Veterinary Anesthesia
When you have completed this chapter, you will be able to:
1 Define anesthesia and differentiate between general and local anesthesia.
2 Differentiate between sedation, tranquilization, and neuroleptanalgesia.
3 Explain the concept of balanced anesthesia and list the factors to consider in developing an anesthetic plan for a patient.
4 List the classes of injectable medications used in anesthetic protocols and give examples of each.
5 List and describe the features of the commonly used inhalant anesthetics.
6 List the parts of the anesthesia machine, describe the function of each part, and list procedures used for verifying proper operation.
7 Differentiate between nonrebreathing and rebreathing systems and explain the proper use, advantages, and disadvantages of each type of system.
8 List the reflexes used in monitoring of anesthetic depth and describe the relationship between vital signs, patient reflexes, and anesthetic depth.
9 List the stages and planes of anesthesia and describe changes in patient physiology and behavior in each stage.
10 Describe the equipment needed and procedures used for placement of an endotracheal tube.
11 Describe procedures used for IV, IM, mask, and chamber induction.
12 Describe general considerations for patient positioning, comfort, and safety during anesthesia.
13 Describe procedures used in monitoring patients during recovery from anesthesia.
14 Differentiate between manual and mechanical ventilation and explain indications, procedures, and complications of ventilation.
WHAT IS ANESTHESIA?
Anesthesia is defined as an absence of sensation that affects either the whole body or an isolated part or region of the body. Tranquilization and sedation are by convention included in the study of anesthesia because these techniques are frequently used in conjunction with anesthesia. The effects appropriate for each patient vary depending on the procedure and may include light to heavy sedation, local anesthesia, general anesthesia, muscle relaxation, analgesia, or a combination thereof.
General anesthesia is characterized by unconsciousness and insensibility to feeling and pain induced by administration of anesthetic agents given alone or in combination. General anesthesia provides an environment in which general surgery or other painful procedures can be performed without the danger of patient movement or injury to personnel. Anesthetic induction is the process used to take the patient from a state of consciousness to general anesthesia. Anesthetic maintenance is the process used to keep the patient under general anesthesia until recovery.
In contrast, local anesthesia is the loss of sensation in a localized body part or region induced by the administration of a drug or other agent without the loss of consciousness. Local anesthesia is used for procedures that do not require the patient to be unconscious and for adjunct pain control. Administration of local anesthetic to remove a skin tumor, a nerve block performed on a horse to localize lameness, or an epidural used to provide analgesia for a patient undergoing an orthopedic procedure are all examples of local anesthesia.
Premedication refers to the administration of an agent or agents before induction of general anesthesia to calm and relax the patient, ease induction and recovery, minimize adverse effects, reduce the amount of general anesthetic needed, provide muscle relaxation, or provide pain control. A variety of tranquilizers, sedatives, anesthetics, and anticholinergics are used alone or in combination for this purpose. Premedication is also referred to as preanesthesia.
Sedation is a state of calm or drowsiness, whereas tranquilization is a state of relaxation and reduction of anxiety. Many tranquilizers also produce some degree of sedation. Consequently, these terms are often used interchangeably, even though they have somewhat different meanings.
Neuroleptanalgesia is a state of profound sedation and analgesia produced by simultaneous administration of an opioid and tranquilizer. Neuroleptanalgesia is commonly used to perform minor procedures, such as wound treatment or radiography, and to induce general anesthesia in sick patients.
The objectives of anesthesia are to produce a loss of sensation in the whole body or a body part or region and to provide muscle relaxation, analgesia, and alteration of consciousness appropriate to the procedure. In addition, patient safety must be preserved and adverse effects minimized, with special attention to respiratory and cardiovascular function. This can seldom be achieved with the use of only one drug. This is why the concurrent administration of two or more anesthetic drugs is commonplace. This use of drugs with complimentary effects, referred to as balanced anesthesia, enables the anesthetist to fulfill these diverse objectives. Although there are many commonly used protocols, premedication with acepromazine, anesthetic induction with a ketamine-diazepam mixture, anesthetic maintenance with sevoflurane gas, and administration of a morphine infusion for pain control is one example of balanced anesthesia.
PATIENT PREPARATION
The technician must accurately identify factors that can compromise a patient and must effectively communicate this information to the veterinarian before beginning any anesthetic procedure. Specifically, careful patient preparation is important for the following reasons:
• To minimize the likelihood of preventable complications, such as aspiration
• To allow treatment of any problems that may endanger the patient, such as dehydration, bleeding, organ dysfunction, or organ failure
• To allow the veterinarian to make anesthetic drug choices based on facts about the patient's condition
• To give the anesthetist awareness of potential problems that the patient may experience during the procedure
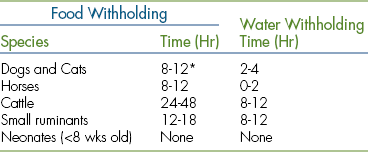
FASTING RECOMMENDATIONS
Swallowing reflexes become sluggish, lower esophageal sphincter tone decreases, and patients may experience nausea or vomiting during anesthetic procedures. This combination of factors may allow stomach contents to reflux into the esophagus and the pharynx resulting in a range of mild to devastating complications, including aspiration of stomach contents into the lungs, postanesthesia esophagitis, and esophageal stricture. Therefore it is vital to observe fasting recommendations before any anesthetic procedure in common domestic species. Although fasting recommendations vary widely from practice to practice, guidelines may be found in Table 27-1.
GATHERING HISTORICAL INFORMATION
In addition to the usual historical information needed for any patient, such as vaccine status and the medical and surgical history, there are several additional pieces of information that must be determined before administering an anesthetic. The following is a list of questions that should be asked of a client at the time of patient drop-off:
• Has the patient been fasting for the recommended time?
• How is the patient feeling today?
• Are there any changes in the patient's condition since the procedure was scheduled?
• Is the patient up to date on routine preventive care?
• What is the patient in for today (including left or right side if the procedure involves a limb, eye, or ear; and including the location of tumors or other localized lesions)?
Unless asked, a client may not reveal this information at the time of drop-off for a variety of reasons. For example, an owner who failed to observe fasting recommendations may be unwilling to admit a lack of willpower to withhold food, may not realize the importance of fasting, or may simply have forgotten to tell you. An owner may not think to inform you that the patient worsened or developed vomiting, coughing, or some other sign of significant illness. If the procedure is not verbally confirmed, the surgeon could mistakenly perform a procedure on the incorrect limb or the wrong surgery altogether. Thus any missing piece of information can make the difference between a successful and a potentially serious outcome.
PHYSICAL ASSESSMENT
A physical assessment should be performed immediately before administering any anesthetic to uncover any problems that may increase patient risk or necessitate a change in patient management (see Box 27-1 for physical findings that should be reported to the veterinarian). The focus of this assessment should be on the nervous, cardiovascular, and pulmonary systems because the health status of these systems is closely associated with the outcome of any anesthetic procedure. Below is a list of important components of a preanesthetic physical assessment:
• Observe the patient's level of consciousness (e.g., bright, alert and responsive; alert but subdued; lethargic; stuporous; comatose).
• Observe the general body condition including body weight and hydration.
• Note weakness, abnormal gait, or recumbency.
• Look for parasites, wounds, tumors, or other external lesions.
• Examine body orifices for external signs of disease, such as diarrhea, nasal discharge, hematuria, or vaginal discharge.
• Determine vital signs (temperature, pulse, respiratory rate [RR]).
• Assess mucous membrane color and capillary refill time (CRT).
• Watch the patient breathe noting respiratory effort.
DIAGNOSTIC TESTING
When preparing a patient for surgery, diagnostic tests are often needed to supplement the history and physical assessment. The purpose of these tests is to uncover abnormalities that may impair the patient's ability to compensate, that may lead to unanticipated complications, or that may impair the patient's ability to eliminate the anesthetics. Testing recommendations vary widely from practice to practice. Most diagnostic testing recommendations are based on the signalment and physical status class and may include blood work, urinalysis, thoracic radiographs, serology, electrocardiography, and other tests as indicated. In general, young, healthy patients receive relatively fewer tests, and patients that are older, sick, or have other risk factors receive more.
PATIENT STABILIZATION
Abnormalities identified during patient evaluation must be treated before administration of the anesthetic. This patient stabilization includes treatment or correction of dehydration, anemia, cardiac arrhythmias, respiratory compromise, major organ failure, electrolyte or acid-base imbalances and may involve administration of antibiotics, analgesics, fluids, blood, oxygen, or a wide variety of other agents. The veterinary technician will be intimately involved in this stabilization process and must be prepared to accurately calculate doses, place intravenous (IV) catheters, set fluid administration rates, and administer drugs, blood, and oxygen as ordered by the veterinarian.
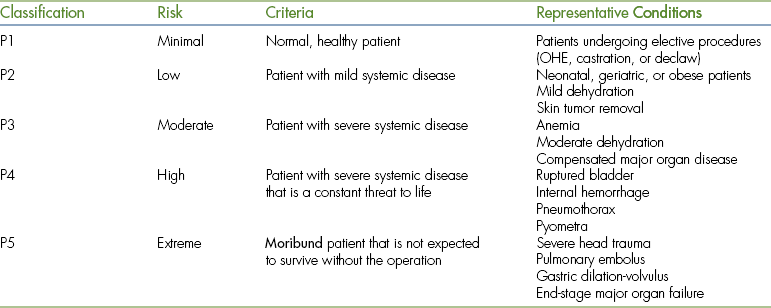
PHYSICAL STATUS CLASSIFICATION
The information gleaned from the patient evaluation is used to determine a physical status classification. This system, developed by the American Society of Anesthesiologists (ASA), is a subjective rating of the patient's condition based on historical, physical, and laboratory findings that places the patient in one of five classes (Table 27-2). This system is a tool that can be used to guide the anesthetist in appropriate patient management because anesthetic protocols are often based on physical status classification. This classification is somewhat subjective, so use your best judgment based on the criteria listed. Any surgery that is an emergency, regardless of ASA class, is additionally assigned the letter “E.”
ANESTHETIC AGENTS
Anesthetic agents used in veterinary patients may be classified in one of several ways. These may be grouped according to the route of delivery (topical, oral, injectable, or inhalant) or by primary use (preanesthetic, sedative, induction agent, or maintenance agent). Anesthetics may also be grouped into drug classes based on chemistry. Agents in any given class tend to have similar actions, properties, uses, and effects, so this system will be used to classify the anesthetic agents described.
Agonists, partial agonists, mixed agonist-antagonists and antagonists: Anesthetic drugs work by binding to specific receptors on or inside the cells of target tissues. In the case of many anesthetics, these target tissues are located in the central or peripheral nervous systems. Most anesthetic agents are agonists (drugs that bind to receptors and exert one or more effects). Some drug classes, such as the opioids and α2-adrenergics, include drugs called antagonists that block or reverse the action of the corresponding agonist. These antagonists are referred to as reversal agents. In the opioid class, there are also agents that are classified as partial agonists and those classified as mixed agonist-antagonists. Partial agonists bind to receptors and exert a partial or milder effect, whereas mixed agonist-antagonists partially reverse the effects of pure agonists.
ANTICHOLINERGICS
Although not true anesthetic agents, anticholinergics are used to counteract effects of parasympathetic nervous system stimulation, such as bradycardia and excess salivation. Although many anesthetics cause these effects to some degree, the barbiturates and dissociatives have a notable tendency to cause excess salivation, and opioids and α2-adrenergic agonists are especially likely to cause bradycardia. Atropine and glycopyrrolate are the most commonly used anticholinergics in veterinary patients.
Anticholinergics have many effects expected to result from a parasympathetic nervous system blockade, including increased heart rate (HR), bronchodilation, reduced tear secretions and salivation, reduced gastrointestinal (GI) activity, and dilation of the pupils, especially in cats. Protective ophthalmic ointment should be applied to patients receiving these agents to prevent drying of the corneas.
There are many potential adverse effects, including tachycardia, cardiac arrhythmias, bronchodilation, mydriasis, and ileus. The bronchodilation caused by these drugs can increase anatomic dead space, which increases the risk of hypoxemia. Mydriasis may render the pupillary light reflex unreliable. Anticholinergics may also cause a thickening of the mucus in the airways, especially in cats, which can result in blockage. Some anesthetists use anticholinergics routinely in small animal (SA) patients, whereas others do not.
In ruminants, copious salivary secretions become more viscid, pool in the pharynx, and may be aspirated, thus predisposing the patient to airway blockage. Horses may develop colic from GI stasis. For these reasons, anticholinergics are avoided in these species unless necessary to treat bradycardia.
Atropine is a rapid-acting agent that comes in SA and large animal (LA) strengths (0.54 mg/ml and 15 mg/ml, respectively). Glycopyrrolate is similar to atropine with the following differences: Glycopyrrolate has a slower onset and a longer duration. It is less likely to cause tachycardia, cardiac arrhythmias, and ileus and suppresses salivation more effectively. Unlike, atropine, it does not cross the placental barrier and will not adversely affect the fetuses if used for cesarean sections (C-sections). It also does not cross the blood-brain barrier and thus has less impact on vision than atropine.
