CHAPTER 65 Abdominal oesophagus and stomach
ABDOMINAL OESOPHAGUS
The abdominal oesophagus is 1–2.5 cm in length, and is slightly broader at the cardiac orifice than the diaphragmatic aperture. It lies to the left of the midline and enters the abdomen through the oesophageal aperture (formed by the two diaphragmatic crura) opposite the level of the tenth thoracic vertebra. It runs obliquely to the left and slightly posteriorly, and ends at the gastro-oesophageal junction/cardiac orifice of the stomach. The abdominal oesophagus lies posterior to the left lobe of the liver, which it grooves slightly, anterior to the left crus, the left inferior phrenic vessels and the left greater splanchnic nerve; its surface is covered in a thin layer of connective tissue and visceral peritoneum which contains the anterior and posterior vagi as well as the oesophageal branches of the left gastric vessels. The anterior vagus may be single or composed of multiple trunks, and is closely related to the outer fibres of the longitudinal muscle coat of the oesophagus. The posterior vagus is usually a single trunk and is less closely applied to the oesophageal muscle within the loose connective tissue, which makes its identification during surgery somewhat easier.
The abdominal oesophagus is effectively tethered to the diaphragm by connective tissue (Fig. 65.1); the phreno-oesophageal ligament. This is formed of two thickened bands of elastin-rich connective tissue; the inferior phreno-oesophageal ligament is effectively an extension of the transversalis fascia extending beneath the parietal peritoneum as it is reflected from the diaphragm onto the abdominal oesophagus. The fibres are only loosely attached to the adventitial tissues and a variable amount of fat often lies beneath it, between the oesophageal wall and the crural sling. This oesophageal fat pad tends to act to tether the oesophagus to the fibres of the crura but tends to regress with age. On the thoracic side of the diaphragm the superior phreno-oesophgeal ligament is similarly formed from an extension of the subpleural endothoracic fascia. It is denser than its inferior counterpart with more elastin present and is tethered much more firmly through the muscle fibres of the oesophageal wall into the submucosal tissues. It may well act to restore lower oesophageal position after the movement engendered by the peristalsis of swallowing (Kwok et al 1999). Anteriorly, the subperitoneal connective tissue is particularly dense and blends with both the outer layer of the oesophageal wall and the apex of the crural fibres of the diaphragm. On the posterior aspect the peritoneal reflection is extremely short since the crura lie steeply angled, and the posterior oesophageal wall has a much shorter ‘effective length’ than the anterior. This short reflection of peritoneum is sometimes referred to as the gastrophrenic ligament and, via the peritoneum over the oesophagus continues directly onto the posterior surface of the stomach. It covers the oesophageal branches of the left gastric vessels and the coeliac branches of the posterior vagus and can thus be said to form an extremely short, wide mesentery to the abdominal oesophagus. In all but the thinnest individuals a pad of adipose tissue is found beneath the peritoneum covering the anterior surface of the lower abdominal oesophagus and the adjacent gastric wall. It is a useful surgical marker for the external location of the gastro-oesophageal junction.
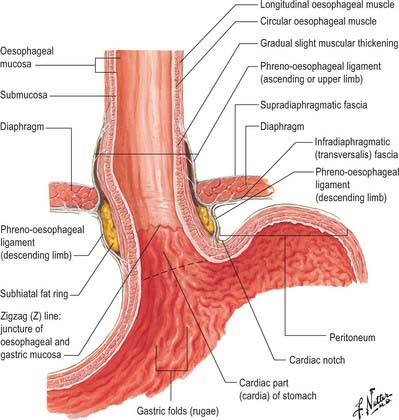
Fig. 65.1 The anatomical structures around the abdominal oesophagus.
(Reprinted from Netter Anatomy Illustration Collection, © Elsevier Inc. All Rights Reserved.)
VASCULAR SUPPLY AND LYMPHATIC DRAINAGE
Arteries
The abdominal oesophagus is supplied by oesophageal branches of the left gastric artery. These ascend as an anterior and posterior branch beneath the visceral peritoneum to supply perforating branches to the intramural and submucosal plexuses. The posterior surface usually receives an additional supply via branches of the upper short gastric arteries, the terminal branches of the oesophageal branches of the thoracic aorta and occasionally an ascending branch of the posterior gastric artery.
Veins
Veins drain via plexuses to the left gastric and upper short gastric veins (see Stomach veins below).
Lymphatic drainage
Lymphatic drainage occurs to the left gastric and left and right paracardial nodes and from the posterior surface directly to the uppermost para-aortic nodes (see Fig. 65.13).
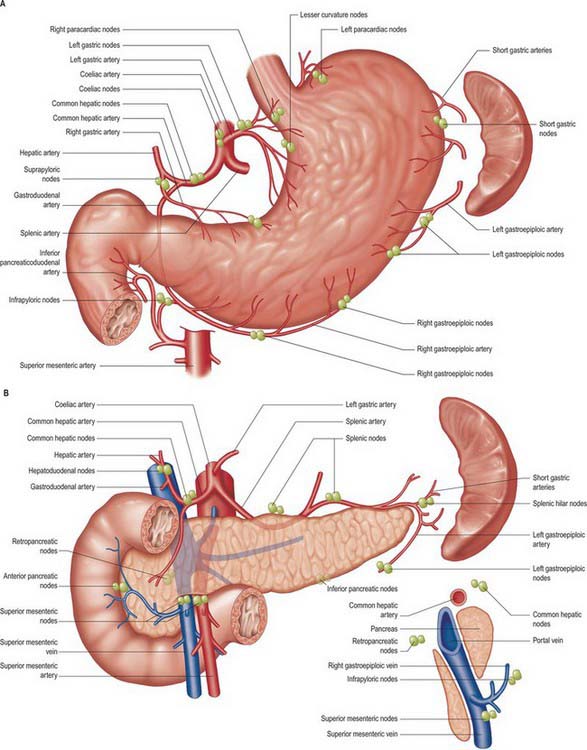
Fig. 65.13 Main lymph node stations of the stomach and upper abdominal viscera. The sagittal relationships of the node groups around the neck of the pancreas are shown bottom right. The para-aortic nodes are among the highest nodes for these viscera but have been removed for clarity (see Ch. 66).
INNERVATION
The abdominal oesophagus is innervated by sympathetic and parasympathetic fibres. The parasympathetic fibres are derived directly from the peri-oesophageal thoracic plexus and to a lesser extent from the anterior and posterior vagi. They are motor to the distal oesophagus and both stimulatory and inhibitory to the functional lower oesophageal sphincter (LOS), maintaining both background tone and co-ordinating distal oesophageal peristalsis with relaxation of the LOS during swallowing. The sympathetic supply originates from the fifth to 12th thoracic spinal segments and is mainly distributed via the greater and lesser splanchnic nerves and the coeliac plexus.
STOMACH
The stomach is the widest part of the alimentary tract and lies between the oesophagus and the duodenum. It is situated in the upper abdomen, extending from the left upper quadrant downwards, forwards and to the right, lying in the left hypochondriac, epigastric and umbilical areas. It occupies a recess beneath the diaphragm and anterior abdominal wall that is bounded by the upper abdominal viscera on either side. The mean capacity of the stomach increases from approximately 30 ml at birth, to 1000 ml at puberty, and approximately 1500 ml in adults. The peritoneal surface of the stomach is interrupted by the attachments of the greater and lesser omenta which define the greater and lesser curvatures that separate two surfaces.
