CARE OF THE NEONATE AND NEONATAL DISEASES
Neonatal camelids are referred to as crias, and the newborn and its dam form a strong family bond (Figure 22-36). The newborn alpaca cria should weigh at least 12 lb at birth, and the normal llama cria should be greater than 15 lb; however, comparison with the average birth weight on any given farm may be of more significance. Crias are born with the eyelids open and the incisors erupted. The neonate is covered with an epidermal membrane that attaches at the mucocutaneous junctions, coronary bands, and the umbilicus. The camelid fetus is not surrounded by an amniotic membrane as occurs in other species, making the newborn much less likely to suffocate after birth. Camelid mothers do not lick the cria to dry it nor do they stimulate the baby to stand. The mother may nuzzle the cria and vocalize with a humming sound.

FIGURE 22-36 The neonatal camelid is referred to as a cria. The dam and cria form strong family bonds. (Courtesy of Ms. Vida Palmer.)
 TECHNICIAN NOTE
TECHNICIAN NOTE
The camelid fetus is not surrounded by an amniotic membrane as occurs in other species, making the newborn much less likely to suffocate after birth.
Following birth, crias should attempt to stand in 30 minutes and be successful by 60 minutes. Newborns should actively try to nurse the dam within the first hour and successfully nurse within 3 to 4 hours. If nursing has not occurred by 6 hours after birth, intervention is essential. Crias usually nurse three to four times per hour. During the first 3 days of life, the newborn may not gain any weight and may lose up to 1 lb; greater weight loss than this should be of concern. After the first few days, the cria should gain 0.5 lb per day for the first 2 weeks. Llama crias then continue to gain at the rate of 1 lb/day, and alpaca crias may gain 0.25 to 0.5 lb/day. The newborn should be alert and have clear eyes and erect ears. Typically, the body temperature is 101° F to 102° F; heart rate, 80 to 100 bpm; and respiratory rate, 10 to 30 breaths/min.
Camelids are obligate nasal breathers, so open-mouth breathing is considered abnormal and may be indicative of respiratory or congenital problems. As with other neonates, the newborn cria should be weighed, examined thoroughly, and the umbilicus dipped in disinfectant. Observation of nursing and assessment of passive transfer is also of great importance in neonatal care. It is not necessary to administer enemas to every neonate. Meconium should be passed within 18 to 24 hours after birth, and failure to do so, especially if the cria is straining, may warrant a gentle enema using 200 to 500 ml of warm water. Newborns should be carefully watched, especially during the first 48 hours of life. Crias that are considered “at risk” include premature crias, crias born to mothers with dystocia, newborns with congenital defects, crias that suffer excessive umbilical bleeding, crias born to the same mating that experienced problems in previous years, and crias that develop FPT. “At-risk” crias often show abnormalities in vital signs, labored respirations, weakness, depression, failure to nurse, failure to stand, and straining with failure to pass meconium.
 TECHNICIAN NOTE
TECHNICIAN NOTE
Camelids are obligate nasal breathers, so open-mouth breathing is considered abnormal and may be indicative of respiratory or congenital problems.
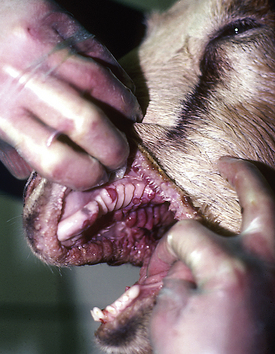
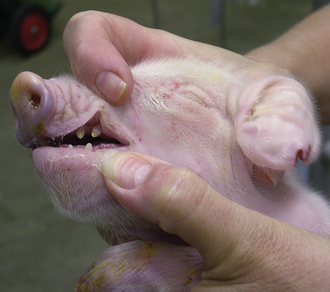
There is considerable variation in gestation in alpacas and llamas (330 to 360 days), with some pregnancies lasting more than 1 year. This variation makes it difficult to determine prematurity based on length of gestation. In addition, in pasture breeding situations, undetected early embryonic death may be followed by another breeding several days later. Prematurity is not based entirely on gestational length. Signs of prematurity in the newborn are of more importance than time in utero. Low birth weight may be the most obvious sign, but premature crias also show signs, such as weakness and inability to stand or hold the head up to nurse. Affected crias also have excessive laxity of tendons and ligaments and may walk on their fetlocks. In addition, premature crias often have nonerect or curled ears as a result of immature cartilage in the ears (Figure 22-37), the hair coat is especially silky, and the rubbery covering of the toe persists for 1 to 2 days in premature babies (disappears in 6 to 12 hours in full-term crias). The incisors are not erupted in premature crias (Figure 22-38), and the mucous membranes are dark red from decreased oxygenation as a result of undeveloped lungs. Prematurity is life threatening and requires immediate and intensive therapy.
