Surface and Equipment Asepsis
After completing this chapter, the student should be able to do the following:
 Determine which operatory surfaces may be involved in the patient-to-patient spread of microbes.
Determine which operatory surfaces may be involved in the patient-to-patient spread of microbes.
 Differentiate between clinical contact surfaces and housekeeping surfaces.
Differentiate between clinical contact surfaces and housekeeping surfaces.
 List the operatory surfaces that should be covered with barriers before patient care.
List the operatory surfaces that should be covered with barriers before patient care.
 Describe how to place and remove surface covers properly.
Describe how to place and remove surface covers properly.
 List the types of surface disinfectants and describe their properties.
List the types of surface disinfectants and describe their properties.
 Differentiate between low-, intermediate- and high-level disinfectants and give examples when each should be used.
Differentiate between low-, intermediate- and high-level disinfectants and give examples when each should be used.
 Read with understanding the labels on disinfectants.
Read with understanding the labels on disinfectants.
 Describe the importance of precleaning before surface disinfection.
Describe the importance of precleaning before surface disinfection.
 Describe how to preclean and disinfect contaminated surfaces and equipment.
Describe how to preclean and disinfect contaminated surfaces and equipment.
 Describe how aseptically to retrieve and distribute clinical supply items.
Describe how aseptically to retrieve and distribute clinical supply items.
TYPES OF ENVIRONMENTAL SURFACES
Two types of dental environmental surfaces are related to disease spread: clinical contact surfaces and housekeeping surfaces. Clinical contact surfaces are surfaces that may be touched frequently with gloved hands during patient care or that may become contaminated with blood, saliva, or other potentially infectious material and subsequently contact instruments, devices, hands, or gloves. Box 12-1 gives examples of such surfaces. Housekeeping surfaces are surfaces that do not come into contact with hands or devices used in dental procedures (e.g., floors, walls, and sinks). The clinical contact surfaces need to be treated properly before they become involved in the care of the next patient. Housekeeping surfaces can be treated at the end of the day.
Different microorganisms may survive on environmental surfaces for different periods, as determined in laboratory studies. For example, Mycobacterium tuberculosis may survive for weeks, whereas the herpes simplex virus dies in a matter of seconds to minutes. Various conditions influence the survival time of microorganisms in the environment, including humidity, temperature, the presence of nutrients and blood or saliva, and the general surface properties of the microorganism. Accurately predicting how long any microorganism actually may survive on a dental office surface is impossible because of these unknown variables. Thus if a surface becomes contaminated with saliva, blood, or other potentially infectious material, the safest approach is to assume that it indeed contains live microorganisms that must be removed or killed before the surface is involved in the treatment of the next patient.
Two general approaches to surface asepsis are these: one is to prevent the surface or item from becoming contaminated by use of a surface cover, and the other is to preclean and disinfect the surface after contamination and before reuse. Each approach has advantages and disadvantages, and usually an office will use a combination of both approaches (Table 12-1).
SURFACE COVERS
The best way to manage surface asepsis from an infection control point of view is to prevent contamination of the surface so that it will not have to be precleaned and disinfected before reuse.
Types of Surface Covers
Contamination can be prevented by proper placement of a surface cover before an opportunity for contamination exists. Surface covers should be impervious to fluids to keep microorganisms in saliva, blood, or other liquids from soaking through to contact the surface. Examples of appropriate material for surface covers include clear plastic wrap, bags, or tubes, and plastic-backed paper. Some plastics are designed specifically for use as surface covers in the office in that they have the shape of the item to be covered (e.g., air/water syringe handle covers, hose covers, and pen covers). Some sheets of plastic also have a slightly sticky substance on one side to hold them on the surface. Other plastics (e.g., some food wraps) have a natural clinging ability on contact with a smooth surface. Some plastic bags are available with drawstrings that hold the bag around an item to be protected. To reduce costs, one can use thin rather than thick plastic sheets or bags as long as they are not punctured by the surface being covered. Patient bibs are made of plastic-backed paper and also can be used to cover flat operatory surfaces, although thin plastic sheets may be less expensive.
Use of Surface Covers
Dental units come in several shapes and sizes with different positioning of the handpiece control system and light and other accessories. Thus the sizes and shapes of surfaces to be covered vary from one office to the next, but general procedures for use of surface covers are the same (Procedure 12-1).
