Chapter 21 Nail surgery
The most common problems for which patients seek the assistance of a podiatrist are disorders of the toenails (Krautz 1970). The disorders that are seen most commonly include those of congenital, traumatic, infectious, inflammatory, acquired and neoplastic aetiology (Shereff 1994). However, it has long been acknowledged that the ingrown toenail (Fig. 21.1) is the most common abnormality of toenails seen in orthopaedic practice, and that ingrowing toenails are a common cause of pain, disability and absence from work (Bartlett 1937, Bose 1971, Dixon 1983, Fowler 1958, Murray 1979). Matricectomy is now the most common surgical procedure performed on the foot (Boberg et al 2002, Espensen et al 2002). For many years the most widely used operation was the wedge excision, as described by Cheyne and Burghard in 1912. However, this method of treatment gives considerable discomfort and has a high recurrence rate (Palmer & Jones 1979, Sykes 1986).
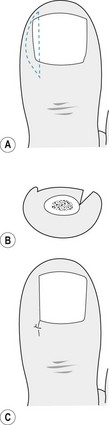
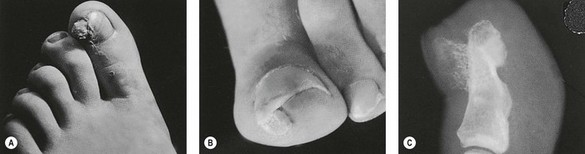
Figure 21.1 Onychocryptosis (ingrowing toenail), showing a splinter of nail within the sulcus and overlying granulation tissue.
PHENOLISATION
Removal of part or the entire toenail with phenolisation of germinal tissue has become a common procedure with high levels of patient satisfaction and very low regrowth rates, particularly when carried out by podiatrists (Bostanci et al 2001, Gabriel et al 1979, Islam et al 2005, Laxton 1995, Morkane 1984). In a comparison of matrix excision and phenolisation, Bos et al (2007) found significantly better results in the phenolisation group. As a method of treatment the phenol and alcohol matricectomy remains pre-eminent in the radical treatment of painful nail conditions.
Phenol
Phenol (C6H5OH, carbolic acid) was originally isolated from coal tar by Friedlieb Runge in 1834. Although naturally occurring as a by-product of decomposition it is a largely man-made product synthesised for use in the manufacture of phenolic resins.
Phenol is uniquely both hydrophilic and lipophilic. It is poorly soluble in water but is highly soluble in isopropyl alcohol, glycerine, ketones and esters (Boberg et al 2002). It has a sweet and acrid odour in its liquid form. Liquefied phenol is colourless, and is particularly stable when stored in an airtight container and protected from light. The solution becomes pink when left exposed to air and light but loses none of its potency or concentration.
Phenol has long been used as a chemical agent against bacteria and fungi and as an anaesthetic in many medicinal preparations (Espensen et al 2002). In 1865, Joseph Lister reduced the mortality rate in amputation surgery by two-thirds by disinfecting his theatre in Glasgow Royal Infirmary with an airborne spray of dilute phenol. Phenol is toxic and has the potential to cause serious local and systemic effects if absorbed or ingested. Reported effects include fluctuating body temperature, cardiac and respiratory changes, convulsions and, ultimately, death. The medical use of phenol has been shown to be very safe, and no systemic complications have been reported in relation to its use in matricectomy (Boberg et al 2002). However, the UK Health and Safety Executive issued a Hazard Alert Notice in 2000 following concerns regarding phenol. This imposed on employers a legal obligation to protect staff by using control measures to limit exposure to phenol or, preferably, to prevent exposure entirely by using a different substance or method.
In the UK, the Medicines and Healthcare products Regulatory Agency (MRHA) considers that phenol as applied to the skin is a medicinal product, and as such is classified as an unlicensed medicine. As only medical and dental practitioners are legally entitled to use an unlicensed medicine, the use of phenol by podiatrists in the UK must be considered illegal. The MRHA has, however, approved a commercial product called EZ Swabs, which is a method of containing and applying phenol, as a Class 11b Medical Device. Approving it in this way enables podiatrists to continue to use phenol within the law. Other regulations may apply in other parts of the world.
