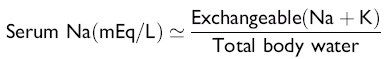Chapter 22 Clinical Chemistry Tests
Laboratory data are discussed here in relation to case management. The focus is on interpretation of an abnormal finding in the typical clinical situation. An in-depth pathophysiologic explanation of these alterations is beyond the scope of this section. Should additional information be required, a textbook on veterinary clinical pathology should be consulted.1,2
All samples should be submitted with specific objectives in mind. In general, these objectives fall into one of the following categories:
SUBMISSION OF LABORATORY SAMPLES
Veterinary Diagnostic Services
The clinician must be aware of inherent limitations of laboratory evaluation in certain clinical settings. In general, veterinary diagnostic laboratories are preferred to general medical laboratories, because human medical laboratories may be less familiar with animal diseases and the responses of animals to disease. There may also be differences in test methodology and interpretation. These species differences can cause confusion when results are evaluated on the basis of human criteria that may not apply to animals.
Various desktop or portable hand-held point-of-care devices are available to veterinarians for determination of serum chemistry, electrolytes, and acid-base balance. Many of these devices use self-contained strips, cartridges, or rotors and thus reduce errors associated with the maintaining, measuring, and mixing of reagents. In addition, some of these devices can use whole blood rather than serum or plasma. Some devices require refrigerated storage of reagent cartridges, which must then be warmed to room temperature for use. There are relatively few independently published data comparing the results obtained with these point-of-care devices and those obtained with standard laboratory procedures. A widely used hand-held device (iSTAT) has been shown to yield comparable results for blood electrolyte concentrations and acid-base balance in dogs and horses.3 However, it was noted that the correlation’ between results from this device and from standard laboratory techniques was poor for sodium in the dog and for hematocrit in the horse. Portable point-of-care devices can provide rapid, accurate, and relatively inexpensive results. As technology continues to improve, it can be anticipated that there will be wider and more general application of these devices in many large animal practice settings. Important requirements are the establishment of normal values with these devices for our large animal species of important age or production groupings as well as clear definition of the limitations and possible idiosyncratic reactions in certain species or clinical settings.
Selection of Procedures
Selection of specific laboratory tests fosters logically integrated thinking and concentrates on evaluation of the primary medical problems. However, the sophisticated autoanalyzers used by large commercial laboratories can perform a wide battery of tests quickly and efficiently with little additional cost. These panels may be broadly defined (e.g., a general large animal health panel) or may offer a more focused evaluation of a specific organ system (e.g., liver, kidney, or muscle). The clinician must ensure that the panel selected contains all the appropriate tests for a thorough evaluation of the individual patient’s medical problems.
The following recommendations for diagnostic panels are intended to provide a clear indication of organ damage and/or dysfunction. The most directly applicable diagnostic procedures are listed under “Recommended,” and additional procedures that may be of benefit in certain circumstances are listed under “Optional.”
GENERAL PANEL
The broadly based general chemistry panel should provide a balanced evaluation of the most likely medical problems.
MUSCLE PANEL
The muscle panel should detect active skeletal and cardiac muscle destruction (rhabdomyolysis) and the degree of secondary renal damage. The possible causative factors are evaluated as optional procedures, depending on the history, clinical findings, or special circumstances. Muscle biopsy with special staining may be critical to the diagnosis of specific muscle diseases such as polysaccharide storage myopathy, immune mediated myopathy, and mitochondria myopathy. Special tests for muscle function and the genetic basis for some myopathies are discussed in the section on muscles (Chapter 42).
LIVER DISEASE PANEL
The liver panel should detect active damage to the hepatic parenchyma, involvement of the biliary system, and alteration in hepatic function.
RENAL DISEASE PANEL
The kidney panel should provide a rough quantitative estimation of compromised renal function and should indicate the location and nature of the damage to the urinary tract.
GASTROINTESTINAL DISEASE PANEL
The gastrointestinal disease panel should include evaluation of acid-base status, fluid and electrolyte balance, and renal function, which are common complicating features of gastrointestinal diseases. Additional optional or special diagnostic procedures may be necessary in calves or foals with neonatal diarrhea, in horses with colic, and in ruminants with gastrointestinal stasis or displacement.
Metabolic Profiling
The health status and productivity of dairy cattle, swine, and other food animals maintained in large confined groups involve a fragile balance between metabolic events, nutrition, agents of disease, management, and environmental factors. In these production units the health status of the herd as a whole is of paramount importance. Subclinical disease or nutritional imbalance may contribute to suboptimal productivity. Most productivity problems in these settings are multifactorial. Defining and finding solutions to these problems can be a difficult and complicated task. Sequential assessment of weight gains, body conditions scores, milk quality, and milk production are useful measures of the presence of subclinical production disorders but do not identify the cause. Metabolic profiling is a tool that has been employed in some situations. Blood samples are drawn from a number of individuals as representative of the group as a whole. Some have recommended the submission of pooled serum samples from representative individuals, much like the use of bulk tank tests as a reflection of the general level of mastitis in a herd. Sampling may be done routinely and sequentially. In dairy cattle this may be done during gestation or lactation but frequently focuses on the periparturient period when a combination of nutritional and metabolic events often contributes to costly production disorders.
Metabolic profiles might include most of the parameters listed under the recommended general panel (p. 376), with the addition of magnesium, total cholesterol, nonesterified fatty acids (NEFA) and β-hydroxybutyrate (BOHB). BOHB, NEFA, and cholesterol may provide an indication of energy balance, whereas BUN, creatinine, total protein, albumen, and CK may be helpful in assessing protein status. In certain settings, trace minerals or fat-soluble vitamins may be important indicators of underlying nutritional problems. Metabolic profiling is not a substitute for careful clinical examination, analysis of husbandry practices, and ration analysis, but it may play a useful role in some modern large- scale operations in which a variety of subclinical problems can quickly translate into financial disaster.
SOURCES OF VARIATION IN NORMAL VALUES
Laboratory
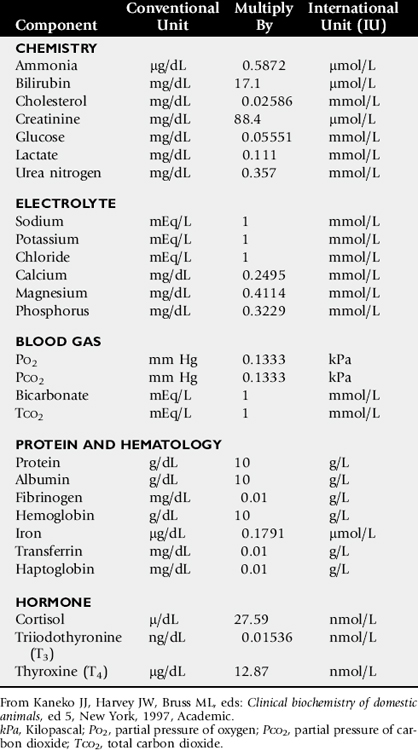
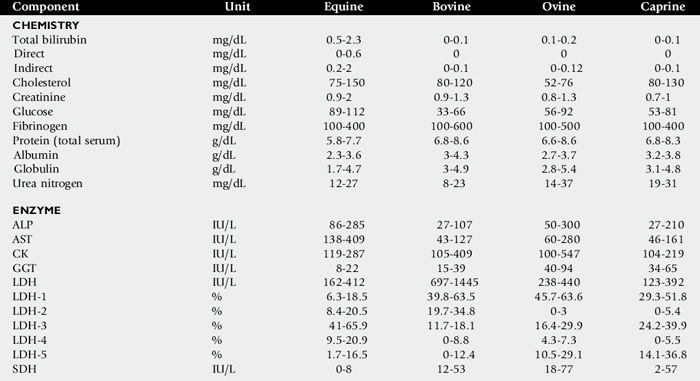
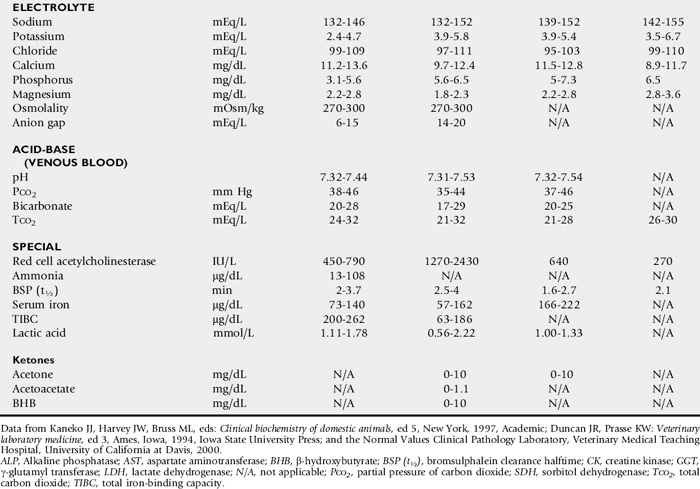
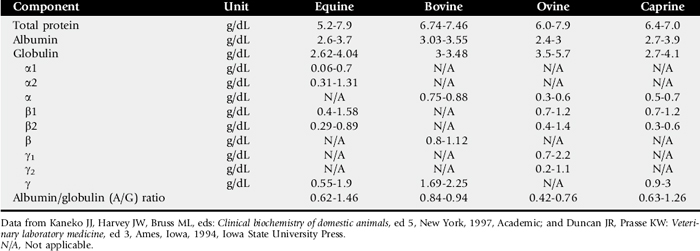
One of the most commonly overlooked sources of variation in clinicopathologic data is the difference in results obtained by different laboratories. This can result in fivefold to tenfold differences in the normal range of certain enzyme activities between laboratories using similar but not identical methodologies. In the past there was marked variation in the units of measure used to express the activities of different serum enzymes. The standard method of representing serum enzyme activity is in international units per liter (IU/L), which is used in this text. Correction factors for converting the commonly used but older units of measure to international units are given in Table 22-1. The normal values given in Tables 22-2 and 22-3 are those used at the University of California Veterinary Medical Teaching Hospital or are from the literature.2,3 It is always best to use normal values and reference intervals established for the species, age, and production type by the diagnostic laboratory to which samples are submitted.
Species
There is relatively modest variation among species for most clinicopathologic parameters. Notable exceptions are plasma electrolyte concentration, erythrocyte potassium concentration in some breeds of sheep, and serum bilirubin concentration, which is higher in horses than in other species. BUN is a less reliable indicator of renal function in ruminants and horses than creatinine because urea nitrogen can be metabolized by the intestinal microflora. Donkeys and burros have a much higher GGT level than horses and cattle.
Breed
Significant differences in hematologic parameters exist between hot-blooded and cold-blooded horses. Hot-blooded horses include most of the athletic breeds of horses (the thoroughbred, quarter horse, standardbred, and Arabian breeds). Cold-blooded horses include the pony and draft breeds. Cold-blooded horses have lower red cell values both at rest and after exercise and maintain a slightly lower leukocyte count; they also have lower resting and fasting indirect bilirubin concentrations.
Age
There are several important differences in hematologic and clinical chemistry between neonatal and adult animals. The effects of age have been studied most carefully in horses and cattle. Suckling neonatal animals tend to have lower BUN, slightly lower total protein and globulin, moderately higher GGT and phosphate, and markedly greater alkaline phosphatase than do adult animals.
Sex
With the obvious exception of sex hormone concentrations, there are few recognized differences in clinical chemistry values between sexes. In most domestic animals the intact male tends to have a slightly higher erythrocyte count, hemoglobin concentration, and PCV than the female or neutered male. This sex-related difference has been demonstrated most clearly in the horse.
