Chapter 46 Neonatal jaundice
Introduction
Neonatal jaundice is common and occurs physiologically in a significant number of healthy full-term babies. Midwifery practice challenges include:
A significant number of babies with acute bilirubin encephalopathy and subsequent kernicterus are being reported (Manning et al 2007). This may further increase as mothers and babies are discharged home earlier before feeding is established, especially if community services are not in place to support a policy of early discharge (Sellwood & Huertas-Ceballos 2008).
Understanding the normal physiology of bilirubin metabolism allows recognition of why jaundice is so common in newborn babies and explains the mechanism of jaundice in many diseases. A basic knowledge of the rare genetic diseases may also allow a greater depth of understanding of normal physiology and these will be mentioned where appropriate.
Physiology
The majority of bilirubin forms from the breakdown of heme, an iron-containing molecule, an essential component of cytochromes, myoglobin and haemoglobin. At the end of their lifespan, red blood cells are sequestered by the spleen and the haemoglobin is broken down into component parts of heme and globin. The iron molecule is then removed from the heme to be recycled and the heme molecule is oxidized to biliverdin, which is then reduced to form unconjugated bilirubin (Dennery et al 2001). Increased red cell breakdown leads to increased levels of unconjugated bilirubin. Bilirubin is a lipid-soluble molecule that easily crosses lipid membranes, such as those within the brain. The insolubility in water means that bilirubin must be transported in the bloodstream linked to albumin. In this protein-bound state, bilirubin is not available to be filtered by the glomerulus or to enter into cell tissues (see website).
In the liver, unconjugated bilirubin is transported into the cells and converted into bilirubin diglucuronide (conjugated bilirubin) by the enzyme UDP glucuronosyltransferase. Conjugated bilirubin is water-soluble and actively excreted by the liver cells into the intrahepatic bile ducts with bile salts, cholesterol and phospholipids. This bile then flows down the extrahepatic ducts and into the small intestine. Within the colon some of the conjugated bilirubin is hydrolysed back to unconjugated bilirubin while the remaining is metabolized into stercobilinogen and urobilinogen. Stercobilinogen is a brown pigment that is excreted within the faeces. Urobilinogen is reabsorbed in the enterohepatic circulation to be converted back to unconjugated bilirubin. A small amount of urobilinogen is carried in the bloodstream and excreted by the kidneys.
Physiological jaundice
Physiological jaundice is jaundice occurring as a consequence of the changeover from intrauterine to extrauterine life. The fetus possesses a large number of red blood cells that contain fetal haemoglobin which facilitates diffusion of oxygen from placental to fetal circulation. The newborn baby begins life with 6–7 million red cells per cubic millimeter, which need to reduce to the adult level of 5 million/mm3. Fetal haemoglobin needs to be replaced by adult haemoglobin, resulting in an increase in red cell breakdown and an increased bilirubin load on the immature liver. Additionally, intestinal transit is slow until enteral feeds have been established, leading to increased reabsorption of urobilinogen via the enterohepatic circulation.
Features of physiological jaundice
Supplementing breastfeeding with water or glucose appears to have no effect on bilirubin levels in healthy newborns (Nicoll et al 1982) and should be avoided. Physiological jaundice may be exacerbated in situations that lead to increased bilirubin production (for example, polycythaemia, bruising) or decreased bilirubin excretion (poor feeding with delayed intestinal transit). In the past it was believed that physiological jaundice could not lead to kernicterus but unfortunately this may not be the case and vigilance is crucial.
Evaluation of jaundice
The practitioner needs as much information as possible to accurately assess the risk of the baby developing jaundice, its significance and management plan. The maternal blood group should be known as well as any familial predisposition to neonatal jaundice. Risk factors include infection during pregnancy and delivery, and any bruising or perinatal trauma to the baby. Babies at high risk of developing jaundice may need to remain under hospital care for longer and research continues into accurately identifying babies at risk (Sanpavat et al 2005).
It is important to assess the behaviour and feeding pattern of the baby – an alert baby feeding well is less of a concern than one who is sleepy and uninterested in feeding. Urine and bowel activity are also strong indicators of wellbeing.
