Large Animal Surgical Nursing
Emergency Situations and Procedures
Anesthesia for the Equine Patient
SURGICAL NURSING OF FOOD ANIMALS
Conditions of the Gastrointestinal Tract
Conditions of the Musculoskeletal System
Conditions of the Respiratory System
Conditions of the Urogenital System
Conditions of the Reproductive System
Conditions of the Ophthalmic System
Surgical Procedures of Young Stock
When you have completed this chapter, you will be able to:
1 Describe the preoperative procedures needed for equine patients.
2 Discuss the responsibilities of the veterinary technician during equine surgery.
3 Describe postoperative monitoring, medication administration, bandage care, and grooming for equine patients.
4 List commonly performed surgical procedures in equine patients.
5 Describe indications and preoperative, intraoperative, and postoperative considerations for common surgical procedures in equine patients.
6 List and describe common emergency situations and procedures in equine patients.
7 List commonly performed surgical procedures in bovine patients.
8 Describe indications and preoperative, intraoperative, and postoperative considerations for common surgical procedures in bovine patients.
9 List commonly performed surgical procedures in small ruminants.
10 Describe indications and preoperative, intraoperative, and postoperative considerations for common surgical procedures in small ruminants.
SURGICAL NURSING OF HORSES
PREOPERATIVE PREPARATION
Numerous procedures are required in preparation of the equine patient for anesthesia and surgery. Many if not all of these procedures involve the veterinary technician. It is probably wise that a checklist be developed that the veterinary technician can use to make sure that all procedures are performed. This is particularly helpful in a hospital where more than one technician is working on the same case. Because of the dense hair coat of horses, thorough grooming is necessary. This may include simply brushing or currying the horse’s coat, or it may require that the horse be bathed. The aim of grooming is to remove as much loose hair, dander, and dirt from the horse’s body as possible, thereby keeping such material out of the operating room (OR). If the horse is shod, the shoes are generally removed before surgery to prevent injury to the horse during recovery from anesthesia or damaging the recovery stall flooring. Some therapeutic shoes may not be removed to prevent damage to the hooves. If the shoes must be left on, wrapping them with gauze and elastic tape will provide some protection from injury from the shoes during recovery. The horse’s feet need to be picked out and cleaned. One of the main responsibilities of the technician will be to clip a wide area of hair in the vicinity of the surgery site before anesthetic induction. If the surgery will be performed on a limb, the hair can be clipped the day before surgery and the limb can be cleaned and a bandage placed to keep the site clean. The final aseptic preparation is performed once the horse is under anesthesia. Clipping the hair and cleaning the surgery site before anesthetic induction will reduce anesthesia time.
It is important that the technician consult the clinician regarding the specific site that should be clipped. Areas of the mane and tail should be clipped only under special circumstances. Most owners are adamant that these areas should not be clipped for cosmetic purposes. The hair of the mane and tail takes months to years to grow out, and unnecessarily clipping these areas may cause needless delay in a show horse’s convalescence. The location of the skin incision and the appropriate part of the horse to clip before surgery can usually be found in equine surgical textbooks. However, because of variation among surgeons, the technician should always consult the surgeon before clipping the patient.
Unlike ruminants and small animals, horses do not regurgitate or vomit. Adult horses are generally held off feed for approximately 12 hours to allow time for emptying of the stomach, which may allow the horse to ventilate more easily. Horses are generally provided water during this time. Young foals that are still nursing are generally not held off feed before anesthesia, but, if they are, it is usually only for 1 to 2 hours. A complete physical examination should be performed, including auscultation of the heart and lungs. In adult horses, a rebreathing bag may need to be used to increase the respiratory effort sufficiently to hear air moving through the lung fields. An electrocardiogram should be performed if there is any evidence of an abnormal heart rhythm detected during auscultation. Preoperative blood work usually includes a complete blood count (CBC) and fibrinogen determination. Some clinicians also perform a chemistry profile depending on the age and health of the horse. A tetanus vaccination should have been given within the last 3 months. If the date is not known, a booster should be given intramuscularly.
Before general anesthesia, an intravenous (IV) catheter is placed in one of the jugular veins. The location of the catheter is important to provide access without compromise to the surgical site. The anesthetic agents for induction are administered through the catheter. Some anesthetic agents (thiobarbiturates) and perioperative medications (phenylbutazone) are irritating if injected perivascularly. Thereforeit is imperative that the veterinary technician place the catheter into the vein and secure it appropriately. Perioperative medications, such as antibiotics and nonsteroidal antiinflammatory drugs (NSAIDs) are usually administered before anesthetic induction. However, if an infectious process is suspected, the surgeon may opt to start antibiotics after a sample has been obtained at surgery for culture and susceptibility testing. In this case the medication can be administered during anesthesia or after recovery; this will depend on the medication and the condition of the patient while under anesthesia. Because horses are generally intubated with an endotracheal tube through the oral cavity, it is important that the mouth be thoroughly washed out before anesthetic induction; this will reduce the chance that feed material will be carried into the airway during intubation. Once the horse is intubated, the cuff should be inflated to prevent saliva and other materials from draining into the lower airway and leading to aspiration pneumonia.
INTRAOPERATIVE NURSING
The OR technician should consult the surgeon regarding which instruments will be required. In a hospital where there are many OR technicians and surgeons, an organized system to delineate the various surgeons’ instrument preferences and glove size should be used. This will allow the technician to know the different requirements of individual surgeons. One common difference among surgeons is the type of suture material chosen to close wounds. The technician must learn to adapt to these individual preferences. It is recommended that the technician have all the available instruments close to the surgery. Even if the instrument is used infrequently, it is better to have it nearby rather than waste time looking for it once it is needed. Time-wasting activities lead to prolonged anesthetic time, which could lead to increased morbidity or mortality. Correctly labeled radiographs are essential for most limb surgery. The radiographs should be placed on a radiographic view box in the OR. The technician should have available gloves, gowns, and drapes and all other supplies that are anticipated to be used. In some lower limb surgeries, an Esmarch bandage (Latex Rubber Bandage/Tourner Wrap, Smiths & Nephew Richards) and tourniquet are used to assist with hemostasis during surgery. An Esmarch bandage is a flat, gum-rubber elastic bandage that is wrapped around the limb in a spiral fashion from distal to proximal to a point above the surgical site. At this point, an inflatable tourniquet is applied and secured. The aim of the Esmarch bandage is to force blood out of the limb, and the tourniquet prevents blood from entering into the site. The Esmarch bandage is removed after the tourniquet is fully inflated. The use of an Esmarch bandage and tourniquet enables the surgeon to operate in a bloodless field and results in a shorter surgery time. Following surgery, a pressure bandage is applied, and the tourniquet is released. It is strongly recommended that the tourniquet only be used for a maximum period of 2 hours to prevent any potentially serious side effects.
Because of their immense body weight, horses are prone to myositis (muscle damage) during recumbency, and this can be life threatening. Therefore the OR technician must ensure that the patient is well padded on the surgery table. The pressure of the horse’s body and the hypotension that can occur during anesthesia can result in hypoperfusion of the muscles. If this condition is prolonged, the muscles can undergo metabolic change, resulting in extreme soreness and pain. In severe cases, muscle pigment (myoglobin) is released into the bloodstream and excreted in the urine (coffee-colored urine); the pigment can lead to kidney damage. The first sign that muscle damage has occurred during anesthesia is manifested during recovery. Usually the front or rear limb, or both, on the side that the horse is lying on will be affected. However, the uppermost limb or any limb in a horse in dorsal recumbency can be involved. The horse may be unable to bear weight on the limb. If a forelimb is involved, the horse will drag the limb in a flexed position and will be unable to bear weight; this is associated with triceps damage. If the hind limb is involved, the horse may knuckle in the lower joints and walk on the dorsal aspect of the fetlock, and the limb will collapse as the horse tries to bear weight. Most horses show some improvement over the first few days, but some horses are unable to rise. Management of a postoperative recumbent patient pre-sents a number of problems to clinicians and technicians. Appropriate padding materials include: an inflatable water bed, semiinflated inner tubes under the shoulder and hip, dunnage bags, and foam rubber pads.
The patient and the surgery site must be positioned so that it is comfortable to the surgeon and safe for the patient. This will help ensure that the surgeon does not become fatigued or frustrated and a subsequent compromise in technique does not occur. It is not wise to overextend, overflex, abduct, or adduct the limbs because of potential complications of myopathy and neuropathy.
Aseptic preparation of the surgery site, surgical instruments, and the surgeon is imperative to a successful and uncomplicated surgery. It is the responsibility of all personnel involved to maintain asepsis, but the OR technicians should assume primary responsibility for ensuring that the surgical site is properly prepared and the instruments are properly sterilized and packaged. The OR technician must be cognizant of all activities in preparation for surgery and during the surgical procedure. If a technician observes a break in aseptic technique, it should be brought to the attention of the surgeon so that the problem can be remedied. The techniques involved in sterilization of surgical instruments and supplies and aseptic preparation of the surgery site are covered in Chapter 28.
POSTOPERATIVE NURSING
Technicians play a vital role in the postoperative care of the equine patient. Although veterinarians are responsible for the patients’ care, technicians are often primarily involved with postoperative monitoring, administering medications, changing bandages, grooming, and other tasks required on postoperative patients. Monitoring the postoperative patient is similar to previously discussed patient monitoring. Although all body systems should be evaluated, the important things to consider in the postoperative patient are the presence and magnitude of postoperative pain, whether the patient is febrile, and whether there are any signs of infection (swelling, erythema, heat, pain) at the incision site. The postoperative patient should be examined for any complications, such as pneumonia, diarrhea, jugular vein thrombophlebitis, or laminitis.
Technicians are generally responsible for administering medications postoperatively. This may involve giving antibiotics or NSAIDs orally, IV, or intramuscularly. Many horses that undergo surgery have an IV catheter that is used in the postoperative period to administer perioperative antibiotics. The duration of antibiotic therapy depends on clinician preference and the type and severity of the underlying disease process. Many horses are administered NSAIDs in the postoperative period for their antiinflammatory and analgesic properties.
Horses undergoing limb surgery generally have a bandage placed on the limb at the conclusion of surgery before recovery from anesthesia. The limbs are often kept bandaged until the skin sutures are removed 10 to 14 days postoperatively. The bandages should probably be changed every 2 to 3 days initially or more frequently if they become wet or soiled from the outside or if wound drainage soaks through from the inside. There are several types of materials used for limb bandages in horses and several methods of application (see Chapter 34). In general, a sterile nonadherent material is usually placed directly against the incision and held in place with sterile, soft roll gauze (Kling, Johnson & Johnson). The next layer of the bandage is usually a sterile, soft combine that covers the circumference of the limb for the entire distance of the bandage, which is also held in place with soft roll gauze. This layer can be skipped if the outer bandage that is placed is a thick, sterile combine material. Next a thick layer of rolled cotton, sheet cottons, or combine material is placed on the limb and secured with soft roll gauze. An Ace bandage, Elasticon (Johnson & Johnson), or Vetwrap (Animal Care Products/3M) can be used as the final layer of the bandage. Elasticon is useful for securing the top of the bandage to the skin above it and the bottom of the bandage to the foot below. This helps seal the bandage and prevents debris from getting between the skin and the bandage. All layers of the bandage should be applied in the same direction (dorsal to palmar or plantar) and with even tension; this should help prevent constriction of the tendons in the metacarpal or metatarsal area and subsequent tendinitis (bandage bow). When the bandages are changed postoperatively, the incision should be examined for swelling, heat, exudate, and pain on palpation. The limb should be monitored for excessive swelling above and below the bandage. The exudate should be removed, the wound gently cleaned, and the bandage reapplied. If there has been an appreciable change in the horse’s gait or in the incision from the last bandage change, this should be brought to the immediate attention of the veterinarian.
SURGICAL CONSIDERATIONS
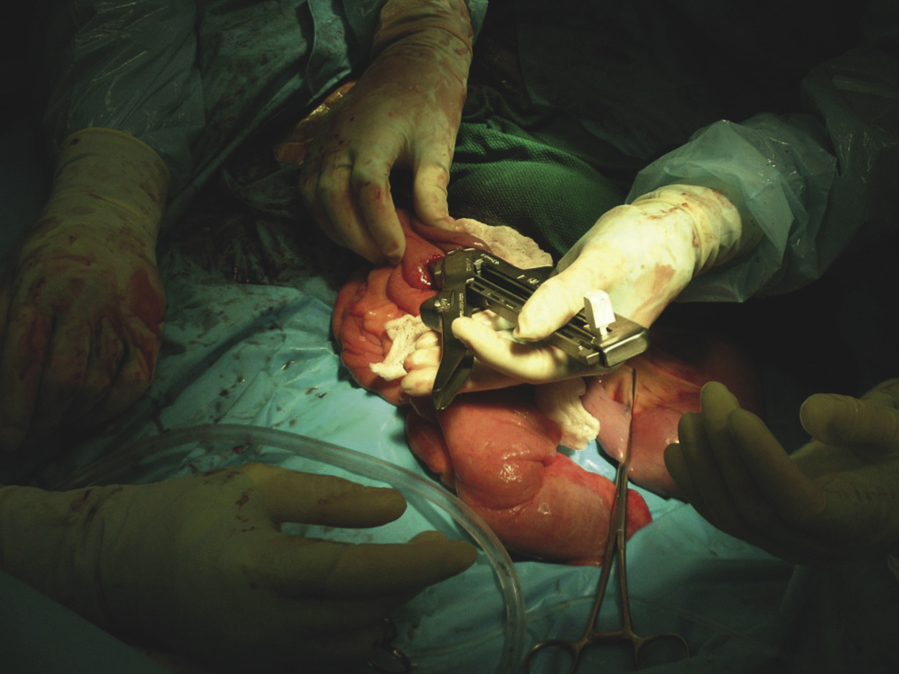
Abdominal surgery is a major undertaking and requires a full team to perform it in an effective and efficient manner. Adult horses and foals frequently undergo abdominal surgery for gastrointestinal and urogenital tract disease. Although a flank incision in a standing, sedated horse is sometimes used for horses with colic or other abdominal disease, the most common approach to the abdominal cavity is through a ventral midline incision with the horse under general anesthesia and positioned in dorsal recumbency (Figure 31-1). Because most horses with colic requiring surgery will be operated on with the patient under general anesthesia, the veterinary technician will be involved in the preparation of the horse for surgery. This will include placing a catheter, administering perioperative medications, passing a nasogastric tube, washing out the mouth, clipping the hair, preparing the anesthetics, aseptically preparing the incision site, and opening surgical packs at the time of surgery. Most colic patients can be clipped before anesthesia, but if the horse is in severe pain, it may be done after anesthetic induction for the safety of the horse and personnel. The hair should be clipped from rostral to the xiphoid area to the udder or preputial area and to the flank folds on either side; clipped hair and other debris can be removed with a vacuum before aseptic preparation. The incision is draped with four small drapes or towels, and a large, water-impermeable drape is placed that covers the entire horse. The incision is usually made from the umbilicus rostrally toward the xiphoid until the necessary exposure is achieved, but the incision can be extended caudal to the umbilicus. This is particularly necessary for urogenital tract surgery, such as a cystotomy for removal of cystic calculi. Once the incision is made, a thorough exploration is usually performed depending on the reason for surgery. Once the abnormality is identified, it is corrected. Suction is often necessary to decompress gas from the gastrointestinal tract or aspirate fluid, such as urine from the bladder during a cystotomy.
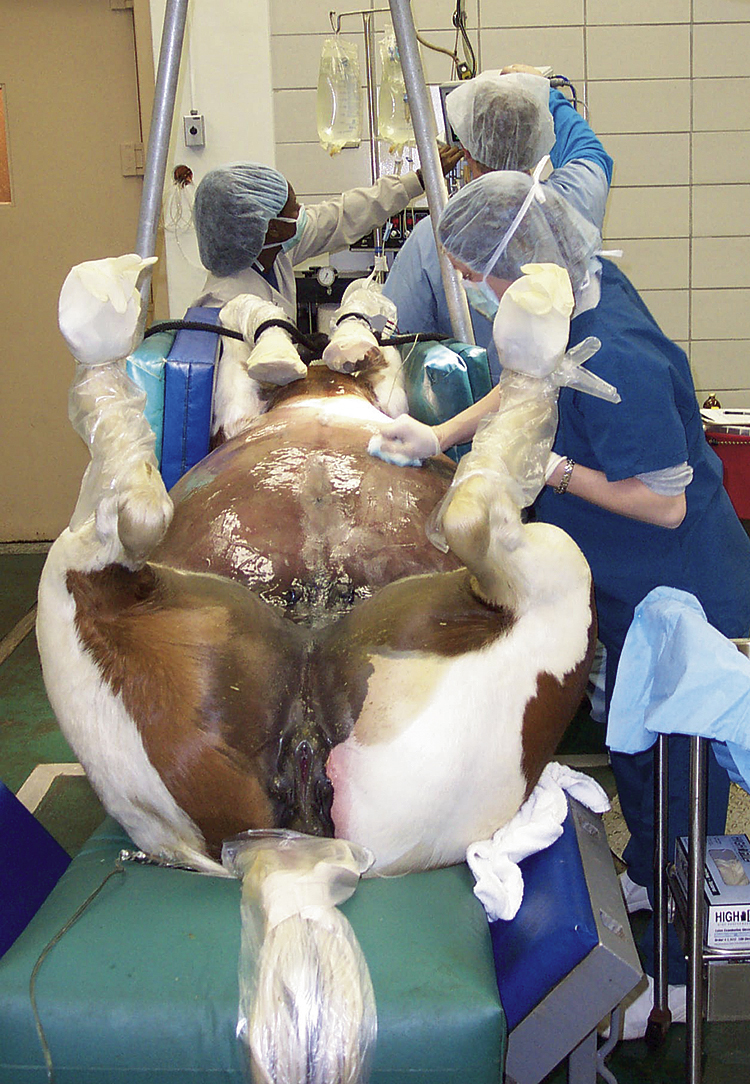
FIGURE 31-1 Preparation of the ventral abdominal area for abdominal surgery in a horse with colic that is under general anesthesia and positioned in dorsal recumbency.
There are numerous surgical techniques and manipulations that the surgeon may perform with which the OR veterinary technician becomes familiar through experience. Many specialized instruments are required for abdominal surgery (see Chapter 28). One group of instruments that has become increasingly popular with veterinary surgeons for use in equine abdominal surgery is gastrointestinal stapling equipment. The technician must become familiar with the different instruments and cartridges. Intestinal resection and anastomosis often require specialized instruments and supplies.
Once the cause of colic or other abdominal problem has been corrected and the horse has recovered from anesthesia, the veterinary technician becomes even more closely involved with patient management. Horses usually require administration of IV fluids, antibiotics, antiinflammatory drugs, and other medications in the postoperative period. The veterinary technician usually administers or oversees administration of these medications. The technician may also perform nasogastric intubation, blood collection, IV catheterization, and changing bandages.
Fortunately, most horses with colic respond to conservative medical treatment, and only a small percentage require surgical intervention. Surgical treatment of colic is necessary for intestinal volvulus and incarceration, enterolithiasis, fibrous foreign body obstruction, and some intestinal displacements. Refer to Chapter 33 for more information about treating horses with medical colic.
Hernia Repair
Herniation of omentum or abdominal viscera through the abdominal wall can occur with an umbilical hernia, inguinal (scrotal) hernia, or incisional hernia. Umbilical hernias are usually congenital and are relatively common in foals. Small hernias may close spontaneously as the foal grows, whereas others require surgical intervention. Umbilical hernias can be repaired using several different methods. Generally the body wall is closed with either interrupted or continuous absorbable suture. Some surgeons open the peritoneum (open herniorrhaphy), and others leave the peritoneum intact (closed herniorrhaphy). If an umbilical hernia is large or it has not closed by several months of age, it should probably be surgically repaired. The owner should be instructed to manually reduce hernial contents at least daily; if at any time the hernia cannot be reduced, the horse should be examined by a veterinarian immediately. If intestine becomes incarcerated in the hernia, vascular compromise can occur, leading to ischemic injury.
