Chapter 43 Infant feeding
At the end of this chapter, you will:
Introduction
This chapter will present the anatomy and physiology of infant feeding, including the maternal breast and hormonal influences, neonatal nutritional needs, and identification of public health policies for breastfeeding. Whilst human milk is the superior food for the neonate and breastfeeding is recommended by the midwife, there are some women who will choose, for a variety of reasons, not to breastfeed. It is the midwife’s role to facilitate a non-judgemental environment for discussing the woman’s views and expectations around infant feeding, providing factual, unbiased, evidence-based information in order that an informed decision about infant feeding can be made. It is vital that women are educated in the principles of safe preparation and administration of artificial feeds should they be unable or choose not to breastfeed.
Gold standard
Human milk is the gold standard for nutrition of the human infant (Lawrence & Lawrence 2005, NICE 2006). It contains unique constituents valuable for brain growth, such as cholesterol, omega-3 fatty acids and the amino acid taurine, together with immune properties that cannot be matched with any substitutes. It is the standard all health professionals should endeavour to achieve for the neonate, through the information given to women and their families.
In 1989, Protecting, promoting and supporting breastfeeding: the special role of maternity services (WHO/UNICEF 1989) was published. This was adopted as a global initiative by policy makers at a meeting in Florence, now referred to as the Innocenti Declaration (Henschel & Inch 1996). In June 1991, the Baby-Friendly Hospital Initiative (BFHI) was launched at the International Paediatric Association Conference, Ankara, providing a global focus for the intent of the Innocenti Declaration. The principles of the declaration were embodied in the ‘Ten steps to successful breastfeeding’ (Box 43.1), designed as a set of standards that can be followed by maternity units all over the world and audited to demonstrate measurable improvements.
Box 43.1
The ten steps to successful breastfeeding (WHO/UNICEF 1989)
All providers of maternity services should:
Institutions which fully implement all ten steps can apply to be assessed and accredited as ‘baby friendly’. A first step towards accreditation is the Certificate of Commitment, which is awarded to units which implement steps 1, 7 and 10 and adopt an action plan to meet the requirements of the remaining steps.
The UK Baby Friendly Initiative (UK BFI), launched in 1994, is a programme of the UK Committee for UNICEF and aims to ensure that all mothers and babies receive the health and social benefits provided by breastfeeding. The UK BFI encourages and assesses hospitals to become ‘baby friendly’ by implementing the ‘Ten steps’.
The WHO Global Strategy for Infant and Young Child Feeding, incorporating the UNICEF recommendations, was published in 2002. This document represented 2 years of evidence and all previous initiatives and statements calling on governments to fully support breastfeeding (WHO 2002).
Public health issues
The National Service Framework for Children, Young People and Maternity Services recommends all Trusts (hospitals) have minimum standards for breastfeeding, early access to support services and specialist advice (DH 2004a).
NICE postnatal guidelines specify that a written policy for breastfeeding must be available in all healthcare settings and communicated to all staff. A lead healthcare professional must be identified to ensure this is implemented, using the Baby Friendly Initiative as a minimum standard in an environment conducive to breastfeeding (NICE 2006).
Maternity matters (DH 2007a) sets out the DH Policy commitment to maternity services and includes the achievement of the Public Service Agreement (PSA) targets (DH 2007b), namely ‘deliver an increase of 2% points per year in breastfeeding initiation rate, focusing especially on women from disadvantaged groups’. Breastfeeding is a key factor in reducing childhood obesity (Cross Government Obesity Unit 2008, Von Kries et al 1999) and diabetes (CEMACE 2007).
A breastfeeding manifesto was launched in May 2007 by a coalition of 33 UK membership organizations, including all the main Royal Colleges, to improve awareness of the health benefits of breastfeeding and its role in reducing health inequalities across the UK (Breastfeeding Manifesto Coalition 2007). Its guiding principles were for women to feel enabled to initiate and continue breastfeeding for as long as they wish, supported to make informed choices about feeding and ensuring awareness of the significant benefits associated with breastfeeding. See Box 43.2.
Box 43.2
Breastfeeding Manifesto Coalition objectives 2007
As part of the drive to promote breastfeeding, a DVD was developed by Best Beginnings, supported by the DH. This illustrates women discussing the practicalities and benefits of breastfeeding, and is distributed to all pregnant women in the UK (Best Beginnings 2008).
The Healthcare Commission’s review of the maternity services in England recommended Trusts pay particular attention to helping women from minority ethnic groups to maintain breastfeeding (Healthcare Commission 2008).
NICE guidelines on maternal and child nutrition recommend encouraging breastfeeding during the antenatal period and ensuring that women are taught positioning and attachment and to continue breastfeeding for at least 6 months (NICE 2008a).
The strategy for children and young people’s health sets out how the DH will minimize health inequalities and cement the standards set through the National Service Framework and Every child matters (DH 2009a). It focuses on expanding support for women antenatally and in the immediate postnatal period within Sure Start Children’s Centres and on the reduction of obesity.
Midwives should expand their public health role in educating women and their families regarding the value of breastfeeding, and in encouraging women to breastfeed. They can reduce inequalities and social deprivation by working closely with health visitors (HVs) and those specialist nurses providing the Family Nurse Partnership Programme to support women who choose to breastfeed to continue to do so (DH 1999). A multidisciplinary and longsighted approach is required, in line with governments’ public health strategies, that commences preconceptually, and develops and supports women during pregnancy and through into the first few months of the baby’s life.
The full-term neonate – nutritional requirements
Calorific requirements were based traditionally on volumes of formula required by artificially fed babies (Riordan 2008). The calorific requirement for term infants is thought to average 440 kJ per kilogram of body weight per day (110–120 kcal/kg/day) depending on gestational age (Blackburn 2007).
Breast milk (or infant formulae) contains approximately 275 kJ (65 kcal) per 100 mL (Blackburn 2007, Riordan 2008). A baby weighing 3.5 kg requires approximately 1540 kJ in 24 hours – about 525 mL of milk per day. This amount varies depending on the gestation and age of the baby, and volume and content will vary from feed to feed. A meta-analysis of the volume of milk secreted by exclusively breastfeeding women around the world found this to be constant at around 800 mL per day. The volume of milk transferred from the breast to the baby is less than 100 mL per day for the first 24–36 hours, gradually increasing to 500 mL from 36 hours (Neville 1999).
There is no evidence to suggest that healthy term infants require larger volumes of fluid any earlier than they are made available (RCM 2009). The low volume of colostrum is important for optimal physiological adaptation of the neonate, and health professionals need to appreciate that bioavailability of breast milk’s 200 known constituents identifies its superiority over formula milk.
In human milk the calorific value is derived from the carbohydrate and fat content which is absorbed easily through the gut, while cows’ milk has a higher proportion of protein which is less easily digestible. The content of breast milk changes throughout a feed and during the day and night, so can never be directly compared with cows’ milk or formula milk (RCM 2009).
Midwives should be conversant with the relevant DH guidelines (Statutory Instrument 77 1995), information and position statements from the Scientific Advisory Committee on Nutrition (SACN) Subgroup on Maternal and Child Nutrition, its parent body, the Food Standards Agency (FSA), and the WHO Global Strategy on Infant and Young Child Nutrition (WHO 2002).