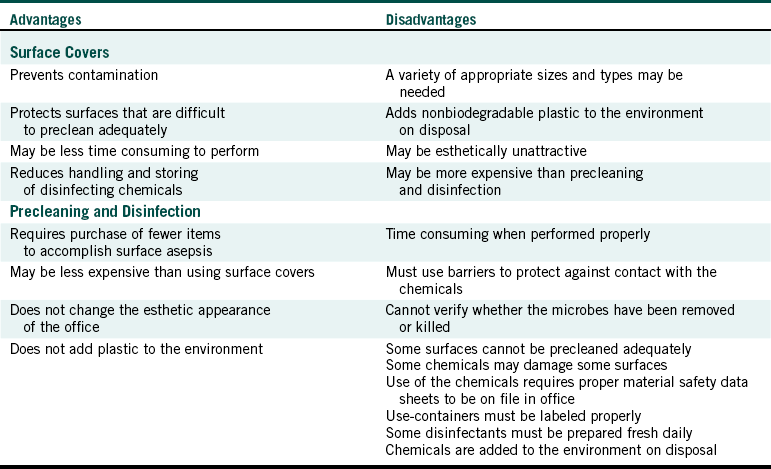
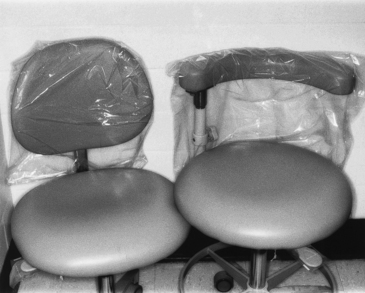
Clear plastic bags are available in various sizes and are easy to use. For example, if a chair has control buttons on the side, one can use one bag to cover the headrest and the buttons (Figure 12-1). Wraparound backs of side chairs used by the dental team may be touched during patient care and can be covered with a large plastic bag (Figure 12-2). On units with a bracket table, one may use one bag to cover the handpiece control unit and the bracket table (Figure 12-3 and Figure 12-4). One can cover hoses and connectors with plastic tubing that is secured with tape or a rubber band at the connector or can force a narrow, long plastic bag over the connector end (Figure 12-5).
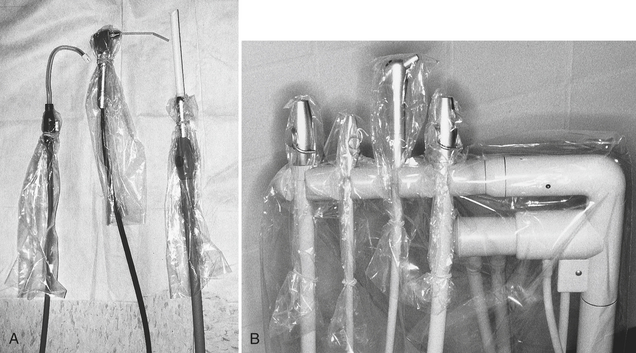
FIGURE 12-5 Surface covers for hoses and connectors. A, Saliva ejector connector, air/water syringe handle, and high volume evacuation connector. B, Various connectors on holders.
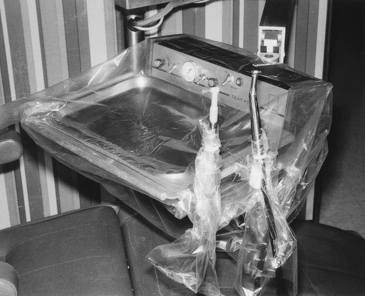
Light handles and light switches commonly are touched during patient care and can be covered with plastic wrap or bags, depending on their shape (Figure 12-6). Some lights have removable handles that can be cleaned and heat sterilized before reuse. Covering the air/water syringe handle with plastic wrap to prevent contamination is better than trying to preclean and disinfect properly around the buttons that tend to retain debris (see Figure 12-5). One can cover the heads of x-ray units and the control panels (Figure 12-7), and if the water at sinks is not controlled by elbow levers, foot pedals, or automatic devices, one can cover faucet handles with plastic bags (Figure 12-8).
PRECLEANING AND DISINFECTION
Precleaning and disinfection best lend themselves to nonelectric surfaces that are smooth and easily accessible for facilitating good contact with the decontaminating chemicals. Procedure 12-2 describes general procedures for precleaning and disinfecting surfaces.
Approaches to Precleaning and Disinfection
Surfaces to be disinfected first must be precleaned. Precleaning reduces the number of contaminating microorganisms and the blood or saliva present (referred to as bioburden) and facilitates action of the disinfecting chemical. Precleaning is an important step that one must not slight. The organic material in blood and saliva insulates microorganisms from contact with a disinfecting chemical and also may inactivate a portion of the active chemical in the disinfectant.
