antibiotics, treatment with immunosuppressive agents, joint prostheses, and intravenous drug abuse.5-7 In the equine literature there is reference to fungal infectious arthritis in only six adult horses and two foals.8-13 Of these, four had a history of previous joint injection with corticosteroids, three were systemically ill (neonatal septicemia [both foals] and gastrointestinal disease [one]), and one horse had a history of trauma.8-13 All horses had had previous therapy with an antimicrobial agent, and six horses had received numerous antimicrobial drugs, including systemic administration of penicillin (five), trimethoprim-sulfamethoxazole (four), amikacin sulfate (three), ceftiofur sodium (three), ticarcillin clavulanate (two), rifampin (two), gentamicin sulfate (one), chloramphenicol (one), streptomycin (one) and metronidazole (one).9-12 Amikacin sulfate was administered intraarticularly before diagnosis of fungal infectious arthritis in two horses.8,12 Although certain fungal diseases have a regional (geographic) distribution, including coccidioidomycosis, histoplasmosis, and blastomycosis, no geographic predilection was suspected in the horses with intraarticular fungal infection.8-13 Rather, the development of fungal infectious arthritis appears to be the result of a combination of factors, including systemic or local immunosuppression (either secondary to systemic disease or the result of corticosteroid administration) and the use of systemic or intraarticular antimicrobial therapy. These likely create an ideal environment for fungi to thrive. Weakened local immune response and lack of competition from bacterial pathogens allow opportunistic organisms such as Candida to colonize a joint that would normally be inhospitable.
Clinical Signs and Diagnosis
The clinical manifestation of fungal infectious arthritis closely resembles that of bacterial infectious arthritis, including severe lameness, joint effusion, and variable degrees of periarticular edema. As is true for horses with bacterial infectious arthritis,4 the metacarpophalangeal and the tarsocrural joints are overrepresented in horses with fungal infectious arthritis.8-13 Although fever (body temperature >38°C) is detected in more than 50% of horses with bacterial infectious arthritis,4 only one of eight horses (12.5%) reported to have fungal infectious arthritis was pyrexic at the time of diagnosis.8-13 Radiological findings in seven horses with fungal infectious arthritis included the presence of focal subchondral radiolucency (six), periarticular bone proliferation (four), osteopenia (two), and joint space narrowing (two).8-13 Nuclear scintigraphy revealed diffuse increased radiopharmaceutical uptake (IRU) of all bones of the metacarpophalangeal joint, with focal intense IRU in the distal, palmar aspect of the third metacarpal bone and the proximal aspect of the proximal phalanx in one horse with Candida utilis infection.8 The reported synovial fluid nucleated cell count ranged from 15,000 to 82,000 cells/mcL (median 45,000 cells/mcL).8-12 Synovial fluid total protein concentrations ranged from 1.8 to 5.4 g/dL (median 4.3 g/dL).8-10,12
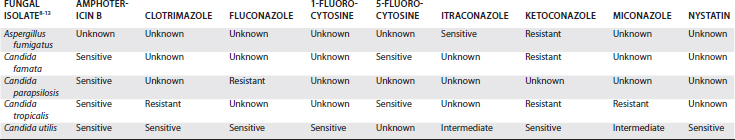
Fungal organisms were observed cytologically in synovial fluid of only one of the eight horses (12.5%) in the previous reports.8-13 Similarly, fungal organisms were identified using Gram stain in only 20% of people with fungal infectious arthritis.5 Definitive diagnosis of fungal infectious arthritis is most frequently by fungal culture from synovial fluid.8-13 Unfortunately, although fungi can be isolated from reduced solidified holding media and blood culture media,8 they are generally slow growing and it frequently takes 7 to 10 days for a positive result. During this time initiation of antifungal therapy is delayed, often resulting in exacerbation of clinical signs and hospital costs. Speciation and susceptibility testing are also slow for fungal organisms, frequently taking an additional 10 to 15 days before results are obtained. Of eight horses reported to have fungal infectious arthritis, six had infections caused by Candida species. There were two horses with Candida albicans and one each with Candida famata, Candida parapsilosis, Candida tropicalis, and C. utilis. The remaining two horses had monoarticular infection with Aspergillus fumigatus or Scedosporium prolificans. A summary of the results of in vitro susceptibility testing of horses reported to have fungal infectious arthritis is provided in Table 65-1.