Physiology of the gastrointestinal tract
The maturation of the neonatal gut is stimulated by initiation of feeding, milk composition, hormonal regulation and genetic encoding (Blackburn 2007). Initiation of early feeding is a major stimulus for the increase of plasma concentrations of peptide hormones, for example, enteroglucagon, which stimulates growth of the intestinal mucosa; gastrin, which stimulates growth of the gastric mucosa and exocrine pancreas; and motilin and neurotensin, which stimulate gut activity. Colostrum stimulates epithelial cell turnover and maturation. Epidermal growth factor and cortisol also assist in the growth and development of the neonatal gastrointestinal system. None of these are available in formula milk.
Breast milk aids the passage of meconium through the gut, whereas formula milk does not. Delayed passage of meconium is associated with elevated bilirubin levels owing to reabsorption of unconjugated bilirubin and recirculation to the liver; therefore, physiological jaundice may be problematic in formula-fed infants.
Until the baby is 9 months old, intake of formula milk stimulates a greater insulin response than intake of breast milk (Blackburn 2007), thus initiating an unnecessary increase in the metabolism of glucose stores.
One of the most notable actions is that of secretory IgA, which has important anti-toxic and anti-allergic properties, protecting the neonatal gut from bacteria, viruses and other harmful organisms, which cannot be replicated in artificial formulae.
Normal neonatal metabolism
The immature neonatal exocrine pancreatic function is a major factor in the digestion of foods in the first few weeks of life. The neonate relies on alternative/additional means for digestion of proteins, carbohydrates and fats, and compensation occurs by use of enzymes in the saliva, intestine and breast milk.
Protein digestion in the neonate is disadvantaged owing to the limited production of gastric pepsin (a mere trace in some) with pancreatic enterokinase output less than 10% of adults. The ratio of whey to casein proteins in breast milk is more easily digested (Hamosh 1998, Xiao-Ming 2008).
Neonatal carbohydrate digestion relies on amylase in breast milk, which remains high during the first 6 weeks of lactation. Neonatal salivary amylase is only one-third of adult levels, while pancreatic amylase represents only 2.5–5% of adult levels.
Fat digestion has been shown to be greater in breastfed versus formula-fed preterm neonates (Hamosh 1998). Though the neonate has raised gastric lipase, there is reduced pancreatic lipase for fat digestion. This is compensated by the stimulus of suckling at the breast, stimulating secretion of lingual lipase in the neonate (Blackburn 2007).
Colostrum and breast milk are uniquely tailored to assist the neonate in independent metabolism and this should be explained to the woman.
Constituents of colostrum and breast milk
Colostrum is produced from 16 weeks’ gestation and continues for the first 3–4 days postpartum, until replaced by milk. It is a yellow-orange, thick sticky fluid that assumes its colour from beta-carotene (Lawrence & Lawrence 2005), with a lower calorific value than breast milk (approx 67 kcal/100 mL versus 75 kcal/100 mL for breast milk).
The daily volume ranges from 2 to 29 mL per feed, and protein, fat-soluble vitamins and mineral percentages are higher than in breast milk, with lower levels of carbohydrate and fat. It is unique in its high concentration of protective constituents – immunoglobulins, macrophages, lymphocytes, neutrophils, and mononuclear cells – giving it a higher protein content. The concentration of growth factors is up to five times higher in colostrum than in mature milk.
Transitional breast milk is produced between colostrum (from 3–4 days) and mature milk and lasts for approximately 10 days to 2 weeks postpartum (Lawrence & Lawrence 2005). During this time, protein and immunoglobulin levels decrease while carbohydrate and fat levels increase. Water-soluble vitamins increase and fat-soluble vitamins decrease.
Mature breast milk contains approximately 90% water with 10% proteins, carbohydrate and fats with vitamins and minerals. The main solid constituent is the fatty acid component that provides 50% of the calorific requirements. Fat content varies at and during each feed according to the neonate’s requirement.
Protein
Approximately 0.9% of breast milk is protein – the more easily digested whey and casein. The ratio is reported to be 9/1 to 6/4 whey/caseins at different lactating periods (Xiao-Ming 2008). Whey is an easily digested antioxidant and can act as an antihypertensive, anticancerous, antiviral, antibacterial and chelating agent (Xiao-Ming 2008). The main components are alpha-lactalbumin, beta-lactoglobulin, serum albumin, immunoglobulins, lactoferrin and lysozyme. Casein constitutes the smaller portion of the protein. In cows’ milk, the protein content is reversed, with an approximately 80% casein to 20% whey ratio (Lawrence & Lawrence 2005). Out of the 20 amino acids present, eight are essential and provide the important nitrogen content required by the neonate. Two of the most abundant amino acids are cystine and taurine. Taurine is absent from cows’ milk but plays an important role in brain maturation and is thought to function as a neurotransmitter. It was originally presumed to be involved only in conjugation of bile acids. Cystine is essential for somatic growth (Riordan 2008).
Carbohydrates
Carbohydrates comprise mainly lactose with small quantities of oligosaccharides, galactose and fructose. Lactose increases calcium absorption and is readily metabolized into galactose and glucose (assisted by the intestinal enzyme lactase), providing the necessary energy to feed the growing brain (Riordan 2008). These levels remain constant and are unaffected in malnourished women (Lawrence & Lawrence 2005).
Some oligosaccharides promote the growth of Lactobacillus bifidus, which increases the acidity of the neonatal gut, protecting it from pathogenic invasion (Kunz et al 1999).
Fats
The fat content varies at different times of the day and during a feed, with higher amounts towards the end of a feed (Kunz et al 1999). Preterm fat concentrations may be 30% higher (Riordan 2008), though others did not detect any major differences in lipid composition between term and preterm breast milk apart from more medium- and intermediate-chain fatty acids (Rodriguez-Palmero et al 1999). Long-chain polyunsaturated fatty acids (LCPUFA) are important for normal visual and brain development and are absent from formula milk. Addition of LCPUFA to formula milk in one very small study was found to improve IQ at 10 months of age (Williatts et al 1998).
The majority of LCPUFA are derived from maternal body stores rather than diet. Maternal diet may directly affect the fatty acid composition of breast milk (Kunz et al 1999, SACN 2007). Vegetarian women are able to maintain a high milk content of arachidonic acid (AA) and docosahexaenoic acid (DHA). DHA is the LCPUFA associated with improved visual and neurological function (Makrides et al 1995, SACN 2007).
Approximately 98% of the fat components are triglycerides that are broken down to fatty acids and glycerol by the enzyme lipase, found in breast milk itself. The remaining fats are phospholipids (0.7%), cholesterol (0.5%) and other lipolysis products. Digestion of triglycerides is initiated in the stomach, where gastric lipase commences lipolysis, and this is continued in the intestine by pancreatic lipase. The resulting monoglycerides have potent bactericidal properties and maintain infection control in the stomach and small intestine (Rodriguez-Palmero et al 1999).
Vitamins
Water-soluble vitamins C (ascorbic acid), B1 (thiamine), B2 (riboflavin and niacin), B6 (pyridoxine), folate (pteroylglutamic acid), B12 (cobalamin), pantothenic acid and biotin are all present in breast milk. Only niacin and B12 can be increased by maternal intake if found to be deficient (Rodriguez-Palmero et al 1999).
Fat-soluble vitamins A (retinol), beta-carotene (carotenoids), D (cholecalciferol), E (alpha-tocopherol) and K (phylloquinone) are all present in breast milk.
Minerals
These include sodium, potassium, chloride, calcium, magnesium, phosphorus, free phosphate and sulphur. Citrate binds some minerals and is soluble in water, so is important though not a mineral. Trace elements such as iron, zinc, copper, manganese, selenium, iodine and fluorine are all present in breast milk, though the latter two are absent from colostrum (Rodriguez-Palmero et al 1999).
