Dental Unit Water Asepsis
PRESENCE OF MICROORGANISMS IN DENTAL UNIT WATER
TYPES AND IMPORTANCE OF MICROORGANISMS IN DENTAL UNIT WATER
BIOFILM IN DENTAL UNIT WATER LINES
NEED TO IMPROVE DENTAL UNIT WATER QUALITY
CURRENT INFECTION CONTROL RECOMMENDATIONS
DENTAL UNIT WATER AND INFECTION CONTROL
After completing this chapter, the student should be able to do the following:
 Define biofilm and describe how it forms inside dental unit water lines.
Define biofilm and describe how it forms inside dental unit water lines.
 List the microbes that may be present in dental unit water.
List the microbes that may be present in dental unit water.
 Describe the concerns of having microbes present in dental unit water.
Describe the concerns of having microbes present in dental unit water.
 Describe the different approaches for reducing the microbial quantity in dental unit water.
Describe the different approaches for reducing the microbial quantity in dental unit water.
 Describe the procedures for monitoring the quality of dental unit water.
Describe the procedures for monitoring the quality of dental unit water.
 Describe what a “boil water” notice means.
Describe what a “boil water” notice means.
The goal of infection control in dentistry is to reduce or eliminate exposures of patients and the dental team to microorganisms. Other chapters have discussed examples of how to accomplish infection control; this chapter describes contamination of the patient and the dental team with microorganisms present in dental unit water and what may be done to control the quality of dental unit water.
DENTAL UNIT WATER
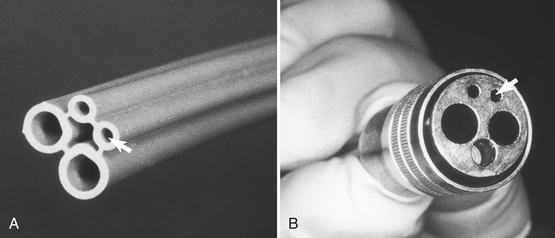
Water enters the dental office from municipal supplies or from wells. As in the homes, water then is routed to various sites, including sink faucets, toilets, water heaters, air conditioners, humidifiers, washers, and, in dental offices, dental units. At the dental unit, water enters plastic water lines that pass through a multichannel control box that allows the water to be distributed to the hoses that feed various attachments such as high-speed handpieces, air/water syringes, and sometimes an ultrasonic scaler. The water lines in dental units have a small bore (about 1⁄16 -inch inside diameter), and, in the standard four-hole handpiece hose, the water line is one of the two smaller lines (Figure 13-1). Thus the water that enters the dental unit is the same water that supplies the entire office.
PRESENCE OF MICROORGANISMS IN DENTAL UNIT WATER
The Environmental Protection Agency standard for the microbial quality of drinking water (called potable water) is no more than a total of 500 CFU/mL (colony-forming units per milliliter) of noncoliform bacteria. A colony-forming unit is considered to be one bacterial cell or a small number of bacterial cells, and a milliliter is about one fourth of a teaspoon. Municipal water that enters the dental unit is not sterile and does have a low number of waterborne microbes present, so the water that enters the dental unit usually contains just a few microorganisms (e.g., 0 to 500 CFU/mL). However, water exiting the dental handpiece, air/water syringe, and ultrasonic scalers may contain more than 100,000 CFU/mL. Table 13-1 lists the results of some studies conducted in the United States that have measured the concentration of bacteria in dental unit water. Maximum reported recoveries from dental unit water in the United States have been 1.2 million and 10 million CFU/mL. Dental unit water contamination occurs worldwide, with more than 35 articles in the scientific literature describing various levels of bacteria from Austria, Canada, Denmark, England, Germany, New Zealand, and the United States.
TABLE 13-1
Presence of Bacteria in Dental Unit Water
| Location | Source | CFU/mL (Mean) |
| San Francisco∗ | 10 dental units from 3 offices | 180,000 |
| Tap water from same offices | 15 | |
| Washington† | 54 air/water syringe hoses | 165,000 |
| California† | 22 high-speed handpiece hoses | 739,000 |
| Oregon† | 10 faucets | <30 |
| 4 water coolers | <30 | |
| 11 rivers and streams | 28,200 | |
| Indianapolis‡ | 5 dental units | 148,000 |
| New Orleans§ | 6 dental units | 188,333 |
| Baltimore¶ | 8 dental units | 110,000 |
∗Able LC et al: Studies on dental aerobiology. IV. Bacterial contamination of water delivered by dental units, J Dent Res 50:1567-1569, 1971.
†Santiago JI et al: Microbial contamination of dental unit waterlines: short- and long-term effects of flushing, Gen Dent 42:528-544, 1994.
‡Unpublished data from the author, 1996.
§Mayo JA, Oertling KM, Abdrieu SC: Bacterial biofilm: A source of contamination in dental air-water syringes, Clin Prev Dent 12:13-20, 1990.