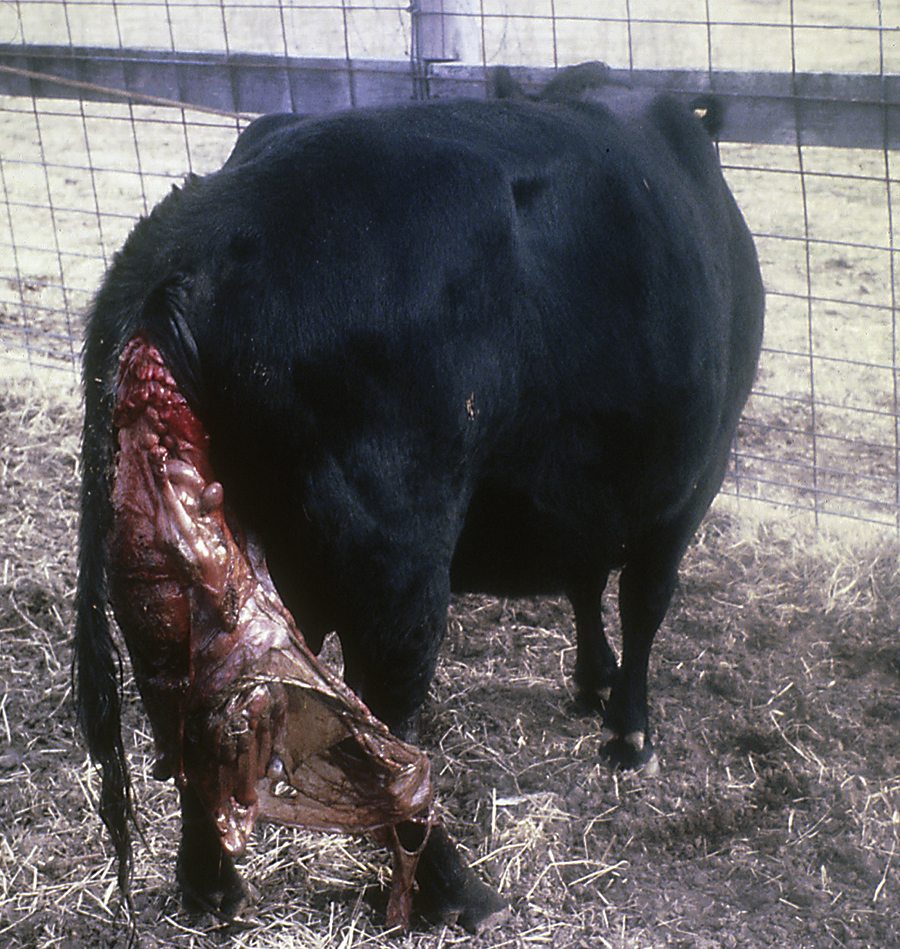
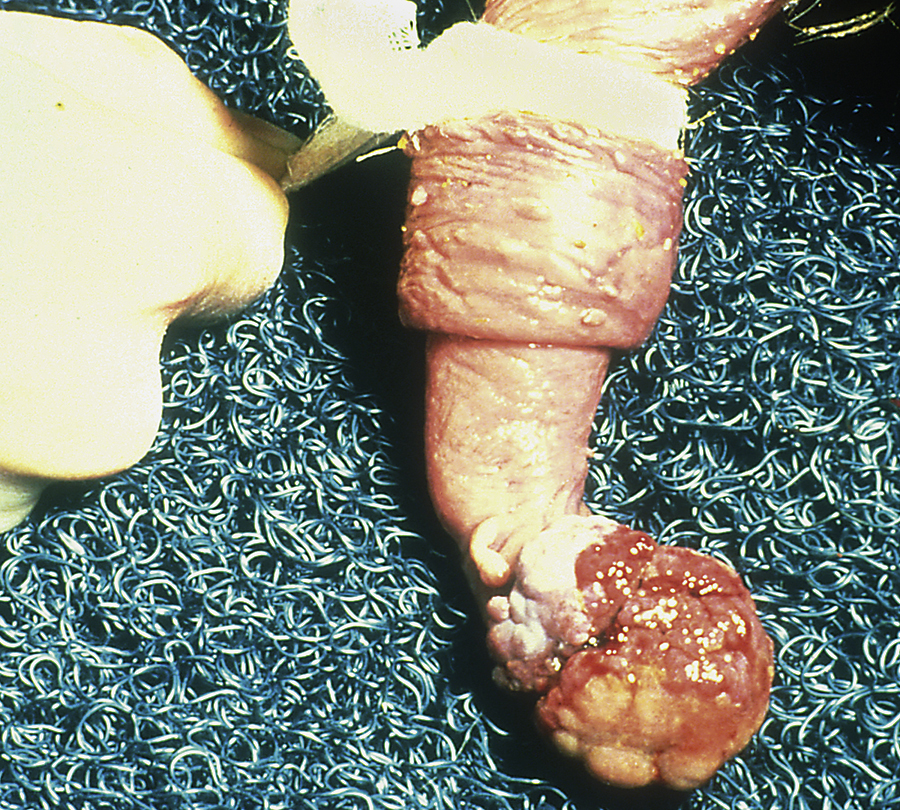
Inguinal or scrotal hernias can occur in horses of any age, but newborn foals and adult breeding stallions are probably the most commonly affected. Frequently the herniated contents do not become incarcerated and can be easily reduced. The hernia should be reduced at least daily in foals because intestine could become incarcerated, which would necessitate emergency surgery. Sometimes these hernias will spontaneously resolve in foals, but many foals require surgical repair. Because the tissues are friable in foals, successful surgical repair can be difficult. Scrotal hernias in adult horses most commonly occur in stallions shortly after breeding. In most instances, the herniated structure or structures become(s) incarcerated (not reducible), which necessitates immediate surgery. Incarceration of intestine within the scrotum will result in a large, firm, and cold scrotum on the affected side secondary to compromised testicular blood flow. The blood supply to the intestine also becomes compromised, resulting in ischemic injury. Generally the testicle on the affected side is removed, and the affected segment of intestine often requires resection. This necessitates preparation of the horse for inguinal and ventral midline surgery.
Acquired body wall herniation occurs in horses subsequent to trauma and following surgery. Blunt trauma, such as a kick, can lead to disruption of the body wall musculature. Body wall hernias occur secondary to abdominal incisions; these occur more frequently in horses that develop incisional infection or other complicating factors. Small body wall hernias can be repaired primarily by suturing the defect. Larger body wall defects require the use of mesh implants. It is critical that there be no residual incisional infection present at the time of mesh herniorrhaphy and that aseptic technique is followed during placement of the mesh.
UROGENITAL TRACT SURGERY
Urinary calculi occur infrequently in horses. Urinary calculi in horses are usually composed of calcium carbonate and have a spicular appearance. These calculi may develop in the kidney or urinary bladder. Small-diameter calculi can be passed during normal urination and go unnoticed. Clinical signs of urinary calculi include stranguria (slow and difficult urination or straining to urinate), pollakiuria (frequent urination), and hematuria (bloody urine). Horses that develop renal calculi will develop signs of abdominal discomfort when the stones become lodged in the ureter. In addition, cystic (urinary bladder) calculi that become lodged in the urethra in male horses cause an inability to urinate and subsequent abdominal pain. Urinary calculi can be diagnosed based on clinical signs, urinalysis, palpation of the urinary bladder per rectum, and endoscopic evaluation of the urethra and urinary bladder. Occasionally a calculus can be palpated in the proximal urethra of male horses at the level of the ischial arch. There are several techniques and certain instruments available for removing urinary tract calculi.
Umbilical Repair
Foals commonly develop diseases of the umbilical remnants, including infection (navel ill) in the umbilical arteries, veins, and urachus. These foals often become depressed, inappetent, and febrile. Many foals also develop secondary septicemia and septic arthritis. Umbilical remnant infection may be diagnosed based on clinical signs of swelling, heat, or drainage in the umbilical area. However, foals can have infection within these structures and be normal on palpation. Transabdominal ultrasonography is also helpful in diagnosing diseases of the umbilical structures. Foals with umbilical remnant infection require treatment with broad-spectrum antibiotics; many of these foals require surgical removal of the affected structures. Surgery for umbilical remnant disease involves a similar approach and instrumentation as for repairing an umbilical hernia. It is necessary to proceed with caution and have suction available and ready while dissecting the umbilical structures to prevent contamination of the abdominal cavity.
Patent urachus is a condition wherein foals dribble urine from the umbilicus because a patent canal between the urachus and urinary bladder is present at birth or develops in the postnatal period. Because those that develop in the postnatal period often occur secondary to an infectious process, it is imperative to rule out umbilical remnant infection and systemic infectious disease. Foals with a patent urachus may be treated nonsurgically by applying an irritant, such as iodine solution, or using silver nitrate sticks on the external surface of the urachus to promote scarification and closure. This is probably most effective in those foals that have a patent urachus at birth. Caution should be used with these agents, and application should be limited to once daily. If a rapid response is not observed or the foal has an infectious process occurring in the umbilical remnants, surgical resection should be performed.
Castration
Castration is one of the most commonly performed surgeries in horses. It is usually performed in the field and does not require extensive surgical facilities or instrumentation. Although under most circumstances castration is performed under short-acting IV general anesthesia, it can be performed in the standing horse with heavy sedation and infiltration of a local anesthetic into the scrotum and spermatic cord. The most common drugs for castration with the horse under IV anesthesia include xylazine-ketamine or xylazine-thiobarbiturate; both combinations can be used with or without guaifenesin. It is important to document that both testicles have descended into the scrotum before commencing with castration in the field. One needs to be prepared for a more extensive surgery requiring entrance into the abdominal cavity (as in a retained testicle); this needs to be planned for because it often takes more time than a routine castration. If both testicles cannot be palpated in the scrotum, the testicle may be located intraabdominally, in the inguinal canal, or immediately outside the external inguinal ring. A horse with a testicle located outside the abdominal cavity but not within the scrotum is referred to as a high flanker. If the testicle cannot be palpated in the scrotum, sedation may relax the horse and the cremaster muscle and allow the examiner to palpate the testicle or a portion of it. If the testicle still cannot be palpated after sedation, a rectal examination with or without ultrasonography may help confirm the location of the testicle. Involvement of the veterinary technician for castration includes general restraint, handling, administering and monitoring anesthesia, preparation of the surgical site, and preparation of instruments.
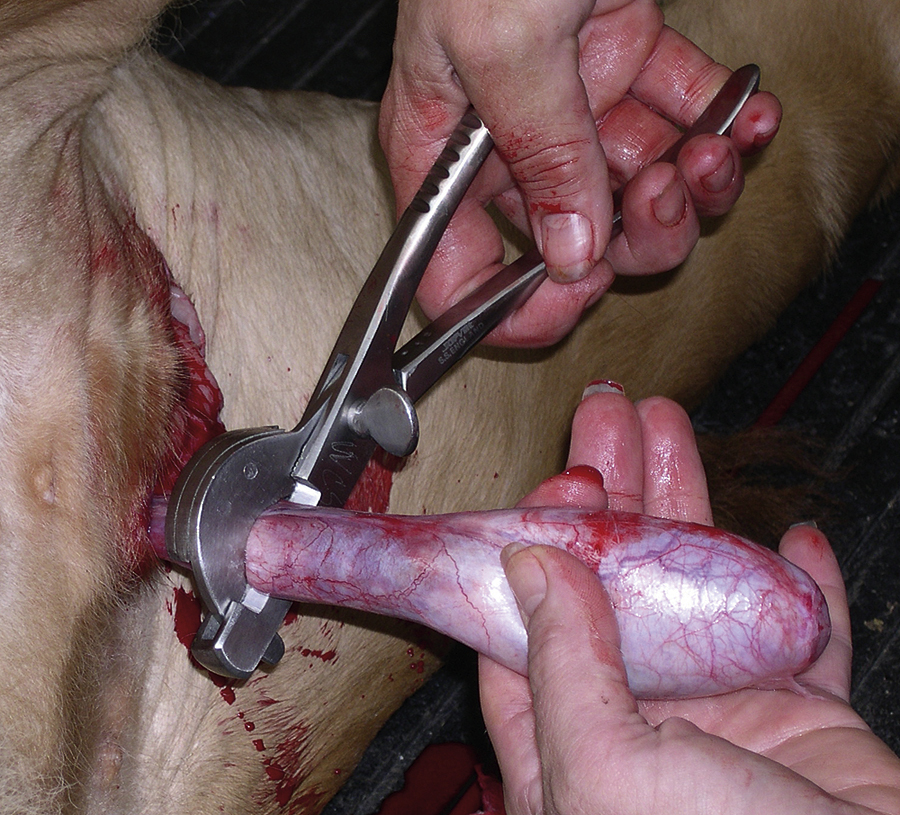
Castration is usually performed with the horse in lateral recumbency with the upper rear limb pulled forward and tied around the horse’s neck. Castration involves making an incision over each testicle parallel to the median raphe through the skin and subcutaneous tissue. The testicles are removed by crushing then cutting the spermatic cord proximal to the testicle and epididymis using emasculators (Figure 31-2, A). The emasculators should be placed on the spermatic cord so that the cord is crushed on the side toward the body wall and cut on the side toward the scrotum (Figure 31-2, B). There are numerous types of emasculators, and each surgeon may have an individual preference. The entire spermatic cord may be crushed and cut simultaneously within the tunic (closed castration), or the tunica albuginea may be opened, and the emasculators can be applied to the vascular structures separately (open castration); this is often done in aged stallions that have an excessively large-diameter spermatic cord. The spermatic cord should be examined after the emasculator is removed to make sure that there is no bleeding. The skin incisions are stretched manually to promote drainage.
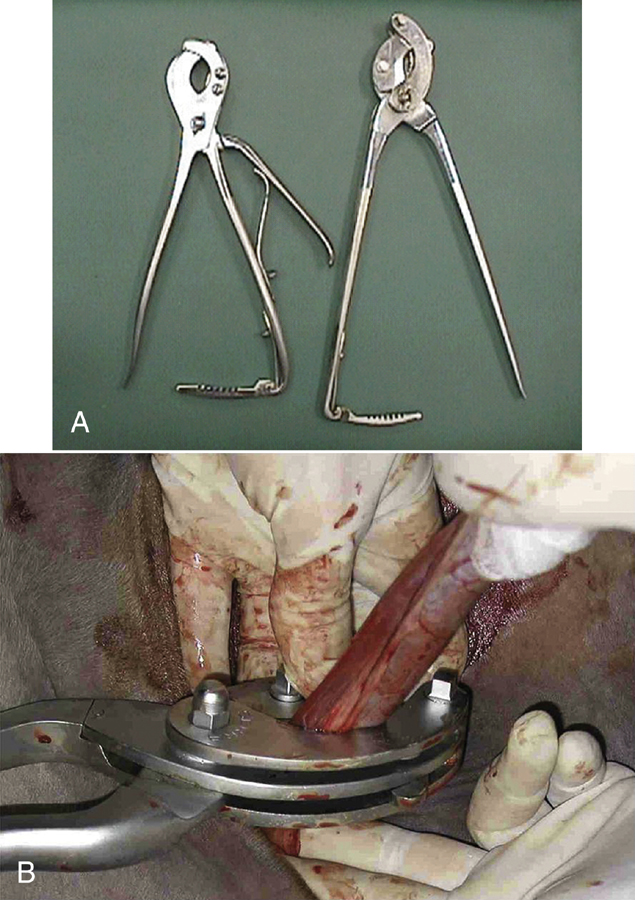
FIGURE 31-2 A, Emasculators used to crush and cut the spermatic cord of horses during castration. B, Use of emasculators during castration of a horse: the emasculators are placed around the spermatic cord so that the nut on the emasculators is located toward the testicle, ensuring that the spermatic cord is crushed toward the body side and the cord is cut toward the testicle side.
Postoperative care usually includes strict stall confinement for 24 hours and then controlled exercise (hand walking) once or twice daily for 1 to 2 weeks to promote drainage, prevent excessive swelling, and prevent or reduce stiffness and soreness. The horse should be monitored closely during the first day after surgery for signs of excessive hemorrhage, evisceration of intestine or omentum (herniation), or excessive swelling.
If the testicle has not descended (cryptorchidism), surgery is more involved and requires anesthesia of longer duration. Cryptorchidectomy (removal of a cryptorchid testicle) also requires the surgeon to use a different surgical technique than for routine castration. The testicle can be approached through various incisions, but an approach through the inguinal ring is most often used. A sponge forceps is used to grasp the structures that lead to the scrotum (gubernaculum), and the testicle is extracted from the inguinal canal. In some horses, the testicle cannot be retrieved in this manner, and the surgeon must manually explore the inguinal canal or caudal abdominal cavity. Once the testicle is retrieved, it is removed using a similar technique as described for routine castration. Following removal of the retained testicle, the other one is removed in a routine manner. Occasionally, horses have both testicles retained. More recently, laparoscopic cryptorchidectomy techniques have been described that can be performed in the standing, sedated horse. The testicle is removed via a flank incision, avoiding any enlargement or damage to the inguinal canal. Laparoscopy can also be used in an anesthetized patient, particularly in cases where there is difficulty in locating an abdominal testicle.
It is believed that cryptorchid horses are more at risk for evisceration after surgery. To prevent this, some surgeons may elect to temporarily pack a length of gauze soaked in sterile saline or an antiseptic into the subcutaneous areas of the inguinal canal. The gauze packing is held in place with large sutures in the skin and is usually removed in 24 to 72 hours. Other surgeons place interrupted absorbable sutures in the external inguinal ring.
Ovariectomy is performed in mares with diseased ovaries, in mares with normal reproductive tracts for use as teaser mares, and in some mares used as performance horses that have unacceptable behavior associated with estrus. An ovariectomy can be performed unilaterally or bilaterally, depending on the reason for the procedure. Laparoscopic techniques have been described that allow an ovariectomy to be performed in the standing horse. This provides excellent visualization of the ovary, good access to the associated artery and vein to ensure that adequate hemostasis is achieved, and a shorter convalescence. Diseased ovaries are usually enlarged and require removal through an incision in the ventral body wall (caudal midline or diagonal paramedian) or the flank. The most common cause of ovarian disease necessitating removal is neoplasia; the most common types of ovarian neoplasia include granulosa theca cell tumors and teratomas. Mares with granulosa theca cell tumors often display abnormal behavior, such as anestrus, persistent estrus or nymphomania, or stallionlike behavior. Ovarian tumors and other ovarian diseases are diagnosed based on clinical signs, rectal examination, and transrectal ultrasonography. Nondiseased ovaries of normal size can usually be removed through a flank incision or via an incision in the vaginal wall (colpotomy) in standing, sedated mares with either local anesthetic infiltration in the body wall or a caudal epidural anesthetic. Hemostasis of the ovarian pedicle is provided either by transfixing with multiple sutures, application of an automatic stapling device, or crushing with a chain écraseur. Complications include hemorrhage, abdominal pain, myositis, and other problems related to anesthesia and abdominal surgery.
Perineal Surgery
Perineal surgery is relatively common in equine practice. Primiparous mares develop rectovaginal and cervical lacerations during foaling. Abnormal perineal conformation can lead to reproductive unsoundness. Mares with abnormal conformation can develop pneumovagina or pneumouterus secondary to aspirating air into the reproductive tract. They also can develop vesicovaginal reflux in which urine pools in the cranial vaginal cavity; this can drain into the uterus during estrus when the cervix is opened, leading to endometrial inflammation. Most surgical procedures to correct these caudal reproductive tract abnormalities are performed in standing mares that have been sedated, and a caudal epidural anesthesia is used.
A caudal epidural anesthesia is performed after clipping the hair over the tail head and aseptically preparing the skin. An 18-gauge, 1.5-inch needle is inserted through the skin between the last sacral and first coccygeal vertebrae or between the first and second coccygeal vertebrae and advanced (Figure 31-3). The correct location can be confirmed by checking to see if local anesthetic placed in the hub of the needle is drawn into the epidural space. Once the correct location has been identified, the local anesthetic is injected. The most commonly used agents for horses are lidocaine, mepivacaine, or xylazine. A caudal epidural anesthetic will desensitize the perineal region. Because horses will also develop incoordination in their rear limbs following the procedure, care should be taken when moving them until the effects of the anesthetic dissipate.
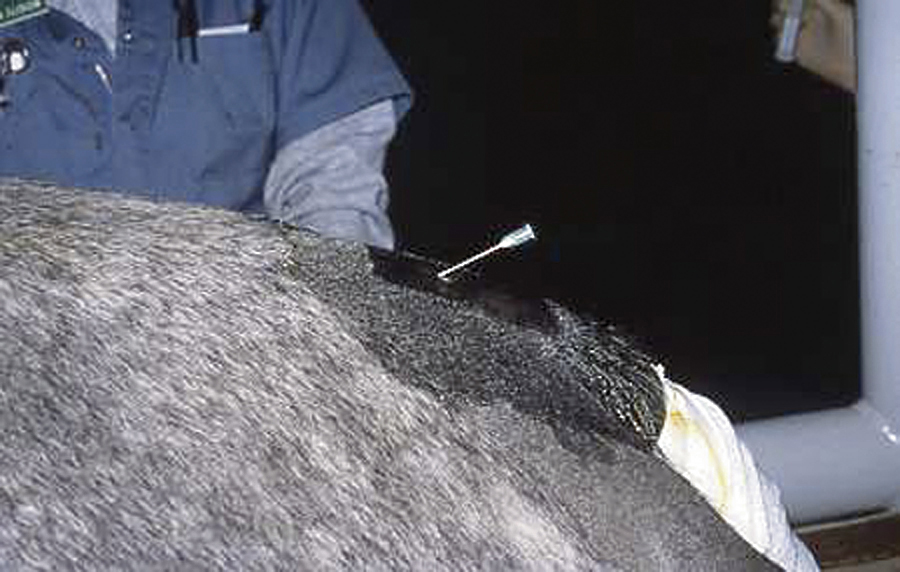
FIGURE 31-3 Technique for injecting a caudal epidural anesthetic between the first and second coccygeal vertebrae in a horse using an 18-gauge, 1½-inch needle.
