CHAPTER 71 Spleen
The spleen consists of a large encapsulated mass of vascular and lymphoid tissue situated in the upper left quadrant of the abdominal cavity between the fundus of the stomach and the diaphragm. Its shape varies from a slightly curved wedge to a ‘domed’ tetrahedron. The shape is mostly determined by its relations to neighbouring structures during development. The superolateral aspect is shaped by the left dome of the diaphragm with the inferomedial aspect being influenced mostly by the neighbouring splenic flexure of the colon, the left kidney and stomach. Its long axis lies approximately in the plane of the tenth rib. Its posterior border is approximately 4 cm from the mid-dorsal line at the level of the tenth thoracic vertebral spine. Its anterior border usually reaches the mid-axillary line.
The size and weight of the spleen vary with age and sex. It can also vary slightly in the same individual under different conditions. In the adult it is usually 12 cm long, 7 cm broad, and 3–4 cm wide. It is comparatively largest in the young child, and although its weight increases during puberty, by adulthood it is relatively smaller in comparison to the neighbouring organs. It tends to diminish in size and weight in senescence. Its average adult weight is about 150 g, although the normal range is wide, between 80 g and 300 g, in part reflecting the amount of blood it contains. The normal-sized adult spleen fits comfortably in a cupped hand; the spleen has to be at least three times its normal size before it can be palpated.
Additional collections of fully functional splenic tissue may exist near the spleen, especially within the gastrosplenic ligament and greater omentum. These accessory spleens, or splenunculi, are usually isolated but can be connected to the spleen by thin bands of similar tissue. They may be numerous and widely scattered in the abdomen. The spleen may retain its fetal lobulated form or show deep notches on its diaphragmatic surface and inferior border in addition to those usually present on the superior border.
RELATIONS
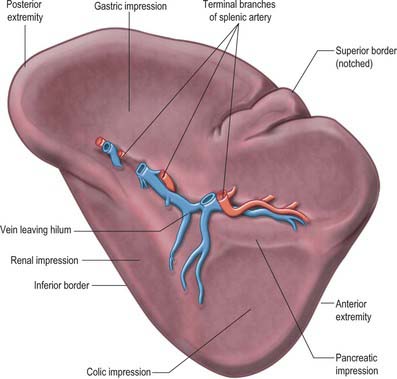
The spleen has a superolateral diaphragmatic and an inferomedial visceral surface (Fig. 71.1). There are superior and inferior borders and anterior and posterior extremities or poles. The diaphragmatic surface is convex and smooth and faces mostly superiorly and laterally although the posterior part may face posteriorly and almost medially as it approaches the inferior border. The diaphragmatic surface is related to the abdominal surface of the left dome of the diaphragm which separates it from the basal pleura, the lower lobe of the left lung and the ninth to eleventh left ribs (Fig. 71.2). The pleural costodiaphragmatic recess extends down as far as its inferior border. The visceral surface faces inferomedially towards the abdominal cavity, and is irregular and marked by gastric, renal, pancreatic and colic impressions. The gastric impression faces anteromedially and is broad and concave where the spleen lies adjacent to the posterior aspect of the fundus, upper body and upper greater curvature of the stomach. It is separated from the stomach by a peritoneal recess, which is limited by the gastrosplenic ligament. The renal impression is slightly concave and lies on the lowest part of the visceral surface, separated from the gastric impression above by a raised strip of splenic tissue and the splenic hilum. It faces inferomedially and slightly backwards, being related to the upper and lateral area of the anterior surface of the left kidney and sometimes to the superior pole of the left suprarenal gland. The colic impression lies at the inferior pole of the spleen and is usually flat. It is related to the splenic flexure of the colon and the phrenicocolic ligament. The pancreatic impression is often small when present and lies between the colic impression and the lateral part of the hilum. It is related to the tail of the pancreas which lies in the splenorenal ligament. The hilum of the spleen is a long fissure pierced by several irregular apertures through which the branches of the splenic artery and vein as well as nerves and lymphatics enter and leave the spleen; it lies in the visceral surface closer to the inferior border and anterior border.
