Chapter 26 Alterations in Blood Proteins
Proteins play an integral role in numerous physiologic processes. Not only are they important to the basic structural integrity of most body tissues, but as enzymes and hormones, they also regulate many of the body’s biochemical reactions. Hemostasis, resistance to infection, and acid-base balance depend on protein metabolism. Plasma proteins also act as carriers for other plasma constituents, and albumin provides osmotic pressure to help maintain proper intravascular volume and prevent edema. Because of the central role proteins play in the body’s homeostasis and the close relationship between plasma proteins and tissue proteins, much information about the body’s response to disease can be obtained by measuring the total plasma protein and its fractions—albumin, the globulins, and fibrinogen.
Filtration between intravascular and extravascular space, metabolic demands, hormonal balance, nutritional status, and water balance determine the plasma protein concentration of an individual at any given time. Through colostrum absorption, passive transfer of immunoglobulins causes a rise in the total protein concentration of the newborn (see Chapter 53). With time, however, the passively absorbed immunoglobulin concentration declines through natural catabolic degradation. The rate of decline varies among species and classes of immunoglobulins. The time required to reach levels that are no longer protective depends on the initial concentration of the immunoglobulin. The total protein concentration also declines over the next several weeks, even though immunoglobulins are actively produced (Fig. 26-1). In adults the protein concentration remains relatively stable. Pregnancy alters plasma proteins because fetal development imposes additional stress on the dam’s protein reserve,1 and the concentration and response of each protein fraction to different stressors vary among species.2,3 In general, however, albumin decreases and globulin (especially α2-globulin) increases in response to stress.
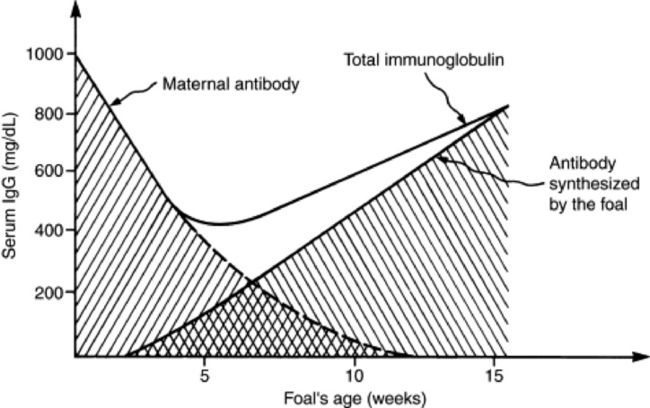
Fig. 26-1 Immunoglobulin in foal serum during the first 15 weeks of life.
From Tizard I: Veterinary immunology, ed 3, Philadelphia, 1987, Saunders.
Several methods are available for determining the concentration of serum or plasma protein. The biuret test is a simple colorimetric technique that has been widely adapted for use in automated chemical analyzers. It is highly specific for protein, especially in the range of 1 to 10 g/dL. Unfortunately the biuret technique is not precise enough for evaluation of very low levels, such as those found in cerebrospinal fluid. Refractometry is a useful method for rapidly determining the protein level in serum, plasma, or other body fluids because the refractive index of a solution is proportional to its protein concentration. Mild hemolysis or icterus of a solution does not interfere with its accuracy; however, turbid or lipemic solutions may alter the transmission of light and provide inaccurate results.
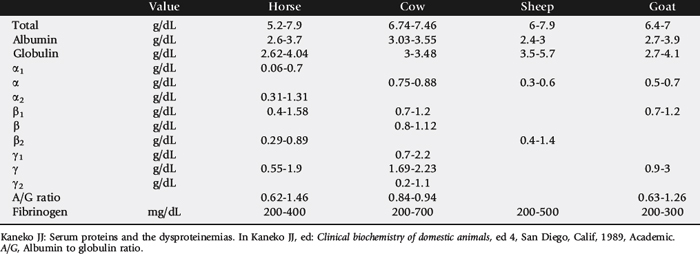
The concentration of the total plasma protein and of the individual fractions varies among species (Table 26-1). When a dysproteinemia is suspected, the total plasma (or serum) protein concentration, albumin-to-globulin (A/G) ratio, serum protein electrophoresis (SPE) results, and plasma fibrinogen concentration should be evaluated. The A/G ratio can easily be calculated from most automated serum chemistry profiles. Changes in the A/G ratio often are the first indication of dysproteinemia. Because this method of albumin measurement can be inaccurate when values are markedly low,4 the A/G ratio is most accurately obtained from serum protein electrophoresis.
