Animal Reproduction
When you have completed this chapter, you will be able to:
1 List the stages of the canine estrous cycle and describe the events that occur in each stage.
2 List the female reproductive hormones and describe their roles in the process of reproduction.
3 Describe the formation and structure of the placenta in domestic animals.
4 List and describe methods used to confirm pregnancy.
5 Describe the events that occur in each of the stages of whelping in the canine.
6 List clinical signs of pyometra, eclampsia, metritis, mastitis, and brucellosis in canines.
7 List the stages of the feline estrous cycle and describe the unique features of the feline reproductive process.
8 Differentiate between polyestrous, seasonally polyestrous, and induced ovulator reproductive cycles.
9 Describe the procedures and tests used for performing a breeding soundness examination in the mare.
10 Describe methods for synchronization of estrous in herd animals.
There are two embryologic tubular systems present in the early embryo that will become either the male genitalia or the female genitalia. If an animal has XY chromosomes, it will become a male, and one tubular system (Wolffian) will persist. In the male, the female tubular system regresses, and the Wolffian system becomes the epididymis and vas deferens. If an individual has an XX karyotype, the other tubular system (Müllerian) will persist and develop into a female reproductive system. In the female, the Müllerian system becomes the uterine tubes, uterus, cervix, and anterior vagina. Because there are two systems, many potential abnormalities can occur when sections of the systems fail to regress or fail to develop fully. An example of an embryonic malformation would be the hermaphrodite, which has developed both male and female gender organs.
In both the male and female, the brain is the initiator of the reproductive cycle. Neural input from higher brain centers results in the release of gonadotropin-releasing hormone (GnRH) from neurons in the hypothalamus into the hypophyseal-portal vessels. The GnRH then enters the anterior pituitary, causing release of the gonadotropins, follicle-stimulating hormone (FSH), and luteinizing hormone (LH).
Both FSH and LH are large, complex hormones that are stored within granules inside cells in the anterior pituitary. These hormones are glycoproteins, a complex of carbohydrates and proteins. Because the gonadotropins are extremely large and complex, they cannot be synthesized in the laboratory. The complexity and protein nature of the gonadotropins make them antigenic, and exogenous administration of these drugs derived from other species can induce antibody formation.
GENERAL FEMALE REPRODUCTION
The normal stages of reproduction are proestrus (the time leading to estrus), estrus (the time of mating), and diestrus (the time when pregnancy is being established). In most species, pregnancy is the normal event that ensures species survival. However, if pregnancy does not occur, the female will return to a sexually active state to entice mating with a male. If pregnancy is not established, the events will recur, thus forming a cycle of proestrus, estrus, diestrus, proestrus, estrus, diestrus, and so forth. The other normal events in the reproductive life of an animal are puberty (the time of first ovulation), pregnancy, and anestrus (time when the animal is not undergoing any reproductive events). The name given to these recurring events is the estrous cycle. (Note that estrus is spelled estrous when it is an adjective, as in estrous cycle.)
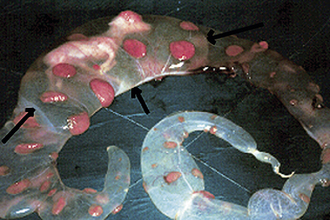
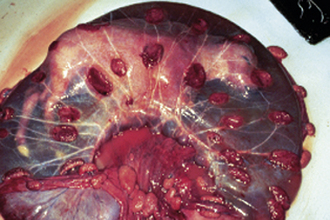
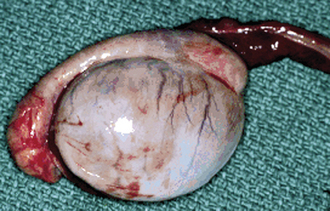
The beginning of an estrous cycle in most females starts with GnRH from the hypothalamus, causing the release of FSH from the anterior pituitary (Figure 14-1). The FSH is released into the bloodstream and is carried to the ovaries where it initiates its follicle-stimulating action, causing the growth of ovarian follicles. Ovarian follicles are structures on the ovary that contain the egg, or oocyte (Figure 14-2). All the oocytes that a female will ever have are present on the ovary at birth, and most of the follicles contain no fluid. Some follicles are selected to grow and start to develop fluid around them (an antrum) in a process that is independent from FSH stimulation. Once a follicle has reached the antral stage, the action of the FSH causes rapid growth of the follicle. The follicle wall is composed of two layers, the thecal cell layer and the granulosa cell layer. As the follicle grows, it produces the steroid hormone estrogen. Estrogen is the hormone that causes the characteristic behaviors and sexual receptivity when the female is in estrus. The oocyte within the follicle also begins to mature so that it will be ready for fertilization after ovulation. As the follicle grows and reaches maturity, the granulosa cells also produce a protein hormone called inhibin. Inhibin inhibits further FSH release so that only a species-specific number of follicles are chosen for final growth and maturation (depending on the species, this may be one or more).
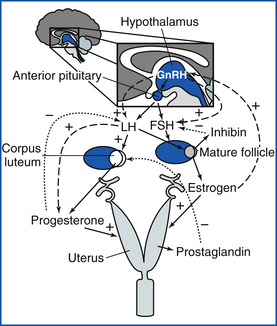
FIGURE 14-1 The general hormonal control of female reproduction: Pulsatile GnRH causes FSH to be released from the anterior pituitary. FSH causes follicular growth and maturation, where estrogen is produced. Inhibin from the follicle feeds back on the anterior pituitary and causes less FSH to be released. A surge of estrogen from the follicle causes GnRH release from the hypothalamus, which causes an LH surge. LH causes ovulation of the follicle and the formation of a CL, which produces progesterone. If a pregnancy signal is not secreted by the early embryo (bovine, equine, ovine, caprine), prostaglandin is released from the uterus, goes to the CL, and causes luteolysis (luteal death). The cycle then starts over with follicular growth.
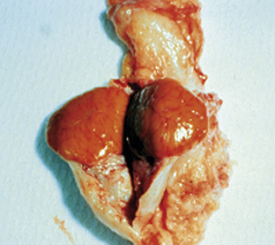
The estrogen surge produced by the developing follicle stimulates the release of GnRH from the hypothalamus, which causes release of LH from the anterior pituitary. At the ovary, LH causes the mature follicle to ovulate. Ovulation is the release of the oocyte into the oviduct. After ovulation, the follicle transforms into a corpus luteum (CL), or yellow body. The CL (Figures 14-2 and 14-3) has been transformed by the LH surge to produce only progesterone, the hormone that maintains pregnancy.
Pregnancy actually begins in the uterine tube. The uterine tube is often called the oviduct and consists of three segments: the infundibulum, the ampulla, and the isthmus (Figure 14-4). When ovulation occurs, the oocyte is picked up by the dilated end of the oviduct called the infundibulum. The oocyte is then transported through the ampulla to the junction of the isthmus and ampulla. In most species, this is where fertilization occurs. Fertilization is the joining of the oocyte, which has half of the chromosome complement, with the sperm cell, which has the other half of the chromosome complement, thus forming the embryo that has both the maternal and paternal chromosome complements. The embryo normally stays within the oviduct for several days before it moves into the uterus.
If the oocyte is not successfully fertilized, the CL still develops and maintains progesterone production for a time that is consistent within each species. This time of progesterone domination is called diestrus. It is the early embryo in most species that signals to the uterus and ovary that pregnancy has been established. If the signal for pregnancy is not received by the uterus, most species (not the dog and cat) will initiate an ending of diestrus so that the animal can return to estrus and have another chance to become pregnant. In most species, this occurs when the hormone prostaglandin is released from the uterus. Prostaglandin is a small molecule derived from arachidonic acid. When prostaglandin is released, it binds to receptors on the CL and lyses it. Because the CL is the source of progesterone, after the CL is destroyed by prostaglandin, the progesterone concentration in the blood falls. When progesterone is not present, there is a rise in FSH that allows new follicles to grow. This time of growing follicles after the death of the CL is called proestrus. As the animal enters proestrus, the follicles grow, estrus follows, the LH surge causes ovulation, and a CL forms. Therefore the animal has estrous cycles until pregnancy is established.
Once the embryo is in the uterus, it must establish itself as a viable pregnancy before prostaglandins destroy the CL. Different species have different mechanisms to do this. Each species appears to have a relatively unique substance produced by the early embryo that prevents the CL from being destroyed by prostaglandins released from the uterus. Dogs and cats appear rather unique in that their corpora lutea (plural for corpus luteum) appear to have preprogrammed life spans without any endogenous destruction by prostaglandins if they are not pregnant.
As pregnancy progresses, the early embryo changes from an embryonic disk to a more complex structure that includes the embryo or fetus and placenta. After all the bodily organs are formed, the embryo is called a fetus. The placenta forms from specialized cells on the embryo. These cells develop into the chorion and allantois. The amnion is a fluid-filled sac that immediately surrounds the fetus, whereas the chorion and allantois fuse to form the chorioallantois. The fetus has two fluid-filled sacs surrounding it (Figure 14-5). The chorion attaches to the uterus and has the function of transferring nutrients from the uterus to the fetus. The structure of the placenta varies from species to species (Figures 14-6 and 14-7). The ruminants have many individual attachment areas called placentomes. Dogs and cats have a zone of the placenta that attaches to the uterus. Horses and pigs have a more generalized attachment (diffuse) of the placenta to the uterus. In some species, such as the ewe and horse, the placenta produces the progesterone that maintains the pregnancy instead of the CL.
Parturition is the act of giving birth. The only domestic species in which the entire parturition mechanism is completely understood is sheep; other species are hypothesized to be similar. As gestation progresses, the fetal hypothalamus and pituitary mature enough to cause the fetal release of corticotropin-releasing hormone from the hypothalamus, which causes the fetal adrenal gland to produce high concentrations of cortisol. The high cortisol concentrations cause a change in placental production of progesterone to estrogen and a release of prostaglandins from the uterus. These hormones cause the cervix to dilate and the uterus to contract, thereby forcing the fetus out. Parturition is normally divided into three stages: I, II, and III. Stage I is the preparatory stage. Maternal pelvic ligaments relax and the cervix softens, preparing for delivery. Behaviorally the female becomes restless and prepares to give birth. The expulsion of the fetus is stage II (Figure 14-8). Stage III is the expulsion of the placenta. The timing of the events of parturition varies by species. A summary of the length of the estrous cycle and the length of gestation is found in Table 14-1.
CANINE REPRODUCTION
The estrous cycle of the bitch has four phases: proestrus (9 to 10 days average), estrus (9 to 10 days average), diestrus (57 to 58 days average), and anestrus (2 to 5 months average) (Figure 14-9). Interestrus consists of diestrus and anestrus combined and usually lasts 4 to 7 months. Interestrus periods of less than 4 months are associated with infertility.
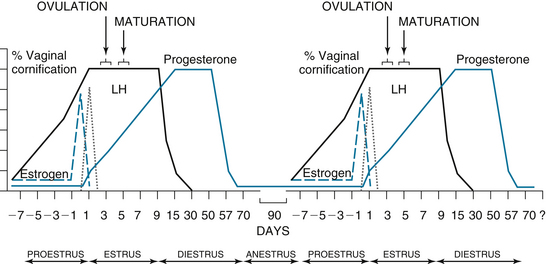
FIGURE 14-9 The canine estrous cycle. Proestrus lasts about 9 days. During proestrus, the vaginal cornification increases about 10% per day, and estrogen peaks at the end of proestrus. There is 100% vaginal cornification throughout a 9-day estrus. At the beginning of estrus, the LH peaks. At the same time the LH peaks, progesterone starts to rise. About 2 days after the LH peak, ovulation occurs, followed by a 2- to 3-day maturation of the oocytes. At the start of diestrus, the vaginal cornification abruptly declines to less than 50% cornified. Diestrus lasts about 57 days and is characterized by high progesterone. At the end of diestrus, progesterone declines, and the bitch enters a 90- to 150-day anestrus. The cycle then starts over again.
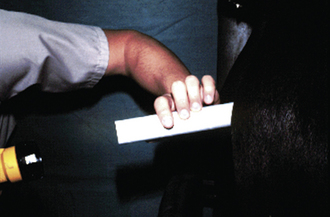
During proestrus, the bitch is attractive to male dogs, but will not allow mating. The vulva appears swollen, and a serosanguineous discharge is present. Estrogen, which is rising during proestrus, causes the epithelial cells in the vagina to cornify. Vaginal cytology is a common tool used to follow a bitch’s cycle. To prepare a vaginal cytology slide, moisten a long cotton swab and pass it through the vulva and vestibule (Figure 14-10). The canine vagina is nearly vertical at the entrance. To get a swab into the cranial vagina, once past the vulvar lips, direct the swab nearly vertical (toward the anus) until it will go no further. Redirect the swab horizontally and twirl it to obtain a sample. Roll the swab on a slide and stain the slide using a modified Wright’s or Giemsa stain. Noncornified cells have a rounded cytoplasm and a large stippled nucleus. Cornified cells have a more angular-shaped cytoplasm, and the nucleus is either pyknotic or not apparent. The percentage of cornified cells increases approximately 10% per day, reaching 90% to 100% (full cornification) by the onset of estrus (Figure 14-11).
