THORAX
1. INTRODUCTION
7. EMBRYOLOGY
1. INTRODUCTION
The thorax lies between the neck and abdomen, encasing the great vessels, heart, and lungs, and provides a conduit for structures passing between the head and neck superiorly and the abdomen, pelvis, and lower limbs inferiorly. Functionally, the thorax and its encased visceral structures are involved in the following:
- Protection: the thoracic cage and its muscles protect the vital structures in the thorax
- Conduit: the thorax provides for a superior thoracic aperture and an inferior thoracic aperture, and a central mediastinum
- Segmentation: the thorax provides an excellent example of segmentation, a hallmark of the vertebrate body plan
- Breathing: movements of the diaphragm and intercostal muscles are essential for expanding the thoracic cavity to facilitate the entry of air into the lungs in the process of breathing
- Pumping blood: the thorax contains the heart, which pumps blood through the pulmonary and systemic circulations
The sternum, ribs (12 pairs), and thoracic vertebrae (12) encircle the thoracic contents and provide a stable thoracic cage that both protects the visceral structures of the thorax and offers assistance with breathing. Because of the lower extent of the rib cage, the thorax also offers protection for some of the abdominal viscera, including the liver and gallbladder on the right side, the stomach and spleen on the left side, and the adrenal (suprarenal) glands and upper poles of the kidneys on both sides.
The superior thoracic aperture (the anatomical thoracic inlet) conveys large vessels, important nerves, the thoracic lymphatic duct, the trachea, and the esophagus between the neck and thorax. The inferior thoracic aperture (the anatomical thoracic outlet) conveys the inferior vena cava (IVC), aorta, esophagus, nerves, and thoracic lymphatic duct between the thorax and the abdominal cavity. Additionally, the thorax contains two pleural cavities laterally and a central “middle septum” called the mediastinum, which is divided as follows (Fig. 3-1):
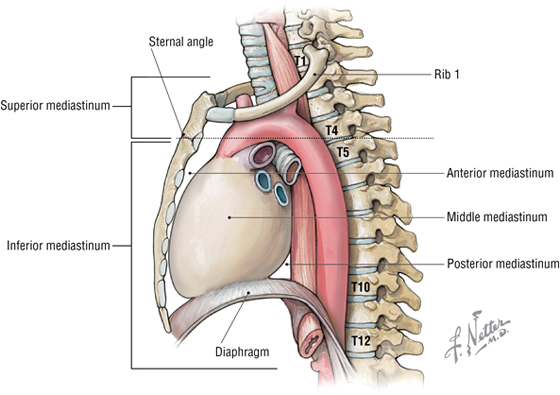
FIGURE 3-1 Subdivisions of the Mediastinum
- Superior mediastinum: a midline compartment that lies above an imaginary horizontal plane that passes through the manubrium of the sternum (“sternal angle of Louis”) and the intervertebral disc between the T4 and T5 vertebra
- Inferior mediastinum: the midline compartment below this same horizontal plane, which is further subdivided into an anterior, middle (contains the heart), and posterior mediastinum
2. SURFACE ANATOMY
Key Landmarks
Key surface landmarks include the following (Fig. 3-2):
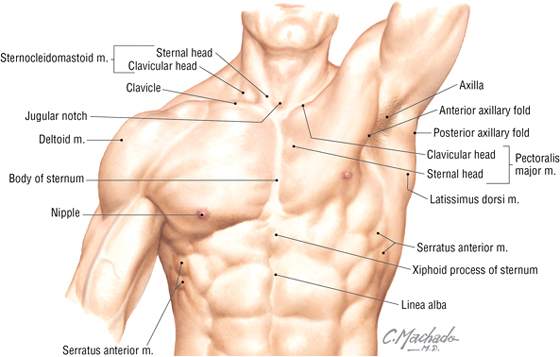
FIGURE 3-2 Surface Anatomy Landmarks
- Jugular (suprasternal) notch: a notch marking the level of the second thoracic vertebra, the top of the manubrium, and the midpoint between the articulation of the two clavicles
- Sternal angle (of Louis): marks the articulation between the manubrium and body of the sternum, the dividing line between the superior and inferior mediastinum, and the site of articulation of the second ribs (useful for counting ribs and intercostal spaces)
- Nipple: marks the T4 dermatome and approximate level of the dome of the diaphragm on the right side
- Xiphoid process: marks the inferior extent of the sternum and the anterior attachment point of the diaphragm
Reference Planes
In addition to the sternal angle of Louis, physicians often use other imaginary planes of reference to assist in locating underlying visceral structures of clinical importance. Important vertical planes of reference include the following (Fig. 3-3):
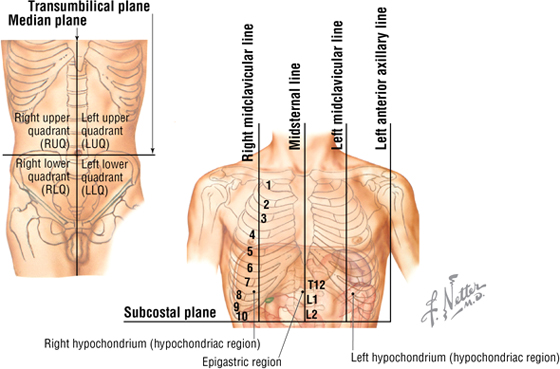
FIGURE 3-3 Planes of Reference
- Midclavicular line
- Anterior axillary line
- Midaxillary line
- Posterior axillary line
- Scapular line
- Midvertebral line
3. THE THORACIC WALL
The Thoracic Cage
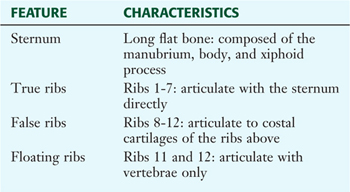
The thoracic cage, which is part of the axial skeleton, includes the midline sternum and 12 pairs of ribs, each with a head, neck, tubercle, and body (ribs 11 and 12, the floating ribs, are short and do not have a neck or tubercle) (Fig. 3-4). This bony framework provides the scaffolding for attachment of the chest wall muscles and the pectoral girdle, which includes the clavicle and scapula and forms the attachment of the upper limb to the thoracic cage at the shoulder joint (Table 3-1).
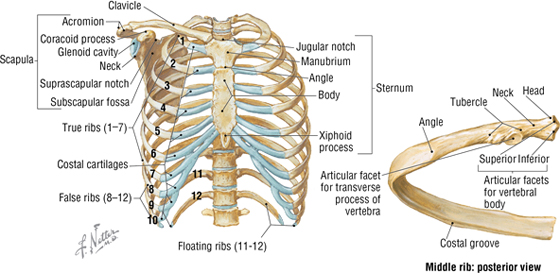
FIGURE 3-4 Thoracic Cage
|
TABLE 3-1 Features of the Thoracic Cage
|
 |
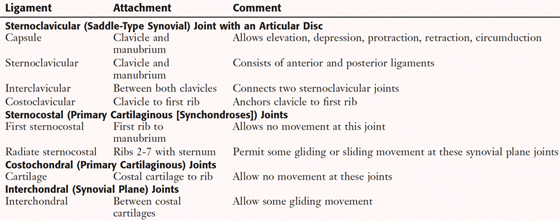
Joints of the Thoracic Cage
Joints of the thoracic cage include articulations between the ribs and the sternum and thoracic vertebrae, and the sternum and the clavicle (Fig. 3-5 and Table 3-2).
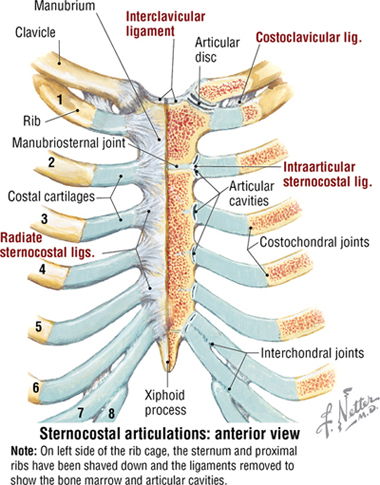
FIGURE 3-5 Joints of the Thoracic Cage
|
TABLE 3-2 Joints of the Thoracic Cage
|
 |
Muscles of the Anterior Thoracic Wall
The muscles of the anterior thoracic wall include several muscles that attach to the thoracic cage but actually are muscles that act on the upper limb (Fig. 3-6). These muscles are as follows (for a review, see Chapter 7, Upper Limb):
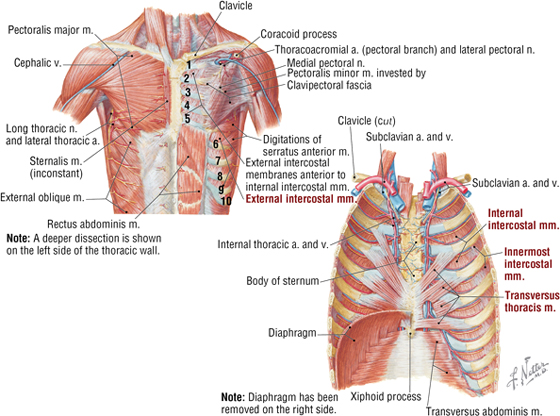
FIGURE 3-6 Muscles of the Anterior Thoracic Wall
- Pectoralis major
- Pectoralis minor
- Serratus anterior
The true anterior thoracic wall muscles fill the intercostal spaces or support the ribs, act on the ribs (elevate or depress the ribs), and keep the intercostal spaces rigid, thereby preventing them from bulging out during expiration and being drawn in during inspiration (see Fig. 3-6 and Table 3-3).
|
TABLE 3-3 Muscles of the Anterior Thoracic Wall
|
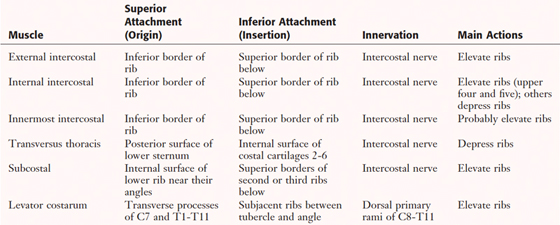 |
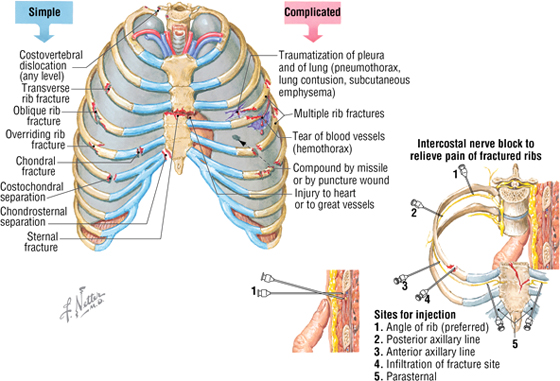
C L I N I C A L F O C U S
Thoracic Cage Injuries
Thoracic cage injuries usually result from trauma and often involve rib fractures (ribs 1 and 2 and 11 and 12 are more protected and often escape being fractured), crush injuries with rib fractures, and penetrating chest wounds (such as gunshot or stab wounds). The pain caused by rib fractures can be intense because of the expansion and contraction of the rib cage during respiration, sometimes requiring palliation by anesthetizing the intercostal nerve (nerve block).
Intercostal Vessels and Nerves
The intercostal neurovascular bundles (vein, artery, and nerve) lie inferior to each rib, running in the costal groove deep to the internal intercostal muscles (Fig. 3-7 and Table 3-4). The veins largely correspond to the arteries and drain into the azygos system of veins or the internal thoracic veins (see Fig. 3-7).
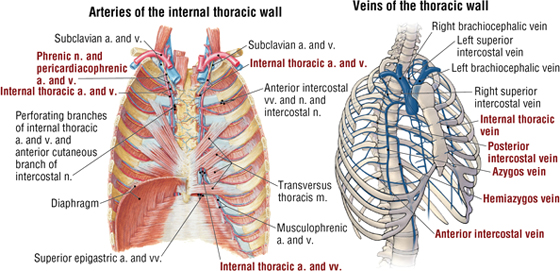
FIGURE 3-7 Intercostal Vessels and Nerves (Contd.)