History
The use of the procedure of phenolisation of symptomatic toenails has grown since Boll first described it in 1945. In 1962, Suppan and Ritchling combined the use of phenol with the more traditional technique of wedge resection of the nail plate, bed and matrix by suggesting that phenol should be applied for 5 minutes without pressure following the resection. Greene (1964) considered that the alcohol used to wash out the phenol delayed healing, may have increased postoperative pain and was unnecessary as it did not neutralise the action of the phenol, which was in effect self-limiting. The need to apply the phenol with pressure was stressed in 1956 by Nyman, who used alternate applications of phenol and alcohol, finishing with an alcohol-soaked dressing postoperatively. In the early 1970s Yale (1974) presented a paper at West Point Military Academy describing the results of 500 phenol and alcohol matricectomies. The study had been carried out over a 2-year period on soldiers who were able to return to light duties on the day of surgery and full duties within a week. He stressed the need for the use of fresh phenol and strict haemostasis. The requirement for the procedure to be carried out in a bloodless field was further stressed by Dagnall in 1981. Bostanci et al (2001) reported a series of 350 phenol matricectomies with a mean follow-up of 25 months. The success rate was found to be 98.8%. Subsequent authors have reported results of varying the length of time of application of phenol and alternative dressing regimens (Dovison & Keenan 2001, Dunlop 1998, Drago et al 1983, Rinaldi et al 1982).
THE PHENOLISATION TECHNIQUE
The phenol and alcohol technique is not an invasive procedure, and therefore does not require a full operating-theatre protocol. However, it should be carried out with a local sterile field around the foot and using sterile instruments and dressings. Following local anaesthesia, normally a digital block (see Ch. 20), and a pre-surgical scrub to the toe and forefoot, a tourniquet is applied to enable the procedure to be carried out in a bloodless field. The tourniquet should be broad and flat, such as an Esmarch bandage, to minimise damage to underlying structures. Using an elasticised bandage style of tourniquet will give good exsanguination of the toe, as it is applied from distal to proximal and allows the application of the phenol in a bloodless field. A long length of Esmarch bandage may appear unsightly when applied, and subsequently ‘dangling’, but it becomes virtually impossible to apply a postoperative bandage whilst leaving the tourniquet in place. This is a useful fail-safe procedure.
The Esmarch bandage is applied in the following manner. One end is doubled back for approximately 2 cm and applied to the digit distally, with the doubled end appearing as a tab. The bandage is then wound around the toe with the material stretched so that each layer overlaps and secures the preceding layer. This overlap should be used for all further layers of the bandage down to the base of the toe, ensuring that the material is stretched so that the pressure prevents the blood from entering the toe. On reaching the base of the toe the bandage and its tension is secured and maintained using forceps. Using the tab at the distal end of the toe, the bandage is loosened to uncover the nail and most of the distal phalanx. This end of the bandage is also secured. The distal end of the toe should now be completely exsanguinated. The time for which a tourniquet is in place should be kept to a minimum so that the risk of swelling after its release is minimised. Ideally, this should be less than 30 minutes.
Currently there are commercially produced devices for exsanguinating the toe and providing a tourniquet effect. One of these is Tournicot.
The time of application of the tourniquet, the time of its release and the return of the blood to the digit after the nail procedure must be noted in the patient’s case record.
Total nail avulsion
A narrow spatula or elevator is used to separate the eponychium from the nail plate. Using steady pressure an elevator is inserted below the nail plate and moved, parallel to the long axis of the toe, until there is separation of the nail plate and the nail bed and matrix (Fig. 21.2A). Under the eponychium the instrument is out of sight and the separation is felt as a sudden reduction in resistance. Care must be taken to ensure that the elevator is inserted into each proximal corner of the posterior nail fold by reinserting the instrument until the nail plate is fully separated. The nail plate is removed by locking mosquito forceps or a haemostat onto the plate half way between the sulcus and the midline of the toe, and rolling the instrument dorsally towards the midline (Fig. 21.2B). A similar procedure will release the other side of the plate and the nail can normally be lifted in one piece.
