CHAPTER 70 Pancreas
The pancreas is the largest of the digestive glands and performs a range of both endocrine and exocrine functions. The major part of the gland is exocrine, secreting a range of enzymes involved in the digestion of lipids, carbohydrates and proteins. The endocrine function of the pancreas is derived from cells scattered throughout the substance of the gland: they take part in glucose homeostasis and are also involved in the control of upper gastrointestinal motility and function.
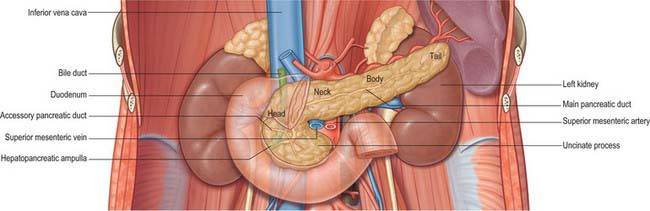
The pancreas is salmon pink in colour with a firm, lobulated smooth surface. The main portion is divided into four parts, head, neck, body and tail, purely on the basis of anatomical relations: there are only very minor functional or anatomical differences between each part (Figs 70.1 and 70.2). The pancreas also possesses one accessory lobe (the uncinate process), which is anatomically and embryologically distinct. In adults the pancreas measures between 12 and 15 cm long and is shaped as a flattened ‘tongue’ of tissue, thicker at its medial end (head) and thinner towards the lateral end (tail). With age, the amount of exocrine tissue tends to decline, as does the amount of fatty connective tissue within the substance of the gland, and this leads to a progressive thinning atrophy which is particularly noticeable on CT scanning. The pancreas lies within the curve of the first, second and third parts of the duodenum, and extends transversely and slightly upwards across the posterior abdominal wall to the hilum of the spleen, behind the stomach. It does not lie in one plane but is effectively ‘draped’ over the other structures in the retroperitoneum and the vertebral column and so forms a distinct shallow curve, of which the neck and medial body are the most anterior parts. Because of its flattened shape, the parts of the pancreas, particularly the body, are often referred to as having surfaces and borders.
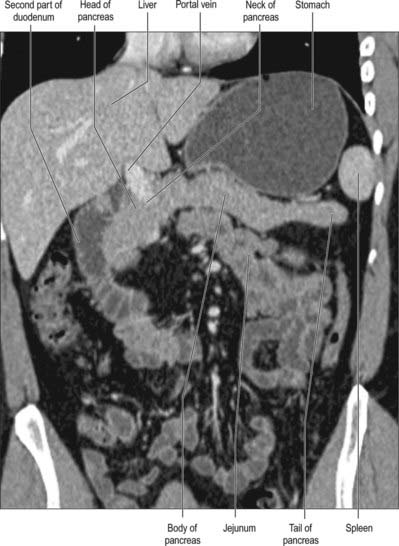
Fig. 70.2 Coronal reformat CT of the pancreas
(by courtesy of Dr Louise Moore, Chelsea and Westminster Hospital).
HEAD
The head of the pancreas lies to the right of the midline, anterior and to the right side of the vertebral column, within the curve of the duodenum. It is the thickest and broadest part of the pancreas but is still flattened in the anteroposterior plane. Superiorly it lies adjacent to the first part of the duodenum, but close to the pylorus the duodenum is on a short mesentery, and here the duodenum lies anterior to the upper part of the head. The duodenal border of the head is flattened and slightly concave, and is firmly adherent to the second part of the duodenum; occasionally a small part of the head is actually embedded in the wall of the second part of the duodenum. The superior and inferior pancreaticoduodenal arteries lie between the head and the duodenum in this area. The inferior border lies superior to the third part of the duodenum and is continuous with the uncinate process. Close to the midline, the head is continuous with the neck. The boundary between head and neck is often marked anteriorly by a groove for the gastroduodenal artery and posteriorly by a similar but deeper deep groove that contains the union of the superior mesenteric and splenic veins as they form the portal vein.
The anterior surface of the head (Fig. 70.3) is covered in peritoneum and is related to the origin of the transverse mesocolon.