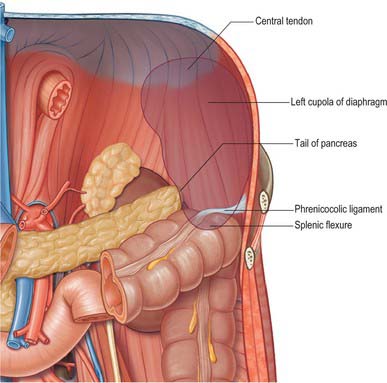
Fig. 71.2 The posterior relations (‘bed’) of the spleen.
(Adapted from Drake, Vogl and Mitchell 2005.)
The superior border separates the diaphragmatic surface from the gastric impression and is usually convex. Near the anterior extremity there may be one or two notches that have persisted from the lobulated form of the spleen in early fetal life. However, they are often absent and are not a reliable guide to the identification of the spleen during clinical examination. The inferior border separates the renal impression from the diaphragmatic surface and lies between the diaphragm and the upper part of the lateral border of the left kidney. It is more blunt and rounded than the superior border and corresponds in position to the lower margin of the eleventh rib. The posterior extremity, or superior pole, usually faces the rounded vertebral column. The anterior extremity, or inferior pole, is larger and less angulated than the posterior extremity and connects the lateral ends of the superior and inferior borders: it is related to the colic impression and may lie adjacent to the splenic flexure and the phrenicocolic ligament.
PERITONEAL CONNECTIONS OF THE SPLEEN
The spleen is almost entirely covered by peritoneum that adheres firmly to its capsule, and is separated from the stomach and left kidney by recesses of the greater sac. It develops in the upper dorsal mesogastrium (see Figs 73.6 to 73.7), and remains connected via folds of peritoneum to the posterior abdominal wall (by the splenorenal, phrenicocolic and phrenicosplenic ligaments) and to the anterolateral abdominal wall and stomach (by the gastrosplenic ligament) (Fig. 71.3A). The splenorenal ligament is formed from two layers of peritoneum. The anterior layer is continuous with the peritoneum of the posterior wall of the lesser sac over the left kidney and with peritoneum of the splenic hilum where it runs into the posterior layer of the gastrosplenic ligament. The posterior layer of the splenorenal ligament is continuous with the peritoneum over the inferior surface of the diaphragm and runs onto the splenic surface over the renal impression. The splenic vessels lie between the layers of the splenorenal ligament: the tail of the pancreas is usually present in its lower portion (see Fig. 64.5B). The length of the splenorenal ligament may vary. Longer ligaments tend to make the spleen more mobile and may predispose the spleen to injury due to rotational shear forces during trauma, however they also make the mobilization of the spleen easier during surgery. The presence of the pancreatic tail within the splenorenal ligament must be remembered because it can be injured during ligation of the splenic vessels, causing pancreatitis or a pancreatic duct fistula to form.
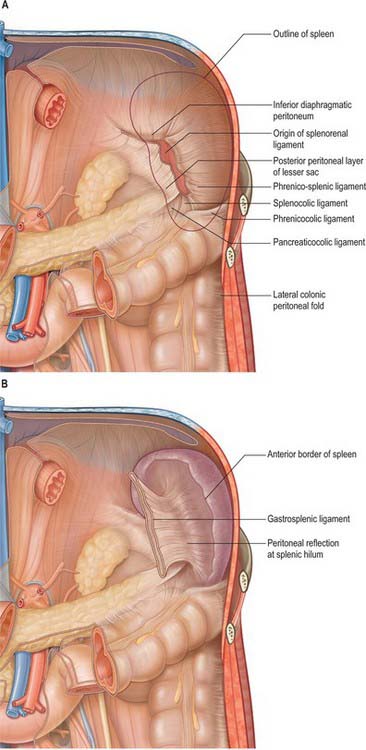
Fig. 71.3 The peritoneal connections of the spleen. A, The posterior peritoneal connection seen as if the spleen has been removed with the folds fixed in their ‘normal’ positions. B, The anterior peritoneal connections seen with the spleen in place as if the stomach and greater omentum have been removed with the folds fixed in their ‘normal’ positions.
(A and B adapted from Drake, Vogl and Mitchell 2005.)
The gastrosplenic ligament also has two layers (Fig. 71.3B). The posterior layer is continuous with the peritoneum at the splenic hilum and over the posterior aspect of the stomach. The anterior layer is formed from the peritoneum that is reflected off the gastric impression and reaches the greater curvature of the stomach anteriorly. The short gastric and left gastroepiploic branches of the splenic artery pass between its layers. Division of the gastrosplenic ligament during surgery may be hazardous if the ligament is short because ligation of the short gastric vessels may risk injury to the greater curvature of the stomach.
