Chapter 10 Management of high-risk patients
All patients are at varying levels of risk of acquiring complications following podiatric treatment, the potential being minimised by the podiatrist adopting stringent aseptic/antiseptic techniques before, during and after treatment. Various pathological conditions reduce an individual’s healing potential or produce an increased susceptibility to infection and/or necrosis and ulceration. The patient group frequently cited as being at high risk is those with diabetes, but there are other systemic and local pathologies that place patients in the high-risk category (Box 10.1).
Diabetes mellitus affects approximately 750 000 individuals in the UK (1–2% of the population). It is estimated that 6% of people over 65 years of age suffer from diabetes. Four per cent of hospital beds are occupied by people affected by the complications of diabetes, and 20% of those will be hospitalised because of foot pathologies. Fifty per cent of lower-limb amputations are performed due to the complications of diabetes mellitus, and after 3 years only 50% of diabetics who have undergone amputation will still be alive. It is important to note that 15% of the diabetic population has a foot ulcer, and that 84% of leg amputations are preceded by an ulcer. The podiatrist is part of the multidisciplinary diabetic team caring for the patient. Diabetes is a multisystem disease, and without close liaison between all those involved in the patient’s care treatment will be submaximal. The diabetic care team ideally comprises a podiatrist, diabetologist, specialist nurse, vascular and orthopaedic surgeons, radiologist, orthotist, microbiologist, general practitioner (GP) and the patient and his or her carers. ‘Shared care’ of diabetic patients, between the hospital and the general GP should still allow multidisciplinary cooperation between professionals. The complications associated with diabetes that can affect the feet are summarised in Box 10.2.
Box 10.2 Complications associated with diabetes mellitus that can affect the feet and lower limbs
The Saint Vincent Declaration (1990), aimed to reduce all diabetic lower-limb amputations (resulting from gangrene) by 50% over 5 years. Since the publication of this document it has been shown that diabetic complications, in type 1 diabetics, can be reduced substantially by strict control of blood glucose concentrations (Diabetes Control and Complications Trial Research Group 1993).
In late 1998 the results from the United Kingdom Prospective Diabetes Study (UKPDS) were published. The UKPDS was the world’s biggest and most comprehensive study of type 2 diabetes. It took 20 years to complete and studied 5000 patients in 23 centres in the UK. The results from this study showed that glycaemic control and the control of hypertension have a marked effect on morbidity and mortality in people with type 2 diabetes. Its major conclusions, using existing treatment to establish intensive glycaemic control, were that:
The study also demonstrated that:
As stated earlier, the UKPDS demonstrated that a combination of hypertension and diabetes represents a substantially increased risk of morbidity and mortality. It is thought that the prevalence of hypertension in people with diabetes is much greater than in the non-diabetic population; the prevalence may be as high as 40%.
The link between diabetes and hypertension is not fully understood, and may differ between type 1 and type 2 diabetes. Therefore, identifying those at risk will become increasingly important, and the National Service Frameworks for type 1 and type 2 diabetes mellitus will have major impacts on foot care and podiatric provision. Establishing screening programmes to target those people at high risk of developing foot pathologies will be of vital importance and, with diminishing resources, podiatrists and their colleagues from other medical disciplines will be under increasing pressure to preserve tissue viability in diabetics.
AIMS IN MANAGING HIGH-RISK PATIENTS
The main aims when managing high-risk patients, be they diabetic or not, are:
PREVENTION OF COMPLICATIONS
History taking and assessment
Obtaining a detailed history is paramount, accompanied by a vascular, neurological and biomechanical assessment. History taking includes the recording of medical and surgical details. Any drug therapy (including non-prescription medication) taken by the patient is noted and checked using the current edition of the British National Formulary. It is important to make enquiries regarding drug therapy as the underlying condition for which the drug is being administered may impair wound healing (e.g. vitamin B injections for pernicious anaemia). Alternatively, the drug itself may impair wound healing potential or compromise the patient’s immune system (e.g. corticosteroids).
Investigation of vascular and neurological systems does not need to be complicated (see Chs 1 and 5). Non-invasive methods of vascular assessment, such as calculation of ankle–brachial systolic pressure indices, are relatively simple to perform. Doppler ultrasonography provides the practitioner with an opportunity to identify abnormalities in the flow patterns of blood within the vessels. The use of pressure plates/systems can be helpful in locating areas of high pressure loading, particularly in the neuropathic foot.
Simple measurement of blood sugar levels, using a correctly calibrated glucometer, is indicated when there is a family history of diabetes, or when the patient presents with signs and symptoms of diabetes (e.g. recurrent episodes of infection).
Patient education regarding the relationship of their primary systemic pathology to foot health is vital: many excellent educational packs are available, but they must be accompanied by explanations appropriate to the individual patient. Whenever possible, carers should attend, and be involved with, education sessions.
General points regarding treatment
The practitioner must pay close attention to aseptic/antiseptic techniques. The use of autoclaves for instrument sterilisation is a minimum requirement. Meticulous preoperative preparation of the patient’s skin is necessary to effect a rapid reduction in the number of pathogenic transient flora, and to remove gross contamination. The most important preoperative cleansing routine is a thorough application of an alcohol-based preparation; any antiseptic added to the alcohol provides limited improvement to the activity of the solution.
