Preventive Health Programs
When you have completed this chapter, you will be able to:
1 Describe storage, handling, reconstitution, and routes of administration for animal vaccines.
2 Differentiate between core and elective vaccines.
3 Name the core vaccines for dogs and cats.
4 Name the elective vaccines for dogs and cats.
5 List recommended anatomic locations for each vaccine commonly administered to dogs and cats.
6 List and describe the potential vaccine adverse reactions in dogs and cats.
7 List commonly used products to prevent parasite infection and infestation in dogs and cats.
8 List and describe commonly performed diagnostic screening tests in dogs and cats.
9 Provide a general outline of a routine preventive health program for horses.
PREVENTIVE HEALTH PROGRAMS FOR DOGS AND CATS
PUPPY AND KITTEN WELLNESS VISITS AFTER WEANING
Multiple veterinary visits are important for puppies and kittens during the postweaning period (Tables 9-1 and 9-2). During these visits, the young animal is examined for abnormalities, including congenital problems. It is also dewormed and immunized, its growth and development charted, and the owner educated about pet-care issues including: parasite control, common behavioral and training techniques (refer to Chapter 11), nutrition and feeding schedules (refer to Chapter 12), neutering, exercise, and shelter requirements. Young pets must be protected from extremes of weather and the dangers of roaming. They must have fresh water available to them, and they should never be left in a car on a hot day, even for a few minutes, because they are particularly susceptible to heat stroke. Owners are grateful to be warned about common household exposures they may not realize could be dangerous for their pet. Examples of household dangers include: potentially toxic food items, such as grapes, raisins, chocolate, onions, and garlic; drugs, such as aspirin and acetaminophen; and poisons, such as antifreeze, rat poison, toxic plants, and lawn-care products. Refer to Chapter 35 for additional information about toxicology. When discussing nutrition, the veterinary health care team can inform owners about public health concerns regarding raw meat and diets or treats that can carry bacteria, such as Salmonella. The early visits are also an opportunity to talk about the pros and cons of neutering versus breeding (refer to Chapter 14 for more information about reproduction). Spaying female dogs prevents unwanted pregnancy, pyometra, ovarian cancer, helps prevent diabetes mellitus, and if done before the second heat, prevents mammary cancer. For breeds predisposed to gastric dilatation and volvulus, a preventive gastropexy may be done at the time of spaying. Spaying female cats not only prevents unwanted pregnancy, but also stops the almost incessant signs of heat because cats are induced ovulators. Neutering male dogs may prevent roaming, aggression toward other male dogs, prostatic hypertrophy, prostatic infections, and testicular cancer. Neutering male cats may prevent roaming, cat fight abscesses, and marking (spraying) of odiferous urine. Female dogs and cats are usually spayed at 6 months of age unless puppy vaginitis or vulvar conformation warrant going through one heat cycle. Male dogs and cats are usually neutered at 6 to 8 months of age to prevent urinary marking, but large-breed dogs may be neutered later (up to 10 to 14 months of age) at their time of puberty. Cryptorchid dogs should be neutered early because an undescended testicle is at risk to develop cancer or torsion. Shelters advocate neutering puppies and kittens even before adoption, as early as 2 months of age, to help stop overpopulation; no adverse effects have been related to spaying and neutering at this young age.
TABLE 9-1
Canine Vaccination Protocol at the Matthew J. Ryan Veterinary Hospital
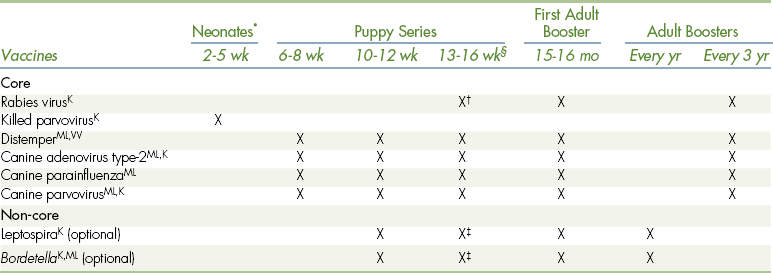
ML,K,VVAvailable as modified-live (ML), killed (K), and/or VV (virus vectored) vaccine.
∗A dog less than 6 wk of age that is lacking colostrum or is at high risk for infectious disease should be given a killed-parvovirus vaccine.
†Check the local laws in your area of practice for the age when rabies vaccine should be first given to puppies.
‡Because of the potential for allergic reactions, we recommend giving leptospirosis vaccine and a killed, injectable Bordetella vaccine 3-4 wk before the last vaccine in the puppy series. Boosters are subsequently given together with the last of the puppy series.
§Try to give the last puppy booster at around 16 weeks of age.
Adapted from Bellwether 61:16, 2005 with gratitude to Dr. Margret Casal and with permission from the University of Pennsylvania School of Veterinary Medicine.
TABLE 9-2
Feline Vaccination Protocol at the Matthew J. Ryan Veterinary Hospital
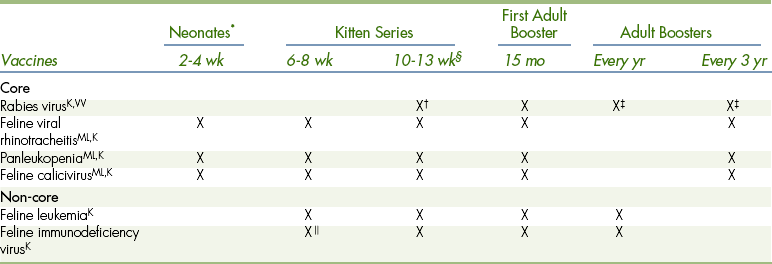
ML,K,VVAvailable as modified-live (ML), killed (K), and/or VV (virus vectored) vaccine.
∗Give a few drops of the MLV (IN vaccine) in each eye and in each nostril as soon as the eyes open. Useful in catteries or during outbreaks in shelters. From 6-8 wk on, either the IN or the injectable FVRCP (feline viral rhinotracheitis, calicivirus, panleukopenia) can be used. Do not inject the IN vaccine.
†Check the local laws in your area of practice for the age when rabies vaccine should first be given to kittens.
‡Repeat yearly if a recombinant vaccine is used.
§Try to give the last kitten booster at 13 weeks of age with the rabies booster.
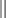 A series of 3 vaccines are recommended between 6 and 12 weeks of age, at least 2 weeks apart.
A series of 3 vaccines are recommended between 6 and 12 weeks of age, at least 2 weeks apart.
Adapted from Bellwether 61:17, 2005 with gratitude to Dr. Margret Casal and with permission from the University of Pennsylvania School of Veterinary Medicine.
Healthy visits are a good time to chat about common warning signs of problems that may occur in the course of a pet’s life, such as a scooting dog rubbing its hind end on the floor may need its anal sacs emptied or may need to be treated for tapeworms. A thirsty dog may not be able to concentrate its urine, or a cat straining in the litter box may not be constipated, but may have a serious urethral obstruction that requires emergency care. The veterinary staff can also educate the owner about genetic predispositions for their pet’s breed and how the staff can help screen or monitor for these.
In the past, after a pet’s adolescent growth period, owners were likely to receive annual postcard reminders to schedule an appointment because annual booster vaccinations were due. Now we know that many of the vaccines we use for dogs and cats produce a longer duration of immunity than just 1 year, and the emphasis of preventive care has shifted from giving vaccines to the importance of routine visits for healthy animals before they become ill. History taking, a physical examination (Chapter 8), and education of the owner can be given the attention they truly deserve. Updates concerning nutrition, behavior, training, and screening for common diseases can be addressed on an individual basis based on the lifestyle and breeding of the animal.
ROUTINE WELLNESS VISITS THROUGHOUT LIFE
Wellness visits are an important part of preventive maintenance care, once or twice a year, throughout the pet’s life (see www.npwm.com/home.htm). The pet’s history needs to be updated concerning new potential health risks, such as those caused by lifestyle changes, travel, the addition of new pets to the home, the administration of new medications, or the development of disease among human family members. Vaccine protocols, nutrition, and parasite control may need adjustment, depending on the individual’s needs. Screening tests and discussions about public health issues may be indicated because asymptomatic pets can be a source of illness for family members, particularly those who are immunocompromised or pregnant.
Subtle deteriorating health changes may be missed by owners. A complete physical examination may detect abnormalities or a change from the animal’s previous “normal.” By tackling problems early, before illness becomes obvious or more serious, intervention has its greatest advantage.
GROOMING MAINTENANCE
Pets are more comfortable when they are groomed and kept clean, and owners can better enjoy time with their pets. Bathing, ear cleaning, removing discharges and matted hair, nail trimming, and anal sac expression are common grooming procedures (see Chapter 8 for anal sacs; Chapter 21 for grooming). Preventive dental care is also important (see Chapter 32).
SMALL ANIMAL IMMUNIZATIONS
Immunity against disease can be acquired passively by maternal antibodies passing from mother to progeny via the placenta (in some species, but not dogs or cats) during gestation or via colostrum shortly after birth. Immunity can also be acquired actively as animals develop antibodies to antigens present in their environment. Antigens are usually proteins that are a part of the pathogenic organism. Vaccines include antigens from a pathogen that causes a particular disease. When the vaccine is introduced to the immune system, the animal forms antibodies to the antigen. If the immunization is successful, a protective level of humoral (B-cell) circulating antibodies and/or a cell-mediated (T-cell) lymphocyte response will be elicited. Subsequently, if the animal is exposed naturally to the pathogen, immunologic memory will cause a rapid and heightened immune response that protects the animal from illness.
MATERNAL ANTIBODY INTERFERENCE
Antibodies present in colostrum can be absorbed through the neonate’s intestine for only about 1 day. Therefore it is important that neonates nurse within the first 24 hours of birth. The passive immunity gained from consuming colostrum wanes over time. For example, the half-life of maternal antibodies against distemper or the infectious hepatitis virus is roughly 8.5 days. In general, the neonate is protected against diseases by maternal antibodies for up to 14 to 16 weeks, depending upon the amount of colostrum the neonate ingests and the type and concentration of antibodies present in the colostrum. Interestingly, strong protective antibody titers in neonates render immunization ineffective. The passively acquired antibodies from the dam or queen block the neonate’s ability to mount an active immune response from the vaccine. Without doing antibody titers on each young animal, we do not know how long the maternal antibodies are present for each disease in each individual. In addition, we do not know when the titer will still be high enough to interfere with the vaccine antigens, and most importantly, we do not know when the titer will be too low to protect the animal from disease due to natural exposure. We do know that there is a period when the antibody level is high enough to interfere with immunization yet, at the same time, is too low to be protective against environmental pathogens. This is a vulnerable and precarious time for the health of the young animal. For this reason, it is important to keep puppies and kittens away from other animals and their excrement until they have received a full series of immunizations. Thus the administration of a series of vaccinations every few weeks after weaning is recommended. In this way, immunization occurs during the earliest possible opportunity to confer active immunity (see Tables 9-1 and 9-2). Vaccinating more often than every few weeks is not recommended because of interferon interference (see later discussion). An adult animal, such as a stray, with no known history of being immunized does not need to complete a repetitive series of vaccines as does a puppy or kitten because adults lack maternal antibody interference. Adults without prior immunizations should receive two sets of vaccines given 3 weeks apart or may receive one set followed by natural exposure to the antigens, which acts as and has the same effect as the second set of immunizations. One year later, the animal may begin to receive adult boosters.
