Chapter 3 Dermatological conditions of the foot and leg
Laboratory diagnosis of dermatophyte infection
Systemic treatment of foot and nail infections
Inflammatory skin diseases
Several chronic inflammatory skin diseases commonly involve the feet. A podiatrist should be able to recognise the clinical features of the most common skin diseases and be aware of appropriate management and referral criteria.
PSORIASIS AND RELATED DISORDERS
Psoriasis is a chronic inflammatory skin condition with a prevalence of about 2% of the UK population (Griffiths & Barker 2007). It can affect any age group, including children, but onset is commonest in early adulthood and late middle age. The disease is characterised by increased turnover of the epidermis, but the pathogenesis involves abnormalities in T-lymphocyte function and increased vascularity, as well as proliferation and altered differentiation of keratinocytes. There is clear evidence to suggest that susceptibility to psoriasis is inherited; a number of genetic loci have been implicated but the precise genetic basis of the disease is not yet known (Ortonne 1999).
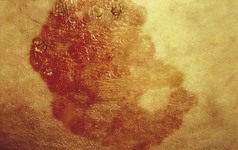
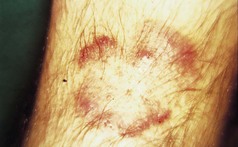
Chronic plaque psoriasis is the most characteristic form. Patients present with circumscribed itchy patches of thick, scaly, red skin often prominent on the elbows, knees and scalp, although any body site can be affected (Fig. 3.1). Other variants include:
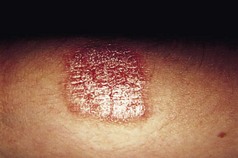
Figure 3.1 A typical plaque of chronic plaque psoriasis. These plaques are normally found on the limbs.
Psoriasis can be triggered or exacerbated by a number of factors, which include: trauma (psoriasis appearing at sites of injury such as operative scars is called the Köbner phenomenon); drugs, such as lithium or hydroxychloroquine; infection, particularly the streptococcal pharyngitis, which triggers guttate psoriasis (see above); and stress, which does appear to be important in disease exacerbations in some patients. As with many chronic diseases, patients with severe psoriasis have increased cardiovascular morbidity, and are more prone to psychological problems, including alcoholism. About 40% of women with psoriasis improve during pregnancy while 15% deteriorate.
The severity of psoriasis can be assessed by the PASI (psoriasis area and severity index) score. This is useful in measuring objective response in clinical trials, in which 75% improvement (PASI 75) is a commonly used an endpoint. However, the PASI is mainly a measure of extent and fails to take account of many other factors; it is often supplemented by the Dermatology Life Quality Index (DLQI).
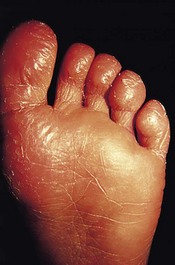
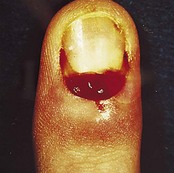
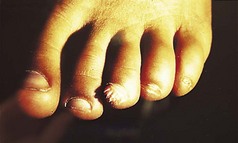
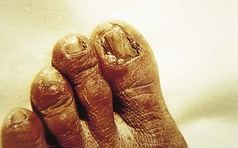
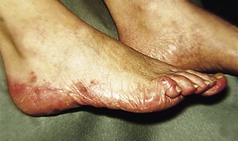
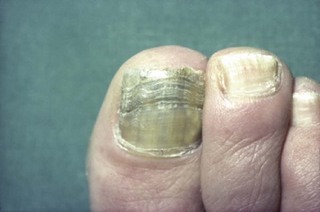
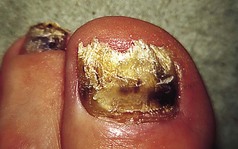
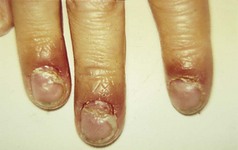
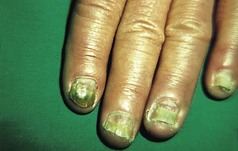
The feet are commonly involved in psoriasis. Typical pink or red plaques with a superficial layer of fine silvery white scale may be seen on the dorsum of the foot, while more hyperkeratotic, fissured skin is seen on the plantar surface, particularly at sites of pressure. Psoriasis commonly also causes nail dystrophy, including separation of the nail plate from the nail bed (onycholysis) or subungual hyperkeratosis. Fine indentations or pitting of the nail plate may also be seen; this is more prominent on fingernails. Psoriatic arthritis may be an additional problem, and can present with pain and decreased mobility in the axial skeleton and the small joints of the hands and feet. A rare mutilating form of arthritis can result in significant resorption of bone in the digits.
Differentiating plantar psoriasis from other causes of acquired plantar keratoderma (thickening of the plantar skin), such as eczema, lichen planus and fungal infection, is often difficult. It is therefore important to look for typical signs of psoriasis at other sites and to enquire about a positive family history of the disease.
Treatment
Regular emollients reduce scaling and fissuring, and keratolytics such as 5% salicylic acid in Vaseline or 50% propylene glycol in water treat hyperkeratosis. The numerous active topical treatments available include vitamin D analogues such as calcipotriol, topical steroids, coal-tar-based preparations, dithranol (anthralin), an anthraquinone compound, and vitamin A derived drugs (retinoids).
Patients may benefit from treatment with ultraviolet B (UVB) phototherapy, or photochemotherapy using an oral or topical photoactive drug (a psoralen) in combination with ultraviolet A (PUVA). This treatment can be localised by using hand and foot irradiation units. More severe cases may require treatment with drugs such as the oral retinoid acitretin, or immunosuppressive agents such as methotrexate or ciclosporin (Warren & Griffiths 2008). The latter two agents in particular carry significant toxicity risks for bone marrow, liver or kidney, and all long-term systemic agents for psoriasis necessitate monitoring. Patients with severe psoriasis not suitable for these drugs or who respond poorly may be treated with biological agents (Smith et al 2005). These include the TNF-alpha antagonists etantercept, infliximab, and adalimumab. These agents carry the risks of immunosuppression, and are currently very expensive. Biological agents with other targets are licensed or under development for psoriasis and psoriatic arthropathy.
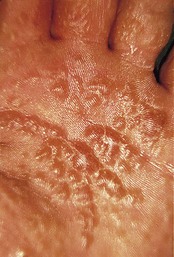
Palmoplantar pustular psoriasis (PPP)
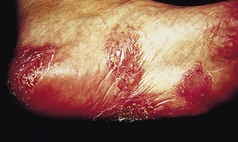
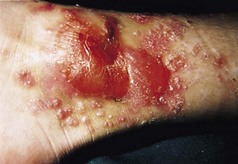
Many patients with this chronic inflammatory disorder of the palms and soles have no other features of psoriasis and hence there is some debate as to whether it is a true subtype of the disorder. It typically presents with red scaly hyperkeratotic palmar and plantar skin studded with sterile pustules (Fig. 3.2). Fresh pustules initially appear yellow and subsequently dry to leave brown discoloration. Extensive plantar disease can result in pain on weight-bearing. PPP is more common in middle-aged women and is strongly associated with cigarette smoking. The differential diagnosis includes acute infected eczema, dermatophyte fungal infection and Reiter’s disease (see below). Treatment of PPP is similar to that of psoriasis but the disease is often resistant to many of the available therapeutic options (Eriksson et al 1998).
REITER’S DISEASE
This is a reactive disorder in which arthritis, urethritis, conjunctivitis and inflammatory mucocutaneous disease are triggered by urogenital or gut infections. It is most commonly seen in young men in whom it often follows Chlamydia trachomatis urethritis.
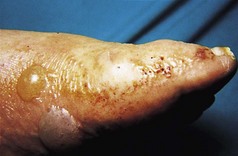
The classic cutaneous finding is keratoderma blenorrhagicum, a psoriasis-like eruption that usually affects the soles of the feet and may not appear for several months after the onset of arthritis and conjunctivitis. Affected patients have thick, ‘limpet-like’ hyperkeratoses with a dull yellow discoloration or a more acute pustular rash.
Patients with active systemic disease may require treatment with immunosuppressive drugs such as methotrexate or azathioprine, which may also improve the cutaneous features of the disease. Application of emollients and keratolytics may reduce the plantar keratoderma.
PITYRIASIS RUBRA PILARIS (PRP)
This is a rare group of disorders characterised by hyperkeratosis of hair follicles, widespread erythema and palmoplantar keratoderma. The cause of PRP is unknown.
In the classic form, erythema surrounding individual hair follicles spreads out to become confluent, although there are typically islands of spared normal-appearing skin. The erythema has a distinctive orange-yellow hue, which is particularly prominent in the hyperkeratotic skin of the palms and soles. The disorder is often difficult to differentiate from psoriasis. Patients are generally treated with bland emollients, although many subsequently require the addition of systemic agents such as acitretin or methotrexate. Response to treatment is often poor but the disease generally resolves spontaneously (Griffiths 1980).
ECZEMA (DERMATITIS) AND RELATED DISORDERS
Dermatitis is an inflammatory skin disease that may be caused by a number of factors. For most purposes the terms ‘eczema’ and ‘dermatitis’ are synonymous. Acute dermatitis is characterised by redness, scaling and weeping, often with vesiculation (tiny fluid-filled blisters). In contrast, chronic dermatitis is characterised by excoriation (scratch marks) and thickening of the skin known as ‘lichenification’. Common categories of eczema include those described below.
CASE STUDY 3.1 ECZEMA/DERMATITIS – OUTLINE OF DIFFERENTIAL DIAGNOSIS AND MANAGEMENT
A boy aged 8 years and a keen swimmer had to stop this activity as he developed thin plantar skin on both feet which fissured easily, becoming painful and prone to infection. There was no family history of similar problems, but his mother recalled that he developed a red itchy rash (urticaria) the previous summer. He had treated it with a number of topical preparations without success. Previously the skin problem had occurred around the toes but now the site and skin changes followed the shoe/skin contact line, which suggested an allergy to the dye and/or adhesive in the footwear. Differential diagnosis included tinea pedis, hyperhidrotic skin (sweaty skin that dries quickly on exposure, becoming dry and scaly – xerosis), juvenile plantar dermatosis and atopic dermatitis (Leung 1998). Laboratory microscopy and culture of scrapings showed no evidence of fungal infection.
The treatment was symptomatic, as no clear diagnosis was evident. Fissures were closed with adhesive skin closures or medical-grade acrylic glue (with prior patch testing, although this cannot be relied upon). While waiting for the laboratory report, the patient’s mother spent some time isolating the suspected allergen. The patient’s socks were washed in water only, all topical applications were stopped and the shoes suspected of being implicated were not worn for 5 weeks to allow the apparent hypersensitivity to subside. There was a marked improvement after 2 weeks and a gradual introduction of the suspected allergens identified a particular pair of shoes, which were discarded. The patient could have been referred to the allergy clinic for tests, but this simple method of identifying a potential allergen was an attractive option given the time delay in referral.
Further aspects of management to prevent recurrence included distancing the patient’s skin from potential allergens, and the control of any hyperhidrosis. Water enhances percutaneous absorption of many topical substances, and a sweaty environment provides an opportunity for enhanced bacterial colonisation of the skin and increased potential for bacterial superantigen associated with atopy (Leung 1998). The boy’s mother contacted the shoe manufacturers to enquire about products used in manufacture; a barrier cream was applied to the patient’s feet (e.g. silicone). Sweat-absorbing socks and charcoal-impregnated insoles were also used by the patient.
It is difficult to say whether one or all of these management methods were responsible for the success in the relief of the patient’s symptoms. In this particular case it was neither good clinical practice nor ethical to withhold treatment to determine which was the most successful.
Atopic eczema
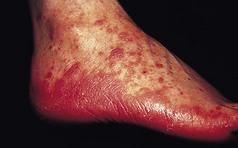
This is a common, chronically relapsing skin disease with a genetic predisposition and a rising prevalence in the population. It is often associated with the other atopic diseases, asthma and hay fever. Onset is usually in infancy, and about 60% of children are clear of dermatitis by the age of 10 years. In a minority, eczema persists into adult life, and others may present for the first time later in life. The cause is complex and multifactorial, but a major underlying factor is a defective skin barrier. Many patients with atopic eczema carry null mutations in the epidermal barrier protein filaggrin (Sandilands et al 2007). Defects in the skin barrier may be responsible for the altered immune reactivity to common environmental allergens such as the house dust mite. Atopic eczema can affect any body site but is particularly common on the flexural surfaces of limbs and on the face. Involvement of the feet is less common, although the ankles are often affected (Fig. 3.3). Patients with defects in filaggrin are more likely to show hyperlinearity and hyperkeratosis of the palms and soles.
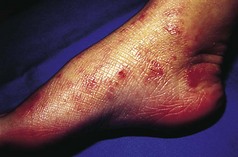
Figure 3.3 A 3-year-old child of with severe atopic eczema showing erythema and coarse skin markings (lichenification) of the feet.
Treatment
The mainstay of treatment is a combination of regular emollients (moisturisers) and topical corticosteroid ointments or creams. These vary in potency from the mild hydrocortisone, to the more potent synthetic fluorinated corticosteroids, which in prolonged use cause skin atrophy. Topical immunosuppressive agents (tacrolimus, pimecrolimus) are a more recent alternative to topical corticosteroids. Anti-inflammatory pastes containing tars such as ichthammol, or zinc, are particularly effective in settling eczema of the feet and ankles. Affected skin is often infected by Staphylococcus aureus, and antiseptic or topical or oral antibiotic may be needed. Patients with severe chronic eczema may require treatment with immunosuppressive drugs such as azathioprine or ciclosporin; phototherapy is also used (Brehler et al 1997).
Contact dermatitis
Contact dermatitis can be subdivided into patients who have a hypersensitivity reaction to specific allergens (allergic contact dermatitis) and those who have a non-specific reaction to irritants (irritant contact dermatitis), but the two often coexist.
Irritant contact dermatitis is usually the result of non-allergic, chemical damage to the skin. This may occur acutely, for instance after exposure to acids or alkalis, but is more commonly the result of cumulative exposure to a variety of irritants such as soaps, detergents and even water. Workers in jobs where wet work is common are particularly prone to irritant contact dermatitis. Some workers are far more prone to the effects of irritants than others, although the reason for this is not clear. The hands are the most common site of irritant contact dermatitis.
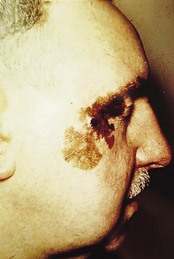
Allergic contact dermatitis is an important cause of dermatitis of the feet. Patients may become sensitised (allergic) to a wide variety of allergens present in footwear, hosiery and even topical medicaments (Fig. 3.4). In footwear dermatitis, the commonest sources of allergens are chrome (used in leather tanning), rubber vulcanising agents, adhesives (e.g. colophony) and textile dyes. On repeated exposure to such allergens, a contact dermatitis develops. Sometimes a distinctive pattern of dermatitis, with a sharp demarcation between normal and affected skin, will point to allergic contact hypersensitivity as a possible cause.
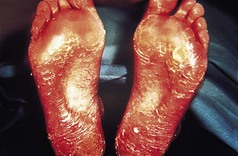
Figure 3.4 Allergic contact dermatitis of the soles of the feet due to sensitivity to mercaptobenzothiazole, a rubber additive.
Patch testing with standardised concentrations of allergens may identify the source of allergic contact dermatitis. This procedure involves application of a series of allergens to the back under adhesive tape. Patches are removed after 48 hours and reactions read at 48 and 96 hours; positive tests are identified as individual red papular or vesicular reactions. In assessing footwear dermatitis, patch tests with samples from different parts of a patient’s shoe may be useful (Cockayne et al 1998).
CASE STUDY 3.2 SENSITIVITY TO COLOPHONY
This man was treated with adhesive strapping support for a sprained ankle. Within 48 hours he developed an acute vesicular and weeping eczema (here being treated with potassium permanganate soaks). Patch testing revealed sensitivity to colophony, a common resinous material found in adhesives.
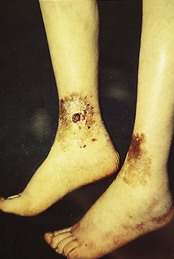
Stasis and varicose eczema
Chronic venous or lymphatic insufficiency may cause dermatitis. The classic distribution of venous or varicose eczema is on the gaiter area of the leg (lower shin and calf), although it can also extend onto the foot. There is often a history of varicose veins or of deep vein thrombosis. Oedema, increased pigmentation (haemosiderin deposition), purpura and ulceration are often present with the dermatitis. Chronic venous hypertension causes distended veins to develop a fibrin cuff, which inhibits movement of fluid, nutrients and metabolites (Hoffman 1997), resulting in poor tissue viability and mechanical weakness (Dealey 2005). Atrophie blanche (white, scarred skin) may be present, and such skin is especially vulnerable to ulceration.
Dermatitis may also complicate chronic oedema due to cardiac or renal insufficiency, obesity, immobility or lymphatic disease. Often these are present in combination. In lymphoedema a firm non-pitting oedema is present, and dilated lymph vessels may give a cobbled appearance to the skin; there may also be a wary hyperkeratosis of the feet. Lymphoedematous legs are vulnerable to recurrent cellulitis, which in turn causes further lymphatic damage.
Allergic contact dermatitis may complicate up to 50% of cases of stasis dermatitis, with hypersensitivity to preservatives and other ingredients in topical medicaments and rubber in bandages being common. The treatment of choice is graduated elastic compression in the form of bandaging or stockings.