The uptake of iron in breast milk is facilitated by the high levels of lactose and vitamin C, enabling up to 70% of absorption to take place. Absorption of exogenous iron from formula milk is limited and can adversely affect the action of lactoferrin from breast milk in the gut if the woman is mixed-feeding (see Table 43.1).
Table 43.1 Defence agents in breast milk (Also see RCM 2009)
| Substance and production | Action |
|---|---|
| 1. Antimicrobial agents | |
| Immunoglobulins | |
| Neonate produces minimal amounts of these in the first few months of life | Proteins produced by plasma cells in response to an antigen – located in the lactoglobulin fraction of breast milk |
| Secretory IgA – most abundant immunoglobulin | Important in providing passive immunity More resistant to proteolytic enzymes Provides resistance to a range of pathogens in gastrointestinal and respiratory tracts Neutralizes viruses and toxins from microorganisms such as Escherichia coli, Salmonella, Clostridium difficile, rotavirus and Vibrio cholerae (Riordan 2008) |
| IgG, IgM, IgD and IgE | These are other immunoglobulins found in breast milk in small amounts |
| Lactoferrin – an iron-binding glycoprotein | Competes with bacteria for iron, thus depriving bacteria of nutrients for proliferation Enhances iron absorption in neonate’s intestinal tract An essential growth factor for B and T lymphocytes (Riordan 2008) |
| Lysozyme – a whey protein | Acts with peroxide and ascorbate to destroy Gram-positive and other bacteria in the gut and respiratory system Increases progressively after 6 months’ lactation |
| Bifidus factor – nitrogen-containing carbohydrate | Promotes growth of anaerobic lactobacilli in the neonatal gut, providing a protective acid medium (Riordan 2008) |
| B12-binding protein | Deprives bacteria of vitamin B12 |
| Oligosaccharides – carbohydrates (monosaccharides) | Act by blocking antigens from attaching to the epithelium of the gastrointestinal tract Prevent the attachment of pneumococci (Riordan 2008) |
| Fatty acids | Disrupt membranes surrounding certain viruses and destroy them |
| Complement (C3 and C4 components) | Has the ability to fuse bacteria bound to a specific antibody and destroy them through lysis (Lawrence & Lawrence 2005) |
| Fibronectin | Facilitates the uptake of bacteria by mononuclear phagocytic cells |
| Mucins – protein and carbohydrate molecules | Adhere to bacteria and viruses (including HIV) and prevent them from attaching to mucosal surfaces (Lawrence & Lawrence 2005) |
| 2. Anti-inflammatory factors | |
| Secretory IgA, lactoferrin and lysozyme | Multipurpose anti-inflammatory role Lactoferrin inhibits the complement system and suppresses cytokine release from macrophages that have been stimulated by bacteria (Rodriguez-Palmero et al 1999) |
| Antioxidants (alpha-tocopherol, beta-carotene cystine, ascorbic acid) | Absorbed into the circulation and have systemic anti-inflammatory effects (Rodriguez-Palmero et al 1999) |
| Epithelial growth factors | Enhance maturation of the neonatal gut and limit entry of pathogens |
| Other anti-inflammatory factors include platelet-activating factor, antiproteases (alpha-antichymotrypsin and alpha-antitrypsin) and prostaglandins | |
| 3. Immunomodulators | |
| Nucleotides, cytokines and anti-idiotypic antibodies | Appear to promote development of the neonatal immune system |
| 4. Leucocytes (white blood cells) | |
| Approximately 90% of leucocytes in breast milk are neutrophils and macrophages | Eliminate bacteria and fungi by phagocytosis |
| 80% of the lymphocytes are T cells, though the cytotoxic capacity of these cells is low | |
Unabsorbed iron is a contributory factor to the increased incidence of gastroenteritis in formula-fed infants.
Advantages of breastfeeding
The normal neonate
The recommendation of ‘exclusive’ breastfeeding is promoted by the DH (Kramer & Kakuma 2006, NICE 2006, Shribman & Billingham 2009) and its value to the neonate is well documented by the WHO (WHO 2007) and on the NHS website (see website) and in other publications (Britton et al 2007, Horta et al 2007, Lawrence & Lawrence 2005, NHS Centre for Reviews and Dissemination 2000, NICE 2006, Riordan 2008).
Breastfeeding has beneficial effects on the psychological and physical wellbeing of mother and baby. The action of sucking at the breast helps to initiate production of saliva that increases absorption of carbohydrate and fat. Neonatal saliva contains amylase that assists in glucose absorption and lipase that increases uptake of fatty acids (Blackburn 2007). These enzymes will be reduced if the baby is preterm and unable to suckle, as tube-feeding bypasses this process, so it is important for the midwife to assist the woman to initiate suckling as soon as the reflex is present. In addition, pancreatic secretory trypsin inhibitor is a major motogenic and protective factor in human breast milk as its presence influences gut integrity and repair (Marchbank et al 2009).
Breast milk’s immune properties have been specifically highlighted (AAP 2005, Hanson 1998a, Mannick & Udall 1996, Newman 1995, Orlando 1995). It provides protection from leukaemia (Davis 1998, Shu et al 1999); rotavirus infection (Newburg et al 1998); gastrointestinal infections (Dewey et al 1995, Golding et al 1997, Mannick & Udall 1996); respiratory tract infection (Lopez-Alarcon et al 1997, Repucci 1995); Haemophilus influenzae meningitis (Silfverdal et al 1999); urinary tract infection (Pisacane et al 1992); otitis media (Dewey et al 1995, Duncan et al 1993); and necrotizing enterocolitis (Lucas & Cole 1990). The Millenium Cohort Study estimated that a 53% reduction of re-admisssions of children to hospital with diarrhoea and lower respiratory tract infections could have been made if women exclusively breastfed for 6 months (Quigley et al 2007).
Other benefits include: improved motor/personal and social development (Michaelsen et al 2009, Wang & Su 1996); improved IQ (Florey et al 1995, Lucas et al 1992); and protection from non-insulin-dependent diabetes mellitus (Cavallo et al 1996, Chertok et al 2009, Drash et al 1994, Pettitt et al 1997); eczema, asthma, and food allergies (Coutts 1998, Hanson 1998b, Oddy 2009, Saarinen & Kajosaari 1995); and from cardiovascular disease in later life (Horta et al 2007, Leon & Ronalds 2009, Ravelli et al 2000).
Further benefits include possible protection from schizophrenia (McCreadie 1997); juvenile rheumatoid arthritis (Mason et al 1995); inflammatory bowel disease (Mikhailov & Furner 2008); Crohn’s disease and coeliac disease (Hanson 1998a, Koletzko et al 1989); development of the physiological integrity of the oral cavity, ensuring alignment of teeth and fewer problems with malocclusions (Palmer 1998); and possible protection from sudden infant death (McVea et al 2000). The action of breastfeeding has beneficial effects on dental caries, and mouth and jaw development, and reduces the risk of childhood obesity (Arenz & Von Kries 2009, Horta et al 2007, O’Tierney et al 2009).