Jaundice progresses from head to toe and resolves in the opposite direction, from toe to head, therefore observation of the colour of the sclera is not useful in assessing improvement. Clinical assessment of severity by experienced practitioners can be highly accurate (Riskin et al 2003), though caution is required in babies with dark skin where visual estimation alone may lead to error (AAP 2004, NICE 2010).
Clinical evaluation of jaundice requires that the baby is undressed, and cephalocaudal progression of jaundice evaluated using the five zones as in Figure 46.1. The Kramer (1969) tool facilitates assessment of the advancement of dermal icterus, and if the baby rapidly advances down the scale, swift referral to the neonatologist for diagnosis and treatment can be achieved. Jaundice becomes clinically apparent when the serum bilirubin rises above 85 µmol/L (bilirubin can be measured as a concentration recorded in µmol/L or mg/dL). Any baby with significant jaundice at 48 hours should have a further assessment by a health professional. Transcutaneous bilirubinometer devices have been developed that correlate well with serum bilirubin measurements, which may reduce the number of blood tests required (Briscoe et al 2002, NICE 2010, Rubaltelli et al 2001, Wong et al 2002) (Box 46.1; also see website). These devices should be used in infants over 35 weeks’ gestation and a postnatal age of greater than 24 hours, and a reading greater than 250 µmol/L is an indication for serum bilirubin testing.
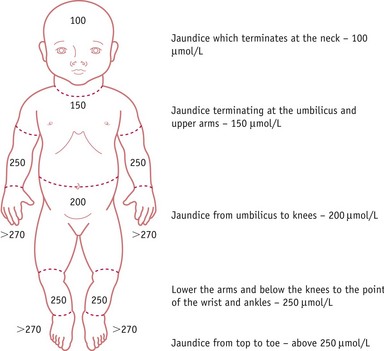
Figure 46.1 The Kramer tool. Estimates of serum bilirubin (mmol/L) are obtained by assessing distal progression of jaundice.
(Adapted from Kramer, 1969.)
Box 46.1
Taking blood for serum bilirubin measurement – preparation
In order to take a heel specimen correctly, the baby’s foot needs to be lower than the body and the heel needs to be warm, and this can be achieved by use of a gauze swab soaked in warm water wrapped around the heel and secured with cling film or a gel-based heel warmer. Both should be tested against the inner aspect of the practitioner’s arm.
Records
To facilitate effective management, bilirubin results should be sequentially recorded. Graphs are available for preterm and term babies, allowing bilirubin levels to be plotted against the time the blood sample was taken, enabling the measurement to be plotted against the baby’s age in hours (AAP 2004). This helps inform the practitioner at which level phototherapy should be commenced and when exchange transfusion may be indicated. This is crucial for babies with an unconjugated hyperbilirubinaemia. In these babies, a urine dipstick will also show a positive urobilinogen (secondary to normal enterohepatic circulation of urobilinogen) and a negative bilirubin result (conjugated bilirubin will have been excreted via the liver and bowel). The National Institute for Health and Clinical Excellence (NICE) has also developed the Bili-Wheel, which measures the bilirubin level alongside the gestation to highlight interventions required (NICE 2010) (see website).
Unconjugated hyperbilirubinaemia
Unconjugated hyperbilirubinaemia has three main causes:
Increased red cell breakdown
This occurs most commonly in the neonate because of infection, bruising (for example, after ventouse or forceps delivery), polycythaemia or haemolytic disease of the newborn, or, more rarely, following localized haemorrhage or thrombosis.
Haemolytic disease of the newborn
Haemolytic disease of the newborn is the immune-mediated red cell breakdown which occurs in rhesus disease and ABO incompatibility (not to be confused with haemorrhagic disease of the newborn – vitamin K deficiency). In haemolytic disease of the newborn, the maternal immune system has been ‘immunized’ against aspects of the baby’s blood group (see Fig. 46.2). This ‘immunization’ usually occurs because of a previous pregnancy, miscarriage, or following blood transfusion when fetal blood cells have passed to the maternal circulation.