The phrenicocolic ligament extends from the splenic flexure of the colon to the diaphragm at the level of the eleventh rib. It extends inferiorly and laterally and is continuous with the peritoneum of the lateral end of the transverse mesocolon at the lateral margin of the pancreatic tail, and the splenorenal ligament at the hilum of the spleen.
If the peritoneal attachments of the spleen are not recognized during surgery, the splenic capsule may be injured, with subsequent serious bleeding. Downward traction on the phrenicocolic ligament during handling of the descending colon, especially during mobilization of the splenic flexure, may cause rupture of the splenic capsule. This is less likely if traction on the phrenicocolic ligament is made laterally or medially. The superior border and anterior diaphragmatic surface are often adherent to the peritoneum of the greater omentum. Medial traction on the omentum during surgery may cause capsular injury, but this is less likely if any limited traction required is applied inferiorly. The diaphragmatic surface of the spleen is occasionally adherent to the peritoneum over the inferior surface of the diaphragm: these adhesions often occur after inflammation in the spleen, but may also be present congenitally.
SPLENOMEGALY
Splenic enlargement may accompany any massive immune response and occurs in many other systemic inflammatory and degenerative conditions. In splenomegaly, the anterior border, anterior diaphragmatic surface and notched superior border may become clearly palpable below the left costal margin. The notches are often exaggerated and may be clearly palpable, and the transverse colon and splenic flexure are displaced downward.
SPLENIC TRAUMA
The spleen is particularly prone to rotational injury during rapid deceleration or compression. Because it has relatively mobile peritoneal connections, tearing injuries to the splenic vessels at the hilum or burst injuries of the splenic pulp may occur. Fractures of the overlying lower left ribs may cause sharp penetrating injuries to the splenic capsule and pulp. The spleen may also be injured during surgical procedures by tearing of its capsule through peritoneal adhesions and connections (see above). Minor capsular tears may be treated by conservative management if from blunt trauma or by application of various haemostatic substances to the exposed pulp. Direct sutured repair of more extensive tears of the spleen is rarely successful because the splenic pulp is fragile. Moderately severe injuries may be treated by conservative management if from blunt trauma or by compression of the splenic tissue until haemostasis occurs if encountered at surgery, but extensive burst injuries or major injuries to the hilar vessels usually require splenectomy.
SPLENECTOMY
Partial splenectomy is followed by rapid regeneration of lost tissue and there is no significant loss in any of the functions of the spleen. Total splenectomy has few haematological consequences since the functions of the spleen are largely assumed by the liver. There is, however, a loss in immune function, particularly in the antibody response to systemic infections with encapsulated bacteria. This is referred to as ‘overwhelming post-splenectomy sepsis syndrome’, and is particularly a problem when the spleen is lost in early childhood, but is still a significant risk even if the spleen is lost in late adult life. Splenectomy in adults is usually followed by an increased white blood cell count with increased lymphocytic, neutrophil, eosinophil and platelet counts in the peripheral blood: these effects fade and disappear within a few weeks.
VASCULAR SUPPLY AND LYMPHATIC DRAINAGE
Splenic artery
The spleen is supplied exclusively from the splenic artery (see Fig. 70.7). This is the largest branch of the coeliac axis and its course is among the most tortuous in the body. From its origin the artery runs a little way inferiorly, then turns rapidly to the left to run initially horizontally above the level of the neck of the pancreas, before ascending as it passes more laterally. It is less steeply inclined than the body and tail of the pancreas and so comes to lie posterior to the superior border of the gland. It lies in multiple loops or even coils which appear above the superior border of the pancreas and descend to lie behind the gland. The splenic artery lies anterior to the left kidney and left suprarenal gland and runs in the splenorenal ligament posterior to the tail of the pancreas. It divides into two or three main branches before entering the hilum of the spleen (Fig. 71.1). As these branches enter the hilum they divide further into four or five segmental arteries that each supply a segment of the splenic tissue. There is relatively little arterial collateral circulation between the segments, which means that occlusion of a segmental vessel often leads to infarction of part of the spleen. There is, however, considerable venous collateral circulation between the segments, making segmental resection of the spleen practically impossible. The splenic artery gives off various branches to the pancreas in its course and gives off short gastric arteries to the stomach just prior to dividing or from its terminal branches.