Factors Influencing Results or Their Interpretation
Many factors influence the reliability and interpretation of results obtained by laboratory analysis. Sample collection and handling are very important factors. The sample collection site (e.g., jugular vein, mammary vein, tail vein, or carotid artery) can have an important effect on the results of tests such as blood gas evaluation, glucose, or ketones. The choice of anticoagulants depends on whether the samples are to be submitted for serum, plasma, or whole blood determinations. The specific sample requirements for the most commonly ordered clinical chemistry determinations are listed in Table 22-4. Serum is required for most chemistry determinations, and serum separator tubes work very well in most settings. There have been some indications that results of some serum hormone assays may be influenced by collection of blood in serum separator tubes. Heparin is the anticoagulant of choice for most chemical determinations requiring plasma. In former times fluoride-oxalate was the anticoagulant of choice for blood glucose determination, because it halts glycolysis by the red cells. However, fluoride may interfere with certain chemical procedures (specifically the glucose oxidase method for blood glucose determination) and should be used only for blood lactate -determination or in selected circumstances in which glucose determinations are required and samples must be held for some period of time without refrigeration. Citrate is the anticoagulant of choice for clotting tests and blood typing. Ethylenediaminetetraacetic acid (EDTA) is the anticoagulant most often used for hematologic evaluation. Both citrate and EDTA are chelating agents, which may interfere with a wide variety of chemical determinations.
Table 22-4 Recommended Anticoagulants for Hematologic or Clinical Chemistry Evaluation
| Anticoagulant | Specimen | Test or Procedure |
|---|---|---|
| Ethylenediamine tetraacetic acid (EDTA) | Whole blood Whole blood Plasma |
Complete blood count, cross-match, platelet count Blood selenium Refractometric protein and fibrinogen |
| Peritoneal fluid | Fluid analysis | |
| Bone marrow | Hematologic evaluation | |
| Synovial fluid | Fluid analysis | |
| Heparin | Whole blood | Blood pH, blood gases |
| Plasma | Electrolytes, osmolality | |
| Synovial fluid | Mucin clot test | |
| Fluoride and oxalate | Plasma | Lactate |
| Citrate | Whole blood | Blood typing |
| Plasma | Coagulation tests (PT, PTT, factor analysis) | |
| None, serum separator tubes | Serum | Most chemistries, electrolytes, osmolality Protein electrophoresis |
| Hormones (cortisol, T3, T4) | ||
| Immunoglobulins (IgG, IgM, IgA) |
PT, Prothrombin time; PTT, partial thromboplastin time; T3, triiodothyronine; T4, thyroxine.
Modified from Brobst DF, Parry BW: Normal clinical pathology data. In Robinson NE, ed: Current therapy in equine medicine, ed 2, Philadelphia, 1987, Saunders.
Samples should be submitted as soon after collection as possible, but circumstances may require storage of some samples for 12 to 24 hours. For collection of serum, whole blood should be allowed to clot before refrigeration. Serum should be separated from the red cells immediately after clot formation and then kept refrigerated. Samples should be stored in clean containers free from exposure to sunlight, medications, or chemicals. If whole blood is left at room temperature for longer than 60 minutes, blood glucose will be falsely low as a result of red cell glycolysis. Storage of whole blood may result in in vitro hemolysis, with the potential for misleading increases in the serum or plasma enzymes AST and lactate dehydrogenase (LDH) due to hemolysis. Failure to separate serum or plasma from the red cells within an hour of collection may lead to leakage of erythrocyte potassium and a falsely elevated serum or plasma potassium concentration.
Stress, transportation, excitement, and handling produce physiologic responses in animals that affect a variety of hematologic and biochemical parameters. This is most evident in the horse, which shows marked increases in red cell mass and to a lesser extent plasma protein concentration in response to excitement, exercise, or catecholamine administration. The red cell count and hemoglobin concentration can increase by as much as 50%, whereas plasma protein concentration may increase by 1 to 2 g/dL. Leukocytosis is induced as the marginating leukocyte pool is mobilized into the general circulation. Prolonged stress results in the release of endogenous corticosteroids, which produce the typical “stress response” in the leukogram. A similar leukogram is found in race horses some 4 to 6 hours after racing. The combination of catecholamine and glucocorticoid release associated with stress, transport, and excitement, as well as with many gastrointestinal catastrophes, may result in markedly elevated blood glucose concentrations (up to 400 mg/dL). Modest elevations (twofold to fourfold increase) in muscle-derived enzymes occur in association with prolonged transport or endurance exercise.
Large losses or compartmentalization of sodium-containing fluid accompanies many systemic disorders, particularly digestive problems such as diarrhea, colic, displacement of viscera, excessive sweat losses, and some urinary tract diseases. These forms of dehydration lead to decreases in plasma volume, which are indicated by moderate-to-marked increases in the PCV and TPP concentration. The concentration of other compounds dissolved in the plasma may also increase as a result of decreases in the plasma volume. The concentrations of compounds that are largely protein-bound, such as calcium, are generally closely related to protein concentration. More than 50% of serum calcium is bound to albumin. Increases or decreases in plasma protein concentration normally result in proportional changes in total serum calcium concentration, whereas the physiologically active ionized calcium may remain unchanged.
Diseases that cause a reduction in effective circulating fluid volume often also cause alterations in renal function. This so-called “prerenal azotemia” results in moderate to marked elevation in BUN and creatinine. Although this is generally considered primarily a prerenal azotemia, real pathologic changes in the kidneys often are associated with the systemic processes initiated by these disorders. Reevaluation of renal function (urinalysis BUN, creatinine) in these patients during the course of disease is important because it affects prognosis, response to fluid administration, and the potential for nephrotoxicity and systemic toxicity of a variety of chemotherapeutic agents.
Fasting laboratory data are important for evaluation of many disease conditions in human and small animal patients. Truly fasting conditions are rather difficult given the large and complex gastrointestinal tract of most herbivores and are thus seldom used. The feeding of animals in relation to sample collection can, however, have an impact on the data obtained. Hay feeding in horses is reported to affect sodium, potassium, and protein concentrations within the first few hours after feeding. Animals feeding on lush green pasture or large amounts of silage may have slightly different parameters from those fed high-concentrate rations. The anion-cation balance of the ration has an impact on relative serum electrolyte concentration, acid-base balance, and urine pH and urinary electrolyte excretion. Lactescent (cloudy) plasma may be observed in samples from nursing foals or calves. The fluid intake of the normal nursing neonate may range from 100 mL/kg/day to more than 250 mL/kg/day. This high fluid intake is reflected by a commensurately high output of urine with a low specific gravity and low osmolar content.
The administration of certain medications may have an impact on some laboratory parameters. Tranquilization may be necessary for restraint and safe sample collection. The practitioner should be aware that tranquilizers often decrease red cell mass and plasma protein concentration. This is particularly true of the phenothiazine-derivative tranquilizers when used in the horse. Xylazine administered to large animals produces a modest catecholamine release, which may be evidenced by the slight sweating response seen in many horses sedated with this drug. Glucose concentration will increase modestly in response to the xylazine-induced catecholamine release. Repeated intramuscular injections with certain antibiotics (especially erythromycin and tetracycline) or other preparations that are locally irritating may produce slight-to-moderate elevations in muscle-derived serum enzyme activities. Intravenous administration of certain drugs and compounds such as dimethyl sulfoxide (DMSO) can produce intravascular hemolysis and hematuria. The amount of hemolysis in these circumstances is relatively small and of little consequence, except that it can cause confusion as to why hemoglobinuria occurred.
FLUID AND ELECTROLYTE BALANCE
Packed Cell Volume and Total Plasma Protein
Changes in the plasma volume generally are reflected by changes in the PCV and the TPP concentration. In dehydrated humans, changes in the PCV are believed to be the more reliable guide to changes in plasma volume because substantial protein fluxes into and out of the circulation have been shown to occur. However, in most animal species the range of normal for the PCV is much wider than for the TPP concentration. This is particularly true of horses, in which excitement, pain, or catecholamine release can produce variable mobilization of splenic erythrocytes, making it difficult to obtain a truly resting PCV. For these reasons, precise quantitative estimation of a change in plasma volume using these parameters is more complex and less reliable in large animal species. As plasma volume increases or decreases, the change in the PCV is always less than the change in the TPP concentration. However, a large disparity in the changes in the PCV and the TPP concentration in a patient with a history of loss of sodium-containing fluid and clinical evidence of reduced effective circulating fluid volume suggests blood or protein loss. Marked increases in the PCV with a normal-to-low TPP concentration frequently are encountered in animals with acute protein-losing enteropathies such as salmonellosis or equine toxic enteritis. In horses undergoing treatment for diarrhea, the excessive administration and retention of sodium-containing fluids is a key factor in the development of edema and hypoproteinemia. Blood loss generally results in a decrease in both PCV and TPP concentration.
Serum Sodium
The serum sodium concentration is a function of the exchangeable cation content (i.e., the exchangeable sodium [Na] in the extracellular fluid [ECF] volume plus the exchangeable potassium [K] in the intracellular fluid [ICF] volume relative to total body water), as indicated in the following formula:
Changes in the sodium concentration reflect the net changes in this relationship and often do not represent accurately the changes in sodium balance. Changes in water balance are thus primarily responsible for changes in the serum sodium concentration. Hyponatremia is an indication of a relative water excess, whereas hypernatremia is an indication of a relative water deficit.
Dehydration is defined as a loss of body water (fluid volume contraction). It occurs in a variety of clinical circumstances. The serum sodium concentration provides a means of categorizing dehydration in a physiologically meaningful way. Hypertonic dehydration, which occurs when water losses exceed the losses of sodium and potassium, is indicated by hypernatremia. The response of horses to feed and water deprivation is an example of this form of dehydration. Isotonic dehydration occurs with a balanced loss of water and electrolytes—that is, approximately 140 to 150 mEq of sodium plus potassium (Na + K) for each liter of water lost. Because the relative water balance has not changed, the serum sodium concentration remains unchanged despite the accumulation of what may have been a substantial sodium deficit. The early stages of acute diarrhea and the dehydration of heavily sweating endurance horses are examples of isotonic dehydration. Hypotonic dehydration occurs when the losses of exchangeable cations (Na + K) exceed the net change in water balance; this condition is indicated by hyponatremia. Hypotonic dehydration often is seen in animals with subacute or chronic diarrhea that develop substantial water and electrolyte deficits but then replace part of the water deficit through water consumption. Fig. 22-1 shows the compartmental distribution of fluid between the ECF volume and the intracellular fluid (ICF) volume in four situations.
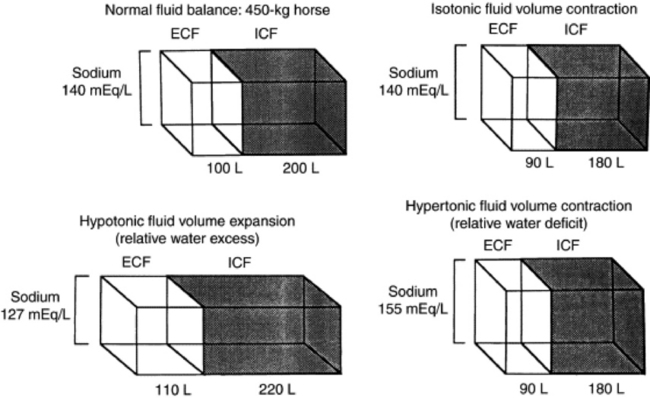
Fig. 22-1 Compartmental distribution of fluid between the extracellular fluid (ECF) volume and the intracellular fluid (ICF) volume in a 450-kg horse with normal fluid balance; with isotonic fluid volume contraction; with hypotonic fluid volume expansion; and with hypertonic fluid volume contraction.