TYPES OF VACCINES
Vaccines work because they contain a pathogen that is either killed or altered. In this way, the vaccine is able to stimulate an immune response in an animal without actually causing the disease. Microbes are cultured, harvested, and inactivated by either killing them with heat or chemicals or modifying (attenuating) them by growing them in a nonhost species, such as birds (usually their eggs), or in the environment where the temperature is below body temperature. Vaccines do not need to include the entire pathogenic organism. Subunits of the organisms make effective antigens for use in vaccines. Modern vaccines may be produced using recombinant technology and include one or more synthesized antigens in each preparation. Viral- or DNA-vectored vaccines induce production of an antigen within the vaccinated animal’s own cells because the DNA is incorporated within the host’s genome and is transcribed.
Modified-live–virus (MLV) vaccines stimulate interferon production better than killed-virus vaccines do. Interferon helps protect the animal quickly, even before antibody production occurs. However, interferon from a previous vaccine may negate the animal’s ability to mount a response to another vaccine if given within 2 weeks. That is why the 3- to 4-week interval between boosters for puppies and kittens is recommended. Theoretically the microbes in an MLV vaccine could be virulent if the attenuation process is incomplete or unsuccessful. In addition, the vaccine can be dangerous to immunosuppressed and pregnant animals. An MLV should only be given to healthy individuals that are not immunosuppressed or pregnant (fetuses may be affected). For example, a puppy recovering from a parvovirus is immunosuppressed because of its illness and should not receive an MLV distemper vaccine, lest it become ill from the distemper virus in the vaccine. Animals vaccinated with an MLV vaccine may shed virus particles for a few days. This can be beneficial because other animals in the house may be indirectly vaccinated, or it can be problematic because immunosuppressed and pregnant animals can be exposed. Immunosuppressed or pregnant animals can be safely vaccinated with killed-virus vaccines (if available), but the animals may not be able to mount protective titers. It is best to check the dam’s or queen’s vaccination status and boost if necessary before she is bred so that her colostrum will contain protective antibodies for the neonates. Bacterins (killed bacterial vaccines) in general do not stimulate immunity as well as MLVs, both in duration and efficacy. Bacterins tend to cause more postvaccinal side effects than MLVs because they possess a higher antigen load and contain adjuvants that, on one hand, enhance the immune response, but on the other hand, may be associated with more inflammation, including autoimmune, allergic, or even neoplastic complications.
STORAGE, RECONSTITUTION, AND ROUTE OF ADMINISTRATION
Vaccines need to be stored according to the manufacturer’s directions (e.g., refrigerated). Lyophilized (freeze-dried) powders need to be reconstituted and gently mixed with the proper type and amount of diluent provided by the manufacturer. If an animal is to receive a DA2PP (distemper, hepatitis [adenovirus-2], parvovirus, and parainfluenza) vaccine without adding a leptospirosis vaccine as the diluent, another sterile diluent should be used that is manufactured specifically for this purpose with the appropriate pH and preservatives.
The quantity of vaccine used for a small Chihuahua puppy is the same as for an adult Great Dane; vaccines should not be divided into smaller quantities because of the animal’s age or size. Vaccines in multiuse vials must be gently mixed before drawn into a syringe, and care must be taken to ensure the continued sterility of the vial.
Most vaccines today are administered subcutaneously (SQ) rather than intramuscularly (IM), although some local laws require the rabies vaccine to be given IM in specific areas of the body. Refer to Chapter 20 for a description of injection techniques. When giving any SQ injection, make sure the vaccine is indeed placed SQ and not dripping down the side of the animal. It is not recommended to vaccinate in the tail or between the shoulder blades; there is poor drainage in the interscapular area, and if a postvaccinal lump develops, it is difficult to surgically remove it. Vaccines are generally given as SQ injections on the distal thigh or shoulder area so that if in the rare event a vaccine-induced mass should form at the injection site, amputation would be possible.
Transdermal vaccines are given without needles, using air-powered special devices, such as Merial’s VET JET. Some vaccines are administered intranasally (IN) to stimulate the development of local mucosal immunoglobulins at the site where pathogens commonly gain entrance to the body. IN vaccines may overcome maternal antibody interference and may induce immunity faster, but for shorter duration. In addition, IN vaccines may have single or combined components. For example, an IN canine kennel cough complex includes a combination of modified-live adenovirus-2, parainfluenza, and Bordetella. An IN feline combination vaccine includes FVRCP (feline viral rhinotracheitis, calicivirus, and panleukopenia). IN-attenuated products may cause mild upper respiratory signs, such as sneezing for a few days, which assists the shedding of antigens to other animals. These vaccines should not be used in households with immunosuppressed or pregnant animals. These products are also available as SQ vaccines. An IN vaccine should never be injected SQ; animals where such a mix-up occurred have suffered severe illness, including hepatic necrosis.
CORE VERSUS ELECTIVE VACCINATIONS
Manufacturers have produced vaccines against more that 20 different types of infectious diseases in dogs and cats. In an effort to decrease possible side effects from overvaccinating, an individual’s lifestyle and risk of exposure should be considered along with the benefits of vaccinating to design the best vaccination protocol for each animal. Elective vaccines may be indicated in some situations, but are not necessary for all dogs and cats. In contrast, core vaccines are necessary because they protect against highly contagious and dangerous viruses that are ubiquitous in the environment. Even indoor pets can become exposed to these dangerous viruses, and there is no specific therapy for viral infections. In addition, core vaccines are both safe and effective in preventing these illnesses. The core vaccines are listed below:
DURATION OF IMMUNITY
Some vaccines produce long-acting immunity for 3 or more years, whereas others last only 6 to 12 months and even during this time may have questionable efficacy (Table 9-3). The DA2PP and FVRCP vaccines work well and have a longer duration of immunity than previously thought. Although manufacturers’ inserts may recommend annual boosters, the American Veterinary Medical Association (AVMA), American Animal Hospital Association (AAHA), and American Association of Feline Practitioners (AAFP) have established guidelines for the frequency of immunizations (refer to appendix). They recommend that canine DA2PP and feline FVRCP combination boosters be given every 3 years rather than annually to adult pets that have received the puppy or kitten series and first adult booster.
TABLE 9-3
Duration of Immunity and Efficacy for Canine Vaccines Commercially Available in the United States
| Vaccine | Minimum Duration of Immunity | Estimate of Relative Efficacy (%) |
| Core | ||
| Canine distemper | ≥7 yr∗ | >90 |
| Canine parvovirus-2 | ≥7 yr∗ | >90 |
| Canine adenovirus-2 | ≥7 yr∗ | >90 |
| Rabies virus | ≥3 yr∗ | >85 |
| Noncore | ||
| Canine coronavirus | “Lifetime”‡,¶ | — |
| Canine parainfluenza | ≥3 yr∗ | >80 |
| B. bronchiseptica | ≤1 yr∗,† | <70 |
| L. canicola | ≤1 yr† | ≤50 |
| L. grippotyphosa | ≤1 yr§ | — |
| L. icterohaemorrhagiae | ≤1 yr† | ≤75 |
| L. pomona | ≤1 yr§ | — |
| B. burgdorferi (Lyme disease) | ≥1 yr∗ | ≤75 |
| Giardia | ≤1 yr§ | — |
Schultz RD: Considerations in designing effective and safe vaccination programs for dogs. In Carmichael L, editor: Recent advances in canine infectious diseases. Ithaca, NY, 2000, International Veterinary Information Service (www.ivis.org). Document No. A0110.0500 available at http://www.ivis.org/advances/Infect_Dis_Carmichael/schultz/chapter_frm.asp?LA=1. Accessed October 4, 2008.
∗Experimental challenge studies and/or serologic studies have been performed. Field experience during outbreaks also confirm experimental challenge studies.
†Based on field experience and observations from outbreak studies and clinical records. Reliable experimental or controlled studies often not available.
‡Not available; cannot be determined. CCV has not been shown to cause significant disease.
§Vaccines recently licensed; information not available except from company data.
Reprinted with permission from www.ivis.org.
SITES OF SUBCUTANEOUS VACCINE ADMINISTRATION
Because there is a small risk of the pet developing a localized vaccine reaction, the site of administration should always be recorded. A consensus of veterinary groups has recommended that rabies vaccines be given SQ over the right rear leg area, the combination vaccines (DA2PP, DA2LPP, or FVRCP) should be given SQ over the right shoulder area, and elective vaccines should be given over the left rear leg. Should a lump, alopecia, or some other local problem appear, the specific vaccine involved is known.
IMMUNIZATIONS FOR DOGS
Rabies (Core Vaccine for Dogs and Cats)
Rabies is a most serious zoonotic rhabdoviral disease of all mammals that is usually transmitted by the saliva of an infected animal via a bite wound. The virus enters distal nerves near the wound and travels proximally toward the spinal cord and brain. This migration can take months during which time the animal is asymptomatic. Once in the brain and salivary glands, the infected animal generally dies of encephalitis within 10 days despite treatment. The clinical signs of rabies include 1 to 3 days of prodromal abnormal behavior followed by 3 to 4 days of hyperactivity, which may include aggressiveness, vicious behavior and biting (“furious” rabies), or motor neuron paralysis, which not only hampers normal movement in the legs, but also affects facial muscles and the ability to swallow (paralytic or “dumb” rabies). Dogs are more likely to become wildly aggressive and “foam at the mouth,” whereas cats are more likely to have hind leg paralysis.
Rabies vaccine is a core vaccine for all dogs and cats. Proof of up-to-date rabies immunization is necessary for dog licensure in many locations. Rabies vaccines are killed-virus vaccines and may be approved as 1-year or 3-year vaccines. Local laws govern whether puppies require their first rabies vaccine at 13 weeks or 16 weeks and may require boosters more frequently than the manufacturer’s recommendations. Local statutes may also insist on IM administration, which would override the manufacturer’s recommendation for SQ administration. In areas where rabies is endemic in wildlife populations, such as among raccoons, skunks, and bats, additional protection may be warranted for the pet and the human family with which it lives. Giving a 3-year vaccine every 2 or 2.5 years may provide this added security. Stray animals with an unknown history of vaccination should receive a booster 1 year after the initial vaccine is administered. In some areas, stray animals are quarantined for a period of time since they could have been exposed to rabies and would be asymptomatic (and noninfectious) during an incubation period. Education is important for owners of new stray animals that have an unknown vaccination history and the possibility of previous bite wounds. There is no test available to diagnose rabies premortem.