¶Williams HN, Brockington AM: Quantitation of bacteria in water delivered by dental units as determined by plate-count and direct-count methods, Quintessence Int 26:31-36, 1995.
TYPES AND IMPORTANCE OF MICROORGANISMS IN DENTAL UNIT WATER
Although human oral microorganisms have been found in dental unit water, the vast majority of those present are waterborne microorganisms (Table 13-2). Most of the waterborne microorganisms are of low pathogenicity or are opportunistic pathogens causing harmful infections only under special conditions or in immunocompromised persons. Microorganisms of main concern are species of Pseudomonas, Legionella, and Mycobacterium.
TABLE 13-2
Microbes Isolated from Dental Unit Water
| Microorganism | Probable Source | Pathogenicity |
| Bacteria | ||
| Achromobacter xylosoxidans | Water | Opportunistic |
| Acinetobacter sp. | Mouth | Low |
| Actinomyces sp. | Water | Opportunistic |
| Alcaligenes denitrificans | Water | Low |
| Bacillus sp. | Water | Low |
| Bacillus subtilis | Mouth | Low |
| Bacteroides sp. | Water | Low |
| Flavobacterium sp. | Mouth | Low |
| Fusobacterium sp. | Water | Low |
| Klebsiella pneumoniae | Mouth | Low |
| Lactobacillus sp. | Water | Opportunistic |
| Legionella pneumophila | Water | Opportunistic |
| Legionella sp. | Water | Low |
| Methylobacterium mesophilicum | Water | Low |
| Micrococcus luteus | Water | Low |
| Moraxella sp. | Water | Low |
| Mycobacterium gordonae | Water | Low |
| Norcardia sp. | Water | Low |
| Ochrobactrum sp. | Water | Low |
| Pasteurella haemolytica | Water | Low |
| Pasteurella sp. | Water | Low |
| Peptostreptococcus sp. | Mouth | Low |
| Pseudomonas aeruginosa | Water | Opportunistic |
| Pseudomonas cepacia | Water | Opportunistic |
| Pseudomonas paucimobilis | Water | Opportunistic |
| Pseudomonas sp. | Water | Opportunistic |
| Serratia marcescens | Water | Opportunistic |
| Staphylococcus aureus | Mouth | Intermediate |
| Staphylococcus sp. | Mouth | Low |
| Streptococcus sp. | Mouth | Low |
| Veillonella alcalescens | Mouth | Low |
| Xanthomonas sp. | Water | Low |
| Fungi | ||
| Alternaria sp. | Water | Low |
| Cephalosporium sp. | Water | Low |
| Cladosporium sp. | Water | Low |
| Exophiala mesophila | Water | Low |
| Penicillium sp. | Water | Low |
| Scopulariopsis sp. | Water | Low |
| Protozoa | ||
| Acanthamoeba sp. | Water | Low |
| Naegleria sp. | Water | Low |
Adapted from Miller CH: Dental unit waterline contamination, Operatory Infection Control Updates 2:1-8, 1994.
Pseudomonas
Pseudomonas aeruginosa and P. cepacia are common inhabitants of the environment, existing in soil and natural waters. Many strains can survive and even multiply in water of low nutrient content such as distilled water. Thus to find Pseudomonas species in almost any type of domestic water supply, storage tanks, and drain lines is not unusual. Pseudomonas cepacia is an important respiratory pathogen in patients with cystic fibrosis. Pseudomonas aeruginosa is usually opportunistic in causing urinary tract infections, wound infections, pneumonia, and septicemia in burn patients and, along with P. cepacia, usually has a higher degree of resistance than many bacteria to killing by disinfecting chemicals and by antibiotics. The only scientific report that directly implicates any microorganism from dental unit water as a health risk has involved Pseudomonas. The report from England implicated P. aeruginosa from dental unit water as the cause of oral infections in two medically compromised dental patients.
Legionella
Legionella pneumophila and other species are gram-negative bacteria that naturally occur in water and may gain some protection against the chlorine present in domestic water because they can exist inside certain free-living amebae also present in the water. Legionella pneumophila is the causative agent of a type of pneumonia called legionnaires’ disease, which was first recognized in 1976 when 182 attendees at an American Legion convention in Philadelphia became infected with this bacterium, which was present in water in the convention hotel. The bacterium usually is transmitted by inhalation of aerosolized contaminated water or by aspiration of organisms that have colonized the oropharynx. Specific examples of how L. pneumophila may have been transmitted to human beings from various sources of water involve cooling towers, heat exchange apparatuses, a mist machine spraying produce in a grocery store, humidifiers, shower heads, hot-water faucets, tap water used to clean medical equipment, and whirlpools in hospitals. However, the route of spread has not been identified for all cases detected. Legionella pneumophila also may cause a nonpulmonary infection called Pontiac fever, and rarely, wound infections follow irrigation with Legionella-contaminated water. Although L. pneumophila is the principal pathogen in this genus, 30 other species of Legionella exist and may cause up to 20% to 30% of all Legionella infections.