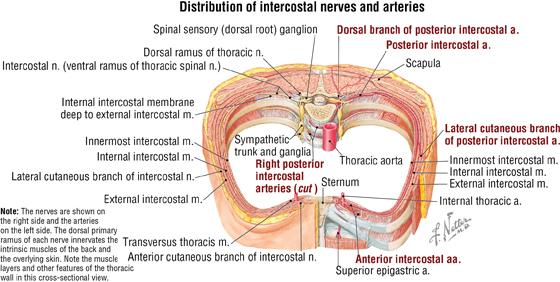
FIGURE 3-7 (Contd.) Intercostal Vessels and Nerves
|
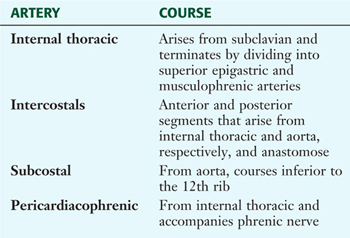
TABLE 3-4 Arteries of the Internal Thoracic Wall
|
 |
The intercostal nerves are the primary ventral rami of the first 11 thoracic spinal nerves. The 12th thoracic nerve gives rise to the subcostal nerve, which courses inferior to the 12th rib. The nerves give rise to lateral and anterior cutaneous branches and branches innervating the intercostal muscles (see Fig. 3-7).
The Female Breast
The female breast extends from approximately the second to the sixth ribs and from the sternum medially to the midaxillary line laterally. Mammary tissue is composed of compound tubuloacinar glands organized into about 15 to 20 lobes, which are supported and separated from each other by fibrous connective tissue septae (the suspensory ligaments of Cooper) and fat. Each lobe is divided in lobules of secretory acini and their ducts. Features of the breast include the following (Fig. 3-8):
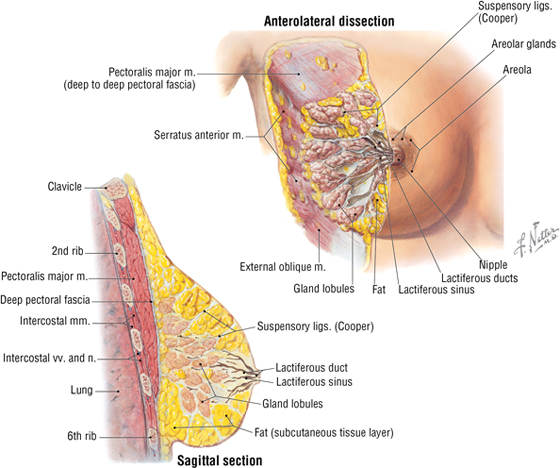
FIGURE 3-8 Anterolateral and Sagittal Views of the Female Breast
- Breast: fatty tissue containing glands that produce milk; lies in the superficial fascia above the retromammary space, which lies above the deep pectoral fascia enveloping the pectoralis major muscle
- Areola: circular pigmented skin surrounding the nipple; it contains modified sebaceous and sweat glands that lubricate the nipple and keep it supple
- Nipple: site of opening for the lactiferous ducts; usually lies at about the level of the fourth intercostal space
- Axillary tail (of Spence): extension of mammary tissue superolaterally toward the axilla
- Lymphatic system: lymph is drained from breast tissues; about 75% of lymphatic drainage is to the axillary lymph nodes (Figs. 3-9 and 7-11), and the remainder drains to infraclavicular, pectoral, or parasternal nodes.
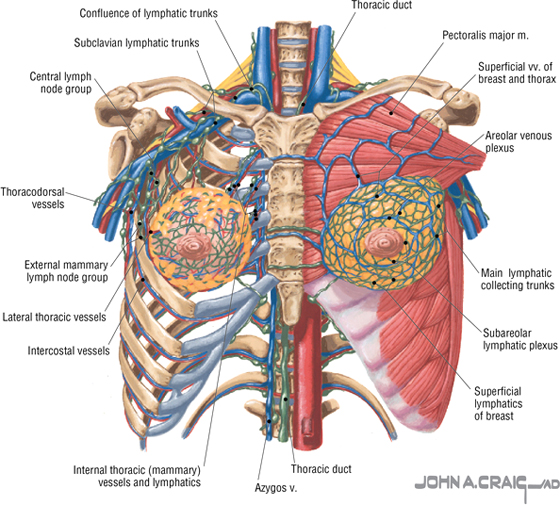
FIGURE 3-9 Veins and Lymphatics of the Female Breast
The arterial supply to the breast includes the following:
- Anterior intercostal branches of the internal thoracic (mammary) arteries (from the subclavian artery)
- Lateral thoracic artery (branch of the axillary artery)
- Thoracodorsal artery (branch of the axillary artery)
The venous drainage (Fig. 3-9) largely parallels the arterial supply, finally draining into the internal thoracic, axillary, and adjacent intercostal veins.
C L I N I C A L F O C U S
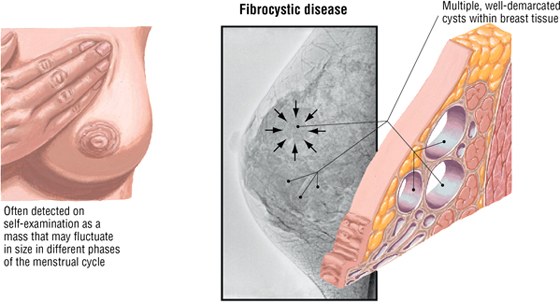
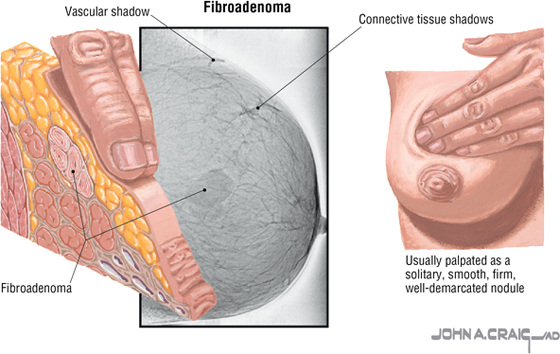
Fibrocystic Breast Disease
Fibrocystic change (disease) is a general term covering a large group of benign conditions occurring in about 80% of women that are often related to cyclic changes in maturation and involution of glandular tissue. Fibroadenoma, the second most common tumor of the breast after carcinoma, is a benign neoplasm of glandular epithelium and is usually accompanied by a significant increase in connective tissue stroma. Both conditions present as palpable masses and warrant follow-up evaluation.
C L I N I C A L F O C U S
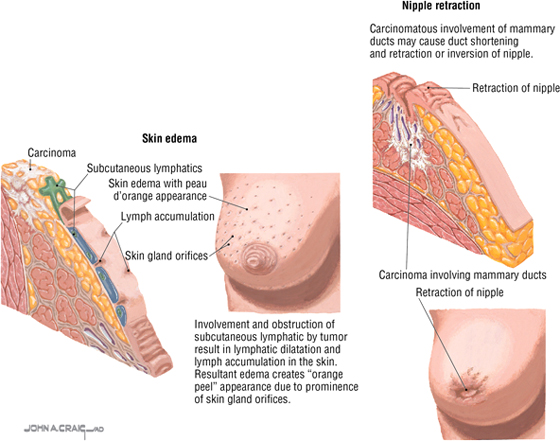
Breast Cancer
Breast cancer is the most common malignancy in women; approximately two thirds of all cases occur in postmenopausal women. Invasive carcinoma may involve the suspensory ligaments, causing retraction of the ligaments and dimpling of the overlying skin. Additionally, invasion and obstruction of the subcutaneous lymphatics can result in dilatation and skin edema, creating an “orange peel” appearance (peau d'orange). About 50% of cancers develop in the upper outer quadrant (quadrant closest to the axilla, which includes the axillary tail). Distant sites of metastasis include the following:
- Lungs and pleura
- Liver
- Bones
- Brain
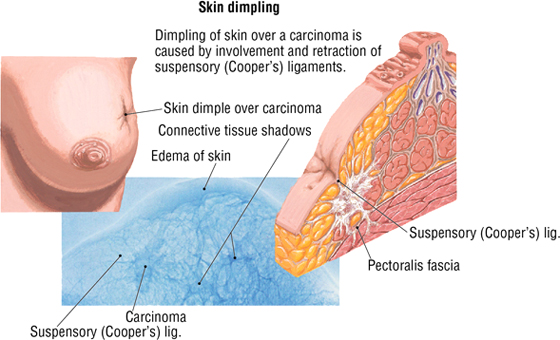
4. THE PLEURA AND LUNGS
The Pleural Spaces (Cavities)
The thorax is divided into the following three compartments:
- Right pleural space
- Left pleural space
- Mediastinum (a “middle septum” lying between the pleural spaces)
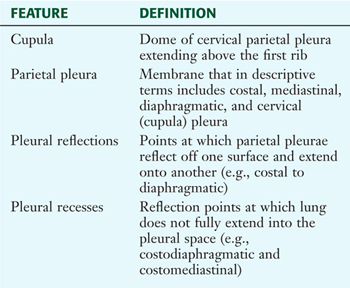
The lungs lie within the pleural cavity (right and left) (Fig. 3-10), which is a “potential space” between the investing visceral pleura (which closely envelops each lung) and the parietal pleura (which reflects off each lung and lines the inner aspect of the thoracic wall) (Table 3-5). Normally, the pleural cavity contains a small amount of serous fluid, which lubricates the surfaces and reduces friction during respiration. The parietal pleura is richly innervated with pain fibers that course in the somatic intercostal nerves; the visceral pleura has few, if any, pain fibers.
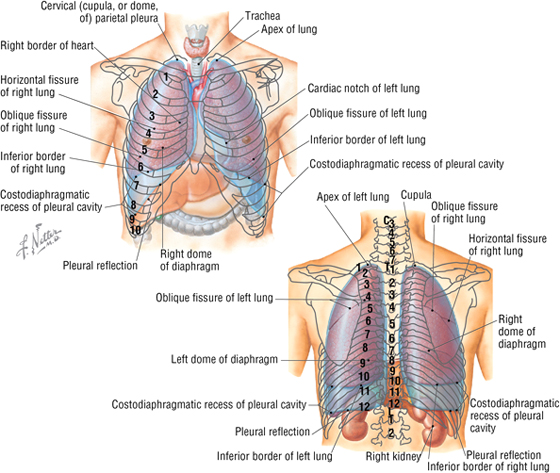
FIGURE 3-10 Anterior and Posterior Topography of the Pleura and Lungs
|
TABLE 3-5 Pleural Features and Recesses
|
 |
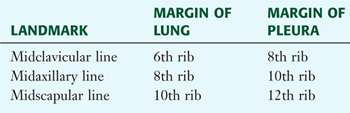
Clinically, it is important for physicians to be able to “visualize” the extent of the lungs and pleural cavities topographically on the surface of their patients (see Fig. 3-10). The lungs lie adjacent to the parietal pleura inferiorly to the sixth costal cartilage. (Note the presence of the cardiac notch on the left side.) Beyond this point, the lungs do not occupy the full extent of the pleural cavity during quiet respiration. These points are important to know if one needs access to the pleural cavity without injuring the lungs (Table 3-6), such as to drain inflammatory exudate (pleural effusion), hemorrhage into the cavity (hemothorax), or air (pneumothorax). In quiet respiration, the lung margins reside two ribs above the extent of the pleural cavity at the midclavicular, midaxillary, and midscapular lines (see Table 3-6 and Fig. 3-10).
|
TABLE 3-6 Surface Landmarks of the Pleura and Lungs
|
 |
The Lungs
The paired lungs are invested in the visceral pleura and are attached to mediastinal structures (trachea and heart) at their hilum. Each lung possesses the following surfaces:
- Apex: superior part of the upper lobe that extends into the root of the neck (above the clavicles)
- Hilum: area located on the medial aspect through which structures enter and leave the lung
- Costal: anterior, lateral, and posterior aspects of the lung in contact with the costal elements of the internal thoracic cage
- Diaphragmatic: inferior part of the lung in contact with the underlying diaphragm
The right lung has three lobes and is slightly larger than the left lung, which has two lobes. Both lungs are composed of spongy and elastic tissue, which readily expands and contracts to conform to the internal contours of the thoracic cage (Fig. 3-11 and Table 3-7).