Canine Distemper (Core Vaccine for Dogs)
Canine distemper (CD) killed countless numbers of puppies before vaccinations became available in the last century. It is caused by a paramyxovirus, which can be spread via direct contact between dogs or their excretions and by contact with wildlife reservoirs, such as coyote, wolf, raccoon, skunk, weasel, and otter. Because the disease is not easily treatable, this vaccine is considered a core vaccine. Clinical signs may include oculonasal discharge, coughing, dehydration, vomiting, diarrhea, seizures, and inflammation of the chorion and retina in the eye. Later-onset manifestations may include thickening of the footpads and planum nasale, enamel hypoplasia of the teeth, neurologic tics, or epilepsy. The CD paramyxovirus shares some antigens with the human measles virus; therefore a measles vaccine made for dogs can be used in young puppies because it can override colostral distemper antibodies and be protective. CD vaccines are currently modified-live or viral-DNA–vectored vaccines; the killed-virus CD vaccine proved ineffective and is no longer used. Remember that an MLV vaccine should not be given to immunosuppressed animals, such as puppies recovering from a parvovirus, lest they become sickened by the virus in the vaccine.
Infectious Canine Hepatitis (Core Vaccine for Dogs)
Adenovirus-1 causes infectious canine hepatitis, but it also causes a host of other problems, such as glomerulonephritis, vomiting, diarrhea, bleeding, and blue eye in dogs and wild canids. Unfortunately, the vaccine for adenovirus-1 could cause an immune-mediated side effect called “blue eye,” which makes it unfavorable for use, and it is no longer recommended. The vaccine for adenovirus-2 (a virus in the kennel cough complex), on the other hand, does not cause blue eye and will confer protection against both adenovirus-1 and adenovirus-2. It is recommended as a core vaccine and is usually given SQ in combination with other vaccines, such as DA2PP, or it is given IN together with kennel cough complex vaccines.
Canine Parvovirus (Core Vaccine for Dogs)
Parvovirus type-2 is now the most common viral cause of mortality in young dogs. The parvovirus particles are highly resilient in the environment and are not easily killed by common cleaning agents. They are ubiquitous and can be inadvertently brought into the home on the shoes and clothing of pet owners. In this way, even strictly indoor dogs can be exposed and succumb to this deadly disease. Kennels and contaminated areas, bedding, and clothing must be cleaned with either a 1:30 solution of bleach and water or with special parvocidal disinfectants. The illness causes vomiting, diarrhea (sometimes bloody, often foul smelling), fever, severe dehydration, and protein loss from the gastrointestinal tract. Infected animals have leukopenia with low neutrophil counts and are susceptible to secondary bacterial infections. Complete immunization confers a long duration of immunity (7 years or more). The virus in the MLV vaccine may be shed for a time and can cause a false-positive reaction on the fecal Parvocite test.
Parainfluenza (Core Vaccine for Dogs)
The canine parainfluenza virus, like the adenovirus-2, is one of the many viruses that make up the kennel cough complex. As with other viral diseases, it is not treatable with antibiotics, but fortunately does not cause a high degree of mortality, as does canine parvovirus. The vaccine is safe and effective (for 3 years or more) and is often given as a part of the core vaccine combo DA2PP. It may also be given IN as part of the kennel cough complex vaccine.
Leptospirosis (Elective Vaccine for Dogs)
In many areas where leptospirosis is endemic, this vaccine is considered core. However, in hot, arid regions where leptospirosis is unlikely to occur, it should not be used because the vaccine has been associated with adverse reactions, such as anaphylaxis. These reactions are particularly prevalent among small-dog breeds and puppies under 12 weeks of age. Leptospirosis is caused by the bacteria Leptospira spp., which may induce acute or chronic disease in the kidney, liver, and eye. Any one of a number of types (serovars) of Leptospira spp. can cause disease in dogs. L. canicola, L. icterohaemorrhagiae, L. pomona, and L. grippotyphosa have been particularly implicated and the new four-serovar bacterins hope to protect against these bacterial types. Bivalent bacterins that protect against L. canicola and L. icterohaemorrhagiae do not protect against the other serovars and are no longer recommended. There are other pathogenic serovars against which there are no vaccines available; even the new four-serovars subunit vaccine is ineffective. Because the leptospirosis vaccines may not protect all dogs to the same extent, may not protect against the carrier state, and do not produce long-lasting immunity, they must be given annually. The vaccine comes as a liquid and can be given alone each year (over the left hind leg) or given as part of the combination vaccine DA2LPP (over the right front leg). In the combination vaccine DA2LPP, it is used as the diluent and is mixed with the lyophilized DA2PP powder.
Leptospira spp bacteria are shed in the urine of affected animals, such as dogs, rats, cattle, and many types of wildlife. Infected animals can become carriers and shed the bacteria intermittently for years. Leptospirosis is a zoonotic disease and can cause illness in humans. It is important to protect yourself from exposure. Be sure to use universal precautions, such as wearing gloves when working with undiagnosed dogs with renal failure or icterus and those known to have leptospirosis.
Bordetella bronchiseptica (Elective Vaccine for Dogs)
This bacterium in the kennel cough complex is often given during the month before exposure (e.g., before the dog is boarded). Both killed bacterins are available, which are given SQ, and live-attenuated preparations, which are given IN. Immunization may not be completely effective in all vaccinates, and protection is not long lasting. Annual and biannual boosters therefore may be needed, depending upon the dog’s risk for and frequency of potential exposures.
Other Elective Vaccines for Dogs
There are many elective annual boosters for dogs that are rarely necessary, controversial, or not recommended. The inactivated, canine Giardia vaccine, for example, does not protect against infection, but may help decrease shedding of oocysts. This vaccine may be useful in colony situations and may help decrease the risk of exposure to immunosuppressed owners or other animals. Canine Lyme (Borrelia burgdorferi) vaccines are either recombinant subunit OspA or killed bacterins and are controversial even in Lyme-endemic regions. One study showed that Lyme bacterin caused more adverse reactions than any other vaccine when used alone, including leptospirosis vaccine. The efficacy and duration of immunity are not strong, so annual boosters would be necessary. Because 95% of dogs exposed to B. burgdorferi remain asymptomatic, the use of Lyme vaccine appears unwarranted. Dogs with signs of Lyme-induced arthritis typically respond quickly to doxycycline. Rarely, dogs that may be genetically predisposed, such as Labradors, Golden retrievers, and Shetland sheepdogs, may acquire a serious form of protein-losing nephropathy called “Lyme nephropathy,” which is an immune-mediated glomerulonephritis triggered by Lyme antigens. There are concerns that Lyme vaccines may sensitize an individual to this immune-mediated disease and may cause excessive amounts of antigen-antibody immune complexes to be deposited in glomeruli. Tick control is paramount in Lyme-endemic areas to prevent not only Lyme disease, but other tick-borne diseases, such as anaplasmosis, ehrlichiosis, Rocky Mountain spotted fever, babesiosis, and bartonellosis. Canine coronavirus vaccine, which is a killed-virus or MLV vaccine, is not recommended because the virus does not appear to cause disease in dogs by the age they would be seen for vaccination. Recommendations for other vaccines, such as Crotalus atrox toxoid (rattlesnake vaccine) and Porphyromonas sp. (periodontal disease vaccine) are still pending.
IMMUNIZATIONS FOR CATS
Feline Panleukopenia (Core Vaccine for Cats)
Panleukopenia is caused by a parvovirus that is similar, but not identical, to the one that infects dogs. Panleukopenia used to be called “feline distemper” because it was a common infectious feline disease when CD was a common infectious disease among dogs. Like canine parvoviral enteritis, panleukopenia causes severe dehydration, vomiting, diarrhea, fever, and leukopenia. It can also cause cerebellar hypoplasia in neonates if they are exposed during late gestation or early life. Killed-virus vaccines are safer to use than MLV vaccines in pregnant queens and immunosuppressed cats. Because viral particles can be shed from cats vaccinated with MLV vaccines, it is recommended that only killed-virus vaccines be used for animals that live with pregnant or immunosuppressed cats. Immunization with the kitten series followed by the first adult booster produces excellent protection and long-lasting immunity, probably as long as 7 years or more. This core vaccine is represented by the “P” (for “panleukopenia” or “parvo”) in the FVRCP combination.
Feline Viral Rhinotracheitis (Herpesvirus) and Calicivirus (Core Vaccine for Cats)
These two viruses cause upper respiratory disease, including oculonasal discharges, ulceration of the mouth and nose (calicivirus), and sometimes serious ocular disease (herpes) or polyarthritis (calicivirus). The FVRCP combination vaccine can be given either SQ or IN and is produced as both an MLV or killed-virus product. The efficacy of the vaccine is not 100% because it may not protect against the carrier state and not all calicivirus vaccines protect against the rare serious systemic form of calicivirus. However, immunity lasts about 3 years in fully immunized adult cats. It is therefore recommended to be given every 3 years, not annually, after the kitten series and annual booster are completed. A risk factor worth noting is that vaccines for calicivirus may cause immune-mediated polyarthritis.
Feline Leukemia Virus (FeLV)) and Feline Immunodeficiency Virus (FIV) (Elective Vaccines for Cats)
These viruses are shed in the saliva and excretions of seropositive cats. The risk of exposure is greatest among young cats that go outdoors or that live in catteries. The FeLV virus is a retrovirus that can cause lymphoma, bone marrow dyscrasias, and immunosuppression in cats usually within 2 to 4 years of their becoming seropositive. The FIV is also a retrovirus and is responsible for causing “feline AIDS,” characterized by immunosuppression and the predisposition for secondary infections. All cats should be tested for FeLV and FIV before immunization. Ideally, cats would be quarantined for 8 weeks and would be tested at the beginning and at the end of this period before having contact with virus-free cats. The FeLV vaccine may be used to protect outdoor cats from sporadic exposure to the virus, but will not protect cats that have constant exposure such as those that live in the same home or cattery with one or more FeLV-positive cats. Vaccination with an FeLV vaccine does not interfere with testing for FeLV antigens. However, vaccination with FIV vaccine does interfere with FIV testing because the test screens for anti-FIV antibodies and cannot distinguish antibodies produced during immunization from those made in response to natural infection. Thus cats immunized against FIV will have a positive test result. It is recommended that FIV-immunized cats have an identity chip placed at the time of vaccination to facilitate identification and return of the cat to its home should it become lost. In this way, the cat would be spared euthanasia in a shelter or veterinary hospital that mistook it for an FIV-infected animal. The FIV and FeLV vaccines do not confer long-lasting immunity, so annual boosters are recommended for cats that go outdoors. Some veterinarians suggest that all cats receive the kitten and first adult FeLV boosters because exposure during early life is most problematic. In addition, adult cats may develop natural immunity to FeLV making it less important to vaccinate adult animals than cats less than 1 year old.
