 CHAPTER 78 Asthma
CHAPTER 78 Asthma
ETIOLOGY
Inflammatory cells (mast cells, eosinophils, T lymphocytes, neutrophils), chemical mediators (histamine, leukotrienes, platelet-activating factor, bradykinin), and chemotactic factors (cytokines, eotaxin) mediate the underlying inflammation found in asthmatic airways. Inflammation contributes to airway hyperresponsiveness, which is the tendency for the airways to constrict in response to allergens, irritants, viral infections, and exercise. It also results in edema, increased mucus production in the lungs, influx of inflammatory cells into the airway, and epithelial cell denudation. Chronic inflammation can lead to airway remodeling, which results from a proliferation of extracellular matrix proteins and vascular hyperplasia and may lead to irreversible structural changes and a progressive loss of pulmonary function.
EPIDEMIOLOGY
Asthma is the most common chronic disease of childhood in industrialized countries, affecting nearly 6 million children younger than 18 years of age in the United States. The 2003 National Health Interview Survey of the Centers for Disease Control and Prevention yielded a lifetime asthma prevalence of 12.5% and current asthma prevalence of 8.5% in children younger than 18 years of age. Between 1996 and 2004, asthma attack rates and asthma prevalence have stabilized when compared with the previous 12 months.
CLINICAL MANIFESTATIONS
Children with asthma have symptoms of coughing, wheezing, shortness of breath or rapid breathing, and chest tightness. The history should elicit the frequency, severity, and factors that worsen the child’s symptoms. Exacerbating factors include viral infections, exposure to allergens and irritants (smoke, strong odors, fumes), exercise, emotions, and change in weather/humidity. Nighttime symptoms are common. Rhinosinusitis, gastroesophageal reflux, and sensitivity to nonsteroidal anti-inflammatory drugs (especially aspirin) can aggravate asthma. Treatment of these conditions may lessen the frequency and severity of the asthma. Obtaining a family history of allergy and asthma is useful.
During acute episodes, the physical examination may reveal tachypnea, tachycardia, cough, wheezing, and a prolonged expiratory phase. Physical findings may be subtle. Classic wheezing may not be prominent if there is minimal air movement. As the attack progresses, cyanosis, diminished air movement, retractions, agitation, inability to speak, tripod sitting position, diaphoresis, and pulsus paradoxus (decrease in blood pressure with inspiration of >15 mm Hg) may be observed. Physical examination may show evidence of other atopic diseases such as eczema or allergic rhinitis.
LABORATORY AND IMAGING STUDIES
Objective measurements of pulmonary function (spirometry) help establish the diagnosis and treatment of asthma. Spirometry is used to monitor response to treatment, assess degree of reversibility with therapeutic intervention, and measure the severity of an asthma exacerbation. Generally, children older than 5 years of age can perform spirometry maneuvers. Spirometry is preferred to peak flow measures in the diagnosis of asthma because of the variability in predicted peak flow reference values. For younger children who cannot perform spirometry maneuver or peak flow, a therapeutic trial of controller medications helps in the diagnosis of asthma.
Allergy skin testing should be included in the evaluation of all children with persistent asthma but not during an exacerbation of wheezing. An allergist offers the special skill of administering and interpreting skin tests to determine immediate hypersensitivity to aeroallergens (airborne allergens such as tree and grass pollens, and dust). Positive skin test results correlate strongly with bronchial allergen provocative challenges. In vitro serum tests, such as radioallergosorbent test (RAST) and enzyme-linked immunosorbent assay, are generally less sensitive in defining clinically pertinent allergens, are more expensive, and require several days for results compared with several minutes for skin testing (see Table 77-4).
A chest radiograph should be performed with the first episode of asthma or with recurrent episodes of undiagnosed cough or wheeze or both to exclude anatomic abnormalities. Repeat chest radiographs are not needed with new episodes unless there is fever, which suggests pneumonia, or localized findings on physical examination.
Two novel forms of monitoring asthma and airway inflammation directly include exhaled nitric oxide analysis and quantitative analysis of expectorated sputum for eosinophilia. Currently, both are used mainly in research settings.