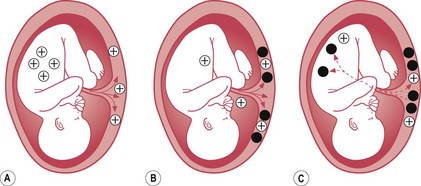
Figure 46.2 Antibody formation. A. The crosses represent fetal blood cells crossing over into the maternal circulation. B. An antibody response (black circles) is mounted by the mother. C. The antibodies cross into the fetal circulation where they will break down the fetal blood cells.
Rhesus factor is the rhesus C, D and E antigens expressed on red blood cells. It is the D antigen that is most likely to cause isoimmunization. An individual who is ‘rhesus negative’ does not express the D antigen and has the genotype dd. An individual who is ‘rhesus positive’ does express the D antigen and can be heterozygous (Dd) or homozygous (DD) for the rhesus antigen D genes. Isoimmunization in a rhesus-negative mother can occur with a heterozygous or homozygous fetus or baby and the risk of this occurring can be predicted from a knowledge of the father’s genotype as shown in Figure 46.3.
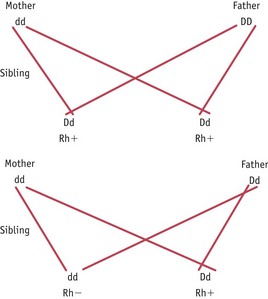
Figure 46.3 Inheritance of rhesus factor. D represents rhesus positivity, which is a dominant trait, and d represents rhesus negativity. In this instance the infant that has Dd will be rhesus positive and may result in the rhesus negative mother mounting an immune reponse.
Rhesus isoimmunization can cause a severe haemolysis that may result in fetal anaemia and a need for intrauterine blood transfusions to prevent the development of hydrops fetalis.
In order to prevent isoimmunization, anti-D immunoglobulin is administered to women at risk, antenatally and/or postnatally forming complexes with the fetal red cells to prevent the women’s immune system from mounting its own immune response. Current advice from NICE is to routinely administer anti-D prophylaxis to all rhesus-negative women antenatally at least once at 28 weeks’ gestation (exact regimen depends on dose used) (NICE 2008). It can be administered at times when the women are at increased risk of isoimmunization, such as after miscarriage or after the birth of a rhesus-positive baby. Women and babies at risk can be identified by taking cord and maternal blood after delivery to determine the baby’s blood group, and measure the presence of fetal blood cells and antibodies in the maternal system, as in Box 46.2.
Box 46.2
Coombs and Kleihauer tests
Anti-D is a blood product; therefore, prior to administration, informed consent must be obtained. The anti-D must be prescribed on the woman’s drug chart by a medical practitioner (NMC 2008).
Severe haemolysis is less common with ABO incompatibility than with rhesus D incompatibility but the principle of isoimmunization is the same. The ABO blood group refers to the pattern of expression of the A and B antigens on the red blood cells as shown below:
A mother of blood group O may develop antibodies against the A and B antigens, a mother with blood group A against the B antigen, and a mother of blood group B against the A antigen. These last two examples are extremely rare and ABO incompatibility is most common in a mother who has blood group O with a fetus that has blood group A or B.
ABO incompatibility usually manifests at less than 36 hours of age, although it may not become obvious until after 48 hours. A history of mother’s blood group being O positive should alert the midwife as to this possibility.
A baby diagnosed and treated for ABO incompatibility needs to be closely observed for signs of ‘late’ anaemia, which may occur due to ongoing haemolysis by antibodies that may persist in the baby’s circulation for several weeks. Symptoms include lethargy, pallor and poor feeding history. Folate and iron may be prescribed to encourage red blood cell production in the bone marrow but it is not unusual for a baby to develop a severe anaemia requiring blood transfusion. Continuity of care by the midwife providing care up to 28 days can be very helpful.
Failure of conjugation
The ability to conjugate bilirubin varies in individuals depending on differing levels of the enzyme UDP glucuronosyltransferase (secondary to individual variation in gene expression) present in the liver, and immaturity of the newborn’s enzyme levels.
Prolonged unconjugated hyperbilirubinaemia may occur in some breastfed babies as the enzyme may also be inhibited by breast milk, as illustrated by a study of Taiwanese babies (Huang et al 2004). There is no specific test for breast milk jaundice and diagnosis is made when all other causes have been excluded. Management is dependent on the baby’s condition: usually the baby is active and feeds well but jaundice is prolonged and can take up to 6 weeks to resolve.