There are several surgical procedures for correcting caudal reproductive tract abnormalities. The most important factor in the eventual success of repairing a rectovaginal tear is that the mare’s feces be made soft (cow patty consistency) and kept soft for at least 30 days after surgery. This decreases the straining and tension placed on the repaired rectal shelf. The most effective method for getting the feces soft is to remove hay and other coarse roughage from the diet and feed the mare on lush pasture or a complete pelleted feed. Administration of mineral oil or magnesium sulfate to the diet also helps soften the feces.
Caslick’s Procedure: The most commonly performed perineal surgery is Caslick’s operation. This is performed in many fillies on the racetrack and in mares with poor vulvar conformation to prevent pneumovagina and fecal contamination of the vagina, respectively. This procedure is usually performed with sedation and local anesthetic infiltration of the edge of the vulva. The edges of the dorsal vulvar labia are incised and then sutured using a continuous suture pattern. The closure is extended down to the level of the pelvic floor. The suture should not be any lower than this because it may interfere with urination and contribute to urine pooling.
Dystocia: Fetotomy and C-Section: Dystocia means “difficult birth” and is relatively uncommon in horses compared with cattle. However, when dystocia occurs in mares, it is usually a serious problem. Because parturition is rapid in horses and the expulsive efforts of the mare are violent, veterinary obstetric manipulations are difficult and exhausting. Care must be taken at all times to prevent injuring the reproductive tract of the mare. There are many causes of dystocia in the mare; the most frequent ones include premature placental separation and abnormal presentation of the fetus, especially when either the head or limbs or both are deviated. Because the neck of the foal is relatively long, it can easily become twisted. Sometimes the foal may come hind feet first (rare), or if the hind feet are retained, the tail comes first. This latter situation is true breech position. Transverse presentation is also rare in mares. Other occasional causes of dystocia include an excessively large fetus or fetal monsters (e.g., hydrocephalus). An anatomic or physiologic abnormality in the mare herself may cause dystocia. For example, a mare that has sustained a pelvic fracture can develop callus formation, which impairs the shape and size of the birth canal. Another cause of dystocia is torsion of the uterus. This may occur during gestation, particularly during the last trimester.
Dystocia in mares is corrected using a variety of methods, depending on the cause of the dystocia, the status of the foal, and the condition of the mare. Sometimes the dystocia can be corrected by manipulating fetal position or presentation with the mare standing, with or without the use of sedation or an epidural anesthetic. Placement of a nasotracheal tube will prevent the mare from exerting an abdominal press and will relieve straining. Sometimes a short-acting anesthetic protocol combined with rolling the mare on her back or hoisting her hind limbs is enough to relieve the dystocia and provide the veterinarian with sufficient relaxation in the mare to deliver the fetus. Fetotomy is sometimes performed to relieve dystocia, particularly if the fetus is dead. Fetotomy is a process in which a dead foal is cut into pieces while within the uterus and removed. Caution must be taken while performing a fetotomy to prevent serious injury to the reproductive tract of the mare.
Most C-section deliveries are performed in the mare with general anesthesia. Generally a C-section delivery is performed through a caudal ventral midline or flank incision in mares. Time is usually critical for saving the foal and for the overall health and well-being of the mare. The technician must be prepared for the surgery and have necessary equipment, personnel, and drugs ready for reviving the foal if necessary. The same instruments that are used for colic surgery are often used for C-section delivery, but additional instruments may be necessary. If the foal is alive, the technician or other personnel need to be prepared and equipped to revive it. The foal will usually be depressed from the effects of general anesthesia and may need vigorous rubbing and drying. Oxygen should be available and heat lamps and a nasotracheal tube and Ambu bag to ventilate the foal. Forceps to clamp the umbilicus should be readily available if excessive bleeding occurs. A suction device to remove mucus and stomach contents from the airway should be attended to by a technician while the other technicians continue to be cognizant and attentive to the needs of the surgeons.
ORTHOPEDIC SURGERY
Horses frequently sustain severe musculoskeletal injuries, such as long-bone fractures or disruption of tendons or ligaments. These injuries often require stabilization with the use of bandages, splints, or casts before transport to a referral hospital. Successful stabilization of these injuries and safety of transport are important considerations in the outcome of these cases. Most severe injuries should be bandaged and splinted or casted to a level at least one joint above the injury. A heavy Robert Jones bandage should be applied and rigid splints placed on the lateral and either the dorsal or palmar aspects of the limb to provide appropriate support. Splints can be made out of rigid materials, such as wood, steel, or aluminum. The splints should not be excessively heavy or bulky, but must provide appropriate support. Horses with phalangeal fractures can be casted with their distal limb in flexion or can be placed in a commercially available device, such as a Kimsey splint (Figure 31-4). Horses with limb injuries should be hauled in a trailer with partitions to provide some support for them to balance themselves. The head should be tied loosely enough to enable the horse to use the head and neck for balance. Horses with front limb injuries should be transported with their head toward the rear of the trailer, and those with rear limb injuries should be transported with their head toward the front of the trailer.
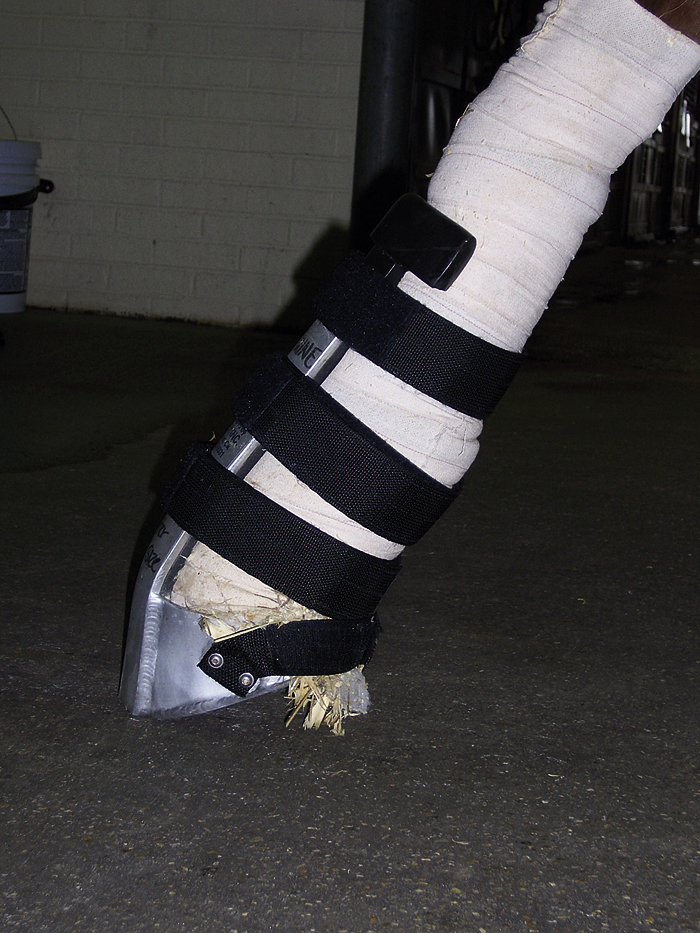
FIGURE 31-4 Use of Kimsey splint to stabilize fractures or joint subluxations in the lower limb of horses.
Orthopedic surgery has become more common in horses. Athletic horses develop numerous orthopedic conditions that are amenable to surgical correction. Historically, fractures of long bones in adult horses were considered irreparable. However, with advanced techniques and more rigid surgical implants, many of these injuries are potentially correctable.
Major fractures of long bones in horses are best repaired with screws and bone plates to prevent movement at the fracture site while the bone heals under rigid fixation (Figure 31-5). Although aseptic technique is imperative for all surgical procedures, it is especially crucial to the overall success of orthopedic surgery in horses. If bony infection develops, it can lead to instability of the implants and fixation failure, which often necessitates euthanasia. It is the responsibility of all personnel to follow aseptic protocol. The technician should strive to maintain asepsis by monitoring the activities of all personnel involved in surgery. Orthopedic surgery requires the use of several specialized instruments and implants; because many of these surgeries are performed on an emergency basis, it is imperative that the technician make sure that instruments are available and ready for use. Many orthopedic injuries that are surgically repaired require the use of external coaptation (cast) for anesthetic recovery or for longer periods postoperatively (see Chapter 34). Therefore the technician should anticipate this need and have the appropriate materials available at the conclusion of surgery. The technician may also be needed to assist with anesthetic recovery of the orthopedic equine patient.

FIGURE 31-5 A proximal phalanx fracture in a horse repaired with cortical bone screws placed in lag fashion to compress the fracture line.
Postoperative monitoring of the orthopedic patient is vital for early detection of potential problems. It is particularly important to observe how the horse is using the affected limb in the stall; any dramatic change in use of the limb may signal an impending problem (infection or cast sores). The cast should also be monitored for heat, odor, or exudate, which would indicate the development of cast sores. The most common locations for sores to develop in association with a half-limb cast are at the proximal, dorsal aspect of the metacarpus or metatarsus, at the palmar or plantar aspect of the fetlock over the sesamoid bones, and over the heel bulbs. Bandages need to be changed frequently, and the incision sites should be monitored for swelling, erythema, and discharge. Drains are commonly used in orthopedic surgery following repair of a long bone. Drains can be useful in preventing seroma formation, but they can serve as potential routes for inoculation of the surgery site. Therefore it is important to keep these drains sterile by keeping a clean, sterile bandage on the leg. This may require changing the bandage more frequently than once daily.
Arthroscopic Surgery
Arthroscopy is commonly performed for the diagnosis and treatment of joint disease. It is commonly performed for removing osteochondral chip fractures, treating cartilaginous and bony abnormalities associated with osteochondrosis, treating septic arthritis, and evaluating causes of joint lameness that have no definitive radiographic abnormalities. Depending on the joint evaluated and the type and location of the lesion, the horse may be positioned in dorsal or lateral recumbency. It is necessary to have the radiographs on a view box in the OR so that the surgeon can evaluate them intraoperatively. During arthroscopy, the technique of triangulation is used whereby the lesion forms one corner of the triangle and the arthroscope and surgical instruments serve as the other two corners of the triangle. Generally the arthroscope is placed in the joint on the side opposite the lesion, and the surgical instrument is placed in the joint on the same side as the lesion. The portal for placement of the arthroscope is usually made by making a small (1 cm) incision in the skin and subcutaneous tissue and then using a sharp trocar to advance the arthroscopic cannula through the fibrous joint capsule and synovial lining. Once the cannula has penetrated the joint cavity, the sharp trocar is replaced with a blunt obturator to pass the cannula across the joint; this prevents iatrogenic damage to the cartilage. The skin incisions are usually made before joint distention in the carpus, but after joint distention in other joints. The joint is distended with sterile polyionic fluid to facilitate placement of the arthroscope. Once the arthroscope is in place, the joint is evaluated; once the lesion is identified, the most appropriate location for the instrument portal is determined by using a needle to triangulate the lesion with the arthroscope. Once the appropriate location for the instrument portal is identified, the instrument portal is made with a scalpel blade (No. 11 or 15). The appropriate instrument is placed into the joint. The instruments commonly used in arthroscopy include a blunt probe for palpating intraarticular structures, rongeurs for removing osteochondral fragments, and curettes for débriding diseased cartilage and bone. A fenestrated cannula is often used at the end of surgery to facilitate removal of cartilage and bone debris via lavage. Motorized equipment is available and is sometimes necessary for débridement of large areas of diseased bone.
The surgeon uses specific instruments for arthroscopy, and these may vary depending on the joint involved and the individual surgeon’s preference. Generally, there will be a standardized set of arthroscopy instruments that are packaged together. Instruments are steam sterilized, but if they are to be used on more than one case per day, they are sterilized with a cold sterilization solution before each use. Following sterilization, the instruments are packed in a sterile stainless steel pan that is later used to rinse disinfecting solution off the arthroscopy instruments. One of the most important and most expensive instruments is the arthroscope; it should be handled carefully to prevent damage. Additional items necessary are a sterile needle (usually 18-gauge) and syringe, which are used for distending the joint. During arthroscopic surgery, the joint is kept distended with sterile physiologic solution; this solution is usually delivered with a pump through a sterile IV set.
Many hospitals perform arthroscopy using a video camera so that the entire procedure can be viewed on a television screen (Figure 31-6). This causes less strain on the surgeon’s eye, makes the procedure more educational for surgery assistants and technical staff, provides an opportunity to videotape the procedure, and probably allows the procedure to be performed with fewer breaks in aseptic technique. To provide the intense light required to illuminate the inside of the joint, a fiber-optic light source and light cable are required. It is essential that the technician be familiar with the assembly and function of the arthroscopic equipment and the proper care, cleaning, and disinfecting of the instruments. The arthroscopy instruments are disinfected using a cold sterilization solution, such as activated dialdehyde (Cidex, Surgikos); the instruments, arthroscope, and light cables are soaked for a minimum of 10 minutes. One should read the manufacturer’s recommendations regarding the time required for disinfecting. To prevent delays, the instruments can be placed in the sterilizing solution at the start of anesthesia. This will also ensure adequate sterilization time. Before using the instruments, they are transferred sterilely into an empty sterile tray. The instruments are then rinsed with sterile saline to remove the sterilization solution.

FIGURE 31-6 Use of arthroscopy for evaluating joint disease in horses. The arthroscope is inserted into the joint and attached to a camera that projects the image on a television screen for easy viewing by the surgeon and other personnel.
After the surgical site has been aseptically prepared and draped and the instruments removed from the sterilizing solution, the technician will be responsible for attaching the fiber-optic cable to its light source. The system that delivers the fluid to distend the joint must also be connected to the appropriate fluid source. Once the system is connected to the fluid source, the surgeon must run fluid through the system to flush all air bubbles out of the tubing so that they do not enter the joint. Electric fluid pumps are generally used to maintain joint distention; these may be manually or pressure controlled.
Following surgery, all specialized arthroscopy equipment and instruments need to be cleaned. The arthroscope lens should be examined for scratches, and the video camera should be dried carefully. If several arthroscopy surgeries are scheduled for the day, the instruments are placed in the cold sterilization solution in preparation for the next surgery.
Flexural Deformities: Flexural and angular limb deformities (crooked legs) are abnormalities of the limbs that arise from abnormal development of bones and musculotendinous structures in the limbs. Flexural limb deformities result in overflexion of certain joints. There are three main manifestations of flexural limb deformities in horses. These can be present at birth or develop during the first few months or years of life. Carpal flexural deformities result in front limbs that are flexed or buckled forward at the carpus. This may range from mild deformity to a severe deformity that prevents the foal from standing. Mild to moderate cases are often amenable to treatment with controlled exercise combined with application of bandages and splints that extend from the ground to the elbow or tube casts that extend from just above the fetlock to the middle portion of the antebrachium. IV administration of oxytetracycline may be beneficial to help relax the musculotendinous structures.
The second type involves flexural deformity of the distal interphalangeal (coffin) joint, which results in a characteristic clubfoot-shaped hoof (Figure 31-7). This often is first noticed when the foal is a few months of age and can progress to the point that the foal walks on the toe or the dorsum of the hoof wall. Mild to moderate cases (those in which the foot has not passed the vertical plane) often respond to corrective trimming (lower heel) and application of an extended toe shoe, which helps to stretch out the deep digital flexor tendon. More advanced cases usually require surgical transection of the inferior check ligament, which lengthens the deep digital flexor musculotendinous unit.

FIGURE 31-7 Flexural deformity of the distal interphalangeal (coffin) joint of the right front limb in a horse.
The third type of flexural deformity involves the metacarpophalangeal joint and is characterized by an increased steepness to the pastern and fetlock (Figure 31-8). This usually begins to develop around 1 year of age, but may occur as late as 2 years. It can progress until the horse knuckles over at the fetlock. This condition commonly occurs in rapidly growing heavily muscled horses, such as 1- to 2-year-old quarter horses. Conservative treatment involves controlled exercise, dietary management (balanced minerals, low energy and protein), management of pain (arising from osteochondrosis or physitis) with NSAIDs, and application of bandages and splints that extend from the ground to the elbow. More severely affected horses or those that do not respond to conservative treatment may be successfully treated surgically by performing a superior check or inferior check ligament desmotomy or both, depending on whether the superficial digital flexor or deep digital flexor tendons or both are involved.
Angular Limb Deformities: Angular limb deformities are deformities that develop in the appendicular skeleton in a medial-to-lateral direction. These deviations can be present at birth or develop during the first few months of life. Mild deformities may self-correct, others may persist but not worsen, and still others may become more severe with time. These deformities are named in reference to the joint involved and the direction of the deviation. The most common deviation is carpal valgus, where the limb distal to the carpus deviates laterally (Figure 31-9). Other common deviations include fetlock varus, where the limb distal to the fetlock deviates medially (Figure 31-10), and tarsal valgus. These deviations can occur because of disproportionate growth of bone on either side of the growth plate, incompletely ossified cuboidal bones in the carpus and tarsus, or ligamentous laxity. The deviations in foals with incompletely ossified cuboidal bones or ligamentous laxity can usually be manually straightened, whereas those with disproportionate growth at the physis cannot.
Treatment of mild to moderate angular deviations may include stall rest with controlled exercise, depending on the age of the foal. Successful surgical procedures have been developed to treat moderate to severe deformities. Transection and elevation of the periosteum near the affected growth plate on the concave (short) side of the limb will stimulate more rapid bone growth, which usually leads to correction of the disproportionate growth. Periosteal transection and elevation can be repeated in 4 to 6 weeks if the deformity has not been completely corrected. The deformities do not overcorrect with this procedure. In more severe deformities or in older foals with less growth potential, the growth on the convex (or long) side of the bone can be slowed by performing transphyseal bridging. This is usually performed by placing a screw on either side of the growth plate and then tightening a figure-eight wire around the screw heads to provide compression of the growth plate. Use of transphyseal bridging can lead to correction of more severe deformities, but it is imperative that these implants be removed at the correct time to prevent overcorrection leading to the opposite type of deformity. Foals with deviations of the carpus or tarsus subsequent to ligamentous laxity or incompletely ossified cuboidal bones are best treated with stall rest with controlled exercise combined with application of full-limb bandages and splints or tube casts extending from the distal cannon bone to the proximal radius or tibia.
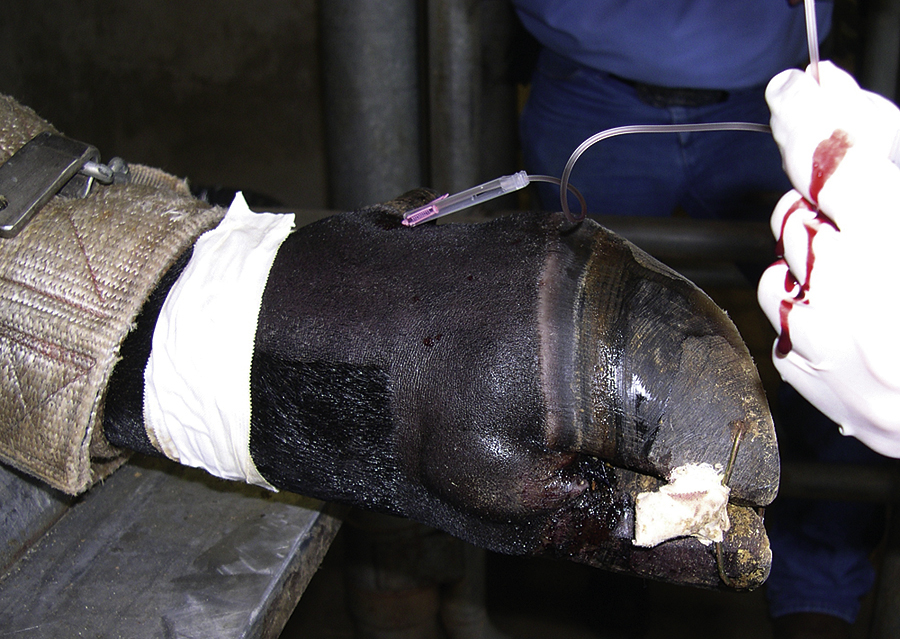
Laminitis: Laminitis (founder) is a serious, often life-threatening disease of horses involving inflammation of the sensitive laminae of the feet. It often involves both front feet or all four feet. However, it can occur in only one forefoot or rear foot if there is a severe lameness in the opposite limb. The exact cause of laminitis is unknown, but horses with serious infectious or inflammatory diseases resulting in endotoxemia, such as ischemic or inflammatory bowel disease, pleuropneumonia, septic metritis, and grain overload, are predisposed. Laminitis occurs almost exclusively in adult horses; it rarely occurs in horses less than 1 year of age.