Legionella pneumophila or other Legionella species have been detected in dental unit water. Legionella pneumophila was found in the water from about 10% of 42 units in 35 practices in Austria, from 3 of 5 units in a hospital dental clinic in London, from 4% of 194 dental units at levels greater than 100 CFU/mL in a London teaching hospital, and from several dental units at the University of Dresden in Germany. In the United States, L. pneumophila has been detected in dental unit water in an Ohio dental school clinic and in 8% of the water samples taken from 28 dental facilities in California, Massachusetts, Michigan, Minnesota, Oregon, and Washington. In the latter study, L. pneumophila was never detected at concentrations greater than 1000 CFU/mL, but other species of Legionella were found in 68% of the water samples tested and at levels of at least 10,000 CFU/mL in 19% of the samples.
Although L. pneumophila and other Legionella species may be present in some dental unit water in the United States, no documentation indicates that dental unit water has ever caused legionnaires’ disease in patients or in dental team members. However, a comment about unpublished data in a report about Legionella in dental unit water infers that a dentist in California who died of legionellosis may have contacted the causative agent from his dental unit water. Indirect evidence that dental team members may have occupational exposure to legionellae comes from two studies that showed higher rates of seroconversion with antibodies to legionellae in dental personnel than in nondental personnel. One of the studies also showed that seroconversion rates increased as the years of experience in dentistry increased. This information suggests that dental workers at least are exposed to Legionella.
Mycobacterium
Nontuberculous mycobacteria (e.g., Mycobacterium chelonae) have been detected in some domestic water supplies. These bacteria are somewhat resistant to chemical killing, have caused infections in dialysis patients, and have been detected in the water used to process dialyzers. A case report describes an intraoral infection with M. chelonae, but the source of this bacterium was not known.
Other Bacteria
Acinetobacter, Alcaligenes, Klebsiella, and Serratia (see Table 13-2) are gram-negative opportunistic pathogens that may cause harmful infections in compromised hosts. No specific documentation exists that these bacteria from dental unit water have caused any infections in patients or in dental team members. The oral bacteria of Bacteroides, Fusobacterium, Lactobacillus, Peptostreptococcus, and Streptococcus are involved in causing dental caries or periodontal diseases and have opportunistic pathogenicity if allowed to accumulate on tooth surfaces in plaque. The majority of microbes present in dental unit water are bacteria, but fungi such as Cladosporium and Exophiala mesophila and protozoa such as Acanthamoeba and Naegleria also have been detected.
Endotoxins
One study has shown that the dental unit water tested contained 1000 units of endotoxin per milliliter. Endotoxin is a component of the cell walls of gram-negative bacteria and also is known as lipopolysaccharide (see Chapter 2). Endotoxin can cause inflammation and shock and has been implicated in a variety of harmful infections involving gram-negative bacteria, including periodontal diseases. Although no endotoxin standard for drinking water exists, United States Pharmacopeia sterile water cannot have more than 0.25 units of endotoxin per milliliter.
BIOFILM IN DENTAL UNIT WATER LINES
Water entering dental units usually has a low number of microorganisms present, but the water that passes out of the dental unit through handpieces, scalers, and air/water syringes is highly contaminated. Thus the incoming water becomes highly contaminated when inside the dental unit. This contamination comes from biofilm that forms on the inside of the dental unit water lines.
Microorganisms exist in dental unit water lines in two types of communities. One bacterial community exists in the water itself and is referred to as the planktonic (free-floating) microorganisms. The other exists in a sessile form attached to the inside walls of the water lines called biofilm.
Biofilm is defined as a mass of microorganisms attached to a surface exposed to moisture. Biofilms are common; they form just about anywhere one finds a moist, nonsterile environment, including the surfaces of rocks, plants, and fish associated with natural water environments in streams, lakes, and oceans and those associated with “domestic/industrial” water environments such as water lines, sewer systems, drain lines, wells, septic tanks, sewage treatment facilities, water storage containers, humidifiers, and spray heads. Biofilms also form on biomedical materials implanted in or associated with the human body, including many types of catheters, sutures, wound drainage tubes, endotracheal tubes, mechanical heart valves, and intrauterine contraceptive devices.
The best example of biofilm in dentistry is dental plaque, also referred to as oral biofilm. Thus a type of plaque develops inside of dental unit water lines that causes a permanent infection of the water delivery system.
Mechanisms of Biofilm Formation
Biofilm forms when bacterial cells adhere to a surface using cell surface polymers. Many of these polymers are highly hydrated exopolysaccharides, referred to as glycocalyx polymers, that give the biofilm a “slimy” nature. As the attached cells multiply within the glycocalyx, the new cells remain embedded and form microcolonies on the surface. Continued multiplication results in the joining of microcolonies, and this with the continual recruitment of additional bacteria from the planktonic phase can result in a covering of the surface.