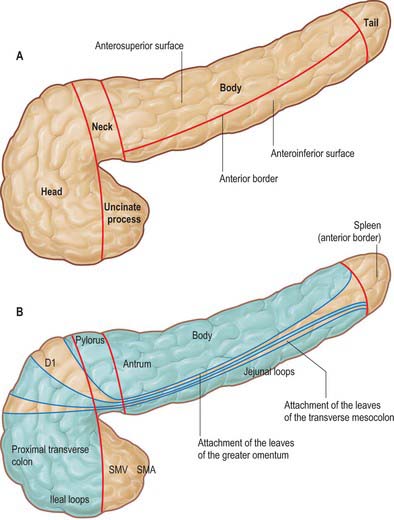
Fig. 70.3 A, Regions and anterior surfaces and borders of the pancreas. B, Anterior relations of the pancreas. Areas covered in peritoneum are shown in blue and structures overlying these areas are separated from the pancreas by peritoneal ‘spaces’. The spleen in relation to the tail lies anterior to the anterior leaf of the splenorenal ligament and not in direct contact with the pancreatic tissue. D1, first part of the duodenum; SMA, superior mesenteric artery; SMV, superior mesenteric vein.
NECK
The neck of the pancreas is approximately 2 cm wide and links the head and body. It is often the most anterior portion of the gland and is defined as the portion of the pancreas that lies anterior to the portal vein, which is closely related to the upper posterior surface (see Fig. 70.8). The lower part of the neck lies anterior to the superior mesenteric vein just before the formation of the portal vein. These are important relations during surgery for pancreatic cancer because malignant involvement of these vessels may make resection impossible. The anterior surface of the neck is covered with peritoneum and lies adjacent to the pylorus just inferior to the epiploic foramen. The gastroduodenal and anterior superior pancreaticoduodenal arteries descend in front of the gland in the region of the junction of the neck and head.
BODY
The body of the pancreas is the longest portion of the gland and runs from the left side of the neck to the tail. It is slightly triangular in cross-section, becoming progressively thinner and less broad towards the tail. The body is described as having three surfaces, anterosuperior, posterior and anteroinferior.
The anterosuperior surface of the pancreas makes up most of the anterior aspect of the gland close to the neck. Laterally, it narrows and lies slightly more superiorly to share the anterior aspect with the anteroinferior surface. It is covered by peritoneum, which runs anteroinferiorly from the surface of the gland to be continuous with the anterior, ascending layer of the greater omentum (see Fig. 64.4). The superior leaf of the transverse mesocolon is reflected and is continuous with the descending, posterior leaf of the greater omentum just above the anterior border and so forms virtually none of the covering of the anterosuperior surface. It is separated from the stomach by the lesser sac.
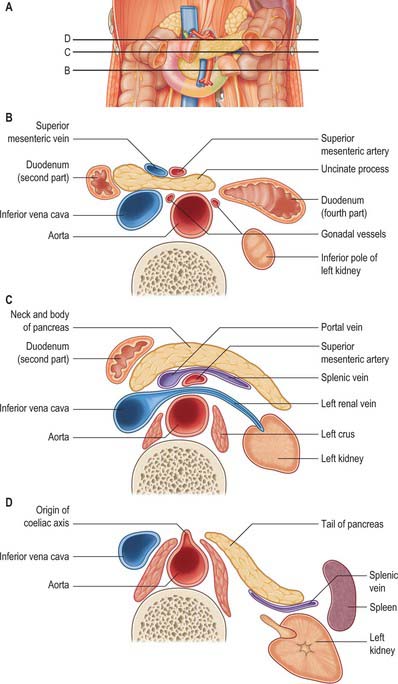
The posterior surface of the pancreas is devoid of peritoneum. It lies anterior to the aorta and the origin of the superior mesenteric artery, the left crus of the diaphragm, left suprarenal gland and the left kidney and renal vessels, particularly the left renal vein (Fig. 70.4). It is closely related to the splenic vein which lies between the posterior surface and the other posterior relations and runs from left to right forming anything from a shallow groove to a near enveloped ‘tunnel’ in the substance of the gland. The left kidney is also separated from the posterior surface by perirenal fascia and fat.
The anteroinferior surface of the pancreas begins as a narrow strip just to the left of the neck. As the body runs laterally, it broadens out to form more of the anterior aspect of the body. It is covered by peritoneum which is continuous with that of the posteroinferior layer of the transverse mesocolon. The fourth part of the duodenum, the duodenojejunal flexure and coils of jejunum all lie inferiorly. The lateral end of the inferior border often lies superior and posterior to the splenic flexure. The peritoneum of the anterosuperior layer of the transverse mesocolon is reflected onto the upper part of the anteroinferior surface at the line of the anterior border where it is continuous with the posterior leaf of the greater omentum. The apex of the ligament of Treitz may form an anterior relation of this surface of the body laterally.
