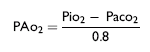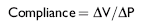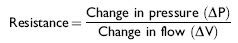Chapter 31 Diseases of the Respiratory System
GENERAL EVALUATION OF THE PATIENT WITH RESPIRATORY DISEASE
History
As with any disease process, acquisition of an accurate and appropriate history is the first step undertaken in evaluating the patient with a complaint thought to be related to the respiratory tract. Animals with respiratory disease may have widely varied histories, and it is important to gather as much information as possible. Age and breed may play a role in the development of respiratory disease such as congenital defects, neoplastic disease, or inherited or acquired immuno-deficiency syndromes seen in certain breeds. The environment in which the animal is maintained can contribute to the development or severity of respiratory disease—heaves in horses for example—and respiratory disease may become manifest after a change to new environment. In horses the work they are expected to perform can lead to important diagnostic clues, and recent events, such as long-distance transport, can predispose to diseases such as pleuropneumonia. It is important to know if certain diseases are either endemic or epidemic where the horse is kept or has recently moved from; diseases such as strangles and Rhodococcus equi bronchopneumonia of foals come to mind. Any recent traumatic or potentially traumatic event should be noted. If possible, a thorough vaccination history should be obtained, as should an accurate history of any administered treatments or supplements and the patient’s response to those treatments.
Presenting Signs or Chief Complaints
Many presenting signs or chief complaints should lead to more thorough evaluation of the respiratory system; some are more directly associated with either the upper or the lower respiratory tract. Findings or complaints associated with respiratory disease include nasal discharge, either bilateral or unilateral. Respiratory noise at rest or during exercise is commonly associated with abnormalities of the upper airway, as may be inequalities of airflow present at the nares. Normal animals may periodically cough or sneeze, but an increase in either activity may indicate involvement of the respiratory tract. Exercise intolerance or apparent decrease in the ability of the animal to exercise should prompt evaluation of the respiratory system. Other clinical signs that indicate thorough evaluation of the respiratory tract include but are not limited to abnormal breathing patterns (tachypnea, hyperpnea, dyspnea), cyanosis, hemoptysis, epistaxis, unusual swellings (facial, pharyngeal, cervical), lymphadenopathy, ataxia or reluctance to move, foul smell to the breath, weight loss and ventral abdominal, and sternal or limb edema.
Physical Examination
The initial physical examination occurs at some distance from the patient and involves evaluation of the demeanor, posture, mental status, and way of movement of the patient. It is important to note whether the patient has an abnormal stance, such as standing with the head and neck extended; is unwilling to move; or is standing with elbows abducted, suggesting pleural pain. Ideally the respiratory rate can be determined by observation, as can the respiratory pattern. Although some respiratory diseases are not manifested at rest, important clues can be gained from observation of the patient at rest in many others. The normal resting respiratory rate of an adult horse is between 8 and 16 breaths/min; for adult cattle, 15 to 35 breaths/min; and for sheep and goats, 12 to 20 breaths/min. There is some small abdominal component during the expiratory phase, which is, along with inspiration, an active process for horses. The normal rate for neonates is up to 60 breaths/min at birth and less than 30 breaths/min by 1 month of age; respiratory rate decreases toward the adult rate with age. High ambient temperature, fever, and excitement can all increase respiratory rate. Normal breathing is quiet, is apparently effortless, and is termed eupnea. The term dyspnea refers to a breathing pattern that is inferred by the observer to reflect difficulty in breathing; the animal will appear distressed, and the work of breathing is obviously increased, although the actual rate may be within normal limits. Other terms used to describe breathing patterns include tachypnea (characterized by rapid rate and shallow depth or low tidal volume), hyperpnea (increased frequency and depth of breathing [e.g., postexercise recovery]), and apnea (no discernable breathing). Two additional terms are hypoventilation and hyperventilation, both of which require a change in arterial carbon dioxide partial pressure as a component of their definitions. Hyperventilation is a pattern that increases alveolar ventilation and causes arterial hypocapnia, whereas hypoventilation alters gas exchange in such a way as to cause arterial hypercapnia, or retention of carbon dioxide.
Closer examination can reveal some of the physical manifestations of the presenting complaints listed earlier. Beginning with the head, the clinician should determine that airflow is even from both nostrils, as differences can indicate either congenital or acquired abnormalities ranging from choanal atresia to the presence of upper airway masses. Abnormal respiratory sounds can sometimes be present at rest and may be heard at the nares; abnormal breath odors may be particularly prominent at the nares. The frontal and maxillary sinuses should be percussed; identification of abnormal resonance, usually dullness, may be made easier by performing this with the patient’s mouth held open. Palpation of the submandibular regions, larynx, and pharyngeal and cervical regions should be performed to identify any abnormal lymph node enlargement, masses, or areas of muscular atrophy. Both jugular veins should be checked for both patency and the presence of any evidence of injection sites or infections that may contribute to abnormal upper airway function by interfering with normal recurrent laryngeal nerve or vagosympathetic trunk function.
Coughing represents a nonspecific irritation of receptors in the airway and can be induced by many mechanisms. It can be, and usually is, a normal protective reflex that allows the animal to clear material from the airway. Cough can be associated with increased mucus production, production of other respiratory secretions, or decreased mucociliary clearance. In older horses cough is most commonly associated with heaves; in younger horses an association has been made both with infectious diseases and small airway inflammatory disease. Normal animals should not cough when the larynx or trachea is palpated.
Nasal discharge can be unilateral or bilateral, scant or copious, clear, mucoid, mucopurulent, or even bloody. The nature and character of nasal discharge can provide some information about a possible source of the discharge but should not be overinterpreted. Horses, for example, have a tendency to swallow excess airway secretions, and the volume of secretions may be underestimated. Although unilateral nasal discharge seems to suggest a source in front of the larynx, bilateral nasal discharge can be of either upper or lower airway origin. Skin depigmentation of the ventral nares or presence of mucoid material in feed or water containers is a clue to presence of a nasal discharge.
Hemoptysis is the coughing up of blood from the airways or lungs. It is important to determine conclusively that the blood has come from the respiratory system. Epistaxis is defined as blood seen at the nares and often originates in the nasal passages, sinuses, turbinates, nasopharynx, or equine guttural pouches, although the lung can be, and is, a source on occasion, as in exercise-induced pulmonary hemorrhage (EIPH) or after lung biopsy. Bilateral epistaxis generally indicates bleeding caudal to the choanae. Because animals tend to swallow excessive respiratory secretions, bleeding can be occult and may not be seen unless the animal drops its head toward the ground. Significant blood loss can occur in this manner, unseen by owners.
Examination of the oral mucous membranes may reveal cyanosis—bluish discoloration of the oral, nasal, or vulvar mucous membranes. Cyanosis does not become apparent until a level of 5 mg/100 mL of deoxygenated hemoglobin, about one third of the total normal hemoglobin, is present, reflecting a profound decrease in oxygen saturation of hemoglobin and suggestive of severe hypoxemia. As it is the total quantity of deoxygenated hemoglobin that lends the mucous membranes the bluish color, very anemic patients may lack sufficient deoxygenated hemoglobin to appear blue, making appreciation of cyanosis impossible in these patients. One caveat is that all newborns are cyanotic for the first few breaths and become pink only when they have established neonatal, as opposed to fetal, cardiorespiratory circulation and opened their lungs to allow for gas exchange.
It is important that auscultation of the thorax take place in as quiet an environment as possible. In addition, auscultation of the lung fields should be performed under two breathing conditions: eupnea and hyperpnea, with hyperpnea induced by the use of a rebreathing bag. Some common misconceptions regarding the use of a rebreathing bag exist. Simply occluding the animal’s nostrils or using a rectal sleeve as a rebreathing bag are both inadequate methods of fully examining the patient. The purpose is to cause the animal to rebreathe its own expired carbon dioxide, not to necessarily deprive it of oxygen. Rebreathing expired carbon dioxide results in increased PaCO2, which stimulates deeper and more frequent breathing efforts, making recognition of abnormal lung sounds simpler. The bag used should be large enough to accommodate two to three times the normal tidal volume of the animal and should be held in such a manner as to prevent the bag from occluding the patient’s nostrils. Once the bag is removed, the animal will usually take several very deep breaths and the examiner should take advantage of these very large breaths to reexamine areas where suspicious sounds were heard during rebreathing. Animals with significant lung pathology will not tolerate the bag well, may cough when the bag is removed, and may require more time to return to baseline respiratory patterns when the bag is removed.
Normal breath sounds are those produced by turbulence within the tracheobronchial tree and may vary considerably depending on location within the lung, breathing pattern, and condition of the animal.1 Only airways from the larynx to segmental bronchi contribute to sound generation; bronchial and vesicular sounds both represent larger airway flow events. Vesicular sounds are the quietest sounds, heard over the middle and diaphragmatic lung regions, correlate best with regional ventilation, and mainly represent segmental bronchial sounds; they do not represent air flow in terminal conducting airways and alveoli, which is silent because of the nature of its flow. Bronchial sounds are louder and are heard best over the trachea and base of the lung. Common abnormalities found during auscultation include ventral areas of dullness if pleural effusion is significant, dorsal areas of dullness or hyperresonance with pneumothorax, and dorsal harsh lung sounds. The degree of variation in normal regarding lung sounds is large, and auscultatory findings do not always correlate well with the degree of lung abnormality. That said, abnormal lung sounds are always potentially clinically important.
Adventitious lung sounds are divided into short discontinuous sounds called crackles and longer continuous sounds called wheezes, replacing the older terms rales and rhonchi, respectively. Crackles are most commonly generated by sudden pressure equalization when collapsed airway segments open. Although an air-fluid interface is required, crackles do not necessarily imply excessive secretions or pulmonary edema. They are often end-inspiratory and associated with reinflation of atelectatic lung. Crackles may be normal when ausculted in the previously down lung of a laterally recumbent neonate. Disease processes that generate crackles include pneumonia, interstitial fibrosis, chronic obstructive lung disease, congestive heart failure, and atelectasis.2
Wheezes commonly represent oscillation of airway walls before complete closing (expiratory) or opening (inspiratory). Intrathoracic airways are usually involved in expiratory wheezes and include the lower trachea and main, lobar, and segmental bronchi. Disappearance of a wheeze after coughing indicates secretory rather than tissue-component origin. Disease processes responsible for wheezes include airway stenosis or external compression; airway luminal compromise by foreign body, purulent material, cyst, or neoplasm; airway wall thickening as in chronic bronchitis; and bronchoconstriction. Expiratory wheezes are a hallmark of obstructive lung diseases such as heaves. Crackles and wheezes may be variably present. A final category of adventitious sounds includes the “rubbing” or “creaking” sounds generated by sliding or stretching of inflamed pleural surfaces, commonly termed pleural friction rubs.
Percussion of the thorax is performed by methodic tapping over the intercostal spaces of the thorax using a variety of instruments, including plexors, pleximeters, spoons, fingers, neurologic hammers, and hands. It is an inexpensive and useful component of the physical examination and should be performed in all patients in which the respiratory system is suspect. Percussion of the thorax can reveal hyporesonance (dullness) ventrally when pleural effusion is present and hyperresonance dorsally in pneumothorax and can cause some patients to exhibit pleurodynia during the examination. Other conditions that can alter resonance of the thorax include but are not limited to diaphragmatic hernia with intrathoracic intestine, pericardial effusion, pulmonary and pleural abscessation, and consolidated lung. The point at which a change occurs from resonant to dull can be marked with adhesive tape. Thus the outline of aerated lung immediately beneath the chest wall is delineated. It is usually impossible to fully delineate the lung field cranially because of body fat and triceps musculature. There is a distinct region of cardiac dullness for all species on the left side.
Percussion allows delineation of pleural effusion and intrathoracic masses or consolidated lung up to 7 cm beneath the pleural surface but cannot distinguish between them. The procedure should be performed whenever pleural effusion is suspected on the basis of auscultatory findings and in all ruminants as part of the physical examination to uncover occult pneumonia.
ADDITIONAL DIAGNOSTIC EVALUATION OF THE RESPIRATORY TRACT
Endoscopy
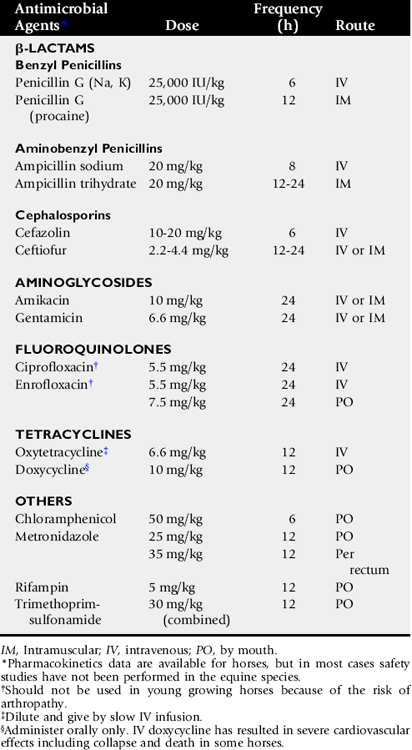
The upper airway can be directly examined with the aid of an endoscope, the only limitations being the size of the patient, the patency of the airway, and the size of the available equipment. Standard flexible fiberoptic endoscopes, available to most practitioners and present now in virtually all referral hospitals, allow direct examination of the nasal passages, ethmoid turbinates, nasal maxillary opening of the sinuses, pharynx, guttural pouch openings, larynx, and cranial trachea (Fig. 31-1). Smaller (8- to 10-mm-diameter) endoscopes can be readily introduced into the equine guttural pouches with the aid of a biopsy instrument, and longer endoscopes (more than 150 cm long with diameters greater than 10 mm) are commonly employed to examine mainstem bronchi and their initial branches in large animals.3 Small brushes, used for collecting exfoliated cells for cytologic study, and a variety of biopsy instruments can be used for sampling the airway. The use of airway endoscopy has evolved to include videoendoscopy of the equine upper airway during treadmill exercise at high speed (12 to 14 m/sec) to evaluate dynamic respiratory function and make objective measurements by use of freeze-frame features.4
Fig. 31-1 Normal equine larynx. The larynx is directly visualized by endoscopy, with both structure and symmetry evaluated.
Courtesy Dr. Corinne Sweeney, University of Pennsylvania, New Bolton Center, Kennett Square, Penn.
Sedation or tranquilization will facilitate many endoscopic examinations, but examinations aimed at evaluating pharyngeal and/or laryngeal function are best performed without any form of chemical restraint that might alter function. Most horses will allow standing examination of the upper airway with only physical restraint, such as judicious use of a nose twitch. Introduction of the endoscope into the trachea may elicit coughing, particularly in horses but less so in cattle. Small ruminants, such as sheep and goats, may require local tracheal anesthetic administration in the form of 2% lidocaine administered through small tubing passed through the biopsy channel of the endoscope. If used, care must be taken that lidocaine is diluted and does not reach a toxic dose in small ruminants. Diluted topical 2% lidocaine can similarly be used in horses and cattle if needed for evaluation of the distal trachea, main stem bronchi, and larger bronchial tree branches. Horses are more sensitive to tracheal and bronchial stimulation and are more likely to require topical anesthesia than cattle.
Airway abnormalities such as pharyngeal lymphoid hyperplasia, laryngeal hemiplegia, epiglottic entrapment by arytenoepiglottic folds, dorsal displacement of the soft palate, pharyngeal cysts, retropharyngeal masses, and epiglottic deformities are all best diagnosed by endoscopic examination. Guttural pouch diseases and EIPH are also best evaluated using this technique. The degree and nature of airway secretions accumulating in the trachea can be easily assessed using an endoscope, and accumulated secretions may be sampled by aspirating the secretions through small tubing introduced into the trachea via the biopsy channel. Because the endoscope has passed through the nonsterile upper airway, these samples are best suited for cytologic, not microbiogic, evaluation but may be fully compatible with evaluation using newer molecular diagnostic techniques.5-8 Endoscopy has also been used to facilitate removal of foreign bodies from the airway, generally aided by the biopsy instrument.
Radiography
Radiographs are indicated when the clinician suspects a congenital anomaly involving any thoracic structure; infectious disease of the pleura, pulmonary parenchyma, racheobronchial tree, or mediastinum; pneumothorax or pneumomediastinum; thoracic neoplasia of any origin; or trauma. Radiographs are frequently coupled with thoracic ultrasonographic evaluation. If significant accumulation of pleural fluid is suspected based on physical examination findings, the ultrasonographic portion of the examination should be performed first and radiographs obtained after drainage of excess fluid, as fluid may obscure potentially important parenchymal disease. The equipment needed to perform radiographic evaluation of the upper airway is available in most private practices, and most large referral and university practices have the equipment needed to perform thoracic radiography in larger patients such as adult horses and cattle. Digital radiography is becoming more commonplace and may replace more convention radiography in many practices and referral clinics over the next few years. Because of its configuration, the thorax in adult horses and cattle is filmed in the standing lateral position, generally requiring a series of three or four separate but overlapping images; thus the benefit of the ventrodorsal view in which the two lungs may be compared is lost. Neonates and small ruminants can be more readily handled and retained in recumbent positions, allowing for multiple recumbent views.
Skull and cervical radiographs offer diagnostic information for evaluation of the upper respiratory tract. For large animal species, standing lateral skull films are easily obtained, and, with practice and adequate sedation, ventrodorsal and oblique projections can also be obtained in most patients. Certain difficult patients may require general anesthesia in order to obtain radiographs of diagnostic quality. In these cases other imaging modalities such as computed tomography (CT) and magnetic resonance imaging (MRI) might also be considered if available. Skull radiographs image the sinuses, pharynx, and larynx, allowing for assessment of anatomic dimensions of the pharyngeal and laryngeal structures. Sinuses affected by neoplasia or inflammation may show abnormal tissue density, a horizontal fluid line on a standing lateral film, bone lysis around the affected sinus, or alveolar periostitis. Thorough evaluation of the sinuses and nasal passages requires lateral, dorsoventral, and oblique views. Foreign bodies can be assessed in many cases. The equine guttural pouches are evident on lateral skull projection, and abnormal fluid accumulation, distortion by enlarged retropharyngeal lymph nodes, or emphysema can be radiographically apparent.
Radiographic assessment of the thorax of large animals remains preferable to ultrasonographic examination for detection of diffuse parenchymal diseases such as interstitial pneumonia, pulmonary edema, equine multinodular pulmonary fibrosis (EMPF), fungal pneumonia, acute lung injury (ALI), acute respiratory distress syndrome (ARDS), chronic disorders, and deep parenchymal or mediastinal abscesses. Unfortunately, many radiographic changes in equine respiratory disorders tend to be nonspecific or, in certain disease such as EIPH, inflammatory airway disease (IAD), or heaves, minimal to nonexistent.
Four types of radiographic patterns are described for the thorax: alveolar (airspace), interstitial, bronchiolar, and vascular. Opaque areas coalesce and fully obliterate vessels and bronchi in the alveolar pattern; air bronchograms may be prominent. This pattern is common in pulmonary edema, pulmonary hemorrhage, EMPF, ALI, ARDS, lung consolidation, and neoplasia. Interstitial patterns are the most common patterns noted in equine thoracic radiographs and are characterized by a blurring of the edges of pulmonary vessels, a diffuse increase in lung density, and variable reticular, linear, and nodular opacities. The reticular pattern is most commonly associated with more diffuse infectious lung diseases, pulmonary edema, interstitial pneumonia, and pulmonary fibrosis, whereas the irregular linear pattern is seen most commonly with resolving bronchopneumonia. A nodular pattern is seen with abscesses, granulomata, and neoplasms. It is rare to see a pure bronchial pattern in a horse, and it usually seen in association with an interstitial pattern. An exception is paired linear opacities or numerous small circular opacities (donuts) representing thickening of large or medium airways in equine bronchitis and bronchiolitis. The vascular pattern is seen in horses radiographed immediately postexercise or in animals with left-to-right cardiac shunts. Finally, extraparenchymal problems such as pleural effusions or free gas may be seen on thoracic radiographs of large animals. Thoracic radiology may be used for evaluation of potential rib fracture but is far less sensitive than thoracic ultrasonography in this regard.
Ultrasonography
Thoracic ultrasonography, a companion to thoracic radiography, is useful for diagnostic, therapeutic, and prognostic evaluation of the extraparenchymal thorax, the pleural space, and the peripheral (superficial) parenchyma of the lung. Unlike thoracic radiography, in which specialized equipment is needed to image the adult large animal, thoracic ultrasonography is an imaging technique readily available to most practitioners. In many instances it is superior to thoracic radiography as an imaging method; examples include evaluation of pleural effusions, assessment of thoracic trauma, evaluation of neoplasms or granulomata, detection of mediastinal masses or abscesses, and guidance of transthoracic lung biopsy.9,10 Ultrasonography is considered greatly superior to thoracic radiography in the detection of rib fractures.11 This imaging technique should be considered for complete evaluation of any large animal with suspected or diagnosed pulmonary disease.
Ultrasonography is generally performed with the patient standing, although in neonates lateral recumbency may be preferred or even necessary, and sound waves are generated by piezoelectric crystals and transmitted to the area of interest through a skin coupling gel, with subsequently reflected echoes detected by the same crystal. Echo signals from all tissue interfaces are displayed on a screen; the image can be photographed for a permanent record or stored digitally. Air trapped beneath the haired skin can interfere with the process, as can excessive skin dirt, so preparation of the acoustic window usually involves hair removal and cleansing in order to get the best image possible.
Although ultrasound waves will not penetrate the aerated portion of the lung, limiting the examination to extraparenchymal surfaces in normal horses, ultrasonography is superior to thoracic radiography in evaluation of these areas of the chest. Small amounts of pleural fluid that would be missed on auscultation, percussion, or thoracic radiographs can be detected, and the amount and character of pleural effusion in each hemithorax can be separately evaluated.9 Clear fluid is anechoic, but inflammatory cells, gas, and fibrin are echogenic, causing opacities that can be seen floating in pleural fluid and altering the general echogenicity of the fluid. Because of this, ultrasound is the method of choice for diagnosis and monitoring of pleural space disease. Ultrasonography should be used to guide catheter placement for drainage of accumulated fluid in the pleural space. The pleural surfaces are imaged well by ultrasound, with thickened or roughened areas easily detected. Lack of normal independent movement of the visceral and parietal pleural surfaces during the respiratory cycle, suggestive of adhesion formation, can be readily monitored.9,10
Consolidated lung is a better acoustic medium than aerated parenchyma and can be well visualized. If there is pleuropneumonia with consolidation or atelectasis caused by compression of the ventral lung by pleural effusion, it will be evident. Pulmonary abscesses or masses extending to the lung surface can be imaged, and ultrasound can be used for guidance for transthoracic biopsy.9,10 Thoracic radiography remains superior to ultrasound in diagnosis of pulmonary parenchymal disease and pneumothorax, but combined the two techniques will improve patient management diagnostically and therapeutically.
Nuclear Medicine Imaging
Nuclear medicine imaging is a very specialized technique available at a few university and private specialty referral practices. Gamma-emitting radioisotopes such as krypton-81 m or technetium-99 m can be used with an external detector (gamma camera) to assess regional pulmonary ventilation and perfusion in the horse. The procedure is safe and painless. Anesthesia is not needed, and the only requirement is that the patient stands quietly in front of the gamma camera. After the study the patient must be kept in an isolated area to allow decay and excretion of the radiopharmaceutical (normally no more than 48 hours), and, of course, all pertinent radiation regulations must be strictly adhered to.