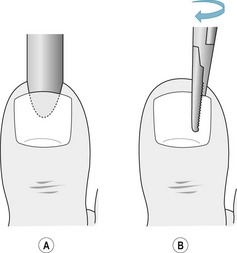
Figure 21.2 (A) The instrument to separate the nail plate from the nail bed is inserted under the nail in a central location. The nail is completely detached from the nail bed, with the elevator moving towards the sides of the nail plate. (B) The forceps are used to grip the detached nail plate and apply a rolling motion towards the midline of the toe.
Partial nail avulsion
A partial nail avulsion is designed to remove the involuted section of nail, which is causing painful symptoms within the sulcus. The remaining nail plate should be flat and must be sufficiently wide to give an acceptable cosmetic appearance. It is entirely possible to carry out partial nail avulsions in both sulci and leave a nail plate with good cosmesis.
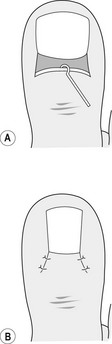
The toe is prepared as for a total nail avulsion and a fine spatula inserted to free the eponychium from the nail plate. There should be as little separation as necessary to limit the tracking of the liquid phenol. A narrow elevator is inserted below the nail plate to separate it from the nail bed, as with a total avulsion, but being careful to separate only the section of nail to be avulsed (Fig. 21.3A). The section of nail plate to be removed is split by introducing a pair of Thwaite’s single-bladed nail nippers (Fig. 21.3B), a nail chisel, or a combination of both. The nippers are moved proximally to split the nail with as few cuts as possible and, preferably, if possible, only one. Alternatively, a nail chisel may be used with gentle controlled force. In either case, the most proximal part of the cut takes place below the eponychium, out of sight of the operator, and care must be taken to avoid damage to the underlying distal phalanx. A pair of mosquito or artery forceps is locked onto the full section of nail, to reduce the possibility of splintering, and rotated dorsally towards the midline of the toe (Fig. 21.3C).
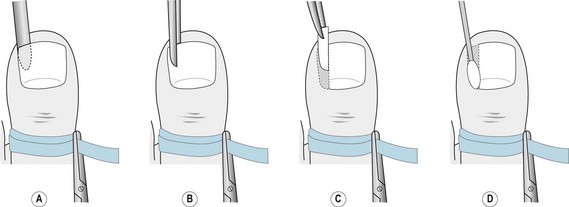
Figure 21.3 (A) Separation of the section of nail plate to be removed using a fine elevator. (B) Cutting the section of nail using Thwaite’s nippers. (C) Removal of the section of nail using fine artery forceps. (D) Application of phenol using a fine cotton wool bud.
The proximal edge of the nail plate must be checked to ensure that all the plate has been removed and to exclude the possibility that fragments have been left in situ. The proximal nail fold and sulci must also be checked for fragments that would interfere with the phenolisation procedure and become a focus for infection.