Padding must be accurately shaped, avoiding creases and irregularities. Strapping should be non-constrictive. If caustic medicaments are used they must be applied with great care and their actions monitored closely. Detailed advice regarding suitable footwear and hosiery is essential, and is considered in other chapters. Minor surgical procedures are performed as a last resort, and only after consultation with the patient’s GP.
MANAGEMENT OF ESTABLISHED WOUNDS, INFECTION OR NECROSIS
An ulcer is an example of a wound that, for various reasons, will not heal. Most research pertinent to wound healing is not restricted to ulcers; the general term ‘wound’ is used in preference to ‘ulcer’ in the following discussion.
The following wound management strategies are considered:
Examination of the wound
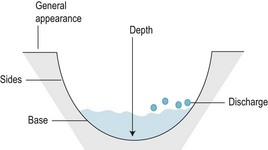
A detailed history and examination of the wound occurs at the initial consultation (Box 10.3). At each subsequent visit the wound and the patient’s general condition are assessed, any changes being recorded meticulously. The points that will be observed by the podiatrist are summarised in Box 10.4 and Figure 10.1. Photographs provide a detailed permanent record, although this recording method is not always practicable during routine clinical practice. However, pieces of sterile transparent film, often as part of wound-dressing systems, are available. The pieces of film have a grid system to allow accurate measurement and record of the wound, without the risk of contamination and cross-infection. At each visit a subjective, as well as objective, assessment of the wound and the whole patient are made.
Box 10.4 Points to observe when examining a wound
Figures 10.2 and 10.3 illustrate plantar wounds proximal to the toe webbing and under the first metatarsal head, respectively.
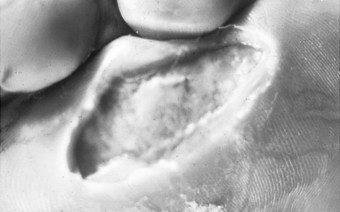
Figure 10.2 A plantar wound proximal to the toe webbing. This wound has walls that undermine surrounding tissue, making its true size difficult to ascertain. Slough covers its base. The surrounding tissues are macerated.
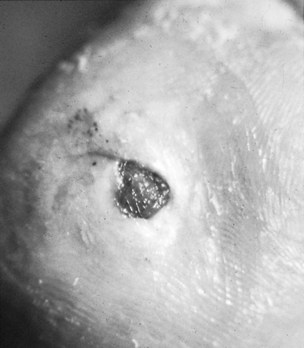
Figure 10.3 A plantar wound under the first metatarsal head. The wound is shallow and its walls are not undermining surrounding tissue; its base is covered with granulation tissue.
Infection
Diagnosing infection is vital, particularly in the high-risk foot. Without prompt and adequate intervention, tissue, limbs and ultimately the patient his or herself may be severely compromised by severe infection. Swabs are frequently taken from wounds, sent for microbiological examination to diagnose and identify infection and the causative microorganisms, and help in management. Many definitions of ‘infection’ exist (historically 105 bacteria per gram of tissue has been identified as the point at which wound healing is inhibited), and the podiatrist needs to understand the principles of what constitutes infection as opposed to wound contamination and wound colonisation:
It should be understood that there is a continuum, and the balance between wound colonisation and wound infection is delicate, particularly in the high-risk foot and when ulceration has become chronic.
Although wound swabbing is often considered the ‘gold standard’ in diagnosing infection, recent opinion advises caution, and swab results may identify only surface colonisation of the wound (Cutting & Harding 1994). It is advocated that, unless attention is paid to ‘associated host reaction’ (Ayton 1985), treatment may be inappropriate. The signs and symptoms of inflammation, the presence of pus, a change in pain, a change in the appearance of granulation tissue and odour are some of the clinical criteria of ‘associated host reaction’ used to help diagnose infection.
However, it is important that microbiological specimens, when indicated, are collected in the correct manner, and that all paperwork is completed accurately. As suggested above, all wound surfaces are populated by microorganisms, but not all of these contaminated wounds are clinically infected. Microorganisms obtained from swabs taken from the deepest part of the wound are more likely to be of clinical significance than are those obtained by superficial swabbing. When free pus is unavailable for collection, swabs moistened with sterile saline are generally considered preferable to specimens collected with a dry swab, and the swab should be rotated 360° to ensure that all parts of the collecting head have made contact with the wound. Inappropriate transportation (either by using the incorrect transport medium, keeping the specimen at the wrong temperature, or delaying transport) of the specimen to the laboratory can cause damage. Clinical information provided to the laboratory needs to be full and accurate, including facts such as the patient is diabetic. This latter point is important, as the microenvironment of ulcers associated with diabetes often supports a mixed range of infecting agents, many of which act synergistically, and knowing the patient’s medical status will help the laboratory in providing the most accurate report. Advice from the local microbiologist will be invaluable in all these matters.