The radioisotope is bound to albumin aggregates of 10 to 15 μm diameter. When injected into a peripheral vein (e.g., for a perfusion scan), the aggregates become trapped in the pulmonary arterial vasculature. Given even and thorough mixing in the right ventricle, the resulting image illustrates the perfusion distribution of the pulmonary arterial system. The ventilation scan is generated when the horse inhales aerosolized radioisotope particles through a close circuit system.12 The particles have a small enough diameter to be deposited in the alveoli and small conducting airways with the gamma camera recording the sites of deposition. Together, the ventilation and perfusion scans allow for evaluation of the ventilation/perfusion (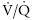 ) ratio, important in evaluation of certain respiratory problems such as EIPH (high
) ratio, important in evaluation of certain respiratory problems such as EIPH (high 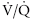 areas), pulmonary thromboembolism (high
areas), pulmonary thromboembolism (high  areas), and heaves (low
areas), and heaves (low  areas).13 One final use is in the evaluation of mucociliary clearance or tracheal mucous transport. The time a bolus of radioisotope requires to cover a given tracheal distance is recorded in millimeters per minute and compared with normal ranges.14
areas).13 One final use is in the evaluation of mucociliary clearance or tracheal mucous transport. The time a bolus of radioisotope requires to cover a given tracheal distance is recorded in millimeters per minute and compared with normal ranges.14
This technology is specialized, expensive, and not readily available as of this writing. It has greatest potential application in the equine athlete or the valuable equine patient.
Arterial Blood Gas Analysis
Arterial blood gas determinations are the most sensitive indicator of respiratory function readily available to the clinician. The most accessed arteries for sampling are the metatarsal, temporal, facial, and brachial arteries (Fig. 31-2). In cattle the coccygeal artery on the ventral aspect of the tail head is easily accessible. Heparin is the only acceptable anticoagulant for blood gas samples, and all gas bubbles must be removed and the syringe capped to prevent equilibration of the sample with room air. Use a short (1-inch), small-gauge (25-gauge generally) needle and a 1- to 3-mL syringe for most samples. The syringe and needle can be purchased preheparinized especially for arterial blood gas sampling, or regular syringes and needles may be heparinized by aspirating a small volume of heparin into the syringe via the needle and then forcefully expelling the air and heparin from the syringe three times. This minimizes the effect heparin might have on any reported values from the blood gas analyzer. Pulsation of blood from the needle, spontaneous filling of the syringe, and bright color of the blood all confirm a successful arterial puncture. If successful arterial puncture is questionable, then a comparison sample may be drawn from the jugular vein. Once the sample has been drawn, the vessel should be manually compressed for 2 to 5 minutes to prevent hematoma formation. If the sample will not be analyzed within 10 minutes, it should be placed on ice to slow metabolism of blood cells. The patient’s body temperature at the time of sampling should also be recorded, as results are frequently reported at both 37° C and at the actual temperature of the patient, called temperature-corrected values, for pH, PO2, and PCO2, as these values are known to be temperature variable.
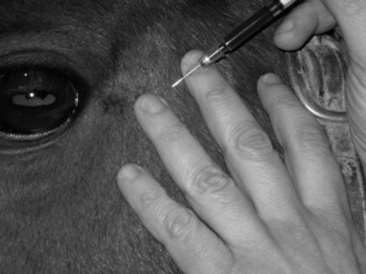
Fig. 31-2 Arterial blood sample drawn from temporal artery for arterial blood gas analysis.
Courtesy Dr. Eric Birks, University of Pennsylvania, New Bolton Center, Kennett Square, Penn.
Portable arterial and venous blood gas analyzers are now making arterial blood gas analysis more practical for use in the field, and the technique is no longer reserved for large institutions or referral practices.15 It is virtually impossible to manage severe respiratory disease without knowledge of arterial blood gas parameters. Pulse oximetry is also being more commonly employed in some institutions and referral centers, but these monitors measure only oxygen saturation of hemoglobin, useful for severe hypoxemia but giving no measurement of actual arterial oxygen and carbon dioxide partial pressures. The most common abnormalities recognized with arterial blood gas analysis in animals breathing room air are hypoxemia with normocapnia or hypocapnia and hypoxemia with hypercapnia. There are five primary means by which hypoxemia develops in any animal with a heart and lungs. For our purposes, hypoxemia is defined as decreased oxygen tension of the arterial blood (decreased PaO2) and hypoxia is defined as decreased oxygen concentration at the level of the tissue, with or without hypoxemia. Hypoxia results from hypoxemia, decreased perfusion of the tissue bed in question, or decreased oxygen-carrying capacity of the blood as a result of anemia or hemoglobin alteration.
Hypoxemia develops from (1) low content or concentration of oxygen in the inspired air (FiO2) such as seen in high altitude or when an error is made during the mixing of ventilator gas; (2) hypoventilation; (3) ventilation-perfusion mismatch; (4) diffusion limitation; or (5) intrapulmonary or intracardiac right-to-left shunting of blood. Mild to moderate hypoxemia is not an uncommon finding in neonates but must be evaluated in terms of the current age of the foal and its position. The difficulty encountered obtaining the sample must also be considered, as severe struggling can variably affect the arterial blood gas results. If the lung is significantly involved in the underlying pathology, such as with severe pneumonia, ALI, or ARDS, increased PaCO2 may very well be present, representing respiratory failure.
Hypoxemia is usually treated with intranasal humidified oxygen insufflation at 4 to 10 L/min in neonates and 10 to 15 L/min in adults. Hypercapnia is not a simple matter to treat. It is important to try to distinguish between acute and chronic hypercapnia. Acute hypercapnia is usually accompanied by a relatively dramatic decrease in blood pH of 0.008 pH units for each 1-mm Hg increase in PaCO2. This respiratory acidosis can promote circulatory collapse, particularly in the concurrently hypoxemic and/or hypovolemic patient. The effects of more chronic CO2 retention are less obvious, as the time course allows for adaptation. The pH change is less, about 0.003 pH units per 1-mm Hg increase in PaCO2, as it is balanced by enhanced renal absorption of bicarbonate by the proximal renal tubule. Most patients in acute respiratory distress are in the acute stages of respiratory failure, but chronic adaptation will begin to occur within 6 to 12 hours and will be maximal in 3 to 5 days. An increase in bicarbonate will be noted, particularly if the acidosis is primarily respiratory in origin.
Alveolar gas exchange is readily estimated by determining the alveolar-arterial (A-a) gradient for oxygen, computed by subtracting the PaO2 measured by the arterial blood gas from the calculated alveolar oxygen partial pressure (PAO2). The PAO2 is effectively estimated using the partial pressure of inspired oxygen (PiO2) as follows16:
The PiO2 equals the total barometric pressure (760 mm Hg) minus the partial pressure of water vapor (42 mm Hg) multiplied by the fraction of room air that is oxygen (0.21), and thus equals 150 mm Hg for room air. For patients on supplemental inspired oxygen, the practitioner must remember to recalculate the PiO2 with the new oxygen fraction (FiO2) in the inspired gas, possible only in patients receiving inspiratory gas through a closed system. The PaCO2 is obtained from the arterial blood gas measurement. The A-a gradient is normally only 4 to 10 mm Hg; an increase beyond this indicates impaired gas exchange within the lungs, most often the result of ventilation-perfusion mismatching. The A-a gradient can be estimated only in patients receiving intranasal insufflation of oxygen.
A second useful measure is the PaO2/FiO2 ratio, a component of most definitions of both ALI and ARDS.17 The PaO2/FiO2 ratio equals the PaO2 obtained from the arterial blood gas divided by the FiO2, the oxygen fraction in the inspired gases. The normal PaO2/FiO2 ratio is >300 mm Hg; a ratio <300 mm Hg is consistent with a potential diagnosis of ALI, and a ratio <200 mm Hg suggests ARDS, a more severe form of ALI. The ranges of normal arterial blood gas values for various species are listed in Table 31-1.
Table 31-1 Normal Arterial pH and PCO2 Values for Various Species (Nonneonate)
| Species | Blood pH | PCO2 (mm Hg) |
|---|---|---|
| Bovine | 7.32–7.45 | 35–53 |
| Ovine | 7.32–7.54 | 37–46 |
| Equine | 7.32–7.44 | 38–46 |
| Caprine | 7.42–7.46 | 33–38 |
Respiratory Function Testing
The major functions of the lungs are to transport gas from the periphery to the site of gas exchange (i.e., the “bellows” function) and to provide gas exchange with the blood, facilitating gas transport to the tissues. The first of these is assessed by means of pulmonary function tests, and the second by arterial blood gas evaluation, discussed earlier.
Pulmonary function tests have historically primarily been used in horses and in most cases as a research tool in veterinary teaching hospitals. However, newer, portable technologies are beginning to allow use of some of the less invasive techniques in the field, and practitioners are becoming aware of the potential utility of these testing techniques.18 Pulmonary function testing (PFT) involves measurement of pressure, flow, and volume during breathing to allow computation of ventilatory functional values. They are valuable in assessment of equine athletes, especially those suspected of inflammatory obstructive airway disease, and a portion of this section is dedicated to this subject. Baseline measurements can be compared, and airway hyperreactivity (AHR) can be evaluated using histamine or methacholine bronchoprovocation protocols.19 Responses to environmental changes or therapy can be noninvasively evaluated.20-22
Collection and Evaluation of Respiratory Secretions
TRACHEAL ASPIRATES AND BRONCHOALVEOLAR LAVAGE
Various spaces in the respiratory system can undergo aspiration or lavage for diagnostic or therapeutic purposes. The most commonly performed procedure is the tracheobronchial aspiration. By aspirating from the airways caudal to the larynx, a sample without pharyngeal contamination is obtained.
In both the horse and the ruminant the procedure is performed with the animal standing. Sedation or restraint may be needed. A small area over the trachea in the middle third of the neck is clipped and routinely sterilely prepared. The skin is anesthetized using a local block of 2% lidocaine, generally less than 3 mL is given as a “bleb” subcutaneously, and a small stab incision is made. A trocar or angiocatheter needle is introduced on the midline between muscle bundles, with the beveled edge facing ventrally to decrease the opportunity for inadvertent cutting of the tubing when the needle is introduced or manipulated, and the ventral tracheal wall is punctured between two cartilaginous rings. The distal end of the trocar or needle is then advanced distally in the trachea, taking care not to lacerate the dorsal tracheal mucosa. Sterile polyethylene tubing or the catheter from the angiocatheter is introduced through the trocar or needle for about 30 cm. A needle or sharp trocar should be withdrawn to prevent severing the tubing or catheter, but a cannula with rounded edges may be left in place. Approximately 20 to 30 mL of nonbacteriostatic sterile saline solution is introduced quickly. Intermittent aspiration is performed as the tubing is gradually withdrawn. The tubing can be advanced again if a guarding cannula has been left in place to prevent introduction of skin contamination. Additional saline solution aliquots can also be introduced. Once an adequate sample has been obtained, the tubing is completely withdrawn. Injectable antimicrobial solution or suspension can be infiltrated at the skin incision site if a septic sample is suspected, and in horses and small ruminants a sterile dressing can be applied for 24 hours if desired. Possible complications include subcutaneous (SC) emphysema (usually peritracheal but may extend into the mediastinum), local cellulitis, or cutting of the catheter at the needle and loss into the airway. The latter is usually resolved because the catheter is rapidly coughed up, but good technique should prevent this complication. The sample should be cultured for aerobic bacteria. Anaerobic colonization is possible, and appropriate cultures should be made if these organisms are suspected (evidence of pleural effusion, consolidation, abscessation, history of aspiration fetid breath). For patients with prior antimicrobial therapy, it is advised to discontinue antibiotics for 72 to 96 hours before culture, although a recent study has shown reliable recovery of bacteria using bronchoalveolar lavage (BAL) fluid from foals receiving therapy.6
Airway aspiration can also be performed during routine endoscopy of the trachea using an aspiration catheter advanced through the endoscope biopsy channel, but there is potential for pharyngeal contamination. Results comparing culture from a protected aspiration catheter passed through an endoscope compared favorably with culture from traditional percutaneous tracheobronchial aspiration (TBA).5
A direct smear and Gram stain can be used as an initial guide for antimicrobial therapy pending culture results. Cytologic evaluation can be extremely valuable in differentiating among infectious, allergic, parasitic, and neoplastic processes. Transtracheal aspirates (TTAs) from clinically normal horses contain columnar ciliated epithelial cells, a few neutrophils, and multiple mononuclear cells. Increased percentages of neutrophils and the presence of mast cells, eosinophils, giant cells, and hemosiderophages have been demonstrated in aspirates from normally performing thoroughbred racehorses, indicating some airway inflammation in “normal” equine athletes.8 Mucus, large spores, and fungal hyphae may be found in the absence of airway disease and must not be overinterpreted. Heaves, or recurrent airway obstruction (RAO), is characterized by increased numbers of nondegenerate neutrophils and occasional eosinophils. In cases of pneumonia, neutrophils may constitute 40% to 90% of the cellular sample. Bacterial pneumonia causes a more degenerate appearance of neutrophils, and intracellular bacteria may be found. Equine lungworm is characterized by finding large numbers of eosinophils and occasionally a larva. In ruminants the most important information gathered in patients with bronchopneumonia is usually the result of culture and antimicrobial sensitivity testing.
BAL involves obtaining a sample from the terminal airways and alveolar region. It is performed using a long endoscope or double-lumen tube introduced through the nares. Endoscopic BAL allows for more exact placement of the end of the endoscope, so a clear understanding of the anatomic location of the distal airway lavage is available. Use of the double-lumen tube is essentially a blind technique, but most frequently the dorsal lung of one hemithorax is sampled. The outer tube or the endoscope is wedged in a bronchus, and the smaller tube advanced. Saline solution aliquots of 60 to 300 mL are introduced, followed by continuous aspiration using low suction pressure. The procedure has the advantage of sampling the airways nearest the parenchymal region, but only a limited area of the lung is sampled instead of the pooled secretions from a tracheobronchial aspirate. Thus BAL may be superior to tracheobronchial aspiration in evaluation of horses with chronic lung diseases, but false-negative results can be obtained from horses with pneumonia or pleuropneumonia. BAL cytology is valuable in evaluation of fungal infections and IAD and assessment of therapeutic response.
Thoracocentesis
Aspiration from the pleural space is a simple, easily performed, inexpensive procedure that can be both diagnostic and therapeutic. In the horse with septic or neoplastic effusions, sedation is often unnecessary because the procedure causes only minimal additional discomfort. After ultrasonographic evaluation of the thorax, a point is chosen at which drainage or fluid sampling would seem most appropriate—frequently in the sixth or seventh intercostal space 10 cm dorsal to the olecranon and above the lateral thoracic vein. The area should be clipped, if it was not clipped for the thoracic ultrasound examination, and surgically prepared. Multiple sites may be needed in horses with loculated pockets of fluid in the pleural cavity, and these sites should also be chosen using ultrasonography. The skin and intercostal tissue down to the pleura are anesthetized with lidocaine, and a stab incision is made. A sterile 2- to 3-inch teat cannula or bitch catheter is introduced immediately cranial to the rib border to avoid the intercostal nerve and vessel along the caudal aspect of the ribs. The cannula should be attached to sterile intravenous (IV) extension tubing and a three-way stopcock. When the cannula is advanced bluntly through the parietal pleura, a sudden loss of the force required to advance is felt. Aspiration should be attempted at this point. The orientation of the cannula can be varied to reach as much fluid as possible. Normally only a few milliliters of straw-colored fluid are obtained. In cases of pleural effusion, as much as 30 L may be removed from each side of the chest (Fig. 31-3). If fluid is excessive, the tubing can be extended over a bucket for gravity drainage, or a vacuum pump with fluid trap can be attached. Once the procedure is complete, a purse-string suture is placed around the stab incision, and the cannula is withdrawn while the suture is tightened. In cases in which the effusion is large and expected to continue forming for several days, the initial drainage can be performed by placing a chest tube instead of puncturing the pleural space with a teat cannula. If a chest tube is to be left in place it should be secured with a Chinese finger trap suture and the end covered by a Heimlich valve to prevent aspiration of air into the thorax through the tube. If the thorax is being drained rapidly, the patient should be watched carefully for signs of distress, as draining of large volumes can alter cardiovascular parameters significantly.

Fig. 31-3 Thoracocentesis and therapeutic drainage in the horse. Pleural effusion can be large and bilateral. Samples should be obtained for culture and cytologic examination at the time the chest is drained.
Courtesy Dr. Corinne Sweeney, University of Pennsylvania, New Bolton Center, Kennett Square, Penn.
Increasing opacity, presence of fibrin clumps, and malodor of pleural fluid all suggest relative progression from transudate to septic exudate containing inflammatory cells and debris. A putrid odor suggests the presence of anaerobic bacteria. Samples should be cultured for aerobic and anaerobic organisms. A white blood cell (WBC) count of 10,000/μL or less is considered normal; fewer than 60% are normally neutrophils, the remainder being lymphocytes and macrophages. The proportion and total number of neutrophils increase with pleuritis. Erythrocytes are normally not present in the absence of a traumatic tap. The protein concentration is normally less than 3.5 g/dL, and pH should be approximately 7.4. Additional metabolic values that give early indication of sepsis can be obtained on pleural fluid samples collected after filtration through a blood administration set to remove fibrin and debris potentially detrimental to analytic equipment. Pleural fluid pH, PCO2, and concentration of glucose, lactate, and bicarbonate can be directly compared with similar analysis of venous blood from the patient. A septic pleural exudate is acidic, with decreased glucose and bicarbonate but increased lactate and PCO2 compared with venous blood concentrations or tensions, apparently reflecting metabolic activity of phagocytic cells and bacteria and development of an anaerobic environment.23 Of these values, low pleural fluid glucose concentration (<40 mg/dL) has the best correlation with sepsis.24
Neoplastic cells may be found in cases of lymphosarcoma, adenocarcinoma, or other neoplasms. Equine gastric squamous cell carcinoma occasionally manifests with neoplastic pleural effusion. If neoplastic effusion is suspected but diagnostic cells do not exfoliate into the pleural fluid, pleuroscopy with the patient under sedation and local anesthesia can be used directly to visualize and obtain biopsy samples of intrathoracic lesions. The technique of pleuroscopy is beyond the scope of this chapter.
Mediastinal fenestrations may be occluded by fibrin and cell debris; therefore each side of the thorax should be evaluated separately. In the horse a transtracheal aspiration for culture should also be performed because of the common association of pleuritis with bacterial pneumonia and pulmonary abscessation. Although identical organisms are generally isolated from both samples, this is sometimes not the case.
Sinus Trephination
Sinus trephination is performed with some frequency in horses and ruminants. Clinical signs indicating a need for sinus trephination include foul-smelling purulent nasal discharge (the most consistent sign with dental disease or invasive tumors), facial malformation, exophthalmos, stertorous breathing, and epistaxis. Sinus cysts, neoplasms, and hematomas occasionally occur and result in serosanguineous discharge. When the physical examination, especially percussion, and radiographic findings indicate, the sinus should be trephined for diagnostic aspiration, drainage, and flushing, if necessary. In some cases sinoscopy may be indicated, particularly when the true extent of the disease process is difficult to determine or if biopsy samples are needed.
In the horse the frontal, sphenopalatine, and ethmoidal sinuses all communicate with the posterior chamber of the maxillary sinus and drain through the nasal maxillary opening into the middle meatus. The anterior chamber of the maxillary sinus is separated by an osseous septum that often breaks down with infection, making the posterior chamber of the maxillary sinus the most productive site for diagnostic aspiration. A line is drawn from the medial canthus of the eye perpendicularly to the facial crest. After tranquilization and local anesthesia, the sinus is approached with a Steinmann pin midway on this line. Once the sinus has been entered, aspiration should be performed by using a sterile 16-gauge needle or canine urinary catheter. One skin suture will suffice for closure. If purulent material or fluid within the sinus is under pressure, some leakage into the SC space may occur, with resulting cellulitis. The sample should be cultured for aerobic and anaerobic bacteria and examined cytologically for signs of septic inflammation or neoplastic cells.
The frontal sinus is trephined more often for flushing in chronic cases than for diagnostic purposes. The approach is 2.5 cm lateral to the midline of the face and 2.5 cm caudal to the point at which the nasal bones begin to diverge.
In cattle the frontal sinus is most often affected with septic inflammation as a consequence of dehorning. Purulent material frequently accumulates in the postorbital diverticulum of the sinus. This site is approached for trephination 4 cm from the edge of the orbital cavity just dorsal to the temporal (lateral) canthus of the eye.
Postdehorning sinusitis in goats can be a severe condition, especially in animals dehorned when mature. The frontal sinus contains numerous septa, creating poor drainage; the bony plate protecting the brain is thin, so that septic necrosis of bone leading to meningitis may occur. Therefore, in mature goats with sinusitis, appropriate systemic antimicrobial therapy and vigorous curettage of the affected areas should be used. A bone flap similar to the technique used in chronic maxillary sinusitis may be required to expose the frontal sinuses to curettage adequately.
Guttural Pouch Catheterization
When indicated by radiography and/or endoscopy, equine guttural pouches are easily catheterized for diagnostic sampling and flushing. Sampling can also be achieved by placing thin tubing through the biopsy channel and directly aspirating or aspirating after introduction of sterile saline, as TBA and lavage are performed through the biopsy channel. The patient should be tranquilized so that the head drops, facilitating drainage of the secretions by gravity. A Chambers mare catheter can be passed through the ventral meatus into the pharynx if the endoscope is not to be used for the sampling or lavage. The curved end is directed beneath the flap of the medial lamina of the pouch ipsilateral to the nostril used for passage. Successful passage is indicated by lack of resistance while the catheter is inserted deeper than if it were in the pharynx. The position of the catheter tip in the pharynx can be observed through an endoscope placed up the opposite nasal passage. Once the catheter is within the pouch, it can be used to obtain a sample, to drain excessive secretions, or to act as a conduit for flushing. A self-retaining uterine catheter can be left in place for repeated flushing, but the Chambers catheter can be passed repeatedly with no complications.
