Chapter 45 Respiratory and cardiac disorders
Introduction
Developments in the organization of the maternity services means that midwives are taking on greater responsibility for the care of the newborn at birth and in the postnatal period. Midwives need to be able to respond to the challenge of resuscitating newborn babies, examining babies to confirm normality and detect abnormality, and stabilizing sick infants while awaiting transfer to tertiary facilities. This chapter provides an overview of fetal anatomy and physiology and relates this to resuscitation of the newborn (see Ch. 29). The chapter also offers an overview of some of the common respiratory and cardiac disorders midwives may encounter in practice.
Respiratory and cardiac development in the fetus
Midwives need to appreciate respiratory and cardiac development in utero as this facilitates understanding of normal adaptation at birth. It provides a basis for care provision in some respiratory and cardiac disorders.
Respiratory development (Table 45.1)
Fetal lungs are filled with fluid secreted by the lungs – rather than amniotic fluid. This fluid is important in facilitating the maturation and development of the fetal lungs. Approximately 300–350 mL of fetal lung fluid is produced daily by the fetus at term. In utero, it can move up the trachea, where some is swallowed by the fetus and some escapes into the amniotic fluid. At birth, a small amount of this fluid drains from the nose but most is moved out of the alveoli into the lymphatic system with the first breaths (Greenough & Milner 2005).
Table 45.1 Respiratory development in the fetus
| Post conception | |
|---|---|
| 3–6 weeks | Fetal lungs start to develop from the foregut The division of the foregut and the respiratory system is complete by the end of this period. Disruption at this time can lead to abnormalities such as tracheo-oesophageal fistula (Blackburn 2007) |
| 7–16 weeks | Respiratory system continues to grow and differentiate |
| 16 weeks | Tracheobronchial tree is formed Cilia and mucus-producing glands are present |
| 16–26 weeks | Primitive bronchioles start to develop rich vascular network required for gaseous exchange in extrauterine life |
| 20–24 weeks | Lung is lined with epithelium composed of Type I and Type II pneumatocytes Type II pneumatocytes start to appear Type II pneumatocytes produce surfactant, a pulmonary lipoprotein which decreases surface tension, thus reducing the work of breathing As gestational age increases, more surfactant is synthesized (Blackburn 2007) |
| 24 weeks | Vascular system proliferates Leads to thinning of the vascular epithelium and the capillaries come into close contact with the developing airways – eventually becomes the blood–gas barrier |
| 26 weeks onwards | Terminal air sacs appear, which then develop into the alveoli (Note: Despite the lack of alveoli in babies born between 24 and 26 weeks’ gestation, the vascular bed is sufficient to allow some gaseous exchange and this can, with support, sustain extrauterine life [Hodson 1998]) |
| 29–35 weeks | Proliferation of alveoli starts and increases dramatically (Hodson 1998) Development of alveoli continues after birth |
| 30 weeks onwards | Significant increase of total lung surface and lung volume |
| 35 weeks | Fetus has sufficient surfactant and functional alveoli to support extrauterine life |
Fetal breathing movements are rapid irregular movements, which may be seen on ultrasound as early as 10 weeks’ gestation. The strength and frequency of fetal breathing movements increase with gestational age. By the third trimester, breathing movements can be detected about 30% of the time, at a rate of 30–70 breaths per minute (bpm). It is thought that fetal breathing movements are important in enhancing lung development and growth. Fetal breathing patterns can be altered during periods of hypoxia, sometimes ceasing for several hours. Monitoring fetal breathing movements by ultrasound is used as part of biophysical profiling to assess fetal wellbeing.
Cardiac development
The cardiovascular system is the first system to develop in the embryo. The rapidly developing embryo requires an efficient and effective way of transporting oxygen and nutrients and excreting waste products (Blackburn 2007). The heart begins to develop from the neural plate at around 3 weeks post conception, at first appearing like two long strands. The cords undergo a process known as canalization to become two hollow endocardial tubes which fold back on themselves and fuse to become a single tube. This becomes the endocardium. The tissue around the outside of the endocardial tube becomes thicker and eventually becomes the myocardium.
The single tube is essentially upside down at this stage, with the structures that will become the atria at the lower (caudal) end and the ventricular structures at the upper (cephalic) end. By 22 days post conception, the single cardiac tube starts to beat and blood moves from the bottom of the tube to the top. As the heart enlarges, it has to fold back on itself in order to be accommodated. As the tube folds from top to bottom, it twists round so the single atrium moves to the cephalic position and the single ventricle moves to the caudal position. Between the fourth to sixth week post conception, septation occurs and divides the atrium and ventricle into two. During the septation process, the foramen ovale is formed, enabling movement of blood between the atria.
The process of cardiac development is complex, must take place in a specific sequence over a very short period of time and is controlled by cardiac genes. Alterations in the genetic material can lead to failure in development or altered growth patterns, giving rise to congenital cardiac malformations.
The fetal circulation
The placenta provides the fetus with oxygen and nutrients and also disposes of waste products. In order to achieve this, the fetal circulation has a number of unique features, including temporary structures, to allow shunting of blood, allowing the mixing of oxygenated and deoxygenated blood, high pulmonary vascular resistance and a low systemic circulation (see Ch. 29 and website).