Splenic vein
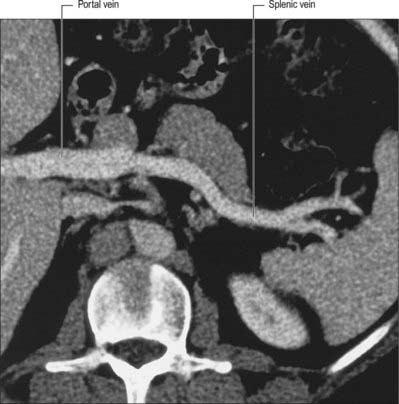
The splenic vein is formed within the splenorenal ligament, close to the tip of the tail of the pancreas, by five or six tributaries that emerge from the hilum of the spleen (Fig. 71.4). The tributaries are thin walled and often spread over several centimetres because the hilum is long and thin (Fig. 71.1). This must be remembered during surgical removal of the spleen because the venous tributaries must be divided close to the hilum to avoid injury to the pancreatic tail. They should be ligated in several groups to prevent the risk of avulsion of the veins from the splenic hilum and consequent profuse bleeding before the resection is complete.
The splenic vein runs in the splenorenal ligament below the splenic artery and posterior to the tail of the pancreas. It descends to the right, and crosses the posterior abdominal wall inferior to the splenic artery and posterior to the body of the pancreas, receiving numerous short tributaries from the gland as it does so. It crosses anterior to the left kidney and renal hilum and is separated from the left sympathetic trunk and left crus of the diaphragm by the left renal vessels, and from the abdominal aorta by the superior mesenteric artery and left renal vein. It ends behind the neck of the pancreas, where it joins the superior mesenteric vein to form the portal vein. The short gastric and left gastro-epiploic veins drain into the splenic vein through the folds of the gastrosplenic ligament near its origin.
Lymphatics
Lymphatic vessels drain along the splenic trabeculae and pass out of the hilum into lymphatic vessels that accompany the splenic artery and vein. They run posterior to the pancreas, close to the splenic artery, and drain into nodes at the hilum and along the splenic artery and into the coeliac nodes.
INNERVATION
The spleen is innervated by the splenic plexus, which consists of branches of the coeliac plexus, left coeliac ganglion and right vagus that accompany the splenic artery. The fibres are mainly sympathetic and terminate around blood vessels and in the non-striated muscle of the splenic capsule and trabeculae. Most appear to be noradrenergic, vasomotor, and are concerned with the regulation of blood flow through the spleen. Adrenergic agonists inhibit the concentration of red cells in the splenic blood (so called ‘plasma skimming’) indicating that sympathetic activity causes an increase in the ‘fast’ circulation of the spleen as opposed to slow filtration (Reilly 1985).
Referred pain
The sensation of pain arising from the pulp of the spleen is poorly localized and referred to the central epigastrium, as is the case for other structures derived from the foregut. Distension of the splenic capsule stretches the parietal layers of the peritoneum and produces pain that is localized to the posterior left upper quadrant.
MICROSTRUCTURE
The spleen is essentially concerned with phagocytosis and immune responses. In the fetus it is also an important site of haemopoiesis. Postnatally it may become haemopoietic in certain pathological conditions. Although important to the defence of the body it is not absolutely essential: if the spleen is removed, many of its functions can be assumed by the liver and by other lymphoid tissues.
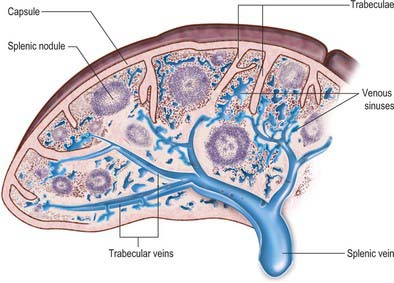
Microscopically, the parenchymal tissue of the spleen consists of two major components, white pulp and red pulp, named on the basis of their appearance when a fresh spleen is transected (Fig. 71.5). The white pulp is composed of lymphoid tissue in which B and T lymphocytes mature and proliferate under antigenic stimulation (Fig. 71.6). The red pulp is a unique filtration device that enables the spleen to clear particulate material from the blood as it perfuses the spleen. It is composed of a complex system of interconnected spaces populated by large numbers of phagocytic macrophages that remove effete red blood cells, microorganisms, cellular debris and other particulate matter from the circulation.