PARTS OF THE STOMACH
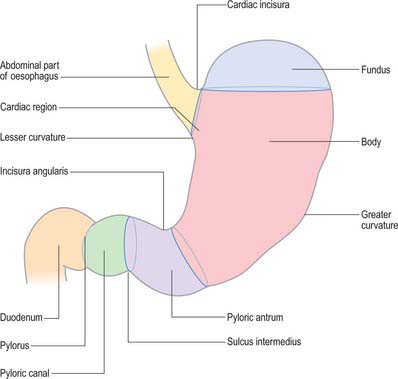
For descriptive purposes, the stomach is divided by arbitrary lines drawn on its external surface into a fundus, body, pyloric antrum and pylorus (Fig. 65.2). The internal appearance and microstructure of these regions varies to some degree. The fundus is dome shaped and projects above and to the left of the cardiac orifice to lie in contact with the left dome of the diaphragm. It lies above a line drawn horizontally from the incisura cardiaca to the greater curvature. The body extends from the fundus to the incisura angularis, which is a constant external notch at the lower end of the lesser curvature. A line drawn from the incisura angularis to an indentation on the greater curvature defines the lower boundary of the body. The pyloric antrum extends from this line to the sulcus intermedius, where the stomach narrows to become the pyloric canal (1–2 cm long), which terminates at the pyloric orifice.
GASTRIC RELATIONS
Gastric curvatures
Lesser curvature
The lesser curvature extends between the cardiac and pyloric orifices and forms the medial (posterior and superior) border of the stomach. It descends from the medial side of the oesophagus in front of the decussating fibres of the right crus of the diaphragm, curves downwards and to the right and lies anterior to the superior border of the pancreas (Fig. 65.3). It ends at the pylorus just to the right of the midline. In the most dependent part there is typically a notch, the incisura angularis, whose position and appearance vary with gastric distension. The lesser omentum is attached to the lesser curvature and contains the right and left gastric vessels.
Greater curvature
The greater curvature is four or five times longer than the lesser. It starts from the incisura cardiaca formed between the lateral border of the abdominal oesophagus and the fundus of the stomach. It arches upwards, posterolaterally and to the left. Its highest convexity, the apex of the fundus, is approximately level with the left fifth intercostal space just below the left nipple in males, but varies with respiration. From this level it sweeps inferiorly and anteriorly, slightly convex to the left, almost as far as the tenth costal cartilage in the supine position, where it turns medially to end at the pylorus. There is frequently a groove, termed the sulcus intermedius, in the curvature close to the pyloric constriction. The start of the greater curvature is covered by peritoneum which continues over the anterior surface of the stomach. Laterally the greater curvature gives attachment to the gastrosplenic ligament and beyond this to the greater omentum, which contains the gastroepiploic vessels. The gastrosplenic ligament and the greater omentum, together with the gastrophrenic and splenorenal ligaments, are continuous parts of the original dorsal mesogastrium: their names merely indicate regions of the same continuous sheet of peritoneum and its associated connective tissue.
Gastric volvulus
Volvulus of the stomach is much less common than volvulus of either the sigmoid colon or caecum. Two types of gastric volvulus may occur. The first, organoaxial volvulus, occurs about a line of rotation running from below the cardiac orifice to the pylorus. The antrum, body and fundus rotate upwards, with the greater curvature coming to lie above the lesser curvature as the volvulus progresses. The second, mesenteroaxial volvulus, occurs about a line drawn ‘across’ the body of the stomach, usually just above the incisura angularis. This type of volvulus is perpendicular to the line of organoaxial volvulus. The distal body and antrum rotate anteriorly, superiorly and laterally while the upper body and fundus rotate posteriorly, medially and inferiorly. Although relatively mobile within the upper abdomen, the stomach is normally tethered to the oesophagus at the gastro-oesophageal junction (see above), to the duodenum at the pylorus, to the spleen by the gastrosplenic omentum, and to the liver by the lesser omentum. The attachment to the transverse colon via the gastrocolic omentum also restrains the stomach but is the most mobile of all. For either type of gastric volvulus to occur, it is necessary for some or all of these points of tethering to be loosened either by previous surgical division or by chronic lengthening and loosening of their connective tissue. Organoaxial volvulus is most common because the lesser omentum, gastrosplenic ligament and gastrocolic omentum are more likely to undergo chronic lengthening by traction than the other attachments of the stomach. Mesenteroaxial volvulus requires the gastro-oesophageal junction and pylorus to be sufficiently mobile as to come into close approximation. These structures are firmly tethered and consequently this form of gastric volvulus is much less common. Despite the profuse gastric arterial supply, either type of volvulus may compromise the vascularity of the stomach.
Gastric surfaces
When the stomach is empty and contracted, the two surfaces tend to lie facing almost superiorly and inferiorly, but with increasing degrees of distension they come to face progressively more anteriorly and posteriorly (Fig. 65.4).
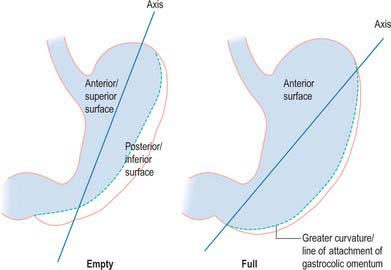
Fig. 65.4 Axes of the empty and full stomach. As the stomach distends, the greater curvature ‘rolls’ downwards and the anterosuperior surface comes to lie almost completely vertical as the anterior surface.
Anterior (superior) surface
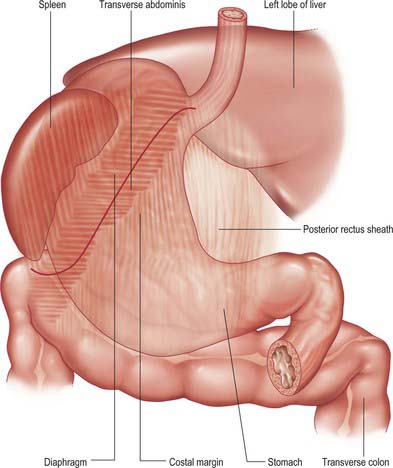
The lateral part of the anterior surface is posterior to the left costal margin and in contact with the diaphragm, which separates it from the left pleura, the base of the left lung, the pericardium and the left sixth to ninth ribs (Fig. 65.5). It lies posterior to the costal attachments of the upper fibres of transversus abdominis, which separate it from the seventh to ninth costal cartilages. The upper and left part of this surface curves posterolaterally and is in contact with the gastric surface of the spleen. The right half of the anterior surface is related to the left and quadrate lobes of the liver and the anterior abdominal wall. When the stomach is empty, the transverse colon may lie adjacent to the anterior surface. The entire anterior (superior) surface is covered by peritoneum.
Posterior (inferior) surface
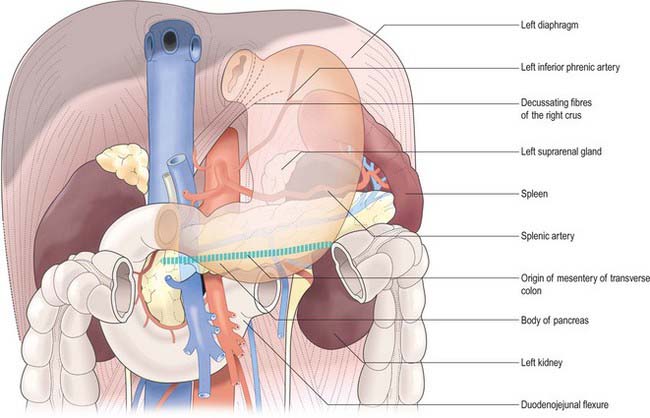
The posterior surface lies anterior to the left crus and lower fibres of the diaphragm, the left inferior phrenic vessels, the left suprarenal gland, the superior pole of the left kidney, the splenic artery, the anterior pancreatic surface, the splenic flexure of the colon and the upper layer of the transverse mesocolon (Fig. 65.3). Together these form the shallow stomach bed: they are separated from the stomach by the lesser sac (over which the stomach slides as it distends). The upper left part of the surface curves anterolaterally and lies in contact with the gastric surface of the spleen. The greater omentum and the transverse mesocolon separate the stomach from the duodenojejunal flexure and ileum. The posterior surface is covered by peritoneum, except near the cardiac orifice, where a small, triangular area contacts the left diaphragmatic crus and sometimes the left suprarenal gland. The left gastric vessels reach the lesser curvature at the right extremity of this bare area in the left gastropancreatic fold. The gastrophrenic ligament passes from the lateral aspect of this bare area to the inferior surface of the diaphragm.
