Chapter 32 Diseases of the Alimentary Tract
DIAGNOSTIC PROCEDURES IN THE EXAMINATION OF THE EQUINE ALIMENTARY SYSTEM
A thorough physical examination is compulsory, and tests that provide a minimum database (complete blood count [CBC], serum chemistries, and urinalysis) are often indicated in horses with suspected alimentary tract disease. Once a list of differential diagnoses is compiled, a number of ancillary diagnostic tests are available to narrow the possibilities. Each diagnostic test or procedure is limited in the type and extent of information that can be obtained, and therefore the clinician should select the complement of procedures that is most likely to provide the information required to make a proper diagnosis and determine the appropriate therapy.
RECTAL EXAMINATION
A systematic approach to examining the abdominal and retroperitoneal viscera should be established and applied during each examination to ensure that all pertinent regions and structures are examined. When feasible and if required, the patient should be sedated to allow a more thorough examination. In some cases, epidural anesthesia is required to obtain adequate access to structures during rectal examination. The principal goal of a rectal examination is to identify changes in size, texture, shape, or location of visceral organs, peritoneum, mesentery, vasculature, or objects that are normally not present.
In the pelvic region of the normal horse, the urethra and accessory sex glands (male) or the vaginal vault and cervix (female) can be palpated. The urethra is usually not discernible in the female, but abnormalities such as uroliths may be felt. In the caudal abdominal cavity the bladder, the uterus in females, and the pelvic flexure and small colon typically should be felt. The pelvic flexure and left ventral and left dorsal colons are normally located ventrally, on midline or toward the left side of the abdomen. The small colon, with formed fecal balls palpable, courses throughout the caudal abdomen, mostly on the left side. In females the left ovary can be felt in the left dorsal, caudal region of the abdomen. Both ovaries should be palpated in conjunction with palpation of the uterus. The peritoneal surface should be felt along the surface of the abdominal wall and the surfaces of the viscera. It should feel smooth, with no crepitus or irregularities. Advancing along the left side of the abdomen, the spleen can be felt as a smooth structure, with the caudal border having a well-delineated, tapered border. The size and location of the spleen are variable, because it can extend from the left body wall to the right ventral region of the abdomen. Advancing cranially and dorsally, the left kidney can be palpated. The kidney should feel smooth with the renal pelvic fissure discernible, although in the overweight horse extensive perirenal fat may obscure this detail.
From the left kidney, moving toward midline and extending from the abdominal aorta, the cranial mesenteric and ileocecocolic arteries may be felt. Palpation of fremitus in these arteries may be associated with arteritis and thrombus formation secondary to Strongylus vulgaris larval migration, although this association has been very inconsistent. Fremitus is frequently absent when severe arteritis exists, or the arteries may be entirely normal and fremitus felt. Fremitus can often be elicited by compressing the wall of the normal artery, thus accelerating flow through the compressed lumen. The mesenteric root of the colon can be felt ventral to the cranial mesenteric artery. This should palpate as a mildly taut band of tissue extending from the dorsal midline ventrally. Excessive tension, displacement, thickening, or masses within the mesentery should be considered abnormalities. It may be possible to palpate an enterolith, fecalith, or gravel impaction in the transverse colon, although this may be beyond the reach of the examiner because the transverse colon is located cranial and medial to the left kidney.
Sweeping to the right side of the abdomen, the base and cupola of the cecum can be felt. The body of the cecum can be followed partially by sweeping along the medial aspect of the cecum, cranially toward midline. The cecum has a prominent ventral band and sacculations. Gas, together with ingesta that is soft and mainly of a fluid consistency, can be felt within the cecum. Firm or excessive ingesta suggests an abnormality.
Findings that are different from normal often must be differentiated as being variations of normal or truly abnormal. Some common abnormal findings include abnormalities of the peritoneal surface. Crepitus, or a “plastic wrap” texture, is indicative of gas secondary to trauma or infection. An irregular or rough surface may be indicative of fibrin on a visceral surface or neoplasia, or with a perforated intestine there may be ingesta adhered to a visceral surface. There are many abnormal presentations of the large colon, most of which are associated with signs of colic. Thickening of the wall of the colon may be appreciated on rectal palpation and is indicative of edema or cellular infiltration of the colon. Palpation of abnormal masses in the wall of the colon or associated with the colonic mesentery is indicative of infection, infarction, granulomatous colitis, or neoplasia.
Normally the small intestine is not discerned by palpation. Occasionally, though, peristaltic contractions may be felt in the small intestine as it courses across midline toward the base of the cecum. In some cases this will cause the small bowel to palpate as a firm, tubular structure. Relaxation of the peristaltic contraction should be discerned in such cases. Distention of small intestine is abnormal. In some cases the bowel may feel thickened, which can occur with ileal muscular hypertrophy, edema, or inflammatory disorders of the small bowel.
Other abnormal findings that may accompany disorders of the abdominal alimentary system include masses, adhesions, enlarged and thickened mesenteric arteries, and caudal displacement of the spleen (secondary to gastric distention or neoplasia).
PARACENTESIS
Abdominal paracentesis is performed routinely in patients with suspected disorders of the abdominal viscera. Cytologic examination of peritoneal fluid; white blood cell (WBC) and red blood cell (RBC) counts; protein, fibrinogen, lactate, phosphate, and glucose concentrations; lactate dehydrogenase (LDH), creatine kinase (CK), and alkaline phosphatase (ALP) activity; and pH can be quantitated. The results of peritoneal fluid analysis may help establish a specific diagnosis, but, more important, may reflect inflammatory, vascular, or ischemic injury to the intestine requiring surgical intervention.
ENDOSCOPY
There are two basic types of endoscopic equipment available: equipment based entirely on a fiberoptic system and equipment based on a video chip system. A typical endoscope found in many private practices is a fiberoptic endoscope that has an insertion tube that is 100 to 110 cm in length and 10 to 14.5 mm in outer diameter. The larger-diameter tube can be inserted only through the nasal passages of older yearlings and adults and is not suitable for alimentary endoscopy, whereas a diameter of 10 mm allows passage through the turbinates of young foals. An insertion tube of 100 cm is sufficient for esophagoscopy in foals up to approximately 3 months of age. For older animals, an insertion tube length of 150 to 180 cm is required for esophagoscopy.
An insertion tube length of 110 cm is sufficient to reach the stomach of foals up to 30 to 40 days of age. A length of 150 to 180 cm is required for weanlings, and 200 cm is usually required for yearlings and adults. An insertion tube length of 200 cm is sufficient to reach the stomach of all adults of warm-blooded breeds, although 280 to 300 cm is required to examine the pylorus in adult horses. A 280- to 300-cm-long insertion tube permits duodenoscopy in adult horses.
Before a gastroscopic examination, suckling foals up to 20 days of age are not routinely kept from nursing for more than 1 hour. Older foals and mature horses should not have solid feed for 6 to 10 hours so that ingesta from the stomach may be adequately emptied. Longer duration of feed deprivation (18 hours) is desirable to view the antrum and pylorus of horses. Many foals less than 30 days old do not require sedation for gastroscopy, although sedation with 0.5 mg of xylazine per kilogram may facilitate the procedure. Sedation is required if the foal is to be placed in a recumbent position so that the entire glandular portion of the stomach can be examined. Sedation of older foals and horses is required. Xylazine (0.5 mg/kg given intravenously [IV]) usually provides adequate sedation. For greater sedation, detomidine (0.005 to 0.05 mg/kg IV) or a combination of xylazine and butorphanol (0.01 mg/kg) are effective.
After insertion of the endoscope, the stomach is distended with air until the nonglandular and glandular regions of the gastric surface can be observed. Distention with air is tolerated by foals and horses and has been associated only rarely with adverse effects. Occasionally, sick neonates with poor intestinal motility developed small intestinal distention and experienced discomfort after gastroscopy.
More complete descriptions of techniques of gastroduodenal endoscopy can be found elsewhere.1,2
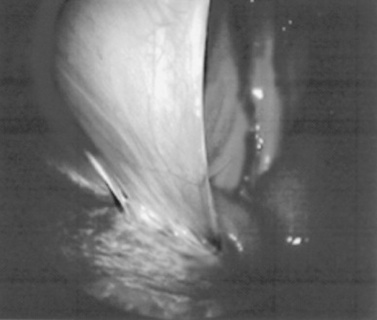
Endoscopy of the rectum and distal small colon can be performed with most flexible endoscopes in use in equine practice and should be preceded, as much as possible, by evacuation and saline lavage of the rectum and distal small colon. The mucosal surface should appear pink to pale red and should have a smooth, “velvety” appearance. Mucosal edema or thickening, hyperemia, irregularities, defects, tears, and intraluminal masses are abnormal findings. Because of the concern for trauma to the rectum and small colon, the horse should be adequately sedated and restrained before preparation and examination of the distal alimentary tract.
LAPAROSCOPY
Laparoscopy can offer valuable diagnostic information regarding the abdominal cavity and is only minimally invasive.3,4 It should always be preceded by a thorough physical examination, including abdominal palpation per rectum, paying particular attention to the sites for trocar insertion to ascertain that there are no adherent masses or viscera in the area. If abdominocentesis is to be part of the diagnostic workup, it should be performed before laparoscopy because of the effect of laparoscopy on abdominal fluid values. In experimental animals undergoing diagnostic laparoscopy with carbon dioxide insufflation, both the abdominal WBC count and the abdominal total protein increased.5
The indications for laparoscopy include palpable abdominal masses, enlarged viscera, adhesions, acute or chronic colic, weight loss, or the desire to obtain visceral biopsy specimens. Contraindications include adherent viscera or masses at the site of laparoscopic trocar insertion, diaphragmatic hernias, or extreme bloating. Horses with acute colic can be safely examined laparoscopically if one is careful when inserting the trocars and telescopes.
The basic instruments for laparoscopic examinations include a laparoscopic telescope, laparoscopic cannula and trocar assembly, fiberoptic light source and cable, insufflator, and biopsy and manipulation instruments. The 30-degree laparoscope allows better visualization of the less accessible areas compared with the 0-degree telescopes. Video cameras make visualization easier with less eyestrain but require more powerful light sources (250 watts). The cost of laparoscopic instrumentation has decreased recently as a result of the explosion of popularity of laparoscopy in humans, increasing the supply and availability of used instruments.
Horses should be fasted for 18 to 24 hours before most laparoscopic procedures; water is allowed on an ad libitum basis. Fasting increases intraabdominal visualization and decreases the possibility of penetrating a gas-distended viscus. The animal is restrained in standing stocks if the procedure is to be done while it is standing. Preoperative antibiotics, antiinflammatory drugs, tetanus prophylaxis, and a sedative analgesic combination are administered. It is important to administer the analgesics before abdominal insufflation. The flank areas are prepared for aseptic surgery. Local anesthetic agents are infiltrated subcutaneously (SC) and intramuscularly (IM) in the middle of the paralumbar fossa slightly above the crus of the internal abdominal oblique muscle for the insertion of the laparoscopic telescope. If additional instruments are to be used, their insertion sites are similarly anesthetized.
It is preferable to begin the laparoscopic procedure on the left side of the abdomen to minimize the chance of penetrating the cecal base. The horse is then draped and a stab incision is made. The laparoscopic cannula and trocar assembly are inserted through the musculature and into the abdominal cavity. It is useful to orient the trocar toward the opposite coxofemoral joint when inserting it. The trocar is exchanged for the telescope, and confirmation of entry into the abdominal cavity is made before insufflation is commenced. If the abdominal cavity has not been penetrated, a quick thrust with the telescope will usually penetrate the peritoneum. Insufflation of the abdomen with CO2 to 8 to 10 mm Hg will usually be sufficient for most examinations.
Systematic examination of the abdominal cavity is then carried out. On the left side of the abdomen the spleen, left kidney, nephrosplenic ligament (Fig. 32-1), stomach, left side of liver, diaphragm, and ventral colon may be visualized cranially. Looking caudally, the examiner will see the root of the mesentery, the isolated small intestinal and small and large colon sections, the urogenital tract, the bladder, and the terminal rectum. The procedure is repeated on the right side of the abdomen. Looking cranially, the examiner will see the liver, epiploic foramen, right kidney, descending duodenum, cecal base, and large colon. Caudally, the urogenital tract, root of mesentery, and isolated pieces of intestine are visible. Liver biopsies and right kidney biopsies are taken from the right side. Left kidney and spleen biopsies are taken from the left side of the abdomen. Mesenteric lymph node biopsies are usually obtained via the left flank. Other masses are biopsied from the more accessible side. At the end of the procedure the abdomen is deflated, and the skin is closed with skin sutures only. Closure of the skin incision should wait until examination of both sides is completed in order to minimize subcutaneous emphysema.
In some horses with ventral or cranial abdominal masses as determined with ultrasound, it is useful to anesthetize the animal in dorsal recumbency for better characterization of the mass. Biopsies may be readily obtained. In horses with acute colic but without obvious signs indicating the necessity for surgery, laparoscopy can help in making the decision to continue medical therapy or proceed to surgery. Strangulated sections of small intestine can be seen, proximal enteritis can be diagnosed, and edema and vascular compromise to the large colon can be seen. No abnormalities may be detected in some animals with very localized lesions, or lesions may be inaccessible, depending on location.
Laparoscopic complications are similar to those of any other abdominal exploratory procedure. Inadvertent penetration of a viscus may occur. The left kidney may be perforated if the laparoscope is inserted too far dorsally. The spleen may be penetrated if the laparoscope is inserted too far ventrally or is not aimed toward the opposite coxofemoral joint. The cecum may be perforated when entering from the right side. Fasting the horses and carefully inserting the laparoscopic trocars will minimize the occurrence of these problems. Subcutaneous emphysema occurs commonly but has not caused any clinical problems. The peripheral WBC count increased but stayed within normal limits in experimental animals undergoing laparoscopy.5
IMAGING OF THE ALIMENTARY TRACT
Radiography
In the horse the alimentary tract is a dynamic and complex environment to evaluate with any modality. Because of the size of the animal, as well as the distinct difference between air and soft tissue, radiographs are a useful diagnostic tool to evaluate the teeth, pharynx, esophagus, stomach, and intestinal tract. Portable x-ray units with maximal kVp settings up to 100 and the upper limits of the mAs settings at 30 make it possible for ambulatory practitioners to obtain diagnostic images of the head and cranial esophagus. However, to obtain images of the thoracic esophagus and abdomen, a referral clinic with a more powerful x-ray generator is usually required.
For the average foal, abdominal radiographs have been described using exposures ranging from 80 to 88 kVp and 20 to 26 mAs for the standard abdomen.6 In adult horses, exposures range from 60 to 140 kVp and 20 to 70 mAs.7,8 In order to completely evaluate the abdomen, it has been recommended that the abdomen be divided into four quadrants (cranioventral, midabdominal, caudodorsal, and caudoventral).7 Large cassettes (35 cm × 43 cm) and fast screens are also needed to ensure a diagnostic image is obtained. Because of the large amount of scatter radiation produced from the high exposure and thickness of tissue penetrated, an 8:1 to 10:1 grid should be used.6-10 Alternatively, an air gap technique can be used to prevent image degradation.
Availability of computed radiography (CR) and digital radiography (DR) has greatly increased the diagnostic capabilities of conventional radiographic examinations. Both of the systems available to the equine practitioner are considered indirect imaging modalities in which the x-ray photon interacts with an intensifying screen to convert the x-ray photons to light. This light then interacts with an imaging plate: film, as with a conventional radiographic system; within a photostimulable phosphor, as with CR; or within a flat-panel detector, as with DR. Regardless of the method, these images are considered “indirect” because the x-ray photon is first changed to light and then detected by the imaging medium.11 The main benefits of CR and DR are the increased latitude of the film. It is possible to change the contrast and grayscale levels after the exposure if an adequate number of photons are available to the detector. The DR systems also offer a rapid evaluation of the image because the cassette is directly connected to the computer. This makes the portable systems able to show radiographic images within 10 seconds after the exposure is made. In contrast, the CR system requires the cassette to be placed into a reader in order to display the image. Finally, because both CR and DR are generally Digital Imaging and Communications in Medicine (DICOM) compliant, this allows any specialists with standard medical imaging software to view the images via a compact disk (CD) or via the Internet.
Survey radiography is generally helpful to evaluate the cervical esophagus for evidence of rupture as well as to evaluate the abdomen. Esophageal ruptures secondary to an obstruction or vigorous placement of a nasogastric tube result in a small volume of gas that tracks just dorsal to the trachea (Fig. 32-2). This can be confused with a tracheal laceration; however, with tracheal lacerations generally the gas accumulation will surround the trachea and the volume of gas within the subcutaneous tissues and the cranial mediastinum will be severe. In addition, esophageal obstructions, also called choke, can sometimes be identified on survey radiographs depending on the material that is causing the obstruction and the amount of air or contrast medium that is able to surround the structure (Fig. 32-3). Although the nature of the obstruction cannot be determined, the extent of the abnormality can sometimes be identified.
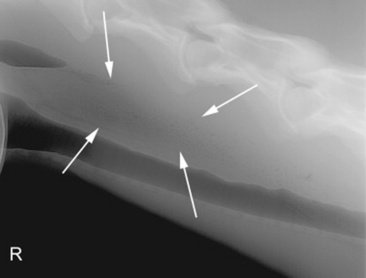
Fig. 32-2 Standing lateral radiograph of a 13-year-old Morgan gelding with an esophageal tear. Note the tubular region of small gas opacities caused by air trapped around the outer border of the esophagus (arrows). An esophageal perforation secondary to an ingested foreign body was confirmed with endoscopy.
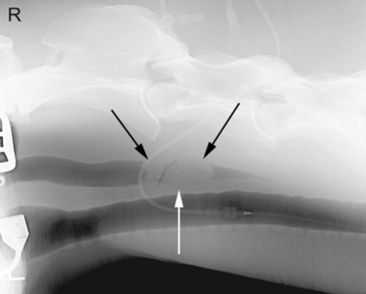
Fig. 32-3 Standing lateral radiograph of a 12-year-old quarter horse mare. Note the ovoid mass surrounded by gas just dorsal to the trachea (arrows). This lesion was a mixture of hay and grass.
Abdominal radiography is useful to evaluate the small and large intestines for sand accumulation, enterolithiasis, impactions, or small intestinal disorders in foals. When sand is ingested, it generally will accumulate within the large colon along the ventral abdomen8 (Fig. 32-4). Radiography has been found to be a useful method to monitor the resolution of sand impactions after medical management; however, sequential examinations are needed to verify that the volume of sand has reduced.8 If the volume of sand is large enough, it is difficult to determine if an enterolith is present because of summation of the two lesions. Enteroliths are a solid concretion of mineral that usually forms around a nidus, such as a metallic foreign body (Fig. 32-5). The mineral composition is varied, as illustrated by the different opacities present within the enterolith. Radiographs have a 96.4% positive predictive value to detect enteroliths in high-prevalence areas. These enteroliths were generally found to be within the midabdominal radiograph, and 67% of small colon enteroliths caused large colon distention, which was also identified on radiographs.7 Impactions are more difficult to diagnose because usually there is just increased feed accumulation within the abdomen. Although no enterolith or obstruction is identified, granular material can be seen, usually within the ventral colon near the sternal flexure. This is because pelvic flexure impactions will cause the feed material to accumulate orad, causing distention of the left ventral colon (Fig. 32-6). Intestinal disorders such as functional ileus secondary to enteritis (Fig. 32-7) or obstruction secondary to intussusception or meconium impaction (Fig. 32-8) in foals can also be identified on abdominal radiographs. These images show large dilation of the small intestine, and differentiation between functional and mechanical ileus in foals is generally based on the size of the intestine and the volume of gas that is present.9 Evaluation of the abdomen using ultrasound may aid in qualifying the small or large intestinal motility as well as identifying the source of an obstruction if the determination on radiographs cannot be made.

Fig. 32-4 Standing lateral radiograph of a 4-year-old Arab mare with a history of colic. Note the large amount of opaque material within the ventral colon, likely secondary to sand accumulation.
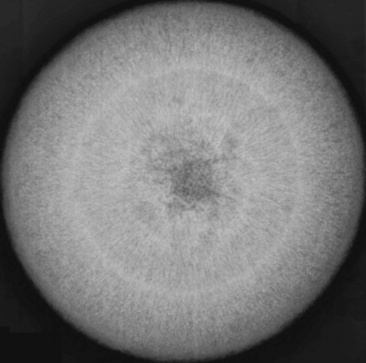
Fig. 32-5 Radiograph of enterolith obtained after surgical removal from the small colon. Note the variation in opacities caused by the various types of mineral that are contained within the enterolith.
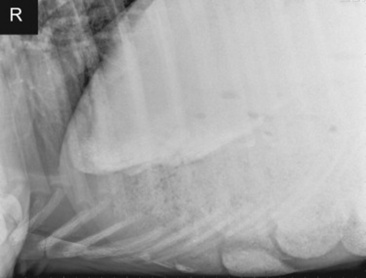
Fig. 32-6 Standing lateral radiograph of a 3-year-old Paint horse gelding with a pelvic flexure impaction. The radiograph shows the sternal flexure with a large amount of granular material and a small amount of sand accumulation in the ventral colon.
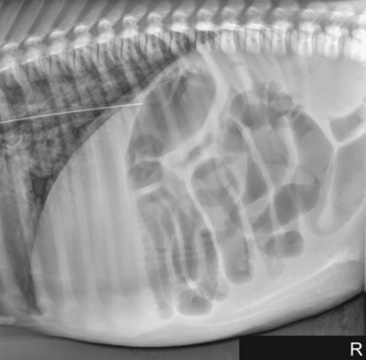
Fig. 32-7 Standing lateral radiograph of a 1-day-old, premature quarter horse filly. Note the large amount of gas-distended intestine. Because of the large amount of small intestinal distention, functional ileus is the primary differential diagnosis.
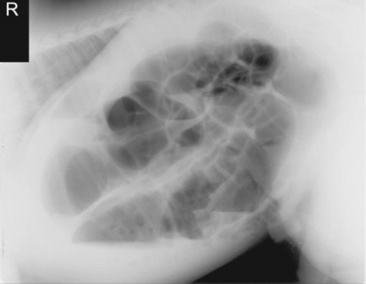
Fig. 32-8 Standing lateral radiograph of a 2-day-old thoroughbred colt with a meconium impaction. Note the large amount of gas distention of the colon.
Radiography also allows for the use of contrast medium administration to further outline the alimentary tract as well as evaluate pharyngeal function and esophageal motility.12-14 In my opinion, first administering approximately 60 mL of barium sulfate paste or liquid orally via a 60-mL dosing syringe and obtaining radiographs of the laryngeal region and esophagus provide useful information about swallowing as well as large obstructions. If the barium liquid is identified dorsal to the soft palate, within the larynx or trachea, abnormal pharyngeal function is likely present.12 The barium paste will coat the pharynx and esophagus and is useful to identify any ulcerations or irregularities in the mucosal surface. After those procedures have been performed or if there is no evidence of an oropharyngeal dysphagia, then an esophagram is performed. This procedure is done using approximately 200 to 500 mL of barium sulfate liquid diluted 1:1 or 2:1 with water to bring the total volume to 500 to 1000 mL. This liquid is administered through a nasogastric tube or cuffed endotracheal tube placed within the cranial esophagus to the level of C2 to C3. If a cuff is available, the cuff can be inflated with approximately 10 mL of room air. A radiograph is then made to verify that the tube is not within the trachea, and when it has been confirmed that the tube is within the esophagus, the dose of barium is administered using a stomach pump. Toward the end of the dose, while still pumping the liquid, radiographs of the cranial, mid, and caudal esophagus are obtained (Fig. 32-9). The use of the pump provides distention of the esophagus to help identify strictures or irregularities in the esophageal wall.
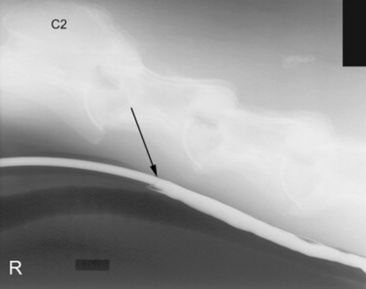
Fig. 32-9 Standing lateral radiograph showing a normal esophagram using barium liquid. The arrow marks the region where the nasogastric tube ends. This is approximately at the level of C3.
Positive (barium sulfate) and negative (room air) contrast medium radiography have also been used to evaluate the stomach and intestinal tract through oral administration of contrast medium,6,15,16 and the rectum and colon have been evaluated via retrograde administration of contrast medium.16 These methods allow for the evaluation of the stomach, intestinal tract, and rectum for regions of obstruction as well as ulcerations, tumors, motility disorders, and/or malformations. Although these methods have been described, ultrasound has virtually eliminated the need to expose personnel and patients to the repetitive, high doses of radiation needed to obtain sequential radiographs of the abdomen.