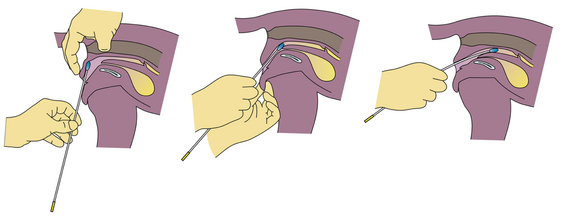
FIGURE 14-10 A diagram demonstrating cell collection for vaginal cytology preparation and examination. (From Eilts, BE: Determining estrous status, NAVC Clinician’s Brief 5:40-41, 2007.)
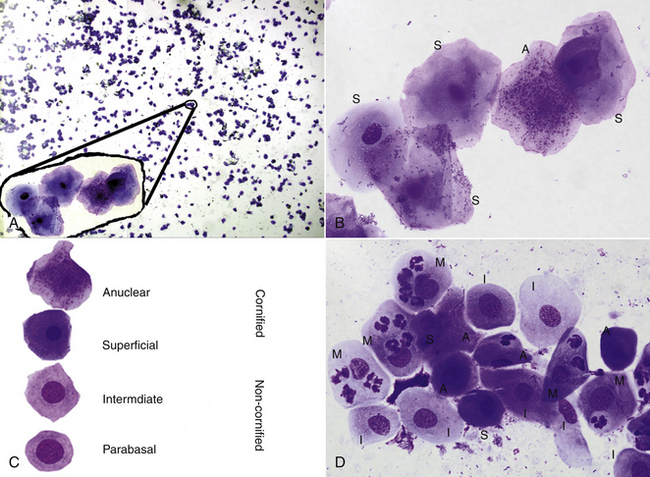
FIGURE 14-11 A low-power (A) and a high-power inset from the low-power view (B), of a vaginal cytology sample of a dog in estrus. Cell types demonstrated in a canine vaginal cytology (C). A high-power view (D) of a vaginal cytology of a dog in diestrus. The upper right and lower right cells are labeled with the cell type from the lower left (M, metestrum cell; I, intermediate; S, superficial; A, anuclear). (From Eilts, BE: Determining estrous status, NAVC Clinicians Brief 5:40-41, 2007.)
Estrus is the period of receptivity when the bitch will allow mating. During estrus, the vulvar swelling typically decreases slightly, and the bloody discharge changes to a straw color, although a bloody discharge may continue throughout estrus. Vaginal cytology is fully cornified, and the background of the slide is clear. No white blood cells should be present. Occasionally, bacteria may be seen on the slide.
The end of estrus, or the first day of diestrus, is typified by an abrupt decline in the percentage of cornified cells. This day is important to detect because whelping can be accurately predicted from day 1 (D1) of diestrus. D1 of diestrus also correlates well with the LH peak (8 days before D1) and ovulation (approximately 6 days before D1).
To maximize fertility, viable sperm must be present when the oocyte is ready to be fertilized. If the male is readily available, mating every other day is usually practiced. If performing artificial insemination (AI) and male availability is not limited, breeding three times per week (e.g., Monday, Wednesday, Friday) for as long as the vaginal cytology is fully cornified provides equally good results. However, when the number of breedings is reduced to one or two during a single estrus, such as with frozen or fresh cooled semen, timing insemination to coincide with ovulation becomes critical. Ovulation occurs approximately 2 days after the LH surge. The bitch ovulates immature oocytes that require 2 to 3 more days for maturation. Mature oocytes are then viable for another 2 to 3 days. Therefore the fertile period is 4 to 8 days after the LH surge, with peak fertility occurring 5 to 6 days after the LH surge.
To best estimate the day of ovulation, hormone assays should be used. Vaginal cytology does not give a precise prediction of ovulation. The LH peak may occur anywhere from the same day of or up to 2 days after full cornification. Unlike most species, serum progesterone rises before ovulation in the bitch. Therefore progesterone is useful to predict the LH surge because the increase in serum progesterone is closely associated with the LH peak. Before the LH surge, serum progesterone is less than 1 ng/ml. On the day of the LH surge, serum progesterone rises to 1.5 to 2.0 ng/ml (2 ng progesterone = 3.18 nmol progesterone) and after that continues to rise during diestrus or pregnancy. By identifying this initial rise in progesterone, the day of the LH surge can be estimated and insemination performed during the period of peak fertility. Although LH can be measured semiquantitatively with in-house test kits (Figure 14-12), the peak only lasts 1 day, so serum must be tested daily. Progesterone assays provide some advantages because after the initial rise (on the same day as the LH surge), progesterone continues to rise, so the day of initial rise can be estimated even if a day is missed. Ovulation occurs 2 days after the LH rise and is associated with a progesterone concentration of 5 ng (15.90 nmol)/ml.
In-house, semiquantitative kits for progesterone analysis are available (Figure 14-13). The in-house tests are easy to perform, and results are available in about 20 minutes. Some aspects of the tests require attention to detail to achieve meaningful results. The kits need to be at room temperature before use, and the manufacturer’s recommend that the kits be placed at room temperature for approximately 2 hours before use. If the test is run using a cold kit, results will be incorrect, often giving a false high progesterone. Blood should be allowed to clot at a cool temperature (in the refrigerator), and cells should be separated from serum or plasma as soon as possible (within 20 minutes of collection is the manufacturer’s recommendation). If serum is allowed to remain in contact with the red blood cells, progesterone will be bound by them, and test results will be artificially low. Hemolyzed or lipemic samples may also cause erroneously low results. Serum samples may also be frozen for analysis later. If a laboratory is available that can give rapid turnaround time and provide quantitative results, this is preferable when breeding with frozen semen or any time breedings are limited.
If only two breedings are performed, such as with fresh cooled semen, insemination should be performed on either days 3 and 5 or days 4 and 6 after the LH peak or initial rise in progesterone, keeping in mind that the viability of fresh chilled semen is reduced, and the timing of insemination is more critical. The viability of frozen semen is reduced even further, and the timing is even more critical. When frozen semen is used, usually a single surgical insemination is conducted. Insemination with frozen semen should be performed on day 5 or 6 after the LH peak or initial rise in progesterone or 3 days after a progesterone level of 5 ng (15.90 nmol)/ml. Insemination with frozen semen is performed either surgically or by a transcervical endoscopic method. It is beneficial to continue to monitor vaginal cytology and identify D1 of diestrus, which usually occurs 8 days after the LH peak or 6 days after ovulation. Significant variations of the first day of diestrus from this expected time frame are associated with decreased fertility.
VAGINAL CULTURES
Vaginal cultures can be taken from the bitch for various reasons, including prepubertal or postpubertal vaginitis, postparturient discharge, discharges during pregnancy, postabortion discharge, and prebreeding in normal or infertile bitches. Vaginal cultures are best performed with a guarded swab to prevent contamination as the culture swab is passed through the vestibule. If a culture is to be performed as part of a prebreeding examination and the client needs a negative culture before breeding, the performance and interpretation of the culture become critical. Bacteria, including mycoplasma, can be cultured from the vagina of the majority of fertile and infertile bitches. Once a culture has been obtained, it must be interpreted. If the culture was obtained as part of a work-up for a clinical problem, a pure culture is probably significant. However, a vaginal culture as part of a prebreeding examination in the absence of clinical signs often produces results of little significance.
CANINE PREGNANCY DIAGNOSIS
Pregnancy diagnosis can be performed by abdominal palpation, hormone assay, ultrasonography, or radiography. Palpation can be performed during a 7- to 10-day window beginning around day 21 after D1. After approximately day 30 post-D1, the embryonic vesicles become confluent and the ability to diagnose pregnancy by palpation is lost until late in gestation. Ultrasound can be used after approximately day 20 post-D1, possibly earlier, depending on the machine and probe used, until parturition. Radiography can be used after day 45 post-LH (day 37 post-D1). An in-house assay for relaxin is available and is reliable after about day 28 post-LH (day 20 post-D1) (Figure 14-14). When pregnancy testing, it is helpful to know the day of progesterone rise or D1 of diestrus to be able to estimate gestation length. Whereas gestation length seemingly can be as short as 55 days or as long as 70 days when timed from breeding, it is a reliable 57 to 58 days when timed from D1 of diestrus. Because a bitch is receptive to the male for 9 or 10 days, the timing from breeding is quite variable, resulting in errors (false-negative results) when examining for pregnancy or predicting whelping.
PARTURITION AND DYSTOCIA
Stage I of whelping averages 6 to 12 hours, but can be as long as 36 hours. The bitch is usually restless and may show nesting behavior. She often appears nervous, pants, and may tremble or shiver. The body temperature drops to 99° F about 24 hours before stage II in approximately 85% of bitches. This temperature drop is related to the abrupt decline in progesterone and can be useful for the dog owner to signal that whelping is imminent. To be reliable, the temperature should be taken at the same time each day, preferably in the morning before any activity. Stage II, when the bitch pushes the puppies out, lasts approximately 20 to 60 minutes per puppy (Figure 14-15). However, no more than 2 hours should elapse between each delivery. Stage II usually lasts a total of 3 to 6 hours, but may be as long as 24 hours total. The presentation of the puppies is 60% anterior in the bitch. A blackish-green discharge is normal during parturition and comes from the site of placental attachment to the uterus.
Guidelines for recognizing dystocia (difficult birth) are strong continual contractions for 30 minutes without progress; weak, infrequent contractions for 2 hours without progress; or a prolonged interval between puppies. If any of these criteria are met, a veterinary examination is warranted. Ultrasound can be used to assess fetal viability, but radiography is the only reliable method to accurately determine the number of pups in utero, their relative size, and their position.
POSTPARTUM PROBLEMS
The bitch has the longest postpartum uterine involution period of the domestic species. A nonodorous hemorrhagic vulvar discharge is normal for 8 to 10 weeks after whelping and does not indicate metritis. Clients are often concerned when a bloody discharge persists for that length of time, but should be reassured that it is normal. When the discharge persists for a prolonged period, such as 12 weeks or more, the bitch is considered to have subinvolution of the placental sites (SIPS). Treatment in these cases can be medical (ergonovine), surgical (ovariohysterectomy), or conservative (monitoring).
Indications that a bitch is suffering from postpartum problems include signs of discomfort, such as crying and whining, by the pups. Metritis is characterized by a foul-smelling vaginal discharge. Retained placenta results in a green discharge. Clinical signs of mastitis in the dam include fever, lethargy, and swollen mammary glands. The glands may be discolored. In many cases of metritis or mastitis, the pups will need to be hand fed or at least supplemented.
Eclampsia, or hypocalcemia, is characterized by tremors and excitation and is more common in smaller breeds. It most commonly occurs in the postpartum period. It is a true emergency. Hyperthermia and convulsions may require sedation or short-term anesthesia in addition to calcium treatment.
PSEUDOPREGNANCY
All bitches experience a 57- to 58-day period of elevated serum progesterone after estrus, whether pregnant or not. As progesterone declines at the end of a nonpregnant diestrus, many bitches, even if not pregnant, will experience mammary development, lactation, and maternal behavior. Clients may be concerned that the bitch is uncomfortable, or the behavioral changes may be unacceptable. With time, clinical signs will fade, and the bitch will return to normal. Alternatively, hormonal treatment with the prolactin inhibitor cabergoline or the androgen mibolerone can be used. Treatment with a progestogen, such as megestrol acetate, is contraindicated because signs of pseudopregnancy will return when therapy is halted.
Pyometra (uterine infection) can be a life-threatening situation in the bitch. It is progesterone related, usually occurring during diestrus, and may follow inappropriate estrogen therapy. The bitch may have a vaginal discharge depending on whether the cervix is open or closed. A bitch with pyometra is often lethargic, depressed, febrile, and exhibits polyuria and polydipsia. A leukocytosis is found on a complete blood count (CBC). Palpation or ultrasonography reveals an enlarged, fluid-filled uterus. If the breeding potential of the bitch is to be preserved, medical treatment with prostaglandins, cabergoline, and antibiotics is indicated; otherwise, surgical treatment (ovariohysterectomy) is usually recommended.