Modified from Kaneko JJ, Harvey JW, Bruss ML, eds: Clinical biochemistry of domestic animals, ed 5, New York, 1977, Academic.
HYPONATREMIA
Hyponatremia is often but not invariably associated with conditions that cause sodium depletion such as vomiting, diarrhea, excessive sweat losses, and adrenal insufficiency. The fluid losses in these conditions are most often hypotonic or isotonic, and initial fluid and electrolyte deficits do not result in hyponatremia until water intake, renal water retention, or both disturb the balance between the remaining exchangeable cations and the total body water.
The accumulation of sodium-containing fluid in body cavities or the gut lumen caused by ascites, peritonitis, or rupture of the bladder or by displacement, torsion, or volvulus of the gut is referred to as a third-space problem. When such accumulations develop rapidly, the plasma volume is reduced, and the serum sodium concentration subsequently may decrease as compensating renal responses cause water retention. Rupture of the bladder in neonatal foals is associated with marked hyponatremia and hypochloremia. As fluid intake continues and dilute urine accumulates in the abdomen, sodium, chloride, and other ions are drawn from the rest of the ECF into this accumulating fluid. No sodium or chloride has been lost from the body, and the observed decreases in the electrolyte concentration are caused by changes in the relative water balance. The neurologic signs seen in these foals are largely caused by the effects on the central nervous system of the rapidly developing and marked hypotonic hyponatremia. Progressively severe neurologic disturbances may be seen as the serum sodium concentration falls below 115 mEq/L and then below 100 mEq/L. The severity of the neurologic abnormalities is a function of both the rate at which hyponatremia develops and the absolute degree of hyponatremia. Neurologic disturbances can occur iatrogenically if excessive amounts of free water (usually given as 5% dextrose) are administered to patients with altered renal function.
Mastitis results in an increased loss of sodium in the milk, and a low-grade mastitis problem in a dairy herd on a marginal dietary salt intake may result in sodium depletion and medullary washout. Decreased milk production, polyuria, hyposthenuria, and a low urine sodium level may be noted, although the serum sodium concentration may remain within the lower range of normal.
The most common causes of hyponatremia are listed in Box 22-1. Marked hyperlipidemia or hyperproteinemia produces a falsely low sodium concentration value because lipid or protein occupies a significant volume in the serum or plasma sample and because sodium is present only in the aqueous phase. This potential cause of hyponatremia is indicated by an increase in the osmolar gap between measured and calculated osmolality. The use of direct ion-specific electrodes for electrolyte determinations avoids this potential cause of a falsely low sodium concentration value.
Marked hyperglycemia causes a reduction in the measured serum sodium concentration of approximately 1.6 mEq/L for every 100 mg/dL increase in the glucose concentration. Increases in the plasma glucose concentration generate osmotic forces that result in the movement of cellular water into the ECF, diluting the plasma sodium concentration.
HYPERNATREMIA
Hypernatremia can occur in the initial stages of diarrhea, vomiting, or renal disease if water loss exceeds electrolyte loss (Box 22-2). When water losses are replaced by increased water consumption, enhanced renal water retention, or both, the serum sodium concentration decreases. Food and water deprivation in normal horses and cattle is associated with substantial reduction of renal and fecal output, but continued cutaneous and respiratory insensible water loss may result in hypernatremia. In this case the hypernatremia is the result of a primary water loss. Hypernatremia may occur transiently as a result of sodium excess after administration of hypertonic saline or sodium bicarbonate if water intake is restricted or impaired. Hypernatremia has been reported in calves fed an inappropriately mixed oral electrolyte replacement solution as their only fluid intake.4 The hypernatremia observed with salt poisoning in cattle and swine is the result of water restriction in animals that have been maintained on a high-salt intake.
Serum Potassium
The serum potassium concentration is influenced by factors that alter internal balance (the distribution of potassium -between the ECF and the ICF) and those that change external balance (potassium intake and output). Changes in the serum potassium concentration occur in a wide variety of clinical circumstances and have profound neuromuscular effects that are largely the result of changes in cell membrane potential. The responses to dehydration and acid-base imbalance often complicate the evaluation of the potassium concentration. For example, calves with acute diarrhea often develop potassium depletion because of excessive losses and inadequate intake, but the serum potassium concentration of these animals usually is normal to increased as the result of renal shutdown and the metabolic acidosis induced by dehydration, sodium depletion, and hypovolemia. Hypokalemia may become evident only as other fluid and electrolyte losses are replaced.
Measuring the erythrocyte potassium concentration is relatively easy and has been suggested as an aid in assessing the need for potassium supplementation in racehorses with recurrent muscle disease. However, experimental studies in horses indicate that the erythrocyte potassium concentration does not always accurately reflect potassium deficits.
HYPOKALEMIA
Hypokalemia may result from depletion of the body’s potassium stores or from a redistribution of potassium from the ECF into the ICF space (Box 22-3). Hypokalemia is most commonly seen with altered intake and absorption and with excessive potassium losses from the gastrointestinal tract caused by vagal indigestion, torsion of the abomasum, ileus, or diarrhea. Excessive renal loss may result from mineralocorticoid excess, certain diuretics, or altered renal function, as reported in horses with renal tubular acidosis. Marked hypokalemia develops when reduced dietary intake caused by anorexia is associated with excessive potassium losses.
Box 22-3 Causes of Hypernatremia
* External balance refers to the relative changes in potassium intake and output; internal balance refers to the distribution of potassium between the extracellular and the intracellular fluid compartments.
Hypokalemia without potassium depletion results from the movement of extracellular potassium to the intracellular space. This form of hypokalemia occurs in response to an acute alkalosis and to the administration of insulin or glucose. Overzealous and rapid administration of sodium bicarbonate can produce an alkalosis with a profound and rapidly developing hypokalemia. Animals with moderate potassium deficits that are vigorously treated with sodium bicarbonate to correct a coexisting mild metabolic acidosis may be particularly prone to this problem. The initial -response to catecholamine administration is a modest, transient increase in potassium caused by α-adrenergic stimulation, which often is followed by hypokalemia caused by β-adrenergic receptor responses.
HYPERKALEMIA
Hyperkalemia may develop in vitro as a result of hemolysis or leakage of erythrocyte potassium after storage of whole blood (Box 22-4). The release of potassium from leukocytes or platelets into the serum after clot formation is a potential cause of hyperkalemia if marked leukocytosis or thrombocytosis is present. Hyperkalemia also results from renal potassium retention in Addison’s disease, acute renal failure, and renal shutdown. A number of factors contribute to the movement of intracellular potassium into the ECF, resulting in hyperkalemia. Hyperkalemia often is associated with metabolic acidosis, particularly when the acidosis results from volume depletion and is complicated by renal shutdown. Hyperkalemia has been reported in animals with massive muscle necrosis, but neither hyperkalemia nor metabolic acidosis is a common feature in horses with exertional rhabdomyolysis. Vigorous short-term exercise of horses at high intensity results in a marked but transient hyperkalemia (9 to 10 mEq/L) that may be associated with the profound lactic acidosis seen with anaerobic workloads.5 Potassium returns to normal within minutes, and often a modest hypokalemia occurs later in the recovery period. Episodic hyperkalemia and muscular weakness are associated with the condition known as hyperkalemic periodic paralysis (HYPP).6 HYPP is inherited as an autosomal-dominant trait in horses, with a specific quarter horse lineage7 (see Chapter 42 for a more complete discussion of this disorder). The disease is the result of a single DNA base pair substitution that leads to the production of an abnormal voltage-regulated sodium channel at the cell membrane.8 Sudden marked increases in the serum potassium concentration, up to 8 to 9 mEq/L, are -the result of transcellular movement of potassium and are associated with profound electrocardiographic abnormalities and fluid shifts.
Serum Chloride
Alterations in the chloride concentration usually are associated with nearly proportional changes in the sodium concentration as the result of changes in relative water balance. In addition, the chloride concentration tends to vary inversely with the bicarbonate concentration; therefore, when disproportionate changes in the chloride concentration relative to sodium occur, significant acid-base imbalances should be anticipated. Disproportionate increases in chloride are associated with a normal-to-low anion gap hyperchloremic metabolic acidosis, but they also are seen as a result of the compensating responses for a primary respiratory alkalosis (Box 22-5). A striking hyperchloremic metabolic acidosis has been reported in horses with renal tubular acidosis.9,10 Disproportionate decreases in chloride relative to sodium characteristically are seen in metabolic alkalosis but also may be seen as part of the compensating response for chronic primary respiratory acidosis (Box 22-6). Hypochloremic metabolic alkalosis is a common feature in many digestive disorders of ruminants and is caused by loss of chloride-rich fluids or sequestration of such fluids in the abomasum and forestomachs.
Osmolality
Measurement of the serum osmolality provides an indication of relative water balance in much the same way as the serum sodium concentration does. In most circumstances these parameters are closely correlated. Comparing the measured osmolality with the calculated osmolality, as determined from the measured concentrations of the major solutes in serum (sodium, glucose, and urea), provides a means of determining if the serum water content deviates widely from normal or if foreign, low—molecular-weight substances are present in the blood. The difference between the measured osmolality and the calculated osmolality is called the osmolar gap. Decreases or increases in the osmolar gap could indicate laboratory error, but increases of more than 10 mOsm/kg generally are the result either of a decrease in the serum water content (caused by hyperlipidemia or hyperproteinemia) or of the presence of abnormally high concentrations of low—molecular-weight substances in the serum. These substances can include a variety of exogenous and potentially toxic compounds such as mannitol, ethanol, methanol, propylene glycol, ethylene glycol, isopropanol, ethyl ether, acetone, trichloroethane, and paraldehyde.
Serum Calcium
Calcium plays a vital role in many of life’s processes, including maintenance of neuromuscular excitability, permeability of cell membranes, conduction of nerve impulses, muscle contraction, and blood clotting. For these reasons the serum calcium concentration or, more correctly, the ionized calcium concentration normally is maintained within a relatively narrow range, despite wide variation in intake and output. Calcium metabolism is regulated by dietary factors, vitamin D and its active metabolites, and the hormones parathormone and calcitonin. The serum calcium concentration is maintained by adjusting intestinal absorption, renal excretion, and mobilization of available calcium from the large stores in bone. Calcium exists in the serum in three forms: ionized calcium, complexed calcium, and protein-bound calcium. Ionized calcium, which normally constitutes 40% to 60% of the total calcium, is the physiologically active form of calcium. Protein binding (protein-bound calcium normally constitutes 40% to 50% of the total calcium) can cause confusion. Hyperalbuminemia may result in a modest hypercalcemia, whereas hypoproteinemia, especially hypoalbuminemia, regularly results in a moderate hypocalcemia. The ionized calcium concentration generally remains within normal limits, despite increases or decreases in total calcium associated with the change in protein concentration. The acid-base balance has additional influence on the amount of ionized and protein-bound calcium. Alkalosis reduces ionized calcium and increases protein binding, whereas acidosis produces the opposite effect. Ion-specific electrodes are available for determining the ionized calcium level, which can be very useful if blood samples are handled appropriately. Most diagnostic laboratories provide the total serum calcium value, which is composed of ionized, complexed, and protein-bound calcium.