The recent increase in multiple-antibiotic-resistant strains of microorganisms, such as methicillin-resistant Staphylococcus aureus (MRSA), dictate that systemic antibiotics must not be prescribed indiscriminately (Duckworth et al 1988). However, for wounds infected by multiple bacterial strains (particularly diabetic neuropathic ulcers) antibiotic coverage will include drugs active against Gram-positive staphylococci and streptococci, Gram-negative species and anaerobes (e.g. Bacteroides fragilis). Where infection has become chronic, or when deeper structures are involved, investigations are required to exclude soft-tissue infection or osteomyelitis. It should be noted that plain film radiographs will not show changes until approximately 2 weeks after bone infection has become established. Bone scans such as 111In-labelled white-cell scan are much more specific and will show changes early on. However, this method has poor resolution between bone and soft tissues. Other investigations, such as 99mTc diphosphonate bone scans, can be used; however, this method has low specificity, as it demonstrates increased osteoblastic activity and increased blood flow to bone. Both these events occur in other pathologies, such as during the active stage of Charcot neuroarthropathy. Magnetic resonance imaging (MRI) is a very sensitive technique for imaging, and distinguishing between bone and soft tissues, but it is not very specific for osteomyelitis. It should also be noted that many imaging techniques are expensive to perform and not always easy to access.
Management of wounds
There has been much debate about the use of surgical masks, sterile gloves, hats and plastic aprons while treating wounds. Historically, face masks were worn to prevent contamination of wounds by droplets disseminated from the practitioner’s nasopharynx. Surveys demonstrate that the incidence of hospital-acquired infection is not affected by the use of face masks (Orr 1981). It is concluded that masks contribute little to protecting wounds, and may increase transmission of Staphylococcus aureus by encouraging shedding of colonised skin squames through the rubbing action of the mask on the face (Report of the Infection Control Nurses Association 1984). The most effective method of preventing contamination is to reduce conversation while attending to the wound and to carry out well-organised treatment.
Sterile surgical gloves may be worn, particularly when working on deep wounds, but clean latex gloves are acceptable, providing that they are replaced frequently, and worn only at appropriate times. However, there are problems with latex sensitivity. Most workers in this field advise avoiding the use of powdered latex gloves in order to reduce the number of airborne latex particles. Authorities also recommend wearing gloves for as short a period of time as possible, and advise that hands should be washed after gloves are removed (Ayliffe et al 1999).
Since the insistence of Semmelweis over 150 years ago, it has been well recognised that the practitioner’s hands are one of the major vehicles of cross-infection (Rotter 1997). Three levels of hand-washing are recognised: social hand-washing (using soap and water), hygienic hand-washing (with antiseptic hand-wash preparation), and finally surgical hand-washing (a 3-minute hand-wash using an antiseptic). At all levels washing must be performed so that all surfaces of the hands are treated (Taylor 1978).
Many studies confirm that when doctors decontaminate their hands between patient examinations, the rate of hospital infection is also reduced (Larson 1995). An editorial in the British Medical Journal (BMJ Editorial 1999) described continuing problems with members of the medical and allied health professions in complying with straightforward hand-washing protocols. In one study doctors were asked to estimate the number of hand-washes they performed prior to patient examination. The practitioners were observed, and although the doctors’ own perception of their hand-washing rate was 73% the actual observed rate of hand-washing was only 9% (Tibballs 1996). By adopting a standardised hand-wash technique, using soap and water, the number of pathogenic transient organisms is reduced. Hand rinses containing 70% ethanol are very effective in removing transient organisms (Rotter 1984). Paper hats can prevent skin squames from the scalp contaminating wounds.
Single-use plastic aprons may be worn to prevent contamination of permeable cotton clinic coats. Studies demonstrate a 50% reduction in the number of organisms recovered from clean plastic aprons when compared to the number isolated from a clean cotton garment; the moisture content of cotton supports the growth of bacterial colonies (Report of the Infection Control Nurses Association 1984).
The use of prepacked, sterile instruments, medicaments and dressings is part of normal practice, and aseptic techniques should be employed correctly and with care. Interestingly, papers question the lack of reliable evidence to support many of the expensive and possibly overcomplicated aseptic practices used by the nursing profession, which podiatry has adapted for its own specialised use (Bree-Williams & Waterman 1996). It has also been shown that such techniques can become ritualised, and potentially dangerous, unless employed logically and with some thought (Merchant 1988).
Disposal of soiled dressings and instruments, unless performed with care, is a major potential source of cross-infection, and local infection-control guidelines must be followed.
The use of instruments on any wound, and the amount of tissue debrided, will depend on the aetiology and clinical state of the lesion at the time of treatment. Ischaemic ulcerations (whether or not they are associated with diabetes) must be treated conservatively; conversely, diabetic neuropathic ulcers (without associated ischaemia) require maximum debridement to encourage healing. It is important when treating neuropathic ulcers (assuming there is an adequate arterial blood supply) with undermining walls to ensure full exploration of the site and to identify the full extent of the wound. In long-standing neuropathic wounds debridement is purported to help stimulate healing to an acute process, thus aiding repair. However, although debridement is widely advocated in neuropathic ulcerations there still needs to be more research to prove and understand its precise role in the wound-healing process.
Desloughing and wound cleansing agents
Desloughing agents
Slough (a collection of necrotic material, leucocytes and microorganisms) can become a medium for further bacterial growth; in many cases it is important to try to remove it. In ischaemic wounds, slough tends to be firmly attached to the base of the wound, whereas slough in neuropathic wounds may be less adherent and easier to remove. It is important to recognise the intimate physical relationship that exists between slough and granulation tissue; the problems of disturbing the latter while removing the former must be considered before deciding upon a treatment method.