Pompholyx
This is a striking acute vesicular type of dermatitis that affects the palms (cheiropompholyx) and the soles of the feet (podopompholyx). The aetiology of pompholyx is unclear, although some studies have suggested a prevalence of atopy in up to 50% of sufferers. Patients complain of intense itching and discomfort and examination reveals multiple tiny vesicles that look like sago grains (Fig. 3.5). The role of contact allergy in pompholyx is debated, although some investigators believe that ingested nickel may be a factor in nickel-sensitive patients. Treatment is aimed at drying the acute, weeping vesicles with potassium permanganate soaks and reducing the inflammatory component with potent topical steroid creams (Burton & Holden 1998).
Juvenile plantar dermatosis
This is probably a type of irritant plantar dermatitis that occurs almost exclusively in children aged between 3 and 14 years and is more common in atopics. It presents with fissured, dry dermatitis on the forefoot and heel, with a typical glazed red appearance (Fig. 3.6). Again, the cause is not clear although there have been suggestions that excess humidity in shoes made from modern non-porous materials, such as trainers, might be responsible. The differential diagnosis includes allergic contact dermatitis and fungal infection. Most cases clear spontaneously but some patients benefit from regular emollients and avoidance of synthetic footwear. Cork insoles are anecdotally of value (Lemont & Pearl 1992).
LICHEN PLANUS
Lichen planus (LP) is an intensely itchy inflammatory skin condition which appears to be immunologically mediated, although the exact cause is not clear. Some cases are associated with adverse reactions to drugs such as gold, while other cases have been reported in patients infected with hepatitis B and C viruses.
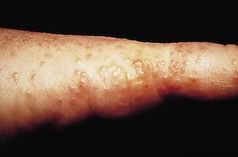
Patients classically present with itchy, polygonal, violaceous, flat-topped papules covered with superficial white lines known as Wickham’s striae. Papules may coalesce into plaques, and lesions can appear in lines of trauma (another example of the Koebner phenomenon). The rash can affect any body site, although lesions on the wrists and ankles are particularly common. In addition, LP can cause nail dystrophy, scarring alopecia and can affect oral and genital mucosa.
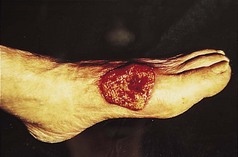
LP can affect plantar skin and the lesions are often not characteristic of LP lesions at other sites. A spectrum of disease may be seen ranging from a few discrete papules at the margin of the foot to widespread hyperkeratosis with fissuring and ulceration (Fig. 3.7).
LP is usually a self-limiting disease, although it may occasionally persist for years. The treatment of choice for widespread, severe disease is systemic steroids, although more limited disease may respond well to potent topical steroids. Hyperkeratotic plantar involvement should be treated with combinations of keratolytics, such as salicylic acid, and potent topical steroids. Oral steroids or ciclosporin can be added in difficult cases (Boyd & Neldner 1991).
ICHTHYOSIS
‘Dry skin’, or ichthyosis, of varying degrees is common in the population. Skin becomes more rough and scaly with age due to a reduction in lipid synthesis, and this may be exacerbated by medications or disease. Genetically determined types of ichthyosis also occur, of which the commonest is ichthyosis vulgaris, due to homozygosity for null mutations in the stratum corneum keratin filament aggregating protein filaggrin (Sandilands et al 2007). Other genetic causes include a lack of cholesterol processing due to steroid sulfatase deficiency in X-linked ichthyosis. Ichthyosis affecting the feet may be manifest by both hyperkeratosis and hyperlinearity.
KERATODERMAS
Plantar skin is characterised by a markedly thickened outer horny layer, or stratum corneum, that provides a functional advantage in protecting the foot against the effects of chronic trauma. This becomes particularly apparent when comparing the feet of people who walk barefoot with those who do not. In addition to this physiological spectrum in the thickness of plantar skin, there are a number of pathological disorders that result in plantar hyperkeratosis or keratoderma. It is not uncommon for podiatrists to see patients presenting with plantar keratoderma, and this section will attempt to classify this heterogeneous group of disorders and define their clinical features and the options available for treatment.
Broadly speaking, the palmoplantar keratodermas (PPKs) can be divided into inherited or acquired varieties, the latter being associated with inflammatory skin diseases such as eczema or psoriasis (see above), or a variety of other or uncertain aetiologies.
Inherited palmoplantar keratodermas
This is a group of rare genetic skin diseases that are characterised by variable degrees of palmar and plantar hyperkeratosis, often in association with other clinical features that make up a syndrome. There have been a number of attempts to classify these diseases based on the pattern of hyperkeratosis (diffuse, focal or punctate), histological features, mode of inheritance and, more recently, by identifying the underlying gene mutation. The latter technique may provide evidence for the molecular basis of these diseases and may have practical implications for both future prenatal diagnosis and gene-targeted therapy.
A detailed review of all the inherited PPKs is beyond the scope of this text, and interested readers are directed to reviews such as that by Kimyai-Asadi et al (2002).
For practical, clinical purposes it is helpful to classify inherited PPKs according to the pattern of hyperkeratosis and the presence or absence of other associated diseases (Table 3.1).
Table 3.1 Examples of inherited palmoplantar keratoderma and their causes (Judge et al 2004)
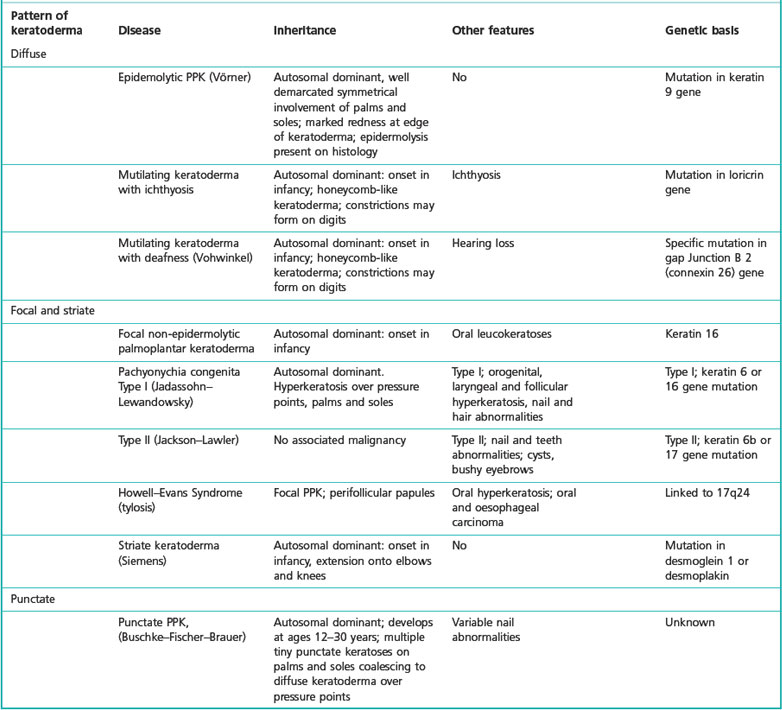
Aetiology
Recent research has identified mutations in a variety of mainly structural genes of the epidermis that have been found in different patterns of keratoderma. These include keratins, a group of structural proteins with a range of properties that are expressed in specific patterns in different areas of the skin. Other keratodermas are due to mutations in proteins involved in cell adhesion, and in forming the cornified envelope, the waterproof outer layer of the epidermis.
Clinical features
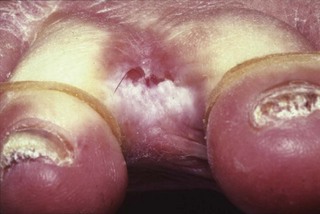
The features of some inherited PPKs are outlined in Table 3.1, and some examples are shown in Figure 3.8. The severity of these diseases varies both within and between affected families. Keratoderma tends to be more prominent in the weight-bearing areas of plantar skin and can result in discomfort on walking and abnormalities of gait. In addition, patients may complain of malodour as a result of bacterial degradation of the thick keratin layers. Keratoderma which extends to the dorsa of the digits may result in constricting bands and even autoamputation of digits (Fig. 3.9).
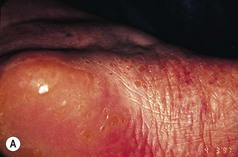
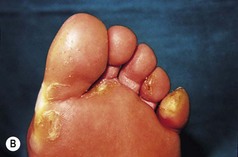
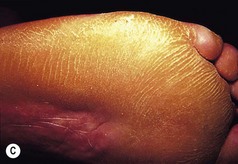
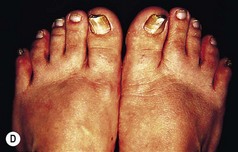
Figure 3.8 Patterns of keratoderma: (A) punctate; (B) focal; (C) diffuse. (D) Focal keratoderma is seen with a hypertrophic nail dystrophy in pachyonychia congenita.
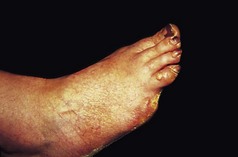
Figure 3.9 Vohwinkel’s keratoderma (mutilating keratoderma with deafness). A toe has been lost due to the formation of a constricting band (‘pseudo-ainhum’).
Although thickening of plantar skin is common, features that suggest a keratoderma of genetic origin include: early onset or a positive family history of keratoderma; an extensive or unusual pattern; thickening of the skin of other areas such as the elbows, knees and wrists; and other clinical features such as abnormalities of the hair, nails and teeth or a history of deafness. If an inherited PPK is likely then referral to a dermatologist may be appropriate.
In a few families with an inherited PPK there is an associated increased risk of developing some internal malignancies. In a few families, focal keratoderma is associated with a high risk of oesophageal cancer (the Howel–Evans syndrome).
Treatment
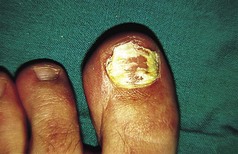
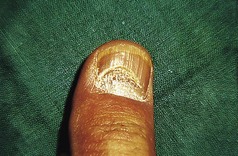
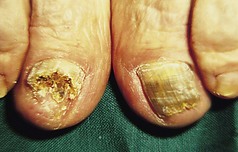
Patients should be advised on the regular use of topical keratolytics such as salicylic acid 5–10% in white soft paraffin or 35–70% propylene glycol. Occlusion with polythene increases the efficacy of keratolytics and can be used in combination with manual paring or abrasion with a pumice stone. Oral vitamin A derived drugs (retinoids) are particularly helpful for some patients but may result in increased pain in affected skin. Surgery has an occasional role for patients with focal keratoderma. Keratoderma may be complicated by dermatophyte fungal infections, and skin scrapings should be sent for mycological culture if this is suspected.
CASE STUDY 3.3 KERATIN MUTATION
A 6-month-old child developed blistering and hyperkeratosis of the feet as she began to weight bear. Her mother was affected by life-long hard skin of the hands and feet. Genetic investigation showed them to have a mutation in keratin 9. This intermediate filament protein is specifically expressed in palmoplantar epidermis, and the mutation causes structural weakness and a hyperkeratotic response.
Acquired keratoderma
Keratoderma climactericum
This disorder occurs predominantly in obese postmenopausal women. It presents with erythema and hyperkeratosis over the weight-bearing areas of the heel and forefoot. Patients are often asymptomatic, although pain on walking can be a problem if there is extensive involvement. The disorder has been described in younger women following oophorectomy, suggesting that reduction in endogenous oestrogens may be important in the aetiology. Treatment with emollients and keratolytics may be of some benefit (Deschamps et al 1986).
Acrokeratosis paraneoplastica (Bazex’s syndrome)
This is a rare cause of acquired keratoderma that is associated with some internal malignancies. It is more common in men and typically presents with erythema and scaling on the extremities (ears, nose, hands and feet). With time the eruption becomes more generalised and more hyperkeratotic, with keratoderma on the hands and feet. Successful treatment of the underlying malignancy often leads to resolution of the disorder (Bolognia 1995).
Keratoderma and hypothyroidism
Palmar and plantar hyperkeratosis have been reported in association with hypothyroidism. Treatment with thyroxine replacement may result in clinical improvement (Hodak et al 1986).
Blistering disorders
Blistering has many causes, including bacterial or viral infections, insect bites and inflammatory skin disorders. Traumatic blistering of the feet is common and is particularly prevalent in recreational sports, such as hill walking and jogging, which impart recurrent shearing mechanical forces to the plantar skin. Poorly fitting or inappropriate footwear may exacerbate the problem. In addition, there is a group of rare skin disorders that are characterised by blistering as a result of inherited abnormalities in a variety of key structural skin proteins.
EPIDERMOLYSIS BULLOSA: THE INHERITED MECHANOBULLOUS DISORDERS
Epidermolysis bullosa (EB) encompasses a spectrum of disorders characterised by skin fragility and blistering following mild mechanical trauma. Recent research into EB has identified the molecular basis for a number of the diseases, opening up the possibility for prenatal diagnosis and gene-targeted therapy in the future.
EB can be broadly divided into three groups of diseases on the basis of the ultrastructural level of the blister formation.
AUTOIMMUNE BLISTERING DISORDERS
Disease mediated by autoantibodies against target antigens in the epidermis or its basement layer results in increased skin fragility or blistering. The commonest autoimmune blistering disorder is bullous pemphigoid, in which proteins of the basement membranes are attacked. This causes the whole epidermis to lift off, producing an intact firm blister. On the feet, the blisters may be more vesicular, resembling those of pompholyx (Fig. 3.11). In the various forms of pemphigus, antibodies are directed against adhesion molecules within the epidermis, and the result is friable blisters or erosions. Both of these conditions are commonest in the elderly, and require aggressive treatment with high doses of oral corticosteroids or other immunosuppressive agents.
Tumours
‘Tumor’ in Latin simply means a swelling. This, on the foot, could include some corns, verruca plantaris, boils, etc. By usage and wont, the anglified version ‘tumour’ (American ‘tumor’) is applied to new growths or neoplasms (Table 3.2). Customarily, these are divided into benign or malignant, which is obviously a vitally important distinction. In the skin, perhaps more so than other systems, these margins can be indistinct. Some tumours are initially premalignant but inexorably transform if neglected. Some normally benign lesions may become malignant. Some apparently aggressive malignancies may spontaneously involute. Malignancy usually implies the ability to spread, or metastasise, to distant sites by blood or lymphatic vessels. However, lesions such as basal cell carcinoma in the skin may be locally malignant and destructive, but virtually never metastasise.
Table 3.2 Classification of skin tumours
| Tumour type | Degree of malignancy | Examples |
|---|---|---|
| Epidermal tumours | Benign | Seborrhoeic keratoses |
| Premalignant | Bowen’s disease | |
| Malignant | ||
| Pigmented skin tumours | Benign | |
| Premalignant | ||
| Malignant | Malignant melanoma | |
| Vascular tumours | Benign | |
| Malignant | Kaposi’s sarcoma | |
| Fibrous tumours | Benign | |
| Malignant | Dermatofibrosarcoma protuberans | |
| Adnexal tumours | Benign | Eccrine poroma |
| Other structures | Benign |
Nevertheless, the concept of benign or malignant is a useful working one, provided that the limitations are appreciated and the compartments are not considered too watertight.
Tumours of cells in the epidermis, dermis, appendages, etc. are numerous. Some are very rare. Some virtually never affect the feet. Thus the ones discussed in this section are not exhaustive and selection has been made. Some ‘tumours’, that is swellings, have been included because they might be mistaken for true neoplasms. Some tumours of deeper structures are described, as they may present as a foot swelling.
Any podiatric procedure should include visual and clinical examination of the foot skin and subcutaneous structures (see Ch. 1). The podiatrist is often the person most likely to diagnose foot tumours.
For the patient, ageing, if it brings eyesight problems, obesity and/or arthritis, can make their feet a ‘lost world’. Doctors can be curiously reluctant to examine the feet. Most medical examination couches and their lighting are better geared to visualisation of the upper half of the patient. The daunting prospect of the time involved in the removal of the patient’s tights, multi-laced boots, etc. in a busy surgery can be a deterrent.
CAUSES OF SKIN TUMOURS
The cause of many skin tumours is unknown. Understandably, research has mainly concentrated on malignant tumours. A number of factors have been identified as being important. These include external agents and the host’s genetic makeup.
In 1775, Sir Percival Pott noted an increase in scrotal cancers in chimney sweeps. This was the first realisation of the skin carcinogenicity of soot, pitches and tars. This finding has been confirmed in animal experimentation and in other occupations over the centuries. Other substances, including arsenic, nitrogen mustard and psoralens, have been added. It has been discovered that the agent may have the same property even if taken systemically. This is especially so with arsenic, immunosuppressants, etc.
Areas of long-continued skin damage, such as leg ulcers, burns and tuberculosis infections, may undergo malignant transformation. More recently, the damage due to radiation has been appreciated. Many of the early medical pioneers of x-radiation developed skin tumours, usually on the hands, because they were unaware of the need for protection. A number of skin tumours are linked to excess exposure to ultraviolet (UV) radiation, mainly in the 280–320 nm wavelength range.
Much more recently, the importance of some infecting viruses has been appreciated. A number can produce tumours in animals, either in the natural situation or experimentally. In the human, the papilloma virus (especially types 16/18) promotes malignancy in the female genitalia and cervix. A herpes virus is established in some cases of Kaposi’s sarcoma and a retrovirus in some T-cell lymphomas. This is a developing field and other associations may soon be found.
The host’s resistance depends on genetic individuality, modified by lifestyle, occupation, etc. The Celtic races have skin that tends to burn rather than tan, rendering them more vulnerable to UV-radiation-induced tumours. UV radiation, inter alia, can damage the cellular DNA. Normally, there is a repair system that mops up this damaged DNA. When this is defective, cancer can develop. This happens predominantly in the disease xeroderma pigmentosum, which is rare in the UK but more common in Asia.