Tranquilizers and sedatives are commonly used to provide patient restraint for minor procedures, such as grooming, diagnostic imaging, blood draws, nail trims, and wound treatment. They are used as premedications and also to produce specific effects, such as analgesia and muscle relaxation. Each of these drugs has unique properties and must be chosen based on the particular effects desired. For instance medetomidine (an α2-adrenergic agonist) produces analgesia and muscle relaxation, whereas acepromazine minimizes vomiting and development of cardiac arrhythmias. Often tranquilizers and sedatives are used in combination or with other anesthetic agents to produce a combination of effects that cannot be achieved by using one drug alone.
PHENOTHIAZINE TRANQUILIZERS
Phenothiazine tranquilizers (also classified as major tranquilizers) are used to calm and sedate patients before general anesthesia. This helps improve the quality of anesthetic induction and recovery by reducing anxiety. The phenothiazine tranquilizer used most commonly for this purpose is acepromazine. In addition to its use alone, it is also often used in combination with other agents. Combinations include “RAT” (Rompun, generic name xylazine, acepromazine, and Torbugesic, generic name butorphanol) and “BAG” (butorphanol, acepromazine, and glycopyrrolate).
Acepromazine induces mild to moderate sedation and is antiemetic and antiarrhythmic. Unlike many other agents, it causes little respiratory or cardiac depression. It has a relatively long duration.
Acepromazine blocks α1-adrenergic receptors in the sympathetic nervous system resulting in dose-dependent peripheral vasodilation. Consequently the main adverse effect of acepromazine is hypotension, which can lead to cardiovascular collapse. It also can cause hypothermia or hyperthermia, changes in the HR, prolapse of the third eyelid, and paradoxical excitement or aggression. Adverse effects specific to horses include excitement, sweating, tachypnea, and protrusion of the penis, which can lead to permanent injury. Therefore some clinicians will not use this drug in breeding stallions.
Give acepromazine IM at least 15 minutes before induction and allow the patient to remain in a quiet area because the tranquilizing effect may be overridden, especially in excited patients. Note that the commonly used dose of the injectable form of acepromazine is significantly less than the dose on the label. Use of higher doses will not increase the level of sedation, but will cause increased hypotension.
When administering acepromazine IV, give it slowly and avoid intraarterial injection. Boxers, greyhounds, and giant-breed dogs and debilitated, young, or geriatric patients may be sensitive to this drug, whereas terriers and cats are relatively resistant. Acepromazine should not be given to patients with seizure disorders because it may lower the seizure threshold. Inform owners to use caution when handling patients that have received this drug because personality changes may occur resulting in aggression.
BENZODIAZEPINE TRANQUILIZERS
Benzodiazepines (also classified as minor tranquilizers) are most often used in combination with other agents, such as opioids and dissociatives, to produce a range of effects from sedation to general anesthesia. Benzodiazepines are controlled substances. Diazepam, midazolam, and zolazepam are benzodiazepines commonly used in veterinary patients. Zolazepam is one of the two components in the product Telazol. The other component of this product is tiletamine (see Dissociatives for a discussion of this drug). Although benzodiazepine antagonists (flumazenil and sarmazenil) are available, they are seldom used because of the expense.
Effects of benzodiazepines include anxiety reduction and mild to moderate sedation, although young healthy dogs are resistant to these drugs, and sedation in cats is often unpredictable. Other effects include appetite stimulation in cats, skeletal muscle relaxation, and anticonvulsant activity. The effects generally last no more than a few hours.
Benzodiazepines are relatively safe and minimally affect the cardiopulmonary system, but can produce adverse effects that vary among species. Dogs tend to experience CNS excitement, anxiety, and fear, especially if young and healthy and may become more difficult to control. Aggressive animals may lose inhibition, prohibiting use in these patients. Horses may experience muscle fasciculations, weakness, and mild ataxia.
Following intramuscular (IM) injection, diazepam is erratically absorbed and causes pain. It can also cause bradycardia, apnea, hypotension, and pain if given rapidly intravenously as a result of the propylene glycol vehicle.
Do not store diazepam in syringes or IV bags because it is soluble in plastic and will lose potency. Diazepam is commonly mixed with ketamine (a dissociative anesthetic) in equal volumes and given intravenously to induce general anesthesia in small animals (see Boxes 27-7 and 27-8).
Midazolam is more potent than diazepam, but can be used with ketamine in place of diazepam for anesthetic induction. Midazolam is water soluble and is compatible with a variety of agents, unlike diazepam which is not water soluble and therefore cannot be mixed with other agents except ketamine.
α2-ADRENERGIC DRUGS
α2-Adrenergic agonists are sedatives that are used alone or in combination with opioids, dissociatives, and other agents to produce a wide spectrum of effects from mild sedation to general anesthesia, including analgesia and muscle relaxation. Xylazine and medetomidine are α2-agonists most commonly used in SA patients. Xylazine, detomidine, and romifidine are most commonly used in LA patients.
The main therapeutic effects of α2-agonists are CNS depression, analgesia, muscle relaxation, and sedation. These agents may cause vomiting in dogs and cats. Effects on the cardiovascular system include an initial hypertension followed by prolonged hypotension. Bradycardia is common, and cardiac output decreases. At high doses, these drugs cause a decrease in both respiratory rate (RR) and depth. In horses, xylazine causes lowering of the head and relaxation of the facial muscles, leading to drooping of the ears and lower lip. Relaxation of limb muscles leads to ataxia and a wide-based stance.
α2-Agonists can cause significant bradycardia, reduced cardiac output, heart block, cardiac arrhythmias, and hypotension. They can cause respiratory depression, especially when administered with other agents, and respiratory distress in brachycephalic dogs. Other adverse effects include pain upon IM injection, muscle tremors, and changes in body temperature. Dogs may bloat secondary to aerophagia, and horses may sweat. Ruminants may experience profound respiratory depression, hypersalivation, bloat, diarrhea, premature delivery, or abortion. Because sedated patients are sensitive to auditory stimuli, horses may kick, small animals may move, and aggressive patients may bite in response to loud noises.
The sedation produced by these drugs can be profound and prolonged, as can cardiovascular depression. Therefore give standard doses only to young, healthy patients and use cautiously in geriatric, diabetic, pregnant, pediatric, or sick patients. Most clinicians do not recommend routine use of anticholinergics with α2-agonists because they can predispose the patient to development of arrhythmias and hypertension.
Xylazine is used in many domestic and exotic species as one component of anesthetic mixtures and is also used to induce vomiting in cats following ingestion of toxins. Xylazine comes in SA (2% or 20 mg/ml) and LA (10% or 100 mg/ml) concentrations. Ruminants are extremely sensitive to this drug, requiring about one tenth of the dose used for horses. Swine require a high dose, so xylazine is usually given in combination with other drugs in this species.
Medetomidine is used primarily in small and exotic animal species. When compared with xylazine, it is more potent and less likely to produce side effects. It can be used in equal volumes with the reversal agent atipamezole to sequentially sedate and awaken patients undergoing minor procedures. Sudden arousal has been reported in patients heavily sedated with medetomidine, resulting in bites.
Detomidine (Dormosedan) and romifidine (Sedivet) are α2-agonists used in horses to produce sedation for procedures, such as dental work, and as a part of anesthetic combinations. Detomidine has a longer duration of action and provides more profound sedative and analgesic activity than xylazine. Romifidine generally causes less muscle relaxation than xylazine or detomidine.
α2-Adrenergic antagonists increase the HR, increase the blood pressure, and stimulate the CNS. These are used to “wake” patients following sedation or anesthesia and to reverse adverse effects of α2-agonists. Unfortunately, these agents also reverse desirable effects, such as analgesia. Therefore an alternative analgesic must be administered if pain control is warranted. Adverse effects include apprehension as a result of rapid arousal, excitement, muscle tremors, and salivation.
Yohimbine is an α2-antagonist used to reverse the effects of xylazine. Although it is labeled for use in dogs, it is used in other species. It should be used cautiously in patients with seizure disorders. Atipamezole (Antisedan) is used to reverse the effects of medetomidine (Domitor). Both the dose and concentration of this drug is five times that of medetomidine, so these two drugs are administered in equal volumes. Cats are more sensitive to this drug and are given a reduced dose. In addition to the general adverse effects of α2-antagonists listed earlier, this drug can also cause vomiting or diarrhea. Atipamezole may also be used to reverse detomidine. Tolazoline is used to reverse the effects of α2-agonists in a variety of species.
α2-Antagonists may be given IM in all species. When given IV, α2-antagonists should be given slowly to effect to avoid excitation and aggression that is sometimes seen with rapid reversal. Their IV use in cats may cause unwanted severe salivation and excitement and is thus contraindicated.
OPIOIDS
Opioids (also known as narcotics) are drugs related to morphine. Opioids are classified as agonists, partial agonists, mixed agonist-antagonists, and antagonists. Morphine, fentanyl, oxymorphone, and hydromorphone are examples of pure agonists. Buprenorphine is a partial agonist, butorphanol is a mixed agonist-antagonist, and naloxone is an antagonist. Opioids provide analgesia and sedation and are combined with tranquilizers to produce neuroleptanalgesia. With the exception of naloxone, most opioids commonly used in veterinary patients are controlled substances.
Opioids work by stimulating specific opioid receptors in the brain and spinal cord, each of which produces distinct effects. For instance, stimulation of the μ-opioid receptors produces analgesia, euphoria, dependence, miosis, hypothermia, and respiratory depression. κ-Receptor stimulation produces analgesia, miosis, and sedation. σ-Receptor stimulation produces dysphoria, hallucinations, respiratory and cardiac stimulation, and mydriasis. The effects of each opioid depend on its affinity for each of the various receptors.
Opioid agonists primarily stimulate the μ-receptors. They are used as sedatives, analgesics, and—when combined with tranquilizers—neuroleptanalgesics. The effects of opioid agonists vary according to the species. Cats and large animals tend to experience anxiety, excitement, hyperthermia, and mydriasis, whereas dogs and primates experience sedation, hypothermia, and miosis. High doses of opioid agonists can induce narcosis in dogs, a state in which the patient appears profoundly sedated, but can be aroused by loud noises or other stimulation. CNS effects may include euphoria and dysphoria. Euphoria is an exaggerated sense of well-being, whereas dysphoria is restlessness or discomfort. The opioid agonists are among the best analgesics available and are often used to control moderate to severe pain. Opioid agonists cause a variety of other effects, including cough suppression, respiratory depression, bradycardia, hypotension, and changes in urination and GI tract activity, which vary agent to agent.
There are many adverse effects of opioid agonists, including significant CNS and respiratory depression, which are of concern, especially when using morphine. Peristalsis initially increases then decreases in dogs, causing defecation followed by constipation. Dogs may pant, or—if not in pain—may whine or bark. Other adverse effects of opioids include vomiting, excessive salivation, and in horses sweating and increased locomotor activity. Special caution must be used in neonatal, geriatric, and debilitated patients; patients with head trauma or respiratory disease; or patients with a number of other major organ diseases. Rapid IV administration of morphine may cause histamine release and bronchoconstriction.
Morphine, hydromorphone, fentanyl, and many other pure agonists are class II controlled substances and consequently are subject to special record-keeping requirements. As a result of respiratory depression, monitor patients closely and be prepared to provide ventilatory support. Opioid agonists must be used with caution and in lower doses in cats and horses. GI activity should be monitored closely in horses receiving μ-agonist opioids because decreased motility may lead to colic.
Most opioid agonists have a duration of only a few hours necessitating administration by constant rate infusion for a sustained effect. When used for analgesia, morphine may also be administered by epidural injection, and fentanyl may be administered via transdermal patch (Duragesic). Oxymorphone and hydromorphone are used in a similar fashion to morphine.
Opioid partial agonists exert partial activity at the μ-receptors, but cannot stimulate the receptors to the same degree as a full or pure agonist, and thus cannot provide the same degree of analgesia. Buprenorphine, the drug example in this class, is available only as a solution for injection, but also can be given orally for pain control in cats. Buprenorphine must be given only every 8 to 12 hours because it has a long duration of action. At high doses, this drug can cause respiratory depression that is difficult to reverse with naloxone because buprenorphine binds tightly to the μ-receptors.
Opioid mixed agonist-antagonists exert agonist activity at the κ-receptors and antagonist activity at the μ-receptors. This results in many of the same effects as opioid agonists (sedation, analgesia, and cough suppression) but to a lesser degree. If given following a pure opioid agonist, they will partially reverse the effects of these agents including the analgesic effects. In other words, if given following a pure agonist, they will decrease the beneficial effects, not increase them.
Butorphanol, the representative drug in this class, is used as a premedicant, an analgesic for mild to moderate pain, an antitussive, and when given with tranquilizers, neuroleptanalgesia. In small animals, adverse effects of this agent are rare, but may include transient sedation and ataxia. Horses may also experience excitement at high doses. Because the analgesic effects of butorphanol last only about 1 to 2 hours, frequent administration is necessary for adequate pain control.
Butorphanol is available in oral and injectable forms. The injectable form is available in multiple strengths: 0.5 mg/ml, 2 mg/ml, or 10 mg/ml. It is important to be sure that you select the correct strength.