During the precleaning process, one may use regular soap and water, but use of a surface cleaner/disinfectant that contains detergents is best for the precleaning step and the disinfecting step. Using a disinfectant/cleaner for the precleaning step starts the killing process early and reduces the chances of spreading the contamination to adjacent surfaces. This (along with wearing heavy utility gloves, a mask, protective eyewear, and protective clothing) provides a little more protection to the person cleaning the surface. Water-based disinfectant-detergents (e.g., some synthetic phenolics, iodophors, and quaternary ammonium compounds—alcohols) solubilize organic materials such as blood and saliva and facilitate their removal. One also can use these same products in the disinfection step.
There are two approaches to precleaning and disinfection (see Procedure 12-2). One (using a liquid disinfectant/cleaner) is spray-wipe-spray and the other (using a disinfectant towelette) is wipe-discard-wipe. For spray-wipe-spray (Figure 12-9) spray the cleaner/disinfectant on the surface and wipe (preclean) the surface vigorously with a paper towel or gauze pad. Then respray the disinfectant/cleaner on the precleaned surface and let it remain moist for the longest contact time indicated on the disinfectant label (usually 10 minutes). If moisture remains, one should wipe it away with a paper towel. Some disinfectants are promoted as “one-step” products (i.e., spray and wipe). Although it is usually best to follow manufacturer’s directions on all products, it won’t hurt to use the one-step products in the two-step procedures (spray-wipe-spray). The two-step procedure becomes even more important should the postcleaning disinfecting contact time stated on the product label be shortened for some reason. If this is the case, at least the precleaning step will have removed/killed some of the microbes in the bioburden on the surface.
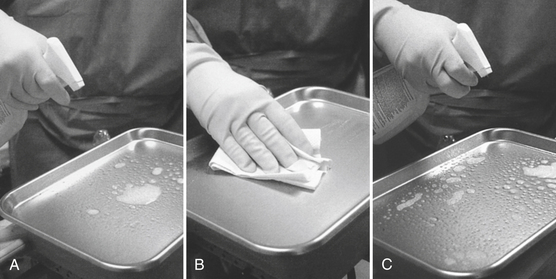
FIGURE 12-9 Surface precleaning and disinfecting by the spray-wipe-spray technique. Precleaning consists of A, spraying with a detergent-disinfectant and B, wiping the surface to clean it. Disinfection is C, reapplying the detergent-disinfectant followed by the appropriate contact time.
Do not store paper towels or gauze pads in a disinfectant and use as wipes to avoid spraying. Wet the towels or pads just before use. Paper towels may be held behind some surfaces to catch overspray, if desired.
An alternative to spray-wipe-spray is the wipe-discard-wipe procedure using disinfectant towelettes. Pull a towelette from its container and wipe (preclean) the surface. Then discard that towelette. Obtain another towelette and wipe (disinfect) the surface, let the surface remain wet for the prescribed time, and discard the towelette. Dry off the surface if necessary. When using containers of disinfectant towelettes, keep the lid closed when not in use to prevent evaporation of the alcohol. Also periodically invert the container to redistribute any settled liquid.
If it is difficult to remove all of the bioburden from a given surface, then consider protecting it with a surface cover during future use. This is usually better than attempting to brush it clean and risk spreading contaminations to nearby surfaces.
Unfortunately, one cannot determine the effectiveness of disinfecting surfaces. Thus performing the procedures carefully and using the disinfectant as described on the product label is important.
Characteristics of Disinfectants
There are four general types of antimicrobial chemicals.
• Antibiotics (for killing microorganisms in or on the body)
• Antiseptics (for killing microorganisms on the skin or other body surfaces)
• Disinfectants (for killing microorganisms on environmental/inanimate surfaces or objects)
• Sterilants (for killing all microorganisms on inanimate objects)
The Centers for Disease Control and Prevention has categorized disinfectants based on their microbial spectrum of activity. This categorization follows and is described further in Table 12-2:
TABLE 12-2
Categories of Disinfecting/Sterilizing Chemicals
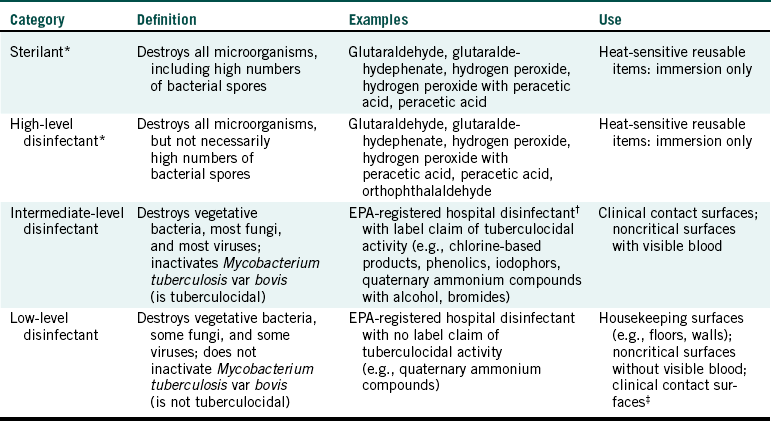
EPA, Environmental Protection Agency.