Transition to extrauterine life
At birth, the newborn infant is exposed to changes in temperature and tactile stimulation, which with the hypoxic and hypercapnic changes that take place as labour progresses, stimulates the first breath. This breath inflates the lungs and forces the fetal lung fluid out into the lymphatic system (Strang 1977). Pulmonary vascular resistance decreases dramatically and the pressure in the right side of the heart falls. Because gas exchange now occurs in the lungs, alveolar oxygenation concentration levels increase.
The dramatic fall in pulmonary vascular resistance, and the increase in oxygen concentration, facilitates the closure of the ductus arteriosus. Blood flows from the lungs to the left atrium, increasing the pressure in the left side of the heart and causing the flap-like opening of the foramen ovale to close. Blood then passes from the left atrium to the left ventricle and from there into the aorta. Clamping of the umbilical cord prevents blood flowing back into the placenta and this increases the systemic circulatory pressure. The reduced blood flow through the umbilical cord vessels causes constriction of the ductus venosus.
The changes in the temporary structures may take some time to become permanent. It is recommended that auscultation of the fetal heart to elicit cardiac murmurs should be delayed until at least 6 hours after birth to allow for the closure of the ductus arteriosus and the foramen ovale (Onuzo 2006).
The changes may also be reversed in adverse conditions – hypoxia can cause the ductus arteriosus to remain patent, particularly in preterm infants. Some infants are less likely to make a successful transition to extrauterine life. Preterm infants may experience difficulty in establishing adequate lung volume or oxygenation because of poor muscle tone or lack of surfactant. Babies born at term by elective caesarean section are more likely to encounter problems clearing fetal lung fluid because of the lack of stress response associated with labour, leading to the development of transient tachypnoea of the newborn (Morrison et al 1995).
Resuscitation of the newborn
Although hypoxia is a stimulus for the onset of breathing at birth, profound hypoxia can depress the respiratory centre in the brain and prevent or inhibit the successful transition to extrauterine life. Neonatal hypoxia may be characterized by the absence of breathing or by the presence of profound irregular gasping movements. It has been suggested that primary apnoea is caused by a period of acute hypoxia. During this period, breathing ceases. Initially the heart rate remains the same, but soon falls to about 60 beats per minute (bpm). If steps are not taken to correct the hypoxia, primitive spinal centres take over and produce deep, irregular agonal gasps. Eventually the lack of oxygen causes cessation of cardiac activity and the baby enters terminal apnoea. At birth it is not possible to tell which stage the baby has reached, so the approach to newborn resuscitation is the same in all these situations.
The need for resuscitation may be anticipated in certain situations (Box 45.1), but is not always predictable; therefore, all midwives must have skills in resuscitation of the newborn. ‘Fire drills’ and participation at courses, including the Newborn Life Support (NLS) course, are useful ways of maintaining skills in resuscitation of the newborn.
Equipment for newborn resuscitation (Box 45.2)
In an emergency, all that is required for newborn resuscitation is a flat surface and a pair of lungs to give mouth to mouth and nose breaths. However, midwives must be well prepared for resuscitation in home or the community (see website). In hospitals, community maternity units and birthing centres, a resuscitaire may be used as this provides a stable surface, warmth, and adequate lighting for effective resuscitation. The resuscitaire has suction and a source of oxygen – either piped or cylinder – and a pressure-limiting device so that the pressure used to deliver breaths can be measured and limited. Some resuscitaires incorporate a ventilator circuit so that a sick baby does not need to be moved to a separate system after being stabilized.
All equipment for neonatal resuscitation must be checked regularly and also rechecked immediately before use.
Personnel
When the need for complex resuscitation is anticipated, a senior member of the neonatal team should be summoned. In situations where the need for resuscitation is unexpected, help should be obtained as quickly as possible. In out-of-hospital situations, this may involve calling for an ambulance, general practitioner or neonatal emergency team, depending on local circumstances. It is always best to call for help early. If the situation has resolved by the time help arrives, they are very unlikely to complain if the baby has recovered.
Thermal control
Babies are born into an environment considerably cooler than the intrauterine environment, are wet and have a large surface area (see Chs 42, 44 and website). Prior to resuscitation, term babies should be dried and wrapped in warm towels to prevent heat loss, and their thermoregulation needs to be monitored during resuscitation.
Good practice, based on current evidence, for term babies with suspected birth asphyxia is to maintain their body temperature at 37°C.
Induced hypothermia as a possible method of reducing neurological damage in babies with birth asphyxia is currently under investigation, and results are awaited (Edwards & Azzopardi 2006).
Assessment
A baby who is blue and floppy with a heart rate above 100 bpm is likely to respond quickly to airway opening and minimal resuscitative measures. A baby who is pale, floppy, not breathing with a slow heart rate (<60 bpm) is likely to be seriously compromised and in need of urgent resuscitation. Depending on the assessment of the baby at this stage, it may be necessary to call for additional help.
It is good practice to note the time or start the clock when the baby is born. This provides a benchmark against which treatment can be measured.