Computed tomography (CT) and magnetic resonance imaging (MRI) are of little use for evaluation of the alimentary tract (except for the head). This is mainly because of the size of the patient compared with the size of the gantry and bore in CT and MR units, respectively. Dental disorders such as abscesses and fractures can be clearly seen on CT images, especially after three-dimensional reconstructions (Fig. 32-10), and CT is also useful to detect pharyngeal and esophageal masses that may not be fully identified with conventional radiographs. CT and MRI can be used in foals that are able to be placed within the gantry or bore of the magnet; however, because of the motion of the gastrointestinal tract and the long acquisition times used with respiratory gating sequences, MRI has not been used widely to evaluate the thorax or abdomen. A single case report has been published that described the use of contrast esophagraphy and CT to aid in the surgical planning of a persistent right fourth and left sixth aortic arch that caused a vascular ring anomaly in a foal.17 However, the applications for these technologies have yet to be realized.
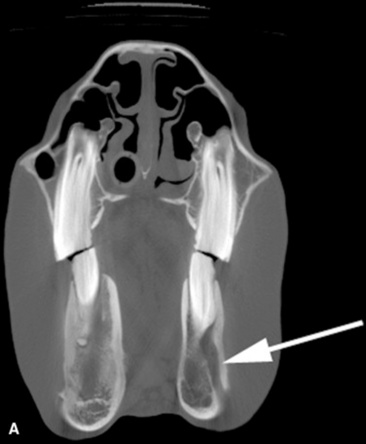
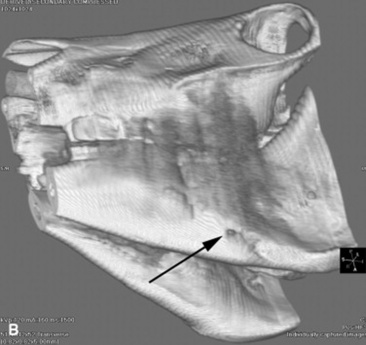
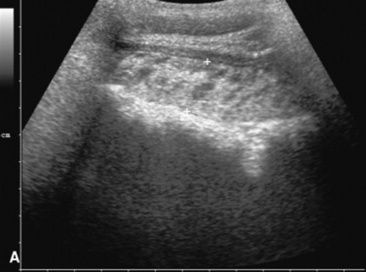
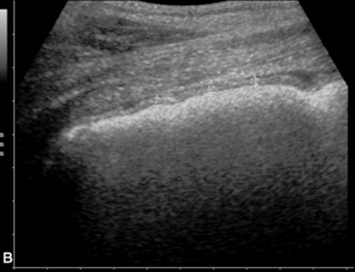
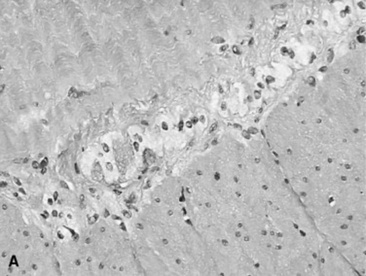
Fig. 32-10 These are transverse (A) and three-dimensional reconstructed computed tomography images (B) of the head of a 4-year-old pony mare with chronic draining tracts from the mandible. The arrows illustrate the tract through the mandible that communicates with the apical portion of the left mandibular first molar (tooth #309).
Ultrasound
Ultrasound machines have become lightweight, extremely portable, and affordable to the general practitioner. Although the machine is affordable, image quality is highly dependent on the ultrasound probes available for the examination and experience of the sonographer. These ultrasound probes come in various shapes and sizes including linear array probes, curvilinear probes, and sector-phase array probes. The physics behind the probe technology is beyond the scope of this text, but the main generalizations are that phase array probes are mainly used for cardiac examination, curvilinear probes give a large field of view, and linear array probes give exceptional superficial detail. For abdominal evaluation, a curvilinear probe is the most practical choice. The second choice offered with probe technology is the frequency. Frequency of ultrasound probes determines both the resolution and the penetration that can be achieved. The higher the frequency of the probe selected, the better the image quality (resolution). However, the increased resolution comes at the cost of penetration. A 10-MHz probe can generally image only approximately 6 cm into the abdomen, whereas a 1-MHz probe can image approximately 30 to 36 cm. For this reason, one should select the highest frequency probe possible to image to the desired depth. For example, if the ventral colon were examined, because it is relatively close to the skin surface (approximately 5 cm), then an 8- to 10-MHz probe would give the best detail for the desired depth. If the nephrosplenic space were to be imaged, this structure is approximately 12 to 15 cm deep from the skin surface, and a 5-MHz probe would be needed. This will cause a reduction in image quality, but the sacrifice is needed to gain the desired depth. The ultrasound examination requires the use of large volumes of isopropyl alcohol to wet the hair and to serve as a coupling medium to provide airtight contact between the skin and the probe. Acoustic coupling gel and clipping can be performed to enhance image quality, but in my experience, using isopropyl alcohol provides a good image, and clipping can be done in limited areas as needed to enhance the image quality.
In the last 5 years ultrasound has come to the forefront of evaluation of the equine alimentary tract, primarily centering on the abdomen. Ultrasound has been used in foals to determine the growth rate and normal appearance of thoracic and abdominal organs18 and in adult horses to evaluate the gastrointestinal tract for causes of pain including torsion, small intestinal obstruction, colon impaction, large colon displacement, intussusception, strangulating lesions, and enteritis and colitis.10,19-23 This modality is even more useful because it provides real-time information to help assess contractility of the intestine. Although this has been explored using both standard two-dimension imaging, also called B-mode or brightness-mode imaging, and spectral Doppler imaging,24 the presence of gas within the bowel and the fact that the bowel is usually perpendicular to the image plane makes quantitative evaluation of intestinal contractility difficult at best. When compared with radiography to identify intestinal sand accumulations, ultrasound was found to be 87.5% sensitive and specific using radiography as the gold standard.10 The main limitations are the artifacts secondary to gas within the colon and the fact that gas and mineral are both echogenic on ultrasound, whereas they have opposite opacities on radiographs.
Ultrasound evaluation of horses with abdominal pain (colic) provides a rapid method to identify abnormalities within the gastrointestinal tract. Distention of the small intestine to a diameter greater than 5 cm has been strongly associated with strangulating or obstructing lesions19 (Fig. 32-11). In foals with intussusception, the small intestine appears enlarged and there is generally distended small intestine orad to the lesion; however, at the site of the intussusception there is a normal-appearing small intestinal wall (intussuscipiens) surrounded by a larger structure that appears to surround the inner small intestinal wall (called the intussusceptum)19 (Fig. 32-12). Large colon torsion occurs when the large colon rotates 360 degrees or more around the root of the mesentery to cause occlusion of venous drainage while maintaining arterial flow. This causes the wall to become thick and edematous. If ultrasound is performed in the cranioventral abdomen, just caudal to the xiphoid process, then a colon wall size greater than 9 mm is 100% specific for a large colon torsion21 (Fig. 32-13). A large colon displacement would have minimal to no vascular compromise, so it would be an ultrasound diagnosis based on exclusion. Chronic displacements did have a mild amount of edema in the colon wall, causing the size to be approximately 7 mm thick but never greater than 9 mm in the one study described.21 The colon and small intestinal wall will also become thick with inflammation. Small intestinal wall thickness greater than 4 mm is indicative of inflammation.19 The right dorsal colon can be imaged in the right tenth to twelfth intercostal space around the region of the costochondral junction, and a focal wall thickness of 9 to 12 mm has been identified with right dorsal colitis.23
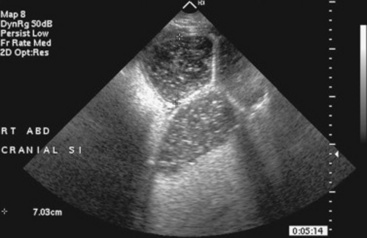
Fig. 32-11 Transabdominal ultrasonographic image of a 6-year-old thoroughbred gelding with acute onset of colic. The small intestine is 7 cm in diameter and was noted to have minimal to no contractility. This is consistent for mechanical ileus. A strangulating lipoma was identified at surgery.
Courtesy of Cornell University.
Scintigraphy
Nuclear medicine is a widely used modality to image the gastrointestinal tract in humans and animals. Although physical obstruction can be assessed with endoscopy, ultrasound is unable to identify the pylorus in the normal horse because of the peripheral nature of the colon and colonic gas. Nuclear medicine provides a functional evaluation of the pylorus to determine if delayed gastric emptying compared with normal horses is present. This modality works better than gastric emptying with barium sulfate because of the minimal invasive nature and ease of acquisition of images. However, the need for a gamma camera to generate the images and the licensing requirements to handle radioactive material make this modality less universally available. The primary use for evaluating the gastrointestinal tract with scintigraphy is to evaluate gastric emptying time.25-28 Two protocols have been outlined in the references provided. The first uses the readily available technetium-99m pertechnetate (99mTc) bound to disofenin or sulfur colloid.26,29,30 This combination of radioisotope and radiopharmaceutical is fed in a pelleted ration alone or mixed with radiolabeled eggs. The normal range for the t½ gastric emptying has been reported to be 1.49±0.17 hours30 and 1.56±1.08 hours.29 The other method is the carbon-13 (13C) bound to octanoic acid breath test (13C-OABT). This method was validated compared with the 99mTc sulfur colloid and found to have similar results compared with the solid phase gastric emptying t½.24 The main difference is that the 13C-OABT is measured in the exhaled breath of the horse and with spectroscopy rather than using a gamma camera. The rationale for 13C-OABT is that because a gamma camera is not needed, this test may be more portable and useful for field investigations.29
Another nuclear medicine procedure in the realm of alimentary tract evaluation involves the use of 99mTc hexamethylpropyleneamine oxime (99mTc-HMPAO). This procedure allows for radiolabeling WBCs in order to determine areas of inflammation.31,32 The use of 99mTc is a matter of convenience because it is also used in equine bone imaging. Thus, detection equipment, such as a low-energy, all purpose collimator, is readily available in many practices. HMPAO is used because it binds to granulocytes and therefore should travel to areas of increased inflammation.32 HMPAO also associates with the reticuloendothelial system, enabling localization within the lungs, liver, spleen, kidneys, and urinary bladder. The main drawback is that with the activity bound to WBCs there is a general lack of anatomic information, which can cause lesion localization to be difficult.32 Although this technique is expensive and labor-intensive, the results from the limited studies available appear encouraging.
BIOPSY
The decision about whether to obtain a biopsy is often based on the ease of obtaining a sample and the relative value of the evaluation that can be made. Very small samples, such as those obtained with an endoscope biopsy instrument, are relatively easy to obtain, but they provide limited information. Full-thickness bowel specimens, obtained by means of ventral midline or flank laparotomy, are more difficult to obtain, but they provide much more information.
Taking a biopsy sample by endoscopy allows the practitioner to choose the biopsy site on the basis of the appearance of the mucosal surface, which most frequently reflects an inflammatory disorder. Conversely, when a biopsy sample is obtained through laparotomy, the serosal surface of the bowel may not reflect a disorder within the bowel wall. In such instances it may be useful to obtain several biopsy specimens. Rectal mucosal biopsies are easily performed. Many instruments can be used to obtain the biopsy specimen, and a uterine biopsy forceps works well. A fold of mucosa can readily be pinched between two fingers, and a sample of this tissue is obtained. The size of the sample is adequate for histologic or bacteriologic examination.
FECAL EXAMINATION
Cytologic, biochemical, bacteriologic, immunologic, and electron microscopic evaluations can be performed on fecal samples. In addition, observation of the consistency and color, the presence of foreign material such as sand or gravel, and the presence of parasites should be included in the examination of the alimentary system. In addition to fecal consistency, fecal particle size can be used to evaluate the efficiency of mastication or the colonic transit time. Increased particle size, with loose or watery stool, is suggestive of decreased colonic transit time.
Cytologic examinations are primarily used to evaluate the parasite burden of the animal. Ova of large and small strongyles, tapeworms, round worms, and Strongyloides westeri are most common. Coccidia are occasionally observed but are clinically unimportant. Examination of fecal WBCs has been advocated in the evaluation of horses and foals with enterocolitis. Because these cells are very labile, their presence in large numbers indicates that an inflammatory process is present and that the inflammation is in the distal colon or is associated with decreased transit time.
Determination of fecal occult blood has been recommended to diagnose gastric ulcers, duodenal ulcers, and other potentially hemorrhagic disorders of the alimentary tract. However, the usefulness of this test has been shown to be quite limited, because negative results can be obtained when blood is present in the proximal portion of the gastrointestinal tract.33 The sensitivity of most commercially available tests is poor, giving negative results in the face of severe gastric bleeding.
Fecal culture is an essential component in the evaluation of many patients. In bacteriologic culture techniques for fecal samples, selective media that are designed to isolate Salmonella are routinely used. These media include selenite broth, tetrathionate broth, brilliant green agar, XLD agar, and Salmonella-Shigella agar. Less selective media, McConkey’s and eosin methylene blue agars are desirable to culture other potential gram-negative bacterial pathogens such as Escherichia coli, but the mere presence of E. coli in the feces does not determine its pathogenicity. Enterotoxigenic E. coli have been isolated from foals with diarrhea, but special tests, such as polymerase chain reaction (PCR) assays, must be performed to determine whether an isolate produces enterotoxin.
Tests for detection of enterotoxins of Clostridium difficile* and Clostridium perfringens† in fecal specimens are available at diagnostic laboratories or can be performed using enzyme-linked immunosorbent assay (ELISA) kits.
The presence of rotavirus in a fecal sample can be determined by use of an ELISA or an agglutination test. Both assays test for the presence of viral antigen in the feces. The ELISA is reported to be more sensitive than the agglutination test but is less specific. Therefore the agglutination test is likely to give more false-negative results, and the ELISA test is likely to give more false-positive results. The ELISA test is more time-consuming and inconvenient to perform than the agglutination test. When rotavirus is a concern, particularly as a farm problem, a reasonable approach is to screen fecal samples with the agglutination test and repeat testing of samples that yield negative findings with the ELISA.
ABSORPTION AND DIGESTION TESTS
Tests that evaluate the ability of the equine intestinal tract to digest and absorb nutrients have a more limited clinical application than in human or small animal medicine, but they can be useful in the evaluation of horses with chronic weight loss, suspected small intestinal inflammation or neoplasia, gastric and small intestinal partial obstruction, and postoperative small intestinal malabsorptive disorders. For absorption tests to be diagnostic, the intestinal disorder must either be diffuse or affect the delivery to and transit through the small intestine.
Maldigestion tests are performed to evaluate exocrine pancreatic function and small intestinal mucosal brush border disaccharidase activity. Pancreatic exocrine deficiencies have not been described in the horse, probably because equine pancreatic secretions consist primarily of water and bicarbonate and have less enzymatic activity than in monogastric omnivorous species. Mucosal brush border disaccharidase-related maldigestion is relevant in viral and bacterial enteritides of foals, particularly rotavirus and coronavirus enteritides. As a result of these viral infections, there is loss of the superficial villous epithelial cells of the small intestine, in which the disaccharidases lactase, cellobiase, maltase, sucrase, and trehalase are located.34 Lactase levels are greatest in young suckling foals, and loss of this enzyme activity, secondary to loss of the mucosal villous cells, leads to lactose maldigestion. Lactose tolerance can be tested by administering a 20% solution of D-lactose at a dose of 0.5 to 1 g/kg. This dose should result in an approximate doubling of the serum glucose level within 60 minutes of administration.35
Clinically applicable absorption tests include the D-glucose and D-xylose absorption tests. The glucose absorption test has the advantage of being relatively easy and inexpensive to perform. However, cellular uptake and metabolism of glucose, as well as intestinal absorption, influence the results and thus are undesirable variables. The xylose absorption test is therefore advantageous because it more directly measures intestinal absorptive capacity. The results of both tests, though, are affected by gastric emptying rate and small intestinal transit time. In the United States, D-xylose is available only through chemical suppliers and only for research purposes; its availability for clinical diagnostic use is restricted.
The D-glucose and D-xylose tests are performed similarly. After an 18- to 24-hour fast, a 10% solution of D-glucose or D-xylose, 0.5 to 1 g/kg, is administered through a nasogastric tube. For the measurement of glucose, blood is collected in sodium fluoride tubes; and for the measurement of D-xylose, blood is collected in heparinized tubes. Samples are taken at 0, 30, 60, 90, 120, 150, 180, 210, and 240 minutes after administration. Peak levels, which normally range from 20 to 25 mg/dL, occur 60 to 120 minutes after administration, and levels thereafter should decrease. The normal curve resembles an inverted V. Variability in absorption curves occur as a function of age and type of feed the horse is given.36 Delay or flattening of the absorption curve may reflect delayed gastric emptying, increased intestinal transit time, or impaired intestinal absorption.37 Accurate interpretation of the results of these tests depends on the results of other diagnostic evaluations. In addition, different types of diet have been shown to affect the height, although not the shape, of the absorption curves significantly. In general, diets that have a higher digestible energy content result in a lower peak in the curve.
BREATH TESTS
In humans, dogs, and cats, breath tests are used to assess a variety of intestinal disorders. The urea breath test is used as part of an assessment of Helicobacter status of the patient,38 but this is not an issue for horses. The hydrogen breath test is used in assessments of intestinal bacterial overgrowth and in determination of carbohydrate digestion and absorption in the intestine. In patients with an abnormal intestinal bacterial population or carbohydrate malabsorption, there will be excessive bacterial fermentation of carbohydrate, with one byproduct being hydrogen. Because hydrogen is freely diffusible from the bowel into the blood, and from the blood into the alveoli, measurement of exhaled hydrogen gas can be used to assess the status of intestinal bacterial fermentation of carbohydrates. In horses, the hydrogen breath test is most applicable to conditions in which there is carbohydrate malabsorption and thus increased delivery of soluble carbohydrate to the large intestine for bacterial fermentation to occur. There are two reports of this technique in horses, one in which different carbohydrate substrates were evaluated in ponies39 and another in which the hydrogen breath test was used in conjunction with D-xylose absorption in nine horses with a variety of clinical disorders.40 In the study with ponies (N = 7), fasting resulted in negligible levels of breath hydrogen excretion. Sustained increases in breath hydrogen concentration greater than 10 ppm were observed for all ponies after the ingestion of oats or the administration of wheat flour, for three ponies after the administration of glucose and xylose, and for two ponies after the administration of lactulose and lactose. The pattern of breath hydrogen excretion was subject to variation among animals after the ingestion of identical test meals. In the clinical study the diseased horses showed higher fasting breath hydrogen (H2) levels (range 7.5 to 61.5 ppm) than normal horses (range 0 to 5 ppm). After xylose administration, none of the healthy animals showed an increase in breath H2 production, and five of diseased animals showed increases in breath hydrogen. In this group of patients, abnormalities in hydrogen breath measurement were more apparent than abnormalities in D-xylose absorption.
DENTISTRY AND ORAL DISEASE
The horse has evolved over millions of years to become a continuously grazing animal and in doing so has developed its own dental form and function. The horse’s oral and dental structures provide it with the ability to detect, prehend, masticate, and begin the digestion of forage. As we have domesticated and confined the horse, we have altered its diet to consist of less continual grazing and more interval feeding of dry hay, grain, processed forages, and other concentrates. Selective breeding and domestication have increased the incidence of equine dental and oral disease in today’s equine population.
DENTAL AND ORAL ANATOMY
The structures the horse uses in eating include tactile and prehensile lips; hypsodont incisors, premolars, and molars; facial bones; muscles of mastication; tongue and hard and soft palates; buccal mucosa; cheeks; olfactory organs; taste buds; salivary glands and ducts; and blood vessels and nerves that support these structures. The equine mouth is a long cylindric cavity that is the beginning of the alimentary canal and is commonly referred to as the oral cavity. The muscular lips make up the entrance to the mouth, which is bounded laterally by the cheeks, dorsally by the hard palate and ventrally by the body of the mandible and the mylohyoid muscles. The caudal aspect of the mouth is composed of the soft palate, root of the tongue, and epiglottis and is continuous with the oropharynx.41,42
The blood supply to the mouth is derived from the maxillary, mandibular, labial, and sphenopalatine arteries. The venous drainage is chiefly through the linguofacial veins. Sensation to the mouth and cheeks is derived from the trigeminal nerve (cranial nerve V), and motor function from the facial nerve (cranial nerve VII). The hard palate has a central raphe that divides the surface into right and left halves. The flat portion of the palatal mucosa just caudal to the upper incisors may appear swollen in the young horse when permanent incisors are erupting. This normal mucosal enlargement seen in 2- to 5-year-old horses has been referred to as lampas. Farther caudally, the hard palate becomes more concave and contains paired transverse ridges, which are instrumental in moving a food bolus caudally, in a spiral fashion, as the horse masticates forage. The muscular tongue sits in the bottom of the mouth, supported in a sling formed by the mylohyoideus muscles, between the paired hemimandibular rami. The root of the tongue is attached to the lateral aspect of the soft palate, the pharynx, and hyoid bone. The lingual muscles receive their motor innervation from the hypoglossal nerve (cranial nerve XII) and sensory innervation from the lingual branch of the mandibular nerve and glossopharyngeal nerve (cranial nerve IX).
The mandible is the largest bone of the face and is formed by paired hemimandibles that fuse rostrally at the mandibular symphysis when the horse is approximately 2 to 3 months old. Each hemimandible is composed of a horizontal and a vertical ramus. The dental alveoli are contained within the horizontal ramus. The vertical ramus terminates with the coronoid process rostrally and the mandibular condyle caudally. The temporalis muscle inserts on the coronoid process.
Between the incisors and the rostral aspect of the mandibular cheek teeth, on the horizontal rami of the mandible, are the “bars” of the mouth. This large interdental space or natural diastema is the resting area for the bit. Canine teeth, if present, are located in these spaces. The ventral border of the mandible of the young horse is wide and round, but as the horse ages and the mandibular cheek teeth continue to erupt, the ventral border of the mandible becomes more sharply angled. Eruption swellings or “bumps” often develop along the ventral border of the mandible of young horses as the permanent mandibular cheek teeth erupt.
The paired incisive (premaxillary) bones form the rostral part of the upper jaw and contain the alveoli of the upper incisors. Caudally the incisive bone becomes thinner and forms the rostral part of the hard palate. The suture line between the incisive bones and the maxillary bones is an anatomically weak area and a common site of fracture. The upper canines, if present, are situated just caudal to this suture line.
The paired, large maxillary bones extend from the incisive bone rostrally to the nasal bones dorsally and lacrimal bones caudally. The facial crest is a prominent ridge of bone on the lateral aspect of the maxillae. This crest continues caudally as the zygomatic process and joins the malar and temporal bones to form the zygomatic arch. The ventromedial aspects of the maxillary bones join to form a horizontal shelf that provides rigid support to the majority of the hard palate. The alveoli of the upper canines, premolars, and molars are embedded in the maxillae. The position of the alveoli of the upper cheek teeth is somewhat variable, but usually the alveoli of the first two cheek teeth lay rostral to the sinuses. The apices of the third and fourth cheek teeth lie within the rostral maxillary sinus in the young to middle-aged horse, and the apices of the caudal two cheek teeth lie within the caudal maxillary sinus. Each alveolus is separated by transverse interalveolar bony septa.
The horse has five paired paranasal sinuses: the conchofrontal, sphenopalatine, caudal maxillary, rostral maxillary, and ethmoidal sinuses. The rostral and caudal maxillary sinuses are contained within the maxillae and are usually separated by a thin bony septum, although this septum often breaks down in the presence of sinus disease. The infraorbital canals (one on each side of the head) traverse longitudinally through the maxillary sinuses.
Bacterial sinusitis can occur secondary to disease of the third, fourth, fifth, and sixth upper cheek teeth and classically results in a unilateral, malodorous, nasal discharge. Almost the entire lumen of the maxillary sinuses of a young horse is occupied by dental alveoli, but as the facial bones grow and reserve crowns of the upper cheek teeth erupt, the cheek teeth move rostrally and ventrally, causing the sinus compartments to enlarge. The paranasal sinuses drain into the caudal aspect of the nasal cavity via a slitlike opening, the nasomaxillary aperture. The medial compartment of the rostral maxillary sinus, or ventral conchal sinus, has poor drainage and is a common site for inspissated exudate to accumulate. Its secretions must drain into the lateral compartment of the rostral maxillary sinus before draining into the caudal aspect of the middle meatus through the nasomaxillary aperture.
The temporomandibular joint is a synovial joint formed by the articulation of the squamous temporal bone with the condylar process of the mandible. The joint lies approximately 15 cm above the level of the occlusal surface of the cheek teeth. The cavity of the joint is large and divided by a cartilaginous, intraarticular disk. The joint is bound by a tight capsule and lateral and caudal ligaments. The equine temporomandibular joint has a wide range of lateromedial movements, which allows for the medially directed power stroke of mastication, but limited vertical and rostrocaudal movements.