Opioid antagonists are used to “wake” patients following sedation with opioid agonists, partial agonists, or mixed agonist-antagonists and to reverse undesirable effects of these agents. Naloxone is the opioid antagonist most commonly used in veterinary patients. Adverse effects of this drug are uncommon. Naloxone may be administered by giving the calculated dose slowly intravenously to effect and the remainder by the subcutaneous route. The duration of effect is about 1 to 2 hours; so if the opioid you are reversing is of a longer duration than this, repeat doses may be needed. One to two drops under the tongue can be used to revive neonates delivered by C-section if the dam received opioids.
PROPOFOL
Propofol is a short-acting IV anesthetic used to induce general anesthesia in a variety of species. It may also be used to maintain general anesthesia by administering repeat boluses to effect or a constant rate infusion. Propofol is a phenolic compound that is chemically unlike any other anesthetic. It is not a controlled substance.
Following slow IV injection, it induces a state of general anesthesia in 30 to 60 seconds with a duration of approximately 2 to 5 minutes. The patient rapidly recovers when drug administration is stopped, will sit up within about 10 minutes, and stand within about 15 to 30 minutes. Propofol decreases intracranial and intraocular pressure, provides muscle relaxation, and exhibits antiemetic and anticonvulsant properties. It does not provide significant analgesia. Although propofol is safe when used as directed, it has a relatively narrow therapeutic index and so must be given with caution.
Respiratory depression including apnea may occur and can be severe following rapid injection or with high doses. Therefore it is important to monitor the RR and depth carefully, especially during the first 1 to 2 minutes following initial injection. Cardiac effects include bradycardia and decreased strength of contraction. It causes hypotension, which can be significant following rapid injection. Propofol should be given with caution to patients with preexisting hypotension.
Propofol can cause seizurelike symptoms following induction and allergic reactions in some patients. Transient excitement and muscle tremors may occur during induction if the drug is given slowly or if the patient is not premedicated. Cats can develop Heinz body anemia (a specific type of anemia induced by exposure to certain drugs or toxins), anorexia, lethargy, and diarrhea following repeat doses or use on a daily basis.
Unlike barbiturates, propofol is not cumulative and may be used in greyhounds and other sight hounds. It is available only as an IV injectable in the form of a milky emulsion containing soybean oil and egg lecithin and is an exception to the rule that cloudy liquids should never be administered IV.
Propofol will support bacterial growth. Therefore it is important to handle containers with strict aseptic technique. The manufacturer recommends discarding unused portions more than 6 hours old, although some clinicians believe that propofol can be kept in the refrigerator for up to 24 hours if sterile technique is observed. It must be well mixed before use. If the patient experiences apnea or is given an overdose, treat with supportive care (intubation, oxygen administration, manual or mechanical ventilation, fluid therapy, and diuresis) until vital signs normalize.
DISSOCIATIVES
Dissociatives (also known as cyclohexamines) are a unique group of injectable anesthetic agents used alone to immobilize patients for minor or brief procedures. They are also used in combination with opioids and tranquilizers to induce and maintain anesthesia and to provide analgesia or other specific effects. Ketamine and tiletamine (one of two agents contained in Telazol) are the dissociatives most often used in veterinary patients. Both are controlled.
When used alone, dissociatives produce immobilization, but not surgical anesthesia. Ketamine and other dissociatives induce a state known as “catalepsy” or “dissociative anesthesia,” in which the patient appears awake, but is immobilized and does not respond to its surroundings. Catalepsy is also characterized by open, central, and dilated eyes; nystagmus; increased muscle tone; increased sensitivity to light and sound; an exaggerated palpebral reflex; and intact pedal and laryngeal reflexes.
Dissociatives increase HR and blood pressure without the decrease in cardiac output characteristically seen with most other agents. Other effects include increased CSF pressure and intraocular pressure and superficial analgesia. They may also induce apneustic breathing, a pattern in which there is a prolonged pause following inspiration and a short pause following expiration.
Dissociatives can induce respiratory depression, cardiac arrhythmias, hypersalivation, vomiting, vocalization, prolonged recoveries, jerking movements, tremors, and pain upon IM injection. Increased salivation can be prevented by premedicating with a low dose of an anticholinergic. Dissociatives may induce seizurelike activity during recovery and should not be used in patients with seizure disorders. Ketamine may also cause behavioral changes that can in some cases last for days or weeks.
Tranquilizers or sedatives should be used with or before administration of dissociatives in dogs and horses to decrease adverse effects. Because the eyes remain open, use a corneal lubricant during dissociative anesthesia. Ketamine can be given at a rate of 100 mg/5 kg orally for restraint of fractious cats.
The product Telazol contains tiletamine in combination with zolazepam (a benzodiazepine) and when reconstituted, contains 50 mg of each drug/ml. Once reconstituted, Telazol is stable for 14 days if refrigerated. Telazol is used as an induction agent in healthy dogs and cats, especially if aggressive. Because it causes hypothermia, body temperature must be closely monitored.
A mixture of ketamine and diazepam (or midazolam) is commonly used to induce general anesthesia in dogs, cats, and horses. This combination has an onset of about 30 to 90 seconds and 5 to 10 minutes of working time. In small animals, the two drugs are mixed in a 1:1 volume ratio and given to effect at a rate of about 1 ml of the mixture/20 lb IV slowly over 30 to 90 seconds. Diazepam or midazolam (0.03 to 0.05 mg/kg) can be added to ketamine (2.2 mg/kg) for induction of anesthesia in horses.
Ketamine-α2-adrenergic agonist mixtures are used to provide anesthesia in dogs, cats, horses, and exotics. These mixtures cause significant cardiovascular and respiratory depression and must be used cautiously. Other dissociative mixtures include TKX (Telazol-ketamine-xylazine) and TKD (Telazol-ketamine-Domitor).
BARBITURATES
Barbiturates, a class of drugs developed in the early 1900s, are used for a variety of purposes, including induction of general anesthesia, treatment of seizures, and euthanasia. All barbiturates are controlled. These agents are classified as ultrashort-, short-, intermediate-, and long-acting based on their duration of action.
The ultrashort-acting barbiturates, thiopental sodium and methohexital, are used in SA patients for induction of general anesthesia. The use of these drugs for induction of anesthesia in large animals is now rare, having largely been replaced by ketamine.
Thiopental sodium is rapidly absorbed into the brain, more slowly redistributed into muscle and fat, and later is metabolized and excreted. The patient recovers as the drug redistributes to the muscle and fat, thus decreasing the amount in the brain tissue. This results in rapid onset (30 to 60 seconds) and short duration (5 to 20 minutes) following a single dose. The effects of thiopental are cumulative if multiple doses are given, however, because the fat and muscle become saturated with the drug until it is metabolized and excreted. The result of this saturation is a prolonged recovery time. Because sight hounds have low body fat levels, saturation occurs more quickly, resulting in even longer recovery times than other animals.
Thiopental sodium causes a dose-dependent decrease in cardiac output, blood pressure, RR, and tidal volume (VT). It does not produce significant analgesia, however, and is a poor muscle relaxant. Adverse effects include hypotension, bradycardia, and arrhythmias, particularly ventricular premature contractions (VPCs) and bigeminy (alternating normal complexes and VPCs). It can also cause severe respiratory depression including a period of apnea following induction, which may require ventilatory support. Because neonates delivered by C-section also experience respiratory depression, this drug should not be used for this purpose.
Thiopental and other barbiturates cause swelling, pain, and tissue irritation if injected outside the vein. Treat perivascular injections by infusing the area with large volumes of normal saline with or without a local anesthetic.
If injected too slowly or without premedications, the patient may experience undue excitement during induction or recovery. The use of this drug alone in horses will induce ataxia and excitement and is not recommended. Other adverse effects include salivation, coughing, and laryngospasm, which can be decreased by concurrent use of anticholinergics. Thiopental should not be used in sight hounds because of the likelihood of prolonged recovery, in horses with leukopenia, or patients without suitable veins.
Barbiturates should be dosed based on lean body weight, patient condition, and concurrent administration of other preanesthetic and anesthetic drugs. Thiopental has a relatively narrow therapeutic index and must be administered cautiously. In healthy patients, give one half of the calculated dose intravenously as a bolus and the remainder to effect. Debilitated, hypothermic, geriatric patients or patients with major organ disease may require a much lower dose and may experience prolonged recoveries. Treat any overdose with supportive care.
Thiopental is packaged as a dry powder that must be reconstituted to a concentration ranging from 2% to 2.5% in small animals to 5% to 10% in large animals. The reconstituted solution has a shelf life of 7 days when refrigerated. Use the lowest concentration that is practical, and do not use thiopental if the solution is not clear.
Methohexital is an ultrashort-acting barbiturate used for induction and maintenance of anesthesia that has an onset of about 15 to 60 seconds and duration of about 5 to 10 minutes. This drug is reconstituted to a 1% solution and has a shelf life of 6 weeks at room temperature. It is considered safe in sight hounds because it is rapidly metabolized, and therefore repeat doses are not cumulative. Because it can cause excitement and seizures during induction or recovery, patients should be premedicated before administration.
Pentobarbital sodium is an intermediate-acting barbiturate that, although no longer used for routine anesthesia in clinical settings, is given intraperitoneally to induce general anesthesia in lab animals. It is also given intravenously to treat status epilepticus in small animals and is used in a concentrated form as a euthanasia agent.
IMIDAZOLE DERIVATIVES
Etomidate is a short-acting, injectable, imidazole-derivative sedative-hypnotic used for induction of anesthesia in dogs and cats. It is not a controlled substance. Etomidate causes minimal changes in cardiovascular and respiratory function and decreases both intracranial and intraocular pressure. It has a significantly wider therapeutic index than propofol and thiopental sodium. It is therefore the agent of choice for patients with severe heart disease or shock. Etomidate also produces good muscle relaxation, but no analgesia.
Although etomidate has a wide margin of safety, adverse effects include vomiting, muscle movements, sneezing, and excitement during induction and recovery. It also suppresses adrenocortical function. Premedication is recommended to reduce these adverse effects. IV injections may be painful and cause phlebitis, and rapid injection or repeat doses can cause hemolysis in cats. Administration through a running IV fluid line will decrease pain and hemolysis. Etomidate is not in common use because of its relative higher cost and adverse effects.
GUAIFENESIN
Guaifenesin, also known as “GG” or glyceryl guaiacolate, is an injectable muscle relaxant and sedative used in combination with other agents for short procedures or to improve the quality of induction and recovery in large animals. It is also used as an expectorant to treat respiratory conditions.
Adverse effects on the cardiovascular, respiratory, and GI systems are mild and of little consequence. If injected out of the vein, guaifenesin is irritating to the tissues. Solutions more than 7% in strength cause hemolysis in ruminants, whereas solutions more than 15% cause hemolysis in horses.
Guaifenesin is often administered as a 5% to 10% solution in dextrose by rapid IV infusion before ketamine or barbiturate induction to produce muscle relaxation in LA species. Several combinations are also commonly used. “Double drip” (ketamine and GG) is used for induction and/or maintenance of anesthesia in ruminants. “Triple drip” (xylazine, ketamine, and GG) is used for IV maintenance of anesthesia in horses.
INHALANT ANESTHETICS
Inhalant anesthetics are liquid agents that are vaporized in oxygen and administered via an anesthetic breathing system by endotracheal tube, mask, or chamber. Vapor pressure, blood-gas solubility coefficient, and minimum alveolar concentration measure properties of these agents that influence the way they are used. Therefore a basic knowledge of these concepts is necessary to use inhalant anesthetics effectively and safely.
Vapor pressure is a measurement of the tendency of a liquid to evaporate. Agents with a high vapor pressure evaporate readily, reaching dangerously high concentrations if not regulated and therefore must be administered using an agent-specific precision vaporizer. Conversely, low vapor pressure agents may be administered with a nonprecision vaporizer.
The blood-gas solubility coefficient is a measurement of the tendency of an agent to dissolve in blood. It is associated with the speed of induction, recovery, and change in anesthetic depth, each of which are faster when using agents with a low solubility coefficient and slower when using agents with a high solubility coefficient.
The minimum alveolar concentration (MAC) is the percent concentration of an agent required to prevent a response to surgical stimulation in 50% of patients and therefore is a measurement of the potency of an agent. An agent with a high MAC is less potent (more of the agent is required to attain surgical anesthesia) than an agent with a low MAC. Typically a dial setting of approximately 1.5 to 2 times the MAC is required to reach surgical anesthesia in a majority of patients.
HALOGENATED ANESTHETICS
These inhalant agents are used in a wide variety of species to induce and maintain general anesthesia. Isoflurane and sevoflurane are the halogenated anesthetics most commonly used in veterinary patients.
Halogenated anesthetics cause CNS depression, hypothermia, respiratory depression, hypotension, and muscle relaxation. Although they cause myocardial depression, cardiac function is maintained close to that of preanesthetic levels. These agents have little or no analgesic effect postoperatively.
Both isoflurane and sevoflurane have high vapor pressures (240 mm Hg and 160 mm Hg, respectively) and must be administered via a precision vaporizer. Both agents also have low solubility coefficients (1.4 and 0.6, respectively) resulting in relatively rapid inductions, recoveries, and changes in anesthetic depth.