Airway management
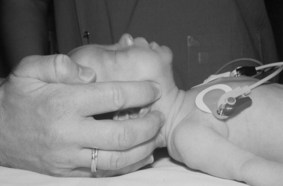
The baby is placed on his back on a flat surface. The prominent occiput tends to encourage the neck to flex and this can lead to airway obstruction. To correct this, the baby’s head is placed in the neutral position (Fig. 45.1). To help maintain this position, a small roll may be placed under the shoulders.
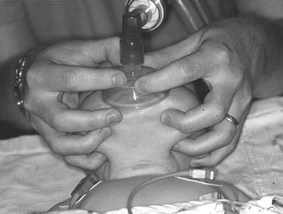
If the baby has poor tone, the tongue may fall back and block the airway. To overcome this, jaw thrust can be employed. This involves placing the fingers under the angle of the jaw to move it forward. This can be done with one hand or two (Fig. 45.2).
Alternatively, a Guedel airway can be inserted. The airway is measured from the middle of the lips to the angle of the jaw, and should be inserted with the curved surface upwards. Care must be taken to ensure that it goes over the tongue and does not force the tongue back. A laryngoscope or tongue depressor can help with this manoeuvre. At the same time, the airway can be examined for any blockages such as vernix or blood clots, which can be removed under direct vision with a large-bore suction catheter.
Once the airway is open, the situation is reassessed. If oxygen is entering the lungs, the heart rate may increase even before the chest moves. If there is no response to airway opening manoeuvres, then the next step is to provide inflation breaths.
Breathing
A face mask which covers the nose and mouth without leaving gaps is selected and attached to either a T-piece or 500 mL self-inflating bag. If using a T-piece, the pressure setting should be checked and the blow-off valve on the self-inflating bag should be checked to ensure it works. There is debate as to whether resuscitation of the newborn requires oxygen or if air can be just as effective. Current evidence suggests that oxygen is required for preterm babies but there is a lack of good-quality information in relation to term babies (Wang et al 2008). If oxygen is available, then it should be used. If a T-piece is used, the pressure limiter should be set to a maximum of 30 cmH2O initially.
The face mask is applied to the baby’s face and held firmly in place, ensuring that there are no leaks which would cause a reduction in the pressure being delivered. Five inflation breaths are then given. These ‘breaths’ are delivered by squeezing the self-inflating bag or occluding the T-piece for about 3 seconds. Following this, the baby is reassessed. It is very common for the heart rate to increase, even before chest movement is detected. If the chest does not move and the heart rate does not increase, then it should be assumed that the manoeuvre has been unsuccessful. The position of the baby’s head should be rechecked and the inflation breaths delivered again and the situation reassessed.
Once the chest has been seen to move or the heart rate has increased, ventilation breaths are used to sustain breathing. These breaths are shorter and faster – about 30 per minute – and the pressure can be reduced to about 20 cmH2O.
The baby should be reassessed every 30 seconds to ensure that resuscitation continues to be effective. If the baby has been in terminal apnoea, the first signs of spontaneous respiration may be gasping breaths. Ventilation breaths should continue to be delivered until regular breathing is sustained.
Circulation
Once the chest has been seen to move, the heart rate will normally increase. However, in some babies, the lack of oxygenated blood in the coronary circulation means that the cardiac muscle is unable to respond to the lung inflation and the circulation of oxygenated blood. In this case, cardiac compressions may help ‘bump start’ the heart.
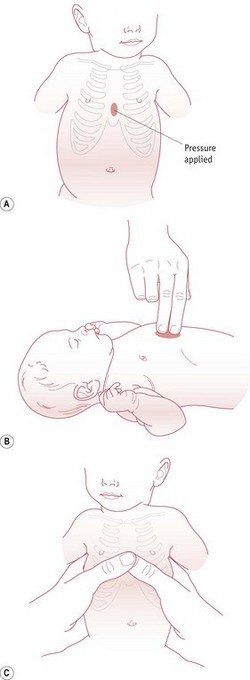
The most effective method of chest compressions is to encircle the chest with both hands, placing the thumbs just below the nipples on the sternum (David 1988). Alternatively, though less effective, a two finger technique can be used (Fig. 45.3). The chest is then compressed by about a third. Three chest compressions should be carried out for every ventilation breath. The heart rate should be assessed after 30 seconds. Once the heart rate is above 60 bpm, cardiac compressions can be stopped.
Drugs
If the heart rate fails to respond, despite the delivery of effective ventilation breaths and cardiac compressions, it may be necessary to consider administering drugs. In newborn babies, the umbilical vein can be cannulated easily using a feeding tube. This delivers drugs and fluids directly into the inferior vena cava via the ductus venosus. There is a significant amount of dead space in the umbilical venous catheter (up to 2 mL), so each drug administration should be followed by a 2 mL flush of normal saline to ensure that the drug reaches the circulation.
Resuscitating preterm babies
Preterm babies usually manage to breathe at birth and have a good heart rate, though face major challenges in maintaining their body temperature due to their comparatively large surface area. Hypothermia can cause hypoxia and hypoglycaemia and may interfere with surfactant production, leading to a worsening of respiratory distress syndrome (Gluck et al 1972, Stephenson et al 1970).