MISMATING
It is not unusual to have a client bring in a dog that has been bred accidentally or escaped while in estrus and request that she be “mismated” (aborted). In the past, few options were available other than estradiol cypionate (ECP), ovariohysterectomy, or allowing her to whelp. The use of ECP is not without risks. If given late in estrus or at the beginning of diestrus, there is a significant risk of pyometra. Another serious potential complication with the use of ECP is aplastic anemia. Further, many bitches seen for mismating may actually not have been bred or will not become pregnant. In one report, more than half of the bitches seen for pregnancy termination were not pregnant and would have been treated needlessly. For these reasons and the availability of suitable alternatives, the use of ECP for mismating is no longer recommended, and it is no longer manufactured in the United States. In many cases, the preferred method is to wait until such time as pregnancy diagnosis can be performed. If the bitch is pregnant, therapeutic options include prostaglandins, cabergoline, bromocriptine, or dexamethasone.
Prostaglandins effectively terminate pregnancy and are safe in dogs if used properly. Prostaglandins should be given subcutaneously, rather than intramuscularly. Further, a single dose will be ineffective in lysing the corpora lutea, so multiple small doses are used, usually two or three times per day for 5 to 7 days. Side effects include vomiting, diarrhea, and urination. An uncommon complication is cardiovascular collapse. Therefore an intravenous catheter is often recommended during the initial phases of treatment in case fluid therapy is necessary.
An alternative to prostaglandins is cabergoline (or a related compound, bromocriptine). Both are dopamine agonists, and administration will result in progesterone decline and pregnancy loss. Bromocriptine may be associated with vomiting, whereas cabergoline causes few side effects.
Dexamethasone is an attractive alternative to the previously mentioned drugs. It is administered orally, so it can be given at home. With any of these drugs, it is important to monitor pregnancy loss and continue therapy until abortion is complete. In addition, if the pregnancy is advanced when therapy is begun, it is important to inform the client that fetal discharge may be observed.
BRUCELLOSIS
Canine brucellosis, although typically thought of as a disease characterized by abortion and infertility, may manifest itself in a variety of ways. Although usually considered a venereal disease, Brucella canis is also spread through oronasal routes. Aborted fetuses and vaginal discharges are rich sources of B. canis organisms. Infected males shed the organism in their urine in addition to their semen. Transmission can occur between adult dogs in the absence of aborted material or sexual contact.
Because B. canis is an intracellular organism, the disease is extremely difficult to treat effectively, and cures are nearly impossible to achieve. No treatment has been found that is 100% effective in achieving a cure, although serum titers may decrease. Because of the nature of the disease and the potential for spread, euthanasia is commonly recommended for breeding animals in a kennel situation. A less drastic choice for pets is neutering and antibiotic therapy, although a persistent infection is still likely.
Because of the finality of neutering or euthanasia, a correct diagnosis is imperative. Unfortunately, many testing methods result in a high incidence of false-positive results and can be regarded as screening tests only. For example, antibodies against antigens of Pseudomonas aeruginosa, Bordetella bronchiseptica, and some Staphylococcus spp. can cause a false-positive result in a brucellosis test. Therefore a positive reaction on a screening test does not mean a dog is infected, but does suggest the need for a further, more definitive diagnosis.
The in-house screening test (D-Tec CB, Synbiotics) is rapid and sensitive. False-positive results are common (20% to 50% of dogs with positive test results do not have brucellosis, and some breeds, such as English sheepdogs, have exceptionally high false-positive rates) (Figure 14-16). A negative result is highly accurate, provided the infection did not occur within 3 to 4 weeks before testing. Screening tests can give a false-negative result when a dog is infected for less than 4 weeks or is chronically infected and has recently received antibiotic treatment. If a dog has a positive result on the in-house test, samples should then be submitted to a diagnostic laboratory for further testing with a more specific test.
FELINE REPRODUCTION
Although most species have a spontaneous LH surge that causes ovulation during every estrous cycle, the queen must have vaginal stimulation to induce an LH surge. The queen is therefore an induced ovulator. Because the queen does not necessarily ovulate during each estrous cycle, there are unique aspects in the queen’s estrous cycle that are not seen in other species. When a queen comes into estrus, there are three potential outcomes after estrus: the queen can ovulate and become pregnant, the queen can ovulate and not become pregnant, or the queen may not ovulate (Figure 14-17). Each of these outcomes results in a different series of subsequent events and time sequences for a return to estrus. The outcomes also depend on the season of the year because queens are seasonal breeders.
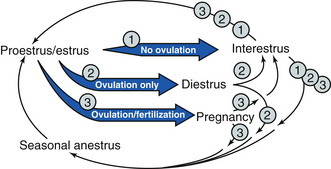
FIGURE 14-17 The feline estrous cycle. Depending on the season and whether ovulation occurs, the queen can do one of six things on entering an 8-day estrus. 1. If ovulation is not induced, the queen can go into a 2- to 14-day interestrus or seasonal anestrus. 2. If the queen ovulates but is not pregnant, a 36-day diestrus followed by either a 2- to 14-day interestrus or seasonal anestrus can occur. 3. If the queen ovulates and is pregnant, a 65-day pregnancy followed by either a 2- to 6-week interestrus or seasonal anestrus may occur.
SEASONALITY
Most queens have estrous cycles during the spring and are considered long-day breeders. This may be masked by the fact that most domestic cats are kept indoors under artificial lighting. The artificial lighting may be sufficient to stimulate estrous cycles all year long. The season and effect of artificial lighting must always be taken into account when discussing the queen’s estrous cycle.
ESTROUS CYCLE
Proestrus in the queen is only 1 to 3 days long and may not be apparent. Estrus in the queen lasts 8 to 10 days, and the queen shows distinctive outward signs of estrus. These signs include rolling and assuming an exaggerated lordosis when petted. The lordosis results in an elevation of the queen’s hindquarters. These signs are so intense that queens in estrus may be brought in for neurologic problems. Unlike other species that automatically enter a diestrus period of progesterone domination after estrus, the queen must have adequate vaginal stimulation to ovulate. The vaginal stimulation must come after the third day of estrus, and there must be multiple stimulations at least 2 to 3 hours apart to induce an LH rise of sufficient magnitude and duration to cause ovulation. Even if breeding does occur, vaginal stimulation will not necessarily be adequate to elicit a sufficient LH rise to cause ovulation.
If the queen ovulates and becomes pregnant, the gestation period is 63 to 66 days after mating. After parturition, the queen undergoes an anestrous period of variable duration. Anestrus is a time when nothing is happening on the ovaries, and the queen does not come into estrus. Postpartum anestrus may be as short as 2 weeks or as long as 6 weeks. Depending on the season and/or lighting conditions, the queen may return to proestrus or go into seasonal anestrus.
If a queen is bred, ovulates, but does not become pregnant, a diestrus (or pseudopregnancy) of approximately 40 days follows the estrus. During diestrus, the queen has a high concentration of progesterone in the blood that is produced by the corpora lutea on the ovaries that prevents the return to estrus. These corpora lutea apparently have a finite life span and cannot be lysed with exogenous prostaglandins, as in the bitch. After a nonpregnant diestrus, a short anestrus occurs. This anestrus is approximately 2 weeks long. Depending on the season and/or lighting conditions, the queen may return to proestrus or enter a seasonal anestrus.
If the queen is not bred or is bred and has insufficient stimulation to cause an LH surge, ovulation will not take place. If there is no ovulation, the follicles on the ovary regress. At this time, there are no structures on the ovaries (follicles or CLs), so this is called anestrus. This is a transitory anestrus, so a better term is interestrus. Depending on the season and/or lighting conditions, the interestrus may be 3 to 14 days or may extend into a long seasonal anestrus.
Pregnancy can be diagnosed in the queen 16 to 30 days postcoitus by abdominal palpation, after 14 days postcoitus to term by ultrasound, and after 43 days postcoitus to term by radiography. Palpation must be done within a limited time frame because the gestational sacs are too small before day 16 and they become too confluent after day 30 to distinguish. Ultrasound can detect if the fetus is alive by seeing the fetal heartbeat, but it is difficult to count the number of fetuses and to estimate their size. Radiography is the best method to count the number of conceptuses and to estimate their size, but fetal mineralization of the skeleton must be adequate to detect them radiographically.
COMMON REPRODUCTIVE PROBLEMS
Queens are generally fertile and have few infertility problems. Because most pet queens have ovariohysterectomies performed at an early age, few of these queens are seen for reproductive problems.
The most common questions arise from the clinical signs that accompany estrus in the queen and irritate the owner. Queens that have not undergone an ovariohysterectomy may be seen for prolonged estrus. Although cystic ovaries do occur in queens, they are not often the cause of the prolonged estrus. More commonly a queen with persistent estrus is undergoing normal estrus, with a short 1- to 2-day interestrus, followed by another estrus. This makes it seem like the queen is in constant estrus, when there are normal periods of estrus and interestrus occurring. Treatment for this condition is either performing an ovariohysterectomy or induction of ovulation.
Ovulation can be induced by vaginal stimulation with a cotton swab, glass rod, or thermometer. The stimulations must meet the same criteria as breeding to be effective: the vaginal stimulation must come after the third day of estrus, and there must be multiple stimulations at least 2 to 3 hours apart to induce an LH rise sufficient to cause ovulation. An alternative to vaginal stimulation is the administration of GnRH or human chorionic gonadotropin (hCG). The GnRH will cause an endogenous release of LH from the anterior pituitary, thereby resulting in ovulation. The drug hCG has LH action and will directly cause ovulation. After ovulation induction, estrus will not be shortened; however, the queen will then enter diestrus and interestrus phases, so estrus will not recur for approximately 2 months. There are no drugs approved to control the estrous cycle in the queen.
Another common complaint is the queen that comes into estrus after an ovariohysterectomy. It is important to document the presence of extra ovarian tissue by hormone analysis. This is most easily done using the same techniques to induce ovulation. If the queen goes out of estrus and has high progesterone, then extra ovarian tissue must be sought surgically. If not, then the adrenal glands may be the source of the estrogen causing the signs of estrus.
Parturition in the queen (queening) can last as long as 36 hours. The following criteria can be used to diagnose dystocia (abnormal birth): 20 minutes of intense labor with no kitten, 10 minutes of intense labor when a kitten is present, acute depression, or the presence of fresh blood for more than 10 minutes. If any of these criteria are noted, it is advised that the queen be examined. Although most queens do not have problems queening, an examination and radiographs will help rule out if a cesarean delivery is required.
The normal postpartum discharge is a red-black, nonodorous fluid that can be seen as long as 3 weeks after queening. If the queen appears depressed or the kittens are dying, the queen should be examined for mastitis or metritis.
Pyometra in the queen has similar signs as seen in the bitch, including depression and vaginal discharge. Bitches with pyometra tend to be older, whereas queens can be any age.
EQUINE REPRODUCTION
Mares are seasonally polyestrous, meaning that during the breeding season they cycle repeatedly. The natural breeding season centers around the period of long day length. Under natural conditions, mares begin cycling in late March or early April and continue until September or October. Some mares may continue to cycle year round. In the northern hemisphere, many breed associations have designated Jan. 1 as the birth date for all horses born in a given year. This means that a horse born in January and a horse born in June will both be considered 1 year old the following January when they are actually 12 months and 7 months old, respectively. This is a large difference for horses competing as 2 year olds. Therefore there is a great deal of pressure to have foals born early in the season (January or February), but many mares are not cycling at this time. The most cost-effective method to stimulate earlier cyclicity is to “trick” the mare into perceiving that the days are lengthening by providing artificial lighting and mimicking a 16-hour daylight period. This should be started by December 1 to get the most benefit. A 200-watt bulb in a 12 × 12-foot stall is sufficient. For an outdoor situation, eight 1000-watt metal halide floodlights at a height of 20 feet will provide sufficient light for an 84 × 66-foot paddock. Artificial lighting should be added in the evening, rather than in the morning. Turning the lights on earlier in the morning is less effective than leaving them on later in the evening.
During the breeding season, the mare’s estrous cycle averages 21 to 22 days in length. Diestrus, the period when a CL is present and producing progesterone, is a consistent 15 days in length based on hormone levels (Figure 14-18). During this time, the mare will tease “out,” resisting the stallion’s advances by kicking, squealing, pinning her ears back, and clamping her tail between her legs. Based on behavioral signs, diestrus is shorter (approximately 14 days) because she does not tease out until 1 day or so after ovulation. Estrus, or the period of receptivity, is shown by teasing “in.” The mare squats, urinates, lifts her tail, and “winks” (everts her clitoris) on the approach of the stallion.