GASTRIC ORIFICES
Cardiac orifice and gastro-oesophageal junction
The opening from the oesophagus into the stomach is the cardiac orifice (Fig. 65.1). It is typically situated to the left of the midline behind the seventh costal cartilage at the level of the eleventh thoracic vertebra. It is typically 10 cm from the anterior abdominal wall and 40 cm from the incisor teeth. The abdominal oesophagus is continuous with the cardiac orifice; the right side is continuous with the lesser curvature, the left side with the greater curvature. There is no specific anatomically discernible cardiac sphincter related to the orifice.
Internally, the transition between oesophagus and stomach is difficult to define because mucosa of gastric fundal pattern extends a variable distance up into the abdominal oesophagus. It usually forms a ‘zig-zag’ squamo-columnar epithelial junction with the oesophageal epithelium above this Z line: for histological and endoscopic purposes, this is often referred to as the gastro-oesophageal junction. A sling of longitudinal and oblique gastric muscle fibres forms a loop on the superior, left, side of the gastro-oesophageal junction between the oesophagus and the lesser curvature, and this is taken as the external boundary of this junction. Bands of thickened circular smooth muscle in the upper wall of the greater curvature and the distal oesophagus are sometimes confusingly referred to as ‘clasp’ or ‘sphincter’ fibres.
Lower oesophageal sphincter and gastro-oesophageal reflux
At rest there is a gastro-oeosophageal pressure gradient due to the presence of negative intra-thoracic pressure (transferred to the thoracic oesophagus) and positive intra-abdominal pressure (transferred to the stomach and augmented by any contraction of the stomach wall itself). Several anatomical and physiological factors normally prevent gastro-oesophageal reflux. Minor factors include the folds of gastric mucosa present in the gastro-oesophageal junction, the mucosal rosette, which contribute to the formation of a fluid- and gas-tight seal and also help to ensure that even low levels of tone within the lower oesophageal wall muscles may occlude the lumen of the junction against low pressures of gastric gas; the angle of the cardiac orifice, which is formed, in part, by the pull of the long oblique fibres of the inner layer of the gastric smooth muscle and may help to form a type of ‘flap valve’; and the length of the abdominal oesophagus, which is buttressed externally by pads of adipose connective tissue at and below the level of the diaphragmatic hiatus. However, the major anti-reflux mechanisms are the tonic contractions of the specialized smooth muscle of the wall lower oesophageal and the encircling fibres of the right diaphragmatic crus, which, together, exert a radial pressure that can be measured by electromyography or manometric testing (Paterson 2001, Mittal 2006), and form an effective high pressure zone (HPZ). At and just below the level of the entry of the abdominal oesophagus into the stomach, the circular fibres of the intermediate layer of the muscularis externa lying over the upper lesser curvature are particularly pronounced and sometimes referred to as ‘clasp’ fibres and exert fairly constant myogenic tone (Fig. 65.6). Since the oesophagus passes obliquely into the stomach, with increasing gastric distension the tone in the clasp fibres rises and they may act to draw the anterior and posterior surfaces together, increasing the tone at the gastro-oesophageal junction, contributing to the HPZ. These anatomical and physiological features are together referred to as the lower oesophageal sphincter (LOS). If reflux is to be prevented, the pressure within the HPZ must exceed the difference between the pressures on either side of the junction. The oesophageal part of the LOS is controlled by the intramural plexuses of the enteric nervous system via the neural release of nitric oxide which relaxes the smooth muscle of the LOS. Tone is reduced in advance of the oesophageal peristaltic wave during swallowing and raised again after the food bolus has passed. During inspiration, the greater negative intrathoracic pressure which increases the gastro-oesophageal pressure gradient is offset by increased pressure in the HPZ due to contraction of the peri-oesophageal fibres of the right diaphragmatic crus. Activation of the crural diaphragm slightly before the costal fibres ensures that this increase precedes the increase in the gradient.
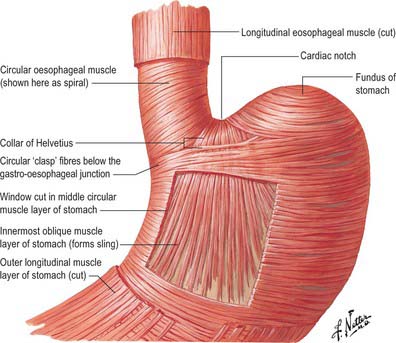
Fig. 65.6 Detail of the arrangements of the muscle layers of the oesophageal and gastric walls.
(Reprinted from Netter Anatomy Illustration Collection, © Elsevier Inc. All Rights Reserved.)
Reflux of gastric contents into the abdominal and lower thoracic oesophagus as a result of transient relaxation of the LOS occurs as a normal event in most individuals for a small percentage of their daily life. It also occurs as a result of a weak LOS, or of hiatus hernia which disrupts the normal anatomical barriers. Surgical procedures to prevent excessive reflux mainly focus on the restoration of a normal length of intra-abdominal oesophagus, by reduction of any hiatus hernia which carries the gastro-oesophageal junction into the thorax, and an increase in the tonic contraction surrounding the intra-abdominal oesophagus, which is usually achieved by partial wrapping of the fundus of the stomach around the intra-abdominal oesophagus to provide greater tone, ‘fundoplication’.
Barrett’s oesophagus
The squamous epithelium lining the lower oesophagus may be pathologically replaced by islands, strips, or circumferential patches of columnar, gastric type epithelium. This process is most likely to be the result of chronic reflux of gastric contents, acid or alkali, into the oesophagus, inducing a change in mucosal cell type. The abnormal columnar type epithelium, which may extend for a variable length up the lower part of the oesophagus, is referred to as Barrett’s epithelium.
Pyloric orifice
The pyloric orifice is the opening from the stomach into the duodenum, and typically lies 1–2 cm to the right of the midline in the transpyloric plane when the body is supine and the stomach empty. The pyloric sphincter is a muscular ring formed by a marked thickening of the circular gastric muscle interlaced with some longitudinal fibres. The circular pyloric constriction on the surface of the stomach usually indicates the location of the pyloric sphincter, and is often marked by a prepyloric vein which crosses the anterior surface vertically downwards.
GASTRIC FORM AND INTERNAL APPEARANCES
Numerous factors influence both the form and position of the stomach, including the posture and build of the individual, the extent of filling of the stomach, the position of the surrounding viscera, and the tone of the abdominal wall and gastric musculature. The empty stomach is most commonly J-shaped, the fundus usually contains gas, and, in the erect posture, the pylorus descends to the level of the second or the third lumbar vertebra. The lowest part of the antrum often lies below the level of the umbilicus, and the overall axis of the organ is slightly inclined from the vertical (Fig. 65.7). In short, obese individuals, the axis of the stomach lies more towards the horizontal as a ‘steer-horn’ shape.