To affect the wide lateromedial range of motion in the temporomandibular joint during the power stroke of mastication, the masseter and pterygoid muscles have evolved into most highly developed muscles of mastication in the horse. The powerful masseter muscle originates along the full length of the facial crest and zygomatic arch and has wide insertions along the caudolateral aspect of the mandible. The superficial muscle fibers of the masseter run almost vertically, whereas the deep fibers course in a ventrocaudal direction. The masseter pulls the jaw to the ipsilateral side and contributes to closure of the jaw. The origins and insertions of the medial and lateral pterygoid muscles are similar to those of the masseter, but these muscles lie on the medial aspect of the mandible. The digastricus muscle originates on the occipital bone and attaches to the caudal aspect of the mandible. This muscle assists in opening the mouth, but because gravity also assists in opening the mouth, this muscle is small. The temporalis muscle functions to close the jaw, but because the temporomandibular joint of the horse is capable of only slight vertical movement, this muscle is also small and poorly developed. The muscles of mastication are all innervated by the trigeminal nerve (cranial nerve V).
TEETH
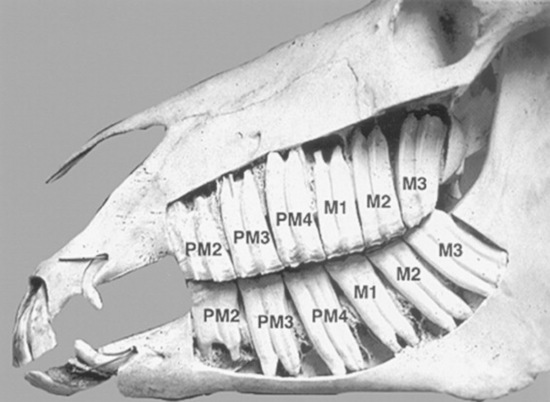
The foal has erupted 24 deciduous teeth by the time it is approximately 6 months old. Expansion of the cranial and facial bones during the first 2 to 3 years of life allows room for the expansion of the dentition from 24 deciduous teeth to the 36 to 44 permanent teeth present in the adult horse. The mouth of the mature horse contains six incisors in both the upper and lower jaws and six permanent upper cheek teeth and six permanent lower cheek teeth on each side of the mouth (Fig. 32-14). The rostral three cheek teeth are premolars, and the caudal three cheek teeth are molars. Incisors and premolars have deciduous and permanent sets. Molars erupt later than the deciduous premolars and do not have deciduous counterparts. The occlusal surface of the cheek teeth of the upper jaw is broad and square, and that of the lower cheek teeth is narrower and rectangular.
The cheek teeth in each quadrant of the horse’s mouth are commonly referred to by number (1 to 6), from rostral to caudal. The vestigial and inconsistently present first premolar, often referred to as a wolf tooth, is not included in the 1 to 6 nomenclature. To help avoid confusion, the American Veterinary Dental College Nomenclature and Classification Committee has endorsed the use of the Modified Triadan Tooth Numbering System for the horse.43 This three-digit tooth numbering system is based on a full phenotypic dentition composed of 44 teeth. The first digit designates the location of the quadrant, or arcade, and whether the dentition is deciduous or permanent. The permanent teeth in a quadrant are designated using numbers 1 to 4, and the deciduous teeth in a quadrant are designated using numbers 5 to 8. The numbering sequence for the permanent teeth starts with #1 for the upper right teeth, #2 for the upper left teeth, #3 for the lower left teeth, and #4 for the lower right teeth. In each dental quadrant, the incisors are numbered 01 to 03, and the first or central incisor is always 01. The canines, whether present or not, make up the 04 position in this formula. The premolars are numbered 05 to 08, and the molars are numbered 09 to 11.
The equine incisors and molarized cheek teeth are hypsodont and have long anatomic crowns. The tooth crown is the enamel-containing portion of the tooth. When these hypsodont teeth erupt, their occlusal surface is covered with thin layers of cementum and enamel, which wear away from masticatory forces and abrasive forage, to expose the true or functional occlusal surface of the tooth. This process is termed coming into wear. The functional occlusal surface of hypsodont teeth is composed of thin, brittle sheets of hard enamel sandwiched between softer layers of cementum and dentin. This three-textured occlusal surface is self-sharpening and resistant to wear and fracture. The occlusal surfaces of the molar arcades wear in an undulating fashion with 13 loph basins (food channels) between transverse ridges.
The incisors and upper cheek teeth have enamel invaginations in the crown, termed infundibula. These enamel invaginations are partially filled with cementum, which receives its blood supply from the soft tissue covering the tooth before eruption. The shallow infundibulum present on each incisor has a wide opening at the occlusal surface, referred to as a “cup.” As the incisor wears, the small apical portion of the infundibulum eventually becomes exposed at the occlusal surface and is termed “the spot.” Each upper cheek tooth has a rostral and a caudal infundibulum. These enamel invaginations, or cones, give the central area of these teeth a hard wear surface. The center of the cement lake that fills the infundibulum contains a hole, which is the remnant (i.e., a “ghost”) of the central blood vessel that once supplied nutrition to the now dead infundibular cementum.
Much of the crown of the hypsodont teeth of the horse is held in reserve subgingivally, in the alveolar bone. The apex of the tooth slowly completes its development by forming roots for several years after the tooth erupts. The interior of the tooth is composed primarily of dentine, with primary dentine lining the common pulp chamber of the newly erupted hypsodont tooth. The pulp chambers of hypsodont teeth are active throughout the horse’s life and continuously produce secondary dentine within the pulp cavity. Continuous production of secondary dentine prevents the vital pulp from being exposed at the occlusal surface as the tooth wears. The depth of secondary dentine at the occlusal surface of the pulp horns varies in horses but is at least 5 to 7 mm thick and generally increases in thickness as the tooth ages. Secondary dentine absorbs pigment from feed and is seen as a brown area on the occlusal surface of the tooth.
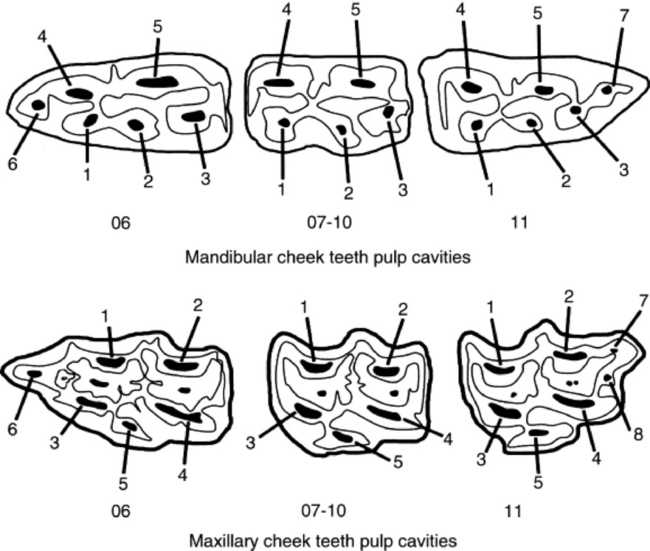
The pulp cavity of the young, permanent cheek tooth is large, but as the tooth ages the pulp divides into smaller pulp chambers, or horns, by deposition of secondary dentine. From 2 to 4 years after eruption, mandibular cheek teeth have a distinct, apically located, common pulp chamber that communicates with the pulp horns. Five pulp horns are present in the 07s to 10s, six pulp horns are present in the 06s, and six or seven pulp horns are typically present in the 11s (Fig. 32-15). By 6 to 8 years after a mandibular cheek tooth erupts, production of secondary dentine divides the endodontic system of the tooth into two distinct compartments or roots. Each compartment consists of a root canal, a pulp chamber, and two or three pulp horns. Each maxillary cheek tooth has three roots. Because of the continuous production of cementum around the apical or root portion of the tooth and continuous wear of the crown, old equine teeth are primarily composed of cementum. When most of the enamel of the crown has worn away, the softer dentine and cementum are quickly worn flat, leading to the condition known as “smooth mouth.”44
Innervation of the dental structures is supplied by the trigeminal nerve, which exits the skull just below the ear. This nerve traverses rostrally and then divides into ophthalmic, maxillary, and mandibular branches. The maxillary nerve enters the caudal aspect of the maxilla ventral to the orbit via the maxillary foramen and runs through the maxilla in the infraorbital canal, giving off branches that supply the maxillary cheek teeth. The nerve then exits the maxilla at the infraorbital foramen, located just rostral and dorsal to the facial crest. As the mandibular nerve runs medially along the horizontal ramus of the mandible, it branches into smaller nerves, including the mandibular alveolar nerve, which enters the mandibular canal at the mandibular foramen on the caudomedial aspect of the mandible and innervates the mandibular cheek teeth. The inferior alveolar nerve exits the mandibular canal at the mental foramen at the rostrolateral aspect of the mandible just rostral to the mandibular cheek teeth to become the mental nerve, which supplies the ipsilateral soft tissues over the incisive portion of the mandible.
The horse is anisognathic, which means that the bottom jaw is narrower (by about 25%) than the upper jaw. The molar tables are sloped at a 10- to 15-degree angle from dorsal lingual to buccal ventral (Fig. 32-16). Lateral excursion of the jaw during mastication favors occlusal wear of the buccal aspect of the lower molar arcades and the lingual aspect of the upper molar arcades. As the horse chews, the jaw moves in a rotary motion from side to side with limited rostral to caudal excursion. The molars are constructed so that the enamel, cementum, and dentine interdigitate to provide a sharp, serrating surface that allows for uneven, continuous wear when the horse is eating.
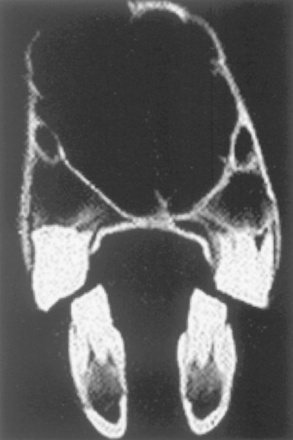
Fig. 32-16 Computed tomographic scan of the equine skull at the level of the first molars. Dorsal is at the top. Note that because the upper molars are offset laterally from the lower molars, the molar tables are sloped at a 10- to 15-degree angle from dorsal lingual to buccal ventral.
The extent of lateral excursion of the mandible during normal mastication is affected by the length of stem or roughage in the horse’s ration. Horses on pasture or hay have a wide area of mandibular excursion, whereas horses eating pellets or concentrates have a more limited range of lateral jaw excursion. Horses fed predominantly pellets or only a small quantity of long-stemmed roughage tend to have incomplete wear of the molar surface, predisposing the arcades to development of sharp enamel edges, a vaulted ceiling of occlusion, or the serious problem of shear mouth. Malocclusion of the incisors or the molar arcades perpetuates abnormal wear patterns that can eventually lead to severe dental disorders.
Rostral or caudal molar malocclusions or problems with eruption (e.g., displaced, deformed, missing, or supernumerary teeth and delayed eruption) lead to uneven dental wear. Horses with asymmetry between the upper and lower molar arcades, such as from mandibular fracture, facial injury, congential deformities such as brachygnathism (parrot mouth) and prognathism (sow mouth), or an abnormally narrow mandible in relation to the maxillae, often develop abnormal dental wear, resulting in sharp enamel points, dental overgrowths, shear mouth, step mouth, or wave mouth.
Equine males normally have two upper and two lower canines (or bridle teeth). The upper canines erupt just caudal to the suture between the incisive and maxillary bones. The lower canines are located further rostrally, producing a long lower diastema or interdental space. Canines of mares are rudimentary or absent.
The rudimentary first premolars, or wolf teeth, are constant in fetal life in both the upper and lower jaws. Many never develop to the point of eruption but instead degenerate and become incorporated in the maxilla or mandible. The upper first premolars erupt in 20% to 80% of horses, whereas the lowers erupt in only 1% to 5% of horses.
The dynamic changes that take place in the horse’s head continue at a slower pace throughout life after the horse matures. The hypsodont premolars, molars, and incisors with their large reserve crowns and slowly forming short roots continually erupt and wear. With continuous eruption and wear of the hypsodont teeth, all horses eventually wear their cheek teeth to the roots.
DENTAL EXAMINATION
A horse’s dentition should be examined biannually as a routine part of a health maintenance program. Eating efficiency and oral hygiene are the most important considerations from a medical standpoint when providing dental care, but often owners are more enthusiastic about dental care because of its positive effects on the horse’s athletic performance. Written documentation of findings during dental examination is necessary to formulate a problem-oriented treatment plan and to follow progress after the horse receives routine maintenance and/or treatment for dental abnormalities. A consistent routine on the part of the examiner increases the efficacy and quality of the examination (Box 32-1).
Box 32-1 Recommended Timetable for Routine Dental Examinations and Common Corrective Procedures
2 TO 3 YEARS OF AGE
Signs of dental disease may be obtained from the history or observed in a horse suffering from dental problems. A history of abnormal head carriage or head tossing while being ridden or during eating, prolonged time of eating, halitosis, dysphagia, drooling, dribbling feed (i.e., quidding) or eating hay before grain should lead one to consider that a dental problem exists.45 Indicators of a dental problem in performance horses include tail wringing, head shaking, lugging in or out on the track, and fighting the bit (i.e., refusing to collect the head). In addition, intermittent dorsal displacement of the soft palate in performance horses may be a sign of a dental abnormality.
Good dental health is extremely important to the equine digestive system. Chronic colic or choke can result from improper mastication of feed, and reluctance to drink cold water may be caused by dental pain. Proper mechanical digestion of feed allows better carbohydrate absorption in the small intestine and improved fiber fermentation in the cecum and large colon. Improper mastication of roughage and concentrate produces large feed particles with decreased surface area per mass. The large particles are poorly digested in the small and large intestine because decreased surface area of the feed does not allow proper enzymatic degradation and bacterial fermentation. Finding whole grain or stem particles more than 5 mm long during examination of the manure indicates that the horse suffers from improper mastication.
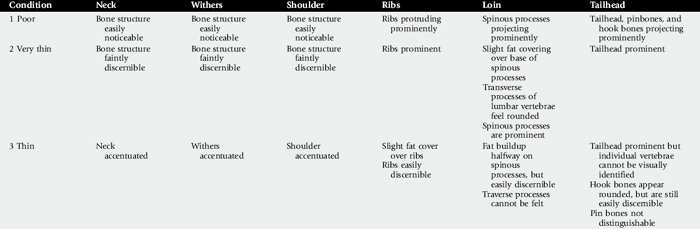
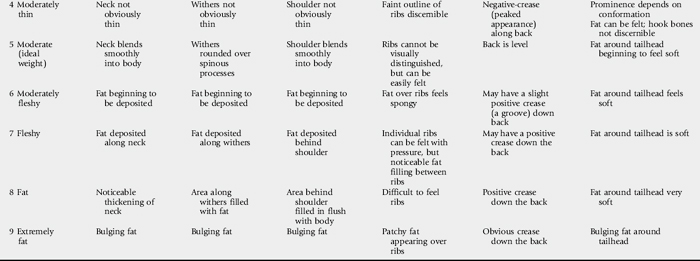
The physical examination begins with observation of the horse’s body condition, attitude, and temperament. The horse’s overall condition should be evaluated in light of its use and dietary intake. Assigning a body score is an accurate way to subjectively record body condition (Table 32-1).46 Objective assessment of body condition using a scale, a weight tape, or photographs is also beneficial and provides reliable data to evaluate the effects of treatment. The age of the horse should be considered during evaluation, because different conditions need to be addressed as the horse ages. The use of the horse should be considered during evaluation, because horses that wear a bit might require dental care not required by horses that do not wear a bit. Stable surroundings should be carefully observed for evidence of vices, such as cribbing or poor eating habits, such as dribbling hay or grain.
Body and head conformation should be considered in evaluating the masticatory system. The head should be observed from both sides and the front to detect asymmetries, protuberances, or swellings. Horses with small heads have more of an angle in the curve of the mandibular ramus (i.e., the curvature of Spee) and are predisposed to dental crowding and ramps on the lower dental arcades.
The horse should be approached at its left shoulder. The tongue should be checked for proper movement, abnormal swelling, and signs of trauma, and the horse’s ability to swallow should be evaluated. Excessive lacrimation, abnormal nasal discharge, or halitosis should be noted. The horse should receive a neurologic evaluation if any cranial nerve deficits are detected. Finally, the mandibular rami, masseter muscles, temporomandibular joints, and submandibular lymph nodes should be palpated to detect enlargements or asymmetry.
The frontal and maxillary sinuses should be percussed with the horse’s mouth open. The width between the mandibular rami should be noted, because this width correlates with the room in the mouth for the bit. The sides of the head lateral to the upper dental arcades should be compressed, starting at the orbit and moving forward to the first cheek tooth at the level of the nasomaxillary notch, to detect protuberances, depressions, asymmetry, or evidence of pain. The commissures of the lip should be observed and palpated for signs of trauma inflicted by sharp teeth or improperly fitting bits. The incisor arcades should be visually evaluated from the front and both sides. The occlusal surfaces should make good contact and be level. The horse’s age should be assessed by the dentition and compared with its actual age.47 In movement of the lower jaw from side to side, the normal slide and separation of the incisor arcades as the jaw moves through normal lateral excursion should be observed.
For the oral cavity to be examined in detail, the horse should be fitted with a loose-fitting halter. If the horse is fractious or resists examination, it should be sedated before proceeding with the oral examination. Sedation should be given only after the horse’s signalment (i.e., breed, age, body condition), temperament, and health (i.e., mucous membrane appearance, capillary refill time (CRT), heart rate, and rectal temperature) have been assessed.
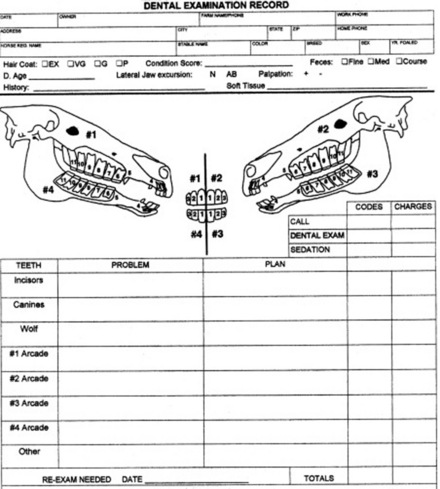
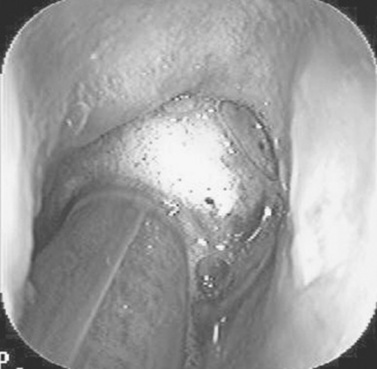
The mouth should be rinsed thoroughly with clean water. Trapped feed in the mouth should be removed manually or with a dental pick and irrigation. Horses with sharp buccal points on the upper dental arcade often resist application of a full-mouth speculum; therefore floating the upper arcades before applying the speculum may decrease resentment to the speculum. With a full-mouth speculum in place and the head properly restrained, a detailed visual and tactile oral examination should be carried out. A bright light source is necessary to illuminate the entire oral cavity for complete visual inspection. The examiner should manually inspect all hard and soft tissue in the oral cavity. The use of a dental mirror and pick is often necessary to see and probe the occlusal surfaces and pockets between the cheek teeth. Endoscopic examination of the oral cavity using a videoendoscope or an endoscope with a camera aids in identification of obscure lesions. Because rabies is a potential cause of dysphagia in the horse, the examined should have an adequate titer for rabies antibodies.
DENTAL RADIOLOGY
Diagnostic radiology is a valuable aid in the diagnosis of equine dental disease. The excellent contrast among air, bone, soft tissue and teeth provides good radiographic detail. Good-quality films can be obtained using a portable x-ray machine and rare-earth intensifying screens without a grid. Digital radiology provides high-quality radiographic images and allows for these high-quality images to be shared electronically for consultation with colleagues.
Indications for radiographic examination of the head include a suspicion of dental infection, maleruption, or a diastema and oral pain of unknown origin. The skull should be examined radiographically before and after dental extraction. Any facial swelling, deformity, neoplasm, trauma, or fracture may warrant radiographic evaluation to aid in diagnosis and treatment.
Radiographs can be taken with the horse standing and sedated. The head and radiographic cassette can each be placed on a stand to decrease distortion caused by motion. A lateral projection centered over the rostral edge of the facial crest should be taken to demonstrate fluid lines within the sinuses. The lateral view superimposes the dental arcades and should not be relied on to diagnose diseases involving the dental reserve crown and roots. Lesion-oriented oblique projections demonstrate the apical portion of the upper or lower cheek teeth and are helpful in diagnosing periapical dental disease. Open-mouth, oblique radiographic projections are beneficial in evaluating the exposed crown of the cheek teeth. Special, 4- × 8-inch, flexible dental cassettes can be used to obtain intraoral radiographic projections of the maxillary dental arcades. Dorsoventral radiographic projections centered over the tooth in question or area of concern can demonstrate periodontal disease on the buccal aspect of the upper cheek teeth or a large area of infundibular decay. Intraoral, occlusal radiographic projections are useful in demonstrating fractures of the incisors or other lesions rostral to the bars of the mouth. Other imaging techniques, such as ultrasonography, nuclear scintigraphy, CT, and MRI are helpful in the diagnosis of many oral and dental-related diseases.48
TREATMENT
A plan for treatment based on the results of history, clinical findings, and oral examination should be outlined to the owner and/or trainer before proceeding with a dental procedure. The owner and/or trainer should be informed of any abnormalities and given a plan for treatment, as well as an estimate of the cost, before any corrective procedure is performed.
Therapy must be planned to ensure that all equipment necessary to complete the task is at hand. The horse should be properly restrained, and adequate help should be present to assist in completing the procedure. A dental record aids in documenting findings during examination and the procedures performed (Fig. 32-17).
Routine Dental Maintenace
FLOATING
Dental floating is an age-old and routine method to correct abnormalities associated with dental eruption and occlusal wear. Floating also allows sculpting of the teeth to accommodate the bit. The main purpose of molar floating, or leveling, is to remove points or sharp edges from the buccal aspect of the upper molar arcades and lingual aspect of the lower molar arcades. Floating may also entail removing minor hooks or ramps from the rostral or caudal aspect of one or more arcades or leveling of minor elevations on the occlusal surface of the arcades. Routine floating and other corrective measures in the mouth may require both the added physical restraint provided by a dental halter and mild, chemical sedation.
Proper equipment is required to float all aspects of the exposed crowns of the cheek teeth, regardless of horse’s size. mall instruments are often needed to access the oral cavity of miniature horses or ponies, and extra long and heavy instruments are often required to work effectively in the mouth of the large warmblood or draft horses. Sharp float blades made of carbide chips or sharp tungsten carbide planing blades make the work of floating more efficient and less strenuous than in the past, when steel blades were used.
The outward curve of the upper arcade makes the central buccal area (i.e., the area involving premolar 3 [PM3] through molar 2 [M2]) the easiest to reach with the float. To reach all areas of PM2 and M3, the head of the float should be offset or angled. In most cases the lower arcade can be floated to remove the lingual enamel points using a flat, long-handled float. To remove rostral and caudal hooks, special equipment such as carbide planing blades, power burrs, sliding chisels, or single action molar cutters may be required. (Note: Using sliding chisels and molar cutters to remove a hook can result in serious damage to the tooth). The use of dental equipment requires special training and skill to prevent iatrogenic injury to the horse’s mouth.
The use of a mouth speculum or a dental wedge aids not only the oral examination, but also floating. (Note: The use of a dental wedge can result in serious damage to the teeth.) A mouth speculum ensures that the horse’s mouth remains open, increasing safety for both the horse and the examiner. For horses with slightly ramped back teeth caused by a greater than normal curvature of Spee, a mouth speculum and a slightly curved or swivel-headed float may be needed to float the occlusal surface of the last molar.
To increase a horse’s comfort while it wears a bit, the rostral aspect of the first upper and lower cheek teeth are sometimes rounded and the buccal cusps of the upper PM2s and PM3s are reduced. A horse that has received these procedures is said to have received a “bit seat.” In theory, a bit seat is created to prevent the soft tissue of the mouth from pressing against sharp points on the PM2s. To prevent exposing the pulp cavity when forming bit seats or correcting overgrowths, care should be taken not to “overfloat” the occlusal surface of a tooth. An offset float or an S-shaped rasp is usually necessary to create a bit seat.
WOLF TEETH
Most horses that are worked with a bit in the mouth benefit from having wolf teeth extracted. Some wolf teeth exfoliate naturally when the horse is approximately 3 years old, at the same time the cap of the first cheek tooth (i.e., 06 or PM2) is shed and the permanent tooth erupts. Although not all wolf teeth cause discomfort, a loose or sharp wolf tooth can be a distraction or even cause pain of such severity that it leads to bad bitting habits. Determining if the wolf teeth are responsible for a bitting problem is often difficult. Sometimes a wolf tooth does not erupt in a normal downward path, but instead migrates rostrally beneath the gum, causing a subgingival enlargement that irritates the horse. Such unerupted first premolars have been referred to as “blind” or “occult” wolf teeth and should be removed. Most wolf teeth are easily elevated with the horse sedated. Infiltrating a few milliliters of local anesthetic solution around the wolf teeth may aid in their removal.