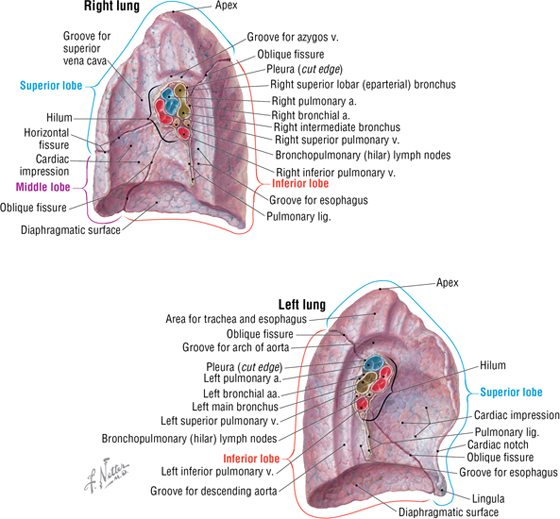
FIGURE 3-11 Features of the Medial Aspect of the Lungs
|
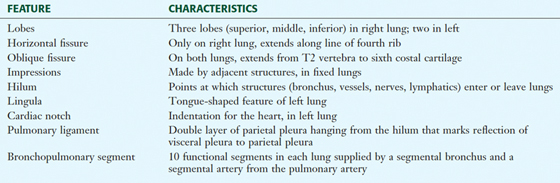
TABLE 3-7 External Features of the Lungs
|
 |
C L I N I C A L F O C U S
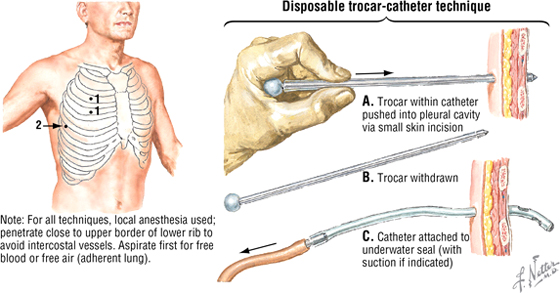
Chest Drainage Tubes
A chest tube provides a way to evacuate air or fluids (blood, pus) from the pleural cavity, thus reapposing the parietal and visceral pleura and enhancing the patient's ability to breathe normally. Following administration of a local anesthetic, the tube is inserted close to the upper border of a rib to avoid the neurovascular bundle, which runs in the costal groove at the inferior margin of each rib.
Preferred sites
1. For pneumothorax (2nd or 3rd interspace at midclavicular line)
2. For hemothorax (5th interspace at midaxillary line)
The lungs are supplied by several small bronchial arteries that arise from the proximal portion of the descending thoracic aorta. Although much of this blood returns to the heart via the pulmonary veins, some also collects into small bronchial veins that drain into the azygos system of veins.
The lymphatic drainage of both lungs is to pulmonary (intrapulmonary) and bronchopulmonary (hilar) nodes, which then drain into tracheobronchial nodes (Fig. 3-12).
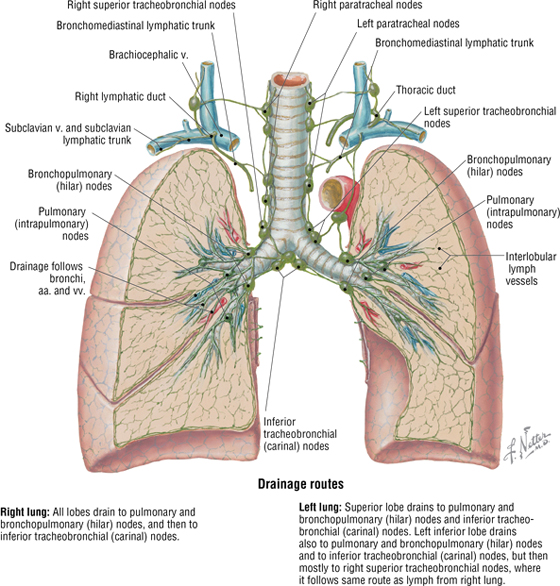
FIGURE 3-12 Lymphatic Drainage Routes of the Lungs
As visceral structures, the lungs are innervated by the autonomic nervous system. Sympathetic bronchodilator fibers, which relax smooth muscle, arise from upper thoracic spinal cord segments. Parasympathetic bronchoconstrictor fibers, which contract smooth muscle and increase mucus secretion, arise from the vagus nerve.
C L I N I C A L F O C U S
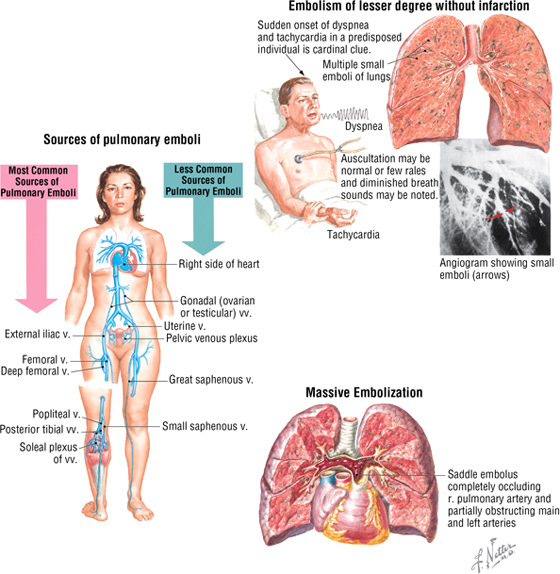
Pulmonary Embolism
The lungs naturally filter venous clots larger than circulating blood cells and can usually accommodate small clots because of their fibrinolytic (“clot-buster”) mechanisms. However, pulmonary embolism (PE) is the cause of death in 10% to 15% of hospitalized patients. Thromboemboli originate from deep leg veins in approximately 95% of cases. Major causes are called Virchow's triad and include the following:
- Venous stasis (e.g., caused by extended bed rest)
- Trauma (e.g., fractures or tissue injury)
- Coagulation disorders (inherited or acquired)
Other contributors to PE include postoperative and postpartum immobility, and some hormone medications that increase the risk of blood clots. About 60% to 80% of PEs are “silent” because they are small; larger emboli may obstruct mediumsized vessels and lead to infarction or even obstruction of a vessel as large as the pulmonary trunk (saddle embolus). PE without infarction is common and presents as tachypnea, anxiety, dyspnea, and vague substernal pressure. Saddle embolus, on the other hand, is an emergency that can precipitate acute cor pulmonale (right-sided heart failure) and circulatory collapse.
C L I N I C A L F O C U S
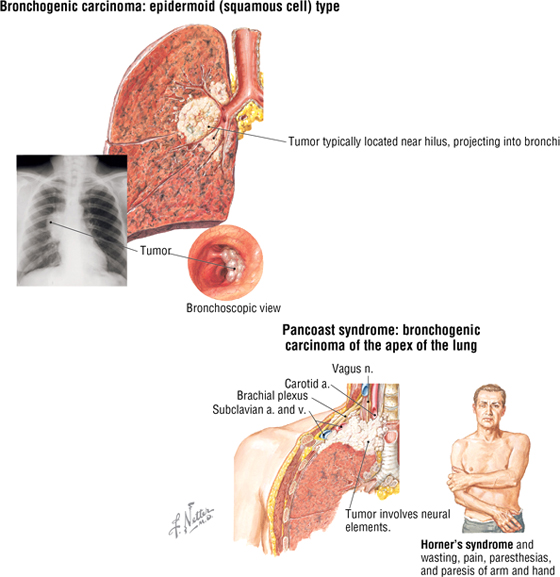
Lung Cancer
Lung cancer is the leading cause of cancer-related death. It arises either from alveolar lining cells of the lung parenchyma or from the epithelium of the tracheobronchial tree. While there are a number of types, squamous cell (bronchiogenic) carcinoma and adenocarcinoma (from intrapulmonary bronchi) are the most common types. Bronchiogenic carcinoma may impinge on adjacent anatomical structures. For example, in Pancoast syndrome, the tumor may spread to involve the sympathetic trunk and compromise the sympathetic tone to the head. This may lead to Horner's syndrome, which is char acterized by the following symptoms:
- Miosis: constricted pupil
- Ptosis: minor drooping of the upper eyelid
- Anhidrosis: lack of sweating
- Flushing: subcutaneous vasodilation
Additionally, the neurovascular components passing into the upper limb (trunks of the brachial plexus and subclavian artery) may be affected, resulting in paresthesia in the neck, head, shoulder, and limb, with 90% affecting areas of ulnar nerve distribution (C8-T1).
C L I N I C A L F O C U S
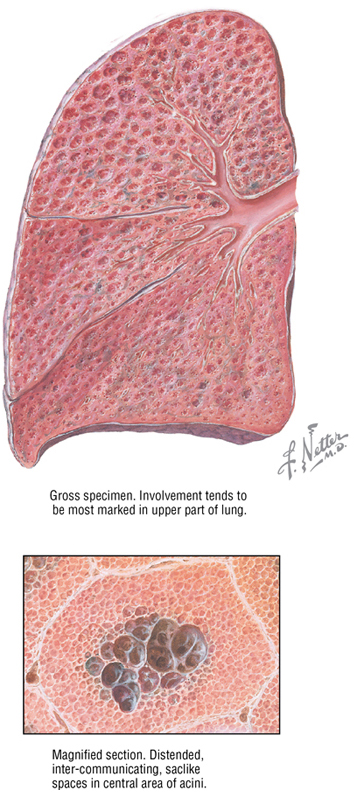
Chronic Obstructive Pulmonary Disease
Chronic obstructive pulmonary disease (COPD) is a broad classification of obstructive lung diseases, the most familiar being chronic bronchitis, asthma, and emphysema. Emphysema is characterized by permanent enlargement of airspaces at and distal to the respiratory bronchioles, with destruction of the bronchiole walls by inflammation. As a result, lung compliance increases because the elastic recoil of the lung decreases, causing collapse of the airways during expiration. This increases the work of expiration as patients try to force air from their diseased lungs. This can lead to a “barrel-chested” appearance owing to hypertrophy of the intercostal muscles. Smoking is a major risk factor for COPD.
C L I N I C A L F O C U S
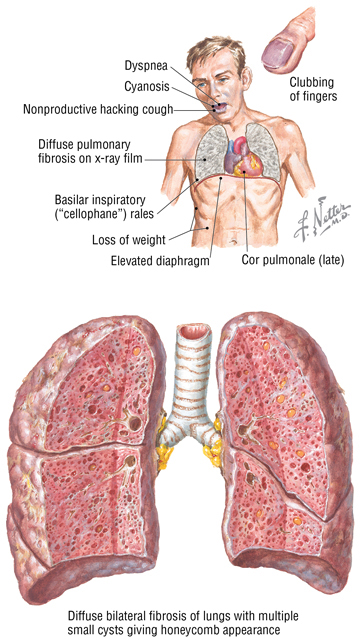
Idiopathic Pulmonary Fibrosis
Idiopathic pulmonary fibrosis is a chronic restrictive lung disease. Chronic restrictive lung diseases comprise approximately 15% of noninfectious lung diseases and include a diverse group of disorders with reduced compliance that cause chronic inflammation, fibrosis, and the need for more pressure to inflate the stiffened lungs. This is a poorly understood interstitial fibrotic disorder, perhaps caused by an injurious environmental or occupational agent, which leads to hypoxemia and cyanosis. Men are affected more often than women, and most patients are diagnosed between the ages of 30 and 50 years.
The Trachea and Bronchi
The trachea is a single midline airway that extends from the cricoid cartilage to its bifurcation at the sternal angle of Louis. It lies anterior to the esophagus and is rigidly supported by 16 to 20 C-shaped cartilaginous rings (Fig. 3-13 and Table 3-8).