DIFFERENTIAL DIAGNOSIS
Many childhood conditions can cause wheezing and coughing of asthma (Table 78-1) but not all cough and wheeze is asthma. Misdiagnosis delays correcting the underlying cause and exposes children to inappropriate asthma therapy (Table 78-2).
TABLE 78-1 Differential Diagnosis of Cough and Wheeze in Infants and Children
| Upper Respiratory Tract | Middle Respiratory Tract | Lower Respiratory Tract |
|---|---|---|
| Allergic rhinitis | Bronchial stenosis | Asthma |
| Adenoid/tonsillar hypertrophy | Enlarged lymph nodes | Bronchiectasis |
| Foreign body | Epiglottitis | Bronchopulmonary dysplasia |
| Infectious rhinitis | Foreign body | Chlamydia trachomatis |
| Sinusitis | Laryngeal webs | Chronic aspiration |
| Laryngomalacia | Cystic fibrosis | |
| Laryngotracheobronchitis | Foreign body | |
| Mediastinal lymphadenopathy | Gastroesophageal reflux | |
| Pertussis | Hyperventilation syndrome | |
| Toxic inhalation | Obliterative bronchiolitis | |
| Tracheoesophageal fistula | Pulmonary hemosiderosis | |
| Tracheal stenosis | Toxic inhalation, including smoke | |
| Tracheomalacia | Tumor | |
| Tumor | Viral bronchiolitis | |
| Vascular rings | ||
| Vocal cord dysfunction |
From Lemanske RF Jr, Green CG: Asthma in infancy and childhood. In Middleton E Jr, Reed CE, Ellis EF, et al, editors: Allergy: Principles and Practice, 5th ed, St Louis, 1998, Mosby–Year Book, p 878.
Allergic bronchopulmonary aspergillosis is a hypersensitivity type of reaction to antigens of the mold Aspergillus fumigatus. It occurs primarily in patients with steroid-dependent asthma and in children with cystic fibrosis.
TREATMENT
Optimal medical treatment of asthma includes several key components: environmental control; pharmacologic therapy; and patient education, including attainment of self-management skills. Because many children with asthma have coexisting allergies, steps to minimize allergen exposure should be taken (Table 78-3). For all children with asthma, exposures to tobacco and wood smoke and to persons with viral infections should be minimized. Asthma medications can be divided into long-term control medications and quick-relief medications.
TABLE 78-3 Controlling Factors Contributing to Asthma Severity
| Major Indoor Triggers for Asthma | Suggestions for Reducing Exposure |
|---|---|
| Viral upper respiratory tract (RSV, influenza virus) | |
| Tobacco smoke, wood smoke | |
| Dust mites | |
| Animal dander | |
| Cockroach allergens | |
| Indoor mold |
RSV, respiratory syncytial virus.
From American Academy of Allergy, Asthma and Immunology: Pediatric asthma: promoting best practice. Milwaukee, Wisconsin, 1999, American Academy of Allergy, Asthma, and Immunology, p 50.
Long-Term Control Medications
Inhaled Corticosteroids
Inhaled corticosteroids are the most effective anti-inflammatory medications for the treatment of chronic, persistent asthma and are the preferred therapy when initiating long-term control therapy. Early intervention with inhaled corticosteroids reduces morbidity but does not alter the natural history of asthma. Regular use reduces airway hyperreactivity, the need for rescue bronchodilator therapy, risk of hospitalization, and risk of death from asthma. Inhaled corticosteroids are available as an inhalation aerosol, dry powder inhaler (DPI), and nebulizer solution.
The potential risks of inhaled corticosteroids are favorably balanced with their benefits. A reduction in growth velocity may occur with poorly controlled asthma or inhaled corticosteroids use. Low-to-medium dose inhaled corticosteroids may decrease growth velocity, although these effects are small (approximately 1 cm in the first year of treatment), generally not progressive, and may be reversible. Potential growth suppression should be monitored by regularly scheduled height measurements. Inhaled corticosteroids do not have clinically significant adverse effects on hypothalamic-pituitary-adrenal axis function, glucose metabolism, or subcapsular cataracts or glaucoma when used at low-to-medium doses in children. Rinsing the mouth after inhalation and using spacers help lessen the local adverse effects of dysphonia and candidiasis and decrease systemic absorption from the gastrointestinal tract. Inhaled corticosteroids should be titrated to the lowest dose needed to maintain control of a child’s asthma. For children with severe asthma, higher dose inhaled corticosteroids may be needed to minimize the oral corticosteroid dose.