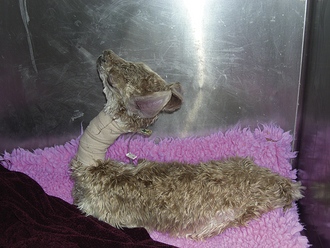
FIGURE 22-37 Premature crias often have nonerect or curled ears as a result of immature cartilage in the ears.
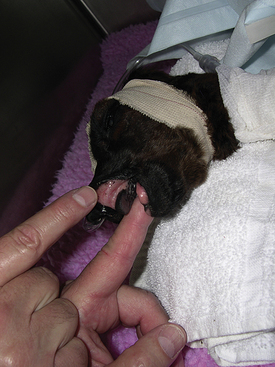
FIGURE 22-38 The incisors (bottom only) are not erupted in premature crias, and the mucous membranes appear dark red in color.
 TECHNICIAN NOTE
TECHNICIAN NOTE
There is considerable variation in gestation in alpacas and llamas (330 to 360 days), with some pregnancies lasting more than 1 year thus making it difficult to determine prematurity based on length of gestation.
Once the condition of prematurity has been established, the cria should be provided supplemental heat and oxygen. Most premature crias are incapable of nursing the dam, so it is suggested that a warm plasma transfusion using camelid plasma (Triple J Farms, Redmond, Wash.) be administered. Following plasma transfusion, fluids, such as Normasol with 5% to 10% dextrose, can be given to meet any existing fluid deficits and prevent hypoglycemia. Premature crias are susceptible to infection, so administration of broad-spectrum antibiotics is usually initiated. In addition, thiamine and cimetidine are often given to promote neurologic development and prevent C3 ulcers, respectively. If the cria is strong enough to nurse, it should be fed milk at the rate of 10% to 12% of its body weight divided into four feedings a day. If the cria is incapable of nursing, tube feeding may be necessary via an orogastric tube (a stallion urinary catheter works well). Intensive care is continued until the cria matures appropriately or is capable of survival without additional support. Daily weights are helpful in assessing the health of the neonate and ensuring adequate oral nutrient intake.
 TECHNICIAN NOTE
TECHNICIAN NOTE
Most premature crias are incapable of nursing the dam, so it is suggested that a warm plasma transfusion using camelid plasma be administered.
Monitoring mucous membrane color, respiratory rate, and blood gases helps determine the point at which the cria can be weaned from oxygen supplementation. Other signs of maturation, such as eruption of the incisors and straightening of the ears, are important to assess. Any angular limb deformities may be addressed by application of light support splints; often these deformities improve as the neonate matures. The cria should be housed in close association or contact with the dam.
To prevent FPT, the newborn should be observed closely for the first 3 to 4 hours to make sure it nurses. It is desirable to get colostrum from the dam into the cria; however, if that is not possible, cow, goat, or sheep colostrum can be substituted. The cria should receive 20% of its body weight in colostrum in four to six feedings during the first 24 hours after birth. The use of commercial colostrum supplements or replacers should be avoided. It may be beneficial to feed colostrum to premature or sick crias for 3 to 4 days after birth. In the event the cria will not nurse, orogastric intubation should be performed to make certain that the cria receives adequate colostral immunity. Radial immunodiffusion is one of the most accurate tests used to check for passive transfer, but it is only useful if the cria received llama or alpaca colostrum, not cow, goat, or sheep colostrum. Sodium sulfite turbidity and total serum protein can be useful to access passive transfer status. These tests are most beneficial in crias that are from 24 hours to 7 days of age. If colostrum is not available, an IV plasma transfusion is strongly recommended. “At-risk” crias should be watched closely for any signs of septicemia for the first several months of life.
Orphaned crias may be bottle fed goat’s milk, lamb milk replacer, kid milk replacer, or whole cow’s milk. The cria should receive milk at 10% to 12% of its body weight per day. Initially, this amount can be divided into four to six feedings per day, but feedings can gradually be reduced to two to four feedings per day. Weighing the bottle-fed cria to document adequate weight gain is important. It is extremely important that orphans receive minimal human contact and are left with the herd at times other than feeding to prevent the development of “bezerk llama syndrome.”