Lung Biopsy
Lung biopsy is most often done in the horse and should be used in conjunction with other, less invasive diagnostic techniques such as ultrasonography, radiography, and transtracheal aspiration. Lung biopsy is indicated to obtain a histologic diagnosis or prognostic information primarily in cases of diffuse lung disease, because the sample obtained is generally very small. A more useful parenchymal sample is obtained by means of percutaneous rather than endobronchial biopsy; complications of percutaneous biopsy are uncommon but do occur. A large gauge (14-gauge) 15-cm or longer biopsy needle, with a sample “slot” length of 22 mm, provides an excellent sample. Lung biopsy is easier with newer spring-loaded biopsy instruments than with older hand-operated biopsy instruments. Discomfort is minimal, and sedation may or may not be needed. The site for biopsy should be determined after ultrasonographic evaluation of the thorax, and the procedure should be performed away from common locations of major pulmonary vessels. Caudal and dorsal locations are generally chosen. The site should be widely clipped, surgically prepared, and infiltrated with local anesthetic down to the pleura. The biopsy needle is inserted through a stab incision just cranial to the rib, similar to catheter placement for pleural fluid sampling and drainage, and directed medially through the intercostal muscles and parietal pleura. The needle should be advanced the distance indicated by ultrasonographic measurement and then sharply advanced <1 cm to enter the lung parenchyma. The biopsy is obtained by “firing” the spring-loaded instrument, and the biopsy instrument is then withdrawn. If no specimen is obtained, the procedure can be repeated. After sufficient biopsy specimens have been obtained and the biopsy instrument withdrawn, a single skin suture can be placed at the incision site, but no additional aftercare is needed. The specimen should be placed directly in 10% formalin or glutaraldehyde for fixation. If additional samples can be safely obtained, they should be submitted for bacterial and fungal culture and potentially for more advanced molecular diagnostic techniques and handled appropriately for the desired test. Complications of lung biopsy have been reported to range from transient epistaxis or hemoptysis, which is to be expected, to more severe pleural and parenchymal hemorrhage. Lung biopsy is not indicated for pleuropneumonia cases but is generally required to differentiate among EMPF, discrete fungal lesions, and potential neoplasia, when accurate diagnosis is required for therapeutic and prognostic purposes.
Molecular Techniques
Infectious pulmonary disease represents a diagnostic challenge for the equine practitioner. A presumptive diagnosis can often be made based on the history, physical examination, clinical impression, complete blood cell count, radiography, and endoscopy. Definitive identification of the causative agent(s) is necessary to ensure that the appropriate therapeutic and prophylactic protocols are instituted.
Culture of infectious pathogens is an indispensable diagnostic technique and should be attempted in all cases of pulmonary disease. However, cultivating infectious pulmonary pathogens from clinical specimens is time-consuming and may require from days to months before an organism is identified, potentially delaying appropriate management. Molecular recognition systems that can be used for rapid identification can improve response time and reduce the number of susceptible animals exposed to the infectious pathogen.
Several technologic innovations have improved the rapidity and sensitivity with which microorganisms are identified.25 Immunologic assays and nucleic acid—based methods for the identification of bacterial, viral, and fungal pathogens have found clinical application in the diagnosis of pulmonary disease.
IMMUNOLOGIC TECHNIQUES
Immunologic detection of bacterial, viral, and fungal pulmonary pathogens have been developed. Immunoassays rely on interaction between the bacterial, viral, and fungal antigens and enzyme- or fluorescent-labeled antibody. The use of polyclonal antibodies tends to increase the sensitivity of the assay, as the preparation may contain antibodies to multiple epitopes on the target antigen, thus increasing the chance of antigen detection, but tends to decrease assay specificity because of their heterogenous nature. Test specificity can be improved by the use of monoclonal antibodies, as these antibodies interact with only a single well-defined epitope or very similar epitopes.
Immunohistochemistry (IHC) is rapidly becoming a standard diagnostic tool for the identification of viral, bacterial, and protozoal pathogens in tissue sections. This technique depends on polyclonal or monoclonal antibodies binding to a target antigen and the demonstration of this interaction by colored histochemical reactions visible by light microscopy or by emittance of fluorescence detectable after ultraviolet light illumination. IHC is highly versatile, and assays have been developed to detect a variety of equine pulmonary pathogens including, but not limited to, equine herpesviruses (EHVs), equine viral arteritis (EVA) virus, Hendra virus (HeV), R. equi, and Pneumocystis carinii, in tissue sections. Recent advancements in methodology have increased the ability to detect antigens in formalin-fixed tissues.
NUCLEIC ACID-BASED TECHNIQUES
Nucleic acid—based techniques for the diagnosis of equine pulmonary disease are becoming widely accepted because of the sensitivity, specificity, and speed with which results can be obtained. These assays can detect nucleic acid of both live and dead pathogens at very low concentrations, often less than 100 copies per microliter. Bacterial, viral, and fungal pulmonary pathogens can be discriminated based on nucleic acid sequences unique to those particular organisms. Polymerase chain reaction (PCR) testing is the most widely used molecular diagnostic technique in both research and clinical laboratories. PCR testing involves the enzymatic replication of a target region of deoxyribonucleic acid (DNA) as defined by a set of oligonucleotide primers. DNA polymerase synthesizes each complementary strand of the target region in the 5’ to 3’ direction, and the amount of DNA that is synthesized increases exponentially. For viruses whose genome is composed of ribonucleic acid (RNA), an initial reverse transcriptase (RT) step is required to generate a complementary strand of nucleic acid because the DNA polymerase requires a double-stranded template to amplify the target sequence.
Real-time PCR combines PCR amplification with detection of the amplified products, allowing quantification of PCR products. In real-time PCR the PCR reaction is carried out in a reaction tube to which an optical device is attached to read the fluorescent signal generated during each cycle of PCR reaction. Increases in the reporter fluorescence are proportional to the increase in PCR product. By monitoring the changes in degree of fluorescence, the time in which a transition from exponential to log phase amplification occurs can be determined and compared with that of a standard control to determine the initial template concentration.
Hydrolysis probes, such a TaqMan (Applied Biosystems, Foster City, Calif.), are commercially available and can be tailored for use in diagnostic tests to detect specific pathogens. In the TaqMan assay a probe is labeled with fluorescence and binds to a nucleic acid sequence within the target region. During amplification the Thermus aqaticus (Taq) polymerase hydrolyses the probe that separates the fluorescein from a quenching dye and allows the emission of fluorescence. The amount of fluorescence emitted is proportional to the accumulation of specific PCR product. TaqMan assays reduce the risk of contamination, as there is less sample handling compared with traditional PCR techniques, and the results are produced rapidly.
With the increased use of PCR assays as diagnostic tests, there is increased demand for standardized techniques and internal control measures. PCR is a highly sensitive test and may produce false-positive results, commonly attributed to contamination. False-negative results may occur as a result of the presence of enzyme inhibitors in the sample that suppress DNA amplification. Control plasmids, which contain a DNA sequence that is amplified at the same time as the target DNA but is sufficiently mutated that it can be distinguished from the target DNA sequence after PCR testing, are increasingly being used to identify the presence of inhibitory molecules within the assay either resulting from tissue or sample handling problems or associated with collected tissues.
These assays do not substitute for careful clinical evaluation but can shorten the time to confirmed, accurate diagnosis and thus allow for early initiation of therapeutic strategies and prevention protocols. With further understanding of the molecular biology and immunology of pulmonary disease, diagnostic and management techniques will become further refined.
PULMONARY FUNCTION TESTING
PFT has emerged as an essential tool in equine referral practice and is largely aimed at describing the severity, anatomic pattern, stability (reactivity), and reversibility of bronchoconstriction caused by noninfectious airway obstruction and inflammation, such as is seen in heaves and airway inflammatory disease.26 Clinical indications for noninvasive PFT include assessment of horses with intermittent cough, exercise intolerance, abnormal breathing pattern, and excess mucus, as well as early detection of subclinical disease, evaluation of treatment response, intensive care monitoring, and prediction of outcome. PFT is generally divided into the assessment of respiratory mechanics (mechanical properties of the respiratory system) and gas exchange. Analysis of gas exchange investigates ventilation-perfusion matching, shunt, diffusion capacity, and dead space—to—tidal volume ratio (VD/VT). Lung mechanics, on the other hand, determines the static and dynamic properties of the lung, including resistance, compliance, functional residual capacity (FRC), and ventilatory parameters.26
Mechanics of Breathing
The mechanical function of the lung is essentially defined by static and dynamic properties. Tests that are performed with the respiratory system at equilibrium and zero flow are referred to as static tests. Examples include measurement of lung volume subdivisions (e.g., FRC) and compliance of the lung and chest wall.26
FRC is a measure of the amount of gas that remains in the lungs at end-expiration (end-expiratory volume). FRC is lower in patients with increased “lung stiffness” (reduced elastic recoil of the lung) as well as airway inflammation, whereas FRC increases in patients with expiratory airway obstruction and gas trapping.27 This test can be easily performed in awake clinical patients via a helium dilution technique.28 In short, the patient is connected to a reservoir bag at end-expiration to rebreathe a standard, commercial breathable gas mixture of 10% helium (He), 20% oxygen, and the remainder nitrogen for 90 seconds. The dilution of He (a nonexchangeable gas) gives a measure of end-expiratory lung volume.28
Measurements of static compliance or “elastic recoil of the lung” require breath holds and have applications only to the anesthetized patient. So-called pressure-volume curves are generated in the relaxed patient during lung deflation from total lung capacity (TLC), using an esophageal balloon technique. Compliance is defined as the lung volume change per unit of pressure change27:
Reduced lung compliance (i.e., increased lung stiffness) may be associated with increased fibrous tissue (pulmonary fibrosis), atelectasis (e.g., underventilated lung), or an increased pulmonary venous pressure, in which the lung becomes engorged with blood. Emphysema and normal aging of the lung, which leads to alteration in elastic tissue, are causes of increased lung compliance.27
In contrast to static tests, tests that are performed with the respiratory system in motion (e.g., during quiet breathing) are referred to as dynamic. An example of a dynamic measurement is resistance, a measurement that requires flow. Resistance arises from friction of air molecules against airway walls (see formula26).
The measurement of pulmonary resistance (RL) using a flow meter attached to a face mask and an esophageal balloon catheter to measure transpulmonary pressure changes is conventional in the horse but is rarely used in the clinical setting.26 This technique allows computation of both RL and dynamic compliance (Cdyn) at spontaneous breathing frequencies. Maximal transpulmonary pressure change (ΔPPLmax) and RL both increase in cases of obstructive airway disease, whereas Cdyn decreases. However, this classic technique is fairly insensitive in detecting subclinical disease29,30 and has greater utility in the diagnosis of IAD if coupled with a challenge test (i.e., histamine bronchoprovocation [see later]).31,32
Forced Oscillation Techniques
In contrast to the conventional methods, noninvasive measurements of total respiratory system resistance via forced oscillation techniques (FOT) are used in the diagnosis of IAD in horses. In short, oscillometry is the study of lung mechanical function via the application of external forces to the respiratory system.33 Either a loudspeaker (e.g., Impulse Oscillometry System [IOS]) or air pressure (e.g., Monofrequency Forced Oscillatory Mechanics [FOM]) is used to superimpose pulses of flow (4 to 5 L/sec peak) through a face mask on the horse’s respiratory system during spontaneous breathing. The generated reciprocal pressure waves are subsequently recorded at the airway opening (i.e., face mask). The magnitude and phase relationship between the input of flow and output of pressure are then used to perform the calculations of impedance (total opposition to airflow) and its components, resistance and reactance, at a variety of oscillatory frequencies (generally 1 to 7 Hz).26 In most horses with IAD there is a frequency dependence of resistance, with higher values for resistance recorded at the lower oscillatory frequencies (1 to 2 Hz), indicative of bronchoconstriction.34-36 Higher frequencies (≥2 Hz) provide information concerning central airway resistance (Raw). Baseline respiratory resistance measurements using FOT are commonly combined with bronchoprovocation tests for the early diagnosis of IAD (see later).
Histamine Bronchoprovocation
Bronchoprovocation is a challenge test that assesses the response of the respiratory system to a bronchoconstrictor agonist (e.g., inhaled histamine).26 The provocatory concentration necessary to cause a 100% increase in baseline respiratory system resistance is commonly termed PC100RRS. Horses with a low PC100RRS (e.g., less than 6 mg/mL of histamine) are designated as having hyperreactive airways. Airway hyperreactivity (AHR) is an exaggerated narrowing response to a bronchoconstrictive stimulus, was first found in horses with heaves,37 and is considered a hallmark of IAD. There is a correlation between airway reactivity and mast cell percentage in BAL fluid (BALF) in horses with a history of exercise intolerance.38 The clinical symptoms associated with AHR are thought to include coughing (increased sensitivity) and exercise intolerance, whereas bronchoconstriction causes uneven ventilation and hypoxemia.39
Respiratory Inductive Plethysmography
The objective assessment of respiratory function and breathing pattern may also be obtained through the concurrent application of respiratory inductive plethysmography (RIP) and pneumotachography, which has been validated for the use in humans,40,41 horses,42 and sheep.43 In summary, two elastic bands, each containing a single sinusoidal conducting wire, are temporarily placed around the animal’s thorax and abdomen (Fig. 31-4). Stretch and contraction of the bands resulting from normal respiratory movements are measured as voltage changes, which are proportional to the change in circumference and volume of the thorax and abdomen.44 Simultaneous measurements of nasal flow are obtained at the airway opening (face mask). This technique rapidly assesses the synchronicity of breathing pattern (e.g., thoracoabdominal asynchrony due to diaphragmatic paralysis)44 and the individual contribution of the thorax and abdomen to respiration. It further quantifies changes in flow resulting from airway obstruction.
Forced Maneuvers
Forced expiratory maneuvers are commonly performed in humans with suspected asthma. Simplistically, the patient is asked to blow out hard from TLC until the lung is empty. The volume of air expelled in 1 sec (FEV1) corrected for vital capacity (FVC) is used as a measure of lower airway obstruction.26 Couetil adapted the method of forced maneuvers for routine clinical application in heavily sedated, nasotracheally intubated horses.45 He further demonstrated flow limitation in horses with early signs of obstructive airway disease (IAD) that was worse in animals with heaves. Remission from heaves showed improvement in flow limitation.26
PFT is considered instrumental in the understanding of the pathogenesis, pathophysiology, epidemiology, diagnosis, and treatment of lung disease in the horse.26 The described tests are aimed at quantifying airway obstruction, airway reactivity, and lung stiffness in the clinical setting. PFT will thus facilitate the diagnosis of subclinical disease and improve the clinician’s assessment with regard to aggressive management of lung disease, decisions for ventilation, intensity of monitoring, and assessment of treatment response and prognosis.
DISORDERS OF THE LUNGS
BACTERIAL PNEUMONIA AND PLEUROPNEUMONIA IN ADULT HORSES
Bacterial infections of the lower respiratory tract are common in adult horses. Lower respiratory tract infections may be localized to the lumen of the airways (termed bacterial bronchitis or septic inflammatory airway disease) or may involve the pulmonary parenchyma (termed pneumonia). In equine medicine the term bronchopneumonia is often used to refer to lower respiratory tract infection regardless of whether the infection is localized to the bronchi or involves both the bronchi and the lung parenchyma. When there is subsequent extension of the infection from the pulmonary parenchyma to the visceral pleura and pleural space, the disease is referred to as pleuropneumonia. The spectrum of clinical signs shown by horses with bronchopneumonia is broad and reflects the severity of the disease process. Early identification of affected animals and immediate initiation of appropriate therapy are essential to prevent mortality and functional impairment of the respiratory system. Expenses incurred by owners of affected horses include cost of medical care, loss of income during the illness and the recovery period, cost of training after a prolonged period of inactivity, and financial loss resulting from death of some animals or diminished performance of others after recovery.
Infectious Agents Involved
Adult horses most commonly acquire bacterial pneumonia by aspiration of microorganisms that normally inhabit their nasopharynx or oral cavity.46,47 β-hemolytic streptococci, particularly Streptococcus equi subsp. zooepidemicus, are by far the most common bacterial pathogens isolated from adult horses with bronchopneumonia.48 Nonenteric gram-negative bacteria such as Pasteurella species and Actinobacillus species are also frequently isolated, either alone or in combination with S. zooepidemicus. Enteric gram-negative bacteria such as Klebsiella species, Escherichia coli, Enterobacter species, and Salmonella enterica may also be isolated. Other aerobic gram-positive organisms such as Staphylococcus species and R. equi or gram-negative organisms such as Pseudomonas species and Bordetella bronchiseptica are occasionally isolated. Pseudomonas species are rarely a primary cause of pneumonia in horses, and their presence often reflects contamination of equipment used for taking airway samples (such as endoscopes). Streptococcus pneumoniae, a common pathogen of humans, has been positively correlated with lower airway inflammation in young thoroughbred racehorses in the United Kingdom.49 The microorganism can also induce pneumonia in ponies after heavy intrabronchial challenge.50 However, S. pneumoniae is rarely isolated from pneumonic horses in the United States.
Anaerobic bacteria are isolated from approximately one third of adult horses with severe bronchopneumonia, pleuropneumonia, or pulmonary abscessation. The most common anaerobes isolated are Bacteroides species., particularly Bacteroides fragilis, Clostridium species, and Peptostreptococcus species; Fusobacterium and Eubacterium species may also be isolated.48,51 Isolation of anaerobes from horses with pneumonia or pleuropneumonia has been associated with a less favorable prognosis in some studies. In one study the survival rate for 221 pneumonic horses with strictly aerobic isolates from tracheobronchial aspirates was 81.4% compared with 38.3% for the 81 horses in which anaerobes were cultured.48 Mixed bacterial infections are very common and may represent synergy between aerobic or facultative aerobic and anaerobic bacteria.
The importance of Mycoplasma species in the development of equine bronchopneumonia and pleuropneumonia is controversial. Several Mycoplasma species have been isolated from the respiratory tract of both diseased and healthy horses, with Mycoplasma felis and Mycoplasma equirhinis being the most common isolates. In one study, isolation of M. equirhinis was positively correlated with lower airway inflammation in a group of young thoroughbred racehorses in the United Kingdom,52 whereas isolation of Mycoplasma species was not significantly associated with disease in a similar population in Australia.53 An outbreak of lower respiratory tract disease caused by M. felis infection has been described.54M. felis has also been isolated from horses with pleuropneumonia, and at least in one instance experimental infection with M. felis has resulted in pleuropneumonia.55,56
Epidemiology
Bacterial bronchopneumonia may affect horses of any age and breed. In one retrospective study of 327 horses with pneumonia or pleuropneumonia, there was no sex predilection but 82% of the horses were less than 5 years of age.48 In a retrospective case-control study of risk factors for development of pleuropneumonia, thoroughbreds were at greater risk whereas standardbreds were at lower risk for developing the disease.57 In the same study the most significant risk factor for development of pleuropneumonia was long-distance transport within the week before the onset of clinical signs.57 In another study, 24.4% of 90 horses with pleuropneumonia had recently been transported over long distances, and 12.2% had recently undergone general anesthesia.58 Five of the postsurgical cases had undergone upper airway surgery.58 Whether these horses had preexisting lung disease or whether they developed aspiration pneumonia as a result of surgery and general anesthesia could not be ascertained.
Other factors significantly associated with increased risk of developing pleuropneumonia include recent viral respiratory tract infection or exposure to horses with viral infections and racing within 48 hours before developing clinical signs.57 One study in the United Kingdom identified a higher incidence of pneumonia and pleuropneumonia in show jumpers, presumably reflecting the greater distance over which these horses are transported compared with racehorses in that country.59
Pathophysiology
Colonization of the lungs by opportunistic bacteria occurs when the pulmonary defense mechanisms are compromised or are overwhelmed by massive numbers of bacteria. Several factors can contribute to causing increased numbers of bacteria in the lower airways. Dysphagia or esophageal obstruction will lead to aspiration of large numbers of pharyngeal bacteria, and these disease processes often result in pneumonia. However, the vast majority of horses with bacterial pneumonia or pleuropneumonia do not have a history of dysphagia or esophageal obstruction. Other factors that have been shown to significantly increase bacterial contamination of the lower respiratory tract include confinement with the head elevated, transportation, and high-intensity exercise.60-62 In one study, confinement of horses with the head elevated resulted in a significant increase in bacterial numbers as well as neutrophilic inflammation in the lower respiratory tract as early as 6 hours after initiation of confinement.61Actinobacillus species, Pasteurella species, and β-hemolytic streptococci were the predominant bacterial isolates. Lowering the head for 30 minutes every 6 hours to facilitate postural drainage during a 24-hour confinement did not prevent multiplication of bacteria.61 Clearance of accumulated secretions and bacteria occurred within 8 to 12 hours after release from confinement.61 In similar confinement experiments, pretreatment with penicillin considerably reduced the number of β-hemolytic streptococci but did not reliably reduce total bacterial numbers.63 Cilia of many other species such as dogs and humans can transport mucus effectively against gravity, and posture has no effect on tracheal mucociliary transport in these species.64,65 In contrast, periods of lowered head posture are absolutely essential for normal mucociliary clearance in horses.66
In one study, long-distance transport by road over 12 hours resulted in increased bacterial contamination and neutrophilic inflammation in tracheal aspirate fluid when the horses’ head were restrained in an elevated position.67 This is in contrast to another study in which there was no significant cytologic or bacteriologic change in BALF from mares whose heads not restrained in an elevated position during transportation for 12 hours.68 Collectively, these findings suggest that duration of time with a raised head position is more important than the stress of transport alone in development of airway colonization. Finally, the last factor shown to increase bacterial contamination of the large airways of horses is exercise. In one study a single bout of high-intensity exercise resulted in a tenfold increase in aerobic and a hundredfold increase in anaerobic bacterial counts in tracheal aspirate samples compared with preexercise values.60
Pulmonary defense mechanisms can be compromised by numerous factors. These include stress (e.g., transport and intense exercise), viral infections, malnutrition, exposure to dust or noxious gases, immunosuppressive therapy, immunodeficiency disorders, and general anesthesia. Infections with influenza virus and EHV-4 have been shown to significantly decrease mucociliary clearance for up to approximately 30 days after infection in horses.69 In one study, long-distance transportation resulted in a significant reduction in phagocytosis by peripheral blood neutrophils for approximately 36 hours after transportation.67 The effect of long-distance transport on number, viability, and function of cells in BALF has given conflicting results with no clear pattern emerging.68,70-72 Similarly, the effects of exercise on the immune system are complex and depend on a multitude of factors including the intensity or duration of exercise, the specific immune function being analyzed, and the timing of the measurement in relation to exercise. In general, moderate exercise enhances immune function whereas strenuous exercise tends to be detrimental to immune function.73 High-intensity exercise in horses leads to a significant decrease in blood neutrophil and bronchoalveolar macrophage phagocytic activity, which may lead to decreased bacterial clearance from the lungs.74-76 Exercise also affects the adaptive immune system, as lymphocyte proliferation responses and interferon (IFN)-γ production are decreased after high-intensity exercise.77-79 Strenuous exercise has also been shown to increase susceptibility to experimental influenza virus infection in ponies compared with rested animals, indicating that alterations of the immune function after exercise is a real phenomenon and not just an in vitro artifact.77
Regardless of the exact mechanism predisposing to bacterial colonization, the inflammatory response induced by bacterial invasion results in infiltration of neutrophils and other inflammatory cells into the airways and pulmonary parenchyma. Inflammatory cells and their mediators cause damage to the airway epithelium and capillary endothelium, leading to flooding of the terminal airways with inflammatory cells, serum cellular debris, and fibrin. This process is generally more severe in the ventral portions of the lung. In early stages the lungs may be simply edematous, reflecting inflammation and early exudation, whereas in chronic severe cases the airways may be filled with purulent material and necrotic debris. Affected areas may show various degrees of consolidation, although in some cases focal abscesses may develop. These lesions interfere with gas exchange, and if the condition is severe enough the resulting ventilation-perfusion mismatch leads to hypoxemia and clinical signs of respiratory disease.