Desloughing/cleansing agents include the hypochlorite group of chemicals, and enzymes that allow autolytic breakdown of slough by-products generated from the patient’s own leucocytes. The hypochlorites interact with protein and it is this reaction that imparts their antibacterial role. It should be noted that the British National Formulary no longer recommends the use of chlorinated solutions such as Dakin’s solution (Chlorinated Soda Solution, Surgical BPC) for wound cleansing due to their irritant effects.
Preparations containing enzymes are used for desloughing wounds (Forsling 1988). Enzymes, such as streptokinase and streptodornase, degrade fibrin and remove DNA from cell nuclei. Most enzyme preparations are presented as dry powders, which are refrigerated until application, when they are reconstituted with sterile isotonic saline (e.g. Varidase, Lederle). The solution can be held in contact with the wound using gauze and a film dressing, or it can be injected under tough necrotic slough using a syringe. Enzymatic preparations seem to be used less frequently in the UK than elsewhere; however, some workers have found successful results using these products in a small randomised, controlled, double-blinded study (Martin et al 1996).
Cadexamer iodine (Iodosorb, Smith and Nephew UK), contains 0.9% w/w of iodine and exerts a hydrophilic action, acting as an absorbent. The product also helps to remove debris and bacteria from the wound surface by capillary action. The beads swell under the influence of exudate and release the iodine.
Intrasite gel can be used to help remove slough and absorb excess exudate.
Sterile larvae of Lucilia sericata (the common greenbottle) are used to deslough wounds (Rayman et al 1998). The way in which the larvae work is not fully understood; however, it is thought they may produce changes in the wound pH, produce natural antimicrobial substances (Pavillard & Wright 1997), and remove necrotic material as part of their normal feeding mechanisms. They reduce pain and odour caused by the wound, and some workers suggest that they may produce growth factors (Prete 1997). Another important attribute is the ability of larval therapy to treat wounds colonised or infected by MRSA (Wise et al 1998).
The larvae are only 2–3 mm long when placed on the wound. They are held in situ by masking the area with a hydrocolloid sheet. The larvae are placed in a carefully shaped hole within the hydrocolloid sheet and then covered with a secondary dressing and left in situ for several days.
Wound-cleansing agents and antiseptics
The skin around the wound must be cleaned prior to treatment to reduce the number of transient microorganisms present. Solutions of antiseptics, such as chlorhexidine gluconate in alcohol base, are satisfactory. Chlorhexidine gluconate has activity against a wide range of both Gram-positive and Gram-negative bacteria; however, chlorhexidine is not active against fungi, spores or viruses. Iodophors are complexes of iodine and solubilisers (e.g. povidone iodine) and are found in many products, including preoperative skin preparation. Povidone iodine has a wide range of activity against microorganisms, including a sporicidal action.
Wound-cleansing agents are used prior to the application of a dressing. Most authorities advocate that antiseptic wound cleansers are not necessary in a wound management programme and that sterile isotonic saline (0.89%) is the preferred solution for wound cleaning. Wound-cleansing agents are available in sterile, single-use sachets, the use of which is strongly advocated. Solutions are applied to the wound site using sterile gauze. Cotton wool should not be used as fibres are shed onto the wound surface where they act as foreign bodies. Solutions may be applied to difficult sites via a sterile syringe barrel, without the needle attached.
Experiments have demonstrated that wound-cleansing agents, with the exception of isotonic saline, can produce transient closure of capillaries (Brennan et al 1986). Chlorhexidine’s effect was minimal. However, in clinical practice, the reduction of capillary blood flow is of very short duration, although the use of sterile isotonic saline is suggested for cleaning the majority of wounds. Interestingly, some recent studies suggest that tap water may be an acceptable alternative to sterile normal saline for cleansing of open wounds (Riyat & Quinton 1997).
Antiseptics
Over recent years the use of antiseptics in the management of ulceration has been controversial. Most antiseptics have a deleterious effect on the wound microenvironment; they can interfere with wound healing, produce resistance in some microorganisms and produce skin sensitivities if used for long periods of time. However, the debate has been re-opened, and with a newer understanding of the way in which microorganisms behave in a chronic wound environment it is feasible that certain antiseptics may have a role in the management of chronic wounds (Gilchrist 1997).
The use of topical antibiotics is contraindicated; antibiotics should be delivered systemically, but only after the causative microorganism has been identified and its sensitivity to a specific antibiotic ascertained.
Dressings
There is a continued abundance of new wound dressings, and as technology moves forward more are to be anticipated. It should be stressed that, in many chronic wounds, the failure of a wound-care product to work is the result of underlying pathologies – emphasising the need to assess the patient’s general health before deciding on a management strategy. No dressing will heal a wound while the local microenvironment is unsuitable (e.g. oedema and local infection). It is unrealistic to expect all chronic wounds to heal; Turner encapsulates this concept by emphasising the need to consider, before predicting the likely prognosis for wound healing:
‘factors which will produce a microenvironment associated with the wound that will allow healing to proceed at a maximum rate commensurate with the age and physiological condition of the patient’
The literature accompanying a wound-care product must be consulted to ensure the suitability of the dressing for individual patients and their wounds. Unfortunately, the price and availability may restrict the use of some products.
The properties of the ‘ideal wound dressing’ are considered before describing the various groups of products.
1. The ability to remove exudate
The removal of excess exudate from the surface of the wound is important for three main reasons:
Exudate also possesses desirable actions. Various substances found in exudate (e.g. growth factors) are necessary for successful wound healing.