CANINE TEETH
Canine teeth are present in most male horses over 4 years old. The unopposed positions of the canine teeth in the diastema make them unlikely to cause or develop problems. Some mares have small or rudimentary canines that can become loose or accumulate tartar, necessitating their removal. Canines of a stallion or gelding, especially canines that are long or sharp, can interfere with bitting and can be a nuisance or danger to the groomer or handler. Canines are more likely to accumulate tartar than are other teeth. Crowns of the canines can be ground 2 to 3 mm and polished to remedy any of these problems. Erupting canines in the 4- to 6-year-old horse may cause subgingival pain, especially when overlying tissue is contacted by the bit, which in turn may cause head shaking or other bad habits. The thin layer of tender mucosa over the canine should be removed. The root of a canine of the male is long and curved, making this tooth extremely difficult to extract. If a canine tooth must be removed because of injury or infection, it is usually removed by creating a bone flap over the root.
DENTAL CAPS
As the developing permanent premolar pushes to the surface, it presses on the roots of the worn down deciduous tooth, gradually cutting off that tooth’s nutrition. The deciduous tooth dies as its blood supply diminishes, causing it to become loose and displaced. The osseous alveolar walls adjust to these changes by producing and reabsorbing bone, to provide a new socket for the embedded portion of the newly formed permanent tooth. The remaining portion of the deciduous premolar has up to four “legs” (i.e., root slivers) that straddle the crown of the permanent tooth. If these slivers are fractured, they may remain embedded in the gingival tissue after the cap is shed, causing gingivitis or periodontal disease.
The eruption pattern of the permanent molarized dentition follows a sequence that predisposes to entrapment of the deciduous PM3 and PM4. Delayed shedding of deciduous premolars can predispose to gingivitis, periodontal irritation, or apical infection. Retained, split, or displaced deciduous premolars can be distracting to a young horse in training and have been implicated as a cause of intermittent dorsal displacement of the soft palate.
Retained caps can cause lingual displacement or delayed eruption of the permanent premolars. Horses with retained caps may develop bony enlargements called “eruption cysts,” on the ventral aspect of the horizontal ramus of the mandible or on the dorsal aspect of the maxillae, rostral to the facial crest. Often such swellings are benign, but if eruption is severely inhibited, blood-borne bacteria can colonize in the inflamed dental pulp, leading to anachoretic pulpitis and periapical dental infection, which lead to more severe facial or mandibular swelling. A draining tract often accompanies swellings caused by periapical infection, especially those on the mandible. Caps should be evaluated by palpation as well as visually. A crease or neck can be seen or felt just above the gumline at the juncture separating the deciduous and permanent tooth. Radiographic examination of the premolars may be necessary to identify retained caps.
If a cap has been shed, the caps that remain on the same tooth in the other three arcades should be considered retained and should be removed. By placing a molar forceps on the tooth and rotating the forceps lingually, the cap is easily removed. The cap should be extracted in a manner that ensures that the roots, especially the buccal roots, are removed, because slivers of roots of a retained cap are irritating and can predispose the horse to development of periodontal disease.
INCISORS
After the cheek teeth have been evaluated and treated, the horse’s mouth should be completely reexamined, both visually and by palpation, to ensure that all sharp or uneven edges have been smoothed and that no teeth have been broken or loosened during floating. The speculum is then removed, the mouth closed, and excursion of the jaw reevaluated to confirm that the mandible is capable of full excursion.
With the jaw in the resting state, normally only the incisors are in occlusion. The incisors should meet evenly and slide to the side unobstructed until the cheek teeth begin to contact. As the mandible moves laterally, the upper and lower arcades contact, and the incisors are lifted apart as the mandibular cheek teeth slide up the sloped occlusal surface of the upper cheek teeth. Uneven or excessively long incisors may need to be aligned or reduced. Minor incisor leveling can be performed with a flat carbide float or a motorized burr. Care should be taken not to reduce the crown to the extent that that the pulp is damaged. When floating has been completed, the horse should have a full, comfortable range of motion of the jaw. As the horse chews, the upper and lower dental arcades should be in proper contact with each other.
EQUINE DENTAL DEVELOPMENTAL ABNORMALITIES
Equine dental developmental abnormalities can involve tooth number, morphology, or position in the dental arcades. Abnormalities of dental development and eruption occur quite commonly in the horse and result in a wide range of clinical conditions. Some developmental abnormalities of the teeth of a young horse may not cause the horse to exhibit clinical signs of dental disease until the horse reaches middle age. A congenital or developmental problem present at the time of tooth eruption often leads to acquired dental problems as the teeth continue to erupt and wear. Consequently, several different dental abnormalities, the origins of which are interrelated, are often present by the time the horse is presented because of signs of dental disease. A detailed oral examination should include checking for the proper number and position of teeth. Because only the exposed crown of a tooth can be visualized in the oral cavity, many developmental defects may not be recognized simply by oral examination. If a developmental abnormality is suspected, the dentition should be examined radiographically to further delineate abnormalities.
Supernumerary Teeth
Supernumerary teeth are teeth in excess of the normal, expected number in any of the dental arches. This disorder has been referred to as polydontia or hyperdentition. Supernumerary teeth can be loosely categorized morphologically into two categories: (1) supplemental teeth that resemble teeth of the normal series in crown and root morphology but not always in size, and (2) rudimentary or dysmorphic teeth that are abnormally shaped and smaller than normal teeth.
These extra teeth are usually encountered at the caudal aspects of the arcades, but supernumerary teeth can also occur lingually, buccally, or rostrally to the arcades. Clinical signs caused by supernumerary cheek teeth are most commonly associated with dental overgrowths and diastemata, which often cause periodontal disease. Examination of a radiograph that encompasses the entire affected dental arcade is often necessary to recognize a supernumerary tooth.
Supernumerary incisors are reported more commonly in horses than are supernumerary cheek teeth. The main differential diagnosis for supernumerary incisors or cheek teeth is retained deciduous teeth, and in some cases, determining whether an extra tooth is a retained deciduous tooth or a supernumerary tooth is difficult. Radiographic examination of the affected jaw may be indicated to determine the identity of an extra incisor. A retained deciduous incisor has a more mature root and a shorter reserve crown than those of the adjacent permanent incisors.
Management of horses with supernumerary teeth is generally limited to regular assessment of the dentition, coupled with aggressive floating to minimize the opportunity for soft-tissue damage caused by unopposed dental elongations or sharp enamel points. If complications occur, such as severe periodontal disease or paranasal sinusitis, the supernumerary tooth or displaced adjacent tooth should be extracted, and appropriate therapy undertaken to manage associated disease.
Oligodontia
Oligodontia is the condition in which the number of teeth is less than normal. Oligodontia can be caused by congenital absence of a tooth germ or by traumatic loss of a tooth. Absence of a tooth in the dental arcade, regardless of the cause, leads to dental drift, or tipping of adjacent teeth. Lack of wear of the antagonist to the missing tooth can lead to dental elongations and abnormal mastication. Radiographic examination of the dentition is often necessary to confirm a diagnosis of oligodontia. Oligodontia may be associated with other epidermal defects, such as faulty development of hair and hooves.
Dental Dysplasia or Hypoplasia
Dental dysplasia (i.e., abnormal growth and/or development of a tooth or teeth) may result in an irregularly shaped tooth that does not fit properly into a dental arch. The poor fit may lead to entrapment of food and periodontal disease. Enamel hypoplasia can be caused by certain drugs or chemicals administered to the dam during gestation, or it may be idiopathic. Dental dysplasia can involve the abnormal formation of all tissues of the tooth or only a single tissue. When enamel is dysplastic, however, the other calcified tissues of the teeth, cementum and dentine, also become dysplastic because enamel acts as the scaffolding and template for their deposition. Abnormal morphology of enamel has been associated with branched pulp horns and abnormally shaped teeth. Cemental hypoplasia usually involves the infundibular portion of the tooth but can be seen as a defect of the peripheral cement of the coronal or reserve crown or the roots.
Abnormal Dental Eruption
Abnormal dental eruption, or maleruption, is often seen after trauma to developing teeth or surrounding bones but has also been reported to be congenital or idiopathic. Cheek teeth can become vertically impacted when dental buds develop in crowded areas in the dental arcades. Teeth may become rotated or displaced because of developmental malpositioning of tooth buds or overcrowding before, during, or after eruption.
Dixon and colleagues reported that 70% of displacements of cheek teeth were developmental and caused by overcrowding of the cheek tooth arcade at the time of eruption. These researchers frequently found that if a cheek tooth was displaced in one arcade, the same tooth in the contralateral arcade was also displaced. They concluded that the remaining 30% of displacements were caused by abnormal positioning of the dental bud.44 They found that a tooth might fail to erupt if it is displaced horizontally to the adjacent teeth. Developmental diastemata, or abnormal spaces or gaps between cheek teeth, are often the result of insufficient angulation of rostrally and caudally located teeth toward the center of the arcade to achieve good compression of adjacent teeth. Dental buds with normal angulation that develop too far apart can also result in diastemata.
Malocclusion of incisors can be congenital, developmental, or acquired. Mandibular brachygnathism (i.e., parrot mouth or overjet) is a congenital incisor malocclusion, the origin of which is usually genetic. Many horses have some degree of overjet of the premaxillary incisors, but the overjet rarely causes a problem with prehension unless the premaxillary and mandibular incisors totally lack occlusion. If brachygnathism is discovered when the foal is young, orthodontic treatment may correct or at least improve the condition.
A main consideration with an incisor overjet in the adult horse is the malocclusions of the cheek teeth that accompany this condition. The maxillary cheek teeth arcades of horses with an overjet are usually positioned rostral to the mandibular cheek teeth arcades, causing a rostral overgrowth of the upper PM2s (i.e., 106 and 206) and a caudal overgrowth of the lower M3s (i.e., 311 and 411). These overgrowths must be reduced to allow proper lateral excursion of the jaw and mastication.
Prognathism (i.e., sow mouth or undershot jaw) occurs with less frequency in the horse and is seen most commonly in miniature or dwarf breeds. Early detection and correction of the malocclusion in the foal may prevent the condition from worsening. The cheek teeth of a horse with prognathism should be evaluated for malocclusion caused by overgrowth of the upper M3s (i.e., 111 and 211) and the lower PM2s (i.e., 306 and 406).
Bony malformation or curvature of the skull can result in malocclusion of both the incisors and the cheek teeth. The most common malocclusion caused by bony malformation of the skull is an offset or diagonal incisor bite. Some bony malformations, such as campylorrhinus lateralis (i.e., wry nose), are obvious, but subtle changes to the large bony plates in the head can be difficult to recognize.
Dental overgrowths associated with malocclusions should be corrected gradually to prevent dysphagia and pain caused by inadvertent exposure of pulp horns. Often, incisor malocclusion cannot be completely resolved, but regular maintenance may prevent it from worsening.
DENTAL DISEASE
Dental disease is grouped into four basic types: abnormal occlusal wear pattern, periodontal disease, dental caries, and disease of the dental pulp. All of these types of basic dental disease are interrelated, and horses with one of these types of disease also have, to varying degrees, the other types of disease.
Proper alignment of the dental arcades is critical to the normal wear of the dentition. Historically, abnormal dental wear patterns have been described as elongations of the crown, descriptive terms for which include hooks, ramps, waves, step mouth, tall teeth, and excessively high transverse ridges.49 Elongations are usually found on a normal tooth that opposes an abnormal tooth in the opposite arcade, such as a damaged, misplaced, or missing tooth. This abnormal tooth, as well as the elongation, should be evaluated. Most elongations are reduced with float or a grinding instrument. When reducing an elongation, care should be exercised not to cause iatrogenic damage to the tooth, such as pulpal exposure, thermal injury to the pulp or fracture of the crown.
Periodontal disease is often a painful dental condition and is described as the leading cause of “quidding” in horses. A mild form of periodontal disease primarily affects young horses, 2½ to 5 years old, that are shedding deciduous teeth (caps) and erupting permanent incisors and premolars. A more severe and progressive form of periodontal disease is seen in mature horses and is the result of the chronic effects of diastemata, or spaces that develop between teeth that are not aligned properly in the arcade. Diastemata promote periodontal disease by allowing feed to become trapped between teeth. Diastemata can form secondary to developmental disease, such as when dental buds are spaced too far apart or are abnormally angulated so that the teeth in the dental arcade are not properly compressed. This is usually a progressive condition that when left untreated worsens. Spaces between teeth or teeth out of alignment also predispose to abnormal wear patterns on the affected and opposing dental arcades.
Treatment of horses with periodontal disease involves treating both the cause and the effects of the disease. Periodontal disease caused by feed trapped at the gingival margin of a diastema often improves after the diastema is thoroughly cleansed using dental picks and/or irrigation. Correction of any associated abnormal wear pattern is also indicated. In more refractory cases, opening or widening of the diastema with a special burr or a right angle grinder may be necessary. This procedure may allow the horse’s masticatory actions to more easily channel food in and out of the diastema, thus preventing or reducing entrapment, stagnation, and decay of feed.
The severity of periodontal disease can be decreased using high-pressure irrigation to clean periodontal pockets. After the pockets have been irrigated, the diastema is packed with a perioceutic agent such as doxycycline gel or a powdered antibiotic. This technique places a high concentration of antibiotic in contact with the infected and inflamed tissues, and the packing acts as a temporary barrier to recontamination. This form of therapy may need to be repeated regularly to produce long-term, positive results.
Caries usually involve the cemental layer of the tooth. Peripheral cemental caries is seen secondary to periodontal disease. The most common type of cemental caries involves the infundibular portion of the incisors or maxillary cheek teeth (Fig. 32-18). The incisors have one infundibulum and the upper cheek teeth have two, and each infundibulum has a cup, or open portion, at the occlusal surface. The occlusal surface of the tooth is covered with cementum for several months after the tooth erupts, and as the tooth wears, the cementum-filled infundibulum is exposed at the occlusal surface. Some degree of decay is always present at the occlusal surface of an infundibulum, because feed and other products of mastication are compressed into the ghost of the vascular canal.
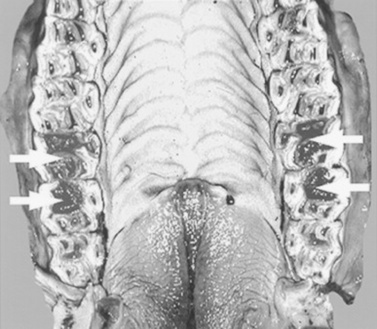
Fig. 32-18 Cadaver specimen of the maxillary dental arcade. Note the black areas of dental decay on the occlusal surfaces of M1 and M2 (arrows).
An infundibulum contains dead cementum that is completely encased in a layer of enamel, which prevents the infundibular caries from causing widespread inflammation or infection. True dental pulp infection secondary to infundibular caries occurs only if caries penetrate this protective enamel layer. Developmental malformations of the infundibulum may weaken this enamel barrier, predisposing the dentine and pulp to exposure from infundibular caries. Infundibular caries may weaken a tooth whose infundibula are congenitally deformed or have an abnormally large vascular channel, predisposing the tooth to excessive attrition or midsagittal fracture of the crown.
Infundibular caries is usually innocuous but can predispose to dental elongations on the opposing arcades (e.g., wave mouth,Fig. 32-19). These abnormal wear patterns should be reduced regularly. To strengthen the tooth and delay the progression of infundibular caries, abnormally large infundibular vascular channels can be cleaned, partially packed, and sealed with a dental composite material. As advanced diagnostic methods, such as CT, become more readily available to the equine practitioner, these abnormal infundibula can be more easily recognized.
Infection of the dental pulp occurs primarily in horses 4 to 10 years old. Horses with infection of the pulp are presented because of clinical signs of associated inflammatory changes at the apical region of the tooth. The clinical signs of pulpal infection vary and depend on the involvement of structures adjacent to the apex of the affected tooth. Horses with infection of one of the lower first four cheek teeth develop swelling on the ventral aspect of the mandible over the apex of the affected tooth, and within this swelling, a draining tract usually develops. The last two lower cheek teeth are embedded in the portion of the mandible surrounded by the large muscles of mastication, so infection of one of these teeth causes the surrounding muscles to swell. Exudate accumulated between the mandible and musculature may be seen during ultrasonographic evaluation of the soft tissues of the mandible.
The apices of the upper two or three cheek teeth are closely associated with the facial bones; therefore when one of these teeth becomes infected, facial swelling usually results. The apices of the caudal three or four upper cheek teeth reside within the maxillary sinuses; when one of these teeth becomes infected, purulent nasal discharge caused by secondary sinusitis usually results.
Because pulpal infection destroys the tissue responsible for the production of secondary dentin, the pulp horns and root canals of the affected teeth fail to fill with dentine as they normally would. In the later stages of pulpal infection, the affected tooth usually exhibits some degree of decay at the site of the pulp horn on the occlusal surface. This weakened, decayed area may predispose to fracture of the crown.
Administration of antimicrobial drugs has been successful in the treatment of horses with apical dental infection in its early stages, but the most common treatment of horses with an apically infected tooth is removal of the affected tooth and treatment of associated bone or sinus infection. Teeth can be removed by one of three methods: extraction via an oral approach, repulsion via an apical approach, or elevation via a lateral buccotomy approach.
SALIVARY GLANDS AND DUCTS
Saliva hydrates and lubricates the oral cavity, facilitates swallowing, prevents tooth demineralization, and regulates oral microbial flora.50 Diseases of the salivary glands of horses are uncommon but include sialoadenitis, salivary calculi, salivary mucocele, trauma and neoplasia. During oral examination the openings of the salivary ducts should be noted. The ducts of the paired parotid salivary glands enter the mouth at the parotid papillae, located next to the upper last premolars (i.e., 109 and 209). The ducts of the paired mandibular salivary glands open into the mouth on the lateral aspect of the sublingual caruncles. The ducts (approximately 30 in number) of the paired sublingual salivary glands are seen as small pores in the sublingual recess.
Slobbering or drooling may indicate excessive production of saliva (i.e., ptyalism) or accumulation of saliva in the mouth from dysphagia. Heavy metal toxicity, poisoning with a parasympathomimetic agent, neurologic disease, or stomatitis may cause ptyalism. Dysphagia can be caused by esophageal obstruction (choke), an oral foreign body, or some neurologic diseases, such as rabies.
Sialoadenitis, or inflammation of a salivary gland, may be the result of obstruction of a salivary duct from accumulated exudate or mucus, feed particles such as grass awns, an orally introduced foreign body, or a sialolith. Sialoliths occur infrequently but are found almost exclusively in the parotid salivary duct. Obstruction of the duct with a sialolith causes salivary retention, which can lead to glandular atrophy or acute sialoadenitis, swelling of the gland, and rupture of the duct. A sialolith causes a hard, usually smooth enlargement at some point along the course of the parotid salivary duct. The horse usually shows no signs of pain when the enlargement is palpated. The sialolith is usually apparent during radiographic evaluation of the skull, but it can be obscured by adjacent bone. Diagnosing the presence of a sialolith is usually straightforward because the condition is easily differentiated from other conditions that produce similar clinical signs, such as trauma, apical dental infection, or facial tumor. Surgical removal of the sialolith and primary closure of the duct and surrounding tissue usually yield good results.
A salivary mucocele is an accumulation of salivary secretions in a single or multiloculated cavity adjacent to a ruptured salivary duct. A ranula is type of mucocele that develops secondary to obstruction of a sublingual salivary duct. Treatment of horses with a mucocele consists of creating a salivary fistula into the oral cavity or excising the mucocele and destroying salivary gland, either with a chemical inserted into the duct or by ligation of the duct.
Laceration or iatrogenic injury to a salivary duct can lead to a salivary cutaneous fistula. The fistula can be resolved by reapposing the severed duct with sutures, by creating a new oral opening for the duct, or by destroying the salivary gland. The parotid salivary gland can be ablated by flushing a solution of 10% formalin into the duct or by ligating the duct proximal to the fistula. Wounds involving the salivary glands can usually be resolved successfully by cleansing, debriding, and suturing the wound.
Primary neoplasms of the salivary glands of horses include benign mixed tumors, adenocarcinomas, and acinar cell tumors. Neoplasms that invade the salivary gland from an adjacent area or melanomas of old grey horses that metastasize to the salivary gland are more common than primary salivary neoplas
EQUINE ORAL NEOPLASMS
Equine oral neoplasms are rare, making up a very small percentage of facial or mandibular neoplasms. Oral neoplasms can be divided into three basic types: odontogenic, osteogenic, and secondary (soft tissue).51
Odontogenic neoplasms are derived from remnants of dental epithelium. The five types that have been reported in the oral cavity of horses are ameloblastomas, ameloblastic odontomas, complex odontomas, compound odontomas, and cementomas. Histologic examination of odontogenic neoplasms can be confusing because of variation in appearance of tissue obtained at different sampling sites and age-related changes in the neoplasm’s appearance. Because of their rarity, ill-defined biologic behavior, poorly defined radiographic features, and histologic variations, odontogenic neoplasms can be difficult to classify.
Primary bone neoplasms of horses are rare, and most (osteoma, ossifying fibromyoma) are benign. More than 80% of equine osteosarcomas occur in the head region. Like odontogenic neoplasms, primary bone neoplasms of horses are difficult to classify. In addition to gross and histologic examination, correlating history and clinical, radiologic, and biochemical findings is often essential to establish a diagnosis.
Secondary neoplasms of the oral cavity include squamous cell carcinoma, lymphosarcomas, papilloma, and melanomas that have extended into the oral cavity from an adjacent site or metastasized into the oral cavity from a remote site. Squamous cell carcinoma is the most frequently reported oral neoplasm in the horse. Generally these neoplasms are seen in old horses; there is no gender or breed predilection. Neoplasms of the tongue are rare and include lymphosarcomas, multiple myeloma, rhabdomyosarcoma, and paraneoplastic bullous stomatitis.
Options available to control progression of oral neoplasms include radiotherapy, hyperthermia, chemotherapy, cryosurgery, immunotherapy, autogenous vaccines, photodynamic therapy, laser therapy, and surgical resection. Results of these treatments vary according to the type of tumor and circumstances, such as whether the neoplasm has invaded bone.
ANATOMIC AND PHYSIOLOGIC CONSIDERATIONS
The most cranial aspect of the esophagus is located on the median plane immediately dorsal to the trachea. However, at approximately the midcervical region (C4 to C5), the esophagus typically shifts to the left of the trachea and lies just deep to the external jugular vein.52,53 It is here that intraluminal obstructions or the tip of a stomach tube may be visualized, and this is the region where trauma can readily result in esophageal perforation. When a stomach tube is passed, it is critical that the tube be palpated to ensure the tube is in the esophagus because jugular pulses can be confused with the appearance of the tip of the tube. In addition to its proximity to the external jugular vein, the esophagus is also located adjacent to the vagosympathetic trunk and the common carotid artery.52 The esophagus is innervated by branches of the vagosympathetic trunk, and blood is supplied to the cervical esophagus by branches of the carotid arteries. The thoracic esophagus, which lies ventral to the trachea until the tracheal bifurcation, where it resumes a dorsal position, receives its blood supply from the bronchoesophageal artery. Venous drainage is via the external jugular veins in the cervical esophagus and the esophageal vein in the thoracic esophagus.
The muscular wall of the esophagus increases in thickness as the esophagus courses distally, while the lumen gets smaller.52 The esophagus is not covered by a serosa except for a very short segment that traverses the abdominal cavity between the diaphragm and the stomach.53 Instead, the outer wall of the esophagus is composed of adventitia that is loosely attached to surrounding tissues. This loose connection allows movement of the esophagus during swallowing and during movement of the neck. The cranial two-thirds of the esophageal wall consists of skeletal muscle, whereas the distal third of the esophagus is composed of smooth muscle. Although the muscular layers are composed of an outer and an inner layer, similar to the remainder of the gastrointestinal tract, the skeletal muscle layers are oriented obliquely to one another.52 This, and an abundant submucosa, enables extensive dilation of the esophagus as a bolus of food moves toward the stomach. In addition, the velocity of esophageal contraction is faster in the skeletal muscle segment of the esophagus compared with the distal smooth muscle segment.54 The muscle layers become oriented in more of an outer longitudinal and inner circular configuration in the caudal esophagus.52 In the resting collapsed state, redundant esophageal mucosa and submucosa become oriented in longitudinal folds. The mucosa is composed of stratified squamous epithelium that is continuous with the stratified epithelium of the cardiac portion of the stomach.52
The cranial esophageal sphincter is formed by the cricopharyngeus muscle and maintains a resting intraesophageal pressure of approximately 85 mm Hg and a postdeglutition pressure as high as 200 mm Hg. Although the caudal esophageal sphincter is anatomically indistinct, resting intraesophageal pressure in this region is maintained at approximately 13 mm Hg, and postdeglutition pressure in the caudal esophagus may be as high as 100 mm Hg. The pressure in the caudal esophagus is maintained at approximately 10 mm Hg higher than the intraluminal pressure of the stomach.54,55 Although the higher pressure in the distal esophagus has been implicated as the cause for the inability of most horses to vomit and for gastric rupture, other factors such as a poorly developed vomiting reflex may be more important.56
DIAGNOSTIC CONSIDERATIONS
Esophageal disease should be a differential diagnosis in any horse that demonstrates excessive salivation. Such signs also indicate the need to assess hydration, electrolyte levels, and acid-base status. In a study in which horses had continual loss of saliva via an experimentally placed esophagotomy, abnormalities included hypochloremia, hyponatremia, and hypokalemia.57 This results from the relatively high levels of these electrolytes in saliva. Furthermore, because horses depend on dietary intake of potassium, hypokalemia would be exacerbated in a horse that was also unable to eat because of esophageal obstruction. Loss of salivary fluid and bicarbonate also results in dehydration and metabolic acidosis. However, metabolic alkalosis subsequently occurs presumably as a result of renal compensation for electrolyte loss, particularly chloride.57
Further examination of horses with esophageal disease may reveal evidence of swelling or emphysema in the region of the cranial or cervical esophagus that should prompt a thorough oral examination and further diagnostics such as radiography and endoscopy to define the nature of any esophageal abnormalities. If the esophagus has been perforated or ruptured, subcutaneous emphysema is usually evident. The lungs should be carefully auscultated for evidence of aspiration pneumonia. Radiographs of the chest are required for a full pulmonary assessment.