Biofilm forms on the inside of the dental unit water lines as the water is flowing through the unit. Several factors allow this to occur (Box 13-1). The water in the dental unit water lines moves at normal line pressures, which is more slowly than one might imagine. The water is not pressurized into the form of the handpiece spray until the water and air mix inside the handpiece. Intermittent stagnation of the water inside the units commonly occurs between patients, overnight, and over the weekends. This facilitates attachment of bacteria from the planktonic community. The dynamics of fluid flowing through a line are such that the maximum flow rate occurs in the center of the stream of fluid and the minimum flow rate occurs near the surfaces of the wall of the tubing. Thus the water is moving more slowly near the surface of the walls, facilitating attachment of bacteria. Another key factor in water line biofilm formation is that most waterborne bacteria have developed the ability to attach to surfaces more efficiently than most nonwaterborne bacteria. This feature allows these bacteria to become stabilized on a surface and let the nutrients in the water come to them. The small diameter of dental unit water lines causes a large amount of biofilm to form. As the diameter of pipes or tubings decreases, the surface-to-volume ratio increases. So the smaller the diameter, the more surface relative to volume there is for biofilm to form. In smaller lines, more bacteria have a chance to contact and attach to a surface than they have to remain in the fluid. Thus water coming out of a water line in a home that has a ½- to ¾-inch diameter will have less biofilm than the 1⁄16-inch diameter line in a dental unit.
Rate of Biofilm formation
The rate at which biofilm forms depends on the aforementioned factors. As we all know, the biofilm on our teeth (dental plaque) begins to reform immediately after we brush them. By the end of the day most persons can even see this plaque. Dental unit water line biofilm forms more slowly but begins in a new dental unit within hours. One study has shown that microbial levels in dental unit water reached 200,000 CFU/mL within 5 days after installation of new dental unit water lines.
Figure 13-2 shows what mature biofilm in dental handpiece water lines looks like under the scanning electron microscope. This particular water line was used in a dental unit for at least 5 years. Figure 13-3 shows scanning electron microscope photomicrographs of biofilm that formed in an air/water syringe water line of a new dental unit that was in operation for just 5.3 months.
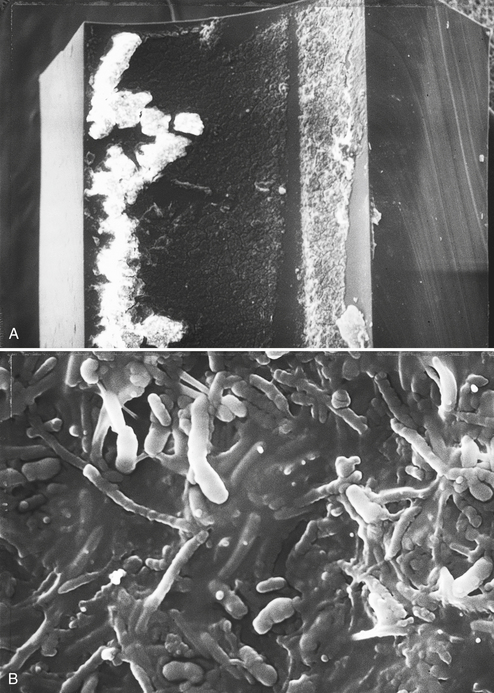
FIGURE 13-2 Scanning electron micrographs of the inside of a small section of high-speed dental handpiece water line. A ½-inch long section of handpiece hose attached to a dental unit was removed, and the water line was separated from the other three lines. The water line section was cut longitudinally with a clean sterile scalpel to expose the inner lumen. The water line section then was fixed in 5% glutaraldehyde in cacodylate buffer containing 0.15% ruthenium red. After rinsing with buffer, the section was treated with 4% osmium tetraoxide for 2 hours at room temperature, rinsed again, dehydrated in increasing concentrations of ethanol, incubated for 2 hours in hemamethyl-disilizane, dried for 3 days, exposed to colloidal graphite, and sputter-coated with gold-palladium. A, Low-power magnification (80×) with cut edges of the water line on the right and left sides and the lumen with biofilm in the middle. B, High-power magnification (6000×) of the same sample showing biofilm in the lumen. (From Miller CH: Infection control, Dent Clin North Am 40:437-456, 1996. With permission).