Halogenated anesthetics induce a dose-dependent hypotension, which is more prominent with sevoflurane than isoflurane. They can also cause vomiting, nausea, and ileus in addition to a dose-dependent respiratory depression that can progress to apnea. Although the primary route of excretion for these agents is through the lungs, the amount that is metabolized by the liver and excreted by the kidneys varies agent to agent. About 3% of sevoflurane is metabolized, whereas only about 0.2% of isoflurane is metabolized, making it an excellent anesthetic choice for patients with kidney or liver disease.
There have been reports of fire or extreme heat production when sevoflurane is used with desiccated carbon dioxide (CO2) absorbent. This problem is more common when using low oxygen flow over a long period of time. To prevent this complication, turn off the machine when not in use, replace absorbent granules regularly, avoid the use of low oxygen flow for protracted periods, and monitor the temperature of the absorbent canister. Sevoflurane also reacts with chemicals in CO2 absorbent to produce compound A, a chemical that causes renal damage in rats. This effect has not been found to be clinically significant.
Although isoflurane and sevoflurane are similar, there are subtle differences in the way they are used. Because isoflurane is irritating to mucous membranes, patients may struggle and hold their breath during mask or chamber induction. In contrast, sevoflurane is not irritating, making it ideal for anesthetic inductions. One of the chief advantages of sevoflurane is the rapid inductions, recoveries, and changes in anesthetic depth associated with this agent. Therefore safe use of this agent requires subtle dial changes and vigilant monitoring on the part of the veterinary anesthetist.
Halothane was used extensively in veterinary anesthesia for many years, although use has gradually decreased as isoflurane and sevoflurane have gained wide acceptance. Halothane has a similar vapor pressure to isoflurane, necessitating use of a precision vaporizer. It can be used for mask or chamber induction, but has a higher solubility coefficient (2.4) than isoflurane. Consequently, inductions, recoveries, and changes in anesthetic depth take somewhat longer. Halothane has similar effects to isoflurane, but causes less respiratory depression, has a greater tendency to induce cardiac arrhythmias, and is a more potent cardiac depressant.
Methoxyflurane is currently off the market, but was used for many years to maintain anesthesia. Because of its low vapor pressure (23 mm Hg), methoxyflurane can be administered from a nonprecision vaporizer in-circle (VIC). It cannot be used for mask or chamber inductions, however, because of a high solubility coefficient (15). Methoxyflurane is 50% metabolized and has been linked to organ damage.
Desflurane is similar to isoflurane, but has an extremely high vapor pressure and low solubility coefficient. Because the boiling point of this agent is near room temperature, desflurane requires a special, expensive electronic vaporizer. Inductions and recoveries are even more rapid than with sevoflurane, producing what is sometimes referred to as “one-breath anesthesia.” Desflurane is not arrhythmogenic, but causes a dose-related respiratory depression.
Enflurane has not found wide acceptance in veterinary medicine as a result of adverse effects.
NITROUS OXIDE
Nitrous oxide (N2O), sometimes referred to as “laughing gas,” is a gas at room temperature and is stored in compressed gas cylinders identified by a deep blue color. N2O is administered along with oxygen through a gas-specific flowmeter that is blue in color.
N2O is one of the oldest inhalant anesthetics, its use dating back to the mid-1800s. N2O will not produce general anesthesia alone, but speeds the uptake of other agents into the bloodstream and allows lower doses to be used because it has analgesic properties. In the past, it was administered in conjunction with older halogenated agents, such as methoxyflurane. When used with newer agents, these benefits are of less clinical importance. It is therefore seldom used in practice.
Because N2O displaces oxygen in the lungs and breathing circuit, it can cause hypoxemia during anesthesia or recovery. It also diffuses into air spaces, such as the chest cavity of patients with pneumothorax and the stomach of patients with gastric dilation-volvulus, thus worsening these conditions. Its use is contraindicated in ruminants and horses because of the large amount of air in the rumen and large intestine, respectively. Specific flow rates must be used to maintain oxygenation, and other precautions must be taken, which are detailed in most veterinary anesthesia textbooks.
ANESTHETIC EQUIPMENT
An endotracheal tube is a device that is placed inside the trachea of an unconscious patient, attached to a breathing circuit, and used to administer oxygen and inhalant anesthetics. Endotracheal tubes increase patient safety because they maintain an open airway; minimize the likelihood of pulmonary aspiration of blood, stomach contents, and other substances; facilitate administration of supplemental oxygen; and allow the anesthetist to ventilate the patient when necessary. Because of these benefits, many veterinarians prefer to place an endotracheal tube in all patients undergoing general anesthesia even if not receiving inhalant anesthetics.
Endotracheal tubes come in a variety of sizes, lengths, and types needed to accommodate the wide size variation of veterinary patients and may be made of red rubber (Figure 27-1, D), silicon rubber (Figure 27-1, A), or polyvinyl chloride (PVC) (Figure 27-1, C and E). Murphy tubes have a side hole called the Murphy eye (Figure 27-2, J) at the beveled end that permits airflow in the event of blockage of the tip. The patient end of a Cole tube is tapered and has no cuff (Figure 27-1, B). This type is used for small patients and birds, which do not have an expandable trachea.
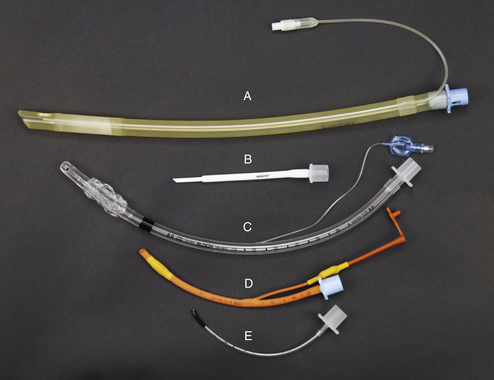
FIGURE 27-1 Endotracheal tube type, material, and size comparison. A, Cuffed 11-mm silicone rubber tube. B, 2.5-mm Cole tube. C, Cuffed 8-mm PVC tube. D, Cuffed 4-mm red rubber tube. E, Uncuffed 2-mm PVC tube.
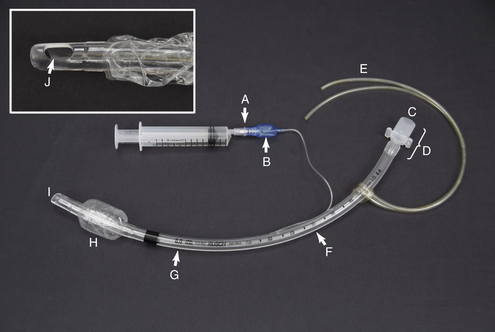
FIGURE 27-2 Endotracheal tube parts. A, Valve with syringe attached. B, Pilot balloon. C, Patient end. D, Connector. E, Tie. F, Measurement of length from the patient end (cm). G, Measurement of ID (mm). H, Inflated cuff. I, Patient end. J, Murphy eye.
An endotracheal tube consists of the following parts: The connector (Figure 27-2, D) is attached to the breathing circuit of the anesthetic machine or to an Ambu bag. The cuff (Figure 27-2, H) is a balloonlike part at the beveled patient end (Figure 27-2, I), which when inflated, creates a seal between the tube and the tracheal mucosa. This prevents mixing of room air and anesthetic gases and prevents aspiration of liquid or solid materials around the tube. The cuff is connected by a small tube to the pilot balloon (Figure 27-2, B) and a valve (Figure 27-2, A), which is used to inflate the cuff. The pilot balloon allows the anesthetist to monitor cuff inflation.
Although there are several different scales used to measure the diameter of an endotracheal tube, internal diameter (ID) is most common (Figure 27-2, G). Tubes for dogs and cats range in size from 3.0 to 14 mm ID. Small ruminants, swine, and foals require tubes between 6 and 18 mm ID. Small exotic animals may require tubes as small as 1.0 mm ID.Horses and mature cattle require sizes ranging from 16 to 30 mm ID.
LARYNGOSCOPES
Laryngoscopes are used to visualize the larynx while placing endotracheal tubes. A laryngoscope consists of a handle and a blade that is used to depress the tongue and includes a light-source to illuminate the throat (Figure 27-3). Common blade sizes range from 0 (small) to 5 (large), although longer blades are available for use in swine and some exotics. Miller blades are straight (Figure 27-3, A and C) and McIntosh blades are curved (Figure 27-3, B and D). Laryngoscopes are often used in small ruminants, camelids, and swine, may be helpful in dogs and cats, but are not used in adult cattle, which are intubated by digital palpation, and horses, which are intubated blindly.

FIGURE 27-3 Laryngoscope handles and blades. A, Size-4 Miller blade. B, Size-4 McIntosh blade. C, Size-2 Miller blade. D, Size-1 McIntosh blade. E, Laryngoscope handle with size-00 Miller blade in unlocked position. F, Laryngoscope handle with size-3 McIntosh blade in locked position (note that the light turns on when the blade is locked).
MASKS
Masks are cone-shaped devices used to administer oxygen and anesthetic gases to patients that are not intubated (Figure 27-4). They are usually made of plastic or rubber, come in a variety of sizes, and have a rubber gasket designed to create a seal around the patient's muzzle. The smallest mask that comfortably fits the patient should be selected. Masks may be used to induce or maintain anesthesia. They are frequently used to administer anesthetic gases to small patients in which intubation is difficult. Masks may also be used to administer oxygen during the preanesthetic and postanesthetic periods. Masks do not maintain an open airway, do not protect against aspiration, nor do they afford the ability to ventilate the patient as does an endotracheal tube.
ANESTHETIC CHAMBERS
Anesthetic chambers are solid boxes used to induce general anesthesia in small patients that are feral, vicious, intractable, or cannot be handled without undue stress (Figure 27-5). Chambers are usually clear to allow the anesthetist to observe the patient. They have two ports, one of which is attached to a fresh gas source and the other that allows exit of waste gas. A common way to set up a chamber is to attach the inhalation tube and exhalation tube of a semiclosed rebreathing system to each port in place of the Y-piece. Chambers prevent close monitoring of the patient during induction, thus necessitating extreme care when anesthetizing patients using this method.
THE ANESTHETIC MACHINE
Anesthetic machines are used to deliver inhalant anesthetics and oxygen to patients during general anesthesia. These machines are complex and have many specialized and distinct parts that must be properly used and maintained to ensure patient safety. Many different makes and models are in common use, ranging from state-of-the-art machines to those that have been in service for many years, so the veterinary technician may encounter a wide variety of machines in appearance, size, and age. The basic function and use is similar, however, and has not changed significantly over the past several decades. For this reason, a complete knowledge of anesthetic machine systems and associated equipment along with a review of the owner's manual will prepare the technician for operation of any machine he or she may encounter.
An anesthetic machine consists of the following general systems:
1. The carrier gas supply (Figure 27-6, A) delivers oxygen and other carrier gases to the patient at a controlled flow rate. Compressed gas cylinders, the pressure reducing valve, the tank and line pressure gauges, the flowmeters, and the oxygen flush valve are part of this system.

FIGURE 27-6 Anesthetic machine systems. A, Carrier gas supply: Note the two size-E compressed gas oxygen cylinders beside the “As” at the bottom of this image. B, Anesthetic vaporizer. C, Breathing circuit. Note that the scavenging system (see Figure 27-8) is not visible in this view.
2. The anesthetic vaporizer (Figure 27-6, B) vaporizes a precise concentration of liquid inhalant anesthetic and mixes it with the carrier gases.
3. The breathing circuit (Figure 27-6, C) delivers the anesthetic and oxygen mixture to the patient via an endotracheal tube, mask, or chamber and conveys expired gases away from the patient. Breathing circuits are classified as either rebreathing systems (see Figure 27-6) or nonrebreathing systems (Figure 27-7).
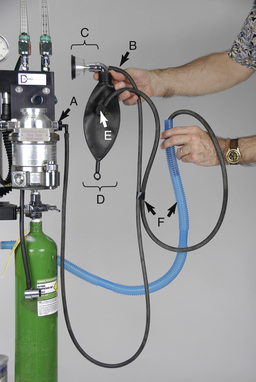
FIGURE 27-7 Parts of a nonrebreathing circuit. A, Outlet port of the vaporizer with keyed fitting. B, Fresh gas inlet. C, Connector with mask attached. D, Reservoir bag. E, Pressure relief valve. F, Scavenging hose.
4. The scavenging system disposes of waste and excess anesthetic gases (Figure 27-8).

FIGURE 27-8 Scavenging system: The waste gas exits from the pop-off valve (A) of this rebreathing system (or the discharge hose of a nonrebreathing system), flows through the vacuum regulator (B), and finally into either a charcoal canister (C) or alternatively into an outlet pipe in the ceiling or wall.
PREPARING THE MACHINE
Box 27-2 highlights the steps required to prepare an anesthetic machine for use.
Machine Assembly
Before using any anesthetic machine, attach all necessary parts including the vaporizer inlet and outlet port hoses, the reservoir bag, the corrugated breathing tubes, the scavenging system hoses, and any other parts required for the machine that you are using.
Checking for Leaks
To check the low-pressure system of a nonrebreathing system for leaks, occlude the patient connector and the scavenging hose or pressure relief valve. Turn the oxygen on to fill the bag. When the bag is full, turn the flowmeter off. The system has no leaks if the bag remains inflated for at least 10 seconds.