Radiographs of the esophagus should initially include plain films that may reveal evidence of an obstruction or areas of gas opacity within facial planes indicative of esophageal perforation.58 However, facial and subcutaneous emphysema must be differentiated from other causes, including tracheal perforation.56 The esophagus is often gas-distended cranial to an obstruction up to the cranial esophageal sphincter. Plain films may be diagnostic, but contrast radiographs are frequently required to fully define the nature of esophageal abnormalities.58 Administration of barium paste or liquid will reveal linear opacifications as a result of the linear mucosal folds and may help outline intraluminal obstructions or strictures (Fig. 32-20).58 A double-contrast study is a useful radiographic technique for defining esophageal wall abnormalities, particularly postobstruction mucosal ulceration, and is performed by placing a cuffed nasogastric tube in the cranial esophagus and injecting 300 to 500 mL of liquid barium followed by a similar volume of air. Care should be taken when evaluating such radiographs, because swallowing can create the false impression that there is a stricture.59 The incidence of swallowing can be decreased by administration of xylazine. Liquid barium is preferable if swallowing function is compromised because it is less harmful to pulmonary tissues than paste, and water-soluble iodinated contrast material is particularly damaging to the lung because of its hypertonicity.56 However, if an esophageal perforation is suspected, water-soluble contrast material is preferable.56
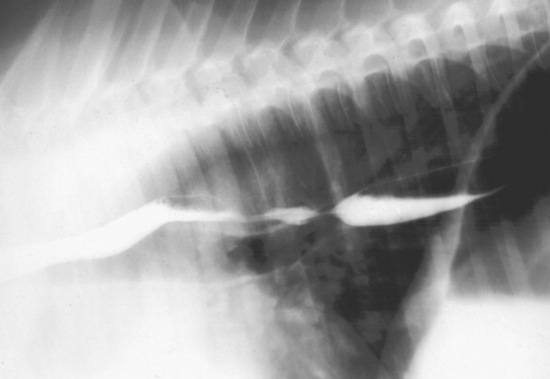
Fig. 32-20 Barium contrast esophagram outlining esophageal luminal structure.
Courtesy Dr. K.E. Sullins.
Endoscopic evaluation of the esophagus should be performed as part of a complete evaluation of esophageal injuries and abnormalities. After sedation of the patient, the endoscope should be passed all the way into the stomach before the esophagus is examined; it will be more readily viewed as the endoscope is withdrawn.58 Inflation of the esophagus must also be performed intermittently as the wall of the esophagus collapses around the end of the endoscope. The longitudinal folds of the esophageal mucosa will be readily appreciated and can be flattened out as the esophagus is distended to more clearly view the entire circumference of the esophagus. Swallowing may create artifacts such as the appearance of strictures, so the esophagus should be carefully reinflated after each swallow to carefully evaluate such findings.59
A recent study evaluated structures that could be evaluated via thoracoscopy of the mediastinum, including the thoracic component of the esophagus. Although this procedure caused pneumothorax, a 15-minute period of evaluation allowed observation of a number of structures including the esophagus.60 Portals placed at the eighth, tenth, and twelfth intercostal spaces were useful for completion of this procedure and could provide additional information aside from radiographic and endoscopic evaluation of the thoracic esophagus.
ESOPHAGEAL OBSTRUCTION
Esophageal obstruction, either primary (simple choke) or secondary to other disease processes, is the most common esophageal disorder seen in horses. Although primary obstructions may be caused by foreign bodies, including corncobs, potatoes, apples, carrots, medicinal boluses, stones, riding crops, or wood fragments, primary obstructions are most often caused by roughage, particularly leafy alfalfa hay, coarse grass hay, bedding, and even grass.61-71 Prior esophageal trauma or poor mastication caused by dental abnormalities may predispose horses to esophageal impaction. Obstructions from roughage may be precipitated by wolfing or gulping food, particularly if the horse is exhausted and/or mildly dehydrated such as after a long ride, or weakened from chronic debilitation. Secondary impactions are caused by intramural or extramural abnormalities that mechanically impede food passage. Examples of intramural obstructions include tumors (squamous cell carcinomas), strictures, diverticula, cysts, and vascular ring anomalies.72-78 Mediastinal or cervical masses (tumors or abscesses) may cause extramural obstructions.
The clinical signs associated with esophageal obstructions are similar whether they are classified as primary or secondary and are rarely specific. Horses with esophageal obstruction are often anxious and stand with the neck extended. Gagging or retching may be noted, particularly with acute proximal obstructions. Bilateral frothy nasal discharge containing saliva and food material, coughing, odynophagia, ptyalism, and dysphagia are usually the primary clinical signs, the severity of which varies with the degree and location of the obstruction. Distention of the cervical esophagus may be evident at the site of obstruction. Other clinical signs may be observed related to complications stemming from the obstruction, such as dehydration, weight loss, aspiration pneumonia, or esophageal rupture.
Thorough physical examination, including a complete oral examination, must be performed to rule out other causes of hypersalivation, dysphagia, and nasal discharge. Palpation of the jugular furrow may reveal a mass associated with the impaction. In most horses the esophagus is located in the left jugular furrow, but it may be found in the right furrow in some animals. Crepitus or cellulitis may be evident, suggesting rupture of the esophagus. Auscultation of the lungs is important to determine whether pneumonia or pleural fluid is present because of aspiration or intrathoracic esophageal rupture. Passage of a nasogastric tube is a good way to determine whether and where an obstruction is present but provides little information about the nature of the obstruction or the condition of the esophagus.
Ultrasonography of the cervical region is extremely useful not only to confirm the presence of a cervical esophageal impaction, but also to provide critical information about the location and extent of the impaction and esophageal wall thickness and integrity. Ultrasonography may also provide information about the cause of the obstruction. Radiography, particularly air or barium contrast studies, may be useful to assess an esophageal impaction but may be more useful for evaluating the esophagus after rather than before relief of the impaction to demonstrate stricture, dilation, diverticula, esophageal rupture, or masses.79,80 Care should be taken when interpreting radiographic studies in sedated horses, particularly after passage of a nasogastric tube or other esophageal manipulations that may contribute to esophageal dilation.81 Impacted food material can be detected in the esophagus by a typical granular pattern, and gas is often observed to accumulate proximal to the obstruction. Foreign bodies may be identified by contrast radiographic studies.
Definitive evaluation of esophageal obstructions often requires endoscopic examination. Most cases of esophageal obstruction occur at sites of natural narrowing of the esophageal lumen, such as the cervical esophagus, the thoracic inlet, the base of the heart, or the terminal esophagus. Therefore an endoscope longer than 1 meter may be required for complete evaluation. Endoscopic evaluation is useful before relief of an impaction to localize the impaction and to investigate the nature of the impaction if a foreign body is suspected. Foreign bodies may be retrievable via transendoscopic tethering.69 Critical diagnostic and prognostic information is obtained after resolution of the impaction to determine whether mucosal ulceration, esophageal rupture, masses, or strictures are present.
The primary goal of treatment for esophageal impaction is to relieve the obstruction. Parenteral administration of acepromazine (0.05 mg/kg IV), xylazine (0.25 to 0.5 mg/kg IV) or detomidine (0.01 to 0.02 mg/kg IV), oxytocin (0.11 to 0.22 IU/kg IM), and/or esophageal instillation of lidocaine (30 to 60 mL of 1% lidocaine) may help reduce esophageal spasms resulting from pain or increased esophageal tone.66,81-83 However, recent conclusive studies revealed that detomidine, acepromazine, or a combination of xylazine and butorphanol had the greatest effect on esophageal motility when evaluated by a monomer in conscious horses.84 However, in vitro studies revealed a relaxant effect of oxytocin on esophageal muscle, suggesting it may be useful for relief of esophageal obstruction.85
Resolution of an impaction may require physical dispersal of the material. A nasogastric tube can be used to displace the impacted material in conjunction with external massage if the obstruction is in the cervical region. Often it is necessary to carefully lavage the esophagus with water via an uncuffed or a cuffed nasogastric tube while the head is lowered to aid in breaking up the impaction. Some clinicians advocate a dual tube method whereby a tube is placed through each nasal passage into the esophagus for ingress and egress of the lavage fluid. Because of the risk of aspiration of water and/or food material, esophageal lavagfe is sometimes done under general anesthesia with a cuffed nasotracheal tube.
In refractory cases, intravenous administration of isotonic fluid containing 0.9% NaCl and KCl (10 to 20 mEq/L) for 24 hours at a rate of 50 to 100 mL/kg/day in conjunction with esophageal relaxants such as oxytocin may promote hydration and softening of the impaction and will help prevent or alleviate any electrolyte or acid-base imbalances resulting from salivary losses of chloride, sodium, and potassium. Refractory cases may require esophagotomy to relieve the impaction. Strict restriction of access to food and water, including access to bedding material, must be enforced until the obstruction is resolved and the esophagus has regained function.
Dilation proximal to the site of obstruction, mucosal injury from trauma, and esophagitis are sequelae to esophageal impaction that predispose patients to reobstruction. The rate of reobstruction may be as high as 37%. Depending on the duration of the obstruction and the degree of trauma or dilation, the risk of reobstruction is high for 24 to 48 hours or longer; therefore food should be withheld for at least 24 to 48 hours after resolution of the obstruction. After 48 to 72 hours or when the esophageal mucosa has recovered as assessed by endoscopy, soft food (moistened pellets and bran mashes) can be fed. The patient can be gradually returned to a high-quality roughage diet over a period of 7 to 21 days depending on the degree of esophageal damage induced by the impaction and the nature of any underlying disease. The prognosis for survival is good (78%), but some horses may require permanent dietary modification if persistent chronic obstruction is a problem.68
Complications of esophageal impaction include metabolic alkalosis from prolonged loss of salivary chloride and sodium, esophageal ulceration, stricture, perforation, aspiration pneumonia, and megaesophagus. Esophageal endoscopy and/or ultrasonography should be performed immediately after the impaction is relieved to determine whether any complications of the impaction have developed or if an inciting cause of the obstruction is present. Endoscopic evaluation is critical to determine the postobstruction treatment and followup. Reevaluation should be performed intermittently every 2 to 4 weeks after resolution of the impaction if esophageal dilatation or mucosal injury is noted. Additional evaluation via radiography may be warranted to assess motility and transit times.
If the obstruction was present for 48 hours or longer, dehydration, hyponatremia, hypochloremia, and hypokalemia may occur and should be corrected via oral electrolyte solutions or intravenous administration of 0.9% NaCl and KCl (10 to 20 mEq/L). If aspiration is suspected, administration of broad-spectrum antibiotics that are effective against gram-positive and gram-negative organisms, including metronidazole for anaerobes, is advisable. Sucralfate (20 mg/kg orally [PO] q6h) may hasten healing if esophageal ulceration is evident, but this is controversial. Some clinicians suggest that administration of a nonsteroidal antiinflammatory drug such as flunixin meglumine (1 mg/kg PO or IV q12h) or phenylbutazone (1.1 mg/kg PO or IV q12h) for 2 to 4 weeks after resolution of the impaction may reduce the development of strictures.
ESOPHAGITIS
Inflammation occurs during many conditions of the esophagus. Esophagitis refers to a clinical syndrome of esophageal inflammation, which may or may not be ulcerative. Causes of esophagitis in horses include trauma (foreign bodies, nasogastric tube), infection (mural abscesses), and chemical injury (medicines, cantharidin).86,87 An important category of esophagitis is reflux esophagitis, caused by reflux or delayed clearing of gastric contents into the distal esophagus and subsequent chemical injury to the mucosa (Fig. 32-21). Similar to ulceration of the squamous portion of the stomach in horses, a major cause of ulcerative esophagitis is epithelial damage resulting from exposure to acid, which is synergistically exacerbated by bile salts.62,88 The major protective mechanisms of the esophageal mucosa include salivary and food material buffers, normal peristaltic motility, and the barrier formed by the gastroesophageal sphincter. Thus, esophagitis may be seen in conjunction with gastric ulcer disease, motility disorders, increased gastric volume from gastric outflow obstructions, gastric paresis, intestinal ileus, or impaired lower esophageal sphincter function.
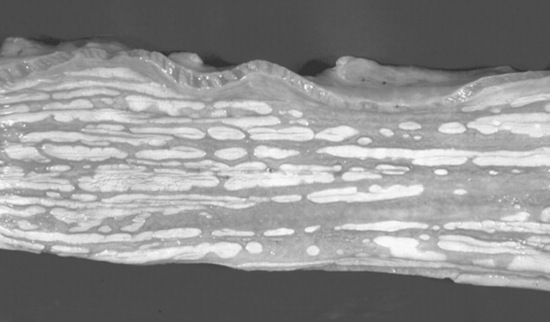
Fig. 32-21 Severe ulceration of the esophagus in a weanling foal that had severe duodenitis and gastric outflow obstruction.
Courtesy Dr. M.J. Murray.
The clinical signs of esophagitis are nonspecific and similar to those of esophageal obstruction and gastric ulcer disease. In fact, esophagitis may occur concurrently with esophageal obstruction or gastric ulcer disease, so clinical signs may overlap extensively with these diseases. Gagging, or discomfort when swallowing, may be evident, and hypersalivation and bruxism are signs of esophageal pain. Partial or complete anorexia may be noted such that horses with chronic esophagitis may have significant weight loss. Motility dysfunction secondary to esophagitis may cause recurrent esophageal impaction. Clinical signs of underlying disease that predispose to esophagitis may predominate or mask the signs of esophagitis. Horses with gastrointestinal motility disorders such as anterior enteritis are at high risk for developing reflux esophagitis because of the presence of both gastric acid and bile salts in the fluid reflux. However, signs attributable to esophagitis secondary to ileus may not be noted because of the profound signs caused by the intestinal disorder. Foals with gastric outflow obstructions commonly have reflux esophagitis.
Diagnosis requires endoscopic examination of the esophagus. Diffuse, patchy, linear, or coalescing erosion or ulcerations may be noted. Significant edema or hyperemia may also be observed. It is important to determine whether underlying disease, such as infection, neoplasia, diverticula, or esophageal stricture is present. In addition, the stomach must be examined because reflux esophagitis is commonly accompanied by gastritis or gastric ulcer disease. Contrast radiography may be helpful if endoscopy is not available to detect esophageal ulceration and can be used to assess esophageal motility and transit time.
The principles of therapy for reflux esophagitis include control of gastric acidity and correction of any underlying disorder that is contributing to gastroesophageal reflux. Thus, treatment with histamine-2 (H2)–receptor antagonists such as ranitidine or proton pump antagonists such as omeprazole is important for resolution of the disease. Some clinicians advocate sucralfate administration to aid healing of esophageal ulcers. However, the efficiency of sucralfate binding to ulcerated mucosa in the squamous epithelium of the gastrointestinal tract has recently been brought into question.
Foals with reflux esophagitis secondary to delayed gastric outflow caused by gastroduodenal ulcer disease or gastric paresis may benefit from prokinetic drugs that act on the proximal gastrointestinal tract. Metoclopramide (0.02 to 0.1 mg/kg SC q4-12h) reduces gastroesophageal reflux by increasing lower esophageal sphincter tone, gastric emptying, and gastroduodenal coordination. Caution should be exercised when giving metoclopramide to horses because they are prone to extrapyramidal neurologic side effects. Cholinergic drugs such as bethanechol (0.025 to 0.035 mg/kg SC q4-24h or 0.035 to 0.045 mg/kg PO q6-8h) may improve gastric emptying and are effective for treating reflux esophagitis. For esophagitis from trauma or pressure injury after esophageal impaction, judicious use of nonsteroidal antiinflammatory drugs may be warranted to reduce esophageal inflammation and pain.
Dietary modification may be necessary in patients with esophagitis, depending on the degree of ulceration or if motility is impaired. Horses with less severe esophagitis should be fed frequent small meals of moistened pellets and fresh grass. Severe esophagitis may necessitate withholding food and complete esophageal rest for several days. Although the prognosis for esophagitis is good in the absence of underlying disease, the risk of stricture formation is high if severe circumferential or coalescing ulcerations are present. Animals with esophagitis from severe trauma or infection may also be prone to stricture formation.
MOTILITY DISORDERS OF THE ESOPHAGUS
Esophageal hypomotility is the most common motility dysfunction of the equine esophagus and results in esophageal dilation or megaesophagus. Although megaesophagus in horses is most commonly acquired, there are reports of idiopathic megaesophagus in young horses that is likely congenital (Fig. 32-22 and Color Plate 1).89-92 Acquired megaesophagus in adult horses is usually caused by either primary or secondary esophageal obstruction. Esophageal impactions of relatively short duration cause proximal dilation of the esophagus that is generally reversible. However, if the duration of the obstruction is long, the motility of the esophagus may be permanently impaired. Acquired megaesophagus in foals is often secondary esophagitis resulting from gastric outlet or duodenal obstruction. Other causes of acquired megaesophagus include extraesophageal obstruction by tumors or abscesses, pleuropneumonia, and vascular ring anomalies. In addition, acquired megaesophagus may result from neurologic, neuromuscular, or muscular disorders. Neurologic diseases that cause vagal neuropathy, such as equine protozoal myeloencephalitis, equine herpesvirus myeloencephalitis, and idiopathic vagal neuropathy, have been associated with megaesophagus in horses. Pleuropneumonia may be associated with vagal neuropathy, resulting in megaesophagus. Megaesophagus is also an early sign of equine dysautonomia93 and may be noted in patients with botulism. Myasthenia gravis is a well-known cause of megaesophagus in other species but has not been reported in horses. Also in other species, electrolyte disorders, cachexia, primary myopathies, myositis, and Addison’s disease may affect esophageal motility but have not been associated with megaesophagus in horses.
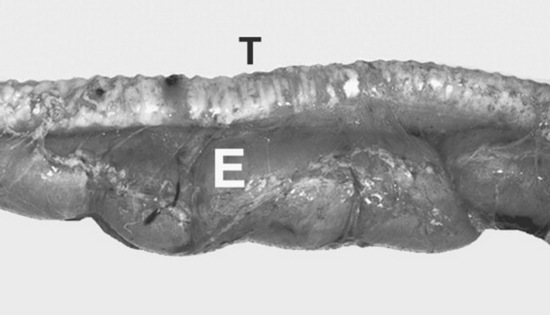
Fig. 32-22 Megaesophagus in a 10-month-old Paint horse foal that had two duodenal strictures that appeared to have been present for several months. The trachea (T) lies dorsal to the dilated esophagus (E).
Courtesy Dr. M.J. Murray.
Esophageal inflammation, particularly reflux esophagitis, may affect motility and cause megaesophagus. However, because esophageal hypomotility may predispose to reflux esophagitis, it may be difficult to determine whether the esophagitis or the megaesophagus is the causative disorder. Iatrogenic megaesophagus can be induced by the α2-adrenergic agonist detomidine, but this is transient and reversible.94 However, the use of this drug may complicate clinical evaluation of esophageal motility. Because esophageal hypomotility is a functional obstruction, the clinical signs of esophageal hypomotility or megaesophagus are similar to those of esophageal obstruction. Thus, the clinical signs include ptyalism, dysphagia, and nasal discharge of saliva and food material. The cervical esophagus may be sufficiently dilated to be evident externally. Weight loss is a common sign, and clinical signs attributable to an underlying disease may be evident.
Diagnosis of esophageal hypomotility requires transit studies. Transit time of a bolus from the cervical esophagus to the stomach can be measured by fluoroscopy or contrast radiography.58,93 Other signs of esophageal hypomotility and megaesophagus include pooling of contrast material and an absence of peristaltic constrictions. Endoscopy may reveal a dilated esophagus and an absence of peristaltic waves. Evidence of underlying disease causing obstruction or esophageal dilation may be observed. The esophagus should be evaluated for evidence of esophagitis that is either causing esophageal motility dysfunction or is a result of impaired esophageal clearance of gastric fluid. Esophageal manometry may be useful to document abnormal postdeglutition contraction pressures, contraction time, and propagation times.55,89 Other diagnostic tests such as a CBC and chemistry to help identify an underlying cause should be performed. A careful neurologic evaluation should be performed. Signs of neurologic disease and abnormal cerebrospinal fluid analysis suggest an underlying neurologic disorder. Myopathy may be detected by electromyography.
Treatment of esophageal hypomotility or megaesophagus should be aimed at treating the underlying cause. Dietary modification should be aimed at improving esophageal transit of food. Slurries of pellets should be fed. In addition, it may be beneficial to feed from an elevated position to promote transit. In patients with reflux esophagitis associated with megaesophagus, metoclopramide or bethanechol may be beneficial to increase lower esophageal tone, promote gastric emptying, and reduce gastroesophageal reflux. The prognosis depends on the underlying cause and the degree of dilation. Although many cases of megaesophagus associated with reflux esophagitis respond well to treatment, many other forms of megaesophagus including congenital megaesophagus have a poor prognosis.
CONGENITAL DISORDERS
Congenital disorders of the esophagus are rare. Reported congenital abnormalities include congenital stenosis,95 persistent right aortic arch, congenital strictures, esophageal duplication cysts,96,97 and idiopathic megaesophagus.98 In the one report on congenital stenosis, double-contrast radiography revealed concentric narrowing of the thoracic esophagus in the absence of any vascular abnormalities at the base of the heart. Successful treatment included having the foal stand with the forelimbs elevated off the ground after each feeding.
Persistent right aortic arch is a congenital anomaly in which the right fourth aortic arch becomes the definitive aorta instead of the left aortic arch, which results in constriction of the esophagus by the ligamentum arteriosum as it extends between the anomalous right aorta and the left pulmonary artery.75 Clinical signs may include dysphagia, drooling of saliva, and distention of the cervical esophagus as a result of partial obstruction of the thoracic esophagus.99 Endoscopic examination typically reveals dilatation of the esophagus cranial to the obstruction with evidence of diffuse esophagitis. In addition, evaluation of the thorax usually reveals the presence of aspiration pneumonia. Successful surgical treatment of persistent right aortic arch has been reported in one foal.
Esophageal duplication cysts cause typical signs of esophageal obstruction, including salivation, dysphagia, and swelling of the cervical esophagus as they enlarge. Such signs can make them difficult to differentiate from simple obstruction (choke). However, an aspirate of the mass may aid in the diagnosis by revealing the presence of keratinized squamous cells.100 Cysts may communicate with the lumen of the esophagus. Surgical treatments have included complete surgical resection and surgical marsupialization. The latter appears to be more successful and to result in fewer complications. Complications of surgical resection have included laryngeal hemiplegia secondary to surgical trauma to the recurrent laryngeal nerve in the region of the esophagus, and esophageal fistula formation.
ESOPHAGEAL PERFORATION
Perforation typically occurs in the cervical region in response to external trauma or rupture of an esophageal lesion such as an impacted diverticulum. The esophagus is particularly vulnerable to external trauma in the distal third of the neck because it is covered by only a thin layer of muscle at this point.101 Iatrogenic perforation may occur in response to excessive force with a stomach tube against an obstruction or a compromised region of the esophagus. Esophageal perforations may be open or closed and tend to cause extensive necrosis of tissues surrounding the wound because of drainage of saliva and feed material within fascial planes (Fig. 32-23). This may lead to extensive cellulitis and endotoxemia. Closed perforations of the esophagus are particularly troublesome, as wound discharge may migrate all the way to the mediastinum and pleural space via fascial planes and may cause abscessation (Fig. 32-24). In addition, extensive subcutaneous and fascial emphysema frequently develops and is usually evident on cervical radiographs.