In severely affected animals, inflammation extends to the pleural space. The first stage of bacterial pleuropneumonia is an exudative stage, which is characterized by rapid outpouring of sterile pleural fluid into the pleural space in response to inflammation of the pleura. The associated pneumonic process is usually contiguous with the visceral pleura and results in increased permeability of the capillaries in the visceral pleura. If appropriate antimicrobial therapy is initiated at this stage, the pleural effusion may not become septic and may resolve. With progression, the bacteria invade the pleural fluid from the contiguous pneumonic process and the second, fibropurulent, stage evolves. This stage is characterized by the accumulation of large amounts of pleural fluid with many degenerate neutrophils, bacteria, and cellular debris. Fibrin is deposited in a continuous sheet covering both the visceral and parietal pleura adjacent to affected areas. As this stage progresses, there is often loculation and formation of limiting membranes. These loculations prevent dissemination of the infection but make drainage of the pleural space with chest tubes increasingly difficult. The last stage is the organization stage, in which fibroblasts grow into the exudate from both the visceral and parietal pleura surfaces and produce the membrane called the pleural peel. This inelastic pleural peel encases the lung and renders it virtually functionless. At this stage the exudate is generally thick.
Clinical Signs and Physical Examination
The spectrum of clinical signs shown by horses with bacterial lung infection is broad and usually reflects the severity of the disease process. Horses with septic IAD and no or minimal involvement of the lung parenchyma may be completely normal at rest. Clinical signs may be limited to exercise intolerance and poor performance, or affected horses may cough or have bilateral nasal discharge during or immediately after exercise. Even in cases of early bronchopneumonia, clinical signs may not be obvious. As the disease progresses clinical signs may include any combination of fever, anorexia, depression, bilateral nasal discharge, cough, weight loss, tachypnea, and respiratory distress. Nasal discharge is usually mucopurulent but may be hemorrhagic in some cases with pulmonary infarction and necrotizing pneumonia.80 Halitosis and a foul-smelling nasal discharge may be present and has been associated with infection by anaerobic bacteria. However, the absence of a putrid odor does not rule out anaerobic infections.
Because the parietal pleura are highly innervated and painful when inflamed, horses with acute pleuropneumonia often exhibit pleurodynia (pleural pain). Pleurodynia can often be detected by applying digital pressure to the intercostal space, resulting in grunts, intercostal muscle spasm, or even escape maneuvers by the patient when pain is present. Pleurodynia may also be manifested as pawing, stiff forelimb gait, abducted elbows, and reluctance to move. The condition may easily be mistaken for colic, exertional rhabdomyolysis, or laminitis. As more fluid accumulates in the pleural space and the disease becomes chronic, pain is less evident. A plaque of sternal edema is a common clinical finding in horses with pleuropneumonia. This is not a specific finding, as it can occur with many other disease processes.
Careful auscultation after application of a rebreathing bag (if not precluded by respiratory distress) is extremely valuable in defining the presence and sometimes extent of lung involvement. Horses with a large amount of secretions in the trachea often have an audible tracheal rattle. Most horses with bronchopneumonia cough when the rebreathing bag is applied, whereas normal horses do not. Occasional inspiratory or expiratory crackles and/or wheezes may be heard over affected areas, which are more commonly located ventrally. Because consolidated lung parenchyma is a good acoustic medium, mild consolidation sometimes results in only increased bronchial sounds. In contrast, the lung sounds may be diminished in areas of severe consolidation, extensive abscess formation, or pleural effusion.
Auscultation of horses with pleuropneumonia reveals normal lung sounds in the dorsal lung fields with no or considerably decreased lung sounds ventrally. Pleural friction rubs are often not heard because they are present only in the acute stage of the disease. If they are heard, friction rubs are present predominantly at the end of inspiration and the early part of expiration. They disappear as inflammation decreases or as pleural fluids accumulate. Cardiac sounds are often heard over a wider area of the chest than normal, probably as a result of enhanced conduction of sound through the pleural fluid. Thoracic percussion is useful to detect and delineate pleural effusion with resonant sounds dorsal and dull sounds ventral to the horizontal line of effusion.
Differential Diagnosis
When physical examination and auscultation indicate pulmonary disease, the major clinical task is differentiating infectious from noninfectious causes. Among infectious causes, bacterial pneumonia is most common. Viral infections are usually confined to the upper respiratory tract. Pulmonary aspergillosis most often follows severe gastrointestinal (GI) disease that resulted in mucosal compromise. Severe respiratory disease unresponsive to antimicrobial therapy should arouse suspicion of fungal pneumonia.81 Parasitic pneumonitis is rare in adult horses but may be seen in horses pastured with donkeys or mules. The two major noninfectious causes of pulmonary disease that may be confused with bacterial pneumonia include heaves and nonseptic IAD. Other uncommon causes of noninfectious pulmonary disease that may result in similar clinical signs include pneumothorax, pulmonary edema, smoke inhalation, neoplasia, and idiopathic interstitial pneumonia. The diagnostic tests described in the following sections aid in the differentiation between infectious and noninfectious causes of lower respiratory tract disease.
When pleural effusion is present, several differential diagnoses should be considered. Bacterial pleuropneumonia is by far the most common cause of pleural effusion in horses. In one study, 90 of 122 horses (73.8%) with pleural effusion had pleuritis secondary to bacterial pneumonia or lung abscessation.58 Other, less common causes of pleural effusion in horses include hemothorax, penetrating chest wounds, esophageal ulceration or rupture, neoplasia, fungal pneumonia, pericarditis, congestive heart failure, diaphragmatic hernia, hypoproteinemia, and chylothorax.
Diagnostic Approach
Presumptive diagnosis of lower respiratory tract infection is generally based on clinical signs and careful auscultation of the lungs with a rebreathing bag. The need for additional diagnostic procedures is determined by the severity and duration of clinical signs, the number of affected animals, and the value of the affected animal(s), as well as prior treatment used and response to such therapy. Bacterial pneumonia or pleuropneumonia most commonly affects an individual horse on a given farm. In horses suspected of having bacterial pneumonia, the goal of diagnostic evaluation is to rule out diseases of the upper respiratory tract and to determine the cause and severity of lung involvement.
HEMATOLOGY AND BIOCHEMISTRY
Horses with bacterial bronchopneumonia commonly have an inflammatory hematologic profile. Leukocytosis and absolute neutrophilia with or without a left shift are supportive of a diagnosis of bacterial infection. Neutropenia with a toxic left shift may also be evident in the acute stages in severely affected animals. Increased fibrinogen concentrations, hyperglobulinemia, and mild hypoalbuminemia are common. Anemia of chronic inflammation may develop in chronically affected animals. There does not seem to be a good correlation between the severity of clinical signs and the presence or the magnitude of hematologic changes. When plasma fibrinogen concentrations are elevated, their sequential measurement provides a useful means of monitoring response to treatment and is a useful guide in the decision to discontinue treatment. It must be remembered that a normal hematologic profile does not rule out bacterial bronchopneumonia.
ENDOSCOPY
Endoscopy may be useful to rule out upper airway infection when physical examination and auscultation of the lungs are not conclusive. Presence of mucopurulent secretions in the trachea and bronchi confirms lower respiratory tract disease. However, the presence of mucopurulent secretions does not necessarily indicate a bacterial infection, as this is also a common finding in horses with heaves and horses with nonseptic IAD. Bronchoscopy may also be useful in locating the affected lung segment in horses with focal pneumonia or draining pulmonary abscesses. It should be noted that exploration of the trachea and lower airways via bronchoscopy may contaminate subsequent samples taken for culture.
TRACHEOBRONCHIAL ASPIRATION FOR CYTOLOGY AND CULTURE
TBA for cytologic examination and bacterial culture is one of the most helpful diagnostic procedures available when bronchopneumonia is suspected. BAL is not as useful as tracheobronchial aspiration in cases of bronchopneumonia, because with BAL only a small portion of a lung is sampled. In contrast to BAL, TBA yields a pooled sample of secretions from all portions of the lung, thus increasing chances of culturing the pathogen of interest. In one study involving 22 horses diagnosed with pneumonia or pleuropneumonia based on thoracic radiographs or ultrasonography, cytology of BAL was abnormal in only 10 horses despite attempts at selectively sampling the affected area.82 In contrast, all horses had evidence of septic inflammation based on tracheobronchial aspirate cytology.82 Whenever possible, antimicrobial therapy should be discontinued at least 24 hours before tracheobronchial aspiration is performed. Tracheobronchial aspirate is preferably obtained by sterile percutaneous transtracheal aspiration to avoid contamination from the upper airway. Alternatively, the sample can be collected with a sterile guarded aspiration catheter passed through the biopsy channel of a flexible endoscope.83,84 Endoscopy has the advantage of allowing selective aspiration of exudate when it is present, which may therefore enhance recovery of bacteria. However, bacterial contamination of nasal or pharyngeal origin more commonly occurs in tracheobronchial aspirate samples collected via endoscopy. Airway fluid specimens should be submitted for cytology and Gram stain, as well as for aerobic and anaerobic bacterial culture. The fluid used for anaerobic cultures should be transferred to the laboratory immediately after collection in a manner that prevents or minimizes exposure to air. Anaerobic transport media are commercially available and should be routinely used. Specimens submitted for isolation of anaerobes should not be refrigerated, because many anaerobes are intolerant to cold. In horses with pleural effusion, a tracheobronchial aspirate should be obtained even if pleural fluid is available for bacterial culture. In one study, culture of the pleural fluid was negative in 43% of 111 horses with pleuropneumonia, whereas tracheobronchial fluid yielded growth in all cases.48 Only approximately 5% of cases had growth from the pleural fluid but not from the tracheobronchial aspirate.48
Macrophages and columnar ciliated epithelial cells predominate in tracheobronchial aspirates from healthy horses. The percentage of neutrophils in tracheobronchial aspirates from apparently healthy horses can be quite variable. In one study approximately 75% of apparently healthy thoroughbred racehorses in training had less than 20% well-preserved neutrophils.85 Occasionally plant spores and fungal hyphae may be present, either free or in large mononuclear cells. Their presence does not necessarily indicate fungal infection and probably reflects the horse’s environment. Degenerated neutrophils displaying karyolysis and cytoplasmic vacuolization are the predominant cell type in horses with bacterial lower respiratory tract infections. Bacteria may be found intracellularly or extracellularly. Presence of squamous epithelial cells indicates contamination with the upper respiratory tract or pharynx and is more common in samples obtained via the endoscope. The trachea is not a perfectly sterile site, and potentially pathogenic bacteria can be isolated from the trachea of normal horses.83 Therefore culture results should always be interpreted in the context of clinical signs and cytologic examination. If small numbers of bacteria are cultured in the absence of cytologic evidence of sepsis, it is unlikely that they are the cause of the respiratory problem. Similarly, growth of various molds is common in tracheobronchial aspirate cultures, and the clinical signs and cytologic findings should be considered before treatment with antifungal agents is initiated.
THORACIC ULTRASONOGRAPHY
Thoracic ultrasonography can be performed using a range of transducers and machine types. The normal aerated lung parenchyma is not penetrated by the ultrasound beam, rendering only the pleural space and superficial lung surface available for study. Ultrasonography is a helpful diagnostic tool when lung involvement includes peripheral areas but may not be as useful as radiography to evaluate the full extent of lung lesions because lesions with overlying aerated lung will not be detected. However, in most horses with bronchopneumonia the periphery of the lung is affected, enabling the clinician to successfully image some of the lesions. Early ultrasonographic lesions are nonspecific and may include only irregularities of the pleural surface. These lesions may progress to form focal areas of consolidation of various sizes. Consolidated lung varies in appearance from dimples of the pleural surface to large wedge-shaped areas of sonolucent lungs (Fig. 31-5).
Fig. 31-5 Sonogram of the left thorax obtained from a 3-year-old thoroughbred gelding. The ventral aspect of the lung is completely consolidated. Note the liver-like appearance of the wedge-shaped area of the lung. Fluid bronchograms are indicated by an arrow.
Ultrasonography offers a considerable advantage over radiographs in the study of the pleural surfaces and space. Small amount of pleural effusion that would otherwise be missed clinically or by radiography can be easily detected by ultrasonography. Ultrasonography can also be used to assess the nature and approximate volume of fluid, to select the optimal site and depth for thoracocentesis, and to detect sequelae such as fibrin deposition, pleural adhesion, abscess formation, and pneumothorax. Pleural effusion appears as hypoechoic to anechoic fluid between the parietal pleural surface and the lungs (Fig. 31-6). The echogenicity of the fluid typically reflects the degree of cellularity. Fibrin appears as filamentous strands floating in the effusion (Fig. 31-7). The presence of small (<1 mm) bright echoes (gas echoes) often indicates anaerobic infections (see Fig. 31-7).86 Gas echoes may also be seen with gangrenous pneumonia or bronchopleural fistulas, or they may result from leakage of air during thoracocentesis.
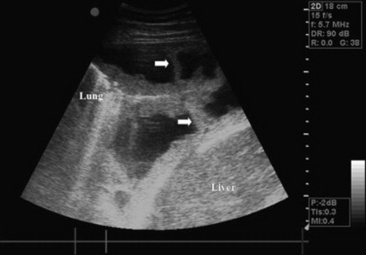
Fig. 31-6 Sonogram of the thorax obtained from a 4-year-old horse with pleuropneumonia. The ventral tip of the lung is atelectatic and covered with fibrin. There are adhesions between the tip of the lung and the diaphragm and chest wall (arrows).
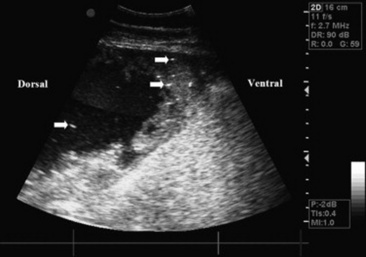
Fig. 31-7 Sonogram of the ventral thorax obtained from a horse being treated for pleuropneumonia. The sonogram shows a pleural abscess filled with fluid dorsally and with hyperechoic material ventrally. There are multiple gas echoes (some indicated by arrows). Multiple anaerobes were obtained on culture of an aspirate.
RADIOGRAPHY
In horses with mild septic IAD, thoracic radiographs may be normal or may reveal only mild bronchial or bronchointerstitial patterns. In more severely affected animals, radiographs demonstrate irregular opacities in the ventral thorax that may obscure the normal vasculature and cardiac silhouette (Fig. 31-8). Air bronchograms are sometimes visible. Abscesses are often present as circular soft-tissue opacities of varying sizes. In some cases they may be cavitated with thick walls and a distinct horizontal line representing fluid-gas interface (Fig. 31-9). Pleural effusion appears as a horizontal line demarcating a ventral soft-tissue opacity that obscures the heart and ventral lung fields (Fig. 31-10). When severe pleural effusion is present, it is most efficient to perform thoracic radiographs after the pleural fluid has been drained. Although ultrasonography is superior to radiographs in horses with pleuropneumonia, radiography should still be performed when possible to detect and monitor resolution of deep pulmonary abscesses that may be missed during ultrasonography.
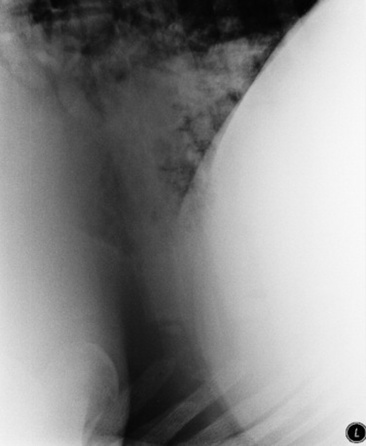
Fig. 31-8 Lateral thoracic radiograph of the caudoventral lung field from a 4-year-old thoroughbred mare showing a pronounced alveolar pattern with air bronchograms. The caudal silhouette of the heart is almost completely obscured.
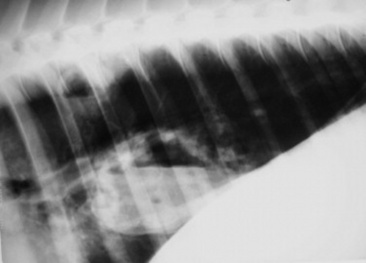
Fig. 31-9 Lateral thoracic radiograph of the caudodorsal lung field from a 3-year-old thoroughbred filly showing two cavitary abscesses with distinct horizontal lines representing fluid-gas interface.
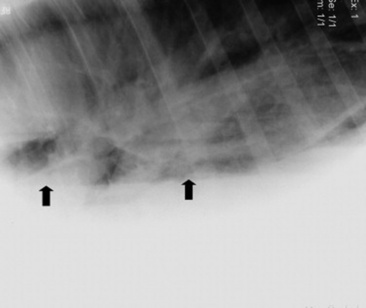
Fig. 31-10 Lateral thoracic radiographs from a four-year-old thoroughbred gelding with pleuropneumonia. There is a large amount of pleural effusion obscuring the ventral lung fields. The dorsal aspect of the fluid line is indicated by arrows. An interstitial pattern is noted in the caudodorsal lung fields.
THORACOCENTESIS FOR CYTOLOGY AND CULTURE
Thoracocentesis should be considered in horses with pleural effusion. The procedure can be of diagnostic value by allowing differentiation of septic pleuritis from effusion caused by other disease processes. It can also be of therapeutic value via removal of excessive chest fluid, which allows pulmonary reexpansion and a reduction in respiratory distress. Thoracocentesis is best performed using ultrasound guidance to determine the most appropriate site. A blunt teat cannula is typically used if a small volume of effusion is being sampled strictly for diagnostic purposes. Depending on the volume of effusion, a 16-, 24-, or 32-French chest tube is used when pleural drainage is also indicated (see later). An aliquot of pleural fluid is placed into tubes containing anticoagulant solution (ethylenediaminetetraacetic acid [EDTA]) so that cytologic evaluation may be performed. Part of the fluid should be saved in sterile containers with appropriate transport media for subsequent Gram stain and aerobic as well as anaerobic bacterial culture.
Normal equine pleural fluid is clear to light yellow and odorless. Protein concentration is <3.4 g/dL (range 0.2 to 4.7 g/dL), and nucleated cell count is <8000/μL (range 800 to 12,100/μL) in approximately 95% of apparently healthy horses.87 In horses with bacterial pleuropneumonia the effusion is typically exudative, cloudy, and yellow to red, with increased nucleated cell count and protein concentration. Such effusion usually contains greater than 90% neutrophils, most of which exhibit degenerative changes. Putrid-smelling pleural fluid is a hallmark of anaerobic infection; however, the absence of odor does not exclude anaerobic infection. There is no association between the WBC count or protein concentration in pleural fluid and survival.
Biochemical analysis of the pleural fluid may also provide a rapid assessment of the likelihood of sepsis, hence the need for pleural drainage. Values for pH, PO2, PCO2, bicarbonate, lactate, and glucose in pleural fluid from horses with nonseptic pleural effusion are similar to those in concurrent venous blood samples.88 Horses with septic pleural effusion have significantly lower pH, bicarbonate, and glucose in their pleural fluid compared with paired venous blood samples. In contrast, their pleural fluid has a significantly higher PCO2 and lactate concentration.88 Pleural fluid pH <7.1 and glucose concentration <40 mg/dL are suggestive of septic effusion in horses.89
THORACOSCOPY
Thoracoscopy is rarely necessary in the initial evaluation of horses with pleuropneumonia. However, the technique may be of diagnostic and therapeutic value in more chronic cases in which pleural abscesses or pleural adhesions develop (see section on treatment).90
Treatment
SYSTEMIC ANTIMICROBIAL THERAPY
Administration of appropriate antimicrobial agents is the most important part of the therapeutic plan. The choice of the antimicrobial agent depends on the severity of the clinical signs, cost, ease of administration, and, when available, results of bacteriologic culture and susceptibility testing of a tracheobronchial aspirate. Doses for commonly used antimicrobial agents are presented in Table 31-2. In early cases of mild bronchopneumonia, the practitioner is justified in suspecting S. zooepidemicus as the causative organism and treating accordingly. S. zooepidemicus is almost invariably susceptible to penicillin, ampicillin, and cephalosporins such as ceftiofur (Table 31-3). The possibility of a mixed infection, especially involving gram-negative organisms, must be kept in mind when such empiric therapy is unsuccessful. Ceftiofur offers the advantage of having a good spectrum of activity against common nonenteric gram-negative pathogens such as Pasteurella species and Actinobacillus species. Therapy of horses with mild lower respiratory tract infections should be continued for a minimum of 10 days or until clinical signs resolve. In some cases the duration needed may preclude continuous intramuscular (IM) therapy because of muscle soreness. Trimethoprim-sulfonamide (TMS) combinations offer the advantage of oral administration. Unfortunately, the usefulness of TMS combinations for the treatment of bacterial respiratory tract infections in horses is limited by their lack of in vivo activity against S. zooepidemicus. In contrast to penicillin, TMS is ineffective in eradicating S. equi subsp. zooepidemicus in a tissue chamber model of infection in horses.91,92 This failure of in vivo response was observed despite in vitro susceptibility of the isolate and high concentrations of TMS in the tissue chamber fluid.91,92
Table 31-3 In Vitro Antimicrobial Susceptibility of Aerobic Bacterial Isolates That Are Commonly Isolated from Horses with Bronchopneumonia or Pleuropneumonia*
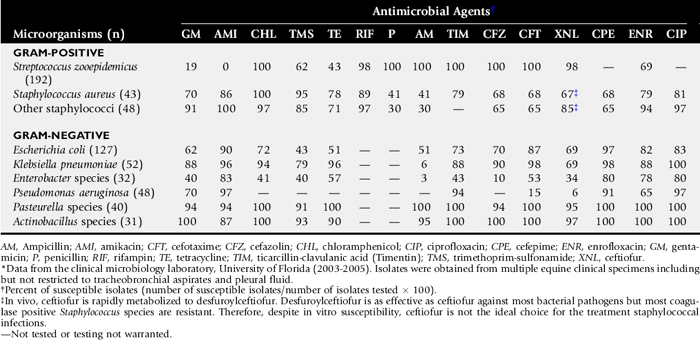
In more severe cases of bronchopneumonia and in all cases of pleuropneumonia, antimicrobial therapy should ultimately be selected based on results of culture and in vitro susceptibility testing. Before these results are obtained, selection should be based on knowledge of the prevalence and susceptibility pattern of bacteria commonly isolated from affected horses (see Table 31-2). Polymicrobial and mixed aerobic and anaerobic infections are common; thus broad-spectrum antimicrobial therapy is initially required. A combination of gentamicin for gram-negative coverage and penicillin for gram-positive and anaerobic coverage is commonly used as initial broad-spectrum therapy for moderate to severe bronchopneumonia. Enrofloxacin can be used as a substitute for gentamicin for gram-negative coverage in adult horses. Advantages of enrofloxacin over gentamicin include greater activity against Enterobacteraceae, better penetration in phagocytic cells and tissues, and better activity in purulent material. However, enrofloxacin should never be used as standalone initial therapy in horses with bronchopneumonia because of its lack of activity against anaerobes and against streptococci such as S. zooepidemicus. Ampicillin or cefazolin can replace penicillin for gram-positive coverage and offers the supplementary advantage of providing additional gram-negative coverage.