On the right side, the superior border of the pancreas is initially blunt and somewhat flat, but as the gland is followed to the left, the surface changes to become narrower and sharper. An omental tuberosity usually projects from the right end of the superior border above the level of the lesser curvature of the stomach, in contact with the posterior surface of the lesser omentum. The superior border is related to the coeliac artery. The common hepatic artery runs to the right just above the gland. The splenic artery runs a course that is often highly tortuous to the left along the superior border; it tends to rise above the level of the superior border at several points along its course.
The anterior border of the pancreas separates the anterosuperior from the anteroinferior surfaces. The two layers of the transverse mesocolon diverge along this border. The upper is reflected inferiorly after a very short distance into the descending (posterior) leaf of the greater omentum whilst the other runs downwards and backwards over the anteroinferior surface.
The inferior border of the pancreas separates the posterior from the anteroinferior surfaces. At the medial end of the inferior border, adjacent to the neck of the pancreas, the superior mesenteric vessels emerge from behind the gland. More laterally, the inferior mesenteric vein runs under the border to join the splenic vein on the posterior surface. This is a useful site of identification of the inferior mesenteric vein during left-sided colonic resections and on CT imaging.
TAIL
The tail of the pancreas is the narrowest, most lateral portion of the gland and lies between the layers of the splenorenal ligament. It is continuous medially with the body and is between 1.5 and 3.5 cm long in adults. It may finish at the base of the splenorenal ligament or extend up nearly as far as the splenic hilum, in which case it is prone to injury at splenectomy during ligation of the splenic vessels. Posteriorly it is related to the splenic branches of the splenic artery and the splenic vein and its tributaries. The tip of the tail may lie in contact with the splenic hilum.
UNCINATE PROCESS
The uncinate process of the pancreas extends from the inferior lateral end of the head of the gland (Fig. 70.5). Embryologically it is separate from the rest of the gland, and so it lies posterior to the superior mesenteric vein and occasionally the artery as they descend and run forward into the root of the ileal mesentery, in close contact with its anterior surface. Posteriorly it lies in front of the aorta, and inferiorly it lies on the upper surface of the third part of the duodenum. Tumours of the uncinate process do not cause obstruction to the common bile duct, but frequently compress the third part of the duodenum as a result of this close relationship.
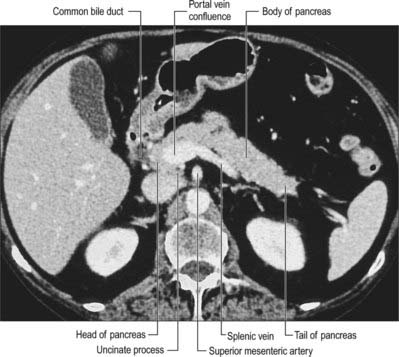
Fig. 70.5 Cross sectional CT scan of pancreas at the level of the uncinate process (cross sectional level between those shown in Fig. 70.4B,C).
PANCREATIC DUCTS
The exocrine pancreatic tissue drains into multiple small lobular ducts. The arrangement of the main ducts draining the pancreas is subject to some variation but the commonest arrangement is a single main and a single accessory duct (Fig. 70.6). This arrangement reflects the embryological development of the dorsal and ventral pancreatic ducts (see Ch. 73). The main duct is derived from the distal part of the dorsal duct in the body and tail but fuses with the more posteriorly placed ventral duct in the region where the dorsal bud fuses with the ventral bud (because the latter forms the posterior portion of the head of the pancreas and uncinate process). The accessory duct is the remnant of the duct of the dorsal pancreatic bud lying in the anterior pancreatic head after the principle part has joined with the ventral bud duct.
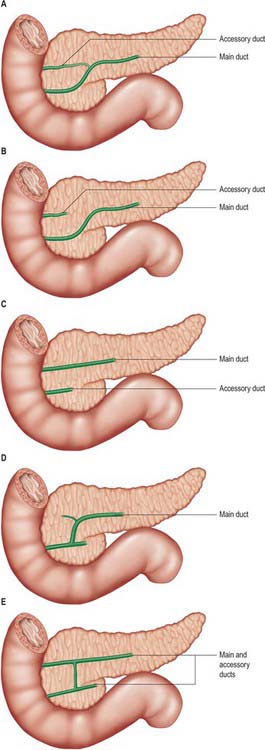
Fig. 70.6 Variations in the ductal anatomy of the pancreas. A, Normal (50%). B, Absence of communication between normally sited accessory duct and main ducts (10%). C, Persistance of complete ventral and dorsal ducts with separate drainage (5%). B and C are both forms of ‘pancreas divisum’. D, Absence of accessory duct (20%). E, Conjoined drainage of persistant ventral and dorsal ducts (<5%).