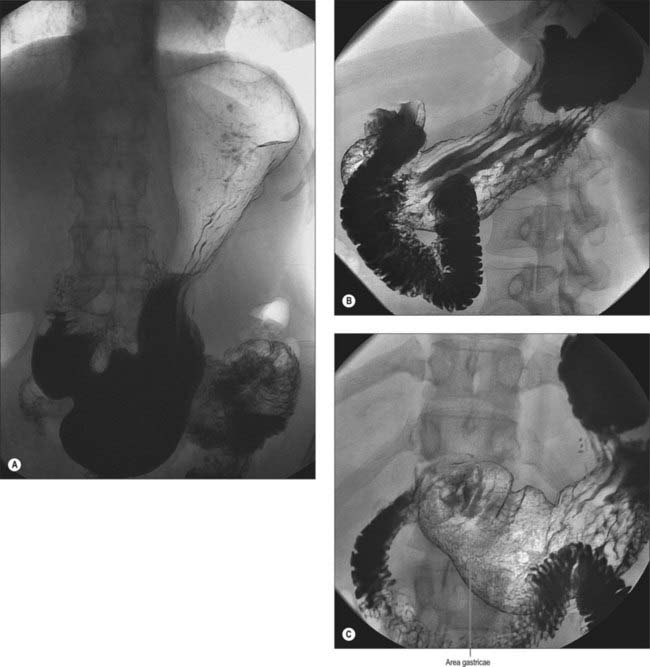
Fig. 65.7 Double contrast barium meal. A, In the erect position the stomach has a more ‘J’-shaped configuration. B, Initial stomach filling demonstrates a horizontally lying stomach with prominent gastric rugal folds. C, The area gastricae within the antrum are clearly identified on distension of the stomach.
The extent of filling mainly affects the shape and position of the body, because the pyloric part usually remains contracted during digestion. As the stomach fills, it expands forwards and downwards but, when the colon or small bowel is distended, the fundus enlarges towards the liver and diaphragm. As stomach capacity increases, the pylorus is displaced to the right and the axis of the whole organ lies in a more oblique direction. In this position the anterior and posterior surfaces tend to face forwards and backwards and the lowest part is the pyloric antrum, which extends below the umbilicus. When intestinal distension interferes with downward expansion of the body, the stomach retains a horizontal position.
Internal appearances
During endoscopic examination (Fig. 65.8), the stomach is typically at least partially distended by air. The cardiac orifice and the lowest portion of the abdominal oesophagus viewed from above are typically closed at rest by tonic contraction of the lower oesophageal musculature. The gastric mucosa lining the orifice is puckered into ridges and is present for a short but variable distance up the abdominal oesophagus; the transition between columnar and squamous epithelium is usually clearly visible. The presence of abnormal columnar epithelium within the oesophagus is referred to as Barrett’s oesophagus but the precise definition of this condition is difficult (see above). From within the distended stomach, the cardiac orifice appears in the medial wall of the fundus and is asymmetrical. The medial edge of the cardiac orifice is continuous with the medial wall of the body of the stomach. The mucosa is slightly thickened at this point with a raised profile, forming part of the ‘mucosal rosette’ that lines the orifice. The ‘rosette’ aids closure of the cardiac orifice and helps prevent reflux of stomach contents into the oesophagus. The medial edge of the orifice is more clearly visible than the lateral edge because it forms a more acute angle with the mucosal lining of the abdominal oesophagus.
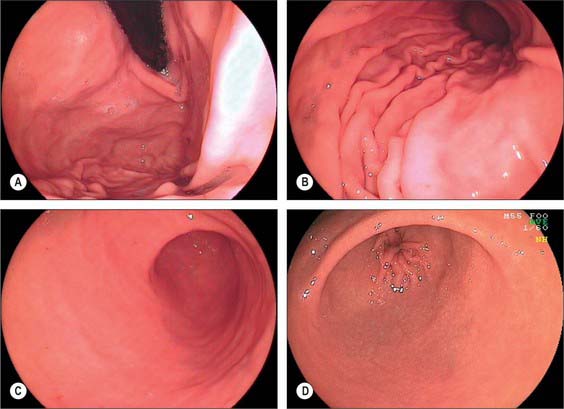
Fig. 65.8 Endoscopic appearances of the stomach. A, Cardiac orifice and fundus from below showing the pronounced lateral mucosal fold at the cardiac orifice; B, Body and greater curvature showing mucosal folds (‘magenstrasse’); C, Antrum showing the internal appearance of the incisura angularis; D, Prepyloric antrum and opening of the pyloric canal.
In the partly distended stomach, the mucosa of the fundus is thrown into gentle folds with no particular pattern (rugae). As the stomach fills towards capacity, these folds rapidly become less pronounced, and the wall is nearly smooth when the stomach is over-inflated. The body of the stomach has the most pronounced mucosal folds. Even in moderate distension, they appear as long, broad mucosal ridges running in sinuous strips from fundus to pyloric antrum (Fig. 65.7). They are seen on all mucosal surfaces of the body but are most obvious on the anterolateral, lateral and posterolateral parts (which correspond to the inner surface of the anterior and posterior external surfaces and to the greater curvature). Here they are occasionally called the magenstrasse, a reference to their possible role in directing liquid entering the stomach immediately down into the pyloric antrum. These folds are least prominent on the medial surface (corresponding to the inner surface of the lesser curvature), which is much smoother, particularly when the stomach distends.
The areae gastricae within the antrum are small elevations or puckerings of the mucosal surface that are readily seen on double contrast barium meal (Fig. 65.7). The few folds present in the antrum when the stomach is relaxed disappear with distension. The antrum adjacent to the pyloric canal, the prepyloric antrum, has a smooth mucosal surface that culminates in a slight puckering of the mucosa at the pyloric orifice caused by the contraction of the pyloric sphincter.
Gastrostomy
Since the lower body and antrum of the stomach is related to the posterior aspect of the left anterior abdominal wall, it may usefully be accessed to form a gastrostomy. Its mobility enables the anterior surface of the stomach to be readily approximated to the parietal peritoneum on the posterior surface of the abdominal wall, and a communication to be established between the lumen of the stomach and the surface of the skin. Although this may be performed as a direct open surgical procedure under general anaesthetic, it is much more commonly performed using a percutaneous puncture guided by either endoscopic visualization of the stomach or radiological imaging. The procedure is made easier by the fact that the anterior surface of the stomach lies most nearly in the vertical plane when the stomach is distended. One of the main hazards of the procedure results from the occasional interposition of the transverse colon between the stomach and anterior abdominal wall, which may lead to inadvertent transfixion of the colon by the needle puncture system. The variable length of the transverse colonic mesentery means that it may sometimes lie adjacent to the anterior gastric surface when a subject is recumbent. These risks may be reduced by radiological guidance.
VASCULAR SUPPLY AND LYMPHATIC DRAINAGE
Arteries
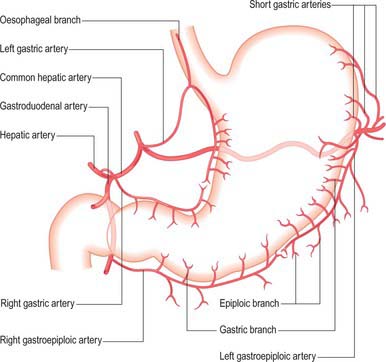
The arterial supply to the stomach comes predominantly from the coeliac axis although intramural anastomoses exist with vessels of other origins at the two ends of the stomach (Figs 65.9, 65.10, 65.11). The left gastric artery arises directly from the coeliac axis. The splenic artery gives origin to the short gastric arteries as well as the left gastroepiploic artery and may occasionally give origin to a posterior gastric artery. The hepatic artery gives origin to the right gastric artery and to the gastroduodenal artery, which in turn gives origin to the right gastroepiploic artery.