Cell growth is a very complicated procedure. Normally genes accelerate it forward at an appropriate speed. If these are altered, they can change to transforming genes or oncogenes (i.e. cancer-producing genes). If viruses are involved they may hijack the normal genes. The oncogenes, formed for whatever reason, may thus inappropriately speed the cells along, proliferating in abnormal forms. The brakes applied in the normal system are via the suppressor genes. Of the tumour suppressor genes involved in human skin cancer, p53 tumour suppressor gene is possibly the most important. When this is inactivated by limitation or inhibition, the brakes fail and the lesion progresses abnormally quickly.
Because of their easy accessibility, skin tumours are easier to investigate than systemic ones. There are bound to be many discoveries in the future.
EPIDERMAL TUMOURS
Seborrhoeic keratosis
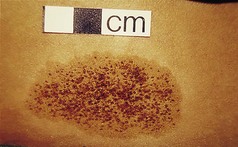
Seborrhoeic keratosis (synonyms: senile or seborrhoeic wart, basal cell papilloma) is one of the commonest tumours found. They are benign but can mimic malignant tumours Their incidence increases with advancing age. They tend to cluster on the trunk, but any body surface, including the feet, can be involved. There are several varieties. One type, stucco keratoses, generally affects the legs and ankles.
Clinical features
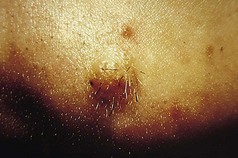
The commonest type of seborrhoeic keratosis is found on the trunk. There are usually a number of lesions at various stages of development. Initially, they are small papular lesions with a slight increase in pigmentation. They become larger, usually up to about 1 cm in diameter, tending to darken, sometimes until they are almost black. Some can become very large, up to 10 cm or more. The edge is distinct and may slightly overhang the surrounding normal skin. The degree of pigmentation is variable but is usually homogeneous throughout any individual lesion. The surface is rough and wart-like, and at times can become extremely heaped up and thickened. Small circumscribed round areas on the surface are characteristic and known as horn cysts. The surface colour is matt and lacklustre (Fig. 3.12).
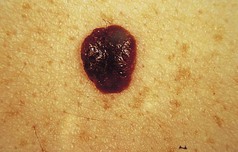
Figure 3.12 Seborrhoeic keratosis. Note distinct margin, homogeneous colour and scattered horn cysts.
On palpation they can feel rather greasy – hence the ‘seborrhoeic’ in the name. Horn cysts give them a simultaneous warty feel. On the extremities they tend to be flatter. Some can be irritated by clothing, etc., and secondary infection can supervene.
Some may spontaneously involute, but the natural history is for slow development to a certain size. Normally at least several other lesions can be found. At times, massive numbers can erupt rapidly. This is called the sign of Leser–Trélat. It has been much disputed as to whether this is a sign of underlying malignancy (Lindelöf et al 1992).
Stucco keratosis is the name given to a type usually seen on the legs and ankles. They are usually white or grey and smaller. They rarely exceed 3–4 mm. The crusting is easily detachable.
Seborrhoeic keratoses can usually be easily diagnosed by careful examination, but may be confused with viral warts and other hyperkeratotic lesions. The various pigmented lesions may at times need to be excluded.
Histology
Keratinocytes resembling basal cells gather in the epidermis. Increased numbers of melanocytes may contribute to the darkening colour. Concentric whorls of keratin form the keratin cysts.
Treatment
This is usually indicated for cosmetic reasons or because the lesion is catching and becoming irritated.
Seborrhoeic keratoses are superficial and usually entirely epidermal. They are ideally suited for treatment with liquid nitrogen and usually require only a short application of this. They will curette off easily, and this has the merit of being able to obtain histology, which is always needed if there is diagnostic doubt. The reddish area left will slowly return to normal.
Bowen’s disease
Bowen’s disease (Kossard & Rosen 1992) is an intradermal carcinoma in situ which can slowly progress towards a squamous cell carcinoma. Sun damage, arsenic exposure and papilloma virus have all been suggested as causes.
Clinical features
Bowen’s disease as described in the foreign literature has a predilection for the head and neck but the general view in the UK is of a preponderance to affect the lower leg. The usual picture is of a slowly enlarging reddish, scaly patch with definite margins (Fig. 3.13). This can be mistaken for psoriasis, but a careful history and examination should differentiate. Some of the patches may be more crusted or raised (Fig. 3.14).
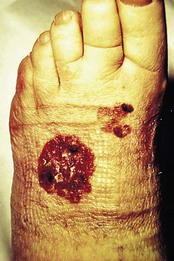
Figure 3.14 Bowen’s disease on the dorsum of the foot. Well-demarcated area. Somewhat unusually superadded infection has caused increased crusting.
The differential diagnosis includes basal cell carcinoma, squamous cell carcinoma, malignant melanoma or eczema. If there is doubt, histological examination is essential. Rarely, a nail bed or periungual variant has been described (Sau et al 1994). If neglected, there is a slow progression to squamous cell carcinoma, which is reckoned to occur in approximately 5%.
Basal cell carcinoma
Basal cell carcinoma (synonyms: rodent ulcer, basal cell epithelioma) (Leffell & Fitzgerald 1999) is the commonest skin tumour, certainly of the white races. Close on one million occur annually in the USA. They are, however, extremely rare on the foot, and especially the plantar surface, where only about 22 have been described (Roth et al 1995). Even fewer peri- or subungual ones have been noted. A certain number occur on the leg.
Clinical features
The incidence of basal cell carcinoma increases with age. The commonest site is on the face, which may suggest a solar aetiology. However, many occur in areas where there is little or no UV exposure.
The typical history is of a small lesion developing and extending very slowly over months or years. From time to time it crusts and the patient thinks it is healing. The crust comes off with a little bleeding and this cycle often repeats itself, with slow peripheral growth. The outline is round or oval, with a raised or rolled border. This is often pinkish and slightly nodular and is likened to a string of pearls. Over this edge are usually seen dilated telangiectatic vessels (Fig. 3.15). The centre may ulcerate or be more raised and dome-shaped. Locally, they may be very large and destructive, eroding down to the deep structures – hence ‘rodent’ ulcer.
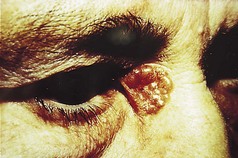
Figure 3.15 Basal cell carcinoma (rodent ulcer). Typical site showing the raised border, like a string of pearls, with telangiectasic vessels crossing it.
Those on the lower leg can be less characteristic (Fig. 3.16), and the differential diagnosis between them and Bowen’s disease, squamous cell carcinoma, malignant melanoma or vascular lesions may be difficult. They virtually never metastasise.
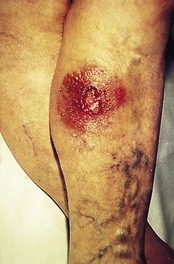
Figure 3.16 Basal cell carcinoma (rodent ulcer) on the leg. Surrounding redness is atypical and due to a contact dermatitis.
Some patients have multiple lesions and some of those suffer from the naevoid basal cell carcinoma syndrome (Gorlin’s syndrome). There are several features of this, but with reference to the feet and hands, small pits or depressions may be seen on the plantar and palmar surfaces.
Histology
Cells resembling those of the basal cell layer or appendages appear to ‘bud’ down from that layer. They form spherical masses of very uniform cells in the dermis. Characteristically the cells at the periphery of these spheres line up in a regular arrangement known as pallisading. The masses are surrounded by a fibrous stroma.
Squamous cell carcinoma
Cutaneous squamous cell carcinoma (Kwa et al 1992, Salasche et al 1993) arises from the skin keratinocytes. It is the second commonest skin tumour after basal cell carcinoma. It may affect any area of the skin. It is relatively rare on the foot, although an orthopaedic study suggested that, of the soft-tissue tumours encountered, it was the commonest (Ozedmir et al 1997). A rare variant, verrucose carcinoma, largely targets the foot.
Aetiology
Numerous factors leading to squamous cell carcinoma have been described (Kwa et al 1992). The host’s age, natural skin colour, and immune and genetic status are all important. Thus there is an increased incidence of squamous cell carcinoma in those immunosuppressed by drugs or human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS). Similarly, those unable to naturally protect their skin, such as albinos with no pigment or those whose DNA repair mechanisms are impaired, as in xeroderma pigmentosum, are at risk. External agents are also important. Scrotal carcinoma was described in 1775 in chimney sweeps from ingrained soot and tar. Over the ensuing centuries, many other outside factors that may contribute to squamous cell carcinoma have been described. Some skin lesions are known to be premalignant, for example Bowen’s disease (carcinoma in situ) or actinic keratoses. An area of chronic irritation, including leg ulcers, may progress to squamous cell carcinoma (Hill et al 1996).
Human papilloma virus is a powerful carcinogen in genital carcinoma, but its role in the skin is more uncertain (Sasaoka et al 1996). Contamination with tars, heavy mineral oils, hydrocarbons, etc. continues to be a problem in some industries.
Exposure to UV radiation is a definite factor, as witnessed by the problems encountered by fair-skinned immigrants to very sunny climates, as found in Australia, etc. The feet are usually relatively protected, but one variant of squamous cell carcinoma was described in elderly Japanese on the dorsa of unprotected feet (Toyama et al 1995).
X-radiation is also carcinogenic. Repeated damage from radiant heat may provoke erythema ab igne, and rarely may progress to squamous cell carcinoma. Arsenic exposure has been incriminated.
Clinical features
Squamous cell carcinoma may present in very many different ways. Thus, in any unusual or non-responsive skin condition the possibility of it should be borne in mind, otherwise it may be missed.
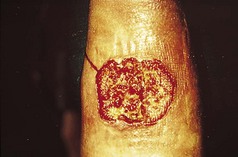
Figure 3.18 Squamous cell carcinoma on the shin. There is an irregular outline with an undermined edge and a dirty, sloughy base.
The vast majority of squamous cell carcinomas are only locally aggressive, but if neglected, they can spread to the draining lymph nodes and thence to other areas (Lund & Greensboro 1965) (Fig. 3.19). This takes a variable time. Head and neck, genital and large tumours all spread more quickly.
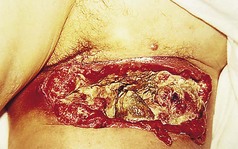
Figure 3.19 Metastasis of squamous cell carcinoma to the groin lymph nodes with subsequent breakdown.
CASE STUDY 3.4 DIAGNOSIS OF ULCERATED AREA
A 70-year-old man presented with a symptomless, irregular, ulcerated area on the dorsum of his left foot. He was of Celtic extraction, but had spent most of his working life as a lifeguard on an Australian beach. What is the likeliest diagnosis and appropriate treatment?
The answer is squamous-cell carcinoma. The Celtic races are more likely to suffer sun damage. The dorsum of the foot is a relatively unusual area, but in his work he is likely to have been more exposed to the sun. It would be essential that a biopsy and surgical excision be undertaken as soon as reasonably possible.
Squamous cell carcinoma variant: verrucose carcinoma of the foot
Verrucose carcinoma (synonyms: epithelioma or carcinoma cuniculatum) (Seehafer et al 1979) may appear at any site but it has a special predilection for the plantar aspect of the foot. Initially it may resemble a simple verruca plantaris. It progresses relatively slowly and may become nodular. At this stage the differential diagnosis would include many nodular foot lesions. With time, the tumour bulk increases and becomes soggy and foul smelling, having been likened to an over-ripe orange. It has a marked tendency to burrow under the skin surface, the sinuses appearing at a slightly distant site from the main lesion – hence the ‘cuniculatum’ denoting a resemblance to rabbit burrows. These rarely metastasise.
Histology
In squamous cell carcinoma the epidermal cells are malignant and manifest in a variety of ways, from large, well-differentiated cells with some individual cell keratinisation, to bizarre, abnormal cells with little or no resemblance to epidermal cells. While in situ, they are contained by the basement membrane to within the epidermis. Sooner or later they ‘break through’ into the dermis, initially often in fine filaments, but later in broad masses.
Verrucose carcinoma is a well-differentiated tumour, but it may have minimal changes suggesting malignancy at the start.
With all suspected squamous cell carcinomas, clinical suspicion should override an apparently normal biopsy. The cancer may not have manifested itself histologically or the sample may have missed the diagnostic part. Therefore, always keep an open mind and be prepared to re-biopsy on several occasions if clinical doubt persists.
Treatment
Prevention should be practised. Measures would include control of UV exposure, radiation, etc. If possible, potentially premalignant lesions should be treated. Particular care in monitoring those at risk for genetic reasons is desirable. This is especially expedient in the immunosuppressed and transplanted patient. Immunisation programmes for young girls in Scotland have commenced. Hopefully, this will reduce the future incidence of genital skin and cervical cancers. The programme may be extended to boys and others later.
In the patient with established squamous cell carcinoma, surgical excision of the region with histological confirmation is therefore the best option. If there is suspicion of lymph node involvement, a suitable dissection should also be undertaken.
In the few patients where surgery is not an option, cryotherapy, laser, photodynamic therapy or intralesional injections have all been suggested. If the patient continues to be at risk, long-term follow-up is necessary.
CASE STUDY 3.5 SEQUELAE OF LONG-TERM VARICOSE ULCERATION
A 58-year-old man asks you about a vascular lesion on his ankle during a podiatry session. He has gross varicose veins with pigmentation around the ankle. He has had a small varicose ulcer near the area for years. He works as a forester. What might be developing?
There are a number of possibilities. Trauma from his work might lead to a pyogenic granuloma. The long-standing ulcer may undergo malignant transformation to a squamous-cell carcinoma. Malignant melanoma on the lower leg may be hypomelanotic or amelanotic. Ideally, histology examination should be undertaken in the near future.
CUTANEOUS METASTATIC DISEASE
Neoplasms beginning in another tissue may spread to the skin by direct extension or as local or distant metastases (Lookingbill et al 1990, Schwartz 1995). These lesions may occur in a patient with known malignant disease or be the first manifestation of the underlying tumour. Skin malignancies themselves, notably melanoma, may also metastasise to other areas of the skin.
Clinical features
There have been several very large surveys of many thousands of patients. Skin involvement as the first sign of cancer is rare (0.8%), and this was equally divided between direct extension, local and remote metastases.
In patients presenting with a tumour elsewhere, skin involvement at that time was found in 1.3%. In all patients with cancers, the skin involvement seems to be of the order of 5%. The skin involvement may come from many initial sites and, in a significant number, no primary site can be identified.
The non-skin tumours that most commonly spread to the skin are breast, colon and rectum, ovary and prostate. There is a wide range of skin lesions. With direct spread from, for example, breast, there may be an apparent nipple or areolar eczema in Paget’s disease, which arises from an intraduct carcinoma (Fig. 3.20). Other breast tumours may spread and grow into the skin, hardening it to resemble a metal breastplate (carcinoma en cuirasse). This may occur in tumours from other sources. Many other types of lesion occur. Small nodules are perhaps the commonest. They often appear to be under the skin, pushing up from below and splaying out the normal cutaneous markings (Fig. 3.21). Indurated erythema, telangiectic plaques or non-healing ulcers are among descriptions of other presentations.
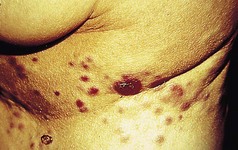
Figure 3.21 Cutaneous nodules pushing up from below. These are secondaries from a primary breast cancer.
When the skin metastasis comes from another skin tumour, malignant melanoma is the commonest cause. The metastases will often mimic the original tumour.
PIGMENTED SKIN LESIONS
Skin colour is largely, although not entirely, due to melanin pigment produced by melanocytes (Bleehen 1998, MacKie 1998, Rhodes 1999). These cells originate from the neural crest and are found in the skin from the eighth fetal week onwards. The melanocytes are the basic factory unit. The rate of production of pigment is influenced by many factors. Ethnic background and sun exposure are two obvious influences.
The melanocyte produces a pigment-containing package – the melanosome – which is transferred to the keratinocyte. The melanocyte is a dendritic cell, which can interdigitate with and ‘service’ about three dozen keratinocytes. This entire complex is called the melanin unit.
The melanocytes are normally situated in the basal epidermal layer and comprise approximately 6% of these cells in covered sites. This rises to 15% in sun-exposed areas.
Naevus
A naevus is the term used to designate a non-malignant growth. Its use is mainly, although not exclusively, confined to skin lesions. They can be classified under headings such as epidermal, dermal, subcutaneous, vascular, etc. Pigmented naevi (in lay terminology, moles) form a large group.
Freckles
Freckles (synonyms: ephelis, ephelide) are common, especially in red-haired persons of Celtic extraction. In size, they are up to 2–3 mm. They tend to be familial. Freckles require UV radiation to develop and usually appear in sun-exposed areas at the age of 3–5 years. Thereafter, they fluctuate in colour intensity in response to sunlight. There is no increase in melanocyte numbers, but these produce more and slightly unusual melanosomes.
Treatment is unnecessary, although these populations are statistically more liable to malignant melanomas. This may be due to them having a similar phenotype and pigmentation profile to adults at risk (McLean & Gallagher 1995).
Lentigo
Lentigos are flat, brownish/black lesions, of which the common ‘age’ or ‘liver’ spots on the dorsa of the hands are examples. They may be induced by sun (solar or actinic changes) but, unlike freckles, tend to fade with UV protection. The lesions can get quite large, up to 25 mm or more and, although the margins can be somewhat ragged, the overall pigmentation is homogeneous with no clumping. They may respond to cryotherapy.
Variants occur in relation to other UV stimuli. Photochemotherapy plus PUVA leads to PUVA lentigos, and sun-bed usage to very large, dark, freckle-like lesions with a slightly irregular edge.