The destruction of the nail matrix is best achieved using liquefied phenol BP, although a number of alternatives have been used with acceptable results. There has been much debate surrounding the application of the phenol, but it is now generally acknowledged that a rigid adherence to time of contact is not sufficient. The liquefied phenol should be fresh, free from contamination and colourless, and can be used as a saturated solution of 80% BP or 89% USP. It is best to apply the phenol with a cotton wool bud lightly moistened with the liquid and discarded after each application (Fig. 21.3D). Care should be taken to limit the quantity of phenol used, as it is can be absorbed and cause tissue toxicity (Shepherdson 1977). The phenol is worked into the nail matrix, sulcus and bed with pressure. For partial nail avulsions it may be necessary to remove much of the cotton wool from the bud to facilitate its insertion below the eponychium. It is important to avoid phenol tracking onto adjacent tissue and, although with judicious use this is unlikely, the application of tincture of benzoin compound or petroleum jelly to the surrounding tissue may prevent unnecessary skin contact. As a general guide, the phenol should be applied for three separate 1-minute applications. However it is normally the case that older tissue will respond more quickly than younger, moister tissue and that chronic toenail dystrophies that exhibit increased fibrous tissue will require longer application. There is a change in the colour of the nail bed and matrix tissues from pinkish to a dirty white, and in texture from firm to softer during phenolisation, and the observation of these changes should be used as the main determinant of the total time of application. The site is flushed with alcohol, taking care to avoid contaminating the surrounding area, to wash out the phenol, although it should be noted that this does not neutralise or terminate the action of the acid. The area is dried and the tourniquet removed, with the return of arterial blood flow being noted when the colour returns to the digit.
Sodium hydroxide has been used with success for many years. The nail plate is removed in a similar manner as in the phenolisation technique, with a pellet of 10% sodium hydroxide being rubbed into the nail bed and matrix until the capillaries are seen to coagulate. Travers and Ammon (1980) observed this to take from 3 seconds to 3 minutes, depending on the patient. Kocyigit et al (2005) experimented with application times of 30, 60 and 120 seconds. Their success rates of 71%, 93% and 94%, respectively, indicate that a single application of 30 seconds is insufficient, but they noted a prolonged healing time with the 2-minute applications as a disadvantage. Those who advocate the use of sodium hydroxide report it to have a higher success rate, lower recurrence rate, less drainage and faster healing time when compared with phenolisation. However, Cumming et al (2005)compared phenol and sodium hydroxide matricectomies and found no significant differences between the groups in terms of healing time, postoperative pain, regrowth or satisfaction rates. Travers and Ammon (1980) analysed the results of 1000 sodium hydroxide procedures carried out over a 6-year period. They reported 15 (1.5%) cases of regrowth, of which 12 required revision.
Several authors (Abbott & Geho 1980, Gardner 1958, Polokoff 1935, Zuber 2002) have reported the successful use of negative galvanism, with little postoperative infection or pain. The direct current when applied to the nail bed and matrix causes sodium ions to migrate towards the negative pole and chlorine ions towards the positive pole. When the sodium ions reach the negative pole they break down to form sodium hydroxide and hydrogen gas, producing an alkali chemical burn and cauterisation of the germinal tissue. The effect at the positive pole (anode) is negated by using a flat dispersive plate with a large surface area (in excess of 150 cm2). The negative pole (cathode), in contrast, concentrates its current by using an electrode of 2-5 mm. Despite its reported success, negative galvanism is not widely used as experience is required to adopt an adequate current and length of application. In addition, there is the potential electrical hazard to both the patient and the operator.
Yang and Li (2002) attempted to assess the effectiveness of a carbon dioxide laser for matricectomy. In a small-scale study of 18 partial matricectomies (14 patients) they found that use of the SharPulse CO2 laser had a high cure rate, short postoperative pain duration and low risk of postoperative infection. Orenstein et al (2007) treated 40 patients and achieved 94% success when measuring the recurrence rate. Laser, however, remains an expensive option in the management of ingrown toenails.
Radiosurgery (radiowave surgery, high-frequency electrosurgery) is a relatively new modality in the surgical management of skin lesions, including ingrowing toenails. By using high-frequency radiowaves at 4 MHz, and using the tissues rather than the electrode to provide resistance, the electrode tip remains cool and causes less damage to surrounding tissues than traditional electrocautery. The resulting wound heals faster and with less postoperative pain (Sperli 1998).
Postoperative management
It is essential that the patient is given clear verbal and written advice regarding the signs (and symptoms) of immediate postoperative problems. The written advice should contain a contact telephone number so that the patient can speak to a podiatrist in case of emergencies (see Ch. 27).