If the baby is well and all other causes have been excluded, the management is to encourage frequent breastfeeds as ‘breastfeeding jaundice’ rarely requires phototherapy treatment. Previously, practitioners have advised the discontinuation of breastfeeding in order to confirm the diagnosis, but this can provide negative feedback to the mother regarding her ability to feed and nurture her baby, so should be avoided.
Increased enterohepatic circulation
Delayed intestinal transit will increase the enterohepatic circulation of bilirubin and lead to an increased level of unconjugated bilirubin. In a normal newborn baby, intestinal peristalsis develops over the first few days as enteral feeds are established and a delay in establishing feeds can exacerbate jaundice. The most common medical reason for delayed intestinal transit is congenital hypothyroidism. Babies diagnosed with congenital hypothyroidism must be commenced on thyroxine as soon as the diagnosis has been confirmed, to minimize the complication of neurodevelopmental delay. Babies are normally screened for this by measuring thyroid-stimulating hormone (TSH) on the Guthrie card.
The enzymes in colostrum encourage the passage of meconium (see Ch. 43), and the midwife can do much to support the woman in successfully breastfeeding, thereby ensuring that the baby receives colostrum and avoids dehydration. The midwife also notes the passage of meconium as an important part of the baby’s progress and wellbeing.
Complications of unconjugated hyperbilirubinaemia
Kernicterus
Kernicterus describes the yellow staining of the basal ganglia – usually seen on autopsy in babies who have had severe jaundice (Hachiya & Hayashi 2008). It also describes the chronic long-term clinical effects of a severe hyperbilirubinaemia (AAP 2004).
The risk of kernicterus is influenced by factors including the rate of rise of the bilirubin level as well as its maximum level. Other factors that increase an individual’s susceptibility are:
There are also likely to be individual genetic factors that affect the risk of jaundice as well as sensitivity to hyperbilirubinaemia, some of which have been described above (Hansen 2000a). Kernicterus is rare in Europe and the United States (Dodd 1993, Newman & Maisels 1992) but may be increasing in incidence (Manning et al 2007). A further increase may parallel the less aggressive management of jaundice and earlier discharge into the community of newborn babies, especially those born at less than 37 weeks’ gestation.
Consequences of a severe hyperbilirubinaemia can be acute or long-term complications. Acute symptoms may be reversible and without long-term complications if appropriately managed (Harris et al 2001) (Table 46.1). Up to 15% of babies who develop long-term complications may be asymptomatic in the acute stage. (See website.)
Table 46.1 Symptoms of acute bilirubin encephalopathy
| Age | Acute symptoms |
|---|---|
| 1–2 days | Poor suck, hypotonia, stupor, seizures |
| 3–7 days | Increased tone in extensor muscles, opisthotonos, fever |
| >1 week | Hypertonia |
Management of unconjugated hyperbilirubinaemia
The recent trend towards home deliveries and early hospital discharge has been associated with increased readmission for jaundice and an increased reporting of babies with kernicterus. In some cases, high-risk babies can be identified (Box 46.3) and their discharge delayed. Undertaking a predischarge newborn bilirubin screening programme has been shown to reduce the rate of readmissions to hospital with significant jaundice (Eggert et al 2006). Bilitool is a web-based tool (www.bilitool.org) which facilitates this prediction of risk and allows evidence-based planning of follow-up and further bilirubin measurement (Bhutani 1999, Longhurst et al 2004). In the community there should be active breastfeeding support and careful monitoring of the jaundice by parents and community staff with early hospital review where necessary (Bhutani & Johnson 2000). Transcutaneous bilirubinometers to screen for significant hyperbilirubinaemia should also be encouraged (Briscoe et al 2002, Engle et al 2005).
Box 46.3
Risk factors for developing significant unconjugated jaundice post discharge
Initial assessment establishes the general condition and the most appropriate investigations and management that the baby requires. Supportive measures may include the administration of antibiotics and the correction of dehydration. These may be required in the management of unconjugated hyperbilirubinaemia as meningitis and septicaemia are both more common during the first month of life than at any other time during childhood, and dehydration commonly exacerbates jaundice. It may be necessary to correct acid–base defects and hypoxia since both may increase the risk of kernicterus and the administration of albumin may also reduce the amount of free bilirubin available to cross the blood–brain barrier (Ahlfors 1994).