Other Elective Vaccines for Cats
The following vaccines are rarely necessary, controversial, or not recommended. They do not confer long-term immunity and must be given annually, if given at all. Chlamydophila felis (previously called Chlamydia) is a bacterial infection that causes upper respiratory disease and conjunctivitis. The disease is treatable with antibiotics and is not a common problem in cats in the United States. Thus vaccination with either the killed bacterin or attenuated vaccine is unnecessary unless cultures prove it is problematic in a particular cattery or colony. Feline Bordetella is also not a common pathogen among cats and may exist in normal flora of the feline respiratory tract. For this reason, the use of the attenuated vaccine is controversial. Feline Giardia killed-virus vaccine may help to decrease oocyst shedding and may be of use in catteries or in situations in which a Giardia-carrying cat lives with an immunosuppressed human. Feline infectious peritonitis (FIP) vaccine is a doubly mutated, modified-live coronavirus vaccine for cats that may induce a systemic sensitization to the virus and the local immunity it is designed to give. For this reason, it is a controversial vaccine. Circulating antibodies against FIP from first generation SQ vaccines were shown to cause increased immune-mediated vasculitis, faster illness, and death in vaccinates compared with nonvaccinates when both were challenged with a virulent FIP virus. Feline ringworm (fungal) vaccine is not currently available. This vaccine helped decrease skin lesions from ringworm in cats, but a cat could become an asymptomatic carrier and be a source of infection for owners or other animals.
POSTVACCINAL ADVERSE EVENTS
Postvaccinal adverse events should be reported both to the manufacturer and to the U.S. Pharmacopeia Veterinary Practitioner’s Reporting Program (1-800-487-7776).
Anaphylaxis
Most postvaccinal side effects are not life threatening, such as local pain, transient swelling at the vaccination site, or mild systemic signs of lethargy or fever for a day or 2. However, sometimes an allergic reaction to microbial antigens, adjuvant, inactivators, or preservatives in a vaccine can cause severe reactions, such as anaphylaxis. Anaphylaxis is an immediate hypersensitivity response that may include respiratory arrest, cardiovascular collapse, and death within 30 minutes of immunization. Less severe, but still alarming symptoms are: hives, facial edema, and periocular swelling. Emergency treatment for anaphylaxis may include the administration of:
1. An antihistamine, such as Benadryl (diphenhydramine) at a dose of 2 to 4 mg/kg t.i.d. to q.i.d. PO, IM, or IV.
2. A short-term corticosteroid, such as dexamethasone at a dose of 0.25 mg/kg IV, or for milder reactions, discharge the animal with a short course of prednisone tablets at 0.5 to 1 mg/kg b.i.d. PO.
3. Severe cases may also require epinephrine at a dose of 0.5 to 1.5 ml IV of a 1:10,000 solution, to be repeated in 30 minutes.
Sometimes it is difficult to determine the cause of the allergic reaction. For example, if a dog had a reaction after receiving a combined DA2LPP and rabies vaccine, it is not clear which of the vaccines caused the problem. Because bacterins are most likely to cause anaphylaxis, and since they are elective vaccines, it is not necessary to subject an animal to the possibility of a second reaction by giving the product again. A prudent approach may be to give only the rabies vaccine as it is required by law and to pretreat the patient with a dose of antihistamine 30 minutes before the immunization is given. Close monitoring for 24 hours postinjection would also be needed. If there is no reaction, the DA2PP vaccine without the leptospirosis bacterin might be administered at a later time using this pretreatment and monitoring protocol.
Other Immune-Mediated Reactions
Delayed hypersensitivity reactions may occur days or weeks after vaccination, such as immune-mediated hemolytic anemia, immune-mediated thrombocytopenia, polyarthritis, hypertrophic osteodystrophy, or thyroiditis, possibly triggered by vaccine antigens in genetically predisposed individuals. Breeds with predispositions for vaccine reactions include small-dog breeds, white dogs, or dogs with a diluted coat color, Old English sheepdogs, Weimaraners, and Akitas. It is difficult to prove, but a causal relationship between an immune-mediated illness and the receipt of vaccine within 30 to 45 days has empirically been observed and has caused concern. Even harder to prove is immune-mediated damage to organs months to years after vaccination. For instance, when the FVRCP vaccination is administered SQ, but not IN, it has been shown to induce antibodies against feline kidney cells and against the vaccine viruses. This is not entirely surprising because the vaccine viruses are first grown in feline kidney cell cultures before they are inactivated or attenuated. Laboratory studies of renal function were not found to be impaired 56 weeks postvaccination, but more long-term studies are needed, particularly those that include vaccine boosters.
For animals that have had reactions to vaccines and for immunosuppressed animals that should not receive MLV vaccines, serologic titers for the core vaccine diseases, such as distemper, parvovirus, rabies, and FVRCP, can be quantified to offer some reassurance that an animal has protective humoral antibodies and does not need a booster. A negative titer does not necessarily mean that an animal is unprotected, since long-lasting immunity may exist as a result of cell-mediated immunity or local immunity, which are not easily measured.
Postvaccinal Sarcomas in Cats
A serious postvaccinal adverse effect is the development of fibrosarcoma at the injection site. Fortunately, this is fairly rare and occurs in 1 to 10 cats per 10,000 doses. It is usually associated with the administration of adjuvanted vaccines, such as rabies and FeLV vaccines. First described by veterinary pathologists Dr. Mattie Hendrick and Dr. Michael Goldschmidt at the University of Pennsylvania in 1991, this invasive cancer is the main reason why veterinarians have reevaluated and adjusted vaccine protocols for pets. Further scrutiny regarding the duration of immunity has led to triannual rather than annual protocols, checking titers, vaccinating at particular sites on the body, increased use of IN vaccines (despite postvaccinal sneezing or shedding), and the development of new nonadjuvanted vaccines.
The “1-2-3 rule” reminds us to carefully monitor lumps that occur at an injection site. Many masses are benign granulomas and resolve by 2 to 3 months postvaccination, but some may be serious invasive fibrosarcomas and need to be handled aggressively. All lumps should be recorded (location, size, shape) and assumed to be malignant until proven otherwise. A cytologic examination of an aspirate may not be definitive, so a biopsy or complete removal are recommended if the mass is still growing after 1 month, is greater than 2 cm in diameter, or if the mass persists for more than 3 months.
PARASITE PREVENTION
The treatment of endoparasites and ectoparasites is further discussed in Chapter 17 and Chapter 25. Typically, all puppies are dewormed with a product such as pyrantel pamoate (Nemex) two to three times, 3 weeks apart, to treat roundworms and hookworms that may have been acquired from the mother. After treatment to prevent new infestations, owners are educated about how parasite eggs are shed in the feces of infected dogs and cats. They learn that the ova can be infective for many months, even after weather has removed the bulk of the feces in lawn areas and parks. To prevent reinfection, a monthly preventative may be used, which prevents both heartworm and a variety of intestinal parasite infections (Table 9-4). Flea bites may cause dermatitis, provoke allergies, cause blood loss, and may transmit infectious agents, such as Bartonella, Mycoplasma, and tapeworms. A variety of available flea-prevention products include insecticides that kill fleas, repellants that repel fleas, and growth inhibitors that render the flea unable to produce viable eggs after it has taken a blood meal (see Chapter 25). Tick exposure may transmit diseases, such as Lyme disease, anaplasmosis, babesiosis, ehrlichiosis, Rocky Mountain spotted fever, bartonellosis, and mycoplasmosis. Products available (see Table 9-4) include those that repel or prevent ticks from attaching and products that kill ticks within hours after they have attached. Since some types of organisms may be transmitted soon after tick attachment, products that prevent ticks from attaching may be preferred. Regional risks and the pet’s lifestyle need to be considered. All topical products are somewhat waterproof in the rain or an occasional swim, but frequent shampooing decreases efficacy and may even require reapplication (e.g., imidacloprid products). Since products often distribute in the lipid layer of the skin, the application should not be done within a day of bathing. Products containing permethrins should not be used on or near cats. If a pet eats an amitraz collar, a special antidote called yohimbine needs to be administered.
TABLE 9-4
Some Commonly Used Products for Prevention of Canine Parasites
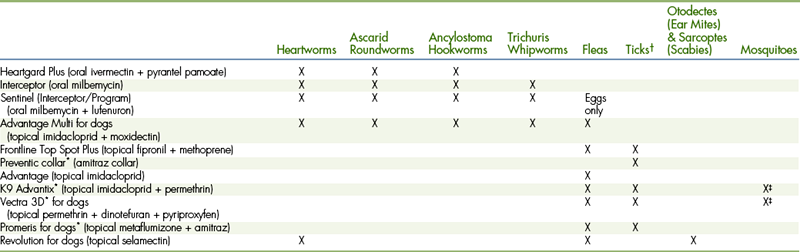
∗Repels as well as kills.
†Including ticks which can transmit Lyme disease, Ehrlichiosis, Anaplasmosis, Babesiosis, Rocky Mountain Spotted Fever, etc.
‡Repels most mosquitoes but should not be considered a heartworm preventive.
ROUTINE HELPFUL SCREENING TESTS
SCREENING FOR REGIONAL INFECTIOUS DISEASES
In regions where certain infectious diseases are found, in-house screening tests may be a valuable aid to help monitor the prevalence of each disease, particularly occult disease that is not showing outward, clinical signs. See Table 9-5 for a list of recommendations for addressing positive and negative test results using the SNAP 4Dx (IDEXX) test.
TABLE 9-5
Recommendations for Snap-4DX (IDEXX) Test Results
Case Signalment: 6 yr old Male Castrated Labrador Retriever
History: appears healthy to owner
Physical examination: no remarkable abnormalities
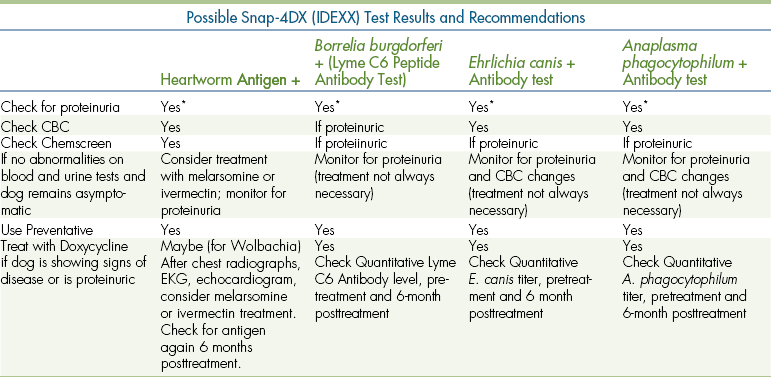
∗Further recommendations concerning proteinuria:
1. Check for proteinuria (complete urinalysis plus E.R.D. [HESKA], microalbuminuria [MA] test, or urine protein/creatinine ratio). If negative, recheck periodically (2-4 times/year).
2. If proteinuric, monitor blood pressure measurements, check for target organ damage (retinal examination, etc), consider antihypertensive medication.
3. If very mildly proteinuric, recheck monthly for trend; quantify urine protein/creatinine ratio.
4. If moderately proteinuric, quantify, monitor, and investigate (consider diagnostic work-up including CBC, biochemical profile, urine culture, chest radiographs, abdominal ultrasound, tick serology, renal biopsy, etc). You are checking CBC and biochemical profile for changes associated with protein-losing nephropathy, anemia, leucopenia or thrombocytopenia due to immune-mediated or infectious disease, hypoalbuminemia, hypercholesterolemia, azotemia, etc. Consider checking for co-infections that may not be Doxycycline-responsive (e.g., Babesia spp., Bartonella spp., etc.).
5. If moderately proteinuric, intervene with treatment including an ACE (angiotensin-converting enzyme) inhibitor, omega-3 fatty acid supplement; if hypertensive, treat with amlodipine; if hypoalbuminemic, treat with an antithrombotic low dose of aspirin; consider immunosuppressive therapy for protein-losing nephropathy cases.