The main pancreatic duct (of Wirsung) usually runs within the substance of the gland from left to right. It tends to lie more towards the posterior than the anterior surface and is formed by the junction of several lobular (secondary) ducts in the tail. It increases in calibre as it runs within the body because it receives further lobular ducts which join it almost at right angles to its axis, forming a ‘herringbone pattern’. On ultrasound the duct can often be demonstrated, measuring approximately 3mm in diameter in the head, 2mm in the body, and 1mm in the tail in adults. As it reaches the neck of the gland it usually turns inferiorly and posteriorly towards the bile duct, which lies on its right side. The two ducts enter the wall of the descending part of the duodenum obliquely and unite in a short dilated hepatopancreatic ampulla (see Ch. 69).
The accessory pancreatic duct (of Santorini) usually drains the upper part of the anterior portion of the head of the pancreas. Much smaller in calibre than the main duct, it is formed within the substance of the head from several lobular ducts and ascends anterior to the main duct; it usually communicates with it through several small branches although these are rarely large enough to fill the accessory duct on a pancreatogram via the main ampulla. The accessory duct usually opens onto a small rounded minor duodenal papilla, which lies about 2cm anterosuperior to the major papilla. If the duodenal end of the accessory duct fails to develop, the lobular ducts drain via a small channel(s) into the main duct.
The main and accessory pancreatic ducts demonstrate some variability in their anatomy reflecting variations in the development and fusion of the dorsal and ventral ducts (Fig. 70.6). One clinically important variation is ‘pancreas divisum’, where entirely separate drainage occurs for different portions of the pancreas and which may allow some forms of pancreatitis to affect only one portion of the gland.
VASCULAR SUPPLY AND LYMPHATIC DRAINAGE
Arteries
The pancreas has a rich arterial supply derived from the coeliac axis and superior mesenteric artery via both named vessels and multiple small un-named vessels (Fig. 70.7).
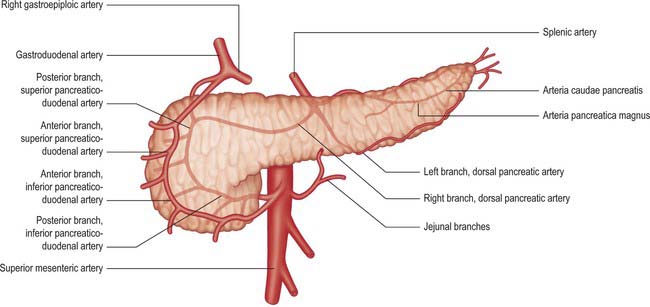
Inferior pancreaticoduodenal artery
The inferior pancreaticoduodenal artery arises from the superior mesenteric artery or its first jejunal branch, near the superior border of the third part of the duodenum. It usually divides directly into anterior and posterior branches. The anterior branch passes to the right, anterior to the lower border of the head of the pancreas, and runs superiorly to anastomose with the anterior superior pancreaticoduodenal artery. The posterior branch runs posteriorly and superiorly to the right, lying posterior to the lower border of the head of the pancreas and anastomoses with the posterior superior pancreaticoduodenal artery. Both branches supply the pancreatic head, its uncinate process and the second and third parts of the duodenum.
Superior pancreaticoduodenal artery
The superior pancreaticoduodenal artery is usually double. The anterior artery is a terminal branch of the gastroduodenal artery and descends in the anterior groove between the second part of the duodenum and head of the pancreas. It supplies branches to the head of the pancreas and anastomoses with the anterior division of the inferior pancreaticoduodenal artery. The posterior artery is usually a separate branch of the gastroduodenal artery arising at the upper border of the first part of the duodenum. It descends to the right, anterior to the portal vein and common bile duct, where the duct passes behind the first part of the duodenum. The artery next runs posterior to the head of the pancreas and crosses posterior to the common bile duct embedded in the head of the pancreas. It enters the duodenal wall and anastomoses with the posterior division of the inferior pancreaticoduodenal artery. The posterior superior artery supplies branches to the head of the pancreas and the first and second parts of the duodenum. (See Kimura 2000.)