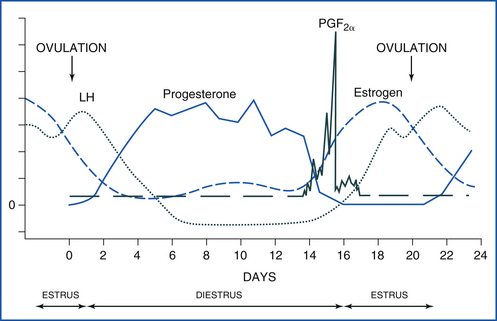
FIGURE 14-18 The equine estrous cycle. Estrus is 4 to 7 days long, and LH peaks after ovulation. Diestrus begins around 2 days after ovulation. Progesterone is high throughout a 14- to 15-day diestrus. If a pregnancy signal is not secreted by the early embryo by day 14 to 16, prostaglandin is released from the uterus, goes to the CL, and causes luteolysis (luteal death). The cycle then starts over.
Between the noncycling period (anestrus) and the cycling period, a period called transition occurs. Transition varies in length and is characterized by irregular periods of estrous behavior, but without ovulation. A mare may exhibit estrous behavior for 2 or 3 days, then stop for a few days, and then begin again. Alternatively the mare may exhibit estrous behavior continuously without ovulation for 3 weeks or more. Although follicles may be present during these periods, they do not ovulate. The transitional period is a physiologically normal occurrence. Although cyclicity can be induced earlier in the year, a transitional period will still precede the onset of cyclicity. An awareness of this can help to reduce needless breedings and help mare owners understand this sometimes aggravating period. The administration of altrenogest, a synthetic progestogen, will stop the estrous behavior and is often used during transition.
During the breeding season, the duration of estrus is variable (4 to 7 days). Estrus tends to be shorter near the peak of the breeding season (June) and longer further away from June, such as March or September. Follicular development can be monitored by palpation per rectum and ultrasonography during estrus to decide optimal breeding time.
BREEDING SOUNDNESS EXAMINATION
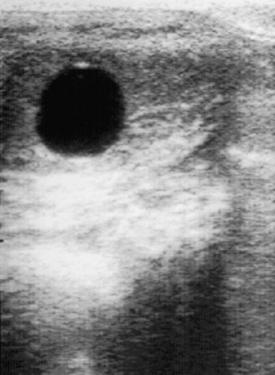
Breeding soundness examinations are commonly performed at the sale or purchase of a mare or when a mare fails to become pregnant after breeding. A typical breeding soundness examination consists of palpation per rectum, ultrasonography, a vaginal speculum examination, uterine culture and cytology, and a uterine biopsy. During the ultrasonographic examination, structures on the ovaries and, more importantly, features of the uterus can be observed. Fluid-filled structures, such as follicles (Figure 14-19) and endometrial cysts will be black. Corpora lutea have a variable appearance, ranging from a rather homogeneous gray to a “spider web” trabecular pattern with a brighter thick-walled circumference. An important indication of the stage of the estrous cycle is the ultrasonographic appearance of the uterus. During diestrus, it has a homogeneous hyperechoic appearance, but in estrus it has a characteristic appearance caused by edema in the endometrial folds. It has been described as looking like the spokes of a cartwheel, a sliced orange, or pizza (Figure 14-20).
A vaginal speculum examination, uterine culture and cytology, and uterine biopsy are performed aseptically. First, wrap the tail with a clean tail wrap or gauze. Prepare a clean bucket with clean water and add small (approximately 10 cm × 10 cm) pieces of cotton. Rather than placing disinfectant in the bucket, it is preferable to place the soap on the cotton itself before scrubbing the vulva, thereby leaving clean water to rinse the vulva. Otherwise, disinfectant residues may remain on the vulva and be carried into the vagina, with a potential spermicidal or tissue-irritating effect. Cleansing of the perineal area should be done using the “clean hand-dirty hand” technique. One hand (the clean hand) is used to retrieve clean pieces of cotton from the bucket. The cotton is then transferred to the other hand (the dirty hand), which is then used to scrub the perineal area. This method keeps the bucket with clean water and cotton from being contaminated. The vulvar labia should be scrubbed in a manner similar to that for surgical preparation (i.e., from the center of the area being cleaned to the perimeter and repeated until clean). Check the inside of the labia during the procedure to be sure no feces have entered as a result of the palpation and ultrasonography procedures.
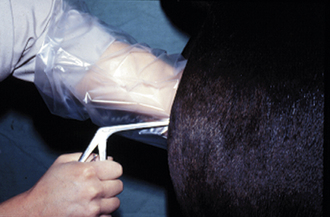
A vaginal speculum examination can discern trauma to the cervix; discharge emanating from the uterus, cervix, or vagina; urine pooling in the cranial vagina; and other conditions associated with subfertility. After aseptically preparing the vulva, a small amount of sterile lubricant is placed on the end of a speculum, the labia are parted, and the speculum is placed into the vagina. A light source is then used to look through the speculum and examine the vagina and cervix (Figure 14-21).
The next procedure usually performed is a uterine culture and cytology. The importance of performing a cytologic examination in conjunction with the culture cannot be overstated. Without a cytologic specimen, it is impossible to differentiate between an infectious process and a contaminant resulting from improper sampling or mare preparation. When obtaining a culture and cytology specimen, only guarded swabs should be used. After aseptically preparing the vulva, a sterile sleeve is used to introduce the culture-cytology instrument into the vagina, through the cervix, and into the uterus. After the swab is withdrawn, the sample can be used to prepare a cytology specimen. Stain the cytology slide using a modified Wright’s or Giemsa stain. The slide should contain numerous epithelial cells and be examined for inflammatory cells, primarily neutrophils. A positive cytology slide will contain numerous inflammatory cells, whereas a negative slide will not. Bacteria isolated from a culture with a negative cytology finding can be considered contaminants. An argument can be made to not submit the culture if the cytology slide is negative. However, if the cytology slide is positive, not only do culture results reveal the causative agent, but also sensitivity results aid in making a therapeutic choice. Excess lubricant used during the procedure can interfere with the interpretation by staining darkly and obscuring the field.
The next, and often final, step in the breeding soundness examination is to obtain an endometrial biopsy. As with the previous procedures, it is done in an aseptic manner. The closed instrument is carried into the uterus, wearing a sterile sleeve (Figure 14-22). The instrument is held in the uterus while the hand is withdrawn and placed in the rectum. The instrument is then opened to permit endometrial tissue to enter the jaws and then closed to snip off a piece of endometrium. The tissue is next placed in a fixative and processed through a laboratory.
BREEDING MANAGEMENT
Monitoring follicular development by palpation and ultrasonography per rectum allows the mare to be inseminated close to ovulation. This has numerous benefits, including making sure that sperm are present when the oocyte is released, minimizing overuse of a stallion, and reducing the number of times a mare is bred during an estrus. Reducing the number of breedings per estrus becomes more important as a mare’s fertility declines. When breeding a mare with fresh cooled or frozen semen, daily or even more frequent examinations are usually performed. With fresh cooled semen use, the semen is usually ordered after a follicle reaches 35 mm in diameter, and semen is shipped overnight to the mare’s location. With frozen semen, the semen is stored in liquid nitrogen, and insemination must occur within 24 hours before to within 6 hours after ovulation.
After a mare is bred, an ultrasonographic examination is usually preformed the following day (possibly sooner or later depending on the mare and the particular situation) to confirm that ovulation has occurred and to examine the uterus for the presence of fluid. The failure of a mare to clear the fluid from the uterus after breeding has been associated with decreased fertility and is the reason for many postbreeding treatments.
Artificial Insemination (Uterine Infusion)
Breeding a mare by artificial insemination is a relatively simple procedure. It is an aseptic procedure, and the same procedure is used for uterine infusion as for AI. A sterile sleeve is donned and a sterile pipette carried through the vagina and cervix into the uterus. The semen or intrauterine medication is then deposited directly into the uterus.
Postbreeding Treatments
If fluid is detected in the uterus by ultrasonography 8 hours or more after breeding, a treatment to aid uterine clearance is indicated. Oxytocin or cloprostenol are the two most commonly used drugs to stimulate uterine clearance.
In addition to oxytocin or cloprostenol injections, common postbreeding treatments include intrauterine infusion of antibiotics and uterine lavage. Intrauterine infusion and lavage are performed in the same aseptic manner using sterile equipment as AI.
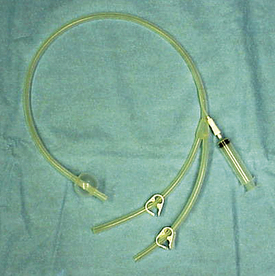
Uterine lavage is performed using a large-bore catheter with an inflatable cuff (Figure 14-23). The catheter is placed in the uterus, and the cuff is inflated and seated against the internal cervical os to provide a good seal. Sterile saline, usually 1 L at a time, is infused into the uterus and then retrieved. This is repeated until the saline retrieved is clear. This procedure helps to clear the uterus of debris.
EQUINE PREGNANCY DIAGNOSIS
Pregnancy diagnosis is usually done with the aid of ultrasonography 2 weeks after ovulation. With experience, the characteristic ultrasonographic appearance of the conceptus at various stages can be easily recognized and used to evaluate its growth and health (Figure 14-24). The term conceptus refers to everything derived from the fertilized ovum, including the fetal membranes and fluids the embryo or fetus. Fetal sexing is most commonly performed between 60 and 70 days by identifying the position of the genital tubercle.
Hormonal methods for pregnancy diagnosis exist. They can be useful in cases where palpation per rectum is not feasible, such as with small miniature horses or wild, fractious mares. Pregnant mares will have a positive result for equine chorionic gonadotropin (eCG), formerly called pregnant mare serum gonadotropin (PMSG), from 35 days until approximately 120 days of gestation. False-positive results happen if fetal death occurs during that period. Another hormone used for pregnancy testing is estrone sulfate. The benefit of estrone sulfate compared with eCG is that it is produced in high quantities by a viable fetoplacental unit beginning around 60 days of gestation. If the pregnancy is lost, estrone sulfate drops off rapidly, so false-positive results are uncommon. Progesterone cannot be used to test for pregnancy because it is elevated during diestrus in nonpregnant mares and during gestation in pregnant mares.
VACCINATIONS
Equine herpesvirus can be a significant cause of abortion in horses. Vaccines are available, and a good program consists of vaccination at 5, 7, and 9 months of gestation. Either a killed-virus or modified live-virus vaccine may be used. Repeated vaccinations at these intervals are necessary each time a mare is pregnant to provide good protection. In addition, it is advisable to minimize stress, and it is important to keep pregnant mares separated from new additions to the herd or horses that have significant outside contact.
It is also recommended to give an annual booster for flu, encephalitis (Eastern, Western, West Nile), and tetanus at 10 months of gestation. This practice will help ensure good-quality colostrum and increase the protection level provided to the foal by passive transfer of antibodies through the colostrum. Additional information on preventive health programs is found in Chapter 9.
Induction of Parturition
The gestation length in mares is generally quoted as 330 to 340 days. However, there is a wide variation in normal gestation length, ranging from 320 to 400 days. It is important to remember that the fetus determines the gestation length. For this reason, chemical induction of parturition based on the gestation length alone is risky and can result in the birth of premature foals. The decision to induce parturition is based on the following criteria:
1. Gestation length greater than 330 days
2. Relaxation of pelvic ligaments
3. Presence of colostrum in the udder
4. Relaxation or softening of the cervix
5. Most importantly, milk calcium more than 400 ppm with milk potassium higher than sodium
The electrolyte changes are well correlated with fetal maturity and are the best way to assess readiness for birth. Calcium can be measured with a water hardness test kit, but be sure to use one that measures only calcium, not just divalent cations. Magnesium, another divalent cation present in colostrum, rises slowly as parturition approaches and can interfere with the interpretation of the results. Milk calcium greater than 400 ppm and potassium less than sodium can occur in cases of placentitis or twins, so induction of parturition should not be considered unless potassium is greater than sodium, along with the increase in calcium.
PARTURITION
Parturition is a rapid process in horses. In the normal course of events as the mare begins labor (stage I), she will lie down, get up, roll, and so forth. During these maneuvers, the foal is getting into the correct position. Finally, at the end of stage I, the chorioallantois ruptures (“water breaks”), signaling the onset of stage II. Usually within 10 minutes, a bluish white membrane (amnion) appears at the vulvar opening. Within another 10 minutes, the feet will protrude through the vulva, still within the amnion. After another 10 minutes, the head protrudes, and delivery will be completed soon thereafter (Figure 14-25).
A red membrane protruding from the vulva, the chorioallantois, is an indication of premature placental separation, or “red bag” (Figure 14-26). This is an emergency, and the chorioallantois must be ruptured manually and delivery assisted or the foal will quickly die.
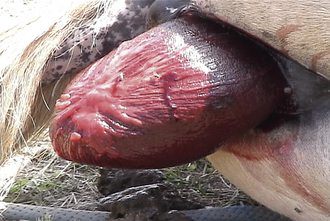
FIGURE 14-26 Premature placental separation (“red bag”) during parturition in the mare. The chorioallantois is observed protruding through the vulvar opening.