6. Educate owner about using better tick control products, landscaping techniques for tick avoidance, and public health issues.
Many cats carry Bartonella henselae, which is the causative agent of cat scratch fever in humans. Because of the potential health risk to owners, some veterinarians recommend that cats be tested for the presence of antibodies to Bartonella spp. A popular screening test for this purpose is the FeBart Bartonella Western blot (National Veterinary Laboratories, N.J.).
OTHER ANNUAL SCREENING TESTS
It is recommended that all pets be tested annually for proteinuria as a part of their wellness exam. Proteinuria can be quickly assessed by acquiring a free-catch urine sample and testing it for microalbuminuria using the in-house E.R.D. (HESKA) test or reference laboratory MA (microalbuminuria) test (Antech). This would be done in conjunction with a complete urinalysis. A thorough annual checkup would include a CBC, biochemical profile, and fecal examination. In addition, after 7 years of age, cats are typically screened for hyperthyroidism by requesting a serum T4 test as a part of the annual routine blood work.
SCREENING FOR GENETIC DISEASE PREDISPOSITIONS
Every breed is somewhat inbred and predisposed to certain genetic problems. For a list of dog breeds and the diseases to which they are predisposed, refer to the Inherited Diseases in Dogs website at the University of Cambridge, School of Veterinary Medicine (www.vet.cam.ac.uk/idid/). In addition, the National Breed Club websites (available via the American Kennel Club at www.akc.org/clubs/search/index.cfm?action=national&display=on) have specific information about pet health and suggested screening tests for each breed. An ophthalmologic examination to check for congenital cataracts, PennHIP radiographs to check for hip dysplasia, and von Willebrand’s testing for an abnormal tendency to bleed are examples of recommended screening tests for particular breeds. There are specific DNA tests available for a variety of genetic diseases. Testing laboratories are listed at www.akcchf.org/research/genetic_tests.pdf. For diseases in which a phenotypic change occurs (either grossly or by a laboratory abnormality), there may be a recommended age to check for it. Some breeds may have genetic predispositions with no age limit for when illness may occur. The Soft Coated Wheaten Terrier Club of America, for example, has an informative website (www.scwtca.org) that describes annual screening tests recommended for all Wheatens (Box 9-1).
Educating owners about their breed’s predispositions helps them to avoid risky behavior and helps them be more observant for early warning signs of abnormalities. For instance, a dachshund is predisposed to intervertebral disk disease so catching objects in midair is not the best game to play with them. Screening tests that are available for genetic diseases should be done before breeding an animal, but it is also important for the individual so that intervention can be started as early in life as possible. For instance, Persian cats may have polycystic kidney disease (PKD), which can be detected after 10 months of age by ultrasonography, but otherwise would not be discovered for years because the average age of renal failure detection is at 7 years of age. Maine coon and rag doll cats may carry the gene for hypertrophic cardiomyopathy (with autosomal dominant inheritance). Therefore an annual echocardiogram is recommended in these breeds to detect it early before heart failure causes the cat distress.
PREVENTIVE HEALTH PROGRAM FOR HORSES
A preventive health program for horses should be designed to meet the specific needs of the individual animal or herd. Such programs generally vary from one stable to another and from one veterinary practice to another, depending on expected exposures, management styles, and personal preferences of attending veterinarians and horse owners.
An example of one preventive health program for horses is outlined in Box 9-2.
PHYSICAL EXAMINATION
All new additions to a stable or an established herd should have a negative Coggins test result for equine infectious anemia before arrival. Ideally upon arrival, the horse(s) should immediately be placed in quarantine for 1 month before entering the general population. During this time, the first physical examination of the preventive health program can be performed (refer to Chapters 8 and 22). If quarantine facilities are not available, at the very least, a thorough physical examination should be performed before the horse is allowed contact with any animals from the resident population. Any signs of illness or a parasite infection should be addressed before the new horse is turned in with resident horses.
VACCINATIONS
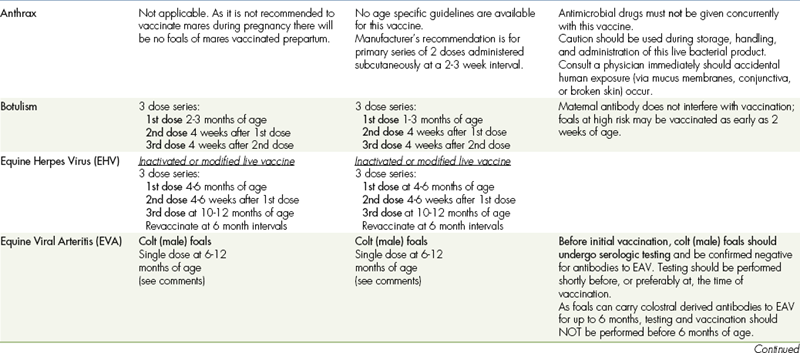
Vaccination schedules are based on the age of the horse, anticipated exposure to infectious organisms, and duration of immunity provided by the vaccine. Tables 9-6 and 9-7 list the vaccination guidelines provided by the American Association of Equine Practitioners. A variety of commercially available vaccines are approved for use in healthy horses, and the choice of product often depends on geographical location and personal experience and familiarity.
TABLE 9-6
VACCINATIONS FOR FOALSa,b
CORE VACCINATIONS Protect Against Diseases that are Endemic to a Region, Those with Potential Public Health Significance, Required by Law, Virulent/highly Infectious, and/or Those Posing a Risk of Severe Disease. Core Vaccines have Clearly Demonstrated Efficacy and Safety, and thus Exhibit a High Enough Level of Patient Benefit and Low Enough Level of Risk to Justify Their Use in all Equids.
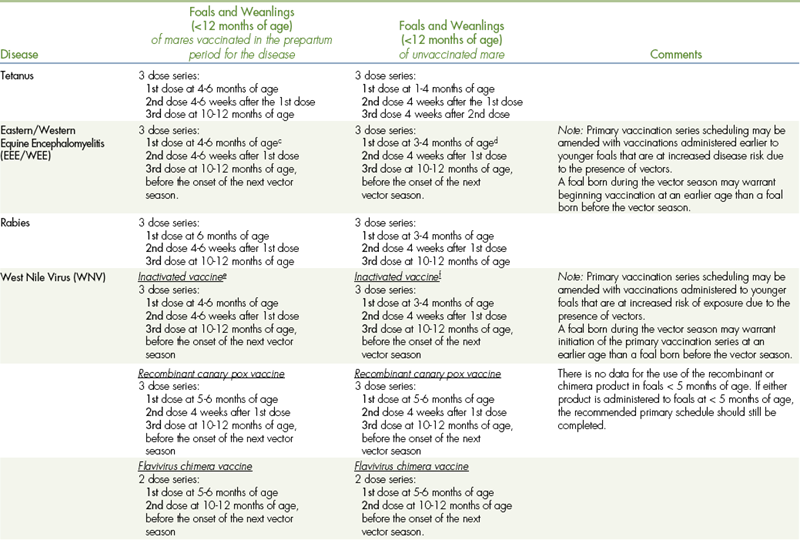
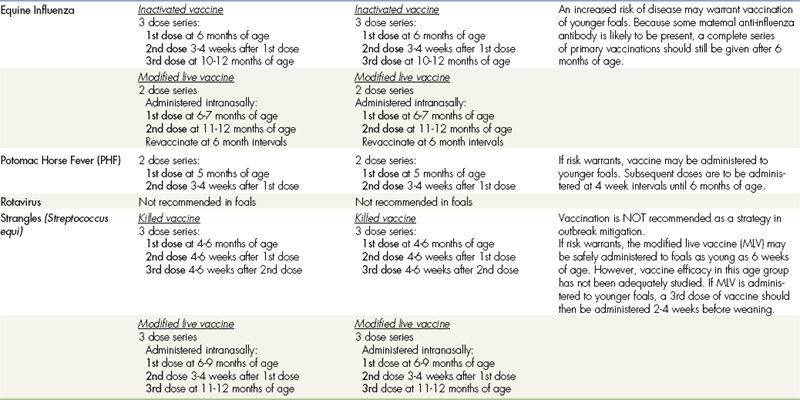
aALL vaccination programs should be developed in consultation with a licensed veterinarian.
bThe two categories (core and risk-based vaccinations) reflect differences in the foal’s susceptibility to disease and ability to mount an appropriate immune response to vaccination based on the presence (or absence) of maternal antibodies derived from colostrum. The phenomenon of maternal antibody interference is discussed in the text portion of these guidelines.
cFoals in the Southeastern USA: The primary vaccination series should be initiated with an additional dose at 3 months of age due to early seasonal vector presence.
dFoals in the Southeastern USA: The primary vaccination series should be initiated at 3 months of age due to early seasonal vector presence.
eFoals in the Southeastern USA: Due to early seasonal vector presence, the primary vaccination series should be initiated earlier with the addition of a dose at 3 months of age.
fFoals in the Southeastern USA: Due to early seasonal vector presence, the primary vaccination series should be initiated at 3 months of age.
TABLE 9-7
VACCINATIONS FOR ADULT HORSES∗
CORE VACCINATIONS Protect Against Diseases That Are Endemic to a Region, Are Virulent/Highly Contagious, Pose a Risk of Severe Disease, Those Having Potential Public Health Significance, and/or Are Required by Law. Core Vaccines Have Clearly Demonstrable Efficacy and Safety, with a High Enough Level of Patient Benefit and Low Enough Level of Risk to Justify Their Use in All Equids.