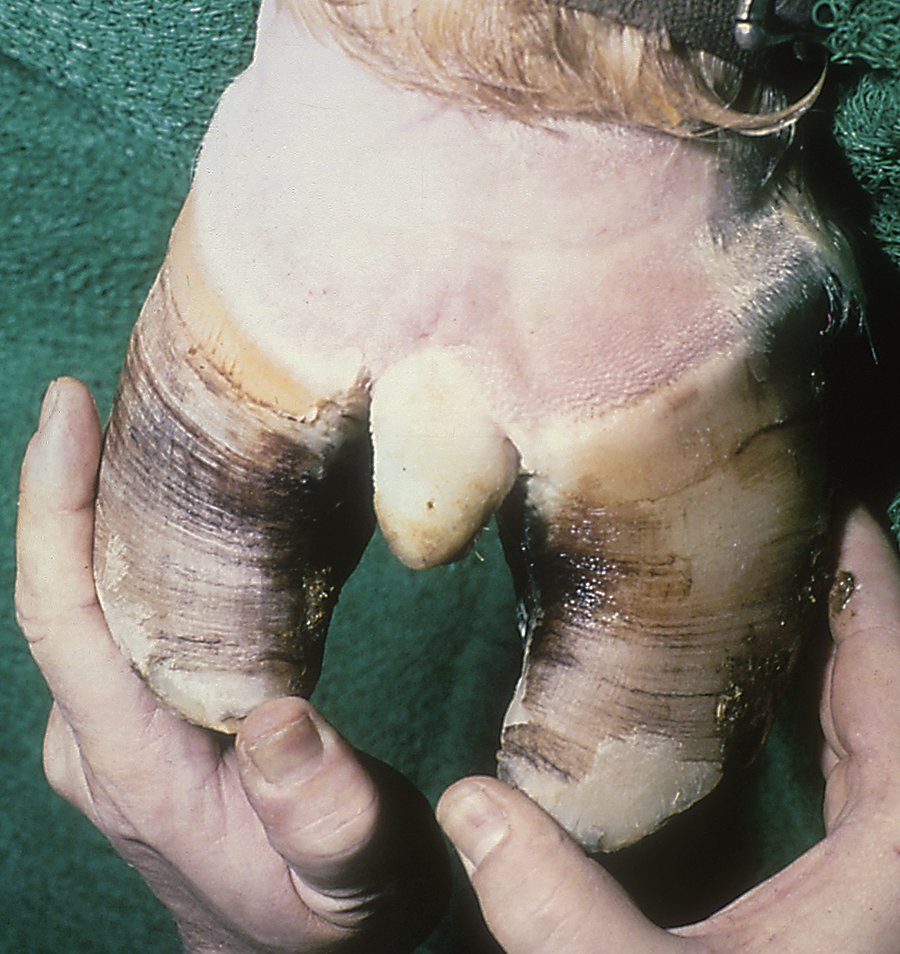
Acute laminitis occurs in the initial stages of the disease, resulting in extreme pain and reluctance to move. Horses often have increased heat in the hooves and have a pronounced or bounding digital pulse. They are reluctant to walk, turn, or allow their feet to be picked up. They stand with a characteristic stance with their rear legs camped underneath their torso and their front feet camped out in front (Figure 31-11). Chronic laminitis occurs when, because of degeneration of the sensitive laminae on the coffin bone (distal phalanx), the dorsal laminar attachments to the insensitive laminae of the hoof detach and the coffin bone rotates. In severe chronic laminitis, the rotated coffin bone may protrude through the sole of the foot. A lateral radiograph of the foot is usually required to determine whether coffin bone rotation has occurred (Figure 31-12, A and B). In more severe cases, all laminar attachments may become detached, and the coffin bone is displaced distally within the hoof wall. Horses that have distal displacement of the coffin bone develop a characteristic depression at the coronary band and are termed sinkers. Horses with chronic laminitis develop characteristic concentric rings on the hooves and an abnormal shape of the hooves (Figure 31-13).
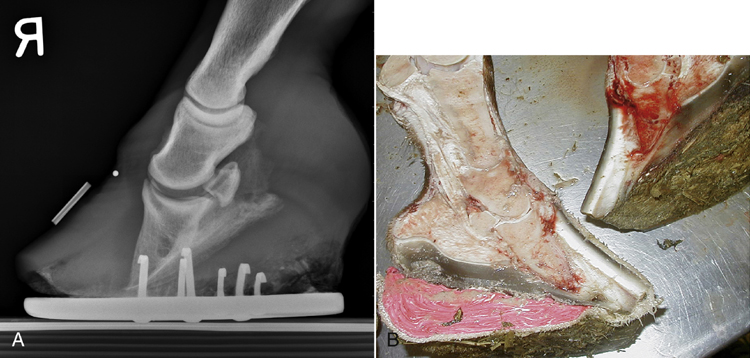
FIGURE 31-12 A, Lateral radiograph of the front foot of a horse with laminitis that has evidence of coffin bone rotation. B, Gross pathologic photograph of sagittal section of both front feet of a horse with bilateral laminitis that has undergone coffin bone rotation.
The main focus of treatment of horses with laminitis involves reducing inflammation and providing analgesia with antiinflammatory drugs (phenylbutazone), promoting digital blood flow with vasodilator drugs (acepromazine, isoxsuprine, topical glyceryl trinitrate), and mechanically supporting the distal phalanx by providing frog support (frog pads or heart bar shoes). Nursing care is also an important component of the therapeutic regimen, particularly in chronic laminitis. Because laminitis is extremely painful, horses often spend long periods of time lying down. This necessitates care of decubital ulcers. Deep bedding is necessary, and using straw on top of shavings, padded mats, or a water bed can help prevent the development of these ulcers. In addition, they often develop subsolar abscesses that require daily soaking and bandaging. The prognosis for return of the horse to athletic competition depends on the occurrence and severity of rotation or sinkage of the coffin bone. Most horses that have appreciable rotation do not return to athletic function. The prognosis for horses that develop distal displacement of the coffin bone is poor.
Bog Spavin: Bog spavin is a term used to describe the accumulation of synovial fluid (effusion) in the tarsocrural joint of the hock (Figure 31-14). Fluid can accumulate secondary to osteochondrosis, synovitis, and arthritis. Degenerative joint disease(arthritis) is a common performance-limiting condition of horses and can affect numerous joints. Bone spavin refers to arthritis in the distal intertarsal and tarsometatarsal joints of the hock. High ring-bone and low ring-bone refer to arthritis in the proximal interphalangeal (pastern) and distal interphalangeal (coffin) joints, respectively. Osselet is a term to describe arthritis in the metacarpophalangeal or metatarsophalangeal (fetlock) joint.
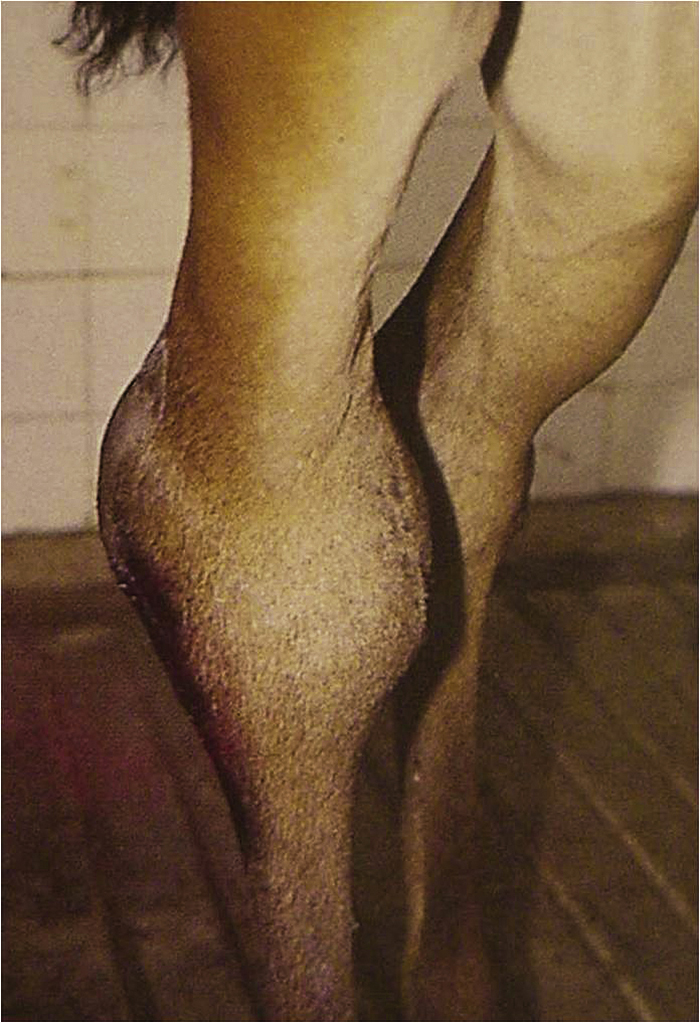
Tendinitis: Tendinitis (bowed tendons) is an injury involving primarily the superficial digital flexor tendon and occasionally the deep digital flexor tendon of the front limbs. This injury is usually sustained secondary to racing or other strenuous activity. There are different degrees of tendinitis ranging from mild edema and inflammation to tendon fiber separation to tendon fiber tearing or disruption. When tendon fibers tear, the result is hemorrhage and inflammatory debris accumulating in a cavity within the tendon, which is known as a core lesion. Treatment of tendinitis includes hydrotherapy, NSAIDs, support bandages, topical antiinflammatory agents (sweats, poultices), and exercise restriction or controlled exercise. Several surgical procedures have been used to either treat tendinitis or prevent its recurrence. The most commonly performed surgery is tendon splitting, which evacuates the core lesion and allows more rapid vascularization and healing of the area. The prognosis for return to athletic function depends on the severity of the injury; some horses with severe core lesions can return to athletic function if given appropriate treatment and time for convalescence.
Osteochondrosis: Osteochondrosis is a form of developmental orthopedic disease in which the articular cartilage and underlying subchondral bone do not develop appropriately. This can result in the formation of osteochondritis dissecans (cartilage flaps), osteochondral fragments, cartilage erosion, and subchondral bone cysts. These abnormalities often manifest as joint effusion and lameness when young horses are first put into strenuous exercise. Many of these lesions are amenable to treatment via arthroscopy, resulting in the horse returning to athletic function.
Subsolar Abscess: Subsolar abscess is a common cause of severe lameness. Horses usually will not bear weight on the limb. There is palpable heat in the hoof and a bounding digital pulse similar to that in a horse with laminitis. However, the difference is that subsolar abscesses usually occur only in one foot. Pain can be localized by applying focal pressure to the sole of the foot with hoof testers. Occasionally, purulent debris will accumulate and migrate, and an area breaks open at the coronary band and drains (gravel). Treatment involves paring out the sole until the abscess is located to provide drainage. The foot should be kept bandaged to keep it dry and clean. The affected foot can be soaked daily in a solution of povidone-iodine (Betadine) and magnesium sulfate (Epsom salts) and then rebandaged. The horse should be given analgesics (phenylbutazone) for a few days. Appropriate tetanus prophylaxis should be administered. The foot needs to be protected from dirt and debris until the area fills in with granulation tissue and is covered with cornified tissue.
Septic Arthritis: Septic arthritis is a common occurrence in adult horses secondary to iatrogenic inoculation of joints during arthrocentesis or joint surgery or subsequent to traumatic joint injuries. It occurs commonly in foals subsequent to hematogenous spread from a focus of infection, such as the umbilicus (navel ill), lungs (pneumonia), or intestinal tract (enteritis). The cornerstone of treatment of septic arthritis includes broad-spectrum antibiotics administered systemically, intraarticular antibiotics, NSAIDs, and joint drainage and lavage.
UPPER RESPIRATORY TRACT SURGERY
Abnormalities of the upper respiratory tract can be performance limiting to athletic horses and, if severe, can also be life threatening. Many obstructive diseases of the upper respiratory tract are amenable to surgical correction. The most common of these are left laryngeal hemiplegia, epiglottic entrapment, dorsal displacement of the soft palate (DDSP), and arytenoid chondritis. Others are subepiglottic cysts, guttural pouch empyema, guttural pouch tympany, and guttural pouch mycosis.
Left laryngeal hemiplegia (“roarer”) is a condition resulting in paralysis of the left arytenoid cartilage, which prevents it from being abducted during inspiration. This results in the arytenoid collapsing and being pulled into the airway secondary to the negative pressure that is generated during inspiration. The cause of this condition is unknown, but it results in a recurrent laryngeal neuropathy. Because this nerve normally provides innervation to the major abductor muscle of the arytenoid cartilage, the cricoarytenoideus dorsalis, a neuropathy results in muscle atrophy and an inability to abduct the arytenoid. As the name implies, this condition occurs almost exclusively on the left side (95%); it is believed that this is related to the longer length of the nerve on the left side and that it may become damaged from the vibrations as it courses around the aortic arch. This condition is diagnosed using endoscopy at rest or during exercise on a high-speed treadmill; the left arytenoid cartilage is not fully abducted during inspiration and in severe cases actually collapses into the airway. Horses with this condition make a characteristic inspiratory noise (roaring) and develop exercise intolerance. Surgical treatment is a prosthetic laryngoplasty, which involves placing a suture between the cricoid cartilage and the muscular process of the arytenoid cartilage to mimic the action of the cricoarytenoideus dorsalis and abduct the arytenoid cartilage (tieback). The laryngeal ventricles (saccules) are also everted and resected (ventriculectomy or sacculectomy) through either a ventral laryngotomy or by use of an endoscopically guided laser. Approximately 70% of horses treated with a prosthetic laryngoplasty and sacculectomy return to athletic function. Most horses will continue to make some noise, and in some, the noise may not improve. The laryngotomy incision is usually left open to heal by second intention. This requires daily cleaning with gauze sponges with saline or water followed by application of petrolatum to the skin around the incision and on the mandible and neck to prevent skin scald from the drainage. It usually takes approximately 3 weeks for the incision to heal. Some clinicians partially close the incision, which reportedly shortens the time required to heal.
Epiglottic entrapment is a condition where the aryepiglottic membrane that extends from the arytenoid cartilage to the ventral surface of the epiglottis hypertrophies and rolls upward to envelope the rostral and abaxial portions of the epiglottis. Normally the epiglottis should have a serrated edge and a distinct vascular pattern present on the dorsal surface. When the epiglottis becomes entrapped, the serrated edge and vascular pattern can no longer be seen. The shape or outline of the epiglottis can still be observed (unlike that seen with a DDSP), but the tip appears more rounded and the abaxial surface is smooth rather than serrated. In more chronic cases, the tip of the epiglottis may become ulcerated. The cause of epiglottic entrapment is unknown, but it is believed that these horses have an instability between the caudal edge of the soft palate and the epiglottis and that the aryepiglottic membrane hypertrophies and makes the epiglottis more rigid. Epiglottic entrapment can be intermittent or permanent. Some horses can continue to perform athletically with an entrapped epiglottis, but it does appear to affect performance in most horses. Treatment of epiglottic entrapment includes transecting the aryepiglottic membrane to release the epiglottis. This can be done using several techniques. First, it can be performed with a hooked bistoury placed through the nasal passages in a standing, sedated horse with or without endoscopic guidance; care must be taken to prevent trauma to other structures and to prevent laceration of the soft palate. Second, it can be performed in an anesthetized horse with a mouth speculum by manually guiding a hooked bistoury and transecting the membrane on midline. Third, it can be performed using an endoscopically guided laser in a standing, sedated horse. Finally, in more severe or chronic recurring cases, the aryepiglottic membrane can be resected through a ventral laryngotomy. The prognosis for return to athletic performance is good, but entrapment can recur. Some of these horses may develop DDSP after the entrapment is released. Horses that have the entrapment released using the hooked bistoury or laser can generally resume training in a few days, whereas those treated via resection through a laryngotomy require approximately 3 weeks before resuming training.
DDSP is generally a dynamic obstructive disease of the upper respiratory tract that occurs during exercise. Normally the soft palate remains ventral to the epiglottis. However, if the epiglottis is small or flaccid or the caudal edge of the soft palate is flaccid, the soft palate can become displaced dorsal to the epiglottis during strenuous exercise. The cause of this condition is unknown, but it is believed that the factors listed previously predispose the palate to become displaced during inspiration when negative pressure is generated in the upper airway. This condition usually is intermittent, occurring during strenuous exercise and dissipating once exercise has stopped and the horse swallows. Because horses are obligate nasal breathers, DDSP interferes with the horse’s breathing. Horses with DDSP usually make a characteristic gurgling or snoring type of noise, which will dissipate as soon as they swallow and replace the palate into its normal position.
Treatment options for a horse with DDSP include placing a cloth or leather tie on the horse’s tongue and pulling the tongue rostrad and tying the tongue to the mandible in the interdental space. The epiglottis, tongue, and sternothyrohyoideus muscles are attached to the hyoid apparatus. Because the tongue is attached at the rostral aspect of the hyoid apparatus and the sternothyrohyoideus muscles are attached at its caudal aspect, a tongue tie prevents caudal retraction of the hyoid apparatus, including the epiglottis. This seems to help approximately 50% of horses with DDSP because it prevents caudal retraction of the epiglottis and maintains normal epiglottic-palate alignment. Because of its noninvasive nature, the tongue tie is generally the first thing attempted in horses with DDSP. If this does not work, a section of the sternothyrohyoideus muscles can be resected in the midcervical region; this also prevents caudal retraction of the hyoid apparatus. This myectomy procedure helps in approximately 50% of horses with DDSP that fail to respond to a tongue tie. If this procedure does not work, the caudal margin of the soft palate can be resected (staphylectomy). There are two theories as to why this may help prevent DDSP. First, it is believed that the caudal edge of the palate becomes more fibrous as it heals with scar tissue; this makes the caudal edge more rigid and therefore more resistant to displacement. The other theory is that, if the palate does displace, it enables the palate to be replaced more easily. Regardless of the mechanism, it seems that it helps prevent DDSP in approximately half of the horses that do not respond to the tongue tie or myectomy. A laryngeal tie forward procedure reportedly has achieved a higher success rate in horses with DDSP and should now be considered the surgery of choice if no other source of inflammation is present in the upper respiratory tract. However, it requires general anesthesia, so some of the alternative procedures may be performed initially in the standing patient.
Arytenoid chondritis is an inflammatory, degenerative condition of the arytenoid cartilagines resulting in a proliferative mass on one or both arytenoids. This usually results in an obstructive disease of the upper airway with signs similar to the conditions described earlier. These cartilagines are usually enlarged and more fibrous than normal, which prevents them from being effectively treated with a tieback. The treatment of choice is to remove the affected arytenoid cartilage through a ventral laryngotomy. Because of the time required for dissection in the laryngeal region during an arytenoidectomy, a tracheotomy is usually performed in the middle or proximal trachea to provide a mechanism for ventilation during anesthesia. The tracheotomy can be performed either before anesthetic induction or once the horse is anesthetized. These horses are prone to upper airway obstruction postoperatively and need to be closely monitored. The tracheotomy tube is usually left in place, at least for a couple of days, until it is believed the horse has an airway of adequate diameter for breathing. It is imperative that these horses be monitored closely while the tracheotomy tube is in place to make sure that it does not become dislodged or obstructed with mucus or other discharge. The laryngotomy and tracheotomy sites require daily cleaning and application of petrolatum on the skin around the incisions. Both these incisions will heal by second intention in approximately 3 weeks.
Bacterial infection of the guttural pouch (empyema) usually is a sequela to strangles or retropharyngeal lymph node abscesses. Clinical signs include swelling in the throat-latch region and a bilateral mucopurulent nasal discharge. Horses with guttural pouch empyema can be treated conservatively with antibiotics and guttural pouch lavage; this may be effective in many horses that are treated early in the course of the disease. However, in more chronic cases, the mucopurulent material becomes inspissated and forms gelatinous concretions (chondroids) that lie in the floor of the guttural pouches. Resolution of empyema requires removal of the chondroids, and long-term effective drainage can usually only be achieved with surgical drainage. Several approaches are reported for surgical drainage of the guttural pouches, but the most common surgical approach for guttural pouch empyema is the modified Whitehouse technique; the incision is made in the skin on the ventrum of the throat region just axial to the linguofacial vein and is followed by blunt dissection into the pouch. The guttural pouch is lavaged intraoperatively. Indwelling catheters can be placed into the guttural pouches in standing, sedated horses under endoscopic guidance; these catheters enable frequent lavage of the pouches. The guttural pouches should not be lavaged with irritating solutions because of the proximity of blood vessels and nerves coursing through the area. The incision is managed similarly to a laryngotomy or tracheotomy incision.
Guttural pouch tympany is an accumulation of air in the guttural pouches; this occurs in foals and weanlings and is usually associated with an abnormality of the opening to the pouches. It can occur on one or both sides and is characterized by a fluctuant, nonpainful swelling in the throat-latch region. If unilateral guttural pouch tympany is present, then it is usually treated by surgically creating an opening in the septum between the left and right pouches; this is usually approached through an incision in Viborg’s triangle on the affected side. If bilateral tympany is present, creating an opening in the septum will not effectively drain the two sides. Therefore the opening to one or both of the guttural pouches is surgically revised through a Viborg’s triangle approach. Surgical revision of the guttural pouch opening may be performed on only one side with creation of an opening in the septum to enable both pouches to evacuate the air through one opening.