Pancreatic branches
The pancreas is supplied by numerous small arterial branches which usually run into the gland directly from their arteries of origin. They are particularly numerous in the region of the neck, body and tail. Most originate from the splenic artery as it runs along the superior border of the gland and supply the left part of the body and tail. A dorsal branch descends posterior to the pancreas, dividing into right and left branches. It sometimes arises from the superior mesenteric, middle colic, hepatic or rarely, the coeliac artery. The right branch is often double and runs between the neck and uncinate process to form a prepancreatic arterial arch as it anastomoses with a branch from the anterior superior pancreaticoduodenal artery. The left branch runs along the inferior border to the pancreatic tail where it anastomoses with the greater pancreatic artery (arteria pancreatica magna) and the artery to tail of the pancreas (arteria caudae pancreatis).
Small un-named branches also arise from the first jejunal arcade of the superior mesenteric artery and the arterial branches of the retroperitoneal vessels.
Small arteries characteristically run along the inferior and superior borders of the gland, either lying in a deep groove or within the tissue of the gland. They receive contributions from many of the arteries supplying the gland, but mainly from the inferior and superior pancreaticoduodenal arteries, and supply branches which penetrate the substance of the gland at right angles to the vessel. They may bleed profusely on cutting the parenchyma of the gland during resection and usually require ligation.
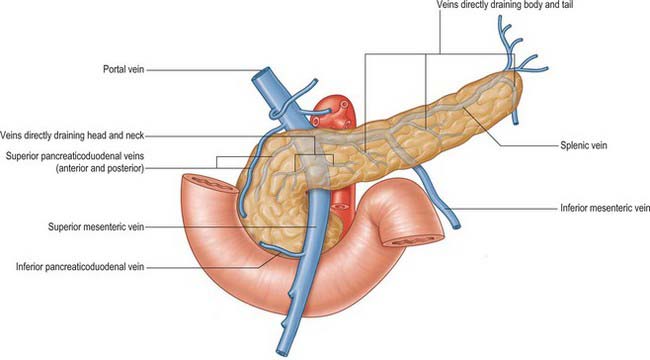
Veins
The venous drainage of the pancreas is primarily into the portal system (Fig. 70.8). The head and neck drain primarily via superior and inferior pancreaticoduodenal veins. The body and tail drain mostly via small veins that run directly into the splenic vein along the posterior aspect of the gland, or occasionally directly into the portal vein. The veins are numerous and short, features that may lead to troublesome bleeding during surgical mobilization of the head and neck of the gland. Small venous channels exist between all parts of the gland and the retroperitoneal veins, and ultimately drain into the lumbar veins: they may hypertrophy and become clinically significant in cases of portal hypertension.
Lymphatics
The lymphatic drainage of the pancreas is extensive; multiple groups of nodes may receive drainage from each region of the gland (see Fig. 65.12B) which in part explains the poor prognosis following resection of pancreatic tumours. Lymph capillaries commence around the pancreatic acini. The larger lymph vessels follow the arterial supply and drain into the lymph nodes around the pancreas and adjacent node groups. Lymphatics from the tail and body drain mostly into the pancreaticosplenic nodes, although some drain directly to pre-aortic nodes. Lymphatics from the neck and head drain more widely into nodes along the pancreaticoduodenal, superior mesenteric and hepatic arteries, and some also drain to the pre-aortic nodes and coeliac axis nodes. There is no evidence of lymphatic channels within the pancreatic islets.
INNERVATION OF THE EXOCRINE PANCREAS
The exocrine lobules of the pancreas are innervated by a fine network of sympathetic and parasympathetic fibres. The sympathetic supply originates from the sixth to tenth thoracic spinal segments and is mainly distributed to the pancreas via the sympathetic contribution to the coeliac ganglia. The postganglionic fibres are distributed to the gland via the arterial supply as periarterial plexuses. The parasympathetic supply is from the posterior vagus nerve and the parasympathetic component of the coeliac plexus. The supply to the gland is both vasomotor (sympathetic) and parenchymal (sympathetic and parasympathetic). The exocrine lobules are innervated by a fine network of parasympathetic and sympathetic fibres. Sensory fibres running from the gland run in both the sympathetic and parasympathetic systems: they mediate the sensation of pain arising from the gland and may also carry other sensory information. In chronic inflammation or inoperable tumours of the gland, thermal or chemical ablation of the coeliac plexus may be required to control chronic pain mediated by these fibres.