When the practitioner is confronted with dysproteinemia, SPE is necessary to quantitate the individual protein fractions that make up the total. Fig. 26-2 shows normal equine and bovine SPE results. Albumin is identified as a discrete molecular compound by a sharp, narrow-based peak nearest the anode. The sharpness of the albumin peak is a measure of the quality of the SPE procedure and is used to differentiate polyclonal globulin peaks. The α-, β-, and γ-globulins form broad-based peaks during their migration in the electrical field, and, depending on the species, one or two types of the individual fraction normally are present.
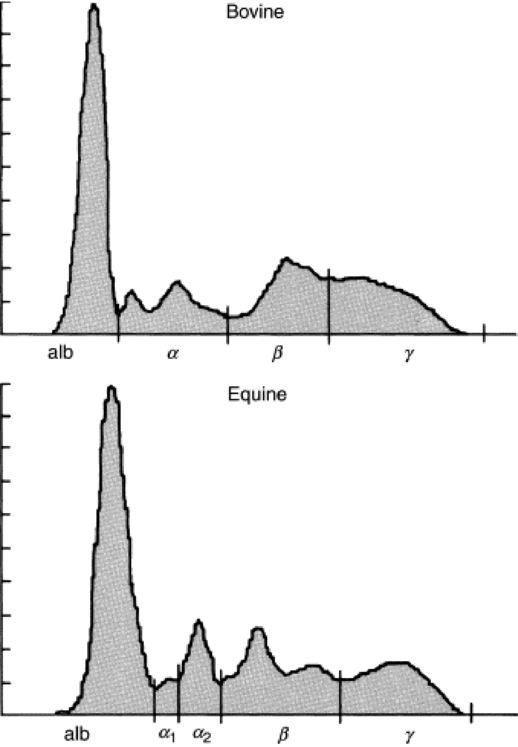
Fig. 26-2 Normal bovine and equine serum protein electrophoresis. alb, Albumin.
Courtesy Dr. Dennis DiNicola, Purdue University, West Lafayette, Ind.
HYPERPROTEINEMIA
Hyperproteinemia can result from an elevation in the concentration of all plasma proteins (panhyperproteinemia) or an absolute increase in globulins (hyperglobulinemia) (Boxes 26-1 and 26-2).
Panhyperproteinemia
An increase in the concentration of all blood proteins most commonly results from loss of the fluid component of the blood. Dehydration (decreased fluid intake or excessive fluid loss or both) causes hyperproteinemia with an associated increase in packed cell volume (PCV); however, a dehydrated, anemic animal will have hyperproteinemia with a normal or subnormal PCV. The A/G ratio will be normal. In large animals a total plasma protein concentration above 8 g/dL can be expected with severe dehydration. Initially dehydration causes withdrawal of tissue fluid into the intravascular space as the body attempts to maintain adequate blood volume. As dehydration proceeds, intravascular fluid is lost; hemoconcentration results, with a relative increase in total protein and progressive peripheral circulatory failure. If renal function is adequate, urine concentration increases and output decreases in an attempt to compensate for the fluid loss; water is absorbed from the gastrointestinal (GI) tract, assuming that GI function is normal.
A decrease in fluid intake can result from unavailability of water, lack of thirst caused by depression or toxemia, or dysphagia.
In rare cases, polyuria with renal failure, exudation from extensive skin wounds, and excessive sweating can cause dehydration. Dehydration most commonly occurs after excessive fluid loss, especially from diarrhea. Other causes of increased fluid loss from the blood include fluid sequestration with an intestinal obstruction, vagal indigestion with internal vomiting, and grain engorgement.
Clinical signs of dehydration include tachycardia, an increase in the capillary refill time, and a decrease in pulse pressure, skin elasticity, and urine output. Improvement with appropriate fluid therapy is evidenced by improvement in clinical signs and a decrease in the PCV and the plasma protein concentrations. A decline in the plasma proteins while the PCV remains elevated often indicates protein loss into a third space (this occurs most commonly in horses with severe colitis) and is a poor prognostic sign. Massive plasma transfusions are indicated in such patients.