Lentigos show an increase in normal melanocytes, which are arranged along the basal layer.
The sun-bed and PUVA lentigos indicate skin damage and such patients may be at later risk of developing malignant melanoma.
Congenital melanocytic naevus
Congenital melanocytic naevus (synonym: congenital naevomelanocytic naevus) should, by definition, be present at birth, and about 1% of neonates have these. However, rather similar lesions can develop up to a few years postpartum and may be included under this title. It may be that some of these contain the naevus cells, but for whatever reason they delay in producing the pigment. The lesion probably forms between the second and sixth uterine months.
It is traditional to classify these naevi according to size: under 1.5 cm is small, 1.5 cm to 20 cm is medium, and over 20 cm is large.
Clinical features
Congenital melanocytic naevi are usually brown to black in colour. The skin markings can be seen traversing the lesion and may be more pronounced than normal. In many cases, hair of an inappropriate terminal type will develop with time. The surface of the lesion may be smooth, ranging to warty or lobular. An irregular edge may cause concern, but the pigmentation in this area, and throughout the main lesion, is relatively homogeneous. They grow disproportionately slowly with age, compared with the increase in body size (Fig. 3.22).
Histology
Melanocytes are increased in the basal cell area. There is often a gap with a relatively normal area and clumps of naevoid melanocytes in the lower dermis. These seem to have a predilection for skin appendages.
Treatment
The true incidence of congenital melanocytic naevi developing malignancy is disputed, but it is generally agreed that the larger they are the more likely is this complication. However, the large congenital melanocytic naevi affecting the extremities seem to be immune from this (DeDavid et al 1997). Nonetheless, monitoring of all these lesions is advised. Serial photography is advocated.
Small lesions may be excised for cosmetic reasons. The larger the lesion, the more disfiguring it is, but perversely, the more difficult it is to remove. Regular monitoring and attempts at cosmetic camouflage are perhaps the best options.
Acquired melanocytic naevus
An acquired melanocytic naevus (synonym: acquired naevocytic naevus) is an abnormality, but it is an abnormal person who never has any of these. They affect both sexes and appear at intervals throughout life, from shortly after birth. Small numbers present, peaking in incidence around the teens, then falling off in old age.
The melanocytic naevus cells have been the subject of long debate as to their origin. They may be entirely located at the dermoepidermal junction forming junctional naevi. Junctional naevi may represent a stage in the development of compound naevi. This progression is often arrested in areas such as the palms, soles and genitalia, where they can remain for a long time. If junctional changes persist and dermal ones develop, the lesions are called ‘compound naevi’, of which there are various histological variants. There may be closely packed melanocytic naevus cells in the dermis, mainly in the upper papillary layers, in contradistinction to the appearance in congenital naevi.
If the junctional changes are absent, the lesion is termed an ‘intradermal naevus’.
Clinical features
Acquired melanocytic naevi occur in both sexes, with an increased prevalence in black skin (Fig. 3.23). Junctional naevi are the commonest on palms, soles and genitalia. They are usually flat and only a few millimetres in diameter, being round or oval. The colour varies from brown to dark black. Occasionally, stippling can be seen, often with the aid of magnification. The individual, darker areas have a smooth outline. The skin markings are not disturbed. With time, in areas other than the palms and soles, etc., they usually progress toward compound naevi.
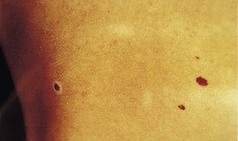
Figure 3.23 Typical benign acquired naevi. They are multiple with a clearly demarcated edge and the colour is homogeneous.
Compound naevi are usually dome-shaped papules with a light to dark colour, which is usually homogeneous. Coarse hair may grow through the lesion. Occasionally, there can be a circle of increased pigmentation around the periphery, which may be postinflammatory hyperpigmentation from trauma. The nail matrix may be affected by junctional or compound naevi, with a marked preponderance of the former. Longitudinal melanonychia may occur (Tosti et al 1996).
CASE STUDY 3.6 POSSIBLE MELANOCYTIC NAEVUS
A 20-year-old woman noted a brown spot on her right sole. It measured about 2 mm in diameter. She was reasonably sure that it had not been there 4 months ago. She was on the oral contraceptive. On examination, the pigmentation was homogeneous, the margin was distinct, the lesion round and the skin markings uninterrupted. What should you do?
The answer is to reassure her. The history and examination suggest a benign acquired melanocytic naevus. In view of the apparently fairly rapid history, she should be reviewed in a few months to see if any change has occurred. The oral contraceptive pill is not relevant.
Intradermal naevi
With the disappearance of the junctional component, pigmentation tends to fade. In intradermal naevi, the lesion is often a flesh-coloured papule. Hair may grow through it (Fig. 3.24).
Histology
In the junctional naevus, small collections of melanocytes or ‘packets’ seem almost to be inserted between the epidermis and dermis. Thereafter, some appear to travel into the upper dermis, either as packets or columns. This forms the compound naevus. When the junctional activity fades, the lesion becomes an intradermal naevus. There is often a zone of normal dermis between the lesion and the epidermis.
The melanocytic naevus cells have nuclei of similar size to normal melanocytes. They have abundant, slightly eosinophilic cytoplasm. There are many histological variants.
Treatment
It is manifestly impossible to remove all naevi and, indeed, this would be undesirable. Surgical removal can be carried out for cosmetic reasons or if the physical location is such that constant irritation occurs. The patient must be aware that formal surgical excision will inevitably result in a scar. This scar may be more noticeable and disfiguring than the original lesion. This is particularly so in the young teenager or adult, with firm elastic skin. It is especially relevant to lesions on the trunk, and in particular on the back.
Some of the protuberant lesions may be improved by a shave biopsy. This will not remove any hair growth and, if there is deeper pigmentation, it may recur and darken. That the lesion will not regrow cannot be guaranteed.
Acceptance of the lesion as a ‘beauty spot’ or cosmetic camouflage techniques are other options.
If there is any doubt whatsoever that the lesion may be a malignant melanoma, excision, or at least a diagnostic biopsy, should be carried out. If there is less doubt and these procedures are deemed to be liable to be mutilating, then assiduous monitoring is essential.
Speckled and lentiginous naevus
Speckled and lentiginous naevus (synonym: naevus spilus) (Rhodes 1999, Stewart et al 1978) is considered relatively rare, although an Australian survey found a prevalence of 2% (Rivers et al 1995). Originally called naevus spilus (from spilus = dot), this name has become confused in the literature, especially when tardus (i.e. late developing) is added as naevus spilus tardus. Some reckon this to be equal to Becker’s naevus.
Clinical features
The lesion usually develops in childhood on the extremities, but other areas can be affected. It is a light brown macular area that may measure up to 10 cm in diameter. Spots, which may be very dark, develop within this (Fig. 3.25).
Histology
The macular area has increased melanocytes, like a lentigo, and the dark areas show melanocytic naevoid cells in the epidermis and dermis.
Becker’s naevus
Becker’s naevus (Monckton et al 1965, Vincent 1999) is a fairly common lesion, occurring in about 1 in 2000 young men and a fifth of that in females. It is pigmented, but there are few or no changes in the melanocytes.
Clinical features
It usually appears in the late teens or early adult life, gradually becoming more obvious. The usual site to be involved is the shoulder, but other areas, including the leg, have been described.
It is often large in area (over 20 cm diameter) with a markedly indented geographic outline. Within it, there can be white island areas about the size of a fingertip. Normally, coarse, darkish hairs develop within the patch. They remain for life.
Spitz naevus
Spitz naevus (Casso et al 1992) is a benign melanocytic lesion, akin to a compound naevus. However, the histology can be very similar to that of malignant melanoma, and pathologists asked to look at it without an adequate history can be forgiven for diagnosing the latter. In the past, benign juvenile melanoma was used to describe these naevi, but it is a term best not used, to avoid confusion.
Clinical features
Spitz naevi usually occur in young people up to the age of 20 years. The head, neck and leg are common locations, although anywhere may be affected. They are usually asymptomatic, dome-shaped, firm nodules, which are pink, red or tan in colour (Fig. 3.26). The natural history is unclear.
Dysplastic naevi
Synonym: clinically atypical naevus. Some patients have naevi, often multiple, which exhibit clinical and/or histological features that are unusual and worrying (Sagebiel 1989, Slade et al 1995). These can occur in some families or in isolation. Many different names have been applied to these. They prove a difficult management problem.
Clinical features
By definition, these are clinically atypical lesions and thus the great concern is to try to differentiate them from malignant melanoma. It is difficult to convey a word picture. They may occur in any sites, either singly or sometimes in vast numbers. They tend to be larger than benign naevi, often more than 5 mm in diameter. They are roughly round or oval, with some asymmetry. The border is irregular, but often fuzzy, rather than sharper, larger projections of the malignant melanoma. There may be a collarette of increased pigmentation. Colour variegation within the lesion is common, but more ‘twin tone’ in contradistinction to the multiple variations seen in malignant melanoma (Fig. 3.27).
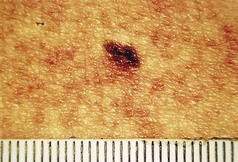
Figure 3.27 Dysplastic naevus. This shows the difficulty in distinguishing this lesion from a malignant melanoma. The borders and colour heterogeneity are not so marked as in malignant melanoma. If in any doubt, further investigation or excision is necessary.
The actual areas of pigmentation have more regular edges. Skin markings are usually uninterrupted.
The natural history is difficult to assess. Some may disappear, but all in all there is an increased chance of melanoma developing, which may be up to 70 times (Halpern et al 1993). This is most marked in those with multiple lesions and a family history of malignant melanoma.
Histology
The microscopic changes may be found with or without clinical atypia and may be reported by the pathologist as dysplastic. As with the clinical situation, there is a kaleidoscope of variation within the histology. The cardinal changes are an irregular proliferation of melanocytes, singly or in packets along the basal cell layer. There is elongation of the rete ridges. The melanocytes themselves show variable atypia.
Treatment
Any dysplastic naevi that cause clinical concern should be excised, along the same lines as for malignant melanoma. If there is less concern, then they may be left. If they are multiple and there is a family history of malignant melanoma, regular careful supervision is required. General photographic views plus individual close-ups are invaluable.
Malignant melanoma of the skin
A cutaneous malignant melanoma (Grin-Jorgensen et al 1991, Langley et al 1999, MacKie 1998) arises from melanocytes in the epidermis. Similar tumours may occur in other tissues (e.g. the eye). It is vitally important for the patient and/or practitioner to diagnose the melanoma early, when a cure can easily be effected. Missing it until it is in later stages will greatly increase the chances that it will lead to the patient’s death. There has been a tremendous increase in the incidence of the tumour in the last 50–60 years. It has been reckoned that in the USA the individual risk of an invasive melanoma increased from 1 in 1500 in 1935 to 1 in 75 in 2000.
Aetiology
The main causative factor for malignant melanoma seems to be sun exposure, although some still seem less convinced. A single or a few burning episodes in the child or the young adult seems to be more provocative than longer term outdoor unprotected activity. Thus the culture in the western world of sun tanning by natural or artificial means, more holidays in the sun and scantier clothing may all play a part.
Some people are more at risk than others. The red-headed, freckled, Celtic races are a particular example. They tend to burn in the sun easily and their voluntary or enforced migration to sunnier climates has been responsible for an ‘epidemic’ of malignant melanoma in, for example, Australia.
There are differences in incidence in different occupations and socio-economic groups. Some of these may be dependent upon affordability of holidays, outdoor exposed working, etc.
In a few cases there is an apparent hereditary tendency to develop malignant melanoma. Skin type, lifestyle, geographical domicile, etc. all influence this.
There was an initial marked increase in malignant melanoma in females compared with males, although the incidence is now rising disproportionately in the latter. Differences in dress patterns may have been important.
Patients with some pre-existing pigmented lesions are at more risk of malignant melanoma. These include some dysplastic naevi and acral pigmented lesions. A personal history of a previous malignant melanoma renders the patient more at risk of developing a second primary lesion.
Clinical features
Some malignant melanomas produce little or no pigment and are termed hypomelanotic or amelanotic melanomas. They pose particular difficulties in diagnosis. Most, however, retain the facility of pigment production. A careful history and meticulous examination in good light are essential. A changing lesion should be paid particular attention. There are many devices marketed to help visualisation, some of which are very expensive. Much can be achieved with a spotlight and some magnification, even using a hand lens. In most medical locations an auroscope is available and, used without the ear piece, this gives light and some magnification. Sometimes the application to the surface of the lesion of a little mineral oil will alter the refraction and allow the clinician to see a little deeper into the epidermis. Appearances on plantar surfaces may be misleading (Akasu & Sugiyama 1996).
Features that would cause concern in the pigmented cutaneous malignant melanoma are mainly related to irregularity. This irregularity of the outline, the border and its pigmentation, colour of the lesion and the skin marking are all ominous features. Increasing size to more than a pencil thickness (i.e. about 7 mm), bleeding, discharging and itching, can all be added.
An ABCD checklist has been suggested:
It must be stressed that no system will be foolproof in the diagnosis of malignant melanoma. Very early lesions will not have developed the above features. A practitioner must realise that an evolving lesion may not be diagnosable at that moment in time, and the patient should not be deterred from seeking further advice if the lesion appears to change.
The prognosis depends vitally on the thickness of the melanoma. Those with a horizontal growth pattern are termed ‘superficial spreading melanomas’ and have a reasonably good outlook in general. On the other hand, those that grow vertically spread more rapidly into the lymphatics and blood vessels, metastasising earlier, with a consequently poorer prognosis. These are termed ‘nodular melanomas’.
These and some other clinical types of malignant melanoma are recognised.
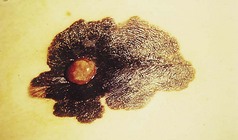
Figure 3.29 Malignant melanoma. This exhibits asymmetry, an irregular border and colour variation. The main lesion is in a superficial spreading phase but the nodule represents vertical growth.
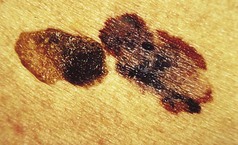
Figure 3.30 Malignant melanoma on the left with irregularities of border and pigmentation. The serendipitous juxtaposition of a seborrhoeic keratosis on the right allows a comparison of features.
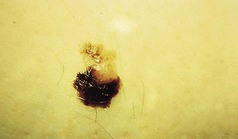
Figure 3.31 Malignant melanoma on the ankle of a 21-year-old female. Jagged irregular border. over half of the lesion is almost amelanotic.
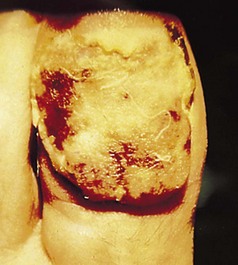
Figure 3.34 Neglected subungual malignant melanoma. Hypomelanotic. Only a small rim of pigmentation can be seen.
Malignant melanoma can be an enigmatic and unpredictable disease. Very early lesions may spread. Nonetheless, there is usually a close correlation to the thickness of the tumour. The deeper the lesion penetrates the more liable it is to spread.
Metastases may take the form of nearby local nodules or spread to the local lymph nodes. Distant areas of skin, subcutaneous tissue and nodes may be affected. The visceral metastases affect, in descending frequency, the lungs, liver, brain, bone and intestines. All of these may occur many years after apparent cure.
Malignant melanoma may present as a distant metastasis. Sometimes the initial primary cannot be found. This may be due to the fact that, rarely, primary melanoma can spontaneously heal.
In the examination of patients with pigmented lesions in general and malignant melanoma in particular, the keeping of meticulous records is mandatory. The history of the patient’s observations and symptoms, a family history and a lifestyle for risk factors should be taken. On examination, measurements of the horizontal and vertical sizes, comments about the border, irregularity and colour variegation should be noted. If possible, the regional lymph nodes should be palpated.
Histology should be obtained if there is the slightest doubt about a lesion. There has been much controversy as to whether an incisional biopsy is likely to provoke spread and, to a certain extent, this still continues. Many authorities feel that there is no evidence for this. If possible, an excisional biopsy should be done, but there may be occasions when it is more expedient to send a part for diagnosis. Some feel that in thicker melanomas a biopsy of the main draining (sentinel) lymph node should be done. This can be identified by dye or radiation methods. This approach still remains controversial (Garcia & Poletti 2007).
CASE STUDY 3.7 AREAS OF PIGMENTATION
A 40-year-old labourer notices a brown subungual area on his right, second toe. He cannot remember any specific injury. On examination, a band of pigmentation is seen, extending throughout the length of the nail and onto the posterior nail cuticle. What action would you take?
The history and examination are highly suggestive of a subungual melanoma, and immediate fast-track referral for surgery is imperative.
Histology
Malignant melanocytic cells invade the dermis. The cells vary in the degree of atypia, with cellular and nuclear enlargement and variation. There is often upward spread into other areas.
Stress has been placed on the importance of the thickness of the malignant melanoma, and the pathologist has a vital role to play in the measurable assessment of this. Originally, this was done by measuring the invasion of the tumour in relation to other anatomical structures of the dermis. These were known as Clark’s levels I–V:
However, this can be difficult on certain sites, such as acral ones, and a micrometer measurement known as the Breslow thickness is now more often used. Using this, melanomas under 0.75 mm are considered to have a good prognosis. Over that, the outlook steadily declines with increasing thickness.
Treatment
Prevention by removing all removable known causes is desirable. There have been many campaigns to warn of the dangers of sun exposure and to promote methods of sun protection. These need to continue.
Persons at risk from genetic problems, past history or due to at-risk pigmented lesions require careful monitoring.
In the suspected malignant melanoma, fast-track referring is essential.