To check the low-pressure system of a rebreathing system for leaks, assemble the machine and secure all connections. Close the pop-off valve completely. Place your thumb over the Y-piece, and use the oxygen flush valve and the oxygen flowmeter to fill the reservoir bag until the pressure manometer indicates a pressure of 30 cm of water. Turn off the flowmeter. No leaks are present if the pressure decreases no more than 5 cm of water (to the 25 cm of water mark) in 10 seconds. As an alternative, when the pressure in the system reaches 30 cm of water, you may turn the flowmeter back on just enough to maintain pressure. A leak is present if greater than 200 ml/min is necessary.
Setting the Pop-off Valve
When using a semiclosed rebreathing system, adjust the pop-off valve immediately after checking the low-pressure system for leaks. With the Y-piece occluded, turn the oxygen flow back on to the anticipated maximum for that procedure. A general rule of thumb is about 1 to 3 L/min for patients under 30 lb and about 3 to 5 L/min for patients more than 30 lb. Then open the pop-off valve gradually until the pressure manometer indicates a pressure of 1½-2 cm of water.
THE CARRIER GAS SUPPLY
The gases into which the liquid inhalant anesthetic evaporates and that carry the vaporized anesthetic to the patient are referred to as carrier gases. Oxygen is the carrier gas used during all anesthetic procedures. Oxygen administration is necessary throughout anesthesia not only to carry the anesthetic, but also to compensate for the diminished RR, depth, and available oxygen that most patients experience during anesthesia. In some circumstances, N2O may be used with oxygen, although in recent years, use of N2O in veterinary patients has declined.
Compressed gas cylinders store carrier gases at high pressure (see Figure 27-6, A). They are attached to the yoke (Figure 27-9, A) of the anesthetic machine or alternatively may be stored in a remote location and connected to the machine via gas lines. An outlet valve is located on the top of all compressed gas cylinders (Figure 27-9, C). This valve must be opened when the cylinder is in use by turning the valve stem counterclockwise until it is fully open. When opened, gas will flow through the yoke and into the anesthetic machine. The valve is closed by turning it clockwise (see Figure 27-9, right side).
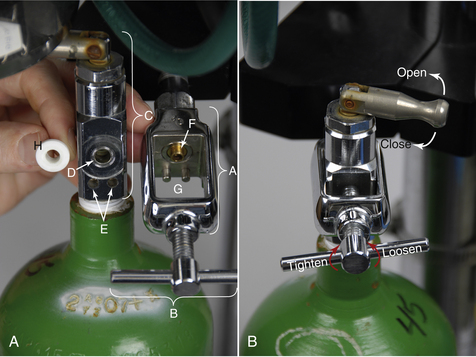
FIGURE 27-9 A (right image), Parts of compressed gas cylinder and yoke. A, Yoke. B, Wing nut. C, Outlet valve. D, Valve port. E, Pin holes. F, Nipple of yoke. G, Index pins. H, Nylon washer. B, Opening-closing the outlet valve; loosening-tightening the wing nut.
Compressed gas cylinders are usually owned by a supplier that will pick up and refill them as needed. Always be sure that you have at least one spare full tank before commencing any anesthetic procedure. If the primary tank runs out, you will always have a second tank available.
Three holes are visible on the face of the outlet valve. The large hole is the valve port where the gas exits the cylinder (Figure 27-9, D). This port fits onto the nipple of the yoke (Figure 27-9, F) with a nylon washer in between (Figure 27-9, H). The two smaller holes (Figure 27-9, E) fit onto index pins (Figure 27-9, G), which hold the cylinder in place. These holes and pins are a specific distance apart for each gas, a feature that prevents a cylinder containing the wrong gas from being attached to the yoke.
When removing a cylinder from the machine, be sure the outlet valve is closed and the oxygen is evacuated (“bled off”) from the system (see Tank Pressure Gauge for a review of this procedure). Support the cylinder, loosen the wing nut (Figure 27-9, B) and back the valve port off of the yoke. Carefully lower the tank until the valve clears the yoke. When attaching a full cylinder, first inspect the valve port for cleanliness then place a clean, undamaged nylon washer between the valve port and the nipple. Gently raise the tank into place, lining up the valve port and the pin holes with the corresponding structures on the yoke. Tighten the wing nut as securely as you are able to by hand. Open the valve slowly and listen for leaks. If there is a leak, recheck the holes for proper alignment, tighten the wing nut further, or use a new washer.
Compressed gas cylinders may contain different gases. To prevent confusion, cylinders are color coded as follows: Green (United States) or white (international) is oxygen; blue is N2O. These cylinders come in two sizes called E tanks (see Figure 27-6, A at the bottom) and H tanks. E tanks are stored either on the yoke of the anesthetic machine or in a rack. H tanks are much larger and are stored on a movable cart or chained to the wall and are often used to feed centralized oxygen sources. A centralized oxygen source is one in which the oxygen from an H tank is piped to outlets at various points around the hospital. The outlets are then connected to anesthetic machines via quick-release connectors.
Some larger practices use a concentrator as a primary oxygen source. An oxygen concentrator is a machine that extracts oxygen from room air.
The tank pressure gauge (Figure 27-10, B) indicates the pressure in a compressed gas cylinder. When full, a cylinder contains oxygen at a pressure of approximately 2200 pounds per square inch (psi). As the oxygen is used, the cylinder pressure gradually decreases. Oxygen cylinders should be changed when the pressure reaches 100 to 200 psi or a level at which the tank does not contain enough gas to last the anticipated length of the procedure. It is important to check the pressure before and throughout the anesthetic period to be sure that there is enough oxygen to complete the procedure.

FIGURE 27-10 A, Line pressure gauge (registering 48 psi). B, Tank pressure gauge (registering 800 psi). C, Pressure reducing valve.
The volume of oxygen contained in an E tank (expressed in liters) can be estimated by multiplying the pressure in psi by a factor of 0.3. Therefore a full tank contains about 660 L of oxygen (2200 psi × 0.3 = 660). At a flow rate of 1 L/min, this will last about 660 minutes, or 11 hours. The volume of oxygen in an H tank in liters is about three times the pressure in psi. Therefore an H tank with a pressure of 1000 psi contains about 3000 L of oxygen. When a compressed gas cylinder is turned off, the tank pressure gauge will continue to register pressure until the system is evacuated or “bled off.” This is accomplished by depressing the oxygen flush valve until the gauge reads 0 psi.
The pressure reducing valve (Figure 27-10, C) reduces the pressure of the gas exiting the compressed gas cylinder to 40 to 50 psi. This pressure is maintained regardless of the pressure in the cylinder. The line pressure gauge (Figure 27-10, A) indicates the pressure in the line connecting the pressure reducing valve and the flowmeter(s). When the oxygen is turned on, this gauge should read 40 to 50 psi. Both of these parts function passively and require no action on the part of the machine operator.
The flowmeter (Figure 27-11) controls the rate at which the carrier gas is delivered to the patient, and reduces the pressure from 40-50 psi to 15 psi. Carrier gas flow rates are expressed in liters per minute (L/min). Flowmeters are gas specific and color coded to match the compressed gas cylinders (green for oxygen and blue for N2O). Therefore if using N2O in addition to oxygen, there will be flowmeters to adjust the flow separately for each carrier gas. Some machines, such as the one pictured, have two oxygen flowmeters. The meter on the right is used for flow rates greater than 1 L/min, and the meter on the left is used for flow rates less than 1 L/min.

FIGURE 27-11 Oxygen flowmeters with ball indicators: The flowmeter on the left is adjusted to 0.5 L/min, and flowmeter on the right is adjusted to 1.5 L/min for a total oxygen flow of 2 L/min.
Turn on a flowmeter by turning the dial counterclockwise. All flowmeters have a ball or rotor indicator that rises to a height proportional to the flow of gas. Read the center of a ball indicator or the top of a rotor indicator. When turning off these meters, turn clockwise just until the ball or rotor drops to zero. Even though the knob can still be turned, do not turn it any further to prevent damage to the valve.
Oxygen flow rates must be carefully chosen to ensure patient safety, produce desired changes in anesthetic depth, and to conserve carrier and anesthetic gases. Although in some practices it is common practice to use a standard rate of 1 to 2 L/min for most SA patients, using specific rates will improve patient response, cost savings, and safety. Oxygen flow rates depend on the type of equipment and system used. When using a rebreathing system, higher rates should be used during induction, recovery, and when changing anesthetic depth. Lower rates may be used during maintenance (see Box 27-3 for recommended oxygen flow rates).
The oxygen flush valve (Figure 27-12, F) delivers pure oxygen at 35 to 75 L/min directly to the breathing circuit, bypassing the flowmeter and vaporizer. The oxygen flush valve is used to quickly fill an empty reservoir bag with fresh oxygen, but will dilute the concentration of inhalant anesthetic in the breathing circuit when it is used. It is also used to deliver fresh oxygen to a critically ill patient or to flush inhalant anesthetic out of the circuit during anesthetic recovery or during a crisis. To flush the circuit, turn off the vaporizer, force the gases out of the reservoir bag using gentle hand pressure, and press the valve to refill the bag with fresh oxygen. When using this valve, use only short bursts to avoid overfilling the bag and damaging the patient's lungs as a result of a buildup of pressure.
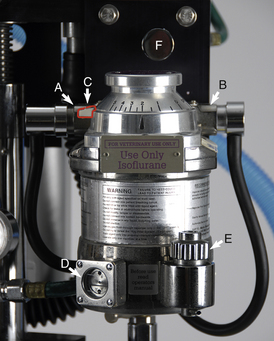
FIGURE 27-12 Precision anesthetic vaporizer for isoflurane set on 2%. A, Inlet port with keyed fitting leading from the flowmeters. B, Outlet port with keyed fitting leading to the fresh gas inlet. C, Safety lock. D, Indicator window. E, Fill port. F, Oxygen flush valve (part of the compressed gas supply).
ANESTHETIC VAPORIZERS
The anesthetic vaporizer holds liquid inhalant anesthetic and adds controlled amounts of vaporized anesthetic to the carrier gas. Vaporizers may be classified as precision or as nonprecision.
Precision Vaporizers
The inhalant anesthetics isoflurane and sevoflurane require the use of a precision vaporizer designed and color coded specifically for the agent used (purple is isoflurane; yellow is sevoflurane) (see Figure 27-12). Turn on the vaporizer by disengaging the safety lock (Figure 27-12, C) and turning the dial to the desired percent concentration.
With the exception of some older models, the amount of inhalant anesthetic gas vaporized by a precision vaporizer is independent of variables such as ambient temperature, oxygen flow rate, RR, depth, and back pressure. This allows precise delivery of high vapor pressure inhalant agents, such as isoflurane and sevoflurane. Precision vaporizers are located out of the breathing circuit because of their high resistance to gas flow and are therefore known as vaporizer out-of-circle or VOC (Figure 27-13).
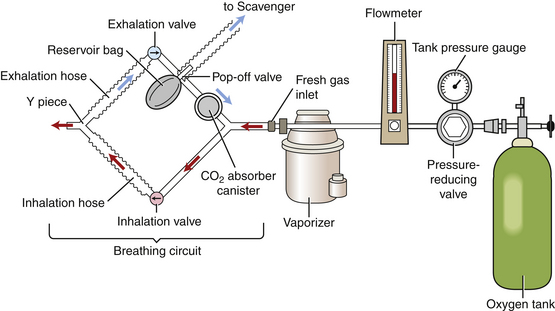
FIGURE 27-13 Diagram of an anesthetic machine with a rebreathing circuit and VOC. Note that the vaporizer is located outside of the breathing circuit.
All precision vaporizers will be somewhat affected by very high or very low carrier gas flow rates. Specifically, oxygen flows in excess of 10 L/min or lower than 500 ml/min may affect output. When flows are significantly less than the patient's respiratory minute volume (200 ml/kg/min), the output will decrease slightly as a result of a dilution effect by expired gases. Therefore higher dial settings may be needed under these circumstances.
Nonprecision Vaporizers
Nonprecision vaporizers are intended to be used only with low vapor pressure anesthetics, such as the discontinued agent methoxyflurane and, in contrast with precision vaporizers, are located in the breathing circuit (VIC) because they do not impede the flow of gases around the circuit as the patient breathes. These vaporizers do not measure a precise concentration and are affected by ambient temperature, oxygen flow rate, back pressure, and the patient RR and depth. Although infrequently used, these vaporizers may be used with high vapor pressure anesthetics, such as isoflurane, if specifically designed for this purpose.
Vaporizer Inlet Port, Outlet Port, and the Fresh Gas Inlet
The vaporizer inlet port (see Figure 27-12, A) is the point where oxygen and other carrier gases enter the vaporizer from the flowmeters. The vaporizer outlet port (see Figure 27-12, B) is the point where the oxygen, inhalant anesthetic, and other carrier gases exit the vaporizer. The point at which these gases enter the breathing circuit is referred to as the fresh gas inlet. The vaporizer outlet and inlet ports are connected to hoses with keyed fittings that prevent the operator from inadvertently attaching the wrong hose to the wrong vaporizer port.
BREATHING CIRCUITS
Breathing circuits circulate fresh gases to the patient and convey waste gases to the scavenging system. During inhalation and exhalation, the patient's lungs act like a bellows to move air through the breathing circuit. Nonrebreathing systems do not resist air movement and are generally used for smaller patients because they minimize the work required to breathe. In contrast, rebreathing systems resist air movement, thus impairing the ability of small patients to move the gases through the circuit.