The preterm neonate
Breastfeeding confers all of the above advantages and, because of the reduced capability of the immune system, is vital for early protection against infection. Preterm infants are particularly vulnerable to necrotizing enterocolitis, so it is very important that women are supported to breastfeed fully (Lucas & Cole 1990). Women who give birth prematurely provide perfectly balanced breast milk for their babies – the non-protein nitrogen content is 20% higher than in those who give birth at term, providing the necessary free amino acids essential for growth (Riordan 2008). Preterm breast milk contains higher concentrations of polymeric immunoglobulin A (pIgA), lactoferrin, lysozyme and epidermal growth factor. In addition, the numbers of macrophages, neutrophils and lymphocytes are higher in the colostrum (Xanthou 1998). Lingual lipases will be reduced if the baby is preterm and unable to suckle, as tube-feeding bypasses this process (see website).
The woman
Breastfeeeding confers significant health benefits on women, such as protection against several cancers, including premenopausal breast (Lee 2003) and ovarian cancer, improved bone density and reduction of anaemia. It can also be an effective postpartum contraceptive during ‘total’ breastfeeding (WHO Task Force 1999), having the added advantage of delaying menstruation and reducing anaemia (Wang & Fraser 1994). (For more information, see website.)
Contraindications to breastfeeding
There are very few absolute contraindications to breastfeeding.
Neonatal conditions (WHO/UNICEF 2009)
Maternal conditions
HIV
Breastfeeding should be avoided as part of a programme of interventions to reduce the risk of mother-to-child HIV transmission (DH 2004b, RCM 1998). WHO/UNICEF advise against breastfeeding if replacement feeding is acceptable, feasible, affordable, sustainable and safe (AFASS) as is the case in the UK and developed countries (WHO/UNICEF 2009).
However, exclusive breastfeeding for the first 4–6 months continues to be advised in ‘resource-constrained’ settings, such as in sub-Saharan Africa, where HIV transmission has been found to be reduced by exclusive breastfeeding in comparison with mixed feeding (Coovadia et al 2007, Gray & Saloojee 2008, WHO/UNICEF 2009, WHO/UNICEF/UNAIDS/UNFPA 2007).
Drugs – maternal medication
Certain drugs pass through breast milk and may be harmful to the neonate, and temporary or permanent avoidance of breastfeeding may be recommended, depending on the prescription of drugs currently in use by the woman (for example, antipsychotic, anticarcinogenic, iodides, anti-epileptics). Midwives can update their knowledge using the most recent British National Formulary (BNF) or the local pharmacy drug information service within their Trust (see website).
Substance misuse
Substances such as nicotine, alcohol, ecstasy, amphetamines and cocaine are known to have harmful effects on the baby through breast milk. Opioids, benzodiazepines and cannabis can all cause sedation in the mother and baby. Women should be asked to abstain and cease to breastfeed while under the influence of these substances (WHO/UNICEF 2009).
Conditions where a woman can continue to breastfeed but health problems may be of concern
Hepatitis B
The baby should be given a hepatitis B vaccine within the first 48 hours or as soon as possible thereafter (WHO/UNICEF 2009). The woman can continue breastfeeding.
Hepatitis C
A small study of the breast milk of seropositive women indicated a very low risk of transmitting the virus to the baby (Zimmermann et al 1995). The advice is to continue breastfeeding.
Pollutants in breast milk
A variety of pollutants in the environment may arise in breast milk, which should not prevent breastfeeding or its promotion, as breastfeeding itself offers a degree of protection against many pollutants.
The Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment examined the high quantities of polychlorinated biphenyls (PCBs) and dioxins present in breast milk and concluded that the advantages of breastfeeding still outweighed the risks (Mitchell 1997). Intake of organochlorines measured in breast milk set against the WHO acceptable daily intakes failed to demonstrate an unacceptable intake for the baby (Quinsey et al 1996). Examination of the reported levels of the pesticide DDT in breast milk fat demonstrated a decline in most areas of the world (Smith 1999), and guidance on the implications of exposure to cadmium, lead and mercury resulted in encouragement of breastfeeding under ‘most circumstances’ (Abadin et al 1997).
The role of the midwife
Knowledge of breastfeeding
Box 43.3
The seven-point plan for the protection, promotion and support of breastfeeding in community healthcare settings (UNICEF UK BFI 2001)
All providers of community healthcare should:
The seven-point plan is the result of a widespread consultation procedure involving health professionals, service providers, mother support groups, professional organizations and other interested parties. It therefore reflects consensus in the UK as to what constitutes best practice in the care for and support of breastfeeding mothers and babies by the community health services.
Physiology of lactation
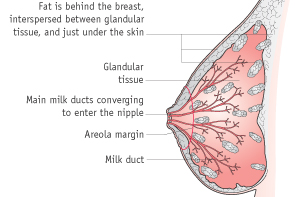
Puberty to pregnancy (mammogenesis)
Oestrogen and growth hormone stimulate the growth of the mammary ducts during puberty. In the second half of the menstrual cycle, progesterone stimulates development of the lactiferous ducts and alveoli. Proliferation of the epithelial tissue is a gradual process at each menstrual cycle.
Prolactin is a single-chain peptide hormone released from the anterior pituitary gland, and serum levels increase during pregnancy. It is thought to be essential for the development and final stages of the differentiation of the alveoli and ducts in pregnancy (Blackburn 2007, Neville 1999). Prolactin-inhibiting factor, produced by the hypothalamus, maintains low prolactin levels to prevent milk secretion in pregnancy.
Oxytocin is an octapeptide hormone produced in the hypothalamus and stored and secreted in the posterior pituitary gland (Blackburn 2007). It is produced in low levels during pregnancy (possibly due to the action of a placental enzyme). It stimulates electrical activity and muscle contractions in the myometrium during labour and is critical in the milk ejection reflex postpartum.
Other hormones, such as human placental lactogen (hPL), human chorionic gonadotrophin (hCG), growth hormone and adrenocorticotrophic hormone (ACTH), act synergistically with prolactin and progesterone to influence the growth of the glandular tissues of the alveoli to promote mammogenesis (Blackburn 2007). hPL assists in mobilization of free fatty acids and inhibition of peripheral glucose utilization and stimulates mammary growth. ACTH stimulates the adrenals to secrete corticosteroids.
Initiation of lactation (lactogenesis)
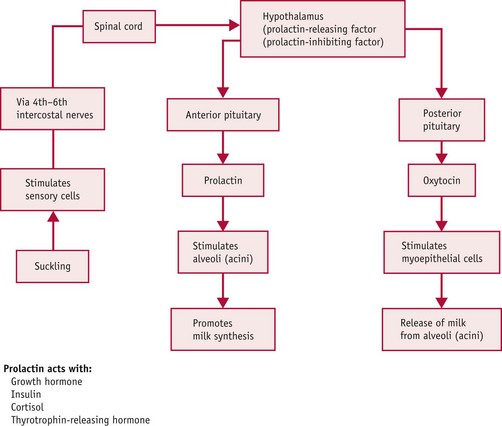
Initiation of milk production involves a complex interaction of several hormones and factors. Following the birth, oestrogen and progesterone levels decline rapidly, allowing a rise in prolactin and oxytocin levels. Prolactin, released from the anterior pituitary gland, stimulates alveolar cells to produce milk while acting synergistically with growth hormone, insulin, cortisol, and thyrotrophin-releasing hormone (TRH) (Blackburn 2007).
Oxytocin stimulates contraction of the myoepithelial cells surrounding the alveoli, causing an ejection reflex, and milk is propelled down the lactiferous ducts to the ampulla.