HYPOCALCEMIA
Large increases or decreases in the serum calcium concentration generally are the result of a failure in the normal mechanisms of calcium homeostasis rather than a reflection of absolute calcium deficits or calcium-phosphorus imbalances. Hypocalcemia occurs with some frequency in domestic animals, particularly in high-producing dairy cattle near the onset of lactation. In cattle the serum calcium concentration normally decreases to less than 8 mg/dL with the stress of parturition and the onset of lactation (Box 22-7). Failure to mobilize sufficient calcium to maintain serum calcium results in the clinical syndrome of parturient paresis (see Chapter 41 for a more detailed discussion of this syndrome). Most animals are recumbent with a calcium level of 6 mg/dL or less, and fatalities may occur if the level drops below 4 mg/dL. Parturient paresis is associated with a normal to increased serum magnesium level, hypophosphatemia, and hypocalcemia. Change in the cation-anion balance in the diet of dairy cattle during the periparturient period has modest effects on the acid-base balance and enhances calcium mobilization from storage sites, thus reducing the incidence of milk fever in cows at high risk. Grass tetany is associated with marked hypomagnesemia and modest hypocalcemia, whereas the inorganic phosphorus level remains within the normal range.
Systemic diseases resulting in anorexia (e.g., traumatic reticuloperitonitis, ketosis, and displaced abomasum) or acute toxemic conditions (e.g., coliform mastitis, septicemia, or aspiration pneumonia) that produce anorexia in lactating cattle frequently result in hypocalcemia. Hypocalcemia also is seen in sheep on marginal rations if stressed by inclement weather or when being moved. Hypocalcemia is seen in cattle with fat necrosis, presumably as the result of incorporation of calcium with the fat as a form of soap. Horses with exhaustive disease syndrome or transit tetany often develop decreases in ionized calcium with resultant muscle cramps and synchronous diaphragmatic flutter, which generally respond to intravenous calcium administration. Horses, cattle, and sheep usually respond initially to acute renal tubular damage with mild hypocalcemia and hyperphosphatemia.
HYPERCALCEMIA
Marked hypercalcemia, with a serum calcium level ranging from 14 to 20 mg/dL, and modest hypophosphatemia frequently are observed in horses with chronic renal failure that are fed a high-calcium diet such as alfalfa hay (Box 22-8). In these horses, blood samples -collected in standard EDTA tubes may actually clot. This occurs because the serum calcium concentration is so high that there is insufficient EDTA to bind all the calcium, and some free calcium is available to complete the clotting process. Vitamin D intoxication can develop as a result of excessive dietary supplementation or the ingestion of certain plants such as Cestrum diurnum (day blooming jasmine) and Solanum malacoxylon, which contain toxic quantities of vitamin D analogs. Primary hyperparathyroidism is exceedingly rare in large animals, but pseudohyperparathyroidism with hypercalcemia occasionally can develop in animals with tumors that produce protein substances with parathormone-like biologic activity. This has been reported in a few animals with lymphosarcoma or gastric squamous cell carcinoma.
Serum Phosphorus
Phosphorus is found primarily in the skeleton and teeth in close association with calcium in the intricate and dynamic crystalline structure of bone. Intracellularly, phosphate plays an essential role in the degradation and synthesis of many compounds. Also, as adenosine triphosphate (ATP), adenosine diphosphate (ADP), and adenosine monophosphate (AMP), it is the primary form of energy storage and transfer required for almost all of life’s processes. Phosphorus in the ECF exists primarily as the buffer pair H2PO−3 and HPO−4 and plays a role in acid-base balance. Like calcium, phosphorus is regulated by dietary factors, the active metabolites of vitamin D, and the hormones parathormone and calcitonin. Imbalances of calcium and phosphorus or the presence of compounds that bind these substances in the gut can produce serious imbalances that are not always evident on analysis of serum samples. Measurement of urinary output or creatinine clearance ratios for calcium and phosphate are simple and helpful procedures. They provide an indication of an imbalance while more definitive procedures such as ration analysis are contemplated.
HYPOPHOSPHATEMIA
Serum phosphorus concentrations are not always an accurate guide to phosphate balance, but dietary deficiencies of phosphorus are frequently manifested by hypophosphatemia. Hypophosphatemia is a common feature in cattle with parturient paresis (see also Chapter 41) and horses with chronic renal failure (Box 22-9). It has been reported in animals with experimental oxalate toxicity, in chronic wasting states, or with starvation. Postparturient hemoglobinuria is a disorder of cattle, primarily lactating dairy cattle, that often is associated with diets low in phosphorus. Although marked hypophosphatemia is often reported, it is not an invariable feature of this disorder.
HYPERPHOSPHATEMIA
Age-related differences exist in the normal range of serum phosphorus concentration. Young animals have much higher values than adults, with values for neonates commonly up to 7 to 9 mg/dL. The serum phosphate concentration declines progressively with age. Hyperphosphatemia is seen in animals with vitamin D toxicity, transiently in horses after long-distance endurance rides, and initially in horses with acute renal failure (Box 22-10).
Serum Magnesium
Disturbances of magnesium metabolism occur principally in cattle and sheep. Complex nutritional and environmental interactions contribute to a variety of clinical syndromes attributed to magnesium deficiency and the onset of tetany in grazing animals.
HYPOMAGNESEMIA
Hypomagnesemia is reported in cattle with grass tetany and in sheep with grass staggers (Box 22-11) (see also Chapter 41). A serum magnesium level below 1.8 mg/dL is considered low; values below 1 mg/dL are considered severe and are likely to be associated with clinical signs. Hypomagnesemia caused by dietary magnesium deficiency has been reported in calves reared in confinement and fed a milk diet exclusively.
HYPERMAGNESEMIA
Hypermagnesemia occurs infrequently but may be seen with overzealous administration of Epsom salts (MgSO4), either orally as a drench by means of nasogastric intubation or as an enema for the treatment of digestive disorders (Box 22-12). Intravenous administration of magnesium produces muscle relaxation but does not alter consciousness, and hypertonic MgSO4 solutions are not considered a humane means of euthanasia.
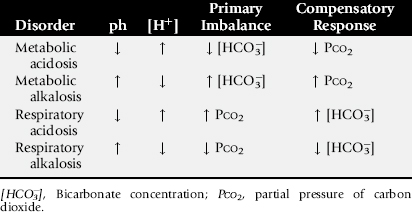
ACID-BASE IMBALANCE
Using the traditional approach to acid-base balance, the four primary acid-base imbalances and their compensating responses are presented in Table 22-5. Acidosis is associated with an increase in the hydrogen ion concentration (decreasing pH), whereas alkalosis is caused by a decrease in the hydrogen ion concentration (increasing pH). When the primary imbalance is associated with a change in the bicarbonate concentration, the acid-base imbalance is called a metabolic disorder. The compensating response for a metabolic acid-base imbalance is mediated by the respiratory tract, which alters the partial pressure of carbon dioxide (PCO2) to counterbalance the primary imbalance and to partly restore the pH toward normal. Primary respiratory imbalances are related to changes in alveolar ventilation, which result in an increased PCO2 in respiratory acidosis and a decreased PCO2 in respiratory alkalosis. The compensating response for these primary respiratory acid-base imbalances is mediated by the kidneys through alterations in the excretion or retention of hydrogen ions and bicarbonate. Heparinized blood samples for acid-base evaluation must be drawn anaerobically and sealed immediately. Arterial blood samples should be submitted for evaluation of primary respiratory disorders and for evaluation of ventilation in patients under general anesthesia. Venous blood samples are easier to obtain and provide reliable data for most primary metabolic acid-base abnormalities.
Blood gas analysis should be done as soon after collection as possible. However, appropriately collected blood samples yield reliable results for as long as 4 hours if held in ice water. The patient’s rectal temperature should be provided to the laboratory so that corrections can be made for variation in body temperature. Changes in body temperature have a major impact on the partial pressure of oxygen (PO2) as well as the PCO2 but relatively little effect on estimations of bicarbonate or base balance. During brief exercise at maximal intensity, the temperature of the central venous blood may exceed the rectal temperature by as much as 3° C. In these circumstances, the central blood temperature is more appropriate than the rectal temperature for correcting blood gas determinations. The blood sampling site (arterial, venous, or capillary blood) has a significant impact on the blood gas values obtained.11 Arterial blood samples yield higher values for pH and lower values for PCO2 than venous blood, but the bicarbonate is higher in venous blood. The use of blood gas data for evaluation of -acid-base imbalances in animals has been reviewed.12,13 In addition, an excellent review of the traditional and nontraditional approaches to evaluation of acid-base balance as related to fluid therapy in small animals has application to many large animal situations and is well worth reading.14
Metabolic Acidosis
Metabolic acidosis is characterized by a decrease in pH and bicarbonate concentration. Metabolic acidosis is traditionally thought to be produced by the addition of hydrogen ions or the loss of bicarbonate ions. The most common causes include the lactic acidosis of ruminal overload, hypovolemia associated with loss or compartmentalization of sodium-containing fluid, ketoacidosis in ketosis and pregnancy toxemia, loss of bicarbonate-rich saliva with oral diseases or esophagostomy in cattle, gastrointestinal loss of bicarbonate as a result of diarrhea, and renal failure, which may result in decreased ability to excrete hydrogen and thus to retain bicarbonate (Box 22-13). Other causes include ingestion of certain drugs or toxic compounds such as salicylate, methanol, ethylene glycol, or paraldehyde. Increased ventilation provides the compensating respiratory response for metabolic acidosis, and a decline in the PCO2 generally begins within minutes. This temporarily minimizes the fall in blood pH, but long-term correction of metabolic acidosis requires renal bicarbonate retention and enhanced acid excretion. Complete correction of metabolic acidosis may be difficult in patients with intrinsic renal disease or those with diseases such as renal tubular acidosis that impair the ability of the kidneys to excrete acid or retain bicarbonate, or both.
Metabolic Alkalosis
Metabolic alkalosis is characterized by an increase in pH and bicarbonate. It occurs fairly commonly in domestic animals, particularly in association with digestive disturbances in ruminants. An initiating process capable of generating alkalosis is necessary and must be coupled with additional factors to maintain metabolic alkalosis. Generation of metabolic alkalosis is traditionally thought to be caused by excessive hydrogen loss, bicarbonate retention, or contrac-tion alkalosis (Box 22-14). Contraction alkalosis occurs when the ECF volume is reduced because of loss or sequestration of fluids high in sodium and chloride but without proportionate loss of bicarbonate. This is a contributing mechanism for the generation of the metabolic alkalosis reported in heavily sweating endurance horses and in response to the diuretic furosemide in the horse. The most common causes of increased hydrogen ion loss are the gastrointestinal losses caused by salivary secretions in ponies after surgical esophagostomy15; massive gastric reflux associated with anterior enteritis, ileus, or small bowel obstruction in horses; and sequestration of fluid in the abomasum and forestomach associated with a variety of gastrointestinal displacements or functional disturbances (vagal indigestion) of ruminants. Continuous salivary losses in horses after surgical esophagostomy result in transient metabolic acidosis followed by progressive metabolic alkalosis. Most of these disorders cause significant dehydration and sodium, chloride, and potassium deficits.