Specific treatments for unconjugated hyperbilirubinaemia are phototherapy and exchange transfusion. The exact level of bilirubin that causes bilirubin encephalopathy and kernicterus and at what stage these treatments should be commenced remain contentious. Bilirubin encephalopathy is rare in full-term babies without underlying pathology but relaxation of treatment guidelines has led to an increase in the number of babies developing this complication (Hansen 2000b, Manning et al 2007). The aim of treatment is to identify those babies at specific risk (Bhutani & Johnson 2000) and to prevent kernicterus without exposing many more babies to unnecessary or potentially harmful treatment.
The NICE guidelines (2010) and the American Academy of Pediatrics, updating its 2001 guidelines, suggest that babies should be considered within the following groups when assessing the need for phototherapy, and the Bilitool™ uses these risk groups (AAP 2004, www.Bilitool.org):
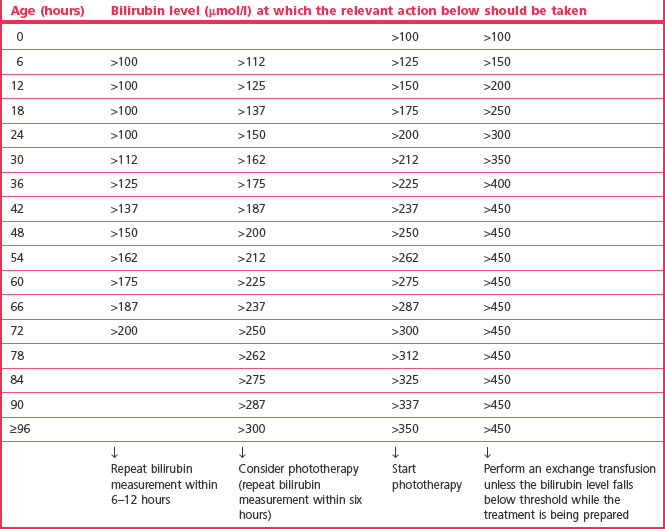
Risk factors include G6PD deficiency, evidence of hypoxia or ischaemia, haemolytic disease, infection, lethargy, and temperature instability. Table 46.2 can still be considered as a guide for babies >38 weeks but local policies and recent evidence should be consulted.
Table 46.2 Consensus-based bilirubin thresholds for management of babies 38 weeks or more gestational age with hyperbilirubinaemia (NICE 2010)
Phototherapy
The effect of light on the excretion of bilirubin has been known since the 1950s (Cremer et al 1958). Phototherapy is an artificial method that provides light of a specific wavelength to enhance bilirubin excretion. Fat-soluble unconjugated bilirubin is mainly converted into water-soluble lumirubin that can be excreted through the kidneys. The most effective light spectrum for converting the yellow bilirubin pigment to the photoisomer lumirubin is blue light and the wavelength of blue light is in the 425–475 nm range.
Other factors affecting the effectiveness of phototherapy are:
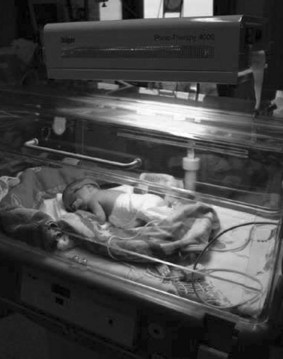
There are a number of ways of delivering phototherapy, including conventional phototherapy (Fig. 46.4), 360 degree units, blue light units and fibreoptic biliblanket (Figs 46.5 and 46.6) (not used as first line in term babies).


Figure 46.5 The Ohmeda BiliBlanket. A. Light Source. B. The Biliblanket. C. The new Bilisoft LED phototherapy system.
(Courtesy of GE Healthcare Clinical Systems UK Ltd.)

Figure 46.6 The Medela BiliBed. A. Phototherapy source, light-permeable support and baby in therapy blanket. B. Baby in crib with unit in position.
(Courtesy of Medela AG.)
