CHAPTER 77 Assessment
CHAPTER 77 Assessment
Atopy is a result of a complex interaction between multiple genes and environmental factors. It implies specific IgE-mediated diseases, including allergic rhinitis, asthma, and atopic dermatitis. An allergen is an antigen that triggers an IgE response in genetically predisposed individuals.
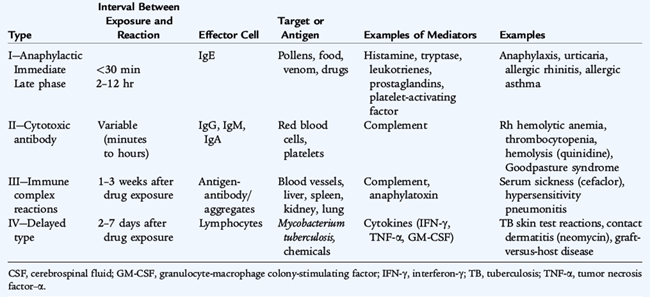
Hypersensitivity disorders of the immune system are classified into four groups based on the mechanism that leads to tissue inflammation (Table 77-1). Type I reactions are triggered by the binding of antigen to high-affinity IgE receptors on the surface of tissue mast cells, circulating basophils, or both, causing the release of preformed chemical mediators, such as histamine and tryptase, and newly generated mediators, such as leukotrienes, prostaglandins, and platelet-activating factor, These mediators contribute to the development of allergic symptoms, with anaphylaxis as the most profound symptom. Several hours after the initial response, a late-phase reaction may develop with an influx of other inflammatory cells such as basophils, eosinophils, monocytes, lymphocytes, and neutrophils, and their inflammatory mediators. Recruitment of these cells leads to more persistent and chronic symptoms.
Type II (antibody cytotoxicity) reactions involve IgM, IgG, or IgA antibodies binding to the cell surface and activating the entire complement pathway resulting in lysis of the cell or release of anaphylatoxins, such as C3a, C4a, and C5a (see Chapter 75). These anaphylatoxins trigger mast cell degranulation, resulting in inflammatory mediator release. The target can be cell surface membrane antigens, such as red blood cells (hemolytic anemia); platelet cell surface molecules (thrombocytopenia); basement membrane molecules in the kidney (Goodpasture syndrome); the alpha chain of the acetylcholine receptor at the neuromuscular junction (myasthenia gravis); and thyroid-stimulating hormone receptor on thyroid cells (Graves’ disease).
Type III (immune complex) reactions involve the formation of antigen-antibody or immune complexes that enter into the circulation and are deposited in tissues such as blood vessels and filtering organs (liver, spleen, kidney). These complexes initiate tissue injury by activating the complement cascade and recruiting neutrophils that release their toxic mediators. Local reactions caused by the injection of antigen into tissue are called Arthus reactions. Administration of large amounts of antigen leads to serum sickness, a classic example of a type III reaction. Other type III–mediated reactions include hypersensitivity pneumonitis and some vasculitic syndromes, such as Henoch-Schönlein purpura.
Type IV (cellular immune–mediated or delayed-hypersensitivity) reactions involve recognition of antigen by sensitized T cells. Antigen-presenting cells form peptides that are expressed on the cell surface in association with major histocompatibility complex class II molecules. Memory T cells recognize the antigen peptide/major histocompatibility complex class II complexes. Cytokines, such as interferon-γ, tumor necrosis factor-α, and granulocyte-macrophage colony-stimulating factor, are secreted from this interaction, which activates and attracts tissue macrophages. Contact allergies (nickel, poison ivy, topical medications) and immunity to tuberculosis are type IV reactions.
HISTORY
A family history of allergic disease is often present in affected patients. Multiple genes predispose to atopy. If one parent has allergies, the risk that a child will develop allergic disease is 25%. If both parents have allergies, the risk increases to 50% to 70%. Similar allergic diseases tend to occur in families.
PHYSICAL EXAMINATION
Children with allergic rhinitis exhibit frequent nasal itching and rubbing of the nose with the palm of the hand, the allergic salute, which can lead to a transverse nasal crease found across the lower bridge of the nose. Allergic shiners, blue-gray to purple discoloration below the lower eyelids that is attributed to venous congestion, are often present in children along with swollen eyelids or conjunctival injection. Dermatologic findings of atopy include hyperlinearity of the palms and soles, white dermatographism, pityriasis alba, prominent creases under the lower eyelids (Dennie-Morgan folds or Dennie lines), and keratosis pilaris (asymptomatic horny follicular papules on the extensor surfaces of the arms).