The factors responsible for maintaining metabolic alkalosis involve impaired renal bicarbonate excretion. These factors are associated with the renal response to decreases in the effective circulating fluid volume, chloride depletion, or potassium depletion. Renal tubular sodium resorption is enhanced in response to hypovolemia. Maintenance of electroneutrality requires that sodium resorption in the proximal tubule be accompanied by a resorbable anion, whereas in the distal tubule, sodium resorption is associated with the secretion of another cation, usually hydrogen or, to a lesser extent, potassium. Chloride is the only resorbable anion normally present in appreciable quantities in the proximal tubular fluid. In metabolic alkalosis, plasma bicarbonate is increased and the chloride concentration is generally decreased as the result of disproportionate chloride losses. The relative lack of the resorbable anion chloride in the proximal tubule thus allows a larger amount of sodium to reach the distal tubule, where aldosterone and other factors enhance hydrogen or potassium loss into the tubular lumen in exchange for sodium. Potassium depletion reduces or eliminates potassium exchange as a means of sodium retention, thus placing greater emphasis on hydrogen ion exchange. Because renal hydrogen excretion is linked with bicarbonate resorption, the excess bicarbonate cannot be eliminated, and metabolic alkalosis is maintained. This is the reason for the paradoxic aciduria seen in some patients with metabolic alkalosis, and it is the reason these patients respond when given intravenous fluids containing chloride -and potassium. The compensating respiratory response to metabolic alkalosis is hypoventilation, resulting in an increase in the PCO2. Excessive bicarbonate administration is an additional potential cause of metabolic alkalosis. Most normal animals can tolerate large doses of bicarbonate, and excesses are rapidly eliminated by renal excretion.16 However, patients with decreases in effective circulating fluid volume, particularly when coupled with potassium or chloride deficits, may not tolerate a bicarbonate load because renal clearance of excess bicarbonate is likely to be impaired. Attempts to alter the acid-base balance, and thereby affect the athletic performance of racing horses, by prerace administration of high doses of sodium bicarbonate—containing “milk shakes” has become a major concern around the world. This has stimulated substantial research on the acid-base balance of horses before and after racing. In many racing jurisdictions stringent prerace or postrace standards for blood pH or bicarbonate levels (or both) have been enacted to control this practice. The prerace administration of the diuretic furosemide is allowed in some racing jurisdictions. This drug results in a mild metabolic alkalosis with increased bicarbonate, and allowances for this may be included in some regulations. Racing supplements (which may contain sodium bicarbonate), bicarbonate precursors, and diets that supply a high cation-anion balance also have the potential to produce a significant metabolic alkalosis.
Respiratory Acidosis
Respiratory acidosis is characterized by a decrease in pH and an increase in PCO2, which develop because of decreased effective alveolar ventilation. CO2 diffuses through the lungs much more readily than oxygen; thus diseases that compromise ventilation normally result in decreases in Po2 before significant increases in PCO2 develop. The most common causes are acute upper respiratory obstruction and primary pulmonary diseases, including pneumonia, pneumothorax, and chronic obstructive lung disease (Box 22-15). Diseases -or drugs that affect the respiratory center of the central nervous system also may produce respiratory acidosis, as can general anesthesia. The compensating response for respiratory acidosis is renal retention of bicarbonate. This response requires days to develop and is seen only in chronic respiratory acidosis. Exogenous bicarbonate does not correct respiratory acidosis and should not be administered to affected patients.
Respiratory Alkalosis
Respiratory alkalosis is caused by hyperventilation, which may be stimulated by hypoxemia associated with pulmonary disease, congestive heart failure, severe anemia, or neurologic disorders (Box 22-16). The initial compensating response to acute respiratory alkalosis is a modest decline in the ECF bicarbonate concentration, the result of cellular buffering. Subsequent renal responses result in a decrease in the ECF bicarbonate concentration through reduced renal bicarbonate resorption. The decline in bicarbonate may be offset by chloride retention; thus hyperchloremia and decreased PCO2 may be associated with compensated respiratory alkalosis, as well as with compensated metabolic acidosis. Compensating responses for chronic respiratory alkalosis that lasts several weeks actually may be sufficient to return pH to normal.
Mixed Acid-Base Imbalances
Mixed acid-base disorders occur when several primary acid-base imbalances coexist.17 Metabolic acidosis and alkalosis can coexist, and either or sometimes both may occur with either respiratory acidosis or respiratory alkalosis. The following factors should be considered when evaluating possible mixed acid-base disorders:
Mixed acid-base abnormalities occur with some frequency in domestic animals and often are overlooked. The practitioner must be aware of the potential for mixed acid-base imbalances to correctly interpret blood gas data in complex clinical situations.
Anion Gap
The anion gap can be an extremely helpful tool for categorizing causal factors in acid-base imbalances and may prove a useful prognostic guide in animals with serious digestive disorders. The anion gap can be calculated as the difference between the major cations (sodium plus potassium) and the measured anions (chloride plus bicarbonate). The anion gap normally is 12 to 16 and provides an approximation of the so-called “unmeasured anions.” These are anions that are not measured routinely in the clinical laboratory; they include the anionic equivalents of plasma proteins (particularly albumin), sulfate, phosphate, lactate, ketones, and a variety of inorganic anions. Significant differences exist in the normal range of the anion gap among species, and there also may be age-related differences. Foals are reported to have a larger anion gap than adult horses.
Hypoalbuminemia and hyperchloremic metabolic acidosis are the most common causes of a decrease in the anion gap resulting from decreases in unmeasured anions. The cause of normal to low anion gap hyperchloremic metabolic acidosis often can be differentiated on the basis of the serum potassium concentration. Animals with hyperchloremic metabolic acidosis associated with gastrointestinal fluid losses or renal tubular acidosis most often manifest hypokalemia, whereas hyperkalemia generally is seen in patients with decreased mineralocorticoid secretion (Addison’s disease) or renal failure with renal shutdown. Decreases in the anion gap can be seen with increases in cationic proteins associated with polyclonal gammopathy or multiple myeloma. Decreases in the anion gap also result from overhydration caused by decreases in the protein concentration and changes in the relative concentration of plasma sodium and chloride.
Most commonly, high anion gap acidosis is associated with accumulation of a metabolizable acid such as lactic acid associated with anaerobic exercise, grain overload, or hypovolemic shock. The commonly employed laboratory procedures for the determination of lactate only measure the L form of lactate. Microbial fermentation in the gastrointestinal tract may result in the production of both the D and L forms of lactate. It has recently been shown in milk-fed calves and kids that accumulation of D-lactate is a major factor in the profound acidosis associated with certain digestive disorders. Unfortunately, at present, determination of D-lactate requires special procedures that may not be readily available. Ketoacidosis, uremic acidosis, and poisoning with a variety of anionic poisons result in increases in nonmetabolizable acids that are also causes of an increased anion gap. When a high anion gap metabolic acidosis is found, a thorough search for the potential causes of the accumulated unmeasured anions is indicated. The anion gap also is useful for identifying mixed acid-base imbalances. A mixed metabolic acid-base imbalance should be suspected when the change in the anion gap does not approximate the change in bicarbonate. Increases in the anion gap can be associated with dehydration and contraction alkalosis caused by changes in the protein concentration and the relative concentration of plasma sodium and chloride.
Bicarbonate and Total Carbon Dioxide
Bicarbonate accounts for approximately 95% of the measured CO2; thus the total CO2 (TCO2) or “CO2 content” of serum or plasma provides a measure of metabolic changes in the acid-base balance. Most automated chemistry profiles now provide the bicarbonate level directly, whereas some may still provide the TCO2. Bicarbonate or TCO2 is decreased in metabolic acidosis and increased in metabolic alkalosis. However, the bicarbonate or TCO2 values provide only a crude indication of acid-base status. When acid-base abnormalities are suspected, appropriate samples should be submitted for blood gas determination.
Buffer Base, Standard Bicarbonate, and Base Excess or Base Deficit
The terms buffer base, standard bicarbonate, and base excess (or base deficit) represent derived calculated estimates of the metabolic component of acid-base balance. The buffer base indicates the sum of all the buffer anions in blood under standardized conditions. The standard bicarbonate is the plasma bicarbonate concentration that would be found under specific conditions that eliminate respiratory influences on the values obtained. The base excess or base deficit often is supplied in routine assessment of acid-base balance; it indicates the deviation of bicarbonate from normal. The calculated base deficit provides a means of estimating the amount of bicarbonate required to correct metabolic acidosis. The bicarbonate estimate is calculated by multiplying the base deficit by the probable bicarbonate space (about 40% to 60% of body weight), as in the following equation:
NONTRADITIONAL OR STRONG ION APPROACH TO ACID-BASE BALANCE
Peter Stewart first described the quantitative physiochemical approach to acid-base balance over 25 years ago.18,19 In this approach acid-base balance is determined by three independent variables: strong ion difference (SID), the partial pressure of CO2, and the total concentration of nonvolatile weak acids (Atot), the principal components of which are plasma proteins and inorganic phosphate. Bicarbonate and pH are dependent variables determined by these three independent variables. Several studies have attempted to adapt Stewart’s approach for practical application in human and veterinary medicine.14,20-27 Most of these studies used rather old human values for Atot and Ka. Experimentally determined species-specific Atot and Ka data are now available for horses, cattle, dogs, and humans.28-30
Peter Constable refined Stewart’s model and developed an approach that he called the simplified strong ion model of acid-base equilibrium.31 Constable assumed that plasma ions act either as strong ions, volatile buffer ions (HCO−3), or nonvolatile buffer ions. Plasma pH is determined by five independent variables: PCO2, SID, the concentration of individual nonvolatile plasma buffers (albumin, globulin, and phosphate), ionic strength, and temperature. The simplified strong ion model explains many of the anomalies when the Henderson-Hasselbalch equation is applied to plasma and is algebraically simpler than Stewart’s model.
Strong electrolytes are assumed to be completely dissociated in aqueous solution and chemically nonreactive. The SID is simply the difference between the total concentration of strong cations (sodium, potassium, and magnesium) and the total concentration of strong anions (chloride, sulphate, lactate, acetoacetate, and B-hydroxybutyrate). Sodium, potassium, and chloride are normally the principal determinants of SID. The SID is synonymous with buffer base and as such can be considered as roughly equivalent to the metabolic component of the traditional approach to acid-base balance. In fluids with little or no protein, such as cerebrospinal fluid (CSF), bicarbonate concentration is the same as the SID. Abnormalities in PCO2 are viewed in essentially the same manner in both the traditional and nontraditional approaches to acid-base balance. Plasma albumen makes up the majority of Atot. The Atot exists in both dissociated A− and undissociated HA forms. A decrease in Atot resulting from hypoalbuminemia causes an alkalosis with an increase in bicarbonate, whereas hyperalbuminemia has the opposite effect. Increases in A− can cause an increase in anion gap, whereas decreases in A− result in a decrease in anion gap. Change in protein concentration may potentiate or ameliorate the effects of alterations in SID on acid-base balance.
When protein and inorganic phosphate remain within the normal range, acid-base balance is controlled by changes in PCO2 mediated by the respiratory system, whereas changes in SID are largely under the control of the kidneys. Heavy sweat loss in an endurance horse, displaced abomasum in a cow, or the prerace administration of the diuretic furosemide in a race horse produces metabolic alkalosis. In each case the alkalosis is the result of a disproportionate loss of chloride relative to sodium, yielding hypochloremia and an increase in SID. Correction of the alkalosis is brought about by the provision of chloride, generally as sodium chloride or potassium chloride. This results in a decrease in SID and thus a return of the dependent variables (bicarbonate and pH) toward normal. Metabolic acidosis with a large base deficit is generally treated with sodium bicarbonate. In the strong ion approach to treatment the rationale for the administration of sodium bicarbonate is to provide the strong cation, sodium, without a strong anion. Other metabolizable anions could be substituted for bicarbonate and achieve similar effects.