In the event of malignant melanoma being diagnosed clinically or histologically, rapid and adequate excision at the primary site is of paramount importance. The exact surgical clearance margins are disputed but, in general, the thicker the lesion, the wider should be the margins.
If the regional lymph nodes are involved they should be excised.
Once the tumour has spread further, the prognosis is dire. Much work continues on the best chemotherapeutic regimen.
Epidermal naevi
These are a collection of hamartomas or benign abnormalities. They are present from birth, but may not be clinically recognisable until later. Various classifications are suggested. Many lesions do not conform to these headings. Many are warty.
Clinical features
Appearing at or after birth, the lesions develop slowly. Many are linear, probably along the path of embryonic development lines (Blaschko’s lines).
Usually the surface becomes warty and pigmented, especially so on the limbs and palms and soles (Fig. 3.35).
Some become inflamed and intensely itchy. These may be designated inflammatory linear verrucose epidermal naevi (ILVEN).
Dermal and subcutaneous naevi
Hamartomas may affect any of the dermal or subcutaneous structures. They may be isolated lesions or part of a syndrome (e.g. the tuberose sclerosis complex). Classification of dermal and subcutaneous naevi can be difficult, and infinite variations occur.
Usually they present clinically as a swelling. Often the diagnosis is obtained from the histology.
VASCULAR TUMOURS
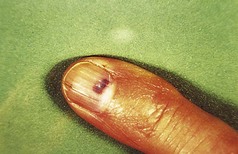
Pyogenic granuloma
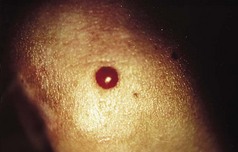
Pyogenic granuloma (synonyms: granuloma pyogenicum, lobular capillary haemangioma) is a common lesion that usually follows an injury, which may or may not be remembered by the patient. In a paediatric survey, most gave no history of trauma (Patrice et al 1991). The injury may rarely be iatrogenic (e.g. after cryotherapy) (Kolbusz & O’Donoghue 1991).
Clinical features
A friable vascular lesion develops rapidly at the site of a previous injury, or apparently spontaneously. There are, rarely, generalised forms of the disease that cannot be due to trauma and occasionally may have an underlying malignancy (Strohal et al 1991). Multiple ones may be triggered in severe acne on the site of active lesions where treatment is inaugurated with retinoids (Exner et al 1983). Paronychia has been described as a cause (Bouscarat et al 1998).
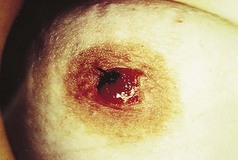
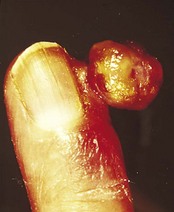
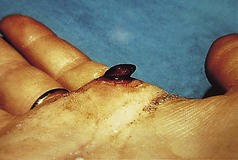
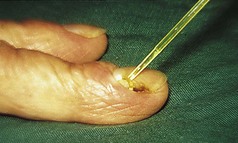
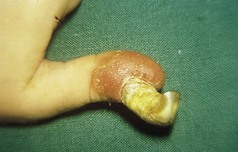
However, the commonest form is a single one. A usual history is of a thorn or pinprick injury followed by the development of a mushroom-type lesion, which bleeds on the slightest touch. Because of this, it is common to see evidence that the patient has covered the lesion with a protective dressing (Figs 3.36, 3.37).
Lesions occurring in restricted anatomical sites are constrained and may lose their mushroom-like appearance. An example of this would be in a nail fold (Fig. 3.38).
The lesion reaches a variable size, usually up to 1 cm, and then ceases growing. In long-established pyogenic granulomas there may be an attempt at epithelialisation, which may lessen the tendency to bleed. Some spontaneously involute. Some develop surrounding or satellite lesions of more pyogenic granulomas locally.
Pyogenic granuloma is usually an easy diagnosis to make and usually responds to treatment. However, it is vital not to miss a more sinister diagnosis, which some tumours may mimic. The greatest pitfall is the malignant melanoma, especially amelanotic or hypomelanotic ones (Elmets and Ceilley 1980).
Other tumours, such as Bowen’s disease or squamous cell carcinoma (Mikhail 1985), verrucose carcinoma (Brownstein & Shapiro 1976), eccrine poroma (Pernia et al 1993) and metastatic lesions may confuse (Giardina et al 1996). Never remove tissue without first obtaining histological confirmation.
Histology
The lesion is circumscribed and contains multiple capillaries, some very dilated (Lever & Schaumberg-Leuer 1990). There is considerable proliferation of endothelial cells. At the base, the epidermis tends to grow, leading to a collarette constricting the lesion and contributing to the ‘mushroom stalk’.
Treatment
The pyogenic granuloma is usually easy to curette. The tissue obtained should always be sent for histological examination. Curettage may need to be repeated on one or two occasions. Formal excision can be carried out.
CASE STUDY 3.8 FRIABLE NODULES
A 15-year-old girl presents with a bleeding friable nodule at the side of her right hallux. She is keen on a martial art form, which necessitates her working out barefoot on a wooden floor. She says that she has noted some small glands on both groins and she has a family history of malignant melanoma. What is the likeliest diagnosis?
The answer is pyogenic granuloma from a minor injury to her foot. Malignant melanoma is rare at this age, but it would be important to consider this. Pyogenic granuloma should have a small stalk, like a mushroom, and it should be possible to curette this off. It would be imperative to send the tissue for histological examination. The finding of glands in the groins of young active persons is not uncommon, and the fact that she stated that these occurred in both groins is less worrying than if the glands had been on one side only.
Glomus tumour
Glomus tumour (synonym: glomangioma) (Requena & Sangueza 1997) is rare and originates from the cells of smooth muscle, the normal function of which is to regulate temperature by acting as valves on arteriovenous anastomotic shunts. These are most profuse on the digits. Glomus tumours are commonest on the digits, although a multiple variety occurs.
Clinical features
Preceding trauma has been implicated, but evidence is vague. There may be an increased incidence in musicians (Harvell & Maibach 1992).
A solitary glomus tumour is usually located in the periungual area, especially of the hands. It may occur at any age, although mostly in early adult life, and affects either sex. A small purplish nodule develops. It is usually only 5 mm in diameter and, if under the nail, can give it a purplish colour. The usual cardinal symptom is pain, which may be agonising and paroxysmal, sometimes triggered by minor pressure or temperature reduction.
The clinical history should suggest the diagnosis, but it can be confirmed in early stages by the use of magnetic resonance imaging (Drapé et al 1996), which may also be able to differentiate other tumours (Goettman et al 1994). Solitary lesions have been reported in other areas, such as the volar aspects of the hands and feet and also on the head and neck.
Multiple lesions are usually called ‘glomangiomas’ and occur as scattered blue dermal nodules. They are more common in childhood, although overall are very rare. Pain is not a feature of these.
Histology
The single glomus tumour is composed of a nodule encapsulated in fibrous tissue. The glomus cells are filled with a large nucleus and a little cytoplasm surrounding vascular channels.
Multiple glomangiomas are not encapsulated and are more vascular.
Treatment
Removal of a single lesion by surgery is the best option. Multiple lesions may be considered for laser treatment (Goldman 1978).
Kaposi’s sarcoma
Kaposi’s sarcoma was originally described in 1872. This remained a rare tumour until almost a century later. It is now much more common and various types are recognised. These include a sporadic (or classic) type, African endemic, immunosuppressive-drug-induced and HIV-related. Whatever the type, it results in a tumour arising from mesenchymal cells of vascular or lymphocytic endothelium (Tappero et al 1993).
Aetiology
Classic Kaposi’s sarcoma was initially considered idiopathic. With the description of the African endemic disease, it was wondered if there was an infecting agent akin to that in Burkitt’s lymphoma. The relative avalanche of cases in association with immunosuppression due to drugs or HIV infection led to further study, and human herpes virus number 8 has been found in all forms of the disease. It is not unique to this condition and it is likely that other cofactors are needed to enhance its propensity to lead to the disease (Dictor et al 1996, Kemény et al 1997).
Clinical features
The classic or sporadic form mainly affects males over 60 years old, often of Jewish or Mediterranean extraction. The commonest affected site is the leg or foot, although lymph node and internal organs can be affected (Tappero et al 1993). It remains rare, one study finding only 163 cases in 20 years (Lospalluti et al 1995). In this series, the survival was 6–13 years. The disease often causes few clinical problems.
The African type has various forms, ranging from one similar to the classic type, through to a florid rapidly disseminating systemic variety. It can affect all ages.
Kaposi’s sarcoma associated with immunosuppression was recognised in 1964 (Klein et al 1974), and there have been many reports since that time (La Parola et al 1997).
Kaposi’s sarcoma associated with AIDS/HIV tends to be a very aggressive disease, with a survival time of up to 36 months (Lemlich et al 1987). Three-quarters had systemic involvement, especially affecting lymph nodes, the gastrointestinal tract and, to a lesser extent, the lungs.
The original skin changes of multiple bluish tumours looked rather like a bunch of grapes, located mainly on the extremities. As the new variants have been described, the spectrum of described skin lesions has extended considerably. Macular, papular, nodular and plaque forms have all been noted. Most of the forms have a purplish colour.
The differential diagnoses include malignant melanoma, glomus tumour, pyogenic granuloma, especially with satellites, sarcoid, etc. (Schwartz 1996).
Histology
In early lesions, the changes may be subtle and difficult to discern. When fully developed, there are proliferations of small vessels, bundles of spindle cells and slit-like spaces, into which red blood cells haemorrhage (Lemlich et al 1987).
Treatment
In the classic form in the elderly, if slow-growing, it may be expedient to do little or nothing. Local treatment for single or few lesions may include surgical removal, cryotherapy, laser, radiation or intralesional cytotoxic therapy (Tappero et al 1993). More aggressive systemic therapies with single or multi-drug therapy may be necessary.
Kaposi’s sarcoma due to immunosuppression or drugs may resolve if these can be withdrawn (La Parola et al 1997). If the Kaposi’s sarcoma is associated with AIDS/HIV, control of superinfection is well worthwhile, as is treatment of the HIV.
CASE STUDY 3.9 NODULES OF THE INSTEP
A 30-year-old homosexual male comes in for treatment by a podiatrist on account of a plantar wart. Nearby you notice a group of purplish nodules, near his instep. What is the likely diagnosis?
The answer is Kaposi’s sarcoma, related to HIV infection. Care should be taken for the operative’s personal protection if there is any chance of contact with blood.
FIBROUS TUMOURS
Acquired fibrokeratoma
There is a group of fibrous lesions occurring on the distal extremities that have been reclassified and renamed over the years, resulting in a number of different names. An early description of the lesions, called ‘acquired digital fibrokeratoses’, was challenged in the ensuing discussion on account of their occurrence elsewhere and ‘acral fibromatosis’ suggested as a preferable name, but this does not seem to have caught on (Bart et al 1968). Other authors make the same point (Hare & Smith 1969, Kint et al 1985, Verallo 1968).
Some are designated ‘periungual fibromatas’ if around the nail (Baran et al 1996) where, if multiple, they can be called ‘garlic clove tumours’ (Steel 1965). The best term at present would appear to be ‘acquired fibrokeratoma’.
Perhaps a separate heading should be kept for those periungual lesions in association with tuberose sclerosis (Koenen’s tumours), although individually they are clinically and histologically similar to others (Kint & Baran 1988).
Aetiology
On rather tenuous grounds, trauma is suggested as a cause (Bart et al 1968, Herman & Datnow 1974, Verallo 1968). However, in most recorded cases, no such history is obtainable.
Clinical features
The lesions have a predilection for the digits, often near the joint, with the periungual area being very common (Verallo 1968). The rate of growth is usually slow, but they can appear in a few months (Kint et al 1985). The lesions are usually flesh-coloured or slightly translucent. They are usually projections slightly narrower at the base, widening out and then coming to a rather pointed end, which often has a hyperkeratotic tip with a reddish base. (Figs 3.39, 3.40) A hyperkeratotic collarette may be seen. Some are more plateau-like (Verallo 1968). Those around the nails commonly cause a groove or sulcus on the nail plate (Kint & Baran 1988) (Fig. 3.41). The differential diagnosis includes a supernumerary digit, dermatofibroma, osteoma or a pyogenic granuloma.
Histology
There are variations, but often there are collagen bundles in the long axis of the lesion, interspersed with numerous capillaries (Kint et al 1985). There may be a mucopolysaccharide deposit between the collagen bundles. This may account for the clinical translucent appearance.
Treatment
Surgical excision is usually successful. Spontaneous clearing in an infantile variant has been described (Duran-McKinster et al 1993).
Koenen’s tumours
These are periungual fibromas that form one part of the condition now known as the ‘tuberous sclerosis complex’ (synonyms: epiloia, Bourneville disease). There are many features of the complex, which can be grouped into cutaneous, mental retardation and epilepsy. Skin problems include facial angiofibromas (adenoma sebaceum), pigmented leathery lesions (shagreen patches), hypopigmented areas (ash-leaf white macules) and Koenen’s tumours. Infinite degrees of severity of the condition occur, and the Koenen’s tumours may be the only manifestation at times. They appear more likely to recur after removal (Verallo 1968).
Dermatofibroma
A dermatofibroma (synonyms: histiocytoma cutis, fibrous histiocytoma, sclerosing haemangioma) is a very common benign fibrous tumour, usually occurring on the extremities.
Aetiology
These tumours have usually been attributed to minor trauma. A long-held belief that dermatofibromas were due to insect bites and a foreign-body reaction to the residual parts has not been confirmed (Evans et al 1989). They seem to be negative for p53 expression and CD34 (HPCA-1), which may help to distinguish them from malignant lesions (Diaz-Cascajo et al 1995, Kutzner 1993).
Clinical features
Dermatofibromas can occur anywhere on the skin, but the limbs are the usual site. They may be flat, but more often are slightly protuberant (Fig. 3.42). Usually slow-growing and less than 1 cm in diameter, they can, on occasion, be giant and over 3 cm (Requena et al 1994). They are usually flesh-coloured, but can have a yellowish tinge, mimicking xanthomata. This may be accentuated by cholesterol deposits (Hunt et al 1990). At times they are slightly pigmented. They may have a ring of enhanced pigmentation, probably in the nature of postinflammatory hyperpigmentation. On palpation, they are firm to hard, with the hardness extending beyond the visual lesion. In other words, the part seen is rather like the tip of an iceberg.
Normally they remain static for years, but some can become atrophic. Multiple lesions on the palms and soles have been described (Bedi et al 1976). Overlying basal cell carcinomas have been noted (Fujisawa et al 1991).
Dermatofibrosarcoma protuberans
This is a rare tumour (Gloster 1996) that can be very aggressive locally.
Clinical features
The dermatofibrosarcoma protuberans (DFSP) usually occurs in the trunk and only rarely on the hands and feet.
It commences as an indurated purplish-reddish or flesh-coloured plaque. Over time, which varies from several months to years, nodules develop. Locally malignant, it rarely metastasises.
ADNEXAL TUMOURS
Eccrine poroma
This was described as a benign tumour of the epidermal portion of the eccrine sweat gland in 1956 (Pinkus et al 1956). The same group described a malignant variant in 1963 (Pinkus & Mehregan 1963).
Aetiology
An immunohistochemical study of cytokeratins helped to find associations between certain normal adnexal structures and the tumour cells. However, it could not determine whether the tumour cells were derived from the adnexal structures or undifferentiated pluripotent cells (Demirkesen et al 1995). The malignant tumours tended to be high expressors of the p53 protein (Tateyama et al 1995).
Clinical features
The eccrine poroma is a relatively common tumour. It can occur at any age, although usually over 40 years, and affects both sexes (McKee 1994). More than a half are found in the sole or plantar surface of the toes. It usually appears as a solitary, non-tender, slightly red nodule. It may protrude or be pedunculated. The surface may be warty or ulcerated and bleeding (Moeller et al 1987). Rare variants, such as multiple lesions (eccrine poromatosis) (Goldner 1970) or linear lesions (Ogino 1976), have been described. Malignant forms are also rare. Only 27 cases were found in 31 years in a retrospective study in a large reference centre (Shaw et al 1982).
The common benign form should be easy to diagnose but may be confused with verrucae plantaris, fibromas, amelanotic melanoma, basal cell carcinoma or squamous cell carcinoma (Moeller et al 1987). It may become as large as 3 cm in diameter (McKee 1994). They may persist and remain benign for a very long time (Morris et al 1968).
Histology
The tumour cells come from the outer layer of the upper dermal eccrine duct. They grow down as uniform cells with central nucleoli and prominent intercellular connections. There is a sharp boundary between the tumour cells and the epidermal cells(McKee 1994). The rare malignant forms range from showing Bowenoid change to frank malignancy with perineural and lymphatic infiltration (Shaw et al 1982).
Treatment
In the benign form, surgical excision with an adequate margin is the preferred option (Goldner 1970). If the lesion is symptomless, the patient frail or the site surgically difficult, treatment need not be insisted upon if the tumour is benign. Any clinical or histological suspicion of malignancy necessitates a wide excision.
OTHER STRUCTURES
Leiomyoma
Leiomyomas (Fisher & Helwig 1963) are benign tumours that arise from smooth muscle cells. As such, they may occur in association with the muscle cells around hairs (piloleiomyoma), genitalia or veins (angioleiomyoma). They may be simple or multiple.