Fig. 65.10 Coeliac axis and its branches. Demonstrated on digital subtraction angiography (A) and digital reformatted multislice CT angiogram
(B and C). B and C by courtesy of GE Worldwide Medical Systems. (by courtesy of Dr James McCall, Chelsea & Westminster Hospital, London)
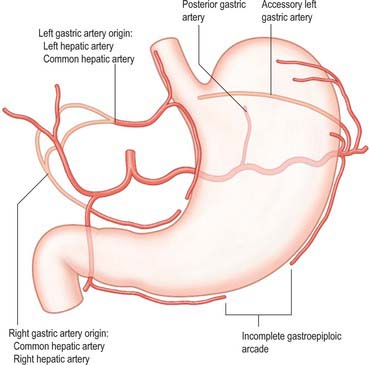
Fig. 65.11 Principle variations in the gastric arterial supply. Accessory vessels or possible replaced origins of vessels are shown by pale pink lines.
Left gastric artery
The left gastric artery is the smallest branch of the coeliac axis. It ascends to the left of the midline and crosses either just over the lower end of or below the level of the origin of the left crus of the diaphragm beneath the peritoneum of the upper posterior wall of the lesser sac. Here it lies adjacent to the left inferior phrenic artery and medial or anterior to the left suprarenal gland. It runs forwards into the superior portion of the lesser omentum adjacent to the superior end of the lesser curvature, and turns anteroinferiorly to run along the lesser curvature between the two peritoneal leaves of the lesser omentum. At the highest point of its course, it gives off one or more oesophageal branches. In its course along the lesser curvature, it gives off multiple branches that run onto the anterior and posterior surfaces of the stomach and anastomose with the right gastric artery in the region of the incisura angularis.
The left gastric artery rarely arises from the common hepatic artery or its branches. The most common of these rare variations is an origin from the left hepatic artery, when the left gastric artery passes between the peritoneal layers of the superior lesser omentum to reach the lesser curvature of the stomach. Other variants include a common origin with the common hepatic artery. (A replaced/accessory left hepatic artery origin from the left gastric artery is more common than a replaced/accessory left gastric origin.)
Short gastric arteries
There is no universally accepted definition of what constitutes the short gastric arteries but a practical definition is ‘vessels which supply the fundus of the stomach above the level of the splenic artery and which arise to the left of the greater curvature of the stomach’. The short gastric arteries are variable in number, commonly between five and seven. They arise from the splenic artery or its divisions, or from the proximal left gastroepiploic artery, and pass between layers of the gastrosplenic ligament to supply the cardiac orifice and gastric fundus. They anastomose with branches of the left gastric and left gastroepiploic arteries. An accessory left gastric artery may arise with these vessels from the distal splenic artery.
Left gastroepiploic artery
The left gastroepiploic artery is the largest branch of the splenic artery. It arises near the splenic hilum and runs anteroinferiorly between the layers of the gastrosplenic ligament into the upper gastrocolic omentum. It lies between the layers of peritoneum close to the greater curvature, running inferiorly to anastomose with the right gastroepiploic artery. It gives off gastric branches to the fundus of the stomach through the gastrosplenic ligament, and to the body of the stomach through the gastrocolic omentum. These are necessarily longer than the gastric branches of the right gastroepiploic artery and may be 8–10 cm long. Epiploic (omental) branches arise along the course of the vessel and descend between the layers of the gastrocolic omentum into the greater omentum. A particularly large epiploic branch commonly originates close to the origin of the left gastroepiploic artery, descends in the lateral portion of the greater omentum and provides a large arterial supply to the lateral half of the omentum.
Posterior gastric artery
A distinct posterior gastric artery may occur. When present, it arises from the splenic artery in its middle section posterior to the body of the stomach (Fig. 65.11). It ascends behind the peritoneum of the lesser sac towards the fundus and reaches the posterior surface of the stomach in the gastrophrenic fold.
Right gastric artery
The right gastric artery arises from the hepatic artery as it passes forwards from the posterior wall of the lesser sac into the lower border of the lesser omentum above the first part of the duodenum. It runs between the peritoneal layers of the lesser omentum just above the medial end of the lesser curvature, passes superiorly along the lesser curvature, giving off multiple branches onto the anterior and posterior surfaces of the stomach, and anastomoses with the left gastric artery.
The origin of the right gastric artery often varies: the most common alternative origins are from the common hepatic, left hepatic, gastroduodenal or supraduodenal arteries.
Gastroduodenal artery
The gastroduodenal artery arises from the common hepatic artery posterior and superior to the first part of the duodenum. It gives origin to the right gastroepiploic and superior pancreaticoduodenal arteries at the lower border of the first part of the duodenum. (For details of the gastroduodenal artery and its relevance to clinical situations, see Chapter 66.)
Right gastroepiploic artery
The right gastroepiploic artery originates from the gastroduodenal artery behind the first part of the duodenum, anterior to the head of the pancreas. It passes inferiorly towards the midline between the layers of the gastrocolic omentum, lies inferior to the pylorus, then runs laterally along the greater curvature and ends by anastomosing with the left gastroepiploic artery. It is adjacent to the pylorus but, more distally, lies approximately 2 cm from the greater curvature of the stomach. The right gastroepiploic artery gives off gastric branches that ascend onto the anterior and posterior surfaces of the antrum and lower body of the stomach, epiploic branches that descend into the greater omentum, and branches that contribute to the supply of the inferior aspect of the first part of the duodenum.
Arterial anastomoses of the stomach
There is an anastomosis between the oesophageal arteries originating from the thoracic aorta and the vessels that supply the fundus in the region of the cardiac orifice. At the pyloric orifice the extensive network of vessels supplying the duodenum allows for some anastomosis between vessels of superior mesenteric artery origin and the pyloric vessels. The major named vessels supplying the stomach form extensive arterial anastomoses both on the serosal surface and around the curvatures. The right and left gastroepiploic arteries and the left and right gastric arteries anastomose freely with each other along the greater and lesser curvatures respectively. Anastomoses also form between the short gastric and left gastric arteries in the region of the fundus, and between the right gastric and right gastroepiploic arteries in the region of the antrum. In addition to the extensive serosal anastomoses, networks form within the stomach wall at intramuscular, submucosal and mucosal levels. A true plexus of small arteries and arterioles is present within the submucosa: it supplies the mucosa and shows considerable regional variation both in the gastric wall and in the proximal duodenum. The rich arterial supply to the stomach ensures that the high mucosal blood flow required for physiological functioning is maintained even if one or more vessels become occluded: the stomach exhibits considerable resistance to ischaemia even when multiple arterial supplies are lost.
The pyloric arteries are rami of the right gastric and right gastroepiploic arteries. They pierce the duodenum distal to the sphincter around its entire circumference and pass through the muscular layer to the submucosa where they divide into two or three rami. The latter turn back into the pyloric canal beneath the mucosa and run to the end of the pyloric antrum: they supply the entire mucosa of the pyloric canal (Fig. 65.12). Branches of the pyloric submucosal arteries may anastomose close to their origin with the duodenal submucosal arteries, and their terminal rami also anastomose with gastric arteries from the prepyloric antrum. The pyloric sphincter is supplied by the gastric and pyloric arteries via rami that leave their parent vessels in the subserosal and submucosal levels to penetrate the sphincter.
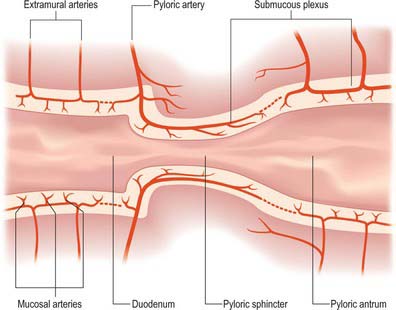
Fig. 65.12 Blood supply of the stomach and the proximal duodenum. A scheme of arterial arrangements at the gastroduodenal junction. Dotted lines indicate sites where the submucous plexus may be non-continuous. Shaded areas represent the muscular layer of the visceral wall.