Treatment of anaerobic pleuropneumonia is usually empiric, because antimicrobial susceptibility testing of anaerobes is difficult owing to their fastidious nutritive and atmospheric requirements. Thus familiarity with antimicrobial susceptibility patterns is helpful in formulating the treatment regimen when an anaerobe is suspected. The majority of anaerobic isolates are susceptible to relatively low concentrations of penicillin. However, B. fragilis, a frequently encountered anaerobe in horses with pleuropneumonia, is routinely resistant to penicillin. Other members of the Bacteroides family are known to produce β-lactamases and are potentially penicillin resistant. Metronidazole has excellent in vitro activity against a variety of obligate anaerobes including B. fragilis. Oral administration rapidly results in adequate serum levels and thus is an acceptable route of administration for horses with pleuropneumonia. Therefore, if anaerobic infection is suspected, oral metronidazole is usually added to the combinations mentioned previously. Malodorous nasal discharge, halitosis, or the presence of gas echo in pleural effusion on ultrasonographic examination suggests the presence of an anaerobic infection. However, the absence of these findings does not rule out anaerobic bacterial infection. Horses with extensive lung consolidation and horses with pleuropneumonia may benefit from adding metronidazole to the treatment regimen. Metronidazole is not effective against aerobes and therefore should always be used in combination therapy. Chloramphenicol is active against most aerobes and anaerobes cultured from horses with bronchopneumonia or pleuropneumonia. However, because of human health concerns, its use should be limited to the treatment of horses with severe anaerobic bacterial infections refractory to metronidazole therapy in countries where its use is allowed. Rifampin is bactericidal and active against streptococci and most species of Bacteroides and Clostridium. It penetrates well into abscesses and may be helpful in anaerobic infections with walled-off abscesses. Rifampin should always be used in combination with another antimicrobial agent to decrease the likelihood of emergence of resistant mutants.
In cases of severe bronchopneumonia, lung abscesses, or pleuropneumonia, long-term antimicrobial therapy ranging from 3 weeks to sometimes several months may be required. In the initial stages of therapy IV antimicrobials are preferred to achieve higher serum concentrations. Oral antimicrobial agents can be used later in the course of the disease if appropriate based on susceptibility testing. Clinical signs, lung auscultation, fibrinogen concentrations, and repeated ultrasonographic and radiographic examination are useful in assessing response to therapy and deciding when to discontinue antimicrobial therapy. Stall rest must be enforced during therapy of pneumonia, and return to exercise should be gradual and permitted only after the horse is clinically normal and antibiotic therapy has been completed.
AEROSOLIZED ANTIMICROBIAL AGENTS
Aerosolized antimicrobial agents may be a useful adjunct to oral or systemic administration, particularly in horses with chronic septic IAD and no or minimal involvement of the lung parenchyma. The rate and extent of penetration of a drug into most sites outside the vascular space such as lung tissue are determined by the drug’s concentration in plasma, molecular charge and size, extent of plasma protein binding, and blood flow.93 In other tissues such as the central nervous system (CNS) and the eye, a lipid membrane provides a barrier to drug diffusion.93 There is a similar barrier between blood and the bronchial epithelium, restricting penetration of some drugs into bronchial secretions and epithelial lining fluid of the lower airways.94 Aerosol administration of antimicrobial agents can result in high drug concentrations in the respiratory tract while minimizing systemic concentrations and their resulting toxicity.
Antimicrobial delivery by inhalation is greatly influenced by the product formulation and type of nebulizer. Aerosol use of IV formulations can lead to exposure to potentially irritant or toxic additives and inappropriate pH or osmolality ranges. In one study the particle size distribution and particle density of gentamicin sulfate and ceftiofur sodium aerosols were affected by the antimicrobial concentration of the solution.95 Gentamicin concentrations of 50 mg/mL or ceftiofur concentrations of 25 mg/mL produced the optimal combinations of particle size and aerosol density with the use of a medical ultrasonic nebulizer.95 In healthy horses, aerosolization of 20 mL of the commercially available IV gentamicin sulfate solution (diluted to 50 mg/mL) using an ultrasonic nebulizer resulted in bronchial lavage fluid concentrations approximately 12 times higher than concentrations achieved by IV administration at a dose of 6.6 mg/kg.96 In the same study, serum concentrations after aerosol administration were below 1 μg/mL at all times.96 Once-daily aerosol administration of gentamicin to healthy horses for 7 consecutive days did not result in pulmonary inflammation or drug accumulation in the respiratory tract.97 The major limitation to the use of aerosolized gentamicin in horses is its lack of activity against S. zooepidemicus, the most common bacterial pathogen of the equine respiratory tract. Additional studies are required to assess the efficacy of aerosolized antimicrobial agents for the treatment of bacterial respiratory tract infections in horses.
ANCILLARY TREATMENTS
The need for ancillary treatments depends on the severity of the disease and is most often necessary in horses with pleuropneumonia. Nonsteroidal antiinflammatory drugs (NSAIDs) such as flunixin meglumine (1 mg/kg IV or by mouth [PO] once or twice daily) or phenylbutazone (2.2 mg/kg IV or PO once or twice daily) may be beneficial to minimize inflammation, provide analgesia, and control high fevers. Additional analgesia may be necessary in horses with severe pleurodynia. Adequate hydration should be maintained in patients receiving these agents for extended periods, especially if aminoglycosides are used concurrently. IV fluid therapy may be necessary to correct hypovolemia in acute stages, but it is rarely required in chronic cases. Intrapharyngeal insufflation of oxygen is indicated in horses that remain severely hypoxemic despite adequate drainage of the pleural cavity. Adequate parenteral or preferably enteral nutritional support via a nasogastric tube (NGT) is beneficial in horses that remain anorectic for several days. In horses presented with severe systemic illness, additional therapies aimed at treating endotoxemia may be beneficial (see section on endotoxemia).
PLEURAL DRAINAGE
Small amounts of pleural effusion may be resorbed quite readily with appropriate antimicrobial therapy. Therefore although a small sample of pleural fluid is diagnostically useful, pleural drainage is not necessarily indicated in all cases of pleuropneumonia. Drainage of pleural effusion results in removal of exudate and debris and allows for reexpansion of the lungs. Indications for drainage include a poor response to conservative therapy or the presence of pleural fluid with at least one of the following characteristics: sufficient volume to cause respiratory distress, empyematous character, fetid odor, or cytologic or biochemical evidence of sepsis. If indicated, the procedure should be attempted as early as possible before deposition of fibrin results in loculations and impairs drainage. The ventral mediastinum is perforated in normal horses in such a way that the left and right pleural spaces communicate. During severe inflammation the small perforations in the ventral mediastinum may be become obstructed by fibrin. Therefore, depending on the severity of the inflammatory response present, draining one side of the thorax may or may not be sufficient to resolve bilateral pleural effusion. Repeat ultrasonography of the opposite hemithorax should always be performed before the placement of a chest tube to determine if draining one side has actually emptied both sides of the thorax. Pleural drainage is best performed using ultrasound guidance to determine the most appropriate site. Pleural drainage can be accomplished either with intermittent thoracocentesis or indwelling chest tubes. Thoracocentesis is easily accomplished in the field and may not need to be repeated unless considerable pleural effusion reaccumulates. Indwelling chest tubes are indicated when continued pleural fluid accumulation makes intermittent thoracocentesis impractical. If properly placed and managed, indwelling tubes provide a method for frequent fluid removal and do not exacerbate the underlying pleuropneumonia or increase the production of pleural effusion. A one-way valve may be attached to allow for continuous drainage without leakage of air into the thorax. A Heimlich valve or an unlubricated condom with the tip cut off may be used for that purpose. As an alternative to continuous drainage, the tube can be temporarily occluded and periodically attached to a one-way valve for intermittent drainage. Heparinization of tubing after drainage may help maintain patency. The chest entry site and end of the drainage tube must be maintained aseptically. If a chest tube is placed aseptically and managed correctly, it can be maintained for several days to weeks. Local cellulitis may occur at the site of entry into the chest but is considered a minor complication. Pneumothorax is another potential complication of indwelling chest tubes. It should be removed as soon as it is no longer functional. A purse-string suture must be preplaced and tied as the tube is removed to prevent pneumothorax.
PLEURAL LAVAGE
Pleural lavage may be helpful to dilute thick viscous pleural fluid and remove fibrin, debris, and necrotic tissue. Pleural lavage is most helpful in subacute stages before loculated pockets of pleural fluid develop. However, pleural lavage may help break down fibrous adhesions and establish communication between loculae. Pleural lavage is typically performed by using the same chest tube for infusion and drainage of lavage fluid. Alternatively, lavage can be performed by infusing fluid through a dorsally positioned tube and draining it through a ventrally positioned tube. Five to 10 liters of sterile, warm isotonic fluid solution is infused by gravity flow. After infusion, the chest tube is reconnected to the unidirectional valve for drainage. Allowing the horse to walk once the continuous flow stops will often result in drainage of additional fluid. Pleural lavage is probably contraindicated in horses with bronchopleural communications because it may result in spread of septic debris up the airways and into normal areas of the lungs.98 Coughing and drainage of lavage fluid from the nose during infusion suggest the presence of a bronchopleural fistula (see discussion of complications, later).
THORACOSCOPY
Thoracoscopy allows direct evaluation of the lungs and pleural cavity. In selected cases thoracoscopy may be a useful tool to facilitate placement of thoracic drains in abscesses, transect pleural adhesions, and disrupt loculations.90 The technique can also be used to perform biopsy or aspiration of specific lesions affecting the periphery of the lungs. The procedure can be performed in the standing sedated horse with local anesthesia and is usually very well tolerated.99
Adhesions between the lung and the diaphragm or body wall may prevent complete collapse of the lung, making insertion of the instruments more difficult and sometimes limiting the view of the pleural cavity. Adhesions are easier to disrupt during the first week after formation when the tissue is fibrinous rather than fibrous. Transection of mature adhesion is more difficult and can result in severe hemorrhage. Creation of a pneumothorax is a necessary feature of thoracoscopy, and horses should be monitored carefully throughout the procedure. Transient exacerbation of clinical signs of pulmonary disease caused by pneumothorax can be alleviated by reinflation of the lungs.
THORACOTOMY
Surgical intervention in horses with pleuropneumonia is not a substitute for adequate medical management, but in carefully selected cases it can save the lives of horses that would have to be euthanized otherwise. The criteria for surgical intervention include: (1) failure to respond to antimicrobial therapy, pleural drainage, and pleural lavage; (2) stable systemic medical condition; (3) presence of a large amount of fibrin, debris, or pus in the pleural space; and (4) presence of either a walled-off lesion or a complete mediastinum to avoid creation of a bilateral pneumothorax. Surgical intervention is most beneficial in chronic cases with large unilateral localized pockets of thick debris, especially if there is resolution or at least a significant improvement of the disease in the opposite hemithorax. Before surgical exploration, the nature and location of the lesion must be thoroughly characterized by ultrasonography or thoracoscopy to determine the ideal surgical site. When there is bilateral disease, thoracotomy is performed on the most severely affected side. If necessary a second thoracotomy can be performed on the opposite side at a later time. Thoracotomy is typically performed with the horse standing. It is common practice to place a large chest tube into the targeted cavity and leave it open to atmospheric air for at least 2 hours before thoracotomy.98 The onset of respiratory distress indicates development of bilateral pneumothorax, in which case standing thoracotomy is contraindicated and pneumothorax must be corrected. Unilateral pneumothorax is usually well tolerated.
The standing surgical procedures most commonly performed include thoracotomy via an intercostal approach and thoracotomy and rib resection. For the intercostal approach the surgical site is prepared and infiltrated with local anesthetic. The lateral thoracic vein must be identified to avoid inadvertent incision. A vertical incision is made through the skin, intercostal musculature, and pleura. The length of the incision is dictated by the size of the pleural abscess and consistency of its content. When necessary, partial excision of the intercostal muscles will facilitate manual exploration of the cavity and removal of fibrin and necrotic debris (Fig. 31-11). The major advantage of the intercostal approach over rib resection is preservation of thoracic wall integrity and compliance. Thoracotomy with rib resection is elected when the cavity is very large and it is anticipated that extensive manual debridement of fibrin and necrotic debris will be necessary. The advantage of the thoracotomy with rib resection over the intercostal approach is improved access to the thorax, allowing manual removal of large fibrin clumps and necrotic debris (Fig. 31-12). With both rib resection and intercostal approaches, the incision is left open and irrigated once or twice daily with a sterile isotonic fluid solution. The cavity is periodically debrided via gentle massage, taking care not to disrupt mature adhesions. After adequate formation of granulation tissue, tap water may be used. Depending on the size of the incision, it may take a few weeks to 2 to 3 months for complete closure by second intention. Complications during thoracotomy may include bilateral pneumothorax (if the cavity is not walled off and the mediastinum is not complete) and cardiac arrhythmias (if the lesion is in close proximity to the heart). The most common long-term complication is the formation of a chronic draining fistula, but by itself, this complication does not prevent the horse from returning to its usual occupation.
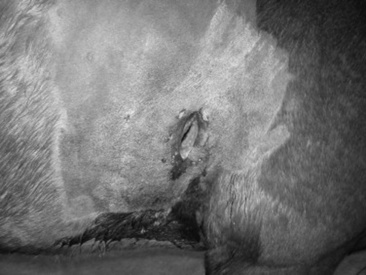
Fig. 31-11 Intercostal thoracotomy with partial excision of the intercostal muscles in a 7-year-old horse with a large pleural abscess. The image was obtained 3 weeks after surgery.
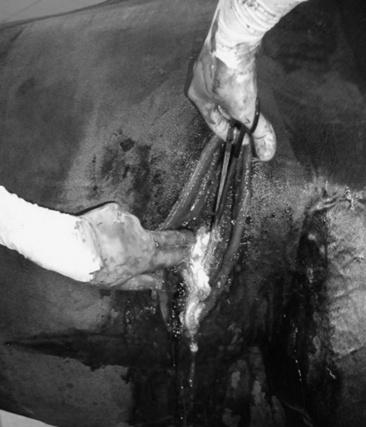
Fig. 31-12 Thoracotomy and rib resection in a thoroughbred filly with a large pleural abscess in the right hemithorax communicating with another abscess cranial to the heart. In this case a rib resection was performed to facilitate manual removal of purulent material and necrotic debris from both abscesses.
Complications of Pleuropneumonia
Several complications may occur during medical therapy of severe pneumonia and pleuropneumonia. These complications include jugular vein phlebitis or thrombosis from catheter placement, diarrhea resulting from antimicrobial therapy, pneumothorax or cellulitis secondary to thoracocentesis, endotoxemia, coagulopathies, and laminitis. In a retrospective study of 153 horses with pleuropneumonia presented to a referral hospital, complications included pleural abscesses (21.6%), cranial thoracic masses (7.2%), bronchopleural fistulas (6.5%), pericarditis (2.6%), and laminitis (1.3%).
Pleural abscesses refractory to antimicrobial therapy are treated by thoracocentesis or thoracotomy (see earlier). In some cases of pleuropneumonia, the heart may act as a valve to trap effusion and inflammatory debris in the cranial thorax. Small cranial thoracic masses may result in nonspecific clinical signs such as fever, tachycardia, and sternal edema. Larger abscesses may result in jugular vein distention, forelimb extension (pointing), and caudal displacement of the heart. Diagnosis is made by ultrasonography (Fig. 31-13). It is necessary to pull a forelimb forward to successfully image the cranial thorax. Most horses with cranial thoracic masses will respond to conservative therapy with antimicrobial agents.100 Drainage of the abscess should be performed in cases refractory to medical therapy or when the mass interferes with normal cardiac function. Cranial thoracic abscesses may be sampled in the standing horse with a front limb pulled forward. However, when drainage and lavage are required, the procedure is best performed under short-term general anesthesia to avoid damage to the heart and major blood vessels present in the cranial thorax. It is not recommended to leave an indwelling tube because the triceps musculature often causes the tube to kink. Repeated drainage and lavage may be necessary in some cases.
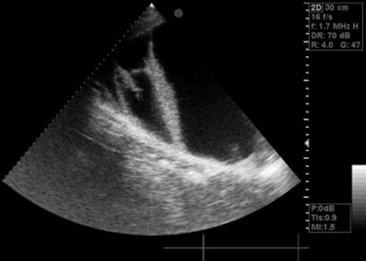
Fig. 31-13 Sonogram of the cranial thorax obtained from the third intercostal space of a 2-year-old thoroughbred filly with distended jugular veins and pitting edema of the ventral thorax. A large fluid-filled loculated abscess was imaged.
Bronchopleural fistulas develop when necrosis of lung tissue leads to direct communication between the airways and the pleural cavity. Diagnosis may be confirmed by thoracoscopy or by injecting sterile fluorescein dye into the pleural fluid and looking for the presence of dye at the nostrils or within the trachea by endoscopy. Horses with bronchopleural fistula often cough during pleural lavage, and lavage fluid may be noted at the nostrils. Most bronchopleural fistulas spontaneously seal as a result of adhesions to the chest wall or fibrin deposition on the visceral pleura. Sealing may take several weeks to months. In one case partial pneumonectomy was successful in resolving a chronic bronchopleural fistula and pulmonary abscess.101
Prognosis
The prognosis for survival and return to normal athletic function depends on the severity and duration of clinical signs before therapy. Horses with septic IAD or mild to moderate bronchopneumonia have a very good prognosis for a return to previous athletic performance. The prognosis for horses with pulmonary abscesses and without concurrent pleuritis is also good. In one study, 45 of 50 (90%) adult horses with pulmonary abscesses survived.102 In the same study, 92% of standardbreds and 52% of thoroughbreds raced after treatment of pulmonary abscesses.102 For horses that returned to racing, performance after successful treatment of lung abscesses was not significantly different from that before the illness.102
In a retrospective study of 327 horses with pneumonia or pleuropneumonia, the overall survival rate was 75%.93 In the same study, the survival rate for 81 horses from which anaerobic bacteria were cultured was only 4.3%.93 However, another study failed to identify an association between the presence of anaerobic bacteria and decreased survival.103 In cases of pleuropneumonia, retrospective studies have shown survival rates ranging from 43.3 to 87.6%.103,104 Differences in survival rates among studies may reflect differences in referral populations as well as advances in therapy in more recent years. Many horses that would have been euthanized owing to chronicity and lack of response to medical therapy several years ago are now successfully treated with the surgical approaches described earlier. This is evidenced by a retrospective of 153 horses with pleuropneumonia in which the survival rate was 95.7% when horses electively euthanized were excluded.104
The effect of pleuropneumonia on subsequent racing performance has not been examined extensively. In one retrospective study, 43 of 70 (61%) horses that had recovered from pleuropneumonia returned to racing, and 24 of the 43 (56%) won at least one race.104 In the same study, horses that required placement of an indwelling thoracic drain did not have a worse prognosis for return to performance compared with horses that did not require placement of a drain.104 In contrast, horses that developed complications such as pulmonary abscesses, cranial thoracic masses, or bronchopleural fistulas were significantly less likely to return to racing.104
RHODOCOCCUS EQUI INFECTIONS
R. equi, a gram-positive facultative intracellular pathogen, is one of the most common causes of pneumonia in foals between 3 weeks and 5 months of age. R. equi has also emerged as a significant opportunistic pathogen in immunosuppressed people, especially those infected with the human immunodeficiency virus.105,106 Although R. equi can be cultured from the environment of virtually all horse farms, the clinical disease in foals is endemic and devastating on some farms, sporadic on others, and unrecognized on most. On farms where the disease is endemic, costs associated with veterinary care, long-term therapy, and mortality of some foals may be very high. This section reviews the clinical manifestations, pathogenesis, epidemiology, diagnosis, treatment, and prevention of R. equi infections in foals.
Clinical Manifestations
The most common manifestation of R. equi infections in foals is a chronic suppurative bronchopneumonia with extensive abscessation (Fig. 31-14). The slow spread of the lung infection combined with the remarkable ability of foals to compensate for the progressive loss of functional lung make early clinical diagnosis difficult. Early clinical signs often consist of only a mild fever or a slight increase in respiratory rate that may not be apparent unless foals are exercised or stressed by handling. As the pneumonia progresses, clinical signs may include decreased appetite, lethargy, fever, tachypnea, and labored breathing characterized by nostril flaring and increased abdominal effort. Cough and bilateral nasal discharge are inconsistent findings. A smaller percentage of affected foals may have a more devastating subacute form. These foals may be found dead or more commonly are presented in acute respiratory distress with a high fever and no previous history of clinical respiratory disease. Foals with the subacute form of the disease have a poor prognosis despite appropriate therapy.
Fig. 31-14 Left lung from a foal with severe pneumonia caused by R. equi. There is marked consolidation of the cranial portion of the lung, and multiple abscesses are present caudodorsally.
Courtesy Dr. William Castleman.
Extrapulmonary manifestations of rhodococcal infections may also occur. Intestinal lesions are present in approximately 50% of foals with R. equi pneumonia presented for necropsy.107 However, the majority of foals with R. equi pneumonia do not show clinical signs of intestinal disease. In the same study, only 4% of the foals had intestinal lesions without pneumonia.107 The intestinal form of R. equi infection is characterized by a multifocal ulcerative enterocolitis and typhlitis over the area of the Peyer’s patches, with granulomatous or suppurative inflammation of the mesenteric and/or colonic lymph nodes.107 In some cases, the only abdominal lesion noted may be a single large abscess (usually in a mesenteric lymph node) that often adheres to the large or small bowel. Clinical signs associated with the abdominal form of the disease may include fever, depression, anorexia, weight loss, colic, and diarrhea.108 Marked GI lymphatic obstruction associated with increased protein concentration in the peritoneal fluid and hypoproteinemia may lead to ascites, giving affected foals a pot-bellied appearance. Such foals have a poor prognosis because of the extensive granulomatous inflammation of the colonic mucosa and submucosa and mesenteric lymph nodes.