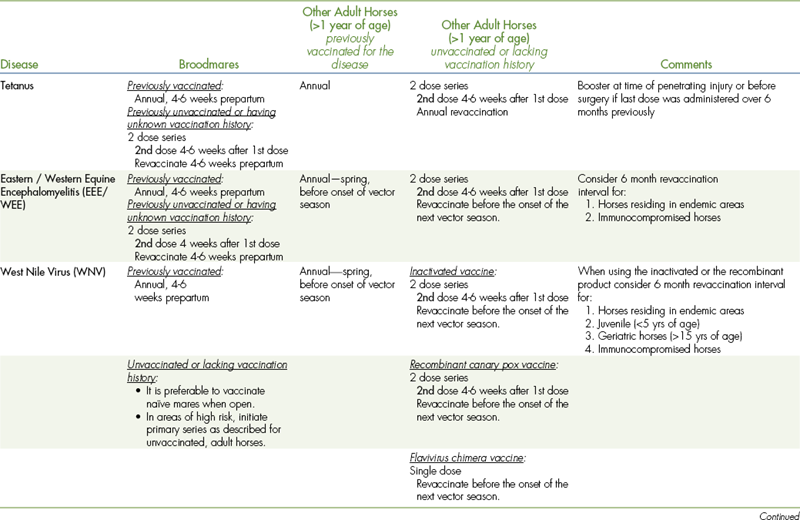
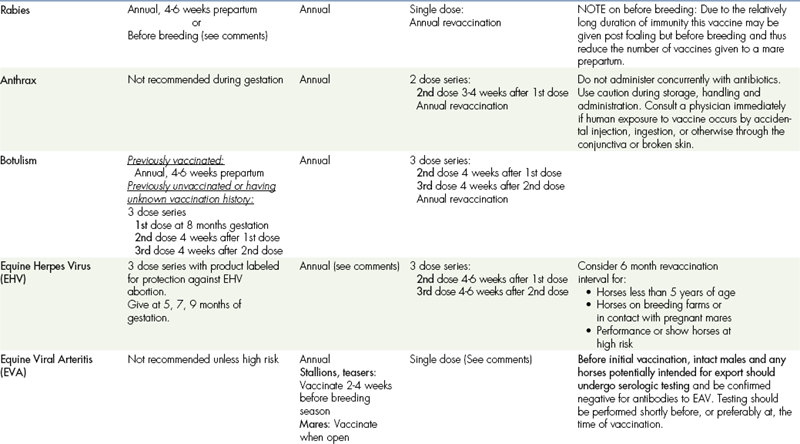
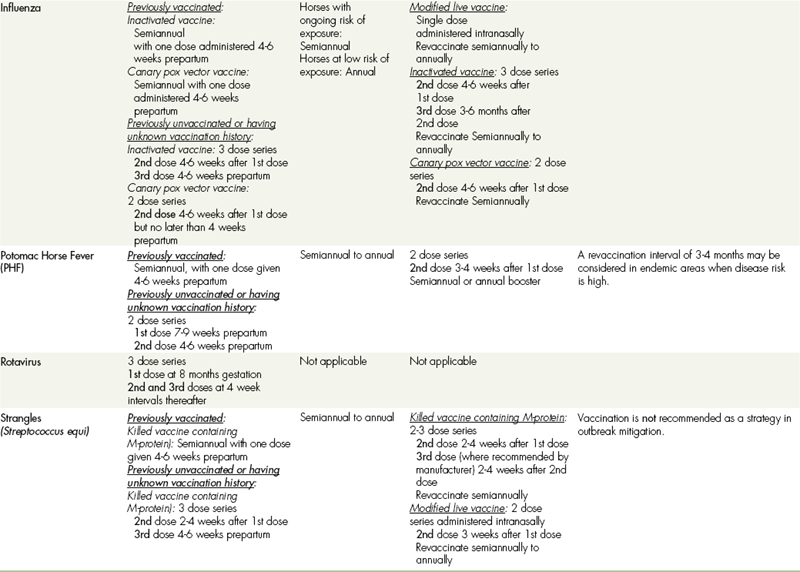
∗ALL vaccination programs should be developed in consultation with a licensed veterinarian.
‡Note: Vaccines are listed in this table in alphabetical order, not in order of priority for use.
Young horses that are immunologically naive or any horse that has an unknown immunization history should receive an initial immunization followed by a second booster immunization. The length between initial and booster vaccinations can vary based on the type of vaccine and manufacturer, but is generally 4 weeks.
In rare instances, anaphylactoid reactions can occur with the use of any vaccine. These life-threatening crises must be handled quickly. Accordingly, it is essential that epinephrine be available for the treatment of anaphylactoid reactions. Other complications, such as fever, lameness, and swelling or abscess formation at the injection site, may also occur with the routine use of the vaccines. The horse owner should always be informed of these possibilities before any vaccine is administered.
Common diseases and vaccines used as an aid in disease prevention are discussed in the following sections.
TETANUS VACCINES
Tetanus, or lockjaw, is a disease characterized by muscular rigidity that may culminate in death from respiratory arrest or convulsions. Tetanus is caused by toxins produced by the anaerobic bacterium Clostridium tetani. Active immunity to tetanus is produced by the administration of a tetanus toxoid, which is a purified, inactivated toxin of C. tetani. C. tetani is routinely found in the environment, and yearly vaccinations are recommended for all horses. Tetanus toxoid booster vaccinations are also routinely given by many veterinarians when treating horses with penetrating injuries or at surgery.
Tetanus antitoxin is produced by hyperimmunization of donor horses with tetanus toxoid. Tetanus antitoxin provides protection by binding to the C. tetani toxin and can be used locally at the site of infection or given parenterally. The administration of tetanus antitoxin to unvaccinated horses induces immediate protection, which lasts approximately 2 weeks, but its use should be restricted to high risk cases because it can cause acute hepatitis.
Tetanus antitoxin and tetanus toxoid should never be mixed in the same syringe and should be injected at distant sites if administered at the same time.
WESTERN, EASTERN, AND VENEZUELAN ENCEPHALITIS VACCINES
Equine encephalomyelitis is a viral neurologic disease of horses caused by eastern, western, and Venezuelan viruses. These viruses are maintained in nature by bird and animal reservoirs and are transmitted to horses by biting insects. Venezuelan equine encephalomyelitis occurs primarily in South and Central America and has not been diagnosed in the United States for many years. The trivalent vaccine is commonly used for horses in states bordering Mexico to create a buffer zone, which may prevent the spread of Venezuelan equine encephalomyelitis into the United States.
The equine encephalomyelitis vaccines currently used for active immunization are inactivated-virus vaccines. They should be administered annually before the biting-insect season. Vaccine protection lasts approximately 6 to 8 months, and in areas where winter freezes are uncommon or in endemic areas, semiannual vaccinations are advisable.
EQUINE HERPESVIRUS VACCINES
Equine herpesvirus (EHV), also known as rhinopneumonitis, frequently causes respiratory disease, but can also cause abortion, neurologic disease, and neonatal illness. Although multiple herpesviruses have been identified, current vaccines offer protection against EHV-1 and EHV-4. These viruses cause respiratory disease; however, EHV-1 is also associated with infections of the central nervous system and the reproductive tract. EHV-4 is most frequently associated with upper respiratory tract disease in young horses and is rarely a cause of abortion.
Both inactivated and MLV vaccines are available for protection against the respiratory form of EHV; no currently available vaccines are licensed for protection against neurologic disease. Because the duration of immunity is short lived, high-risk animals should be vaccinated every 6 months. Pregnant mares should be vaccinated during the fifth, seventh, and ninth months of gestation with an approved vaccine to aid in the control of abortion.
EQUINE INFLUENZA VACCINES
Equine influenza is a highly contagious viral disease with worldwide distribution. Influenza is contracted through inhalation and infects the upper and lower airways. Influenza is frequently seen in mobile populations of horses, and disease outbreaks usually occur in horses 1 to 3 years of age after mixing with infected horses at racetracks, training barns, or show grounds.
Both inactivated and MLV vaccines are available. MLV vaccines for influenza are administered IN and provide a greater duration of immunity. Booster vaccines are recommended every 6 months in high-risk animals.
STRANGLES VACCINES
Strangles is a respiratory disease caused by infection with the bacterium Streptococcus equi. Strangles is easily transmitted through direct contact with mucopurulent discharge from infected horses or from contaminated fomites, such as feeding utensils, buckets, or other equipment. Strangles is characterized by a sudden onset of fever and nasal discharge followed by acute swelling and abscess formation in submaxillary, submandibular, and retropharyngeal lymph nodes.
Several inactivated injectable vaccines and one low-virulence live strain IN vaccine are available to aid in the control and prevention of strangles. IM strangles vaccinations may cause postinjection reactions or abscesses at the site of administration. Because of these adverse effects, vaccination against strangles is recommended only for horses with a high likelihood of exposure. Vaccination is not 100% effective for preventing disease, but does often reduce the severity and incidence of disease. Purpura hemorrhagica (immune-mediated vasculitis) is a possible adverse effect of all strangles vaccines.
EQUINE VIRAL ARTERITIS VACCINE
Equine viral arteritis (EVA) is a contagious viral disease. Although an infection is rarely serious in healthy adult horses, it is concerning to horse breeders because it can lead to abortion, neonatal death, and render stallions as permanent carriers of the virus.
Only one commercially available MLV vaccine is available against EVA. Vaccination is recommended for colts intended to be breeding stallions and for broodmares with no evidence of previous exposure to the virus before being bred to carrier stallions. Vaccination is tightly controlled in some states, and seropositive horses may have problems with import or export to certain countries.
POTOMAC HORSE FEVER VACCINES
Potomac horse fever (PHF) is caused by Neorickettsia risticii (formerly known as Ehrlichia risticii). It is most prevalent in the eastern United States, particularly near large waterways, but has been identified throughout the United States and in other countries.
Approved vaccines are available for use in the control and prevention of PHF, and their use should be considered in areas where the disease is known to occur. The antibody response to vaccination is reportedly poor; however, vaccinated animals may exhibit reduced severity of clinical signs.
BOTULISM VACCINE
Botulism is caused by toxins produced by the bacterium Clostridium botulinum and results in gradual progressive muscular weakness. Multiple types of C. botulinum exist, although type B is most common in horses and is associated with the consumption of decaying forage.
The currently available equine botulism vaccine is a C. botulinum type B toxoid and is recommended for use in endemic areas. This vaccine requires an initial three-dose series followed by annual vaccination. Foals from unvaccinated mares may benefit from vaccination beginning as early as 2 weeks of age.
ANTHRAX VACCINE
Anthrax is caused by the bacterium Bacillus anthracis. Infection results from ingestion of soil, forage, or water contaminated with spores.
The currently available vaccine is an avirulent live-spore vaccine. Because swelling and abscesses have been associated with vaccination, its use is generally limited to high-risk areas.
RABIES VACCINES
Rabies is a viral disease affecting the nervous system and resulting in death. Approved killed-virus vaccines are available for use in horses and should be used annually. Rabies vaccines induce a strong immunologic response; therefore only a single dose is required annually in adult horses.
WEST NILE VIRUS VACCINES
West Nile virus (WNV) was a foreign animal disease before 1999 when the disease was detected in humans and horses on the East Coast of the United States; however, WNV is currently prevalent throughout the United States. The disease is caused by a flavivirus that infects numerous species of birds and mosquitoes; humans and horses are dead-end hosts.
Several vaccines are available for protection against WNV. They should be administered annually before the biting-insect season. Vaccine protection lasts approximately 6 months, and in areas where winter freezes are uncommon, semiannual vaccinations are advisable.
PARASITES
A good preventive health program should also account for the control of internal and external parasites. Heavy parasite burdens decrease athletic and reproductive performance and can cause weight loss and colic. A good deworming program should target ascarids, small and large strongyles, bots, and tapeworms (see Chapter 17).
It is important that all horses maintained at a facility be on an effective deworming program. If all horses pastured together are not properly dewormed, the parasite control program for all horses will be ineffective. There is no standard program concerning the frequency of administration or anthelmintic of choice; therefore it is important to discuss available options with owners.
Although the standard has been to recommend deworming of all horses every 8 to 12 weeks, studies have demonstrated that a small number of horses on each farm are usually responsible for carrying the majority of all worms. Why some horses carry high worm burdens and some do not is still unknown. Although treating all horses similarly is easiest, it is not ideal. If possible, fecal flotations should be evaluated on 10% of the herd immediately before and 7 days after dewormer administration. Egg counts greater than 150 eggs/g before deworming indicate that the interval between treatments is too long. The presence of ova after treatment indicates resistance to the anthelmintic used.