(Redrawn courtesy of C Piasecki, Department of Anatomy, Royal Free Hospital School of Medicine, London and the Journal of Anatomy.)
Dieu la Foy lesions
Abnormalities of the intramural vascularity of the stomach are a rare cause of bleeding from the upper gastrointestinal tract. So-called ‘Dieu la Foy’ lesions commonly occur in the proximal body or fundus. Originally thought to be small arteriovenous malformations of the submucosal plexus, they are now considered to be caused by a larger than normal penetrating arterial vessel running through the muscular coat of the stomach into the submucosa before branching into the submucosal plexus.
Veins
The veins draining the stomach ultimately empty into the portal vein. A rich submucosal and intramural venous network gives rise to veins that usually accompany the corresponding named arteries and drain into either the splenic or superior mesenteric veins, although some pass directly into the portal vein. The course and distribution of the veins is highly variable even up to the level of the major named vessels.
Short gastric veins
Four or five short gastric veins drain the gastric fundus and the upper part of the greater curvature into the splenic vein or one of its large tributaries.
Left gastroepiploic vein
The left gastroepiploic vein drains both anterior and posterior surfaces of the body of the stomach and the adjacent greater omentum via multiple tributaries. It runs superolaterally along the greater curvature, between the layers of the gastrocolic omentum, and drains into the splenic vein within the gastrosplenic ligament.
Right gastroepiploic vein
The right gastroepiploic vein drains the greater omentum, distal body and antrum of the stomach. It passes medially, inferior to the greater curvature, in the upper portion of the gastrocolic omentum. Just proximal to the pyloric constriction it passes posteriorly to drain into the superior mesenteric vein below the neck of the pancreas. The right gastroepiploic vein may receive the superior pancreaticoduodenal vein close to its entry into the superior mesenteric vein.
Left gastric vein
The left gastric vein drains the upper body and fundus of the stomach. It ascends along the lesser curvature to the oesophageal opening where it receives several lower oesophageal veins, then curves posteriorly and medially behind the posterior peritoneal surface of the lesser sac. It drains into the portal vein directly at the level of the upper border of the first part of the duodenum.
Right gastric vein
The right gastric vein is typically small and runs along the medial end of the lesser curvature, passing under the peritoneum as it is reflected from the posterior aspect of the pylorus and first part of the duodenum onto the posterior wall of the lesser sac. It drains directly into the portal vein at the level of the first part of the duodenum. It receives the prepyloric vein as it ascends anterior to the pylorus at the level of the pyloric opening.
Posterior gastric veins
Distinct posterior gastric veins may occur, sometimes as multiple small vessels. When present, they accompany the posterior gastric artery from the middle of the posterior surface of the stomach and drain into the splenic vein.
Oesophageal and gastric varices
Blood from the mucosa normally drains into a superficial venous plexus, then into a deeper intrinsic venous plexus and finally into the peri-oesophageal veins via perforating veins. In the abdominal oesophagus the perforating veins drain into tributaries of the left gastric veins; in the lower thoracic oesophagus they form tributaries of the azygos and hemiayzgos systems. Bidirectional flow is possible in this region, and accommodates pressure changes that occur during breathing and Valsalva manoeuvres.
Varices are progressive dilatations which occur predominantly in the submucosal plexus of the distal abdominal oesophagus and occasionally on the gastric side of the gastric orifice at portal venous pressures above 15 mmHg. Portal venous hypertension, of any cause, predisposes to the opening up of pre-existing embryonic venous channels between venous tributaries of the portal system and the systemic venous circulation (Paquet 2000), such as the venous plexuses which exist in the lower abdominal oesophagus and gastric orifice. In portal hypertension, the valves within the perforating vessels become incompetent, permitting retrograde flow, and this causes variceal dilatation of the deep and superficial veins. Varices in the distal oesophagus are often easily visible at endoscopy, because they are situated superficially in the lamina propria and protrude into the oesophageal lumen; they are a frequent cause of substantial upper gastrointestinal bleeding. Spontaneous cessation of the bleeding is less common than at other sites, in part because the pressure and the flow rate within the veins is high, but also because there is a relative lack of submucosal supporting connective tissue to allow for venous contraction and thrombosis. Gastric varices are often present on the inferior aspect of the gastric orifice. They present less commonly in clinical practice than oesophageal varices, are less easy to diagnose because of their location, and occasionally exist without the presence of oesophageal varices. In these circumstances, it may be that the embryonal ‘point of meeting’ between portal and systemic venous systems is lower than usual and occurs in the upper stomach rather than the lower oesophagus.
Lymphatic drainage
The stomach has a rich network of lymphatics that connect with lymphatics draining the other visceral organs of the upper abdomen. At the gastro-oesophageal junction the lymphatics are continuous with those draining the lower oesophagus, and in the region of the pylorus they are continuous with those draining the duodenum. In general, they follow the course of the arteries supplying the stomach. However, many separate groups of nodes are now recognized (Fig. 65.13), and their relationship to the regions of the stomach and the vascular territories supplied, is of great importance during resection of the stomach, particularly for malignancy. Pancreatic and hepatic lymphatics play a significant role in draining areas of the stomach during disease.
Gastrectomy and gastric lymphadenectomy
Surgery for gastro-oesophageal malignancy involves varying degrees of radical resection of the associated lymph nodes that potentially drain the tumour area. The wide ramification of the gastro-oesophageal lymph node drainage and the multiple possible nodes which may be involved in a tumour inevitably mean that the extent of lymphadenectomy undertaken may be wide. The extent of possible/actual nodal involvement by the tumour is classified as N1 (loco-regional nodes specific to the tumour site), N2 (regional and major named vessel nodes draining the tumour) and N3 (wider draining nodes including para-aortic nodes). Gastrectomies can be classified according to the node groups excised for the tumour: D1 (e.g. removal of the portion of the stomach involved and the local lymph nodes directly related to the arteries ligated), D2 (e.g. total gastrectomy including all gastric lymph node groups often including the spleen) and D3 (e.g. total gastrectomy plus extensive lymphadenectomy that includes the associated upper abdominal lymph nodes, namely pancreatic, superior mesenteric, coeliac, hepatic and transverse colic). (For details of the lymph node stations relevant to surgical gastric resection, see Japanese Gastric Cancer Association 1998 and UICC 1997.)
INNERVATION
The stomach is innervated by sympathetic and parasympathetic fibres.
Sympathetic innervation
The sympathetic supply to the stomach originates from the fifth to 12th thoracic spinal segments, and is mainly distributed to the stomach via the greater and lesser splanchnic nerves via the coeliac plexus. Periarterial plexuses form along the arteries and supply the stomach from the coeliac axis. Additional innervation comes from fibres of the hepatic plexus, which pass to the upper body and fundus via the upper limit of the lesser omentum and via direct branches from the greater splanchnic nerves.
The gastric sympathetic nerves are vasoconstrictor to the gastric vasculature and inhibitory to gastric musculature. The sympathetic supply to the pylorus is motor, and brings about pyloric constriction. The sympathetic supply also conducts afferent impulses that mediate sensations, including pain.