Calculation of SID is simple and provides useful insight in patients with metabolic acid-base disturbances. Factors that influence SID range from changes in free water, to sodium-chloride imbalances that result from excessive losses or disproportionate retention of sodium or chloride, to the accumulation of strong organic anions. Organic acidosis can be produced by the accumulation of exogenous as well as endogenous anions. The anion gap does not always accurately predict the presence of unmeasured strong anions. Mathematic methods have been developed as a means for the detection of unmeasured anions18,32 and more recently for the calculation of the simplified strong ion gap.33
Both the traditional and the nontraditional or strong ion approaches to acid-base balance have proven useful to address practical problems in both research and medical settings. The traditional approach to acid-base balance is more widely accepted and user-friendly. The strong ion approach may provide a better understanding as to why the bicarbonate concentration is changing as it integrates acid-base and electrolyte disorders. The strong ion approach has recently gained wider acceptance from members of the human critical care community, who have found it useful for the analysis of the complex fluid, electrolyte, and acid-base problems of patients in intensive care units. Several easy-to-use computer or calculator programs have been developed for the mathematically challenged that facilitate implementation of the strong ion approach to acid-base balance.
SERUM ENZYMES
Some of the common and less common causes of elevated serum enzyme activity are listed in Box 22-17.
Box 22-17 Causes of Elevated Serum Enzymes
ELEVATION OF LACTATE DEHYDROGENASE (LDH)
Sorbitol Dehydrogenase
SDH is a liver-specific enzyme in all large animal species, and increases in this enzyme indicate hepatocellular damage and leakage of enzymes. Increases in SDH also are seen with obstructive or strangulating gastrointestinal lesions and with acute toxic enteritis as a result of liver damage associated with absorption of bacteria or their toxins (or both) from the damaged bowel into the portal circulation. This enzyme is a sensitive indicator of liver damage, and modest increases may be seen with anoxia, acute anemia, or general anesthesia. The half-life of SDH in the circulation is short (a matter of hours), and elevations indicate active and ongoing liver damage. This enzyme is not stable when stored at room temperature, but refrigerated samples may yield useful results after several days of storage.
Creatine Kinase
CK is a highly sensitive and specific indicator of muscle damage in domestic animals. Although CK is found in both cardiac and skeletal muscle, elevations of this enzyme most commonly are associated with exertional myopathies (rhabdomyolysis) and also are seen as musculoskeletal manifestations of systemic disease. Intramuscular injections, vigorous exercise, or prolonged shipping may result in modest releases (up to a fourfold increase over resting values) of CK into the circulation without producing histologic evidence of muscle damage. Endurance exercise may lead to moderate elevation of CK (2000 to 15,000 IU/L) in some horses that show no recognizable sign of exertional myopathy. The half-life of this enzyme in the circulation is very short (2 hours in horses and 4 hours in cattle), and even marked elevations in CK may return to normal within 12 to 24 hours after a single muscle insult. Although marked elevation of CK can be a guide to the extent of muscle damage, the short half-life and the potential for continuing myonecrosis have a marked influence on the enzyme activity observed at any point in time. A persistent elevation of CK suggests a process resulting in active and continuing muscle damage and provides grounds for resting athletic horses. Elevated CK provides no information on the factors responsible for the rhabdomyolysis. Hemolysis may produce falsely high values for CK.
Aspartate Aminotransferase
AST is found in high concentration in a variety of tissues, including skeletal and cardiac muscles, the erythrocytes and kidneys, and the liver. This enzyme is a nonspecific indicator of tissue damage and tends to be less sensitive to mild insults than the tissue-specific enzymes SDH and CK. The half-life of AST in the circulation is relatively long, and elevations may persist for as long as 10 days after an episode of myonecrosis or liver damage. As a general rule, extensive muscle necrosis tends to produce much higher elevations of AST than severe liver necrosis. This enzyme is most useful when compared with the tissue-specific enzymes as determined sequentially over the time course of a disease process. Elevations of CK and AST indicate muscle damage, whereas elevations of SDH and AST indicate liver damage. Marked but transient elevations of CK and SDH are associated with a single insult to the muscles and liver, respectively, whereas AST increases gradually and remains elevated for a much longer time. Thus a moderate to marked increase in AST in an animal with progressively declining SDH or CK indicates that some tissue damage occurred within the past 7 to 10 days but also that the process may no longer be active. This often is a favorable prognostic indicator. Persistent elevation of or a progressive increase in CK or SDH and AST over time indicates an active, continuing process of tissue damage, and the prognosis is more guarded. AST is relatively stable at room temperature, but hemolysis or lipemia may interfere with the assay.
γ-Glutamyltransferase
GGT is an important marker of hepatobiliary disorders and cholestasis in large animals. GGT is quite stable, and reliable results can be obtained from samples submitted several days after blood samples have been drawn, provided the serum is refrigerated. The activity of this enzyme is highest in the cells of the periportal region of the liver, in the pancreas, and in the renal tubular cells. Pancreatic diseases resulting in inflammation and necrosis are relatively rare in large animal species. Damage to the renal tubular cells leads to a release of GGT into the tubular lumen and the urine. Because this enzyme is a relatively large molecule, it is not resorbed into the systemic circulation, and renal tubular damage does not result in elevated serum GGT activity. Increases in GGT relative to creatinine in the urine have been used as an index of acute renal tubular damage. However, the validity of the normal range for this ratio in horses has been questioned.
In large animal species an elevation in serum GGT is one of the more reliable indicators of damage to the liver and biliary obstruction. Disease processes such as pyrrolizidine alkaloid intoxication, chronic active hepatitis, cholangiohepatitis, and cholelithiasis produce liver damage, primarily in the periportal region, leading to marked and persistent elevation of GGT activity in the serum. In these instances, elevations in serum ALP activity generally are associated with the increase in GGT. Two syndromes, fatty liver syndrome in dairy cows and hyperlipemia syndrome of periparturient mares of pony and miniature horse breeds, are associated with liver damage, which is often reflected by elevation of GGT.
Most suckling neonatal large animals have high levels of GGT activity in their serum. This is the result of absorption of maternal GGT, which is present in relatively high levels in the colostrum. Elevation of GGT in neonates should be regarded as a normal finding unless it is associated with other evidence of liver disease. The normal range of serum GGT activity for burros, donkeys, and asses may be substantially higher (two to three times) than the normal range of serum GGT activity for horses. Caution should therefore be used when evaluating this enzyme in these species. Elevation in serum GGT activity has been reported in thoroughbred racehorses that are performing below expectations. The reasons for the increase in GGT are not known, but horses often respond to a period of rest or reduction in workload. These horses show little histologic evidence of liver damage, and other indices of liver damage and dysfunction usually are within normal limits. The stress of training may be associated with an elevated GGT. Certain trainers, often highly successful trainers, appear to have a disproportionately large number of horses with elevations of this enzyme. The normal range for GGT of thoroughbreds in race training may be slightly higher than that of normal sedentary horses.
Alkaline Phosphatase
ALP is used in most species as a marker for intrahepatic or extrahepatic obstruction of the biliary system. The enzyme is also released by osteoblasts from metabolically active bone. This may be the reason that young, rapidly growing animals normally have high levels of serum ALP. Elevations in ALP are also reported in cases of rickets and healing fractures. The intestinal isoenzyme of ALP is very similar to the ALP isoenzyme found in neutrophils. Elevations in the ALP activity of abdominal fluid in horses with intraabdominal disease may reflect the release of this enzyme from the neutrophils rather than being a specific marker of damage to the bowel.
ALP has been useful for evaluating liver disease in large animals, particularly in horses with pyrrolizidine alkaloid intoxication, chronic active hepatitis, and cholangiohepatitis, and in some patients with cholelithiasis. A profound elevation in ALP activity is thought to reflect periportal liver damage and biliary obstruction in these patients. A moderate to marked elevation in ALP may be observed in a wide range of disorders resulting in hepatic necrosis and intrahepatic cholestasis. Because this enzyme is not organ specific in large animals, elevations in ALP activity must be interpreted in relation to more organ-specific enzymes such as SDH and GGT.
Other Enzymes
Lactate dehydrogenase is found in relatively high concentrations in a variety of organs and tissues of the body from the heart, liver, muscle, and kidney to the erythrocytes and leukocytes. An elevation in serum LDH enzyme activity must be evaluated in relation to other, more organ-specific enzymes. LDH isoenzyme analysis can be helpful in differentiating organ system damage, but the analysis is time-consuming and not always available. An elevation in LDH activity is expected in hepatic necrosis and serves as an indicator of an active disease process. Extensive muscle damage and rhabdomyolysis tend to result in a more massive release of enzyme and much higher serum enzyme activity. A modest elevation in LDH may be seen in some hemolytic disorders and some cases of leukemia. Blood samples must be handled with care, because hemolysis results in falsely elevated serum LDH activity.
Glutamic dehydrogenase and ornithine carbamoyltransferase are two enzymes that are reported to be sensitive indicators of hepatic necrosis in ruminants. Alanine aminotransferase (ALT) is an important liver-specific enzyme that has wide application in small animals and is often included in automated chemistry profiles. This enzyme has not been useful for evaluation of liver disease in large animal species, and occasionally horses with marked rhabdomyolysis and no other evidence of liver disease show an elevation in serum ALT activity.
BILIRUBIN
Bilirubin is a breakdown product of the heme component of the hemoglobin molecule. Bilirubin exists in the serum in two forms and is responsible for the yellow color known as icterus, or jaundice, of the mucous membranes. Unconjugated, prehepatic, albumin-bound bilirubin is also known as “indirect-reacting” bilirubin, as determined by the van den Bergh reaction. Indirect-reacting bilirubin must be taken up by the liver cells, where it is conjugated and then excreted in the bile. Conjugated bilirubin is known as “direct-reacting” bilirubin, as determined by the van den Bergh reaction. Horses normally have a much higher serum bilirubin level than ruminants, and hot-blooded horses have a higher bilirubin level than cold-blooded horses of the pony and draft breeds. The horse also differs from ruminants in that horses often develop moderate icterus in response to fasting or anorexia associated with many systemic diseases. The increase in the bilirubin concentration in these horses is caused almost entirely by an increase in unconjugated (indirect-reacting) bilirubin, and within a few days the bilirubin can increase from the normal range up to 6 to 8 mg/dL. Therefore the total serum bilirubin concentration is of little diagnostic value in the ill horse unless both the direct- and indirect-reacting bilirubin values are determined.
Total serum bilirubin is elevated in animals with hemolytic anemia, and this increase is caused largely by an increase in indirect-reacting bilirubin (Box 22-18). The degree to which bilirubin is elevated in hemolytic anemia is a function of the rate of red cell destruction and the capacity of the liver to excrete the newly formed bilirubin. The total bilirubin rarely exceeds 10 mg/dL in hemolytic anemia. An exception is the hemolytic anemia of neonatal isoerythrolysis in newborn foals, which often is associated with marked clinical icterus. In these foals the serum bilirubin may exceed 25 mg/dL, a variable but substantial proportion of which (40% to 60%) is likely to be direct-reacting bilirubin.
The second major cause of clinical icterus and increased serum bilirubin is liver failure. Liver failure results in impaired uptake and excretion of bilirubin. Acute liver failure caused by hepatic necrosis results in marked to moderate increases in both direct- and indirect-reacting bilirubin. In horses with acute liver failure, bilirubin often exceeds 10 mg/dL, and this increase is caused primarily by increases in indirect-reacting bilirubin. Direct-reacting bilirubin rarely exceeds 25% of the total bilirubin in the horse, and increases of this magnitude suggest an intrahepatic or extrahepatic biliary obstruction. With chronic liver failure, icterus is more variable, and total bilirubin rarely exceeds 10 mg/dL. Liver failure in ruminants, particularly chronic liver failure, is associated with a much less striking elevation in serum bilirubin than occurs in horses. In the absence of hemolytic anemia, a bilirubin value above 2 mg/dL indicates impaired hepatic function in ruminants.