Referred pain
Pain arising in the pancreas is poorly localized. In common with other foregut structures, the majority of pain arising from the pancreas is referred to the epigastrium. Inflammatory or infiltrative processes arising from the gland rapidly involve the tissues of the retroperitoneum that are innervated by somatic nerves: pain is referred to the posterior paravertebral region around the lower thoracic spine.
PANCREATITIS AND PSEUDOCYST
Pancreatitis is one of the major pathological processes affecting the pancreas. Gallstones lying within the common bile duct are associated with pancreatitis. The presence of a common drainage for the common bile duct and the pancreatic duct may allow reflux of bile or pancreatic enzymes into the pancreatic duct during the passage of a gallstone through the ampulla. Reflux may also occur if the wall of the common bile duct becomes oedematous even though the gallstones have not entered the common ampulla.
Inflammation in the pancreas may cause a range of secondary pathologies. The course of the superior mesenteric artery and vein behind the neck and between the inferior border and uncinate process makes these vessels vulnerable to compression and secondary inflammation, which may result in an inflammatory aneurysm of the superior mesenteric artery or thrombosis of the superior mesenteric vein. Inflammatory aneurysms may rupture producing major haemorrhage. Thrombosis of the superior mesenteric vein may cause potentially lethal venous ischaemia of the small intestine. Thrombosis of smaller arterial branches such as the origin of the middle colic artery may cause ischaemia of individual organs such as the transverse colon.
In acute episodes, the profuse arterial and venous supply to the gland makes it particularly prone to haemorrhage. The extravasated blood collects in the retroperitoneal tissues because the neck and body of the gland lie largely in the loose connective tissue of the retroperitoneum. The pancreas lies anterior to the thoracolumbar and perirenal fasciae and the blood-stained fluid can track freely in the retroperitoneal tissues to appear in either the flanks (Grey–Turner’s sign), groins, or above the iliac crest where the iliac fascia is attached. Blood-stained fluid tracking laterally from the head of the pancreas may enter the lesser omentum and the ‘bare area’ of the liver, from where it may run forward into the falciform ligament and appear around the skin of the umbilicus (Cullen’s sign).
During acute episodes of inflammation, the close anterior relationship of the stomach may contribute to gastric stasis and vomiting. The origin of the superior mesenteric plexus also lies close to the pancreas and secondary inflammation in the tissues around the pancreas may affect the autonomic supply to the midgut and contribute to the paralytic ileus that frequently develops.
In severe cases, pancreatic inflammation may cause the collection of fluid within and around the pancreatic tissue. Intrapancreatic collections frequently resolve spontaneously over time, but coalescence of the fluid may occur anterior to the pancreas beneath the layer of peritoneum covering its anterior surfaces (although this actually lies beneath the posterior wall of the lesser sac). If this collection persists and grows, the peritoneum anterior and superior to the pancreas is stretched and comes to lie in contact with the anterior wall of the lesser sac. This collection is referred to as a pseudocyst. The anterior wall of the pseudocyst is formed of the twin layers of peritoneum lying adjacent to the posterior wall of the stomach, the lesser omentum and, occasionally, the gastrosplenic ligament. The lateral wall of the pseudocyst includes the splenorenal ligament. The posterior wall is a mixture of fibrous tissue resulting from previous inflammation, the anterior surface of the pancreas and the retroperitoneal tissues. Treatment of the pseudocyst usually involves drainage of the contents into the lumen of the stomach. This may be established endoscopically by placement of a drain through the posterior stomach wall and the two thickened layers of peritoneum into the cyst. Alternatively, the drain may be placed using radiological guidance, via the anterior abdominal wall and the anterior stomach wall.
PANCREATIC RESECTION
Resection of the pancreas is complicated by several factors. The extensive vascular supply requires careful haemostasis. Spleen-preserving resections may be undertaken if the underlying pathology does not involve the splenic vein where it lies in the groove on the posterior surface of the gland, although the multiple small pancreatic veins draining into it may cause troublesome bleeding. Resection of the head and neck is possible provided the plane between the neck and the portal vein has not been involved by disease. Occasional small venous branches may enter the portal vein directly and may also cause bleeding during this mobilization. Resection of the head and neck are almost always accompanied by resection of the distal first and second parts of the duodenum because of the dense adherence between the two and the common arterial supply. Resection without removal of the proximal part of the first part of the duodenum and pylorus, pylorus-preserving pancreatectomy, may be possible provided there is an adequate arterial supply to the pylorus from the stomach, and directly from the pre-pyloric vessels.