A variety of anthelmintics are available, with benzimidazoles (fenbendazole, oxfendazole, and oxibendazole), pyrantel salts, ivermectin, and moxidectin as the most common. No anthelmintic is effective against all internal parasites, and a few differences should be pointed out:
1. Moxidectin and ivermectin are the only approved boticides.
2. Praziquantel is the only FDA-approved product for tapeworms and is available in combination with moxidectin and ivermectin.
3. Moxidectin and fenbendazole (fenbendazole given for 5 days at double dose) are approved for removing encysted small strongyles.
Daily deworming products are available that are added to a horse’s grain and fed each day. The products currently available are ineffective at controlling all the species of internal parasites. Many horse owners incorrectly assume that because their horses are on daily dewormer internal parasites are not a problem.
Feed additives are also available that are lethal to developing housefly and stable fly larvae in treated horse feces (but not effective against existing adult flies). These types of feed additives should be used with caution because they are organophosphate larvicides with possible adverse effects if used concomitantly with other pharmaceutical products.
DENTAL CARE
The routine examination and care of the teeth is an important part of any horse’s preventive health program. It is estimated that as many as 80% of horses have dental problems. Signs of a dental problem can range from obvious to subtle and include: weight loss, bad breath, excessive drooling, swelling of the face or jaw, dropping feed while eating, head tossing, excessive chewing of the bit, and problems while being ridden (bucking, tail ringing, fighting against the bit). The proper and thorough examination of the oral cavity usually requires sedation, a light source (such as headlamp or flashlight), and a mouth speculum.
In the horse, the teeth are continually erupting, and the lower jaw is narrower than the upper jaw. As the horse grinds its food from side to side, the teeth are worn down unevenly. The inside (near the tongue) of the upper teeth is worn as is the outside (near the cheek) of the lower teeth. Thus sharp points develop on the outside of the upper teeth and the inside of the lower teeth. These sharp enamel points can become severe and result in lacerations of the tongue and cheek. Most enamel points can be removed by floating (rasping). The cheek teeth of the upper jaw are often positioned slightly forward of the teeth in the lower jaw, and because of the offset positioning, hooks and ramps can form. Hooks are sharp points found on the first upper cheek teeth. Ramps are sharp points found on the last lower cheek teeth.
Wolf teeth are the small, pointed, rudimentary first premolars located just in front of the first cheek teeth. Wolf teeth do not appear in all horses, are more common in the upper jaw than in the lower jaw, and will vary in size. In some horses, the position of these teeth causes interference with the position and function of the bit. For this reason, wolf teeth are often removed before a horse enters training (around 12 to 18 months of age). Wolf teeth are generally removed while the horse is standing and sedated.
Normally the deciduous premolars are replaced by the permanent premolars between the ages of 2 to 4 years without a problem. Occasionally a deciduous premolar fails to fall out, a condition known as a retained cap. This can result in discomfort leading to decreased feed consumption and lowered performance. Caps are easily removed in standing, sedated horses.
Feed that becomes trapped around a tooth can lead to bacterial growth, resulting in an infection. Other causes of an infection are a fractured jaw and inflammation of the periodontal ligament (ligament that holds a tooth to the bone). An infected tooth usually leads to the more obvious clinical signs of dental disease, such as a swollen face or jaw, a draining abscess, trouble eating, and foul breath. Because the upper teeth are closely associated with the nasal sinuses, nasal discharge and sinusitis can also be a sign of an infection. Depending on the site of the infection and the length of the tooth root, the infected tooth may be pulled from the oral cavity or removed by accessing the roots via the maxillary sinus or mandible and driving the tooth forward into the oral cavity.
Routine dental maintenance can prevent or minimize many of the dental problems observed in horses. Yearly examinations are recommended for mature horses. Young horses (2 to 5 years) are losing deciduous teeth and gaining their permanent teeth. During this time, 24 teeth are lost and replaced, providing ample opportunity for dental problems to occur. Young horses should have a thorough dental examination before starting training and then twice yearly until all permanent teeth are in. Finally, horses with a history of dental problems should also have their teeth examined biannually or even more frequently if required.
HOOF CARE
The role of the veterinarian and veterinary technician in hoof care is largely advisory for most routine hoof care is provided by farriers. However, education of the client on the importance of proper and frequent hoof care for the prevention of lameness is important.
Horse hooves grow an average of one quarter inch per month depending on ground surface, exercise frequency, nutrition, and individual growth rates. Based on the average growth rate, hooves should be trimmed every 6 to 8 weeks to maintain proper shape, balance, and movement. Keeping the hooves trimmed short and maintaining the correct hoof-pastern axis helps prevent excess stress on tendons and ligaments of the limb. In foals, some minor conformation problems, such as splayfoot or pigeon toe, can be corrected or minimized with frequent hoof trimming.
Cleaning out the bottom of the foot is also important. Hoof cleaning not only removes rocks and debris from the foot, but also helps in the prevention of thrush. Thrush is caused by anaerobic bacteria that grow in moist and dark conditions, such as in the sulci of the frog and under dirt that has accumulated and packed into the sole. Thrush appears as a moist, malodorous accumulation in the sulci of the frog and sometimes over the sole. Frequent cleaning removes dirt and exposes these bacteria to drying, aerobic conditions. Copper- or iodine-based solutions can be applied to the sulci and frog to treat thrush.
NUTRITION
Proper nutrition is the foundation for any preventive health program. Many health issues, such as laminitis, colic, and ulcers, can be directly related to nutritional problems. Owners should be encouraged to feed a balanced diet and to work closely with their veterinarian or equine nutritionist to develop proper diets for their horse(s). Equine nutrition is discussed in more detail in Chapter 13.
PREVENTIVE HEALTH PROGRAM FOR LIVESTOCK SPECIES
Preventive medicine is especially important in livestock to maintain the productivity of the herd. Management, nutrition, and vaccination each play a role in minimizing the incidence of disease in livestock species. This section is not intended to provide a comprehensive review of all of the vaccines available in livestock; the goal is to describe typical preventive management procedures and commonly used vaccination programs.
SWINE
Preventive medicine in swine herds begins with piglets, which must be kept in a warm, draft-free environment. When pigs are piled up on top of each other, they are too cold, and piling up increases the risk of rectal prolapse. Within the first week of life, piglets have their needle (canine) teeth trimmed and tails docked to decrease chewing on each other. Baby pigs are commonly given a shot of iron at the time of teeth and tail trimming, and male piglets that will not be used for breeding are castrated.
GROWING PIGS
Pigs are vaccinated against erysipelas at weaning, when they are removed from the sow and placed into groups of growing pigs. Erysipelas, which is caused by Erysipelothrix rhusiopathiae, is characterized by fever, skin lesions, and sudden death in infected pigs. Animals that survive the acute infection may develop chronic arthritis or endocarditis and consequently grow poorly. Pens into which weaned pigs are moved must be cleaned and disinfected, and they must be well ventilated without being cold or drafty. Newly grouped weaned pigs should not be mixed in pens or buildings with older pigs. Overcrowding must also be avoided. Because weaning is a stressful time for pigs, some farms will add antibiotics to the feed for a few weeks after weaning. Pigs may also be dewormed at weaning, if necessary, and some farms will vaccinate pigs at weaning against pathogens that may cause pneumonia, such as Mycoplasma bacteria.
Biosecurity (a protocol to prevent the introduction of disease organisms onto the farm) is practiced more commonly and more strictly in swine production than any other type of animal agriculture. On some farms, all visitors, including veterinarians and their staff, are asked to shower and change into clothing provided by the farm before coming into contact with any animals. The risk of spreading disease may also be minimized by working with the youngest pigs first then proceeding through progressively older groups of pigs.
BREEDING ANIMALS
Pigs are commonly vaccinated for leptospirosis, parvovirus, and again for erysipelas before entering the breeding herd. Leptospirosis, an infection with L. pomona, L. bratislava, or other members of the genus Leptospira, may cause infertility, abortion, stillbirth, or the birth of weak piglets. Animals purchased for breeding should be tested for brucellosis and pseudorabies if the animals are not from a pseudorabies-free area (most of the United States is now pseudorabies free). Brucellosis may cause abortion or infertility and it is zoonotic. Pseudorabies causes death in young pigs and respiratory disease with the possibility of chronic infection in older animals. Animals entering a herd free of porcine reproductive and respiratory syndrome (PRRS) should also be tested for the PRRS virus, which causes reproductive failure, respiratory disease, and chronic infections. Depending on their origin, animals may need to be treated for internal and external parasites. New additions to the herd should always be quarantined away from the herd for 30 days or more before introduction to the herd. Quarantine prevents new animals from spreading diseases they may have been carrying asymptomatically when they were purchased or diseases, such as pneumonia, that they may have developed during transport to the farm.
Sows in the breeding herd should have booster vaccinations against erysipelas and leptospirosis when their litters are weaned; boars may be given the same vaccines every 6 months. Sows and gilts (young sows) may also be vaccinated against Escherichia coli bacteria to diminish the occurrence of diarrhea in their offspring and against parvovirus, which may cause infertility and abortion.
CATTLE
Although beef and dairy production systems have many differences, they will be discussed together here because many of the principles of disease control and diseases of concern are the same in both systems.
BIRTH TO WEANING
Preventive medicine in cattle actually starts before birth because many pregnant cows are vaccinated against E. coli, rotavirus, and coronavirus to protect their calves from developing diarrhea. Colostrum from vaccinated cows provides extra protection to calves against diseases for which the cow has been vaccinated. Calves should be born into a dry, draft-free environment. It is essential that calves receive an adequate amount of good quality colostrum soon after birth. In beef herds, this is ensured by the frequent monitoring of cows during calving season, whereas dairy herds typically hand-feed colostrum to newborn calves. Dairy farms will keep frozen colostrum or colostrum replacer on hand to feed orphan calves or calves from dams that fail to produce adequate colostrum or leak colostrum before calving.
Vaccination in beef calves should ideally begin before weaning because weaning is a stressful time for calves that increases their risk for developing disease. Weaning in dairy calves is done at a younger age than in beef calves (less than 2 months of age versus about 6 months), so vaccination is commonly delayed until after weaning. For any young cattle, vaccination at less than 3 months of age is likely to be incompletely effective as a result of interference from maternal antibodies obtained in colostrum. Calfhood vaccination programs usually include a clostridial vaccine, a viral respiratory and reproductive pathogen vaccine, and brucellosis vaccination (often called “Bangs” vaccination). Clostridial diseases, such as tetanus, blackleg, and malignant edema, are caused by bacteria from the genus Clostridium, occur primarily in young animals, and are often rapidly fatal. Viral respiratory and reproductive diseases are commonly included in one “five-way” or “six-way” product, usually with a brand name connoting strength rather than what the vaccine is designed to protect against. The viral reproductive and respiratory pathogens commonly included in combination vaccines are bovine diarrhea virus (BVD), which may cause diarrhea, mucosal ulcers, abortion, and immunosuppression, plus infectious bovine rhinotracheitis (IBR), parainfluenza 3 (PI3), and bovine respiratory syncytial virus (BRSV), which are primarily respiratory pathogens. These combination vaccines also frequently include the bacterial pathogens Histophilus somnus, which may cause sudden death or fever and depression, and/or Leptospira species, which cause infertility and abortion. Bangs vaccination protects against brucellosis, an infection with Brucella abortus, which is associated with abortion and infertility. Brucellosis vaccination is reported to the United States Department of Agriculture, and vaccinates are marked with an orange ear tag and tattoo in the right ear. Only heifers under 1 year of age may be legally vaccinated against brucellosis, and in some states, the maximum age for vaccination is less than 1 year. Vaccination against brucellosis is especially important for animals that may be sold as breeding stock. Special care is taken in the handling and administration of RB51, the brucellosis vaccine, because the vaccine contains live organisms and the disease is zoonotic. Unlike the clostridial and viral vaccines, the brucellosis vaccine is given only one time.