The action required to stimulate both of these hormones is known as the neurohormonal reflex (or ‘let-down’ reflex). This stimulus is controlled by the effect of the neonate sucking at the breast, but may also be stimulated by the mother seeing, smelling, touching and hearing her baby.
Suckling stimulates prolactin release from the anterior pituitary gland, and therefore it is imperative that the midwife assists the woman to breastfeed as soon as possible after the birth. It is suggested that sucking movements reach a peak at 45 minutes and decline within 2–2.5 hours after the birth (Righard & Alade 1990, WHO/UNICEF 1989) in line with a physiological reduction in adrenaline levels (Widstrom et al 1990). Lack of ‘priming’ the alveolar prolactin receptor cells may result in shut-down and reduction of milk supply. Sensory nerve endings are activated in the nipple and areola area and this stimulates the hypothalamus via the spinal cord. As a result, oxytocin is released, prolactin-inhibiting factor is suppressed, and prolactin is released.
The levels of prolactin are increased towards the end of a feed, after approximately 20–30 minutes following a feed and at night, thus maintaining a diurnal increase (Blackburn 2007). The midwife needs to explain this to women so they understand that breastfeeding at night promotes and stimulates the production of prolactin for the next day and should be encouraged.
Prolactin release works on a supply and demand principle. When the baby suckles at the breast, prolactin-releasing factor is released by the hypothalamus and stimulates prolactin release from the anterior pituitary gland. When the baby stops suckling, a negative feedback prolactin-inhibiting factor (PIF) is released by the hypothalamus, usually by half an hour after a feed (Prentice et al 1989, Wilde et al 1995), and this inhibits prolactin supply (Fig. 43.2).
Prolactin-inhibiting factor (PIF) is a protein secreted in the breast milk itself which increases in amount as breast milk accumulates in the breast. Its function is to exert negative feedback to block future milk production when there is ineffective milk removal from the breast. Whilst prolactin and oxytocin are released systemically, therefore influencing milk production in both breasts, PIF build-up can occur in one breast, only affecting milk production in that breast. Therefore, if a baby is incorrectly attached and unable to effectively remove milk from the breast, the build-up of PIF will ultimately result in a reduced milk supply. Milk production can be ‘stepped up’ again by effectively removing milk from the breast, thereby reducing the amount of circulating PIF in the breast milk.
Prolactin can be inhibited by oestrogen. A woman wishing to use oral contraception following the birth must be advised to take the ‘progesterone-only’ pill while she continues to breastfeed.
If the women ‘complements’ with formula milk following breastfeeds, the baby’s suckling may be reduced at subsequent feeds, which ultimately interferes with the body’s ability to produce the required amount of breast milk.
It is vital that the midwife discusses the physiology of lactation with the woman to dispel any fears or myths about breastfeeding.
Maintenance of established lactation (galactopoiesis)
This phase is reliant on an intact hypothalamic–pituitary axis regulating prolactin and oxytocin levels and maintenance of frequent sucking and removal of milk by the neonate (Blackburn 2007). Growth hormone, corticosteroids, thyroxine and insulin continue to play an important part in maintaining established lactation.
The volume of milk produced commences at approximately 50 mL per day and increases to around 500 mL per day (double for twins) by 36 hours and up to 800 mL per day by 3 months (Neville 1999). Sodium and chloride levels in breast milk fall in the first few days, followed by an increase in lactose concentrations. Lactoferrin and secretory IgA rise immediately, then fall with the increase in volume in the first few days.
Regular suckling suppresses luteinizing hormone (LH) while follicle-stimulating hormone (FSH) may return to normal due to the pulsatile secretions of gonadotrophin-releasing hormone (GnRH) by 4 weeks postpartum (Neville 1999). This may have an impact on the woman’s fertility.
Assisting breastfeeding
Midwives’ contribution to the support and education of women in the antenatal and immediate postnatal period has an enormous effect on the woman’s satisfaction, on breastfeeding success and on breastfeeding rates overall (NICE 2006, 2008b).
Additional support has a positive effect on the duration of breastfeeding (Britton et al 2007, Dyson et al 2005, Kramer & Kakuma 2006). This includes a range of activities, with face-to-face support in the antenatal and postnatal periods demonstrating the most positive effect 2 months postnatally.
Early initiation of breastfeeding
It is important that skin-to-skin contact takes place in an unhurried environment for an unlimited period immediately (or as soon as possible) after delivery (Moore et al 2007, NICE 2006, WHO 2002). It increases the duration of breastfeeding, maternal–infant interaction, neonatal temperature, and glucose levels at 90 minutes, and reduces crying of the neonate (Moore et al 2007, NICE 2006). Separation of a woman and her baby within the first hour of birth for routine procedures should be avoided (NICE 2006).
The initiation of breastfeeding is increased and promoted by ‘needs-based, one-to-one, informal education or support sessions, delivered either before or before and after the birth by a trained breastfeeding professional or peer counsellor’ (Dyson et al 2005).
Midwives should ensure that they document and audit the timing and initiation of breastfeeding following all births.
Positions for feeding and good attachment/latching-on
These have been cited as the key to successful breastfeeding (NICE 2006, 2008a). The midwife needs to provide the woman with simple, helpful advice on positioning and attachment. These two aspects of breastfeeding can cause major problems if they are not addressed from the birth.
Preparation of the mother is important, either sitting or lying down using cushions or pillows to support the back. Preparation of the baby so that he or she is unwrapped, with hands free (and mittens removed) enables the baby to experience touch, stimulating the neurological system and myelinization (Blackburn 2007).
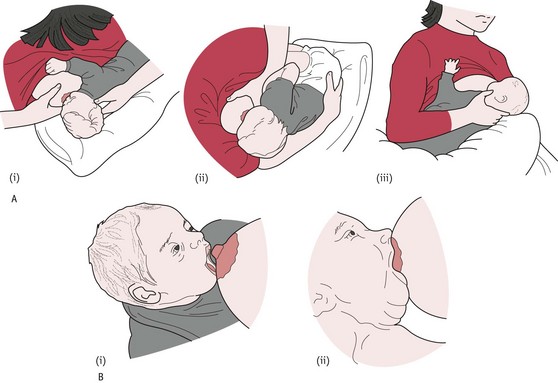
Positioning (‘nose to nipple’)
The baby is supported to face the mother, parallel to the mother’s chest. The baby’s neck (rather than head) should be supported enough to allow the head to extend backwards as necessary (Fig. 43.3A). The mother should then bring the baby’s nose in line with her nipple and ensure the rooting reflex is triggered, causing the mouth to ‘gape’ (DH 2010a, NICE 2006, RCM 2002).
The consequences of ineffective suckling owing to poor attachment on the breast have been linked to ‘failure to thrive’ (Morton 1992) and early cessation of breastfeeding (Campbell 1997, Righard & Alade 1992).
Using a pacifier (dummy) can cause confusion and should be discouraged, as the baby is more likely to ‘nipple-suck’, leading to reduced milk production and breastfeeding (Koosha et al 2008, Neifert et al 1995, Righard & Alade 1992, UNICEF UK BFI 1998a).
Attachment (‘baby to breast’)
The baby should be brought up to the breast quickly to ensure correct attachment, rather than the breast brought down to the baby, which encourages bad maternal posture and poor attachment. The baby approaches the breast leading with the chin, which enables use of the tongue and lower jaw (DH 2010a, NICE 2006).