Hyperglobulinemia
Hyperproteinemia in a patient with apparently normal hydration usually is caused by hyperglobulinemia, because hyperalbuminemia is a result of dehydration. The most common cause of hyperglobulinemia is a generalized increase in γ-globulins (polyclonal gammopathy). This represents the activity of plasma cells in response to chronic antigenic stimulation. Chronic infection, abscess, amyloidosis, and neoplasia typically result in a generalized increase in γ-globulins. Some immunoglobulins (particularly IgM) migrate in the β-globulin region, and polyclonal increases in β-globulins usually are associated with an increase in γ-globulins. A concomitant decrease in albumin synthesis commonly occurs. Chronic hepatitis, hepatic abscess, and suppurative diseases are usually accompanied by an increase in the γ-globulin concentration. Immune-mediated disease processes (e.g., autoimmune hemolytic anemia and autoimmune thrombocytopenia), lymphosarcoma, and other tumors of the reticuloendothelial system typically demonstrate polyclonal increases in γ-globulin.
An abnormal increase in a single immunoglobulin class is known as monoclonal gammopathy. On SPE, the monoclonal peak is as sharp as or sharper than the albumin peak and is the result of a single clone of plasma cells producingan increased amount of immunoglobulin. Monoclonal gammopathies can be caused by multiple myeloma, lymphocytic leukemia, and other tumors of the reticuloendothelial system (e.g., lymphosarcoma). The clinical signs depend on the degree of organ involvement, plasma cell proliferation, and protein production. Increased susceptibility to infection can be expected as a result of decreased production of normal immunoglobulins, leukopenia, and/or impaired granulocyte function. Internal parasitism, especially strongylosis, may cause an associated β-globulin spike that does not usually cause hyperglobulinemia. An increase in both β- and γ-globulin fractions (β-bridging) can occur with intense antigenic stimulation, chronic active hepatitis, or lymphosarcoma.
The α-globulins are divided into α1 and α2 fractions in most species except ruminants. α-Globulins are known as acute-phase reactants because their concentration rapidly increases after tissue injury or inflammation. α2-Antiplasmin rapidly increases,5 whereas ceruloplasmin6 increases several days after the onset of inflammation.7 An increase in C-reactive protein has been associated with pneumonitis, enteritis, and arthritis in horses.8 The increase does not generally cause hyperglobulinemia.
HYPOPROTEINEMIA
Hypoalbuminemia
Hypoalbuminemia often exists despite normal total plasma protein levels. The three most common causes of hypoalbuminemia are a decrease in production, an increase in loss by the gut, and renal loss (Boxes 26-3 and 26-4).
Albumin is produced by the liver, has the lowest molecular weight, and is the most abundant of the plasma proteins, accounting for 75% of plasma osmotic activity. In addition to maintaining osmotic pressure, a major function of albumin is to bind and transport plasma components that do not have a specific transport protein. Hypoalbuminemia causes a decrease in the A/G ratio.
Starvation, malnutrition, and chronic GI disorders that interfere with digestion and absorption may lead to inadequate provision of amino acid substrate for general protein production. Hypoalbuminemia often precedes the development of panhypoproteinemia with dietary protein deficiencies. In certain cases, diets that appear to be well balanced and to provide adequate protein nutrition may actually be inadequate in demanding conditions.
Although albumin is produced by the liver, synthesis does not usually decrease in acute liver disease. Chronic, diffuse liver diseases such as chronic hepatitis, fibrosis, and hepatic neoplasia may cause hypoalbuminemia. Because the half-life of albumin is prolonged in horses and cattle compared with that in dogs and humans,9 hypoalbuminemia rarely occurs with large animal hepatic disease.10 If it does, it often is accompanied by increases in β- and γ-globulins. Because these changes occur late in the course of the disease, they may be of more prognostic than diagnostic value.
Increased metabolic demands such as occur with fever, trauma, surgery, and neoplasia can lead to a state of negative nitrogen balance with excessive albumin breakdown. Chronic antigenic stimulation can also result in increased albumin catabolism to provide necessary amino acids for immunoglobulin production; however, this increased albumin catabolism typically does not result in a change in the total plasma protein concentration.
Excessive protein loss usually occurs through the urinary and GI tracts. Normally urine contains little or no protein, but transient physiologic proteinuria occurs with exercise, stress, convulsions, and excessive protein intake, as well as in neonates; however, none of these factors causes hypoproteinemia.