The use of a nonrebreathing system (Figure 27-14) is recommended for patients under 7 kg in body weight. A nonrebreathing circuit is attached to the outlet port of the anesthetic vaporizer in place of the keyed fitting of a rebreathing circuit (see Figure 27-7, A). Fresh oxygen and inhalant anesthetic are delivered to the patient through a fresh gas inlet (see Figure 27-7, B) while exhaled gases pass into the scavenging system (see Figure 27-7, F), often after passing through a reservoir bag (see Figure 27-7, D). These systems flush out expired gases by use of a relatively high oxygen flow rate (200 to 300 ml/kg/min) and consequently do not require a CO2 absorbent canister or unidirectional valves. Nonrebreathing circuits are available in a variety of configurations in which the position of some parts including the fresh gas inlet, the reservoir bag, and the scavenger outlet varies. The Ayre's T-piece; Mapleson A, D, E, or F circuits; and Magill, Jackson-Rees, and Bain circuits are names of some of the nonrebreathing circuits available.
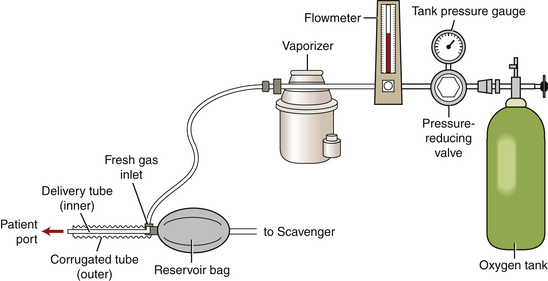
FIGURE 27-14 Diagram of an anesthetic machine with a nonrebreathing system attached to the vaporizer outlet port.
Nonrebreathing systems have disadvantages. They do not conserve gases, moisture, or body heat, thus increasing the
vigilance required to maintain patient body temperature. Manual ventilation and waste gas scavenging are more difficult.
Rebreathing systems deliver anesthetic gases to the patient, remove CO2, and recirculate carbon dioxide–free exhaled gases to the patient. These systems are also called “circle systems” because the gases move in a modified circular pattern (see Figure 27-13). Fresh oxygen enters the breathing circuit through the fresh gas inlet, and excess and waste gases exit the circuit through the pop-off valve. A rebreathing system may be used for patients with more than 2 kg of body weight providing it is fitted with pediatric breathing tubes when used to anesthetize patients between 2 and 7 kg.
Rebreathing systems have a number of advantages. They may be operated using lower gas flow rates and are therefore more economical than nonrebreathing systems. They allow waste anesthetic gas to be efficiently and easily scavenged. These systems also minimize body heat and moisture loss, and allow observation and control of patient ventilation. These systems also have disadvantages. Infectious agents may be transferred from patient to patient via microbe-laden moisture that condenses inside the machine parts and is inhaled by subsequent patients. Because the parts of these systems restrict air movement, they are not intended for use in small patients.
A semiclosed rebreathing system (partial rebreathing system) is a safe, practical, and economical system to use in general practice in all patients with a body weight greater than or equal to 2 kg. When using a semiclosed rebreathing system, the pop-off valve is partially open, the oxygen flow rate is higher than the metabolic needs of the patient (greater than 15 ml/kg/min), and waste gases exit through the pop-off valve.
A closed rebreathing system (total rebreathing system) is identical to the semiclosed system with two exceptions. The pop-off valve is nearly or entirely closed, and the oxygen flow rate is just enough to meet metabolic needs of the patient (7 to 15 ml/kg/min). In other words, in this system, approximately the same amount of fresh gas is added to the circuit as the patient consumes. With the exception of equine and bovine anesthesia, closed systems are infrequently used in practice because of the constant monitoring required, although most veterinary anesthesia texts include a detailed protocol for using these systems.
REBREATHING CIRCUIT PARTS
Unidirectional flow valves keep the flow of gases in a rebreathing circuit going one way as the patient breathes. The inhalation (inspiratory) valve (Figure 27-15, C) opens to allow gas to flow through the corresponding corrugated breathing tube and into the patient's lungs during inspiration. During expiration, the exhalation (expiratory) valve (Figure 27-15, A) opens to allow expired gases to flow through the corresponding corrugated breathing tube into the CO2 absorbent canister. Thus inhalation of expired gases containing CO2 is prevented.
FIGURE 27-15 Parts of a rebreathing circuit. A, Exhalation unidirectional flow valve. B, Pop-off valve. C, Inhalation unidirectional flow valve. D, Pressure manometer. E, 2-L reservoir bag. F, CO2 absorbent canister. G, SA corrugated breathing tubes.
The reservoir bag or rebreathing bag (Figure 27-15, E) is a storage reservoir for anesthetic gases. It holds gases that will fill the patient's lungs during inspiration, receives gases breathed out by the patient during expiration, and therefore should deflate during inspiration and inflate during expiration. The reservoir bag also allows the anesthetist to visually observe patient respirations and to manually ventilate for the patient, when necessary. Reservoir bags come in a variety of sizes.
The bag should contain enough gas to fill the patient's lungs during an inhalation, but should not be so large as to prevent visualization of respiratory movements. The following rules of thumb can be used when selecting a bag: 500 ml for up to 5 kg; 1 L for 6 to 10 kg; 2 L for 11 to 25 kg; 3 L for 26 to 45 kg; 5 L for greater than 45 kg. Patients more than 200 kgor that are intubated with at least an 18-mm endotracheal tube require an LA anesthesia machine that uses 30-L bags.
When in use, the reservoir bag should be an average of three-fourths full. The amount of gas in the bag is influenced by a variety of factors, including oxygen flow, pop-off valve adjustment, and scavenging system adjustment. Low oxygen flows, a full-open pop-off valve, or a maladjusted scavenging system can cause the bag to empty. This will prevent the patient from filling its lungs with anesthetic gases during inspiration and will impair the ability to manually ventilate for the patient. In contrast, high oxygen flows, a closed pop-off valve, or malfunctioning scavenging system may result in an overfilled bag. This may impair the patient's ability to expire or cause a buildup of pressure within the lungs and will impair the ability to monitor respirations by sight.
The pop-off valve or pressure relief valve (Figure 27-15, B)allows excess gases to exit the breathing circuit, transfers these waste gases to the scavenging system, and prevents buildup of excess pressure within the circuit. The pop-off valve allows a range of settings from fully closed to fully open to maintain optimum volume in the reservoir bag. When fully open, it releases when the gas pressure in the circuit exceeds 0.5 to 1 cm of water. As the valve is tightened, more pressure is required for release. When using a semiclosed rebreathing system, the valve is kept partially open when the patient is spontaneously breathing. It is closed only when providing manual ventilation so that gases may be forced into the patient's lungs using hand pressure. Between each breath provided by manual ventilation, it must be opened again to allow the escape of gases and to prevent excess pressure in the chest, which can lead to decreased cardiac output and death.
The CO2 absorbent canister (Figure 27-15, F) is connected to the exhalation valve and receives expired gases. The canister holds absorbent granules, such as calcium hydroxide, which passively remove CO2 from the expired air. Be sure to purchase an absorbent intended specifically for the inhalant agent that you are using.
The absorbent granules in the canister must be fresh to prevent the patient from rebreathing toxic levels of CO2. When saturated, the granules will no longer absorb the waste CO2 and therefore should be changed after 6 to 8 hours of use or when one third to one half of the granules become saturated. Fresh absorbent granules are able to be crushed and are white in color, but when saturated, become hard and turn an off-white color that is visibly distinguishable from the original. Most absorbents also contain a pH indicator that will cause a color change to blue or violet when saturated. The color reaction does not always occur, however, and will dissipate after a few hours if not noted.
The pressure manometer (Figure 27-15, D) indicates pressure (expressed in centimeters of water) in the breathing circuit and the patient's lungs. This pressure is influenced primarily by oxygen flow and pop-off valve adjustment. The pressure manometer should read 0 to 2 cm of water when the patient is breathing spontaneously. It should read no more than 20 cm of water in small animals or 40 cm of water in large animals when providing manual or mechanical ventilation unless the chest cavity is open, in which case the pressure can be somewhat higher. Excessive pressure in the circuit can result in dyspnea, lung damage, pneumothorax, and decreased cardiac output. Therefore frequent monitoring of the pressure is critical during any anesthetic procedure.
The negative pressure relief valve admits room air into the breathing circuit if a vacuum is detected, thus preventing patient asphyxiation. A vacuum may occur if the scavenging system exerts excessive suction, if the oxygen flow is too low, or if the oxygen cylinder is empty.
The corrugated breathing tubes (Figure 27-15, G) complete the breathing circuit by carrying the anesthetic gases to and from the patient. The Y-piece connects the inhalation and exhalation corrugated breathing tubes together. The opposite ends of the breathing tubes attach to the unidirectional valves. The remaining port of the Y-piece is then connected to a mask or to an endotracheal tube. LA tubes are 50 mm in diameter. SA tubes (Figure 27-16, A) are 22 mm in diameter. Pediatric tubes (Figure 27-16, B), which are shorter and smaller than conventional SA tubes, decrease mechanical dead space and are intended for patients between 2 and 7 kg of body weight. The Universal F-circuit (Figure 27-16, C) is a type of SA breathing tube in which the inhalation tube is located within the exhalation tube. This arrangement is designed to conserve body heat. As the cold inspired gases travel through the inner turquoise tube, the warm expired gases travel through the outer, transparent tube, warming the inspired gases.
SCAVENGING SYSTEM
This system of hoses and pipes is connected to the pop-off valve (see Figure 27-8, A) or other breathing circuit outlet and transfers waste gas outside the building through a system of pipes. Active scavenging systems use a fan to remove waste gas, whereas passive scavenging systems work by gravitational flow. Some active scavenging systems have a vacuum regulator (see Figure 27-8, B) that can be adjusted to prevent inadequate or excessive vacuum.
All scavenging systems must be checked periodically to be sure that the tubes are not blocked and that the vacuum is properly adjusted. Excess vacuum from an active scavenging system will draw all the gas out of the breathing circuit. This may lead to asphyxiation and can be recognized by a collapsed reservoir bag. Obstruction of the scavenging system will have the same effect as a closed pop-off valve and is indicated by a full reservoir bag or pressure buildup in the circuit.
An activated charcoal cartridge (see Figure 27-8, C) is attached to the discharge hose of the scavenging system and may be used as an alternative when a conventional scavenging system is not available. The activated charcoal will absorb most commonly used inhalant anesthetics (except N2O) as the waste gas is filtered through the cartridge. Activated charcoal cartridges should be weighed before use and must be replaced after a weight gain of 50 g.
ANESTHETIC MACHINE MAINTENANCE
All anesthetic machines require regular maintenance to keep them functioning properly. Whereas much of the maintenance of individual parts can be performed by the anesthetist as detailed later, the machine should be inspected and maintained by a repair professional at least once a year to ensure proper operation.
The tank pressure gauge, line pressure gauge, pressure reducing valve, flowmeters, oxygen flush valve, pop-off valve, negative pressure relief valve, and the pressure manometer do not require regular maintenance, but should be checked by a repair professional for proper function annually or when a problem is suspected. Corrugated breathing tubes, reservoir bags, and other detachable rubber parts should be cleaned periodically with a mild disinfectant, such as chlorhexidine, rinsed, dried, inspected for holes or defects, and replaced as necessary.
Unidirectional flow valves should be disassembled, cleaned with 70% isopropyl alcohol or mild disinfectant, dried, and inspected before reassembly to ensure that neither the valve nor valve seat is damaged or warped. An incompetent valve will allow rebreathing of expired CO2—a serious and potentially fatal complication.
To change the CO2 absorbent, disassemble the canister, dispose of the exhausted granules, check the gaskets for damage, and clean each part with mild soap and water. Rinse, dry, and reassemble the parts. Fill the canister loosely with fresh absorbent granules, leaving at least one half inch of air space at the top. Following reassembly, the canister must be airtight.
ANESTHETIC MONITORING
There is a common perception that modern anesthetic agents are safe. This belief can lead to the erroneous assumption that careful monitoring is not as important as it once was. Anesthetic monitoring is and always has been one of the cornerstones of the successful practice of anesthesia. Conditions that may lead to serious complications are often subtle, but must be recognized and corrected without delay. Serious anesthetic complications often develop rapidly and if not prevented, are devastating for the patient, the owner, and the anesthetist. Consequently the anesthetist must develop the ability to endure long periods of relative boredom in a state of readiness to manage periods of urgency and crisis. This requires that the anesthetist be knowledgeable, alert, and watchful for subtle changes in the condition of the patient.
During any general anesthetic procedure, the anesthetist must strike a delicate balance of sufficient depth of anesthesia to produce unconsciousness and insensitivity to pain without endangering the life of the patient by compromising cardiovascular and respiratory system function. Monitoring allows the anesthetist to achieve this balance through careful observation and precise regulation of the amount of anesthetic administered.