 TECHNICIAN NOTE
TECHNICIAN NOTE
It is extremely important that orphan crias receive minimal human contact and are left with the herd at times other than feeding to prevent the development of “bezerk llama syndrome.”
Crias that do not nurse may need total parenteral nutrition (TPN). The recipe described for use in the foal (Smith) seems to work well for crias, and the TPN can be reduced incrementally as the cria begins to nurse on its own.
Congenital abnormalities are relatively common among camelids. This high prevalence is blamed on the narrow genetic pool available to breeders before importation of native South American camelids during the 1980s. Even with greater genetic diversity, congenital defects continue to plague breeders. Some common congenital defects include choanal atresia, atresia ani, wry face (maxillofacial dysgenesis), patent urachus, and cleft palate. Choanal atresia is the presence of a membranous or osseous separation of the nasal and pharyngeal cavities. Since camelids are obligate nasal breathers, the primary clinical sign in affected newborns is open-mouth breathing. Since this condition is probably hereditary and the prognosis for life is poor, euthanasia is recommended.
Diarrhea is an important cause of morbidity in neonatal camelids. Many factors may be involved in the cause of neonatal diarrhea, including management and nutritional factors and a variety of pathogens. The most common pathogens causing diarrhea in neonates are coronavirus, E. coli, Cryptosporidium spp., Giardia spp., and coccidia. If diarrhea in the young is not treated effectively, it may lead to the development of chronic diarrhea, which may ultimately result in chronic renal failure.
 TECHNICIAN NOTE
TECHNICIAN NOTE
Diarrhea is an important cause of morbidity in neonatal camelids and if not treated effectively, it may lead to the development of chronic diarrhea, which may ultimately result in chronic renal failure.
Coccidiosis is most frequently diagnosed in neonates and juveniles since adults are more resistant to infection as a result of their mature immune systems and prior exposure. Coccidiosis is typically associated with conditions of overcrowding and poor hygiene. The oocysts cause direct damage to the small intestinal epithelium resulting in diarrhea, enteritis, and sometimes straining. Chronic coccidiosis may cause nutrient malabsorption and subsequent poor growth in affected individuals. Sulfadimethoxine (Albon, Pfizer) dosed at 15 mg/kg orally twice daily for 5 days is an effective treatment for coccidiosis. Amprolium may also be used at the rate of 10 mg/kg orally once daily for 5 days. The correct dosing of amprolium is crucial because overdosing may produce clinical signs of polioencephalomalacia caused by thiamine deficiency. Ionophore antibiotics, such as monensin and salinomycin, which are commonly used to treat coccidiosis in cattle, are toxic to camelids and should therefore not be used in these species.
Diarrhea caused by E. coli often occurs in combination with neonatal septicemia secondary to FPT. Neonates are affected between 3 to 7 days of age and often exhibit profuse, watery diarrhea, lethargy, dehydration, and abdominal distention. Leukopenia with a degenerative left shift neutrophilia is often present in these crias. The treatment should include a good broad-spectrum antibiotic with gram-negative coverage along with fluid therapy. Sick camelids are often hypernatremic, so low-sodium IV fluids, such as 0.45% sodium chloride with 2.5% dextrose, are indicated.
Cryptosporidiosis (Cryptosporidium parvum) is a zoonotic disease that can cause severe and sometimes fatal diarrhea in neonates and immunocompromised individuals. The infection occurs by the fecal-oral route and may occur when contaminated feed or water are ingested. The diagnosis of cryptosporidiosis is accomplished by the examination of fecal smears using modified acid-fast stains. There is no specific treatment for cryptosporidiosis, so supportive therapy using IV fluids and/or TPN are important, especially since the disease results in malabsorption and maldigestion.
Giardiasis, also a zoonotic disease, primarily stems from contaminated water sources. The organism, which affects the small intestine causing villous atrophy, results in a malabsorptive diarrhea with dehydration and weight loss. Oral fenbendazole dosed at 50 mg/kg once daily for 5 days is an effective treatment for Giardia.
Salmonella spp. do not appear to be common causes of diarrhea in camelids.