Guttural pouch mycosis can be life threatening. Fungal plaques form in the lining of the guttural pouches; if the plaques involve vascular structures, such as the internal carotid artery, severe fatal hemorrhage can occur. Fatal hemorrhage is often preceded by several episodes of substantial epistaxis. However, once the diagnosis is made, surgery should not be delayed. The most accepted method of surgical treatment is vascular occlusion of either the internal carotid artery, external carotid artery, or both, depending on which vessels are affected. This can be done following placement of an intraarterial balloon-tipped catheter or by placement of newer springs or coils that stimulate local occlusion. Both the internal and external carotid arteries can be ligated unilaterally with no untoward effects. The major potential complication of external carotid artery occlusion is blindness. Once the affected vessels are ligated, the fungal infection is treated by lavage of the guttural pouches and instillation of antifungal medication into the pouch via indwelling catheters or via the endoscope.
EMERGENCY SITUATIONS AND PROCEDURES
Several emergency situations can arise that necessitate immediate action on the part of a technician or clinician to prevent death of a horse. One of the most common emergency situations is the development of upper airway obstruction leading to dyspnea. Obstructive diseases involving the nasal passages, nasopharynx, and larynx can be alleviated by a tracheotomy. A tracheotomy is generally performed at the junction of the middle and proximal thirds of the neck on the ventral midline. An incision is made on the ventral cervical midline through the skin, subcutaneous tissue, and cutaneous colli muscle parallel to the trachea. The paired sternothyrohyoideus muscles are then split on midline to expose the tracheal rings. The membrane between two adjacent rings is then cut with a scalpel on the ventral surface for a distance of approximately one third of the circumference of the tracheal rings. Care should be taken not to cut the tracheal rings and not to cut vital structures adjacent to the trachea (carotid artery, recurrent laryngeal nerve, jugular vein). Many times the tracheotomy must be performed on an extremely anxious horse or after the horse has collapsed from insufficient oxygen. Therefore one should be careful not to get into a situation where injury occurs.
Veterinary technicians should become familiar and comfortable with the dosages and indications for drugs commonly used in emergency situations. A list of drugs and doses along with the drugs and syringes should be kept readily available in several locations throughout the hospital. These can be prepared in small emergency packs.
Occasionally, horses develop reactions to certain drugs. These may be anaphylactic reactions resulting in shock or death or allergic type of reactions resulting in skin wheals. Horses may develop a reaction to procaine penicillin, which usually results in an anaphylactoid reaction. These horses usually require treatment with corticosteroids and epinephrine. They may recover or die subsequent to pulmonary edema. Horses often develop skin wheals in response to drugs or environmental allergens (Figure 31-15, A and B). The drugs that most commonly cause these wheals in horses are NSAIDs and trimethoprim-sulfa antibiotics.
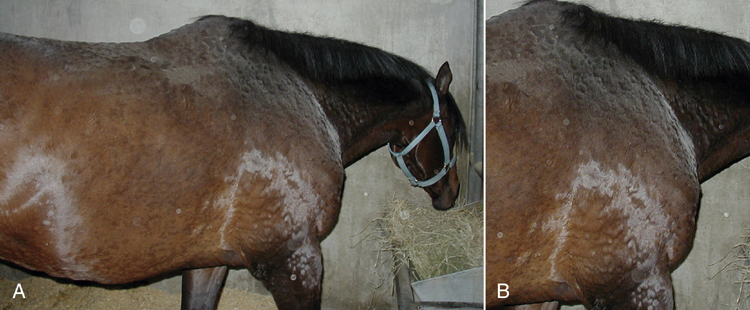
FIGURE 31-15 A, Horse with wheals throughout the body indicating an anaphylactic reaction. B, Closer view of the wheals (raised areas of pitting edema).
Intracarotid injection of drugs can cause seizurelike activity. This can be life threatening to the horse and is potentially injurious to the handler and other personnel in the vicinity. The chance for this can be minimized by using an 18-gauge needle that is unattached from the syringe and directed down the jugular vein. Normally, if the needle is in the jugular vein, blood will slowly ooze out of the needle hub only if the jugular vein is occluded. If the carotid artery is inadvertently entered with the needle, blood will exit in a pulsatile manner. If this occurs, do not inject the medication. The needle should be removed, and compression should be applied to decrease hematoma formation. The needle should be reinserted into a different location using the same technique.
ANESTHESIA FOR THE EQUINE PATIENT
The veterinary technician may be directly or indirectly involved in anesthesia of horses. Frequently the technician is primarily responsible for all aspects of anesthesia, including selection of induction and maintenance anesthetic agents, instrumentation, monitoring, and recovery of patients. It is important that the technician be familiar with the properties and recommended doses of the anesthetic agents administered and the equipment (ventilator, blood pressure monitor, anesthetic machine, etc.) used (see Chapter 27). Numerous complications can arise, and it is important that the technician be familiar with the methods of treating these complications, including the correct drugs and doses for treating hypotension and cardiac arrhythmias. Because it is important to maintain mean arterial blood pressure at 70 mm Hg or greater to help prevent myopathies and neuropathies, blood pressure should be monitored via an indirect or direct method. Hypotension is usually treated by decreasing the depth of anesthesia, increasing the rate of administration of IV fluids, and administration of vasoactive drugs, such as dobutamine, dopamine, or phenylephrine. It is important to monitor how well the horse is oxygenated and ventilated during anesthesia; this can be done most effectively by monitoring arterial blood gases. The technician should monitor recovery from anesthesia and be prepared for any potential complications.
SURGICAL NURSING OF FOOD ANIMALS
As in equine practice, veterinary technicians are vital team members in food animal practices. Their roles and responsibilities are identical to those in other fields of veterinary nursing; the principle goal is to safely provide optimal nursing care to patients. Familiarity with food animal species (the emphasis of this section of the chapter will be bovine) is important because they act and behave differently from each other and particularly from horses. To ensure personnel safety, the practice must be equipped with appropriate chutes and stocks so that animals can be examined and restrained. Additional equipment, such as tilt tables, will allow further surgical or diagnostic procedures to be performed, either using sedation or general anesthesia. This equipment is invaluable to allow veterinarians and technicians to examine and work on even the largest bulls in relative safety. Smaller stocks and restraint devices are available for small ruminants, but should only be used for these species.
PREOPERATIVE PREPARATION
In nonemergency situations, the following should be routinely performed before any surgical intervention. A physical examination must be performed, and a packed-cell volume (PCV) and total protein are recommended if there is a risk of significant blood loss or if general anesthesia will be used. Further blood work, such as a CBC and full biochemistry panel, is indicated for most complicated gastrointestinal disturbances since electrolyte abnormalities or infectious processes can influence the surgical outcome and postoperative convalescence. The position of the patient during surgery needs to be considered so that feed can be withheld for an appropriate time to reduce the risks of regurgitation and aspiration pneumonia. If the adult patient will be in dorsal or lateral recumbency or under general anesthesia, 36 to 48 hours of fasting is indicated. Because of the capacity of the rumen, water should be removed 12 hours preoperatively. For standing procedures, no fasting is required. IV catheterization should be performed shortly before surgery if a catheter is necessary. Either jugular vein can be used, but because of the thickness of cow skin, a stab incision is often made with a scalpel blade to prevent burring of the edges of the catheter. IV catheters can be displaced or pulled out, even when sutured or superglued to the skin. In most cases where IV fluid therapy is indicated, the cow will remain quiet and will usually not move around excessively or rub at the catheter site.
If antibiotics, analgesics, or anesthetics are to be used, care should be taken to ensure that all of the drugs administered are licensed for use in this species (refer to Chapters 25, 26, and 27). The technician and veterinarian must be conscious of withdrawal times for all drugs that are used because these may influence postoperative management. Penicillin or ceftiofur tend to be the most widely used antibiotics, and high doses of ceftiofur will provide reasonable Gram-negative coverage. Other alternatives include oxytetracycline, but it has only bacteriostatic properties. Flunixin is the only licensed NSAID; the use of phenylbutazone should be avoided. Anesthetic drugs are used off label in cattle (see Chapter 27).
Considering the environment in which cattle live, they are often dirtier or dustier than animals that are maintained on pasture. If possible, clipping the hair before entering the OR or area is preferable to minimize the risk of contamination of the surgery suite. Skin preparation is performed initially to remove any gross skin contaminants and dander, and immediately before surgery, a sterile prep should be applied with either povidone-iodine or chlorhexidine scrub solutions, using a standard technique, once the cow is restrained for the surgery (stocks, tilt table, etc.). Iodine scrub should be rinsed with alcohol, and chlorhexidine should be rinsed off with saline.
Preparation of the surgical room or area is important. Having all of the necessary supplies nearby will improve efficiency and prevent a surgery from beginning with some important instrument or piece of equipment unavailable. If the animal is to be recumbent for the procedure, adequate padding needs to be present for the patient to be placed on to prevent any anesthetic-related complications, such as a myopathy or myositis and neuropathies, (particularly radial nerve paresis or paralysis) from developing. If in lateral recumbency, the distal forelimb should be pulled cranially, and the uppermost hind limb should be elevated off the most dependant hind limb by pads or a bale of straw. Even with appropriate padding and support, complications can develop and need to be addressed.
Surgical preparation for the veterinarian must include an appropriate surgical scrub of their hands and forearms. Sterile gloves should be worn, and, where possible, the patient should be draped, and the surgeon should be wearing a sterile gown, cap, and mask.
CONDITIONS OF THE GASTROINTESTINAL TRACT
Abnormalities in the gastrointestinal tract make up the majority of surgeries performed in cattle. For a discussion of actinomycosis and pharyngeal injuries, refer to Chapter 22.
ORAL LACERATIONS
Cattle, especially calves, are not discriminate eaters and often consume debris, such as hardware, found in the pasture. Lacerations or injuries can occur on the tongue, cheeks, or palate when pieces of wire or other sharp objects are chewed and potentially swallowed. Excess salivation or bloody oral discharge may be seen along with varying degrees of dysphagia and malodorous breath if feed is accumulating within the wound. Diagnosis is usually easy once an oral examination has been performed. With appropriate wound care, many of these will heal without much treatment because of the excellent blood supply to the oral cavity. Flushing with copious amounts of water (usually via a hose) will prevent feedstuff accumulating within the wound. Changing the diet to softer feed rather than course roughage will limit feed buildup within the wound. Severe lacerations or punctures may require surgical débridement and repair, and if the tongue is involved, application of a tourniquet may help limit blood loss during the repair.
MANDIBULAR FRACTURES
Fractures are either traumatic or secondary to other pathologic processes, such as lumpy jaw. Fracture location, type, and the ability of the animal to eat and drink will usually dictate whether surgical repair is necessary. Clinical signs observed include dysphagia, salivation, and often crepitus or palpable instability. Oral examination is also important to identify any communication with the oral cavity because this is a route for secondary bacterial infection, which can complicate the healing process. Radiographs will help confirm the diagnosis and aid identification of the optimal therapy. If there is marked displacement or instability of the mandible and if the animal is having difficulty eating and drinking, some form of stabilization is indicated.
Surgical options include figure-eight wiring with orthopedic cerclage wire, screw fixation, or application of an external fixation device. The choice is made depending upon the location of the fracture, the degree of comminution, involvement of tooth roots, if the fracture is open or closed, and the cost of the procedure. External fixators are inexpensive, effective, and relatively easily applied.
Most fractures will heal with some form of stabilization, and many cows will resume eating immediately following fixation. If the cow remains anorexic, offering different types of feed is recommended because some cows will only eat their usual ration. If necessary, the oral cavity can be bypassed completely by performing a rumenostomy. Potential complications of mandibular fractures include osteomyelitis, sequestrum formation, or abscessation of involved tooth roots. Failure or loosening of the implants is often not a problem because these injuries will heal rapidly. Removal of the implants can be performed using sedation once healing has occurred.
LAPAROTOMIES
Numerous surgical approaches to the bovine abdomen have been described, and all have various pros and cons. The majority of procedures can be performed via a flank laparotomy in the standing patient, but ventral midline or paramedian approaches are necessary in specific situations. Clipping a much larger area than is necessary will prevent any problems if the incision has to be extended and will limit any contamination of tissue if any drapes used should slip. Local anesthetic techniques can be used to facilitate surgery in the standing patient. IV sedation is sometimes required so that the patient tolerates this, but once the area is successfully anesthetized, most patients will settle down, often to the point that some will begin to ruminate.
REGIONAL ANALGESIC TECHNIQUES FOR ABDOMINAL SURGERY
For a flank incision, lidocaine can be injected directly over the line of the incision. Local anesthetic must be placed under the skin, into the muscle, and to the level of the peritoneum if complete analgesia is to be achieved. This is effective and simple, but may lead to an increased risk for incisional complications, such as infection or dehiscence. An alternative that does not involve injection directly into the surgical site is an inverted-L block. Local anesthetic is injected into all of the layers of tissue in an inverted-L pattern, vertically behind the last rib and then horizontally below the transverse processes of the lumbar vertebrae. This blocks the nerve fibers at a site distant to the location of the incision, and for this reason, the block must be continued at least a few centimeters distal to and caudal to the edge of the incision. If these blocks do not provide adequate analgesia, more local anesthetic can be placed easily and quickly, but since a large volume of local anesthetic can be used, care must be taken not to exceed the toxic dose of lidocaine (6 to 8 mg/kg).
Paravertebral analgesia can be performed, which requires the use of less local anesthetic and can provide a larger area of surgical analgesia than the other techniques. Two techniques are described, and the simplest to perform in dairy cattle (tend to be thinner and the landmarks are easily palpable) is the distal paravertebral technique. Here the transverse processes of lumbar vertebrae 1, 2, and 4 are palpated through the skin. An 18-gauge, 1½-inch needle is then inserted completely so that it lies parallel to and just below the palpable tip of the transverse process of L1. About 20 ml of 2% lidocaine is injected in a fan pattern, then the needle is withdrawn so that it can be relocated in the same fashion above the transverse process. Ten to 15 ml of lidocaine can be injected in the same fanlike pattern in the area. This process is repeated over the transverse processes of L2 and L4. This technique anesthetizes the nerves T13, L1, and L2 at a site distal to where they exit the vertebral column.
The proximal paravertebral technique requires a longer needle; an 18-gauge, 5-inch spinal needle passed through a 14-gauge, 1-inch guide needle is used for this technique. The 14-gauge needle is placed 2 cm lateral to midline at the cranial edge of the transverse process of L1. The spinal needle is then inserted through the 14-gauge needle down onto the transverse process. The spinal needle is then walked cranially, off the edge of the process. A “pop” will be felt as the needle penetrates the thick fascial layer, and 15 ml of lidocaine should be injected in this location. Withdrawing the needle roughly 1 to 2 cm will place it above the fascia, and another 15 ml of lidocaine should be injected at this site. The technique should be repeated over the transverse processes of L2 and L3.
Successful placement of either paravertebral block will result in analgesia of the flank, but this can take between 15 to 30 minutes. This can be confirmed by observing scoliosis (the side that is blocked will relax, making the spine bend laterally the other way); vasodilation, which will make the side palpably warmer (particularly in cool weather); and a lack of response to a noxious stimuli. Incomplete analgesia may resolve if more time is allowed for diffusion of the anesthetic to occur.
SURGICAL APPROACHES TO THE ABDOMEN
Most abdominal exploratory laparotomies are performed through the right flank (Figure 31-16). This approach provides access to most of the abdominal viscera, although only certain structures can actually be exteriorized. The proximal extent of the incision is approximately one handbreadth below the transverse processes and one handbreadth behind the caudal edge of the ribs. A 15-cm vertical incision will allow passage of an arm into the abdominal cavity. Keeping the incision located higher in the flank is desirable to limit the ability of intestine to prolapse out of the incision. Procedures commonly performed from the right flank include abdominal exploratories and correction of abomasal displacements and torsions. An approach through the left flank is used for C-sections, rumenotomies, or rumenostomies.
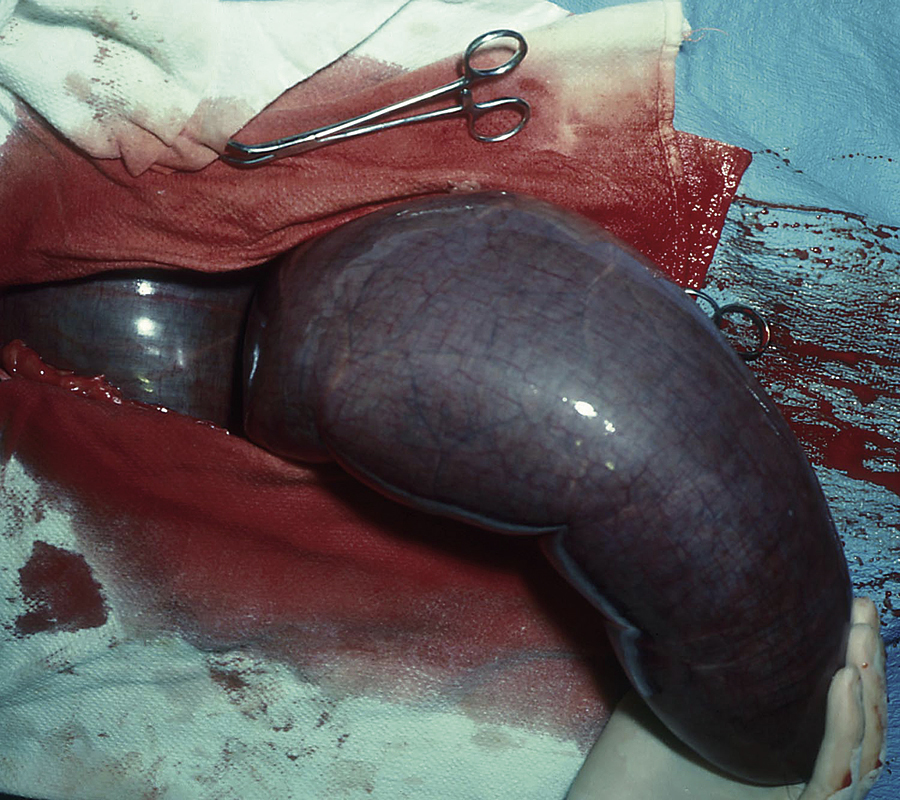
FIGURE 31-16 A right flank laparotomy approach for correction of an intestinal obstruction (cecal volvulus) in a cow.
Certain procedures may require a ventral midline or paramedian celiotomy. Sedation is essential for these as is some form of restraining device to maintain the cow’s position. Tilt tables can be used, or the cow can be propped up in dorsal recumbency using bales of straw or ropes. Abomasopexies, C-sections, or umbilical procedures sometimes require this approach. Instead of paravertebral analgesia, local infiltration is often used or lidocaine is infiltrated on either side of the incision with a portion that converges together at the most cranial aspect of the incision.
RUMENOTOMY FOR GRAIN OVERLOAD
A rumenotomy is performed via a left flank laparotomy with the cow standing. A broad area should be clipped and prepped for surgery. The skin incision is oriented vertically and often needs to be bigger than usual flank laparotomy incisions (may need to be 20 cm) so that the rumen can be exteriorized and then secured. The rumenotomy site is the dorsal sac of the rumen. This is secured using one of two recommended rumenotomy procedures: use of a rumen board (Weingarth apparatus) or suturing the rumen wall to the skin. This is important to prevent contamination of either the peritoneal cavity or the skin and muscle layers with rumen contents. Ensuring that enough rumen is exposed beyond the site of fixation will help make closure easier. The contents of the rumen have to be evacuated manually, which is time consuming. Having specially designed feed bins to place the rumen contents into can be helpful. A normal trash can with multiple holes drilled through its bottom and a chicken-wire mesh insert to hold fiber in the can be used to allow fluid to drain out and hold in most of the fibrous portion of the rumen contents. This will make movement and subsequent disposal of the rumen contents easier. Siphoning off the fluid can be performed with a larger-bore tube, such as a Kingman tube, placed into the rumen via the rumenotomy. Once the rumen is emptied, transfaunation of ruminal fluid from a normal cow can be beneficial. This can either be given orally or via the rumenotomy before closure. Removing any gross contamination from the edges of the rumenotomy is important before suturing it closed in two layers. The body wall and skin can then be closed in a routine fashion.
A rumenostomy, or permanent rumen fistula, can be created using a similar technique. Commercial rubber rumen fistulae and plugs are available, and if one of these is to be used, close attention to the size of the surgical incision is necessary. The skin incision needs to be longer and should be about 15 cm. This will allow the fistula to form a tight seal and prevent leakage of rumen contents. The skin incision is continued through the external abdominal oblique muscle; then the deeper muscles are separated in the direction of their fibers. Following opening of the peritoneum, a three-layer closure technique can be used. The peritoneum is sutured to the abdominal muscles using an absorbable suture. Following exteriorization of part of the rumen, the rumen is sutured to the subcutaneous tissue, then the rumen is incised and the mucosa is sutured to the skin. Ensuring that the second layer is tight before incising the rumen will help minimize the risk of contaminating the abdomen with rumen contents. Placement of the rubber fistula and plug will now allow the rumen to be sealed, but accessible when necessary. This is usually used for research purposes, but can be helpful when treating sick cattle because it makes collection of ruminal contents for rumen transfaunation considerably easier.