Parasympathetic innervation
The parasympathetic supply to the stomach is from the anterior and posterior vagus nerves (Fig. 65.14). The anterior vagus (formed mainly from fibres from the left vagus originating from the oesophageal plexuses) is often double or even triple and supplies filaments to the cardiac orifice. The nerve lies very closely applied to the outer muscle of the abdominal oesophagus and divides near the oesophageal end of the lesser curvature into gastric and pyloric/hepatic branches. The upper anterior gastric branches radiate on the anterior surface of the upper body and fundus. The main gastric branch is termed the greater anterior gastric nerve and lies in the lesser omentum near the lesser curvature; branches from this nerve pass to the body and antrum with the gastric arterial supply branches. Hepatic/pyloric branches (generally one main and possibly one accessory) originate below the cardiac orifice. The main hepatic/pyloric nerve runs between the peritoneal layers of the lesser omentum almost horizontally towards its free edge to reach the hilum of the liver where hepatic branches ramify and pyloric branches turn down on the left side of the hepatic artery to reach the pylorus. The second nerve usually arises from the greater anterior gastric nerve during its course and runs inferomedially to the pyloric antrum where pyloric (and antral) branches form and hepatic branches (one or two) run superiorly to contribute to the hepatic plexus. Variations in the anterior nerve include accessory pyloric branches, long gastric branches with a high lying nerve trunk and a low origin of the pyloric branch with a low lying nerve trunk.
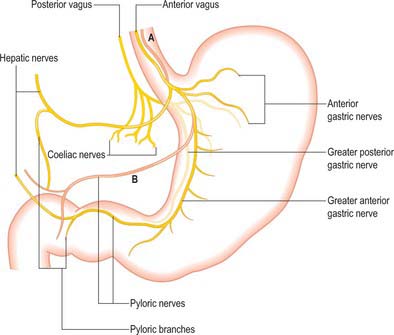
Fig. 65.14 Distribution of the vagal nerves to the stomach. The two commonest variations in the anterior vagus are shown in pink. A, Multiple main trunks. B, Low origin of the hepatic/pyloric branch lying close to the lesser curvature.
The posterior nerve tends to lie more medial than the anterior and is often a short distance from the wall of the abdominal oesophagus. It produces two main groups of branches, gastric and coeliac. Gastric branches arise and then run behind the cardiac orifice and upper body of the stomach. They extend over the posterior surface of the body and fundus to the proximal antrum but do not normally reach the pyloric sphincter. The largest is termed the greater posterior gastric nerve and runs posteriorly along the lesser curvature. Large coeliac branches (one or more) arise from the posterior nerve as thick bundles which carry the majority of fibres forming the nerve to the coeliac plexus (and onwards). Hepatic branches (one or two) are often small and originate from the coeliac branches. No true plexus occurs on either the anterior or posterior gastric surfaces, but plexuses are present in the submucosa and between the layers of the muscularis externa.
The parasympathetic gastric supply is secretomotor to the gastric mucosa and motor to the gastric musculature. It is responsible for coordinated relaxation of the pyloric sphincter during gastric emptying.
Referred pain
The majority of the sensation of pain arising from the stomach is poorly localized, and in common with other structures of foregut origin, is referred to the central epigastrium. Pain arising from the region of the gastro-oesophageal junction is commonly referred to the lower retrosternal and subxiphoid areas.
MICROSTRUCTURE
The gastric wall consists of the major layers found elsewhere in the gut, i.e. mucosa, submucosa, muscularis externa and serosa, together with gastric vessels and nerves (Figs 65.15, 65.16). The microstructure reflects the functions of the stomach as an expandable muscular sac lined by secretory epithelium, although there are local structural and functional variations in this pattern.
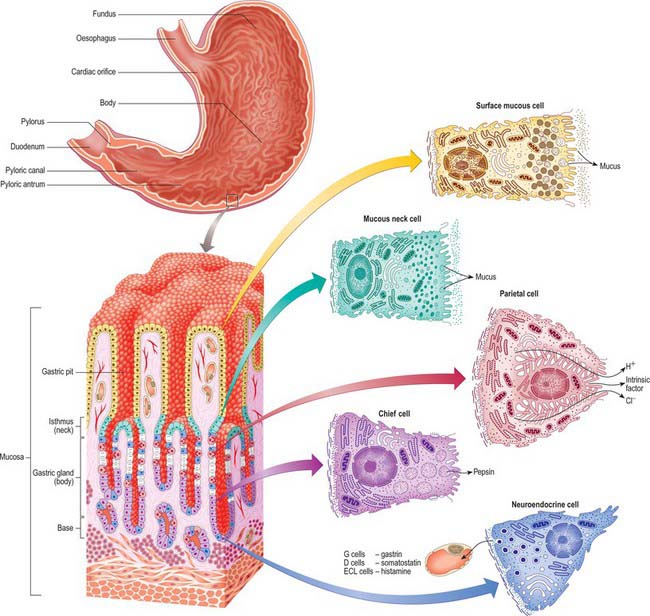
Fig. 65.15 Principal regions of the interior of the stomach and the microstructure of tissues and cells within its wall. Undifferentiated, dividing cells are shown in white.
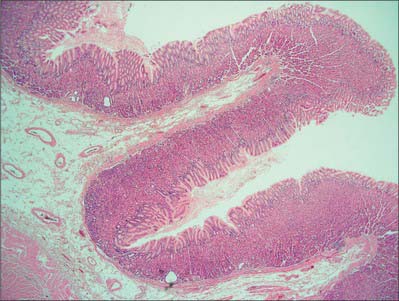
Fig. 65.16 Low power micrograph showing the stomach wall, thrown into longitudinal folds or rugae which are visible macroscopically. The surface epithelium is infolded microscopically to form gastric pits. Gastric glands extend through the thickness of the mucosal lamina propria and open into the bases of the gastric pits. A muscularis mucosae layer and submucosa follow the contours of the rugae. Part of the external muscularis layers is seen below left.
(By courtesy of Mr Peter Helliwell and Dr Joseph Mathew, Department of Histopathology, Royal Cornwall Hospitals Trust, UK.)
Mucosa
The mucosa is a thick layer with a soft, smooth surface that is mostly reddish brown in life but pink in the pyloric region. In the contracted stomach the mucosa is folded into numerous folds or rugae, most of which are longitudinal. They are most marked towards the pyloric end and along the greater curvature. The rugae represent large folds in the submucosal connective tissue (see below) rather than variations in the thickness of the mucosa covering them, and they are obliterated when the stomach is distended. As elsewhere in the gut, the mucosa is composed of a surface epithelium, lamina propria and muscularis mucosae.
Epithelium
When viewed microscopically at low magnification, the internal surface of the stomach wall appears honeycombed by small, irregular gastric pits approximately 0.2 mm in diameter (Figs 65.15, 65.16). The base of each gastric pit receives several long, tubular gastric glands that extend deep into the lamina propria as far as the muscularis mucosae. Simple columnar mucus-secreting epithelium covers the entire luminal surface including the gastric pits, and is composed of a continuous layer of surface mucous cells which release gastric mucus from their apical surfaces to form a thick protective, lubricant layer over the gastric lining. This epithelium commences abruptly at the cardiac orifice, where there is a sudden transition from oesophageal stratified squamous epithelium.
Gastric glands
Although all gastric glands are tubular, they vary in form and cellular composition in different parts of the stomach. They can be divided into three groups – cardiac, principal and pyloric. The most highly specialized are the principal glands.
Principal gastric glands
The principal glands are found in the body and fundus, three to seven opening into each gastric pit. Their junction with the base of the pit is the isthmus, immediately basal to this is the neck, and the remainder is the base. The walls of the gland contain at least five distinct cell types: chief, parietal, mucous neck, stem and neuroendocrine.