Clinically significant proteinuria consists primarily of albumin, resulting in subsequent hypoalbuminemia. Because of its small size and low molecular weight, albumin is readily filtered through defects in the glomerular basement membrane. Glomerulonephritis, amyloidosis, and less commonly pyelonephritis cause albuminuria, which may lead to hypoproteinemia.7
Protein-losing enteropathy refers to the excessive loss of plasma proteins into the GI tract, with resultant hypoproteinemia. The diagnosis of protein-losing enteropathy usually is made after ruling out protein loss through other routes (urine), increased protein catabolism, and inability to produce protein (liver disease). The clinically important mechanisms of GI protein loss are defective lymphatic drainage, increased mucosal permeability, exudation as a result of inflammation, and ulceration. In a study of horses with diarrhea, albumin was lower in horses that died than in those that survived.11 Panhypoproteinemia eventually develops, especially when inflammation is a cause.
The most common cause of protein-losing enteropathy in the horse is idiopathic granulomatous enteritis.12 Tuberculosis and histoplasmosis also cause “granulomatous changes.” Lesions are most commonly located in the small intestine, and weight loss results. Other causes of chronic protein-losing enteropathy in the horse include eosinophilic gastroenteritis, intestinal lymphosarcoma, and strongyle larval migrans. Salmonellosis, nonsteroidal antiinflammatory drug (NSAID) toxicity, and other causes of acute colitis and enteritis may result in hypoalbuminemia and a general loss of all plasma proteins. A decreasing plasma protein level that occurs with an elevated PCV indicates acute protein loss from the gut.
The most common cause of chronic protein-losing enteropathy in ruminants is Johne’s disease. Hypoalbuminemia causes hypoproteinemia. Trichostrongylus infection, intestinal lymphangiectasia, and intestinal lymphosarcoma can cause a primary hypoalbuminemic hypoproteinemia.
Clinical signs of hypoalbuminemia include edema of the distal extremities, ventral body wall, and face. The albumin level generally must be below 1.5 g/dL in horses and below 1 g/dL in ruminants before these clinical signs occur. Pharyngeal and laryngeal edema may result in upper airway obstruction, necessitating a tracheostomy.
Panhypoproteinemia
Vigorous fluid therapy or excess water intake can cause dilution of the plasma proteins, with subsequent panhypoproteinemia. Panhypoproteinemia occurs most often in animals that have acute protein-losing colitis or enteritis and that are receiving intravenous fluid therapy. Similarly, animals that lose large amounts of sodium through diarrhea and then drink fresh water may become hyponatremic as a result of a relative water excess.
Acute blood loss results in loss of plasma proteins and a dilution of the remaining protein by rapid movement of interstitial fluid into the intravascular space to help maintain intravascular volume. This dilutional effect is intensified by the excess water intake that commonly occurs after acute blood loss. Acute hemorrhage resulting from trauma, severe epistaxis, or internal vascular rupture should be ruled out in a hypoproteinemic, anemic animal.
GI blood loss can result from abomasal or gastric ulcers, blood-sucking parasites (particularly Haemonchus contortus in ruminants), viral or bacterial infection, azotemia, neoplastic invasion, or exposure to caustic chemicals. NSAID toxicosis and strangulating GI obstructions and infarctions can result in mucosal necrosis and leakage of plasma proteins into the gut lumen. Although protein-losing enteropathy initially results in hypoalbuminemia, it eventually results in panhypoproteinemia.
Blood loss from the urinary tract can result from congenital vascular disorders, renal trauma, renal calculi, pyelonephritis, neoplasia, or cystic calculi. Coagulation dysfunction, such as disseminated intravascular coagulation (DIC) or immune-mediated thrombocytopenia, may cause blood loss by way of the GI or urinary tract.
Congestive heart failure may cause hypoproteinemia by a number of mechanisms. Extracellular fluid is diluted by the retained sodium and water, and plasma protein is lost into interstitial spaces, ascitic fluid, and the GI tract. Hypoproteinemia also can occur as a result of acute severe peritonitis with massive protein exudation, as is seen with a ruptured GI viscus.