∗Some, but not all, of these products can serve as high-level disinfectants and sterilants depending on the immersion time used.
†A hospital disinfectant is one that has been shown to kill Staphylococcus aureus, Pseudomonas aeruginosa, and Salmonella choleraesuis.
‡The Centers for Disease Control and Prevention indicates that low-level disinfectants can be used on clinical contact surfaces if the product has a label claim of killing human immunodeficiency virus and hepatitis B virus in addition to being an EPA-registered hospital disinfectant.
Adapted from Centers for Disease Control and Prevention: Guideline for infection control in dental health-care settings, 2003, MMWR 52 (No. RR-17):66, 2003.
• Sterilant/high-level disinfectant (for killing all microorganisms on submerged, inanimate, heat sensitive objects)
• Intermediate-level disinfectant (for killing vegetative bacteria, most fungi, viruses, and Mycobacterium tuberculosis var bovis)
• Low-level disinfectant (for killing most vegetative bacteria, some fungi, and some viruses)
Labels on antimicrobial products can be confusing, but reading these labels carefully before using the product is very important. These labels commonly include the type of antimicrobial agent (active ingredients) and general properties such as the following:
• Virucidal (kills at least some viruses)
• Bactericidal (kills at least some bacteria)
• Fungicidal (kills at least some fungi)
• Tuberculocidal (kills the Mycobacterium tuberculosis var bovis bacterium)
• Sporicidal (kills bacterial spores, which means it is a sterilant)
• Hospital disinfectant (shown to kill the three representative bacteria: Staphylococcus aureus, Salmonella choleraesuis, and Pseudomonas aeruginosa)
The labels also commonly include the following:
• Specific microorganisms (genus and species) shown to be killed in laboratory testing (along with the necessary contact time)
• Directions for use of the product, including the need for precleaning
• Precautionary statements on handling the product
• Warnings such as toxic, poisonous, or flammable
• Treatment for accidental contact
• Storage and disposal information
• How to activate or dilute the product
• Name and address of the manufacturer/distributor, volume of the container, and Environmental Protection Agency (EPA) registration number
No perfect disinfectant exists that will kill all types of disease-producing microorganisms rapidly, have no toxic properties, be unaffected by organic materials, be odorless, not cause any damage to any surface, and be economical. Nevertheless, several classes of disinfecting chemicals have gained wide use in dentistry and medicine. These classes are identified by the type of antimicrobial agents present in the product, which are listed on the product label as active ingredients (Table 12-3). Because M. tuberculosis var bovis is more difficult to kill than most other microorganisms, disinfectants with tuberculocidal activity are considered as strong disinfectants (see Chapter 11, Figure 11-1, Mycobacterium species). Stronger disinfectants are active against nonenveloped hydrophilic viruses also, as described in Table 12-4. Use of a water-based disinfectant is reported to provide better cleaning of biologic material such as blood than use of an alcohol-based disinfectant. For dental infection control, a water-based surface disinfectant that is EPA-registered and tuberculocidal (such as iodophors, phenolics, or chlorines) is appropriate if used as directed by the manufacturer and careful precleaning is performed.