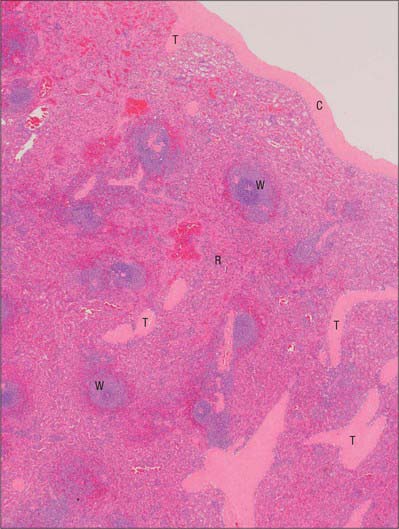
Fig. 71.5 A section through the spleen. White pulp (W) is present as ovoid areas of basophilic tissue. Red pulp (R) lies between white pulp tissue and consists of splenic sinusoids and intervening cellular cords. Part of the capsule (C) is seen top right, from which trabeculae (T) extend into the splenic tissue.
(By courtesy of Mr Peter Helliwell and Dr Joseph Mathew, Department of Histopathology, Royal Cornwall Hospitals Trust, UK.)
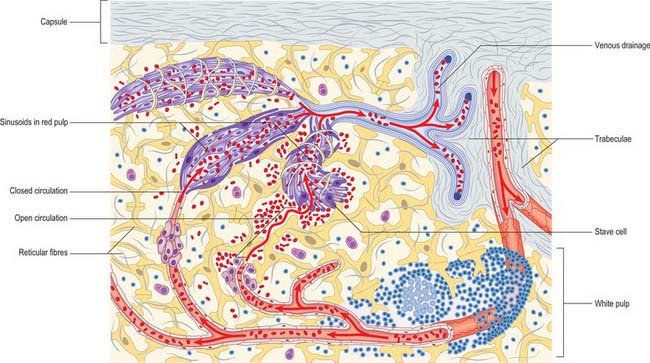
Fig. 71.6 The main features of splenic structure, not to scale. Shown are the capsule, trabeculae, reticular fibres and cells, the perivascular lymphoid sheaths and follicles (white pulp), and the cellular cords and venous sinusoids of the red pulp. The ‘open’ and ‘closed’ theories of splenic circulation are illustrated: it is likely that most of the circulation is of the open form. The venous sinusoids are lined by specialized endothelial ‘stave’ cells, the intercellular gaps of which have been over-emphasized for clarity.
Fibrous framework of the spleen
The serosa of the peritoneum covers the entire organ except at its hilum and along the lines of reflexion of the splenorenal and gastrosplenic ligaments. Deep to this layer is the connective tissue capsule, a continuous layer approximately 1.5 mm thick, containing abundant type I collagen fibres and some elastin fibres. The capsule has an outer and an inner lamina in which the directions of the collagen fibres differ, thereby increasing its strength. Numerous trabeculae extend from the capsule into the substance of the spleen, where they branch to form a supportive framework. The largest trabeculae enter at the hilum and provide a conduit for the splenic vessels and nerves, dividing into branches in the splenic pulp (Figs 71.6 and 71.7). Within the spleen, these branches become continuous with a delicate network of type III (reticular) collagen fibres that pervades both red and white pulps and is secreted by fibroblasts within its meshes.
White pulp
In the spleen parenchyma, branches of the splenic artery radiate out from the hilum within trabeculae, ramifying and narrowing to arterioles. In their terminal few millimetres, their connective tissue adventitia is replaced by a sheath of T-lymphocytes, the periarteriolar lymphatic sheath (PALS). This sheath is enlarged in places by lymphoid follicles, which are aggregations of B-lymphocytes, 0.25–1 mm in diameter and visible to the naked eye on the freshly cut spleen surface as white semi-opaque dots: they contrast with the surrounding deep reddish-purple of the red pulp. Follicles are usually situated near the terminal branches of arterioles and typically protrude to one side of a vessel, which consequently appears eccentrically placed within the follicle. Arterioles branch laterally within follicles to form a series of parallel terminal arterioles, called penicilli, or penicillar arterioles, from their resemblance to the penicillium mould.