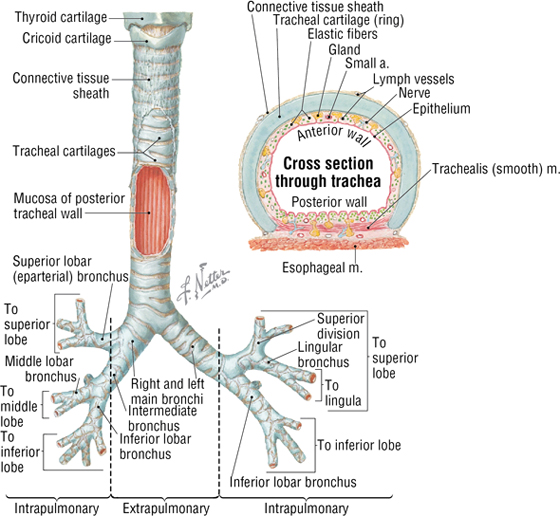
FIGURE 3-13 The Trachea and Bronchi. (Reprinted with permission from Major NM: A Practical Approach to Radiology. Philadelphia, Saunders, 2006.) (Contd.)
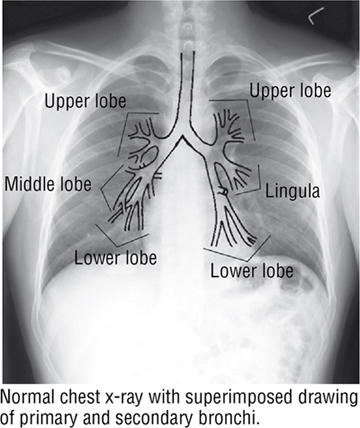
FIGURE 3-13 (Contd.) The Trachea and Bronchi. (Reprinted with permission from Major NM: A Practical Approach to Radiology. Philadelphia, Saunders, 2006.)
|
TABLE 3-8 Features of the Trachea and Bronchi
|
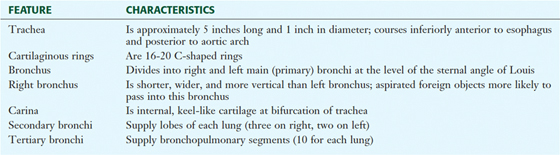 |
The trachea bifurcates inferiorly into a right and a left main bronchus, which enter the hilum of the right and left lungs, respectively, and immediately divide into lobar (secondary) bronchi (see Fig. 3-13). Each lobar bronchus then divides again into tertiary bronchi supplying the 10 bronchopulmonary segments of each lung (sometimes the left lung may have 8 to 10 segments) (see Fig. 3-13 and Tables 3-7 and 3-8). The bronchopulmonary segments are lung segments that are supplied by a tertiary bronchus and a segmental artery of the pulmonary artery that passes to each lung. The bronchi and respiratory airways continue to divide into smaller and smaller passageways until they terminate in alveolar sacs (about 23 divisional generations from the right and left main bronchi). Gas exchange occurs only in these most distal respiratory regions.
C L I N I C A L F O C U S
Aspiration of Foreign Objects
The right main bronchus is shorter, more vertical, and wider than the left main bronchus, so aspirated foreign objects often pass more easily into the right main bronchus and lung.
5. PERICARDIUM AND HEART
The Pericardium
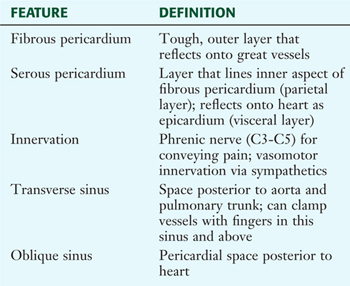
The pericardium and heart lie within the middle mediastinum. The heart is enclosed within a fibroserous pericardial pouch that extends and blends into the adventitia of the great vessels that enter or leave the heart. The pericardium has a fibrous outer layer that is lined internally by a serous layer (parietal serous layer), which then reflects and is continuous with a visceral serous layer that is the outer covering of the heart itself (also known as the epicardium) (Fig. 3-14 and Table 3-9. These two serous layers form a potential space known as the pericardial sac (cavity).
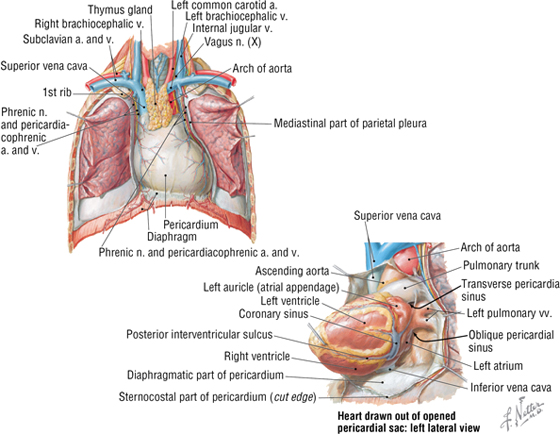
FIGURE 3-14 The Pericardium and Pericardial Sac
|
TABLE 3-9 Features of the Pericardium
|
 |
C L I N I C A L F O C U S
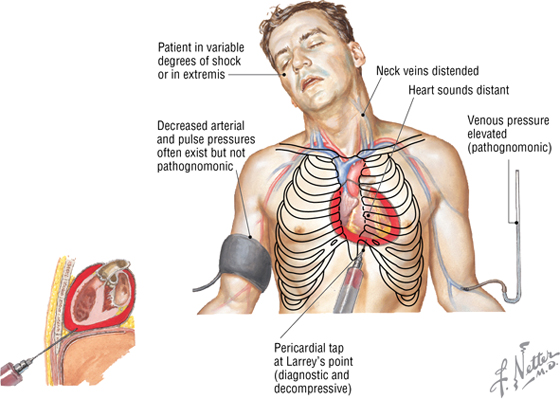
Cardiac Tamponade
Cardiac tamponade can result from fluid accumulation or bleeding into the pericardial sac. Bleeding may be caused by a ruptured aortic aneurysm, a ruptured myocardial infarct, or a penetrating injury that compromises the beating heart and decreases venous return and cardiac output. The fluid can be removed by a pericardial tap (i.e., withdrawn by a needle and syringe).
The External Heart
The heart is essentially two muscular pumps in series. The atria contract in unison, followed by contraction of the two ventricles. The right side of the heart receives the blood from the systemic circulation and pumps it into the pulmonary circulation supplying the lungs. The left side of the heart receives the blood from the pulmonary circulation and pumps it into the systemic circulation, thus perfusing the organs and tissues of the entire body, including the heart itself. In situ, the heart is oriented in the middle mediastinum and has the following descriptive relationships (Fig. 3-15):
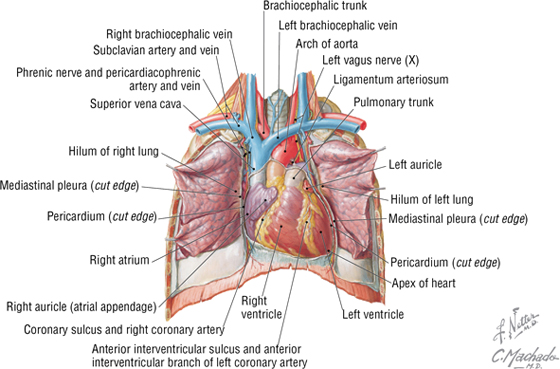
FIGURE 3-15 Anterior In Situ Exposure of the Heart (Contd.)
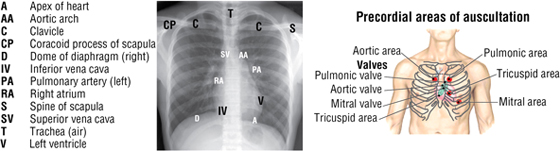
FIGURE 3-15 (Contd.) Anterior In Situ Exposure of the Heart
- Anterior (sternocostal): the right atrium, right ventricle, and part of the left ventricle
- Posterior (base): the left atrium
- Inferior (diaphragmatic): mostly the left ventricle
- Acute angle: the sharp right ventricular margin of the heart
- Obtuse angle: the more rounded left margin of the heart
- Apex: the inferolateral part of the left ventricle at the fourth to fifth intercostal space
The atrioventricular groove (coronary sulcus) separates the two atria from the ventricles and marks the locations of the right coronary artery and the circumflex branch of the left coronary artery. The anterior and posterior interventricular grooves mark the locations of the left anterior descending (anterior interventricular) branch of the left coronary artery and the posterior descending (posterior interventricular) artery, respectively.
The Coronary Arteries and Cardiac Veins
The right and left coronary arteries arise immediately superior to the right and left cusps, respectively, of the aortic semilunar valve (Fig. 3-16). Corresponding great, middle, and small cardiac veins parallel the left anterior descending (LAD) branch, the posterior descending artery (PDA), and the marginal branch of the right coronary artery, with each emptying into the coronary sinus on the posterior aspect of the atrioventricular groove (Table 3-10). Additionally, numerous small cardiac veins (thebesian veins) empty venous blood into the chambers of the heart.
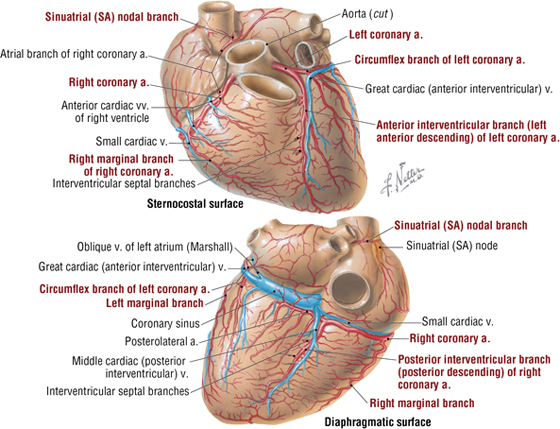
FIGURE 3-16 Coronary Arteries and Cardiac Veins
|
TABLE 3-10 Coronary Arteries and Cardiac Veins
|
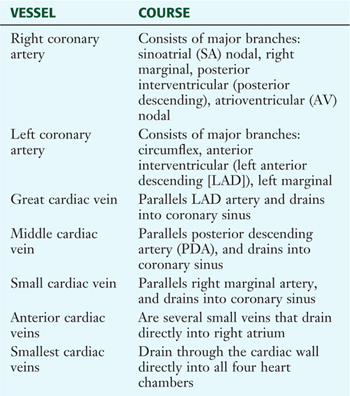 |
C L I N I C A L F O C U S
Dominant Coronary Circulation
About 70% of individuals have a “right dominant” coronary circulation. This means that the right coronary artery gives rise to the PDA and the posterolateral artery, as shown in Figure 3-16. When these two arteries arise from the left coronary artery’s circumflex branch, then the heart is considered “left dominant.” If both the right and left coronary arteries contribute to these two branches, then the circulation is considered “balanced.”
C L I N I C A L F O C U S
Angina Pectoris (The Referred Pain of Myocardial Ischemia)
Angina pectoris is usually described as pressure, discomfort, or a feeling of choking or breathlessness in the left chest or substernal region that radiates to the left shoulder and arm, as well as the neck, jaw and teeth, abdomen, and back. The pain also may radiate to the right arm. This radiating pattern is an example of referred pain, in which visceral afferents from the heart enter the upper thoracic spinal cord along with somatic afferents, both converging in the spinal cord’s dorsal horn. The higher brain center’s interpretation of this visceral pain may initially be confused with somatic sensations from the same spinal cord levels.
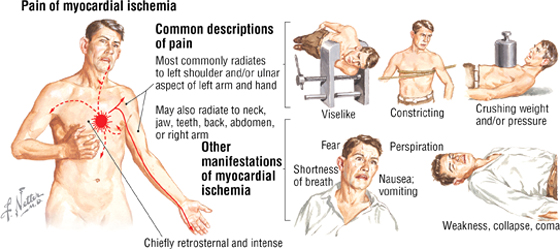
C L I N I C A L F O C U S
Coronary Angiogenesis
Angiogenesis occurs by the budding of new blood vessels. Hypoxia and inflammation are the two major stimuli for new vessel growth. Revascularization of the myocardium after an ischemic episode, bypass surgery, or percutaneous coronary intervention is vital for establishing new vessels (angiogenesis) and for creating anastomoses (interconnections) with existing vessels.