If the guidelines for the time frame of delivery are not being met, an examination for possible dystocia is indicated. It is critical to perform any obstetric manipulations in as clean a manner as possible and with sufficient good-quality obstetric lubricant. The failure to adhere to guidelines of strict cleanliness and ample lubrication invites complications in the postpartum period and can compromise future fertility.
POSTPARTUM PROBLEMS
Mares do not experience postpartum problems as commonly as cows, but when they do, they can be life threatening. A retained placenta for more than 6 hours duration deserves veterinary attention. Various methods, such as large-volume saline distention or oxytocin therapy, can be used to stimulate placental release. Manually detaching the placenta should not be done because of the potential for causing damage to the uterus and hemorrhage. Systemic antibiotics, antiinflammatories, and tetanus toxoid are usually administered in cases of placentas retained more than 6 hours.
A prolapsed uterus is a true emergency and must be dealt with as quickly as possible. Unfortunately, even with proper treatment, mortality can approach 50%. The mare should be restrained and the uterus protected from trauma until the veterinarian arrives.
The clinical signs of postpartum hemorrhage as a result of a ruptured uterine artery, usually either into the broad ligament or the abdomen, include signs of abdominal pain and pale mucous membranes. Mares exhibiting such signs in the postpartum period should be kept calm and quiet and be confined to a stall until examined by a veterinarian.
Postpartum metritis, often a sequela to retained placenta or contamination during obstetric procedures, can lead to septicemia and laminitis. As a result, treatment should not be delayed in a postpartum mare that has a foul-smelling vaginal discharge or is febrile or depressed.
HORMONE USE IN MARES
Prostaglandin F2a and its analog cloprostenol can be used to lyse a CL and return a mare to estrus. It is effective beginning about 5 or 6 days after ovulation, when the CL has matured sufficiently to respond. Mares will return to estrus in 2 to 7 days after prostaglandin administration. Prostaglandins should not be used to induce parturition because of the high incidence of premature placental separation, dystocia, and fetal death.
Human Chorionic Gonadotropin
Human chorionic gonadotropin (hCG) is used to induce ovulation in mares that have a follicle 35 mm or larger. Doses commonly used range from 2000 to 3500 IU given intravenously, and ovulation can be expected to occur in 36 to 48 hours in approximately 80% of mares. Although concerns about antibody production have been expressed, no correlation between antibodies and the failure to induce ovulation has been shown. Nevertheless, some mares fail to ovulate after receiving HCG repeatedly.
Deslorelin
An alternative to hCG for ovulation induction is an analog of GnRH, deslorelin. Ovulation occurs within approximately the same time frame as with hCG. However, deslorelin appears to be more effective on slightly smaller (30-mm) follicles than hCG, and the failure of the mare to ovulate is reportedly less common.
Progestins
Altrenogest (Regu-Mate, Intervet) is a progesterone-like compound that is administered orally. It is used primarily for pregnancy maintenance and estrous cycle control. Its use for pregnancy maintenance is empiric in that true progesterone deficiency as a cause of pregnancy loss has not been documented. However, anecdotal reports of mares failing to maintain pregnancy unless supplemented with altrenogest are not uncommon. Because the fetoplacental unit eventually takes over progestogen production, altrenogest therapy can usually be discontinued at about 4 months of gestation.
The other reason to use altrenogest is estrous cycle control. Altrenogest mimics progesterone in the mare. Therefore mares given altrenogest act as if they are in diestrus. Altrenogest can be used for estrus synchronization in embryo transfer programs and to manipulate the estrous cycle to prevent or control the onset of estrus in performance mares. Altrenogest is also used in mares in late gestation when there is concern about possible impending abortion. Through its action, uterine motility is inhibited, thereby supporting maintenance of pregnancy.
Oxytocin
A common cause of infertility is persistent mating-induced endometritis. An inflammatory response to sperm cells normally occurs after breeding, whether by natural service or AI. This inflammatory response is necessary to remove excess semen and debris and to prepare the uterus for pregnancy. Uterine clearance mechanisms, such as uterine motility and lymphatic drainage, are critical in this process. Infertility results when uterine clearance mechanisms fail and fluid remains in the uterus after breeding. An ultrasound examination 12 hours or more after breeding should reveal no fluid in the uterus. If fluid is still present, oxytocin therapy (20 IU intravenously or intramuscularly) should be instituted. Oxytocin is effective in aiding uterine clearance and can be given as often as every 2 or 3 hours.
Oxytocin is also the only drug available to safely and reliably induce parturition. If the mare has met the criteria and is ready to give birth, a small dose (10 IU intravenously) of oxytocin is sufficient to initiate parturition. Lower doses result in a more natural process, whereas higher doses result in a faster and more forceful delivery.
Domperidone
Domperidone is a dopamine antagonist used to alleviate the effects of fescue toxicosis and stimulate lactation. It has also shown some promise to stimulate cyclicity in mares with lactational anestrus and may be beneficial in hastening the onset of cyclicity in the spring, but this has not yet been well documented.
BOVINE REPRODUCTION
The cow is a nonseasonal polyestrous species, meaning that cows have estrous cycles all year around. The entire estrous cycle averages 21 days long, but it can be as short as 18 days and as long as 24 days (Figure 14-27). Estrus lasts 18 to 20 hours, but may be shorter in hot, humid weather because of heat stress. Estrus is the time the cow is in “standing heat” and will stand with all four legs firmly braced to be mounted by a bull or another cow. Ovulation occurs 12 to 18 hours after the end of estrus. Estrus is followed by metestrus, which is 3 to 5 days long and is the time of luteal development. A bloody discharge from the vulva may be noticed in nearly half of all cows and heifers 1 or 2 days after they are in estrus. This bloody discharge is no indication of fertility or infertility, but merely a sign that the individual was in estrus 1 or 2 days earlier. During metestrus, a corpus hemorrhagicum (CH) is formed at the site of ovulation. This structure will develop into a CL over the next few days. However, during metestrus, the CL is not yet mature and not yet susceptible to the luteolytic action of prostaglandins. The next phase is called diestrus and lasts from day 5 or 6 until day 17 of the estrous cycle and is the time that the mature CL (“yellow body”) is present and producing progesterone. During diestrus, there are waves of follicular growth that are important in understanding a cow’s response to estrous cycle synchronization. At the end of diestrus, if an embryo is not present in the uterus to provide a pregnancy signal, the uterus releases a prostaglandin that lyses the CL, resulting in a decline of progesterone, and the cow returns to proestrus.
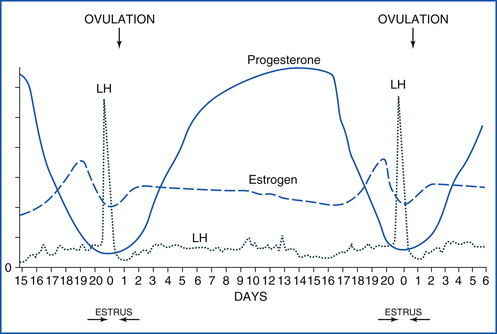
FIGURE 14-27 The bovine estrous cycle. Estrus is 12 to 18 hours long, and LH peaks during estrus. Diestrus lasts until about day 17, and progesterone is high throughout diestrus. If a pregnancy signal is not secreted by the early embryo by day 16 or 17, prostaglandin is released from the uterus, goes to the CL, and causes luteolysis (luteal death). The cycle then starts over.
BOVINE PREGNANCY DIAGNOSIS
Various methods of pregnancy diagnosis exist, but the most commonly employed is palpation per rectum. An experienced person can diagnose pregnancy by 30 days of gestation. Ultrasonography is becoming more popular for pregnancy diagnosis. Machines have become more affordable, and accurate pregnancy diagnosis can be performed by 24 days or earlier in some cases. Fetal viability can be assessed, and fetal sexing is possible between 55 and 70 days.
Progesterone tests should not be regarded as pregnancy tests. Although a progesterone test can detect the presence of a CL, many scenarios exist where a CL is present and progesterone is high, but the cow is not pregnant.
BREEDING/ARTIFICIAL INSEMINATION
Most dairy cattle, and increasing numbers of beef cattle, are bred by AI. There are numerous advantages to AI, with rapid genetic improvement being the primary one. However, the need for estrus detection to time insemination is a major drawback. Fortunately, cows, especially dairy cows, exhibit “standing heat” when in estrus, and observation of this behavior is the best method of estrus detection. “Teaser” bulls, bulls that have been surgically altered to prevent vaginal penetration with the penis and have had vasectomies to render them sterile, can be used to help with estrus detection.
Estrus synchronization, through the use of hormones, also helps improve estrus detection. Many schemes have been devised using prostaglandins to lyse the CL. Other schemes use progestogen to mimic the luteal phase and suppress follicular growth. An ear implant, Synchro-Mate B, containing the progestogen named norgestomet, has been successfully used for years in cattle, but it is no longer available in the United States as of the time of writing this chapter. Recently an intravaginal device has been approved for synchronization of estrus in dairy heifers and for synchronization of return to estrus in lactating dairy cattle. The product is called EAZI-BREED CIDR and contains 1.38 g of progesterone in silicone molded over a nylon spine. EAZI-BREED CIDR is the first and only approved source of progesterone for use in dairy cattle. EAZI-BREED CIDR can be used in association with prostaglandin F2a (PGF2a). Another popular method for synchronization of estrus involves an injection of GnRH at a random stage of the estrous cycle, followed by an injection of PGF2a 7 days later, followed by a second injection of GnRH 48 hours later, and insemination 16 to 18 hours after that.
ABNORMALITIES OF THE ESTROUS CYCLE
The most common reason for the failure of a cow to show estrus is pregnancy, whether as a result of poor record keeping or an unknown visit by a neighboring bull. For this reason, it is always advisable to have a cow examined for pregnancy before attempting to “bring her into heat” with prostaglandins. After calving, a period of postpartum anestrus is common. This period is usually shorter for dairy cows (2 to 4 weeks depending on nutrition and environmental conditions) than for beef cows. Beef cows nursing calves have a longer period of postpartum anestrus, usually 45 to 90 days. The postpartum anestrus period in beef cattle is prolonged if the cows are not in good body condition when they calve.
Pathologic reasons for anestrus include pyometra, luteal cysts, and cystic ovarian disease (COD). Pyometra may be caused by a number of factors, including trichomoniasis, bovine viral diarrhea (BVD) virus, and postpartum uterine infection. It is characterized by a pus-filled uterus and a persistent CL. The treatment consists of simply administering a prostaglandin, although two injections 24 hours apart may be needed to improve response.
Luteal cysts arise from follicles that fail to ovulate, but do form luteal tissue. Progesterone is elevated, and a period of prolonged diestrus results. The treatment consists of a prostaglandin injection. COD results from follicles that fail to ovulate and do not form true luteal tissue. Progesterone secreted by thecal cells in the cyst is low, and although some cows may exhibit persistent or frequent estrus, anestrus is much more common. The treatment consists of GnRH or hCG administration.
ABNORMALITIES OF PREGNANCY
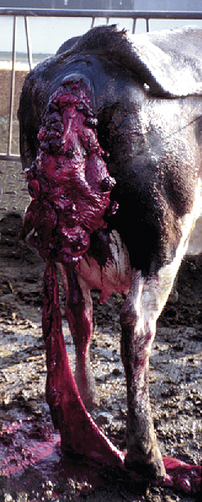
Uterine prolapse is easily recognized by the presence of the mucosal, or inner, surface of the uterus, with its characteristic caruncles, hanging from the vulva (Figure 14-28). It is more common in dairy than beef cattle. It occurs in the immediate postpartum period and is often associated with hypocalcemia or dystocia. Uterine prolapse should be considered an emergency, and the uterus should be replaced to its normal position as soon as possible. Uterine prolapse is not hereditary and is not considered likely to recur.
Vaginal Prolapse
Although seemingly similar to uterine prolapse, vaginal prolapse is actually quite different. This is a hereditary problem; therefore affected individuals should not be kept as breeding stock. It occurs under conditions associated with elevated estrogen, most commonly seen during late gestation, although it may also be associated with cystic follicular degeneration or the ingestion of certain plants. Typically the affected animal will be a beef cow or heifer in late gestation, and the vagina and possibly the cervix will be protruding from the vulva. Various methods have been described to treat the condition, but all consist of cleaning the exposed tissue, replacing it, and preventing recurrence. It must be remembered that in most cases, the cow will not have calved yet, so care will need to be taken to monitor her to reduce the chance of dystocia and mortality.
Dystocia
Dystocia is much more common in cattle than in mares. It is most commonly caused by fetal-maternal disproportion in size. Uterine torsion is also a fairly common cause of dystocia. As with mares, cleanliness and lubrication are essential for successful management of dystocia.