STAGES AND PLANES OF ANESTHESIA
In the early 1900s, a system of stages and planes of general anesthesia was developed to describe patient responses to diethyl ether, an early inhalant anesthetic that is no longer used in clinical practice. Changes in patient behavior, body movements, ocular signs, reflexes, and vital signs in response to the progression from consciousness to deep surgical anesthesia were observed and documented. Under this system, general anesthesia was divided into four stages (I to IV), and stage III was subdivided into four planes (1 to 4).
Stage I—The Period of Voluntary Movement
During this stage, the patient gradually loses consciousness. It is usually characterized by fear, excitement, and struggling. The HR increases, and the patient may hold its breath, urinate, or defecate. Near the end of stage I, the patient loses the ability to stand and becomes recumbent.
Stage II—The Period of Involuntary Movement
During this stage, also known as the excitement stage, the patient loses voluntary control and assumes a regular breathing pattern. It is usually characterized by involuntary reactions in the form of vocalizing, struggling, or paddling. The HR and RRs are often elevated, pupils are dilated, muscle tone is marked, and reflexes are present.
Stage III—The Period of Surgical Anesthesia
During this stage, the patient is unconscious and progresses gradually from light to deep surgical anesthesia. It is characterized by progressive muscle relaxation, decreasing heart and respiratory rates, and loss of reflexes. The pupils gradually dilate, tear production decreases, and the pupillary light reflex is lost. The increase in heart and respiratory rates seen in response to surgical stimulation during light anesthesia is gradually lost. Many authors now divide this stage into three planes corresponding to light (stage III, plane 1), medium (stage III, plane 2), and deep (stage III, plane 3) surgical anesthesia.
Stage IV—The Period of Anesthetic Overdose
During this stage, the nervous, cardiovascular, and respiratory systems are extremely depressed. Breathing stops, muscle tone is flaccid, pupils are widely dilated, and all reflexes are absent. The heart stops, and death follows quickly unless rapid action is taken.
PRINCIPLES OF MONITORING
Healthy patients (physical status class P1) should be monitored at least every 5 minutes during any general anesthetic procedure. Higher-risk patients (physical status classes P2 to P5) must be monitored more frequently and in some cases continuously. Ultimately the anesthetist must judge the frequency of monitoring that is appropriate for each patient. An anesthesia record should be used to document monitoring parameters, drug administration, and other information pertinent to the procedure (Figure 27-17).
FIGURE 27-17 Anesthesia record: The form is used to document the anesthetic protocol, monitoring parameters, treatments, and other information pertinent to the procedure.
At any given time during anesthesia, the patient should be somewhere between consciousness and deep surgical anesthesia (stage III, plane 3). Careful observation of the patient based on expected responses enables the anesthetist to determine the stage that the patient is in. For instance, unconsciousness, intact reflexes, eyes in a central position, marked jaw tone, and movement in response to stimulation are all expected responses from a patient in light surgical anesthesia (stage III, plane 1), a point inappropriate to perform surgery. In contrast, absent palpebral, pedal, and swallowing reflexes; moderate jaw tone; eyes in a ventromedial position; and an intact corneal reflex are all expected responses from a patient in medium surgical anesthesia (stage III, plane 2), an appropriate stage to begin.
There are many factors that influence progression through the stages of anesthesia and interpretation of physical signs. For instance, patients induced using IV agents often pass through stages I and II so quickly that they are minimally noticeable. In contrast, patients induced with an inhalant agent take longer to reach surgical anesthesia and may be difficult to control while passing though these stages. The administration of premedications generally decreases excitement seen during stages I and II and therefore eases passage into surgical anesthesia.
In addition, the anesthetic protocol will influence interpretation of physical signs. For example, a patient induced with an α2-adrenergic agonist will often have a much lower HR than one induced with an inhalant agent. A patient may have dilated pupils if given a dissociative agent, but conversely may have constricted pupils if given an opioid. Consequently, physical signs must be interpreted in light of the agents administered.
After considering each of these factors, the anesthetist must ultimately answer two questions:
1. Is the patient safe or in danger?
2. Is the anesthetic depth inadequate, excessive, or appropriate for the procedure performed?
Monitoring parameters are the physical signs used to answer these questions. They are subdivided into vital signs, reflexes, and other indicators of anesthetic depth. Although all monitoring parameters are evaluated together, some are more useful for answering the first question, whereas others are more useful for answering the second.
VITAL SIGNS
Vital signs are used primarily to evaluate the cardiovascular and pulmonary systems. Vital signs include the HR and rhythm, RR and depth, mucous membrane color, CRT, blood pressure, and temperature. These parameters are used principally to answer the question: “Is the patient safe or in danger?” They are only loosely correlated with the depth of anesthesia. Specifically, patients in lighter planes of anesthesia tend to have higher HRs, RRs, and blood pressure; pinker mucous membranes; and more rapid CRT, whereas patients in deeper planes experience opposite effects (Table 27-3). However, other factors may change this association. For example, a patient in light surgical anesthesia given an opioid agonist may have a decreased HR. Conversely a patient in deep surgical anesthesia may have an elevated HR if in shock. Although vital signs are helpful in determining anesthetic depth, other parameters are better suited to this purpose.
Heart rate measured in beats/min (bpm), and heart rhythm (Table 27-4) may be monitored by palpation of the chest wall, palpation of the pulse, auscultation, or use of monitoring equipment. The HR generally decreases gradually in response to increasing anesthetic depth because most anesthetic agents are cardiovascular depressants. This effect is extremely variable, however, and is influenced by the specific agents used, blood pressure, preexisting illness, and other factors. Although bradycardia is caused to some degree by most anesthetic agents, the opioids and α2-adrenergic agonists are particularly likely to have this effect. Conversely, dissociatives and anticholinergics may cause tachycardia.
TABLE 27-4
Normal and Abnormal Heart Rate and Rhythm

∗In addition to the rates listed, any arrhythmia should be reported to the veterinarian.
†Because of the extreme variability of size, large dogs tend to have lower rates, whereas small dogs and puppies have higher rates.
During anesthesia, the normal heart rhythm is normal sinus rhythm (NSR) or sinus arrhythmia (SA) in dogs and NSR in cats. Large animals typically exhibit NSR, but SA may also be observed. Cardiac arrhythmias can be induced by anesthetic agents, particularly the α2-adrenergic agonists, barbiturates, anticholinergics, and dissociatives, but can also be caused by other conditions, including hypoxia, gastric dilation-volvulus, hypercarbia, preexisting heart disease, and trauma.
Mucous membrane color (Table 27-5) is monitored by observing the color of the oral mucous membranes. Capillary refill time (Table 27-5) is the time it takes (in seconds) for the normal color to return following application of digital pressure to the gums near the base of a tooth. If the oral tissues are pigmented, the tongue, conjunctiva, or mucous membranes of the prepuce or vulva can be used as alternatives.
Pale mucous membranes indicate any cause of poor capillary perfusion or anemia. Prolonged CRT indicates poor capillary perfusion. Cyanosis indicates low oxygen saturation and is a medical emergency.
Blood pressure is monitored by indirect measurement using a Doppler or oscillometric monitor or by direct measurement via an arterial catheter. Pulse strength (see Table 27-5) as determined by palpation of a peripheral artery (such as the lingual, femoral, carotid, or dorsal pedal artery in small animals or the facial, auricular, digital, or dorsal pedal artery in large animals), gives the anesthetist a crude indication of blood pressure. A strong pulse is suggestive of normal blood pressure, and a weak one is suggestive of hypotension. The pulse strength varies widely among individuals, so the anesthetist should assess the pulse before anesthetic administration as a point of reference.
Respiratory rate measured in breaths/min (bpm), respiratory effort, and VT (Table 27-6) are monitored by observing movement of the chest wall, expansion and contraction of the reservoir bag, or movement of the unidirectional flow valves or with monitoring equipment. Auscultation—an important tool for evaluating lung sounds—does not work well to monitor RR and depth. It is advisable to observe the patient's respiratory depth and quality while awake as a point of reference.
Since many anesthetic agents are respiratory system depressants, anesthetized patients will often experience a decrease in RR and about a 25% decrease in VT directly related to anesthetic depth. This hypoventilation can lead to atelectasis. Atelectasis results in decreased gas exchange, which may lead to hypoxemia. This effect is common during anesthesia, particularly in the dependent lung (the one nearest the table). Many clinicians recommend gentle inflation of the lungs by periodic manual ventilation about every 5 minutes during anesthesia to prevent this complication. This technique is referred to as “bagging” or “sighing” the patient.
In addition to the effects of anesthetic agents, there are many other potential causes of hypoventilation, including postinduction apnea. As the name implies, postinduction apnea is a phenomenon that commonly occurs following anesthetic induction for the following reason. During induction, all patients pass through lighter stages of anesthesia during which hyperventilation occurs for a period of time ranging from several seconds to as much as a few minutes. Hyperventilation of a sufficient length will cause the patient to expire excessive quantities of CO2 resulting in a decreased concentration of CO2 in the blood. In response to the decreased concentration, the patient then hypoventilates or stops breathing until a normal CO2 level is reestablished.
Postinduction apnea can be frightening and dangerous if not understood and must be managed promptly with supportive care. Supportive care involves careful monitoring of other parameters, including oxygen saturation, and periodic manual ventilation about 2 to 4 times/min to maintain adequate oxygen levels until the patient begins to breathe spontaneously. When managing postinduction apnea, avoid ventilating too frequently so that the normal CO2 level can be reestablished. The use of a capnograph allows precise measurement of CO2 levels and so is a useful tool for managing this problem.
During anesthesia, the patient should exhibit normal respiratory effort. Dyspnea indicates a serious patient problem or machine malfunction that must be addressed promptly. Abdominal breathing is a unique breathing pattern associated with dangerously excessive anesthetic depth. It is characterized by a rocking motion of the abdomen and chest as a result of paralysis of the respiratory muscles and must be acted upon immediately.
Body temperature (Table 27-7) should be monitored about every 15 to 30 minutes with a rectal thermometer or probe. Although body temperature can be estimated by touching the skin of the paw or ear, it cannot be accurately determined this way. Hypothermia is experienced by most patients during anesthesia, but can lead to prolonged recovery and predisposition to anesthetic overdose if severe. Hypothermia usually occurs rapidly following induction and is often of a relatively large magnitude. Therefore the following steps should be taken to reduce heat loss during all anesthetic procedures:
• Do not allow the patient's body to contact stainless steel.
• Place a heat-retaining surface under the patient, such as a warm-water circulating blanket, blanket, towel, or lamb's wool.
• Warm IV fluids before administration.
• During preparation of the surgery site, avoid the use of alcohol as a rinsing agent or wetting the hair excessively.
• Avoid excessively low ambient temperatures in the surgical suite.
Malignant hyperthermia is a complication of general anesthesia in which the body temperature progressively rises to dangerous levels. Some animals, such as Pietrain, Landrace, and Poland China swine are genetically predisposed, but it may also be a reaction to certain drugs including the inhalant anesthetics. Malignant hyperthermia may occur in a variety of species, is a medical emergency, and must be promptly recognized, reported, and treated. Early signs include muscle rigidity and excessive production of CO2, which leads to exhaustion of the CO2 absorbent.
REFLEXES AND OTHER INDICATORS OF ANESTHETIC DEPTH (TABLE 27-8)
Reflexes are involuntary protective responses to stimuli (such as the “kick” response when your physician taps your knee with a neurologic hammer). Reflexes and other indicators of anesthetic depth are used to answer the question “Is the anesthetic depth inadequate, excessive, or appropriate for the procedure being performed?”
TABLE 27-8
Interpretation of Reflexes and Other Indicators of Anesthetic Depth
∗Absent when using halogenated inhalant agents (small animals); may be sluggish when other agents are used to maintain anesthesia or in large animals.
†Nystagmus may be caused by severe hypoxia or hypercarbia in horses.
The palpebral reflex is induced by gently tapping the skin at the medial or lateral canthus of the eye with your finger. This reflex is present when the patient is too light, progressively diminishes with increasing depth, and is generally absent in surgical anesthesia in small animals, but may still be present in horses and ruminants until they are in a moderately deep plane of anesthesia.
The swallowing reflex is a normal reflex that occurs in response to the presence of saliva or food in the pharynx. It is detected by watching the throat for swallowing motions. The swallowing reflex is present when anesthetic depth is inadequate, but is absent in surgical and deeper stages of anesthesia and is therefore used to determine whether or not the patient is too light.
The pedal reflex is the withdrawal of a limb in response to a painful stimulus. To induce this reflex, place a limb in a relaxed position and vigorously pinch a toe. Withdrawal of the limb indicates inadequate depth of anesthesia.
The corneal reflex is induced by placing a drop of sterile artificial tears on the cornea. When the reflex is present, the eyeball will retract slightly within the orbit. When in lighter planes of anesthesia, a blink response may also occur. This response is difficult to interpret when the eyes are in a ventromedial position, but should be present during all planes of surgical anesthesia and therefore helps to determine if the anesthetic depth is excessive.
Muscle tone is most frequently determined in small animals by assessing jaw tone. Open the jaw with the fingers and feel for resistance. Muscle tone is high when the patient is too light, gradually diminishes with increasing anesthetic depth, and is described using the terms marked, moderate, loose, and flaccid. Muscle tone may alternatively be assessed by observing the size of the anal opening, which will be closed at lighter planes and progressively more open at deeper planes.