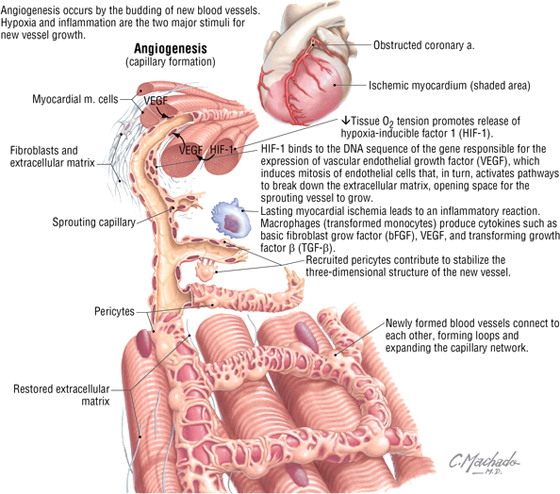
C L I N I C A L F O C U S
Coronary Bypass
A coronary artery bypass graft (CABG), also called “the cabbage procedure,” offers a surgical approach for revascularization. Veins or arteries from elsewhere in the patient’s body are grafted to the coronary arteries to improve blood supply. In a saphenous vein graft, a portion of the great saphenous vein is harvested from the patient’s lower limb; other alternatives are the internal thoracic artery graft and the radial artery graft.
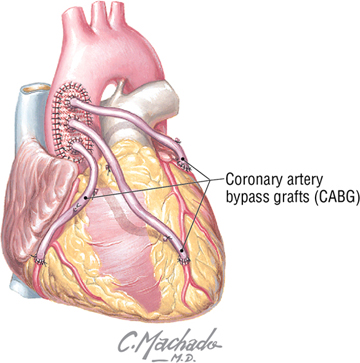
C L I N I C A L F O C U S
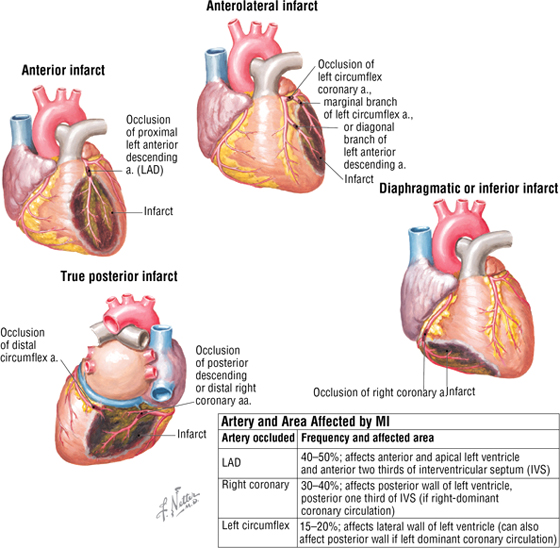
Myocardial Infarction
Myocardial infarction (MI) is a major cause of death. Coronary artery atherosclerosis and thrombosis, the major causes of MI, precipitate local ischemia and necrosis of a defined myocardial area. Necrosis usually occurs approximately 20 to 30 minutes after coronary artery occlusion. Usually MI begins in the subendocardium. This is because the subendocardial region is the most poorly perfused part of the ventricular wall.
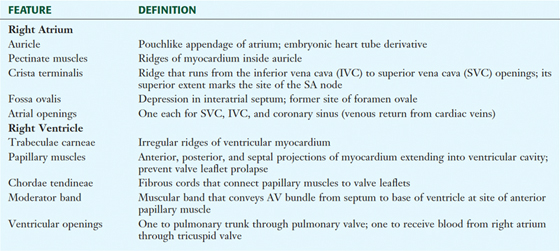
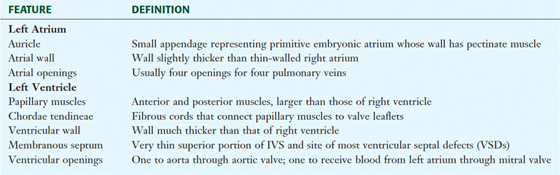
The Chambers of the Heart
The human heart has four chambers, each with unique internal features related to their function (Fig. 3-17 and Table 3-11). The right side of the heart is composed of the right atrium and right ventricle. These chambers receive blood from the systemic circulation and pump it to the pulmonary circulation for gas exchange.
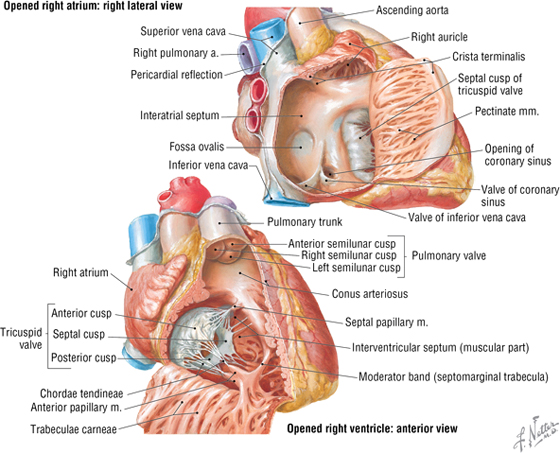
FIGURE 3-17 Right Atrium and Ventricle Opened
|
TABLE 3-11 General Features of the Right Atrium and Ventricle
|
 |
The left atrium and left ventricle receive blood from the pulmonary circulation and pump it to the systemic circulation (Fig. 3-18 and Table 3-12).
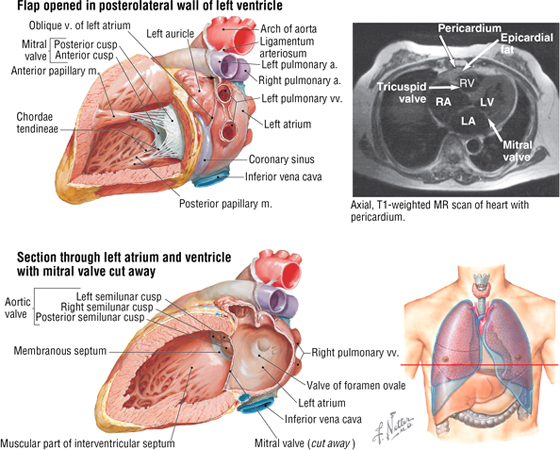
FIGURE 3-18 Left Atrium and Ventricle Opened. (MR reprinted with permission from Kelley LL, Petersen C: Sectional Anatomy for Imaging Professionals. Philadelphia, Mosby, 2007.)
|
TABLE 3-12 General Features of the Left Atrium and Ventricle
|
 |
In both ventricles, the papillary muscles and their chordae tendineae provide a structural mechanism that prevents the atrioventricular valves (tricuspid and mitral) from everting (prolapsing) during ventricular systole. The papillary muscles (actually part of the ventricular muscle) contract as the ventricles contract and pull the valve leaflets into alignment. This prevents them from prolapsing into the atrial chamber above as the pressure in the ventricle increases. During ventricular diastole, the muscle relaxes and the tricuspid and mitral valves open normally to facilitate blood flow into the ventricles.
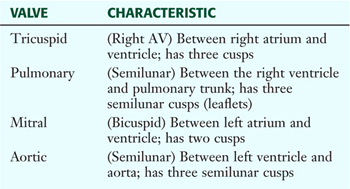
The Cardiac Skeleton and Valves
The heart has four valves that along with the myocardium are attached to fibrous rings of dense collagen that make up the fibrous skeleton of the heart (Fig. 3-19 and Table 3-13). The following heart sounds result from valve closure:
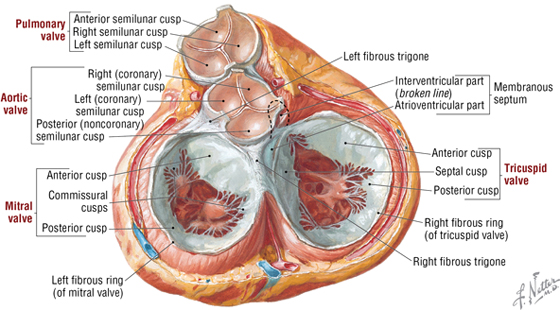
FIGURE 3-19 Heart in Ventricular Diastole Viewed from Above with Atrial Chambers Removed
|
TABLE 3-13 Features of the Heart Valves
|
 |
- First sound (S1): results from the closing of the mitral and tricuspid valves
- Second sound (S2): results from the closing of the aortic and pulmonary valves
C L I N I C A L F O C U S
Cardiac Auscultation
Auscultation of the heart requires not only an understanding of normal and abnormal heart sounds but also knowledge of the optimal location to detect the sounds. Sounds are best heard by auscultating the area where turbulent blood flow radiates (i.e., distal to the valve through which the blood has just passed).
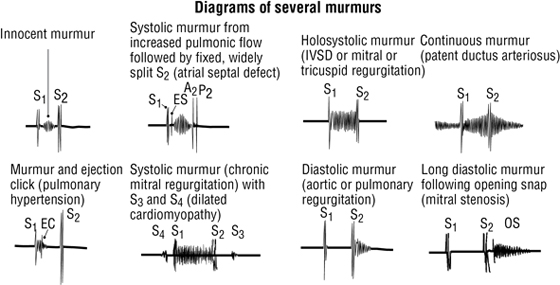
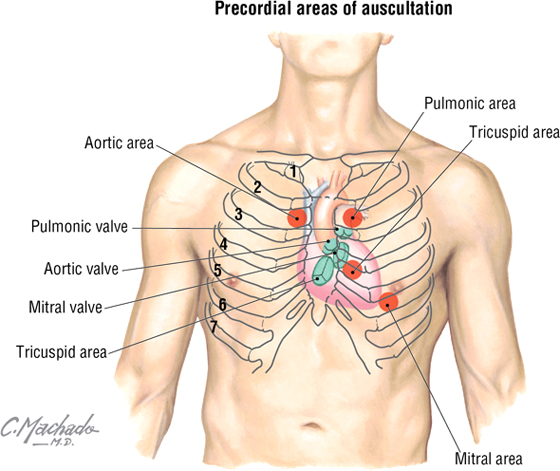
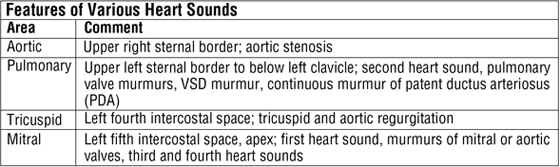
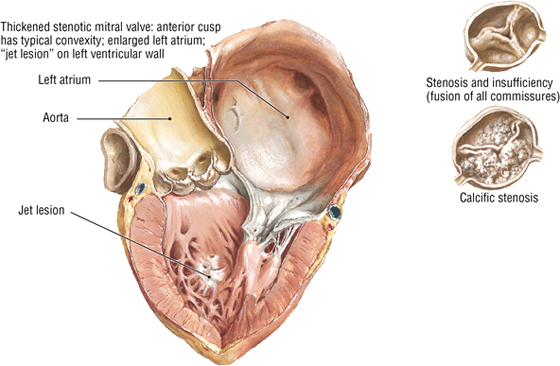
C L I N I C A L F O C U S
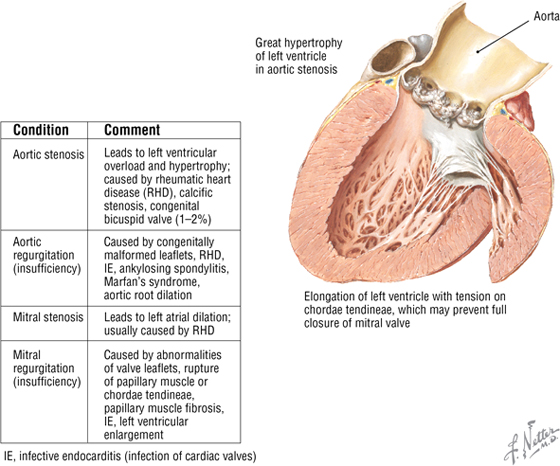
Valvular Heart Disease
Although each valve may be involved in disease, the mitral and aortic valves are the most commonly involved. Major problems include stenosis (narrowing) or insufficiency (compromised valve function, often leading to regurgitation).