Polysynovitis is present in approximately one third of cases of R. equi infection; in some cases effusion of multiple joints may even be the presenting complaint.109,110 The tibiotarsal and stifle joints are most commonly affected. Occasionally all joints are affected. The degree of joint effusion is variable, and in most cases lameness is not apparent or is limited to a stiff gait. Cytologic examination of the synovial fluid usually reveals a nonseptic mononuclear pleocytosis, and bacteriologic culture of the synovial fluid is negative.110 Histologic examination of the synovial membrane of a few affected foals revealed lymphoplasmacytic synovitis.109,111 Fluorescein-labeled antiequine immunoglobulin G (IgG) staining of the synovial membrane of three affected foals revealed evidence of immunoglobulin within the synovial membrane, and rheumatoid factors (i.e., antibodies directed against autologous or heterologous Fc portion of immunoglobulin) were identified in the synovial fluid of a foal with nonseptic joint effusion and R. equi pneumonia.109,111 These findings are suggestive of an immune-mediated process. However, experimental infection with a heavy intrabronchial inoculum of virulent R. equi consistently results in polysynovitis without associated lameness.112 Culture of the synovial fluid within a few days of the onset of synovial effusion in these foals often yields R. equi, and histologic examination of the synovial membrane reveals suppurative inflammation.112 Therefore it is possible that the synovitis is septic in origin but that the infection is rapidly cleared from the synovial structures, resulting in chronic nonseptic inflammation at the time of diagnosis. Regardless of the inciting cause, local therapy of the affected joints in clinical cases of R. equi–associated polysynovitis is usually not indicated because the effusion resolves without any apparent consequences as the primary infection responds to appropriate antimicrobial therapy. The presence of a nonseptic polysynovitis in a foal between 3 weeks and 6 months of age is highly suggestive of R. equi infection and deserves further investigation (see section on diagnosis). Immune-mediated processes may also contribute to the development of uveitis, anemia, and thrombocytopenia in some foals infected with R. equi.113
Bacteremic spread of the organism from the lungs or GI tract may occasionally result in septic arthritis and, more commonly, osteomyelitis. However, foals can occasionally develop R. equi septic arthritis or osteomyelitis without apparent lung or other source of infection. The degree of lameness of foals with septic arthritis distinguishes them from foals with nonseptic polysynovitis. In equivocal cases bacterial culture and cytologic examination of the synovial fluid should be performed. In addition to appropriate antimicrobial therapy (see treatment), foals with R. equi septic arthritis and osteomyelitis often require aggressive local therapy. R. equi vertebral osteomyelitis or diskospondylitis resulting in spinal cord compression has also been reported.114-116
Other rare extrapulmonary manifestations of R. equi infections in foals include panophthalmitis, guttural pouch empyema, sinusitis, pericarditis, nephritis, and hepatic, renal, and intracranial abscessation.113,117 Ulcerative lymphangitis, cellulitis, and SC abscesses have also been reported. Disease caused by R. equi is rare in adult horses. Sporadic reports of infection involving primarily lungs or abdominal lymph nodes (similar to infection observed in foals), or rarely of wound infection, exist. In one adult horse, acquired combined immunodeficiency of unknown origin led to R. equi septicemia and lung abscessation.118 Rarely the organism has also been isolated from infertile mares and aborted fetuses.107,119,120
R. equi has been isolated from many species other than humans and horses. R. equi is frequently cultured from the submaxillary lymph nodes of pigs with granulomatous lymphadenitis. However, R. equi can also be isolated from the submaxillary lymph nodes of 3% to 5% of apparently healthy pigs, and experimental infection studies in pigs have failed to reproduce the lesions.121,122 The causative role of R. equi in granulomatous lymphadenitis in pigs thus remains unproven. Isolation of R. equi from other domestic animal species is rare. R. equi can be isolated from lymph node granulomas in 0.008% of cattle at abattoir postmortem inspection.123R. equi has also been cultured in rare cases of bronchopneumonia, mastitis, metritis, ulcerative lymphangitis, and septic arthritis in cattle. In goats R. equi has a tendency to cause liver abscesses with concurrent bronchopneumonia or pulmonary abscessation.124R. equi has been isolated from cases of pneumonia as well as from wound infections, SC abscesses, vaginitis, hepatitis, osteomyelitis, myositis, and joint infections in dogs and cats.125
Virulence
The ability of R. equi to induce disease in foals likely depends on both host and microbial factors. R. equi is a facultative intracellular pathogen, and its ability to persist in and eventually destroy alveolar macrophages seems to be the basis of its pathogenicity. Knowledge of the virulence mechanisms of R. equi were largely speculative until the discovery of a virulence plasmid.126,127 Unlike most environmental R. equi, isolates from pneumonic foals typically contain an 80- to 90-kb plasmid. Plasmid-cured derivatives of virulent R. equi strains lose their ability to replicate and survive in macrophages.112 Plasmid-cured derivatives also fail to induce pneumonia and are completely cleared from the lungs of foals within 2 weeks after heavy intrabronchial challenge, confirming the absolute necessity of the large plasmid for the virulence of R. equi.112,128
Nucleotide sequencing of the large plasmid of two foal isolates revealed the presence of 69 open reading frames (ORFs).129 Comparisons of the plasmid sequence with genes previously identified in other microorganisms has identified three functional regions. Two of these regions contain genes encoding proteins involved in conjugation, plasmid replication stability, and segregation.129 The third region of 27.5 kb bears the hallmark of a pathogenicity island and contains the genes for a family of eight closely related virulence-associated proteins designated VapA and VapC to VapI.127,129-133 With the exception of vapF and vapI, which are not functional, all vap genes encode proteins with a clear signal sequence, indicating that they are extracellular proteins.129,133,134 VapA is expressed on the bacterial surface, and its expression is temperature regulated, occurring between 34° C and 41° C.135 VapC, VapD, and VapE are secreted proteins concomitantly regulated by temperature with VapA.136 In a recent study, an R. equi mutant lacking a 7.9-kb DNA region spanning six vap genes (vapA, vapC, vapD, vapE, vapF, vapI) was attenuated for virulence in mice and failed to replicate in macrophages.137 Complementation with vapA alone could restore full virulence, whereas complementation with vapC, vapD, or vapE could not. Conversely, a recombinant plasmid-cured derivative expressing wild-type levels of VapA failed to survive and replicate in macrophages and remained avirulent in foals, showing that expression of VapA alone is not sufficient to restore the virulence phenotype.112 These findings show that although VapA is essential for virulence, other plasmid-encoded products also contribute to the ability of R. equi to cause disease. Consistent with these findings, it was recently demonstrated that two R. equi mutants lacking expression of pathogenicity island genes (ORF4 and ORF8) were fully attenuated despite enhanced transcription of vapA.138 All vap genes as well as five other ORFs within the pathogenicity island are upregulated when R. equi is grown in macrophage monolayers.139 Regulation of expression of the genes of the pathogenicity island is complex and depends on at least five environmental signal including temperature, pH, oxidative stress, magnesium, and iron.135,139,140 The precise role of each of these genes in the pathogenesis of R. equi infections remains to be determined.
R. equi isolates are often classified as virulent, avirulent, or intermediately virulent based on their ability to induce disease or death in mice. Virulent R. equi isolates contain the large plasmid described previously and express VapA. Intermediately virulent R. equi isolates contain one of four distinct large plasmids encoding a 20-kDa antigen (VapB) that is related to, but distinct from, VapA. In contrast, avirulent R. equi isolates do not express Vap antigens. All three categories of R. equi have the ability to cause disease in immunosuppressed humans. Analysis of R. equi isolates from immunocompromised human patients with and without acquired immunodeficiency syndrome (AIDS) reveals that only approximately 20% of isolates contain an 80- to 90-kb plasmid and express VapA.141,142 Therefore the pathogenesis of R. equi infection in immunocompromised human patients appears to be different from the pathogenesis in foals, in which the virulence plasmid is always found. To my knowledge, intermediately virulent isolates (expressing VapB) have never been isolated from foals with naturally acquired R. equi infections. Experimentally, heavy intrabronchial challenge of foals with intermediately virulent R. equi results in pneumonia but at a dose much higher than that required for induction of pneumonia with VapA-expressing strains.143 Almost all isolates from the submaxillary lymph nodes of pigs produce VapB and are intermediately virulent to mice, suggesting that pigs or their environment may be the source of infection for some human cases.144 Most isolates from cattle, goats, and dogs are avirulent and do not contain plasmids encoding vapA or vapB.123-125 In contrast, most isolates from cats contain a large plasmid and express VapA.125
Although it has been firmly established that the virulence plasmid is essential for infection of foals, it is also clear that chromosomally encoded factors, such as regulatory genes and metabolic pathways are also important for allowing the pathogen to thrive within the host.145-148R. equi is closely related to Mycobacterium tuberculosis as evidenced by a partial genome sequence of R. equi, which showed that the majority of R. equi genes have most homology with M. tuberculosis.149 The unique cell wall of R. equi and other mycolata such as Mycobacterium, Corynebacterium, and Nocardia species is completely different from those of gram-negative and other gram-positive bacteria. Mycolata are characterized by a unique cell envelope that consists of mycolic acid anchored to an arabinogalactan wall polysaccharide.150 The mycolic acid—containing cell wall is likely to be of importance for survival of R. equi under harsh environmental conditions such as those that occur within macrophages. Consistent with this theory, R. equi isolates with a longer carbon chain and mycolic acid are more virulent than those with shorter chains as determined by lethality and granuloma formation in mice.151
Epidemiology and Pathogenesis
R. equi is a soil organism with simple growth requirements. The highest numbers of R. equi are found in surface soil, whereas the organism cannot be found at depths exceeding 30 cm.152R. equi can be isolated from the feces of most adult horses at concentrations ranging between 10 and 103 colony-forming units (CFUs) per gram of feces. The number of R. equi in the feces of mares on endemic farms does not increase in the weeks before or after foaling.153 In the same study, R. equi could be isolated from the feces of only 19% of the foals at 1 week of age and from all foals by 4 weeks of age.153 In another study, virulent R. equi could be isolated from the feces of all sampled mares during at least one sampling period, indicating that mares can be an important source of virulent R. equi for their surrounding environment.154 However, dams of affected foals did not shed more R. equi in feces than did dams of unaffected foals, indicating that heavier shedding by particular mares does not explain infection in their foals.154
Intestinal carriage in adult herbivores is mainly passive, as the organism cannot replicate in the anaerobic environment of the adult large intestine. However, the organism multiplies in the intestine of the foal up to about 3 months of age, reaching numbers up to 105 CFUs per gram of feces.155 Virulent R. equi present in the sputum of pneumonic foals will be swallowed and thus can multiply in the intestine. Therefore the manure of R. equi–affected foals is likely a major source of progressive contamination of the environment with virulent organisms. Under suitable conditions of high summer temperatures, R. equi can multiply in the environment by 10,000-fold in only 2 weeks.156 A single gram of soil contaminated with foal manure may therefore, under favorable conditions, contain millions of virulent R. equi organisms. R. equi can also be cultured from the air in the stalls on endemic farms, particularly on dry and windy days.153 In a recent study, the odds of detecting airborne virulent R. equi in stables were approximately 17 times greater than in paddocks of a given farm, and concentrations of virulent R. equi were also significantly higher in stables than in paddocks.157
Inhalation of virulent R. equi is the major route of pneumonic infection. The incubation period after experimental intrabronchial challenge varies from approximately 9 days after administration of a heavy inoculum to approximately 2 to 4 weeks when a lower inoculum is administered.112,158 Lung consolidation can be detected as early as 3 days after heavy intrabronchial challenge.112 The incubation period under field conditions likely depends on the number of virulent bacteria in the environment as well. Ingestion of the organism is a significant route of exposure, and likely also of immunization, but rarely leads to hematogenously acquired pneumonia unless the foal has multiple exposures to large numbers of bacteria.159 Indeed, oral administration of live virulent R. equi to foals on day 2 and day 7 of life is highly effective in preventing development of pneumonia after subsequent heavy intrabronchial challenge.160 Clearly, oral vaccination with live R. equi would not be an acceptable way to immunize foals because it would lead to progressive contamination of the environment with virulent bacteria. However, it undoubtedly shows that newborn foals have the ability to mount protective immune responses against R. equi (see section on immunity).
Epidemiologic evidence suggests that most foals on endemic farms become infected early in life.161 The median age at the time of diagnosis is approximately 37 days on endemic farms with a screening program to promote early diagnosis.162 Given the fairly long incubation period of the disease, this finding would also support the fact that many foals become infected within the first few weeks of life. In one study, foals aged 3 to 13 days (mean 6.4 days) were more susceptible to experimentally induced R. equi pneumonia than foals aged 14 to 36 days (mean 25 days). Collectively these findings indicate that many foals on endemic farms become infected at a young age and that younger foals may be more susceptible to infection caused by R. equi. However, these findings do not indicate that foals are susceptible to R. equi only during the neonatal period. Much older foals are still highly susceptible to infection by R. equi, particularly if they have never been exposed to R. equi. In one study, intatracheal administration of R. equi to 10 foals aged 27 to 67 days (mean 49 days) resulted in disease in all foals, including those receiving a low-dose challenge.128 In addition, some foals are diagnosed with R. equi pneumonia at 3 or 4 months of age. There is no evidence to support a 90- to 120-day incubation period, which would be required if all foals were infected shortly after birth.
Although R. equi can be cultured from the environment of virtually all horse farms, the clinical disease in foals is endemic and devastating on some farms, sporadic on others, and unrecognized on most. This probably reflects differences in environmental and management conditions, as well as differences in the virulence of isolates, but the exact basis for the difference in the prevalence of the disease among farms remain unknown. Breeding farms with a large acreage, a large number of mares and foals, a high foal density, and a population of transient mares and foals have greater odds of being affected by R. equi pneumonia.163,164 In contrast, R. equi pneumonia does not appear to be associated with lack of attention to routine preventative health practices.165 Although the total numbers of R. equi isolates in the environment may be similar in farms with and without a history of R. equi infections, farms with endemic disease may be more heavily infected with virulent R. equi.166 In a survey of the prevalence of R. equi at horse-breeding farms in Japan, the organism was isolated from almost all soil samples, at numbers of 102 to 105 CFUs per gram of soil. The vast majority of these isolates did not contain plasmids and were avirulent. Virulent R. equi isolates containing 80- to 90-kb plasmids and expressing VapA were cultured from 24 of the 31 farms examined. On those farms, virulent R. equi represented 1.7% to 23.3% of all isolates.167 However, quantification of virulent R. equi from a single soil sample cannot be used to determine whether foals on a given farm are at increased risk for developing disease caused by R. equi.168 There is considerable chromosomal variability among isolates of R. equi obtained from the same farm and among isolates from various continents.169 However, a small number of strains on a given farm are often responsible for the majority of clinical cases.170 It is interesting to note that multiple R. equi strains were isolated in five of the six cases in which more than one isolate from a single foal was examined, indicating that disease is commonly caused by simultaneous infection with multiple strains.170 Differences in disease prevalence among farms cannot be explained by soil geochemistry factors, as there is no association between soil pH, salinity, nitrate, phosphorus, potassium, calcium, magnesium, sodium, sulfur, zinc, iron, manganese, and copper and R. equi disease status on a given farm.171
R. equi can also be cultured from the soil of areas not inhabited by horses. In one study, R. equi was isolated from approximately 73.9% of soil samples collected from 115 parks and 49 household yards in Japan. The number of R. equi in those samples ranged from 10 to 105 CFUs per gram of soil. None of the 1294 isolates from those samples expressed VapA or VapB, suggesting that the human environment is not a significant route of exposure to virulent or intermediately virulent R. equi.122
Rhodococcus equi-Phagocytic Cell Interactions
The infectivity of R. equi in vitro is largely limited to cells of the monocyte-macrophage lineage.172 Optimal binding of R. equi to mouse macrophages in vitro requires complement and is mediated by MAC-1, a leukocyte complement receptor type 3 (CR3, CD11b/CD18).172 After entry through Mac-1, virulent R. equi can survive and even replicate in macrophages.173 In contrast, opsonization of R. equi with specific antibody significantly enhances killing of R. equi by equine macrophages.174 These findings suggest that cellular entry by non-Fc receptors may allow R. equi to avoid antibody-associated killing pathways. Entry of several microorganisms into macrophages after adherence to complement receptors, rather than Fc receptors, has been shown to allow them to avoid the toxic consequences of the oxidative burst.175 The lipoarabinomannan, an important component of the cell envelope of R. equi, has the ability to bind to mannose-binding protein.176 Binding of mannose-binding protein by lipoarabinomannan in vivo may activate complement C3b deposition onto R. equi via the lectin pathway, thereby promoting Mac-1 mediated uptake into macrophages. Alternatively the lipoarabinomannan of R. equi, like that of M. tuberculosis, may mediate bacterial entry through the macrophage mannose receptors. Recent studies have shown that R. equi can also activate macrophages through Toll-like receptor 2 (TLR2), leading to the release a variety of proinflammatory mediators.177 In the same study VapA was identified as one of the bacterial components leading TLR2-mediated macrophage activation.177 However, plasmid-cured derivatives of R. equi induced cytokine release by macrophages to the same degree as the parent strain, indicating that the presence of VapA is not necessary for activation of the innate immune response.178 Consistent with these findings, the lipoarabinomannan of R. equi has been shown to be a potent inducer of cytokine induction by equine macrophages.176
During phagocytosis, bacteria are wrapped in a portion of the plasma membrane and taken up into macrophages, forming a new organelle called a phagosome. The phagosome matures over time by consecutive interactions with early endocytic, late endocytic, and lysosomal vesicles, leading to the formation of a phagolysosome. During phagosome maturation, bacteria are exposed to a number of microbicidal products, such as acid, reactive oxygen and nitrogen intermediates, and lysosomal hydrolases, that all contribute to killing and degradation of ingested bacteria. Intracellular persistence of R. equi in equine macrophages is associated with absent or decreased phagosome-lysosome fusion, and phagocytosis of R. equi is not associated with a functional respiratory burst.174,179,180 Recent studies in mouse macrophages have shown that R. equi has the ability to block maturation of endocytic organelles after completion of the early endosome stage but before a fully mature late endosome compartment is reached.181 After ingestion by macrophages both virulent strains of R. equi and their avirulent plasmid-cured derivatives replicate in single membrane vacuoles for approximately 6 hours. Thereafter, virulent R. equi suppresses acidification of the vacuole and replicates intracellularly. In contrast, the vacuoles containing plasmid-cured derivatives progressively acidify, leading to complete eradication of the bacteria.181,182 These findings indicate the importance of plasmid-encoded products in preventing acidification of R. equi–containing vacuoles within macrophages. Similarly, necrotic death of R. equi–infected macrophages is also regulated by plasmid-encoded products.183
As stated earlier, R. equi can survive and even replicate in resident or nonactivated macrophages. Killing of R. equi by mouse macrophages is dependent on the presence of IFN-γ, which activates macrophages to produce both reactive oxygen and reactive nitrogen intermediates. These two radicals combine to form peroxynitrite, which efficiently kills R. equi.184 Neither reactive oxygen nor reactive nitrogen intermediates alone are sufficient to mediate killing of R. equi.184 Additional cytokines such as TNF-α may have a similar effect on macrophages, as both IFN-γ and tumor necrosis factor (TNF)-α are both required for clearance of virulent R. equi in mice.185
Neutrophils play an important role in early host defense against virulent R. equi.186 As opposed to macrophages, neutrophils from foals and adult horses are fully able to kill R equi.187-189 As seen with macrophages, killing of R. equi by neutrophils is considerably enhanced by specific opsonizing antibody.187-191
Immunity
ANTIBODY-MEDIATED IMMUNITY
Immunity to R. equi pneumonia in foals likely depends on both the antibody and cell-mediated components of the immune system, but its exact basis remains to be determined. The strongest evidence for a role of antibody in protection against R. equi is the partially protective effect of passively transferred anti—R. equi hyperimmune (HI) equine plasma (see section on prevention). The mechanisms by which HI plasma confers protection are not completely understood. The list of possible effector molecules includes antibody and nonspecific factors such as fibronectin, complement components, collectins, cytokines, and acute phase proteins. Opsonization of R. equi with specific antibody has been shown to promote phagocytosis and killing of R. equi by alveolar macrophages, identifying antibody as a critical component of HI plasma.174 In most studies evaluating the protective effect of HI plasma, plasma donors were immunized with whole cell vaccines or a mixture of several soluble antigens, making it impossible to determine the role of antibody against defined antigens of R. equi.
Recent studies have focused more specifically against the role of antibody against plasmid-encoded virulence-associated proteins (Vap). First, a monoclonal antibody to VapA and serum from horses immunized with partially purified VapA have opsonizing activity.192 Moreover, purified immunoglobulins obtained from horses vaccinated with partially purified VapA protected mice against intraperitoneal challenge with virulent R. equi compared with mice administered immunoglobulins from nonimmunized horses.193 More recently, IV administration of purified immunoglobulins obtained from horses immunized with recombinant VapA and VapC to foals was found to reduce the severity of pneumonia after heavy experimental challenge with R. equi.194 In the same study the degree of protection conferred by purified anti-VapA and anti-VapC immunoglobulins was similar to that provided by commercially available HI plasma.194
In adult horses the concentrations of R. equi-specific and VapA-specific IgGa and IgGb antibodies (the IgG isotypes that preferentially opsonize and fix complement in horses) are dramatically enhanced after intrabronchial administration with virulent R. equi. This occurs in conjunction with clearance of the bacteria from the lungs.195 In foals, production of antibody to VapA and VapC but not to other Vap proteins, increases after natural exposure to R. equi.194 Characterization of the subisotype response in naturally infected foals revealed mainly an increase in IgGa, IgGb, and IgG(T).194,196
CELL-MEDIATED IMMUNITY
Because of the facultative intracellular nature of R. equi, cell-mediated immune mechanisms are thought to be of major importance in resistance to infection. A large part of the knowledge of cell-mediated immunity to R. equi infections comes from infection of mice. Deficiencies in the complement component C5 and natural killer (NK) cells in mice do not impair the pulmonary clearance of virulent R. equi.197 In contrast, functional T lymphocytes are absolutely required for the clearance of virulent (plasmid and VapA positive) R. equi in mice.198-200 However, athymic nude mice (lacking functional T lymphocytes) clear plasmid-cured derivatives from their lungs within 1 week of infection, suggesting that, as opposed to virulent organisms, clearance of avirulent plasmid-negative strains in mice does not require functional lymphocytes and depends mainly on innate defense mechanisms.198
The two major mechanisms by which T lymphocytes mediate clearance of intracellular pathogens are secretion of cytokines and direct cytotoxicity. Although both CD4+ (helper) and CD8+ (cytotoxic) T cells contribute to host defense against R. equi in mice, CD4+ T lymphocytes play the major role and are absolutely required for complete pulmonary clearance.199,201,202 The mouse CD4+ Th cells can be divided into two subsets based on the cytokines they produce. The Th1 subset produces primarily IFN-γ and IL-2 and is mainly responsible for macrophage activation and cell-mediated immunity. The Th2 subset produces mainly IL-4, IL-5, and IL-10, which primarily promotes humoral immunity. Studies in mice have clearly shown that a Th1 response is sufficient to effect pulmonary clearance of R. equi, whereas a Th2 response is detrimental.200,203
Clearance of virulent R. equi in adult horses is associated with a significant increase in BALF CD4+ and CD8+ lymphocytes, lymphoproliferative responses to R. equi antigens, development of R. equi–specific cytotoxic CD8+ T lymphocytes, and IFN-γ but not IL-4 induction by CD4+ and CD8+ lymphocytes.195,204-206 How these findings in mice and adult horses relate to the foal is currently being elucidated. Even on endemic farms on which exposure to virulent R. equi is high, most foals do not develop clinical signs of respiratory disease, suggesting that most foals develop protective immune responses that continue to operate throughout adult life. Age-related deficiencies in R. equi—specific cytotoxic T lymphocyte activity have been documented in 3-week-old foals.206 Cytotoxic T lymphocyte activity was improved by 6 weeks of age and was similar to that in adult horses by 8 weeks.206 In another study, foals with a peripheral blood CD4:CD8 T lymphocyte ratio <3 between 2 and 4 weeks of age were more likely to develop R. equi pneumonia than foals with a higher ratio.207 These findings may represent important immunologic mechanisms associated with increased susceptibility of individual foals to R. equi infections.
The fact that young foals are deficient in their ability to produce IFN-γ in response to mitogens has led to the hypothesis that IFN-γ deficiency may be at the basis of their peculiar susceptibility to R. equi infections.208 A comparison between cell-mediated immune responses of foals and adult horses after intrabronchial challenge with virulent R. equi has recently been completed. The results indicate that foals can mount an appropriate IFN-γ response to a low inoculum of virulent R. equi but that their immune system is naive compared with that of adult horses.209 A heavy intrabronchial challenge overwhelms their immune system, leading to a significant increase in IL-4 induction.209
In another study, foals infected intrabronchially with a heavy inoculum of a virulence plasmid—containing strain of R. equi had significantly lower IFN-γ mRNA expression by bronchial lymph node CD4+ T lymphocytes than foals infected with an avirulent plasmid-cured derivative of the same strain.210 In addition, IL-10 (a cytokine known to downregulate Th1 responses in other species) and many inflammatory cytokines (IL-1β, TNF-α) were expressed at significantly higher levels in the lungs of foals infected with the virulent strain.210 The cytokine response in the lungs of foal infected with virulent R. equi is at least partially mediated by NF-κB activation.211 Taken together, these findings suggest that plasmid-encoded products of R. equi may have an immunomodulating effect in the respiratory system of foals.