Milk Fever
Milk fever, or parturient hypocalcemia, most commonly occurs during the postpartum period, although it may also occur during parturition. It is more common in dairy cattle than beef cattle. Milk fever is characterized by flaccid paralysis. The incidence is increased when cattle are fed high levels of calcium prepartum. This condition should be viewed as an emergency and is treated by a slow intravenous infusion of calcium gluconate or oral administration of a calcium-containing gel.
SWINE REPRODUCTION
Gilts usually reach puberty at 4.5 to 6 months of age. Social environment and nutrition are important factors determining the onset of cyclicity in gilts. The onset of puberty is commonly seen in gilts weighing 82 kg or more. Direct contact between boars and gilts is important in swine reproduction. Boars have pheromone-secreting salivary glands that sexually stimulate female pigs. This “boar effect” (stimulating or detecting estrus) is even more evident if mature, experienced boars are used. Daily exposure of 5- to 6-month-old gilts to a mature boar will hasten the onset of cyclicity. Season probably influences the onset of puberty because gilts born in the fall start to cycle earlier than their spring-born counterparts. In addition, all factors that contribute to good management, such as number of gilts per pen, adequate physical space per gilt, ambient temperature, and health status (diseases, parasites) in general, will ensure that gilts reach puberty around 5 to 6 months of age.
Pharmacologic agents can also induce puberty. Fertile estrus and ovulation can be induced using exogenous gonadotropins. eCG in association with hCG is effective in inducing estrus in gilts. These two gonadotropins are marketed in a combination to induce estrus and ovulation. Estrogen administration is also effective, but does not yield as reliable results as the eCG-hCG combination. GnRH is effective, but it is expensive and needs to be delivered in a pulsatile fashion (every hour for 3 to 4 days), making this difficult to be done in a commercial unit.
ESTROUS CYCLE
Domestic pigs are nonseasonal polyestrous animals. Female pigs exhibit estrus at 21-day intervals after they reach puberty. Longer estrous cycles, such as 26 days, have been associated with early embryonic mortality.
During proestrus, follicular development intensifies as the corpora lutea in the nonpregnant pig start to regress around day 15 of the estrous cycle. Initial behavioral signs of estrus are not always easily recognized. They include increased restlessness, reduced appetite, mounting other animals, homosexual behavior (malelike sexual activity), and lordosis. The vulva swells and becomes more pink and moist. A cloudy mucous discharge may be present during this phase.
Estrus is the period when the female pig is responsive to the boar’s approach. If a male and female are put together during this stage, they start to show a precopulatory behavior (foreplay) that includes sniffing each other and head-to-head contact; the male starts to compulsively follow the female pig and initiate mounting attempts. Courtship culminates with the female pig standing still and allowing the boar to mount. Estrus also can be detected by checking the female for lordosis. Lordosis occurs when pressure is applied on the pig’s back by someone sitting on it and the female pig stands still, quiet, and passive, assuming a mating position. The duration of estrus can be variable among individuals of different breeds or age or during different times of the year (longest in summer and shorter in winter). Estrus lasts on average 40 to 60 hours (2 to 3 days), but it can vary from 1 to 4 days. Ovulation usually takes place during the second day of estrus (36 to 44 hours after the onset of estrus), and mated females reportedly ovulate 4 hours before unmated female pigs. Follicular rupture of all follicles present in the ovary (10 to 20) may take 1 to 9 hours.
Diestrus is behaviorally characterized by a lack of sexual receptivity and lasts for 18 days if the female pig is not pregnant. Anestrus is mainly seen when sows are lactating.
PREGNANCY
The deposition of semen in pigs is intracervical. The presence of cartilaginous rings in the sow’s cervix in association with the fibroelastic spiral tip (corkscrew) of the boar’s penis provides a natural and strong lock of the penis inside the cervix. Subsequently, during mating, strong contractions of the cervix are potentiated by oxytocin release in response to coitus. Several billion sperm cells are released during coitus in an average of 200 to 250 ml of semen. Uterine contractions allow a controlled number of sperm cells to reach the oviduct, where fertilization occurs. It seems that a minimum of four embryos must be present in the uterus to cause appropriate maternal recognition of pregnancy. The average length of gestation is 114 days (3 months + 3 weeks + 3 days). Piglets are born weighing on average 1.4 to 1.6 kg. A tail-first presentation is not abnormal. The interval time between piglets averages 10 to 15 minutes. Intervals greater than 20 minutes are associated with an increasing number of stillbirths. All fetal membranes should be delivered in 4 to 6 hours.
CONTROL OF FARROWING
Farrowing can occur at any time of the day or night. Each sow may take several hours to deliver all the piglets and fetal membranes. It is important to assist sows during delivery because the early detection and correction of potential problems during farrowing prevent piglet losses. Assistance during farrowing can be facilitated by pharmacologically inducing parturition. Accordingly, prostaglandin administration after day 112 of gestation will induce parturition in 20 to 30 hours. Combining a prostaglandin with oxytocin or xylazine will help to improve the precision of response to prostaglandin administration.
PHARMACOLOGIC CONTROL OF THE ESTROUS CYCLE
Exogenous hormones can be used to alter or manipulate the estrous cycle in pigs. Estrus and ovulation can be induced by different means either in cycling pigs or in early pregnant pigs to synchronize estrus (Table 14-2).
TABLE 14-2
Hormones Used in Swine Reproduction
| Desired Effect | Drug | Regimen |
| Induction of puberty | P.G. 600 | 5 ml IM |
| eCG/hCG | 400-1000 IU/200-1000 U IM | |
| Estrus/ovulation | P.G. 600 | 5 ml IM |
| eCG/hCG | 500-1000 IU/500-1000 U IM | |
| Regu-Mate | 15 mg/gilt/day for 18 days orally | |
| Abortion | PGF2a | 10 mg Lutalyse or 500 mg Estrumate 12-45 days after breeding |
eCG, Equine chorionic gonadotropin; hCG, human chorionic gonadotropin; IM, intramuscularly; PGF2a, prostaglandin.
The preparation P.G. 600 contains 400 IU of eCG and 200 units of hCG. It can be used to induce estrus in prepubertal gilts or in sows showing anestrus after weaning. It can also be used in cycling sows after day 16 of the estrous cycle. Administering P.G. 600 will cause estrus and ovulation in 3 to 5 days following treatment. Estrus and ovulation can also be induced by administering ECG and HCG 2 days apart. Prostaglandins can be used to terminate pregnancy or diestrus only if given 12 days after ovulation. Before day 12, multiple injections (twice daily for 5 days) of prostaglandins are necessary to interrupt diestrus (short cycling) or to terminate pregnancy. Weaned sows usually show spontaneous signs of estrus in 11 to 12 days after weaning. Administering P.G. 600 on the day of weaning will also induce estrus in 3 to 5 days. Sows that do not come into estrus in 2 weeks after weaning will show signs of estrus if P.G. 600 is administered.
Artificial Insemination
AI with fresh or cooled semen is commonly performed in the swine industry. The boar is easily trained to mount a dummy and have semen manually collected. After collecting the sperm-rich portion of the ejaculate, semen is evaluated to determine its quality and how much extender to add. A sow should be inseminated with 3 to 4 billion sperm cells in a volume of 80 to 100 ml. The number of total insemination doses from an ejaculate will depend on the frequency of collection, age of boar, breed, and some individual variation. Farrowing rates from sows artificially inseminated are comparable with those observed in sows naturally mated. Artificially inseminating twice 12 to 24 hours apart is likely to improve pregnancy rates. In a commercial unit, heterospermic insemination (mixing semen from two or more boars) is sometimes used and reportedly increases pregnancy rates.
Swine Pregnancy Diagnosis
Ultrasonography procedures are used to diagnose pregnancy in pigs. Doppler and amplitude-depth ultrasound were the first methods to be employed and are accurate between 30 and 90 days of gestation. Real-time (B-mode) ultrasound is being used with more frequency in the swine industry as equipment cost decreases. Accuracy is greater than 90% if used after 20 days of gestation.
Postpartum Complications and Diseases
Retained placenta is not a common occurrence in pigs. Fetal membranes from all piglets are usually expelled after the last piglet is born. A manual examination of the birth canal is warranted if the placenta is not passed out after sows apparently deliver their last piglet.
Obstetric problems are also not common, and the great majority of sows and gilts deliver without any technical or veterinary assistance. Nevertheless, persistent and forceful abdominal contractions without delivery of a piglet for longer than 1 hour suggest potential complications. A problem will be even more evident if it is accompanied by vaginal discharge but not expulsion of a fetus. An increased time interval between deliveries is also suggestive of dystocia. The reason may be a primary absence of uterine contractions (primary inertia) or secondary to the presence of either an oversized, malpositioned, or malformed fetus. Oxytocin can be administered for primary uterine inertia. The removal of piglets is also helpful for stimulating progression of delivery. Occasionally a cesarean delivery is needed to deliver the piglets.
Prolapse of the uterus can occur during parturition or after all piglets have been delivered. Excessive straining or a large pelvic inlet may predispose to uterine prolapse. Uterine prolapse is likely fatal. Vaginal prolapse generally happens a few days before parturition. It requires veterinary intervention to reposition it, and sows should be watched for any problems during labor.
Metritis and mastitis are the main diseases of the postpartum period and consequently lead to a disturbance in milk production. Hypogalactia (low milk production) and agalactia (absence of milk production) are often associated with mastitis and metritis. Any signs of abnormal, fetid vaginal discharge or abnormal enlargement of the udder warrant veterinary assistance. Oxytocin can be used to stimulate milk let-down during treatment.
OVINE AND CAPRINE REPRODUCTION
Sheep and goats are seasonally polyestrous, short-day breeders. Estrous cycles start to occur in the late summer and autumn. The photo period is the primary environmental cue controlling seasonal breeding in the ewe. Exposure of sheep and goats to increasing or long day length induces anestrus, whereas short or decreasing day length initiates estrous cycles. Perception of the day length by the eye is signaled to the pineal gland, which will cause melatonin release. Melatonin will induce the secretion of GnRH and LH, which initiates cyclicity. Low ambient temperature also cues small ruminants to start to cycle. Sheep and goats kept in tropical or subtropical areas do not display marked seasonality and can cycle almost year-round.
The geographic origin of a specific breed influences the length of the breeding season. For example, some breeds have a 2- to 4-month breeding season, and some cycle all year long. Suffolk and Suffolk crosses average 6 months of breeding season. Dorset and Finn sheep have an extended breeding season (8 months), whereas Merino ewes cycle almost year-round.
Dairy goats (Saanen, Toggenburg) cycle from August to February in the northern hemisphere. Most meat or crossbred-type goats have a more extended breeding season and undergo anestrus in late spring and summer.
ESTROUS CYCLE
Ewes cycle regularly every 17 (range 14 to 19) days and does every 21 (range 18 to 22) days during the breeding season. During proestrus, ewes and does are not sexually receptive, but attract the male’s attention, and courtship is initiated.
Estrus lasts 24 to 48 hours in ewes and 24 to 36 hours in does. Estrogen causes the vulva to be edematous and moist. Does show overt signs of estrus more often than ewes. Accordingly, does may exhibit homosexual behavior, but ewes do not. Estrus detection is efficiently achieved using males that have undergone a vasectomy. Both species actively seek the male as they advance into estrus. Multiple copulations during the same estrus are correlated with higher pregnancy rates. A cloudy vaginal discharge may be seen at the end of estrus and should not be mistaken for an infectious vaginal discharge. Ovulation occurs 24 to 30 hours after the onset of estrus in both species. Diestrus lasts 15 to 17 days in ewes and 18 to 20 days in does.
BREEDING MANAGEMENT
Puberty of ewes occurs at 6 to 9 months, but it can be as late as 1 to 2 years of age depending on breed, nutrition, and the time of birth during the year (e.g., lambs and kids born in the fall come into puberty later than spring-born offspring). Pubertal ewes and does should be bred only if they have attained 65% of their mature body weight. The male/female ratio should be 1:50 in natural breeding situations or 1:10 for synchronized breeding. Fresh or frozen semen can be used for AI. The deposition of semen can be intravaginal, intracervical, or intrauterine (Table 14-3). Laparoscopic procedures have become common practice in the sheep and goat industry.
TABLE 14-3
Artificial Insemination (AI) Breeding in Ewes
| Semen | Semen Dose (Number of Spermatozoa) | Lambing Rate (%) |
| Laparoscopic IU | ||
| Fresh or frozen | 20-40 × 106 | 40-100 |
| Transcervical IU | ||
| Fresh or frozen | 50-100 × 106 | 30-80 |
| Cervical | ||
| Fresh only | 200 × 106 | 40-80 |
| Vaginal | ||
| Fresh only | 400 × 106 | 20-60 |
Modified from Youngquist RS, editor: Current therapy in large animal theriogenology, St Louis, 1997, WB Saunders.