2. The ability to maintain humidity at the wound–dressing interface
Until Winter’s seminal work in the 1960s, wounds were kept dry. The rationale for this regimen was to discourage bacterial invasion. In 1962, Winter showed that epithelial movement across a wound was compromised by thick scab formation. Epithelial cells seek a moist surface for movement, and in dry wounds the suitable environment is deep under the scab; in the latter case re-epithelialisation is a slow, energy-consuming process (Winter 1962). It should be noted that dressings that allow ‘strike through’ can dehydrate wound surfaces.
3. Permeability of the dressing to gases
The importance of atmospheric oxygen varies with different stages of wound healing. Early dressings, such as Op-Site (Smith & Nephew), were gas permeable. Under this type of dressing the number of neutrophils in the exudate increased, and the regeneration of epithelial cells increased ten-fold – both these events being beneficial to wound healing.
The effects of hypoxia (reduced oxygen levels) can aid the healing process. Neoangiogenesis (the production of new capillaries, a major component of granulation tissue) is increased in hypoxic environments. Reduced oxygen concentration stimulates macrophages to produce molecules able to stimulate new vessel growth (Silver 1994). Hypoxia is reported to decrease pain, possibly by interfering with the production of prostaglandins and other chemicals.
4. The ability to be impermeable to microorganisms
The dressing should be impermeable to the entry of pathogenic microorganisms onto the wound surface; this situation is most likely to occur during ‘strike through’.
5. The ability to maintain a suitable temperature at the wound surface
After routine cleansing it takes 40 minutes for the surface of a wound to regain its original temperature, and 3 hours before normal cell mitotic function returns (Myers 1982). It is therefore advisable to leave wounds exposed for the minimum time possible during re-dressings (also reducing the risk of cross-infection). It is also wise to avoid using cold solutions for irrigation and cleaning of lesions.
Oxygen dissociation from haemoglobin is impaired when the temperature is reduced by 10°C. Thus, dressings that allow ‘strike through’ not only allow contamination, and encourage dehydration of wounds, but can cause temperature loss by convection.
6. The ability to maintain low adherence at the wound–dressing interface
Dressings that adhere to wound surfaces damage delicate granulation tissue and epithelium when removed. These dressings can become incorporated into granulation tissue and produce foreign body reactions. In addition, patients may experience distress and pain during the change of dressings if the material has become adherent.
Some low-adherence dressings can be responsible for autolytic damage to tissues around the wound site. This is because exudate is unable to travel through the pore structure of the plastic film interface of the dressing.
7. The ability to be free from contaminants
The dressing must be constructed from material that can be sterilised and kept in that condition until it is used. Dressings should not contain substances able to cause toxic reactions, or adversely interact with the wound surface. Particulates or fibres shed from a dressing can become incorporated into granulation tissue and produce foreign body reactions.
8. The ability to maintain a suitable pH
Oxygen dissociates from haemoglobin most efficiently in an acidic environment (Bohr effect). A low pH (acidic) is important in stimulating neoangiogenesis.
9. Other factors, including patient acceptability, ease of application and comfort, and cost
Some early dressings (particularly the occlusive hydrocolloids) were unacceptable to patients because of malodour and exudate formed by the interaction of the dressing components and the wound.
Some dressings are difficult to apply to the contours of the foot as they are designed for use on larger, flatter body surfaces. The judicious use of strapping and conforming bandages helps to overcome these problems.
The cost and availability of some dressings may prohibit their use; however, the cost-effectiveness of a product should always be fully researched.
Types of dressing for use in podiatric practice
The introduction, and accessibility, of new dressings is changing at such a prodigious rate that a detailed description of individual dressings is inappropriate. This section describes the features of groups of products currently available. It is stressed that, before using any wound-care product, practitioners should make themselves fully aware of the product’s indications and contraindications, either by consulting the manufacturer or the current edition of the British National Formulary.
Conventional dressings
The majority of podiatrists use gauze swabs, mainly because of their availability and low cost. The disadvantages associated with gauze dressings include: ‘strike through’, the shedding of fibres, adherence and incorporation into the wound surface. Gauze is woven from cotton, but newer developments have led to the production of fabrics made from non-woven viscose. Swabs made from viscose are more absorptive and less liable to shed fibres. Filmated swabs have layers of cotton wool between either traditional woven, or newer non-woven, gauze; this improves absorption, but can lead to shedding of fibres onto the wound surface.
The use of paraffin gauze (the older name for which is tulle gras) reduces some of the adverse effects of using gauze; however, granulation tissue can grow through its structure and incorporate the paraffin gauze into the wound.
Primary wound-dressing films
Semipermeable adhesive film dressings
Examples: Bioclusive (Johnson & Johnson), Tegaderm (3M), Opsite (Smith & Nephew UK, Cutifilm) (Beiersdorf).
The first low-adherence dressing was Opsite; it was originally developed as an adhesive incise drape for general surgery. These types of film dressing are permeable to gas and water vapour but are impermeable to water. They have no fibres that can be shed into wounds; they are transparent and therefore allow monitoring of the wound site. However, semipermeable adhesive films are non-absorbent.
Such dressings may be used to reduce shear stress over vulnerable areas, such as heels.
Perforated film absorbent dressings
Examples: Melolin (Smith & Nephew), Release (Johnson & Johnson).