The Conduction System of the Heart
The heart’s conduction system is formed by specialized cardiac muscle cells that form nodes, and unidirectional conduction pathways that initiate and coordinate excitation and contraction of the myocardium (Fig 3-20). It includes the following four elements:
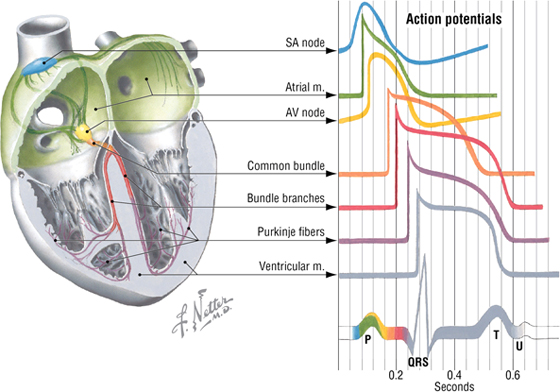
FIGURE 3-20 Conduction System and Electrocardiogram
- SA (sinoatrial) node: the “pacemaker” of the heart, where initiation of action potential occurs; located at the superior end of the crista terminalis near the superior vena cava (SVC) opening
- AV (atrioventricular) node: the area of the heart that receives impulses from the SA node and conveys them to the common atrioventricular bundle (of His); located between the opening of the coronary sinus and the origin of the septal cusp of the tricuspid valve
- Common AV bundle and bundle branches: a collection of specialized heart muscle cells; divides into right and left bundle branches, which course down the interventricular septum
- Subendocardial (Purkinje) system: the ramification of bundle branches in the ventricles of the heart’s conduction system; distributes into a subendocardial network of conduction cells that supply the ventricular walls and papillary muscles
Autonomic Innervation of the Heart
Parasympathetic fibers from the vagus nerve (CN X) course as preganglionic nerves that synapse on postganglionic neurons in the cardiac plexus or within the heart wall itself (Fig. 3-21). Parasympathetic stimulation does the following:
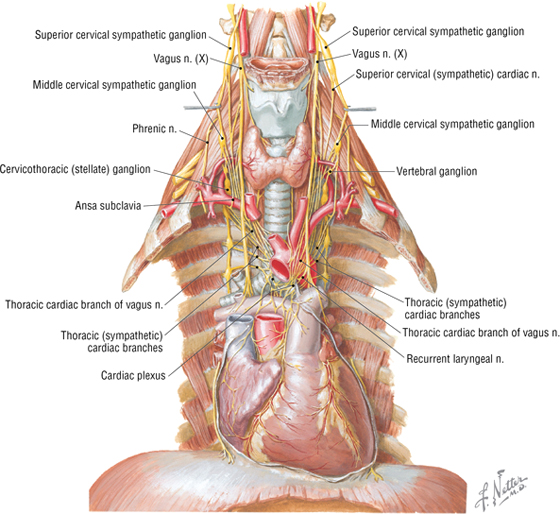
FIGURE 3-21 Autonomic Innervation of the Heart
- Decreases heart rate
- Decreases the force of contraction
- Vasodilates coronary resistance vessels (however, most vagus effects are restricted directly to the SA nodal region)
Sympathetic fibers arise from the upper thoracic cord levels (intermediolateral cell column of T1-T4/T5) and enter the sympathetic trunk (see Fig. 3-21). These preganglionic fibers synapse in the upper cervical and thoracic sympathetic chain ganglia, and then postganglionic fibers pass to the cardiac plexus. Sympathetic stimulation does the following:
- Increases the heart rate
- Increases the force of contraction
- Vasoconstricts the coronary resistance vessels (via alpha adrenoceptors), but this is masked by a powerful and very important metabolic coronary vasodilation (mediated by adenosine release from myocytes); important because coronary arteries must dilate to supply blood to the heart as it increases its workload
Visceral afferents for pain are conveyed back to the upper thoracic spinal cord via the sympathetic fiber pathways; see the clinical focus box, “Angina Pectoris (The Referred Pain of Myocardial Ischemia),” earlier in this chapter.
Visceral afferents mediating cardiopulmonary reflexes (stretch receptors, baroreflexes, and chemoreflexes) are conveyed back to the brainstem via the vagus nerve.
C L I N I C A L F O C U S
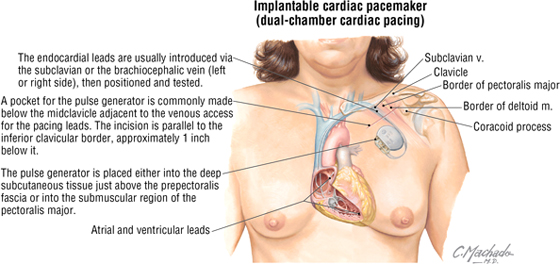
Cardiac Pacemakers
Cardiac pacemakers consist of a pulse generator and one or two endocardial leads with an electrode (passive or active fixation lead). The lead is threaded through the subclavian vein, brachiocephalic vein, SVC, and right atrium and is either embedded there or threaded in the trabeculae carnae of the right ventricular wall. Depending on the device and its programming, the lead may sense as well as pace the cardiac chamber in which it is embedded. In pacing, the electrode impulses generated by the pulse generator depolarize the myocardium and initiate contractions at a prescribed rate.
C L I N I C A L F O C U S
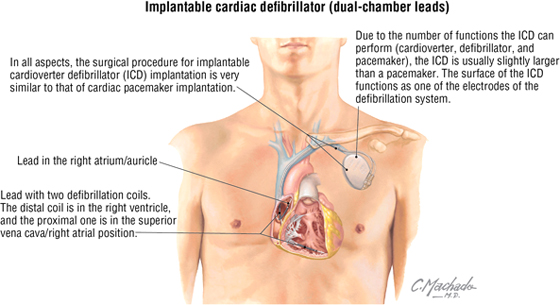
Cardiac Defibrillators
An implantable cardioverter defibrillator is used for survivors of sudden cardiac death, patients with sustained ventricular tachycardia (a dysrhythmia originating from a ventricular focus with a heart rate typically greater than 120 beats/minute), those at high risk for developing ventricular arrhythmias (ischemic dilated cardiomyopathy), and other indications. In addition to sensing arrhythmias and providing defibrillation to stop them, the device can function as a pacemaker for postdefibrillation bradycardia or atrioventricular dissociation.
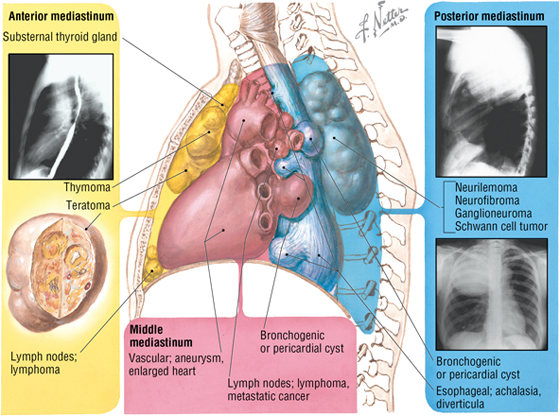
6. THE MEDIASTINUM
The mediastinum (“middle septum”) is the middle region of the thoracic cavity and is divided into a superior and inferior mediastinum by an imaginary horizontal line extending from the sternal angle of Louis to the intervertebral disc between T4 and T5 (Fig. 3-22). The superior mediastinum contains the following (see Fig. 3-22):
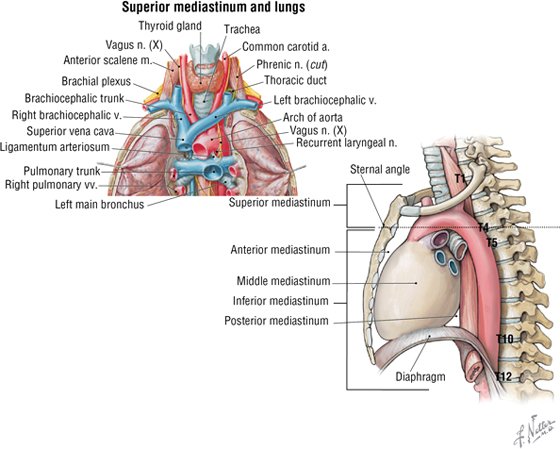
FIGURE 3-22 Mediastinum (Contd.)
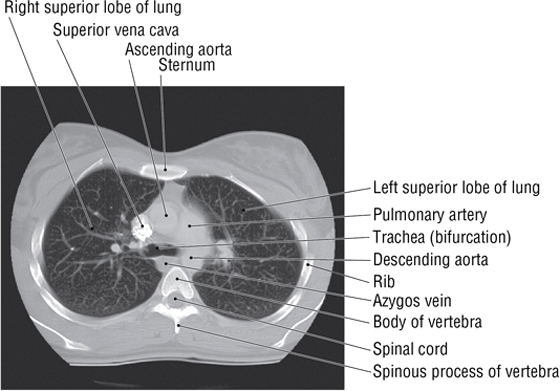
FIGURE 3-22 (Contd.) Mediastinum
- Thymus gland (largely involuted and replaced by fat in older adults)
- Brachiocephalic veins
- SVC
- Aortic arch and its three arterial branches
- Trachea
- Esophagus
- Phrenic and vagus nerves
- Thoracic duct and lymphatics
The inferior mediastinum is further subdivided into the following categories (Fig. 3-23):
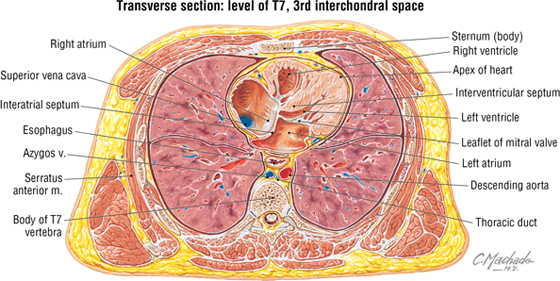
FIGURE 3-23 Inferior Mediastinum
- Anterior mediastinum: the region posterior to the body of the sternum and anterior to the pericardium (substernal region); contains a variable amount of fat
- Middle mediastinum: the region containing the pericardium and heart
- Posterior mediastinum: the region posterior to the heart and anterior to the bodies of the thoracic vertebrae; contains the esophagus and its nerve plexus, thoracic aorta, azygos system of veins, sympathetic trunks, lymphatics, and thoracic duct
The Esophagus and Thoracic Aorta
The esophagus extends from the pharynx (throat) to the stomach and enters the thorax posterior to the trachea. As it descends, it gradually inclines to the left of the median plane, lies anterior to the thoracic aorta (Fig. 3-24), and pierces the diaphragm at the T10 level. The thoracic aorta descends alongside and slightly to the left of the esophagus and gives rise to the following arteries before piercing the diaphragm at the T12 level:
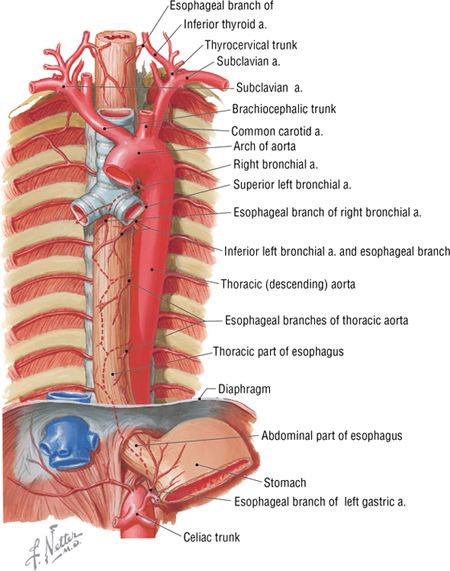
FIGURE 3-24 Esophagus and Thoracic Aorta
- Pericardial arteries: small arteries that branch from the thoracic aorta and supply the posterior pericardium; variable in number
- Bronchial arteries: arteries that supply blood to the lungs; usually one artery to the right and two to the left, but variable in number
- Esophageal arteries: arteries that supply the esophagus; variable in number
- Mediastinal arteries: small branches of the internal thoracic artery that supply the lymph nodes, nerves, and connective tissue of the posterior mediastinum
- Posterior intercostal arteries: paired arteries that supply blood to the lower nine intercostal spaces
- Superior phrenic arteries: small arteries to the superior surface of the diaphragm; anastomose with the musculophrenic and pericardiacophrenic arteries (which arise from the internal thoracic artery)
- Subcostal arteries: paired arteries that lie below the inferior margin of the last rib; anastomose with superior epigastric, lower intercostal, and lumbar arteries.