Eye position and pupil size are also useful in determining anesthetic depth. Eye position is central (straight forward) in light anesthesia, gradually shifts to a ventromedial position (toward the chin), and finally shifts back to a central position in deep anesthesia. In most patients, ventromedial deviation is common during surgical anesthesia. In some horses, eye position may change from ventromedial to central and back again. This is usually indicative of surgical anesthesia. The presence of nystagmus usually indicates a light plane of anesthesia in any patient.
Response to Surgical Stimulation
When stimulated by cutting or manipulation of viscera, the HR, RR, VT, or blood pressure may increase. A sudden marked increase in any of these parameters indicates inadequate depth of anesthesia. Mild changes in HR may occur, however, even in surgical anesthesia. Realize that the appropriate depth for each patient may vary somewhat according to the degree of surgical stimulation and pain associated with the procedure.
MONITORING EQUIPMENT
Monitoring equipment is a useful addition to physical assessment, but should never be used alone. The main advantages of this equipment are to give early warning of impending problems before serious consequences develop and to provide a measurement of certain physical parameters, such as blood pressure, oxygen saturation, or expired CO2, that is not possible with physical assessment. Monitoring equipment allows the anesthetist to assess the patient more precisely than would be otherwise possible. For instance, a pulse oximeter can detect hypoxemia long before cyanosis is visible. A capnograph can warn of inadequate or excessive ventilation accurately and rapidly, and an oscillometric monitor can detect subtle changes in blood pressure before a change in pulse strength is evident. In this section, the use of this equipment will be reviewed.
An esophageal stethoscope (Figure 27-18) is a device designed to amplify the sound of the heartbeat so that the anesthetist can monitor the heart from a distance. Although this device is not capable of determining the heart rhythm, changes in rate, irregularity, or interruption in the heart sounds can alert the anesthetist to a possible arrhythmia. Esophageal stethoscopes are inexpensive, easy to operate, and easily maintained.
FIGURE 27-18 A, Esophageal stethoscope. A, Catheter. B, Sensor. C, Base unit. B, Measurement of the catheter to the level of the fifth rib or the caudal border of the scapula (arrow).
Parts include esophageal catheters of various sizes, a sensor, and base unit, which amplifies and converts the heart sounds into an audible electronic signal. To use an esophageal stethoscope, lubricate the closed end of an appropriately sized catheter and insert it into the esophagus to the level of the heart (about the fifth rib). Attach the free end to the electronic base via the sensor. Adjust the position of the catheter and the volume until the signal is audible. Care is minimal. The base should be cleaned as needed and batteries must be changed periodically. Catheters should be washed with a disinfectant and dried following use. Avoid immersion or introducing water inside the catheter.
An electrocardiographic (ECG) monitor is used to monitor the HR and rhythm. Electrodes are attached to specific locations on the patient's skin, and the electrical activity of the heart and the HR are displayed on a screen in real time. These monitors generally require little care. When using an ECG monitor, realize that it is possible for the heart to stop beating and the electrical activity to continue for a time after the heart has stopped. Therefore never depend on this monitor alone as a guarantee of patient safety.
A pulse oximeter (Figure 27-19) is a device designed to detect changes in the oxygen saturation of hemoglobin. Red and infrared wavelength light is passed through or reflected off of a tissue bed, detected by a sensor, and analyzed. The sensor is sensitive to blood pulsation in the arteries, so it also determines the HR. The machine determines the percent oxygen saturation (%SpO2) by calculating the difference between levels of oxygenated and deoxygenated hemoglobin. Both the HR and the oxygen saturation are digitally displayed.

FIGURE 27-19 Pulse oximeter with transmission lingual probe: The upper number (97) represents the percent oxygen saturation (%SpO2). The lower number (70) represents the HR in beats per minute.
Normally, when breathing pure oxygen, hemoglobin in the lungs is at least 97% saturated with oxygen. Therefore during oxygen administration, the oxygen saturation should be greater than 95%. Saturation between 90% and 95% indicates a problem that must be acted upon. Saturation less than 90% indicates hypoxemia. Saturation less than 85% for longer than 30 seconds is a medical emergency.
Parts include a computerized base unit and a variety of probes, each of which analyzes light either passed through or reflected off a tissue bed. Pulse oximeter probes are classified as transmission or reflective. Transmission probes are constructed in a clamplike configuration. One of the jaws houses a light source, and the other houses a sensor that detects the transmitted light. Transmission probes must be applied over a nonpigmented tissue bed that is thin enough to allow light transmission, such as the tongue, lip, ear, flank fold, prepuce, vulva, digital web, nasal septum, or foot of smaller patients. Although these probes are able to function through a thin hair coat, excessive hair will prevent operation. The lingual probe and the “C” probe are examples of transmission probes (see Figure 27-20 for examples of probe types and placement).
FIGURE 27-20 Examples of pulse oximeter probes and locations for placement. Red, Transmission probe on the ear flap. Additional red dots show alternate placement locations for this probe (tongue, lip, and flank fold). Green, Reflective probe taped to the ventral surface of the tail base. Blue, “C-probe” (a transmission probe) on the toe web. The other blue dot shows an alternate placement location for this probe (the skin fold between the Achilles tendon and the tibia).
Reflective probes are often long and narrow. The light source and sensor are located next to each other on one side of the probe. These probes are placed inside a hollow organ, such as the esophagus or rectum, with the side housing the light source and sensor in contact with a tissue bed. When placing a reflective probe in the rectum, care must be taken to digitally displace the feces from the wall of the rectum and place the correct side of the probe against the tissue. A reflective probe may also be taped against the ventral surface of the tail (see Figure 27-20).
Pulse oximeter probes can be frustrating to work with because of the high incidence of signal loss. When this happens, values will no longer appear on the display, the numbers will be incorrect, or an alarm may sound, and the probe must be readjusted or moved to a different location. Probe function is adversely affected by many factors, including tissue pigmentation, motion, excessive pressure, orientation in relation to ambient light, and patient conditions, such as anemia, icterus, vasoconstriction, or edema. Box 27-4 contains suggestions for trouble-shooting signal loss.
Pulse oximeters require little maintenance, but must be handled with care. Probes should be cleaned with alcohol or other mild disinfectant, but must not be immersed, scrubbed, or autoclaved.
An ultrasonic Doppler monitor (Figure 27-21) is a device that detects the flow of blood through small arteries and converts this motion into an audible signal. The blood flow is converted to a continuous sound similar to that of a heart murmur. Parts include an electronic base unit that processes the signal and a probe that emits and receives an ultrasonic wave. The hair overlying a small artery must be clipped and the skin cleaned and covered with a generous amount of ultrasonic gel. The probe is positioned over the artery, adjusted until an audible signal is detected, and then taped in place.

FIGURE 27-21 Doppler monitor: Base unit with the probe positioned over the ventral surface of the metacarpus proximal to the metacarpal pad.
The placement of an ultrasonic Doppler probe requires patience and finesse. It must be oriented parallel to and precisely over the artery and must make firm, but not excessive contact. Sometimes subtle differences in position of only a millimeter or two can make the difference between success and failure in acquiring a signal.
Ultrasonic Doppler probes are delicate and expensive and must be handled carefully. They should be cleaned by wiping gently with a gauze sponge. Gentle cleaning with tap water is acceptable, but the probe must not be immersed, scrubbed, or autoclaved.
In SA patients, ultrasonic Doppler probes may be placed on the ventral surface of a paw proximal to the metacarpal or metatarsal pad, on the ventral surface of the tail base, on the dorsomedial surface of the hock, or on the medial surface of the thigh in patients less than 10 lb. In LA patients, the ventral tail is the most frequently used site (see Figure 27-22 for common locations to place the probe).
FIGURE 27-22 Locations for Doppler probe placement. Red, Determination of systolic blood pressure by use of a sphygmomanometer with the cuff placed around the tail base and the probe placed on the ventral surface of the tail distal to the cuff. Green, Probe over the dorsomedial surface of the hock. Blue, Probe proximal to the metatarsal pad or metacarpal pad.
When used with a sphygmomanometer, the Doppler monitor can also be used to determine blood pressure (Figure 27-22, top left). Place a properly fitted cuff on the foreleg, metatarsus, or tail base with the cuff balloon centered over the artery. The width of the cuff should be 30% to 50% of the circumference of the extremity (Figure 27-23, inset). After establishing a good Doppler signal, inflate the cuff until the artery is occluded (the signal can no longer be heard). Gradually decrease the pressure until the audible signal can first be heard again. This represents the systolic pressure. These instruments tend to underestimate blood pressure in cats by about 15 mm Hg, but are fairly accurate in dogs. All indirect blood pressure measurements are subject to many inaccuracies, however, and must be interpreted in light of other signs (see Table 27-9 for normal blood pressure values during anesthesia).
TABLE 27-9
Normal Blood Pressure Values During Anesthesia
| Blood Pressure Value (mm Hg) | Small Animal | Equine |
| Systolic | 100-160 | 100-120 |
| Mean | 80-120 | 80-100 |
| Diastolic | 60-100 | 60-80 |
| Minimum acceptable mean during anesthesia | 60 | 70 |
| Hypertension | Systolic >160 | Systolic >140 |
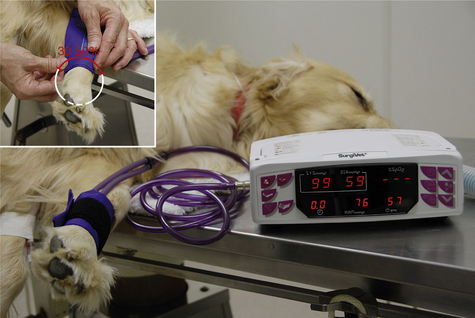
FIGURE 27-23 Oscillometric blood pressure monitor with a cuff placed on the metacarpus. The following measurements are indicated: Systolic BP: 99 mm Hg; diastolic BP: 59 mm Hg; mean arterial pressure (MAP): 76 mm Hg; HR: 57 bpm. Inset, Selecting an appropriately sized blood pressure cuff, the width of which should be 30% to 50% of the circumference of the extremity.
An oscillometric blood pressure monitor (see Figure 27-23) is a device that is used to measure blood pressure and HR. Parts include a blood pressure cuff and a computerized base unit that inflates and deflates the cuff and analyzes the signals received by the cuff.
A cuff is placed around a leg or tail with the balloon centered over an artery, and the unit is turned on. The cuff is inflated and deflated automatically by the machine. The unit detects oscillations within the cuff bladder caused by pulsations of the arteries. Based on changes in the intracuff pressure, systolic, mean, and diastolic pressures are calculated. The cuff can be placed around the foreleg, metatarsus, metacarpus, or tail of small and large animal patients (see Figure 27-24 for example locations to place the cuff). For proper operation, the cuff should be at the same horizontal plane as the heart. This device is more accurate in patients more than 10 kg.
FIGURE 27-24 Locations for placement of a blood pressure cuff: Red, Base of the tail. Green, Metatarsus. Blue, Metacarpus.
As its name implies, an apnea monitor (Figure 27-25) is used to warn the anesthetist of apnea. Parts include a base unit and a sensor. The sensor, which is placed between the endotracheal tube connector and the breathing circuit, detects temperature changes between the warm expired and cold inspired air. It emits an audible beep when the patient breathes and will sound an alarm when no breath is detected for a preset time period.
FIGURE 27-25 Apnea monitor. The probe (circled) is located between the breathing circuit and the endotracheal tube connector. The lapse time indicates the time in seconds since the previous breath. This alarm is set to sound if the interval between breaths exceeds 10 seconds.
The sensor increases mechanical dead space, which can be significant, especially in small patients. For this reason, special endotracheal tube connectors are available that accommodate the sensor and minimize dead space. Although apnea monitors do not warn of inadequate respiratory depth, they may have difficulty detecting respirations if the patient's VT is significantly decreased or the patient becomes hypothermic and will sound the apnea alarm. Consequently, as with any monitor, alarm signals must be confirmed by physical examination of the patient.
A capnograph, also known as an end-tidal CO2 monitor (Figure 27-26), is a device that measures the level of CO2 present in the inspired and expired air. Parts include a computerized base unit and a fitting that is placed between the endotracheal tube connector and the breathing circuit.
FIGURE 27-26 Capnograph registering an end-tidal CO2 level of 38 mm Hg and a RR of 10 bpm (upper right). The sensor (circled) is located between the breathing circuit and the endotracheal tube connector. The graph indicates the CO2 levels throughout the respiratory cycle, which in normal patients is 35 to 40 mm Hg during expiration and 0 mm Hg during inspiration.
The capnograph enables the anesthetist to estimate the partial pressure of CO2 in the patient's bloodstream and is one of the best indicators of adequate respiration. Because CO2 is produced at the tissue level, carried by the vascular system, and eliminated by the lungs, abnormal readings may be caused by disease of the lungs, cardiovascular system, or tissues and equipment malfunctions.
In normal animals during inspiration, the level should be 0 mm Hg, and at the end of expiration, the level should rise to 35 to 40 mm Hg. Any change in the configuration of the curve can indicate a problem and should be explored. The interpretation of capnograph tracings is complex and beyond the scope of this chapter. The student is encouraged to explore the recommended readings for more information.
 TECHNICIAN NOTE
TECHNICIAN NOTE