TABLE 12-3
Active Ingredients in Surface Disinfectants
| Type of Disinfectant | Example of Active Ingredient(s) Listed on Product Label |
| Chlorines∗ | Sodium hypochlorite |
| Chlorine dioxide | |
| Iodophors∗ | Butoxypolypropoxypolyethoxyethanol-iodine complex |
| Water-based phenolics∗ | |
| Triphenolics | o-Phenylphenol, o-benzyl-p-chlorophenol, and tertiary amylphenol |
| Dual phenolics | o-Phenylphenol and o-benzyl-p-chlorophenol |
| PCMX | p-Chloro-m-xylenol |
| Alcohol-based phenolics∗ | Ethyl or isopropyl alcohol plus |
| o-phenylphenol or o-phenylphenol and tertiary amylphenol | |
| Isopropyl alcohol | |
| Alcohols∗ | |
| Quaternary ammonium compounds† | |
| First generation | Benzalkonium chloride |
| Second generation | Alkyldimethylethylbenzyl ammonium chloride or alkyl- dimethyl-3,4-dichloro-benzyl ammonium chloride |
| Third generation | Combination of first and second generation |
| Fourth generation | Dioctyldemethyl ammonium bromide or didecyldemethyl ammonium bromide |
| Fifth generation | Combination of first and fourth generation |
| Alcohol–quaternary ammonium compound∗ | A dimethyl-benzyl ammonium chloride plus isopropyl alcohol |
TABLE 12-4
Types of Viruses Used in Testing Disinfectant
| Class | Virus | Solubility |
| Nonenveloped viruses | Poliovirus type 1 | Hydrophilic∗ |
| Coxsackievirus B1, B2 | Hydrophilic | |
| ECHO type 6 | Hydrophilic | |
| Rhinovirus type 17 | Hydrophilic | |
| Adenovirus type 2 or 7 | Intermediate† | |
| Reovirus | Intermediate | |
| Rotavirus | Intermediate | |
| V-40 virus | Intermediate | |
| Enveloped viruses | Herpes simplex 1 | Lipophilic‡ |
| Influenza A2 | Lipophilic | |
| Vaccinia | Lipophilic | |
| Human immunodeficiency virus | Lipophilic |
∗Hydrophilic viruses do not have an envelope, and this makes them more difficult to kill with disinfecting chemicals and makes them more soluble in water.
†Intermediate viruses do not have an envelope, but their susceptibility to killing by disinfecting chemicals is intermediate between lipophilic and hydrophilic viruses, and they are more soluble in lipid materials than other nonenveloped viruses.
‡Lipophilic viruses have a lipid envelope that makes them easier to inactivate with disinfecting chemicals and makes them more soluble in lipid materials than in water.
Manufacturers of disinfectants must submit testing data on the antimicrobial activity and safety of their products to the EPA. If the data are consistent with claims stated on the product labeling and other requirements are met, the EPA registers the product. The U.S. Food and Drug Administration also must grant marketing clearance to liquid chemical sterilants/high-level disinfectants labeled for use on medical devices such as heat-sensitive dental items.
CHLORINE COMPOUNDS
Chlorine compounds have been used for many years to disinfect drinking water, swimming pool water, and various inanimate surfaces. These agents are intermediate-level disinfectants, kill a wide variety of microorganisms, and are tuberculocidal. Sodium hypochlorite (which is the main chemical in bleach) is an example of a chlorine compound used to disinfect surfaces. One should note that commercial bleach (which contains about 5.25% sodium hypochlorite) is a good surface disinfectant at a 1:10 to 1:100 dilution with water, even though the bleach product is not an EPA-registered disinfectant. However, EPA-registered disinfectants containing sodium hypochlorite or other chlorine compounds are available. Sodium hypochlorite can damage fabrics and metal surfaces (particularly aluminum), and its activity is reduced in the presence of organic material. If one uses diluted commercial bleach as a disinfectant, one should prepare the solution fresh daily. One should wear gloves, protective eyewear, a mask, and protective clothing when using any disinfectant.
IODOPHORS
Iodine and iodine-alcohol mixtures (known as tinctures of iodine) are well-known killing agents but have some undesirable properties of corrosiveness, staining, irritation of tissues, and allergenicity. When iodine is complexed with certain organic materials, the compound is referred to as an iodophor (see Table 12-3). Most iodophors are intermediate-level disinfectants and retain the broad-spectrum antimicrobial activity (including tuberculocidal activity) of iodine, but they are less corrosive, are less irritating to tissues, and have reduced staining activity. Detergents are added to iodophor preparations used as surface disinfectants to enhance the cleaning ability of the solution. One may purchase iodophor disinfectants as concentrated solutions that must be diluted correctly before use. The diluted solution that is used should be prepared fresh daily, unless the manufacturer’s label indicates otherwise. Because hard water may reduce antimicrobial activity, iodophor disinfectants should be diluted with distilled or deionized water. Iodophors still may be slightly corrosive to some metals and may cause slight staining with repeated use on light-colored surfaces. However, one usually can remove this stain by wiping with alcohol. One should wear gloves, protective eyewear, a mask, and protective clothing when using any disinfectant.