The Azygos System of Veins
The azygos venous system drains the posterior thorax and forms an important venous conduit between the IVC and SVC (Fig. 3-25). This system represents the deep venous drainage characteristic of veins throughout the body. Its branches, although variable, largely drain the same regions supplied by the thoracic aorta’s branches described earlier in the chapter. The key veins include the azygos vein, with its right ascending lumbar, subcostal, and intercostal tributaries (sometimes the azygos vein also arises from the IVC before the ascending lumbar and subcostal tributaries join it), the hemiazygos vein, and the accessory hemiazygos vein. (If present, it usually begins at the fourth intercostal space.) Ultimately, these veins drain into the azygos vein, which ascends right of the midline to empty into the SVC.
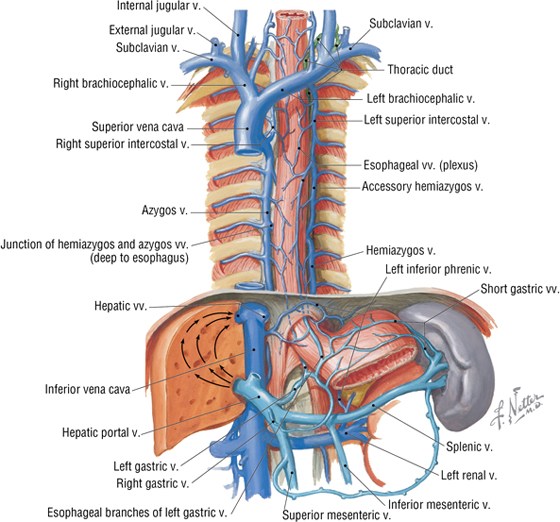
FIGURE 3-25 Azygos System of Veins
Mediastinal Lymphatics
The thoracic lymphatic duct begins in the abdomen at the cisterna chyli, ascends through the posterior mediastinum posterior to the esophagus, crosses to the left of the median plane at approximately the T5-T6 vertebrae, and empties into the venous system at the junction of the left internal jugular and left subclavian veins (Fig. 3-26).
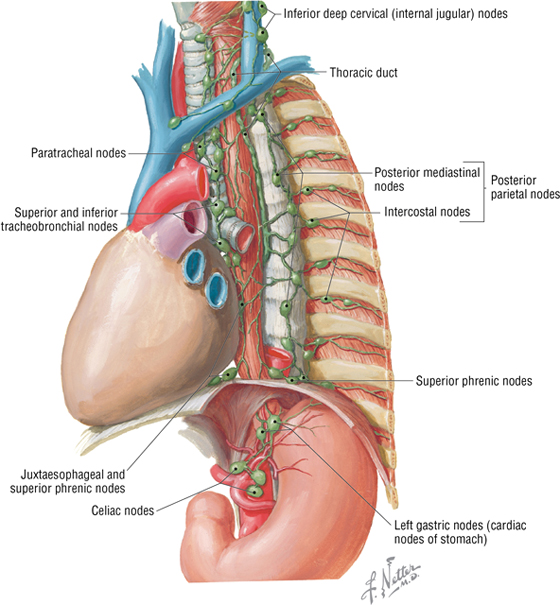
FIGURE 3-26 Mediastinal Lymphatics
C L I N I C A L F O C U S
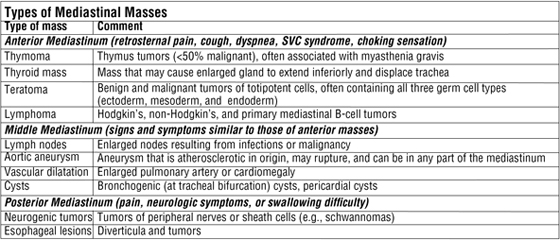
Mediastinal Masses
7. EMBRYOLOGY
The Respiratory System
The airway and lungs begin developing during the fourth week of gestation. Key features of this development include the following (Fig. 3-27):
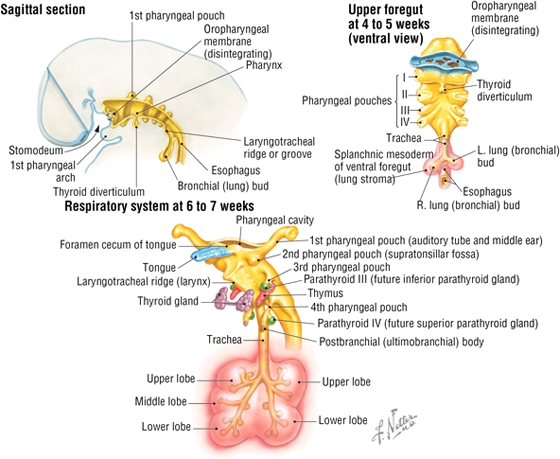
FIGURE 3-27 Embryology of the Respiratory System
- Formation of the laryngotracheal diverticulum from the ventral foregut, just inferior to the last pair of pharyngeal pouches
- Division of the laryngotracheal diverticulum into the left and right lung (bronchial) buds, each with a primary bronchus
- Division of the lung buds to form the definitive lobes of the lungs (three lobes in the right lung, and two lobes in the left lung)
- Formation of segmental bronchi and 10 bronchopulmonary segments in each lung (by weeks 6 to 7)
The airway passages are lined by epithelium derived from the endoderm of the foregut, while mesoderm forms the stroma of each lung. By 6 months of gestation, the alveoli are mature enough for gas exchange, but the production of surfactant, which reduces surface tension and helps prevent alveolar collapse, may not be sufficient to support respiration. A premature infant’s ability to keep its airways open often is the limiting factor if the premature birth occurs before adequate surfactant cells (type II pneumocytes) are present.
Early Embryonic Vasculature
Toward the end of the third week of development, the embryo establishes a primitive vascular system to meet its growing needs for oxygen and nutrients (Fig. 3-28). Blood leaving the embryonic heart enters a series of paired arteries called the aortic arches, which are associated with the pharyngeal arches. The blood then flows from these arches into the single midline aorta (formed by the fusion of two dorsal aortae), coursing along the length of the embryo. Some of the blood enters the vitelline arteries to supply the future bowel (still the yolk sac at this stage), and some passes to the placenta via a pair of umbilical arteries, where gases, nutrients, and metabolic wastes are exchanged.
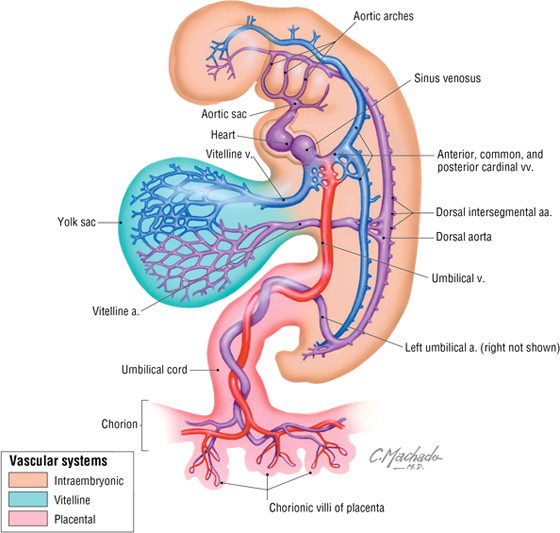
FIGURE 3-28 Early Embryonic Vasculature
Blood returning from the placenta is oxygenated and carries nutrients back to the heart via the single umbilical vein. Blood also returns from two additional sources to the heart via the following veins:
- Vitelline veins: drain blood from yolk sac; will become the portal system draining the gastrointestinal tract through the liver
- Cardinal veins: form SVC and IVC (and azygos system of veins) and their tributaries; will become the caval system of venous return
The Aortic Arches
Blood pumped from the primitive embryonic heart passes into aortic arches that are associated with the pharyngeal arches (Fig. 3-29). The right and left dorsal aortas caudal to the pharyngeal arches fuse to form the single midline aorta, while the aortic arches give rise to the arteries summarized in Table 3-14.
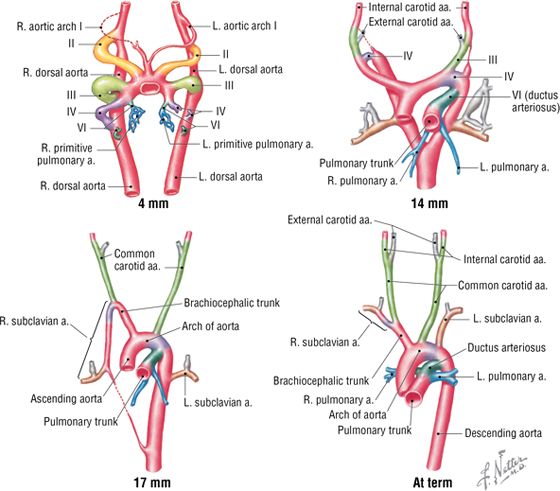
FIGURE 3-29 Sequential Development of Aortic Arch Derivatives
|
TABLE 3-14 Aortic Arch Derivatives
|
 |
Development of the Embryonic Heart Tube and Heart Chambers
The primitive heart begins its development as a single unfolded tube, much like an artery develops (Fig. 3-30). The heart tube receives blood from the embryonic body, which passes through its heart tube segments in the following sequence:
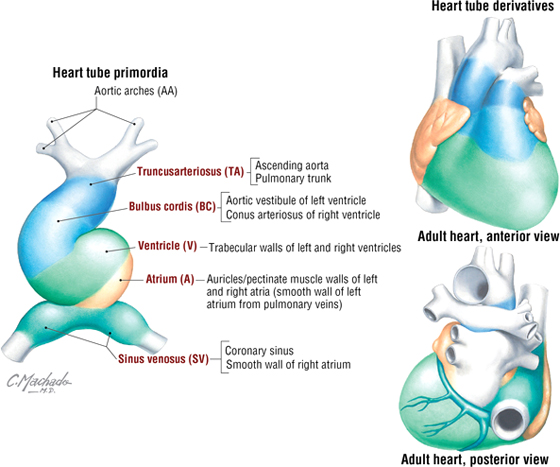
FIGURE 3-30 Primitive Heart Tube Formation
- Sinus venosus: receives all the venous return from the embryonic body to the heart tube
- Atrium: receives blood from the sinus venosus and passes it to the ventricle
- Ventricle: receives atrial blood and passes it to the bulbus cordis
- Bulbus cordis: receives ventricular blood and passes it to the truncus arteriosus
- Truncus arteriosus: receives blood and passes it to the aortic arch system for distribution to the body
This primitive heart tube soon begins to fold upon itself in an “S-bend.” The ventricle folds downward and to the right, and the atrium and sinus venosus fold upward and to the left, thus forming the definitive positions of the heart’s future chambers (atria and ventricles) (see Fig. 3-30 and Table 3-15).
|
TABLE 3-15 Adult Heart Derivatives of the Embryonic Heart Tube
|
 |
*The smooth part of the left atrium is formed by incorporation of parts of the pulmonary veins into the atrial wall. The junction of the trabeculated and smooth parts of the right atrium is called the crista terminalis.