Diagnosis
The distinction between lower respiratory tract infections caused by R. equi and those caused by other pathogens is problematic, especially on farms with no previous history of R. equi infections. Many diagnostic tests including complete blood count (CBC), measurement of fibrinogen concentrations, ultrasonography, radiographs, and serology may help distinguish R. equi pneumonia from that caused by other pathogens. However, bacteriologic culture and/or PCR amplification combined with cytologic examination of a tracheobronchial aspirate are necessary to make a definitive diagnosis of R. equi pneumonia.
CLINICAL LABORATORY TESTS
Hyperfibrinogenemia is the most consistent laboratory finding in foals with R. equi pneumonia, although in rare cases patients may have normal fibrinogen concentrations. Neutrophilic leukocytosis with or without monocytosis is also common. One study showed significantly higher fibrinogen concentrations and WBC counts in nonsurvivors than in survivors,212 whereas other studies showed no difference between the two groups.213,214 In a review of 40 cases of lung abscesses, there was a trend toward higher fibrinogen concentrations and WBC counts for the foals from which R. equi was isolated from a tracheobronchial aspirate as opposed to foals from which another pathogen was isolated.214 However, in all these studies there was a considerable overlap in ranges, which precludes the use of fibrinogen concentrations and WBC as diagnostic tests or prognostic indicators for an individual animal.
IMAGING TECHNIQUES
Thoracic radiography is useful in evaluating the severity of pneumonia and in assessing response to therapy. A prominent alveolar pattern characterized by ill-defined regional consolidation is the most common radiographic abnormality.212 The consolidated lesions are often seen as more discrete nodular and cavitary lesions consistent with pulmonary abscessation. Although in one study nonsurvivors tended to have more severe radiographic lesions than survivors, many survivors have very severe radiographic lesions; thus radiographs should not be used as the sole criterion for prognostication and euthanasia.110,212,213 In foals less than 4 months of age radiographic evidence of nodular lung lesions and tracheobronchial lymphadenopathy is highly suggestive of R. equi infections. However, in foals of 4 months and older S. zooepidemicus is another common cause of lung abscesses.214 Ultrasonography is a helpful diagnostic tool when lung involvement includes peripheral areas but may not be as useful as radiography to evaluate the full extent of lung lesions because abscesses with overlying aerated lung will not be detected. However, in most horses and foals with pulmonary abscessation the periphery of the lung is affected, enabling the ultrasonographer to successfully image some of the abscesses.215 Early ultrasonographic lesions are nonspecific and may include only irregularities of the pleural surface. These lesions may progress to form focal areas of consolidation of various sizes (Fig. 31-15, A). In more chronic cases, well-circumscribed, encapsulated abscesses can be detected (Fig. 31-15, B). Ultrasonography is very useful in evaluating the severity of pneumonia and in assessing response to therapy, especially for equine practitioners who do not have access to thoracic radiography. Ultrasonography is also a useful tool for detection of some abdominal abscesses (Fig. 31-16) and for screening for R. equi–infected foals on farms where the disease is endemic (see section on control).
Fig. 31-15 A, Sonogram of the right thorax in a foal with mild pneumonia caused by R. equi. There is an approximately 1 cm2 area of focal consolidation. B, Sonogram of the left thorax in a foal with severe pneumonia caused by R. equi. There is consolidation of the ventral aspect of the lung surrounding a large encapsulated abscess.
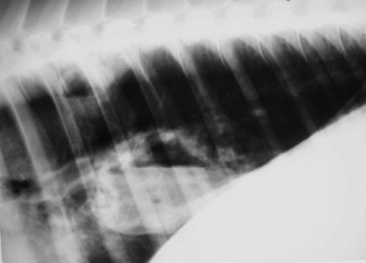
Fig. 31-16 Radiograph of the caudodorsal lung of a foal with severe Rhodococcus equi pneumonia demonstrating the classically described “Easter Basket” abscess. The appearance of the abscess results from the thick capsule, the fluid within the abscess, and the gas cap above the fluid, creating the gas-fluid interface line contained within the capsule. This radiographic appearance is not as common as a more usual radiographic appearance of bronchointerstitial pneumonia with associated variably sized abscesses.
SEROLOGY
A number of workers have investigated serologic approaches to diagnose R. equi infections in foals. The two most commonly used types of assays are agar gel immunodiffusion (AGID) and the enzyme-linked immunosorbent assay (ELISA). Serologic diagnosis of R. equi infections is problematic because the widespread exposure of foals to this organism at a young age leads to antibody production without necessarily producing clinical disease. In addition, maternally derived antibody causes positive reactions with sensitive ELISA assays, which further confound the interpretation of the test. Several independent studies have recently evaluated the performance of available serologic tests for diagnosis of infection caused by R. equi on endemic farms.186,216-218 The serologic tests evaluated were found to have low sensitivity, low specificity, or both. Improving either sensitivity or specificity of ELISA by changing the cutoff value of the tests could be done only at the detriment of the other. The performance of each assay was not improved by sequential sampling of each foal at 2-week intervals over time.186 Reliance on serology as the sole diagnostic test for R. equi infections results in overdiagnosis of the disease and in missing infections in the early stages. Serologic tests may be more useful at the farm level to detect overall exposure, but they have little value in establishing or excluding a diagnosis of R. equi pneumonia.
CYTOLOGY, CULTURE, AND POLYMERASE CHAIN REACTION AMPLIFICATION
Bacteriologic culture or PCR amplification combined with cytologic examination of a tracheobronchial aspirate is the only acceptable way of making a definitive diagnosis of R. equi pneumonia. In one study only 7 of 11 foals (64%) with positive R. equi culture at necropsy and 57 of 89 foals (64%) with radiographic evidence of lung abscessation yielded R. equi on culture of a tracheobronchial aspirate.219 However, in two other studies all 17 foals in which R. equi was isolated from the lung parenchyma at necropsy had previously undergone culture of a tracheobronchial aspirate that had yielded the organism, suggesting that culture of a tracheobronchial aspirate is a consistent and reliable method of diagnosing R. equi pneumonia.214,220 Larger case series are required to determine the exact sensitivity of tracheobronchial aspirate culture for the diagnosis of R. equi pneumonia in foals. Multiple other pathogens are often isolated along with R. equi. Foals without clinical disease exposed to contaminated environments may have R. equi in the trachea as a result of inhalation of contaminated dust. In one study conducted on a farm with endemic R. equi pneumonia, 77 of 216 foals sampled (36%) had positive tracheobronchial aspirate cultures but no signs of respiratory disease.221 For this reason, bacteriologic culture of a tracheobronchial aspirate should be interpreted in the context of cytologic evaluation, physical examination, and laboratory results. A light growth of R. equi from a foal with no clinical signs of respiratory disease, normal fibrinogen concentrations and WBC count, and no cytologic evidence of airway inflammation is likely an incidental finding. The use of PCR amplification based on the VapA gene sequence is a more sensitive mean of identifying R. equi in tracheobronchial aspirate samples than bacterial culture, especially if the foal sampled is concurrently being treated with antimicrobial agents.222 However, increased sensitivity may also result in a higher incidence of false-positive results owing to the detection of very small numbers of R. equi present as environmental contaminants. Several conventional and real-time PCR assays have been developed for the detection of virulent R. equi.222-225 PCR amplification may be done in association with, but should not replace, bacterial culture because it does not permit identification of concurrent bacterial pathogens and in vitro antimicrobial susceptibility testing of R. equi isolates.
Positive R. equi culture from nasal or fecal swabs cannot be taken as evidence of infection. R. equi can be cultured from the feces of normal horses even if they live on farms with no history of R. equi pneumonia.155,226,227 The quantitative culture of the feces of foals at weekly intervals has been advocated as an aid in early diagnosis of R. equi infections in foals because the bacterial count per gram of feces increased at the same time as clinical signs appeared.226 However, a single fecal sample from a foal has no diagnostic value because of individual as well as farm-to-farm variation in the number of R. equi in the feces.155,226,227 Furthermore, a negative fecal culture may not be helpful in ruling out R. equi infection; in one study only 5 of 30 foals (17%) with confirmed R. equi pneumonia had positive fecal cultures.221 Similarly, bacterial culture and PCR amplification of nasal swabs are poorly sensitive for the diagnosis of R. equi pneumonia.222,228
Treatment
A wide variety of antimicrobial agents are active against R. equi in vitro. However, because R. equi is a facultative intracellular pathogen surviving and replicating in macrophages and therefore causes granulomatous lesions with thick caseous material, many of these drugs are ineffective in vivo. For example, in one study all 17 foals with R. equi pneumonia treated with the combination of penicillin and gentamicin died despite the fact that all isolates were sensitive to gentamicin.214 The combination of rifampin and erythromycin became the treatment of choice in the 1980s and has dramatically reduced foal mortality since its introduction.110,219 In recent years clarithromycin or azithromycin, two newer generation macrolides, often replaces erythromycin in the combination with rifampin.229 Macrolides and rifampin are highly active against R. equi in vitro but exert only bacteriostatic activity.230 As a result, macrolides exert time-dependent activity against R. equi in vitro. Of the three macrolides listed previously, clarithromycin is the most active against R. equi in vitro. The minimum inhibitory concentrations at which 90% of R. equi isolates are inhibited (MIC90) are 0.12, 0.25, and 1 μg/mL for clarithromycin, erythromycin, and azithromycin, respectively.231 The combination of a macrolide and rifampin is synergistic both in vitro and in vivo, and the use of the two classes of drugs in combination reduces the likelihood of R. equi resistance to either drug.230,232 Rifampin and macrolides are lipid soluble, allowing them to penetrate cell membranes and caseous material.
The recommended doses are listed in Table 31-4. Several formulations of erythromycin are commercially available. Although they all show slight differences in bioavailability and elimination, they all result in therapeutic concentrations at recommended doses. Advantages of azithromycin and clarithromycin over erythromycin in foals include enhanced oral bioavailability, prolonged half-lives, and much higher concentrations in bronchoalveolar cells and pulmonary epithelial lining fluid.233-235 These properties of the newer generation macrolides contribute to their lower doses and longer dosing intervals. Concentrations of clarithromycin in pulmonary epithelial lining fluid and bronchoalveolar cells of foals at steady state are considerably higher than concentrations reported after daily administration of azithromycin to foals.234,235 However, clarithromycin concentrations at these sites decrease rapidly, whereas the release of azithromycin from cells is much slower, resulting in sustained concentrations of azithromycin in tissues for days after discontinuation of therapy.233-235 In a retrospective study, the combination clarithromycin and rifampin was significantly more effective than erythromycin-rifampin or azithromycin-rifampin, especially in foals with severe radiographic lesions.229
Table 31-4 Recommended Doses, Oral Bioavailability, and Serum Half-Lives of Antimicrobial Agents Commonly Used for the Treatment of R. equi Infections in Foals
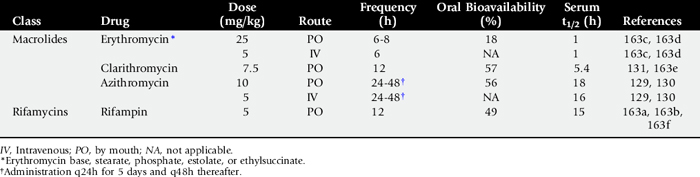
Because polymicrobial infections are common, a third antimicrobial agent may be necessary if another pathogen resistant to macrolides and rifampin is isolated in significant number along with R. equi. The combination of gentamicin or amikacin with erythromycin or rifampin in vitro gives significant antagonistic activity against R. equi compared with either drug alone.230,232 However, the clinical significance of this in vitro finding has not been established. Resolution of clinical signs, normalization of plasma fibrinogen concentrations, and radiographic or ultrasonographic resolution of lung lesions are commonly used to guide the duration of therapy, which generally ranges between 2 and 12 weeks depending on the severity of the initial lesions.
Although well tolerated by most foals, macrolides commonly cause diarrhea. Most of the time the diarrhea is self-limiting and does not necessitate cessation of therapy, but affected foals should be monitored carefully because some may develop severe diarrhea, leading to dehydration and electrolyte loss that necessitate intensive fluid therapy and cessation of oral macrolides. The incidence of diarrhea in foals treated with erythromycin-rifampin has ranged from 17% to 36%.229,236 In one study, foals treated with clarithromycin had a higher incidence of diarrhea (28%) than those treated with azithromycin (8%).229 In most cases diarrhea was mild and self-limiting. In the same study the incidence of severe diarrhea necessitating administration of IV fluids was not significantly different among groups of foals treated with azithromycin-rifampin, clarithromycin-rifampin, or erythromycin-rifampin.229 During surges of very hot weather an idiosyncratic reaction characterized by severe hyperthermia and tachypnea has been described in foals treated with erythromycin.236 Anecdotal reports suggest that these reactions may occasionally occur with newer macrolides as well. Administration of antipyretic drugs and placement of the foal in a cool environment will treat this problem. Severe enterocolitis has also been reported in mares whose foals are being treated with erythromycin, presumably resulting from disruption of the mare’s normal colonic microflora after ingestion of small amounts of active drug during coprophagia or after contamination of feeders or water buckets with drug present on the foal’s muzzle.237
Even though the vast majority of R. equi isolates from foals are highly susceptible to macrolides and rifampin, strains resistant to each have been encountered. The percentage of R. equi isolates resistant to either macrolides or rifampin has ranged between 0% and 4% depending on the studies.231,238 Rifampin should not be used in monotherapy because this increases the chance of resistance developing.230,239 Rifampin resistance is conferred by mutations in the RNA polymerase β subunit encoded by the rpoB gene.240,241 Progressive development of resistance to both erythromycin and rifampin during treatment is extremely rare, but it has been reported.215 The molecular mechanisms of macrolide resistance in R. equi isolates have not been determined, but R. equi isolates resistant to one of the three macrolides listed previously are almost invariably resistant to the other two as well. Therapy of foals developing severe diarrhea during macrolide therapy or therapy of foals infected with resistant isolates is problematic because of the limited range of alternative effective drugs.
Oral doxycycline in combination with rifampin has been used successfully for the treatment of foals with pneumonia caused by R. equi. The recommended dose of doxycycline in foals is 10 mg/kg q12h PO. This dose results in serum, pulmonary epithelial lining fluid, and bronchoalveolar cell concentrations above the MIC90 of R. equi isolates (1 μg/mL) for the entire dosing interval. Doxycyline is bacteriostatic against R. equi, but the drug is highly synergistic with rifampin and with macrolides in vitro. Therefore doxycycline could also be combined with a macrolide for the treatment of rifampin-resistant isolates. Chloramphenicol can be administered orally and achieves high concentrations within phagocytic cells in other species. The recommended dosage regimen is 50 mg/kg q6h PO. However, the fact that only 70% of R. equi isolates are susceptible to this drug and the potential human health risk make this drug a less attractive alternative. High doses of a TMS combination (30 mg/kg of combination every 8 or 12 hours orally) have been used alone or in combination with rifampin in foals with mild or early R. equi pneumonia or for continued therapy in foals responding well to other antimicrobials.110 However, TMS is not nearly as effective as the combination macrolide-rifampin.
In an experimental model of R. equi infection in immunosuppressed mice, the most effective drugs in monotherapy were found to be, in order of effectiveness, vancomycin, imipenem, and rifampin.242 Amikacin, erythromycin, ciprofloxacin, or minocycline in monotherapy did not lead to a significant decrease in bacterial counts. The most active drugs in combination were those including vancomycin or rifampin, but these combinations were not significantly different from vancomycin monotherapy.242 Vancomycin and imipenem should be used in foals only for the treatment of life-threatening R. equi infections caused by isolates confirmed to be resistant to all other possible alternatives.
Nursing care, provision of adequate nutrition and hydration, and maintenance of the foal in a cool and well-ventilated environment are important. Oxygen therapy using humidified oxygen by pharyngeal insufflation in moderately hypoxemic foals, or by percutaneous transtracheal oxygenation in severely hypoxemic animals, is indicated.243 Judicious use of NSAIDs is of value in reducing fever and improving attitude and appetite in febrile depressed anorectic foals. Nebulization may be helpful in selected animals with tenacious secretions and nonproductive cough. However, most foals with R. equi pneumonia do not benefit from nebulization, and the procedure is stressful to some. Similarly, bronchodilators are rarely helpful clinically in foals with pneumonia caused by R. equi. In addition to appropriate systemic antimicrobial therapy, foals with R. equi septic arthritis or osteomyelitis often require aggressive local therapy such as joint lavage, surgical debridement, and IV or intraosseous regional limb perfusion with antimicrobial agents. The prognosis of foals with abdominal abscesses is poor, although rare patients will respond to long-term antimicrobial therapy. Surgical removal or marsupialization has been attempted in some foals, but abdominal adhesions usually result in the inability to resect the lesion or in chronic intermittent episodes of colic.
Prognosis
Before the introduction of the combination of erythromycin and rifampin as the treatment of choice in the early 1980s, the prognosis of R. equi–infected foals was poor, with reported survival rates as low as 20%.244 Using erythromycin and rifampin, Hillidge reported a successful outcome (as assessed by survival) in 50 of 57 foals (88%) with confirmed R. equi pneumonia.219 Studies in referral centers, more likely to see the most severely affected cases, have revealed survival rates ranging from 59% to 72%.9,213,229 Recent studies have shed light on the impact of R. equi infections on future athletic performance. Ainsworth and colleagues245 performed radiography, hematologic and BAL analysis, and pulmonary function tests in five horses (6 to 18 months) with prior R. equi pneumonia and compared the results with age-matched controls with no history of respiratory disease. There was no significant difference between the two groups. In another study the gas exchange of seven standardbred horses previously diagnosed with R. equi pneumonia as foals was not compromised during intense treadmill exercise compared with reference values for healthy fit standardbreds.246 Bernard and co-workers247 evaluated the influence of R. equi pneumonia on future racing performance. Thirty horses that previously had R. equi pneumonia were compared with the affected foals’ dams’ progeny. There were no significant differences in total earnings, average earning index, and age at the first race between horses that previously had R. equi pneumonia and controls or the North American average. These results suggest that horses with previous R. equi pneumonia are likely to perform as well as expected if they race, but because only foals registered with the Jockey Club were considered, it does not indicate if they are as likely to race as a control population. More recently, the records of 219 foals diagnosed with R. equi pneumonia and treated with erythromycin and rifampin were reviewed.213 The survival rate was 72%. Of the survivors 54% had at least one racing start, as opposed to 65% for the control population, suggesting that horses contracting R. equi pneumonia as foals may be slightly less likely to race as adults. However, as also shown by Bernard and colleagues,247 the racing performance of foals that raced was not significantly different from that of the U.S. racing population.213
Control of Rhodococcus Equi Infections on Farms where the Disease Is Endemic
Current approaches for the control of R. equi infections on endemic farms depend on (1) decreasing the size of infective challenge, (2) screening for earlier recognition and therapy of affected foals, and (3) passive immunization. Recent advances in the area of chemoprophylaxis and immunoprophylaxis are also discussed.
DECREASING THE SIZE OF INFECTIVE CHALLENGE
There seems to be a progressive buildup of infection on horse farms with prolonged use. Thus, endemic farms are often those used for breeding horses for many years, those with heavy concentrations of mares and foals, and those located where summer temperatures are high, where the soil type is sandy, and where dust is extensive. Large numbers of foals kept on bare, dusty, manure-containing paddocks may result in heavy environmental challenge, with clinical disease maintaining the population of environmental virulent bacteria. General recommendations that would be expected to decrease dust formation and inhalation of virulent R. equi may help in reducing the incidence of the disease. However, solid evidence demonstrating the efficacy of the following general recommendations in decreasing clinical disease is lacking. Common sense dictates that foals should be housed in well-ventilated, dust-free areas and that dirt paddocks should be avoided. Pneumonic foals should be isolated, because they may contribute the most to contamination of the environment with virulent organisms. When possible, pasture could be rotated to decrease grass destruction, dust formation, and consequent inhalation of R. equi. Any sandy or dirt areas should ideally be planted with grass or made “off limits” to foals, or, alternatively, irrigation may be useful in decreasing dust formation and encouraging grass propagation. Because mares and foals tend to congregate around water sources and under shade in hot summers, reduction in the size of mare-foal bands or mobile troughs and shade may reduce the destruction of grass and exposure to barren soil. Dispersal of foals onto grass paddocks will reduce dust formation and therefore the number of inhaled bacteria. Ingestion of low numbers of organisms via grazing on contaminated grass may actually have the beneficial effect of immunizing foals orally.160,248 Manure should be regularly removed from stalls, pens, and paddocks and composted. The fact that the concentrations of airborne virulent R. equi are higher in stables than in paddocks157 suggests that frequent cleaning and disinfection of stalls may help to decrease contamination and opportunity for infection. Avoiding dirt-floored foaling stalls should be considered, because R. equi is a soil saprophyte and epidemiologic evidence suggests that dirt floors may increase the risk of development of R. equi pneumonia.163
SCREENING FOR EARLIER DETECTION OF AFFECTED FOALS
R. equi pneumonia is often not recognized until it is well advanced and therefore difficult to treat. Even severely affected foals may appear to suckle and behave normally to a casual observer. Screening for early recognition of R. equi–induced pneumonia before development of clinical signs and appropriately treating infected foals reduce losses and limit the costs associated with long-term therapy of severely affected animals. It is important to emphasize that screening methods are not diagnostic tests. A useful screening test is one in which the probability of disease is high with a positive test result (high positive predictive value) and very low with a negative test result (high negative predictive value). The higher the prevalence of disease at a given farm, the higher the positive predictive value of a given test will be. Therefore, depending on the prevalence of R. equi infections on a given farm, a positive result of a screening test could be a basis to perform a diagnostic test (low to moderate prevalence) or to initiate therapy (high prevalence). Screening methods can be divided into three categories: physical examination findings, imaging techniques, and laboratory tests (Box 31-1). In one study on a large breeding farm with endemic R. equi problems, use of twice-weekly complete physical examinations with auscultation of the lungs was apparently successful in promoting early diagnosis and preventing mortality.249 However, thoracic auscultation is a fairly insensitive tool, and many foals will not show clinical signs of respiratory disease or develop a fever until lung lesions are well established.
Box 31-1 Screening Methods for Earlier Detection of Foals Infected with R. equi on Endemic Farms
* Preferred method when the resources or expertise required are available.