Both rotavirus and coronavirus have been identified as causing diarrhea in neonatal camelids; however, of the two viruses, coronavirus appears to occur more commonly. Electron microscopy and fecal ELISA tests are the most useful for diagnosis of these viruses. There is no specific treatment for viral diarrhea, so supportive therapy, such as IV fluids, is useful. Monoclonal antibody vaccines, such as those available for oral use in calves and lambs, can be safely given to camelids on farms experiencing outbreaks of viral diarrhea, although the efficacy in these species is unknown.
Nematodes are capable of causing diarrhea in crias as young as 2 months of age because of the inherent lack of acquired resistance. Clinical signs include ill thrift, inappetence, anorexia, emaciation, and diarrhea. Fecal parasitology is useful in the diagnosis, and a 5-day course of oral fenbendazole at 20 mg/kg is usually effective.
Diarrhea in crias less than 7 days of age is likely due to nutritional factors in bottle-fed babies or gram-negative infections, especially in cases of inadequate colostrum ingestion. Viral diarrhea usually affects crias older than 7 days and is most often due to a coronavirus infection in the United States. Cryptosporidium and Giardia also tend to affect crias older than 7 days of age, and these infections are often due to overcrowding or sanitation problems on larger farms. Coccidiosis is unlikely to occur in crias less than 3 weeks of age, and it may be indicative of a herd problem. Diarrhea caused by GI parasites in crias less than 2 months of age is rare. It should be kept in mind that diarrhea may be multifactorial and can involve more than one pathogen. There are numerous diagnostic tests available to help determine the cause of neonatal diarrhea so that the clinician can initiate an appropriate treatment for affected individuals and control the spread of disease through the rest of the group.
 TECHNICIAN NOTE
TECHNICIAN NOTE
Diarrhea may be multifactorial and can involve more than one pathogen. Numerous diagnostic tests are available to help determine the cause of neonatal diarrhea so that the clinician can initiate an appropriate treatment for affected individuals and control the spread of disease through the rest of the herd.
RESTRAINT AND HANDLING OF CAMELIDS
Effective restraint requires knowledge of camelid behavior. Llamas and alpacas have been domesticated for thousands of years, and if they are accustomed to handling, they are docile and pleasant. Only rarely is an individual aggressive or a “spitter.” Camelid ear and tail position expresses important information. The ears of a content, unaroused animal are in a vertical position and turned slightly forward. In an alarmed animal, the ears are pointed forward. Varying degrees of aggressiveness are manifest by ears that are positioned from barely behind the vertical to flattened on the neck. The tail position also reflects the emotional state of the animal. In an unaroused camelid, the tail lies flat against the perineum. With alarm, the tail position rises to horizontal or as much as 45 degrees above horizontal. Aggressive behavior is displayed by the tail in a completely vertical position. An accurate reading of body language is important to prevent injury to the handler and the animal.
Llamas and alpacas are usually calm, but the most common behavioral response to annoyance is spitting of regurgitated stomach contents. Once a spitter is restrained, the head can be turned away from handlers to redirect ingesta, or a towel or rag that covers the mouth can be tucked into the nosepiece of a halter to discourage continued spitting. A muzzle that hooks onto the halter can also be used to prevent spitting. Camelids may kick and generally “cowkick,” although they can also kick directly backward. Biting is usually restricted to fighting between intact males, although llamas have been known to occasionally bite humans. Mature males have two upper and one lower canine teeth (“fighting teeth”) on each side of the mouth that are sharp. These teeth occasionally need to be blunted or cut short for the safety of other animals and human handlers.
Dam-raised male camelids are usually no more difficult to handle than females (in contrast with bulls, stallions, bucks, and rams); however, bottle-fed orphan males or neonates that receive too much human contact may imprint on humans creating a dangerous behavioral problem. An imprinted male treats a human as if it is another male and can therefore become quite aggressive, especially when the male reaches puberty. A number of persons have been seriously injured as a result of this behavioral problem.
 TECHNICIAN NOTE
TECHNICIAN NOTE
Llamas and alpacas are usually calm, but the most common behavioral response to annoyance is spitting of regurgitated stomach contents.