MICROSTRUCTURE
The pancreas is composed of two different types of glandular tissue. The main tissue mass is exocrine, in which pancreatic islets of endocrine cells are embedded (Figs 70.9-70.11).
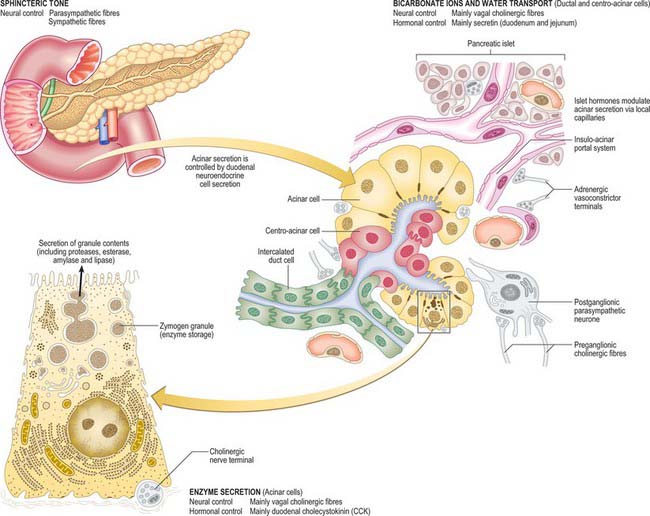
Fig. 70.9 Microstructure of the exocrine pancreas and the mechanisms by which its secretion is controlled. Pancreatic stellate cells (see text) are not shown.
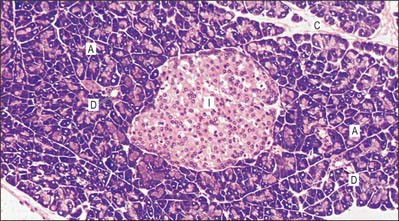
Fig. 70.10 Pancreatic tissue. Exocrine acinar cells (A) are deeply stained basally, indicating the high ribosomal concentration. Small ducts (D) are shown. An endocrine islet (of Langerhans) (I) is shown centrally, with pale-staining cells surrounded by a network of capillaries, seen as clear spaces. Connective tissue septa (C) separate lobules.
(By permission from Dr JB Kerr, Monash University, from Kerr JB 1999 Atlas of Functional Histology. London: Mosby.)
Exocrine pancreas
The exocrine pancreas isa branched acinar gland, surrounded and incompletely lobulated by delicate loose connective tissue. It is formed of pyramidal, secretory cells arranged mainly as spherical clusters, or acini. A narrow intercalated, intralobular duct originates within each secretory acinus, lined initially by flattened or cuboidal centro-acinar cells. These small ductules form branching links which run within and between adjacent acini, explaining why structurally distinct intralobular pancreatic ducts are infrequent (see Kerr 1999, for details). More distally, these are replaced by taller cuboidal and eventually columnar epithelium in the larger interlobular ducts. The latter are surrounded by loose connective tissue of the septa, containing smooth muscle and autonomic nerve fibres. Neuroendocrine cells are present amongst the columnar ductal cells and mast cells are numerous in the surrounding connective tissue.
Acinar cells of the exocrine pancreas have a basal nucleus and, in their basal cytoplasmic domain, abundant rough endoplasmic reticulum which results in their basophilic staining characteristics. Dense secretory zymogen granules stain deeply with eosin in the apical region. A prominent supranuclear Golgi complex is surrounded by large, membrane-bound granules containing the proteinaceous constituents of pancreatic secretion, including enzymes which are only active after release. Ganglionic neurones and cords of undifferentiated epithelial cells are also found within the acini. The structure of the exocrine pancreas and its functional regulation are summarized in Fig. 70.9.
Pancreatic stellate cells, PaSCs, are one of several resident cells in the exocrine pancreas. They are regulated by autocrine and paracrine stimuli and share many features with their hepatic counterparts. PaSCs are myofibroblast-like cells and are found in the periacinar space, where their long cytoplasmic processes encircle the base of the acinus, and in perivascular and periductal regions of the pancreas. They have been implicated as key players in the pathobiology of the major disorders of the exocrine pancreas, including chronic pancreatitis and pancreatic cancer (Bishr Omary et al 2007).