Patients must be advised to expect some postoperative bleeding, and indeed this is a good indication that the wound is freely draining. Elevation of the limb(s) should be encouraged for the remainder of the day to minimise any bleeding and local swelling.
There is normally little postoperative pain if phenol is used because of its neurolytic action on local nerve endings, and discomfort can be controlled by the patient’s preferred choice of analgesics obtained over the counter in any pharmacy.
The first redressing should be carried out at 3–5 days. Earlier redressing does not allow the wound to epithelialise and later can cause the postoperative dressing to harden and irritate the wound.
Strict antiseptic precautions, a ‘no-touch’ technique and sterile dressings must be employed. There should be evidence of early granulation and a serous exudate, which occurs as a result of the chemical-burn effect of the phenol. The discharge will be noted for 2–6 weeks. Nails that were locally infected before the procedure drain for a slightly longer period postoperatively. Prolonged drainage may be caused by individual hypersensitivity to phenol, the use of too much phenol, matrix or nail spicule left in situ, the formation of an epidermoid inclusion cyst or indolent infection (Yale 1987).
Opinions vary as to whether the toe should be kept dry or bathed in saline. In either case, however, ointments or pastes, which impede wound drainage, are contraindicated. Dovison and Keenan (2001) found there to be no clinical difference between dressings of povidone iodine and amorphous hydrogel in terms of rate of healing or infection. However, other complications such as hypergranulation were more likely in the amorphous hydrogel group.
Patients can be taught to redress their toe by taking time at the first redressing to explain each step of the procedure and by giving written instructions, which must include a contact telephone number for help or advice.
Redressing by the patient should be carried out every 5–7 days whenever 30 minutes of uninterrupted time is available. Using a basin, lined with a polythene bag or bin liner to reduce the chances of cross-infection, enough tepid water to cover the foot is added. A tablespoon of household salt (sodium chloride) is dissolved and the foot immersed. After 10 minutes the foot is removed and dried, but care is taken not to dry the toe, which should be left to dry in the air before covering with a clean gauze dressing using a minimal-touch technique. Redressing should continue until there is no staining on the dressing when it is removed.
In a 1998 audit of post-phenolisation wound care, Dunlop suggested that patients should be seen 1, 2 and 4 weeks postoperatively. In the study the vast majority of patients required either shorter or longer periods between their appointments, demonstrating the flexibility that is required and the individuality of patient needs.
Several studies (Felton & Weaver 1999, Giacalone 1997) have sought to add evidence to the debate surrounding the use of phenol in patients with diabetes. The theory would suggest that such patients cannot tolerate the chemical burn and would have difficulty healing, with increased infection and postoperative complications. However, no significant difference has been noted in healing time or postoperative infection rate in studies to date. Felton and Weaver (1999), however, noted a 10.3% infection rate in their diabetic group (12.2% in non-diabetic group) and the potential for serious complications must be considered in preoperative planning.
SURGICAL PROCEDURES
Phenol and alcohol ablation of the nail matrix is noted for its considerable advantages. However, the potential for an increased rate of infection and delayed wound healing must be borne in mind. Thus, there remains a place for incisional matricectomy in patients whose history or circumstances do not favour phenol ablation.
Winograd procedure
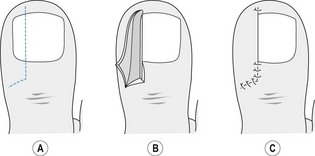
The Winograd procedure, originally described in 1929, allows the excision of the medial or lateral nail sulcus with its adjacent nail plate, bed and proximal nail root matrix. A linear incision, from the free edge of the nail plate to about 5 mm beyond the eponychium, is deepened to bone (Fig. 21.4A). A narrow elevator is inserted distally to proximally to free and remove the border of the nail plate. A second elliptical incision joins either end of the first incision, creating a wedge of tissue that includes any hypertrophied soft tissue, nail bed and nail matrix (Fig. 21.4B). Following removal of the wedge, non-absorbable sutures or skin closures are used to approximate the wound edges (Fig. 21.4C).