TRAUMATIC RETICULOPERITONITIS
For the diagnosis and medical treatment for this condition, please see Chapter 22. Surgical intervention may be necessary if traumatic reticuloperitonitis (TRP) fails to respond to conservative treatment, if a foreign body is observed outside the reticulum on radiography, or if an intraabdominal or thoracic abscess is suspected. The approach of choice is a left flank exploratory laparotomy and rumenotomy using transruminal exploration. The technique required for rumenotomy is the same as that described for treating grain overload, with the exception that the incision is located a little more cranially to ensure that the reticulum can be reached by the surgeon. Most abscesses that form secondary to TRP are located on the medial wall of the reticulum and are usually tightly adhered. These abscesses are the most common causes of vagal indigestion associated with TRP. These tightly adhered abscesses can be lanced and drained into the reticulum or omasum and then explored for the presence of a foreign body. Lancing these abscesses can be difficult and requires a blind technique. Providing a scalpel blade on a loop of suture will allow the loop to be placed over the surgeon’s wrist to reduce the risk of dropping it into the rumen.
ABOMASAL DISPLACEMENTS AND VOLVULUS
Abomasal displacement is a common problem of high-producing dairy cows fed high-concentrate, low-roughage diets. Displacements are most likely to occur in the first 6 weeks after calving. Predisposing factors include high-grain diets with an increased amount of volatile fatty acids in the abomasum; hypocalcemia; concurrent diseases, such as mastitis, metritis, and ketosis; and lack of exercise. Increased volatile fatty acids, histamine release with concurrent diseases, and hypocalcemia may lead to abomasal dilation and atony and subsequent displacement of the abomasum from its normal right paramedian position to the left or right paralumbar area. Left displaced abomasum (LDA) is much more common than right displaced abomasum (RDA). Abomasal volvulus (AV) may occur after displacement to the right and carries a poorer prognosis than LDA or RDA because of compromise of the innervation and blood supply that occurs when the abomasum twists. AV can quickly lead to development of shock and toxemia if not diagnosed and treated early.
LDA, RDA, or AV can be diagnosed by auscultation of a distinct “ping” in the left or right paralumbar fossa area since the gas trapped in the abomasum produces a characteristic “metallic pinging” sound when percussing the area over the gas cap while simultaneously auscultating with a stethoscope.
The Liptak test can also be used as an aid in diagnosing LDA. After percussion of the abomasum on the left side under the last few ribs, an area just below the gas ping, which corresponds to the fluid level in the abomasum, is clipped and surgically prepared. Centesis is performed using an 18-gauge, 10- to 12-cm needle. Fluid with a pH less than 4.5 confirms the presence of an LDA. Aspiration of gas with a characteristic “burnt almond” odor is indicative of an LDA.
Surgery is usually necessary to correct abomasal displacements, and AV requires immediate surgical intervention. The choices of surgical approach and technique depend on the direction of the displacement, the presence of volvulus, the condition of the animal, and the surgeon’s preference. Traditionally a right flank laparotomy is performed to correct the displacement. The most helpful piece of equipment is a piece of surgical tubing (must be long enough to reach to the far side of the cow and then extend well clear of the surgical incision) attached to a large needle. This is taken into the abdomen, guarded by the surgeon’s hand, and carried round the caudal edge of the rumen and omentum. This is then tunneled through the abomasal wall to decompress it. Once decompressed, the abomasum can be swept or pulled back to the left side where either an omentopexy or pyloropexy can be performed to the abdominal wall. A right sided displacement or volvulus needs to be decompressed and repositioned before pexying to the body wall. Surgical approaches using a left and right flank laparotomy or laparoscopic approaches have been described and are beyond the scope of this text. If a cow has had a recurrence of an abomasal displacement following a left-sided approach, a paramedian abomasopexy may be indicated. In this situation, the cow is sedated and rolled into dorsal recumbency either on a tilt table or by casting her with ropes. The ventral abdomen is clipped and prepped for a paramedian incision roughly 10 cm behind the xyphoid process and 10 cm to the right of midline. The surgical site is anesthetized using local infiltration of local anesthetic and the site is sterilely prepped. An incision is made into the body wall, and the abomasum is exposed through this incision. An abomasopexy is performed, by suturing the abomasum and the body wall closed simultaneously, resulting in a strong adhesion that will prevent future displacements. Postsurgically the diet should be restricted to roughage only, and grain should be introduced gradually into the diet once recovery is complete.
AFTERCARE FOLLOWING A LAPAROTOMY
Feed can be reintroduced once the cow has recovered from any sedation or general anesthesia. It is usually ideal to withhold feed and water until they are no longer showing any signs of sedation, which with xylazine can be prolonged. The goal is to return them to full feed, but this should be done gradually over a few days. Cows that are relatively healthy will often eat well after surgery and will require little further management. If they have been anorectic for a prolonged period, offering different feed is important to try and tempt the cow to eat something. If necessary, force feeding can be performed via a large-bore stomach tube. Slurries made from alfalfa meal or a pelleted feed can be used, but can be difficult to pump through the tube. Constant stirring or increasing the fluid content of the feed can be helpful to ease its passage through the tube. Rumen fluid transfaunation can also be helpful for these cows, if possible. Keeping the cow in a well-bedded, dry, draft-free environment will ensure that they are comfortable and should help them to recover. The incision will usually need little if any care. An infection of the surgical site can be identified by swelling, discomfort upon palpation of the area, and usually some discharge. Flank incisions can be treated by creating ventral drainage and allowing any purulent material to drain out. This is often all that is needed, and the incision will heal by second intention with routine wound management.
OTHER GASTROINTESTINAL CONDITIONS
The remainder of the gastrointestinal tract can also require surgical therapy. In the small intestine, intussusceptions, volvulus, or hemorrhagic bowel syndrome can occur. Cecal tympany or displacement can sometimes be mistaken for an RDA (ping in this case will be further caudal and dorsal on the flank than for an abomasal problem), or this can even become intussuscepted into itself. Obstructions or intussusceptions of the spiral colon can also occur. These often are evaluated surgically, and an approach through the right flank is most commonly used. Exposure of the entire gastrointestinal tract is not always possible, so if the incision needs to be elongated ventrally, general anesthesia may be indicated to prevent sudden movement or the cow becoming sternal, which could allow (uncontrolled evisceration) parts of the intestines to fall onto nonsterile surfaces.
Adhesion formation can be a sequela of abdominal surgery or following a separate disease process. This will often lead to signs of an abomasal outflow obstruction, general colic, or weight loss. An exploratory laparotomy can be performed to evaluate this, but treatment options are limited. Adhesions can be broken down, but the risk of recurrence is high.
CONDITIONS OF THE MUSCULOSKELETAL SYSTEM
Lameness is commonly encountered in cattle and is most often (88%) caused by lesions or problems in the foot. The majority of these conditions have been discussed in Chapter 22. Upper leg problems, such as anterior cruciate ligament rupture, coxofemoral (hip) luxation, fractures, and arthritis, account for the remaining 12% of lameness seen. When foot problems occur, they are most often seen in the claws that bear the most weight, front medial and hind lateral claws. It is important to examine all foot problems early since many conditions can progress to osteomyelitis and/or septic arthritis if not properly treated. Regardless of the cause of lameness, it can lead to loss of production as a result of decreased milk production, weight loss, delayed breeding or anestrus, and culling. Intensive housing and feeding of large groups of animals has lead to an increased incidence of lameness.
REGIONAL ANALGESIA AND ANTIBIOTIC PERFUSION TECHNIQUES AND PMMA IMPLANTS
Regional analgesia (IV retrograde analgesia) of the foot and/or distal limb is commonly performed before claw amputation or corn removal, although the regional block can also be used for other surgeries or painful techniques of the foot or distal limb or as a diagnostic aid in lameness examinations. A tourniquet is applied distal to the hock or carpus in the midmetatarsal or midmetacarpal area. A superficial vein is located, either the common dorsal metacarpal (metatarsal) vein or the palmar or plantar metacarpal (metatarsal) vein, and surgically prepared. An IV injection of 15 to 30 ml of 2% lidocaine will provide analgesia in 5 minutes, which will persist until the tourniquet is released (the tourniquet should not be left in place more than an hour) (Figure 31-17). It is ideal to have the cow’s leg secured to prevent extravascular placement of the local anesthetic. Even with restraint, movement is often still possible, so the use of a butterfly catheter with a short extension can be helpful. An alternative to this technique involves placing the tourniquet above the tarsus or carpus to achieve analgesia more proximally. Accordingly, more lidocaine should be used to block this larger area (may need 30 to 60 ml of perfusate). This same technique can be used to perform regional perfusion of antibiotics to the distal limb for the purpose of treating localized infections, such as foot rot with cellulitis. An antibiotic with an IV formulation should be used and can be diluted with sterile saline to produce a perfusate volume of at least 20 to 30 ml. Cephalosporins can be injected through the catheter and allowed to perfuse the limb distal to the tourniquet for approximately 45 minutes. Both of these techniques are primarily used in cattle, but can be applied to all food animal species. The implantation of antibiotic-impregnated polymethylmethacrylate (PMMA) beads subcutaneously near infected joints has shown promise for treatment of septic arthritis in calves. The beads slowly release antibiotics into the joint and appear to be more effective than systemic antibiotic treatment or joint flushing alone. Techniques such as joint flushing, PMMA bead implants, regional perfusion, and systemic antibiotics used in combination work well to resolve septic arthritis. Following resolution of the sepsis, the beads can be removed, but should not cause any problem if left in place.
SURGICAL DISEASES OF THE HOOF AND PHALANGES
Interdigital hyperplasia (interdigital fibroma, corn) is a thickening of the interdigital skin, which causes a mass to protrude between the claws (Figure 31-18). One or more feet may be involved, but the hind feet are more commonly affected. Beef breeds, especially bulls, have a higher incidence of corns. Fibromas develop in response to chronic irritation between the claws. Hereditary predisposition is suspected. Spreading of the toes and other conformational problems probably contribute to irritation of the interdigital skin.
The size of the mass varies from a noticeable thickening of the skin to a size of 3 cm or more. A large mass can cause pain, and the fibroma may become eroded, ulcerated, and even infected leading to more swelling and pain. Lameness varies, depending on the size of the mass, from absent to severe. The size of the corn and degree of lameness are guides in determining whether removal is necessary. Surgical excision is accomplished using IV retrograde analgesia, and care should be taken to ensure that all of the hyperplastic tissue is excised. Excision of part of the fat pad in the interdigital space will also help improve wound healing by preventing the fat from protruding between the healing skin edges. To reduce the discomfort associated with the outward displacement of the toes, the toes can be wired together by drilling holes at the toe region and securing them using cerclage wire. Placement of the wires on the abaxial side of the claws will reduce the risk of the wire pulling through the hoof capsule prematurely.
Claw Amputation
Diseases of the foot may become so severe that they cannot be treated, thus necessitating amputation of the affected digit. Any of the previously discussed conditions of the foot (see Chapter 22) and other diseases may lead to infection of deeper tissues with resulting osteomyelitis of the first phalanx (P1), second phalanx (P2), or third phalanx (P3) and/or septic arthritis of the proximal or distal interphalangeal joints (pastern or coffin joint). In cases of advanced infection, removal of the infected claw may be the only treatment option. This procedure is performed with IV retrograde analgesia (described earlier). A full-thickness skin incision is made on the axial aspect of the claw, perpendicular to the bone’s long axis, down to the bone of the distal first phalanx. The affected claw is then removed at a level necessary to remove all infected tissue using OB wire (it should be noted that amputation can only be performed distal to the fetlock joint). The claw should be removed at a cosmetic angle and through bone rather than through a joint. The foot is then bandaged snuggly after topical antibiotic application. Bandage changes should be done every 3 days until any exposed bone is covered with granulation tissue. The entire healing process takes about 6 weeks. Cattle can support weight on one claw, but will eventually experience breakdown of supporting structures of the remaining claw. The time it takes for breakdown depends on the claw removed, the weight and use of the animal, and the surface on which the animal must stand. It is undesirable to remove any claw in a bull, except for salvage purposes, and it is not generally a good choice to remove a weight-bearing claw (front medial or hind lateral) in cows, although sometimes there is no choice.
Foot Block Application and Casting
Wooden or acrylic blocks or commercial rubber shoes may be glued to the bottom of healthy claws to reduce or eliminate weight bearing on a diseased or painful claw. These devices are adhered to the claw using an acrylic material (Technovit, Jorgensen Laboratories, Inc.) or products, such as Equithane adhesives. Wooden blocks may last as long as 6 weeks and may be allowed to wear off naturally unless they are wearing abnormally, in which case they may be manually removed earlier. Blocks or shoes help promote healing of affected claws and make the animal more comfortable when standing or moving.
An additional method of immobilizing the claws is application of a hoof cast. A wound dressing should be applied to the surgery site, if one is present, and a layer of cast padding material should be placed between the claws. The claws can be wired together, stockinette can be applied up to the distal metacarpal or metatarsal bone, and then cast padding material can be applied to the proximal extent of the area to be cast (usually level of mid-P1). This material should overlap approximately 50% with the previous layer. Fiberglass cast material can then be applied to immobilize the distal limb. Applying the material while it is still wet will prevent it from curing too rapidly because this can prevent it from adhering to itself, which can result in a weaker cast. The cast can remain in place for 10 to 14 days at which time it should be removed. Cast removal can be performed with a cast saw or using Gigli wires if they were placed between the cast padding and the cast tape at the time of application.
Obturator and Sciatic Nerve Paresis and Paralysis
The obturator and/or sciatic nerves may sustain damage during dystocia or forced fetal extraction resulting in “calving paralysis.” Treatment consists of the use of NSAIDs early, good nursing care, housing the animal on a soft surface with good footing, flotation devices, lifting the cow or heifer for short periods of time at least a couple of times a day, rolling the cow from side to side several times daily to prevent severe muscle compression, and hobbling cows or heifers that can stand, but cannot adduct their hind legs.
Radial Nerve Paralysis
Radial nerve paralysis is most commonly seen following a period of lateral recumbency with the most dependent forelimb being exposed to either excessive or prolonged pressure (the weight of the animal itself can be enough) over the upper forelimb. The most obvious clinical sign is an inability to bear any weight on the affected limb if an attempt to stand is made. The elbow will appear to be dropped, and the cow will be unable to maintain its carpus or elbow in extension, preventing weight bearing. This often does not appear to be painful, but can be stressful if multiple attempts to stand or move are made. Treatment is supportive. Systemic NSAIDs should be administered, and a Robert Jones bandage and splint should be applied to the limb. The bandage should extend from the proximal radius to the hoof, and the splint can be placed on the dorsal or palmar surface. The goal is to lock the carpus in extension with the splint, which will allow weight bearing to resume while limiting mobility. This is often all that is necessary, and this condition usually will show signs of improvement within 24 hours of onset. The duration of neurologic deficits will depend on the severity of injury, but a full recovery is usually possible. The most important other cause of radial nerve deficits is an upper limb fracture (humerus, proximal radius or ulna), but these cases can often be distinguished because there is marked local swelling, pain upon palpation, or manipulation and often crepitus.
Fractures
Trauma, either from a kick or a fall, can result in long-bone, spinal, or pelvic fractures. An acute-onset, severe lameness is observed, with the animal often either non–weight bearing in the affected limb or recumbent. Soft tissue swelling tends to be marked, and as a result of limited soft tissue coverage, many fractures will be open. Before attempting any fracture repair, the weight, size, age, disposition, and financial value of the animal need to be taken into consideration. Radiographs should be taken to allow accurate classification of the fracture (displaced or nondisplaced, open or closed, simple or comminuted). The amount of injury to the associated soft tissues can delay healing if the vasculature has been compromised, or if excessive tissue is injured, it can predispose to infections. Some form of support should be applied to the limb until fixation is attempted, and these can range from a Robert Jones bandage with or without splints, cast material, or for some fractures, simply stall confinement (femur, humerus, and pelvis).
Feed should be withheld if anesthesia is going to be necessary for fracture stabilization, systemic antibiotics should be initiated, and analgesia should be provided. If the cow is dehydrated or showing signs of shock, IV fluids may be administered via a jugular catheter.
Fracture stabilization can be achieved using either internal fixation devices (screws and bone plates) or using external fixation (casts, Thomas splints, or transfixation casts). If stabilization is adequate and no infection is present, the bones will often be able to heal with time.
Osteomyelitis should be considered if following fracture stabilization there is a sudden increase in the degree of lameness. Signs of inflammation and swelling are often present at the surgical site (assuming this is visible and not underneath a cast), but radiographs are often necessary to confirm the presence of infection. Radiographic evidence of osteomyelitis is usually only observed once the infection is well established, roughly 10 to 14 days after its onset. Systemic or local antibiotics should be administered, drainage should be established to prevent accumulation of purulent material, and in severe cases, internal implants may need to be removed or replaced. Before radiographic changes become evident, the patient may be febrile or have an altered leukogram. Aggressive therapy is necessary if there is to be any chance of success, but the prognosis is often poor.
Strict stall rest until radiographic evidence of fracture healing occurs is necessary, and the patient may still be reluctant or unable to move easily. Ensuring that the cow has easy access to feed and water is essential to prevent it from struggling or moving excessively and risking damage to the healing bone. Keeping these cows on deep bedding is important to reduce the risk for decubital ulcers, so rolling the cow may be required to prevent or reduce their occurrence. Excessively deep bedding can actually make it more difficult for the cow to stand or move, especially if they are wearing a cast. Where possible, slings or flotation devices may be helpful to prevent prolonged periods of recumbency.
Septic Arthritis
Septic arthritis is a common cause of severe, acute-onset lameness in cattle. In calves, it is usually secondary to an infection elsewhere in the body and spreads hematogenously. In adults, it is often secondary to either a puncture wound or trauma. Diagnosis is based on severe lameness, local swelling, and often an obvious wound. Confirmation of synovial involvement can be obtained by performing a sterile arthrocentesis at a site distant from any puncture or wound. If possible, a sample of the synovial fluid should be obtained for cytology, white blood cell count, total protein, and culture and sensitivity. In a septic joint, the white blood cells will be preponderantly neutrophils (greater than 75%), the white blood cell count is greater than 3000/μl, total protein is greater than 40 g/L, and bacteria may or may not be cultured. Attempts to distend the joint with saline will not succeed if the joint is open and it communicates with a wound. In this case, when saline is injected, it will be seen exiting the joint via the wound, confirming involvement of the joint. If the joint is intact, sterile saline can be injected into the joint to distend it, and the increase in pressure should allow the syringe to fill back up if it remains attached and pressure is removed from the plunger. Care should be taken when identifying a site for arthrocentesis so as to prevent iatrogenic infection of the joint; the needle should not be passed through any infected tissue. Identification of infected tissue can be difficult, so if there is any doubt, arthrocentesis may be contraindicated.
Treatment revolves around removing the bacteria and inflammatory mediators from within the joint. Flushing the joint using sterile fluids and through-and-through lavage with multiple needles placed within the joint can be performed under sedation. Large needles often need to be used (14 to 16 gauge) because smaller needles will often become blocked by fibrin or other debris from the joint. If this occurs or the infection is chronic, arthrotomies may be necessary to establish adequate drainage. These may need to be protected by bandages or stent dressings between joint flushes. If arthroscopic lavage is performed, general anesthesia may be indicated to prevent damage to the equipment. Following lavage, antibiotics can be placed directly into the joint to achieve high concentrations that will persist. Ceftiofur or penicillin can be used for this purpose. Joint lavage is usually performed once daily and should be repeated until no lameness is evident. Other cost-effective methods of antibiotic delivery include regional IV antibiotic perfusion or insertion of PMMA antibiotic-impregnated beads. These techniques are beneficial, but if they cannot be used, systemic antibiotics should be administered in conjunction with lavage. In joints that do not respond to therapy or that develop secondary osteoarthritis following resolution of the infection, arthrodesis or claw amputation may be treatment options to be considered to try and improve the level of comfort.
CONDITIONS OF THE RESPIRATORY SYSTEM
Surgical conditions of the respiratory tract are rare in cattle. Tracheostomies may be the most useful techniques for this body system, but they will be performed infrequently. Any obstruction of the upper respiratory tract may necessitate this, and surgical preparation, such as clipping, cannot often be performed. In true emergencies, reestablishing a patent airway is more important than a neatly clipped or scrubbed surgical site. The most appropriate location to perform a tracheostomy is on the ventral midline, roughly halfway between the larynx and the thoracic inlet. The ability to palpate the tracheal rings in this region that have the least soft tissue overlying them will help locate the ideal surgical site. Placing a line block with local anesthesia under the skin will aid the procedure. If possible, the trachea should be grasped with one hand through the skin and stabilized before incising the skin over it (about 8 to 10 cm). Ensuring that the incision is on midline will expose a muscle layer that should be split, either sharply or bluntly, until the trachea is exposed. The trachea should be opened by making a stab incision (just big enough to insert a tracheostomy tube) into it through the membrane between the cartilaginous tracheal rings. A temporary tracheotomy tube, piece of stomach tube, or other semirigid, hollow piece of tubing can then be used to establish a patent airway. If the skin or deeper incision is off the ventral midline, exposing the trachea can be difficult, and other vital structures can be damaged (jugular veins, carotid artery, etc.). In most situations, a temporary tracheotomy should be performed, and a permanent tracheostomy can be performed, if indicated, in a more controlled manner at a later time.