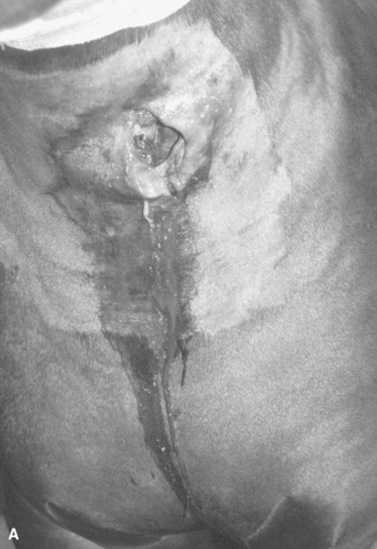
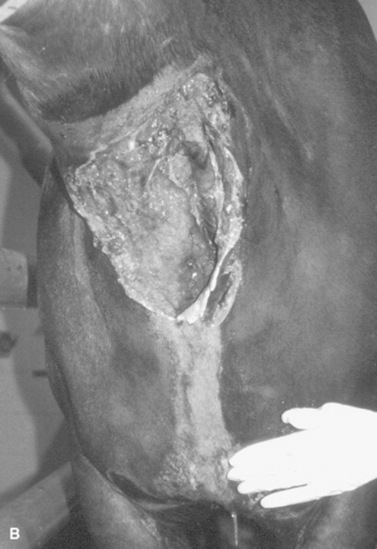
Fig. 32-23 Esophageal perforation in a horse. A, An open esophageal laceration was detected on presentation in the midcervical region. The wound was treated by lavage and debridement, and the horse was fed via a tube inserted into the esophagus through the wound. B, Approximately 14 days later, dissection of esophageal contents within surrounding fascial planes has resulted in extensive sloughing of tissue.
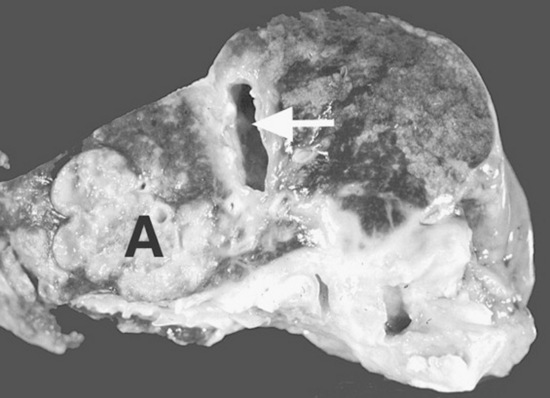
Fig. 32-24 Esophageal abscess (A) and cellulitis that developed secondary to an esophageal obstruction. The esophageal lumen is indicated by the white arrow.
Courtesy Dr. M.J. Murray.
Treatment should include conversion of closed perforations to open perforations if possible,102 extensive debridement and lavage of affected tissues, broad-spectrum antibiotics, tetanus prophylaxis, and esophageal rest. The latter may be achieved by placing a feeding tube into the esophagus via the wound. Alternatively, a nasogastric tube should be placed using a small tube (12-Fr diameter). For open perforations, once the wound has granulated and contracted to a small size, oral feeding may be attempted. Extensive loss of saliva via esophageal wounds may lead to hyponatremia and hypochloremia. In addition, transient metabolic acidosis occurs because of salivary bicarbonate loss, followed by progressive metabolic alkalosis. Although there are reports of esophageal wounds healing well by second intention, it takes a prolonged period of time.103 In addition, some perforations never completely heal and form permanent esophagocutaneous fistulas that may require surgical correction. The development of esophageal strictures is not common because wounds are usually linear and not circumferential. However, traction diverticula may develop. Other complications of esophageal wounds include Horner’s syndrome and left laryngeal hemiplegia.
In a retrospective study on esophageal disorders, only 2 of 11 horses with esophageal perforations survived long term, and in a report on esophageal trauma secondary to nasogastric intubation, 4 of 5 horses were euthanized. The prognosis is therefore poor in horses with esophageal perforations, largely because of the extent of cellulitis, tissue necrosis, shock, and local wound complications.
ESOPHAGEAL STRICTURE
Strictures most commonly occur as sequelae to esophageal obstructions that result in circumferential erosion or ulceration of the esophageal mucosa, although strictures may result from oral administration of corrosive medicinal agents and trauma to the neck.104 Congenital strictures have also been reported. Strictures that result from mucosal and submucosal trauma are termed esophageal webs or rings. Strictures may also originate in the muscular layers and adventitia of the esophagus (mural strictures) or in all of the layers of the esophagus (annular stenosis).105 Horses with these lesions have a presentation similar to that of horses with simple obstructions because strictures result in partial obstruction and accumulation of feed material in the lumen. Esophageal webs or rings can be observed endoscopically, whereas mural strictures or annular stenosis may require double-contrast esophagrams to confirm their presence.
In one study on esophageal stricture after simple obstruction, maximal reduction in esophageal lumen occurred within 30 days of esophageal obstruction. Although surgery has been employed to reduce such strictures, initial medical management is warranted because strictures may resolve with conservative therapy, and the esophagus continues to remodel for up to 60 days after ulceration. In one report, seven horses with esophageal obstruction—induced stricture were treated conservatively by feeding a slurry diet and administering antiinflammatory and antimicrobial medications, and five of seven were clinically normal within 60 days. One of the five successfully treated horses had a 10-cm area of circumferential ulceration, suggesting extensive mucosal injury may resolve without permanent stricture formation. If there is insufficient resolution of strictures within 60 days, other methods to increase esophageal diameter should be investigated. Bougienage has been used successfully in small animal patients and human beings. The technique involves passage of a tubular dilatable instrument down the esophagus and stretching of the stricture. Some authors have suggested that this may be accomplished by passing a nasogastric tube with an inflatable cuff (Fig. 32-25).106 However, the procedure has to be performed frequently to have any success and is not well tolerated in the horse. Alternatively, several surgical techniques have been used to resolve strictures, including resection and anastomosis,107,108 temporary esophagostomy with fenestration of the stricture, esophagomyotomy for strictures of the muscularis and adventitia,109,110 and patch grafting with local musculature.111 However, such surgeries are fraught with complications, largely because of the propensity of the traumatized esophagus to restricture. The esophagus lacks a serosal layer and does not rapidly form a fibrin seal, as does the remainder of the intestinal tract, so anastomoses tend to leak. In addition, tension on the esophagus during swallowing and movement of the neck impairs healing of anastomoses.
ESOPHAGEAL DIVERTICULA
There are two types of diverticula: traction (true) diverticula and pulsion (false) diverticula. Traction diverticula result from wounding and subsequent contraction of periesophageal tissues, with resultant tenting of the wall of the esophagus. Pulsion diverticula arise from protrusion of esophageal mucosa through defects in the muscular wall of the esophagus and usually result from trauma or acute changes in intraluminal pressure (Fig. 32-26). Traction diverticula appear as a dilatation with a broad neck on contrast esophagography, whereas pulsion diverticula typically have a flask shape with a small neck on an esophagram.112 Whereas traction diverticula are usually asymptomatic and of little clinical significance, pulsion diverticula may fill with feed material, ultimately leading to esophageal obstruction.113 However, a movable mass in the midcervical region may be noticed before onset of complete obstruction.56 Pulsion diverticula may be surgically corrected by inverting or resecting prolapsed mucosa and closing the defect in the wall of the esophagus.77,112,113 Inversion of excessive mucosa may predispose horses to esophageal obstruction and should therefore be reserved for small diverticula.
NEOPLASIA
Neoplasia of the esophagus is rare, but squamous cell carcinoma114,115 and leiomyosarcoma116 have reportedly affected the esophagus either as the primary site or in association with a lesion in the squamous portion of the stomach. The predominant clinical signs are weight loss, colic, and recurrent esophageal obstruction. The tumor is typically detected antemortem on esophagoscopy and radiography, but a definitive diagnosis may require a biopsy during laparotomy.72,116 When neoplasia affects the lower esophageal sphincter, gastroesophageal reflux may contribute to ulceration of esophageal mucosa. The prognosis for malignant neoplasia of the esophagus is grave.
GASTRIC ULCERATION
Just as the term colic describes a clinical presentation and encompasses a large number of disorders, the term equine gastric ulcer syndrome (EGUS) describes a clinical finding, the cause of which is likely to be multifactorial and different from case to case. The umbrella of EGUS includes lesions in the squamous or glandular mucosal linings of the stomach, focal or multifocal ulceration, generalized gastritis, gastric emptying disorders, gastroesophageal reflux disorders, and obstructive disorders. In foals, signs attributable to gastric disease can be the result of primary duodenal disease (see Color Plates 1-12).
Prevalence and Incidence
Gastric ulceration is a widespread phenomenon, affecting a large number of foals and horses. The overall prevalence of gastric ulceration in foals up to 60 days old has been reported to range from 25% to 50%, and most lesions were observed in the squamous mucosa.117,118 In the majority of foals with lesions, clinical signs of ulcers were not apparent, and in one study119 most lesions observed in foals less than 60 days old healed without treatment.
More than half of apparently normal horses had gastric lesions in one endoscopic study,120 and lesions were more prevalent and severe in horses exhibiting clinical signs consistent with gastric ulceration (poor appetite, poor body condition, recurrent abdominal discomfort). Horses in training for racing appear to be at particular risk for developing gastric lesions, because 70% to 90% of racehorses have had gastric lesions documented endoscopically120-125 or at necropsy.126
The incidence of gastric ulcers in horses exposed to ulcerogenic stresses has been reported to range from 70% to 86%.127-129 Ulcers developed within 5 to 7 days in some reports. In one report ulcers developed in the majority of horses in simulated race training within 7 days,130 and it was reported that 11 of 15 horses exercised using a mechanical exerciser developed gastric ulcers within 7 days.131 Most studies have been done in horses in race training, but in one study that simulated conditions of traveling to a recreational horse show, 7 of 10 horses developed gastric ulcers within 5 days.129 The latter two studies demonstrated a high incidence rate and rapid onset of gastric ulcers in horses not in race training regimens.
In the endoscopic studies referenced in the previous paragraphs, the majority of gastric lesions in adult horses reportedly occurred in the squamous mucosa. To some extent this observation has been influenced by the limitations of the endoscopic examination, in which only the portion of the stomach not obscured by ingesta or gastric secretions was observed. In studies in which the antrum and pylorus were specifically examined, lesions were found in the antral mucosa in 47% and 58% of horses.132,133 In some horses with moderate to severe antral ulcers, the gastric squamous mucosa was normal.
Pathophysiology
Damage to the gastric lining results from a combination of the physical properties of the gastric mucosa, the physiology of gastric acidity in the equine stomach, and the horse’s response to potentially ulcerogenic stress factors. The predominant factor in peptic injury to alimentary mucosa is hydrochloric acid, although the proteolytic enzyme pepsin,134 bile acids,135 and short-chain fatty acids136 may facilitate or exacerbate hydrochloric acid—induced mucosal injury.
The dorsal portion of the equine stomach is lined by a stratified squamous epithelial mucosa, which, like esophageal mucosa, has minimal intrinsic resistance to peptic injury (Fig. 32-27).137 The equine gastric squamous mucosa is highly susceptible to acid-induced injury, with damage to this tissue detected within 30 minutes of in vitro exposure to solutions acidified by HCl.138 The equine gastric glandular epithelium (Fig. 32-28) is histologically and physiologically similar to the lining of the stomach of other animals and human beings, and this mucosa has evolved elaborate mechanisms to protect itself from peptic injury. These include the mucus-bicarbonate barrier, prostaglandins, nitric oxide, growth factors, mucosal blood flow, and cellular restitution.139,140
Fig. 32-27 Photomicrograph of equine gastric squamous epithelial mucosa. There are multiple layers of epithelium arranged in parallel with the luminal surface. The most superficial layers of cells are cornified, and superficial to these cells are layers of keratin. (Hematoxylin and eosin stain.)
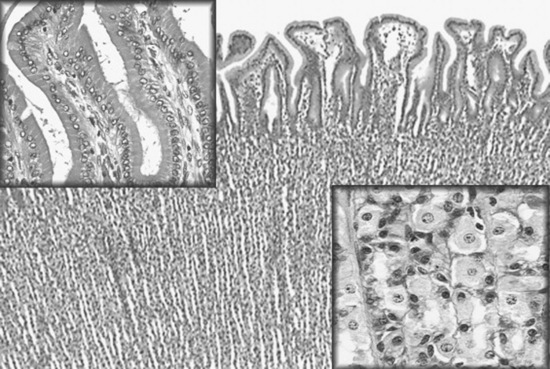
Fig. 32-28 Photomicrograph of equine gastric glandular mucosa. In contrast to the squamous mucosa, the glands are parallel to one another and perpendicular to the luminal surface. There are multiple cell types within the mucosa, with surface epithelial cells and mucus-secreting cells toward the lumen, and parietal cells, chief cells, enterochromaffin-like cells, G-cells, and D-cells deeper in the mucosa. The insert at the top left shows a high-power magnification of cells lining gastric pits on the surface of the epithelium. The insert at the lower right shows a high-power magnification of cells lining the gastric glands deeper in the mucosa.
Hematoxylin and eosin stain.
Perhaps the key factor in whether the gastric squamous mucosa is injured by HCl is the duration of contact of HCl with the mucosa, which is influenced by eating behavior, exercise, and perhaps other factors, which collectively are recognized as “ulcerogenic stresses.” Risk factors, acting singly or interacting together, contribute to the ulcerogenic stress experienced by a horse, and the expression of the ulcerogenic stress as a gastric ulcer depends on individual characteristics of the animal as well as the risk factors themselves.
Foals and horses and secrete HCl continuously,141 and gastric acidity is greatest when foals do not nurse142 or horses do not eat.143 Gastric pH can fall to highly acidic levels (<2) within minutes of cessation of nursing or eating hay. Any disruption of feeding or nursing, whether it is imposed or caused by a clinical disorder, will prolong gastric acidity and increase the time that the squamous mucosa is exposed to HCl.
Prolonged periods of high gastric acidity (pH < 2) were created in horses using a protocol of alternating 24-hour periods of feed deprivation with free-choice timothy hay,144 which consistently resulted in erosion and ulceration, often severe, in the gastric squamous epithelial mucosa in horses.143 Erosions, sometimes bleeding, were seen after 48 hours cumulative feed deprivation, and ulcers were consistently seen after 96 hours. Concurrent administration of the H2-receptor antagonist ranitidine during feed deprivation significantly minimized the area of lesions in the gastric squamous epithelial mucosa, demonstrating the direct contribution of HCl in the pathogenesis of these lesions.
Feeding practices and management of horses can influence gastric acidity and peptic injury to the gastric squamous mucosa. Changing horses from pasture to stall confinement with free choice timothy hay for 7 days resulted in erosion and ulceration of gastric squamous mucosa.143 Imposed feed deprivation in the management of cases of colic can result in erosion and ulceration of the gastric squamous mucosa. Similarly, horses that are partly or completely anorectic because of their illness will likely develop erosions or ulcers in their gastric squamous mucosa.
Given the very high prevalence of gastric ulcers in horses in training, exercise is an important risk factor for EGUS. The reasons for the high prevalence and incidence of EGUS in association with exercise have not been fully explained, but in one report it was shown that treadmill exercise was associated with increased exposure of the dorsal part of the stomach to highly acidic gastric contents.145 This occurred secondary to, and as a possible consequence of, increased intraabdominal pressure during exercise. These changes were observed when horses increased speed from a walk to a trot. It is interesting to note that several horses in that study developed gastric ulcers.
The high prevalence and severity of gastric ulcers in racehorses has contributed to the perception that EGUS is primarily a disorder of racehorses and other horses in intensive training, However, results of a recent study demonstrated that activities considered to be normal by the recreational horse enthusiast are associated with an increased incidence of gastric squamous mucosal ulcers.131 Ten horses were exposed over 5 days to conditions that simulated activities that are typical for the recreational use of horses, including transportation to an unfamiliar stable environment, twice-daily feeding, and light exercise (lunge line), and return transportation to the premises of origin. Ten age-matched herdmates remained on the premises of origin during the trial. Gastroscopy was performed at the beginning (day −1) and the end of the trial (day 5). The appearance of all horses’ stomachs was normal at the beginning of the trial. Two control horses and seven transported horses developed ulcers in the squamous mucosa of the stomach by day 5. The ulcer scores of the transported horses increased significantly from day −1, whereas ulcer scores in the control horses did not change significantly from day −1.
The pathophysiology of lesions in the gastric glandular mucosa of foals and horses is not well understood. Nonsteroidal antiinflammatory drugs can induce gastric ulcers, but this is an infrequent cause of ulcers in most horses. Most lesions in the gastric glandular mucosa are observed in the antrum and adjacent to the pylorus. The role of hydrochloric acid in the development of ulcers in the glandular mucosa, particularly in the antrum, is unclear. In the feed deprivation model, in which prolonged increased gastric acidity induces gastric squamous mucosal ulcers, lesions were not induced in the glandular mucosa.143 In human beings, Helicobacter pylori is considered to be the predominant cause of gastric erosions and ulcers,146 but Helicobacter organisms have not been reported to have been cultured from equine gastric mucosa. However, antibodies to Helicobacter proteins have been demonstrated in sera from foals and horses,147 and through use of PCR techniques the Helicobacter-specific 16s rRNA gene was identified in equine gastric mucosa.148 Spiral organisms were identified in equine gastric mucosa by immunofluorescence, using a highly specific antibody to a Helicobacter protein (David Scott, personal communication, 2005). The role of an equine Helicobacter in the pathophysiology of ulcers in the mucosa of the antrum and pylorus is speculative, but consideration must now be given to this organism as a potential pathogen in the horse.
Clinical Syndromes
The magnitude of adverse health effects in the majority of foals and horses with gastric lesions remains somewhat speculative because the disease often goes unrecognized. In one report, though, the prevalence of gastric ulceration was significantly greater in horses with clinical problems such as colic, poor appetite, and poor bodily condition than in asymptomatic horses.120
FOALS
The clinical signs that typically are associated with gastric ulcers in foals (e.g., bruxism, dorsal recumbency, salivation, interrupted nursing, diarrhea, and colic) are, in fact, observed in the minority of foals with endoscopically observed ulcers.118 Therefore when clinical signs are seen, the clinician should consider that severe ulceration exists. Diarrhea was the most frequently associated clinical signs in one report.149 Colic, bruxism, dorsal recumbency, or ptyalism should alert the veterinarian to the probability of severe ulceration. Ptyalism occurs as a result of esophagitis, which often results from gastroesophageal reflux caused by gastric outlet obstruction or pseudoobstruction. Thus, ptyalism in foals often reflects a serious problem in the stomach and/or duodenum. Weanlings and older foals with chronic gastric ulceration often have intermittent diarrhea and abdominal discomfort, poor growth, rough hair coat, and a pendulous abdomen.
In foals with clinical signs, squamous mucosal lesions often are severe (see Plate 12). Most glandular lesions that result in clinical signs are located in the vicinity of the pylorus, although in young foals (<30 days old) with stress ulcers lesions are often located in the glandular mucosa in the body of the stomach (see Plate 8).
ADULTS
Although most adult horses with gastric lesions do not demonstrate overt clinical signs, low-grade discomfort that results in subtle signs may go unnoticed. Indeed, horses that I have treated solely on the basis of endoscopic findings have frequently demonstrated improved attitude and appetite, yet attitude and appetite were not considered problems before treatment began.
In horses, gastric lesions have most frequently been associated with colic, poor appetite, and poor bodily condition.120,150 Other signs associated with ulcers have included attitude changes, stiffness, a tucked-up abdomen, and poor performance.
It should be noted that all the signs attributable to gastric ulcers have also been reported in horses that were referred for gastroscopy but did not have ulcers. Thus, although many signs appear typical for horses with ulcers, none is pathognomonic. Nonetheless, gastric ulcers should be strongly considered in horses with recurrent colic and other vague disorders for which a diagnosis has not been determined.
Hemorrhage (i.e., either active bleeding or darkened, coagulated blood) can occur with deep gastric ulcers in horses. However, bleeding from ulcers in the gastric squamous mucosa does not cause anemia or hypoproteinemia in adult horses, and if these abnormalities are present, another cause must be determined.
Diagnosis
Diagnosis of gastric ulceration is based on the presence of age-related characteristic clinical signs, endoscopic findings, and response to treatment. The diagnosis of gastric ulceration in the majority of foals and horses can be definitively determined only by gastroscopic examination. In young foals (<30 days) with an immature colonic flora, the presence of fecal occult blood may be indicative of gastroduodenal ulceration. In older animals, hemoglobin is too extensively degraded by colonic microorganisms for blood originating in the stomach to be detected by fecal occult blood tests.
The use of sucrose as a marker of equine gastric mucosal permeability has been reported in research studies in horses. As a disaccharide, ingested sucrose is digested to its constituent sugars, glucose and fructose, which are absorbed through the small intestine. The presence of ingested sucrose in the blood reflects absorption of the disaccharide through a breach in the gastric mucosa. In one report, horses were administered 454 g of table sugar by nasogastric tube, and urine sucrose was increased after 120 minutes in horses with gastric ulcers, particularly moderate to severe ulcers.151 Because the need for collection of urine for this test will restrict its utility, a method for detection of sucrose in equine plasma has been developed.152 To date, a validation of this method as a diagnostic test for gastric ulcers in horses has not been reported.
Treatment
Processes that promote ulcer healing are stimulated by injury to the gastric mucosa. These include increased capillary blood flow, generation of new capillaries (neovascularization), induction of growth factor receptors, and proliferation of epithelium along a capillary scaffold.153,154 With erosions in the stomach lining, some epithelium remains in the erosion bed and healing can occur within a few days because the epithelium is regenerated relatively rapidly. With severe ulcers that extend through the entire epithelium and lamina propria, a bed of granulation tissue forms in the ulcer bed, and healing results primarily from contraction of the ulcer margins. This type of healing can require weeks to complete.
Gastric ulcers can heal without treatment, even in the face of continued ulcerogenic stress, but in such conditions new ulcers typically form as old ulcers heal.155 Also, the healing is often incomplete. For example, in racehorses it is typical to observe ulcers with proliferative margins and granulation tissue in the ulcer beds mixed with what appear to be newly formed, bleeding ulcers. In most cases, unless the ulcerogenic stress is removed the stomach will remain ulcerated. This means taking the horse out of training and probably turning it out onto pasture, because even taking the horse out of training but maintaining stall confinement can be ulcerogenic.155 Successful treatment of gastric ulcers is predicated on addressing the underlying cause and treating with medications that create an environment that is favorable to ulcer healing. Acid-suppressive treatment often is required to break the cycle of inappetence that causes increased gastric acidity, which results in ulceration, which then prolongs and exacerbates the inappetence (Fig. 32-29).
Fig. 32-29 Diagram representing the pathophysiology of ulceration of the equine gastric squamous mucosal epithelium and the permissive effect of acid suppression on ulcer healing.
Decisions concerning whether to treat gastric lesions, what medication to use, and for how long are best made on the basis of results of a gastroscopic examination, the type of activity for which the horse is used, and whether the horse will continue in that activity or be rested. If response to treatment is used as a diagnostic aid, clinical conditions such as poor appetite, colic, or diarrhea (foals) that result from gastric ulcers should improve in 24 to 48 hours of initiating acid-suppressive therapy. If improvement in clinical signs is not observed, gastric ulceration, if present, should be considered to be a secondary, not a primary, problem.
The primary objective in the treatment of gastric ulcers is to alleviate discomfort, and this is best done by inhibiting or neutralizing acid secretion. Decreasing gastric acidity also creates an environment that is permissive for healing of the gastric mucosal epithelium. Once ulcers form, there are changes in the tissue that promote healing. Suppressing acidity creates an environment within the stomach that permits ulcer healing.
Several acid-suppressing drugs are approved for treating gastric ulcers in horses (Box 32-2).
Box 32-2 Therapeutic Agents Registered for Use in Treating Gastric Ulcers in Foals and Adult Horses
The H2 antagonists cimetidine, ranitidine, famotidine, and nizatidine interfere with histamine stimulation of HCl secretion by competitively blocking the H2 receptor on the parietal cell. Both cimetidine and ranitidine have been shown to suppress gastric acid secretion in horses and neonatal foals.141,142,144,156,157 Effectiveness of these drugs in blocking acid secretion is dependent on plasma concentrations, and the magnitude and duration of acid inhibition are highly variable in horses. Both the degree and duration of suppression of gastric acidity by H2 antagonists vary among horses,157,158 presumably as a result of differences in drug absorption. A dose of 6.6 mg of ranitidine per kilogram given PO every 8 hours provided adequate suppression of acidity in most horses in research studies. This dosage schedule resulted in a median 24-hour gastric pH of 4.6 in horses with free access to hay (compared with a pH of 3.1 in horses fed, but not given ranitidine).144 However, because of the individual variability in response among horses, the duration of effect may be only 1 to 2 hours. Using lower doses often results in no response.