PREGNANCY
Gestation lasts approximately 150 days in both species. Ewes are dependent on luteal function only during the first 2 months of gestation. Because pregnancy maintenance in ewes is accomplished by placental hormones after day 50 of gestation, administration of prostaglandin after 2 months of gestation does not induce abortion or parturition. Does are dependent on luteal function throughout gestation, and prostaglandin administration interrupts pregnancy at any stage. Parturition can be safely induced by prostaglandin administration in goats after day 146 of gestation. Does go into labor an average of 28 to 36 hours after prostaglandin administration. Dexamethasone induces parturition in ewes within 36 to 48 hours if administered after day 144 of gestation. Twinning is common, and lambs and kids should be standing within 15 minutes and nursing within 1 hour after birth.
RAM/BUCK EFFECT
Male pheromones produced under androgenic stimulation dramatically influence cyclicity in ewes and does. The introduction of a new, mature, odoriferous male during the transition from the anestrus season into the breeding season will induce estrus in most females. Ewes ovulate in 3 to 6 days, but the CL of this first cycle is short lived. After the second ovulation, regular cyclicity is established. The buck effect is more efficient in does inasmuch as cyclicity is regularly initiated with the first ovulation.
PHARMACOLOGIC CONTROL OF THE ESTROUS CYCLE
Intravaginal sponges delivering progestogen are used to synchronize estrus in ewes and does. Controlled intravaginal drug-releasing devices (CIDRs) are widely used internationally, but are not yet commercially available in the United States. Recently a vaginal sponge impregnated with flurogestone acetate (45 mg/sponge) has been approved by the Food and Drug Administration (FDA) for synchronizing estrus in cycling adult ewes during their normal breeding season. This vaginal sponge has not been approved for use in ewes that have not had lambs. Prostaglandins (extralabel use) are also used to synchronize estrus alone or in combination with progestogen.
OVINE AND CAPRINE PREGNANCY DIAGNOSIS
Pregnancy diagnosis can be performed by checking for returning to estrus, ballottement (palpation) of the fetus in the abdomen, or ultrasonography. The return to estrus can be observed by using a marking harness and crayon on the male (Figure 14-29). Doppler ultrasonography is 90% accurate after 75 days of gestation. Real-time (B-mode) ultrasonography using a 5-MHz probe is 100% accurate after 60 days.
PERIPARTURIENT PROBLEMS
Dystocia usually results from an abnormal fetal disposition or fetopelvic disproportion. It is important to recognize dystocia because the cervix will close after 2 to 3 hours of nonproductive labor. A cesarean delivery is the treatment of choice. Ringwomb refers to the failure of the cervix to dilate during parturition.
Pregnancy toxemia is a common problem seen in ewes in the last 6 weeks of gestation. It is associated with multiple fetuses and inadequate nutrition. Hypoglycemia in these cases may lead to neurologic signs and incoordination. Vaginal prolapse usually occurs in late gestation (3 to 6 weeks before parturition), and dystocia is likely to result in a cesarean delivery.
Hypocalcemia is a condition of pregnant ewes and does leading to cool extremities, failure of the cervix to dilate, and generalized weakness. The treatment is intravenous calcium administration. The hypocalcemic female should be examined 3 hours after calcium treatment. If no progress in delivery is observed, a cesarean delivery is indicated.
Pseudopregnancy is a common condition in goats. It is characterized by a collection of fluid inside the uterus without pregnancy (hydrometra). If not treated with prostaglandins, the natural expulsion of the fluid is called a cloudburst.
INFERTILITY
The natural absence of horns in goats is associated with abnormal sexual development. This condition is called polled (hornless) intersex. The polled condition is determined by an autosomal-dominant gene that is the same or closely linked to a recessive gene causing infertility. Homozygous polled genes cause sex reversal in the female. Affected animals may be genetically female, but exhibit male, female, or mixed characteristics and sexual behavior. The polled gene is dominant, but fortunately the intersex condition is seen only in homozygous animals. Therefore this condition can be prevented by having at least one horned parent.
CAMELID REPRODUCTION
New world camelids include the llama, alpaca, vicuna, and guanaco. They developed from a common ancestor in South America before the arrival of Europeans. Many aspects of reproduction are similar, but there are differences that can be attributed to speciation.
ESTROUS CYCLES
Puberty occurs at 10 to 12 months of age when the animal reaches approximately 60% of the adult body weight. Although llamas have peak fertility during the summer, they cycle year-round. Environmental and endocrinologic factors responsible for the onset and cessation of sexual activity are not yet clearly defined.
Camelids do not have regular estrous cycles. They are induced ovulators, similar to cats. Sexual receptivity can vary from 1 to 36 days. Coitus lasts 5 to 50 minutes with an average of 18 minutes. One mating is sufficient to induce ovulation, which occurs 1 to 3 days later. Delayed ovulation occurs in 30% and absence of ovulation in 10% of females after a single copulation. Treatment with hCG (500 to 700 IU) or GnRH (800 mg) is effective to induce ovulation. The use of a male that has had a vasectomy is more effective to induce ovulation. The ovulatory follicle can be on either ovary, but the pregnancy is invariably in the left horn. Luteolysis is mediated by PGF2a. The CL remains functional throughout gestation. Prostaglandin F2a can be used to induce parturition.
The gestation length averages 344 days (range 331 to 347 days). Parturition lasts 1 to 2 hours, and few complications occur. Approximately 90% of crias (baby camelids) are born between 7:00 am and 1:00 pm.
GENERAL MALE REPRODUCTION
The male differs greatly from the female in the production of gametes. Whereas in the female only 1 to 10 oocytes ovulate during an estrous cycle, males are continually producing and excreting millions of sperm cells. Testicular anatomy differs significantly from ovarian anatomy. The testis is made up of many tubules, each of which connects to a central collecting duct. Between the tubules are the interstitial cells, which continually produce testosterone (Figure 14-30). Each tubule is lined by primordial germ cells (immature sperm cell precursors) and Sertoli cells. The Sertoli cells surround all the developing sperm cells, leaving them with no other contact to the body. This is critical in that the developing sperm are recognized as a foreign substance to the male and would be destroyed by the immune system if they were not protected. As the sperm cells mature, they leave their attachment to the Sertoli cell and are moved through the tubules.
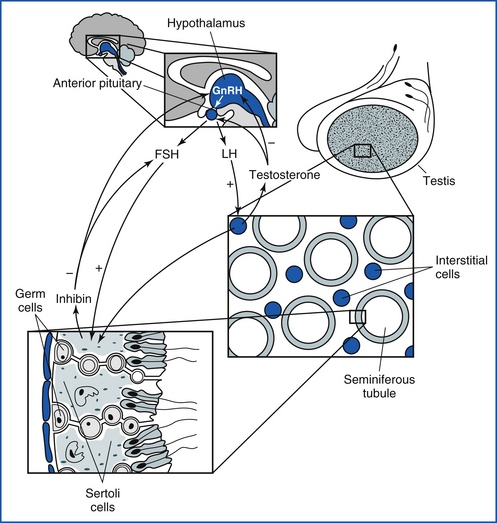
FIGURE 14-30 The general hormonal control of male reproduction: Pulsatile GnRH causes FSH to be released from the anterior pituitary. FSH causes increased sperm growth, maturation, and release. Inhibin from the Sertoli cells in the tubules feeds back on the anterior pituitary and causes less FSH to be released. GnRH secretion from the hypothalamus also results in LH release, which causes testosterone production by the interstitial cells of Leydig. The rise in testosterone causes less LH and GnRH to be released.
The entire testis is covered by a tight capsule, the tunica albuginea (Figure 14-31). The paired testes are contained within the scrotum. The scrotum maintains the testes at a lower body temperature than the rest of the body. If the testes are not kept at a lower temperature, sperm cell production will cease. However, even though sperm cell production ceases, the interstitial cells still produce testosterone. A common example of these consequences is in a cryptorchid animal. A cryptorchid animal has one or both of the testes retained in the abdomen. If the testes are in the abdomen, the animal is sterile, but will still show masculine behavior because testosterone is still produced by the testes.
Although the anatomy of the testes differs from that of the ovary, the control of sperm cell production is quite similar to that of oocyte production; however, it is more continuous and does not occur in cycles. In the male, LH from the anterior pituitary causes an increase in testosterone production (see Figure 14-30). As testosterone production rises, it causes a decrease in GnRH and LH release. The decreased GnRH and LH release causes less testosterone to be produced. As less testosterone is produced, it follows that more GnRH and LH are produced, thus resulting in a balanced feedback mechanism and a relatively constant testosterone production. Testosterone is essential for the production of sperm cells. If testosterone is not present, sperm cells will not be produced. The concentration of testosterone within the testis is 10 times that in the systemic circulation. The administration of testosterone decreases endogenous testosterone production because of the negative feedback on the anterior pituitary and hypothalamus. This will result in lower testosterone concentrations within the testis. Because testosterone is needed for sperm cell production, the exogenous testosterone will eventually decrease sperm cell production.
The other hormone involved in sperm cell production is FSH. Just as in the female, FSH release is triggered by GnRH from the hypothalamus. The FSH acts on the Sertoli cell to increase the division of primordial sperm cells and to release more sperm cells that are embedded in the Sertoli cells. As sperm cell production rises, the hormone inhibin feeds back on the hypothalamus and anterior pituitary to decrease GnRH and FSH, respectively. This causes fewer sperm cells to be produced. As fewer cells are produced, the FSH will increase to produce more sperm cells, thereby keeping sperm cell production relatively constant. In general, FSH causes production of the gamete (oocyte in the female and sperm cell in the male), and LH causes production of the dominant hormone (progesterone in the female and testosterone in the male).
After the sperm cells are released, they move through the tubules and into the head of the epididymis. Within the epididymis, the sperm cells attain motility and the ability to fertilize. The movement through the epididymis to the tail of the epididymis is relatively constant and cannot be increased by increasing the number or frequency of ejaculates. The sperm cells are finally stored in the tail of the epididymis, where they are either ejaculated or voided in the urine if they are not ejaculated (Figure 14-32).
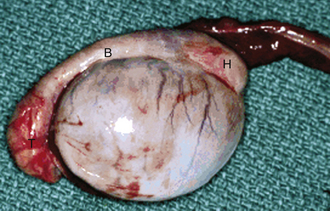
FIGURE 14-32 Lateral view of the right canine testis and epididymis with the head (H), body (B), and tail (T) of the epididymis.
Ejaculation through the penis is the final process in sperm production and delivery. In most domestic species, the penis comprises cavernous blood tissue surrounded by a firm covering, or tunic. An erection occurs when the male is sexually stimulated. During sexual stimulation, parasympathetic innervation causes a blood flow increase into the cavernous portions of the penis. As blood flow increases to the penis, muscles around the proximal penis also contract to prevent blood outflow. Because the cavernous portions of the penis are contained within the tunic, the pressure increases, resulting in a penile erection. Any disruption of the cavernous tissue or the tunic can result in an erection failure.
During an erection, the sperm cells in the tail of the epididymis are moved to the end of the ductus deferens into the ampullae in the process called emission. Once the sperm cells are present in the ampullae, the stimulation to the penis during mating causes ejaculation. Ejaculation is the forceful expulsion of the semen through the penis. The force comes from sympathetic nerves causing smooth muscle contractions in the urethra. During ejaculation, the sperm cells are mixed with fluid from the accessory sex glands. The accessory sex glands include the ampullae, prostate, vesicular glands, and bulbourethral glands. Each species has one or all of these glands, and different glands have different clinical problems in each species. When the sperm cells are mixed with the accessory sex gland fluid, the result is now termed semen. Secretions from the accessory sex gland fluid add various components to the ejaculate, increase the volume, and stabilize the sperm cell membrane. Once the sperm cells enter the female reproductive tract, the sperm cell membrane undergoes a physical and biochemical change called capacitation. Capacitation is required before the sperm cells are capable of fertilization. In the uterus, the sperm cells are quickly moved to the oviduct, where fertilization occurs. Sperm cells are moved to the oviduct by uterine contractions.
CLINICAL EXAMINATION OF THE MALE
Depending on the species, an evaluation of the male as a sound potential breeder may employ different techniques to collect semen and evaluate sperm output. Although semen collection procedures may differ among species, analysis of the semen is quite similar.