Chief (peptic) cells (Fig. 65.15) are the source of the digestive enzymes pepsin and lipase. They are usually basal in position and cuboidal in shape, and their nuclei are rounded and euchromatic. They contain secretory zymogen granules and their abundant cytoplasmic RNA renders them strongly basophilic. Parietal (oxyntic) cells are the source of gastric acid and of intrinsic factor, a glycoprotein necessary for the absorption of vitamin B12. They are large, oval and strongly eosinophilic, and have centrally placed nuclei. Parietal cells occur intermittently along the walls of the more apical half of the gland, but can reach as far as the isthmus; they bulge laterally into the surrounding connective tissue. They have a unique ultrastructure related to their ability to secrete hydrochloric acid. The luminal side of the cell is deeply invaginated to form a series of blind-ended channels (canaliculi) that bear numerous irregular microvilli covered by a plasma membrane rich in H+/K+ ATPase antiporter channels. The latter actively secrete hydrogen ions into the lumen; chloride ions follow along the electrochemical gradient. The mitochondria-rich cytoplasm facing these channels contains a tubulo-vesicular system of abundant fine membranous tubules directed towards the canalicular surface. The precise structure of the cell varies with its secretory phase: when stimulated, the number and surface area of the microvilli increases up to five-fold, probably as a result of the rapid fusion of the tubulo-vesicular system with the plasma membrane. This process is reversed at the end of stimulated secretion, when the excess membrane retreats back into the tubulo-alveolar system and microvilli are lost.
Mucous neck cells are numerous at the necks of the glands and are scattered along the walls of the more basal regions. They are typical mucus-secreting cells, displaying apical secretory vesicles containing mucins, and basally displaced nuclei: their products are distinct histochemically from those of the superficial mucous cells.
Stem cells are relatively undifferentiated mitotic cells from which the other types of gland cell are derived. They are relatively few in number, and are situated in the isthmus of the gland and the bases of the gastric pits. Stem cells are columnar and possess a few short apical microvilli. They periodically undergo mitosis; their progeny migrate either apically, to differentiate into new surface mucous cells, or basally, to form mucous neck, parietal, chief or neuroendocrine cells. All of these cells have a limited lifespan, especially the mucus-secreting types, and so they are constantly replaced. The typical replacement period for surface mucous cells is 3 days, and for mucous neck cells is 1 week. Other cell types appear to live much longer.
Neuroendocrine (enteroendocrine) cells occur in all types of gastric gland, but more frequently in the body and fundus of the stomach. They are situated mainly in the deeper parts of the glands, among the chief cells. They are basally situated, pleomorphic cells and display irregular nuclei surrounded by granular cytoplasm that contains clusters of large (0.3 μm) secretory granules. Neuroendocrine cells synthesize a number of biogenic amines and polypeptides that are important in the control of gut motility and glandular secretion. They are part of the system of dispersed neuroendocrine cells: in the stomach they include G cells, which secrete gastrin; D cells, which secrete somatostatin; and ECL (enterochromaffin-like) cells, which secrete histamine.
Cardiac glands
Cardiac glands are confined to a small area near the cardiac orifice: some are simple tubular glands, others are compound branched tubular. Mucus-secreting cells predominate; parietal and chief cells are present, but sparse.
Pyloric glands
Pyloric glands empty via groups of two or three short convoluted tubes into the bases of the deep gastric pits of the pyloric antrum: the pits occupy about two-thirds of the mucosal depth (Fig. 65.17). The glands are populated mainly by mucus-secreting cells, but they also contain neuroendocrine cells, especially G cells, which secrete gastrin when activated by appropriate mechanical stimulation (causing increased gastric motility and secretion of gastric juices). Although parietal and chief cells are scarce, parietal cells are always present in both fetal and postnatal pyloric glands, and may also appear in the duodenal mucosa, proximally near the pylorus, in adult tissue.
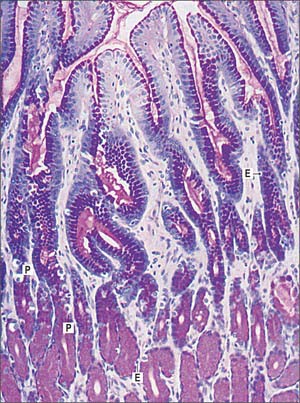
Fig. 65.17 Micrograph showing the pyloric region of the stomach. Pyloric glands are stained with the periodic acid-Schiff (PAS) technique to show mucin (magenta) in the gastric pits and glands. Pale staining cells are the larger parietal cells (P) and smaller enteroendocrine cells (E).
(By permission from Dr JB Kerr, Monash University, from Kerr JB 1999 Atlas of Functional Histology. London: Mosby.)
Lamina propria
The lamina propria forms a connective tissue framework between the glands. It contains small masses of lymphoid tissue, gastric lymphatic follicles, which resemble solitary intestinal follicles (especially in early life). It also contains a complex periglandular vascular plexus involved in the maintenance of the mucosal environment, e.g. the removal of bicarbonate produced in the tissues as a counterpart to acid secretion, and neural plexuses rich in both sensory and motor terminals.
Muscularis mucosae
The muscularis mucosae is a thin layer of smooth muscle fibres lying external to the layer of glands, arranged as continuous inner circular and outer longitudinal layers, and a discontinuous external circular layer. The inner layer sends strands of smooth muscle cells between the glands: their contraction probably assists in emptying into the gastric pits.
Submucosa
The submucosa is a variable layer of loose connective tissue. It contains thick bundles of collagen, numerous elastin fibres, blood vessels and nervous plexuses, including the ganglionated submucosal (Meissner’s) plexus.
Muscularis externa
The muscularis externa is a thick muscle coat immediately under the serosa, with which it is closely connected by subserous loose connective tissue. From innermost outwards, it contains oblique, circular and longitudinal layers of smooth muscle fibres. The layers are not always easily separated: the circular layer is poorly developed in the oesophageal region, but is thickened at the distal pyloric antrum to form the anular pyloric sphincter; the outer longitudinal layer is most pronounced in the upper two-thirds of the stomach; the inner oblique layer is most obvious in the lower half.
The actions of the muscularis externa of the stomach produce a churning movement that mixes food with the gastric secretions. When the muscles contract, they reduce the volume of the stomach and throw the mucosa into longitudinal folds or rugae (see above). The folds flatten as the stomach distends with food and the musculature relaxes and thins. Muscle activity is controlled by a network of unmyelinated autonomic nerve fibres and their ganglia which lie between the muscle layers in the myenteric (Auerbach’s) plexus.
Serosa or visceral peritoneum
The serosa is an extension of the visceral peritoneum. It covers the entire surface of the stomach other than along the attachments of the greater and lesser omenta to the greater and lesser curvatures respectively, where the peritoneal layers are separated by vessels and nerves, and over a small posteroinferior area near the cardiac orifice, where the stomach contacts the diaphragm at the reflections of the gastrophrenic and left gastropancreatic folds.
DiDio LJ, Anderson MC. The ‘Sphincters’ of the Digestive System. Baltimore: Williams and Wilkins, 1968.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma. Gastric Cancer. 1998;1:10-24. 2nd English edition.
Japanese Research Society for Gastric Cancer. Japanese Classification of Gastric Carcinoma. Tokyo: Kanehara & Co Ltd, 1998. and Gastric Cancer 1: 10–24, 25–30
A detailed, widely accepted, description of the lymph node fields of the upper abdominal viscera, particularly in relation to malignancy..
Kwok H, Marriz Y, Al-Ali S, Windsor JA. Phrenoesophageal ligament revisited. Clin Anat. 1999;12:164-170.
Mittal RK, Goyal RK 2006 Sphincter mechanisms at the lower end of the esophagus. GI Motility online.
Paquet KJ. Causes and mechanisms of oesophageal varices development. Med Sci Monit. 2000;6:915-928.
Paterson WG. The normal anti-reflux mechanism. Chest Surg Clin N Am. 2001;11:473-483.
Silverstein FE, Tytgat GNJ. Atlas of Gastrointestinal Endoscopy, 2nd edition. New York: Gower Medical Publishing, 1991.
UICC, Sobin LH, Wittekind CH. TNM classification of malignant tumours, 5th edition, Berlin: Springer-Verlag, 1997.