GLUCOSE
The concentration of glucose in the blood normally is regulated by the hormones insulin and glucagon, but it is influenced by several other factors as well.
Hypoglycemia
Fasting usually does not result in hypoglycemia except in neonatal animals (Box 22-19). Newborns have limited energy reserves, and any disease, injury, congenital defect, maternal rejection, agalactia, or management error that limits energy intake can result in marked hypoglycemia associated with profound depression, even coma. Rapid, semiquantitive field tests for blood glucose (Dextrostix* and Chemstrip BG†) provide a practical means of early recognition of this problem. Hypoglycemia may be seen in animals with acute toxic enteritis, coliform mastitis, septicemia, and colic associated with strangulated bowel, as well as in the later stages of endotoxemia and in some horses with exhaustion after prolonged exercise. Hypoglycemia is a reasonably consistent feature with primary ketosis and fat cow syndrome in cattle, with pregnancy toxemia in sheep and goats, and in hyperlipemia syndrome, which is seen primarily in pregnant or lactating ponies.
Hyperglycemia
Hyperglycemia may be seen with excitement, transportation, or stress and probably is mediated by increases in catecholamine and glucocorticoid hormones (Box 22-20). The stress and pain of acute severe colic in horses frequently results in hyperglycemia, and elevations of blood glucose above 250 mg/dL are associated with a poor prognosis in such cases. Endotoxemia initially results in a transient hyperglycemia that may be followed by marked hypoglycemia in the terminal stages of the toxemia. The later stages of Cushing’s syndrome in horses generally are associated with a non—insulin-responsive hyperglycemia and glycosuria. Similar changes can be induced transiently when exogenous glucocorticoid hormones are administered at a high dose rate. Insulin-responsive diabetes mellitus rarely occurs in large animals but has been reported in association with destructive pancreatic lesions.
CREATININE
Creatinine is derived from the cyclic use of phosphocreatine, the muscle energy store, resulting in the production of inorganic phosphate and creatinine. In the resting animal this process occurs at a relatively constant rate. The absolute muscle mass and level of physical activity may influence the rate of creatinine production and thus the serum concentration. Starvation, with loss of muscle mass, may result in a slightly reduced serum creatinine level, whereas serum creatinine may be slightly higher in muscular, athletic individuals than in sedentary animals. Creatinine is distributed throughout the body water and is not reused. Creatinine is normally excreted by the kidneys, primarily by glomerular filtration. In azotemic patients a substantial part of creatinine is metabolized and excreted by nonrenal routes. Serum or urine creatinine concentrations determined by the standard alkaline picrate reaction may be falsely elevated by the presence of noncreatinine chromogenic compounds in the serum. These chromogens include glucose, fructose, ascorbic acid, hippuric acid, urea, ketones, and several other compounds. The contribution of these compounds can be reduced or eliminated, and most automated chemical laboratories use such methodology. However, if inappropriately high creatinine is reported in patients without other evidence of renal failure, the method of creatinine measurement should be ascertained.
Alterations in renal blood flow caused by decreases in effective circulating fluid volume (hypovolemia) produce an elevation in serum creatinine and BUN; this can be considered a prerenal azotemia (Box 22-21). It occurs with some frequency in animals with acute enteritis, peritonitis, acute heart failure, massive blood loss, and some forms of colic and in horses with exhaustive disease syndrome. An important point is that many of these disorders initiate the release of inflammatory mediators, which may cause renal damage and impaired renal function above and beyond that associated with impaired renal blood flow and hypovolemia. Prerenal azotemia usually is seen in animals that are dehydrated and volume depleted and that have a history of loss or compartmentalization of sodium-containing fluid. Prerenal azotemia often can be marked (creatinine level above 6 mg/dL), but if uncomplicated, it generally responds rapidly to fluid replacement therapy. Urine production, the urine sodium concentration, and the fractional excretion of sodium usually are low, and the urine specific gravity usually is elevated. The ratio of urine to plasma urea or creatinine, as well as the urine-to-plasma ratio of osmolality, are reported to be higher in horses with prerenal azotemia compared with renal azotemia.16
The serum creatinine concentration provides a crude measure of the glomerular filtration rate. However, serum creatinine, like BUN, is not a very sensitive or early indicator of changes in renal function. In ruminants, creatinine is a more reliable indicator of renal failure than BUN. Urea nitrogen can be secreted in saliva and metabolized by the ruminal microflora; this frequently results in a disparity between BUN and creatinine levels in ruminants with renal -failure. Although small increases in creatinine may be seen with progressively compromised renal function, nearly two thirds to three fourths of the nephrons must be nonfunctional before the serum creatinine level clearly exceeds the normal range. Both acute and chronic renal failure are usually associated with elevated creatinine. Acute renal failure, especially in animals with anuria or oliguria, is usually associated with progressive daily changes in blood parameters. In contrast, blood parameters of animals with chronic renal failure tend to remain relatively constant. A transient but markedly elevated serum creatinine has been observed in some newborn foals that have no other evidence of compromised renal function. Many of these foals are born to mares that had medical problems before parturition. Alterations in placental function may allow the accumulation of creatinine in the foal’s circulation. In most of these otherwise normal foals this marked elevation in serum creatinine resolves within the first few days of life.
BLOOD UREA NITROGEN
The term blood urea nitrogen is ingrained in the veterinary literature, despite the fact that urea nitrogen determinations usually are performed on serum or plasma samples. This is of little consequence, because the actual difference in urea concentrations of whole blood and serum or plasma is relatively small. Urea provides a nontoxic means of excreting ammonia generated by amino acid catabolism and the intestinal microflora. It is distributed throughout the body water. In the intestine, urea is broken down by urease, which is produced by the intestinal microflora, and the nitrogen, as ammonium ion, is recycled to the liver. Urea is excreted by the kidneys, primarily by glomerular filtration.
Urea production occurs almost exclusively in the liver, and liver failure is frequently associated with a decrease in BUN (Box 22-22). Urea nitrogen tends to be low in nursing animals because of their high fluid intake and urine output and their anabolic state of rapid growth. Urea nitrogen may be reduced slightly in animals given anabolic steroids or fed diets low in protein but of adequate calorie content.
Starvation or other processes that result in rapid tissue catabolism such as fever, burns, or corticosteroid administration may result in modest increases in BUN (Box 22-23). BUN, along with creatinine, provides a crude index of altered renal function in most animal species. BUN is influenced more directly by dietary factors than is creatinine, and creatinine is generally a better guide to renal failure. This is particularly true in ruminants, in which BUN may remain within normal limits in animals with marked impairment of renal function. With these considerations in mind, the discussion of prerenal, renal, and obstructive causes of azotemia in the section on creatinine apply to BUN.
SERUM PROTEIN
The serum or plasma proteins consist of albumin and globulin fractions. These are discussed in Chapter 26.
URINALYSIS
Urinalysis can be an extremely useful diagnostic tool, providing information on many systemic disorders. It is essential in the evaluation of primary renal disease. Urine normally is collected as a voided sample or after catheterization. Voided urine samples are safe and easy to collect but are easily contaminated. Catheterization is preferred for bacteriologic evaluation, but the resultant mild trauma may result in a slight increase in urine red cells and protein. Because of the urethral diverticulum at the level of the pelvis, it is difficult to successfully catheterize male ruminants. Urethral obstruction is common in male small ruminants, and percutaneous aspiration may be the only way to obtain a urine sample in affected animals. Urinalysis should be performed as soon after collection as possible (within 30 minutes) to avoid degeneration of cellular elements, changes in pH, or bacterial overgrowth. If this is not possible, samples should be refrigerated. Urine volume and composition are influenced by feed and water intake, salt supplementation, environmental factors, exercise, stress, systemic disease, and drug administration. Urine samples collected after administration of a diuretic are dilute and unsuitable for routine urinalysis. The tranquilizer xylazine, which is often used to assist in the catheterization of male horses, may alter the results of the urinalysis because it produces diuresis as a result of glycosuria.
Specific Gravity
Specific gravity is defined as the weight of urine relative to the weight of distilled water. The specific gravity of serum is approximately 1.008 to 1.012. The urine specific gravity is an indicator of renal function, because it reflects the action of the renal tubules and collecting ducts on the glomerular filtrate by providing an estimation of the number of particles dissolved in the urine. It is usually measured by refractometry. Urine dipsticks with reagent pads for estimating the urine specific gravity in humans should not be used for this purpose in domestic animals, because a poor correlation has been reported compared with refractometry in large animals.26 Normal animals have the capacity to dilute their urine specific gravity to less than 1.010 and to concentrate to greater than 1.050. The normal range reported for most adult large animals is 1.020 to 1.050. Suckling neonatal animals normally produce a very dilute urine with a specific gravity below 1.010. Although this could represent immature renal function, it more likely reflects their high-volume fluid intake, because normal neonates can concentrate their urine if fluids are withheld.
Failure to produce a concentrated urine, as reflected by a specific gravity below 1.020, in the face of dehydration is an indication of altered renal function, which may be caused by primary renal disease, diabetes insipidus, medullary washout, or nephrogenic diabetes insipidus. With severe and chronic sodium depletion, tubular sodium may be insufficient to sustain normal countercurrent mechanisms for water resorption. This process is called medullary washout and has been reported as a cause of polyuria in lactating dairy cattle on a salt-deficient diet. At least 50% of renal function must remain for highly concentrated urine to be produced. Isosthenuria, a urine specific gravity that remains around 1.010 despite variation in hydration, occurs when renal disease progresses to the point at which renal function is reduced to less than a third of normal. Altered renal function in these animals is usually reflected by a moderate to marked elevation in BUN and creatinine levels and by other changes in the urinalysis. Hyposthenuria occurs when the specific gravity remains below 1.010; this indicates altered release of or response to antidiuretic hormone, as occurs with diabetes insipidus, medullary washout with chronic sodium depletion, nephrogenic diabetes insipidus, psychogenic polydipsia, and chronic liver failure in some horses.
pH
The normal range of urine pH for adult herbivores is alkaline, with values ranging from 7 to 9. Neonatal foals tend to have a slightly acid urine with a pH below 7. Aciduria in adults may be seen in postrace samples collected from racehorses, after prolonged fasting, with ketosis in ruminants, or in response to metabolic acidosis. A paradoxic aciduria frequently is seen in ruminants with hypochloremic, hypokalemic metabolic alkalosis, as was explained earlier. Urine pH is also influenced by the cation-anion balance of the diet.
Protein
Protein normally is not detected in urine, although a false-positive protein reaction may be noted on urine dipsticks if the sample is strongly alkaline. Urine with a positive reaction for protein on dipsticks should be checked for protein by a chemical method. Proteinuria should be evaluated in relation to the other findings in the urinalysis. Persistent and strongly positive reactions for protein in the absence of leukocytes, red cells, bacteria, or casts suggest glomerular protein loss, as in glomerulonephritis or amyloidosis. The presence of bacteria and leukocytes with proteinuria suggests sepsis in the urinary tract, whereas hemorrhage or inflammation in the urogenital tract often is associated with proteinuria.