SEMEN ANALYSIS
When evaluating semen, it is important to have all equipment at 37° C. Sperm cells are susceptible to cold shock and osmotic shock. Semen is best handled with a thin wooden stick because the wood is thermoneutral and will not cold shock the sperm cells. The first step in semen analysis is to examine the motility. The motility will decline with time because of changes in the semen temperature and pH. The gross motility is examined by placing a drop of semen on a warm slide and examining it at 100 times magnification (10 × objective). The light on the microscope needs to be reduced greatly to see the cells. The sample is only evaluated for movement; however, the concentration can be estimated to help prepare other samples.
After the gross motility is evaluated, the percent of progressively motile sperm is assessed. Individual motility slides are made by placing a drop of semen on the slide and then placing a coverslip over the sample. The sample should be evaluated at 400 times magnification (40 × objective). It is desirable to have approximately 10 cells per high-power field. If there are more cells, the motility cannot be estimated accurately. The motility is estimated by determining the percent of cells that move progressively across the field. Cells that move in tight circles are not progressively motile. If more than 10 cells are seen per high-power field, the sample can be diluted. Dilution can be done by placing a drop of warm saline on a slide and then placing a small amount of semen into the saline (concentrated bull and ram semen usually only requires a quick touch of the saline with a wooden stick dipped into the semen). If the cells were alive on the gross motility examination but are dead on the individual motility examination, the saline may be hypertonic. As saline remains opened, the water evaporates and increases the osmolarity of the solution. Replace the saline and repeat the motility evaluation.
Sperm morphology is generally examined after staining with an eosin-nigrosin stain (Lane Manufacturing). Slides are made by painting a line of the stain across one end of the slide. A small amount of semen is placed in the stain. A second slide is then used to push the stain across the length of the slide. The objective of making the slide is to have a dark background to highlight the cells. If there are lighter and darker areas on the slide, it may be easier to find a more suitable area to examine the cells. To examine the morphology slide, always use 1000 times magnification (100 × objective, oil). Lower magnification will not allow adequate assessment of the sperm cells. A total of 100 cells are counted, and they are classified as normal or abnormal. A spermiogram can be performed to differentiate the different type of sperm abnormalities, which include proximal droplets, distal droplets, kinked tails, coiled tails, acrosome abnormalities, midpiece abnormalities, and misshaped heads.
Sperm concentration is also performed in situations where a physiologic ejaculate is obtained, such as with a stallion or dog. In those species in which the sperm cell output is estimated using scrotal circumference (SC), a sperm count is not done. The concentration of sperm is determined by diluting the sample 1:100 and then counting the diluted sample on a hemacytometer. The easiest way to make a 1:100 dilution is to prepare two tubes of 0.9-ml formal buffered saline. Add 0.1 ml of raw semen to the first tube and mix. Take 0.1 ml of diluted semen from the first tube and add it to the second tube. The second tube now has a 1:100 dilution. Place the 1:100 dilution on a hemacytometer and count all the sperm cells in the middle big square surrounded by triple lines (it has 25 smaller squares). The total number of sperm cells counted and multiplied by 106 gives the concentration per milliliter. The volume of the sample multiplied by the concentration gives the total number of sperm cells in the ejaculate.
BULL, RAM, AND BUCK
The bull, ram, and buck have a fibroelastic penis that has a low blood volume in the cavernous space, but there is high pressure within the penis. The stimulus for ejaculation in these species is temperature. When sensors on the penis encounter the correct temperature in the vagina, the male ejaculates. It is important to note that even though there is no pain response on the penis, the temperature sensors may still be able to signal ejaculation. However, it is also difficult to ascertain whether the temperature sensors are functional. To obtain a physiologic ejaculate, an artificial vagina (AV) needs to be used to collect the semen. Most AVs consist of a hard shell with a rubber liner inserted. Warm water is placed between the rubber liner and the shell. The temperature of the water is critical in inducing ejaculation.
The animal is allowed to become sexually stimulated and mount an estrual female, restrained male, or immobile object; the collector then diverts the penis into the AV. Because this is not easy to do and most ruminants are not trained to breed an AV, most semen collections in the field are performed using an electroejaculator (Figure 14-33). An electroejaculator consists of a probe inserted into the rectum. On the ventral side of the probe are electrodes that stimulate the sympathetic and parasympathetic nerves. The probe is connected to a control box. The box controls how much stimulation the animal receives. The stimulation is low at first and is gradually increased until the animal has an erection, protrudes the penis, and ejaculates. The ejaculate is then collected into a receptacle. Some machines have an automatic progression of power settings, whereas other machines have to be stepped up manually to control the power.
Although commonly used, electroejaculation may be a painful process for the animal. As long as the power is applied, that animal will produce fluid (from the accessory sex glands), so the “ejaculate” is not truly physiologic. Because the ejaculate is not physiologic, other estimates must be used to estimate sperm output. Sperm output is generally estimated by measuring the SC. When measuring the SC, the testes and epididymides are also palpated for size and consistency. A normal bull testis has the consistency of a flexed human bicep. Standards have been set for the desired SC of different ruminant species of different ages.
In the ram, it is important to palpate the epididymides carefully because B. ovis causes infertility and epididymitis. In the bull, a rectal examination is performed to evaluate the vesicular glands. Other body systems to evaluate are vision, teeth, and locomotion. The animal must be able to see, eat, and move around to be a successful breeder. Guidelines have been set by the Society for Theriogenology regarding the minimal criteria needed for an animal to be acceptable for breeding. Breeding soundness evaluation forms and criteria are available for veterinarians from the Society for Theriogenology (Figure 14-34).

FIGURE 14-34 Bull breeding soundness examination form. These forms are copyrighted and available from the Society for Theriogenology. (Society for Theriogenology, PO Box 3007, Montgomery, AL 36109)
Bulls commonly get seminal vesiculitis, and white cells will be seen in the semen. Other common problems in bulls are penile hematomas and preputial injuries (Figure 14-35). A penile hematoma usually occurs when a bull is breeding and the penis bends. This increases the pressure in the penis and causes a rupture of the penile tunic. The rupture almost always happens at the distal sigmoid flexure, and a blood clot forms. Preputial injuries are common in Bos indicus bulls. These bulls have a redundant prepuce that often is everted or hangs out. When the prepuce is damaged, it swells, and the bull cannot retract it. Conservative therapy is common in these cases, but a reefing surgery or a circumcision may be needed.
STALLION
Semen is collected most commonly with an AV (Figure 14-36). The temperature and pressure of the AV are the criteria that contribute to the stallion ejaculating. A final temperature of 45° C to 48° C is generally needed, and the pressure must be adequate. Some stallions prefer hotter and some colder; some prefer more pressure, and some prefer less pressure. Once the AV is prepared, the stallion is teased with an estrual mare until attaining an erection. The penis should be washed with clean warm water, avoiding the use of disinfectants because they can disrupt the normal commensal organisms on the penis and are spermicidal. If a mare is used for a mount, the stallion is led to the side of the mare and allowed to mount. The stallion will then position himself on the mare. As the stallion begins to thrust, the penis is diverted into the AV, and the stallion is allowed to thrust and ejaculate into the AV. As the stallion dismounts, the AV is held vertical to prevent semen from draining out the open end. Immediately after the dismount, the water in the AV should be drained out to prevent heat shock to the cells and to allow the semen to drain into the collection bottle.

FIGURE 14-36 Three models of equine artificial vaginae. From the top, Missouri, Hannover, ARS/Colorado.
Stallions can also be trained to mount dummies, or phantoms. A dummy allows the collection of a stallion’s semen without an estrual mare present and is a safer way to collect semen. Stallion semen often has a large gel fraction that must be removed before an analysis can be performed. This is usually done with an in-line filter attached to the collection bottle. Alternatively the ejaculate can be filtered in the laboratory after collection. Motility and morphology assessments are performed the same as in other species. Stallion semen may be diluted and counted manually or using a densitometer. A densitometer measures the amount of light that passes through the semen sample. The higher the concentration of cells, the less light passes through, the less the percent transmittance. Commercially available machines are calibrated to read out the sperm cell concentration based on internal calculations made from the percent transmittance (Figure 14-37).
If semen is being collected as a part of a breeding soundness evaluation, two ejaculates are obtained 1 hour apart. If the second sample has about half of the number of sperm as the first ejaculate, the ejaculates can be considered representative. Stallions are also seasonal, and both sperm production and libido decrease in the winter when day length is short and increase in the summer.
The transport of cooled stallion semen is becoming more and more popular with certain breeds. Commercially available shipping containers cool the extended semen at a specific rate and keep it cool for up to 48 hours. Extenders are liquids added to the semen to help the longevity of shipped semen. Most extenders are made from skim milk and glucose and have some antibiotics added. When extending stallion semen, the final concentrations should be between 25 × 106 and 50 × 106 cells/ml; however, the final dilution ratio should be at least four parts of extender to one part of semen. If the semen cannot be extended to that concentration and ratio, the semen must be centrifuged and the resulting pellet resuspended to the desired 25 to 50 million/ml concentration. Many stallions, despite having semen that appears good, do not have semen that withstands even the best cooling and shipping procedures.
Stallions may have problems with a decreased libido, hind limb lameness resulting in breeding difficulties, blood in the semen (hemospermia), or urine in the semen (urospermia). These may be challenging problems for the veterinarian to diagnose and treat. The most common breeding injury in the stallion is when the mare kicks the penis or scrotum. The sequel to a kick on the penis is often a hematoma. This is a large blood clot on the outside of the penile tunic, which results in paraphimosis (the penis will not go back into the sheath). If the scrotum is kicked, the testes can swell and be damaged by both the heat from the inflammation and pressure necrosis of the testicular parenchyma swelling within the confined testicular tunic. Conservative therapy for these conditions includes hydrotherapy and antiinflammatories.
CANINE
Canine semen evaluation is performed as in other species, which includes motility, morphology, and a semen count. The main difference is the way the semen is collected. Dog semen is collected by a manual massage of the penis. It is best to have an estrual bitch present when the collection is attempted. Once the dog is somewhat aroused, the prepuce is pushed caudal to the bulbus glandis with a rubber or plastic cone. Circumferential manual pressure is then applied proximal to the bulbus glandis. Pressure is applied while the dog ejaculates. The initial portion of the ejaculate is clear, followed by the sperm-rich fraction. The final portion of the ejaculate is the clear prostatic portion. Normally, it is not necessary to collect the prostatic fraction. It is common for the dog to step over the collector’s hand during the collection process, thereby resulting in the male and female “tied” and facing opposite directions. The criteria for a dog to be a good potential breeder have been published by the Society for Theriogenology. The most common reproductive disorder in the dog is prostatitis. A dog with prostatitis will have white blood cells in the ejaculate and possibly show pain during ejaculation.
TOM
It is uncommon to collect semen from the tom. However, semen can be collected using a small AV or an electroejaculator. A tom must be extensively trained to use an AV and must be anesthetized to use an electroejaculator. This is why semen evaluations are rarely performed in the tom. One option is to use a vaginal swab to detect the presence of sperm cells in a queen that has just been bred. The tom will also have retrograde ejaculation into the bladder, so after breeding, a cystocentesis can be performed in an attempt to find sperm cells as another option. The most common causes of infertility in the tom are poor teeth (because the tom bites the queen’s neck during breeding) and hair rings around the penis.
BOAR
Semen from the boar is collected by manual pressure of the distal penis. The boar is led to an estrual sow or can be trained to mount a dummy. As the boar mounts and extends the penis, the collector grasps the penis “backhand” such that the tip of the penis is grasped mainly with the little finger. It is the manual pressure on the penis that elicits ejaculation in the boar. The tip of the penis is diverted to an open container. The bottle should be covered with gauze or cheesecloth to filter out the gel fraction of the ejaculate. The boar takes approximately 10 to 20 minutes to ejaculate and will continue to ejaculate as long as pressure is applied to the penile tip (the collector’s hand usually tires before the boar). The most common breeding problems in boars are bite wounds to the penis and infections of the preputial diverticulum.
CAMELID
Llamas and alpacas can have semen collected by electroejaculation or with an AV, or they can have semen recovered from the vagina of the female after natural mating. The AV is the best method to obtain a semen sample. The copulatory pattern of the llama is somewhat unique when compared with other domestic species. The female llama lies down, and the male llama will lie on top of the female during copulation. Copulation can take as long as 30 minutes, and the male may ejaculate several times. Semen is evaluated as with other species.
Knottenbelt, D.C., et al. Equine stud farm medicine and surgery. Edinburgh: WB Saunders; 2003.
Root Kustritz M.V., ed. Small animal theriogenology. St Louis: Butterworth-Heinemann, 2003.
Samper J.C., Pycock J.F., McKinnon A.O., eds. Current therapy in equine reproduction. St Louis: Saunders, 2007.
Younquist R.S., ed. Current therapy in large animal theriogenology, ed 2, St Louis: Saunders, 2007.
 TECHNICIAN NOTE
TECHNICIAN NOTE