When the baby is attached to the breast properly, ‘his mouth is wide open and he has a big mouthful of breast; his chin is touching the breast; his bottom lip is curled back’ (NICE 2006). The correctly attached baby will take long deep sucks with pauses, and the ear will be seen visibly moving during the process and audible swallowing is heard (Fig. 43.3B).
If the baby is attached correctly (Fig. 43.4) there should be no friction of the tongue or gum on the nipple and no movement of the breast tissue in and out of the baby’s mouth (DH 2010a, RCM 2002). The midwife should share this knowledge with women and help to reduce the confusion that can occur from poor positioning, or if bottle feeds are given as a complement to breast milk.
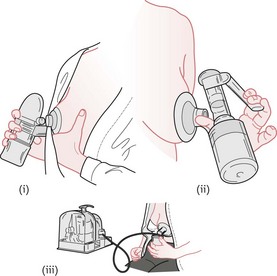
Expressing
Women should also be shown ‘how to breastfeed and maintain lactation even if they should be separated from their infants’ (NICE 2006). This includes providing information and advice on hand expression of their breast milk and storage of expressed breast milk (NICE 2006, WHO/UNICEF 1989). See Figures 43.5 and 43.6.
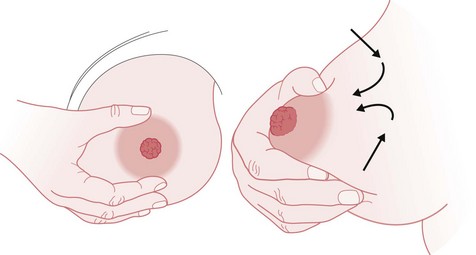
Figure 43.5 Hand-expressing breast milk. A. Technique 1. B. Technique 2. See Box 43.4 for explanatory text.
(From UNICEF UK BFI 1998b.)
Box 43.4
Techniques for manual expression (UNICEF UK BFI 1998b)
Technique 1
Technique 2
A Cochrane review on methods of expressing breast milk concluded there was no evidence that simultaneous pumping obtained more milk overall than sequential pumping (Becker et al 2008). However, assisting women to relax during pumping appeared to increase yield and ensuring the woman had a choice in the method of pumping was important. The study stated that the effectiveness of the method of expressing was dependent on the reason for obtaining breast milk, for example, if the baby was in the neonatal unit (see website).
Neonatal nutrition
The midwife must have adequate knowledge of the constituents of breast milk (RCM 2009). A term breastfed baby should have adequate nutrition through breastfeeding on demand. A preterm neonate (less than 37 completed weeks of gestation) or small-for-gestational-age baby will have different demands and the midwife should seek guidance from a neonatologist on frequency and volume of feeds, as this is outwith normal midwifery practice (NMC 2004). The advantages of breast milk for a preterm baby include protection and development of the immature gut due to antibodies and other substances that are not available in formula milk (Henderson et al 2007); and the fat content of breast milk regulates blood flow, blood clotting and immune responses (RCM 2009).
Public health issues
Smoking carries higher risks for the infant, and smoking during pregnancy and in the postnatal period reduces the volume of breast milk in addition to reducing the birthweight of the baby. Midwives need to discuss this with women in early pregnancy (Shribman & Billingham 2009).
Obesity risk by school age is reduced by 20% if a baby is breastfed, according to the European Childhood Obesity Study Group (Koletzko et al 2009), and the Cross Government Obesity Unit Strategy recommends breastfeeding to reduce childhood obesity (Cross Government Obesity Unit 2008).
Bed sharing is not recommended, especially if the parents are heavy smokers, have drunk alcohol or have taken drugs that make them drowsy (DH 2009b). It is recommended that the baby is placed in its own cot after a feed and remains in the parents’ room until at least 6 months of age.
Maternal nutrition during breastfeeding may vary according to cultural and religious differences, such as the use of ‘hot’ and ‘cold’ foods across cultures (Vincent 1999), vegetarianism and fasting.
The lactating woman should aim for a diet containing a minimum of 1800 kcal with a recommended average intake of at least 2200 kcal (Lawrence & Lawrence 2005). Women have enhanced metabolic efficiency due to hormonal influences during pregnancy and in the early postnatal period, which enables them to utilize their normal diet to produce an adequate milk supply for their baby.
Dietary restriction during lactation should be discouraged (Butte 2000) but midwives should be cautious about assuming that a poor diet will result in insufficient breast milk, which can undermine a woman’s belief in her own body.
Common problems
Insufficient milk
The most common cause for insufficient milk is poor attachment, ineffective milk removal and infrequent feeding
(Neifert 2004).
Women who have received continuous support are more likely to have a positive attitude to breastfeeding (Hillervik-Lindquist et al 1991). Lack of support and advice from the midwife, or immediate family and friends, can undermine the woman and lead to psychological and ‘iatrogenic’ problems. It is helpful for the midwife to have discussed breastfeeding in the antenatal period and be aware of whether the woman planned to breastfeed or if she is ‘under duress’ from family members. Stress can inhibit oxytocin but should not be severe enough to inhibit lactation (Powers 1999).
There are few physical causes for inadequate milk supply and these need to be identified and rectified by midwives and the multiprofessional team. The principles of the physiology of lactation should be discussed with the woman to ensure she is positioning the baby for adequate and frequent milk ‘removal’ (Powers 1999).
Trauma to the fetal skull during instrumental birth or difficult caesarean section can disrupt alignment of the fetal skull, cause damage to the cranial nerves and affect sucking and swallowing mechanisms (Kroeger & Smith 2004). This should gradually resolve over time with appropriate explanation, positioning advice and support.
Certain drugs reduce lactation (oral contraceptives containing oestrogen, bromocriptine, thiazide diuretics) and should therefore be avoided. Smoking and alcohol consumption can reduce the milk supply (Horta et al 1997, Mennella 1997). Women at risk should be identified in the antenatal period and offered appropriate advice and support.
Anaemia may affect the milk supply, shorten the length of breastfeeding, and lower the age of weaning (Henly et al 1995). Women who experienced postpartum haemorrhage of between 500 and 1500 mL were found to have insufficient milk supply, with infants showing failure to thrive (Willis & Livingstone 1995). Early review and treatment of such women is recommended.
Breast surgery may cause decreased milk production where the ducts have been severed, as in breast reduction (Neifert et al 1990). Surgery for breast enhancement involving silicone implants does not normally cause nerve and duct damage as the prosthesis is implanted beneath the pectoral muscle (Berlin 1994). The type of surgery should normally be identified by the midwife in the antenatal period and a plan of action documented.
Nipple shields, especially the non-silicone variety, prevent the neonate from applying the stimulus required for effective milk removal from the breast and may therefore reduce the milk supply.
Medical disorders, such as hyperthyroidism or hypothyroidism, can affect milk supply. Hypopituitarism (Sheehan syndrome) affects the supply of hormones from the anterior pituitary gland and therefore prevents prolactin production. All such medical conditions require prompt referral and investigation.
Engorgement – venous/milk
Venous retention may occur because of the increase in blood and lymph circulation when the milk ‘comes in’, causing tenderness (Hill & Humenick 1994), but this should not be confused with ‘engorgement’.
Engorgement of the breast is a pathological condition whereby oedema causes poor milk flow by constricting the milk ducts. This is caused by infrequent, ineffective milk removal and is preventable by good breastfeeding practices, including correct positioning and attachment. Secondary vascular and lymph stasis may occur (Hill & Humenick 1994).