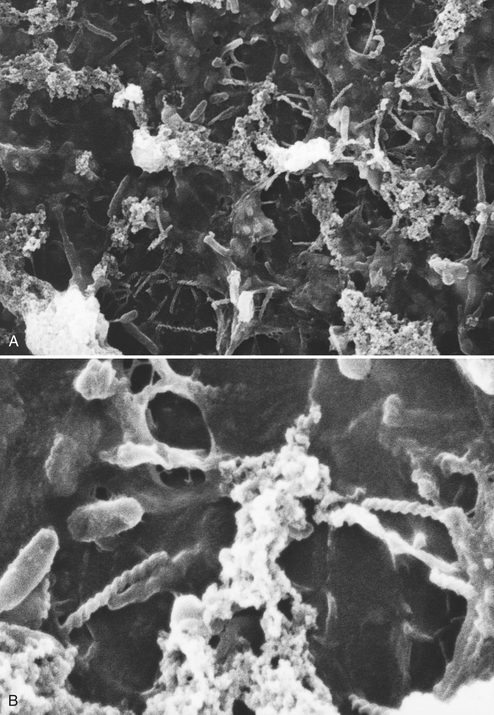
FIGURE 13-3 Scanning electron micrograph of biofilm on the inside of an air/water syringe water line. The samples were prepared as described in the legend of Figure 13-2. A, Original magnification, 1500×. B, Original magnification, 6000×.
Biofilm can serve as a continuous source of contamination of the flowing water as cells or chunks dislodge naturally or from physical stress placed on the line. The fact that biofilm serves as a source of microorganisms in the exiting water has been demonstrated. When water lines containing biofilm were flushed to remove planktonic bacteria and the lines were filled with sterile water, the sterile water became heavily contaminated after a few hours.
Although human beings cannot live without water, we commonly think of water as having little nutritional value. However, tap water contains low concentrations of inorganic and organic material that can serve as a source of nutrients for microorganisms. In fact biofilm in water lines serves as a great mechanism by which bacteria can gain continuous access to the low levels of nutrients in a never-ending flow of water. The waterborne bacteria also are conditioned to an existence in a low-nutrient environment. For example, strains of Pseudomonas aeruginosa and P. cepacia have been shown to multiply to high levels in water taken from distilled water reservoirs and commercially prepared distilled water.
NEED TO IMPROVE DENTAL UNIT WATER QUALITY
No evidence indicates the occurrence of any widespread public health problem from exposure to dental unit water. However, the sources of the microorganisms causing low levels of infectious diseases in the community are not always identified, and the presence of potential pathogens in dental unit water is of concern. Also, the goal of infection control is to eliminate or reduce exposure to microorganisms. Because infectious diseases may occur when human beings and microorganisms come into contact with each other, all health care providers have a responsibility to reduce this possible contact, particularly when it may occur between patients and microorganisms in a health care facility. Using dental unit water that is contaminated heavily with microorganisms of any kind for dental treatment is contrary to the goals of infection control.
Thus improving the quality of dental unit water as means become available is a natural part of maintaining the high quality of patient care and staff protection for which dentistry is well noted.
CURRENT INFECTION CONTROL RECOMMENDATIONS
Centers for Disease Control and Prevention
Current (2003) recommendations from the Centers for Disease Control and Prevention (CDC) related to microorganisms in dental unit water are as follows:
• Dental offices use water that meets regulatory standards set by the Environmental Protection Agency for drinking water (fewer than 500 CFU/mL of heterotrophic water bacteria) for routine dental treatment output water.
• Consult with the dental unit manufacturer for appropriate methods and equipment to maintain the recommended quality of dental water.
• Follow recommendations for monitoring water quality provided by the manufacturer of the unit or water line treatment product.
• Discharge water and air for a minimum of 20 to 30 seconds after each patient from any dental device connected to the dental water system that enters the patient’s mouth (e.g., handpieces, ultrasonic scalers, and air/water syringe).
• Consult with the dental unit manufacturer on the need for periodic maintenance of antiretraction mechanisms.
One should flush high-speed handpieces to discharge water and air for a minimum of 20 to 30 seconds after use on each patient. This procedure is intended to aid in physically flushing out patient material that may have entered the turbine and air or water lines. One should consider use of an enclosed container or high-velocity evacuation to minimize the spread of spray, spatter, and aerosols generated during discharge procedures. Additionally, evidence indicates that overnight or weekend microbial accumulation in water lines can be reduced substantially by removing the handpiece and allowing water lines to run and discharge water for several minutes at the beginning of each clinic day. However, flushing of the water lines will not remove the biofilm in the lines. One should use sterile saline or sterile water as a coolant/irrigator when performing surgical procedures involving the cutting of bone.
American Dental Association
In 1995 the American Dental Association (ADA) board of trustees approved the following statement prepared by the ADA Council on Scientific Affairs:
The Council recommends an ambitious and aggressive course to encourage industry and the research community to improve the design of dental equipment so that by the year 2000, water delivered to patients during nonsurgical dental procedures consistently contains no more than 200 colony forming units per milliliter (CFU/mL) of aerobic mesophilic heterotrophic bacteria at any point in time in the unfiltered output of the dental unit; this is equivalent to an existing quality assurance standard for dialysate fluid that ensures that the fluid delivery systems in hemodialysis units have not been colonized by indigenous waterborne organisms. Manufacturers of dental equipment are encouraged to develop accessory components that can be retrofitted to dental units currently in use, whatever the water source (public or independent), to aid in achieving this goal. Further, the ADA should urge industry to ensure that all dental units manufactured and marketed in the USA in the future have the capability of being equipped with a separate water reservoir independent of the public water supply. In this way, dentists will not only have better control over the quality of the source water used in patient care, but also will be able to avoid interruptions in dental care when “boil water” notices are issued by local health authorities. At the present time commercially available options for improving dental unit water quality are limited and will involve some additional expense. They include the use of independent water reservoirs, chemical treatment regimens, daily draining and air purging regimens, and point of use filters.