Reprinted with permission from Dudek R: High-yield Embryology: A Collaborative Project of Medical Students and Faculty. Philadelphia, Lippincott William & Wilkins, 2006.
The four chambers of the heart (two atria and two ventricles) are formed by the internal septation of the single atrium and ventricle of the primitive heart tube. Because most of the blood does not perfuse the lungs in utero (the lungs are filled with amniotic fluid and are partially collapsed), blood in the right atrium passes directly to the left atrium via a small opening in the interatrial septum called the foramen ovale. The interatrial septum is formed by the fusion of a septum primum and a septum secundum (develops on the right atrial side of the septum primum) (Fig. 3-31). This fusion occurs after birth when the left atrial pressure exceeds that of the right atrium (blood now passes into the lungs and returns to the left atrium, raising the pressure on the left side) and pushes the two septae together, thus forming the fossa ovalis of the postnatal heart. The ventricular septum forms from the superior growth of the muscular interventricular septum from the base of the heart toward the downward growth of a thin membranous septum from the endocardial cushion (Fig. 3-32). Simultaneously, the bulbus cordis and truncus arteriosus form the outflow tracts of the ventricles, pulmonary artery, and aorta.
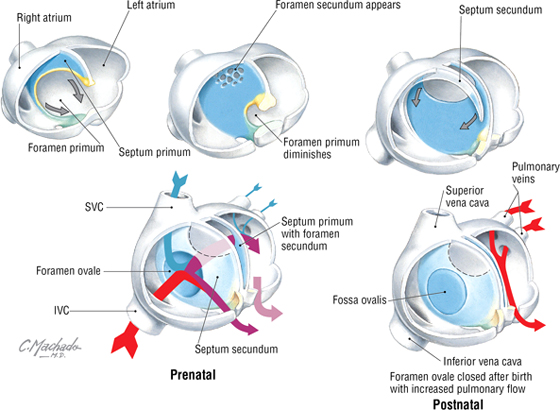
FIGURE 3-31 Atrial Septation
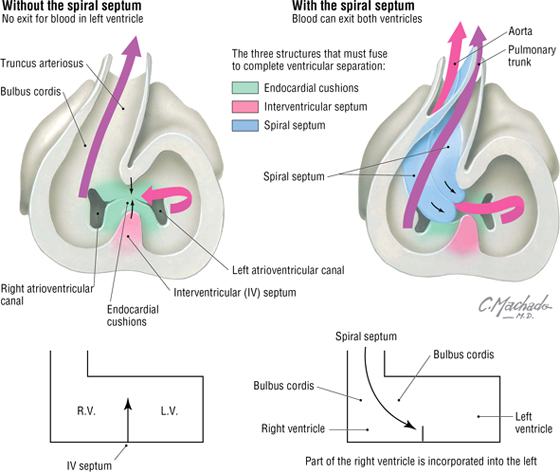
FIGURE 3-32 Ventricular Septation
C L I N I C A L F O C U S
Ventricular Septal Defect
Ventricular septal defect (VSD) is the most common congenital heart defect, representing about 30% of all heart defects. Approximately 80% of cases are perimembranous (occur where the muscular septum and membranous septum of the endocardial cushion should fuse). This results in a left-to-right shunt, which may precipitate congestive heart failure. The repair illustrated in the following figure is via the right atrial approach.
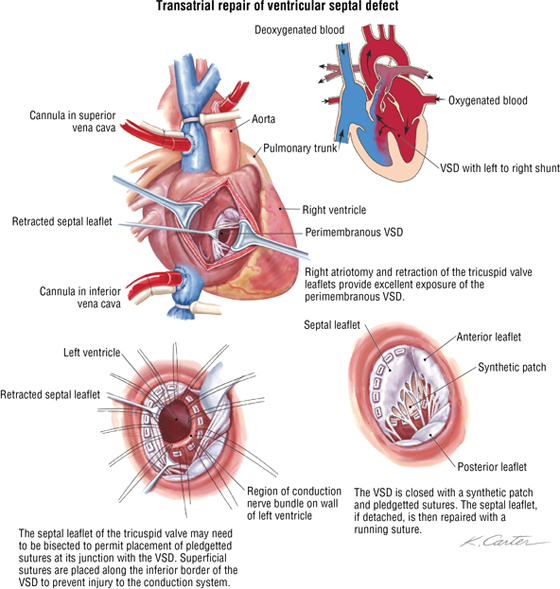
C L I N I C A L F O C U S
Atrial Septal Defect
Atrial septal defects make up approximately 10% to 15% of congenital cardiac anomalies. Repair of these defects (other than fossa ovalis defects) can be achieved surgically by using a relatively new transcatheter approach through the IVC and into the atria, where a septal occluder is deployed and secured. By threading the catheter through the IVC, it is positioned perfectly to pass directly into the defect, which mimics the direction of flow of the fetal blood passing from the IVC through the foramen ovale and into the left atrium.
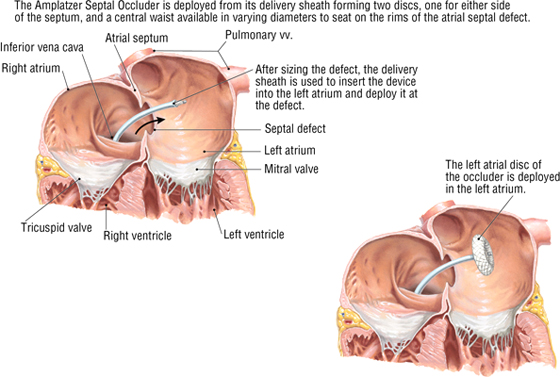
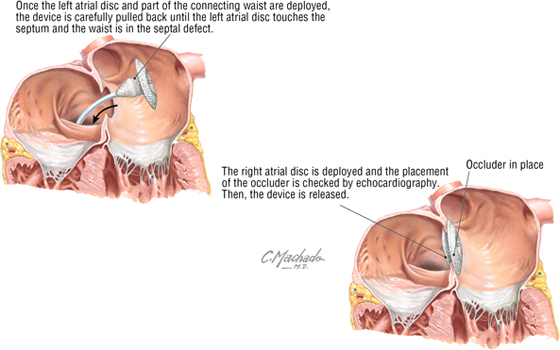
C L I N I C A L F O C U S
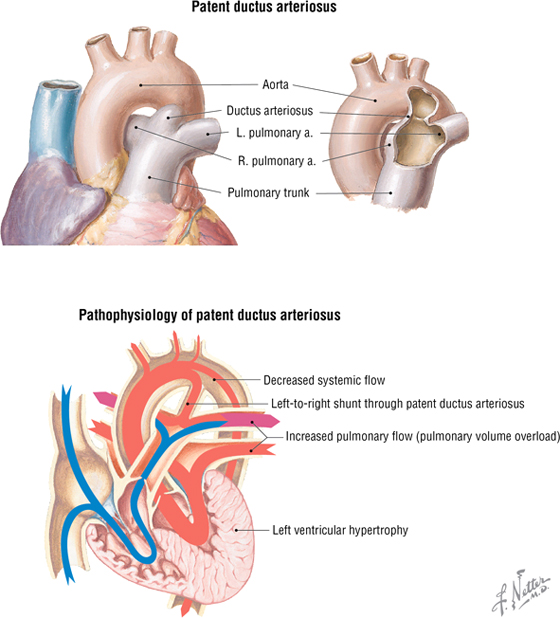
Patent Ductus Arteriosus
Patent ductus arteriosus (PDA) is failure of the ductus arteriosus to close shortly after birth. This results in a shunt of blood from the aorta into the pulmonary trunk, which may lead to congestive heart failure. PDA accounts for approximately 10% of congenital heart defects and can be treated medically (or surgically if necessary). The latter treatment is by direct surgical ligation or via a less invasive catheter-based device that is threaded through the vasculature and positioned to occlude the PDA. Often, children with a PDA may be fine until they become more active and then experience trouble breathing when exercising and demonstrate a failure to thrive. A continuous murmur usually is evident over the left sternal border to just below the clavicle (see the clinical focus box, “Cardiac Auscultation,” earlier in this chapter).
C L I N I C A L F O C U S
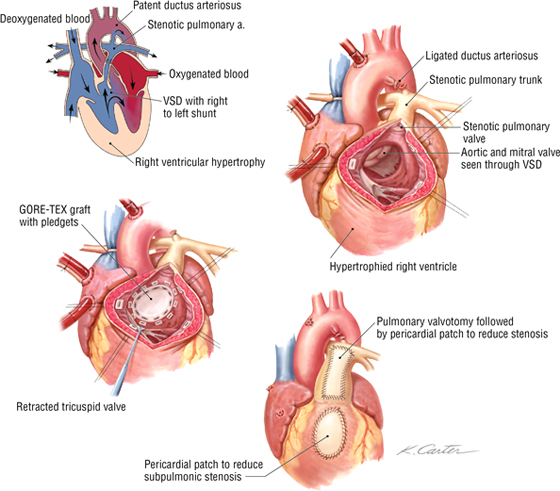
Repair of a Tetralogy of Fallot
Tetralogy of Fallot usually results from a maldevelopment of the spiral septum that normally divides the truncus arteriosus into the pulmonary trunk and aorta. This defect involves the following:
- Pulmonary stenosis or narrowing of the right ventricular outflow
- Overriding (transposed) aorta
- Right ventricular hypertrophy
- Ventricular septal defect (VSD)
Surgical repair is done on cardiopulmonary bypass with an aim to close the VSD and provide unobstructed flow into the pulmonary trunk. The stenotic pulmonary outflow tract is widened by inserting a patch into the wall (pericardial), thus increasing the volume of the subpulmonic stenosis and/or the pulmonary artery stenosis.
Fetal Circulation
The pattern of fetal circulation is one of gas exchange and nutrient/metabolic waste exchange across the placenta with the maternal blood (but not the exchange of blood cells), and distribution of oxygen and nutrient-rich blood to the tissues of the fetus (Fig. 3-33). Various shunts allow fetal blood to largely bypass the liver (not needed for metabolic processing in utero) and lungs (not needed for gas exchange in utero) so that the blood may gain direct access to the left side of the heart and be pumped into the fetal arterial system. At or shortly following birth, these shunts close, resulting in the normal pattern of pulmonary and systemic circulation (see Fig. 3-33).
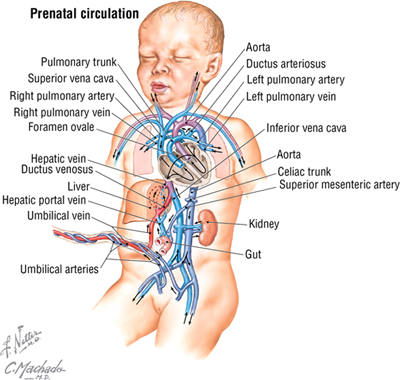
FIGURE 3-33 Fetal Circulation Pattern and Changes at Birth (Contd.)
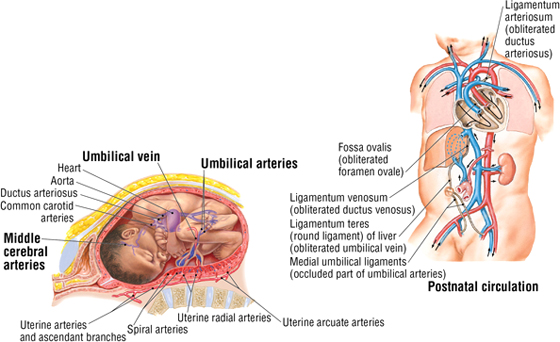
FIGURE 3-33 (Contd.) Fetal Circulation Pattern and Changes at Birth
C L I N I C A L F O C U S
Online Figures
Hemothorax
Common cough
Pneumonia
Cardiovascular disease
Saphenous vein graft
Infective endocarditis
Mitral valve prolapse
Ventricular tachycardia
Chylothorax
Coarctation of aorta
Additional figures available online. See Table of Contents for Instructions for online access.