ALCOHOLS
Isopropyl alcohol (isopropanol, sometimes referred to as rubbing alcohol) and ethyl alcohol (ethanol) have been used as antiseptics and disinfectants for many years because they are relatively nonirritating to the skin, although alcohol does “sting” when placed on mucosa (e.g., eye, mouth, and nostrils). Although alcohols at 50% to 70% concentration rapidly kill many microorganisms and are tuberculocidal, they evaporate rapidly when sprayed or wiped on surfaces. To counteract this drawback, extenders that retard evaporation of the alcohol after it is placed on a surface have been added to some isopropanol preparations sold as surface disinfectants. Nevertheless, alcohol has other properties that make it less desirable than other agents as a disinfectant. These properties include a reduction in activity by organic material, corrosiveness, and destruction of some plastic surfaces. Alcohols also do not solubilize protein material in blood or saliva well and have been reported to be poor cleaners. Alcohols dry out the skin because they tend to dissolve fat and oil that serve as natural skin moisteners. The “waterless” alcohol-containing hand rubs also contain special skin moisteners to reduce these drying effects. Although other agents are more appropriate than alcohol as surface cleaners/disinfectants, alcohol is an important component of some disinfectant preparations (see Table 12-3).
SYNTHETIC PHENOLICS
Phenol (also known as carbolic acid) has the distinction of being the first widely recognized disinfectant used in hospitals. Lord Joseph Lister suggested the use of phenol as an antiseptic during surgical procedures and as an environmental surface disinfectant more than 100 years ago. Although phenol clearly reduced postoperative infections, it was toxic to tissues, and its use on human beings was stopped. Several phenol-related (phenolic) compounds have been synthesized since and used for microbial killing. Thus these compounds are referred to as synthetic phenolics, with some use today as active ingredients in surface disinfectants (see Table 12-3) and in mouth rinses and handwashing agents.
Most of the synthetic phenolic disinfectants are intermediate-level tuberculocidal agents and may contain one, two (dual phenolics), or three phenolics (tirphenolics), as well as detergents to facilitate cleaning. Some of these disinfectants also contain alcohol. Some preparations must be diluted before use and should be prepared fresh daily unless otherwise stated on the product label. Some preparations are packaged in aerosol cans, and others are contained in pump-spray bottles. Some preparations may leave a film on the disinfected surface, degrade plastic surfaces with prolonged contact, or etch glass surfaces. One should wear gloves, protective eyewear, a mask, and protective clothing when using any disinfectant.
QUATERNARY AMMONIUM COMPOUNDS
ALCOHOL-FREE QUATERNARY AMMONIUM COMPOUNDS: Quaternary ammonium compounds are also called “quats.” These compounds are cationic detergents categorized as low-level disinfectants. A wide variety of quats has been developed over the years, and each time a new type of quat is developed, it is referred to as the next generation (see Table 12-3). All of the alcohol-free quat disinfectants have a low level of antimicrobial activity, and none are tuberculocidal. These disinfectants also may be inactivated by organic materials and soaps. Although they may be appropriate for disinfection of floors and walls, they are less desirable for items more directly involved in patient treatment that may be contaminated with body fluids. Because of these drawbacks, the American Dental Association recommended in 1978 that quaternary ammonium compounds (free of alcohol) not be used in dentistry. However, the Centers for Disease Control and Prevention has indicated that low-level disinfectants such as alcohol-free quats may be used on clinical contact surfaces if the product also has claims for killing human immunodeficiency virus and hepatitis B virus.
EQUIPMENT DECONTAMINATION
Some basic considerations for equipment decontamination exist. In general, one should try to prevent the equipment from becoming contaminated in the first place. If this is not possible, one should select heat sterilization first. If the equipment cannot be heat sterilized, then one should use a liquid sterilant or an appropriate disinfectant for decontamination. Following the manufacturer’s directions for preventing contamination or for cleaning, sterilization or disinfection of dental equipment is important.
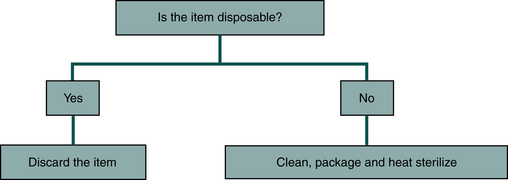
One should use the following principles to reduce the spread of microbes from dental equipment used in the mouth that will penetrate soft tissue or tooth structure (Figure 12-10).
• If the item is disposable, one should dispose of it properly after use on one patient.
• If the item is not disposable, one should clean it, package it, and heat sterilize it.
One should use the following approach to decontamination if the item will be used in the patient’s mouth but will not penetrate soft tissue or tooth structure (Figure 12-11).