Other low-adherence dressings incorporate an absorptive backing, covered with a perforated plastic film. Although they can absorb wound discharge, they are not suitable for heavily exuding wounds. However, a secondary absorbent dressing can be used over the top of perforated film absorbent dressings.
Low-adherent wound contact layers
Unmedicated
Examples: N-A Dressing (Johnson & Johnson), Tricotex (Smith & Nephew).
These dressings are made from knitted viscose; they are non-absorbent, providing a non-adherent primary wound dressing.
Medicated
Examples: Bactigras (Smith & Nephew), Serotulle (Seton).
Some low-adherent wound contact dressings incorporate an antiseptic, such as chlorhexidine acetate, in a paraffin or polyethylene glycol base. Research indicates that chlorhexidine is not easily released from its paraffin base. However, it is suggested that dressings with polyethylene glycol more effectively liberate antiseptics; for example, Inadine (Johnson & Johnson) which contains povidone iodine.
Semipermeable hydrogels
Example: Intrasite Gel (Smith & Nephew).
At present Intrasite Gel is the only hydrogel available on the Drug Tariff. It is composed of a low percentage of carboxymethylcellulose, 80% water and 20% propylene glycol. Structurally, these are hydrophilic polymers that contain a high percentage of water.
There are two basic presentations of semipermeable hydrogels: the first type is in a sheet form (not dissimilar to a thin slice of table jelly); the second is known as amorphous hydrogel and resembles wallpaper paste in texture. Both forms are absorptive; the first type retains its gross structure but swells, the second type absorbs exudate until its substance becomes dispersed in water.
The sheet form of hydrogel consists of approximately 96% water (the percentage varies with individual products), and is transparent, flexible and easily moulded, with mechanical properties that protect delicate granulation tissue. It is gas-permeable but impermeable to water. This form of hydrogel will dehydrate and must be either replaced or rehydrated with sterile isotonic saline to prevent fragmentation of the product. The percentage of water in the original dressing dictates its absorptive power.
The amorphous hydrogels can remove slough by rehydrating dry, necrotic tissue. This type of hydrogel is effective in absorbing exudate and used as a carrier for medicaments such as metronidazole. Hydrogels are kept in situ by applying gauze, or another secondary dressing, over them.
Hydrocolloids
Examples: Granuflex (ConvaTec), Comfeel (Coloplast), Tegasorb (3M).
These dressings are composed of substances that form a gel when in contact with a wound surface. The constituents vary from manufacturer to manufacturer, but they contain polysaccharides and protein. Thus constituents adhere to, and interact with, the wound surface; these are held on a water-repellent, flexible foam backing, which should not require a secondary dressing. In the early versions of hydrocolloid dressings, interaction between the dressing and the wound surface produced a yellow, semi-liquid and malodorous substance. Contemporary products are more sophisticated. The semi-liquid produced at the dressing–wound interface is protective and absorptive, providing a moist and insulated environment; finally, it forms an impermeable layer. The latter results in an acidic and hypoxic environment conducive to neoangiogenesis. The occlusive environment contraindicates the use of hydrocolloids when a wound is clinically infected – particularly when anaerobe species are isolated. The use of hydrocolloids may also be contraindicated for treating diabetic ulcers.
Hydrocolloids are available in other presentations (e.g. pastes); these are used in wounds supporting a heavy slough. Most manufacturers produce ‘wound-management systems’; these involve using desloughing agents, and specific types of dressing at different stages of wound healing.
Alginate dressings
Examples: Kaltostat (BritCair ConvaTec), Sorbsan (Maersk).
These products are manufactured from calcium and sodium salts of alginic acid, which is derived from seaweed. They represent a very old treatment: sailors used seaweed for dressing wounds and effecting haemostasis centuries ago.
When in contact with blood or exudate, alginate fibres convert, via calcium/sodium ion exchange, into a hydrophilic gel. The gel is absorbent, providing a protective, moist interface to the wound. The relative amounts of mannuronic acid and guluronic acid present in alginate formulations determine the characteristics of the gel that is finally formed once the dressing is in contact with the wound. Dressings with higher concentrations of mannuronic acid form softer gels (Sorbsan); those with higher concentrations of guluronic acid form firmer end products. Generally, alginates are relatively easy to remove, provided the area is irrigated with sterile isotonic saline; however, some situations will require the use of forceps to complete the removal of the dressing. Theoretically, the fibres associated with the dressing present no hazard, as they are biodegradable. There have been some reports of patients experiencing a mild burning sensation when alginates are applied; this could be due to the intensely hydrophilic properties of the product causing a rapid dehydration. The effect can be minimised by moistening the dressing with sterile isotonic saline before application.
Polyurethane foams
Examples: Lyofoam (Seton), Allevyn (Smith & Nephew).
Marine sponges were probably the earliest forms of foams used as wound dressings: in 1884 Joseph Gamgee introduced an artificial absorbent sponge.
Foams are indicated for treating wounds with moderate amounts of exudate. The manufacturing process produces a dressing with a smooth, low-adherent hydrophilic inner layer and an outer layer of untreated hydrophobic foam. The construction of the outer layer helps reduce ‘strike through’, although a secondary dressing may be required. Foam dressings are permeable to gases and allow adequate hydration of the wound surface and provide effective thermal insulation.
Other dressings – the way forward?