When a preterm birth is imminent, senior help should be available. All surfaces that the baby will touch should be warmed. At birth the baby is placed into a food-grade plastic bag up to their neck, without drying the skin, and the head should be covered with a hat. The baby should be intubated and exogenous surfactant delivered via the endotracheal tube (Verder et al 1999). Following stabilization, the baby can be transferred to the neonatal unit for ongoing care.
Post-resuscitation care
If resuscitation is prolonged, the baby’s temperature and blood glucose levels must be checked to ensure that they are within normal limits and, if not, appropriate treatment given. Most babies requiring resuscitation respond readily and can remain with the mother. If there are ongoing concerns, the baby may be transferred to a Transitional Care Unit or Neonatal Unit. The parents need accurate and up-to-date information about what is happening and care should be taken not to provide information that is unduly reassuring – especially if the baby’s condition does not warrant this. Documentation should be descriptive, not opinionated, noting the condition of the baby at birth and response to any interventions.
Discontinuing resuscitation
In some situations, the baby may not respond to resuscitation. The decision to stop resuscitation is usually taken by a senior member of the clinical team, after reviewing the situation, and in some units this might be the midwife. Protocols should be in place in community maternity units and birth centres to ensure that midwives can have access to expert advice in this situation.
Respiratory disorders in the newborn – principles of care
A normal term neonate has a respiratory rate of between 20 and 40 bpm. Breathing is effortless and quiet. Chest movement should be equal on both sides of the chest. The mucous membranes should be pink. The expected oxygen saturation level in a newborn baby is 95–99% (Lyon 2005).
Signs of respiratory compromise may include:
Any baby showing signs of respiratory compromise should be carefully observed and monitored after transfer to a neonatal unit. For midwives working in community maternity units and birth centres, it may be necessary to care for the baby for several hours until specialist assistance arrives. Oxygen saturation monitoring should be used if available. The baby’s respiratory rate should be monitored continuously, using either continuous monitoring or an apnoea monitor. In order to facilitate observation of the baby’s respiratory function and temperature, the baby should be nursed naked in an incubator.
If the baby has a rapid respiratory rate, breastfeeding or formula feeding may be difficult and may increase the risk of aspiration. Nasogastric feeding of small volumes of milk may be possible, though a full stomach pressing on the diaphragm may cause respiratory embarrassment. If this occurs, enteral feeds are withheld and intravenous fluids given by an umbilical venous cannula to maintain glycaemic control.
The parents will be very anxious about their baby, especially if separated. It is essential that they are given accurate information and kept fully informed about the baby’s condition. If the baby is to be transferred to a different hospital, the mother should be transferred to the same hospital as soon as possible.
Meconium aspiration syndrome (MAS)
Meconium-stained liquor is associated with fetal hypoxia and asphyxia, and is seen primarily in term or post-term infants. It is believed that when the fetus becomes hypoxic, the anal sphincter relaxes and meconium is released into the amniotic fluid.
When meconium-stained liquor was present at birth in the past, practices such as vigorous suction of the nose and mouth and thoracic pressure during delivery were used to prevent meconium aspiration. As the pathophysiology of MAS is more complex than this, these practices are now no longer used (see website).
Meconium has been found in the alveoli of stillborn infants, which suggests that aspiration may take place in utero (Brown & Gleicher 1981). It is believed that the hypoxic fetus starts to gasp in utero and this moves meconium-stained amniotic fluid into the fetal lungs. Fetal hypoxia is associated with a rise in pulmonary artery pressure and MAS is associated with an increased incidence of persistent pulmonary hypertension of the newborn (PPHN). In PPHN, the pressure in the pulmonary vasculature is elevated, causing the temporary fetal structures to remain partially open after birth, causing newborn hypoxaemia. The presence of meconium in the lung may cause a chemical pneumonitis which leads to inactivation of surfactant. Meconium is a viscous substance and may also cause obstruction of the airways.
Resuscitation when meconium is present in the liquor
A multicentre randomized controlled trial compared outcomes for babies who had their nose and mouth suctioned while the head was on the perineum with those who were born without suctioning. The data concluded that prophylactic suctioning conferred no benefits (Wiswell et al 2000). This study also compared routine intubation and suctioning for all infants born through meconium-stained liquor with a selective policy where the intervention was used only for babies who were not breathing at birth. The conclusion was that babies who breathe at birth do not require routine suctioning, and therefore this intervention should be reserved for babies with no respiratory effort.
If meconium-stained liquor is present at birth – particularly if it is thick, indicating a lack of liquor – expert assistance should be called so that the baby can be intubated, if necessary. If expert help is unavailable, the baby should be handled gently at birth, kept warm and assessed. If the baby is breathing and crying, then no further resuscitation is needed but the baby’s respiratory rate must be observed over the next 4–6 hours for signs of respiratory distress (see website).
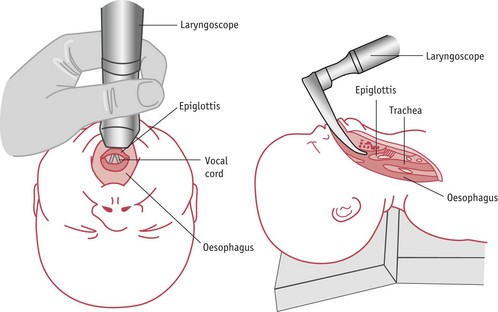
If the baby is unresponsive, a laryngoscope should be used to facilitate suction under direct vision (see Fig. 45.4). A large-bore catheter should be used to suck out particulate matter. Suctioning should stop when all visible meconium has been aspirated or the heart rate falls below 60 bpm. If the heart rate is slow, the baby’s head should be placed in the neutral position and inflation breaths given. Once the heart rate rises above 60 bpm, any remaining meconium may be removed with suction.