Both milk and venous engorgement cause distension of the breast tissue, impeding the release of milk, and preventing the baby from ‘latching on’. Engorgement may occur at any time during the first 2 weeks postpartum but is likely to peak at around the third to sixth day after delivery, or may occur following transfer home from hospital (Hill & Humenick 1994). It may last for 48 hours, during which time the woman will experience discomfort and is at risk from development of mastitis and subsequent breast abscess.
Warm flannels or a hot shower or bath may improve the milk flow by increasing the blood supply around the alveoli. Gentle hand (or pump) expression will help to release the milk flow and therefore the tension around the nipple and areola, in order to attach the baby more easily. If the baby is separated from the woman, then hand-expressing or a breast pump needs to be used regularly.
A Cochrane review reported that treatments using cold cabbage leaves or cabbage leaf extract, ultrasound versus a placebo, and oxytocin and cold packs had no demonstrable effect on the symptoms. Use of Danzen (serrapeptase – an anti-inflammatory agent) and bromelain/trypsin complex improved the overall symptoms compared to a placebo (Snowden et al 2002).
There is limited evidence surrounding the practical relief of engorgement. Midwives should be aware that it is imperative to prevent such a condition occurring in the first place, by providing advice and support with regard to correct positioning, flexible feeding arrangements, and completion of suckling on one breast at a time in order to ensure adequate milk removal (Renfrew et al 2000).
Sore/cracked nipples
The most common cause of sore or cracked nipples is incorrect attachment – preventable by good midwifery support, education and advice (NICE 2006, RCM 2002, Renfrew et al 2000). Poor attachment and soreness are created as the neonate compresses the end of the nipple against the hard palate, causing damage, instead of taking the entire nipple in as far as the soft palate (NICE 2006).
Women with sore or cracked nipples are often prompted to wean their babies, as the pain can be unbearable. The psychological impact of nipple pain can cause high levels of emotional distress, and may affect the mother–child relationship, though both will resolve once the pain is removed (NICE 2006).
Problems in the neonate such as a short frenulum (ankyloglossia), or breast engorgement, should be identified by the midwife as these will create difficulties for the neonate in latching on (NICE 2005, 2006). See Neonatal problems below.
Occasionally, the woman may suffer from a condition known as Raynaud’s phenomenon (see website).
Other conditions causing pain may present as eczema or psoriasis on the nipple or areola. Fungal infections, such as Candida albicans (thrush), can cause burning or shooting pain sensations, and need to be identified and treated with the appropriate medication (Amir et al 1996). Aggressive treatment with antibiotics to treat colonization of cracked nipples with Staphyloccocus aureus was found to improve healing and decrease the risk of developing mastitis in a randomized comparative study of 84 breastfeeding mothers in Canada (Livingstone & Stringer 1999). Midwives must be vigilant in identifying and screening for differential diagnoses rather than assuming conservative treatment is all that is required.
Management
The midwife should assess and record the damage to the nipple for:
Knowledge and application of current evidence-based practice reduces conflicting advice to women (Huml 1999).
Past methods including drying the nipples through exposure to air or using a hair-dryer are now known to cause scab formation and delayed healing by unnaturally drying the skin in an area that is normally moist (Inch & Fisher 2000). See website for further information.
Other suggested treatments using moisture though not demonstrating significant benefits include:
The effectiveness of silicone nipple shields has been demonstrated in case studies and retrospective data following the establishment of the milk supply, but only as a last resort (Bodley & Powers 1996, Brigham 1996, Elliott 1996, Pessl 1996). They may cause a diminishing milk supply and exacerbate engorgement (Inch & Fisher 2000) so should be treated with caution and never used as a substitute for teaching correct attachment.
Treatments that have been proven to be not effective include chlorhexidine spray (Renfrew et al 2000) and other creams (Inch & Fisher 2000).
Mastitis
Mastitis is an inflammatory condition of the breast which may or may not be accompanied by infection (Jones 2006).
Mastitis can be incorrectly diagnosed in the first few days but is usually caused by engorgement or milk stasis producing increased pressure in the alveoli due to non-removal of milk. The pressure builds up, forcing the milk out into the surrounding tissues.
Mastitis most commonly occurs in the second or third week postpartum (Jones 2006).
Infective mastitis is caused by bacterial invasion, usually via a cracked nipple. Staphylococci or streptococci are the most common organisms and these act on the milk forced outside the alveoli into the surrounding cells.
Neonatal problems
Tongue tie (ankyloglossia)
A short frenulum may present the neonate with difficulty in attaching and suckling at the breast. The tongue is unable to move forward and cup the nipple and thus stimulate release of milk from the breast. One of the first signs is sore nipples and poor weight gain due to lack of adequate milk supply, in spite of regular feeds (see website).
Cleft lip and palate
Cleft lip and palate are congenital malformations characterized by incomplete fusing of the lip and upper jaw (Riordan 2008). This may involve the lip, or may extend to the soft and hard palate, and may be unilateral or bilateral (see website).
Down (or Down’s) syndrome
This congenital anomaly, characterized by heart defects, a protruding or large tongue and hypotonicity, can be challenging for breastfeeding. Manual expressing or pumping is often required because of inadequate stimulation of the let-down reflex by the baby.
The woman will need encouragement and advice for positioning the baby, with the emphasis on firm support of the breast and baby’s head with use of pillows. Success of breastfeeding is most likely to be dictated by the severity of the cardiac abnormality, which will affect the respirations and tire the baby easily (Renfrew et al 2000).
Breastfeeding the preterm baby
Breast milk is the optimum nutrition for the preterm infant (Henderson et al 2007). It provides additional immunity to the immature system, such as IgA, lactoferrin, lysozyme and oligosaccharides, stimulates maturation of the gastrointestinal tract and reduces necrotizing enterocolitis (RCM 2009). Different strategies, including expression and cup feeding, often need to be employed to support the development of breastfeeding. See website for more information.
Twins and triplets
The Multiple Births Foundation produces a booklet for professionals and parents with advice and information on breastfeeding for multiples. This includes the importance of getting help and support for domestic chores as well as extra help for positioning the babies at each feed and especially at night. Cup feeding is suggested if the babies are born early (from 30 weeks’ gestation) and are unable to feed at the breast initially (see Fig. 43.8).
Going back to work
The current recommendation is for all babies to be exclusively breastfed for 6 months (WHO 2002). Many women need to return to work before this time and should be encouraged and supported by the midwife to continue breastfeeding before and after work, and to express either at work (dependent on facilities) or home and leave the expressed breast milk (EBM) with the childminder or carer. It is also useful to provide information on breastfeeding support groups and counsellors (see website).
There is no statutory right to paid breastfeeding breaks or a shorter working day in the UK but there is some legal protection under the health and safety laws – that is, the Management of Health and Safety at Work Regulations 1992 and the Employment Rights Act 1996 (Maternity Alliance 1999). If women work in the public sector, they are protected under European law. The Pregnant Workers Directive (1992) states: ‘if your work affects your breastfeeding’ the public sector employer must temporarily alter the working conditions and/or hours of work to protect breastfeeding or give alternative work (Maternity Alliance 1999).
Artificial feeding
The 2005 Infant Feeding Survey was the first time breastfeeding rates were provided for each separate country. Initial breastfeeding rates were 78% for England, 70% for Scotland, 67% for Wales and 63% in Northern Ireland (Bolling et al 2007). Highest incidences were found among women in the professional and managerial groups. By 6 weeks the breastfeeding rate across the UK had fallen to 48% and by 6 months only 25% were still breastfeeding.