Hyaluronic acid, a glycosaminoglycan, promotes cell proliferation (including fibroblasts), and Hyafill has been used with success by some podiatrists.
Platelet-derived growth factors and cytokines (e.g. transforming growth factors α and β2) have been used to treat diabetic ulcers (Holloway et al 1993). Recent work shows that the chronic wound environment contributes to an increase in the amount of proteases: the relative overproduction of these substances is thought to break down normal growth factors. Hence the development of products that aim to protect available growth factors from protease degradation – one example being Promogran (Smith & Nephew).
Bioengineered dressings such as Dermagraft (Smith & Nephew) have aroused much interest. This product is a human, fibroblast-derived dermal replacement, and early results appear promising. Other approaches involve seeding the patient’s own epithelial cells onto a suitable culture medium and using the resultant culture on their wounds (Shakespeare 1991).
Almost any substance imaginable has been used as a wound dressing at some point in history. Some of the older remedies are currently being reassessed using modern scientific methods. Honey, for example, is acidic (approximate pH 3.7), and it may encourage wound neoangiogenesis and may also be bactericidal.
OTHER ASPECTS OF MANAGEMENT
In people with diabetes, education and advice about preventing secondary complications of the disease is imperative and part of the podiatric management. Despite general consensus among podiatrists and other healthcare professionals that health education plays a vital role in management plans, there is minimal statistically sound evidence to show the most satisfactory method of delivering health education to people with diabetes. This disappointing lack of evidence must not of course stop education programmes, but must motivate podiatrists to find the optimum ways of disseminating information in the most effective manner to people with diabetes and other pathologies that impair tissue viability. It is a matter of urgency that the delivery and content of foot health education is reviewed in the light of evidence-based practice.
The use of padding and orthoses to protect wounds may be indicated; each patient will require a specific prescription, and therefore only general comments can be made.
Soft, cushioning, padding materials are indicated rather than firm, redistributive materials; the use of the latter can compromise capillary blood flow to the edges of the wound. Accurate positioning of pads is vital, and is not always an easy task, particularly when large dressings are applied. As little adhesive as is practicably possible should be placed on the skin; conforming bandages, for example, Kling (Johnson & Johnson) or plastic film sprays can be used to protect vulnerable areas of skin from adhesives.
Replaceable silicone pads (Silipos) of various shapes and sizes are useful in cases where the patient can reach his or her feet, and where there is no danger of the pad constricting a digit, or part of the foot.
When specific areas of high pressure loading are identified, casted insoles constructed from composites of low-, medium- and high-density thermoplastics may be required. These customised insoles are valuable in preventing initial damage occurring. During the last decade many new orthotic materials have been specifically developed for the diabetic foot, and they show promise in the overall management plan.
Footwear and hosiery must be carefully selected and, if necessary, modifications such as stretching or balloon patching executed. Slippers, often a popular choice with patients, are not recommended unless well fitting, as they produce shearing stresses that cause movement of dressings, predispose to falls and reduce activity of the calf muscle pump. Boots made from low-density thermoplastics may be suitable for an immobile patient. Bespoke or semi-bespoke footwear is the answer for many chronic foot problems, or they can be worn as a preventive measure. As a temporary measure, commercially available postoperative boots or sandals can be modified by the addition of felts and sponges. The orthotist is an important link in the provision of special footwear when caring for patients in a hospital setting.
Total contact casting may be indicated when treating neuropathic ulcers. The technique has several variations that allow removal of the cast for inspection of the wound site. The technique must be taught correctly by someone with experience in the technique, and the podiatrist must be confident in the correct application of the cast before attempting to use total contact casts on patients. The introduction of Aircast pneumatic walkers has proved beneficial for some patients; these transfer pressure over the whole of the plantar surface of the foot and support the leg, and they also have a rocker sole. When these methods are used the patient must be provided with an address or telephone number that they may access 24 hours a day, 7 days a week, in case advice is needed.
The patient with an ulcer, open lesion or infection is advised to rest and elevate the affected limb, but the danger of immobility producing a deep vein thrombosis cannot be overlooked. Other problems of immobility are the development of pressure sores and, if the limb is insufficiently elevated, oedema may ensue.
Patients must be discouraged from smoking. Smoking adversely affects wound healing in several ways: it causes vasoconstriction, reduces macrophage and epithelial cell function and reduces immunoglobulin G (IgG) levels (Siana et al 1992).
However, the patient’s quality of life, and their compliance, may be adversely affected unless a compromise between ideal and realistic advice is given.
Consideration has been given to the role of multidisciplinary care of the patient, which is crucial during treatment of wounds. The same approach is important once improvement of the wound occurs, so the patient is monitored by professionals, such as district nurses and the GP, who can liaise with each other and the podiatrist in providing patient care.
CONCLUSIONS
The high-risk patient and their wounds provide a challenge to the podiatrist; the adage ‘prevention is preferable to cure’ holds true for this group of patients. The challenge is minimised by adopting a multidisciplinary approach to patient care, using expertise gained by other healthcare professionals and by colleagues. It is important that other professions understand the role of podiatry in the care of the high-risk patient, and it is incumbent on the podiatrist to ensure that this information is forthcoming.
Patient education is important, but only if the patient and their carers understand the rationale behind the information that is provided – the responsibility for this lies with the podiatrist.