Leukotriene Modifiers
Leukotriene modifiers are oral, daily-use, asthma medications. Leukotrienes, synthesized via the arachidonic acid metabolism cascade, are potent mediators of inflammation and smooth muscle bronchoconstriction. Leukotriene modifiers inhibit these biologic effects in the airway. Two classes of leukotriene modifiers include cysteinyl leukotriene receptor antagonists (zafirlukast and montelukast) and leukotriene synthesis inhibitors (zileuton). The leukotriene receptor antagonists have much wider appeal than zileuton. Zafirlukast is approved for children older than 5 years of age and is given twice daily. Montelukast is dosed once daily at night as 4-mg granules or chewable tablets for children 6 months to 5 years, 5-mg chewable tablets for children 6 to 14 years, and 10-mg tablets for adolescents 15 years of age or older. Pediatric studies show the usefulness of leukotriene modifiers in mild asthma and the attenuation of exercise-induced bronchoconstriction. These agents may be helpful as steroid-sparing agents in patients with asthma that is more difficult to control.
Long-Acting β2-Agonists
Long-acting β2-agonists, formoterol and salmeterol, have twice-daily dosing and relax airway smooth muscle for 12 hours, but they do not have any significant anti-inflammatory effects. Adding a long-acting bronchodilator to inhaled corticosteroid therapy is more beneficial than doubling the dose of inhaled corticosteroids. Formoterol, available in the DPI form, is approved for use in children older than 5 years of age for maintenance asthma therapy and for prevention of exercise-induced asthma. Formoterol has a rapid onset of action similar to albuterol (15 minutes), whereas salmeterol has onset within 30 minutes. Salmeterol is available in the DPI form and is approved for children 4 years of age or older. A budesonide and formoterol combination product (Symbicort) is available as an inhalation aerosol in two doses, varying by the corticosteroid dosage (80 μg or 160 μg). Each of these strengths contains 4.5 μg of formoterol.
A fluticasone/salmeterol combination product is available either as a DPI (Advair Diskus) or as an inhalation aerosol (Advair HFA). The Advair Diskus is available as 100/50, 250/50, or 500/50, with the dose of corticosteroid listed first (μg) and the dose of salmeterol listed second (μg). The dosing of this medication is usually one puff twice daily. The Advair HFA is available as 45/21, 115/21, or 231/21 dosages and is administered as two inhalations twice daily. Because combination agents administer two medications simultaneously, compliance is generally improved.
Theophylline
Theophylline was more widely used previously, but because current management is aimed at inflammatory control, its popularity has declined. It is mildly to moderately effective as a bronchodilator and is considered an alternative, add-on treatment to low- and medium-dose inhaled corticosteroids. Theophylline is available in syrup, tablet, and capsule formulations. Serum levels must be monitored and maintained generally between 5 and 15 μg/mL. Levels can be affected by febrile illnesses; diet; and medications, such as macrolide antibiotics, cimetidine, and oral antifungal agents. Adverse effects associated with elevated theophylline levels include nausea, insomnia, headaches, hyperreactivity, and seizures.
Omalizumab
Omalizumab (Xolair) is a humanized anti-IgE monoclonal antibody that prevents binding of IgE to high-affinity receptors on basophils and mast cells. It is approved for moderate to severe allergic asthma in children 12 years of age and older. Xolair is delivered by subcutaneous injection every 2 to 4 weeks, depending on body weight and pretreatment serum IgE level.
Quick-Relief Medications
Short-Acting β2-Agonists
Short-acting β2-agonists, such as albuterol, levalbuterol, and pirbuterol, are effective bronchodilators that exert their effect by relaxing bronchial smooth muscle within 5 to 10 minutes of administration. They last for 4 to 6 hours. Generally, a short-acting β2-agonist is prescribed for acute symptoms and as prophylaxis before allergen exposure and exercise. The inhaled route is preferred because adverse effects—tremor, prolonged tachycardia, and irritability—are fewer. Overuse of β2-agonists implies inadequate control and that a change in medications may be warranted. The definition of “overuse” depends on the severity of the child’s asthma; use of more than one metered dose inhaler canister per month or more than eight puffs per day suggests poor control.