It is always desirable to use the least amount of restraint necessary to perform a procedure. Many alpacas have been halter broken and can be restrained with the use of the halter. Untrained individuals are best controlled by pulling the head and neck close to the handler’s chest with one hand while the other hand rests on the top of the shoulders with slight pressure (Figure 22-39). As an alternative, the tail may be grasped and held upright by the second hand. Crias less than 20 kg (45 lb) may be lifted with one arm around the chest and the other arm supporting the abdomen in front of the rear legs, which has a calming influence on the cria and reduces struggling (Figure 22-40). Camelids can also be restrained in the kushed (sternal recumbency) position (Figure 22-41).

FIGURE 22-39 Restraint of the adult camelid is best accomplished by holding the head and neck close to the handler’s chest while the other hand rests on the animal’s shoulders with slight pressure.

FIGURE 22-40 Crias may be held with one arm around the chest and the other arm supporting the abdomen in front of the rear legs.

FIGURE 22-41 Adult camelids can also be safely restrained in sternal recumbency or the kushed position.
NERVOUS SYSTEM
Meningeal Worm (Parelaphostrongylus tenuis)
One parasite that is of great importance to llama and alpaca producers is the meningeal worm, Parelaphostrongylus tenuis. The llama and alpaca and other animals, such as wild cervids and domestic small ruminants, are aberrant hosts of this parasite, whereas the white-tailed deer is the normal host. This parasite does not cause clinical disease in white-tailed deer, but in camelids, it causes high morbidity and mortality. The P. tenuis larvae migrate through the spinal cord of aberrant hosts causing neurologic deficits. Clinical signs appear around 45 to 53 days after infection. Most commonly, clinical signs reflect asymmetrical, focal spinal cord lesions, including hypermetria, ataxia, stiffness, muscular weakness, posterior paresis, paralysis, head tilt, arching neck, circling, blindness, gradual weight loss, apparent depression, seizures, and death. Clinical signs generally begin in the hind limbs and progress to the front limbs. The course of disease may be acute to chronic, ranging from death within days to ataxia that lasts months to years.
 TECHNICIAN NOTE
TECHNICIAN NOTE
P. tenuis larvae migrate through the spinal cord of aberrant hosts, such as the llama and alpaca, causing neurologic deficits that may be acute or chronic in nature.
Although consistent clinical signs and CSF eosinophilia are highly suggestive of a meningeal worm infection, the antemortem diagnosis of aberrant P. tenuis migration is often a diagnosis based on exclusion and response to therapy. The definitive diagnosis of a meningeal worm infection is made at necropsy. A confirmed diagnosis requires microscopic demonstration of the larvae within the brain or spinal cord.
A treatment regimen that has proven successful at Ohio State University involves fenbendazole (20 to 50 mg/kg body weight, PO, q 24 hours for 5 days) and flunixin meglumine (1 mg/kg, IV, IM, or SC, q 12 hours for 5 days) or dexamethasone in nonpregnant females and males (0.1 mg/kg, IV, IM, or SC, q 24 hours for 3 days). DMSO (1g/kg given in 500 ml of 5% dextrose solution, IV, q 24 hours) given to effect is useful in some cases, but may cause severe appetite suppression. DMSO should be discontinued if inappetence or anorexia occurs. Vitamin E, selenium, Vitamin B-complex, and Vitamin A are useful to assist healing of neural tissues.
Dexamethasone should not be administered to pregnant females because this drug may induce abortion. Alternatively, prednisolone sodium succinate (0.5 to 1.0 mg/kg, IV, IM, or SC, q 12 hours) has been used, but for no more than 3 days in pregnant females without subsequent abortion. Ivermectin is most effective against larval stages before the entrance into the spinal cord since it does not readily cross the blood-brain barrier; however, damage to nervous system tissues during larval migration may alter the permeability of the blood-brain barrier. The antiinflammatory drugs are critical to reduce the inflammation associated with the presence of the migrating larvae and the subsequent inflammatory response to the killed larvae. Use of antiinflammatory drugs is important to prevent the clinical signs from becoming more severe after instituting treatment.
In addition to drug therapy, supportive care and physical therapy are essential to aiding recovery. Using slings to support llamas that are unable to stand and performing physical therapy for muscles are beneficial (Figure 22-43). Hydroflotation therapy to facilitate recovery after prolonged recumbency may also help. A great deal of perseverance is required to care for severely affected camelids because recovery may take several weeks to months to years.

FIGURE 22-43 Camelids that are unable to stand may benefit from the use of slings and physical therapy. (Courtesy of Dr. Christine Navarre.)