The ADA reaffirmed this statement in 1999, and later indicated support of the 2003 CDC recommendations as described in Appendix G.
DENTAL UNIT WATER AND INFECTION CONTROL
The dental professional should follow CDC guidelines by not using dental unit water as an irrigant for oral surgery. The CDC defines oral surgical procedures as involving the “incision, excision, or reflection of tissue that exposes the normally sterile areas of the oral cavity. Examples include biopsy, periodontal surgery, apical surgery, implant surgery, and surgical extractions of teeth (e.g., removal of erupted or non-erupted tooth, requiring elevation of the mucoperiosteal flap, removal of bone and/or section of tooth, and suturing if needed.” Such surgeries may involve the use of sterile water delivery systems as mentioned in the next section or hand irrigation using sterile water in a sterile disposable syringe. Specialties, including oral surgery, endodontics, and periodontics, may have other recommendations concerning the use of irrigants.
Flushing of the Water Lines
One should flush water lines and handpieces between patients as recommended by the CDC (see the foregoing discussion). Although flushing will not remove biofilms from the lines (biofilm forms while water is moving through the lines), it may reduce the planktonic microbial count in the water temporarily and help clean the handpiece water lines of materials that may have entered from the patient’s mouth. Flushing also brings into the dental unit a fresh supply of chlorinated water from the main water lines.
Minimization of Sprays and Spatter
The routine use of high-volume evacuation with the high-speed handpiece, ultrasonic scaler, and air/water syringe reduces exposure of the dental team to aerosol and spatter from the patient’s oral fluids and from contamination with the water spray from handpiece, scaler, and syringe. This evacuation also may reduce exposure of the patient to these waterborne microorganisms.
Barriers for the Patient and Dental Team
The rubber dam serves as a protective barrier for the patient from dental unit water. The dam does not eliminate exposure totally but greatly reduces direct contact. The dam also greatly reduces the aerosolizing and spattering of patient microorganisms onto the dental team but does not reduce exposure of the dental team to dental unit water. However, protective barriers of eyewear, masks, and face shields do serve as barriers for the dental team against microorganisms coming from the patients’ mouths and from the aerosols and sprays of dental unit water.
APPROACHES TO IMPROVE DENTAL UNIT WATER QUALITY
Developing approaches to improve the quality of water used for patient treatment is a rapidly advancing field, and additional breakthroughs could occur at any time. Box 13-2 gives basic considerations for designing approaches to improve dental water quality. Current approaches include independent water reservoirs, antimicrobial agents, filters, and sterile water delivery systems. In some instances, more than one of these approaches are combined. A listing of the specific products representing these approaches with links to the manufacturers can be found at the Organization for Safety and Asepsis Procedures Web site (http://www.osap.org). One must remember the importance of checking with dental unit manufacturers for their recommendations to maintain the water quality in their units.
Independent water reservoirs
Although drinking water is not supposed to contain any more than 500 CFU/mL, contamination may be greater by the time the water passes though all of the distribution lines leading from the city water treatment facility to the dental office. Because accurately predicting the quality of municipal water when it enters dental units is not possible, one approach has been to disconnect from city water and supply another source of water. This process can involve installation of a water reservoir (e.g., a bottle) filled with good-quality water (e.g., distilled water) for patient treatment. This system also allows for the use of decontaminating or cleared antimicrobial agents (see below) for cleaning of the water lines. Some independent reservoir systems have dual bottles, one for treatment water and one for a cleaner. These independent water reservoirs (also known as self-contained water systems or clean water systems) provide a means for delivering treatment water and water line cleaning agents.
Attacking the biofilm is important just before one starts using an independent reservoir system or any system designed to improve the quality of the incoming water. One also must periodically decontaminate the lines after installing the new water delivery systems, depending on the product/system used. One must remember that it is not enough to use good-quality incoming water. If one does not treat the biofilm in the water lines, even sterile water placed in the bottles will come out highly contaminated. Thus the water lines need to be treated with an agent that will control the biofilm so as to maintain the quality of the water placed into the bottles as it passes through the lines. One also should not touch the pickup tube in the bottle (Figure 13-4) with contaminated fingers when changing the bottle, and one needs to clean the bottles with soap and water every day.