Anticholinergic Agent
Ipratropium bromide is an anticholinergic bronchodilator that relieves bronchoconstriction, decreases mucus hypersecretion, and counteracts cough-receptor irritability by binding acetylcholine at the muscarinic receptors found in bronchial smooth muscle. It seems to have an additive effect with β2-agonists when used for acute asthma exacerbations. Long-term use of anticholinergic medications is not supported by the literature.
Oral Corticosteroids
Short bursts of oral corticosteroids (3 to 10 days) are administered to children with acute exacerbations. The initial starting dose is 1 to 2 mg/kg/day of prednisone followed by 1 mg/kg/day over the next 2 to 5 days. Oral corticosteroids are available in various liquid or tablet formulations. Prolonged use of oral corticosteroids can result in systemic adverse effects such as hypothalamic-pituitary-adrenal suppression, cushingoid features, weight gain, hypertension, diabetes, cataracts, glaucoma, osteoporosis, and growth suppression. Children with severe asthma may require oral corticosteroids over extended periods. The dose should be tapered as soon as possible to the minimum effective dose, preferably administered on alternate days.
Approach to Therapy
Current therapy is based on the concept that chronic inflammation is a fundamental feature of asthma and that the processes underlying asthma can vary in intensity over time, requiring treatment to be adjusted accordingly. Based on the 2007 National Asthma Education and Prevention Program guidelines, classification of asthma severity is emphasized for initiation of therapy in patients not currently receiving controller medications. Assessing control is emphasized for monitoring and adjusting therapy. A stepwise approach is used for management of infants, young children 0 to 4 years, children 5 to 11 years (Fig. 78-1), youths 12 years or older, and adults (Fig. 78-2). Medication type, amount, and scheduling are determined by the level of asthma severity or asthma control. Therapy is then increased (stepped up) as necessary and decreased (stepped down) when possible. A short-acting bronchodilator should be available for all children with asthma. A child with intermittent asthma has asthma symptoms less than two times per week. To determine if a child is having more persistent asthma, the rule of twos is helpful: daytime symptoms occurring two or more times per week or nighttime awakening two or more times per month implies a need for daily anti-inflammatory medication.
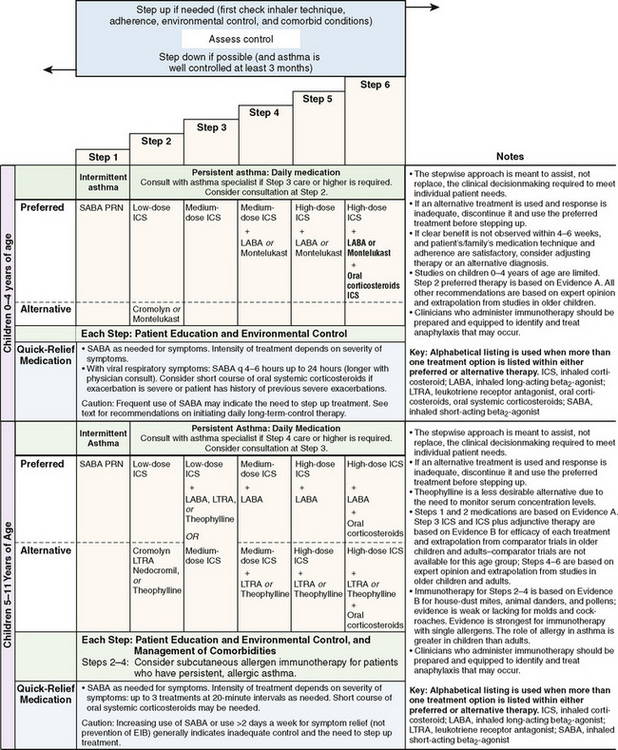
FIGURE 78-1 Stepwise long-term approach for managing asthma in children, 0–4 years of age and 5–11 years of age.