Aftercare
The majority of babies born through meconium will be well and will suffer no ill effects. It is advisable to undertake ‘meconium observations’ (assessing the baby’s colour, tone and respiratory rate) for up to 6 hours after birth (see website). Signs of illness in the baby include a rise in the respiratory rate (>60 bpm) and increasing cyanosis.
A baby with signs of increasing respiratory distress will need to be admitted to the neonatal unit for continuous observation. Babies with mild respiratory distress usually respond well to the administration of warmed humidified oxygen and intravenous antibiotics.
Babies with MAS who require prolonged resuscitation must be admitted to the neonatal unit for monitoring and treatment. Traditional treatments for MAS included intubation and ventilation using high concentrations of oxygen. However, the high pressures required to deliver the breaths (because of the high intrapulmonary pressures) can damage the lungs. Newer technologies for the management of MAS include the administration of exogenous surfactant. Because meconium inactivates surfactant, exogenous surfactant may reduce the need for more invasive treatments (Findlay et al 1996).
Nitric oxide is a gas which is delivered directly into the lungs via an endotracheal tube. It is a potent vasodilator and can reverse pulmonary hypertension, reducing hypoxaemia, and has been shown to be effective in treating some babies with MAS (Gupta et al 2002).
In the most severe cases of MAS, extracorporeal membrane oxygenation (ECMO) may be required (UK Collaborative ECMO Trial Group 1996). ECMO is a highly invasive form of treatment during which the blood is pumped out of the body, oxygenated in an oxygenator (essentially an artificial lung), and then the oxygenated blood returned to the baby (see website). Few centres in the UK provide this treatment, so a baby with MAS requiring ECMO may have to be transported many miles from the ‘home’ unit for treatment, resulting in separation from the parents.
Mortality and morbidity rates for babies with severe MAS are high. Mortality rates can be as high as 20% due to pulmonary complications. Some survivors of severe MAS will go on to develop chronic lung disease as a consequence of the treatment for respiratory failure. A few babies with severe MAS will have neurodevelopmental delay. It is believed that this happens as a result of cerebral damage in utero, rather than as a direct consequence of meconium inhalation (Greenough & Milner 2005).
Transient tachypnoea of the newborn (TTN)
Transient tachypnoea of the newborn (also known as ‘wet’ lung’) is believed to be due to a failure to clear fetal lung fluid during transition to extrauterine life. It almost always affects term babies and is more common in babies born by elective caesarean section. The baby presents with:
The clinical features of TTN are similar to those of pneumonia and the two conditions may coexist.
Pneumonia
This may be acquired congenitally as a result of contamination with infective agents during labour or birth, or late onset – acquired after 7 days. Acquired pneumonia is most commonly seen in babies who are already intubated. The most common causative organisms are Streptococcus A or Streptococcus B, Klebsiella and E. coli.
Clinical features are similar to those for TTN:
In severe cases, the Group B streptococcus is associated with rapid collapse in the newborn period.
Respiratory distress syndrome
Respiratory distress syndrome (RDS) is caused by a deficiency of surfactant (see Ch. 44). The severity of the condition is associated with gestational age: the lower the gestational age of the baby, the more severe the RDS. Although primarily a disease affecting the lungs, the condition impacts on all body systems as impaired oxygenation and pulmonary hypoperfusion affects blood pressure, thus inhibiting tissue oxygenation.
RDS presents soon after birth – usually within 4 hours, characterized by:
Administration of corticosteroids before birth reduces the incidence and severity of RDS (Ward 1994).
Exogenous surfactant is recommended following birth to stabilize the lungs (Verder et al 1999) and has been associated with:
Surfactant is given via an endotracheal tube at birth – and further doses according to the baby’s condition. Once stabilized, the baby may remain intubated with a ventilator delivering a mixture of air and oxygen directly using positive pressure to inflate the lungs. Alternatively, the baby may be extubated and placed on continuous positive airways pressure (CPAP) via nasal prongs. CPAP allows the baby to breathe on his own but maintains a low pressure (about 4–6 cmH2O) to prevent the lungs collapsing between breaths (Greenough & Milner 2005). This facilitates gas exchange and also reduces damage to the surfactant-producing alveolar cells.
Babies with severe RDS are nursed in an incubator and have continuous monitoring of oxygenation, blood pressure, heart rate and respiratory rate. Their vulnerable state means that rapid fluctuations in blood pressure, arterial oxygen and carbon dioxide levels can affect the brain and kidneys, so care is directed at ensuring stability and detecting and correcting any abnormality.
Very preterm babies may remain in the neonatal unit for many months (see Ch. 44). Some go on to develop chronic lung disease (CLD), sometimes known as bronchopulmonary dysplasia (see website).