Endocrine pancreas
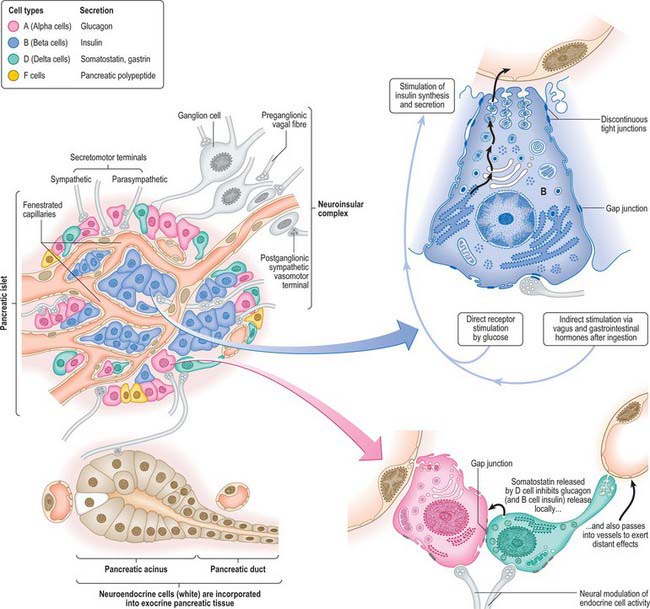
The endocrine pancreas consists of pancreatic islets of Langerhans, composed of spherical or ellipsoid clusters of cells embedded in the exocrine tissue. The human pancreas may contain more than a million islets, usually most numerous in the tail. An islet is a mass of polyhedral cells, each in close proximity to fenestrated capillaries and a rich autonomic innervation. Specialized staining procedures or immunohistochemical techniques are necessary to distinguish the three major types of cell, designated alpha, beta and delta. Their general organization is shown in Fig. 70.11.
The most numerous cells, types alpha and beta, secrete glucagon and insulin respectively. Alpha cells tend to be concentrated at the periphery of islets, and beta cells more centrally. A third type, the delta cell, secretes somatostatin and gastrin, and like alpha cells, is peripherally placed within the islets. A minor cell type, the F cell, secretes pancreatic polypeptide (PP), which is stored in smaller secretory granules. The autonomic neurotransmitters acetylcholine (ACh) and noradrenaline affect islet cell secretion: ACh augments insulin and glucagon release, noradrenaline inhibits glucose-induced insulin release, and they may also affect somatostatin and PP secretion.
Innervation of endocrine pancreas
The innervation of the endocrine islets is almost exclusively from the parasympathetic system. Fine branches ramify among the cells and form plexuses around the islets. Fibres frequently synapse with acinar cells before innervating the islets, suggesting a close linkage between neural control of exocrine and endocrine components. Many fibres enter the islets with the arterioles. Parasympathetic ganglia lie in the connective tissue within and between lobules, and in the former case are frequently associated with islet cells, forming neuroinsular complexes. Both alpha and beta cells are involved in these neuroinsular complexes. Three types of nerve terminal are seen in islets. Cholinergic terminals have agranular vesicles with a diameter of 30–50 nm, adrenergic terminals have dense-cored vesicles with a diameter of 30–50 nm and a third, uncharacterized, type have dense-cored vesicles with a diameter of 60–200 nm (Smith & Porte 1976).
No selective link with any one type of insular cell has been found. Sometimes more than one type of terminal contacts a single cell and some of the chemical synapses between axon terminals and islet cells show narrow areas in the synaptic clefts suggesting an electrical synapse or gap junction. Such junctions also occur between islet cells, and electrical coupling of nerve supply to a functional network of islet cells may occur (Orci 1974).
Bishr Omary M, Lugea A, Lowe AW, Pandol SJ. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest. 2007;117:50-59.
Kerr JB. Atlas of Functional Histology, Chapter 14. London: Mosby, 1999.
Kimura W. Surgical anatomy of the pancreas for limited resection. J Hepatobiliary Pancreat Surg. 2000;7:473-479.
Nagai H. Configurational anatomy of the pancreas: its surgical relevance from ontogenetic and comparative-anatomical viewpoints. J Hepatobiliary Pancreat Surg. 2003;10:48-56.
Orci L. A portrait of a pancreatic B-cell. Diabetologia. 1974;10:163-187.
Smith PH, Porte DJr. Neuropharmacology of the pancreatic islets. Annu Rev Pharmacol Toxicol. 1976;16:269-285.