The tracheostomy site will need to be cleaned frequently because the majority of the normal respiratory secretions will exit through this site. Application of petroleum jelly on the skin around the site will prevent scalding. Following resolution of the inciting cause, the tube can be removed, and the tracheostomy site will heal by second intention. A permanent tracheostomy can be performed in a similar location, but instead of incising between the tracheal rings, the opening into the tracheal lumen removes a portion of three to four tracheal rings to allow the tracheal mucosa to be sutured to the skin edges. Aftercare of this stoma is the same as for a temporary tracheostomy.
CONDITIONS OF THE UROGENITAL SYSTEM
ANESTHESIA OF THE UROGENITAL AND REPRODUCTIVE SYSTEM
A caudal or low epidural provides loss of sensation to the anus, vulva, perineum, and caudal aspects of the thighs. It is used for relief of tenesmus and obstetric straining; vaginal, rectal, and uterine prolapse repair; and surgical procedures of the perineal area. Injection is made between the first and second coccygeal (Cy1 to Cy2) vertebrae or in the sacrococcygeal space. The space is located by moving the tail up and down and while palpating for the first obvious articulation caudal to the sacrum. Using aseptic technique, an 18-gauge, 1½-inch needle is inserted through the space on the midline at a 10-degree angle (tip of needle directed cranially) until a drop of lidocaine placed in the hub of the needle is sucked into the space. An alternative approach involves insertion of the needle until it hits the floor of the spinal canal at which time the needle is backed out slightly to enter the epidural space. There should be no resistance to injection. The dose of lidocaine used is 0.5 to 1 ml of 2% lidocaine per 45 kg body weight with which the animal should have adequate analgesia but remain standing. If sedation is helpful or longer duration of action is indicated, xylazine can be given alone (0.05 mg/kg diluted to 5 ml in sterile saline) or in combination (use a lower dose, 0.03 mg/kg) with the lidocaine.
A bilateral, internal pudendal nerve block can be performed to facilitate examination or surgery of a bull’s penis. This nerve can be palpated via a rectal examination, on either side of the pelvic canal, dorsal to the pudendal artery where it is associated with the lesser sciatic foramen. Care must be taken to only inject the area around the nerve, and about 15 ml is injected in the region of the nerve and slightly caudally.
UROLITHIASIS
Urolithiasis is the result of formation of calculi (uroliths) within the urinary tract. The result of these uroliths varies from minor urinary tract irritation to complete obstruction of urine flow. The nonclinical manifestation of this condition is referred to as urolithiasis, whereas the clinical form is termed obstructive urolithiasis. When complete obstruction occurs, there is marked distention of the urinary bladder with eventual rupture of the bladder, the urethra, or both. Depending on the site of rupture, urine accumulates in the ventral subcutaneous tissues (urethra) or in the abdomen (bladder) resulting in swelling commonly referred to as water belly.
Although there is no sex predilection for development of calculi, males are much more likely to become obstructed as a result of the length, shape, and size of their urethra. The most common site of obstruction in cattle is the distal sigmoid flexure of the penis (Figure 31-19). Feedlot animals receiving grain rations with high phosphorus levels are predisposed to developing phosphate calculi.
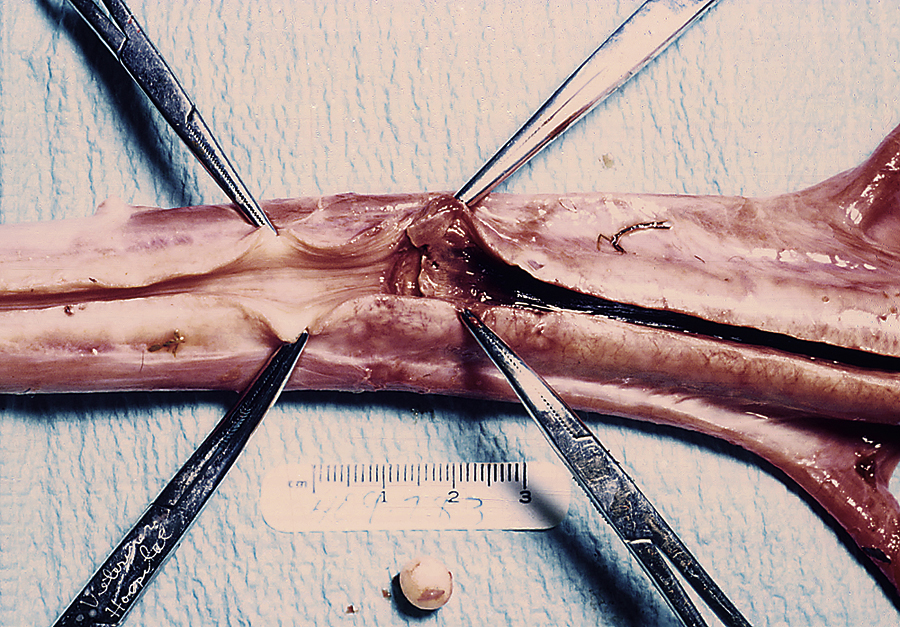
FIGURE 31-19 Identification of a calculus causing obstructive urolithiasis at the distal sigmoid flexure in a bull. Note urethral necrosis at the site where the calculus was lodged.
Clinical signs of acute urethral obstruction are attributable to trauma to the urinary tract epithelium and bladder distention. Early in the course, the animal repeatedly assumes a posture for urination, but little or no urination results from these attempts to void. As bladder distention progresses, the animal may tread, stretch, tail swish, and kick at its abdomen. Blood and/or crystals may be present on the preputial hairs. Nonspecific signs such as anorexia, mild bloat, and lethargy are also common. Owners often misinterpret these signs as evidence of acute gastrointestinal disorders, especially since affected animals may show signs similar to colic. After the bladder or urethra ruptures, straining ceases, and the animal may go through a brief phase of euphoria; however, azotemia (increased blood urea nitrogen [BUN] and creatinine) and dehydration quickly develop. If urethral obstruction is diagnosed early, before azotemia develops or before the bladder or urethra rupture, the animal may be immediately slaughtered. If not, medical and/or surgical treatment should be initiated quickly.
Medical management involves the use of muscle relaxants to facilitate passage of the calculi, antimicrobial therapy for urinary tract infection, and the use of a urinary acidifier, such as ammonium chloride. Medical therapy alone is rarely successful, so it may be necessary to consider surgery in some cases. Perineal urethrostomy may be chosen as a salvage procedure, particularly for feedlot steers or bulls of low economic value (Figure 31-20). This surgical approach allows for relief of obstruction and resolution of the uremia before slaughter. Long-term, urethral stricture has been a problem with this technique. The procedure is performed with the animal standing and analgesia provided by a caudal or low epidural block.
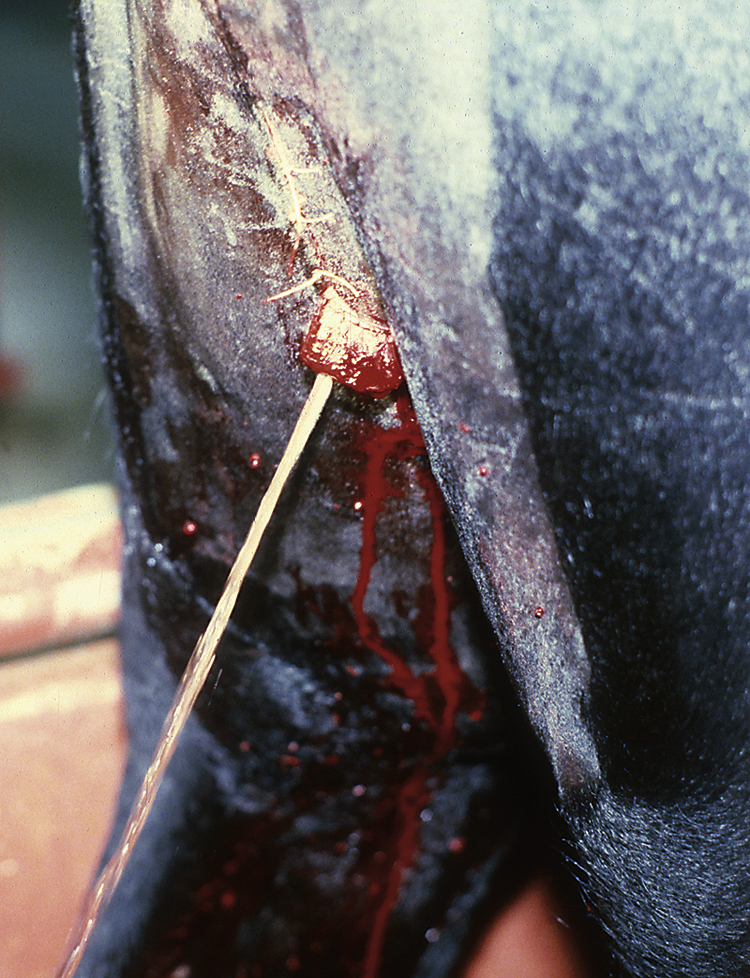
FIGURE 31-20 Perineal urethrostomy performed as a salvage procedure for treatment of obstructive urolithiasis in a steer.
The low approach is often preferred for perineal urethrostomy. This allows the penis to be diverted caudally at such an angle as to prevent urine scald to the hind legs. It also makes it possible to perform repeated procedures higher, if necessary. The skin incision is made beginning at the dorsal aspect of the scrotum or scrotal remnant and extending dorsally on the midline for 10 to 15 cm. The incision is continued until the penis is encountered. The retractor penis muscles may be the first structures seen and are frequently mistaken for the penis. These muscles are superficial to the penis, pink, soft, and easily separated into two structures. The penis is a relatively firm, single structure covered by the white tunica albuginea. Once the penis has been located, it is bluntly dissected from the surrounding tough fascia and pulled caudally out of the incision. The penis is transected at a length adequate to allow the transected end to exit the perineal incision without tension leaving a 2- to 3-cm stump exposed. The stump is sutured to the skin of the lower part of the incision using a mattress suture that surrounds the corpus cavernosum penis (CCP) and passes under the urethra. This suture limits hemorrhage from the CCP. In addition, the urethra can be split for several centimeters and spatulated by suturing the urethral mucosa and tunica albuginea to the skin using 2-0 or 3-0 absorbable suture material. This optional technique is intended to limit stricture of the urethral opening. The remaining skin incision is closed with simple interrupted sutures of nonabsorbable suture material. Urethral obstruction may recur as a result of additional calculi or because of stricture of the urethrostomy site.
An alternative surgical procedure that can be used for valuable breeding bulls or “pets” is the ischial urethrostomy, a temporary urethrostomy with catheter placement. This technique allows for urine egress through a Foley catheter placed in the urethra at the level of the ischium, just below the anus, and antegrade or retrograde flushing of the distal urethra to remove calculi. When the tube is pulled, the urethrostomy usually heals by second intention without stricture formation. This procedure can be useful for preservation of fertility in valuable breeding bulls.
Dietary management is the key to control and prevention of obstructive urolithiasis. The calcium to phosphorus ratio of the overall diet should be in the range of 2:1 to 2.5:1. A continuous supply of fresh, clean water should be available at all times, and salt may be added to the ration up to 4% to promote water intake and diuresis. In addition, vitamin A may be added to the ration to help prevent desquamation of epithelial cells in the bladder. Prophylactic use of urinary acidifiers is advocated; administration of ammonium chloride at 1% to 1.5% of the ration is recommended.
RUPTURED BLADDER
This is seen as a secondary complication of obstructive urolithiasis that is not treated promptly enough. In adults, this complication is diagnosed by the clinical signs of progressive abdominal distention, dehydration, anorexia, and depression. Confirmation often requires abdominocentesis, which produces a clear, yellow fluid with a peritoneal:serum creatinine ratio of 2:1 or greater. Urinary catheterization is reported to be an effective treatment to allow the bladder time to heal if a dorsal tear is present. Ventral tears usually require surgical repair, but gaining access to this region of the bladder can sometimes be challenging via a flank laparotomy, necessitating a ventral approach.
UROVAGINA
Older cows with poor perineal conformation or those in poor condition may be more susceptible to urovagina. Urine accumulates in the vagina, resulting in marked inflammation, which can lead to infertility. Performing a vaginal examination will reveal the presence of urine in the vagina, which is considered to be diagnostic. If the cow is to be treated, surgical correction is necessary, to either elongate the urethra or to elevate the transverse fold to redirect the urine externally. Anesthesia can be provided using a caudal or low epidural or by locally infiltrating lidocaine along the site of the incisions. Restraining the cow in stocks is preferable to limit their movement. Before beginning surgery, the rectum should be emptied, and the tail should be restrained so that it is clear from the surgery site. Self retaining retractors can be helpful for this procedure, or alternatively, stay sutures can be placed to hold the vulva open against the perineum. Adequate lighting is best provided by the surgeon wearing a head lamp. Other forms of lighting are difficult to aim properly, and the surgeon’s head usually obstructs the beam. Urethral extensions can be created by splitting the transverse fold, which is located above the urethral orifice in the vagina, and then creating a new shelf by continuing this incision laterally along the vaginal wall on both sides. Dissection of these shelves distally to allow them to meet in the midline with no tension is performed bluntly, and then the mucosal sheets are sutured in a Y pattern. Leaving a urinary catheter in place for a period postoperatively is helpful to ensure that urination can occur because some postoperative swelling may occur.
CONDITIONS OF THE REPRODUCTIVE SYSTEM
Removal of extra teats is of most value in young dairy heifers, but it is also performed in beef heifers intended for show. Often this procedure is performed at the time of brucellosis vaccination. The extra teats are removed flush with the skin and parallel to the normal folds of the udder using curved scissors. In young calves, suturing the skin is not usually necessary. Care must be taken not to remove any of the four normal teats.
OVARIAN DISEASE
Ovarian cysts, abscesses, or neoplasms occur in cattle. Other indications for ovariectomy include preventing pregnancy or to try and improve fattening of feedlot cows. To minimize the risk for hemorrhage, ovariectomy should ideally be performed when the ovary is in the follicular or early luteal phase. A flank approach is often used if a unilateral ovariectomy is performed or in situations where the ovary is enlarged. A colpotomy can be used for a bilateral ovariectomy if both are a normal size, but will require a caudal epidural. Achieving good hemostasis is essential, especially in pathologic ovaries. To minimize discomfort during this process, applying a gauze or lap sponge soaked in lidocaine to the ovarian pedicle may reduce any movement by the patient. This gauze or sponge should be attached to the surgeon to prevent it from being lost inside the abdomen. Holding this sponge over the pedicle for at least a minute may be beneficial. Transfixation sutures, application of a chain écraseur or emasculators can be used if a flank approach is used. Specific instruments, such as a Kimberly-Rupp or a Willis rod, should be reserved for normal ovaries.
Postoperatively, these patients should be monitored for signs of hemorrhage because inadequate hemostasis can be life threatening.
DYSTOCIA
C-section is indicated when there is a chance of delivering a live calf during a dystocia, malposition, or presentation or when the calf is excessively large. Several approaches are available for C-section, including flank approaches (right or left), paramedian approaches (right or left), or the ventral midline approach. The technique used is determined by the size, temperament, and physical condition of the cow and the veterinary surgeon’s preference. Most commonly an approach is made in the standing patient via an incision through the left paralumbar fossa, using the landmarks described earlier in this chapter for a right-sided flank laparotomy. One of the benefits of using a left flank approach is that the rumen will obstruct other viscera, such as small intestine, from eviscerating through the incision. This is of particular concern if the incision has to be extended distally as a result of the size of the fetus (this incision will be considerably larger than that necessary for an abdominal exploratory). The limbs of the fetus are used to maneuver the uterus to the body wall. The limb is then pulled through the incision and used to lock the uterus and calf in place. The uterus is then incised over the metacarpus or metatarsus, and the calf can be exteriorized with minimal abdominal contamination. Closure of the uterus is performed in two layers, with at least one layer in an inverting pattern. The uterus is then lavaged and replaced into the abdomen before routine closure of the laparotomy site. If available, oxytocin can be administered at this stage to aid uterine involution and to minimize hemorrhage. This will also encourage passage of the placenta, assuming that it has not been sutured during the uterine closure. If the calf is not viable or is emphysematous, alternative surgical approaches may be indicated, but are often limited by available facilities, equipment, or personnel.
UTERINE TORSION
This is an infrequent cause of dystocia, but, if present, will prevent parturition from proceeding. It is most commonly seen during early labor and is identified when an animal that is having a prolonged labor is evaluated rectally or rarely during a vaginal examination. The torsion is identified by the location of the broad ligaments because one of these is pulled tight as the uterus rotates away from its origin, whereas the other broad ligament is less taut. This also provides information on the direction of the torsion, either clockwise or counterclockwise (when viewed from behind the cow). If the calf’s limbs can be palpated through the cervix, attempts can be made to swing the calf and uterus to reduce the torsion. This can be difficult when the calf is large or the cow is small. Rolling the cow while placing pressure on the flank (usually with an assistant standing on a plank of wood placed over the flank and paralumbar fossa) to unwind the torsion can be attempted, but only if the direction of the torsion has been correctly identified. For example, to reduce a clockwise torsion, the cow should be placed in right lateral recumbency and then rolled onto her left side while pressure is applied to try and stabilize the calf. The rectal examination should then be repeated to see if the torsion has been reduced. If this is not successful, it can be repeated, but a flank celiotomy and C-section may be necessary.
VAGINAL AND UTERINE PROLAPSE
Vaginal prolapse is a fairly common occurrence in cattle (Figure 31-21). It usually occurs in pluripara cows during the last 2 months of gestation and tends to recur during subsequent pregnancies. Hereford, Santa Gertrudis, and Holstein breeds seem to be more commonly affected. Factors that influence the development of vaginal prolapse include increased estrogen levels in late pregnancy, increased fetal size with increased intraabdominal pressure in late pregnancy, bulky diets causing increased intraabdominal pressure, recumbency that forces the urinary bladder and other organs into the pelvic cavity and places pressure on the constrictor vestibuli muscle, and obesity with proliferation of pelvic fat (vaginal prolapse can occur in overconditioned, nonpregnant heifers). A new population of vaginal prolapse cows is emerging in embryo donor cows that are superovulated. Hormonal extremes are a suggested cause of vaginal prolapse in these cows.
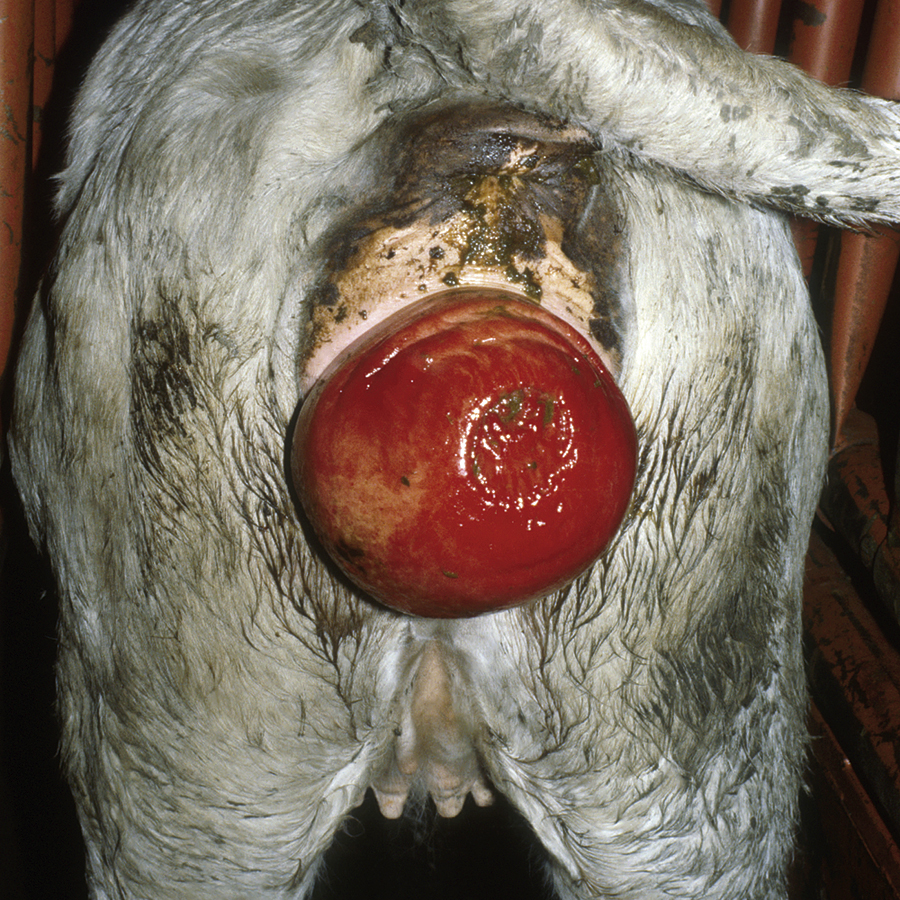
FIGURE 31-21 Vaginal prolapse in a prepartum cow. Recurrence during subsequent pregnancies is common.