(From National Heart Lung Blood Institute, National Asthma Education and Prevention Program: Expert panel report 3: guidelines for the diagnosis and management of asthma. Summary report 2007, NIH Publication No. 08-5846, Bethesda, MD, 2007, U.S. Department of Health and Human Services, p 42, http://www.nhlbi.nih.gov/guidelines/asthma/asthsumm.pdf)
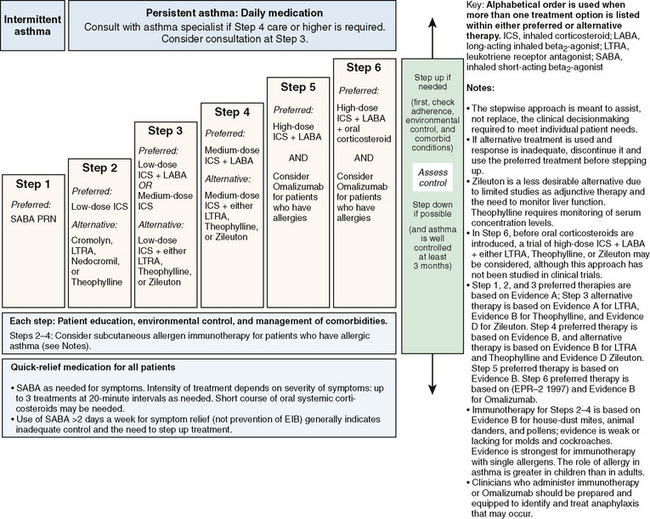
FIGURE 78-2 Stepwise approach for managing asthma in youths 12 years of age or older and adults.
(From National Heart Lung Blood Institute, National Asthma Education and Prevention Program: Expert panel report 3: guidelines for the diagnosis and management of asthma. Summary report 2007, NIH Publication No. 08-5846, Bethesda, MD, 2007, U.S. Department of Health and Human Services, p 45, http://www.nhlbi.nih.gov/guidelines/asthma/asthsumm.pdf)
Inhaled corticosteroids are the preferred initial long-term control therapy for children of all ages (Fig. 78-3). For infants and young children 0 to 4 years of age, daily long-term control therapy is recommended for those who had four or more episodes of wheezing in the previous year that lasted more than 1 day and affected sleep and who have a positive asthma predictive index. For children older than 5 years of age with moderate persistent asthma, combining long-acting bronchodilators with low-to-medium doses of inhaled corticosteroids improves lung function and reduces rescue medication use. For children with severe persistent asthma, a high-dose inhaled corticosteroid and a long-acting bronchodilator are the preferred therapy. The guidelines also recommend that treatment be reevaluated within 2 to 6 weeks of initiating therapy. Once the patient’s asthma is under control, control should be assessed on an ongoing basis every 1 to 6 months. The asthma should be well controlled for at least 3 months before stepping down therapy. Note that asthma management guidelines are based on review of the published evidence, not solely on the age recommendations and dosages approved by the U.S. Food and Drug Administration.
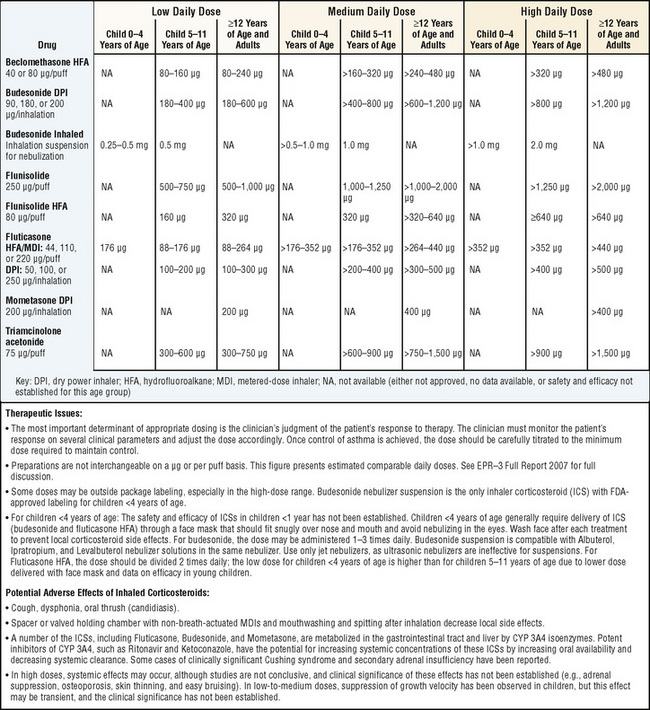
FIGURE 78-3 Estimated comparative daily dosages for inhaled corticosteroids.