The prognosis for survival depends upon how severe the clinical signs become. Clinical experience suggests that camelids that are unable to stand have a poor prognosis (10% to 20% recovery); those that are able to stand unaided have a fair to good prognosis (75% to 85% recovery). Animals that survive clinical disease do not seem to develop patent infections and are unlikely to pose a health risk to other animals. Many animals suffer permanent neurologic deficits. but may remain productive members of the herd for breeding and pets.
The prevention of a meningeal worm infection may be difficult. Ideally, llamas and alpacas should not graze the same pasture as white-tailed deer; however, in many areas of the United States, it may not be feasible to separate the two species. Placing a deerproof fence may offer some protection to prevent movement of deer. Additionally, thick ground cover can be removed to expose the environment to fluctuations in temperature, and vegetation-free buffer zones (i.e., gravel, limestone) can be placed around fence lines to reduce migration of snails and slugs into the pasture. Molluscicides may be considered to destroy snails and slugs that serve as intermediate hosts, thereby interrupting the life cycle of the meningeal worm and preventing infection in aberrant hosts. Drainage should be established in low-lying areas, and access to swampy areas may be restricted by fencing. These compounds present a potential environmental risk from contamination of ground water and may be toxic if consumed by camelids or other animals. The prophylactic treatment against migrating larvae may be achieved by administration of ivermectin (0.2 mg/kg) every 30 to 45 days during the high-risk periods or throughout the year in regions that have mild summers and winters. Anthelmintic resistance is unlikely to become a problem in the meningeal worm because these infections do not become patent. However, meningeal worm infection has occurred in some herds that maintain vigilant prophylaxis. These “breaks” in the prevention of larval migration may have been caused by insufficient dosing of anthelmintic, accidental failure to administer the anthelmintic, or some unknown mechanism.
 TECHNICIAN NOTE
TECHNICIAN NOTE
The prognosis for survival of the meningeal worm depends upon how severe the clinical signs become; camelids that are unable to stand have a poor prognosis, whereas those that are able to stand unaided have a fair to good prognosis for recovery.
METABOLIC CONDITIONS
Hepatic Lipidosis
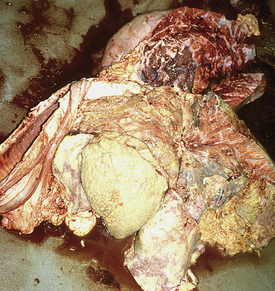
When excessive fat accumulates in liver cells, the disease process is termed hepatic lipidosis, fatty infiltration, or fatty liver disease. This syndrome has been well defined in cats, cows, sheep, goats, ponies, and humans. Although there are differences in conditions that initiate hepatic lipidosis between these species, usually a period of inadequate energy intake (i.e., negative energy balance [NEB]) initiates body fat mobilization. Unfortunately, in camelids, the disease outcome is nearly always fatal if not recognized early and treated aggressively.
Although hepatic lipidosis in llamas and alpacas has not been frequently reported in the veterinary literature, it has commonly been recognized in cases of camelid illness and death. Veterinary diagnostic laboratories report some degree of fatty liver infiltration in the majority of llamas and alpacas submitted for necropsy; however, it may not always be clear whether hepatic lipidosis was the primary lesion causing the death of the animal or secondary to some other disease process.
One study at Oregon State University revealed a mostly middle-aged, pregnant, or lactating female population to be affected. In contrast to hepatic lipidosis in other species, males ranging from 5 months to 18 years accounted for 22.6% of the cases. The most common factor documented in histories from these affected camelids was a recent significant loss of appetite or severe weight loss varying from a couple of days to several weeks. Affected animals had a variety of BCSs (thin to obese). In some cases, there were other medical problems or changes in social or environmental conditions, such as uncharacteristically hot weather or movement of animals in or out of certain pastures or pens, evident around the time the condition developed. Some llamas were reported to be clinically normal less than 24 hours before they were found ill or dead. Most affected animals had elevations in enzymes that indicate liver disease. These are not, however, specific for hepatic lipidosis and may be increased with any cause of liver disease. A definitive diagnosis of hepatic lipidosis is only accomplished by microscopic or analytic measurement of fat content of liver biopsy specimens.