Clinical features
Leiomyomas are uncommon. Only about 100 were reported in a review of the literature over a century and a quarter. However, they are often missed by the clinician and diagnosed by the pathologist. There appears to be a familial incidence (Verma et al 1973). Multiple lesions are more common than single ones. Leiomyomas are commonest on the extremities, especially the legs. A subungual leiomyoma has been described (Requena & Baran 1993) but is rare. Pain and tenderness are reported as the characteristics of the condition, but may be absent in some. Also, other conditions may be painful. The pain in leiomyomas may be spontaneous with a stabbing or burning sensation, or provoked by pressure. The severity is variable, but may be agonisingly severe. On examination, firm smooth, subcutaneous nodules are found, usually several millimetres in diameter, but rarely over a centimetre. They are pink to red in colour, fixed to the skin, but moveable over deeper structures.
Histology
The tumour consists of muscle cells, which may be apparently normal in appearance (Mann 1970). There is evidence of nerve damage and distortion.
Treatment
Solitary lesions may be able to be excised. Phenoxybenzamine (Venencie et al 1982), an alpha-adrenergic blocking agent, or nifedipine (Pulimood & Jacob 1997), a calcium-channel blocker, have been advocated to alleviate pain.
Subungual exostosis
This lesion (Zimmerman 1997) is not a skin tumour but it is included here as it is commonly referred to as such. It is a benign, bony outgrowth from a terminal phalanx.
Clinical features
Females are affected twice as often as males. The hallux is the usual digit, but others can be affected. The lesion usually arises from the dorsal aspect of the terminal phalanx and appears under the distal edge of the nail plate, which it elevates but does not distort. There may be pain from pressure. On palpation, it is very hard. X-ray of the affected digit should be diagnostic, but should include an anteroposterior and a lateral view. In its early stages it may be cartilaginous, non-ossified and thus radiotranslucent.
Treatment
Surgical removal is usually permanently curative. Outpatient procedures have been described (De Berker et al 1994).
Myxoid cyst
Myxoid cyst (synonyms: mucoid cyst, pseudocyst) is a lesion that usually occurs around the distal aspect of a digit. They are very much more common on the fingers than on the toes.
Aetiology
Myxoid cysts tend to be associated with degenerative joint disease. Thus, Heberden’s nodes are commonly seen in association. A tiny thread-like channel connects the cyst to the joint space.
Clinical features
The lesions appear usually on the skin covering the terminal phalanx. Toes can be affected, although much less commonly than the fingers.
While the commonest site is near the proximal nail folds, they can occur elsewhere round the nail, or at other sites. A small, translucent nodule appears (Fig. 3.43). If near the nail, it can cause pressure distortion with a sulcus developing. At times, some discharge, either spontaneously or after trauma, producing a rather sticky fluid.
The symptoms are those related to the pressure effects. The distorted nail may catch or the lesion may cause pain on touch.
At times, depending on the site, they may transilluminate. Ultrasound or magnetic resonance imaging may help to resolve any diagnostic doubt.
Treatment
Radical excision necessitates the identification of the connection to the joint, otherwise recurrence will occur. Local destructive means, such as cryotherapy (Dawber et al 1983), infrared coagulation (Kemmett & Colver 1994) or multiple needling, have been tried.
Ganglia
A ganglion is a cystic swelling commonly formed near the wrist. They also occur on the feet. There is dispute as to whether they are a degenerative process or a benign tumour of the tendon sheath or joint capsule.
Clinical features
On the foot, these are usually on the dorsum. In general, ganglia appear in early adult life. They may be symptomless or cause problems due to pressure. If a nerve is compressed, neurological symptoms may ensue.
Bursae
These are closed sacs, lined with synovial membrane, which are present in many parts of the body. Their aim is to prevent friction, usually between a tendon and a bony prominence. They may be present naturally or develop in response to a new challenge of this type. If such a sac becomes inflamed or infected it is termed ‘bursitis’.
Clinical features
A number of bursae are present normally around the foot, and others may develop. A common problem area is at the first metatarsophalangeal joint in the patient with hallux valgus. This can become inflamed and infected, causing considerable pain. Similar problems can occur in bursae situated in other areas. Another common site is near the Achilles tendon. Bursitis may complicate some autoimmune disorders.
Piezogenic pedal papules
Piezogenic pedal papules (synonym: painful piezogenic pedal papules) (Cohen et al 1970) are small papular lesions on the heel, provoked by pressure of standing (piezogenic = pressure generated). They can be painful.
Clinical features
The flesh-coloured nodules typically appear on the heels when the patient stands and disappear when the pressure is removed. Usually they occur on the medial aspect of the heel, although they can affect the lateral aspect (Fig. 3.44).
Neurofibromatosis
Neurofibromatosis (synonym: von Recklinghausen’s neurofibromatosis) (Zvulunov & Esterly 1995) is one of a mixed group of conditions affecting the skin and nerves – the neurocutaneous disorders. Since von Recklinghausen’s original description, the condition has been split, mainly into neurofibromatosis 1 and neurofibromatosis 2, although other numbers are being added. The main skin involvement is in neurofibromatosis 1.
Clinical features
Neurofibromatosis 1 is an autosomal-dominant, familial condition. It occurs in about 1 in 4000 persons. In neurofibromatosis 1 many systems can be affected. The skin changes can be divided into pigmentary and tumour ones.
The pigmentary changes start in childhood as smooth patches like milky coffee (café au lait), measuring over 15 mm in adults and numbering six or more. These changes can affect any area. Smaller, similar lesions resembling freckles aggregate in the axillae or groin. Axillary freckling is considered pathognomic of the condition. The tumorous lesions can be small and button-like. On palpation, they feel as though they can be pushed through the deep fascia. Larger subcutaneous lesions occur, which may be painful. (Fig. 3.45) Also, much larger nodular or diffuse plexiform neurofibromas can be present. These can all affect any area, including the foot.

Figure 3.45 Neurofibromatosis. Type 1 (von Recklinghausen) nodules, shagreen and café au lait patches are seen.
The eye, skeletal structures and many other areas may be affected. The lesions can cause problems, either due to pressure effects or due to malignant transformation. The severity and extent of neurofibromatosis 1 can vary from minor to major involvement, even within one family.
Neurofibromatosis 2 is the other main variant, but is much rarer, affecting only 1 in 40 000. Skin lesions are rarer, but do include café au lait spots and tumours (Mautner et al 1997). The main problem is neurofibromas of the acoustic nerves.
Histology
The café au lait spots are densely pigmented melanocytes. The neurofibromas are composed of loosely arranged spindle cells with pale cytoplasms. The cells are elongated and wavy.
Treatment
Monitoring and genetic counselling form much of the management (Eichenfield et al 1997, Wolkenstein et al 1996).
Small numbers of neurofibromas may be amenable to surgical removal procedures for cosmetic reasons. Some cutaneous or visceral ones may need surgery due to pressure effects or suspicion of malignant transformation.
Fungal infections of the feet and nails
Fungal infections constitute the most common dermatoses of the feet. About 20% of the population are likely to be affected at some time or other during their lives. Fungi are ubiquitous organisms, and well over 100 000 species have been described and are widely distributed in nature. They are very successful microorganisms and form a diverse group known as eukaryotes, which have definite cell walls but, unlike plants, contain no chlorophyll and are therefore incapable of producing their own nutrients. They thus exist as either parasites or saprophytes, which respectively depend upon living or dead organic material for nutrition. The majority of fungi are moulds, which are composed of a network of branching filaments and look rather like cotton wool in culture (Fig. 3.46). Yeasts, on the other hand, exist as single cells and their colonies are smooth-surfaced on culture.
Figure 3.46 Culture of the fungus Trichophyton rubrum showing the typical cotton-wool-like appearance.
Fungi are classified on the basis of their method of sexual reproduction and are divided into five groups or phyla, namely: chytridiomycetes, zygomycetes, ascomycetes, basidiomycetes and a group known as fungi imperfecti. Many human pathogens belong to this last group.
Fewer than 200 of the many thousands of species of fungi are recognised as human pathogens and many of these are pathogenic only in the face of a diminished host response. Therefore, many new fungal pathogens have been identified in recent times because of the advent of new diseases such as AIDS. In immunocompetent individuals only dermatophytes and Candida yeasts usually cause infection. A number of other saprophytic moulds and some yeasts may invade nail, but their role as primary pathogens is controversial and they may only affect previously damaged nail. However, this will be considered later in this chapter.
DERMATOPHYTE INFECTION
Dermatophyte species are often classified according to their primary host. Hence, dermatophytes, which have man as a primary host, are called anthropophilic species; those which have an animal as a primary host are zoophilic; and those which primarily affect the soil but may infect either humans or animals are known as geophilic species. Fungal infections of the feet are almost always caused by anthropophilic species in developed societies. However, geophilic infection may occur in countries where the population regularly goes barefoot, but even then such infections are uncommon because geophilic dermatophytes are not so well adapted to survival on keratin as are their anthropophilic relatives. Zoophilic infections, which usually have a single animal species as a host, are relatively common in humans but usually affect the scalp, trunk or limbs but very rarely the feet. Indeed, if a zoophilic species is reported from a specimen taken from the foot or nail it is more likely to be due to laboratory error than a true infection.
Three varieties of dermatophyte, Trichophyton rubrum, Trichophyton mentagrophytes var. interdigitalae and Epidermophyton floccosum, are common pathogens of feet. E. floccosum generally accounts for less than 2% of all cases of foot infection and even a smaller number of nail infections. Many acute infections are caused by T. mentagrophytes var. interdigitalae but this variety often produces a brisk inflammatory response and may therefore be self-resolving. T. rubrum is by far the commonest pathogen seen in chronic infection because it is particularly well adapted to life on the human host and produces very little in the way of inflammatory response, and is therefore not likely to resolve spontaneously. Well over 80% of all nail infections are caused by T. rubrum.
Epidemiology
Dermatophytes cause athlete’s foot, or tinea pedis, which affects about 15% of the population. The prevalence rises to 25% or more in sportsmen who regularly use communal bathing facilities.
Although dermatophytes are not commensal organisms as such, they may produce very little in the way of clinical signs and can therefore be carried for long periods of time without the patient’s knowledge. They are deposited on the floors of communal bathing places protected by small pieces of keratin and the average contamination rate in public swimming baths has been identified as being >100 fungal fragments/m2 of floor space. In some areas, particularly steps, this has risen to figures as high as 700 fungal fragments/m2 of floor. It is therefore easy to understand why fungal foot infection is so common, as each one of these fragments is capable of causing human infection. Although not highly pathogenic, dermatophytes are especially well adapted to life in the human host, and are therefore naturally difficult to eradicate. Disinfection of communal bathing places would seem an obvious method of disease control, but fungal elements are protected in small pieces of keratin and, therefore, a disinfectant that would penetrate keratin would be also likely to cause an acute dermatitis of the feet in users of such places and is thus not a feasible option. Swimming baths and other facilities that encourage users to walk through a trough of disinfectant are unlikely to contribute to solving the problems in that such a disinfectant is never going to be strong enough to do the job because of the damage it would cause to the feet. Regular hosing down of changing rooms and the surrounds of swimming pools etc. will reduce the carriage rate, but all floors can only be ‘as clean as the users’ feet’ so this will not eradicate infection either.
Dermatophyte infection nearly always becomes established first in the toe clefts, although punctate infection of the sole can occur. The fourth toe cleft is nearly always infected first, and individuals with closed toe clefts are much more prone to infection than those with open clefts and this anatomical variation is well recognised. From the fourth toe cleft the disease can spread to all other toe clefts onto the soles and sides of the feet and sometimes onto the dorsum of the foot. From there, infection – of usually one hand – is often seen, although hand infection is much less usual than foot infection.
Clinical types
Toe cleft infection
The most common variety of fungal foot infection is tinea pedis or athlete’s foot, which affects only the toe clefts. It is often confined to the fourth toe clefts and consists of maceration of the skin, often with the development of a single central fissure. Sometimes it is asymptomatic, but equally may produce itching and burning in the toe cleft. It may remain confined to the fourth toe cleft almost indefinitely or can spread to other clefts. There is little evidence as to why this is or is not so, and it is not easy to predict which individuals will develop spread out of the fourth cleft, but host defence mechanisms or frequency of use of communal bathing facilities probably have some part to play.
Secondary infections with yeasts or bacteria are common, although these will generally disappear with eradication of the dermatophyte alone and are therefore secondary pathogens. Occasionally a Gram-negative bacterial infection may become established and often can persist alone.
Moccasin tinea pedis
Spread from outwith the toe clefts onto the sole and sides of the foot may occur and this is known as moccasin tinea pedis because it is often confined to the sole and the sides of the foot in the distribution of a moccasin or loafer-type shoe (Fig. 3.47). This usually takes the form of a dry-type dermatitis reaction where scaling is often more pronounced than inflammation. In its early stages the disease is seen only in the region of the toe clefts as spread is relatively slow. Occasionally, a vesicopustular reaction can take place on the soles. Although moccasin tinea pedis is often bilateral, a unilateral picture raises greater suspicion of a fungal infection than, say, a dermatitis reaction secondary to contact. In advanced cases the dorsum of the foot can also become involved and even the lower part of the leg. In such cases classic anular lesions with raised scaly edges and healing centres are seen, hence the name ringworm.
Allergic reactions, often known as dermatophyte or id reactions, are well recognised. The commonest is a simple desquamation of the skin of the palms, which is usually asymptomatic, and non-inflamed. If a patient presents with sudden spontaneous peeling of the skin of the hands, the feet should always be examined. Treatment of the foot infection usually clears the hands as well. A blistering reaction on the soles, hands, or both is known as a podopompholyx, a cheiropompholyx (Fig. 3.48) or a cheiropodopompholyx, respectively. Again, fungi are rarely isolated from these blisters and this is an allergic response. The condition is sometimes so severe as to require a short course of systemic steroids but will usually settle down with treatment of the primary fungal infection.
CASE STUDY 3.10 MOULD INFECTION AFFECTING THE NAIL
A 56-year-old male presents with a history of nail dystrophy of many years duration affecting both first nails
On examination the affected nails are grey-green in colour and are both ridged and thickened. He has no evidence of disease affecting the toe clefts or soles. The clinical appearance and lack of any obvious skin infection suggest that this may not be a dermatophyte infection and will therefore not respond to antifungal treatment. A specimen of nail should be sent to the mycology laboratory.
Culture of the specimen revealed infection with a mould – Scopulariopsis brevicaulis, which does not respond to currently available antifungal agents. The infected nails should be removed in the hope that the new nail will grow in normally. If this fails to occur, then nail avulsion with phenolisation of the nail bed is the only option.
DIFFERENTIAL DIAGNOSIS OF FOOT INFECTION
As mentioned previously, bacterial infection alone is an unusual reaction in the toe clefts but may occur with some Gram-negative species, and a specific infection called erythrasma caused by a corynebacterium can also occur. This has the unique quality of coral pink fluorescence under a Wood’s light, and particularly inflamed toe clefts should always be examined in this fashion to exclude this infection, which responds to systemic antibiotics rather than antifungals. Psoriasis, pustular psoriasis, various types of dermatitis, plantar keratodermas and pitted keratolysis may all be confused with fungal infections of the soles, although, in truth, the clinical appearances are not especially similar and confusion is usually only to the uneducated eye.
Because the fourth toe cleft is nearly always initially affected, it can be said with some confidence that a normal fourth cleft precludes a fungal foot infection whatever else is wrong with the feet. This rule of thumb probably holds good in more than 99% of cases.
NAIL INFECTION
Fungal nail infection may be caused by dermatophyte yeasts or non-dermatophyte moulds. It is accepted that dermatophytes are by far the predominant pathogens and probably account for more than 85% of all cases of fungal nail infection. This percentage is even higher in the toenails, where yeast infections are rarely seen. Yeasts, however, do much more often affect fingernails. The place of non-dermatophyte moulds in the pathogenesis of fungal nail infection is unclear. Scytilidium dimidiatum, previously known as Hendersonula toruloidea, is the only certain primary pathogen of nails in that it can also cause tinea pedis. This is a non-dermatophyte geophilic mould which is a tropical plant pathogen. It is generally seen in immigrants or visitors to the tropics and produces a typical black discoloration of the nails (Fig. 3.49). Other non-dermatophyte moulds that may occasionally be reported from mycology laboratories are probably secondary pathogens to previously damaged nail. It must be remembered that such previous damage is most likely to be due to a dermatophyte infection but other causes, notably onychogryphosis in the elderly, must be considered.
Fungal nail infection is a disease of insidious onset but thereafter there is relentless progression and it does not resolve spontaneously. Various surveys have found a prevalence of between 3% and 10% in western countries, and it is therefore likely to occur in 5–10% of the population. This makes it an especially common disease, and treatment is an important pharmaco-economic issue because of its high prevalence.
Clinical types
There are various published clinical classifications of onychomycosis but the following is probably the most widely accepted:
Some authorities add a further type, namely endonyx onychomycosis (EO). This variety is caused exclusively by Trichophyton soudanense and is exceedingly rare in the UK.
Distal and lateral subungual onychomycosis (DLSO)
This is the commonest variety of onychomycosis and is almost exclusively caused by dermatophytes. Infection is initially a disease of the hyponichium, resulting in hyperkeratosis of the distal nail bed. It generally begins at the lateral edge of the nail, rather than the central portion, and spreads progressively proximally down the nail bed producing further hyperkeratosis and thus onycholysis (Fig. 3.50). Ultimately the underside of the nail is involved, which results in a thickening of the nail, which sometimes becomes friable and crumbles away. Sometimes the fungus proliferates in the space between the nail plate and nail bed, and this is known as a dermatophytoma. Such lesions are either round or linear and are important in that such an appearance is the most common cause of treatment failure.
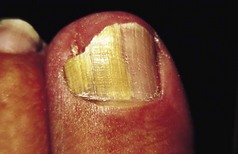
Figure 3.50 Distal and lateral subungual onychomycosis (DLSO) showing spread to the proximal nail fold.