ALTERATIONS IN PLASMA FIBRINOGEN
Fibrinogen is a large—molecular-weight protein produced by the liver. Its primary function is to serve as substrate for thrombin in the formation of fibrin during hemostasis. Fibrinogen, as an acute-phase reactant protein, increases its concentration during active inflammatory disease and is a useful marker in assessment of the inflammatory response.
Hyperfibrinogenemia
Plasma fibrinogen is nearly always increased during severe inflammatory conditions and may increase with milder inflammation that is not associated with leukocytosis or neutrophilia (Boxes 26-5 and 26-6). After surgical treatment for subchondral bone cysts and osteochondrosis desiccans, horses still had hyperfibrinogenemia 15 days after surgery.13 Hyperfibrinogenemia generally occurs with infectious, suppurative, traumatic, and neoplastic diseases and subsides as the condition improves. Chronic inflammation is associated with hyperfibrinogenemia, but the degree of hyperfibrinogenemia is not always directly correlated with the severity of the disease. Fibrinogen is an especially useful indicator of inflammation in cattle because of their greater capacity to produce fibrinogen,14 which is a more sensitive indicator of inflammation than the leukocyte count (see Table 26-1).
Hypofibrinogenemia
A decrease in the fibrinogen concentration may result from increased consumption of fibrinogen or decreased synthesis. Severe, diffuse liver damage, such as occurs with severe pyrrolizidine alkaloid toxicity, causes a decrease in the fibrinogen concentration, whereas mild to moderate inflammatory liver disease can result in an increase in plasma fibrinogen. With DIC and fibrinolysis a decrease in the fibrinogen concentration would be expected; however, hypofibrinogenemia is not common in horses with DIC. Inflammatory disorders often are the cause of DIC, and a compensatory increase in production masks the increased consumption. In rare cases, rapid removal of fibrinogen from the circulation may occur as a result of primary hyperfibrinolysis. An erroneous finding of hypofibrinogenemia may result if the fibrinogen concentration is quantitated from samples containing clotted blood.
1 Larson BL, Kendall KA. Changes in specific blood serum protein levels associated with parturition in the bovine. J Dairy Sci. 1957;40:659.
2 Kaneko JJ. Serum proteins and the dysproteinemias. In Kaneko JJ, editor: Clinical biochemistry of domestic animals, ed 4, San Diego: Academic, 1989.
3 Cornelius CE, Baker NF, Kaneko JJ, Douglas JR. Distribution and turnover of iodine-131—tagged bovine albumin in normal and parasitized cattle. Am J Vet Res. 1962;23:837.
4 Matteeuws DR, Kaneko JJ, Loy RG, et al. Compartmentalization and turnover of I-labeled albumin and globulin in horses. Am J Vet Res. 1966;27:699.
5 Topper MJ, Prasse KW. Analysis of coagulation proteins as acute phase reactants in horses with colic. Am J Vet Res. 1998;59:542.
6 Okumura M, Fujinaga T, Yamashita K, et al. Isolation, characterization, and quantitative analysis of ceruloplasmin. Am J Vet Res. 1991;52:1979.
7 Morris DD, Lee JW. Renal insufficiency due to chronic glomerulonephritis in two horses. Equine Pract. 1982;4:21.
8 Takiguchi M, Fujinaga T, Naiki M, et al. Isolation, characterization, and quantitative analysis of C-reactive protein from horses. Am J Vet Res. 1990;51:1215.
9 Dixon FJ. Half-lives of homologous serum albumins in several species. Proc Soc Exp Biol Med. 1953;83:287.
10 Parraga ME, Carlson GP, Thurmond M. Serum protein concentrations in horses with severe liver disease: a retrospective study and review of the literature. J Vet Intern Med. 1995;9:154.
11 Mair TS, Cripps PJ, Ricketts SW. Diagnostic and prognostic value of serum protein electrophoresis in horses with chronic diarrhea. Equine Vet J. 1993;25:324.
12 Eades SC. Diseases affecting plasma proteins. In Colahan PT, Merritt AM, Moore JN, Mayhew IG, editors: Equine medicine and surgery, ed 5, St Louis: Mosby, 1999.
13 Allen BV, Kold SE. Fibrinogen response to surgical tissue trauma in the horse. Equine Vet J. 1988;20:441.
14 McSherry BJ, Horney FD, DeGroot JJ. Plasma fibrinogen levels in normal and sick cows. Can J Comp Med. 1970;34:191.