Glucose
Glucose is not found in the urine of normal large domestic animals unless the blood glucose level increases above the renal threshold, which is thought to be approximately 100 to 140 mg/dL in ruminants and approximately 160 to 180 mg/dL in horses. The causes of hyperglycemia and glycosuria were described in a previous section; they include Cushing’s syndrome, stress, and catecholamine or glucocorticoid hormone release. Hyperglycemia and glycosuria can be created iatrogenically when glucose-containing fluids are administered at an excessive rate. Glycosuria is a fairly consistent finding in sheep with enterotoxemia type D (pulpy kidney). The presence of glycosuria without hyperglycemia strongly suggests renal tubular damage resulting from a toxic or ischemic insult.
Occult Blood
Both myoglobin and hemoglobin give a positive reaction on urine occult blood dipsticks. In most instances proteinuria shows a positive result as well. A tentative clinical impression sometimes can be formed to differentiate myoglobin from hemoglobin in dark urine if the sample is shaken vigorously in a closed, transparent container. Myoglobin tends to produce a brown foam, whereas hemoglobin produces a reddish foam. Hemoglobinuria caused by intravascular hemolysis is generally associated with clinical and hematologic evidence of a hemolytic anemia. Hematuria may result in a positive occult blood reaction if lysis of some of the intact red blood cells has occurred. This is likely to happen if the urine is very dilute or if it is held at room temperature for an extended period before analysis. False-positive reactions can occur if microbial peroxidase or oxidizing contaminants are present.
Myoglobin
Myoglobinuria should be associated with clear clinical and clinicopathologic evidence of extensive muscle damage. The ammonium sulfate precipitation method of differentiating hemoglobin from myoglobin is imprecise and frequently fails to detect myoglobin in the dark, coffee-colored urine of horses with severe rhabdomyolysis. Accurate differentiation of these compounds in urine requires more sophisticated procedures.
Cells
The normal range for cells generally is considered to be up to five red cells or leukocytes per high-power field. An increased number of erythrocytes indicates hematuria, which may be caused by neoplasia, trauma, inflammation, or coagulopathy. Pyuria, an increase in urine leukocytes, indicates an inflammatory process, and when associated with bacteriuria it indicates a septic process in the urinary tract. The presence of sheets or rafts of transitional cells suggests neoplasia.
Casts
Casts are accumulations of protein and cellular material that form in the renal tubules, and when present in the urine, they indicate renal damage or tubular disease. Casts can consist of erythrocytes, leukocytes, or renal tubular cells. As cellular degeneration proceeds, the cell type is more difficult to determine, and the casts become granular and then waxy. Hyaline casts are noncellular and are formed from mucoprotein. They may be seen with glomerulonephritis, fever with passive congestion, or severe dehydration with altered renal blood flow.
Crystals
The crystalline structures in the urine of large animals are those usually associated with an alkaline urine. Calcium carbonate crystals normally are found in abundance in horse urine, particularly if the horse has been fed alfalfa hay. Triple phosphate and calcium oxalate crystals are frequently observed in relatively small numbers. The major crystals involved in urolithiasis of feedlot cattle is struvite (Mg NH4PO4·6H2). In the western United States silicate stones are most common in livestock under range conditions. Carbonate and oxalate stones are common causes of urolithiasis in small flocks of backyard sheep and goats fed alfalfa hay.
Bacteria
Bacteria sometimes are seen in small numbers in voided urine samples and may represent surface contaminants. Bacterial infections of the urinary tract usually are associated with significant pyuria. When this is noted, a catheterized sample (horse) or clean midstream catch (ruminant) should be obtained for Gram stain, culture, and sensitivity testing. Results from ruminant samples must be carefully interpreted, because male ruminants routinely urinate within the prepuce, thus heavily contaminating samples. Fortunately, urinary tract infections in male ruminants and horses are rare.
Urine Creatinine Clearance Ratio
Urinary electrolyte excretion is affected by a variety of factors, including dietary intake, alterations in renal function, and specific hormones that regulate renal electrolyte excretion Determination of electrolyte concentrations from randomly collected urine samples is easily done and can be clinically useful when these concentrations are compared with serum concentrations. The presence of substantial amounts of sodium in the urine of an animal with hyponatremia suggests excessive renal sodium loss caused by altered renal function or hormonal control. However, marked variation in the rate of urine production can lead to serious problems in the interpretation of urine electrolyte concentrations. The standard physiologic methods of determining renal electrolyte clearance are complicated by the need for quantitative urine collection and are not well suited to most practical clinical situations. One means of overcoming these difficulties is expression of the renal electrolyte clearance relative to the endogenous creatinine clearance. Expression of the renal clearance as a ratio eliminates the need for quantitative urine collection. This derived value is known as the creatinine clearance ratio or the fractional excretion (FE) and is calculated by the following formula:
where X represents the electrolyte concentration and C represents the creatinine concentration.
The FE fluctuates somewhat during the day in relation to physical activity and feed and water intake. However, under standardized conditions, there is close agreement between the FE determined from single random samples of blood and urine and that based on samples quantitatively collected during a 12- to 24-hour period.16 The FE of electrolytes has a very wide range of normal values. The principal sources of this variation are differences in dietary intake and environmental or experimental conditions. The FE of electrolytes has been useful for detecting specific dietary deficiencies or imbalances. Dietary salt deficiency is associated with an extremely low FE of sodium and chloride, whereas the plasma concentration of these ions generally remains within normal limits. In a similar fashion, dietary calcium and phosphorus imbalances seldom are reflected by the serum concentrations of these ions. Calcium deficiency and phosphorus excess are relatively common dietary problems in large animals and result in a low FE for calcium and a high FE for phosphorus. There are technical problems with the determination of the urine calcium concentration in horses because of their normally alkaline urine and the resultant precipitation of calcium carbonate in the urine. Mixed urine samples from horses must be acidified (hydrochloric acid can be used) to solubilize the calcium.
Increases in the FE of sodium are noted with renal tubular damage and impaired sodium resorption. The sodium FE increase has been a useful indicator for the differential diagnosis of prerenal azotemia, which almost invariably results in a low sodium FE, from the azotemia caused by primary renal disease, in which the FE for sodium often is markedly increased.
1 Duncan JR, Prasse KW. Veterinary laboratory medicine, ed 3. Ames, Iowa: Iowa State University Press, 1994.
2 Kaneko JJ, Harvey JW, Bruss ML. Clinical biochemistry of domestic animals, ed 5. New York: Academic, 1997.
3 Looney AL, Ludders J, Erb HN, et al. Use of a handheld device for analysis of blood electrolyte concentrations and blood gas partial pressures in dogs and horses. J Am Vet Med Assoc. 1998;213:526.
4 Pringle JK, Berthiaume LMM. Hypernatremia in calves. J Vet Intern Med. 1988;2:66.
5 Harris P, Snow DH. Alterations in plasma potassium concentrations during and following short-term strenuous exercise in the horse. J Physiol. 1986;376:46P.
6 Cox JH. An episodic weakness in four horses associated with intermittent serum hyperkalemia and the similarity of the disease to hyperkalemic periodic paralysis in man. Proceedings of the Thirty-first Annual Meeting of the American Association of Equine Practitioners. 1985:383.
7 Spier SJ, Carlson GP, Harrold D, et al. Genetic study of hyperkalemic periodic paralysis in horses. J Am Vet Med Assoc. 1993;202:933.
8 Rudolph JA, Spier SJ, Byrns G, et al. Periodic paralysis in quarter horses: a sodium channel mutation disseminated by selective breeding. Nat Genet. 1992;2:144.
9 Ziemer EL, Parker HR, Carlson GP, Smith BP. Clinical features and treatment of renal tubular acidosis in two horses. J Am Vet Med Assoc. 1987;190:294.
10 Ziemer EL, Parker HR, Carlson GP, et al. Renal tubular acidosis in two horses: diagnostic studies. J Am Vet Med Assoc. 1987;190:289.
11 Speirs VC. Arteriovenous and arteriocentral venous relationships for pH, PCOM2, and actual bicarbonate in equine blood samples. Am J Vet Res. 1980;41:199.
12 Carlson GP. Fluid-electrolyte and acid-base balance. In Kaneko JJ, Harvey JW, Bruss ML, editors: Clinical biochemistry of domestic animals, ed 5, New York: Academic, 1997.
13 DiBartola SP. Introduction to acid-base disorders. In: DiBartola SP, editor. Fluid therapy in small animal practice. Philadelphia: Saunders, 2000.
14 deMorais HAS. A nontraditional approach to acid-base disorders. In: DiBartola SP, editor. Fluid therapy in small animal practice. Philadelphia: Saunders, 1992.
15 Stick JA, Robinson NE, Krehbiel JD. Acid-base and electrolyte alterations associated with salivary loss in the pony. Am J Vet Res. 1981;42:733.
16 Rumbaugh GE, Carlson GP, Harrold DR. Clinicopathologic effects of rapid infusion of 5% sodium bicarbonate in 5% dextrose in the horse. J Am Vet Med Assoc. 1981;178:267.
17 Wilson EA, Green RA. Clinical analysis of mixed acid-base disturbances. Compend Cont Educ Pract Vet. 1985;7:S364.
18 Stewart PA. How to understand acid-base: a quantitative acid-base primer for biology and medicine. New York: Elsevier, 1981.
19 Stewart PA. Modern quantitative acid-base chemistry. Can J Physiol Pharmacol. 1983;61:1444.
20 Fencl V, Rossing TH. Acid-base disorders in critical care medicine. Annu Rev Med. 1989;40:17.
21 Leith DE. The new acid-base: power and simplicity. Proc Am Coll Vet Intern Med. 1990;8:449.
22 Frischmeyer KJ, Moon PF. Evaluation of quantitative acid-base balance and determination of unidentified anions in swine. Am J Vet Res. 1994;55:1153.
23 Gilfix BM, Bique M, Magder S. A physical chemical approach to the analysis of acid-base balance in the clinical setting. J Crit Care. 1993;8(4):187.
24 Heigenhauser GJF, et al. The role of the physiochemical systems in plasma in acid-base control in exercise. In: Taylor AW, Gollnick PD, Green HJ, et al, editors. International series on sports sciences. Champaign, Ill: Human Kinetics, 1990.
25 Carlson GP. The interrelationships between fluid, electrolyte, and acid-base balance during maximal exercise. Equine Vet J Suppl. 1995;18:261.
26 Kowalchuk JM, Scheuermann BW. Acid-base regulation: a comparison of quantitative methods. Can J Physiol Pharmacol. 1994;72:818.
27 Whitehair KJ, Haskins SC, Whitehair JG, Pascoe PJ. Clinical applications of quantitative acid-base chemistry. J Vet Intern Med. 1995;9:1.
28 Stampfli HR, Misiaszek S, Lumsden JH, et al. Weak acid concentration Atot and dissociation constant Ka of plasma proteins in racehorses. Equine Vet J Suppl. 1999;30:438.
29 Staempfli HR, Constable PD. Experimental determination of net protein charge and A(tot) and K(a) of nonvolatile buffers in human plasma. J Appl Physiol. 2003;95:620.
30 Constable PD, Stampfli HR. Experimental determination of net protein charge and A(tot) and K(a) of nonvolatile buffers in canine plasma. J Vet Intern Med. 2005;19:507.
31 Constable PD. A simplified strong ion model for acid-base equilibria: application to horse plasma. J Appl Physiol. 1997;83:297.
32 Constable PD, Hinchcliff KW, Muir WW. Comparison of anion gap and strong ion gap as predictors of unmeasured strong ion concentration in plasma and serum from horses. Am J Vet Res. 1998;59:881.
33 Constable PD, Stämpfli HR, Navetat H, et al. Use of a quantitative strong ion approach to determine the mechanism for acid-base abnormalities in sick calves with or without diarrhea. J Vet Intern Med. 2005;19:581.