Double phototherapy is the combination of two phototherapy units (BiliBlanket and overhead or two overheads) placed at different positions above and/or below the infant. This can significantly increase the excretion of bilirubin (Holtrop et al 1992). There are now phototherapy units that can deliver 360° of phototherapy by completely surrounding the infant within a tunnel of light.
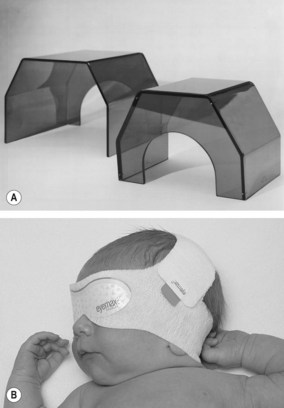
Overhead units have incorporated or attached UV light filters designed to protect the infant from harmful rays. Manufacturer guidelines for use of different phototherapy units (such as distance of the overhead from the infant) may vary. There have been reports of babies suffering UV burns as a direct consequence of phototherapy.
The overhead phototherapy unit requires consideration of the following aspects of care:
Once phototherapy has commenced, it is difficult to assess the degree of jaundice by looking at the skin, and serum bilirubin should initially be measured after 4–6 hours and then 6–12 hourly once stable or falling (NICE 2010). After stopping phototherapy, it is important to check the serum bilirubin at 12 hours (NICE) to monitor for significant rebound in hyperbilirubinaemia which may require further treatment.
Exchange transfusion
Exchange transfusion involves removing the baby’s blood, with maternal antibodies and bilirubin within it, and replacing it with fresh, rhesus-negative blood. During this procedure, up to 90% of the blood may be replaced. This was the first treatment to be successfully used for severe neonatal jaundice. It is particularly useful in haemolytic disease, as both red blood cells and red cell antibodies causing their breakdown are removed from the neonatal circulation. Removal of the antibodies prevents late-onset anaemia.
The main indications for exchange transfusion are:
The exact level of hyperbilirubinaemia at which exchange transfusion should be performed remains difficult to define. It is not recommended that the ratio of bilirubin to albumin should influence the decision (NICE 2010). Phototherapy should be continued throughout the procedure (AAP 2004, Hulzebos et al 2008).
In most cases, a two-volume exchange is performed: 160 mL/kg of the circulating blood volume is removed and replaced with transfused whole blood (commonly packed red cells and 0.9% saline or 4.5% albumin as whole blood is difficult to obtain). The procedure must be conducted slowly under strict aseptic conditions with detailed recording of the amount of blood removed and amount infused. Ideally two practitioners should undertake this in order to maintain high safety standards. There are two methods: single site or two site.
The preferred two-site method involves aspirating blood from a peripheral or umbilical artery at a similar rate to the infusion rate of a transfusion that is being delivered through a peripheral vein.
The single-site method involves cannulation of the umbilical vein with aspiration of 5–10 mL of blood through a three-way tap followed by infusion of the same quantity of donor blood. This can result in significant changes in central venous pressure and intravascular volume and is also associated with the complications of umbilical venous cannulation.
Once the transfusion is completed, the baby should continue to be monitored under phototherapy until the hyperbilirubinaemia has begun to decrease and phototherapy is no longer needed. In most cases, an exchange transfusion followed by double phototherapy is effective, however, some babies may require additional exchange transfusion.
Complications
An exchange transfusion should only be performed in a baby at significant risk of kernicterus in whom the benefits of transfusion outweigh the risks of complications (Ahlfors 1994). The risks can be minimized by intensively monitoring electrolytes and platelets throughout the procedure and by minimizing the changes in circulating blood volume during the procedure.
Immunoglobulin
The use of intravenous immunoglobulin to reduce ongoing haemolysis as an additional form of treatment in haemolytic jaundice has also been described, and there is now good evidence for this (NICE 2010, Alpay et al 1999, Ergaz & Arad 1993).
Prolonged jaundice
Prolonged jaundice is jaundice after 2 weeks of age in a term and/or in a preterm baby who remains jaundiced at 3 weeks. Both situations require paediatric assessment with some investigations. Investigation should normally include:
The urgent diagnosis is to identify/exclude biliary atresia, in which case the baby may not initially be severely jaundiced but will have pale stools and dark urine (see below).