Congenital abnormalities
The majority of congenital abnormalities affecting the respiratory system are usually detected antenatally. Decisions about the place of birth can be made well in advance and expert neonatal care can be available at birth. Congenital abnormalities that impact on the respiratory system include diaphragmatic hernia, tracheo-oesophageal fistulae (TOF), oesophageal atresia, and facial abnormalities, such as Pierre Robin syndrome. Depending on the severity of the condition, respiratory difficulty may present at birth or within a few hours of birth.
Diaphragmatic hernia
Diaphragmatic hernia can range from a small hole in the muscle to complete agenesis which allows the gut contents or liver to protrude through into the thoracic cavity. This reduces the space for lung development, thus the lungs are hypoplastic and, in extreme cases, are incapable of supporting respiration. Other congenital abnormalities may be present, including cardiac and renal abnormalities. The prognosis for the baby depends largely on the extent of the diaphragmatic defect and the coexisting abnormalities. Babies diagnosed early in the antenatal period tend to have a poor prognosis (Sebire et al 1997), which may be due to the severity of the defect. Babies presenting soon after birth are more likely to have a large defect and have a significantly higher morbidity rate. Infants who present 24 hours after birth are more likely to survive.
The baby may present with increasing tachypnoea and cyanosis. Breath sounds may be reduced on the side of the hernia as the lungs are displaced by the viscera. The abdomen may have a flat appearance as the intestinal contents protrude into the thorax (Davenport 2005). Diagnosis can be confirmed by X-ray or ultrasound, which shows the presence of viscera in the thoracic cavity.
If the defect is diagnosed antenatally, arrangements should be made for the birth to take place in a maternity unit with a neonatal service capable of providing specialist care for the baby. Usually the baby will be electively intubated at birth. The extended use of a bag and mask can cause intestinal distension. A large-bore nasogastric tube should be passed and left on free drainage to decompress the bowel. The baby is then ventilated and stabilized prior to surgery to repair the defect. Delayed surgery is associated with an enhanced survival rate. The whole process is extremely worrying for the parents and intensive support from staff is required.
Tracheo-oesophageal fistula (TOF) and oesophageal atresia
Tracheo-oesophageal atresia and fistulae are thought to occur because of abnormal separation of the primitive trachea and oesophagus in the first trimester (see Ch. 48). Oesophageal atresia may exist on its own or coexist alongside a fistula which communicates with the trachea. The defects are also associated with chromosomal abnormalities, especially trisomy 13 or 18, and may be detected antenatally on ultrasound. Oesophageal atresia is associated with maternal polyhydramnios. If diagnosed in the antenatal period, arrangements are made for the birth to take place in a maternity unit with access to a neonatal surgical unit. There may be other abnormalities present, and typically these include lower gastrointestinal abnormalities, such as duodenal atresia or anorectal abnormalities.
At birth the baby may present with frothy saliva dribbling from the mouth and may appear to choke or have frequent colour changes. In some cases, the baby may aspirate the secretions, which causes a form of respiratory distress. The initial management is to keep the airway clear. The baby should be nursed in a slightly head-up position. A Replogle tube (see website) has a double lumen and this is placed in the pouch at the blind end of the atresia and kept on continuous low-pressure suction. This is usually sufficient to keep the baby’s airway clear. Intravenous fluids are required to maintain the baby’s blood glucose level within normal limits and to maintain fluid balance prior to surgery.
If birth occurs in the birth centre or at home, the baby should be kept warm and supported in a slight head-up position. Suction can be gently applied to the mouth to keep the airway free of secretions until transfer to a neonatal unit. If there is likely to be a delay of several hours, then an umbilical venous catheter should be inserted and 10% dextrose given to maintain hydration and blood glucose levels.
Surgery is usually performed soon after birth to correct the defect. In a few cases, a wide gap between the oesophageal segments may delay surgery, to enable the two pouches to elongate. If this happens, the baby will have a gastrostomy and be fed directly into the stomach until surgery to repair the defect (Stringer et al 2005).
Cardiac abnormalities
The cardiovascular system is the first of the body’s systems to develop and function. It is believed that the rapid development which takes place in this system makes it susceptible to teratogens. Congenital heart defects (CHD) account for about 30% of all reported congenital anomalies. Causes include the following:
Accurate antenatal history taking is fundamental in identifying CHD. Women who report a family history of CHD or have diabetes mellitus should be advised of the increased risk of CHD to the fetus and referred for counselling and specialized screening. General anomaly screening with a four-chamber view of the heart at 18 weeks’ gestation will identify less than 50% of affected fetuses (Wyllie et al 1994). Cardiac abnormalities detected at this time are more likely to be complex and have poor outcomes. Detailed transvaginal and transabdominal ultrasound can detect cardiac abnormalities at 13–14 weeks’ gestation, providing sufficient information to make accurate interpretations of the images in women with a high risk of having a fetus with CHD (Bronshtein & Zimmer 2002) (see website).
Some cardiac abnormalities may be detected in the early postnatal period when carrying out routine examinations. The family history should be reviewed and checked with the mother to ensure that nothing has been overlooked. Dysmorphic features may lead the examiner to suspect a chromosomal abnormality: in these cases, careful evaluation of the cardiovascular system should be carried out. The femoral pulses should be palpable. Absent or weak pulses are suspicious and babies in whom pulses cannot be felt should be referred for further examination.