Scytilidium dimidiatum produces a similar appearance, although there is a notable black discoloration of the nail in this infection and it is seen only in patients from the tropics or, occasionally, travellers to the tropics. Scopulariopsis brevicaulis and some Aspergillus species are occasionally isolated but they are probably secondary pathogens to previously damaged nail in that they are saprophytic moulds that will thrive in dead material but not produce proteases capable of invading the nail themselves. Most non-dermatophyte moulds are resistant to antifungal drugs, and nail removal is usually necessary as treatment.
CASE STUDY 3.11 SKIN DERMATOPHYTE INFECTION
A 19-year-old paraplegic girl presents in the clinic with severe maceration of her toe clefts, which are notably soggy and white looking. She has both sensory and motor loss affecting her feet and is therefore asymptomatic. She uses a hydrotherapy pool for physiotherapy purposes on a regular basis.
It is likely that she has a primary dermatophyte infection, with a secondary infection consisting of both yeasts and bacteria.
Topical Terbinafine should take care of both dermatophytes and yeasts, and in addition Betadine ointment should be used for any bacterial overlay. Potassium permanganate (Condy’s crystals) footbaths for 10 minutes each day are always helpful in such cases.
A laboratory specimen for both fungi and bacteria should be submitted before continuing treatment.
Superficial white onychomycosis (SWO)
This is a less common variety and is usually the result of a T. mentagrophytes infection. However, some moulds, such as Acremonium and Fusarium, may also be causal, and this is the only type of onychomycosis that affects the dorsal surface of the nail (Fig. 3.51) and thus sometimes responds to topical treatment.
An especially dense, white appearance occurs in patients with AIDS. Although this is not a true SWO, in that the whole thickness of the nail plate is involved, it is certainly white in appearance and is appropriately classified here for the sake of clinical convenience.
Proximal subungual onychomycosis (PSO)
Yeast infection is the commonest cause of a proximal nail infection, but this is dealt with under the heading of candidal onychomycosis (see below). T. rubrum occasionally causes PSO where the disease begins at the proximal end of the nail (Fig. 3.52). This is often seen in patients with intercurrent disease such as AIDS and peripheral vascular disease.
Total dystrophic onychomycosis (TDO)
All of the above varieties will eventually produce total dystrophy of the nail, which is really a classification for end-stage infection (Fig. 3.53).
Candidal onychomycosis (CO)
Candidal infection of the nail can exist in four forms. The commonest variety is a proximal infection of the nail secondary to a chronic paronychia. This is generally seen in patients with wet occupations where the cuticle becomes detached from the nail plate and infection occurs in the subcuticular space (Fig. 3.54). This is often the result of a mixed infection of yeasts and bacteria, resulting in further swelling of the posterior nail fold, thereby enhancing the cuticular separation. Ultimately, a proximal nail dystrophy will occur because of inflammation in the region of the nail matrix (Fig. 3.55).
Distal infection with yeasts is almost exclusively seen in patients with Raynaud’s phenomenon. It is unclear whether the peripheral vasospasm produces onycholysis and a secondary yeast infection, or whether there is a primary yeast infection resulting in onycholysis. The former is logically more likely in that yeasts do not produce proteolytic enzymes capable of dissolving keratin. It is nearly always seen in the fingernails and is not very common (Fig. 3.56). Total candidal nail dystrophy secondary to chronic mucocutaneous candidosis is even less common. Chronic mucocutaneous candidosis is an inborn defect of cell-mediated immunity, of which there are various types that vary with the severity of the immune defect. In the most severe types there is gross thickening and hyperkeratosis of the whole nail, which is packed with yeasts (Fig. 3.57). Such patients are usually children, who often do not survive into adult life because of the degree of immunodeficiency.
The fourth variety of yeast infection is purely secondary to intercurrent disease, and psoriatic nails are often colonised by yeasts but treatment of the yeast will rarely significantly improve the clinical appearance because psoriasis is the primary defect.
Tinea incognito
It is appropriate to mention this variety of fungal infection here, although it is a poor name that legitimises misdiagnosed and mistreated infection with topical steroids (Fig. 3.58). When the classic ring-like appearance is seen this disease results in loss of integrity of the ring, a reduction of scaling and the development of nodules, which makes diagnosis even more difficult. It can be seen on the feet and lower legs, when fungal infection is mistaken for a dermatitis reaction and treated with topical steroids. If there is clinical doubt and treatment must be instituted then it is always safer to begin with a topical antifungal, which will do no harm to a dermatitis, whereas a topical steroid will enhance a fungal infection.
LABORATORY DIAGNOSIS OF DERMATOPHYTE INFECTION
There is no doubt that many cases of fungal foot infection are treated without laboratory confirmation of diagnosis. Many antifungal preparations are now available on an over-the-counter basis and self-treatment is common. Even when patients visit the general practitioner it is rare for the diagnosis to be confirmed by laboratory methods. The clinical appearances of interdigital infection are relatively typical and this is probably fair enough. However, in order for a therapeutic trial of this sort to be effective it is necessary to use the most effective antifungal preparation. Allylamines are generally much more effective antidermatophyte drugs than azoles, and they are the treatment of choice (see below). It is generally accepted that infections of the sole, and certainly of the nails, should be treated systemically, and in such cases it is always wise to confirm the diagnosis by sending a specimen to a laboratory with expertise in the diagnosis of fungal infections.
In cases of skin infection a skin scraping should be taken from the edge of the lesion, which can either be done dry or following dampening of the area with saline. A blunt scalpel blade can then be used to scrape away the scales, or the slurry when saline is used, and the specimen placed on black paper. The advantages of using the saline method are that it stops skin scales flying around, which makes the specimen difficult to obtain and there is a theoretical risk of inhaling fungal spores thus producing allergy in medical and podiatric practitioners. One should not take the view that this risk is very great, but it is a theoretical one. Because nail infection is primarily a disease of the nail bed rather than the nail plate, collection of hyperkeratotic material from beneath the nail is always best and, of course, a specimen from the most proximal part of the infection contains the most active fungus. A small probe is ideal for removing this material from beneath the nail. Nail clippings are less likely to yield fungus and are more difficult to handle in a laboratory. Scrapings from the nail surface are of no use at all in cases other than SWO.
When the specimens arrive at the laboratory they are cleared with 20% potassium hydroxide and examined directly microscopically. It requires some expertise to differentiate fungal elements from cell walls, and such microscopy should only be carried out by an experienced technician who will usually be able to differentiate a dermatophyte from a non-dermatophyte mould and certainly from a yeast. Culture is carried out in Sabaraud’s dextrose agar and a properly taken specimen will usually grow in culture. However, about 30% of nail specimens that are positive on microscopy fail to grow in culture simply because the specimen is taken from an area where the infection is old and the fungus is dead. However, direct microscopy is entirely adequate for diagnosis of infection and institution of treatment because dermatophytes are not commensal organisms and when the fungus is seen microscopically it is indicative of active disease. In a clinical-trial situation, of course, positive microscopy and culture is demanded and greater care should be taken in the collection of specimens.
It is certainly mandatory to confirm the diagnosis of infection in nail disease. Although modern antifungal drugs do produce cure rates in the region of 80% they have to be given for 3 months and thereafter the nail is left to grow out normally. In the case of toenails this takes at least 12 months before the result of the therapeutic trial is known, and this is quite unacceptable where the drugs are costly and possibly even toxic. Although fungal nail infection is the commonest cause of nail dystrophy it still accounts for only 50% of all cases of dystrophic nails that present, and this confirms the need for accurate laboratory diagnosis. If the disease is strongly suspected on clinical grounds and the laboratory test is negative in microscopy and culture, the test should be repeated at least once, and possibly twice, before fungal infection is excluded simply because nails are difficult specimens for the laboratory to handle and no blame can be attached to even the best laboratory if an infection is missed at first pass, especially if the specimen is taken from the wrong area.
TREATMENT OF FUNGAL FOOT AND NAIL INFECTIONS
Interdigital tinea pedis, the commonest variety of fungal infection, is best treated topically. An antifungal cream should always be used rather than antifungal powder. Antifungal foot powders are useful prophylactically but have no therapeutic role. There are many proprietary brands of topical antifungal but they mainly fall into two drug groups: the azoles and the allylamines. Both azoles and allylamines inhibit sterol biosynthesis in the fungal cell wall. Azole drugs inhibit a cytochrome P450 enzyme, 14 alpha-demethylase, which results in ergosterol depletion. This causes the cell wall to leak; the cell will stop growing and will eventually die. Azoles are generally considered to be less potent in their mode of action, and when used topically should be given for the time taken for the keratin to turn over, which in the case of skin is 4 weeks. Allylamines act on a non-cytochrome P450 enzyme called squalene epoxidase. This results in ergosterol depletion but also in squalene accumulation, and this double mode of action is fungicidal. Topical allylamines therefore need to be used for only one week, and indeed clinical trials have demonstrated that they are more effective over one week of treatment than are azole drugs used over 4 weeks. Furthermore, relapse rates in ‘cured’ patients are much lower than in those treated with azoles. More recently, topical terbinafine in a more adherent base has been developed for once-only use on skin and the results are encouraging.
Topical allylamines should, therefore, be considered the treatment of choice in interdigital foot infection, and topical terbinafine is now available over the counter in both regular and once-only formulations. Although it is more costly than topical azoles, its higher cure rates over a shorter treatment duration, together with its lower relapse rates make it a much more attractive option. The problem with 4 weeks’ treatment is that symptoms will resolve long before the period ends and compliance with the full course of therapy using azole drugs is much less likely. Their failure rate is thus enhanced even further. Although some studies have shown topical terbinafine to be effective in moccasin tinea pedis, it is likely that systemic treatment will be required in some cases for infection of the palms and soles, where the keratin is much thicker and there may be difficulty in penetration by topical agents. Creams have no part to play in the treatment of nail infection, and nail lacquers are generally ineffective other than in the case of superficial white onychomycosis where the dorsal surface of the nail is affected. There are no topical allylamine preparations designed specifically for nails, and amorolfine nail lacquer (Loceryl) is probably the most effective agent, but again it should only be recommended for superficial white onychomycosis or, possibly, as an adjunct to systemic therapy. There are a small number of studies that show that adjunctive topical and systemic treatment works better than systemic treatment alone in nail infections but this requires confirmation in larger properly blinded series.
Systemic treatment of foot and nail infections
There are currently five oral agents available for oral use in the treatment of superficial fungal infections:
Griseofulvin is the oldest of these drugs and is a weakly fungistatic antidermatophyte agent. It must be given for the whole of the time the skin at the infected site takes to turn over, and this is 4–6 weeks at least in infections of the palms and soles, 6–12 months in fingernails and 12–18 months in toenails. Even then, relapse rates are high and the drug no longer has a place in treating infections of the feet. It remains the only drug licensed for use in children and is still used in scalp infections. Although it can still be used in children with toenail infections, the results are so poor as to make it hardly worthwhile. Ketoconazole is no longer licensed for use in the normal run of skin and nail infections because of hepatotoxicity. It was not in any case very much more effective than griseofulvin in dermatophyte infections. Fluconazole is a useful drug in yeast infections and is the most bioavailable of all oral preparations, but it only has relatively weak antidermatophyte activity. It is unlikely ever to become licensed for use in nail disease and its only value in foot infection would be if other agents are contraindicated for some reason.
Itraconazole and terbinafine are the leading players in the treatment of foot and nail infection and terbinafine is the most potent antidermatophyte agent in that it has the lowest minimal inhibitory concentration (MIC) of all of these drugs against dermatophytes, and furthermore the MIC is equivalent to the minimal fungicidal concentration (MFC), making this a truly fungicidal drug in vitro.
Itraconazole can achieve fungicidal concentrations, but its MFC is about ten times greater than its MIC and about 100 times greater (in µg/ml) than the MFC of terbinafine. These in vitro data would suggest that terbinafine is the most potent antidermatophyte agent in vivo, and this is certainly borne out in clinical studies in nail infection. Itraconazole is given in nail infection in a pulsed fashion where 400 mg/day of the drug is given for one week per month and repeated three or four times. Terbinafine, on the other hand, is given in a dose of 250 mg/day for 6 weeks in fingernails and 3 months in toenails, and a large study comparing continuous terbinafine with pulsed itraconazole has revealed terbinafine to be the superior agent by some significant margin.
Terbinafine is, therefore, the treatment of choice in toenail infection, and a number of studies have shown that treatment courses longer than 3 months are not statistically superior. The nail at that stage is not clear of fungus and remains abnormal but studies show that it should thereafter grow out normally in about 80% of cases. If itraconazole is chosen it should be given in four pulses of 400 mg/day for 1 week each over 4 months and again regrowth awaited. An additional month of treatment in the case of terbinafine or an additional pulse in the case of itraconazole may well enhance cure rates in selected cases. Continuing growth of the fungus in culture at the end of the prescribed treatment period is certainly an indication that treatment should be continued for longer.
The two drugs are much closer in terms of efficacy in skin infection, and terbinafine 250 mg/day for 2 weeks is likely to be equivalent to itraconazole 400 mg/day for 1 week in infections of the palms and soles. This is based on historical data and no direct clinical study of these two regimens has been carried out. More importantly there have been no studies of relapse rate, which is likely to be lower with fungicidal agents.
Safety
Itraconazole and terbinafine do have some potential side-effects, as do all drugs. However, minor side-effects such as nausea and itch are seen in only about 5% of cases, which is the same as for most commonly used drugs. Terbinafine does cause taste disturbance in about 1 in 400 cases; this is reversible on cessation of therapy but should be recognised and the drug stopped should it occur. This disturbance varies from a vague diminution in taste sensation to almost complete loss of taste. Hepatotoxicity occurs in about 1 in 50 000 cases in terbinafine-treated patients, and this usually is in the form of an idiosyncratic cholestasis, which again is reversible. Itraconazole causes abnormality of liver enzymes somewhat more frequently, but this rarely becomes symptomatic and again should not give rise to any problems providing it is recognised and the drug stopped when this side-effect does occur. Although the incidence of significant side-effects with systemic antifungal agents compares favourably with many other drugs, there is no doubt that there is some resistance on the part of both patients and medical attendants to their prescription in nail infection. Topical agents, which are largely ineffective, continue to maintain a significant market share, and ongoing development of effective topical agents for nail infections remains a priority.
Prophylaxis
Treatment of systemic infection is relatively expensive and, of course, attention should be paid to prophylactic measures after successful treatment to try to prevent reinfection. Nail infection is secondary to toe-cleft disease, and any evidence of recurrence of toe-cleft infection should be treated enthusiastically. Prevention of toe-cleft infection is possible with antifungal foot powders or the wearing of small plastic socks while swimming. The former is probably a more feasible option, and any antifungal foot powder should be applied after swimming or using communal bathing places. Particular attention must be paid to the toe clefts, and such use of powder has been shown to be useful in preventing reinfection in regular users. The degree of spread via contaminated footwear is unknown but it is theoretically possible, and powder may be applied to the inside of shoes as well.
Akasu R, Sugiyama H. Dermatoscopic and videomicroscopic features of melanocytic plantar nevi. American Journal of Dermatopathology. 1996;18:10-18.
Baran R, Dawber R, Haneke E, Tosti A. Onychomycosis and its treatment. In: A text atlas of nail disorders. London: Martin Dunitz; 1996:155-167.
Bart RS, Andrade R, Kopf AW, Leider M. Acquired digital fibrokeratomas. Archives of Dermatology. 1968;97:120-129.
Bedi TR, Pandhi RK, Bhutani LK. Multiple palmoplantar histiocytomas. Archives of Dermatology. 1976;112:1001-1003.
Bleehen SS. Textbook of dermatology. vol 2, Ch. 39, 6th edn. Rook Wilkinson, Ebling; 1998:pp 1753–1815
Bolognia JL. Bazex syndrome: acrokeratosis paraneoplastica. Seminars in Dermatology. 1995;14(2):84-89.
Bouscarat F, Bouchard C, Bouhour D. Paronychia and pyogenic granuloma of the great toes in patients treated with indinavir. New England Journal of Medicine. 1998;338(24):1776-1777.
Boyd AS, Neldner KH. Lichen planus. Journal of the American Academy of Dermatology. 1991;25:593-619.
Brownstein MH, Shapiro L. Verrucous carcinoma of skin: epithelioma cuniculatum plantare. Cancer. 1976;38(4):1710-1716.
Brehler R, Hildebrand A, Luger TA. Recent developments in the treatment of atopic eczema. Journal of the American Academy of Dermatology. 1997;36:983-994.
Burton JL, Holden CA. Pompholyx. In: Textbook of dermatology. Oxford: Blackwell; 1998.
Casso EM, Grin-Jorgensen CM, Grant-Kels JM. Spitz nevi. Journal of the American Academy of Dermatology. 1992;27:901-913.
Cockayne SE, Shah M, Messenger AG, Gawkrodger DJ. Foot dermatitis in children: causative allergens and follow-up. Contact Dermatitis. 1998;38:203.
Cohen HJ, Gibbs RC, Minkin W, Frank SB. Painful piezogenic pedal papules. Archives of Dermatology. 1970;101:112.
Dalle S, Depape L, Phan B, et al. Squamous cell carcinoma of the nail apparatus: clinicopathological study of 35 cases. British Journal of Dermatology. 2007;156:871-874.
Dawber RPR, Sonnex T, Leonard J, Ralfs I. Myxoid cysts of the finger: treatment by liquid nitrogen spray cryosurgery. Clinical and Experimental Dermatology. 1983;8:153-157.
Dealey C. The Care of Wounds, 3rd ed. Oxford: Blackwell Scientific Press; 2005.