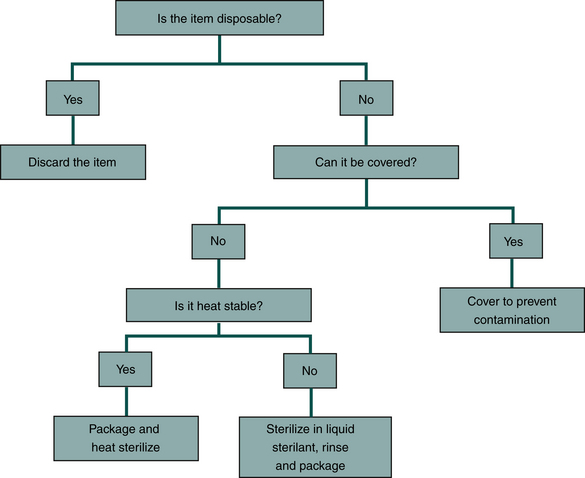
FIGURE 12-11 Management of equipment that will be used in a patient’s mouth but will not penetrate soft tissue or tooth structure.
• If the item is disposable, one should dispose of it properly after use on one patient.
• If the item is reusable, one should cover the parts that may become contaminated with an impervious barrier to prevent contamination.
• If the item is not disposable, cannot be covered completely, and becomes contaminated, one should clean it, package it, and heat sterilize it.
• If the item cannot be heat sterilized, one should clean and submerge it in a liquid sterilant/high-level disinfectant.
• If the item is not disposable and cannot be covered, heat sterilized, or submerged in a liquid agent, do not use the item.
One should use the following approach for items that are not used in the patient’s mouth (Figure 12-12).
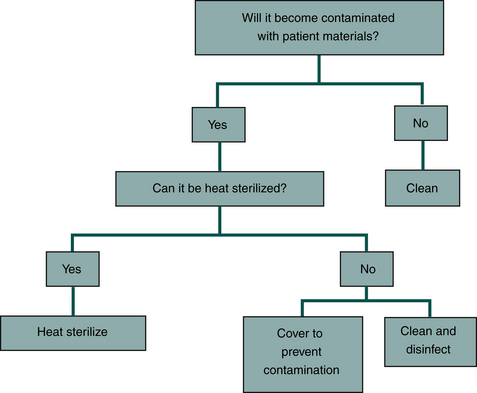
FIGURE 12-12 Management of equipment that will not be used in a patient’s mouth but that may become contaminated by touching or by spatter.
• If an item will not become contaminated with patient materials, one should just keep it clean.
• If an item will become contaminated with patient materials, one should clean it and when possible heat sterilize it.
• If the item cannot be heat sterilized because of its size or composition, one should clean and disinfect it.
Management of High-tech Equipment
New technologies are developed continually that aid the dental profession in treating patients. These technologies must be compatible with infection control or their use will be limited, but some technologies may present special infection control challenges, such as computers, cameras, and digital x-ray sensors.
For computers, one should avoid their contamination by performing hand hygiene before use. If this does not occur, recent studies have shown that the keyboards can be successfully decontaminated by using disinfectant wipes such as those containing quats. Also the same study showed that no damage occurred to the keyboards after 300 disinfecting procedures. Care should be taken that disinfectant wipes used should not contain excess fluid that might that might enter the base of the keyboard.
For 35-mm, video, and digital cameras, one should try to avoid their contamination. They are not designed to be disinfected. One should cover them with plastic sheeting or operate them only with clean hands.
Computer-aided design/computer-aided manufacturing devices (CAD/CAMS) use close-up intraoral pictures to design and manufacture restorations. These devices use a small camera lens on the end of a wire that transmits pictures from the patient’s mouth to a TV-type monitor. One should check with the manufacturer for proper decontamination of the camera and wire. One model has an outer removable prismatic device that slides over the camera lens, so the prism rather than the camera lens becomes contaminated. The prism can be sterilized in a dry heat sterilizer but not in a steam sterilizer, for the moist heat can damage the prism.
Digital radiography equipment needs special attention. This equipment uses intraoral sensors to provide a digital image that can be manipulated, viewed on a monitor, printed, or stored electronically. Because the sensors are reusable, one must handle them properly to prevent cross-contamination during subsequent use. One type of sensor, the charge-coupled device (CCD), is attached to a wire and should not be heat sterilized. Instead, one should cover the CCD with a plastic barrier that extends down the cable to prevent any contact with patient materials or contaminated hands. If the CCD does become contaminated, one may disinfect it by the spray-wipe-spray technique according to the manufacturer’s directions. Another type of digital x-ray sensor is the complementary metal-oxide semiconductor with active pixel sensors. This device is wired and should be treated with plastic barriers just like the CCD sensor. A third type of sensor, the photostimulable phosphor plate sensor, is wireless and is placed in the patient’s mouth much like a regular film packet. One must cover the device with a plastic barrier; it cannot become contaminated because it cannot be heat sterilized or chemically disinfected.