Animals that naturally have horns are commonly dehorned to protect other animals from injury. In dairy calves, this is usually done in the first few weeks of life, whereas in beef animals, dehorning may be done at the time of weaning.
GROWING CATTLE
Disease in growing cattle is best prevented by appropriate nutrition and clean, well-ventilated housing (or good pasture) in addition to vaccination. Growing cattle that are at risk will need to be treated for external and internal parasites, as necessary. Adequate fly control will help prevent infectious bovine keratoconjunctivitis (pinkeye), an infection of the eye caused by Branhamella ovis and/or Moraxella bovis bacteria, which are carried between animals on flies. Heifers commonly receive another dose of viral respiratory and reproductive vaccine before being bred for the first time. Beef cattle are also typically given a repeat vaccination against viral respiratory pathogens upon entering the feedlot; in addition, such animals are commonly vaccinated against the bacterial respiratory pathogens Histophilus somnus, Mannheimia hemolytica, and Pasteurella multocida.
BREEDING ANIMALS
To protect the health of the herd, care must be taken when obtaining breeding bulls. Beef herds are more likely to purchase bulls because they generally employ natural breeding throughout the herd, whereas dairy herds tend to use artificial insemination extensively. In addition to a breeding soundness examination for fertility, purchased bulls should at least have a negative test result for BVD, which may cause infertility, abortion, diarrhea, death, or a chronic poor condition. It is also advisable to test bulls for the venereal disease trichomoniasis, which is passed to cows during breeding and causes fertility problems. It is preferable to purchase the bull from a herd that is free of Johne’s disease, which causes chronic diarrhea and weight loss, and bovine leukosis virus, which causes cancer. Bulls should be vaccinated before the breeding season against the viral respiratory and reproductive pathogens; campylobacteriosis, which is passed to cows when breeding and causes infertility; and leptospirosis. Cows should also be revaccinated against these diseases before the breeding season.
In dairy cows, mastitis (an infection of the mammary gland) is an important disease and a key focus of preventive efforts. Dairy cows are commonly vaccinated against mastitis caused by E. coli, which can be severe and life threatening. The vaccine does not prevent E. coli mastitis, but it does reduce the frequency and severity of the disease on the farm. Most mastitis cannot be prevented by vaccination; prevention relies on management steps. These include keeping cow housing and milking areas clean, cleaning the teats well before milking, dipping the teats into disinfectant before and after milking (called predipping and postdipping), and proper nutrition.
Only the most commonly used vaccines are mentioned previously, but many more are available. Not all available vaccines are considered effective. Vaccine protocols should be tailored to each herd specifically based on the needs and risks in the herd. It must also be kept in mind that even an excellent vaccination protocol cannot overcome poor management and nutrition.
Hoof trimming is especially important in dairy animals. They may not wear their hooves down as quickly as they grow, and animals that have suffered an episode of lameness may not wear their hooves evenly. Dairy cows should have their feet trimmed at least once a year and more often if problems develop. Because they walk more, beef animals do not usually need routine trimming, but it may be necessary for animals with abnormal hoof growth patterns, such as “corkscrew claws,” in which the wall of the hoof spirals under the sole. This is thought to be a hereditary problem, so cows who display corkscrew claws should probably be removed from a breeding program.
As with bulls, purchased cows should be tested and quarantined before joining a herd. It is preferable to buy cows from a herd that is free of Johne’s disease and bovine leukosis virus, and all animals should have a negative test result for BVD virus. In addition, the milk of purchased dairy cows should have a negative test result for the presence of contagious or incurable mastitis infections.
SMALL RUMINANTS: SHEEP AND GOATS
As with cattle, preventive health programs for sheep and goats begin before birth, when pregnant ewes and does are vaccinated against C. perfringens type C and D and C. tetani, which cause the usually fatal diseases lamb dysentery, overeating disease, and tetanus. Protection for lambs and kids is provided through the colostrum of their vaccinated dams. Ewes and does should be vaccinated at least 4 weeks before their due date; if they have not previously been vaccinated against clostridial diseases, they should be vaccinated twice during pregnancy. If the ewes and does do not get vaccinated during pregnancy, kids or lambs should be vaccinated just after birth and again at 2 to 3 weeks of age, and tetanus antitoxin should be administered at the time of castration, dehorning, and tail-docking during the first week of life. The offspring of vaccinated dams should be vaccinated twice between 6 and 10 weeks of age. In selenium-deficient areas, ewes should be supplemented with selenium orally or by an injection to prevent weakness caused by selenium deficiency (white muscle disease) in the lambs.
Small ruminants may also be vaccinated against contagious ecthyma, also called orf or sore mouth. It is a viral disease that causes painful lesions of the skin on the mouth of young animals and the mouth and teats of ewes and does, resulting in decreased nursing by young animals. Sore mouth vaccination is performed by scratching the skin in an area without wool (inner ear or under the tail in older animals, inner thigh in young animals) and introducing a live virus into the scratch. This must be done well before lambing or kidding so that newborn animals will not be affected. In addition, great care must be taken by the person administering the vaccine because it contains a live virus and the disease is zoonotic. The vaccine should not be used in herds that have not had problems with the disease.
Coccidia are present in all sheep and goats, and they may cause diarrhea, weight loss, and illness in young animals under stress. Coccidia are controlled by management steps such as preventing overcrowding, good sanitation, and feeding off the ground. In addition, a coccidiostatic drug, such as lasalocid or decoquinate, may be added to the feed to help prevent outbreaks of coccidiosis.
THE BREEDING HERD
For small ruminants that are kept as pets, rabies vaccination is probably worthwhile, though it is not used in commercial flocks. As in other species, many other vaccines are available for use in sheep and goats; decisions about whether to use them in particular animals or herds should be made in consultation with a veterinarian and in light of specific needs and risks.
Parasitism is a serious problem in sheep and goats, particularly infestations with Haemonchus contortus, also called the barber pole worm. H. contortus attaches to the wall of the abomasum and consumes the animal’s blood. If left untreated, infestation may lead to severe anemia and death. There is no strategy that completely eliminates internal parasites in sheep and goats, but there are a number of ways to reduce the burden. One aspect is good nutrition; well-nourished animals are less susceptible to parasitic illness. Animals should be fed off the ground in a trough into which young animals cannot climb to reduce the contamination of feed with feces. Goats that are able to browse plants above the ground, instead of just grazing as sheep do, will have reduced parasite burdens. Parasitism in sheep may be reduced by rotating them through pastures that have been kept empty or used by cattle or horses (not goats) or crops for the previous 3 to 6 months. Culling of animals with chronic severe parasitism is recommended because such animals have low parasite resistance, a trait that may be passed on to the offspring. Oral dewormers of the benzimidazole (e.g., thiabendazole) and avermectin (e.g., ivermectin) families are used in small ruminants, but these drugs must be used strategically because overuse promotes resistance. Some experts have recommended deworming only animals that show signs of disease. Sheep may also develop external parasites, such as ticks and lice, which should be treated as necessary with sprays, dips, or ivermectin-type drugs.
SUMMARY
There are many aspects of preventive medicine in livestock species. Vaccination is important, but it is not a substitute for proper management. Disease is also prevented through proper nutrition, good hygiene, appropriate housing, and parasite control. New animals must be tested for disease and quarantined before mixing them in with the herd to prevent the introduction of disease into the herd.
Kitchell BE: Feline vaccine-associated sarcomas, WSAVA Congress, 2005. Available at www.vin.com/proceedings/Proceedings.plx?CID=WSAVA2005&PID=10915&O=Generic. Accessed October 4, 2008.
Klingborg, D.J., et al. AVMA council on biologic and therapeutic agents’ report on cat and dog vaccines. J Am Vet Med Assoc. 2002;221:1401–1407.
Littman, M.P. The Lyme test is positive—now what? Proc 25th Ann Vet Med Forum. ACVIM. 2007:501–503.
Littman, M.P., et al. ACVIM small animal consensus statement on Lyme disease in dogs: diagnosis, treatment, and prevention. J Vet Intern Med. 2006;20:422–434.
Paul, M.A., et al. Report of the American Animal Hospital (AAHA) canine vaccine task force: 2006 AAHA canine vaccine guidelines. J Am Anim Hosp Assoc. 2006;42:80–89.
Richards, J.R., et al. The 2006 American Association of Feline Practitioners feline vaccine advisory panel report. J Am Vet Med Assoc. 2006;229:1405–1441.
Schultz, R.D., Considerations in designing effective and safe vaccination programs for dogs. Carmichael L., ed. Recent advances in canine infectious diseases. International Veterinary Information Service: Ithaca, NY, 2000. (www.ivis.org). Document No. A0110.0500 available at www.ivis.org/advances/Infect_Dis_Carmichael/schultz/chapter_frm.asp?LA=1. Accessed October 4, 2008..
Shelter medicine website from the University of California (Davis) Koret Shelter Medicine Program. Available at www.sheltermedicine.com/portal/is_vaccination.shtml#top3 and www.sheltermedicine.com/portal/portal.shtml#top3. Accessed October 4, 2008.
Ensminger, M.E., Hammer, C.J., Ensminger’s equine science, ed 8, Upper Saddle River, NJ, Pearson Prentice Hall, 2004.
Love, S. Treatment and prevention of intestinal parasite-associated disease. Vet Clin North Am Equine Pract. 2003;3:791–806.
Smith, B.P. Large animal internal medicine, ed 4. St Louis: Mosby; 2009.
Bagley C V : Vaccination program for beef calves . Available at http://extension.usu.edu/files/publications/factsheet/AH_Beef__40.pdf. Accessed October 4, 2008.
Bagley C V : Vaccination programs for dairy young stock . Available at http://extension.usu.edu/files/publications/factsheet/AH_Dairy_06.pdf . Accessed October 4, 2008.
Maryland small ruminant page : Available at www.sheepandgoat.com. Accessed October 4, 2008.
Tubbs R C : Herd health programs for swine seedstock production. Available at http://extension.missouri.edu/explore/agguides/ansci/g02508.htm . Accessed October 4, 2008.
Tubbs R C, Floss J L : Herd management for disease prevention. Available at http://extension.missouri.edu/explore/agguides/ansci/g02507.htm . Accessed October 4, 2008.
 TECHNICIAN NOTE
TECHNICIAN NOTE