Since deficient energy intake is a hallmark factor in initiating hepatic lipidosis, therapy must be focused on increasing energy intake immediately. Offering a variety of browse and fresh grass clippings has been beneficial to stimulating feed intake. Blackberry leaves are particularly appealing to camelids. Injections of B-vitamins can be beneficial for appetite stimulation. If more aggressive oral supplementation is required, a liquid gruel can be administered via tube, if feasible. Soaking alfalfa pellets in hot water and mixing in calf electrolytes, calcium propionate, propylene glycol, and other ingredients can provide energy sources and fermentable material. Camelids are obligate nasal breathers, so indwelling nasogastric tubes are not practical. Rumen transfaunation can be used to repopulate the microbial fauna and restimulate fermentation. Collected rumen fluid from cattle, sheep, or goats can be used in llamas or alpacas.
In more severe cases, intensive supportive care and dietary management, including TPN, may be used. Since camelids may have insulin resistance, administration of an appropriate dose of insulin in conjunction with glucose therapy is warranted; never administer insulin without concurrent glucose therapy because this may result in hypoglycemia. The prognosis is always guarded in the more severe cases of hepatic lipidosis, even with aggressive nutritional support. All sick camelids should be considered at risk for developing hepatic lipidosis, especially those with anorexia or metabolic demands of pregnancy and lactation. Close monitoring of feed intake in sick animals is absolutely essential to prevent deaths.
 TECHNICIAN NOTE
TECHNICIAN NOTE
Deficient energy intake is a hallmark factor in initiating hepatic lipidosis, therefore therapy must be focused on increasing energy intake immediately.
The prevention of hepatic lipidosis is based on ensuring adequate energy and protein intake, especially in pregnant and lactating females, by feeding good-quality forage and appropriate supplementation. Forage testing is the only true way to know the quality of forage that is fed. Most cases of hepatic lipidosis are associated with the feeding of mature grass forage (less than 9% crude protein). The addition of some alfalfa or clover forage to grass forage will improve the quality of the diet. Grain supplements with some protein will be required to support lactation, though the amount and composition required will vary by production level and forage quality. Lactating dams have the highest nutrient requirements and should be fed the best-quality forage and potentially supplemented with a grain product containing energy and protein sources.
Given the strong association between significant weight loss and hepatic lipidosis, one can use routine (monthly or bimonthly) body weight determinations to assess potential risk. Body weight loss exceeding 15% over a short (1 to 2 weeks) period of time is a high-risk factor for this disease. Pregnant animals should gain approximately 10% to 15% of their body weight over the last 3 months of pregnancy to account for fetal growth. Lactating animals will be expected to lose body weight in support of lactation. This weight loss will vary by individuals and the amount of milk produced. A typical weight loss should be less than 10% of body weight following birthing. Excessive weight loss in early lactation is an indicator of inadequate dietary amounts or quality and can predispose to hepatic lipidosis problems. By far, the single best and simplest method of evaluating your nutritional program is body condition scoring. Body condition scoring is a method that subjectively grades animals by the amount of subcutaneous fat stores into defined “fatness” categories. A five-point system covering physical states of emaciated (1), thin (2), average (3), fat (4), and obese (5) has been developed. The ideal body condition is 3.0, having a moderate amount of body fat. Although some individuals will maintain a lower or higher BCS and remain healthy, this is just inherent individual differences in metabolism. BCSs 2.0 and below or 4.0 and above are considered abnormal and represent extremely thin or fat animals, respectively. Most animals other than those in late pregnancy or lactation should maintain a BCS between 2.5 and 3.25. Late-pregnant animals should have a slightly higher body condition (3.25 to 3.5) to have reserves to support impending lactation. Lactating animals will lose body condition rapidly as they produce milk. Lactating animals should not lose more than 0.5 to 0.75 condition scores. Important times to assess a BCS would be during early to mid pregnancy, early to mid lactation, and periodically (four to six times per year) for other animals of the herd to assess energy status.
 TECHNICIAN NOTE
TECHNICIAN NOTE
The best and easiest method of evaluating a camelid nutritional program is by body condition scoring using a five-point system defining the physical states of emaciated (1), thin (2), average (3), fat (4), and obese (5).
Heat Stress
Heat stress is a common occurrence for llamas and alpacas during the summer season. Since these animals originate from the Andes Mountains of South America, where high heat and humidity are not as common as in many areas of the United States, llamas and alpacas are not adapted to handle these conditions. It is critical to manage them in a way to protect them from heat stress because it can lead to illness and even death of the animal.