When wounds develop they must be treated on an individual basis, and only after a full medical history and physical examination of the patient, and their lesion, have been carried out. When selecting wound-care products the practitioner is aided by an understanding of the normal wound healing process and by frequent consultation of the medical and nursing press for new developments.
Ayliffe GAJ, Babb JR, Taylor LJ. Nursing aspects of prevention of infection. Hospital-acquired infection – principles and prevention, 3rd edn. Oxford: Butterworth & Heinemann; 1999. p. 104
Ayton M. Wounds that won’t heal. Nursing Times. 1985;81(46 Suppl):16-19.
BMJ Editorial. Hand-washing. A modest measure with big effects. BMJ. 1999;318:686.
Bree-Williams FJ, Waterman H. An examination of nurses’ practices when performing aseptic technique for wound care. Journal of Advanced Nursing. 1996;23(1):48-54.
Brennan S, Foster ME, Leaper DJ. Antiseptic toxicity in wounds healing by secondary intention. Journal of Hospital Medicine. 1986;8:263-267.
Cutting KF, Harding KG. Criteria for identifying wound infection. Journal of Wound Care. 1994;3(4):198-201.
Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development of long-term complications in insulin dependent diabetes mellitus. New England Journal of Medicine. 1993;329:977-986.
Duckworth GJ, Lothian JLE, Williams J. Methicillin resistant Staphylococcus aureus: report of an outbreak in a London teaching hospital. Journal of Hospital Infection. 1988;II:1-5.
Forsling E. Comparison of saline and sreptokinase–streptodornase in the treatment of leg ulcers. European Journal of Clinical Pharmacology. 1988;33:637-638.
Gilchrist B. Should iodine be reconsidered in wound management? Journal of Wound Care. 1997;6(3):148-150.
Holloway GA, Steed DL, De Marco M, et al. A randomised, controlled, multicentre, dose response trial of activated platelet supernatent, topical CT-102 in chronic, nonhealing diabetic wounds. Wounds. A Compendium of Clinical Research and Practice. 1993;5(4):198-206.
Larson EL. APIC Guidelines for hand-washing and hand antisepsis in healthcare settings. American Journal of Infection Control. 1995;23:251-269.
Martin SJ, Corrado OJ, Kay EA. Enzymatic debridement for necrotic wounds. Journal of Wound Care. 1996;5(7):10-311.
Merchant J. Aseptic technique reconsidered. Care – Science and Practice. 6(3), 1988.
Myers JA. Modern plastic surgical dressings. Health and Social Services Journal. 1982;18 March:336-337.
Orr N. Is a mask necessary in the operating theatre? Annals of the Royal College of Surgeons. 1981;63:390.
Pavillard ER, Wright EA. An antibiotic from maggots. Nature. 1997;180:916-917.
Prete P. Growth effects of Phaenicia sericata larval extracts on fibroblasts: mechanism for wound healing by maggot therapy. Life Science. 1997;60:505-510.
Rayman A, Stansfield G, Woolard T, et al. Use of larvae in the treatment of the diabetic necrotic foot. Diabetic Foot. 1998;1:7-13.
, Report of the Infection Control Nurses Association Working Party on Ward Protective Clothing 1984. Infection Control Nurses Association.
Riyat MS, Quinton DN. Tap water as a wound cleaning agent in accident and emergency. Journal of Accident and Emergency Medicine. 1997;14(3):165-166.
Rotter M. Hygienic hand disinfection. Infection Control. 1984;5:18.
Rotter ML. 150 years of hand disinfection – Semmelweis’ heritage. Hygienic Medicine. 1997;22:332-339.
Saint Vincent Declaration. Diabetes Care and Research in Europe. Workshop Report. Diabetic Medicine 1990;7:370.
Shakespeare P. Cultured human skin epithelium for wound repair. Journal of Tissue Viability. 1991;1:19-20.
Siana JE, Frankid S, Gottrup F. The effect of smoking on tissue function. Journal of Wound Care. 1992;1(2):37-41.
Silver IA. The physiology of wound healing. Journal of Wound Care. 1994;3(2):106-109.
Taylor LJ. An evaluation of hand-washing techniques. Nursing Times. 1978;74(54):108.
Tibballs J. Teaching hospital medical staff to hand-wash. Medical Journal of Australia. 1996;164:395-398.
Turner TD 1979 Products and their development in wound management. Symposium on wound healing, Espoo, Finland, 1–3 November 1979.
Winter GD. Formation of the scab and the rate of epithelialisation of superficial wounds in the skin of the young domestic pig. Nature. 1962;193:293-294.
Wise R, Hart T, Cars O, et al. Antimicrobial resistance. BMJ. 1998;317:609-610.
Ayliffe GAJ, Lowbury EJL, Geddes AM, Williams JD. Control of hospital infection, 3rd edn. London: Chapman and Hall; 1992.
Levin ME, O’Neal LW, Bowker JH. The diabetic foot, 5th edn. St. Louis, MI: Mosby; 1993.
Pickup JC, Williams G. Chronic complications of diabetes, 1st edn. Oxford: Blackwell Scientific; 1994.
Underwood JCE. General and systemic pathology, 1st edn. Edinburgh: Churchill-Livingstone; 1992.
Watkins PJ, Drury PL, Taylor KW. Diabetes and its management, 4th edn. Oxford: Blackwell Scientific; 1990.