Some babies with CHD may present with cyanosis, though this can be difficult to detect as babies may have ‘dusky’ episodes that pass without any ill effect. Prolonged ‘duskiness’ is abnormal and should be investigated. Other signs of CHD are less specific, including:
Heart murmurs are quite common in babies as a result of the transition to extrauterine life. In the absence of any other features, the baby may be followed up after a period of 4–6 weeks to assess the possible causes of the murmur.
Many cardiac lesions present after the baby and the parents have been transferred from the care of the maternity services to the primary health care team. Parents should be advised of the importance of continuing to assess their baby’s wellbeing and reporting the non-specific features of CHD to their GP or health visitor if they are concerned.
Investigations
If CHD is suspected, blood pressure should be checked in the upper limbs and one leg. A difference of 20 mmHg in the systolic BP between the arms and leg is suggestive of CHD – coarctation of the aorta in particular (Archer 2005). However, the absence of a gradient does not confirm normality and babies who are unwell must continue to be observed closely and referred for further screening.
In some cases, a hyperoxia test can be carried out to differentiate between cyanosis that is respiratory in origin and that which is cardiac (see website).
Chest X-rays and electrocardiograms can provide information about the heart in terms of size and function. However, transthoracic echocardiography is more effective in diagnosing the type and extent of the cardiac defect (Archer 2005). Doppler ultrasound can assess blood velocity and can assist in identifying shunts and stenosis – areas of narrowing in the cardiac pathway.
Treatment
The treatment of a baby with CHD depends on the type of defect and their general condition. Cardiac catheterization can be used for interventions such as widening stenosed vessels. Surgery may take place in stages. In the initial stage, treatment is palliative and designed to alleviate symptoms. Later, as the baby grows, corrective surgery may be carried out. In complex cases, several operations may be required.
Depending on the results of the initial investigations, the baby may be referred to a specialist centre for further investigation and treatment. In some cases, even when a defect is diagnosed, it is possible for the baby to remain in the care of the parents and be referred for treatment at a later date. Whether the baby is transferred to a specialist centre or discharged, the parents need accurate information about their baby and what the future might hold. In particular, the parents of the baby who has a delayed referral require information about the clinical features of deterioration and how they should obtain assistance if they are concerned about their baby’s condition.
Forms of congenital heart defects
Congenital heart disease is commonly divided up into two groups – cyanotic and acyanotic, which can be confusing because not all babies with cyanotic heart disease will be cyanosed initially.
Acyanotic heart defects
These include patent ductus arteriosus, ventricular and atrial septal defects, pulmonary stenosis, aortic stenosis and coarctation of the aorta.
Patent ductus arteriosus (PDA)
The ductus arteriosus is a temporary structure which exists to divert blood away from the lungs to the aorta in the fetal circulation (see Chs 29 and 41). In term babies, the ductus arteriosus normally closes within 12–24 hours of birth in response to the circulating high partial pressure of oxygen and reduction in circulating maternal prostaglandins. Preterm babies are more likely to experience periods of hypoxia and an increase in circulating prostaglandins, which makes the ductus arteriosus more likely to remain open.
Deoxygenated blood from the pulmonary arteries shunts through the ductus arteriosus into the aorta, bypassing the lungs. Preterm babies are likely to show decreasing levels of oxygenation and worsening of RDS. Term babies may be reluctant to feed and have poor growth patterns. The baby may have tachypnoea and tachycardia. On auscultation of the heart, a murmur may be heard.
Atrial septal defect (ASD)
This defect allows communication between the left and right side of the heart with mixing of oxygenated and deoxygenated blood. A simple ASD is a hole in the atrial septum and is rarely symptomatic. Closure is best performed before the onset of pulmonary hypertension. In many cases this is when the child is around 5 years of age. A complex ASD (often associated with an underlying chromosomal disorder) involves other structures, such as the mitral valve or the ventricular septum and tricuspid valve. Surgery is more complicated and there is a higher mortality rate associated with this condition.
Ventricular septal defect (VSD)
Ventricular septal defects are the most commonly occurring cardiac defect, and may occur on their own or as part of a complex heart defect. The septal defect allows mixing of blood between the two ventricles. Typically, blood from the left ventricle passes through to the right ventricle during systole. The blood is then recirculated. The flow of blood from the left to the right side of the heart can lead to elevated right ventricular pressure and pulmonary hypertension. This occurs if the defect is large. The baby may show signs of respiratory distress and cyanosis, as well as failure to thrive. Babies with a small defect may be asymptomatic. Surgical correction of the defect may be carried out. Small defects may close on their own.
Coarctation of the aorta
Coarctation of the aorta is narrowing of the aorta at the point where the ductus arteriosus joins, and may occur on its own or as part of a complex CHD. Mild forms of the defect may be undetectable and there may be no symptoms until the child is older. In more severe cases, femoral pulses may be weak or absent. Depending on the position of the coarctation, rapid collapse may follow closure of the ductus arteriosus.