It is important to know when llamas and alpacas are most in danger for heat stress. Commonly used is the heat index, which is simply a formula to estimate the risk of heat stress. The heat index can be estimated by adding the temperature (F) and percent humidity (%). Typically a heat index of less than 120 is safe, 120 to 180 creates possible problems, and greater than 180 is the range where animals are in the most danger. During the warmer months of the year, there are many ways to keep animals cool. Shade is an easy way to keep them from getting too hot. The shade provided by trees is a great place for camelids to relax and stay cool during the heat of the day. If there are no trees available, artificial shade, such as tents, barns, and shelters, can be provided, keeping in mind that ventilation in these structures is important. Fans are an excellent way to keep the air moving and keep the animals cool. Tunnel ventilation barns are the most desirable because the “tunnel effect” maximizes cooling of the air. Fans placed in series (e.g., all facing the same direction) can create this effect and cool the barn. If available, having an air-conditioned room or area of the barn can help keep animals cool or be used as a place to move animals that begin to show signs of heat stress. Giving llamas and alpacas plenty of fresh water also helps prevent heat stress. There should be multiple sources of cool, clean water so that all the animals have a place to drink.
 TECHNICIAN NOTE
TECHNICIAN NOTE
The heat index can be used to determine when llamas and alpacas are at the most risk for developing heat stress, and the heat index can be estimated by adding the temperature (F) and percent humidity (%).
Shearing is one of the most important ways to help llamas and alpacas keep cool. Since the fibers work to trap the heat close to the animal’s body, shearing helps the animal to lose heat through evaporation more effectively. If possible, shearing from head to toe (leaving about 1 to 3 inches of fiber on the body) is most effective, but barrel cuts (e.g., abdomen and thorax only) will also help. Differences are observed among the various camelids (e.g., llama, suri alpaca, huacuya alpaca, guanaco, vicuña) with respect to tolerance of hot and cold.
Proper management and husbandry can help prevent heat stress. For example, if the animals need to be worked or handled for any reason, it should be done early in the morning in the coolest part of the day. Breeding to have crias born in the spring is important since gestation and parturition can cause stress for the female and during the warmer months can cause considerable heat stress. Crias born in the warmer months are often born weak and can become dehydrated soon after birth. Weaning should also take place during the cooler months because it is a stressful time for both the cria and its mother.
The body condition of the animal also plays an important role in heat stress. Obese animals are more prone to the effects of the heat, so proper management of weight is a good way to help these animals cool themselves. On the other hand, emaciated animals also have increased susceptibility to extremes of environment. Proper nutrition of the animals is also important. In particular, providing adequate selenium, vitamin E, copper, zinc, and B vitamins, such as thiamine, can increase tolerance of environmental extremes. Monitoring the animals is important during the summer months so that any signs of heat stress can be caught early. Signs to watch for are nasal flaring, open-mouth breathing (Figure 22-44), tachypnea, dyspnea, drooling, depression or dullness, not eating feed, scrotal swelling in intact males (Figure 22-45), weakness, trembling, a rectal temperature greater than 104° F, a heart rate more than 90 bpm, or a respiratory rate more than 40 breaths/min. Taking temperatures often is a good way to learn what the normal temperatures of the animals are in the morning and afternoon so that abnormal temperatures are more easily recognized. Treatment of llamas and alpacas with heat stress should first be to cool the animal down using water or alcohol. Additional cooling with the use of a fan or air conditioner may be useful. If the animal has not been shorn, this may considered in the treatment regimen, but only if it does not cause further stress. Other therapies include cool IV fluids with electrolytes, steroids or NSAIDs, and good nursing care including lifting the animal periodically if it is unable to stand. Water flotation tanks are especially useful for this purpose (Figure 22-46). The most important aspect of heat stress is prevention.

FIGURE 22-44 Monitoring of camelids during the hot summer months for signs of heat stress, such as open-mouth breathing is important. (Courtesy of Dr. David Pugh.)

FIGURE 22-45 Another sign of heat stress in camelids is scrotal swelling in intact males. (Courtesy of Dr. David Pugh.)

FIGURE 22-46 Animals that are recumbent as a result of heat stress may benefit from the use of water flotation tanks. (Courtesy of Dr. Christine Navarre.)
 TECHNICIAN NOTE
TECHNICIAN NOTE
Shearing camelids before the onset of hot weather and careful monitoring of the animals during the summer months are important for the prevention of heat stress.
 TECHNICIAN NOTE
TECHNICIAN NOTE