Effective doses of cimetidine have not been examined as extensively as those of ranitidine, but an effective oral dose may be as high as 50 mg/kg/day. Lower doses are often given, and sometimes with clinical improvement, but in my experience ulcers have often persisted.
Formulations for parenteral administration of H2 antagonists are available but are approximately three times the cost of oral products. Ranitidine should be given IV at 1 to 1.5 mg/kg every 8 hours, and cimetidine at 6.6 mg/kg three to four times daily. The parenteral route of administration can be very beneficial in foals and horses that cannot tolerate or use orally administered medications.
Omeprazole is a proton pump inhibitor that blocks gastric acid secretion by inhibiting the parietal cell H+,K+-ATPase (proton pump) that secretes HCl.159 Omeprazole is a potent inhibitor of gastric acidity in horses. The antisecretory effects of omeprazole persist far longer than the drug’s plasma level, because binding to the H+,K+-ATPase of the parietal cell persists for up to 24 hours after a single dose.160 This prolonged duration of action enables once-daily dosing.
A paste formulation of omeprazole* has been registered in the United States, Canada, Europe, and elsewhere for use in treating gastric ulcers in foals and horses. In clinical trials, ulcer healing in horses and foals treated once daily with omeprazole paste at a dose of 4 mg of omeprazole per kilogram of body weight was substantially superior to healing in sham-treated horses.161,162 It is important to note that in one set of these trials,162 ulcer healing occurred in more than 77% of omeprazole-treated horses that were in race training, a result that has not been noted in horses treated with H2 antagonists.163 Thus, omeprazole brings a new perspective to the treatment of gastric ulcers in horses. Once-daily treatment and a paste formulation should enhance treatment compliance, and the potency of acid suppression permits horses to remain in their activities while being effectively treated for ulcers.
Another important feature of omeprazole paste was the confirmation of its ability to prevent both occurrence and recurrence of ulcers in horses in race training, in which conditions are most ulcerogenic.164,165 The daily dose used for prevention was one fourth that used for treatment (1 mg of omeprazole per kilogram vs. 4 mg/kg, respectively). In an 8-day study of horses in mild to heavy exercise, once-daily omeprazole paste† at a dose of 1 mg/kg prevented gastric ulcers in 45 of 51 treated horses (12% incidence), compared with an incidence of 73% in 51 untreated horses.168a
Antacids can effectively reduce gastric acidity, but only briefly. In a study examining administration of 180 mL of Maalox‡, gastric pH was increased for at most 45 minutes.157 In another study 240 mL of Maalox TC§ increased gastric pH for 2 hours.166 Thus, liquid antacid products must be given both in large volumes (240 mL) and very frequently, and these products are not suitable for ulcer treatment. Feed additives that contain antacids are popularly considered to be helpful in controlling gastric ulcers in horses, but there are no supportive data. Also, an acid-neutralizing effect is most desirable when the stomach is empty, not when it is full, because gastric pH naturally is high when horses ingest feed. Antacids containing aluminum may have some effect on healing of gastric glandular lesions, because aluminum hydroxide has been shown to enhance gastric mucosal nitric oxide, which should promote mucosal blood flow.167
Sucralfate, the major components of which are sucrose octasulfate (SOS) and aluminum hydroxide, is helpful in the treatment of peptic ulcers in people,168 although healing rates for sucralfate in treatment of duodenal ulcers were much longer than for H2 antagonists. The mechanism of action likely involves adherence to ulcerated mucosa, stimulation of mucous secretion, enhanced mucosal blood flow, and enhanced prostaglandin E synthesis. These are all factors relevant to glandular mucosa, and it is doubtful that sucralfate is effective in treating ulcers in the equine gastric squamous mucosa. In fact, I have observed lesions to develop in the squamous mucosa while horses were being treated with sucralfate.
Sucralfate can be administered concurrently with an H2 antagonist. Concurrent administration may reduce H2 antagonist absorption by 10%, but this has not appeared to affect efficacy in horses.169 It is important to note that sucralfate can substantially interfere with the absorption of other drugs, particularly fluoroquinolones, and therefore its use with other medications should be determined on a case-by-case basis.
Few studies have directly compared the effectiveness of different ulcer treatments in horses. In a study of racehorses at a California race track, treatment with cimetidine was compared with treatment with registered omeprazole paste on healing and prevention of gastric ulcers.124 Horses were treated with cimetidine 20 mg/kg three times daily for treatment or prevention of ulcer recurrence or omeprazole 4 mg/kg once daily for treatment and 2 mg/kg once daily for prevention of recurrence in a randomized controlled trial. There was significant reduction in ulcer severity in horses treated with omeprazole, and ulcer severity did not significantly increase after horses received the prevention dose of omeprazole. Cimetidine did not reduce ulcer severity or prevent recurrence of ulcers after treatment with omeprazole. In a randomized controlled trial conducted in Australia with 60 racehorses in training, ulcer healing was significantly better in horses treated daily with omeprazole paste* (4 mg of omeprazole per kilogram) than in horses treated with a commercial ranitidine product† (6.6 mg of ranitidine per kilogram three times daily).170 Furthermore, there was additional ulcer healing in horses treated with omeprazole paste after 28 days of treatment with ranitidine.
A study that included 798 racehorses examined the effect of several medications used to treat EGUS.171 Two hundred and twenty-seven horses had been treated for at least 2 weeks at the time of gastroscopy with registered omeprazole pastea (4 mg/kg/day or 2 mg/kg/day), omeprazole in unregistered compounded products, H2 antagonists (drugs and dosages not specified), sucralfate, or “buffers.” The risk of having moderate to severe EGUS in horses treated with registered omeprazole pastea at 4 mg/kg/day or 2 mg/kg/day (0.18) was significantly less (P < .001) than in untreated horses, but the risk of having moderate to severe EGUS in horses treated with the other products was no different from that in untreated horses.
In some cases prokinetic drugs may be required to enhance gastric emptying. Use of a prokinetic drug is indicated when there is suspected gastroesophageal reflux, gastric outlet obstruction or pseudoobstruction, or duodenal ulceration or inflammation. Drugs available for this purpose include bethanechol and metoclopramide.
I have used bethanechol successfully to enhance gastric emptying and minimize gastroesophageal reflux with few mild adverse effects. Bethanechol was reported to enhance gastrointestinal motility while not increasing gastric acid output in horses.172 In cases of acute gastric atony, 0.025 mg/kg SC every 4 to 6 hours has been effective in promoting gastric motility and emptying, followed by oral maintenance doses of 0.35 to 0.40 mg/kg three or four times daily. Adverse effects can include diarrhea, inappetence, salivation, and colic, but they occur infrequently. Bethanechol can be administered chronically (weeks to months) in horses with pyloric fibrosis and stenosis, although there are no data regarding its long-term effectiveness.
Reported experience with metoclopramide in the equine is limited to its use in postoperative ileus (POI). Doses ranging from 0.10 to 0.25 mg/kg three or four times daily are used by some clinicians to treat suspected delayed gastric emptying, although one report indicated that constant infusion at a rate of 0.04 mg/kg/hr was superior to interval dosing in enhancing postsurgical small intestinal motility.173 Sudden neurologic excitation is an adverse reaction to metoclopramide but is more common toward 0.25 mg/kg. In one foal I treated, administration of metoclopramide over several days resulted in tachycardia, bilateral facial sweating, miosis, and enophthalmos. These signs resolved when the drug was discontinued.
REFLUX GASTRITIS
Reflux gastritis frequently accompanies conditions in which there is small intestinal ileus with large volumes of enterogastric reflux that must be evacuated by nasogastric intubation. This fluid typically has a pH of 5 to 7 and contains substantial biliary and pancreatic secretions. Often gastroscopy reveals that extensive erosion of the squamous mucosa occurs in association with this reflux. The erosions extend from the margo plicatus dorsally, primarily along the cranial and right sides of the stomach. Presumably squamous mucosal erosion results from the effect of bile acids on the gastric squamous mucosa combined with accumulation of gastric hydrochloric acid.138 Often in such cases, islands of regenerative squamous epithelium can be observed in the eroded or ulcerated fundus within 2 to 3 days, and in another 2 to 3 days the squamous epithelium can completely regenerate. Horses with reflux gastritis may be slow to regain a normal appetite after feeding resumes, and in some cases administration of an acid-suppressive drug has resulted in a quick improvement in appetite. Intravenous administration of an H2 antagonist may prevent these lesions from developing or hasten healing, but treatment may not be necessary in all cases.
GASTRIC IMPACTION
True gastric impaction occurs infrequently and may result from ingestion of certain feed stuffs or eating when there is impaired intestinal motility. Potential predisposing feeds include beet pulp, bran, straw, wheat, and barley. Beet pulp and bran can become desiccated within the stomach and may not become rehydrated by water or gastric secretions. Dental disorders may predispose some horses to gastric impaction if roughage is incompletely masticated. Feeding a horse that has signs of colic may predispose to gastric impaction because there may be poor gastric emptying associated with generalized decreased gastrointestinal motility.
Definitive diagnosis of gastric impaction is difficult. Gastric impaction is occasionally diagnosed during exploratory laparotomy as the primary cause of colic in horses.174 Other than at surgery, definitive diagnosis of gastric impaction can be difficult. If the horse has not eaten for several hours, yet poorly macerated or digested feed material is recovered from the nasogastric tube, a gastric impaction may be suspected. On rectal examination, the spleen may be displaced caudally and medially, but this finding is not specific for gastric impaction or dilation. Gastric impactions can be confirmed by gastroscopy, although one cannot differentiate a normally full stomach from an impacted stomach. The key to making this diagnosis is the failure of the stomach to empty appreciably in 12 to 24 hours. Radiography may also reveal a distended stomach that distorts the diaphragm cranially.
Gastric impactions can be effectively treated medically by administering dioctyl sodium succinate (DSS), 5% solution, 4 to 8 oz, in 4 to 6 L of water. The DSS acts as a surfactant and allows water to penetrate the impacted, desiccated ingesta, facilitating their removal from the stomach. Alternatively, one can lavage the stomach with water repeatedly by pumping 2 to 4 L of water via nasogastric tube and recovering the infused water and ingesta by gravity flow or aspiration through the nasogastric tube. This is particularly effective in treating bran mash impactions, because of the small particle size of the bran. Mineral oil is less effective in treating gastric impactions because the interior of the impacted ingesta is desiccated and compacted. The mineral oil slides around the impaction and does not penetrate it; thus it does not facilitate passage of the impacted ingesta through the pylorus. When diagnosed at surgery, gastric impactions can be effectively treated by the injection of 2 to 4 L of saline transmurally into the stomach, followed by gentle massage of the stomach and the impacted mass. The impaction usually resolves within 12 to 24 hours. Treatment with bethanechol, 0.02 mg/kg SC every 6 to 8 hours to promote gastric emptying may be helpful in conjunction with these therapies. Bethanechol may have reduced effectiveness in a distended stomach, but it should not contribute to stomach rupture and therefore can be used safely.
Gastric impaction can also accompany grass sickness, in which case the prognosis for survival is poor. Grass sickness occurs in the United Kingdom and in areas of South America. The disease does not occur in the United States, but horses recently imported from the United Kingdom have been diagnosed with grass sickness in the United States.
GASTRIC RUPTURE
Gastric rupture occurs as a sequela to gastric distention from ingesta, fluid, or gas. The adult equine stomach can hold 20 to 25 L when maximally distended. Gastric rupture can occur from simple excessive distention, but also the integrity of the wall of the stomach may become compromised because of decreased blood flow. Distention of the small intestine has been demonstrated to significantly reduce mural blood flow, and this likely occurs in the stomach with distention. In some cases, it has appeared that rupture occurred as a result of an infarction of a portion of the stomach wall, without apparent substantial distention. Gastric perforation from ulceration happens rarely in adult horses. Because of extensive contamination of the peritoneal cavity with stomach contents, treatment is not possible and humane destruction of the horse is required.
ABSCESSES
Abscesses in the wall of the stomach are infrequent findings and occur most frequently in foals. Abscesses can form secondary-to-severe gastric ulceration, Rhodococcus equi bacteremia, foreign body penetration, or septic peritonitis. Signs of gastric abscessation are variable and similar to those of abscessation in other organs: fever, neutrophilia, hyperfibrinogenemia, anemia, weight loss, and possibly colic. Diagnosis may be made endoscopically, radiographically, or ultrasonographically. In some cases use of labeled WBC scintigraphy may identify an intraabdominal abscess in the region of the stomach. Usually by the time a diagnosis is made, the abscess is very advanced and often it is adhered to multiple abdominal viscera. Treatment should include long-term antimicrobial drugs, but outcomes are usually poor.
GASTRIC TUMORS AND MASSES
Gastric tumors, neoplastic and nonneoplastic, occur infrequently. Squamous cell carcinoma is the most common neoplastic disorder that affects the equine stomach (Fig. 32-30).175 The tumor originates from the gastric squamous epithelium and can metastasize to the abdominal cavity and viscera and/or extend into the esophagus. It typically affects horses in their teens or older. Presenting signs include chronic weight loss, anemia, nasal reflux, or colic. Diagnosis can be made by gastroscopy, laparoscopy, barium contrast radiography, or peritoneal fluid analysis when the tumor has metastasized into the abdomen. Metastatic masses may be felt on rectal palpation. When the tumor obstructs the cardia, it is difficult, if not impossible, to pass a nasogastric tube, and saliva and ingesta accumulate within the esophagus. There is no effective therapy.
Primary gastric adenocarcinoma has been described, and metastatic lymphosarcoma, mesothelioma, and bile duct carcinoma have involved the stomach.
Nonneoplastic masses that have been observed in the equine stomach include Draschia megastoma masses, proliferative granulation tissue, and adenomatous masses in the antrum and pylorus. Rarely, proliferative squamous mucosa may appear as a mass attached to a stalk (Fig. 32-31). These can occur in a healing ulcer as an ulcer bed contracts while there is rapid proliferation of granulation tissue.
Fig. 32-31 Endoscopic view of the stomach of a horse in which there is a mass (arrow) protruding from the squamous mucosa along the lesser curvature. The mass appeared to be a proliferation of granulation tissue, around which margins of a healing ulcer had contracted.
Photo courtesy of Dr. Guy Lester, Murdoch University, Murdoch, Western Australia.
Adenomatous masses in the antrum and pylorus have been reported,176 and I am aware of several more cases. These appear as large, irregular polypoid lesions (Fig. 32-32) and can obstruct most of the antrum. The cause of these lesions is unknown. Horses with these lesions have been presented with mild, intermittent colic. There is no effective treatment.
Fig. 32-32 Postmortem photograph of several masses in the pyloric antrum that were identified on histologic examination as gastric polypous adenomas. The masses almost completely obstructed the antrum and pylorus, and there was muscular hypertrophy of the pyloric canal, resulting in stenosis.
Photo courtesy of Dr. Udo Hetzel, University of Liverpool, Liverpool, England.
PYLORIC STENOSIS
Pyloric stenosis can occur secondary to chronic ulceration and fibrosis or muscular hypertrophy. The majority of cases occur as a result of chronic ulceration at the pylorus. I have diagnosed pyloric stenosis in foals, yearlings, and adult horses up to 20 years old.177
Diagnosis of pyloric stenosis is best made by endoscopy. Usually there will be active ulceration and inflammation, and the pyloric opening will appear small and fixed. If a biopsy forceps is pushed into the stomach lining at the pylorus, the wall of the stomach will appear to be rigid owing to scar tissue in the stomach wall. With primary muscular hypertrophy, no ulceration of mucosal inflammation should be present.
Treatment of pyloric stenosis caused by fibrosis from chronic ulceration is difficult. The fibrosis, and therefore the stenosis, may be permanent, although I have treated cases in which the pylorus became less rigid and more compliant when the primary ulceration and associated inflammation were resolved. The treatment objectives are to promote ulcer healing and enhance gastric emptying. Long-term acid-suppressive treatment may be required. In some cases vigorous debridement of the chronically ulcerated mucosa may promote generation of healthy granulation tissue, leading to ulcer healing. Gastric emptying can be promoted by bethanechol (0.02 mg/kg SC q8h or 0.35 mg/kg PO q8h).
If medical management is not effective, surgical bypass of the pylorus (gastroenterostomy) is indicated. Whereas pyloromyotomy has been effective in treating cases with primary muscular hypertrophy, the degree of fibrosis that is present in most cases precludes this approach.
INTESTINAL INJURY AND HEALING IN THE HORSE
There are numerous causes of intestinal injury during equine acute abdomen. Classic pathophysiologic explanations use ischemia, ulceration, and inflammation from infection, parasites, trauma, toxins, or immune complexes to categorize and explain intestinal injury. For the clinician, understanding the underlying pathophysiology of injury is important for recognition and treatment of horses with colic. For many of these intestinal disorders the signs can be similar, making clinical differentiation difficult.
Cellular injury was once characterized by the morphologic change it caused. The new paradigm includes cell injury, which is as much functional as it is structural. The response to a stimulus is mediated by numerous autocrine and paracrine messengers, which include cytokines, chemokines, prostaglandins, neuropeptides, and proinflammatory substances such as interleukins (ILs), tumor necrosis factor alpha (TNF-α) complement, histamine, bradykinin, serotonin, and interferon (IFN).178-180 In the intestine, inflammatory responses to these messengers can be orchestrated by mucosal cells, fibrocytes, macrophages, mast cells, endothelial cells, neurons, muscle cells, and polymorphonuclear cells.178 The chain of events is complex, with the relationship of all the different cell responses not yet fully understood. It is clear that the inflammatory response from any insult initiates a multitude of chemical and immune reactions, which, depending on the severity of the insult, can cause both local and systemic effects.
Several mechanisms can stimulate an inflammatory response, including ischemia, reperfusion after ischemia, inflammation from bacterial or viral infection, and inflammation from parasites, trauma (surgical), or toxins. The resulting tissue injuries vary and may be differentiated by their effect on the different layers of the intestine and by the vascular and nervous response. Though the mucosa or serosa is often affected first, the inflammatory response frequently involves the remaining layers of the intestine, the submucosa, and smooth muscle. The inflammatory response may also vary depending on the specific cause. For example, ischemia can be caused by strangulation of the blood supply, distention of the intestinal wall, or poor perfusion resulting from systemic shock. Although the response of individual intestinal cells may be similar for each, each stimulus appears to evoke different sequences of cell response, thereby causing different clinical signs and varying response to treatment. In some instances injury to the intestine may be secondary to another disease process, but the damage to the intestine can still initiate a systemic response resulting in a systemic inflammatory response syndrome (SIRS) with multiple organ failure.
INTESTINAL INFLAMMATION: GENERAL CONCEPTS
The cascade of events initiated by infection or ischemia has been extensively studied. Inflammatory mediators increased during bowel inflammation are released from numerous cell types, including mucosal cells, endothelial cells, fibrocytes, myocytes, mesothelial cells, and neurons.178 There is also evidence that the compounds making up the tissue ground substance and cytoskeleton can also initiate an inflammatory response and help transmit signals or cells between immunocytes and afferent neurons. Theoretically all cells in the intestine can act as effector cells, both producing cytokines to send messages to other cells and being activated to respond to the insult by chemokines.178,180,181 Cytokines, growth factors, and adhesion molecules can all initiate an inflammatory response.178,179 This response also alters cell apoptosis, resulting in delayed removal of mucosal cells and neutrophils or early death of immunocytes or specific cells in organs.182 Cytokines such as IL-1β and TNF-α, platelet-activating factor (PAF) complement (C5a), IFN-γ, and histamine are all reported to be involved in inciting intestinal inflammation.183 Describing all the effects and interactions of the inflammatory cytokines and eicosanoids is beyond the scope of this chapter, but it is apparent these substances can stimulate and inhibit the inflammatory reaction by directing cell communication and cell response to injury. As a result the disease process should be viewed as a sequence of altered cell functions, which are integrated and designed to protect the intestine from permanent injury.
The cells primarily observed to take part in intestinal injury are mucosal cells, endothelial cells, neurons, fibroblasts, mast cells, eosinophils, neutrophils, and macrophages. Rather than responding independently, these cells likely all respond to the initial insult, each with its inherent cytokine production or cell activation, which stimulates or suppresses other cell responses. Envisioned as a group acting simultaneously, the sequence likely proceeds from an initial stimulus to mucosal or serosal cells or in the case of ischemia the vascular endothelium or enteric neurons.
Mucosal cells react by releasing cytokines to activate macrophages and lymphocytes in the lamina propria.181 These cells release cytokines and adhesion molecules, which in turn activate other cells while initiating local defenses. Simultaneously, endothelial cells respond to the cytokine message by releasing cytokines to attract neutrophils and eosinophils and subsequently enable them to migrate through the endothelium into the interstitium. Afferent neurons detect cytokine increases and initiate neuropeptide, cytokine, or eicosanoid release from the efferent neurons, resulting in activation of numerous cells including those already activated by the initial cell messengers.184-188 Fibrocytes, responding to the initial cytokine messages and growth factors, subsequently release cytokines and growth factors such as Il-1α, Il-1β, TNF-α, transforming growth factor, and platelet-derived growth factor. All can be proinflammatory or can help to repair the mucosa.189,190 Metalloproteinases released in response to inflammatory cytokines alter the basement membranes and collagen in the different layers, allowing migration of cells to the extracellular space and stimulating other cells to release chemoattractants.178 Muscle cells, once thought to be neutral in the inflammatory cycle, appear to be able to release cytokines, thereby participating in the inflammatory reaction.178,191 Increased adhesion molecule production in the muscle after surgical manipulation has been linked to neutrophil infiltration and subsequent bowel dysfunction.192-194
Although all the cells in the intestine participate in the inflammatory response as effector cells, they can also act as suppressors. Each appears to communicate with other cells locally to cause or suppress the inflammatory response. Production of nitric oxide by endothelial cells can reduce neutrophil adhesion, whereas increased release of growth factor by fibroblasts and the vascular endothelium speeds healing of the mucosa.189 The response to receptor activation can take only seconds to minutes to upregulate cytokine production. Lack of suppression, perhaps because of chronic or overwhelming stimulation or severe damage to cells, which release the inhibitor messengers, allows amplification of inflammation and permanent cell damage.
The role of each cell type as effectors and messengers is slowly being unraveled. The role of some cells is better understood than that of others. Reperfusion, cytokines, and complement initiate endothelial cell changes. Subsequent production of cytokines and prostanoids by endothelial cells attracts neutrophils and macrophages.195 Endothelial cells also stimulate the inflammatory response by altering capillary permeability, promoting neutrophil adhesion, and altering blood flow. The interaction between the endothelial cell and neutrophils or eosinophils is a pivotal response in causing intestinal injury. This response is made possible by PAF, leukotrienes (LTB4), and adhesion molecules produced by endothelial cells and neutrophils.180,195 Neutrophil migration into affected tissues subsequently causes severe damage including damage to cells and tissue ground substance, which further promotes the inflammatory response.
Although all intestinal cells can be involved in the inflammatory process, the nervous system is now known to be integral in the inflammatory response. Release of potassium, adenosine triphosphate (ATP), bradykinin, and prostaglandin E2 all stimulate afferent neurons.184,196 Neuropeptides released from neurons in response to afferent signals caused by products of cell injury including cytokines, eicosanoids, and histamine.184,197 Substance P, neurokinin, calcitonin gene-related protein (CGRP), and vasoactive peptide (VIP) have all been found increased levels in inflamed tissues, suggesting that they act as messengers to histiocytes or immunocytes, which subsequently release cytokines.184,186 The paracrine response differs in different tissues, but inflammatory responses by mast cells, neutrophils, T cells (cytokine release), B cells, macrophages, fibroblasts, and muscle cells are in part caused by neuropeptide stimulation. The coordination of this response is not totally understood, but neuropeptides serve as proinflammatory mediators or suppressors.188,198 This system allows for immediate response of cells to a stimulus without humoral involvement and likely can cause persistent inflammatory reactions in response to inflamed tissue.184
Local inflammation of the intestine is known to cause changes in other organs distant to the intestinal damage, specifically the lung.199 Circulating cytokines and activated neutrophils rapidly initiate an inflammatory response in the lung after intestinal inflammation. This response is well known in experimental models and humans but has not been reported during intestinal disease in the horse. Other organs are likely affected, creating signs of multiple organ involvement as part of SIRS.200
Ischemia and Reperfusion
Ischemia is a deficiency of blood flow in tissue or an organ. The lack of energy production in the cell starts a degenerative process. Within 5 minutes there are alterations in mitochondria, characterized by swelling and disorganization of cristae.201 Mitochondrial changes precede cytoplasmic and membrane changes, which occur in the first 30 minutes by activation of phospholipases, cytokine production, and accumulation of arachidonic acid. If ischemia persists, the cell degradation continues with failure of the membrane ion pumps, allowing calcium to move into the cytoplasm.202 This calcium accumulation within the cell activates proteases, which cause cell membrane damage and nuclear clumping. Calcium uptake in the mitochondria is increased, which inhibits oxidative phosphorylation, thereby decreasing the cell’s source of energy.202
In the intestine, microscopic changes become evident at 30 minutes when the mucosal epithelial cells and serosal mesothelial cells separate from their basement membranes.203 This appears to be a mechanical separation caused by water movement from the vasculature into the subepithelial space. Metalloproteinases may also be involved in altering the basement membrane. The space, created by the initial separation, named Grunehagan’s space, occurs at the tip of the villus in the small intestine (Fig. 32-33).203 If ischemia continues, the cell damage progresses as the mucosal cells progressively slough off the lamina propria toward the intestinal crypts (see Fig. 32-33). The change is similar in the colon, with epithelial cells sloughing off the surface of the mucosa (see Fig. 32-33). However, the slough is somewhat slower in the colon as it proceeds into the crypts. The serosa reacts in a similar fashion, with mesothelial cells lifting off the basement membrane before there is visible cell membrane or cytoplasmic change. Other than vascular congestion, there is minimal change in the architecture of the supporting tissues in the mucosa or the serosa for the first 60 minutes of total ischemia. After 180 minutes of ischemia the lamina propria and mucosal vascular tuft have lost their architecture. The tissue is necrotic and becoming homogeneous, with lack of nuclear definition and cell structure.