Zadik’s procedure
In the belief that excision of the nail bed was not necessary to prevent regrowth of the nail, Zadik described a procedure in 1950, which involved excision of the nail matrix only. Using an elevator the nail plate is removed and a full-thickness flap created by extending oblique incisions from both corners of the proximal nail fold. This flap allows good access to the matrix, which is carefully excised from the bone. Regrowth of spicules of nail is a common consequence of inadequate dissection, and the periosteum should be removed to ensure complete removal of nail matrix cells. For closure, the eponychial flap is replaced and the lateral incisions sutured (Fig. 21.5).
Frost procedure
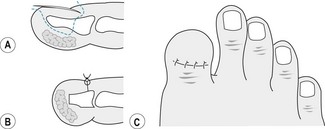
In a development of Winograd’s procedure, Frost (1950) used the initial incision of the original operation to which he added an incision posteriorly to give an L-shaped tissue flap for better exposure of the nail matrix (Fig. 21.6A,B). With deep dissection the nail plate, bed and matrix are excised. Sutures or skin closures may be used for closure. Tissue necrosis of the flap has proved to be the most common complication, and a number of modifications have been developed in an effort to reduce damage to the blood supply of the flap by altering the right angle of the L to give a curved or diagonal incision (Fig. 21.6C).
Terminal Syme’s amputation
In 1951 Thompson and Terwilliger described an amputation of the distal half of the distal phalanx that significantly reduced the rate of recurrence following toenail surgery. Using an elliptical incision around the entire nail plate and matrix, which is deepened to bone, the nail folds are excised. The distal phalanx is cut distal to the insertions of the long tendons and released from the soft tissue pulp of the toe in a similar fashion to that used when the calcaneum is dissected in a Syme’s ankle amputation (hence the name ‘terminal Syme’s amputation’). The soft tissues are sutured to give good pulp cover of the remaining bone. There remains a potential for recurrence, and complications include growth of nail spicules and inclusion cysts at the suture line. Some women regard the resultant shortening of the hallux as cosmetically unsatisfactory, and women particularly should be counselled thoroughly preoperatively (Fig. 21.7).
AVULSION USING UREA
Not all patients are suitable for surgical treatment of symptomatic nail conditions, and the use of urea has advantages, particularly in cases of uncontrolled diabetes, vascular disease or in the immunosuppressed. The urea softens the nail plate while also dissolving the bond between the nail bed and the nail plate. Its use, however, is time consuming and requires good patient compliance in the application of the urea and in maintaining a dry dressing. With the surrounding skin protected, 40% urea is applied to the nail plate and occluded with adhesive tape and/or a finger cut from a surgical glove. The patient is instructed to change the dressing and reapply the urea once or twice a week, with the necrotic nail being debrided at regular visits until symptomatic relief is obtained (Faber & Smith 1978, Port & Sanicola 1980, South & Faber 1980).
TREATMENT OF SUBUNGUAL EXOSTOSIS
A subungual exostosis is a small outgrowth of bone under the nail plate or near its free edge. It generally occurs singly and unilaterally and will most frequently affect the hallux. While the aetiology is not fully understood, it is associated with either a single traumatic event or, more likely, multiple minor trauma. The lesion is slow growing, rarely exceeding 5 mm in diameter, and becomes progressively more painful as it increases in size. It is more commonly seen in patients aged 20–40 years, with a female/male ratio of 2:1 (Dochery 1987, Evison 1966).
In the later stages tenderness is experienced when even mild pressure is exerted over the nail plate. The epidermis covering the exostosis, which becomes stretched and thinned and takes on a bright red colour, will blanch with the application of pressure and will present a hard resistance upon palpation. These observations are useful in the differential diagnosis with subungual heloma durum and other soft-tissue lesions, including glomus tumour, pyogenic granuloma, subungual verruca and inclusion cyst. Accurate diagnosis of subungual exostosis is by means of a lateral-projection radiograph with the involved digit isolated (Fig. 21.8).