Imaging techniques such as radiography and ultrasonography are relatively specific because they detect lung lesions. As stated in the diagnosis section earlier, radiography offers the advantage of detecting either central or peripheral lesions. However, radiography has several disadvantages that limit its use as a practical screening test. Disadvantages of radiography include cost, need for special equipment, need for more personnel, and exposure of personnel to radiation. In addition, early radiographic lesions can be subtle and less typical of R. equi pneumonia than more advanced cases. Ultrasonography of the chest offers several advantages over radiography as a screening test. The entire chest can be scanned in just a few minutes, and when the periphery of the lung is affected ultrasonography is more sensitive than radiography. Biweekly ultrasonographic examination of the chest of all the foals on endemic farms (starting at 3 to 4 weeks of age) has been extremely useful in preventing mortality by promoting early identification of pneumonic foals before development of clinical signs.250 Using this approach, as many as 50% of foals on a farm were found to have subclinical lung lesions.250 Many foals with mild lung lesions may recover without therapy. However, it is impossible to discriminate between foals in which the infection will successfully resolve without treatment and foals that will eventually develop clinical signs. Foals with no clinical signs and ultrasonographic lesions consisting of only irregularities of the pleural surface (comet tail artifact) do not require therapy.250 However, foals with focal areas of consolidation ≥1 cm in diameter or in depth (see Fig. 31-15, A) and foals with clinical signs of respiratory disease are usually treated.251 Ultrasonography also offers the advantage of allowing evaluation of the severity of lung involvement, and follow-up examinations permit a more objective assessment of response to therapy. The duration of therapy is proportional to the initial severity of the lesions.250
Many farms do not have access to the resources or expertise required for periodic ultrasonographic evaluation. Laboratory tests may be more practical on farms where blood can be collected by farm workers. Recent studies have shown that currently available serologic assays and measurement of serum amyloid A are not useful screening tests.252,253 In contrast, combination of careful daily observation and measurement of WBC counts and plasma fibrinogen concentrations in all foals at 2- to 4-week intervals, although labor intensive, may be a useful alternative to ultrasonography for early identification of R. equi–infected foals on endemic farms. In one study 165 foals were monitored during the entire breeding season, and diagnosis of R. equi pneumonia was confirmed in pneumonic foals by culture of a tracheobronchial aspirate. Total WBC count was found to be a significantly better screening method than measurement of fibrinogen concentrations.216 Using a cutoff value of 14,000 cells/μL, sensitivity and specificity for early identification of foals with R. equi pneumonia were 88% and 81%, respectively.216 On a farm where the disease prevalence is 40%, the predictive value of a positive test result (WBC >14,000 cells/μL) would be 75% and the predictive value of a negative test result (WBC <14,000 cells/μL) would be 91%. In contrast, the same cutoff value used on a farm with a disease prevalence of 5% would result in a predictive value of a positive test result of only 20%. This example underscores the fact that such screening methods are valid only when the prevalence of the disease is high. Because increases in WBC counts and fibrinogen concentrations are nonspecific findings, it is advisable to subject foals identified by such screening methods to additional diagnostics (e.g., ultrasonography or TBA) before initiation of therapy to reduce the rate of false-positive results. However, some farms where the prevalence of the disease is particularly high and well documented may elect to initiate therapy based on these screening methods alone.
PASSIVE IMMUNIZATION
IV administration of HI plasma obtained from horses vaccinated against R. equi using various antigens has consistently proved effective in significantly reducing the severity of R. equi pneumonia in foals after experimental challenge.192,254 However, studies evaluating the efficacy of various HI plasma preparations under field conditions have given equivocal results, with only two of five controlled clinical trials demonstrating a statistically significant reduction in the incidence of R. equi pneumonia.162,220,255-257 In one randomized controlled prospective study involving 165 foals on an endemic farm, the incidence of pneumonia caused by R. equi was 19% in the group that had received plasma versus 30% in the control group. Although the decrease in incidence did not quite reach significance (P = .09), such a decrease would be relevant to many farm managers.
The ideal time for administration of HI plasma and the minimal effective dose require further research. Administration of HI plasma before infection with R. equi is important.258 However, early administration may result in the decline of passively transferred antibody to a nonprotective level at a time when foals are still susceptible to R. equi and environmental challenge is high.256 Therefore IV administration of 1 L of HI plasma within the first few days of life followed by a second administration at approximately 25 days of age, although expensive, may be the best approach on farms with high morbidity rates. U.S. Department of Agriculture (USDA)–licensed R. equi antibody (HI plasma) for the prevention of R. equi pneumonia is commercially available in North America (Lake Immunogenics Inc., Ontario, NY; Plasvacc USA, Templeton, Calif.). HI plasma is expected to slightly decrease the incidence of the disease (by 30% to 40%) but will not prevent infection in all foals and should not lull farm owners into a false sense of security and reduce the need for continued vigilance. Whether this strategy is cost-effective will vary from farm to farm. When used for the control of R. equi infections on endemic farms, administration of HI plasma should always be combined with screening techniques aimed at early identification and treatment of infected foals (see earlier).
CHEMOPROPHYLAXIS
Another suggested approach for the prevention of R. equi infections on endemic farms is the prophylactic administration of antimicrobial agents to all the foals during the time period when they are most susceptible to infection. Azithromycin is an attractive choice for prophylactic administration because of good oral bioavailability, long half-life, and high and sustained concentrations in pulmonary epithelial lining fluid, bronchoalveolar cells, and neutrophils. In a prospective randomized clinical trial, azithromycin was administered at a dose of 10 mg/kg q48h for the first 2 weeks of life. Preliminary data analysis suggests an overall significant benefit, although protection was not absolute.259 However, there are several potential problems with the mass administration of azithromycin or other antimicrobial agents for the prevention of R. equi infections. First, the period of susceptibility to R. equi is much longer than 2 weeks. As a result, the ideal time period for antimicrobial prophylaxis remains unknown. Second, administration of azithromycin to foals has occasionally resulted in life-threatening adverse effects such as enterocolitis and possibly hyperthermia. Prophylactic administration to all the foals on a farm will increase the likelihood of occurrence of adverse effects. Finally and most importantly, the long-term effects of this practice on selection of resistant bacterial isolates are unknown. Given the fact that R. equi isolates resistant to azithromycin are usually resistant to erythromycin and clarithromycin also, development of resistance could have devastating consequences for the foal crop.
A novel approach that may prove beneficial in the prevention of R. equi pneumonia is the administration of gallium maltolate. Gallium is a trivalent semimetal that shares many similarities with ferric iron. Most bacteria require iron for survival. Recent studies have shown that iron acquisition is essential for the survival of R. equi and that the organism can acquire and use iron bound to transferrin and lactoferrin.260 Gallium readily binds to plasma transferrin and lactoferrin.261 Bacteria acquire gallium and incorporate it into essential iron-dependant enzyme systems, which leads to inactivation of those systems and bacterial death. A recent study has shown that gallium inhibits in vitro growth of R. equi and that oral treatment of mice with gallium maltolate may decrease the number of R. equi in tissues after experimental infection.262 Recent pharmacokinetic and safety studies in neonatal foals have indicated that gallium maltolate at a dose of 20 mg/kg results in adequate serum concentrations and that no adverse clinical or clinicopathologic effects were apparent when gallium was administered for 5 days.263 Larger numbers of foals treated over longer periods of time will be needed to more fully characterize safety issues. Clinical trials to critically evaluate the prophylactic effectiveness of gallium in foals on endemic farms are awaited.
ACTIVE IMMUNIZATION
It would be considerably more convenient to control R. equi pneumonia on endemic farms by the active immunization of mares or foals with a protective antigen. Such attempts have so far been unrewarding in foals. The role of antibody in partial protection against R. equi infection would suggest that vaccination of mares would be effective at conferring at least some degree of protection. However, in both a field study and an experimental challenge, vaccination of mares did not provide protection against R. equi pneumonia despite a significant increase in colostral specific R. equi antibody and transfer of passive immunity to foals.257,264 More recently, a vaccination of a small number of mares with VapA protein antigen associated with a water-based nanoparticle adjuvant led to high anti-VapA IgG concentrations in mares and foals and may have conferred protection against natural challenge compared with nonvaccinated controls.265 However, a more extensive study on an endemic farm will be necessary to confirm these preliminary findings before widespread vaccination of mares can be recommended.
Because cell-mediated immunity is of paramount importance in protection against R. equi, active immunization of foals will likely be required for complete protection. Recent studies in mice indicated that DNA immunization against vapA protects against R. equi infection and that the IgG subisotype response is consistent with a Th1-based immune response.266 A similar DNA vaccine containing the vapA gene has been shown to induce strong cell-mediated immune responses in adult horses, but responses were poor in foals.267 Immunization in foals will need to be initiated very early in life, and an effective vaccine will have to overcome the relative immaturity and possible inherent bias of the naive neonatal immune system. The fact that oral administration of live virulent R. equi confers almost complete protection against heavy intrabronchial challenge248,264 and the fact that most foals on endemic farms do not develop disease or develop subclinical disease and eventually clear the infection provide the proof of concept regarding the potential for active immunization in foals. Further work is needed to examine active immunization strategies based on a better understanding of the antigens of importance and the method of administration or adjuvant required to produce the effective Th1 immune response required for protection against R. equi pneumonia in neonatal foals.
PNEUMONIA IN FOALS
Equine pneumonia is a significant cause of morbidity and mortality in both the newborn and the older foal.268-271 Although the causative agents and the mode of lung infection are generally different for each age-group, foal pneumonia is commonly associated with a compromise in the immunologic protection of the host. The exact immunologic basis of the foal’s inherent susceptibility to opportunistic respiratory pathogens needs to be further elucidated.
Failure of passive transfer of immunity (FPT) is a well known risk factor of neonatal infection, including respiratory disease,271 because not only are affected foals deprived of specific maternal antibody protection, but their neutrophil function is also seriously impaired.272 Although foals can respond immunologically in utero to bacterial or viral infections, their ability to do so is less than that of adults. Reduced complement values and defective chemotaxis (directed migration of neutrophils or macrophages) and killing capacity of the neonatal neutrophil contribute to a relatively decreased defense against invading bacteria. Furthermore, an immature ciliary apparatus and the presence of fewer alveolar macrophages in neonates in comparison with adult horses lead to decreased bacterial clearance from the lungs.273 The number and distribution of the bronchoalveolar cells approach adult levels only at approximately 3 to 6 weeks of age.274
It has been suggested that a natural cellular immunodeficiency may also occur in foals at 2 to 4 months of age.275 Although the exact association between age-related cellular immunodeficiency and the defense against intracellular pathogens such as P. carinii (Pneumocystis jiroveci) and R. equi of foals still needs to be clarified, CD4+ and CD8+ T lymphocytopenia was documented in a filly with P. carinii pneumonia.276
Etiology
NEONATAL PNEUMONIA (<1 MONTH OF AGE)
In contrast to the older foal, infectious lung disease in the neonate is usually part of a multiple-organ, systemic infection or sepsis, which may be acquired via the placenta in utero or perinatally through environmental bacteria leading to hematogenous (ascending) infection (e.g., omphalophlebitis). E. coli, Klebsiella species, Actinobacillus equuli, Salmonella species, and Streptococcus species are some of the more common bacteria involved in neonatal foal pneumonia.277
Descending lung infections may be caused by inhalation of viral, bacterial, or opportunistic fungal agents. Furthermore, iatrogenic aspiration (oil, medication, oral supplements) or aspiration of gastric reflux, milk, meconium, and contaminated amniotic fluid after placental infection may seed the foal’s lung. Meconium aspiration occurs in utero in foals that experience fetal distress (perinatal asphyxia). Although the meconium is commonly sterile at this time, it creates mechanical airway obstruction, surfactant inactivation, and pulmonary inflammation (caused by vasoactive mediators and chemotactic and inflammatory cytokines, including TNF-α, interleukin [IL]-1β, and IL-8).278 The resulting edema and vasoconstriction may lead to hypoperfusion of the pulmonary parenchyma, with damage to type II pneumocytes and decreased production of surfactant.279 This type of “secondary surfactant deficiency” may also be induced by other forms of generalized pulmonary inflammation, including diffuse bacterial pneumonia. Neurologic dysfunction, severe inflammation, or structural abnormalities of the upper airways (congenital cysts, dorsal displacement of the soft palate, laryngeal paralysis, pharyngeal weakness, etc.) may predispose to aspiration pneumonia postpartum.
EVA and herpes viral infections (EHV-1 and EHV-4) have been implicated in in utero viral infections leading to lung disase.280 Although involvement of adenovirus in the pathogenesis of pneumonia has also been reported in a thoroughbred foal, fatal adenoviral pneumonia is primarily associated with combined immunodeficiency (SCID) in Arabian foals.281,282 In addition, a recent report documents the isolation of influenza A virus from a seven-day-old foal with bronchointerstitial pneumonia.282
PNEUMONIA IN OLDER FOALS (1 TO 6 MONTHS OF AGE)
S. zooepidemicus and R. equi are the most important pathogens associated with pneumonia in older foals, although plurimicrobial infections (Actinobacillus species, B. bronchiseptica, E. coli, Klebsiella pneumoniae, Pasteurella species, Pseudomonas species, Salmonella species, and Staphylococcus species) are not uncommon.283,284S. zooepidemicus was isolated from 87% of uncomplicated lower respiratory tract infections in foals (1 to 8 months old) on selected farms285 and was documented as the most common cause of pulmonary abscesses in a second study.286 In contrast, anaerobes appear to be infrequently involved in primary pneumonia of foals.285Corynebacterium pneumonia due to aerosol infection is a rare cause of diffusely increased interstitial and peribronchial radiographic densities, indicative of pulmonary abscesses in foals.287
Although respiratory viruses are often believed to be primary offending agents that lead to secondary bacterial lung invasion, they have limited importance in nursing foals.285,288 Equine adenoviruses (EAdVs) and EHV-2 are ubiquitous in the general equine population, with little pathogenicity in immunocompetent foals. Although adenoviruses are the most common respiratory pathogens in Arabian foals with severe combined immunodeficiency (SCID), the role of EHV-2 in the respiratory disease complex of foals remains unclear. The latter has been implicated as a predisposing factor for R. equi pneumonia.
Because of the complexity of the disease, in-depth discussions of Rhodococcus pneumonia and ALI and ARDS are presented elsewhere in Chapter 31.
INTERSTITIAL PNEUMONIA
Acute, severe interstitial or bronchointerstitial pneumonia is a sporadic, rapidly progressive disease of foals of 1 to 6 months of age that is characterized by a sudden onset of respiratory distress, tachypnea, fever, high mortality, and histopathologic evidence of severe, diffuse, necrotizing bronchiolitis, alveolar septal necrosis, and neutrophilic alveolitis on necropsy.289-291 Although no definitive causative agent(s) or factor(s) of this disease have yet been identified, suspected causes include viruses, P. carinii, heat stroke, environmental toxins, and bacteria.289-292 Enteric gram-negative organisms, R. equi, Pseudomonas aeruginosa, and P. carinii have all been cultured from the lungs of affected foals.
The reported cases are usually sporadic, affecting a single foal within a herd,289 although rare clusters of cases have also been described.291 The disease is generally rapidly progressive and may lead to sudden death as a result of fulminant respiratory failure. Severe respiratory distress, hypoxemia, hypercapnia, and respiratory acidosis are the most striking clinicopathologic signs. The hypoxemia of bronchointerstitial pneumonia is relatively resistant to supplemental oxygen therapy, indicating a pulmonary shunt. Thoracic radiographs commonly demonstrate caudodorsally distributed interstitial and bronchointerstitial pulmonary opacities. With advanced disease, the radiographic pattern progresses to include patches of a coalescing alveolar nodular pattern with air bronchograms.293 As with bacterial pneumonia, many foals demonstrate hyperfibrinogenemia and neutrophilic leukocytosis on hematology.
Therapy is symptomatic, including antiinflammatory therapy, broad-spectrum antibiotics, thermoregulatory control, bronchodilation, and supplemental oxygen. Surviving foals may develop a proliferative epithelial and interstitial response including bronchiolar and alveolar epithelial hyperplasia, type II cell hyperplasia, and hyaline membrane formation.293
Chronic pulmonary disease (1 to 3 months’ duration) with marked radiographic interstitial opacities was reported in 12 foals, age 3 to 9 months, that were examined at a veterinary teaching hospital.294 Evaluation failed to yield a consistent pathogen from tracheobronchial aspirates. The authors proposed that chronic interstitial pneumonia occurring in foals is associated with a good prognosis and considered corticosteroid therapy as a potentially useful treatment.
PNEUMOCYSTIS JIROVECI (CARINII) INFECTION
Rare opportunistic fungal pathogens of the lung may include P. carinii (renamed P. jiroveci in humans), Aspergillus, Candida, and Mucor species. P. carinii is a unicellular eukaryote that has been classified as a fungus after DNA studies.295 It is most commonly seen in humans and animals that suffer from a concurrent immunodeficiency. It was first recovered in Arabian foals with SCID.296 It has since been recovered from foals of other breeds with no specific history of immunodeficiency, although for the most part no direct testing for immunocompetence was performed in the reported cases. However, one more recently documented case demonstrated a low number of circulating CD4+ and CD8+ lymphocytes in a filly with P. carinii.276
In several reports the onset of clinical signs in affected foals ranged from a 3-week history of weakness, weight loss, and nasal discharge to acute dyspnea. Half of the reported non-Arabian cases had concurrent infections with R. equi, Enterobacter cloaca, E. coli, or S. zooepidemicus. A severe interstitial, sometimes miliary and alveolar pattern was seen on radiographs of the lungs. The majority of the reported foals died, and the diagnosis of P. carinii was obtained at necropsy.276,292,296-299 On postmortem examination the lungs were uniformly heavy and consolidated, and a diffuse interstitial pneumonia was found. Trophozoites and cysts were observed in the alveolar epithelial cells, and macrophages within the alveoli. Silver staining and streptavidin-biotin immunolabeling of histologic sections provide a better visualization of the organisms in the pulmonary tissue.297 A few foals were diagnosed by visualization of the organism on a BAL sample. Two surviving foals responded to trimethoprim-sulfamethoxazole (TMS) therapy.
Clinical Presentation and Diagnosis of Foal Pneumonia
The clinical manifestation of pneumonia in the newborn foal can be variable and depends on the disease severity and underlying or associated problems. Early in life, localizing clinical signs of respiratory infection may be absent even in the presence of extensive disease. Dyspnea may be seen in severely affected foals and may manifest as an increase in respiratory rate, effort, or thoracoabdominal asynchrony (paradoxic breathing). However, signs of respiratory distress and hypoxemia are frequently vague. Even some severely hypoxemic foals may show only restlessness, considerable resistance, and struggling when being handled or restrained. Abnormal respiratory sounds (crackles or wheezes) may be heard on auscultation of ill foals. However, even normal foals may show crackles on the down lung after having remained in lateral recumbency for a prolonged period of time. Furthermore, foals with no auscultable abnormalities may still have extensive pulmonary disease.300,301 Similar to other species, cyanosis is a sign of severe hypoxemia in foals. The arterial PaO2 (partial pressure of oxygen), however, must reach very low levels (35 to 45 mm Hg) before clinical cyanosis is observed. Approximately 5 g/dL of unoxygenated hemoglobin in the capillaries generates the dark-blue color that is appreciated as cyanosis in humans. Therefore severely anemic foals may never appear cyanotic even in the face of profound hypoxemia. Weakness, depression, anorexia, weak or absent suckle reflex, dehydration, and fever may also be noted in newborn foals with respiratory disease. Cough and nasal discharge are usually absent in the early stages of neonatal pulmonary disorders.277 The older foal, on the other hand, will generally exhibit clinical signs that focus on the respiratory tract, such as abnormal auscultation of the lungs, nasal discharge, cough, fever, tachypnea, and increased respiratory effort.
Thoracic radiographs and arterial blood gas analysis are important in confirming the presence and evaluating the extent and distribution of foal pneumonia. Because radiographic findings may precede or lag behind alteration of respiratory function, repeated diagnostic imaging is recommended. A recent study documented that radiographic abnormalities involving the caudodorsal lung region were most common in neonatal foals admitted to a referral center.302 Bedenice and colleagues showed that the presence of dyspnea, tachypnea, a fibrinogen concentration >400 mg/dL, systemic inflammatory response syndromes (SIRS), hypoxemia, or FPT in neonatal foals may be a predictor of underlying respiratory disease and should prompt an early radiographic evaluation of the thorax, even in the absence of other localizing clinical signs.271,302 Thoracic ultrasonography has become a popular diagnostic tool for the assessment of equine lung and pleural disease and is particularly useful in determining the side(s) of the thorax affected, as well as the precise location of the lesion in most cases.271
Diagnostic sampling of the respiratory tract is less commonly performed in neonatal foals compared with older foals and adult horses. However, TBA, BAL, protected catheter brush (PCB), or protected aspiration catheter sampling (PAC) is important in stabilized patients, irrespective of age, to direct antimicrobial therapy and evaluate therapeutic efficacy. Lung biopsies are rarely performed and are limited to patients with chronic disease or suspected fungal pneumonia.301
Treatment
The treatment goals in dealing with bacterial pneumonia in either age-group are similar and directed at maintaining adequate gas exchange to ensure patient survival, limit progressive pulmonary damage, and ultimately eradicate infection. Long-term goals should aim to maintain optimal pulmonary function and conserve adult athletic performance. Specific treatment strategies for septic pneumonia may include goal-directed antimicrobial therapy, treatment of inflammation independent of antimicrobial therapy, respiratory therapy (oxygen therapy, ventilation, bronchodilation), and cardiovascular support.300
Broad-spectrum antimicrobial treatment, with consideration of the drugs’ anticipated sensitivity and lung penetration, should be initiated before culture results are available. Third- or fourth-generation cephalosporins (ceftiofur and cefepime, respectively) show good penetration into lung tissues.303 Alveolar delivery of antibiotics typically occurs via diffusion of a free, nonprotein-bound drug and is usually satisfactory if plasma concentrations and alveolar perfusion are adequate.304 Aminoglycosides penetrate lung tissue at 10% to 45% of serum levels. However, the acidic environment of infected airways may reduce the drug’s activity below levels sufficient to eradicate the offending bacteria in some cases.305,306 Antibiotic therapy should not be discontinued prematurely in neonatal foals, because pulmonary infection is commonly well established before its diagnosis.307
Outcome
The survival of neonatal foals with septic pneumonia is contingent on the underlying disease conditions, a prompt diagnosis, and the cause and severity of lung dysfunction. Few studies have objectively evaluated the outcome of foal pneumonia.300 One report documented that 65% of foals with radiographic evidence of pulmonary disease were discharged alive from the hospital, in contrast to 86% survival of foals without respiratory illness. The presence of diffuse radiographic infiltrates (caudodorsal, caudoventral, and cranioventral lung involvement) or concurrent alveolar patterns within the caudodorsal and caudoventral lung indicated lower survival rates.271 Pneumonia is also one of the greatest causes of morbidity and mortality in foals greater than 1 month of age.269
The long-term effect on athletic performance has thus far been evaluated only in older foals that survived rhodococcus pneumonia. R. equi infection in foals was associated with a decreased chance of racing as adults. However, the performance of foals that went on to race was not significantly different from that of the general U.S. racing population.308 It remains speculative whether severe equine neonatal pneumonia will most likely limit future peak athletic performance. Human studies have shown that many hosts develop long-lasting or permanent lung changes after recovery from neonatal pneumonia, which may significantly affect the quality of life and susceptibility to later infections. Case-based evaluations in horses have demonstrated that functional and structural pulmonary abnormalities may occur in survivors of severe neonatal pneumonia. However, the clinical significance of this finding currently remains unclear.