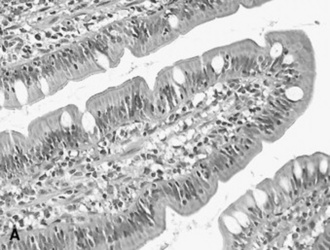
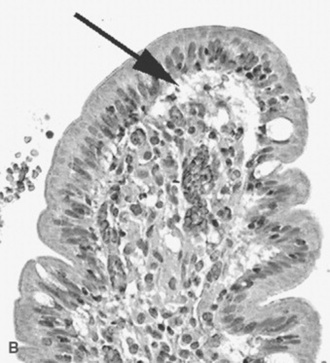
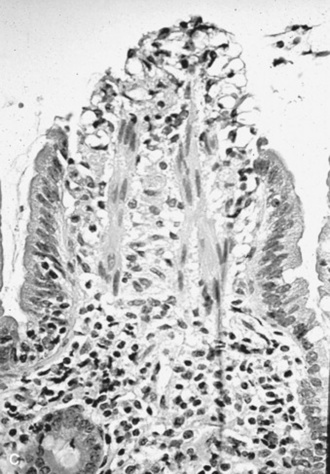
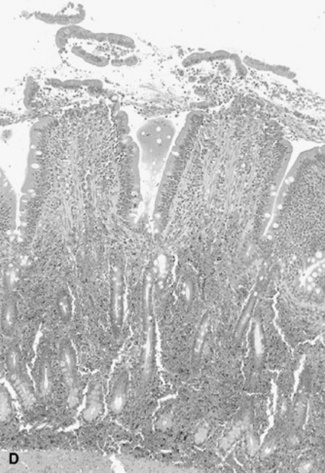
Fig. 32-33 Photomicrographs of A, normal small intestinal villus (hematoxylin and eosin stain [H&E]); B, grade I epithelial lesion with formation of Grunehagen’s space (arrow) at the top of the villus (H&E); C, grade III lesion with mucosal slough off the sides of the villus (H&E); and D, mucosal slough and red blood cell accumulation in the mucosa caused by venous strangulation obstruction of the small intestine (H&E).
Estimating the time required to create ischemic lesions depends on the experimental methods used. Different types of anesthesia in the horse and other animals have resulted in different rates of mucosal degeneration during ischemia. For example, experiments using animals anesthetized with inhalant anesthetics are different from those that required animals breathing air, most likely from higher tissue oxygen concentrations at the beginning of the experiment.203-205 Also, some cell types, such as muscle, appear more resistant to ischemia, which probably depends on the intracellular energy reserves of the cells. There are also differences in the response to low-flow ischemia versus total arteriovenous obstruction. Nevertheless, lesions caused by ischemia progress with reperfusion.
If reperfusion—either resumption of blood flow or increased flow after low-flow conditions—occurs while cells are still viable, a cascade of events is set in motion by the delivery of oxygen to the previously ischemic tissue.195,206 The resultant injury is called reperfusion injury and relies on renewed oxygen in the tissue, with participation of endothelial cells and afferent receptors to create the subsequent inflammatory response. Although it makes sense that oxygen is needed to resuscitate the previously ischemic cells, the innate defense system in many cell types is to respond to the ischemic change with an inflammatory response. This response appears to help initiate a defense against bacterial invasion and is aimed at removing the damaged cells from the system. If enough cells have been damaged, the reperfusion effect can cause enough inflammation to prevent cells from surviving, thereby delaying healing.
Reperfusion injury starts as a change in intracellular metabolism in previously ischemic tissue. It does not require exposure to a microbe or toxin, but it does rely on cell production of cytokines and leukotrienes as signals to numerous blood and tissue cells required to complete the process. One primary initiator of reperfusion injury in the intestine is the production of oxygen radicals (O2•). Oxygen has the ability to take on an extra electron during enzymatic processes in the cell. Most cells including endothelial cells and the small intestinal mucosal cells contain xanthine dehydrogenase, which when converted to xanthine oxidase catalyzes hypoxanthine to xanthine.206 Hypoxanthine is increased in the cytoplasm of cells during ischemia. Calcium and proteases initiate xanthine dehydrogenase conversion to xanthine oxidase, a process that can be stimulated in endothelial cells by IL-1, TNF-α, and C5a, as well as neutrophil adherence. Oxygen is used in the reaction and ends up with an extra electron, making an O2• or superoxide.205
Once released, O2• can initiate a number of chemical reactions that cause cell membrane damage directly and by stimulating phospholipase activity. During O2• production nitric oxide (NO) production is decreased, thereby allowing neutrophil adhesion and migration. Superoxide interaction with iron or catalase causes production of hydroxyl radicals (OH•) or hydrogen peroxide (H2O2) respectively. Both are cytotoxic. The OH• alters or destroys cell membranes. Affected cells rapidly express cytokines and leukotrienes, which act as chemoattractants for neutrophils. After the initial free radical production, cell injury progresses as two main events. Calcium, already increased in the cell, increases further, effectively blocking further energy production in the mitochondria and activating proteases, causing further degradation of the cell nucleus and cytoplasm.202 Second, radicals formed from molecular oxygen can cause cell membrane damage, thereby initiating a similar cellular response. The formation of chemical mediators takes only seconds to minutes after reperfusion and rapidly sets the inflammatory cascade in motion.
Although the mucosal cells and serosal mesothelial cells are damaged early in ischemia and are able to release cytokines to alert other cells locally, endothelial cells appear to be the primary initiators of reperfusion injury.179 They participate in cytokine production and are involved with neutrophil adhesion and migration from blood into tissues. Endothelial cells are also involved with the changes in blood flow after reperfusion.188 The platelets and neutrophils accumulate in and obstruct capillaries, altering blood flow. Endothelial cell swelling or contraction causes constriction of blood vessel lumens (Fig. 32-34). Changes in endothelial cells in response to histamine, complement, leukotrienes, and PAF increases capillary permeability, allowing fluid and protein to move into the interstitium.188 Even though the metabolic state of the tissue during initial reperfusion initially results in overall increased blood flow, the increased vascular permeability increases interstitial pressure, causing capillary collapse. Combined with endothelial cell change, vascular constriction and collapse of the tissue vasculature results in a “no-reflow phenomenon,” promoting further tissue ischemia.
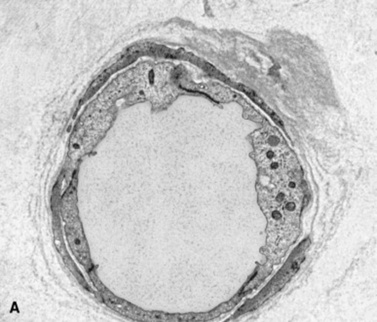
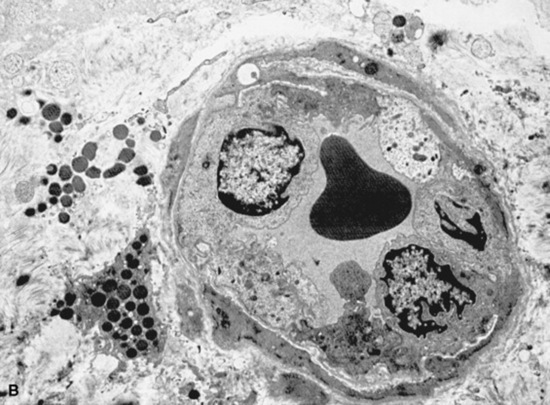
Fig. 32-34 Transmission electron photomicrographs of small vessels in the serosa (A) with normal endothelial cells before ischemia and (B) with swollen endothelial cells and accumulation of neutrophils narrowing the vessel lumen during reperfusion.
Endothelial cell membrane changes allow expression of adhesion molecules and receptors, which are necessary for neutrophil adhesion and migration through the capillary and venule endothelium (Fig. 32-35). This migration of neutrophils causes the most prominent inflammation in the tissue, because activated neutrophils release elastase, oxygen radicals, and other serine proteases, which attack collagen, ground substance, and cell membranes.207 The respiratory burst, a buildup of oxygen free radicals in neutrophils during activation, is responsible for most of the inflammation and tissue damage seen during reperfusion. It is also apparent that reperfusion injury can expand the initial injury into the surrounding viable tissue and that it can cause irreversible tissue damage.208-210 Reperfusion injury can also cause injury distant from the local damage and has been observed in the lung because of cytokine and activated neutrophil circulation.211 Although not reported in the literature, the resulting lesion can be seen in the lungs of horses that succumbed to severe intestinal strangulation, obstruction, and shock.
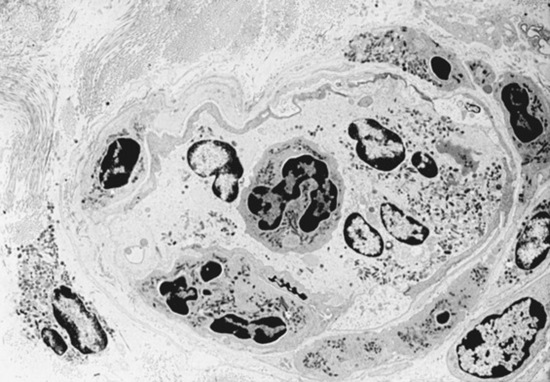
Fig. 32-35 Transmission electron photomicrograph of neutrophils adhering to the endothelium and accumulating in the perivascular space after migrating through the endothelium.
Cell necrosis is recognized as a pathologic feature during ischemia and reperfusion. Recently, however, alterations in apoptosis (programmed cell death) have been found to increase during ischemia and reperfusion in experimental studies of the heart, brain, liver, adrenal glands, kidney, and intestine.212-214 Alternatively, epithelial cells undergoing hypoxia and reoxygenation modulate neutrophils to delay apoptosis, suggesting a role in initiating SIRS.215 Significantly increased apoptotic cells in muscle, mucosa, neurons, and glia in naturally obstructed or strangulated equine intestine suggests apoptosis is stimulated by and may promote the inflammatory response.216,217 Apoptosis is also increased in intestine distant to the primary lesion, supporting systemic stimulation by cytokines.218 The amount of apoptosis observed in experimental animals and in horses with intestinal obstruction suggests this process is excessive during reperfusion and may result in both morphologic and functional changes.
The importance of oxygen radical generation as a mechanism of injury in strangulating lesions has been questioned in the horse.219 However, both clinical signs and lesions from clinical cases suggest that intestinal damage progresses after reperfusion. Use of a low-flow to no-flow model of ischemia with subsequent reperfusion responded to treatment to counteract injury from superoxide production and neutrophil accumulation.220 This suggests that free radical production is part of the cascade of events causing an inflammatory response in the small intestine after bowel strangulation. Lack of malondialdehyde and conjugated diene production in the large intestine suggests less of a role for tissue-generated oxygen radicals and that reperfusion injury is primarily caused by the oxygen radical release from migrating neutrophils as seen in xanthine oxidase—deficient rats.207,221,222
Recent research suggests that endothelial cells are not the only cells that act as “local effectors” of inflammation during reperfusion. Signs of inflammation are present in all parts of the intestine including the muscle and myenteric plexus.191,223-225 Alterations in muscle mitochondrial morphology have been identified in equine jejunum after low-flow ischemia.226 Similarly, during large colon volvulus the neurons in the myenteric plexus undergo degeneration and decrease in number compared with normal conditions.224 Evidence of ischemic injury and inflammation occurs in both muscle and myenteric plexuses. This is relevant in horses with colon volvulus, in which survivors had significantly more neurons than nonsurvivors.224 Perhaps of greater importance is the long-term effect of the inflammation in the myenteric plexus after ischemia and reperfusion. One can speculate that the high rates of repeat colic episodes in horses after colon torsion or large colon impaction are related to damage to the enteric nervous system.227
Reperfusion injury occurs after low-flow and no-flow ischemia. These models appear to emulate the clinical event, although the onset of ischemia in strangulation obstruction most likely starts as low-flow progressing to no-flow ischemia. Although the time frame of reperfusion injury is somewhat understood from the experimental models, the functional effects of dynamic events set in motion by reperfusion such as “no reflow” as well as persistent inflammation in the serosa and around the intestinal nerve ganglia are not well understood. The relevance of these myenteric plexus lesions is also questioned, because they do not always correlate with severity of clinical signs.224
Low-Flow Ischemia
The effect of ischemia-reperfusion differs with the type of ischemia. Low-flow ischemia is defined as flow less than 25% of normal blood flow. At this flow rate, vascular and cell damage is minimal, with degeneration occurring over a long time period. When normal flow is returned by increasing vascular volume or release of the arterial obstruction, there is a hyperemic response doubling the normal blood flow to the affected intestine.226 Cell damage accelerates during reperfusion, with marked changes in vascular permeability, mucosal damage, serosal damage, and signs of fluid accumulation in the submucosa, muscle, and serosa.206,226,228,229 Edema in the submucosa and serosa causes vascular collapse, leading to decreased blood flow and eventually a “no-reflow” phenomenon in the serosa and mucosa, with flow to the affected segment decreased below normal.230,231 The decreased flow is partially caused by endothelial cell swelling and neutrophil margination in the vasculature. Neutrophils migrate into all tissues but predominately the mucosal and serosal layers. As neutrophils migrate through the capillary or venule, they accumulate around the vessels. As reperfusion progresses, the greatest injury is observed in the serosa, with marked accumulation of neutrophils beneath the serosal basement membrane. Although neutrophil accumulation is most marked in the serosa, neutrophils can be found in all layers of the intestine. This type of low-flow ischemia is rarely documented in equine abdominal disease, but it likely exists in distended intestine in which there is low flow to the affected bowel wall. The flow is directly related to the intraluminal pressure, with pressures causing greater than 50% reduction in blood flow common in severe small intestinal distention.232
When examined in experimental models or when observed in clinical cases, most of these changes from acute low-flow ischemia are reversible and the affected intestine survives and heals. The effect varies greatly and is dependent on the length of ischemia. It is suspected that function is compromised temporarily, resulting in ileus, endotoxin absorption, or enteritis with excess secretion.233 In the small intestine serosal scarring and adhesions are the most common long-term result and may increase the risk of colic episodes in the future. Even if lesions are not grossly evident and appear minor on microscopic examination, horses with colic, particularly those requiring surgery, likely have intestinal injury, which can ultimately result in permanent intestinal dysfunction. Hypothetically this is one possible reason for increased risk of colic after previous colic episodes or previous surgery for colic.227,234
Nonstrangulating infarction is the classic example of low-flow ischemia. In the horse, thromboembolic colic is reported to be caused by thrombosis of the mesenteric artery and its branches in horses infected with fourth stage larvae of S. vulgaris.235 The mechanism of infarction is most likely reduction of blood flow from a thrombus in the artery or thromboembolism obstructing the peripheral vasculature. In nonstrangulating infarction the intestinal injury most likely results from long-term low-flow ischemia or low-flow ischemia with episodes of reperfusion injury. In clinical cases the injury is severe, often resulting in necrosis in all layers of the bowel, with obvious infarction.
Low-flow ischemia may also occur during shock.194 Intestinal injury in bowel not directly involved in the primary lesion is suggestive that this injury is frequently present in horses with bowel strangulation obstruction.194 In horses with obstructing or strangulating lesions, biopsies from nonaffected, grossly normal intestine have evidence of some degree of bowel injury including neutrophil infiltration.209,210,216,236 Endotoxin administration also caused an inflammatory reaction in the intestines, with loss of mucosa and neutrophil infiltration in the mucosa.237 This may be relevant to assessing horses in shock.
Total Arteriovenous Occlusion
Total ischemia caused by arteriovenous occlusion causes total vascular stasis. The most common cause of this form of ischemia is the mesenteric infarction caused by incarceration of bowel in hernias or constricted spaces. Early in the occlusion, capillary congestion is observed in all layers of the intestine. The intestine undergoes degeneration as previously described for ischemia. When total ischemia exceeds 2 hours in the small intestine and 3 hours in the large colon, the tissue changes during blood flow occlusion are extensive, and cell necrosis may preclude a response to reperfusion.203-205,238-241 If the capillary damage is not too extensive during total ischemia, reperfusion causes further degeneration including mucosal cell loss, increased edema, and neutrophil migration (see Fig. 32-33). However, oxygen radical production seen with low-flow ischemia and reperfusion may not be responsible for the continued damage during total arteriovenous occlusion.242 Furthermore, these events cannot be separated from the systemic shock, which can be cause secondary lesions seen in intestine distant to the lesion.209 Despite the questions about the validity of reperfusion in the total ischemic event, continued intestinal injury during reperfusion is seen in both the small intestine and the large colon after total ischemia.203,208,243 The epithelial cell loss after reperfusion is progressive and is likely caused by either countercurrent exchange creating a decreased oxygen concentration in the distant mucosa or by inflammation resulting from oxygen radical—induced inflammation from neutrophil activation.207,223,244 The response in the serosa includes marked edema with initiation of neutrophil migration. Eventually a massive collection of neutrophils fills the outer border of the serosa.245,246 During this type of ischemia and reperfusion, the damage from the inflammatory reaction can continue for days and if severe enough can prevent healing of the mucosa and serosa and is consistent with persistent ileus and shock.
Venous Occlusion
Venous obstruction without arterial obstruction causes increased pressure at the capillary, resulting in decreased blood flow and RBC and fluid extravasation into the interstitium. The result is separation of the tissues with an increased diffusion distance for oxygen and nutrients. The RBC accumulation causes tissue damage, and if it is severe enough a continuation of cell hypoxia becomes irreversible even with reperfusion. Injury during venous obstruction appears more severe than observed during subsequent reperfusion.247 Small intestine can survive 2 hours of venous obstruction, although the resultant degeneration is severe and the intestine permanently scarred after healing.204 The gross lesion is marked with obvious thickening of the bowel, which at first is red and eventually purple. With time the color turns black or green with subsequent necrosis and alterations in hemoglobin.
Based on both experimental work and observations in clinical cases, irreversible death of a segment of the intestine occurs after 2 hours in the small intestine and 3 hours in the large colon, although this obviously varies depending on the amount of residual blood flow to the affected tissue.204,205,239 For the surgeon venous occlusion is the most difficult type of lesion to assess, as intestinal thickness or amount of hemorrhage may not correlate with viability. Recent reports of survival of horses with hemorrhagic lesion at surgery suggest that this injury can be reversed more readily than a lesion caused by total arteriovenous occlusion. The sequence of mucosal degeneration remains the same in this type of ischemia, making the biopsy the most reliable method for determining if the intestine can survive and heal.205
Distention
Although distended intestine may appear to have normal color and motility after decompression, alterations in blood flow in the wall of the distended intestine are a form of low-flow ischemia. During intestinal distention intraluminal pressure causes collapse of the veins and capillaries, thereby decreasing the vascular capacity. This occurs even when blood pressure is normal. When small intestinal pressure is increased to 18 cm of water, as previously measured in clinical disease,248 mesenteric blood flow to the intestine is decreased by at least 50%.232,249 Increasing capillary back pressure while maintaining arterial pressure alters Starling forces in the intestinal wall. The result is secretion of fluid from the vasculature even with an increase in wall tension. Water and some protein escapes into the interstitium, causing submucosal and serosal edema. At higher intraluminal pressures there is more secretion than absorption of water, creating a cyclic increase in intraluminal pressure after bowel compliance has reached its limit.250 Fluid and eventually protein leaks through the serosa into the peritoneal cavity.
If the pressure is maintained, intestinal compliance allows blood flow to gradually increase, acting as a form of reperfusion. Serosal and submucosal edema progresses during the period of distention. The mucosal and serosal lymphatics dilate, and RBCs and WBCs migrate into the serosa and submucosa (Fig. 32-36). There is minimal mucosal injury at pressures seen in clinical cases during simple obstruction of the small intestine or colon.
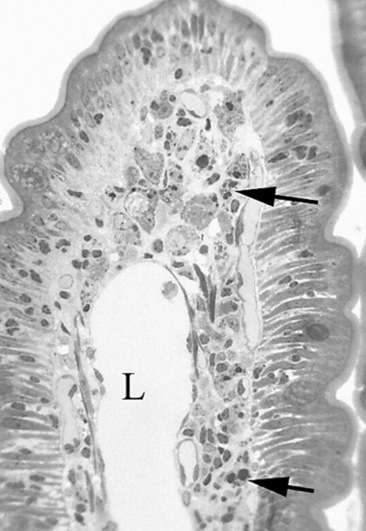
Fig. 32-36 Photomicrograph of edema, lymphatic dilatation, and RBC and WBC accumulation in the serosa after 18 cm H2O distention for 2 hours and reperfusion for 1 hour.
Hematoxylin and eosin stain.
Subsequent decompression of the bowel causes reperfusion with a hyperemic response similar to that seen after low-flow or total ischemia. Blood flow to the affected bowel can initially double, but this effect is temporary, with subsequent blood flow decreasing below normal.232 The mucosa appears relatively resistant to short-term distention, whereas the edema in the serosa causes capillary closure and increased vascular permeability. Serosal edema increases and more neutrophils migrate into the serosa, causing destruction of collagen and ground substance.232,245 Intestinal smooth muscle is also affected with edema, and neutrophil migration is evident in the fascial planes around the myenteric plexuses (Fig. 32-37). Although bowel can frequently heal after this type of ischemic insult, the serosa is often thickened by fibrous tissue with the possibility of adhesion formation and mesenteric constriction.251 The mucosa appears relatively resistant to the low-flow ischemia experienced with bowel distention, but the response is time dependent. Rarely does prolonged distention cause bowel necrosis. Bowel wall necrosis is more common when foreign bodies or impactions cause focal distention. Because the response to distention is both time and pressure dependent, the time frame for permanent damage is difficult to determine. The small intestine is more susceptible to distention injury compared with the large colon. Clinical measurement of intraluminal pressure during obstruction indicates that the large colon can tolerate at least twice the distention pressure with no adverse effects.252,253
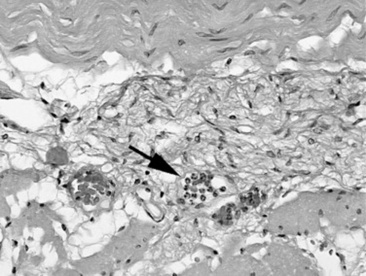
Fig. 32-37 Photomicrograph of neutrophil infiltrate (arrow) in the muscle and around a myenteric plexus after low-flow ischemia and reperfusion.
Hematoxylin and eosin stain.
Intestinal dysfunction occurs after decompression of bowel that was previously obstructed. Ileus with gastric reflux is common in horses with previously distended small intestine, and the bowel pressure measured at surgery can predict the severity.252,253 Abnormal motility with lack of response to prokinetic drugs is also observed after small intestine distention in vitro.254 Although the injury from distention cannot be separated from conditions in the peritoneal cavity or failure of the cardiovascular system, the correlation of increased intraluminal pressures and survival indicates the importance of intestinal distention in causing injury.230,232,248,251,252
The enteric nervous system also appears to respond to intestinal distention. Chronic obstruction caused by impaction of the colon or cecum or colon displacement has been associated with a significant decrease in the number of neurons in the myenteric plexuses, whereas the number of myenteric plexuses was similar to that in normal horses.224 This change in neuron number was also associated with increased thickness of the longitudinal muscle in the pelvic flexure or both circular and longitudinal muscle hypertrophy in the cecum.255 This appears similar to pseudoobstruction in humans and to experimental denervation of intestinal segments in rats. The lack of nervous inhibition is hypothesized to allow constant and uncoordinated muscular contractions with resulting hypertrophy and eventually poor transit of ingesta.
Myenteric plexuses from distended or obstructed large colons also had an increase in the number of glial cells (Fig. 32-38). This appears to be an inflammatory response by the enteric nervous system to the conditions involved with bowel distention. Alternatively the inflammation and neuron dropout may have been responsible for colon dysfunction in these horses examined because of colon obstruction.