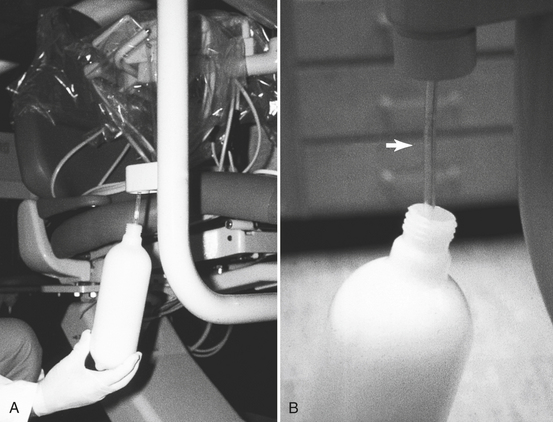
FIGURE 13-4 A, An independent water reservoir (bottle) being attached to the dental unit. B, Arrow shows the pickup tube that guides the water through the dental unit when the bottle is pressurized.
Procedure 13-1 gives an example of how one can use an independent water reservoir. Although this approach is labor intensive, it can yield high-quality water if one follows the steps faithfully.
Decontaminating and Antimicrobial Agents
Decontaminating and antimicrobial agents are available to use in independent water reservoirs to attack the biofilm. Some agents are placed in the bottles periodically (e.g., once a week) and flushed into the water lines, held there for various periods of time, and flushed out. Other chemical agents are added directly to the treatment water to provide continuous antimicrobial activity in the lines. Other antimicrobial systems such as ultraviolet light, high heat, or antimicrobial chemicals (e.g., iodine, silver ions, and ozone) are used to treat municipal water before it enters the dental units. Chemical agents with antimicrobial claims are to be registered by the Environmental Protection Agency. Devices attached to the dental unit to deliver decontaminating agents should be cleared by the FDA as an accessory to a medical device.
Filters
Microbial filters are designed to remove free-floating microbes, and in one instance endotoxin, from the water. Placing a microbial filter in the water line just before the water enters the handpiece or air/water syringe can improve the quality of the treatment water greatly. Some of the concerns for this approach include knowing when to change the filter, positioning the filter in the line to avoid cross-contamination from any retraction of patient materials through the handpiece or air/water syringe, and the need to disinfect any part of the water line downstream from the filter. Even though filtering the output water does not address the biofilm problem, a filter at the end of the line may be an important safety measure to use along with other water quality improvement approaches. Some filters are used in combination with antimicrobial agents.
Sterile Water Delivery Systems
Oral surgical procedures (see the foregoing discussion) present an opportunity for microorganisms to enter the vascular systems and other normally sterile areas of the mouth, increasing the potential for a localized or systemic infection. Because dental units cannot reliably deliver sterile water, systems have been developed and cleared by the FDA that completely bypass the dental unit and deliver sterile solutions (e.g., water or saline) through sterile disposable or autoclavable lines to the patient. A sterile solution for irrigation also can be delivered through a hand syringe.
WATER QUALITY MONITORING
Because numerous studies have demonstrated that most, if not all, untreated dental units produce water that is below the standard for drinking water, routinely testing the water from unmodified dental units is not necessary. However, if equipment is being installed or products are being used to improve the microbial quality of water, baseline and periodic testing of the microbial levels in the exiting water may provide useful information as to whether the changes are working. Monitoring can detect not only how the system is functioning but also how the products/equipment are being used. The tests should determine total bacterial counts in the water after inoculation of diluted chlorine-neutralized water samples onto R2A agar plates and incubation of the plates at room temperature for at least 1 week. One should keep water samples cold during transport if they are to be shipped to a laboratory for analysis. One should recognize that these counts may not always specifically reflect the degree of biofilm present in the lines, for this relationship has not been determined scientifically. Nevertheless, if one obtains high counts (above 500 CFU/mL), then the intended improvements are not working or are not being performed properly. If one obtains low counts, the improvements are working or the water analyzed did not reflect the true state of the system. Thus one may need multiple samples to confirm low counts. Commercial microbiology laboratories, hospital laboratories, some dental companies, and some dental schools can perform these water quality tests.
BOIL WATER NOTICES
Water treatment facilities in our cities and towns are charged with providing safe drinking water to the public. Occasionally problems occur in the water treatment plant or with the water distribution system that brings the treated water to our homes and offices. One of the most common problems is a break in a water main that allows groundwater (contaminated with various microorganisms) to leak into the water distribution system and expose all sites downstream of the leak. Other problems are power failures or mechanical failures at the treatment plant that interrupt purification systems. When this occurs, the water company or health authorities issue a boil water notice indicating that the water should not be consumed or should be boiled before use. Such water also should not be used for patient treatment of any type or for handwashing. This means that one must use syringe irrigation with nonmunicipal water when water is needed for treatment, or, if a dental unit has a self-contained water system and does not use municipal water, then no problem exists except with the office tap water. Otherwise, the dentist should see no patients until the water problem is solved. One can use alcohol hand rubs during a boil water notice if the hands have no visible soil, or one can use an antimicrobial handwashing agent and bottled water if visible soil is present.