Conjugated hyperbilirubinaemia
Conjugated hyperbilirubinaemia is always pathological and refers to a situation where >15% of the total bilirubin or 25 μmol/L is in conjugated or ‘direct reacting’ form. This occurs as a direct consequence of interruption in the normal conjugation and hepatic excretion of bilirubin diglucuronide and results from an obstruction at any point along the pathway from the hepatocyte to the intestine. Bilirubin conjugation within the liver is often able to continue even in the presence of significant liver damage but the excretion of the conjugated bilirubin into intrahepatic bile ducts may become obstructed. Consequently, the concentration of bilirubin diglucuronide within the hepatocytes will continue to increase and eventually it diffuses into the bloodstream.
Conjugated jaundice can be suspected clinically in a baby with pale stools (absent stercobilinogen) and dark urine (bilirubin present). A urine dipstick test will be positive for bilirubin. These are critical clinical features as this jaundice may initially be subtle and easily missed, and always requires further assessment.
The main causes of a conjugated jaundice are as follows:
In a term baby without perinatal concerns, conjugated jaundice most commonly presents as prolonged jaundice. Any term baby who remains jaundiced at 10 days to 2 weeks of age should be investigated for conjugated jaundice by examining the stools and taking blood for a ‘split’ bilirubin (direct and indirect bilirubin). Infants with extrahepatic biliary atresia must be identified as early as possible, as surgery performed at 4–6 weeks improves prognosis. Short-term outcome has not been found to be improved by steroids (Vejchapipat et al 2007) and long-term outcome remains guarded with regard to long-term liver disease (Hadzic et al 2003, Hartley et al 2009).
Complications
Complications may be general or specific complications of the underlying disease itself rather than direct complications of the jaundice.
Management of conjugated hyperbilirubinaemia
The main objective in the management is to establish the cause of the jaundice. Phototherapy is ineffective because conjugated bilirubin is already water-soluble and the inappropriate use of phototherapy may lead to the ‘bronzed baby’ syndrome. Exchange transfusion is not indicated for a conjugated hyperbilirubinaemia because conjugated bilirubin is not lipid-soluble in the same way as unconjugated bilirubin, and will not cause kernicterus.
Signs may include the following:
First-line investigations should include a blood glucose, liver function tests and clotting studies. Early advice should be sought from a paediatric hepatologist so that the most appropriate investigations and subsequent management can be arranged.
Follow-up
All babies who have had significant unconjugated hyperbilirubineamia should be reviewed at least once following discharge. This enables results of investigations to be reviewed, further investigations to be arranged if appropriate, and the baby’s clinical condition to be reassessed. Since one of the complications of hyperbilirubinaemia is sensorineural hearing loss, these babies should have formal hearing tests (see website).
Neonates who have had haemolytic jaundice require early review and regular follow-up for the first 3 months of life since the other effect of haemolysis is anaemia (especially when the treatment was phototherapy without an exchange transfusion). The baby should be seen at less than 2 weeks of age to review its clinical condition and to perform a full blood count. This investigation will provide information about the extent of the ongoing haemolysis (haemoglobin) and the baby’s bone marrow response and ability to compensate (reticulocyte count). Some babies may require a later blood transfusion.
Babies that have had clinical evidence of acute bilirubin encephalopathy require continued neurodevelopmental follow-up and further investigations such as MRI to establish a guide to prognosis.
Conclusion
Jaundice is a common problem of the newborn baby. An understanding of the normal physiology of bilirubin metabolism enables the midwife to predict risk factors for developing unconjugated hyperbilirubinaemia and be aware of the clinical signs suggestive of a conjugated hyperbilirubinaemia. The aim in unconjugated hyperbilirubinaemia is to prevent babies from developing severe jaundice that may lead to bilirubin encephalopathy and kernicterus. Infants with prolonged jaundice should be referred to a paediatrician to swiftly identify conjugated hyperbilirubinaemia. The midwife will often be the person who will identify the jaundice, and continue to provide care and support to the woman, baby and family. She needs to be knowledgeable about the different aspects of care and management, be able to provide accurate and evidence-based information to the family, and ensure that they feel informed and confident in the professionals providing care to the baby.