Treatment options include the insertion of a stent or angioplasty to relieve the stenosis. Alternatively, surgery may be performed to remove the stenosed part of the aorta or a patch inserted to make the narrow section of the aorta wider.
Pulmonary stenosis
Pulmonary stenosis occurs when the pulmonary valve becomes narrowed. This causes an obstruction to blood leaving the right ventricle and can lead to a reduction in blood going to the lungs. The stenosis may be relieved by surgery.
Aortic stenosis
Aortic stenosis is a narrowing of the valve leading from the left ventricle into the aorta, which usually occurs alongside other heart defects. In simple cases, the baby will be asymptomatic and there may be no sign other than a cardiac murmur. In severe cases, the baby will collapse suddenly and require urgent surgery to relieve the stenosis.
Cyanotic defects
This group of defects includes complex conditions such as transposition of the great arteries, tetralogy of Fallot and hypoplastic left heart syndrome.
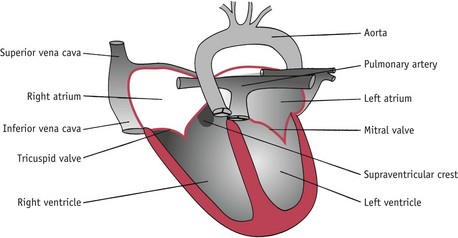
Transposition of the great arteries (TGA)
In this condition, the major heart arteries are transposed. The aorta arises from the right ventricle and the pulmonary artery arises from the left ventricle (Fig. 45.5). TGA is usually associated with other cardiac defects such as VSD. These babies usually present within the first few hours of life – especially if there is no shunt. Cyanosis may be marked and accompanied by tachypnoea and tachycardia. Initial treatment involves an infusion of prostaglandins to open the ductus arteriosus. The foramen ovale may be surgically enlarged so that well-oxygenated blood can flow from the left ventricle into the right ventricle. Surgery to correct the defect is an arterial switch procedure and is performed within 3 weeks of birth. Survival rates for surgery are around 90%.
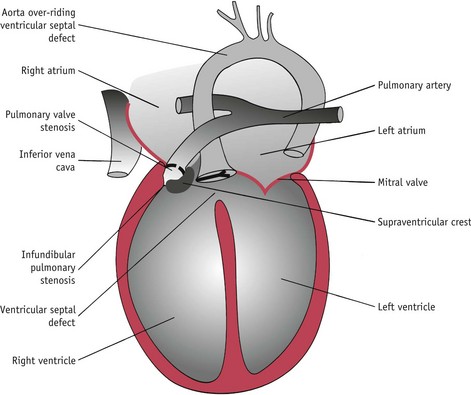
Tetralogy of Fallot
This condition comprises four abnormalities (Fig. 45.6):
The baby develops a right-to-left shunt. There is mixing of oxygenated and deoxygenated blood at the level of the ventricles and blood flows preferentially through the aorta, rather than the pulmonary arteries, because the pressure is lower in the larger vessel. Cyanosis may be present soon after birth or may develop in the first year of life. The baby may exhibit poor growth and failure to thrive and become breathless when engaged in any activity. The condition is treated by surgery.
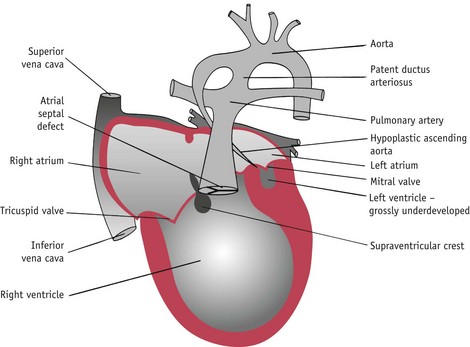
Hypoplastic left heart syndrome
In this condition the left side of the heart is underdeveloped (hypoplastic) (Fig. 45.7). There is atresia of the mitral and aortic valves. The left ventricle and the aorta are underdeveloped. In contrast, the right side of the heart is hypertrophied and the pulmonary artery is enlarged.
Blood passes through the ductus arteriosus into the aorta. When the ductus arteriosus closes, death follows soon afterwards. A prostaglandin infusion can be used to keep the ductus arteriosus open. Palliative surgery can be carried out so that the right ventricle is used to supply the systemic circulation. Ultimately, heart transplantation may be carried out to correct the problem.
Parental care
Congenital cardiac defects present major challenges for families. The baby may be acutely unwell and need urgent treatment in a specialist centre located many miles away from home. Even after initial treatment, the baby may require many years of follow-up with an uncertain prognosis. Families who have babies with less complex cardiac defects may find themselves coping with feelings of uncertainty as they await treatment. Babies with CHD may be difficult to feed and may exhibit poor growth, causing further concern for the parents. Health professionals working with parents should ensure that they receive accurate, consistent information. Parents of babies with CHD may find it helpful to be given contact details of specialist support groups and voluntary organizations.
Conclusion
The move towards midwife-led care means that midwives have taken on a greater responsibility for screening and detecting cardiac and respiratory disorders in the antenatal and postnatal periods. A knowledgeable and skilled midwife is able to facilitate an effective partnership with parents; the midwife should be able to provide information and support to enable them to confirm normality in their newborn infant and to have the confidence to seek support and help if they are concerned about their baby’s wellbeing.