(Adapted from National Heart Lung Blood Institute, National Asthma Education and Prevention Program: Expert panel report 3: guidelines for the diagnosis and management of asthma. Summary report 2007, NIH Publication No. 08-5846, Bethesda, MD, 2007, U.S. Department of Health and Human Services, p 49, http://www.nhlbi.nih.gov/guidelines/asthma/asthsumm.pdf)
COMPLICATIONS
Most asthma exacerbations can be managed at home successfully. Status asthmaticus is an acute exacerbation of asthma that does not respond adequately to therapeutic measures and may require hospitalization. Exacerbations may progress over several days or occur suddenly and can range in severity from mild to life-threatening. Significant respiratory distress, dyspnea, wheezing, cough, and a decrease in peak expiratory flow rates characterize deterioration in asthma control. During severe episodes of wheezing, pulse oximetry is helpful in monitoring oxygenation. In status asthmaticus, arterial blood gases may be necessary for measurement of ventilation. As airway obstruction worsens and chest compliance decreases, carbon dioxide retention can occur. In the face of tachypnea, a normal PCO2 (40 mm Hg) indicates impending respiratory arrest.
First-line management of asthma exacerbations includes supplemental oxygen, repetitive or continuous administration of short-acting bronchodilators, and oral or intravenous corticosteroids (Fig. 78-4). Administration of anticholinergic agents (ipratropium) with bronchodilators decreases rates of hospitalization and duration of time in the emergency department. Early administration of oral corticosteroids is important in treating the underlying inflammation. The use of intravenous magnesium sulfate is gaining use in the emergency department in children with severe exacerbations and in children with moderate exacerbations who have clinical deterioration despite treatment with β2-agonists, ipratropium, and systemic glucocorticoids. The typical dose is 25 to 75 mg/kg (maximum 2.0 g) intravenously administered over 20 minutes. Epinephrine (intramuscular) or terbutaline (subcutaneous) is rarely used except when severe asthma is associated with anaphylaxis or unresponsive to continuous administration of short-acting bronchodilators.
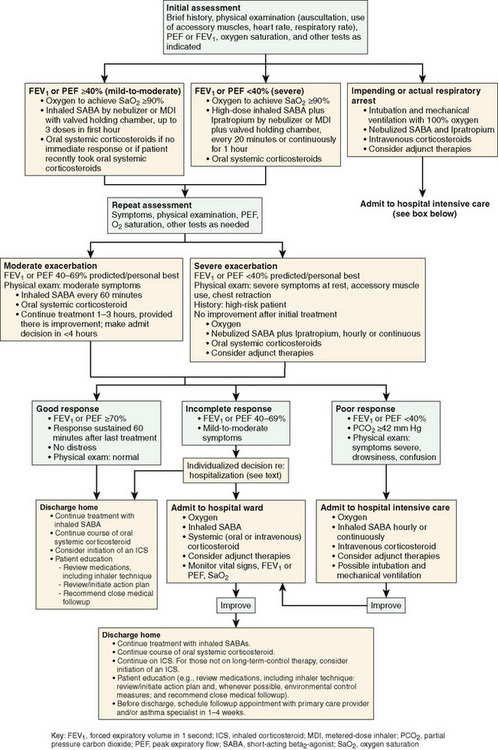
FIGURE 78-4 Management of asthma exacerbations: emergency department and hospital-based care.
(From National Heart Lung Blood Institute, National Asthma Education and Prevention Program: Expert panel report 3: guidelines for the diagnosis and management of asthma. Summary report 2007, NIH Publication No. 08-5846, Bethesda, MD, 2007, U.S. Department of Health and Human Services, p 55, http://www.nhlbi.nih.gov/guidelines/asthma/asthsumm.pdf)
PROGNOSIS
For some children, symptoms of wheezing with respiratory infections subside in the preschool years, whereas other children have more persistent asthma symptoms. Prognostic indicators for children younger than 3 years of age who are at risk for asthma include eczema; parental asthma; or two of the following: allergic rhinitis, wheezing with a cold, or eosinophilia of greater than 4%. The strongest predictor for wheezing continuing into persistent asthma is atopy (Table 78-4).