Temporary relief may be obtained with the use of protective padding or avulsion of the nail plate. More permanent relief is afforded by surgical excision, either using a minimal incision distally (fish mouth) or by an incision at the hyponychium and raising a proximally based flap. In either case, the lesion is removed and its base curetted.
In addition to regrowth of the exostosis, subsequent nail deformity must be considered as a possible postoperative complication.
Abbott WW, Geho H. Partial matricectomy via negative galvanic current. Journal of the American Podiatry Association. 1980;70:239-243.
Bartlett R. A conservative operation for the cure of so-called ingrown toenail. JAMA. 1937;108:1257.
Boberg JS, Frederiksen MS, Haron FM. Scientific analysis of phenol nail surgery. Journal of the American Podiatric Medical Association. 2002;92:575-579.
Bos AM, van Tilburg MW, van Sorge AA, Klinkenbijl JH. Randomized clinical trial of surgical technique and local antibiotics for ingrowing toenail. British Journal of Surgery. 2007;94:292-296.
Bose B. A technique for excision of nail fold for ingrowing toenail. Surgery, Gynecology and Obstetrics. 1971;132:511.
Bostanci S, Ekmekci P, Gurgey E. Chemical matricectomy with phenol for the treatment of ingrowing toenail: a review of the literature and follow-up of 172 treated patients. Acta Dermatologica Venereologica. 2001;81:181-183.
Cumming S, Stewart S, Harborne D, Smith J, Broom H, Abbott A, Barton A. A randomised controlled trial of phenol and sodium hydroxide in nail surgery. British Journal of Podiatry. 2005;8(4):123-127.
Dagnall JC. The history, development and current status of nail matrix phenolisation. Chiropodist. 1981;36:315-324.
Dixon GL. Treatment of ingrown toenail. Foot and Ankle. 1983;3:254-260.
Dochery GL. Nails: fundamental conditions and procedures. In: McGlamry ED, editor. Comprehensive textbook of foot surgery. Baltimore, OH: Williams & Wilkins; 1987:5-10.
Dovison R, Keenan A. Wound healing and infection in nail matrix phenolisation wounds. Journal of the American Podiatric Medical Association. 2001;91:230-233.
Drago JL, Jacobs AM, Oloff L. A comparative study of postoperative care with phenol nail procedure. Journal of Foot Surgery. 1983;22:332-334.
Dunlop GM. Clinical audit of a patient teaching programme in the care of wounds following toenail removal. The Foot. 1998;8:85-88.
Espensen EH, Nixon BP, Armstrong DG. Chemical matricectomy for ingrown toenails. Journal of the American Podiatric Medical Association. 2002;92:287-295.
Evison G, Price CHG. Subungual exostosis. British Journal of Radiology. 1966;39:451.
Faber EM, Smith DA. Urea ointment in the nonsurgical avulsion of nail dystrophies. Cutis. 1978;22:689.
Felton PM, Weaver TD. Phenol and alcohol chemical matricectomy in diabetic versus nondiabetic patients. Journal of the American Podiatric Medical Association. 1999;89:410-412.
Fowler AW. Excision of the germinal matrix: a unified treatment for embedded toenail and onychogryphosis. British Journal of Surgery. 1958;45:382.
Frost L. Root resection for incurvated nail. Journal of the National Chiropractic Association. 1950;40:19.
Gabriel SS, Dallas V, Stephenson DL. The ingrowing toenail; a modified segmental matrix excision operation. British Journal of Surgery. 1979;66(4):285-286.
Gardner P. Negative galvanic current in the surgical correction of onychocryptotic Nails. Journal of the American Podiatry Association. 1958;48:555-560.
Giacalone VF. Phenol matricectomy in patients with diabetes. Journal of Foot and Ankle Surgery. 1997;36:264.
Greene AA. A modification of the phenol–alcohol technique for toenail correction. Current Podiatry. 1964;13:20-23.