A dental office involved in a boil water notice needs to contact the manufacturer of their dental units and determine exactly how to disinfect and flush the inside of the unit water lines after the “all clear” notice is given. If such directions are not available, a guideline is to flush the lines and faucets for 5 minutes.
Abel, L.C., et al. Studies on dental aerobiology. IV. Bacterial contamination of water delivered by dental units. J Dent Res. 1971;50:1567–1569.
American Dental Association. Council on Scientific Affairs: Dental unit waterlines: approaching the year 2000. J Am Dent Assoc. 1999;130:1653–1664.
Atlas, R.M., et al. Legionella contamination of dental-unit waters. Appl Environ Microbiol. 1995;61:1208–1213.
Centers for Disease Control and Prevention. Guidelines for infection control in dental health care settings, 2003. MMWR. 2003;52(No. RR-17):1–.68.
Challacombe, S.J., Fernandes, L.L. Detecting Legionella pneumophila in water systems: a comparison of various dental units. J Am Dent Assoc. 1995;126:603–608.
Coan, L., Hughes, E.A., Hudson, J.C., Palenik, C.J. Sampling water from chemically treated dental units with detachable power scalers. J Dent Hyg. 2007;80:80.
Cochran, M.A., Miller, C.H., Sheldrake, M.A. The efficacy of the dental dam as a barrier to the spread of microorganisms during dental treatment. J Am Dent Assoc. 1989;119:141–144.
Costerton, W.J., et al. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41:435–464.
Fotos, P.G., et al. Prevalence of Legionella-specific IgG and IgM antibody in a dental clinic population. J Dent Res. 1985;64:1382–1385.
Martin, M.V. The significance of the bacterial contamination of the dental unit water systems. Br Dent J. 1987;163:152–154.
Miller, C.H. Microorganisms in dental unit water. Calif Dent Assoc J. 1996;24:47–52.
Mills, S.E. The dental unit waterline controversy: defusing the myths, defining the solutions. J Am Dent Assoc. 2000;131:1427–1441.
O’Donnell, M.J., Shore, A.C., Russell, R.J., Coleman, D.C. Optimization of the long-term efficacy of dental chair waterline disinfection by the identification and rectification of factors associated with waterline disinfection failure. J Dent. 2007;35:438–451.
Palenik CJ, Burgess K, Miller CH: Methods for microbial analysis of dental unit water. Annual conference proceedings: Organization for Safety and Asepsis Procedures, Tucson, AZ, June 19–22, (abstract 0308), 2003.
Palenik, C.J., Burgess, K.H., Miller, C.H. Effects of delayed microbial analysis of dental unit water line specimens. Amer J Dent. 2005;18:87–90.
Pankhurst, C.L., Coulter, W.A. Do contaminated dental unit waterlines pose a risk of infection? J Dent. 2007;35:712–720.
Porteous, N.B., et al. Isolation of an unusual fungus in treated dental unit waterlines. J Am Dent Assoc. 2003;134:853–858.
Reinthaler, F.F., Mascher, F., Stunzer, D. Serological examination for antibodies against Legionella species in dental personnel. J Dent Res. 1988;67:942–943.
Tullner JB, Miller CH, Sheldrake MA, Gonzalez-Cabezas C: Accumulation of biofilm inside of dental unit waterlines. Annual conference proceedings: Organization for Safety and Asepsis Procedures, Dallas, June 13–16, (abstract 9602), 1996.
Walker, J.T., Marsh, P.D. Microbial biofilm formation in DUWS and their control using Disinfectants. J Dent. 2007;35:721–730.
Review Questions
______1. Legionella bacteria can cause what type of disease in susceptible persons?
______2. Besides Legionella, what other two bacteria that may be present in dental unit water are of the most concern in causing infections in compromised persons?
______3. What is the maximum acceptable level of bacteria in dental unit water as recommended by the Centers for Disease Control and Prevention?
______4. What role does dental unit water line biofilm play in the microbial contamination of dental unit water?
b. Biofilm traps all the bacteria in the incoming water and keeps the levels of bacteria in the outgoing water low.
c. Biofilm sheds bacteria into the water, causing increased levels of bacteria in the outgoing water.
______5. The level of microbes in water coming out of an unmodified dental unit is almost always:
______6. When does the Centers for Disease Control and Prevention recommend that dental unit water lines with the attached handpiece should be flushed?
______7. What type of water should be used to irrigate during oral surgical procedures?
______8. All of the following are reasonable approaches to improve dental unit water quality except one. The exception is:
a. replacing the water lines once a week
b. using an independent water reservoir
______9. Potable water is the same as:
______10. Which of the following attacks dental unit water line biofilm?