Management
Successful management of horses with fungal infectious arthritis is dependent on the attending veterinarian’s ability to make a timely diagnosis and respond with aggressive medical and surgical therapy. Most horses with fungal infectious arthritis have chronic synovitis, fibrin accumulation within affected joints, and thickened periarticular tissues, making lavage in standing horses or using needles difficult. Arthroscopic debridement and lavage are mandatory in most horses; additional information about the integrity of articular cartilage and samples of synovial membrane and fibrin can also be obtained at the time of surgery. Although antiseptics such as povidone-iodine9 or antifungal agents such as miconazole9 are occasionally added to lavage fluids, there is no evidence that these improve outcome.
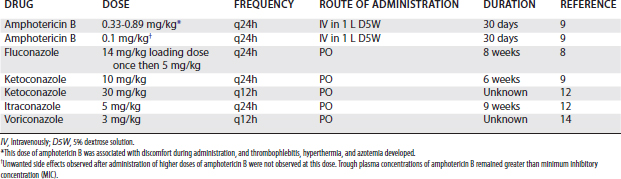
Whenever possible, medical therapy should include prolonged administration (6 to 8 weeks) of appropriate systemic antifungal agents. Antifungal drugs reported to be administered systemically in horses include amphotericin B,9-11 fluconazole,8-10 ketoconazole,9,12 and itraconazole.12 Systemic administration regimens previously used for amphotericin B, fluconazole, ketoconazole, and itraconazole and a suggested regimen for voriconazole are provided in Table 65-2. Although there is no published information regarding clinical use of voriconazole, pharmacokinetics after intravenous and oral administration have been established in the horse.14
Because in vitro susceptibility results may not be available for some time, initiation of empiric antifungal chemotherapy while awaiting final culture results is highly recommended. Although more fungal organisms isolated from equine joints are sensitive to amphotericin B than any other antifungal chemotherapeutic agent, use of this drug (both systemic and local) has been associated with severe undesirable side effects, making it a poor choice for initial empiric antifungal therapy. Rather, I recommend systemic administration of fluconazole or itraconazole combined with local administration of additional drugs (see later). In my experience, lameness will usually dramatically improve within 24 hours of administration of appropriate antifungal chemotherapeutic drugs even in horses with well-established infection.
Local administration of antifungal agents by intraarticular injection and regional limb perfusion are important adjuncts to parenteral therapy.8,10 Intraarticular administration of fluconazole (20 mg)8 and voriconazole (100 mg)15 has been used in the successful management of fungal infectious arthritis without known complications. Although intraarticular administration of amphotericin B has been reported in at least one horse10 and is regularly performed in people with fungal infectious arthritis,16 the drug is a well-established model for inducing chemical arthritis in the horse and its use is questionable. In horses in which amphotericin B is deemed necessary, based on the results of in vitro culture and susceptibility testing, local administration by regional limb perfusion may be preferred to parenteral or intraarticular administration. I gave amphotericin B at a dose of 5 mg, diluted in 50 mL of 5% dextrose, by regional limb perfusion on several occasions with minimal untoward side effects. Clinical improvement in conjunction with systemic and intraarticular administration of fluconazole was achieved in a horse with C. utilis infectious arthritis.8 Severe phlebitis and cellulitis were observed after regional limb perfusion with higher doses (100 mg) of amphotericin B.17 Pharmacokinetic studies examining the synovial fluid, tissue, and plasma concentrations of amphotericin B after regional limb perfusion have not been performed.
Prognosis
Even with an aggressive multipronged approach to therapy, the prognosis for horses with fungal infectious arthritis must be considered guarded at best. Of the eight reported horses with fungal infectious arthritis, only five survived to discharge. Of these five horses, one returned to athletic function, three were retired and used for breeding, and one was retired to pasture. However, most surviving horses had no radiological changes in the affected joints at the time of initiation of antifungal treatment, and earlier detection and treatment may have improved prognosis for athletic function. Careful cytologic examination for fungi in synovial fluid and submission of fluid specifically for isolation of fungi early in the management of horses with refractory infectious arthritis may help to diagnose fungal infectious arthritis.