ASEPTIC DISTRIBUTION OF DENTAL SUPPLIES
Numerous supplies are used for patient care, and their storage and distribution present a major challenge to infection control. Examples of such items include cotton balls and rolls, gauze pads, floss, articulating paper, retraction cord, orthodontic wire, and tubes or bottles of dental materials, to mention only a few. The major problems relate to surface asepsis and how these supplies are obtained for use at chairside without cross-contamination.
Aseptic Retrieval
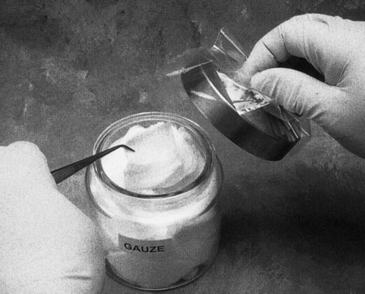
If one stores supply items in bulk, such as a container of cotton rolls, one must use an aseptic retrieval system (rather than saliva-coated gloved fingers) to avoid contamination of unused items in the container. Providing sterile forceps (to retrieve the supply item) with the instruments needed for each patient is one approach to this problem (Figure 12-13). Storing supplies (or instruments) in drawers at chairside lends itself to cross-contamination of the drawer handle (if not covered or precleaned and disinfected) or of bulk items inside (if aseptic retrieval is not used).
Unit Dosing
The dental professional may protect supply containers, bottles, and tubes of materials used at chairside on more than one patient (multidose containers) with a surface cover for each patient or in some instances may preclean and disinfect these between patients. Many types of disposable supplies can be unit dosed, which means that the supplies are distributed or packaged in small numbers sufficient for care of just one patient and are placed at chairside before care begins. For example, a package may contain four cotton rolls, three cotton balls, two gauze pads, articulating paper, or whatever one anticipates for a single patient. Whatever is not used with a patient is discarded. Some supply items are unit dosed by the manufacturers, saving office staff time. Although unit dosing can solve some cross-contamination problems, unfortunately it can be expensive and wasteful if not organized properly.
Centers for Disease Control and Prevention. Guideline for infection control in dental health-care settings, 2003. MMWR. 2003;52(No. RR-17):1–68.
Miller, C.H. High-tech equipment: keeping it germ-free. Dent Prod Rpt. 2003;37:68–71.
Miller, C.H. Infection control. Dent Clin North Am. 1996;40:437–456.
Miller, C.H. High-tech equipment: keeping it germ-free. Dent Prod Rpt. 2003;37:68–71.
Miller, C.H. Labels, a must read. Dent Prod Rpt. 2006;40:82–86.
Miller, C.H., Strategies for infection control in dentistry. ADA guide to Dental Therapeutics, ed 3, Chicago, ADA Publishing, 2003. 551–566
Miller, C.H., Palenik, C.J. Sterilization, disinfection and asepsis in dentistry. In: Block S.S., ed. Sterilization, disinfection and preservation. ed 5. Philadelphia: Lea & Febiger; 2001:1049–1068.
Molinari, J.A., et al. Cleaning and disinfectant properties of dental surface disinfectants. J Am Dent Assoc. 1988;117:179–182.
Rutala, W.A., White, M.S., Gergen, M.F., Weber, D.J. Bacterial contamination of keyboards: Efficacy and functional impact of disinfectants. Inf Cont Hosp Epidemol. 2006;27:372–377.
Williams, H.N., Singh, R., Romberg, E. Surface contamination in the dental operatory. J Am Dent Assoc. 2003;134:325–330.
Review Questions
______1. Which of the following microbes are not killed by intermediate-level disinfectants?
______2. Which of the following types of antimicrobial agents should be used on floors?
______3. Which of the following antimicrobial agents should not be used for surface disinfection in dentistry?
______4. Which of the following agents is not tuberculocidal?
______5. What types of surfaces should be covered rather than disinfected?
______6. Why should disinfectants used on clinical contact surfaces in dentistry contaminated with blood or saliva have tuberculocidal activity?
a. Tuberculosis is a common occupational disease of dental personnel, so a tuberculocidal agent is needed to prevent this type of spread in the office.
b. The tuberculosis agent is spread most commonly by touching contaminated surfaces.
c. Because the tuberculosis agent is one of the more resistant microbes to kill, tuberculocidal activity indicates sufficient potency to kill most other vegetative microbes.
______7. Liquid sterilants such as a glutaraldehyde should be used only on reusable items that can be submerged and are:
______8. One should not use ______________ as a surface cover.
______9. Use of a disinfectant-type cleaner to preclean a contaminated operatory surface:
b. starts the microbial killing process and helps protect the person doing the cleaning
c. eliminates the need to follow up with a disinfectant wipe
______10. When can a liquid sterilant/high-level disinfectant achieve sterilization?