The midwife needs to be conversant with the equipment and information for parents regarding artificial feeding (DH 2009c, 2009d, 2010b).
For information regarding constituents of artificial milk formula, see Westland & Crawley in Infant Milks in the UK table (RCM 2009).
Reasons why some women may artificially feed their baby
Some women may be unable to breastfeed their baby if they have HIV or are taking certain drugs (see Contraindications to breastfeeding above). Other women may be unsupported by their families, be suffering from postnatal depression or post-traumatic stress disorder, or simply be subjected to ‘peer’ pressure to artificially feed their babies.
Regulations surrounding infant formulae
Infant formulae are artificial feeds that are manufactured to take the place of human milk in providing a sole source of nutrition for the young infant. The essential composition of these formulae was set down as a Parliamentary Statute, or law, which came into force in March 1995 (Statutory Instrument 77 1995).
Midwives need to be aware of the WHO International Code of Marketing of Breastmilk Substitutes (WHO 1981), the aim of which is to ensure that infants receive safe and adequate nutrition, through the marketing and practices surrounding breast milk substitutes, advertising and donation of free samples or equipment directly to the general public.
The majority of infant formula brands can be divided into two groups: whey dominant and casein dominant. When whey is the dominant protein the whey:casein ratio is closer to that of human milk, as in ‘first’ milks (UNICEF UK BFI 2010). Casein-dominant formulae have a whey:casein ratio closer to that of cows’ milk, as in ‘follow-on’ milks (RCM 2009, UNICEF UK BFI 2010).
Types of feed available
Examples of formulae available in the UK for infant feeding are available (RCM 2009; and see website).
Methods of artificial feeding
The most common method of feeding a term baby with formula milk is via the bottle (Fig. 43.7). There is a range of bottles to choose from and teats that mimic the shape of the nipple. The midwife needs to have a good knowledge of how to teach women to make up feeds correctly (see Fig. 43.9).
Other methods
For babies who required additional help in feeding, such as babies with feeding problems and preterm babies, there is a range of strategies, including the use of syringe feeding, cup-feeding (Fig. 43.8) and supplementary feeding. For more information, see website.
Complementary/supplementary feeds
Supplementary feeds (breast milk replacements) and complementary feeds (breast milk substitutes) have no place in the care of a healthy term baby who is breastfeeding, as they interfere with the breastfeeding physiology (MIDIRS/NHS Centre for Reviews and Dissemination 1999). It is important that women understand the physiology of breastfeeding.
Disadvantages of artificial feeding
Artificial feeding has been described as a risk behaviour (Minchin 2000). The scientific dangers of artificial feeding are well known amongst health professionals and the midwife should share the information with women before a decision is made to feed artificially. Babies who are artificially fed are at risk of several infections and disorders as shown in Box 43.5.
Box 43.5
Risk factors for infections/disorders requiring hospital admission or medical treatment
Other risks of bottle-feeding currently being researched include childhood lymphomas, inflammatory bowel disease, multiple sclerosis, dental occlusions, coronary heart disease, autoimmune thyroid disease, coeliac disease, Kawasaki disease, lower IQ, risk of sudden infant death syndrome, and obesity (MIDIRS/NHS Centre for Reviews and Dissemination 1999).
Advantages of artificial feeding
The role of the midwife in artificial feeding
Preparation of feeds
All parents should be shown how to prepare artificial feeds and how to clean and sterilize the utensils. Ideally there should be an opportunity to practise with the midwife present so that any queries can be discussed. Women who have other children to care for may be rushed into taking shortcuts whilst making up their babies’ feeds. Mistakes such as using the wrong scoop for the brand of milk, over- or underfilling the scoop, adding too little or too much water, and adding sugar or cereals to the feed are more common in parents with more than one child than in first-time parents and occur in all social classes. Hence, it is not safe to assume that multiparae or those in the higher social groups will make up feeds accurately. All parents need careful teaching and demonstrations for making up feeds by the midwife. See Figures 43.9 and 43.10.
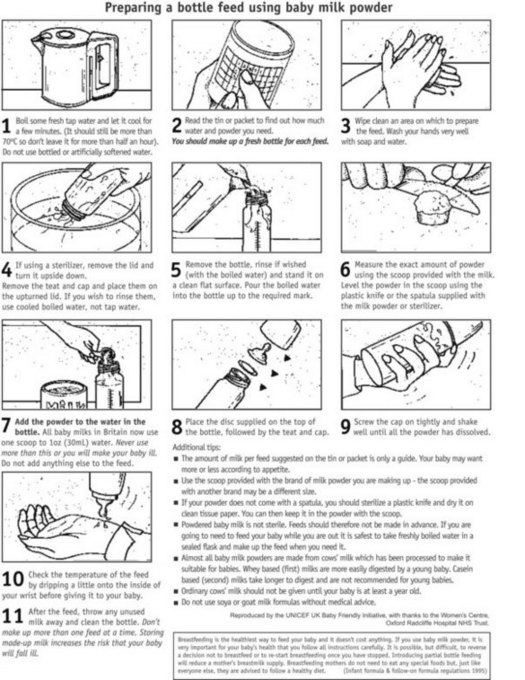
Figure 43.9 Preparing a bottle feed using formula milk powder.
(From UNICEF UK BFI 2001, with permission of UNICEF UK.)
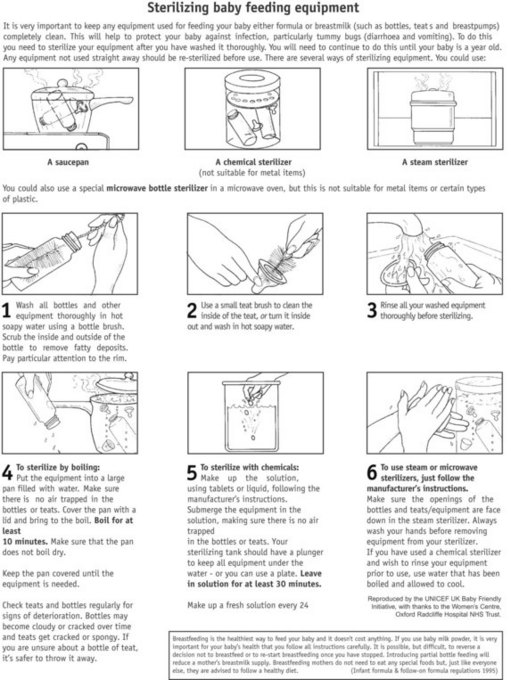
Figure 43.10 Sterilizing baby feeding equipment.
(From UNICEF UK BFI 2001, with permission of UNICEF UK.)
The midwife should work in tandem with the health visitor and GP to provide a seamless service with regard to advice on infant feeding. Depending on the practice within the primary care trust, the midwife will probably ‘transfer’ the care of the mother and baby to the health visitor 10 days after the birth. The mother is invited to attend ‘baby’ clinics at the local health centre or GP surgery, where she will get advice about feeding, weaning and immunizations. The midwife may continue visiting and providing breastfeeding advice postnatally for as long as she considers neccessary (NMC 2004).
Conclusion
The mother’s choice of feeding method, and her success with her chosen method, have long-term implications for the health of herself and her baby, and therefore for the health of the community as a whole. The importance of providing evidence-based, up-to-date and accurate information and sensitive support to women prior to the birth of the baby, and during the neonatal period, is fundamental to the woman’s physical and psychological wellbeing and to her view of herself as a mother. Time invested by the midwife can pay huge dividends and must be seen as a priority.