TABLE 78-4 Risk Factors for Persistent Asthma
From Liu A, Martinez FD, Taussig LM: Natural history of allergic diseases and asthma. In Leung DYM, Sampson HA, Geha RS, Szefler SJ, editors: Pediatric Allergy: Principles and Practice. St Louis, 2003, Mosby, p 15.
PREVENTION
Education plays an important role in helping patients and their families adhere to the prescribed therapy and needs to begin at the time of diagnosis. Successful education involves teaching basic asthma facts, explaining the role of medications, teaching environmental control measures, and improving patient skills in the use of spacer devices for metered dose inhalers and peak flow monitoring. Families should have an asthma management plan (Fig. 78-5) for daily care and for exacerbations.
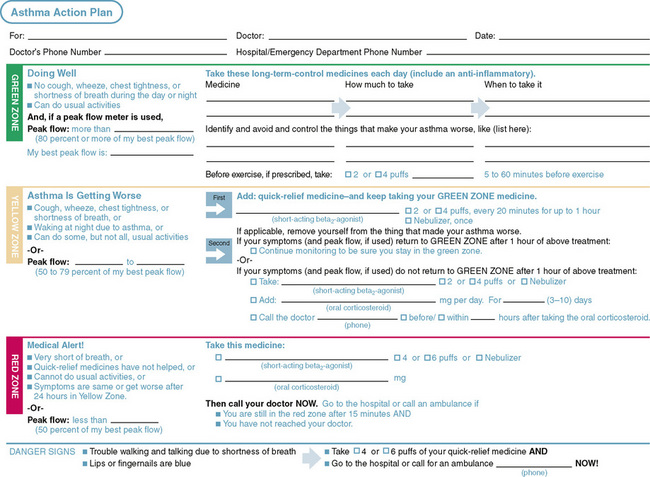
FIGURE 78-5 Asthma self-management guideline.
(From National Heart Lung Blood Institute, National Asthma Education and Prevention Program: Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Summary Report 2007. NIH Publication No. 05-5251. Bethesda, MD, 2007, U.S. Department of Health and Human Services, p 119. http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf)
Peak flow monitoring is a self-assessment tool that is helpful for children older than 5 years of age. It is advisable for children who are poor perceivers of airway obstruction, have moderate to severe asthma, or have a history of severe exacerbations. Peak flow monitoring also can be useful in children who are still learning to recognize asthma symptoms.
To use a peak flow meter, a child should be standing with the indicator placed at the bottom of the scale. The child must inhale deeply, place the device in the mouth, bite down on the mouthpiece, seal the lips around the mouthpiece, and blow out forcefully and rapidly. The indicator moves up the numeric scale. The peak expiratory flow rate (PEFR) is the highest number achieved. The test is repeated three times to obtain the best possible effort. Peak flow meters are available as low range (≤300 L/sec) and high range (≤700 L/sec). It is important to provide the appropriate range meter so that accurate measurements can be obtained, and children do not become discouraged because their exhalations barely move the indicator.
A child’s personal best is the highest PEFR achieved over a 2-week period when stable. Based on the child’s personal best, a written action plan can be established, which is divided into three zones, similar to a stoplight. The green zone indicates a PEFR 80% to 100% of the child’s personal best value. In this zone, the child is likely asymptomatic and should continue with medications as usual. The yellow zone indicates a PEFR 50% to 80% of the child’s personal best value, which generally coincides with more asthma symptoms. Rescue medications, such as albuterol, are added, and a telephone call to the physician may be warranted if the peak flows do not return to the green zone within the next 24 to 48 hours or if asthma symptoms are deteriorating. The red zone indicates a PEFR below 50% and is a medical emergency. Rescue medication should be taken immediately. If the PEFR remains in the red zone or the child has significant airway compromise, a phone call to the physician or emergency care is needed.
A key message for families is that children with asthma should be seen not only when they are ill but also when they are healthy. Regular office visits allow the health care team to review adherence to medication and control measures and to determine if doses of medications need adjustment.