Initially the vaginal prolapse may be intermittent, only protruding when the animal is lying down, but eventually it progresses to the point that it remains prolapsed at all times. Although vaginal prolapse is not an emergency, it should be repaired soon after it is noticed by the owner. The most common method of repair is the Buhner technique (see later). Since this condition usually occurs before calving, the cow will need to be observed closely for signs of impending parturition. Owners should be advised to cull these cows since this is likely to recur with subsequent pregnancies.
Uterine prolapse is common in the cow as a result of the anatomic suspension of the uterus (Figure 31-22). It occurs at parturition or shortly thereafter while the cervix is fully dilated. The uterus invaginates, and the uterine mucosa protrudes through the vulvar lips. Uterine prolapse is more likely to occur in first-calf heifers and is not likely to recur at subsequent calvings. Factors playing a role in the development of uterine prolapse include concurrent hypocalcemia, recumbency, such as that resulting from obturator nerve paralysis, dystocias with excessive straining, excessive force used during fetal extraction, and unnecessary traction on retained placentas.
Uterine prolapse is considered an emergency because of the possibility of shock from exposure of uterine mucosa, fatal hemorrhage from rupture of the middle uterine arteries, and concurrent hypocalcemia. In addition, the urinary bladder and/or intestines may also be involved within the prolapse.
To prevent uterine prolapse, it is wise to force the animal to stand as soon as possible after calving and administer oxytocin to begin uterine involution.
Treatment consists of replacement of the prolapsed portion and application of a retention suture (Buhner) to prevent recurrence. This is best accomplished with the animal in a standing position with the aid of a caudal epidural. If the cow is already down and cannot get up, attempt to elevate the hindquarters or pull the cow’s hind legs straight out behind her as she lies in sternal recumbency; an epidural helps maintain this position. Before replacement of the uterus, the placenta is removed atraumatically, if possible; the uterus is cleansed with warm water and a mild disinfectant; and the uterus is lubricated to facilitate replacement. Elevation of the uterus makes it easier to replace. The uterus is inserted a little at a time, making certain that the apical end of each horn has been completely returned to its normal position. Oxytocin (40 IU), calcium, if necessary, intrauterine antibiotics, and systemic antibiotics should be administered. The vulva is then sutured using the Buhner suture technique.
The Buhner suture technique is useful for retention of both vaginal and uterine prolapse. It is a buried purse-string suture that simulates the action of the constrictor vestibuli muscle. To begin the suture pattern, a 1-cm horizontal skin incision is made midway between the dorsal commissure of the vulva and the anus. Another horizontal incision is made at the ventral commissure of the vulva. The Buhner needle is inserted into the ventral incision, driven deeply (5 to 8 cm) and directed out of the dorsal skin incision. The eye of the needle is threaded with Buhner suture tape, and the needle is pulled out through the ventral incision. The procedure is repeated on the opposite side resulting in two free ends of tape from the ventral incision. The suture is tightened so that only two to three fingers can be inserted into the vagina. This allows for normal urination, but prevents reprolapse of the vagina or uterus. This suture may be completely buried and left in place indefinitely. The Buhner suture tape is particularly strong, will not disintegrate, and is well tolerated by the tissues. The tape may be tied such that it can be untied as the cow begins to calve, as in the case of prepartum vaginal prolapse.
FIBROPAPILLOMAS OF THE PENIS
Fibropapillomas of the penis in bulls are caused by the bovine papillomavirus (Figure 31-23). They tend to occur in young bulls housed together and are contracted from warts on other parts of the body when the bulls display homosexual behavior by “riding” each other. The warts may result in hesitancy or refusal to breed and may become large enough that they prevent extension (phimosis) or retraction (paraphimosis) of the penis. Surgical removal of the wart(s) is one treatment option and may be performed in conjunction with vaccination with either a commercial or autogenous wart vaccine. These can be removed in the standing animal using local anesthetic (either bilateral internal pudendal nerve block or anesthesia of the dorsal penile nerve) and restraint. The fibropapilloma can be excised, and the penile mucosa should be sutured using an absorbable suture. Recurrence is not uncommon.
PREPUTIAL PROLAPSE
Preputial prolapse tends to occur in bulls of Bos indicus influence (i.e., Brahman, zebu) as a result of several predisposing breed-related factors, including a pendulous sheath, long prepuce, large preputial orifice, and the absence of retractor prepuce muscles. The prepuce may become traumatized as a result of environmental exposure because of the inability of the bull to keep the prepuce within the preputial cavity, or trauma may occur as an accident during breeding. Once traumatized, the prepuce begins to swell and prolapses further, making it susceptible to further injury (Figure 31-24). The affected prepuce is treated initially by soaking in warm water with Betadine and Epsom salts to reduce swelling and control infection. The prepuce is returned to the preputial cavity and wrapped with a tube in place to prevent further trauma. Once the inflammation and infection are under control, surgery (reefing) to remove scar tissue and shorten the prepuce may be considered if the bull is valuable. Without surgery, recurrence rate for preputial trauma and reprolapse is high.
CONDITIONS OF THE OPHTHALMIC SYSTEM
OCULAR SQUAMOUS CELL CARCINOMA (CANCER EYE)
Ocular squamous cell carcinoma (OSCC), the most common tumor of cattle, is estimated to cause annual losses of $20 million in beef cattle in the United States. Losses result from condemnation of affected carcasses and loss of prime breeding stock.
The cause is multifactorial; there appears to be a genetic predisposition for development of ocular squamous cell tumors, and exposure to ultraviolet radiation (amount and intensity) and lack of protective pigmentation around the eye play an important role. Tumors occur predominantly in Herefords, but also other breeds with similar patterns of periocular pigmentation, such as Simmentals and Holsteins. It is seldom seen in animals less than 4 years of age, and peak age of occurrence is 8 years. The tumors begin as benign plaques or papillomas that often progress quickly to squamous cell carcinoma. Common sites for development of malignancy in decreasing order of prevalence are the lateral and medial limbus (Figure 31-25), eyelids (especially lower), third eyelid, and medial canthus.
Treatment modalities include cryotherapy, radiofrequency hyperthermia, immunotherapy, chemotherapy, radiation therapy, and surgery (keratectomy, lid resection, or extirpation). Although commonly referred to as enucleation, the term extirpation is more accurate. Extirpation is removal of all the contents of the bony orbit. Since this procedure is commonly performed for advanced cases of OSCC where removal of all ocular tissue is crucial and because cosmetic appearance is not as important in cattle, we are more likely to perform extirpation rather than evisceration or enucleation of the eye.
Extirpation is performed with the aid of a retrobulbar or Peterson eye block. To perform the Peterson block, an 18-gauge, 12-cm needle bent to a slight curve is used to block cranial nerves II, IV, V, and VI as they emerge from the round foramen. The needle enters the skin at the angle produced by the supraorbital process and the zygomatic arch and is directed medially. The concavity of the needle is directed caudally so that the point of the needle will pass around the cranial border of the coronoid process of the mandible and to the pterygopalatine fossa of the skull. A reliable indication of proper position is a severe twitching of the eyelids. Once the proper position is located, 5 ml of local anesthetic is injected. The needle is repositioned slightly two more times with injection of 5 ml of local anesthetic each time. Aspiration is essential before injection to prevent depositing lidocaine in the cerebrospinal fluid (CSF), possibly resulting in sudden death. Before withdrawing the needle completely, it is redirected caudally just beneath the skin along the zygomatic arch to block the auriculopalpebral nerve. The four-point retrobulbar block is performed by injecting through the eyelids, both dorsally and ventrally, and at the medial and lateral canthi using a slightly curved, 18-gauge, 12-cm needle that is directed to the apex of the orbit. Five to 10 ml of local anesthetic are injected at each site. Exophthalmos, corneal anesthesia, and mydriasis indicate a satisfactory retrobulbar block.
The eye is surgically prepared, and the lids are sutured or clamped together. A transpalpebral incision is made approximately 1 cm from the lid margins (unless the disease extends beyond this margin). The skin incision is full thickness, but does not penetrate the palpebral conjunctiva; the conjunctival sac helps contain contaminated ocular structures during surgery. Sharp dissection is continued 360 degrees around the bony orbit. The orbital ligament is incised at the medial canthus, and the muscles, adipose, lacrimal glands, and fascia are removed. The optic artery can be ligated, significantly reducing hemorrhage, or the lids can be tightly closed and a gauze stent placed over the incision to provide pressure and hemostasis. The lids are closed with appositional or everting interrupted sutures using nonabsorbable No. 3 suture material. If excessive skin must be removed such that the incision cannot be closed, the orbit can be packed with gauze that is removed in 48 to 72 hours. The incision is left to heal by second intention. NSAIDs and antibiotics may be administered before surgery.
SURGICAL PROCEDURES OF YOUNG STOCK
If possible, calves should be dehorned within the first month of life (or when the horn buds are first palpable) using a dehorning iron. The electrothermal dehorning technique is easy to perform when the calf is young; produces desirable, cosmetic results; and is much less stressful to the young calf. Dehorning of beef calves is commonly performed at the time of weaning in conjunction with castration, vaccination, and other management procedures. This age group is usually dehorned using a Barnes dehorner or scoop to remove the horn (Figure 31-26). Hemostasis, which is crucial, is provided by pulling or twisting the cornual artery. Calves older than 6 months may have exposed frontal sinuses after dehorning and may be at greater risk for development of sinusitis. Dehorning mature cattle often requires analgesia via a cornual nerve block. The block is performed by injecting 10 ml of 2% lidocaine under the frontal crest halfway between the lateral canthus of the eye and the base of the horn. Large horns are removed with a dehorning saw or Gigli wire. Another method of dehorning cattle is surgical or cosmetic dehorning, which is usually performed on show cattle. This method requires local or regional analgesia. An elliptical incision is made around the base of the horn, the horn is removed, and the skin incision is closed. This surgical technique allows for a more cosmetic appearance to the poll and reduces the chances of postoperative hemorrhage or infection.
CASTRATION
Several techniques are available for castration of calves. As with dehorning, castration is best performed when the calf is young because it is easier and less stressful to the calf at that time. Castration of young calves is often done without analgesia. Small calves can be adequately restrained on the ground, whereas larger calves are restrained in a chute with the tail pushed tightly up over the back. A technique commonly employed is the “open” method in which the bottom one third to one half of the scrotum is excised exposing the testicles. In young calves, the testicles are pulled until the cords break. In older calves, the cord can be sharply transected or an emasculator that crushes and cuts can be used to separate the cord (Figure 31-27). Another less commonly used technique is a “closed” castration using an emasculotome, which crushes the cord within the scrotum without cutting the scrotal skin. This technique is also referred to as bloodless castration or pinching. Castration in young calves has also been performed by application of an elastrator band. It is important to make certain that both testicles are below the band when the procedure is completed. It takes 2 to 3 weeks for the scrotum and testicles to slough. This technique has been associated with development of tetanus; therefore vaccination for tetanus may be advisable.
UMBILICAL HERNIAS AND INFECTIONS
As in other species, the umbilicus consists of paired umbilical arteries, a urachus, and an umbilical vein. The foramen where these exit the body wall is a common site of herniation, and this is considered to be a hereditary defect in some breeds, such as Holsteins. Any increase in size of the umbilical stalk, discharge from it, or swelling associated with the adjacent tissue warrants closer investigation. Simple umbilical hernias do occur and if small can be managed with application of belly wraps, hernia clamps, or elastrator bands. Close examination and palpation of the hernia and its ring is vital to ensure that it is completely reducible, no intestines appear to be in the hernia sac, there is no evidence of infection, and that the hernia is small (less than 5 cm in length). Ultrasonographic examination can be useful to identify structures within the hernia sac. Hernias that are greater than 5 cm in length will often benefit from an umbilical herniorrhaphy.
The majority of hernias or umbilical swellings are associated with infection in one or more of the umbilical remnants. Palpation of the mass is often of limited use diagnostically because it is firm, hot, swollen, and the patient usually resents palpation. Ultrasonographic examination allows the umbilical structures to be visualized and any enlargement or involvement of bladder or liver to be identified before initiating treatment. Medical treatment can be started in an attempt to treat the infection, but surgical resection can be necessary for severely affected patients.
Omphalectomy or umbilical herniorrhaphy can be performed with the calf in dorsal recumbency using sedation and a local block or general anesthesia. Feed does not need to be withheld for as long as for adult patients, but this should be removed approximately 6 to 8 hours before surgery. A large area should be clipped and prepped for surgery as if the umbilical vein or urachus is infected; the surgical incision may have to extend as far cranially as the xiphoid process or caudally to the pelvis. If the vein is infected up to the liver, a marsupialization may need to be performed that will require a second incision lateral to the main site. Attempt to remove the infected tissue intact; this may necessitate resection of the apex of the bladder. Following removal of the infected tissue, the body wall will be repaired. Some defects may be large enough to require a mesh herniorrhaphy, but most can be sutured closed.
Following surgery, the calf must be maintained in a stall for a month. Allowing excessive exercise will often lead to incisional complications, such as edema, seroma formation, or dehiscence. If umbilical vein marsupialization was performed, the stoma must be cleaned daily, and low pressure lavage with sterile fluid can be performed to try and treat the infected structures that could not be removed. This stoma will often need to be repaired in the future because it will often form a small hernia when the infection has resolved.
SELECTED CONDITIONS OF SMALL RUMINANTS
Male small ruminants are at high risk for developing urolithiasis as a result of the feeding of excessive grain in the diet. The cause and clinical presentation is similar to that described in cattle. Goats that are obstructed tend to vocalize because of pain. Small ruminants are small enough that it is possible to palpate and/or ultrasound their urinary bladders, which further aids diagnosis of the condition. Management of this condition is somewhat different in small ruminants. Small ruminants possess a urethral process or vermiform appendage, which is usually the first place obstruction occurs. The second most common site of obstruction is the distal sigmoid flexure. Oftentimes calculi resemble sand, which may block most of the distal portion of the urethra.
The urethral process should be examined in all cases of suspected urolithiasis in small ruminants since this is the most common location for calculi to lodge. The penis and urethral process can be exteriorized by placing the animal in a sitting position on its rump (Figure 31-28). If necessary, a lumbosacral epidural can be performed. Epidural anesthesia provides analgesia and prevents resistance to exteriorization of the penis caused by the retractor penis muscles. Lidocaine (2%, 1 ml/20 lb not to exceed a total dose of 15 ml in any small ruminant) is injected into the epidural space at the lumbosacral junction (Figure 31-29). Loss of motor control to the hind legs lasts from 1 to 3 hours, so the patient should be recovered on a well-bedded surface to prevent trauma to the rear legs. Once the penis is exteriorized, it can be grasped with dry gauze. If the urethral process is obstructed, it can be amputated close to its attachment to the glans. If the urethral process is not present, it may mean that it had already been amputated or it may have necrosed and sloughed by itself during a previous episode of obstruction. Immediate urethral patency may occur after urethral process amputation; however, reobstruction is common. Consequently, it appears that urethral process amputation alone rarely results in a long-term cure.

FIGURE 31-28 Examination of the urethral process in a wether for the presence of calculi (urolithiasis).
FIGURE 31-29 Administration of a lumbosacral epidural in a goat results in loss of motor control to the hind legs.
Urethral catheterization with saline flushing may be attempted to relieve obstruction; however, the bladder is difficult to catheterize because of the presence of the suburethral diverticulum in ruminants, and complications associated with catheterization include urethritis, urethral rupture, and urethral damage leading to stricture.
Perineal urethrostomy may be suitable in cattle as a salvage procedure (Figure 31-30). Urethrostomy frequently results in stricture formation in the urethra and is probably not a good choice for pets because of a shortened life span.

FIGURE 31-30 Perineal urethrostomy, a surgical treatment for obstructive urolithiasis, is prone to stricture formation in small ruminants.
Cystotomy and/or tube cystostomy have become the procedures of choice for treatment of obstructive urolithiasis and often result in prolonged life and preservation of breeding capability in breeding males. Using this procedure, calculi can be removed from the bladder, and normograde and retrograde urethral flushing can be attempted. In addition, a Foley catheter placed in the bladder allows urine egress while the urethra and bladder heal (Figure 31-31). The catheter is then removed when it is determined that the animal can urinate a normal stream consistently from the urethra.
OPHTHALMIC SYSTEM
Entropion, or inward rolling of the eyelid, has been reported to be the most common ocular disease of neonatal lambs (Figure 31-32). The congenital or primary form involves only the lower lid, but is usually bilateral. Clinical signs of blepharospasm, photophobia, eye rubbing, and keratoconjunctivitis are ordinarily observed in lambs during the first few days to weeks of life. Initial treatment is conservative and involves administration of ophthalmic antibiotics and manually rolling the lower lid outward. If this is unsuccessful, other treatment options include injection of penicillin or tetracycline in a linear fashion parallel to the lower lid or clamping of the skin of the lower lid below and parallel to the lid margin with mosquito forceps for 30 seconds. Both techniques should create sufficient inflammation and fibrosis to keep the lower lid rolled out. Another technique involves placement of two or three vertical mattress sutures in the lower lid to roll out the lid margin. Congenital entropion is considered to be a heritable trait, so affected animals should not be kept for breeding.
COMMON SURGICAL PROCEDURES PERFORMED ON SMALL RUMINANTS
Lumbosacral epidurals are useful for alleviating pain during procedures performed caudal to the umbilicus. The animal will lose motor control to the hind legs and should be confined to a small, well-bedded area to prevent injury. A lumbosacral epidural is performed at the lumbosacral junction that can easily be palpated in small ruminants. A 20-gauge, 3.8-cm needle is used for this technique. The needle is inserted perpendicular to the dorsal midline until a slight popping sensation is encountered. If the epidural space has been entered, injection should be easy. An alternative is to advance the needle until bone is felt, then back the needle out slightly and attempt injection. If CSF is encountered, the subarachnoid space rather than the epidural space has been entered. It is acceptable to inject lidocaine into this area if aseptic technique has been used during the procedure, but the lidocaine dose should be reduced to half of that used for epidural injection. Analgesia will take several minutes if in the epidural space and will be almost immediate if injected into the subarachnoid space. The dose of lidocaine used for this procedure is 5 to 8 ml/45 kg body weight.
The optimal time for disbudding or dehorning kids is 3 to 5 days in buck kids and 5 to 7 days in doe kids. At this age, the procedure is less invasive because the horn buds have not yet attached to the underlying bone, and there is less chance of regrowth if dehorned properly at this age. Many owners perform this procedure with restraint only; however, the kid may be sedated with xylazine and butorphanol. In addition, a ring block of 1% lidocaine around the base of the horn will provide local analgesia. The horn bud is removed by first burning with the dehorning iron and either allowing the bud to slough or by excising the bud after burning around its base. Care should be taken not to leave the iron on too long (5 to 10 seconds per side) to prevent thermal meningitis. The burning procedure may be repeated, allowing the area to cool between burns, until a uniform ring of copper-colored skin is apparent around the entire horn bud bases (Figure 31-33).
FIGURE 31-33 Disbudding kids is performed by burning the horn buds with an electric dehorning iron before 2 weeks of age.
C-sections are indicated in cases of dystocia. A left flank laparotomy can be used to approach the uterus and, using sedation and an inverted-L block, can be rapidly performed. Following incision of the skin, the flank muscles can be bluntly separated along the direction of their fibers, and the uterus can be brought into the surgical field. It is important that the uterus is carefully palpated before closure to prevent leaving a fetus in the contralateral horn. Alternatively a ventral midline or ventral paramedian approach can be used.
Factors causing rectal prolapse in sheep include short tail docking, overconditioning of lambs (increased pelvic fat), straining as a result of urolithiasis, diarrhea, dystocia, or conditions that increase abdominal pressure, such as coughing. Short tail docks are performed strictly for cosmetic reasons in show lambs and may result in loss of innervation to the rectum and anal sphincter, which comes from S3 to Cy5. A resolution to the American Veterinary Medical Association (AVMA) suggests that lamb tails not be docked shorter than the distal end of the caudal tail fold. Methods for rectal prolapse repair include purse-string suture after replacement (strictly a salvage procedure), injection of irritating solutions (tetracycline, strong iodine in oil) at three to four points perirectally, and rectal amputation.
Auer J.A., Stick J.A., eds. Equine surgery, ed 3, Philadelphia: Saunders, 2006.
Divers, T.J., Peek, S.F. Rebhun’s diseases of dairy cattle, ed 2. St Louis: Saunders; 2008.
Fubini, S.L., Ducharme, N.C. Farm animal surgery. St Louis: Saunders; 2004.
Hanie, E.A. Large animal clinical procedures for veterinary technicians. St Louis: Mosby; 2006.
Koterba A.M., Drummond W.H., Kosch P.C., eds. Equine clinical neonatology. Philadelphia: Lea & Febiger, 1990.
Orsini, J.A., Divers, T.J. Manual of equine emergencies, ed 3. St Louis: Saunders; 2008.
Pugh, D.G. Sheep and goat medicine. St Louis: Saunders; 2002.
Reed S., Bailey W., Sellon D., eds. Equine internal medicine, ed 2, St Louis: Saunders, 2003.
Smith, B.P. Large animal internal medicine, ed 4. St Louis: Mosby; 2009.
 TECHNICIAN NOTE
TECHNICIAN NOTE