COMMON MANIFESTATIONS
Cutaneous manifestations are most common and range from generalized xerosis (dry skin) to urticaria to the pruritic, erythematous papules and vesicles of atopic dermatitis. There may be involvement of the upper respiratory tract with allergic rhinitis and the lower respiratory tract with asthma. Allergic disease may involve only the skin or the nose, eyes, lungs, and gastrointestinal tract alone or in combination. It is distinguished by environmental exposure to an inciting trigger and usually a history of previous similar disease or development of symptoms after a suspected trigger. Many patients have more than one allergic symptom.
INITIAL DIAGNOSTIC EVALUATION
Diagnosis begins with a thorough history with description of all symptoms, exposure to common allergens, and responses to previous therapies. In vivo skin testing and in vitro serum testing are crucial to accurate diagnosis.
SCREENING TESTS
Atopy is characterized by elevated levels of IgE (Table 77-2) and eosinophilia (3% to 10% of white blood cells or an absolute eosinophil count of >250 eosinophils/mm3) with a predominance of Th2 cytokines, including interleukin (IL)-4, IL-5, and IL-13. Extreme eosinophilia suggests a nonallergic disorder such as infections with tissue-invasive parasites, drug reactions, or malignancies (Table 77-3). A classic example of a type IV reaction is the tuberculin skin test. A small amount of purified protein derivative from Mycobacterium tuberculosis is injected intradermally (see Chapter 124). In a previously sensitized individual, a type IV inflammatory reaction develops over the next 24 to 72 hours.
TABLE 77-2 Disorders Associated with Elevated Serum Immunoglobulin E
Allergic disease
Atopic dermatitis (eczema)
Tissue-invasive helminthic infections
Hyperimmunoglobulin-E syndrome
Allergic bronchopulmonary aspergillosis
Wiskott-Aldrich syndrome
Bone marrow transplantation
Hodgkin disease
Bullous pemphigoid
Idiopathic nephrotic syndrome
TABLE 77-3 Disorders Associated with Eosinophilia
ALLERGIC DISEASE
GASTROINTESTINAL
INFECTIOUS
NEOPLASTIC
RESPIRATORY
SYSTEMIC
IATROGENIC
There are two methods for identifying allergen-specific IgE: in vivo skin testing and in vitro serum testing (Table 77-4). In vivo skin testing introduces allergen into the skin via a prick/puncture or intradermal injection. The allergen diffuses through the skin to interact with IgE that is bound to mast cells. Cross-linking of IgE causes mast cell degranulation, which results in histamine release; this prompts the development of a central wheal and erythematous flare. The wheal and flare are measured 15 to 20 minutes after the allergen has been placed. Properly performed skin tests are the best available method for detecting the presence of allergen-specific IgE.
TABLE 77-4 Comparison of In Vivo Skin Tests and In Vitro Serum IgE Antibody Immunoassay in Allergic Diagnosis
| In Vivo—Skin Test | In Vitro—Serum Immunoassay |
| Less expensive | No patient risk |
| Greater sensitivity | Patient/physician convenience |
| Wide allergen selection | Not suppressed by antihistamines |
| Results available immediately | Preferable to skin testing for Dermatographism Widespread dermatitis Uncooperative children |
From Skoner DP: Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol 108, S2–S8, 2001.
In vitro serum testing, such as the radioallergosorbent test (RAST) and enzyme-linked immunosorbent assay, measures levels of antigen-specific IgE. The CAP-RAST method uses a newer type of solid phase and shows higher sensitivity, specificity, and reproducibility. These tests are indicated for patients who have dermatographism or extensive dermatitis; who cannot discontinue medications, such as antihistamines, that interfere with test results; who are very allergic by history, where anaphylaxis is a possible risk; or who are noncompliant for skin testing. The presence of specific IgE antibodies alone is not sufficient for the diagnosis of allergic diseases. Diagnosis must be based on the physician’s assessment of the entire clinical picture, including the history and physical examination, the presence of specific IgE antibodies, and the correlation of symptoms to IgE-mediated inflammation.
DIAGNOSTIC IMAGING
Diagnostic imaging has a limited role in the evaluation of allergic disease. Chest radiography is helpful with the differential diagnosis of asthma. Sinus radiography and computed tomography may be useful, but when these images are abnormal, they do not distinguish allergic disease from nonallergic disease.