Islam S, Lin EM, Drongowski R, et al. The effect of phenol on ingrown toenail excision in children. Journal of Pediatric Surgery. 2005;40:290-292.
Kocyigit P, Bostanci S, Ozdemir E, Gurgey E. Sodium hydroxide matricectomy for the treatment of ingrown toenails: comparison of three different application periods. Dermatologic Surgery. 2005;31:744-747.
Krautz CE. Nail survey (1942–1970). British Journal of Chiropody. 1970;35:117.
Laxton C. Clinical audit of forefoot surgery performed by registered medical practitioners and podiatrists. Journal of Public Health and Medicine. 1995;17:311-317.
McGlamry ED. Comprehensive textbook of foot surgery. Williams & Wilkins. Baltimore, OH. 1987
Morkane AJ, Robertson RW, Inglis GS. Segmental phenolisation of ingrowing toenails: a randomised controlled study. British Journal of Surgery. 1984;71:526-527.
Murray WR. Onychcryptosis: principles of non-operative and operative care. Clinical Orthopaedics and Related Research. 1979;142:96.
Nyman SP. The phenol–alcohol technique for toenail excision. Journal of the New Jersey Chiropodists Society. 1956;5:4.
Orenstein A, Goldan O, Weissman O, et al. A comparison between CO2 laser surgery with and without lateral fold vaporization for ingrowing toenails. Journal of Cosmetic Laser Therapy. 2007;9:97-100.
Palmer BV, Jones A. Ingrowing toenails: the results of treatment. British Journal of Surgery. 1979;66:575-576.
Polokoff M. Negative galvanic current used to destroy nail matrix. Cited in McGlamry (1987). 1935.
Port M, Sanicola KF. Non surgical removal of dystrophic nails utilising urea ointment occlusion. Journal of the American Podiatry Association. 1980;70:521-523.
Rinaldi R, Sabia M, Gros J. The treatment and prevention of infection in phenol–alcohol matricectomies. Journal of the American Podiatry Association. 1982;72:453.
Shepherdson A. Nail matrix phenolisation, a preferred method to surgical excision. Practitioner. 1977;219:725-728.
Shereff MJ. Disorders of toenails. In: Gould JS, editor. Operative foot surgery. Philadelphia, PA: WB Saunders, 1994.
South DA, Faber EM. Urea ointment in the nonsurgical avulsion of nail dystrophies. A reappraisal. Cutis. 1980;25:609-612.
Sperli AE. The use of radiosurgery in plastic surgery and dermatology. Surgery Technology International. 1998;7:437-442.
Suppan RJ, Ritchlin JD. A non-disabilitating surgical procedure for ingrown nail. Journal of the American Podiatry Association. 1962;52:90.
Sykes PA. Ingrowing toenails: time for critical appraisal? Journal of the Royal College of Surgeons Edinburgh. 1986;31:300-304.
Thompson TC, Terwilliger C. The terminal Syme’s operation for ingrown toenail. Surgical Clinics of North America. 1951;31:575-584.
Travers GR, Ammon RG. The sodium hydroxide chemical matricectomy procedure. Journal of the American Podiatry Association. 1980;70:476.
Winograd AM. A modification in the technique of operation for ingrown toenail. JAMA. 1929:229-230.
Yang KC, Li YT. Treatment of recurrent ingrown great toenail associated with granulation tissue by partial nail avulsion followed by matricectomy with SharPulse carbon dioxide laser. Dermatologic Surgery. 2002;28:419-421.
Yale JF. Phenol–alcohol technique for correction of infected ingrown nail. Journal of the American Podiatry Association. 1974;64:46-53.
Yale JF. Yale’s podiatric medicine, 3rd edn. Baltimore, OH: Williams and Wilkins; 1987.
Zadik FR. Obliteration of the nail bed of the great toe without shortening of the terminal phalanx. Journal of Bone and Joint Surgery (British Edition). 1950;32:66-67.
Zuber TJ. Ingrown toenail removal. American Family Physician. 2002;65:2547-2558.