De Berker D, Lawrence CM, Dahl MGC. Outpatient surgery for subungual exostoses. British Journal of Dermatology. 1994;131(Suppl 44):44.
DeDavid M, Orlow SJ, Provost N. A study of large congenital melanocytic nevi and associated malignant melanomas: review of cases in the New York University Registry and the world literature. Journal of the American Academy of Dermatology. 1997;36:409-416.
Demirkesen C, Hoede N, Moll R. Epithelial markers and differentiation adnexal neoplasms of the skin: an immunohistochemical study including individual cytokeratins. Journal of Cutaneous Pathology. 1995;22:518-535.
Deschamps P, Leroy D, Pedailles S, Mandard JC. Keratoderma climactericum (Haxthausen’s disease): clinical signs, laboratory findings and etretinate treatment in 10 patients. Dermatologica. 1986;172(5):258-262.
Diaz-Cascajo C, Bastida-Inarrea J, Borrego L, Carretero-Hernández G. Comparison of p53 expression in dermatofibrosarcoma protuberans and dermatofibroma: lack of correlation with proliferation rate. Journal of Cutaneous Pathology. 1995;22:304-309.
Dictor M, Rambech E, Way D. Human herpesvirus 8 (Kaposi’s sarcoma-associated herpesvirus) DNA in Kaposi’s sarcoma lesions, AIDS Kaposi’s sarcoma cell lines, endothelial Kaposi’s sarcoma simulators, and the skin of immunosuppressed patients. American Journal of Pathology. 1996;148:2009-2016.
Drapé JL, Idy-Peretti I, Goettmann S, et al. Standard and high resolution magnetic resonance imaging of glomus tumours of toes and fingertips. Journal of the American Academy of Dermatology. 1996;35:550-555.
Duran-McKinster C, Herrera M, Reyes-Mugica M, Ruiz-Maldonado R. Infantile digital fibromatosis: spontaneous regression in three cases. European Journal of Dermatology. 1993;3:192-194.
Eichenfield LF, Levy ML, Paller AS, Riccardi VM. Guidelines of care for neurofibromatosis type 1. Journal of the American Academy of Dermatology. 1997;37:625-630.
Elmets CA, Ceilley RL. Amelanotic melanoma presenting as a pyogenic granuloma. Cuts. 1980;25(2):164-166. 168
Eriksson M-O, Hagforsen E, Lundin IP, Michaelsson G. Palmoplantar pustulosis: a clinical and immunohistological study. British Journal of Dermatology. 1998;138:390-398.
Evans J, Clarke T, Mattacks CA, Pond CM. Dermatofibromas and arthropod bites: is there any evidence to link the two? Lancet. 1989;2:36-37.
Exner JH, Dahod S, Pochi PE. Pyogenic granuloma-like zone lesions during isotretinoin therapy. Archives of Dermatology. 1983;119:808-818.
Fine J-D, Bauer EA, Briggaman RA. Revised clinical and laboratory criteria for subtypes of inherited epidermolysis bullosa. Journal of the American Academy of Dermatology. 1991;24:119-135.
Fisher WC, Helwig EB. Leiomyomas of the skin. Archives of Dermatology. 1963;88:510-520.
Fujisawa H, Matsushima Y, Hoshino M, et al. Differentiation of the basal cell epithelioma-like changes overlying dermatofibroma. Acta Dermatologica Venereologica (Stockholm). 1991;71:354-357.
Garcia C, Poletti E. Sentinel node biopsy for melanoma is still controversial. Journal of the American Academy of Dermatology. 2007;56:347-348.
Giardina VN, Morton BF, Potter GK, et al. Metastic endometrial adenocarcinoma to the skin of a toe. American Journal of Dermatopathology. 1996;18(1):94-96.
Gloster HM. Dermatofibrosarcoma protuberans. Journal of the American Academy of Dermatology. 1996;35:355-374.
Goettman S, Drapé JL, Idy-Peretti I, et al. Magnetic resonance imaging: a new tool in the diagnosis of tumours of the nail apparatus. British Journal of Dermatology. 1994;130:701-710.
Goldman L. Laser treatment of multiple progressive glomangiomas. Archives of Dermatology. 1978;114:1853-1854.
Goldner R. Eccrine poromatosis. Archives of Dermatology. 1970;101:606-608.
Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263-271.
Griffiths WAD. Pityriasis rubra pilaris. Clinical and Experimental Dermatology. 1980;5:105-112.
Grin-Jorgensen C, Kopf AW, Maize JC. Cutaneous malignant melanoma. Periodic Synopsis. 1991:712.
Halpern AC, Guerry DIV, Elder DE, et al. Natural history of dysplastic nevi. Journal of the American Academy of Dermatology. 1993;29:51-57.
Hare PJ, Smith PA. Acquired (digital) fibrokeratoma. British Journal of. Dermatology. 1969;81:667-670.
Harvell J, Maibach HJ. Skin disease among musicians. Medical Problems in the Performing Arts. 1992;7:114-120.
Herman PS, Datnow B. Acquired (digital) fibrokeratomas. Acta Dermato-Veneriologica (Stockholm). 1974;54:73-76.
Hill BB, Sloan DA, Lee EY, et al. Marjolin’s ulcer of the foot caused by nonburn trauma. Southern Medical Journal. 1996;89(7):707-710.
Hodak E, David M, Feuerman EJ. Palmoplantar keratoderma in association with myxoedema. Acta Dermato-Venereologica. 1986;66:243-245.
Hoffman D. Leg ulceration with mixed arterial and venous disease. Journal of Wound Care. 1997;6(2):53-55.
Hunt SJ, Santa Cruz DJ, Miller CW. Cholesterotic fibrous histiocytoma. Archives of Dermatology. 1990;126:506-508.
Judge MR, McLean WHI, Munro CS. Disorders of keratinisation. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook’s textbook of dermatology. 7th edn. Oxford: Blackwell; 2004:34.1-34.111.
Kemény L, Gyulai R, Kiss M, et al. Kaposi’s sarcoma-associated herpesvirus/human herpesvirus-8: a new virus in human pathology. Journal of the American Academy of Dermatology. 1997;37:107-113.
Kemmett D, Colver GB. Myxoid cysts treated by infra-red coagulation. Clinical and Experimental Dermatology. 1994;19:118-120.
Kimyai-Asadi A, Kotcher LB, Jih MH. The molecular basis of hereditary palmoplantar keratodermas. Journal of the American Academy of Dermatology. 2002;47:327-343.
Kint A, Baran R. Histopathologic study of Koenen tumors. Journal of the American Academy of Dermatology. 1988;18:369-372.
Kint A, Baran R, De Keyser H. Acquired (digital) fibrokeratoma. Journal of the American Academy of Dermatology. 1985;12:816-821.
Klein MB, Pereira FA, Kantor I. Kaposi sarcoma complicating systemic lupus erythematosus treated with immunosuppression. Archives of Dermatology. 1974;100:602-605.
Kolbusz RV, O’Donoghue MN. Pyogenic granuloma following treatment of verruca vulgaris with cryotherapy and Duoplant. Cutis. 1991;47(3):204.
Kossard S, Rosen R. Cutaneous Bowen’s disease. Journal of the American Academy of Dermatology. 1992;27:406-410.
Kukita A, Ishihara K. Clinical features and distribution of malignant melanoma and pigmented nevi on the soles of the feet in Japan. Journal of Investigative Dermatology. 1989;92:210S-213S.
Kutzner H. Expression of the human progenitor cell antigen CD34 (HPCA-1) distinguishes dermatofibrosarcoma protuberans from fibrous histiocytoma in formalin-fixed, paraffin-embedded tissue. Journal of the American Academy of Dermatology. 1993;28:613-617.
Kwa RE, Campana K, Moy RL. Biology of cutaneous squamous cell carcinoma. Journal of the American Academy of Dermatology. 1992;26:1-26.
Langley RGB, Barnhill RL, Mihm MC, et al. Fitzpatrick’s dermatology in general medicine. vol 1, Ch. 92, 5th edn. McGraw Hill, New York. 1999:pp 1080–1116
La Parola IL, Masini C, Nanni G. Kaposi’s sarcoma in renal-transplant recipients: experience at the Catholic University in Rome 1988–1996. Dermatology. 1997;194:229-233.
Leffell DJ, Fitzgerald DA. Fitzpatrick’s dermatology in general medicine. vol 1, Ch. 81, 5th edn. McGraw Hill, New York. 1999.
Lemlich G, Schwam L, Lebwohl M. Kaposi’s sarcoma and acquired immunodeficiency syndrome: Post-mortem findings in twenty four cases. Journal of the American Academy of Dermatology. 1987;16:319-325.
Lemont H, Pearl B. Juvenile plantar dermatosis. Journal of the American Podiatric Medical Association. 1992;82(3):167-169.
Leung AKC. Pruritis in children. Journal of the Royal Society for Promotion of Health. 1998;118(5):280-286.
Lever WF, Schaumberg-Leuer G. Histopathology of the skin, Ch. 30. Philadelphia: Lippincott; 1990. p. 696
Lindelöf B, Sigurgeirsson B, Melander S. Seborrheic keratoses and cancer. Journal of the American Academy of Dermatology. 1992;26:947-950.
Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma. Journal of the American Academy of Dermatology. 1990;22:19-26.
Lospalluti M, Mastrolonardo M, Loconsole F, et al. Classical Kaposi’s sarcoma: a survey of 163 cases observed in Bari, South Italy. Dermatology. 1995;191:104-108.
Lund HZ, Greensboro NC. How often does squamous cell carcinoma of the skin metastasise? Archives of Dermatology. 1965;92:635-637.
McKee PH. Pathology of the skin with clinical correlations. London: Mosby-Wolfe; 1994. pp 15.50–15.51
MacKie RM. Textbook of dermatology. vol 2, Ch. 38, 6th edn. Rook Wilkinson, Ebling. 1998:pp 1717–1752
McLean DI, Gallagher FP. ‘Sunburn’ freckles, café au lait macules, and other pigmented lesions of schoolchildren: the Vancouver mole study. Journal of the American Academy of Dermatology. 1995;32:565-570.
Mann PR. Leiomyoma cutis: an electron microscope study. British Journal of Dermatology. 1970;82:463-469.
Mautner VF, Lindenau M, Baser ME. Skin abnormalities in neurofibromatosis 2. Archives of Dermatology. 1997;133:1539-1543.
Mellerio JE. Molecular pathology of the cutaneous basement zone. Clinical and Experimental Dermatology. 1999;24:25-32.
Mikhail GR. Subungual basal cell carcinoma. Journal of Dermatologic Surgery & Oncology. 1985;11(12):1222-1223.
Moeller CA, Welch RH, Kaplan DL. An enlarging tumor of the foot. Archives of Dermatology. 1987;123(5):653-654.
Monckton Copeman PW, Jones EW. Pigmented hairy epidermal nevus (Becker’s nevus). Archives of Dermatology. 1965;92:249-251.
Morris J, Wood MG, Samitz MH. Eccrine poroma. Archives of Dermatology. 1968;98:162-165.
Ogino A. Linear eccrine poroma. Archives of Dermatology. 1976;112:841-844.
Ortonne JP. Recent developments in the understanding of the pathogenesis of psoriasis. British Journal of Dermatology. 1999;140:1-7.
Ozedmir HM, Yildiz Y, Yilmaz C, Saglik Y. Tumors of the foot and ankle: analysis of 196 cases. Journal Foot and Ankle Surgery. 1997;36(6):403-408.
Patrice SJ, Wiss K, Mullien JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Paediatric Dermatology. 1991;8(4):267-276.
Pernia LR, Guzman-Stein G, Miller HL. Surgical treatment of an aggressive metastasized Eccrine poroma. Annals of Plastic Surgery. 1993;30:257-259.
Pinkus H, Mehregan AH. Epidermotropic eccrine carcinoma. Archives of Dermatology. 1963;88:597-606.
Pinkus H, Rogin JR, Goldman P. Eccrine poroma: tumors exhibiting features of the epidermal sweat duct unit. Archives of Dermatology. 1956;74:511-521.
Pulimood GS, Jacob M. Pain in multiple leiomyomas alleviated by nifedipine. Pain. 1997;73:101-102.
Requena L, Baran R. Digital angioleiomyoma: an uncommon neoplasm. Journal of the American Academy of Dermatology. 1993;29:1043-1044.
Requena L, Sangueza OP. Cutaneous vascular proliferations. Part II: hyperplasias and benign neoplasms. Journal of the American Academy of Dermatology. 1997;37:887-920.
Requena L, Farina C, Fuente C, et al. Giant dermatofibroma: a little known clinical variant of dermatofibroma. Journal of the American Academy of Dermatology. 1994;30:714-718.
Rhodes AR. Fitzpatrick’s dermatology in general medicine. vol 1, Ch. 90. 5th edn. McGraw Hill, New York. 1999:pp 1018–1059
Rivers JK, MacLennan R, Kelly JW. The eastern Australian childhood nevus study: prevalence of atypical nevi, congenital nevus-like nevi, and other pigmented lesions. Journal of the American Academy of Dermatology. 1995;32:957-963.
Roth MJ, Stern JB, Haupt HM, et al. Basal cell carcinoma of the sole. Journal of Cutaneous Pathology. 1995;22(4):349-353.
Sagebiel RW. The dysplastic melanocytic nevus. Journal of the American Academy of Dermatology. 1989;20:496-501.
Salasche S, Dinehart SM, Pollack SV, Skouge JW. Guidelines of care for cutaneous squamous cell carcinoma. Journal of the American Academy of Dermatology. 1993;23:628-631.
Sandilands A, Smith FJ, Irvine AD, McLean WH. Filaggrin’s fuller figure: a glimpse into the genetic architecture of atopic dermatitis. Journal of Investigative Dermatology. 2007;127:1282-1284.
Sasaoka R, Morimura T, Mihara M, et al. Detection of human papillomavirus type 16 DNA in two cases of verrucous carcinoma of the foot. British Journal of Dermatology. 1996;134:983-984.
Sau P, McMarlin SL, Sperling LC, Katz R. Bowen’s diseases of the nail bed and periungual area: a clinicopathologic analysis of seven cases. Archives of Dermatology. 1994;130:204-209.
Schwartz RA. Cutaneous metastatic disease. Journal of the American Academy of Dermatology. 1995;33:161-182.
Schwartz RA. Kaposi’s sarcoma: advances and perspectives. Journal of the American Academy of Dermatology. 1996;34:804-814.
Seehafer JR, Rahman D, Soderstrom CW. Epithelioma cuniculatum: verrucous carcinoma of the foot. Cutis. 1979;23:287-290.
Shaw M, McKee PH, Lowe D, Black MM. Malignant eccrine poroma: a study of twenty seven cases. British Journal of Dermatology. 1982;107:675-680.
Slade J, Marghoob AA, Salopek TG, et al. Atypical mole syndrome: risk factor for cutaneous malignant melanoma and implications for management. Journal of the American Academy of Dermatology. 1995;32:479-494.
Smith CH, Anstey AV, Barker JN, et al. British Association of Dermatologists guidelines for use of biological interventions in psoriasis. British Journal of Dermatology. 2005;153:486-497.
Steel HH. Garlic clove fibroma. Journal of the American Medical Association. 1965;191:1082-1083.
Stewart DM, Altman J, Mehregan AH. Speckled lentiginous nevus. Journal of the American Academy of Dermatology. 1978;114:895.
Strohal R, Gillitzer R, Sinzits E, Stingl G. Localised vs generalised pyogenic granuloma. A clinicopathologic study. Archives of Dermatology. 1991;127(6):856-861.
Tappero JW, Conant MA, Wolfe SF, Berger TG. Kaposi’s sarcoma. Journal of the American Academy of Dermatology. 1993;28:371-395.
Tateyama H, Eimoto T, Tada T. p53 protein and proliferating cell nuclear antigen in eccrine poroma and porocarcinoma: an immunohistochemical study. American Journal of Dermatopathology. 1995;17:457-464.
Tosti A, Baran R, Piraccini BM, et al. Nail matrix nevi: a clinical and histopathologic study of twenty two patients. Journal of the American Academy of Dermatology. 1996;34:765-771.
Toyama K, Hashimoto-Kumasaka K, Tagami H. Acantholytic squamous cell carcinoma involving the dorsum of the foot of elderly Japanese: clinical and light microscopic observations in five patients. British Journal of Dermatology. 1995;133:141-142.
Venencie PY, Puissant A, Boffa GA, et al. Multiple cutaneous leiomyomata and erythrocytosis with demonstration of erythropoietic activity in the cutaneous leiomyomata. British Journal of Dermatology. 1982;107:483-486.
Verallo VVM. Acquired digital fibrokeratomas. British Journal of Dermatology. 1968;80:730-736.
Verma KC, Chawdhry SD, Rathi KS. Cutaneous leiomyomata in two brothers. British Journal of Dermatology. 1973;90:351-353.
Vincent CY. Fitzpatrick’s dermatology in general medicine. vol 1, Ch. 83, 5th edn. McGraw Hill, New York. 1999;pp 873–881
Warren RB, Griffiths CE. Systemic therapies for psoriasis: methotrexate, retinoids, and cyclosporine. Clinics in Dermatology. 2008;26:438-447.
Wolkenstein P, Freche B, Zeller J. Usefulness of screening investigations in neurofibromatosis type 1: a study of 152 patients. Archives of Dermatology. 1996;132:1333-1336.
Zimmerman EH. Subungual exostosis. Cutis. 1997;19:185-188.
Zvulunov A, Esterly NB. Neurocutaneous syndromes associated with pigmentary skin lesions. Journal of the American Academy of Dermatology. 1995;32:915-935.