Like the periarteriolar sheaths, follicles are centres of lymphocyte proliferation as well as aggregation, and when antigenically stimulated, the white pulp increases in size as lymphocytes proliferate. The primary follicles become intensely active in B-cell proliferation, and they develop germinal centres similar to those found in lymph nodes: the presentation of antigen by follicular dendritic cells is involved in this process. The germinal centres regress when the infection subsides. Follicles generally atrophy with increasing age and may be absent in the very elderly.
Red pulp
The red pulp constitutes the majority (75%) of the total splenic volume. It contains large numbers of venous sinusoids that ultimately drain into tributaries of the major splenic veins. The sinusoids are separated from each other by a fibrocellular network consisting of small bundles of delicate collagen type III fibres, the reticulum, and numerous reticular fibroblasts and splenic macrophages. Seen in two-dimensional sections, these intersinusoidal regions appear as strips of tissue, the splenic cords, which alternate with splenic sinuses (Fig. 71.6). In reality, they form a three-dimensional continuum around the venous spaces.
Venous sinusoids
Venous sinusoids are elongated ovoid vessels approximately 50 μm in diameter, lined by a characteristic, ‘incomplete’ endothelium that is unique to the spleen. Their endothelial cells are long and narrow, and aligned with the long axis of the sinusoid; they are often called stave cells, because they are reminiscent of planks in a barrel (Fig. 71.6). Stave cells are attached to their neighbours at intervals along their length by short stretches of intercellular junctions. These junctions alternate with intercellular slits which allow blood cells to squeeze into the lumen of the sinusoid from the surrounding splenic cords. A discontinuous basal lamina is present on the abluminal aspect of the sinus. The sinusoids are supported externally by circumferentially and longitudinally disposed reticular fibres, which are components of the fibrous reticulum.
Reticular tissue of the splenic cords
Large, stellate fibroblasts called reticular cells lie around the sinusoids. They synthesis the matrix components of the reticulum, including collagen and proteoglycans, and their cytoplasmic processes compartmentalize the reticular space. Blood is released from the ends of capillaries originating from penicillar arterioles and percolates through the reticular spaces within the splenic cords. As it does so, macrophages within the reticulum remove blood-borne particulate material, including ageing and damaged erythrocytes. When numbers of damaged erythrocytes increase, the reticular cells proliferate and the size of the red pulp increases considerably: this causes enlargement of the whole spleen, and in extreme cases, splenomegaly.
Marginal zone
The marginal zone lies at the interface between the white and red pulp. It is a region of great functional importance because it is where blood is delivered into the red pulp, and where many lymphocytes leave the circulation to migrate into their respective T- and B-lymphocyte areas in the white pulp. The lymphocytes are more loosely arranged than they are in the white pulp, and are held in a dense network of reticular fibres and cells. The arterioles leaving the white pulp are surrounded by a small aggregation of macrophages, the periarteriolar macrophage sheath.
Splenic microcirculation
The segmental splenic arteries enter the hilum and ramify in the trabeculae throughout the organ. The splenic vein forms in the splenorenal ligament from tributaries emerging from the hilum; they are similar in number to the arterial branches. Small arteries tapering to arterioles pass through the white pulp then turn abruptly to form penicillar branches which, after a course of approximately 0.5 mm, pass out of the white pulp into the marginal zone and red pulp. The passage of blood through the vascular compartments between the arterioles and splenic veins is referred to collectively as the intermediate circulation of the spleen. Ultimately, blood is passed to the venous sinusoids from which it enters venules leading to small veins that run within trabeculae, and thence into larger veins that drain the spleen at its hilum
Open and closed splenic circulations
Views on the intermediate circulation of the spleen differ on whether blood passes from the arterioles (or their terminal capillaries) directly into the venous sinuses (a closed circulation), or is instead discharged into a network of spaces in the splenic cords before entering the sinuses through the minute slits in their walls (an open circulation). In humans, evidence favours the presence of an anatomically and physiologically open circulation, in which blood percolates slowly through the reticular tissue of the splenic cords and filters through slits in the sinus walls before joining the majority of the blood flow (see Fig. 71.6). There is thought to be an additional closed vascular route, but this is likely to provide only a minor contribution to splenic circulation.